Abstract
Worldwide, populations face significant burdens from neurodegenerative disorders (NDDs), especially Alzheimer's and Parkinson's diseases. Although there are many proposed etiologies for neurodegenerative disorders, including genetic and environmental factors, the exact pathogenesis for these disorders is not fully understood. Most patients with NDDs are given lifelong treatment to improve their quality of life. There are myriad treatments for NDDs; however, these agents are limited by their side effects and difficulty in passing the blood–brain barrier (BBB). Furthermore, the central nervous system (CNS) active pharmaceuticals could offer symptomatic relief for the patient's condition without providing a complete cure or prevention by targeting the disease's cause. Recently, Mesoporous silica nanoparticles (MSNs) have gained interest in treating NDDs since their physicochemical properties and inherent ability to pass BBB make them possible drug carriers for several drugs for NDDs treatment. This paper provides insight into the pathogenesis and treatment of NDDs, along with the recent advances in applying MSNs as fibril scavengers. Moreover, the application of MSNs-based formulations in enhancing or sustaining drug release rate, and brain targeting via their responsive release properties, besides the neurotoxicity of MSNs, have been reviewed.
Keywords: Neurodegenerative disorders, Alzheimer's disease, Parkinson's disease, Blood–brain barrier, Mesoporous silica, Drug delivery
1. Introduction
Neurodegenerative diseases (NDDs) are permanent damage and loss of neurons in various parts of the central nervous system (CNS). This deterioration, especially in the intellectual or cognitive locus, can negatively affect the patient's clinical condition and lifestyle. Recently, genetic and environmental factors have been identified as potential risk factors for certain types of NDDs (Manoharan et al., 2016). NDDs encompass a variety of debilitating conditions that affect the nervous system and lead to progressive deterioration of mental and physical abilities. These include well-known disorders such as Alzheimer's disease (AD) and Parkinson's disease (PD), and other less common conditions, such as Huntington's disease, and ataxia (Migliore and Coppedè, 2009).
AD is significantly prevalent among older people and responsible for dementia in 60–80 % of patients (DeTure and Dickson, 2019), which may be either sporadic or familial. Alzheimer's patients experience mood swings, irritability, aggressiveness, memory problems, and a moderate decline in vital functions (Petrovic et al., 2007). The main pathological hallmarks of AD include the formation of extracellular amyloid plaques, composed of aggregates of amyloid-β peptides, and intracellular neurofibrillary tangles, composed of hyperphosphorylated tau protein (Castellani and Perry, 2013, Castellani et al., 2022). Additionally, these lesions can be induced by the mutations of certain genes, such as PSEN1 and PSEN2 (Kabir et al., 2020, Weggen and Beher, 2012). On the other hand, PD is the second most common neurodegenerative disorder, characterized by age-related progressive degeneration of dopamine-producing neurons in the substantia nigra, leading to motor dysfunction such as tremors, stiffness and difficulty with movement (Bahbah et al., 2021, Magrinelli et al., 2016). The incidence of PD increases with age, while its prevalence is about 1900 cases per 100,000 individuals over 80 years (Pringsheim et al., 2014). The degeneration of dopaminergic neurons in the substantia nigra pars compacta region of the brain is a defining characteristic of PD, and is closely linked to the accumulation of Lewy bodies. Moreover, the overexpression or mutation of the α-syn gene is a major contributing factor to the development and progression of PD (Ouerdane et al., 2022).
Treatment of Alzheimer's and Parkinson's disease poses significant challenges due to the complex nature of these neurodegenerative disorders. Treating AD includes Acetyl cholinesterase inhibitors, which block the degradation of Acetylcholine (Ach) by cholinesterase enzymes, leading to increased Ach concentration within the synaptic cleft (Eldufani and Blaise, 2019, Sharma, 2019). Besides, the N-methyl d-aspartate receptors antagonist prevents glutamate receptors from being overactivated, as a result, restoring the influx of Ca2+ ions to the normal. Unfortunately, regardless of the effectiveness of the previously mentioned therapies, they can only provide symptomatic relief but are inept in treating the underlying causes or preventing the recurrent episodes of these diseases (Carvajal et al., 2016). Whereas the treatment of PD includes levodopa (l-Dopa) that is a dopamine agonist, and monoamine oxidase B inhibitor. Despite being the most potent oral drug, l-Dopa often leads to motor fluctuations with doses of ≥ 600 mg/day (Parkinson Study Group, 2004). The fluctuations observed with dopamine agonist monotherapy are much less common than on l-Dopa monotherapy. Nevertheless, dopamine agonists may also cause additional side effects such as hallucinations, somnolence, and leg edema. However, the existing approaches to treat PD are based on symptomatic alleviation that attempts to adjust dopamine levels in the brain or correct movement impairments (Dietrichs and Odin, 2017, Group, P.S., 2004, Holloway et al., 2004, Reichmann, 2016).
Several drug discovery studies have been concerned with developing novel treatments for PD (Green et al., 2019). Meanwhile, other drug development studies were concerned with encapsulating different CNS-acting agents, such as apomorphine in solid lipid nanoparticles (SLNs) (Loureiro et al., 2017) and nanostructured lipid carriers (NLCs) (Cunha et al., 2021). For instance, Hsu et al. (2010) studied three different formulations (NLCs, SLNs, and lipid emulsions) containing entrapped apomorphine. The study results confirmed that lipid emulsions were the best at providing sustained delivery of apomorphine. However, in vivo real-time bioluminescence proved that NLCs could build up in specific areas in the brain, unlike the other formulations (Tapeinos et al., 2017). Since many drugs for AD and PD treatment are incapable of crossing the Blood-Brain Barrier (BBB), lipid-based formulations have gained substantial interest as candidates for treating NDDs because of their small size and the feasibility of passing the BBB.
Although polymeric nanostructures offer numerous advantages, for example, tunable particle size, shape, and high loading capacity, they also exhibit some demerits, such as immunogenicity, biocompatibility, and the presence of organic solvent residuals. Nevertheless, numerous modification approaches have been developed to curtail these issues (Patel et al., 2012, Tapeinos et al., 2017). Recently, drug delivery systems (DDSs) are becoming increasingly essential to minimize the limitations of conventional therapies, such as lacking selectivity and their poor bio-distribution (Bhatia, 2016). A well-designed DDS can greatly enhance the therapeutic outcome of a drug by precisely delivering it to the targeted site while preserving the integrity of the drug molecule, thus minimizing the undesired side effects. Furthermore, current development in nanotechnology has shown the merits of nanoparticles (particles smaller than 100 nm) as effective drug carrier due to their unique physicochemical and biological properties (Yetisgin et al., 2020).
Several types of novel DDSs include a wide range of materials such as silica- and carbon-based porous nanoparticles (Wang et al., 2018, Zhang et al., 2018), responsive liposomes (Lee and Thompson, 2017), SLNs (Geszke-Moritz and Moritz, 2016), dendrimers (Chauhan, 2018), self-emulsifying emulsions (Singh, 2021), polymeric and magnetic nanoparticles (Assa et al., 2017, Tabatabaei Mirakabad et al., 2014). The nanoparticle-based DDSs were employed for brain targeting and have shown to be good candidates for improving the efficacy of the active ingredient and limiting its adverse effect. Biomedical applications of silica have expanded rapidly in recent years (Huang et al., 2022). For example, mesoporous bioactive glass (MBG), provides good biocompatibility and bone regeneration ability via loading different proteins or drugs within the surfaces of MBG (Zhang et al., 2012). Additionally, MSNs have been thoroughly investigated in controlled drug release due to their physicochemical properties, such as homogeneous pore network, large surface area, low toxicity, and improved bio-distribution of the loaded cargo (Vallet-Regí, 2010).
Silica nanoparticles (SiNPs) are inert and can be easily loaded with various fluorescent probes or surface-modified with functionalizing agents (Ow et al., 2005, Qian et al., 2008). For instance, Schmidt et al., (2018) loaded MSNs with brain-derived neurotrophic factor, which were able to remainin the neurons of the ganglia and achieve a sustained release of the neurotrophic factor for 80 days. Furthermore, the in vivo studies conducted by Barandeh et al., (2012) showed that MSNs can penetrate neurons without exerting any cytotoxic effect on drosophila. Also, MSNs can cross the BBB in mice, and their transport efficiency is reliant on size and not affected by drug loading (Jampilek et al., 2015, Liu et al., 2014). To the best of our knowledge, there are no reviews on the applications of MSNs as scavengers of neurotoxic fibrils precipitating the progression of NDDs. In addition, the ability of MSNs to control drug release through enhancing, sustaining, and targeting the delivery of the active principals to CNS has been discussed.
2. Pathogenesis of Parkinson’s disease
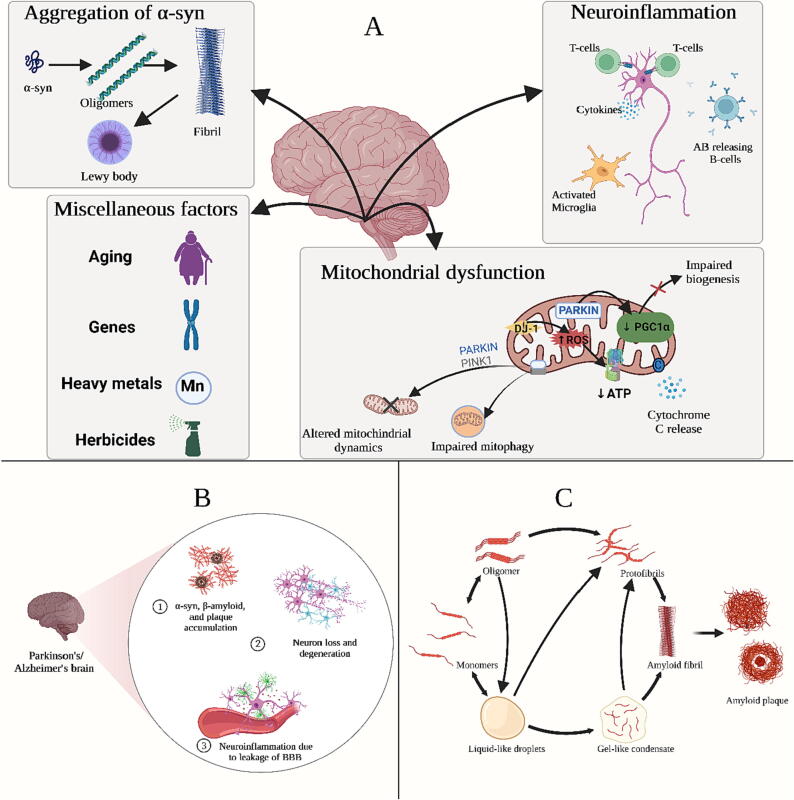
Various key molecular events have been identified in in vitro and in vivo modelling studies, along with the findings of the post-mortem analysis of PD patients. These events include α-synuclein (α-syn) misfolding and accumulation, dysregulation of protein homeostasis, impaired mitochondrial function, neuroinflammation, and oxidative stress, resulting in a vicious cascade of insults that eventually cause irreversible cellular damage (Fig. 1B) (Kumar and Singh, 2015, Soares Martins et al., 2021). The synaptic protein alpha-synuclein (α-syn) is considered the primary constituent of Lewy bodies and neurites, which are abnormally accumulated inclusions in the dopaminergic neurons in the substantia nigra pars compacta and cause neuronal death (Fig. 1A) (Kalia and Lang, 2015, Raza and Anjum, 2019). α-syn is not only a pathogenic hallmark of PD but also of dementia with Lewy bodies and multiple system atrophy (Rocha et al., 2018). In the early stages, PD patients exhibit motor dysfunction, and misfolded α-syn is mainly found in areas responsible for motor function. While at the late stages of the disease, misfolded α-syn is located in cortical structures controlling higher cognitive processing (Henderson et al., 2019). Besides, Masuda-Suzukake et al., (2013) support the belief of α-syn migration between neurons via synaptic terminals in a prion-like manner, where misfolded α-syn can propagate the formation of Lewy bodies in the recipient neuron in mice.
Fig. 1.
(A) Key caustive factors in Parkinson’s disease, (B) Neuropathological features of Alzheimer and parkinsons diseases, (C) Steps of amyloid plaque formation. This Figure was created by Biorender.com with permission number: ES24P6SJ3E.
Mutation of the human α-syn gene leads to an autosomal dominant form of PD through overexpression of α-syn. Furthermore, dysregulation of protein homeostasis mechanisms induces the accumulation of misfolded α-syn. For instance, the mutation in GBA1 causes the loss of-enzymatic activity of GCase (essential in autophagy), resulting in lysosomal substrate accumulation. In addition, the overexpression of mutant GBA1 results in glucosylceramide accumulation and promotes the p62 and α-syn aggregation (Bae et al., 2014, Stojkovska et al., 2018). Moreover, loss-of-function mutations in PARK2 impair parkin E3 ligase activity and promote the aggregation of α-syn and the formation of Lewy bodies (Madsen et al., 2021). This is ascribed to the failure of interaction between mutant Parkin and α-syn, causing early-onset autosomal recessive PD.
Certain species of α-syn are involved in inhibiting mitochondrial protein import by binding to the TOM20 receptor and preventing its co-receptor TOM22 from binding to it, resulting in oxidative stress and impaired mitochondrial functions (Di Maio et al., 2016). These species might also potentiate neuroinflammation via microglial activation and cause elevated production of reactive oxygen species (ROS) (Duffy et al., 2018). Mitochondrial dysfunction originating from exposure to neurotoxins has a potential role in PD pathogenesis. For example, MPTP exposure causes a rapid onset of the parkinsonian phenotype and deterioration in the dopaminergic neurons in the substantia nigra, mainly through inhibiting mitochondrial complex I activity. Additionally, mutations in specific genes such as PARKIN, and PINK1, responsible for the clearance of damaged mitochondria through mitophagy, lead to early-onset autosomal recessive PD (Borsche et al., 2021, Malpartida et al., 2021, Mani et al., 2021).
