Abstract
Glucose metabolism is impaired in brain aging and several neurological conditions. Beneficial effects of ketones have been reported in the context of protecting the aging brain, however, their neurophysiological effect is still largely uncharacterized, hurdling their development as a valid therapeutic option. In this report, we investigate the neurochemical effect of the acute administration of a ketone d-beta-hydroxybutyrate (d-βHB) monoester in fasting healthy participants with ultrahigh-field proton magnetic resonance spectroscopy (MRS). In two within-subject metabolic intervention experiments, 7 T MRS data were obtained in fasting healthy participants (1) in the anterior cingulate cortex pre- and post-administration of d-βHB (N = 16), and (2) in the posterior cingulate cortex pre- and post-administration of d-βHB compared to active control glucose (N = 26). Effect of age and blood levels of d-βHB and glucose were used to further explore the effect of d-βHB and glucose on MRS metabolites. Results show that levels of GABA and Glu were significantly reduced in the anterior and posterior cortices after administration of d-βHB. Importantly, the effect was specific to d-βHB and not observed after administration of glucose. The magnitude of the effect on GABA and Glu was significantly predicted by older age and by elevation of blood levels of d-βHB. Together, our results show that administration of ketones acutely impacts main inhibitory and excitatory transmitters in the whole fasting cortex, compared to normal energy substrate glucose. Critically, such effects have an increased magnitude in older age, suggesting an increased sensitivity to ketones with brain aging.
Subject terms: Neuroscience, Preclinical research
Introduction
Human brain energetics are primarily dependent on glucose metabolism. Steady glucose supply and metabolism ensure the maintenance of resting potentials and generation of action potentials, synthesis of neurotransmitters and neuromodulators, cerebral production of adenosine triphosphate (ATP), and enables general functional adequacy [1, 2]. Disturbances in glucose metabolism are a main hallmark of metabolic conditions (e.g. insulin resistance, Type-2 diabetes mellitus) but glucose hypometabolism is also generally accepted as an effect of normal physiological brain aging [3]. Imaging studies show that older chronological age in cognitively normal adults is correlated with lower glucose utilization [4]. The expression of glucose transporter proteins is reduced in healthy aging and correlates with decreased glucose utilization [5]. In human physiology, frontal glucose hypometabolism is correlated to decreases in cognitive performance in non-demented healthy older adults [6, 7] and hypotheses suggest it may be linked to the onset [8, 9] and development [10] of Alzheimer’s disease (AD) and other neurodegenerative disorders [11–13].
Historically, restoring impaired glucose metabolism through insulin supplementing has had limited success mainly because brain aging and glucose hypometabolism are associated with hyperinsulinemia and reduced insulin signaling in the brain [14, 15]. An alternate way of alleviating physiological consequences of impaired glucose metabolism is to supplement the brain with an alternative fuel source. Evidence suggests that ketones beta-hydroxybutyrate (d-βHB) and acetoacetate (AcAc), whether endogenously produced or supplemented, may represent such an alternative fuel source [16]. Although the metabolism of ketones in fasting conditions has long been established [17], more recent works have shown that ketone supplements in the absence of glucose can supply up to a third of brain energy requirements in short ketosis [18]. In prolonged fasting, studies have shown that the human liver can produce ketones at a rate of 100–150 g/day, enough to supplement the brain’s daily energy needs [19, 20]. Interestingly, metabolism of ketones is not significantly affected by aging [21] and remains relatively stable, suggesting a different metabolic pathway for ketones in the aging brain. Moreover, studies suggest that ketones may exert neuroprotective properties in the aging brain. Recent evidence suggest ketones can reverse functional brain network instability [22], a functional correlate of decreased cognitive acuity considered a potential marker for brain aging. Other results show blood oxygen level-dependent signal changes in older people after administration of ketones, showing cerebral consumption of ketones and improving cognitive performance [23].
Ketones also seem to demonstrate potential benefits in disease. Introduction of the ketogenic diet shows improvements in cognitive function in AD [24, 25], mild cognitive impairment [26–29] and healthy older adults with memory deficits [30]. Importantly, preliminary results suggest that metabolism of ketones remains unaffected by AD pathology, suggesting a route to supplement the brain’s energy needs and potentially delay the evolution of the disease [21, 31, 32]. Additionally, several works have shown that ketones promote antiepileptic effects [33–36] in children [37, 38] and adults [39] with drug-resistant epilepsy. Results in pediatrics are most striking, with multiple clinical trials showing significant seizure frequency reduction [37] and lasting improvements [40]. Preclinical works also suggest the administration of ketones promotes anti-inflammatory effects in stress [41, 42] and caloric restriction [43] paradigms.
The mechanisms by which ketones alleviate symptoms associated with glucose hypometabolism are not well understood. Specifically, the neurochemical characterization of ketones in humans is incomplete and warranted to further assess its therapeutic potential in physiological brain aging. In this perspective, proton magnetic resonance spectroscopy (1H-MRS) allows the non-invasive estimation of cerebral metabolites in a region of interest. MRS can assess static and dynamic changes in brain metabolism following an intervention or physiological challenge [44, 45]. Metabolites that can be reliably estimated at higher field strength include main inhibitory neurotransmitter gamma-aminobutyric acid (GABA), excitatory neurotransmitter glutamate (Glu), and glutamine (Gln). Glu is also a known substrate for energy and is critical in the astrocytic and neuronal synthesis of GABA, Gln and glutathione. The cycling of Glu and Gln depends on essential precursors glucose and lactate and is thus particularly relevant in the study of brain energy metabolism [46].
The overarching aim of this study is to provide an understanding of the acute effect of a single administration of ketones in the human brain. Here, we investigate the neurochemical effect of an acute administration of d-beta-hydroxybutyrate (d-βHB) in healthy, cognitively normal adults. Specifically, in Experiment 1, we investigate the effect of an acute administration of d-βHB on metabolites in the anterior cingulate cortex, using a within-subject design. In Experiment 2, using a similar design in a larger sample size with a broader age-range, we investigate the effect of separate administrations of d-βHB and glucose on metabolites in the posterior cingulate cortex, and the relationships between effects of d-βHB and glucose with age. This will provide a better understanding of regional (anterior and posterior MRS acquisitions), temporal (pre-and-post-d-βHB) and functionally comparative (d-βHB and glucose) effect of ketones on brain metabolic compounds.
Methods and materials
Population
In Experiment 1, data were obtained from 16 healthy participants (eight males, age range 20–50). In Experiment 2, data were obtained from 26 healthy participants (13 males, age range 25–79). Cognitively normal adults were recruited through ad-placement for the Boston (MA, USA) metropolitan area. Participants were screened for contraindications for MRI and metabolic challenge procedure. Participants with a history of insulin resistance and diabetes mellitus were excluded; screening of participants included testing of homeostatic model assessment for insulin resistance (HOMA-IR), assessment of blood glucose (HbA1c) and Standardized Mini-Mental State Examination (SMMSE) to ensure cognitive normality. Participants with neurological or psychiatric conditions, brain injury, or currently battling life-threatening illness (e.g. cancer) were excluded. Participants were also excluded if they were following or had followed in the previous six months a low-carbohydrate or ketogenic diet. Participants were matched for sex independently in Experiments 1 and 2 (see Table 1 for participants’ characteristics). Study design and recruitment effort were approved by the Internal Review Board at Massachusetts General Hospital and informed consent was obtained.
