Abstract
Aim
To perform a systematic review of administration of calcium compared to no calcium during cardiac arrest.
Methods
The search included Medline (PubMed), Embase, Cochrane, Web of Science, and CINAHL Plus and was conducted on September 30, 2022. The population included adults and children in any setting with cardiac arrest. The outcomes included return of spontaneous circulation, survival, survival with favourable neurologic outcome to hospital discharge and 30 days or longer, and quality of life outcome. Cochrane Risk of Bias 2 and ROBINS-I were performed to assess risk of bias for controlled and observational studies, respectively.
Results
The systematic review identified 4 studies on 3 randomised controlled trials on 554 adult out-of-hospital cardiac arrest (OHCA) patients, 8 observational studies on 2,731 adult cardiac arrest patients, and 3 observational studies on 17,449 paediatric in-hospital cardiac arrest (IHCA) patients. The randomised controlled and observational studies showed that routine calcium administration during cardiac arrest did not improve the outcome of adult OHCA or IHCA or paediatric IHCA. The risk of bias for the adult trials was low for one recent trial and high for two earlier trials, with randomization as the primary source of bias. The risk of bias for the individual observational studies was assessed to be critical due to confounding. The certainty of evidence was assessed to be moderate for adult OHCA and low for adult and paediatric IHCA. Heterogeneity across studies precluded any meaningful meta-analyses.
Conclusions
This systematic review found no evidence that routine calcium administration improves the outcomes of cardiac arrest in adults or children.
PROSPERO Registration: CRD42022349641.
Keywords: Advanced Life Support, Basic Life Support, Cardiac Arrest, Calcium, Systematic Review, ILCOR
Introduction
Calcium administration during cardiac arrest has been shown to have variable results on the outcome of cardiac arrest patients. Older small randomised controlled trials1, 2 did not demonstrate beneficial effects of calcium on survival after in-hospital (IHCA) and out-of-hospital cardiac arrest (OHCA), limited by small study size and no survival to discharge in either trial. Observational studies have been limited by high risk of bias. The American Heart Association and European Resuscitation Council currently recommend against routine administration of calcium for treatment of cardiac arrest.3, 4 The most recent International Liaison Committee on Resuscitation (ILCOR) review on this topic was in 2010, at which time the treatment recommendation for adults was: “Routine administration of calcium for treatment of in-hospital and out-of-hospital cardiac arrest is not recommended.”5 The recommendation in 2010 for children was: “Routine use of calcium for infants and children with cardiopulmonary arrest is not recommended in the absence of hypocalcemia, calcium channel blocker overdose, hypermagnesemia, or hyperkalemia.”6 In spite of these recommendations, calcium administration remains common during cardiac arrest.7, 8 The publication of a recent and larger randomised controlled trial9, 10 adds substantial new data to the existing evidence on this topic, prompting this updated systematic review to compare the outcomes of routine calcium administration to no calcium administration for cardiac arrest in adults or children.
Methods
Protocol and registration
The protocol for this review was prospectively submitted to the International Prospective Register of Systematic Reviews (PROSPERO) on July 8, 2022 (registration number CRD42022349641). The protocol is provided in the Supplementary Materials. This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.11 The PRISMA checklist is provided in the Supplementary Materials. This review was commissioned at no cost by ILCOR and was carried out by ILCOR Task Force members and other volunteers. Given that the data reviewed were publicly available, this systematic review did not require the approval from the institutional review board.
Eligibility criteria and outcomes
The study question was framed using the PICOST (Population, Intervention, Comparison, Outcome, Study Design, Time frame) format: in adults and children in any setting (in-hospital or out-of-hospital) with cardiac arrest (P), does administration of calcium (intravenous or intraosseous) during cardiac arrest (I) as compared to no administration of calcium during cardiac arrest (C) improve clinical outcomes (O).
Relevant outcomes were prioritised by the ILCOR Advanced Life Support and Paediatric Life Support Task Forces and based on the available literature. We included return of spontaneous circulation (ROSC), health-related quality of life, survival and survival with favourable neurologic outcome to hospital discharge, 30 days or longer.
Randomised controlled trials (RCTs) and non-randomized studies (non-randomized controlled trials, interrupted time series, controlled before-and-after studies, cohort studies) with a control group were eligible for inclusion. Ecological studies, case series, case reports, reviews, abstracts, editorials, comments, letters to the editor, and unpublished studies were excluded. All years and all languages were included if there is an English abstract.
