Abstract
Ischemic stroke is a leading cause of morbidity and mortality, with limited treatments that can facilitate brain regeneration. Neural progenitor cells (NPCs) hold promise for replacing tissue lost to stroke, and biomaterial approaches may improve their efficacy to overcome hurdles in clinical translation. The immune response and its role in stroke pathogenesis and regeneration may interplay with critical mechanisms of stem cell and biomaterial therapies. Cellular therapy can modulate the immune response to reduce toxic neuroinflammation early after ischemia. However, few studies have attempted to harness the regenerative effects of neuroinflammation to augment recovery. Our previous studies demonstrated that intracerebrally transplanted NPCs encapsulated in a chondroitin sulfate-A hydrogel (CS-A + NPCs) can improve vascular regeneration after stroke. In this paper, we found that CS-A + NPCs affect the microglia/macrophage response to promote a regenerative phenotype following stroke in mice. Following transplantation, PPARγ-expressing microglia/macrophages, and MCP-1 and IL-10 protein levels are enhanced. Secreted immunomodulatory factor expression of other factors was altered compared to NPC transplantation alone. Post-stroke depression-like behavior was reduced following cellular and material transplantation. Furthermore, we showed in cultures that microglia/macrophages encapsulated in CS-A had increased expression of angiogenic and arteriogenic mediators. Neutralization with anti-IL-10 antibody negated these effects in vitro. Cumulatively, this work provides a framework for understanding the mechanisms by which immunomodulatory biomaterials can enhance the regenerative effects of cellular therapy for ischemic stroke and other brain injuries.
Keywords: Stroke, Hydrogel, Neural progenitor cells, Microglia, Regeneration, Glycosaminoglycans, Immunomodulation
1. Introduction
Ischemic stroke is one of the leading causes of mortality and morbidity worldwide, and a major cause of long-term disability in the US (Powers et al., 2018). Currently, there are limited therapeutic options for facilitating recovery after stroke. Cellular therapies hold promise for improving functional recovery after ischemia via trophic support and cell replacement. Neural progenitor cells (NPCs) may differentiate into functional neurons and integrate into existing host circuitry, although the extent to which this occurs is under study (Wei et al., 2017; Xiong et al., 2021; Thompson and Bjorklund, 2015). NPCs may also promote endogenous recovery mechanisms such as the recruitment of progenitor cells and immunomodulation. Previous work has demonstrated that transplantation of NPCs can induce vascular recovery and encourage the recruitment of subventricular zone neuroblasts (Wei et al., 2017; McCrary et al., 2020; Chau et al., 2014; Moshayedi and Carmichael, 2013; Savitz and Cox Jr., 2016). Biomaterial approaches can enhance these effects by promoting transplanted cellular retention in the stroke region and by facilitating a regenerative microenvironment (Jendelova et al., 2016; Rajkovic et al., 2018).
Chondroitin-4-sulfate (CS-A) has been identified as a beneficial augmenter of cellular and trophic therapies in the injured brain (McCrary et al., 2020; Betancur et al., 2017; Karumbaiah et al., 2015; Kwok et al., 2012; Latchoumane et al., 2021). Recently, we tested intracranial transplantation of NPCs encapsulated in hydrogels composed predominantly of CS-A for ischemic stroke in mice (McCrary et al., 2020). Encapsulated cells exhibited significantly increased post-transplantation survival and increased neuronal differentiation. Treatment with CS-A encapsulated NPCs (CS-A + NPCs) increased vascular density, improved angiogenesis, promoted large vessel remodeling, augmented cerebral blood flow recovery, and improved post-stroke sensorimotor deficits. These effects were dependent on fibroblast growth factor 2 (FGF2), a pro-angiogenic molecule released by microglia/macrophages in the brain.
CS proteoglycans and their associated sulfated glycosaminoglycan (GAG) sidechains are abundantly expressed in the brain extracellular matrix. CS-A is abundantly expressed in the brain extracellular matrix and in neurogenic niches (Ida et al., 2006; Akita et al., 2008), where it is an important mediator of endogenous neural stem cell dynamics, neural plasticity, and angiogenesis (Kwok et al., 2012; Purushothaman et al., 2012; Rauvala et al., 2017; Nishimura et al., 2010; Maeda, 2010). The diverse sulfation patterns of extracellular CS-GAGs are responsible for the sequestration of soluble factors and mediation of downstream signal transduction pathways (Gama et al., 2006), including the binding, retention, and signal transduction of important immunoregulatory molecules (Karumbaiah et al., 2014; Sirko et al., 2007). Using surface plasmon resonance specific binding affinity assays, we previously demonstrated the high affinity of several soluble neurotrophic and immunomodulatory factors including interleukin 10 (IL-10) to predominantly CS-A containing GAGs (Karumbaiah et al., 2015).
In this current work, we sought to elucidate the microglia/macrophage immunomodulatory response to the CS-A biomaterial and NPCs in the ischemic mouse brain to better understand its potential for regenerative therapy. Inflammation plays an important role in ischemic stroke. Microglia/macrophages exhibit a diverse range of biological functions, both pro-inflammatory and also regenerative, following brain damage (Savitz and Cox Jr., 2016; Jin et al., 2010; Hu et al., 2015; Xiong et al., 2016; Kriz, 2006; Santos Samary et al., 2016; Amantea et al., 2009). Harmful neuroinflammation is associated with brain injury-related neuropsychiatric deficits, such as post-stroke depression (PSD), defined as the development of symptoms following stroke including depressed mood or loss of interest along with other major criteria for depression, lasting for >2 weeks (Wu et al., 2018; Kronenberg et al., 2014; Robinson and Jorge, 2016; Pietra Pedroso et al., 2016). Microglia/macrophages cells can enhance recovery by promoting neurogenesis, axon growth, and vascular regeneration and remodeling (Jin et al., 2010; Kriz, 2006; Pei et al., 2015; Jin et al., 2013). These mechanisms are possibly induced by the release of factors such as monocyte chemoattractant protein-1 (MCP-1), growth factors such as FGF2, and chemokines including CCL3, CCL4, and SDF-1 (Hu et al., 2015; Heil et al., 2006; Liu et al., 2014; Prakash and Carmichael, 2015). These factors promote proliferation of endothelia and smooth muscle cells to facilitate vessel remodeling, expansion of collateral diameter, and angiogenesis, which are critical for tissue restoration after ischemic stroke (Semenza, 2007; Carmeliet, 2000).
The specific roles of CS-A in brain inflammation after stroke are largely unknown. Previous research suggests that CS-A might activate monocytes cultured in vitro (Stephenson and Yong, 2018; Rachmilewitz and Tykocinski, 1998). One group used a combination of CS and hyaluronan with incorporated SDF-1 and FGF2 in a rat stroke model, which showed reduced astrogliosis (Jian et al., 2018). However, they did not incorporate cellular therapy in their treatment and the macrophage/microglial response was not examined. Our previous study demonstrated that CS-A hydrogels attenuated reactive astrogliosis in a rat TBI model (Betancur et al., 2017). However, the microglial/macrophage responses to CS-A and CS-A encapsulated NPCs following transplantation into the infarcted brain remains uncharacterized.
CS-A is known to enrich IL-10, and IL-10 is associated with reduced neural toxicity after stroke and TBI (Karumbaiah et al., 2015; Garcia et al., 2017). Based on our own previous results confirming vascular recovery and sensorimotor benefits in multiple rodent models (McCrary et al., 2020; Betancur et al., 2017), we hypothesized that CS-A + NPC treatment will modulate microglial/macrophage responses and promote regeneration following ischemic stroke. This study sought to characterize the effects of CS-A + NPC treatment on microglia/macrophage phenotype and secreted immunomodulatory factor expression following intracerebral transplantation. Additionally, knowing that brain inflammation is associated with post-stroke depression, we tested whether our combinatorial treatment may provide therapeutic psychiatric effects in a rodent model. Furthermore, we interrogated the likely underlying mechanisms using in vitro studies of stroke-tissue-derived microglia/macrophages.
2. Materials and methods
2.1. Overview of experimental design
We used an established focal ischemic stroke mouse model which targets the right sensorimotor cortex to study the effects of CS-A encapsulation of NPCs for the treatment of stroke (Wei et al., 1995). CS-A hydrogels were synthesized to match brain tissue mechanical properties as previously described, and mouse-induced pluripotent stem cells underwent the 4−/4+ retinoic acid differentiation protocol which produces neuronal lineage committed NPCs (Karumbaiah et al., 2015; Bain et al., 1996). Intracranial transplantations (2 μL, 100 k cells/ μL) were performed into the ischemic core and peri-infarct regions 7d after stroke, and animals were sacrificed 7d or 14d after transplantime pointsese timepoints were selected to avoid the initial early wave of inflammation thought to result in blood-brain-barrier disruption, edema, neuronal death, and hemorrhagic transformation, and to instead encourage regenerative processes of neurovascular recovery which peaks over the course of 1–3 weeks (Jin et al., 2010; Kriz, 2006; Jin et al., 2013; Perera et al., 2006; Nakase et al., 2008). Adult male mice (C57BL/6 J) were treated with sham transplantations (stroke control), NPCs, CS-A gel loaded with NPC media, or the combined CS-A encapsulated NPCs (CS-A + NPCs).
