Abstract
Purpose of Review
Preeclampsia and related hypertensive disorders of pregnancy affect up to 10% of pregnancies. Neurological complications are common and neurologists often become involved in the care of obstetric patients with preeclampsia. Here, we review the definition(s), epidemiology, clinical features, and pathophysiology of preeclampsia, focusing on maternal neurological complications and headache as a common presenting symptom of preeclampsia.
Recent Findings
Neurological symptoms are early and disease-defining features of preeclampsia. Neurological complications of preeclampsia may include headaches, visual symptoms, cerebral edema, seizures, or acute cerebrovascular disorders such as intracerebral hemorrhage or reversible cerebral vasoconstriction syndrome. A history of migraine is an independent risk factor for vascular diseases during pregnancy, including preeclampsia and maternal stroke. The pathophysiology of both preeclampsia and migraine is complex, and the mechanisms linking the two are not fully understood. Overlapping clinical and pathophysiological features of migraine and preeclampsia include inflammation, vascular endothelial dysfunction, and changes in vasoreactivity.
Summary
Neurological complications are recognized as a major contributor to maternal morbidity and mortality. Pregnant and postpartum women commonly present with headache, and red flags in the clinical history and examination should prompt urgent neuroimaging and laboratory evaluation. A focused headache history should be elicited from patients as part of routine obstetrical care to identify patients at an increased risk of preeclampsia and related hypertensive disorders of pregnancy. Collaborative models of care and scientific investigation in the emerging field of neuro-obstetrics have the common goal of reducing the risk of maternal neurological morbidity and mortality from preeclampsia.
Keywords: Preeclampsia, Neuro-obstetrics, Headache, Stroke, Maternal mortality
Introduction
Preeclampsia and related hypertensive disorders of pregnancy are among the commonest of adverse pregnancy outcomes, affecting up to 10% of pregnancies in the USA [1]. Preeclampsia accounts for 6.9% of maternal mortality in the USA [2] and 14% worldwide [3]. Among women with preeclampsia, autopsy and detailed case series suggest that neurological complications are the direct cause of 30–70% of deaths, most often due to intracerebral hemorrhage or cerebral edema [4–7]. Despite this, unfortunately, neurological red flags in pregnant and particularly postpartum women are often missed. At least half of maternal deaths due to preeclampsia are thought to have been preventable with earlier recognition, diagnosis, and treatment [8–12].
The international obstetrics community now recognizes that neurological symptoms, including headache, can be disease-defining features of preeclampsia [13••, 14••]. However, the American College of Obstetricians and Gynecologists (ACOG) notes that “using headache as a diagnostic criterion for preeclampsia with severe features is unreliable and nonspecific” [13••]. Unsurprisingly, neurologists are frequently called to assist in the diagnostic evaluation of women who are being “ruled out” for preeclampsia. The diagnosis has major implications for blood pressure management, timing and mode of delivery, and discharge disposition. For this reason, it is critically important that neurologists understand the pathophysiology, clinical presentation, and neurological complications of preeclampsia and related disorders.
Here, we review the definitions, epidemiology, and complex pathophysiology of preeclampsia and related disorders as they affect the maternal brain, focusing on recent translational and clinical research findings. We discuss the relationship between migraine and preeclampsia, as well as the overlap between preeclampsia, the reversible cerebral vasoconstriction syndrome (RCVS), and the posterior reversible encephalopathy syndrome (PRES). Lastly, we discuss the evaluation of headache in pregnant and postpartum women at high risk for preeclampsia, focusing on headache features that should prompt escalation of care and involvement of a multidisciplinary neuro-obstetrics team.
Definitions and Epidemiology
Neurological Symptoms Are Early and Disease-Defining Features of Preeclampsia
Preeclampsia is a heterogeneous syndrome whose definition has evolved over centuries. However, headache and other neurological symptoms were recognized since the early days of medicine as a high-risk feature which can presage development of eclamptic seizures. Hippocrates noted in 400 BCE that “in pregnancy, the onset of drowsy headaches with heaviness is bad; such cases are perhaps liable to some sort of fits at the same time” [15], an apt description of eclampsia with prodromal neurological symptoms. Similarly, in 1843, Dr. Robert Johns of the Dublin Lying-In Hospital described a prodrome of “headache, weight, or giddiness in the head, ringing in the ears, [or] a temporary loss of vision… [In] most, if not all the cases… these very premonitory symptoms had been present before labour, and I argue, that had they attracted the requisite attention at that period, the subsequent convulsions might have been avoided” [16]. In 1994, Douglas and Redman reported that, among 383 women with eclampsia, half reported headache prior to their convulsions, and 19% reported visual disturbances [17]. These symptoms often preceded the recognition or diagnosis of hypertension.
In 2013, ACOG re-defined preeclampsia, specifying that the disorder may be diagnosed in the absence of proteinuria, in women with new-onset hypertension after 20 weeks gestation together with evidence of other organ dysfunction, which includes neurological symptoms such as severe headache [18]. The International Society for the Study of Hypertension in Pregnancy similarly considers neurological features to be preeclampsia-defining when coupled with new hypertension [14••]. These features include severe headache, altered mental status, eclamptic seizures, stroke, persistent visual scotomata, or cortical blindness. Currently accepted diagnostic criteria for preeclampsia are detailed in Table 1, with neurological features highlighted. Neurologists must be familiar with these criteria, as the diagnosis has significant implications for management and disposition. Neurologists should also keep in mind that preeclampsia can occur in the postpartum period, as is discussed further in the following section.
Table 1.
Defining characteristics of preeclampsia and related disorders
| ACOG (2019 revision) | ISSHP (2018 revision) | |
|---|---|---|
| Preeclampsia | ||
| New-onset, persistent hypertension at or after 20 weeks pregnancy in a woman with previously normal blood pressure AND |
|
|
| Proteinuria |
|
|
| OR In absence of proteinuria |
New onset of any of the following:
|
|
| Preeclampsia variants and related disorders | ||
| Preeclampsia superimposed on chronic hypertension |
|
|
| HELLP syndrome (Hemolysis, Elevated Liver enzymes, Low Platelets) |
|
|
| Eclampsia |
|
|
Precise definitions of preeclampsia have been debated for decades. Note there are important differences between the ACOG and ISSHP definitions, but both organizations consider severe headache or visual symptoms to be preeclampsia-defining in the setting of hypertension. ACOG notes that women may not exhibit other signs of preeclampsia (e.g., hypertension, proteinuria) before presenting with seizures. Of note, ISSHP also considers other neurological complications, including eclamptic seizures, stroke, altered mental status, and clonus, to be preeclampsia-defining in the setting of hypertension
ACOG American College of Obstetricians and Gynecologists, ISSHP International Society for the Study of Hypertension in Pregnancy, SBP systolic blood pressure, DBP diastolic blood pressure, ALT alanine aminotransferase, AST aspartate aminotransferase, LDH lactate dehydrogenase, DIC disseminated intravascular coagulation
Neuro-epidemiology of Preeclampsia and Related Disorders
Preeclampsia occurs in approximately 2–8% of pregnancies [13••]. Since many women have more than one pregnancy, the per-woman prevalence of preeclampsia may be substantially higher. A review of medical records from 9862 deliveries in Olmstead County, MN between 1976 and 1982 found that, while the incidence of hypertensive disorders of pregnancy and preeclampsia on a per-pregnancy basis was 7.3% and 3.5%, respectively, the incidence doubled when assessed on a per-woman basis [19]. Black, Indigenous, and women of color are disproportionally affected by preeclampsia as a result of systemic racism and structural inequities in healthcare [20, 21]. Preeclampsia rates are also far higher in low- and middle-income countries where access to prenatal care is limited [22]. Among women with chronic hypertension, up to 50% will develop superimposed preeclampsia [23].