Information sources and search strategy
The search was conducted on July 8, 2022 and updated on September 30, 2022. Databases searched were Medline (PubMed), Embase, Cochrane, Web of Science, and CINAHL Plus. Clinicaltrials.gov, the International Clinical Trials Registry Platform (https://www.who.int/ictrp/en/), and PROSPERO were searched for ongoing or other completed studies. The search strategy is provided in the Supplementary Materials.
Study selection
Following removal of duplicates, two reviewers, using pre-defined screening criteria, independently screened all titles and abstracts retrieved from the search. Any disagreements regarding inclusion or exclusion were resolved by discussion between the reviewers and with a third reviewer if needed. The Kappa-value for interobserver variance was calculated. In case of only weak or moderate agreement between reviewers (i.e. a Kappa <0.60),12 a third reviewer reviewed all excluded titles and abstracts to ensure optimised sensitivity. Two reviewers then independently reviewed the full-text reports of all potentially relevant publications passing the first level of screening. Any disagreement regarding eligibility was resolved by discussion. The final report includes a Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) diagram showing the number of studies remaining after each stage of the selection process and reasons for exclusion of full-text articles.
Data collection
Two reviewers, using a pre-defined standardised data extraction form, independently extracted data as pertinent to the PICO, which included details of the study design, population, intervention, comparator, and outcomes. Any missing statistical parameters (e.g. relative risk, odds ratio) of importance and variance measures (e.g. confidence intervals) were calculated if data permitted. Any discrepancy regarding the extracted data was identified and resolved via discussion.
Risk of bias in individual studies
Two investigators independently assessed risk of bias for the included studies. Risk of bias was assessed by the Cochrane Risk of Bias 2 (RoB2) tool13 for randomised controlled trials and the ROBINS-I tool14 for observational studies. The RoB2 tool involves assessment of the risk of bias from each of five domains including (1) randomisation process, (2) deviations from intended deviation, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported result.13
In the ROBINS-I tool, risk of bias is assessed within specified domains, including (1) bias due to confounding, (2) bias in selection of participants into the study, (3) bias in classification of interventions, (4) bias due to deviations from intended interventions (5) bias due to missing data, (6) bias in measurement of outcomes, (7) bias in selection of the reported result, and (8) overall bias.14 Bias assessments were tabulated with detailed explanations when studies were judged at high risk of bias.
Heterogeneity and data synthesis
Studies were assessed for clinical (i.e., participants, interventions, and outcomes), methodological (i.e., study design or risk of bias), and potentially statistical heterogeneity (i.e., forest plots, Chi-squared statistics, and I2 statistics). A narrative synthesis was planned if heterogeneity (i.e., clinical, methodological, or statistical) was deemed too substantial across studies to allow for meaningful meta-analyses. Randomised trials and non-randomized studies were not combined in meta-analyses. Consistent with the I-ROBINS recommendations, observational studies with a critical risk of bias were not included in meta-analyses.14
Confidence in cumulative evidence
