Abstract
Frailty, an age-related condition of increased vulnerability to acute endogenous or exogenous stressors, is a key barrier to successful treatment of cancer in older people. In this group of patients, assessment of frailty is required before starting a new treatment. According to guidelines, the gold standard to assess frailty in older adults with cancer is geriatric screening followed by geriatric assessment (GA) across essential GA-domains (social status, physical function, nutrition, cognition, emotion, co-morbidity, polypharmacy). GA enables tailoring of both oncological therapy and non-oncological interventions to the patient’s vulnerabilities. Large clinical trials recently have demonstrated that the feasibility and tolerability of systemic cancer treatment in older patients are significantly improved by such GA-guided management. Indications and optimal tools for frailty monitoring during the course of cancer treatment have not yet been defined in greater detail. New technologies such as wearable sensors or apps offer promising new opportunities to further develop frailty monitoring. This review describes the current standards and perspectives for the assessment and monitoring of frailty in elderly patients with cancer.
Keywords: cancer, frailty, geriatric screening, geriatric assessment
Introduction
Every year, 20 million new cancer cases occur worldwide.1 Incidence rates are low in younger people, but show a steep increase in older adults.2 In the United States, for example,3 the incidence in people up to the age of 50 years is less than 500 per 100,000 inhabitants a year. For those over 70 years, however, it is four times higher. Approximately 30% of US patients newly diagnosed with cancer are 65 to 74 years old. Another 25% are 75 years or older. Comparable incidence rates across age groups have been reported for other regions and countries.2
Unlike younger patients with cancer, vulnerable older subjects are more susceptible to unfavorable health events and medical complications during the clinical course. “Frailty” is an established term to describe aging-associated vulnerability,4–7 and it has been recognized as a main obstacle of cancer therapy in patients of advanced age.8,9 With frailty, longer lasting therapeutic success is more difficult to achieve. For example, frailty increases the risk of chemotherapy intolerance and of poorer treatment response.10,11 Patients with frailty undergoing cancer surgery have an increased likelihood of post-operative complications.5,12 Advanced frailty may also pose competing risks of morbidity and mortality independent of cancer and its treatment. The prevalence of frailty in older adults with cancer is around 40–50% with a wide range from 5% to 90% depending on the patient population and the method used to assess frailty.8,13
Over the past two decades, huge efforts have been made in order to optimize the detection and quantification of frailty in such patients. The basic underlying idea was to determine a patient’s individual degree of frailty at baseline (ie, before the start of cancer therapy) and to use this information to adjust the oncological treatment.14 This includes the decision whether the patient should receive tumor therapy or not as well as the choice of the most adequate treatment modality and regimen (eg, standard versus gentler therapy).15 The principle of using information from a frailty evaluation to therapeutically target this condition with suitable interventions has been established in geriatric medicine for a long time but just recently adopted to the oncological context.16
International and national medical societies (eg, International Society of Geriatric Oncology [SIOG], American Society of Clinical Oncology [ASCO]) have developed detailed recommendations for the assessment of frailty in older adults prior to the initiation of cancer therapy.17–19 There is a growing understanding that frailty in older patients with cancer is not a static biomarker that just needs to be recorded at a single point in time to make final treatment decisions.20,21 Instead, frailty in such individuals is subject to dynamic changes throughout a patient’s remaining lifespan. This raises the question whether and for what specific purposes frailty should be recorded repeatedly during cancer therapy. Compared to the amount of guidance that is available for the initial frailty assessment in older adults with cancer, there is surprisingly little advice on frailty monitoring so far.
This narrative review makes the effort to summarize the latest advances in the conceptualization of frailty in the context of cancer. The focus is on the increasingly important link between frailty evaluation and frailty interventions as well as the re-evaluation of frailty during cancer treatment. A PubMed search was performed by using variations of the following global search term: [cancer OR tumor] AND [frailty OR geriatric] AND [screening OR assessment OR management OR evaluation OR intervention]. Articles published from January 2005 to January 2023 considered relevant to the topic were examined in greater detail. Additionally, we examined guidelines and consensus recommendations that have been published by SIOG and ASCO or other medical societies (eg, National Comprehensive Cancer Network [NCCN])17–19,22 on the assessment and management of frailty in older adults with cancer. This literature served as the basis for preparing this review.
General Definition and Identification of Frailty
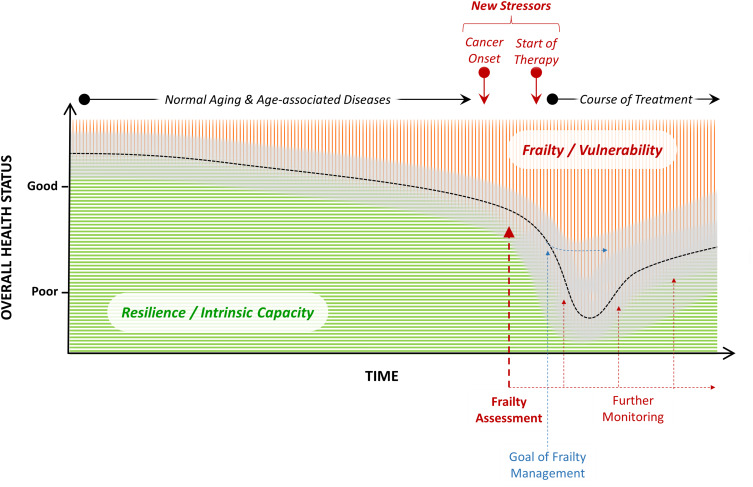
Frailty is generally defined as an age-related clinical condition of increased vulnerability to acute endogenous or exogenous stressors.4–7 Older adults with frailty are at increased risk to experience worsening of their overall health status due to adverse health events emerging from interactions between existing frailty features and new stressor events (Figure 1). Frailty arises primarily from normal aging and age-related diseases.7,23,24 Ordinary aging processes at the molecular and cellular level including senescence and stem cell exhaustion cause a physiological decrease of the reserve capacity of organs and organ systems.25,26 Coincidently, these processes may promote the creation of an environment that fosters the development of pathological tissue degeneration (eg, vascular, musculoskeletal) and subclinical inflammation which lead to common aging-associated diseases (eg, coronary heart disease, osteoarthritis)27 followed by a possible decline in physical and mental function and disability. Later, the cancer disease and damage caused by its treatment may contribute to frailty.28,29 In older patients with cancer, comorbidity (ie, the burden of chronic illness), disability (ie, the loss of function and autonomy), and frailty often co-exist.9 They are considered as overlapping although not identical phenomena. There is also significant overlap between the concepts of frailty and “intrinsic capacity”.30 From a simplified view, intrinsic capacity and resiliency can be understood as the opposite to vulnerability and frailty (Figure 1).
Figure 1.
Illustration of the general concept, impact, assessment, management, and monitoring of frailty in older adults with cancer (red area represents the magnitude of a patient’s frailty and green area represents his/her intrinsic capacity at a given point in time, respectively; the black dotted curve reflects the overall health status at specific time points, with the gray band indicating inter-individual variance).
Two methods are generally recognized as the gold standard to identify frailty in older people.6,7 The “Fried frailty criteria” are based on a phenotype model. In this model, presence of at least 3 of the following 5 criteria in a patient indicate frailty: low physical activity, poor endurance (self-reported exhaustion), weakness (reduced grip strength), slowness (decreased walking speed), and unintentional weight loss.31 However, the use of this approach to identify frailty in routine clinical care has remained uncommon, because there is no generally accepted and clinically easily applicable operationalization of the individual criteria. The “Rockwood frailty index” is based on a deficit-accumulation model.32 The index is calculated by dividing the number of deficits diagnosed by the total number of 70 pre-defined deficits. Deficit items include various diseases, signs from clinical examinations, and impairments of activities of daily living. In contrast to the phenotype model, deficit-accumulation models not just allow to determine whether frailty is present or not (categorical variable), but also to quantify the extent of frailty in a patient (continuous variable). With the 70-item model, an index of 0.25 and above may indicate frailty. In routine care, however, an index calculation with 70 or even with fewer deficit items (eg, 50 or 20) has proven to be too cumbersome to capture frailty. This approach has therefore not become more widely established in clinical practice. In the oncology context, a minority of studies have used Fried criteria or the Rockwood index to identify and measure frailty in older cancer patients.8,9
Techniques other than Fried criteria or the Rockwood index have been accepted as appropriate for detecting frailty in older people.33 These include frailty screenings and geriatric assessment (GA). Both methods are easier to implement in everyday clinical care.