Inflammatory processes were identified as the main contributors to the pathogenesis of PD. Although neuroinflammation is supposed to be a neuroprotective process and compensate for neuronal damage, its neurotoxic effects can exacerbate the dopaminergic neuronal damage. Neuronal loss is usually accompanied by inflammatory changes in microglia, astrocytes, innate immune cells, and infiltrating peripheral immune cells (Gelders et al., 2018, Mani et al., 2021, Park et al., 2018). Even though the trigger of the immune system is still unknown, debris of degenerating neurons such as human neuro-melanin is suspected of inducing neuroinflammation (Badanjak et al., 2021, Vila, 2019).
According to the Braak hypothesis, the pathological hallmarks of intra-neuronal α-syn aggregations arise from the olfactory bulb and the nerves in the gut. Then α-syn spreads to the brain through the olfactory tract and the vagus nerve. The occurrence of some clinical symptoms, such as constipation ten years before motor symptoms, can further support this hypothesis (Braak et al., 2006, Henderson et al., 2019). Furthermore, bowel inflammation induced by E. coli-producing amyloid protein curli or bacterial products, such as lipopolysaccharide, allows α-syn build up in the gut or brain (Baizabal-Carvallo and Alonso-Juarez, 2020, Chiang and Lin, 2019). Several studies have shown that resecting the vagus nerve and appendix may limit the probability of developing PD (Jankovic and Tan, 2020).
3. Pathogenesis of Alzheimer’s disease
Pathogenesis of AD starts with the sequential processing of amyloid precursor protein by β-secretases (BACE1) and γ-secretases to produce two main types of Aβ polymers, Aβ40 and Aβ42. Aβ42 is more toxic, less soluble, and more aggregation-prone than Aβ40. Gene mutations like PSEN1 and PSEN2 were found to participate in early-onset autosomal-dominant AD through excessive production of the more toxic forms of amyloid as Aβ42 leading to faster progression of neurodegeneration. Aβ undergoes oligomerization and then polymerization into insoluble amyloid fibrils that aggregate into plaques, resulting in neurotoxicity (Fig. 1C) (Shen and Kelleher, 2007, Tiwari et al., 2019). Furthermore, this polymerization results in tau pathology induction through activating kinases, leading to hyperphosphorylation of τ protein and aggregation into insoluble neurofibrillary tangles (NFTs). NFTs are highly insoluble patches in the neuronal cytoplasm that impair communication between neurons and lead to apoptosis (Fan et al., 2020, Grundke-Iqbal et al., 1986, Vergara et al., 2019). The accumulation of plaques and tangles is followed by microglia recruitment around plaques, leading to microglial activation and triggering innate immune responses against Aβ plaques and NFTs (Heneka et al., 2015, Tiwari et al., 2019).
ACh significantly affects cognitive functions and other physiological processes, including memory. The progressive degeneration of cholinergic neurons occurring in the brains of AD patients consequently leads to impaired cognitive function and memory loss. It was also demonstrated that β-amyloid could reduce ACh release and choline uptake. This is possibly due to interactions between ACh esterase and Aβ peptide as well as the neurotoxicity of Aβ oligomers. Additional factors involving the usage of cholinergic receptor antagonists (scopolamine) and reduction in glutamate concentration and d-aspartate uptake might lead to the progression of neuronal damage (Breijyeh and Karaman, 2020, Ferreira-Vieira et al., 2016, Monczor, 2005).
4. Classification of silica
Mesoporous silica is a versatile class of silica with a pore size range between 2 and 50 nm, making it useful for applications such as drug delivery, catalysis, and sensing (Jafari et al., 2019). There are several different types of mesoporous silica, including MCM-41, SBA-15, and SBA-16, each with unique pore sizes and structures that can be tailored for specific applications (Wang, 2009). A typical approach in synthesizing MSNs typically involves a template-assisted approach using surfactants or block copolymers or the evaporation-induced self-assembly method, which combines a silica precursor, surfactant, and solvent to form the desired mesoporous structure (Kumar et al., 2017). The structure of MSNs can be tailored using various structure-directing agents during synthesis, including cetrimonium bromide and poloxamers. For instance, incorporating cetrimonium bromide as a templating agent results in the formation of mobile crystalline material (MCM) systems, while poloxamers lead to the formation of Santa Barbara amorphous (SBA) systems (Narayan et al., 2018). These methods are simple and efficient in creating the ordered mesoporous silica structure. Additionally, there are other types of silica materials, such as nonporous silica, microporous silica, and hierarchical porous silica, each with unique properties and applications. This section will delve into the various types of silica as follows:
4.1. Silica gel
Silica gels can be defined as porous and granular forms of amorphous silicas, consisting of a complex net of microscopic pores that can adsorb water and other organic solvents; as a result, silica gel has been used as a drying agent in dehumidification operations. In addition, silica gel can be applied in chromatography and separation techniques (Loy, 2003, Pourhakkak et al., 2021).
4.2. Fumed silica
The fumed silica manufacturing process includes the combustion of volatile silanes, for example, silicon tetrachloride, in an oxygen-hydrogen flame. The pyrogenic origin nature of the manufacturing process allows fumed silica to have a structure of finely dispersed, aggregated particles and a large surface area with high activity (silanol group). As a result, fumed silica is used in various applications, such as a free-flowing additive in powder-like solids and as an active filler and thickening agent of liquids (Barthel et al., 2005).
4.3. Precipitated silica
Precipitated silica can be produced from a water glass solution followed by adding sulphuric acid under specific conditions. Large particles and tiny particles can be generated by tuning the desired specific surface area. Precipitated silica is mainly used for food manufacture as a flow regulator (e.g. SIPERNAT®) and recently in the pharmaceutical industry (Müller et al., 2008). Furthermore, precipitated silica has been employed in water purification because of its heavy metal adsorption capacity (Agaba et al., 2018).
4.4. Spherical silica
Nonporous amorphous SiNPs are usually used for cosmetics manufacturing and printer toners. Nevertheless, spherical silica has been implemented in developing DDSs in recent years (Xu et al., 2019). Because of their characteristics, including large surface area-to-volume ratio, chemical stability, and ease of surface modification, it was also employed in chromatography and separation techniques (Wei et al., 2012), besides molecular imaging as a platform for contrast agent incorporation (Yong et al., 2009).
4.5. Colloidal silica
Colloidal SiNPs can be produced through the base-catalyzed hydrolysis and polycondensation of tetraethyl orthosilicate (TEOS) in an alcoholic medium with variation in the concentration and ratios of water, ammonia, and TEOS to control the particle size (Arantes et al., 2012). Recently, colloidal nanoparticles have been involved in many technological applications, for instance, the development of opal photonic crystals (Santamaría Razo et al., 2008) and coating material (Rubio et al., 2005).
4.6. Mesoporous silica
MSNs can be synthesized with a template made of micellar rods reacting with TEOS, forming nanosized spheres or rods with a regular arrangement of pores. MSNs exhibit numerous attractive physicochemical properties, including uniform adsorption, biocompatibility, large pore volume to the surface area, easy surface modification and low cytotoxicity. Because of these attributes, MSNs were employed in drug delivery, biosensing and bioimaging (Grumezescu et al., 2013, Lakshmi and Pola, 2020). In Table 1, we discuss several important classes of mesoporous silica that can be used in different drug delivery applications.
Table 1.
Characteristics of different classes and subtypes of mesoporous silica.
| Class | Subtype | Pore volume (cc/g) |
Pore diameter (nm) |
Symmetry | Reference |
|---|---|---|---|---|---|
| MCM | MCM-41 | >1.0 | 1.5–8 | Hexagonal | (Karaman and Kettiger, 2018) |
| MCM-48 | >1.0 | 2–5 | Cubic | (Kruk et al., 2000) | |
| MCM-50 | >1.0 | 2–5 | Lamellar | (Kruk et al., 2000) | |
| SBA | SBA-11 | 0.68 | 5.8 | Cubic (3D) | (Kruk et al., 2000) |
| SBA-12 | 0.83 | 3.1 | Hexagonal (3D) | (Karaman and Kettiger, 2018, Kruk et al., 2000) | |
| SBA-15 | 1.17 | 6–10 | Hexagonal (2D) | (Kruk et al., 2000) | |
| SBA-16 | 0.91 | 5–15 | Cubic | (Karaman and Kettiger, 2018) | |
| FDU | FDU-2 | 0.98 | 2.3–3 | Cubic | (Chircov et al., 2020, Farjadian et al., 2019) |
| FDU-11 | 1.88 | 2.7 | Tetragonal | (Chircov et al., 2020, Poyatos-Racionero et al., 2020) | |
| FDU-12 | 0.27–0.48 | 36 | Cubic | (Chircov et al., 2020, Huang et al., 2010) | |
| FDU-13 | 1.83 | 1.7 | Orthorhombic | (Chircov et al., 2020, Poyatos-Racionero et al., 2020) | |
| KIT | KIT-5 | 0.45 | 9.3 | Cubic | (Narayan et al., 2018) |
| KIT-6 | 1.37 | 10.5 | Cubic (3D) | (Hochstrasser et al., 2020, Kleitz et al., 2003) | |
| Other types | COK-12 | 0.45–1.23 | 5.5–6 | Hexagonal | (Chircov et al., 2020, Karaman and Kettiger, 2018) |
| FSM-16 | 0.96 | 3.2–3.9 | Hexagonal (2D) | (Chircov et al., 2020, Zimowska et al., 2016) | |
| HMM-33 | – | 4–15 | Disordered | (Chircov et al., 2020, Shen et al., 2002) | |
| TUD-1 | 0.5–1.7 | 2.5–25 | Disordered | (Chircov et al., 2020, Heikkila et al., 2007) |
MCM: Mobil Composition of Matter, SBA: Santa Barbara amorphous; FDU: Fudan University; KIT: Korean Advanced Institute of Science and Technology, COK: Centrum voor Oppervlaktechemie en Katalyse/Centre for Research Chemistry and Catalysis, FSM: folded sheets of mesoporous materials, HMM: Hiroshima Mesoporous Material, TUD: Technical Delft University.
Although l-Dopa is considered the agent with high efficacy in treating PD symptoms, its efficacy decreases over time; therefore, larger doses are necessary to maintain the desired response. For this reason, alternative DDSs were developed to achieve a sustained action, slow and continuous release of l-Dopa, and extend its effectiveness (Bardajee et al., 2020, López et al., 2015).
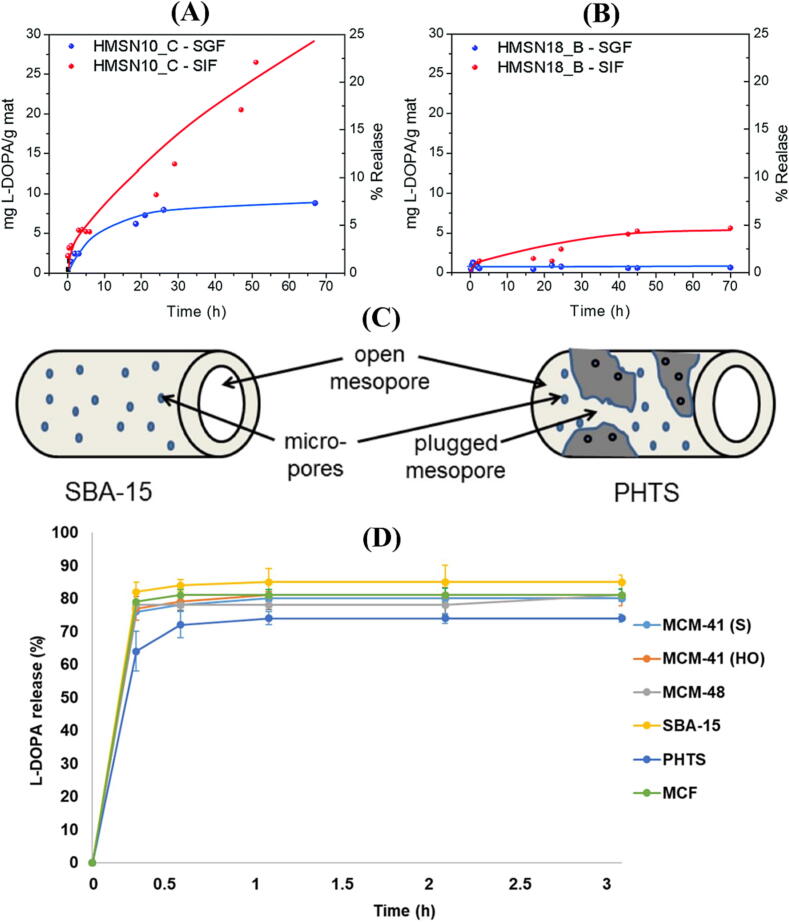
Morales and his colleagues (2021) demonstrated that l-Dopa-loaded MSNs of different shapes and sizes could be prepared by amidation of l-Dopa with fatty acids such as decanoyl chloride and oleoyl chloride to form anionic surfactants. These novel surfactants were designed to form nanomicelles containing l-Dopa as drug-based structured-directing agents. As an advantage of this synthetic process, several steps of surfactant removal and subsequent drug incorporation have been avoided. Additionally, findings of drug release revealed that the developed MSNs-based DDS were pH-responsive since there was hardly any release under acidic conditions in the stomach pH (1.2) (Fig. 2A and B). However, under neutral conditions in the intestinal pH (7.4), l-Dopa was released in a continuous and sustained manner.
Fig. 2.
l-Dopa % release profile from the HMSNs synthetized based on the templates (A) N-decanoyl-l-Dopa, (B) and N-oleyl-l-Dopa. (C) Diagram depicting the pore structure of SBA-15 and PHTS; (D) The l-DOPA % release profile for six different types of MSNs. This Figure was reproduced under Creative Commons license from ref. (Morales et al., 2021, Swar et al., 2019).
