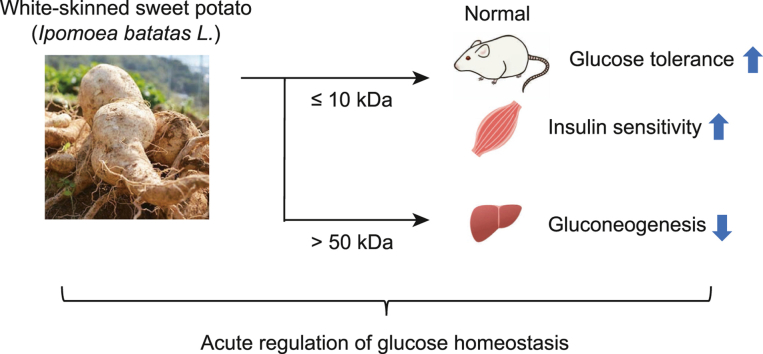
Abstract
Long-term administration of Ipomoea batatas L. (white-skinned sweet potato, WSSP) has been reported to help manage type 2 diabetes mellitus (T2DM) in humans and animals; however, the mechanisms of blood glucose regulation by WSSP remain unclear. Therefore, we aimed to investigate the acute effects of WSSP on blood glucose homeostasis under normal conditions and the underlying mechanisms. Three fractions of WSSP (≤10, 10–50, and >50 kDa) were obtained via ultracentrifugation. Rats were subjected to an oral glucose tolerance test (OGTT) after a single administration of WSSP. The insulin tolerance test (ITT) and pyruvate tolerance test (PTT) were performed to evaluate insulin sensitivity and gluconeogenesis, respectively. Single WSSP administration markedly reduced blood glucose levels as revealed by the OGTT. Serum insulin levels were not increased by WSSP treatment. Blood glucose levels during ITT were significantly reduced due to WSSP treatment. WSSP treatment activated the phosphorylation of Akt, thereby activating insulin signaling in the skeletal muscles and liver. The ≤10 kDa fraction considerably reduced blood glucose levels per the OGTT and ITT. In contrast, gluconeogenesis in PTT and the expression of key enzymes in hepatocytes were suppressed by the >50 kDa fraction. This study demonstrated that WSSP acutely reduced postprandial blood glucose levels by improving insulin sensitivity in skeletal muscles in normal rats, which was attributed to constituents with a molecular weight of ≤10 kDa. Moreover, WSSP treatment suppressed gluconeogenesis in the liver, for which constituents of >50 kDa were responsible. Thus, WSSP can acutely regulate blood glucose homeostasis via multiple mechanisms. Since postprandial hyperglycemia leads to the onset of T2DM, WSSP, as a functional food, may possess potential active compounds that prevent T2DM.
Keywords: White-skinned sweet potato, Postprandial blood glucose, Insulin sensitivity, Gluconeogenesis
Graphical abstract
Highlights
-
•
WSSP suppressed postprandial blood glucose levels via improved insulin sensitivity.
-
•
The ≤10 kDa fraction of WSSP improved glucose tolerance and insulin sensitivity.
-
•
The >50 kDa fraction of WSSP suppressed gluconeogenesis both in vivo and in vitro.
-
•
WSSP can exhibit hypoglycemic activity via multiple constituents in normal rats.
1. Introduction
The incidence of type 2 diabetes mellitus (T2DM) is rapidly increasing, and is one of the most serious global problems. In 2021, 537 million adults (age, 20–79 years) suffered diabetes worldwide. This number has been predicted to increase to 643 million by 2030 and 783 million by 2045 [1]. Moreover, over 75% of adults with diabetes live in low- and middle-income countries [1]. The etiology of T2DM involves lifestyle factors, such as dietary and exercise habits, as well as aging and genetic predispositions. These factors comprehensively affect insulin secretion from pancreatic β-cells and insulin sensitivity in peripheral tissues [[2], [3], [4]]. Elevated postprandial blood glucose levels usually indicate an early metabolic change in T2DM development [5,6]. Postprandial hyperglycemia is an independent risk factor for T2DM complications, such as microangiopathy and macroangiopathy [7,8]. Since the suppression of postprandial hyperglycemia can reduce the incidence of T2DM, the control of postprandial blood glucose levels via dietary therapy may be effective in the prevention of T2DM [9,10]. Regarding dietary therapy, beneficial effects of meal sequence have been reported for the control of postprandial glucose level elevation and body weight [11]. The identification of food and natural materials that can improve postprandial hyperglycemia and T2DM represents an area of growing interest [12,13].
Various plants and natural resources have been investigated for their effects on T2DM, few of which have been used in medicine since ancient times. Ipomoea batatas L. (white-skinned sweet potato, WSSP), also known as Caiapo, belongs to the Convolvulaceae family. WSSP, originating in Brazil, has been used as a folk medicine for treating diabetes and other diseases in the Shikoku region of Japan [14]. Previous studies have shown that WSSP reduces fasting blood glucose and cholesterol levels and improves glucose tolerance in patients with T2DM after treatment for 6 weeks [15,16]. WSSP has also been reported to improve glucose tolerance after treatment for approximately 30 days in diabetic and obese animals such as KKAy and db/db mice [17]. In Zucker fatty rats, 7 weeks of WSSP treatment lowered blood glucose, insulin, triglyceride, and free fatty acid levels and improved glucose tolerance [14]. Thus, long-term administration of WSSP may be effective in T2DM management; however, the mechanism of blood glucose regulation by WSSP remains unclear.
