Abstract
Domestic and wild felids are considered suitable hosts for the parasitic mite Sarcoptes scabiei, and sarcoptic mange is reported in several felid species in the scientific literature. However, the historic classification of Sarcoptes mites into host-specific varieties does not include S. scabiei var. felis. It is unclear whether sarcoptic mange transmission in felids involves canids, other sympatric species, or exclusively felids. This study aimed to characterize the genetic structure of S. scabiei mites from domestic cats (Felis catus) and Eurasian lynx (Lynx lynx carpathicus), comparing them with Sarcoptes mites from sympatric domestic and wild carnivores. Ten Sarcoptes microsatellite markers were used to genotype 81 mites obtained from skin scrapings of 36 carnivores: 4 domestic cats, one dog (Canis lupus familiaris), 4 Eurasian lynx, 23 red foxes (Vulpes vulpes), and 4 grey wolves (Canis lupus lupus) from either Italy, Switzerland or France. Two genetic clusters of S. scabiei with a geographical distribution pattern were detected: mites from cats originating from Central Italy clustered with those from sympatric wolves. In contrast, all the other mites from Switzerland, France and Northern Italy clustered together. These results strengthen the previously advanced hypothesis that genetic variants of S. scabiei have a predominant geographic-related distribution with cryptic transmission patterns. These patterns may rely on the interactions between different hosts living in the same ecological niche rather than a simple infection among hosts belonging to the same taxon, reinforcing the idea that the S. scabiei historic classification into “var” might have little ongoing relevance.
Keywords: Sarcoptic mange, Scabies, Felid, Carnivore, Host-specificity, Genetic structure
Abstract
Les félidés domestiques et sauvages sont considérés comme des hôtes appropriés pour l'acarien parasite Sarcoptes scabiei, et la gale sarcoptique est signalée chez plusieurs espèces de félidés dans la littérature scientifique. Cependant, la classification traditionnelle des acariens du genre Sarcoptes en variétés spécifiques à l'hôte n'inclut pas S. scabiei var. felis. On ne sait pas si la transmission de la gale sarcoptique chez les félidés implique des canidés, d'autres espèces sympatriques ou exclusivement des félidés. Cette étude visait à caractériser la structure génétique des acariens S. scabiei des chats domestiques (Felis catus) et du lynx eurasien (Lynx lynx carpathicus), en les comparant aux Sarcoptes des carnivores domestiques et sauvages sympatriques. Dix marqueurs microsatellites de Sarcoptes ont été utilisés pour génotyper 81 acariens issus de grattages cutanés de 36 carnivores : 4 chats domestiques, un chien (Canis lupus familiaris), 4 lynx eurasiens, 23 renards roux (Vulpes vulpes) et 4 loups gris (Canis lupus lupus) d'Italie, de Suisse ou de France. Deux groupes génétiques de S. scabiei, qui suivent un modèle de distribution géographique, ont été détectés. Les acariens des chats originaires du centre de l'Italie se regroupent avec ceux des loups sympatriques. En revanche, tous les autres acariens de Suisse, de France et d'Italie du Nord sont groupés ensemble. Ces résultats renforcent l'hypothèse précédemment avancée selon laquelle les variants génétiques de S. scabiei ont une distribution géographique prédominante avec des schémas de transmission cryptiques. Ces modèles peuvent reposer sur les interactions entre différents hôtes vivant dans la même niche écologique plutôt que sur une simple transmission parmi des hôtes appartenant au même taxon, renforçant l'idée que la classification historique de S. scabiei en “var” a peu de pertinence.
Introduction
Sarcoptic mange, also referred to as scabies, is a highly contagious skin disease caused by the burrowing mite Sarcoptes scabiei, affecting 200 million people each year (WHO, 2020 [45) [13, 17] and more than 150 mammal species [29]. Concerning free-ranging felids, cases have been reported in the Eurasian lynx (Lynx lynx carpathicus) [35], Iberian lynx (Lynx pardinus) [27], and European wild cat (Felis silvestris silvestris) [26] in Europe; Himalayan lynx (Lynx lynx isabellinus) [11] and snow leopard (Uncia uncia) (although only clinically suspected) [28] in Asia; leopard (Panthera pardus), lion (Panthera leo) and cheetah (Acinonyx jubatus) [10] in Eastern Africa, though not in wild felids in the Americas (Table 1).
