Background:
High-dose, adjuvanted, and recombinant influenza vaccines may offer improved effectiveness among older adults compared with standard-dose, unadjuvanted, inactivated vaccines. However, the Advisory Committee on Immunization Practices (ACIP) only recently recommended preferential use of these “higher-dose or adjuvanted” vaccines. One concern was that individuals might delay or decline vaccination if a preferred vaccine is not readily available.
Methods:
We mathematically model how a recommendation for preferential use of higher-dose or adjuvanted vaccines in adults ≥65 years might impact influenza burden in the United States during exemplar “high-” and “low-”severity seasons. We assume higher-dose or adjuvanted vaccines are more effective than standard vaccines and that such a recommendation would increase uptake of the former but could cause (i) delays in administration of additional higher-dose or adjuvanted vaccines relative to standard vaccines and/or (ii) reductions in overall coverage if individuals only offered standard vaccines forego vaccination.
Results:
In a best-case scenario, assuming no delay or coverage reduction, a new recommendation could decrease hospitalizations and deaths in adults ≥65 years by 0%–4% compared with current uptake. However, intermediate and worst-case scenarios, with assumed delays of 3 or 6 weeks and/or 10% or 20% reductions in coverage, included projections in which hospitalizations and deaths increased by over 7%.
Conclusions:
We estimate that increased use of higher-dose or adjuvanted vaccines could decrease influenza burden in adults ≥65 in the United States provided there is timely and adequate access to these vaccines, and that standard vaccines are administered when they are unavailable.
Keywords: Adjuvanted vaccine, High-dose vaccine, Mathematical model, Seasonal influenza, Vaccine recommendation
Vaccination against seasonal influenza is an important public health tool, averting an estimated 39,000–100,000 hospitalizations and 3,500–12,000 deaths annually.1 In the United States, there are many influenza vaccines licensed for use, including standard-dose inactivated, high-dose inactivated, adjuvanted inactivated, recombinant, and live attenuated vaccines. However, the effectiveness of each type of vaccine in preventing influenza-associated illness depends on many factors, including the age of the recipient.2
The effectiveness of three types of influenza vaccine—the high-dose inactivated, adjuvanted inactivated, and recombinant vaccines—has been studied among adults ≥65 years in comparison to standard-dose unadjuvanted inactivated formulations. These “higher-dose or adjuvanted” vaccines may be more effective in preventing influenza-like illness, hospitalizations, and deaths than standard-dose unadjuvanted inactivated vaccines.2–6 Observational studies of the relative effectiveness of different influenza vaccines found higher-dose or adjuvanted vaccines were administered to 74% of community-dwelling Medicare beneficiaries in the 2017/2018 influenza season, 80% in 2018/2019, and 81% in 2019/2020.7–9 Increasing coverage further could reduce influenza-associated morbidity and mortality in this age group. However, the Advisory Committee on Immunization Practices (ACIP) only recently recommended the preferential use of these vaccines over standard vaccines in adults ≥65.2 One potential concern was that a preferential recommendation may result in individuals delaying or foregoing vaccination if a higher-dose or adjuvanted vaccine is unavailable.
Here we use a mathematical model of influenza transmission and disease progression to evaluate the potential impact of changes in uptake of standard vaccines and higher-dose or adjuvanted vaccines by adults ≥65 on influenza-associated burden in the United States. We explore different trade-off scenarios that range from no delay or reduction in overall vaccine coverage (best-case scenario) to a delay of 6 weeks and a 20 percentage point reduction in vaccine coverage (worst-case scenario). We report the number of symptomatic cases, hospitalizations, and deaths averted in adults ≥65 relative to a baseline scenario reflecting current vaccine uptake and assess the sensitivity of these outcomes to our parameter inputs.
