SUMMARY
Some people are more attractive to mosquitoes than others, but the mechanistic basis of this phenomenon is poorly understood. We tested mosquito attraction to human skin odor and identified people who are exceptionally attractive or unattractive to mosquitoes. These differences were stable over several years. Chemical analysis revealed that highly attractive people produce significantly more carboxylic acids in their skin emanations. Mutant mosquitoes lacking the chemosensory co-receptors Ir8a, Ir25a, or Ir76b, were severely impaired in attraction to human scent but retained the ability to differentiate highly and weakly attractive people. The link between elevated carboxylic acids in “mosquito magnet” human skin odor and phenotypes of genetic mutations in carboxylic acid receptors suggests that such compounds contribute to differential mosquito attraction. Understanding why some humans are more attractive than others provides insights into what skin odorants are most important to the mosquito and could inform the development of more effective repellents.
Keywords: Aedes aegypti, mosquito, behavior, olfaction, chemosensory receptors, skin, sebum, metabolomics
Summary
Chemical analysis of the skins of humans who are exceptionally attractive or unattractive over time to mosquitoes reveals higher levels carboxylic acids in the former. These compounds are sensed by mosquito chemosensory ionotropic receptors.
Graphical Abstract
INTRODUCTION
The globally invasive mosquito Aedes aegypti is a highly efficient vector of viruses including yellow fever, dengue, chikungunya, and Zika among human populations (Bhatt et al., 2013). A single female mosquito will bite multiple humans during her 3 to 6-week lifetime to obtain sufficient protein to produce a new batch of eggs as often as every four days (Ponlawat and Harrington, 2005). This repetitive human-directed feeding behavior allows the mosquito to contract and transmit pathogens in successive bites. Aedes aegypti are efficient vectors of disease because they specialize on human hosts (McBride et al., 2014; Rose et al., 2020; Zhao et al., 2022), thereby focusing pathogen transmission on our species. Female Aedes aegypti mosquitoes have a strong innate drive to hunt humans, using sensory cues including exhaled CO2, body heat, and skin odor. While CO2 and heat are generic stimuli that signify a living warm-blooded animal, skin odor provides information about whether the target is a human or non-human animal (McBride et al., 2014; Rose et al., 2020; Zhao et al., 2022). It is well-documented that mosquitoes are more strongly attracted to some humans than others (Acree et al., 1968; Ansell et al., 2002; Bernier et al., 2002; Brady et al., 1997; Braks and Takken, 1999; Brouwer, 1960; Fernandez-Grandon et al., 2015; Gilbert et al., 1966; Harrington et al., 2014; Himeidan et al., 2004; Knols et al., 1995; Konopka et al., 2021; Mukabana et al., 2002; Qiu et al., 2006; Verhulst et al., 2011b), but the underlying mechanisms for this phenomenon remain unclear. The observation that some people are “mosquito magnets” is a topic that captivates the general public and scientific community alike. There is much speculation about possible mechanisms, but only some have a scientific basis. A common explanation offered by non-experts is that differences in ABO blood type “explain” attractiveness to mosquitoes, but experimental data that address this belief are contradictory (Anjomruz et al., 2014; Khan et al., 2022; Shirai et al., 2004; Thornton et al., 1976; Wood et al., 1972). The widely quoted efficacy of eating garlic (Rajan et al., 2005) or B vitamins (Ives and Paskewitz, 2005) as a home remedy to repel mosquitoes is similarly unclear. Although a twin study documented a strong heritable component (Fernandez-Grandon et al., 2015), non-genetic factors also contribute to selective attractiveness to mosquitoes. A given person can become more attractive to mosquitoes in contexts including pregnancy (Ansell et al., 2002; Lindsay et al., 2000), malaria parasite infection (Busula et al., 2017; De Moraes et al., 2014; De Moraes et al., 2018; Lacroix et al., 2005; Robinson et al., 2018), and beer consumption (Lefevre et al., 2010; Shirai et al., 2002). The most widely accepted explanation for these differences is that variation in skin odors produced by different humans, related in part to their unique skin microbiota (Braks and Takken, 1999; Verhulst et al., 2011b), governs their attractiveness to mosquitoes (Acree et al., 1968; Bernier et al., 2002; Brady et al., 1997; Brouwer, 1960; Qiu et al., 2006). However, the specific chemical mechanism for differential attractiveness to mosquitoes remains unclear.
Human skin odor is a blend of many organic compounds (Bernier et al., 1999; Bernier et al., 2000; Gallagher et al., 2008), the composition of which has not been exhaustively inventoried. It remains unclear how consistent human skin odor is over time within an individual. Whereas much work has focused on characterizing human axillary (armpit) malodor, there is relatively little information about the composition of the markedly less intense skin odor that emanates from body sites commonly bitten by mosquitoes. Furthermore, additional work is needed before we can fully appreciate the extent of interindividual variation in human skin odor. Thus, it is not known which specific components are most relevant for mosquito attraction to humans, nor do we understand which odorants cause mosquitoes to choose to bite some people over others. Humans who are highly attractive to mosquitoes may produce more attractant odors than other people (McBride et al., 2014). Alternatively, less attractive humans may emit compounds that repel mosquitoes (Logan et al., 2008; Logan et al., 2010). To date no single molecule obtained from human skin can be said to be sufficient to explain how attractive a person is to mosquitoes. Blends of odorants can be more or less attractive depending on the composition of the blend and the concentration of a specific molecule. For example, the binary blend of ammonia and lactic acid strongly synergizes to elicit mosquito attraction (Geier et al., 1999; Steib et al., 2001). Although carboxylic acids are neutral or repellent when presented individually or in combination with each other, they strongly increase mosquito attraction when combined with ammonia and lactic acid (Smallegange et al., 2009; Smallegange et al., 2005). Mosquito attraction behavior is elicited much more reliably using live human hosts or natural odor blends collected directly from humans, than it is by mixing pre-specified compounds, despite improvements in synthetic odor blends as lures for attract-and-kill traps for use in the field (Bernier et al., 2007; Njiru et al., 2006; Verhulst et al., 2011a). Moreover, the current absence of a complete reference metabolome of chemical compounds found on human skin, and the lack of commercially available standard molecules for many skin compounds also limits the effectiveness of human odor blend reconstitution approaches for studying mosquito attraction.
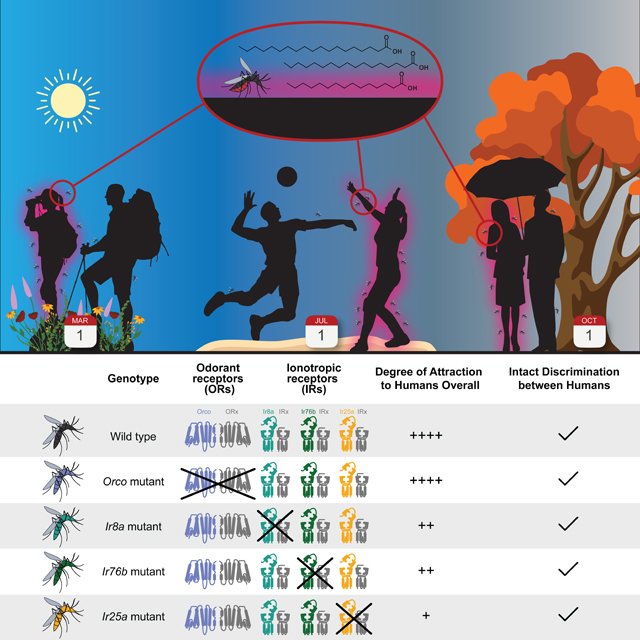
Mosquitoes use two large multigene families to detect olfactory cues that each encode odor-gated ion channels, the odorant receptors (ORs) and the ionotropic receptors (IRs) (Abuin et al., 2011; Benton et al., 2009; Butterwick et al., 2018; Del Mármol et al., 2021; Rytz et al., 2013; Sato et al., 2008; Silbering et al., 2011). ORs and IRs are evolutionarily unrelated, but both assemble into multi-subunit complexes with a ligand-selective subunit and a co-receptor subunit that does not respond to odorants (Abuin et al., 2011; Benton et al., 2009; Butterwick et al., 2018; Del Mármol et al., 2021; Rytz et al., 2013; Sato et al., 2008; Silbering et al., 2011). There are hundreds of ligand-selective ORs and IRs in a given insect species, but only one OR co-receptor (Orco) and three IR co-receptors (Ir8a, Ir76b, Ir25a). In Aedes aegypti there are 116 ligand-selective ORs and 132 ligand-selective IRs (Matthews et al., 2018). Together these large gene families of odor-gated ion channels sense a vast number of chemical ligands. Although there is some overlap in ligand tuning, ORs generally respond to esters, alcohols, ketones, and aldehydes, and IRs respond to carboxylic acids and amines (Hallem and Carlson, 2006; Silbering et al., 2011). Because of this co-receptor organization, mutating a single co-receptor gene leads to profound deficits in the ability of an insect to detect whole classes of odorants (Abuin et al., 2011; Benton et al., 2009; Larsson et al., 2004; Neuhaus et al., 2005). Nevertheless, mosquitoes are remarkably resilient in the face of such genetic manipulations. Animals lacking the major receptor for carbon dioxide, Gr3, continue to be attracted to humans in semi-field conditions (McMeniman et al., 2014). Mosquitoes with a loss of function mutation in Orco lose strong preference for humans over non-human animals but retain strong attraction to humans overall (DeGennaro et al., 2013; Raji et al., 2019). Finally, Ir8a mutants show severe deficits in detecting lactic acid, a major human skin odor, but nevertheless remain partially attracted to humans (Raji et al., 2019). The recent discovery of extensive co-expression of ORs and IRs in single olfactory sensory neurons may explain this functional redundancy (Herre et al., 2022; Task et al., 2022).
In this paper we analyzed the skin-derived compounds that differentiate highly from weakly attractive humans and asked which mosquito sensory pathways are required to distinguish such people. We developed a two-choice behavioral assay that allowed us to test mosquito attraction with higher throughput, allowing for frequent, repeat sampling of human subjects. We collected human skin odor samples on nylon stockings worn on the forearms and profiled the attractiveness of 64 human subjects to mosquitoes. We identified a cohort of highly and weakly attractive people and discovered that the Orco co-receptor is not required for discriminating between them. Mutants lacking Ir8a, Ir76b, and Ir25a retained a preference for “mosquito magnets,” but showed an overall reduced attraction to human skin odor (Raji et al., 2019). Therefore, although neither the OR nor the IR pathway is solely required for discriminating among different people, mutating the IR pathway produced significantly stronger effects on overall mosquito attraction to humans than the OR pathway. We used gas chromatography/quadrupole time of flight-mass spectrometry (GC/QTOF-MS) to identify skin odor molecules that are associated with attractiveness to mosquitoes. Because carboxylic acids have been shown to be attractive to mosquitoes (Smallegange et al., 2009), we focused our chemical analysis on detecting acids in the human skin odor blend by using specialized sample preparation to enrich for highly polar acids. Since these are otherwise difficult to detect using standard analytical chemistry approaches, their contribution to differential mosquito attraction to humans is relatively understudied (Bernier et al., 2000). We determined that highly attractive humans have higher levels of several carboxylic acids on their skin than less attractive humans. When we substantially diluted nylons from the most highly attractive subject, mosquitoes were no longer able to distinguish this subject from the least attractive subjects. Our results strongly suggest a link between the known function of IRs in acid-sensing and our observation that skin-derived carboxylic acids are associated with a person being a “mosquito magnet.”
RESULTS
Mosquitoes show strong preferences for individual humans
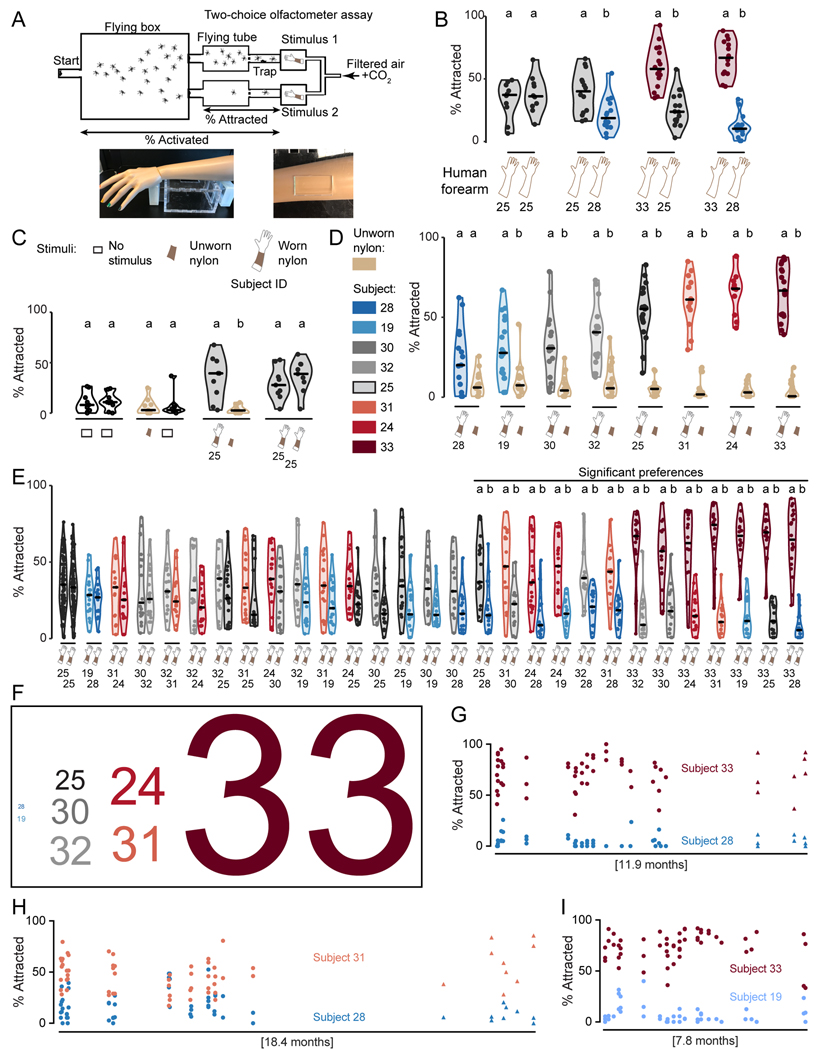
In previous studies, we used a two-choice Gouck olfactometer (Gouck, 1972) to characterize female Aedes aegypti preferences for a human or non-human animal (DeGennaro et al., 2013; McBride et al., 2014). This large apparatus was not suitable for the higher-throughput analysis of mosquito preference for humans required for this study. We therefore adapted a previously described single-stimulus olfactometer (Basrur et al., 2020) to reconfigure it as a two-choice olfactometer (Figure 1A), allowing us to test mosquito preferences between the forearms of two different live human subjects or their forearm skin odor collected on nylon sleeves. In this assay, a mixture of air and carbon dioxide (CO2) was passed over each stimulus to convey volatile odors to mosquitoes downwind (Figure 1A). Mosquitoes flew upwind and those that entered either of the two cylindrical traps in front of each stimulus were scored as attracted. This assay tests attraction to stimuli that are ~0.91 m away, meaning that to reach the attraction trap, mosquitoes must travel ~610 times their body length, assuming an average female mosquito thorax length of 1.5 mm (Yeap et al., 2013). During the 3.1-year course of this study, we carried out >2,330 behavior trials on 174 experimental days. There was no difference in attraction when mosquitoes were offered the left and right forearms of the same subject (Figure 1B). In pilot experiments comparing mosquito attraction among all possible pairings of three live human subjects, we identified one (Subject 33) that was significantly more attractive than two others (Subjects 25 and 28) (Figure 1B). When the two less attractive subjects were competed against each other, Subject 25 attracted significantly more mosquitoes than Subject 28 (Figure 1B).