Table 1.
Cases of sarcoptic mange previously reported in felids.
|
| ||||
| Country | Host species | Number of individuals | Suspected origin | Reference |
|---|---|---|---|---|
|
| ||||
| UK | Domestic cat (Felis catus) | 1 | Fox | [12] |
| Indonesia | Domestic cat (Felis catus) | 9* | NA | [15] |
| Sweden | Domestic cat (Felis catus) | 25 | Dog | [4, 29, 40] |
| Eurasian lynx (Lynx lynx carpathicus) | ||||
| Taiwan | Domestic cat (Felis catus) | 5* | NA | [14] |
| Australia | Domestic cat (Felis catus) | 4* | Dog, fox, wombat | [20] |
| India | Domestic cat (Felis catus) | 1 | Dog | [38] |
| India | Domestic cat (Felis catus) | 1 | NA | [37] |
| Switzerland | Eurasian lynx (Lynx lynx carpathicus) | 2* | Fox/lynx | [35] |
| Norway | Eurasian lynx (Lynx lynx carpathicus) | NA | NA | [25] |
| Germany | Eurasian lynx (Lynx lynx) | NA | Fox | [34] |
| Spain | Iberian lynx (Lynx pardinus) | 1 | Fox | [27] |
| Pakistan | Himalayan lynx (Lynx lynx isabellinus) | 1 | Fox livestock | [11] |
| Pakistan | Snow leopard (Uncia uncia) | NA | Blue sheep | [28] |
| Spain | European wild cat (Felis silvestris silvestris) | 1 | Fox/cat/dog/rabbit | [26] |
| Kenya | Cheetah (Acinonyx jubatus) | 3 | Thomson’s gazelle | [10] |
| Kenya | Lion (Panthera leo) | 3 | Wildebeest | [10] |
| South Africa | Leopard (Panthera pardus) | NA | NA | [29] |
NA: information not available; *Multiple cases reported in the same article.
Sarcoptic mange is considered a rare disease in domestic cats (Felis catus), more frequently affected by “feline mange”, a similar condition caused by the related burrowing mite Notoedres cati [19]. However, several confirmed sarcoptic mange cases in domestic cats have appeared in the scientific literature in the last two decades [12, 14, 15, 20, 37, 38] (Table 1), including an epidemic involving 25 animals [4]. The origin of these unusual episodes has been empirically attributed to contact with affected dogs living in the same household [20, 38] or, more rarely, with foxes visiting neighbouring gardens [12, 20].
Similarly, in wild felids, the source of S. scabiei transmission has been associated with sympatric hosts within carnivore communities (e.g., Eurasian lynx and red foxes (Vulpes vulpes) in continental Europe [5]) or in a prey-to-predator context (e.g., gazelle Eudorcas thomsonii and cheetahs in Eastern Africa [10]). Molecular evidence of the robustness of the latter association has been obtained using molecular markers in the case of lion, cheetah and the respective favourite ruminant preys in Masai Mara, Kenya [10].
Sarcoptes scabiei has been traditionally classified into host-specific varieties. Still, growing molecular evidence shows that the mere taxon-oriented approach is insufficient to embrace the issue’s complexity [7]. In this regard, various molecular tools have become available to deepen our understanding of the genetic differences between Sarcoptes strains affecting different host species and to track transmission pathways more efficiently and objectively. Amongst these tools, microsatellite markers have been shown to be more informative than other markers in characterizing the populations’ “strains” of S. scabiei affecting wildlife in Oceania [43], Europe [5, 23, 31, 36, 41], Africa [10], Asia [21], and South and North America [33, 42], sometimes revealing unexpected patterns of spread.
In line with the infrequent occurrence of sarcoptic mange in domestic cats, Sarcoptes isolates from feline hosts have never been characterized at a molecular level. This leaves open speculation on the possible transmission pathways and reservoir hosts and possible “strain”-specific diversity regarding pathogenicity. This study aims to investigate the molecular profile of Sarcoptes mites obtained from domestic and wild felids from different European countries and to compare them with Sarcoptes mites from sympatric and allopatric wild carnivores (Felidae and Canidae families), using microsatellite markers.
