Abstract
Cosmetics made from natural ingredients are increasingly popular because they contain bioactive compounds which can provide many health benefits, more environmentally friendly and sustainable. The health benefits obtained from natural-based ingredients include anti-aging, photoprotective, antioxidant, and anti-inflammatory. This article reviewed the potential of selected flavonoids from bajakah tampala (Spatholobus littoralis Hassk.) as the native plant in Indonesia. We present in silico, in vitro, in vivo, and clinical research data on the use of selected flavonoids that have been reported in other extracts.
Keywords: Cosmetic properties, Selected flavonoids, Indonesia, Spatholobus littoralis Hassk.
1. Introduction
The trend in the cosmetic industry from natural ingredients has been proliferating since the 21st century. This increasing is encouraged by environmental and health awareness (FAO and Non-Timber Forest Products-Exchange Programme, 2020). In the forecast period (2020–2021), the global market for cosmetics is estimated to grow by 4.75 %, surpassing $716 billion in 2025 and reaching $784.6 billion in 2027. The North American and Asia Pacific regions account for more than 60 % of the global market for cosmetics (Roberts, 2022). Indonesia is one country located in the Asia Pacific region. Its national cosmetic industry growth grew by 7.36 % in the first quarter of 2018 (Ministry of Industry of The Republic of Indonesia, 2018). In 2020, exports of cosmetics from Indonesia totaled USD 784.9 thousand (a 1.5 % increase over 2019) (Trade Attache Indonesian Embassy in Tokyo, 2021).
The principle of “back to nature” has been widely used in the research world and the development of the research cosmetic industry, such as using plant extracts that have been well received by consumers (Sim and Nyam, 2021). Natural materials can be obtained from various plant parts, such as stems, flowers, leaves, fruit, and root (Pandurangan et al., 2018). Cosmetics from natural ingredients are simply absorbed into the body, hypoallergenic, and environmentally friendly (Amberg and Fogarassy, 2019, Carvalho et al., 2021). Bioactive compounds in plants have caught the attention of scientists because of their broad range of health advantages, including their anti-inflammatory, antioxidant, anti-gout, and anticarcinogenic characteristics (Gouvinhas et al., 2020, Sianipar et al., 2022).
The consultancy firm McKinsey & Company (2020) stated that the global income for the beauty industry (including skin care, color cosmetics, hair care, fragrances, and personal care) might decline by up to 30 % during the coronavirus disease (COVID-19) pandemic. However, the beauty industry has faced intense competition and innovation pressure after this pandemic. The beauty industry must innovate, transform, understand cosmetic consumers, and adapt rapidly, which keeps companies growing and reaching new market potential opportunities. Beauty products shift towards wellness, pampering, and natural ingredients (Farisha and Safari, 2021, Wang, 2022). Therefore, cosmetic products made from natural ingredients will have the opportunity to be popular among consumers due to the impact of COVID-19 (Embassy of The Republic of Indonesia in Brussels, 2021).
Environmental variables, including radiation and pollution, produce reactive oxygen species (ROS) and the beginning of the body's response to inflammation (Zhang et al., 2020). This condition is characterized by the extracellular matrix proteins collagen and elastin and the activity of aging-related enzymes such as tyrosinase, elastase, hyaluronidase, and collagenase (Aguilar-Toalá et al., 2019, Buhren et al., 2016, Taghouti et al., 2018). Tyrosinase is an enzyme that helps melanocytes produce melanin; however, pigmentation problems result when too much melanin is produced in the skin (Liyanaarachchi et al., 2018). Elastase will hydrolyze elastin and affect the mechanical properties of connective tissue (Aguilar-Toalá et al., 2019).
Indonesia is a mega-biodiversity country with about 30,000 species that have been recognized and 950 species: plants, animals, and microbes-that have medicinal properties (Embassy of The Republic of Indonesia in Brussels, 2021). This potential includes Indonesia's strength in developing cosmetic products from natural ingredients. The bajakah tampala plant (Spatholobus littoralis Hassk.) comes from the Leguminosae family (Numan, 1998). It is important to analyze and review because of the large amount of research data that shows its biological activity scientifically and empirically. For the Dayak community in Central Kalimantan, Indonesia, it is a local wisdom ingredient for aches, and diarrhea, reducing lumps in the body and lowering uric acid. The ethanol extract of S. littoralis Hassk. has high antioxidant activity (IC50 8.25 μg/mL) (Iskandar and Warsidah, 2020). This plant also shows anti-breast cancer activity (Iskandar et al., 2022). Additionally, flavonoids, saponins, steroids, terpenoids, tannins, and phenolic compounds have all been scientifically demonstrated to be present in bajakah tampala's phytochemical composition. Furthermore, Sianipar (2022) reported that phenolic compounds dominated by more than 50 % in the 1-butanol stem fraction of a 70 % ethanol and water extract of S. littoralis Hassk.
The flavonoids of S. littoralis Hassk. have similarities with their application in antioxidant, anti-inflammatory, and photoprotective, as well as in cosmetic products such as anti-aging. It also inhibits the activity of tyrosinase, elastase, collagenase, and hyaluronidase enzymes. However, to the best authors' knowledge, no review has been found on applying some selected flavonoids of S. littoralis Hassk. plant as an active ingredient in cosmetics. Therefore, based on the literature study, the potential of this indigenous plant in cosmetic properties has been reviewed.
2. Compound composition of S. littoralis Hassk. stem fraction
Using liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, Sianipar (2022) conducted a preliminary study to identify the putative compounds contained in the most active fraction to inhibit xanthine oxidase (XO) activity of S. littoralis Hassk. stem, namely 1-butanol stem fraction of water extract and 1-butanol stem fraction of 70 % ethanol extract. The analysis revealed that groups of phenolics dominated the two most active fractions. In addition, the two most active fractions had XO inhibitory activity (antigout agent) with IC50 of 116.91 ± 3.51 µg/mL for the 1-butanol stem fraction of 70 % ethanol extract and IC50 of 137.15 ± 5.00 µg/mL for the 1-butanol stem fraction of water extract (Sianipar, 2022).
The flavonoid sub-class contained in the two most active fractions (1-butanol fraction of 70 % ethanol extract and 1-butanol fraction of water extract) consisted of flavan-3-ol, isoflavone, flavone, flavanone, and flavonol. 1-butanol fraction of 70 % ethanol extract contains flavanone as the largest percentage of flavonoid sub-class composition (36.36 %); while isoflavone (75.00 %) in 1-butanol fraction of water extract is the largest flavonoid sub-class. The list of flavonoid compounds contained in each of the most active fractions can be observed in Table 1 (Sianipar, 2022).
Table 1.
The list of flavonoid compounds contained in 1-butanol stem fraction of 70% ethanol extract and 1-butanol stem fraction of water extract (Sianipar, 2022).
| No. | Sub-class of flavonoids | Compounds | 1-butanol stem fraction of 70 % ethanol extract |
1-butanol stem fraction of water extract |
||||
|---|---|---|---|---|---|---|---|---|
| Presence | Quantity | Percentage | Presence | Quantity | Percentage | |||
| 1. | Flavan-3-ol | Catechin | ✔ | 1 | ||||
| sub-total | 1 | 9.09 % | sub-total | 0 | 0.00 % | |||
| 2. | Isoflavone | Daidzein | ✔ | 1 | ✔ | 1 | ||
| Formononetin | ✔ | 1 | ✔ | 1 | ||||
| Glycitein | ✔ | 1 | ||||||
| sub-total | 2 | 18.18 % | sub-total | 3 | 75.00 % | |||
| 3. | Flavone | Luteolin | ✔ | 1 | ||||
| Apigenin | ✔ | 1 | ||||||
| Negletein | ✔ | 1 | ||||||
| Apigetrin | ✔ | 1 | ||||||
| sub-total | 3 | 27.27 % | sub-total | 1 | 25.00 % | |||
| 4. | Flavanone | Naringenin | ✔ | 1 | ||||
| Hesperetin | ✔ | 1 | ||||||
| (-)-8-Prenylnaringenin | ✔ | 1 | ||||||
| 6,8-Diprenylnaringenin | ✔ | 1 | ||||||
| sub-total | 4 | 36.37% | sub-total | 0 | 0.00 % | |||
| 5. | Flavonol | Kaempferide | ✔ | 1 | ||||
| sub-total | 1 | 9.09 % | sub-total | 0 | 0.00 % | |||
| Total | 11 | 100.00 % | Total | 4 | 100.00 % | |||
3. Application of S. littoralis Hassk. in cosmetic properties
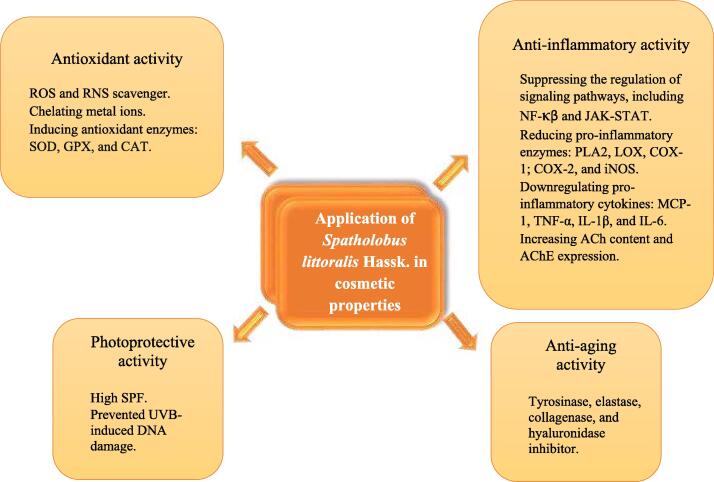
We explore the application of S. littoralis Hassk. in cosmetic properties, as summarized in Fig. 1.There are four main activities: antioxidant activity, anti-inflammatory activity, photoprotective activity, and anti-aging activity. The activity of selected flavonoids is divided based on in silico (Table 3), in vitro (Table 4), in vivo (Table 5), and clinical data (Table 6) associated with cosmetic properties.
Fig. 1.
Schematic diagram on application of Spatholobus littoralis Hassk. in cosmetic properties.
Table 3.
Selected flavonoids of S. littoralis Hassk. in cosmetic properties based on in silico activity.
| Cosmetic properties | The main mechanism | Selected flavonoids | In silico activity | Reference(s) |
|---|---|---|---|---|
| Antioxidant activity |
|
Catechin | Catechin binds five enzymes that are responsible for ROS: NADPH oxidase (PDB: 2CDU), cytochrome P450 (CP450) (PDB: 1OG5), myeloperoxidase (MP) (PDB: 1DNU), and XO; the docking scores are -6.75; -5.78; -5.19; and -7.83 kcal.mol-1 respectively. Those docking scores are lower than dextromethorphan (DEX) as the positive control. The interaction patterns of catechin and the binding site of the enzyme are: 1. NADPH oxidase→ hydrogen bonds: Asp179, Tyr188, Val214; and pi–c: Lys187. 2. Cytochrome P450 →hydrogen bond: Ser365; pi–pi: Phe476. 3. Myeloperoxidase →hydrogen bond: Asn186, Arg188, Asn189, Phe213. 4. Xanthine oxidase →HB: Glu802, Thr1010; pi–pi: Phe914, Phe1009. |
(Kritsi et al., 2022) |
| Daidzein | The binding energy of docked complex with Catalase as an antioxidant protein was found to be -100.665 kcal.mol-1. The interaction residues for Catalase were ARG-72, ARG-112, TYR-358, HIS-75, GLY-147, ASN-148, PHE-153, ARG-354, TYR-358, ASP-389, ASN-403, ARG-68, GLU-330, GLU-71, GLU-119, and ARG-170. | (Tidke et al., 2018) | ||
| Formononetin | Docking score for formononetin from vegetal extract to bind glutathione peroxidase 4 (GPX4) (protein target) is -6.547 kcal.mol-1. | (Costea et al., 2022) | ||
| Glycitein | The binding energy of docked complex with Catalase as an antioxidant protein was found to be -97.5342 kcal.mol-1. The interaction residues for Catalase were ARG-72, ARG-112, TYR-358, HIS-75, GLY-147, ASN-148, PHE-153, ARG-354, TYR-358, ASP-389, ASN-403, ARG-68, GLU-330, GLU-71, GLU-119, and ARG-170. | (Tidke et al., 2018) | ||
| Luteolin | Luteolin binds ROS with a docking score of -8.3 kcal.mol-1. Residue interactions were LYS1980, ALA1978, LEU2026, LEU2086, VAL1959. | (Syamsul et al., 2022) | ||
| Apigenin | Docking score for apigenin from vegetal extract to bind GPX4 (protein target) is -6.918 kcal.mol-1. | (Costea et al., 2022) | ||
| Hesperetin | Hesperetin from citrus demonstrated good binding energies for the target enzymes, such as β-site amyloid precursor protein (APP) cleaving enzyme 1 (BACE1) and AChE with binding energies -8.3; -8.4 kcal.mol-1 respectively. | (Lee et al., 2018) | ||
| Naringenin | Docking score for naringenin from vegetal extract to bind GPX4 (protein target) is -6.048 kcal.mol-1. | (Costea et al., 2022) | ||
| Anti-inflammatory activity |
|
Catechin | Catechin binds four target proteins:
|
(Khan et al., 2022b) |
| Daidzein | Daidzein binds COX-1 with binding energy -110.38 kcal.mol-1. The main residues of interactions for COX-1 were GLN-44, GLN-42, HIS-43, THR-206, ASN-382, ALN-203, GLN-203, HIS-207, PHE-210, HIS-388, LYS-468, ARG-469. | (Tidke et al., 2018) | ||
| Formononetin | Formononetin binds TNF-α with a docking score of -7.626 kcal.mol-1. | (Costea et al., 2022) | ||
| Glycitein | Glycitein binding COX-1 with binding energy -104.35 kcal.mol-1. The main residues of interactions for COX-1 were GLN-44, GLN-42, HIS-43, THR-206, ASN-382, ALN-203, GLN-203, HIS-207, PHE-210, HIS-388, LYS-468, ARG-469. | (Tidke et al., 2018) | ||
| Luteolin | Following docking investigations, luteolin binds to the catalytic iron atom in 5-LOX and generates stabilizing hydrogen bonds with His367 and Thr364. Luteolin binding ACE-2 with binding energy -8.9 kcal.mol-1. |
(Kutil et al., 2014) (Alzaabi et al., 2022) |
||
| Apigenin | Apigenin binding ACE-2 with binding energy -8.5 kcal.mol-1. | (Alzaabi et al., 2022) | ||
| Hesperetin | Hesperetin functions as an in silico inhibitor of the SARS spike glycoprotein-Human ACE2 complex with an affinity of -9.2 kcal.mol-1. | (Cheke et al., 2021) | ||
| Naringenin | Naringenin binds ACE-2 with binding energy -8.5 kcal.mol-1. | (Alzaabi et al., 2022) | ||
| Tyrosinase inhibitor |
|
Catechin | Catechin had a lower docking score (-9.58 kcal.mol-1) than the inhibitor kojic acid (-7.99 kcal.mol-1) when it came to binding to the active site of the tyrosinase enzyme. Tyrosinase was the target protein, and catechin's binding energy to it was -7.64 kcal.mol-1 via H-bond (HIS296). Compared to kojic acid, the energy value was lower (-5.03 kcal.mol-1). It proved that kojic acid and catechin have different levels of affinity. |
(Abdelfattah et al., 2022) (Laksmiani et al., 2020) |
| Daidzein | Daidzein isolated from the root of Pueraria lobata inhibited mushroom tyrosinase activity with a docking score of -7.09 kcal.mol-1. The docking score is lower than the positive control (kojic acid), -5.5 kcal.mol-1. | (Wagle et al., 2019) | ||