METHODS
Mathematical Model
We used a deterministic mathematical model of influenza transmission and infection progression to simulate disease dynamics during a single season in the United States (eFigure 1, http://links.lww.com/EDE/C18). The model is a system of ordinary differential equations that distinguish between susceptible, exposed (but not infectious), asymptomatic (or presymptomatic) and infectious, symptomatic and infectious, and recovered individuals (SEAIR).10,11 A fraction of symptomatic individuals develop severe disease requiring hospitalization, and a fraction of those hospitalizations are ultimately fatal. A proportion of the population remain immune for the duration of the season (reflecting pre-existing immunity) and we assumed individuals who recover from infection in the current season are immune to reinfection.12,13
To capture age-specific differences in contact patterns, vaccine effectiveness (VE), and risks of developing severe disease and dying, we stratified the population into six age groups: 0–4, 5–12, 13–17, 18–49, 50–64, and ≥65. Vaccination occurred in all age groups throughout the season; all vaccinees <65 received a standard vaccine, whereas vaccinees ≥65 received either a standard vaccine or a higher-dose or adjuvanted vaccine. To align the model with data used for calibration (see below), we did not partition the 0–4 age group into infants less than or greater than 6 months. Although the former are ineligible for influenza vaccination, they constitute less than 1% of the US population and thus have minimal impact on our analysis.14 Due to uncertainty in the magnitude of indirect effects provided by influenza vaccination,15–17 we initially assumed that standard vaccines and higher-dose or adjuvanted vaccines reduced the probability of developing symptoms if infected but did not protect against infection or onward transmission.18 However, we incorporated vaccine protection against infection and/or onward transmission at 10% of protection against symptoms in sensitivity analyses. Finally, we assumed asymptomatic and presymptomatic individuals were as infectious as symptomatic individuals and did not explicitly model the use of antivirals or other mitigation measures. Further details on the modeling methods and assumptions can be found in the eAppendix, http://links.lww.com/EDE/C18.
Baseline Model Calibration
We first calibrated the model to recent seasonal influenza dynamics from 2011/12 to 2018/19, reflecting vaccine uptake without a specific preferential recommendation in place. This provided a “baseline” model to which all other scenarios could be compared. We used publicly available data to inform model inputs where possible. The US population and the fraction in each of our six age groups were obtained from US census data, and age-specific contact patterns were determined using a synthetic contact matrix (eFigure 2, http://links.lww.com/EDE/C18).10,14,19 The proportion vaccinated in each age group and month, July to May, from 2011/12 to 2018/19 (all products combined) were obtained from FluVaxView (eFigure 3, http://links.lww.com/EDE/C18).20 We calculated average vaccine coverage rates for each month and age group across all seasons, and then translated these values to daily rates by dividing by the number of days in that month, assuming vaccination was evenly distributed within each month. These rates were used to calculate the number of individuals vaccinated daily in each age group before model simulation, and then proportionally distributed among the infection classes during each simulation. We approximated standard VE for each age group as the average VE reported by the CDC VE network from 2011/12 to 2018/19 (eTables 1–2, http://links.lww.com/EDE/C18).21 Finally, age-specific risks of influenza hospitalization (case-hospitalization ratios, CHRs) and death (hospitalization-fatality ratios, HFRs) were informed by the ratios of total hospitalizations to illnesses and deaths to hospitalizations, respectively, reported by CDC from 2011/12 to 2018/19.22 Again, we used average values across all seasons as model inputs (eTables 1–2, http://links.lww.com/EDE/C18).
We obtained the remaining epidemiologic parameter values through literature review (eTable 1, http://links.lww.com/EDE/C18).22–31 To capture variation in the timing and severity of seasonal influenza epidemics, we calibrated the basic reproduction number R0, the timing of peak transmission, and the proportion of individuals with pre-existing immunity to generate two representative influenza seasons: (i) a moderate- to high-severity season with total burden outcomes (symptomatic cases, hospitalizations, and deaths) in the upper range of reports from 2011/12 to 2018/19 and a relatively early peak in incidence (henceforth referred to as the high-severity season); and (ii) a moderate- to low-severity season with burden outcomes in the lower range of previous reports and a later peak in incidence (henceforth referred to as the low-severity season; eTable 1 and eFigure 4, http://links.lww.com/EDE/C18).32,33 Because lower VE could contribute to increased season severity, we assumed standard vaccines were less effective in the high-severity season (VE = 25%) than the low-severity season (VE = 40%). We then fixed the relative effectiveness of higher-dose or adjuvanted vaccines compared with standard vaccines (rVE) in adults ≥65 at 15%, where rVE is given by (VEHDAV − VE)/(1 − VE) × 100% and VEHDAV is the absolute effectiveness of higher-dose or adjuvanted vaccines.4 We also assumed 75% of vaccinees ≥65 received a higher-dose or adjuvanted vaccine.7–9,34 Both parameters were varied in subsequent analyses.