Material and methods
Skin scrapings from four domestic cats and a dog were collected in Italy, France and Switzerland during a dermatological examination due to severe itch and crusted skin lesions. In contrast, skin samples were also obtained from wildlife with skin lesions compatible with sarcoptic mange and submitted for post-mortem examination (Table 2).
Table 2.
Origin and sample size of the animals affected by sarcoptic mange included in this study.
|
| |||
| Sampling site | Host species | N | n |
|---|---|---|---|
|
| |||
| France | Domestic cat (Felis catus) | 1 | 3 |
| Central Italy | Domestic cat (Felis catus) | 2 | 8 |
| Switzerland | Domestic cat (Felis catus) | 1 | 6 |
| Switzerland | Eurasian lynx (Lynx lynx) | 4 | 8 |
| France | Eurasian lynx (Lynx lynx) | 1 | 5 |
| Switzerland | Red fox (Vulpes vulpes) | 11 | 11 |
| North Italy | Red fox (Vulpes vulpes) | 12 | 28 |
| North Italy | Domestic dog (Canis lupus familiaris) | 1 | 3 |
| France | Wolf (Canis lupus) | 2 | 5 |
| Central Italy | Wolf (Canis lupus) | 2 | 4 |
N: number of sampled animals; n, number of mites used for microsatellite analysis.
All samples were stored at −20 °C in 70% ethanol tubes until mite isolation and later shipped to the Department of Veterinary Sciences of Turin, Italy. Morphological criteria were applied for the preliminary identification of collected mites [8]. For each skin sample, one to six mites were isolated and individually stored in 70% ethanol [3].
DNA was extracted from individual mites following the HotSHOT Plus ThermalSHOCK technique [2]. Then, a 10x multiplex PCR was performed using ten validated primers extracted from the previously published panel [44] to target S. scabiei mites (Sarms 33, 34, 35, 36, 37, 38, 40, 41, 44, 45) following the PCR protocol of Soglia et al. [39]. Capillary electrophoresis was performed with an Applied Biosystems SeqStudioTM. The software GeneMapper 4.0 (Applied Biosystems, Foster City, CA, USA) allowed the allele calls and microsatellite visualization. After molecular analysis, mites that did not fulfil the required criteria (eight detectable loci out of the ten analyzed) were excluded from the genetic analysis. Two population genetics analyses were applied to the 81 mite microsatellite outputs: i) Bayesian clustering and ii) principal component analysis (PCA). The first one requires Hardy-Weinberg equilibrium (HWE), while no assumptions are required for the PCA. Descriptive statistics, such as observed and expected heterozygosis (Ho and He, respectively), allelic richness (R) and HWE analysis, were carried out with software R 4.0 using the packages Adegenet 2.1.3 [16]. P-values for the HWE test were based on Monte Carlo permutations of alleles. The Bayesian assignment test was computed with the software STRUCTURE 2.3.4. Burn-in and run lengths of Markov chains were 10,000 and 100,000, respectively, and five independent runs for each K (for K = 1–20) were run. The ancestry model selected was the admixture type. Clusters were estimated as suggested by Evanno [6], using the DK method.
Results
A total of 53 alleles were detected. Allele count ranged from 2 (Sarms 34) to 11 (Sarms 45). Eleven private alleles were found across the ten microsatellite loci, distributed among six loci (Sarms 33, 34, 38, 41, 44, 45). Deviation from HWE was detected only in Sarms 34 (supplementary material, Table S1). Observed heterozygosity ranged between 0.07 (Sarms 37) and 0.88 (Sarms 41) (Table 3).
Table 3.
