Abstract
Texture analysis is a quantitative imaging analysis that provides novel biomarkers beyond conventional image reading. Our aim was to use texture analysis of pulmonary emboli derived from thoracic computed tomography for prediction of mortality and prognosis of acute pulmonary embolism (PE). Overall, 216 patients (116 female, 53.7%) were included in the analysis. Texture analysis was calculated on axial slices of the contrast enhanced pulmonary angiography of the proximal embolus. Clinical scores, serological parameters, need for intubation, intensive care unit (ICU) admission and mortality was assessed and correlated with the texture features. In the correlation analysis, there were several associations with mortality in days, the highest for the parameter S(0,5)SumVarnc (r = −0.43, P < 0.001). Another parameter, S(3,−3)AngScMom correlated with sepsis-related organ failure assessment score (SOFA)-score (r = 0.31, P < 0.001). Several texture features correlated with venous lactate and glucose levels. In discrimination analysis, there were significant differences in regard to texture features between survivors and non-survivors and between patients with and without the need for ICU admission (P = 0.02, respectively). These results highlight the potential clinical benefit of texture features in patients with acute PE as novel imaging biomarkers. Further studies are needed to validate these results.
Keywords: texture analysis, computed tomography, pulmonary embolism
Introduction
Acute pulmonary embolism (PE) is life-threatening with 30‐day mortality rates ranging from 0.5% to over 20% depending on symptoms at presentation.1,2 However, there are also low-risk clinical courses without severe complications.3 That is why, an immediate risk stratification of patients with acute PE at presentation is crucial for patient care.3
Computed tomography pulmonary angiography (CTPA) is the diagnostic clinical gold standard for the diagnosis of PE with a reported sensitivity and specificity of up to 100%.4-6 It is the first imaging performed in patients suspected of PE, most commonly on hospital admission.4 Therefore, risk stratification based upon CTPA is important.5 Established imaging signs for severe course are the right ventricle diameter to left ventricle (LV) diameter-ratio, which has the strongest predictive value and contrast media reflux into the inferior vena cava, which is a significant prognostic marker in acute PE.5,7,8
Texture analysis is an emergent research field to provide novel quantitative biomarkers derived from radiological images.9-11 Various applications of texture analysis have been investigated throughout clinical medicine, predominantly in the field of oncology.9-11 Different spatial characteristics of tumors were used for better discrimination purposes, treatment prediction and prognosis stratification.9-11 Texture analysis by this approach can provide information from different images, which is beyond the scope of the clinical observation carried out by a radiologist.
Regarding thrombus imaging, a plethora of investigations were performed to better characterize cerebral thrombi in acute ischemic stroke patients.12,13 Briefly, direct correlation analyses between histologic characteristics and imaging features were performed on unenhanced CT images and CT angiography.12 Noteworthy, imaging could predict thrombus permeability, which could aid in mechanical thrombectomy in cerebral stroke.13 That is of clinical importance, as clot composition shows statistically significant associations with treatment outcome.14,15
Albeit of these promising reports in ischemic stroke patients, the possible benefit of embolus imaging has not been used in PE. There is no doubt that images analyzed with texture analysis can provide insight into the microstructure of the embolus.
Therefore, the present study investigated whether texture analysis parameters of pulmonary emboli derived from CT images show associations with mortality and clinically relevant factors in patients with acute PE.
Patients and Methods
Patient Acquisition
This retrospective study was approved by the institutional review board (118/19-ck, Ethics Committee, University of Leipzig, Leipzig, Germany).
All patients with acute PE were retrospectively assessed within the time period 2014–2019.
Inclusion criteria
- sufficient CT images with clearly visible pulmonary emboli on admission to the hospital;
- available clinical data regarding clinical signs, serological parameters and follow-up.
- no thrombolysis before and/or during the CT acquisition.
Exclusion artifacts were
- severe image artifacts (ie, due to implants or motion artifacts) as well as any form of treatment;
- missing clinical data/follow-up;
- thrombolysis before CT imaging;
- chronic PE.
Overall, 216 patients (116 females, 53.7%) were included in the analysis. The median age at the time of CT acquisition was 65 years ranging from 17 to 99 years. The patient sample was based on a previous study, which elucidated the associations between clinical parameters, survival, and pulmonary CT obstruction scores in patients with acute PE.16
Clinical Parameters
The following clinical parameters were retrieved on hospital admission:
- relevant clinical comorbidities (active malignant disease, surgery performed within the last 4 weeks, chronic lung disease, chronic heart failure).
- blood pressure (mmHg), heart rate (beats/min), need for intubation, vasopressor, or intensive care unit admission.
- following clinical scores were calculated according to the proposed standards: Wells score,17 revised Geneva score,18 and simplified Pulmonary Embolism Severity Index (sPESI) score.19
- serological parameters: hemoglobin (mmol/L), hematocrit (L/L), platelet count (109/L), fibrinogen (g/L), INR (1), PTT (s), D-dimer level (μg/mL), lactate (venous blood, mmol/L), pH (venous blood), glucose (mmol/L), troponin T (μg/L), and N-terminal natriuretic peptide (BNP, pg/mL).
- Mortality, assessed in days after diagnosis of PE.
A risk stratification of PE was performed according to the American Heart Association (AHA) as follows: low-risk PE, submassive PE and massive PE.3
Embolus localization was classified as follows: pulmonary trunk, main pulmonary arteries, lobar and segmental arteries according to the most proximal located embolus.
Imaging Technique
Computed tomography was performed on admission for every patient without any previous treatment.
Computed tomography pulmonary angiography was performed with a 128-slice CT scanner (Ingenuity 128, Philips, Hamburg, Germany). Intravenous administration of an iodine-based contrast medium (60 mL Imeron 400 MCT, Bracco Imaging Germany GmbH, Konstanz, Germany) at a rate of 4.0 mL/s via a peripheral venous line. Automatic bolus tracking was performed in the pulmonary trunk with a trigger of 100 Hounsfield units (HU). Typical imaging parameters were: 100 kVp; 125 mAs; slice thickness, 1 mm; pitch, 0.9. Computed tomography pulmonary angiography was performed in every case in deep inspiration level.
Texture Analysis
Computed tomography images were processed with the free available texture analysis software MaZda (version 4.7, available at http://www.eletel.p.lodz.pl/mazda/).20,21 A polygonal region of interest (ROI) was placed on the largest, representative slide of the pulmonary embolus on the largest, proximal position. The ROI was drawn clearly within the margin of the embolus with at least 2 mm distance to the surrounding contrast media to address possible artifacts. The measurements were performed in a blinded manner to the clinical results by a resident of radiology with 2 years of general experience.
