Abstract
Insulin resistance (IR) and cardiovascular disease (CVD) represent two universal public health hazards, especially in today’s Western societies. A causal-effect relationship has been established that links IR with CVD. The mediating mechanisms are perplexing, under ongoing, rigorous investigation and remain to be fully elucidated. IR is a condition encompassing hyperglycemia and compensatory hyperinsulinemia. It occurs when insulin is not capable of exerting its maximum effects on target tissues, including skeletal muscles, liver and adipose tissue. This alteration of insulin signaling pathways results in the development of cardiometabolic disorders, including obesity, dyslipidemia, low-grade inflammation, endothelial dysfunction and hypertension, all of which are predisposing factors for atherosclerosis and CVD. The management of IR can be achieved through dietary modifications, the inclusion of regular exercise routines in everyday life, pharmacological agents and other interventions tailored to each individual patient’s needs. It is important to underline though that, although various antidiabetic drugs that may improve IR are available, no medications are as yet specifically approved for the treatment of IR. This narrative review will focus on the current scientific and clinical evidence pertaining to IR, the mechanisms connecting IR with CVD, as well as plausible strategies for a holistic, personalized approach for IR management.
Keywords: Insulin, insulin resistance, inflammation, dyslipidemia, oxidative stress, endothelial dysfunction, hypertension, cardiovascular disease
Introduction
Insulin is a hormone involved in the metabolism of carbohydrates, lipids and proteins and is involved in cell growth and differentiation.1,2 Insulin resistance (IR) constitutes a significant health hazard in a global aspect. IR, which is closely related to metabolic syndrome (MetS), has been associated with the so-called Western lifestyle, characterized by high-calorie food consumption, limited physical activity and excessive stress.3 IR is a clinical condition in which tissues sensitive to insulin, namely skeletal and cardiac muscle, adipose tissue and the liver, demonstrate a diminished ability of glucose uptake due to a reduced biological effect of insulin in comparison with healthy individuals.4,5 The excess glucose in the bloodstream leads to hyperglycemia and indirectly to hyperinsulinemia with subsequent disruption of glucose metabolism.4–7 IR has been extensively linked with chronic low-grade inflammation and production of proinflammatory cytokines, such as tumor necrosis factor α (TNF-α), interleukin (IL)-6, IL-8, plasminogen activator inhibitor-1 (PAI-1) and monocyte chemoattractant protein-1 (MCP-1); their increased production is accompanied by elevated levels of C-reactive protein (CRP), a widely used inflammatory biomarker.8–11 IR can be assessed with various indices, such as fasting insulin levels, homeostasis model assessment-insulin resistance (HOMA-IR), serum-triglyceride-to-serum-high-density-lipoprotein-cholesterol-ratio, as well as by the most recently proposed, clinically valuable IR indices, triglyceride-glucose-neck circumference and triglyceride-glucose-neck circumference to height ratio.11,12 The gold standard for IR measurement is the glucose clamp technique. IR alone has been established as a separate risk factor for cardiovascular events, even in patients without diabetes mellitus.13–15
Cardiovascular disease (CVD) was responsible for 17.9 million lives lost in 2019; by 2030, this number is expected to increase to >22.2 million,16 making CVD the leading cause of death globally. The vast majority of cardiovascular events are attributed to atherosclerosis, in which lipid plaques form in the vessel walls.17 CVD has been extensively associated with chronic low-grade inflammation and involvement of both innate and adaptive immunity with macrophages being the protagonists of this process;17,18 thus, it becomes clear that the pathogenesis of CVD has an immunoinflammatory background. During the initial phase of atherosclerosis, endothelial injury triggers the production of proinflammatory cytokines like MCP-1, ILs, TNF-α and adhesion molecules, thus laying the foundation for increased production of CRP.19 As mentioned earlier, these same events occur in the setting of IR. Impaired insulin cellular signaling in IR alters glucose metabolism and endothelial dysfunction and may contribute to the acceleration of atherosclerosis.19,20 Moreover, numerous studies link IR with other health conditions, including obesity, non-alcoholic fatty liver disease (NAFLD), hypertension, polycystic ovary syndrome (PCOS) and heart failure (HF).21,22 IR and HF form a vicious cycle; IR is an independent risk factor for HF development, while HF may exacerbate whole body IR.22
This narrative review article shall attempt to present and discuss the current clinical and scientific data illustrating the pathophysiological connection between IR and CVD. In addition, potential therapeutic strategies for the treatment of IR will also be discussed.
Insulin: physiology, signaling and metabolism
In 1921, Frederick Banting and Charles Best made a groundbreaking discovery: a specific substance secreted from the pancreas, later named insulin, lowered blood glucose levels in canines. The first purification of insulin by James Collip and its use for the treatment of the first human patient with diabetes mellitus took place in 1922.23 The discovery of insulin paved the way for the subsequent effective treatment of type 1 diabetes (T1D), an incurable cause of death at that time, and endless scientific research. Since 1960, studies and experiments have demonstrated that biologically active human insulin is a 51 amino-acid protein composed of two peptide chains, A and B. There are 21 amino acids in chain A and 30 amino acids in chain B. The two chains are interlinked with two disulphide bonds, located in A7-B7 and A20-B19. An additional disulfide bond links amino acids 7 and 11 in chain A. In the presence of zinc, insulin forms hexamers.24,25
Insulin is derived from proinsulin, an inactive protein produced by beta cells in the islets of Langerhans. Its biological activity is precipitated by the cleavage of C-peptide in proinsulin, an event that further allows insulin to connect to the appropriate receptor.26 Once active, it is stored in granules inside the cells and then released via glucose signaling-induced exocytosis; glucose enters the cell and the pathway of glycolysis produces adenosine triphosphate (ATP) and leads to the closure of ATP-dependent potassium (K+) channels. Membrane depolarization is followed by the opening of voltage-gated calcium (Ca2+) channels, an increase in intracellular Ca2+ concentration and, finally, the exocytosis of insulin-containing granules and the release of insulin in the bloodstream.27,28 Insulin is then released into the portal vein system and enters the hepatocytes. A transmembrane glycoprotein, carcinoembryonic antigen-related cell adhesion molecule 1, is responsible for the clearance of 80% of the secreted insulin; its degradation seems to be reduced in IR, resulting in hyperinsulinemia and suggesting a possible correlation or even causation through desensitization. The remaining insulin molecules are transferred to insulin-sensitive tissues and exert their action via binding to insulin receptors.29
Insulin is an anabolic hormone with both metabolic and mitogenic effects. It exerts its function during postprandial hyperglycemia, mediates glucose uptake and storage in muscle cells and adipocytes, promotes glycogen synthesis, inhibits lipolysis and decreases gluconeogenesis in hepatocytes.1,6,30 Its function is exerted through binding to insulin receptors. The insulin receptor belongs to the subfamily of tyrosine kinase receptors, similar to the insulin growth factor 1 (IGF-1) receptor, a tetrameric glycosylated protein with two α and two β subunits integrated with the cell membrane.21,24 The receptor is activated when insulin binds to the α subunits, leading to autophosphorylation of tyrosine residues in β subunit domains and subsequent activation of various intracellular kinase cascades, including phosphorylation of phosphatidylinositol 3-kinase (PI3K), Akt-1 and Akt-2, and mammalian target of rapamycin.21,24 This leads to increased transport of glucose transporter 4 (GLUT4) to the surface of the membrane and glucose uptake by skeletal muscle cells and adipocytes.24 A reference regarding its action on vascular walls ought to be made; insulin can either protect or adversely affect the vessels via different kinase cascades.6 Its protective action is related to the activation of the PI3K cascade and the production of endothelial nitric oxide synthase (eNOS) and nitric oxide (NO), whereas its detrimental impact pertains to the activation of mitogen-activated protein kinase (MAPK) and vascular smooth muscle cell proliferation, inflammation and atherosclerosis.6
When insulin signaling malfunctions, as in the case of IR, glucose is not being appropriately used and cellular metabolism shifts to alternative pathways to obtain energy and preserve homeostasis. These alterations may actually represent some of the mechanisms responsible for the development of CVD.
Insulin resistance
Insulin resistance refers to the diminished response of insulin-sensitive tissues to insulin signaling.4,5,7 Insulin cannot exert its full biological activity, which is the facilitation of glucose entrance in cells to be utilized as the primary energy substrate. The excess glucose remains in the blood circulation, a condition named hyperglycemia, and tissues shift their metabolic pathways in response to inadequate energy uptake, underpinning disorders such as impaired adipose tissue function and obesity, inflammation, dyslipidemia, production of reactive oxidative species (ROS), atherosclerosis, endothelial dysfunction and hypertension, all of which are associated with the promotion of CVD.31,32
Obesity and impaired adipose tissue function
White adipose tissue (WAT) was once known to serve the sole purpose of storing energy in the form of lipids.33 However, over time, this perception has changed radically: adipose tissue is an endocrine tissue as well.34 WAT is either subcutaneous (SCAT) or visceral (VAT), which vary in distribution, morphology and function.35 Obesity is defined as a body mass index (BMI) ≥30 kg/m2.36 In obese individuals, excess fat is stored in the adipocytes of WAT, increasing their size and thus the overall tissue mass. The size of adipocytes is indicative of their function, as large adipocytes tend to be insulin-resistant, hyperlipolytic and resistant to the anti-lipolytic effect of insulin.37,38 Many comorbidities accompany obesity.39 In particular, IR and impairments in glucose metabolism are associated with increased lipid storage in VAT, although increased SCAT percentage also accounts for IR development. Nonetheless, obesity contributes to low-grade inflammation, activating the immune cells residing in the adipose tissue.40–43
Insulin promotes fatty acid and triacylglycerol synthesis, decreases the rate of lipolysis, increases the uptake of triglycerides from the blood circulation and decreases the rate of fatty acid oxidation in muscle and liver.44 As previously mentioned, obesity leads to IR and hyperlipolysis in adipocytes. IR activates hormone-sensitive lipase (HSL),45 leading to an increase of free fatty acids (FFA) plasma levels along with high dietary fat accumulation, namely lipotoxicity. Lipotoxicity is considered a risk factor for IR; high levels of FFA and, in particular, two specific metabolites, ceramide and diacylglycerol (DAG), intervene in proper insulin signaling by generating a negative feedback loop and suppression of insulin receptor substrate-1 (IRS-1).44 The contribution of elevated FFA plasma levels to IR is also clearly demonstrated in late-term pregnant women, who commonly develop IR in the presence of increased FFA levels.46 Clearly, IR and lipotoxicity create a vicious cycle and a proatherogenic profile.20,47,48 Mitochondrial dysfunction in obese WAT is also producing lipid metabolites, ultimately contributing to IR.49
Inflammation
Insulin resistance and an excess lipid pool also trigger inflammatory signaling pathways like c-Jun N-terminal kinase (JNK), IκBα kinase β and nuclear factor KB (NF-kB), resulting in the production of proinflammatory cytokines, such as TNF-α, IL-6, IL-1β, PAI-1, MCP-1, leptin and resistin, and a reduction of adiponectin levels.7,20,47,50 Proinflammatory cytokines shift adipose tissue macrophage polarization,51 especially in immune cell-rich VAT. Proinflammatory M1 macrophages are abundant in obese mice, while anti-inflammatory M2 are more prominent in lean subjects,52,53 the former further inducing the production of cytokines and thus maintaining the inflammatory state.
Tumor necrosis factor-α affects the expression of other inflammatory cytokines from adipocytes, such as IL-6, MCP-1 and PAI-1, inhibits peroxisome proliferator-activated receptor-γ2, GLUT454 and IRS-1, leading to IR, and increases FFA in the circulation through stimulation of lipolysis and inhibition of triglyceride (TG) synthesis.55
Interleukin-6 is a pleiotropic cytokine with its levels being elevated in obese individuals. IL-6 increases lipolysis and suppresses the expression of the IRS1 and GLUT4 genes. Hence, it is linked to IR, reduced glucose uptake in adipose tissue and impaired glycogen synthesis in the liver, while, on the other hand it sensitizes muscle cells for the effects of insulin.56,57
Interleukin-1β is secreted by macrophages as the final product of the NLR family pyrin domain-containing 3 (NLRP3) inflammasome activation. This cytokine has been related to type 2 diabetes (T2D) and atherosclerosis. Its deleterious effects on pancreatic β-cells in the islets of Langerhans are notable, along with its action upon endothelial cells, macrophages and smooth muscle cells. The NLRP3 inflammasome possesses a pivotal role in obesity-related insulin resistance. IL-1β is also a mediator for the production of other proinflammatory cytokines.58–60
Plasminogen activator inhibitor-1 is a serine protease inhibitor with pleiotropic functions. It is primarily involved in thrombosis, also mediating a connection between obesity, CVD and IR. The literature pertaining to studies regarding the latter is extensive. The gene that encodes for PAI-1 is upregulated in atherosclerotic plaques.61 TNF-α, IL-1β, very-low density lipoprotein (VLDL-C) and lipoprotein (a) constitute specific mediators, and PAI-1 itself is an acute phase protein, elevated in inflammatory states.61 A large cohort study revealed that healthy non-diabetic subjects who developed T2D in a 5-year interval presented significantly elevated PAI-1, fibrinogen and CRP levels compared with those who did not develop T2D.62 Elevated plasma PAI-1 levels and hypofibrinolysis are interconnected via IR and obesity.63 All of the aspects of the MetS, namely insulin, glucocorticoids, VLDL, FFA, glucose and angiotensin II, are associated with increased PAI-1 production. VAT secretes PAI-1, thus explaining its connection with obesity-related insulin resistance.64,65 The PAI-1 genotype seems to affect the vascular risk linked to hyperinsulinemia.66 A previous study also described a causality relationship between elevated PAI-1 plasma levels and coronary artery disease (CAD).67 Finally, a cross-sectional analysis based on the Framingham Offspring Study showed that elevated fasting insulin levels in impaired glucose tolerance conditions were associated with elevated PAI-1 plasma levels and, subsequently, impaired fibrinolysis.68
Monocyte chemoattractant protein, or chemokine (CC motif) ligand 2 (CCL2), is a chemokine secreted by skeletal muscle cells, smooth muscle cells, adipocytes and endothelial cells. When bound in its receptor, C-C chemokine receptor type 2, it promotes the recruitment of monocytes and T-lymphocytes in tissues. MCP-1 plays a prominent role in the pathogenesis of atherosclerosis69 and its high plasma levels have been correlated with plaque vulnerability. Injured endothelium secretes MCP-1 for its renewal, which amongst other effects, facilitates the migration of monocytes to the site of the lesion; these cells later become foam cells.70 Circulating MCP-1 levels are elevated in subjects with increased adipose tissue mass and T2D, but weight loss, exercise and thiazolidinediones (TZD) lower these levels.69 CCL2 seems to exert a direct effect on adipocytes, decreasing insulin-regulated glucose uptake and altering the expression of certain adipogenic genes.71
Leptin is a peptide produced and secreted from WAT and mediates appetite and feeding behavior. Its plasma levels are elevated in obese individuals and patients with CVD. Leptin reduces insulin synthesis, leading to a condition known as leptin resistance, hyperleptinemia and hyperphagia. Leptin increases proinflammatory cytokines’ production from immune cells and stimulates inflammatory pathways, such as MAPK and PI3. These facts confirm a clear correlation between leptin, IR and CVD.72,73
Resistin is another peptide secreted by macrophages, monocytes and bone marrow cells in humans. There is evidence that resistin contributes to IR and inflammation, although the association between resistin levels and IR remains controversial.72
Adiponectin is the only anti-inflammatory adipokine produced by adipose tissue during inflammation. Overall, it increases insulin sensitivity. Its levels tend to be lower in obese individuals. Inflammation is possibly the main factor suppressing adiponectin levels in IR. The anti-inflammatory effects of adiponectin extend to the cardiovascular system, as adiponectin seems to protect the endothelium and the smooth muscle cells. In a study, male patients with hypoadiponectinemia had a two-fold increased risk of developing CAD.74 Adiponectin also seems to modify the macrophage phenotype via a plethora of mechanisms.72–75
In addition to the aforementioned mediators, other less familiar yet significant hormones appear to influence IR, some of them acting as potential novel markers of metabolic disturbances.76 Irisin is a recently discovered myokine, highly expressed in skeletal muscles after exercise. Irisin enters the circulatory system and is able to convert WAT into brown adipose tissue. Other target tissues include skeletal muscles, the pancreas, the liver, the kidneys and the brain, especially the hypothalamus, although most of the mechanisms involved remain unknown. The net effect of irisin in tissues results in normoglycemia and normal lipid levels.77 Irisin alleviated IR related to FFA and lipotoxicity, ameliorated PI3K/Akt insulin signaling and impeded the Toll-like receptor 4/NF-κB inflammatory pathways in murine models.78 Another in vitro study concluded that PI3K/Akt activation by irisin inhibits autophagy in rat H9c2 cells, thus improving IR.79 Circulating levels of irisin are elevated in obese patients, diminished in patients with diabetes, and are positively correlated with enhanced insulin sensitivity.77,79 Moreover, irisin plays a significant role in atherosclerosis.80 It interferes with a plethora of intracellular pathways, potentiating direct or indirect vascular repair and alleviating vascular inflammation and crucial related disorders, discussed in the upcoming paragraphs, including dyslipidemia and oxidative stress.80
Apelin is another relatively recently discovered peptide hormone, initially extracted from bovine stomachs. Apelin is an endogenous ligand for the apelin receptor (APJ), a G-coupled protein receptor distributed abundantly in the human body. Apelin is mainly secreted by endothelial cells and adipose tissue and the apelin/APJ system exerts multiple systemic functions. Apelin improves insulin resistance and insulin secretion and diminishes serum glucose, glycosylated hemoglobin (HbA1c) and low-density lipoprotein cholesterol (LDL-C) levels. Apelin and pro-inflammatory factors also participate in an endless regulatory cycle, with apelin downregulating the activity of macrophages and decreasing MCP-1 and other chemotactic proteins and TNF-α, overall improving inflammatory states, although, under certain circumstances, it may potentiate inflammation.81 Serum apelin levels are found to be elevated in IR states and its direct administration is related to improvements in insulin sensitivity, while its secretion is largely regulated by insulin. Molecular mechanisms involve augmentation of glucose uptake via adenosine monophosphate-activated protein kinase (AMPK) and eNOS and inhibition of lipolysis via phosphorylation of HSL, leading to a decrease of FFA levels and, finally, reduced IR.82 Further basic and clinical evidence suggest that apelin is an insulin sensitizer and its elevated levels are linked with T2D rather than obesity.83 Concerning atherosclerosis, a case–control study on 60 Egyptian patients demonstrated a powerful correlation between serum apelin and the degree of carotid intima-media thickness, suggesting a plausible future application for apelin as a clinically useful prognostic biomarker.84
