This pooled analysis of 2 nonrandomized uncontrolled trials investigates the association between epicranial electrical focal cortex stimulation and seizure frequency in drug-refractory focal epilepsy.
Key Points
Question
Is long-term treatment using epicranial electrical focal cortex stimulation associated with a reduction in seizure frequency in drug-refractory focal epilepsy?
Findings
In 2 nonrandomized trials, 33 adult participants with uncontrolled unifocal epilepsy were implanted with a pulse generator and an electrode array placed above the individual focus region without experiencing device- or procedure-related serious adverse events. A pooled analysis showed a 52% reduction in seizure frequency and at least a 50% response in the sixth month of neurostimulation compared with the prospective baseline.
Meaning
Results suggest that focal cortex stimulation with an epicranial electrode array may offer a safe and effective new treatment option for patients with drug-refractory focal epilepsy.
Abstract
Importance
For the large population of people with drug-refractory epilepsy, alternative treatment approaches are needed. Clinical trial outcomes of a novel stimulation device, which is newly available in Europe for the treatment of patients with a predominant seizure focus, are reported for the first time.
Objective
To perform a pooled analysis of the results of 2 prospective, multicenter, single-arm trials, A Pilot Study to Assess the Feasibility of Neurostimulation With the EASEE System to Treat Medically Refractory Focal Epilepsy (EASEE II) and A Pilot Study to Assess the Feasibility of Patient-Controlled Neurostimulation With the EASEE System to Treat Medically Refractory Focal Epilepsy (PIMIDES I), assessing the safety and efficacy of epicranial focal cortex stimulation (FCS) with a novel implantable device (EASEE [Precisis]) as adjunctive treatment for adult patients with drug-refractory focal epilepsy.
Design, Setting, and Participants
This study was a pooled analysis of 2 nonrandomized uncontrolled trials, EASEE II and PIMIDES I, which began on January 15, 2019, and January 14, 2020, respectively, and ended on July 28, 2021. EASEE II and PIMIDES I were the first in-human, prospective, single-arm trials with an 8-month evaluation period. Patients were recruited at 7 European epilepsy centers. Consecutive participants with drug-refractory focal epilepsy were enrolled. Study data were analyzed from September 29, 2021, to February 2, 2022.
Interventions
After a 1-month prospective baseline period, patients were implanted with the neurostimulation device. After a 1-month postimplantation recovery period, unblinded FCS was activated using both high-frequency and direct current (DC)–like components performed via electrode arrays placed epicranially above the individual epileptic focus region.
Main Outcomes and Measures
Efficacy was prospectively assessed by the responder rate in the sixth month of stimulation compared with baseline; safety and additional end points were assessed after device implantation and during the stimulation period.
Results
Of the 34 adult patients enrolled at 6 German and 1 Belgian investigational site, 33 (mean [SD] age, 34.6 [13.5] years; 18 male patients [54.5%]) received the neurostimulation device implant. A total of 32 patients underwent combined high-frequency direct current–like stimulation at least until the 8-month postimplant follow-up visit. After 6 months of stimulation, 17 of 32 patients (53.1%) were responders to treatment with at least a 50% reduction in seizure frequency compared with baseline, corresponding to a significant median seizure reduction by 52% (95% CI, 0.37%-0.76%; P < .001). No device- or procedure-related serious adverse events were reported (0; 95% CI, 0%-10.58%). There were no significant alterations in cognition, mood, or overall quality of life.
Conclusions and Relevance
Results of this pooled analysis of 2 nonrandomized uncontrolled trials suggest that FCS with a novel neurostimulation device was associated with an effective reduction in seizure frequency in patients with drug-refractory focal epilepsy and may offer a promising treatment option for patients with a predominant epileptic focus.
Trial Registration
German Clinical Trials Register: DRKS00015918 and DRKS00017833, respectively, and jointly under PROSPERO: CRD42021266440
Introduction
Epilepsy is one of the most common neurologic disorders, affecting approximately 1% of the world’s population.1 Despite the availability of new antiseizure medications (ASMs), more than one-third of patients with epilepsy do not sufficiently respond to drug therapy,2 particularly those with focal epilepsy. Many of these patients are not eligible for epilepsy surgery; thus, alternative treatment strategies are urgently needed. Neuromodulation using electrical brain stimulation either in the periphery or directly targeted to the brain via implanted electrodes has been shown to be an effective tool to reduce seizure burden,3 both with interference at a thalamic network hub4 or with direct stimulation of the epileptogenic brain area.5 Furthermore, both direct current (DC) stimulation6 and alternating current (AC) stimulation7 of the epileptic focus have been reported to reduce seizure frequency.
This article presents the results of a participant-level pooled analysis including 2 prospective pilot clinical trials on a novel epicranial neurostimulation device with a specially designed electrode placed epicranially over the epileptic focus region. This concept is referred to as focal cortex stimulation (FCS). Using a combination of 2 stimulation modes (high-frequency stimulation [HFS]; direct current–like stimulation [DLS]), the neurostimulation device aims at modulating epileptic activity at the origin of ictal discharges. The pooled analysis protocol was fixed prior to obtaining results from the clinical efficacy studies, defining the percentage of responders to the novel device as a primary end point.