Descriptive statistics of the Sarcoptes populations arranged by Sarms locus.
|
| ||
| Mst locus | Ho | He |
|---|---|---|
|
| ||
| Sarms 33 | 0.09 | 0.25 |
| Sarms 34 | 0.01 | 0.01 |
| Sarms 35 | 0.03 | 0.52 |
| Sarms 36 | 0.04 | 0.52 |
| Sarms 37 | 0.01 | 0.27 |
| Sarms 38 | 0.09 | 0.54 |
| Sarms 40 | 0.04 | 0.23 |
| Sarms 41 | 0.05 | 0.42 |
| Sarms 44 | 0.05 | 0.33 |
| Sarms 45 | 0.06 | 0.47 |
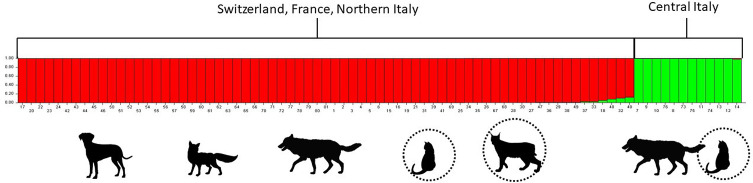
The Bayesian assignment test revealed the presence of two geographically separated clusters (Fig. 1). One cluster included mites from the cats and wolves from Central Italy (green cluster), and the other from foxes, lynx, wolves, dog and cats from France, Switzerland and Northern Italy (red cluster).
Figure 1.
Barplot of Sarcoptes-derived genetic clusters generated with the software Structure 2.3.4 with maximum likelihood K = 2. Each mite is represented by a single bar, and the height of each coloured segment is proportional to the membership fraction in each cluster. Felids are marked with a dotted circle.
The results of the PCA are displayed in Figure 2. The multivariate analysis revealed two main clusters, mainly scattered along the first axis: (i) mites collected on cats and wolves from Central Italy; and (ii) mites collected on cats, dog, wolves, foxes and lynx from France, Switzerland and Northern Italy (see also Table 2).
Figure 2.
Principal components analysis (PCA) of microsatellite loci representing cat-, dog-, lynx-, wolf- and fox-derived mite populations in France, Switzerland, North Italy and Central Italy. Each population is labelled with the host species and the geographical origin. The eigenvalues of the two axes are displayed in the barplot on the left. Components PCA 1 and PCA 2 explained 11.3% and 7.9% of the variance, respectively (black bars of the eigenvalues, inset).
Discussion
The main finding of this study is the identification, using microsatellite markers, of two genetic clusters of European S. scabiei with a geographical distribution pattern. Cat-derived mites from Central Italy clustered with those obtained from sympatric wolves. By contrast, all the other mites from Switzerland, France and Northern Italy clustered together.
These results align with previous evidence, though referring to different animal models, that genetic lineages of S. scabiei have a geographic-related distribution adding to the expected host-related distribution [23]. Interspecific transmission within broad taxa (e.g., carnivores) might thus rely on direct or indirect interactions between different hosts sharing the same ecological niche [21, 23, 31].
This finding differs from the classical idea that S. scabiei exclusively embraces host-specific varieties. It instead supports the view that various mammal species sharing the same environment have opportunities for direct or indirect contact (through predation, scavenging, territorial fights, denning, mating, etc.), which may allow the sharing of pathogens [7, 32].
While numerous species-related varieties of S. scabiei have been traditionally associated with various hosts, a felid-specific variety (e.g., a putative S. scabiei var. “felis”) has never been described. The explanation is possibly the infrequent occurrence of sarcoptic mange in domestic cats, in which notoedric mange, caused by Notoedres cati, is predominant. Nonetheless, in the last few decades, an increasing number of sarcoptic mange cases in both domestic and wild felids have been reported [10, 12, 15, 20, 35]. These cases referred to different epidemiological scenarios in which infected dogs, foxes or wild felids’ natural prey were pinpointed as the likely source of infection (Table 1).
Recently, nuclear molecular markers such as microsatellites have been proven valuable for investigating population genetic differences and putative transmission pathways within S. scabiei [21, 23, 31, 33]. To the authors’ knowledge, this is the first study to apply such tools to domestic/wild felid-derived mites, indicating, on a molecular basis, what is historically reported, namely that a var. felis of S. scabiei may not exist. This conclusion needs confirmation by further molecular analyses based, in parallel, on microsatellite markers and innovative genomic techniques [9, 18, 22, 46]. A larger dataset of mites from diversified geographical areas and carnivore communities is similarly recommended.