Among numerous frailty screening tools (eg, Identification of Seniors at Risk [ISAR], Groningen Frailty Indicator [GFI], Vulnerable Elders Survey-13 [VES-13], Triage Risk Screening Tool [TRST]),34–37 the Clinical Frailty Scale (CFS) has recently received greater attention and increasingly been used in clinical settings during the Covid pandemic.38–40 This pictogram-driven screening tool summarizes the overall level of frailty of an older person and is easy for clinicians to use as part of their medical history taking and physical examination. Due to its simple structure, CFS is also suitable in situations with acute or new illness to record the previous level of frailty before the new stressor disease has occurred (eg, a symptomatic Covid-19 infection).39 Such information is highly relevant and helps to avoid under-treatment or over-treatment of older patients when it comes to far-reaching treatment decisions (eg, for or against ventilation therapy in the example of Covid-19). Independent of the pandemic, this principle can also be applied to the oncological context. In old patients with newly diagnosed cancer who present in poor general condition, knowledge of frailty before the onset of the tumor disease is very important when anticipating the prospect of tumor-specific therapy of re-improving the condition. The number of studies examining CFS in the oncology setting has increased over the past 1–2 years. A majority was conducted in the context of tumor surgery. Results were promising regarding the usefulness of this tool to predict outcomes (Table 1).
Table 1.
Key Findings from Recently Published Studies Investigating the Clinical Frailty Scale (CFS)38 in Older Adults with Cancer
| Author (Year) | N | Median Age | Setting | Key Results |
|---|---|---|---|---|
| Pearce et al (2022)86 | 514 | 76 years | Gastro-esophageal cancer, 1L palliative chemotherapy (multi center) | Higher CFS scores were associated with poor overall treatment utility, progression, and death |
| Philip et al (2022)87 | 820 | ≥ 65 years | Various cancers, surgical resection (single center) | Higher CFS scores were associated with longer stay, post-op mortality, morbidity, and readmission rate |
| Stamatakos et al (2022)88 | 52 | 76 years | Bladder cancer, with radical cystectomy (single center) | Higher CFS scores were associated with 1-year-mortality, longer hospital stays, and respiratory complications |
| Osatnik et al (2022)89 | 269 | 69 years | Critically ill patients with cancer on ICU (single center) | CFS scores predicted hospital mortality |
| Niemeläinen et al (2021)90 | 161 | 85 years | Colorectal cancer, elective surgery (multi center) | CFS scores ≥ 3 were correlated with more postoperative complications |
| Mima et al (2021)91 | 142 | ≥ 60 years | Pancreatic cancer, surgical resection (single center) | Higher CFS scores predicted poor survival |
Abbreviations: CFS, clinical frailty scale; 1L, first line; ICU, intensive care unit.
GA is a core methodology in geriatric medicine.41,42 Its use for a systematic and comprehensive recording of vulnerabilities in older patients across geriatric domains (social support - activities of daily living - mobility and falls - nutrition - cognition - emotion - sleep - vision and hearing - pain and wounds - co-morbidity - polypharmacy) is firmly anchored in routine geriatric care. GA makes use of traditional assessment tools that have been tried and tested over many years. Among these are scores and scales (eg, Lawton scale for instrumental activities of daily living, Katz scale for basal activities of daily living, Charlson score for comorbidities) as well as performance tests (eg, Timed-Up&Go test for mobility, Mini Mental State Exam for cognition).43–47 Notably, new approaches are currently emerging to perhaps replace parts of a GA using modern sensor-based diagnostics (eg, wearable sensors, apps).
Over recent years, medical disciplines other than geriatrics, such as cardiology or trauma surgery, have begun to discover GA as a potentially useful technique specific to their field.48,49 Oncology is at the forefront of this development.
Frailty Evaluation and Interventions in Older Cancer Patients
A fundamental goal of frailty assessment and management in older adults with cancer is to protect them from adverse health outcomes as well as possible (Figure 1). Unfavorable outcomes to be prevented in general must be distinguished from those that are specific to the oncological context (Table 2). Practical recommendations on frailty evaluation and interventions in older patients with cancer are based on clinical studies examining whether an assessment was able to predict the occurrence or reduced the incidence of such outcomes.17–19 Approaches that were subject to these studies included performance scores, frailty screening, and geriatric assessment. The validity of these approaches can be summarized as follows.
Table 2.
Potential Adverse Outcomes Related to Frailty in Older Adults with Cancer
| General Frailty Outcomes | Oncology-Specific Frailty Outcomes (by Setting) |
|---|---|
| Early death | Systemic drug treatment (incl. chemotherapy) |
| Care dependency |
|
| Nursing home admission |
|
| Hospital admission |
|
| Permanent bedrest |
|
| Falls |
|
| Delirium |
|
| Exacerbation / Progression of chronic diseases | Radiotherapy |
| Onset of acute illnesses |
|
| Adverse drug interactions |
|
|
|
|
|
|
|
| Surgical treatment | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cancer survivor | |
|
Validity of Performance Scores
In routine care, oncologists often use Eastern Cooperative Oncology Group or Karnofsky performance score (ECOG PS, KPS) to roughly estimate the general condition of their patients.50,51 ECOG PS and KPS are tools to describe the overall health status and global activity level of cancer patients. However, poor ECOG PS or KPS numbers may be observed in already chronically ill patients with severe pre-existing diseases (eg, terminal COPD, progressive Parkinson disease) as well as in otherwise healthy patients but with acute symptomatic cancer illness (eg, cancer fatigue, cancer pain, acute infection). However, good ECOG PS or KPS numbers do not rule out the existence of chronic and clinically relevant vulnerabilities in older patients (eg, tendency to fall, mild cognitive impairment, inappropriate polypharmacy).52 The capacity of ECOG PS to predict chemotherapy toxicity in older cancer patients is low.53 Performance scores such as ECOG PS or KPS are therefore insufficient to comprehensively surrogate frailty in older adults with cancer. Neither do these tools allow for differentiated oncological treatment decisions nor for the selection of meaningful frailty interventions.
Validity of Frailty Screenings
Most recommendations of international and national medical societies have in common that frailty assessment in older adults with cancer should start with a quick screening in order to identify those patients who are presumably vulnerable and could benefit from a comprehensive GA. Table 3 lists the screening tools proposed by SIOG and ASCO.17–19 The advice is based on study results. The available evidence has been compiled in several systematic reviews (Table 4). The most frequently applied tool is the so-called G8 (Geriatrics 8) screening.54 Numerous studies in older patients with cancer demonstrated associations between abnormal G8 scores and poor frailty outcomes such as shortened survival, increased treatment toxicity and complications after tumor surgery (Table 4).55,56 The G8 tool queries key vulnerabilities (mobility issues, nutritional issues, cognitive issues and mood problems, and polypharmacy) together with age and subjective health perception. During routine oncological work-ups, such frailty features are usually not checked systematically. Meanwhile, a self-reported version of the G8 has been made available to facilitate its implementation in busy clinics.57 A G8 screening score of ≤14 identifies patients with possibly increased vulnerability and should prompt for a comprehensive GA. Importantly, abnormal results of G8 or other frailty screenings such as VES-13 should not be used alone to mark a patient as being “frail”, because false positive screening may occur. G8 has a high sensitivity albeit lower specificity. In contrast, VES-13 has lower sensitivity but higher specificity than G8.58 Of note, frailty must not be considered as an absolute measure, but always judged in relation to the level of stress burdened on the patient by an offered cancer treatment. The level of stress may vary depending on the type of the tumor therapy (eg, major surgery, high-dose chemotherapy, hematopoietic stem cell transplantation vs mild chemotherapy, immunotherapy, oral hormone therapy etc.). Therefore, G8 or VES-13 are well suited in distinguishing between presumably robust and vulnerable patients. However, these tools have not been sufficiently validated regarding their utility to tailor final oncological treatment or frailty intervention plans.59 For these purposes, greater knowledge about single vulnerabilities is required including information on their severeness, underlying causes, possible consequences in the course of the cancer disease and treatment, and intervenability.
Table 3.