The certainty in the overall evidence was assessed using Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, ranging from very low to high certainty of evidence.15 Detailed assessment of overall risk of bias, inconsistency, indirectness, imprecision and other issues such as publication bias were tabulated using the GRADEpro software (McMaster University, 2014).
Results
Study selection
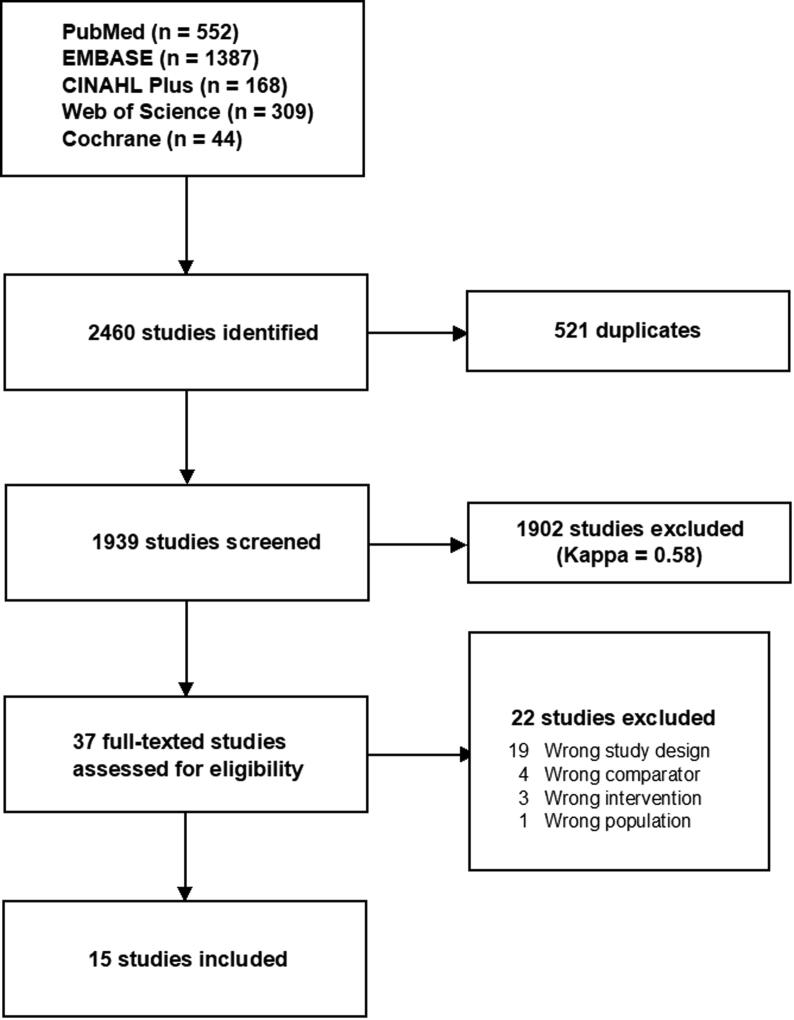
The search identified 1939 unique records, and 37 full-text manuscripts were assessed for eligibility (kappa = 0.58; Fig. 1). A total of 15 articles were eligible for inclusion, including 4 papers reporting on 3 controlled trials on 554 adult OHCA patients,1, 2, 9, 10 8 observational studies on 2,731 adult cardiac arrest patients (4 on OHCA,16, 17, 18, 19 2 on IHCA,20, 21 and 2 combined,22, 23 and 3 observational studies on 17,449 paediatric IHCA patients.24, 25, 26 No additional studies were identified after reviewing the references of included studies. The search for ongoing or unpublished randomised controlled trials did not identify any studies. Clinical and methodological heterogeneity precluded any meaningful meta-analyses for both randomised trials and observational studies. A descriptive overview of the studies is provided. Additional study and patient characteristics and study results are provided in the Supplementary Materials.
Fig. 1.
PRISMA Diagram, Chart illustrating the flow of articles. Of 1939 titles and abstracts, 36 full-text articles were assessed for eligibility, and 15 articles were included in the review.
Randomised controlled trials
Three trials comparing calcium administration to no calcium administration during adult OHCA were identified (Table 1, Table 2). There was heterogeneity in patient populations and intervention between these trials. Stueven et al. conducted two trials in 1982–1983, during which they randomised 90 OHCA patients in refractory electromechanical dissociation1 and 73 patients in refractory asystole2 to receive intravenous calcium chloride or saline. Electromechanical dissociation was defined as any electrical complex without pulses, and ventricular fibrillation and ventricular tachycardia were excluded. The rate of successful resuscitation (defined as “conveyance of patient with pulse in the emergency department”) was 16.7% (8/48) in the exposed group and 4.8% (2/42) in the unexposed group for patients with electromechanical dissociation,1 and 7.7% (3/39) in the exposed group and 1/34 (2.9%) in the unexposed group for patients with asystole.2 There were no survivors to hospital discharge in either group from both trials.1, 2 Risk of bias was assessed as high for both trials due to the method of randomization (Table S1).
Table 1.
Characteristics of randomised trials for adult out-of-hospital cardiac arrest.
| Study | Country | Year of inclusion | Main inclusion criteria | Patients | Location of cardiac arrest | Age group | Time of exposure | Intervention | Control | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Stueven, 1985 (EMD)1 | USA | 1982–1983 | OHCA patient in refractory EMD at time of randomisation (had adrenaline and bicarbonate). | 90 | OHCA | Adult | Unclear | IV calcium chloride | Saline | High |
| Stueven, 1985 (Asystole)2 | USA | 1982–1983 | OHCA patient in asystole at time of randomisation; refractory asystole (had adrenaline, atropine and bicarbonate) | 73 | OHCA | Adult | Unclear | IV calcium chloride | Saline | High |
| Vallentin, 20219 | Denmark | 2020–2021 | ≥18 years OHCA adults who received at least 1 dose of epinephrine during cardiac arrest | 391 | OHCA | Adult | During CPR, after first dose of epinephrine | IV or IO calcium chloride | Saline | Low |
| Vallentin, 202210 |
EMD: Electromechanical dissociation; OHCA: Out-of-hospital cardiac arrest; VF: Ventricular fibrillation; VT: Ventricular tachycardia.
Table 2.