In the present study, we evaluated glucose tolerance after a single WSSP administration in normal rats to investigate the acute effect of WSSP on blood glucose regulation. We further examined the mechanisms underlying blood glucose regulation. Additionally, the bioactive constituents in WSSP were investigated by fractionating the WSSP solution. Here, we show that WSSP acutely suppresses postprandial blood glucose elevation by improving insulin sensitivity in skeletal muscles in a non-diabetic normal state, a property attributed to constituents with a molecular weight of ≤10 kDa.
2. Materials and methods
2.1. Preparation and fractionation of WSSP
WSSP powder, obtained by squeezing WSSP tubers, centrifuging the juice at 6000×g for 30 min, and lyophilizing the supernatant, was provided by Fujisangyo (Kagawa, Japan). The powder (0.5 g) was suspended in 10 mL of distilled water at a concentration of 5% for ultracentrifugation. The WSSP solution (10 mL) in Vivaspin 20–50 K (Cytiva, Tokyo, Japan) was centrifuged (5500×g, 4 °C, 15 min) several times to prevent membrane clogging. The ≤50 kDa fraction was then placed in Vivaspin 20–10 K (Cytiva) and centrifuged as described above. Subsequently, the WSSP solution was separated into three fractions (>50 kDa: 0.10 mL, 10–50 kDa: 0.22 mL, and ≤10 kDa: 9.68 mL) based on molecular weight. Each fraction of the WSSP solution was totaled up to 10 mL using distilled water.
2.2. Animals
The experimental procedures used for animals were approved by the Ritsumeikan University Biwako-Kusatsu Campus Animal Care Committee (BKC2019-034 and BKC2021-018). Male Wistar ST rats (Japan SLC, Hamamatsu, Japan) were housed in a temperature-controlled environment under a 12 h light–dark cycle with free access to water and standard laboratory chow (CRF-1; Oriental Yeast, Tokyo, Japan). The experiments were performed using rats aged 6–7 weeks (180–230 g body weight).
2.3. Oral glucose tolerance test (OGTT)
OGTT was performed as described previously [18]. Briefly, the rats were fasted for 16 h and then orally administered with glucose (2 g/kg body weight) at 9:00 a.m. The WSSP solution or its fractions (10 mL/kg body weight) were orally administered 15 min before glucose injection. Blood samples were collected from the tail vein, and glucose levels were measured using the glucose oxidase method (Glucocard G Black; Arkray, Kyoto, Japan). The incremental area under the curve (iAUC) was calculated using the trapezoidal rule. Serum insulin levels were determined using ELISA (Morinaga Institute of Biological Science, Yokohama, Japan). Experiments using the same protocol were repeated two or three times to ensure reproducibility.
2.4. Insulin tolerance test (ITT)
ITT was performed as described previously [19], with minor modifications. Briefly, the rats were fasted for 6 h and then intraperitoneally administered with human insulin (Lilly, Indianapolis, IN, USA) (0.75 U/kg body weight) at 3:00 p.m. The WSSP solution or its fractions (10 mL/kg body weight) were orally administered 15 min before insulin injection. Blood glucose levels were measured as described above.
2.5. Pyruvate tolerance test (PTT)
PTT was performed as described previously [20]. Briefly, the rats were fasted for 16 h and then intraperitoneally administered with sodium pyruvate (1 g/kg body weight) at 9:00 a.m. The WSSP solution or its fractions (10 mL/kg body weight) were orally administered 15 min before pyruvate injection. Blood glucose levels were measured as described above.
2.6. Hepatocyte isolation and hepatic glucose production
Hepatocytes were isolated from rats via collagenase digestion as described previously [21], with minor modifications. Briefly, rats were perfused through the inferior vena cava with washing solution (142 mM NaCl, 2.7 mM KCl, 0.28 mM Na2HPO4·12H2O, 10 mM HEPES, and 0.1 mM EGTA [pH 7.4]) for 5 min (11.6 mL/min) and then with isolation solution (142 mM NaCl, 2.7 mM KCl, 0.28 mM Na2HPO4·12H2O, 10 mM HEPES, 0.1 mM CaCl2, 5 mM glucose, and 200 μg/mL collagenase type II (Worthington Biochemical, Lakewood, NJ) [pH 7.4]) for 9 min (11.6 mL/min). The isolated hepatocytes were incubated for 1 h at 37 °C in DMEM (without glucose and pyruvate) (GIBCO, Rockville, MD). For gluconeogenesis analysis, the hepatocytes were treated for 2 h at 37 °C in 48-well plates (5.1 × 105 cells/well) with Krebs-Ringer bicarbonate buffer (119.4 mM NaCl, 3.7 mM KCl, 2.7 mM CaCl2, 1.3 mM KH2PO4, 1.3 mM MgSO4, and 24.8 mM NaHCO3) containing 0.25 mM 3-isobutyl-1-methylxanthine and gluconeogenic substrates (1 mM pyruvate and 10 mM lactate) with WSSP or its fractions. Glucose concentration in aliquots of the supernatant was measured using the Gopod method (Megazyme, Huissen, Netherlands). Protein concentrations in cell lysates were determined using the BCA method (FUJIFILM Wako Pure Chemical, Osaka, Japan).
