Abstract
STUDY QUESTION
In women with threatened miscarriage, does progesterone supplementation until the completion of the first trimester of pregnancy increase the probability of live birth?
SUMMARY ANSWER
In women with threatened miscarriage, 400 mg vaginal progesterone nightly, from onset of bleeding until 12 weeks, did not increase live birth rates.
WHAT IS KNOWN ALREADY
Limited evidence has indicated that vaginal micronized progesterone may make little or no difference to the live birth rate when compared with placebo in women with threatened miscarriage. Subgroup analysis of one recent randomized trial reported that in women with bleeding and at least one previous miscarriage, progesterone might be of benefit.
STUDY DESIGN, SIZE, DURATION
We performed a randomized, double-blinded, placebo-controlled trial between February 2012 and April 2019. Eligible pregnant women under 10 weeks gestation, experiencing a threatened miscarriage as apparent from vaginal bleeding were randomized into two groups in a 1:1 ratio: the intervention group received 400 mg progesterone as vaginal pessaries, the control group received placebo vaginal pessaries, both until 12 weeks gestation. The primary endpoint was live birth. We planned to randomize 386 women (193 per group). The study was stopped at a planned interim analysis for futility after randomization of 278 women.
PARTICIPANTS/MATERIALS, SETTING, METHODS
This trial was conducted at the Mater Mothers’ Hospital, a tertiary centre for maternity care in South Brisbane, Queensland, Australia. We randomized 139 women to the intervention group and 139 women to the placebo group. Primary outcome data were available for 136 women in the intervention group and 133 women in the placebo group.
MAIN RESULTS AND THE ROLE OF CHANCE
The live birth rates were 82.4% (112/136) and 84.2% (112/133) in the intervention group and placebo group, respectively (risk ratio (RR) 0.98, 95% CI 0.88 to 1.09; risk difference −0.02, 95% CI −0.11 to 0.07; P = 0.683). Among women with at least one previous miscarriage, live birth rates were 80.6% (54/67) and 84.4% (65/77) (RR 0.95, 95% CI 0.82–1.11; P = 0.550). No significant effect was seen from progesterone in women with two (RR 1.28, 95% CI 0.96–1.72; P = 0.096) or more (RR 0.79, 95% CI 0.53–1.19; P = 0.267) previous miscarriages. Preterm birth rates were 12.9% and 9.3%, respectively (RR 1.38; 95% CI 0.69 to 2.78; P = 0.361). Median birth weight was 3310 vs 3300 g (P = 0.992). There were also no other significant differences in obstetric and perinatal outcomes.
LIMITATIONS, REASONS FOR CAUTION
Our study was single centre and did not reach the planned sample size because it was stopped prematurely at an interim analysis.
WIDER IMPLICATIONS OF THE FINDINGS
We did not find evidence supporting the treatment effect of vaginal progesterone in women with threatened miscarriage. Progesterone in this setting should not be routinely used for threatened miscarriage. The treatment effect in women with threatened miscarriage after previous miscarriages warrants further research.
STUDY FUNDING/COMPETING INTEREST(S)
Mothers’ and babies Golden Casket Clinical Fellowship (L.A.M.). Progesterone and placebo pessaries were provided by Perrigo Australia.
B.W.J.M. reports grants from NHMRC, personal fees from ObsEva, personal fees from Merck KGaA, personal fees from Guerbet, personal fees from iGenomix, outside the submitted work.
TRIAL REGISTRATION NUMBER
ACTRN12611000405910
TRIAL REGISTRATION DATE
19 April 2011
DATE OF FIRST PATIENT’S ENROLMENT
06 February 2012
Keywords: threatened miscarriage, progesterone, miscarriage prevention, live birth, early pregnancy
Introduction
Threatened miscarriage, apparent from vaginal bleeding with or without lower abdominal pain, affects ∼25% of all clinical pregnancies (Hasan et al., 2009). Around one-quarter of threatened miscarriages will proceed to a complete miscarriage over the ensuing weeks of pregnancy (Dede et al., 2010; Duan et al., 2011). When the pregnancy continues, women who experience a threatened miscarriage are at increased risk of adverse pregnancy outcomes including pregnancy loss, antepartum haemorrhage, preterm delivery, perinatal mortality and low-birthweight babies (Jauniaux et al., 2010; Saraswat et al., 2010).
Miscarriage can be a significant loss for a woman and her partner and can be related to longer-term sequelae such as depression, anxiety, and delay in pursuing pregnancy (Thapar and Thapar, 1992). With the exception of anticoagulants for women with persistent antiphospholipid antibodies (Hamulyák et al., 2020) and perhaps progesterone for women with multiple miscarriages (Haas et al., 2019), there is presently no agreed therapeutic approach which has been shown to reduce the risk of pregnancy loss in women who present with a threatened miscarriage.