Figure 1: Mosquitoes show strong preferences among individual humans.
(A) Schematic of two-choice olfactometer assay (top). Photographs (bottom) of a mannequin arm modeling the position of a live human forearm on top of the stimulus box in the two-choice olfactometer (left) and the opening in the stimulus box lid used to expose an area of human skin (5.08 cm x 2.54 cm) in the assay (right).
(B-E) Wild-type mosquitoes attracted to live human forearms (B) or a 5.08 cm x 2.54 cm piece of human-worn nylons and controls (C-E) of the indicated subjects in the two-choice olfactometer assay. Subject pairs in E are ordered by nonparametric effect size.
(F) Attractiveness scores for human subjects derived from data in E, with subject ID font size scaled to the attractiveness score: Subject 33 (score=144), Subject 24 (score=34), Subject 31 (score=32), Subject 32 (score=26), Subject 30 (score=23), Subject 25 (score=18), Subject 19 (score=1), Subject 28 (score=0).
(G-I) Longitudinal two-choice olfactometer data for two wild-type Aedes aegypti mosquito strains, Orlando (circles) and Liverpool (triangles), showing attraction to the indicated subject pairs. The total time elapsed between the first and last experiment shown is indicated, and corresponded to July 12, 2018 to July 3, 2019 (G), February 1, 2018 to August 6, 2019 (H), and July 30, 2018 to March 21, 2019 (I).
In B-E, data are displayed as violin plots with median indicated by horizontal black lines and the bounds of the violin corresponding to the range (30–40 mosquitoes/trial). B: n=10–16 trials, C: n=7–8 trials, D: n=12–21 trials, E: n=14–20 trials (except n=81 trials for the Subject 25 vs 25 comparison). Data corresponding to adjacent violin plots labeled with different letters are significantly different (p<0.05, Wilcoxon rank-sum tests with Bonferroni correction).
The two-choice olfactometer as configured for live human subjects requires participants to be physically present for competitions that take place in a warm, humid room. To make it feasible to carry out hundreds of competitions between two subjects over many months, we collected human forearm odor on nylon sleeves (DeGennaro et al., 2013; McBride et al., 2014). Empty stimulus traps did not attract mosquitoes, and mosquitoes did not prefer unworn nylon over an empty trap (Figure 1C). However, a 7.62 cm x 10.16 cm swatch cut from nylons worn by Subject 25 was significantly more attractive than the same-sized swatch from an unworn nylon (Figure 1C). There was no evidence for side bias when we used nylons from Subject 25 in both stimulus traps (Figure 1C).
To study interindividual differences in attractiveness to mosquitoes, we recruited an additional 5 human subjects who provided skin-scented nylon samples frequently over a period of several months. Nylons worn by each of these 8 subjects were significantly more attractive than an unworn nylon (Figure 1D). However, there were remarkable differences in the attractiveness of the 8 subjects. Nylons from Subject 28 attracted few mosquitoes, Subject 25 showed intermediate attractiveness, whereas Subject 33 was highly attractive (Figure 1D), mirroring the results of the live human experiments in Figure 1B. This suggests that skin odor is the primary driver of differential mosquito attraction to humans, since temperature and CO2 cues were held constant in all nylon experiments. It also suggests that skin odor captured on forearm-worn nylon is a good approximation of the odor emanating from a live human forearm. When we tested the 5 additional subjects, we found one additional low attractor (Subject 19) two additional high attractors (Subjects 24 and 31), and two subjects with intermediate attractiveness (Subjects 30 and 32) (Figure 1D).
We reasoned that in a real-world situation, mosquitoes would choose among multiple different humans in a local area, such that the absolute attractiveness of a single human would not necessarily predict their attractiveness relative to another person. To systematically determine the relative attractiveness of these 8 humans to mosquitoes, we performed a round-robin style “tournament”, competing nylons from all possible subject pairings from this group of 8 subjects, for a total of 28 separate competitions using the two-choice olfactometer assay (Figure 1E). We sampled each pair of humans on 6 separate days over a period of several months (558 trials, performed over 42 experimental days). Among 28 subject pairs tested, we found 13 pairs for which mosquitoes significantly preferred one subject’s odor over the other (Figure 1E). Subject 33 attracted significantly more mosquitoes than every other subject in essentially every trial performed, usually by a large margin. Subjects 19 and 28 were significantly less attractive than several other subjects. Mosquitoes did not have a preference between the two low attractors, Subjects 19 and 28 (Figure 1E). To rank subjects from most to least attractive, we devised an attraction score based on how many more mosquitoes each subject attracted when competed against all 7 other subjects. By this metric, Subject 33 was the most attractive, yielding an attractiveness score that was 4 times the attractiveness score of the next most attractive subject, and over 100 times greater than that of the two least attractive Subjects 19 and 28 (Figure 1F). These differences in attraction to specific pairs of humans were remarkably stable over many months and were seen with two different wild-type strains of Aedes aegypti (Figure 1G-I). We provide empirical evidence that mosquitoes strongly prefer some people over others, and that the olfactory cues that make some people “mosquito magnets” are stable over many months.
Orco and Ir8a mutant mosquitoes retain individual human preferences
We have shown that small swatches of human-scented nylon provide enough information for mosquitoes to distinguish between and prefer one person over another. What sensory mechanisms do mosquitoes rely on to detect these interindividual differences in skin odor? We tested the preference of mosquitoes lacking the OR co-receptor Orco, which retain strong attraction to humans, but show deficits in discriminating humans from non-human animals (DeGennaro et al., 2013). We also tested mosquitoes lacking the co-receptor Ir8a, which is expressed in the antenna and necessary for detection of several acids, including lactic acid, a component of human sweat (Raji et al., 2019).
We carried out control trials where nylons from Subject 25 were placed in both stimulus boxes (Figure 2A) and found that Orco mutant mosquitoes showed wild-type levels of activation and attraction (Figure 2B). Ir8a mutants showed decreased overall attraction to Subject 25 in the two-choice olfactometer assay, despite normal levels of activation, as defined by entry into the flying chamber (Figure 2C).
Figure 2: Mosquitoes lacking Orco or Ir8a retain individual human preferences.
(A) Schematic of two-choice olfactometer assay indicating the location of mosquitoes that were not activated, activated but not attracted, or attracted in response to a control trial in which Subject 25 nylons were placed in both stimulus boxes.
(B,C) Stacked bar plots indicate the mean total percent of mosquitoes that were in each category in all trials for Orco (30–40 mosquitoes/trial, n=30–34 trials, *p<0.01, Wilcoxon rank-sum tests with Bonferroni correction comparing each category across the two genotypes) (B) or Ir8a (30–40 mosquitoes/trial, n=31–33 trials, *p<0.0001, Wilcoxon rank-sum tests with Bonferroni correction comparing each category across the two genotypes) (C).
(D) Schematic of two-choice olfactometer assay, indicating the location (purple shading) of all mosquitoes attracted to either stimulus in a control trial in which Subject 25 nylons were placed in both stimulus boxes.
(E,F) Pie charts of trials in which 9 or fewer animals entered either trap were excluded for Orco (E) or Ir8a (F).
(G,H) Percent of mosquitoes of the indicated genotype attracted to the indicated stimuli in the two-choice olfactometer assay. Data from trials that met the inclusion criteria are displayed as violin plots with median indicated by horizontal black lines and the bounds of the violin corresponding to the range (30–40 mosquitoes/trial, n=11–18 trials, except n=29–31 for the Subject 25 vs 25 comparison). Data corresponding to adjacent violin plots labeled with different letters are significantly different (p<0.05, Wilcoxon rank-sum tests with Bonferroni correction).
(I, J) Percent of mosquitoes of the indicated genotype attracted to the indicated stimuli in the two-choice olfactometer assay. Data from trials that met the inclusion criteria are displayed as violin plots with median indicated by horizontal black lines and the bounds of the violin corresponding to the range (30–40 mosquitoes/trial, n=11–22 trials, except n=24–29 for the Subject 25 vs 25 comparison). Data corresponding to adjacent violin plots labeled with different letters are significantly different (p<0.05, Wilcoxon rank-sum tests with Bonferroni correction).
In the course of carrying out these control experiments we noted low participation in some trials. Therefore, we put inclusion criteria in place such that trials in which 9 or fewer mosquitoes entered either trap were excluded (Figure 2D-F). We note that low levels of participation preclude the accurate calculation of attraction preferences, such as an instance in which only three mosquitoes entered either trap. A high percentage of excluded trials may reflect an overall deficit in locomotor activity or general decreases in activation and attraction to human sensory cues or a combination of both factors.
More than 90% of all such control trials using both wild type and Orco mutants resulted in at least 10 total mosquitoes being attracted to either stimulus (Figure 2E). In experiments examining Ir8a, 94% of trials using wild-type mosquitoes resulted in 10 or more total mosquitoes being attracted to either stimulus, but this was reduced to 73% for trials using Ir8a mutants (Figure 2F).
Among trials that met the inclusion criteria, Orco mutants did not differ from wild-type controls, retaining the ability to distinguish 5 pairs of highly and weakly attractive humans (Figure 2G-H). Ir8a mutants largely retained the same preferences as wild-type controls, despite their diminished overall attraction to human odor (Figure 2I-J). This suggests that mosquitoes with significant olfactory deficits are still able to tell the difference between individual people.
Generation and behavioral characterization of Ir76b and Ir25a mutants
We next used CRISPR-Cas9 to generate mosquitoes that lack the two other IR co-receptors Ir76b and Ir25a (Figure 3A-B, Supplemental Figure S1). We generated 2 mutant alleles of each gene (Ir76b32, Ir76b61, Ir25aBamHI, and Ir25a19) and tested the behavior of heterozygous animals of all 4 strains and the heteroallelic Ir76b32/61 and Ir25aBamHI/19 null mutants. Homozygous Ir76b and Ir25a mutants had difficulty blood-feeding and Ir25a homozygous mutants generally laid fewer eggs. Notably, Anopheles coluzzii Ir76b null mutants show normal attraction to human host cues but do not blood-feed or produce eggs (Ye et al., 2022). To characterize the response of these strains and wild-type control mosquitoes to human cues, we first used a single-stimulus olfactometer (Basrur et al., 2020) to examine activation and attraction levels (Figure 3C). Ir76b32/61 mutants displayed reduced general activity levels in response to an unworn nylon or a nylon worn by Subject 33 (Figure 3D). However, this defect was readily overcome when Ir76b mutants were presented with the forearm of Subject 33, indicating the absence of gross motor defects (Figure 3D). Ir25aBamHI/19 mutants showed wild-type levels of activation across all stimuli tested (Figure 3E). Ir76b32/61 mutants showed normal levels of attraction to nylons worn by Subject 33 and to the forearm of Subject 33 (Figure 3F). In contrast, Ir25aBamHI/19 mutants displayed significant defects in their attraction to both Subject 33 nylons and to the forearm of Subject 33 (Figure 3G). This suggests that the Ir25a co-receptor, along with one or more of the ligand-selective IRs with which it assembles a functional receptor, plays an important role in detecting human skin emanations.
Figure 3: Generation and characterization of Ir25a and Ir76b mutants.
(A-B) Schematic of the Aedes aegypti Ir76b (A) and Ir25a (B) genomic loci, detailing sgRNA sites and modified protein products of the indicated mutant alleles superimposed on Ir76b and Ir25a protein snake plots, which were generated using Protter v1.0 (Omasits et al., 2014).
(C) Schematic of single stimulus olfactometer assay.
(D-G) Percent of mosquitoes of the indicated genotypes activated to leave the start canister (D-E) or attracted to the indicated stimuli (F-G) in the single stimulus olfactometer assay. Data are displayed as violin plots with median indicated by horizontal black lines and the bounds of the violin corresponding to the range (10–20 mosquitoes/trial, n=6–16 trials). Kruskal-Wallis test was used to compare each mutant allele to wild-type controls (ns, not significant; *p<0.05). See also Supplemental Figure S1.
Ir76b and Ir25a mutant mosquitoes retain individual human preferences
Given the dramatic decrease in attraction of Ir25aBamHI/19 mutant mosquitoes to Subject 33, we next asked if these mutants along with Ir76b32/61 mutants could distinguish between nylons worn by highly and weakly attractive human subjects, when these were presented simultaneously in the two-choice olfactometer assay. The two-choice assay is substantially larger than the single-choice assay, and thus may represent a more difficult behavioral task, so we expected that it might reveal additional phenotypes not seen in Figure 3D-G.
In control two-choice assay trials in which Subject 25 nylons were placed in both stimulus boxes, Ir76b32/61 mutants showed lower activation in response to human worn nylons, and both Ir76b32/61 and Ir25aBamHI/19 mutants showed significantly decreased attraction compared to wild-type controls (Figure 4A). Furthermore, only 25% of Ir76b32/61 and 38% of Ir25aBamHI/19 mutant trials had at least 10 mosquitoes attracted to either stimulus, compared to 100% of wild type trials (Figure 4B).
Figure 4: Mosquitoes lacking Ir76b or Ir25a show reduced attraction to humans but retain individual human preferences.
(A) Schematic of two-choice olfactometer assay indicating the location of mosquitoes that were not activated, activated but not attracted, or attracted in response to a control trial in which Subject 25 nylons were placed in both stimulus boxes. Stacked bar plots indicate the mean total percent of mosquitoes that were in each category (30–40 mosquitoes/trial, n=13–16 trials, *p<0.05, Wilcoxon rank-sum tests with Bonferroni correction comparing each category across the two genotypes).
(B) Top: schematic of two-choice olfactometer assay, indicating the location (purple shading) of all mosquitoes attracted to either stimulus in a control trial in which Subject 25 nylons were placed in both stimulus boxes. Bottom: trials in which 9 or fewer animals entered either trap were excluded.
(C-E) Left: Schematic of Ir76b and Ir25a and ligand-specific subunit (IRx). Right: percent of mosquitoes of the indicated genotype attracted to the indicated stimuli in the two-choice olfactometer assay. Data from trials that met the inclusion criteria are displayed as violin plots with median indicated by horizontal black lines and the bounds of the violin corresponding to the range (30–40 mosquitoes/trial, n=8–13 trials, except n=4–13 for the Subject 25 vs 25 comparison). Data corresponding to adjacent violin plots labeled with different letters are significantly different (p<0.05, Wilcoxon rank-sum tests with Bonferroni correction).
Analysis of trials that met these inclusion criteria showed that despite substantial defects in overall attraction to human odor, both Ir76b32/61 and Ir25aBamHI/19 mutants distinguished highly and weakly attractive human subjects (Figure 4C-E). These data indicate that mosquitoes have evolved highly redundant sensory systems permitting them to retain attraction to humans even with significant genetic disruption of their olfactory system (Herre et al., 2022). Nevertheless, mutating the IR pathway produced stronger effects on overall mosquito attraction to humans than the OR pathway.