| Luteolin | The binding energy of luteolin on tyrosinase enzyme was -6.19 kcal.mol-1 which was lower than kojic acid (-5.5 kcal.mol-1). The interactions that occur are H-bond interaction (Cys83, Gly245, Ala246, Val248), electrostatic interaction (Glu322), and hydrophobic interaction (His85 and Val248). Luteolin binds the mushroom tyrosinase with a docking score-7.9 kcal.mol-1. The hydroxyl group on Ring B of luteolin formed H-bond with Glu322. |
(Wagle et al., 2018) (Jakimiuk et al., 2021) |
||
| Hesperetin | Hesperetin chelates a copper ion that combines with three histidine residues (HIS259, HIS85, and HIS61) within the active site to inhibit tyrosinase in the competitive pathway (KI=4.030±26 mM). | (Si et al., 2012) | ||
| Naringenin | Naringenin is a similar compound to 2S-Pinocembrin which has one H-bond on the active site of the tyrosinase enzyme. | (Lall et al., 2016) | ||
| Elastase inhibitor |
|
Catechin | Catechin had the best docking score (-20.36 kcal.mol-1) on binding the active site of elastase enzyme compared to -13.32 kcal.mol-1 of kojic acid as an inhibitor. Hydrogen bonding interactions with Pro232 and Arg 249 and hydrophobic contacts to Lys233, Lys241, and Val243.Catechin binds MMP1 with binding affinity -8.5 kcal.mol-1. Possible binding sites were ARG214, HIS218, ASN180, SER239. Common residues were HIS218, GLU219, SER239, PRO238, ARG21. Catechin also binds MMP8 with binding affinity -8.8 kcal.mol-1. Possible binding sites were ALA161, VAL194, HIS197, LEU214, TRY219, ASN218, PRO217. Common residues were LEU160, TRY216, ASN218, LEU214, GLU198, PRO217, HIS197, GLU198, ALA161. | (Abdelfattah et al., 2022) (Kose et al., 2020) |
| Luteolin | Luteolin binds MMP8 with a binding affinity was -10.1 kcal.mol-1. Possible binding sites were LEU160, LEU214, PRO217, TYR216, ARG222, GLU198, ALA161. Common residues were LEU160, TRY216, ASN218, LEU214, GLU198, PRO217, HIS197, GLU198, ALA161. Luteolin also binding MMP11 with a binding affinity was -10 kcal.mol-1. Possible sites were HIS219, LEU181, LEU236, SER238, PHE240, TYR241, VAL216, GLN215. Common residues were THR202, ASP200, GLU201, GLN12, GLN209, ASN208, TYR241. | (Kose et al., 2020) | ||
| Apigenin | Apigenin binds MMP2 with a binding affinity was -9 kcal.mol-1. Possible binding sites were VAL198, ILE222, ALA220, PRO215, LEU218, HIS201, TRY223. Common residues were VAL198, ILE222, ALA220, LEU218, LEU197, HIS201, TRY223. | (Kose et al., 2020) | ||
| Naringenin | Naringenin binds MMP8 with binding affinity was ALA112, ASP115, THR224, ARG111, GLU108, SER105. Common residues were LEU160, TRY216, ASN218, LEU214, GLU198, PRO217, HIS197, GLU198, ALA161. | (Kose et al., 2020) | ||
| Collagenase inhibitor |
|
Catechin | Catechin showed strong binding at the active site of collagenase enzyme with a docking score of -12.71 kcal.mol-1. The docking score of catechin is lower than quercetin as the positive control (-12.20 kcal.mol-1) Epigallocatechin gallate binds collagenase with a docking score of -9.93 kcal.mol-1. Hydrogen bonds: Gly158, Leu160, Ala161, Tyr189, Tyr219, Ala220. Hydrophobic interactions: Leu160, His197. Electrostatic interaction: Glu198. |
(Abdelfattah et al., 2022) (Priani and Fakih, 2021) |
| Luteolin | Luteolin binds collagenase with a docking score of -11.0 kcal.mol-1. Residue interactions were LEU235, SER239, VAL215, HIS218, LEU181. | (Syamsul et al., 2022) | ||
| Hyaluronidase inhibitor |
|
Catechin | Catechin contained in Warburgia salutaris bark aqueous extract binding active site on hyaluronidase enzyme with docking score -13.73 kcal.mol-1. The docking score of catechin is lower than kojic acid as the positive control (-9.10 kcal.mol-1). Epigallocatechin gallate binds hyaluronidase enzyme with a docking score of -8.9 kcal.mol-1 via H-bonds (ASP56, ASP111, TRP301, SER304) and hydrophobic interactions (ASP111, GLU113, TRP301). |
(Abdelfattah et al., 2022) (Younis et al., 2022) |
| Daidzein | Daidzein binds energy with an active site in hyaluronidase (-27.08 kJ mol-1). Besides, daidzein binding amino acids via hydrophobic interaction, hydrophilic interaction, and hydrogen bond. | (Zeng et al., 2015) | ||
| Luteolin | Luteolin binds energy with an active site in hyaluronidase (-25.03 kJ mol-1). Besides, daidzein binding amino acids via hydrophobic interaction, hydrophilic interaction, and hydrogen bond. Luteolin binds hyaluronidase with a docking score -6.8 kcal.mol-1. Residue interactions were ASP111, GLU113, TYR55. |
(Zeng et al., 2015) (Syamsul et al., 2022) |
||
| Apigenin | Apigenin binds hyaluronidase enzyme with binding energy-56.15 kcal.mol-1. | (Mumpuni and Mulatsari, 2017) | ||
| Naringenin | Naringenin binds energy with an active site in hyaluronidase (-24.28 kJ mol-1). Besides, daidzein binding amino acids via hydrophobic interaction, hydrophilic interaction, and hydrogen bond. | (Zeng et al., 2015) |
Table 4.
Selected flavonoids of S. littoralis Hassk. in cosmetic properties based on in vitro activity.
| Cosmetic properties | The main mechanism | Selected flavonoids | In vitro activity | Reference(s) |
|---|---|---|---|---|
| Antioxidant activity |
|
Catechin | Green tea contains (−)-catechin and (−)-Epigallocatechin-3-gallate (EGCG), compounds that work directly to scavenge ROS and chelating agent metal ions. | (Bernatoniene and Kopustinskiene, 2018) |
| Daidzein | Daidzein from Thai fermented soybean as ROS scavenger. | (Kulprachakarn et al., 2021) | ||
| Formononetin | Formononetin has a scavenging capacity against reactive oxygen (94.79%, IC50 4.27 µg/mL) and reactive nitrogen species (94.33%, IC50 5.0 μg/mL). | (Vishnuvathan et al., 2017) | ||
| Glycitein | Glycitein from Thai fermented soybean as ROS scavenger. | (Kulprachakarn et al., 2021) | ||
| Luteolin | Luteolin as antioxidant has IC50 DPPH = 2.099±0.0587 μg/mL and IC50 ABTS = 0.59±0.0208 μg/mL. | (Tian et al., 2021) |
||
| Apigenin | Apigenin as antioxidant has IC50 ABTS = 0.8243±0.0044 μg/mL. | (Tian et al., 2021) | ||
| Hesperetin | By upregulating the development of the transcription nuclear factor-erythroid factor 2 (Nrf2) and heme oxygenase-1 (HO-1), hesperetin acts as a ROS scavenger and enhances the body's natural antioxidant defence systems. Hesperetin also prevented apoptotic cell death and elevated GSH, CAT, and SOD synthesis in retinal pigment epithelia 19 (RPE-19) cells, protecting them from oxidative stress. | (Khan et al., 2020) | ||
| Naringenin | Naringenin at 5 and 10 μM concentrations can inhibit NADPH oxidase (the cell interval levels decreased to 2.0 and 1.3) which produces superoxide anion and increases HO-1 gene expression (the cell increased to 0.7 and 0.9) thereby naringenin can reduce aging effects. | (Lim and Kim, 2018) | ||
| Negletein | Negletein at 10 μM concentration can be a strong radical scavenger in the DPPH mechanism. | (Lombardo et al., 2013) | ||
| Kaempferide | Kaempferide isolated from Alpinia galanga L. showed the highest superoxide scavenging property with an EC50 value of 868 ppm. | (Divakaran et al., 2013) | ||
| Anti-inflammatory activity |
|
Catechin | Catechin at 100 μM inhibited 56.25±0.99% NO production in RAW264.7 macrophage cells. | (Divakaran et al., 2013) |
| Daidzein | The in vitro results showed that daidzein suppressed MAPK signalling pathways, reducing NO release, inhibiting secretions of inflammatory cytokines (IL-6 and TNF-α), and down-regulating expression of inflammatory indicators (iNOS and COX-2) in RAW264.7 macrophages. | (Tan et al., 2022) | ||
| Formononetin | Formononetin inhibits inflammatory responses (IL-1β, IL-6, and TNF-α) and suppressed NF-κB activity in mast cells-mediated allergic inflammation. | (Xu and An, 2017) | ||
| Glycitein | Glycitein from soybean cultivar inhibits nitric oxide (NO) production in RAW264.7 cells. | (Eum et al., 2020) | ||
| Luteolin | Considering respective IC50 values of 54:45 2:89, 93:62 3:04, and 56:60 2:34 μg/mL, in vitro anti-inflammatory tests utilizing membrane stabilization, protein denaturation, and proteinase activities demonstrated the efficiency of the dietyl-ether fraction of Thespesia garckeana with rich of luteolin. | (Alozieuwa et al., 2022) | ||
| Apigenin | Apigenin inhibits pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) in macrophages | (Zhang et al., 2014b) | ||
| Hesperetin | Hesperetin at a concentration of up to 100 μM reduced NO and prostaglandin E2 (PGE2) production and at a concentration of 50 μM reduced TNF-α and IL-6 levels in RAW264.7 cells. | (Choi et al., 2022) | ||
| Naringenin | Naringenin has inhibitory activity on NF-κB and decreases matrix metalloproteinase expression level. | (Lim and Kim, 2018) | ||
| Negletein | Negletein from Actinocarya tibetica Benth. has promising anti-inflammatory in inhibition of TNF-α (IC50 16.4 μM) and IL-1β (IC50 6.4 μM). | (Singh et al., 2013) | ||
| Photoprotective activity |
|
Catechin | Catechin (homolog) as UVB photoprotector with minimal sun protection factor value (SPF=7.3). | (Stevanato et al., 2014) |
| Daidzein | A combination of 30 μM daidzein and 60 μM genistein more effectively prevented UVB-induced DNA damage. | (Bevilacqua et al., 2011) | ||
| Formononetin | Formononetin is very effective in reducing erythema through treatment and irradiation procedures. | (Lin et al., 2008) | ||
| Glycitein | The glycitein nanoemulsion which is calculated in the skin layer can be maintained after 8 hours of skincare with a formulation that can protect the skin from UV exposure. | (Nemitz et al., 2019) | ||
| Luteolin | During UV radiation absorption, luteolin prevented the development of cyclobutane pyrimidine dimers (CPD in cell culture (the human keratinocyte cell line HaCaT). | (Wölfle et al., 2011) | ||
| Apigenin | Apigenin (homolog) as UVB photoprotector with moderate sun protection factor value (SPF=28.8). | (Stevanato et al., 2014) | ||
| Hesperetin | Hesperetin with D-limonene and lecithin reduced skin erythema. | (Saija et al., 1998) | ||
| Naringenin | Naringenin is a UVB photoprotector with a high sun protection factor value (SPF=12.3). | (Stevanato et al., 2014) | ||
| Tyrosinase inhibitor |
|
Catechin | Hop Tannin's catechin prevents tyrosinase by attaching to its active site and creating a hydrogen bond with that as well. | (Liu et al., 2022) |
| Daidzein | Daidzein from Aspergillus oryzae acted competitive inhibition toward the L-tyrosinase binding site of tyrosinase (KI= 19.4±0.4 μM). | (Chang et al., 2007) | ||
| Formononetin | From Sophora flavescens, formononetin was extracted and showed potent in vitro tyrosinase inhibitory effects. (IC50=24.1±2.3 μM). | (Kim et al., 2018b) | ||
| Glycitein | Glycitein from Aspergillus oryzae showed competitive inhibition toward the L-tyrosinase binding site of tyrosinase (KI= 50.6±8.76 μM). | (Chang et al., 2007) | ||
| Luteolin | Kinetic studies showed that luteolin followed reversible noncompetitive inhibition on tyrosinase activity. | (Zhang et al., 2017) | ||
| Apigenin | Apigenin in Artocarpus heterophyllus inhibited mushroom tyrosinase (IC50 656 μM) | (Arung et al., 2006) | ||
| Hesperetin | Tyrosinase was competitively and reversibly inhibited by hesperetin with a KI of 4.03±0.26 mM. | (Si et al., 2012) | ||
| Naringenin | (-)-naringenin from Prunus persica has 57% tyrosinase inhibitory activity at 500 μM (recommendation for skin-whitening agent). | (Murata et al., 2022) | ||
| Elastase inhibitor |
|
Catechin | Catechin contained in crude grape pomace extract showed a dose-independent inhibition of elastase activity with an IC50 value of 14.7 μg/mL. | (Wittenauer et al., 2015) |
| Daidzein | Daidzein has elastase inhibition activity with IC50 57.35±5.64 μg/mL. | (Juliana et al., 2020) | ||
| Formononetin | Formononetin contained in Pisa sulla extract at 250 μg/mL concentration reduced >20% elastase activity. | (Burlando et al., 2017) | ||
| Glycitein | Glycitein significantly inhibited >90% of the expression of MMPs in glioma cells. | (Lee et al., 2010) | ||
| Luteolin | Luteolin has anti-elastase activity with IC50 12.7±0.5 μM. Luteolin inhibited MMP-1 activity in HacaT cells. |
(Ryu et al., 2017) (Hwang et al., 2011) |
||
| Apigenin | Apigenin has anti-elastase activity with IC50 46.1 ± 0.9 μM. | (Ryu et al., 2017) | ||
| Hesperetin | Hesperetin has 47.71% MMPs inactivation | (Liu et al., 2017) | ||
| Naringenin | Naringenin has anti-elastase activity with IC50 84 μM. Naringenin has 64.69% MMPs inactivation. |
(Jakimiuk et al., 2021) (Liu et al., 2017) |
||
| Kaempferide | Kaempferide at 1 μM significantly decreases the release of elastase by neutrophils. | (Granica et al., 2013) | ||
| Collagenase inhibitor |
|
Catechin | Unfermented cocoa from Malaysia contains epicatechin can inhibit 62.99% collagenase activity. The roots bark of Ulmus davidiana var. japonica contains (-)-catechin, which greatly inhibited collagen formation. |
(Abdul Wahab, 2014)(Lee et al., 2020) |
| Daidzein | Daidzein has anti-collagenase activity with IC50 98.18 μg/mL. | (Alqodri et al., 2020) | ||
| Formononetin | Formononetin contained in Pisa sulla extract at 2.5 mg/mL concentration and Ventimiglia sulla extracts at 25 μg/mL concentration reduced >50% collagen type I activity. | (Burlando et al., 2017) | ||
| Apigenin | In RAW264.7 macrophage cells, apigenin at 500 M reduced collagenase activity by 85.3%. | (Lee et al., 2007) | ||
| Naringenin | Citrus fruits containing naringenin can prevent the degradation of dentin collagen (80%). | (Liu et al., 2017) | ||
| Hyaluronidase inhibitor |
|
Catechin | In green tea (Camellia sinensis)-derived HaCaT cells, EGCG boosted the synthesis of skin-hydrating genes by acting as a hyaluronidase inhibitor. | (Kim et al., 2018a) |
| Daidzein | Daidzein has effectively inhibited the hyaluronidase enzyme with IC50 95.80±3.98 μg/mL. | (Asan et al., 2019) | ||
| Luteolin | Luteolin has 29.10±1.27% hyaluronidase inhibition at 50 μg/mL. | (Süntar et al., 2012) | ||
| Apigenin | Apigenin has hyaluronidase inhibition with IC50 162.86 mg/mL. | (Yusuf et al., 2021) | ||
| Hesperetin | In the presence of hyaluronidase, hesperetin defends oocytes against oxidative damage during in vitro aging. | (Kim et al., 2019) | ||
| Naringenin | Naringenin has 9.58±0.25% hyaluronidase inhibition. | (Moon et al., 2009) |
Table 5.