Visfatin is an insulin-mimetic adipocytokine and its function depends on nicotinamide adenine dinucleotide biosynthesis. Mainly, but not exclusively, produced by visceral fat, visfatin aids in maintaining insulin sensitivity and exerts its actions via phosphorylation of insulin receptors and activation of the PI3K and MAPK signaling pathways. Its levels appear to be elevated in obesity, compensating for abnormal serum glucose levels. Nonetheless, visfatin levels exceeding a certain value may act detrimentally and precipitate development of IR, endothelial dysfunction and inflammation, as it is widely expressed in foam cells.85 Thus, visfatin presents dose-dependent beneficial or proinflammatory properties.85 An in vitro study concluded that visfatin favors proinflammatory cytokine production and inhibits insulin signaling via the signal transducer and activator of transcription 3 (STAT3) and NF-kB pathways,86 while a meta-analysis of 14 articles demonstrated a positive correlation between serum circulating visfatin levels and IR, thus rendering it a valuable predictor of metabolic disturbances, amongst which IR, MetS and CVD.87
Fetuin-A is a hepatokine, which has been demonstrated to affect MetS and atherosclerosis and may act as either a positive or a negative acute phase protein. In patients with MetS, where the SCAT percentage is increased, an overproduction of fetuin-A occurs. This protein possibly contributes to adipose tissue inflammation acting as a chemoattractant and macrophage-polarizing agent. Concerning CVD, from a clinical perspective, decreased fetuin-A levels are associated with subclinical as well as with clinical CAD. On the other hand, fetuin-A levels have been shown to be significantly lower in the setting of ischemic cardiomyopathy, as compared with that of dilated cardiomyopathy, potentially providing fetuin-A with a discriminative power between these different types of HF.88 Results of different studies regarding fetuin-A confirm its contribution to the pathogenesis of IR; it seemingly impedes the maturation of β cells in the pancreatic islets,89 enhances apoptosis and damages pancreatic β cells when oversecreted,90 and is overexpressed at the initiation of a high-fat diet, concurrent with IR, in murine models.91 Additionally, fetuin-A along with a Western lifestyle are held accountable for the development of IR, vascular inflammation and atherosclerosis,92 as well as for the development of T2D in people of Pakistani origin.93 In addition, a previous study suggested that a pro-inflammatory diet might aide IR and T2D development via fetuin-A.94 Moreover, a thorough meta-analysis comprising more than 110 00 participants concluded that increased circulating levels of fetuin-A were linked to an elevated risk of T2D.95 Serum fetuin-A levels have been proposed to be used as a potential biomarker regarding IR and PCOS.96 Elevated serum levels of fetuin-B, a second member of the fetuin family in mammals, are also linked to impaired glucose metabolism, inducing IR and chronic inflammation, with hepatic steatosis apparently being the common denominator.97–100 Its expression is modified via upregulation of the leptin-STAT3 signaling cascade.101 Along with fetuin-A, it can also be a potentially useful biomarker in PCOS.102 Interestingly, other studies suggest that fetuin-A and fetuin-B may alter glucose metabolism in a different manner,103 or may present inconsistent findings regarding their metabolic effects.104 There is also some evidence suggesting a non-causal relationship between fetuin-B and IR.105
Adropin is a peptide with pleiotropic functions. Interest has been focused on glucose metabolism, as literature reports evidence of a reduction of hepatic gluconeogenesis, activation of the GLUT4 receptor, stimulation of insulin sensitizing cascades, such as Akt, improvement of lipid profile and most importantly, inhibition of inflammation. TNF-α and IL-6 are reduced and the endothelium is protected via effects exerted on eNOS.106 With regard to liver glucose synthesis, researchers demonstrated that adropin sensitizes the AMPK pathway by acting on protein phosphatase 2 (PP2A).107 A few studies also demonstrated the association between elevated plasma adropin levels and glucose and lipid homeostasis and insulin sensitivity, whereas its absence predisposes to obesity and IR.108–110
Sodium-glucose cotransporter 2 inhibitors (SGLT2-i), which will be discussed later, are instrumental in regulating atherosclerosis-related inflammation. Their mechanism of action includes attenuation of M1 macrophages and foam cell formation, enhancement of anti-inflammatory M2 macrophages and peroxisome proliferator-activated receptor-gamma (PPAR-γ) signaling, reduced oxidation of LDL-C and reduced production of pro-inflammatory cytokines, tipping the balance in favor of an anti-inflammatory state and regression of atherosclerotic plaque formation.111
Dyslipidemia
Type 2 diabetes mellitus and IR are associated with dyslipidemia; the combination of these entities may further increase the risk for CVD. For example, the Framingham Heart Study (FHS) and later associated studies have demonstrated a correlation between abnormal circulating cholesterol levels and CVD for more than half a century;112,113 setting the foundation for determining certain risk profiles and scores, including the well-known Framingham Risk Score for coronary heart disease (CHD).114 Total cholesterol and high-density lipoprotein cholesterol (HDL-C) plasma levels are among the score parameters. On the other hand, the ARIC study has also demonstrated an increased relative risk of CHD, and therefore CVD, with increased LDL-C and especially small dense LDL-C (sdLDL-C) levels.115 IR is the culprit behind diabetic dyslipidemia and is characterized by decreased levels of HDL-C and increased levels of sdLDL-C, both metabolically linked to each other through hypertriglyceridemia or elevated VLDL-C plasma levels.116 Approximately 75% of T2D patients present with dyslipidemia;117 it precedes T2D and constitutes an early event in the pathogenesis of atherosclerotic CVD.118 In the Look AHEAD study, a higher risk of adverse CVD outcomes was found in overweight and obese patients with T2D and dyslipidemia.119 Studies suggest causality between hypertriglyceridemia and CVD;120–122 TG levels >500 mg/dl (>5.7 mmol/l) are associated with increased CVD risk and mortality.123 Another study demonstrated a strong correlation between hypertriglyceridemia and sdLDL-C with myocardial infarction (MI) and peripheral atherosclerosis.124
In the IR state, insulin, despite its already high levels, cannot exert its inhibitory effects toward VLDL-C secretion from the hepatocytes or degradation of apolipoprotein B (apoB), leading to the elevation of triglycerides in the circulation; these levels are also sustained at a high value due to the concurrent release of FFA, as part of IR-related adipose tissue dysfunction. Lipoprotein lipase (LPL) activity is also affected and the clearance of VLDL-C is impaired. Hypertriglyceridemia ultimately leads to the generation of the highly atherogenic sdLDL-C, although the total LDL-C levels appear to increase only mildly, and also leads to lower quality and quantity of HDL-C. The role of cholesteryl ester transfer protein is crucial in enriching LDL-C and HDL-C with TG, further inducing their lipolysis by hepatic lipase. As a result, sdLDL-C plasma levels increase, whereas HDL-C plasma levels and apolipoprotein A-I diminish.125 In fact, TG to HDL-C ratio is a widely used index indicative of IR development,126 although it may not necessarily represent an independent prognostic factor for CVD development after taking into account the already established traditional risk factors, as demonstrated by a cohort study conducted on postmenopausal women.11 The sdLDL-C particles are particularly proatherogenic for various reasons: their smaller size facilitates their entrance into the vessel walls; they have impaired affinity with the LDL-C receptor; a longer half-life and lower resistance to oxidation.127,128
Oxidative stress
A contemporary definition of oxidative stress focuses on the incapability of endogenous cellular mechanisms to maintain redox homeostasis, mainly because of the disruption of redox signaling. Abundant reactive oxygen species (ROS) and nitrogen species, either radical or non-radical, and deficiency of antioxidant mechanisms are the main components of oxidative stress. Focusing on ROS, inflammation majorly contributes to their genesis, enhanced by the accumulation of polymorphonuclear cells and macrophages.129 Odd electrons derived from cellular respiration and other metabolic processes generate superoxide and hydrogen peroxide, the primary oxidants that cause cellular and molecular damage, also giving rise to reactive species like peroxynitrite, singlet oxygen and hypochlorous acid.129 Oxidative stress has a detrimental impact on DNA, proteins and lipids and participates in the formation of advanced glycation end-products (AGEs) in hyperglycemic conditions, while being also an essential contributing factor to the formation of atherosclerotic plaques.130
Oxidized LDL-C (oxLDL-C) is produced when ROS indirectly oxidize apoB-100 and modify the original LDL-C, rendering it a crucial factor in atherogenesis. Smoking also directly oxidizes LDL-C. OxLDL-C is capable of binding to specific receptors on endothelial cells, known as lectin-like oxidized LDL receptors-1. The uptake of oxLDL-C by endothelial cells promotes matrix metalloproteinase (MMP) production, downregulates eNOS, thus impairing vasodilation ability, induces leukocyte adhesion to the endothelium and renders the endothelium prothrombotic. OxLDL-C also binds to scavenger receptors (SR) on macrophages, yielding lipid-rich foam cells, a core element of plaques. Excess ROS are additionally held accountable for stimulating smooth muscle cell migration and SR expression, as well as collagen deposition to the injured endothelial site, acting as a link between innate and adaptive immunity and inducing the release of MMPs, which ultimately lead to rupture of the plaques’ fibrous caps.131,132 They also lead to dismantled insulin function and adipokine dysregulation.31 ROS are generated by various systems, namely nicotinamide adenine dinucleotide phosphate oxidase (NOX), xanthine oxidase, mitochondrial enzymes, myeloperoxidases and uncoupled eNOS.131,132 All systems contribute to atherosclerosis. However, NOX is the most crucial ROS generator system in the cardiovascular system, with the NOX2 subunit being of significant importance in atherosclerosis, increasing superoxide production and reducing the bioavailability of NO.133 On the other hand, dysfunction of the myeloperoxidase system and smoking potentiate alterations in MAPK signaling, which mediates inflammation, cell proliferation and atherosclerosis;131 MAPK is associated with the mitogenic effects of insulin, as previously mentioned.6
Advanced glycation end-product generation occurs when cellular protein and lipid molecules are constantly exposed to elevated glucose levels, as in IR and diabetes; and AGEs are the cause of diabetic complications, such as diabetic retinopathy, nephropathy and CVD.134,135 Hyperglycemia favors their formation, induces chronic inflammation biomarkers and participates in ROS formation.6,136 AGEs activate NOX, inhibit eNOS activity and interact with the extracellular matrix to promote ROS generation after binding to AGE receptors, inducing further endothelial damage and reduced NO production.135 AGEs also stimulate oxidative pathways, such as the protein kinase C signaling cascade.137 Ultimately, AGEs are core elements in the generation of excess ROS levels.
Endothelial dysfunction
The endothelium comprises the innermost single-cell layer of the arterial wall and its functions are a cornerstone in maintaining vascular integrity. Vascular homeostasis ensures oxygen and nutrient transportation to tissues, conservation of optimal vascular tone, regulation of hemostasis and the inflammatory response. When endothelial cells cannot exert their function properly, conditions like atherosclerosis and hypertension rise. NO is a small lipophilic molecule that mediates vascular tone and its bioavailability determines vascular homeostasis, being the most potent vasodilator. It also has anti-inflammatory, antioxidant and anti-coagulant properties and inhibits leukocyte adhesion and smooth muscle cell proliferation.138–140 NO is produced from L-arginine by nitric oxide synthase (NOS) isoforms with the aid of various co-factors. NOS3, or eNOS, is abundant in endothelial cells. Post-translational modifications of eNOS along with ROS create the uncoupled form of eNOS and thus decrease NO production, which is also diminished by reduced availability of substrates or co-factors and increased NO breakdown.141,142 NOS2, or inducible NO synthase (iNOS), is widely expressed in macrophages, stimulated by inflammatory signals and regulated by pathways and agents linked to IR, namely MAPK and NF-kB. Finally, NOS1, or neuronal NO synthase, is found mainly in the ventromedial hypothalamus.143–145
Insulin mediates cardiovascular events via the L-arginine/NO pathway, and these elements appear to be part of a vicious, never-ending cycle.146 Insulin enhances endothelial NO production and vasodilation via the PI3K cascade activation; however, in IR, insulin’s action is shifted towards vasoconstriction, hypertrophy of smooth muscle cells and accelerated atherosclerosis via activation of the MAPK pathway.146 Vascular IR is prominent in obese and diabetic patients and increased vascular resistance related to obesity and diabetes is mainly attributed to diminished NO bioavailability. Defective NO synthesis in the vessels and impaired vasodilation are linked to hyperinsulinemia and IR, a state in which oxLDL is also elevated, as previously mentioned. In addition, elevated LDL-C levels extensively contribute to downregulating eNOS expression.147,148 Obesity, accompanied by IR, diminishes the expression of eNOS. Anti-obesogenic and insulin-sensitizing abilities are attributed to eNOS-derived NO; eNOS additionally mediates insulin and glucose transport in skeletal muscle and adipocytes and regulates gluconeogenesis. Absence of iNOS has been linked with ameliorated glucose tolerance and insulin sensitivity, whereas its overexpression in liver promotes hyperglycemia, hyperinsulinemia and hepatic IR. NOS1-derived NO is related to appetite stimulation and hyperphagia.149 Nevertheless, glucose metabolism and insulin-stimulated NO production share common signaling pathways, underpinning a coupling between insulin’s metabolic and vascular effects.150 Interestingly, a previous study demonstrated that glucosamine is a potent inhibitor of endothelial NO production; glucosamine production is enhanced by chronically elevated plasma glucose levels.148 Overall, inadequate bioavailability of NO in the endothelium abolishes vasodilation and results in hypertension and inflammation while facilitating arterial stiffness, a precursor and predictor of CVD.151
Hypertension
Hypertension is one of the most common clinical predicaments that stem from increased peripheral vascular resistance and, in some cases, increased cardiac output. Increased basal sympathetic tone activity and overactivation of the renin–angiotensin–aldosterone system (RAAS) characterize hypertension.152 Hypertensive patients tend to be hyperinsulinemic and glucose intolerant.153 As suggested by a meta-analysis of 10 230 hypertension patients, fasting insulin levels and IR constitute independent factors for hypertension development; the relative risk of hypertension for fasting insulin concentrations was 1.54, with a higher risk in women compared with men, and the RR for HOMA-IR, an IR index, was 1.43.153 A previous study used the euglycemic hyperinsulinemic clamp method to demonstrate that 25% of the hypertensive subjects had concurrent IR.154 On the other hand, other estimations predict that 50% of hypertensive patients are insulin resistant,155 not to mention the recent finding that the triglyceride-glucose (TyG) index is a potentially useful index for IR screening in Asian patients with hypertension.156 Association between increased plasma insulin levels and elevated plasma catecholamine levels, evidence demonstrating IR development in rodents fed a high-carbohydrate diet, as well as prospective epidemiological studies linking hyperinsulinemia with CAD have been enforcing the establishment of a relationship between IR, hypertension and CVD for more than 30 years.157
Insulin regulates endothelial NO production and the secretion of endothelin-1 (ET-1), a strong vasoconstrictor agent. The balance between vasorelaxation and vasoconstriction in IR tilts towards the latter, leading to hypertension and endothelial dysfunction. Decreased NO production interferes with renal vascular tone, inducing vasoconstriction and increased sodium reabsorption.155,158 RAAS consists of hormones essential to maintaining homeostasis in arterial blood pressure. Renin converts angiotensinogen to angiotensin (Ang) I, which is in turn converted to Ang II by angiotensin converting enzyme (ACE). Ang II induces an increase in blood pressure via AT1 and AT2 receptors. In short, Ang II stimulates vasoconstriction and promotes sodium retention, acting on proximal tubules and the adrenal zona glomerulosa and releasing aldosterone, which also retains sodium and water in the distal tubules. Increased RAAS expression in WAT, especially in VAT, is associated with an increase in BMI. RAAS activity and increased body weight are positively correlated, and RAAS activity is decreased after weight loss. Adipose RAAS induces and exacerbates IR; Ang II and aldosterone disturb insulin-dependent glucose uptake and generate ROS, further promoting IR.159 Hyperinsulinemia is involved in a vicious cycle of vasculopathy, smooth muscle cell proliferation, atherogenesis, cellular calcium overload and renal sodium reabsorption.160 In addition, it might synergistically activate the MAPK pathway along with RAAS.161 In diabetic patients, RAAS is upregulated with prominent elevations in plasma renin, arterial pressure and renal vascular resistance. On the other hand, losartan had a better antihypertensive result in hyperglycemic than normoglycemic patients. Hyperglycemia aids local Ang II production and enhances the tissue response to it, produces AGEs, which also cause stimulation of the Ang II/AT1R pathway, and finally downregulates ACE2, an enzyme producing Ang 1-7, causing a further imbalance in RAAS. In general, RAAS antagonists can reverse the diabetes-induced RAAS activation and its effects on hypertension and vasculopathy.162 Furthermore, certain antidiabetic mediations exerting protective cardiovascular effects, such as SGLT2-i and glucagon-like peptide-1 (GLP-1) receptor agonists, appear to favorably interact with RAAS.163 Finally, 10–15% of hypertensive patients, >50 years old, present with atherosclerotic renal artery stenosis (ARAS), a clinical condition predisposed by classic atherosclerotic risk factors, including MetS and diabetes mellitus. As the prevalence of MetS is increased in both patients with ARAS and patients with peripheral artery disease, and as patients with ARAS frequently have co-existing peripheral atherosclerotic lesions, it appears safe to postulate that both IR states and renal artery atherosclerosis are contributing to hypertension.164–166
A very recent large Chinese nationwide, prospective, cohort study, which was conducted on 111 576 adults without CVD at baseline, elucidated the causal relationship between IR and CVD in relation to the glucose tolerance status.167 The study reached the following conclusions: (i) glucose intolerance status exacerbated the association between IR and CVD; (ii) prediabetic obese adults with IR were at a higher risk for CVD; (iii) in diabetic patients, IR increased the risk for CVD; however, this risk was not further increased by the presence of obesity.167
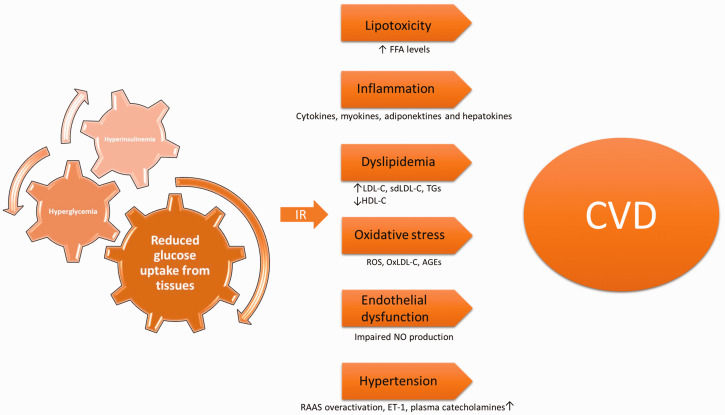
A brief summary of the mechanisms by which IR promotes CVD is shown in Table 1. In addition, a schematic depiction of the pathophysiological factors linking IR with CVD is shown in Figure 1.