Progesterone is important for the establishment and maintenance of pregnancy. Its presence creates a mature endometrium and a favourable immune environment for early embryonic development. Women with recurrent miscarriage have particularly low endometrial progesterone levels (Salazar and Calzada, 2007). Also, progesterone levels have been observed to be lower in pregnancies that subsequently end in miscarriage (Arck et al., 2008), but it is not known whether the lower levels are merely predicting a poor pregnancy outcome or are causative (Duan et al., 2011).
Caution needs to be exercised in using hormones in the early embryological and organogenesis stage of development. There has been concern regarding the use of progestins in pregnancy, particularly with respect to the potential for genital (hypospadias in males and female virilization) and non-genital anomalies (Carmichael et al., 2005). On the other hand, progesterone could be effective in decreasing the miscarriage rate.
A recent Cochrane review that included only two placebo-controlled randomized trials found that in women with threatened miscarriage vaginal micronized progesterone increased the live birth rate, although the treatment effect was small and non-significant (risk ratio (RR) 1.03, 95% CI 1.00 to 1.07) (Devall et al., 2021). The treatment effect was more evident in women with one or more previous miscarriages. These findings were predominantly driven by one large randomized trial that yielded the same conclusions (Coomarasamy et al., 2019).
We performed a placebo-controlled randomized trial to further elucidate the treatment effect of vaginal progesterone in women with threatened miscarriage.
Materials and methods
Study design
This study was a single-centre, doubled blinded randomized placebo-controlled clinical trial performed in the Department of Obstetrics and Gynaecology, Mater Mothers’ Hospitals, Brisbane, Australia and coordinated by staff of the Fertility Assessment and Research Clinic (FAR Clinic).
This trial was registered at the Australian New Zealand Clinical Trials Registry (ACTRN12611000405910). All trial participants provided written informed consent. The study was conducted following the Helsinki Declaration after approval by the local ethic committee (Mater MREC/16/MHS/90). The reporting followed the CONSORT guidelines.
Study population
Women were eligible if they were pregnant <10+0 weeks, over 18 years of age, diagnosed as having threatened miscarriage as apparent from bleeding with or without pain. Women bleeding for other diagnoses such as vulvar, vaginal, or cervical trauma or lesion were not eligible. Only singleton pregnancies were eligible. A live intrauterine pregnancy was determined by foetal heart rate on ultrasound. Women were not eligible if they were pregnant as a result of ART often being on luteal phase/early pregnancy hormonal support, or if participating in another clinical trial.
Recruitment, randomization, and blinding
Potential participants were identified by clinical staff based on threatened miscarriage presentation with live intrauterine pregnancy. Eligible women were given a generic flyer outlining the trial and contact details. They were referred to the research team and had a doctor or research midwife consultation that explained the trial details as soon as possible, usually on the same day or the next day of the diagnosis. Medical history and recent results were reviewed before signing the consent form and allocating study number. Then, a ‘script’ including study number was written for the participant by a member of the research team and sent with patient to the hospital pharmacy. After obtaining the written prescription, pharmacist allocated the next ‘treatment’ as per the random allocation sheet.
The random allocation sheet was prepared using computer-generated variable block randomization in a ratio of 1:1 by an independent statistician. Identical pessaries with progesterone or placebo were prepared by Perrigo Australia, Balcatta (Western Australia) and stored in consecutive, serial numbered containers. Participants, investigators, and dispensing pharmacists were blinded to active and placebo treatments.
Procedures
Participants administered to themselves vaginal pessaries containing either 400 mg progesterone or placebo nightly, from the day of randomization until 12+0 weeks of gestation or earlier if pregnancy ended before 12 weeks. Participants were able to contact the research midwives at any time and were instructed to report events and discomforts. Compliance was passively checked as scripts were dispensed in 20 night packs and returns for further therapy were recorded. If a woman declined further participation, this was recorded, and a short withdrawal questionnaire completed if the woman agreed. Lost to follow-up was recorded and the reason for loss was noted.
Outcomes
The primary outcome was live birth. Live birth is defined as a delivery of one or more living babies ≥23+0 week’s gestation. Secondary outcomes were gestation at birth, preterm birth (birth below 37+0), preterm birth before 35+0 weeks, preterm birth before 32+0 weeks, preterm birth before 28+0 weeks, preterm premature rupture of membranes (clinically confirmed rupture of membranes at <37+0), threatened preterm labour (requiring presentation to hospital and positive foetal fibronectin, or steroids and tocolysis or MgSO4 given for neuroprotection), antepartum haemorrhage (any significant per vaginum loss requiring hospital presentation), birth weight, small for gestational age (<10th centile for gestation based on standard population curves), congenital anomaly, nursery admission (consideration given to diagnoses recorded as per Perinatal Society of Australia & NZ Classification, with particular analysis of intraventricular haemorrhage, necrotizing enterocolitis, retinopathy of prematurity, respiratory distress syndrome), neonatal mortality (death recorded up until 28 days after live birth), mode of delivery, complete miscarriage (sub-categorized by gestation <12 weeks and 12–20 weeks), pregnancy loss between 20 and 23 weeks, postnatal depression (diagnosed by medical doctor or psychologist, whether medicated or not), gestational diabetes (antenatal Oral Glucose Tolerance Test meeting diagnostic criteria, fasting blood glucose >5.5 mmol/l, and 2 h level >8.0 mmol/l), venous thromboembolism (deep venous thrombosis or pulmonary embolus, requiring anti-coagulant therapy), pre-eclampsia, gestational hypertension (as per the Society of Obstetric Medicine of Australia and New Zealand, 2014 guidelines (Lowe et al., 2015)), and Depression Anxiety Stress Scale (DASS)-21 questionnaire scores (Antony et al., 1998). DASS-21 is a set of three self-report scales designed to measure the emotional states of depression, anxiety and stress. Each scale contains seven items that are rated between 0 and 3 according to how much a statement applies to the patient over the past week (never, sometimes, often and almost always). A total score for each scale is derived from the items and a higher score suggests more symptoms.