Table 1.
Participants’ characteristics and demographics.
| Experiment 1 | Experiment 2 | |
|---|---|---|
| Age (mean ± SD) | 39.6 ± 18.8 years | 50.7 ± 16.1 years |
| Age range (years) | 20–50 | 25–79 |
| Total sample size | 16 | 26 |
| Sex | 8 Women | 13 Women |
| 8 Men | 13 Men | |
| Baseline SMMSE | 29.9 ± 0.31 | 29.8 ± 0.73 |
| Baseline HOMA-IR | 3.43 ± 1.37 | 4.15 ± 1.80 |
| Baseline HBA1C | 5.19 ± 0.30 | 5.21 ± 0.30 |
Study design
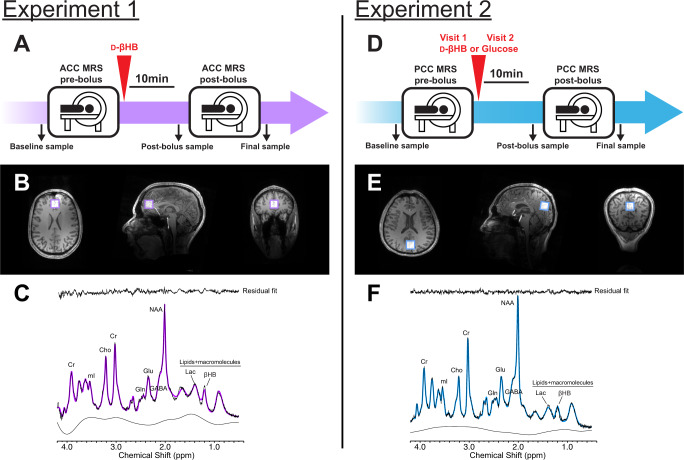
Experiments 1 and 2 were designed as within-subject metabolic interventions, where data were obtained before and after intake of a bolus. Participants were asked to come in a fasted state (i.e., following overnight fast). Procedures were performed at the same time of day, from 8:00 to 11:00, with bolus intake scheduled at 9:30. For Experiment 2 participants came in for two visits and allocation of the d-βHB or glucose bolus was randomized. Participants gave a blood sample at baseline, followed by pre-bolus MR acquisition. Participants were taken out of the MR scanner and administered the bolus, followed by a blood sample 10 min later. This timing allowed for repositioning in the scanner and acquisition of anatomical data in time to acquire MRS past the 30 min time point, which corresponds to peak brain βHB concentration observed in previous study [22]. After post-bolus MR acquisition, participants gave a final blood sample (see Fig. 1).
Fig. 1. Study design and magnetic resonance spectrocopy (MRS) representative voxel placement and sample spectra.
Study design for (A) Experiment 1 and (D) Experiment 2. Positioning of the MRS voxel on the (B) anterior cingulate cortex in Experiment 1 and (E) posterior cingulate cortex in Experiment 2, on the axial, sagittal and coronal planes. Sample MRS spectra from (C) Experiment 1 and (F) Experiment 2.
Bolus
Boluses were administered as liquid drinks in unlabeled containers and prepared with concentrations of d-βHB (0.4 g/mL) and glucose (0.25 g/mL) relative to the participants’ weight (395 mg d-βHB per kg of body weight). The d-βHB bolus was prepared with a transparent ketone (R)−3-hydroxybutyl (R)−3-hydroxybutyrate monoester (Pure ΔG Ketone Ester, HVMN Inc., Miami FL USA) diluted with water (volume ratio 1:1.6). d-βHB is produced endogenously from the metabolism of fatty acids in a state of hypoglycemia or fasting, and supplemented d-βHB is quickly metabolized and elevates blood d-βHB levels to millimolar levels [47–49]. In Experiment 2, the glucose drink was prepared with an orange flavor (Fisherbrand Glucose Tolerance Test Beverage, Fisher Scientific Inc., Pittsburg PA USA) and calorie-matched with the d-βHB drink to ensure within-subject control of caloric intake.
Blood sampling
Blood was drawn with a pin-prick needle and blood sample strips were analyzed with a Precision Xtra monitoring system (Abbott Laboratories, IL USA).
Magnetic resonance imaging (MRI)
Anatomical images were obtained from an ultrahigh-field 7 T Siemens MR scanner (Siemens Healthineers, Erlangen, Germany) with a homebuilt 32-channel head coil. High resolution T1-weighted images were acquired with a T1 (MEMPRAGE) sequence with TR 2530 ms, TE 1.61 ms, TI 1100 ms, slice thickness 1.00 mm, 190 slices, field of view 256 x 256 mm2, flip angle 7 degrees, isotropic resolution 1 x 1 x 1 mm.
Proton magnetic resonance spectroscopy (MRS)
MRS was performed with a single-voxel stimulated echo acquisition mode (STEAM) sequence with TE 5.00 ms, TR 4500 ms, TM 75 ms, water suppression bandwidth 132 Hz, 80 averages in a 20x20x20mm2 voxel, acquisition bandwidth 4000 Hz, vector size 2048 points, RF pulse duration 3200 ms and with 3D outer volume suppression interleaved with variable power and optimized relaxation (VAPOR) water suppression. Unsuppressed water acquisition was obtained with similar parameters in four averages. Frequency and eddy current corrections were performed prior to data processing. Concentration levels were estimated with LC Model [50] and metabolites were excluded when Cramer-Rao Lower Bounds (CRLBs) exceeded 20% to ensure reliability in analyses. Metabolites were analyzed in absolute concentration levels (i.e. relative to water) and normalized to creatine+phosphocreatine (Cr + PCr; tCr) to control for potential tissue inhomogeneity [51]. Segmentation of the voxel was performed with Gannet 3.0 [52] and SPM 12 prior to statistical analyses to obtain tissue fraction of gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF), and to perform partial volume correction using a two-compartment model and GABA alpha-correction [53]. The LC Model basis set used for quantification was described previously and used in prior works with ultrahigh-field MRS [54]. Averaged spectra macromolecules using metabolite nulling inversion-recovery experiments (acquisition parameters: TR = 2 s and TI = 675 ms) were also included in the 7 T LC Model basis sets as previously described [54].