2.7. Immunoblotting of tissue and hepatocytes
The rats were fasted for 16 h and then intraperitoneally administered insulin (3 U/kg body weight) at 9:00 a.m. The WSSP solution (10 mL/kg body weight) was orally administered 15 min before insulin injection. Skeletal muscles and liver tissues were isolated from rats euthanized 15 min after insulin administration. The tissues were homogenized in lysis buffer (RIPA buffer containing 1 mM EDTA [pH 8.0]), and the lysates were centrifuged at 3,000 rpm for 1 min at 4 °C. Hepatocytes treated under the same conditions as those used for hepatic glucose production were homogenized in a lysis buffer. The supernatants (20 μg of protein content) were heated at 95 °C for 5 min and subjected to electrophoresis on SDS-polyacrylamide (8%) gels and then transferred onto polyvinylidene difluoride membranes (Immobilon-P; Merck-Millipore, Darmstadt, Germany). The primary antibodies used were anti-phospho-Akt (Thr308), anti-Akt, anti-pan-actin, anti-phosphoenolpyruvate carboxykinase (PEPCK) 1, and anti-β-actin (Cell Signaling Technology, Danvers, MA), along with anti-glucose-6-phosphatase (G6Pase) catalytic subunit 3 (Abcam, Tokyo, Japan). The secondary antibody used was HRP-conjugated anti-rabbit antibody (Cell Signaling Technology). The fluorescent bands were visualized and quantified using Amersham Imager 600 (Cytiva).
2.8. Statistical analysis
Statistical analyses were performed using GraphPad Prism for Windows (version 7; GraphPad Software, San Diego, CA). Data are expressed as the mean ± standard deviation. Significant differences were evaluated using Student’s t-test or ANOVA, followed by Tukey’s multiple comparison test. P < 0.05 was considered statistically significant.
3. Results
3.1. Improvement of glucose tolerance upon WSSP treatment
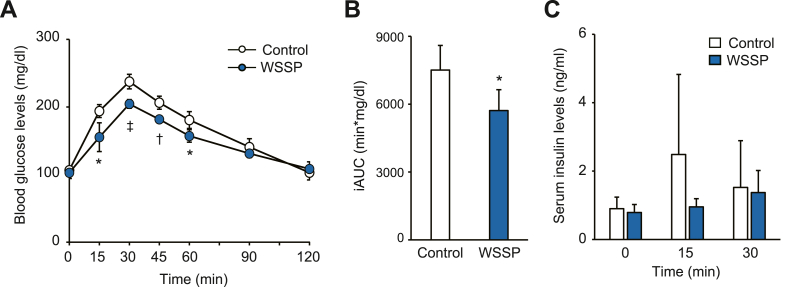
To determine the acute effect of WSSP on blood glucose regulation, an OGTT was performed in normal rats after a single administration of the WSSP solution. In the WSSP treatment group, blood glucose levels during the OGTT were significantly lower than those in the control group at 15 min (155.4 ± 21.2 mg/dL vs 194.0 ± 9.5 mg/dL, P < 0.05), 30 min (204.6 ± 6.4 mg/dL vs 237.6 ± 10.8 mg/dL, P < 0.001), 45 min (181.8 ± 4.4 mg/dL vs 206.4 ± 9.6 mg/dL, P < 0.005), and 60 min (157.2 ± 9.2 mg/dL vs 180.8 ± 12.5 mg/dL, P < 0.05) (Fig. 1A). The iAUC was also markedly lower in the WSSP treatment group than in the control group (Fig. 1B). There was no change in the serum insulin levels between the WSSP treatment group and the control group during the OGTT (Fig. 1C). Therefore, WSSP may acutely suppress the blood glucose response after glucose loading independent of insulin secretion.
Fig. 1.
WSSP treatment improves glucose tolerance in normal rats. (A) Blood glucose levels during OGTT after oral administration of WSSP. (B) The iAUC of blood glucose levels in (A). Data are expressed as the mean ± standard deviation (n = 5). *P < 0.05, †P < 0.005, and ‡P < 0.001 vs control. (C) Serum insulin levels during the OGTT after oral administration of WSSP. Data are expressed as the mean ± standard deviation (n = 6).
