Abstract
Objectives
The risk of adverse events and prognostic factors are changing in different time phases after acute myocardial infarction (AMI). The incidence of adverse events is considerable in the early period after AMI hospitalisation. Therefore, dynamic risk prediction is needed to guide postdischarge management of AMI. This study aimed to develop a dynamic risk prediction instrument for patients following AMI.
Design
A retrospective analysis of a prospective cohort.
Setting
108 hospitals in China.
Participants
A total of 23 887 patients after AMI in the China Acute Myocardial Infarction Registry were included in this analysis.
Primary outcome measures
All-cause mortality.
Results
In multivariable analyses, age, prior stroke, heart rate, Killip class, left ventricular ejection fraction (LVEF), in-hospital percutaneous coronary intervention (PCI), recurrent myocardial ischaemia, recurrent myocardial infarction, heart failure (HF) during hospitalisation, antiplatelet therapy and statins at discharge were independently associated with 30-day mortality. Variables related to mortality between 30 days and 2 years included age, prior renal dysfunction, history of HF, AMI classification, heart rate, Killip class, haemoglobin, LVEF, in-hospital PCI, HF during hospitalisation, HF worsening within 30 days after discharge, antiplatelet therapy, β blocker and statin use within 30 days after discharge. The inclusion of adverse events and medications significantly improved the predictive performance of models without these indexes (likelihood ratio test p<0.0001). These two sets of predictors were used to establish dynamic prognostic nomograms for predicting mortality in patients with AMI. The C indexes of 30-day and 2-year prognostic nomograms were 0.85 (95% CI 0.83–0.88) and 0.83 (95% CI 0.81–0.84) in derivation cohort, and 0.79 (95% CI 0.71–0.86) and 0.81 (95% CI 0.79–0.84) in validation cohort, with satisfactory calibration.
Conclusions
We established dynamic risk prediction models incorporating adverse event and medications. The nomograms may be useful instruments to help prospective risk assessment and management of AMI.
Trial registration number
Keywords: myocardial infarction, coronary heart disease, coronary intervention, prognosis
STRENGTHS AND LIMITATIONS OF THIS STUDY.
We developed and validated a dynamic risk prediction tool using data from a large, prospective, multicentre registry.
We analysed the incremental prognostic value of in-hospital and postdischarge adverse events as well as medications over traditional predictors in patients following acute myocardial infarction.
We compared the predictive performance of our models with existing risk prediction tools, including the Global Registry of Acute Coronary Events 1.0 and 2.0 scores.
The predictive performance of the dynamic risk prediction tool can be further improved if including more follow-up information.
The dynamic risk prediction tool should be further validated in external cohorts.
Introduction
Although in-hospital mortality of acute myocardial infarction (AMI) has been decreased in many countries,1 2 risk of adverse events remains considerable in survivors after AMI hospitalisation.3 Previous studies have indicated unsatisfactory and imbalanced quality of secondary prevention medications in clinical practice,4 5 which could cause negative impact on prognosis of patients with AMI. Individualised risk assessment may aid in decision-making of long-term therapeutic strategies for patients after AMI. However, the existing risk prediction tools, which are mainly based on predictive indexes collected at admission, fail to consider the changing nature of adverse events and medications after AMI hospitalisation,6 7 and therefore may not be appropriate to guide long-term management. Dynamic risk assessment may help improve the quality of long-term management for patients following AMI.
Although several studies sought to forecast mortality dynamically in patients with acute coronary syndromes,8–11 the prognostic components of these models were obtained during hospitalisation, without taking follow-up adverse events and medications into consideration. Dynamic prognostic instruments designed to help risk reassessment should include postdischarge information which is associated with outcomes. This study aimed to develop and validate dynamic risk prediction models, visualised by nomograms, which included in-hospital and postdischarge adverse events and medications, to assist in prognostic evaluation and decision-making of secondary prevention strategies in patients following AMI.
Methods
Study population
The data for the present study were from the China Acute Myocardial Infarction (CAMI) Registry. The design of the CAMI Registry has been described and published elsewhere.12 Briefly, 108 hospitals from 31 provinces and municipalities throughout Mainland China were included in this prospective, nationwide, multicentre registry. Consecutive patients with AMI were enrolled in the registry and the final diagnosis of patients must meet the third Universal Definition of Myocardial Infarction.13 All types of AMI were eligible for the CAMI Registry, except type 4a and type 5. Presenting characteristics, medical history, laboratory results, medications and clinical outcomes were collected according to the American College of Cardiology/American Heart Association Task Force on clinical data standards and NCDR-ACTION-GWTG element dictionary.
Patients registered in the CAMI Registry from January 2013 to September 2014 were included in this study. Those with invalid diagnosis (n=1312), who were transferred out (n=1181) or died during hospitalisation (n=1690) were excluded. The remaining population (n=23 887) was divided randomly according to 2:1 ratio into derivation (n=15 925) and validation (n=7962) cohorts for developing and validating a 30-day risk prediction model. After further excluding patients who died within 30 days after discharge (n=293) and those with missing data on 30-day medication use (n=5391), the remaining derivation (n=12 136) and validation (n=6067) cohorts were used for establishing and testing a 2-year risk prediction model (online supplemental figure 1).
bmjopen-2022-069505supp001.pdf (2.3MB, pdf)
Definitions
Standard definitions of adverse events have been described elsewhere in detail.14 Taking a medication within 30 days means using the medication during this period after discharge without discontinuation.
Follow-up and endpoints
Patients were followed by clinical visits or telephone call at 30 days, 6 months, 12 months, 18 months and 24 months. Adverse events (such as death, recurrent myocardial infarction and heart failure worsening) and medications at follow-up time points were collected by trained cardiologists or cardiovascular fellows. The primary endpoints of this analysis were all-cause mortality within 30 days after AMI hospitalisation (for establishing the 30-day risk prediction model) and mortality between 30 days and 2 years (for establishing the 2-year risk prediction model).