Main results of randomised trials for adult out-of-hospital cardiac arrest.
| Study | Patients | Treatment | Control |
ROSCa |
Mid-term survival (30 and 90 days) |
Mid-term favourable neurological outcome (30 and 90 days) |
Long-term survival (6 and 12 months) |
Long-term favourable neurological outcome (6 and 12 months) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | ||||
| Stueven, 1985 (EMD)1 | 90 | Calcium | Saline | 8/48 (16.7%) | 2/42 (4.8%) | NR | NR | NR | NR | NR | NR | NR | NR |
| RR 3.5 (0.79–15.58) | |||||||||||||
| Stueven, 1985 (Asystole)2 | 73 | Calcium | Saline | 3/39 (7.7%) | 1/34 (2.9%) | 0/39 (0%)b | 0/34 (0%)b | NR | NR | NR | NR | NR | NR |
| RR 2.43 (0.26–22.31) | |||||||||||||
| Vallentin, 20219 & Vallentin, 202210 | 391 | Calcium | Saline | 37/193 (19%) | 53/198 (27%) | 10/193 (5.2%)c | 18/198 (9.1%)c | 7/193 (3.6%)c | 15/198 (7.6%)c | 10/193 (5.2%)e | 18/198 (9.1%)e | 8/193 (4.2%)e | 17/198 (8.6%)e |
| RR 0.72 (0.49–1.03) | RR 0.57 (0.27–1.18)c | RR 0.48 (0.20–1.12)c | RR 0.57 (0.27–1.18)e | RR 0.48 (0.22–1.07)e | |||||||||
| 10/193 (5.2%)d | 18/198 (9.1%)d | 7/193 (3.6%)d | 18/198 (9.1%)d | 9/193 (4.7%)f | 18/198 (9.1%)f | 7/193 (3.6%)f | 17/198 (8.6%)f | ||||||
| RR 0.57 (0.27–1.18)d | RR 0.40 (0.17–0.91)d | RR 0.51 (0.24–1.09)f | RR 0.42 (0.18–0.97)f | ||||||||||
EMD: Electromechanical dissociation; NR: Not reported; ROSC: Return of spontaneous circulation; RR: Risk ratio.
Defined as outcomes at ROSC.
Defined as outcomes at hospital discharge.
Defined as outcomes at 30 days.
Defined as outcomes at 90 days.
Defined as outcomes at 6 months.
Defined as outcomes at 12 months.
Vallentin et al.9 randomised 397 adult OHCA patients from 2020-2021 to receive up to 2 doses of 5 mmol intravenous or intraosseous calcium chloride or saline (Table 1, Table 2). The first dose of calcium chloride was administered immediately after the first dose of epinephrine. The trial was terminated early based on suggestions of harm in a pre-planned interim analysis. Of the 397 patients randomised, 391 were included in the analyses (193 in the calcium group and 198 in the saline group; mean age 68 [SD 14] years; 114 [29%] were female). For the primary outcome of ROSC, 19% (37/391) of the exposed group and 27% (53/198) of the unexposed group achieved ROSC (risk ratio 0.72 [0.49–1.03], risk difference −7.6% [95% CI −16% to 0.8%]; p = 0.09).9 For midterm survival outcomes, 5.2% (10/193) from the exposed group and 9.1% (18/198) from the unexposed group (RR 0.57 [0.27–1.18]) survived to 30 days, 90 days, and 6 months.9, 10 For long-term survival outcome, 4.7% (9/193) from the exposed group and 9.1% (18/198) from the unexposed group survived to 1 year (RR 0.51 [95% CI 0.24–1.09]).10
Survival with favourable neurologic outcomes are highlighted in Table 2. Survival with favourable neurologic outcome at 30 days was observed in 3.6% (7/193) of the exposed group and in 7.6% (15/198) in the unexposed group (risk ratio, 0.48 [95% CI, 0.20 to 1.12]; risk difference − 4.0% [95% CI −8.9% to 0.7%]; p = 0.12).9 Survival at 90 days with a favourable neurological outcome occurred in 3.6% (7/193) of the exposed group and 9.1% (18/198) in the unexposed group (risk ratio 0.40 [95% CI, 0.17–0.91]).9 Survival at 6 months with a favourable neurological outcome occurred in 5.2% (10/193) of the exposed group and 9.1% (18/198) in the unexposed group (risk ratio 0.57 [95% CI, 0.27–1.18]; risk difference −3.9% [95% CI −9.4% to 1.3%]). At 1 year, 3.6% (7/193) survived with a favourable neurological outcome in the exposed group while 8.6% (17/198) survived with a favourable neurological outcome in the unexposed group (risk ratio 0.42 [95% Cl 0.18–0.97]; risk difference −5.0% [95% CI −10% to −0.2%]).10 The quality of life (5-dimensional, 5-level EuroQol score) was lower for the exposed group at 30-days, 90-days, 6-months, and 1-year,9, 10 but the results were imprecise with wide confidence intervals (Table S2). Risk of bias of this trial was assessed as low (Table S1).