Our calibrated baseline model (with no recommendation in place and maintaining baseline higher-dose or adjuvanted vaccine uptake) captured epidemic dynamics typically observed in moderate- to high- and moderate- to low-severity seasons, such as the timing, age distribution, and total burden of influenza (eFigures 5–7 and eTable 3, http://links.lww.com/EDE/C18).20,35
Incorporating Changes in Vaccine Preference
We modeled the potential impacts of a new preferential recommendation for higher-dose or adjuvanted vaccines in adults ≥65 through changes in vaccine uptake. First, we defined a range of trade-off scenarios that accounted for (i) delays in the administration of additional higher-dose or adjuvanted vaccines relative to the timing of baseline vaccination administration; and/or (ii) decreases in overall vaccine uptake. Such scenarios could occur if there were impediments to finding locations that offered higher-dose or adjuvanted vaccines; if physicians only recommended higher-dose or adjuvanted vaccines to patients; or if individuals only offered standard vaccines elected to forego vaccination. We considered pairwise combinations of best, intermediate, and worst-case values for both parameters relative to baseline vaccination, with delays of 0, 3, and 6 weeks in the administration of additional higher-dose or adjuvanted vaccines to those formerly receiving a standard vaccine, and reductions in overall vaccine coverage of 0, 10, or 20 percentage points to represent former standard vaccine recipients who forego vaccination entirely. We did not consider differences in vaccine safety or monetary costs. In tandem, we incorporated the potential benefits of a new recommendation by allowing the proportion of vaccinees ≥65 who receive a higher-dose or adjuvanted vaccine to increase 0–20 percentage points relative to baseline. The ranges defined above reflect our uncertainty in how a preferential recommendation will impact vaccine uptake.
In addition to the uncertainty incorporated through the trade-off and benefit parameters, we incorporated uncertainty through two other key vaccine parameters from the baseline model: we varied the fraction of vaccinees ≥65 receiving a higher-dose or adjuvanted vaccine at baseline between 60% and 80% and rVE between 5% and 35%.3,6–9,34 The different sources of uncertainty were combined by first constructing 1,000 uniform Latin hypercube samples from the baseline higher-dose or adjuvanted vaccine uptake, the increase in higher-dose or adjuvanted vaccine uptake relative to baseline, and rVE. We ensured that the fraction of vaccinated and unvaccinated adults ≥65 summed to 1 but assumed the parameters were otherwise independent. We then simulated the model for each Latin hypercube sample (1,000 total), trade-off scenario combination (nine total), and season severity (high and low).36,37 This resulted in 18,000 simulations spanning a wide range of possible outcomes. For each simulation, we compared the number of symptomatic cases, hospitalizations, and deaths in adults ≥65 with the new recommendation in place to that of the corresponding baseline model (with no recommendation and with baseline higher-dose or adjuvanted vaccine uptake). Changes in burden in other age groups were minimal and are not presented.
Sensitivity Analyses
We explored the sensitivity of the model to five key parameters: baseline higher-dose or adjuvanted vaccine uptake, rVE, the increase in higher-dose or adjuvanted vaccine uptake relative to baseline, the delay in additional higher-dose or adjuvanted vaccine administration relative to baseline, and the reduction in overall vaccination coverage relative to baseline (eTable 1, http://links.lww.com/EDE/C18). First, we conducted a one-way sensitivity analysis by varying each parameter in turn and recalculating the impact of the new recommendation while keeping all other parameters fixed. Second, we performed a two-way sensitivity analysis by simultaneously varying the two trade-off scenario parameters while keeping all other parameters fixed. We calculated the impact of the recommendation at each point in this 2D space and then identified the