Methods
Study Design
This was a pooled analysis of 2 nonrandomized uncontrolled trials: A Pilot Study to Assess the Feasibility of Neurostimulation With the EASEE System to Treat Medically Refractory Focal Epilepsy (EASEE II) and A Pilot Study to Assess the Feasibility of Patient-Controlled Neurostimulation With the EASEE System to Treat Medically Refractory Focal Epilepsy (PIMIDES I), which began on January 15, 2019, and January 14, 2020, respectively, and ended on July 28, 2021 (Supplement 1). EASEE II and PIMIDES I are prospective, interventional, unblinded, multicenter pilot studies using the EASEE System (Precisis GmbH), an innovative neuromodulation method for patients with drug-refractory focal epilepsy. Study designs and patient recruitment were almost identical for both trials (Figure 1A)8,9 and were described previously (Supplement 2 and Supplement 3).10 After assuring the feasibility of data pooling and finalization of the meta-analysis protocol, a participant-level meta-analysis project was prospectively defined to consider all clinical data available from both trials on the safety and performance of the neurostimulation device, based on the fact that the studies were homogeneous in terms of focal epilepsy and drug resistance of participants, interventions, and assessed outcomes.
Figure 1. Trial Design, Neurostimulation Device Lead and Stimulation Modes, and Electrode Localization.
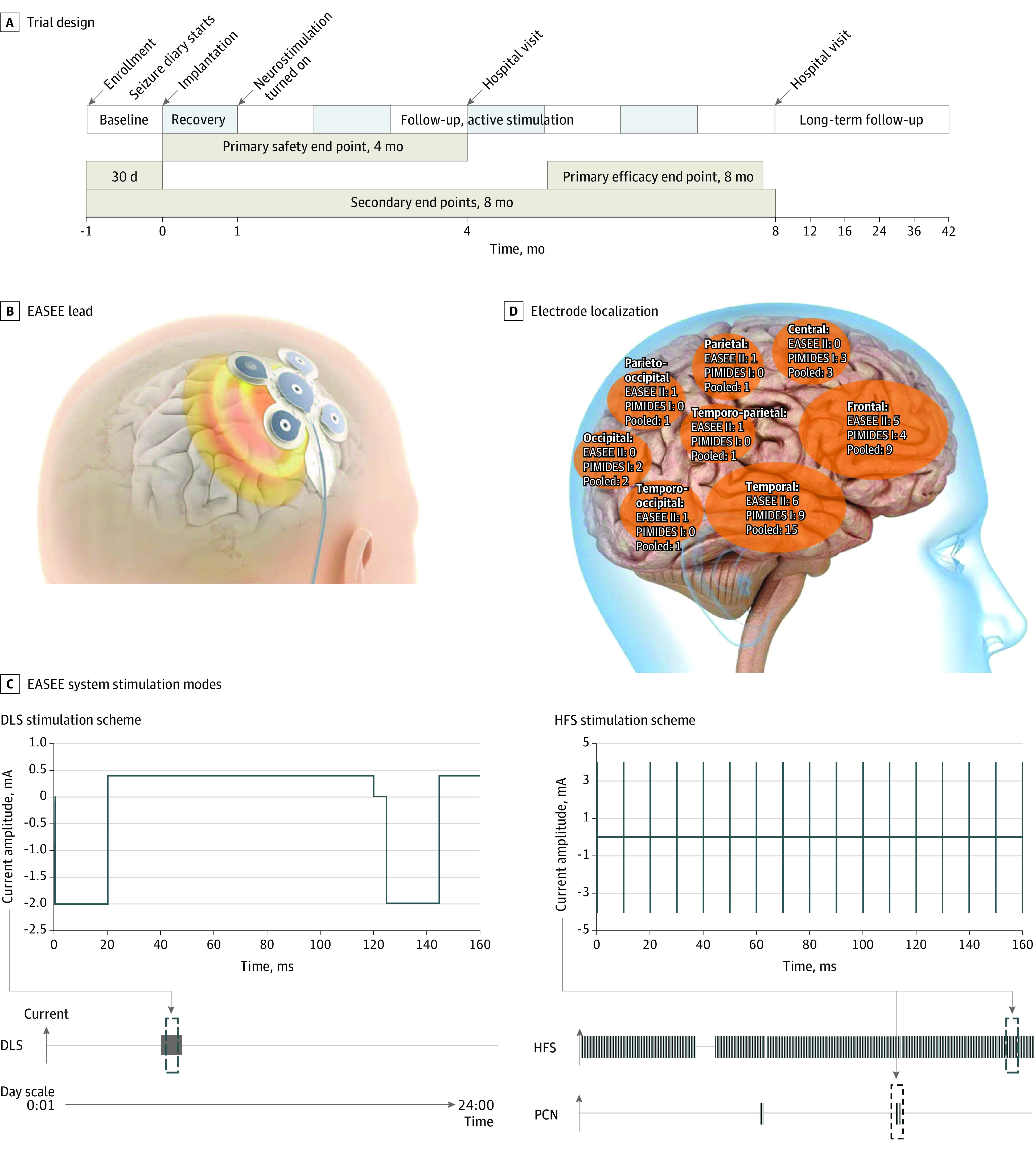
A, Trial time line for the A Pilot Study to Assess the Feasibility of Neurostimulation With the EASEE System to Treat Medically Refractory Focal Epilepsy (EASEE II) and A Pilot Study to Assess the Feasibility of Patient-Controlled Neurostimulation With the EASEE System to Treat Medically Refractory Focal Epilepsy (PIMIDES I) studies. B, The neurostimulation device consists of a lead placed epicranially above the respective epileptic focus and connected to the pulse generator in the chest. The lead includes a 5-channel pseudo-Laplace electrode (a central electrode surrounded by 4 peripheral electrodes; total diameter, 77 mm) and a connecting cable. C, Device stimulation modes are direct current–like stimulation (DLS; cathodal pulses of 2 mA, duration 20 microseconds, with equilibrating pulses of 100-microseconds’ duration for 20 minutes per day), high-frequency stimulation (HFS; bursts of 4 mA, rectangular biphasic symmetric, at 100 Hz, pulse width 160 microseconds; trains of 500-microseconds’ duration applied every 2 minutes), and on-demand patient-controlled neurostimulation (PCN) for 10 seconds to 60 seconds according to settings (available to PIMIDES I cohort). Stimulation intensity was based on finite element method modeling and experimental data from Liu et al8 (repetitive direct-current stimulation available in Lu et al9). D, Implantation of the device electrode was tailored to each patient and placed above the respective epileptic foci. Shown here is the distribution of electrode locations in the study population, projected to the right hemisphere.