Data collection
Baseline characteristics were recorded based on medical history form, enrolment form, and pre-trial DASS-21 questionnaire (Lovibond and Lovibond, 1995). Treatment information and pregnancy outcome were collected from telephone follow-ups (at 12 weeks, 32 weeks of gestation, and 4 weeks after delivery), pregnancy outcome form, completion questionnaire, and post-trial DASS-21 questionnaire. We tried to collect outcome data for all participants, irrespective of compliance to the trial protocol.
Sample size
Threatened miscarriage was estimated to induce complete miscarriage in 25.9% of the placebo population. In four previous trials, the progestin-treated population quote a combined complete miscarriage rate of 13.8% (Gerhard et al., 1987; Palagiano et al., 2004; El-Zibdeh and Yousef, 2009; Pandian, 2009). Assuming type-1 error of 0.05 and power of 80%, and 10% of women being non-compliant or lost to follow-up, in a 1:1 ratio, a sample size of 193 women per arm was calculated.
Statistical analysis
Analysis was performed by intention to treat. Baseline characteristics were described by descriptive analysis. For continuous variables, normality was estimated using frequency histograms and the Shapiro test initially. If the variables were normally distributed, they were presented as mean with SD, otherwise, their medians and interquantile ranges (IQRs) were reported. Categorical variables were presented with the proportions of the two arms. RRs and their 95% CIs for binary outcomes were computed between the two treatment arms with the log-binomial regression or Poisson regression with robust variance estimate. Continuous outcomes, in view of their non-normality, were compared between the two treatment arms with the Wilcoxon rank-sum test. Subgroup analyses included: history of miscarriage, infertility prior to current pregnancy, maternal age (<40 years, ≥40 years), baseline progesterone level (<25 nmol/l, 25–50 nmol/l, >50 nmol/l), and gestation at recruitment (<8 weeks, 8–10 weeks). All analyses were two-sided. We used Stata 16.1 (Stata Corp, College Station, TX, USA) software for all statistical analyses.
Data monitoring and interim analysis
A Data Safety Monitoring Committee (DSMC) met initially at 3 months, thereafter on an annual basis, or urgently as required. The committee was comprised of a speciality-related clinician, a researcher, a statistician, and a consumer representative. A prespecified blinded third-party interim analysis was performed after enrolment had reached 200 participants. The DSMC reviewed the results of the interim analysis and made recommendations.
Results
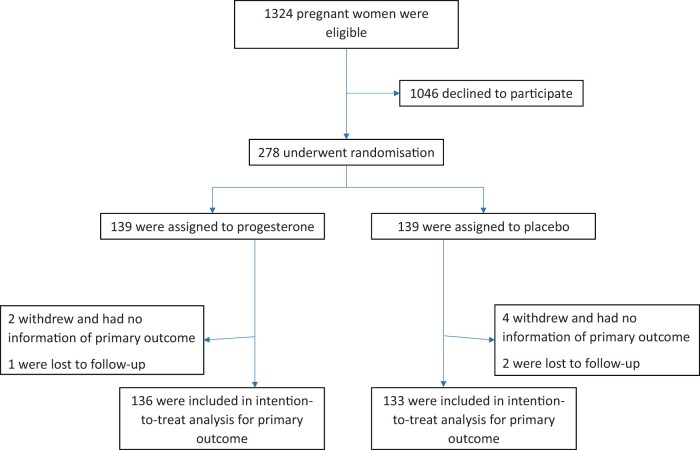
Between February 2012 and April 2019, we screened a total of 1324 women who were eligible and 278 were consented and randomly assigned to progesterone or placebo (Fig. 1). At the interim analysis, in view of the low likelihood to achieve statistical significance for the comparison and the difficulty of further recruitment, the DSMC recommended to stop the trial prematurely. When checking compliance, 154 (55.4%) were fully compliant as per protocol, while 105 (37.8%) were partially compliant, which includes 27 women who did not collect a subsequent script for a further 20 pessaries when expected to. Finally, 14 participants never used any pessaries after recruitment (6 of these women withdrew from trial).