Carboxylic acids are elevated in skin odor of highly attractive humans
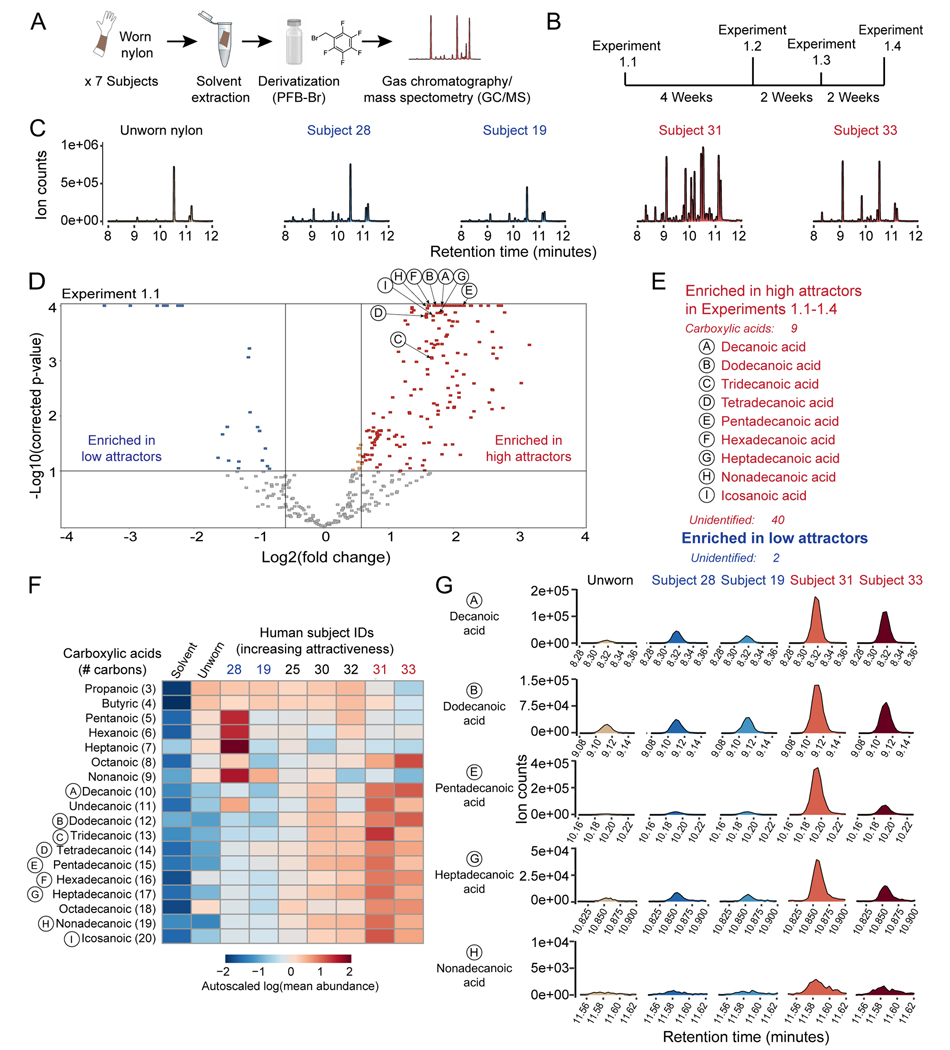
We used gas chromatography/quadrupole time of flight-mass spectrometry (GC/QTOF-MS) to identify compounds on human-worn nylon that were associated with mosquito attractiveness (Figure 5A). Because IR co-receptor mutants showed significantly reduced attraction to humans, we focused our chemical analysis on acidic compounds, which are detected by the IR pathway (Ai et al., 2010; Prieto-Godino et al., 2017; Raji et al., 2019). We collected nylons from 7 of 8 human subjects in our initial subject cohort on 4 days spaced at least 1 week apart, and then performed 4 independent analyses (Experiments 1.1–1.4; Figure 5B; Supplemental Figure S2A). Samples were derivatized with pentafluorobenzyl bromide (PFB-Br) and analyzed using negative methane chemical ionization on a Q-TOF instrument, allowing formula prediction from the mass of detected ions.
Figure 5: Carboxylic acids are enriched on the skin of humans who are highly attractive to mosquitoes.
(A) Overview of experimental procedure for gas chromatography/quantitative time of flight mass spectrometry (GC/QTOF-MS) experiments.
(B) Timeline of 4 replicate GC/QTOF-MS experiments in initial human subject cohort.
(C) Representative chromatograms from the indicated sample groups, including merged extracted ion chromatograms from a set of ~200 features enriched on worn nylons versus unworn nylons and solvent controls in Experiments 1.1–1.4 (Supplemental Table S1).
(D) Volcano plot of features enriched on worn nylons versus unworn nylons and solvent controls in Experiment 1.1. Nine identified compounds that were differentially abundant between high and low attractor groups in Experiments 1.1–1.4 are indicated.
(E) Table of differential features in Experiments 1.1–1.4.
(F) Heatmap quantifying abundance of carboxylic acids with 3–20 carbons in the indicated human subjects, averaged across 4 experiments.
(G) Representative extracted ion chromatograms of several carboxylic acids in the two most and least attractive subjects from the initial cohort.
See also Supplemental Figure S2 and Supplemental Figure S4.
Using unbiased feature detection and data filtering we identified 204 molecular features that were enriched on subject nylons versus unworn nylons and method blanks, across all 4 experiments (Supplemental Table S1). High attractor subjects appeared to have more of these putative “human-derived” peaks overall than low attractor subjects (Figure 5C). Of these, ~50 features were differentially present in samples from the two most attractive subjects, Subjects 33 and 31, versus the two least attractive subjects, Subjects 19 and 28, in all 4 replicate experiments (Figure 5D,E, Supplemental Table S1). Nearly all differentially enriched features (49/51) were more abundant in the 2 highly attractive subjects, although we did find 2 features that were enriched in the 2 low attractors (Figure 5E). We were able to predict chemical formulas for about 40 of these features and ultimately identified 9 as straight chain fatty acids by matching their mass and retention time to that of authentic standards (Figure 5E). We then extracted the signals for all the straight chain acids with acyl chain lengths between 3–20 carbons (Figure 5F). Consistent with the untargeted analysis described above, these compounds were all low or absent in unworn nylons and solvent controls, but only fatty acids with >10 carbons appeared to be enriched in the most highly attractive subjects (Figure 5F-G, Supplemental Figure S2B-C). Several control compounds were similarly abundant in the high and the low attractor samples, including 2 deuterated internal standards present in the extraction solvent and an unknown “nylon-derived” entity that was present in all nylon containing-samples, and absent in solvent-alone controls (Supplemental Figure S2D,E).
Association of elevated skin-derived carboxylic acids and mosquito attraction confirmed in a validation cohort
To confirm our finding that highly mosquito-attracting humans produced more abundant carboxylic acids on their skin, we enrolled 56 new human subjects in a validation study (Supplemental Figure S4A) and used the higher-throughput single-stimulus olfactometer assay to screen the attractiveness of nylons from a given new subject compared to an unworn nylon (Figure 6A). Alongside the 56 new subjects, we also tested nylons from 7 subjects from the initial cohort in this assay (Figure 6B). Consistent with our initial behavioral studies, performed over 1 year earlier, Subject 28 was the least attractive of all 64 subjects tested over the course of the whole study, and Subject 33 was among the most attractive (Figure 6B, Figure 1G). We moved 18 subjects (4 from the initial cohort and 14 from the validation cohort) forward to metabolite profiling with GC/QTOF-MS, comprising 11 highly attractive and 7 weakly attractive subjects. Subjects provided 4 more odor samples, spaced 1 week apart that were used for additional behavioral testing to confirm their high/low attractor status, and for GC/QTOF-MS analysis. In Figure 6B we plot all data collected from the low and high attractor groups that were included in the GC/QTOF-MS analysis. Behavioral data from 45 additional subjects, 42 subjects from the validation cohort and three subjects from the initial cohort, who were not included in the GC/QTOF-MS validation study because of sample size limitations are available on Zenodo (DOI: 10.5281/zenodo.5822538).
Figure 6: Carboxylic acids are enriched in a validation cohort of highly mosquito attractive humans.
(A) Schematic of single stimulus olfactometer assay.
(B) Mosquitoes attracted to nylons from 18 subjects at the extremes of low and high attraction in the single stimulus olfactometer assay, comprising 14 subjects from the GC/QTOF-MS validation study and 4 subjects from the initial cohort. Single stimulus olfactometer assay data from 45 additional subjects, comprising 42 subjects from the GC/QTOF-MS validation study and three subjects from the initial cohort are available on Zenodo (DOI: 10.5281/zenodo.5822538). Data are displayed as violin plots with median indicated by horizontal black lines and the bounds of the violin corresponding to the range (14–24 mosquitoes/trial, n=13–28 trials)
(C) Timeline of 4 replicate GC/QTOF-MS experiments (Experiments 2.1–2.4), performed approximately one year after Experiments 1.1–1.4.
(D) Volcano plot of features enriched on worn nylons versus unworn nylons and solvent controls in Experiment 2.3. Identified compounds that were differentially abundant between high and low attractors in all Experiments 2.1–2.4 are indicated with an arrow, and labeled with an uppercase letter, corresponding to the table in E.
(E) Table describing features that were consistently differentially abundant in high versus low attractors in Experiments 2.1–2.4.
(F) Heatmap quantifying abundance of carboxylic acids with 10–20 carbons, averaged across 4 experiments, in the 18 subjects in B.
(G) Representative extracted ion chromatograms of three carboxylic acids in the 3 most and 3 least attractive subjects of the 18 subject validation cohort in B.
(H) Quantified abundance (median peak areas) of three carboxylic acids in high attractors (n=11) versus low attractors (n=7) across Experiments 2.1–2.4. Data are displayed as violin plots with median indicated by horizontal black lines and the bounds of the violin corresponding to the range. Each plotted point represents the overall median abundance of the compound in one subject across Experiments 2.1–2.4.
Data corresponding to adjacent violin plots labeled with different letters are significantly different (Wilcoxon rank-sum test followed by FDR correction p≤0.1).
See also Supplemental Figure S3 and Supplemental Figure S4.
We again performed 4 replicate metabolomic experiments (Figure 6C; Experiments 2.1–2.4) and found 161 molecular features that were likely to be human-derived (Supplemental Table S1). We then filtered features that were differentially abundant in high versus low attractor groups in all 4 replicate experiments, resulting in a list of 13 features enriched in the high attractor group (Figure 6D-E). We identified 3 of these as pentadecanoic acid, heptadecanoic acid, and nonadecanoic acid (Figure 6E, Supplemental Figure S3C). We were not able to identify the remaining 10 features definitively, although in some cases we were able to predict a chemical formula. Of note, many of these features had the same predicted formula as the identified straight-chain fatty acids but eluted at different retention times, making it likely these are branched chain isoforms of the identified carboxylic acids. Deuterated internal standards and a nylon-derived entity showed no difference in abundance between the high and low attractor groups (Supplemental Figure S3A,B).
We next performed a targeted re-analysis of carboxylic acids with 10–20 carbons in individual human subjects and control samples (Figure 6F-H). The abundance of carboxylic acids on individual subjects from the initial cohort was remarkably consistent with results obtained about 1 year earlier. Low attractors Subjects 19 and 28 had much lower levels of many carboxylic acids than high attractors Subjects 31 and 33 (Figure 5F, Figure 6F, Supplemental Figure S4B). The carboxylic acid pattern was consistent from week to week for individual subjects in the larger cohort in Experiments 2.1–2.4 (Supplemental Figure S4C). Overall, the high attractor group had significantly higher levels of 3 carboxylic acids (pentadecanoic, heptadecanoic, nonadecanoic) than the low attractor subjects in this targeted re-analysis of the data (Figure 6G-H). However, not all individual subjects fit this pattern. Low attractor Subject 90 had high levels of all carboxylic acids examined, in contrast to the 6 other low attractors (Figure 6F). In both the initial and validation cohorts, we documented an association between high levels of skin carboxylic acids and attractiveness to mosquitoes.
Dilution of highly attractive human odor eliminates mosquito preferences
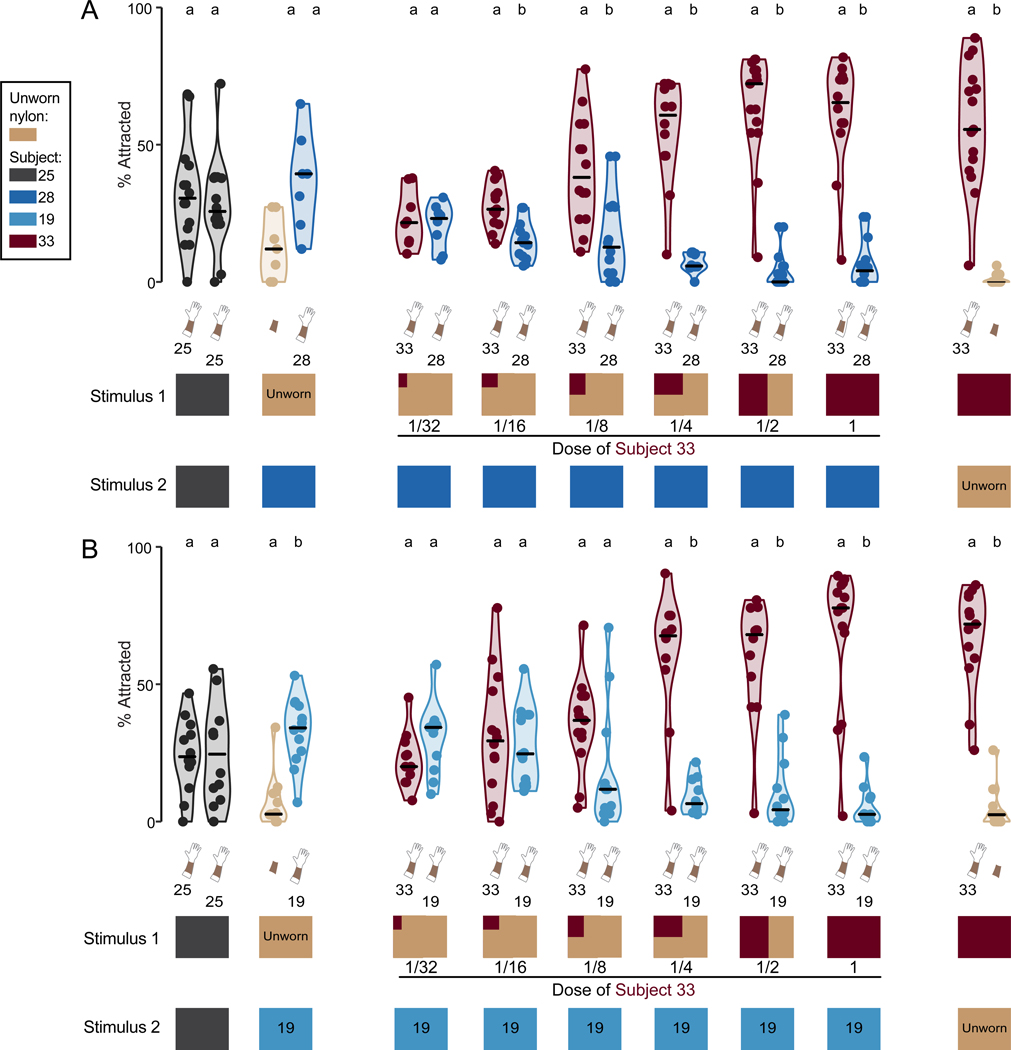
To test the hypothesis that high attractors have higher levels of mosquito attractant compounds on their skin, we performed a dose-response experiment, in which we competed different sized swatches of high attractor Subject 33 nylon against a standard 5.08 cm x 2.54 cm-sized swatch of low attractor worn nylon from Subject 19 or Subject 28 (Figure 7). We found that mosquitoes preferred the odor blend of high attractor Subject 33 to that of both low attractor subjects, even when presented with a substantially smaller swatch of Subject 33 nylon than the less attractive subject’s nylon (Figure 7). Subject 33 nylon only became indistinguishable from low attractor nylons from Subject 28 and Subject 19 nylon, when competed against a swatch of Subject 33 nylon that was 32-fold or 8-fold smaller, respectively (Figure 7). Therefore, the skin odor blend of highly attractive Subject 33 provided a much more potent attractive stimulus than that of weakly attractive humans, which is consistent with our GC/QTOF-MS findings that highly attractive subject nylons contained more human-derived compounds, including carboxylic acids, than those of less attractive subjects.
