Abstract
Introduction:
Exhaled breath condensate (EBC) sampling has been suggested as a less-invasive and cost-effective method to detect biological macromolecules, including miRNA. To explore the feasibility of its use as a biomarker of early effects of asbestos exposure, we conducted a preliminary test on male volunteers by comparing the miRNA profile in the EBC and the plasma using 2 different sequencing platforms.
Methods:
Six male volunteers, all retired and unexposed to dust or fumes, participated in the test. RNA was extracted from 200 μL EBC samples and same-size plasma samples. Sample aliquots were processed in 2 laboratories using 2 different sequencing platforms: a MiSeq Illumina® platform and a more performing HiSeq Illumina® platform.
Results:
The HiSeq3000® sequencing platform identified twice as many unique molecular indexes (UMI)-validated miRNA as the MiSeq® platform. The Spearman’s correlation coefficient between EBC counts and plasma counts was significant in 5/6 subjects with either platform (MiSeq® = 0.128-0.508, P = .026-<.001; HiSeq® = 0.156-0.412, P = .001-<.001). The intraclass correlation coefficient confirmed the consistency of the miRNA profile over the 6 participants with both biospecimens. Exploring the agreement between the EBC and plasma samples with Bland-Altman plots showed that using the HiSeq3000® platform substantially improved the EBC miRNA detection rate.
Conclusion:
Our preliminary study confirms that, when using the HiSeq® sequencing platform, EBC sampling is a suitable, non-invasive method to detect the miRNA profile in healthy subjects.
Keywords: miRNA, next generation sequencing, biomarkers, exhaled breath condensate
Introduction
Thirty years after its ban, asbestos-related disorders are still an occupational health concern in Europe and its continuing use is a most severe occupational threat in large parts of the world.1 The International Agency for Research on Cancer (IARC) links asbestos exposure to the risk of malignant mesothelioma (MM) and cancer of the lung, larynx and ovary. Limited evidence of carcinogenicity exists for cancer of the pharynx, stomach and colon-rectum.2 Non-malignant conditions following occupational asbestos exposure include diffuse pleural thickening and asbestosis, a specific form of pulmonary fibrosis.1
The initial non-specific symptoms in MM cases are often the reason for a late diagnosis and the consequent fatal prognosis. Currently, the early detection of asbestos-related neoplasms, and specifically MM of the pleura and lung cancer, relies on screening programmes using CT-scan or standard, B-reader classified chest X-rays,3 which have slightly improved the 5-year cancer survival rate.4 However, so far, accurate, non-invasive biomarkers capable of early detecting deadly asbestos-related diseases in high-risk subpopulations, such as subjects formerly exposed to asbestos, are simply unavailable. Their development would be of utmost importance.1
MicroRNAs (miRNAs) are endogenous, non-coding ribonucleic acids approximately 21 nucleotides in length,2 offering promising opportunities in this sense. A number of miRNAs intervene in regulating key cellular processes and signalling pathways involved in lung tumourigenesis, including cell proliferation, differentiation, angiogenesis, apoptosis, invasion and metastasis,5 through regulating gene expression at the post-transcriptional level by acting on the 3′ untranslated region (UTR) of messenger RNAs.6 Because of their critical role, introducing the miRNA profile might substantially improve the early diagnosis and prognosis of diseases involving multiple modifications in gene expression, such as cancer.7 Recently, numerous miRNAs have been detected as overexpressed or underexpressed in patients affected by malignant mesothelioma (MM).8 Also, over expression of miR-21, miR-96, miR-155, miR-182 and miR-183 and under expression of miR-148a, miR-148b and miR-let-7 reportedly indicate poor prognosis in non-small-cell lung cancer (NSCLC) patients.9,10 For instance, miR-21 promotes the proliferation of lung cancer cells and invasion through increasing the expression of the matrix proteases MMP-2 and MMP-9, and inhibits apoptosis through the Caspase 3 signalling pathway11; miR-155 targets Rb1 tumour suppressor gene12; mirR-183 5p also inhibits apoptosis through the Caspase 2/7 pathway and is inversely related to the activity of several genes implicated in the synthesis of cell adhesion molecules.13 Instead, miR-148b exerts the opposite effect and acts as a tumour suppressor through regulating invasion-, apoptosis- and proliferation-related oncogenes.14 A case-control study confirmed the NGS ability to detect hotspot mutations in the EBC of lung cancer patients.15 Another case-control study reported an increase in NSCLC risk associated with overexpression of miR-21 and under expression of miR-486 in plasma and in the exhaled breath condensate (EBC), which would pave the way towards a more extensive use of these biomarkers acquired through a non-invasive method.16
However, further studies are warranted to estimate the repeatability and to calculate the sensitivity, specificity and negative and positive predictive values of miRNA as biomarkers in the surveillance of subjects exposed to asbestos or in the early diagnosis of malignant mesothelioma of the pleura.17
Three methods exist to detect miRNA expression levels: real-time reverse transcription PCR (qRT-PCR), microarray hybridization and, more recently, next-generation sequencing (NGS).18-20 The high throughput NGS technology has shown a higher sensitivity in respect to the real-time reverse transcription PCR (qRT-PCR) and microarray techniques. It is more cost-efficient, allows a much larger sequence coverage and depth, and can detect new, previously unidentified miRNAs21 resulting in advancements well beyond the possibility of the available alternative techniques.
