Abstract
Background and Aims
As more therapeutic options with their own characteristics become available for inflammatory bowel disease [IBD], drug development and individual treatment decision-making needs to be tailored towards patients’ preferences and needs. This study aimed to understand patient preferences among IBD patients, and their most important treatment outcomes and unmet needs.
Methods
This qualitative study consisted of [1] a scoping literature review, [2] two focus group discussions [FGDs] with IBD patients [n = 11] using the nominal group technique, and [3] two expert panel discussions.
Results
IBD patients discussed a multitude of unmet needs regarding their symptoms, side-effects, and psychological and social issues for which they would welcome improved outcomes. In particular, IBD patients elaborated on the uncertainties and fears they experienced regarding the possible need for surgery or an ostomy, the effectiveness and onset of action of their medication, and the medication’s long-term effects. Furthermore, participants extensively discussed the mental impact of IBD and their need for more psychological guidance, support, and improved information and communication with healthcare workers regarding their disease and emotional wellbeing. The following five characteristics were identified during the attribute grading as most important: prevent surgery, long-term clinical remission, improved quality of life [QoL], occurrence of urgency and improved labour rate.
Conclusions
This study suggests that IBD drug development and treatment decision-making are needed to improve IBD symptoms and adverse events that significantly impact IBD patients’ QoL. Furthermore, this study underlines patients’ need for a shared decision-making process in which their desired treatment outcomes and uncertainties are explicitly discussed and considered.
Keywords: Inflammatory bowel disease, patient, centric decision, making, qualitative patient preference study
1. Introduction
Inflammatory bowel disease [IBD] is a collective term for a range of clinical phenotypes caused by chronic, idiopathic, and remitting inflammation of the gastrointestinal tract. Crohn’s disease [CD] and ulcerative colitis [UC] are the two most common forms.1,2 IBD is a lifelong disease affecting more than 10 million people worldwide, without any cure currently being available.3,4 Symptoms include abdominal pain, chronic and relapsing episodes of [bloody] diarrhoea, urgency, nausea, vomiting, weight loss, anorexia, and fatigue, which negatively impact patients’ health and quality of life.5
Over the past few decades, a transition has taken place in the therapeutic landscape of IBD; where IBD patients were previously highly dependent on conventional therapies such as aminosalicylates, corticosteroids, and immunosuppressants, more advanced therapies are being developed and entering the market [e.g., biologicals and small molecules].6 IBD treatments in development and use today differ in terms of benefits, side-effects, route of administration, cost, mechanism and speed of action, treatment schedule, and uncertainties related to their treatment outcomes in the long and short term. As more therapeutic options with their own characteristics come on the market and are in the pipeline, it is increasingly being recognized that drug development and evaluation need to be tailored towards patients’ preferences and needs. In addition, it is increasingly expected that IBD patients are involved in individual treatment decision-making, whereby shared decision making [SDM] is considered the ideal model.7,8 This model encourages patients, in consultation with their clinician, to [1] consider the available screening, treatment or management options including their associated benefits, risks and uncertainties, [2] communicate their preferences, and [3] express their desire for involvement in decision-making.9 However, to facilitate SDM in the clinical encounter, a better understanding of the factors that IBD patients find important is needed.
Patient preference studies, usually consisting of a qualitative and a quantitative phase, can help to identify which treatment characteristics are important to patients, how important they are, which trade-offs patients are willing to make between various characteristics, how preferences may vary according to individual patient characteristics, and how much uncertainty patients will accept.10 The qualitative phase of a patient preference study can generate information about patients’ experiences and perspectives to gain in-depth and broad knowledge on the value of medical products and characteristics associated with them.11–13 In addition, qualitative research is recommended to inform the identification of attributes [treatment features] used in subsequent quantitative research.14,15
A systematic literature review by Bewtra et al.16 showed that previous patient preference studies among IBD patients were mostly quantitative in nature, did not use a particular qualitative data collection method [such as individual interviews or group discussions] and/or resulted in limited evidence on the perceived value of treatment attributes according to patients. In addition, these studies were conducted before newer treatments such as biologicals and Janus kinase [JAK] inhibitors were available, which have been described to affect patients’ preferences.16 Moreover, other published patient preference studies had a specific focus on preferences for surgery and medical management vs colectomy, had the goal to identify preferences for the administration of treatments, or were focused on a specific drug formulation.17–22
Given the lack of comprehensible and recent qualitative research on IBD patients’ preferences that considers different [novel] treatments and their associated characteristics, the present qualitative patient preference study aimed to investigate which patient-relevant treatment characteristics of both treatments in development and in use today IBD patients find important and why. More specifically, this study aimed to understand: [1] IBD patients’ unmet needs regarding their disease and treatment, and [2] treatment outcomes considered important by IBD patients. The findings from this study were furthermore intended to support the identification and development of attributes and levels for inclusion in a subsequent quantitative patient preference study to obtain more representative quantitative results in a larger sample of IBD patients. Furthermore, the findings may inform the development of a patient decision aid to facilitate SDM in clinical practice.