Interestingly, Bornstein et al., 2004 performed a genetic characterization (although not specifying the molecular markers employed) of Sarcoptes mites from six out of 25 infected cats involved in a single outbreak in Sweden. These authors noted that “the mites had DNA sequences identical to S. scabiei from naturally infected dogs and Swedish wildlife”. These results agree with our findings (Figs. 1 and 2) and confirm that S. scabiei taxonomy cannot be simplified in clear-cut host-specific varieties or subspecies, as already outlined [7, 41]. Empirical information in previous studies (Table 1) suggests that dogs and foxes play a key role in the transmission of S. scabiei to domestic and wild felids in various scenarios, from urban to remote natural areas.
Unlike what was observed in two large wild felids in Eastern Africa (Table 1), no prey-to-predator pattern of S. scabiei transmission was identified in the present study. For example, the lynx mites came from the Western Alps (Table 2), where sarcoptic mange has not been reported in the main lynx prey, the roe deer (Capreolus capreolus), nor in the Northern chamois (Rupicapra rupicapra). In these areas, the disease has been detected only in smaller and rarely preyed animals, such as the red fox [25, 30] and mustelids [1].
Despite the limited sample size and the use of a single (although particularly informative) class of genetic markers, this study may be considered baseline data in the unexplored field of felid Sarcoptes epidemiology in felid hosts. Our results, far from suggesting that domestic cats and wolves from Central Italy infected each other by direct contact (which can be considered a rare, though possible event), show that the same S. scabiei strain circulates in different carnivore hosts living in the same geographical area. It seems reasonable to assume that infected dogs or red foxes represent the missing link between wolves and cats in Central Italy (see Figs. 1 and 2). Interestingly, the owners of these cats reported that they had free outdoor access and that foxes were often seen roaming near the house.
While sarcoptic mange may not always represent a threat to domestic animals, numerous cases resulted in zoonotic transmission to the owners or members of the family in contact with the infected cat [24]. Moreover, infection by Sarcoptes mites circulating in European carnivore communities can put additional pressure on species or populations of conservation concern, such as the Iberian lynx and the European wild cat (not included in this study). In these wild felids and other European wild felids not included in this study, the recognition of canids (and most likely the red fox) as the expected source of infection may have practical consequences in the planning of delicate conservation and management interventions, such as restocking and reintroductions.
In conclusion, our results suggest that domestic and wild felids in Italy and neighbouring countries are affected by the same Sarcoptes strains as those involving sympatric canids. A specific geographical pattern may reveal the transmission pathways of Sarcoptes mites in the investigated carnivore hosts rather than a “host species-related only” pattern. Thus, the continued use of the term “var” in the past scientific literature referring to Sarcoptes host-specific subspecies or “strains” may be outdated or even misleading, appearing of little relevance. Accordingly, we believe that the contemporary understanding of the broad and sometimes unexpected host associations of S. scabiei should cautiously lead to the dismission of the historic nomenclature based on questionable mite morphological differences and the exclusive view of strict host association [46].
Supplementary material
The supplementary material of this article is available at https://www.parasite-journal.org/10.1051/parasite/2023012/olm.
Results of the Hardy–Weinberg equilibrium test at locus (rows) and populations (columns) showing P values from the Monte Carlo test. P values less than 0.05 (*) and 0.01 (**) are considered significant.
Acknowledgments
The authors are grateful to Christian Braglia and Noëlle Cochet-Faivre who visited and collected skin scrapings from the cats in Italy and France, respectively. We would like to thank Gianni Perugini for providing mites from one of the wolves.
Cite this article as: Moroni B, Albanese F, Rita Molinar Min A, Pasquetti M, Guillot J, Roberto Rolando Pisano S, Ryser-Degiorgis M,-P Rüfenacht S, Gauthier D, Cano-Terriza D, Scaravelli D, Rossi L & Peano A. 2023. Sarcoptic mange in Felidae: does Sarcoptes scabiei var. felis exist? A first molecular study. Parasite 30, 11.