In Experiment 1, the voxel was positioned over the anterior cingulate cortex, with the horizontal midline of the voxel aligned with the top of the corpus callosum on the sagittal plane and centered on the interhemispheric fissure on the axial plane (see Fig. 1). In Experiment 2, the voxel was positioned in the posterior cortex, encompassing parts of the median posterior cingulate cortex and precuneus. Post-bolus MRS data in Experiment 1 and 2 were obtained 45 min after intake of bolus. This timeframe is based on a pharmacokinetics time-course of brain d-βHB with 3 T MRS showing brain levels of d-βHB peak 30 min after administration and remain stable up to 90 min post-bolus [22].
Statistical analyses
Normality of distribution and heterogeneity of variances were verified with the Shapiro–Wilk test and Levene’s test. When these could not be verified, analyses of variance were computed with the Kruskal-Wallis nonparametric test and Wilcoxon test for post-hoc comparisons. Repeated-measures analyses of variance (RM-ANOVA) were computed to assess the effect of 1) Treatment (d-βHB) and 2) Time (pre-bolus and post-bolus) on metabolites in the anterior voxel (Experiment 1) and effect of 1) Treatment (d-βHB and glucose), and 2) Time (pre-bolus and post-bolus) on metabolites in the posterior voxel (Experiment 2). Post-hoc pairwise comparisons with Bonferroni correction were computed when the RM-ANOVA proved significant.
In Experiment 2, linear regression models were used to assess the effect of 1) Age and 2) blood levels of d-βHB (as predicting variables) on metabolites levels (as dependent variables) in the posterior voxel. Results are reported significant at α = 0.05 after correcting for false discovery rate with the Hochberg-Benjamini procedure [55]. All statistical analyses were computed with R statistical packages [56].
Results
Experiment 1: acute d-βHB modulates anterior cingulate metabolites
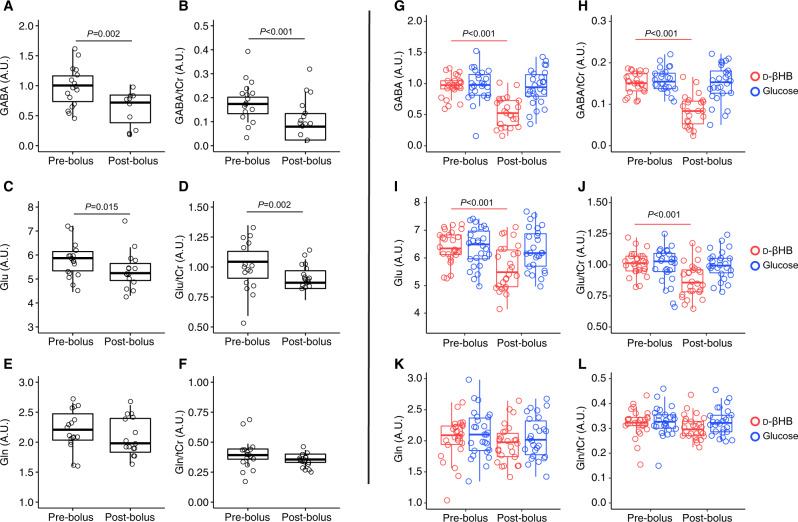
Normality of distribution could not be verified in levels of GABA (P = 0.013) and Glu (P = 0.021). Nonparametric Kruskal-Wallis analysis shows that GABA (Χ2(1, N = 15) = 10.02, P = 0.002) and Glu (Χ2(1, N = 15) = 5.95, P = 0.015) levels were significantly reduced in the anterior voxel after administration of d-βHB. Levels of GABA/tCr (Χ2(1, N = 15) = 21.98, P < 0.001) and Glu/tCr (Χ2(1, N = 15) = 10.52, P = 0.002) were also significantly reduced. Levels of tCr were not significantly changed after d-βHB (P = 0.66). Levels of Gln and other metabolites did not significantly change (see Fig. 2).
Fig. 2. MRS levels of metabolites.
Box and whisker diagrams representing anterior cingulate distribution of (A) GABA, (B) GABA/tCr, (C) Glu, (D) Glu/tCr, (E) Gln and (F) Gln/tCr in Experiment 1; and similar diagrams representing posterior cingulate distribution of (G) GABA, (H) GABA/tCr, (I) Glu, (J) Glu/tCr, (K) Gln and (L) Gln/tCr in Experiment 2. Results are given in arbitrary units (A.U.).
Experiment 2: acute d-βHB, but not glucose, modulates posterior cingulate metabolites
RM-ANOVA with factors Time (pre-and post-bolus) and Treatment (d-βHB and glucose) showed a significant interaction between factors in the effect on levels of GABA (F(1,21) = 20.16, P < 0.001) and Glu (F(1,25) = 15.0, P < 0.001). For levels of posterior GABA, post-hoc comparisons showed that differences between pre-and post-bolus were significant in the d-βHB condition (P < 0.001) but not glucose (P = 0.685). Similarly, differences in levels of Glu between pre-and post-bolus were significant in the d-βHB condition (P < 0.001) but not glucose (P = 0.685). No other metabolites we significantly modulated (see Fig. 2).
Similar results were obtained in levels of GABA/tCr (F(1,21) = 27.7, P < 0.001) and Glu/tCr (F(1,25) = 34.76, P < 0.001). Post-hoc pairwise comparisons showed that levels of GABA/tCr decreased in the d-βHB condition (P < 0.001) but not glucose (P = 0.755). Levels of Glu/tCr decreased in the d-βHB condition (P < 0.001) but not glucose (P = 0.872) (see Fig. 2).
Experiment 2: acute d-βHB age-dependently modulates posterior GABA and Glu
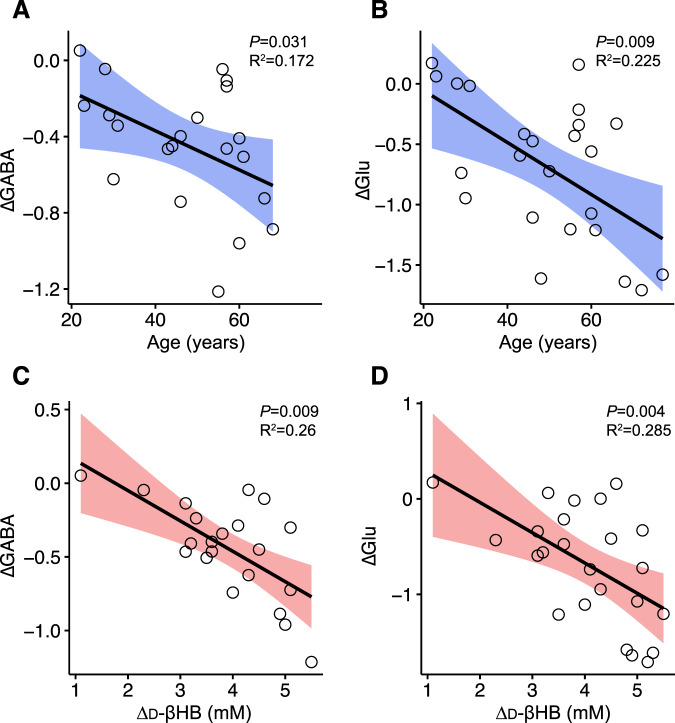
Linear regression models showed that age predicted changes in levels of posterior GABA (ΔGABA; adjR2 = 0.172, β = −0.46, P = 0.031) and Glu (ΔGlu; adjR2 = 0.225, β = −0.51, P = 0.009) with administration of d-βHB (see Fig. 3).