Neurological complications seen in association with preeclampsia include seizures (eclampsia), arterial ischemic stroke, RCVS, PRES, cervical artery dissection, cerebral venous sinus thrombosis, subarachnoid hemorrhage (SAH), and intracerebral hemorrhage (ICH). Of these, ICH is the most devastating, directly causing up to 70% of deaths from preeclampsia [7]. While hemorrhagic strokes due to rupture of vascular lesions such as arteriovenous malformations and cerebral aneurysms have been reported in association with preeclampsia [24–27], there is often no underlying vascular lesion to account for the bleed [28]. Preeclampsia-associated maternal stroke has high morbidity and mortality. Half of strokes associated with hypertensive disorders of pregnancy are hemorrhagic [29] (for comparison, 87% of strokes are ischemic in the general population [30]), and 13% are fatal [29]. Stroke is associated with a 100-fold increase in mortality in women with hypertensive disorders of pregnancy [31].
Risk factors for neurovascular complications of preeclampsia include older age, non-white race, heart disease, chronic hypertension, infections, prothrombotic and hypercoagulable states, and history of migraine [29]. Migraine is associated with an increased risk of both hypertensive disorders of pregnancy and maternal stroke [32••], an association which will be discussed further in the following sections.
Once thought to occur only in pregnancy and be cured by delivery of the placenta, it is now clear that preeclampsia and related disorders can occur in the postpartum period, including in women who had no history of gestational hypertension [33, 34]. In a study of 121 women with delayed-onset postpartum preeclampsia, 68% had headache as the presenting symptom [35]. In addition, antepartum diagnosis of, gestational hypertension or preeclampsia diagnosed antepartum can worsen after delivery and develop neurological sequelae, including eclamptic seizures [33]. In fact, the highest risk period for maternal stroke is postpartum [36], particularly in the first 2 weeks after delivery but with risk extending up to 12 weeks postpartum [28, 37•]. Furthermore, women with a history of preeclampsia have increased cardiovascular and stroke risk and earlier onset of clinical cardiovascular disease [38••, 39]. For this reason, a thorough obstetric history should be elicited from women presenting with cerebrovascular symptoms at any age.
Pathophysiology of Preeclampsia and the Maternal Brain
Pathophysiology of Preeclampsia
Like its definition, the cause of preeclampsia has been a subject of hot debate for decades, and a comprehensive discussion of its complex pathophysiology is beyond the scope of this review. The obsolete term “toxemia” reflects initial hypotheses that the syndrome was caused by a pregnancy-induced toxic or inflammatory state [40]. Subsequently, placental insufficiency or ischemia was recognized as a key factor in the pathogenesis of preeclampsia [41]. This is thought to occur due to poor implantation of the trophoblast, incomplete remodeling of spiral arteries in the placental decidua, or failure of the maternal cardiovascular system to meet the demands for increased cardiac output during pregnancy. Imbalance of angiogenic factors such as soluble fms-like tyrosine kinase-1 (sFlt-1), soluble endoglin, and placental growth factor (PlGF), leading to systemic maternal endothelial dysfunction, has been implicated in the pathogenesis of preeclampsia [42]. Several animal models of preeclampsia have been developed, including surgical models based on reduction of uterine perfusion pressure (RUPP), genetic models based on overexpression of sFlt-1, and environmentally induced models making use of inflammation-inducing infusions [43•]. More recently, the role of maternal inflammasomes expressed on the placenta has been recognized as a major contributor to the pathophysiology of preeclampsia. Inflammasomes are multiprotein signaling complexes composed of pattern recognition receptors and pro-inflammatory caspases, which are activated in response to danger-associated molecular patterns (DAMPs) and other stress- or pathogen-induced triggers [44]. The emergence of these innate inflammatory pathways as a key component of preeclampsia pathogenesis suggests that the perennial preeclampsia debate has come full circle back to the original “toxemia” hypothesis.
Preeclampsia and Cerebral Autoregulation
Cerebral autoregulation is the process by which cerebral arterioles react dynamically to changes in systemic blood pressure to ensure a constant flow of blood to the brain and prevent hyperperfusion injury. Both animal and clinical studies have demonstrated impaired cerebral autoregulation in the preeclampsia syndrome [45•, 46, 47]. Impaired dynamic cerebral autoregulation was demonstrated in women with chronic hypertension and women with preeclampsia, but not in women with normal pregnancy or gestational hypertension [48, 49]. Another study showed paradoxically increased cerebral autoregulatory capacity in women with preeclampsia [50]. A study using a RUPP preeclampsia rat model found severely impaired autoregulation compared to controls [51•]. The same group later showed this effect to be associated with changes in the renin-angiotensin system [52]. However, another recent study showed paradoxically enhanced cerebral autoregulation in a preeclampsia rat model [53], and long-term autoregulatory dysfunction after preeclampsia was not seen in humans [54]. The complex relationship between cerebral autoregulation and preeclampsia is an area of active investigation.
Blood–Brain Barrier Dysfunction
Increased blood–brain barrier permeability has been demonstrated in several animal models of preeclampsia [51•, 55–57]. This impairment may account for the cerebral edema seen frequently in women with eclampsia and occasionally in women with preeclampsia, particularly those with neurological symptoms [58–61]. Radiographically, the pattern of edema shares some features with the posterior reversible encephalopathy syndrome (PRES) and is usually reversible if treated early and aggressively [62]. The mechanisms of blood–brain barrier dysfunction in preeclampsia are not fully understood but may be mediated in part by neuroinflammation [53, 63]. Preeclampsia is associated with elevations in serum markers of inflammation, including C-reactive protein, platelet and complement activation, and elevated pro-inflammatory cytokines including interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-17 [64, 65]. Animal models have shown that TNF-α may play a critical role in preeclampsia-associated blood–brain barrier dysfunction [56]. Proteomic analyses of cerebrospinal fluid (CSF) from women with preeclampsia have shown clear differences compared with normotensive women, including increased markers of neuroinflammation and heme-binding proteins [66, 67]. Persistent cerebral edema and neuroinflammation were demonstrated several months postpartum in a RUPP rat model [68]. However, a recent study of women with and without preeclampsia did not show evidence of increased inflammatory markers in CSF from the preeclampsia group [69]. Preeclampsia also causes shedding of microvesicles from the stressed placenta into the maternal circulation, triggering a pro-inflammatory cascade due to recognition of DAMPs by maternal inflammasomes [70–72]. However, the effect of preeclampsia-associated alarmins and their receptors on neurovascular unit function in the maternal brain is not well understood. In addition, changes in angiogenic pathways likely play a role in preeclampsia-associated neurovascular unit dysfunction [56, 73, 74]. Bevacizumab, a monoclonal vascular endothelial growth factor (VEGF) inhibitor with a mechanism analogous to that of sFlt-1, has been shown to induce a preeclampsia-like state, including headache, cerebral edema, and seizures in nonpregnant humans [75, 76].
Hyperexcitability and Vasoconstriction
Preeclampsia is associated with increased sympathetic activity [77], hyperreflexia (no longer considered a defining feature), and, in the case of eclampsia, seizures. Preeclampsia’s very name indicates its status as a precursor to eclampsia, which is defined by new generalized seizures in the setting of preeclampsia. However, preeclampsia does not always lead to eclampsia, and eclamptic convulsions may occur with little to no preeclampsia prodrome. Pregnancy itself has been shown to increase seizure susceptibility [78•]. In the setting of cerebral hyperperfusion due to impaired autoregulation, as well as increased blood–brain barrier permeability, this may contribute to the development of eclampsia. The postpartum state, similar to sympathomimetic medications, is considered a vasoconstrictive trigger for RCVS [79•]. Magnesium infusion reduces the risk of seizures in women with preeclampsia; hypothesized mechanisms include NMDA receptor antagonism, blood–brain barrier stabilization, systemic vasodilation leading to rapid blood pressure reduction, and prevention or reversal of cerebral vasospasm [80, 81]. Interestingly, intravenous magnesium has been suggested as an effective treatment for both PRES [82, 83] and RCVS [84] outside of pregnancy, but this strategy has not been tested in a clinical trial.