threshold that partitioned the regions where these impacts were positive or negative. Finally, we conducted a multiway sensitivity analysis using a partial rank correlation coefficient framework.38 We first expanded our Latin hypercube sampling approach to include the trade-off scenario parameters, allowing them to vary uniformly between their lower and upper limits (eTable 1, http://links.lww.com/EDE/C18). We recalculated the impact of the new recommendation for each season severity (high and low) and new Latin hypercube sample (1,000 total), resulting in 2,000 additional simulations. We then identified the independent influence of each parameter by quantifying its nonparametric partial rank correlation with the number of hospitalizations in adults ≥65 averted by the new recommendation.38 All analyses were performed in R 4.0.3 using the deSolve, flumodels, tidyverse, lhs and sensitivity packages.10,39–43 Visualizations were created using the gplots, ggforce, ggpubr, patchwork, viridis, and scico packages.44–49
RESULTS
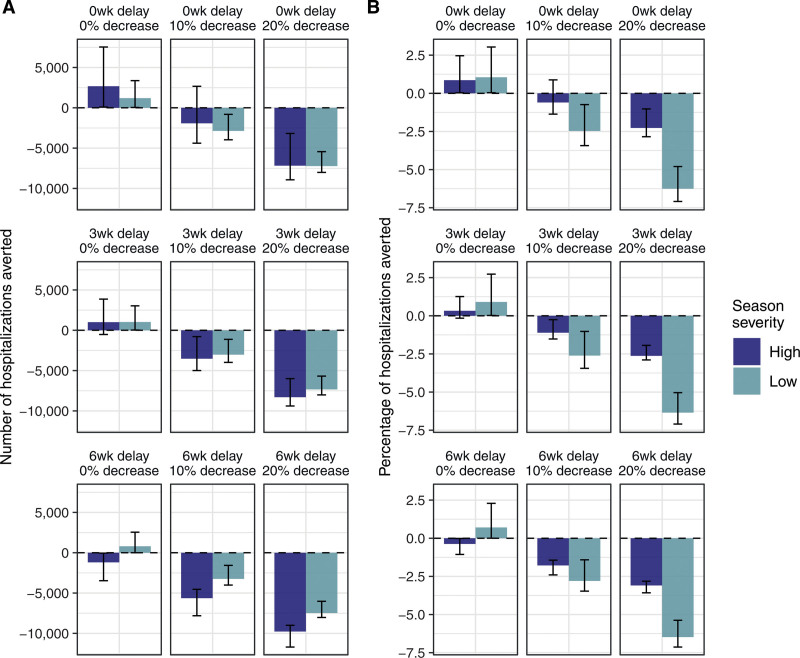
In the best-case scenario, which allowed higher-dose or adjuvanted vaccine uptake to increase but assumed no delay or reduction in overall coverage, the preferential recommendation always resulted in fewer symptomatic cases, hospitalizations, and deaths relative to baseline (Figure 1 and eFigure 8, http://links.lww.com/EDE/C18, where positive values indicate a decrease in burden relative to the corresponding baseline simulation and therefore a positive impact of the recommendation). However, in the intermediate and worst-case scenarios, the assumed increase in uptake of higher-dose or adjuvanted vaccines was often insufficient to outweigh the trade-offs of delayed or reduced vaccine coverage. For example, introducing delays of 3 or 6 weeks in additional higher-dose or adjuvanted vaccine administration (without a reduction in overall coverage) allowed burden to increase in the high-severity season, and reducing overall coverage by 10 or 20 percentage points (with or without a delay in administration of higher-dose or adjuvanted vaccines) allowed burden to increase in both seasons.
FIGURE 1.
Averted hospitalizations in adults ≥65 under different trade-off scenarios. Bars indicate the mean number (A) and percentage relative to baseline (B) of averted hospitalizations from 1,000 uniform Latin hypercube parameter combinations; error bars are the 95th percentiles. Each panel shows a different combination of the delay in administration of additional higher-dose or adjuvanted vaccines (0, 3, or 6 weeks), and reduction in overall vaccine coverage (0, 10, or 20 percentage points). Positive values indicate a decrease in burden relative to the corresponding baseline simulation and therefore a positive impact of the new recommendation. Results for the number of averted symptomatic cases and deaths are shown in eFigure 8, http://links.lww.com/EDE/C18, and results assuming a logit-normal distribution for rVE with a median value of 15% are shown in eFigure 12, http://links.lww.com/EDE/C18. rVE indicates relative vaccine effectiveness.
Within each trade-off scenario, there was substantial variation in the potential impacts of a new recommendation due to parameter uncertainty: the predicted change in deaths, hospitalizations, and symptomatic cases varied by as much as 1,000, 9,000 and 100,000, respectively, within a particular scenario and season (Figure 1A, eFigure 8, http://links.lww.com/EDE/C18). There were also differences between the high- and low-severity seasons, with the latter generally experiencing smaller magnitude changes in absolute burden (Figure 1A) but greater changes in the percentage relative to baseline (Figure 1B). Incorporating indirect protection by allowing vaccines to protect against infection and/or onward transmission did not impact our overall findings (eFigure 9, http://links.lww.com/EDE/C18). In general, our results highlight the large degree of uncertainty in the potential direction (positive or negative) and magnitude of changes in burden following a preferential recommendation for higher-dose or adjuvanted vaccines.