SIOG Recommendations and ASCO Guidelines for the Assessment of Frailty in Older Adults with Cancer
| SIOG Recommendations17,18
(for All Cancer Patients ≥ 70 Years) |
ASCO Guidelines19
(for Cancer Patients ≥ 65 Years with Chemotherapy) |
|---|---|
| Frailty Screening | |
|
|
|
|
|
|
| Geriatric Assessment | |
| 0. Social Status | |
|
|
| 1a. Functional Status - Autonomy | |
|
|
|
|
| 1b. Functional Status - Mobility | |
|
|
|
|
|
|
|
|
| 2. Nutritional Status | |
|
|
|
|
| 3. Cognitive Status | |
|
|
|
|
|
|
|
|
| 4. Emotional Status | |
|
|
| 5. Comorbidity | |
|
|
|
|
|
|
| 6. Polypharmacy | |
|
|
|
|
| 7. Other Domains / Tools | |
|
|
|
|
|
Note: () = To be considered, ePROGNOSIS, www.eprognosis.ucsf.edu (calculators for remaining life expectancy).
Abbreviations: SIOG, International Society of Geriatric Oncology; ASCO, American Society of Clinical Oncology; G8, Geriatrics 8; TRST, Triage Risk Screening Tool; VES-13, Vulnerable Elders Survey 13; IADL, Instrumental Activities of Daily Living; ADL, (Basal) Activities of Daily Living; TUG, Timed-Up-And-Go; SPPB, Short Physical Performance Battery; MNA, Mini Nutritional Assessment; BMI, Body Mass Index; MMSE, Mini Mental State Exam; MOCA, Montreal Cognitive Assessment; BOMC, Blessed Orientation Memory Concentration; GDS, Geriatric Depression Scale; CIRS(-G), Cumulative Illness Rating Scale (Geriatric); ACE-27, Adult Comorbidity Evaluation 27; CCS, Charlson Comorbidity Score; BEERS, Beers criteria; START/STOP, Start/Stop criteria; CARG, Cancer Aging Research Group Chemotoxicity Calculator; CRASH, Chemotherapy Risk Assessment Scale for High-Age Patients; QLQ-C30, Quality of Life Questionnaire C30; WHO ICOPE, World Health Organization Integrated Care for Older People.
Table 4.
Systematic Reviews* of Studies Examining Frailty Screening and Geriatric Assessment in Older Adults with Cancer
| Author | Year | No. of Included Studies | Major Findings |
|---|---|---|---|
| Shaw et al12 | 2022 | 71 | Review included studies of cancer surgery. Frailty was associated with 30-day and long-term mortality, postoperative complications, length of stay, and adverse discharge disposition. |
| Chuang et al92 | 2022 | 6 | Review included 6 RCTs exploring GA plus geriatric interventions. GA was associated with lower incidence of grade 3–5 treatment-related toxicity and dose reduction compared with standard of care. |
| Hamaker et al61 | 2022 | 61 | GA led to changes of oncologic treatment plans in one third of patients, recommendations of non-oncologic frailty interventions in more than two thirds of patients, improved communication about treatment goals, lower treatment toxicity, more frequent treatment completion, improved physical function, and better quality of life. |
| Garcia et al58 | 2021 | 17 | G8 and VES-13 were the most frequently evaluated screening tools. G8 had higher sensitivity, VES-13 had higher specificity. |
| Scheepers et al93 | 2020 | 44 | Review included only studies in hematologic malignancies. Frailty as assessed by screening or GA was predictive for poor overall survival and, with more variation between studies, treatment complications, treatment non-completion, and hospitalization. |
| Van Walree et al56 | 2019 | 46 | Sensitivity for G8 screening was high, but specificity was low. Abnormal G8 results were associated with shorter overall survival and more treatment-related complications. |
| Bruijnen et al94 | 2019 | 46 | Physical function and nutritional status were the GA domains most often associated with adverse outcomes. |
| Hamaker et al95 | 2018 | 35 | After GA, oncologic treatment plans were altered in about one third of patients, and non-oncologic frailty interventions were recommended in more than two thirds of patients. |
| Handforth et al8 | 2015 | 20 | Presence of frailty was associated with increased all-cause mortality, postoperative mortality, and treatment complications. |
| Hamaker et al96 | 2014 | 15 | Review included only studies in hematologic malignancies. Impairments assessed by GA were associated with poor overall survival and (in 2 studies) chemotherapy-related toxicity. |
| Puts et al97 | 2014 | 34 | Abnormal GA results were associated with adverse outcomes. |
| Ramjaun et al98 | 2013 | 9 | Results of GA predicted mortality, chemotherapy-related toxicity, and (in 1 study) complications post-surgery. |
| Puts et al99 | 2012 | 73 | GA time was 10–45 minutes. Some GA domains were associated with adverse outcomes. |
| Hamaker et al59 | 2012 | 14 | G8 and TRST screenings had the highest sensitivity (but poor specificity) for frailty as assessed by GA. Frailty screenings appear to have insufficient discriminative power to select patients for GA. |
Note: *Table excludes systematic reviews of studies examining frailty assessment in single cancer entities (eg, lung cancer, colorectal cancer etc.).
Utility of Geriatric Assessment
SIOG and ASCO strongly recommend to perform comprehensive GA in cancer patients ≥ 65–70 years who were identified as presumably vulnerable by prior frailty screening.17–19 The SIOG recommendations are not targeted to a specific subset of older cancer patients.17,18 The ASCO guideline refers to the subset of older adults receiving chemotherapy.19 Both guidelines uniformly recommend that the GA should cover essential geriatric domains. The minimum is physical function including instrumental and basic activities of daily living (IADL, ADL), mobility, nutrition, cognition, mood, co-morbidity, and co-medications. For each geriatric domain, the guidelines propose a set of GA instruments (Table 3).
To date, a plethora of retrospective and prospective, non-comparative and comparative GA studies in older patients with cancer has been published. The accumulated study evidence has been summarized in several systematic reviews (Table 4) and is the basis for the current recommendations made by SIOG and ASCO (Table 3) or for other country-specific guidelines (eg, NCCN). In general, studies of GA in older adults with cancer were highly heterogenous regarding oncological settings, patient populations, sizes, and endpoints (Table 4). Many of these studies examined one of the following aspects:
Feasibility of GA in older cancer patients.
Prevalence of geriatric impairments (as assessed by GA) in older cancer patients.
Association of geriatric impairments with treatment complications such as toxicity, dose modifications, treatment discontinuation, length of hospital stay, or unplanned hospitalization.
Association of geriatric impairments with cancer treatment efficacy endpoints such as response rates or progression-free survival.
Association of geriatric impairments with overall survival (mostly all-cause mortality).
Impact of performing a GA (vs not performing GA) on communication with patients and their caregivers.
Impact of performing a GA (vs not performing GA) on oncological or non-oncological treatment decisions.
Impact of performing GA (vs not performing GA) on outcomes such as treatment complications or survival.
Impact of performing GA with vs without geriatric management (frailty interventions) on outcomes.
Overall, it can be concluded from these studies that GA uncovers geriatric impairments, predicts treatment tolerability and feasibility, predicts (all-cause) mortality, facilitates communication about treatment goals and preferences, results in changes of oncological treatment plans, and enables targeted non-oncological treatment of geriatric impairments in older cancer patients (Table 4).
The ability of GA to predict treatment complications was exploited by developing chemotoxicity risk calculators. These tools incorporated GA elements and allow to calculate the likelihood of grade 3–5 toxicity during chemotherapy of older patients with cancer. Use of such calculators is strongly recommended by the ASCO guideline addressing the subset of chemotherapy-treated older patients.19 The CARG (Cancer Aging Research Group) and the CRASH (Chemotherapy Risk Assessment Score for High Age Patients) tool are easily accessible online.53,60 Both tools take functional impairments of the patient into account and thus require careful geriatric examination of the patient. Results of selected studies investigating CARG or CRASH in older patients with cancer are outlined in Table 5. It should be noted that neither the CARG nor the CRASH score have been validated in greater detail for their capacity to predict toxicity of non-chemotherapeutic agents (eg, kinase inhibitors, immune checkpoint inhibitors).
Table 5.