However, apart from the above-mentioned issues of repeatability, specificity and sensitivity, finding the most suitable specimen for the analysis of miRNAs biomarkers is challenging. The EBC sampling would be less-invasive, rapid, cost-effective and easily repeatable.22 EBC sampling has also provided recent progress in identifying other biological macromolecules, such as lipids, proteins, DNA and markers of oxidative stress,23 extending its potential applications in clinics, prevention and epidemiology. The low yield of miRNA from the EBC is a major obstacle, which was successfully overcome in 2 studies using the NGS technology.22,24 Also, its potential for discovering previously unidentified miRNAs21 would make it more suitable for the purposes of the project we are about to launch on detecting new molecular signatures as early biomarkers of asbestos-related health effects. Based on such assumptions, we conducted a preliminary test on male volunteers to compare of the EBC miRNA profile in the EBC to that in the plasma using 2 sequencing platforms, different in terms of cost and performance.
Material and Methods
Study subjects
Six volunteers, all males and retired, aged 65 to 73, who had never been exposed to respiratory hazards, as judged by an expert occupational physician on the basis of their working histories, unaffected by malignant or nonmalignant respiratory diseases, took part in this test, preliminary to the launch of a project of investigating new biomarkers of early effects of past exposure to asbestos, which was approved by the Ethics Committee of the University of Cagliari on 1 October 2021 (protocol No. 889). All participants signed a written informed consent form.
Exhaled breath condensate (EBC) collection
EBC specimens were collected at the study site with the TURBO-DECCS system (Medivac, Parma, Italy), with the temperature set at −5°C. The subjects breathed into a Volmet20® exhaled air meter (Medivac, Parma, Italy), calibrated at 100 L, for 15 to 20 minutes, at tidal volume, through a mouthpiece equipped with a 2-way non-rebreathing valve that separates inspiratory and expiratory air and traps saliva. The condenser respected the standards for EBC collection recommended by the American Thoracic Society/European Respiratory Society (ATS/ERS).25 The collected samples (1-2 mL) were subsequently labelled.
Plasma collection
Both serum and plasma samples have been profitably used to investigating the miRNA profile. We opted for plasma as a comparison term with EBC results because of its reportedly higher miRNA detection rate.26 The participants donated a 5 mL blood sample in an EDTA Vacutainer tube. Trained professional nurses performed blood withdrawals at the study site. Samples were delivered within 2 hours to the laboratory, where they were centrifuged at 3000 rpm and 4°C for 10 minutes. Then, the plasma was extracted and centrifuged again at 140 00 rpm to remove any residual cellular contamination. The supernatant was collected for immediate RNA extraction. All the RNA aliquots were stored at −80°C until analysis.
RNA extraction and miRNA library preparation
RNA was extracted from 200 μL of EBC sample and from a same size sample of plasma using the miRNeasy Serum/Plasma Advanced Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. MiRNA libraries were prepared from 5 μL of total RNA using the QIAseq miRNA Library Kit according to the manufacturer’s recommendations for biofluid samples. A key advantage of this method is that unique molecular indexes (UMIs) are incorporated during cDNA synthesis, tagging each strand prior to amplification, which more accurately reflects endogenous miRNA levels by controlling for library amplification bias.
