Abstract
Our experience of time can feel dilated or compressed, rather than reflecting true “clock time.” Although many contextual factors influence the subjective perception of time, it is unclear how memory accessibility plays a role in constructing our experience of and memory for time. Here, we used a combination of behavioral and functional MRI measures in healthy young adults (N = 147) to ask the question of how memory is incorporated into temporal duration judgments. Behaviorally, we found that event boundaries, which have been shown to disrupt ongoing memory integration processes, result in the temporal compression of duration judgments. Additionally, using a multivoxel pattern similarity analysis of functional MRI data, we found that greater temporal pattern change in the left hippocampus within individual trials was associated with longer duration judgments. Together, these data suggest that mnemonic processes play a role in constructing representations of time.
Keywords: time estimation, memory, event boundaries, neuroimaging
An hour, once it lodges in the queer element of the human spirit, may be stretched to fifty or a hundred times its clock length; on the other hand, an hour may be accurately represented on the timepiece of the mind by one second.
On a busy vacation, time may escape you—by the time you go to the museum, grab lunch in the park, shop for souvenirs, and visit a historical site, the day may seem to have flown by. Yet when recalling the trip to a friend, that same day may feel like a week; all of those events could not have possibly occurred within the same few hours. This puzzle raises a fundamental question of how the structure of experience can paradoxically influence subjective impressions of time in experience and in reflection.
A great deal of work has focused on the latter: how the structure of experience influences how we remember elapsed time. In particular, abrupt shifts in context, or event boundaries, influence memory for time. Memory for the temporal order of events is disrupted across event boundaries (DuBrow & Davachi, 2013; Heusser et al., 2018; Horner et al., 2016). Further, intervals that contain an event boundary are remembered as longer than equivalently timed intervals without a boundary (Clewett et al., 2020; Ezzyat & Davachi, 2014), and mnemonic duration judgments scale with the number of events (Faber & Gennari, 2015, 2017; Lositsky et al., 2016). Such findings converge with the intuition that busy days feel long in memory: Events may serve to dilate time in memory. How, though, do events also result in time feeling subjectively shorter in the moment?
Event boundaries also influence memory on the more immediate time scale of working memory by reducing access to information prior to the boundary (Ezzyat & Davachi, 2011; Rinck & Bower, 2000; Speer & Zacks, 2005; Zwaan, 1996). Thus, perhaps this decreased accessibility to mnemonic information translates into a contraction of time for intervals containing event boundaries. Models of time perception posit an “integrator” or “accumulator” that sums across experience to determine how much time has passed (e.g., Wittmann, 2013). By reducing the contents of working memory, event boundaries may reduce the information integrated into duration judgments, leading to an underestimation of time. Consistently, there is some evidence that in-the-moment time judgments are compressed after one or more discrete boundaries (Bangert et al., 2020; Liverence & Scholl, 2012; Yousif & Scholl, 2019). However, whether this effect of event boundaries on more immediate time judgments is indeed due to a decreased accessibility to the prior event remains an open question.
A separate body of literature has examined the interplay of time and memory by asking whether the hippocampus—a structure critical for episodic memory (Scoville & Milner, 1957)—plays a role in tracking time. In one seminal study, Meck and colleagues (1984) found that although disrupting hippocampal function did not result in impairments in perceiving duration, it critically impaired temporal working memory and led to underestimation of the “reference memories.” This study suggests that the hippocampus may be involved in duration processing, but it leaves open the question of how, precisely, mnemonic information is incorporated into representations of time.
A plethora of subsequent work has established that the medial temporal lobe—in particular, the hippocampus—indeed is sensitive to temporal duration information (Davachi & DuBrow, 2015; Eichenbaum, 2013). Individual hippocampal neurons are sensitive to temporal information, firing during delays (e.g., MacDonald et al., 2011; Reddy et al., 2021; Sakon et al., 2014; Umbach et al., 2020) or at specific temporal moments (Sun et al., 2020; Terada et al., 2017). Further, memories acquired close in time are coded by overlapping neural populations (Cai et al., 2016; Rubin et al., 2015). Such overlapping representations may have consequences for the subjective representation of time in long-term memory: Events remembered as further apart in time are associated with greater functional MRI pattern change in the medial temporal lobe (Deuker et al., 2016; Ezzyat & Davachi, 2014; Lositsky et al., 2016; Nielson et al., 2015). However, whether and how such hippocampal representations influence more immediate judgments of time remain unclear.
Statement of Relevance.
Our everyday experiences convey a powerful truth: Our perception of time often diverges from the reality of time. When enjoying an active vacation with family, time moves quickly: Hours go by in minutes. When sitting through an unnecessary meeting, time moves slowly: Minutes go by in hours. What is the origin of these phenomenologically compelling illusions of time perception? Past research has examined how a range of specific factors, from emotions to blinking, contributes to the distortion of time. Here, in contrast, we evaluated how the content and accessibility of our memories shape time perception. We show that context shifts, known to disrupt memory processing, also lead to robust contractions of perceived time. We discuss how both effects—memory disruptions and time distortions—may be linked via the hippocampus.
In the present study, we examined the role of memory accessibility and hippocampal representations on subjective judgments of time. Critically, we induced context shifts by inserting event boundaries, allowing us to assess the role of memory representations while holding true duration constant. First, in three behavioral experiments, we extended prior work demonstrating that event boundaries reduce estimates of duration and provide evidence that these reductions are due to decreased mnemonic accessibility. In a final functional MRI experiment, we used pattern similarity to track changes in representations within a single temporal interval and found that pattern change in the left hippocampus supports duration judgments, suggesting that the hippocampus may carry behaviorally relevant temporal information on the order of seconds.
Experiment 1
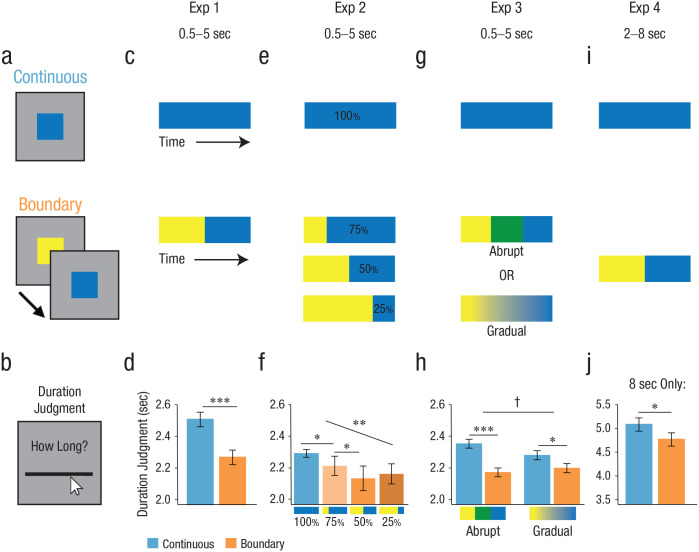
To examine how event boundaries affect subjective duration judgments, we designed a paradigm in which participants viewed a colored square (0.5–5 s in Experiments 1–3; 2–8 s in Experiment 4; see Fig. 1a), which remained a single color for the duration of the trial (continuous condition) or switched colors during the interval (boundary condition). Participants then judged how long the square, regardless of color, was presented on the screen using a continuous timeline (see Fig. 1b). We hypothesized that the color switches act as event boundaries, thus decreasing accessibility of the preboundary interval in memory. Critically, if memory for information from across the entire interval is integrated into a duration estimate, then reduced memory access for preboundary information would lead to shorter duration judgments in the boundary condition.
Fig. 1.
Behavioral task and data. (a) Participants viewed a colored square, which either stayed the same color for the total duration (continuous condition) or switched colors during the interval (boundary condition). (b) Participants then estimated how long the square was on the screen (regardless of color change). (c) Schematic of trial structures. In Experiment (Exp) 1, boundaries always occurred halfway through the total interval of 0.5 s to 5 s. (d) Experiment 1 results: Boundary trials were judged as significantly shorter than continuous trials. (e) In Experiment 2, color switches in boundary conditions occurred either one quarter, one half, or three quarters through the total interval of 0.5 s to 5 s, such that the second event was 75%, 50%, or 25% of the total duration, respectively. (f) Experiment 2 results: There was a significant linear effect of boundary placement; pairwise comparisons showed that 75% trials were judged to be reliably shorter than 100% trials, and 50% trials were judged as reliably shorter than 75% trials. (g) In Experiment 3, boundary trials were either abrupt (two color switches, each color presented for one third of the interval of 0.5-5 s) or gradual (smoothly morphing through color space for the entire interval of 0.5-5 s). (h) Experiment 3 results: In both groups, boundary trials were judged as reliably shorter than continuous trials, although this effect was weaker in the gradual change group. (i) As in Experiment 1, boundaries occurred halfway through the interval, although the interval was extended to be 2 s to 8 s. (j) Experiment 4 results: Boundary trials were judged as reliably shorter than continuous trials. †p < .07, *p < .05, **p < .005, ***p < .001 (all two-tailed). Error bars denote the within-participants standard error of the mean.
Method
Participants
Twenty-one individuals (13 female; age: M = 21.5 years, range = 18–33 years) were recruited from New York University and the larger community and participated for either course credit or payment ($10/hour). Informed consent was obtained in a manner approved by the University Committee on Activities Involving Human Subjects at New York University. We aimed to collect data from 20 usable participants; one participant was excluded for reporting that they explicitly counted the intervals during the task, and thus one additional participant was collected.
