Abstract
Introduction
Spinal infusions of either fentanyl or sufentanil have been reported in international reports, articles, and scientific events worldwide. This study aimed to determine whether intrathecal fentanyl or sufentanil offers safety in mortality and perioperative adverse events.
Methods
MEDLINE (via PubMed), EMBASE, CENTRAL (Cochrane library databases), gray literature, hand-searching, and clinicaltrials.gov were systematically searched. Randomized controlled trials with no language, data, or status restrictions were included, comparing the effectiveness and safety of adding spinal lipophilic opioid to local anesthetics (LAs). Data were pooled using the random-effects models or fixed-effect models based on heterogeneity.
Results
The initial search retrieved 4469 records; 3241 records were eligible, and 3152 articles were excluded after reading titles and abstracts, with a high agreement rate (98.6%). After reading the full texts, 76 articles remained. Spinal fentanyl and sufentanil significantly reduced postoperative pain and opioid consumption, increased analgesia and pruritus. Fentanyl, but not sufentanil, significantly reduced both postoperative nausea and vomiting, and postoperative shivering; compared to LAs alone. The analyzed studies did not report any case of in-hospital mortality related to spinal lipophilic opioids. The rate of respiratory depression was 0.7% and 0.8% when spinal fentanyl or sufentanil was added and when it was not, respectively. Episodes of respiratory depression were rare, uneventful, occurred intraoperatively, and were easily manageable.
Conclusion
There is moderate to high quality certainty that there is evidence regarding the safety and effectiveness of adding lipophilic opioids to LAs in spinal anesthesia.
KEYWORDS: Anesthesia, spinal; Fentanyl; Sufentanil; Safety; Drug-related side effects and adverse reactions
Introduction
Anesthesiologists have been using the spinal route for fentanyl and sufentanil for many years. However, these drugs have not been approved either by the United States Food and Drug Administration (FDA) or the National Health Surveillance Agency of Brazil (Anvisa) for this goal. In FDA's labels, only intravenous or intramuscular routes are predicted for fentanyl citrate ampoules and intravenous or epidural routes for sufentanil. Anvisa approved both fentanyl and sufentanil for the epidural route, but there is no recommendation regarding their use in spinal anesthesia. The recent emergence of electronic health records in Brazil turned this practice evident to the local pharmacovigilance committee, because electronic prescriptions had shown that anesthesiologists prescribed and injected fentanyl or sufentanil in subarachnoid space. Later, it also became evident to Anvisa, because such a committee notified the agency about this fact, which raised concerns due to the lack of prediction of the spinal route in fentanyl's or sufentanil's label. The Brazilian Society of Anesthesiology (SBA) was asked for solid evidence about the safety and effectiveness of intrathecal (IT) fentanyl and sufentanil, and an expert team was formed to investigate it.
The concerns of Anvisa are relevant as specialists know that both sufentanil and fentanyl can produce respiratory depression1 by reducing the responsiveness of the brainstem respiratory centers and causing increases in carbon dioxide tension.2,3 Intravenous routes produce dose-dependent respiratory depression. Only a few case reports of respiratory depression after spinal infusion of fentanyl or sufentanil were found. However, doses described in these cases are now considered overdoses for the analgesic effects in daily practice and guidelines, which changed after some dose-response studies. There is lack of good evidence for the incidence of respiratory depression related to the addition of low doses of those opioids to spinal anesthesia due to its rarity.
Potential respiratory depression, as a single argument, should not be a reason to abandon spinal fentanyl or sufentanil since Anvisa has also approved morphine for spinal route use. Morphine can produce even more respiratory depression than lipophilic opioids due to its higher cephalad spread, but its safety has been better documented. Therefore, benefits and risks should be balanced to make a sound decision for its clinical use. Indeed, even two systematic reviews with meta-analysis could not provide evidence of a significant increase in the incidence of respiratory depression when the IT morphine dose was lower than 0.3 mg.4,5 For cesarean delivery under spinal anesthesia, a systematic review comparing morphine in low doses (50–100 μg) to morphine in higher doses (> 100–250 μg) did not detect any case of respiratory depression regardless of the dose used.6
In one study, the addition of opioids, either hydrophilic or lipophilic, to local anesthetics (LAs) in spinal anesthesia prolonged postoperative analgesia, decreased postoperative pain intensity, and reduced the number of patients requiring postoperative rescue analgesia.7 However, opioid-based anesthesia can increase the rates of postoperative complications other than respiratory depression and hypoxemia, such as hyperalgesia, nausea and vomiting, pruritus, ileus, constipation, urinary retention, tolerance by desensitization, dizziness, and drowsiness. All risks and benefits must be known, balanced, and individualized.
This systematic review and meta-analysis aimed to determine whether IT fentanyl or sufentanil offers safety in terms of mortality or perioperative adverse events. It is hypothesized that the addition of fentanyl or sufentanil to spinal anesthesia can enhance recovery after surgery, with modest side effects during the postoperative period.
Objectives
To perform a systematic review and meta-analysis of the evidence related to the addition of fentanyl or sufentanil to spinal anesthesia and subsequent respiratory depression, postanesthesia care unit (PACU) length of stay, risk of needing additional analgesics, and other secondary outcomes.
Methods
The systematic review was conducted by a team of content specialists (NMF, JPJP, LMTAA, and GMNG) and method specialists (GMNG and RAO). The recommendation methods of the Cochrane Handbook for Systematic Reviews of Interventions8 were followed, and Preferred Reporting Items for Systematic Reviews and Meta-Analyses9 guidelines were complied with.
Protocol and registration
All authors actively participated in the study plan phase. The authors included a search in the COMET database with no core outcome set suitable for use prior to the study plan. After the planning phase, the protocol was registered on the PROSPERO database (CRD42020219474) (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=219474).
Eligibility criteria
Study design
Only randomized controlled trials (RCTs) were included in this study. No language, publication status, or year of publication restrictions were made.
Participants/population
Participants were adult patients who received spinal anesthesia for surgery.
Interventions
Addition of spinal fentanyl or sufentanil to LAs, regardless of the dose used.
Control
LA (including lidocaine, bupivacaine, or levobupivacaine) alone or combined with saline solution for spinal anesthesia was used.
Outcomes
No set recommendation of outcomes was found. We included death (present or absent), PACU length of stay (in minutes), postoperative pain (assessed by visual analog scale), respiratory depression (present or absent), urinary retention (present or absent), pruritus (present or absent), postoperative nausea and vomiting (PONV), nausea alone (present or absent), vomiting alone (present or absent), need for additional analgesia (present or absent), time to first rescue analgesia (in minutes), and shivering (present or absent) as the most important outcomes, considering clinical relevance. We initially included data for outcome definitions and time points, for example: worst and resting pain, 12 h and 24 h PONV.
Exclusion criteria
The following were excluded in this study: (1) indirect comparisons, (2) pediatric patients, (3) addition of general anesthesia, (4) combined/different regional anesthesia, and (5) other LAs besides lidocaine, bupivacaine, or levobupivacaine.
When a study met the eligibility criteria but included a group with drugs other than fentanyl/sufentanil, the study was included, and this particular group (which used other drugs) was excluded from the comparison.
Information sources and search
The team developed the search strategy, and one author (RAO) ran it on 2020/11/17. The search included MEDLINE, CENTRAL, and EMBASE databases using the MeSH terms "Injections, Spinal" OR "Anesthesia Epidural" OR "Anesthesia Spinal" AND "fentanyl" OR "sufentanil" OR "Piperidines" OR "Bupivacaine" OR "Levobupivacaine" OR "Lidocaine" OR "Anesthetics, Local" (see search strategy for PUBMED). A search was done for ongoing studies on the clinicaltrials.gov database, and for gray literature on the opengray.eu database. The authors also searched reference lists from included studies and looked for specialists’ knowledge of any publication of the title.
Study selection
The output of all included databases was exported to files that were imported to a research software (Rayyan QCRI). Two reviewers (JPJP and LMTAA) used the software blinded to other choices to select included studies and to exclude duplicates. Discordances were solved by a third reviewer (NMF).
Data items
From the full text of each included trial, characteristics of the randomization, the sample size for each group, group characteristics (drugs and doses), presence of any exclusion criteria, clinical features including demographics (age and gender), type of surgery, and outcomes measured were extracted.
Data collection and analysis
One author (GMNG) developed software to collect data in a web app (https://www.appsheet.com/start/83632bce-4cf4-42c5-8136-1f783bb8747d#appName=SpinalopioidsSRDatacollector-443302&page=detail&table=Article&row=Chandra%3A%202008). Two authors (GMNG and NMF) pilot tested it using ten random articles and refined the software before all authors used it. Four authors (NMF, LMTAA, JPJP, and GMNG) filled the structured web app forms asynchronously, and then, the data was exported to a spreadsheet file. The relational database helped the authors to check for similar outcome measures. One author (NMF) checked the exported data consistency by manually verifying studies’ data.
Risk of bias in individual studies
Two reviewers (NMF and RAO) independently evaluated the methodological quality of the studies. The “risk of bias tool” from the Cochrane Handbook for Systematic Reviews of Interventions version 5.4 was used to assess the risk of bias of the included studies in terms of the assessment criteria.8 Each of the seven domains of bias was rated as follows: low risk of bias, if the study met the quality criteria; unclear risk of bias, if one or more of the quality criteria were only partially completed or imprecise; or high risk of bias, if one or more of the criteria were not met or not included. Disagreements were resolved by discussing them with the team. Reasons for judgment are described.
For every study with at least one domain classified as unclear, the corresponding author of the respective study was contacted by two reviewers (JPJP and LMTAA) through electronic correspondence. The content of the correspondence was a standard letter pointing out the method's issues that raised concern about the risk of bias and giving the authors the opportunity to clarify them.
Summary measures
Planned methods of analysis
Data were analyzed using the Revman 5.4 package. Mean difference (MD) for continuous outcomes with 95% confidence intervals (CIs) was used. Dichotomous outcomes were expressed as RR with 95% CI. A test for heterogeneity was conducted. A value of I2 more significant than 50% was assumed to indicate substantial heterogeneity, and potential sources of heterogeneity were investigated. If significant heterogeneity occurred (I2 > 50% or p < 0.05), a random-effect model was used to calculate the pooled MD or RR. Publication/reporting biases were investigated using funnel plots when possible (more than ten studies included).
Assessment of methodological quality
Confidence in the estimated effect assessment was carried out using Grading of Recommendations, Assessment, Development, and Evaluations (GRADE).10
Supplemental files
The detailed description of the additional analyses, including data and statistical calculations, can be found in the Appendix Supplementary materials.
Results
Study selection
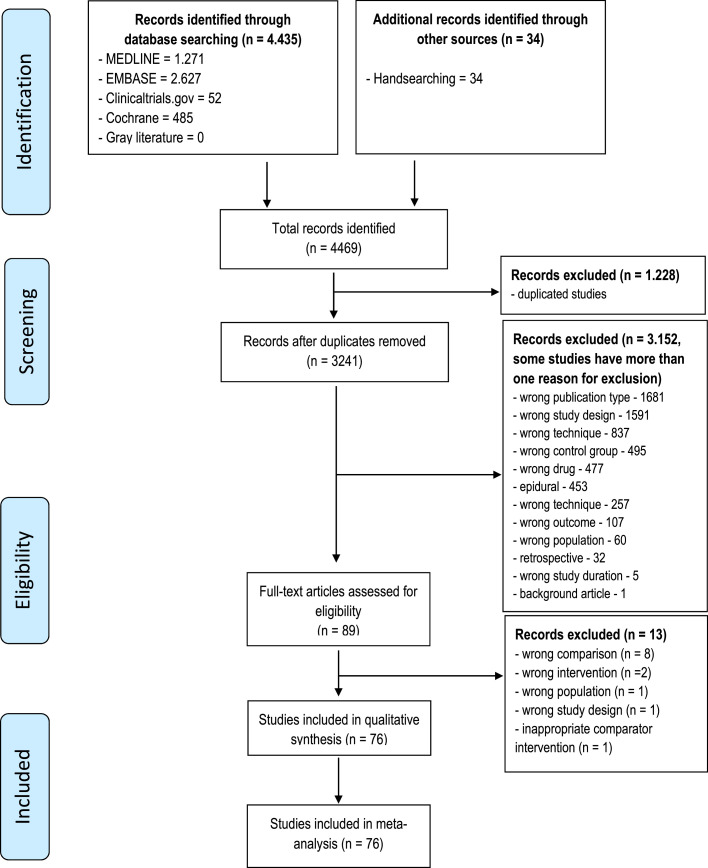
A total of 4469 records were retrieved from the initial database search. After removing duplicate articles, 3241 records were eligible. After a simple reading of the titles and abstracts, with a high agreement rate (only 46 conflicts), 3152 studies were excluded. The authors considered 89 full-text studies for eligibility.