Figure 7: Dilution of highly attractive human odor eliminates mosquito preferences.
(A,B) Percent of mosquitoes attracted to the indicated stimuli in the two-choice olfactometer assay. Mosquitoes were presented with a constant size of nylon worn by a low attractor, either Subject 28 (A) or Subject 19 (B), and decreasing amounts of nylon worn by high attractor Subject 33, corresponding to the indicated fraction of the low attractor nylon size. The total amount of nylon was balanced by adding unworn nylon. Data are displayed as violin plots with median indicated by horizontal black lines and the bounds of the violin corresponding to the range (30–40 mosquitoes/trial, n=11–20 trials). Data corresponding to adjacent violin plots labeled with different letters are significantly different (p<0.05, Wilcoxon rank-sum tests with Bonferroni correction).
DISCUSSION
Why are some people more attractive to mosquitoes than others?
In this work, we establish that the differential attractiveness of individual humans to mosquitoes is a stable over many months and is associated with the abundance of skin-associated carboxylic acids. This finding is consistent with the discovery that mice at a specific stage of infection with malaria parasites were more attractive to mosquitoes, and that infected mice showed an overall increase in concentration of many emitted volatile compounds (De Moraes et al., 2014). Highly attractive subjects produced significantly higher levels of three carboxylic acids—pentadecanoic, heptadecanoic, and nonadecanoic acids—as well as 10 unidentified compounds in this same chemical class. The specific blend of these and other carboxylic acids varied between different high attractive subjects. Therefore, there may be more than one way for a person to be highly attractive to mosquitoes. We did not identify any compounds that were reproducibly enriched on the skin of the least attractive humans, consistent with the idea that these individuals lack mosquito attractants, rather than emitting a shared set of repellent compounds. A previous study that analyzed interindividual differences in mosquito attractiveness focused on different classes of compounds and identified five—6-methyl-5-hepten-2-one, octanal, nonanal, decanal, and geranylacetone—that were enriched on the skin of weakly attractive humans (Logan et al., 2008). Individually these compounds reduced mosquito flight activity and/or attraction, suggesting that some people may release natural repellents that make them less attractive to mosquitoes. One of our subjects, Subject 90, had high levels of carboxylic acids in their skin emanations but was only weakly attractive to mosquitoes. It is plausible that Subject 90 produces higher levels of a natural repellent that would counteract the elevated levels of carboxylic acids, but this was not tested in our study.
Mosquito attractiveness of a given person is stable over years
Our behavioral assay results corroborate anecdotal evidence that context matters for how attractive a person is to mosquitoes in a real-world setting, since mosquitoes feed opportunistically. If one human walks into a highly mosquito-infested environment alone, they may receive many bites, regardless of their overall attractiveness level because they are the only feeding option. Mosquito preferences matter more in group settings. The “mosquito-magnet” in the group may receive the most bites, leaving the less attractive humans largely untouched. This suggests that mosquitoes distinguish the scent of two human samples using cues that exist along a continuum. We propose that exceptionally high or low attractiveness to mosquitoes is a “fixed” trait, caused by factors that remain constant over a period of several years, even when environmental factors are not strictly controlled. It has been shown that identical twins are more similarly attractive to mosquitoes than fraternal twins (Fernandez-Grandon et al., 2015), suggesting a genetic component to mosquito attractiveness. Moreover, the blend of carboxylic acids that characterizes individual human body odor types is more similar in monozygotic twins than unrelated subjects (Kuhn and Natsch, 2009). We speculate that genetically determined skin characteristics and/or other very stable inter-individual differences contribute to making someone highly or weakly attractive to mosquitoes.
Sensory mechanisms of mosquito discrimination between humans
Because no single mutation of Ir8a, Ir76b, or Ir25a, or Orco was able to disrupt the ability of mosquitoes to discriminate between two subjects, we speculate that there is extensive redundancy in the detection of human-derived skin odors. This may be due to central olfactory coding mechanisms or to the recently described co-expression of ORs and IRs in the same olfactory sensory neuron (Herre et al., 2022; Task et al., 2022). One possible mechanism of IR redundancy could lie with their use of three and not one co-receptor as for the OR system. Removing any one IR co-receptor might have only a partial effect on the ability of the mosquito to detect odorants sensed by IRs. One possibility is that the three IR co-receptors together with ligand-selective IRs collaborate to tile the chemical space of carboxylic acids, such that removing any single IR co-receptor reduces the overall attraction to humans but allows the mutants to retain the ability to detect differences in levels of carboxylic acids. Our findings argue against the idea that mosquitoes distinguish between highly and weakly attractive humans using a single odor, such as lactic acid, as has been suggested (Acree et al., 1968). If this were the case, we would expect that Ir8a mutants, which cannot sense lactic acid (Raji et al., 2019), would lose their preference for highly attractive humans. Instead, we propose that “mosquito magnets” produce elevated levels of multiple mosquito-attractant compounds, and that this drives mosquito preferences.
Microbiota influence over human skin acid production
Humans have more abundant free fatty acids on their skin surface than non-human animals (Nicolaides et al., 1968), and these may signal to mosquitoes that there is a human nearby. Human skin is unique among mammals because it has relatively little hair and numerous eccrine sweat glands across most of its surface. Some animals, including humans, produce a specialized waxy substance from sebaceous glands called sebum. In humans, sebum is triglyceride-rich, producing a characteristic surface lipid composition that contains about 25% free fatty acids (Nicolaides et al., 1968). This is thought to have protective effects, such as limiting sun damage in the absence of protective hair, and emulsifying eccrine sweat, preventing its overly rapid evaporation to regulate body temperature (Huang et al., 2009; Nicolaides, 1974). Human skin acids are astonishingly diverse, with branched, odd-chain, and esterified fatty acids reported, along with skin-specific patterns of desaturation (Nicolaides, 1974). Given the vast array of acid types found on the skin, it is unlikely that two individual humans will possess the same exact complement of acids in the exact same ratios, potentially giving each human a unique chemical signature. Skin bacteria contribute to the pool of free fatty acids found on human skin by producing several types of fatty acid synthetase enzymes that allow them to produce diverse types of acids themselves (Kaneda, 1991; Lu et al., 2004) and by cleaving free fatty acids from human sebum triglycerides using lipase enzymes (Nicolaides et al., 1968). Additionally, recent work has shown that skin microbiota composition is remarkably stable within an individual over time, even though skin is exposed to a constantly fluctuating environment (Oh et al., 2016). Most viable skin bacteria reside in the pores, where they are protected from external factors, such as hygiene habits and seasonal weather changes (Acosta et al., 2021). It is reasonable to think that an individual’s skin microbiota contributes to their skin acid composition, which we have shown to be remarkably stable over time.
Limitations of the study
We have identified an association between skin carboxylic acid abundance and attractiveness of individual humans to mosquitoes. It is important to note that our results do not allow us to conclude that skin carboxylic acid abundance directly causes specific humans to be highly attractive to mosquitoes. Demonstrating causality would require experiments to either specifically remove carboxylic acids from the skin of highly attractive human subjects which is not technically feasible, or supplement the skin odor of weakly attractive human subjects with specific carboxylic acids, provided in naturalistic ratios to the other components of the human odor blend. Our study found 13 molecular “features” enriched on nylons worn by high attractors, but we were only able to identify three of these definitively, so we reason that it would not be informative to spike a small subset of the enriched compounds onto low attractor nylons. Human skin odor is a complex blend of several classes of chemical compounds, each of which requires its own specialized analytical detection methods. Our study exclusively focused on compounds with carboxylic acid groups, thus, we have not exhaustively catalogued all human skin metabolites that differ between humans of varying attractiveness to mosquitoes. Thus, we cannot rule out the possibility that other types of compounds not detected by our methods, may contribute to differences in human attractiveness to mosquitoes. Notably, the carboxylic acids we identified are not especially volatile, so it is unclear whether they are important for differential mosquito attraction to humans across long distances. It is possible that the compounds we identified may give rise to more volatile components that are also enriched on the skin of “mosquito magnet” subjects, but which were not found in our study due to the analytical methods used.
Closing remarks
The attraction preferences of disease-vectoring mosquitoes have important public health implications, since it is estimated that in disease endemic areas a small fraction of humans is more frequently targeted, and these individuals serve as a reservoir of pathogens (Harrington et al., 2014; Muirhead-Thomson, 1951). Moreover, recent work has shown that flavivirus infections alter the microbiome of humans and make them more attractive to mosquitoes (Zhang et al., 2022). Understanding the mechanistic basis for mosquito biting preferences will suggest ways to reduce mosquito attraction to humans and curb the spread of dangerous arboviruses. Studies in humans (Busula et al., 2017; De Moraes et al., 2018; Lacroix et al., 2005; Robinson et al., 2018) and mice (De Moraes et al., 2014) have demonstrated that malaria infection enhances human attractiveness to mosquitoes by altering the chemistry of human skin odor, leading to greater pathogen transmission. Understanding what makes someone a “mosquito magnet” will suggest ways to rationally design interventions such as skin microbiota manipulation to make people less attractive to mosquitoes. We propose that the ability to predict which individuals in a community are high attractors would allow for more effective deployment of resources to combat the spread of mosquito-borne pathogens.
STAR Methods
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Leslie B. Vosshall (leslie@rockefeller.edu).
Materials availability
Mosquito lines generated in this study are freely available on request.
Data and code availability
Data and code have been deposited in Zenodo and are publicly available. The DOI is listed in the key resources table.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental Models: Organisms/Strains | ||
| Aedes aegypti (Liverpool) wild type | Vosshall Lab | |
| Aedes aegypti (Orlando) wild type | Vosshall Lab | |
| Ir25a 19/19 | Vosshall Lab | This paper |
| Ir25a BamHI/BamHI | Vosshall Lab | This paper |
| Ir76b 32/32 | Vosshall Lab | This paper |
| Ir76b 61/61 | Vosshall Lab | This paper |
| Orco 5/16 | Vosshall Lab | PMID: 23719379 |
| Ir8a dsRed/dsRed | DeGennaro Lab | PMID: 30930038 |
| GC/QTOF-MS | ||
| DB5ms column (30 m × 250 μm, 0.25 μm film thickness | Agilent | #19091S-433 |
| Data Analysis | ||
| Behavioral and GC/QTOF-MS raw data and analysis | Vosshall Lab | DOI: 10.5281/zenodo.5822538 |
| Other | ||
| Quattroport olfactometer assay | Vosshall Lab | DOI: 10.5281/zenodo.5822538 |
| Two-choice assay | Vosshall Lab | DOI: 10.5281/zenodo.5822538 |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human and animal ethics statement
Blood-feeding procedures and mosquito behavior with live human hosts were approved and monitored by The Rockefeller University Institutional Review Board (IRB protocol LVO-0652) and the Rockefeller University Institutional Animal Care and Use Committee (IACUC protocol 20068-H). Human subjects gave their written informed consent to participate in this study.
Human subject information
All subjects who participated in our studies were selected solely based on their availability and willingness to participate in frequent nylon wearing experiments. The initial cohort of 8 subjects was recruited from scientists working in or nearby the Vosshall lab. Over several months, as we collected the nylon preference dataset shown in Figure 1E, we noticed that Subject 33 and Subject 28 and were exceptionally attractive and unattractive, respectively, so we decided to specifically examine whether mosquitoes preferred the actual forearm of Subject 33 to that of Subject 28 (Figure 1B). Subject 25 was included in these experiments as a “moderate/average” attractor for comparison.
64 healthy human subjects participated in this study. Age at inception of study on 12/7/2017: mean 29.8, median 29, range 19–57 years. Self-identified gender: 37 female, 26 male, 1 non-binary. Due to sample size limitations, we intentionally did not subdivide these groups further to investigate the contribution of demographic factors such as sex, age, and ethnicity, or behavioral factors such as diet, personal care, or activity levels on mosquito attractiveness, which we determined empirically. The behavior experiments in Figures 1–4, and Figure 7 included only subjects from the initial cohort (Subjects 19, 24, 25, 28, 30, 31, 32, 33). Seven of these subjects were tested in GC/QTOF-MS Experiments 1.1–1.4 in Figure 5. Subject 24 had moved away before we performed the experiments in Figures 5–7. These 7 subjects from the initial cohort (Subjects 19, 25, 28, 30, 31, 32, 33) were also analyzed about a year later alongside 56 newly recruited subjects (Figure 6B). The full single stimulus olfactometer behavior dataset for all 63 subjects is available on Zenodo (DOI: 10.5281/zenodo.5822538), including data for the 18 subjects whose data are plotted in Figure 6B, comprising 4 subjects from the initial cohort (Subjects 19, 28, 31, and 33) and 14 subjects from the validation cohort. These 18 subjects were selected for inclusion in GC/QTOF-MS Experiments 2.1–2.4, based on their overall level of attractiveness to mosquitoes in initial screening experiments (a subset of the data presented in (Figure 6B), and their availability to participate in additional behavior and GC/QTOF-MS experiments.
Mosquito rearing and maintenance
Aedes aegypti wild-type laboratory strains (Orlando and Liverpool) were reared in an environmental room maintained at 70–80% relative humidity and 25–28°C, as previously described (DeGennaro et al., 2013). All animals were maintained with a photoperiod of 14 hours light: 10 hours dark throughout larval, pupal, and adult life stages. Adult mosquitoes were provided constant access to 10% sucrose. Female mosquitoes were fasted for 14–24 hours without sucrose in the presence of water prior to behavioral experiments. For stock maintenance, females were blood fed on live mice.