Statistical analysis
Categorical variables were summarised as frequencies and percentages, and compared by χ2 or Fisher’s exact test, as appropriate. Continuous variables were expressed as mean±SD or medians (IQR) according to data distributions, and compared by Student’s t-test or non-parametric test. Kaplan-Meier curve and density plots were used to depict the changing nature of risk after AMI hospitalisation. In the derivation cohort, 190 deaths occurred within 30 days after discharge, which could ensure at most 19 predictor parameters (greater than 12 predictor parameters finally included) in the 30-day risk prediction model based on the rule of thumb of 10 events per candidate predictor parameter. Similarly, 740 deaths occurred between 30 days and 2 years, which could ensure at most 74 predictor parameters (greater than 15 predictor parameters finally included) in the 2-year risk prediction model.15
The derivation cohort was used to identify predictors of 30-day mortality and mortality between 30 days and 2 years. The associations between variables, including age, sex, body mass index, diabetes, hypertension, hyperlipidaemia, smoking, prior angina pectoris, prior myocardial infarction, prior heart failure, prior stroke, prior peripheral artery disease, prior percutaneous coronary intervention (PCI), prior coronary artery bypass graft (CABG), prior renal dysfunction, chronic obstructive pulmonary disease (COPD), symptom onset to admission time, heart rate, systolic blood pressure, Killip class, cardiac arrest at admission, diagnosis, anterior wall involvement, creatinine clearance, haemoglobin, leucocyte count, left ventricular ejection fraction (LVEF), in-hospital PCI, in-hospital CABG, heart failure, recurrent myocardial ischaemia, recurrent myocardial infarction, stroke, bleeding events (not including cerebral haemorrhage) during hospitalisation, antiplatelet therapy at discharge, statins at discharge, β blockers at discharge, and ACE inhibitor (ACEI) or angiotensin II receptor blocker (ARB) at discharge, and 30-day mortality, were first assessed in univariable Cox regression models. For obtaining prognostic factors of mortality between 30 days and 2 years, the associations of adverse events within 30 days (recurrent myocardial infarction and heart failure worsening) and 30-day medications (antiplatelet therapy, statins, β blockers and ACEI/ARB) with mortality were also assessed in univariable Cox models besides variables mentioned previously. Subsequently, the least absolute shrinkage and selection operator (LASSO) method was adopted to select predictors of short-term and long-term mortality, respectively, from variables with p≤0.1 in univariable analysis. The selected predictors were used to establish dynamic risk prediction models by multivariable Cox regression model. The relative importance of these variables was ranked according to the proportion of explainable log-likelihood ratio χ2 statistics.
To analyse the incremental prognostic value of adverse events and medications over traditional predictive indexes, we compared the predictive performance between models with or without adverse events and medications using C index, net reclassification index (NRI), integrated discrimination improvement index (IDI), likelihood ratio test and Bayesian information criterion (BIC). The clinical utility of models was compared by decision curve analysis. Predictive performance was also compared between 30-day risk model or 2-year risk model and the Global Registry of Acute Coronary Events (GRACE) risk scores (versions 1.0 and 2.0) to analyse the additional value of the dynamic models beyond existing prognostic tools.6 16
Two prognostic nomograms, which could make complex predictive formulas friendly to use in clinical practice, were constructed based on the regression coefficients of predictors for mortality using the ‘rms’ package of R software. Discrimination and calibration of the nomograms were assessed by C index and calibration curves presenting the relationship between observed and predicted survival probabilities in both derivation and validation cohorts. Subgroup analyses were performed in patients with complete data on model predictors in the validation cohort according to age, sex, diabetes, AMI classification, in-hospital PCI and hospital level (province level, prefecture level and county level).
Before regression analysis, we used Martingale residual plots to check the linearity assumption for continuous variables (online supplemental figures 2 and 3). We also calculated variance inflation factor to examine multicollinearity issue. The proportional hazards assumptions were tested by inspection of Schoenfeld residual plots (online supplemental figures 4 and 5). Multiple imputation was used to generate five data sets without missing values. The LASSO method was performed in each data set. Only variables selected by LASSO method in all five data sets were included in final dynamic risk prediction models. Number of missing values for selected predictors was shown in online supplemental table 1. Results of Cox regression models were reported as hazard ratio (HR) with 95% confidence intervals (CIs). A two-tailed p<0.05 was considered statistically significant. Statistical analyses were performed using R V.4.0.5 (R Foundation for Statistical Computing, Vienna, Austria) and SAS V.9.4 (SAS Institute).
Patient and public involvement
Patients or the public were not involved directly in the design, conduct, reporting or dissemination plans of our research.
Results
Baseline characteristics, medications and outcomes of derivation and validation cohorts were summarised in table 1 and online supplemental tables 2 and 3. Rates of 30-day mortality among hospital survivors in the derivation and validation cohorts were 1.2% (190/15 925 patients) and 1.3% (103/7962 patients), respectively. Rates of mortality between 30 days and 2 years were 6.1% (740/12 136 patients) and 5.7% (345/6067 patients). The Kaplan-Meier curve and density plots showed the changing risk of death and recurrent myocardial infarction within 2 years after AMI hospitalisation (online supplemental figures 6 and 7).
Table 1.