Key Findings from Studies Investigating the CARG and CRASH Chemotherapy Toxicity Risk Calculators in Older Adults with Cancer
| Author (Year) | N | Median Age | Setting | Key Results |
|---|---|---|---|---|
| Suto et al (2022)100 | 76 | 71 years | Solid tumors treated with new anticancer regimen | Incidence of grade 3–5 AE during first treatment course correlated with risk predicted by the CARG tool |
| Cavdar et al (2022)101 | 208 | 70 years | Solid tumors treated with chemotherapy | CARG tool predicted incidence of grade 3–5 toxicity |
| Mittal et al (2021)102 | 100 | 68 years | Tumors treated with new chemotherapy | CRASH tool predicted severe chemotherapy toxicity |
| Ostwal et al (2021)103 | 270 | 69 years | Solid tumors treated with curative intent with chemotherapy | CARG tool predicted incidence of grade 3–5 toxicity |
| Alibhai et al (2021)104 | 175 | 73 years | Metastatic cancer of the prostate treated with chemotherapy or androgen-receptor targeted therapy | CARG tool predicted incidence of grade 3–5 toxicity in patients receiving chemotherapy as well as patients treated with antihormone drugs |
| Chan et al (2021)105 | 259 | 73 years | Solid tumors treated with chemotherapy or targeted tumor drugs | CARG tool did not predict severe treatment-related AE |
| Ortland et al (2020)106 | 120 | 77 years | Patients with systemic cancer therapy | CARG and CRASH tools showed similar performance in predicting grade 3–5 AE |
| Zhang et al (2019)107 | 106 | Years | Solid tumors treated with chemotherapy | CARG and CRASH scores were correlated and predicted grade 3–5 toxicity |
| Kotzerke et al (2019)108 | 104 | 73 years | Solid tumors treated with chemotherapy | CARG tool (but not G8) predicted severe (grade 4) chemotherapy-related toxicity |
| Alibhai et al (2017)109 | 46 | 75 years | Patients with prostate cancer treated with chemotherapy | Observed incidence of grade 3–5 AE was lower than predicted by CARG tool |
| Hurria et al (2016)110 | 250 | 73 years | Tumors treated with new chemotherapy | Rate of grade 3–5 toxicity increased with increasing CARG score |
| Extermann et al (2012)60 | 518 | 76 years | Cancers treated with chemotherapy | Development and initial validation of the CRASH tool |
| Hurria et al (2011)53 | 500 | 73 years | Cancers treated with chemotherapy | Development and initial validation of the CARG tool |
Abbreviations: CARG, Cancer Aging Research Group; CRASH, Chemotherapy Risk Assessment Scale for High-Age Patients; AE, adverse events.
Recent pivotal randomized-controlled trials (RCTs) investigating the impact of GA with and without subsequent geriatric management added very compelling evidence that GA is a powerful frailty assessment for older patients with cancer.61,62 These RCTs demonstrated that GA-directed management of vulnerabilities is able to reduce the risk of these patients to experience toxicity or premature discontinuation of systemic cancer treatment. In the two largest RCTs (GAP70+ and GAIN study) with more than 600 patients each, rates of grade 3–5 toxicity were reduced by 20% and 10%, respectively.63,64 The smaller INTEGERATE trial reported lower chemotherapy discontinuation rates with integrated GA-guided oncogeriatric care compared with usual care (33% vs 53%).65 In a RCT in colorectal cancer patients with tumor surgery followed by chemotherapy (GERICO),66 more patients in the oncogeriatric intervention arm completed scheduled chemotherapy compared with patients of the control arm (45% vs 28%). More details for RCTs examining GA with or without geriatric management are shown in Table 6. Of note, there have also been trials which did not meet their primary endpoint.67–69
Table 6.
Selection of Randomized-Controlled Trials (RCTs) Examining Geriatric Assessment (GA) with Frailty Interventions in Older Adults with Cancer
| RCT (Author, Year) | N | Med. Age | Setting | Intervention vs. Control | Key Results |
|---|---|---|---|---|---|
| GAP70+ (Mohile et al 2021)63 |
718 | 77 y | 40 oncology practices (USA), patients with solid tumors or lymphomas starting a new systemic therapy | Oncologists provided with tailored geriatric assessment summary plus recommendations for management of geriatric issues vs usual care | 51% vs 71% grade 3–5 AE, similar OS; fewer falls and more medications discontinued with intervention |
| GAIN (Li et al 2021)64 |
605 | 71 y | Single cancer center (USA), patients with solid tumors starting a new chemotherapy | GA at baseline (in both arms) ± geriatric intervention provided or organized by an MDT | 51% vs 61% grade 3–5 AE, 28% vs 13% completion of advance directive |
| INTEGERATE (Soo et al 2022)65 | 154 | 76 y | Three cancer centers (AUS), patients with solid tumors or aggressive lymphoma | GA at baseline with geriatric consultation, creation of a personal management plan and according interventions vs usual care | Better HRQOL and lower rate of hospital admissions (unplanned) with the intervention, 33% vs 53% treatment discontinuations |
| GERICO (Lund et al 2020)66 |
142 | 75 y | Two oncology clinics (DK), patients with colorectal cancer post-surgery treated with adjuvant or palliative chemotherapy | GA with GA-directed interventions vs usual care | 45% vs 28% completion of the chemotherapy as scheduled, better HRQOL and better mobility in the intervention arm |
| 5C (Puts et al 2021)67 |
351 | 76 y | Eight hospitals (CAN), patients with solid or hematological cancer referred for new chemotherapy | GA by nurse and geriatrician with monthly follow-up by nurse vs usual care | No difference in HRQOL scores |
Abbreviations: RCT, randomized-controlled trial; y, years; AE, adverse events; OS, overall survival; GA, geriatric assessment; MDT, multidisciplinary team; HRQOL, health-related quality of life.
Comprehensive delivery of GA-guided frailty interventions ideally happens within a multidisciplinary approach involving social workers, nurses/nurse practitioners, physiotherapists, occupational therapists, dieticians, psychologists, pharmacists, and geriatricians in addition to oncologists, radiotherapists, and surgeons.70,71 Table 7 shows a list of interventions used in GAP70+, GAIN, INTEGERATE, and GERICO with the intention to improve single vulnerabilities and hence to modify the overall frailty level of older cancer patients over time.63–66 Next to the measurement of oncological outcome improvements (eg, decreased treatment toxicity), successful frailty intervention may also be validated by measuring whether vulnerabilities captured at baseline improve during further follow-up. However, except for the GAP70+ trial,63 none of the randomized frailty intervention trials shown in Table 6 included a geriatric re-assessment during or after the cancer treatment.
Table 7.
Proposal for the Monitoring of Frailty Interventions as Used to Target Single Vulnerabilities in Older Adults with Cancer in Pivotal Randomized-Controlled Trials (GAP70+, GAIN, INTEGERATE, GERICO)63–66
| Domain | Vulnerabilities | Used Interventions in Trials | Proposal for Monitoring |
|---|---|---|---|
| Social status | Loneliness Poverty |
Referral to social worker, implementation of visiting nurse service or transportation service, assistance for economic and social needs, involvement of health care proxy | Re-assessments of HRQOL using questionnaires |
| Functional status Autonomy |
Impairment of IADL Impairment of ADL |
Referral to aide service, referral to occupational therapist, implementation of nurse service, implementation of home service | Re-assessments using IADL or ADL questionnaires |
| Functional status Mobility |
Walking slowness Frequent falls Weakness / Sarcopenia |
Referral to aide service, referral to physical therapist, prescription of exercise, deprescription of orthostatic or psychoactive drugs, education on fall risk evaluation of home safety | Re-assessments using TUG or SPPB etc.; use of activity tracker (eg, footstep count etc.) |
| Nutritional status | Low body mass index / Cachexia Low food or fluid intake Swallowing or teeth problems |
Referral to dietician, referral to specialist for swallowing, referral to dentist, use of anti-emetics, recommendation of diet, education on diet and nutrition | Re-assessments of body mass index; use of a calorie-counting apps |
| Cognitive status | Mild cognitive impairment (MCI) Dementia |
Referral to memory clinic, referral to psychiatry, optimization of pharmacological treatment with psychoactive drugs, shared information with health care proxy, written instructions for appointments and medications, prevention of delirium, use of simple cancer treatment regimens | Re-assessments using MMSE etc.; use of wearable activity tracker, recording of activity using an electronic diary |
| Emotional status | Depression Anxiety |
Referral to psychologist, referral to spiritual services, optimization of pharmacological treatment with antidepressants, appointment with local support groups | Re-assessments using GDS; use of activity tracker, recording of mood using an electronic diary |
| Comorbidity | Co-existing chronic disease | Communication with primary care physician, review of medication, initiation of investigations, modification of cancer treatment regimen | Disease-specific re-assessment (physical exam, laboratory etc.) |
| Polypharmacy | High number of drugs Potentially inadequate drugs |
Referral to pharmacist, recommendation of pill box and medication calendar, communication with primary care physician, deprescribing, education on polypharmacy | Re-assessments of medication risks using BEERS list or electronic prescription tools |
Abbreviations: SIOG, International Society of Geriatric Oncology; ASCO, American Society of Clinical Oncology; G8, Geriatrics 8; TRST, Triage Risk Screening Tool; VES-13, Vulnerable Elders Survey 13; IADL, Instrumental Activities of Daily Living; ADL, (Basal) Activities of Daily Living; TUG, Timed-Up-And-Go; SPPB, Short Physical Performance Battery; MNA, Mini Nutritional Assessment; BMI, Body Mass Index; MMSE, Mini Mental State Exam; MOCA, Montreal Cognitive Assessment; BOMC, Blessed Orientation Memory Concentration; GDS, Geriatric Depression Scale; CIRS(-G), Cumulative Illness Rating Scale (Geriatric); ACE-27, Adult Comorbidity Evaluation 27; CCS, Charlson Comorbidity Score; BEERS, Beers criteria; START/STOP, Start/Stop criteria; CARG, Cancer Aging Research Group Chemotoxicity Calculator; CRASH, Chemotherapy Risk Assessment Scale for High-Age Patients; QLQ-C30, Quality of Life Questionnaire C30; WHO ICOPE, World Health Organization Integrated Care for Older People; RCT, randomized-controlled trial; y, years; AE, adverse events; OS, overall survival; GA, geriatric assessment; MDT, multidisciplinary team; HRQOL, health-related quality of life.