Stimuli
Stimuli were blue (R = 0, G = 0, B = 255), green (R = 0, G = 255, B = 0), and yellow (R = 260, G = 200, B = 80) squares presented centrally on a mid-gray background. Each square was presented on screen for an interval of 0.5 s to 5 s (sampled equally in increments of 0.5 s). For half of the trials, the color remained the same for the entire duration (continuous condition), and for the other half of the trials, the color switched halfway through the total duration (boundary condition).
Each participant viewed each color or color pair once for each time point. For each participant, color pairings were randomly assigned such that the pairing of the three colors in the boundary condition was fixed (e.g., the boundary trials for one participant would always be blue–green, green–yellow, and yellow–blue, and this pairing would be randomly assigned for each participant). The order of presentation was pseudorandomized such that condition-duration combinations were not repeated back to back, and no condition or duration appeared more than 4 times consecutively.
In total, the task consisted of three trials per condition and time point (60 trials total).
Duration judgment task
On each trial, participants were instructed to attend to the square and keep track of the time. Importantly, participants were explicitly verbally instructed not to count while the square was on the screen (Rattat & Droit-Volet, 2012), and debriefing questionnaires suggested that compliance with this instruction was high.
Participants subsequently were presented with a continuous timeline with the prompt “How long was the square on the screen?” This timeline was bounded by 0.5 s and 5 s. Participants were instructed to disregard any color changes and estimate the total duration of the square presentation, regardless of color. Participants responded using a computer mouse. Responses were self-paced.
Results
To assess accuracy of participants’ duration judgments, we calculated a Spearman rank correlation between the actual duration of the trial and the participant’s subjective judgment (response made on the timeline) for each participant. For the purposes of statistical evaluation, rho values were Fisher transformed. All participants exhibited a positive Spearman rank correlation value (mean correlation, or z = 1.29, 95% confidence interval, or CI = [1.15, 1.43]), t(19) = 19.14, p < .001, d = 4.28, 95% CI = [2.93, 5.84] (see Fig. S1a in the Supplemental Material available online).
To further confirm that each participant performed above chance relative to their own response distribution (i.e., irrespective of response bias), we also computed a permutation test for each participant: Across 10,000 iterations, each participant’s full distribution of responses was shuffled, and the rank correlation between the time of each trial and this shuffled response was computed. Significance was determined as being higher than 95% of this null distribution. All participants in all experiments demonstrated accuracy reliably above chance relative to their own distributions.
Next, we addressed our key question that event boundaries influence temporal duration judgments. In this experiment, in which boundary trials switched colors halfway through (see Fig. 1c), we found that participants judged boundary trials to be reliably shorter than continuous trials (mean difference, M = −0.24, 95% CI = [−0.34, −0.14]), t(19) = −5.07, p < .001, d = −1.13, 95% CI = [−1.73, −0.57] (see Fig. 1d). This finding conceptually replicates prior findings demonstrating a compression of duration judgments for experiences that contain a boundary (Bangert et al., 2020; Liverence & Scholl, 2012; Yousif & Scholl, 2019).
Experiment 2
After establishing an influence of event boundaries on duration judgments in this task, we next aimed to understand why boundaries lead to the compression of time. One reason that boundary trials might be judged as shorter is that the preboundary color is less accessible in working memory (Zwaan, 1996), leaving mostly the postboundary memory to be integrated into the duration judgment, thus leading to a shorter estimate. If this “flushing” account is true, then judgments of time not only should be influenced by the presence of a boundary but also should be sensitive to when in the interval the boundary occurred. In other words, boundaries that occur earlier in the trial will have more mnemonic content (because relatively less information was flushed at the boundary) and thus may be judged as longer than trials in which the boundary occurred later in the sequence.
Method
Participants
Twenty-nine individuals (18 female; age: M = 19.4 years, range = 18–24 years) were recruited from New York University for course credit. Informed consent was obtained in a manner approved by the University Committee on Activities Involving Human Subjects at New York University. The sample size for this experiment was chosen to be larger than that of Experiment 1 to increase sensitivity, given that more conditions were present in this experiment.
Stimuli
Stimuli were blue (R = 0, G = 0, B = 200), green (R = 75, G = 205, B = 75), and yellow (R = 260, G = 200, B = 80) squares presented centrally on a mid-gray background. Each square was presented on screen for an interval of 0.5 s to 5 s (sampled equally in increments of 0.5 s).
For half of the trials, as in Experiment 1, the color remained the same for the entire duration (continuous condition). The other half of the trials were one of three boundary conditions: For one third of the boundary trials (one-sixth of the total experiment), the color switched one-quarter of the way through the total duration (such that the second event was 75% of the total duration); for one third, the color switched one-half of the way through (second event was 50% of total duration); and for one third, the color switched three-quarters of the way through the total duration (second event was 25% of total duration).
Each participant viewed each color or color pair an equal number of times for each time point (here, twice over the entire experiment). Color pairings and pseudorandomization of trial sequences were conducted using the same procedure as in Experiment 1.
In total, the task consisted of six trials per condition and time point (120 trials total).
Duration judgment task
The duration judgment task was identical to that described in Experiment 1.
Results
Again, we first assessed accuracy of participants’ duration judgments and found that all participants exhibited a reliable correlation (mean z = 1.18, 95% CI = [1.08, 1.27]), t(28) = 24.66, p < .001, d = 4.58, 95% CI = [3.39, 5.93] (see Fig. S1b).
In this experiment, we manipulated the placement of the boundary such that the last event composed 100% (continuous condition), 75%, 50%, or 25% of the trial (see Fig. 1e). First, we replicated the result of Experiment 1 such that boundary trials were judged on average to be shorter than continuous trials (M = −0.12, 95% CI = [−0.17, −0.07]), t(28) = −5.00, p < .001, d = −0.93, 95% CI = [−1.38, −0.49]. To test our primary hypothesis that the extent of compression is modulated by the duration of the second color event, we examined whether the position of the boundary significantly modulated participants’ duration judgments (see Fig. 1f). We found a significant main effect of boundary placement (continuous or 100%, 75%, 50%, 25%) on duration judgment, F(3, 84) = 7.69, p < .001, ηp2 = .22, 95% CI = [0.06, 0.35]. To assess whether this main effect reflected the scaling of duration judgments with the length of the postboundary event, we asked whether duration judgments were linearly related to the length of the last event. For each participant, we computed the Pearson correlation between their duration judgments and the relative placement of the boundary and found a reliable negative correlation across participants (mean Fisher-transformed correlation = −0.57, 95% CI = [−0.90, −0.25]), t(28) = −3.66, p = .001, d = −0.68, 95% CI = [−1.10, −0.27]. To further probe this relationship, we conducted planned pairwise comparisons between neighboring conditions. The 75% boundary trials were rated as significantly shorter than 100% (continuous) trials (M = −0.07, 95% CI = [−0.14, −0.02]), t(28) = −2.64, p = .013, d = −0.49, 95% CI = [−0.88, −0.10]. The pairwise difference between 75% and 50% trials also reached significance; 50% trials were judged as significantly shorter than 75% trials (M = −0.08, 95% CI = [−0.16, −0.00]), t(28) = −2.09, p = .046, d = −0.39, 95% CI = [−0.78, −0.010]. However, we did not find a significant difference between 50% and 25% trials (M = 0.03, 95% CI = [−0.05, 0.11]), t(28) = 0.77, p = .446, d = 0.14, 95% CI = [−0.23, 0.52]. These data suggest that subjective time scales with the length of the most recent event up to the halfway point. Thus, when the most accessible information becomes too short (e.g., half the length of the trial), participants may rely on additional heuristic information (e.g., that multiple events occurred).
To further probe this memory accessibility account, we assessed the relative contributions of the first and second event durations on the resulting duration judgments. Because first and second event durations were modulated independently in this experiment, we were able to model duration judgments as a function of both first and second event duration. The strongest version of our account—that event boundaries completely disrupt access to preboundary information, leading to reliance on only the second event—would predict that duration judgments would scale only with the duration of the second event, regardless of first event duration. We thus ran a linear model on the group-averaged data, predicting mean duration judgments as a linear combination of the first and second events. Overall, this model proved an excellent fit to the data (R2 = .99). There were significant contributions of both first (β = 0.59, p < .001) and second (β = 0.66, p < .001) event durations, although notably the weight on the second event was numerically higher. To assess the reliability of this model fit as well as the difference in weights on the first event versus the second event, we conducted bootstrap resampling across participants (Efron & Tibshirani, 1986). Briefly, we resampled our participants with replacement 1,000 times; with each iteration, we recomputed the group-averaged data as well as the model fits. We then derived an empirical p value by assessing for what proportion of resamples the weight on the second event was higher than that on the first event. The weight on the second event was higher than the first in all 1,000 resamples (p < .001), indicating that although both first and second event durations influence duration judgments, participants overweight the duration of the second event. This finding is consistent with the interpretation that event boundaries lead to reduced (although not completely eliminated) accessibility of the first event and thus an increased reliance on the postboundary event.