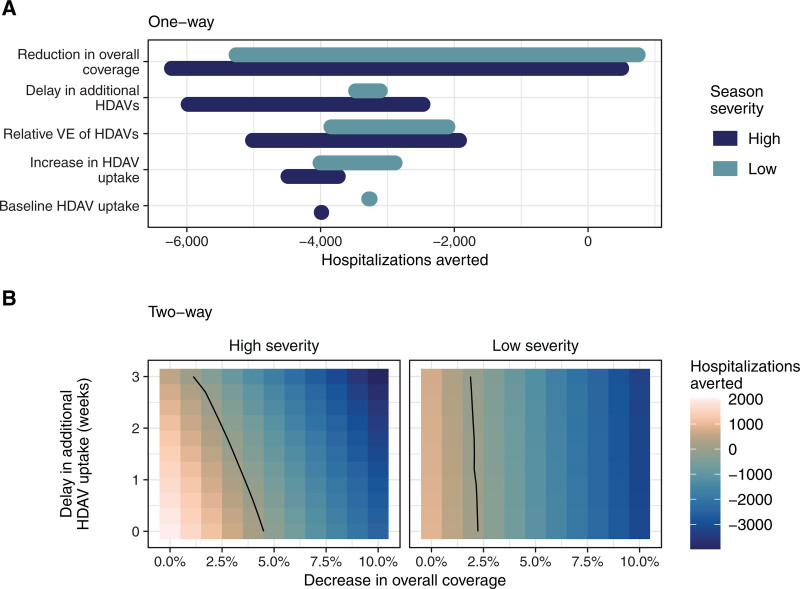
Given this large uncertainty, we conducted sensitivity analyses to identify parameters that were most influential in determining the potential change in influenza burden associated with a preferential recommendation for higher-dose or adjuvanted vaccines. The one-way sensitivity analysis identified the potential reduction in overall vaccine coverage as having the strongest influence on the eventual change in disease burden (Figure 2A). The next most influential parameters in the high-severity season were the delay in additional higher-dose or adjuvanted vaccine uptake and rVE. The latter was also important in the low-severity season, as was the increase in uptake of higher-dose or adjuvanted vaccines following the new recommendation. The greater importance of increasing higher-dose or adjuvanted vaccine uptake in the low-severity season is likely due to the greater assumed absolute effectiveness of higher-dose or adjuvanted vaccines in this season: there is more to gain for each additional individual who receives a higher-dose or adjuvanted vaccine instead of a standard vaccine. Conversely, the greater importance of the delay in higher-dose or adjuvanted vaccine uptake in the high-severity season is due to our assumptions on season timing: the high-severity season occurs earlier than the low-severity season, and thus is more likely to have begun before the completion of a delayed vaccination campaign (eFigure 5B, http://links.lww.com/EDE/C18). The partial rank correlation coefficient results supported the one-way sensitivity analysis (eFigure 10, http://links.lww.com/EDE/C18).
FIGURE 2.
The reduction in overall vaccine coverage has the greatest impact on predicted outcomes. One-way (A) and two-way (B) sensitivity analyses assessing the impact of key parameters on the number of hospitalizations averted in adults ≥65. Positive values indicate a positive number of hospitalizations were averted relative to the corresponding baseline simulation and thus the new recommendation had a positive impact (i.e., reduced the total number of hospitalizations). Parameter ranges are given in eTable 1, http://links.lww.com/EDE/C18. In (B), rVE is 15%, 75% of vaccinees ≥65 receive an HDAV at baseline, and there is a 10 percentage point increase in HDAV uptake following the new recommendation. rVE indicates relative vaccine effectiveness and HDAV indicates higher-dose or adjuvanted vaccine.
Finally, we conducted a two-way sensitivity analysis of the two trade-off parameters to identify thresholds where the burden averted by a recommendation changed from positive to negative. Strikingly, we found that in a scenario with relatively optimistic parameter values (10 percentage point increase in higher-dose or adjuvanted vaccine uptake, 75% higher-dose or adjuvanted vaccine uptake at baseline, and 15% rVE), we could afford a maximum reduction in overall coverage of four percentage points before the new recommendation increased the estimated disease burden relative to baseline (Figure 2B). Increasing higher-dose or adjuvanted vaccine uptake by 15 percentage points following the recommendation only marginally improved this threshold (eFigure 11, http://links.lww.com/EDE/C18). Overall, our findings reveal the importance of the trade-off parameters, particularly the reduction in overall vaccine coverage, in determining the impact of a new preferential recommendation.
DISCUSSION
We evaluated the potential impact of changes in vaccine uptake following a preferential recommendation for higher-dose or adjuvanted vaccines in adults ≥65 on influenza-associated burden in the United States. We found a wide range of outcomes that were mediated by the relative delay and reduction in overall vaccine coverage, rVE, and the timing and severity of the influenza season. In a best-case scenario, with no assumed delay or coverage reduction, a preferential recommendation could decrease the number of hospitalizations and deaths in adults ≥65 years by 0%–4% compared with current uptake (Figure 1B). However, intermediate and worst-case scenarios, with assumed delays of 3 or 6 weeks and/or 10 or 20 percentage point reductions in coverage, included projections in which hospitalizations and deaths increased by over 7%. Thus, except in a best-case scenario with no delay or reduction in vaccine coverage, our results always included the potential for increased disease burden following a preferential recommendation.
Our analyses illustrate that higher-dose or adjuvanted vaccines can decrease influenza burden in adults ≥65 if there is timely and adequate access to these vaccines, and if standard vaccines are used when they are unavailable. Notably, the ACIP preferential recommendation includes the caveat that “any other age-appropriate influenza vaccine should be used” if a higher-dose or adjuvanted vaccine is unavailable at the time of vaccination, which may encourage standard vaccine use when necessary. This may be especially important for the approximately 20% of community-dwelling adults ≥65 years who receive a standard vaccine rather than a higher-dose or adjuvanted vaccine.7–9 More generally, a better understanding of the factors that determine whether a standard vaccine or a higher-dose or adjuvanted vaccine is received could help increase use of the latter in upcoming seasons and thus enhance the impact of the preferential recommendation.
