Abstract
Background
A neurocognitive phenotype of post-COVID-19 infection has recently been described that is characterized by a lack of awareness of memory impairment (i.e., anosognosia), altered functional connectivity in the brain’s default mode and limbic networks, and an elevated monocyte count. However, the relationship between these cognitive and brain functional connectivity alterations in the chronic phase with the level of cytokines during the acute phase has yet to be identified.
Aim
Determine whether acute cytokine type and levels is associated with anosognosia and functional patterns of brain connectivity 6–9 months after infection.
Methods
We analyzed the predictive value of the concentration of acute cytokines (IL-1RA, IL-1β, IL-6, IL-8, IFNγ, G-CSF, GM-CSF) (cytokine panel by multiplex immunoassay) in the plasma of 39 patients (mean age 59 yrs, 38–78) in relation to their anosognosia scores for memory deficits via stepwise linear regression. Then, associations between the different cytokines and brain functional connectivity patterns were analyzed by MRI and multivariate partial least squares correlations for the whole group.
Results
Stepwise regression modeling allowed us to show that acute TNFα levels predicted (R2 = 0.145; β = −0.38; p = .017) and were associated (r = −0.587; p < .001) with scores of anosognosia for memory deficits observed 6–9 months post-infection. Finally, high TNFα levels were associated with hippocampal, temporal pole, accumbens nucleus, amygdala, and cerebellum connectivity.
Conclusion
Increased plasma TNFα levels in the acute phase of COVID-19 predict the presence of long-term anosognosia scores and changes in limbic system functional connectivity.
Keywords: Anosognosia, Cytokine, Cognition, TNFα, SARS-CoV-2, Post-COVID-19 condition, Immunology
1. Introduction
Follow-up studies of patients with COVID-19 have highlighted the prevalence of long-term cognitive disorders (Voruz et al., 2022d), relatively independently of the severity of the respiratory disease in the acute phase (Mazza et al., 2022, Voruz et al., 2022b). Memory and executive deficits are commonly reported, in addition to fatigue and psychiatric symptoms (Alnefeesi et al., 2021, group, C.-B.O.C.S., 2021, Voruz et al., 2022a, Voruz et al., 2022b). Intriguingly, research has shown that some patients are unaware of their memory impairment (i.e., anosognosia for memory dysfunction) (Voruz et al., 2022b). This symptom is associated with poorer cognitive performances and changes in the functional connectivity of the limbic system among patients with COVID-19, but also in other pathologies (Starkstein, 2014, Voruz et al., 2022b, Voruz et al., 2022c). Long-term anosognosia for memory dysfunction after COVID-19 has also been associated with leukocyte variations during the acute phase (Nuber-Champier, 2022), with the monocyte count in the acute phase predicting anosognosia for memory loss 6–9 months after infection. The inflammatory cascade triggered by SARS-CoV-2 infection involves the secretion of numerous cytokines including tumor necrosis factor (TNFα), a key cytokine in signaling and COVID-19-related protein cascades (Karki et al., 2021). One emerging hypothesis following observations of persistent long-term cognitive difficulties and physiological patterns after COVID-19 suggests a potential trigger or accelerator of neurodegenerative process (Beauchet and Allali, 2022, McAlpine et al., 2021). Indeed, different studies have shown that COVID-19 associates with neurotoxicity, hippocampal degeneration, and increased risk of Alzheimer's disease (Bayat et al., 2022, Chiricosta et al., 2021, Li et al., 2022). However, although the hypothesis of an initiation (or acceleration) of neurodegenerative cascades is central after COVID-19 infection, other hypotheses that limbic encephalitis after COVID-19 (Franke et al., 2023, Franke et al., 2021) would result in persistent cognitive symptoms could potentially be another interesting avenue (Shnayder et al., 2022).
Finally, previous research underlined the role of cytokine markers in the persistence of psychiatric symptoms three months after infection (Mazza et al., 2021), while others highlighted the existence of relationships between immune, cognitive and neuropsychiatric data in pathologies such as depression, lupus, and Alzheimer's disease (Barcik et al., 2021, Diamond et al., 2006; Kronfol and Remick, 2000; McAfoose and Baune, 2009). Therefore, it would be interesting to explore whether blood cytokine levels reflecting the immune response in the acute phase of COVID-19 are associated with cognitive disorders following COVID-19.
In this context, the aim of the present study was twofold: i) to explore whether cytokine synthesis is associated with (and predictive of) long-term anosognosia, and ii) to investigate the relationship between functional brain connectivity and cytokine markers (Contreras et al., 2020; Jayaraman et al., 2021).
Based on previous results indicating that variation of monocytes to total leukocytes is predictive of anosognosia in the chronic phase (Nuber-Champier, 2022), together with the observation of an association between cytokines and cognitive disorders in other diseases (Kronfol and Remick, 2000, McAfoose and Baune, 2009), we hypothesized that innate immune cytokine synthesis in the acute phase of COVID-19 predicts anosognosia scores for memory dysfunction in the chronic phase of COVID-19 (6–9 months after infection). In addition, and in line with known associations between brain structures and inflammatory processes in both Alzheimer’s disease (Bayat et al., 2022, Jayaraman et al., 2021) and COVID-19 (Douaud et al., 2022), we expected to observe patterns of brain connectivity involving the hippocampal, para-hippocampal and subcortical structures related to inflammatory markers (e.g., TNFα).