Ir25a and Ir76b mutant strain generation
Ir25a and Ir76b mutants were generated using methods described previously (Kistler et al., 2015). sgRNA sequences were designed with the CRISPOR v4.3 sgRNA design tool (http://crispor.tefor.net/) (Concordet, 2018) using the following parameters: Genome, Aedes aegypti – yellow fever mosquito – NCBI GCF_002204515.2 (AaegL5.0); Protospacer Adjacent Motif (PAM), 20bp-NGG - Sp Cas9, SpCas9-HF1, eSpCas9 1.1. For each gene, two pairs of sgRNA with predicted MIT Specificity Scores ≥95 were selected for targeted double stranded break-induced mutagenesis, with each pair flanking roughly 250 base pairs within exon 2 for Ir76b and exons 2 and 3 for Ir25a. sgRNA DNA templates were prepared by annealing oligonucleotides as previously described using the following target sequences:
Ir25a-sgRNA1: GTTGAGCTACTAACCGTCGA
Ir25a-sgRNA2: TACTGACAGCAAAGGGCTGT
Ir25a-sgRNA3: CCTACGGTTTCCGCATCAAC
Ir25a-sgRNA4: AAGAAGGCGACTTGAGGCAA
Ir76b-sgRNA1: GTTACACCGAACGTCAGAA
Ir76b-sgRNA2: TACTCTGGTCGGACGCGGTG
Ir76b-sgRNA3: CTCCTTTCAATCGGGACGTG
Ir76b-sgRNA4: CAACGGCCAGCAGCGATACC
In vitro transcription was performed using HiScribe Quick T7 kit (NEB E2050S) following the manufacturer’s protocol. Following in vitro transcription and DNAse treatment for 15 minutes at 37°C, sgRNA was purified using RNAse-free SPRI beads (Ampure RNAclean, Beckman-Coulter A63987), and eluted in Ultrapure water (Invitrogen, 10977–015). To further facilitate isolation of loss-of-function mutants, 200 bp single-stranded DNA oligodeoxynucleotide (ssODN) donors were designed as a template for homology-directed repair (Kistler et al., 2015). The ssODN donor had homology arms of 88–90 bases on either side of the inner most sgRNA target sites (sgRNA2 and sgRNA3 for both Ir25a and Ir76b), flanking an insert with stop codons in all three frames of translation and a BamHI restriction site (IDT). Since homology arms contained sgRNA target sites for the outer most sgRNA target sites (sgRNA1 and sgRNA4 for both Ir25a and Ir76b), PAM motifs for the respective sgRNAs were mutated to avoid ssODN donor cleavage. The sequences for each ssODN follow below with left and right homology arms italicized, stop codons underlined, and BamHI restriction site highlighted in bold:
Ir25a-ssODN:
TTGCGCTGAACTATATAAGAAAGAACCCAAGCCTCGGACTTTCAGTTGAGCTACTAACCGTCGAATGAAACCGTACTGACAGCAAAGGGCTAATAAGGATCCATAACTAAGGAACCGGCCAAGAAGGCGACTTGAGGCAATAGCGATCTCTATCAAACGTAAAAAGCAACTATCTGTTGCAGGTTTGCTACAAAACTTTA
Ir76b-ssODN:
TCTAATTGCATCGAACTCTCTTTCCCACGTTCAACAGGACTGGCCGCTGAGTTACACCGAACGTCAGAATAGTACTCTGGTCGGACGCGTAATAAGGATCCATAACTAAGGGTGTGGATTTTGATTCTGGTATCGCTGCTGGCCGTTGGTCCAATCATCTACGGAATGCTGATTGTGCGGTACAAAATGACCAAAGACAA
For each target gene, approximately 500 wild-type Aedes aegypti (Liverpool LVP-IB12 strain) embryos were injected with a mixture containing recombinant Cas9 protein (PNA Bio, CP01) at 300 ng/μl, 4 sgRNAs at 40 ng/μl each, and donor ssODN at 125 ng/μl. Embryos were injected by the Insect Transformation Facility at the University of Maryland Institute for Bioscience & Biotechnology Research. Embryos were hatched and G0 females were crossed to wild-type Liverpool males, and their G1 offspring were screened for germline mutation by PCR amplification and Sanger sequencing the regions flanking the sgRNA target sites. Two unique stable mutant lines, each resulting in an early stop codon due to a frameshift mutation, were isolated from each injection. For Ir25a, one isolated mutant line had a 160-bp deletion with an ssODN integration (Ir25aBamHI), and the other had a 19-bp deletion (Ir25a19). For Ir76b, one isolated mutant line had a 61-bp deletion (Ir76b61), and the other had a 32-bp deletion (Ir76b32). Virgin females from each mutant line were backcrossed to wild-type Liverpool males for 8 generations prior to establishment of stable homozygous lines. To control for potential CRISPR-Cas9 off-target effects on behavior, homozygous mutant lines were intercrossed to generate heteroallelic mutants that were tested in all behavior experiments alongside appropriate genetic controls. It was previously shown that although Ir76b mutant Anopheles coluzzii females show normal attraction to human host cues, they fail to blood feed and therefore produce no offspring (Ye et al., 2022). We also observed deficits in blood-feeding and egg-laying in homozygous Aedes aegypti Ir25a and Ir76b strains, albeit far less severe than the Anopheles coluzzii Ir76b mutants. All homozygous Ir25a and Ir76b mutant strains were maintained by blood-feeding on a human arm. We determined empirically that these mutant mosquitoes fed more avidly when they were 14 days post eclosion, rather than 7 days, which is the standard timepoint at which we blood feed to propagate other strains. These strains did not feed effectively on live mice or on an artificial membrane blood feeder. A volunteer inserted an arm into a standard BugDorm rearing cage (30 cm3) cage for 30 minutes at ambient temp/humidity. These cages contained a mixed population of males and females, which had been allowed to mate freely since eclosion. Mosquitoes had access to the entire hand and forearm of the subject. For context, normal strains feed to repletion on a human arm after 5–10 minutes, even when the arm is placed outside of the cage netting and not inside the cage. Feeding of these mutants was unsuccessful when the human arm was placed against the netting on the outside of the cage. About 50% of females were engorged at the end of a given 30-minute feeding session, while the rest were partially fed or unfed. Notably, a given female took much longer to probe the arm repeatedly before successfully feeding. No attempt was made to refeed females that did not feed during a given 30-minute feeding session. Four days after feeding, an oviposition cup was placed into the cage and as many eggs as possible were collected over a 4-day period. After this stage the entire process was repeated once or twice with the same cage to obtain enough eggs to propagate the strains and carry out experiments. It is our impression that Ir25a females but not Ir76b females laid fewer eggs than wild type, but we did not investigate this further in the course of this study. Unlike the observed difficulties with the homozygous mutants, heterozygous mutants fed normally and laid normal numbers of eggs.
Ir25a and Ir76b mutant genotyping
Genotypes were confirmed using Phire Tissue Direct PCR Master Mix (Thermo Fisher, F170L) followed by gel electrophoresis and Sanger sequencing (Genewiz) using the following primer combination for each mutant allele:
Ir25aBamHI, Ir25a19
Forward: AATACTTGAGGAGTCGTTGAAT
Reverse: GAAGCAATGCCTTGTACTTATG
Ir76b61
Forward: AGCCGAATATGAAGGTCAAGC
Reverse: CAGCACCTGTTCCTTGTCTT
Ir76b32
Forward: TGCATCGAACTCTCTTTCCC
Reverse: CGATAGCTAAGATGCCAGTACAT
The Ir25aBamHI allele was detected by either a 160 bp deletion or presence of an exogenous BamHI restriction site from the donor ssODN. BamHI restriction digest of the PCR product generated a single 764 bp fragment in wild-type animals and two fragments (275 bp and 329 bp) in the Ir25aBamHI mutant. For mutants with small deletions, the presence or absence of endogenous restriction enzyme target sites was used to distinguish between mutant and wild-type alleles. PCR products were generated and digested with the indicated enzyme, producing the indicated bands in mutant and wild type:
Ir25a19 with MspI
Wild type: 502 and 262 bp; Ir25a19: 764 bp
Ir76b61 with BstUI
Wild type: 367 and 397 bp; Ir76b61: 764 bp
Ir76b32 with BstNI
Wild type: 392 and 365bp; Ir76b61: 757 bp
All genotyping experiments were performed with a no DNA control as well as fragment size validation using 1Kb Plus DNA ladder (ThermoFisher Scientific, 10787026). See Supplemental Figure S1.
Given the severe host-seeking and blood-feeding deficits displayed by Ir25a and Ir76b female homozygous mutants, it is difficult to maintain these as homozygous strains. For the benefit of scientists wishing to work with these new strains, we have devised a crossing scheme that uses heterozygous mutant females to propagate the mutant alleles. An important aspect of this approach is that Ir25a and Ir76b mutants do not carry a fluorescent marker at their respective gene loci. This strategy consists of first crossing homozygous Ir25a or Ir76b mutant males to heterozygous females from the corresponding Ir25a-QF2 and Ir76b-QF2 gene-sparing knock-in driver lines (Herre et al., 2022). These strains contain the 3xP3-dsRed marker integrated at the Ir25a or Ir76b genetic locus. This initial cross will generate both fluorescent and non-fluorescent heterozygous mutants at a 1:1 ratio. Then, taking the fluorescent heterozygous mutant females, these animals will again be crossed to the homozygous mutant males. This cross will generate non-fluorescent homozygous mutants and fluorescent heterozygous mutants at a 1:1 ratio. At this point, mutant alleles can easily be maintained by collecting eggs from the cross between non-fluorescent homozygous mutant males with fluorescent heterozygous females isolated at each generation. Since the mutations and the locations of the inserted fluorescent markers are tightly linked, we expect the recombination rates between the mutation sites and the markers to be extremely rare. However, occasional genotyping is recommended to ensure proper propagation of each of the mutant alleles.
METHOD DETAILS
Human skin odor collection
Human subjects washed their forearms with Dove unscented soap and water, dried them with clean laboratory paper towels, and then wore nylons sleeves on both forearms between the wrist and elbow for 6 hours. Nylon sleeves were prepared by using scissors to remove 2” of fabric from the tip of the stocking foot of knee highs (L’eggs brand Everyday, Amazon), so that the modified stocking was open on both ends. Subjects wore 2 nylon sleeves on each arm: a brown experimental nylon was worn next to the skin, and a black outer nylon was placed over the experimental nylon to minimize contamination of the inner nylon. Subjects wore nylons during the day and were allowed to perform typical daytime activities but were asked not to exercise or drink alcohol while wearing the nylons. After the 6-hour wearing period, nylons were deposited in Whirl-Pak bags and kept at −20°C for 1–10 days before behavioral and chemical analysis. For the “round-robin” two-choice assay experiment in Figure 1E, we competed 2 nylon sleeves that had been worn on the same day by 2 different human subjects, to strictly control the “age” of the nylons being used to determine if mosquitoes preferred one subject over the other. We relaxed the requirement that subjects wear the nylons on the same day for subsequent two-choice assay experiments, since small differences in nylon age did not change the preference of mosquitoes for specific human subjects. In Figures 2,3,5, and 8, we compared nylons that had been worn within 2 days of each other by two different subjects. This allowed us to better accommodate the schedules of human subjects, who were not always available on the same day over the extended duration of the study.
Behavioral assays
The single choice olfactometer assay referred to in this work (Figure 3C, Figure 6B) is the same assay previously referred to as the “Quattroport” in an earlier publication (Basrur et al., 2020), because the assay allows 4 independent single stimulus olfactometer trials to be run in parallel. In this work, we refer to the Quattroport assay as the “single stimulus olfactometer assay” to avoid confusion about the number of stimuli being presented to each group of mosquitoes in a single trial. We repurposed components of the Quattroport assay to create the two-choice olfactometer assay, which allows us to compete 2 different stimuli against each other in the same trial. We performed two separate preference trials in parallel, increasing throughput over the Gouck olfactometer assay (Gouck, 1972). Details of fabrication and operation of the two-choice assay are available on Zenodo (DOI: 10.5281/zenodo.5822538). Air flow and CO2 conditions for the two-choice olfactometer assay were carried as described for the Quattroport (Basrur et al., 2020). Human forearm-worn nylon sleeves were used as the stimulus in all behavior figures with the two-choice olfactometer assay, except Figure 1B in which human subjects placed their forearm over a hole in the stimulus box lid, exposing 12.9 cm2 of skin to mosquitoes (demonstrated with a mannequin arm in Figure 1A). We shuffled the order in which different stimuli were assessed over the course of the day, and we randomized the position of different stimuli across all stimulus boxes of the assays, to reduce time of day and position effects. All behavioral experiments were carried out in an environmental room set to 25°C, 70–80% relative humidity. The day before behavior was measured, 20 female mosquitoes (aged 7–14 days post-eclosion, mated) were sorted under cold anesthesia into each start canister and given access only to water for 18–22 hours. The same canisters were used for the single stimulus and two-choice assays. However, twice as many females were tested in each two-choice assay trial (40 females per trial, 20 in each of 2 canisters) as in each single stimulus trial (20 females per trial). In both assays, mosquitoes were acclimated to a carbon-filtered air stream for 10 minutes (Donaldson Ultrac-A). CO2 was then introduced into the air stream for 30 seconds, at which point mosquitoes were released, and given 5 minutes to assess the stimulus or stimuli. Mosquitoes were prevented from contacting stimuli by a mesh divider. Sliding doors between assay compartments allow the experimenter to count the number of mosquitoes that were not activated, or activated but not attracted, or both activated and attracted to the stimulus. In the single stimulus assay, mosquitoes that entered an attraction trap were scored as attracted to the stimulus. In the two-choice assay, mosquitoes that entered a cylindrical flying tube or the adjacent trap, both downwind of the stimulus, were scored as attracted to that stimulus. In both assays, all mosquitoes that left the start canister were scored as activated. We are confident that mosquitoes use olfactory information to detect nylon stimuli, since mosquitoes cannot contact or taste the nylon in our assays due to the presence of a mesh barrier, and there are no visual cues that differentiate nylons worn by different subjects.
Cleaning behavioral assay apparatus
Between trials, the assay apparatus was vacuumed to remove live and dead mosquitoes, and air was flowed through the assay for 5–10 minutes to flush out residual CO2 and odor. Two-choice assay parts were cleaned before use in every experiment, as described below. In some cases, assay parts needed to be cleaned between trials, so that they could be re-used during the same behavior experiment. Whenever possible, we constructed enough replicate assay parts to reduce the need to wash parts between trials, which slows down assay throughput. Experimenters always wore gloves when cleaning and handling clean assay parts. Detailed procedures are described here for how and when we cleaned each part of the two-choice assay (going from left to right in the schematic in Figure 1A: 1) start canisters, 3D-printed connector joints, and accompanying acrylic sliding doors were washed in a dishwasher (Miele Optimal Series dishwasher, Cascade Original “Actionpacs” detergent pods) at least 2 days before behavior and allowed to air dry completely before female mosquitoes were loaded into the canisters on the day before behavior; 2) the flying box was too big to be washed in the sink or dishwasher, so the inside of the box was sprayed down with 70% ethanol from a laboratory spray bottle and this was wiped down with laboratory paper towels, and allowed to air dry; 3) two cylindrical flying tubes were washed in the dishwasher as described above and allowed to air dry; 4) Acrylic stands that support the cylindrical flying tubes were washed in the dishwasher as described above; 5) the complete supply of attraction traps and accompanying 3D printed joins and acrylic sliding doors were washed in the dishwasher as described above at least one day before the experiment and allowed to air dry. When we performed more trials than we had traps available, we hand washed the traps used in the first few trials using hot water and soap (Bac Down Handsoap, Decon Labs, Inc., Catalog #: 7001), and allowed them to air dry before re-using them in trials later in the day. 6) For stimulus boxes and lids, the cleaning procedure was the same as that for the traps. The complete supply of stimulus boxes and lids was washed in the dishwasher as described above at least one day before the experiment and allowed to air dry. When we performed more trials than we had stimulus boxes available, we hand washed the stimulus boxes used in the first few trials using hot water and soap and allowed them to air dry before re-using them in trials later in the day. 7) The 3D printed stop piece which connects the air/CO2 supply to the stimulus box was wiped down with 70% ethanol that had been sprayed onto a laboratory paper towel before the first trial, and between every trial. Similarly, the single stimulus olfactometer assay parts were cleaned before every experiment. This means that, going from left to right in the schematic in Figure 3C: 1) start canisters, 3D-printed connector joints, and accompanying acrylic sliding doors were washed in a dishwasher at least 2 days before behavior and allowed to air dry completely before female mosquitoes were loaded into the canisters on the day before behavior; 2) the two cylindrical flying tubes were hand washed in the sink with hot water and soap and a bottle brush (Dr. Brown’s, Amazon, ASIN: B01NCUKCC0), and allowed to air dry; 3) Acrylic stands that support the cylindrical flying tubes were washed in the dishwasher as described above; 4) the complete supply of attraction traps and accompanying 3D printed joins and acrylic sliding doors were washed in the dishwasher as described above at least one day before the experiment and allowed to air dry. When we performed more trials than we had traps available, we hand washed the traps used in the first few trials using hot water and soap and allowed them to air dry before re-using them in trials later in the day. 5) For stimulus boxes and lids, the cleaning procedure was the same as that for the traps. The complete supply of stimulus boxes and lids were washed in the dishwasher as described above at least one day before the experiment and allowed to air dry. When we performed more trials than we had stimulus boxes available, we hand washed the stimulus boxes used in the first few trials using hot water and soap and allowed them to air dry before re-using them in trials later in the day. 6) The 3D printed stop piece which connects the air/CO2 supply to the stimulus box was wiped down with 70% ethanol that had been sprayed onto a laboratory paper towel before the first trial, and between every trial.