Swar et al. (2019) evaluated the efficiency of l-Dopa loading and its in vitro release via UV–vis spectroscopy from six types of mesoporous silica materials [MCM-41(HO), MCM-41 (S), MCM-48, SBA-15, PHTS, and MCF] which are varying in pore diameters and morphologies. As for drug loading capacity per 10 mg of the prepared mesoporous silica materials, SBA-15 had the highest loading capacity amongst all other mesoporous silica particles. MCF was found to have the second-highest loading capacity due to its large pore size. The loading capacity of MCM-41(S), MCM-41(HO) and MCM-48 were similar as their pore sizes were relatively similar, while PHTS had the least loading capacity among all types, possibly because of plugged pores, as seen in Fig. 2C. The release of l-Dopa was sustained, and SBA-15 released about 85 % in 1 hr, which was the highest amount. The l-dopa release from MCF and the other three types of MCM was (83 %, 81 %, 80 %, and 78 %, respectively), while l-Dopa was released in the least amount from PHTS (74 %) (Fig. 2D).
5. Neurotoxicity of mesoporous silica nanoparticles
BBB is a highly effective barrier that protects the most delicate and complex organ in the human body, the brain (Nair et al., 2018). This complex system consists of endothelial cells, pericytes, astroglia, perivascular mast cells and basal lamina and controls the flow of ions, chemicals, and macromolecules from the blood to the brain. This barrier protects brain cells from toxic substances and circulating medications in the blood and other body fluids (Urayama and Banks, 2006). There are several ways in which nanoparticles can cross the BBB, including i) By expanding tight junctions between endothelial cells or imparting local toxicity resulting in penetrating the BBB and delivering the drug either bound or free of NPs; ii) by transcytosis; iii) by endocytosis, or iv) NPs may follow one mechanism or a combination of the previously mentioned mechanisms, however mechanisms (ii), (iii), and (iv) are considered to be the prevailing ones (Mendiratta et al., 2019, Saraiva et al., 2016).
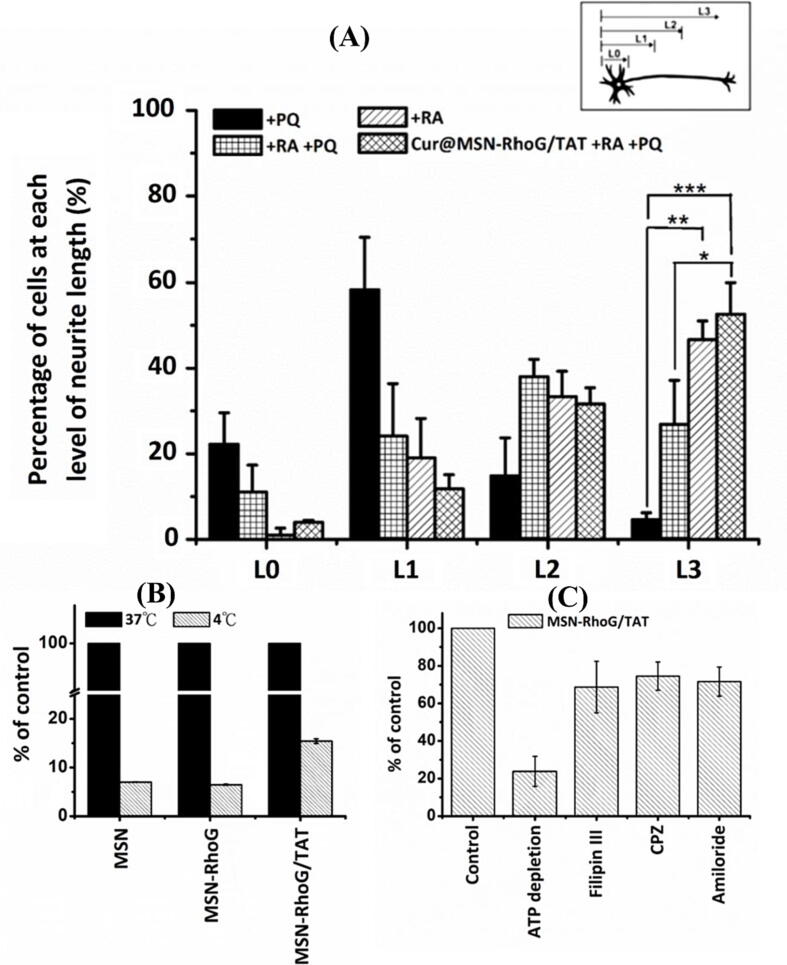
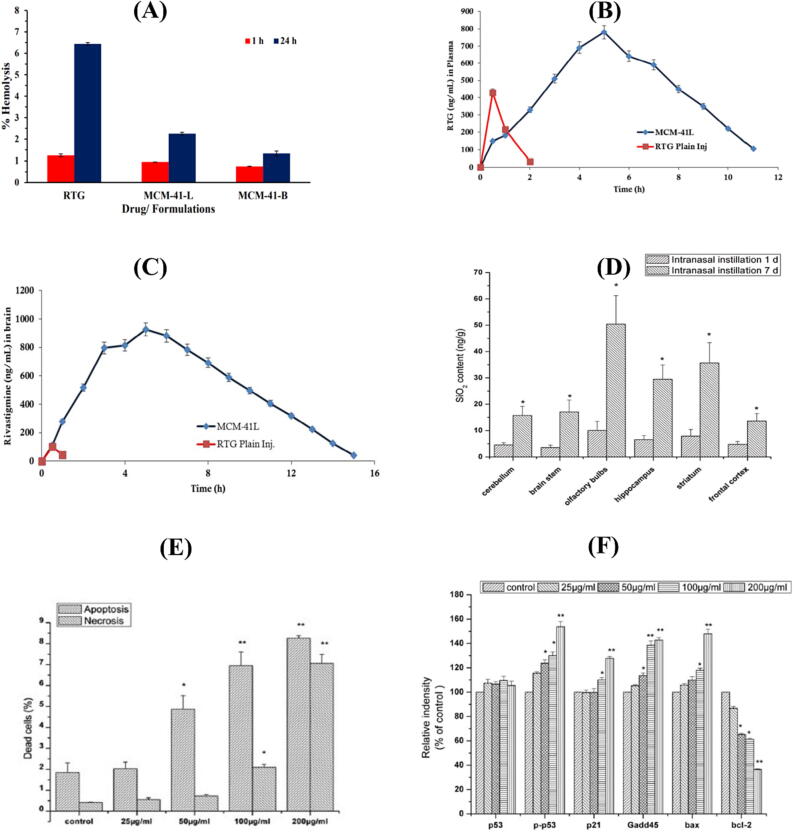
Due to their adequate biocompatibility, silica-based nanostructures were proposed for many biomedical applications (Rosenholm et al., 2010). MSNs exhibit well-defined structures with high specific surface area and large pore sizes. Despite their characteristic physicochemical properties, MSNs also raise concerns about their safety (Tang et al., 2012). In light of the scant evidence, evaluating the extent of neurotoxicity caused by such tiny nanoparticles is not feasible. It is still essential to assess the safety and toxicity of MSNs prior to any clinical applications (Landsiedel et al., 2012). A study by Cheng et al. (2019) examined neuron-related disorders by exposing Neuro-2a cells to various concentrations of MSN for 4 hr. MSN-treated N2a cells were then evaluated by neurite length distribution and flow cytometry to assess the cellular absorption effectiveness, and the outcomes demonstrated increased neurite growth and dose-dependent cell uptake (Fig. 3A and B). Based on the water-soluble tetrazolium-1 assay results, MSNs demonstrated no substantial impact on cell viability. Pandey et al. (2018) investigated the effect of Rivastigmine and MCM-41L nanoparticles on hemolysis in a time-dependent manner. Nonetheless, the toxicity was significantly lower than that of Rivastigmine in its pure form (Fig. 4A). This result was ascribed to the MCM-41L NPs shielding the drug after encapsulation and/or the encountered steric hindrance. This system improved the bioavailability and brain delivery of Rivastigmine without causing substantial hemolysis (Fig. 4B and C).
Fig. 3.
(A) Neurite length distribution following treatment with paraquat (PQ), Retinoic acid (RA), their combination alone or in conjugation with curcumin loaded-MSNs (*, p < 0.05; **, p < 0.01; ***, p < 0.001). (B) A flow cytometric analysis for cellular uptake of MSNs, MSNs-RhoG, and MSN-RhoG/TAT at 37 and 4 °C. (C) Investigation of the cellular uptake mechanism of MSN-RhoG/TAT in N2a cells in the presence of chemical inhibitors for ATP depletion (sodium azide and 2-deoxyglucose), and endocytosis inhibitors (filipin III, chlorpromazine, and amiloride). This Figure was reproduced under Creative Commons license from ref. (Cheng et al. 2019).
Fig. 4.
(A) Observations of the percentage hemolysis as a function of time for Rivastigmine, blank (MCM-41B) and Rivastigmine-loaded MCM-41 (MCM-41L); (B) In vivo pharmacokinetic plasma profile of Rivastigmine and MCM-41L and (C) In vivo brain distribution of Rivastigmine and MCM-41L in Wistar rats. (D) An assessment of the content of SiNPs in the brains of rats (n = 6) following 1 and 7 days of intranasal instillation of SiNPs. (E) Dose-dependent apoptosis and necrosis in PC12 cells induced by SiNPs. (F) A western blot densitometric analysis of SiNP-induced expression of p53, p-p53, p21, Gadd45, bax, and bcl-2 in PC12 cells. This Figure was reproduced under Creative Commons licenses from ref. (Pandey et al., 2018, Wu et al., 2011).
In their study, Wu and his colleagues (2011) evaluated the toxic effect of SiNPs on the brain. In the in vivo studies, they administered SiNPs to adult rats via nasal instillation. SiNPs exhibited a unique distribution in the brain in which the striatum seems to be a major accumulation site for these particles (Fig. 4D). This can increase ROS production in the striatum leading to functional damage. Additionally, SiNPs may be involved in depleting dopaminelevels. On the other hand, SiNPs were proven to induce oxidative stress and exert cytotoxic damage in PC-12 cells via apoptosis rather than necrosis at a concentration beginning from 50 µg/ml (Fig. 4E) (Wu et al., 2011). Besides, SiNPs were involved in causing apoptosis via the activation of the p53 pathway (Fig. 4F).
Moreover, several studies highlighted that MSNs could cause microglia activation, which in turn could induce an inflammatory response and generate ROS and reactive nitrogen species, which might negatively affect cell viability (Choi et al., 2010, Tambuyzer et al., 2009). Further, MSNs are able escape the BBB and accumulate in specific brain regions, including the striatum and hippocampus, where it could trigger certain deleterious effects in the in vivo studies (Halliwell and Gutteridge, 2015, Kishido et al., 2007, Zhou et al., 2016). The accumulation of MSN in these brain regions led to oxidative stress and inflammatory changes, which could cause substantial lipid peroxidation and are believed to possess deleterious effects on dopaminergic neurons.
6. Implications of MSNs as toxic fibrils scavengers
The imbalance between the high production of ROS and the scavenging activities leads to oxidative stress, thus could cause damage to DNA, RNAs, and proteins leading to several chronic and degenerative disorders (Avramouli et al., 2015). Meanwhile, this can cause neurological degeneration and toxicity in dopaminergic neurons in PD (Guo et al., 2018). In a recent study, dopamine-containing polymers were formulated using Michael-type addition, and then the polymer was photo-crosslinked via the water-in-oil emulsion technique to form microspheres (Newland et al., 2016). In non-toxic concentrations, these microspheres showed excellent scavenging activity and reduced the dissolved oxygen in physiological solutions.
In addition to facilitating intercellular communication, exosomes were proven to have scavenging capability, which could be harnessed to reduce the burden of Aβ-peptide and increase its clearance in mice brains suffering from AD (Yuyama et al., 2014). Additionally, exosomes counteract Aβ-induced disruptions and apoptosis in the genetically modified neuroblastoma cell line (N2a cells); this scavenging effect is due to the binding of its glycosphingolipid with Aβ-glycan (Soliman et al., 2021).
Viswanathan et al. (2014) modified SBA-15 mesoporous silica by covalently linking thioflavin-S with the iron oxide in its matrix. In their in vitro studies, they applied a magnetic field (fishing) strategy to the previously mentioned nano-formulation, in which the process of targeting and separating the plaque was achieved. This magnetic nanoconjugate exhibited the ability to eradicate the KLVFF peptide, which is a recognition motif in Aβ that has been involved in plaque formation. This treatment strategy cannot be considered a complete cure for AD since another drug must be loaded into the mesoporous matrix to disrupt aggregation.
Wang et al. (2019) formulated silica–cyclen nanocomposite using cyclen as a metal chelator and SiNPs as a carrier, and it was tested on PC12 cells. The results showed that the SiO2–cyclen effectively inhibited Aβ aggregation by reducing ROS produced by the Cu–Aβ40 complex and protected cells from metal-induced Aβ toxicity. Moreover, the in vivo results showed that the SiO2–cyclen nanocomposite could pass BBB.
Recently, Sant et al. (2021) applied silica nanobowls (NBs) with a lipid-polymer coating to facilitate the isolation of transient and soluble Aβ aggregates. Silica NBs were composed of a silica core coated with a lipid polymer coating of a mixture of 1, 2-dioleoyl-sn-glycero-3-phosphoethanol-amine and polymer (Poly (N-isopropyl acrylamide)) to prevent nonspecific protein adsorption. When NBs were incubated with neuroblasts containing Aβ, 75 % of Aβ was adsorbed to NBs. However, the adsorption to NBs was shown to be reversible by mechanical agitation, and the Aβ aggregation driving domain was unaffected by NBs during scavenging. In this way, NBs can be implemented to characterize and diagnose AD and other NDDs by time-resolved separation of toxic Aβ species from biological samples.
Jung and his colleagues (2020) targeted Aβ peptides via anti-Aβ single-chain variable fragments entrapped into ultra-large pore MSNs with attenuated toxicity. The in vitro investigations showed that Aβ nanodepletors could cause a notable decline in the Aβ-monomers aggregation in a concentration-dependent manner and effectively mitigate Aβ-induced neurotoxicity. Furthermore, the in vivo evaluation of weekly stereotaxic injections of Aβ nanodepletors into the brain of AD mice showed a reduction in Aβ levels by 70 % (Jung et al., 2020). Moreover, Ma and his colleagues (2018) formulated a redox-activated near-infrared (NIR) responsive polyoxometalates-based nanoplatform and examined it in PC12 cells. During the study, polyoxometalates were able to absorb NIR lasers and generate local hyperthermia to disaggregate Aβ fibrils. The polyoxometalates could reach to the brain via receptor-mediated transcytosis and showed antioxidant activity via scavenging the Aβ-induced ROS.