Table 1.
A brief summary of the mechanisms by which insulin resistance promotes cardiovascular disease.
| Mechanism | Mediators and systems involved |
| Lipotoxicity | Increased free fatty acid plasma levels |
| Inflammation | Involved cytokines: tumor necrosis factor-α, interleukin-6, plasminogen activator inhibitor-1, monocyte chemoattractant protein-1, leptin, adiponectin |
| Dyslipidemia | High levels of low-density lipoprotein cholesterol, small dense low-density lipoprotein cholesterol, hepatic triglycerides |
| Low levels of high-density lipoprotein cholesterol | |
| Oxidative stress | Increased production of reactive oxygen species and nitrogen species |
| Oxidized low-density lipoprotein cholesterol | |
| Advanced glycation end-products | |
| Endothelial dysfunction | Disrupted vascular homeostasis due to impaired nitric oxide production |
| Hypertension | Renin–angiotensin–aldosterone system overactivation |
| Endothelin-1 | |
| Elevated plasma catecholamines |
Figure 1.
Schematic depiction of the pathophysiological factors linking insulin resistance (IR) with cardiovascular disease (CVD). FFA, free fatty acids; LDL-C, low-density lipoprotein cholesterol; sdLDL-C, small dense LDL-C; TGs, triglycerides; HDL-C, high-density lipoprotein cholesterol; ROS, reactive oxygen species; oxLDL-C, oxidized LDL-C; AGEs, advanced glycation end-products; NO, nitric oxide; RAAS, renin–angiotensin–aldosterone system; ET-1, endothelin-1.
Management of insulin resistance
Modification in lifestyle choices along with medications constitute the core of the management of IR states. Nutritional guidelines and meta-analyses propose a low glycemic index diet, preferably low-carbohydrate, rich in fibers (both soluble and insoluble), with an adequate protein and low-fat content, combined with at least 150 minutes of moderately intensive aerobic exercise, or 75 min of strenuous aerobic activity, or a combination of them, per week. These nutritional routines have been proved to improve IR, decrease BMI due to fat loss, reduce HbA1c and render the gut microbiota more balanced. Plant-based proteins and carbohydrates induced more notable effects toward improvement of IR and achievement of target weight. Undoubtedly, clinicians ought to personalize and tailor dietary and exercise plans to each patient’s metabolic profile, preferences, habits and lifestyle for maximum efficacy.168,169
Dietary adjustments and bariatric surgery
A 24-week randomized, open, parallel study on 74 patients with T2D, assigned an isocaloric, calorie-restricted vegetarian diet versus a conventional diabetic diet to the experimental and control groups, respectively, along with exercise in the second half of the study.170 The findings demonstrated that insulin sensitivity improved significantly with vegetarian meals compared with the control group: 30% (95% confidence interval [CI] 24.5, 39) versus 20% (95% CI 14, 25), respectively (P = 0.04).170 The reduction of IR may be attributed to the concurrently observed weight loss, visceral fat loss and the amelioration of oxidative stress markers, as the beneficial adaptations in these factors were also greater in the experimental group.170 An excellent example of a primarily plant-based and holistically balanced diet is the Mediterranean diet (MedDiet), which has copious amounts of vegetables, whole-grain meals, seeds, fruits and extra virgin olive oil (which alone has been studied and solidly proven to prevent CVD),168 moderate consumption of white meat, eggs and dairy and low consumption of red meat and animal-derived fat. Its structure provides polyunsaturated fatty acids, anthocyanins, resveratrol and polyphenols, all of which contribute to a decrease of inflammation and lipotoxicity, an increase of insulin-dependent glucose uptake, neuroprotection, improvement of hyperinsulinemia and hyperandrogenemia, and even mediate gene transcriptions with favorable effects on glycose homeostasis, atherosclerosis and tumorigenesis.171 The PREDIMED study, a multicentered, randomized, nutritional intervention trial enrolling 7447 subjects, was conducted in Spain and confirmed that the MedDiet is a valuable tool in the primary prevention of CVD, providing a 30% risk reduction in the incidence of major cardiovascular events with a per-protocol (adherence-adjusted) reduction of 58% over a median follow-up of 4.8 years.172 Assessment of the effects of the MedDiet has also been made in obese pediatric and adolescent patients. For example, an open-label study in Mexico selected 49 children from another study investigating the management of T2D in pediatric subjects and randomly assigned them to the MedDiet versus a standard diet.173 Children that followed the MedDiet presented a 10.5% decrement (95% CI –13.1, –7.7) in glucose levels compared with a 4.9% decrease (95% CI –8.1, –1.7) in the standard diet group, as well as a significant decrease in the frequency of glucose >100 mg/dl, a 45% decrease in MetS and a significant decrease in BMI.173
Nevertheless, another study disputes the supremacy of the MedDiet, suggesting that high-protein dietary patterns achieve better results in minimizing IR.174 In 16 women that completed the 21-day randomized, controlled, inpatient crossover feeding trial, the high-protein diet reduced IR more effectively than the MedDiet, while improving glycemic variability and favorably altering the gut microbiota.174 Either way, low-carbohydrate intake is generally well tolerated in humans who can utilize ketone bodies as an alternative fuel. Ketotic states maintain low insulin levels, improve insulin sensitivity, potentiate the breakdown of fat stores and spare lean muscles, while improving metabolic and inflammatory parameters; hence, ketosis could also be helpful for lean weight loss, fat loss and improvement of insulin resistant states.175 Finally, a systematic review pertaining to a ubiquitous IR manifestation in females, PCOS, assessed dietary interventions on 1193 participants with PCOS.176 Results revealed that IR markers and body composition might be optimally benefited from the Dietary Approaches to Stop Hypertension diet and calorie-restricted diets, respectively, with results comparable with metformin.176
Typical eating patterns in modern societies comprise at least three meals a day. Routines based on intermittent fasting (IF), namely early-time restricted feeding (eTRF), have been the main subject of interest for various studies. IF and periodic fasting in rodents may be able to delay the development and evolution of various diseases, including diabetes, CVD, Alzheimer’s disease and stroke. IF has also been proven to be efficient in humans, prompting weight loss and favorably affecting IR and other risk factors for CVD. A randomized controlled trial tested the efficacy of eTRF in prediabetic men compared with a control diet for 5 weeks.177 This pattern suggests a 6-h eating period with dinner prior to 15:00, followed by an extended fasting period; all patients that completed the study exceeded expectations with regard to compliance. Results concluded that eTRF is useful in lowering insulin levels and arterial blood pressure, improving insulin sensitivity, and reducing appetite and oxidative stress, thus rendering it an efficient strategy for prediabetic and prehypertensive patients with no apparent significant impact on lipid profile, arterial stiffness or inflammatory markers. Overall health improvement relies on signaling cascades related to mitochondrial function, DNA repair mechanisms and autophagy.177,178 Another randomized controlled trial studied the impact of intermittent and continuous energy restriction (IER and CER, respectively) on metabolic disease markers and weight loss in 107 premenopausal women with a BMI varying from 24 to 40 kg/m2.179 Both IER and CER were equally effective in weight loss (–6.4 kg [95% CI –7.9, –4.8 kg] for IER versus –5.6 kg [95% CI –6.9, –4.4 kg] for CER).179 There was a more significant impact of IER on IR reduction and fasting insulin levels: the difference between the groups for fasting insulin was –1.2 µU/ml (95% CI –1.4, –1.0 µU/ml); and for insulin resistance it was –1.2 μU/mmol/l (95% CI –1.5, –1.0) (both comparisons P = 0.04).179 Reductions in leptin, androgens, high-sensitivity CRP, TC and LDL-C, TGs and arterial blood pressure were comparable.179
Gut microbiota alterations in IR, diabetes and obesity is a significant, ongoing field of investigation. There is some evidence suggesting that overweight people present an altered Bacteroidetes/Firmicutes ratio, with an increase in Firmicutes and a decrease in Bacteroidetes, diminished bacteria with anti-inflammatory properties, an abundance of pathogens and less microbial diversity. Diet modifications may change the gut microbiota rapidly and may even predispose to inflammation. Intestinal permeability is disturbed in overweight conditions. High-fat and low-fiber intake, as in the Western diet, enhances the translocation of lipopolysaccharides due to increased growth of Gram-negative bacteria and subsequent endotoxemia; this metabolic endotoxemia triggers toll-like receptor 2-induced inflammation from adipocytes, rendering it a pathogenic factor for IR. These acknowledgements lay the foundation for a potential therapeutic approach based on an appropriately structured diet, possibly enriched with probiotic and prebiotic supplements to form a healthy gut microbiota. However, evidence to date is not convincing.180
Vitamin D deficiency characterizes patients with T1D and T2D, and numerous observational and preclinical trials underline the importance of this nutrient in their pathogenesis; 70% of prediabetic patients with hypovitaminosis D eventually become diabetic. Vitamin D receptors are abundant in pancreatic beta cells and in immune cells, plausibly explaining why vitamin D potentiates improvement in insulin sensitivity and inflammatory states. Hence, nutritional vitamin D supplementation might retard the conversion of prediabetes to diabetes and improve IR by up to 60%, also favorably affecting other conditions related to IR, such as PCOS and MetS. Dosage must be calibrated accordingly, taking into account parameters like the degree of deficit, age, possible bariatric surgery status and other malabsorption factors.181 Nonetheless, the hypothesis that vitamin D supplementation may potentiate insulin sensitivity, thus improving IR, is supported mainly by observational studies, whereas pertinent relative clinical evidence to this day has not been conclusive.181 In a randomized clinical trial, 2423 prediabetic adults were assigned to receive either vitamin D3 (4000 IU daily) or placebo on a daily basis.182 Within 2.5 years, 9.39 and 10.66 events of diabetes per 100 person-years occurred, respectively, with the hazard ratio for vitamin D being 0.88 compared with placebo (P = 0.12 not significant), suggesting that vitamin D supplementation, even at the maximum recommended daily dose, does not necessarily contribute to the prevention of T2D development.182
Metabolic or bariatric surgery is the most effective therapeutic choice for obese patients leading to weight loss and amelioration of diabetes and IR. Meta-analyses report that, along with weight loss, diabetes remitted after bariatric surgery, with a chance for remission being as high as 65%, and simultaneous favorable 5-year changes in glycated hemoglobin were also noted. These improvements were noticed shortly after the performance of the operation; some suggested mechanisms include alterations in gut microbiota and decreased caloric intake with depletion of liver fat and improvement of IR and the associated inflammatory profile. A systematic review of the literature, which assessed 19 543 patients subjected to metabolic surgery, demonstrated a reduction in the incidence of cardiovascular risk factors, such as T2D, hypertension and dyslipidemia, in those patients, which would potentially lead to a reduction in the occurrence of adverse cardiovascular events.183 Notably, in this study, 73% of the patients with T2D presented resolution or remission of diabetes.183 Additionally, following bariatric operations, adaptive immunity shifts in favor of an anti-inflammatory state, along with an increase of anti-oxidant properties and reduction of oxidative stress, thus establishing an overall low-inflammation phenotype and a reduction in IR.184
Exercise
The link between exercise, longevity and delayed onset of diseases has been noted for more than 2000 years by Hippocrates and was proven epidemiologically for the first time in the 1950s when sedentary lifestyle was correlated to increased prevalence of CHD. T2D development can be delayed or even reversed in prediabetic patients with systematic exercise, regardless of race. Studies in China, Finland and the US concluded that the onset of T2D was reduced by 46% in 6 years with exercise. On the other hand, diet alone reduced T2D by 31%. A combination of diet and exercise increased that percentage to 58%, which was more efficacious than metformin alone. During exercise, muscle fiber contraction generates metabolic and mitochondrial adaptations, facilitating insulin-dependent glucose uptake by skeletal muscles, thus improving hyperinsulinemia and promoting cross-talk between tissues through myokine secretion. Myokines released from muscles after bouts of exercise have an endocrine function and seem to increase GLP-1 secretion, lipolysis and glucose uptake by adipocytes, all of which are related to the promotion of insulin sensitivity.185 High-intensity interval training and high-intensity functional training effectively improve all health markers, induce the secretion of vascular endothelial growth factor, an enhancing factor for NO production, and stimulate anti-oxidant enzymes and IGF-1, all favorably affecting insulin sensitivity.186
Exercise has been shown to play a strong beneficial role in patients with MetS. For example, a study of 19 223 men demonstrated that the relative risk (RR) for all-cause and CVD mortality was 1.29 and 1.89, respectively, for men with MetS compared with healthy men.187 However, this difference was smoothed out and became non-statistically significant once cardiorespiratory fitness (CRF) was included in lifestyle, with RR being 0.98 for all-cause and 1.23 for CVD mortality.187 CRF impact was dose-dependent.187 Another trial on the same group demonstrated the alleviation of statistical significance of RRs for all-cause and CVD mortality in normal, overweight and obese people with and without MetS after the inclusion of CRF in their lifestyles.188 Time spent on physical activity is another determinant, with 60 minutes a week of leisure-time vigorous physical activity significantly reducing rates of MetS among unfit and fit men.189 A meta-analysis of the impact of structured exercise programs on IR in 846 diabetic patients demonstrated clear evidence for the effectiveness of structured exercise programs in reducing IR in patients with T2D.190 Another randomized controlled trial confirmed the effectiveness of an 8-week aerobic exercise protocol on diabetic women in decreasing glucose plasma levels, insulin levels and IR with HOMA-IR being the evaluation tool.191
Current literature involving numerous studies suggests that resistance training enhances insulin sensitivity and glucose tolerance among different population groups, including young and old subjects, postmenopausal women and hypertensive or diabetic patients. Emerging evidence also suggests that aerobic exercise and resistance training provide benefits through distinct mechanisms of action, which are worthy of further investigation.192 Resistance training in diabetic adults was found to be more effective than aerobic training at increasing maximal oxygen consumption within 12 weeks or longer with no apparent differences in HbA1c, BMI and lipid profile.193 Exercise intervention is also strongly recommended with regard to juvenile IR, as it can moderately improve insulin levels and IR.194 Another meta-analysis involving obese children and adolescents showed that physical exercise improved fasting insulin levels and HOMA-IR (fasting insulin: –3.37 μU/ml [95% CI –5.16, –1.57 μU/ml]; I2 = 54%; P = 0.003; HOMA-IR: –0.61 [95% CI –1.19, –0.02]; I2 = 49%; P = 0.040), but not fasting glucose levels.195 Evaluation of aerobic training, resistance training or their combination showed that aerobic exercise was clearly more efficient in reducing insulin levels and HOMA-IR (fasting insulin: –4.52 μU/ml [95% CI –7.40, –1.65 μU/ml]; I2 = 65%; P = 0.002); HOMA-IR: –1.33 [95% CI –2.47, –0.18]; I2 = 73%; P = 0.005).195
Medications
It ought to be mentioned that there are currently no medications approved specifically for the treatment of IR. However, various studies confirm the efficacy of certain antidiabetic drugs in reducing IR, including metformin, TZDs, SGLT2-i and GLP-1 receptor agonists, which will be described below.