Footnotes
Edited by: Jean-Lou Justine
References
- 1.Akdesir E, Origgi FC, Wimmershoff J, Frey J, Frey CF, Ryser-Degiorgis MP. 2018. Causes of mortality and morbidity in free-ranging mustelids in Switzerland: Necropsy data from over. BMC Veterinary Research, 14, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alasaad S, Rossi L, Maione S, Sartore S, Soriguer RC, Pérez JM, Rasero R, Zhu XQ, Soglia D. 2008. HotSHOT Plus ThermalSHOCK, a new and efficient technique for preparation of PCR-quality mite genomic DNA. Parasitology Research, 103, 1455–1457. [DOI] [PubMed] [Google Scholar]
- 3.Alasaad S, Soglia D, Maione S, Sartore S, Soriguer RC, Pérez JM, Rasero R, Rossi L. 2009. Effectiveness of the postponed isolation (post-frozen isolation) method for PCR-quality Sarcoptes mite gDNA. Experimental and Applied Acarology, 47, 173–178. [DOI] [PubMed] [Google Scholar]
- 4.Bornstein S, Gidlund K, Karlstam E, Bergstrom K, Zakrisson G, Nikkila T, Bergvall K, Renstrom L, Mattsson JG. 2004. Sarcoptic mange epidemic in a cat population. Veterinary Dermatology, 15, 34. [Google Scholar]
- 5.Cardells J, Lizana V, Martí-Marco A, Lavín S, Velarde R, Rossi L, Moroni B. 2021. First description of sarcoptic mange in an Iberian hare (Lepus granatensis). Current Research in Parasitology & Vector-Borne Diseases, 1, 100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Earl DA, vonHoldt BM. 2012. Structure harvester: A website and program for visualizing structure output and implementing the Evanno method. Conservation Genetics Resources, 4, 359–361. [Google Scholar]
- 7.Escobar LE, Carver S, Cross PC, Rossi L, Almberg ES, Yabsley MJ, Niedringhaus KD, Van Wick P, Dominguez-Villegas E, Gakuya F, Xie Y, Angelone S, Gortázar C, Astorga F. 2021. Sarcoptic mange: An emerging panzootic in wildlife. Transboundary and Emerging Diseases, 69, 927–942. [DOI] [PubMed] [Google Scholar]
- 8.Fain A. 1968. Étude de la variabilité de Sarcoptes scabiei avec une révision des Sarcoptidae. Acta Zoologica et Pathologica Antverpiensia, 47, 1–196. [Google Scholar]
- 9.Fraser TA, Shao R, Fountain-Jones NM, Charleston M, Martin A, Whiteley P, Holme R, Carver S, Polkinghorne A. 2017. Mitochondrial genome sequencing reveals potential origins of the scabies mite Sarcoptes scabiei infesting two iconic Australian marsupials. BMC Evolutionary Biology, 17, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gakuya F, Rossi L, Ombui J, Maingi N, Muchemi G, Ogara W, Soriguer RC, Alasaad S. 2011. The curse of the prey: Sarcoptes mite molecular analysis reveals potential prey-to-predator parasitic infestation in wild animals from Masai Mara. Kenya. Parasites & Vectors, 4, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hameed K, Angelone-alasaad S, Din JU, Nawaz MA, Rossi L. 2016. The threatening but unpredictable Sarcoptes scabiei: first deadly outbreak in the Himalayan lynx, Lynx lynx isabellinus, from Pakistan. Parasites & Vectors, 9, 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy JI, Fox MT, Loeffler A, Sinclair G. 2013. Feline sarcoptic mange in the UK. Veterinary Record Case Reports, 171, 351. [DOI] [PubMed] [Google Scholar]
- 13.Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, Marks R, Naldi L, Weinstock MA, Wulf SK, Michaud C, Murray CJL, Naghavi M. 2014. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. Journal of Investigative Dermatology, 134, 1527–1534. [DOI] [PubMed] [Google Scholar]
- 14.Huang HP, Lien YH. 2013. Feline sarcoptic mange in Taiwan: A case series of five cats. Veterinary Dermatology, 24, 457–9. [DOI] [PubMed] [Google Scholar]
- 15.Iqomah M, Suwarno N, Yuliani P. 2020. Cat scabies at the animal health clinic of Salatiga Agriculture Service on August to November 2020. Journal of Parasite Science, 4, 45. [Google Scholar]
- 16.Jombart T. 2008. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics, 24, 1403–1405. [DOI] [PubMed] [Google Scholar]