Frailty Monitoring in Older Cancer Patients
Following the initial frailty assessment at the start of a cancer therapy, the overall frailty level as well as single vulnerabilities may undergo significant changes during treatment and throughout a patient’s further life (Figure 1). Over time, alterations of the social situation, the physical and mental functionality, and co-morbidities may occur. Observations from studies suggest that both deterioration and improvements are possible. For example, in a study of 144 over 50 years old breast cancer patients examined for frailty by using modified Fried criteria before and after chemotherapy, the proportion of subjects with a Fried score of 3 or 4 increased from 13% to 46%.72 Another study with 439 older adults with cancer (≥70 years) found functional declines in IADL and ADL in about one third of the patients during chemotherapy.21 The large GOSAFE study, which included more than 1000 participants, explored functional recovery after cancer surgery.73 In individual patients of this study, both loss and gain of function were observed at 3 and 6 months follow-up. There have also been some studies suggesting loss of cognitive capacity (memory function) in patients after chemotherapy.74 Other analyses demonstrated that cancer survivors develop frailty earlier and more frequently compared to non-cancer survivors.29 In the GAP70+ trial, delivery of targeted frailty interventions to older patients receiving chemotherapy resulted in lower numbers of falls and lower numbers of prescribed drugs while physical functioning (IADL, mobility) and mood remained unchanged.63
However, the total number of studies exploring frailty over time in older cancer patients have remained rather low and the available data are of descriptive nature. Therefore, underlying mechanisms of worsening or improvement of frailty must be inferred from clinical observations rather than from systematic study evidence. Clinical experience suggests that progressive tumor disease, toxicity by the tumor treatment, and exacerbation or progression of chronic conditions or acute intercurrent diseases independent of the cancer could drive deterioration of frailty, whereas improvements may occur in response to the remission of a tumor or targeted frailty interventions. Although frailty is subject to dynamic changes and actively modifiable the currently available recommendations by SIOG and ASCO on frailty assessment in older cancer patients are lacking further advice on how to monitor this condition during or after the tumor treatment.17–19 At present, this topic must be discussed in the absence of broader evidence or guidance.
Potential Indications for Frailty Monitoring
Frailty monitoring may play a role in clinical research as well as in clinical practice:
In RCTs investigating cancer treatments, oncology-specific outcomes such as response rates, progression-free and overall survival, adverse events, and quality of life are typical study endpoints. In contrast, re-evaluation of geriatric domains such as physical or cognitive functioning or nutritional status post-treatment have remained uncommon in such trials, even when studying a population of older patients.75 Nevertheless, the inclusion of these endpoints in future RCTs is essential to better understand the risks and benefits of oncological therapies in vulnerable older patients.76
In routine care, re-performing frailty assessments after the start of an oncological treatment together with non-oncological frailty interventions (Table 7) would allow an evaluation of whether and to what degree a patient is responsive to such management. This would enable a multidisciplinary team to decide whether frailty interventions should be continued, escalated, de-escalated, or stopped, or whether the focus of frailty interventions must be shifted towards other vulnerabilities than those considered crucial at the beginning of the tumor treatment. Furthermore, frailty monitoring over time may guide oncologists to increase or decrease the intensity (eg, dosing) of the cancer treatment. To date, however, there has been no study exploring the utility of repeated frailty assessments to guide continuous adaptation of cancer treatment after its initiation.
In routine practice, re-doing a frailty assessment also appears useful if the overall health status of an old patient suddenly deteriorates during systemic cancer treatment and the patient gets hospitalized in an unplanned manner (Figure 1). In addition to the standard investigations of the primary cause of the hospitalization (eg, an infection), a careful holistic view on vulnerabilities at this time point could support decision-making about the need of early rehabilitation measures in the hospital, for example on an acute geriatric ward. Finally, re-assessment of frailty after the end of cancer treatment could inform rehabilitation measures as well as reasonable rehabilitation goals.77,78 This approach seems particularly useful after tumor surgery, radiotherapy, or adjuvant systemic therapy.
Traditional Tools for Frailty Monitoring
Various tools may be used to monitor a patient’s general frailty level as well as his or her individual vulnerabilities (Table 7). Performance scores (ECOG PS, KPS) are commonly used in oncology to follow the general condition and activity level of patients.50,51 However, these tools do not provide deeper insight into the course of single vulnerabilities. The same applies to frailty screenings such as G8 that was not designed to monitor frailty.36,54 However, some screening tools (eg, CFS) may be usable and easy to implement in workflows. Repeated GA using standard assessments (Table 7) are another option for frailty monitoring in older cancer patients. There is no consensus whether the entire GA should be repeated or monitoring of selected GA domains (eg, those that showed abnormal results on initial assessment) is sufficient. Of note, neither the CARG nor the CRASH tool have been studied with regards to monitoring the risk of chemotherapy toxicity over time (eg, between treatment cycles or prior and after rehabilitation).
Smart Digital Tools for Frailty Monitoring
Frailty monitoring in older adults with cancer could be an application for wearable sensors and other digital assessment technology (Table 7). Meanwhile, the technological progress allows for the collection of a multitude of data by portable devices such as wristwatches, foot pods, breast belts, and smartphones worn on the body and equipped with apps. Numerous manufacturers offer such devices fully configured and ready to use for end users. Using commercial activity trackers, physical activity and movement behavior including number of steps, falls, etc. can be tracked in real time. Moreover, these devices are increasingly capable of recording sleeping behavior as well as circulatory and respiratory parameters as for example heart rate, oxygen saturation, and body temperature. Surrogates of cardiopulmonary capacity (eg, VO2max) can be calculated and monitored over time. Downloadable apps for smart phones and tablets offer new opportunities to follow frailty domains other than physical activity and cardiopulmonary reserve. For example, users can repeatedly enter information about their drinking and eating habits, calorie intake or body weight into apps offered commercially in app stores. In the future, apps might also allow us to remotely query mood and drive, or to follow cognitive function through repeated app-embedded cognitive testing.
A growing number of studies examine whether these features of new smart technologies can help to measure frailty in patients at least as well or even better than standard GA instruments or than the Fried criteria or the Rockwood index.79,80 The majority of these studies is aimed to develop digital biomarkers for physical frailty,81 but there have also been some studies exploring the role of digital sensors to assess other aspects of frailty (eg, cognitive frailty).82 Unfortunately, the feasibility and usefulness of such approaches have only been little investigated in oncological settings. So far, there is only rudimentary data available. For example, in a study with 84 older cancer patients (median age 71 years), gait and balance parameters assessed by wearables were degraded when compared with age-matched non-cancer patients as well as in patients with chemotherapy-induced peripheral neuropathy (CIPN) versus without CIPN.83 Another trial explored a wearable activity tracker in somewhat younger patients with cancer undergoing chemotherapy. This study reported a correlation between unplanned health encounters and tracker-recorded activity data, but not ECOG PS.84
Advantages and disadvantages must be weighed against each other when using wearable sensors and apps to assess and monitor frailty in older patients with cancer. Although many products might be available off-the-shelf and consumer-ready (eg, smart watches, app stores), there is no broader accepted standard device and no consented protocol regarding the processing, safe storage and transmission of these digital data. For the moment, the lack of technical standards as well as data protection rules may limit a broader application of these tools for assessing and monitoring frailty. However, if such hurdles are overcome, many new possibilities open up. For example, the data might be transmitted to oncological practitioners or a multiprofessional team responsible for frailty interventions. These caregivers could be alerted in real-time if a patient’s frailty level deteriorates or improves, and may enable them to immediately adjust therapeutic approaches to the new frailty situation.