Metformin is an oral biguanide and one of the oldest antidiabetic drugs in use. Its glucose lowering effects rely on the inhibition of hepatic glucose production, reduction of lipid secretion from intestinal cells and increased fatty acid oxidation in muscle cells and adipocytes. Therapeutic doses are beneficial in hepatic cellular respiration. Its action in intestinal cells is notable, affecting oxidative phosphorylation, glycolysis, lactate production and the gut microbiota, though doses exceeding the recommended range interfere with mitochondrial respiration.196 Metformin potentiates the increase of GLUT4 production and expression, as confirmed by numerous trials on animals and humans, including women with PCOS, thus facilitating glucose uptake. Involved mechanisms include interference with the insulin signaling pathway, activation of AMPK signaling pathways and GLUT4 transport mediators and epigenetic modification, suggesting that metformin improves IR via AMPK dependent and independent mechanisms and delays or prevents T2D development with a concomitant improvement in cardiovascular outcomes.197,198
An ongoing double-blind, randomized controlled trial on 40 adults, the INTIMET study, aims to quantify the beneficial effects of metformin on liver, muscle and adipose tissue IR in patients with T1D and may also identify factors that predict an individual's response to metformin in T1D.199 Metformin has been found to improve endothelial function by reducing PAI-1, CRP and ET-1 levels, improving NO synthesis and positively affecting oxidative stress conditions. Most importantly, a 32.5% reduction in IR, as measured by HOMA-IR, during metformin administration was noted.200 Another double-blind, placebo-controlled clinical trial on 37 overweight or obese young T1D patients favored metformin in terms of improving IR, both whole-body and peripheral, but it did not affect endogenous glucose release.201 A particular reference ought to be made for metformin and PCOS, a prediabetic state that affects 5–10% of women of reproductive age and has a negative impact on body weight and overall cardiovascular risk factors. Metformin can modify hyperandrogenemia and irregular menstrual cycles in PCOS by exerting its effects on IR, and its combination with myoinositol and TZDs exert a superior effect.202–205 However, neither exogenous insulin administration nor metformin can prevent pancreatic beta cells from losing their function in young patients with impaired glucose tolerance or recently diagnosed T2D.206
Thiazolidinediones, rosiglitazone, pioglitazone and troglitazone, are oral antidiabetic drugs, known to be beneficial in IR conditions. Their mechanism of action comprises activation of PPAR-γ, a nuclear receptor that modifies the transcription of various genes, namely those encoding GLUT4 receptors, LPL and other enzymes involved in energy homeostasis. IR is reduced in adipose tissue, muscle cells and the liver. PPAR-γ is abundant in adipocytes, suggesting endocrine communication with skeletal muscles and hepatocytes; molecules like FFAs and TNF-α might also be a part of signaling. Along with the improvement of dyslipidemia, IR amelioration leads to a consequent reduction of CVD risk. Pioglitazone has also been found to reduce MI and ischemic strokes. Thus, after extensive comprehension of the risk for side-effects, clinicians are now more capable of selecting patients eligible for TZD prescription, balancing benefits and risks.207–212 Administration of TZDs has been proven to delay beta cell dysfunction, as measured by certain specific indices, by exerting protective effects against oxidative stress and preserving the composition of the islets.213,214
Several studies including randomized controlled trials confirm the value of TZDs in IR management. For example, in the DREAM study, 8 mg of rosiglitazone daily reduced the incidence of T2D by 60% and increased normoglycemia by 70% in people free of CVD.215 A DREAM substudy revealed reduction of hepatic and visceral fat and an increase in subcutaneous fat and adiponectin levels; however, these changes cannot explain the normoglycemic effects of TZDs.216 The ADOPT study indicated that glucose levels were better regulated with rosiglitazone compared with metformin and glibenclamide (risk reduced by 32% and 63% compared with metformin and glibenclamide, respectively; P < 0.001).217 The CHICAGO, PROactive and PERISCOPE studies showed a significant delay in the formation of atherosclerotic plaques and amelioration of other traditional CVD risk factors, underpinning the favorable effect of TZDs in prediabetic and diabetic patients.218 Research supports the benefits provided by TZDs in T2D or MI when combined with metformin or sulfonylureas.218 In a randomized controlled trial conducted on prediabetic or diabetic patients with non-alcoholic steatohepatitis (NASH) on TZDs, resolution of NASH and improved peripheral insulin sensitivity was documented in 51% of the patients.219 Finally, a meta-analysis of randomized clinical trials involving patients with NASH also concluded that inflammation regressed following a combination of TZDs and lifestyle changes but paradoxically found no effect of TZDs alone on IR.220 Of course, weight gain, water retention and HF are well documented side-effects of TZDs, not to be taken lightly.218,220
Glucagon-like peptide-1 is an incretin secreted by the distal ileus, colon, pancreas and central nervous system. Its half-time is narrowed to 2 minutes, mainly due to its catabolism by dipeptidyl-peptidase 4 (DPP-4). GLP-1 binds to GLP-1 receptors, which are abundant throughout the body and exert a variety of actions, amongst which effects that contribute to energy homeostasis, namely reduced hepatic gluconeogenesis and steatosis, increased survival, proliferation and decreased apoptosis of pancreatic beta cells, increased insulin sensitivity and glucose uptake from muscle cells, increased lipolysis and glucose uptake from adipocytes and reduction of appetite. GLP-1 receptor agonists, liraglutide, exenatide, semaglutide, lixisenatide, dulaglutide, albiglutide, were manufactured to reproduce the multisystemic actions of GLP-1 via binding to GLP-1 receptors. The aforementioned effects of GLP-1 explain the wide use of GLP-1 receptor agonists in the management of IR and coexisting conditions, namely T2D, NASH and PCOS, with encouraging data concerning protection from CVD. Studies have demonstrated improved IR parameters and reduced lipotoxicity related to NASH, reduced hepatic and visceral fat accumulation and improved glucose permeability through the blood–brain barrier.221–225 In addition, these receptor agonists have been proven to be useful treatment agents in PCOS patients with IR.226 Administration of liraglutide as monotherapy or combined with metformin also induced weight loss and reduction of testosterone but with mixed results concerning IR improvement.222
Many clinical studies confirm the efficacy of GLP-1 receptor agonists in glycemic control and weight loss management with insignificant risks of hypoglycemia. Different GLP-1 receptor agonists may exhibit different pharmacokinetic and clinical effects, with convincing evidence regarding their cardioprotective capacity.227 Liraglutide in particular inhibits kinase pathways, such as PI3K/AKT and ERK 1/2 on one hand, reducing vascular smooth muscle cell proliferation related to hyperglycemia, and MKK4/JNK on the other hand, leading to the improvement of the hypoadiponectinemia-induced inflammatory stress in NASH.227 Other beneficial effects of GLP-1 receptor agonists related to atherosclerosis include the moderation of inflammation in plaques, as well as the amelioration of dyslipidemia and endothelial dysfunction.225,228 Liraglutide, semaglutide and albiglutide reduce the risk of major adverse cardiac events, while exenatide and lixisenatide exhibit neutral effects.229 Another meta-analysis suggested that all GLP-1 receptor agonists are capable of reducing cardiovascular incidents, cardiovascular mortality and all-cause mortality to different degrees, with no significant adverse effects, allowing for the personalization of drugs and regimens.230 A dual gastric inhibitory peptide and GLP-1 receptor agonist, tirzepatide, favorably modulated metabolites related to IR and future T2D risk with more significant reductions of HbA1c levels, HOMA-IR and amelioration of dyslipidemic profile, as compared with dulaglutide and placebo, leading to an overall improvement of metabolic health.231 GLP-1 receptor agonists enhance NO production and activate several kinases in cardiomyocytes, including Akt-1, PI-3K and MAPK, potentiating glucose uptake and further cardioprotection against ischemia; additionally, NO production by GLP-1 receptor agonists in endothelial cells promotes vasodilation.232
Sodium-glucose cotransporter 2 inhibition constitutes a novel and effective therapeutic strategy for managing T2D with concomitant cardiovascular benefits. Empagliflozin, dapagliflozin, canagliflozin and ertugliflozin are currently approved and used in everyday clinical practice. These medications inhibit the glucose reabsorption by the S1 segment of the proximal tubules in nephrons where SGLT2 are located. SGLT2 are responsible for the reabsorption of 80–90% of glucose.233 Hence, SGLT2-i achieve glycemic control via glucosuria; HbA1c levels decrease by 0.5–1.0% and there are also prominent improvements in BMI, lipid profile, endothelial function and a reduction of arterial blood pressure.233 Empagliflozin is an SGLT2-i, ideal as monotherapy or as an add-on antidiabetic agent due to its once-a-day dosage regimen, preferred and prescribed for T2D patients with elevated CV risk in the EU, USA, Japan and other countries.234 Empagliflozin increases adipose tissue utilization and browning in WAT and attenuates IR and obesity-derived inflammation via activation of M2 macrophages.235 Furthermore, it reverses brain IR by increasing responsiveness of the hypothalamus to insulin, a plausible mechanism concerning the regulation of energy metabolism and reduction of fasting glucose levels and hepatic fat.236 The action of empagliflozin is superior to that of sitagliptin, a DPP-4 inhibitor, with regard to amelioration of IR and improvement of cardiometabolic health in early-stage T2D.237 Additionally, glucosuria induced by empagliflozin has been shown to improve beta cell function and IR, regardless of elevated endogenous glucose production and decreased insulin secretion, with a further reduction in fasting and postprandial hyperglycemia.238 Several systematic reviews and meta-analyses provide data in favor of SGLT2-i for the management of NAFLD, the hepatic component of IR and precursor to NASH, and PCOS.239–243 Empagliflozin and dapagliflozin, along with hepatic fat reduction, also aid in reducing total body weight, alanine aminotransaminase and aspartate aminotransaminase levels, IR and liver fibrosis; and in women with PCOS, these agents have been shown to reduce body weight, fasting plasma glucose levels, HOMA-IR and androgen levels.239–243 Literature suggests that several molecular mechanisms favor the promotion of insulin sensitivity by SGLT2-i:244,245 (i) suppression of glucotoxicity, attributed to the decrease of chronically elevated blood glucose levels via excretion of glucose through urine; (ii) enhanced caloric disposition and weight reduction, which is a consequence of reduction in the absolute number of adipocytes, reduction in the levels of adipocytokines and amelioration of lipotoxicity; (iii) attenuation of inflammation, including regulation of RAAS and immune responses, decrease of proinflammatory cytokines and increase of anti-inflammatory cytokines (IL-10), lowered activity of NLRP3 inflammasomes, modulation of expression of inflammatory-related genes and modification of the redox state; (iv) improvement of pancreatic beta cell function, via interference with the cascades responsible for cell apoptosis; (v) amelioration of oxidative stress, favoring proper mitochondrial function and RAAS regulation, decreasing pro-oxidant enzymes, free radicals and AGEs.
Literature confirms the favorable effects of SGLT2-i in the prevention of the cardiovascular complications attributed to T2D. SGLT2-i promote the alteration in the metabolic preferences of the heart, liver and kidneys to ketone bodies and short-chain fatty acids rather than glucose, along with water preservation and improvement of glomerular hemodynamics, thus contributing to cardiac, hepatic and renal protection in patients with or without T2D.246,247 Cardiovascular protection is also attributed to decreased plasma volume due to natriuresis and decreased oxidative stress, as well as decreased plasma uric acid levels, decreased proteinuria and reduced formation of AGEs, along with improved vascular and endothelial function. At the same time, in addition to the aforementioned favorable changes in myocardial metabolism, SGLT2-i have been also shown to improve calcium handling and myocardial energetics within the cardiomyocytes, effects that may lead to an improvement of HF outcomes.248,249 Evidence indicates that SGLT2-i moderately affect the incidence of major adverse cardiovascular events related to atherosclerosis but robustly favor the reduction of hospitalizations due to HF and delay of renal disease, regardless of patient history, with an associated reduction of cardiovascular and all-cause mortality.250,251 Several studies concerning SGLT2-i and GLP-1 receptor agonists suggest their tailored use in diabetic patients with CVD and chronic kidney disease, according to properly structured clinical guidelines.252–256
A summary of the results of the main trials discussed in this review is shown in Table 2 (non-pharmacological interventions) and Table 3 (pharmacological interventions).
Table 2.
A summary of the clinical trials reporting on the non-pharmacological interventions used in the management of insulin resistance (IR).
| Trial | Design | Intervention | Results |
|---|---|---|---|
| Kahleova et al.170 | 24-week, randomized, open, parallel, metabolically controlled design enrolling 74 subjects with T2D | Isocaloric, calorie-restricted vegetarian or conventional diabetic diet for the first 12 weeksplusAerobic exercise in the second 12 weeks | Insulin sensitivity improved significantly with vegetarian meals, compared with the control group (30% [95% CI 24.5, 39] versus 20% [95% CI 14, 25], P = 0.04).Body weight decreased more in the experimental group than in the control group (–6.2 kg [95% CI –6.6, –5.3] versus –3.2 kg [95% CI –3.7, –2.5]; interaction group X time P = 0.001). Reduction in visceral and subcutaneous fat was greater in the experimental group than in the control group (P = 0.007 and P = 0.02, respectively). Alterations in insulin sensitivity and oxidative stress markers correlated with visceral fat loss. Adiponectin increased (P = 0.02) and leptin decreased (P = 0.02). Vitamin C, superoxide dismutase and reduced glutathione increased in the experimental group (P = 0.002, P < 0.001 and P = 0.02, respectively). Greater changes when exercise was added. |
| PREDIMED study172 | Multicentered, randomized, nutritional interventional trial involving 7447 subjects free of CVD at baseline but with high risk for CVD from 2003 to 2011 | MedDiet plus extra virgin olive oil (EVOO) orMedDiet plus nutsorControl diet, low in fat content | 288 CVD events during a median follow-up time of 4.8 years; hazard ratios: 0.69 (95% CI 0.53, 0.91) for the MedDiet plus EVOO and 0.72 (CI 0.54, 0.95) for the MedDiet plus nuts compared with the control group. 30% risk reduction in the incidence of major cardiovascular events with a per-protocol (adherence-adjusted) reduction of 58%. |
| Velazquez-Lopez et al.173 | Open-label, randomized controlled interventional trial on 49 children from another study related to the management of T2D in pediatric patients | MedDiet or standard diet for 16 weeks | 10.5% decrement (95% CI –13.1, –7.7) in glucose levels compared with a 4.9% decrease (95% CI –8.1, –1.7) in the standard diet. Decrease in glucose levels and frequency of glucose >100 mg/dl (P < 0.05). 45% decrease in metabolic syndrome, BMI, lean and fat mass. |
| Tettamanzi et al.174 | 21-day randomized controlled inpatient crossover feeding trial in 20 IR women, 16 of whom completed the trial | MedDiet versus high protein (HP) diet | HP diet: more effective in: (i) reducing insulin resistance (insulin: Beta = −6.98 (95% CI –12.30, –1.65) µIU/ml, P = 0.01; HOMA-IR: –1.78 (95% CI –3.03, –0.52), P = 9 × 10−3); and (ii) improving glycemic variability (–3.13 (95% CI –4.60, –1.67) mg/dl, P = 4 × 10−4), a risk factor for T2D development. Favorable alteration in gut microbiota composition |
| Shang et al.176 | Systematic review of 19 trials with 1193 participants with PCOS | Dietary interventions | The Dietary Approaches to Stop Hypertension diet and calorie-restricted diets might be the optimal choices for reducing IR and improving body composition, respectively, in women with PCOS. |
| Sutton et al.178 | 5-week, proof-of-concept, isocaloric and eucaloric randomized controlled feeding trial in prediabetic men | Early-time restricted feeding (eTRF) versus control | eTRF decreased fasting insulin by 3.4 ± 1.6 mU/l (P = 0.05) and decreased insulin levels at t = 60 min and 90 min post-load (P ≤ 0.01) eTRF increased the insulinogenic index (b cell responsiveness) by 14 ± 7 U/mg (P = 0.05) and decreased insulin resistance (3-h incremental AUC ratio) by 36 ± 10 U/mg (P = 0.005). Decreased morning levels of systolic and diastolic blood pressure by 11 ± 4 mmHg (P = 0.03) and 10 ± 4 mmHg (P = 0.03), respectively, compared with the control schedule. No effects on arterial stiffness (unaltered augmentation index [Δ = −1.4% ± 2.1%; P = 0.53] or pulse wave velocity [Δ = −0.5 ± 0.4 m/s; P = 0.23]), HDL-C (Δ = −0.6 ± 0.9 mg/dl; P = 0.48) or LDL-C (Δ = 2 ± 6 mg/dl; P = 0.75). Decreased plasma levels of 8-isoprostane, a marker of oxidative stress to lipids, by 11 ± 5 pg/ml (P = 0.05) or approximately 14%.No effects on hs-CRP (Δ = −0.3 ± 1.0 mg/l; P = 0.77), cortisol (Δ = −0.1 ± 1.3 mg/dl; P = 0.95) or IL-6 (Δ = 0.45 ± 0.27 pg/ml; P = 0.12). No effects on appetite. |
| Harvie et al.179 | 6-month randomized controlled interventional trial in 107 premenopausal young overweight women | IER versus CER | Greater impact of IER in IR reduction and fasting insulin levels: the difference between the groups for fasting insulin was –1.2 μU/ml (95% CI –1.4, –1.0 µU/ml); and for insulin resistance it was –1.2 μU/mmol/l (95% CI –1.5, –1.0) (both comparisons P = 0.04). Both IER and CER were equally effective in weight loss (–6.4 kg [95% CI –7.9, –4.8 kg] for IER versus –5.6 kg [95% CI –6.9, –4.4 kg] for CER). Percentage of fat loss in the IER and CER groups was 79% (±24%) and 79% (±26%), respectively (P = 0.99). Modest increase in adiponectin in the IER group, but not in the CER group (mean difference [95% CI] +9 [–2, 21%], P = 0.08). Modest decreases in the inflammatory marker hs-CRP. Oxidation protein products appeared to decrease in the IER group and to have a slight increase in the CER group (mean difference between groups at 6 months [95% CI]: –10 [–19, 2%], P = 0.12). |
| Villarreal-Calderón et al.184 | Systematic review assessing 19 543 patients | Metabolic surgery | CVD factors were improved. 73% of subjects presented resolution or remission of T2D. |
| Katzmarzyk et al.187 | Observational cohort study with clinical evaluation of 19 223 men from 1979 to 1995 with mortality follow-up through to December 1996 | Cardiorespiratory fitness (CRF) inclusion | No CRF: RR for all-cause and CVD mortality was 1.29 and 1.89, respectively, for men with metabolic syndrome compared with healthy men. CRF: RR 0.98 for all-cause and 1.23 for CVD mortality, when metabolic syndrome was present. |
| Katzmarzyk et al.188 | Observational cohort study including 19 173 men | CRF inclusion | Risks of all-cause mortality were 1.11 (0.75, 1.17) in normal weight, 1.09 (0.82, 1.47) in overweight and 1.55 (1.14, 2.11) in obese men with MetS compared with normal weight healthy men. Risks for CVD mortality were 2.06 (0.92, 4.63) in normal weight, 1.80 (1.10, 2.97) in overweight and 2.83 (1.70, 4.72) in obese men with the MetS compared with normal weight healthy men. After the inclusion of CRF in the model, the risks associated with obesity and metabolic syndrome were no longer significant. |
| Laaksonen et al.189 | Prospective population-based cohort study of 612 middle-aged men, 4-year follow-up | Leisure-time physical activity (LTPA) | Men engaging in 3 h/week of moderate or vigorous LTPA were half as likely as sedentary men to have the MetS after adjustment for major confounders or potentially mediating factors. Men in the upper third of VO2max were 75% less likely than unfit men to develop the MetS, even after adjustment for major confounders. Vigorous LTPA had an even stronger inverse association, particularly in unfit men. |
| Sampath Kumar et al.190 | Meta-analysis involving 846 diabetic patients | Structured exercise programs | The standardized mean difference in the intervention group for fasting insulin levels, HOMA-IR, fasting blood sugar, HbA1c and BMI was: –1.64, 0.14, –5.12, 0.63 and –0.36, respectively, compared with the control group. |
| Motahari-Tabari et al.191 | Randomized controlled clinical trial enrolling 53 diabetic women for 8 weeks | Aerobic exercise protocol versus control | Intervention group: lower plasma glucose (P = 0.05), insulin levels (P = 0.000) and insulin resistance (P = 0.02) compared with the control group. |
| Nery et al.193 | Meta-analysis of eight articles with 336 subjects in total | Protocols of aerobic and resistance exercise, varying in duration and frequency | Resistance training in diabetic adults was found to be more effective at increasing maximal oxygen consumption (mean difference: –2.86; 95% CI –3.90, –1.81; random effect) within 12 weeks or longer, with no apparent differences concerning HbA1c, BMI and lipid profile when compared with aerobic training. |
| Fedewa et al.194 | Meta-analysis of 24 studies | Exercise training on fasting insulin | A small-to-moderate effect was found for exercise training on fasting insulin and improving insulin resistance in youth (Hedges’ d effect size = 0.48 [95% CI 0.22, 0.74], P < 0.001 and 0.31 [95% CI 0.06, 0.56], P < 0.05, respectively). |
| Marson et al.195 | Meta-analysis of 17 studies | Exercise training in general and evaluation of aerobic, resistance exercise or their combination | Physical exercise improved fasting insulin levels and HOMA-IR (fasting insulin: –3.37 μU/ml; 95% CI –5.16, –1.57 μU/ml; I2 54%, P = 0.003); HOMA-IR: –0.61; 95% CI –1.19, –0.02; I2 49%, P = 0.040), but not fasting glucose levels. Evaluation of aerobic, resistance training or their combination showed that aerobic exercise is clearly more efficient in reducing insulin levels and HOMA-IR (fasting insulin: –4.52 μU/ml; 95% CI –7.40, –1.65; I2 65%, P = 0.002); HOMA-IR: –1.33; 95% CI –2.47, –0.18; I2 73%, P = 0.005). |
T2D, type 2 diabetes mellitus; CI, confidence interval; CVD, cardiovascular disease; MedDiet, Mediterranean diet; BMI, body mass index; HOMA-IR, homeostasis model assessment-insulin resistance; PCOS, polycystic ovary syndrome; AUC, area under the curve; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; IER, intermittent energy restriction; CER, continuous energy restriction; RR, relative risk; MetS, metabolic syndrome; HbA1c, glycosylated hemoglobin.
Table 3.