Figure 1.
Recruitment, randomization, and follow-up of participants.
Baseline characteristics were comparable between the two treatment arms (Table I). Mean maternal age at randomization was 30.7 ± 5.0 years in the progesterone group and 30.4 ± 5.2 in the placebo group. Median numbers of miscarriages were 0 (IQR 0–1) and 1 (IQR 0–2) in the two groups, respectively. Median gestational age at randomization was also comparable (7.4 vs 7.4 weeks).
Table I.
Baseline characteristics of participants.
| Progesterone (N = 139) | Placebo (N = 139) | |
|---|---|---|
| Female age (years) | 30.7 ± 5.0 | 30.4 ± 5.2 |
| Female age (years) min to max | 18.5 to 44.5 | 18.2 to 48.3 |
| BMI | 24.9 (21.3 to 30.5) | 24.4 (21.8 to 28.0) |
| Gravidity | 3 (1 to 4) | 3 (2 to 4) |
| Parity | 0 (0 to 1) | 0 (0 to 1) |
| Infertility | 25/134 (18.7%) | 24/133 (18.1%) |
| Number of full term deliveries | 0 (0 to 1) | 0 (0 to 1) |
| Number of miscarriages | 0 (0 to 1) | 1 (0 to 2) |
| Smoker | 5/135 (3.7%) | 10/134 (7.5%) |
| Regular menstrual cycle | 110/137 (80.3%) | 101/132 (76.5%) |
| Cycles to conception | 3 (1 to 8) | 3 (1 to 8) |
| Gestational age at baseline | 7.4 (6.4 to 8.6) | 7.4 (6.6 to 8.6) |
| Beta hCG (IU/l) | 76 000 (42 366.0 to 130 302.0) | 76 149 (40 699.5 to 119 145.0) |
| Progesterone level (ng/ml) | 16.6 (12.7 to 21.7) | 16.3 (12.6 to 20.1) |
| <10 | 13/136 (9.6%) | 16/133 (12.0%) |
| ≥10 | 123/136 (90.4%) | 117/133 (88.0%) |
| Foetal heart rate (mph) | 146 (122 to 169) | 149 (118 to 168) |
| Vitamins or supplements intake | 117/134 (87.3%) | 117/131 (89.3%) |
| DASS 21 score baseline | ||
| Depression score | 4 (0 to 10) | 4 (2 to 8) |
| Anxiety score | 4 (2 to 10) | 4 (2 to 10) |
| Stress score | 10 (4 to 16) | 10 (4 to 16) |
Data are presented as mean ± SD, median (interquartile range), or n/N (%) unless specified.
For the primary outcome of live birth, the rates in the progesterone group (82.4%) and placebo group (84.2%) were similar (RR 0.98, 95% CI 0.88–1.09). The rates for complete miscarriage were also comparable (14.7% vs 15.8%, RR 0.93, 95% CI 0.53–1.64). There were no significant differences in the rates of preterm birth (RR 1.38, 95% CI 0.69–2.78) and small for gestational age (RR 0.32, 95% CI 0.07–1.53), and no significant differences were found for maternal outcomes (gestational diabetes, gestational hypertension, pre-eclampsia, venous thromboembolism, and postnatal depression) and neonatal outcomes (congenital anomaly, nursery admission, and mortality). Gestation at birth and birth weight between groups were also comparable (Table II).
Table II.
Treatment outcomes—intention-to-treat analysis.
