Abstract
The World Health Organization has proposed that a search be made for alternatives to vaccines for the prevention and treatment of COVID-19, with one such alternative being selective serotonin reuptake inhibitors (SSRIs). This study thus sought to assess: the impact of previous treatment with SSRI antidepressants on the severity of COVID-19 (risk of hospitalisation, admission to an intensive care unit [ICU], and mortality), its influence on susceptibility to SARS-CoV-2 and progression to severe COVID-19. We conducted a population-based multiple case-control study in a region in the north-west of Spain. Data were sourced from electronic health records. Adjusted odds ratios (aORs) and 95%CIs were calculated using multilevel logistic regression. We collected data from a total of 86,602 subjects: 3060 cases PCR+, 26,757 non-hospitalised cases PCR+ and 56,785 controls (without PCR+). Citalopram displayed a statistically significant decrease in the risk of hospitalisation (aOR=0.70; 95% CI 0.49–0.99, p = 0.049) and progression to severe COVID-19 (aOR=0.64; 95% CI 0.43–0.96, p = 0.032). Paroxetine was associated with a statistically significant decrease in risk of mortality (aOR=0.34; 95% CI 0.12 – 0.94, p = 0.039). No class effect was observed for SSRIs overall, nor was any other effect found for the remaining SSRIs. The results of this large-scale, real-world data study indicate that, citalopram, could be a candidate drug for being repurposed as preventive treatment aimed at reducing COVID-19 patients’ risk of progressing to severe stages of the disease.
Keywords: Serotonin and noradrenaline reuptake inhibitors, Citalopram, Antidepressants, COVID-19, Hospitalisation, Drug repositioning
1. Introduction
Despite the fact that vaccines have greatly advanced the fight against SARS-CoV-2, it is not envisaged that vaccination alone will succeed in eradicating the pandemic (Callaway, 2021; Callaway and Ledford, 2021; Fred et al., 2021). Vaccine hesitancy (Marcec et al., 2021; Wang and Liu, 2021), low vaccination rates in countries with fewer resources (Saha et al., 2021), the waning of protection with time (Israel et al., 2021; Shrotri et al., 2021), and the appearance of new variants (Planas et al., 2021; del Rio and Malani, 2022) all render it necessary to seek effective alternative treatments (Plotkin et al., 2017; Smith et al., 2011; Torres et al., 2020). The development of new drugs entails high financial and time-related costs, and serves to further widen the gap between countries in terms of access to COVID-19 treatment (Alsabhan and Alshammari, 2022; Calusic et al., 2022; Sachs et al., 2022). The World Health Organization (WHO) therefore recommends that consideration be given to the strategy of repurposing existing drugs (Solidarity Trial Consortium et al., 2021) that are widely available and have well understood safety profiles.
A number of studies have suggested that selective serotonin reuptake inhibitors (SSRIs) may have a beneficial effect for early-stage subjects with COVID-19 (Fritz et al., 2022; Hoertel et al., 2021a; Hoertel et al., 2022a, 2022b; Kumar et al., 2020; Meikle et al., 2021; Pashaei, 2021; Rajpoot et al., 2021; Xiao et al., 2020; Zimniak et al., 2021) whereas few others have not (Rauchman et al., 2021). Fluvoxamine had been proposed as the principal molecule responsible for this beneficial effect, causing a number of clinical trials to be undertaken to evaluate its effectiveness (Lenze et al., 2020; Reis et al., 2022). Initially, doubts about its efficacy on hospitalisation, mechanical ventilation and mortality led the WHO to advise against its use (World Health Organization, 2022), although a recent meta-analysis has found that medium doses are associated with lower mortality and hospitalisation (Deng et al., 2023). That said, however, the possible effect of other molecules of this group has still to be established.
The lack of available scientific data based on real patients highlights the importance of conducting observational studies with a low risk of bias, which would allow for a body of evidence to be created while minimising the risk of exposure to potentially harmful or ineffective treatments (Hoertel et al., 2021a; Sherman et al., 2016). Hence, taking advantage of the availability of a regional healthcare database in north-west Spain, which covers close on 2.7 million beneficiaries, as well as the medications dispensed to them, their comorbidities, and the services provided to them in primary and hospital care, we carried out a case-control study to evaluate the association between use of SSRIs -both overall and by active ingredient- and severity of COVID-19, defined as: (1) risk of hospitalisation; (2) risk of intensive care unit (ICU) admissions and (3) risk of mortality. As secondary objectives, we assessed their influence on (4) susceptibility to the virus and (5) progression to severe COVID-19.
2. Material and methods
2.1. Study population and setting
The study took place in Galicia, a region in north-west Spain with a population of approximately 2.7 million, 98% of which is covered by the Galician Health Service (GHS). The study period comprised the months of March to December 2020.
2.2. Real world data: the Galician integrated healthcare database
Data were mainly sourced from the GHS health record system. This database contains all the clinical information generated at the different levels of the healthcare process, including both primary and specialised care (medical visits, diagnostic tests, surgical interventions, hospital admissions, etc.). Data were also collected from other healthcare registers (drug prescriptions and dispensing, laboratory data, the National Health System hospital discharge registry [Minimum Basic Data Set/Conjunto Mínimo Básico de Datos]). These data were collected by an independent information technology company.
2.3. Cases and controls
We used a population-based multiple case-control design (Rothman et al., 2008). This design is characterised by using data from a representative sample of all cases (in this instance, with exhaustive sampling) in a precisely defined and identified population (i.e., the population attended by the GHS). These data are then compared to data on persons (controls) randomly extracted from the same population in which the cases appear (population-based case-controls), an approach that would provide a valid estimate of the prevalence of exposure and covariates in the population of origin (Abajo et al., 2020). According to Rothman, this design can be regarded as the most desirable option for case-control designs (Rothman et al., 2008).
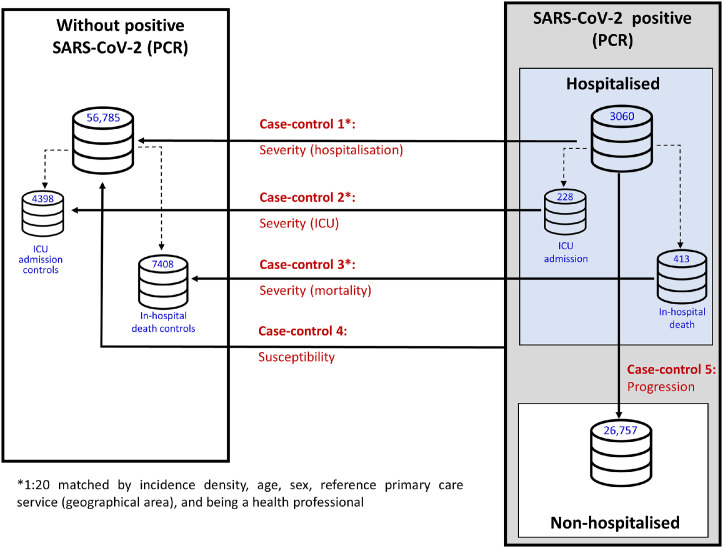
A total of 5 case-control substudies were conducted, differing in their respective definitions of cases and controls in order to respond to each of the designated study objectives (Fig. 1 and Supplementary Table S1), i.e., severity (hospitalisation, ICU admissions, mortality), susceptibility to the virus, and progression to severe COVID-19.
Fig. 1.
Population-based multiple case-control design.
2.3.1. Case-control 1: severity (hospitalisation)
The effect on risk of hospitalisation was assessed by defining cases as all subjects over the age of 18 years with diagnosis of COVID-19, confirmed by PCR, and admitted to a GHS hospital. To rule out subjects admitted due to causes other than COVID-19 infection, a maximum of 10 days’ difference was established between the date of the PCR+ and that of hospital admission. Controls were randomly selected from the population that did not present with a PCR+, and were matched (up to 20 controls per case) by incidence density, age, sex, primary care service of reference, and health-professional status, so as to ensure the same risk of exposure to SARS-CoV-2.
2.3.2. Case-control 2: severity (ICU)
The cases in this substudy were all subjects over the age of 18 years with diagnosis of COVID-19, confirmed by PCR, who required admission to an ICU at a GHS hospital. As controls, we included the subgroup of controls from the Case-control 1 substudy, who were then matched with cases from this substudy.