- 17.Karimkhani C, Colombara DV, Drucker AM, Norton SA, Hay R, Engelman D, Steer A, Whitfeld M, Naghavi M, Dellavalle RP. 2017. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infectious Diseases, 17, 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korhonen PK, Gasser RB, Ma G, Wang T, Stroehlein AJ, Young ND, Ang CS, Fernando DD, Lu HC, Taylor S, Reynolds SL, Mofiz E, Najaraj SH, Gowda H, Madugundu A, Renuse S, Holt D, Pandey A, Papenfuss AT, Fischer K. 2020. High-quality nuclear genome for Sarcoptes scabiei – A critical resource for a neglected parasite. PLoS Neglected Tropical Diseases, 14, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraabøl M, Gundersen V, Fangel K, Olstad K. 2015. The taxonomy, life cycle and pathology of Sarcoptes scabiei and Notoedres cati (Acarina, sarcoptidae): a review in a fennoscandian wildlife perspective. Fauna Norvegica, 35, 21–33. [Google Scholar]
- 20.Malik R, McKellar Stewart K, Sousa CA, Krockenberger MB, Pope S, Ihrke P, Beatty J, Barrs VRD, Walton S. 2006. Crusted scabies (sarcoptic mange) in four cats due to Sarcoptes scabiei infestation. Journal of Feline Medicine and Surgery, 8, 327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuyama R, Yabusaki T, Senjyu N, Okano T, Baba M, Tsuji- T, Yokoyama M, Kido N, Kadosaka T, Kato T, Suzuki M, Asano M. 2019. Possible transmission of Sarcoptes scabiei between herbivorous Japanese serows and omnivorous Caniformia in Japan: a cryptic transmission and persistence? Parasites & Vectors, 12, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mofiz E, Holt DC, Seemann T, Currie BJ, Fischer K, Papenfuss AT. 2016. Genomic resources and draft assemblies of the human and porcine varieties of scabies mites, Sarcoptes scabiei var. hominis and var. suis. GigaScience, 5, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moroni B, Angelone S, Pérez JM, Molinar AR, Pasquetti M, Tizzani P, López-Olvera JR, Valldeperes M, Granados JE, Lavín S, Mentaberre G, Camacho-Sillero L, Martínez-Carrasco C, Oleaga A, Candela M, Meneguz PG, Rossi L. 2021. Sarcoptic mange in wild ruminants in Spain: solving the epidemiological enigma using microsatellite markers. Parasites & Vectors, 14, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moroni B, Rossi L, Bernigaud C, Guillot J. 2022. Zoonotic episodes of scabies: a global overview. Pathogens, 11, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munson L, Terio K, Lane E, Robert N, Courchamp F. 2007. Wild felid. Felid Biology and Conservation, 31, [Google Scholar]
- 26.Najera F, Crespo E, Garc A, Grande-Gomez R, Herrera-Sanchez F, Gentil M, Cort C, Müller E, Calero R, Revuelta L. 2021. First description of Sarcoptic mange in a free-ranging European wildcat (Felis silvestris silvestris) from Spain. Animals, 11, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oleaga A, García A, Balseiro A, Casais R, Mata E, Crespo E. 2019. First description of sarcoptic mange in the endangered Iberian lynx (Lynx pardinus): clinical and epidemiological features. European Journal of Wildlife Research, 65, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostrowski S, Gilbert M. 2016. Diseases of free-ranging snow leopards and primary prey species. Snow Leopards., 2016, 97–112. [Google Scholar]
- 29.Pence DB, Ueckermann E. 2002. Sarcoptic mange in wildlife. Revue Scientifique et Technique (International Office of Epizootics), 21, 385–398. [PubMed] [Google Scholar]
- 30.Pisano SRR, Zimmermann F, Rossi L, Capt S, Akdesir E, Bürki R, Kunz F, Origgi FC, Ryser-Degiorgis MP. 2019. Spatiotemporal spread of sarcoptic mange in the red fox (Vulpes vulpes) in Switzerland over more than 60 years: lessons learnt from comparative analysis of multiple surveillance tools. Parasites & Vectors, 12, 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasero R, Rossi L, Soglia D, Maione S, Sacchi P, Rambozzi L, Sartore S, Soriguer RC, Spalenza V, Alasaad S. 2010. Host taxon-derived Sarcoptes mite in European wild animals revealed by microsatellite markers. Biological Conservation, 143, 1269–1277. [Google Scholar]