Experiment 3
Experiment 2 provided initial evidence that memory accessibility may contribute to duration judgments. Next, to approach this question in a complementary way, we examined whether abrupt changes (associated with flushing of working memory contents) were necessary to elicit boundary-related temporal compression. In a between-participants design, participants were tested on continuous and either “abrupt” boundary trials (with two color switches, equally spaced over the interval) or “gradual” boundary trials (in which the color smoothly morphed over the entire interval; see Fig. 1g). We hypothesized that if the context drifted gradually, rather than abruptly, over the course of the trial, we would observe a reduced effect of color change on duration judgments. Such gradual shifts may be associated with some forgetting of early information, but we would not necessarily expect a full “flushing” on the basis of prior work showing that prior memories persist when change is gradual (Gershman et al., 2014).
Method
Participants
Eighty individuals (62 female; age: M = 19.9 years, range = 18–27 years) were recruited from New York University and the larger community and participated for either course credit or payment ($10/hour). We assigned 20 individuals to each of the four experimental groups (described below). Informed consent was obtained in a manner approved by the University Committee on Activities Involving Human Subjects at New York University. Post hoc power analyses of Experiment 1 revealed an achieved power of 0.998, lending confidence to the choice of 20 participants per group.
Stimuli
Stimuli were colored squares presented centrally on a mid-gray background. Each square was presented on screen for an interval of 0.5 s to 5 s (sampled equally in increments of 0.5 s).
For all participants, for half of the trials, the color remained the same for the entire duration (continuous condition), as in Experiment 1. The trials of the other half were in the boundary condition, which differed by experimental group.
In the abrupt change groups, there were two color switches, which occurred one third and two thirds of the way through the total duration. To test whether the boundary duration compression effect was driven by the number of changes, or change of the final color specifically, we had an “ABC” group, in which the color changed at each switch, and an “ABA” group, in which the final segment of the trial returned to the color of the first segment.
In the gradual change groups, the color slowly morphed from the initial color to the final color without an abrupt boundary. Because both rate of color change and ending color (if rate of change was held constant) could be cues to duration, we included two groups: one that had a constant rate of change and different end colors for different durations (rate constant), and one that had constant end colors and different rates of change for different durations (rate changing). For a depiction of the four conditions, see Figure S2 in the Supplemental Material.
For the abrupt change groups, square stimuli were blue (R = 0, G = 0, B = 255), green (R = 0, G = 255, B = 0), and yellow (R = 260, G = 200, B = 80). For each participant, color triplets were randomly assigned such that the order of the three colors in the boundary condition was fixed. Each participant viewed each color or color triplet twice for each time point.
For the gradual change groups, square stimuli morphed between red (R = 250, G = 0, B = 0) and blue, blue (R = 0, G = 0, B = 250) and red, and blue and green. In the rate changing group, the step size between colors per screen refresh was calculated separately for each time point such that the end color was always the same, for example, starting at (250, 0, 0) and ending at (0, 0, 250). In the rate constant group, the step size between colors per screen refresh was fixed such that one RGB value was subtracted from the start color and one added to the end color for each refresh.
Pseudorandomization of trial sequences was conducted using the same procedure as in Experiment 1. In total, the task consisted of six trials per condition and time point (120 trials total), broken up into two runs.
Duration judgment task
The duration judgment task was identical to that described for Experiment 1.
Results
Again, we first assessed accuracy of participants’ duration judgments and found that all participants exhibited a reliable correlation (mean z = 1.06, 95% CI = [0.99, 1.13]), t(79) = 29.31, p < .001, d = 3.28, 95% CI = [2.60, 3.96] (see Fig. S1c).
Next, we assessed our key hypothesis that gradual, as opposed to abrupt, shifts in context will be associated with a reduced influence of event boundaries on temporal duration judgments. First, we examined each of the four experimental groups separately. Because there were no differences in the boundary effect for the two abrupt change and two gradual change groups, respectively (see the Stimuli section for Experiment 3; see Fig. S2), these groups were collapsed. In the abrupt change group, we robustly replicated the boundary compression effect (M = −0.17, 95% CI = [−0.24, −0.11]), t(39) = −5.14, p < .001, d = −0.81, 95% CI = [−1.18, −0.46]. In the gradual change group, we also replicated the effect (M = −0.08, 95% CI = [−0.15, −0.01]), t(39) = −2.30, p = .027, d = −0.36, 95% CI = [−0.69, −0.043], although the effect was numerically weaker. Consistent with our hypothesis and reflective of the difference in effect sizes across the two groups, an analysis of variance (ANOVA) revealed a marginal interaction between condition (continuous vs. boundary) and group (gradual change vs. abrupt change), such that there was reduction in the boundary effect for the gradual group, F(1, 78) = 3.63, p = .060, ηp2 = .04, 95% CI = [0.00, 0.16] (see Fig. 1h). These data suggest that abrupt changes in context (associated with decreased memory accessibility) result in stronger boundary effects and provide further support that the extent of duration distortion across events is modulated by accessibility to the event representations.
Experiment 4
Across the three behavioral experiments presented thus far, we have extended prior work demonstrating that event boundaries reduce estimates of duration and provided novel insight into why event boundaries may lead to reduced duration judgments; specifically, we provided evidence that such reductions may be due to decreased mnemonic accessibility. Next, we ran a functional MRI experiment designed to assess how hippocampal pattern change within a single trial relates to temporal duration judgments.
Method
Participants
Eighteen right-handed native English speakers (seven female; age: M = 27.1 years, range = 22–34 years) were recruited from New York University and the larger community and participated for payment ($25/hour). We planned to recruit 20 individuals, as in Experiment 1 (in which achieved power was 0.998), but we had only 18 usable participants because of attrition. However, this experiment had more trials than the previous one, and the neural analyses used a within-participants approach to brain-behavior correlations. Informed consent was obtained in a manner approved by the University Committee on Activities Involving Human Subjects at New York University. The first eight out of 15 runs were lost for one participant because of equipment failure; thus, only the usable runs of this data set were included in analyses. The verbal temporal perception task and localizer task were not collected for two participants because of time constraints.
Stimuli
Stimuli were blue (R = 0, G = 0, B = 160), red (R = 160, G = 0, B = 0), and yellow (R = 195, G = 175, B = 35) squares presented centrally on a mid-gray background. In contrast to Experiments 1 to 3, each square was presented on screen for an interval of 2 s, 4 s, 6 s, or 8 s. For half of the trials, the color remained the same for the entire duration (continuous condition), and for the other half of the trials, the color switched halfway through the total duration (boundary condition).
Each participant viewed each color or color pair an equal number of times for each time point (here, 10 times over the entire experiment). Color pairings and pseudorandomization of trial sequences were conducted using the same procedure as in Experiment 1.
During each delay period (see below), a colored noise mask was presented over the entire display. A single mask image was created by randomly sampling R, G, and B values independently for each pixel on a 13-in. MacBook Pro.
In total, the task consisted of 30 trials per condition and time point (240 trials total). These were broken into 15 functional MRI runs of 16 trials each, and each condition-duration combination was presented twice per run.
Duration judgment task
The duration judgment task was identical to that of Experiment 1, with the following changes. The timeline was bounded by 0 s and 10 s. Participants responded using an MRI-compatible trackball. Additionally, there was a variable delay interval (2, 4, or 6 s) between square presentation and time judgment, which was included to orthogonalize the functional MRI regressors associated with the presentation and judgment periods. During this delay, a colored noise mask was presented over the entire screen to prevent any afterimages that may have been caused by a lingering percept of the square.
There was a variable intertrial interval (4, 6, or 8 s) during which participants completed an odd/even task after the time judgment period. Each number was presented for a maximum of 1.9 s with an interstimulus interval of 0.1 s. Participants responded using the left and right buttons on the sides of the trackball. The intertrial periods were jittered to orthogonalize the functional MRI regressors associated with the presentation and judgment periods, and this interval served as the baseline in functional MRI analyses.
Verbal duration judgment task
We additionally included a single behavioral run following completion of the functional MRI study, in which participants—instead of indicating their responses on the timeline—verbally reported how long they thought the square was on the screen, in seconds (see Figs. S3c and S3d in the Supplemental Material). Participants were given no numerical bounds or constraints on how precise their responses had to be. No functional data were collected during this session. The goal of this additional run was to assess participants’ duration estimates in an unconstrained way (i.e., without giving them a bounding timeline) and to verify that our effects could not be explained by idiosyncrasies in how individual participants use the timeline.
Localizer
At the end of the functional MRI session, participants engaged in a blocked color localizer in which each of the three colors was flashed repeatedly (0.9 s on, 0.1 s blank interstimulus interval) for 32 s. Each color was presented twice per run, and there were two runs of this task. The data from this run are not reported in this article.
Functional MRI parameters
Functional images were acquired on a Siemens Allegra head-only 3T scanner. Data were collected using an echo-planar-imaging pulse sequence (34 contiguous slices oriented parallel to the anterior commissure-posterior commissure axis; repetition time, or TR = 2,000 ms; echo time, or TE = 15 ms; flip angle = 82°; voxel size = 3 × 3 × 3 mm). Additionally, a high-resolution T1-weighted anatomical scan (magnetization-prepared-rapid-acquisition gradient echo sequence, 1 × 1 × 1 mm) was obtained for each participant. During functional scans, stimuli were viewed through a mirror attached to the head coil.