Observational studies in adults
There were 8 observational studies in adult cardiac arrest: 4 studies on OHCA,16, 17, 18, 19 2 studies on IHCA,20, 21 and 2 studies including both IHCA and OHCA22, 23 (Table S3). Years of patient inclusion ranged from 1980 to 2020. The number of patients analysed ranged from 30 to 773. The number of patients exposed to calcium administration ranged from 4 to 105, and the proportion of patients exposed to calcium ranged from 7.1% to 36.4%. The mean age of exposed patients ranged from 43 to 66 years. All 8 studies16, 17, 18, 19, 20, 21, 22, 23 reported on ROSC, successful resuscitation, or survival to hospital admission. Three studies reported on survival to hospital discharge,16, 22, 23 and one study23 reported on survival to hospital discharge with favourable neurologic outcome. The results of individual studies were inconsistent and imprecise with wide confidence intervals. Six studies favoured no calcium administration for ROSC16, 17, 18, 20, 22, 23 and two studies favoured no calcium for survival to hospital discharge.16, 22 Two studies favoured calcium administration for ROSC,19, 21 and one study favoured calcium administration for survival to hospital discharge and survival to discharge with favourable neurologic outcome23 (Figure S1). The risk of bias was assessed as critical for all adult observational studies due to confounding (Table S4).
Observational studies in children
We identified 3 observational studies in paediatric IHCA24, 25, 26 (Table S5). Years of patient inclusion ranged from 2000 to 2019. The number of patients analysed ranged from 51 to 15,921. The number of patients exposed to calcium administration ranged from 16 to 1986, and the proportion of patients exposed to calcium ranged from 12.5% to 45%. All 3 studies reported on ROSC and survival to hospital discharge. Two studies reported on survival to hospital discharge with favourable neurologic outcome.24, 26 The results of individual studies were imprecise with wide confidence intervals. All three studies favoured no calcium administration for paediatric IHCA (Figure S2). The risk of bias was assessed as critical for all paediatric observational studies due to confounding (Table S6).
Certainty of evidence
The certainty of evidence was assessed as moderate for the Vallentin randomised trial and very low for the Steuven trials. Reasons for downgrading are summarised in Table 3. Observational studies in adults were not used to assess the certainty in evidence for OHCA given that evidence from randomised trials was available. The certainty of evidence was assessed as low for adult IHCA (Table S7) and paediatric IHCA (Table S8) based on observational studies.
Table 3.
Certainty of evidence for randomised trials in adults with out-of-hospital cardiac arrest.
| Studies | Risk of Bias | Inconsistency | Indirectness | Imprecision | Othera | Overall |
|---|---|---|---|---|---|---|
| ROSC | ||||||
| Stueven, 1985 (EMD)1 | Serious | N/Ab | Not serious | Seriousc | None | Moderate |
| Stueven, 1985 (Asystole)2 | Serious | N/Ab | Not serious | Very seriousd | None | Very low |
| Vallentin, 20219 | Not serious | N/Ab | Not serious | Very seriousd | None | Very low |
| Survival to 1 months and 3 months | ||||||
| Vallentin, 20219 | Not serious | N/Ad | Not serious | Seriousc | None | Moderate |
| Favourable neurological outcome at 1 months and 3 months | ||||||
| Vallentin, 20219 | Not serious | N/Ae | Not serious | Seriousc | None | Moderate |
| Survival at 6 months and 12 months | ||||||
| Vallentin, 202210 | Not serious | N/Ae | Not serious | Seriousc | None | Moderate |
| Favourable neurological outcome at 6 months and 12 months | ||||||
| Vallentin, 202210 | Not serious | N/Ae | Not serious | Seriousc | None | Moderate |
EMD: Electromechanical dissociation; ROSC: Return of spontaneous circulation.
Includes assessment of publication bias and magnitude of the effect.
Cannot judge inconsistency because meta-analysis was not performed.
Confidence interval that includes one, and smaller than planned sample size as trial was stopped early.
Very small sample size, wide confidence interval.
Cannot judge inconsistency due to single trial.