A summary of the clinical trials reporting on the pharmacological interventions used in the management of insulin resistance (IR).
| Trial | Design | Intervention | Results |
|---|---|---|---|
| INTIMET study199 | Ongoing, double-blind, multicentered, phase 3 randomized placebo-controlled trial on 40 adults with T1D for 26 weeks | Metformin 1500 g daily versus placebo | Quantification of the beneficial effects on the liver, muscles and adipose tissue in T1D. Results awaited. |
| Cree-Green et al.201 | Double-blind, multicentered placebo-controlled clinical trial on 37 young T1D patients for 3 months | Metformin gradually titrated up to 1000 mg twice daily versus placebo | Between-group difference in change in the primary outcome of whole-body insulin sensitivity during the 80 mU/m2/min phase, expressed as glucose infusion rate (GIR) adjusted for baseline GIR, sex and random center effects, was significant and favored the metformin group (1.3 [0.1, 2.4] mg/kg/min, P = 0.03) at 3 months). The change in glucose metabolic clearance rate from the 80 mU/m2/min phase was 0.923 (–0.002, 1.867) dl/kg/min (P = 0.05) and favored metformin. Post-treatment change in glucose rate of appearance (Ra) during the 16 mU/m2/min phase representing hepatic insulin sensitivity was not significantly different between groups (P = 0.12). The change in glycerol Ra, representing adipose insulin sensitivity, was not different (P = 0.69). |
| Fruzzetti et al.204 | Randomized controlled trial enrolling 50 young women with PCOS | Metformin 1500 mg/daily versus myoinositol plus folic acid for 6 months | BMI significantly decreased from 28.4 ± 5.2 kg/m2 to 26.8 ± 5.8 kg/m2 in the metformin group (P < 0.01) and from 27.3 ± 4.5 kg/m2 to 25.3 ± 3.9 kg/m2 in the inositol group (P < 0.01). In the metformin group, after 6 months of treatment, the HOMA-IR decreased from 2.4 ± 0.3 to 2.0 ± 0.3 (P < 0.01) and the AUC-insulin decreased from 10 870 ± 3555 to 8140 ± 2125 (P < 0.05); the Matsuda index improved from 4.9 ± 0.9 to 9.6 ± 3.9 (P < 0.005). In the inositol group, there was a decrease in HOMA-IR from 2.1 ± 0.5 to 1.5 ± 0.4 (P < 0.05) and the AUC-insulin from 11 890 ± 4830 to 7392 ± 5277 (P < 0.01); the Matsuda index improved from 5.96 ± 1.9 to 10.6 ± 3.4 (P < 0.05). |
| RISE Consortium206 | Randomized controlled trial enrolling 91 obese or overweight adolescents and young adults with impaired glucose tolerance or T2D for 12 months | 3 months of insulin glargine followed by 9 months of metformin versus 12 months of metformin alone | In both groups, clamp measured beta cell function was significantly lower at 12 and 15 months versus baseline; neither treatment prevented beta cell dysfunction in youth. BMI declined from baseline within the metformin alone group over the first 9 months, resulting in a significantly lower BMI across the 12-month intervention period in the metformin alone versus glargine followed by metformin group (P = 0.008) and specifically at 3 (P < 0.001), 6 (P < 0.02), and 9 (P < 0.04) months. By 15 months, HbA1c had increased significantly from baseline in the glargine followed by metformin group (P = 0.03), ending at ∼6% (42 mmol/mol) in both treatment groups. |
| DREAM study215 | Double-blind randomized placebo-controlled clinical trial in 5269 adults for 3 years | Ramipril 15 mg/day and/or rosiglitazone 8 mg/day | Treatment with rosiglitazone reduced by almost 60% the incidence of T2D and increased the likelihood (+70%) of regression to normoglycemia. |
| DREAM sub-study216 | Double-blind, randomized placebo-controlled trial including 190 participants from the DREAM study, for 3.5 years | Rosiglitazone 8 mg/day versus placebo | Visceral abdominal fat area was 23 cm² lower (95% CI 5, 41; P = 0.01) in the rosiglitazone group. Rosiglitazone was also associated with a lower visceral:subcutaneous abdominal fat ratio by 0.104 (95% CI 0.027, 0.181; P = 0.008) compared with placebo and a higher liver:spleen ratio by 0.08 (95% CI 0.01, 0.15; P = 0.02). Rosiglitazone raised adiponectin levels 14.9 μg/ml more than placebo (95% CI 11.1, 18.7; P < 0.0001). Rosiglitazone lowered fasting glucose by 0.36 mmol/l more than placebo (95% CI 0.16, 0.56; P = 0.0004) and 2-h post-load glucose by 1.21 mmol/l (95% CI 0.51, 1.91; P = 0.0008) more than placebo. |
| ADOPT study217 | Double-blind, controlled clinical trial in 4360 patients with a follow-up of 4.0 years | Rosiglitazone versus metformin versus glibenclamide | The cumulative incidence of monotherapy failure averaged at 5 years: 15% with rosiglitazone, 21% with metformin and 34 % with glibenclamide. Risk reduction for rosiglitazone of 32 % as compared with metformin and 63 % as compared with glibenclamide (P < 0.001 for both comparisons). |
| PROactive, CHICAGO, PERISCOPE studies218 | Randomized controlled trials | Effects of pioglitazone and glimepiride (CHICAGO, PERISCOPE) in CVD risk modification | PROactive: benefit regarding mortality, non-fatal MI and stroke. CHICAGO: Intima/media thickness regressed with pioglitazone. PERISCOPE: atheroma did not progress with pioglitazone but progressed with glimepiride. |
| Cusi et al.219 | Double-blind randomized placebo-controlled trial enrolling 101 prediabetic or diabetic patients with NASH, with 18-month duration | Hypocaloric diet and pioglitazone 45 g/day or placebo | 58% of the intervention group achieved a reduction of at least 2 points in the NAFLD score (treatment difference, 41 percentage points [95% CI 23, 59 percentage points]) and 51% had resolution of NASH (treatment difference, 32 percentage points [95% CI 13, 51 percentage points]) (P < 0.001 for each). Pioglitazone was associated with an improvement in individual histologic scores, including the fibrosis score (treatment difference, –0.5 [95% CI –0.9, 0.0]; P = 0.039); reduced hepatic triglyceride content from 19% to 7% (treatment difference, –7 percentage points [95% CI, –10, –4 percentage points]; P < 0.001); and improved adipose tissue, hepatic, and muscle insulin sensitivity (P < 0.001 versus placebo for all). |
| He et al.220 | Meta-analysis of five studies | TZD administration | No significant difference noted between the change in HOMA-IR for patients treated with a TZD and that of their corresponding control group (MD 1.37; 95% CI –0.06, 2.80; P = 0.06). No significant difference noted in the change in HOMA-IR of patients treated with a TZD alone compared with that of the placebo groups (MD 0.87; 95% CI –0.58, 2.31; P = 0.24). TZD therapy and lifestyle changes caused a significantly greater reduction in their fasting insulin level than that of patients who underwent lifestyle changes alone or lifestyle changes with placebo (SMD 0.65; 95% CI 0.24, 1.06; P = 0.002). Patients treated with TZDs alone did not experience a significantly greater reduction in their fasting insulin level than that of patients who received a placebo (SMD 0.51; 95% CI –0.34, 1.35; P = 0.24). No significant difference noted in the change in BMI between the TZD-treated patients and the control patients (MD 0.85; 95% CI –0.24, 1.95; P = 0.13). Effects of TZD on fibrosis were unclear. TZD resulted in a significantly greater reduction in lobular inflammation than that in the corresponding placebo groups (OR 1.72; 95% CI 1.33, 2.22; P < 0.0001). |
| Bethel et al.230 | Meta-analysis of four trials, including 33 457 participants | GLP-1 receptor agonists versus placebo | Significant relative risk reduction in cardiovascular mortality of 13% compared with placebo (HR 0.87; 95% CI 0.79, 0.96; P = 0.007) and a significant relative risk reduction in all-cause mortality of 12% (HR 0.88; 95% CI 0.81, 0.95; P = 0.002). No significant effects of GLP-1 receptor agonists were seen for fatal and non-fatal MI, fatal and non-fatal stroke, hospital admission for unstable angina, or hospital admission for heart failure. No significant effect of GLP-1 receptor agonists was seen on the proportions of patients who had severe hypoglycemia (OR 0.93; 95% CI 0.74, 1.18; P = 0.56). |
| Pirro et al.231 | Randomized, placebo-controlled, phase 2b trial enrolling 259 patients with T2D for 26 weeks | Weekly administration of subcutaneous tirzepatide (1–15 mg), dulaglutide (1.5 mg) or placebo | Branched-chain amino acids, direct catabolic products glutamate, 3-hydroxyisobutyrate, branched-chain ketoacids, and indirect byproducts such as 2-hydroxybutyrate, decreased compared with baseline and placebo. Changes were significantly larger with tirzepatide. |
| ASSET study236 | Prospective, randomized, open-label, blinded-endpoint, parallel-group trial involving 44 Japanese patients with T2D for 12 weeks | Empagliflozin versus sitagliptin | Cardiometabolic markers, HDL-C and ketone bodies including β-hydroxybutyric acid were significantly increased, whereas uric acid, plasma glucose, plasma insulin and HOMA-IR were significantly lower in the empagliflozin group than in the sitagliptin group (P < 0.05). |
| Ferrannini et al.238 | Prospective single intervention study enrolling 66 patients with T2D, 34 of whom on metformin and 32 of whom drug-naive | Empagliflozin | Both single-dose and chronic empagliflozin treatment caused glycosuria during fasting (median 7.8, interquartile range [IQR] 4.4 g/3 h; median 9.2, IQR 5.2 g/3 h) and after meal ingestion (median 29.0, IQR, 12.5 g/5 h and median 28.2, IQR 15.4 g/5 h). After 3 h of fasting, endogenous glucose production was increased 25%, while glycemia was 0.9 ± 0.7 mmol/l lower (P < 0.0001 versus baseline). After meal ingestion, glucose and insulin-AUC decreased, whereas the glucagon response increased (all P < 0.001). Tissue glucose disposal was reduced (median 75, IQR 16 g and 70, IQR 21 g versus 93, IQR 18 g, P < 0.0001) due to a decrease in both glucose oxidation and nonoxidative glucose disposal, with a concomitant rise in lipid oxidation after chronic administration (all P < 0.01). Beta cell glucose sensitivity increased (median 55, IQR 35 pmol·min–1·m–2·mM–1 and 55, IQR 39 pmol·min–1·m–2·mM–1 versus median 44, IQR 32 pmol·min–1·m–2·mM–1; P < 0.0001). Insulin sensitivity was improved. |
| Wei et al.239 | Meta-analysis of 10 randomized controlled trials with 573 patients with NAFLD | SGLT2 inhibitors administration versus placebo | Reduced levels of ALT (WMD –5.36, 95% CI –8.86, –1.85, P = 0.003) and AST (WMD –2.56, 95% CI –3.83, –1.29, P < 0.0001). Significant reduction of liver proton density fat fraction (WMD –2.20, 95% CI –3.67, –0.74, P = 0.003), visceral fat mass area (WMD –20.71, 95% CI –28.19, –13.23, P < 0.00001), subcutaneous fat areas (WMD –14.68, 95% CI: –26.96, –2.40, P = 0.02). |
| Zhang et al.240 | Meta-analysis of four articles involving 244 patients with NAFLD | Empagliflozin versus placebo | Significantly reduced BMI (MD –0.98, 95% CI –1.87, –0.10, P = 0.03), liver stiffness measurement (MD –0.49, 95% CI –0.93, –0.06, P = 0.03), AST (MD –3.10, 95% CI –6.18, –0.02, P = 0.05), HOMA-IR (MD –0.45, 95% CI –0.90, 0.00, P = 0.05) of the treatment group. |
| He et al.241 | Meta-analysis of 11 studies enrolling 839 patients | Dapagliflozin versus placebo | Dapagliflozin led to a greater reduction in AST, ALT, TG, body weight, BMI, HbA1c and fasting plasma glucose compared with control. No difference was found between the dapagliflozin and control groups in HOMA-IR, TC, LDL-C and HDL-C. |
| Sun et al.242 | Meta-analysis of seven trials with 390 subjects | Dapagliflozin versus placebo | Compared with the placebo or control group, dapagliflozin significantly reduced levels of ALT (WMD –6.62 U/l, 95% CI –12.66, –0.58, P = 0.03) and AST (WMD –4.20 U/l, 95% CI –7.92, –0.47, P = 0.03). Dapagliflozin favorably affected HOMA-IR (WMD –0.88, 95% CI –1.43, –0.33, P = 0.002). Dapagliflozin reduced bodyweight (WMD –3.79 kg, 95% CI –4.63, –2.95, P < 0.00001), BMI (WMD –1.33 kg/m2, 95% CI –2.37, –0.28, P = 0.01), LDL-C (WMD –2.66 mg/dl, 95% CI –3.99, –1.32, P < 0.00001) and TG (WMD –16.77 mg/dl, 95% CI –31.93, –1.61, P = 0.03). Dapagliflozin increased total cholesterol (WMD 9.77 mg/dl, 95% CI 1.58, 17.97, P = 0.02). |
| Sinha et al.243 | Meta-analysis of four trials involving 158 women with PCOS | SGLT2 inhibitor therapy versus placebo | SGLT2 inhibitor therapy led to a reduction in body weight (SMD –0.68, 95% CI –1.16, –0.19, P < 0.01), fasting plasma glucose (SMD –0.59, 95% CI –0.99, –0.19, P < 0.01) and insulin resistance as assessed with the HOMA-IR (SMD –0.39, 95% CI –0.76, –0.03, P = 0.03). SGLT2 inhibitor therapy led to a reduction of dehydroepiandrosterone sulphate levels (SMD –0.55, 95% CI –0.94, –0.16, P < 0.01). |
T1D, type 1 diabetes mellitus; PCOS, polycystic ovary syndrome; BMI, body mass index; HOMA-IR, homeostasis model assessment-insulin resistance; AUC, area under the curve; T2D, type 2 diabetes mellitus; HbA1c, glycosylated hemoglobin; CI, confidence interval; CVD, cardiovascular disease; MI, myocardial infarction; NASH, non-alcoholic steatohepatitis; NAFLD, non-alcoholic fatty liver disease; TZD, thiazolidinediones; MD, mean difference; SMD, standardized mean difference; OR, odds ratio; GLP-1, glucagon-like peptide-1; HR, hazard ratio; HDL-C, high-density lipoprotein cholesterol; SGLT2, sodium-glucose cotransporter 2; ALT, alanine aminotransaminase; WMD, weighted mean difference; AST, aspartate aminotransaminase; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol.
Conclusions and future directions
In conclusion, insulin constitutes an anabolic hormone with a pivotal role in energy homeostasis, exerting numerous effects in muscle cells, hepatocytes and adipocytes. Its overall action involves the facilitation of glucose uptake via insulin-dependent mechanisms that promote GLUT4 potentiation, thus maintaining normal circulating blood glucose levels. Different signaling pathways involving various kinases are activated and vessels can be affected positively or negatively, depending on the affected cascade. When insulin cannot fully exert its effects on target tissues, a clinical condition known as IR develops. IR is characterized by hyperglycemia and compensatory hyperinsulinemia, which can exhaust beta cells in the long term. Its measurement and quantification rely on various indices, mainly fasting insulin levels, HOMA-IR and glucose clamp technique.257 IR is a precursor to many pathological conditions that characterize Western societies and are considered risk factors for the development of CVD, namely obesity, inflammation, dyslipidemia, endothelial dysfunction, oxidative stress and hypertension. In addition, IR accompanies prediabetes, NAFLD and NASH, PCOS and HF; all conditions associated with an increased CVD risk. Thus, IR is a condition predisposing to CVD via various complex, as yet not fully elucidated, mechanisms. Its management involves primary prevention strategies, including abandoning the Western lifestyle with proper diet modifications and avoidance of a sedentary lifestyle. Specific pharmacological agents have also been demonstrated to improve insulin sensitivity, such as metformin, TZDs, GLP-1 receptor agonists and SGLT-2-i, with a concomitant proven efficacy regarding the prevention of cardiovascular events, especially in the case of the latter classes of agents.
Future directions regarding IR have endless potential. Firstly, further in vitro and in vivo studies are required, in order to attempt to elucidate the incredibly complex mechanisms that lead to IR. New biomarkers for the early detection of IR along with metabolomics and gut microbiota analysis seem to be afar; however, they ought to constitute very straightforward approaches,258 based on precision medicine. IR in children is a critical field of research, as potential future protocols for its, currently non-existent, screening and holistic, personalized management would reduce child and adult obesity as a long-term consequence.259 Interestingly, branched-chain amino acids have been linked to IR and are therefore worthy of further research.260 Equally interesting is the conduction of further research involving the role of mitochondrial dysfunction, micro RNAs and autophagy.261–265 In addition, establishment of novel biomarkers and therapeutic targets involving less known myokines, hepatokines and adipocytokines, as previously analysed, ought to be considered.
The search term ‘Insulin Resistance’ in ClinicalTrials.gov revealed over 3000 studies with different perspectives. Indicatively, researchers have focused on identifying novel urinary biomarkers for IR screening, the effect of specific diet modifications and dietary supplements, gut microbiota alterations and exercise in people according to age and sex, novelties concerning maternal IR screening and further investigation of classic antidiabetic drug use in IR. A recent phase 1b randomized controlled clinical trial studied an agent known by the name of BFKB8488A, a bispecific agonist antibody targeting fibroblast growth factor receptor 1c and Klothoβ.266 In this study, BFKB8488A proved its safety and tolerability and showed encouraging effects in T2D and NAFLD, improving the patients’ lipid profile at the same time.266 This sets the ground for further conduction of related trials and assessment of its potential as a novel pharmacological agent in the management of IR. Nevertheless, the future can move in a myriad of different directions. Undoubtedly, the scientific community still has a long way ahead on the journey of clarifying the mechanisms involved in IR, as well as its association with CVD. Vast efforts shall be made to discover and add new pharmacological agents in the armamentarium of IR management.
Footnotes
Author contributions: Conceived the concept: Constantine E. Kosmas; analyzed the data: Constantine E. Kosmas; wrote the first draft of the manuscript: Constantine E. Kosmas and Maria D. Bousvarou; contributed to the writing of the manuscript: Christina E. Kostara, Evangelia J. Papakonstantinou, Evdokia Salamou and Eliscer Guzman; made critical revisions of the manuscript: Constantine E. Kosmas and Maria D. Bousvarou; agreed with the manuscript results and conclusions: all authors; reviewed and approved the final version of the manuscript: all authors.