Preprocessing of functional MRI data
Functional MRI data processing was carried out using FEAT (fMRI Expert Analysis Tool, Version 6.00), part of FSL (FMRIB’s Software Library, available at www.fmrib.ox.ac.uk/fsl). Blood-oxygen-level-dependent (BOLD) images were first skull-stripped using the Brain Extraction Tool (Smith, 2002). The first four volumes of each run were discarded to allow for T1 stabilization. Additionally, images underwent motion correction using MCFLIRT (Jenkinson et al., 2002), grand-mean intensity normalization by a single multiplicative factor, and high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 50.0 s). No spatial smoothing was applied to the data. Each run was realigned to the final functional MRI run of the session. Motion outliers were detected using FSL Motion Outliers; TRs containing motion outliers were excluded from pattern similarity analyses (see the Pattern Similarity Analysis section below).
Region of interest definition
The primary regions of interest (ROIs) in this study were the hippocampus and visual cortex. However, given that many other regions have been implicated in temporal duration judgments (Buhusi & Meck, 2005; Lositsky et al., 2016), we also examined prefrontal regions, striatal regions, and the entorhinal cortex.
Anatomical hippocampal ROIs (left and right) were defined for each participant via FSL’s FIRST automated segmentation tool (Patenaude et al., 2011). We examined the bilateral hippocampus as well as each hemisphere separately. However, given prior findings that patterns specifically in the left hippocampus relate to memory and time estimates across boundaries (DuBrow & Davachi, 2014; Ezzyat & Davachi, 2014; Hsieh et al., 2014; Nielson et al., 2015), we hypothesized that our effects may be driven by the left hippocampus.
For our visual cortex ROI, we created a mask of V1 and V4. Specifically, we used probabilistic masks for V1 and hV4 (Wang et al., 2015); these masks were thresholded to 75%, concatenated together, and then transformed into each participant’s native space. This choice was motivated by prior work demonstrating that patterns of activity in V1 and V4 carry information about color (Brouwer & Heeger, 2009).
Anatomical caudate and putamen ROIs were defined for each participant via FSL’s FIRST automated segmentation tool (Patenaude et al., 2011).
Prefrontal cortical regions were defined using the Harvard-Oxford Cortical Structural Atlas. Specifically, ROIs were created in standard space for the middle frontal gyrus (MFG); inferior frontal gyrus (IFG), pars triangularis; and IFG, pars opercularis. Each of these three ROIs was then transformed into the participants’ functional spaces and thresholded at 75%. The IFG pars triangularis and IFG pars opercularis were then concatenated together to create one IFG ROI.
The entorhinal cortex was manually segmented on every participant’s T1-weighted anatomical scans, using published anatomical landmarks (Insausti et al., 1998; Pruessner et al., 2002).
Pattern similarity analysis
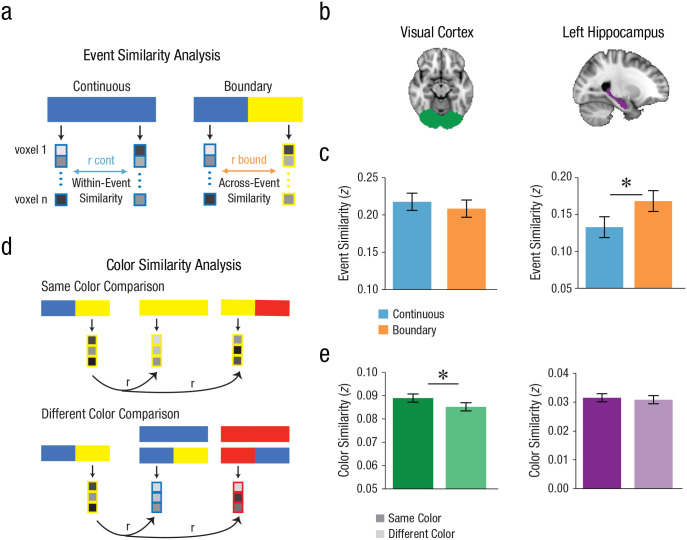
Pattern similarity analyses were conducted on 8-s trials only to maximize separation between the hemodynamic responses to the beginning and end of each trial. The data were modeled via an approach adapted from the work by Turner et al. (2012). This approach is well suited for within-trial analyses because it uses a multiparameter single-trial general linear model, which allows for the unmixing of temporally adjacent BOLD responses. Because our stimuli were temporally extended, we extracted the activation pattern across voxels for TRs 2 to 7 (4–14 s) after the true stimulus onset. A design matrix was constructed in which each of these “trial-related” TRs was a separate regressor of interest; in addition, separate nuisance regressors of no interest included separate TRs for every other trial collapsed across each condition/color combination as well as TRs from the intertrial-interval and delay periods. This design matrix was regressed against the activation of each voxel in each ROI to compute a separate beta estimate for each TR of the trial. To avoid making strong assumptions about where the true onset, midpoint, and offset of each trial would fall, we estimated the beginning and end of the stimulus period by averaging the first two and last two betas, respectively. Specifically, from trial onset, the second and third TRs (corresponding to approximately 0–2 s after stimulus onset) were averaged as an estimate of the “beginning,” and the fifth and sixth TRs (corresponding to approximately 6–8 s after stimulus onset) were averaged as an estimate of the “end” (see Fig. 2).
Fig. 2.
Pattern similarity approaches. (a) Event similarity was computed as the Fisher-transformed Pearson correlation between the estimated evoked activity at the beginning of an 8-s trial and the estimated evoked activity at the end of that 8-s trial. Correlations were computed separately for continuous and boundary trials. (b) Regions of interest: visual cortex (left) and left hippocampus (right). (c) Left: The visual cortex did not show differential event similarity by condition. Right: The left hippocampus exhibited greater event similarity across boundary trials than across continuous trials. (d) Color similarity was computed as the Fisher-transformed Pearson correlation between the estimated evoked activity at the end of an 8-s trial and the estimated evoked activity at the beginning of another trial that shares the same color. As a comparison, different-color trials were computed as the correlation between the patterns at the end of one trial and the beginning of all other trials that did not share its color. (e) Left: The visual cortex exhibited greater similarity for trials of the same color than trials of a different color. Right: The left hippocampus did not show differential similarity as a function of trial color. *p < .05 (two-tailed). Error bars denote the within-participants standard error of the mean.
TRs containing a motion outlier were excluded from subsequent analyses; furthermore, if there was more than one motion outlier within the trial-related TRs, the entire trial was excluded from subsequent analyses. On average, five (SD = 3.33) individual time points per participant were excluded from our analysis. Further, an average of 2.83 (SD = 1.34) trials per participant were excluded from our analysis because there was more than one motion outlier in a given trial.
Event similarity
Event similarity was computed by correlating the patterns corresponding to the beginning and end of every trial separately. Pearson correlation values were Fisher transformed and averaged for the two conditions to produce a similarity score.
Event dissimilarity predicting behavior
To assess whether within-trial event similarity in the visual cortex and hippocampus influences duration judgments, we conducted a mixed-effects analysis implemented via the lme4 package in the R programming environment (http://cran.r-project.org/web/packages/lme4; R version 4.1.3; lme4 version 1.1-28; R Core Team, 2022). To account for individual differences in timeline usage, we z scored participants’ duration judgments across all trials. Instead of event similarity, we ran these models with event dissimilarity (1 - event similarity), such that we quantified how the amount of pattern change relates to duration judgments. Mixed-effects linear models were run, and significance was assessed using iterative model comparisons, which resulted in chi-square values and corresponding p values. Normalized duration judgment was the dependent measure; visual cortical dissimilarity, hippocampal dissimilarity, and condition, as well as interactions between them, were included as fixed-effects predictors. Our general approach consisted of running the simplest form of the model and then including only significant predictors from these simple models in subsequent models. Model comparisons were also used to determine which variables were included as by-participant random factors; as a result of these comparisons, the condition factor was included as a by-participant random effect, and a slope and intercept were calculated for each participant.
Color similarity
To isolate responses to color, irrespective of temporal position information, we assessed similarity of the end of a boundary trial to the beginning of all other trials, conditionalized on whether the color at the end of the trial of interest was the same as or different from the color at the beginning of the other trials. This across-trial color similarity metric was computed as the Fisher transformation of the Pearson correlation between the end of the trial of interest and the beginning of every other trial separately. Trials from the same functional MRI run were excluded because of temporal autocorrelation. For each trial, the mean of the similarity values to all other trials was computed. The mean color similarity for same and different color comparisons was then calculated.
Results
Behavior
First, we again assessed accuracy of participants’ duration judgments and found that all participants exhibited a reliable correlation (mean z = 1.08, 95% CI = [0.96, 1.20]), t(17) = 18.98, p < .001, d = 4.47, 95% CI = [2.99, 6.20] (see Fig. S1d).