For each ROI, gray-level (μ) normalization was performed, using the limitation of dynamics to μ ± 3 standard deviations to minimize the influence of contrast and brightness variation, as in similar studies utilizing texture analysis.22,23 Table 1 provides a detailed description of the investigated texture features. Last, 279 texture features were retrieved for every patient. To ensure intraobserver reliability, measurements were carried out by the same radiologist for a second time in a blinded manner. Intraclass correlation coefficients (ICC) were then calculated for the area of the measured emboli and for four of the most relevant texture features.
Table 1.
Overview of the Texture Features in the Present Analysis.
| Texture group | Texture feature name | Abbreviation |
|---|---|---|
| Gray-level histogram | Mean, variance, skewness, kurtosis, percentiles (1, 10, 50, 90, 99%) | Mean |
| Variance | ||
| Skewness | ||
| Kurtosis | ||
| Perc.01%, perc.10%, perc.50%, perc.90%, perc.99% | ||
| Co-occurrence matrix | Angular second moment, contrast, correlation, entropy, sum entropy, sum of squares, sum average, sum variance, inverse difference moment, difference entropy, difference variance; (for four directions and 5 interpixel distances (offsets; n = 1–5)) | S(2,-2)AngScMom, S(3,-3) AngScMom, S(1,-1) AngScMom, S(5.5) AngScMom, S(0.2) AngScMom |
| S(3.0) contrast, S(3.3) contrast, S(2.2) contrast, S(4.4) contrast, S(5.0) contrast, S(0.1) contrast | ||
| S(5.0) correlat, S(5,-5) correlat, S(2,-2) correlat, S(1,-1) correlat, S(0.1) correlat, S(3,-3) correlate | ||
| S(3.0) entropy, S(5,-5) entropy, S(2,-2) entropy, S(4,-4) entropy, S(0.5) entrop, S(3,-3) entropy | ||
| S(1.0) SumEntrp, S(0.2) entropy, S(0.5) SumEntrp, S(4.0) SumEntrp, S(3.0) SumEntrp | ||
| S(3.0) SumOfSqs, S(4.0) SumOfSqs, S(0.5) SumOfSqs, S(5,-5) SumOfSqs | ||
| S(2,-2) SumAverg, S(3,-3) SumAverg, S(0.3) SumAverg, S(0.5) SumAverg, S(0.4) SumAverg | ||
| S(1.1) SumVarnc, S(3.0) SumVarnc, S(5,-5) SumVarnc | ||
| S(0.3) InvDfMom, S(1.0) InvDfMom, S(4,-4) InvDfMom, S(5.0) InvDfMom, S(0.1) InvDfMom | ||
| S(5.5) DifEntrp, S(4.4) DifEntrp, S(1,-1) DifEntrp, S(0.1) DifEntrp, S(2.0) DifEntrp, S(1.0) DifEntrp | ||
| S(3.3) DifVarnc, S(1,-1) DifVarnc, S(2,-2) DifVarnc, S(5,-5) DifVarnc, S(2.0) DifVarnc | ||
| Run-length matrix | Run-length non-uniformity, gray-level non-uniformity, long run emphasis, short run emphasis, fraction of image in runs; (for 4 angles) | Vertl_GLevNonU,45dgr_GLevNonU, horzl_GLevNonU, 135dr_GLevNonU, Z_GLevNonU |
| Vertl_RLNonUni, 45dgr_RLNonUni,135dr_RLNonUni | ||
| Vertl_LngREmph, 45dgr_LngREmph, horzl_LngREmph, 135dr_LngREmph | ||
| Vertl_ShrtREmp, 45dgr_ShrtREmp, horzl_ShrtREmp, 135dr_ShrtREmp | ||
| Vertl_fraction, 45dgr_fraction, horzl_fraction, 135dr_fraction, Z_fraction | ||
| Absolute gradient | Gradient mean, variance, skewness, kurtosis, non-zeros | GrMean |
| GrVariance | ||
| GrSkewness | ||
| GrKurtosis | ||
| GrNonZeros | ||
| Autoregressive model | Theta 1–4, sigma | Teta1, teta2, teta3, teta4 |
| Sigma | ||
| Wavelet transform | Energies of wavelet transform coefficients in sub-bands LL, LH, HL, HH; (for 3 subsampling factors) | WavEnLL_s-1, WavEnLL_s-2, WavEnLL_s-3, WavEnLL_s-4 |
| WavEnLH_s-1, WavEnLH_s-2, WavEnLH_s-3 | ||
| WavEnHL_s-1, WavEnHL_s-2, WavEnHL_s-3, WavEnHL_s-4 | ||
| WavEnHH_s-1, WavEnHH_s-2, WavEnHH_s-3 |
Figures 1 and 2 display 2 representative cases of the patient sample for illustration purposes.
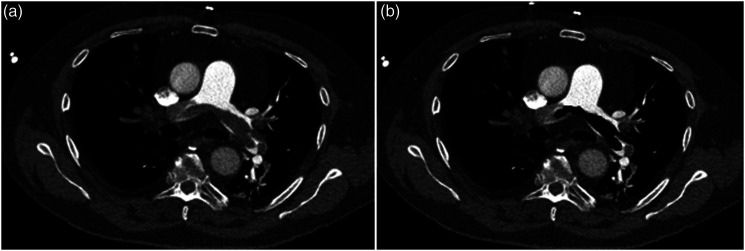
Figure 1.
A. Representative case of the patient sample with a central acute pulmonary embolism with saddle thrombus of the truncus pulmonalis. The patient died after 1 day. B. Region of interest (ROI) drawn within the central embolism to measure the texture features. Some smaller emboli were not included into the ROI.
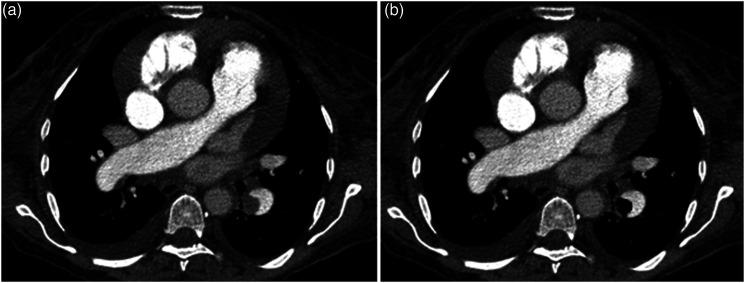
Figure 2.
A. Another representative case with a lobar pulmonary embolism in the left lower lobe. One can also appreciate global cardiomegaly, a pleural effusion on the right side as well as a pericardial effusion. The patient survived. B. ROI drawn within the embolism.