Cerebral Small Vessel Disease
Preeclampsia is associated with a long-term increased risk of stroke [38••, 39] and cognitive impairment [85, 86]. The reasons for this may be complex, as preeclampsia and cerebrovascular disease share many risk factors. However, animal studies have demonstrated microvascular dysfunction as a direct effect of preeclampsia [45•]. Several studies have shown higher white matter hyperintensity volumes in women with a history of preeclampsia [87–90]. Whether preeclampsia directly causes long-term cerebral small vessel disease is an area of active investigation.
Migraine and Preeclampsia: Missing Links
Patients with migraine have an elevated risk of developing preeclampsia, with an odds ratio ranging from 1.08 to 3.5 [32••]. One retrospective case series of pregnant women with well-phenotyped migraine diagnoses showed a preeclampsia rate of 21.3%, fivefold higher than the risk in the general population; however, this study had no comparison group [91]. While migraine with aura is a known vascular risk factor in women [92], the risk of preeclampsia appears to be elevated in women with migraine regardless of the presence of aura. Women with active or frequent migraine attacks during pregnancy may represent a higher risk phenotype [93]. The connection between migraine and preeclampsia is not fully understood. While migraine is considered primarily a brain disorder, there is evidence that peripheral components, including trigeminal nerve afferents, dural immune cells, and vascular endothelial cells, play a major role in the pathophysiology of migraine pain [94]. The mechanisms that initiate migraine pain remain elusive and are likely complex and multifaceted. An overlap of neuroinflammation, vascular endothelial and smooth muscle cell dysfunction, platelet dysfunction, and changes in vascular reactivity may provide the link between migraine and preeclampsia (Fig. 1).
Fig. 1.
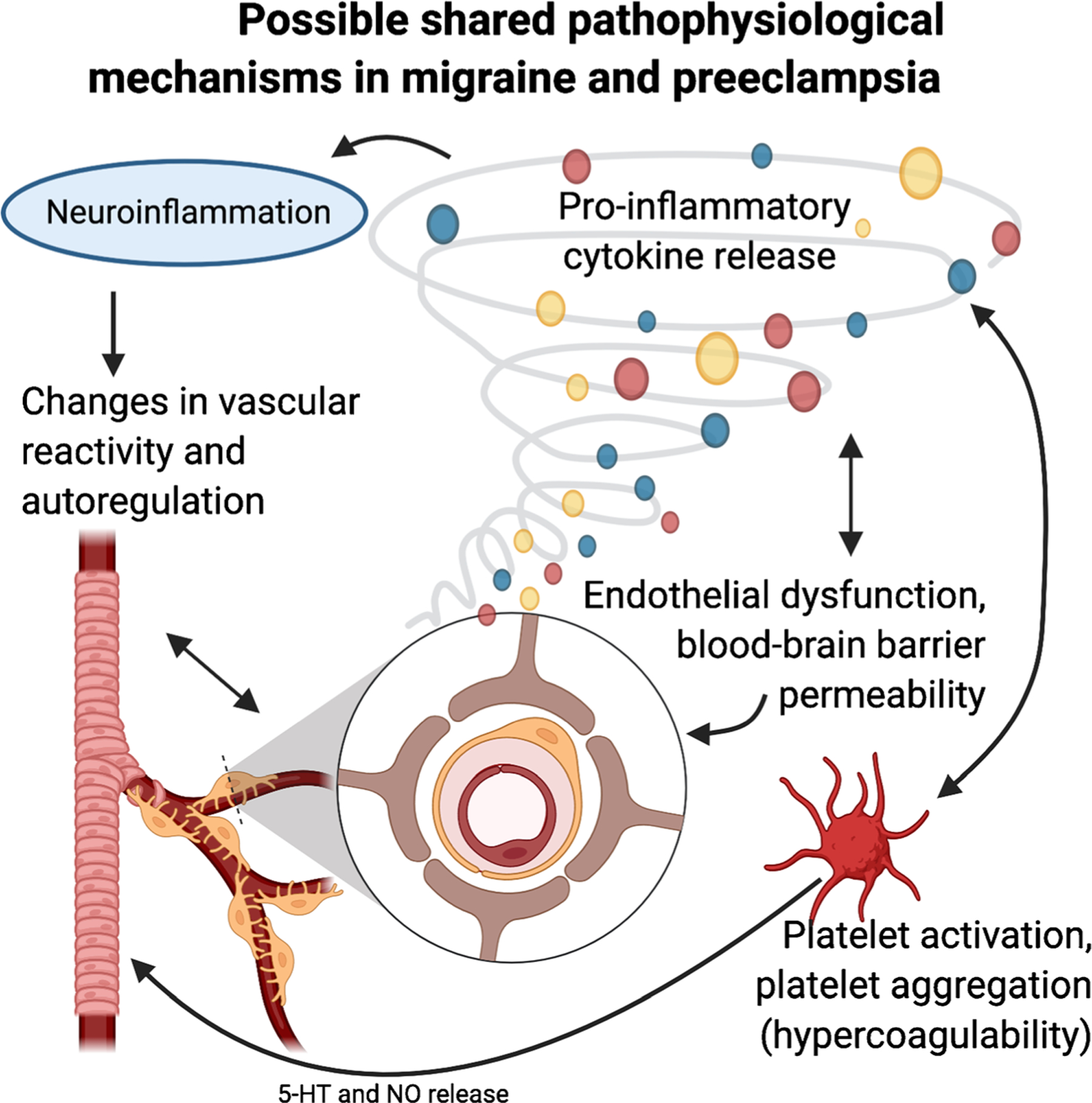
Possible shared pathophysiological mechanisms in migraine and preeclampsia. Preeclampsia and migraine share overlapping pathophysiology, including inflammation and endothelial dysfunction. Like migraine, the cerebral effects of preeclampsia include neuroinflammation, alterations in angiogenic pathways, endothelial cell dysfunction, changes in vascular reactivity, blood–brain barrier dysfunction, and platelet activation. This may account for the epidemiological association between migraine and risk of preeclampsia; however, the specific mechanisms of this association are not well understood. Figure created with BioRender.com
The headache phase of migraine is thought to involve sterile neurogenic inflammation, which leads to dural plasma protein extravasation and increased meningeal vascular permeability, arterial vasodilatation, and activation of local immune cells. Trigeminal nerve activation releases vasoactive neurotransmitters and neuropeptides, such as substance P and calcitonin gene–related peptide, adjacent to meningeal blood vessels. These vasoactive neurotransmitters and neuropeptides, in turn, trigger meningeal arterial vasodilatation, dural plasma protein extravasation, and mast cell activation and degranulation [94]. Neurogenically mediated dural plasma protein extravasation following trigeminal ganglion stimulation has been demonstrated in animal models, and levels of the pro-inflammatory mast cell components TNF-α, IL-1β, IL-10, and histamine are increased during migraine attacks, supporting this hypothesis [95–97].
Factors outside of neurogenic inflammation likely also contribute to migraine pathogenesis. Genome-wide association studies have identified multiple genetic susceptibility loci that are independently associated with migraine. Most of the identified loci are involved in genes regulating vascular and smooth muscle tissues, suggesting that vascular and smooth muscle dysfunction plays a role in migraine [98]. The release of substances from the meningeal vascular endothelial and smooth muscle cells, such as endothelin-1, nitric oxide, pros-tacyclin, and IL-1β, may play a role in activating meningeal nociception in migraine [94].
The role of platelets in migraine remains disputed, and it is unclear if platelet dysfunction is a cause or a consequence of migraine pathophysiology. It has been shown that migraineurs have abnormalities in platelet membranes that lead to decreased membrane fluidity, increased platelet activation, and increased formation of platelet-platelet and platelet-leukocyte aggregates. Platelet-platelet aggregates may contribute to a prothrombotic tendency, and platelet-leukocyte aggregates are thought to stimulate the release of inflammatory mediators, such as IL-1, IL-6, IL-8, and TNF-α, which can promote sensitization and activation of meningeal nociceptors. Platelet activation also appears to lead to serotonin release from platelet dense granules and increase the activity of platelet nitric oxide synthase, which, in turn, can influence vascular reactivity [99].