2.2. Neural progenitor cell culture
Mouse-induced pluripotent stem cell-derived neural progenitor cells (iPSC-NPCs) were differentiated from iPSCs originally generated from mouse embryonic fibroblasts (Stemgent Inc., Cambridge, MA) as previously described (Chau et al., 2014). This line of iPSC-NPCs expresses GFP to permit tracking following transplantation. iPSCs were cultured in the N2/B27 supplemented serum-free medium at 20% O2, 5% CO2, at 37 °C. The medium was prepared with 45% Dulbecco’s modified eagle medium: nutrient mixture F-12 (DMEM/F12; Sigma Aldrich, St Louis, MO), 45% Neurobasal (Thermo Fisher Scientific), 0.5% N2 supplement (Thermo Fisher Scientific), 1% B27 supplement (Thermo Fisher Scientific), 1% GlutaMAX, 1% nonessential amino acids (Sigma Aldrich), 0.1 mM β-mercaptoethanol (β-ME; Sigma Aldrich), 100 U/mL penicillin/streptomycin (Sigma Aldrich), 5% knockout serum replacement (KSR; Thermo Fisher Scientific). The small molecules were recombinant leukemia inhibitory factor (LIF; 10 ng/mL; Millipore, Billerica, MA), CHIR 99021 (3 μM; Tocris), (S)-(+)-Dimethindene maleate (2 μM; Tocris) and minocycline hydrochloride (2 μM; Santa Cruz Biotechnology, Santa Cruz, CA). Before differentiation, the iPSCs were cultured in DMEM (Sigma Aldrich), 10% ES-FBS (Thermo Fisher Scientific), GlutaMAX, nonessential amino acids, nucleoside mix, LIF, β-ME (Sigma Aldrich), LIF, b-ME, and penicillin/streptomycin. All cells used in this study were harvested and ready for transplantation after an established ‘4−/4+’ retinoic acid (RA, 1 μM; Sigma Aldrich) neural differentiation protocol.
2.3. Mouse focal ischemic stroke model
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Emory University and are in accordance with the NIH and Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Before and after surgery, the animals were housed at 4–5 animals per cage, with ad libitum access to food and water. The mouse focal ischemic stroke model was performed as previously described using C57BL/6 J mice (8–12 weeks) from Jackson Laboratories (Wei et al., 2017; Wei et al., 1995). Cortical ischemia was achieved by permanent occlusion of the distal branches of the right middle cerebral artery (MCA) supplying the sensorimotor cortex. The MCA occlusion was paired with 7-min ligation of both common carotid arteries (CCAs) to cause sufficient reduction of local cerebral blood flow (LCBF) in the sensorimotor region and followed by partial reperfusion. This creates a focal area of ischemic in the right sensorimotor cortex which has been employed successfully in our other stroke studies (McCrary et al., 2020; Jiang et al., 2017; Lee et al., 2016; Choi et al., 2012). Body temperature was monitored during surgery and recovery period using a rectal probe. The temperature was maintained at 37 °C on a homoeothermic blanket for 1 h after emergence from anesthesia. In this stroke model, necrosis, apoptosis, and autophagy occur at various timepoints up to a week after infarction (Jiang et al., 2017). At 7 days after stroke, there is an increase in microglia/macrophages in the ischemic core region. Additionally, at this same time point, released factors such as BDNF and VEGF could be detected in the stroke core region.
2.4. Intracerebral transplantations
Seven days after stroke surgery, mice skulls were thinned over the visible region of the stroke core, and 200,000 cells suspended in 2 μL medium or 200,000 cells loaded into 2 μL CS-A hydrogel, or 2 μL CS-A hydrogel with medium only were loaded into a Hamilton syringe (Hamilton, Reno, NV, USA) and injected at a depth of 1 mm from the skull. As previously described in other works, lyophilized 5% (w/v) CS-A hydrogels that were synthesized by 2-hydroxy-4′-(2- hydroxyethoxy)-2-methylpropiophenone (Irgacure-2959, Sigma-Aldrich) initiated photo-crosslinking of methacrylate chondroitin sulfate (mCS), were rehydrated in either media or cell suspension prior to intracerebral injections. SAX-HPLC analysis of CS used in these studies was previously determined to consist primarily of CS-A (86%), with minor traces of other sulfated CS-GAG species (McCrary et al., 2020; Karumbaiah et al., 2015). The number and volume of cells injected was shown to be safe and effective in our published studies (McCrary et al., 2020; Chau et al., 2014).
2.5. Immunohistochemistry and image analysis
Animals were sacrificed by decapitation and brains were immediately removed and immersion fixed in 10% buffered formalin for 72 h, dehydrated in 30% sucrose, and sliced into 10 μm thick coronal sections using a cryostat (Leica Microsystems, Buffalo Grove, IL). Cell counting followed the stereology principle to avoid repeated cell counts. For systematic random sampling in design-based stereological counting, every 10th (90 μm apart) brain section across the entire region of interest was counted. For multi-stage random sampling, six fields per brain section were randomly chosen under 10, 20, or 40× magnification of an epifluorescence microscope or in confocal images. The sections were air dried and fixed with 10% buffered formalin (Fisher Scientific, Pittsburgh, PA, USA). Brain sections were then submerged in an ethanol/acetic acid solution (2:1) for 10 min, washed 3 times with 1× PBS solution, and incubated with 0.2% Triton X-100 for 45 min. Slides were then blocked with 1% fish gelatin (diluted in PBS; MilliporeSigma) for 1 h at room temperature. Slides were incubated with, IBA-1 (1:400, Cell Signaling, Danvers, MA, USA), and PPAR-gamma (1:400, Sigma-Aldrich, St. Louis, MO) primary antibodies diluted in PBS at 4 °C overnight. Primary antibodies were washed with PBS and replaced with secondary antibodies Alexa Fluor488 goat anti-mouse (1:300; Life Technologies, Grand Island, NY), and Cy3-conjugated donkey anti-rat (1:300; Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h at room temperature before rinsing with PBS. After a final PBS wash, slides were mounted with Vectashield fluorescent mounting medium (Vector Laboratory, Burlingame, CA), and cover-slipped for microscopy and image analysis. Blinded stereological counting was performed on 6 randomly chosen microscope images of the stroke core region per section, and 6–8 sections were analyzed per animal. The number of PPAR-gamma and IBA-1 co-labeled cells were counted.
2.6. Sholl analysis of microglia/macrophages
Stereologically matched images of coronal brain sections stained with IBA1 were used to compare microglia morphology using Sholl analysis via the Image J plugin (Image J, NIH). Briefly, concentric circles were manually drawn around the center of the soma using the concentric circles plugin (Image J, NIH). Cell soma area was measured and recorded (Image J Analysis, NIH).
2.7. Western blot analysis
The stroke region was homogenized using Mammalian Protein Extraction Reagent (M-PER) Lysis buffer with phosphatase and proteinase inhibitors (Pierce, Rockford, IL). Tissue samples were centrifuged, and the supernatant was isolated. Protein concentration was determined using the Bicinchoninic Acid Assay (Sigma Aldrich, St. Louis, MO). Protein samples were loaded into 12% polyacrylamide gels and separated via sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in a Hoefer Mini-Gel system (Amersham Bioscience, Piscataway, NJ). Protein was then transferred onto a polyvinylidene fluoride (PVDF) membrane (BioRad, Hercules, CA). Membranes were blocked using 5% BSA diluted with TBS and 0.05% Tween 20 (TBST) at room temperature for 1 h, then incubated overnight with primary antibodies for bFGF (1:500, Cell Signaling, Danvers, MA, USA), IBA-1 (1:400, Cell Signaling, Danvers, MA, USA), VEGFR2 (1:1000, Cell Signaling, Danvers, MA, USA), TIE-2 (Cell Signaling, Danvers, MA, USA) Cylin D1, (Cell Signaling, Danvers, MA, USA), Cyclin E1, (Cell Signaling, Danvers, MA, USA), and the loading control Tubulin (1:5000, Sigma-Aldrich, St. Louis, MO). After washing with TBST, membranes were incubated with AP-conjugated secondary antibodies (GE Healthcare, Piscataway, NJ) for 1 h, then washed with TBST, and exposed to bromochloroidolylphosphate/nitroblue tetrazolium. Signal intensity was quantified using ImageJ (NIH) and normalized to tubulin intensity.
2.8. U-PLEX (protein multiplex)
U-PLEX is a protein multiplex assay which utilizes biotinylated antibodies coupled with proprietary linkers with electrochemiluminescent labels. This allows for detection and quantification of proteins. The U-PLEX Assay platform was purchased from Meso Scale Diagnostics (MSD, Rockville, MD, USA) and used according to the manufacturer’s protocol. Sample preparations and measurements were performed by the Emory Multiplexed Immunoassay Core (EMIC). After 1 h of incubation at room temperature with 5% MesoScale Discovery Blocker A (R93AA-1) and 3 washes with 150 μL PBS/0.05% Tween, 25 μL of brain homogenate or standard were added, and the plate was incubated for 1 h at room temperature, 500 rpm. After 3 PBS Tween washes, 25 μL of secondary antibody was added to each well and the plate was incubated 1 h at room temperature. Finally, 25 μL of streptavidin Sulfo-TAG/well was added after 3 PBS Tween washes, and the plate was incubated 1 h at room temperature. A MesoScale Quickplex Plate Scanner was used for protein quantification. The analytes tested included the following proteins from both the ischemic core and the peri-infarct regions: MCP-1, MIP-1a, MIP-1b, TNF, IL-4, IL-6.