Statistical Analysis
The statistical analysis and graphics creation were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). Collected data were evaluated by means of descriptive statistics (absolute and relative frequencies). Spearman’s correlation coefficient (r) was used to analyze associations between investigated scores after testing for normality distribution. Group differences were calculated with Mann-Whitney test and Fisher exact test, when suitable. ICC was used to calculate intraobserver agreement. Multivariate regression analysis was used to predict ICU admission. Multivariate Cox regression analysis was used to assess mortality outcome. In all instances, a 2-sided P < 0.05 was considered statistically significant.
Results
Clinical Signs and Scores
Overall, 57 patients (26.4%) died with a median duration of day of 2 (IQR 10), ranging from 1 to 60 days. Furthermore, 172 patients (79.6%) needed ICU admission; 56 patients (25.8%) showed clinical signs of deep venous thrombosis.
Table 2 displays the investigated clinical and serological parameters divided by survivors and non-survivors.
Table 2.
Overview of the Investigated Demographic and Clinical Parameters. Parameters Marked with * Show Values as Median and Interquartile Range. The Other Parameters Values are Shown as Mean ± Standard Deviation.
| Parameter | Non-survivors (n = 57) | Survivors (n = 159) | P-value |
|---|---|---|---|
| Age (years) | 67.6 ± 16.3 | 62.9 ± 17.1 | 0.06 |
| Gender (male, n, %) | 32 (54.7) | 84 (52.8) | 0.67 |
| Systolic blood pressure (mmHg) | 105.5 ± 32.9 | 131.9 ± 22.4 | <0.0001 |
| Heart rate (beats/min) | 116.3 ± 27.1 | 102.7 ± 19.3 | 0.05 |
| Respiratory rate (/min) | 23.0 ± 8.7 | 23.4 ± 7.4 | 0.95 |
| Body temperature (°C) | 36.6 ± 1.2 | 36.7 ± 0.9 | 0.79 |
| BMI (kg/m2) | 29.0 ± 7.7 | 28.3 ± 5.9 | 0.83 |
| Troponin T (μg/L)* | 74.70 (93.3) | 55.29 (77.6) | 0.34 |
| D-dimer (μg/ml)* | 13.11 (20.9) | 7.31 (12.8) | 0.19 |
| Venous pH | 7.34 ± 0.08 | 7.37 ± 0.12 | <0.0001 |
| Venous lactate (mmol/L)* | 3.5 (4.0) | 2.2 (1.6) | 0.001 |
| Wells score | 6.2 ± 1.1 | 6.1 ± 1.9 | 0.95 |
| Geneva score | 7.3 ± 2.2 | 8.1 ± 3.7 | 0.31 |
| sPESI score | 3.2 ± 1.1 | 1.7 ± 0.9 | <0.0001 |
| Intubation (n, %) | 42 (73.6) | 27 (17.1) | <0.0001 |
Abbreviations: BMI = body mass index, sPESI score = simplified Pulmonary Embolism Severity Index.
Systolic blood pressure, pH, sPESI score and need for intubation were highly significantly different between survivors and non-survivors (each P < 0.0001).
As relevant comorbidities, 59 patients (27.2%) had active malignant disease, 51 patients (23.5%) underwent surgery within 4 weeks before the CT scan, 26 patients (11.9%) had chronic lung diseases, and 39 (17.9%) patients had chronic heart failure.
Localization and Texture Features of Thrombotic Emboli
In 13 patients (6.0%), the embolus was located within the pulmonary trunk. In 97 patients (44.6%), the embolus was located within the main pulmonary arteries. In the other 107 patients (49.4%), the emboli were in the lobar and/or segmental arteries. Table 3 gives an overview of the investigated texture features in accordance with thrombus localization. Most texture features showed statistically significant differences between centrally located (pulmonary trunk and main pulmonary arteries) compared with peripheral located emboli.
Table 3.
Comparison of the Clinically Relevant Texture Features in Accordance to Thrombus Localization.
| Parameter | Pulmonary trunk | Main arteries | Lobar and segmental arteries | P-value |
|---|---|---|---|---|
| S(1,1) Sumvarnc | 388.8 ± 25.9 | 382.9 ± 25.5 | 369.8 ± 36.9 | 0.025 |
| S(1,0) InvDfMom | 0.30 ± 0.09 | 0.29 ± 0.06 | 0.26 ± 0.06 | <0.001 |
| S(1,1) SumEntrp | 1.88 ± 0.02 | 1.83 ± 0.08 | 1.75 ± 0.10 | <0.001 |
| S(2,0) SumEntrp | 1.87 ± 0.02 | 1.82 ± 0.08 | 1.72 ± 0.11 | <0.001 |
| S(2,0) DifEntrp | 1.16 ± 0.17 | 1.15 ± 0.10 | 1.19 ± 0.10 | 0.091 |
| S(2,2) SumEntrp | 1.83 ± 0.04 | 1.79 ± 0.08 | 1.68 ± 0.13 | <0.001 |
| S(3,0) DifEntrp | 1.27 ± 0.15 | 1.27 ± 0.09 | 1.27 ± 0.10 | 0.911 |
| S(3,3) Correlat | 0.36 ± 0.30 | 0.32 ± 0.23 | 0.16 ± 0.33 | 0.001 |
| S(3,3) SumEntrp | 1.80 ± 0.04 | 1.76 ± 0.08 | 1.60 ± 0.24 | <0.001 |
| S(4,4) Correlat | 0.32 ± 0.29 | 0.26 ± 0.23 | 0.06 ± 0.32 | <0.001 |
| S(4,-4) InvDfMom | 0.11 ± 0.03 | 0.10 ± 0.03 | 0.09 ± 0.04 | 0.019 |
| S(5,5) InvDfMom | 0.11 ± 0.03 | 0.10 ± 0.03 | 0.08 ± 0.04 | <0.001 |
| S(5,0) InvDfMom | 0.12 ± 0.04 | 0.11 ± 0.03 | 0.10 ± 0.06 | 0.002 |
| WavEnLL_s-1 | 17898.10 ± 604.9 | 17863.71 ± 711.2 | 18604.57 ± 1473.9 | 0.001 |
| Horzl_LngREmph | 1.52 ± 0.25 | 1.61 ± 0.38 | 1.44 ± 0.25 | <0.001 |
| Horzl_ShrtREmp | 0.91 ± 0.03 | 0.90 ± 0.04 | 0.92 ± 0.03 | 0.001 |
| Horzl_fraction | 0.88 ± 0.05 | 0.86 ± 0.06 | 0.89 ± 0.05 | <0.001 |
| 135dr_ShrtREmp | 0.94 ± 0.02 | 0.94 ± 0.03 | 0.95 ± 0.03 | <0.001 |
| 135dr_fraction | 0.92 ± 0.03 | 0.92 ± 0.04 | 0.94 ± 0.03 | <0.001 |
Intraclass Correlation Coefficient
The calculation of intraclass correlation coefficient for the measured area of the emboli resulted in an ICC of 0.99. For all included texture features, the ICC was over 0.80 showing that agreement was good to excellent. For example, for both S(3,-3)AngScMom and S(0,5)SumVarnc, ICC was 0.83. For S(4,0)InvDfMom, ICC was 0.86 and for S(3,3)Correlat it was 0.88.