2.3.3. Case-control 3: severity (mortality)
To assess the effect on risk of mortality in patients with COVID-19, cases were defined as subjects over the age of 18 years with diagnosis of COVID-19, confirmed by PCR, who had been admitted to a GHS hospital and had died due to COVID-19 during their hospitalisation. The controls were the subgroup of controls from the Case-control 1 substudy who were then matched with cases from this substudy.
2.3.4. Case-control 4: susceptibility to the virus
All subjects over the age of 18 years with diagnosis of COVID-19, confirmed by PCR (both hospitalised and non-hospitalised) were defined as cases in the substudy on risk of infection. As a control group, we used the same controls as those in the Case-control 1 substudy, characterised by the absence of a PCR-confirmed COVID-19 diagnosis. Although the controls are not matched, this does not affect the validity of the study, since their absence does not produce biases, but a decrease in the efficiency of the study (Rose and van der Laan, 2009, Rothman et al., 2008).
2.3.5. Case-control 5: progression to severe COVID-19
To establish the effect of SSRIs on progression to severe COVID-19, defined as requiring hospital admission among COVID-19 patients, we used the same cases as those in the Case-control 1 substudy, characterised by having required admission to a GHS hospital. The controls, on the other hand, were all patients with diagnosis of COVID-19 confirmed by PCR, who did not require hospitalisation. As in the susceptibility sub-study, controls were also not matched (Rose and van der Laan, 2009; Rothman et al., 2008).
2.4. Drug exposure
Based on the drug prescription/dispensing database, we measured the use of SSRI antidepressants (code ATC N06AB) prescribed and dispensed during the 6 months immediately preceding the index date. The index date was set as 10 days prior to the PCR+ date, to prevent the possibility of the presence of disease symptoms altering exposure to the medication. The index date was the same for controls as for cases, thereby ensuring that the two were matched. Separate analyses were performed by treatment group and by active ingredient, i.e., fluoxetine (N06AB03), citalopram (N06AB04), paroxetine (N06AB05), sertraline (N06AB06), escitalopram (N06AB10) and fluvoxamine (N06AB08).
2.5. Covariates
The demographic variables and comorbidities (hypertension, diabetes, chronic obstructive pulmonary disease/COPD, obesity, ischaemic heart disease, cerebrovascular accident, heart failure, atrial fibrillation, chronic renal failure, cancer, asthma) were sourced from hospital and primary care records. Based on drug prescription/dispensing records, we assessed exposure to classes of drugs (antihypertensives, diuretics, nonsteroidal anti-inflammatory drugs/NSAIDs, hypolipidemic agents, anticoagulants, antiaggregants and glucocorticoids) other than those specifically targeted by this study. Also, as a proxy for the degree of chronicity of patients, we considered the number of different drugs prescribed and dispensed for chronic conditions in the 6 months immediately preceding the index date (Huber et al., 2013).
2.6. Statistical analysis
Risk of hospitalisation, ICU admission, mortality, susceptibility to the virus, and progression to severe COVID-19 were assessed using multilevel logistic regression (Brown and Prescott, 2006). These models were used because of the structure of the data and because they have many advantages over conditional regression (Brown and Prescott, 2006; Pinheiro and Bates, 2000; Stroup, 2021)(1) they allow the analysis of matched and unmatched models; (2) they permit the introduction of random terms to control for heterogeneity of initial clusters and time periods; (3) strata where cases match exposures with controls still count as events for the calculation and for the estimates; among others. To construct the models, the following four levels were considered: patient; case and control strata (for severity sub-studies); health centre; and pandemic wave. We used random-effects to assess the effect of the pandemic wave and nested random-effects for patients, case and control strata, and health centre. Complementary analyses were performed to assess the effect of dose, aetiological window and the contribution of different mechanisms of action (inhibition of acid sphingomyelinase and sigma-1 receptor activation). Results were expressed as adjusted odds ratios (aORs) with their 95% confidence intervals (CIs), with adjustments being made for the above-mentioned covariates. Adjusted estimates were obtained for the effect of antidepressant treatment dispensed as compared to the absence of any antidepressant drug treatment.
Statistical significance was set at 0.05, and all statistical analyses were performed using the free R Statistical Software environment (version 4.1.2).
2.7. Ethics statement
The study was approved by the Galician Clinical Research Ethics Committee (reference 2020–349) and was conducted according to the Helsinki Declaration, and Spanish legislation on biomedical studies and respect for human rights. The study protocol was registered at the European Union Electronic Register of Post-Authorisation Studies (EU PAS, reg. no. EUPAS44587). All data were extracted on an anonymised basis, thereby ensuring subjects’ confidentiality and privacy at all times.
3. Results
Data were collected on 86,602 subjects, made up of: 3060 cases (subjects with positive PCR who required hospitalisation), 228 of whom required admission to an ICU and 413 who died during hospitalisation; 26,757 non-hospitalised cases (subjects with positive PCR who did not require hospitalisation); and 56,785 subjects with positive PCR.
The demographic and clinical characteristics of cases and controls are shown in Tables 1 and 2 . The median age (interquartile range/IQR) of hospitalised cases was 74 (59–84) years; of ICU admitted cases, 69 (60–76) years; of deceased cases, 85 (77–89) years; and of non-hospitalised COVID+, 47 (33–63) years. The percentage of persons older than 65 years among hospitalised cases was 66.7%; in cases admitted to the ICU, 65.8%; in deceased cases, 94.2%; and in non-hospitalised COVID+, 23.4%. The most prevalent comorbidities in cases were hypertension, diabetes and obesity. The most commonly consumed active ingredients were escitalopram and sertraline.
Table 1.
Demographic and clinical characteristics of COVID-19 cases and matched controls (severity: hospitalisation, ICU admissions, mortality).
| Severity |
||||||
|---|---|---|---|---|---|---|
| Hospitalisation |
ICU admissions |
Mortality |
||||
| Characteristic | Cases (N = 3060) |
Controls (N = 56,785) |
Cases (N = 228) |
Controls (N = 4398) |
Cases (N = 413) |
Controls (N = 7408) |
| Sex; n (%) | ||||||
| Male | 1552 (50.7) | 28,729 (50.6) | 160 (70.2) | 3078 (70.0) | 242 (58.6) | 4394 (59.3) |
| Female | 1508 (49.3) | 28,056 (49.4) | 68 (29.8) | 1320 (30.0) | 171 (41.4) | 3014 (40.7) |
| Age, median (IQR) | 74 (59–84) | 73 (59–84) | 69 (61–76) | 69 (60–76) | 85 (77–89) | 84 (75–88) |
| Health professional; n (%) | 81 (2.6) | 1260 (2.2) | 8 (3.5) | 84 (1.9) | — | — |
| Comorbidities; n (%) | ||||||
| Hypertension | 1754 (57.3) | 28,020 (49.3) | 126 (55.8) | 2060 (46.8) | 304 (73.6) | 4870 (65.7) |
| Diabetes | 841 (27.5) | 10,920 (19.2) | 75 (33.2) | 900 (20.5) | 160 (38.7) | 1826 (24.6) |
| COPD | 398 (13.0) | 4569 (8.0) | 34 (15.0) | 392 (8.9) | 89 (21.5) | 904 (12.2) |
| Obesity | 889 (29.1) | 10,817 (19.0) | 82 (36.3) | 809 (18.4) | 115 (27.8) | 1592 (21.5) |
| Ischaemic heart disease | 359 (11.7) | 4768 (8.4) | 32 (14.2) | 408 (9.3) | 91 (22.0) | 942 (12.7) |
| Cerebrovascular accident | 306 (10.0) | 3874 (6.8) | 17 (7.5) | 243 (5.5) | 75 (18.2) | 763 (10.3) |
| Heart failure | 469 (15.3) | 4030 (7.1) | 24 (10.6) | 205 (4.7) | 110 (26.6) | 831 (11.2) |
| Atrial fibrillation | 466 (15.2) | 5769 (10.2) | 33 (14.6) | 329 (7.5) | 90 (21.8) | 1186 (16.0) |
| Chronic renal failure | 437 (14.3) | 4316 (7.6) | 31 (13.7) | 251 (5.7) | 102 (24.7) | 912 (12.3) |
| Cancer | 529 (17.3) | 7770 (13.7) | 34 (15.0) | 620 (14.1) | 104 (25.2) | 1378 (18.6) |
| Asthma | 285 (9.3) | 3388 (6.0) | 16 (7.0) | 227 (5.2) | 26 (6.3) | 387 (5.2) |
| Current smoker | 809 (26.4) | 8532 (15.0) | 82 (36.0) | 875 (19.9) | 88 (21.3) | 890 (12.0) |
ICU = intensive care unit; IQR= interquartile range; COPD = chronic obstructive pulmonary disease.