3.2. Improvement of insulin sensitivity upon WSSP treatment
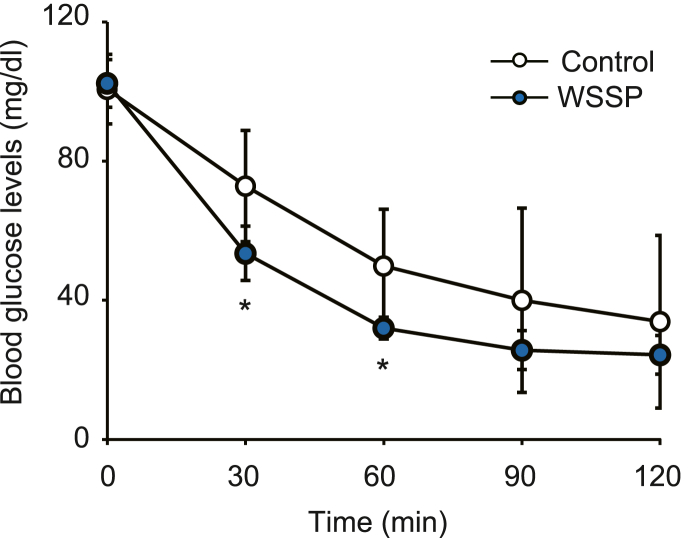
Next, we examined the effect of WSSP on insulin sensitivity in peripheral tissues, such as the liver, skeletal muscle, and adipose tissue, via the ITT. Blood glucose levels during the ITT in the WSSP treatment group were significantly lower than those of the control group at 30 min (53.5 ± 7.8 mg/dL vs 72.8 ± 16.0 mg/dL, P < 0.05) and 60 min (32.0 ± 3.1 mg/dL vs 49.8 ± 16.4 mg/dL, P < 0.05) (Fig. 2). This finding suggests that WSSP acutely improves insulin sensitivity, resulting in the reduction of postprandial blood glucose levels.
Fig. 2.
WSSP treatment improves insulin sensitivity in normal rats. Blood glucose levels during ITT after oral administration of WSSP are shown. Data are expressed as the mean ± standard deviation (n = 6). *P < 0.05 vs control.
3.3. Activation of insulin signaling in skeletal muscle and liver upon WSSP treatment
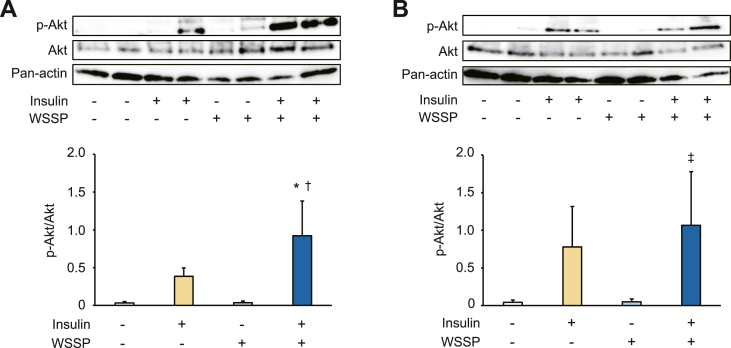
We investigated the effect of WSSP on insulin signaling based on the phosphorylation of Akt in insulin-acting tissues. In the skeletal muscles, insulin considerably increased Akt phosphorylation in the WSSP treatment group, and the increase was significantly higher than in the control group (Fig. 3A). A significant increase in Akt phosphorylation by insulin in the WSSP treatment group was also observed in the liver and was higher than in the control group (Fig. 3B). Therefore, WSSP may activate insulin signaling in peripheral tissues, including the skeletal muscles and liver.
Fig. 3.
Effects of WSSP on insulin signaling in skeletal muscle and liver. The expression of phosphorylation of Akt in the skeletal muscles (A) and liver (B). The tissues were isolated after oral administration of WSSP and intraperitoneal injection of insulin. Subsequently, the lysates were subjected to Western blot analysis. Representative blot panels of each independent experiments are shown. Intensities of the bands were quantified, and the bar graphs show p-Akt levels corrected by Akt levels. Data are expressed as the mean ± standard deviation (n = 4). *P < 0.05 vs insulin. †P < 0.001 vs WSSP. ‡P < 0.05 vs WSSP.
3.4. Effects of WSSP fractions on glucose tolerance and insulin sensitivity
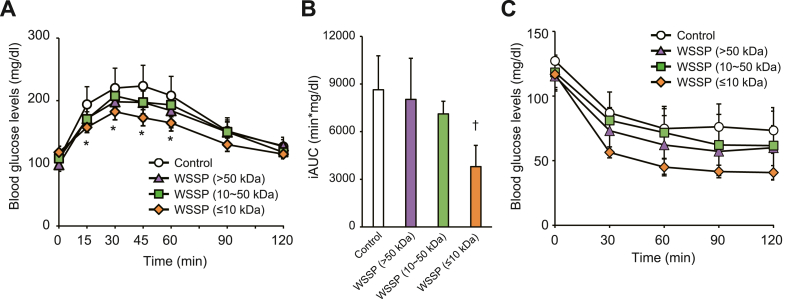
To determine the active constituents in WSSP that improved glucose tolerance, the WSSP solution was separated into three fractions (≤10, 10–50, and >50 kDa) via ultracentrifugation. Then, OGTT was performed after a single administration of each fraction. Blood glucose levels in the ≤10 kDa fraction group were significantly lower than those of the control group at 15 min (157.5 ± 8.3 mg/dL vs 194.0 ± 28.3 mg/dL, P < 0.05), 30 min (182.3 ± 12.8 mg/dL vs 220.2 ± 31.9 mg/dL, P < 0.05), 45 min (172.8 ± 12.9 mg/dL vs 223.5 ± 33.0 mg/dL, P < 0.05), and 60 min (164.5 ± 12.9 mg/dL vs 208.2 ± 30.7 mg/dL, P < 0.05) (Fig. 4A). The iAUC was significantly lower in the ≤10 kDa fraction group than in the control group (P < 0.001) (Fig. 4B). In the ITT, Blood glucose levels in the ≤10 kDa fraction group among three fraction groups were considerably lower than those of the control group (Fig. 4C). These results indicate that the active constituents involved in improving glucose tolerance have a molecular weight of ≤10 kDa.