The authors excluded 13 studies due to a lack of pertinent study design. Non-RCTs, systematic reviews, studies with inappropriate comparator intervention, studies with no control group, and studies with combination specifics were excluded (supplementary material contains the reasons for exclusion). Finally, 76 RCTs were included for qualitative and quantitative analyses. The selection process is shown in a flowchart (Fig. 1).
Figure 1.
Flow diagram.
Study characteristics
The essential characteristics of the included studies are listed in Table 1. The 76 included studies consisted of 4734 patients (control group: 1970 patients in 76 studies; fentanyl group: 1895 patients in 60 studies11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68; and sufentanil group: 869 patients in 26 studies12,21,24,26,29,31,43,48,49,64,69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83). There were ten studies investigating the effect of both fentanyl and sufentanil.12,21,24,26,29,31,43,48,49,84
Table 1.
Basic characteristics of trails included.
| Study | Surgery | Patient gender | Country | Number of patients | Control | Experiment | Opioid | Dose (µg) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Control | Experiment | LA | Dose (mg) | LA | Dose (mg) | ||||||
| 01 | Abdollahpour et al. 2015 | Cesarean section | 0 | 50 | Iran | 25 | 25 | Bupivacaine 0.5% | 12,5 | Bupivacaine 0.5% | 12,5 | Sufentanil | 1.5 |
| 02 | Acharya et al. 2019 | Cesarean section | 0 | 60 | Nepal | 30 | 30 | Bupivacaine 0.5% | 10 | Bupivacaine 0.5% | 10 | Fentanyl | 12.5 |
| 03 | Agrawal et al. 2016 | Cesarean section | 0 | 40 | India | 20 | 20 | Bupivacaine 0.5% | 12.5 | Bupivacaine 0.5% | 12.5 | Fentanyl | 25 |
| 04 | Akan et al. 2013 | TURP | 60 | 0 | Turkey | 20 | 20/20 | Levobupivacaine 0.5% | 10 | Levobupivacaine 0.5% | 7.5/7.5 | Fentanyl/Sufentanil | 25/2.5 |
| 05 | Asokumar et al. 1998 | Labor analgesia | 0 | 41 | United States of America | 19 | 22 | Bupivacaine 0.25% | 2.5 | Bupivacaine 0.25% | 2.5 | Fentanyl | 25 |
| 06 | Atallah et al. 2003 | Transurethral Cystoscopic Surgery | NS | NS | Egypt | 40 | 40 | Bupivacaine 0.1% | 5 | Bupivacaine 0.1% | 5 | Fentanyl | 20 |
| 07 | Atallah et al. 2006 | Percutaneous nephrolitotomy | 64 | 41 | Egypt | 54 (ITT) | 54 (ITT) | Bupivacaine 0.5% | 7.5 | Bupivacaine 0.5% | 7.5 | Fentanyl | 10 |
| 08 | Attri et al. 2015 | Infraumbilical surgeries | 83 | 17 | India | 50 | 50 | Levobupivacaine 0.5% | 10 | Levobupivacaine 0.5% | 10 | Fentanyl | 25 |
| 09 | Aydın et al. 2011 | Arthroscopic knee surgery | 25 | 25 | Turkey | 25 | 25 | Bupivacaine 0.5% | 10 | Bupivacaine 0.5% | 5 | Sufentanil | 2.5 |
| 10 | Bang et al. 2012 | Cesarean section | 0 | 105 | South Korea | 35 | 35/35 | Bupivacaine 0.5% | 6.5-11 | Bupivacaine 0.5% | 6.5-11 | Sufentanil | 2.5/5 |
| 11 | Ben-David et al. 1997 | Arthroscopic knee surgery | 40 | 10 | Israel | 25 | 25 | Bupivacaine 0.5% | 5 | Bupivacaine 0.5% | 5 | Fentanyl | 10 |
| 12 | Ben-David et al. 2000 | Surgery repair of hip fracture | 2 | 18 | Israel | 10 | 10 | Bupivacaine 0.5% | 10 | Bupivacaine 0.5% | 4 | Fentanyl | 20 |
| 13 | Bidikar et al. 2017 | Cesarean section | 0 | 60 | India | 30 | 30 | Levoupivacaine 0.5% | 10 | Levoupivacaine 0.5% | 7.5 | Fentanyl | 12.5 |
| 14 | Biswas et al. 2002 | Cesarean section | 0 | 40 | India | 20 | 20 | Bupivacaine 0.5% | 10 | Bupivacaine 0.5% | 10 | Fentanyl | 12.5 |
| 15 | Braga et al. 2003 | Cesarean section | 0 | 80 | Brazil | 20 | 20/20/20 | Bupivacaine 0.5% | 12.5 | Bupivacaine 0.5% | 12.5 | Sufentanil | 2.5/5/7.5 |
| 16 | Braga et al. 2012 | Cesarean section | 0 | 48 | Brazil | 24 | 24 | Bupivacaine 0.5% | 10 | Bupivacaine 0.5% | 10 | Sufentanil | 5 |
| 17 | Chandra et al. 2008 | Cesarean section | 0 | 60 | India | 20 | 20/20 | Bupivacaine 0.5% | 7.5 | Bupivacaine 0.5% | 7.5 | Fentanyl/Sufentanil | 25/5 |
| 18 | Chilvers et al. 1997 | Gynecological laparoscopy | 0 | 63 | Canada | 21 | 21/21 | Lidocaine 1% | 20 | Lidocaine 1% | 20 | Fentanyl | 10/25 |
| 19 | Cowan et al. 2002 | Cesarean section | 0 | 50 | United Kingdom | 25 | 25 | Bupivacaine 0.5% | 13.75 | Bupivacaine 0.5% | 13.75 | Fentanyl | 20 |
| 20 | Dahlgren et al. 1997 | Cesarean section | 0 | 80 | Sweden | 20 | 20/20/20 | Bupivacaine 0.5% | 12.5 | Bupivacaine 0.5% | 12.5 | Fentanyl/Sufentanyl | F10/S2.5/S5 |
| 21 | Demiraran et al. 2006 | Cesarean section | 0 | 100 | Turkey | 25 | 25/25/25 | Bupivacaine 0.5% | 12.5 | Bupivacaine 0.5% | 12.5 | Sufentanil | 1.5/2.5/5 |
| 22 | Derakhshan et al. 2018 | Lower limb surgery | 32 | 28 | Iran | 30 | 30 | Bupivacaine 0.5% | 15 | Bupivacaine 0.5% | 15 | Sufentanil | 5 |
| 23 | Desai et al. 2019 | Femur surgery | 39 | 21 | India | 30 | 30 | Bupivacaine 0.5% | 12.5 | Bupivacaine 0.5% | 7.5 | Fentanyl | 25 |
| 24 | Doger et al. 2014 | TURP | 20 | 0 | Nigeria | 20 | 20 | Bupivacaine 0.5% | 10 | Bupivacaine 0.5% | 7.5 | Sufentanil | 5 |
| 25 | Donadoni et al. 1987 | Urological procedures | 35 | 3 | Belgium | 20 (ITT) | 20 (ITT) | Lidocaine 5% | 75 | Lidocaine 5% | 75 | Sufentanil | 5 |
| 26 | Farzi et al. 2017 | Cesarean Section | 0 | 92 | Iran | 33 (ITT) | 33 (ITT)/33(ITT) | Bupivacaine 0.5% | 12.5 | Bupivacaine 0.5% | 12.5 | Fentanyl/Sufentanil | 25/2.5 |
| 27 | Gauchan et al. 2014 | Cesarean Section | 0 | 70 | Nepal | 35 | 35 | Bupivacaine 0.5% | 12 | Bupivacaine 0.5% | 10 | Fentanyl | 20 |
| 28 | Girgin et al. 2008 | Inguinal herniorrhaphy | 31 | 7 | Turkey | 20 (ITT) | 20 (ITT) | Levoupivacaine 0.5% | 7.5 | Levoupivacaine 0.5% | 5 | Fentanyl | 25 |
| 29 | Gupta et al. 2013 | Urological procedures | 66 | 24 | India | 30 | 30/30 | Bupivacaine 0.5% | 7.5 | Bupivacaine 0.5% | 7.5 | Fentanyl/Sufentanil | 25/10 |
| 30 | Gurbet et al. 2008 | Anorrectal surgery | 24 | 11 | Turkey | 20 (ITT) | 20 (ITT) | Bupivacaine 0.5% | 5 | Bupivacaine 0.5% | 2.5 | Fentanyl | 25 |
| 31 | Hakkim et al. 2015 | Lower abdomen, urological, and lower extremities | 55 | 45 | India | 50 | 50 | Bupivacaine 0.5% | 12.5 | Bupivacaine 0.5% | 12.5 | Sufentanil | 5 |
| 32 | Hassani et al. 2014 | Lower Extremity Surgery | 67 | 23 | Iran | 30 | 30/30 | Bupivacaine 0.5% | 15 | Bupivacaine 0.5% | 15 | Fentanyl/Sufentanil | 25/2.5 |
| 33 | Hoda et al. 2007 | Hip fracture surgery | NS | NS | Pakistain | 30 | 30/30 | Bupivacaine 0.5% | 10 | Bupivacaine 0.5% | 8/6 | Fentanyl | 20/20 |
| 34 | Hunt et al. 1989 | Cesarean section | 0 | 55 | United States of America | 9 | 6/8/7/7/6/7/5 | Bupivacaine 0.5% | 7.5-11 | Bupivacaine 0.5% | 7.5-11 | Fentanyl | 2.5/5/6.25/12.5/25/37.5/50 |
| 35 | Jain et al. 2004 | Cesarean section | 0 | 45 | India | 15 | 15/15 | Bupivacaine 0.5% | 7.5 | Bupivacaine 0.5% | 7.5 | Fentanyl | 10/20 |
| 36 | Kararmaz et al. 2003 | TURP | 40 | 0 | Turkey | 20 | 20 | Bupivacaine 0.5% | 7.5 | Bupivacaine 0.5% | 4 | Fentanyl | 25 |
| 37 | Kaur et al. 2011 | Urological procedures | 55 | 5 | India | 30 | 30 | Bupivacaine 0.5% | 12.5 | Bupivacaine 0.5% | 7.5 | Sufentanil | 10 |
| 38 | Kezri et al. 2014 | Cesarean section | 0 | 60 | Iran | 30 | 30 | Bupivacaine 0.5% | 10 | Bupivacaine 0.5% | 10 | Fentanyl | 25 |
| 39 | Korhonen et al. 2003 | Knee arthroscopy | 47 | 51 | Finland | 50 (ITT) | 50 (ITT) | Bupivacaine 0.5% | 4 | Bupivacaine 0.5% | 3 | Fentanyl | 10 |