Like migraine, preeclampsia is mediated, in part, by neuroinflammation, alterations in angiogenic pathways, endothelial cell dysfunction, changes in vascular reactivity, and blood–brain barrier dysfunction. Aberrant platelet activation has also been demonstrated as part of the preeclampsia syndrome, and inhibition of platelet activation with aspirin has been shown in randomized trials to reduce the risk of preterm preeclampsia [100, 101]. It is possible that patients with migraine have a genetically preexisting dysfunction of platelets, vascular endothelial cells, and smooth muscle cells, which makes cerebral and meningeal blood vessels more receptive to the neuroinflammation and the resultant increased blood–brain barrier permeability and vasoreactivity linked to preeclampsia. Decreased levels and impaired function of circulating endothelial progenitor cells have been demonstrated in both migraine [102] and preeclampsia [103, 104], suggesting an overlapping pathophysiology of vascular endothelial dysfunction. Increases in pro-inflammatory cytokines, such as TNF-α and IL-1β, and increased white matter hyperintensity volume identified in both migraine and preeclampsia support the idea that the two disorders may share similar pathways of neurovascular unit dysfunction and neuroinflammation. Given the risk inherent in migraine patients, a thorough headache and migraine history should be a standard part of obstetrical care to identify patients at an elevated risk of developing preeclampsia.
Clinical Considerations When Evaluating Pregnant and Postpartum Women with Headache
Migraine and tension-type headache are the most common primary headache disorders worldwide, contributing to more disability-adjusted life years than all other neurologic disorders combined [105, 106]. This burden is particularly high for young adult and middle-aged women, accounting for 11.2% of all years of life lived with disability in women between the ages of 15 and 49 according to the Global Burden of Disease Study 2016 [107]. Migraine is three times more common in women than in men, and women have the highest prevalence during their childbearing years, when up to 28% suffer from migraine [108]. Tension-type headache has a lifetime prevalence approaching 80% and has only a slight female predominance [109]. Tension-type headache is less influenced by pregnancy and has not been shown to increase the risk of hypertensive disorders of pregnancy [110•]. Migraine, on the other hand, is associated with an increased risk of vascular diseases during pregnancy, including gestational hypertension, preeclampsia, ischemic stroke, acute myocardial infarction and heart disease, and thromboembolic events [32••].
Headache is a common complaint leading to neurologic consultation, and an acute headache in a pregnant or postpartum patient is worrisome. Pregnancy and the puerperium itself are considered red flags that indicate a higher likelihood of diagnosing a secondary cause of headache [111]. While most pregnant patients presenting for neurologic consultation with an acute headache will have a primary headache disorder, more than one-third will be discovered to have an underlying secondary cause of their headache, the majority of which will be a hypertensive disorder of pregnancy [112, 113]. A recent retrospective study of postpartum patients requiring inpatient neurologic consultation for acute headache suggests even more reasons to be concerned in the puerperium, as 73% had a secondary headache disorder, most commonly postdural puncture headache (45.7%) and postpartum preeclampsia (26.1%) [114]. Other secondary headaches seen in pregnancy and the puerperium include headache due to cervical artery dissection (often associated with preeclampsia), RCVS, ischemic stroke (particularly in the posterior circulation), intracerebral or subarachnoid hemorrhage, and cerebral venous thrombosis.
Despite the heightened concern for secondary headache disorders, migraine and tension-type headache are the most common causes of headache during pregnancy [110•]. Large studies estimate that 67–89% of women will have a significant improvement in migraine attacks throughout pregnancy, most notably in the second and third trimesters [115]. Many women, however, will continue to have attacks throughout pregnancy. Migraine with aura is less likely to improve and more likely to start or worsen during pregnancy than migraine without aura. Patients who have migraine with aura may also develop new aura symptoms during pregnancy [110•]. Headache remains common in the puerperium, and up to 40% of women, regardless of migraine history, develop postpartum headache within the first week after delivery, largely resolving within 5 weeks. More than 75% of these postpartum headaches are primary headache disorders, and migraine can present for the first time in the postpartum period in 5% of patients [110•, 116, 117]. More than half of patients with migraine will revert to their pre-pregnancy headache pattern within 1 month after delivery, a recurrence delayed only by breastfeeding and maternal age over 30 years [110•, 118].
Preeclampsia and related disorders make up the majority of secondary headache disorders in pregnancy. The headache of preeclampsia is often progressive and bilateral, pulsating in quality, intractable to treatment, and aggravated by physical activity. Patients can have associated visual changes, such as blurry vision and scotomas, which can be mistaken for symptoms of migraine aura [119]. PRES, a clinical-neuroradiological syndrome characterized by predominantly parietooccipital white matter lesions suggestive of vasogenic edema and various neurological symptoms, is often associated with preeclampsia and eclampsia [120]. Headache is the most common neurologic symptom in 2/3 of patients with PRES and eclampsia and generally is of insidious onset, dull quality, and bilateral occipital location [60]. RCVS is more common in the puerperium and presents as a sudden-onset (thunderclap), severe, diffuse headache that is recurrent over 1–2 weeks. Neuroimaging, which can be negative at headache onset, shows segmental cerebral vasoconstriction that starts at the periphery and progresses toward the central blood vessels and spontaneously resolves within 3 months [119, 121]. It can be difficult to differentiate the headache of preeclampsia and related disorders from migraine, as individual headache features are not significantly different and clinical features often overlap [114]. In patients with a history of a primary headache disorder, longer attack duration is suggestive of the diagnosis of a secondary headache disorder, whereas psychiatric comorbidity and phonophobia are more likely associated with a primary headache disorder [112].
Given the difficulty in distinguishing between primary and secondary headache disorders in pregnancy and the puerperium, a thorough history and examination, along with a high index of suspicion, are required. Red flags in the clinical history should prompt further neuroimaging and laboratory evaluation. Lack of headache history, elevated blood pressure, prior history of a secondary headache disorder, fever, very severe pain, and an abnormal neurological examination have all been found to be independent risk factors for secondary headache in pregnancy [112, 113, 122]. Laboratory evaluations associated with a secondary headache disorder in pregnancy include abnormal platelets, elevated C-reactive protein and liver function tests, proteinuria, and abnormal lumbar puncture results [123]. Independent risk factors for postpartum secondary headache disorders include lack of headache history, orthostatic headache, and abnormal neuroimaging [114].
When and How to Obtain Neuroimaging
Primary headache disorders, specifically migraine and tension-type headache, are highly prevalent in women of childbearing age. Even though most acute headaches in pregnancy and the puerperium will be a primary rather than a secondary headache, acute headache is one of the most common neurologic symptoms heralding preeclampsia. It can be difficult to distinguish between the headache of migraine and preeclampsia. Rapidly identifying headache features that should prompt emergent neuroimaging evaluation and early diagnosis and treatment is essential to reduce morbidity and mortality.
Various mnemonics have been proposed to describe red flag features of headache indicating a possible secondary cause. We propose the use of the mnemonic SCAN ME (Table 2) to help non-neurologists identify which pregnant and postpartum patients presenting with acute headache should be discussed with a neurologist and, in some cases, have urgent neuroimaging. We recognize this would be a significant change in practice from the current standard, and evidence to support this approach is lacking. Certainly, not all pregnant/postpartum women with severe headache require neuroimaging. However, given the potentially catastrophic consequences of missing a diagnosis (including the diagnosis of preeclampsia), in our opinion, decisions about neuroimaging are best made in collaboration with a neurologist if SCAN ME features are present.
Table 2.