Individual libraries were quantified using a fluorometric high sensitivity double strand (ds) DNA assay (Qubit 3 Invitrogen). Library quality was assessed on a high sensitivity DNA chip on the Bioanalyzer (Agilent, Santa Clara, CA, USA); library batches were pooled, re-quantified, denatured and diluted to 10 pM. Library pools were sequenced on MiSeq® (Illumina, San Diego, CA, USA) using a MiSeq reagent kit V3 150 cycles. A 75 cycles SR run was applied yielding about 0.5 M of reads for EBC samples and 2 M for plasma samples. An aliquot of the same samples was delivered to a second laboratory to create independent libraries and to sequence the same library preparation protocol with an HiSeq3000® sequencing platform (Illumina, San Diego, CA, USA), which provided about 2 M of 75 bp single reads allowing the identification of the UMI sequences. We explored the library quality results with the criteria proposed by Aparicio-Puerta et al.27 Using the HiSeq3000® platform, the percentage of short reads ranged 52% to 67% in the EBC and 6% to 16% in the plasma, and that of ribosomal RNA ranged 1.2% to 2.1% in the EBC and 0.5% to 3.3% in the plasma. The respective percentages with the MiSeq® platform for short reads were 2% to 26% in the EBC and 5% to 18% in the plasma, and the figures for ribosomal RNA were 0.004% to 1.4% in the EBC and 0.8 to 3.0% in the plasma.
Sequencing data alignment and counts generation
After demultiplexing, the FASTQ files containing the sequence data were analysed using the GeneGlobe Qiagen Bioinformatics Analysis Portal. Briefly, reads were first processed by trimming off the 3′ adapter and low-quality bases using cutadapt (https://cutadapt.readthedocs.io/en/stable/guide.html). Then, the insert sequences and UMI sequences were identified. Reads with less than 16 bp insert sequences or less than 10 bp UMI sequences (UMI_defective_reads) were discarded.
A single set of sequences was used to annotate the insert sequences for all reads/samples sets. A sequential alignment strategy was followed to map them to different databases (perfect match to mature miR-Base, miRBase hairpin, non-coding RNA, mRNA and other RNAs). Finally, a second mapping to mature miRBase was performed using bowtie (https://bowtie-bio.sourceforge.net/bowtie2/index.shtml), with tolerance up to 2 mismatches. At each step, only unmapped sequences pass to the next step. Read counts for each RNA category (miRBase mature, miRBase hairpin, piRNA, tRNA, rRNA, mRNA and otherRNA) are calculated from the mapping results. miRBase V21 is used for miRNA, and piRNABank is used for piRNA. To identify possible novel miRNA molecules, all remaining unmapped sequences are aligned to the Genome Reference Consortium GRCh38.
For each sample, all reads assigned to a particular miRNA or piRNA ID are counted, and the associated unique molecular indexes (UMIs) are aggregated to count unique molecules.
Based on the Aparicio-Puerta et al27 criteria on sequencing quality, with the HiSeq3000® platform, the percentage of valid reads ranged 22% to 42% in the EBC and 82% to 93% in the plasma. The respective percentages with the MiSeq® platform were 15% to 37% in the EBC and 30% to 70% in the plasma.
Statistical methods
Overall, the analysis with the HiSeq3000® platform resulted in detecting 600 UMI-validated miRNA reads versus 303 detected with the MiSeq® sequencing system, a difference expected due to the different sensitivity of the 2 instruments.
Comparison of the miRNA expression in the EBC and plasma
To compare the miRNA expression in the EBC and the plasma, for each participant, we first assumed the distribution of the UMI-validated miRNA counts that were detected and then categorized them using the following cutoffs: not detected, less than the median count, above the third quartile, above the fourth quartile and above the 90th percentile. The ‘below the median’ category combined the first and the second quartile because, when using the MiSeq® platform, EBC miRNA reads represented by 1 count only exceeded or were close to the 50th percentile of the count distribution in the 6 participants. For consistency, we kept the same categories for plasma samples and for the analyses with the HiSeq3000® platform.
Correlation analyses
We used the Spearman’s correlation coefficient to test the correlation between the UMI-validated miRNA reads in the EBC and that in the plasma of the 6 participants, separately by sequencing platform. The same correlation was tested for the total miRNA.
Between participants agreement
To explore the inter-individual agreement between the miRNA profiles, we calculated the intraclass correlation coefficient for the UMI-validated miRNA counts and the respective categories in the EBC and in the plasma, separately by sequencing platform.28
EBC-plasma agreement
We used Bland-Altman plots on the normalized miRNA count distribution to visualize the agreement between results in the EBC and plasma samples with each of the 2 sequencing platforms.29 Briefly, we set the paired difference between the log2 count in the plasma and the log2 count in the EBC of each individual miRNA detected in the y-axis and their mean value (Log2 P + Log2 E)/2 in the x-axis.