Behavior inclusion criteria
Two-choice olfactometer assay data indicating the overall percent mosquito activation and attraction in response to a single human subject stimulus, are presented in Figure 2B-C, Figure 4A for all control trials performed (i.e. all trials examining Subject 25 vs Subject 25, before the application of any inclusion criteria). In Figure 2E-F, and Figure 4B, we report the percent of control trials that met the inclusion criteria used in later parts of these Figures, which required that there were: 1) at least 30 live mosquitoes at the end of the assay and 2) at least 10 mosquitoes attracted to either stimulus. We chose these inclusion criteria because IR mutants displayed large defects in overall attraction to human subjects, and we wished to examine preferences specifically using trials which passed a minimal threshold of overall attraction to human odor. Trials with very low overall attraction to either stimulus, could give misleading results, because they are subject to “jackpotting” effects. For example, if 2 mosquitoes were attracted to Subject A, and 1 mosquito was attracted to Subject B, this is not a meaningful difference in preference, so we would exclude this trial for having <10 animals attracted to either subject. To assay mosquito preferences between genotypes in Figure 2G-J and Figure 4C-E, we present only trials which passed the inclusion criteria. We discarded trials in which substantially fewer mosquitoes were loaded into the assay (unintentionally), and which very few mosquitoes (<10) were attracted to either stimulus. There were some exceptions to the application of inclusion criteria, described here: the experiment in Figure 1C compared mosquito preferences between several stimulus pairs that were expected to (and did) result in very low levels of attraction. These stimuli were: 1) unworn nylons versus no stimulus, and 2) no stimulus versus no stimulus. For this experiment, we did not require that more than 10 mosquitoes were attracted to either stimulus. We only required that there were >30 live animals at the end of the trial. For single stimulus olfactometer experiments (Figure 3D-G, Figure 6B), we included trials with >14 live mosquitoes at the end of the trial.
Nylon sleeve behavioral assay stimuli
For human odor host-seeking assays, a 7.62 cm x 10.16 cm piece of the brown experimental nylon was cut and used as a stimulus in either the two-choice olfactometer assay or the single stimulus olfactometer assay. This was the largest size of material that could be laid flat on the bottom of the stimulus boxes used in both assays. By using this size of nylon stimulus, we could cut three pieces of nylon from each nylon sleeve, yielding a total of 6 pieces of nylons from each day of nylon wearing, or enough for 6 trials of that subject. Experimenters always handled nylon sleeves with gloves and cleaned scissors with 70% ethanol between samples. Subjects wore 2 nylon sleeves on each of their 2 forearms (one brown experimental nylon next to their skin, covered by one black protective nylon, as described above). We asked subjects to remove both nylon sleeves they wore on each arm as a single “unit” (keeping the black protective nylon outside the brown experimental nylon) and to place these in a Whirl-Pak bag (Nasco, Catalog #: B01062) in the freezer. This allowed us to determine which side of the brown experimental nylon had touched the subject’s skin (the side that was not touching the black outer nylon), and we marked this side with a fine point permanent marker. The side of the nylon that touched the subject’s skin was placed facing upwards in the stimulus box, to ensure consistency between trials. In all behavior experiments, nylon samples were de-identified before being cut and presented to mosquitoes in the behavioral assay (either the two-choice assay or the single stimulus olfactometer assay), such that experimenters were blinded to the Subject ID. Each piece of nylon was attached to a flexible plastic rectangle of the same size (cut with scissors from plastic file folders: Letter size, Office Depot, Catalog #: 700259), using small binder clips (3/4” size, Office Depot Catalog #: 808857). The color of the plastic rectangle indicated the de-identified label given to that nylon, corresponding to the source of the nylon. For instance, in a given experiment, Subject 33 was designated as Subject “A” by someone other than the experimenter, and then the experimenter cut the de-identified nylon pieces and attached all Subject “A” nylons to red plastic cards. The color of the card was chosen randomly for each experiment, and colors rotated between subjects. Each nylon piece was used for only one trial and then discarded. Binder clips and plastic cards were washed with soap and water as described above and set out to air dry at the end of each experiment.
Statistical analysis of behavior
Statistical analyses were performed using R. Two-choice assay data were analyzed by comparing the percent of mosquitoes attracted to each of two stimuli, for several pairs of stimuli, using Wilcoxon rank-sum tests with Bonferroni correction (Figure 1B-E, Figure 2G-J, Figure 4C-E). Nonparametric effect size (ES) was calculated as ES= Z/sqrt(N) where N is the number of observations (Figure 1E) (Fritz et al., 2012). A power analysis was used to predetermine the approximate sample size needed for Figure 1E (G*Power software). Wilcoxon rank-sum tests with Bonferroni correction were used to compare the percent of mosquitoes in each of three categories (attracted, activated but not attracted, or not activated) between wild type and each mutant genotype in Figure 2B-C, Figure 4A,. Single stimulus olfactometer assay data in Figure 3D-G were analyzed by using a Kruskal-Wallis test to compare the percentage of mosquitoes activated or attracted across 4 genotypes: including wild-type controls, 2 heterozygous mutants, and the heteroallelic null mutants. When indicated, post hoc analysis used pairwise Wilcoxon rank-sum tests with Bonferroni correction to compare wild-type mosquitoes to each of the other 3 genotypes for a given stimulus (Figure 3D-G).
Calculation of the “Attraction Score”
The attraction score reported in Figure 1F was calculated as follows. For each of the 28 subject pairs in the “round-robin tournament”, we calculated the total number of mosquitoes attracted to each subject in the pair, by adding the number that were attracted across every trial performed for that specific pair in Figure 1E. For example, for the Subject 33 vs Subject 28 pair, we summed the total number of mosquitoes attracted to Subject 33 or to Subject 28 across the 18 trials in which they were compared to each other: Subject 33 attracted 468 mosquitoes, whereas Subject 28 attracted only 35 mosquitoes. We took the difference between these values (468–35=433) and divided it by 18 trials to get the average “margin of victory” per trial for this subject pair (433/18=24). Since Subject 33 attracted 24 more mosquitoes than Subject 28 per trial, on average, Subject 33 was “awarded” 24 points, and Subject 28 was awarded 0 points. Subject 33 also attracted more mosquitoes than the remaining 6 other subjects, with average “margin of victory scores” of 15, 18, 20, 21, 23, and 23. The “attraction score” for each subject, reported in Figure 1F, represents the sum of 7 average “margin of victory” values. In the example above, the “attraction score” is calculated by summing the average “margin of victory scores” for Subject 33, compared to the 7 other subjects: 15+18+20+21+23+23+24=144.
Sample preparation for gas chromatography/quadrupole time of flight-mass spectrometry (GC/QTOF-MS)
Nylon sleeves that had been stored at −20°C for 1–10 days were thawed and cut into 5.08 cm x 2.54 cm pieces. Experimenters always handled nylon sleeves with gloves and cleaned scissors with 70% ethanol between samples. For each subject, 6 separate pieces (5.08 cm x 2.54 cm each) of the same nylon sleeve were analyzed in each of the 4 experiments shown in Figure 5 (Experiments 1.1–1.4), and 5 pieces of the same nylon sleeve were analyzed in each of the 4 experiments shown in Figure 6 (Experiments 2.1–2.4). Extraction solvent was 80% methanol (CID: 67–56-1, Methanol, Fisher Chemical, Catalog #:A456–4) prepared with Millipore water and spiked with deuterated isotope-labeled internal standards sourced from Cambridge Isotope Laboratories: nonanoic acid-D17 (CID: 130348–94-6, Catalog #: DLM-9501–0.5), propanoic acid-D5 (CID: 60153–92-6, Catalog #: DLM-1919–5), phenol-D5 (CID:4165–62-2, Catalog #:DLM-695–1), acetate-D4 (CID:1186–52-3, Catalog #: DLM-12–10 ), butyrate-D7 (CID: 73607–83-7, Catalog #: DLM-1508–5) and valerate-D9 (CID: 115871–50-6, Catalog #:DLM-572–1). Each piece of nylon was extracted in 1 mL of extraction solvent in a 5 mL Eppendorf tube (Fisher Scientific Catalog #: 14–282-301). 5 mL Eppendorf tubes containing extraction solvent and nylon were kept overnight at −20°C. Then, each tube was vortexed for 20 seconds to ensure thorough extraction of the nylon. A P1000 pipet with standard 1000 μL pipet tip was used to press the nylon against the side of the 5 mL Eppendorf tube, so that the extraction solvent was squeezed out of the nylon. Before doing this, the plunger of the pipet had been pressed down, so that it could be released after squeezing the nylon, to pick up the extraction solvent, before it could be reabsorbed into the nylon sleeve. Typically, 700–900 μL of nylon “extract” (a cloudy mixture of extraction solvent plus nylon compounds) was recovered from each sample, and this was immediately transferred to a 2 mL glass GC vial (Wheaton μL MicroLiter autosampler vials; 12×32mm, Catalog #: 11–1200) and secured with a septum cap.
Pentafluorobenzyl bromide (PFB-Br) derivatization
100 μL of the liquid nylon extract was manually transferred using a pipet to a new glass autosampler vial, containing 100 μL of borate buffer (100mM boric acid CID: 10043–35-3, Sigma Aldrich, Catalog #: 339067, adjusted to pH 10 using 10 M NaOH (CID: 1310–73-2, Fisher Chemical, Catalog #: S318500. Subsequent steps were performed using a single needle liquid handling system (Gerstel MPS). First 400 μL of 100 mM pentafluorobenzyl bromide (PFB-Br, CID: 1765–40-8, Sigma-Aldrich, Catalog number: 101052), prepared in acetonitrile (Fisher Chemical; Catalog #: A955–4) and 400 μL cyclohexane (Sigma-Aldrich, Catalog #: 650455–4L) were added sequentially to the reaction vials. Samples were then sealed using crimp caps (Wheaton MicroLiter Catalog #: 11–0040A) and heated to 65°C with shaking for 1 hour to esterify carboxyl and hydroxyl groups (Supplemental Figure S2A). Samples were allowed to cool to room temperature and centrifuged for 2 minutes at 2,000 rpm, 20°C to promote phase separation, then returned to the robotic sample preparation instrument. Finally, the upper cyclohexane layer was transferred to clean autosampler vials (Wheaton MicroLiter, Catalog #: 11–1200) and 50 μL further transferred into a second clean autosampler vial pre-filled with 450 μL cyclohexane, creating a 10-fold dilution of the derivatized nylon extracts that was subsequently used for GC/QTOF-MS analysis.
GC/QTOF-MS data collection
GC/QTOF-MS data was collected using an Agilent 7890B gas chromatograph (GC) and an Agilent 7200 quadrupole time-of-flight (QTOF) mass spectrometer (MS), fitted with a Gerstel MPS Robotic Autosampler. 1 μL of the sample volume was injected in splitless mode onto a DB5ms column (30 m × 250 μm, 0.25 μm film thickness; Agilent Technologies, Catalog #:19091S-433). GC conditions were as follows: initial oven temperature 60°C (hold 1 minute), ramp at 25°C/minute to 300°C (hold 2.5 minutes), ramp at 120°C/minute to 60°C (hold 1 minute); the total run time was 16.1 minutes. The inlet, transfer line, chemical ionization (CI) source, and quadrupole temperatures were set to 280°C, 300°C, 150°C, and 150°C respectively. The emission current was set to 4.2 μA and data collection was in 2 GHz mode, mass range m/z 50–650. For all GC/QTOF-MS experiments, the sample injection order was randomized using the “RAND” function in Microsoft Excel. The same GC column was used for data acquisition shown in Figure 5 and Figure 6, column trimming as part of routine maintenance was responsible for slightly shorter retention times shown in Figure 6. The QTOF mass spectrometer was operated in negative chemical ionization (nCI) mode with methane as the reagent gas (1 mL/minute); in nCI mode, derivatized molecules undergo electron capture dissociation (ECD) and are detected as deprotonated (M-H) ions.
GC/QTOF-MS data analysis
The workflow for GC/QTOF-MS data analysis is summarized in Supplemental Table S1. Raw data (Agilent “.D” files) were analyzed using Agilent MassHunter software. Untargeted feature finding was performed using Agilent Unknowns Analysis software (v10.1) (Supplemental Table S1, step 1). Specifically, data was first converted using SureMass peak detection (absolute height filter >1000 counts, RT window size factor=50, Extraction window +−50ppm, use base peak shape=yes, sharpness threshold 25%, Ion peaks minimum=1, maximum=1). Subsequently, feature finding was performed on all worn nylon samples across 4 replicate experiments for Experiments 1.1–1.4 (Figure 5) and, again for Experiments 2.1–2.4 (Figure 6). Putative features, denoted as accurate mass @ retention time, were exported from Unknowns Analysis as a .csv file, and imported into R. A custom deduplication script was used to remove most “fuzzy” duplicate features with similar mass and retention time values, and features detected in <10 data files, resulting in a list of 1494 features for the experiments in Figure 5, and 1925 features for the experiments in Figure 6 (Supplemental Table S1, step 2). This feature list was imported into Agilent Quantitative Analysis software (v10.1) and a targeted data re-extraction performed (settings: +Gaussian smoothing, +area filter ≥ 0, RT window: ± 0.05 minutes extraction window ± 100ppm) (Supplemental Table S1, step 3), resulting in a fully aligned dataset across 4 replicate experiments. Raw peak areas were then exported as a .csv file, and a custom R script was again used to remove features present in <10% of samples, impute missing values with 1/2 the lowest value for that feature, and log2 transform data (Supplemental Table S1, step 4). The resulting .csv file was imported into Agilent Mass Profiler Professional software (v15.1). We considered high quality features to be those found in at least 50% of samples in at least 1 subject group, with a coefficient of variation <40% in at least 1 subject group. Putative human skin-derived features were considered those at least 2-fold enriched (false discovery rate, FDR<0.05) in any Subject’s worn nylons versus unworn nylons and solvent blank controls (Supplemental Table S1, step 5). Volcano plots were used to filter on features differentially abundant in the high versus low attractor subjects in each individual experiment of 4 replicate experiments (Supplemental Table S1, step 6; Figure 5D, Figure 6D). Venn diagrams were used to identify hits that met the differential abundance criteria in high versus low attractors across all 4 replicate experiments (Supplemental Table S1, step 7): Experiments 1.1–1.4 (Figure 5E) and Experiments 2.1–2.4 (Figure 6E). Redundant features with closely matching mass and retention times, that were missed by the earlier deduplication step were removed manually, after inspecting the raw data in Agilent Qualitative Analysis software (Supplemental Table S1, step 8). Data File S1 contains lists of mass and retention times for unknown features that were found in 4 replicate experiments, either Experiments 1.1–1.4 or Experiments 2.1–2.4. For all targeted data re-extractions, 100 ppm mass accuracy was used to extract the detected pseudomolecular [M-H]− ion, unless the peak was considered saturated, in which case the [(M+1)-H]− isotope was used consistently across all data files. For Experiments 1.1–1.4, we calculated the median abundance of each compound in each of 4 experiments for each of 2 high attractor subjects and 2 low attractor subjects (Supplemental Figure S2C). Then we compared these values for each compound between the high and low attractor groups using a nonparametric linear mixed effects model for repeated measures based on ranks, with group as a fixed effect and subject as a random effect. Benjamini-Hochberg correction was applied for multiple comparisons (FDR<0.1). For Experiments 2.1–2.4, we calculated the median abundance value for each compound in each subject across 4 replicate experiments, and then we compared these values between the high (n=11) vs low attractor (n=7) groups (Figure 6H) using a Wilcoxon rank-sum test. Benjamini-Hochberg correction was applied for multiple comparisons (FDR<0.1). Statistical analysis was performed in R version 4.0.5. Heatmaps were generated using Metaboanalyst (v5.0; https://www.metaboanalyst.ca/) (Pang et al., 2021) using autoscaled data.
