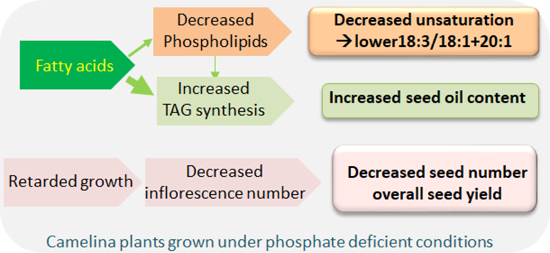
Abstract
Camelina (Camelina sativa) is an emerging industrial oilseed crop because of its potential for double cropping, fallow year production, growth on marginal lands, and multiple uses of seed oils and meals. To realize the potential for sustainable production of camelina, a better understanding of how camelina seed oil production and composition respond to low input environments is desired. Phosphorus (P) is one of the least available essential macronutrients to plants with finite worldwide supply. This study investigated seed oil production and lipid composition of camelina in field settings and under greenhouse conditions in response to P deficiency. Lipidomic profiling reveals that P deficiency in field settings triggered extensive leaf lipid remodeling that decreased the ratio of phospholipids to non-P-containing galactolipids from 30% to 5% under P sufficient to deficient conditions. P deficiency increased seed oil content per seed weight by approximately 25% and 20% in field and greenhouse settings, respectively. In addition, P deficiency altered seed fatty acid composition, with increases in monounsaturated 18:1 and 20:1 and decreases in polyunsaturated 18:3. Total seed production was decreased by 10- to 15-fold under P deficiency and the decrease resulted from reduced seed numbers without affecting seed weight. The results from field and greenhouse conditions indicate that P deficiency increases seed oil content, alters fatty acid composition, and decreases greatly seed production, suggesting that achieving a high yield and quality of camelina seed oil is positively linked to P status of soil.
Keywords: Camelila sativa, phosphate deficiency, fatty acid composition, lipid remodeling, seed oil content
Graphical abstract
1. INTRODUCTION
Camelina (Camelina sativa) was an ancient oilseed crop, and its cultivation was later replaced by more productive Canola. The interest in camelina as an industrial and bioenergy crop has increased in recent years because of its potential for fallow year production, growth on marginal lands, and versatile applications of the oil seeds (Gebauer et al., 2006; Ruiz-Lopez et al., 2014; Neupane et al., 2022). Camelina oil is favored as an alternative feedstock for non-food applications, such as soaps, varnishes, and biodiesel production due to its low-cost, high-energy content, and fatty acid composition (Moser and Vaughn, 2010). The oil content of camelina seeds ranges from 30% to 46%, and the majority (80–90%) of fatty acids of the oil is unsaturated, which includes a high level of α-linolenic acid (30–40% of total oil content), an omega-3 fatty acid valuable for human and animal nutrition (Moser, 2014; Campbell et al., 2013). Camelina can be grown with few input costs and under marginal conditions further enhances interest in camelina production as an industrial oil crop. However, how nutrient limitations in fields affect seed oil content and fatty composition, the critical value traits of camelina, remains to be characterized.
Phosphorus (P) is one of the least available essential macronutrients to plants, and it is not a renewable resource due to the finite supply (Gilbert, 2009; López-Arredondo et al., 2013; Heuer et al., 2017). Approximately 70% of global cultivated land is deficient in available P (López-Arredondo et al., 2014). The widespread application of P fertilizers not only increases crop production costs, but also aggravates the environmental burden and accelerates the depletion of finite P reserves (Cordell et al., 2009; Scholz et al., 2013; Hartley et al., 2013). Camelina was reported to produce moderate yields on poor soils (Putnam et al., 1993; Wojtkowiak et al., 2009), and field tests suggested that camelina responded to P fertilizer when soil P levels were 12 ppm (part per million) or less under Montana field conditions (Jackson, 2008; McVay and Lamb, 2008). However, the effect of P deficiency on camelina seed oil production remains largely unknown.
Phospholipids contain a significant pool of cellular P, constituting approximately 1/3 of organic P in plants (Gaude et al., 2008; Tjellström et al., 2008). Under P shortage, one of the overt metabolic changes in plants is membrane lipid remodeling by which phospholipids decrease to allow limited P available for other vital cellular needs, while non-P-containing lipids increase to maintain the membrane function (Härtel et al., 2000; Andersson et al., 2003; Li et al., 2006; Moellering and Benning, 2011; Okazaki et al., 2013; Su et al., 2018). Specifically, the common phospholipids, such as phosphatidylcholine (PC), decrease whereas non-phospholipids, such as digalactosyldiacylglycerol (DGDG), increase (Härtel et al., 2000; Li et al., 2006; Moellering and Benning, 2011; Okazaki et al., 2013). Fatty acids in plants are synthesized in chloroplasts, exported to the endoplasmic reticulum (ER), and incorporated into PC (Bates et al., 2012; Tjellström et al., 2015). PC may provide diacylglycerol (DAG) directly for triacylglycerol (TAG) synthesis catalyzed by PC: DAG cholinephosphotransferase. In addition, PC serves as the substrate in ER for producing polyunsaturated fatty acids (PUFAs), such as linoleic (18:2) and linolenic (18:3) acids, which constitute more than 50% fatty acids of camelina seed oil (Haslam et al., 2016). The multi-faceted roles of PC in fatty acid desaturation and trafficking, and TAG synthesis could mean that the PC decrease under P deficiency might affect the seed oil content and fatty acid composition that determines the value of seed oil. Therefore, this study was undertaken to determine the effect of P deficiency on lipid remodeling, seed oil production, and fatty acid composition under P-deficient field and controlled greenhouse conditions.
2. MATERIALS AND METHODS
2.1. Field experiment conditions
The field experiment was conducted at the Haskell Agricultural Laboratory of the University of Nebraska–Lincoln, Concord, NE. The study was planted in a field located at 42°23’40” N/96°57’21” W, which was in a long-term P management study since 1989. The soils at the site are from the Crofton silt loam (Fine-silty, mixed, superactive, calcareous, mesic Udic Ustorthents) and Nora silty clay loam (Fine-silty, mixed, superactive, mesic Udic Haplustolls) soil series. Prior to this study, the field was planted with corn (Zea mays.) except for 2006 and 2016 when the field was planted with soybeans (Glycine max.). In 2002 a factorial arrangement of P application method was initiated with Starter (s) (in the row next to the seed), Knife (k) (applied 75 mm in the soil midway between two rows), and broadcast (b) (applied evenly across the whole field) was applied in the spring at two P rates, 16.6 and 33.2 kg ha−1 elemental P as either ammonium polyphosphate (10–34-0, N-P2O5-K) or superphosphate (0–46-0). The starter and knife treatments were the ammonium polyphosphate treatments and the broadcast was the superphosphate. There were two controls, a no nitrogen (N) with 16.6 P Starter treatment and a no N, no P control. N was applied so that a total of 168 kg ha−1 as ammonium nitrate, adjusted to include the N in the P treatments to all N receiving treatments. When corn or soybean were grown, the field was not tilled. In 2017 the field was disked and rolled after the herbicide Trifluralin (a,a,a-trifluoro-2,6-dinitro-N, N-dipropyl-p-toluidine) was applied (April 11, 2017.) No P was applied in 2017 and based on recommendations for camelina 40 kg ha−1 of ammonium nitrate was applied on April 17, 2017.