Conclusions
The evidence for benefits of a frailty assessment in older adults with cancer has significantly increased in recent years. Most importantly, recent prospective, randomized-controlled studies have demonstrated that frailty assessment improves the outcome of such patients.63–66 Frailty assessment followed by frailty interventions significantly enhances the treatment tolerability and feasibility, particularly in elderly patients receiving systemic cancer therapy. The number needed to treat is relatively low at around 5–10.63,64 These new data underscore that frailty assessment is not meant to exclude patients with pre-identified vulnerabilities from cancer therapy, but to make oncological treatments in these patients as safe as possible through additional supportive measures. Based on these new data, performing a comprehensive GA (ie, GA with GA-guided interventions) is at the edge of becoming mandatory in older adults with cancer. However, despite the high level of evidence, only a minority of cancer centers worldwide have integrated GA and GA-led interventions into the routine care of elderly cancer patients so far.85 The implementation barriers are diverse and include lack of knowledge, limited human, temporal and spatial resources, and billing and reimbursement problems.
In addition to a broad implementation of frailty assessments before starting cancer therapy, there is also a growing need to follow-up frailty in older cancer patients in the course of their disease and treatment. Modern digital technologies such as wearable sensors and apps may offer new ways to simplify and advance frailty assessment and monitoring in this patient population. However, evidence in the oncology context remains low. In the future, such approaches could perhaps replace or supplement parts of a GA, thereby reducing the need of resources.
This review is the first to address the issue of continuous frailty assessment in elderly patients with cancer in more detail. Further studies are needed to expand the evidence base. In such studies, the following key questions should be examined as a matter of priority:
Which frailty trajectories are particularly common and typical in older cancer patients receiving a particular treatment?
How do frailty interventions modify such trajectories and how can the success or failure of these interventions be predicted in individual patients?
What tools should be used as a standard to determine changes in frailty in individual patients during cancer treatment and frailty interventions?
How can frailty assessment and monitoring be improved by new smart technologies in older patients with cancer?
Funding Statement
There is no funding to report.
Disclosure
VG received advisory board or speaker fees and travel support from Astra Zeneca, AbbVie, Janssen, Gilead, Roche, Heel, Berlin Chemie, and Merck.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Matsuda T, Saika K. Age-specific cancer incidence rate in the world. Jpn J Clin Oncol. 2020;50(5):626–627. doi: 10.1093/jjco/hyaa057 [DOI] [PubMed] [Google Scholar]
- 3.National cancer institute Surveillance, Epidemiology, and End Results Program (SEER). Available from: https://seer.cancer.gov/. Accessed November 6, 2022.
- 4.Doody P, Lord JM, Greig CA, Whittaker AC. Frailty: pathophysiology, theoretical and operational definition(s), impact, prevalence, management and prevention, in an increasingly economically developed and ageing world. Gerontology. 2022. doi: 10.1159/000528561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeiro AR, Howlett SE, Fernandes A. Frailty-A promising concept to evaluate disease vulnerability. Mech Ageing Dev. 2020;187:111217. [DOI] [PubMed] [Google Scholar]
- 6.Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394(10206):1376–1386. [DOI] [PubMed] [Google Scholar]
- 7.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26(6):1091–1101. [DOI] [PubMed] [Google Scholar]
- 9.Ethun CG, Bilen MA, Jani AB, Maithel SK, Ogan K, Master VA. Frailty and cancer: implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin. 2017;67(5):362–377. [DOI] [PubMed] [Google Scholar]
- 10.Jespersen E, Winther SB, Minet LR, Moller S, Pfeiffer P. Frailty screening for predicting rapid functional decline, rapid progressive disease, and shorter overall survival in older patients with gastrointestinal cancer receiving palliative chemotherapy - a prospective, clinical study. J Geriatr Oncol. 2021;12(4):578–584. doi: 10.1016/j.jgo.2020.10.007 [DOI] [PubMed] [Google Scholar]
- 11.Narasimhulu DM, McGree ME, Weaver AL, et al. Frailty is a determinant of suboptimal chemotherapy in women with advanced ovarian cancer. Gynecol Oncol. 2020;158(3):646–652. doi: 10.1016/j.ygyno.2020.05.046 [DOI] [PubMed] [Google Scholar]
- 12.Shaw JF, Budiansky D, Sharif F, McIsaac DI. The association of frailty with outcomes after cancer surgery: a systematic review and metaanalysis. Ann Surg Oncol. 2022;29(8):4690–4704. doi: 10.1245/s10434-021-11321-2 [DOI] [PubMed] [Google Scholar]
- 13.Overcash J, Cope DG, Van Cleave JH. Frailty in older adults: assessment, support, and treatment implications in patients with cancer. Clin J Oncol Nurs. 2018;22(6):8–18. doi: 10.1188/18.CJON.S2.8-18 [DOI] [PubMed] [Google Scholar]
- 14.Extermann M. Integrating a geriatric evaluation in the clinical setting. Semin Radiat Oncol. 2012;22(4):272–276. doi: 10.1016/j.semradonc.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 15.Balducci L, Extermann M. Management of the frail person with advanced cancer. Crit Rev Oncol Hematol. 2000;33(2):143–148. doi: 10.1016/S1040-8428(99)00063-3 [DOI] [PubMed] [Google Scholar]
- 16.Magnuson A, Allore H, Cohen HJ, et al. Geriatric assessment with management in cancer care: current evidence and potential mechanisms for future research. J Geriatr Oncol. 2016;7(4):242–248. doi: 10.1016/j.jgo.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Society of Geriatrie Oncology (SIOG). Comprehensive geriatric assessment. Available from: https://siog.org/resources/resources-siog/comprehensive-geriatric-assessment/. Accessed November 6, 2022.
- 18.Wildiers H, Heeren P, Puts M, et al. International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595–2603. doi: 10.1200/JCO.2013.54.8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326–2347. doi: 10.1200/JCO.2018.78.8687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goede V, Neuendorff NR, Schulz RJ, Hormigo AI, Martinez-Peromingo FJ, Cordoba R. Frailty assessment in the care of older people with haematological malignancies. Lancet Healthy Longev. 2021;2(11):e736–e745. [DOI] [PubMed] [Google Scholar]
- 21.Kenis C, Decoster L, Bastin J, et al. Functional decline in older patients with cancer receiving chemotherapy: a multicenter prospective study. J Geriatr Oncol. 2017;8(3):196–205. [DOI] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network (NCCN). Older adult oncology. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=4&id=1452. Accessed November 6, 2022.