2. Methods
2.1. General procedure
From the COVID-COG cohort, this study consisted of extracting patients with viable acute cytokine data, and neuropsychological assessment data; in particular regarding memory anosognosia observed in the long term 6–9 months after infection. In this way, a regression analysis was performed to highlight the predictive value of acute cytokines on long-term cognitive impairment, in particular for anosognosia for memory deficits. Finally, functional imaging analyses were performed to highlight functional differences in relation to cognitive phenotypes and immune markers. Additional analyses for comparison and prediction of anosognosia status are presented in the supplementary informations E-J.
2.2. The COVID-COG cohort
In the COVID-COG cohort (Voruz et al., 2022d), 121 patients were included following strict inclusion criteria. Notably, patients had no history of neurological, psychiatric, cancer, neurodevelopmental pathology, pregnancy or age over 80 years, and a confirmed COVID-19 infection by positive polymerase chain reaction (PCR) test from a nasopharyngeal swab and/or positive serological results. The patients were divided according to the severity of their infection in the acute phase, i.e., a so-called mild group of infected but not hospitalized patients (n = 49), a so-called moderate group of hospitalized patients without the need for mechanical ventilation (n = 48) and a so-called severe group of patients coming from intensive care and requiring mechanical ventilation (n = 24). Each group was matched and comparable with respect to age, socio-cultural level, language (interview in French) and clinical variables such as diabetes or medical history (e.g., sleep apnea). Each patient was assessed using a battery of validated cognitive tests, 6–9 months after SARS-CoV-2 infection, during a neuropsychological interview in the neurology department of the University Hospitals of Geneva. The battery consisted of tests assessing memory, executive, perceptual, praxis, emotional, language, attention, and reasoning processes. In addition, psychiatric questionnaires were completed (Voruz et al., 2022a), a neurological examination was also performed by two certified neurologists, and an magnetic resonance imaging (MRI) (see below).
The brain MRI acquisition procedure is extensively described in Voruz et al. (2022b), and details of the MRI sequences are listed in supplementary information A-C. Preprocessing of the functional and structural images was performed with fMRIPrep 20.2.3 (Esteban et al., 2019), which is based on Nipype 1.6.1 (Gorgolewski et al., 2011). Functional connectivity was measured as the Fisher-transformed Spearman’s correlation coefficient between timeseries averaged within a custom brain parcellation. We considered 100 cortical (Schaefer et al., 2018), 34 cerebellar (Diedrichsen et al., 2009) and 22 subcortical (Amunts et al., 2013) regions associated with 19 resting-state networks: 17 cortical, 1 cerebellar, and 1 subcortical (Yeo et al., 2011).
After being given a full description of the study, participants provided their written informed consent. The study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the cantonal ethics committee of Geneva (CER-02186).
2.3. Objective cognitive measurement of memory processes
In accordance with the MNESIS model (Eustache et al., 2016), we measured different dimensions of memory. We used 4 memory tasks to highlight verbal episodic memory, visuo-spatial episodic memory, verbal and visuo-spatial working and short-term memory processes. We used the following tools: the numerical span (Drozdick et al., 2018) measuring verbal and short-term working memory, the Corsi test (Kessels et al., 2000) measuring visuo-spatial and short-term working memory, the RLRI 16 (Grober and Buschke, 1987) measuring verbal episodic memory and the Rey figure (Meyers and Meyers, 1995) measuring visuo-spatial episodic memory. These tests are commonly used in clinical practice to assess memory processes. Measurements of symptom validity and noncredible symptoms using the Behavior Rating Inventory of Executive Function (Abeare et al., 2021) were satisfactory for all participants (Voruz et al., 2022d).
2.4. Measurement of anosognosia of memory deficits
In order to quantify anosognosia, cognitive Complaints Self-Report (QPC) (Thomas-Anterion et al., 2004) scores (QPC internal consistency, Cronbach's Alpha=0.80) were first standardized and divided as follows: 0 = normal behavior, 1 = minor influence on daily life, 2 = considerable influence on daily life, and 3 = substantial influence on daily life. Each standardized subjective measure was then subtracted from patients’ scores on standardized objective memory measures (number span, Corsi test, free/cued recall of 16 items, and Rey's complex figure). This yielded self-appraisal discrepancy scores (SAD) scores ranging from − 3–3, with scores below 0 indicating anosognosia. For example, if a patient did not report any memory problems (cognitive complaints score = 3), but failed objective memory tests (e.g., delayed free recall RL/RI 16 score = 0), we assumed that this patient was anosognosic for memory dysfunction: 0 (standardized episodic memory test score) - 3 (cognitive complaints score) = −3. This SAD method has been previously used in Voruz et al., 2022 and Nuber-Champier et al. (2022). Furthermore, the group allocation according to nosognosia is available in supplementary information D-E.