After 27 years of P treatments the soil in November 2016 varied by P treatments. The soil Olsen P content ranged from 4 ppm for the No N, No P treatment to 21 ppm for the Knife 33 kg ha−1 P treatment. Other P treatments were 7, 17, 6, 14, 8, 21, and 4 ppm for the 34s, 68s, 34b, 68b, 34k, 68k, and the N but no P control treatments. Other soil parameters for the control treatment were 3.4% soil organic matter, pH 7.6, and 161 ppm potassium (ammonium acetate extraction). The experiment was a completely randomized block design with four replications in field plots at the University of Nebraska – Lincoln Haskell Agricultural Laboratory, near Concord, NE. The GPS coordinates location of the field are 42°23’40” N and 96°57’21” W. Individual plot areas were 6.1 m (8 rows) wide × 12.2 m in length. The experiment was planted on 4/14/17 with the camelina cultivar Midas with a Tye drill. The crop was harvested on July 24th. The most recently matured whole leaves were collected from each treatment to measure lipid composition. C. sativa seeds were harvested, dried, and measured seed yield per hectare when plant totally matured. Yields were reduced due to drought conditions with growing season precipitation being 222 ml between April 1 and July 31, 2017.
2.2. Plant materials and growth conditions in greenhouse
For greenhouse experiments, seeds of Licalla and Suneson ecotypes were sown in pot (8.5 × 8.5 × 8.5 cm; length × width × height) filled with soil of 1:1(v/v) vermiculite and perlite. Plants were grown in the greenhouse at Donald Danforth Plant Science Center, St. Louis, MO, under the following conditions: 20–21°C, 50% humidity, 16-hr light/8-hr dark cycle, supplemental light threshold of 566 pmol/m2/s (supplemental lights turned off when outside solar radiation was 566 pmol/m2/s or above), 100% shading with shade cloth when solar radiation at 1415 pmol/m2/s or above, and 50% shading at 1132 pmol/m2/s or above (Li et al., 2015). Six plants were randomly harvested as a repeat. Seedlings were watered using half-strength Hoagland nutrition solution (Hoagland and Arnon, 1950) with varying P concentrations (25, 50, and 500 μM) during the first 3 weeks, then with 50, 100, and 1000 μM P afterwards, respectively. Trays were dipped in the nutrition solution for 10 min as one fertilization treatment. Seedlings were fertilized once a week during the first three weeks, and every three days for the rest of time until the end of the experiments. Plant materials were collected and analyzed at 21 days for vegetative growth parameters. Fresh weight of shoots (leaf and stem) was measured, and then the samples were dried in an oven set at 105°C for 30 minutes, and at 80°C for 48 hours. The biomass was measured as dry weight. When plants grew completely matured, plant height and branch number were measured and counted.
2.3. Membrane lipid profiling
The newest fully expanded leaves were collected in the field-grown plants from each treatment to measure lipid composition. Leaf samples were frozen in liquid nitrogen immediately and subjected to lipid extraction afterwards in the lab as described previously (Welti et al., 2002; Su et al., 2018) with some modifications. Samples were immersed into 3 ml of pre-heated isopropanol solution with 0.01% butylated hydroxytoluene (BHT) in screw-cap tubes at 75°C. The samples were incubated at 75°C for at least 15 minutes, and then 1.5 ml of chloroform and 0.6 ml of water were added and mixed. After agitated (120 rpm) at room temperature for 1 hour, each sample solution was transferred to another fresh glass tube, and 4 ml of chloroform: methanol (2:1 v/v) with 0.01% BHT was added into the original glass tubes and agitated for 30 minutes. The extraction process was repeated until the leaf samples turned completely bleached (3~4 times), followed by one-time wash with 1 ml of 1M KCL and 2 ml of water, respectively. Lipid extracts were then dried under streams of nitrogen gas. Leaf samples after lipid extraction were dried in oven at 105°C overnight and dry weight was measured. The dried lipid samples were dissolved in chloroform proportionally based on the dry weight of leaf samples. Lipid extracts with a mixture of internal lipid standards were introduced into the electrospray source of a triple quadrupole mass spectrometer (API4000; Applied Biosystems, Foster City, CA) by continuous infusion, and data were processed and analyzed using Analyst software (v1.5.1) as described previously (Welti et al., 2002).
2.4. Seed oil content and fatty acid measurements
For one sample, five camelina seeds were put in labeled glass tubes with Teflon-lined screw caps, and then 2 mL of methanol with 1.5% H2SO4 and 0.01% BHT was added using a glass pipette. Twenty-five microliters of 16.2 μmol/ml heptadecanoic acid (C17:0) were added as the internal standard for each sample. The samples were incubated in 90°C for 2 hours for oil extraction and transmethylation. After cooling down, 1 ml of autoclaved ddH2O and 1 ml of hexane were added using a glass pipette. After vortexing, the samples were centrifuged at 900 rpm for 5 minutes. The supernatant was transferred into a labeled vial, and stored in a box at −20°C until ready for gas chromatography (GC) analysis. Fatty acid methyl esters (FAMEs) were loaded onto a GC equipped with a SUPELCOWAX-10 (0.25 mm × 30 m) column using helium as a carrier gas at 20 ml/min and underwent flame ionization. The column temperature was maintained at 170°C for 1 min and gradually increased to 210°C at 3°C per minute. FAMEs from seed lipids were identified by comparing their retention times to those of standards. Six biological repeats were performed for all samples. Oil content was expressed as % per seed weight and fatty acid composition was expressed in mol% of total fatty acids detected.
2.5. Statistical analysis
One-way ANOVA with Duncan’s new multiple range test (IBM SPSS Statistics 24.0) was performed to analyze the data from the field, lipid profiling, and greenhouse experiment data. Values are means ± standard deviation (SD) with biological repeats ranging from 3 to 6, depending on the experiments as specified in figure legends. Different letters indicate significantly differences at p < 0.05 by one-way ANOVA.
3. RESULTS AND DISCUSSION
3.1. Impaired camelina growth and seed production in P-deficient fields
To test the effect of soil P levels on camelina production, we used a well-managed P-deficient field site at University of Nebraska-Lincoln field station. Except for the low P level, other soil parameters, such as total nitrogen (N) level, pH, soil organic matter, or potassium level, were comparable between P-deficient and -sufficient fields. Camelina was planted in plots: 1) P0 – no P applied; 2) P38 – 38 kg/ha P applied, and 3) P76 – 76 kg/ha P applied. P76 was regarded as sufficient P level as suggested by Montana field trials showing that camelina yield responded with up to 70 kg/ha P (Jackson, 2008; McVay and Lamb, 2008). The b, k, and s following the level of P, such as P38b and P76s (Fig. 1B), were used to designate the methods for P application, b for broadcast, k for knife, and s for starter.
Figure 1. Camelina growth and seed yield from plants harvested from fields with different P applications.