Selected flavonoids of S. littoralis Hassk. in cosmetic properties based on in vivo activity.
| Cosmetic properties | The main mechanism | Selected flavonoids | In vivo activity | Reference(s) |
|---|---|---|---|---|
| Antioxidant activity |
|
Catechin | Catechins isolate of Uncaria gambier (Roxb.) with a dose of 20 mg/kg.bw decreased the levels of MDA by 57.63% in male rats. It indicated catechins gave a strong antioxidant effect. | (Musdja et al., 2018) |
| Daidzein | Daidzein contained in soy isoflavones at the rate of 100 mg/day increased feed dry matter intake, enhanced accumulation of SOD and T-AOC, and inhibited the serum MDA and GSH in Xinong Saanen goats. Daidzein 50 mg/kg added to the diet through an extended period increased SOD and CAT activity while lowering MDA levels in the pig plasma.Daidzein significantly elevated the levels of CAT (6%) and SOD (P<0.01) in rats' livers.Injections of vehicle H2O (1 mL/kg bw) and isoflavones (daidzein and genistein) for 1 week each significantly reduced the levels of reactive nitrogen species, such as serum nitrite, nitrate, and nitrotyrosine in lipopolysaccharide (LPS)-induced rats. |
(He et al., 2021 (Li et al., 2021) (Banz et al., 2004) (Yen and Lai, 2003) |
||
| Formononetin | The Nrf2-driven antioxidant defense system was promoted by formononetin in the skin flap of male mice, and active Nrf2 was controlled through the phosphoinositide 3-kinase (PI3K/Akt) signal pathway. In the ethanol-induced rat ulcer model, red propolis (250 and 500 mg/kg) and formononetin (10 mg/kg) lowered total lesion areas, and in the indomethacin-induced rat ulcer model, they decreased ulcer indices thereby inducing SOD and nitric oxide enzymes; and reduce the release of MDA.Formononetin was able to improve antioxidant enzymes (CAT, SOD, and GSH). Formononetin additionally provided a defense against lipid peroxidation (LPO). |
(Li et al., 2022) (de Mendonça et al., 2020) (Jain et al., 2020) |
||
| Glycitein | Following a single dose of glycitein, three oxidative and two bacterial metabolites were found in rat urine as a result of the in vivo metabolism. | (Rüfer et al., 2007) | ||
| Luteolin | PbAc-treated rats displayed significantly reduced levels of antioxidant enzyme expression and activity (SOD, CAT, glutathione reductase/GR, and GPX,) as well as elevated MDA levels when compared to the control rats. | (Jameel et al., 2020) | ||
| Apigenin | After three days of spinal cord injury, apigenin administration (20 mg/kg dosage) restored the decline in SOD and GPX activity and the rise in MDA levels. | (Zhang et al., 2014a) | ||
| Hesperetin | In the rat hippocampus region, hesperetin and nano-hesperetin elevated the activity of antioxidant enzymes (SOD, glutathione GPX, GR, and CAT), GSH levels, and lowered MDA. Hesperetin 20 mg/kg b.w. given orally significantly improved the level of antioxidants (SOD, CAT, and GPX) in 7,12-dimethylbenz(a)anthracene (DMBA) painted group 3 hamsters. |
(kheradmand et al., 2018) (Babukumar et al., 2017) |
||
| Naringenin | Catalase activity carried on by UVB exposure in mice could be inhibited by formulations containing naringenin. | (Martinez et al., 2016) | ||
| Anti-inflammatory activity |
|
Catechin | In a high-fat diet (HFD) produced by diabetic rats with cognitive impairment, catechin was discovered to raise ACh product, ChAT production, and inhibit AChE performance. In mice treated with D-galactose, epigallocatechin substantially reduced the levels of TNF-α, IL-1β, and IL-6. |
(Kim et al., 2020, Kim et al., 2021) (Zeng et al., 2020) |
| Daidzein | Pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and MCP-1 were dramatically lowered in rats by daidzein at a level of 20 mg/kg. In Xinong Saanen goats, the soy isoflavone daidzein reduced the NF-κB pathway. |
(Deng et al., 2021) (He et al., 2021) |
||
| Formononetin | The inflammatory response (IL-6, TNF-α, and IL-1β) to cerebral ischemia-reperfusion injury (CIRI) in mice can be inhibited by the formononetin from Trifolium pratense L. Red propolis' formononetin suppressed the expression of inflammatory cytokines such as TNF-α, NO, and IL-6. |
(Wang et al., 2022) (de Mendonça et al., 2020) |
||
| Glycitein | In dietary soybean extract treatments including glycitein, UV-induced pro-inflammatory cytokines (TNFα-, IL-1β) were reduced in hairless mice. | (Cho et al., 2019) | ||
| Luteolin | Luteolin prevented mice from suffering from acute lung injury (ALI) brought on by LPS by inhibiting the production of TNF-α, IL-6, iNOS, and COX-2. In male Wistar rats given 10 mg/kg of lipopolysaccharide (LPS) and 50 mg/kg of luteolin, luteolin reduced LPS-induced NF-κB expression in the lung but not in the heart or brain cortex. |
(Li et al., 2012) (Rostoka et al., 2010) |
||
| Apigenin | In the liver of mice treated with carbon tetrachloride (CCl4), apigenin treatment was able to reduce the messenger ribonucleic acid (mRNA) expression of inflammatory mediators such as iNOS, COX-2, and TNF-α. Apigenin treatment In rats, blood levels of IL-1β, TNF-α, and intercellular adhesion molecule-1 were lower 3 days after spinal cord injury. |
(Zhao et al., 2014) (Zhang et al., 2014a) |
||
| Hesperetin | Pretreatment with hesperetin substantially reduced the mRNA levels of TNF-α and IL-6 in mice lung tissues injured by LPS.Treatment with hesperetin inhibited the activation of the NF-κB pathway and reduced the production of TNF-α, IL-1β, and IL-6 in ventilator-induced acute lung injury in rats. | (Ye et al., 2019)(Ma et al., 2015) | ||
| Naringenin | The topical application containing naringenin inhibited cytokine production (TNF-α, IL-1β, IL-6, and IL-10) in mice. Naringenin at a dose of 100 mg/kg bw significantly decreased inflammatory responses (NF-κB and COX-2) in rats-induced benzo[a]pyrene. Naringenin uses its anti-inflammatory capabilities, such as lowering NF-κB, iNOS, and TNF-α, to protect rats from LPS-induced acute lung damage. |
(Martinez et al., 2016) (Ali et al., 2017) (Fouad et al., 2016) |
||
| Photoprotective activity |
|
Catechin | The UVB-induced expression of COX-2 in the skin of the wild-type mice was decreased substantially after drinking green tea with catechins. | (Meeran et al., 2009) |
| Daidzein | In a hairless mouse model, oral administration of soybean extract containing daidzein demonstrated photoprotective benefits (protection against UVB irradiation). | (Cho et al., 2019) | ||
| Formononetin | Red propolis extract containing formononetin, which has photoprotective action against UVB-induced dermatitis in rats. Topical application of isoflavones containing formononetin had effective photoprotection against UV photodamage in pig skin. |
(Batista et al., 2018) (Lin et al., 2008) |
||
| Glycitein | Oral intake of soybean extract containing glycitein showed photoprotective advantages in a hairless mice model (protection against UVB irradiation). | (Cho et al., 2019) | ||
| Luteolin | Luteolin alleviates UV-induced skin damage in Wistar albino rats.Luteolin suppressed the in vivo production of UVB-induced cyclobutane pyrimidine dimers. | (Abbas et al., 2022) (Wölfle et al., 2011) |
||
| Hesperetin | Hesperetin prevented UVA-induced MMP-1 in mouse skin from aging. Hesperetin was able to against UVB-induced skin damage. |
(Chaiprasongsuk and Panich, 2022)(Saija et al., 1998) | ||
| Naringenin | Naringenin was effective in preventing skin damage from UVB rays. | (Saija et al., 1998) | ||
| Tyrosinase inhibitor |
|
Catechin | The catechin-rich Warburgia salutaris extract was able to inhibit HSP16 expression and promote DAF-16 nuclear localization in a dose-dependent manner. These results support the extract Elegans nematodes model's in vivo anti-aging potential. | (Abdelfattah et al., 2022) |
| Hesperetin | In comparison to the non-treatment group, the hesperetin-loaded microemulsion in male guinea pigs significantly reduced skin irritation and promoted topical whitening. | (Tsai et al., 2010) | ||
| Elastase inhibitor |
|
Daidzein | Dietary phytoestrogens containing daidzein educed MMPs activity in male mice. | (Lu et al., 2014) |
| Collagenase inhibitor |
|
Daidzein | Daidzein from soy isoflavones demonstrated good collagen breakdown in a hairless mice model. Daidzein activates the transforming growth factor β (TGF-beta/Smad) signal pathway, which promotes collagen synthesis. |
(Kim et al., 2004) (Zhao et al., 2015) |
| Glycitein | In a model using hairless mice, glycitein from soy isoflavones showed good collagen degradation. | (Kim et al., 2004) | ||
| Hyaluronidase inhibitor |
|
Luteolin | Luteolin inhibited the hyaluronidase activity of venom dosage when administered to mice. | (Kuppusamy and Das 1991) |
| Apigenin | Apigenin inhibited the hyaluronidase activity of venom dosage when administered to mice. | (Kuppusamy and Das 1991) |
Table 6.
Selected flavonoids of S. littoralis Hassk. in cosmetic properties based on clinical activity.
| Cosmetic properties | The main mechanism | Selected flavonoids | Clinical activity | Reference(s) |
|---|---|---|---|---|
| Antioxidant activity |
|
Catechin | Catechin-rich Yabukita and Benifuuki teas increased radical scavenging activity in human skin by 28 and 29%, respectively. For two weeks, each participant drank 600 mL of tea every day. | (Megow et al., 2017) |
| Daidzein | Tofu containing daidzein has been shown to reduce coronary heart disease in both men and women in the United States, assuming that daidzein is an excellent antioxidant.In six healthy women between the ages of 18 and 35, consumption of isoflavones containing glycitein (1.0 and 2.0 mg total isoflavones/kg bw/day for 3 weeks) was performed as an effective antioxidant. | (Ma et al., 2020) (Fritz et al., 2003) |
||
| Glycitein | Consumption of isoflavones containing glycitein (1.0 and 2.0 mg total isoflavones/kg bw/day for 3 weeks) acted as a good antioxidant in 6 healthy women 18-35 years old. | (Fritz et al., 2003) | ||
| Luteolin | The antioxidant effects of luteolin and palmitoylethanolamide on olfactory function recovery in corona virus (COVID-19) patients. | (D’Ascanio et al., 2021) | ||
| Apigenin | Consuming 40 mg of apigenin is less than 1% of what is required for a significant therapeutic result on cancer cell behavior, including free radical scavenging. | (DeRango-Adem and Blay, 2021) | ||
| Hesperetin | Hesperetin was quickly absorbed when taken orally and acted as an antioxidant in six healthy volunteers. | (Kanaze et al., 2007) | ||
| Naringenin | In patients with level I hypertension, consumption of naringenin glycoside 677 mg/L for 5 weeks in sweet juice decreased diastolic blood pressure and was related to higher plasma antioxidant levels. In six healthy volunteers, naringenin was promptly absorbed when administered orally and worked as an antioxidant. |
(Reshef et al., 2005) (Kanaze et al., 2007) |
||
| Anti-inflammatory activity |
|
Catechin | In a 12-week open oral intervention research, 16 healthy people were given low-dose green tea catechin (540 mg) and vitamin C (50 mg) daily. The results in human skin contributed to protecting against sunburn inflammation. | (Rhodes et al., 2013) |
| Daidzein | In the United States, it has been demonstrated that tofu containing daidzein lowers inflammation related to coronary heart disease in both men and women. | (Ma et al., 2020) | ||
| Formononetin | When compared to the control diet, consuming soy nuts with formononetin (an isoflavone) reduced IL-18 levels in postmenopausal women over eight weeks. | (Azadbakht et al., 2007) | ||
| Glycitein | For eight weeks, postmenopausal women who consumed soy nuts together with glycitein (an isoflavone) had lower IL-18 levels than those who had a control diet. | (Azadbakht et al., 2007) | ||
| Luteolin | 37 children between the ages of 4 and 14 years ingested 2 capsules/20 kg (each capsule containing 200 mg of total luteolin and quercetin) over 4 weeks, which demonstrated a significant reduction in inflammation-related disorders in humans. | (Theoharides et al., 2012) | ||
| Photoprotective activity |
|
Catechin | Consuming green tea polyphenols with a total catechin content of 1402 mg per day for 12 weeks in a group of 60 female volunteers showed that it might protect skin from damaging UV rays and enhance women's overall skin quality. | (Heinrich et al., 2011) |
| Daidzein | Daidzein-containing soy products were consumed orally by thirty healthy women over eight weeks, and the results demonstrated significant photoprotection with minimum erythema dosage to UVA increased by 60% and to UVB increased by 87%. | (Haron et al., 2020) | ||
| Glycitein | Over eight weeks, thirty healthy women consumed soy products containing glycitein orally, and the results showed considerable photoprotection with minimum erythema dosage to UVA increased by 60% and to UVB increased by 87%. | (Haron et al., 2020) | ||
| Hesperetin | Hesperetin formulations with enhancers (D-limonene and lecithin) were effective as topical photoprotective agents on six volunteers (25-35 years old). | (Saija et al., 1998) | ||
| Naringenin | As topical photoprotective agents in six participants (25-35 years old), naringenin formulations with enhancers (D-limonene and lecithin) proved successful. | (Saija et al., 1998) | ||
| Tyrosinase inhibitor |
|
Daidzein, Formononetin, Glycitein | For two months, the stabilized soy extracts reduced melanin production or suppressed the expression of tyrosinase in 50 South-East Asian women. | (Petit and Piérard, 2002) |
| Apigenin | After 28 days of treatment with an apigenin-based regimen, the skin was brighter (81%) and dark circles under the eyes were minimized (53%) in 25 healthy females. | (Arterbery and Gupta, 2018) | ||
| Elastase inhibitor |
|
Daidzein, Formononetin, Glycitein | After eight weeks of oral intake of 10 mg of isoflavone aglycones, Japanese women (30-40 years old) in the test food group showed considerably greater skin elasticity recovery than those in the control group. For six months, 100 mg/day of an isoflavone-rich supplement enhanced the number of elastic fibers in 22 postmenopausal women (75.8%). |
(Izumi et al., 2007) (Accorsi-Neto et al., 2009) |
| Luteolin | The cream containing luteolin extracted from Artemisia iwayomogi showed effective anti-wrinkle results. | (Kim et al., 2019) | ||
| Apigenin | Apigenin-based regimen enhanced skin elasticity (81%) after 56 days of treatment on healthy 25 females. The cream contained apigenin increased skin elasticity (43.4%) and dermal density (24.75%) in 40 women (30 years old) for 4 weeks of treatment |
(Arterbery and Gupta, 2018) (Choi et al., 2016) |
||
| Hesperetin | A topical formulation containing 0.1% hesperetin and 0.1% sodium cyclic lysophosphatidic acid (NcPA) used for 12 weeks could enhance skin elasticity in 35 females. | (Sheen et al., 2021) | ||
| Collagenase inhibitor |
|
Daidzein, Formononetin, Glycitein | During six months, 100 mg/day of an isoflavone-rich supplement enhanced the amount of collagen in the dermis in 25 postmenopausal women (86.2%). | (Accorsi-Neto et al., 2009) |
| Luteolin | The cream containing luteolin extracted from Artemisia iwayomogi showed effective anti-wrinkle results. | (Kim et al., 2019) | ||
| Apigenin | On healthy 25 females, an apigenin-based regimen resulted in skin that was younger (67%) and softer (90%) after 28 days of treatment. | (Arterbery and Gupta, 2018) | ||
| Hesperetin | On 35 females, a topical composition containing 0.1% hesperetin and 0.1% NcPA used for 12 weeks could prevent collagen degradation and keep the structure of the dermal extracellular matrix. | (Sheen et al., 2021) | ||
| Hyaluronidase inhibitor |
|
Apigenin | Throughout 28 days of treatment, the skin of 25 healthy females was moisturized because of an apigenin-based regimen. During a 4-week treatment period, apigenin cream enhanced skin moisture (51.38%), skin texture evenness (19.65%), and transepidermal water loss (TEWL) (27.61%) in 40 women (30 years old). |
(Arterbery and Gupta, 2018) (Choi et al., 2016) |
| Hesperetin | Over 12 weeks, a topical composition combining 0.1% hesperetin and 0.1% NcPA might enhance stratum corneum hydration and make skin moisturized in 35 females. | (Sheen et al., 2021) |
Application of S. littoralis Hassk. in cosmetic properties are antioxidant activity, anti-inflammatory activity, photoprotective activity, and anti-aging activity, including tyrosinase, elastase, collagenase, and hyaluronidase inhibitor. ROS = reactive oxygen species; RNS = reactive nitrogen species; SOD = superoxide dismutase; GPX = Glutathione peroxidase, CAT = catalase; NF-κβ = nuclear factor-kappa B; JAK-STAT = The Janus kinase/signal transduction and activator of transcription; PLA2 = phospholipase A2; LOX = lipoxygenase; COX-1 = cyclooxygenase-1; COX-2 = cyclooxygenase-2; iNOS = inducible nitric oxide synthase; MCP-1 = monocyte chemoattractant protein-1; TNF-α = tumor necrosis factor-α; IL-1β = interleukin-1β; IL-6 = interleukin-6; ACh = acetylcholine; AChE = acetylcholinesterase; SPF = Sun protection factor; UVB = Ultraviolet B; DNA = deoxyribonucleic acid (Alqodri et al., 2020, Ganesh et al., 2014, Jakimiuk et al., 2021, Lin et al., 2018, Liu et al., 2022, Martemucci et al., 2022, Permana et al., 2020, San Miguel-Chávez, 2017, Sifaki et al., 2019, Stevanato et al., 2014, Yusuf et al., 2021).