2.5. Retrospective dataset extraction from the COVID-COG cohort
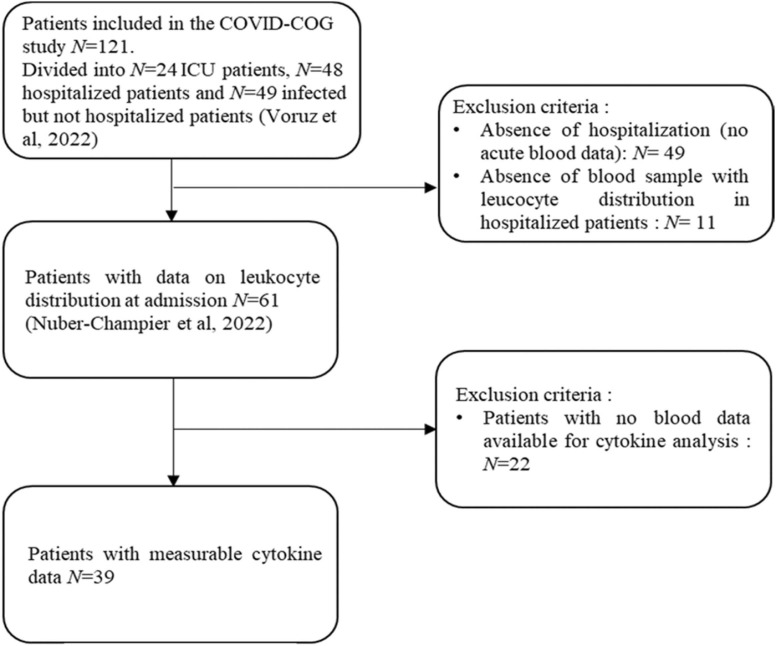
On the 121 patients included in the cohort, 61 hospitalized patients had blood samples for leukocyte distribution (Nuber-Champier, 2022). Of the 61 hospitalized patients with blood samples, 39 patients had analyzable samples for cytokine analysis. The present study sample therefore consisted of 39 patients enrolled in the COVID-COG cohort (Voruz et al., 2022d) who presented blood samples and leukocyte distribution at the time of hospitalization, as described previously (Nuber-Champier et al., 2022) (see Fig. 1 for the flowchart). Blood samples for plasma cytokine analysis were collected between 16 March 2020 and 8 February 2021 during the first days after hospitalization for COVID-19, no patient had received any medication that could impact on cytokines levels. Of these, 15 patients benefited from brain MRI data.
Fig. 1.
Study flowchart. Note. Of the 121 patients initially included in the COVID-COG cohort (Voruz et al., 2022d), we retained only those hospitalized patients who presented blood sample data. Thus, 61 hospitalized patients presented venous blood data (Nuber-Champier, 2022), including 39 patients' samples suitable for cytokine analysis. Regarding the MRI data a total of 41 hospitalized patients presented data (Voruz et al., 2022c). However, of these 41 patients only 15 patients had both analyzable blood samples for cytokine quantification and MRI data. Abbreviation: ICU, intensive care unit.
2.6. Retrospective cytokine analysis
The analysis of cytokines from the biobank of the Geneva University Hospitals' laboratories including TNFα, interleukin (IL)− 1RA, IL-1β, IL-6, IL-8, interferon gamma (IFNγ), granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF), were measured (pg/ml) using commercially available multiplex bead immunoassays (Fluorokine MAP Multiplex Human Cytokine Panel, R&D Systems, Minneapolis, USA) and read using a Bioplex 200 Array Reader (Bio-Rad Laboratories, Hercules, CA, USA) and Luminex® xMAP™ technology (Luminex Corporation, Austin, TX, USA). It is to be noted that the short time between sample processing and freezing did not cause any alteration of the cytokines.
2.7. Statistical analysis
2.7.1. Predictive analyses of long-term anosognosia
We performed a stepwise linear regression analysis to predict anosognosia scores for memory deficits (6–9 months post infection) by acute cytokines (TNFα, IL-1RA, IL-1β, IL-6, IL-8, IFNγ, G-CSF and GM-CSF). We included age, gender, education level and duration of hospitalization in the regression model. We applied false discovery rate (FDR) correction.
2.7.2. Relationship between brain functional connectivity networks and cytokine markers
We computed multivariate correlations between functional connectivity measures and cytokine data using partial least squares correlation (PLSC) analysis (Griffa et al., 2021, Kebets et al., 2019, Krishnan et al., 2011, McIntosh and Lobaugh, 2004). This approach served to identify linear combinations (latent components) of whole-brain functional connectivity patterns and acute-phase cytokines with maximum covariances across patients. The analyses were performed on the whole sample, regardless of whether patients were anosognosic. The significance of the multivariate correlations was evaluated with permutation testing (1000 permutations), and the reliability of the features’ contributions (loadings) to the correlation components with bootstrapping (500 resamples). PLSC analyses were performed using the myPLS toolbox (https://github.com/danizoeller/myPLS).
The significance threshold was set at p < .05 with Bonferroni correction (Sedgwick, 2012) for multiple testing at p < .05. All analyses apart from neuroimaging were performed with the statistical software SPSS 28.0.1.
3. Results
3.1. Socio-demographical and cytokine variables
Of the 39 patients, 17 (43%) had symptoms requiring intermediate hospitalization without mechanical ventilation in the acute phase of the infection (moderate respiratory symptoms) and 22 (56%) required hospitalization in an intensive care unit (ICU) with mechanical ventilation (severe respiratory symptoms). The clinical and sociodemographic data of this sample, extracted from the Geneva COVID-COG cohort, are presented in Table 1. COVID-19 infection was detected by a reverse transcription polymerase chain reaction (RT-PCR) test using the LightCycler® 480 system (Roche, Switzerland) and blood samples were taken 1.37 ± 3.10 days on average after a positive PCR test. From these samples cytokine concentration analyses were performed and are listed in Table 2 (differences of cytokine concentration according to (a-)nosognosia subgroup are presented in supplementary information F-H).(Table 1, Table 2).
Table 1.