Fig. 3. Associations of age and levels of d-βHB with levels of MRS metabolites.
Scatterplots representing (A) the association of age with ΔGABA and (B) the association of age with ΔGlu levels, (C) the association of blood levels of d-βHB with ΔGABA and (D) the association of blood levels of d-βHB with ΔGlu, in the context of administration of d-βHB in Experiment 2. Age is given in years, Δd-βHB is given in mM and metabolite levels are given in arbitrary units (A.U.). Confidence interval are 95% for the regression lines.
Experiment 2: blood levels of d-βHB predict modulations of posterior GABA and Glu
Linear regression models controlled for age showed that blood levels of d-βHB predicted changes in levels of posterior GABA (ΔGABA; adjR2 = 0.26, β = −0.54, P = 0.009) and Glu (ΔGlu; adjR2 = 0.285, β = −0.56, P = 0.004) with administration of d-βHB. The significant relationships observed show that a larger increase in blood levels of d-βHB (Δd-βHB; d-βHBFinal -d-βHBBaseline) correlated with ΔGABA and ΔGlu (see Fig. 3). Levels of blood glucose were not correlated with changes in levels of GABA (P = 0.12) or Glu (P = 0.82) following administration of d-βHB.
Experiment 2: blood levels of d-βHB predict posterior MRS d-βHB levels
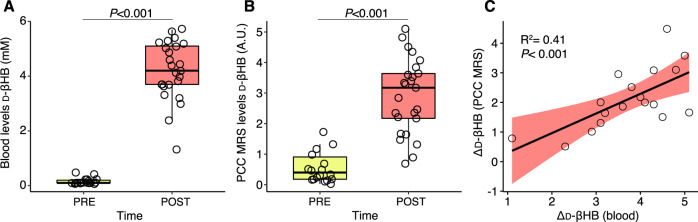
Linear regression showed that blood levels of d-βHB predicted MRS d-βHB (adjR2 = 0.41, β = 0.66, P < 0.001); the relationship remained significant when controlled for age (adjR2 = 0.37, β = 0.64, P = 0.011) (see Fig. 4).
Fig. 4. Blood- and brain-derived levels of d-βHB.
Box and whisker diagrams representing (A) blood levels of d-βHB pre- and post-d-βHB in Experiment 2, (B) MRS levels of d-βHB pre- and post-d-βHB in Experiment 2, and (C) scatterplot representing the association of blood- and MRS-derived levels of Δd-βHB in Experiment 2. Blood Δd-βHB is given in mM and MRS Δd-βHB is given in arbitrary units (A.U.). Confidence interval is 95% for the regression line.
Discussion
Beneficial effects of ketones have been reported in brain aging, AD and drug-resistant epilepsy. While such results are promising, the neurophysiological effect of ketones remains largely uncharacterized, hurdling its development as a therapeutic option in glucose hypometabolism. Here, we investigated the neurochemical effect of an acute administration of d-βHB in fasting healthy participants with ultrahigh-field MRS. Our main results show that levels of GABA and Glu were significantly reduced in the anterior and posterior cortices after administration of d-βHB but not glucose. Importantly, the magnitude of the effect was significantly correlated with older age. Moreover, blood levels of d-βHB predicted cerebral levels of d-βHB.
Our results show that levels of GABA and Glu were significantly reduced after administration of d-βHB. This effect seems generalized to the entire cortex, as results were significant in voxels located in the anterior and posterior cingulate regions, in two different cohorts. Previous works support the idea of decreased glutamatergic activity after the administration of ketones and several mechanisms have been proposed. Studies have reported that d-βHB inhibits vesicular glutamate transporters responsible for storing glutamate into synaptic vesicles at glutamatergic synapses [57]. Other works have shown that d-βHB also acutely activates synthesis of kynurenic acid, an endogenous glutamatergic antagonist [58]. Recent works also showed that administration of d-βHB, at physiological concentration levels similar to human ketosis, resulted in inhibitory action at NMDA receptors, preventing receptor activation in the presence of agonists [59]. Such synaptic effects are supported by the antiepileptic literature, as authors suggest that ketones alter brain handling of glutamate [60]. Additional evidence suggests that astrocyte metabolism is more active in ketosis, resulting in enhanced conversion of Glu to Gln, which leads to more efficient removal of synaptic Glu and, hypothetically, to the upregulated conversion of Gln to GABA [60], which is conflicting to our results on GABA post-d-βHB. Others suggest that when d-βHB replaces glucose as the primary fuel in cultured glutamatergic neurons, glutamate transmission becomes downregulated which reduces glutamatergic excitatory output and thus reduces glutamate-related seizures [61].
A second hypothesis to explain the reduced levels of Glu in hypoglycemia and ketosis resides in the utilization of Glu as a second-choice anaplerotic substrate in the TCA cycle. Oxidation of Glu for energy has been argued before [62, 63] and works suggest astrocytes oxidize Glu at a higher rate than glucose [64]. In hippocampal slices during prolonged hypoglycemia, levels of Glu decreased significantly and were restored to normal levels by administration of dextrose [65]. The authors suggest that decreases in Glu demonstrate its use as substrate for anaplerosis when glucose remains unavailable for a prolonged period. In hypoglycemia, the pyruvate carboxylase reaction is the main anaplerotic reaction to support the TCA cycle but only for a certain period as pyruvate reserves become exhausted. A second anaplerotic reaction producing oxaloacetate from α-ketoglutarate recruits Glu to enter the TCA cycle and synthesize aspartate. Previous MRS results also support the idea of elevated oxidation of Glu in hypoglycemia [66, 67], but this potential explanation for decreased Glu is unlikely within the present study where overnight fasting lowered blood glucose levels but did not induce hypoglycemia.
Importantly, d-βHB is a source of acetyl-CoA and oxidative energy but cannot serve as a precursor of anaplerotic pathway via pyruvate carboxylase [68]. At the cellular level, d-βHB administration inhibits glucose metabolism in astrocytes in cell cultures and hippocampal slices, but this decrease in glycolysis is paralleled by an increased astrocytic metabolism of pyruvate [69]. This suggests that ketone administration may not restore levels of amino acids, including Glu, in hypoglycemia and perhaps partly explains reduced levels of Glu we observed in the post-d-βHB condition, and unchanged levels of Glu post-glucose in Experiment 2. It is possible that administration of glucose quickly normalized levels of Glu, preventing us from measuring a significant difference with MRS, similarly to the results of Rao and colleagues (2010) where dextrose administration restored levels of Glu in rodents. Previous evidence also suggests that brain glucose may be harder to quantify with MRS [22].