Furthermore, Taebnia et al. (2015) assessed the influence of surface chemistry and concentration of MSNs on the fibrillation of recombinant human α-syn protein in PC12 cell line. They found that α-syn fibril formation can be reduced by using positively charged MSNs, such as 3-(2-aminoethyl amino) propyl trimethoxysilane and polyethyleneimine-MSNs. The results were primarily influenced by electrostatic interactions between the positive charge on PEI-MSNs and the negative charge on α-syn protein preventing the protein aggregation. In contrast, aggregation and fibril formation were induced by the repulsion between negatively-charged carboxyl-MSNs and α-syn protein. It was also shown that increasing MSNs charges and concentrations resulted in greater inhibition or acceleration of fibrillation of α–syn.
7. Mesoporous silica nanoparticles as a drug carrier in NDDs
Various barriers exist to CNS drug delivery, including the BBB, limited dissolution, insufficient bioavailability, and a lack of targeting ligands. On the other hand, nano-formulation-based DDSs provide several advantages as improving dissolution, absorption, bioavailability, and lowering side effects as a result of reaching the desired site of action (Kumar et al., 2012, Mendiratta et al., 2019). In Table 2, potential applications of MSNs in treating Alzheimer's and Parkinson's disease are presented.
Table 2.
The application of MSNs as nanocarriers in Alzheimer’s and Parkinson’s disease.
| Drug | MSN type | Loading technique | Comment | Ref |
|---|---|---|---|---|
| Rivastigmine | MCM-41 | Solvent-adsorption equilibrium | MCM-41 nanoparticles improved the delivery of Rivastigmine to Wistar rats brain by 127-fold in vivo and improved bioavailability by 12.3-fold (Fig. 3B). Also, the formulation achieved brain concentration of Rivastigmine was 9-times greater than the untreated drug (Fig. 3C) | (Pandey et al., 2018) |
| Quercetin | N/A | The release of Quercetin from PEG3000-modified silica NPs was higher than CTAB-modified silica NPs | (Nday et al., 2015) | |
| Magnetic silica nanospheres | Nano-encapsulation does not affect the multifaceted biological action of quercetin, which is an antioxidant and anti-amyloid | (Halevas et al., 2020) | ||
| l-Dopa | MCM-41, MCM-48, SBA-15, PHTS, MCF. | SBA-15 showed the fastest l-Dopa release profile | (Swar et al., 2019) | |
| N/A | SDA removal and drug-loading steps were avoided | (Morales et al., 2021) | ||
| Berberine | MCM-41 | Passive method | Berberine inhibited the expression of BACE1, which resulted in curbing Aβ40/42 formation | (Singh et al., 2021) |
| Pramipexole | MCM-41 | Solvent impregnation with post-coating step | Pramipexole loading to surface-coated MCM-41 exhibited efficiency in reducing H2O2-induced toxicity in neuronal SH-SY5Y cells as well as oxidative damage in PD | (Tzankov et al., 2019a) |
| l-Dopa methyl ester HCl | N/A | Rotary evaporation | When the hydrophobicity of silica was increased, the drug release rate was significantly reduced (p < 0.05) | (Kiss et al., 2021) |
N/A: Not applicable (The specific type of silica nanoparticle was not stated).
7.1. Enhancing the drug dissolution rate
Many pharmaceutical products are poorly water-soluble and are limited by their poor dissolution rate and low oral bioavailability (Boyd et al., 2019; Attia et al., 2021a). In order to overcome the drawbacks of these pharmaceuticals, nanomaterials with non-ordered porous structure were employed as carriers (Baumgartner and Planinšek, 2021). The mechanism beyond these nanomaterials acts by confinement of the active principals into nanopores; therefore, their dissolution rate is enhanced (Baumgartner and Planinšek, 2021). Nday et al., (2015) loaded Quercetin as a poorly water-soluble antioxidant into MSNs and tested the effect of this nano-formulation on Cu+2-induced oxidative stress in AD through hybrid nanoparticles. Two functionalized types of silica xerogels (CTAB and PEG3000) were used for Quercetin loading and observed for targeting the viability of neuronal and glial rat primary hippocampal cell cultures against CuGly, as a source of Cu (II). The Quercetin-specific release mechanism was not determined, but it was suggested that the burst release might be due to the irregularity in the xerogels' shape and attributable to the increased surface area of xerogels exposed to the surrounding release medium (Prokopowicz, 2007). However, the fast release of Quercetin was detected in the first 60 min, and then the release rate declined. The internal and external structure of the pores and the CTAB- or PEG3000-modified silica matrix interaction determined the cumulative Quercetin release. The modified silica had internal pores and surface area, so the drug particle resides approximately on its surface (Liong et al., 2008). Moreover, it was assumed that Quercetin-PEG3000 interaction was lower than Quercetin-CTAB interaction; as a result, a high release of Quercetin from PEG3000-modified silica particles was detected. This system led to several advantages, including the bioavailability enhancement of Quercetin, and a featured result was the protective effect of Quercetin-PEG against the CuGly in a low concentration.
7.2. Sustaining the drug release rate
The physicochemical properties of nanoparticles, such as size, shape, surface charge, and composition, play a crucial role in determining their pharmacokinetics within the human body, including factors such as the circulation time course and absorption rate (Ernsting et al., 2013). Sometimes, it could be preferable to lessen the absorption rate to get a more acceptable clinical response (Jani et al., 2009). When it is applicable to produce a prodrug on a molecular level, a product requiring less frequent administration to achieve the desired biological activity within the target time profile. Additionally, limited side effects, fluctuation of drug levels in blood and plasma, and enhanced patient compliance are other advantages of sustained drug delivery (Kumar et al., 2012). The desired efficacy may arise from more localization of the drug, enhanced drug bioavailability, or a sustained period of action (Dixit et al., 2013). The sustained-release delivery systems were intensely studied for many years and yielded promising solutions within the pharmaceutical area (Shargel et al., 1999). Furthermore, a drug delivery approach can attain an extended therapeutic effect via the gradual release of the active ingredient over a prolonged period (days to months) after certain modifications to the formulation (Reddy and Rao, 2015). For these reasons, there is increasing demand for novel sustained-release strategies to replace traditional therapies (Natarajan et al., 2014).
MSNs were reported to achieve higher loading capacity, inhibit the variation of drug concentration in the blood and decrease side effects. In theory, when a cap covers MSNs at the molecular level, the capping molecule demonstrates the ability to control drug release (Vivero‐Escoto et al., 2010). Therefore, upon exposure to suitable conditions (light, enzymatic, pH variations), the susceptible linkages will be broken and liberate entrapped molecules (Attia et al., 2021b). Singh et al., (2021) synthesized MCM-41 via the Stöber process, loaded them with Berberine, an isoquinoline alkaloid, using the “passive method”, and finally covered them with liposomes coating following the thin-film hydration method. The lipid coating on these MSNs was believed to introduce a physical barrier for Berberine against the physiological buffer, thus leading to a delayed drug release (Zhou et al., 2017). The synthesized MSNs-Berberine displayed a significantly higher (p < 0.05) Acetylcholinesterase inhibitory activity than the MCM-41 and pure berberine solutions. Through the down-regulation of BACE1 expression, the lipid-coated Berberine-loaded MCM-41 exhibited antioxidative activities, decreased the malondialdehyde level, and inhibited the amyloid fibrillation in AD.
Similarly, Tzankov et al., (2019a) studied the release of pramipexole loaded by solvent impregnation method into MCM-41 particles and coated with chitosan and/or sodium alginate. Chitosan, a polysaccharide, facilitates the transportation of polar drugs across epithelial membranes (Illum, 2002). Pramipexole prevents H2O2-induced oxidative damage without free radical formation in human neuroblastoma SH-SY5Y cells (Tzankov et al., 2019b). In uncoated MCM-41, the total amount of pramipexole was released within the first 15 min. The double-coated particles reached full release after 300 min, which was explained by electrostatic interaction between components with a negative charge on the surface of MCM-41 with the positively charged chitosan. At the same time, chitosan-coated particles haved attracted an extra coating with negatively charged sodium alginate.
Kiss et al. (2021) loaded l-Dopa methyl ester hydrochloride into surface-modified mesoporous silica (Syloid) using a rotary evaporator. To tune the surface hydrophobicity, trimethylchlorosilane was used, which led to a significant reduction in l-Dopa release (p < 0.05). The hydrophobized silica particles can be applied for the active ingredients with a narrow therapeutic index since the resulting optimized formulations could possess zero order release kinetics, which can achieve steady blood levels of l-Dopa. This would be purposeful for shortening wearing-off periods and reducing the side effects for PD patients (Antonini et al., 2007, Karkossa and Klein, 2019).
7.3. Brain-targeted delivery
Although nanomaterials enabled researchers to develop DDS with desired physicochemical properties; however, surface functionalization and surface coating flexibility are essential features of an ideal nanocarrier used in targeted drug delivery (ud Din et al., 2017). A mesoporous nanoparticle is one of these nanocarriers, with a particle size of 50–200 nm and a surface area from 700 to 1000 m2/g. Additionally, these particles have internal mesopores of 2–6 nm, resulting in a high pore volume from 0.6 to 1 cm3/g. As a result, they are considered ideal candidates for targeted drug delivery (Vallet-Regí et al., 2017). There are active and passive targeting strategies for nanoparticles; passive targeting mainly depends on the particle's properties, including its size, charge, and permeability (Rabanel et al., 2012). While in active targeting approaches, the targeting moieties are employed to deliver the cargo to their target sites (Castillo et al., 2019).
A nanoplatform designed by Cheng et al. (2019) to deliver Curcumin and RhoG, a type of the Rho family GTPase involved in lamellipodia and filopodia creation that enhances neurite outgrowth, so they formulated an MSN-based nanocarrier and loaded both Curcumin, and adsorbed plasmid RhoG-DsRed/TAT peptide complex. In addition to promoting its uptake through cells via energy-independent non-endocytosis routes and endocytosis (Fig. 3C), TAT peptide was introduced to the plasmid by electrostatic interaction because it enhances gene expression through nuclear delivery. The Plasmid RhoG-DsRed/TAT complex served as a non-covalent gatekeeper by inducing Curcumin release when the complex is dissociated. In this study, Curcumin@MSN-RhoG/TAT was found to perform in a manner that protected N2a cells from Paraquat-induced ROS damage through the delivery of Curcumin as an anti-inflammatory agent. Further, Cur@MSN-RhoG/TAT has successfully increased the percentage of cells with longer neurite and protected against ROS damage in N2a cells (Fig. 3A).
A sol–gel process was used by Halevas et al. (2020) to develop modified magnetic core–shell mesoporous silica nano-formulations (MMSNs) loaded with Quercetin. The magnetic core was produced using surface-modified monodispersed magnetite colloidal superparamagnetic nanoparticles. The surface was then modified with PEG3000. Physicochemical characteristics and Quercetin biological activity of the hybrid nanocarriers were evaluated. Results showed that these nano-formulations were exceptionally stable in biological conditions. They also exhibited improved solubility and bioavailability of the loaded Quercetin, which retained its antioxidant and anti-amyloid properties. The generated MMSNs also retained the superparamagnetic properties of the employed surface-modified magnetic NPs core, which makes magnetic targeting applications possible.
7.3.1. Internally triggered drug release
Researchers have been developing novel delivery systems for drugs so as to overcome the limitations of conventional drugs. The drug is usually released in an uncontrolled manner; however, the release behaviour in the responsive DDS is initiated and then modulated by the action of external factors, including physical, chemical, and biological stimuli (Kalhapure et al., 2015). Several mesoporous materials were designed to respond to changes in pH, redox potential, and enzymatic activity, providing “on-demand” drug release (Attia et al., 2021b). Therefore, the designed systems that respond to stimuli show promising results in controlling drug release and bio-distribution.
7.3.1.1. ROS-responsive DDS
ROS is an unavoidable byproduct that is imperative in regulating biological and physiological processes. Therefore, these oxygen species can cause oxidative stress inside cells, which was used as the cornerstone for a specific class of responsive DDSs with a high potential to distribute the drug to target tissues with high ROS levels. In ROS-responsive DDS, the major mechanism of release can be attributed to changes in carrier solubility, carrier cleavage, or prodrug linker cleavage (Liang and Liu, 2016).
Numerous studies have been ingestigated the probability of using Clioquinol to reduce Aβ deposits. Geng et al., (2012) loaded Clioquinol into MSNs modified with arylboronic acids derivative and covered it with human IgG. The H2O2 resulting from Aβ aggregates causes breakage in the arylboronic esters; therefore, the capping IgG and the confined Clioquinol escape. Following its release from nanopores, Clioquinol chelates Cu2+ to disassemble Aβ plaques. However, the release of Clioquinol was limited to high H2O2 levels, such as in Aβ plaques. To avoid this limitation, Yang et al., (2016) formulated MSNs that were capped with gold nanoparticles and loaded them with Clioquinol for targeting purposes. In PC12 cells, this nano-formulation inhibited Cu2+-induced Aβ40 aggregation and exhibited a controlled release of Clioquinol.
7.3.1.2. Enzyme-responsive DDS
In most biochemical reactions, enzymes play a significant role by acting as biological catalysts. On the other hand, many diseases have been linked to abnormal enzyme expression levels (Shahriari et al., 2019). As a result, enzyme-triggered nanomaterials have recently gained considerable attention because of their various applications in the medical field. For instance, if certain enzymes are present, the nanosized DDS can exhibit a high sensitivity by releasing their active ingredients (Guo et al., 2012).