| 40 | Kuberan et al. 2018 | Cesarean section | 0 | 36 | India | 18 (ITT) | 18 (ITT) | Bupivacaine 0.5% | 2,5 | Bupivacaine 0.5% | 2 | Fentanyl | 15 |
| 41 | Kuusniemi et al. 2000 | Urological procedures | 35 | 45 | Finland | 20 | 20/20/20 | Bupivacaine 0.5% | 10 | Bupivacaine 0.5% | 10/7.5/7.5 | Fentanyl | 25/25/25 |
| 42 | Lauretti et al. 1998 | Abdominal hysterectomy | 0 | 50 | Brazil | 10 | 10/10/10 | Bupivacaine 0.5% | 15 | Bupivacaine 0.5% | 15 | Fentanyl | 25/10/25 |
| 43 | Lee et al. 2005 | Urological procedures | NS | NS | Hong Kong | 25 | 25 | Levobupivacaine 0.5% | 13 | Levobupivacaine 0.5% | 11.5 | Fentanyl | 15 |
| 44 | Lee et al. 2009 | Lower extremity surgery | 19 | 16 | Korea | 20/20 | 20 | Bupivacaine 0.5% | 10/5 | Bupivacaine 0.5% | 5 | Fentanyl | 10 |
| 45 | Lee et al. 2011 | Cesarean section | 0 | 72 | Korea | 24 | 24/24 | Bupivacaine 0.5% | 6.5-12 | Bupivacaine 0.5% | 6.5-12 | Fentanyl/Sufentanil | 20/2.5 |
| 46 | Mahajan et al. 2005 | Cesarean section | 0 | 24 | India | 12 | 12 | Bupivacaine 0.5% | 7.5 | Bupivacaine 0.5% | 7.5 | Fentanyl | 20 |
| 47 | Makwana et al. 2014 | Abdominal hysterectomy | 0 | 60 | India | 30 | 30 | Bupivacaine 0.5% | 15 | Bupivacaine 0.5% | 15 | Fentanyl | 25 |
| 48 | Martin et al. 1999 | Oocyte retrieval procedures | 0 | 78 | EUA | 40 | 38 | Lidocaine 1,5% | 45 | Lidocaine 1,5% | 45 | Fentanyl | 10 |
| 49 | Martyr et al. 2001 | Hip fractures | 9 | 33 | Australia | 25 (ITT) | 23 (ITT) | Bupivacaine 0.5% | 12.5 | Bupivacaine 0.5% | 7.5 | Fentanyl | 20 |
| 50 | Neeta et al. 2015 | Urologic, Gynecologic, Orthopedics and general surgery | NS | NS | India | 20 | 20/20 | Bupivacaine 0.5% | 15 | Bupivacaine 0.5% | 15 | Fentanyl/Sufentanil | 25/5 |
| 51 | Ngiam et al. 1998 | Cesarean section | 0 | 60 | Singapore | 20 (ITT) | 20/20 (ITT) | Bupivacaine 0.5% | 7.5 | Bupivacaine 0.5% | 7.5 | Fentanyl/Sufentanil | 15/10 |
| 52 | Olofsson et al. 2004 | Hip fractures | NS | NS | Sweden | 25 | 25 | Bupivacaine 0.5% | 15 | Bupivacaine 0.5% | 7.5 | Sufentanil | 5 |
| 53 | Ozyilkan et al. 2013 | Cesarean section | 0 | 93 | Turkey | 31 | 31/31 | Levobupivacaine 0.5% | 2.2 ± 0.2 mL | Levobupivacaine 0.5% | 2.2 ± 0.2 mL | Fentanyl/Sufentanil | 10/2,5 |
| 54 | Palmer et al. 1995 | Cesarean section | 0 | 28 | EUA | 14 | 14 | Hyperbaric lidocaine 1,5% | 80 | Hyperbaric lidocaine 1,5% | 80 | Fentanyl | 15 |
| 55 | Rajbhandari et al. 2020 | Emergency appendectomy | 52 | 68 | Nepal | 30 | 30/30/30 | Bupivacaine 0.5% | 15 | Bupivacaine 0.5% | 15/15/15 | Fentanyl | 10/20/30 |
| 56 | Randalls et al. 1991 | Cesarean section | 0 | 48 | UK | 12 | 12/12/12 | Bupivacaine 0.5% | 12.5 | Bupivacaine 0.5% | 12.5 | Fentanyl | 10 |
| 57 | Sadegh et al. 2012 | Cesarean section | 0 | 80 | Iran | 40 | 40 | Bupivacaine 0.5% | 12.5 | Bupivacaine 0.5% | 12.5 | Fentanyl | 25 |
| 58 | Seewal et al. 2007 | Inguinal hernia repair | 60 | 0 | UK | 12 | 12/12/12/12 | Bupivacaine 0.5% | 11 | Bupivacaine 0.5% | 11/11/11/11 | Fentanyl | 10/20/30/50 |
| 59 | Sertoz et al. 2014 | Knee arthroscopy | 33 | 26 | Turkey | 33 (ITT) | 29 | Bupivacaine 0.5% | 5 | Bupivacaine 0.5% | 5 | Sufentanil | 2,5 |
| 60 | Seyhan et al. 2006 | Cesarean section | 0 | 45 | Turkey | 15 | 15/15 | Bupivacaine 0.5% | 9 | Bupivacaine 0.5% | 9 | Fentanyl | 10/20 |
| 61 | Shahriari et al. 2007 | Cesarean section | 0 | 40 | Iran | 20 | 20 | Lidocaine 5% | 80 | Lidocaine 5% | 80 | Fentanyl | 15 |
| 62 | Shende et al. 1998 | Cesarean section | 0 | 40 | UK | 20 | 20 | Bupivacaine 0.5% | 12.5 | Bupivacaine 0.5% | 12.5 | Fentanyl | 15 |
| 63 | Shim et al. 2018 | Anorectasl surgery | 35 | 45 | Korea | 40 | 40 | Bupivacaine 0.5% | 5 | Bupivacaine 0.5% | 5 | Fentanyl | 15 |
| 64 | Singh et al. 1995 | Genitourinary surgery | 43 | 0 | USA | 22 | 21 | Bupivacaine 0.5% | 13.5 | Bupivacaine 0.5% | 13.5 | Fentanyl | 25 |
| 65 | Sung et al. 2013 | Inguinal hernia repair | 68 | 4 | Korea | 38 (ITT) | 36 | Bupivacaine 0.5% | 14 | Bupivacaine 0.5% | 13 | Fentanyl | 10 |
| 66 | Techanivate et al. 2004 | Appendectomy | NS | NS | Thailand | 20 | 20 | Bupivacaine 0.5% | 20 | Bupivacaine 0.5% | 20 | Fentanyl | 20 |
| 67 | Tyagi et al. 2013 | Cesarean section | 0 | 90 | India | 30 | 30/30 | Bupivacaine 0.5% | 10 | Bupivacaine 0.5% | 10/10 | Fentanyl | 12,5/25 |
| 68 | Unal et al. 2012 | Knee arthroscopy | 18 | 25 | Turkey | 15 | 15 (ITT)/15 (ITT) | Bupivacaine 0.5% | 4 | Bupivacaine 0.5% | 4/3 | Fentanyl | 25/25 |
| 69 | Venkata et al. 2015 | Cesarean section | 0 | 50 | India | 25 | 25 | Bupivacaine 0.5% | 10 | Bupivacaine 0.5% | 7,5 | Fentanyl | 25 |
| 70 | Vyas et al. 2010 | Cesarean section | 0 | 60 | India | 30 | 30 | Bupivacaine 0.5% | 11 | Bupivacaine 0.5% | 11 | Sufentanil | 5 |
| 71 | Wang et al. 2019 | Total Hip Arthroplasty | 55 | 5 | China | 33 (ITT) | 32 (ITT) | Bupivacaine 0.5% | 10 | Bupivacaine 0.5% | 7,5 | Fentanyl | 20 |
| 72 | Walsh et al. 2003 | TURP | 30 | 0 | Ireland | 15 (ITT) | 15 (ITT) | Bupivacaine 0.5% | 15 | Bupivacaine 0.5% | 10 | Fentanyl | 25 |
| 73 | Waxler et al. 2004 | Rectal ambulatory surgery | 37 | 12 | USA | 21 | 28 | Hyperbaric lidocaine 1,5% | 15 | Hyperbaric lidocaine 5% | 50 | Sufentanil | 10 |
| 74 | Weigl et al. 2016 | Cesarean section | 0 | 59 | Poland | 30 (ITT) | 30 (ITT) | Bupivacaine 0.5% | 7.5-15 | Bupivacaine 0.5% | 7.5-15 | Fentanyl | 25 |
| 75 | Yi-chun et al. 2006 | TURP | 90 | 0 | China | 30 | 30/30 | Bupivacaine 0.5% | 15 | Bupivacaine 0.5% | 7.5/7.5 | Sufentanil | 5/7,5 |
| 76 | Zohar et al. 2007 | Transurethral procedures | 100 | 0 | Israel | 25 | 25/25/25 | Bupivacaine 0.5% | 7.5 | Bupivacaine 0.5% | 5/4/3 | Fentanyl | 20/20/20 |
Characteristics and reasons for exclusion of studies were described in Supplementary Table 1.
Methodological quality of included studies
The risk of bias is summarized in Supplementary Figures 1 and 2. Most of the included RCTs were found to have a low to unclear risk of bias. Low or unclear random sequence generation methods were considered. No study described the allocation concealment method, and it was deemed as an unclear risk. The blinding of participants and personnel, outcome assessment, and outcome data provided detailed information and were judged as low risk. Moreover, some studies had selective reporting bias, and some studies had incomplete outcome data. Reasons for judgment are described in Supplementary Table 2.
Effect estimates for outcomes
In case of dropouts or loss of follow-up, intention-to-treat analysis was used, considering the worst-case scenario. It was deemed that if an intervention worked in the worst scenario, it would probably do it in the best one.
Primary outcomes
Rescue analgesia up to 24 h
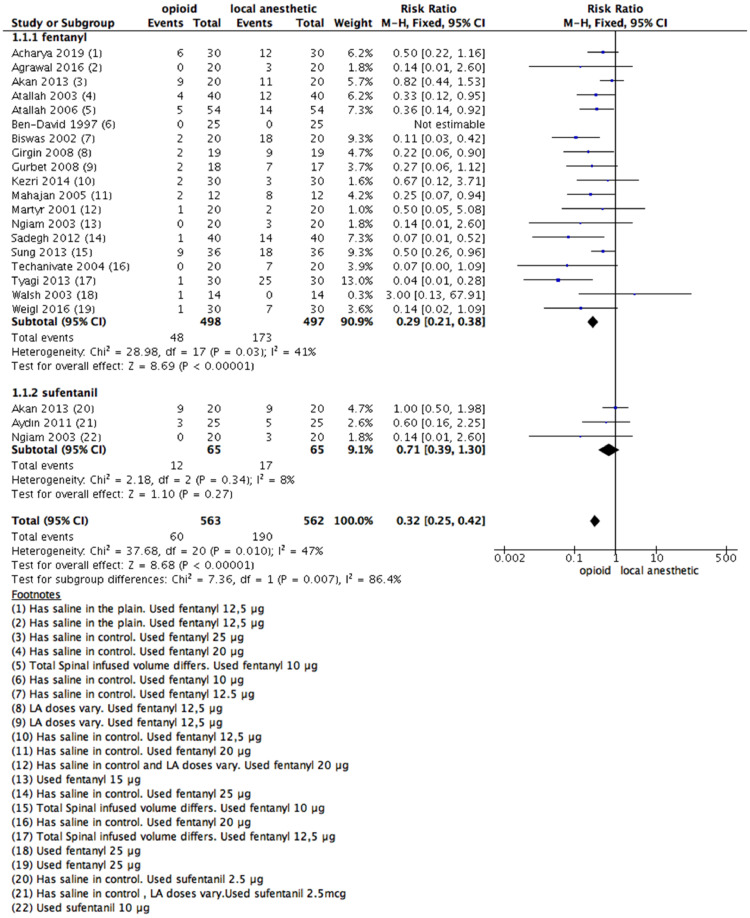
Nineteen studies provided data on rescue analgesia up to the first postoperative day. Seventeen studies involving 995 patients, provided data for comparing the use of IT fentanyl versus LA, and three studies, involving 130 patients, compared the use of IT sufentanil.
Compared with LAs, spinal coadministration of both fentanyl or sufentanil decreased patients' need for rescue analgesia. The RR was 0.29 (0.21–0.38); 95% CI for fentanyl and 0.71 (0.39–1.30); 95% CI for sufentanyl (Fig. 2).