2. Materials and Methods
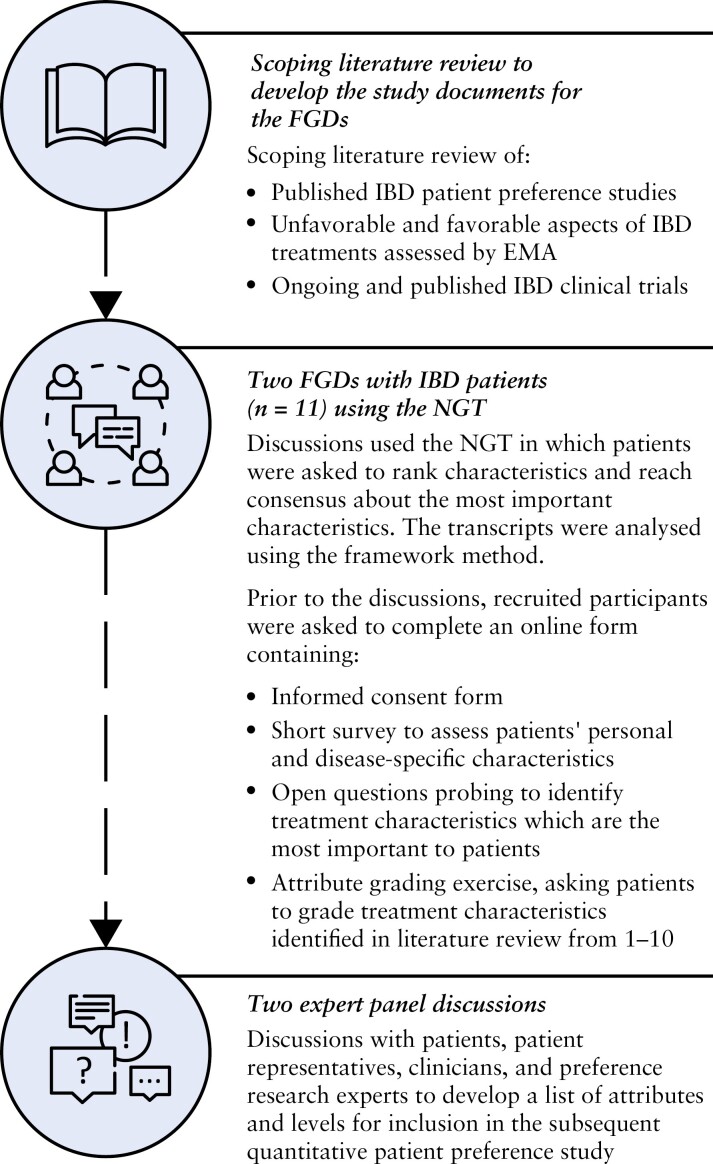
A qualitative study consisting of three phases was designed following the general steps described in the PREFER framework.23 The three phases [Figure 1] consisted of [1] a scoping literature review, [2] two focus group discussions [FGDs] with IBD patients [n = 11] using the nominal group technique [NGT], and [3] two expert panel discussions with patients [n = 2], patient representatives [n = 4], clinicians [n = 3], and preference research experts [n = 2]. The methodology and results of the FGDs were reported according to the recommendations of Hollin et al.14 for reporting qualitative preference research, and the recommendations of Coast et al.15 and Bridges et al.24 on collecting and analysing methods for initial attribute development.
Figure 1.
Design of the qualitative study in three phases, where each phase informed the subsequent phase. FGD, focus group discussion; IBD, inflammatory bowel disease; NGT, nominal group technique; EMA, European Medicines Agency.
2.1. Scoping literature review to develop the study documents for the FGDs
To develop the attribute grading exercise and other study documents for the FGDs, a scoping literature review was performed of [1] attributes and key results of previous published preference studies conducted among IBD patients, [2] favourable and unfavourable aspects of IBD treatments already being prescribed to patients as assessed by the European Public Assessment Reports [EPARs] of the European Medicines Agency [EMA] or as indicated on the products’ leaflet, and [3] primary and secondary endpoints and adverse events of phase 3 IBD clinical trials in the European Union, to ensure also potential ‘future’ treatment outcomes and adverse events could be integrated in the discussion. A detailed description of the databases, search queries, and inclusion and exclusion criteria can be found in Supplementary Appendices 1–3.
2.2. Focus group discussions
2.2.1. Participant recruitment
Ethical approval was obtained from the Ethische Commissie Onderzoek UZ/KU Leuven in Belgium [S65034]. Participants were recruited in April 2021 by an IBD nurse at the University Hospitals of Leuven. Patients who were 18 years or older, diagnosed with IBD [CD or UC] and able to give informed consent were eligible to participate. Because individual patient characteristics could have an influence on participants’ opinions and gradings, and the goal was to identify characteristics important across the IBD patient population, purposive sampling was used to reach heterogeneity among participants with regard to age, subtype of IBD, disease stage [active disease/remission] and treatment experience [including surgery].25 Following recommendations of McMillan et al.,26 recruitment sought to include between five and seven IBD patients per FGD.
2.2.2. Completion of the online form
As preparation for the FGDs, participants were invited to read the information sheet [Appendix 4] and to complete the online form, which consisted of [1] the informed consent form [Appendix 5 Part A], [2] a short survey to assess patients’ personal and disease-specific characteristics, and health literacy [Appendix 5 Part B], [3] open questions to identify treatment characteristics important to participants [Appendix 5 Part C], and [4] a grading exercise in which participants needed to grade the treatment characteristics identified in the scoping literature review from 1 to 10, with 10 being the most important [Appendix 5 Part D]. All participants completed the online form and gave their informed consent prior to the discussion. There was no drop-out due to the length of the survey.
2.2.3. Conduct of the FGDs using the NGT
FGDs using the NGT were chosen as the preference exploration method in close dialogue with clinicians and patient representatives because there was interest in group dynamics and the subject of the discussion was not deemed to be too sensitive to discuss with others. The NGT is a consensus methodology where participants prioritize their thoughts and perspectives, both as a group and individually, with the advantage of reaching a consensus in a short period of time. As the NGT is structured in four stages [idea, round-robin, clarification and finalization, grading and consensus], different techniques are combined including open questions to detect new characteristics not identified during the literature review [bottom-up] and a grading exercise and discussion to question participants about the importance of characteristics identified in the literature [top-down]; the grading allows researchers to select and understand which treatment characteristics are most important and should be integrated in a subsequent preference survey.26
During the FGDs, a predefined FGD guide [Appendix 6] was followed taking into account the four steps of the NGT. Each discussion was executed by the same moderator [E.S.] and in the native language of the participants [Flemish]. Considering the implications of the coronavirus [COVID-19] pandemic, the discussions were organized online. The discussions lasted around 1 h 45 min and were audio-recorded. The content of the online form and the FGD guide was provided with written feedback from a gastroenterologist and a member of a patient organization.
2.2.4. Analysis of the focus group discussions
2.2.4.1. Quantitative descriptive analysis
Participants’ self-reported personal and disease-specific characteristics, and their scores on the attribute grading exercise were analysed descriptively. Health literacy was determined using the Chews’ set of brief screening questions.27 To obtain a final rank of the treatment characteristics, the average, standard deviation, minimal score, and maximal score were calculated for each characteristic by combining the grades of all participants.