Fig. 4.
WSSP (≤10 kDa) improves the glucose tolerance and insulin sensitivity in normal rats. (A) Blood glucose levels during OGTT after oral administration of each fraction of WSSP. (B) The iAUC of blood glucose levels in (A). Data are expressed as the mean ± standard deviation (n = 6). *P < 0.05 and †P < 0.001vs control. (C) Blood glucose levels during ITT after oral administration of each fraction of WSSP. Data are expressed as the mean ± standard deviation (n = 5–6).
3.5. Effects of WSSP fractions on gluconeogenesis in vivo
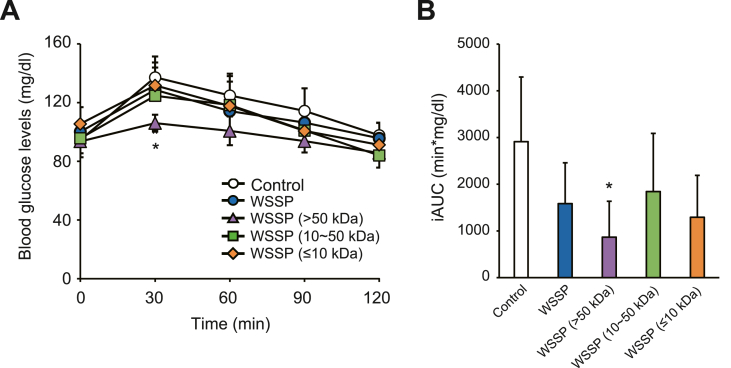
Since WSSP treatment activated insulin signaling in the liver (Fig. 3B), the effect of WSSP on gluconeogenesis was investigated using a PTT. In the >50 kDa fraction group, blood glucose level was significantly lower than that of the control group at 30 min (106.0 ± 5.7 mg/dL vs 137.2 ± 14.2 mg/dL, P < 0.05) (Fig. 5A). The iAUC of the >50 kDa fraction group was significantly lower than that of the control group (P < 0.05) (Fig. 5B). Thus, WSSP may also suppress gluconeogenesis, wherein constituents with a molecular weight of >50 kDa are involved.
Fig. 5.
WSSP (>50 kDa) suppresses gluconeogenesis in normal rats. (A) Blood glucose levels during PTT after oral administration of WSSP or each of its fractions. (B) The iAUC of blood glucose levels in (A). Data are expressed as the mean ± standard deviation (n = 6). *P < 0.05 vs control.
3.6. Effects of WSSP fractions on gluconeogenesis in vitro
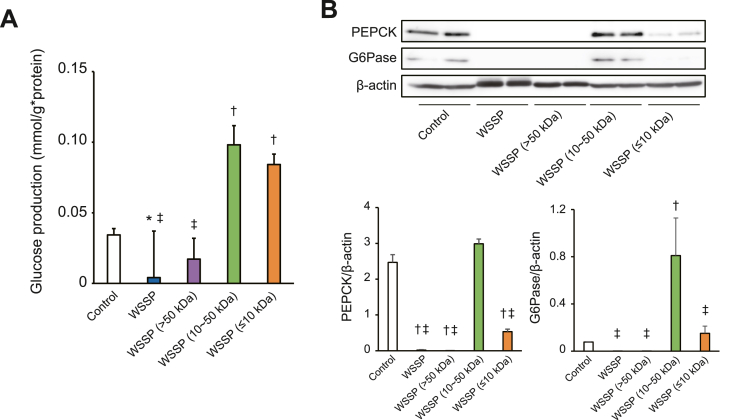
We further investigated the effects of the WSSP fractions on gluconeogenesis in vitro using isolated hepatocytes. Hepatic glucose production in the WSSP treatment and >50 kDa fraction groups was decreased when compared with that of the control group (Fig. 6A). The expression of a key gluconeogenic enzyme, PEPCK, in the WSSP treatment and >50 kDa fraction groups was significantly decreased when compared with the control group (Fig. 6B). The expression of G6Pase in the WSSP treatment and >50 kDa fraction groups was also decreased (Fig. 6C). However, glucose production and the expression of G6Pase in the 10–50 kDa fraction group were significantly increased when compared with those of the control group. These findings suggest that the 10–50 kDa fraction may enhance gluconeogenesis and suppress the anti-gluconeogenic effects of WSSP (Fig. 5), although the >50 kDa fraction had an obvious anti-gluconeogenic effect.
Fig. 6.
Effects of the WSSP fractions on gluconeogenesis in rat hepatocytes. (A) Glucose production after exposure to WSSP or each of its fractions in isolated hepatocytes. Hepatocytes were incubated for 2 h in the presence of gluconeogenic substrates (pyruvate and lactate) with WSSP or each fraction. Data are expressed as the mean ± standard deviation (n = 6). (B) Expression of PEPCK and G6Pase after exposure to WSSP or each of its fractions in isolated hepatocytes. The lysates of hepatocytes incubated as described above were subjected to Western blot analysis. Representative blot panels of each independent experiment are shown. Intensities of the bands were quantified, and the bar graphs show PEPCK and G6Pase levels corrected by β-actin levels. Data are expressed as the mean ± standard deviation (n = 4). *P < 0.05 vs control. †P < 0.001 vs control. ‡P < 0.001 vs WSSP (10–50 kDa).