2.9. Enzyme-linked immunoassay (ELISA)
Human iPSC derived neural stem cells (hNSCs) were seeded at a density of 100 × 103 cells /cm2 in triplicate in two 6-well plates coated with Geltrex and grown in neurobasal media supplemented with 2% B-27 and FGF2 (20 ng/ml). Geltrex coated wells containing media only were used as controls. Media from hNSC and control wells was collected at Days 3 and 7 and stored at −80 °C until required for ELISAs. A human IL-10 ELISA assay (Quantikine, R&D Systems™ D1000B) was performed after Day 7 media collection. Human recombinant IL-10 standard and media from hNSC and control treatments were run in duplicates. Samples were diluted at 1:10 as per vendor recommendation. The ELISA plate was read on a microplate reader at 450 nm, while wavelength correction was performed at 540 nm. Optical density (O⋅D.) values for each treatment were plotted as an average obtained from total number of wells per sample. The detection limit (indicated by a blue dotted line in Supplemental Fig. 2B.) indicates the O.D value obtained for the lowest recombinant human IL-10 standard concentration tested.
2.10. Primary stroke core macrophage/microglia collection and cell culture
Mice were subjected to stroke surgeries as discussed above. 3 days after stroke, the visible infarct core regions from the mice were carefully dissected, pooled, and then dissociated in trypsin + EDTA. The pooled tissue suspension was centrifuged, washed with PBS, and resuspended with DMEM + 10% FBS, and plated on polystyrene tissue culture plates or into CS-A hydrogel. Following culture overnight, non-adherent cells and stroke core debris were removed, and the media was replaced. For the IL-10 neutralization experiments, 1 μg/μL anti-IL-10 antibody (R&D Systems, MAB417) was added to the cell culture media.
2.11. Post-stroke neuropsychiatric behavioral testing
The tail suspension test was used to test depression-like behavior. All experimental mice were habituated in the waiting room for at least 30 min before testing. In the test, mice were suspended by their tails with adhesive tape in a position about 1 cm away from the tail tips. The tests were video recorded for 6 min and the last 5 min were analyzed. Immobility was considered only if the mice completely stopped any initiated movements (Misrani et al., 2019). The open field test was performed to evaluate anxiety. In the tests, each animal was introduced in the center of an open arena sized 50 cm × 50 cm × 50 cm (L × W × T) and allowed to move freely for 10 min. Behaviors, such as travel distances and locations, were recorded using TopScan Clever Sys (Clever Sys, Inc.) and the videos were analyzed by the TopScan Realtime Option Version 3.0 (Clever Sys Inc.). The arena was cleaned with 70% ethanol after each test.
2.12. Statistics, randomization, and blinding
Data were expressed as the means +/− SEM. GraphPad Prism 6 (GraphPad Software, San Diego, CA) was used for statistical analysis and graphic presentation. Microsoft Excel with the Analyse-It add-in was used for principal component analysis (PCA) and linear regression analysis. Briefly, PCA was used to develop composite variables consisting of the U-PLEX data, PPARγ + macrophages counting, and IL-10 levels from Western blotting. Similarly, biochemical and immunohistochemical data were used to construct a statistical model to test the relationship between these data and behavioral outcomes. Statistical tests with corrections for multiple comparisons are presented with each figure. All animals were assigned a number and box label and randomly assigned to treatment groups evenly. All researchers performing stroke surgeries, injections, microscopy, histological analysis, and data analysis were blinded to the treatment groups until collection and analyses were completed.
3. Results
3.1. Effects of CS-A + NPC transplantation on immune system markers in the post-stroke brain
We probed the expression of immune mediators within the stroke region after treatment with CS-A hydrogel encapsulated NPCs by measuring the expression of a variety of immunomodulatory factors, including monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1a and 1b (MIP-1a, MIP-1b), tumor necrosis factor (TNF), and interleukins 4 and 6 (IL-4, IL-6) (Fig. 1). Expression of these factors were interrogated at both the infarct core and the peri-infarct regions (Fig. 1 A–B). We found that at the stroke core region, MCP-1 was significantly increased by CS-A + NPC treatment compared to all other treatments (Fig. 1 A). MIP-1a, MIP1b, and IL-4 levels were significantly reduced by NPC transplantation alone compared to the stroke control and CS-A + NPC group. There was no significant increase in MIP-1a/b expression in the CS-A + NPC group compared to the stroke and hydrogel only controls. The expression of the same proteins was also measured in the peri-infarct region. We found that MCP-1 was also significantly upregulated in the peri-infarct region of mice treated with CS-A + NPCs (Fig. 1 B). There was no significant difference in expression of other factors in the peri-infarct region.
Fig. 1.
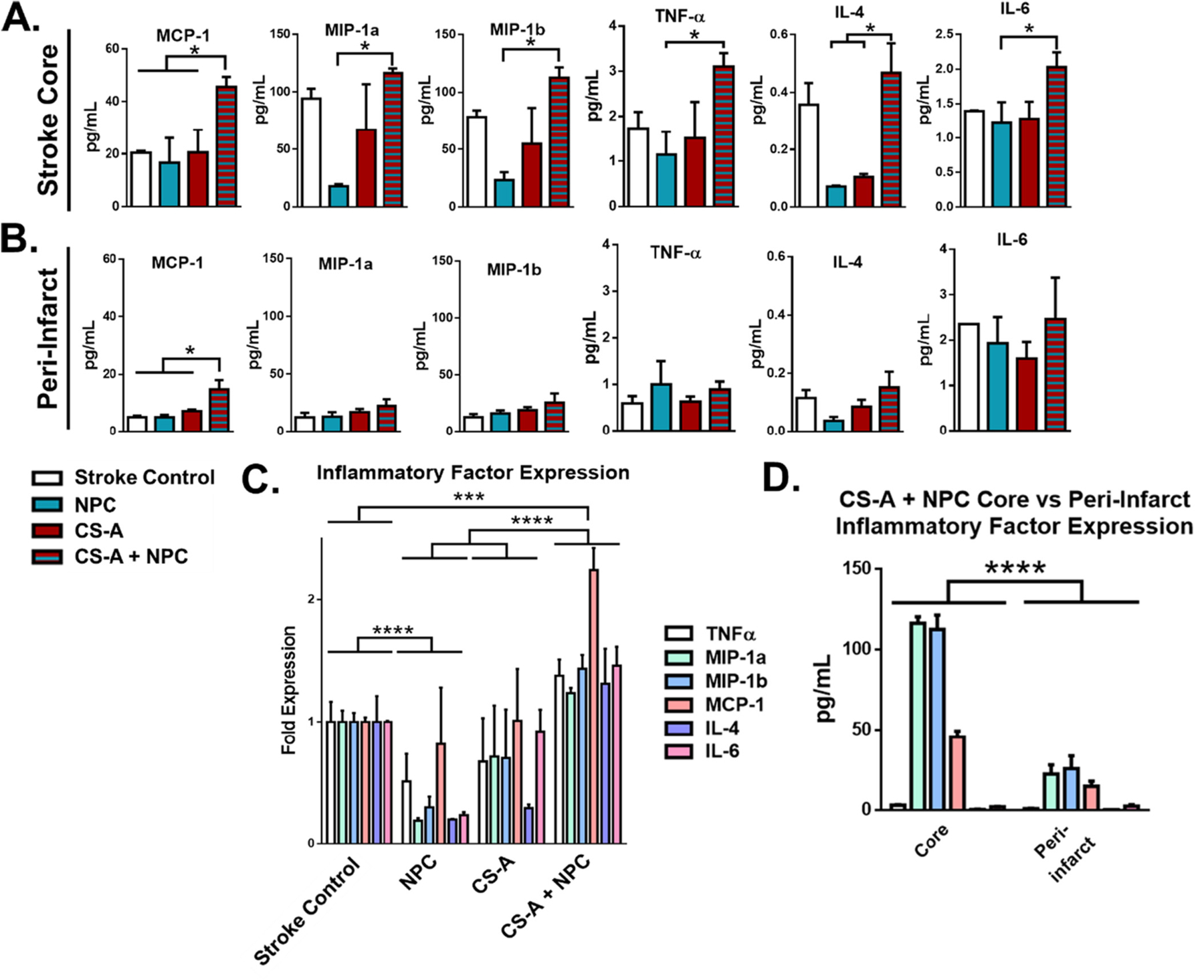
Expression of inflammatory markers following transplantation in stroke mice. A-B. Analysis of expression of immune factors 1 week following intracranial transplantation at the infarct core (A) and peri-infarct region (B). * indicates p < 0.05. Two-way ANOVA. C. Analysis of group expression of chemokines/cytokines in the across treatment groups. D. Comparison of immune factors in the stroke core versus peri-infarct region in CS-A + NPC treated mice. n = 3–5. ***, and **** indicate p < 0.0005 and p < 0.0001, respectively. Two-way ANOVA with Holm-Sidak correction.
We compared the overall relative expression of these factors by treatment group to ascertain whether or not the group expression profiles as a whole were significantly different from each other (Fig. 1 C). We found that tissue obtained from CS-A + NPC treated animals demonstrated the significantly increased expression of immunomodulatory factors when compared to all other treatments and controls. Furthermore, the tissue from NPCs only treated animals showed significantly reduced expression of these factors when compared to the stroke control. We also compared the expression of the immunomodulatory factors in the stroke core region versus the peri-infarct region in mice treated with CS-A + NPCs (Fig. 1 D), and found that group expression of immunomodulatory factors was also found to be significantly reduced in the peri-infarct region compared to the stroke core region in CS-A + NPC treated animals.