| Progesterone (N = 139) | Placebo (N = 139) | ||||
|---|---|---|---|---|---|
|
| |||||
| Binary outcomes | |||||
| n/N (%) | n/N (%) | RR* | 95% CI* | P-value* | |
| Live birth | 112/136 (82.4) | 112/133 (84.2) | 0.98 | 0.88–1.09 | 0.683 |
| Miscarriage | 20/136 (14.7) | 21/133 (15.8) | 0.93 | 0.53–1.64 | 0.805 |
| <12 weeks | 11/130 (8.5%) | 11/130 (8.5) | 1 | 0.45–2.22 | 1 |
| 12–20 weeks | 3/130 (2.3%) | 7/130 (5.4%) | 0.43 | 0.11–1.62 | 0.212 |
| Pregnancy loss between 20 and 23 weeks | 4/136 (2.9) | 0/133 (0.0) | NA | NA | NA |
| Preterm birth | 17/132 (12.9) | 12/129 (9.3) | 1.38 | 0.69–2.78 | 0.361 |
| Preterm birth before 35 weeks | 10/132 (7.6) | 7/129 (5.4) | 1.4 | 0.55–3.56 | 0.484 |
| Preterm birth before 32 weeks | 8/132 (6.1) | 4/129 (3.1) | 1.95 | 0.60–6.33 | 0.264 |
| Preterm birth before 28 weeks | 7/132 (5.3) | 4/129 (3.1) | 1.71 | 0.51–5.70 | 0.382 |
| Small for gestational age | 2/111 (1.8) | 6/105 (5.7) | 0.32 | 0.07–1.53 | 0.152 |
| PPROM | 11/125 (8.8) | 8/123 (6.5) | 1.35 | 0.56–3.25 | 0.499 |
| Threatened preterm labour | 16/126 (12.7) | 14/122 (11.5) | 1.11 | 0.56–2.17 | 0.768 |
| Antepartum haemorrhage | 13/124 (10.5) | 21/123 (17.1) | 0.61 | 0.32–1.17 | 0.138 |
| Gestational diabetes | 16/126 (12.7) | 19/123 (15.5) | 0.82 | 0.44–1.52 | 0.534 |
| Gestational hypertension | 7/126 (5.6) | 10/123 (8.1) | 0.68 | 0.27–1.74 | 0.424 |
| Pre-eclampsia | 2/126 (1.6) | 7/123 (5.7) | 0.28 | 0.06–1.32 | 0.107 |
| Venous thromboembolism | 3/99 (3.0) | 1/103 (1.0) | 3.12 | 0.33–29.50 | 0.321 |
| Postnatal depression | 4/91 (4.4) | 7/99 (7.1) | 0.62 | 0.19–2.05 | 0.436 |
| Vaginal birth | 70/132 (53.0) | 61/130 (46.9) | 1.13 | 0.89–1.44 | 0.324 |
| Assisted vaginal birth | 14/132 (10.6) | 12/130 (9.2) | 1.15 | 0.55–2.39 | 0.71 |
| Elective caesarean | 25/132 (18.9%) | 27/130 (20.8%) | 0.91 | 0.56–1.48 | 0.711 |
| Emergency caesarean | 18/132 (13.6) | 24/130 (18.5) | 0.74 | 0.42–1.29 | 0.29 |
| Congenital anomaly | 9/121 (7.4) | 3/113 (2.7) | 2.8 | 0.78–10.09 | 0.115 |
| Nursery admission | 27/127 (21.3) | 20/118 (17.0) | 1.25 | 0.74–2.11 | 0.394 |
| Neonatal mortality | 1/134 (0.8) | 0/130 (0.0) | NA | NA | NA |
|
| |||||
| Continuous outcomes | |||||
|
| |||||
| Median (IQR) | Median (IQR) | P-value# | |||
|
| |||||
| Gestational age at birth (weeks) | 38.9 (38.0–39.6) | 39.0 (38.0–40.0) | 0.167 | ||
| Birth weight (g) | 3310 (2926–3710) | 3300 (2910–3760) | 0.992 | ||
| Depression score (at end of intervention) | 2 (0–4) | 0 (0–4) | 0.666 | ||
| Anxiety score (at end of intervention) | 2 (0–6) | 2 (0–8) | 0.904 | ||
| Stress score (at end of intervention) | 4 (2–10) | 4 (2–10) | 0.472 | ||
| Depression score (postpartum) | 2 (0–2) | 0 (0–4) | 0.903 | ||
| Anxiety score (postpartum) | 0 (0–2) | 0 (0–4) | 0.599 | ||
| Stress score (postpartum) | 6 (2–8) | 4 (0–8) | 0.311 | ||
Results of log-binomial regressions.
Results of Wilcoxon rank-sum test.
RR: risk ratio; PPROM: preterm prelabour rupture of membranes; IQR: interquartile range.
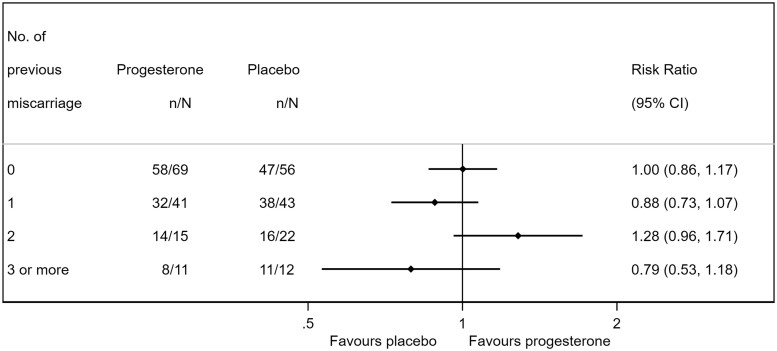
No significant differences were found in the subgroup analysis of live birth for history of miscarriage, infertility for current pregnancy, maternal age (<40 years, ≥40 years), baseline progesterone level (<25 nmol/l (7.9 ng/ml), 25–50 nmol/l, >50 nmol/l (15.7 ng/ml)), and gestation at recruitment (<8 weeks, 8–10 weeks) (Table III). In women with a history of miscarriage, live birth occurred in 80.6% of women in the progesterone group and 84.4% in the placebo group (RR 0.95, 95% CI 0.82–1.11). A further breakdown by the number of miscarriages did not suggest a dose–response pattern for the treatment effect of progesterone (Fig. 2).