Here we considered trade-off scenarios that covered a spectrum of possible outcomes regarding changes in vaccine timing and uptake, from optimistic (no delay or reduction in coverage) to pessimistic (6-week delay and 20 percentage point reduction in coverage), and investigated the sensitivity of our results to these values. We also included other important sources of uncertainty (such as season timing and severity, rVE, and the increase in higher-dose or adjuvanted vaccine uptake) to fully explore the range of plausible outcomes that could occur. Although previous modeling studies have considered similar trade-offs when exploring how the timing of influenza vaccination influences burden within a season,50,51 we are not aware of any studies that assess such trade-offs within the context of changing uptake of standard vaccines and higher-dose or adjuvanted vaccines. Therefore, our results are useful in highlighting key issues that may impact the benefits of the preferential recommendation—such as attitudes toward, and access barriers to, higher-dose or adjuvanted vaccines—that can be evaluated and potentially mitigated if present.
Our analyses are subject to several limitations. First, we only stratify individuals by age and not by other factors that may influence VE and/or uptake, such as medical comorbidities, location of residence, the infecting influenza strain, vaccination history, or race and ethnicity. In particular, racial and ethnic disparities in uptake of higher-dose or adjuvanted vaccines suggest there may be underlying heterogeneity that we have not considered.52 Incorporating such heterogeneity would require additional data describing vaccine uptake within each subgroup but should be continued in future work to address these disparities.
Second, we omit the use of nonpharmaceutical interventions and antivirals to avoid additional uncertainty that is secondary to the focus of this study. We, therefore, assume these factors are independent of vaccination and the preferential recommendation, and thus should impact the model equally in the baseline and recommendation scenarios and have minimal impact on our overall conclusions.
Finally, we made several simplifying assumptions regarding vaccine type and effectiveness. First, we did not stratify higher-dose or adjuvanted vaccines within the model as high-dose, adjuvanted, or recombinant. Instead, differences in their effectiveness and uptake are implicitly incorporated through the wide parameter ranges we have explored. We also did not explicitly consider cell-based vaccines as these are not included in the preferential recommendation and thus should impact the model equally in the baseline and recommendation scenarios. Second, we primarily assumed that vaccines only protect against symptoms and do not reduce an individual’s risk of infection or onward transmission given infection. Incorporating additional protection against infection or onward transmission changed the magnitude of predicted outcomes but did not affect our overall conclusions. Exploring these additional facets of vaccine-mediated protection more fully would increase the parameter uncertainty propagating into model outcomes as these quantities are not well known.15–17 Third, we assumed the vaccine parameters included in our Latin hypercube sampling procedure (baseline higher-dose or adjuvanted vaccine uptake, rVE, and the increase in higher-dose or adjuvanted vaccine uptake following a preferential recommendation) were independent and uniformly distributed. Although estimates of these parameters from a representative sample of seasons would be required to inform different distribution assumptions, we did find that employing a nonuniform distribution for rVE did not impact our conclusions (eFigure 12, http://links.lww.com/EDE/C18). Last, we assumed everyone <65 received a standard vaccine when in fact recombinant vaccines are licensed for adults ≥18. However, our assumed values for standard VE are taken from studies that consider all vaccines administered to each age group and thus account for the use of recombinant vaccines in adults <65.21
In this study, we explored how a preferential recommendation for higher-dose or adjuvanted vaccines over standard vaccines in adults ≥65 could impact seasonal influenza burden in the United States. Our findings indicate that greater use of higher-dose or adjuvanted vaccines might decrease influenza burden in adults ≥65 years but reveal the potential for increased burden if delays or reductions in vaccine coverage occur. Timely and adequate access to higher-dose or adjuvanted vaccines, and the use of standard vaccines when these vaccines are unavailable, are therefore critical to ensure that the preferential recommendation for higher-dose or adjuvanted vaccines in adults ≥65 does not inadvertently increase seasonal influenza burden in the United States.
Supplementary Material
Footnotes
The authors report no conflicts of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Data and code availability: There are no data associated with this paper. All code required to recreate the analysis are publicly available at https://github.com/CDCgov/flu-vaccine-model.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
REFERENCES
- 1.CDC. Past Seasons Estimated Influenza Disease Burden Averted by Vaccination. 2021. https://www.cdc.gov/flu/vaccines-work/past-burden-averted-est.html.