Table 2.
Demographic and clinical characteristics of COVID-19 cases and matched controls (susceptibility and progression to severe COVID-19).
| Susceptibility to the virus |
Progression to severe COVID-19 |
|||
|---|---|---|---|---|
| Characteristic | Cases (N = 29,817) |
Controls (N = 56,785) |
Cases (N = 3060) |
Controls (N = 26,757) |
| Sex; n (%) | ||||
| Male | 12,674 (42.5) | 28,729 (50.6) | 1552 (50.7) | 11,122 (41.6) |
| Female | 17,143 (57.5) | 28,056 (49.4) | 1508 (49.3) | 15,635 (58.4) |
| Age, median (IQR) | 49 (34–67) | 73 (59–84) | 74 (59–84) | 47 (33–63) |
| Health professional; n (%) | 1316 (4.4) | 1260 (2.2) | 81 (2.6) | 1235 (4.6) |
| Comorbidities; n (%) | ||||
| Hypertension | 7847 (26.3) | 28,020 (49.3) | 1754 (57.3) | 6093 (22.8) |
| Diabetes | 3301 (11.1) | 10,920 (19.2) | 841 (27.5) | 2460 (9.2) |
| COPD | 1128 (3.8) | 4569 (8.0) | 398 (13.0) | 730 (2.7) |
| Obesity | 4790 (16.1) | 10,817 (19.0) | 889 (29.1) | 3901 (14.6) |
| Ischaemic heart disease | 1191 (4.0) | 4768 (8.4) | 359 (11.7) | 832 (3.1) |
| Cerebrovascular accident | 1144 (3.8) | 3874 (6.8) | 306 (10.0) | 838 (3.1) |
| Heart failure | 1108 (3.7) | 4030 (7.1) | 469 (15.3) | 639 (2.4) |
| Atrial fibrillation | 1501 (3.7) | 5769 (10.2) | 466 (15.2) | 1035 (3.9) |
| Chronic renal failure | 2437 (8.2) | 4316 (7.6) | 437 (14.3) | 678 (2.5) |
| Cancer | 2230 (7.5) | 7770 (13.7) | 529 (17.3) | 1701 (6.4) |
| Asthma | 2437 (8.2) | 3388 (6.0) | 285 (9.3) | 2152 (8.0) |
| Current smoker | 4845 (16.2) | 8532 (15.0) | 809 (26.4) | 4036 (15.1) |
ICU = intensive care unit; IQR= interquartile range; COPD = Chronic obstructive pulmonary disease.
3.1. Risk of hospitalisation
Risk of hospitalisation was assessed on the basis of 3060 cases and 56,785 controls. While no statistically significant differences were observed for SSRIs overall, they were found for citalopram (aOR=0.70; 95% CI 0.49 – 0.99, p=0.049), which displayed a decreased risk of hospitalisation (see Table 3).
Table 3.
Severity of COVID-19: risk of hospitalisation, ICU admission and mortality.
| Severity |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk of hospitalisation |
Risk of ICU admission |
Risk of mortality |
||||||||||
| CASES: PCR+hospitalised (n = 3060) | CONTROLS: no PCR+ (n = 56,785) | Adjusted ORa (95%CI) |
P-value | CASES: PCR+admitted to ICU (n = 228) | CONTROLS: no PCR+ (n = 4398) | Adjusted ORa (95%CI) |
P-value | CASES: PCR+deceased (n = 413) | CONTROLS: no PCR+ (n = 7408) | Adjusted ORa (95%CI) |
P-value | |
| SSRIs (N06AB) |
366b (12.0%) |
5893b (10.4%) |
0.91 (0.79–1.04) |
0.148 |
24b (10.5%) |
361b (8.2%) |
1.01 (0.59–1.72) |
0.967 |
45b (10.9%) |
826b (11.2%) |
0.62 (0.42–0.91) |
0.015 |
| Fluoxetine (N06AB03) |
41 (1.3%) |
597 (1.1%) |
0.89 (0.64–1.24) |
0.497 |
3 (1.3%) |
39 (0.9%) |
0.99 (0.28–3.48) |
0.988 |
7 (1.7%) |
62 (0.8%) |
1.22 (0.53–2.79) |
0.643 |
| Citalopram (N06AB04) |
36 (1.2%) |
663 (1.2%) |
0.70 (0.49–0.99) |
0.049 |
0 (0.0%) |
35 (0.8%) |
— |
— |
5 (1.2%) |
106 (1.4%) |
0.43 (0.17–1.13) |
0.087 |
| Paroxetine (N06AB05) |
76 (2.5%) |
1058 (1.9%) |
1.07 (0.84–1.37) |
0.594 |
5 (2.2%) |
64 (1.5%) |
1.24 (0.46–3.33) |
0.670 |
4 (1.0%) |
146 (2.0%) |
0.34 (0.12–0.94) |
0.039 |
| Sertraline (N06AB06) |
96 (3.1%) |
1773 (3.1%) |
0.81 (0.59–1.12) |
0.205 |
7 (3.1%) |
102 (2.3%) |
1.11 (0.29–4.20) |
0.874 |
15 (3.6%) |
272 (3.7%) |
0.66 (0.27–1.61) |
0.361 |
| Fluvoxamine (N06AB08) |
3 (0.1%) |
69 (0.1%) |
0.60 (0.19–1.92) |
0.390 |
1 (0.4%) |
2 (0.0%) |
4.95 (0.34–71.42) |
0.240 |
1 (0.2%) |
7 (0.1%) |
2.03 (0.24–17.43) |
0.518 |
| Escitalopram (N06AB10) | 118 (3.9%) |
1823 (3.2%) |
0.94 (0.77–1.15) |
0.561 |
9 (3.9%) |
127 (2.9%) |
1.18 (0.57–2.46) |
0.657 |
14 (3.4%) |
242 (3.3%) |
0.71 (0.40–1.27) |
0.244 |
SSRIs = selective serotonin reuptake inhibitors; ICU = intensive care unit; OR = odds ratio.
Adjusted for: sex, age, and comorbidities: hypertension, diabetes, COPD, obesity, ischaemic heart disease, cerebrovascular accident, heart failure, atrial fibrillation, chronic renal failure, cancer, asthma, current smoker, current use of other pharmacological treatment and number of treatments for chronic diseases.
The overall number of subjects exposed to SSRIs is lower than the sum of those exposed to the active ingredients of individual SSRIs, due to the fact that some subjects were exposed to more than one SSRI across the study period.
3.2. Risk of ICU admission
The effect on risk of ICU admission was ascertained on the basis of 228 cases and 4398 controls. No statistically significant differences were found for SSRIs overall or for any of the active ingredients individually. It should be noted that no results relating to the use of citalopram were obtained, since none of the patients who had used this drug required admission to an ICU (see Table 3).
3.3. Risk of mortality
The effect on risk of mortality was assessed on the basis of 413 cases and 7408 controls. Statistically significant differences were found for SSRIs overall (aOR=0.62; 95% CI 0.42–0.91, p=0.015) and for paroxetine (aOR=0.34; 95% CI 0.12–0.94, p=0.039), showing a decrease in risk in both cases (see Table 3).
3.4. Risk of infection
The analysis of the risk of COVID-19 infection included 86,602 patients: of these, 29,817 were COVID-19 cases (subjects with positive PCR, hospitalised and non-hospitalised) and 56,785 were controls. No effect was found for any of the active ingredients individually. For SSRIs overall, there was an aOR of 0.93 (95% CI 0.87–0.99, p=0.030) (see Table 4).