3.2. CS-A + NPC transplantation affects microglia/macrophages phenotype in the post-stroke brain
We further investigated the effects of treatment with CS-A + NPCs with immunohistochemical and protein expression analysis of microglial-related inflammatory markers. The protein peroxisome proliferator-activated receptor gamma (PPARγ) is expressed in microglia/macrophages that tend to participate in regenerative activities (Zhao et al., 2015). Following 2 weeks after transplantation, the number of PPARγ-expressing macrophages/microglia is significantly increased by CS-A + NPC treatment as determined by immunohistochemical quantification of PPARγ and IBA1 (a microglial marker) co-staining in the stroke area compared to the stroke and NPC only treatment controls (Fig. 2 A–B). We used Western blotting to measure IBA1 and IL-10 accumulated in the stroke area (Fig. 2 C–D). IBA1 was significantly reduced in the NPC treatment group compared to the stroke control and the combined CS-A + NPC treatment groups, which is consistent with previous findings that transplanted NPCs can suppress the local inflammatory response. We also found that the IL-10 levels were significantly increased in the CS-A + NPC treatment group compared to all controls (Fig. 2 D). Sholl analysis derived microglia cell size measurement indicated a significant increase in the CS-A group, but no significant differences in the NPC only or CS-A + NPC groups compared to the stroke control (Fig. 2 E).
Fig. 2.
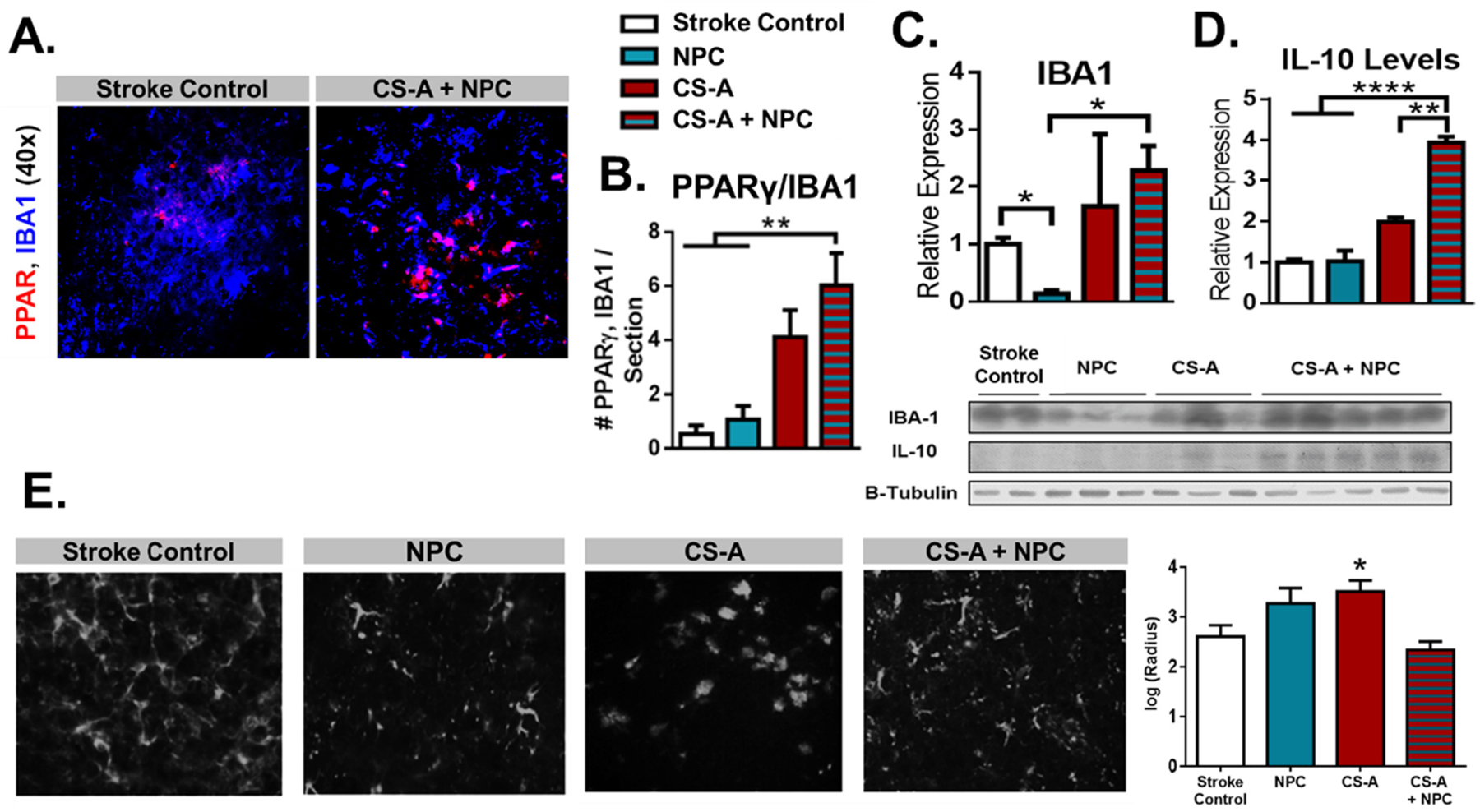
CS-A + NPCs Increases PPARγ Macrophages and IL-10 Expression. A. Representative images of PPARγ and IBA1 co-staining in the stroke area 2 weeks after transplantation. B. Analysis of number of PPARγ/IBA1 co-staining. n = 8–12. **indicates p < 0.005; 1-way ANOVA. C-D. Western blot analyses of IBA1 (C) and IL-10 (D) expression in the stroke area 1 week after transplantation. E. Microglia radius derived from Sholl analysis indicated significant increase in cell size in CS-A treated animals but not in NPC or CS-A + NPC treated groups. n = 4–8. *, **, and **** indicate p < 0.05, p < 0.005, and p < 0.0001, respectively; 1-way ANOVA with Bonferroni correction.
To further clarify the relationship between treatment groups and biochemical phenotypes, we used principal component analysis (PCA) of the protein microarray, Western blot, and PPARγ+ microglia/macrophages (S. Fig. 1). The first 4 principal components (PC) explained nearly 100% of the variability; the first 2 PCs explained 92.8% (S. Fig. 1 A). The largest determinants in the first PC were high levels of PPARγ expressing microglia/macrophages and low IL-4 levels in the core region. The largest determinants in the second PC were high IL-4 levels in the core and low TNF levels in the peri-infarct areas. Treatment groups CS-A + NPC and CS-A correlated positively with PC1 and 2, while the NPC group correlated positively with PC1 but negatively with PC2 (S. Fig. 1 B–C). The stroke control group was weakly positively associated with PC1, and strongly negatively associated with PC2. Graphically, the vector of the first PCs suggests that the NPC-only treatment group is more similar to the stroke control than to the CS-A + NPC treatment group (S. Fig. 1 C).
3.3. Functional benefits of CS-A + NPC transplantation after ischemic stroke
We tested the possibility that CS-A + NPC transplantation may affect neuropsychiatric outcomes following ischemic stroke (Fig. 3). The open field test assesses anxiety-like behavior by monitoring the proportion of time the mice spend in the center of the arena; it can also be used to gather sensorimotor data such as distance traveled and average velocity during the test. We found that all treatment groups showed an increased time spent in the center of the arena, suggesting they have reduced anxiety compared to the stroke control; there were no differences between the transplantation groups (Fig. 3 A). Furthermore, there were no differences in average travel distance or velocity between treatment groups. The tail suspension test assesses apathy-like behavior. We found that mice treated with the CS-A + NPC treatment have reduced immobility time compared to the stroke and NPC-only controls, suggesting that animals treated with the CSA + NPC combination treatment have reduced apathy-like behavior (Fig. 3 B). These results suggest that CS-A scaffolding may improve post-stroke neuropsychiatric deficits. We additionally explored whether the differentially expressed immunomodulatory factors were correlated with post-stroke depressive behaviors. Linear regression analysis revealed that in combination, the number of PPARγ + macrophages (Fig. 2 A–B) and IL-10 protein levels (Fig. 2 D) explained 99.31% of the variance in immobility time in the tail suspension test (R2 = 0.9931, F = 215.38, Significance F = 0.048, p = 0.011). However, neither of these markers significantly explained depressive-like behavior in isolation (PPARγ + macrophages: p value = 0.135, IL-10 protein levels: p value = 0.307).
Fig. 3.
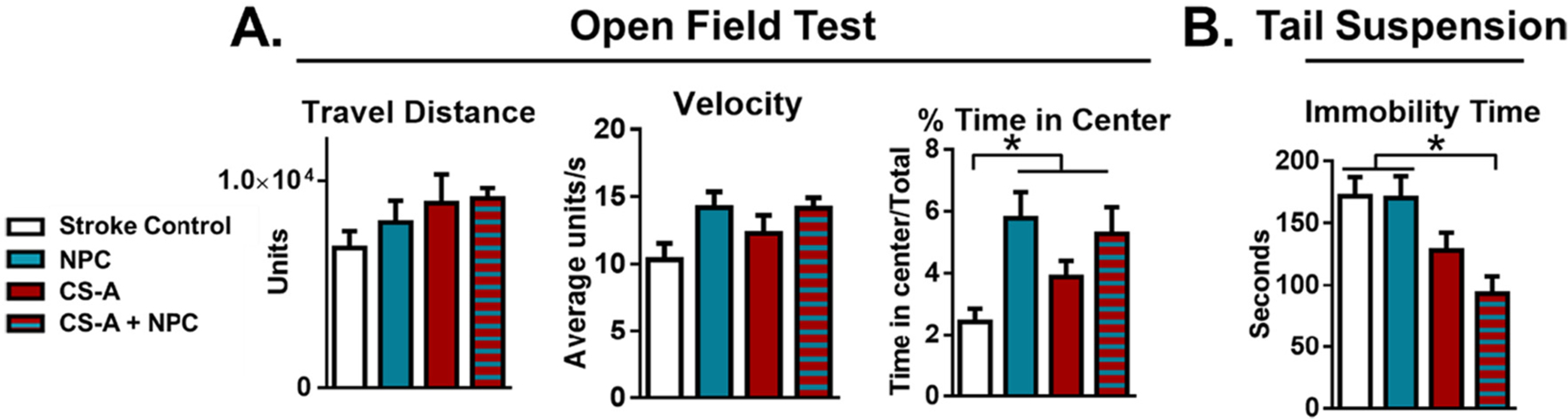
Recovery of post-stroke neuropsychiatric deficits following treatment. A. Analysis of the open field test with travel distance, velocity, and percentage of time spent in center. B. Analysis of tail suspension test. n = 8–12. * indicates p < 0.05; 2-way ANOVA with Bonferroni correction.