Associations Between Thrombus Texture Features and Outcomes
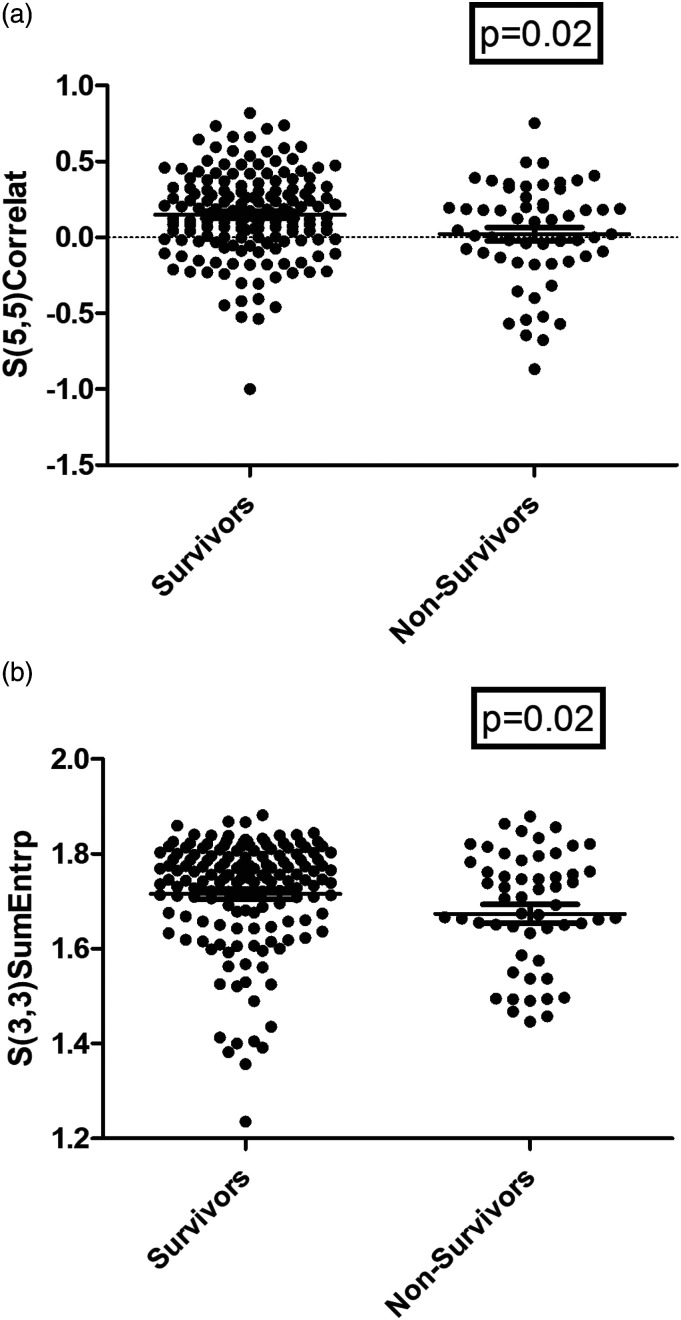
In discrimination analysis, the co-occurrence matrix group of texture features were different between survivors and non-survivors. In detail, S(3,3)Correlat was higher in survivors (0.29 ± 0.27) vs non-survivors (0.16 ± 0.34), P = 0.02 (figure 3A). Also S(3,3)SumEntrp was higher in survivors (1.71 ± 0.13) vs non-survivors (1.64 ± 0.27), P = 0.015 (figure 3B), (Table 4).
Figure 3.
Discrimination between survivors and non-survivors using different texture analysis features. A. Survivors had lower S(5,5)Correlat vs non-survivors, 0.15 ± 0.29 vs 0.02 ± 0.33, respectively (P = 0.02). B. Survivors had higher S(3,3)SumEntrp vs non-survivors, 1.71 ± 0.13 1.67 ± 0.15, respectively (P = 0.02).
Table 4.
Comparison of Texture Features in Survivors and Non-Survivors.
| Parameter | Non-survivors (n = 57) | Survivors (n = 159) | P-value |
|---|---|---|---|
| S(1,1) SumVarnc | 370.1 ± 33.00 | 380.80 ± 30.49 | 0.021 |
| S(1,1) SumEntrp | 1.78 ± 0.10 | 1.81 ± 0.10 | 0.024 |
| S(2,0) SumEntrp | 1.76 ± 0.11 | 1.79 ± 0.10 | 0.029 |
| S(2,2) SumEntrp | 1.71 ± 0.13 | 1.76 ± 0.11 | 0.018 |
| S(3,3) Correlat | 0.16 ± 0.34 | 0.29 ± 0.27 | 0.020 |
| S(3,3) SumEntrp | 1.64 ± 0.27 | 1.71 ± 0.13 | 0.015 |
| S(4,4) Correlat | 0.09 ± 0.31 | 0.21 ± 0.28 | 0.016 |
| S(4,-4) InvDfMom | 0.09 ± 0.03 | 0.10 ± 0.03 | 0.028 |
| S(5,5) InvDfMom | 0.08 ± 0.04 | 0.10 ± 0.03 | 0.017 |
| WavEnLL_s-1 | 18579.74 ± 1439.92 | 18025.18 ± 979.90 | 0.020 |
Also, several texture analysis parameters were different between the patients with and without the need for ICU admission (Table 5). In multivariate regression analysis, these texture features contributed to prediction model including Wells score, GENEVA score, age and heart rate. An example is shown for the texture feature S(2,0)DifEntrp in Table 6.
Table 5.
Comparison of the Texture Features in Patients with ICU Admission and Without ICU Admission.
| Parameter | ICU admission (n = 172) | No ICU admission (n = 44) | P-value |
|---|---|---|---|
| S(1,0) InvDfMom | 0.28 ± 0.07 | 0.26 ± 0.06 | 0.032 |
| S(2,0) DifEntrp | 1.16 ± 0.11 | 1.20 ± 0.11 | 0.022 |
| S(3,0) DifEntrp | 1.26 ± 0.10 | 1.30 ± 0.11 | 0.031 |
| S(5,0) InvDfMom | 0.11 ± 0.05 | 0.10 ± 0.03 | 0.049 |
| Horzl_LngREmph | 1.56 ± 0.36 | 1.42 ± 0.21 | 0.024 |
| Horzl_ShrtREmp | 0.91 ± 0.04 | 0.92 ± 0.03 | 0.007 |
| Horzl_fraction | 0.87 ± 0.05 | 0.89 ± 0.04 | 0.016 |
| 135dr_ShrtREmp | 0.94 ± 0.03 | 0.95 ± 0.02 | 0.028 |
| 135dr_fraction | 0.92 ± 0.04 | 0.94 ± 0.03 | 0.045 |
ICU = intensive care unit.