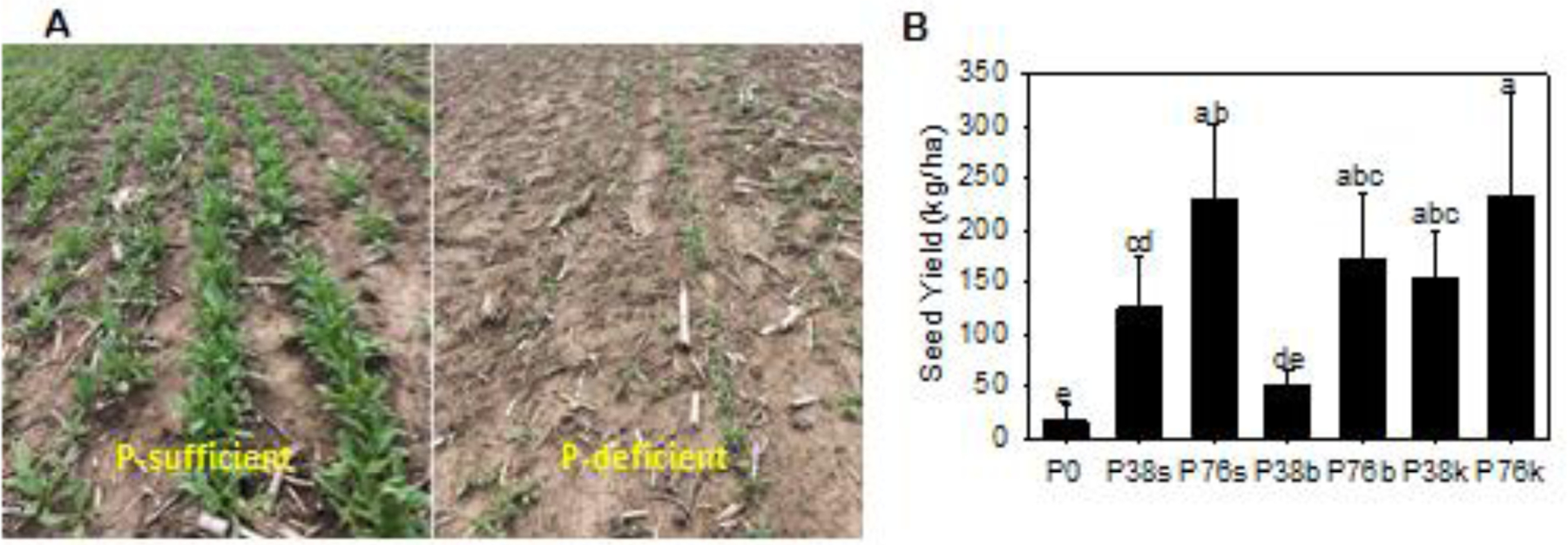
A. Camelina seedlings growing in P-sufficient (left) and –deficient (right) fields. B. Seed yield from camelina plants harvested from field plots with different levels of P fertilizer and placements. P0 refers to no P fertilizer applied; P38 and P76 denote 38 kg/ha and 76 kg/ha P fertilizer applied; and the small b, k, or s following P38 and P76, refer to P fertilized applied by broadcast, knife, or starter, respectively. Values represent the average of four replicates ± standard error. Values followed by the same letters are not statistically different at p < 0:05 by one-way ANOVA.
P levels had profound impacts on camelina growth in fields. Plant growth in the P0 treatment was much slower (Fig. 1A right) than that in fields supplied with P fertilizer (Fig. 1A left). Seed yields ranged from 16 kg/ha in P0 to 231 kg/ha in P76k (Fig. 1B). With P38 application to the field, the seed yield was 52 kg/ha for P38b, 125 kg/ha for P38s, and 155 kg/ha for P38k, representing more than 3-, 7-, and 9-fold increase over P0, respectively. With P76, the seed yield further increased to 172 kg/ha for P76b, 229 kg/ha for P76s, and 232 kg/ha for P76k, constituting approximately 10-, 14-, and 15-fold increase over P0, respectively. The results also showed that the methods of P fertilizer application affected seed yields significantly. Among the three P placements tested, broadcasting was least efficient while knife-in produced the highest seed yield (Fig. 1B). With the same amount of P applied to the field, P38k produced 3-fold more seed yield than P38b (155 vs. 52 kg/ha) whereas P76k yielded 35% more than P76b (232 vs. 172 kg/ha; Fig. 1B).
3.2. Membrane lipid remodeling associated with the level of P deficiency
To assess lipid remodeling of camelina plants grown in fields with different P levels and placements, the most recently expanded leaves of each treatment were harvested from the field for quantitative lipid profiling using tandem mass spectrometry (Fig. 2). Camelina leaves contained relatively high levels of the plastidic lipids DGDG, monogalactosyldiacylglycerol (MGDG), and phosphatidylglycerol (PG) (Fig. 2). In particular, the non-P-containing MGDG and DGDG in samples from P0 constituted approximately 95 mol% of total glycerolipids analyzed (Fig. 2A) whereas the phospholipids, PC, phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylserine (PS), PG, and phosphatidic acid (PA) made up only about 3 mol% (Fig. 2B,C). P applications decreased the level of non-P-containing galactolipids relative to phospholipids. At 38 kg/ha, the mol% of MGDG and DGDG was 82% for P38s, 88% for P38b, and 84% for P38k. At 76 kg/ha, the mol% of galactolipids decreased further to 76% for P38s, 78% for P76b, and 80% for P76k (Fig. 2). Conversely, P0 leaves had a lower level of all phospholipids tested, including PG, PI, PC, PE, PS, and PA, while plants grown at P76 had the highest level of phospholipids regardless of the methods of P applications (Fig. 2)
Figure 2. Membrane glycerolipid composition of camelina leaves from field plots with different levels of P fertilizer and placements.
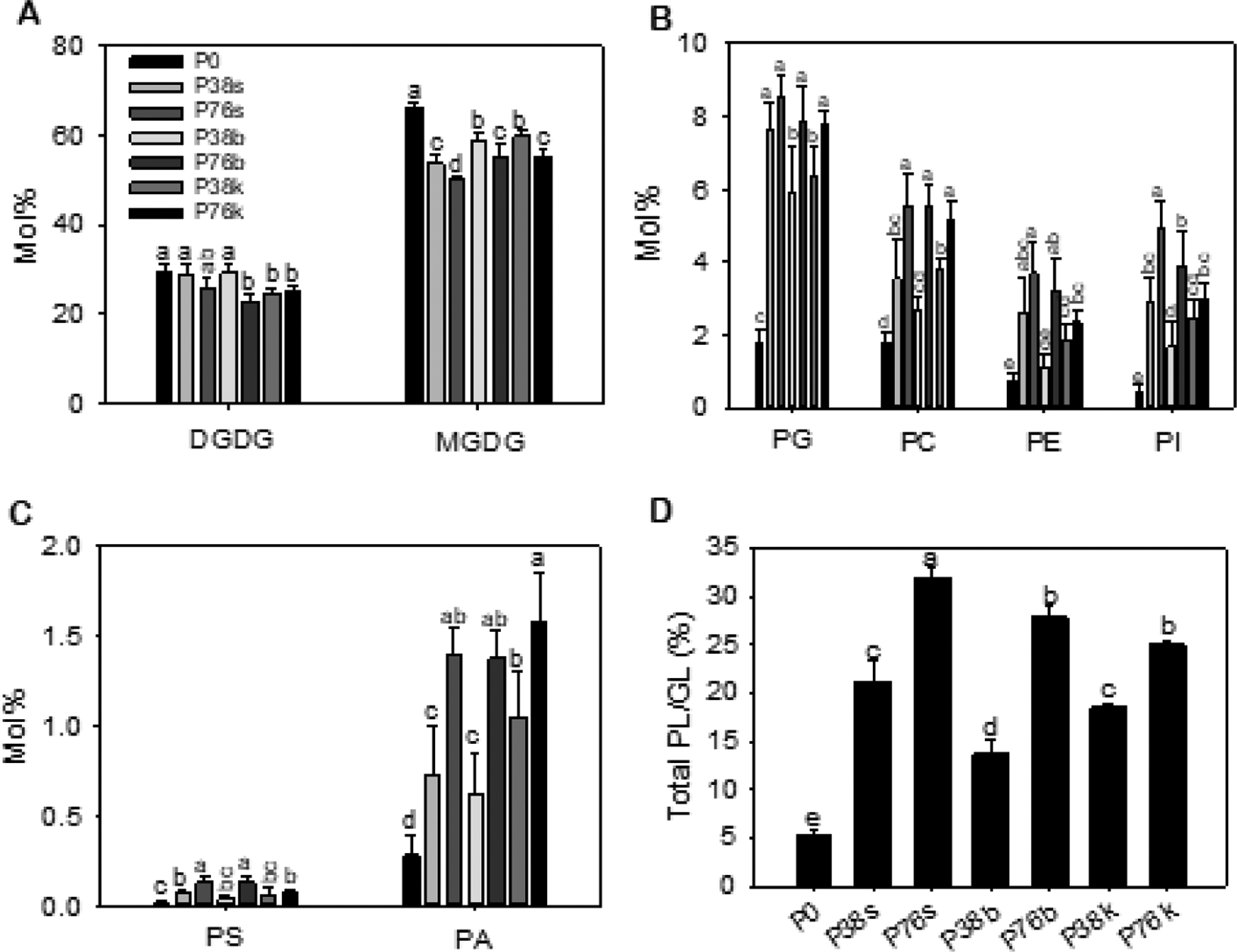
Lipids extracted from newest fully expanded leaves of plants were quantified by ESI-MS/MS. A. Mole% of MGDG and DGDG of all PL (phospholipids) and GL (galactolipids) analyzed. B and C. A. Mole% of different PL classes of all PL and GL analyzed. D. Ratios of PL/GL. PL is the sum of phospholipids analyzed, including phosphatidic acid (PA), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), and phosphatidylserine (PS), whereas GL is the sum of digalactosyldiacylglycerol (DGDG) and monogalactosylglycerol (MGDG). Values are means ± SD (n = 5). Values with different letters show significantly differences at p < 0.05 by one-way ANOVA.