3.4. Potential cellular and molecular mechanisms of CS-A + NPC transplantation
Given the alterations in the expression of inflammatory markers and microglial phenotype following CS-A + NPC treatment (Figs. 1–2), we hypothesized that the CS-A matrix may interact with stroke microglia/macrophages to affect their expression of immunomodulatory proteins. Microglia/macrophages were collected from the stroke core region at 3 days post-infarction and either directly encapsulated in CS-A matrix or plated on tissue culture polystyrene (Fig. 4 A). After 3 days in vitro, we found that microglia grown in the CS-A hydrogel expressed higher levels of VEGFR2, TIE2, PPARγ, bFGF, and IL-10 as determined by Western blotting (Fig. 4 B).
Fig. 4.
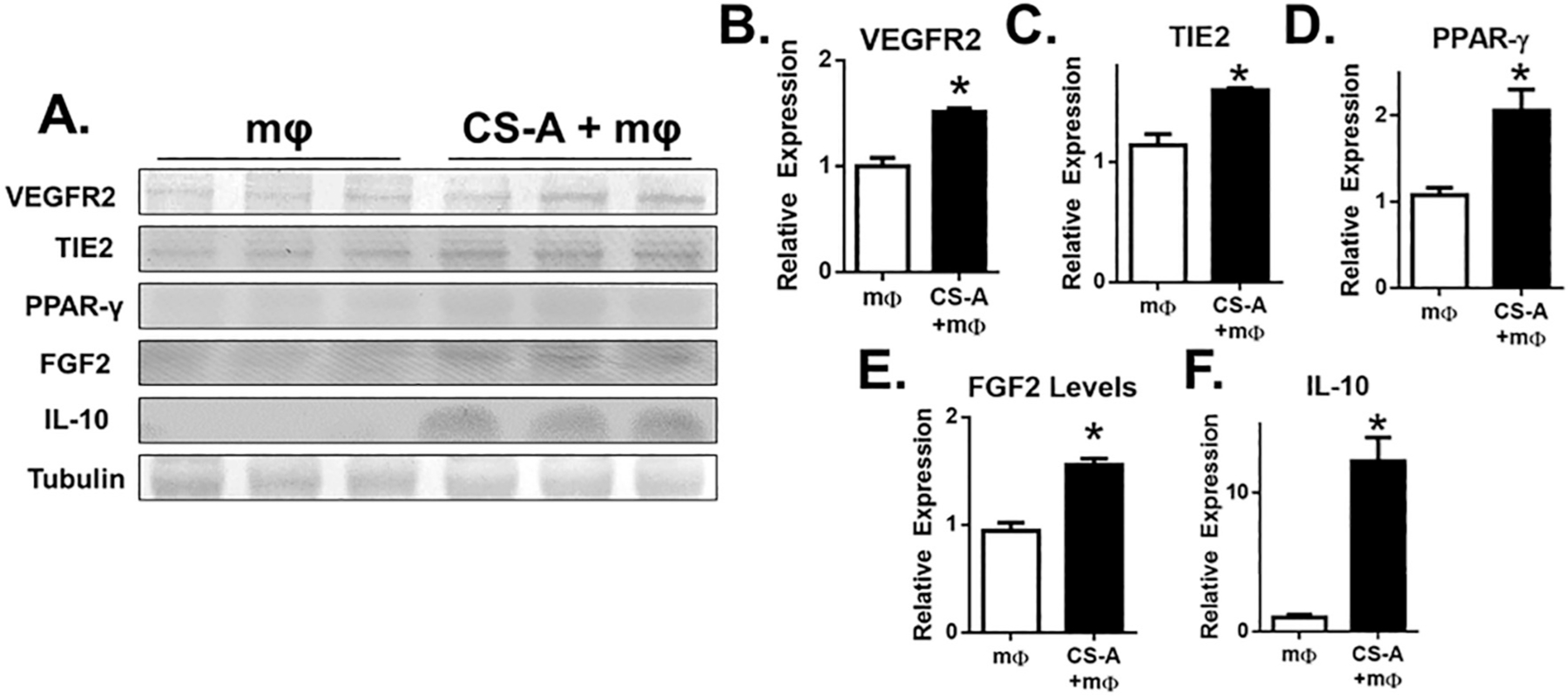
CS-A encapsulation of stroke tissue-derived microglia/macrophages promotes a regenerative macrophage phenotype in vitro. A. Western blotting of VEGFR2, TIE2, PPAR-γ, FGF2, and IL-10. B-F. Analysis of protein expression. n = 3–5 in vitro replicates of tissue pooled from 8 mice. * indicates p < 0.05; two-tailed t-test.
CS-A can regulate IL-10 binding, partitioning, and presentation (Betancur et al., 2017; Karumbaiah et al., 2015; Karumbaiah et al., 2014). Thus, we tested whether IL-10 may play a role in promoting the microglia/macrophage phenotype seen in vitro (Fig. 4) by neutralizing released IL-10 by addition of an anti-IL-10 neutralizing antibody to the cell culture media (Fig. 5). We found that the protein levels of TIE2 and FGF2 were significantly increased by CS-A encapsulation, which is consistent with previous in vitro and in vivo findings (Fig. 5 C–D). Furthermore, IL-10 neutralization resulted in a reduction in TIE2 and FGF2 in both the CS-A encapsulated and non-encapsulated treatment groups. Notably, the levels of TIE2 and FGF2 in the CS-A encapsulated group with IL-10 neutralization were comparable to the non-encapsulated control group (Fig. 5 C–D).
Fig. 5.
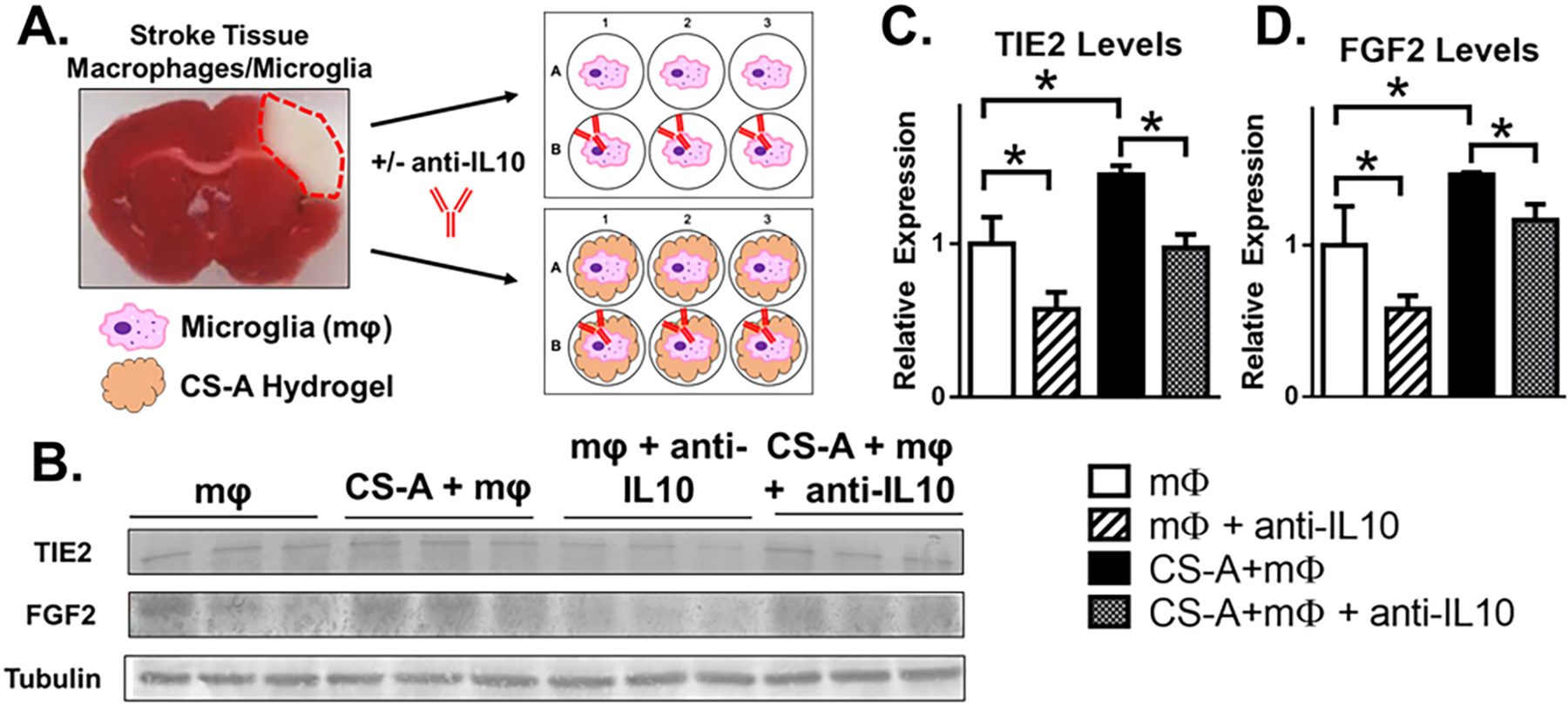
IL-10 Neutralization Reduces Expression of Angiogenic Markers of Stroke Tissue-Derived Macrophages/Microglia in vitro. A. Schematic representation of stroke tissue macrophage/microglia (mφ) culture and IL-10 neutralization in vitro. B. Western blotting of TIE2 and FGF2. C-D. Analysis of TIE2 (C) and FGF2 (D) protein expression. n = 3 in vitro replicates of pooled macrophages from 3 mice per treatment group. * indicates p < 0.05; 1-way ANOVA with Bonferroni correction.