2.2.4.2. Qualitative thematic analysis
The audio-recordings of all FGDs were transcribed ad verbatim in the original language by a member of the research team [E.S.] and pseudonymized. Subsequently, the transcripts were subjected to the framework analysis method [using NVivo software], a qualitative content analysis for text data in which overarching themes are developed.28 Analysis started with familiarization through the conduct, transcription, and reading of the FGDs. Themes of the discussion guide informed the creation of deductive codes. Analytical notes were written in the margins of the transcripts and informed the creation of inductive codes. Based on the deductive and inductive codes, a ‘coding tree’ with characteristics was developed. Regular meetings between the researchers who conducted the FGDs [E.S., R.S., and I.H.] were held to verify the characteristics were interpreted in the same manner. After consensus was reached, the coding tree was uploaded in NVivo, and applied to all transcripts, where literal quotations of transcripts relating to a particular theme were classified under the respective code. NVivo was used for charting [summarizing] the data per code in order to have an oversight off all results, making interpretation and analysis of the results both between participants and between FGDs possible.
2.3. Expert panel discussions
After the conduct of the FGDs, expert panel discussions with patients, patient representatives, clinicians, and preference research experts were held to verify the outcomes of the FGDs and to provide further depth into the identified themes. Furthermore, insights were gathered regarding possible attributes and levels for inclusion in the subsequent quantitative patient preference study, taking into account the criteria and steps as described by Durosini et al.,29 Petrocchi et al.,30 and Janssens et al.31
3. Results
3.1. Scoping literature review to identify characteristics potentially relevant to IBD patients
In total, 22 patient preference studies, 58 phase 3 IBD clinical trials, and 45 IBD treatments fulfilled the inclusion criteria. From the included publications, data were extracted and summarized [Supplementary Appendices 1–3]. Based on the results of the literature review, the attribute grading exercise was created, wherein 55 identified characteristics were grouped into three overarching categories, namely characteristics related to the treatment efficacy, characteristics related to the administration of the treatment, and characteristics related to symptoms of the disease or complications and adverse events of treatments [Appendix 5 Part D].
3.2. FGDs to identify patient-relevant characteristics
3.2.1. Participants’ characteristics
First contact was established with 13 IBD patients of whom 11 joined the FGDs, resulting in a response rate of 84%. The mean age was 37 years (age range: 27–56; median age: 38 years; interquartile range [IQR]: 28.5–42 years) [Appendix 7]. On average, there was a 10.5-year gap between diagnosis and participation in the FGD [age at diagnosis range: 16–35 years, median age at diagnosis: 26 years; IQR: 23.5–30 years]. There was an imbalance in IBD subtype [73% diagnosed with UC]. More than half of the participants [55%] had undergone surgery due to disease complications. Health literacy was adequate in all participants.
Of all participants, three patients were in remission with medical treatment and one participant was not taking any medication on advice of his treating physician. Participants were most frequently treated with biologicals only [n = 7]. Other than biologicals alone, participants were taking a combination of: aminosalicylates with JAK inhibitors [n = 1], corticosteroids with immunosuppressants and biologicals [n = 1], and aminosalicylates with corticosteroids [n = 1]. Table 1 gives an overview of the treatment experience of the participants.
Table 1.
Treatment experience of participants [n = 11]
| Medicinal product classesa | Number of participants taking this class of medication at time of participation | Number of participants who have taken this class of medication [past and present] |
|---|---|---|
| Aminosalicylates | 2 [18%] | 6 [55%] |
| Corticosteroids | 2 [18%] | 11 [100%] |
| Immunosuppressants | 1 [9%] | 8 [73%] |
| Biologicals | 8 [73%] | 9 [82%] |
| Janus kinase inhibitors | 1 [9%] | 1 [9%] |
aTaken alone or in combination with other medicines from the mentioned classes.
3.2.2. Unmet needs and treatment outcomes considered important by IBD patients
IBD patients discussed a multitude of treatment outcomes and unmet needs for which they would welcome improvements both in the context of drug development as well as in the context of decision-making in clinical practice.
3.2.2.1. Gastrointestinal symptoms
Gastrointestinal symptoms [e.g., abdominal pain and cramps, diarrhoea, urgency, incontinence, nocturnal defecation, bowel movements and constipation] had a significant effect on the participants’ life and was seen as detrimental in three ways. First, some participants frequently experienced nocturnal defecation, having an incremental effect on their sleep and therefore causing reduced energy [as further described in ‘Reduced energy’]. Second, work, sports, or social activities could be hampered, limiting their freedom. Last, knowing that gastrointestinal symptoms could appear at any time had an important psychological impact, making participants worrisome about always having a toilet nearby. Therefore, an improvement in both the frequency and the severity of gastrointestinal problems was seen as the most important treatment outcome for all participants: “Something that really comes at 1 actually.” #FG1_E.
3.2.2.2. Reduced energy
The theme of reduced energy caused by participants’ therapy and illness was extensively discussed in both FGDs and was experienced by the majority of the participants. Reduced energy could hinder participants’ ability to participate normally in daily activities, limiting their quality of life. Therefore, participants wanted to avoid reduced energy caused by their therapy: “For me fatigue is something I could not accept as a side-effect.” #FG1_E. Nevertheless, reduced energy could not be avoided for every participant and therefore patients had to adapt their lifestyle around this problem. As a consequence, a couple of participants were not able to exercise, which was seen as another important limiting factor. Reduced energy and fatigue could also result from sleeping problems participants experienced due to their disease or nocturnal defecation, but other causes of reduced energy were also shared [e.g., following psychological problems patients were struggling with, or weight fluctuations].
3.2.2.3. Changes in physical appearance
Patients highlighted that changes in physical appearance, caused both by their altered gut function or as a side-effect of their therapy, made them insecure about their body image. In particular, participants treated with corticosteroids had experienced a spectrum of changes [namely moon face, developing facial hair, hair loss, and weight changes] in their physical appearance, which had a detrimental impact on their mental wellbeing. For these participants, the side-effects outweighed the benefits of their treatment.
Among the several types of physical changes that emerged during the discussions, [excessive] weight fluctuations were seen as the most important, and the topic seemed to receive particular attention and discussion from female participants. Weight fluctuations caused both by participants’ therapy or by altered gut function were found to be detrimental because they made participants uncertain and had a negative effect on their energy levels. Therefore, all participants found it important that their weight was more or less under control. One participant even stated that weight gain was the most important treatment characteristic, and if a choice had to be made between different treatment options, the treatment in which weight gain might occur as a side-effect should be dropped first.
3.2.2.4. Skin manifestations
Several participants previously experienced skin-related issues as a side-effect of their treatment. In general, participants were able to cope with less severe skin manifestations. Nevertheless, the severity in which this side-effect occurred determined how much participants valued this treatment characteristic: “If your whole body gets covered [with skin lesions], you start to look at it differently. So, I also think that it depends a little bit in which degree the side-effect occurs.” #FG1_B.