Godoy-Reyes et al., (2019) developed nanocarriers that are responsive to acetylcholinesterase. MSNs were capped with acetylcholinesterase and linked through boronic ester. In case ACh is present at a sufficient level, the neurotransmitter will be degraded by acetylcholinesterase into choline and acetic acid, which hydrolyze boronic cyclic esters. The designed nanocarrier delivers its payload within <5 min in the presence of ACh. Moreover, the nano-formulation showed selectivity to ACh while keeping the cargo entrapped in the presence of epinephrine and other neurotransmitters. ACh levels in human blood do not cause significant cargo release, whereas a payload release was observed in neuromuscular junctions, synaptic cleft and synaptic vesicles in PD patients, where the ACh concentration ranges from 0.025 to 10 mM. Besides, Llopis-Lorente et al., (2017) developed ACh-controlled Janus-type MSNs modified with acetylcholinesterase on a gold interface and a supramolecular pH-responsive nano valve (β-cyclodextrin-benzimidazole). Providing that ACh is present, the acetylcholinesterase were able to induce the unravelling of the nanovalve, and therefore, the cargo is released. Their cargo delivery was fast and selectively responsive to Ach. This nanodevice responded to Ach concentrations > 10 mm, which is lower than the ACh levels in the blood and within the concentration range present in neuromuscular junctions and the striatum.
Zhou et al., (2014) developed calixarene and pillarene-based DDS with the aim of reducing motor symptoms in PD patients. The cargo release was in response to ACh as a competitive binding agent via operating on the supramolecular nanovalves, while the amount and the speed of the drug release depended mainly on the ACh level or a changing in the sort of gating macrocycle. The study results showed that pyridine-modified MSN was preferred to 3-chloropropyl-modified MSN. That is because pyridine-modified MSN maintained its porosity and was attached to the surface of the silica using a covalent bond. Following that, a florescent dye, Rhodamine 6G, was added to both systems. Release studies proved that Rhodamine 6G-loaded calixarene-based DDS responded well to the external ACh. Nevertheless, Rhodamine 6G-loaded pillarene-based DDS needed a higher ACh concentration to release the drug. Moreover, both systems exhibited negligible premature release of the active ingredient.
7.3.1.3. PH-responsive DDS
pH-Responsive nanoplatforms have gained more focus because of their ability to control the drug release pattern based on a specific stimulus. These synthesized DDSs are designed to respond to changes in pH by releasing the active ingredient in a controlled manner and at its site of action. Consequently, this can result in enhancing the therapeutic efficacy of various drugs. For instance, cancer tissue is known to have a lower pH than normal tissue. Thus, a pH-responsive nanocarrier can detect this change in pH and release the anti-cancer agent via lysis of the acid-labile linkage (Deirram et al., 2019, Song et al., 2017).
The possibility of preparing MSN enclosed with l-Dopa was demonstrated. The concept was applying l-Dopa as a structure-directing agent via amidation with fatty acids such as decanoyl chloride or oleoyl chloride to form anionic surfactants that could be used to produce micelles. The results proved that prepared MSNs were pH-responsive since there was hardly any release under acidic conditions in the stomach (pH = 1.2), while at the neutral conditions in the intestines (pH = 7.4), a continuous and sustained manner was observed (Morales et al., 2021).
7.3.2. Externally triggered drug release
7.3.2.1. Electric-responsive DDS
Electric responsive nanocarriers have recently emerged as a novel approach for the treatment of serious diseases, in particular, cancer and neurodegenerative disorders. These systems can release their load in different ways, for example, sustained, pulsed or on demand when triggered by external electric impulses. Furthermore, using electric impulses can stimulate cell fusion and improve the permeability of cells to the active ingredient (Kolosnjaj-Tabi et al., 2019).
The combination of non-pharmacological and pharmacological approaches (Clioquinol) has improved cells' survival and neurite growth. Wu and his colleagues (2015) synthesized an electrically responsive drug release system by embedding Clioquinol into graphene-MSNs nanohybrids, which possess a high loading capacity and a sustained release profile. An electropolymerized polymer polypyrrole film was then used to seal the pores (Wu et al., 2015). It was demonstrated that electrical stimulation could enhance drug release and achieve better inhibition of Aβ-Cu2+-induced Aβ aggregation. Aside from this, electrochemical stimulation has shown efficacy in vitro and/or in vivo for stimulating neurite and axon extension. Following the Clioquinol release from polypyrrole/graphene-MSNs films, cell death under electrical stimulation and cytotoxicity were reduced.
7.3.2.2. Near-Infrared radiation-responsive DDS
NIR-responsive DDSs are considered one type of the novel smart drug delivery systems. These systems depend on NIR light to achieve drug release at a particular site and time with a high level of convenience. In addition to the on-demand release, NIR-responsive DDS exhibits good dermal penetration. The mechanism of this responsive DDs depends on the absorption of the NIR light and converting NIR to heat by certain photosensitizers such as gold. As a result, the temperature elevation in the surrounding microenvironment triggers drug release (Li et al., 2019).
Liu et al., (2020) developed a novel platform to enhance the permeability of BBB via loading Quercetin on mesoporous silica-encapsulated gold nanorods with yolk-shell structures. In the in vitro studies, a model of BBB was developed using transwell inserts cultured with cerebral endothelial cells and astrocyte-like cells. The study results proved that following NIR-II irradiation, the passage of Quercetin through the BBB indicated that there was an enhancement in the permeability of BBB via photo-thermal conversion. Furthermore, upon I.V. injection of these nanoplatforms followed by NIR-II irradiation, they were not only able to improve the Quercetin accumulation in the brain but also were able to limit the neurological abnormalities in PD model mice in vivo.
8. Future perspective
Mesoporous silica has shown promising potential in various clinical studies, particularly as a drug delivery platform and in dental implant therapy. Clinical research has demonstrated that using tetracycline-loaded MSNs in dental implant therapy can significantly reduce the bacterial count of the implant-abutment junction and improve implant stability (Mirzaali et al., 2023). Furthermore, this approach has shown success in the drug delivery of a single dose of fenofibrate, which was administered to healthy volunteers to tackle solubility-related bioavailability problems with better clinical efficacy (Bukara et al., 2016). Throughout this review, it has been shown that MSNs can effectively serve as carriers for therapeutics in the treatment of AD and PD with the ability to scavenge Aβ peptides and α-syn, which are known to play a significant role in the development and progression of these diseases. Despite these promising results in preclinical studies, the prolonged use of MSNs in treating these chronic conditions is still questionable and might raise concerns about potential toxicity. A recent clinical trial study by Hagman et al. (2020) evaluated the safety of oral dosing of mesoporous silica in male participants. The study found that oral consumption of mesoporous silica up to 9 g daily was safe and did not result in any major adverse events or safety concerns. This suggests that mesoporous silica may be a viable food additive for weight loss in future studies, with a good safety profile. Furthermore, in vivo studies have shown that hollow MSNs are biodegradable with low toxicity and have no significant adverse effects on vital organs, providing a promising avenue for their use in various biomedical applications (Kong et al., 2017). However, further research is required to fully elucidate the applicability and long-term safety of these nanostructured materials and to ensure that the possible risks are minimized.
9. Conclusion
Mesoporous silica particles have been extensively investigated as a viable drug delivery platform for treating Alzheimer's disease and Parkinson's disease, owing to their high surface area-to-volume ratio, tunable pore size, and ability to traverse the blood–brain barrier effectively. Furthermore, mesoporous silica nanoparticles were observed to act as nanoscavengers and have been proposed as a potential therapy for these neurodegenerative disorders as they can bind and remove toxic molecules from the brain, such as amyloid beta peptides in Alzheimer's disease and α-synuclein in Parkinson's disease. However, it is important to note that while these studies have yielded promising results, further research is required to fully elucidate the potential safety risks of this type of structured nanoparticles. As such, caution should be exercised when considering the use of mesoporous silica particles in clinical applications, and toxicity assessments should be conducted prior to any upcoming clinical application.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
The authors acknowledge biorender.com for the design of the figures making.
Author contribution
All authors contributed to the conception of this review and its design. Attia MS and Sabry SA. planned and structured the review. Attia MS, Yahya A. and Nada A. created the first draft and all the illustrations. In previous versions of the manuscript, all authors provided their comments. The final manuscript was read and approved by all authors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agaba A., Cheng H., Zhao J., Zhang C., Tebyetekerwa M., Rong L., Sui X., Wang B. Precipitated silica agglomerates reinforced with cellulose nanofibrils as adsorbents for heavy metals. RSC Adv. 2018;8:33129–33137. doi: 10.1039/C8RA05611K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A., Isaias I.U., Canesi M., Zibetti M., Mancini F., Manfredi L., Dal Fante M., Lopiano L., Pezzoli G. Duodenal levodopa infusion for advanced Parkinson’s disease: 12-month treatment outcome. Mov. Disord. 2007;22:1145–1149. doi: 10.1002/mds.21500. [DOI] [PubMed] [Google Scholar]
- Arantes T.M., Pinto A.H., Leite E.R., Longo E., Camargo E.R. Synthesis and optimization of colloidal silica nanoparticles and their functionalization with methacrylic acid. Colloids Surf. A: Physicochem. Eng. Asp. 2012;415:209–217. doi: 10.1016/j.colsurfa.2012.09.041. [DOI] [Google Scholar]
- Assa F., Jafarizadeh-Malmiri H., Ajamein H., Vaghari H., Anarjan N., Ahmadi O., Berenjian A. Chitosan magnetic nanoparticles for drug delivery systems. Crit. Rev. Biotechnol. 2017;37:492–509. doi: 10.1080/07388551.2016.1185389. [DOI] [PubMed] [Google Scholar]
- Attia M.S., Ali Hasan A., Ghazy F.-E.-S., Gomaa E. Solid dispersion as a technical solution to boost the dissolution rate and bioavailability of poorly water-soluble drugs. IJPER. 2021;55:s327–s339. doi: 10.5530/ijper.55.2s.103. [DOI] [Google Scholar]
- Attia M.S., Hassaballah M.Y., Abdelqawy M.A., Mohamed M.E., Farag A.K., Negida A., Ghaith H., Emam S.E. An updated review of mesoporous carbon as novel drug delivery system. Drug Dev. Ind. Pharm. 2021:1–17. doi: 10.1080/03639045.2021.1988097. [DOI] [PubMed] [Google Scholar]
- Avramouli, A., Theocharopoulou, G., Vlamos, P., 2015. Detection of oxidative stress in neurodegenerative diseases. Presented at the 2015 IEEE International Symposium on Signal Processing and Information Technology (ISSPIT), IEEE, pp. 392–396.
- Badanjak K., Fixemer S., Smajić S., Skupin A., Grünewald A. The contribution of microglia to neuroinflammation in Parkinson’s disease. Int. J. Mol. Sci. 2021;22:4676. doi: 10.3390/ijms22094676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae E.-J., Yang N.-Y., Song M., Lee C.S., Lee J.S., Jung B.C., Lee H.-J., Kim S., Masliah E., Sardi S.P. Glucocerebrosidase depletion enhances cell-to-cell transmission of α-synuclein. Nat. Commun. 2014;5:1–11. doi: 10.1038/ncomms5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahbah E.I., Ghozy S., Attia M.S., Negida A., Emran T.B., Mitra S., Albadrani G.M., Abdel-Daim M.M., Uddin M., Simal-Gandara J. Molecular mechanisms of astaxanthin as a potential neurotherapeutic agent. Mar. Drugs. 2021;19:201. doi: 10.3390/md19040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baizabal-Carvallo J.F., Alonso-Juarez M. The link between gut dysbiosis and neuroinflammation in Parkinson’s disease. Neuroscience. 2020;432:160–173. doi: 10.1016/j.neuroscience.2020.02.030. [DOI] [PubMed] [Google Scholar]
- Barandeh F., Nguyen P.-L., Kumar R., Iacobucci G.J., Kuznicki M.L., Kosterman A., Bergey E.J., Prasad P.N., Gunawardena S. Organically modified silica nanoparticles are biocompatible and can be targeted to neurons in vivo. PLoS One. 2012;7:e29424. doi: 10.1371/journal.pone.0029424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardajee G.R., Khamooshi N., Nasri S., Vancaeyzeele C. Multi-stimuli responsive nanogel/hydrogel nanocomposites based on κ-carrageenan for prolonged release of levodopa as model drug. Int. J. Biol. Macromol. 2020;153:180–189. doi: 10.1016/j.ijbiomac.2020.02.329. [DOI] [PubMed] [Google Scholar]
- Barthel H., Rösch L., Weis J. From Molecules to Materials; Organosilicon Chemistry Set: 2005. Fumed silica-production, properties, and applications; pp. 761–778. [Google Scholar]
- Baumgartner A., Planinšek O. Application of commercially available mesoporous silica for drug dissolution enhancement in oral drug delivery. Eur. J. Pharm. Sci. 2021;167 doi: 10.1016/j.ejps.2021.106015. [DOI] [PubMed] [Google Scholar]
- Bhatia S. Natural polymers vs synthetic polymer. Nat. Polym. Drug Deliv. Syst. Springer. 2016:95–118. [Google Scholar]
- Borsche M., Pereira S.L., Klein C., Grünewald A. Mitochondria and Parkinson’s disease: clinical, molecular, and translational aspects. J. Parkinson’s Disease. 2021;11:45–60. doi: 10.3233/JPD-201981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd B.J., Bergström C.A., Vinarov Z., Kuentz M., Brouwers J., Augustijns P., Brandl M., Bernkop-Schnürch A., Shrestha N., Préat V. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur. J. Pharm. Sci. 2019;137 doi: 10.1016/j.ejps.2019.104967. [DOI] [PubMed] [Google Scholar]
- Braak H., Rüb U., Del Tredici K. Cognitive decline correlates with neuropathological stage in Parkinson’s disease. J. Neurol. Sci. 2006;248:255–258. doi: 10.1016/j.jns.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Breijyeh Z., Karaman R. Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules. 2020;25:5789. doi: 10.3390/molecules25245789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukara K., Schueller L., Rosier J., Daems T., Verheyden L., Eelen S., Martens J.A., Van den Mooter G., Bugarski B., Kiekens F. In vivo performance of fenofibrate formulated with ordered mesoporous silica versus 2-marketed formulations: a comparative bioavailability study in beagle dogs. Journal of Pharmaceutical Sciences. 2016;105:2381–2385. doi: 10.1016/j.xphs.2016.05.019. [DOI] [PubMed] [Google Scholar]
- Carvajal F.J., Mattison H.A., Cerpa W. Role of NMDA receptor-mediated glutamatergic signaling in chronic and acute neuropathologies. Neural Plast. 2016;2016:2701526. doi: 10.1155/2016/2701526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani, R., Perry, G., 2013. Molecular pathology of Alzheimer’s disease. Presented at the Colloquium Series on Neurobiology of Alzheimer’s Disease, Morgan & Claypool Life Sciences, pp. 1–91.