Membrane lipid remodeling in response to P deficiency is well documented in plants grown under laboratory conditions (Dörmann and Benning, 2002; Li et al., 2006). Leaves from field-grown camelina plants displayed both common and unique lipid remodeling. The common changes are decreases in phospholipids with an increase in galactolipids under P deficiency, but the increase in galactolipids was mostly due to the increase of MGDG, rather than DGDG reported for plants in laboratory conditions (Benning and Ohta, 2005, Li et al., 2006; Okazaki et al., 2017). The high levels of plastidic lipids could be a result from field-grown conditions with high levels of sunlight. In addition, our results indicated that the degree of lipid remodeling was associated with the seed yield under different P treatments. Specifically, leaves from P0 had the smallest phospholipids (PL; i.e. PC, PE, PG, PI, PS, and PA) to galactolipids (GL; MGDG and DGDG) ratio which was about 0.05. The PL/GL ratio of samples from P38 ranged from 13% to 21%, while PL/GL ratio of P76 samples varied from 25% to 33% (Fig. 2D), indicating the more severe P scarcity was, the more intense the lipid remodeling was triggered.
At molecular species level, MGDG accumulation mainly came from 36:6 species, while 34:3 DGDG contributed most to the increase of DGDG (Fig. 3A,B). For phospholipids, the reduction of PC, PE, PG and PI levels was due to the decrease of each molecular species in general (Fig. 3C–F). Interestingly, nearly all PC species, particularly the two most abundant ones 34:3 PC and 34:1 PC, displayed incremental decreases in response to P0 vs. P38 vs. P76 whereas the significant decreases of PE, PI, and PG species occurred only on selected species and also depended on P levels. For example, most PG species did not show incremental decreases between P32 and P76, but dramatically dropped between P0 and P38 (Fig. 3C). The results suggest that decreases in PC species may be a better indicator of field P deficiency than other phospholipid species.
Figure 3. Molecular species of glycerolipids of camelina leaves from field plots with different levels of P fertilizer and placements.
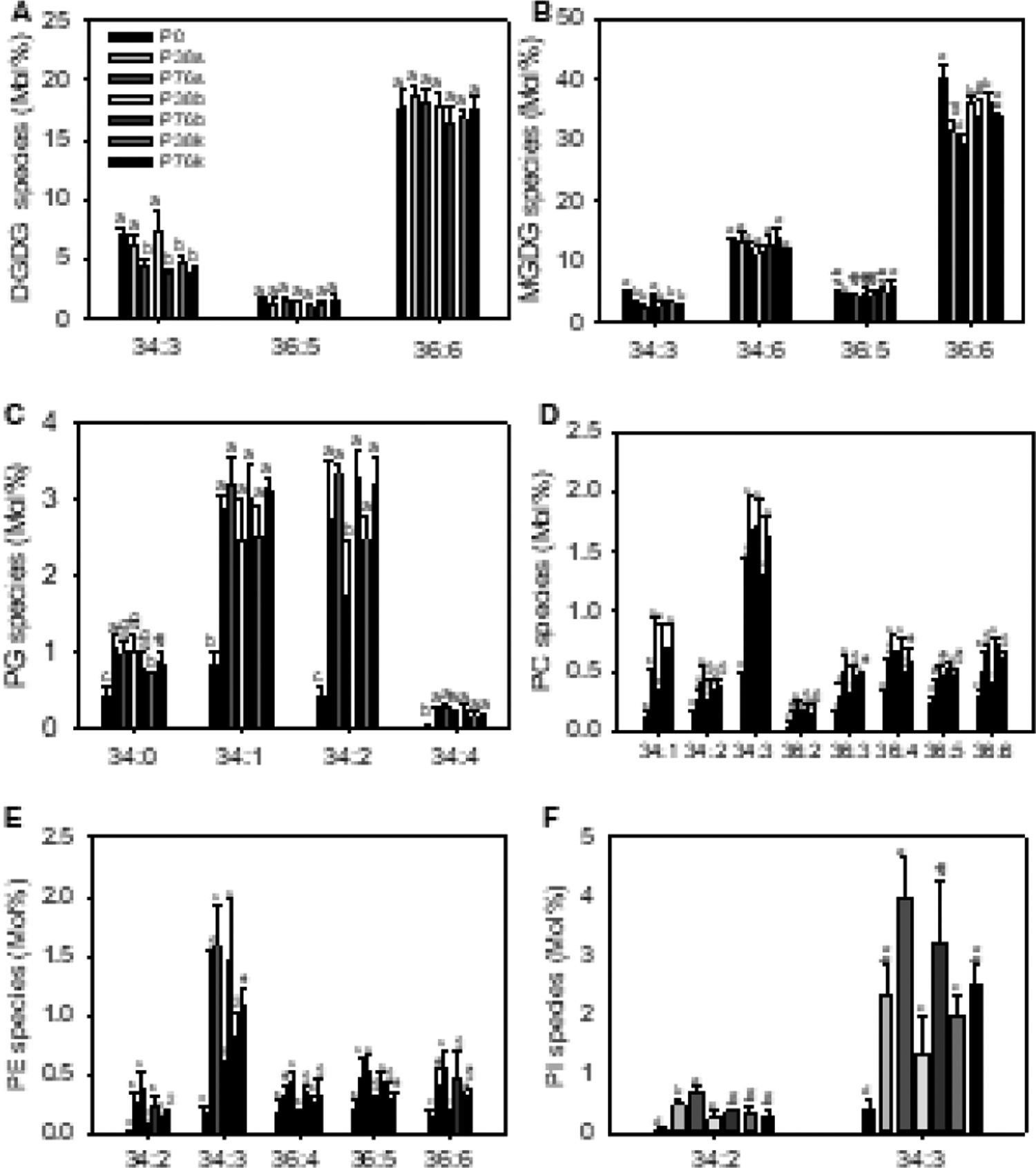
Lipids from fully expanded leaves of camelina plants were quantified by ESI-MS/MS. A to F, Lipid species of various phospholipid classes. Two numbers separated by a colon, such as 36:6. refer to total acyl carbons: total double bonds of two fatty acid esters. Major molecular species are shown. Values are means ± SD (n = 5), and values with different letters denote significantly differences at p < 0.05 by one-way ANOVA.