Next, we sought to replicate the finding from Experiment 1, with longer (8 s) trials in the functional MRI study (see Fig. 1i). Indeed, we found that boundary trials were judged as significantly shorter than continuous trials (M = −0.31, 95% CI = [−0.61, −0.02]), t(17) = −2.26, p = .037, d = −0.53, 95% CI = [−1.05, −0.032] (see Fig. 1j). We additionally ran this analysis for all trials, both within duration and collapsed across, and found a similar pattern of results (see Figs. S3a and S3b). We also separately examined data from the additional run of the task, in which participants responded verbally rather than with the timeline. We found a comparable boundary effect even within that single run (see Fig. S3c) and an across-participants correlation between the boundary effect across the whole functional MRI task (15 runs) and the single verbal run (see Fig. S3d). This additional run demonstrates the robustness of the results, providing evidence that these effects can be observed within a single run and with a different, unconstrained response medium.
Given that in this experiment, unlike Experiments 1 to 3, there was a brief delay between the square presentation and the duration judgment, we also verified that the delay did not have an influence on duration judgments. Indeed, across all trials, although there was a marginal main effect of condition, F(1, 17) = 3.86, p = .066, ηp2 = .19, 95% CI = [0.00, 0.49], there was no main effect of interstimulus interval on duration judgments, F(2, 34) = 0.012, p = .988, ηp2 = .00, 95% CI = [0.00, 0.00], or interaction between condition and interstimulus interval duration, F(2, 34) = 0.52, p = .600, ηp2 = .03, 95% CI = [0.00, 0.17]. The same pattern was true for the 8-s trials only: Although there was a marginal main effect of condition, F(1, 17) = 4.18, p = .057, ηp2 = .20, 95% CI = [0.00, 0.50], there was no main effect of interstimulus interval on duration judgments, F(2, 34) = 0.42, p = .661, ηp2 = .02, 95% CI = [0.00, 0.16], or interaction between condition and interstimulus interval duration, F(2, 34) = 0.21, p = .812, ηp2 = .01, 95% CI = [0.00, 0.12].
Event similarity in the hippocampus
In Experiment 4, our primary interest was how hippocampal representational change within individual trials is related to behavioral duration judgments. To first determine the influence of event structure on neural similarity measures, we examined differences in similarity across individual 8-s trials. Specifically, we extracted the pattern of activity across voxels at the beginning of the trial and at the end of the trial and correlated those two patterns to measure the representational change across the interval. This was done separately for continuous and boundary trials (see Fig. 2a). Thus, in the continuous condition, we examined changes in patterns of activity from the beginning to the end of a single event, whereas in the boundary condition, we probed changes in patterns across two events. Importantly, however, the interval length in the two conditions was matched. This analysis allows us to examine representations that occur at the event level: If a region represents events as a period of neural stability (Baldassano et al., 2017; DuBrow & Davachi, 2014; Ezzyat & Davachi, 2014, 2021), then we would expect to see greater similarity for the continuous condition, which contains one event, versus the boundary condition, which contains two. However, if a region is sensitive to the temporal position information within an interval (e.g., the start of an event), then we may expect to see greater similarity in the boundary condition, akin to a “resetting” of the neural population at a boundary (Ben-Yakov et al., 2013; Sun et al., 2020; Terada et al., 2017; Wallenstein et al., 1998).
We focused on two ROIs: our primary ROI, the hippocampus, and the visual cortex (V1/V4) as a control region (see the Region of Interest Definition section; see Fig. 2b). Specifically, we hypothesized that pattern similarity in the visual cortex would decrease in the boundary condition at a color switch because it should be sensitive to perceptual similarity. Contrary to our hypothesis, we did not find differences in similarity by condition in the visual cortex (M = 0.01, 95% CI = [−0.02, 0.03]), t(17) = 0.79, p = .439, d = 0.19, 95% CI = [−0.29, 0.67] (see Fig. 2c, left). In the bilateral hippocampus, however, there was marginally greater event similarity for the boundary condition, relative to the continuous condition (M = −0.03, 95% CI = [−0.06, 0.00]), t(17) = −1.88, p = .077, d = −0.44, 95% CI = [−0.95, 0.049] (see Fig. S4a in the Supplemental Material, left). This effect was driven by the left hippocampus, which exhibited significantly greater event similarity for the boundary condition (M = −0.04, 95% CI = [−0.07, −0.01]), t(17) = −2.50, p = .023, d = −0.59, 95% CI = [−1.12, −0.082] (see Fig. 2c, right). In contrast, the right hippocampus exhibited no difference in event similarity as a function of condition (M = −0.02, 95% CI = [−0.06, 0.02]), t(17) = −1.21, p = .244, d = −0.28, 95% CI = [−0.77, 0.20] (see Fig. S4a, right).
To assess whether the effects observed in the left hippocampus significantly differed from the pattern observed in the visual cortex, we further conducted an ANOVA examining pattern similarity as a function of both ROI and condition. There was no main effect of condition on pattern similarity, F(1, 17) = 1.81, p = .196, ηp2 = .10, 95% CI = [0.00, 0.40], although there was a main effect of ROI on pattern similarity, F(1, 17) = 11.97, p = .003, ηp2 = .41, 95% CI = [0.07, 0.66]. Critically, there was a significant interaction between condition and ROI, F(1, 17) = 6.88, p = .018, ηp2 = .29, 95% CI = [0.01, 0.57], indicating that the visual cortex and hippocampus exhibited distinct effects.
These results suggest that the left hippocampus is indeed sensitive to event structure, exhibiting greater similarity across two events, relative to one event. To understand whether these patterns were event specific or reflective of more general, stereotyped early versus late event signals, we ran an across-trial version of this analysis, asking whether there is increased similarity between the first event of a boundary trial and the second event of a different boundary trial, relative to the beginning of a continuous trial, to the midpoint of a different continuous trial. We found no differences in similarity (see Fig. S5 in the Supplemental Material), suggesting that these patterns may be event specific. However, we note that an across-trial analysis may not be ideally suited to look at temporal position coding, given the temporal autocorrelation and drift of the BOLD signal across runs of an experiment.
Color similarity in the visual cortex
The event similarity analysis provided evidence that left hippocampal patterns, but not visual cortical patterns, were modulated by the presence of an event boundary. To ensure that the dissociation between the hippocampus and visual cortex was not just due to differences in task sensitivity, we next asked whether patterns of brain activity were modulated by color, irrespective of events. To isolate color representations irrespective of event representations, we correlated the activity patterns from the second event of one (boundary) trial to the first event of all other trials (see Fig. 2d). We then sorted the data by whether the first and second events were the same color or different colors and examined differences in pattern similarity as a function of color match. In the visual cortex, we found that pattern similarity was greater for two events of the same color, compared with two events of different colors (M = 0.004, 95% CI = [0.00, 0.001]), t(17) = 2.14, p = .047, d = 0.51, 95% CI = [0.0070, 1.02] (see Fig. 2e, left), indicating that the visual cortex was sensitive to color information in this task. In contrast, the left hippocampus, which exhibited sensitivity to event structure, showed no such sensitivity to color (M = 0.00, 95% CI = [−0.00, 0.00]), t(17) = 0.48, p = .637, d = 0.11, 95% CI = [−0.36, 0.59] (see Fig. 2e, right). The right hippocampus (M = −0.001, 95% CI = [−0.004, 0.002]), t(17) = −0.81, p = .430, d = −0.19, 95% CI = [00.56, 0.39], and bilateral hippocampus (M = 0.00, 95% CI = [−0.003, 0.002]), t(17) = −0.37, p = .719, d = −0.09, 95% CI = [−0.56, 0.39], exhibited similar null results.
To again assess whether the effects observed in the visual cortex significantly differed from the hippocampus, we further conducted an ANOVA examining pattern similarity as a function of both ROI and condition (same color vs. different color). We ran this analysis in the left hippocampus because this was the ROI that exhibited sensitivity to event structure, but the results are comparable in the right and bilateral hippocampus. There was no main effect of condition on pattern similarity, F(1, 17) = 2.73, p = .117, ηp2 = .14, 95% CI = [0.00, 0.44], although there was a main effect of ROI on pattern similarity, F(1, 17) = 40.52, p < .001, ηp2 = .70, 95% CI = [0.41, 0.83]. There was a marginal interaction between condition and ROI, F(1, 17) = 3.28, p = .088, ηp2 = .16, 95% CI = [0.00, 0.46].
Relationship between event dissimilarity and duration judgments
Finally, we asked whether pattern change over the course of a single 8-s trial could explain variance in duration judgments across trials. To examine how neural pattern similarity influences duration judgments, we ran a series of mixed-effects linear regressions. Specifically, we tested whether event dissimilarity (see Event dissimilarity predicting behavior section above) in the hippocampus and visual cortex predicts duration judgments above and beyond the influence of event boundaries, as well as whether they interacted with condition.