Baseline characteristics of cohorts for developing and validating the 30-day prognostic model
| Variables | Derivation cohort (n=15 925) | Validation cohort (n=7962) |
| Demographics | ||
| Age (years) | 62.27±12.36 | 62.54±12.29 |
| Female | 3878 (24.4) | 1940 (24.4) |
| BMI (kg/m2) | 24.13±3.05 | 24.08±3.04 |
| Medical history | ||
| Diabetes | 2943 (19.6) | 1505 (20.0) |
| Hypertension | 7873 (51.2) | 3897 (50.8) |
| Hyperlipidaemia | 1154 (8.5) | 569 (8.3) |
| Current smoker | 7083 (45.7) | 3483 (44.8) |
| Prior angina pectoris | 4044 (27.8) | 1994 (27.4) |
| Prior myocardial infarction | 1083 (7.4) | 553 (7.5) |
| Prior heart failure | 329 (2.2) | 169 (2.3) |
| Prior PCI | 753 (5.0) | 381 (5.1) |
| Prior CABG | 56 (0.4) | 36 (0.5) |
| Prior renal dysfunction | 197 (1.3) | 94 (1.3) |
| COPD | 279 (1.9) | 142 (1.9) |
| Presenting characteristics | ||
| Symptom onset to admission time | ||
| 0–6 hours | 7398 (47.0) | 3625 (46.0) |
| >6 hours | 8330 (53.0) | 4247 (54.0) |
| Heart rate (beats/min) | 77±18 | 78±18 |
| Systolic blood pressure | 129.48±25.01 | 129.94±25.26 |
| Killip class | ||
| I | 11 836 (76.2) | 5903 (75.8) |
| II–IV | 3694 (23.8) | 1883 (24.2) |
| Cardiac arrest at admission | 128 (0.8) | 77 (1.0) |
| AMI classification | ||
| STEMI | 12 051 (75.7) | 5991 (75.2) |
| NSTEMI | 3874 (24.3) | 1971 (24.8) |
| Anterior wall involvement | 7406 (47.7) | 3696 (47.5) |
Values are shown as mean±SD, median (IQR) or number (%) without imputation of missing data.
AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Predictors of 30-day mortality in patients after AMI
Univariable analysis of the associations between variables and 30-day mortality was presented in online supplemental table 4. In the LASSO-based Cox regression model, age, prior stroke, heart rate, Killip class, LVEF, in-hospital PCI, in-hospital recurrent myocardial ischaemia, in-hospital recurrent myocardial infarction, in-hospital heart failure, antiplatelet therapy and statins at discharge were independently associated with 30-day mortality (online supplemental figures 8–12; table 2). The relative importance of these predictors was ranked and shown in online supplemental figure 13.
Table 2.
Multivariable analysis of 30-day mortality in the derivation cohort
| All-cause death | ||
| Adjusted HR (95% CI) | P value | |
| Age (per 1-year increase) | 1.035 (1.021–1.049) | <0.0001 |
| Prior stroke (vs no) | 1.560 (1.091–2.231) | 0.0148 |
| Heart rate (per 1 beat/min increase) | 1.006 (1.000–1.012) | 0.0497 |
| Killip class II–IV (vs I) | 1.528 (1.115–2.094) | 0.0083 |
| LVEF (per 1% increase) | 0.969 (0.955–0.982) | <0.0001 |
| In-hospital PCI (vs no) | 0.441 (0.307–0.634) | <0.0001 |
| In-hospital recurrent myocardial ischaemia (vs no) | 1.711 (1.019–2.871) | 0.0421 |
| In-hospital recurrent myocardial infarction (vs no) | 4.572 (2.121–9.859) | 0.0001 |
| In-hospital heart failure (vs no) | 2.869 (2.065–3.986) | <0.0001 |
| Antiplatelet therapy at discharge (vs dual antiplatelet therapy) | ||
| Single antiplatelet therapy | 0.791 (0.491–1.275) | 0.3358 |
| None | 2.363 (1.395–4.003) | 0.0014 |
| Statins at discharge (vs no) | 2.009 (1.258–3.208) | 0.0035 |
LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
Predictors of 2-year mortality for 30-day survivors after AMI hospitalisation
Univariable analysis of mortality between 30 days and 2 years after AMI hospitalisation was shown in online supplemental table 5. In the LASSO-based Cox regression model, age, prior renal dysfunction, history of heart failure, AMI classification, heart rate, Killip class, haemoglobin, LVEF, in-hospital PCI, in-hospital heart failure, heart failure worsening within 30 days, antiplatelet therapy, β blocker and statins within 30 days were identified as predictors of mortality (online supplemental figures 14–18; table 3). The relative importance of these predictors was ranked and presented in online supplemental figure 19.
Table 3.
Multivariable analysis of mortality after 30 days in the derivation cohort
| All-cause death | ||
| Adjusted HR (95% CI) | P value | |
| Age (per 1-year increase) | 1.052 (1.045–1.060) | <0.0001 |
| Prior renal dysfunction (vs no) | 1.539 (1.076–2.201) | 0.0181 |
| History of heart failure (vs no) | 1.501 (1.160–1.943) | 0.002 |
| STEMI (vs NSTEMI) | 0.747 (0.639–0.873) | 0.0002 |
| Heart rate (per 1 beat/min increase) | 1.008 (1.005–1.011) | <0.0001 |
| Killip class II–IV (vs I) | 1.330 (1.129–1.566) | 0.0006 |
| Haemoglobin (per 1 g/L increase) | 0.993 (0.990–0.996) | <0.0001 |
| LVEF (per 1% increase) | 0.971 (0.964–0.978) | <0.0001 |
| In-hospital PCI (vs no) | 0.423 (0.351–0.510) | <0.0001 |
| In-hospital heart failure (vs no) | 1.287 (1.085–1.525) | 0.0037 |
| Heart failure worsening within 30 days (vs no) | 1.675 (1.258–2.229) | 0.0004 |
| Antiplatelet therapy within 30 days (vs dual antiplatelet therapy) | ||
| Single antiplatelet therapy | 1.107 (0.911–1.345) | 0.3084 |
| None | 1.430 (1.055–1.937) | 0.0211 |
| β blocker within 30 days (vs yes) | 1.271 (1.085–1.491) | 0.0031 |
| Statins within 30 days (vs yes) | 1.191 (0.908–1.561) | 0.2075 |
LVEF, left ventricular ejection fraction; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Incremental prognostic value of adverse events and medications
The inclusion of in-hospital recurrent myocardial ischaemia, in-hospital recurrent myocardial infarction, in-hospital heart failure, antiplatelet therapy and statins at discharge significantly improved the predictive power of 30-day risk prediction model (table 4; 30-day model 1 vs 30-day model 0: C index, 0.855 (0.830–0.879) vs 0.822 (0.796–0.848); NRI (95% CI), 0.445 (0.339–0.523), p<0.0001; IDI (95% CI), 0.040 (0.025–0.074), p<0.0001; likelihood ratio test p<0.0001; BIC, 3434.091 vs 3350.882). In the 2-year risk prediction model, heart failure worsening within 30 days, antiplatelet, β blocker and statins within 30 days also provided additional prognostic value over predictive indexes obtained during hospitalisation (table 5; likelihood ratio test p<0.0001). The decision curve analysis further demonstrated better clinical utility after adding adverse events and medications into 30-day and 2-year risk models (online supplemental figure 20). Notably, the hospital level provided no incremental value to 30-day or 2-year risk models (the inclusion of hospital level to 30-day model, likelihood ratio test p=0.4174; to 2-year model, likelihood ratio test p=0.5621; online supplemental figure 21).