4. Discussion
The immune response plays a major role in the natural history of stroke pathophysiology and the response to transplanted materials (Jendelova et al., 2016; Nih et al., 2016; Modo et al., 2013; Murray and Wynn, 2011). It is desirable for material approaches to either reduce harmful inflammation, remain immune-inert, or promote a regenerative immune phenotype (Moshayedi and Carmichael, 2013; Cohen and Jensen, 2015). Microglia/macrophages are the major immune cells involved in stroke (Xiong et al., 2016). A pro-regenerative population of macrophages is associated with neurogenesis, angiogenesis, and stroke recovery (Xiong et al., 2016; Taylor and Sansing, 2013).
In this study, the microglial response to the transplanted CS-A hydrogel and CS-A hydrogel encapsulated NPCs was interrogated using immunohistochemical, molecular biological, and cell culture techniques and compared to NPCs only or stroke controls. We found that CS-A + NPC treatment resulted in an increased level of macrophages expressing factors associated with tissue regeneration (Fig. 1–2). The immune response was characterized by analyzing the protein expression of a variety of immunomodulatory cytokines released (Fig. 1). The chemoattractant protein MCP-1, which is important for recruiting vascular-associated macrophages to promote angiogenesis and arteriogenesis, was also significantly increased by CS-A + NPC treatment compared to all other treatments (Fig. 1A). Following ischemic injury and in various treatment paradigms, MCP-1 has been shown to recruit specific subsets of macrophages that participate in regeneration, including angiogenesis, arteriogenesis, and even neurogenesis (Heil et al., 2006; Liu et al., 2014; Semenza, 2007; Yan et al., 2007; Nishijima et al., 2015). MIP-1a and 1b also participate in the recruitment of microglia/macrophages, however, these may be more involved in neurotoxicity and infarct progression during the early phases of stroke pathophysiology (Kriz, 2006; Amantea et al., 2009). These and other molecules were significantly reduced in the cellular treatment, but not affected by CS-A scaffolding (Fig. 1).
The increased levels of MCP-1 in the peri-infarct region suggest that the recruitment of pro-regenerative macrophages, in particular the recruitment of arteriogenic macrophages, extends outside of the stroke core region and into the peri-infarct zone (Fig. 1). The group expression of chemokines/cytokines was found to be different between treatment groups, suggesting that the combination of CS-A + NPCs elicits different immune responses following treatment and that the interaction between the 3D CS-A matrix and NPCs is important to this change (Fig. 1). These findings suggest that transplanted NPCs may reduce microglial infiltration and activity (Fainstein et al., 2013; Koutsoudaki et al., 2016). Interestingly, the combination of the NPCs with the CS-A hydrogel seems to promote rather than inhibit the expression of certain inflammatory factors in the stroke core region. s. Increase in total IBA-1 and IL-10 levels may be attributed to enhanced recruitment of pro-regenerative macrophages observed in the following experiments (Fig. 2). CS-A interacts with NPCs synergistically; while treatment with NPCs alone does not significantly increase the number of PPARγ-expressing microglia/macrophages or IL-10 protein levels, CS-A-encapsulated NPCs show significantly increased levels of these markers (Fig. 2). Microglial morphology is related to phagocytic state. Microglia in the healthy brain often appear ramified with small somas, while actively phagocytic cells after stroke or trauma transition to an ameboid shape (Boche et al., 2013; Savage et al., 2019; Morrison and Filosa, 2013). Treatment with acellular CS-A appears to affect microglial/macrophage morphology (Fig. 2 E). Sholl analysis of microglia/macrophages in the stroke area following treatments revealed that CS-A treated mice were more ameboid in morphology than stroke controls. This suggests the CS-A hydrogel may contribute to the activation of microglia/macrophages. This is consistent with work by others in traumatic spinal cord injury, where endogenous chondroitin sulfate proteoglycans were found to regulate neuroinflammation to promote recovery (Dyck et al., 2018; Lang et al., 2015).
Together, the analysis of secreted immunomodulatory factor expression suggests that CS-A encapsulation of NPCs may promote recruitment of regenerative macrophages via increase of PPARγ, MCP-1, and IL-10. Furthermore, these findings verify that treatment with NPCs can reduce both harmful and beneficial microglial inflammation, and that encapsulation with CS-A may allow expression recovery of some of these proteins during the recovery stage of stroke pathophysiology. These samples were isolated from stroke tissue weeks after infarction, which is long after the initial wave of neurotoxic inflammation during the acute phases of ischemic stroke (Kriz, 2006; Amantea et al., 2009; Taylor and Sansing, 2013). The extent to which CS-A can facilitate beneficial versus harmful microglial inflammation is not easily discernable. However, it should be noted that in all cases except for MCP-1, the expression levels in the CS-A + NPC treatment group were not statistically significantly different than the stroke controls (Fig. 1 A–B). Additionally, our data suggests the microglia/macrophage response elicited a pro-recovery phenotype as demonstrated by improvement in depression-like behavior. The principal component analysis (PCA) also recapitulated the importance of PPARγ-expressing microglia/macrophages as determinants of the CS-A + NPC phenotype (S. Fig. 1). It also highlighted the role of IL-4, a cytokine known to polarize brain microglia towards a pro-regenerative phenotype in stroke (Zhao et al., 2015). Additionally, linear regression analysis indicated that PPARγ-expressing microglia/macrophages and IL-10 levels were strongly related to reductions in post-stroke depression. These results suggest that CS-A does not worsen the inflammatory response compared to the natural history of inflammation in stroke pathology, but rather, promotes regenerative immunomodulation via the recruitment and polarization of arteriogenic macrophages.
Post-stroke neuropsychiatric deficits have been reported in humans and in rodent stroke models and is related to neuroinflammation (Santos Samary et al., 2016; Kronenberg et al., 2014; Zhong et al., 2020). This ischemic stroke model recapitulates some aspects of post-stroke neurocognitive deficits. Previous work by our lab has indicated that early treatment with hypothermia may improve post-stroke depression-like behavior (Zhong et al., 2020). While the mechanisms behind post-stroke psychological deficits are not entirely understood, some theorize that neuroinflammation is a central player (Villa et al., 2018). Thus, we assessed post-stroke neuropsychiatric deficits using tests for anxiety and apathy (Fig. 3 A–B). The tail suspension test indicated that CS-A encapsulated NPCsmay reduce apathy-like behavior (Fig. 3 B). The open-field test was inconclusive as all treatment groups yielded an improvement from baseline post-stroke anxiety-like behavior (Fig. 3 A). However, using linear regression analysis, we found that PPARγ + macrophages and IL-10 in combination were significantly correlated with immobility time in the tail suspension test. This suggests that immunomodulatory macrophages and their released proteins, specifically IL-10, are likely key players in the beneficial immune response and neurocognitive recovery following CS-A + NPC treatment.
The immunomodulatory interleukin 10 is produced primarily by microglia/macrophages and is thought to be a central player in inducing microglia to participate in regenerative rather than harmful neuroinflammation (Savitz and Cox Jr., 2016; Hu et al., 2015; Xiong et al., 2016; Kriz, 2006; Taylor and Sansing, 2013; Wei et al., 2012). We illustrate that human iPSC-derived neural stem cells, which have a high translational value for ischemic stroke and other brain injuries, do not release significant amounts of IL-10 in vitro (S. Fig. 2). This indicates that the source of IL-10 is largely endogenous to the brain. Furthermore, CS-A has a high affinity for IL-10, and in in vitro experiments, was shown to enrich IL-10 following neural stem cell encapsulation (Betancur et al., 2017; Karumbaiah et al., 2015). In experimental stroke, treatment with IL-10 was been found to be neuroprotective and reduced infarct volumes, and also reduced early neuroinflammation (Lambertsen et al., 2019).
We sought to further understand the immunomodulatory mechanisms underlying CS-A + NPC treatment. Given the robust enrichment of IL-10 in the stroke core following treatment with CS-A + NPCs (Fig. 2 D), and knowing that the CS-A sulfation can facilitate IL-10 signaling (Karumbaiah et al., 2015; Karumbaiah et al., 2014), we hypothesized that 3D CS-A matrix may potentiate IL-10 signaling to affect microglia/macrophage phenotype. We tested the specific response of stroke tissue-derived macrophages/microglia to the CS-A hydrogel in a controlled in vitro environment (Figs. 4–5). We found that in vitro culture of primary stroke core macrophages/microglia in the CS-A hydrogel increased the expression of proteins FGF2 and IL-10, and markers such as VEGFR2, TIE2, and PPARγ, which are associated with a regenerative and reparative macrophage phenotype (Fig. 4). Using neutralization studies in vitro, we also found that IL-10 may play a role in promoting a proarteriogenic macrophage/microglial phenotype (Fig. 5). IL-10 neutralization resulted in normalization of TIE2 and FGF2 levels in the CS-A encapsulation group, suggesting that IL-10 is critical for the polarization of microglia towards a regenerative phenotype (Fig. 5 C–D).