The rationale for explaining the reduction in GABA levels after administration of d-βHB is less clear. Previous works in cultured astrocytes have shown that d-βHB suppresses GABA-transaminase time- and dose-dependently, suggesting that d-βHB may transiently reduce catabolism and recapture of GABA. Authors suggest this could potentially lead to increased GABA concentrations and prolonged synaptic GABAergic action, reducing the probability of induction of glutamatergic seizures [70]. Another hypothesis suggests that ketosis increases astrocytic metabolism, leading to a more efficient removal of Glu from the synaptic cleft, an upregulated conversion of Gln to GABA and a higher ratio of GABA to Glu [60]. However, another study showed that administration d-βHB, but not acetone, resulted in inhibitory action at GABAA receptor sites [59]. Concentrations of d-βHB greater than 0.1 mM inhibited the agonist effect of GABA to GABAA receptors, with a maximal effect obtained at concentrations over 5 mM. This suggests that d-βHB could exert an inhibitory effect to both excitatory and inhibitory transmissions, effectively acting as a neuromodulator. Importantly, the results show that although d-βHB showed an inhibitory effect on GABA receptors, for the same dose, its effect was stronger at inhibiting NMDA receptors, suggesting increased efficacy at inhibiting glutamatergic excitatory transmission [59]. Additionally, different doses of d-βHB altered 13C-MRS spectra levels of GABA, Glu and Gln in cortical brain slices, with different effects. The highest dose (+2.5 mmol/L) significantly decreased levels of both GABA and Glu compared to both glucose administration and lower doses of d-βHB (0.25–1.25 mmol/L) [71] consistent with our findings. This physiological concentration of ketones is closest to levels we report in blood samples post-d-βHB (see Table 2 and Table 4 in Supplementary). These results demonstrate how the dosage of d-βHB may affect observed GABA concentrations and support the long-lasting effect of d-βHB observed in a previous MRS study [22].
Importantly, our results show no significant modulation of levels of Gln. Stable levels of Gln suggest unaffected activity of glutamine synthetase, the catalysis enzyme which transforms Glu into Gln in astrocytes, before Gln is transported back to presynaptic neurons for regeneration of Glu. This suggests that production of the main precursor for Glu is not modulated by administration of glucose or d-βHB. Previous MRS works have suggested that Glu-Gln cycling may remain stable in hypoglycemia [66]. Other results have shown a decrease in astrocytic Gln content with the administration of d-βHB compared to glucose, although the effect was small and obtained at high concentrations of d-βHB. Inhibition of glutamine synthetase in the presence of d-βHB (0.25mMol/L) significantly reduced levels of both Gln and GABA [71]. In the present study, it is unlikely that concentrations of d-βHB were not high enough, but it is possible that the timeframe of MRS was suboptimal to capture changes in Gln. Importantly, inhibition of glutamine synthetase is conflicting to previously cited work [60], highlighting that some hypotheses explaining fluctuations of Glu and GABA are mutually exclusive.
Together, results from the literature support our findings on reduced levels of Glu after administration of d-βHB. However, results are not unanimous as to how ketones affect GABA and partly conflict with what we report. Nonetheless, the same results also suggest that the neurophysiological effects of d-βHB are mechanistically complex (i.e. modulation of excitatory and inhibitory transmissions [59]) and dose-dependent [71]. Moreover, the effects of d-βHB are likely compartmentalized and exert differential effects in neurons and astrocytes [72]. Considering the clinical literature, these suggest that antiepileptic effects may depend on inhibiting excitatory glutamatergic output rather than on promoting inhibitory GABAergic mechanisms. MRS literature suggests that modulation of GABAergic outputs can have stimulatory, inhibitory, and neutral net effects on neuronal metabolism, depending on the context [44, 73]. In this perspective, it is possible that ketones and d-βHB modulate both glutamatergic and GABAergic components, with the resulting net effect being a significantly reduced excitatory output [59]. Behavioral measurements are warranted to investigate the potential effect of the modulation of glutamatergic metabolism. Additionally, it should be noted that administration of ketones during moderate fasting may help reach a new transient steady-state, where metabolic rates of GABA, Glu and Gln are preserved.
In exploratory analyses, our results show that the magnitude of the effect of d-βHB on ΔGlu and ΔGABA was predicted by age and blood levels of Δd-βHB, and that cerebral levels of Δd-βHB were predicted by blood levels of Δd-βHB. Previous MRS studies have shown an effect of age on glucose metabolism. Levels of glucose normalized to creatine were higher in older adults compared to young, and even higher in AD patients compared to old, suggesting higher levels of residual glucose and decreased glucose utilization [4]. Ketone metabolism, however, is thought to remain unaffected by normal brain aging [21]. Previous evidence suggests that ketones will compete with glucose and stored glycogen in both neurons [71] and astrocytes [64]. In the d-βHB condition in Experiment 2, ketones were introduced acutely and competed with glucose reserves, and the resulting effect may have depended on individual glucose metabolism, and thus, age. Larger effects on brain levels of GABA and Glu observed in older participants suggest an increased response to ketones, which could be primed by age-related decreased glucose utilization. The decrease in cortical GABA with age is well documented in the MRS literature [74–76] whereas the effect on Glu is more debated [77].
Our results also show that larger increases in blood Δd-βHB predicted larger changes in GABA, Glu and βHB. Prior works have established that plasma levels of d-βHB are highly correlated with their utilization in the brain [32, 78]. This could mean that increased blood Δd-βHB was caused by higher peripheral and cerebral d-βHB utilization, which led to increased effects on cerebral GABA and Glu. This is supported by previous research showing that ketones may reverse network instability in older people [22]. Taken together, our results suggest that relationships between (1) age-induced impaired glucose utilization, (2) age-independent ketone utilization and (3) peripheral and cerebral levels of ketones may explain the observed modulation of cortical levels of GABA and Glu in a ketone administration paradigm.
In this study, we assessed neurochemical characterization of regional (anterior and posterior), temporal (pre- and post-d-βHB) and functionally comparative (d-βHB and glucose) effect of ketones on brain metabolites. Our results demonstrate the limitations of MRS in interpretating cellular mechanisms by which d-βHB alters cerebral metabolism. Future works focusing on deciphering the discrepancies between neuronal and astrocytic metabolism of ketones are warranted, as differences in metabolism of glucose and response to hypoglycemia have been established [79].
Supplementary information
Acknowledgements
The authors wish to thank Dr Dinesh Deelchand from the Center for Magnetic Resonance Research at the University of Minnesota for providing us with the 7T-LCModel basis-set.
Author contributions
AH-B: acquisition, analysis, interpretation of data, drafting the work, final approval of the version to be published. BA: substantial contribution to acquisition of data presented in this work. LMcM: substantial contribution to acquisition data presented in this work. AL: substantial contribution to acquisition of data presented in this work. NAS: substantial contribution to revising the work and interpretation of data. SS: substantial contribution to conception and design and acquisition of data. Y-FY: substantial contribution to revising the work. APL: substantial contribution to revising the work. BGJ: substantial contribution to revising the work. LRM-P: substantial contribution to conception and design, interpretation of data and final approval of the version to be published. E-MR: substantial contribution to conception and design, interpretation of data and final approval of the version to be published.