Results
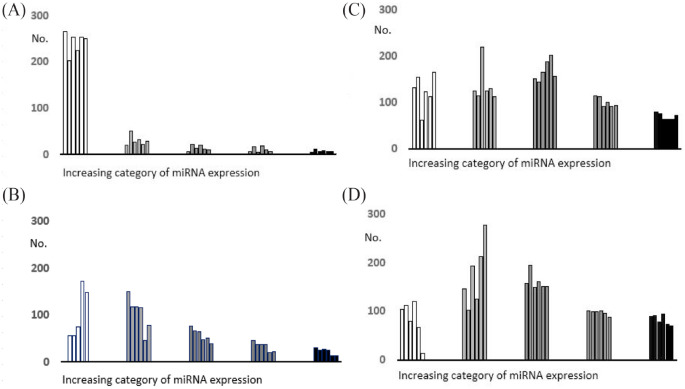
As expected, the HiSeq3000® platform outperformed the MiSeq® in the sequencing yield but, in terms of library quality, the second had a lower percentage of short readings and it was equivalent for the ribosomal RNA percentage. Figure 1 shows the frequency distribution of the miRNA count categories in the EBC and the plasma obtained using either sequencing platform. The HiSeq3000® sequencing platform identified twice as many UMI-validated miRNA (No. = 600) in respect to the MiSeq® platform (No. = 303), and the proportion of null readings among those detected at least once was much lower, ranging 2% to 20% versus 67% to 87% with the MiSeq® platform.
Figure 1.
Frequency distribution of the miRNA counts in the EBC and the plasma (white: undetected; increasing shade of grey from the left: below the median; third quartile; fourth quartile; above the 90th percentile) of the 6 participants: A EBC, MiSeq® platform; B plasma, MiSeq® platform; C EBC, HiSeq3000® platform; D plasma, HiSeq platform®.
Table 1 shows the Spearman’s correlation coefficients between the counts of the UMI validated miRNA in the EBC and in the plasma, by sequencing platform. The regression coefficients varied around fair to moderate values and differed by the platform in most individuals; however, with the exception of 1 subject with either platform, they were all statistically significant, indicating that the 2 biofluids represent a similar pattern of miRNA expression. There was no correlation for the total number of UMI-validated miRNA reads in the EBC versus plasma over the 6 participants with either platform (MiSeq® Spearman’s correlation coefficient: .371, P = .468; HiSeq3000® Spearman’s correlation coefficient: −0.086, P = .872).
Table 1.
Spearman’s correlation coefficients between the UMI-validated miRNA counts in the EBC and the respective counts in the plasma by sequencing platform.
| Subject id | MiSeq® | HiSeq3000® |
|---|---|---|
| Spearman’s correlation P-value | Spearman’s correlation P-value | |
| 1 | .508 <.0001 | .412 <.0001 |
| 2 | .103 .074 | .308 <.0001 |
| 3 | .325 <.0001 | .070 .089 |
| 4 | .334 <.0001 | .169 <.0001 |
| 5 | .396 <.0001 | .239 <.0001 |
| 6 | .128 .026 | .156 .0001 |
The overall agreement between the EBC and the plasma miRNA count was studied by calculating the intraclass correlation coefficient (ICC) for all miRNA over all study participants separately by sequencing platform. As shown in Table 2A, using the less sensitive MiSeq® platform, the ICC was higher for absolute count in respect to the categorized values. Besides, the absolute count in the EBC showed a good correlation over all subjects, while the correlation was moderate for the plasma absolute count. The analysis conducted with the more sensitive HiSeq3000® platform confirms the better correlation using the absolute count; that using the plasma samples scored as excellent and that using EBC samples as fair.
Table 2.
Intraclass correlation coefficients for the miRNA assays over the 6 participants. A: analysis conducted on the 303 UMI-validated miRNA detected with the MiSeq® sequencing platform; B: analysis conducted on the 600 UMI-validated miRNA detected with the HiSeq3000® sequencing platform.