Discussion
This systematic review identified 3 randomised controlled trials on adult OHCA,1, 2, 9, 10 8 observational studies on adult OHCA and/or IHCA,16, 17, 18, 19, 20, 21, 22, 23 and 3 observational studies on paediatric IHCA.24, 25, 26 No meta-analyses were conducted of the three trials included in this review due to heterogeneity, and high risk of bias in the earlier trials, as well as lack of survival outcomes in the earlier trials. The observational studies were all assessed to have a critical risk of bias due to confounding. Many studies provided only unadjusted results or did not adjust adequately for potential confounding factors. Furthermore, very few studies reported on the dose and timing of calcium administration. This systematic review included studies on adult and paediatric cardiac arrest, which differed from a recently published systematic review on this topic that only included adult cardiac arrest studies.27
Results from randomised trials1, 2, 9, 10 and most observational studies16, 17, 18, 20, 22, 23 favoured no routine administration of calcium during cardiac arrest. The majority of available evidence prior to the recent trial by Vallentin and colleagues9 was observational. Although the observational studies generally favour not administering calcium, the interpretations of interventions during cardiac arrest in these studies are often limited by resuscitation time bias.28 Resuscitation time bias accounts for the fact that patients who receive medical interventions during cardiac arrest are often in cardiac arrest for a longer duration. Therefore, they tend to have worse outcomes than those who did not remain in cardiac arrest long enough to receive the intervention being studied. This type of bias confounds the majority of observational studies of medical interventions in this patient population, including the included observational studies of calcium. The publication of a reasonably large and well-done randomised trial9 which also favours not giving calcium, however, strengthens the evidence against routine use of calcium in the setting of cardiac arrest.
Whether routine calcium administration could cause harm remains unclear. The trial by Vallentin et al was stopped early based on suggestions of harm in a pre-planned interim analysis.9 While termination of trials in the face of risk to patients is important, premature termination can also lead to overestimation of effect size. In addition, the number of patients with mid- and long-term survival from that trial was low, and their confidence intervals were therefore wide.9, 10
The effect of calcium administration on cardiac arrest from special circumstances such as hyperkalemia, wide QRS interval on electrocardiogram, hypocalcemia, hypermagnesemia, calcium channel blocker overdose, or haemorrhage remains unknown. Only small trials or observational studies to date have attempted to stratify based on initial rhythm16 or potassium levels,21, 23 which have been limited by critical risk of bias due to confounding. The two observational studies21, 23 that favoured calcium administration stratified their analyses by serum potassium levels during cardiac arrest. Wang et al included adult IHCA patients with serum potassium >6.5 mEq/L during CPR and performed subgroup analyses with potassium levels of 6.5 to 7.4, 7.5 to 9.4, and >9.4 mEq/L.21 The odds ratio for ROSC was 51.11 (95% CI 3.12–1639) for the subgroup with potassium level <9.4 mEq/L who received both calcium and sodium bicarbonate. Wongtanasarasin et al included adult OHCA and IHCA patients from the emergency department and stratified their analyses to low, normal, and high levels of serum potassium and calcium levels.23 They found odds ratio in favour of calcium administration for survival with hospital discharge (OR 1.93 [95%CI 0.43–8.56]) and survival to hospital discharge with favourable neurologic outcome (OR 6.6 [95%CI 0.72–60.74]). The very wide confidence intervals and high risk of bias for findings from both of these trials highlight the lack of certainty in this evidence.
This systematic review has limitations. It is unknown whether the results from this systematic review extends to the special circumstances of cardiac arrest such as hyperkalemia, patients with widened QRS complex on their electrocardiogram, or cardiac arrest from haemorrhage. It is also unknown whether different dosing regimens of calcium administration during cardiac arrest could affect patient outcome in cardiac arrest.
Conclusions
This systematic review did not identify beneficial effects of routine calcium administration during cardiac arrest for adult OHCA and IHCA or paediatric IHCA patients.
CRediT authorship contribution statement
Cindy H. Hsu: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Keith Couper: Methodology, Formal analysis, Investigation, Writing – review & editing. Tyler Nix: Methodology, Resources, Writing – review & editing. Ian Drennan: . Joshua Reynolds: Methodology, Writing – review & editing. Monica Kleinman: Methodology, Writing – review & editing. Katherine M. Berg: Conceptualization, Methodology, Formal analysis, Investigation, Writing – review & editing, Supervision.
Conflicts of interest
Dr. Couper is an Associate Editor of Resuscitation Plus. Drs. Hsu and Berg are Editorial Board Members of Resuscitation Plus. None of the authors have any financial conflicts of interests.