The study protocols were approved by the ethics committees of all participating investigational sites and by the competent authorities in Germany (6 sites) and Belgium (1 site). All patients provided written consent to participate in the study. Both studies were registered in the German Clinical Trials Register and their meta-analysis under PROSPERO. This study followed the clinical investigation guidelines from the European Medical Device Regulation 2017/745.
Participants
Eligible patients were aged 18 to 75 years, with focal-onset seizures uncontrolled by at least 2 ASMs. In addition, patients had 3 or more seizures per month and a predominant epileptic focus that was temporolateral or extratemporal in origin. No invasive recordings were required for focus localization. Patients were not included if they had mesiotemporal or primary generalized epilepsy. In the PIMIDES I trial, patients had to have 1 seizure type with an initial aware phase during which ictal stimulation could be triggered. Further eligibility criteria have been described10 and are available in the eMethods of Supplement 4.
Procedures
Patients fulfilling inclusion criteria and prospective baseline seizure frequency assessment were implanted under general anesthesia with a neurostimulation system consisting of a pulse generator and a stimulation electrode. This system delivers FCS via the stimulation electrode implanted epicranially above the individual epileptic focus region (Figure 1B) and connected to a pulse generator implanted subcutaneously in the pectoral region. Stimulation was activated 1 month after implantation to separate possible surgery-associated outcomes from stimulation outcomes.
After a 1-month recovery period, stimulation was initiated with intermittent bursts of 100-Hz HFS performed every 2 minutes combined with 20 minutes of continuous cathodal DLS per day, with the central electrode acting as a cathode (Figure 1C). The stimulation amplitude was set below the individual perception threshold, up to a maximum of 4 mA, and could be adjusted as needed during the follow-up visits. Stimulation settings were kept constant during the 6 months evaluated except for minor adaptations to ensure that patients did not perceive the stimulation. In the PIMIDES I trial, patients could trigger additional pulses of HFS from 10 seconds to 60 seconds via a handheld device when they perceived early ictal symptoms,10 thereby not affecting the seizure count (primary pooled analysis outcome measure).
Outcomes
Patients recorded seizures in study-specific diaries. Data were collected at each study visit until the 8-month postimplant follow-up visit, which ensured the availability of a full data set for the sixth month of stimulation. Patients were also asked to complete the Quality of Life in Epilepsy–Problems (QOLIE-31-P11) questionnaire, the Seizure Severity Questionnaire (SSQ12), and the Neurological Disorders Depression Inventory for Epilepsy (NDDI-E13). In addition, participant neurocognition was monitored with the EpiTrack (Eisai) screening tool.14,15 All assessments were performed prior to implantation and at prespecified follow-up visits.
Adverse events (AEs) were evaluated by the investigators as either associated with or not associated with the device and/or procedure and were coded according to Medical Dictionary for Regulatory Activities, or MedDRA, coding system. Any serious AE (SAE) was reviewed by an independent data safety and monitoring board. The AE data presented in this article encompass the period from implantation to 8-month postimplant follow-up of each patient.
Statistical Analysis
Sample size, based on the 2 first in-human trials, was to include at least 30 participants. A prospective power calculation for the pooled analysis was performed. For the primary end point, treatment response was defined as a 50% reduction in seizure frequency. For a sample size of 30 participants, a 2-sided 95% CI for the proportion of treatment responders will extend 0.168, ie, 16.8% if the expected proportion of treatment responders is 0.33 (95% CI, 0.16-0.50). nQuery Advisor, version 8.3.1.0 (Statsols) was used for these calculations. Handforth et al11 reported a 16% response rate in the low-stimulation group.11 The prospectively defined primary efficacy end point of the pooled analysis was the responder rate, defined as a reduction of seizure frequency of at least 50% between the sixth month of stimulation and the prospective baseline. All patients in whom stimulation was activated were part of the efficacy analysis. Efficacy of stimulation was based on (1) the estimate of the seizure frequency reduction before and after stimulation in this single-arm trial and (2) a comparison with a historical low-stimulation (sham) group reported for vagus nerve stimulation (VNS).16 Secondary outcomes included reduction in seizure frequency, seizure severity, health-related quality of life, and treatment safety. All patients implanted with the neurostimulation device were part of safety analyses.
As single-arm studies were included, no further aspects for combining different types of studies had to be considered. Seizure frequency was analyzed via the monthly (defined as 30 days) seizure count for each participant and calculated from the seizure diary. A mixed-effects Poisson regression model was used to analyze the monthly seizure counts, including all seizure count measurements postbaseline, with the study center as a covariate and a random-participant effect. The responder rate was given with the corresponding 95% CI. Safety end points of the pooled analysis were summarized with corresponding exact 1-sided 95% CIs based on a binominal distribution. Further safety outcomes included the global incidence of AEs and device- or procedure-associated outcomes. Baseline characteristics and secondary end points were evaluated descriptively. Statistical analysis was performed from February to September 2021, using SAS, version 9.2 (SAS Institute) or later versions.