Table 4.
Comparison of 30-day prognostic models with or without adverse events and medications
| 30-day model 0 | 30-day model 1 | |
| C index | 0.822 (0.796–0.848) | 0.855 (0.830–0.879) |
| NRI (95% CI) | 0.445 (0.339–0.523) | |
| P value | <0.0001 | |
| IDI (95% CI) | 0.040 (0.025–0.074) | |
| P value | <0.0001 | |
| Likelihood ratio test (p value) | <0.0001 | |
| BIC | 3434.091 | 3350.882 |
30-day model 0: age, prior stroke, heart rate, Killip class, LVEF and in-hospital PCI. 30-day model 1: age, prior stroke, heart rate, Killip class, LVEF, in-hospital PCI, in-hospital recurrent myocardial ischaemia, in-hospital recurrent myocardial infarction, in-hospital heart failure, antiplatelet therapy and statins at discharge.
BIC, Bayesian information criterion; IDI, integrated discrimination improvement index; LVEF, left ventricular ejection fraction; NRI, net reclassification index; PCI, percutaneous coronary intervention.
Table 5.
Comparison of 2-year prognostic models with or without adverse events and medications
| 2-year model 0 | 2-year model 1 | |
| C index | 0.822 (0.807–0.836) | 0.825 (0.811–0.839) |
| NRI (95% CI) | 0.119 (−0.045–0.176) | |
| P value | 0.126 | |
| IDI (95% CI) | 0.008 (0.004–0.017) | |
| P value | 0.007 | |
| Likelihood ratio test (p value) | <0.0001 | |
| BIC | 12 792.602 | 12 785.706 |
2-year model 0: age, prior renal dysfunction, prior heart failure, AMI classification, heart rate, Killip class, haemoglobin, LVEF, in-hospital PCI and in-hospital heart failure. 2-year model 1: age, prior renal dysfunction, prior heart failure, AMI classification, heart rate, Killip class, haemoglobin, LVEF, in-hospital PCI, in-hospital heart failure, heart failure worsening within 30 days, antiplatelet therapy, β blockers and statins within 30 days.
AMI, acute myocardial infarction; BIC, Bayesian information criterion; IDI, integrated discrimination improvement index; LVEF, left ventricular ejection fraction; NRI, net reclassification index; PCI, percutaneous coronary intervention.
Comparisons of prognostic models with GRACE risk scores
For predicting 30-day mortality, the 30-day risk prediction model showed significantly better predictive performance than both GRACE 1.0 and 2.0 scores (30-day risk model vs GRACE 1.0 score: C index, 0.855 (0.830–0.879) vs 0.771 (0.740–0.802); NRI (95% CI), 0.412 (0.307–0.485), p<0.0001; IDI (95% CI), 0.048 (0.032–0.090), p<0.0001; BIC, 3267.271 vs 3402.578; 30-day risk model vs GRACE 2.0 score: C index, 0.855 (0.830–0.879) vs 0.752 (0.720–0.784); NRI (95% CI), 0.569 (0.500–0.624), p<0.0001; IDI (95% CI), 0.061 (0.044–0.101), p<0.0001; BIC, 3247.357 vs 3492.004). Similarly, when predicting 2-year mortality, the 2-year risk prediction model also performed better than the GRACE risk scores (2-year risk model vs GRACE 1.0 score: C index, 0.825 (0.811–0.839) vs 0.798 (0.783–0.813); NRI (95% CI), 0.191 (0.147–0.257), p<0.0001; IDI (95% CI), 0.041 (0.031–0.057), p<0.0001; BIC, 12 540.559 vs 12 697.527; 2-year risk model vs GRACE 2.0 score: C index, 0.825 (0.811–0.839) vs 0.769 (0.752–0.786); NRI (95% CI), 0.486 (0.456–0.529), p<0.0001; IDI (95% CI), 0.115 (0.098–0.143), p<0.0001; BIC, 12 257.375 vs 12 934.783). The decision curve analysis further demonstrated better clinical utility of both 30-day and 2-year risk models than GRACE scores (online supplemental figures 22 and 23).