| A. | |||
|---|---|---|---|
| ICC | 95% confidence interval | Correlation | |
| EBC categorized | 0.5168 | 0.4670-0.5677 | Fair |
| EBC absolute counts | 0.8371 | 0.8117-0.8608 | Good |
| Plasma categorized | 0.5168 | 0.4670-0.5677 | Fair |
| Plasma absolute counts | 0.6703 | 0.6281-0.7116 | Moderate |
| B. | |||
| ICC | 95% confidence interval | Correlation | |
| EBC categorized | 0.4781 | 0.4420-0.5151 | fair |
| EBC absolute counts | 0.5700 | 0.5359-0.6043 | fair |
| Plasma categorized | 0.6591 | 0.6287-0.6892 | good |
| Plasma absolute counts | 0.9114 | 0.9009-0.9213 | excellent |
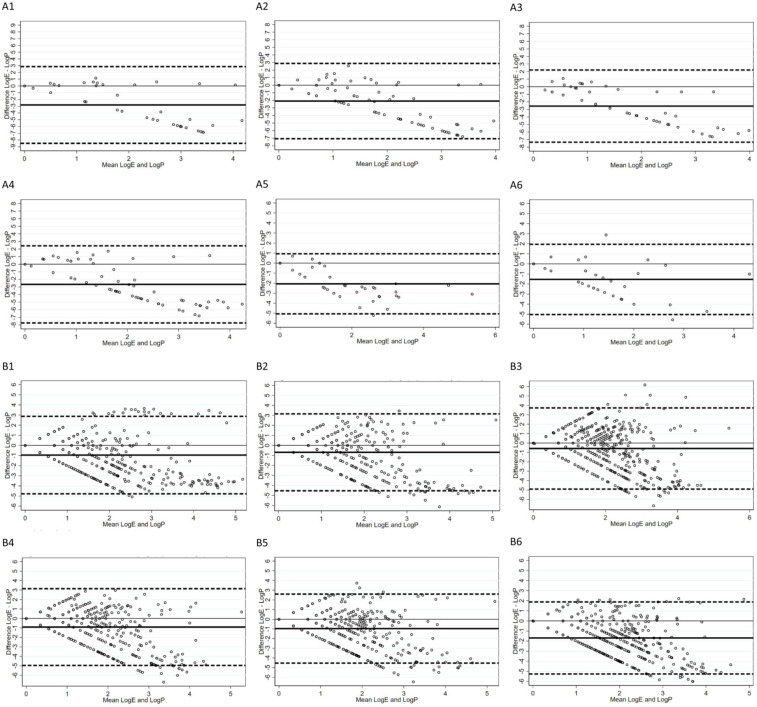
Figure 2 shows the Bland-Altman plots for the 6 participating subjects with the MiSeq® (graphs A1-A6) and the HiSeq3000® (graphs B1-B6) sequencing platforms. In the graphs, the distance between the average difference (thick line) and the line of no difference (thin line) parallel to the x-axis indicates a systematic larger miRNA count in the plasma in respect to the EBC. The A1 to A6 plots also show that the difference between EBC and plasma counts becomes larger with the increasing mean number of reads and is mostly negative, indicating an insufficient detection rate in the EBC using the MiSeq® sequencing platform. In the B1 to B6 plots, the average-difference line is much closer to the line of no difference, and the increasing difference between EBC and plasma count with the increasing number of reads appears approximately symmetric around the line of zero difference. These findings suggest an improvement in the EBC miRNA detection using the HiSeq3000® platform. In all instances, more than 95% of the individual measurements lie within the 95% confidence interval.
Figure 2.
Bland-Altman plots of agreement between miRNA counts in the EBC and plasma using the MiSeq® (a) and the HiSeq3000® (b) platforms. In the y axis is the difference between the log plasma count and the log EBC count; in the x axis is the mean between the log plasma count and the log EBC count; the thick line represents the average difference between the 2 instruments; the upper and lower dashed lines represent the 95% confidence interval of such difference; the thin 0 line is the line of perfect agreement.
With the less sensitive MiSeq® platform, 10 UMI-validated miRNA (3.3%) were detected on all subjects. Five of these (let-7a-5p, let-7b-5p, miR-16-5p, miR-92a-3p and miR-486-5p) had a count above the 75th percentile in all the participants. With the more sensitive HiSeq3000® platform, 239 miRNA (39.8%) were detected in all subjects, and 17 of these had a count above the 90th percentile in all the subjects (let-7a-5p, let-7b-5p, let-7f-5p, let-7i-5p, miR-16-5p, miR-25-3p, miR-92a-3p, miR-93-5p, miR-122-5p, miR-126-3p, miR-197-3p, miR-223-3p, miR-423-5p, miR-486-5p, miR-3915, miR-6083 and miR-6756-3p). All the 5 miRNAs with a count above the 75th percentile in all the participants using the less sensitive platform were included among the 17 miRNA hyper-expressed in all the participants with the more sensitive platform.