Table III.
Subgroup analysis of live birth rate.
| Progesterone (N = 136) | Placebo (N = 133) | |||||
|---|---|---|---|---|---|---|
| n/N (%) | n/N (%) | RR | 95% CI | P | P for interaction | |
| No. of miscarriages | ||||||
| 0 | 58/69 (84.1) | 47/56 (83.9) | 1 | 0.86–1.17 | 0.984 | |
| ≥1 | 54/67 (80.6) | 65/77 (84.4) | 0.95 | 0.82–1.11 | 0.55 | 0.664 |
| Infertility for current pregnancy | ||||||
| No | 90/106 (84.9) | 88/104 (84.6) | 1 | 0.89–1.13 | 0.953 | |
| Yes | 19/25 (76.0) | 19/23 (82.6) | 0.92 | 0.69–1.23 | 0.572 | 0.585 |
| Maternal age | ||||||
| <40 years | 107/131 (81.7) | 108/127 (85.0) | 0.96 | 0.86–1.07 | 0.469 | |
| ≥40 years | 5/5 (100.0) | 4/6 (66.7) | 1.5 | 0.83–2.72 | 0.181 | 0.13 |
| Baseline progesterone level | ||||||
| <25 nmol/l | 2/10 (20.0) | 1/5 (20.0) | 1 | 0.11–9.23 | 1 | |
| 25–50 nmol/l | 40/49 (81.6) | 40/50 (80.0) | 1.02 | 0.84–1.24 | 0.837 | 0.985 |
| >50 nmol/l | 68/74 (91.9) | 66/73 (90.4) | 1.02 | 0.92–1.12 | 0.753 | 0.988 |
| Gestation at recruitment | ||||||
| <8 weeks | 70/89 (78.7) | 66/82 (80.5) | 0.98 | 0.84–1.14 | 0.767 | |
| 8–10 weeks | 42/47 (89.4) | 46/51 (90.2) | 0.99 | 0.87–1.13 | 0.892 | 0.894 |
Figure 2.
Subgroup analysis according to the number of previous miscarriages. Graphical presentation shows risk ratio (point) and 95% confidence interval (bar).
In line with the intention-to-treat analysis, the per-protocol analysis limited to women who were fully compliant showed similar results (Supplementary Table SI).
Discussion
In this double-blinded, placebo-controlled randomized clinical trial in women with threatened miscarriage, 400 mg progesterone applied vaginally and nightly, from onset until 12 weeks, did not increase live birth rates. There were also no significant differences in the rates of miscarriage, preterm birth, and perinatal outcomes.
There are some limitations of our study. First, our study was a single-centre trial, and this may limit the generalizability of the findings. Generalizability may also be restricted by the low participation rate (21%) which may be explained by the fact that women with threatened miscarriage were experiencing immense physical and psychological stress at the time of being asked to consider trial participation. Additionally, this trial was stopped prematurely at the interim analysis and did not reach the planned sample size. However, this decision was justified by the futility of the intervention and ethical considerations. Finally, this trial cannot provide direct evidence with respect to other forms and administration routes of progesterone.
For the primary outcome live birth, our finding is consistent with a large trial in the UK population (Coomarasamy et al., 2019), where vaginal suppositories containing 400 mg progesterone administered twice daily, did not result in a significantly higher live birth rate than placebo. However, while the UK trial suggested that the effect of progesterone differed according to the number of previous miscarriages in a positive dose–response manner, with stronger effect in those who had a higher number of miscarriages, we did not find such a trend in our trial. If this finding in the UK trial was generalizable, we would expect to find a similar pattern even with a smaller sample size.
Apart from chance findings, several differences in the study population and procedure between the two trials may explain this inconsistency. First, our trial included pregnancies <10 weeks, while the UK trial included pregnancies <12 weeks (Coomarasamy et al., 2019). Also, in our study 17 women (6%) were over 39 years old while in the UK study all participants were 39 years old or younger because of more stringent eligibility criteria. Second, we used pessaries containing 400 mg progesterone that applied nightly until 12 weeks of gestation, the UK trial used vaginal suppositories containing 400 mg progesterone twice daily, and the intervention was not planned to stop until 16 completed weeks of gestation. This duration of therapy difference may be critical, as our trial experienced four late mid-trimester losses at 20–23 weeks, all occurring in the progesterone arm raising the possibility that previously sustained pregnancies were provided therapy for too short a duration.
Summing up the findings of this trial and existing evidence, vaginal progesterone makes no benefit to live birth rate for women with threatened miscarriage. Similarly, use of oral progestogen also did not increase live birth (Chan et al., 2021). There is a lack of data assessing the effectiveness of 17-α-hydroxyprogesterone or oral micronized progesterone. Progesterone might be beneficial in selected women, such as those who had two or more miscarriages, but the effect may only be exerted when a high dose is used (e.g. 800 mg daily) and maintained until at least 16 weeks of gestation. This should be the focus of further research and it is desirable to conduct dedicated trials in women with previous miscarriage to generate unequivocal evidence.