GC/QTOF-MS compound identification
To identify compounds of interest, we used the accurate mass of the molecular ion (M-H) to predict chemical formulas using Agilent Qualitative Analysis software (v10.0). Formula prediction was constrained to C, H, O, N atoms, and formulas within 100 ppm mass error of the measured mass were considered (Supplemental Table S1, step 9). Predicted formulas for unknown compounds are included in Data File S1. To achieve positive identification, we spiked nylon samples with authentic standards (details below) and asked whether the area of the unknown peak increased symmetrically (Supplemental Table S1, step 10). Overlaid extracted ion chromatograms generated in Agilent Qualitative Analysis software for three hit compounds are shown in Supplemental Figure S3C. Commercially standards used were: propanoic acid (CID: 5818–15-5, Millipore Sigma; Catalog #: 94425–1ML-F), butyric acid(CID: 107–92-6; Millipore Sigma; Catalog #: 19215–5ML), pentanoic acid (CID: 109–52-4; Millipore Sigma; Catalog #: 75054–1ML), hexanoic acid(CID: 142–62-1; Millipore Sigma; 21529–5ML), heptanoic acid (CID: 111–14-8; Millipore Sigma; Catalog #: 43858), octanoic acid (CID: 124–07-2; Millipore Sigma; Catalog #:21639), nonanoic acid (CID: 112–05-0; Millipore Sigma; Catalog #:73982), decanoic acid (CID: 334–48-5; Millipore Sigma; Catalog #:21409), undecanoic acid (112–37-8; Millipore Sigma; Catalog #:89764), dodecanoic acid (143–07-7; Millipore Sigma; Catalog #:61609), tridecanoic acid (CID: 638–53-9; Millipore Sigma; Catalog #: 91988), tetradecanoic acid (CID: 62217–71-4; Millipore Sigma; Catalog #:70079), pentadecanoic acid (CID: 1002–84-2; Millipore Sigma; Catalog #:91446), hexadecanoic acid (CID: 57–10-3; Millipore Sigma; Catalog #:76119), heptadecanoic acid (CID: 506–12-7; Millipore Sigma; Catalog #:H3500), octadecanoic acid (CID: 38003–60-0; Millipore Sigma; Catalog #:85679), nonadecanoic acid (CID: 646–30-0; Millipore Sigma; Catalog #:72332), icosanoic acid (CID: 506–30-9); Millipore Sigma; Catalog #: 39383).
QUANTIFICATION AND STATISTICAL ANALYSIS
All statistical analyses were performed using R version 3.6.3 (RCoreTeam, 2021). Details of graphical representations and statistical methods are reported in the figure legends.
Supplementary Material
Supplemental Figure S1 - Related to Figure 3
PCR genotyping Ir76b and Ir25a mutant strains
(A-B) Genotyping schematics (left) and agarose gel electrophoresis images (right) of PCR fragments before (left) and after (right) restriction with the indicated restriction enzyme to genotype the indicated Ir76b (A) and Ir25a (B) mutants.
Supplemental Figure S2 - Related to Figure 5
Additional description and validation of GC/QTOF-MS data
(A) Mechanism of pentafluorobenzyl-bromide (PFB-Br) derivatization reaction.
(B) Representative extracted ion chromatograms (EICs) for the indicated compounds.
(C) Abundance of several carboxylic acids in low attractors (Subjects 19, 28) vs. high attractors (Subjects 31,33). Each dot represents abundance of the indicated compound for one subject in one experiment (median of 4 replicate samples). Nonparametric linear mixed-effects model followed by Benjamini Hochberg FDR correction (p<0.1) was used. Violins labeled by different lowercase letters were significantly different. Among 9 compounds analyzed here, all were verified to be significantly more abundant in the high attractors than low attractors, except tridecanoic acid, which was not significantly different (p=0.126).
(D) Representative extracted ion chromatograms (EICs) for three control compounds: two deuterated internal standards and one nylon-derived compound, from the indicated human subjects in Experiment 1.1.
(E) Abundance of control compounds shown in (C). Each dot represents the abundance (median of 4 replicate samples) of the indicated compound for one subject in 1 of 4 experiments: Experiments 1.1–1.4).
Supplemental Figure S4 - Related to Figure 5 and Figure 6
Intra-individual stability of skin chemistry
A) Timeline of behavior (green blocks) and GC/QTOF-MS experiments (purple blocks), relative to one another. 64 human volunteers participated in this study, and nylons from 18 of these were analyzed by GC/QTOF-MS. Nylons from four of these subjects (Subjects 19, 28, 31, 33) were repeatedly tested behaviorally over a three-year period and analyzed using GC/QTOF-MS in 2 sets of 4 replicate experiments (Experiments 1.1–1.4, Experiments 2.1–2.4) that were conducted 1 year apart.
(B) Heatmaps depicting quantified abundance of carboxylic acids with 10–20 carbons, averaged across 4 replicate samples per experiment, in 4 subjects. Each heatmap represents one of 8 independent experiments. Experiments 1.1–1.4 were conducted about a year before Experiments 2.1–2.4.
(C) Heatmaps depicting quantified abundance of carboxylic acids with 10–20 carbons, averaged across 5 replicate samples per experiment, in 18 subjects from the validation cohort. Each heatmap represents one of 4 independent experiments, conducted about a week apart.
Supplemental Figure S3 - Related to Figure 6
Additional validation of GC/QTOF-MS data and confirmation of hit retention times
(A) Representative extracted ion chromatograms (EICs) for three control compounds: two deuterated internal standards and one nylon-derived compound, from the indicated human subjects in Experiment 2.1.
(B) Abundance of control compounds shown in A. Each dot represents the abundance of the indicated compound for one subject in one experiment (median of 4 replicate samples).
(C) Extracted ion chromatograms of three identified hit compounds, overlaid with extracted ion chromatograms of the same sample spiked with a known standard.
HIGHLIGHTS.
Some people are consistently more attractive to mosquitoes than others, due to skin odor
Ionotropic receptor mutants (Ir25a and Ir76b) show reduced overall attraction to humans
Mosquitoes with broad olfactory deficits can distinguish highly and weakly attractive people
Highly attractive people have higher levels of carboxylic acids on their skin
ACKNOWLEDGMENTS
We thank Lindy McBride, Jessica Zung, and members of the Vosshall Lab for comments on the manuscript; all study participants for their generous participation in this research; Gloria Gordon and Libby Mejia for expert mosquito rearing; Ben Matthews and Veronica Jové for discussion and guidance on Ir76b and Ir25a mutagenesis strategies; Maya Davidov and Naquia Unwala for help with pilot behavioral studies to establish the feasibility of this work; Arka Rao, Sara Nunes Violante, Michelle Saio, Ruben Jose Jesus Faustino Ramos, and members of the Donald B. and Catherine C. Marron Cancer Metabolism Center for guidance with metabolomic experiments and GC/QTOF-MS data analysis; and the PIT Crew: Jim Petrillo, Peer Strogies, and Dan Gross of the Rockefeller University Precision Instrumentation Technologies resource center for advice on assay fabrication.
This work was supported in part by grant # UL1 TR001866 from the National Center for Advancing Translational Sciences (NCATS, National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, which also provided a pilot award to M.E.D. Additional funding for this study was provided by a Harvey L. Karp Discovery Award and a Helen Hay Whitney Postdoctoral Fellowship to M.E.D.; a Harvey L. Karp Discovery Award and a Japan Society for Promotion of Science Overseas Research Fellowship to T.M.; and an NIH-NIAID R25 AI140472 Tri-Institutional Metabolomics Training Program grant to J.R.C. L.B.V. is supported by the Howard Hughes Medical Institute (HHMI).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abuin L, Bargeton B, Ulbrich MH, Isacoff EY, Kellenberger S, and Benton R. (2011). Functional architecture of olfactory ionotropic glutamate receptors. Neuron 69, 44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta EM., Little KA., Bratton BP., Mao X., Payne A., Devenport D., and Gitai Z. (2021). Bacterial DNA on the skin surface overrepresents the viable skin microbiome. bioRxiv, DOI 10.1101/2021.1108.1116.455933. [DOI] [PMC free article] [PubMed]
- Acree F Jr., Turner RB, Gouck HK, Beroza M, and Smith N. (1968). L-Lactic acid: a mosquito attractant isolated from humans. Science 161, 1346–1347. [DOI] [PubMed] [Google Scholar]
- Ai M, Min S, Grosjean Y, Leblanc C, Bell R, Benton R, and Suh GS (2010). Acid sensing by the Drosophila olfactory system. Nature 468, 691–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjomruz M, Oshaghi MA, Sedaghat MM, Pourfatollah AA, Raeisi A, Vatandoost H, Mohtarami F, Yeryan M, Bakhshi H, and Nikpoor F. (2014). ABO blood groups of residents and the ABO host choice of malaria vectors in southern Iran. Exp Parasitol 136, 63–67. [DOI] [PubMed] [Google Scholar]
- Ansell J, Hamilton KA, Pinder M, Walraven GE, and Lindsay SW (2002). Short-range attractiveness of pregnant women to Anopheles gambiae mosquitoes. Trans R Soc Trop Med Hyg 96, 113–116. [DOI] [PubMed] [Google Scholar]
- Basrur NS, De Obaldia ME, Morita T, Herre M, von Heynitz RK, Tsitohay YN, and Vosshall LB (2020). Fruitless mutant male mosquitoes gain attraction to human odor. Elife 9, e63982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, and Vosshall LB (2009). Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier UR, Booth MM, and Yost RA (1999). Analysis of human skin emanations by gas chromatography/mass spectrometry. 1. Thermal desorption of attractants for the yellow fever mosquito (Aedes aegypti) from handled glass beads. Anal Chem 71, 1–7. [DOI] [PubMed] [Google Scholar]
- Bernier UR, Kline DL, Allan SA, and Barnard DR (2007). Laboratory comparison of Aedes aegypti attraction to human odors and to synthetic human odor compounds and blends. J Am Mosq Control Assoc 23, 288–293. [DOI] [PubMed] [Google Scholar]
- Bernier UR, Kline DL, Barnard DR, Schreck CE, and Yost RA (2000). Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti). Anal Chem 72, 747–756. [DOI] [PubMed] [Google Scholar]
- Bernier UR, Kline DL, Schreck CE, Yost RA, and Barnard DR (2002). Chemical analysis of human skin emanations: comparison of volatiles from humans that differ in attraction of Aedes aegypti (Diptera: Culicidae). J Am Mosq Control Assoc 18, 186–195. [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. (2013). The global distribution and burden of dengue. Nature 496, 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J., Costantini C., Sagnon N., Gibson G., and Coluzzi M. (1997). The role of body odours in the relative attractiveness of different men to malarial vectors in Burkina Faso. Ann Trop Med Parasitol 91, S121–S122. [Google Scholar]
- Braks MAH, and Takken W. (1999). Incubated human sweat but not fresh sweat attracts the malaria mosquito Anopheles gambiae sensu stricto. J Chem Ecol 25, 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer R. (1960). Variations in human body odour as a cause of individual differences of attraction for malaria mosquitoes. Trop Geogr Med 2, 186–192. [PubMed] [Google Scholar]
- Busula AO, Bousema T, Mweresa CK, Masiga D, Logan JG, Sauerwein RW, Verhulst NO, Takken W, and de Boer JG (2017). Gametocytemia and attractiveness of Plasmodium falciparum-Infected Kenyan children to Anopheles gambiae mosquitoes. J Infect Dis 216, 291–295. [DOI] [PubMed] [Google Scholar]
- Butterwick JA, Del Marmol J, Kim KH, Kahlson MA, Rogow JA, Walz T, and Ruta V. (2018). Cryo-EM structure of the insect olfactory receptor Orco. Nature 560, 447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moraes CM, Stanczyk NM, Betz HS, Pulido H, Sim DG, Read AF, and Mescher MC (2014). Malaria-induced changes in host odors enhance mosquito attraction. Proc Natl Acad Sci U S A 111, 11079–11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moraes CM, Wanjiku C, Stanczyk NM, Pulido H, Sims JW, Betz HS, Read AF, Torto B, and Mescher MC (2018). Volatile biomarkers of symptomatic and asymptomatic malaria infection in humans. Proc Natl Acad Sci U S A 115, 5780–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C, Jasinskiene N, James AA, and Vosshall LB (2013). orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498, 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Mármol J, Yedlin MA, and Ruta V. (2021). The structural basis of odorant recognition in insect olfactory receptors. Nature 597, 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Grandon GM, Gezan SA, Armour JA, Pickett JA, and Logan JG (2015). Heritability of attractiveness to mosquitoes. PLoS One 10, e0122716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz CO, Morris PE, and Richler JJ (2012). Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen 141, 2–18. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Wysocki CJ, Leyden JJ, Spielman AI, Sun X, and Preti G. (2008). Analyses of volatile organic compounds from human skin. Br J Dermatol 159, 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier M, Bosch OJ, and Boeckh J. (1999). Ammonia as an attractive component of host odour for the yellow fever mosquito, Aedes aegypti. Chem Senses 24, 647–653. [DOI] [PubMed] [Google Scholar]
- Gilbert IH, Gouck HK, and Smith N. (1966). Attractiveness of men and women to Aedes aegypti and relative protection time obtained with DEET. Fla Entomol 49, 53–66. [Google Scholar]
- Gouck HK (1972). Host preferences of various strains of Aedes aegypti and Aedes simpsoni as determined by an olfactometer. Bull World Health Organ 47, 680–683. [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, and Carlson JR (2006). Coding of odors by a receptor repertoire. Cell 125, 143–160. [DOI] [PubMed] [Google Scholar]
- Harrington LC., Fleisher A., Ruiz-Moreno D., Vermeylen F., Wa CV., Poulson RL., Edman JD., Clark JM., Jones JW., Kitthawee S., et al. (2014). Heterogeneous feeding patterns of the dengue vector, Aedes aegypti, on individual human hosts in rural Thailand. PLoS Negl Trop Dis 8, e3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herre M, Goldman OV, Lu TC, Caballero-Vidal G, Qi Y, Gilbert ZN, Gong Z, Morita T, Rahiel S, Ghaninia M, et al. (2022). Non-canonical odor coding in the mosquito. Cell 185, 3104–3123.e3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himeidan YE, Elbashir MI, and Adam I. (2004). Attractiveness of pregnant women to the malaria vector, Anopheles arabiensis, in Sudan. Ann Trop Med Parasitol 98, 631–633. [DOI] [PubMed] [Google Scholar]
- Huang ZR, Lin YK, and Fang JY (2009). Biological and pharmacological activities of squalene and related compounds: potential uses in cosmetic dermatology. Molecules 14, 540–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives AR, and Paskewitz SM (2005). Testing vitamin B as a home remedy against mosquitoes. J Am Mosq Control Assoc 21, 213–217. [DOI] [PubMed] [Google Scholar]