Nomograms for dynamic risk prediction
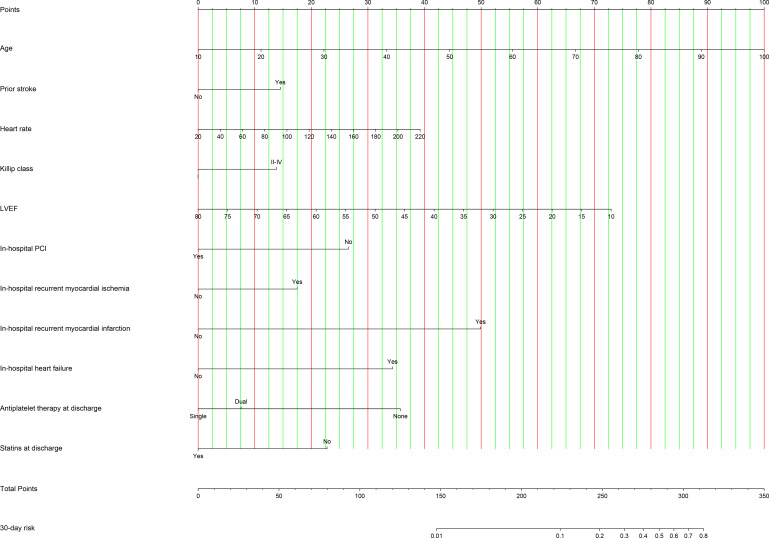
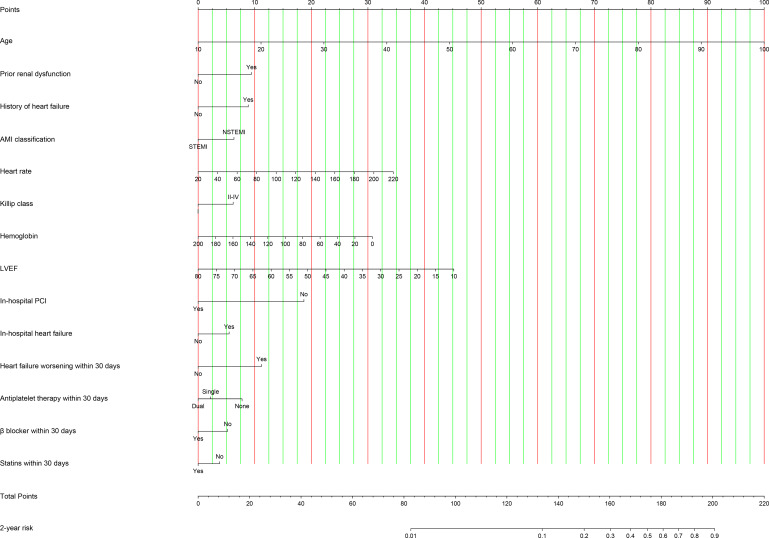
Two nomograms were created by assigning a weighted score based on regression coefficients of each prognostic index for evaluating 30-day and 2-year mortality risk, respectively. All observed values of a prognostic index corresponded vertically to points on the top scale. The sum of points for each index was plotted on the ‘Total points’ scale and corresponded to mortality risk at the bottom (figures 1 and 2).
Figure 1.
Nomogram for predicting 30-day mortality risk. The observed value of a prognostic index was assigned a point by drawing a perpendicular line towards the top scale. The sum of points for each index was plotted on the ‘Total points’ scale, and corresponded to the risk of 30-day mortality at the bottom with a vertical line. LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
Figure 2.
Nomogram for predicting 2-year mortality risk. The observed value of a prognostic index was assigned a point by drawing a perpendicular line towards the top scale. The sum of points for each index was plotted on the ‘Total points’ scale, and corresponded to the risk of 2-year mortality at the bottom with a vertical line. AMI, acute myocardial infarction; LVEF, left ventricular ejection fraction; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
The 30-day prognostic nomogram achieved high discrimination in both derivation and validation cohorts, with C indexes of 0.85 (95% CI 0.83–0.88) and 0.79 (95% CI 0.71–0.86), respectively. The calibration curves presenting the concordance between observed and predicted 30-day survival probabilities in two cohorts also showed satisfactory calibration of the model (online supplemental figure 24). In addition, the 30-day prognostic nomogram achieved moderate to high discrimination (C indexes: 0.74–0.83) in subsets according to age, sex, diabetes, AMI classification, PCI and hospital level (online supplemental table 6).
The C indexes of 2-year prognostic nomogram were 0.83 (95% CI 0.81–0.84) and 0.81 (95% CI 0.79–0.84) in derivation and validation cohorts, respectively. The calibration curves demonstrated excellent calibration of the nomogram in both derivation and validation cohorts (online supplemental figure 25). In aforementioned subgroups of patients, the model discrimination was acceptable (C index: 0.66–0.83, online supplemental table 7).
Discussion
Using data from a large, prospective, multicentre registry, we identified 11 predictors of 30-day mortality, and 14 variables, including heart failure worsening and medications within 30 days, associated with mortality between 30 days and 2 years in patients after AMI hospitalisation. These two sets of predictors were used to develop prognostic nomograms which could predict postdischarge mortality for patients with AMI in different time phases. The nomograms showed satisfactory discrimination and calibration in both derivation and validation cohorts. This is a novel dynamic risk prediction tool which can serve for risk assessment and guide long-term management of patients after AMI.
A series of risk models have been developed to predict mortality in patients with acute coronary syndromes.6 7 17 These models mainly included prognostic factors obtained at admission and provided a fixed estimate of survival probability for a given patient. This working mode of prognostic models was helpful for screening high-risk patients and determining therapeutic strategies after admission. However, the incidence of adverse cardiovascular events remained considerable after AMI hospitalisation. Accumulating evidence has implied that a larger proportion of adverse events occurred in the early phase after AMI hospitalisation,18 which reflected the changing risk following AMI and highlighted the importance of risk reassessment during follow-up. Although some risk prediction models, such as the GRACE risk score and dynamic Thrombolysis in Myocardial Infarction risk score, could be used to assess mortality risk at discharge,6 9 none of them could improve prognostic evaluation during follow-up. Considering the higher risk of adverse events in the early period than that at the late stage after AMI hospitalisation (online supplemental figures 6 and 7), we chose 30 days, which was also one of routine follow-up points after AMI hospitalisation in clinical practice, as the time point of risk reassessment to establish dynamic risk prediction models.
The first model, which was developed to assess 30-day mortality risk at discharge in patients after AMI, included variables related to patients’ demographics, haemodynamics, left ventricular systolic function, treatment, in-hospital adverse events and medications at discharge. Previous studies have established several risk prediction models, such as Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin (eptifibatide) Therapy and Zwolle risk scores,19 20 to predict 30-day mortality in patients with acute coronary syndromes. However, these models mainly used patient characteristics, clinical presentations at admission as well as angiographic features as prognostic indexes. The present study showed that recurrent myocardial ischaemia, recurrent myocardial infarction, heart failure during AMI hospitalisation, antiplatelet therapy and statin use at discharge were independently associated with short-term mortality after discharge, and provided significantly incremental prognostic information over traditional predictive indexes. Therefore, these adverse events and medications were included in the novel 30-day risk prediction model, which might assist in decision-making of postdischarge management.