Table 4.
Susceptibility to the virus and progression to severe COVID-19.
| Susceptibility to the virus |
Progression to severe COVID-19 |
|||||||
|---|---|---|---|---|---|---|---|---|
| CASES: PCR+hospitalised & non-hospitalised (n = 29,817) | CONTROLS: no PCR+ (n = 56,785) | Adjusted ORa (95%CI) |
P-value | CASES: PCR+hospitalised (n = 3060) | CONTROLS: PCR+non-hospitalised (n = 26,757) | Adjusted ORa | P-value | |
| SSRIs (N06AB) |
2330b (7.8%) |
5893b (10.4%) |
0.93 (0.87–0.99) |
0.030 |
366b (12.0%) |
1964b (7.3%) |
0.91 (0.78–1.06) |
0.235 |
| Fluoxetine (N06AB03) |
320 (1.1%) |
597 (1.1%) |
0.90 (0.77–1.05) |
0.174 |
41 (1.3%) |
279 (1.0%) |
0.97 (0.66–1.41) |
0.854 |
| Citalopram (N06AB04) |
227 (0.8%) |
663 (1.2%) |
0.93 (0.79–1.11) |
0.425 |
36 (1.2%) |
191 (0.7%) |
0.64 (0.43–0.96) |
0.032 |
| Paroxetine (N06AB05) |
458 (1.5%) |
1058 (1.9%) |
0.95 (0.84–1.08) |
0.474 |
76 (2.5%) |
382 (1.4%) |
1.06 (0.80–1.42) |
0.679 |
| Sertraline (N06AB06) |
634 (2.1%) |
1773 (3.1%) |
0.94 (0.80–1.09) |
0.404 |
96 (3.1%) |
538 (2.0%) |
0.77 (0.53–1.12) |
0.170 |
| Fluvoxamine (N06AB08) | 28 (0.1%) |
69 (0.1%) |
0.88 (0.54–1.43) |
0.600 |
3 (0.1%) |
25 (0.1%) |
0.68 (0.18–2.50) |
0.560 |
| Escitalopram (N06AB10) | 700 (2.3%) |
1823 (3.2%) |
0.91 (0.82–1.01) |
0.069 |
118 (3.9%) |
582 (2.2%) |
0.98 (0.77–1.24) |
0.860 |
SSRIs = selective serotonin reuptake inhibitors; ICU = intensive care unit; OR = odds ratio.
Adjusted for: sex, age, and comorbidities: hypertension, diabetes, COPD, obesity, ischaemic heart disease, cerebrovascular accident, heart failure, atrial fibrillation, chronic renal failure, cancer, asthma, current smoker, current use of other pharmacological treatment and number of treatments for chronic diseases.
The overall number of subjects exposed to SSRIs is lower than the sum of those exposed to the active ingredients of individual SSRIs, due to the fact that some subjects were exposed to more than one SSRI across the study period.
3.5. Risk of progression to severe COVID-19
Risk of progression to severe COVID-19 infection was determined on the basis of 3060 cases and 26,757 controls (non-hospitalised cases). No effect was observed for SSRIs overall, but effect was in evidence for citalopram (aOR=0.64; 95% CI 0.43–0.96, p=0.032) (see Table 4).
3.6. Complementary analyses
To analyse the window exposure, we considered the following periods: 1, 2 and 3 months. As can be seen in Supplementary Tables S2 and S3, there are no relevant changes between the aORs of the different exposure windows and the significant results obtained for citalopram and paroxetine remain constant, indicating that our findings are robust.
Supplementary Tables S4 and S5 show the association between SSRIs grouped by sigma-1 receptor affinity and COVID-19 outcomes. The low affinity agonist group was associated with a significant reduction in mortality risk (aOR=0.34, 95% CI 0.12–0.94, p=0.038) and the intermediate affinity agonist group showed a slight significant decrease in susceptibility (aOR=0.91, 95% CI 0.83–1.00, p=0.039). Regarding the functional inhibition of acid sphingomyelinase (FIASMA) activity and COVID-19 outcomes, we obtained that the group of SSRIs with reduced FIASMA activity showed a slight significant reduction in susceptibility to the virus (aOR=0.91; 95% CI 0.83–1.00, p=0.039). No other significant associations were observed, and the aORs are very similar between high and low activity, as can be seen in Supplementary Tables S6 and S7.
We assessed the effect of dose in the last month and found that increasing dose leads to a greater reduction in risk, but without a conclusive dose-response relationship, due to the low number of subjects in the strata (see Supplementary Tables S8 and S9).
4. Discussion
This large-scale population-based study using real-world data (RWD) has, for the first time, made it possible to establish that citalopram reduces the risk of hospitalisation being required for COVID-19 (aOR=0.70; 95% CI 0.49–0.99, p=0.049), partly because possibly it reduces the risk of COVID-19 patients progressing to severe stages that might require hospitalisation (aOR=0.64; 95% CI 0.43–0.96, p=0.032). Moreover, a non-significant, but suggestive, association was observed for risk of mortality and none of the patients exposed to this drug required admission to an ICU. The results obtained lead us to believe that citalopram could be a candidate drug for being repurposed as preventive treatment aimed at reducing COVID-19 patients’ risk of progressing to severe stages of the disease.
To our knowledge, this is the first outpatient study to allow for assessment of the effects of the respective active substances that go to make up the SSRI class of drugs. Although there are other studies that have studied some active ingredient in isolation, our study assessed a total of 6 active ingredients, thereby enabling identification of the important magnitude of citalopram's effect in the prognosis of COVID-19 patients. In addition to displaying anti-inflammatory properties (Arteaga-Henríquez et al., 2019; Sacre et al., 2010), this SSRI has been shown to possess in vitro antiviral activity against HIV (Benton et al., 2010; Greeson et al., 2016; Letendre et al., 2007) and SARS-CoV-2 in some cells (Fred et al., 2021). Furthermore, the results of the sub-studies of susceptibility and progression to severe COVID-19 indicate that is associated with a lower risk of hospitalisation is due, not to that fact that it reduces susceptibility to the virus, but rather to the fact that it decreases the risk of progression to severe stages of the disease (aOR=0.64; 95% CI 0.43–0.96, p=0.032). Regarding mortality, we observed a suggestive risk reduction (aOR=0.43; 95% CI 0.17–1.13, p=0.087), close to statistical significance and, strikingly, no patient admitted to the ICU for COVID-19 had previously taken citalopram, which prevented us from calculating aORs. Both the lack of significance (in the mortality sub-study) and the absence of cases (in the ICU admission sub-study) could be due to the fact that citalopram was the second least represented SSRI in the hospitalised PCR+ population. Despite the lack of significant results, these findings with respect to risk of ICU admission and mortality could be in line with a previous study (Hoertel et al., 2021a), which showed that administration of citalopram or escitalopram to patients hospitalised due to COVID-19 was significantly associated with a lower risk of intubation or death (HR=0.60; 95% CI 0.37–0.99, p=0.045). These results, once confirmed in other RWD studies and/or clinical trials, could have a major clinical impact, since citalopram could be repurposed as an economical alternative treatment with a good safety and tolerability profile, widely available for reducing the risk of hospitalisation of patients with COVID-19. In light of these results, it cannot be ruled out that citalopram might slow progression to severe stages in viral diseases, present or future.
Over the course of the pandemic, fluvoxamine has been considered the principal SSRI candidate for management of COVID-19 (Lenze et al., 2020; Reis et al., 2022; Seftel and Boulware, 2021). Initially, doubts about its efficacy on hospitalisation, mechanical ventilation and mortality led the WHO to advise against its use (World Health Organization, 2022), although a recent meta-analysis has found that medium doses are associated with lower mortality and hospitalisation (Deng et al., 2023). The low prevalence of fluvoxamine use in our study rendered it impossible to obtain accurate 95%CIs and conclusive results.