SCAN ME: When to consider neuroimaging in a pregnant or postpartum patient with headache
| S: Sudden/severe/seizure |
| C: Change in position (worse supine) or usual headache quality |
| A: Altered mental status |
| N: Neurological deficits/nausea and vomiting |
| M: Medications without relief |
| E: Elevated blood pressure or temperature |
Used with permission from Obstetric Life Support (OBLS). This mnemonic was developed by the corresponding author (ECM) as a component of an ongoing collaboration with OBLS, a national consortium developing a curriculum for maternal cardiac arrest and acute stroke. OBLS is funded through a grant from the Agency for Healthcare Research and Quality (AHRQ 5R18HS026169–02)
Magnetic resonance imaging (MRI) is preferred over computed tomography (CT) only if immediately available, to avoid radiation exposure; however, fetal radiation from a maternal head CT is extremely low, and CT should be obtained if clinically necessary and MRI is unavailable or contraindicated [124•]. Gadolinium-based contrast should be avoided, as gadolinium crosses the placenta and has teratogenic and embryocidal effects in animal studies. While there are no well-controlled studies of iodine-based contrast agents in pregnant patients, animal studies have not demonstrated an increased risk from intravenous administration [124•]. If there is a suspicion for an acute cerebrovascular disorder, vascular imaging should not be delayed, and the fastest modality should be used [125••]. The American College of Radiology and ACOG both advise that iodinated contrast be administered during pregnancy, including the first trimester, when necessary to maternal care [124•].
Future Directions
Neuro-obstetrics as an Emerging Field of Scholarship and Clinical Practice
Neurological complications are recognized as a major contributor to maternal morbidity and mortality, and women commonly present with headache as the initial symptom. There is an urgent need for more basic, translational, clinical, and population-based research to better understand the mechanisms of preeclampsia-related neurotoxicity. Neurological disorders should be considered as both exposures and outcomes of interest in obstetrical clinical trials aimed at improving maternal health. In addition, neurologists and obstetricians must develop collaborative clinical care models to prevent, diagnose, and effectively treat the neurological complications of preeclampsia and related disorders. Obstetricians should elicit a focused headache history from patients at the first prenatal visit, and neurologists should incorporate a woman’s obstetrical history into their standard neurological consultations, particularly those for headache, cerebrovascular disorders, or cognitive complaints.
The term “neuro-obstetrics” has been introduced to describe this interdisciplinary care model [126]. Building on this, we propose that neuro-obstetrics be defined broadly as a cross-disciplinary field encompassing clinical practice, medical education, scientific investigation, and public health initiatives, with the overarching goal of reducing maternal neurological morbidity and mortality. Preventing neurological complications of preeclampsia and related hypertensive disorders must be a top priority in this emerging field. Other important areas in need of investigation include translational research to understand the mechanisms of the association between migraine and hypertensive disorders of pregnancy, identification of high-risk clinical features of migraine (such as aura status or attack frequency by trimester), and the potential use of preeclampsia serum biomarkers to help rapidly assess acute headache in pregnancy where there is diagnostic uncertainty. Aspirin for preeclampsia prevention is already the standard of care for women with certain high-risk conditions [127]; the use of prophylactic aspirin in pregnant women with migraine could be investigated in clinical trials. Neurologists and neuroscientists can and should play central roles in identifying and closing the many knowledge gaps in this critically important area of neuromedicine.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Centers for Disease Control. Data on selected pregnancy complications in the United States. cdc.gov. 2019. Available from: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-complications-data.htm#hyper. Accessed 29 Dec 2020
- 2.Centers for Disease Control. Pregnancy mortality surveillance system [Internet]. cdc.gov. Atlanta; Available from: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed 14 Feb 2019 [Google Scholar]
- 3.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–33. [DOI] [PubMed] [Google Scholar]
- 4.Hibbard LT. Maternal mortality due to acute toxemia. Obstet Gynecol. 1973;42:263–70. [PubMed] [Google Scholar]
- 5.Gibbs CE, Locke WE. Maternal deaths in Texas, 1969 to 1973. A report of 501 consecutive maternal deaths from the Texas Medical Association’s Committee on Maternal Health. Am J Obs Gynecol. 1976;126:687–92. [PubMed] [Google Scholar]
- 6.Judy AE, McCain CL, Lawton ES, Morton CH, Main EK, Druzin ML. Systolic hypertension, preeclampsia-related mortality, and stroke in California. Obstet Gynecol. 2019;133:1151–9. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa J, Ikeda T, Sekizawa A, Tanaka H, Nakata M, Murakoshi T, et al. Maternal death due to stroke associated with pregnancy-induced hypertension. Circ J. 2015;79:1835–40. [DOI] [PubMed] [Google Scholar]
- 8.Bateman BT, Schumacher HC, Bushnell CD, Pile-Spellman J, Simpson LL, Sacco RL, et al. Intracerebral hemorrhage in pregnancy: frequency, risk factors, and outcome. Neurology. 2006 ed. 2006;67:424–9. [DOI] [PubMed] [Google Scholar]
- 9.Liang C-C, Chang S-D, Lai S-L, Hsieh C-C, Chueh H-Y, Lee TH. Stroke complicating pregnancy and the puerperium. Eur J Neurol. 2006;13:1256–60. [DOI] [PubMed] [Google Scholar]
- 10.Foo L, Bewley S, Rudd A. Maternal death from stroke: a thirty year national retrospective review. Eur J Obstet Gynecol Reprod Biol. 2013;171:266–70. [DOI] [PubMed] [Google Scholar]
- 11.Martin JN Jr, Thigpen BD, Moore RC, Rose CH, Cushman J, May W. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet Gynecol. 2005;105:246–54. [DOI] [PubMed] [Google Scholar]
- 12.Cleary KL, Siddiq Z, Ananth CV, Wright JD, Too G, D’Alton ME, et al. Use of antihypertensive medications during delivery hospitalizations complicated by preeclampsia. Obstet Gynecol. 2018;131:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.••.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 202: Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1–e25 [DOI] [PubMed] [Google Scholar]; Current diagnostic criteria for gestational hypertension and preeclampsia as defined by the American College of Obstetricians and Gynecologists (ACOG).
- 14.••.Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43 [DOI] [PubMed] [Google Scholar]; Current diagnostic criteria for hypertensive disorders of pregnancy as defined by the International Society for the Study of Hypertension in Pregnancy (ISSHP).
- 15.Chesley L Introduction, history, controversies, and definitions. In: Taylor JR, Cunningham FG, Lindheimer M, editors. Chesley’s hypertensive disorders in pregnancy. 4 ed. 2015. pp. 1–24. [Google Scholar]
- 16.Johns R Observations on puerperal convulsions. Dublin J Med. Sci Springer; London. 1843;24:101–15. [Google Scholar]
- 17.Douglas KA, Redman CW. Eclampsia in the United Kingdom. BMJ. BMJ Publishing Group. 1994;309:1395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–31. [DOI] [PubMed] [Google Scholar]
- 19.Garovic VD, White WM, Vaughan L, Saiki M, Parashuram S, Garcia-Valencia O, et al. Incidence and long-term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol. 2020;75:2323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shahul S, Tung A, Minhaj M, Nizamuddin J, Wenger J, Mahmood E, et al. Racial disparities in comorbidities, complications, and maternal and fetal outcomes in women with preeclampsia/eclampsia. Hypertens Pregnancy. 2015;34:506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson JD, Louis JM. Does race or ethnicity play a role in the origin, pathophysiology, and outcomes of preeclampsia? An expert review of the literature. Am J Obs Gynecol. 2020. [DOI] [PubMed] [Google Scholar]
- 22.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol. 2013;170:1–7. [DOI] [PubMed] [Google Scholar]
- 23.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 203: Chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26–50. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Kim Y, Navi BB, Abdelkhaleq R, Salazar-Marioni S, Blackburn SL, Bambhroliya AB, Lopez-Rivera V, Vahidy F, Savitz SI, Medhus A, Kamel H, Grotta JC, McCullough L, Chen PR, Sheth SA Risk of intracranial hemorrhage associated with pregnancy in women with cerebral arteriovenous malformations. J Neurointerv Surg. 2020, neurintsurg-2020–016838. [DOI] [PubMed] [Google Scholar]
- 25.Maor GS, Faden MS, Brown R. Prevalence, risk factors and pregnancy outcomes of women with vascular brain lesions in pregnancy. Arch Gynecol Obstet. 2020;301:665–70. [DOI] [PubMed] [Google Scholar]
- 26.Nussbaum ES, Goddard JK, Davis AR. A systematic review of intracranial aneurysms in the pregnant patient - a clinical conundrum. Eur J Obstet Gynecol. 2020;254:79–86. [DOI] [PubMed] [Google Scholar]
- 27.Desai M, Wali AR, Birk HS, Santiago-Dieppa DR, Khalessi AA. Role of pregnancy and female sex steroids on aneurysm formation, growth, and rupture: a systematic review of the literature. Neurosurg. 2019;47:E8. [DOI] [PubMed] [Google Scholar]
- 28.Meeks JR, Bambhroliya AB, Alex KM, Sheth SA, Savitz SI, Miller EC, et al. Association of primary intracerebral hemorrhage with pregnancy and the postpartum period. JAMA Netw Open. 2020;3:e202769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller EC, Gatollari HJ, Too G, Boehme AK, Leffert LR, Marshall RS, et al. Risk factors for pregnancy-associated stroke in women with preeclampsia. Stroke. American Heart Association, Inc. 2017;48:1752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–596. [DOI] [PubMed] [Google Scholar]
- 31.Wu P, Jordan KP, Chew-Graham CA, Coutinho T, Lundberg GP, Park KE, et al. Temporal trends in pregnancy-associated stroke and its outcomes among women with hypertensive disorders of pregnancy. J Am Heart Assoc. 2020;9:e016182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.••.Wabnitz A, Bushnell C. Migraine, cardiovascular disease, and stroke during pregnancy: systematic review of the literature. Cephalalgia. 2015;35:132–9 [DOI] [PubMed] [Google Scholar]; Systematic review of association between migraine and adverse pregnancy outcomes.