Table 6.
Multivariate Regression Analysis for ICU Admission.
| Parameter | β | 95% CI | P-value |
|---|---|---|---|
| Wells score | 0.88 | 0.62–1.23 | 0.44 |
| Geneva score | 1.15 | 0.97–1.37 | 0.11 |
| Age at embolism | 1.01 | 0.99–1.03 | 0.40 |
| Heart rate | 1.02 | 1.01–1.04 | 0.01 |
| S(2,0) DifEntrp | 0.02 | 0.001–0.56 | 0.02 |
Abbreviation: CI = Confidence interval, β = regression coefficient.
Overall, 21 patients were classified as massive PE (9.7%), 186 patients were submassive (86.1%) and 9 patients were of low-risk (4.2%).
S(4,0)InvDfMom derived from the co-occurrence matrix group could discriminate between massive vs submassive PE (P = 0.02).
In a further analysis, the submassive group was classified with patients with clinical deterioration defined by ICU admission and intubation (n = 54, 29.0%) and patients without clinical deterioration (n = 132, 71.0%).
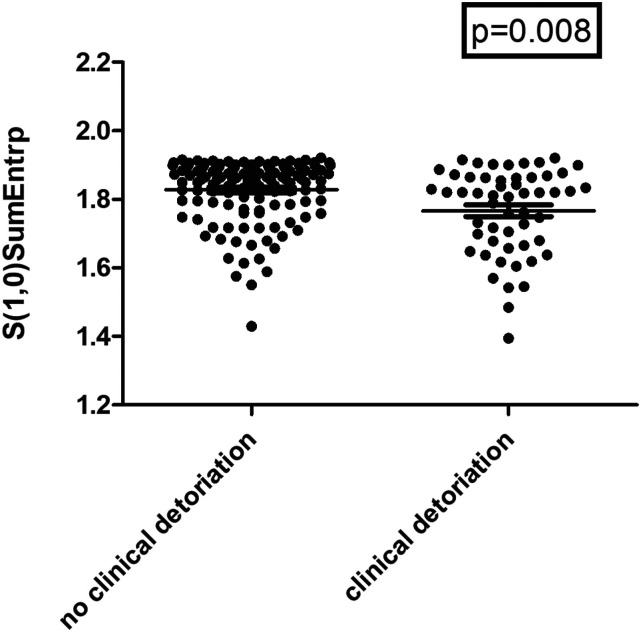
Several texture features were different between these groups. The highest statistically significance achieved S(1,0)SumEntrp (1.83 ± 0.09 vs 1.77 ± 0.12, P = 0.008, figure 4).
Figure 4.
S(1,0)SumEntrp can discriminate patients with clinical deterioration defined by ICU admission and patients without clinical deterioration, 1.83 ± 0.09 vs 1.77 ± 0.12, respectively (P = 0.008).
Correlations Between Texture Features and Clinical and Serological Parameters
In the correlation analysis with mortality in days, the correlations were identified with texture features derived from the co-occurrence matrix group.
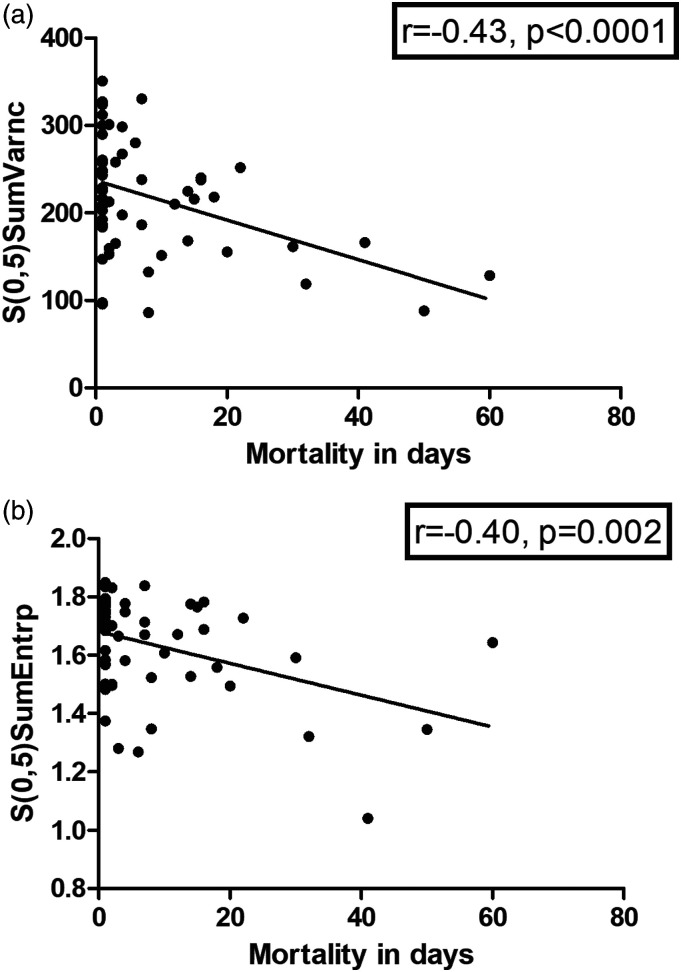
In detail, the following texture features correlated with mortality in days: S(0,5)SumVarnc (r = −0.43, P <0.001) (figure 5A), S(0,5)SumEntrp (r = −0.40, P = 0.002) (figure 5B), S(4,4)Correlat (r = −0.39, P = 0.003), S(5,5)Correlat (r = −0.38, P = 0.004), S(4,-4)SumEntrp (r = −0.38, P = 0.004). In Cox regression analysis including Wells score, GENEVA score, age and heart rate, these texture features contributed to the survival model. An example for S(0,5)SumVarnc is shown in Table 7.
Figure 5.
Correlation analysis between texture features of thrombotic emboli and mortality (days). A. S(0,5)SumVarnc (r = −0.43, P < 0.001). B. S(0,5)SumEntrp (r = −0.40, P = 0.002).
Table 7.