3.3. Increased seed oil content and altered fatty acid composition under P deficiency in fields
To address how P deficiency affects camelina seed oil content and fatty acid composition, we performed the seed oil analysis using camelina seeds collected from the field plots fertilized with different levels of P and different application methods (Fig. 4). The seed oil content was significantly higher in the lower P levels P0 and P38 than that in P76 across three P placements (Fig. 4A). The oil content per seed weight in P38s, P38b, and P38k was 28%, 23%, and 20% higher than that of P76s, P76b, and P76k, respectively (Fig. 4A). The oil content in P0 tended to be higher than that in P38, but the difference was significant only with that of P38b. The results indicate that P deficiency increases seed oil content in camelina seeds.
Figure 4. Seed oil content and fatty acid composition of camelina from field plots with different levels of P fertilizer and placements.
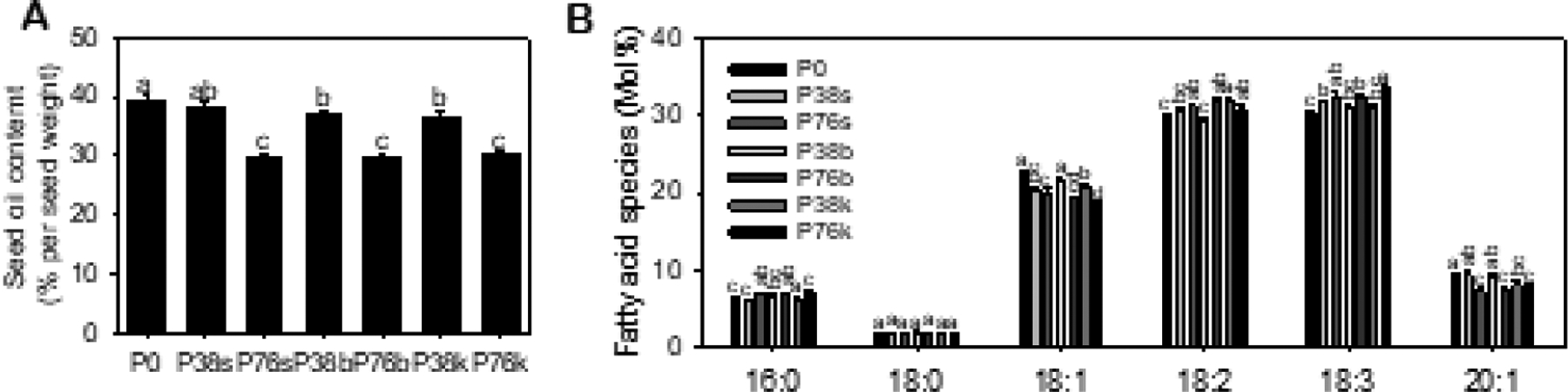
Fatty acid methyl esters were prepared from dry seeds and analyzed using GC to calculate oil content (A) and mol% of fatty acid composition (B). Values are means ± SD (n = 5), and different letters indicate significantly differences at p < 0.05 by one-way ANOVA.
In addition, the P deficiency altered fatty acid composition with an increase in monounsaturated fatty acids (MUFAs), oleic (18:1) and eicosenoic (20:1) acids, and a decrease in PUFAs 18:2 and 18:3 (Fig. 4B). At P0, the mol% of monounsaturated 18:1 and 20:1 fatty acids was approximately 10% and 20% higher than at the high P76 regardless of the P fertilization methods. Conversely, the mol% of polyunsaturated 18:2 and 18:3 fatty acids at P0 was approximately 5% and 10%, respectively, lower than that measured in sufficient P76. The mol% of saturated palmitic acid (16:0) was also lower at P0 than P76. When P38 and P76 were compared within the same P placement, the same held with some deviations. On average, seed oil from the lower P level P38 had higher mol% of 18:1 and 20:1 with a lower mol% of 18:2 and 18:3 fatty acids than that at the higher P level P76. However, depending on the P placement methods, there were some deviations, such as the mol% of 18:2 fatty acid was similar between P38k and P76k and that of 18:1 fatty acid was similar between P38s and P76s (Fig. 4B).
3.4. Effect of P deficiency on seed oil content and composition of camelina in greenhouse
The field results showed that P deficiency increased the seed oil content but decreased the PUFA level. To verify the effect of P deficiency on seed oil traits, we grew two more camelina ecotypes, Suneson and Licalla, at three P levels, 1000 (P1000), 100 (P100), and 50 (P50) μM in greenhouse. Compared to the same genotype at sufficient P (P1000), the oil content at P50 and P100 increased by approximately 18% in Suneson and Licalla (Fig. 5A). These results indicate that P deficiency increases the seed oil content, which is consistent with our data from fields. However, the oil contents of P100 and P50 were similar, suggesting a limit of further P deficiency on further increasing seed oil content.
Figure 5. Oil content and fatty acid composition of camelina seeds from plants grown in greenhouse with different levels of P.
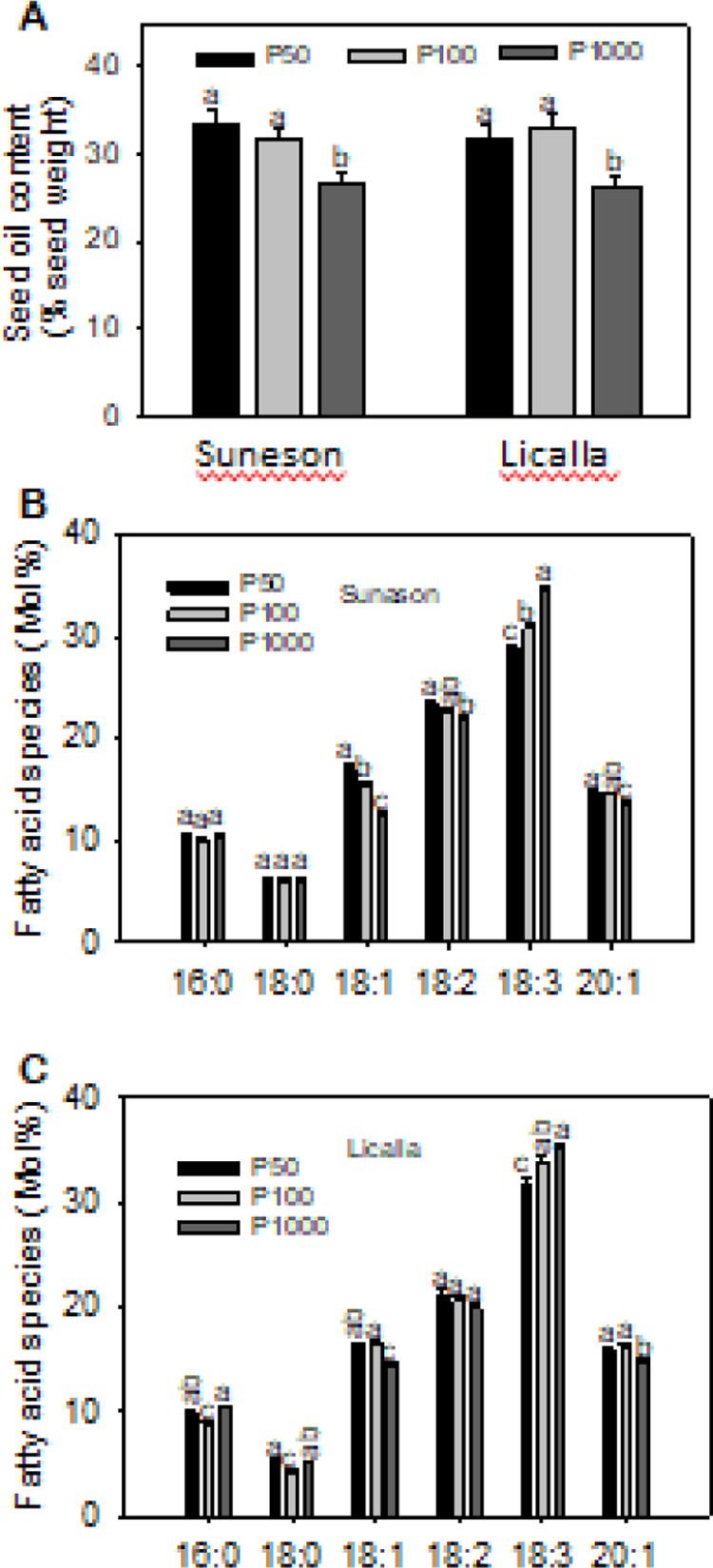
Fatty acid methyl esters from dry seeds were analyzed using GC to calculate oil content (A) and mol% of fatty acid composition of ecotype Suneson (B) and Licalla (C). Values are means ± SD (n = 6), and different letters indicate significantly differences at p < 0.05 by one-way ANOVA.