- 2.Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371:635–645. [DOI] [PubMed] [Google Scholar]
- 4.Lee JKH, Lam GKL, Shin T, Samson SI, Greenberg DP, Chit A. Efficacy and effectiveness of high-dose influenza vaccine in older adults by circulating strain and antigenic match: an updated systematic review and meta-analysis. Vaccine. 2021;39(1 suppl):A24–A35. [DOI] [PubMed] [Google Scholar]
- 5.Lee JKH, Lam GKL, Shin T, et al. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: a systematic review and meta-analysis. Expert Rev Vaccines. 2018;17:435–443. [DOI] [PubMed] [Google Scholar]
- 6.ACIP. GRADE: higher dose and adjuvanted influenza vaccines for persons aged ≥65 years. 2022. https://www.cdc.gov/vaccines/acip/recs/grade/influenza-older-adults.html.
- 7.Izurieta HS, Lu M, Kelman J, et al. Comparative effectiveness of influenza vaccines among U.S. Medicare beneficiaries ages 65 years and older during the 2019-20 season. Clin Infect Dis. 2020;73:e4251–e4259. [DOI] [PubMed] [Google Scholar]
- 8.Izurieta HS, Chillarige Y, Kelman J, et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017-2018. J Infect Dis. 2019;220:1255–1264. [DOI] [PubMed] [Google Scholar]
- 9.Izurieta HS, Chillarige Y, Kelman J, et al. Relative effectiveness of influenza vaccines among the United States elderly, 2018-2019. J Infect Dis. 2020;222:278–287. [DOI] [PubMed] [Google Scholar]
- 10.Asher J, Clay M. ASPR-flumodels. R package version 1.0.7. 2020.
- 11.Walker J, Paul P, Dooling K, et al. Modeling strategies for the allocation of SARS-CoV-2 vaccines in the United States. Vaccine. 2022;40:2134–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnott A, Carville K, Franklin L, Sullivan SG. Consecutive influenza infections in both adults and children. J Infect Dis. 2017;215:658–659. [DOI] [PubMed] [Google Scholar]
- 13.Price O, Birrell FA, Mifsud EJ, Sullivan SG. Epidemiology of repeat influenza infection in Queensland, Australia, 2005-2017. Epidemiol Infect. 2022;150:e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United States Census Bureau. Census Data 2020. 2020. https://www2.census.gov/programs-surveys/popest/.
- 15.Ainslie KEC, Haber M, Orenstein WA. Challenges in estimating influenza vaccine effectiveness. Expert Rev Vaccines. 2019;18:615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman L, Renaud A, Hines D, et al. Exploring indirect protection associated with influenza immunization - a systematic review of the literature. Vaccine. 2019;37:7213–7232. [DOI] [PubMed] [Google Scholar]
- 17.Mertz D, Fadel SA, Lam PP, et al. Herd effect from influenza vaccination in non-healthcare settings: a systematic review of randomised controlled trials and observational studies. Euro Surveill. 2016;21:e30378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malosh RE, Petrie JG, Callear A, et al. Effectiveness of influenza vaccines in the HIVE household cohort over 8 years: is there evidence of indirect protection? Clin Infect Dis. 2021;73:1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prem K, van Zandvoort K, Klepac P, et al. Projecting contact matrices in 177 geographical regions: an update and comparison with empirical data for the COVID-19 era. PLoS Comput Biol. 2021;17:e1009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC. FluVaxView Interactive. 2021. https://www.cdc.gov/flu/fluvaxview/interactive.htm.
- 21.CDC. Past Seasons Vaccine Effectiveness Estimates. 2021. https://www.cdc.gov/flu/vaccines-work/past-seasons-estimates.html.
- 22.CDC. Past Seasons Estimated Influenza Disease Burden. 2021. https://www.cdc.gov/flu/about/burden/index.html.