4. Discussion
Several plants have been reported to serve as functional foods for the treatment of T2DM [12,13]. Among them, WSSP has been used in folk medicine for managing diabetes in a part of Japan [14] and has been shown to improve glucose tolerance after treatment for a few months in humans and animals with T2DM [[14], [15], [16], [17]]. However, the mechanism of blood glucose regulation by WSSP has not been elucidated, although improvements in insulin resistance in patients with T2DM [16] and hyperinsulinemia in T2DM animals with obesity [14] have been observed. The present study revealed that a single administration of WSSP acutely suppressed postprandial blood glucose elevation in healthy animals, which was attributed to improvement in insulin sensitivity, and not insulin secretion, via the phosphorylation of Akt in insulin-acting tissues. Insulin secreted from pancreatic β-cells binds to the insulin receptor on target tissues, such as skeletal muscles, which leads to the phosphorylation of insulin receptor substrate-1 and the subsequent phosphorylation of several signaling molecules, such as Akt. Consequently, glucose transporter 4 (GLUT4) is translocated to the plasma membrane, and glucose is taken up by cells [22,23]. In the liver, insulin suppresses gluconeogenesis and upregulates glycogen synthesis, resulting in increased glucose uptake [23,24]. A recent study showed that an extract of WSSP activates Akt phosphorylation, GLUT4 translocation, and glucose uptake in insulin resistance-induced C2C12 myotubes [25]. Therefore, WSSP can acutely improve insulin sensitivity in targeting tissues, including skeletal muscles, in vivo and suppress postprandial blood glucose elevation in a non-diabetic normal state.
Previous studies have shown that WSSP treatment ameliorates hyperglycemia in streptozotocin- or alloxan-induced diabetic animal models with β-cell deficiency [14,[26], [27], [28]]. In these studies, an increase in serum insulin levels and restoration of pancreatic islets have also been observed. Sweet potato consumption has been shown to possess antioxidant effects in many previous studies [29]. Therefore, it is likely that long-term treatment with WSSP soon after drug administration protects β-cells from drug damage, thereby maintaining blood glucose regulation by insulin. Thus, WSSP treatment may have various long-term effects, in addition to an acute effect on insulin sensitivity, as shown in the present study.
WSSP possesses several bioactive compounds including proteins with different molecular weights [30,31]. Therefore, the WSSP solution was fractionated according to its molecular weight as a first step to determine its active constituents. The present study showed that the fraction with a molecular weight of ≤10 kDa suppressed postprandial blood glucose levels and improved insulin sensitivity. We also found that the >50 kDa fraction suppressed gluconeogenesis. The anti-gluconeogenic effect is not likely to contribute directly to the reduction in blood glucose levels after glucose loading because gluconeogenesis occurs at low levels of blood glucose. Sweet potato contains several proteins that have high water solubility and stabilize emulsions over a wide pH range [30,31]. WSSP has been reported to contain sporamin (25 kDa), the major storage protein in the tuberous roots of sweet potato, which functions as a trypsin inhibitor [30]. We isolated sporamin from WSSP and examined its effect on blood glucose levels via an OGTT; however, sporamin treatment did not affect the blood glucose response (data not shown). A previous study has shown that the active constituents of WSSP that improve glucose tolerance are presumably acidic glycoproteins, estimated to be 22 kDa [32]. Moreover, an arabinogalactan protein estimated to be 126.8 kDa was isolated from WSSP and its continuous administration lowers fasting blood glucose levels in KKAy mice [33] and improves glucose tolerance in db/db mice [34]. Since the predominant constituent of sweet potato is carbohydrates, followed by proteins and ash [29], certain glycoproteins may play a role in suppressing gluconeogenesis. The compounds with hypoglycemic activity of ≤10 kDa included in sweet potato have not yet been reported. Future studies should identify active compounds in the ≤10 kDa and >50 kDa fractions. Orally administered proteins are believed to be degraded into amino acids in the body. However, it has been reported that certain food-derived prolyl and pyroglutamyl peptides containing 2–3 amino acid residues exist in the blood and retain their biological activities [35]. Therefore, peptides derived from active compounds in WSSP may be responsible for the reduction in postprandial blood glucose levels.
We previously showed that 7 days of administration of the hydrophobic fraction of bitter melon fruit extract to ob/ob mice reduces hepatic lipid accumulation and blood glucose levels, and cucurbitacin B has been identified as one of its active components [36]. Furthermore, we reported that bitter melon fruit hydrophobic extract enhances insulin secretion from β-cells, which is not attributed to cucurbitacin B [18]. Thus, multiple constituents with different effects can comprehensively exhibit an antidiabetic effect. Sweet potato contains various nutrients and has multiple health benefits, such as antioxidant, hepatoprotective, anti-inflammatory, antitumor, and antidiabetic effects [29]. Based on these findings, it can be concluded that WSSP exhibits hypoglycemic activity comprehensively via multiple constituents, despite its high carbohydrate content.