- 23.Brivio P, Paladini MS, Racagni G, Riva MA, Calabrese F, Molteni R. From healthy aging to frailty: in search of the underlying mechanisms. Curr Med Chem. 2019;26(20):3685–3701. [DOI] [PubMed] [Google Scholar]
- 24.Weiss CO. Frailty and chronic diseases in older adults. Clin Geriatr Med. 2011;27(1):39–52. [DOI] [PubMed] [Google Scholar]
- 25.Zampino M, Ferrucci L, Semba RD. Biomarkers in the path from cellular senescence to frailty. Exp Gerontol. 2020;129:110750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boccardi V, Mecocci P. The importance of cellular senescence in frailty and cardiovascular diseases. Adv Exp Med Biol. 2020;1216:79–86. [DOI] [PubMed] [Google Scholar]
- 27.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monfardini S, Basso U. Oncological causes of frailty in older cancer patients. Eur J Cancer. 2007;43(8):1230–1231. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D, Mobley EM, Manini TM, et al. Frailty and risk of mortality in older cancer survivors and adults without a cancer history: evidence from the National Health and Nutrition Examination Survey, 1999–2014. Cancer. 2022;128(15):2978–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belloni G, Cesari M. Frailty and intrinsic capacity: two distinct but related constructs. Front Med. 2019;6:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol a Biol Sci Med Sci. 2001;56(3):M146–M156. [DOI] [PubMed] [Google Scholar]
- 32.Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K. The mortality rate as a function of accumulated deficits in a frailty index. Mech Ageing Dev. 2002;123(11):1457–1460. [DOI] [PubMed] [Google Scholar]
- 33.Martin FC, O’Halloran AM. Tools for assessing frailty in older people: general concepts. Adv Exp Med Biol. 2020;1216:9–19. [DOI] [PubMed] [Google Scholar]
- 34.McCusker J, Bellavance F, Cardin S, Trepanier S. Screening for geriatric problems in the emergency department: reliability and validity. Identification of Seniors at Risk (ISAR) Steering Committee. Acad Emerg Med. 1998;5(9):883–893. [DOI] [PubMed] [Google Scholar]
- 35.Steverink N, Slaets JP, Schuurmans H, van Lis M. Measuring frailty: developing and testing the GFI (Groningen Frailty Indicator). Gerontologist. 2001;41(1):236. [Google Scholar]
- 36.Saliba D, Elliott M, Rubenstein LZ, et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49(12):1691–1699. [DOI] [PubMed] [Google Scholar]
- 37.Hustey FM, Mion LC, Connor JT, Emerman CL, Campbell J, Palmer RM. A brief risk stratification tool to predict functional decline in older adults discharged from emergency departments. J Am Geriatr Soc. 2007;55(8):1269–1274. [DOI] [PubMed] [Google Scholar]
- 38.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Church S, Rogers E, Rockwood K, Theou O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20(1):393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kastora S, Kounidas G, Perrott S, Carter B, Hewitt J, Myint PK. Clinical frailty scale as a point of care prognostic indicator of mortality in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2021;36:100896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veronese N, Custodero C, Demurtas J, et al. Comprehensive geriatric assessment in older people: an umbrella review of health outcomes. Age Ageing. 2022;51:5. [DOI] [PubMed] [Google Scholar]
- 42.Ellis G, Gardner M, Tsiachristas A, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2017;9:CD006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 44.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10(1):20–30. [DOI] [PubMed] [Google Scholar]
- 45.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 46.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. [DOI] [PubMed] [Google Scholar]
- 47.Folstein MF, Folstein SE, McHugh PR. ”Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 48.Demurtas J, Ecarnot F, Cernesi S, Solari M, Munoz MA, Cella A. Comprehensive geriatric assessment in cardiovascular disease. Adv Exp Med Biol. 2020;1216:87–97. [DOI] [PubMed] [Google Scholar]
- 49.Eamer G, Taheri A, Chen SS, et al. Comprehensive geriatric assessment for older people admitted to a surgical service. Cochrane Database Syst Rev. 2018;1:CD012485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 51.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of Chemotherapeutic Agents. Columbia, Univ Press; 1949:196. [Google Scholar]
- 52.Jolly TA, Deal AM, Nyrop KA, et al. Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist. 2015;20(4):379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellera CA, Rainfray M, Mathoulin-Pelissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23(8):2166–2172. [DOI] [PubMed] [Google Scholar]
- 55.Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288–300. [DOI] [PubMed] [Google Scholar]
- 56.van Walree IC, Scheepers E, van Huis-Tanja L, et al. A systematic review on the association of the G8 with geriatric assessment, prognosis and course of treatment in older patients with cancer. J Geriatr Oncol. 2019;10(6):847–858. [DOI] [PubMed] [Google Scholar]
- 57.van Walree IC, Vondeling AM, Vink GR, et al. Development of a self-reported version of the G8 screening tool. J Geriatr Oncol. 2019;10(6):926–930. [DOI] [PubMed] [Google Scholar]
- 58.Garcia MV, Agar MR, Soo WK, To T, Phillips JL. Screening tools for identifying older adults with cancer who may benefit from a geriatric assessment: a systematic review. JAMA Oncol. 2021;7(4):616–627. [DOI] [PubMed] [Google Scholar]
- 59.Hamaker ME, Jonker JM, de Rooij SE, Vos AG, Smorenburg CH, van Munster BC. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol. 2012;13(10):e437–e444. [DOI] [PubMed] [Google Scholar]
- 60.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118(13):3377–3386. [DOI] [PubMed] [Google Scholar]
- 61.Hamaker M, Lund C, Te Molder M, et al. Geriatric assessment in the management of older patients with cancer - A systematic review (update). J Geriatr Oncol. 2022;13(6):761–777. [DOI] [PubMed] [Google Scholar]
- 62.Puts M, Soo WK, Szumacher E, Decoster L. Methods for frailty screening and geriatric assessment in older adults with cancer. Curr Opin Support Palliat Care. 2021;15(1):16–22. [DOI] [PubMed] [Google Scholar]
- 63.Mohile SG, Mohamed MR, Xu H, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet. 2021;398(10314):1894–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li D, Sun CL, Kim H, et al. Geriatric Assessment-Driven Intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: a randomized clinical trial. JAMA Oncol. 2021;7(11):e214158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soo WK, King MT, Pope A, Parente P, Darzins P, Davis ID. Integrated Geriatric Assessment and Treatment Effectiveness (INTEGERATE) in older people with cancer starting systemic anticancer treatment in Australia: a multicentre, open-label, randomised controlled trial. Lancet Healthy Longev. 2022;3(9):e617–e627. [DOI] [PubMed] [Google Scholar]
- 66.Lund CM, Vistisen KK, Olsen AP, et al. The effect of geriatric intervention in frail older patients receiving chemotherapy for colorectal cancer: a randomised trial (GERICO). Br J Cancer. 2021;124(12):1949–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Puts N, Alqurini N, Strohschein F, et al. Comprehensive geriatric assessment and management for Canadian elders with Cancer: the 5C study. J Clin Oncol. 2021;39(15_suppl):12011. [Google Scholar]
- 68.Orum M, Eriksen SV, Gregersen M, et al. The impact of a tailored follow-up intervention on comprehensive geriatric assessment in older patients with cancer - a randomised controlled trial. J Geriatr Oncol. 2021;12(1):41–48. [DOI] [PubMed] [Google Scholar]
- 69.DuMontier C, Uno H, Hshieh T, et al. Randomized controlled trial of geriatric consultation versus standard care in older adults with hematologic malignancies. Haematologica. 2022;107(5):1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karnakis T, Gattas-Vernaglia IF, Saraiva MD, Gil-Junior LA, Kanaji AL, Jacob-Filho W. The geriatrician’s perspective on practical aspects of the multidisciplinary care of older adults with cancer. J Geriatr Oncol. 2016;7(5):341–345. [DOI] [PubMed] [Google Scholar]
- 71.Goede V, Stauder R. Multidisciplinary care in the hematology clinic: implementation of geriatric oncology. J Geriatr Oncol. 2019;10(3):497–503. [DOI] [PubMed] [Google Scholar]
- 72.Gilmore N, Kadambi S, Lei L, et al. Associations of inflammation with frailty in patients with breast cancer aged 50 and over receiving chemotherapy. J Geriatr Oncol. 2020;11(3):423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Montroni I, Ugolini G, Saur NM, et al. Quality of life in older adults after major cancer surgery: the GOSAFE International Study. J Natl Cancer Inst. 2022;114(7):969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huehnchen P, van Kampen A, Boehmerle W, Endres M. Cognitive impairment after cytotoxic chemotherapy. Neurooncol Pract. 2020;7(1):11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Le Saux O, Falandry C, Gan HK, You B, Freyer G, Peron J. Changes in the use of end points in clinical trials for elderly cancer patients over time. Ann Oncol. 2017;28(10):2606–2611. [DOI] [PubMed] [Google Scholar]