- 33.Matthys LA, Coppage KH, Lambers DS, Barton JR, Sibai BM. Delayed postpartum preeclampsia: an experience of 151 cases. Am J Obs Gynecol. 2004;190:1464–6. [DOI] [PubMed] [Google Scholar]
- 34.Al-Safi Z, Imudia AN, Filetti LC, Hobson DT, Bahado-Singh RO, Awonuga AO. Delayed postpartum preeclampsia and eclampsia: demographics, clinical course, and complications. Obstet Gynecol. 2011;118:1102–7. [DOI] [PubMed] [Google Scholar]
- 35.Redman EK, Hauspurg A, Hubel CA, Roberts JM, Jeyabalan A. Clinical course, associated factors, and blood pressure profile of delayed-onset postpartum preeclampsia. Obstet Gynecol. 2019;134:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Too G, Wen T, Boehme AK, Miller EC, Leffert LR, Attenello FJ, et al. Timing and risk factors of postpartum stroke. Obstet Gynecol. 2018;131:70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.•.Kamel H, Navi BB, Sriram N, Hovsepian DA, Devereux RB, Elkind MSV. Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med. 2014;370:1307–15 [DOI] [PMC free article] [PubMed] [Google Scholar]; Epidemiological study using administrative data established that risk of thrombotic events after delivery extended beyond the 6 week post-partum period.
- 38.••.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10:e003497. [DOI] [PubMed] [Google Scholar]; Systematic review and meta-analysis of the impact of preeclampsia on future cardiovascular disease in women.
- 39.Miller EC, Boehme AK, Chung NT, Wang SS, Lacey JV, Lakshminarayan K, et al. Aspirin reduces long-term stroke risk in women with prior hypertensive disorders of pregnancy. Neurology. 2019;92:e305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith WT. Parturition and the principles and practice of obstetrics. Lea & Blanchard; 1849. [Google Scholar]
- 41.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obs Gynecol. 1989;161:1200–4. [DOI] [PubMed] [Google Scholar]
- 42.Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost. 2009;7:375–84. [DOI] [PubMed] [Google Scholar]
- 43.•.Gatford KL, Andraweera PH, Roberts CT, Care AS. Animal models of preeclampsia: causes, consequences, and interventions. Hypertension. 2020;75:1363–81 [DOI] [PubMed] [Google Scholar]; Comprehensive review of current animal models of preeclampsia.
- 44.Michalczyk M, Celewicz A, Celewicz M, Woźniakowska-Gondek P, Rzepka R. The role of inflammation in the pathogenesis of preeclampsia. Yi Y-S, editor. Mediators Inflamm. Hindawi; 2020;2020:3864941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.•.Johnson AC, Cipolla MJ. Impaired function of cerebral parenchymal arterioles in experimental preeclampsia. Microvasc Res. 2018;119:64–72 Elsevier; [DOI] [PMC free article] [PubMed] [Google Scholar]; Translational study demonstrating cerebral arteriolar dysfunction in an animal model of preeclampsia.
- 46.Sonneveld MJ, Brusse IA, Duvekot JJ, Steegers EAP, Grune F, Visser GH. Cerebral perfusion pressure in women with preeclampsia is elevated even after treatment of elevated blood pressure. Acta Obstet Gynecol Scand. John Wiley & Sons, Ltd. 2014;93:508–11. 10.1111/aogs.12358. [DOI] [PubMed] [Google Scholar]
- 47.Richards A, Graham D, Bullock R. Clinicopathological study of neurological complications due to hypertensive disorders of pregnancy. J Neurol Neurosurg Psychiatry. 4 ed. 1988;51:416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Veen TR, Panerai RB, Haeri S, Griffioen AC, Zeeman GG, Belfort MA. Cerebral autoregulation in normal pregnancy and preeclampsia. Obstet Gynecol. 2013;122:1064–9. [DOI] [PubMed] [Google Scholar]
- 49.van Veen TR, Panerai RB, Haeri S, Singh J, Adusumalli JA, Zeeman GG, et al. Cerebral autoregulation in different hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2015;212: 513.e1–7. [DOI] [PubMed] [Google Scholar]
- 50.Williams KP, Galerneau F, Small M. Transfer function analysis of dynamic cerebral autoregulation in preeclampsia. Pregnancy Hypertens. 2015;5:322–4. [DOI] [PubMed] [Google Scholar]
- 51.•.Warrington JP, Fan F, Murphy SR, Roman RJ, Drummond HA, Granger JP, et al. Placental ischemia in pregnant rats impairs cerebral blood flow autoregulation and increases blood-brain barrier permeability. Phys Rep. 2014;2:e12134. [DOI] [PMC free article] [PubMed] [Google Scholar]; Translational study demonstrating impaired autoregulation and blood–brain barrier compromise in a preeclampsia animal model.