- Castellani R.J., Plascencia-Villa G., Perry G. Pathogenesis of Alzheimer’s disease. Handbk. Neurotoxic. Springer. 2022:1–20. [Google Scholar]
- Castillo R.R., Lozano D., González B., Manzano M., Izquierdo-Barba I., Vallet-Regí M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: an update. Expert. Opin. Drug Deliv. 2019;16:415–439. doi: 10.1080/17425247.2019.1598375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A. Dendrimers for drug delivery. Molecules. 2018;23:938. doi: 10.3390/molecules23040938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C.-S., Liu T.-P., Chien F.-C., Mou C.-Y., Wu S.-H., Chen Y.-P. Codelivery of plasmid and curcumin with mesoporous silica nanoparticles for promoting neurite outgrowth. ACS Appl. Mater. Interfaces. 2019;11:15322–15331. doi: 10.1021/acsami.9b02797. [DOI] [PubMed] [Google Scholar]
- Chiang H.-L., Lin C.-H. Altered gut microbiome and intestinal pathology in Parkinson’s disease. J. Movement Disorders. 2019;12:67. doi: 10.14802/jmd.18067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chircov C., Spoială A., Păun C., Crăciun L., Ficai D., Ficai A., Andronescu E., Turculeƫ, Ștefan C. Mesoporous Silica Platforms with Potential Applications in Release and Adsorption of Active Agents. Molecules. 2020;25:3814. doi: 10.3390/molecules25173814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Zheng Q., Katz H.E., Guilarte T.R. Silica-based nanoparticle uptake and cellular response by primary Microglia. Environ. Health Perspect. 2010;118:589–595. doi: 10.1289/ehp.0901534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha S., Forbes B., Lobo J.M.S., Silva A.C. Improving drug delivery for Alzheimer’s disease through nose-to-brain delivery using nanoemulsions, Nanostructured Lipid Carriers (NLC) and in situ hydrogels. Int. J. Nanomed. 2021;16:4373. doi: 10.2147/IJN.S305851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deirram N., Zhang C., Kermaniyan S.S., Johnston A.P., Such G.K. pH-responsive polymer nanoparticles for drug delivery. Macromol. Rapid Commun. 2019;40:1800917. doi: 10.1002/marc.201800917. [DOI] [PubMed] [Google Scholar]
- DeTure M.A., Dickson D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019;14:1–18. doi: 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maio, R., Barrett, P.J., Hoffman, E.K., Barrett, C.W., Zharikov, A., Borah, A., Hu, X., McCoy, J., Chu, C.T., Burton, E.A., 2016. α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Science translational medicine 8, 342ra78-342ra78. [DOI] [PMC free article] [PubMed]
- Dietrichs E., Odin P. Algorithms for the treatment of motor problems in Parkinson’s disease. Acta Neurol. Scand. 2017;136:378–385. doi: 10.1111/ane.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit N., Maurya S.D., Sagar B.P. Sustained release drug delivery system. Indian J. Res. Pharm. Biotechnol. 2013;1:305. [Google Scholar]
- Duffy M.F., Collier T.J., Patterson J.R., Kemp C.J., Luk K.C., Tansey M.G., Paumier K.L., Kanaan N.M., Fischer D.L., Polinski N.K. Lewy body-like alpha-synuclein inclusions trigger reactive microgliosis prior to nigral degeneration. J. Neuroinflamm. 2018;15:1–18. doi: 10.1186/s12974-018-1171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldufani J., Blaise G. The role of acetylcholinesterase inhibitors such as neostigmine and rivastigmine on chronic pain and cognitive function in aging: a review of recent clinical applications. Alzheimer’s & Dementia: Transl. Res. Clin. Intervent. 2019;5:175–183. doi: 10.1016/j.trci.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernsting M.J., Murakami M., Roy A., Li S.-D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release. 2013;172:782–794. doi: 10.1016/j.jconrel.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Mao C., Hu X., Zhang S., Yang Z., Hu Z., Sun H., Fan Y., Dong Y., Yang J. New insights into the pathogenesis of Alzheimer’s disease. Front. Neurol. 2020;10:1312. doi: 10.3389/fneur.2019.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Vieira, T.H., M Guimaraes, I., R Silva, F., M Ribeiro, F., 2016. Alzheimer’s disease: targeting the cholinergic system. Current neuropharmacology 14, 101–115 [DOI] [PMC free article] [PubMed]
- Gelders, G., Baekelandt, V., Van der Perren, A., 2018. Linking neuroinflammation and neurodegeneration in Parkinson’s disease. Journal of immunology research 2018. [DOI] [PMC free article] [PubMed]
- Farjadian F., Roointan A., Mohammadi-Samani S., Hosseini M. Mesoporous silica nanoparticles: Synthesis, pharmaceutical applications, biodistribution, and biosafety assessment. Chemical Engineering Journal. 2019;359:684–705. doi: 10.1016/j.cej.2018.11.156. [DOI] [Google Scholar]
- Geng J., Li M., Wu L., Chen C., Qu X. Mesoporous silica nanoparticle-based H2O2 responsive controlled-release system used for alzheimer’s disease treatment. Adv. Healthc. Mater. 2012;1:332–336. doi: 10.1002/adhm.201200067. [DOI] [PubMed] [Google Scholar]
- Geszke-Moritz M., Moritz M. Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies. Mater. Sci. Eng. C. 2016;68:982–994. doi: 10.1016/j.msec.2016.05.119. [DOI] [PubMed] [Google Scholar]
- Godoy-Reyes T.M., Llopis-Lorente A., García-Fernández A., Gaviña P., Costero A.M., Martínez-Máñez R., Sancenón F. Acetylcholine-responsive cargo release using acetylcholinesterase-capped nanomaterials. Chem. Commun. 2019;55:5785–5788. doi: 10.1039/c9cc02602a. [DOI] [PubMed] [Google Scholar]
- Green H., Tsitsi P., Markaki I., Aarsland D., Svenningsson P. Novel treatment opportunities against cognitive impairment in Parkinson’s disease with an emphasis on diabetes-related pathways. CNS Drugs. 2019;33:143–160. doi: 10.1007/s40263-018-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group, P.S., 2004. Levodopa and the progression of Parkinson’s disease. New England Journal of Medicine 351, 2498–2508 [DOI] [PubMed]
- Grumezescu, A.M., Andronescu, E., Ficai, A., Voicu, G., Cocos, O., Chifiriuc, M.C., 2013. EUGENIA CARYOPHYLLATA ESSENTIAL OIL-SiO2 BIOHYBRID STRUCTURE FOR THE POTENTIATION OF ANTIBIOTICS’ACTIVITY. REVISTA ROMANA DE MATERIALE-ROMANIAN JOURNAL OF MATERIALS 43, 160–166
- Grundke-Iqbal, I., Iqbal, K., Tung, Y.-C., Quinlan, M., Wisniewski, H.M., Binder, L.I., 1986. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proceedings of the National Academy of Sciences 83, 4913–4917. [DOI] [PMC free article] [PubMed]
- Guo D.-S., Wang K., Wang Y.-X., Liu Y. Cholinesterase-responsive supramolecular vesicle. J. Am. Chem. Soc. 2012;134:10244–10250. doi: 10.1021/ja303280r. [DOI] [PubMed] [Google Scholar]
- Guo J., Zhao X., Li Y., Li G., Liu X. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease. Int. J. Mol. Med. 2018;41:1817–1825. doi: 10.3892/ijmm.2018.3406. [DOI] [PubMed] [Google Scholar]
- Halevas E., Mavroidi B., Nday C.M., Tang J., Smith G.C., Boukos N., Litsardakis G., Pelecanou M., Salifoglou A. Modified magnetic core-shell mesoporous silica nano-formulations with encapsulated quercetin exhibit anti-amyloid and antioxidant activity. J. Inorg. Biochem. 2020;213 doi: 10.1016/j.jinorgbio.2020.111271. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J.M.C. edition. ed. Oxford University Press; Oxford, United Kingdom: 2015. Free radicals in biology and medicine, Fifth. [Google Scholar]
- Heikkila T., Salonen J., Tuura J., Hamdy M., Mul G., Kumar N., Salmi T., Murzin D., Laitinen L., Kaukonen A. Mesoporous silica material TUD-1 as a drug delivery system. International Journal of Pharmaceutics. 2007;331:133–138. doi: 10.1016/j.ijpharm.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Henderson M.X., Trojanowski J.Q., Lee V.-M.-Y. α-Synuclein pathology in Parkinson’s disease and related α-synucleinopathies. Neurosci. Lett. 2019;709 doi: 10.1016/j.neulet.2019.134316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser J., Svidrytski A., Höltzel A., Priamushko T., Kleitz F., Wang W., Kübel C., Tallarek U. Morphology–transport relationships for SBA-15 and KIT-6 ordered mesoporous silicas. Phys. Chem. Chem. Phys. 2020;22:11314–11326. doi: 10.1039/D0CP01861A. [DOI] [PubMed] [Google Scholar]
- Holloway R.G., Shoulson I., Fahn S., Kieburtz K., Lang A., Marek K., McDermott M., Seibyl J., Weiner W., Musch B. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch. Neurol. 2004;61:1044–1053. doi: 10.1001/archneur.61.7.1044. [DOI] [PubMed] [Google Scholar]
- Hsu S.-H., Wen C.-J., Al-Suwayeh S., Chang H.-W., Yen T.-C., Fang J.-Y. Physicochemical characterization and in vivo bioluminescence imaging of nanostructured lipid carriers for targeting the brain: apomorphine as a model drug. Nanotechnology. 2010;21 doi: 10.1088/0957-4484/21/40/405101. [DOI] [PubMed] [Google Scholar]
- Huang Y., Li P., Zhao R., Zhao L., Liu J., Peng S., Fu X., Wang X., Luo R., Wang R., Zhang Z. Silica nanoparticles: Biomedical applications and toxicity. Biomed. Pharmacother. 2022;151 doi: 10.1016/j.biopha.2022.113053. [DOI] [PubMed] [Google Scholar]
- Huang L., Yan X., Kruk M. Synthesis of Ultralarge-Pore FDU-12 Silica with Face-Centered Cubic Structure. Langmuir. 2010;26:14871–14878. doi: 10.1021/la102228u. [DOI] [PubMed] [Google Scholar]
- Illum L. Nasal drug delivery: new developments and strategies. Drug Discov. Today. 2002;7:1184–1189. doi: 10.1016/s1359-6446(02)02529-1. [DOI] [PubMed] [Google Scholar]
- Jafari S., Derakhshankhah H., Alaei L., Fattahi A., Varnamkhasti B.S., Saboury A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019;109:1100–1111. doi: 10.1016/j.biopha.2018.10.167. [DOI] [PubMed] [Google Scholar]
- Jampilek, J., Zaruba, K., Oravec, M., Kunes, M., Babula, P., Ulbrich, P., Brezaniova, I., Opatrilova, R., Triska, J., Suchy, P., 2015. Preparation of silica nanoparticles loaded with nootropics and their in vivo permeation through blood-brain barrier. BioMed research international 2015. [DOI] [PMC free article] [PubMed]
- Jani G.K., Shah D.P., Prajapati V.D., Jain V.C. Gums and mucilages: versatile excipients for pharmaceutical formulations. Asian J Pharm Sci. 2009;4:309–323. [Google Scholar]
- Jankovic J., Tan E.K. Parkinson’s disease: etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry. 2020;91:795–808. doi: 10.1136/jnnp-2019-322338. [DOI] [PubMed] [Google Scholar]
- Jung H., Chung Y.J., Wilton R., Lee C.H., Lee B.I., Lim J., Lee H., Choi J., Kang H., Lee B., Rozhkova E.A., Park C.B., Lee J. Silica nanodepletors: targeting and clearing Alzheimer’s β-amyloid plaques. Adv. Funct. Mater. 2020;30:1910475. doi: 10.1002/adfm.201910475. [DOI] [Google Scholar]
- Kabir M.T., Uddin M.S., Setu J.R., Ashraf G.M., Bin-Jumah M.N., Abdel-Daim M.M. Exploring the role of PSEN mutations in the pathogenesis of Alzheimer’s disease. Neurotox. Res. 2020;38:833–849. doi: 10.1007/s12640-020-00232-x. [DOI] [PubMed] [Google Scholar]
- Kalhapure R.S., Suleman N., Mocktar C., Seedat N., Govender T. Nanoengineered drug delivery systems for enhancing antibiotic therapy. J. Pharm. Sci. 2015;104:872–905. doi: 10.1002/jps.24298. [DOI] [PubMed] [Google Scholar]
- Kalia L.V., Lang A.E. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- Karaman D.Ş., Kettiger H. Inorganic Frameworks as Smart Nanomedicines. Elsevier; 2018. Silica-based nanoparticles as drug delivery systems: Chances and challenges; pp. 1–40. [Google Scholar]
- Karkossa F., Klein S. Individualized in vitro and in silico methods for predicting in vivo performance of enteric-coated tablets containing a narrow therapeutic index drug. Eur. J. Pharm. Biopharm. 2019;135:13–24. doi: 10.1016/j.ejpb.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Kishido T., Unno K., Yoshida H., Choba D., Fukutomi R., Asahina S., Iguchi K., Oku N., Hoshino M. Decline in glutathione peroxidase activity is a reason for brain senescence: consumption of green tea catechin prevents the decline in its activity and protein oxidative damage in ageing mouse brain. Biogerontology. 2007;8:423–430. doi: 10.1007/s10522-007-9085-7. [DOI] [PubMed] [Google Scholar]
- Kiss T., Katona G., Mérai L., Janovák L., Deák Á., Kozma G., Kónya Z., Ambrus R. Development of a hydrophobicity-controlled delivery system containing levodopa methyl ester hydrochloride loaded into a mesoporous silica. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13071039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitz F., Choi S.H., Ryoo R. Cubic Ia 3 d large mesoporous silica: synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chemical Communications. 2003:2136–2137. doi: 10.1039/b306504a. [DOI] [PubMed] [Google Scholar]
- Kolosnjaj-Tabi J., Gibot L., Fourquaux I., Golzio M., Rols M.-P. Electric field-responsive nanoparticles and electric fields: physical, chemical, biological mechanisms and therapeutic prospects. Adv. Drug Deliv. Rev. 2019;138:56–67. doi: 10.1016/j.addr.2018.10.017. [DOI] [PubMed] [Google Scholar]
- Kong M., Tang J., Qiao Q., Wu T., Qi Y., Tan S., Gao X., Zhang Z. Biodegradable hollow mesoporous silica nanoparticles for regulating tumor microenvironment and enhancing antitumor efficiency. Theranostics. 2017;7:3276. doi: 10.7150/thno.19987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk M., Jaroniec M., Sakamoto Y., Terasaki O., Ryoo R., Ko C.H. Determination of Pore Size and Pore Wall Structure of MCM-41 by Using Nitrogen Adsorption, Transmission Electron Microscopy, and X-ray Diffraction. J. Phys. Chem. B. 2000;104:292–301. doi: 10.1021/jp992718a. [DOI] [Google Scholar]
- Kumar K.S., Bhowmik D., Srivastava S., Paswan S., Dutta A.S. Sustained release drug delivery system potential. The pharma innovation. 2012;1 [Google Scholar]