Progesterone treatment is recommended for the management of recurrent miscarriage. This recommendation is based on a 2019 Cochrane review with ten studies (1684 women) reporting a reduced risk of miscarriage (RR 0.73, 95% CI 0.54–1.00) in unselected women with recurrent miscarriage, and in women with at least three previous miscarriages (four studies including 1334 women; RR 0.59, 95% CI 0.34–1.01) (Haas et al., 2019). This Cochrane review had already excluded a large study that had been retracted following concerns about the accuracy and trustworthiness of the trial data (Ismail et al., 2018). We previously expressed concern on other three trials that were included in the 2019 Cochrane review (Chong et al., 2021). Without these three studies, the treatment effect of progesterone becomes minimal and non-significant (RR 0.95, 95% CI 0.79–1.13). The updated Cochrane review in April 2021 excluded 13 out of 20 eligible studies using a trustworthiness screening tool and concluded that progestogens probably make little or no difference to live birth rate for women with recurrent miscarriage (Devall et al., 2021).
In conclusion, we did not find evidence that vaginal progesterone increases live birth rate in women with threatened miscarriage.
Supplementary Material
Acknowledgements
The research team are grateful to the women and families who participated at an emotionally difficult time for them.
Contributor Information
Lucas A McLindon, Department of Obstetrics and Gynaecology, Mater Mothers’ Hospitals, Mater Health, Brisbane, Queensland, Australia; Department of Obstetrics and Gynaecology, University of Queensland, Brisbane, Queensland, Australia.
Gabriel James, Department of Obstetrics and Gynaecology, Mater Mothers’ Hospitals, Mater Health, Brisbane, Queensland, Australia; Department of Obstetrics and Gynaecology, University of Queensland, Brisbane, Queensland, Australia.
Michael M Beckmann, Department of Obstetrics and Gynaecology, Mater Mothers’ Hospitals, Mater Health, Brisbane, Queensland, Australia; Department of Obstetrics and Gynaecology, University of Queensland, Brisbane, Queensland, Australia.
Julia Bertolone, Department of Obstetrics and Gynaecology, Mater Mothers’ Hospitals, Mater Health, Brisbane, Queensland, Australia.
Kassam Mahomed, Department of Obstetrics and Gynaecology, University of Queensland, Brisbane, Queensland, Australia; Department of Obstetrics and Gynaecology, Ipswich Hospital, Ipswich, Queensland, Australia.
Monica Vane, Department of Obstetrics and Gynaecology, Mater Mothers’ Hospitals, Mater Health, Brisbane, Queensland, Australia.
Teresa Baker, Department of Obstetrics and Gynaecology, Mater Mothers’ Hospitals, Mater Health, Brisbane, Queensland, Australia.
Monique Gleed, Department of Obstetrics and Gynaecology, Mater Mothers’ Hospitals, Mater Health, Brisbane, Queensland, Australia.
Sandra Grey, Department of Obstetrics and Gynaecology, Mater Mothers’ Hospitals, Mater Health, Brisbane, Queensland, Australia.
Linda Tettamanzi, Department of Obstetrics and Gynaecology, Mater Mothers’ Hospitals, Mater Health, Brisbane, Queensland, Australia.
Ben Willem J Mol, Department of Obstetrics and Gynaecology, Monash University, Monash Medical Centre, Clayton, Victoria, Australia; Aberdeen Centre for Women’s Health Research, School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Aberdeen, UK.
Wentao Li, Department of Obstetrics and Gynaecology, Monash University, Monash Medical Centre, Clayton, Victoria, Australia.
Supplementary data
Supplementary data are available at Human Reproduction online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Authors’ roles
L.A.M., M.M.B., and K.M. designed the study. L.A.M. coordinated the trial. M.V. and G.J. collected the data. J.B., M.V., T.B., M.G., S.G., and L.T. managed the trial in the hospital, and commented on the paper. W.L. performed the statistical analyses. W.L., B.W.J.M., and L.A.M. drafted the paper. All authors interpreted the data, critically revised the article, and approved the final version.
Funding
Mothers and babies Golden Casket Clinical Fellowship (L.A.M.). Progesterone and placebo pessaries were provided by Perrigo Australia.
Conflict of interest
B.W.J.M. reports grants from NHMRC, personal fees from ObsEva, personal fees from Merck KGaA, personal fees from Guerbet, personal fees from iGenomix, outside the submitted work.