- 76.Nipp RD, Yao NA, Lowenstein LM, et al. Pragmatic study designs for older adults with cancer: report from the U13 conference. J Geriatr Oncol. 2016;7(4):234–241. [DOI] [PubMed] [Google Scholar]
- 77.Ghignone F, Hernandez P, Mahmoud NN, Ugolini G. Functional recovery in senior adults undergoing surgery for colorectal cancer: assessment tools and strategies to preserve functional status. Eur J Surg Oncol. 2020;46(3):387–393. [DOI] [PubMed] [Google Scholar]
- 78.Pergolotti M, Lyons KD, Williams GR. Moving beyond symptom management towards cancer rehabilitation for older adults: answering the 5W’s. J Geriatr Oncol. 2018;9(6):543–549. [DOI] [PubMed] [Google Scholar]
- 79.Cruz AM, Monsalve L, Ladurner AM, Jaime LF, Wang D, Quiroga DA. Information and communication technologies for managing frailty: a systematic literature review. Aging Dis. 2021;12(3):914–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vavasour G, Giggins OM, Doyle J, Kelly D. How wearable sensors have been utilised to evaluate frailty in older adults: a systematic review. J Neuroeng Rehabil. 2021;18(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park C, Mishra R, Golledge J, Najafi B. Digital biomarkers of physical frailty and frailty phenotypes using sensor-based physical activity and machine learning. Sensors. 2021;21:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou H, Park C, Shahbazi M, et al. Digital biomarkers of cognitive frailty: the value of detailed gait assessment beyond gait speed. Gerontology. 2022;68(2):224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zahiri M, Chen KM, Zhou H, et al. Using wearables to screen motor performance deterioration because of cancer and chemotherapy-induced peripheral neuropathy (CIPN) in adults - Toward an early diagnosis of CIPN. J Geriatr Oncol. 2019;10(6):960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nilanon T, Nocera LP, Martin AS, et al. Use of wearable activity tracker in patients with cancer undergoing chemotherapy: toward evaluating risk of unplanned health care encounters. JCO Clin Cancer Inform. 2020;4:839–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Banna GL, Cantale O, Haydock MM, et al. International survey on frailty assessment in patients with cancer. Oncologist. 2022;27(10):e796–e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pearce J, Swinson D, Cairns D, et al. Frailty and treatment outcome in advanced gastro-oesophageal cancer: an exploratory analysis of the GO2 trial. J Geriatr Oncol. 2022;13(3):287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Philip FA, Jagathnath Krishna KM, Bhargavan RV, Augustine P, Thomas S. Comparison of preoperative assessment tools in older patients undergoing cancer surgery: a prospective study. J Geriatr Oncol. 2022;13(4):420–425. [DOI] [PubMed] [Google Scholar]
- 88.Stamatakos PV, Moschotzopoulos D, Glykas I, et al. Outcomes of radical cystectomy in pT4 bladder cancer frail patients: alpha high-volume single center study. J Frailty Sarcopenia Falls. 2022;7(3):147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Osatnik J, Matarrese A, Leone B, et al. Frailty and clinical outcomes in critically ill patients with cancer: a cohort study. J Geriatr Oncol. 2022;13:1156–1161. [DOI] [PubMed] [Google Scholar]
- 90.Niemelainen S, Huhtala H, Jamsen E, et al. One-year functional outcomes of patients aged 80 years or more undergoing colonic cancer surgery: prospective, multicentre observational study. BJS Open. 2022;6(4):zrac094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mima K, Hayashi H, Nakagawa S, et al. Frailty is associated with poor prognosis after resection for pancreatic cancer. Int J Clin Oncol. 2021;26(10):1938–1946. [DOI] [PubMed] [Google Scholar]
- 92.Chuang MH, Chen JY, Tsai WW, et al. Impact of comprehensive geriatric assessment on the risk of adverse events in the older patients receiving anti-cancer therapy: a systematic review and meta-analysis. Age Ageing. 2022;51:7. [DOI] [PubMed] [Google Scholar]
- 93.Scheepers ERM, Vondeling AM, Thielen N, van der Griend R, Stauder R, Hamaker ME. Geriatric assessment in older patients with a hematologic malignancy: a systematic review. Haematologica. 2020;105(6):1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bruijnen CP, van Harten-Krouwel DG, Koldenhof JJ, Emmelot-Vonk MH, Witteveen PO. Predictive value of each geriatric assessment domain for older patients with cancer: a systematic review. J Geriatr Oncol. 2019;10(6):859–873. [DOI] [PubMed] [Google Scholar]
- 95.Hamaker ME, Te Molder M, Thielen N, van Munster BC, Schiphorst AH, van Huis LH. The effect of a geriatric evaluation on treatment decisions and outcome for older cancer patients - A systematic review. J Geriatr Oncol. 2018;9(5):430–440. [DOI] [PubMed] [Google Scholar]
- 96.Hamaker ME, Prins MC, Stauder R. The relevance of a geriatric assessment for elderly patients with a haematological malignancy--a systematic review. Leuk Res. 2014;38(3):275–283. [DOI] [PubMed] [Google Scholar]
- 97.Puts MT, Santos B, Hardt J, et al. An update on a systematic review of the use of geriatric assessment for older adults in oncology. Ann Oncol. 2014;25(2):307–315. [DOI] [PubMed] [Google Scholar]
- 98.Ramjaun A, Nassif MO, Krotneva S, Huang AR, Meguerditchian AN. Improved targeting of cancer care for older patients: a systematic review of the utility of comprehensive geriatric assessment. J Geriatr Oncol. 2013;4(3):271–281. [DOI] [PubMed] [Google Scholar]
- 99.Puts MT, Hardt J, Monette J, Girre V, Springall E, Alibhai SM. Use of geriatric assessment for older adults in the oncology setting: a systematic review. J Natl Cancer Inst. 2012;104(15):1133–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suto H, Inui Y, Okamura A. Validity of the cancer and aging research group predictive tool in older Japanese patients. Cancers. 2022;14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cavdar E, Iriagac Y, Karaboyun K, Avci O, Seber ES. Prospective comparison of the value of CARG, G8, and VES-13 toxicity tools in predicting chemotherapy-related toxicity in older Turkish patients with cancer. J Geriatr Oncol. 2022;13(6):821–827. doi: 10.1016/j.jgo.2022.03.004 [DOI] [PubMed] [Google Scholar]
- 102.Mittal A, Rangaraju RR, Agarwal A, Chandragouda D, Batra S, Qureshi S. Estimating the risk of chemotherapy toxicity in Indian geriatric patient population and utility of chemotherapy risk assessment scale for high age patients (CRASH) score. South Asian J Cancer. 2021;10(3):161–166. doi: 10.1055/s-0041-1729447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ostwal V, Ramaswamy A, Bhargava P, et al. Cancer Aging Research Group (CARG) score in older adults undergoing curative intent chemotherapy: a prospective cohort study. BMJ Open. 2021;11(6):e047376. doi: 10.1136/bmjopen-2020-047376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alibhai SMH, Breunis H, Hansen AR, et al. Examining the ability of the Cancer and Aging Research Group tool to predict toxicity in older men receiving chemotherapy or androgen-receptor-targeted therapy for metastatic castration-resistant prostate cancer. Cancer. 2021;127(14):2587–2594. doi: 10.1002/cncr.33523 [DOI] [PubMed] [Google Scholar]
- 105.Chan WL, Ma T, Cheung KL, et al. The predictive value of G8 and the Cancer and aging research group chemotherapy toxicity tool in treatment-related toxicity in older Chinese patients with cancer. J Geriatr Oncol. 2021;12(4):557–562. doi: 10.1016/j.jgo.2020.10.013 [DOI] [PubMed] [Google Scholar]
- 106.Ortland I, Mendel Ott M, Kowar M, et al. Comparing the performance of the CARG and the CRASH score for predicting toxicity in older patients with cancer. J Geriatr Oncol. 2020;11(6):997–1005. doi: 10.1016/j.jgo.2019.12.016 [DOI] [PubMed] [Google Scholar]
- 107.Zhang J, Liao X, Feng J, Yin T, Liang Y. Prospective comparison of the value of CRASH and CARG toxicity scores in predicting chemotherapy toxicity in geriatric oncology. Oncol Lett. 2019;18(5):4947–4955. doi: 10.3892/ol.2019.10840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kotzerke D, Moritz F, Mantovani L, et al. The performance of three oncogeriatric screening tools - G8, optimised G8 and CARG - in predicting chemotherapy-related toxicity in older patients with cancer. A prospective clinical study. J Geriatr Oncol. 2019;10(6):937–943. doi: 10.1016/j.jgo.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 109.Alibhai SM, Aziz S, Manokumar T, Timilshina N, Breunis H. A comparison of the CARG tool, the VES-13, and oncologist judgment in predicting grade 3+ toxicities in men undergoing chemotherapy for metastatic prostate cancer. J Geriatr Oncol. 2017;8(1):31–36. doi: 10.1016/j.jgo.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 110.Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366–2371. doi: 10.1200/JCO.2015.65.4327 [DOI] [PMC free article] [PubMed] [Google Scholar]