- Kumar S., Dilbaghi N., Saharan R., Bhanjana G. Nanotechnology as emerging tool for enhancing solubility of poorly water-soluble drugs. Bionanoscience. 2012;2:227–250. [Google Scholar]
- Kumar S., Malik M., Purohit R. Synthesis methods of mesoporous silica materials. Mater. Today:. Proc. 2017;4:350–357. [Google Scholar]
- Kumar A., Singh A. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol. Rep. 2015;67:195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Lakshmi P., Pola S. Nanostructures for Antimicrobial and Antibiofilm Applications. Springer; 2020. Mesoporous silica nanomaterials as antibacterial and antibiofilm agents; pp. 375–397. [Google Scholar]
- Landsiedel R., Fabian E., Ma-Hock L., Wohlleben W., Wiench K., Oesch F., van Ravenzwaay B. Toxico-/biokinetics of nanomaterials. Arch. Toxicol. 2012;86:1021–1060. doi: 10.1007/s00204-012-0858-7. [DOI] [PubMed] [Google Scholar]
- Lee Y., Thompson D. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017;9:e1450. doi: 10.1002/wnan.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Liu J., Li G., Zhang X., Xu F., Fu Z., Teng L., Li Y., Sun F. Near-infrared light-responsive, pramipexole-loaded biodegradable PLGA microspheres for therapeutic use in Parkinson’s disease. Eur. J. Pharm. Biopharm. 2019;141:1–11. doi: 10.1016/j.ejpb.2019.05.013. [DOI] [PubMed] [Google Scholar]
- Liang J., Liu B. ROS-responsive drug delivery systems. Bioeng. Transl. Med. 2016;1:239–251. doi: 10.1002/btm2.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liong M., Lu J., Kovochich M., Xia T., Ruehm S.G., Nel A.E., Tamanoi F., Zink J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Lin B., Shao W., Zhu Z., Ji T., Yang C. In vitro and in vivo studies on the transport of PEGylated silica nanoparticles across the blood–brain barrier. ACS Appl. Mater. Interfaces. 2014;6:2131–2136. doi: 10.1021/am405219u. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhu D., Luo J., Chen X., Gao L., Liu W., Chen T. NIR-II-activated yolk–shell nanostructures as an intelligent platform for Parkinsonian therapy. ACS Appl. Bio Mater. 2020;3:6876–6887. doi: 10.1021/acsabm.0c00794. [DOI] [PubMed] [Google Scholar]
- Llopis-Lorente A., Díez P., de la Torre C., Sánchez A., Sancenón F., Aznar E., Marcos M.D., Martínez-Ruíz P., Martínez-Máñez R., Villalonga R. Enzyme-controlled nanodevice for acetylcholine-triggered cargo delivery based on Janus Au–mesoporous silica nanoparticles. Chem.–A Eur. J. 2017;23:4276–4281. doi: 10.1002/chem.201700603. [DOI] [PubMed] [Google Scholar]
- López T., Esquivel D., Mendoza-Díaz G., Ortiz-Islas E., González R.D., Novaro O. L-DOPA stabilization on sol–gel silica to be used as neurological nanoreservoirs: structural and spectroscopic studies. Mater. Lett. 2015;161:160–163. [Google Scholar]
- Loureiro J.A., Andrade S., Duarte A., Neves A.R., Queiroz J.F., Nunes C., Sevin E., Fenart L., Gosselet F., Coelho M.A. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules. 2017;22:277. doi: 10.3390/molecules22020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy, D.A., 2003. Sol–gel processing.
- Ma M., Gao N., Sun Y., Du X., Ren J., Qu X. Redox-activated near-infrared-responsive polyoxometalates used for photothermal treatment of Alzheimer’s disease. Adv. Healthc. Mater. 2018;7:1800320. doi: 10.1002/adhm.201800320. [DOI] [PubMed] [Google Scholar]
- Madsen D.A., Schmidt S.I., Blaabjerg M., Meyer M. Interaction between Parkin and α-synuclein in PARK2-mediated Parkinson’s disease. Cells. 2021;10:283. doi: 10.3390/cells10020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrinelli F., Picelli A., Tocco P., Federico A., Roncari L., Smania N., Zanette G., Tamburin S. Pathophysiology of motor dysfunction in Parkinson’s disease as the rationale for drug treatment and rehabilitation. Parkinson’s Disease. 2016;2016:9832839. doi: 10.1155/2016/9832839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpartida A.B., Williamson M., Narendra D.P., Wade-Martins R., Ryan B.J. Mitochondrial dysfunction and mitophagy in Parkinson’s disease: from mechanism to therapy. Trends Biochem. Sci. 2021;46:329–343. doi: 10.1016/j.tibs.2020.11.007. [DOI] [PubMed] [Google Scholar]
- Mani S., Sevanan M., Krishnamoorthy A., Sekar S. A systematic review of molecular approaches that link mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurol. Sci. 2021;42:4459–4469. doi: 10.1007/s10072-021-05551-1. [DOI] [PubMed] [Google Scholar]
- Manoharan S., Guillemin G.J., Abiramasundari R.S., Essa M.M., Akbar M., Akbar M.D. The Role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: a mini review. Oxid. Med. Cell. Longev. 2016;2016:8590578. doi: 10.1155/2016/8590578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Suzukake M., Nonaka T., Hosokawa M., Oikawa T., Arai T., Akiyama H., Mann D.M., Hasegawa M. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136:1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiratta S., Hussein M., Nasser H.A., Ali A.A.A. Multidisciplinary role of mesoporous silica nanoparticles in brain regeneration and cancers: from crossing the blood-brain barrier to treatment. Part. Part. Syst. Charact. 2019;36:1900195. doi: 10.1002/ppsc.201900195. [DOI] [Google Scholar]
- Migliore L., Coppedè F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2009;674:73–84. doi: 10.1016/j.mrgentox.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Mirzaali F., Dizaj S.M., Shahi S., Memar M.Y., Parnia F. The antibacterial effect of tetracycline-loaded mesoporous silica nanoparticles in the gingival fluid at implant-abutment junction: a randomized clinical trial study. Pharmaceutical Nanotechnology. 2023 doi: 10.2174/2211738511666230106151403. [DOI] [PubMed] [Google Scholar]
- Monczor M. Diagnosis and treatment of Alzheimer’s disease. Curr. Med. Chem.-Central Nervous Syst. Agents. 2005;5:5–13. [Google Scholar]
- Morales V., McConnell J., Pérez-Garnes M., Almendro N., Sanz R., García-Muñoz R.A. l-Dopa release from mesoporous silica nanoparticles engineered through the concept of drug-structure-directing agents for Parkinson’s disease. J. Mater. Chem. B. 2021;9:4178–4189. doi: 10.1039/d1tb00481f. [DOI] [PubMed] [Google Scholar]
- Müller A.-K., Ruppel J., Drexel C.-P., Zimmermann I. Precipitated silica as flow regulator. Eur. J. Pharm. Sci. 2008;34:303–308. doi: 10.1016/j.ejps.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Nair K.G., Ramaiyan V., Sukumaran S.K. Enhancement of drug permeability across blood brain barrier using nanoparticles in meningitis. Inflammopharmacology. 2018;26:675–684. doi: 10.1007/s10787-018-0468-y. [DOI] [PubMed] [Google Scholar]
- Narayan R., Nayak U.Y., Raichur A.M., Garg S. Mesoporous silica nanoparticles: a comprehensive review on synthesis and recent advances. Pharmaceutics. 2018;10:118. doi: 10.3390/pharmaceutics10030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan J.V., Nugraha C., Ng X.W., Venkatraman S. Sustained-release from nanocarriers: a review. J. Controll. Release, Drug Deliv. Res. Asia Pac. Region. 2014;193:122–138. doi: 10.1016/j.jconrel.2014.05.029. [DOI] [PubMed] [Google Scholar]
- Nday C.M., Halevas E., Jackson G.E., Salifoglou A. Quercetin encapsulation in modified silica nanoparticles: potential use against Cu(II)-induced oxidative stress in neurodegeneration. J. Inorg. Biochem. 2015;145:51–64. doi: 10.1016/j.jinorgbio.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Newland B., Wolff P., Zhou D., Wang W., Zhang H., Rosser A., Wang W., Werner C. Synthesis of ROS scavenging microspheres from a dopamine containing poly(β-amino ester) for applications for neurodegenerative disorders. Biomater. Sci. 2016;4:400–404. doi: 10.1039/C5BM00542F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouerdane Y., Hassaballah M.Y., Nagah A., Ibrahim T.M., Mohamed H.A., El-Baz A., Attia M.S. Exosomes in Parkinson: revisiting their pathologic role and potential applications. Pharmaceuticals. 2022;15:76. doi: 10.3390/ph15010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow H., Larson D.R., Srivastava M., Baird B.A., Webb W.W., Wiesner U. Bright and stable core− shell fluorescent silica nanoparticles. Nano Lett. 2005;5:113–117. doi: 10.1021/nl0482478. [DOI] [PubMed] [Google Scholar]
- Pandey P.K., Sharma A.K., Rani S., Mishra G., Kandasamy G., Patra A.K., Rana M., Sharma A.K., Yadav A.K., Gupta U. MCM-41 nanoparticles for brain delivery: better choline-esterase and amyloid formation inhibition with improved kinetics. ACS Biomater Sci. Eng. 2018;4:2860–2869. doi: 10.1021/acsbiomaterials.8b00335. [DOI] [PubMed] [Google Scholar]
- Park J.-S., Davis R.L., Sue C.M. Mitochondrial dysfunction in Parkinson’s disease: new mechanistic insights and therapeutic perspectives. Curr. Neurol. Neurosci. Rep. 2018;18:1–11. doi: 10.1007/s11910-018-0829-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T., Zhou J., Piepmeier J.M., Saltzman W.M. Polymeric nanoparticles for drug delivery to the central nervous system. Adv. Drug Deliv. Rev. 2012;64:701–705. doi: 10.1016/j.addr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic, M., Hurt, C., Collins, D., Burns, A., Camus, V., Liperoti, R., Marriott, A., Nobili, F., Robert, P., Tsolaki, M., Vellas, B., Verhey, F., Byrne, E.J., 2007. CLUSTERING OF BEHAVIOURAL AND PSYCHOLOGICAL SYMPTOMS IN DEMENTIA (BPSD): A EUROPEAN ALZHEIMER’S DISEASE CONSORTIUM (EADC) STUDY. null 62, 426–432. https://doi.org/10.1179/acb.2007.062. [DOI] [PubMed]
- Pourhakkak P., Taghizadeh M., Taghizadeh A., Ghaedi M. Adsorbent. Interf. Sci. Technol. Elsevier. 2021:71–210. [Google Scholar]
- Poyatos-Racionero E., González-Álvarez I., González-Álvarez M., Martínez-Máñez R., Marcos M.D., Bernardos A., Aznar E. Surfactant-Triggered Molecular Gate Tested on Different Mesoporous Silica Supports for Gastrointestinal Controlled Delivery. Nanomaterials. 2020;10:1290. doi: 10.3390/nano10071290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsheim T., Jette N., Frolkis A., Steeves T.D.L. The prevalence of Parkinson’s disease: a systematic review and meta-analysis: PD PREVALENCE. Mov. Disord. 2014;29:1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- Prokopowicz M. Silica-polyethylene glycol matrix synthesis by sol-gel method and evaluation for diclofenac diethyloammonium release. Drug Deliv. 2007;14:129–138. doi: 10.1080/10717540600812653. [DOI] [PubMed] [Google Scholar]
- Qian J., Li X., Wei M., Gao X., Xu Z., He S. Bio-molecule-conjugated fluorescent organically modified silica nanoparticles as optical probes for cancer cell imaging. Opt. Express. 2008;16:19568–19578. doi: 10.1364/oe.16.019568. [DOI] [PubMed] [Google Scholar]
- Rabanel J.M., Aoun V., Elkin I., Mokhtar M., Hildgen P. Drug-loaded nanocarriers: passive targeting and crossing of biological barriers. Curr. Med. Chem. 2012;19:3070–3102. doi: 10.2174/092986712800784702. [DOI] [PubMed] [Google Scholar]
- Raza C., Anjum R. Parkinson’s disease: Mechanisms, translational models and management strategies. Life Sci. 2019;226:77–90. doi: 10.1016/j.lfs.2019.03.057. [DOI] [PubMed] [Google Scholar]
- Reddy D.V., Rao A.S. A review on oral extended release technology. Res. J. Pharm. Technol. 2015;8:1454–1462. [Google Scholar]
- Reichmann H. Modern treatment in Parkinson’s disease, a personal approach. J. Neural Transm. 2016;123:73–80. doi: 10.1007/s00702-015-1441-1. [DOI] [PubMed] [Google Scholar]
- Rocha E.M., De Miranda B., Sanders L.H. Alpha-synuclein: pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 2018;109:249–257. doi: 10.1016/j.nbd.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Rosenholm J.M., Sahlgren C., Lindén M. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles – opportunities & challenges. Nanoscale. 2010;2:1870. doi: 10.1039/c0nr00156b. [DOI] [PubMed] [Google Scholar]
- Rubio, E., Almaral, J., Ramírez-Bon, R., Castaño, V., Rodríguez, V., 2005. Organic–inorganic hybrid coating (poly(methyl methacrylate)/monodisperse silica). Optical Materials 27, 1266–1269. https://doi.org/10.1016/j.optmat.2004.11.022
- Sant V., Som M., Karkisaval A.G., Carnahan P., Lal R. Scavenging amyloid oligomers from neurons with silica nanobowls: Implications for amyloid diseases. Biophys. J . 2021;120:3329–3340. doi: 10.1016/j.bpj.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaría Razo D.A., Pallavidino L., Garrone E., Geobaldo F., Descrovi E., Chiodoni A., Giorgis F. A version of Stöber synthesis enabling the facile prediction of silica nanospheres size for the fabrication of opal photonic crystals. J. Nanopart. Res. 2008;10:1225–1229. doi: 10.1007/s11051-008-9373-4. [DOI] [Google Scholar]
- Saraiva C., Praça C., Ferreira R., Santos T., Ferreira L., Bernardino L. Nanoparticle-mediated brain drug delivery: overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release. 2016;235:34–47. doi: 10.1016/j.jconrel.2016.05.044. [DOI] [PubMed] [Google Scholar]
- Schmidt N., Schulze J., Warwas D.P., Ehlert N., Lenarz T., Warnecke A., Behrens P. Long-term delivery of brain-derived neurotrophic factor (BDNF) from nanoporous silica nanoparticles improves the survival of spiral ganglion neurons in vitro. PLoS One. 2018;13:e0194778. doi: 10.1371/journal.pone.0194778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahriari M., Zahiri M., Abnous K., Taghdisi S.M., Ramezani M., Alibolandi M. Enzyme responsive drug delivery systems in cancer treatment. J. Control. Release. 2019;308:172–189. doi: 10.1016/j.jconrel.2019.07.004. [DOI] [PubMed] [Google Scholar]
- Shargel, L., Andrew, B., Wu-Pong, S., 1999. Applied biopharmaceutics & pharmacokinetics. Appleton & Lange Stamford.