Discussion
The results of our pilot study indicate that EBC sampling is a non-invasive method capable of detecting early epigenetic changes. EBC miRNA expression is substantially lower than in plasma, but it is fairly correlated with that and consistent across individuals not known to suffer from malignant or non-malignant lung diseases. From the one side, the fair to moderate correlation between EBC and plasma samples we observed would reflect the origin from a single organ, the lungs, while what can be found in the plasma originates from all organs. From the other side, the lower miRNA expression in the EBC is reassuring about the absence of relevant contamination from saliva during sample gathering. Overall, our preliminary test suggests that the miRNA expression in the EBC reflects that in the plasma in absence of known pathological conditions. We also confirmed that the NGS technology is substantially more sensitive than less advanced sequencing platforms in detecting a larger number of miRNA and achieving higher expression levels for those more frequently represented.
A few previous studies monitored specific miRNA in the EBC, which over- or under-expression was assumed to indicate a neoplastic transformation in the lung tissue or the pleura22 and asthma in children.30 More recently, a study performed with GeneChip miRNA Array showed the potential of EBC analysis in identifying new lung cancer biomarkers.31
In respect to the previous positive findings on EBC microarrays, our preliminary test brought 2 novel pieces of information: (1) the assessment of inter-individual variability of several hundred miRNA molecules in subjects not known to suffer from pulmonary diseases and (2) the acceptable correspondence of EBC results to what can be found in the plasma of healthy individuals using deep sequencing, considering the origin of the first from an unique organ.
Our results suggest the feasibility of detecting the miRNA profile in the EBC using a high throughput NGS platform. Next, we aim to assess its prognostic value in the early detection of asbestos-related diseases. A previous case-control study of lung cancer patients identified 3 miRNA up-regulated in the tumoural tissue and the serum, namely miR-96, miR-182 and miR-183.31 Using the MiSeq® platform, we detected these 3 miRNAs in the EBC of 1 (miR-96 and miR-182) and 3 (miR-183) participants, and in the plasma of all of them. Using the HiSeq® platform, miR-96 was detected in the EBC of 4/6 subjects and miR-182 and miR-183 in all of them, while the 3 miRNAs were expressed in the plasma of all the subjects. Another Italian study classified the expression of 24 miRNA in 69 lung cancer cases and 870 individuals without lung cancer into 3 levels of increasing risk (a priori defined) and explored survival using a Cox proportional hazard model.32 The results showed that the microRNA signature classifier they used had predictive, diagnostic and prognostic value and could reduce the false-positive rate of CT scans, so improving the predictive value of lung cancer screening. Examining the plasma samples with the MiSeq® platform, we detected 21/24 and with the HiSeq3000® platform 23/24 miRNA listed in that study. Examining the EBC samples with the MiSeq® platform, only 6/24 miRNA were expressed in all the 6 study participants while, with the HiSeq® platform, these were 16/24.
Several papers evaluated the miRNA expression in MM patients33-39 and, overall, identified 42 mi-RNA which expression was altered. With the MiSeq® platform, we detected 28/42 of those, 6 of which were expressed in all the participating subjects. With the HiSeq® platform, we detected 33 out of the 42 miRNAs, and 16 of these were detected in both the EBC and the plasma of all the participating subjects. According to these results, the use of the HiSeq® platform in the analysis of the EBC would confer greater potential of detecting specific miRNA and panels thereof associated with asbestos related lung diseases and specifically with lung cancer, mesothelioma and interstitial fibrosis.
The present test was preliminary to a vast-scale screening of workers formerly exposed to asbestos to explore the feasibility of using the non-invasive EBC sampling to detect the miRNA profile and possible new miRNA signatures as early biomarkers of malignant and non-malignant asbestos-related diseases. We conducted the analyses with 2 sequencing platforms with different levels of sensitivity, working blindly from each other. In conducting our test, we only relied on a few male volunteers. We acknowledge the small number of participants in our test as a limitation in interpreting the inter-individual variation in the miRNA profile and count in the EBC and plasma as well as the correlation between the 2 bio-fluids. The small study size also did not allow us to explore whether age or gender would have any modifying effect on the miRNA profile in the EBC with respect to the plasma. However, at this stage, we were mainly interested in understanding whether sampling the EBC might be a non-invasive and effective method to detect miRNAs possibly related with lung diseases.