Cox Regression Analysis for Lethal Outcome in Pulmonary Artery Embolism.
| Parameter | HR | 95% CI | P-value |
|---|---|---|---|
| Wells score | 0.79 | 0.59–1.07 | 0.12 |
| Geneva score | 1.13 | 0.96–1.34 | 0.15 |
| Age at embolism | 0.99 | 0.98–1.01 | 0.43 |
| Heart rate | 1.00 | 0.99–1.01 | 0.68 |
| S(0,5)SumVarnc | 1.01 | 1.00–1.01 | 0.01 |
Abbreviation: CI = Confidence interval, HR = hazard ratio.
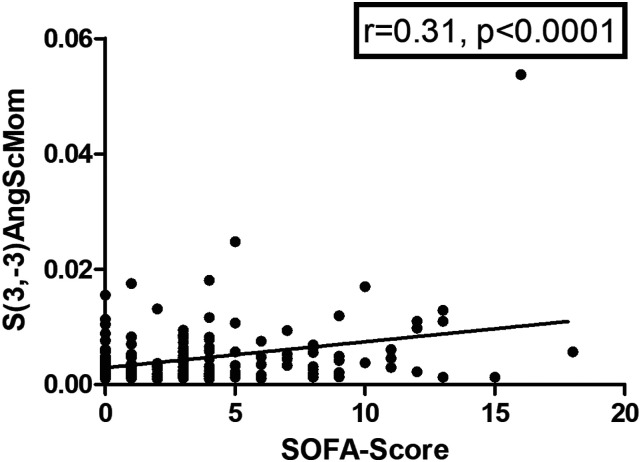
There were no associations between texture features and heart rate or blood pressure. S(3,-3)AngScMom derived from the co-occurrence matrix group showed a statistically significant correlation with SOFA-score (r = 0.31, P < 0.001) (figure 6). There were no correlations with the other investigated scores.
Figure 6.
Correlation between S(3,-3)AngScMom and sepsis-related organ failure assessment score (SOFA)-score (r = 0.31, P < 0.001).
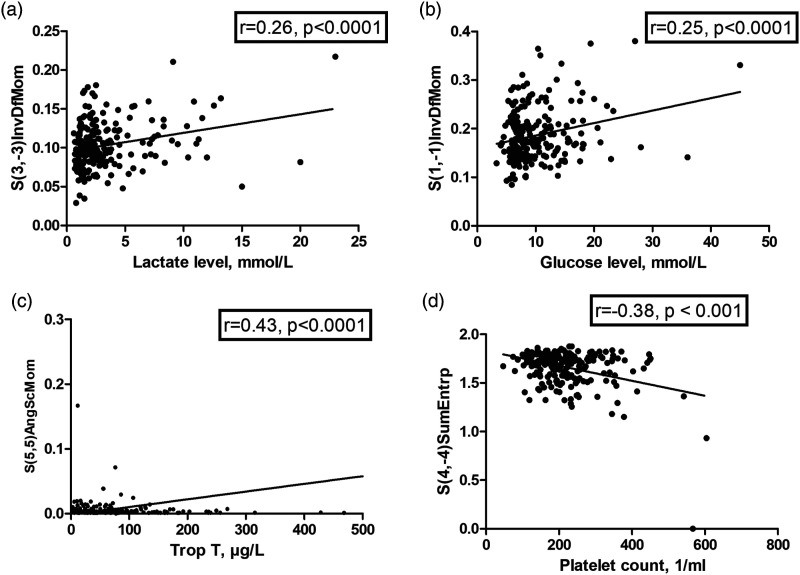
Several texture features correlated with venous lactate levels, for example, S(2,-2)InvDfMom (r = 0.25, P < 0.001) and S(3,-3)InvDfMom (r = 0.26, P < 0.001) (Figure 7A).
Figure 7.
Correlation between several texture analysis features and serological parameters in patients with acute pulmonary embolism. A. Venous lactate levels and S(3,-3)InvDfMom (r = 0.26, P < 0.001). B. Venous glucose levels and S(1,-1)InvDfMom (r = 0.25, P < 0.001). C. Troponin T and S(5,5)AngScmom (r = 0.43, P < 0.001). D. Platelet count and S(4,-4)SumEntrp (r = −0.38, P < 0.001).
Texture features correlated slightly with venous glucose levels: S(1,-1)InvDfMom (r = 0.25, P < 0.001) (Figure 7B), and S(3,0)InvDfMom (r = 0.24, P < 0.001). S(5,5)AngScMom correlated with troponin T (r = 0.43, P < 0.001) (Figure 7C). All identified texture features were of the co-occurrence matrix group.
There were no correlations between texture analysis parameters and D-Dimer levels, BNP, and venous pH.
Several texture features were correlated with platelet count, strongest correlations were found for S(4,-4)SumEntrp (r = −0.38, P < 0.001, Figure 7D), and for S(3,-3)AngScMom (r = 0.34, P < 0.001). Also, several texture features were correlated with the international normalized ratio (INR), the highest correlation coefficient showed S(0,2)SumAverg (r = 0.21, P = 0.002).
For partial thromboplastin time (pTT), WavEnLL_s-3 showed an inverse correlation (r = −0.18, P = 0.013). Fibrinogen level was associated with S(0,3)SumVarnc (r = −0.36, P = 0.049).
Finally, several texture features correlated with hematocrit, for example, S(0,5)AngScMom (r = −0.26, P < 0.001) and S(0,5)SumEntrp (r = 0.25, P < 0.001).
Moreover, several associations were identified between texture features and hemoglobin: S(5,-5)Entropy (r = 0.25, P < 0.001) and S(0,5)AngScMom (r = 0.−26, P < 0.001).
Discussion
The present study sought to establish texture analysis assessment of pulmonary emboli and to elucidate, whether texture features are associated with mortality and clinical parameters in patients with acute PE. This present analysis tried to utilize a possible novel embolism characterization in acute PE based upon CT images.
As presented, there are several statistically significant associations between CT texture features with mortality. With this analysis approach, a novel method can provide prognostic relevant factors in this possible life-threatening disease. Most promising texture features of the present analysis were derived from the co-occurrence matrix group. These matrices determine how often a pixel of intensity finds itself within a certain relationship to another pixel of intensity and therefore quantified the homogeneity or heterogeneity of the analyzed CT image.24
The topic of texture analysis is an emergent field of research with extensive studies in several diseases, predominantly in oncologic imaging.9-11 It has been shown that several texture features derived from CT as well as MRI images reflect distinctive histopathologic characteristics of tumors on a microstructure level.23-26 So far, T1- and T2-weighted texture features to reflect cellularity and nucleic areas in thyroid tumors.23 Furthermore, CT texture features were associated with hypoxia-related immunohistochemical features in head and neck cancer.25 Finally, CT texture analysis was even able to predict the complex immune milieu in lung cancer patients to guide treatment.27
Therefore, the principal hypothesis of this present work was that texture analysis can also reflect relevant structural differences of the embolus of the pulmonary arteries.