References
- Antony MM, Bieling PJ, Cox BJ, Enns MW, Swinson RP.. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol Assess 1998;10:176–181. [Google Scholar]
- Arck PC, Rücke M, Rose M, Szekeres-Bartho J, Douglas AJ, Pritsch M, Blois SM, Pincus MK, Bärenstrauch N, Dudenhausen JW. et al. Early risk factors for miscarriage: a prospective cohort study in pregnant women. Reprod Biomed Online 2008;17:101–113. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Laurent C, Croughan MS, Olney RS, Lammer EJ.. Maternal progestin intake and risk of hypospadias. Arch Pediatr Adolesc Med 2005;159:957–962. [DOI] [PubMed] [Google Scholar]
- Chan DMK, Cheung KW, Ko JKY, Yung SSF, Lai SF, Lam MT, Ng DYT, Lee VCY, Li RHW, Ng EHY.. Use of oral progestogen in women with threatened miscarriage in the first trimester: a randomized double-blind controlled trial. Hum Reprod 2021;36:587–595. [DOI] [PubMed] [Google Scholar]
- Chong K, Li W, Roberts I, Mol BW.. Making miscarriage matter. Lancet 2021;398:743–744. [DOI] [PubMed] [Google Scholar]
- Coomarasamy A, Devall AJ, Cheed V, Harb H, Middleton LJ, Gallos ID, Williams H, Eapen AK, Roberts T, Ogwulu CC. et al. A randomized trial of progesterone in women with bleeding in early pregnancy. N Engl J Med 2019;380:1815–1824. [DOI] [PubMed] [Google Scholar]
- Dede FS, Ulucay U, Kose MF, Dede H, Dilbaz S.. Fetal loss in threatened abortion after demonstration of fetal cardiac activity in a low socioeconomic population. J Obstet Gynaecol 2010;30:622–625. [DOI] [PubMed] [Google Scholar]
- Devall AJ, Papadopoulou A, Podesek M, Haas DM, Price MJ, Coomarasamy A, Gallos ID.. Progestogens for preventing miscarriage: a network meta-analysis. Cochrane Database Syst Rev 2021;4:Cd013792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Yan D, Zeng W, Yang X, Wei Q.. Predictive power progesterone combined with beta human chorionic gonadotropin measurements in the outcome of threatened miscarriage. Arch Gynecol Obstet 2011;283:431–435. [DOI] [PubMed] [Google Scholar]
- El-Zibdeh MY, Yousef LT.. Dydrogesterone support in threatened miscarriage. Maturitas 2009;65(Suppl 1):S43–S46. [DOI] [PubMed] [Google Scholar]
- Gerhard I, Gwinner B, Eggert-Kruse W, Runnebaum B.. Double-blind controlled trial of progesterone substitution in threatened abortion. Biol Res Pregnancy Perinatol 1987;8:26–34. [PubMed] [Google Scholar]
- Haas DM, Hathaway TJ, Ramsey PS.. Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology. Cochrane Database Syst Rev 2019;2019:CD003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamulyák EN, Scheres LJ, Marijnen MC, Goddijn M, Middeldorp S.. Aspirin or heparin or both for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss. Cochrane Database Syst Rev 2020;5:CD012852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan R, Baird DD, Herring AH, Olshan AF, Funk MLJ, Hartmann KE.. Association between first-trimester vaginal bleeding and miscarriage. Obstet Gynecol 2009;114:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AM, Abbas AM, Ali MK, Amin AF.. Peri-conceptional progesterone treatment in women with unexplained recurrent miscarriage: a randomized double-blind placebo-controlled trial. J Matern Fetal Neonatal Med 2018;31:388–394. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Van Oppenraaij RH, Burton GJ.. Obstetric outcome after early placental complications. Curr Opin Obstet Gynecol 2010;22:452–457. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Lovibond SH.. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther 1995;33:335–343. [DOI] [PubMed] [Google Scholar]
- Lowe SA, Bowyer L, Lust K, McMahon LP, Morton M, North RA, Paech M, Said JM.. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust N Z J Obstet Gynaecol 2015;55:e1–e29. [DOI] [PubMed] [Google Scholar]
- Palagiano A, Bulletti C, Pace MC, DE Ziegler D, Cicinelli E, Izzo A.. Effects of vaginal progesterone on pain and uterine contractility in patients with threatened abortion before twelve weeks of pregnancy. Ann N Y Acad Sci 2004;1034:200–210. [DOI] [PubMed] [Google Scholar]
- Pandian RU. Dydrogesterone in threatened miscarriage: a Malaysian experience. Maturitas 2009;65(Suppl 1):S47–S50. [DOI] [PubMed] [Google Scholar]
- Salazar EL, Calzada L.. The role of progesterone in endometrial estradiol- and progesterone-receptor synthesis in women with menstrual disorders and habitual abortion. Gynecol Endocrinol 2007;23:222–225. [DOI] [PubMed] [Google Scholar]
- Saraswat L, Bhattacharya S, Maheshwari A, Bhattacharya S.. Maternal and perinatal outcome in women with threatened miscarriage in the first trimester: a systematic review. BJOG 2010;117:245–257. [DOI] [PubMed] [Google Scholar]
- Thapar AK, Thapar A.. Psychological sequelae of miscarriage: a controlled study using the general health questionnaire and the hospital anxiety and depression scale. Br J Gen Pract 1992;42:94–96. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.