Fatty acid composition was also altered by the P deficiency under the climate-controlled growth condition. Compared to that at sufficient P1000, the mol% of 18:1 fatty acid at P100 and P50 increased significantly by about 20% and 27%, respectively in Suneson, 13% and 11%, respectively, in Licalla, (Fig. 5B, C). Similarly, the mol% of 20:1 fatty acid displayed significant increases at P100 and P50 over P1000. Conversely, the mol% of 18:3 fatty acid at P100 and P50 was significantly lower than that at P1000 in both Suneson and Licalla (Fig. 5B, C). The total amount of unsaturated fatty acids accounted for more than 86% of the total fatty acids in camelina seeds, among which the level of PUFAs was higher than that of MUFAs (sFig. 1). P deficiency increased the proportion of MUFAs by 17% and 10% in seed oil of Suneson and Licalla, respectively, while PUFA levels were decreased by 9% and 5% in Suneson and Licalla, respectively. However, the total level of unsaturated fatty acids remained the same under the sufficient and deficient P conditions. These results suggest that P deficiency affects the percentage of MUFAs and PUFAs and the quality of seed oil.
The results from both field- and greenhouse-grown camelina showed that P deficiency increased seed oil content significantly and the ratio of MUFAs to PUFAs. The increase in 18:3 fatty acid in seeds from plant grown with increased P levels occurred also in other plants, including soybean and flax seeds (Krueger et al., 2013; Xie et al., 2020). The decrease in PUFAs under P deficiency may result from the P deficiency-induced decrease in PC. PC is the substrate for desaturation for producing 18:2 and 18:3 fatty acids in ER during seed oil synthesis. While the effect of P levels on camelina seed oil content was previously unknown, reports on the P effect on other oil seeds varied in other plant species. For example, the seed oil content in soybean tended to decrease with increased P levels (Yin et al., 2016), however, another study reported that soybean oil content was not significantly affected by P levels (Win et al., 2010). In crambe (Crambe abssynica Hoechst), increased P levels had little effect on seed oil content (Rogério et al., 2013). In Arabidopsis, P starvation enhanced the triacylglycerol accumulation significantly in both shoots and roots, although the seed oil situation was not known (Pant et al., 2015). These discrepancies could result from the different species involved and/or experimental conditions used, such as the severity of P deficiency or excess. The results from our current study using different P levels, P placements, and different ecotypes, in the field and under laboratory conditions indicate that P deficiency increases seed oil contents and decreases 18:3 levels.
3.5. Reduced seed number but not seed weight of camelina under P deficiency
P deficiency severely inhibited seedling growth and seed production (Fig 6A, B). Compared to that of P1000, the seed yield at P100 and P50 was reduced by 3.5- and 7-fold in Suneson, and 5- and 10-fold in Licalla, respectively (Fig. 6B). Above-ground fresh weight of 3-week-old plants significantly decreased by 86% and 220% at P100 and P50, respectively in Sunason, and 74% and 241.6%, respectively, in Licalla, compared to those at the sufficient P1000 (sFig. 2). The dry biomass above ground exhibited the same tendency in the two genotypes (sFig. 2). Plant height decreased sharply at P50 and P100 (Fig. 6C).
Figure 6. Camelina growth, branch number, seed yield and seed weight of camelina plants grown under different P levels.
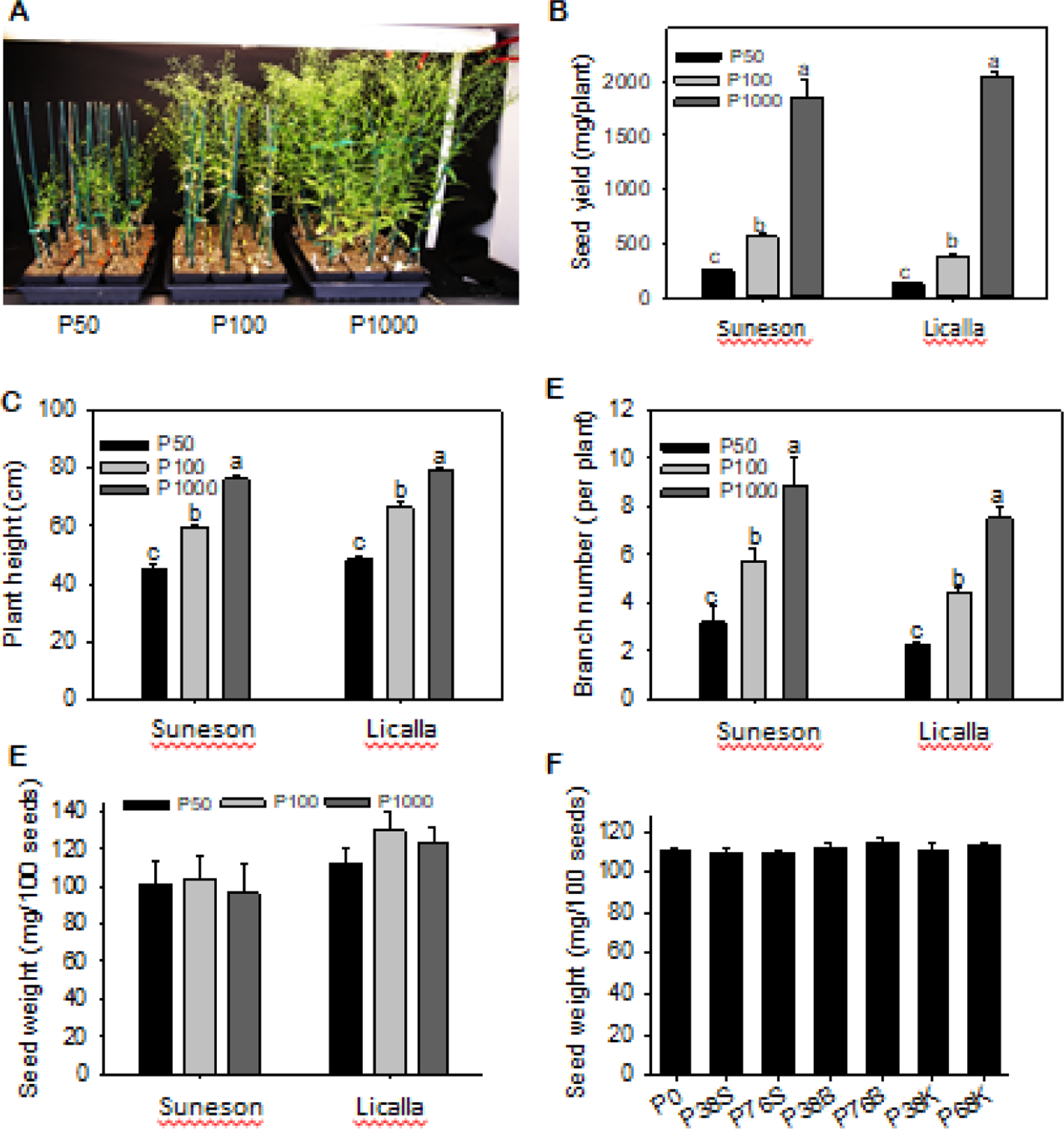
A-E. Data collected from camelina plants grown in greenhouse with 50, 100, and 1000 μM P. Values are means ± SD (n = 3), and different letters indicate significantly differences at p < 0.05 by one-way ANOVA. F. Seed weight of camelina plants from field plots with different levels of P fertilizer and placements. Values are means ± SD (n = 3).