- 23.Punpanich W, Chotpitayasunondh T. A review on the clinical spectrum and natural history of human influenza. Int J Infect Dis. 2012;16:e714–e723. [DOI] [PubMed] [Google Scholar]
- 24.Meltzer MI, Gambhir M, Atkins CY, Swerdlow DL. Standardizing scenarios to assess the need to respond to an influenza pandemic. Clin Infect Dis. 2015;60(1 suppl):S1–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paules C, Subbarao K. Influenza. Lancet. 2017;390:697–708. [DOI] [PubMed] [Google Scholar]
- 26.Suess T, Remschmidt C, Schink SB, et al. Comparison of shedding characteristics of seasonal influenza virus (sub)types and influenza A(H1N1)pdm09; Germany, 2007-2011. PLoS One. 2012;7:e51653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrat F, Vergu E, Ferguson NM, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167:775–785. [DOI] [PubMed] [Google Scholar]
- 28.Leung NHL, Xu C, Ip DKM, Cowling BJ. The fraction of influenza virus infections that are asymptomatic. a review and meta-analysis. Epidemiology. 2015;26:862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biggerstaff M, Cauchemez S, Reed C, Gambhir M, Finelli L. Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature. BMC Infect Dis. 2014;14:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biggerstaff M, Reed C, Swerdlow DL, et al. Estimating the potential effects of a vaccine program against an emerging influenza pandemic–United States. Clin Infect Dis. 2015;60(Suppl 1):S20–S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen C, Kleynhans J, Moyes J, et al. ; PHIRST group. Asymptomatic transmission and high community burden of seasonal influenza in an urban and a rural community in South Africa, 2017–18 (PHIRST): a population cohort study. Lancet Global Health. 2021;9:e863–e874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biggerstaff M, Kniss K, Jernigan DB, et al. Systematic assessment of multiple routine and near real-time indicators to classify the severity of influenza seasons and pandemics in the United States, 2003-2004 through 2015-2016. Am J Epidemiol. 2018;187:1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CDC. How CDC Classifies Flu Severity. 2021. https://www.cdc.gov/flu/about/classifies-flu-severity.htm.
- 34.Mahmud SM, Pabla G, Righolt CH, Loiacono MM, Thommes E, Chit A. What explains racial/ethnic inequities in the uptake of differentiated influenza vaccines? Prev Med. 2022;163:107236. [DOI] [PubMed] [Google Scholar]
- 35.CDC. FluView Interactive: ILI and Viral Surveillance. 2021. https://www.cdc.gov/flu/weekly/fluviewinteractive.htm.
- 36.Manache G, Melching C. Sensitivity of Latin Hypercube sampling to sample size and distributional assumptions. 32nd Congress of the IAHR. Venice, Italy; 2007. [Google Scholar]
- 37.McKay J. In: Sensitivity and Uncertainty Analysis Using a Statistical Sample of Input Values. Ronen Y, ed. Uncertainty Analysis: CRC Press; 1988:145–86. [Google Scholar]
- 38.Blower SM, Hartel D, Dowlatabadi H, Anderson RM, May RM. Drugs, sex and HIV: a mathematical model for New York City. Philos Trans R Soc Lond B Biol Sci. 1991;331:171–187. [DOI] [PubMed] [Google Scholar]
- 39.Carnell R. lhs: Latin Hypercube Samples. R package version 1.1.3. 2021.
- 40.Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. J Open Soft. 2019;4:1686. [Google Scholar]
- 41.R Core Team. R: A Language and Environment for Statistical Computing: R Foundation for Statistical Computing; 2020.
- 42.Iooss B, Da Veiga S, Janon A, Pujol G. Sensitivity: global sensitivity analysis of model outputs. R package version 1.27.0. 2021.
- 43.Soetaert K, Petzoldt T, Setzer RW. Solving differential equations in R: package desolve. J Stat Soft. 2010;33:1–25. [Google Scholar]
- 44.Garnier S, Ross N, Rudis R, Camargo AP, Sciaini M, Scherer C. viridis - Colorblind-Friendly Color Maps for R. R package version 0.6.1. 2021.
- 45.Kassambara A. ggpubr: “ggplot2” Based Publication Ready Plots. R package version 0.4.0. 2020.
- 46.Pedersen TL. patchwork: The Composer of Plots. R package version 1.1.1. 2020.
- 47.Pederson TL. ggforce: Accelerating “ggplot2.” R package version 0.3.3. 2021.
- 48.Warnes GR, Bolker B, Bonebakker L, Gentleman R, Huber W, Liaw A. gplots: Various R Programming Tools for Plotting Data. R package version 3.1.1. 2020.
- 49.Pedersen TL, Crameri F. scico: Colour Palettes Based on the Scientific Colour-Maps. R package version 1.3.0. 2021.
- 50.Ferdinands JM, Alyanak E, Reed C, Fry AM. Waning of influenza vaccine protection: exploring the trade-offs of changes in vaccination timing among older adults. Clin Infect Dis. 2019;70:1550–1559. [DOI] [PubMed] [Google Scholar]
- 51.Newall AT, Chen C, Wood JG, Stockwell MS. Within-season influenza vaccine waning suggests potential net benefits to delayed vaccination in older adults in the United States. Vaccine. 2018;36:5910–5915. [DOI] [PubMed] [Google Scholar]
- 52.Mahmud SM, Xu L, Hall LL, et al. Effect of race and ethnicity on influenza vaccine uptake among older US Medicare beneficiaries: a record-linkage cohort study. Lancet Healthy Longev. 2021;2:e143–e153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.