3.1. Antioxidant activity
UV rays, air pollution, and fluctuations in the outdoor temperature all affect how quickly skin ages (Lin et al., 2018). UV exposure can generate either ROS or RNS. Free radicals are created by an excessive buildup of ROS and RNS. In addition, there are exogenous free radicals among them (Table 2). Free radicals from endogenous sources, released at low concentrations by the mitochondrial electron transport chain, have physiologically essential functions throughout the body (Sekar, 2020, Triawanti and Noor, 2020, Yan et al., 2020).
Table 2.
Free radical and nonradical compounds on ROS and RNS. Modified from San Miguel-Chávez (2017).
| Free radical compounds | Non-radical compounds | ||
|---|---|---|---|
| ROS | RNS | ROS | RNS |
| Hydroxyl (HO⋅) | Nitrogen dioxide (⋅NO2)Nitric oxide (⋅NO) |
Ozone (O3) | Peroxynitrite (ONOO–) |
| Superoxide (O2⋅) | Hydroperoxide (ROOH) | Nitrosyl cation (NO+) | |
| Alkoxy radicals (RO⋅) | Hypochlorous acid (HOCl) | Dinitrogen trioxide (N2O3) | |
| Peroxy radicals (ROO⋅) | Singlet oxygen (1O2) | Nitrous acid (HNO2) | |
| Hydrogen peroxide (H2O2) | Dinitrogen tetroxide (N2O4) | ||
| Hypochlorite (ClO−) | Nitroxyl anion (NO–) | ||
| Organic peroxydes (ROOH) | Nitronium (nitryl) cation (NO2+) | ||
| Aldehydes (HCOR) | Nitrous acid (HNO2) | ||
A free radical is a very reactive atom or molecule because it has unpaired electrons at the outer orbital. The reactivity causes protein denaturation, lipid peroxidation, glucose autooxidation, and fragmentation of DNA. This damage triggers several diseases, such as inflammation, aging, asthma, diabetes mellitus, rheumatoid arthritis, neurodegenerative diseases, and cancer (Martemucci et al., 2022).
Antioxidants are substances that inhibit or slow down the oxidative reactions of lipids, proteins, or nucleic acids. In addition, antioxidants can effectively deactivate radicals based on chemical reaction mechanisms: via a single-electron transfer and a hydrogen atom transfer (San Miguel-Chávez, 2017). Endogenous antioxidants contain triggering antioxidant enzymes such as CAT, GPX, and SOD. Protons are used to create adenosine triphosphate (ATP) from adenosine diphosphate (ADP) during the electron transport chain function. Superoxide is created when 1–3 % oxygen reaches the mitochondria. These free radicals will be transformed into more stable non-radical molecules, such as H2O2, by the presence of SOD in mitochondria. Within mitochondria, GSH action reduces hydrogen peroxide to water. While this occurs, unreduced hydrogen peroxide will leave the mitochondria where CAT subsequently reduces it in the peroxisomes and another group of peroxidases in the cytoplasm (Martemucci et al., 2022, San Miguel-Chávez, 2017). Furthermore, several antioxidants work as metal chelators, converting metal pro-oxidants into more stable chemical states (Vona et al., 2021).
However, antioxidants in the human body are not enough to fight free radicals. It is also necessary to take antioxidants from the outside, namely exogenous antioxidants. Exogenous antioxidants come from foods containing vitamins E, C, and phytochemicals such as polyphenols and flavonoids. Phenolics or polyphenols are a group of compounds from secondary metabolites of natural ingredients produced from the shikimate and phenylpropanoid biosynthetic pathways. An aromatic ring composes the compound, including one or many hydroxyl groups. Antioxidants called phenolic substances are extensively used due to their electron- or hydrogen-donating properties and metal chelating. The quantity and position of hydroxyl groups, glycosylation, and the presence of double bonds (C2 = C3) all affect the structure–activity connection of phenolics as antioxidants. Currently, 8,000 phenolic compounds have been identified as structures. Classes of phenolic compounds that are important in human life are phenolic acids, flavonoids, and tannins (Vuolo et al., 2018). Table 3, Table 4, Table 5, Table 6 summarizes that catechin, daidzein, formononetin, glycitein, luteolin, apigenin hesperetin, naringenin, negletein, and kaempferide were reported as great antioxidants.
3.2. Anti-inflammatory activity
Inflammation occurs in response to normal skin damage (skin aging and inflammatory dermatoses). Injury, pathogenic triggers, and auto-immune reactions require the host to create a powerful immune defense through inflammation. Inflammation is divided into two categories: acute and chronic inflammation. Immediately after tissue damage, acute inflammation usually progresses. Meanwhile, chronic inflammation lasts longer and histologically in the presence of lymphocytes and macrophages, producing fibrosis and tissue necrosis (Goh et al., 2022). The symptoms of inflammation are redness, swelling, and heat (Actor and Smith, 2018, Ganesh et al., 2014).
Initially, external stimuli (microbial infectious organisms, toxins, chemicals, wounds, and allergens) and internal stimuli (ischemia and trauma) will activate immune cells, such as myeloid leukocytes (neutrophil, eosinophil, basophil, mast cells, monocyte, macrophage, dendritic cell), lymphoid leukocytes (B cell, plasma cell, T cell), and non-leukocytes (platelet, fibroblast, endothelial cell). When these immune cells are activated, ROS and RNS are produced, which in turn trigger the activation of NF-κB, activator protein 1 (AP-1), and JAK-STAT. Pro-inflammatory enzymes such as iNOS, PLA2, COX-1, COX-2, and LOX are encouraged to be expressed. Furthermore, the inflammatory cytokines: TNF-α, IL-1β, IL-6, and MCP-1 also result in inflammation (Actor and Smith, 2018, Ganesh et al., 2014, Kim and Heo, 2022, Lin et al., 2018).
The structural element of flavonoids is essential for their anti-inflammatory properties. The flavonoids' planar ring structure and the hydroxyl groups in ring B's locations at 3′ and 4′ impact this. Inhibiting transcriptional signaling circuits like AP-1, JAK-STAT, and NF- κB is indeed a mechanism of flavonoids' anti-inflammatory action. This process will reduce the inflammatory reaction (Al-Khayri et al., 2022). Catechin, daidzein, formononetin, glycitein, luteolin, apigenin, hesperetin, naringenin, and negletein were reported in the regulation of this inflammation, as shown in Table 3, Table 4, Table 5, Table 6.
3.3. Photoprotective activity
ROS and RNS can be generated from endogenous sources and exogenous sources. Endogenous sources are mitochondrial electron transport chain (ETC), endoplasmic reticulum (ER), peroxisomes, membrane-bound nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, lipoxygenase, and cyclooxygenase. Meanwhile, exogenous sources include smoking, UV radiation, ozone, heavy metals, foods, consuming alcohol, and medicines. To defend ROS and RNS, we have internal antioxidants in our body, which are divided into non-enzymatic systems (glutathione/GSH, thioredoxin/TRX, lipoic acid, and ferritin); and enzymatic systems (SOD, CAT, GPX, glutathione reductase/GSR, and glutathione transferase/GST). On the other hand, external antioxidants are vitamins, carotenoids, and polyphenols (flavonoids and phenolic acids). At low concentrations, ROS and RNS are beneficial in regulating processes involving the maintenance of homeostasis and cellular functions. However, if our antioxidant systems work to overcome the defense of ROS and RNS, leading to the release of free radicals and oxidative stress. Oxidative stress can induce DNA oxidative damage, lipid peroxidation, and protein oxidation, which contribute to many diseases such as cardiovascular, cancers, diabetes, and food allergies (Aranda-Rivera et al., 2022, Sharifi-Rad et al., 2020, Vona et al., 2021). Over-exposure to UV radiation is one type of free radical mechanism. These free radicals will reduce collagen and elastin in the skin; thus, the skin becomes wrinkled (de Paula Corrêa et al., 2021, Permana et al., 2020, Stevanato et al., 2014).
UVA (320–400 nm) emission into the atmosphere singlet oxygen and hydroxyl-free radicals by penetrating deeper layers of the skin's dermis and epidermis (by about 1 mm) as the first step for photoaging oxidation of melanin and immediate pigmentation for several hours. Meanwhile, chromophores in the epidermis (160–180 m) absorb 70 % of UVB radiation (290–320 nm). It causes erythema and sunburns, then triggers melanin production by melanocytes and DNA damage. The term “lipid peroxidation” describes UVA as ten times more effective than UVB (de Gálvez et al., 2022).
SPF refers to the lowest erythema dosage ratio between skin protected by sunscreen and skin that is not protected. This SPF measures the effectiveness of UV protection in preventing UVA and UVB sunburn. Quality sunscreen seems to have a high SPF value, and consumers increasingly prefer it. There are three SPF levels: minimum sun protection (SPF value from 2 to under 12), moderate sun protection (SPF value from 12 to under 30), and intense sun protection (SPF value ≥ 30) (Letellier et al., 2022, Stevanato et al., 2014).
Flavonoids from natural plants have been reported as potential sun-protective agents. This is because of its ability to absorb spectrum in the UV region and have antioxidant activity: ROS and RNS scavengers (the ability to delocalize free electrons in molecules) (Rajan et al., 2018). The compound with a high SPF value is naringenin with SPF 12.3 (Stevanato et al., 2014). Several other flavonoids in S. littoralis Hassk. are listed in Table 3, Table 4, Table 5, Table 6.
3.4. Anti-aging activity
Growing to older ages results in biological changes, including changes to the skin (Chalise, 2019). As the largest and outermost organ of the human body, the skin weight between 10 and 15 % of the total body weight (Gu et al., 2020). Skin aging can be affected by intrinsic and extrinsic skin aging. Age-related hormonal changes, such as the sex hormones (estrogens and progesterone's) gradually declining production throughout menopause, impact intrinsic skin aging. Meanwhile, smoking, air pollution, and continuous UV exposure can contribute to extrinsic skin aging (George et al., 2022, Gu et al., 2020, Papakonstantinou et al., 2012, Sifaki et al., 2019). UV radiation exposure can cause ROS and oxidative stress in the skin, a condition known as photoaging, linked to DNA damage and hyperpigmentation in the skin. ROS can activate matrix metalloproteinases (MMPs) by activating AP-1 and mitogen-activated protein kinase (MAPK). The extracellular matrix (ECM) 's collagen and elastin are degraded by MMPs, and the transforming growth factor- β (TGF-β/Smad) signaling pathway is decreased. MAPK activates NF-κB, JAK-STAT, and toll-like receptor (TLR) signaling pathways, as well as the arachidonic acid pathway (COX-1, COX-2, prostaglandin E2, prostaglandin H2, lipoxygenase), and pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6. Dryness, fine wrinkles, decreased elasticity, hyperpigmentation, increased thickness, rough textured skin, brittleness, and decreased collagen are the morphologies of skin aging (Gu et al., 2020, Khan et al., 2022a).
The skin has 3 layers: hypodermis, dermis, and epidermis. Melanocytes in the epidermis layer will produce melanin and play a role in ROS, RNS scavengers, and protection from microbial activity. In the dermis layer, there are ECM proteins. The main components of ECM of the skin are the fibrous proteins, such as elastin, collagen, and glycosaminoglycan. A reduction in protein will affect the health of the skin. Therefore, four enzymes play a role in skin changes: tyrosinase, elastase, collagenase, and hyaluronidase. Each of these enzymes has a different mechanism of action according to its function. For example, inhibition of tyrosinase will prevent the formation of melanin. Therefore, it is used as a whitening agent; elastase, collagenase, and hyaluronidase, contribute to the skin's degree of elasticity and maintain the human tissue's strength (supports the cellular structures), and moisturize the skin. The hypodermis layer also assists in storing energy reserves and shielding the skin from extreme cold and warmth (Pérez-Sánchez et al., 2018, Süntar et al., 2012).
3.4.1. Tyrosinase inhibitor
Two copper ions and three histidines are found in each of the active sites of the metalloenzyme tyrosinase (EC 1.14.18.1). Tyrosinase performs two chemical reactions: the first is the o-hydroxylation of monophenol (l-tyrosine) to o-diphenol (3,4-dihydroxyphenylalanine, l-DOPA), known as monophenolase or cresolase function; the subsequent is diphenolase function or catecholase: the oxidation of diphenol (l-DOPA) to o-quinone (dopaquinone). Dark brown pigments called melanin were also created by the primary reaction followed by polymerization (Fig. 2) (Agarwal et al., 2019, Si et al., 2012).
Fig. 2.
Catalytic cycle of tyrosinase. The formation of melanin in the presence of tyrosinase enzyme. Modified from Agarwal et al. (2019). License number: 5362491270191.
Tyrosinase inhibitors have become whitening agents in cosmetics (Liu et al., 2022). Catechin, daidzein, formononetin, glycitein, luteolin, apigenin, hesperetin, and naringenin have been reported as tyrosinase inhibitors (Table 3, Table 4, Table 5, Table 6).