Figure 2.
Meta-analysis of the rescue analgesia up to 24h. (1.1.1) Rescue analgesia of patients received fentanyl vs local anesthetic alone, fixed-effect model was used. (1.1.2) Rescue analgesia of patients received sufentanil vs local anesthetic alone, fixed- effect model was used.
Based on the analysis, the pooled RR estimate was 0.32 (0.25–0.42) with a 95% CI. The heterogeneity test between subgroups was as follows: Chi2 = 7.36; I2 = 86.4% (Fig. 2).
Four studies used different opioid doses between intervention and control groups.12,28,30,47 Therefore, a sensitivity analysis was performed to investigate different possible doses affecting effect direction. Even in this case, there is no change in effect direction (Supplementary Fig 1).
A funnel plot was made, aiming to look for publication bias. There was no asymmetry.
The time to first rescue analgesia was longer for both IT fentanyl compared to LA alone (Mean difference: 120.3 minutes [81.72 to 158.35]; 95% CI) and for IT sufentanil compared to LA alone (Mean difference: 142.82 minutes [105.06 to 180.52]; 95% CI) (Supplementary Fig 2).
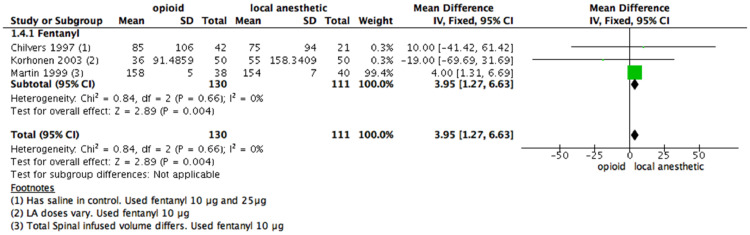
PACU length of stay
Only three studies, all using fentanyl, and totaling 241 patients, provided data on PACU length of stay. Criteria for discharge from the PACU varied among studies, but all of them included stable vital signs and complete resolution of the motor block.
Data from two out of three studies were included. All studies demonstrated longer PACU time with IT fentanyl. There was a significant difference in the PACU length of stay (RR = 3.95 [1.27–6.63]; 95% CI; Fig. 3) for those given IT fentanyl compared to those who were administered a LA alone.
Figure 3.
Meta-analysis of the length to discharge from the PACU (measured in minutes). (1.4.1) Rescue analgesia of the length to discharge from the PACU received fentanyl vs local anesthetic alone, fixed- effect model was used. PACU, post-anesthesia care unit.
The evidence was judged as low certainty due to concerns related to blending37 and attrition bias46 since one study did not describe primary and secondary outcomes before developing the survey. We downgraded the certainty by one level. There is a wide CI. We downgraded the certainty by another level.
The studies used equal doses of LA in both the intervention and control groups. A funnel plot analysis was not performed since there were less than ten studies included in the meta-analysis.
In-hospital mortality
None of the studies described any in-hospital death, and no perioperative deaths were described in the 76 included RCTs. None of the specialists asked could report any death related to the use of intrathecal opioids. The specialists from the team did not know any case report associated with this outcome using these spinal lipophilic opioids.
Secondary outcomes
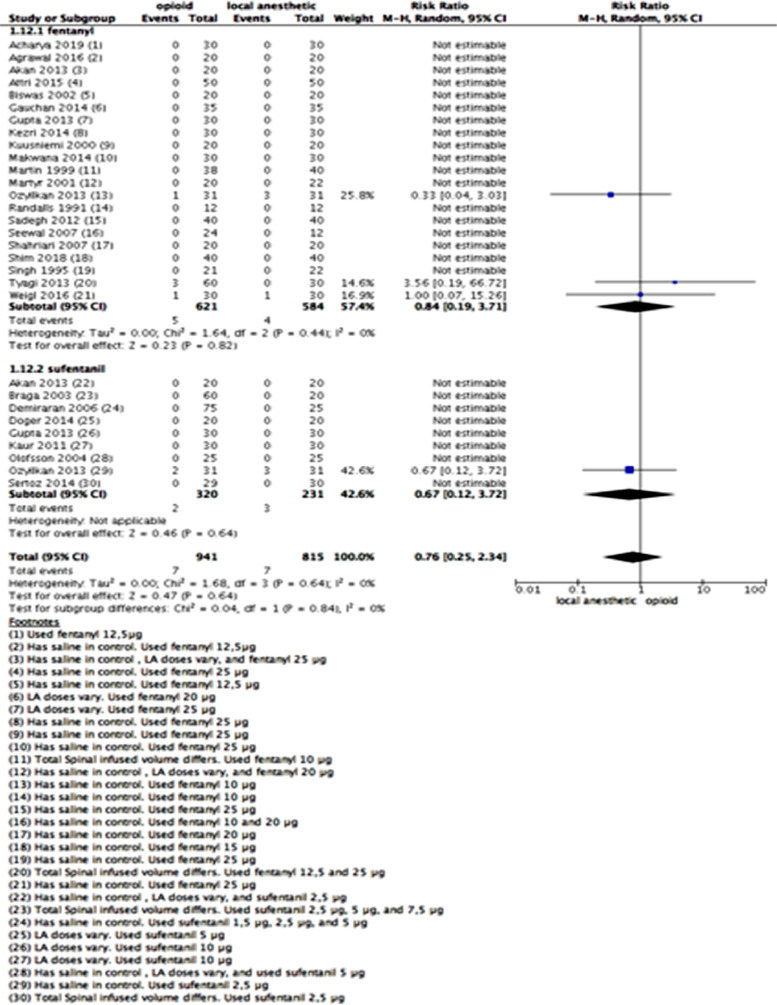
Respiratory depression
Most studies defined respiratory depression as respiratory rate < 8–10 and SpO2 < 90%, and three studies detected this outcome.49,59,63 Twenty eight studies11,12,16,17,20,27,29,36,39,45, 46, 47,49,52, 53, 54, 55, 56,59,67,69,73,75,77,79,80,85 reported respiratory depression and could be joined in a meta-analysis (Fig. 4). The studies reported a total of 14 cases of respiratory depression – 7 out of 941 patients who received spinal fentanyl or sufentanil and 7 out of 815 patients who received LAs. All instances of respiratory depression were uneventful and easily manageable, and all events happened intraoperatively. No study reported late respiratory depression (postoperatively).
Figure 4.
Meta-analysis of respiratory depression events (number of patients) up to 24 h. (1.12.1) Patients who received fentanyl vs local anesthetic alone with respiratory depression up to 24 h; random-effect model was used. (1.12.2) Patients who received sufentanil vs local anesthetic alone with respiratory depression up to 24 h; random-effect model was used.
We did not perform a sensitivity analysis because there were many no-event studies, and those that described rare events of respiratory depression used the same dose of LAs in both intervention and control groups.
Confidence in the estimated effect was judged to be of moderate quality due to imprecision (low number of events with a wide CI). We downgraded it by one level.
Urinary retention
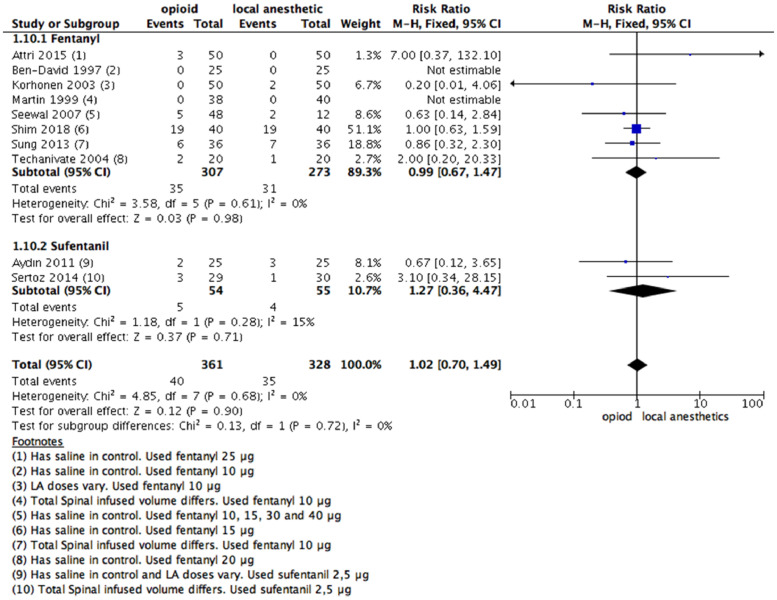
Ten studies consisting of 689 patients (control group: 328 patients; fentanyl group: 307 patients; sufentanil group: 54 patients) provided data from participants with urinary retention up to 24 hours after anesthesia. There was no significant difference in urinary retention (RR = 1.02 [0.70–1.49]; 95% CI; Fig. 5) between intervention and control groups. There were no significant subgroup differences between fentanyl or sufentanil vs. LAs (Chi2 = 0.13; I2 = 0%).
Figure 5.
Meta-analysis of the number of patients with urinary retention up to 24 h. (1.10.1) Patients who received fentanyl vs local anesthetic alone with urinary retention up to 24 h; fixed-effect model was used. (1.10.2) Patients who received sufentanil vs local anesthetic alone with urinary retention up to 24 h; fixed-effect model was used.
We used fentanyl for judgment of the confidence in the estimated effect. It was deemed as high certainty of the evidence considering the high number of patients, low risk of bias, and consistency among studies.
A funnel plot analysis was performed, and no publication bias was observed.
One study included different LA doses between intervention and control groups.37 A sensitivity analysis was made, and there was no change in the effect direction (Supplementary Fig 3).
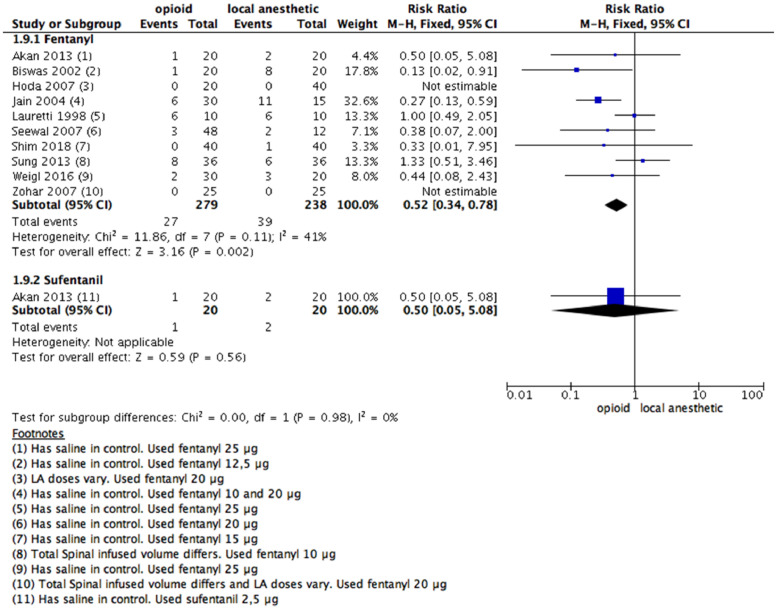
PONV
The number of PONV episodes up to 24 hours postoperatively was explored (number of participants who needed rescue antiemetic). Ten studies provided data on the occurrence of antiemetic treatment after coadministration of fentanyl with an LA (Fig. 6). The authors reported 27/279 in the fentanyl group and 39/238 in LAs (RR = 0.52 [0.34 to 0.78]; 95% CI).
Figure 6.
Meta-analysis of the number of PONV episodes during the 24 hours following surgical procedure. (1.9.1) Patients with PONV episodes during the 24 hours following surgical procedure received fentanyl vs local anesthetic alone, fixed- effect model was used; (1.9.2) Patients with PONV episodes during the 24 hours following surgical procedure received sufentanil vs local anesthetic alone, fixed- effect model was used. PONV, postoperative nausea and vomiting.
The evidence was deemed as having high certainty quality considering the low risk of bias, the number of included studies and patients, and the consistency in the estimated effect between studies.