Funding
The research described in this paper was funded by the W. M. Keck Foundation (to LRM-P), the White House Brain Research Through Advancing Innovative Technologies (BRAIN) Initiative (NSFNCS-FR 1926781 to LRM-P), the National Institutes of Health Grant K01NS110981 (to NAS), the Department of Defense Army Research Office Award W91NF2020189 (to NAS), and the Edward M. Connor Family Endowment for Innovation in Research (to NAS). Scanning at A.A. Martinos Center for Biomedical Imaging enabled by grant P41EB015896. The authors also wish to thank the Baszucki Brain Research Foundation. None of the funding sources played any role in the design of the experiments, data collection, analysis, interpretation of the results, the decision to publish, or any aspect relevant to the study. None of the authors received funding or in-kind support from pharmaceutical and/or other companies to write this manuscript.
Competing interests
AH-B, BA, LMcM, APL, SS, NAS, Y-FY, BGJ and LRM-P declare no conflict of interest. APL is a consultant for Agios Pharmaceuticals, Biomarin Pharmaceuticals, and Moncton MRI; is co-founder of BrainSpec Inc.; and receives research funding from NINDS, NIA, the Department of Defense, and the Alzheimer’s Association. E‐MR is a member on the advisory board for BrainSpec, Inc.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Lilianne R. Mujica-Parodi, Eva-Maria Ratai.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01364-8.
References
- 1.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–45. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36:587–97. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosconi L. Glucose metabolism in normal aging and Alzheimer’s disease: methodological and physiological considerations for PET studies. Clin Transl imaging. 2013;1:217–33. doi: 10.1007/s40336-013-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullins R, Reiter D, Kapogiannis D. Magnetic resonance spectroscopy reveals abnormalities of glucose metabolism in the Alzheimer’s brain. Ann Clin Transl Neurol. 2018;5:262–72. doi: 10.1002/acn3.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011;10:187–98. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wirth M, Bejanin A, La Joie R, Arenaza-Urquijo EM, Gonneaud J, Landeau B, et al. Regional patterns of gray matter volume, hypometabolism, and beta-amyloid in groups at risk of Alzheimer’s disease. Neurobiol aging. 2018;63:140–51. doi: 10.1016/j.neurobiolaging.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Kalpouzos G, Chételat G, Baron JC, Landeau B, Mevel K, Godeau C, et al. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol aging. 2009;30:112–24. doi: 10.1016/j.neurobiolaging.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann N Y Acad Sci. 2008;1147:180–195. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krell-Roesch J, Syrjanen JA, Vassilaki M, Lowe VJ, Vemuri P, Mielke MM, et al. Brain regional glucose metabolism, neuropsychiatric symptoms, and the risk of incident mild cognitive impairment: the Mayo Clinic study of aging. The. Am J Geriatr Psychiatry. 2021;29:179–91. doi: 10.1016/j.jagp.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond TC, Xing X, Wang C, Ma D, Nho K, Crane PK, et al. β-amyloid and tau drive early Alzheimer’s disease decline while glucose hypometabolism drives late decline. Commun Biol. 2020;3:1–13.. doi: 10.1038/s42003-020-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higuchi M, Tashiro M, Arai H, Okamura N, Hara S, Higuchi S, et al. Glucose hypometabolism and neuropathological correlates in brains of dementia with Lewy bodies. Exp Neurol. 2000;162:247–56. doi: 10.1006/exnr.2000.7342. [DOI] [PubMed] [Google Scholar]
- 12.Ciarmiello A, Cannella M, Lastoria S, Simonelli M, Frati L, Rubinsztein DC, et al. Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington’s disease. J Nucl Med. 2006;47:215–22. [PubMed] [Google Scholar]
- 13.Firbank MJ, Yarnall AJ, Lawson RA, Duncan GW, Khoo TK, Petrides GS, et al. Cerebral glucose metabolism and cognition in newly diagnosed Parkinson’s disease: ICICLE-PD study. J Neurol, Neurosurg Psychiatry. 2017;88:310–316. doi: 10.1136/jnnp-2016-313918. [DOI] [PubMed] [Google Scholar]
- 14.Ruud J, Steculorum SM, Brüning JC. Neuronal control of peripheral insulin sensitivity and glucose metabolism. Nat Commun. 2017;8:1–12.. doi: 10.1038/ncomms15259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steculorum SM, Solas M, Brüning JC. The paradox of neuronal insulin action and resistance in the development of aging‐associated diseases. Alzheimer’s Dement. 2014;10:S3–S11. doi: 10.1016/j.jalz.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24:256–68. doi: 10.1016/j.cmet.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF., Jr Brain metabolism during fasting. J Clin Investig. 1967;46:1589–95. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Courchesne-Loyer A, Croteau E, Castellano CA, St-Pierre V, Hennebelle M, Cunnane SC. Inverse relationship between brain glucose and ketone metabolism in adults during short-term moderate dietary ketosis: a dual tracer quantitative positron emission tomography study. J Cereb Blood Flow Metab. 2017;37:2485–93. doi: 10.1177/0271678X16669366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flatt J. On the maximal possible rate of ketogenesis . Diabetes. 1972;21:50–53. doi: 10.2337/diab.21.1.50. [DOI] [PubMed] [Google Scholar]
- 20.Reichard GA, Jr, Owen OE, Haff AC, Paul P, Bortz WM. Ketone-body production and oxidation in fasting obese humans. J Clin Investig. 1974;53:508–15. doi: 10.1172/JCI107584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunnane SC, Courchesne-Loyer A, St-Pierre V, Vandenberghe C, Pierotti T, Fortier M, et al. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Ann N. Y Acad Sci. 2016;1367:12–20. doi: 10.1111/nyas.12999. [DOI] [PubMed] [Google Scholar]
- 22.Mujica-Parodi LR, Amgalan A, Sultan SF, Antal B, Sun X, Skiena S, et al. Diet modulates brain network stability, a biomarker for brain aging, in young adults. Proc Natl Acad Sci. 2020;117:6170–7. doi: 10.1073/pnas.1913042117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yomogida Y, Matsuo J, Ishida I, Ota M, Nakamura K, Ashida K, et al. An fMRI investigation into the effects of ketogenic medium-chain triglycerides on cognitive function in elderly adults: a pilot study. Nutrients. 2021;13:2134. doi: 10.3390/nu13072134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torosyan N, Sethanandha C, Grill JD, Dilley ML, Lee J, Cummings JL, et al. Changes in regional cerebral blood flow associated with a 45 day course of the ketogenic agent, caprylidene, in patients with mild to moderate Alzheimer’s disease: results of a randomized, double-blinded, pilot study. Exp Gerontol. 2018;111:118–21. doi: 10.1016/j.exger.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Ota M, Matsuo J, Ishida I, Takano H, Yokoi Y, Hori H, et al. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neurosci Lett. 2019;690:232–6. doi: 10.1016/j.neulet.2018.10.048. [DOI] [PubMed] [Google Scholar]