3.2.2.5. Vision problems
One participant stated to have a deteriorated eyesight: “I am suffering from tear duct inflammation. [ … ] Whether that’s linked to Crohn’s or my treatment with corticosteroids, I don’t know.” #FG1_B. However, it remained largely unclear whether vision problems could also be an extra-intestinal manifestation associated with having a chronic autoimmune disease such as IBD. As participants knew that some medications could cause eye infections or other visual disturbances, having [permanent] vision problems was seen as an important treatment characteristic.
3.2.2.6. Risk of surgery
Most of the participants saw surgery as their last resort and were resistant to it: “I think surgery is often the last step.” #FG2_D. Despite this, more than half of the participants had undergone surgery in the past because there was no alternative. Participants, especially those diagnosed with CD, knew that repeatedly removing sections of their gut was not sustainable in the long term and were afraid that their bowels would become too short to take in enough nutrients. In addition, they looked at it as ‘a path without an end’. Nevertheless, some participants stated that their symptoms were more or less under control after they underwent surgery.
3.2.2.7. Risk of an ostomy
The need for an ostomy was extensively discussed during the two focus groups, with some participants expressing their fear about ever needing one. An ostomy was something they wanted to avoid at all costs because it would be an additional limitation and would hurt their self-image. Additionally, some participants raised their concerns about needing additional care if they received an ostomy. However, others stated that they did not really think about ever needing one. Nonetheless, one participant with CD had a permanent ostomy and was very pleased with it: “I was 24 when I got my stoma and I have really resisted it. I already had every kind of medication [ … ]. And then I first had a temporary stoma and even then, I felt that burden fell of my shoulders. That I did not have to run to the toilet, that I could go back to places without worrying [ … ]. The only thing is that my disease is still not 100% under control.” #FG2_D. In addition, this participant mentioned that the ostomy enabled him/her to regain a normal life without having the restrictions from the disease.
3.2.2.8. An effective and fast working medication
On several occasions, participants discussed the psychological burden of uncertainties regarding the effectiveness and onset of action of their medication. Participants found it frustrating to start a new treatment, not knowing if the medication was ever going to work for them and for how long it was going to work before losing its efficacy. As a result, most participants had tried various medications until they found [or were still searching for] the one that worked for them. In addition, participants complained about the uncertainty regarding the onset of action of new treatments. This had a negative impact in two ways: [1] they did not know if the medication would eventually work for them, and whether they would need to switch to another medication, or whether they would need to give it more time to be effective, and [2] during this bridging period, participants’ symptoms remained. Therefore, one participant explicitly stated that a fast clinical response was the most important treatment characteristic.
3.2.2.9. Normal social interactions and activities
All participants experienced limitations on their social interactions and activities due to their disease and its unpredictable nature. Making plans, such as going on a holiday, was seen as difficult by some participants. In addition, some participants stated that they frequently needed to cancel activities with their friends because they were not feeling well or were experiencing pain, and were therefore forced to stay at home, resulting in isolation and loneliness. For some participants, this even resulted in deterioration of relationships with their friends or losing their job. Therefore, participants found it key that their treatment enabled them to have a normal social life in which they were able to keep their social contacts, without being restricted by their [gastrointestinal] symptoms. Furthermore, some participants clearly addressed the lack of understanding of people in their surroundings for their situation and disease. Participants attributed this to the unawareness of IBD and all that it entails: “Very often people say, ‘it’s all in your head’, or ‘you ate something bad’, or … there is little understanding of the fact that sometimes you can sit on the toilet for more than half an hour.” #FG2_A.
3.2.2.10. Mental and psychological support
In general, participants tried to maintain a positive mindset: “No matter how sick I’ve been, I did always persevere and believe in it.” #FG1_B. However, many participants suffered greatly from their disease; the disease, to some extent, dictated what they could or could not do in their daily lives. As a result, this had considerable impact on the participants mental wellbeing and some patients were even struggling with mental disorders [e.g., loneliness, anxiety, depression]. Therefore, multiple participants indicated that they needed professional mental and psychological help to cope with their disease: “I have been at the point where I thought: ‘I just can’t get out of here, not mentally, not physically’. That I also sat with very dark thoughts.” #FG2_D. Participants found that too little attention in the clinical encounter was given to the whole mental aspect of the disease and would liked to have had more guidance. They sometimes experienced that clinicians did not have a full understanding of the impact of the disease on their daily lives. As a result, participants extensively discussed the lack of mental support some of them needed. Some participants stated that their clinician did advise them to contact a psychologist if they needed to. However, participants felt that once they left the hospital, they were completely on their own.
3.2.2.11. Information regarding the effect of the disease and treatments in the long and short term
Some participants mentioned the lack of information they received on how their disease would progress and felt uncertain about the long-term effects of their medication, as these effects are relatively unknown. Participants found it very important that they would not struggle with other diseases later in their lives and some participants expressed their fear about the possible emergence of cancer. Despite this, participants still used these medication as they wanted to stay in remission. In addition, some participants had tried so many different medications, they had no other options: “Sometimes you are desperate, and you want to feel better. So, at that moment it was clear to me: I want to get better now, and we’ll see what comes in the future.” #FG2_D. Furthermore, participants would have liked to know more from the beginning on how the disease would affect their life in understandable, layman’s terms: “You do get medical information about what Crohn’s disease is, but you don’t really get to know how to live with it.” #FG2_A. In an ideal situation, most participants would have liked to be put into contact with other patients who could share their experiences. Although participants knew that there was limited literature on the effect of different types of nutrition on their symptoms, they would like to receive more information on what they could or could not eat.
3.2.3. Attribute grading
In addition to the open discussion revealing patients’ unmet needs and treatment outcomes, the attribute grading allowed to explore and trigger further discussion on which treatment and disease-related characteristics matter most to patients. In general, most participants gave a very high score to all the 55 treatment characteristics on the attribute grading exercise [bottom-up] and several participants stated during the discussion of the list that they found all characteristics important. The top ten characteristics, based upon the attribute grading exercise, are shown in Table 2 [full results can be found in Appendix 8]. To a large extent, these characteristics align with the qualitative results; gastrointestinal problems, characteristics related to quality of life, and surgery received very high scores [see Section 3.2.2]. Characteristics that were relatively less important were related to the administration of the treatment, namely treatment frequency, place of treatment, and route of administration, which were also not spontaneously raised by patients during the open discussions. The attribute grading exercise also revealed that the characteristics included in the grading exercise did not include the level of detail and full range of aspects patients freely elaborated on in the open discussion [see Section 3.2.2]; for example, patients indicated and elaborated on the influence of IBD on their mental wellbeing, a characteristic not included in the attribute grading exercise.