In contrast, the data analysed did enable us to find a significant association between use of paroxetine and a reduction in the risk of mortality due to COVID-19 (aOR=0.34; 95% CI 0.12–0.94, p=0.039). Although this finding is in line with previous preclinical (Kutkat et al., 2022) and clinical studies (Hoertel et al., 2021a; Nakhaee et al., 2022), this result should be interpreted with caution, since the absence of association observed in the other sub-studies (risk of ICU admission, mortality, susceptibility, and progression to severe COVID-19) prevents us from identifying what role paroxetine might play in the clinical course of the disease.
Our large-scale, population-based RWD study found an association between use of SSRIs as a whole and a lower risk of mortality (aOR=0.62; 95% CI 0.42–0.91, p=0.015), but no effect on risk of hospitalisation (aOR=0.91; 95% CI 0.79–1.04, p=0.148), ICU admission (aOR=1.01; 95% CI 0.59–1.72, p=0.967) or progression to severe forms of the disease (aOR=0.91; 95% CI 0.78–1.06, p=0.235). In the case of susceptibility, an aOR of 0.93 (95% CI 0.87–0.99, p=0.030) was obtained but, given that the magnitude of the effect is very small, and is, moreover, not linked to a reduced risk of hospitalisation, this result cannot be considered relevant from a clinical and/or public health standpoint. Likewise, the analysis by active ingredient failed to detect any effect (on hospitalisation, ICU admissions, mortality, susceptibility, and progression to severe COVID-19) for the remaining SSRIs (fluoxetine, sertraline and escitalopram). Since SSRIs share their main therapeutic indications, the difference in effect found in the different active ingredients cannot be attributed to confounding by indication bias.
To explain the possible effects of SSRIs on COVID-19, several mechanisms of action have been proposed, which we discuss below based on the results of our study:
-
i)
It has been suggested that serotonin transporter inhibition may decrease platelet aggregation (Sukhatme et al., 2021) and exert direct anti-inflammatory effects (Dong et al., 2016; Hashimoto, 2021; Ohgi et al., 2013; Tynan et al., 2012; Wang et al., 2019), which may be beneficial in patients with COVID-19. However, our findings do not support this proposal, as serotonin transporter inhibition is the common mechanism of action of SSRIs, and in our results we did not observe a class effect against COVID-19.
-
ii)
Another proposed mechanism of action is agonism of the sigma-1 receptor (S1R), as its activation decreases cytokine production and systemic inflammation (Lenze et al., 2020). Although inconclusive, our analyses suggest slightly superior results for low and intermediate affinity agonists, as observed in previous studies (Fritz et al., 2022) that rule out this as the mechanism involved.
-
iii)
Finally, the mechanism that is currently the most widely accepted, is the functional inhibition of acid sphingomyelinase, that prevents the entry of SARS-CoV-2 into host cells. Both biological data (Hashimoto et al., 2022; Hoertel et al., 2021b; Kornhuber et al., 2022) and prior observational data (Fritz et al., 2022; Hoertel et al., 2022a, 2022b) support this hypothesis and, although our results are not conclusive, it is noteworthy that all the molecules tested in our study are FIASMA. Although the involvement of the FIASMA activity of citalopram in COVID-19 has not been previously studied, that of its S enantiomer, escitalopram, has been (Carpinteiro et al., 2020).
Previous studies have also shown that there might be a relationship between some SSRI dose (Fritz et al., 2022; Hoertel et al., 2022b; Lenze et al., 2020; Lu et al., 2022; Reis et al., 2022) and COVID-19 outcomes. Our data indicate that, for citalopram and paroxetine, a suggestive dose-response effect could be observed, although not conclusive due to the low number of subjects in the exposure categories ( Tables S8 and S9)
4.1. Clinical and public health implications
Although the COVID-19 vaccination programme is being continuously developed, the (a) difficulty of access to vaccine in countries with fewer resources (Saha et al., 2021), (b) presence of vaccine hesitancy (Marcec et al., 2021; Wang and Liu, 2021), (c) reduction in immunity with time (Israel et al., 2021; Shrotri et al., 2021), and (d) appearance of new variants with immune evasion properties and/or new viruses (Christie et al., 2021; Imran et al., 2021; Planas et al., 2021; del Rio and Malani, 2022) all underscore the need to find effective, economical and widely available drug treatment options (Calusic et al., 2022). Hence, a repurposing drug strategy affords a crucially significant alternative, especially if the drugs in question have already been approved for other indications and have well-established safety profiles (Venkatesan, 2021). This is the case of SSRIs, which rank among the most prescribed medications worldwide (Thom et al., 2021), and, as a rule, display good tolerance and safety profiles (Devane, 1995). Specifically, citalopram numbers among the SSRIs with greatest acceptability (Hoertel et al., 2021a).
Treatment with repurposed drugs can be geared to: (i) bringing about a reduction in susceptibility to the virus and, by extension, in the number of infections; (ii) reducing the severity of COVID-19 and, in turn, the number of hospital admissions; and (iii) decreasing mortality in hospitalised patients. From a public health point of view, a drug that reduced the risk of progression to more severe stages (decreasing the risk of ICU admission and mortality) rather than susceptibility per se, would have wider applicability, since it would only be necessary to administer it to subjects who were infected and had a higher risk of worse progression.
Accordingly, the association of the use of citalopram with a lower risk of hospitalisation as a consequence of decreasing progression to severe disease stages (rather than susceptibility) acquires special relevance. On confirmation of these results, and bearing in mind the low cost of this drug and the low number of doses needed for early treatment of COVID-19 (taking the disease's mean duration into account) (Brandal et al., 2021; Menni et al., 2022), citalopram could be regarded as an alternative treatment in settings with low vaccine coverage.
5. Strengths and limitations of the study
The main strength of the current study lies in its large sample size, obtained by exhaustive sampling that included all cases with diagnosis of COVID-19 in 2020 across a region having almost 3 million inhabitants. This made it possible to perform an analysis by active substance and rule out an SSRI class effect. Furthermore, to our knowledge, this is the first study to report data jointly on the effect of SSRIs on risk of hospitalisation, ICU admission, mortality, susceptibility and progression to severe stages of COVID-19, thereby making it possible to identify the role played by the various active ingredients of this class of drug in the clinical course of the disease. These five outcome variables were adjusted for a series of confounding variables, including comorbidities, use of other drugs, and socio-demographic factors, something that constitutes another important strength. Lastly, using data on medications that are actually dispensed to measure the variable of exposure (unlike many other studies which use prescription-based data sources) decreases the risk of misclassification of the variable of exposure: even so, the possibility of residual misclassification due to poor adherence to treatment cannot be ruled out (Lam and Fresco, 2015).
Important limitations must also be considered when it comes to interpreting the results of our study. First, being an observational study with secondary databases, the main limitation to be considered is the possible existence of residual confounding due to unmeasured or misclassified study variables, such as the absence of data on the degree of severity of the main comorbidities associated with a higher risk of hospitalisation in COVID-19. In the particular case of admission to the ICU, the data must be taken with caution, since there is an under-registration due to the creation of additional ICU units that were not registered as such. Second, the database we used in our study lacked information regarding the indications of the prescribed treatments so we could not adjust for potential indications that in previous studies were associated with worse COVID-19 outcomes (e.g. dementia, schizophrenia or psychoses) (Hassan et al., 2023; Landes et al., 2022; Liu et al., 2020). However, the existence of a possible indication bias would suggest that the risk reduction associated with the use of citalopram and paroxetine could be even greater than that observed. Third, given that there is a possible correlation between SSRI use and the use of benzodiazepines, antipsychotics and other antidepressants, these could act as a confounding factor in their relationship with COVID-19 (Fico et al., 2023; Hoertel et al., 2022c). Therefore, we performed a sub-analysis in which patients consuming these groups of drugs were excluded and the observed relationships were maintained (data not shown). Fourth, it might be thought that the lack of matching in the susceptibility and progression sub-studies could be a limitation. However, according to Rose and Rothman (Rose and van der Laan, 2009; Rothman et al., 2008) the lack of matching in case-control studies only decreases efficiency but does not influence the risk of bias. Fifth, the data available for study purposes pertain to 2020, a time when (i) vaccines were not yet available and (ii) the alpha variant was predominant, so we do not know to what extent the results of our study can be extrapolated to other variants. Lastly, the limited availability of diagnostic tests in the first months of the pandemic might have given rise to some of the controls in reality being asymptomatic subjects with COVID-19. At all events, both this circumstance and the lack of therapeutic adherence would cause one to underestimate the effect of the drugs studied against COVID-19, a factor that leads us to think that the effect found for citalopram might have been even greater.