However, P deficiency in laboratory or field conditions did not alter seed size or seed weight (Fig. 6E, F; sFig. 3), indicating that P scarcity reduced the number of seeds. Examination of plant morphology indicated that P deficiency sharply decreased the number of branches and seed pods per plant (Fig. 6D). The number of reproductive branches at P50 was decreased by more than 70% in Suneson and Licalla compared to P1000 (Fig. 6D). Thus, P deficiency decreased seed number drastically, but not seed weight, resulting in large decreases in seed yield in camelina.
4. Conclusion
The results from the field and greenhouse conditions indicate that P deficiency increases seed oil content per seed weight and alter fatty acid composition. The level of PUFAs, particularly 18:3, decreases whereas MUFAs, such as 20:1, increase under limited P, thus changing seed oil quality. P deficiency severely impairs camelina growth and seed production, reducing overall oil production. Thus, achieving a high yield of camelina seed oil is strongly linked to P status of soil. In addition, the data indicate that the magnitude of membrane glycerolipid remodeling reflects the degree of P deficiency, and different methods of P applications affected P efficiency for seed production with knife-in being most effective among the methods tested. Taken together, this study suggests that under Pi-deficient conditions, even though seed oil content increases, this is not enough to compensate for the large decrease in seed yield, thereby leading to a decrease in the overall oil production. Increasing soil Pi levels enhance greatly camelina seed and oil production with a high level of PUFA. Thus, achieving a high yield and quality of camelina seed oil is positively linked to soil P levels.
Supplementary Material
HIGHLIGHTS.
P deficiency in field settings decreased the ratio of phospholipids to galactolipids from 30% to 5% under P sufficient to deficient conditions in leaves.
P deficiency increased seed oil content by approximately 25% and 20% in field and greenhouse settings, respectively
P deficiency increased monounsaturated to polyunsaturated fatty acid ratios in seeds.
The results indicate that P deficiency modifies camelina lipid composition, oil content, and seed yield.
Acknowledgements
We thank Mike Mainz, Research Technologist at University of Nebraska-Lincoln, Zachary Cook, Lane Uhing, and Alan Vandenberg for supporting field work, and Sang-chul Kim for critically reading the manuscript.
Funding
This work was supported by grants from Agriculture and Food Research Initiative (AFRI) award [2020–67013-30908/project accession number 1022148] from the USDA National Institute of Food and Agriculture and the National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM141374 to XW, AFRI [2016–67009-25639] to DPS, and University of Nebraska Hatch funding to CAS.
Declaration of Competing Interest
Xuemin Wang reports financial support was provided by US Department of Agriculture. Daniel Schachtman reports financial support was provided by US Department of Agriculture. Xuemin Wang reports a relationship with US Department of Agriculture that includes: funding grants.
Abbreviations:
- DAG
diacylglycerol
- DGDG
digalactosyldiacylglycerol
- MGDG
monogalactosyldiacylglycerol
- P
phosphorus
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PG
phosphatidylglycerol
- PI
phosphatidylinositol
- PS
phosphatidylserine
- TAG
triacylglycerol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Intellectual property
The authors confirm that they have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing they confirm that they have followed the regulations of their institutions concerning intellectual property.
Authorship
All listed authors meet the ICMJE criteria. We attest that all authors contributed significantly to this manuscript, each having fulfilled criteria as established by the ICMJE. The authors confirm that the manuscript has been read and approved by all named authors. The authors confirm that the order of authors listed in the manuscript has been approved by all named authors.
CRediT authorship contribution statement
J. Li: performed experiments in greenhouse conditions, collected data, analyzed seed oil content and fatty acid composition of field- and greenhouse-grown camelina, and wrote the manuscript. Y. Su: did lipidomic profiling, GC performance, data analysis and edited the manuscript. DP. Schachtman: supervised the field experiments and data collection and edited the manuscript. C. Shapiro: developed the multi-P level site and consulted on the field planting, fertilization, seed collection, and edited the manuscript. X. Wang: proposed and oversaw the overall study and edited the manuscript.
Declaration of competing Interest
No conflict of interest exists
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:
REFERENCES
- Andersson MX, Stridh MH, Larsson KE, Liljenberg C, Sandelius AS, 2003. Phosphate-deficient oat replaces a major portion of the plasma membrane phospholipids with the galactolipid digalactosyldiacylglycerol. FEBS Lett 537, 128–132. 10.1016/s0014-5793(03)00109-1. [DOI] [PubMed] [Google Scholar]
- Bates PD, Fatihi A, Snapp AR, Carlsson AS, Browse J, Lu C, 2012. Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiol 160, 1530–1539. 10.1104/pp.112.204438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning C, Ohta H, 2005. Three enzyme systems for galactoglycerolipid biosynthesis are coordinately regulated in plants. J. Biol. Chem, 280, 2397–2400. 10.1074/jbc.R400032200 [DOI] [PubMed] [Google Scholar]
- Campbell MC, Rossi AF, Erskine W, 2013. Camelina (Camelina sativa (L.) Crantz): Agronomic potential in Mediterranean environments and diversity for biofuel and food uses. Crop Pasture Sci 64: 388–398. 10.1071/CP13054. [DOI] [Google Scholar]
- Cordell D, Drangert JO, White Stuart., 2009. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 19, 292–305. 10.1016/j.gloenvcha.2008.10.009. [DOI] [Google Scholar]
- Dörmann P, Benning C, 2002. Galactolipids rule in seed plants. Trends Plant Sci 7, 112–118. 10.1016/s1360-1385(01)02216-6. [DOI] [PubMed] [Google Scholar]
- Gaude N, Nakamura Y, Scheible WR, Ohta H, Dörmann P, 2008. Phospholipase C5 (NPC5) is involved in galactolipid accumulation during phosphate limitation in leaves of Arabidopsis. Plant J 56, 28–39. 10.1111/j.1365-313X.2008.03582.x. [DOI] [PubMed] [Google Scholar]
- Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM, 2006. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am. J. Clin. Nutr 83, 1526s–1535s. 10.1093/ajcn/83.6.1526S. [DOI] [PubMed] [Google Scholar]
- Gilbert N, 2009. Environment: The disappearing nutrient. Nature 461, 716–718. 10.1038/461716a. [DOI] [PubMed] [Google Scholar]
- Hartley TN, Macdonald AJ, McGrath SP, Zhao FJ, 2013. Historical arsenic contamination of soil due to long-term phosphate fertiliser applications. Environ. Pollut 180, 259–264. 10.1016/j.envpol.2013.05.034. [DOI] [PubMed] [Google Scholar]