- Sharma K. Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol. Med. Rep. 2019;20:1479–1487. doi: 10.3892/mmr.2019.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Kelleher R.J., III The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. Proc. Natl. Acad. Sci. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A.K., Singh, S. Sen, Rathore, A.S., Singh, S.P., Mishra, G., Awasthi, R., Mishra, S.K., Gautam, V., Singh, S.K., 2021. Lipid-Coated MCM-41 Mesoporous Silica Nanoparticles Loaded with Berberine Improved Inhibition of Acetylcholine Esterase and Amyloid Formation. ACS Biomaterials Science and Engineering 7, 3737–3753. https://doi.org/10.1021/acsbiomaterials.1c00514. [DOI] [PubMed]
- Singh, D., 2021. Self-nanoemulsifying Drug Delivery System: A Versatile Carrier for Lipophilic Drugs. PNT 9, 166–176. https://doi.org/10.2174/2211738509666210422124023. [DOI] [PubMed]
- Soares Martins, T., Trindade, D., Vaz, M., Campelo, I., Almeida, M., Trigo, G., da Cruz e Silva, O.A., Henriques, A.G., 2021. Diagnostic and therapeutic potential of exosomes in Alzheimer’s disease. Journal of Neurochemistry 156, 162–181. [DOI] [PubMed]
- Shen S., Li Y., Zhang Z., Fan J., Tu B., Zhou W., Zhao D. A novel ordered cubic mesoporous silica templated with tri-head group quaternary ammonium surfactant. Chemical communications. 2002:2212–2213. doi: 10.1039/b206993h. [DOI] [PubMed] [Google Scholar]
- Soliman H.M., Ghonaim G.A., Gharib S.M., Chopra H., Farag A.K., Hassanin M.H., Nagah A., Emad-Eldin M., Hashem N.E., Yahya G., Emam S.E., Hassan A.E.A., Attia M.S. Exosomes in Alzheimer’s disease: from being pathological players to potential diagnostics and therapeutics. IJMS. 2021;22:10794. doi: 10.3390/ijms221910794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Li Y., Xu Q., Liu Z. Mesoporous silica nanoparticles for stimuli-responsive controlled drug delivery: advances, challenges, and outlook. Int. J. Nanomed. 2017;12:87. doi: 10.2147/IJN.S117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojkovska I., Krainc D., Mazzulli J.R. Molecular mechanisms of α-synuclein and GBA1 in Parkinson’s disease. Cell Tissue Res. 2018;373:51–60. doi: 10.1007/s00441-017-2704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swar S., Máková V., Stibor I. Effectiveness of diverse mesoporous silica nanoparticles as potent vehicles for the drug L-DOPA. Materials. 2019;12:3202. doi: 10.3390/ma12193202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabaei Mirakabad F.S., Nejati-Koshki K., Akbarzadeh A., Yamchi M.R., Milani M., Zarghami N., Zeighamian V., Rahimzadeh A., Alimohammadi S., Hanifehpour Y., Joo S.W. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac. J. Cancer Prev. 2014;15:517–535. doi: 10.7314/APJCP.2014.15.2.517. [DOI] [PubMed] [Google Scholar]
- Taebnia N., Morshedi D., Doostkam M., Yaghmaei S., Aliakbari F., Singh G., Arpanaei A. The effect of mesoporous silica nanoparticle surface chemistry and concentration on the α-synuclein fibrillation. RSC Adv. 2015;5:60966–60974. doi: 10.1039/c5ra08405a. [DOI] [Google Scholar]
- Tambuyzer B.R., Ponsaerts P., Nouwen E.J. Microglia: gatekeepers of central nervous system immunology. J. Leukoc. Biol. 2009;85:352–370. doi: 10.1189/jlb.0608385. [DOI] [PubMed] [Google Scholar]
- Tang F., Li L., Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv. Mater. 2012;24:1504–1534. doi: 10.1002/adma.201104763. [DOI] [PubMed] [Google Scholar]
- Tapeinos C., Battaglini M., Ciofani G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release. 2017;264:306–332. doi: 10.1016/j.jconrel.2017.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S., Atluri V., Kaushik A., Yndart A., Nair M. Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019;14:5541. doi: 10.2147/IJN.S200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzankov B., Tzankova V., Aluani D., Yordanov Y., Spassova I., Kovacheva D., Avramova K., Valoti M., Yoncheva K. Development of MCM-41 mesoporous silica nanoparticles as a platform for pramipexole delivery. J. Drug Delivery Sci. Technol. 2019;51:26–35. doi: 10.1016/j.jddst.2019.02.008. [DOI] [Google Scholar]
- Tzankov B., Voycheva C., Yordanov Y., Aluani D., Spassova I., Kovacheva D., Lambov N., Tzankova V. Development and in vitro safety evaluation of pramipexole-loaded hollow mesoporous silica (HMS) particles. Biotechnol. Biotechnol. Equip. 2019;33:1204–1215. [Google Scholar]
- ud Din, F., Aman, W., Ullah, I., Qureshi, O.S., Mustapha, O., Shafique, S., Zeb, A., 2017. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. International journal of nanomedicine 12, 7291 [DOI] [PMC free article] [PubMed]
- Urayama, A., Banks, W.A., 2006. Effects of Stress and Nutrition on Blood-Brain Barrier Functions, in: Nutrients, Stress, and Medical Disorders. Springer, pp. 83–95.
- Vallet-Regí, M., 2010. Nanostructured mesoporous silica matrices in nanomedicine: Symposium: Biomedical applications of nanostructured mesoporous silicas. Journal of Internal Medicine 267, 22–43. https://doi.org/10.1111/j.1365-2796.2009.02190.x [DOI] [PubMed]
- Vallet-Regí M., Colilla M., Izquierdo-Barba I., Manzano M. Mesoporous silica nanoparticles for drug delivery: Current insights. Molecules. 2017;23:47. doi: 10.3390/molecules23010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara C., Houben S., Suain V., Yilmaz Z., De Decker R., Vanden Dries V., Boom A., Mansour S., Leroy K., Ando K. Amyloid-β pathology enhances pathological fibrillary tau seeding induced by Alzheimer PHF in vivo. Acta Neuropathol. 2019;137:397–412. doi: 10.1007/s00401-018-1953-5. [DOI] [PubMed] [Google Scholar]
- Vila M. Neuromelanin, aging, and neuronal vulnerability in Parkinson’s disease. Mov. Disord. 2019;34:1440–1451. doi: 10.1002/mds.27776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan V., Murali G., Gandhi S., Kumaraswamy P., Sethuraman S., Krishnan U.M. Development of thioflavin-modified mesoporous silica framework for amyloid fishing. Micropor. Mesopor. Mater. 2014;197:40–47. [Google Scholar]
- Vivero-Escoto J.L., Slowing I.I., Trewyn B.G., Lin V.S. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small. 2010;6:1952–1967. doi: 10.1002/smll.200901789. [DOI] [PubMed] [Google Scholar]
- Wang S. Ordered mesoporous materials for drug delivery. Micropor. Mesopor. Mater. 2009;117:1–9. [Google Scholar]
- Wang H., Chen Q., Zhou S. Carbon-based hybrid nanogels: a synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem. Soc. Rev. 2018;47:4198–4232. doi: 10.1039/C7CS00399D. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang K., Zhu Z., He Y., Zhang C., Guo Z., Wang X. Inhibition of metal-induced amyloid β-peptide aggregation by a blood–brain barrier permeable silica–cyclen nanochelator. RSC Adv. 2019;9:14126–14131. doi: 10.1039/C9RA02358E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weggen S., Beher D. Molecular consequences of amyloid precursor protein and presenilin mutations causing autosomal-dominant Alzheimer’s disease. Alzheimer’s Res. Ther.apy. 2012;4:1–14. doi: 10.1186/alzrt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B., Rogers B.J., Wirth M.J. Slip flow in colloidal crystals for ultraefficient chromatography. J. Am. Chem. Soc. 2012;134:10780–10782. doi: 10.1021/ja304177m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Wang C., Sun J., Xue Y. Neurotoxicity of silica nanoparticles: brain localization and dopaminergic neurons damage pathways. ACS Nano. 2011;5:4476–4489. doi: 10.1021/nn103530b. [DOI] [PubMed] [Google Scholar]
- Wu L., Wang J., Gao N., Ren J., Zhao A., Qu X. Electrically pulsatile responsive drug delivery platform for treatment of Alzheimer’s disease. Nano Res. 2015;8:2400–2414. [Google Scholar]
- Xu L., Li H.-L., Wang L.-P. PH-sensitive, polymer functionalized, nonporous silica nanoparticles for quercetin controlled release. Polymers. 2019;11:2026. doi: 10.3390/polym11122026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Yin T., Liu Y., Sun J., Zhou Y., Liu J. Gold nanoparticle-capped mesoporous silica-based H2O2-responsive controlled release system for Alzheimer’s disease treatment. Acta Biomater. 2016;46:177–190. doi: 10.1016/j.actbio.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Yetisgin A.A., Cetinel S., Zuvin M., Kosar A., Kutlu O. Therapeutic nanoparticles and their targeted delivery applications. Molecules. 2020;25:2193. doi: 10.3390/molecules25092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong K.-T., Roy I., Swihart M.T., Prasad P.N. Multifunctional nanoparticles as biocompatible targeted probes for human cancer diagnosis and therapy. J. Mater. Chem. 2009;19:4655. doi: 10.1039/b817667c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama K., Sun H., Sakai S., Mitsutake S., Okada M., Tahara H., Furukawa J., Fujitani N., Shinohara Y., Igarashi Y. Decreased Amyloid-β pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J. Biol. Chem. 2014;289:24488–24498. doi: 10.1074/jbc.M114.577213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Guo J., Zhang X., Zhao Y., Cao L., Sun L. Redox- and enzyme-responsive fluorescent porous silica nanocarriers for drug delivery. Sens. Actuators B. 2018;276:370–377. doi: 10.1016/j.snb.2018.08.118. [DOI] [Google Scholar]
- Zhang J., Qu F., Lin H., Wu X., Jiang J. Mesoporous bioactive glass: ideal material for higher uptake and well sustained release of ibuprofen. Mater. Res. Innov. 2012;16:230–235. [Google Scholar]
- Zhou G., Li L., Xing J., Cai J., Chen J., Liu P., Gu N., Ji M. Layer-by-layer construction of lipid bilayer on mesoporous silica nanoparticle to improve its water suspensibility and hemocompatibility. J. Sol-Gel Sci. Technol. 2017;82:490–499. [Google Scholar]
- Zhou Y., Tan L.-L., Li Q.-L., Qiu X.-L., Qi A.-D., Tao Y., Yang Y.-W. Acetylcholine-triggered cargo release from supramolecular nanovalves based on different macrocyclic receptors. Chem. – A Eur. J. 2014;20:2998–3004. doi: 10.1002/chem.201304864. [DOI] [PubMed] [Google Scholar]
- Zhou M., Xie L., Fang C.-J., Yang H., Wang Y.-J., Zhen X.-Y., Yan C.-H., Wang Y., Zhao M., Peng S. Implications for blood-brain-barrier permeability, in vitro oxidative stress and neurotoxicity potential induced by mesoporous silica nanoparticles: effects of surface modification. RSC Adv. 2016;6:2800–2809. doi: 10.1039/C5RA17517H. [DOI] [Google Scholar]
- Zimowska M., Michalik-Zym A., Kryſciak-Czerwenka J., Dula R., Socha R.P., Pamin K.…Serwicka E.M. A comparative study of direct versus post-synthesis alumination of mesoporous FSM-16 silica. Materials Research Bulletin. 2016;83:623–631. [Google Scholar]