Table 2.
Top ten characteristics identified in the attribute grading exercise
| Rank | Characteristic | Mean score | Standard deviation | Maximal score | Minimal score |
|---|---|---|---|---|---|
| 1 | Prevent surgery | 9.55 | 0.82 | 10 | 8 |
| 2 | Long-term clinical remission | 9.45 | 1.21 | 10 | 6 |
| 3 | Improved quality of life | 9.36 | 1.03 | 10 | 7 |
| 4 | Occurrence of urgency | 9.27 | 1.01 | 10 | 7 |
| 5 | Improved labour rate | 9.27 | 1.10 | 10 | 7 |
| 6 | Occurrence of diarrhoea | 9.18 | 1.08 | 10 | 7 |
| 7 | Occurrence of severe infections | 9.18 | 1.25 | 10 | 6 |
| 8 | Prevent hospitalization | 9.09 | 1.45 | 10 | 6 |
| 9 | Occurrence of joint pain | 9.00 | 1.26 | 10 | 6 |
| 10 | Prevent flare-ups | 9.00 | 1.79 | 10 | 4 |
While some participants stated that their reason for scoring an attribute was based on long-term reasoning, other participants only looked at the present. However, scoring could also be influenced by patients’ previous treatment or disease experience. In particular, whether they had experienced a certain symptom or side-effect could have determined their grading. In addition, some patients stated that when side-effects were temporary or could be resolved with other medication, they became manageable and therefore less ‘important’.
3.3. Expert panel discussions to ensure the relevance and correct interpretation of the identified characteristics
Two expert panel discussions were held with [1] patients [n = 2] and patient representatives [n = 4] to verify the importance, relevance, comprehensiveness and understandability of the identified characteristics, [2] clinicians [n = 3] to ensure plausibility from a clinical perspective, and [3] preference research experts [n = 2] to give insights regarding possible attributes and levels compliant with the corresponding rules.14,15 During the expert panel discussions, the unmet needs and treatment outcomes important to IBD patients as identified during the FGDs were presented and the relevance and correct interpretation thereof was confirmed. In particular, in-depth discussions were held regarding the following characteristics: reduced energy, mental disorders that patients may struggle with, and the impact of IBD on patients’ social interactions and activities. As an outcome, the expert panel discussions led to more detail and insights into the identified themes.
4. Discussion
This qualitative study revealed in-depth insights into the symptoms, side-effects, psychological and social issues that IBD patients find important. During the open discussions, patients highlighted gastrointestinal symptoms, reduced energy, changes in physical appearance, skin manifestations, and vision problems as being major issues reducing their quality of life. In addition, they elaborated on the uncertainties and fears they experienced regarding the possible need for surgery and in particular an ostomy. The effectiveness and onset of action of medication, and its long-term effects were further identified as major concerns. Lastly, the mental impact of IBD and the need for more psychological guidance, support, and improved communication with healthcare workers regarding patients’ emotional wellbeing was reported as key.
Our findings show that IBD patients are confronted with both physical and psychological issues that require further support, which has been confirmed in several studies investigating the impact of IBD on patients’ health-related quality of life. For example, Spagnuolo et al.32 assessed the satisfaction with social roles and physical function in IBD patients by evaluating patient-reported outcomes [PROs] and revealed that IBD patients had an impaired ability to perform normal daily activities compared to a control group. Likewise, Iaquinta et al.33 collected PRO data and concluded that IBD patients have increased fatigue and anxiety compared with healthy individuals. The study from Dudley-Brown et al.34 identified the following main themes corresponding to our study findings: uncertainty surrounding exacerbations, the desperate need to find successful treatment, and the feeling of being controlled by the disease.
A possible way to cope with patients’ uncertainties is to improve communication with healthcare workers; patients who are informed about the side-effects of medication and about the possible need for surgery or an ostomy may experience less uncertainty about their future disease progression. In addition, if patients are given the opportunity to ask for help and advice, symptoms and side-effects are probably better managed. Based on patients’ individual needs, there are three possible means to support IBD patients. First, patients may find support in IBD associations where they can receive reliable support from other patients. As confirmed during our FGDs, participants were overall very happy to have participated in the discussion and share their experiences, feelings, and thoughts with other IBD patients as this took away some of their insecurities. Furthermore, Sewitch et al.35 showed that improving social support can have a favourable impact on psychological distress, ultimately improving health outcomes. Second, staff could be trained in IBD centres to provide more psychological support in the IBD medical care pathway. Third, the results of this study can be used to develop patient decision aids [flyers, online tools, etc.] that can be integrated in IBD clinical practice to support SDM. During the discussions, patients mentioned a multitude of treatment outcomes and unmet needs that should be discussed in clinical practice to an enable informed decision-making. For example, patients mentioned the need for more information regarding the effect of the disease and their treatments in the long and short term, suggesting an opportunity for the development of patient decision aids that can inform patients about these aspects. As a result, communication between the patient and the healthcare provider can be facilitated and uncertainties can be addressed. Furthermore, by aligning individual medical treatment decisions with patients’ needs and preferences, better quality decisions, improved patient compliance, and thereby improved patient overall health can be achieved.36,37
Comparing the disease and treatment characteristics found in this study to those identified in previous IBD patient preference studies [Appendix 1] reveals that some attributes overlap, while others were new. Notably, almost all previous studies had a specific scope and involved both patients and gastroenterologists, making it not clear which attributes were raised as important by whom. In contrast, our study identified patient-relevant characteristics across different therapies, with an open scope, directly from IBD patients. Differences can further be explained by the fact that qualitative studies are highly context-specific in view of the research setting. As a result, different variables such as the selected patient sample, the time of the study and the specific questions asked may explain a difference in the identified attributes, making comparison between the results of different qualitative patient preference studies difficult. Therefore, the results need to be interpreted considering the specific period and context under which the study took place, as well as the type of participants who took part. For example, this study took place during the COVID-19 pandemic, wherein the social isolation participants experienced during the pandemic may have amplified the need for and frequency with patients expressed their wish for more mental support. The results should also be viewed in the specific drug therapy context and the influence of the individual drug therapy experience of participants on the identified symptoms and side-effects; the IBD treatment landscape is rapidly evolving, with new therapies that have entered the market or are emerging. These new treatments have different [long-term] side-effects and efficacy profiles from those currently available. This may explain why patients expressed uncertainties regarding the effectiveness and onset of action of their medication, and the long-term side-effects such as the increased risk of cancer, particularly regarding these treatments that have only being marketed and described for a relative short time.