- 52.Warrington JP, Fan F, Duncan J, Cunningham MW, LaMarca BB, Dechend R, et al. The angiotensin II type I receptor contributes to impaired cerebral blood flow autoregulation caused by placental ischemia in pregnant rats. Biol Sex Differ. 2019;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maeda KJ, McClung DM, Showmaker KC, Warrington JP, Ryan MJ, Garrett MR, et al. Endothelial cell disruption drives increased blood brain barrier permeability and cerebral edema in the Dahl SS/jr rat model of superimposed preeclampsia. Am J Physiol Heart Circ Physiol. American Physiological Society; Rockville, MD; 2020;ajpheart.00383.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janzarik WG, Gerber A-K, Markfeld-Erol F, Sommerlade L, Allignol A, Reinhard M. No long-term impairment of cerebral autoregulation after preeclampsia. Pregnancy Hypertens. 2018;13:171–3. [DOI] [PubMed] [Google Scholar]
- 55.Wallace K, Bean C, Bowles T, Spencer S-K, Randle W, Kyle PB, et al. Hypertension, anxiety, and blood-brain barrier permeability are increased in postpartum severe preeclampsia/hemolysis, elevated liver enzymes, and low platelet count syndrome rats. Hypertension. 2018;72:946–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warrington JP, Drummond HA, Granger JP, Ryan MJ. Placental ischemia-induced increases in brain water content and cerebrovascular permeability: role of TNF-α. Am J Phys Regul Integr Comp Phys. 2015;309:R1425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bean C, Spencer S-K, Pabbidi MR, Szczepanski J, Araji S, Dixon S, et al. Peripheral anti-angiogenic imbalance during pregnancy impairs myogenic tone and increases cerebral edema in a rodent model of HELLP syndrome. Brain Sci. 2018;8(12):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Digre KB, Varner MW, Osborn AG, Crawford S. Cranial magnetic resonance imaging in severe preeclampsia vs eclampsia. Arch Neurol. 1993;50:399–406. [DOI] [PubMed] [Google Scholar]
- 59.Shah AK, Rajamani K, Whitty JE. Eclampsia: a neurological perspective. J Neurol Sci. 2008;271:158–67. [DOI] [PubMed] [Google Scholar]
- 60.Brewer J, Owens MY, Wallace K, Reeves AA, Morris R, Khan M, et al. Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am J Obs Gynecol. 2013;208:468.e1–6. [DOI] [PubMed] [Google Scholar]
- 61.Mayama M, Uno K, Tano S, Yoshihara M, Ukai M, Kishigami Y, et al. Incidence of posterior reversible encephalopathy syndrome in eclamptic and patients with preeclampsia with neurologic symptoms. Am J Obs Gynecol. 2016;215:239.e1–5. [DOI] [PubMed] [Google Scholar]
- 62.Postma IR, Slager S, Kremer HPH, de Groot JC, Zeeman GG. Long-term consequences of the posterior reversible encephalopathy syndrome in eclampsia and preeclampsia: a review of the obstetric and nonobstetric literature. Obstet Gynecol Surv. 2014;69:287–300. [DOI] [PubMed] [Google Scholar]
- 63.Fang X, Liang Y, Chen D, Liu Y, Xie M, Zhang W. Contribution of excess inflammation to a possible rat model of eclamptic reversible posterior leukoencephalopathy syndrome induced by lipopolysaccharide and pentylenetetrazol: a preliminary study. Cytokine. 2020;135:155212. [DOI] [PubMed] [Google Scholar]
- 64.Cornelius DC. Preeclampsia: from inflammation to immunoregulation. Clin Med Insights Blood Disord. 2018;11: 1179545X17752325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang Q, Hu B, Han X, Yang J, Di X, Bao J, et al. Cyclosporin A ameliorates eclampsia seizure through reducing systemic inflammation in an eclampsia-like rat model. Hypertens Res. 2020;43: 263–70. [DOI] [PubMed] [Google Scholar]
- 66.van den Berg CB, Duvekot JJ, Güzel C, Hansson SR, de Leeuw TG, Steegers EAP, et al. Elevated levels of protein AMBP in cerebrospinal fluid of women with preeclampsia compared to normotensive pregnant women. Prot Clin Appl. Wiley-Blackwell. 2016;11:1600082–10. [DOI] [PubMed] [Google Scholar]
- 67.Ciampa E, Li Y, Dillon S, Lecarpentier E, Sorabella L, Libermann TA, et al. Cerebrospinal fluid protein changes in preeclampsia. Hypertension. 2018;72:219–26. [DOI] [PubMed] [Google Scholar]
- 68.Clayton AM, Shao Q, Paauw ND, Giambrone AB, Granger JP, Warrington JP. Postpartum increases in cerebral edema and inflammation in response to placental ischemia during pregnancy. Brain Behav Immun. 2018;70:376–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burwick RM, Togioka BM, Speranza RJ, Gaffney JE, Roberts VHJ, Frias AE, et al. Assessment of blood-brain barrier integrity and neuroinflammation in preeclampsia. Am J Obs Gynecol. 2019;221:269.e1–8. [DOI] [PubMed] [Google Scholar]
- 70.Gomez-Lopez N, Motomura K, Miller D, Garcia-Flores V, Galaz J, Romero R. Inflammasomes: their role in normal and complicated pregnancies. J Immunol. 2019;203:2757–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brien M-E, Baker B, Duval C, Gaudreault V, Jones RL, Girard S. Alarmins at the maternal-fetal interface: involvement of inflammation in placental dysfunction and pregnancy complications. Can J Physiol Pharmacol. 2019;97:206–12. [DOI] [PubMed] [Google Scholar]
- 72.Kohli S, Ranjan S, Hoffmann J, Kashif M, Daniel EA, Al-Dabet MM, et al. Maternal extracellular vesicles and platelets promote preeclampsia via inflammasome activation in trophoblasts. Blood Am Soc Hematol. 2016;128:2153–64. [DOI] [PubMed] [Google Scholar]
- 73.Amburgey OA, Chapman AC, May V, Bernstein IM, Cipolla MJ. Plasma from preeclamptic women increases blood-brain barrier permeability: role of vascular endothelial growth factor signaling. Hypertension. 2010;56:1003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hod T, Cerdeira AS, Karumanchi SA. Molecular mechanisms of preeclampsia. Cold Spring Harb Perspect Med. 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cross SN, Ratner E, Rutherford TJ, Schwartz PE, Norwitz ER. Bevacizumab-mediated interference with VEGF signaling is sufficient to induce a preeclampsia-like syndrome in nonpregnant women. Rev Obstet Gynecol. 2012;5:2–8. [PMC free article] [PubMed] [Google Scholar]
- 76.Zuo P-Y, Chen X-L, Liu Y-W, Xiao C-L, Liu C-Y. Increased risk of cerebrovascular events in patients with cancer treated with bevacizumab: a meta-analysis. PLoS One. 2014;9:e102484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yousif D, Bellos I, Penzlin AI, Hijazi MM, Illigens BM-W, Pinter A, et al. Autonomic dysfunction in preeclampsia: a systematic review. Front Neurol. 2019;10:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.•.Johnson AC, Nagle KJ, Tremble SM, Cipolla MJ. The contribution of normal pregnancy to eclampsia. PLoS One. 2015;10: e0133953. [DOI] [PMC free article] [PubMed] [Google Scholar]; Translational study demonstrating increased seizure susceptibility in normal pregnancy as a contributor to eclampsia physiology.
- 79.•.Rocha EA, Topcuoglu MA, Silva GS, Singhal AB. RCVS2 score and diagnostic approach for reversible cerebral vasoconstriction syndrome. Neurology. 2019;92:e639–47 [DOI] [PubMed] [Google Scholar]; Validated diagnostic approach to reversible cerebral vasoconstriction syndrome, regardless of association with pregnancy.