Risk stratification is crucial for patients with acute PE. In the clinical guidelines, an important factor for massive or critical course is hypotension with systolic blood pressure <90 mmHg.3,28-30 However, the absence of hemodynamic instability does not exclude beginning with a possibly progressing right ventricular dysfunction.30 A clinical evaluation includes the Geneva and Wells score for a first assessment of the patients.30 For laboratory biomarkers, elevated troponin concentrations are associated with a worse prognosis.30 Elevated B-type natriuretic peptide indicates right ventricular overload and is also associated with a worse prognosis. In a similar fashion, elevated C-reactive protein levels were associated with right ventricular dysfunction, which could be used as a predictor for prognosis.31
In a recent study, the importance of serum albumin level for short-term mortality was identified in a retrospective study of 269 patients.32 The authors proposed possible explanations that low level of serum albumin may result in deficiency of anti-oxidant function in acute PE patients.32
The imaging modalities echocardiography and CT can provide information regarding right ventricular dysfunction but other reliable prognostic factors are still lacking.3,30 It is a known fact that the sole embolus localization divided into central or peripheral is not a significant predictor of mortality.32 Moreover, total clot burden assessed by CT obstruction scores are not a sufficient method for prognosis prediction and were not translated into clinical routine.16,33 That is why there is need for new methods to characterize the embolus by CT imaging.
The present preliminary results suggest that texture analysis could routinely be used to provide prognostic factors for clinical care.
In vitro analyses investigated different clot compositions of patients with acute PE.34,35 The clot fibrin fibers of patients with fatal PE were significantly lower in diameter compared with the healthy control group.33 Moreover, denser fibrin networks were associated with intermediate risk PE compared with those with low-risk PE.34 It would be very interesting to compare these in vitro analyses with the investigated CT texture features to better characterize clot composition in patients with PE and to assess, which microstructural features of clot composition are reflected by texture features. This can also be interpreted by the fact that the localization of the embolus has an influence on the texture features. Presumably, the different clot characteristics composed of thicker fibers with increased susceptibility, which was shown for central PE compared with peripheral PE might be one reason for these differences.35
Extensive research was undertaken to assess possible differences of the thrombi in acute ischemic stroke.12,13 As such, significant differences were identified between thrombi due to cardioembolic stroke with those due to other stroke causes.36 Moreover, thrombus CT density parameters were associated with thrombus composition defined by fibrin/platelet fractions and red blood cell counts.36 In a similar way, the present results should reflect distinctive differences of emboli composition in patients with ischemic stroke.
Yet, compared with ischemic stroke, there is still lack of direct analyses of the emboli in acute PE, as surgical embolectomy is rarely performed compared with interventional mechanical thrombectomy in patients with ischemic stroke.
Beyond that, no interventional pulmonary thrombectomy was performed for the investigated patients in the present analysis and therefore the present results cannot be translated for those patients. Hypothetically, texture analysis could also aid in treatment planning of pulmonary thrombectomy in a predictive manner. Yet, further studies are needed to elucidate this interesting field for texture analysis.
This is especially of interest, as to date only a combination of right ventricular dysfunction on an echocardiogram or CT with a positive cardiac troponin test was tested as a guide for early therapeutic decisions (anticoagulation plus reperfusion treatment vs anticoagulation alone) in a large randomized controlled trial of acute PE patients.37 Imaging guided treatment decisions could be performed by quantitative CT texture features.
The present analysis has some inherent limitations. First, it is a retrospective study with possible bias. To reduce possible bias, texture analysis was performed blinded to the clinical results. Second, the patient sample is relatively small due to the single center design. Furthermore, selection bias might have influenced the present results. Moreover, it should be acknowledged that the present patient sample has a relatively high mortality rate. Therefore, the identified associations might not transferrable to patient samples with a lower mortality rate. Moreover, no interventional treatment was performed, which could limit the translation of the present results to patients undergoing interventional treatment. Third, texture analysis still lacks standardization. There is a need to employ the investigated texture features in other patient samples scanned with different CT scanner to test for external validation of the present results. This is needed before the presented results can be translated into clinical care. Fourth, the texture analysis measurement only included the most representative embolus of the patients and did not include every embolus of the patients. A measurement of all visible emboli of the patient might result in different results. However, this approach would not be suitable for clinical routine. Fifth, we could not perform correlation analysis for some prognostic relevant parameters, such as C-reactive protein or serum albumin due to lack of data.
Conclusions
Several texture features of pulmonary emboli derived from CTPA were associated with mortality, need for ICU admission, clinical and serological parameters in patients with acute PE. This highlights the possible clinical benefit of those novel imaging biomarkers.
Footnotes
Author Contribution: Study conception and design: HJM and AS; data collection: JL, ML, NB; analysis and interpretation of results: HJM, JL; draft manuscript preparation: HJM, AS, TD. All authors reviewed the results and approved the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Availability of Data and Material: Data available on request from the authors.