Acknowledgement
The authors would like to thank Bernd W. Böttiger and Florian Schmitzberger for their assistance with translation of study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2023.100379.
Appendix 1
International Liaison Committee on Resuscitation’s (ILCOR)
Advanced Life Support Task Force: Lars W. Andersen, Katherine M. Berg, Keith Couper, Charles D. Deakin, Ian R. Drennan, Rakesh Garg, Asger Granfeldt, Karen Hirsch, Mathias Holmberg, Cindy H. Hsu, Peter Kudenchuk, Shinichiro Ohshimo, Tonia C. Nicholson, Jerry P. Nolan, Brian J. O’Neil, Robert W. Neumar, Michael J. Parr, Joshua C. Reynolds, Claudio Sandroni, Jasmeet Soar, Markus Skrifvars, Carolyn Zelop.
Paediatric Life Support Task Force: Richard Aickin, Jason Acworth, Thomaz Bittencourt Couto, Jana Djakow, Raffo Escalante, Anne-Marie Guerguerian, Florian Hoffman, Monica Kleinman, David Kloeck, Hiroshi Kurosawa, Kee-Chong Ng, Gabrielle Nuthall, Tia Raymond, Antonio Rodriguez-Nunez, Steve Schexnayder, Barney Scholefield, Janice Tijssen, Alexis Topjian
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Stueven H.A., Thompson B., Aprahamian C., Tonsfeldt D.J., Kastenson E.H. The effectiveness of calcium chloride in refractory electromechanical dissociation. Ann Emerg Med. 1985;14:626–629. doi: 10.1016/s0196-0644(85)80874-x. [DOI] [PubMed] [Google Scholar]
- 2.Stueven H.A., Thompson B., Aprahamian C., Tonsfeldt D.J., Kastenson E.H. Lack of effectiveness of calcium chloride in refractory asystole. Ann Emerg Med. 1985;14:630–632. doi: 10.1016/s0196-0644(85)80875-1. [DOI] [PubMed] [Google Scholar]
- 3.Soar J., Nolan J.P., Böttiger B.W., Perkins G.D., Lott C., Carli P., et al. European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation. 2015;95:100–147. doi: 10.1016/j.resuscitation.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Panchal A.R., Bartos J.A., Cabañas J.G., Donnino M.W., Drennan I.R., Hirsch K.G., et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142:S366–S468. doi: 10.1161/CIR.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 5.Morrison L.J., Deakin C.D., Morley P.T., Callaway C.W., Kerber R.E., Kronick S.L., et al. Part 8: Advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122:S345–S421. doi: 10.1161/CIRCULATIONAHA.110.971051. [DOI] [PubMed] [Google Scholar]
- 6.Kleinman M.E., de Caen A.R., Chameides L., Atkins D.L., Berg R.A., Berg M.D., et al. Pediatric basic and advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Pediatrics. 2010;126:e1261–e1318. doi: 10.1542/peds.2010-2972A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moskowitz A., Ross C.E., Andersen L.W., Grossestreuer A.V., Berg K.M., Donnino M.W., et al. Trends Over Time in Drug Administration During Adult In-Hospital Cardiac Arrest. Crit Care Med. 2019;47:194–200. doi: 10.1097/CCM.0000000000003506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross C.E., Moskowitz A., Grossestreuer A.V., Holmberg M.J., Andersen L.W., Yankama T.T., et al. Trends over time in drug administration during pediatric in-hospital cardiac arrest in the United States. Resuscitation. 2021;158:243–252. doi: 10.1016/j.resuscitation.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallentin M.F., Granfeldt A., Meilandt C., Povlsen A.L., Sindberg B., Holmberg M.J., et al. Effect of Intravenous or Intraosseous Calcium vs Saline on Return of Spontaneous Circulation in Adults With Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA. 2021;326:2268–2276. doi: 10.1001/jama.2021.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallentin M.F., Granfeldt A., Meilandt C., Povlsen A.L., Sindberg B., Holmberg M.J., et al. Effect of calcium vs. placebo on long-term outcomes in patients with out-of-hospital cardiac arrest. Resuscitation. 2022;179:21–24. doi: 10.1016/j.resuscitation.2022.07.034. [DOI] [PubMed] [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McHugh M.L. Interrater reliability: the kappa statistic. Biochem Med. 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 14.Sterne J.A.C., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stueven H., Thompson B.M., Aprahamian C., Darin J.C. Use of calcium in prehospital cardiac arrest. Ann Emerg Med. 1983;12:136–139. doi: 10.1016/s0196-0644(83)80551-4. [DOI] [PubMed] [Google Scholar]