The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Constantine E. Kosmas https://orcid.org/0000-0003-3926-0304
Christina E. Kostara https://orcid.org/0000-0001-7045-1323
References
- 1.Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat Rev Mol Cell Biol 2018; 19: 31–44. doi: 10.1038/nrm.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Q, Vijayakumar A, Kahn BB. Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol 2018; 19: 654–672. doi: 10.1038/s41580-018-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep 2018; 20: 12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown AE, Walker M. Genetics of Insulin Resistance and the Metabolic Syndrome. Curr Cardiol Rep 2016; 18: 75. doi: 10.1007/s11886-016-0755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebovitz HE. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes 2001; 109: S135–S148. doi: 10.1055/s-2001-18576. [DOI] [PubMed] [Google Scholar]
- 6.Ormazabal V, Nair S, Elfeky O, et al. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol 2018; 17: 122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosmas CE, Silverio D, Tsomidou C, et al. The Impact of Insulin Resistance and Chronic Kidney Disease on Inflammation and Cardiovascular Disease. Clin Med Insights Endocrinol Diabetes 2018; 11: 1179551418792257. DOI: 10.1177/1179551418792257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006; 116: 1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett 2008; 582: 97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112: 1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmiegelow MD, Hedlin H, Stefanick ML, et al. Insulin Resistance and Risk of Cardiovascular Disease in Postmenopausal Women: A Cohort Study From the Women's Health Initiative. Circ Cardiovasc Qual Outcomes 2015; 8: 309–316. doi: 10.1161/CIRCOUTCOMES.114.001563. [DOI] [PubMed] [Google Scholar]
- 12.Mirr M, Skrypnik D, Bogdański P, et al. Newly proposed insulin resistance indexes called TyG-NC and TyG-NHtR show efficacy in diagnosing the metabolic syndrome. J Endocrinol Invest 2021; 44: 2831–2843. doi: 10.1007/s40618-021-01608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gast KB, Tjeerdema N, Stijnen T, et al. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLoS One 2012; 7: e52036. doi: 10.1371/journal.pone.0052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 2010; 56: 1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 15.Ding X, Wang X, Wu J, et al. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol 2021; 20: 76. doi: 10.1186/s12933-021-01268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics – 2020 update: a report from the American Heart Association. Circulation 2020; 141: e139–e596. doi:10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 17.Wolf D, Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res 2019; 124: 315–327. doi: 10.1161/CIRCRESAHA.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taleb S. Inflammation in atherosclerosis. Arch Cardiovasc Dis 2016; 109: 708–715. doi: 10.1016/j.acvd.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, Xian X, Wang Z, et al. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018; 8: 80. doi: 10.3390/biom8030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yazici D, Sezer H. Insulin Resistance, Obesity and Lipotoxicity. Adv Exp Med Biol 2017; 960: 277–304. doi: 10.1007/978-3-319-48382-5_12. [DOI] [PubMed] [Google Scholar]
- 21.Saltiel AR. Insulin signaling in health and disease. J Clin Invest 2021; 131: e142241. doi: 10.1172/JCI142241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopaschuk GD, Karwi QG, Tian R, et al. Cardiac Energy Metabolism in Heart Failure. Circ Res 2021; 128: 1487–1513. doi: 10.1161/CIRCRESAHA.121.318241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attie AD, Tang QQ, Bornfeldt KE. The insulin centennial – 100 years of milestones in biochemistry. J Biol Chem 2021; 297: 101278. doi: 10.1016/j.jbc.2021.101278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Meyts P. Insulin and its receptor: structure, function and evolution. Bioessays 2004; 26: 1351–1362. doi: 10.1002/bies.20151. [DOI] [PubMed] [Google Scholar]
- 25.Klostermeyer H, Humbel RE. The chemistry and biochemistry of insulin. Angew Chem Int Ed Engl 1966; 5: 807–822. doi: 10.1002/anie.196608071. [DOI] [PubMed] [Google Scholar]
- 26.Marques RG, Fontaine MJ, Rogers J. C-peptide: much more than a byproduct of insulin biosynthesis. Pancreas 2004; 29: 231–238. doi: 10.1097/00006676-200410000-00009. [DOI] [PubMed] [Google Scholar]
- 27.In't Veld P, Marichal M. Microscopic anatomy of the human islet of Langerhans. Adv Exp Med Biol 2010; 654: 1–19. doi: 10.1007/978-90-481-3271-3_1. [DOI] [PubMed] [Google Scholar]
- 28.Kojima I, Medina J, Nakagawa Y. Role of the glucose-sensing receptor in insulin secretion. Diabetes Obes Metab 2017; 19: 54–62. doi: 10.1111/dom.13013. [DOI] [PubMed] [Google Scholar]
- 29.Najjar SM, Perdomo G. Hepatic Insulin Clearance: Mechanism and Physiology. Physiology (Bethesda) 2019; 34: 198–215. doi: 10.1152/physiol.00048.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qaid MM, Abdelrahman MM. Role of insulin and other related hormones in energy metabolism – A review. Cogent Food & Agriculture 2016; 2: 1267691. doi: 10.1080/23311932.2016.1267691. [Google Scholar]
- 31.Yaribeygi H, Farrokhi FR, Butler AE, et al. Insulin resistance: Review of the underlying molecular mechanisms. J Cell Physiol 2019; 234: 8152–8161. doi: 10.1002/jcp.27603. [DOI] [PubMed] [Google Scholar]
- 32.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 2010; 53: 1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steiner G, Cahill GF., Jr. Adipose tissue physiology. Ann N Y Acad Sci 1963; 110: 749–753. doi: 10.1111/j.1749-6632.1963.tb15795.x. [DOI] [PubMed] [Google Scholar]
- 34.Esteve Ràfols M. Adipose tissue: cell heterogeneity and functional diversity. Endocrinol Nutr 2014; 61: 100–112 [Article in English, Spanish]. doi: 10.1016/j.endonu.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010; 11: 11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 36.Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care 2016; 22: s176–s185. [PubMed] [Google Scholar]
- 37.Mårin P, Andersson B, Ottosson M, et al. The morphology and metabolism of intra-abdominal adipose tissue in men. Metabolism 1992; 41: 1241–1248. doi: 10.1016/0026-0495(92)90016-4. [DOI] [PubMed] [Google Scholar]
- 38.Misra A, Vikram NK. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition 2003; 19: 457–466. doi: 10.1016/s0899-9007(02)01003-1. [DOI] [PubMed] [Google Scholar]
- 39.Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome. Endocrinol Nutr 2013; 60: 39–43. doi: 10.1016/s1575-0922(13)70026-3. [DOI] [PubMed] [Google Scholar]
- 40.Frayn KN. Visceral fat and insulin resistance–causative or correlative? Br J Nutr 2000; 83: S71–S77. doi: 10.1017/s0007114500000982. [DOI] [PubMed] [Google Scholar]
- 41.Indulekha K, Anjana RM, Surendar J, et al. Association of visceral and subcutaneous fat with glucose intolerance, insulin resistance, adipocytokines and inflammatory markers in Asian Indians (CURES-113). Clin Biochem 2011; 44: 281–287. doi: 10.1016/j.clinbiochem.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 42.Preis SR, Massaro JM, Robins SJ, et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity (Silver Spring) 2010; 18: 2191–2198. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reyes-Farias M, Fos-Domenech J, Serra D, et al. White adipose tissue dysfunction in obesity and aging. Biochem Pharmacol 2021; 192: 114723. doi: 10.1016/j.bcp.2021.114723. [DOI] [PubMed] [Google Scholar]
- 44.Dimitriadis G, Mitrou P, Lambadiari V, et al. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract 2011; 93: S52–S59. doi: 10.1016/S0168-8227(11)70014-6. [DOI] [PubMed] [Google Scholar]
- 45.Lan YL, Lou JC, Lyu W, et al. Update on the synergistic effect of HSL and insulin in the treatment of metabolic disorders. Ther Adv Endocrinol Metab 2019; 10: 2042018819877300. DOI: 10.1177/2042018819877300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sivan E, Homko CJ, Whittaker PG, et al. Free fatty acids and insulin resistance during pregnancy. J Clin Endocrinol Metab 1998; 83: 2338–2342. doi: 10.1210/jcem.83.7.4927. [DOI] [PubMed] [Google Scholar]
- 47.Ye J. Mechanisms of insulin resistance in obesity. Front Med 2013; 7: 14–24. doi: 10.1007/s11684-013-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L, Chen XW, Huang X, et al. Regulation of glucose and lipid metabolism in health and disease. Sci China Life Sci 2019; 62: 1420–1458. doi: 10.1007/s11427-019-1563-3. [DOI] [PubMed] [Google Scholar]
- 49.Bódis K, Roden M. Energy metabolism of white adipose tissue and insulin resistance in humans. Eur J Clin Invest 2018; 48: e13017. doi: 10.1111/eci.13017. [DOI] [PubMed] [Google Scholar]
- 50.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab 2012; 15: 635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Catrysse L, van Loo G. Adipose tissue macrophages and their polarization in health and obesity. Cell Immunol 2018; 330: 114–119. doi: 10.1016/j.cellimm.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 2007; 447: 1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117: 175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tzanavari T, Giannogonas P, Karalis KP. TNF-alpha and obesity. Curr Dir Autoimmun 2010; 11: 145–156. doi: 10.1159/000289203. [DOI] [PubMed] [Google Scholar]
- 55.Ye J. Role of insulin in the pathogenesis of free fatty acid-induced insulin resistance in skeletal muscle. Endocr Metab Immune Disord Drug Targets 2007; 7: 65–74. doi: 10.2174/187153007780059423. [DOI] [PubMed] [Google Scholar]
- 56.Kim JH, Bachmann RA, Chen J. Interleukin-6 and insulin resistance. Vitam Horm 2009; 80: 613–633. doi: 10.1016/S0083-6729(08)00621-3. [DOI] [PubMed] [Google Scholar]
- 57.Hoene M, Weigert C. The role of interleukin-6 in insulin resistance, body fat distribution and energy balance. Obes Rev 2008; 9: 20–29. doi: 10.1111/j.1467-789X.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- 58.Esser N, Legrand-Poels S, Piette J, et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 2014; 105: 141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 59.Libby P. Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J Am Coll Cardiol 2017; 70: 2278–2289. doi: 10.1016/j.jacc.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol 2016; 94: 146–150. doi: 10.1038/icb.2015.101. [DOI] [PubMed] [Google Scholar]
- 61.Iwaki T, Urano T, Umemura K. PAI-1, progress in understanding the clinical problem and its aetiology. Br J Haematol 2012; 157: 291–298. doi: 10.1111/j.1365-2141.2012.09074.x. [DOI] [PubMed] [Google Scholar]
- 62.Festa A, D'Agostino R, Jr, Tracy RP, et al. ; Insulin Resistance Atherosclerosis Study. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 2002; 51: 1131–1137. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 63.Juhan-Vague I, Alessi MC, Morange PE. Hypofibrinolysis and increased PAI-1 are linked to atherothrombosis via insulin resistance and obesity. Ann Med 2000; 32: 78–84. [PubMed] [Google Scholar]
- 64.Juhan-Vague I, Alessi MC, Mavri A, et al. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J Thromb Haemost 2003; 1: 1575–1579. doi: 10.1046/j.1538-7836.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 65.Bastard JP, Piéroni L, Hainque B. Relationship between plasma plasminogen activator inhibitor 1 and insulin resistance. Diabetes Metab Res Rev 2000; 16: 192–201. doi: 10.1002/1520-7560(200005/06)16:3<192::aid-dmrr114>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 66.Alessi MC, Poggi M, Juhan-Vague I. Plasminogen activator inhibitor-1, adipose tissue and insulin resistance. Curr Opin Lipidol 2007; 18: 240–245. doi: 10.1097/MOL.0b013e32814e6d29. [DOI] [PubMed] [Google Scholar]
- 67.Song C, Burgess S, Eicher JD, et al. Causal Effect of Plasminogen Activator Inhibitor Type 1 on Coronary Heart Disease. J Am Heart Assoc 2017; 6: e004918. doi: 10.1161/JAHA.116.004918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meigs JB, Mittleman MA, Nathan DM, et al. Hyperinsulinemia, hyperglycemia, and impaired hemostasis: the Framingham Offspring Study. JAMA 2000; 283: 221–228. doi: 10.1001/jama.283.2.221. [DOI] [PubMed] [Google Scholar]
- 69.Sell H, Eckel J. Monocyte chemotactic protein-1 and its role in insulin resistance. Curr Opin Lipidol 2007; 18: 258–262. doi: 10.1097/MOL.0b013e3281338546. [DOI] [PubMed] [Google Scholar]
- 70.Singh S, Anshita D, Ravichandiran V. MCP-1: Function, regulation, and involvement in disease. Int Immunopharmacol 2021; 101: 107598. doi: 10.1016/j.intimp.2021.107598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology 2014; 147: 577–594.e1. doi: 10.1053/j.gastro.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 72.Farkhondeh T, Llorens S, Pourbagher-Shahri AM, et al. An Overview of the Role of Adipokines in Cardiometabolic Diseases. Molecules 2020; 25: 5218. doi: 10.3390/molecules25215218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol 2014; 63: 250–259. doi: 10.1016/j.jjcc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumada M, Kihara S, Sumitsuji S, et al. ; Osaka CAD Study Group. Coronary artery disease. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 2003; 23: 85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 75.Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011; 11: 85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Polak K, Czyzyk A, Simoncini T, et al. New markers of insulin resistance in polycystic ovary syndrome. J Endocrinol Invest 2017; 40: 1–8. doi: 10.1007/s40618-016-0523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perakakis N, Triantafyllou GA, Fernández-Real JM, et al. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol 2017; 13: 324–337. doi: 10.1038/nrendo.2016.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng S, Chen N, Kang X, et al. Irisin alleviates FFA induced β-cell insulin resistance and inflammatory response through activating PI3K/AKT/FOXO1 signaling pathway. Endocrine 2022; 75: 740–751. doi: 10.1007/s12020-021-02875-y. [DOI] [PubMed] [Google Scholar]
- 79.Song R, Zhao X, Cao R, et al. Irisin improves insulin resistance by inhibiting autophagy through the PI3K/Akt pathway in H9c2 cells. Gene 2021; 769: 145209. doi: 10.1016/j.gene.2020.145209. [DOI] [PubMed] [Google Scholar]
- 80.Cheng ZB, Huang L, Xiao X, et al. Irisin in atherosclerosis. Clin Chim Acta 2021; 522: 158–166. doi: 10.1016/j.cca.2021.08.022. [DOI] [PubMed] [Google Scholar]
- 81.Antushevich H, Wójcik M. Review: Apelin in disease. Clin Chim Acta 2018; 483: 241–248. doi: 10.1016/j.cca.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 82.Xu S, Tsao PS, Yue P. Apelin and insulin resistance: another arrow for the quiver? J Diabetes 2011; 3: 225–231. doi: 10.1111/j.1753-0407.2011.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li C, Cheng H, Adhikari BK, et al. The Role of Apelin-APJ System in Diabetes and Obesity. Front Endocrinol (Lausanne) 2022; 13: 820002. doi: 10.3389/fendo.2022.820002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.El Wakeel MES, Ahmad IH, Mohammed MA, et al. Correlation of serum apelin level with carotid intima-media thickness and insulin resistance in a sample of Egyptian patients with type 2 diabetes mellitus. J Res Med Sci 2022; 27: 13. doi: 10.4103/jrms.JRMS_675_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abdalla MMI. Role of visfatin in obesity-induced insulin resistance. World J Clin Cases 2022; 10: 10840–10851. doi: 10.12998/wjcc.v10.i30.10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heo YJ, Choi SE, Jeon JY, et al. Visfatin Induces Inflammation and Insulin Resistance via the NF-κB and STAT3 Signaling Pathways in Hepatocytes. J Diabetes Res 2019; 2019: 4021623. doi: 10.1155/2019/4021623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang YH, Chang DM, Lin KC, et al. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab Res Rev 2011; 27: 515–527. doi: 10.1002/dmrr.1201. [DOI] [PubMed] [Google Scholar]
- 88.Jirak P, Stechemesser L, Moré E, et al. Clinical implications of fetuin-A. Adv Clin Chem 2019; 89: 79–130. doi: 10.1016/bs.acc.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 89.Gerst F, Kemter E, Lorza-Gil E, et al. The hepatokine fetuin-A disrupts functional maturation of pancreatic beta cells. Diabetologia 2021; 64: 1358–1374. doi: 10.1007/s00125-021-05435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mukhuty A, Fouzder C, Kundu R. Fetuin-A excess expression amplifies lipid induced apoptosis and β-cell damage. J Cell Physiol 2022; 237: 532–550. doi: 10.1002/jcp.30499. [DOI] [PubMed] [Google Scholar]
- 91.Lanthier N, Lebrun V, Molendi-Coste O, et al. Liver Fetuin-A at Initiation of Insulin Resistance. Metabolites 2022; 12: 1023. doi: 10.3390/metabo12111023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee-Ødegård S, Ueland T, Thorsby PM, et al. Fetuin-A mediates the difference in adipose tissue insulin resistance between young adult pakistani and norwegian patients with type 2 diabetes. BMC Endocr Disord 2022; 22: 208. doi: 10.1186/s12902-022-01127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fatima F, Ahsan N, Nasim A, et al. Association of fetuin-A with dyslipidemia and insulin resistance in type-II Diabetics of Pakistani population. Pak J Med Sci 2020; 36: 64–68. doi: 10.12669/pjms.36.2.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Toprak K, Görpelioğlu S, Özsoy A, et al. Does fetuin-A mediate the association between pro-inflammatory diet and type-2 diabetes mellitus risk? Nutr Hosp 2022; 39: 383–392. English. doi: 10.20960/nh.03848. [DOI] [PubMed] [Google Scholar]
- 95.Guo VY, Cao B, Cai C, et al. Fetuin-A levels and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Acta Diabetol 2018; 55: 87–98. doi: 10.1007/s00592-017-1068-9. [DOI] [PubMed] [Google Scholar]