4.1. Implications and further use
The characteristics identified in this study will be useful in informing the design of a subsequent quantitative online patient preference survey to obtain insights from a geographically dispersed and heterogeneous sample of IBD patients. Such a survey will enable to obtain quantitative insights regarding the relative importance of attributes, and whether patient characteristics [e.g., type of treatments received, subtype of IBD, disease state, and experience with symptoms] have a statistically significant impact on patients’ preferences. Furthermore, the results of this qualitative study can aid the development of patient decision aids used to facilitate treatment decision-making in clinical practice by revealing which aspects of treatments [e.g., long-term efficacy and safety of treatments] should be explicitly discussed in clinical practice and communicated to patients.
The identified characteristics important to IBD patients may further be useful to assist and optimize the identification of clinical trial endpoints and outcomes that matter most to IBD patients. Including such outcomes in clinical trials could enable an understanding of how treatments perform regarding patient-relevant outcomes. Obtaining evidence on the extent to which [new] drugs perform on relevant outcomes within and outside the clinical trial setting are two questions which are difficult to resolve at the time of regulatory risk–benefit assessment and Health Technology Assessment [HTA] evaluation. Such evidence could hence support regulators and HTA assessors in understanding whether [novel] IBD treatments address unmet needs and treatment outcomes that matter to patients, and thereby support initial and post-marketing risk–benefit and reimbursement decisions. Qualitative and quantitative preference results could also be considered to inform [joint] scientific advice discussions by HTA bodies and regulators on the relevance of the clinical trial endpoints proposed by industry applicants, for example allowing us to understand whether relevant measures of quality of life are included in clinical trials.23 In addition, resource allocation could be optimized to drugs that target the symptoms and side-effects found important to patients. This could result in a drug development process more tailored to patients’ needs.13,23,38,39 Additionally, the concerns of IBD patients about uncertainties of long-term efficacy and safety can inform future real-world evidence studies.
4.2. Strengths and limitations
By performing discussions with patients as drug end-users to understand their experiences with both their illness and their treatment, this study demonstrates the value of performing a qualitative patient preference study. Furthermore, the use of FGDs with open questions allowed us to obtain both broad and in-depth information on the treatment and disease-related characteristics that IBD patients find most important. In addition, the plausibility, quality, and comprehensiveness of both the patient material and the study, as well as the interpretation of the results, was ensured by collaborating with gastroenterologists and members of patient organizations before and after the conduct of the FGDs.
Patients with different treatment experiences who may have been in remission at the time of the discussions were included in the study. As a result, participant heterogeneity was introduced to avoid biases in the data; it was envisaged that these individual patient characteristics and experiences could have an influence on participants’ opinions. As a consequence, interactions between patients with a different disease and treatment background emerged, ensuring the identified characteristics are important for patients along the IBD spectrum. Likewise, an extensive literature review was performed to ensure patients also discussed potential ‘future’ treatment outcomes and side-effects of novel therapies, as well as favourable and unfavourable aspects associated with therapies already being prescribed to IBD patients, and thus not just treatment outcomes and side-effects participants have already experienced themselves.
We did not differentiate the results according to drug therapy and IBD subtype as the goal was to provide an overarching view of important themes to IBD patients across therapies. Furthermore, note that during the FGDs, it was not always possible to differentiate the raised characteristics between symptoms, side-effects of treatments, or extra-intestinal manifestations associated with having a chronic autoimmune disease such as IBD. This further underlines the complex aetiology of IBD and the limited amount of published information on clinical evidence, resulting in uncertainty regarding the causes of problems that patients experience. Hence, more insights regarding the underlying mechanisms of IBD and the side-effects of different therapies would be useful in order to link adverse events to certain therapies, symptoms of the disease, or extraintestinal manifestations, which could increase treatment adherence and promote the treatment of IBD patients in general.
This study was performed during the COVID-19 pandemic, wherein the social distancing and hospital guidelines in Belgium did not allow face-to-face FGDs. Therefore, the discussions were organized online. This may have resulted in participants withholding from interacting with others. Furthermore, it is likely that participants who were not comfortable with online discussions were less likely to participate [e.g., older participants]. However, the median age of diagnosis of UC patients in this study was 28.3 years and of CD patients 25.3 years, which is respectively 6.6 and 3.7 years younger than the average age of diagnosis.40 Furthermore, as only two FGDs were performed and the discussions only involved participants living in Belgium, it cannot be determined if data saturation was reached and if the results can be extrapolated to patients outside Belgium. Therefore, we speculate that the inclusion of additional patients from other countries could affect the derived themes.
Finally, it should be noted that the qualitative analysis of the FGDs was conducted by one researcher as opposed to multiple researchers, meaning that no cross-check was performed. Nevertheless, regular meetings between the researchers who conducted the FGDs minimized subjective interpretation of the data.
5. Conclusion
This study shows that IBD drug development and individual treatment decision-making does not only need to focus on controlling IBD patients’ gastrointestinal symptoms, but also on other symptoms and adverse events that significantly impact IBD patients’ quality of life. Furthermore, this study underlines the need for shared decision making between healthcare providers and IBD patients, where treatment outcomes and uncertainties about long-term efficacy and safety of medication are explicitly addressed and considered. The findings of this study may serve pharmaceutical industry, regulators, and HTA bodies/payers in understanding patient-relevant unmet needs and treatment outcomes to enable value-based and patient-centric decision-making that is aligned with patients’ actual needs across the IBD drug life cycle.
Supplementary Material
Acknowledgments
The authors are extremely grateful to the patients participating in the focus group discussions, for sharing their experiences and valuable contributions. We would like to thank the Belgian Inflammatory Bowel Disease Research and Development (BIRD) for their support in reaching out to clinicians and patients. Marc Ferrante and João Sabino are Senior Clinical Investigators of the FWO, Belgium.