5. Conclusions
The present study revealed that WSSP acutely reduced postprandial blood glucose levels by improving insulin sensitivity in skeletal muscles in normal rats, which was attributed to constituents with a molecular weight of ≤10 kDa. Moreover, WSSP treatment suppressed gluconeogenesis in the liver, for which constituents of >50 kDa were responsible. Thus, WSSP can acutely regulate blood glucose homeostasis via multiple mechanisms. Suppressing postprandial hyperglycemia leads to prevention of T2DM onset and its complications [7,9,10]. Although further investigation regarding the identification of active compounds and elucidation of the detailed molecular mechanisms of these effects is warranted for clinical applications, this study demonstrated the potential use of WSSP as a functional food material to prevent T2DM.
Author contribution statement
Akito Kinoshita, Takuma Nagata, Futoshi Furuya: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Mikio Nishizawa: Conceived and designed the experiments.
Eri Mukai: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by Fujisangyo Co., Ltd. and the AY2021 Program for Asia-Japan Research Development, Ritsumeikan University.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest’s statement
The authors declare no competing interests.
Acknowledgements
We thank Fujisangyo Co., Ltd. for providing the WSSP powder. We are grateful to Saki Shirako of Medical Chemistry Laboratory, Department of Biomedical Sciences, College of Life Sciences, Ritsumeikan University for her valuable discussion. We thank Editage (www.editage.com) for their English language editing services.
References
- 1.Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B.B., Stein C., Basit A., Chan J.C.N., Mbanya J.C., Pavkov M.E., Ramachandaran A., Wild S.H., James S., Herman W.H., Zhang P., Bommer C., Kuo S., Boyko E.J., Maglian D.J. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183 doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolb H., Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasad R.B., Groop L. Precision medicine in type 2 diabetes. J. Intern. Med. 2018;285:40–48. doi: 10.1111/joim.12859. [DOI] [PubMed] [Google Scholar]
- 4.Galicia-Garcia U., Benito-Vicente A., Jebari S., Larrea-Sebal A., Siddiqi H., Uribe K.B., Ostolaza H., Martin C. Pathophysiology of type 2 diabetes mellitus. Int. H. Mol. Sci. 2020;21:6275. doi: 10.3390/ijms21176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stumvoll M., Goldstein B.J., van Haeften T.W. Type 2 diabetes: principles and pathogenesis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 6.Louis M., Claude C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr. Pract. 2006;12:42–46. doi: 10.4158/EP.12.S1.42. [DOI] [PubMed] [Google Scholar]
- 7.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith-Palmer J., Brandle M., Trevisan R., Orsini Federici M., Liabat S., Valentine W. Assessment of the association between glycemic variability and diabetes-related complications in type 1 and type 2 diabetes. Diabetes Res. Clin. Pract. 2014;105:273–284. doi: 10.1016/j.diabres.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Chiasson J.L., Josse R.G., Gomis R., Hanefeld M., Karasik A., Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 10.Raz I., Ceriello A., Wilson P.W., Battioui C., Su E.W., Kerr L., J ones C.A., Milicevic Z., Jacober S.J. Post hoc subgroup analysis of the HEART2D trial demonstrates lower cardiovascular risk in older patients targeting postprandial versus fasting/premeal glycemia. Diabetes Care. 2011;34:1511–1513. doi: 10.2337/dc10-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubota S., Liu Y., Iizuka K., Kuwata H., Seino Y., Yabe D. A review of recent findings on meal sequence: an attractive dietary approach to prevention and management of type 2 diabetes. Nutrients. 2020;12:2502. doi: 10.3390/nu12092502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alkhatib A., Tsang C., Tiss A., Bahorun T., Arefanian H., Barake R., Khadir A., Tuomilehto J. Functional foods and lifestyle approaches for diabetes prevention and management. Nutrients. 2017;9:1310. doi: 10.3390/nu9121310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patle D., Vyas M., Khatik G.L. A review of natural products and herbs used in the management of diabetes. Curr. Diabetes Rev. 2021;17:186–197. doi: 10.2174/1573399816666200408090058. [DOI] [PubMed] [Google Scholar]
- 14.Kusano S., Abe H. Antidiabetic activity of white skinned sweet potato (Ipomoea batatas L.) in obese Zucker fatty rats. Biol. Pharm. Bull. 2000;23:23–26. doi: 10.1248/bpb.23.23. [DOI] [PubMed] [Google Scholar]
- 15.Ludvik B.H., Mahdjoobian K., Waldhaeusl W., Hofer A., Prager R., Kautzky-Willer A., Pacini G. The effect of Ipomoea batatas (Caiapo) on glucose metabolism and serum cholesterol in patients with type 2 diabetes: a randomized study. Diabetes Care. 2002;25:239–240. doi: 10.2337/diacare.25.1.239. [DOI] [PubMed] [Google Scholar]