Sociodemographic and clinical measures of patients 6–9 months after infection.
| Patients (6–9 months after infection) n = 39 |
|
|---|---|
| Mean age in years ( ± SD) | 59.4 ( ± 11.4) |
| Education level (1/2/3) | 1/14/24 |
| Sex (F/M) | 8/31 |
| Number of patients who required intermediate/intensive care in the acute phase | 17/22 |
| Mean days of hospitalization ( ± SD) | 30.33 ( ± 28.40) |
| Mean days between positive RT-PCR test and collection of immunological data ( ± SD) | 1.37 ( ± 3.10) |
| Diabetes (Yes/No) | 7/32 |
| History of respiratory disorders (Yes/No) | 9/30 |
| History of cardiovascular disorders (Yes/No) | 9/30 |
| History of neurological disorders (Yes/No) | 0/39 |
| History of psychiatric disorders (Yes/No) | 0/39 |
| History of cancer (Yes/No) | 0/39 |
| History of severe immunosuppression (Yes/No) | 0/39 |
| History of developmental disorders (Yes/No) | 0/39 |
Note. Education level: 1 = compulsory schooling, 2 = post-compulsory schooling, and 3 = university degree or equivalent. RT-PCR: reverse transcription polymerase chain reaction allowing RNA to be quantified to determine COVID-19 infection.
Table 2.
Cytokine measures for patients with COVID-19 on admission to hospital.
| Cytokine count on Day 1 of hospitalization | Patients cytokine count in acute phase (n = 39) Mean ( ± SD) |
|---|---|
| TNFα (pg/ml) | 4.72 ( ± 4.08) |
| IL-6 (pg/ml) | 22.26 ( ± 25.72) |
| IL-8 (pg/ml) | 24.13 ( ± 52.37) |
| IL-1Ra (pg/ml) | 6487.94 ( ± 5080.61) |
| Il-1 beta (pg/ml) | 0.88 ( ± 1.21) |
| IFN gamma (pg/ml) | 1.54 ( ± 1.69) |
| G-CSF (pg/ml) | 20.97 ( ± 34.54) |
| GM-CSF (pg/ml) | 0.54 ( ± 0.53) |
Note. G-CSF: granulocyte colony-stimulating factor; GM-CSF: granulocyte-macrophage colony-stimulating factor; IFNγ: interferon gamma; IL: interleukin; TNFα: tumor necrosis factor.
3.2. TNFα level in acute phase predicts scores of anosognosia for memory deficits 6–9 months after COVID-19 infection
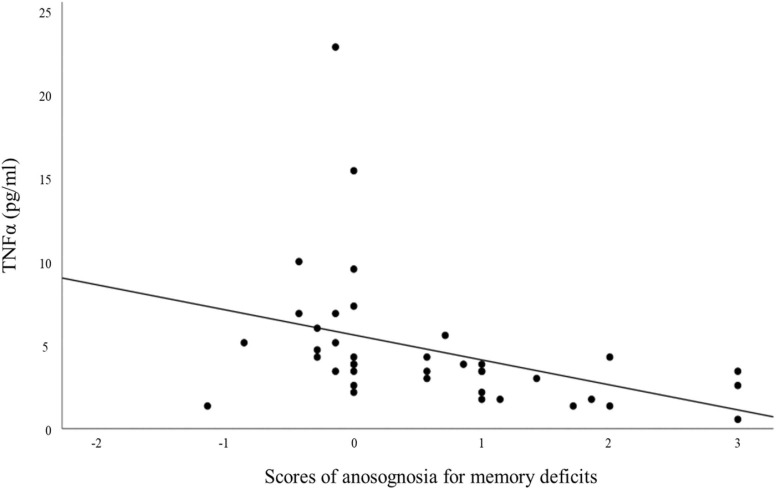
Regression models including cytokines as predictors of anosognosia scores for memory deficits allowed us to show that TNFα levels (pg/ml) collected during the acute phase of the infection significantly predicted (with FDR correction) anosognosia for memory deficits 6–9 months post-infection (R2 = 0.145; β = −0.38; p = .017) and was associated with scores of anosognosia for memory deficits 6–9 months after infection. (r = −0.587; p < .001). Age, gender, education or number of days in hospital did not predict anosognosia scores for memory impairment 6–9 months post infection. In addition, as an exploratory approach, the prediction of anosognosia status compared to non-anosognosia status is presented in supplementary information I-J. ( Fig. 2).
Fig. 2.
Association between TNFα rates and scores of anosognosia for memory deficits. Note. Scores ranging from − 3 (not represented) to < 0 reflect anosognosia for memory disorders, while scores ranging from 0 to + 3 reflects an absence of anosognosia (see also, Voruz et al., 2022 and supplementary information F-J).
3.3. Higher rates of TNFα are associated with reduced functional connectivity in the hippocampus
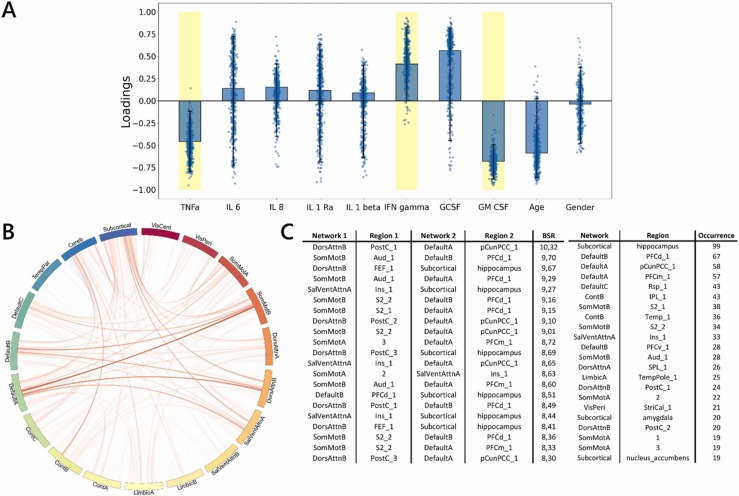
The whole-group PLSC analyses revealed one significant component (p = .01) that explained 35.01% of the covariance between measures of the cytokine markers and functional connectivity. The neuroimaging pattern revealed a positive contribution of the functional connectivity between the default mode, somatosensory motor (SomMot), salience ventral attention (SalVentAttn), and subcortical and cerebellar (Cereb) networks including areas such as the hippocampus, precuneus, and insula. In general, this pattern was associated with lower rates of both TNFα and GM-CSF, as well as higher rates of IFNγ.( Fig. 3).