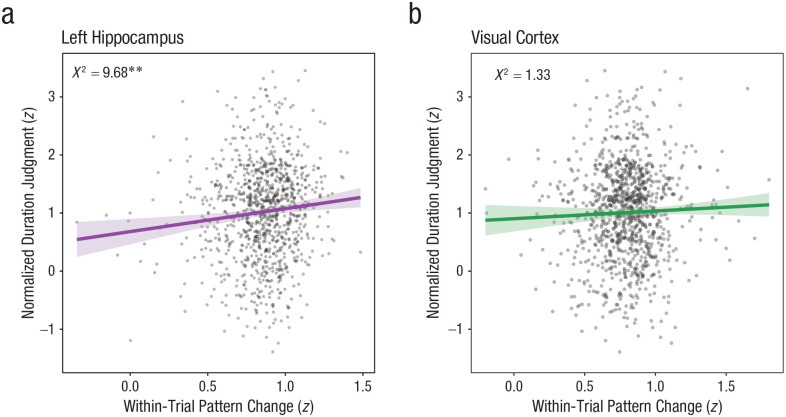
First, we tested a model predicting duration judgment from both condition and hippocampal pattern dissimilarity and found a main effect of bilateral hippocampal dissimilarity, β = 0.29, χ2(1) = 4.67, p = .031 (see Fig. S4b, left); greater dissimilarity—or more pattern change—in the hippocampus predicted longer duration judgments. When examining this effect separately for each hemisphere, we found that right hippocampal dissimilarity did not predict duration judgment, β = 0.14, χ2(1) = 1.17, p = .280 (see Fig. S4b, right), whereas (consistent with the event similarity result) left hippocampal dissimilarity did, β = 0.40, χ2(1) = 9.68, p = .002 (see Fig. 3a). When we included both the right and left hippocampal pattern dissimilarity (in addition to condition) in a model predicting duration judgment, the left hippocampus reliably predicted duration judgment, β = 0.57, χ2(1) = 10.46, p = .001, whereas the right hippocampus did not, β = −0.25, χ2(1) = 2.04, p = .153. Thus, in all subsequent models, we will consider only the left hippocampus.
Fig. 3.
Results of mixed-effects linear model predicting time judgment by event dissimilarity. (a) Event dissimilarity in the left hippocampus predicted duration judgments. Plotted from a model predicting duration judgment as a function of condition, a main effect of hippocampal dissimilarity, and a by-participant random effect of condition. (b) Event dissimilarity in the visual cortex did not predict duration judgment across conditions. Plotted from a model predicting duration judgment as a function of condition, a main effect of visual cortical dissimilarity, and a by-participant random effect of condition. **p < .005.
There was no significant interaction between left hippocampal dissimilarity and condition, χ2(1) = 2.48, p = .115. Although this effect of hippocampal dissimilarity on duration judgments was significant only in the continuous condition, β = 0.59, χ2(1) = 9.10, p = .003, it was numerically in the same direction in the boundary condition, β = 0.21, χ2(1) = 1.59, p = .207.
Next, we constructed a model predicting duration judgment from visual cortical dissimilarity, in addition to condition. The model revealed no main effect of visual cortex dissimilarity on duration judgments, β = 0.17, χ2(1) = 1.33, p = .249 (see Fig. 3b). However, a model predicting duration judgment from the main effect of condition as well as an interaction between visual cortical dissimilarity and condition revealed a significant interaction between visual cortex dissimilarity and condition, χ2(2) = 6.37, p = .041. Specifically, visual cortical dissimilarity predicted duration judgments in the continuous condition, β = 0.48, χ2(1) = 5.08, p = .024, but not the boundary condition, β = −0.17, χ2(1) = 0.78, p = .378.
Given that both the left hippocampus and visual cortex dissimilarity exhibited relations with duration judgments in the continuous condition, we asked whether they contribute independent variance. In a model predicting duration judgment in the continuous condition as a function of left hippocampal and visual cortical dissimilarity, we found that the hippocampus significantly contributed to duration judgment, β = 0.49, χ2(1) = 4.91, p = .027, but the visual cortex did not, β = 0.23, χ2(1) = 0.89, p = .345.
Given that many other brain regions have been implicated in time perception at this time scale (Buhusi & Meck, 2005; Lositsky et al., 2016), we conducted an exploratory analysis to probe other regions. Specifically, we assessed whether pattern dissimilarity in the caudate, putamen, MFG, IFG, and entorhinal cortex predicted duration judgments. We did not find main effects of dissimilarity on duration judgments or interactions with conditions in any of these regions, except for a marginal main effect in the IFG—caudate: main effect, β = −0.12, χ2(1) = 0.63, p = .427, interaction, χ2(2) = 0.89, p = .639; putamen: main effect, β = 0.24, χ2(1) = 0.68, p = .411, interaction, χ2(2) = 0.70, p = .703; MFG: main effect, β = 0.14, χ2(1) = 0.85, p = .355, interaction, χ2(2) = 1.42, p = .493; IFG: main effect, β = 0.27, χ2(1) = 3.38, p = .066, interaction, χ2(2) = 3.83, p = .147; entorhinal cortex: main effect, β = 0.052, χ2(1) = 0.43, p = .510, interaction, χ2(2) = 0.50, p = .778.
Discussion
Using event segmentation to manipulate mnemonic content, we found that duration judgments are influenced by the accessibility of mnemonic representations. After demonstrating that event boundaries lead to reduced duration judgments (Experiment 1), we found that duration judgments scale (to a limit) with the duration of the most recent event (Experiment 2) and that this reduction is attenuated when there is a gradual change in context that may maintain access to previous mnemonic information (Experiment 3). Lastly, trial-by-trial neural pattern change in the left hippocampus predicted longer duration judgments (Experiment 4).
Relation to studies of event boundaries in short- and long-term duration judgments
Under a context change account of duration judgments, in which change is used to infer the passage of time, one might expect boundary trials to be judged as longer than continuous trials (e.g., Poynter, 1983) because the context across two events differs more than the context within a single event. Yet our results show the opposite: Intervals with a boundary are estimated as shorter than equivalent, continuous intervals. Rather, our results point to a memory-based account of duration judgments, in which active mnemonic information is used to infer the passage of time: Event boundaries disrupt access to preboundary information (Zwaan, 1996), leading to decreased time judgments despite greater context change. Notably, we do not propose that the preboundary information is completely lost, but rather, its access is attenuated; such an interpretation is supported by the results of Experiment 2, which found some influence of the first event duration on duration judgments. However, although our experiments were designed to manipulate accessibility to preboundary information, none provide a direct, empirical measure of the accessibility of the preboundary information. Future work employing paradigms that simultaneously measure both memory accessibility/content and duration judgments could provide a more direct link.
In contrast to the current results, work at longer time scales shows precisely the opposite: Intervals spanning a boundary are remembered as longer than equivalent continuous intervals, and duration judgments scale with the number of events (Ezzyat & Davachi, 2014; Faber & Gennari, 2015, 2017; Lositsky et al., 2016). This discrepancy can be explained by the memory-based account because event boundaries may affect mnemonic content differently in working memory versus long-term memory. That is, if event boundaries disrupt memory at encoding, there is less information in working memory, leading to an in-the-moment compression. However, event boundaries are often better remembered (Heusser et al., 2018; Rouhani et al., 2020; Swallow et al., 2009) and may serve as anchor points to within-event content in long-term memory (DuBrow & Davachi, 2016; Michelmann et al., 2019; Shin & DuBrow, 2021). If intervals containing event boundaries have greater mnemonic content in long-term memory, this may lead to the inference that more time has passed. That said, future work using more directly comparable paradigms across short- and long-term judgments will be critical to tease apart these interpretations.
Notably, the differential results across short- and long-term paradigms cannot be explained by a key distinction in the interval timing literature: retrospective timing versus prospective timing (Block et al., 2018). In prospective paradigms, participants know in advance that they will be asked to report temporal information and thus can attend to time. In retrospective paradigms, in contrast, participants are unaware that they will be asked to report temporal duration information. In long-term memory, event boundaries exert a similar influence on temporal memory, regardless of whether the paradigm is prospective (Ezzyat & Davachi, 2014; Faber & Gennari, 2017) or retrospective (Faber & Gennari, 2015; Lositsky et al., 2016). The current study, as well as other short-term studies (e.g., Bangert et al., 2020; Yousif & Scholl, 2019), employed a prospective design. Although—given converging results across prospective and retrospective in long-term memory studies—we do not think that a retrospective design would reverse the behavioral result, future work employing single-trial versions of the experiment could directly test this.
Event representations in the hippocampus
We found increased hippocampal pattern similarity across trials containing a boundary, relative to continuous trials. Given that events are a period of contextual stability (DuBrow et al., 2017), this finding may seem counterintuitive. However, our finding converges with theoretical and empirical work that some hippocampal neurons are sensitive to specific temporal positions within an event (Ginther et al., 2011; Sun et al., 2020; Terada et al., 2017; Wallenstein et al., 1998). If there are neurons that code for the beginnings of events, an event boundary might recruit the same neural population as the beginning of the trial, yielding greater similarity across the two events; in contrast, for a continuous trial, the hippocampal representations would continue to grow dissimilar over time. Although the findings of our across-trial analysis did not support this general position code interpretation because we did not find greater similarity across two events of different trials (see Fig. S5), this analysis was not ideally suited to specifically probe changes occurring at the boundary.
Alternatively, increased pattern similarity across boundaries may reflect enhanced pattern separation within events: Such separation could orthogonalize experiences within an event and reduce within-context interference (Benear et al., 2022; but see Hsieh et al., 2014; Milivojevic et al., 2016). To distinguish between a position sensitivity versus pattern separation account, future work with higher temporal precision could examine whether hippocampal pattern similarity across boundaries is driven by a change occurring specifically at the moment of the boundary or a continuous pattern change across the interval.