6. Conclusion
The COVID-19 pandemic has highlighted the need to evaluate the effect of already marketed drugs on COVID-19 susceptibility, progression and severity. Our RWD study suggests that there is no class effect of SSRIs and that citalopram could reduce the risk of hospitalzation due, fundamentally, to the slowing of progression to severe stages of COVID-19. If these results were to be repeated with other databases and replicated in clinical trials, we feel that, given the magnitude of the effect, this finding might well be relevant for preventing the progression of early-stage subjects with COVID-19 to more severe disease stages. Furthermore, in light of these results, future research could focus on evaluating the potential effect of citalopram on other present or future viral diseases.
Role of funding source
This study was sponsored by the Carlos III Institute of Health via the “COV20/00470” project (co-funded by the European Regional Development Fund, “A way to make Europe”).
Contributors
IVV: wrote paper.
MZC: designed study, wrote paper.
MPL: designed study, analysed data, revised paper.
ECM: designed study, revised paper
MS: analysed data, revised paper.
MTH: designed study, revised paper.
AF: designed study, analysed data, revised paper.
ASB: designed study, analysed data, revised paper.
All authors contributed to and have approved the final manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
We should like to thank the SERGAS General Healthcare Directorate for furnishing the data needed to conduct this study, DXC Technology for its work in extracting the study data, and Michael Benedict for reviewing and revising the English.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.euroneuro.2023.03.011.
Appendix. Supplementary materials
References
- Abajo F.J.de, Rodríguez-Martín S., Lerma V., Mejía-Abril G., Aguilar M., García-Luque A., Laredo L., Laosa O., Centeno-Soto G.A., Gálvez M.Á., Puerro M., González-Rojano E., Pedraza L., Pablo I.de, Abad-Santos F., Rodríguez-Mañas L., Gil M., Tobías A., Rodríguez-Miguel A., Rodríguez-Puyol D., Barreira-Hernandez D., Zubiaur P., Santos-Molina E., Pintos-Sánchez E., Navares-Gómez M., Aparicio R.M., García-Rosado V., Gutiérrez-Ortega C., Pérez C., Ascaso A., Elvira C. Use of renin–angiotensin–aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395:1705–1714. doi: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsabhan J.F., Alshammari T.K. IntechOpen; 2022. New Use of the SSRI Fluvoxamine in the Treatment of COVID-19 Symptoms. [DOI] [Google Scholar]
- Arteaga-Henríquez G., Simon M.S., Burger B., Weidinger E., Wijkhuijs A., Arolt V., Birkenhager T.K., Musil R., Müller N., Drexhage H.A. Low-grade inflammation as a predictor of antidepressant and anti-inflammatory therapy response in MDD patients: a systematic review of the literature in combination with an analysis of experimental data collected in the EU-MOODINFLAME consortium. Front. Psychiatry. 2019;10:458. doi: 10.3389/fpsyt.2019.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton T., Lynch K., Dubé B., Gettes D.R., Tustin N.B., Ping Lai J., Metzger D.S., Blume J., Douglas S.D., Evans D.L. Selective serotonin reuptake inhibitor suppression of HIV infectivity and replication. Psychosom. Med. 2010;72:925–932. doi: 10.1097/PSY.0b013e3181f883ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandal L.T., MacDonald E., Veneti L., Ravlo T., Lange H., Naseer U., Feruglio S., Bragstad K., Hungnes O., Ødeskaug L.E., Hagen F., Hanch-Hansen K.E., Lind A., Watle S.V., Taxt A.M., Johansen M., Vold L., Aavitsland P., Nygård K., Madslien E.H. Outbreak caused by the SARS-CoV-2 Omicron variant in Norway, November to December 2021. Euro. Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.50.2101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H., Prescott R. John Wiley & Sons; London: 2006. Applied Mixed Models in Medicine. [Google Scholar]
- Callaway E. Could new COVID variants undermine vaccines? Labs scramble to find out. Nature. 2021;589:177–178. doi: 10.1038/d41586-021-00031-0. [DOI] [PubMed] [Google Scholar]
- Callaway E., Ledford H. How to redesign COVID vaccines so they protect against variants. Nature. 2021;590:15–16. doi: 10.1038/d41586-021-00241-6. [DOI] [PubMed] [Google Scholar]
- Calusic M., Marcec R., Luksa L., Jurkovic I., Kovac N., Mihaljevic S., Likic R. Safety and efficacy of fluvoxamine in COVID-19 ICU patients: an open label, prospective cohort trial with matched controls. Br. J. Clin. Pharmacol. 2022;88:2065–2073. doi: 10.1111/bcp.15126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpinteiro A., Edwards M.J., Hoffmann M., Kochs G., Gripp B., Weigang S., Adams C., Carpinteiro E., Gulbins A., Keitsch S., Sehl C., Soddemann M., Wilker B., Kamler M., Bertsch T., Lang K.S., Patel S., Wilson G.C., Walter S., Hengel H., Gulbins E. Pharmacological inhibition of acid sphingomyelinase prevents uptake of SARS-CoV-2 by epithelial cells. Cell. Rep. Med. 2020;1(8) doi: 10.1016/j.xcrm.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie A., Mbaeyi S.A., Walensky R.P. CDC interim recommendations for fully vaccinated people: an important first step. JAMA. 2021;325:1501–1502. doi: 10.1001/jama.2021.4367. [DOI] [PubMed] [Google Scholar]
- Deng J., Rayner D., Ramaraju H.B., Abbas U., Garcia C., Heybati K., Zhou F., Huang E., Park Y.J., Moskalyk M. Efficacy and safety of selective serotonin reuptake inhibitors in COVID-19 management: a systematic review and meta-analysis. Clin. Microbiol. Infect. 2023 doi: 10.1016/j.cmi.2023.01.010. S1198-743X(23)00032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane C.L. Comparative safety and tolerability of selective serotonin reuptake inhibitors. Hum. Psychopharmacol. 1995;10(Suppl 3(S3)):S185–S193. doi: 10.1002/hup.470100907. [DOI] [PubMed] [Google Scholar]
- Dong C., Zhang J.C., Yao W., Ren Q., Yang C., Ma M., Han M., Saito R., Hashimoto K. Effects of escitalopram, R-citalopram, and reboxetine on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2016;144:7–12. doi: 10.1016/j.pbb.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Fico G., Isayeva U., De Prisco M., Oliva V., Solè B., Montejo L., Grande I., Arbelo N., Gomez-Ramiro M., Pintor L., Carpiniello B., Manchia M., Vieta E., Murru A. Psychotropic drug repurposing for COVID-19: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2023;66:30–44. doi: 10.1016/j.euroneuro.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fred S.M., Kuivanen S., Ugurlu H., Casarotto P.C., Levanov L., Saksela K., Vapalahti O., Castrén E. Antidepressant and antipsychotic drugs reduce viral infection by SARS-CoV-2 and fluoxetine shows antiviral activity against the novel variants in vitro. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.755600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz B.A., Hoertel N., Lenze E.J., Jalali F., Reiersen A.M. Association between antidepressant use and ED or hospital visits in outpatients with SARS-CoV-2. Transl. Psychiatry. 2022;12(1):341. doi: 10.1038/s41398-022-02109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeson J.M., Gettes D.R., Spitsin S., Dubé B., Benton T.D., Lynch K.G., Douglas S.D., Evans D.L. The selective serotonin reuptake inhibitor citalopram decreases human immunodeficiency virus receptor and coreceptor expression in immune cells. Biol. Psychiatry. 2016;80:33–39. doi: 10.1016/j.biopsych.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Repurposing of CNS drugs to treat COVID-19 infection: targeting the sigma-1 receptor. Eur. Arch. Psychiatry Clin. Neurosci. 2021;271(2):249–258. doi: 10.1007/s00406-020-01231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Suzuki T., Hashimoto K. Mechanisms of action of fluvoxamine for COVID-19: a historical review. Mol. Psychiatry. 2022;27(4):1898–1907. doi: 10.1038/s41380-021-01432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan L., Sawyer C., Peek N., Lovell K., Carvalho A.F., Solmi M., Tilston G., Sperrin M., Firth J. Heightened COVID-19 mortality in people with severe mental illness persists after vaccination: a cohort study of greater manchester residents. Schizophr. Bull. 2023;49(2):275–284. doi: 10.1093/schbul/sbac118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N., Sánchez-Rico M., Vernet R., Beeker N., Jannot A.-S., Neuraz A., Salamanca E., Paris N., Daniel C., Gramfort A., Lemaitre G., Bernaux M., Bellamine A., Lemogne C., Airagnes G., Burgun A., Limosin F., AP-HP /Universities / INSERM COVID-19 Research Collaboration and AP-HP COVID CDR Initiative Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Mol. Psychiatry. 2021;26:5199–5212. doi: 10.1038/s41380-021-01021-4. [DOI] [PubMed] [Google Scholar]