3.4.2. Elastase inhibitor
Photoaging or photodamage due to UV exposure will impact skin elasticity. Elastase is a proteinase enzyme degrades elastin (a component of the vital protein in the ECM) through the MMPs family in the dermis layer by up to 4 %, reducing skin suppleness. Human endothelial cells, neutrophils, monocytes, and macrophages produce elastase (Abdul Wahab, 2014, Ambarwati et al., 2020, Jakimiuk et al., 2021). Thus, inhibition of elastase activity can prevent skin aging. Catechin, daidzein, formononetin, glycitein, luteolin, apigenin, hesperetin, naringenin, and kaempferide as elastase inibitors according to Table 3, Table 4, Table 5, Table 6.
3.4.3. Collagenase inhibitor
The primary component of the dermis is collagen. Structurally, collagen as a macromolecule (∼300 kDa) contains three α peptide chains with a triple-helix structure and repeated sequences (Glycine-Proline-Hydroxyproline) (Fu et al., 2023, Xiao et al., 2023). Collagen types are categorized based on structural variety, the existence of extra, and non-helical domains, and their capabilities. Collagen types I-III, types V, XI, XXIV, and XXVII collagens form fibrils; collagen type IV forms basement membranes; collagen type VI forms microfibrillar connectivity or as unique beaded filament collagen. In addition, collagen type VII forms anchoring fibrils; collagen types VIII and X form hexagonal connectivity; collagen types IX, XII, XIV, XVI, XIX, XX, XXI, and XXII form fibril-associated collagens with interrupted triple helices (FACITs); collagen types XIII, XVII, XXIII, and XXV form membrane-associated collagens with interrupted triple helices (MACITs) or transmembrane networks; collagen types XV and XVIII form multiple triple-helix domains and interruptions (MULTIPLEXINs); and the two unclassified collagen types, the type XXVI and XXVIII (Andriotis et al., 2023, Arseni et al., 2018, Mak and Mei, 2017, Sun et al., 2019, Uitto, 2019).
Type I collagen accounts for well with around 90 % of the organic mass of bone. Types I, III, IV, VI, VII, XIV, XIII, and XVII of collagen are also scattered across the skin layers. Wrinkles and fine lines will appear on the skin when the collagen content decreases. Collagenase plays a role in this degradation. The MMPs family member collagenase is also a member, and prolonged exposure to sunshine stimulates it (Andriotis et al. 2022; Mandrone et al., 2015). Every year, the skin's collagen content declines by roughly 1 % (Warsito and Kusumawati, 2019). Selected flavonoids that can inhibit collagenase activity are catechin, daidzein, formononetin,glycitein, luteolin, apigenin, hesperetin, and naringenin, which can be observed in Table 3, Table 4, Table 5, Table 6.
3.4.4. Hyaluronidase inhibitor
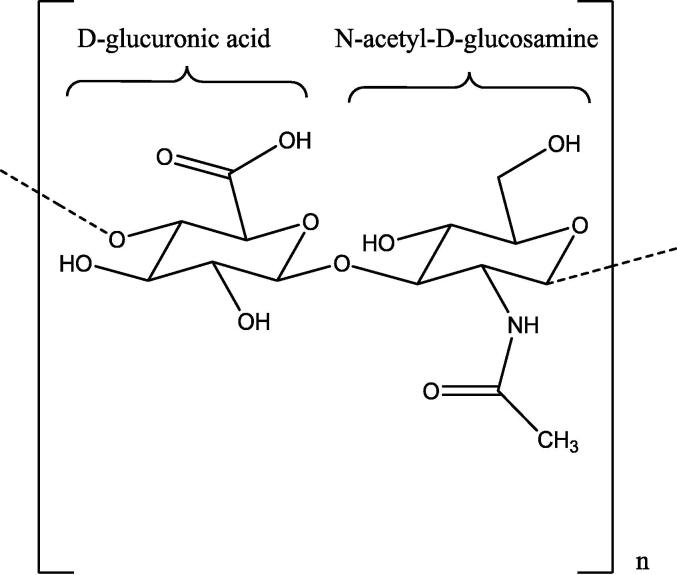
Aging of the skin will also induce dehydration of the skin cells. In order to break down the proteoglycan tissue into smaller hyaluronic acid (HA) fragments, hyaluronidase, an enzyme, hydrolyzes HA. This enzyme is also part of the ECM (Buhren et al., 2016). Disaccharide chains with repeated d-glucuronic acid and N-acetyl-d-glucosamine joined by β 1,3- and 1,4-glycosidic linkages make up the chemical structure of HA (Fig. 3) (Fallacara et al., 2018).
Fig. 3.
Chemical structure of HA disaccharide unit. HA is composed of polymeric disaccharides of d-glucuronic acid, and N-acetyl-d-glucosamine linked together by a glucuronidic bond (Fallacara et al., 2018).
According to its capacity to absorb liquid a maximum of 1000 times the volume, HA is in charge of preserving skin hydration (Warsito and Kusumawati, 2019). However, hyaluronidase activity will cause dry skin and skin allergies (Yusuf et al., 2021), thus increasing skin aging (Kim et al., 2018b, Kim et al., 2018a).
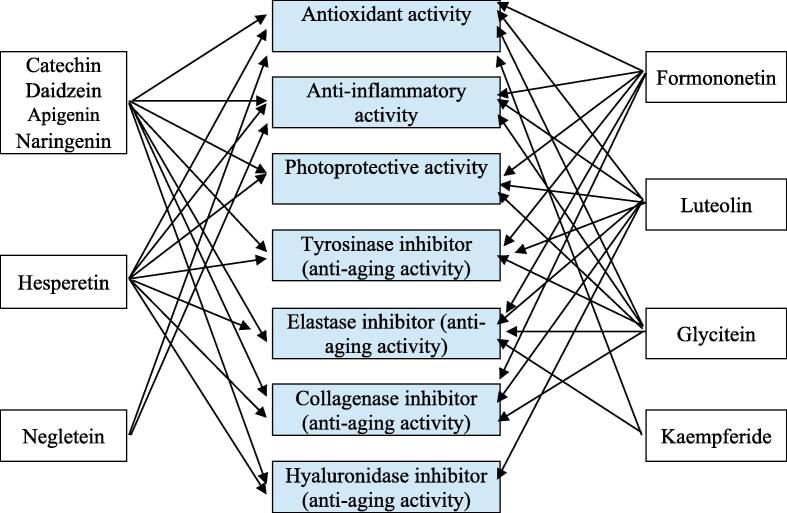
Catechin, daidzein, luteolin, apigenin, hesperetin, and naringenin have been reported as hyaluronidase inhibitors, according to Table 3, Table 4, Table 5, Table 6. Based on data of selected flavonoids of S. littoralis Hassk. in cosmetic properties, then we can describe the relationship between the two as in Fig. 4.
Fig. 4.
The relationship between selected flavonoids of S. littoralis Hassk. and cosmetics properties. There are 10 selected flavonoids that have their activities in cosmetic properties, according to our review.
4. Conclusions
Bajakah tampala (S. littoralis Hassk.) has high potency for herbal cosmetic properties. Some flavonoids, including catechin, daidzein, formononetin, glycitein, luteolin, apigenin, hesperetin, naringenin, negletein, and kaempferide have been reported as cosmetic properties. This data review is based on in silico, in vitro, in vivo, and clinical research on the activities of flavonoids in other extracts. The cosmetic properties are antioxidant activity, anti-inflammatory activity, photoprotective activity, and anti-aging activity, including tyrosinase, elastase, collagenase, and hyaluronidase inhibitor. Further research is recommended to investigate these flavonoids in bajakah tampala as well as the activities against skin aging in cosmetic properties.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors appreciate the funding from the Deputy of Research and Innovation, National Research and Innovation Agency (BRIN) for “Pusat Kolaboratif Riset Kosmetik Berteknologi Nano Berbasis Biomassa” in the Fiscal Year 2022 (Grant number: 398/II/FR/3/2022) and Research Project for “Pengembangan Bioproduk Kosmeseutikal Berbasis Rumput Laut” (RP1WBS3-014). We acknowledge the scientific and technical support provided by the Integrated Laboratory of Bioproducts (iLaB), Research Center for Biomass and Bioproducts, National Research and Innovation Agency (BRIN) via E-Layanan Sains National Research and Innovation Agency (BRIN), Indonesia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Rut Novalia Rahmawati Sianipar, Email: rutnovalia@gmail.com.
Enos Tangke Arung, Email: tangkearung@yahoo.com.
References
- Abbas H., Sayed N.S.E., Ali M.E., Elsheikh M.A. Integrated lecithin-bile salt nanovesicles as a promising approach for effective skin delivery of luteolin to improve UV-induced skin damage in Wistar albino rats. Colloids Surf. B Biointerfaces. 2022;211 doi: 10.1016/j.colsurfb.2021.112299. [DOI] [PubMed] [Google Scholar]
- Abdelfattah M.A.O., Dmirieh M., Ben Bakrim W., Mouhtady O., Ghareeb M.A., Wink M., Sobeh M. Antioxidant and anti-aging effects of Warburgia salutaris bark aqueous extract: Evidences from in silico, in vitro and in vivo studies. J. Ethnopharmacol. 2022;292 doi: 10.1016/j.jep.2022.115187. [DOI] [PubMed] [Google Scholar]
- Abdul Wahab N. Assessment of Antioxidant Capacity, Anti-collagenase and Anti-elastase Assays of Malaysian Unfermented Cocoa Bean for Cosmetic Application. Nat. Prod. Chem. Res. 2014;2 doi: 10.4172/2329-6836.1000132. [DOI] [Google Scholar]
- Accorsi-Neto A., Haidar M., Simões R., Simões M., Soares-Jr J., Baracat E. Effects of isoflavones on the skin of postmenopausal women: a pilot study. Clinics. 2009;64:505–510. doi: 10.1590/S1807-59322009000600004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Actor J.K., Smith K.C. Translational Inflammation. Transl. Inflamm. 2018;1–22 doi: 10.1016/B978-0-12-813832-8.00001-7. [DOI] [Google Scholar]
- Agarwal P., Singh M., Singh J., Singh R.P. Microbial Tyrosinases: A Novel Enzyme, Structural Features, and Applications. Appl. Microbiol. Bioeng. 2019;3–19 doi: 10.1016/b978-0-12-815407-6.00001-0. [DOI] [Google Scholar]
- Aguilar-Toalá J.E., Hernández-Mendoza A., González-Córdova A.F., Vallejo-Cordoba B., Liceaga A.M. Potential role of natural bioactive peptides for development of cosmeceutical skin products. Peptides. 2019;122 doi: 10.1016/j.peptides.2019.170170. [DOI] [PubMed] [Google Scholar]
- Ali R., Shahid A., Ali N., Hasan S.K., Majed F., Sultana S. Amelioration of Benzo[a]pyreneinduced oxidative stress and pulmonary toxicity by Naringenin in Wistar rats: A plausible role of COX-2 and NF-κB. Hum. Exp. Toxicol. 2017;36:349–364. doi: 10.1177/0960327116650009. [DOI] [PubMed] [Google Scholar]
- Al-Khayri J.M., Sahana G.R., Nagella P., Joseph B.V., Alessa F.M., Al-Mssallem M.Q. Flavonoids as potential anti-inflammatory molecules: a review. Molecules. 2022;27:2901. doi: 10.3390/molecules27092901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alozieuwa U.B., Lawal B., Sani S., Onikanni A.S., Osuji O., Ibrahim Y.O., Babalola S.B., Mostafa-Hedeab G., Alsayegh A.A., Albogami S., Batiha G.E.S., Wu A.T.H., Huang H.S., Conte-Junior C.A. Luteolin-Rich Extract of Thespesia garckeana F. Hoffm. (Snot Apple) contains potential drug-like candidates and modulates glycemic and oxidoinflammatory aberrations in experimental animals. Oxid. Med. Cell. Longev. 2022 doi: 10.1155/2022/1215097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqodri L., Girsang E., Napiah A., Ferdinand S. Comparison of Antioxidant and Anti-collagenase Activities Ethanol Extract of Black Soybeans with Daidzein Compounds. Am. Sci. Res. J. Eng. Technol. Sci. 2020;70:90–98. [Google Scholar]
- Alzaabi M.M., Hamdy R., Ashmawy N.S., Hamoda A.M., Alkhayat F., Khademi N.N., Al Joud S.M.A., El-Keblawy A.A., Soliman S.S.M. Flavonoids are promising safe therapy against COVID-19. Phytochem. Rev. 2022;21:291–312. doi: 10.1007/s11101-021-09759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambarwati N.S.S., Elya B., Desmiaty Y., Omar H. Anti-elastase of leaves and stem bark extract of Garcinia daedalanthera pierre. Int. J. Pharm. Res. 2020;12:592–596. doi: 10.31838/ijpr/2020.12.01.126. [DOI] [Google Scholar]
- Amberg N., Fogarassy C. Green consumer behavior in the cosmetics market. Resources. 2019;8 doi: 10.3390/resources8030137. [DOI] [Google Scholar]
- Andriotis, O.G., Nalbach, M., Thurner, P.J., 2023. Mechanics of isolated individual collagen fibrils ✩. 10.1016/j.actbio.2022.12.008. [DOI] [PubMed]
- Aranda-Rivera A.K., Cruz-Gregorio A., Arancibia-Hernández Y.L., Hernández-Cruz E.Y., Pedraza-Chaverri J. RONS and oxidative stress: an overview of basic concepts. Oxygen. 2022;2:437–478. doi: 10.3390/oxygen2040030. [DOI] [Google Scholar]
- Arseni L., Lombardi A., Orioli D. From structure to phenotype: Impact of collagen alterations on human health. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19051407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arterbery V.E., Gupta S. Apigenin as an Anti-Aging Skin Treatment. J. Clin. Cosmet. Dermatology. 2018;2:1–8. doi: 10.16966/2576-2826.128. [DOI] [Google Scholar]
- Arung E.T., Shimizu K., Kondo R. Inhibitory effect of isoprenoid-substituted flavonoids isolated from Artocarpus heterophyllus on melanin biosynthesis. Planta Med. 2006;72:847–850. doi: 10.1055/s-2006-931606. [DOI] [PubMed] [Google Scholar]
- Asan T., Lister I.N.E., Fachrial E., Amalia A., Widowati W., Samin B., Liena L. Potency of black soybean (Glycine max (L.) Merr) extract and daidzein as antioxidant and antihyaluronidase. Maj. Obat Tradis. 2019;24:52. doi: 10.22146/mot.43615. [DOI] [Google Scholar]
- Azadbakht L., Kimiagar M., Mehrabi Y., Esmaillzadeh A., Hu F.B., Willett W.C. Soy consumption, markers of inflammation, and endothelial function: a cross-over study in postmenopausal women with the metabolic syndrome. Diabetes Care. 2007;30:967–973. doi: 10.2337/dc06-2126. [DOI] [PubMed] [Google Scholar]
- Babukumar S., Vinothkumar V., Velu P., Ramachandhiran D., Ramados Nirmal M. Molecular effects of hesperetin, a citrus flavanone on7,12-dimethylbenz(a)anthracene induced buccal pouch squamous cell carcinoma in golden Syrian hamsters. Arch. Physiol. Biochem. 2017;123:265–278. doi: 10.1080/13813455.2017.1317815. [DOI] [PubMed] [Google Scholar]
- Banz W., Hauck S., Gename B., Winters T., Bartke A. Soy isoflavones modify liver free radical scavenger systems and liver parameters in Sprague-Dawley rats. J. Medicinal Food. 2004;7:477–481. doi: 10.1089/jmf.2004.7.477. [DOI] [PubMed] [Google Scholar]
- Batista C.M., Alves A.V.F., Queiroz L.A., Lima B.S., Filho R.N.P., Araújo A.A.S., de Albuquerque Júnior R.L.C., Cardoso J.C. The photoprotective and anti-inflammatory activity of red propolis extract in rats. J. Photochem. Photobiol. B Biol. 2018;180:198–207. doi: 10.1016/j.jphotobiol.2018.01.028. [DOI] [PubMed] [Google Scholar]
- Bernatoniene J., Kopustinskiene D.M. The role of catechins in cellular responses to oxidative stress. Molecules. 2018;23:1–11. doi: 10.3390/molecules23040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M.A., Iovine B., Iannella M.L., Gasparri F., Monfrecola G. Synergic effect of genistein and daidzein on UVB-induced DNA damage: an effective photoprotective combination. J. Biomed. Biotechnol. 2011;2011 doi: 10.1155/2011/692846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhren B.A., Schrumpf H., Hoff N.P., Bölke E., Hilton S., Gerber P.A. Hyaluronidase: from clinical applications to molecular and cellular mechanisms. Eur. J. Med. Res. 2016;21:1–8. doi: 10.1186/s40001-016-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlando B., Pastorino G., Salis A., Damonte G., Clericuzio M., Cornara L. The bioactivity of hedysarum coronarium extracts on skin enzymes and cells correlates with phenolic content. Pharm. Biol. 2017;55:1984–1991. doi: 10.1080/13880209.2017.1346691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho M.J., Oliveira A.L., Pedrosa S.S., Pintado M., Madureira A.R. Potential of sugarcane extracts as cosmetic and skincare ingredients. Ind. Crops Prod. 2021;169 doi: 10.1016/j.indcrop.2021.113625. [DOI] [Google Scholar]