Results
Study Population
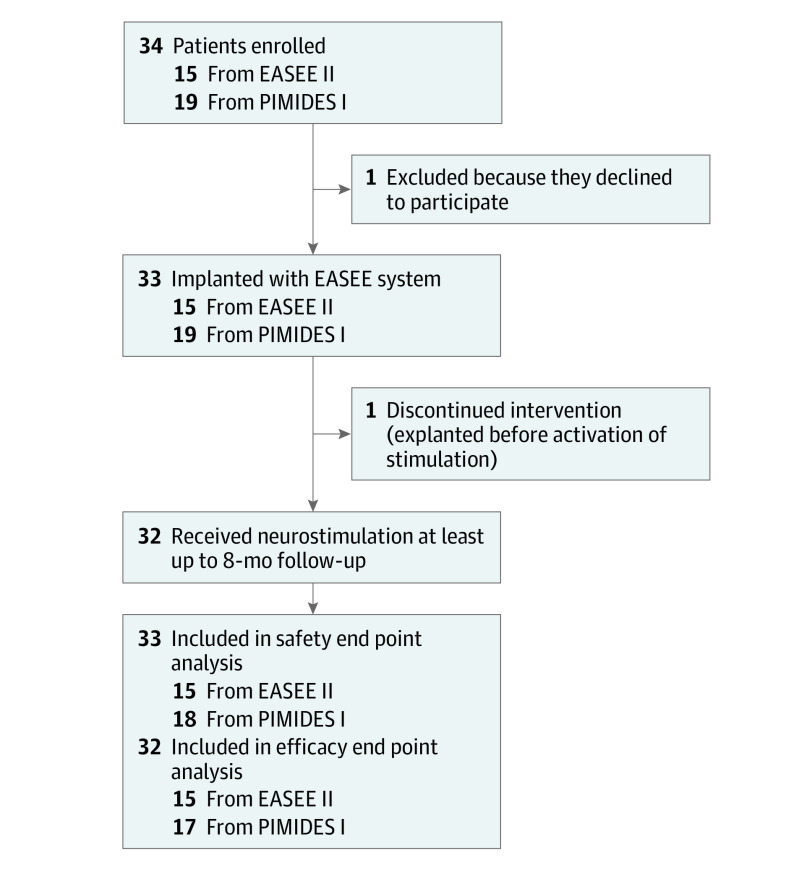
A total of 34 patients were enrolled at 6 German and 1 Belgian investigational site in the EASEE-II and PIMIDES-I trials from January Day, 2019, to November Day, 2020, and 33 (mean [SD] age, 34.6 [13.5] years; 18 male patients [54.5%]; 15 female patients [45.5%]) received the neurostimulation device implant (Figure 2). These 33 patients are considered to be the full analysis set of the pooled analysis in this study; individual trial results are available in eTables 1, 2, and 3 in Supplement 4.
Figure 2. Study Flow Diagram.
EASEE II indicates A Pilot Study to Assess the Feasibility of Neurostimulation With the EASEE System to Treat Medically Refractory Focal Epilepsy; PIMIDES I, A Pilot Study to Assess the Feasibility of Patient-Controlled Neurostimulation With the EASEE System to Treat Medically Refractory Focal Epilepsy.
Demographics for the patients who received the implant are shown in Table 1. The 33 patients had background treatment with 2 to 7 ASMs, of a spectrum of 13 different ASMs with different mechanisms of action. The most common ASMs were lamotrigine (16), brivaracetam (13), levetiracetam (9), and perampanel (6). Etiologies included malformations of cortical development (focal cortical dysplasia, heterotopias, polymicrogyria), postinflammatory and posttraumatic lesions, intracranial bleeding, and nonlesional cases with unknown etiology. Implantation sites included all brain regions on the dorsolateral convexities (Figure 1D). Twenty of 33 patients (60.6%) had placement of a left-sided implant.
Table 1. Demographic and Clinical Data.
| Patient characteristic | Total patients receiving implant (N = 33) |
|---|---|
| Sex, No. (%) | |
| Female | 15 (45.5) |
| Male | 18 (54.5) |
| Age, mean (SD) [range], y | 34.6 (13.5) [18-75] |
| Duration of epilepsy, mean (SD) [range], y | 20.4 (12.4) [3.2-66.2] |
| Baseline seizure count per 30 d, median (IQR) | 12 (2-147) |
| No. of antiseizure medications taken prior to enrollment, mean (SD) [range] | 7.8 (4.3) [3-15] |
| No. of antiseizure medications at baseline, mean (SD) | 3.2 (1.2) |
| ASM, No. (%) | |
| 2 | 12 (36.4) |
| 3 | 7 (21.2) |
| 4 | 11 (33.3) |
| 5 | 2 (6.1) |
| 6 | 0 (0.0) |
| 7 | 1 (3.0) |
| Prior vagus nerve stimulation, No. (%) | 4 (12.1) |
| Location of seizure onset, No. (%) | |
| Temporal | 15 (45.4) |
| Right | 4 |
| Left | 11 |
| Frontal | 9 (27.3) |
| Right | 4 |
| Left | 5 |
| Other | 9 (27.3) |
| Right | 4 |
| Left | 5 |
| Laterlization of seizure onset, No. (%) | |
| Right | 13 (39.4) |
| Left | 20 (60.6) |
Abbreviation: ASM, antiseizure medication.
Thirty-two of 33 patients who received implants underwent combined HFS and DLS stimulation at least until the 8-month postimplant follow-up. In 1 patient, the stimulation was not activated due to the presence of other metal implants in the skull. Intensities applied were set below the individual perception threshold. HFS was configured to a mean (SD) amplitude of 3.51 (0.68) mA and DLS to a mean (SD) amplitude of 1.58 (0.61) mA.