In conclusion, our results confirm that monitoring the miRNA profile in the exhaled breath condensate is feasible. The easy, non-invasive method of collection allows the application in population screenings such as the health screening in workers formerly exposed to asbestos we are planning. Nonetheless, further investigation is warranted to confirm the extent to which the EBC miRNA profile corresponds to that detectable in the plasma. In this regard, the use of high-throughput NGS technologies is recommended.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Regione Autonoma della Sardegna, LR 7/2007, “Promozione della Ricerca Scientifica e dell’Innovazione Tecnologica in Sardegna” year 2020; POR project FESR 2014-2020.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Conceptualization, RCh, RCu, SO and PC; investigation, RCu,SO, PAF, and SDM; data curation, RC, SO, MM, GF and PC.; writing—original draft preparation, SO and PC.; writing—review and editing, SO and PC.; project administration, RCh.; funding acquisition RCh. All authors have read and agreed to the published version of the manuscript.
ORCID iD: Sandro Orrù  https://orcid.org/0000-0001-5085-7975
https://orcid.org/0000-0001-5085-7975
References
- 1. Pal M, Muinao T, Boruah HPD, Mahindroo N. Current advances in prognostic and diagnostic biomarkers for solid cancers: detection techniques and future challenges. Biomed Pharmacother. 2022;146:112488. [DOI] [PubMed] [Google Scholar]
- 2. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843-854. [DOI] [PubMed] [Google Scholar]
- 3. Apostoli P, Boffetta P, Bovenzi M, et al. Position paper on asbestos of the Italian society of occupational medicine. Med Lav. 2019;110:459-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh DK, Bose S, Kumar S. Role of microRNA in regulating cell signaling pathways, cell cycle, and apoptosis in non-small cell lung cancer. Curr Mol Med. 2016;16:474-486. [PubMed] [Google Scholar]
- 6. Lembo A, Di Cunto F, Provero P. Shortening of 3'UTRs correlates with poor prognosis in breast and lung cancer. PLoS One. 2012;7:e31129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu J, Jennings SF, Tong W, Hong H. Next generation sequencing for profiling expression of miRNAs: technical progress and applications in drug development. J Biomed Sci Eng. 2011;4:666-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sturchio E, Berardinelli MG, Boccia P, Zanellato M, Gioiosa S. MicroRNAs diagnostic and prognostic value as predictive markers for malignant mesothelioma. Arch Environ Occup Health. 2020; 75: 471–482. [DOI] [PubMed] [Google Scholar]
- 9. Lamichhane SR, Thachil T, De Ieso P, Gee H, Moss SA, Milic N. Prognostic role of MicroRNAs in human non-small-cell lung cancer: a systematic review and meta-analysis. Dis Markers. 2018;2018: 8309015. doi: 10.1155/2018/8309015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu W, Liu X, He J, Chen D, Hunag Y, Zhang YK. Overexpression of members of the microRNA-183 family is a risk factor for lung cancer: a case control study. BMC Cancer. 2011;11:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li H, Zhao J, Jia X, et al. miR-21 promotes growth, invasion and migration of lung cancer cells by AKT/P-AKT/cleaved-caspase 3/MMP-2/MMP-9 signaling pathway. Int J Clin Exp Pathol. 2020;13:692-700. [PMC free article] [PubMed] [Google Scholar]
- 12. Liu M, Zhou K, Yunchao Y, Cao Y. The candidate oncogene (MCRS1) promotes the growth of human lung cancer cells via the miR-155-Rb1 pathway. J Exp Clin Cancer Res. 2015;34: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He R-Q, Gao L, Ma J, Li Z-Y, Hu X-H, Chen G. Oncogenic role of miR-183-5p in lung adenocarcinoma: a comprehensive study of qPCR, in vitro experiments and bioinformatic analysis. Oncol Rep. 2018;40:83-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dorraki N, Ghale-Noie ZN, Ahmadi NS, et al. miRNA-148b and its role in various cancers. Epigenomics. 2021;13:1939-1960. [DOI] [PubMed] [Google Scholar]
- 15. Youssef O, Knuuttila A, Piirilä P, Böhling T, Sarhadi V, Knuutila S. Hotspot mutations detectable by next-generation sequencing in exhaled breath condensates from patients with lung cancer. Anticancer Res. 2018;38:5627-5634. [DOI] [PubMed] [Google Scholar]
- 16. Mozzoni P, Banda I, Goldoni M, et al. Plasma and EBC microRNAs as early biomarkers of non-small-cell lung cancer. Biomarkers. 2013:18:679-686. [DOI] [PubMed] [Google Scholar]