For the second model assessing 2-year mortality risk in 30-day survivors, we screened new predictors including heart failure worsening within 30 days, antiplatelet therapy, β blocker and statin use within 30 days after discharge. A prior study showed that mortality rate of patients with an early recurrent myocardial infarction (recurrent myocardial infarction within 90 days of discharge) was nearly 50% within 5 years.21 In the present study, we observed that the recurrent myocardial infarction and heart failure worsening within 30 days after discharge were associated with more than threefold and >4.5-fold increase of 2-year mortality risk respectively in univariable analysis, and heart failure worsening, which was included in the 2-year prognostic nomogram, was a statistically significant prognostic index in multivariable analysis. To our best knowledge, this is the first prognostic instrument taking follow-up adverse event after AMI into consideration. In addition, although some studies analysed the prognostic impact of secondary prevention implementation in patients after AMI,22 23 follow-up medications have not been considered as predictive indexes in risk models for AMI so far. For some patients, not taking optimal medical care within 30 days after discharge could be explained by poor medication adherence after AMI, which was a problem for both developed and developing countries.23–25 A study found that about 30% of patients with myocardial infarction who underwent PCI in the USA reported suboptimal adherence to prescribed medications in 6 weeks after hospitalisation.25 Data from the China Patient-centered Evaluative Assessment of Cardiac Events Prospective Study of AMI also showed that a similar percentage of patients with AMI did not take medications as prescribed in the first month after discharge.23 Patients with early medication non-adherence not only suffered a higher risk of early adverse events, but might not comply with long-term secondary prevention measures, and therefore had poorer long-term prognosis. Another situation was that patients were not prescribed with some medications by physicians due to contraindications or high risk of side effects. However, lack of secondary prevention medications after AMI still meant a higher risk of cardiovascular adverse events in these patients. Indeed, our analysis showed that insufficient use of antiplatelet therapy, β blocker and statins within 30 days after AMI hospitalisation had significant negative impact on 2-year survival. The inclusion of follow-up medications and adverse events improved risk prediction of the 2-year risk prediction model.
Although a previous study from CAMI Registry showed that there were significant variations in in-hospital mortality among three levels of hospitals in China,14 hospital level was not used as a predictive index in the present risk prediction models, for the improvement of care quality in low-level hospitals was likely to weaken its prognostic value. Besides, the hospital level was shown to provide no additional prognostic information beyond current predictors in the risk prediction models. Socioeconomic factors, which were known as risk factors for survival following myocardial infarction,26–29 were not included in the present models because we sought to develop models based on predictors directly reflecting patients’ clinical conditions. Notably, socioeconomic factors were also not included in existing risk prediction tools.6 7 9 16 The rates of COPD and prior heart failure in our cohort were lower than the UK population of AMI.30 However, the rates in the present study were similar with data from the Improving Care for Cardiovascular Disease in China-Acute Coronary Syndrome project, which was also a nationwide registry in China.31 32 The distinct prevalence of comorbidity in patients with myocardial infarction between countries highlighted the importance of developing risk prediction models for specific populations.
The present study showed that the dynamic prognostic nomograms achieved satisfactory discrimination and calibration, and performed well in subgroups of patients according to age, sex, diabetes, AMI classification, PCI and hospital level. The clinical utility of nomograms was further confirmed by decision curve analysis. Prognostic nomogram is a graphical presentation format for complex predictive regression models.33 A series of prognostic nomograms have been established for risk prediction in patients with cancer or cardiovascular diseases.34–39 For patients with myocardial infarction, previous prognostic nomograms mainly focused on evaluating short-term risk of mortality or other adverse events.37 38 There also existed nomogram developed to predict risk of adverse events beyond one year.36 However, without consideration of changing nature of event risk or medications, the nomogram might not play roles in postdischarge management of patients. Our prognostic nomograms, which took into account follow-up adverse event as well as medications, could assist in risk reassessment at 30 days after discharge. In detail, using the nomogram for prediction of 30-day mortality, physicians can identify high-risk patients at discharge. At 30-day follow-up, the second nomogram can be used to reassess mortality risk of 30-day survivors, and may guide decision-making of long-term follow-up intensity and strategies of medical care.
Limitations
Several important limitations in this study should be mentioned. First, as a retrospective analysis of a prospective cohort, this study only used data which had been collected in the CAMI Registry. Although the present risk prediction tool has achieved satisfactory discrimination and calibration, it may be further improved by including other prognostic factors of AMI, such as details of angiographic characteristics, which were not available in a large proportion of the cohort. Heart failure worsening and medications within 30 days after discharge were included in the 2-year risk prediction model. However, lifestyle interventions and cardiac rehabilitation programmes, which were associated with lower risk of adverse events in patients with coronary artery disease,40 41 as well as laboratory and echocardiographic indexes were not collected during follow-up. These variables may also improve the predictive performance of the models. Second, although the present study showed the feasibility of assessing 2-year prognosis at 30 days after discharge, risk reassessment is a serial process and ideally performed at more time points beyond the early phase after discharge. Models which can ensure more dynamic and accurate risk prediction are still needed. Third, the distribution of AMI types (types 1, 2, 3, 4b and 4c) was not collected in the CAMI Registry. Results of the present study could have biased if a certain type was more represented than another. However, the CAMI Registry enrolled patients consecutively from 108 hospitals, which meant that it was representative of AMI population in routine clinical practice. It is plausible that the impact of distribution of AMI types is relatively limited. Fourth, there existed some missing values which needed to be imputed before regression analysis. However, almost all predictors had missing values of <6%. Finally, our dynamic models were only internally validated in Chinese patients. Further validations in external cohorts including patients of other races are needed.