- 16.Ludvik B., Waldhaeusl W., Prager R., Kautzky-Willer A., Pacini G. Mode of action of Ipomoea batatas (Caiapo) in type 2 diabetic patients. Metabolism. 2003;52:875–880. doi: 10.1016/s0026-0495(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 17.Kusano S., Abe H., Okada A. Study of antidiabetic activity of white skinned sweet potato (Ipomoea batatas L.); comparison of normal and streptozotocin induced diabetic rats and hereditary diabetic mice. Nippon Nogeikagaku Kaishi. 1998;72:1045–1052. [Google Scholar]
- 18.Shimada T., Kato F., Dwijayanti D.R., Nagata T., Kinoshita A., Okuyama T., Nishizawa M., Mukai E. Bitter melon fruit extract enhances intracellular ATP production and insulin secretion from rat pancreatic β-cells. Br. J. Nutr. 2021;127:377–383. doi: 10.1017/S0007114521001082. [DOI] [PubMed] [Google Scholar]
- 19.Higashida K., Fujimoto E., Higuchi M., Terada S. Effects of alternate-day fasting on high-fat diet-induced insulin resistance in rat skeletal muscle. Life Sci. 2013;93:208–213. doi: 10.1016/j.lfs.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H., Nishikawa Y., Fukushima T., Taniguchi A., Fujita Y., Tsuda K., Inagaki N., Hosokawa M. Lipopolysaccharide inhibits hepatic gluconeogenesis in rats: the role of immune cells. J. Diabetes Invest. 2017;9:494–504. doi: 10.1111/jdi.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujita Y., Hosokawa M., Fujimoto S., Mukai E., Abudukadier A., Obara A., Ogura M., Nakamura Y., Toyoda K., Nagashima K., Seino Y., Inagaki N. Metformin suppresses hepatic gluconeogenesis and lowers fasting blood glucose levels through reactive nitrogen species in mice. Diabetologia. 2010;53:1472–1481. doi: 10.1007/s00125-010-1729-5. [DOI] [PubMed] [Google Scholar]
- 22.Tokarz V.L., MacDonald P.E., Klip A. The cell biology and systemic insulin function. J. Cell Biol. 2018;217:2273–2289. doi: 10.1083/jcb.201802095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen M.C., Shulman G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018;98:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatting M., Tavares C.D.J., Sharabi K., Rines A.K., Puigserver P. Insulin regulation of gluconeogenesis. Ann. N. Y. Acad. Sci. 2018;1411:21–35. doi: 10.1111/nyas.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shyur L.F., Varga V., Chen C.M., Mu S.C., Chang Y.C., Li S.C. Extract of white sweet potato tuber against TNF-α-induced insulin resistance by activating the PI3K/Akt pathway in C2C12 myotubes. Bot. Stud. 2021;62:7. doi: 10.1186/s40529-021-00315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niwa A., Tajiri T., Higashino H. Ipomoea batatas and Agarics blazei ameliorate diabetic disorder with therapeutic antioxidant potential in streptozotocin-induced diabetic rats. J. Clin. Biochem. Nutr. 2011;48:194–202. doi: 10.3164/jcbn.10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akhtar N., Akram M., Daniyal M., Ahmad S. Evaluation of antidiabetic activity of Ipomoea batatas L. extract in alloxan-induced diabetic rats. Int. J. Immunopathol. Pharmacol. 2018;32:1–6. doi: 10.1177/2058738418814678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shih C.K., Chen C.M., Varga V., Shih L.C., Chen P.R., Lo S.F., Shyur L.F., Li S.C. White sweet potato ameliorates hyperglycemia and regenerates pancreatic islets in diabetic mice. Food Nutr. Res. 2020;64:3609. doi: 10.29219/fnr.v64.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S., Nie S., Zhu F. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016;89:90–116. doi: 10.1016/j.foodres.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 30.Senthilkumar R., Yeh K.W. Multiple biological functions of sporamin related to stress tolerance in sweet potato (Ipomoea batatas Lam) Biotechnol. Adv. 2012;30:1309–1317. doi: 10.1016/j.biotechadv.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Mu T.H., Tan S.S., Xue Y.L. The amino acid composition, solubility and emulsifying properties of sweet potato protein. Food Chem. 2009;112:1002–1005. doi: 10.1016/j.foodchem.2008.07.012. [DOI] [Google Scholar]
- 32.Kusano S., Abe H., Tamura H. Isolation of antidiabetic components from white-skinned sweet potato (Ipomoea batatas L.) Biosci. Biotechnol. Biochem. 2001;65:109–114. doi: 10.1271/BBB.65.109. [DOI] [PubMed] [Google Scholar]
- 33.Ozaki S., Oki N., Suzuki S., Kitamura S. Structural characterization and hypoglycemic effects of arabinogalactan-protein from the tuberous cortex of the white-skinned sweet potato (Ipomoea batatas L.) J. Agric. Food Chem. 2010;58:11593–11599. doi: 10.1021/jf101283f. [DOI] [PubMed] [Google Scholar]
- 34.Oki N., Nonaka S., Ozaki S. The effects of an arabinogalactan-protein from the white-skinned sweet potato (Ipomoea batatas L.) on blood glucose in spontaneous diabetic mice. Biosci. Biotechnol. Biochem. 2011;75:596–598. doi: 10.1271/bbb.100711. [DOI] [PubMed] [Google Scholar]
- 35.Sato K. Structure, content, and bioactivity of food-derived peptides in the body. J. Agric. Food Chem. 2018;66:3082–3085. doi: 10.1021/acs.jafc.8b00390. [DOI] [PubMed] [Google Scholar]
- 36.Dwijayanti D.R., Shimada T., Ishii T., Okuyama T., Ikeya Y., Mukai E., Nishizawa M. Bitter melon fruit extract has a hypoglycemic effect and reduces hepatic lipid accumulation in ob/ob mice. Phytother Res. 2020;34:1338–1346. doi: 10.1002/ptr.6600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.