- 17.Gando S., Tedo I., Tujinaga H., Kubota M. Variation in serum ionized calcium on cardiopulmonary resuscitation. J Anesth. 1988;2:154–160. doi: 10.1007/s0054080020154. [DOI] [PubMed] [Google Scholar]
- 18.Crickmer M., Drennan I.R., Turner L., Cheskes S. The association between end-tidal CO2 and return of spontaneous circulation after out-of-hospital cardiac arrest with pulseless electrical activity. Resuscitation. 2021;167:76–81. doi: 10.1016/j.resuscitation.2021.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Thongpitak Huabbangyang P., Tavachai Soion R.N., Acharee Promdee R.N., Kasemchai Nguanjinda R.N., Assanai Chamchan R.N., Ratree Chaisorn P.M.D., et al. Factors associated with successful resuscitation during out-of-hospital cardiac arrest performed by Surgico Medical Ambulance and Rescue Team (SMART), Division of Emergency Medical Service and Disaster, Faculty of Medicine Vajira Hospital, Navamindradhiraj University. J Med Assoc Thai. 2021;1 [Google Scholar]
- 20.van Walraven C., Stiell I.G., Wells G.A., Hébert P.C., Vandemheen K. Do Advanced Cardiac Life Support Drugs Increase Resuscitation Rates From In-Hospital Cardiac Arrest? Ann Emerg Med. 1998;32:544–553. doi: 10.1016/s0196-0644(98)70031-9. [DOI] [PubMed] [Google Scholar]
- 21.Wang C.-H., Huang C.-H., Chang W.-T., Tsai M.-S., Yu P.-H., Wu Y.-W., et al. The effects of calcium and sodium bicarbonate on severe hyperkalaemia during cardiopulmonary resuscitation: A retrospective cohort study of adult in-hospital cardiac arrest. Resuscitation. 2016;98:105–111. doi: 10.1016/j.resuscitation.2015.09.384. [DOI] [PubMed] [Google Scholar]
- 22.Stiell I.G., Wells G.A., Hebert P.C., Laupacis A., Weitzman B.N. Association of drug therapy with survival in cardiac arrest: limited role of advanced cardiac life support drugs. Acad Emerg Med. 1995;2:264–273. doi: 10.1111/j.1553-2712.1995.tb03220.x. [DOI] [PubMed] [Google Scholar]
- 23.Wongtanasarasin W., Ungrungseesopon N., Namsongwong N., Chotipongkul P., Visavakul O., Banping N., et al. Association between calcium administration and outcomes during adult cardiopulmonary resuscitation at the emergency department. Turk J Emerg Med. 2022;22:67–74. doi: 10.4103/2452-2473.342805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivasan V., Morris M.C., Helfaer M.A., Berg R.A., Nadkarni V.M. the American Heart Association National Registry of CPR Investigators. Calcium Use During In-hospital Pediatric Cardiopulmonary Resuscitation: A Report From the National Registry of Cardiopulmonary Resuscitation. Pediatrics. 2008;121:e1144–e1151. doi: 10.1542/peds.2007-1555. [DOI] [PubMed] [Google Scholar]
- 25.Mok Y.H., Loke A.P., Loh T.F., Lee J.H. Characteristics and Risk Factors for Mortality in Paediatric In-Hospital Cardiac Events in Singapore: Retrospective Single Centre Experience. Ann Acad Med Singapore. 2016;45:534–541. [PubMed] [Google Scholar]
- 26.Dhillon G.S., Kleinman M.E., Staffa S.J., Teele S.A., Thiagarajan R.R., for the American Heart Association’s Get With The Guidelines-Resuscitation (GWTG-R) Investigators Calcium Administration During Cardiopulmonary Resuscitation for In-Hospital Cardiac Arrest in Children With Heart Disease Is Associated With Worse Survival—A Report From the American Heart Association’s Get With The Guidelines-Resuscitation (GWTG-R) Registry*. Pediatric Crit Care Med. 2022;23:860–871. doi: 10.1097/pcc.0000000000003040. [DOI] [PubMed] [Google Scholar]
- 27.Messias Hirano Padrao E., Bustos B., Mahesh A., de Almeida C.M., Randhawa R., John Dipollina C., et al. Calcium use during cardiac arrest: A systematic review. Resusc Plus. 2022;12 doi: 10.1016/j.resplu.2022.100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen L.W., Grossestreuer A.V., Donnino M.W. “Resuscitation time bias”—A unique challenge for observational cardiac arrest research. Resuscitation. 2018;125:79–82. doi: 10.1016/j.resuscitation.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.