Conclusions
The dynamic risk prediction tool consisted of a short-term prognostic model and a long-term prognostic model for patients after AMI hospitalisation. Taking the changing nature of adverse events and medications into consideration, the models can serve prospective risk stratification and guide postdischarge management of AMI. Dynamic risk prediction may play an important role in therapeutic decision-making and quality improvement of secondary prevention after AMI hospitalisation.
Supplementary Material
Acknowledgments
We appreciate all the staff and investigators for their contributions to the CAMI Registry.
Footnotes
JL and CW contributed equally.
HX and YY contributed equally.
Contributors: HX and YYa contributed significantly to the conception, design and conduction of this study. JL and CW wrote the initial manuscript. CW, YZ and YW provided strong support in statistical analysis. JL, CW, XG, JY, XZ, YYe, QD, RF, HS, XY, YZ, YW, HX and YYa contributed significantly to revisions of the manuscript, analysis and interpretation of data. HX accepts responsibility for the overall content as a guarantor. The guarantor accepts full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish. All authors contributed substantially to this study and approved its submission.
Funding: This work was supported by the Twelfth Five-Year Planning Project of the Scientific and Technological Department of China (grant number: 2011BAI11B02) and Beijing Nova Program (grant number: Z211100002121063).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data are available upon reasonable request from the corresponding authors (Haiyan Xu, xuhaiyan@fuwaihospital.org; Yuejin Yang, yangyjfw@126.com).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Institutional Review Board Central Committee at Fuwai Hospital, National Center for Cardiovascular Diseases of China (Approval No 2012-431). Participants gave informed consent to participate in the study before taking part.
References
- 1. Peterson ED, Shah BR, Parsons L, et al. Trends in quality of care for patients with acute myocardial infarction in the national registry of myocardial infarction from 1990 to 2006. Am Heart J 2008;156:1045–55. 10.1016/j.ahj.2008.07.028 [DOI] [PubMed] [Google Scholar]
- 2. Yamamoto T, Yoshida N, Takayama M, et al. Temporal trends in acute myocardial infarction incidence and mortality between 2006 and 2016 in Tokyo - report from the Tokyo CCU network. Circ J 2019;83:1405–9. 10.1253/circj.CJ-19-0187 [DOI] [PubMed] [Google Scholar]
- 3. Chaudhry SI, Khan RF, Chen J, et al. National trends in recurrent AMI hospitalizations 1 year after acute myocardial infarction in medicare beneficiaries: 1999-2010. J Am Heart Assoc 2014;3:e001197. 10.1161/JAHA.114.001197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ergatoudes C, Thunström E, Rosengren A, et al. Long-term secondary prevention of acute myocardial infarction (SEPAT) - guidelines adherence and outcome. BMC Cardiovasc Disord 2016;16:226. 10.1186/s12872-016-0400-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao Q, Xu H, Zhang X, et al. Current status and hospital-level differences in care and outcomes of patients with acute non-ST-segment elevation myocardial infarction in China: insights from China acute myocardial infarction registry. Front Cardiovasc Med 2021;8:800222. 10.3389/fcvm.2021.800222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA 2004;291:2727–33. 10.1001/jama.291.22.2727 [DOI] [PubMed] [Google Scholar]
- 7. Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000;284:835–42. 10.1001/jama.284.7.835 [DOI] [PubMed] [Google Scholar]
- 8. Chang W-C, Boersma E, Granger CB, et al. Dynamic prognostication in non-ST-elevation acute coronary syndromes: insights from GUSTO-iib and PURSUIT. Am Heart J 2004;148:62–71. 10.1016/j.ahj.2003.05.004 [DOI] [PubMed] [Google Scholar]
- 9. Amin ST, Morrow DA, Braunwald E, et al. Dynamic TIMI risk score for STEMI. J Am Heart Assoc 2013;2:e003269. 10.1161/JAHA.112.003269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang W-C, Kaul P, Fu Y, et al. Forecasting mortality: dynamic assessment of risk in ST-segment elevation acute myocardial infarction. Eur Heart J 2006;27:419–26. 10.1093/eurheartj/ehi700 [DOI] [PubMed] [Google Scholar]
- 11. Westerhout CM, Pieper KS, James SK, et al. Dynamic modeling of 90-day mortality in ST-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention. Am Heart J 2013;165:354–62. 10.1016/j.ahj.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 12. Xu H, Li W, Yang J, et al. The China acute myocardial infarction (CAMI) registry: a national long-term registry-research-education integrated platform for exploring acute myocardial infarction in China. Am Heart J 2016;175:193–201. 10.1016/j.ahj.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 13. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012;126:2020–35. 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 14. Xu H, Yang Y, Wang C, et al. Association of hospital-level differences in care with outcomes among patients with acute ST-segment elevation myocardial infarction in China. JAMA Netw Open 2020;3:e2021677. 10.1001/jamanetworkopen.2020.21677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riley RD, Ensor J, Snell KIE, et al. Calculating the sample size required for developing a clinical prediction model. BMJ 2020;368:m441. 10.1136/bmj.m441 [DOI] [PubMed] [Google Scholar]
- 16. Fox KAA, Fitzgerald G, Puymirat E, et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open 2014;4:e004425. 10.1136/bmjopen-2013-004425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McNamara RL, Kennedy KF, Cohen DJ, et al. Predicting in-hospital mortality in patients with acute myocardial infarction. J Am Coll Cardiol 2016;68:626–35. 10.1016/j.jacc.2016.05.049 [DOI] [PubMed] [Google Scholar]
- 18. Song J, Murugiah K, Hu S, et al. Incidence, predictors, and prognostic impact of recurrent acute myocardial infarction in China. Heart 2020;107:313–8. 10.1136/heartjnl-2020-317165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boersma E, Pieper KS, Steyerberg EW, et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT investigators. Circulation 2000;101:2557–67. 10.1161/01.cir.101.22.2557 [DOI] [PubMed] [Google Scholar]