- 26.Brandt J, Buchholz A, Henry-Barron B, Vizthum D, Avramopoulos D, Cervenka MC. Preliminary report on the feasibility and efficacy of the modified Atkins diet for treatment of mild cognitive impairment and early Alzheimer’s disease. J Alzheimer’s Dis. 2019;68:969–81. doi: 10.3233/JAD-180995. [DOI] [PubMed] [Google Scholar]
- 27.Fortier M, Castellano CA, Croteau E, Langlois F, Bocti C, St-Pierre V, et al. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimer’s Dement. 2019;15:625–34. doi: 10.1016/j.jalz.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol aging. 2012;33:425–e19-425. doi: 10.1016/j.neurobiolaging.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rebello CJ, Keller JN, Liu AG, Johnson WD, Greenway FL. Pilot feasibility and safety study examining the effect of medium chain triglyceride supplementation in subjects with mild cognitive impairment: a randomized controlled trial. BBA Clin. 2015;3:123–5. doi: 10.1016/j.bbacli.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, et al. Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol aging. 2004;25:311–314. doi: 10.1016/S0197-4580(03)00087-3. [DOI] [PubMed] [Google Scholar]
- 31.Cunnane SC, Courchesne-Loyer A, Vandenberghe C, St-Pierre V, Fortier M, Hennebelle M, et al. Can ketones help rescue brain fuel supply in later life? Implications for cognitive health during aging and the treatment of Alzheimer’s disease. Front Mol Neurosci. 2016;9:53. doi: 10.3389/fnmol.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castellano C-A, Nugent S, Paquet N, Tremblay S, Bocti C, Lacombe G, et al. Lower brain 18F-fluorodeoxyglucose uptake but normal 11C-acetoacetate metabolism in mild Alzheimer’s disease dementia. J Alzheimer’s Dis. 2015;43:1343–53. doi: 10.3233/JAD-141074. [DOI] [PubMed] [Google Scholar]
- 33.Peterman M. The ketogenic diet in epilepsy. J Am Med Assoc. 1925;84:1979–83. doi: 10.1001/jama.1925.02660520007003. [DOI] [Google Scholar]
- 34.Freeman JM, Vining EPG, Pillas DJ, Pyzik PL, Casey JC, Kelly MT. The efficacy of the ketogenic diet—1998: a prospective evaluation of intervention in 150 children. Pediatrics. 1998;102:1358–63. doi: 10.1542/peds.102.6.1358. [DOI] [PubMed] [Google Scholar]
- 35.Wilder RM. The effects of ketonemia on the course of epilepsy. Mayo Clin Bull. 1921;2:307–8.
- 36.Vining EP, Freeman JM, Ballaban-Gil K, Camfield CS, Camfield PR, Holmes GL, et al. A multicenter study of the efficacy of the ketogenic diet. Arch Neurol. 1998;55:1433–7. doi: 10.1001/archneur.55.11.1433. [DOI] [PubMed] [Google Scholar]
- 37.Sourbron J, Klinkenberg S, van Kuijk S, Lagae L, Lambrechts D, Braakman H, et al. Ketogenic diet for the treatment of pediatric epilepsy: review and meta-analysis. Child’s Nerv Syst. 2020;36:1099–109. doi: 10.1007/s00381-020-04578-7. [DOI] [PubMed] [Google Scholar]
- 38.Martin K, Jackson CF, Levy RG, Cooper PN. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database of Syst Rev. 2016;2:CD001903. [DOI] [PubMed]
- 39.Martin-McGill KJ, Bresnahan R, Levy RG, Cooper PN. Ketogenic diets for drug‐resistant epilepsy. Cochrane Database of Syst Rev. 2020;6:CD001903. [DOI] [PMC free article] [PubMed]
- 40.Wijnen B, de Kinderen R, Lambrechts D, Postulart D, Aldenkamp AP, Majoie M, et al. Long-term clinical outcomes and economic evaluation of the ketogenic diet versus care as usual in children and adolescents with intractable epilepsy. Epilepsy Res. 2017;132:91–9. doi: 10.1016/j.eplepsyres.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Yamanashi T, Iwata M, Kamiya N, Tsunetomi K, Kajitani N, Wada N, et al. Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Sci Rep. 2017;7:1–11.. doi: 10.1038/s41598-017-08055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamanashi T, Iwata M, Shibushita M, Tsunetomi K, Nagata M, Kajitani N, et al. Beta-hydroxybutyrate, an endogenous NLRP3 inflammasome inhibitor, attenuates anxiety-related behavior in a rodent post-traumatic stress disorder model. Sci Rep. 2020;10:1–11.. doi: 10.1038/s41598-020-78410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Youm Y-H, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat Med. 2015;21:263–9. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rae CD. A guide to the metabolic pathways and function of metabolites observed in human brain 1 H magnetic resonance spectra. Neurochem Res. 2014;39:1–36. doi: 10.1007/s11064-013-1199-5. [DOI] [PubMed] [Google Scholar]
- 45.Befroy DE, Shulman GI. Magnetic resonance spectroscopy studies of human metabolism. Diabetes. 2011;60:1361–9. doi: 10.2337/db09-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hertz, L and DL Rothman, Glucose, lactate, β-hydroxybutyrate, acetate, GABA, and succinate as substrates for synthesis of glutamate and GABA in the glutamine–glutamate/GABA cycle, in The Glutamate/GABA-Glutamine Cycle. 2016, Springer. p. 9–42. [DOI] [PubMed]
- 47.Cuenoud B, Hartweg M, Godin JP, Croteau E, Maltais M, Castellano CA, et al. Metabolism of exogenous D-beta-hydroxybutyrate, an energy substrate avidly consumed by the heart and kidney. Front in Nutr. 2020;7:1–9. [DOI] [PMC free article] [PubMed]
- 48.Clarke K, Tchabanenko K, Pawlosky R, Carter E, Todd King M, Musa-Veloso K, et al. Kinetics, safety and tolerability of (R)−3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regulatory Toxicol Pharmacol. 2012;63:401–8. doi: 10.1016/j.yrtph.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clarke K, Tchabanenko K, Pawlosky R, Carter E, Knight NS, Murray AJ, et al. Oral 28-day and developmental toxicity studies of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. Regulatory Toxicol Pharmacol. 2012;63:196–208. doi: 10.1016/j.yrtph.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 51.Near J, Harris AD, Juchem C, Kreis R, Marjańska M, Öz G, et al. Preprocessing, analysis and quantification in single‐voxel magnetic resonance spectroscopy: experts’ consensus recommendations. NMR Biomedicine. 2021;34:e4257. doi: 10.1002/nbm.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: a batch‐processing tool for the quantitative analysis of gamma‐aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40:1445–52. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris AD, Puts NA, Edden RA. Tissue correction for GABA‐edited MRS: considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn Reson Imaging. 2015;42:1431–40. doi: 10.1002/jmri.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atassi N, Xu M, Triantafyllou C, Keil B, Lawson R, Cernasov P, et al. Ultra high-field (7tesla) magnetic resonance spectroscopy in Amyotrophic Lateral Sclerosis. PLoS One. 2017;12:e0177680. doi: 10.1371/journal.pone.0177680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc: Ser B (Methodol) 1995;57:289–300. [Google Scholar]
- 56.Team RC, R Core Team R: a language and environment for statistical computing. Foundation for Statistical Computing, 2020.