Efficacy
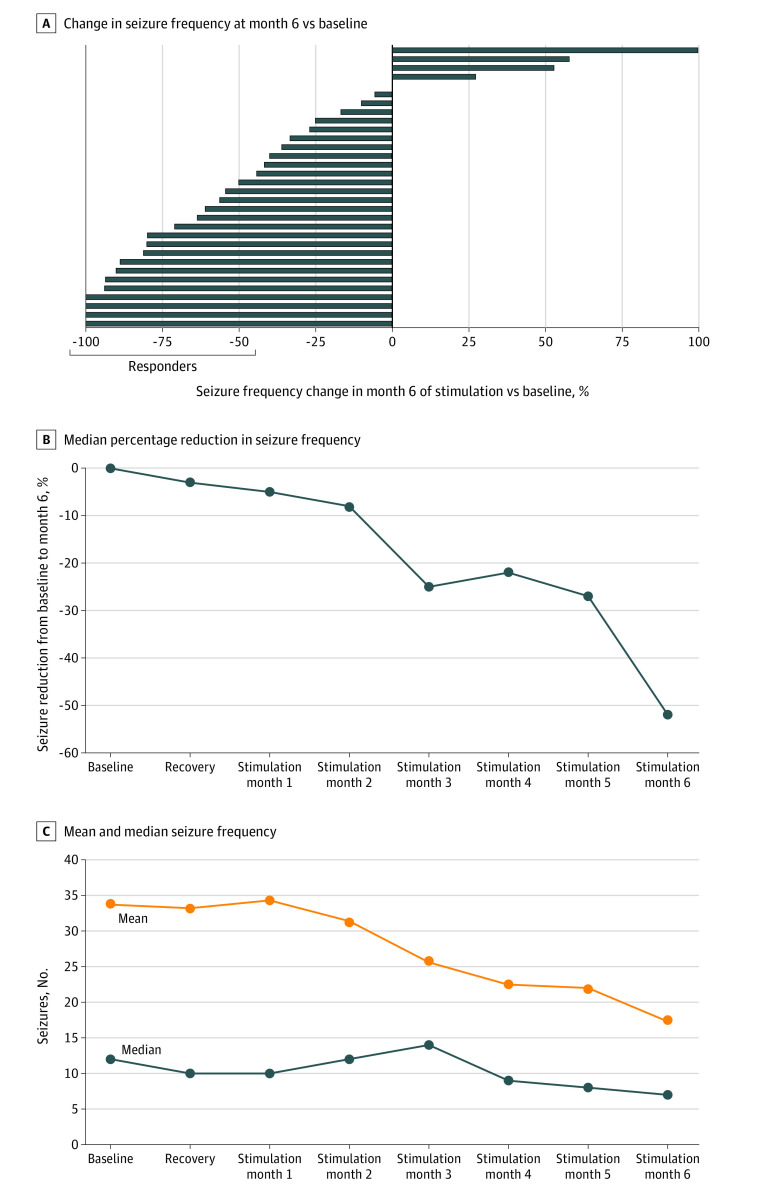
The results of the study suggest that neurostimulation with the novel neurostimulation device was associated with an effective reduction in seizures in patients with drug-refractory focal epilepsy. The primary outcome of the pooled analysis was a responder rate of 53.1% (17 of 32 patients) in the sixth month of stimulation compared with the baseline month (95% CI, 34.7%-70.9%). The lower limit of the CI lies entirely above the 16% response rate observed by Handforth et al16 in the low-stimulation group. Four of 32 patients (12.5%) had no seizures in the sixth month of active stimulation (Figure 3A). The responder rate increased throughout the study period, and all early responders during the first 3 months of FCS treatment (6 [18.8%]) remained responders in the subsequent 3 months. Furthermore, a mixed-effects Poisson regression model analysis of the monthly seizure count showed a statistically significant reduction in seizure frequency in the sixth month of stimulation by 52% (95% CI, 0.37%-0.76%; P < .001) compared with the prospective baseline.
Figure 3. Study Results and Efficacy End Points of the Pooled Analysis.
A, Individual outcomes of seizure frequency in the sixth month of stimulation vs baseline. In month 6, a total of 17 of 32 participants (53%) responded to treatment, and 4 of 17 (23.5%) were seizure free. B, Median percentage reduction in seizure frequency from baseline to the sixth month of stimulation. C, Mean and median seizure frequency on a group level over time.
Seizure frequency was largely unaffected by the device implantation procedure; the median seizure frequency gradually decreased to 52% in the sixth month of stimulation (Figures 3B and C). Changes in drug treatment did not explain treatment efficacy. The mean (SD) daily defined dosage of ASM17 was 4.1 (1.8) at baseline and 4.2 (1.8) during the sixth month of outcome assessment. Ictal stimulation was applied in 16 of 17 patients (94.1%) from the PIMIDES I trial, contributing to a median 0.3% of the total AC stimulation duty cycle. At a group level, no changes from baseline were observed in seizure severity as assessed by the SSQ or in quality of life as indexed by the QOLIE-31-P scores.
Safety
All patients who started neurostimulation continued treatment until the last follow-up. There were no deaths, and no AEs leading to discontinuation of treatment. In total, 11 SAEs were reported in 7 of 33 patients (21.1%) during the first 8 months. None of the 11 SAEs were considered to be associated with the device or procedure. At 4 months after device implantation, the absence of device- or procedure-associated SAEs was within a 95% CI of 0% to 10.58%.
Two patients (6.1%) experienced status epilepticus during the trial: 1 in a patient with a history of status epilepticus and ASM tapering before entering the study, and 1 in a patient who never received stimulation with the study device due to the presence of metal implants in the skull. Worsening of seizures was reported in 2 patients. In addition, 1 patient experienced a seizure cluster, and 1 patient had a seizure with fall. One SAE occurred before the stimulation was turned on (abdominal pain).