Previous studies involving CS-A revealed that transplantation into either the infarcted or post-TBI brain environments can significantly enhance vascular recovery (McCrary et al., 2020; Latchoumane et al., 2021). Macrophage/microglial expression of the VEGFR2 and TIE2 receptors are associated with a subset of immune cells that promote vascular regeneration (Hu et al., 2015; Xiong et al., 2016; Liu et al., 2014; Murray and Wynn, 2011; Hu et al., 2012). Similarly, macrophages can produce FGF2, which can promote both angiogenesis and arteriogenesis (Liu et al., 2014; Arai et al., 2009). The proteins PPARγ and IL-10 were also significantly increased in vivo, suggesting that CS-A may play a role in directing microglia/macrophage phenotype to promote regeneration (Fig. 2). The enhanced expression of VEGFR2, TIE2, and FGF2 by microglia/macrophages (Fig. 4) suggests that CS-A encapsulation may promote microglia/macrophages to assist in vascular regeneration seen in our previous studies (McCrary et al., 2020).
There are several technical and theoretical limitations to with the studies presented above. The macrophage/microglial interaction with the transplanted NPCs was not evaluated in vitro, which appears to be a major influence on the level of chemokines/cytokines in vivo (Fig. 1). However, this would be technically difficult and would not isolate the specific effects of the CS-A scaffolding on microglia/macrophages. Another important limitation is that the dissection and culturing of the stroke core tissue following dissociation does not yield a pure population of microglia/macrophages. Other cell type contaminants from the stroke core such as surviving endothelium or neurons may be present, however, they are very few and likely do not provide a significant contribution to the protein isolated for these experiments (Jiang et al., 2017). We isolated macrophages 3 days after stroke since the infiltration peaks at this timepoint, allowing for the collection of sufficient numbers for in vitro applications. Furthermore, microglia are more active at this time point (Savitz and Cox Jr., 2016; Xiong et al., 2016; Kriz, 2006; Taylor and Sansing, 2013). However, in vivo transplantations were performed at 7 days after stroke. The microglia/macrophages isolated from the stroke core at 3 days and subsequently cultured in vitro may not reflect their phenotype in vivo at 7 days. Additionally, our experiments relied heavily on protein expression, which does not solely define the complex roles of microglia/macrophages in the post-stroke brain. Our base control group was the ischemic stroke disease state; thus, generalization may be limited to this context. Finally, the use of a neutralizing antibody (Fig. 5) may not completely reduce the levels of IL-10 while it could be constantly secreted by cells; alternate strategies such as transgenetic knockdown or knockout may provide more definitive reductions in the bioavailability of IL-10. Future studies may also manipulate other upstream immunomodulatory factors reported herein including MCP-1 both in vitro and in vivo. Despite these limitations, these studies reveal important interactions between macrophages/microglia and the CS-A hydrogel which likely play a role in the profound vascular regeneration seen in our previous work.
The focal ischemic stroke in the sensorimotor cortex for this study generates a well-defined area of infarction (McCrary et al., 2019). Local microglia in the ischemic brain and blood-derived macrophages can respond and accumulate within hours to days and weeks following ischemia (Jin et al., 2010; Hu et al., 2015; Pei et al., 2015). One week after stroke, neuronal death has plateaued and regenerative factor expression in the stroke core is apparent (Wei et al., 2017; Jiang et al., 2017; Zhang et al., 2018). Concurrently, microglia/macrophages and expression of inflammatory markers are elevated at this time point (Jiang et al., 2017; Zhang et al., 2018). Therefore, we hypothesized that one week after stroke is an opportune window for the introduction of immunomodulatory and regenerative therapies that may benefit from synergy with endogenous recovery processes. Additionally, previous studies at these time points illustrated success in outcomes such as improved transplant cell survival and neuronal differentiation, angiogenesis, and sensorimotor recovery (McCrary et al., 2020). Further studies are necessary to corroborate the optimal timing for treatment to maximize the neuropsychiatric effects of CS-A + NPC transplantation. Multiple additional tests, especially for depression and anxiety-like behavior analyses such as the forced swim, sucrose preference, elevated maze, and light/dark box tests are also necessary in future studies.
In summary, CS-A hydrogels may enhance NPC transplantation for stroke by promoting a regenerative microglia/macrophage response via IL-10 accumulation. We also found that MCP-1, an important chemokine integral to arteriogenic macrophages, was upregulated in the infarct and peri-infarct regions in response to CS-A + NPC transplantation. Encapsulation of stroke tissue-derived microglia/macrophages in CS-A scaffolding in vitro resulted in increased expression of FGF2, TIE2, and VEGFR2 which are associated with vascular remodeling and regeneration. These effects culminated in improvements in post-stroke behavior outcomes.
The regenerative and immunomodulatory effects of CS-A scaffolding for cellular therapy may be useful for brain injuries. The effects on angiogenesis and large vessel remodeling, and also the improved sensorimotor recovery documented in our previous studies, are highly desirable for post-stroke brain recovery (McCrary et al., 2020). The microglia/macrophage response documented here, including robust expression of IL-10 within the ischemic stroke region and improvements in post-stroke neuropsychiatric deficits, are also advantageous. However, there are several barriers to translation to human patients. Further experimentation is required to further dissect the immune milieu, including, if and how other types of immune cells may interact with the CS-A hydrogel and encapsulated NPCs. This treatment strategy must also be tested in larger animal models and with different treatment windows, for example, in the acute stroke where treatment effects may result in both neuroprotection and neuroregeneration. Further investigation of other transplanted cell types, cell type-specific alterations, lesion volume effects, BBB permeability, and cell death will aid in the translation of cell encapsulation strategies for the treatment of brain injury. Finally, longer tests detailing the chronic outcomes following transplantation months to years after stroke may prove useful.
Supplementary Material
Acknowledgments
This research was supported by NIH grants NS099596 (LK), NS114221 (LW/SPY), NS091585 (LW), NS085568 (LW/SPY), RX001473 (SPY), and T32GM008169 (MRM). MRM is currently employed by Wellstar Kennestone Hospital where he participated in the revision of the manuscript (2021–2022).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.expneurol.2022.114177.
References
- Akita K, et al. , 2008. Expression of multiple chondroitin/dermatan sulfotransferases in the neurogenic regions of the embryonic and adult central nervous system implies that complex chondroitin sulfates have a role in neural stem cell maintenance. Stem Cells 26 (3), 798–809. [DOI] [PubMed] [Google Scholar]
- Amantea D, et al. , 2009. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J 276 (1), 13–26. [DOI] [PubMed] [Google Scholar]
- Arai K, et al. , 2009. Brain angiogenesis in developmental and pathological processes: neurovascular injury and angiogenic recovery after stroke. FEBS J 276 (17), 4644–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain G, et al. , 1996. Retinoic acid promotes neural and represses mesodermal gene expression in mouse embryonic stem cells in culture. Biochem. Biophys. Res. Commun 223 (3), 691–694. [DOI] [PubMed] [Google Scholar]
- Betancur MI, et al. , 2017. Chondroitin Sulfate glycosaminoglycan matrices promote neural stem cell maintenance and neuroprotection post-traumatic brain injury. ACS Biomater. Sci. Eng 3 (3), 420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boche D, Perry VH, Nicoll JA, 2013. Review: activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol 39 (1), 3–18. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, 2000. Mechanisms of angiogenesis and arteriogenesis. Nat. Med 6 (4), 389–395. [DOI] [PubMed] [Google Scholar]
- Chau MJ, et al. , 2014. iPSC transplantation increases regeneration and functional recovery after ischemic stroke in neonatal rats. Stem Cells 32 (12), 3075–3087. [DOI] [PubMed] [Google Scholar]
- Choi KE, et al. , 2012. A novel stroke therapy of pharmacologically induced hypothermia after focal cerebral ischemia in mice. FASEB J 26 (7), 2799–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LK, Jensen MB, 2015. Scaffolds for intracerebral grafting of neural progenitor cells after cerebral infarction: a systematic review. Arch. Neurosci 2 (4), e25364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck S, et al. , 2018. Perturbing chondroitin sulfate proteoglycan signaling through LAR and PTPsigma receptors promotes a beneficial inflammatory response following spinal cord injury. J. Neuroinflammation 15 (1), 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainstein N, et al. , 2013. Time limited immunomodulatory functions of transplanted neural precursor cells. Glia 61 (2), 140–149. [DOI] [PubMed] [Google Scholar]
- Gama CI, et al. , 2006. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat. Chem. Biol 2 (9), 467–473. [DOI] [PubMed] [Google Scholar]
- Garcia JM, et al. , 2017. Role of Interleukin-10 in acute brain injuries. Front. Neurol 8, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, et al. , 2006. Arteriogenesis versus angiogenesis: similarities and differences. J. Cell. Mol. Med 10 (1), 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, et al. , 2012. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43 (11), 3063–3070. [DOI] [PubMed] [Google Scholar]
- Hu X, et al. , 2015. Microglial and macrophage polarization-new prospects for brain repair. Nat. Rev. Neurol 11 (1), 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida M, et al. , 2006. Identification and functions of chondroitin sulfate in the milieu of neural stem cells. J. Biol. Chem 281 (9), 5982–5991. [DOI] [PubMed] [Google Scholar]