As there were ten included studies for the meta-analysis, a funnel plot analysis was performed, and no significant publication bias was evidenced. Three studies used different doses of LAs.12,32,65 A sensitivity analysis was made, and no difference in the effect direction occurred (Supplementary Fig 4).
Eighteen studies, including 991 patients, provided data on vomiting episodes after fentanyl (15/495) vs. LA (35/496) administration. Eleven studies, including 667 patients, specified data on vomiting episodes induced by sufentanil (9/376) vs. LAs (13/291). Fentanyl decreased the risk of vomiting compared to the LA group (RR = 0.45 [0.26–0.77]; 95% CI) (Supplementary Fig 5).
Twenty-eight studies, including 1404 patients, delivered data on nausea episodes after fentanyl (84/722) vs. LA (98/682) administration (RR = 0.84 [0.65–1.08]; 95% CI), with no statistical difference (Supplementary Fig 6).
The detailed description of both nausea and vomiting as a composite outcome, including data and statistical analysis, can be found in the Supplementary material.
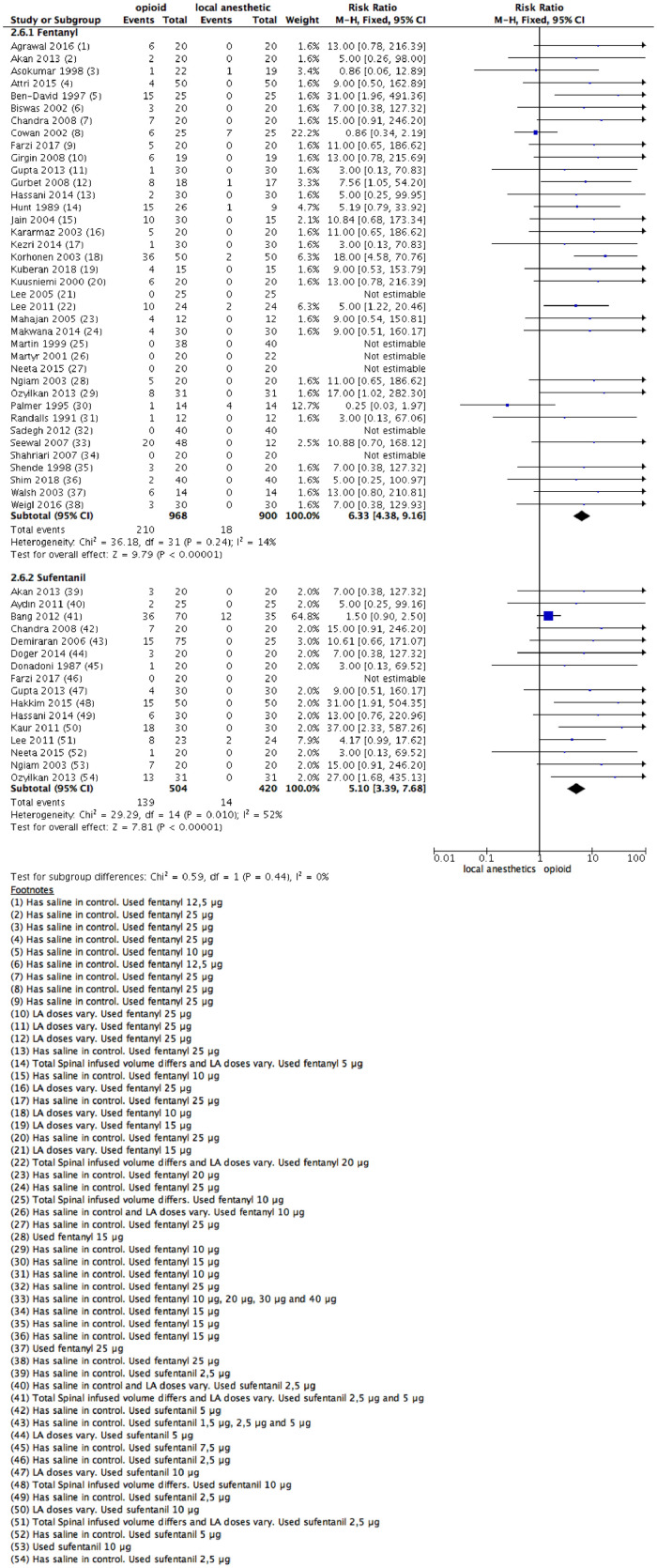
Pruritus
Fifty-four studies offered data on the incidence of pruritus up to the first postoperative day. Thirty-eight studies specified data comparing the use of fentanyl vs. LAs alone, and sixteen studies comparing the use of IT sufentanil with a LA. The studies containing 2684 patients (LAs: 1320 patients; fentanyl group: 968 patients; sufentanil group: 504 patients) specified participants’ data.
There was a significant difference (RR = 6.33 [4.38–9.16]; 95% CI; Fig. 8) between administration of LAs alone and giving fentanyl. For the sufentanil group, an increase in pruritus events also occurred (RR = 5.10 [3.39–7.68]; 95% CI; Fig. 7).
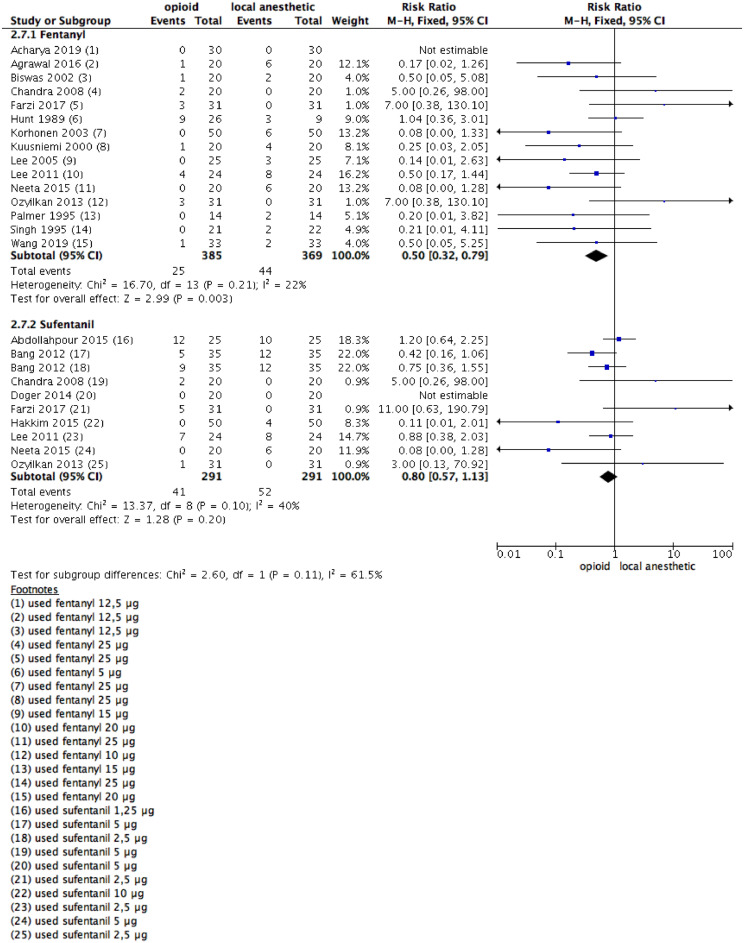
Figure 8.
Meta-analysis of the shivering episodes during the 24 hours following a surgical procedure. (1.8.1) Patients with shivering attacks during the 24 hours following surgical procedure received Fentanyl vs. local anesthetic alone, fixed-effect model was used; (1.8.2) Patients with shivering episodes during the 24 hours following surgical procedure Sufentanil vs. local anesthetic alone, fixed- effect model was used.
Figure 7.
Meta-analysis of the pruritus episodes during the 24 hours following surgical procedure. (1.7.1) Patients with pruritus episodes during the 24 hours following surgical procedure received fentanyl vs local anesthetic alone, fixed- effect model was used; (1.7.2) Patients with pruritus episodes during the 24 hours following surgical procedure received sufentanil vs local anesthetic alone, fixed- effect model was used.
As there were concerns over the risk of bias related to blinding, imbalances between groups, description of loss of follow-up, and poor description of primary and secondary outcomes, the quality of the evidence was downgraded by one level. The evidence was deemed as moderate certainty.
Eleven studies used different LAs doses between groups.12,28,35,37, 38, 39,41,43,47,79,86 A sensitivity analysis was made, and no difference in effect direction was noted. A funnel plot was tested, and no asymmetry occurred.
Shivering
Fifteen studies on patients who were given fentanyl reported postoperative shivering up to 24 hours after anesthesia, with 25/385 and 44/369 patients in the experimental and control groups, respectively (Fig. 8). The rate was significantly lower in the opioid than in the LAs group (RR = 0.5 [0.32–0.79]; 95% CI). Ten studies in which the intervention was sufentanil reported no statistical differences, with 41/291 and 52/291 patients, in the opioid and LAs groups, respectively. The experimental and control groups showed no statistically significant differences (RR = 0.80, 95% CI 0.57 to 1.13).
A funnel plot analysis was performed with no detection of publication bias. Four studies used different LA doses between groups.37,39,41,43 A sensitivity analysis was done, and no statistically significant difference between groups was noted (for fentanyl: RR = 0.68, 95% CI 0.39 to 1.19) (Supplementary Fig 8).
No study was judged as high risk for selection or used different doses of medication between intervention and control groups. Therefore, a sensitivity analysis was not carried out.
Discussion
Lipophilic opioids, including fentanyl and sufentanil, have an excellent pharmacological profile for spinal anesthesia, particularly when compared to hydrophilic opioids. Fentanyl and sufentanil have a faster onset, a shorter duration of action, and a lower rostral spread to the 4th ventricle, lowering the risk of ventilatory depression. They are highly ionized, lipophilic μ-receptor agonists. When dispensed intrathecally, the non-ionized component is rapidly shifted into the spinal cord.87
The main finding of this systematic review was that local IT anesthetic combined with fentanyl or sufentanil prolonged time to first postoperative analgesic administration and created a clinically relevant reduction in postoperative pain with no increase in serious adverse events. The analysis showed that patients receiving spinal fentanyl or sufentanil had a significantly increased analgesia duration of at least 2 hours when compared to LAs, being greater with spinal sufentanil. Moreover, spinal opioids were a protective factor for the need for rescue analgesia. These results confirmed an increase of analgesia quality when adding spinal lipophilic opioids to local anesthetics and are in line with previous investigations.6,7,88
Postoperative pain remains a significant problem in most surgical patients who spend their immediate postoperative period in the PACU, where unsatisfactory pain management delays recovery. The analysis evidenced increased duration and effectiveness of analgesia, and a reduction in the PACU length of stay was hypothesized. However, the results of this study suggest that coadministration of spinal fentanyl with LAs could increase PACU length of stay. This finding is limited due to the number of studies included (only three studies).
The 76 included RCTs did not mention mortality as an outcome. Despite the increasing baseline patient risks and complexity of surgeries, as anesthesia safety has improved over the past decades and perioperative mortality declined, such a low mortality rate could be expected. Maybe mortality was mitigated because it became mandatory to monitor patients during surgery and in a PACU postoperatively, and complications such as respiratory depression were detected and managed earlier.89 Clinical trials evidenced the minimum effective doses of anesthetics for many situations, and lower doses are associated with lower incidence of adverse effects. Therefore, no case of death was detected in this review, including 4734 research participants, aiming at the safety confidence for this outcome.
The definition of opioid-induced respiratory depression has always been controversial.90 Respiratory depression is one of the most dreaded complications associated with opioid administration. When using lipophilic opioids, it generally occurs within the first 30 minutes and has never been described after 2 hours of IT fentanyl or sufentanil.91 Among included studies, there were no differences in the rates of respiratory depression between intervention and control groups. The reported events occurred intraoperatively and were detected by a drop in SpO2. Also, all cases were uneventful, because those studies reported mild respiratory events that were easily manageable with nasal O2 addition. As a limitation, the authors’ definition described for respiratory depression is not a unique condition that could reduce SpO2. Many other common complications might do it as well, such as hypotension, shivering, and hypothermia. So, related events should have been described every time an author would describe respiratory depression to avoid confounding factors. Furthermore, a recent consensus guideline92 from the Society for Obstetric Anesthesia and Perinatology recommends that longer monitoring for respiratory depression is needed when spinal morphine is used, since morphine hydrophilic nature causes a longer half-life than fentanyl and sufentanil.