Contributor Information
Elise Schoefs, Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium.
Séverine Vermeire, Department of Gastroenterology and Hepatology, University Hospitals Leuven, KU Leuven, Leuven, Belgium; Department of Chronic Diseases, Metabolism and Aging, KU Leuven, Leuven, Belgium.
Marc Ferrante, Department of Gastroenterology and Hepatology, University Hospitals Leuven, KU Leuven, Leuven, Belgium; Department of Chronic Diseases, Metabolism and Aging, KU Leuven, Leuven, Belgium.
João Sabino, Department of Gastroenterology and Hepatology, University Hospitals Leuven, KU Leuven, Leuven, Belgium; Department of Chronic Diseases, Metabolism and Aging, KU Leuven, Leuven, Belgium.
Tessy Lambrechts, Department of Gastroenterology and Hepatology, University Hospitals Leuven, KU Leuven, Leuven, Belgium.
Luisa Avedano, European Federation of Crohn’s & Ulcerative Colitis Associations (EFCCA), Brussels, Belgium.
Isabella Haaf, European Federation of Crohn’s & Ulcerative Colitis Associations (EFCCA), Brussels, Belgium.
Maria Stella De Rocchis, European Federation of Crohn’s & Ulcerative Colitis Associations (EFCCA), Brussels, Belgium.
Andrea Broggi, European Federation of Crohn’s & Ulcerative Colitis Associations (EFCCA), Brussels, Belgium.
Magdalena Sajak-Szczerba, European Federation of Crohn’s & Ulcerative Colitis Associations (EFCCA), Brussels, Belgium.
Roberto Saldaña, European Federation of Crohn’s & Ulcerative Colitis Associations (EFCCA), Madrid, Spain.
Rosanne Janssens, Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium.
Isabelle Huys, Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium.
Conference
A part of this work was presented as a poster presentation at DIA Europe 2022 on March 30, 2022.
Funding
This work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of Interest
S.V. reports research support from AbbVie, J&J, Pfizer, Galapagos, Takeda, and consultancy and/or speaker fees from AbbVie, AbolerIS Pharma, AgomAb, Alimentiv, Arena Pharmaceuticals, AstraZeneca, Avaxia, BMS, Boehringer Ingelheim, Celgene, CVasThera, Dr Falk Pharma, Ferring, Galapagos, Genentech-Roche, Gilead, GSK, Hospira, Imidomics, Janssen, J&J, Lilly, Materia Prima, MiroBio, Morphic, MrMHealth, Mundipharma, MSD, Pfizer, Prodigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire, Surrozen, Takeda, Theravance, Tillots Pharma AG, Zealand Pharma. M.F. reports research support from Abbvie, Amgen, Biogen, Janssen, Pfizer, Takeda and Viatris, consultancy fees from Abbvie, Boehringer-Ingelheim, Celltrion, Janssen, Lilly, Medtronic, MSD, Pfizer, Sandoz, Takeda, Thermo Fisher, speaker fees from Abbvie, Amgen, Biogen, Boehringer-Ingelheim, Falk, Ferring, Janssen, Lamepro, MSD, Pfizer, Sandoz, Takeda, Truvion Healthcare, Viatris. JS reports consultancy fees from Janssen and Fresenius, and speaker fees from Abbvie, Takeda, Ferring. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
E.S., R.J. and I.Hu were involved in the design of the study. E.S. designed the study materials. E.S., T.L., S.V., M.F. and J.S. were involved in the recruitment of the participants. E.S., R.J. and I.Hu held the FGDs and analysed results. E.S., R.J., I.Hu, S.V., M.F., J.S., L.A., I.Ha, S.dR., A.B., M.S. and R.S. participated in meetings and reviewed the study materials. E.S. produced the first draft of the manuscript, which was subsequently revised and finalized with all authors. All the authors have read and approved the final manuscript.
Ethics Approval and Consent to Participate
This study was reviewed and approved by the Ethische Commissie Onderzoek UZ/KU Leuven [Belgium; reference S65034]. The patients/participants provided their informed consent to participate in this study.
Data Availability
The datasets presented in this article are not publicly available because they contain information that could compromise participants’ privacy and consent. Requests to access the datasets should be directed to elise.schoefs@kuleuven.be.
References
- 1. Pullen N, Gale JD.. Inflammatory Bowel Disease. Amsterdam: Elsevier; 2007: 613–42. [Google Scholar]
- 2. Damião AOMC., de Azevedo MFC, de Sousa Carlos A, Wada MY, Silva TVM, de Castro Feitosa F.. Conventional therapy for moderate to severe inflammatory bowel disease: a systematic literature review. World J Gastroenterol 2019;25:1142–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lemmens B, de Hertogh G, Sagaert X.. Inflammatory Bowel Diseases. Amsterdam: Elsevier Inc.; 2014. [Google Scholar]
- 4. Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res 2019;2019. doi: 10.1155/2019/7247238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Badia X, Vilaseca J, Casellas F, Lo J.. Influence of inflammatory bowel disease on different dimensions of quality of life. Eur J Gastroenterol Hepatol 2001;567:72. [DOI] [PubMed] [Google Scholar]
- 6. Cohen NA, Rubin DT.. New targets in inflammatory bowel disease therapy: 2021. Curr Opin Gastroenterol 2021;357:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barry MJ, Edggman-Levitan S.. Shared Decision Making-The Pinnacle of Patient-Centered Care. NEJM. 2012. [DOI] [PubMed] [Google Scholar]
- 8. Charles C, Gafnv A, Whelan T.. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44:681–92. [DOI] [PubMed] [Google Scholar]
- 9. Stiggelbout AM, van der Weijden T, de Wit MPT, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ [Online]2012. Doi: 10.1136/bmj.e256 [DOI] [PubMed] [Google Scholar]
- 10. FDA. Patient preference information – voluntary submission, review in premarket approval applications, humanitarian device exemption applications, and de novo requests, and inclusion in decision summaries and device labeling. Guidance for Industry. FDA; 2016. [Google Scholar]
- 11. van Overbeeke E, Whichello C, Janssens R, et al. Factors and situations influencing the value of patient preference studies along the medical product lifecycle: a literature review. Drug Discov Today 2019. doi: 10.1016/j.drudis.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 12. Whichello C, van Overbeeke E, Janssens R, et al. Factors and situations affecting the value of patient preference studies: semi-structured interviews in Europe and the US. Front Pharmacol 2019;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janssens R, Huys I, van Overbeeke E, et al. Opportunities and challenges for the inclusion of patient preferences in the medical product life cycle: a systematic review. BMC Med Inform Decis Mak 2019;19:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hollin IL, Craig BM, Coast J, et al. Reporting formative qualitative research to support the development of quantitative preference study protocols and corresponding survey instruments: guidelines for authors and reviewers. Patient 2020;13:121–36. [DOI] [PubMed] [Google Scholar]