Fig. 3.
Multivariate latent component of PLSC analysis. Note. A) Loadings of behavioral data. PLSC loadings were defined as correlation coefficients between a given feature and its weight in the latent component. Dots represent samples from the bootstrap procedure, and yellow highlights indicate the reliability of the scores’ contributions to the multivariate correlation component. B) Bootstrap sampling ratios for functional connectivity. This network representation illustrates the neuroimaging pattern, with red links indicating a positive influence of functional connectivity on the latent component and darker colors indicating a higher number of significant connections for each pair of resting-state networks involved in the pattern. C) Twenty brain networks and regions whose connections had the greatest impact (BSR: bootstrap sampling ratio) on the latent component.
4. Discussion
In the present study, we showed that TNFα plasma levels measured during the acute phase of COVID-19 can predict scores of anosognosia for memory deficits and are associated with changes in functional connectivity in several networks including the hippocampus, temporal pole, accumbens nucleus, amygdala, and cerebellum. Plasma levels of TNFα measured in hospitalized patients during the acute phase of COVID-19 were reported to be higher in anosognosic patients compared to non-anosognosic patients at the 6–9-month follow-up (see supplementary information F-H).
The results of the present study support the hypothesis that immunological events in the acute phase of COVID-19 are associated with the presence of long-term cognitive sequelae. The cytokine storm observed at the acute phase of COVID-19 is suggested to cause tissue damage in many organs following infection (Ceban et al., 2022, Mangalmurti and Hunter, 2020). Studies have shown that respiratory severity does not sufficiently explain the long-term cognitive effects (Fernández-Castañeda et al., 2022, Mazza et al., 2022, Voruz et al., 2022d), whereas prolonged systemic inflammation may explain the long-term cognitive and psychiatric sequela (Ceban et al., 2022, Fernández-Castañeda et al., 2022, group, C.-B.O.C.S., 2021). As previously described by Voruz et al. (2022b), anosognosia for memory dysfunction could be a useful neurological marker, capable of identifying patients with cognitive deficits after COVID-19.
More specifically, the results of the present study suggest that levels of the cytokine TNFα could be used to predict anosognosia scores for memory deficits. These results were particularly robust, given that the blood samples were taken at entry to hospital, so before any medication was given, and patients had no medical history with potential cognitive implications prior to SARS-CoV-2 infection. Evidence of a relationship between inflammatory markers and cognition has already been found in other contexts. In their review, McAfoose and Baune (2009) described the involvement of the cytokines IL-1β, IL-6 and TNFα in cognitive processes associated with neurophysiological phenomena such as synaptic plasticity and neurogenesis. More specifically, the authors argued that these cytokines have an initial impact on learning and memory by acting on hippocampal regions. Where there is prolonged exposure, they can trigger the development of neurodegenerative molecular cascades. More recently, in a longitudinal study, Lindbergh et al. (2020) showed that TNFα is involved in the reduction of gray matter in older individuals, highlighting associations between TNFα and accelerated brain atrophy and a decline in global cognitive performances.
The release of cytokines such as TNFα following SARS-CoV-2 infection has already been described (Karki et al., 2021). As reported by Lyra e Silva et al. (2022), COVID-related inflammation has an impact on mood disorders and increases the risk of developing dementia. Similarly, Li et al. (2022) suggested that hospitalization with COVID-19 is associated with a significantly higher risk of developing Alzheimer's disease. Research on Alzheimer's disease has revealed potential degenerative effects of TNFα in relation to functional connectivity. These studies have also shown that TNFα mediates hippocampal neuronal loss in Alzheimer's disease (Jayaraman et al., 2021). Curiously, similarities between post-COVID-19 condition and phenotypes of Alzheimer's disease have also emerged at the cognitive level. According to Starkstein (2014) and Mondragón et al. (2019), anosognosia in Alzheimer's disease reflects greater cognitive impairment and is associated with prefrontal, temporal, subcortical and cerebellar anatomical structures, including default mode network which is similar to what is observed in the context of COVID-19 (Voruz et al., 2022b). Taken together, evidence of anosognosia, TNFα plasma level elevation, and hippocampal hypoconnectivity suggest similarities to the markers of Alzheimer's disease and could be long-term risk factors for cognitive impairment following COVID-19.
There are potential treatments to modulate protein and cytokine signaling cascades. Cheng et al. (2014) showed the strategic potential of targeting TNFα and its production via microglia in order to limit cytotoxicity in neurons. According to these authors, this approach could limit neurodegenerative cascades, and learning and memory disorders. Ekert et al. (2018) recently reported beneficial changes in behavior and cognition in all trials where TNFα was manipulated. Therefore, given our results, it might be interesting to replicate the analyses with a larger sample and to test whether limiting innate immune factors in the acute phase has an effect on cognitive impairment in the chronic phase. Furthermore, although an association between anosognosia and inflammatory effects has now been documented in post-COVID-19 condition, it would be relevant to investigate whether similar associations can be found in patients with multiple subjective cognitive complaints as well as neuropsychiatric symptoms (i.e., opposite end of post-COVID-19 condition spectrum).