PONV is a common side effect when using systemic opioids, and its use in the perioperative setting is considered an independent risk factor for this complication.93 Spinal morphine increases the incidence of PONV.4,7 However, unlike common clinician beliefs, spinal lipophilic opioids may reduce nausea and vomiting.87 Indeed, a recent systematic review showed that spinal fentanyl reduced intraoperative nausea and vomiting by 59% when compared to LAs alone after cesarean section,6 which is in line with the findings of our review. Fentanyl decreased the incidence of PONV and vomiting alone. There were few included studies and patients for spinal sufentanil, so there is a need for more studies to answer this question. However, analyzing vomiting, IT sufentanil resulted in lower incidence (2.4% vs. 4.4%) despite not statistically significant. A systematic review of cesarean section showed that spinal sufentanil did not affect nausea incidence, and it can even decrease its incidence when excluding a more heterogeneous study in their analysis leading to an RR = 0.58, 95% CI 0.40 to 0.85.88 The reasons for the reduction of nausea and vomiting with spinal lipophilic opioid could be explained by two effects: 1) inhibition of visceral pain impulses, which may trigger nausea and vomiting and 2) reduction of supplemental intraoperative and postoperative analgesia request with parenteral opioids. Our findings ensure safety for this outcome when using lipophilic IT opioids.
Urinary retention is a critical side effect of IT opioid administration. The mechanism responsible for urinary retention is not entirely defined. Opioids may exert their effects on either the supraspinal or the spinal level canceling the coordination between detrusor and sphincter function.94 However, the present review did not confirm an increased risk of urinary retention with spinal fentanyl or sufentanil. Spinal LAs may delay the return of bladder function beyond the resolution of sensory anesthesia and might lead to distention of the bladder beyond its standard functioning capacity. Therefore, urinary retention could not be attributed to opioid administration.
The incidence of pruritus after the administration of IT opioids was 199/934 (21%) patients for fentanyl and 132/434 (30%) patients for sufentanil. These findings are in line with a previous systematic review that showed a nearly six-fold increase in pruritus incidence when using spinal fentanyl.6 Additionally, when spinal sufentanil was used, the incidence of pruritus could be as high as 33% with an RR of 7.63 when compared to that when LAs alone were used in cesarean section.93 Although the exact mechanism of spinal-opioid-induced pruritus is not well known, the activation of μ receptors at the spinal level is recognized as the trigger for itching.95 Even though pruritus may be uncomfortable and sometimes severe enough to be distressing, the benefits for reducing pain, analgesia rescue, and reducing PONV might outweigh the disadvantage of the opioid side effect. Moreover, due to the higher liposolubility of fentanyl and sufentanil, the induced pruritus is short-lived.
Sufentanil was not effective in decreasing shivering rates despite a statistical tendency. The finding could be attributed to the small number of included and patients’ studies. A previous systematic review6 on IT and epidural sufentanil had similar results, and the authors postulated that the low dose range of IT and epidural sufentanil used (1.5–20 μg) might not be effective in decreasing shivering. The same previous systematic review which evaluated the effects of lipophilic opioids in preventing or reducing shivering after spinal anesthesia reported fentanyl to be more effective than sufentanil. One randomized trial has shown a 30% reduction in shivering by adding spinal sufentanil, but it was excluded from this review because it also included morphine96 as a co-intervention (confounding factor). The present review found a reduction in shivering rates when using fentanyl. Fentanyl, like sufentanil, is a highly ionized, lipophilic μ-receptor agonist, and when it is administered intrathecally, the non-ionized component is rapidly transferred into the spinal cord. It is known that intravenous fentanyl reduces fever, but epidural fentanyl does not.97 Increases in core temperature could be partially explained by the hermetic effect of the low doses of fentanyl used in spinal anesthesia.98 Also, intrathecally administered LAs block thermal regulation by blocking the sympathetic pathways and by causing vasodilation, and the addition of lipophilic opioids is associated with a reduction in LAs doses, which can help mitigate the loss of thermoregulation. Another hypothesis is that the reduction of shivering attributable to fentanyl added to the subarachnoid space is related to a direct effect on the thermoregulation center and in afferent thermal inputs at the spinal cord.
After performing this study, the risk–benefit ratio was found to be unbalanced in relation to the benefits. The risks for serious complications now are quite low, and the benefits are high for clinical outcomes. The spinal route is advised for fentanyl and sufentanil, but the lower recommended doses and monitoring protocols should be considered.
The data obtained demonstrate the safety and effectiveness of lipophilic opioids for IT route. The authors hope that this review can guide stakeholders and specialists to make decisions for clinical practice.
Limitations
The trials involved used various combinations of IT LAs and opioids. The LAs used included bupivacaine, levobupivacaine, or lidocaine, and the opioids used had different doses of fentanyl or sufentanil. This is a possible source of clinical heterogeneity. Fortunately, most of the studies evaluated the combination between bupivacaine and different doses of fentanyl or sufentanil, which had similar clinical effects, ensuring that the trials could be pooled. We performed a sensitivity analysis and did not find any difference in effect direction.
The number of trials and the number of patients recruited in some trials were relatively small, which increased the possibility of random chance and overestimated the beneficial effects. However, pooled data confirm consistency in results.
Some of the endpoints measured in original studies had different definitions. The authors are aware that the limitations of the selected studies were known by the systematic review that included them. For example, many definitions of respiratory depression are described, some associated with low risk while others to a high risk for respiratory arrest. This might hamper the reliability of the final pooled results.
There is a relationship between IT opioid analgesia and side effects, mainly pruritus. The lowest dose of IT lipophilic opioids that will provide adequate analgesia with the least side effects should be analyzed. This dose–response analysis was not an objective of this study. Most IT opioid dose–response studies have been managed in the obstetric population (REF). There are few dose–response studies in the non-obstetric population. Further studies are necessary to determine the effective dose of fentanyl or sufentanil co-administered with a LA.
Most of the studies used fentanyl as the intervention. Therefore, the review will better reflect the effect of fentanyl.
Only three studies considered PACU length of stay a relevant outcome and described it in the paper. A low level of confidence in the estimated effect for this outcome should not be judged as a risk for safety reasons since this is not a clinical outcome. Instead, this outcome is relevant for determining personnel to work in a scenario using IT lipophilic opioids.
Considerable heterogeneity among studies was found in some analyses (e.g., time to rescue analgesia). Possible causes were investigated while considering different patients included in the studies, affecting this outcome.
Strengths
The review detected a large number of studies (76), including 4734 patients.
A rigid methodology was used to analyze the estimated effects, including only RCTs, evaluating the risk of bias, investigating causes of heterogeneity among studies, performing subgroup analysis, and performing sensitivity analysis. A broad search of the literature was made, including EMBASE, CENTRAL (Cochrane), PubMed, handsearching, clinicaltrials.gov, and gray literature.
There were more research participants from the fentanyl group. Therefore, the results of the review imply high confidence in the effect of fentanyl compared to that for LAs.
Studies with higher doses than those considered reasonable and safe for clinical use by the specialists were included. Even these higher doses of the drugs were not able to cause serious adverse events. These facts strengthened the confidence for safety purposes. We included different surgical procedures, which increases external validity of the review, mainly for the surgeries analyzed.
When possible (more than ten studies included in a meta-analysis), we performed a funnel plot analysis, and we did not find any indication of publication bias.
We made a summary of findings table using GRADE to evaluate the confidence in the estimated effects. Were described in Supplementary Table 2. We found moderate to high confidence in the estimated effects, which strengthened the certainty in the findings. We are confident that we are near the real effect of the intervention for fentanyl use.
Conclusion
There is high confidence that the addition of spinal fentanyl to LAs produced a clinically relevant reduction in postoperative pain and analgesic consumption. Moreover, fentanyl reduced both PONV and postoperative shivering more when compared to LAs. Opioid use increased the relative risk of postoperative pruritus in the opioid group. The studies analyzed did not report any case of in-hospital mortality related to spinal lipophilic opioids. Furthermore, respiratory depression episodes were rare, uneventful, occurred intraoperatively, and they were easily manageable. In summary, there is moderate to high quality certainty that there is evidence regarding the safety and effectiveness of adding lipophilic opioids to LAs in spinal anesthesia.
Conflicts of interest
The authors declare no conflicts of interest.
Funding
This study was supported by the Brazilian Society of Anesthesiology (SBA).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.bjane.2021.10.010.