- Hoertel N., Sánchez-Rico M., Cougoule C., Gulbins E., Kornhuber J., Carpinteiro A., Becker K.A., Reiersen A.M., Lenze E.J., Seftel D., Lemogne C., Limosin F. Repurposing antidepressants inhibiting the sphingomyelinase acid/ceramide system against COVID-19: current evidence and potential mechanisms. Mol. Psychiatry. 2021;26(12):7098–7099. doi: 10.1038/s41380-021-01254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N., Sánchez-Rico M., Gulbins E., Kornhuber J., Carpinteiro A., Abellán M., de la Muela P., Vernet R., Beeker N., Neuraz A., Delcuze A., Alvarado J.M., Cougoule C., Meneton P., Limosin F., AP-HP/Université de Paris/INSERM COVID-19 research collaboration/AP-HP COVID CDR Initiative/“Entrepôt de Données de Santé” AP-HP Consortium Association between FIASMA psychotropic medications and reduced risk of intubation or death in individuals with psychiatric disorders hospitalized for severe COVID-19: an observational multicenter study. Transl. Psychiatry. 2022;12(1):90. doi: 10.1038/s41398-022-01804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N., Sánchez-Rico M., Kornhuber J., Gulbins E., Reiersen A.M., Lenze E.J., Fritz B.A., Jalali F., Mills E.J., Cougoule C., Carpinteiro A., Mühle C., Becker K.A., Boulware D.R., Blanco C., Alvarado J.M., Strub-Wourgaft N., Lemogne C., Limosin F., On Behalf Of Ap-Hp/Université Paris Cité/Inserm Covid-Research Collaboration Ap-Hp Covid Cdr Initiative And Entrepôt de Données de Santé Ap-Hp Consortium Antidepressant use and its association with 28-day mortality in inpatients with SARS-CoV-2: support for the FIASMA model against COVID-19. J. Clin. Med. 2022;11(19):5882. doi: 10.3390/jcm11195882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N., Sánchez-Rico M., Gulbins E., Kornhuber J., Vernet R., Beeker N., Neuraz A., Blanco C., Olfson M., Airagnes G., Lemogne C., Alvarado J.M., Arnaout M., Cougoule C., Meneton P., Limosin F., AP-HP/Université de Paris/INSERM COVID-19 Research Collaboration/AP-HP COVID CDR Initiative/‘Entrepôt de Données de Santé’ AP-HP Consortium Association between benzodiazepine receptor agonist use and mortality in patients hospitalised for COVID-19: a multicentre observational study. Epidemiol. Psychiatr. Sci. 2022;31:e18. doi: 10.1017/S2045796021000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber C.A., Szucs T.D., Rapold R., Reich O. Identifying patients with chronic conditions using pharmacy data in Switzerland: an updated mapping approach to the classification of medications. BMC Public Health. 2013;13:1030. doi: 10.1186/1471-2458-13-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran M., Kumar Arora M., Asdaq S.M.B., Khan S.A., Alaqel S.I., Alshammari M.K., Alshehri M.M., Alshrari A.S., Mateq Ali A., Al-Shammeri A.M., Alhazmi B.D., Harshan A.A., Alam M.T., Abida Discovery, development, and patent trends on molnupiravir: a prospective oral treatment for COVID-19. Molecules. 2021;26:5795. doi: 10.3390/molecules26195795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel A., Shenhar Y., Green I., Merzon E., Golan-Cohen A., Schäffer A.A., Ruppin E., Vinker S., Magen E. Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. Vaccines (Basel) 2021;10:64. doi: 10.3390/vaccines10010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J., Hoertel N., Gulbins E. The acid sphingomyelinase/ceramide system in COVID-19. Mol. Psychiatry. 2022;27(1):307–314. doi: 10.1038/s41380-021-01309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Li J., Park J., Hart S.K., Song N.J., Burrow D.T., Bean N.L., Jacobs N.C., Coler-Reilly A., Pendergrass A.O., Pierre T.H., Bradley I.C., Carette J.E., Varadarajan M., Brummelkamp T.R., Dolle R., Peterson T.R. bioRxiv; 2020. Sphingolipid Biosynthesis Inhibition as a Host Strategy Against Diverse Pathogens. 2020.04.10. [DOI] [Google Scholar]
- Kutkat O., Moatasim Y., Al-Karmalawy A.A., Abulkhair H.S., Gomaa M.R., El-Taweel A.N., Abo Shama N.M., GabAllah M., Mahmoud D.B., Kayali G., Ali M.A., Kandeil A., Mostafa A. Robust antiviral activity of commonly prescribed antidepressants against emerging coronaviruses: in vitro and in silico drug repurposing studies. Sci. Rep. 2022;12:12920. doi: 10.1038/s41598-022-17082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landes S.D., Finan J.M., Turk M.A. COVID-19 mortality burden and comorbidity patterns among decedents with and without intellectual and developmental disability in the US. Disabil. Health J. 2022;15(4) doi: 10.1016/j.dhjo.2022.101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam W.Y., Fresco P. Medication adherence measures: an overview. Biomed Res. Int. 2015;2015 doi: 10.1155/2015/217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze E.J., Mattar C., Zorumski C.F., Stevens A., Schweiger J., Nicol G.E., Miller J.P., Yang L., Yingling M., Avidan M.S., Reiersen A.M. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324:2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S.L., Marquie-Beck J., Ellis R.J., Woods S.P., Best B., Clifford D.B., Collier A.C., Gelman B.B., Marra C., McArthur J.C., McCutchan J.A., Morgello S., Simpson D., Alexander T.J., Durelle J., Heaton R., Grant I., CHARTER Group The role of cohort studies in drug development: clinical evidence of antiviral activity of serotonin reuptake inhibitors and HMG-CoA reductase inhibitors in the central nervous system. J. Neuroimmune Pharmacol. 2007;2:120–127. doi: 10.1007/s11481-006-9054-y. [DOI] [PubMed] [Google Scholar]
- Liu N., Sun J., Wang X., Zhao M., Huang Q., Li H. The impact of dementia on the clinical outcome of COVID-19: a systematic review and meta-analysis. J. Alzheimers Dis. 2020;78(4):1775–1782. doi: 10.3233/JAD-201016. [DOI] [PubMed] [Google Scholar]
- Lu L.C., Chao C.M., Chang S.P., Lan S.H., Lai C.C. Effect of fluvoxamine on outcomes of nonhospitalized patients with COVID-19: a systematic review and meta-analysis. J. Infect. Public Health. 2022;15(11):1259–1264. doi: 10.1016/j.jiph.2022.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcec R., Majta M., Likic R. Will vaccination refusal prolong the war on SARS-CoV-2? Postgrad. Med. J. 2021;97:143–149. doi: 10.1136/postgradmedj-2020-138903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle C.K.S., Creeden J.F., McCullumsmith C., Worth R.G. SSRIs: applications in inflammatory lung disease and implications for COVID-19. Neuropsychopharmacol. Rep. 2021;41:325–335. doi: 10.1002/npr2.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menni C., Valdes A.M., Polidori L., Antonelli M., Penamakuri S., Nogal A., Louca P., May A., Figueiredo J.C., Hu C., Molteni E., Canas L., Österdahl M.F., Modat M., Sudre C.H., Fox B., Hammers A., Wolf J., Capdevila J., Spector T.D. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399:1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhaee H., Bayati R., Rahmanian M., Jolfayi A.G., Zangiabadian M., Rakhshanderou S. medRxiv; 2022. The Effect of Antidepressants on Severity of COVID-19 in Hospitalized Patients: A Systematic Review and Meta-Analysis. 2022.04.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgi Y., Futamura T., Kikuchi T., Hashimoto K. Effects of antidepressants on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2013;103(4):853–859. doi: 10.1016/j.pbb.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Pashaei Y. Drug repurposing of selective serotonin reuptake inhibitors: could these drugs help fight COVID-19 and save lives? J. Clin. Neurosci. 2021;88:163–172. doi: 10.1016/j.jocn.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J., Bates D. Springer Science & Business Media; New York: 2000. Mixed-Effects Models in S and S-PLUS. [Google Scholar]
- Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., Planchais C., Porrot F., Robillard N., Puech J., Prot M., Gallais F., Gantner P., Velay A., Le Guen J., Kassis-Chikhani N., Edriss D., Belec L., Seve A., Schwartz O. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- Plotkin S., Robinson J.M., Cunningham G., Iqbal R., Larsen S. The complexity and cost of vaccine manufacturing—an overview. Vaccine. 2017;35:4064–4071. doi: 10.1016/j.vaccine.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajpoot S., Alagumuthu M., Baig M.S. Dual targeting of 3CLpro and PLpro of SARS-CoV-2: a novel structure-based design approach to treat COVID-19. Curr. Res. Struct. Biol. 2021;3:9–18. doi: 10.1016/j.crstbi.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchman S.H., Mendelson S.G., Rauchman C., Kasselman L.J., Pinkhasov A., Reiss A.B. Ongoing use of SSRIs does not alter outcome in hospitalized COVID-19 patients: a retrospective analysis. J. Clin. Med. 2021;11(1):70. doi: 10.3390/jcm11010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis G., dos Santos Moreira-Silva E.A., Silva D.C.M., Thabane L., Milagres A.C., Ferreira T.S., dos Santos C.V.Q., de Souza Campos V.H., Nogueira A.M.R., de Almeida A.P.F.G., Callegari E.D., de Figueiredo Neto A.D., Savassi L.C.M., Simplicio M.I.C., Ribeiro L.B., Oliveira R., Harari O., Forrest J.I., Ruton H., Mills E.J. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Global Health. 2022;10:42–51. doi: 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio C., Malani P.N. COVID-19 in 2022—the beginning of the end or the end of the beginning? JAMA. 2022;327:2389–2390. doi: 10.1001/jama.2022.9655. [DOI] [PubMed] [Google Scholar]
- Rose S., van der Laan M.J. Why match? Investigating matched case-control study designs with causal effect estimation. Int. J. Biostat. 2009;5:1. doi: 10.2202/1557-4679.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman K.J., Greenland S., Lash T.L. Encyclopedia of Quantitative Risk Analysis and Assessment. John Wiley & Sons, Ltd; Chichester: 2008. Case–control studies. [DOI] [Google Scholar]
- Sachs J.D., Karim S.S.A., Aknin L., Allen J., Brosbøl K., Colombo F., Barron G.C., Espinosa M.F., Gaspar V., Gaviria A., Haines A., Hotez P.J., Koundouri P., Bascuñán F.L., Lee J.-K., Pate M.A., Ramos G., Reddy K.S., Serageldin I., Michie S. The Lancet Commission on lessons for the future from the COVID-19 pandemic. Lancet. 2022;400(10359):1224–1280. doi: 10.1016/S0140-6736(22)01585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacre S., Medghalchi M., Gregory B., Brennan F., Williams R. Fluoxetine and citalopram exhibit potent antiinflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis Rheum. 2010;62:683–693. doi: 10.1002/art.27304. [DOI] [PubMed] [Google Scholar]
- Saha S., Tanmoy A.M., Tanni A.A., Goswami S., Sium S.M.A., Saha S., Islam S., Hooda Y., Malaker A.R., Anik A.M., Haq M.S., Jabin T., Hossain M.M., Tabassum N., Rahman H., Hossain M.J., Islam M.S., Saha S.K. New waves, new variants, old inequity: a continuing COVID-19 crisis. BMJ Global Health. 2021;6 doi: 10.1136/bmjgh-2021-007031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seftel D., Boulware D.R. Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19. Open Forum Infect. Dis. 2021;8:ofab050. doi: 10.1093/ofid/ofab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman R.E., Anderson S.A., Dal Pan G.J., Gray G.W., Gross T., Hunter N.L., LaVange L., Marinac-Dabic D., Marks P.W., Robb M.A., Shuren J., Temple R., Woodcock J., Yue L.Q., Califf R.M. Real-world evidence—what is it and what can it tell us? N. Engl. J. Med. 2016;375:2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- Shrotri M., Navaratnam A.M.D., Nguyen V., Byrne T., Geismar C., Fragaszy E., Beale S., Fong W.L.E., Patel P., Kovar J., Hayward A.C., Aldridge R.W., Virus Watch Collaborative Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398:385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J., Lipsitch M., Almond J.W. Vaccine production, distribution, access, and uptake. Lancet. 2011;378:428–438. doi: 10.1016/S0140-6736(11)60478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup W.W. CRC Press; Boca Raton: 2021. Generalized Linear Mixed Models: Modern Concepts, Methods and Applications. 1a. [Google Scholar]
- Sukhatme V.P., Reiersen A.M., Vayttaden S.J., Sukhatme V.V. Fluvoxamine: a review of its mechanism of action and its role in COVID-19. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.652688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom R.P., Alexander J.L., Baron D., Garakani A., Gross L., Pine J.H., Radhakrishnan R., Slaby A., Sumner C.R. Selective serotonin reuptake inhibitors: how long is long enough? J. Psychiatr. Pract. 2021;27:361–371. doi: 10.1097/PRA.0000000000000578. [DOI] [PubMed] [Google Scholar]
- Torres I., Artaza O., Profeta B., Alonso C., Kang J. COVID-19 vaccination: returning to WHO's health for all. Lancet Global Health. 2020;8:e1355–e1356. doi: 10.1016/S2214-109X(20)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynan R.J., Weidenhofer J., Hinwood M., Cairns M.J., Day T.A., Walker F.R. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav. Immun. 2012;26(3):469–479. doi: 10.1016/j.bbi.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Venkatesan P. Repurposing drugs for treatment of COVID-19. Lancet Respir. Med. 2021;9:e63. doi: 10.1016/S2213-2600(21)00270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang R., Liu L., Qiao D., Baldwin D.S., Hou R. Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: a systematic review and meta-analysis. Brain Behav. Immun. 2019;79:24–38. doi: 10.1016/j.bbi.2019.02.021. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu Y. Multilevel determinants of COVID-19 vaccination hesitancy in the United States: a rapid systematic review. Prev. Med. Rep. 2021;25 doi: 10.1016/j.pmedr.2021.101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solidarity Trial Consortium W.H.O., Pan H., Peto R., Henao-Restrepo A.-M., Preziosi M.-P., Sathiyamoorthy V., Abdool Karim Q., Alejandria M.M., Hernández García C., Kieny M.-P., Malekzadeh R., Murthy S., Reddy K.S., Roses Periago M., Abi Hanna P., Ader F., Al-Bader A.M., Alhasawi A., Allum E., Swaminathan S. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N. Engl. J. Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2022. Therapeutics and COVID-19: Living Guideline.app.magicapp.org/#/guideline/nBkO1E/rec/jmYxpV Accessed 26 07 July 2022. [PubMed] [Google Scholar]
- Xiao X., Wang C., Chang D., Wang Y., Dong X., Jiao T., Zhao Z., Ren L., Dela Cruz C.S., Sharma L., Lei X., Wang J. Identification of potent and safe antiviral therapeutic candidates against SARS-CoV-2. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.586572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimniak M., Kirschner L., Hilpert H., Geiger N., Danov O., Oberwinkler H., Steinke M., Sewald K., Seibel J., Bodem J. The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue. Sci. Rep. 2021;11:5890. doi: 10.1038/s41598-021-85049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.