ORCID iDs
Alexey Surov https://orcid.org/0000-0002-9273-3943
Hans-Jonas Meyer https://orcid.org/0000-0001-8489-706X
References
- 1.Hepburn-Brown M, Darvall J, Hammerschlag G. Acute pulmonary embolism: a concise review of diagnosis and management. Intern Med J. 2019;49:15-27. [DOI] [PubMed] [Google Scholar]
- 2.Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060-3073. [DOI] [PubMed] [Google Scholar]
- 3.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788-1830. [DOI] [PubMed] [Google Scholar]
- 4.Albrecht MH, Bickford MW, Nance JW, Jr, et al. State-of-the-art pulmonary CT angiography for acute pulmonary Embolism. Am J Roentgenol. 2017;208:495-504. [DOI] [PubMed] [Google Scholar]
- 5.Meinel FG, Nance JW, Jr, Schoepf UJ, et al. Predictive value of computed tomography in acute pulmonary embolism: systematic review and meta-analysis. Am J Med. 2015;128:747-759.e2. [DOI] [PubMed] [Google Scholar]
- 6.Becattini C, Agnelli G, Germini F, Vedovati MC. Computed tomography to assess risk of death in acute pulmonary embolism: a meta-analysis. Eur Respir J. 2014;43:1678-1690. [DOI] [PubMed] [Google Scholar]
- 7.Bailis N, Lerche M, Meyer HJ, Wienke A, Surov A. Contrast reflux into the inferior vena cava on computer tomographic pulmonary angiography is a predictor of 24-hour and 30-day mortality in patients with acute pulmonary embolism. Acta Radiol. 2021;62:34-41. [DOI] [PubMed] [Google Scholar]
- 8.Bach AG, Nansalmaa B, Kranz J, et al. CT pulmonary angiography findings that predict 30-day mortality in patients with acute pulmonary embolism. Eur J Radiol. 2015;84:332-337. [DOI] [PubMed] [Google Scholar]
- 9.Rogers W, Thulasi Seetha S, Refaee TAG, et al. Radiomics: from qualitative to quantitative imaging. Br J Radiol. 2020;93:20190948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubner MG, Smith AD, Sandrasegaran K, Sahani DV, Pickhardt PJ. CT texture analysis: definitions, applications, biologic correlates, and challenges. Radiographics. 2017;37:1483-1503. [DOI] [PubMed] [Google Scholar]
- 11.Varghese BA, Cen SY, Hwang DH, Duddalwar VA. Texture analysis of imaging: what radiologists need to know. AJR Am J Roentgenol. 2019;212:520-528. [DOI] [PubMed] [Google Scholar]
- 12.Brinjikji W, Duffy S, Burrows A, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J Neurointerventional Surg. 2017;9:529-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luthman AS, Bouchez L, Botta D, Vargas MI, Machi P, Lövblad KO. Imaging clot characteristics in stroke and its possible implication on treatment. Clin Neuroradiol. 2020;30:27-35. [DOI] [PubMed] [Google Scholar]
- 14.Berndt M, Mück F, Maegerlein C, et al. Introduction of CTA-index as simplified measuring method for thrombus perviousness. Clin Neuroradiol. 2021;31:773-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boeckh-Behrens T, Schubert M, Förschler A, et al. The impact of histological clot composition in embolic stroke. Clin Neuroradiol. 2016;26:189-197. [DOI] [PubMed] [Google Scholar]
- 16.Lerche M, Bailis N, Akritidou M, Meyer HJ, Surov A. Pulmonary vessel obstruction does not correlate with severity of pulmonary embolism. J Clin Med. 2019;8:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83:416-420. [PubMed] [Google Scholar]
- 18.Le Gal G, Righini M, Roy P-M, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171. [DOI] [PubMed] [Google Scholar]
- 19.Jiménez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010;170:1383-1389. [DOI] [PubMed] [Google Scholar]
- 20.Strzelecki M, Szczypinski P, Materka A, Klepaczko A. A software tool for automatic classification and segmentation of 2D/3D medical images. Nucl Instrum Methods Phys Res. 2013;702:137-140. [Google Scholar]
- 21.Szczypiński PM, Strzelecki M, Materka A, Klepaczko A. MaZda—a software package for image texture analysis. Comput Methods Prog Biomed. 2009;94:66-76. [DOI] [PubMed] [Google Scholar]
- 22.Fruehwald-Pallamar J, Czerny C, Holzer-Fruehwald L, et al. Texture-based and diffusion-weighted discrimination of parotid gland lesions on MR images at 3.0 Tesla. NMR Biomed. 2013;26:1372-1379. [DOI] [PubMed] [Google Scholar]
- 23.Meyer HJ, Schob S, Höhn AK, Surov A. MRI texture analysis reflects histopathology parameters in thyroid cancer - a first preliminary study. Transl Oncol. 2017;10:911-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Incoronato M, Aiello M, Infante T, et al. Radiogenomic analysis of oncological data: a technical survey. Int J Mol Sci. 2017;18:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer HJ, Hamerla G, Höhn AK, Surov A. CT texture analysis-correlations with histopathology parameters in head and neck squamous cell carcinomas. Front Oncol. 2019;9:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer HJ, Renatus K, Höhn AK, et al. Texture analysis parameters derived from T1-and T2-weighted magnetic resonance images can reflect Ki67 index in soft tissue sarcoma. Surg Oncol. 2019;30:92-97. [DOI] [PubMed] [Google Scholar]
- 27.Sun R, Limkin EJ, Vakalopoulou M, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018;19:1180-1191. [DOI] [PubMed] [Google Scholar]
- 28.Becattini C, Kokorin VA, Lesniak W, et al. Pulmonary embolism - an EFIM guideline critical appraisal and adaptation for practicing clinicians. Eur J Intern Med. 2022;96:5-12. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson A, Davis S, Murch N. Pulmonary embolism in acute medicine: a case-based review incorporating latest guidelines in the COVID-19 era. Br J Hosp Med. 2020;81:1-12. [DOI] [PubMed] [Google Scholar]
- 30.Konstantinides SV, Meyer G, Becattini C, et al. 2019. ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS): the task force for the diagnosis and management of acute pulmonary embolism of the European society of cardiology (ESC). Eur Respir J. 2019;54:1901647. [DOI] [PubMed] [Google Scholar]
- 31.Abul Y, Karakurt S, Ozben B, Toprak A, Celikel T. C-reactive protein in acute pulmonary embolism. J Invest Med. 2011;59:8-14. [DOI] [PubMed] [Google Scholar]
- 32.Tanık VO, Çınar T, Karabağ Y, et al. The prognostic value of the serum albumin level for long-term prognosis in patients with acute pulmonary embolism. Clin Res J. 2020;14:578-658. [DOI] [PubMed] [Google Scholar]
- 33.Jain CC, Chang Y, Kabrhel C, et al. Impact of pulmonary arterial clot location on pulmonary embolism treatment and outcomes (90 Days). Am J Cardiol. 2017;119:802-807. [DOI] [PubMed] [Google Scholar]
- 34.Ząbczyk M, Natorska J, Janion-Sadowska A, Metzgier-Gumiela A, Polak M, Plens K. Prothrombotic fibrin clot properties associated with NETs formation characterize acute pulmonary embolism patients with higher mortality risk. Sci Rep. 2020;10:11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ząbczyk M, Natorska J, Janion-Sadowska A, et al. Loose fibrin clot structure and increased susceptibility to lysis characterize patients with central acute pulmonary embolism: the impact of isolated embolism. Thromb Haemost. 2021;21:529-537. [DOI] [PubMed] [Google Scholar]
- 36.Berndt M, Friedrich B, Maegerlein C, et al. Thrombus permeability in admission computed tomographic imaging indicates stroke pathogenesis based on thrombus histology. Stroke. 2018;49:2674-2682. [DOI] [PubMed] [Google Scholar]
- 37.Steering Committee . Single-bolus tenecteplase plus heparin compared with heparin alone for normotensive patients with acute pulmonary embolism who have evidence of right ventricular dysfunction and myocardial injury: rationale and design of the pulmonary embolism thrombolysis (PEITHO) trial. Am Heart J. 2012;163:33-38. [DOI] [PubMed] [Google Scholar]