Appendix. Supplementary materials
References
- 1.Finch JS, DeKornfeld TJ. Clinical investigation of the analgesic potency and respiratory depressant activity of fentanyl, a new narcotic analgesic. J Clin Pharmacol J New Drugs. 1967;7:46–51. doi: 10.1002/j.1552-4604.1967.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen SW, Maguire PA, Davies MF, et al. Evidence for mu1-opioid receptor involvement in fentanyl-mediated respiratory depression. Eur J Pharmacol. 1996;312:241–244. doi: 10.1016/0014-2999(96)00571-7. [DOI] [PubMed] [Google Scholar]
- 3.Willette RN, Doorley BM, Sapru HN. Activation of cholinergic mechanisms in the medulla oblongata reverse intravenous opioid-induced respiratory depression. J Pharmacol Exp Ther. 1987;240:352–358. [PubMed] [Google Scholar]
- 4.Gehling M, Tryba M. Risks and side-effects of intrathecal morphine combined with spinal anaesthesia: a meta-analysis. Anaesthesia. 2009;64:643–651. doi: 10.1111/j.1365-2044.2008.05817.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Sun H, Sun W-T, et al. Efficacy and safety of intrathecal morphine for pain control after spinal surgery: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25:2674–2684. doi: 10.26355/eurrev_202103_25431. [DOI] [PubMed] [Google Scholar]
- 6.Uppal V, Retter S, Casey M, et al. Efficacy of intrathecal fentanyl for cesarean delivery: a systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. Anesth Analg. 2020;130:111–125. doi: 10.1213/ANE.0000000000003975. [DOI] [PubMed] [Google Scholar]
- 7.Pöpping DM, Elia N, Marret E, et al. Opioids added to local anesthetics for single-shot intrathecal anesthesia in patients undergoing minor surgery: a meta-analysis of randomized trials. Pain. 2012;153:784–793. doi: 10.1016/j.pain.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JPT, Thomas J, Chandler J, et al., Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. 2020 at <https://doi.org/10.1002/9781119536604>
- 9.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal A, Asthana V, Sharma J, et al. Efficacy of lipophilic vs lipophobic opioids in addition to hyperbaric bupivacaine for patients undergoing lower segment caeserean section. Anesth Essays Res. 2016;10:420. doi: 10.4103/0259-1162.176402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akan B, Yagan O, Bilal B, et al. Comparison of levobupivacaine alone and in combination with fentanyl and sufentanil in patients undergoing transurethral resection of the prostate. J Res Med Sci Off J Isfahan Univ Med Sci. 2013;18:378–382. [PMC free article] [PubMed] [Google Scholar]
- 13.Asokumar B, Newman LM, McCarthy RJ, et al. Intrathecal bupivacaine reduces pruritus and prolongs duration of fentanyl analgesia during labor: a prospective, randomized controlled trial. Anesth Analg. 1998;87:1309–1315. doi: 10.1097/00000539-199812000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Atallah MM, Shorrab AA, Abdel Mageed YM, et al. Low-dose bupivacaine spinal anaesthesia for percutaneous nephrolithotomy: the suitability and impact of adding intrathecal fentanyl. Acta Anaesthesiol Scand. 2006;50:798–803. doi: 10.1111/j.1399-6576.2006.01063.x. [DOI] [PubMed] [Google Scholar]
- 15.Atallah MM, Helal MA, Shorrab AA. Hypobaric bupivacaine spinal anesthesia for cystoscopic intervention: the impact of adding fentanyl. Middle East J Anaesthesiol. 2003;17:415–426. [PubMed] [Google Scholar]
- 16.Attri J, Kaur G, Kaur S, et al. Comparison of levobupivacaine and levobupivacaine with fentanyl in infraumbilical surgeries under spinal anaesthesia. Anesth Essays Res. 2015;9:178–184. doi: 10.4103/0259-1162.152148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-David B, Solomon E, Levin H, et al. Intrathecal fentanyl with small-dose dilute bupivacaine: better anesthesia without prolonging recovery. Anesth Analg. 1997;85:560–565. doi: 10.1097/00000539-199709000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Ben-David B, Frankel R, Arzumonov T, et al. Minidose bupivacaine-fentanyl spinal anesthesia for surgical repair of hip fracture in the aged. Anesthesiology. 2000;92:6–10. doi: 10.1097/00000542-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Bidikar M, Mudakanagoudar M, Santhosh MCB. Comparison of intrathecal levobupivacaine and levobupivacaine plus fentanyl for cesarean section. Anesth Essays Res. 2017;11:495–498. doi: 10.4103/aer.AER_16_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biswas B, Rudra A, Bose B, et al. Intrathecal fentanyl with hyperbaric bupivacaine improves analgesia during caesarean delivery and in early postoperative period. Indian J Anaesth. 2002;46:469. [Google Scholar]
- 21.Chandra BJ, Kusum MB, Anurup P, et al. Intrathecal bupivacaine with 5μg of sufentanil or 25μg fentanyl for caesarean delivery in pregnancy induced hypertension. J Anaesthesiol Clin Pharmacol. 2008;24:420. [Google Scholar]
- 22.Chilvers CR, Vaghadia H, Mitchell GW, et al. Small-dose hypobaric lidocaine-fentanyl spinal anesthesia for short duration outpatient laparoscopy. II. Optimal fentanyl dose. Anesth Analg. 1997;84:65–70. doi: 10.1097/00000539-199701000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Cowan CM, Kendall JB, Barclay PM, et al. Comparison of intrathecal fentanyl and diamorphine in addition to bupivacaine for caesarean section under spinal anaesthesia. Br J Anaesth. 2002;89:452–458. [PubMed] [Google Scholar]
- 24.Dahlgren G, Hultstrand C, Jakobsson J, et al. Intrathecal sufentanil, fentanyl, or placebo added to bupivacaine for cesarean section. Anesth Analg. 1997;85:1288–1293. doi: 10.1097/00000539-199712000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Desai D, Bumiya P, Upadhyay M. Spinal anesthesia with low dose bupivacaine and fentanyl for femur surgeries in elderly patients. J Anes Cri Open Access. 2019;11:60–64. [Google Scholar]
- 26.Farzi F, Mirmansouri A, Naderi Nabi B, et al. Comparing the effect of adding fentanyl, sufentanil, and placebo with intrathecal bupivacaine on duration of analgesia and complications of spinal anesthesia in patients undergoing cesarean section. Anesthesiol Pain Med. 2017;7:e12738. doi: 10.5812/aapm.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gauchan S, Thapa C, Prasai A, et al. Effects of intrathecal fentanyl as an adjunct to hyperbaric bupivacaine in spinal anesthesia for elective caesarean section. Nepal Med Coll J NMCJ. 2014;16:5–8. [PubMed] [Google Scholar]
- 28.Girgin NK, Gurbet A, Turker G, et al. The combination of low-dose levobupivacaine and fentanyl for spinal anaesthesia in ambulatory inguinal herniorrhaphy. J Int Med Res. 2008;36:1287–1292. doi: 10.1177/147323000803600616. [DOI] [PubMed] [Google Scholar]
- 29.Gupta S, Sampley S, Kathuria S, et al. Intrathecal sufentanil or fentanyl as adjuvants to low dose bupivacaine in endoscopic urological procedures. J Anaesthesiol Clin Pharmacol. 2013;29:509–515. doi: 10.4103/0970-9185.119158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurbet A, Turker G, Girgin NK, et al. Combination of ultra-low dose bupivacaine and fentanyl for spinal anaesthesia in out-patient anorectal surgery. J Int Med Res. 2008;36:964–970. doi: 10.1177/147323000803600512. [DOI] [PubMed] [Google Scholar]
- 31.Hassani V, Movassaghi G, Safaian R, et al. Bupivacaine-sufentanil versus bupivacaine-fentanyl in spinal anesthesia of patients undergoing lower extremity surgery. Anesthesiol Pain Med. 2014;4:e12091. doi: 10.5812/aapm.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoda MQ, Saeed S, Afshan G, et al. Haemodynamic effects of intrathecal bupivacaine for surgical repair of hip fracture. J Pak Med Assoc. 2007;57:245–248. [PubMed] [Google Scholar]
- 33.Hunt CO, Naulty JS, Bader AM, et al. Perioperative analgesia with subarachnoid fentanyl-bupivacaine for cesarean delivery. Anesthesiology. 1989;71:535–540. doi: 10.1097/00000542-198910000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Jain K, Grover VK, Mahajan R, et al. Effect of varying doses of fentanyl with low dose spinal bupivacaine for caesarean delivery in patients with pregnancy-induced hypertension. Int J Obstet Anesth. 2004;13:215–220. doi: 10.1016/j.ijoa.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Kararmaz A, Kaya S, Turhanoglu S, et al. Low-dose bupivacaine-fentanyl spinal anaesthesia for transurethral prostatectomy. Anaesthesia. 2003;58:526–530. doi: 10.1046/j.1365-2044.2003.03153.x. [DOI] [PubMed] [Google Scholar]
- 36.Khezri MB, Rezaei M, Delkhosh Reihany M, et al. Comparison of postoperative analgesic effect of intrathecal clonidine and fentanyl added to bupivacaine in patients undergoing cesarean section: a prospective randomized double-blind study. Pain Res Treat. 2014;2014 doi: 10.1155/2014/513628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korhonen AM, Valanne JV, Jokela RM, et al. Intrathecal hyperbaric bupivacaine 3 mg + fentanyl 10 μg for outpatient knee arthroscopy with tourniquet. Acta Anaesthesiol Scand. 2003;47:342–346. doi: 10.1034/j.1399-6576.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 38.Kuberan A, Jain K, Bagga R, et al. The effect of spinal hyperbaric bupivacaine-fentanyl or hyperbaric bupivacaine on uterine tone and fetal heart rate in labouring women: a randomised controlled study. Anaesthesia. 2018;73:832–838. doi: 10.1111/anae.14278. [DOI] [PubMed] [Google Scholar]
- 39.Kuusniemi KS, Pihlajamäki KK, Pitkänen MT, et al. The use of bupivacaine and fentanyl for spinal anesthesia for urologic surgery. Anesth Analg. 2000;91:1452–1456. doi: 10.1097/00000539-200012000-00029. [DOI] [PubMed] [Google Scholar]
- 40.Lauretti GR, Mattos AL, Reis MP, et al. Combined intrathecal fentanyl and neostigmine: therapy for postoperative abdominal hysterectomy pain relief. J Clin Anesth. 1998;10:291–296. doi: 10.1016/s0952-8180(98)00030-0. [DOI] [PubMed] [Google Scholar]
- 41.Lee YY, Muchhal K, Chan CK, et al. Levobupivacaine and fentanyl for spinal anaesthesia: a randomized trial. Eur J Anaesthesiol. 2005;22:899–903. doi: 10.1017/S0265021505001523. [DOI] [PubMed] [Google Scholar]
- 42.Lee SJ, Kim SH, Jung JD, et al. The effects of intrathecal fentanyl for spinal anesthesia in lower extremity surgery. Korean J Anesthesiol. 2009;56:280–283. doi: 10.4097/kjae.2009.56.3.280. [DOI] [PubMed] [Google Scholar]
- 43.Lee JH, Chung KH, Lee JY, et al. Comparison of fentanyl and sufentanil added to 0.5% hyperbaric bupivacaine for spinal anesthesia in patients undergoing cesarean section. Korean J Anesthesiol. 2011;60:103–108. doi: 10.4097/kjae.2011.60.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahajan R, Grover V, Jain K, et al. Intrathecal fentanyl with low dose hyperbaric bupivacaine for caesarean delivery in patients with pregnancy induced hypertension. J Anaesthesiol Clin Pharmacol. 2005;21:51–58. [Google Scholar]
- 45.Makwana J, Tn S, Khande A, et al. Comparison between hyperbaric Bupivacaine and hyperbaric Bupivacaine plus Fentanyl intrathecally in major gynaecological surgeries. Int J Med Sci Public Health. 2014;3:319. [Google Scholar]
- 46.Martin R, Tsen LC, Tzeng G, et al. Anesthesia for in vitro fertilization: the addition of fentanyl to 1.5% lidocaine. Anesth Analg. 1999;88:523–526. doi: 10.1097/00000539-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Martyr JW, Clark MX. Hypotension in elderly patients undergoing spinal anaesthesia for repair of fractured neck of femur. A comparison of two different spinal solutions. Anaesth Intensive Care. 2001;29:501–505. doi: 10.1177/0310057X0102900509. [DOI] [PubMed] [Google Scholar]
- 48.Neeta S, Upadya M, Gosain A, et al. A prospective randomized controlled study comparing intrathecal bupivacaine combined with fentanyl and sufentanil in abdominal and lower limb surgeries. Anesth Essays Res. 2015;9:149–154. doi: 10.4103/0259-1162.156287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bozdogan Ozyilkan N, Kocum A, Sener M, et al. Comparison of intrathecal levobupivacaine combined with sufentanil, fentanyl, or placebo for elective caesarean section: A prospective, randomized, double-blind, controlled study. Curr Ther Res - Clin Exp. 2013;75:64–70. doi: 10.1016/j.curtheres.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmer CM, Voulgaropoulos D, Alves D. Subarachnoid fentanyl augments lidocaine spinal anesthesia for cesarean delivery. Reg Anesth. 1995;20:389–394. [PubMed] [Google Scholar]