- 15. Coast J, Al-Janabi H, Sutton EJ, et al. Using qualitative methods for attribute development for discrete choice experiments: issues and recommendations. Health Econ 2012. doi: 10.1002/hec.1739 [DOI] [PubMed] [Google Scholar]
- 16. Bewtra M, Johnson FR.. Assessing patient preferences for treatment options and process of care in inflammatory bowel disease: a critical review of quantitative data. Patient 2013;6:241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Byrne CM, Solomon MJ, Young JM, Selby W, Harrison JD.. Patient preferences between surgical and medical treatment in Crohn’s disease. Dis Colon Rectum 2007;50:586–97. [DOI] [PubMed] [Google Scholar]
- 18. Byrne CM, Tan KK, Young JM, Selby W, Solomon MJ.. Patient and clinician preferences for surgical and medical treatment options in ulcerative colitis. Colorectal Dis 2014;16:285–92. [DOI] [PubMed] [Google Scholar]
- 19. MacKenzie-Smith L, Marchi P, Thorne H, Timeus S, Young R, Le Calvé P.. Patient preference and physician perceptions of patient preference for oral pharmaceutical formulations: results from a real-life survey. Inflamm Intest Dis 2018;3:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Almario C, Keller MS, Chen M, et al. Optimizing selection of biologics in inflammatory bowel disease: development of an online patient decision aid using conjoint analysis. Am J Gastroenterol 2018;113:58–71. [DOI] [PubMed] [Google Scholar]
- 21. Kim ES, Kim KO, Jang BI, et al. Factors contributing to the preference of Korean patients with Crohn’s disease when selecting an anti-tumor necrosis factor agent (Choice study). Gut Liver 2016;10:391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vavricka SR, Bentele N, Scharl M, et al. Systematic assessment of factors influencing preferences of Crohn’s disease patients in selecting an anti-tumor necrosis factor agent (CHOOSE TNF TRIAL). Inflamm Bowel Dis 2012;18:1523–30. [DOI] [PubMed] [Google Scholar]
- 23. The PREFER Consortium. PREFER Recommendations: Why, When and How to Assess and Use Patient Preferences in Medical Product Decision-Making. Zenodo. 2022. doi: 10.5281/zenodo.6592304 [DOI] [Google Scholar]
- 24. Bridges JFP, Hauber AB, Marshall D, et al. Conjoint analysis applications in health – a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health 2011. doi: 10.1016/j.jval.2010.11.013 [DOI] [PubMed] [Google Scholar]
- 25. Wurtz CB. Quantitative sampling. Nautilus 2016;73:131–5. [Google Scholar]
- 26. McMillan SS, King M, Tully MP.. How to use the nominal group and Delphi techniques. Int J Clin Pharm 2016;38:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chew LD, Bradley KA, Boyko EJ.. Brief questions to identify patients with inadequate health literacy. Fam Med 2004;36:588–94. [PubMed] [Google Scholar]
- 28. Gale NK, Heath G, Cameron E, Rashid S, Redwood S.. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013. doi: 10.1186/1471-2288-13-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Durosini I, Janssens R, Arnou R, et al. Patient preferences for lung cancer treatment: a qualitative study protocol among advanced lung cancer patients. Front Public Health 2021;9. doi: 10.3389/fpubh.2021.622154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petrocchi S, Janssens R, Oliveri S, et al. What matters most to lung cancer patients? A qualitative study in Italy and Belgium to investigate patient preferences. Front Pharmacol 2021;12. doi: 10.3389/fphar.2021.602112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Janssens R, Lang T, Vallejo A, et al. Patient preferences for multiple myeloma treatments: a multinational qualitative study. Front Med 2021;8. doi: 10.3389/fmed.2021.686165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spagnuolo R, Iaquinta FS, Mauro D, et al. Satisfaction in social roles and physical function in immune-mediated inflammatory diseases: a cross-sectional study. Rev Recent Clin Trials 2022;17. doi: 10.2174/1574887117666220531162104 [DOI] [PubMed] [Google Scholar]
- 33. Iaquinta FS, Grembiale RD, Mauro D, et al. Fatigue and associated factors in an immune-mediated inflammatory disease population: a cross-sectional study. J Clin Med 2022;11. doi: 10.3390/jcm11092455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dudley-Brown S. Living with ulcerative colitis. Gastroenterol Nurs 1996. doi: 10.1097/00001610-199603000-00004 [DOI] [PubMed] [Google Scholar]
- 35. Sewitch MJ, Abrahamowicz M, Bitton A, et al. Psychological distress, social support, and disease activity in patients with inflammatory bowel disease. Am J Gastroenterol 2001;96:1470–9. [DOI] [PubMed] [Google Scholar]
- 36. Coulter A, Härter M, Moumjid-Ferdjaoui N, Perestelo-Perez L, van der Weijden T.. European Experience with Shared Decision Making. IJPCM. 2015;5:9–14. [Google Scholar]
- 37. Stiggelbout AM, Pieterse AH, De Haes JCJM.. Shared decision making: concepts, evidence, and practice. Patient Educ Couns 2015;98:1172–9. [DOI] [PubMed] [Google Scholar]
- 38. van Overbeeke E, Janssens R, Whichello C, et al. Design, conduct, and use of patient preference studies in the medical product life cycle: a multi-method study. Front Pharmacol 2019;10. doi: 10.3389/fphar.2019.01395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Overbeeke E, Forrester V, Simoens S, Huys I.. Use of patient preferences in health technology assessment: perspectives of Canadian, Belgian and German HTA representatives. Patient 2021;14:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crohn’s & Colitis Foundation of America. The Facts about Inflammatory Bowel Disease. New York: Crohn’s & Colitis Foundation of America; n.d. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this article are not publicly available because they contain information that could compromise participants’ privacy and consent. Requests to access the datasets should be directed to elise.schoefs@kuleuven.be.