- 80.Cotton DB, Hallak M, Janusz C, Irtenkauf SM, Berman RF. Central anticonvulsant effects of magnesium sulfate on N-methyl-D-aspartate-induced seizures. Am J Obs Gynecol. 1993;168:974–8. [DOI] [PubMed] [Google Scholar]
- 81.Euser AG, Cipolla MJ. Resistance artery vasodilation to magnesium sulfate during pregnancy and the postpartum state. Am J Physiol Heart Circ Physiol. 2005;288:H1521–5. [DOI] [PubMed] [Google Scholar]
- 82.Chardain A, Mesnage V, Alamowitch S, Bourdain F, Crozier S, Lenglet T, et al. Posterior reversible encephalopathy syndrome (PRES) and hypomagnesemia: a frequent association? Rev Neurol (Paris). 2016;172:384–8. [DOI] [PubMed] [Google Scholar]
- 83.Fang X, Wang H, Liu Z, Chen J, Tan H, Liang Y, et al. Posterior reversible encephalopathy syndrome in preeclampsia and eclampsia: the role of hypomagnesemia. Seizure. 2020;76:12–6. [DOI] [PubMed] [Google Scholar]
- 84.Mijalski C, Dakay K, Miller-Patterson C, Saad A, Silver B, Khan M. Magnesium for treatment of reversible cerebral vasoconstriction syndrome: case series. Neurohospitalist. 2016;6:111–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Elharram M, Dayan N, Kaur A, Landry T, Pilote L. Long-term cognitive impairment after preeclampsia: a systematic review and meta-analysis. Obstet Gynecol. 2018;132:355–64. [DOI] [PubMed] [Google Scholar]
- 86.Basit S, Wohlfahrt J, Boyd HA. Pre-eclampsia and risk of dementia later in life: nationwide cohort study. BMJ. 2018;363:k4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siepmann T, Boardman H, Bilderbeck A, Griffanti L, Kenworthy Y, Zwager C, et al. Long-term cerebral white and gray matter changes after preeclampsia. Neurology. 2017;88:1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soma-Pillay P, Suleman FE, Makin JD, Pattinson RC. Cerebral white matter lesions after pre-eclampsia. Pregnancy Hypertens. 2017;8:15–20. [DOI] [PubMed] [Google Scholar]
- 89.Postma IR, Bouma A, de Groot JC, Aukes AM, Aarnoudse JG, Zeeman GG. Cerebral white matter lesions, subjective cognitive failures, and objective neurocognitive functioning: a follow-up study in women after hypertensive disorders of pregnancy. J Clin Exp Neuropsychol. 2016;38:585–98. [DOI] [PubMed] [Google Scholar]
- 90.Miller KB, Miller VM, Barnes JN. Pregnancy history, hypertension, and cognitive impairment in postmenopausal women. Curr Hypertens Rep. 2019;21:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grossman TB, Robbins MS, Govindappagari S, Dayal AK. Delivery outcomes of patients with acute migraine in pregnancy: a retrospective study. Headache. 2017;57:605–11. [DOI] [PubMed] [Google Scholar]
- 92.Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener H-C, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006;296:283–91. [DOI] [PubMed] [Google Scholar]
- 93.Sanchez SE, Qiu C, Williams MA, Lam N, Sorensen TK. Headaches and migraines are associated with an increased risk of preeclampsia in Peruvian women. Am J Hypertens. 2008;21: 360–4. [DOI] [PubMed] [Google Scholar]
- 94.Levy D, Labastida-Ramirez A, MaassenVanDenBrink A. Current understanding of meningeal and cerebral vascular function underlying migraine headache. Cephalalgia. 2019;39:1606–22. [DOI] [PubMed] [Google Scholar]
- 95.Heatley RV, Denburg JA, Bayer N, Bienenstock J. Increased plasma histamine levels in migraine patients. Clin Allergy. 1982;12: 145–9. [DOI] [PubMed] [Google Scholar]
- 96.Markowitz S, Saito K, Moskowitz MA. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J Neurosci. 1987;7:4129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perini F, D’Andrea G, Galloni E, Pignatelli F, Billo G, Alba S, et al. Plasma cytokine levels in migraineurs and controls. Headache. 2005;45:926–31. [DOI] [PubMed] [Google Scholar]
- 98.Gormley P, Anttila V, Winsvold BS, Palta P, Esko T, Pers TH, et al. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet. 2016;48:856–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Danese E, Montagnana M, Lippi G. Platelets and migraine. Thromb Res. 2014;134:17–22. [DOI] [PubMed] [Google Scholar]
- 100.Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, dePaco MC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–22. [DOI] [PubMed] [Google Scholar]
- 101.Theilen LH, Campbell HD, Mumford SL, Purdue-Smithe AC, Sjaarda LA, Perkins NJ, et al. Platelet activation and placenta-mediated adverse pregnancy outcomes: an ancillary study to the Effects of Aspirin in Gestation and Reproduction trial. Am J Obs Gynecol. 2020;223:741.e1–741.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee S-T, Chu K, Jung K-H, Kim DH, Kim E-H, Choe VN, et al. Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology. 2008;70:1510–7. [DOI] [PubMed] [Google Scholar]
- 103.Luppi P, Powers RW, Verma V, Edmunds L, Plymire D, HubelCA. Maternal circulating CD34+VEGFR-2+ and CD133+ VEGFR-2+ progenitor cells increase during normal pregnancy but are reduced in women with preeclampsia. Reprod Sci. 2010;17:643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Laganà AS, Giordano D, Loddo S, Zoccali G, Vitale SG, Santamaria A, et al. Decreased endothelial progenitor cells (EPCs) and increased natural killer (NK) cells in peripheral blood as possible early markers of preeclampsia: a case-control analysis. Arch Gynecol Obstet. 2017;295:867–72. [DOI] [PubMed] [Google Scholar]
- 105.Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. [DOI] [PubMed] [Google Scholar]
- 106.Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Buse DC, Loder EW, Gorman JA, Stewart WF, Reed ML, Fanning KM, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53:1278–99. [DOI] [PubMed] [Google Scholar]
- 109.Lyngberg AC, Rasmussen BK, Jørgensen T, Jensen R. Has the prevalence of migraine and tension-type headache changed over a 12-year period? A Danish population survey. Eur J Epidemiol. 2005;20:243–9. [DOI] [PubMed] [Google Scholar]
- 110.•.Negro A, Delaruelle Z, Ivanova TA, Khan S, Ornello R, Raffaelli B, et al. Headache and pregnancy: a systematic review. J Headache Pain. 2017;18:106. [DOI] [PMC free article] [PubMed] [Google Scholar]; A systematic review that summarizes the available data on headache and pregnancy.
- 111.Do TP, Remmers A, Schytz HW, Schankin C, Nelson SE, Obermann M, et al. Red and orange flags for secondary headaches in clinical practice: SNNOOP10 list. Neurology. 2019;92:134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Robbins MS, Farmakidis C, Dayal AK, Lipton RB. Acute headache diagnosis in pregnant women: a hospital-based study. Neurology. 2015;85:1024–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raffaelli B, Siebert E, Körner J, Liman T, Reuter U, Neeb L. Characteristics and diagnoses of acute headache in pregnant women—a retrospective cross-sectional study. J Headache Pain. 2017;18:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vgontzas A, Robbins MS. A hospital based retrospective study of acute postpartum headache. Headache. 2018;58:845–51. [DOI] [PubMed] [Google Scholar]
- 115.Afridi SK. Current concepts in migraine and their relevance to pregnancy. Obstet Med. 2018;11:154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Granella F, Sances G, Zanferrari C, Costa A, Martignoni E, Manzoni GC. Migraine without aura and reproductive life events: a clinical epidemiological study in 1300 women. Headache. 1993;33:385–9. [DOI] [PubMed] [Google Scholar]
- 117.Goldszmidt E, Kern R, Chaput A, Macarthur A. The incidence and etiology of postpartum headaches: a prospective cohort study. Can J Anaesth. 2005;52:971–7. [DOI] [PubMed] [Google Scholar]
- 118.Sances G, Granella F, Nappi RE, Fignon A, Ghiotto N, Polatti F, et al. Course of migraine during pregnancy and postpartum: a prospective study. Cephalalgia. 2003;23:197–205. [DOI] [PubMed] [Google Scholar]
- 119.Burch R Headache in pregnancy and the puerperium. Neurol Clin. 2019;37:31–51. [DOI] [PubMed] [Google Scholar]
- 120.Lee VH, Wijdicks EFM, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65:205–10. [DOI] [PubMed] [Google Scholar]
- 121.Ducros A Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012;11:906–17 [DOI] [PubMed] [Google Scholar]
- 122.Raffaelli B, Neeb L, Israel-Willner H, Körner J, Liman T, Reuter U, et al. Brain imaging in pregnant women with acute headache. J Neurol. 2018;265:1836–43. [DOI] [PubMed] [Google Scholar]
- 123.Sandoe CH, Lay C. Secondary headaches during pregnancy: when to worry. Curr Neurol Neurosci Rep. 2019;19:27. [DOI] [PubMed] [Google Scholar]
- 124.•.Anderson A, Singh J, Bove R. Neuroimaging and radiation exposure in pregnancy. Handb Clin Neurol. 2020;171:179–91 [DOI] [PubMed] [Google Scholar]; Current review of best practices for neuroimaging during pregnancy.
- 125.••.Ladhani NNN, Swartz RH, Foley N, Nerenberg K, Smith EE, Gubitz G, et al. Canadian Stroke Best Practice consensus statement: acute stroke management during pregnancy. Int J Stroke. 2018;49:174749301878661–16 [DOI] [PubMed] [Google Scholar]; Expert consensus-based guidelines for management of acute stroke in pregnancy.
- 126.Brusse IA, Kluivers ACM, Zambrano MD, Shetler K, Miller EC. Neuro-obstetrics: a multidisciplinary approach to care of women with neurologic disease. Handb Clin Neurol. 2020;171:143–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.LeFevre ML. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:819. [DOI] [PubMed] [Google Scholar]