We found no evidence for event sensitivity in the visual cortex. This effect was surprising, given findings that visual areas show greater pattern similarity within, versus across, events (e.g., Baldassano et al., 2017; Ezzyat & Davachi, 2021). However, although we may expect the visual cortex to exhibit enhanced similarity for visually similar information, there may be greater neural adaptation in the continuous condition. Because our stimuli were temporally extended colored squares and the BOLD signal in the visual cortex becomes reduced for prolonged stimuli (Krekelberg et al., 2006), it is possible that adaptation countered any sensitivity to shared visual information.
The role of the hippocampus in tracking time
Our finding that left hippocampal pattern change correlates with subjective duration judgments is consistent with its proposed role in representing time (Eichenbaum, 2013). In humans, left hippocampal pattern stability has been related to remembered temporal proximity; events remembered as farther apart show greater pattern dissimilarity (Ezzyat & Davachi, 2014; Nielson et al., 2015), consistent with our current results. Together, these findings suggest that temporal dissimilarity in hippocampal representations may represent subjective duration, across different time scales of both working and long-term memory—despite the fact that event boundaries have opposing effects on behavior at these two time scales. However, future work directly comparing the hippocampal patterns predicting temporal memory across short and long time scales (e.g., in the same task or same participants) is needed to more comprehensively understand how the left hippocampus may dually support these behaviors.
Notably, one study found that sequences (items + time), but not time alone, could be decoded from the hippocampus (Thavabalasingam et al., 2019). Although these findings suggest that the hippocampus may not be able to represent temporal information in isolation, it is notable that they attempted to decode objective duration. In the current study, we found that hippocampal patterns are related to subjective estimates of time, raising the possibility that the hippocampus codes for subjective, but not objective, duration.
That said, although we found that hippocampal patterns correlate with duration judgments, hippocampal damage does not consistently affect subjective duration judgments (e.g., Melgire et al., 2005; Noulhiane et al., 2007), especially at short durations (Jacobs et al., 2013; Palombo et al., 2016). However, the exact time scale at which processing becomes hippocampal dependent is the subject of ongoing research, may be shorter than previously established (Sabariego et al., 2021), and may depend on the context of the task (Palombo et al., 2019). Our findings contribute to growing evidence that the hippocampus contributes to temporal processing at short time scales, although more work is needed to understand whether and how our findings generalize to other populations, such as those of different ages and neurological conditions.
Prior work has identified a similar role for the entorhinal cortex in representing time (e.g., Lositsky et al., 2016; Umbach et al., 2020). Surprisingly, we did not find that entorhinal patterns predicted duration judgments, although we note that our functional MRI sequences were not optimized to collect signals from this region, which is prone to signal dropout.
Conclusion
One theoretical position is that brains, in fact, do not sense time; activity in a brain region may be correlated with the passage of time, but that does not mean that the region is clocking time per se (Buzsáki & Llinás, 2017; Davachi & DuBrow, 2015; Ezzyat & Davachi, 2014). Although here we report neural measurements that correlate with subjective duration judgments, we suggest that our results are a measure of event memory used to infer the passage of time. Our findings thus raise questions about the extent to which our sense of time arises from pure timing signals, or to what extent time is a product of—or reconstruction from— our memories.
Supplemental Material
Supplemental material, sj-docx-1-pss-10.1177_09567976221129533 for Mnemonic Content and Hippocampal Patterns Shape Judgments of Time by Brynn E. Sherman, Sarah DuBrow, Jonathan Winawer and Lila Davachi in Psychological Science
Acknowledgments
We thank Emily Cowan and Alexa Tompary for helpful conversations and Sami Yousif for feedback on the manuscript. At the initial submission, we dedicated this work to Warren Meck and Howard Eichenbaum, pioneers in the study of time and the brain but, more importantly, both incredible people. Over the course of revising the manuscript, we tragically lost another pioneer: our dear friend, student, mentor, and coauthor Sarah DuBrow. Sarah was incredibly kind, sharp, and fun, and we miss her immensely. We hope that she would be proud of the final manuscript.
Footnotes
ORCID iDs: Brynn E. Sherman  https://orcid.org/0000-0002-5071-7101
https://orcid.org/0000-0002-5071-7101
Jonathan Winawer  https://orcid.org/0000-0001-7475-5586
https://orcid.org/0000-0001-7475-5586
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/09567976221129533
Transparency
Action Editor: Leah Somerville
Editor: Patricia J. Bauer
Author Contribution(s)
B. E. Sherman and S. DuBrow contributed equally to this study.
Brynn E. Sherman: Conceptualization; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft; Writing – review & editing.
Sarah DuBrow: Conceptualization; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft; Writing – review & editing.
Jonathan Winawer: Methodology; Supervision; Writing – review & editing.
Lila Davachi: Conceptualization; Funding acquisition; Methodology; Supervision; Writing – review & editing.
The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This project was funded by the National Institutes of Health (R01 MH074692 award to LD).
Open Practices: All data have been made publicly available via the Open Science Framework and can be accessed at osf.io/n5pkb. None of the studies reported in this article were preregistered.
References
- Baldassano C., Chen J., Zadbood A., Pillow J. W., Hasson U., Norman K. A. (2017). Discovering event structure in continuous narrative perception and memory. Neuron, 95(3), 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangert A. S., Kurby C. A., Hughes A. S., Carrasco O. (2020). Crossing event boundaries changes prospective perceptions of temporal length and proximity. Attention, Perception, & Psychophysics, 82(3), 1459–1472. [DOI] [PubMed] [Google Scholar]
- Benear S. L., Horwath E. A., Cowan E., Camacho M. C., Ngo C. T., Newcombe N. S., Murty V. P. (2022). Children show adult-like hippocampal pattern similarity for familiar but not novel events. Brain Research, 1791, Article 147991. 10.1016/j.brainres.2022.147991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yakov A., Eshel N., Dudai Y. (2013). Hippocampal immediate poststimulus activity in the encoding of consecutive naturalistic episodes. Journal of Experimental Psychology: General, 142(4), 1255–1263. [DOI] [PubMed] [Google Scholar]
- Block R. A., Grondin S., Zakay D. (2018). Prospective and retrospective timing processes: Theories, methods, and findings. In Vatakis A., Balci F., Di Luca M., Correa Á. (Eds.), Timing and time perception: Procedures, measures, and applications (pp. 32–51). Brill. [Google Scholar]
- Brouwer G. J., Heeger D. J. (2009). Decoding and reconstructing color from responses in human visual cortex. Journal of Neuroscience, 29(44), 13992–14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi C. V., Meck W. H. (2005). What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience, 6(10), 755–765. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Llinás R. (2017). Space and time in the brain. Science, 358(6362), 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D. J., Aharoni D., Shuman T., Shobe J., Biane J., Song W., Wei B., Veshkini M., La-Vu M., Lou J., Flores S. E., Kim I., Sano Y., Zhou M., Baumgaertel K., Lavi A., Kamata M., Tuszynski M., Mayford M., . . . Silva A. J. (2016). A shared neural ensemble links distinct contextual memories encoded close in time. Nature, 534(7605), 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett D., Gasser C., Davachi L. (2020). Pupil-linked arousal signals track the temporal organization of events in memory. Nature Communications, 11(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L., DuBrow S. (2015). How the hippocampus preserves order: The role of prediction and context. Trends in Cognitive Sciences, 19(2), 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuker L., Bellmund J. L., Schröder T. N., Doeller C. F. (2016). An event map of memory space in the hippocampus. eLife, 5, Article e16534. 10.7554/eLife.16534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBrow S., Davachi L. (2013). The influence of context boundaries on memory for the sequential order of events. Journal of Experimental Psychology: General, 142(4), 1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBrow S., Davachi L. (2014). Temporal memory is shaped by encoding stability and intervening item reactivation. Journal of Neuroscience, 34(42), 13998–14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBrow S., Davachi L. (2016). Temporal binding within and across events. Neurobiology of Learning and Memory, 134, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBrow S., Rouhani N., Niv Y., Norman K. A. (2017). Does mental context drift or shift? Current Opinion in Behavioral Sciences, 17, 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B., Tibshirani R. (1986). Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statistical Science, 1(1), 54–75. [Google Scholar]
- Eichenbaum H. (2013). Memory on time. Trends in Cognitive Sciences, 17(2), 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzyat Y., Davachi L. (2011). What constitutes an episode in episodic memory? Psychological Science, 22(2), 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzyat Y., Davachi L. (2014). Similarity breeds proximity: Pattern similarity within and across contexts is related to later mnemonic judgments of temporal proximity. Neuron, 81(5), 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzyat Y., Davachi L. (2021). Neural evidence for representational persistence within events. Journal of Neuroscience, 41(37), 7909–7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber M., Gennari S. P. (2015). In search of lost time: Reconstructing the unfolding of events from memory. Cognition, 143, 193–202. [DOI] [PubMed] [Google Scholar]