- 96.Liu S, Hu W, He Y, et al. Serum Fetuin-A levels are increased and associated with insulin resistance in women with polycystic ovary syndrome. BMC Endocr Disord 2020; 20: 67. doi: 10.1186/s12902-020-0538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xia X, Xue S, Yang G, et al. Association of serum fetuin-B with insulin resistance and pre-diabetes in young Chinese women: evidence from a cross-sectional study and effect of liraglutide. PeerJ 2021; 9: e11869. doi: 10.7717/peerj.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qu H, Qiu Y, Wang Y, et al. Plasma fetuin-B concentrations are associated with insulin resistance and first-phase glucose-stimulated insulin secretion in individuals with different degrees of glucose tolerance. Diabetes Metab 2018; 44: 488–492. doi: 10.1016/j.diabet.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 99.Li Z, Lin M, Liu C, et al. Fetuin-B links nonalcoholic fatty liver disease to type 2 diabetes via inducing insulin resistance: Association and path analyses. Cytokine 2018; 108: 145–150. doi: 10.1016/j.cyto.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 100.Meex RC, Hoy AJ, Morris A, et al. Fetuin B Is a Secreted Hepatocyte Factor Linking Steatosis to Impaired Glucose Metabolism. Cell Metab 2015; 22: 1078–1089. doi: 10.1016/j.cmet.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 101.Wang D, Wu M, Zhang X, et al. Hepatokine Fetuin B expression is regulated by leptin-STAT3 signalling and associated with leptin in obesity. Sci Rep 2022; 12: 12869. doi: 10.1038/s41598-022-17000-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adamska A, Polak AM, Krentowska A, et al. Increased serum fetuin-B concentration is associated with HOMA-β and indices of liver steatosis in women with polycystic ovary syndrome: a pilot study. Endocr Connect 2019; 8: 1159–1167. doi: 10.1530/EC-19-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peter A, Kovarova M, Staiger H, et al. The hepatokines fetuin-A and fetuin-B are upregulated in the state of hepatic steatosis and may differently impact on glucose homeostasis in humans. Am J Physiol Endocrinol Metab 2018; 314: E266–E273. doi: 10.1152/ajpendo.00262.2017. [DOI] [PubMed] [Google Scholar]
- 104.Almarashda O, Abdi S, Yakout S, et al. Hepatokines Fetuin-A and Fetuin-B status in obese Saudi patient with diabetes mellitus type 2. Am J Transl Res 2022; 14: 3292–3302. [PMC free article] [PubMed] [Google Scholar]
- 105.Li Z, Liu C, Shi X, et al. Common genetic variants in the FETUB locus, genetically predicted fetuin-B levels, and risk of insulin resistance in obese Chinese adults. Medicine (Baltimore) 2017; 96: e9234. doi: 10.1097/MD.0000000000009234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ali II, D'Souza C, Singh J, et al. Adropin's Role in Energy Homeostasis and Metabolic Disorders. Int J Mol Sci 2022; 23: 8318. doi: 10.3390/ijms23158318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen X, Chen S, Shen T, et al. Adropin regulates hepatic glucose production via PP2A/AMPK pathway in insulin-resistant hepatocytes. FASEB J 2020; 34: 10056–10072. doi: 10.1096/fj.202000115RR. [DOI] [PubMed] [Google Scholar]
- 108.Erman H, Ozdemir A, Sitar ME, et al. Role of serum adropin measurement in the assessment of insulin resistance in obesity. J Investig Med 2021; 69: 1318–1323. doi: 10.1136/jim-2021-001796. [DOI] [PubMed] [Google Scholar]
- 109.Gao S, McMillan RP, Zhu Q, et al. Therapeutic effects of adropin on glucose tolerance and substrate utilization in diet-induced obese mice with insulin resistance. Mol Metab 2015; 4: 310–324. doi: 10.1016/j.molmet.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ganesh Kumar K, Zhang J, Gao S, et al. Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity (Silver Spring) 2012; 20: 1394–1402. doi: 10.1038/oby.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scisciola L, Cataldo V, Taktaz F, et al. Anti-inflammatory role of SGLT2 inhibitors as part of their anti-atherosclerotic activity: Data from basic science and clinical trials. Front Cardiovasc Med 2022; 9: 1008922. doi: 10.3389/fcvm.2022.1008922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Andersson C, Nayor M, Tsao CW, et al. Framingham Heart Study: JACC Focus Seminar, 1/8. J Am Coll Cardiol 2021; 77: 2680–2692. doi: 10.1016/j.jacc.2021.01.059. [DOI] [PubMed] [Google Scholar]
- 113.Andersson C, Johnson AD, Benjamin EJ, et al. 70-year legacy of the Framingham Heart Study. Nat Rev Cardiol 2019; 16: 687–698. doi: 10.1038/s41569-019-0202-5. [DOI] [PubMed] [Google Scholar]
- 114.Mahmood SS, Levy D, Vasan RS, et al. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet 2014; 383: 999–1008. doi: 10.1016/S0140-6736(13)61752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sharrett AR, Ballantyne CM, Coady SA, et al. ; Atherosclerosis Risk in Communities Study Group. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2001; 104: 1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 116.Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism 2014; 63: 1469–1479. doi: 10.1016/j.metabol.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 117.Athyros VG, Doumas M, Imprialos KP, et al. Diabetes and lipid metabolism. Hormones (Athens) 2018; 17: 61–67. doi: 10.1007/s42000-018-0014-8. [DOI] [PubMed] [Google Scholar]
- 118.Ginsberg HN, Zhang YL, Hernandez-Ono A. Metabolic syndrome: focus on dyslipidemia. Obesity (Silver Spring) 2006; 14: 41S–49S. doi: 10.1038/oby.2006.281. [DOI] [PubMed] [Google Scholar]
- 119.Kaze AD, Santhanam P, Musani SK, et al. Metabolic Dyslipidemia and Cardiovascular Outcomes in Type 2 Diabetes Mellitus: Findings From the Look AHEAD Study. J Am Heart Assoc 2021; 10: e016947. doi: 10.1161/JAHA.120.016947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Johansen CT, Hegele RA. Using Mendelian randomization to determine causative factors in cardiovascular disease. J Intern Med 2013; 273: 44–47. doi: 10.1111/j.1365-2796.2012.02586.x. [DOI] [PubMed] [Google Scholar]
- 121.Jørgensen AB, Frikke-Schmidt R, West AS, et al. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J 2013; 34: 1826–1833. doi: 10.1093/eurheartj/ehs431. [DOI] [PubMed] [Google Scholar]
- 122.Peng J, Luo F, Ruan G, et al. Hypertriglyceridemia and atherosclerosis. Lipids Health Dis 2017; 16: 233. doi: 10.1186/s12944-017-0625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet 2014; 384: 626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 124.Duran EK, Aday AW, Cook NR, et al. Triglyceride-Rich Lipoprotein Cholesterol, Small Dense LDL Cholesterol, and Incident Cardiovascular Disease. J Am Coll Cardiol 2020; 75: 2122–2135. doi: 10.1016/j.jacc.2020.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bahiru E, Hsiao R, Phillipson D, et al. Mechanisms and Treatment of Dyslipidemia in Diabetes. Curr Cardiol Rep 2021; 23: 26. doi: 10.1007/s11886-021-01455-w. [DOI] [PubMed] [Google Scholar]
- 126.von Bibra H, Saha S, Hapfelmeier A, et al. Impact of the Triglyceride/High-Density Lipoprotein Cholesterol Ratio and the Hypertriglyceremic-Waist Phenotype to Predict the Metabolic Syndrome and Insulin Resistance. Horm Metab Res 2017; 49: 542–549. doi: 10.1055/s-0043-107782. [DOI] [PubMed] [Google Scholar]
- 127.Hoogeveen RC, Gaubatz JW, Sun W, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol 2014; 34: 1069–1077. doi: 10.1161/ATVBAHA.114.303284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hirano T. Pathophysiology of Diabetic Dyslipidemia. J Atheroscler Thromb 2018; 25: 771–782. doi: 10.5551/jat.RV17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ji LL, Yeo D. Oxidative stress: an evolving definition. Fac Rev 2021; 10: 13. doi: 10.12703/r/10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brieger K, Schiavone S, Miller FJ, Jr, et al. Reactive oxygen species: from health to disease. Swiss Med Wkly 2012; 142: w13659. doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- 131.Kattoor AJ, Pothineni NVK, Palagiri D, et al. Oxidative Stress in Atherosclerosis. Curr Atheroscler Rep 2017; 19: 42. doi: 10.1007/s11883-017-0678-6 [DOI] [PubMed] [Google Scholar]
- 132. Leopold JA, Loscalzo J. Oxidative mechanisms and atherothrombotic cardiovascular disease. Drug Discov Today Ther Strateg 2008; 5: 5–13. doi: 10.1016/j.ddstr.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Senoner T, Dichtl W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019; 11: 2090. doi: 10.3390/nu11092090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nowotny K, Jung T, Höhn A, et al. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015; 5: 194–222. doi: 10.3390/biom5010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res 2010; 106: 842–853. doi: 10.1161/CIRCRESAHA.109.212217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Luc K, Schramm-Luc A, Guzik TJ, et al. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol 2019; 70. doi: 10.26402/jpp.2019.6.01. [DOI] [PubMed] [Google Scholar]
- 137.Ighodaro OM. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharmacother 2018; 108: 656–662. doi: 10.1016/j.biopha.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 138.Botts SR, Fish JE, Howe KL. Dysfunctional Vascular Endothelium as a Driver of Atherosclerosis: Emerging Insights Into Pathogenesis and Treatment. Front Pharmacol 2021; 12: 787541. doi: 10.3389/fphar.2021.787541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Godo S, Shimokawa H. Endothelial Functions. Arterioscler Thromb Vasc Biol 2017; 37: e108–e114. doi: 10.1161/ATVBAHA.117.309813. [DOI] [PubMed] [Google Scholar]
- 140.Tousoulis D, Simopoulou C, Papageorgiou N, et al. Endothelial dysfunction in conduit arteries and in microcirculation. Novel therapeutic approaches. Pharmacol Ther 2014; 144: 253–267. doi: 10.1016/j.pharmthera.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 141.Cyr AR, Huckaby LV, Shiva SS, et al. Nitric Oxide and Endothelial Dysfunction. Crit Care Clin 2020; 36: 307–321. doi: 10.1016/j.ccc.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Konukoglu D, Uzun H. Endothelial Dysfunction and Hypertension. Adv Exp Med Biol 2017; 956: 511–540. doi: 10.1007/5584_2016_90. [DOI] [PubMed] [Google Scholar]
- 143.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 2001; 357: 593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res 2007; 75: 247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sadler CJ, Wilding JP. Reduced ventromedial hypothalamic neuronal nitric oxide synthase and increased sensitivity to NOS inhibition in dietary obese rats: further evidence of a role for nitric oxide in the regulation of energy balance. Brain Res 2004; 1016: 222–228. [DOI] [PubMed] [Google Scholar]
- 146.Artunc F, Schleicher E, Weigert C, et al. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol 2016; 12: 721–737. doi: 10.1038/nrneph.2016.145. [DOI] [PubMed] [Google Scholar]
- 147.Cook S, Scherrer U. Insulin resistance, a new target for nitric oxide-delivery drugs. Fundam Clin Pharmacol 2002; 16: 441–453. doi: 10.1046/j.1472-8206.2002.00130.x. [DOI] [PubMed] [Google Scholar]
- 148.Wu G, Meininger CJ. Nitric oxide and vascular insulin resistance. Biofactors 2009; 35: 21–27. doi: 10.1002/biof.3. [DOI] [PubMed] [Google Scholar]
- 149.Sansbury BE, Hill BG. Regulation of obesity and insulin resistance by nitric oxide. Free Radic Biol Med 2014; 73: 383–399. doi: 10.1016/j.freeradbiomed.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sartori C, Scherrer U. Insulin, nitric oxide and the sympathetic nervous system: at the crossroads of metabolic and cardiovascular regulation. J Hypertens 1999; 17: 1517–1525. doi: 10.1097/00004872-199917110-00003. [DOI] [PubMed] [Google Scholar]
- 151.Brillante DG, O'Sullivan AJ, Howes LG. Arterial stiffness in insulin resistance: the role of nitric oxide and angiotensin II receptors. Vasc Health Risk Manag 2009; 5: 73–78. [PMC free article] [PubMed] [Google Scholar]
- 152.Saxena T, Ali AO, Saxena M. Pathophysiology of essential hypertension: an update. Expert Rev Cardiovasc Ther 2018; 16: 879–887. doi: 10.1080/14779072.2018.1540301. [DOI] [PubMed] [Google Scholar]
- 153.Wang F, Han L, Hu D. Fasting insulin, insulin resistance and risk of hypertension in the general population: A meta-analysis. Clin Chim Acta 2017; 464: 57–63. doi: 10.1016/j.cca.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 154.Lind L, Berne C, Lithell H. Prevalence of insulin resistance in essential hypertension. J Hypertens 1995; 13: 1457–1462. [PubMed] [Google Scholar]
- 155.Manrique C, Lastra G, Gardner M, et al. The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin North Am 2009; 93: 569–582. doi: 10.1016/j.mcna.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Minh HV, Tien HA, Sinh CT, et al. Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J Clin Hypertens (Greenwich) 2021; 23: 529–537. doi: 10.1111/jch.14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988; 37: 1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 158.Lamounier-Zepter V, Ehrhart-Bornstein M, Bornstein SR. Insulin resistance in hypertension and cardiovascular disease. Best Pract Res Clin Endocrinol Metab 2006; 20: 355–367. doi: 10.1016/j.beem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 159.Marcus Y, Shefer G, Stern N. Adipose tissue renin-angiotensin-aldosterone system (RAAS) and progression of insulin resistance. Mol Cell Endocrinol 2013; 378: 1–14. doi: 10.1016/j.mce.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 160.Weidmann P, Böhlen L, de Courten M. Insulin resistance and hyperinsulinemia in hypertension. J Hypertens Suppl 1995; 13: S65–S72. doi: 10.1097/00004872-199508001-00010. [DOI] [PubMed] [Google Scholar]
- 161.Zhou MS, Schulman IH, Raij L. Vascular inflammation, insulin resistance, and endothelial dysfunction in salt-sensitive hypertension: role of nuclear factor kappa B activation. J Hypertens 2010; 28: 527–535. doi: 10.1097/HJH.0b013e3283340da8. [DOI] [PubMed] [Google Scholar]
- 162.Bernardi S, Michelli A, Zuolo G, et al. Update on RAAS Modulation for the Treatment of Diabetic Cardiovascular Disease. J Diabetes Res 2016; 2016: 8917578. doi: 10.1155/2016/8917578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Puglisi S, Rossini A, Poli R, et al. Effects of SGLT2 Inhibitors and GLP-1 Receptor Agonists on Renin-Angiotensin-Aldosterone System. Front Endocrinol (Lausanne) 2021; 12: 738848. doi: 10.3389/fendo.2021.738848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Safian RD. Renal artery stenosis. Prog Cardiovasc Dis 2021; 65: 60–70. doi: 10.1016/j.pcad.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 165.Gupta R, Assiri S, Cooper CJ. Renal Artery Stenosis: New Findings from the CORAL Trial. Curr Cardiol Rep 2017; 19: 75. doi: 10.1007/s11886-017-0894-2. [DOI] [PubMed] [Google Scholar]
- 166.Katsiki N, Athyros VG, Karagiannis A, et al. Metabolic syndrome and non-cardiac vascular diseases: an update from human studies. Curr Pharm Des 2014; 20: 4944–4952. doi: 10.2174/1381612819666131206100750. [DOI] [PubMed] [Google Scholar]
- 167.Wang T, Li M, Zeng T, et al. Association Between Insulin Resistance and Cardiovascular Disease Risk Varies According to Glucose Tolerance Status: A Nationwide Prospective Cohort Study. Diabetes Care 2022; 45: 1863–1872. doi: 10.2337/dc22-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Muscogiuri G, Barrea L, Caprio M, et al. Nutritional guidelines for the management of insulin resistance. Crit Rev Food Sci Nutr 2022; 62: 6947–6960. doi: 10.1080/10408398.2021.1908223. [DOI] [PubMed] [Google Scholar]
- 169.Willems AEM, Sura-de Jong M, van Beek AP, et al. Effects of macronutrient intake in obesity: a meta-analysis of low-carbohydrate and low-fat diets on markers of the metabolic syndrome. Nutr Rev 2021; 79: 429–444. doi: 10.1093/nutrit/nuaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kahleova H, Matoulek M, Malinska H, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet Med 2011; 28: 549–559. doi: 10.1111/j.1464-5491.2010.03209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mirabelli M, Chiefari E, Arcidiacono B, et al. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020; 12: 1066. doi: 10.3390/nu12041066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Estruch R, Ros E, Salas-Salvadó J, et al. ; PREDIMED Study Investigators. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med 2018; 378: e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 173.Velázquez-López L, Santiago-Díaz G, Nava-Hernández J, et al. Mediterranean-style diet reduces metabolic syndrome components in obese children and adolescents with obesity. BMC Pediatr 2014; 14: 175. doi: 10.1186/1471-2431-14-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tettamanzi F, Bagnardi V, Louca P, et al. A High Protein Diet Is More Effective in Improving Insulin Resistance and Glycemic Variability Compared to a Mediterranean Diet-A Cross-Over Controlled Inpatient Dietary Study. Nutrients 2021; 13: 4380. doi: 10.3390/nu13124380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gershuni VM, Yan SL, Medici V. Nutritional Ketosis for Weight Management and Reversal of Metabolic Syndrome. Curr Nutr Rep 2018; 7: 97–106. doi: 10.1007/s13668-018-0235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shang Y, Zhou H, Hu M, et al. Effect of Diet on Insulin Resistance in Polycystic Ovary Syndrome. J Clin Endocrinol Metab 2020; 105: dgaa425. doi: 10.1210/clinem/dgaa425. [DOI] [PubMed] [Google Scholar]
- 177.Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev 2017; 39: 46–58. doi: 10.1016/j.arr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sutton EF, Beyl R, Early KS, et al. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab 2018; 27: 1212–1221.e3. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2011; 35: 714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Festi D, Schiumerini R, Eusebi LH, et al. Gut microbiota and metabolic syndrome. World J Gastroenterol 2014; 20: 16079–16094. doi: 10.3748/wjg.v20.i43.16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wimalawansa SJ. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J Steroid Biochem Mol Biol 2018; 175: 177–189. doi: 10.1016/j.jsbmb.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 182.Pittas AG, Dawson-Hughes B, Sheehan P, et al. ; D2d Research Group. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N Engl J Med 2019; 381: 520–530. doi: 10.1056/NEJMoa1900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pareek M, Schauer PR, Kaplan LM, et al. Metabolic Surgery: Weight Loss, Diabetes, and Beyond. J Am Coll Cardiol 2018; 71: 670–687. doi: 10.1016/j.jacc.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 184.Villarreal-Calderón JR, Cuéllar RX, Ramos-González MR, et al. Interplay between the Adaptive Immune System and Insulin Resistance in Weight Loss Induced by Bariatric Surgery. Oxid Med Cell Longev 2019; 2019: 3940739. doi: 10.1155/2019/3940739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ruegsegger GN, Booth FW. Health Benefits of Exercise. Cold Spring Harb Perspect Med 2018; 8: a029694. doi: 10.1101/cshperspect.a029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ben-Zeev T, Okun E. High-Intensity Functional Training: Molecular Mechanisms and Benefits. Neuromolecular Med 2021; 23: 335–338. doi: 10.1007/s12017-020-08638-8. [DOI] [PubMed] [Google Scholar]