- Chaiprasongsuk A., Panich U. Role of Phytochemicals in Skin Photoprotection via Regulation of Nrf2. Front. Pharmacol. 2022;13:1–22. doi: 10.3389/fphar.2022.823881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalise H.N. Aging: basic concept. Am. J. Biomed. Sci. Res. 2019;1:8–10. doi: 10.34297/ajbsr.2019.01.000503. [DOI] [Google Scholar]
- Chang T.S., Ding H.Y., Tai S.S.K., Wu C.Y. Mushroom tyrosinase inhibitory effects of isoflavones isolated from soygerm koji fermented with Aspergillus oryzae BCRC 32288. Food Chem. 2007;105:1430–1438. doi: 10.1016/j.foodchem.2007.05.019. [DOI] [Google Scholar]
- Cheke R.S., Narkhede R.R., Shinde S.D., Ambhore J.P., Jain P.G. Natural product emerging as potential sars spike glycoproteins-ace2 inhibitors to combat COVID-19 attributed by in-silico investigations. Biointerface Res. Appl. Chem. 2021;11:10628–10639. doi: 10.33263/BRIAC113.1062810639. [DOI] [Google Scholar]
- Cho Y.C., Han J.B., Park S.I. Photoprotective effects of soybean extract against UV-induced damage in human fibroblast and hairless mouse model. J. Animal Reproduction Biotechnnol. 2019;34:20–29. doi: 10.12750/JARB.34.1.20. [DOI] [Google Scholar]
- Choi S.S., Lee S.H., Lee K.A. A comparative study of hesperetin, hesperidin and hesperidin glucoside: antioxidant, anti-inflammatory, and antibacterial activities in vitro. Antioxidants. 2022;11 doi: 10.3390/antiox11081618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S., Youn J., Kim K., Joo D.H., Shin S., Lee J., Lee H.K., An I.S., Kwon S., Youn H.J., Ahn K.J., An S., Cha H.J. WaveApigenin inhibits UVA-induced cytotoxicity in vitro and prevents signs of skin aging in vivo. Int. J. Mol. Med. 2016;38:627–634. doi: 10.3892/ijmm.2016.2626. [DOI] [PubMed] [Google Scholar]
- Costea L., Chițescu C.L., Boscencu R., Ghica M., Lupuliasa D., Mihai D.P., Deculescu-ioniță T., Duțu L.E., Popescu M.L., Luță E.A., Nițulescu G.M., Olaru O.T., Gîrd C.E. The polyphenolic profile and antioxidant activity of five vegetal extracts with hepatoprotective potential. Plants. 2022;11 doi: 10.3390/plants11131680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ascanio L., Vitelli F., Cingolani C., Maranzano M., Brenner M.J., Stadio A.D.I. Randomized clinical trial “olfactory dysfunction after COVID-19: olfactory rehabilitation therapy vs. intervention treatment with Palmitoylethanolamide and Luteolin”: Preliminary results. Eur. Rev. Med. Pharmacol. Sci. 2021;25:4156–4162. doi: 10.26355/eurrev_202106_26059. [DOI] [PubMed] [Google Scholar]
- de Gálvez E.N., Aguilera J., Solis A., de Gálvez M.V., de Andrés J.R., Herrera-Ceballos E., Gago-Calderon A. The potential role of UV and blue light from the sun, artificial lighting, and electronic devices in melanogenesis and oxidative stress. J. Photochem. Photobiol. B Biol. 2022;228 doi: 10.1016/j.jphotobiol.2022.112405. [DOI] [PubMed] [Google Scholar]
- de Mendonça M.A.A., Ribeiro A.R.S., de Lima A.K., Bezerra G.B., Pinheiro M.S., de Albuquerque-Júnior R.L.C., Gomes M.Z., Padilha F.F., Thomazzi S.M., Novellino E., Santini A., Severino P., Souto E.B., Cardoso J.C. Red propolis and its dyslipidemic regulator formononetin: Evaluation of antioxidant activity and gastroprotective effects in rat model of gastric ulcer. Nutrients. 2020;12:1–17. doi: 10.3390/nu12102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula Corrêa M., Germano Marciano A., Carvalho S.B., Bernardo de Souza P.M., da Silveira Carvalho Ripper J., Roy D., Breton L., De Vecchi R. Exposome extrinsic factors in the tropics: The need for skin protection beyond solar UV radiation. Sci. Total Environ. 2021;782 doi: 10.1016/j.scitotenv.2021.146921. [DOI] [Google Scholar]
- Deng T., Zhang N., Liu Y., Li J. Daidzein ameliorates experimental acute reflux esophagitis in rats via regulation of cytokines. Pharmazie. 2021;76:84–91. doi: 10.1691/ph.2021.01003. [DOI] [PubMed] [Google Scholar]
- DeRango-Adem E.F., Blay J. Does oral apigenin have real potential for a therapeutic effect in the context of human gastrointestinal and other cancers? Front. Pharmacol. 2021;12:1–17. doi: 10.3389/fphar.2021.681477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divakaran S.A., Hema P.S., Nair M.S., Nair C.K.K. Antioxidant capacity and radioprotective properties of the flavonoids galangin and kaempferide isolated from Alpinia galanga L. (Zingiberaceae) against radiation induced cellular DNA damage. Int. J. Radiat. Res. 2013;11:81–89. [Google Scholar]
- Embassy of The Republic of Indonesia in Brussels, 2021. Potential of Indonesian Natural Cosmetics and Sustainable Requirements as Market Reference in the European Union. Embassy of The Republic of Indonesia in Brussels, Brussels.
- Eum H.L., Park Y., Yi T.G., Lee J.W., Ha K.S., Choi I.Y., Park N.I. Effect of germination environment on the biochemical compounds and anti-inflammatory properties of soybean cultivars. PLoS One. 2020;15:1–14. doi: 10.1371/journal.pone.0232159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallacara A., Baldini E., Manfredini S., Vertuani S. Hyaluronic acid in the third millennium. Polymers (Basel). 2018;10 doi: 10.3390/polym10070701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO and Non-Timber Forest Products-Exchange Programme, 2020. Naturally beautiful – Cosmetic and beauty products from forests. Available from: ( 10.4060/ca8590en). [DOI]
- Farisha M., Safari A. Transformation of the cosmetic industry due to the COVID-19 pandemic. Int. J. Res. Rev. 2021;8:627–633. doi: 10.52403/ijrr.20211276. [DOI] [Google Scholar]
- Fouad A.A., Albuali W.H., Jresat I. Protective effect of naringenin against lipopolysaccharide-induced acute lung injury in rats. Pharmacology. 2016;97:224–232. doi: 10.1159/000444262. [DOI] [PubMed] [Google Scholar]
- Fritz K.L., Seppanen C.M., Kurzer M.S., Csallany Saari A. The in vivo antioxidant activity of soybean isoflavones in human subjects. Nutr. Res. 2003;23:479–487. doi: 10.1016/S0271-5317(03)00005-8. [DOI] [Google Scholar]
- Fu C., Shi S., Tian J., Gu H., Yao L., Xiao J. Non-denatured yak type I collagen accelerates sunburned skin healing by stimulating and replenishing dermal collagen. Biotechnol. Reports. 2023;37 doi: 10.1016/j.btre.2022.e00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh S., Mukesh G., Ranjan B., Vishal V. A review on some plants having anti-inflammatory activity. J. Phytopharm. 2014;3:214–221. [Google Scholar]
- George, J., Sneed, K., Pathak, Y., College, T., Florida, S., 2022. The skin aging process and anti-aging strategies 33377–33386. 10.26717/BJSTR.2022.42.006712. [DOI]
- Goh Y.X., Jalil J., Lam K.W., Husain K., Premakumar C.M. Genistein: a review on its anti-inflammatory properties. Front. Pharmacol. 2022;13:1–23. doi: 10.3389/fphar.2022.820969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvinhas I., Pinto R., Santos R., Saavedra M.J., Barros A.I. Enhanced phytochemical composition and biological activities of grape (Vitis vinifera L.) Stems growing in low altitude regions. Sci. Hortic. Amsterdam) 2020;265 doi: 10.1016/j.scienta.2020.109248. [DOI] [Google Scholar]
- Granica S., Czerwińska M.E., Zyzyńska-Granica B., Kiss A.K. Antioxidant and anti-inflammatory flavonol glucuronides from Polygonum aviculare L. Fitoterapia. 2013;91:180–188. doi: 10.1016/j.fitote.2013.08.026. [DOI] [PubMed] [Google Scholar]
- Gu Y., Han J., Jiang C., Zhang Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020;59 doi: 10.1016/j.arr.2020.101036. [DOI] [PubMed] [Google Scholar]
- Haron H., Jamil A., Besar N.A.A. Photoprotection and skin whitening effect of dietary soy milk in healthy young female adults. J. Pakistan Assoc. Dermatologists. 2020;30:412–417. [Google Scholar]
- He H., Li J., Xie Y., Li Z., Shi H., Lu C.D. Effects of soy isoflavones on intake, body weight, sex hormones, antioxidant performance, and semen quality in Xinong Saanen goats. J. Appl. Anim. Res. 2021;49:125–132. doi: 10.1080/09712119.2021.1901716. [DOI] [Google Scholar]
- Heinrich U., Tronnier H., De Spirt S., Stahl W. Green tea polyphenols: Provide Photoprotection and improve physiological parameters of human skin. Agro Food Ind. Hi. Tech. 2011;22:38–39. doi: 10.3945/jn.110.136465.green. [DOI] [Google Scholar]
- Hermanns J.F., Petit L., Piérard-Franchimont C., Paquet P., Piérard G.E. Sci-Hub | assessment of topical hypopigmenting agents on solar lentigines of Asian women. Dermatology. 2002;204(4):281–286. doi: 10.1159/000063359. [DOI] [PubMed] [Google Scholar]
- Hwang Y.P., Oh K.N., Yun H.J., Jeong H.G. The flavonoids apigenin and luteolin suppress ultraviolet A-induced matrix metalloproteinase-1 expression via MAPKs and AP-1-dependent signaling in HaCaT cells. J. Dermatol. Sci. 2011;61:23–31. doi: 10.1016/j.jdermsci.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Iskandar D., Warsidah W. Qualitative phytochemical screening and antioxidant activity of ethanol root extract of Spatholobus littoralis Hassk. J. Food Med. Plants. 2020;1:13–15. doi: 10.25077/jfmp.1.1.13-15.2020. [DOI] [Google Scholar]
- Iskandar D., Widodo N., Warsito M., Rollando A. Phenolic content, antioxidant, cytotoxic of fractions of Spatholobus littoralis Hassk from Kalimantan, Indonesia. J. Hunan Univ. Nat. Sci. 2022;49:14–23. doi: 10.55463/issn.1674-2974.49.3.2. [DOI] [Google Scholar]
- Izumi T., Saito M., Obata A., Arii M., Yamaguchi H., Matsuyama A. Oral intake of soy isoflavone aglycone improves the aged skin of adult women. J. Nutr. Sci. Vitaminol. (Tokyo) 2007;53:57–62. doi: 10.3177/jnsv.53.57. [DOI] [PubMed] [Google Scholar]
- Jain P.G., Nayse P.G., Patil D.J., Shinde S.D., Surana S.J. The possible antioxidant capabilities of formononetin in guarding against streptozotocin-induced diabetic nephropathy in rats. Futur. J. Pharm. Sci. 2020;6 doi: 10.1186/s43094-020-00071-9. [DOI] [Google Scholar]
- Jakimiuk K., Gesek J., Atanasov A.G., Tomczyk M. Flavonoids as inhibitors of human neutrophil elastase. J. Enzyme Inhib. Med. Chem. 2021;36:1016–1028. doi: 10.1080/14756366.2021.1927006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameel, A., Roua, A.A., Ahmad, S.B., Ola, M.A., Ehab, A.H., 2020. Luteolin protects against lead acetate ‑ induced nephrotoxicity through antioxidant , anti ‑ inflammatory , anti ‑ apoptotic , and Nrf2 / HO ‑ 1 signaling pathways. [DOI] [PubMed]
- Juliana C., Lister I.N.E., Girsang E., Nasution A.N., Widowati W. Antioxidant and Elastase Inhibitor from Black Soybean (Glycine max L.) and Its Compound (Daidzein) J. Biomed. Transl. Res. 2020;6:11–14. doi: 10.14710/jbtr.v6i1.5540. [DOI] [Google Scholar]
- Kanaze F.I., Bounartzi M.I., Georgarakis M., Niopas I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007;61:472–477. doi: 10.1038/sj.ejcn.1602543. [DOI] [PubMed] [Google Scholar]
- Khan A., Ikram M., Hahm J.R., Kim M.O. Antioxidant and anti-inflammatory effects of citrus flavonoid hesperetin: special focus on neurological disorders. Antioxidants. 2020;9:1–15. doi: 10.3390/antiox9070609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Wang G., Zhou F., Gong L., Zhang J., Qi L., Cui H. Polydeoxyribonucleotide: a promising skin anti-aging agent. Chinese J. Plast. Reconstr. Surg. 2022 doi: 10.1016/j.cjprs.2022.09.015. [DOI] [Google Scholar]
- Khan A., Khan S.U., Khan A., Shal B., Rehman S.U., Rehman S.U., Htar T.T., Khan S., Anwar S., Alafnan A., Rengasamy K.R.R. Anti-Inflammatory and anti-rheumatic potential of selective plant compounds by targeting TLR-4/AP-1 signaling: a comprehensive molecular docking and simulation approaches. Molecules. 2022;27:1–23. doi: 10.3390/molecules27134319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradmand E., Hajizadeh Moghaddam A., Zare M. Neuroprotective effect of hesperetin and nano-hesperetin on recognition memory impairment and the elevated oxygen stress in rat model of Alzheimer’s disease. Biomed. Pharmacother. 2018;97:1096–1101. doi: 10.1016/j.biopha.2017.11.047. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Cho I.S., So Y.K., Kim H.H., Kim Y.H. Kushenol A and 8-prenylkaempferol, tyrosinase inhibitors, derived from Sophora flavescens. J. Enzyme Inhib. Med. Chem. 2018;33:1048–1054. doi: 10.1080/14756366.2018.1477776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.M., Heo H.J. The roles of catechins in regulation of systemic inflammation. Food Sci. Biotechnol. 2022;88 doi: 10.1007/s10068-022-01069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Hwang K., Lee J., Han S.Y., Kim E.M., Park J., Cho J.Y. Skin protective effect of epigallocatechin gallate. Int. J. Mol. Sci. 2018;19:1–13. doi: 10.3390/ijms19010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.Y., Kim S.J., Lee J.Y., Kim W.G., Park W.S., Sim Y.C., Lee S.J. Protective Effects of Dietary Soy Isoflavones against UV-Induced Skin-Aging in Hairless Mouse Model. J. Am. Coll. Nutr. 2004;23:157–162. doi: 10.1080/07315724.2004.10719356. [DOI] [PubMed] [Google Scholar]
- Kim K.Y., Lee E.J., Whang W.K., Park C.H. In vitro and in vivo anti-aging effects of compounds isolated from Artemisia iwayomogi. J. Anal. Sci. Technol. 2019;10:1–8. doi: 10.1186/s40543-019-0193-1. [DOI] [Google Scholar]
- Kim J.M., Lee U., Kang J.Y., Park S.K., Kim J.C., Heo H.J. Matcha improves metabolic imbalance-induced cognitive dysfunction. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/8882763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.M., Kang J.Y., Park S.K., Moon J.H., Kim M.J., Lee H.L., Jeong H.R., Kim J.C., Heo H.J. Powdered green tea (Matcha) attenuates the cognitive dysfunction via the regulation of systemic inflammation in chronic pm2.5-exposed balb/c mice. Antioxidants. 2021;10 doi: 10.3390/antiox10121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kose, A.H.P., Potdar, S.N., Mishra, R.A., Sharma, P.D., 2020. Investigation of phytochemicals as anti-ageing agents aganist matrix metalloproteinases using molecular 8, 3709–3716.