- 20. De Luca G, Suryapranata H, van ’t Hof AWJ, et al. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: implications for early discharge. Circulation 2004;109:2737–43. 10.1161/01.CIR.0000131765.73959.87 [DOI] [PubMed] [Google Scholar]
- 21. Nair R, Johnson M, Kravitz K, et al. Characteristics and outcomes of early recurrent myocardial infarction after acute myocardial infarction. J Am Heart Assoc 2021;10:e019270. 10.1161/JAHA.120.019270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chow CK, Brieger D, Ryan M, et al. Secondary prevention therapies in acute coronary syndrome and relation to outcomes: observational study. Heart Asia 2019;11:e011122. 10.1136/heartasia-2018-011122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shang P, Liu GG, Zheng X, et al. Association between medication adherence and 1-year major cardiovascular adverse events after acute myocardial infarction in China. J Am Heart Assoc 2019;8:e011793. 10.1161/JAHA.118.011793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rymer J, McCoy LA, Thomas L, et al. Persistence of evidence-based medication use after discharge from academic versus nonacademic hospitals among patients with non-ST-segment elevation myocardial infarction. Am J Cardiol 2014;114:1479–84. 10.1016/j.amjcard.2014.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mathews R, Peterson ED, Honeycutt E, et al. Early medication nonadherence after acute myocardial infarction: insights into actionable opportunities from the treatment with ADP receptor inhibitors: longitudinal assessment of treatment patterns and events after acute coronary syndrome (TRANSLATE-ACS) study. Circ Cardiovasc Qual Outcomes 2015;8:347–56. 10.1161/CIRCOUTCOMES.114.001223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Machón M, Aldasoro E, Martínez-Camblor P, et al. Socioeconomic differences in incidence and relative survival after a first acute myocardial infarction in the Basque country, Spain. Gac Sanit 2012;26:16–23. 10.1016/j.gaceta.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 27. Alter DA, Franklin B, Ko DT, et al. Socioeconomic status, functional recovery, and long-term mortality among patients surviving acute myocardial infarction. PLoS One 2014;8:e65130. 10.1371/journal.pone.0065130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bucholz EM, Ma S, Normand S-LT, et al. Race, socioeconomic status, and life expectancy after acute myocardial infarction. Circulation 2015;132:1338–46. 10.1161/CIRCULATIONAHA.115.017009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tetzlaff J, Tetzlaff F, Geyer S, et al. Widening or narrowing income inequalities in myocardial infarction? Time trends in life years free of myocardial infarction and after incidence. Popul Health Metr 2021;19:47. 10.1186/s12963-021-00280-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rothnie KJ, Smeeth L, Pearce N, et al. Predicting mortality after acute coronary syndromes in people with chronic obstructive pulmonary disease. Heart 2016;102:1442–8. 10.1136/heartjnl-2016-309359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cai X, Zhou J, Li W, et al. Potential influential factors of in-hospital myocardial reinfarction in ST-segment elevation myocardial infarction (STEMI) patients: finding from the improving care for Cardiovascular Disease in China- (CCC-) Acute Coronary Syndrome (ACS) project. Oxid Med Cell Longev 2021;2021:9977312. 10.1155/2021/9977312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun H, Li Z, Song X, et al. Revisiting the lipid paradox in ST-elevation myocardial infarction in the Chinese population: findings from the CCC-ACS project. Eur Heart J Acute Cardiovasc Care 2021;10:978–87. 10.1093/ehjacc/zuab053 [DOI] [PubMed] [Google Scholar]
- 33. Bonnett LJ, Snell KIE, Collins GS, et al. Guide to presenting clinical prediction models for use in clinical settings. BMJ 2019;365:l737. 10.1136/bmj.l737 [DOI] [PubMed] [Google Scholar]
- 34. Yan B, Su B-B, Bai D-S, et al. A practical nomogram and risk stratification system predicting the cancer-specific survival for patients with early hepatocellular carcinoma. Cancer Med 2021;10:496–506. 10.1002/cam4.3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ó Hartaigh B, Gransar H, Callister T, et al. Development and validation of a simple-to-use nomogram for predicting 5-, 10-, and 15-year survival in asymptomatic adults undergoing coronary artery calcium scoring. JACC Cardiovasc Imaging 2018;11:450–8. 10.1016/j.jcmg.2017.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao X, Liu C, Zhou P, et al. Estimation of major adverse cardiovascular events in patients with myocardial infarction undergoing primary percutaneous coronary intervention: a risk prediction score model from a derivation and validation study. Front Cardiovasc Med 2020;7:603621. 10.3389/fcvm.2020.603621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guo Q, Wu M, Li H, et al. Development and validation of a prognostic nomogram for myocardial infarction patients in intensive care units: a retrospective cohort study. BMJ Open 2020;10:e040291. 10.1136/bmjopen-2020-040291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zheng X, Huang R, Liu G, et al. Development and verification of a predictive nomogram to evaluate the risk of complicating ventricular tachyarrhythmia after acute myocardial infarction during hospitalization: a retrospective analysis. Am J Emerg Med 2021;46:462–8. 10.1016/j.ajem.2020.10.052 [DOI] [PubMed] [Google Scholar]
- 39. Wang H, Liu D, Liang H, et al. A nomogram for predicting survival in patients with colorectal cancer incorporating cardiovascular comorbidities. Front Cardiovasc Med 2022;9:875560. 10.3389/fcvm.2022.875560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chow CK, Jolly S, Rao-Melacini P, et al. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation 2010;121:750–8. 10.1161/CIRCULATIONAHA.109.891523 [DOI] [PubMed] [Google Scholar]
- 41. Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016;67:1–12. 10.1016/j.jacc.2015.10.044 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-069505supp001.pdf (2.3MB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data are available upon reasonable request from the corresponding authors (Haiyan Xu, xuhaiyan@fuwaihospital.org; Yuejin Yang, yangyjfw@126.com).