- Jendelova P, et al. , 2016. Current developments in cell- and biomaterial-based approaches for stroke repair. Expert. Opin. Biol. Ther 16 (1), 43–56. [DOI] [PubMed] [Google Scholar]
- Jian WH, et al. , 2018. Glycosaminoglycan-based hybrid hydrogel encapsulated with polyelectrolyte complex nanoparticles for endogenous stem cell regulation in central nervous system regeneration. Biomaterials 174, 17–30. [DOI] [PubMed] [Google Scholar]
- Jiang MQ, et al. , 2017. Long-term survival and regeneration of neuronal and vasculature cells inside the core region after ischemic stroke in adult mice. Brain Pathol 27 (4), 480–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G, 2010. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukoc. Biol 87 (5), 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, et al. , 2013. Role of inflammation and its mediators in acute ischemic stroke. J. Cardiovasc. Transl. Res 6 (5), 834–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karumbaiah L, et al. , 2014. Chondroitin sulfate glycosaminoglycans for CNS homeostasis-implications for material design. Curr. Med. Chem 21 (37), 4257–4281. [DOI] [PubMed] [Google Scholar]
- Karumbaiah L, et al. , 2015. Chondroitin Sulfate glycosaminoglycan hydrogels create endogenous niches for neural stem cells. Bioconjug. Chem 26 (12), 2336–2349. [DOI] [PubMed] [Google Scholar]
- Koutsoudaki PN, et al. , 2016. Neural stem/progenitor cells differentiate into oligodendrocytes, reduce inflammation, and ameliorate learning deficits after transplantation in a mouse model of traumatic brain injury. Glia 64 (5), 763–779. [DOI] [PubMed] [Google Scholar]
- Kriz J, 2006. Inflammation in ischemic brain injury: timing is important. Crit. Rev. Neurobiol 18 (1–2), 145–157. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, et al. , 2014. Of mice and men: modelling post-stroke depression experimentally. Br. J. Pharmacol 171 (20), 4673–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok JC, Warren P, Fawcett JW, 2012. Chondroitin sulfate: a key molecule in the brain matrix. Int. J. Biochem. Cell Biol 44 (4), 582–586. [DOI] [PubMed] [Google Scholar]
- Lambertsen KL, Finsen B, Clausen BH, 2019. Post-stroke inflammation-target or tool for therapy? Acta Neuropathol 137 (5), 693–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang BT, et al. , 2015. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature 518 (7539), 404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchoumane CV, et al. , 2021. Engineered glycomaterial implants orchestrate large-scale functional repair of brain tissue chronically after severe traumatic brain injury. Sci. Adv 7 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, et al. , 2016. Regulation of therapeutic hypothermia on inflammatory cytokines, microglia polarization, migration and functional recovery after ischemic stroke in mice. Neurobiol. Dis 96, 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, et al. , 2014. Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Prog. Neurobiol 115, 138–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, 2010. Structural variation of chondroitin sulfate and its roles in the central nervous system. Cent. Nerv. Syst. Agents Med. Chem 10 (1), 22–31. [DOI] [PubMed] [Google Scholar]
- McCrary MR, et al. , 2019. Protective effects of GPR37 via regulation of inflammation and multiple cell death pathways after ischemic stroke in mice. FASEB J 33 (10), 10680–10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrary MR, et al. , 2020. Cortical transplantation of brain-mimetic glycosaminoglycan scaffolds and neural progenitor cells promotes vascular regeneration and functional recovery after ischemic stroke in mice. Adv. Healthc. Mater 9 (5), e1900285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misrani A, et al. , 2019. Differential effects of citalopram on sleep-deprivation-induced depressive-like behavior and memory impairments in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 88, 102–111. [DOI] [PubMed] [Google Scholar]
- Modo M, et al. , 2013. Bioengineering solutions for neural repair and recovery in stroke. Curr. Opin. Neurol 26 (6), 626–631. [DOI] [PubMed] [Google Scholar]
- Morrison HW, Filosa JA, 2013. A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J. Neuroinflammation 10, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshayedi P, Carmichael ST, 2013. Hyaluronan, neural stem cells and tissue reconstruction after acute ischemic stroke. Biomatter 3 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA, 2011. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol 11 (11), 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase T, et al. , 2008. The impact of inflammation on the pathogenesis and prognosis of ischemic stroke. J. Neurol. Sci 271 (1–2), 104–109. [DOI] [PubMed] [Google Scholar]
- Nih LR, Carmichael ST, Segura T, 2016. Hydrogels for brain repair after stroke: an emerging treatment option. Curr. Opin. Biotechnol 40, 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima Y, et al. , 2015. Collaterals: implications in cerebral ischemic diseases and therapeutic interventions. Brain Res 1623, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, et al. , 2010. Opposing functions of chondroitin sulfate and heparan sulfate during early neuronal polarization. Neuroscience 169 (4), 1535–1547. [DOI] [PubMed] [Google Scholar]
- Pei J, You X, Fu Q, 2015. Inflammation in the pathogenesis of ischemic stroke. Front. Biosci. (Landmark Ed.) 20, 772–783. [DOI] [PubMed] [Google Scholar]
- Perera MN, et al. , 2006. Inflammation following stroke. J. Clin. Neurosci 13 (1), 1–8. [DOI] [PubMed] [Google Scholar]
- Pietra Pedroso VS, Rachid MA, Teixeira AL, 2016. Biomarkers in post-stroke depression. Curr. Neurovasc. Res 13 (2), 163–173. [DOI] [PubMed] [Google Scholar]
- Powers WJ, et al. , 2018. 2018 guidelines for the early Management of Patients with Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49 (3), e46–e110. [DOI] [PubMed] [Google Scholar]
- Prakash R, Carmichael ST, 2015. Blood-brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Curr. Opin. Neurol 28 (6), 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman A, Sugahara K, Faissner A, 2012. Chondroitin sulfate “wobble motifs” modulate maintenance and differentiation of neural stem cells and their progeny. J. Biol. Chem 287 (5), 2935–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmilewitz J, Tykocinski ML, 1998. Differential effects of chondroitin sulfates a and B on monocyte and B-cell activation: evidence for B-cell activation via a CD44-dependent pathway. Blood 92 (1), 223–229. [PubMed] [Google Scholar]
- Rajkovic O, Potjewyd G, Pinteaux E, 2018. Regenerative medicine therapies for targeting Neuroinflammation after stroke. Front. Neurol 9, 734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauvala H, et al. , 2017. Inhibition and enhancement of neural regeneration by chondroitin sulfate proteoglycans. Neural Regen. Res 12 (5), 687–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RG, Jorge RE, 2016. Post-stroke depression: a review. Am. J. Psychiatry 173 (3), 221–231. [DOI] [PubMed] [Google Scholar]
- Santos Samary C, et al. , 2016. Immunomodulation after ischemic stroke: potential mechanisms and implications for therapy. Crit. Care 20 (1), 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage JC, Carrier M, Tremblay ME, 2019. Morphology of microglia across contexts of health and disease. Methods Mol. Biol 2034, 13–26. [DOI] [PubMed] [Google Scholar]
- Savitz SI, Cox CS Jr., 2016. Concise review: cell therapies for stroke and traumatic brain injury: targeting microglia. Stem Cells 34 (3), 537–542. [DOI] [PubMed] [Google Scholar]
- Semenza GL, 2007. Vasculogenesis, angiogenesis, and arteriogenesis: mechanisms of blood vessel formation and remodeling. J. Cell. Biochem 102 (4), 840–847. [DOI] [PubMed] [Google Scholar]
- Sirko S, et al. , 2007. Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells. Development 134 (15), 2727–2738. [DOI] [PubMed] [Google Scholar]
- Stephenson EL, Yong VW, 2018. Pro-inflammatory roles of chondroitin sulfate proteoglycans in disorders of the central nervous system. Matrix Biol 71–72, 432–442. [DOI] [PubMed] [Google Scholar]
- Taylor RA, Sansing LH, 2013. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin. Dev. Immunol 2013, 746068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LH, Bjorklund A, 2015. Reconstruction of brain circuitry by neural transplants generated from pluripotent stem cells. Neurobiol. Dis 79, 28–40. [DOI] [PubMed] [Google Scholar]
- Villa RF, Ferrari F, Moretti A, 2018. Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol. Ther 184, 131–144. [DOI] [PubMed] [Google Scholar]
- Wei L, Rovainen CM, Woolsey TA, 1995. Ministrokes in rat barrel cortex. Stroke 26 (8), 1459–1462. [DOI] [PubMed] [Google Scholar]
- Wei L, et al. , 2012. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol. Dis 46 (3), 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, et al. , 2017. Stem cell transplantation therapy for multifaceted therapeutic benefits after stroke. Prog. Neurobiol 157, 49–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, et al. , 2018. Mechanisms and therapeutic targets of depression after intracerebral Hemorrhage. Front. Psychiatry 9, 682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong XY, Liu L, Yang QW, 2016. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol 142, 23–44. [DOI] [PubMed] [Google Scholar]
- Xiong M, et al. , 2021. Human stem cell-derived neurons repair circuits and restore neural function. Cell Stem Cell 28 (1), 112–126 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YP, et al. , 2007. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J. Cereb. Blood Flow Metab 27 (6), 1213–1224. [DOI] [PubMed] [Google Scholar]
- Zhang C, et al. , 2018. Temporal gene expression profiles after focal cerebral ischemia in mice. Aging Dis 9 (2), 249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, et al. , 2015. Neuronal Interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J. Neurosci 35 (32), 11281–11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, et al. , 2020. Neuropsychological deficits chronically developed after focal ischemic stroke and beneficial effects of pharmacological hypothermia in the mouse. Aging Dis 11 (1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