Finally, the present study had three main limitations that must be considered when interpreting the results. First, the blood samples were collected during the acute phase, and other confounding variables may have influenced our observations and predictions on the immuno-cognitive and neuroimaging relationships between the acute and chronic phases (e.g., sedation). Second, although we recruited study participants with strict criteria, we are still susceptible to selectivity bias. Further investigation of the immunological response to COVID-19 and detailed analyses of cytokine markers should be carried out in larger samples to confirm the present hypotheses. Third, data were collected for participants who tested positive for COVID-19 in the earlier stages of the pandemic. This means that we measured the effects of the initial COVID-19 strain, and we cannot speculate whether subsequent SARS-CoV-2 variants would have had the same impact.
5. Conclusion
TNFα plasma levels in patients hospitalized with acute SARS-CoV2 infection predicted scores of anosognosia for memory dysfunction 6–9 months after infection. TNFα levels were significantly associated with hippocampal connectivity. Our results suggest that certain protein cascades, in particular the TNFα, are associated with cognitive and connectivity processes that are reminiscent of neurodegenerative processes. Therefore, the consideration of immunological markers (e.g., TNFα) in the acute phase could be crucial for identifying patients at risk of developing long-term cognitive impairments after COVID-19. Future trials of possible immune interventions for cognitive deficits could have major implications for the cognitive symptoms of post-COVID-19 condition.
Conflict of interest
The authors report no conflicts of interest.
Acknowledgements
The present research was supported by Swiss National Science Foundation (SNSF) funding to JAP (PI) and FA (Co-PI) within the framework of the COVID-19 National Research Program (NRP 78; grant no. 407840_198438).
Implication of the authors
A. Nuber-Champier: Contributed to the writing, analysis and examination of the patients in the study. A. Cionca: Contributed to the writing and analysis in the study. G. Breville: Contributed to the writing and examination of the cytokines in the study. P. Voruz: Contributed to the writing and examination of the patients in the study. I. Jacot de Alcântara: Contributed to the writing and examination of the patients in the study. G. Allali: Contributed to the neurological expertise, writing and proofreading of the analyses and interpretations. P.H. Lalive: Contributed to the neuro-immunological expertise, writing and proofreading of the analyses and interpretations. L. Benzakour: Contributed to the psychiatric expertise, writing and proofreading. K.-O. Lövblad: Contributed to the expertise in neuroimaging, writing and proofreading, as well as the hospital organization in the realization of MRI. O. Braillard: Contributed to the coordination of the patients and the review of this work. M. Nehme: Contributed to the coordination of the patients and the review of this work. M. Coen: Contributed to the coordination of the patients and the review of this work. J. Serratrice: Contributed to the coordination of the patients and the review of this work. J.-L Reny: Contributed to the coordination of the patients and the review of this work. J. Pugin: Contributed to the coordination of the patients and the review of this work. I. Guessous: Contributed to the coordination of the patients and the review of this work. B.N. Landis: Contributed to the coordination of the patients and the review of this work. A. Griffa: Contributed to the neuroimaging expertise, writing and proofreading of the analyses and interpretations. D. Van De Ville: Contributed to the neuroimaging expertise, writing and proofreading of the analyses and interpretations. F. Assal: Contributed to the writing of the overall project, proofreading and scientific direction. J.A. Péron: Contributed to the writing of the overall project, proofreading and scientific direction.
Data availability
Nonsensitive COVID-COG data will be made available at the end of the project on a dedicated open-access platform.
References
- Abeare K., Razvi P., Sirianni C.D., Giromini L., Holcomb M., Cutler L., Kuzmenka P., Erdodi L.A. Introducing alternative validity cutoffs to improve the detection of non-credible symptom report on the BRIEF. Psychol. Inj. Law. 2021;14:2–16. [Google Scholar]
- Alnefeesi Y., Siegel A., Lui L.M., Teopiz K.M., Ho R., Lee Y., Nasri F., Gill H., Lin K., Cao B. Impact of SARS-CoV-2 infection on cognitive function: a systematic review. Front. Psychiatry. 2021:1629. doi: 10.3389/fpsyt.2020.621773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K., Lepage C., Borgeat L., Mohlberg H., Dickscheid T., Rousseau M.-É., Bludau S., Bazin P.-L., Lewis L.B., Oros-Peusquens A.-M. BigBrain: an ultrahigh-resolution 3D human brain model. Science. 2013;340:1472–1475. doi: 10.1126/science.1235381. [DOI] [PubMed] [Google Scholar]
- Barcik W., Chiacchierini G., Bimpisidis Z., Papaleo F. Immunology and microbiology: how do they affect social cognition and emotion recognition? Current Opinion in Immunology. 2021;71:46–54. doi: 10.1016/j.coi.2021.05.001. [DOI] [PubMed] [Google Scholar]