- 187.Katzmarzyk PT, Church TS, Blair SN. Cardiorespiratory fitness attenuates the effects of the metabolic syndrome on all-cause and cardiovascular disease mortality in men. Arch Intern Med 2004; 164: 1092–1097. doi: 10.1001/archinte.164.10.1092. [DOI] [PubMed] [Google Scholar]
- 188.Katzmarzyk PT, Church TS, Janssen I, et al. Metabolic syndrome, obesity, and mortality: impact of cardiorespiratory fitness. Diabetes Care 2005; 28: 391–397. doi: 10.2337/diacare.28.2.391. [DOI] [PubMed] [Google Scholar]
- 189.Laaksonen DE, Lakka HM, Salonen JT, et al. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care 2002; 25: 1612–1618. doi: 10.2337/diacare.25.9.1612. [DOI] [PubMed] [Google Scholar]
- 190.Sampath Kumar A, Maiya AG, Shastry BA, et al. Exercise and insulin resistance in type 2 diabetes mellitus: A systematic review and meta-analysis. Ann Phys Rehabil Med 2019; 62: 98–103. doi: 10.1016/j.rehab.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 191.Motahari-Tabari N, Ahmad Shirvani M, Shirzad-E-Ahoodashty M, et al. The effect of 8 weeks aerobic exercise on insulin resistance in type 2 diabetes: a randomized clinical trial. Glob J Health Sci 2014; 7: 115–121. doi: 10.5539/gjhs.v7n1p115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol 2013; 3: 1–58. doi: 10.1002/cphy.c110062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nery C, Moraes SRA, Novaes KA, et al. Effectiveness of resistance exercise compared to aerobic exercise without insulin therapy in patients with type 2 diabetes mellitus: a meta-analysis. Braz J Phys Ther 2017; 21: 400–415. doi: 10.1016/j.bjpt.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fedewa MV, Gist NH, Evans EM, et al. Exercise and insulin resistance in youth: a meta-analysis. Pediatrics 2014; 133: e163–e174. doi: 10.1542/peds.2013-2718. [DOI] [PubMed] [Google Scholar]
- 195.Marson EC, Delevatti RS, Prado AK, et al. Effects of aerobic, resistance, and combined exercise training on insulin resistance markers in overweight or obese children and adolescents: A systematic review and meta-analysis. Prev Med 2016; 93: 211–218. doi: 10.1016/j.ypmed.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 196.Szymczak-Pajor I, Wenclewska S, Śliwińska A. Metabolic Action of Metformin. Pharmaceuticals (Basel) 2022; 15: 810. doi: 10.3390/ph15070810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Herman R, Kravos NA, Jensterle M, et al. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int J Mol Sci 2022; 23: 1264. doi: 10.3390/ijms23031264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Giannarelli R, Aragona M, Coppelli A, et al. Reducing insulin resistance with metformin: the evidence today. Diabetes Metab 2003; 29: 6S28–6S35. doi: 10.1016/s1262-3636(03)72785-2. [DOI] [PubMed] [Google Scholar]
- 199.Snaith JR, Samocha-Bonet D, Evans J, et al. Insulin resistance in type 1 diabetes managed with metformin (INTIMET): Study protocol of a double-blind placebo-controlled, randomised trial. Diabet Med 2021; 38: e14564. doi: 10.1111/dme.14564. [DOI] [PubMed] [Google Scholar]
- 200.Brame L, Verma S, Anderson T, et al. Insulin resistance as a therapeutic target for improved endothelial function: metformin. Curr Drug Targets Cardiovasc Haematol Disord 2004; 4: 53–63. doi: 10.2174/1568006043481275. [DOI] [PubMed] [Google Scholar]
- 201.Cree-Green M, Bergman BC, Cengiz E, et al. Metformin Improves Peripheral Insulin Sensitivity in Youth With Type 1 Diabetes. J Clin Endocrinol Metab 2019; 104: 3265–3278. doi: 10.1210/jc.2019-00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ben-Haroush A, Yogev Y, Fisch B. Insulin resistance and metformin in polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2004; 115: 125–133. doi: 10.1016/j.ejogrb.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 203.Pugeat M, Ducluzeau PH. Insulin resistance, polycystic ovary syndrome and metformin. Drugs 1999; 58: 41–46; discussion 75–82. doi: 10.2165/00003495-199958001-00010. [DOI] [PubMed] [Google Scholar]
- 204.Fruzzetti F, Perini D, Russo M, et al. Comparison of two insulin sensitizers, metformin and myo-inositol, in women with polycystic ovary syndrome (PCOS). Gynecol Endocrinol 2017; 33: 39–42. doi: 10.1080/09513590.2016.1236078. [DOI] [PubMed] [Google Scholar]
- 205.Zhao H, Xing C, Zhang J, et al. Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with PCOS: a network meta-analysis. Reprod Health 2021; 18: 171. doi: 10.1186/s12978-021-01207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.RISE Consortium. Impact of Insulin and Metformin Versus Metformin Alone on β-Cell Function in Youth With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes. Diabetes Care 2018; 41: 1717–1725. doi: 10.2337/dc18-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hauner H. The mode of action of thiazolidinediones. Diabetes Metab Res Rev 2002; 18: S10–S15. doi: 10.1002/dmrr.249. [DOI] [PubMed] [Google Scholar]
- 208.Vasudevan AR, Balasubramanyam A. Thiazolidinediones: a review of their mechanisms of insulin sensitization, therapeutic potential, clinical efficacy, and tolerability. Diabetes Technol Ther 2004; 6: 850–863. doi: 10.1089/dia.2004.6.850. [DOI] [PubMed] [Google Scholar]
- 209.Lebovitz HE. Thiazolidinediones: the Forgotten Diabetes Medications. Curr Diab Rep 2019; 19: 151. doi: 10.1007/s11892-019-1270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Di Pino A, DeFronzo RA. Insulin Resistance and Atherosclerosis: Implications for Insulin-Sensitizing Agents. Endocr Rev 2019; 40: 1447–1467. doi: 10.1210/er.2018-00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kernan WN, Viscoli CM, Furie KL, et al. ; IRIS Trial Investigators. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med 2016; 374: 1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sunayama S, Watanabe Y, Daida H, et al. Thiazolidinediones, dyslipidaemia and insulin resistance syndrome. Curr Opin Lipidol 2000; 11: 397–402. doi: 10.1097/00041433-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 213.Del Prato S, Marchetti P. Targeting insulin resistance and beta-cell dysfunction: the role of thiazolidinediones. Diabetes Technol Ther 2004; 6: 719–731. doi: 10.1089/dia.2004.6.719. [DOI] [PubMed] [Google Scholar]
- 214.Campbell IW, Mariz S. Beta-cell preservation with thiazolidinediones. Diabetes Res Clin Pract 2007; 76: 163–176. doi: 10.1016/j.diabres.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 215.Scheen AJ. Etude clinique du mois. L'étude DREAM: prévention du diabète de type 2 par le ramipiril et/ou la rosiglitazone chez les personnes dysglycémiques sans maladie cardio-vasculaire [DREAM study: prevention of type 2 diabetes with ramipril and/or rosiglitazone in persons with dysglycaemia but no cardiovascular desease]. Rev Med Liege 2006; 61: 728–732 [Article in French, English abstract]. [PubMed] [Google Scholar]
- 216.Punthakee Z, Alméras N, Després JP, et al. Impact of rosiglitazone on body composition, hepatic fat, fatty acids, adipokines and glucose in persons with impaired fasting glucose or impaired glucose tolerance: a sub-study of the DREAM trial. Diabet Med 2014; 31: 1086–1092. doi: 10.1111/dme.12512. [DOI] [PubMed] [Google Scholar]
- 217.Scheen AJ. L'etude clinique du mois. L'étude ADOPT: quel antidiabétique oral initier chez le patient diabétique de type 2? [ADOPT study: which first-line glucose-lowering oral medication in type 2 diabetes?]. Rev Med Liege 2007; 62: 48–52 [Article in French, English abstract]. [PubMed] [Google Scholar]
- 218.Betteridge DJ. CHICAGO, PERISCOPE and PROactive: CV risk modification in diabetes with pioglitazone. Fundam Clin Pharmacol 2009; 23: 675–679. doi: 10.1111/j.1472-8206.2009.00741.x. [DOI] [PubMed] [Google Scholar]
- 219.Cusi K, Orsak B, Bril F, et al. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med 2016; 165: 305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 220.He L, Liu X, Wang L, et al. Thiazolidinediones for nonalcoholic steatohepatitis: A meta-analysis of randomized clinical trials. Medicine (Baltimore) 2016; 95: e4947. doi: 10.1097/MD.0000000000004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Laurindo LF, Barbalho SM, Guiguer EL, et al. GLP-1a: Going beyond Traditional Use. Int J Mol Sci 2022; 23: 739. doi: 10.3390/ijms23020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cena H, Chiovato L, Nappi RE. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J Clin Endocrinol Metab 2020; 105: e2695–e2709. doi: 10.1210/clinem/dgaa285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Armstrong MJ, Hull D, Guo K, et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol 2016; 64: 399–408. doi: 10.1016/j.jhep.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Armstrong MJ, Gaunt P, Aithal GP, et al. ; LEAN trial team. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016; 387: 679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 225.Zhao X, Wang M, Wen Z, et al. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front Endocrinol (Lausanne) 2021; 12: 721135. doi: 10.3389/fendo.2021.721135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Han Y, Li Y, He B. GLP-1 receptor agonists versus metformin in PCOS: a systematic review and meta-analysis. Reprod Biomed Online 2019; 39: 332–342. doi: 10.1016/j.rbmo.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 227.Aroda VR. A review of GLP-1 receptor agonists: Evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab 2018; 20: 22–33. doi: 10.1111/dom.13162. PMID: 29364586. [DOI] [PubMed] [Google Scholar]
- 228.Ma X, Liu Z, Ilyas I, et al. GLP-1 receptor agonists (GLP-1RAs): cardiovascular actions and therapeutic potential. Int J Biol Sci 2021; 17: 2050–2068. doi: 10.7150/ijbs.59965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Andrikou E, Tsioufis C, Andrikou I, et al. GLP-1 receptor agonists and cardiovascular outcome trials: An update. Hellenic J Cardiol 2019; 60: 347–351. doi: 10.1016/j.hjc.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 230.Bethel MA, Patel RA, Merrill P, et al.; EXSCEL Study Group. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol 2018; 6: 105–113. doi: 10.1016/S2213-8587(17)30412-6. [DOI] [PubMed] [Google Scholar]
- 231.Pirro V, Roth KD, Lin Y, et al. Effects of Tirzepatide, a Dual GIP and GLP-1 RA, on Lipid and Metabolite Profiles in Subjects With Type 2 Diabetes. J Clin Endocrinol Metab 2022; 107: 363–378. doi: 10.1210/clinem/dgab722. [DOI] [PubMed] [Google Scholar]
- 232.Nyström T. The potential beneficial role of glucagon-like peptide-1 in endothelial dysfunction and heart failure associated with insulin resistance. Horm Metab Res 2008; 40: 593–606. doi: 10.1055/s-0028-1082326. [DOI] [PubMed] [Google Scholar]
- 233.Tentolouris A, Vlachakis P, Tzeravini E, et al. SGLT2 Inhibitors: A Review of Their Antidiabetic and Cardioprotective Effects. Int J Environ Res Public Health 2019; 16: 2965. doi: 10.3390/ijerph16162965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Frampton JE. Empagliflozin: A Review in Type 2 Diabetes. Drugs 2018; 78: 1037–1048. doi: 10.1007/s40265-018-0937-z. [DOI] [PubMed] [Google Scholar]
- 235.Xu L, Ota T. Emerging roles of SGLT2 inhibitors in obesity and insulin resistance: Focus on fat browning and macrophage polarization. Adipocyte 2018; 7: 121–128. doi: 10.1080/21623945.2017.1413516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kullmann S, Hummel J, Wagner R, et al. Empagliflozin Improves Insulin Sensitivity of the Hypothalamus in Humans With Prediabetes: A Randomized, Double-Blind, Placebo-Controlled, Phase 2 Trial. Diabetes Care 2022; 45: 398–406. doi: 10.2337/dc21-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hiruma S, Shigiyama F, Hisatake S, et al. A prospective randomized study comparing effects of empagliflozin to sitagliptin on cardiac fat accumulation, cardiac function, and cardiac metabolism in patients with early-stage type 2 diabetes: the ASSET study. Cardiovasc Diabetol 2021; 20: 32. doi: 10.1186/s12933-021-01228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014; 124: 499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wei Q, Xu X, Guo L, et al. Effect of SGLT2 Inhibitors on Type 2 Diabetes Mellitus With Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Front Endocrinol (Lausanne) 2021; 12: 635556. doi: 10.3389/fendo.2021.635556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhang Y, Liu X, Zhang H, et al. Efficacy and Safety of Empagliflozin on Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2022; 13: 836455. doi: 10.3389/fendo.2022.836455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.He K, Li J, Xi W, et al. Dapagliflozin for nonalcoholic fatty liver disease: A systematic review and meta-analysis. Diabetes Res Clin Pract 2022; 185: 109791. doi: 10.1016/j.diabres.2022.109791. [DOI] [PubMed] [Google Scholar]
- 242.Sun L, Deng C, Gu Y, et al. Effects of dapagliflozin in patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol 2022; 46: 101876. doi: 10.1016/j.clinre.2022.101876. [DOI] [PubMed] [Google Scholar]
- 243.Sinha B, Ghosal S. A Meta-Analysis of the Effect of Sodium Glucose Cotransporter-2 Inhibitors on Metabolic Parameters in Patients With Polycystic Ovary Syndrome. Front Endocrinol (Lausanne) 2022; 13: 830401. doi: 10.3389/fendo.2022.830401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yaribeygi H, Sathyapalan T, Maleki M, et al. Molecular mechanisms by which SGLT2 inhibitors can induce insulin sensitivity in diabetic milieu: A mechanistic review. Life Sci 2020; 240: 117090. doi: 10.1016/j.lfs.2019.117090. [DOI] [PubMed] [Google Scholar]
- 245.Lingli X, Wenfang X. Characteristics and molecular mechanisms through which SGLT2 inhibitors improve metabolic diseases: A mechanism review. Life Sci 2022; 300: 120543. doi: 10.1016/j.lfs.2022.120543. [DOI] [PubMed] [Google Scholar]
- 246.Marton A, Kaneko T, Kovalik JP, et al. Organ protection by SGLT2 inhibitors: role of metabolic energy and water conservation. Nat Rev Nephrol 2021; 17: 65–77. doi: 10.1038/s41581-020-00350-x. [DOI] [PubMed] [Google Scholar]
- 247.Hou YC, Zheng CM, Yen TH, et al. Molecular Mechanisms of SGLT2 Inhibitor on Cardiorenal Protection. Int J Mol Sci 2020; 21: 7833. doi: 10.3390/ijms21217833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol 2020; 17: 761–772. doi: 10.1038/s41569-020-0406-8. [DOI] [PubMed] [Google Scholar]
- 249.Fathi A, Vickneson K, Singh JS. SGLT2-inhibitors; more than just glycosuria and diuresis. Heart Fail Rev 2021; 26: 623–642. doi: 10.1007/s10741-020-10038-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019; 393: 31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 251.Zou CY, Liu XK, Sang YQ, et al. Effects of SGLT2 inhibitors on cardiovascular outcomes and mortality in type 2 diabetes: A meta-analysis. Medicine (Baltimore) 2019; 98: e18245. doi: 10.1097/MD.0000000000018245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Brown E, Heerspink HJL, Cuthbertson DJ, et al. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet 2021; 398: 262–276. doi: 10.1016/S0140-6736(21)00536-5. [DOI] [PubMed] [Google Scholar]
- 253.Lee MMY, Petrie MC, McMurray JJV, et al. How Do SGLT2 (Sodium-Glucose Cotransporter 2) Inhibitors and GLP-1 (Glucagon-Like Peptide-1) Receptor Agonists Reduce Cardiovascular Outcomes?: Completed and Ongoing Mechanistic Trials. Arterioscler Thromb Vasc Biol 2020; 40: 506–522. doi: 10.1161/ATVBAHA.119.311904. [DOI] [PubMed] [Google Scholar]
- 254.Bertoccini L, Baroni MG. GLP-1 Receptor Agonists and SGLT2 Inhibitors for the Treatment of Type 2 Diabetes: New Insights and Opportunities for Cardiovascular Protection. Adv Exp Med Biol 2021; 1307: 193–212. doi: 10.1007/5584_2020_494. [DOI] [PubMed] [Google Scholar]
- 255.Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 2021; 372: m4573. doi: 10.1136/bmj.m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Li S, Vandvik PO, Lytvyn L, et al. SGLT-2 inhibitors or GLP-1 receptor agonists for adults with type 2 diabetes: a clinical practice guideline. BMJ 2021; 373: n1091. doi: 10.1136/bmj.n1091. [DOI] [PubMed] [Google Scholar]
- 257.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 258.Park SE, Park CY, Sweeney G. Biomarkers of insulin sensitivity and insulin resistance: Past, present and future. Crit Rev Clin Lab Sci 2015; 52: 180–190. doi: 10.3109/10408363.2015.1023429. [DOI] [PubMed] [Google Scholar]
- 259.Levy-Marchal C, Arslanian S, Cutfield W, et al. ; ESPE-LWPES-ISPAD-APPES-APEG-SLEP-JSPE; Insulin Resistance in Children Consensus Conference Group. Insulin resistance in children: consensus, perspective, and future directions. J Clin Endocrinol Metab 2010; 95: 5189–5198. doi: 10.1210/jc.2010-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yoon MS. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016; 8: 405. doi: 10.3390/nu8070405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Pinti MV, Fink GK, Hathaway QA, et al. Mitochondrial dysfunction in type 2 diabetes mellitus: an organ-based analysis. Am J Physiol Endocrinol Metab 2019; 316: E268–E285. doi: 10.1152/ajpendo.00314.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kumariya S, Ubba V, Jha RK, et al. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy 2021; 17: 2706–2733. doi: 10.1080/15548627.2021.1938914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Luo Y, Cui C, Han X, et al. The role of miRNAs in polycystic ovary syndrome with insulin resistance. J Assist Reprod Genet 2021; 38: 289–304. doi: 10.1007/s10815-020-02019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Li T, Zhu L, Zhu L, et al. Recent Developments in Delivery of MicroRNAs Utilizing Nanosystems for Metabolic Syndrome Therapy. Int J Mol Sci 2021; 22: 7855. doi: 10.3390/ijms22157855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Miao C, Zhang G, Xie Z, et al. MicroRNAs in the pathogenesis of type 2 diabetes: new research progress and future direction. Can J Physiol Pharmacol 2018; 96: 103–112. doi: 10.1139/cjpp-2017-0452. [DOI] [PubMed] [Google Scholar]
- 266.Wong C, Dash A, Fredrickson J, et al. Fibroblast growth factor receptor 1/Klothoβ agonist BFKB8488A improves lipids and liver health markers in patients with diabetes or NAFLD: A phase 1b randomized trial. Hepatology 2022. doi: 10.1002/hep.32742. [DOI] [PubMed] [Google Scholar]