- Faber M., Gennari S. P. (2017). Effects of learned episodic event structure on prospective duration judgments. Journal of Experimental Psychology: Learning, Memory, and Cognition, 43(8), 1203–1214. [DOI] [PubMed] [Google Scholar]
- Gershman S. J., Radulescu A., Norman K. A., Niv Y. (2014). Statistical computations underlying the dynamics of memory updating. PLOS Computational Biology, 10(11), Article e1003939. 10.1371/journal.pcbi.1003939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginther M. R., Walsh D. F., Ramus S. J. (2011). Hippocampal neurons encode different episodes in an overlapping sequence of odors task. Journal of Neuroscience, 31(7), 2706–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusser A. C., Ezzyat Y., Shiff I., Davachi L. (2018). Perceptual boundaries cause mnemonic trade-offs between local boundary processing and across-trial associative binding. Journal of Experimental Psychology: Learning, Memory, and Cognition, 44(7), 1075–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner A. J., Bisby J. A., Wang A., Bogus K., Burgess N. (2016). The role of spatial boundaries in shaping long-term event representations. Cognition, 154, 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh L. T., Gruber M. J., Jenkins L. J., Ranganath C. (2014). Hippocampal activity patterns carry information about objects in temporal context. Neuron, 81(5), 1165–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R., Juottonen K., Soininen H., Insausti A. M., Partanen K., Vainio P., Laakso M. P., Pitkänen A. (1998). MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. American Journal of Neuroradiology, 19(4), 659–671. [PMC free article] [PubMed] [Google Scholar]
- Jacobs N. S., Allen T. A., Nguyen N., Fortin N. J. (2013). Critical role of the hippocampus in memory for elapsed time. Journal of Neuroscience, 33(34), 13888–13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. [DOI] [PubMed] [Google Scholar]
- Krekelberg B., Boynton G. M., van Wezel R. J. (2006). Adaptation: From single cells to BOLD signals. Trends in Neurosciences, 29(5), 250–256. [DOI] [PubMed] [Google Scholar]
- Liverence B. M., Scholl B. J. (2012). Discrete events as units of perceived time. Journal of Experimental Psychology: Human Perception and Performance, 38(3), 549–554. [DOI] [PubMed] [Google Scholar]
- Lositsky O., Chen J., Toker D., Honey C. J., Shvartsman M., Poppenk J. L., Hasson U., Norman K. A. (2016). Neural pattern change during encoding of a narrative predicts retrospective duration estimates. eLife, 5, Article e16070. 10.7554/eLife.16070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald C. J., Lepage K. Q., Eden U. T., Eichenbaum H. (2011). Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron, 71(4), 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck W. H., Church R. M., Olton D. S. (1984). Hippocampus, time, and memory. Behavioral Neuroscience, 98(1), 3–22. [DOI] [PubMed] [Google Scholar]
- Melgire M., Ragot R., Samson S., Penney T. B., Meck W. H., Pouthas V. (2005). Auditory/visual duration bisection in patients with left or right medial-temporal lobe resection. Brain and Cognition, 58(1), 119–124. [DOI] [PubMed] [Google Scholar]
- Michelmann S., Staresina B. P., Bowman H., Hanslmayr S. (2019). Speed of time-compressed forward replay flexibly changes in human episodic memory. Nature Human Behaviour, 3(2), 143–154. [DOI] [PubMed] [Google Scholar]
- Milivojevic B., Varadinov M., Grabovetsky A. V., Collin S. H., Doeller C. F. (2016). Coding of event nodes and narrative context in the hippocampus. Journal of Neuroscience, 36(49), 12412–12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson D. M., Smith T. A., Sreekumar V., Dennis S., Sederberg P. B. (2015). Human hippocampus represents space and time during retrieval of real-world memories. Proceedings of the National Academy of Sciences, USA, 112(35), 11078–11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noulhiane M., Pouthas V., Hasboun D., Baulac M., Samson S. (2007). Role of the medial temporal lobe in time estimation in the range of minutes. NeuroReport, 18(10), 1035–1038. [DOI] [PubMed] [Google Scholar]
- Palombo D. J., Di Lascio J. M., Howard M. W., Verfaellie M. (2019). Medial temporal lobe amnesia is associated with a deficit in recovering temporal context. Journal of Cognitive Neuroscience, 31(2), 236–248. [DOI] [PubMed] [Google Scholar]
- Palombo D. J., Keane M. M., Verfaellie M. (2016). Does the hippocampus keep track of time? Hippocampus, 26(3), 372–379. [DOI] [PubMed] [Google Scholar]
- Patenaude B., Smith S. M., Kennedy D. N., Jenkinson M. (2011). A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage, 56(3), 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynter W. D. (1983). Duration judgment and the segmentation of experience. Memory & Cognition, 11(1), 77–82. [DOI] [PubMed] [Google Scholar]
- Pruessner J. C., Köhler S., Crane J., Pruessner M., Lord C., Byrne A., Kabani N., Collins D. L., Evans A. C. (2002). Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: Considering the variability of the collateral sulcus. Cerebral Cortex, 12(12), 1342–1353. [DOI] [PubMed] [Google Scholar]
- Rattat A. C., Droit-Volet S. (2012). What is the best and easiest method of preventing counting in different temporal tasks? Behavior Research Methods, 44(1), 67–80. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2022). R: A language and environment for statistical computing (Version 4.1.3) [Computer software]. R Foundation for Statistical Computing. http://www.R-project.org [Google Scholar]
- Reddy L., Zoefel B., Possel J. K., Peters J., Dijksterhuis D. E., Poncet M., van Straaten E. C. W., Baayen J. C., Idema S., Self M. W. (2021). Human hippocampal neurons track moments in a sequence of events. Journal of Neuroscience, 41(31), 6714–6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinck M., Bower G. H. (2000). Temporal and spatial distance in situation models. Memory & Cognition, 28(8), 1310–1320. [DOI] [PubMed] [Google Scholar]
- Rouhani N., Norman K. A., Niv Y., Bornstein A. M. (2020). Reward prediction errors create event boundaries in memory. Cognition, 203, Article 104269. 10.1016/j.cognition.2020.104269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin A., Geva N., Sheintuch L., Ziv Y. (2015). Hippocampal ensemble dynamics timestamp events in long-term memory. eLife, 4, Article e12247. 10.7554/eLife.12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabariego M., Tabrizi N. S., Marshall G. J., McLagan A. N., Jawad S., Hales J. B. (2021). In the temporal organization of episodic memory, the hippocampus supports the experience of elapsed time. Hippocampus, 31(1), 46–55. 10.1002/hipo.23261 [DOI] [PubMed] [Google Scholar]
- Sakon J. J., Naya Y., Wirth S., Suzuki W. A. (2014). Context-dependent incremental timing cells in the primate hippocampus. Proceedings of the National Academy of Sciences, USA, 111(51), 18351–18356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville W. B., Milner B. (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry, 20(1), 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y. S., DuBrow S. (2021). Structuring memory through inference-based event segmentation. Topics in Cognitive Science, 13(1), 106–127. [DOI] [PubMed] [Google Scholar]
- Smith S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer N. K., Zacks J. M. (2005). Temporal changes as event boundaries: Processing and memory consequences of narrative time shifts. Journal of Memory and Language, 53(1), 125–140. [Google Scholar]
- Sun C., Yang W., Martin J., Tonegawa S. (2020). Hippocampal neurons represent events as transferable units of experience. Nature Neuroscience, 23(5), 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow K. M., Zacks J. M., Abrams R. A. (2009). Event boundaries in perception affect memory encoding and updating. Journal of Experimental Psychology: General, 138(2), 236–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada S., Sakurai Y., Nakahara H., Fujisawa S. (2017). Temporal and rate coding for discrete event sequences in the hippocampus. Neuron, 94(6), 1248–1262. [DOI] [PubMed] [Google Scholar]
- Thavabalasingam S., O’Neil E. B., Tay J., Nestor A., Lee A. C. (2019). Evidence for the incorporation of temporal duration information in human hippocampal long-term memory sequence representations. Proceedings of the National Academy of Sciences, USA, 116(13), 6407–6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B. O., Mumford J. A., Poldrack R. A., Ashby F. G. (2012). Spatiotemporal activity estimation for multivoxel pattern analysis with rapid event-related designs. NeuroImage, 62(3), 1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach G., Kantak P., Jacobs J., Kahana M. J., Pfeiffer B. E., Sperling M., Lega B. (2020). Time cells in the human hippocampus and entorhinal cortex support episodic memory. Proceedings of the National Academy of Sciences, USA, 117(45), 28463–28474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein G. V., Hasselmo M. E., Eichenbaum H. (1998). The hippocampus as an associator of discontiguous events. Trends in Neurosciences, 21(8), 317–323. [DOI] [PubMed] [Google Scholar]
- Wang L., Mruczek R. E., Arcaro M. J., Kastner S. (2015). Probabilistic maps of visual topography in human cortex. Cerebral Cortex, 25(10), 3911–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M. (2013). The inner sense of time: How the brain creates a representation of duration. Nature Reviews Neuroscience, 14(3), 217–223. [DOI] [PubMed] [Google Scholar]
- Woolf V. (1928). Orlando. Vintage Random House. [Google Scholar]
- Yousif S. R., Scholl B. J. (2019). The one-is-more illusion: Sets of discrete objects appear less extended than equivalent continuous entities in both space and time. Cognition, 185, 121–130. [DOI] [PubMed] [Google Scholar]
- Zwaan R. A. (1996). Processing narrative time shifts. Journal of Experimental Psychology: Learning, Memory, and Cognition, 22(5), 1196–1207. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-pss-10.1177_09567976221129533 for Mnemonic Content and Hippocampal Patterns Shape Judgments of Time by Brynn E. Sherman, Sarah DuBrow, Jonathan Winawer and Lila Davachi in Psychological Science