- 51.Rajbhandari D, Mahato MP, Dahal P, et al. The comparison of effectiveness of different doses of fentanyl added to hyperbaric bupivacaine for spinal anaesthesia in emergency appendectomy: the comparison of effectiveness of different doses of fentanyl added to hyperbaric bupivacaine for spinal anaesthesia in emergency appendectomy. Medico Res Chron. 2020;7:240–249. [Google Scholar]
- 52.Randalls B, Broadway JW, Browne DA, et al. Comparison of four subarachnoid solutions in a needle-through-needle technique for elective caesarean section. Br J Anaesth. 1991;66:314–318. doi: 10.1093/bja/66.3.314. [DOI] [PubMed] [Google Scholar]
- 53.Sadegh A, Tazeh-kand NF, Eslami B. Intrathecal fentanyl for prevention of shivering in spinal anesthesia in cesarean section. Med J Islam Repub Iran. 2012;26:85–89. [PMC free article] [PubMed] [Google Scholar]
- 54.Shahriari A, Khooshideh M. Intrathecal fentanyl added to lidocaine for cesarean delivery under spinal anesthesia - A randomised clinical trial. Middle East J Anesthesiol. 2007;19:397–406. [PubMed] [Google Scholar]
- 55.Shim SM, Park JH, Hyun DM, et al. The effects of adjuvant intrathecal fentanyl on postoperative pain and rebound pain for anorectal surgery under saddle anesthesia. Korean J Anesthesiol. 2018;71:213–219. doi: 10.4097/kja.d.18.27097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh H, Yang J, Thornton K, et al. Intrathecal fentanyl prolongs sensory bupivacaine spinal block. Can J Anaesth J Can Anesth. 1995;42:987–991. doi: 10.1007/BF03011070. [DOI] [PubMed] [Google Scholar]
- 57.Sung TY, Kim MS, Cho CK, et al. Clinical effects of intrathecal fentanyl on shoulder tip pain in laparoscopic total extraperitoneal inguinal hernia repair under spinal anaesthesia: a double-blind, prospective, randomized controlled trial. J Int Med Res. 2013;41:1160–1170. doi: 10.1177/0300060513490083. [DOI] [PubMed] [Google Scholar]
- 58.Techanivate A, Urusopone P, Kiatgungwanglia P, et al. Intrathecal fentanyl in spinal anesthesia for appendectomy. J Med Assoc Thail Chotmaihet Thangphaet. 2004;87:525–530. [PubMed] [Google Scholar]
- 59.Tyagi P, Srivastava A. Comparision of bupivacaine alone and its combination with different doses of fentanyl in spinal anesthesia for cesarean section: a prospective randomized study. Indian J Public Health Res Dev. 2013;4:19. [Google Scholar]
- 60.Unal D, Ozdogan L, Ornek HD, et al. Selective spinal anaesthesia with low-dose bupivacaine and bupivacaine + fentanyl in ambulatory arthroscopic knee surgery. J Pak Med Assoc. 2012;62:313–318. [PubMed] [Google Scholar]
- 61.Venkata HG, Pasupuleti S, Pabba UG, et al. A randomized controlled prospective study comparing a low dose bupivacaine and fentanyl mixture to a conventional dose of hyperbaric bupivacaine for cesarean section. Saudi J Anaesth. 2015;9:122–127. doi: 10.4103/1658-354X.152827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Peng X, Zhan L, et al. Effects of intrathecal bupivacaine and bupivacaine plus fentanyl in elderly patients undergoing total hip arthroplasty. J Coll Physicians Surg Pak. 2019;29:1133–1137. doi: 10.29271/jcpsp.2019.12.1133. [DOI] [PubMed] [Google Scholar]
- 63.Weigl W, Bierylo A, Wielgus M, et al. Analgesic efficacy of intrathecal fentanyl during the period of highest analgesic demand after cesarean section A randomized controlled study. Med U S. 2016;95:e3827. doi: 10.1097/MD.0000000000003827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Guo Q, Wang E, et al. Spinal anesthesia with low dose sufentanil-bupivacaine in transurethral resection of the prostate. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006;31:925–928. [PubMed] [Google Scholar]
- 65.Zohar E, Noga Y, Rislick U, et al. Intrathecal anesthesia for elderly patients undergoing short transurethral procedures: a dose-finding study. Anesth Analg. 2007;104:552–554. doi: 10.1213/01.ane.0000255329.55037.cd. [DOI] [PubMed] [Google Scholar]
- 66.Shende D, Cooper GM, Bowden MI. The influence of intrathecal fentanyl on the characteristics of subarachnoid block for caesarean section. Anaesthesia. 1998;53:706–710. doi: 10.1046/j.1365-2044.1998.329-az0482.x. [DOI] [PubMed] [Google Scholar]
- 67.Seewal R, Shende D, Kashyap L, et al. Effect of addition of various doses of fentanyl intrathecally to 0.5% hyperbaric bupivacaine on perioperative analgesia and subarachnoid-block characteristics in lower abdominal surgery: a dose-response study. Reg Anesth Pain Med. 2007;32:20–26. doi: 10.1016/j.rapm.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 68.Seyhan TÖ, Şentürk E, Şenbecerir N, et al. Spinal anesthesia in cesarean section with different combinations of bupivacaine and fentanyl. Agri. 2006;18:37–43. [PubMed] [Google Scholar]
- 69.Sertöz N, Aysel I, Uyar M. The effects of sufentanil added to low-dose hyperbaric bupivacaine in unilateral spinal anaesthesia for outpatients undergoing knee arthroscopy. Agri. 2014;26:158–164. doi: 10.5505/agri.2014.51422. [DOI] [PubMed] [Google Scholar]
- 70.Abdollahpour A, Azadi R, Bandari R, et al. Effects of adding midazolam and sufentanil to intrathecal bupivacaine on analgesia quality and postoperative complications in elective cesarean section. Anesthesiol Pain Med. 2015;5:e23565. doi: 10.5812/aapm.23565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aydın F, Akan B, Susleyen C, et al. Comparison of bupivacaine alone and in combination with sufentanil in patients undergoing arthroscopic knee surgery. Knee Surg Sports Traumatol Arthrosc. 2011;19:1915–1919. doi: 10.1007/s00167-011-1522-0. [DOI] [PubMed] [Google Scholar]
- 72.Bang YS, Chung KH, Lee JH, et al. Comparison of clinical effects according to the dosage of sufentanil added to 0.5% hyperbaric bupivacaine for spinal anesthesia in patients undergoing cesarean section. Korean J Anesthesiol. 2012;63:321–326. doi: 10.4097/kjae.2012.63.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Braga A de F de A, Braga FS da S, Potério GMB, et al. Sufentanil added to hyperbaric bupivacaine for subarachnoid block in caesarean section. Eur J Anaesthesiol. 2003;20:631–635. doi: 10.1017/s0265021503001017. [DOI] [PubMed] [Google Scholar]
- 74.Braga AA, Frias JAF, Braga FS, et al. Spinal anesthesia for cesarean section. Use of hyperbaric bupivacaine (10mg) combined with different adjuvants. Rev Bras Anestesiol. 2012;62:775–787. doi: 10.1016/S0034-7094(12)70178-2. [DOI] [PubMed] [Google Scholar]
- 75.Demiraran Y, Ozdemir I, Kocaman B, et al. Intrathecal sufentanil (1.5μg) added to hyperbaric bupivacaine (0.5%) for elective cesarean section provides adequate analgesia without need for pruritus therapy. J Anesth. 2006;20:274–278. doi: 10.1007/s00540-006-0437-2. [DOI] [PubMed] [Google Scholar]
- 76.Derakhshan P, Imani F, Koleini ZS, et al. Comparison of adding sufentanil and low-dose epinephrine to bupivacaine in spinal anesthesia: a randomized, double-blind, clinical trial. Anesthesiol Pain Med. 2018;8:e69600. doi: 10.5812/aapm.69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doger C, Yüksel BE, Canoler O, et al. Effects of intrathecal bupivacaine and bupivacaine plus sufentanil in elderly patients undergoing transurethral resection. Niger J Clin Pract. 2014;17:149–153. doi: 10.4103/1119-3077.127423. [DOI] [PubMed] [Google Scholar]
- 78.Donadoni R, Vermeulen H, Noorduin H, et al. Intrathecal sufentanil as a supplement to subarachnoid anaesthesia with lignocaine. Br J Anaesth. 1987;59:1523–1527. doi: 10.1093/bja/59.12.1523. [DOI] [PubMed] [Google Scholar]
- 79.Kaur M, Katyal S, Kathuria S, et al. A comparative evaluation of intrathecal bupivacaine alone, sufentanil or butorphanol in combination with bupivacaine for endoscopic urological surgery. Saudi J Anaesth. 2011;5:202–207. doi: 10.4103/1658-354X.82804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olofsson C, Nygårds EB, Bjersten AB, et al. Low-dose bupivacaine with sufentanil prevents hypotension after spinal anesthesia for hip repair in elderly patients. Acta Anaesthesiol Scand. 2004;48:1240–1244. doi: 10.1111/j.1399-6576.2004.00504.x. [DOI] [PubMed] [Google Scholar]
- 81.Vyas N, Sahu DK, Parampill R. Comparative study of intrathecal sufentanil bupivacaine versus intrathecal bupivacaine in patients undergoing elective cesarean section. J Anaesthesiol Clin Pharmacol. 2010;26:488–492. [PMC free article] [PubMed] [Google Scholar]
- 82.Waxler B, Mondragon SA, Patel SN, et al. Intrathecal lidocaine and sufentanil shorten postoperative recovery after outpatient rectal surgery. Can J Anaesth J Can Anesth. 2004;51:680–684. doi: 10.1007/BF03018425. [DOI] [PubMed] [Google Scholar]
- 83.Hakkim A, Kirubahar R, Kanna V, et al. Comparative study of 0.5% hyperbaric bupivacaine with sufentanil and 0.5% hyperbaric bupivacaine for spinal anesthesia. Int J Res Med Sci. 2015;3:3367–3371. [Google Scholar]
- 84.Ngiam SK, Chong JL. The addition of intrathecal sufentanil and fentanyl to bupivacaine for caesarean section. Singapore Med J. 1998;39:290–294. [PubMed] [Google Scholar]
- 85.Acharya B, Acharya K, Sigdel S, et al. Effect of low dose bupivacaine and fentanyl during elective cesarean section. J Anesth Clin Res. 2019;10:1–6. [Google Scholar]
- 86.Walsh KH, Murphy C, Iohom G, et al. Comparison of the effects of two intrathecal anaesthetic techniques for transurethral prostatectomy on haemodynamic and pulmonary function. Eur J Anaesthesiol. 2003;20:560–564. doi: 10.1017/s0265021503000899. [DOI] [PubMed] [Google Scholar]
- 87.Hamber EA, Viscomi CM. Intrathecal lipophilic opioids as adjuncts to surgical spinal anesthesia. Reg Anesth Pain Med. 1999;24:255–263. doi: 10.1016/s1098-7339(99)90139-6. [DOI] [PubMed] [Google Scholar]
- 88.Hu J, Zhang C, Yan J, et al. Sufentanil and bupivacaine combination versus bupivacaine alone for spinal anesthesia during cesarean delivery: a meta-analysis of randomized trials. PloS One. 2016;11 doi: 10.1371/journal.pone.0152605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bainbridge D, Martin J, Arango M, et al. Perioperative and anaesthetic-related mortality in developed and developing countries: a systematic review and meta-analysis. Lancet Lond Engl. 2012;380:1075–1081. doi: 10.1016/S0140-6736(12)60990-8. [DOI] [PubMed] [Google Scholar]
- 90.Ko S, Goldstein DH, VanDenKerkhof EG. Definitions of “respiratory depression” with intrathecal morphine postoperative analgesia: a review of the literature. Can J Anaesth J Can Anesth. 2003;50:679–688. doi: 10.1007/BF03018710. [DOI] [PubMed] [Google Scholar]
- 91.Rathmell JP, Lair TR, Nauman B. The role of intrathecal drugs in the treatment of acute pain. Anesth Analg. 2005;101:S30–S43. doi: 10.1213/01.ANE.0000177101.99398.22. [DOI] [PubMed] [Google Scholar]
- 92.Bauchat JR, Weiniger CF, Sultan P, et al. Society for Obstetric Anesthesia and Perinatology Consensus Statement: monitoring recommendations for prevention and detection of respiratory depression associated with administration of neuraxial morphine for cesarean delivery analgesia. Anesth Analg. 2019;129:458–474. doi: 10.1213/ANE.0000000000004195. [DOI] [PubMed] [Google Scholar]
- 93.Roberts GW, Bekker TB, Carlsen HH, et al. Postoperative nausea and vomiting are strongly influenced by postoperative opioid use in a dose-related manner. Anesth Analg. 2005;101:1343–1348. doi: 10.1213/01.ANE.0000180204.64588.EC. [DOI] [PubMed] [Google Scholar]
- 94.Kuipers PW, Kamphuis ET, Venrooij GE van, et al. Intrathecal opioids and lower urinary tract function. Anesthesiology. 2004;100:1497–1503. doi: 10.1097/00000542-200406000-00023. [DOI] [PubMed] [Google Scholar]
- 95.Schmelz M. Opioid-induced pruritus. Mechanisms and treatment regimens. Anaesthesist. 2009;58:61–65. doi: 10.1007/s00101-008-1478-8. [DOI] [PubMed] [Google Scholar]
- 96.Figueiredo Locks G de. Incidence of shivering after cesarean section under spinal anesthesia with or without intrathecal sufentanil: a randomized study. Rev Bras Anestesiol. 2012;62:676–684. doi: 10.1016/S0034-7094(12)70166-6. [DOI] [PubMed] [Google Scholar]
- 97.Negishi C, Lenhardt R, Ozaki M, et al. Opioids inhibit febrile responses in humans, whereas epidural analgesia does not: an explanation for hyperthermia during epidural analgesia. Anesthesiology. 2001;94:218–222. doi: 10.1097/00000542-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 98.Smith SW, Hauben M, Aronson JK. Paradoxical and bidirectional drug effects. Drug Saf. 2012;35:173–189. doi: 10.2165/11597710-000000000-00000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.