- 17. Ferrari L, Carugno M, Mensi C, Pesatori AC. Circulating epigenetic biomarkers in malignant pleural mesothelioma: state of the art and critical evaluation. Front Oncol. 2020;10:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi R, Chiang VL. Facile means for quantifying microRNA expression by real-time PCR. Biotechniques. 2005;39:519-525. [DOI] [PubMed] [Google Scholar]
- 19. Li W, Ruan K. MicroRNA detection by microarray. Anal Bioanal Chem. 2009;394:1117-1124. [DOI] [PubMed] [Google Scholar]
- 20. Hafner M, Landgraf P, Ludwig J, et al. Identification of microRNAs and other small regulatory RNAs using cDNA library sequencing. Methods. 2008;44:3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reuter JA, Spacek DV, Snyder MP. High-throughput sequencing technologies. Mol Cell. 2015;58:586-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Youssef O, Knuuttila A, Piirilä P, Böhling T, Sarhadi V, Knuutila S. Presence of cancer-associated mutations in exhaled breath condensates of healthy individuals by next generation sequencing. Oncotarget. 2017;8:18166-18176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Corradi M, Folesani G, Robuschi B, et al. Non-invasive techniques to assess restrictive lung disease in workers exposed to free crystalline silica, Med Lav. 2019;110: 83-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stachowiak Z, Wojsyk-Banaszak I, Jończyk-Potoczna K, et al. MiRNA expression profile in the airways is altered during pulmonary exacerbation in children with cystic fibrosis: a preliminary report. J Clin Med. 2020;9:1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horváth I, Hunt J, Barnes PJ. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Resp J. 2005;26:523-548. [DOI] [PubMed] [Google Scholar]
- 26. Foye C, Yan IK, David W, et al. Comparison of miRNA quantitation by Nanostring in serum and plasma samples. PLOS One. 2017;12:e0189165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aparicio-Puerta E, Gómez-Martín C, Giannoukakos S, Medina JM, Marchal JA, Hackenberg M. mirnaQC: a webserver for comparative quality control of miRNA-seq data. Nucleic Acids Res. 2020;48:W262-W267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fisher RA. Statistical Methods for Research Workers. Oliver & Boyd; 1954. [Google Scholar]
- 29. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135-160. [DOI] [PubMed] [Google Scholar]
- 30. Castro Mendes F, Paciência I, Ferreira AC, et al. Development and validation of exhaled breath condensate microRNAs to identify and endotype asthma in children. PLoS One. 2019;14:e0224983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pérez-Sánchez C, Barbarroja N, Pantaleão LC, et al. Clinical utility of microRNAs in exhaled breath condensate as biomarkers for lung cancer. J Pers Med. 2021;11:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sozzi G, Boeri M, Rossi M, et al. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: a correlative MILD trial study. J Clin Oncol. 2014;32:768-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Balatti V, Maniero S, Ferracin M, et al. MicroRNAs dysregulation in human malignant pleural mesothelioma. J Thorac Oncol. 2011;6:844-851. [DOI] [PubMed] [Google Scholar]
- 34. Santarelli L, Staffolani S, Strafella E, et al. Combined circulating epigenetic markers to improve mesothelin performance in the diagnosis of malignant mesothelioma. Lung Cancer. 2015;90:457-464. [DOI] [PubMed] [Google Scholar]
- 35. Cioce M, Ganci F, Canu V, et al. Protumorigenic effects of mir-145 loss in malignant pleural mesothelioma. Oncogene. 2014;33:5319-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kirschner MB, Cheng YY, Badrian B, et al. Increased circulating miR-625-3p: a potential biomarker for patients with malignant pleural mesothelioma. Thorac Oncol. 2012;7:1184-1191. [DOI] [PubMed] [Google Scholar]
- 37. Kirschner MB, Cheng YY, Armstrong NJ, et al. MiRscore: a novel 6-microRNA signature that predicts survival outcomes in patients with malignant pleural mesothelioma. Mol Oncol. 2015;9:715-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lamberti M, Capasso R, Lombardi A, et al. Two different serum MiRNA signatures correlate with the clinical outcome and histological subtype in pleural malignant mesothelioma patients. PLoS One. 2015;10:e0135331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tomasetti M, Staffolani S, Nocchi L, et al. Clinical significance of circulating miR-126 quantification in malignant mesothelioma patients. Clin Biochem. 2012;45:575-581. [DOI] [PubMed] [Google Scholar]