- Bayat A.-H., Azimi H., Hassani Moghaddam M., Ebrahimi V., Fathi M., Vakili K., Mahmoudiasl G.-R., Forouzesh M., Boroujeni M.E., Nariman Z. COVID-19 causes neuronal degeneration and reduces neurogenesis in human hippocampus. Apoptosis. 2022:1–17. doi: 10.1007/s10495-022-01754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O., Allali G. The prevention of major neurocognitive disorders in the next phase of COVID-19 pandemic: on being proactive. Maturitas. 2022 doi: 10.1016/j.maturitas.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceban F., Ling S., Lui L.M., Lee Y., Gill H., Teopiz K.M., Rodrigues N.B., Subramaniapillai M., Di Vincenzo J.D., Cao B. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Shen Y., Li R. Targeting TNF: a therapeutic strategy for Alzheimer's disease. Drug Discov. Today. 2014;19:1822–1827. doi: 10.1016/j.drudis.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Chiricosta L., Gugliandolo A., Mazzon E. SARS-CoV-2 exacerbates beta-amyloid neurotoxicity, inflammation and oxidative stress in alzheimer’s disease patients. Int. J. Mol. Sci. 2021;22:13603. doi: 10.3390/ijms222413603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras J.A., Aslanyan V., Sweeney M.D., Sanders L.M., Sagare A.P., Zlokovic B.V.…Pa J. Functional connectivity among brain regions affected in Alzheimer's disease is associated with CSF TNF-α in APOE4 carriers. Neurobiology of aging. 2020;86:112–122. doi: 10.1016/j.neurobiolaging.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond B., Kowal C., Huerta P.T., Aranow C., Mackay M., DeGiorgio L.A.…Volpe B.T. Immunity and acquired alterations in cognition and emotion: lessons from SLE. Advances in immunology. 2006;89:289–320. doi: 10.1016/S0065-2776(05)89007-8. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., Balsters J.H., Flavell J., Cussans E., Ramnani N. A probabilistic MR atlas of the human cerebellum. neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P., Lange F., Andersson J.L., Griffanti L., Duff E. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozdick, L.W., Raiford, S.E., Wahlstrom, D., Weiss, L.G., 2018. The Wechsler Adult Intelligence Scale—Fourth Edition and the Wechsler Memory Scale—Fourth Edition, Contemporary Intellectual Assessment: Theories, Tests, and Issues, 4th ed. The Guilford Press, New York, NY, US, pp. 486–511.
- e Silva N.M.L., Barros-Aragão F.G., De Felice F.G., Ferreira S.T. Inflammation at the crossroads of COVID-19, cognitive deficits and depression. Neuropharmacology. 2022;209 doi: 10.1016/j.neuropharm.2022.109023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekert J.O., Gould R.L., Reynolds G., Howard R.J. TNF alpha inhibitors in Alzheimer's disease: a systematic review. Int. J. Geriatr. Psychiatry. 2018;33:688–694. doi: 10.1002/gps.4871. [DOI] [PubMed] [Google Scholar]
- Esteban O., Markiewicz C.J., Blair R.W., Moodie C.A., Isik A.I., Erramuzpe A., Kent J.D., Goncalves M., DuPre E., Snyder M. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods. 2019;16:111–116. doi: 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustache F., Viard A., Desgranges B. The MNESIS model: memory systems and processes, identity and future thinking. Neuropsychologia. 2016;87:96–109. doi: 10.1016/j.neuropsychologia.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Fernández-Castañeda A., Lu P., Geraghty A.C., Song E., Lee M.-H., Wood J., O’Dea M.R., Dutton S., Shamardani K., Nwangwu K. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. 2022;185:2452–2468. doi: 10.1016/j.cell.2022.06.008. e2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C., Ferse C., Kreye J., Reincke S.M., Sanchez-Sendin E., Rocco A., Steinbrenner M., Angermair S., Treskatsch S., Zickler D. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav. Immun. 2021;93:415–419. doi: 10.1016/j.bbi.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C., Boesl F., Goereci Y., Gerhard A., Schweitzer F., Schroeder M., Foverskov-Rasmussen H., Heine J., Quitschau A., Kandil F.I. Association of cerebrospinal fluid brain-binding autoantibodies with cognitive impairment in post-COVID-19 syndrome. Brain Behav. Immun. 2023 doi: 10.1016/j.bbi.2023.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K., Burns C.D., Madison C., Clark D., Halchenko Y.O., Waskom M.L., Ghosh S.S. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front. Neuroinf. 2011:13. doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffa A., Bommarito G., Assal F., Herrmann F.R., Van De Ville D., Allali G. Dynamic functional networks in idiopathic normal pressure hydrocephalus: alterations and reversibility by CSF tap test. Hum. Brain Mapp. 2021;42:1485–1502. doi: 10.1002/hbm.25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E., Buschke H. Genuine memory deficits in dementia. Dev. Neuropsychol. 1987;3:13–36. [Google Scholar]
- Jayaraman A., Htike T.T., James R., Picon C., Reynolds R. TNF-mediated neuroinflammation is linked to neuronal necroptosis in Alzheimer's disease hippocampus. Acta Neuropathol. Commun. 2021;9:1–21. doi: 10.1186/s40478-021-01264-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., Zheng M., Sundaram B., Banoth B., Malireddi R.S. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184:149–168. doi: 10.1016/j.cell.2020.11.025. e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebets V., Holmes A.J., Orban C., Tang S., Li J., Sun N., Kong R., Poldrack R.A., Yeo B.T. Somatosensory-motor dysconnectivity spans multiple transdiagnostic dimensions of psychopathology. Biol. Psychiatry. 2019;86:779–791. doi: 10.1016/j.biopsych.2019.06.013. [DOI] [PubMed] [Google Scholar]