A total of 90 AEs reported in 25 of 33 patients (75.6%) were associated with device implantation (Table 2). Of these, 45 were mild, 39 moderate, and 6 severe. A total of 72 of 90 (80%) had resolved at month 8. There was an increase in the number of seizures (5), new types of seizures (2), the severity of seizures (1), and other worsening (1). Of the 7 patients (21.2%) with headaches, 5 experienced these events within 30 days after device implantation. Psychiatric symptoms included 2 patients (6.1%) with depressive symptoms (1 with worsening of a preexisting condition and 1 with symptoms attributed to COVID-19 pandemic restrictions). Additionally, memory impairment concomitant with an increase in ASM dose was reported in 1 patient (3.0%), and 1 patient each (3%) reported morning tiredness, which eventually resolved, and worsening of inner unrest.
Table 2. Serious and Nonserious Adverse Events Reported in More Than 5% of Patients.
| Type of adverse event | Patient, No. (%) (N = 33) |
|---|---|
| Seizurea | 9 (27.3) |
| Headache | 7 (21.2) |
| Injury, poisoning, procedural complication | 6 (18.2) |
| Infections (eg, nasopharyngitis) | 5 (15.2) |
| Musculoskeletal symptoms | 5 (15.2) |
| Other general disorders and administrative site conditions | 5 (15.2) |
| Dizziness | 4 (12.1) |
| Implant site pain | 4 (12.1) |
| Psychiatric symptoms | 4 (12.1) |
| Abdominal pain | 3 (9.1) |
| Gastrointestinal disorder | 2 (6.1) |
| Nausea | 2 (6.1) |
| Skin disorder | 2 (6.1) |
| Status epilepticus | 2 (6.1) |
| Vascular disorder | 2 (6.1) |
Worsening of seizure situation.
Procedure-associated events were reported in 10 of 33 patients (30.3%) and included headache (3 of 33 [9.1%]), hematoma (2 of 33 [6.1%]), and 1 patient each had a complaint of implant site pain, implant site pruritus, device site discomfort, device site pain, hypoesthesia, procedural headache, procedural pain, musculoskeletal discomfort, and scar pain. During the complete study period, neither a wound nor a device infection occurred.
Device-associated events were reported in 5 of 33 patients (15.2%), with 1 patient each reporting administration site dysesthesia, implant site pruritus, device site discomfort, incision site complication, wound pain, headache, and scar pain.
As part of the safety assessment, questionnaires on mood and a cognitive test battery were applied. No relevant changes in mood as assessed by NDDI-E could be observed, with mean (SD) scores of 13.0 (1.2) at baseline and 13.3 (1.2) at 8-month follow-up visit (mean change +0.30). Cognition as assessed by the EpiTrack tool showed a trend toward improvement, with mean (SD) scores of 27.7 (2.2) at baseline and 29.1 (2.1) at 8-month follow-up visit (mean change +1.30; P < .10).
Discussion
Results of this pooled analysis of 2 nonrandomized, uncontrolled, prospective, open-label studies of a novel neurostimulation device suggest a beneficial association of FCS with seizure frequency in patients with drug-refractory focal epilepsy. The primary goal of this research was to analyze the safety, performance, and efficacy of a novel device delivering epicranial FCS in patients with drug refractory focal epilepsy. The study results suggest class 3 evidence for antiseizure efficacy of this device.18
The observed reduction of seizure frequency was significant with regard to the prospective baseline, and the lower-limit CI of the observed response rate (34.7%) lies entirely above the historical sham response rate (16%) observed in historical controls for sham stimulation in VNS,17 which suggests a better response of patients treated with the novel neurostimulation device. The time course of changes in seizure frequency during epicranial stimulation showed no immediate effects after device implantation, which had been reported as insertional effects following implantation of intracranial stimulators and amounted to 20% to 25% in the respective studies.3,4,5 The progressive seizure reduction during FCS treatment, rather, is in line with a gradually manifesting neuromodulatory effect. Both responder rate (53.1%; 95% CI, 34.7%-70.9%) and the degree of seizure frequency reduction (median of 52.0%) in the sixth month of treatment were comparable with neurostimulation approaches using VNS,16 responsive neurostimulation5 and deep brain stimulation of the anterior nucleus of the thalamus (ANT-DBS)4 applied in similar patient populations with highly drug-resistant focal epilepsy.5 Note that seizure reductions reported in those trials were assessed with blinding at least in studies on intracranial stimulation, whereas in the trials reported here, stimulation occurred without blinding. The seizure reduction observed, however, also compares favorably with results from a recent prospective, unblinded registry of ANT-DBS stimulation, which reports a median seizure frequency reduction by 33.1% after 2 years of stimulation19 and with pharmacological options resulting from the unblinded change in ASM.20
Implantation of the novel neurostimulation device was tailored to each of the study patients with the electrode positioned epicranially according to the location of the respective predominant epileptic focus. Additional data are needed to evaluate if antiseizure outcomes associated with epicranial stimulation are limited to foci on the dorsolateral convexities or also extend to deep foci via network effects.
Subcutaneous electrode placement allows for the exertion of greater effects on neuronal population dynamics when compared with transcutaneous electrical stimulation as shown by both modeling and experimental recordings.8,21 Thus, the intensities chosen for treatment in the trials reported here were assumed to have relevant effects on neuronal firing rates at least on principal neurons in gyral crowns. Stimulation was performed by a combination of HFS and DLS, combining 2 approaches, which each have been shown to exert antiseizure effects in modeling and experimental approaches22,23 as well as under clinical conditions.24,25
Epicranial FCS has low invasiveness, and results suggest a remarkably positive safety profile. The extracranial positioning of the neurostimulation device electrode avoids risks of (1) intracranial hemorrhage with intracranial stimulation and (2) unwanted effects of vagus nerve lesioning or stimulation.4,19,20,26,27 Unlike VNS, FCS from a subgaleal position remains unperceived by patients when appropriate stimulation intensities are chosen and is also imperceptible to others. The tunneling procedure connecting the pulse generator to the lead is similar to that in VNS and DBS.