- Härtel H, Dormann P, Benning C, 2000. DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A 97, 10649–10654. 10.1073/pnas.180320497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam RP, Sayanova O, Kim HJ, Cahoon EB, Napier JA, 2016. Synthetic redesign of plant lipid metabolism. Plant J 87, 76–86. 10.1111/tpj.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer S, Gaxiola R, Schilling R, Herrera-Estrella L, López-Arredondo D, Wissuwa M, Delhaize E, Rouached H, 2017. Improving phosphorus use efficiency: a complex trait with emerging opportunities. Plant J 90, 868–885. 10.1111/tpj.13423. [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI, 1950. The water-culture method for growing plants without soil. California Agricultural Experiment Station Circular 347. [Google Scholar]
- Jackson GD, 2008. Response of camelina to nitrogen, phosphorus, and sulfur. https://landresources.montana.edu/fertilizerfacts/documents/FF49CamelinaNPS.pdf
- Krueger K, Goggi AS, Mallarino AP, Mullen RE, 2013. Phosphorus and potassium fertilization effects on soybean seed quality and composition. Crop Sci 53, 602– 610. 10.2135/cropsci2012.06.0372. [DOI] [Google Scholar]
- Li M, Welti R, Wang X, 2006. Quantitative profiling of Arabidopsis polar glycerolipids in response to phosphorus starvation. Roles of phospholipases D zeta1 and D zeta2 in phosphatidylcholine hydrolysis and digalactosyldiacylglycerol accumulation in phosphorus-starved plants. Plant Physiol 142, 750–761. 10.1104/pp.106.085647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Wei F, Tawfall A, Tang M, Saettele A, Wang X, 2015. Overexpression of patatin-related phospholipase AIIIδ altered plant growth and increased seed oil content in camelina. Plant Biotechnol. J 13, 766–778. 10.1111/pbi.12304. [DOI] [PubMed] [Google Scholar]
- López-Arredondo DL, Leyva-González MA, Alatorre-Cobos F, Herrera-Estrella L, 2013. Biotechnology of nutrient uptake and assimilation in plants. Int. J. Dev. Biol 57, 595–610. 10.1387/ijdb.130268lh. [DOI] [PubMed] [Google Scholar]
- López-Arredondo DL, Leyva-González MA, González-Morales SI, López-Bucio J, Herrera-Estrella L, 2014. Phosphate nutrition: improving low-phosphate tolerance in crops. Annu. Rev. Plant Biol 65, 95–123. 10.1146/annurev-arplant-050213. [DOI] [PubMed] [Google Scholar]
- McVay KA, Lamb PF, 2008. Camelina production in Montana. http://msuextension.org/publications/AgandNaturalResources/MT200701AG.pdf.
- Moellering ER, Benning C, 2011. Galactoglycerolipid metabolism under stress: a time for remodeling. Trends Plant Sci. 16, 98–107. 10.1016/j.tplants.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Moser BR, Vaughn SF, 2010. Evaluation of alkyl esters from Camelina sativa oil as biodiesel and as blend components in ultra-low sulfur diesel fuel. Bioresour. Technol 101, 646–653. 10.1016/j.biortech.2009.08.054. [DOI] [PubMed] [Google Scholar]
- Moser BR, 2014. Biodiesel from alternative oilseed feedstocks: camelina and field pennycress. Biofuels 3, 193–209. 10.4155/bfs.12.6. [DOI] [Google Scholar]; Neupane D, Lohaus RH, Solomon JKQ, Cushman JC, 2022. Realizing the potential of camelina sativa as a bioenergy crop for a changing global climate. Plants 11 (6), 772. 10.3390/plants11060772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki Y, Otsuki H, Narisawa T, Kobayashi M, Sawai S, Kamide Y, Kusano M, Aoki T, Hirai MY, Saito K, 2013. A new class of plant lipid is essential for protection against phosphorus depletion. Nature Commun 4, 1510. 10.1038/ncomms2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki Y, Takano K, Saito K, 2017. Lipidomic analysis of soybean leaves revealed tissue-dependent difference in lipid remodeling under phosphorus-limited growth conditions. Plant Biotechnol 34, 57–63. 10.5511/plantbiotechnology.17.0113a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant BD, Burgos A, Pant P, Cuadros-Inostroza A, Willmitzer L, Scheible W, 2015. The transcription factor PHR1 regulates lipid remodeling and triacylglycerol accumulation in Arabidopsis thaliana during phosphorus starvation, J. Exp. Bot 66, 1907–1918. 10.1093/jxb/eru535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam D, Budin J, Field L, Breene W, 1993. Camelina: a promising low-input oilseed, in: Janick J, Simon JE, (Eds.), New crops, Wiley, New York, pp. 314–322.. [Google Scholar]
- Rogério F, da Silva TRB, dos Santos JI, Poletine JP, 2013. Phosphorus fertilization influences grain yield and oil content in crambe. Ind. Crops Prod 41, 266–268. 10.1016/j.indcrop.2012.04.016. [DOI] [Google Scholar]
- Ruiz-Lopez N, Haslam RP, Napier JA, Sayanova O, 2014. Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. Plant J 77, 198–208. 10.1111/tpj.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz RW, Ulrich AE, Eilittä M, Roy A, 2013. Sustainable use of phosphorus: a finite resource. Sci. Total Environ 461–462, 799–803. 10.1016/j.scitotenv.2013.05.043. [DOI] [PubMed] [Google Scholar]
- Su Y, Li M, Guo L, Wang X, 2018. Different effects of phospholipase Dζ2 and non-specific phospholipase C4 on lipid remodeling and root hair growth in Arabidopsis response to phosphate deficiency. Plant J 94, 315–326. 10.1111/tpj.13858. [DOI] [PubMed] [Google Scholar]
- Tjellström H, Andersson MX, Larsson KE, Sandelius AS, 2008. Membrane phospholipids as a phosphate reserve: the dynamic nature of phospholipid-to-digalactosyl diacylglycerol exchange in higher plants. Plant Cell Environ 31, 1388–1398. 10.1111/j.1365-3040.2008.01851.x. [DOI] [PubMed] [Google Scholar]
- Tjellström H, Strawsine M, Ohlrogge JB, 2015. Tracking synthesis and turnover of triacylglycerol in leaves. J. Exp. Bot 66, 1453–1461. 10.1093/jxb/eru500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Yan Z, Niu Z, Coulter JA, Niu J, Zhang J, Wang B, Yan B, Zhao W, Wang L, 2020. Yield, oil content, and fatty acid profile of flax (Linum usitatissimum L.) as affected by phosphorus rate and seeding rate. Ind. Crops Prod 145, 112087. 10.1016/j.indcrop.2020.112087. [DOI] [Google Scholar]
- Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou HE, Rajashekar CB, Williams TD, Wang X, 2002. Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem 277, 31994–32002. 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- Win M, Nakasathien S, Sarobol E, 2010. Effects of phosphorus on seed oil and protein contents and phosphorus use efficiency in some soybean varieties. Kasetsart J. Nat. Sci 44, 1–9. [Google Scholar]
- Wojtkowiak R, Glazar K, Zembrowski K, Le KT, Przyrodniczy U, Polskiego W, Rolniczych IM, 2009. Production costs in a novel method of manufacture of the methyl esters from false flax (Camelina sativa L.) oil for feed the piston compression-ignition engine. J. Res. Appl. Agric. Eng 54, 164–170. [Google Scholar]
- Yin X, Bellaloui N, McClure AM, Tyler DD, Mengistu A, 2016. Phosphorus fertilization differentially influences fatty acids, protein, and oil in soybean. A. J. Plant Sci 7, 1975–1992. 10.4236/ajps.2016.714180. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