- Kritsi E., Tsiaka T., Ioannou A.G., Mantanika V., Strati I.F., Panderi I., Zoumpoulakis P., Sinanoglou V.J. In Vitro and In Silico Studies to Assess Edible Flowers’ Antioxidant Activities. Appl. Sci. 2022;12 doi: 10.3390/app12147331. [DOI] [Google Scholar]
- Kulprachakarn K., Chaipoot S., Phongphisutthinant R., Paradee N., Prommaban A., Ounjaijean S., Rerkasem K., Parklak W., Prakit K., Saengsitthisak B., Chansiw N., Pangjit K., Boonyapranai K. Antioxidant potential and cytotoxic effect of isoflavones extract from thai fermented soybean (Thua-nao) Molecules. 2021;26 doi: 10.3390/molecules26247432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppusamy U.R., Das N.P. Inhibitory effects of flavonoids on several venom hyaluronidase. Experientia. 1991;47:11–12. doi: 10.1007/bf01918384. [DOI] [PubMed] [Google Scholar]
- Kutil Z., Temml V., Maghradze D., Pribylova M., Dvorakova M., Schuster D., Vanek T., Landa P. Impact of wines and wine constituents on cyclooxygenase-1, cyclooxygenase-2, and 5-lipoxygenase catalytic activity. Mediators Inflamm. 2014;2014:1–9. doi: 10.1155/2014/178931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laksmiani N.P.L., Sanjaya I.K.N., Leliqia N.P.E. The activity of avocado (Persea americana Mill.) seed extract containing catechin as a skin lightening agent. J. Pharm. Pharmacogn. Res. 2020;8:449–456. [Google Scholar]
- Lall N., Mogapi E., de Canha M.N., Crampton B., Nqephe M., Hussein A.A., Kumar V. Insights into tyrosinase inhibition by compounds isolated from Greyia radlkoferi Szyszyl using biological activity, molecular docking and gene expression analysis. Bioorganic Med. Chem. 2016;24:5953–5959. doi: 10.1016/j.bmc.2016.09.054. [DOI] [PubMed] [Google Scholar]
- Lee E.J., Kim S.Y., Hyun J.W., Min S.W., Kim D.H., Kim H.S. Glycitein inhibits glioma cell invasion through down-regulation of MMP-3 and MMP-9 gene expression. Chem. Biol. Interact. 2010;185:18–24. doi: 10.1016/j.cbi.2010.02.037. [DOI] [PubMed] [Google Scholar]
- Lee S., Youn K., Lim G.T., Lee J., Jun M. In silico docking and in vitro approaches towards BACE1 and cholinesterases inhibitory effect of citrus flavanones. Molecules. 2018;23:1–12. doi: 10.3390/molecules23071509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Yu J.S., Phung H.M., Lee J.G., Kim K.H., Kang K.S. Potential anti-skin aging effect of (-)-catechin isolated from the root bark of ulmus davidiana var. Japonica in tumor necrosis factor-α-stimulated normal human dermal fibroblasts. Antioxidants. 2020;9:1–13. doi: 10.3390/antiox9100981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Zhou H.Y., Cho S.Y., Kim Y.S., Lee Y.S., Jeong C.S. Anti-inflammatory mechanisms of apigenin: Inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch. Pharm. Res. 2007;30:1318–1327. doi: 10.1007/bf02980273. [DOI] [PubMed] [Google Scholar]
- Letellier S., Boyer F., Bacqueville D., Duplan H., Perrin L., Lapalud P. How to Ensure Consumers Will Be Satisfied With A New Sustainable Sun Care Product Developed for Extreme Environmental Conditions. Food Qual. Prefer. 2022;102 doi: 10.1016/j.foodqual.2022.104661. [DOI] [Google Scholar]
- Li Y., Jiang X., Cai L., Zhang Y., Ding H., Yin J., Li X. Dietary daidzein supplementeation improved growth performance and antioxidant properties in weaned and growing pigs. Research Square. 2021:1–25. doi: 10.21203/rs.3.rs-152599/v1. [DOI] [Google Scholar]
- Li H., Jiang R., Lou L., Jia C., Zou L., Chen M. Formononetin Improves the Survival of Random Skin Flaps Through PI3K/Akt-Mediated Nrf2 Antioxidant Defense System. Front. Pharmacol. 2022;13:1–13. doi: 10.3389/fphar.2022.901498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.C., Yeh C.H., Yang M.L., Kuan Y.H. Luteolin suppresses inflammatory mediator expression by blocking the Akt/NFκB pathway in acute lung injury induced by lipopolysaccharide in mice. Evidence-based Complement. Altern. Med. 2012;2012 doi: 10.1155/2012/383608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K.H., Kim G.R. Inhibitory effect of naringenin on LPS-induced skin senescence by SIRT1 regulation in HDFs. Biomed. Dermatology. 2018;2:1–9. doi: 10.1186/s41702-018-0035-6. [DOI] [Google Scholar]
- Lin J.Y., Tournas J.A., Burch J.A., Monteiro-Riviere N.A., Zielinski J. Topical isoflavones provide effective photoprotection to skin. Photodermatol. Photoimmunol. Photomed. 2008;24:61–66. doi: 10.1111/j.1600-0781.2008.00329.x. [DOI] [PubMed] [Google Scholar]
- Lin T.K., Zhong L., Santiago J.L. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Chen Y., Zhang X., Zheng J., Hu W., Teng B. Hop Tannins as Multifunctional Tyrosinase Inhibitor: Structure Characterization, Inhibition Activity, and Mechanism. Antioxidants. 2022;11:1–15. doi: 10.3390/antiox11040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Li F., Zhang L., Yu H., Yu F., Chen J. The effect of active components from citrus fruits on dentin MMPs. Arch. Oral Biol. 2017;83:111–117. doi: 10.1016/j.archoralbio.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Liyanaarachchi G.D., Samarasekera J.K.R.R., Mahanama K.R.R., Hemalal K.D.P. Tyrosinase, elastase, hyaluronidase, inhibitory and antioxidant activity of Sri Lankan medicinal plants for novel cosmeceuticals. Ind. Crops Prod. 2018;111:597–605. doi: 10.1016/j.indcrop.2017.11.019. [DOI] [Google Scholar]
- Lombardo E., Sabellico C., Hájek J., Staňková V., Filipský T., Balducci V., De Vito P., Leone S., Bavavea E.I., Silvestri I.P., Righi G., Luly P., Saso L., Bovicelli P., Pedersen J.Z., Incerpi S. Protection of Cells against Oxidative Stress by Nanomolar Levels of Hydroxyflavones Indicates a New Type of Intracellular Antioxidant Mechanism. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G., Su G., Zhao Y., Johnston W.F., Sherman N.E., Rissman E.F., Lau C., Ailawadi G., Upchurch G.R. Dietary phytoestrogens inhibit experimental aneurysm formation in male mice. J. Surg. Res. 2014;188:326–338. doi: 10.1016/j.jss.2013.11.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Feng X., Ding S. Hesperetin attenuates ventilator-induced acute lung injury through inhibition of NF-κB-mediated inflammation. Eur. J. Pharmacol. 2015;769:333–341. doi: 10.1016/j.ejphar.2015.11.038. [DOI] [PubMed] [Google Scholar]
- Ma L., Liu G., Ding M., Zong G., Hu F.B., Willett W.C., Rimm E.B., Manson J.A.E., Sun Q. Isoflavone intake and the risk of coronary heart disease in US men and women: Results from 3 prospective cohort studies. Circulation. 2020;1127–1137 doi: 10.1161/CIRCULATIONAHA.119.041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak K.M., Mei R. Basement Membrane Type IV Collagen and Laminin: An Overview of Their Biology and Value as Fibrosis Biomarkers of Liver Disease. Anat. Rec. 2017;300:1371–1390. doi: 10.1002/ar.23567. [DOI] [PubMed] [Google Scholar]
- Mandrone M., Lorenzi B., Venditti A., Guarcini L., Bianco A., Sanna C., Ballero M., Poli F., Antognoni F. Antioxidant and anti-collagenase activity of Hypericum hircinum L. Ind. Crops Prod. 2015;76:402–408. doi: 10.1016/j.indcrop.2015.07.012. [DOI] [Google Scholar]
- Martemucci G., Costagliola C., Mariano M., D’andrea L., Napolitano P., D’Alessandro A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen. 2022;2:48–78. doi: 10.3390/oxygen2020006. [DOI] [Google Scholar]
- Martinez R.M., Pinho-Ribeiro F.A., Steffen V.S., Silva T.C.C., Caviglione C.V., Bottura C., Fonseca M.J.V., Vicentini F.T.M.C., Vignoli J.A., Baracat M.M., Georgetti S.R., Verri W.A., Casagrande R. Topical formulation containing naringenin: Efficacy against ultraviolet B irradiation-induced skin inflammation and oxidative stress in mice. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey & Company, 2020. How COVID-19 is changing the world of beauty. Available from: (https://www.mckinsey.com/industries/consumer-packaged-goods/our-insights/how-covid-19-is-changing-the-world-of-beauty).
- Meeran S.M., Akhtar S., Katiyar S.K. Inhibition of UVB-induced skin tumor development by drinking green tea polyphenols is mediated through DNA repair and subsequent inhibition of inflammation. J. Invest. Dermatol. 2009;129:1258–1270. doi: 10.1038/jid.2008.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megow I., Darvin M.E., Meinke M.C., Lademann J. A Randomized Controlled Trial of Green Tea Beverages on the in vivo Radical Scavenging Activity in Human Skin. Skin Pharmacol. Physiol. 2017;30:225–233. doi: 10.1159/000477355. [DOI] [PubMed] [Google Scholar]
- Ministry of Industry of The Republic of Indonesia, 2018. National Cosmetic Industry Growth. Available from: (https://ikft.kemenperin.go.id/pertumbuhan-industri-kosmetik-nasional/).
- Moon S.H., Kim K.T., Lee N.K., Han Y.S., Nah S.Y., Cho S.G., Park Y.S., Paik H.D. Inhibitory effects of naringenin and its novel derivatives on hyaluronidase. Food Sci. Biotechnol. 2009;18:267–270. [Google Scholar]
- Mumpuni E., Mulatsari E. QSAR analysis on apigenin derivative compounds as antioxidant using semiempirical austin model 1. Asian J. Chem. 2017;29:1499–1505. doi: 10.14233/ajchem.2017.20535. [DOI] [Google Scholar]
- Murata K., Suzuki S., Miyamoto A., Horimoto M., Nanko S., Mori D., Kanamaru H., Endo Y. Tyrosinase Inhibitory Activity of Extracts from Prunus persica. Separations. 2022;9:4–10. doi: 10.3390/separations9050107. [DOI] [Google Scholar]
- Musdja M.Y., Rahman H.A., Hasan D. Antioxidant Activity of Catechins Isolate of Uncaria Gambier Roxb in Male Rats. LIFE Int. J. Heal. Life-Sciences. 2018;4:34–46. doi: 10.20319/lijhls.2018.42.3446. [DOI] [Google Scholar]
- Nemitz M.C., von Poser G.L., Teixeira H.F. In vitro skin permeation/retention of daidzein, genistein and glycitein from a soybean isoflavone rich fraction-loaded nanoemulsions and derived hydrogels. J. Drug Deliv. Sci. Technol. 2019;51:63–69. doi: 10.1016/j.jddst.2019.02.034. [DOI] [Google Scholar]
- Numan J.W.A.R. Historical biogeography of Spatholobus (Leguminosae-Papilionoideae) and allies in SE Asia. Biogeogr. Geol. Evol. SE Asia. 1998:259–277. [Google Scholar]
- Pandurangan P., Sahadeven M., Sunkar S., Mohana Dhana S.K.N. Comparative analysis of biochemical compounds of leaf, flower and fruit of couroupita guianensis and synthesis of silver nanoparticles. Pharmacogn. J. 2018;10:315–323. doi: 10.5530/pj.2018.2.55. [DOI] [Google Scholar]
- Papakonstantinou E., Roth M., Karakiulakis G. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinol. 2012;4:37–41. doi: 10.4161/derm.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Sánchez A., Barrajón-Catalán E., Herranz-López M., Micol V. Nutraceuticals for skin care: A comprehensive review of human clinical studies. Nutrients. 2018;10:1–22. doi: 10.3390/nu10040403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permana A.D., Utami R.N., Courtenay A.J., Manggau M.A., Donnelly R.F., Rahman L. Phytosomal nanocarriers as platforms for improved delivery of natural antioxidant and photoprotective compounds in propolis: An approach for enhanced both dissolution behaviour in biorelevant media and skin retention profiles. J. Photochem. Photobiol. B Biol. 2020;205 doi: 10.1016/j.jphotobiol.2020.111846. [DOI] [PubMed] [Google Scholar]
- Priani S.E., Fakih T.M. Insights into molecular interaction of flavonoid compounds in citrus peel bound to collagenase and elastase enzymes: a computational study. Pharm. Sci. Res. 2021;8:93–101. doi: 10.7454/psr.v8i2.1202. [DOI] [Google Scholar]
- Rajan V.K., Shameera Ahamed T.K., Muraleedharan K. Studies on the UV filtering and radical scavenging capacity of the bitter masking flavanone Eriodictyol. J. Photochem. Photobiol. B Biol. 2018;185:254–261. doi: 10.1016/j.jphotobiol.2018.06.017. [DOI] [PubMed] [Google Scholar]
- Reshef N., Hayari Y., Goren C., Boaz M., Madar Z., Knobler H. Antihypertensive effect of sweetie fruit in patients with stage I hypertension. Am. J. Hypertens. 2005;18:1360–1363. doi: 10.1016/j.amjhyper.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Rhodes L.E., Darby G., Farrar M.D., Bennett S., Watson R.E.B., Massey K.A., Nicolaou A., Clarke K.A., Dew T.P., Williamson G. Oral green tea catechin metabolites are incorporated into human skin and protect against UV radiation-induced cutaneous inflammation in association with reduced production of pro-inflammatory eicosanoid 12-hydroxyeicosatetraenoic acid. Brit. J. Nutr. 2013;110:891–900. doi: 10.1017/S0007114512006071. [DOI] [PubMed] [Google Scholar]
- Roberts, R., 2022. Beauty Industry Trends & Cosmetics Marketing: Statistics and Strategies for Your Ecommerce Growth. Available from: (https://commonthreadco.com/blogs/coachs-corner/beauty-industry-cosmetics-marketing-ecommerce).