- 32.Rossi L, Tizzani P, Rambozzi L, Moroni B, Meneguz PG. 2019. Sanitary emergencies at the wild/domestic caprines interface in Europe. Animals, 9, 922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudd JL, Clifford DL, Cypher BL, Hull JM, Jane A, Foley JE. 2020. Molecular epidemiology of a fatal sarcoptic mange epidemic in endangered San Joaquin kit foxes (Vulpes macrotis mutica). Parasites & Vectors, 13, 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryser-Degiorgis M-P. 2009. Causes of mortality and diseases of Eurasian lynx (Lynx lynx), in Iberian Lynx Ex Situ Conservation. Vargas A, Breitenmoser-Würsten C, Breitenmoser U, Editors. An Interdisciplinary Approach: , Madrid. p. 275–289. [Google Scholar]
- 35.Ryser-Degiorgis MP, Ryser A, Bacciarini LN, Angst C, Gottstein B, Janovsky M, Breitenmoser U. 2002. Notoedric and sarcoptic mange in free-ranging lynx from Switzerland. Journal of Wildlife Diseases, 38, 228–232. [DOI] [PubMed] [Google Scholar]
- 36.Sannö A, Ander M, Ågren E, Troell K. 2021. Sarcoptic mange in the wild boar, Sus scrofa, in Sweden. Current Research in Parasitology & Vector-Borne Diseases, 1, 100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh R, Turkar S, Dua K, Khan IS. 2019. A rare case of Sarcoptes Scabiei in Persian cat. Indian Journal of Veterinary Medicine, 39, 56–58. [Google Scholar]
- 38.Sivajothi S, Reddy BS. 2015. Cat affected with sarcoptic mange in Y. S. R. District of Andhra Pradesh. India. Comparative Clinical Pathology, 24, 1031–1032. [Google Scholar]
- 39.Soglia D, Rasero R, Rossi L, Sartore S, Sacchi P, Maione S. 2007. Microsatellites as markers for comparison among different populations of Sarcoptes scabiei. Italian Journal of Animal Science, 6, 214–216. [Google Scholar]
- 40.Tryland M, Okeke MI, af CH, Mörner T, Traavik T, Ryser-Degiorgis MP. 2011. Orthopoxvirus DNA in Eurasian Lynx, Sweden. Emerging Infectious Diseases, 17, 626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valldeperes M, Moroni B, Rossi L, López JR, Velarde R, Molinar AR, Mentaberre G, Serrano E, Angelone S, Lavín S, Granados JE. 2021. First report of interspecific transmission of sarcoptic mange from Iberian ibex to wild boar. Parasites & Vectors, 14, 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valle H del, Rudd J, Foley J, Vanstreels RET, Martín AM, Donadio E, Uhart MM. 2022. Sarcoptic mange outbreak decimates South American wild camelid populations in San Guillermo National Park, Argentina. PLoS ONE, 17, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walton SF, Dougall A, Pizzutto S, Holt D, Taplin D, Arlian LG, Morgan M, Currie BJ, Kemp DJ. 2004. Genetic epidemiology of Sarcoptes scabiei (Acari: Sarcoptidae) in northern Australia. International Journal for Parasitology, 34, 839–849. [DOI] [PubMed] [Google Scholar]
- 44.Walton SF, Currie BJ, Kemp DJ. 1997. A DNA fingerprinting system for the ectoparasite Sarcoptes scabiei. Molecular and Biochemical Parasitology, 85, 187–196. [DOI] [PubMed] [Google Scholar]
- 45.WHO. 2020. Scabies. Accessed on 11/10/22. https://www.who.int/news-room/fact-sheets/detail/scabies.
- 46.Xu J, Wang Q, Wang S, Huang W, Xie Y, Gu X, He R, Peng X, Wu S, Yang G. 2022. Comparative genomics of Sarcoptes scabiei provide new insights into adaptation to permanent parasitism and within-host species divergence. Transboundary and Emerging Diseases, 69, 3468–3484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary material of this article is available at https://www.parasite-journal.org/10.1051/parasite/2023012/olm.
Results of the Hardy–Weinberg equilibrium test at locus (rows) and populations (columns) showing P values from the Monte Carlo test. P values less than 0.05 (*) and 0.01 (**) are considered significant.