- Kessels R.P.C., van Zandvoort M.J.E., Postma A., Kappelle L.J., de Haan E.H.F. The Corsi Block-Tapping Task: standardization and normative data. Appl. Neuropsychol. 2000;7:252–258. doi: 10.1207/S15324826AN0704_8. [DOI] [PubMed] [Google Scholar]
- Krishnan A., Williams L.J., McIntosh A.R., Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011;56:455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Kronfol Z., Remick D.G. Cytokines and the brain: implications for clinical psychiatry. Am. J. Psychiatry. 2000;157:683–694. doi: 10.1176/appi.ajp.157.5.683. [DOI] [PubMed] [Google Scholar]
- Li C., Liu J., Lin J., Shang H. COVID-19 and risk of neurodegenerative disorders: a Mendelian randomization study. Transl. Psychiatry. 2022;12:1–6. doi: 10.1038/s41398-022-02052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindbergh C.A., Casaletto K.B., Staffaroni A.M., Elahi F., Walters S.M., You M., Neuhaus J., Rivera Contreras W., Wang P., Karydas A. Systemic tumor necrosis factor-alpha trajectories relate to brain health in typically aging older adults. J. Gerontol. Ser. A. 2020;75:1558–1565. doi: 10.1093/gerona/glz209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalmurti N., Hunter C.A. Cytokine storms: understanding COVID-19. Immunity. 2020;53:19–25. doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladin M., Villa G., De Lorenzo R., Querini P.R., Benedetti F. Prevalence, trajectory over time, and risk factor of post-COVID-19 fatigue. J. Psychiatric Res. 2022 doi: 10.1016/j.jpsychires.2022.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F., group, C.-B.O.C.S. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfoose J., Baune B. Evidence for a cytokine model of cognitive function. Neurosci. Biobehav. Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- McAlpine L.S., Fesharaki-Zadeh A., Spudich S. Coronavirus disease 2019 and neurodegenerative disease: what will the future bring? Curr. Opin. Psychiatry. 2021;34:177. doi: 10.1097/YCO.0000000000000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A.R., Lobaugh N.J. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23:S250–S263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Meyers J.E., Meyers K.R. Psychological Assessment Resources; 1995. Rey Complex Figure Test and Recognition Trial Professional Manual. [Google Scholar]
- Mondragón J.D., Maurits N.M., De Deyn P.P. Functional neural correlates of anosognosia in mild cognitive impairment and Alzheimer’s disease: a systematic review. Neuropsychol. Rev. 2019;29:139–165. doi: 10.1007/s11065-019-09410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuber-Champier A., Voruz P., Jacot de Alcântara I., Breville G., Allali G., Lalive P.H., Assal F., Péron J.A. Monocytosis in the acute phase of SARS-CoV-2 infection predicts the presence of anosognosia for cognitive deficits in the chronic phase. Brain Behav. Immun. Health. 2022;26 doi: 10.1016/j.bbih.2022.100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A., Kong R., Gordon E.M., Laumann T.O., Zuo X.-N., Holmes A.J., Eickhoff S.B., Yeo B.T. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex. 2018;28:3095–3114. doi: 10.1093/cercor/bhx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick P. Multiple significance tests: the Bonferroni correction. Bmj. 2012:344. [Google Scholar]
- Shnayder N.A., Sirbiladze T.K., Demko I.V., Petrova M.M., Nasyrova R.F. Limbic Encephalitis Associated with COVID-19. Encyclopedia. 2022;2(1):26–35. [Google Scholar]
- Starkstein S.E. Anosognosia in Alzheimer's disease: diagnosis, frequency, mechanism and clinical correlates. Cortex. 2014;61:64–73. doi: 10.1016/j.cortex.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Thomas-Anterion C., Ribas C., Honore-Masson S., Million J., Laurent B. Evaluation de la plainte cognitive de patients Alzheimer, de sujets MCI, anxiodépressifs et de témoins avec le QPC (Questionnaire de Plainte Cognitive) NPG Neurol. Psychiatr. Gériatrie. 2004;4:30–34. [Google Scholar]
- Voruz P., Allali G., Benzakour L., Nuber-Champier A., Thomasson M., Jacot de Alcântara I., Pierce J., Lalive P.H., Lövblad K.-O., Braillard O. Long COVID neuropsychological deficits after severe, moderate, or mild infection. Clin. Transl. Neurosci. 2022;6:9. [Google Scholar]
- Voruz P., Cionca A., Jacot de Alcântara I., Nuber-Champier A., Allali G., Benzakour L., Thomasson M., Lalive P.H., Lövblad K.-O., Braillard O. Functional connectivity underlying cognitive and psychiatric symptoms in post-COVID-19 syndrome: is anosognosia a key determinant? Brain Commun. 2022;4 doi: 10.1093/braincomms/fcac057. fcac057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voruz, P., Cionca, A., Jacot de Alcântara, I., Nuber‐Champier, A., Allali, G., Benzakour, L., Lalive, P.H., Lövblad, K.O., Braillard, O., Nehme, M., 2022c. Brain functional connectivity alterations associated with neuropsychological performance 6–9 months following SARS‐CoV‐2 infection. Hum. Brain Mapp. [DOI] [PMC free article] [PubMed]
- Voruz, P., de Alcântara, I.J., Nuber-Champier, A., Cionca, A., Allali, G., Benzakour, L., Lalive, P., Lövblad, K., Braillard, O., Nehme, M., 2022d. Frequency of abnormally low neuropsychological scores in post-COVID-19 syndrome: the Geneva COVID-COG Cohort. Arch. Clin. Neuropsychol. [DOI] [PMC free article] [PubMed]
- Yeo, B.T., Krienen, F.M., Sepulcre, J., Sabuncu, M.R., Lashkari, D., Hollinshead, M., Roffman, J.L., Smoller, J.W., Zöllei, L., Polimeni, J.R., 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Nonsensitive COVID-COG data will be made available at the end of the project on a dedicated open-access platform.