AEs reported in the study were mostly moderate or mild and transient and were frequently associated with the implantation sites (eg, local pain or discomfort in the area of implantation of the electrode or generator). A transient increase in seizure frequency as observed in some patients has also been reported in other neurostimulation trials,28 as well as in trials with add-on treatment with ASM.20,29,30,31 It remains unknown if this transient increase in seizure frequency was associated with the stimulation itself or if it reflects spontaneous fluctuations in seizure frequency. During the trial, 6.1% of patients undergoing FCS reported depressive symptoms, and 3.0% of patients reported memory impairment. Again, it remains unknown if these symptoms are associated with the stimulation. Similar to responsive intracranial neurostimulation of the epileptic focus,32 these incidences are lower than those with stimulation of the anterior nuclei of the thalamus (with 14.8% of patients reporting depression, and 13.0% reporting memory impairment4). With standardized assessment, mood as reflected by the NDDI-E showed almost unchanged group scores, and neurocognitive assessment showed a trend toward improvement. The incidence and type of AEs associated with the procedure and use of the novel treatment method presented here thus appear to be favorable in the context of currently available neuromodulation therapies for patients with drug-refractory epilepsy.33
Limitations
Limitations of the reported data are based on the absence of a control group; thus, placebo effects or the influence of measured or unmeasured confounding variables cannot be ruled out to have contributed to the observed reduction in seizure frequency. Additional data will be needed for response prediction and for a better understanding of the relative contributions of DCL stimulation and HFS and the best stimulation parameters.34 Long-term benefits and effects of FCS will be reported after the completion of ongoing studies.
Conclusions
Results of this pooled analysis of 2 single-arm, nonrandomized, uncontrolled trials suggest that FCS was associated with a significant reduction in the frequency of seizures. The safety profile of implantable epicranial stimulation was favorable compared with published safety profiles of alternative neuromodulation or surgical procedures and compared with the risks associated with uncontrolled seizures. Based on the clinical trial data, FCS has become available as a Conformité Européenne (CE)–certified device in Europe as a new treatment option for patients who experience drug-refractory focal epilepsy.
Trial Protocol
EASEE II Clinical Investigation Plan
PIMIDES I Clinical Investigation Plan
eMethods. Eligibility Criteria
eTable 1. Demographic and Clinical Data
eTable 2. Effectiveness Outcomes
eTable 3. Safety Outcomes
Nonauthor Collaborators
Data Sharing Statement
References
- 1.World Health Organization . Epilepsy. Published February 6, 2022. Accessed March 17, 2022. https://www.who.int/news-room/fact-sheets/detail/epilepsy
- 2.Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 2018;75(3):279-286. doi: 10.1001/jamaneurol.2017.3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulze-Bonhage A. Long-term outcome in neurostimulation of epilepsy. Epilepsy Behav. 2019;91:25-29. doi: 10.1016/j.yebeh.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 4.Fisher R, Salanova V, Witt T, et al. ; SANTE Study Group . Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899-908. doi: 10.1111/j.1528-1167.2010.02536.x [DOI] [PubMed] [Google Scholar]
- 5.Morrell MJ; RNS System in Epilepsy Study Group . Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295-1304. doi: 10.1212/WNL.0b013e3182302056 [DOI] [PubMed] [Google Scholar]
- 6.Fregni F, Thome-Souza S, Nitsche MA, Freedman SD, Valente KD, Pascual-Leone A. A controlled clinical trial of cathodal DC polarization in patients with refractory epilepsy. Epilepsia. 2006;47(2):335-342. doi: 10.1111/j.1528-1167.2006.00426.x [DOI] [PubMed] [Google Scholar]
- 7.Boëx C, Vulliémoz S, Spinelli L, Pollo C, Seeck M. High and low frequency electrical stimulation in nonlesional temporal lobe epilepsy. Seizure. 2007;16(8):664-669. doi: 10.1016/j.seizure.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 8.Liu A, Vöröslakos M, Kronberg G, et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat Commun. 2018;9(1):5092. doi: 10.1038/s41467-018-07233-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu H, Gallinaro JV, Rotter S. Network remodeling induced by transcranial brain stimulation: A computational model of tDCS-triggered cell assembly formation. Netw Neurosci. 2019;3(4):924-943. doi: 10.1162/netn_a_00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kravalis K, Schulze-Bonhage A. PIMIDES I: a pilot study to assess the feasibility of patient-controlled neurostimulation with the EASEE® system to treat medically refractory focal epilepsy. Neurol Res Pract. 2020;2(1):15. doi: 10.1186/s42466-020-00061-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramer JA, Perrine K, Devinsky O, Bryant-Comstock L, Meador K, Hermann B. Development and cross-cultural translations of a 31-item quality of life in epilepsy inventory. Epilepsia. 1998;39(1):81-88. doi: 10.1111/j.1528-1157.1998.tb01278.x [DOI] [PubMed] [Google Scholar]
- 12.Cramer JA, Baker GA, Jacoby A. Development of a new seizure severity questionnaire: initial reliability and validity testing. Epilepsy Res. 2002;48(3):187-197. doi: 10.1016/S0920-1211(02)00003-7 [DOI] [PubMed] [Google Scholar]