- 57.Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, et al. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chmiel-Perzyńska I, Kloc R, Perzyński A, Rudzki S, Urbańska EM. Novel aspect of ketone action: β-hydroxybutyrate increases brain synthesis of kynurenic acid in vitro. Neurotox Res. 2011;20:40–50. doi: 10.1007/s12640-010-9220-0. [DOI] [PubMed] [Google Scholar]
- 59.Pflanz NC, Daszkowski AW, James KA, Mihic SJ. Ketone body modulation of ligand-gated ion channels. Neuropharmacology. 2019;148:21–30. doi: 10.1016/j.neuropharm.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 60.Yudkoff M, Daikhin Y, Horyn O, Nissim I, Nissim I. Ketosis and brain handling of glutamate, glutamine, and GABA. Epilepsia. 2008;49:73–75. doi: 10.1111/j.1528-1167.2008.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lund TM, Risa O, Sonnewald U, Schousboe A, Waagepetersen HS. Availability of neurotransmitter glutamate is diminished when β‐hydroxybutyrate replaces glucose in cultured neurons. J neurochemistry. 2009;110:80–91. doi: 10.1111/j.1471-4159.2009.06115.x. [DOI] [PubMed] [Google Scholar]
- 62.McKenna MC. Glutamate pays its own way in astrocytes. Front Endocrinol. 2013;4:191. doi: 10.3389/fendo.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McKenna MC. The glutamate‐glutamine cycle is not stoichiometric: fates of glutamate in brain. J Neurosci Res. 2007;85:3347–58. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- 64.McKenna MC. Substrate competition studies demonstrate oxidative metabolism of glucose, glutamate, glutamine, lactate and 3-hydroxybutyrate in cortical astrocytes from rat brain. Neurochem Res. 2012;37:2613–26. doi: 10.1007/s11064-012-0901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rao R, Ennis K, Long JD, Ugurbil K, Gruetter R, Tkac I. Neurochemical changes in the developing rat hippocampus during prolonged hypoglycemia. J neurochemistry. 2010;114:728–38. doi: 10.1111/j.1471-4159.2010.06797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Terpstra M, Moheet A, Kumar A, Eberly LE, Seaquist E, Öz G. Changes in human brain glutamate concentration during hypoglycemia: insights into cerebral adaptations in hypoglycemia-associated autonomic failure in type 1 diabetes. J Cereb Blood Flow Metab. 2014;34:876–82. doi: 10.1038/jcbfm.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bischof MG, Brehm A, Bernroider E, Krssák M, Mlynárik V, Krebs M, et al. Cerebral glutamate metabolism during hypoglycaemia in healthy and type 1 diabetic humans. Eur J Clin Investig. 2006;36:164–9. doi: 10.1111/j.1365-2362.2006.01615.x. [DOI] [PubMed] [Google Scholar]
- 68.Kapogiannis D, Avgerinos KI. Brain glucose and ketone utilization in brain aging and neurodegenerative diseases. Int Rev Neurobiol. 2020;154:79–110. doi: 10.1016/bs.irn.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valdebenito R, et al. Targeting of astrocytic glucose metabolism by beta-hydroxybutyrate. J Cereb Blood Flow Metab. 2016;36:1813–22. doi: 10.1177/0271678X15613955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suzuki Y, Takahashi H, Fukuda M, Hino H, Kobayashi K, Tanaka J, et al. β-hydroxybutyrate alters GABA-transaminase activity in cultured astrocytes. Brain Res. 2009;1268:17–23. doi: 10.1016/j.brainres.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 71.Achanta LB, Rowlands BD, Thomas DS, Housley GD, Rae CD. β-Hydroxybutyrate boosts mitochondrial and neuronal metabolism but is not preferred over glucose under activated conditions. Neurochem Res. 2017;42:1710–23. doi: 10.1007/s11064-017-2228-6. [DOI] [PubMed] [Google Scholar]
- 72.Achanta LB, Rae CD. β-Hydroxybutyrate in the brain: one molecule, multiple mechanisms. Neurochem Res. 2017;42:35–49. doi: 10.1007/s11064-016-2099-2. [DOI] [PubMed] [Google Scholar]
- 73.Nasrallah FA, Griffin JL, Balcar VJ, Rae C. Understanding your inhibitions: modulation of brain cortical metabolism by GABAB receptors. J Cereb Blood Flow Metab. 2007;27:1510–20. doi: 10.1038/sj.jcbfm.9600453. [DOI] [PubMed] [Google Scholar]
- 74.Porges EC, Jensen G, Foster B, Edden RA, Puts NA. The trajectory of cortical GABA across the lifespan, an individual participant data meta-analysis of edited MRS studies. Elife. 2021;10:e62575. doi: 10.7554/eLife.62575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hone-Blanchet, A, et al., Frontal metabolites and Alzheimer’s disease biomarkers in healthy older women and women diagnosed with mild cognitive impairment. Journal of Alzheimer’s Disease. 2022;87:1131–41. [DOI] [PMC free article] [PubMed]
- 76.Hone-Blanchet A, Bohsali A, Krishnamurthy LC, Shahid S, Lin Q, Zhao L, et al. Relationships between frontal metabolites and Alzheimer’s disease biomarkers in cognitively normal older adults. Neurobiol Aging. 2022;109:22–30. doi: 10.1016/j.neurobiolaging.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cleeland C, Pipingas A, Scholey A, White D. Neurochemical changes in the aging brain: a systematic review. Neurosci Biobehav Rev. 2019;98:306–19. doi: 10.1016/j.neubiorev.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Pan JW, Rothman TL, Behar KL, Stein DT, Hetherington HP. Human brain β-hydroxybutyrate and lactate increase in fasting-induced ketosis. J Cereb Blood Flow Metab. 2000;20:1502–7. doi: 10.1097/00004647-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 79.Wang F, Smith NA, Xu Q, Goldman S, Peng W, Huang JH, et al. Photolysis of caged Ca2+ but not receptor-mediated Ca2+ signaling triggers astrocytic glutamate release. J Neurosci. 2013;33:17404–12. doi: 10.1523/JNEUROSCI.2178-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.