- Rostoka E., Isajevs S., Baumane L., Line A., Silina K., Dzintare M., Sharipova J., Svirina D., Kalvinsh I., Sjakste N. Effects of lycopene, indole-3-carbinol, and luteolin on nitric oxide production and iNOS expression are organ-specific in rats. Arh. Hig. Rada Toksikol. 2010;61:275–285. doi: 10.2478/10004-1254-61-2010-2012. [DOI] [PubMed] [Google Scholar]
- Rüfer C.E., Maul R., Donauer E., Fabian E.J., Kulling S.E. In vitro and in vivo metabolism of the soy isoflavone glycitein. Mol. Nutr. Food Res. 2007;51:813–823. doi: 10.1002/mnfr.200700013. [DOI] [PubMed] [Google Scholar]
- Ryu H.W., Park Y.J., Lee S.U., Lee S., Yuk H.J., Seo K.H., Kim Y.U., Hwang B.Y., Oh S.R. Potential Anti-inflammatory Effects of the Fruits of Paulownia tomentosa. J. Nat. Prod. 2017;80:2659–2665. doi: 10.1021/acs.jnatprod.7b00325. [DOI] [PubMed] [Google Scholar]
- Saija A., Tomaino A., Trombetta D., Giacchi M., De Pasquale A., Bonina F. Influence of different penetration enhancers on in vitro skin permeation and in vivo photoprotective effect of flavonoids. Int. J. Pharm. 1998;175:85–94. doi: 10.1016/S0378-5173(98)00259-2. [DOI] [Google Scholar]
- San Miguel-Chávez R. Phenolic antioxidant capacity: a review of the state of the art. Phenolic Compd. - Biol. Act. 2017 doi: 10.5772/66897. [DOI] [Google Scholar]
- Sekar, M., 2020. Natural products in aging skin. Aging (Albany. NY). 267–273. https://doi.org/10.1016/b978-0-12-818698-5.00026-2.
- Sharifi-Rad M., Anil Kumar N.V., Zucca P., Varoni E.M., Dini L., Panzarini E., Rajkovic J., Tsouh Fokou P.V., Azzini E., Peluso I., Prakash Mishra A., Nigam M., El Rayess Y., Beyrouthy M.E., Polito L., Iriti M., Martins N., Martorell M., Docea A.O., Setzer W.N., Calina D., Cho W.C., Sharifi-Rad J. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020;11:1–21. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen Y.S., Huang H.Y., Liao Y.H. The efficacy and safety of an antiaging topical serum containing hesperetin and sodium cyclic lysophosphatidic acid: a single-center clinical trial. J. Cosmet. Dermatol. 2021;20:3960–3967. doi: 10.1111/jocd.14063. [DOI] [PubMed] [Google Scholar]
- Si Y.X., Wang Z.J., Park D., Chung H.Y., Wang S.F., Yan L., Yang J.M., Qian G.Y., Yin S.J., Park Y.D. Effect of hesperetin on tyrosinase: Inhibition kinetics integrated computational simulation study. Int. J. Biol. Macromol. 2012;50:257–262. doi: 10.1016/j.ijbiomac.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Sianipar R.N.R. Stem Extract And Fraction Toward XanthIne Oxidase Through In Vitro And Its Metabolite Profile (Master thesis) IPB University; Bogor: 2022. Inhibition Kinetics of Bajakah Tampala (Spatholobus littoralis Hassk.) [Google Scholar]
- Sianipar R.N.R., Sutriah K., Iswantini D., Achmadi S.S. Inhibitory Capacity of Xanthine Oxidase in Antigout Therapy by Indonesian Medicinal Plants. Pharmacognosy J. 2022;14:470–479. [Google Scholar]
- Sifaki M., Calina D., Docea A., Tsioumas S., Katsarou M., Papadogiorgaki S., Fragkiadaki P., Branisteanu D., Kouskoukis K., Tsiaoussis J., Spandidos D. A novel approach regarding the anti-aging of facial skin through collagen reorganization. Exp. Ther. Med. 2019 doi: 10.3892/etm.2019.8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim Y.Y., Nyam K.L. Application of Hibiscus cannabinus L. (kenaf) leaves extract as skin whitening and anti-aging agents in natural cosmetic prototype. Ind. Crops Prod. 2021;167 doi: 10.1016/j.indcrop.2021.113491. [DOI] [Google Scholar]
- Singh B., Sidiq T., Joshi P., Jain S.K., Lawaniya Y., Kichlu S., Khajuria A., Vishwakarma R.A., Bharate S.B. Anti-inflammatory and immunomodulatory flavones from Actinocarya tibetica Benth. Nat. Prod. Res. 2013;27:2227–2230. doi: 10.1080/14786419.2013.805334. [DOI] [PubMed] [Google Scholar]
- Stevanato R., Bertelle M., Fabris S. Photoprotective characteristics of natural antioxidant polyphenols. Regul. Toxicol. Pharmacol. 2014;69:71–77. doi: 10.1016/j.yrtph.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Sun, S., Genovese, F., Karsdal, M.A., 2019. Type VI collagen, Second Edi. ed, Biochemistry of Collagens, Laminins and Elastin: Structure, Function and Biomarkers. Elsevier Inc. https://doi.org/10.1016/B978-0-12-817068-7.00006-9.
- Süntar I., Küpeli Akkol E., Keles H., Yesilada E., Sarker S.D., Arroo R., Baykal T. Efficacy of Daphne oleoides subsp. kurdica used for wound healing: identification of active compounds through bioassay guided isolation technique. J. Ethnopharmacol. 2012;141:1058–1070. doi: 10.1016/j.jep.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Syamsul E.S., Umar S., Wahyuni F.S., Martien R., Hamidi D. Anti-aging activity, in silico modeling and molecular docking from Sonneratia Caseolaris. Open Access Maced. J. Med. Sci. 2022;10:1471–1477. doi: 10.3889/oamjms.2022.10558. [DOI] [Google Scholar]
- Taghouti M., Martins-Gomes C., Schäfer J., Félix L.M., Santos J.A., Bunzel M., Nunes F.M., Silva A.M. Thymus pulegioides L. as a rich source of antioxidant, anti-proliferative and neuroprotective phenolic compounds. Food Funct. 2018;9:3617–3629. doi: 10.1039/c8fo00456k. [DOI] [PubMed] [Google Scholar]
- Tan Y., Zhang X., Cheang W.S. Isoflavones daidzin and daidzein inhibit lipopolysaccharide-induced inflammation in RAW264.7 macrophages. Chinese Med. (United Kingdom) 2022;17:1–10. doi: 10.1186/s13020-022-00653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides T.C., Asadi S., Panagiotidou S. A case series of a luteolin formulation (neuroprotek®) in children with autism spectrum disorders. Int. J. Immunopathol. Pharmacol. 2012;25:317–323. doi: 10.1177/039463201202500201. [DOI] [PubMed] [Google Scholar]
- Tian C., Liu X., Chang Y., Wang R., Lv T., Cui C., Liu M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. South African J. Bot. 2021;137:257–264. doi: 10.1016/j.sajb.2020.10.022. [DOI] [Google Scholar]
- Tidke S.A., Mahajankat A., Devasurmut Y., Devappa R., Vasist K.S., Kosturkova G., Aswathanar R.G. Assessment of Anticancer, Anti-inflammatory and Antioxidant Properties of Isoflavones Present in Soybean. Res. J. Phytochem. 2018;12:35–42. doi: 10.3923/rjphyto.2018.35.42. [DOI] [Google Scholar]
- Trade Attache Indonesian Embassy in Tokyo, 2021. Cosmetic Products Business Intelligence Analysis Report (Skincare) HS: 330499. Trade Attache Indonesian Embassy in Tokyo, Tokyo.
- Triawanti S., Noor M.S. The supplementation of pasak bumi (Eurycoma longifolia Jack.) in undernourished rats to increase spatial memory through antioxidant mechanism. Clin. Nutr. Exp. 2020;33:49–59. doi: 10.1016/j.yclnex.2020.08.002. [DOI] [Google Scholar]
- Tsai Y.H., Lee K.F., Huang Y.B., Huang C.T., Wu P.C. In vitro permeation and in vivo whitening effect of topical hesperetin microemulsion delivery system. Int. J. Pharm. 2010;388:257–262. doi: 10.1016/j.ijpharm.2009.12.051. [DOI] [PubMed] [Google Scholar]
- Uitto J. Toward treatment and cure of epidermolysis bullosa. Proc. Natl. Acad. Sci. U. S. A. 2019;116:26147–26149. doi: 10.1073/pnas.1919347117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnuvathan V.J., Lakshmi K.S., Srividya A.R. Study of Antioxidant Activity of Formononetin By in Vitro Method. Int. J. Pharm. Pharm. Sci. 2017;9:273. doi: 10.22159/ijpps.2017v9i2.13964. [DOI] [Google Scholar]
- Vona R., Pallotta L., Cappelletti M., Severi C., Matarrese P. The impact of oxidative stress in human pathology: Focus on gastrointestinal disorders. Antioxidants. 2021;10:1–26. doi: 10.3390/antiox10020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuolo, M.M., Lima, V.S., Maróstica Junior, M.R., 2018. Phenolic Compounds: Structure, Classification, and Antioxidant Power, Bioactive Compounds: Health Benefits and Potential Applications. Elsevier Inc. 10.1016/B978-0-12-814774-0.00002-5. [DOI]
- Wagle A., Seong S.H., Joung E.J., Kim H.R., Jung H.A., Choi J.S. Discovery of a Highly Potent Tyrosinase Inhibitor, Luteolin 5- O-β- d -Glucopyranoside, Isolated from Cirsium japonicum var. maackii (Maxim.) Matsum., Korean Thistle: Kinetics and Computational Molecular Docking Simulation. ACS Omega. 2018;3:17236–17245. doi: 10.1021/acsomega.8b02694. [DOI] [Google Scholar]
- Wagle A., Seong S.H., Jung H.A., Choi J.S. Identifying an isoflavone from the root of Pueraria lobata as a potent tyrosinase inhibitor. Food Chem. 2019;276:383–389. doi: 10.1016/j.foodchem.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Wang S., Yang M., Yin S., Zhang Y., Zhang Y., Sun H., Shu L., Liu Y., Kang Z., Liu N., Li J., Wang Y., He L., Luo M., Yang X. A new peptide originated from amphibian skin alleviates the ultraviolet B-induced skin photodamage. Biomed. Pharmacother. 2022;150 doi: 10.1016/j.biopha.2022.112987. [DOI] [PubMed] [Google Scholar]
- Wang, Y., 2022. Risk and Scenario Analysis of Cosmetics Business Recovery and Development in the Post-Covid-19 Pandemic Period- The Case of Estee Lauder. In: Proc. 2022 2nd Int. Conf. Enterp. Manag. Econ. Dev. (ICEMED 2022) 656, 180–190. 10.2991/aebmr.k.220603.032. [DOI]
- Warsito, M.F., Kusumawati, I., 2019. The impact of herbal products in the prevention, regeneration and delay of skin aging. Chapter 9. Paul, C. Guest (Ed.). Reviews on Biomarker Studies in Aging and Anti-Aging Research. Advances in Experimental Medicine and Biology 1178. 10.1007/978-3-030-25650-0. [DOI] [PubMed]
- Wittenauer J., MäcKle S., Sußmann D., Schweiggert-Weisz U., Carle R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia. 2015;101:179–187. doi: 10.1016/j.fitote.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Wölfle U., Esser P.R., Simon-Haarhaus B., Martin S.F., Lademann J., Schempp C.M. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic. Biol. Med. 2011;50:1081–1093. doi: 10.1016/j.freeradbiomed.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Xiao L., Lv J., Liang Y., Zhang H., Zheng J., Lin F., Wen X. Structural, physicochemical properties and function of swim bladder collagen in promoting fibroblasts viability and collagen synthesis. Lwt. 2023;173 doi: 10.1016/j.lwt.2022.114294. [DOI] [Google Scholar]
- Xu N., An J. Formononetin ameliorates mast cell-mediated allergic inflammation via inhibition of histamine release and production of pro-inflammatory cytokines. Exp. Ther. Med. 2017;14:6201–6206. doi: 10.3892/etm.2017.5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Zhong Y., Duan Y., Chen Q., Li F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020;6:115–123. doi: 10.1016/j.aninu.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Guan M., Lu Y., Zhang D., Li C., Li Y., Zhou C. Protective effects of hesperetin on lipopolysaccharide-induced acute lung injury by targeting MD2. Eur. J. Pharmacol. 2019;852:151–158. doi: 10.1016/j.ejphar.2019.02.042. [DOI] [PubMed] [Google Scholar]
- Yen G.C., Lai H.H. Inhibition of Reactive Nitrogen Species Effects in Vitro and in Vivo by Isoflavones and Soy-Based Food Extracts. J. Agric. Food Chem. 2003;51:7892–7900. doi: 10.1021/jf034876b. [DOI] [PubMed] [Google Scholar]
- Younis M.M., Ayoub I.M., Mostafa N.M., Hassab M.A.E., Eldehna W.M., Al-rashood S.T., Eldahshan O.A. Stenocarpus sinuatus Leaves Extract. Plants. 2022;11:1–19. doi: 10.3390/plants11070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf L., Girsang E., Nasution A.N., Elvira C., Wibowo S.H.B., Widowati W. H2O2-Scavenging Activity and Hyaluronidase Inhibition Scutellarin and Apigenin in Basil Leaf Extract. J. Kedokt. Brawijaya. 2021;31:133. doi: 10.21776/ub.jkb.2021.031.03.1. [DOI] [Google Scholar]
- Zeng H.J., Ma J., Yang R., Jing Y., Qu L.B. Molecular Interactions of Flavonoids to Hyaluronidase: Insights from Spectroscopic and Molecular Modeling Studies. J. Fluoresc. 2015;25:941–959. doi: 10.1007/s10895-015-1576-3. [DOI] [PubMed] [Google Scholar]
- Zeng Y., Song J., Zhang M., Wang H., Zhang Y., Suo H. Comparison of in vitro and in vivo antioxidant activities of six flavonoids with similar structures. Antioxidants. 2020;9:1–14. doi: 10.3390/antiox9080732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Li F., Chen G. Neuroprotective effect of apigenin in rats after contusive spinal cord injury. Neurol. Sci. 2014;35:583–588. doi: 10.1007/s10072-013-1566-7. [DOI] [PubMed] [Google Scholar]
- Zhang H., Lu M., Jiang H., Wu Z.Y., Zhou D.D., Li D.Q., Yang F.Q. Tyrosinase-mediated dopamine polymerization modified magnetic alginate beads for dual-enzymes encapsulation: Preparation, performance and application. Colloids Surfaces B Biointerfaces. 2020;188 doi: 10.1016/j.colsurfb.2020.110800. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang G., Gurley E.C., Zhou H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in Macrophages. PLoS One. 2014;9:1–18. doi: 10.1371/journal.pone.0107072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhao X., Tao G.J., Chen J., Zheng Z.P. Investigating the inhibitory activity and mechanism differences between norartocarpetin and luteolin for tyrosinase: A combinatory kinetic study and computational simulation analysis. Food Chem. 2017;223:40–48. doi: 10.1016/j.foodchem.2016.12.017. [DOI] [PubMed] [Google Scholar]
- Zhao D., Shi Y., Dang Y., Zhai Y., Ye X. Daidzein stimulates collagen synthesis by activating the TGF-β/smad signal pathway. Australas. J. Dermatol. 2015;56:e7–e14. doi: 10.1111/ajd.12126. [DOI] [PubMed] [Google Scholar]
- Zhao J., Zhang Z., Dai J., Wang L., Zhang C., Ye Y., Li L. Synergistic protective effect of chlorogenic acid, apigenin and caffeic acid against carbon tetrachloride-induced hepatotoxicity in male mice. RSC Adv. 2014;4:43057–43063. doi: 10.1039/c4ra07261h. [DOI] [Google Scholar]