- Kaneda T. (1991). Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev 55, 288–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Ombugadu A, and Ahmad S. (2022). Host-seeking behavior and fecundity of the female Aedes aegypti to human blood types. Pest Manag Sci 78, 321–328. [DOI] [PubMed] [Google Scholar]
- Kistler KE, Vosshall LB, and Matthews BJ (2015). Genome engineering with CRISPR-Cas9 in the mosquito Aedes aegypti. Cell Rep 11, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knols BG, de Jong R, and Takken W. (1995). Differential attractiveness of isolated humans to mosquitoes in Tanzania. Trans R Soc Trop Med Hyg 89, 604–606. [DOI] [PubMed] [Google Scholar]
- Konopka JK, Task D, Afify A, Raji J, Deibel K, Maguire S, Lawrence R, and Potter CJ (2021). Olfaction in Anopheles mosquitoes. Chem Senses 46, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn F, and Natsch A. (2009). Body odour of monozygotic human twins: a common pattern of odorant carboxylic acids released by a bacterial aminoacylase from axilla secretions contributing to an inherited body odour type. J R Soc Interface 6, 377–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix R, Mukabana WR, Gouagna LC, and Koella JC (2005). Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol 3, e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MC., Domingos AI., Jones WD., Chiappe ME., Amrein H., and Vosshall LB. (2004). Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714. [DOI] [PubMed] [Google Scholar]
- Lefevre T, Gouagna LC, Dabire KR, Elguero E, Fontenille D, Renaud F, Costantini C, and Thomas F. (2010). Beer consumption increases human attractiveness to malaria mosquitoes. PLoS One 5, e9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay S, Ansell J, Selman C, Cox V, Hamilton K, and Walraven G. (2000). Effect of pregnancy on exposure to malaria mosquitoes. Lancet 355, 1972. [DOI] [PubMed] [Google Scholar]
- Logan JG, Birkett MA, Clark SJ, Powers S, Seal NJ, Wadhams LJ, Mordue Luntz AJ, and Pickett JA (2008). Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. J Chem Ecol 34, 308–322. [DOI] [PubMed] [Google Scholar]
- Logan JG, Stanczyk NM, Hassanali A, Kemei J, Santana AE, Ribeiro KA, Pickett JA, and Mordue Luntz AJ (2010). Arm-in-cage testing of natural human-derived mosquito repellents. Malar J 9, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YJ, Zhang YM, and Rock CO (2004). Product diversity and regulation of type II fatty acid synthases. Biochem Cell Biol 82, 145–155. [DOI] [PubMed] [Google Scholar]
- Matthews BJ, Dudchenko O, Kingan SB, Koren S, Antoshechkin I, Crawford JE, Glassford WJ, Herre M, Redmond SN, Rose NH, et al. (2018). Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature 563, 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride CS, Baier F, Omondi AB, Spitzer SA, Lutomiah J, Sang R, Ignell R, and Vosshall LB (2014). Evolution of mosquito preference for humans linked to an odorant receptor. Nature 515, 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, and Vosshall LB (2014). Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156, 1060–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muirhead-Thomson RC (1951). The distribution of anopheline mosquito bites among different age groups; a new factor in malaria epidemiology. Br Med J 1, 1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukabana WR, Takken W, Coe R, and Knols BG (2002). Host-specific cues cause differential attractiveness of Kenyan men to the African malaria vector Anopheles gambiae. Malar J 1, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus EM, Gisselmann G, Zhang W, Dooley R, Stortkuhl K, and Hatt H. (2005). Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat Neurosci 8, 15–17. [DOI] [PubMed] [Google Scholar]
- Nicolaides N. (1974). Skin lipids: their biochemical uniqueness. Science 186, 19–26. [DOI] [PubMed] [Google Scholar]
- Nicolaides N, Fu HC, and Rice GR (1968). The skin surface lipids of man compared with those of eighteen species of animals. J Invest Dermatol 51, 83–89. [DOI] [PubMed] [Google Scholar]
- Njiru BN., Mukabana WR., Takken W., and Knols BG. (2006). Trapping of the malaria vector Anopheles gambiae with odour-baited MM-X traps in semi-field conditions in western Kenya. Malar J 5, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Byrd AL, Park M, Program NCS, Kong HH, and Segre JA (2016). Temporal stability of the human skin microbiome. Cell 165, 854–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omasits U, Ahrens CH, Muller S, and Wollscheid B. (2014). Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 30, 884–886. [DOI] [PubMed] [Google Scholar]
- Pang Z, Chong J, Zhou G, de Lima Morais DA, Chang L, Barrette M, Gauthier C, Jacques PE, Li S, and Xia J. (2021). MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res 49, W388–W396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponlawat A, and Harrington LC (2005). Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol 42, 844–849. [DOI] [PubMed] [Google Scholar]
- Prieto-Godino LL, Rytz R, Cruchet S, Bargeton B, Abuin L, Silbering AF, Ruta V, Dal Peraro M, and Benton R. (2017). Evolution of acid-sensing olfactory circuits in Drosophilids. Neuron 93, 661–676.e666. [DOI] [PubMed] [Google Scholar]
- Qiu YT, Smallegange RC, JJ VANL, Ter Braak CJ, and Takken W. (2006). Interindividual variation in the attractiveness of human odours to the malaria mosquito Anopheles gambiae s. s. Med Vet Entomol 20, 280–287. [DOI] [PubMed] [Google Scholar]
- Rajan TV, Hein M, Porte P, and Wikel S. (2005). A double-blinded, placebo-controlled trial of garlic as a mosquito repellant: a preliminary study. Med Vet Entomol 19, 84–89. [DOI] [PubMed] [Google Scholar]
- Raji JI, Melo N, Castillo JS, Gonzalez S, Saldana V, Stensmyr MC, and DeGennaro M. (2019). Aedes aegypti mosquitoes detect acidic volatiles found in human odor using the IR8a pathway. Curr Biol 29, 1253–1262 e1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RCoreTeam (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://wwwR-projectorg/. [Google Scholar]
- Robinson A, Busula AO, Voets MA, Beshir KB, Caulfield JC, Powers SJ, Verhulst NO, Winskill P, Muwanguzi J, Birkett MA, et al. (2018). Plasmodium-associated changes in human odor attract mosquitoes. Proc Natl Acad Sci U S A 115, E4209–e4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose NH, Sylla M, Badolo A, Lutomiah J, Ayala D, Aribodor OB, Ibe N, Akorli J, Otoo S, Mutebi JP, et al. (2020). Climate and urbanization drive mosquito preference for humans. Curr Biol 30, 3570–3579 e3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytz R, Croset V, and Benton R. (2013). Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem Mol Biol 43, 888–897. [DOI] [PubMed] [Google Scholar]
- Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, and Touhara K. (2008). Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452, 1002–1006. [DOI] [PubMed] [Google Scholar]
- Shirai O, Tsuda T, Kitagawa S, Naitoh K, Seki T, Kamimura K, and Morohashi M. (2002). Alcohol ingestion stimulates mosquito attraction. J Am Mosq Control Assoc 18, 91–96. [PubMed] [Google Scholar]
- Shirai Y, Funada H, Seki T, Morohashi M, and Kamimura K. (2004). Landing preference of Aedes albopictus (Diptera: Culicidae) on human skin among ABO blood groups, secretors or nonsecretors, and ABH antigens. J Med Entomol 41, 796–799. [DOI] [PubMed] [Google Scholar]
- Silbering AF., Rytz R., Grosjean Y., Abuin L., Ramdya P., Jefferis GS., and Benton R. (2011). Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J Neurosci 31, 13357–13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallegange RC, Qiu YT, Bukovinszkine-Kiss G, Van Loon JJ, and Takken W. (2009). The effect of aliphatic carboxylic acids on olfaction-based host-seeking of the malaria mosquito Anopheles gambiae sensu stricto. J Chem Ecol 35, 933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallegange RC, Qiu YT, van Loon JJ, and Takken W. (2005). Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae). Chem Senses 30, 145–152. [DOI] [PubMed] [Google Scholar]
- Steib BM, Geier M, and Boeckh J. (2001). The effect of lactic acid on odour-related host preference of yellow fever mosquitoes. Chem Senses 26, 523–528. [DOI] [PubMed] [Google Scholar]
- Task D, Lin CC, Vulpe A, Afify A, Ballou S, Brbic M, Schlegel P, Raji J, Jefferis G, Li H, et al. (2022). Chemoreceptor co-expression in Drosophila melanogaster olfactory neurons. Elife 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton C, Doré CJ, Willson JOC, and Hubbard JL (1976). Effects of human blood group, sweating and other factors on individual host selection by species A of the Anopheles gambiae complex (Diptera, Culicidae). Bull Entomol Res 66, 651–663. [Google Scholar]
- Verhulst NO, Mbadi PA, Kiss GB, Mukabana WR, van Loon JJ, Takken W, and Smallegange RC (2011a). Improvement of a synthetic lure for Anopheles gambiae using compounds produced by human skin microbiota. Malar J 10, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst NO, Qiu YT, Beijleveld H, Maliepaard C, Knights D, Schulz S, Berg-Lyons D, Lauber CL, Verduijn W, Haasnoot GW, et al. (2011b). Composition of human skin microbiota affects attractiveness to malaria mosquitoes. PLoS One 6, e28991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CS, Harrison GA, Doré C, and Weiner JS (1972). Selective feeding of Anopheles gambiae according to ABO blood group status. Nature 239, 165. [DOI] [PubMed] [Google Scholar]
- Ye Z, Liu F, Sun H, Ferguson ST, Baker A, Ochieng SA, and Zwiebel LJ (2022). Discrete roles of Ir76b ionotropic coreceptor impact olfaction, blood feeding, and mating in the malaria vector mosquito Anopheles coluzzii. Proc Natl Acad Sci U S A 119, e2112385119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap HL., Endersby NM., Johnson PH., Ritchie SA., and Hoffmann AA. (2013). Body size and wing shape measurements as quality indicators of Aedes aegypti mosquitoes destined for field release. Am J Trop Med Hyg 89, 78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhu Y, Liu Z, Peng Y, Peng W, Tong L, Wang J, Liu Q, Wang P, and Cheng G. (2022). A volatile from the skin microbiota of flavivirus-infected hosts promotes mosquito attractiveness. Cell 185, 2510–2522.e2516. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Zung JL, Hinze A, Kriete AL, Iqbal A, Younger MA, Matthews BJ, Merhof D, Thiberge S, Ignell R, et al. (2022). Mosquito brains encode unique features of human odour to drive host seeking. Nature 605, 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1 - Related to Figure 3
PCR genotyping Ir76b and Ir25a mutant strains
(A-B) Genotyping schematics (left) and agarose gel electrophoresis images (right) of PCR fragments before (left) and after (right) restriction with the indicated restriction enzyme to genotype the indicated Ir76b (A) and Ir25a (B) mutants.
Supplemental Figure S2 - Related to Figure 5
Additional description and validation of GC/QTOF-MS data
(A) Mechanism of pentafluorobenzyl-bromide (PFB-Br) derivatization reaction.
(B) Representative extracted ion chromatograms (EICs) for the indicated compounds.
(C) Abundance of several carboxylic acids in low attractors (Subjects 19, 28) vs. high attractors (Subjects 31,33). Each dot represents abundance of the indicated compound for one subject in one experiment (median of 4 replicate samples). Nonparametric linear mixed-effects model followed by Benjamini Hochberg FDR correction (p<0.1) was used. Violins labeled by different lowercase letters were significantly different. Among 9 compounds analyzed here, all were verified to be significantly more abundant in the high attractors than low attractors, except tridecanoic acid, which was not significantly different (p=0.126).
(D) Representative extracted ion chromatograms (EICs) for three control compounds: two deuterated internal standards and one nylon-derived compound, from the indicated human subjects in Experiment 1.1.
(E) Abundance of control compounds shown in (C). Each dot represents the abundance (median of 4 replicate samples) of the indicated compound for one subject in 1 of 4 experiments: Experiments 1.1–1.4).
Supplemental Figure S4 - Related to Figure 5 and Figure 6
Intra-individual stability of skin chemistry
A) Timeline of behavior (green blocks) and GC/QTOF-MS experiments (purple blocks), relative to one another. 64 human volunteers participated in this study, and nylons from 18 of these were analyzed by GC/QTOF-MS. Nylons from four of these subjects (Subjects 19, 28, 31, 33) were repeatedly tested behaviorally over a three-year period and analyzed using GC/QTOF-MS in 2 sets of 4 replicate experiments (Experiments 1.1–1.4, Experiments 2.1–2.4) that were conducted 1 year apart.
(B) Heatmaps depicting quantified abundance of carboxylic acids with 10–20 carbons, averaged across 4 replicate samples per experiment, in 4 subjects. Each heatmap represents one of 8 independent experiments. Experiments 1.1–1.4 were conducted about a year before Experiments 2.1–2.4.
(C) Heatmaps depicting quantified abundance of carboxylic acids with 10–20 carbons, averaged across 5 replicate samples per experiment, in 18 subjects from the validation cohort. Each heatmap represents one of 4 independent experiments, conducted about a week apart.
Supplemental Figure S3 - Related to Figure 6
Additional validation of GC/QTOF-MS data and confirmation of hit retention times
(A) Representative extracted ion chromatograms (EICs) for three control compounds: two deuterated internal standards and one nylon-derived compound, from the indicated human subjects in Experiment 2.1.
(B) Abundance of control compounds shown in A. Each dot represents the abundance of the indicated compound for one subject in one experiment (median of 4 replicate samples).
(C) Extracted ion chromatograms of three identified hit compounds, overlaid with extracted ion chromatograms of the same sample spiked with a known standard.
Data Availability Statement
Data and code have been deposited in Zenodo and are publicly available. The DOI is listed in the key resources table.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental Models: Organisms/Strains | ||
| Aedes aegypti (Liverpool) wild type | Vosshall Lab | |
| Aedes aegypti (Orlando) wild type | Vosshall Lab | |
| Ir25a 19/19 | Vosshall Lab | This paper |
| Ir25a BamHI/BamHI | Vosshall Lab | This paper |
| Ir76b 32/32 | Vosshall Lab | This paper |
| Ir76b 61/61 | Vosshall Lab | This paper |
| Orco 5/16 | Vosshall Lab | PMID: 23719379 |
| Ir8a dsRed/dsRed | DeGennaro Lab | PMID: 30930038 |
| GC/QTOF-MS | ||
| DB5ms column (30 m × 250 μm, 0.25 μm film thickness | Agilent | #19091S-433 |
| Data Analysis | ||
| Behavioral and GC/QTOF-MS raw data and analysis | Vosshall Lab | DOI: 10.5281/zenodo.5822538 |
| Other | ||
| Quattroport olfactometer assay | Vosshall Lab | DOI: 10.5281/zenodo.5822538 |
| Two-choice assay | Vosshall Lab | DOI: 10.5281/zenodo.5822538 |