Abstract
Members of the Numb-associated kinase family of serine/threonine kinases play an essential role in many cellular processes, such as endocytosis, autophagy, dendrite morphogenesis, osteoblast differentiation, and the regulation of the Notch pathway. Numb-associated kinases have been relevant to diverse diseases, including neuropathic pain, Parkinson’s disease, and prostate cancer. Therefore, they are considered potential therapeutic targets. In addition, it is reported that Numb-associated kinases have been involved in the life cycle of multiple viruses such as hepatitis C virus (HCV), Ebola virus (EBOV), and dengue virus (DENV). Recently, Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to threaten global health. Studies show that Numb-associated kinases are implicated in the infection of SARS-CoV-2 which can be suppressed by Numb-associated kinases inhibitors. Thus, Numb-associated kinases are proposed as potential host targets for broad-spectrum antiviral strategies. We will focus on the recent advances in Numb-associated kinases-related cellular functions and their potential as host targets for viral infections in this review. Questions that remained unknown on the cellular functions of Numb-associated kinases will also be discussed.
Keywords: NAK, Endocytosis, Autophagy, COVID-19, AAK1
Introduction
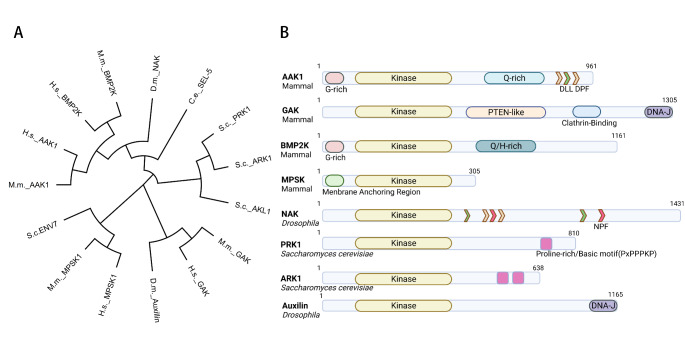
The Numb-associated kinase family is ubiquitous in mammals, yeast, Drosophila, and other model organisms (Fig. 1A). It consists of diverse serine/threonine kinases sharing about 30% sequence identity within their kinase domains at N terminal but little conservation in other domains (Fig. 1B). In mammals, NAK family has four members: cyclin G-associated kinase (GAK), adaptor-associated kinase 1(AAK1), BMP-2-inducible kinase (BMP2K, also known as BIKE), and myristoylated and palmitoylated serine/threonine kinase 1 (MPSK1, also named STK16/TSF1/PKL12/Krct). Among them, AAK1 and BMP2K exhibit high homology in structure, while some functions of AAK1 and GAK are redundant [1]. In Saccharomyces cerevisiae, members of actin-regulating kinases (Ark family), such as Ark1, Prk1, and Akl1, belong to the Numb-associated kinase family. Ark1 and Prk1 exhibit high identity in their kinase domains, but almost no similarity in their non-kinase C terminal domains exists. In addition, the homology of the kinase domain between Saccharomyces cerevisiae Ark1 and human AAK1 is 38% [2]. Saccharomyces cerevisiae Env7 is an ortholog of human MPSK1 [3]. Drosophila melanogaster has two known homologs: Numb-associated kinase (NAK) and the clathrin-disassembly factor auxilin. Drosophila melanogaster NAK shares 33% sequence homology to Human MPSK1 across the kinase domain [1]. The ortholog of NAK in Caenorhabditis elegans is Sel-5. As Serine /threonine kinases, the Numb-associated kinases usually perform their functions by phosphorylating the serine and threonine residues of their substrates. Many phosphorylated substrates of Numb-associated kinases have been identified so far (Table 1).
Fig. 1.
(A) Phylogenetic tree of NAK family produced from alignments generated using MEGA7 software. C.e., Caenorhabditis elegans; D.m., Drosophila melanogaster; H.s., Homo sapiens; M.m., Mus musculus; S.c., Saccharomyces cerevisiae
(B) Domain organization of NAKs. The kinase domain is located at N-terminal. The length of the region downstream of the kinase domain is variable. DPF motif (yellow) is an α-adaptin–interacting motif. NPF motif (red) confers binding to EH domains. DLL motif (green) is known to mediate clathrin binding (CBD2). Only two proteins in the family have DNA J domains (purple), located at their C-terminus. Ark1 contains two conserved motifs PxPPPKP, and Prk1 contains one copy, which closes to the C-terminus of both proteins
Table 1.