- 13.Metternich B, Wagner K, Buschmann F, Anger R, Schulze-Bonhage A. Validation of a German version of the neurological disorders depression inventory for epilepsy (NDDI-E). Epilepsy Behav. 2012;25(4):485-488. doi: 10.1016/j.yebeh.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 14.Lutz MT, Helmstaedter C. EpiTrack: tracking cognitive side effects of medication on attention and executive functions in patients with epilepsy. Epilepsy Behav. 2005;7(4):708-714. doi: 10.1016/j.yebeh.2005.08.015 [DOI] [PubMed] [Google Scholar]
- 15.Meschede C, Witt JA, Brömling S, et al. Changes in cognition after introduction or withdrawal of zonisamide versus topiramate in epilepsy patients: a retrospective study using Bayes statistics. Epilepsia. 2020;61(7):1481-1490. doi: 10.1111/epi.16576 [DOI] [PubMed] [Google Scholar]
- 16.Handforth A, DeGiorgio CM, Schachter SC, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51(1):48-55. doi: 10.1212/WNL.51.1.48 [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization (WHO) . Defined daily dose (DDD)—definition and general considerations. Accessed November 30, 2022. https://www.who.int/tools/atc-ddd-toolkit/about-ddd
- 18.Centre for Evidence-Based Medicine . OCEBM levels of evidence. Accessed November 30, 2022. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
- 19.Peltola J, Colon JA, Pimentel J, et al. Deep brain stimulation of the anterior nucleus of the thalamus for drug-resistant epilepsy in a real-world setting: MORE registry 2-year results. Published November 22, 2021. Accessed March 6, 2023. https://www.aesnet.org/abstractslisting/deep-brain-stimulation-of-the-anterior-nucleus-of-the-thalamus-for-drug-resistant-epilepsy-in-a-real-world-setting--more-registry-2-year-results
- 20.Janmohamed M, Lawn N, Spilsbury K, Chan J, Dunne J. Starting a new anti-seizure medication in drug-resistant epilepsy: add-on or substitute? Epilepsia. 2021;62(1):228-237. doi: 10.1111/epi.16765 [DOI] [PubMed] [Google Scholar]
- 21.Vöröslakos M, Takeuchi Y, Brinyiczki K, et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat Commun. 2018;9(1):483. doi: 10.1038/s41467-018-02928-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mina F, Benquet P, Pasnicu A, Biraben A, Wendling F. Modulation of epileptic activity by deep brain stimulation: a model-based study of frequency-dependent effects. Front Comput Neurosci. 2013;7:94. doi: 10.3389/fncom.2013.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikkelsen R, Andreasen M, Nedergaard S. Suppression of epileptiform activity by a single short-duration electric field in rat hippocampus in vitro. J Neurophysiol. 2013;109(11):2720-2731. doi: 10.1152/jn.00887.2012 [DOI] [PubMed] [Google Scholar]
- 24.Jarosiewicz B, Morrell M. The RNS System: brain-responsive neurostimulation for the treatment of epilepsy. Expert Rev Med Devices. 2021;18(2):129-138. doi: 10.1080/17434440.2019.1683445 [DOI] [PubMed] [Google Scholar]
- 25.Sudbrack-Oliveira P, Barbosa MZ, Thome-Souza S, et al. Transcranial direct current stimulation (tDCS) in the management of epilepsy: a systematic review. Seizure. 2021;86:85-95. doi: 10.1016/j.seizure.2021.01.020 [DOI] [PubMed] [Google Scholar]
- 26.Ramsay RE, Uthman BM, Augustinsson LE, et al. ; First International Vagus Nerve Stimulation Study Group . Vagus nerve stimulation for treatment of partial seizures: 2. Safety, side effects, and tolerability. Epilepsia. 1994;35(3):627-636. doi: 10.1111/j.1528-1157.1994.tb02483.x [DOI] [PubMed] [Google Scholar]
- 27.Révész D, Rydenhag B, Ben-Menachem E. Complications and safety of vagus nerve stimulation: 25 years of experience at a single center. J Neurosurg Pediatr. 2016;18(1):97-104. doi: 10.3171/2016.1.PEDS15534 [DOI] [PubMed] [Google Scholar]
- 28.Mao H, Chen Y, Ge Q, Ye L, Cheng H. Short- and long-term response of vagus nerve stimulation therapy in drug-resistant epilepsy: a systematic review and meta-analysis. Neuromodulation. 2022;25(3):327-342. [DOI] [PubMed] [Google Scholar]
- 29.European Medicines Agency . Briviact (in Italy: Nubriveo). Accessed August 22, 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/briviact-italy-nubriveo
- 30.The Vagus Nerve Stimulation Study Group . A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology. 1995;45(2):224-230. doi: 10.1212/WNL.45.2.224 [DOI] [PubMed] [Google Scholar]
- 31.Nair DR, Laxer KD, Weber PB, et al. ; RNS System LTT Study . Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology. 2020;95(9):e1244-e1256. doi: 10.1212/WNL.0000000000010154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loring DW, Kapur R, Meador KJ, Morrell MJ. Differential neuropsychological outcomes following targeted responsive neurostimulation for partial-onset epilepsy. Epilepsia. 2015;56(11):1836-1844. doi: 10.1111/epi.13191 [DOI] [PubMed] [Google Scholar]
- 33.Simpson HD, Schulze-Bonhage A, Cascino GD, et al. Practical considerations in epilepsy neurostimulation. Epilepsia. 2022;63(10):2445-2460. Published online August 9, 2022. doi: 10.1111/epi.17329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozák G, Berényi A. Sustained efficacy of closed loop electrical stimulation for long-term treatment of absence epilepsy in rats. Sci Rep. 2017;7(1):6300. doi: 10.1038/s41598-017-06684-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
EASEE II Clinical Investigation Plan
PIMIDES I Clinical Investigation Plan
eMethods. Eligibility Criteria
eTable 1. Demographic and Clinical Data
eTable 2. Effectiveness Outcomes
eTable 3. Safety Outcomes
Nonauthor Collaborators
Data Sharing Statement