Phosphorylated substrates of NAK family. Substrates and their phosphorylation sites reported for Numb-associated kinases are listed. Furthermore, the related physiological functions are also summarized in this table
| Protein | Alias | Substrates | P-sites/motifs of the substrates |
Physiological function | Reference |
|---|---|---|---|---|---|
| AAK1 | AP-1 | T154 | Regulation of clathrin-dependent endocytosis; Endosomal pathway |
[10] [8] [27] |
|
| AP-2 | T156 | ||||
| Numb | T102 | Dendritic morphogenesis of developing mammalian neurons | |||
| Eps15 | - | Positive regulation of Notch signaling pathway | |||
| GAK | Auxilin | AP-2 | T156 |
Process clathrin coat disassembly; Receptor-mediated endocytosis |
[55] [56] [57] [58] |
| AP-1 | - | ||||
| Atp1a3 | T705 | Na+/K+-ATPase | |||
| CHC | T606 | Mitosis; Microtubule generation and outgrowth | |||
| Protein phosphatase 2 A | T104 | ||||
| BMP2K | BIKE | AP-2 | T156 | Clathrin-dependent endocytosis |
[14] [59] |
| CLINT1 | T294 | Cell signaling | |||
| MPSK1 | Krct, EDPK, STK16, TSF-1, PKL12 | MBP | - | Secretory vesicle trafficking |
[60] [61] [62] [63] |
| Histone H1 | - | ||||
| PHAS-1 | - | - | |||
| DRG1 | - | Cellular growth | |||
| PRK1 | Prk1p | Pan1 | The LxxQxTG motifs | Actin cortical patch assembly; Endocytosis; TORC2-Dependent Signaling Network in yeast |
[64] [65] [66] [18] [67] |
| Sla1 | The LxxQxTG motifs | ||||
| Ent1/2 | T394, T416 | ||||
| Scd5 | The LxxTxTG motifs | ||||
| Bni1 | The LxxQxTG motifs | Exocytosis | |||
| ARK1 | Ark1p | Pan1 | The LxxQxTG motifs | Actin cortical patch assembly; Clathrin-dependent endocytosis; TORC2-Dependent Signaling Network in yeast |
[64] [65] [18] |
| Sla1 | The LxxQxTG motifs | ||||
| AKL1 | Akl1p | Sal1 | The LxxQxTG motifs | Clathrin-dependent endocytosis; TORC2-Dependent Signaling Network in yeast |
[68] [22] [23] |
| Pan1 | - | ||||
| Ent1 | - | ||||
| Fpk1 | - | ||||
| Nak | AP-2 | T156 | Endocytosis in Drosophila |
[69] [70] |
|
| Numb | - | Asymmetric cell division | |||
| Auxilin | AP-2 | - | Clathrin coat disassembly; Receptor-mediated endocytosis; Compound eye morphogenesis; Notch signaling pathway | [55] | |
| AP-1 | - |
It is reported that the Numb-associated kinases are implicated in diverse cellular processes, including endocytosis, autophagy, dendrite morphogenesis, osteoblast differentiation, and the regulation of the Notch pathway. Therefore, they have been associated with a diversity of diseases and disorders such as neuropathic pain, Alzheimer’s disease, Parkinson’s disease, and prostate cancer. So far, members of the Numb-associated kinases have been considered as potential drug targets for related disorders including neuropathic pain and Parkinson’s disease [4, 5]. Recent researches indicate that Numb-associated kinases play a crucial role in viral replication. Human Numb-associated kinases are demonstrated to be implicated in the viral infection of HCV, DENV, EBOV, and rabies virus (RABV) [6]. COVID-19, caused by SARS-CoV-2, is still a threat to global health. Although vaccines can reduce the severity of COVID-19, vaccination for some special populations is limited, and the appearance of new viral variants is also a challenge. Thus, therapeutic antiviral drugs are needed. Silencing AAK1, BIKE, GAK, and MPSK1 individually with siRNA is reported to suppress SARS-CoV2 infection in Calu-3 cells. Moreover, inhibitors of Numb-associated kinases such as sunitinib, erlotinib, and gefitinib are demonstrated to suppress SARS-CoV-2 infection [7]. So Numb-associated kinases are also proposed as possible therapeutic targets for broad-spectrum antiviral strategies. We will focus on the recent advances in Numb-associated kinases-related cellular functions, and their potential as host targets for viral infections in this review.
Numb-associated kinases associated cellular functions
The role of Numb-associated kinases in endocytosis
Endocytosis is a fundamental cellular process that transports extracellular molecules, membrane proteins, and lipids into cells, and plays an essential role in nutrient uptake, receptor internalization, and signaling transduction. Depending on the cargo type and internalization mechanism, endocytosis has been divided into three major types: phagocytosis, pinocytosis, and receptor-mediated endocytosis. Numb-associated kinases play important roles in receptor-mediated endocytosis which includes clathrin-mediated endocytosis (CME), caveolae-mediated endocytosis, and clathrin and caveolae-independent endocytosis [10].
In mammals, AAK1 is a key regulator in receptor-mediated endocytosis. It has two physiological substrates: Numb and the plasma membrane adaptor complex AP2 (adaptor protein 2). On the one hand, AAK1 can phosphorylate Numb on Thr-102, thereby regulating the endocytic activity of Numb in clathrin-mediated endocytosis [8]. On the other hand, AAK1 is a binding partner for the medium subunits of the AP2 complex. AP2 acts as the bridge between cargo membrane proteins and clathrin lattice as a key component of the clathrin-mediated endocytic mechanism. Phosphorylation of the µ2 subunit of the AP2 complex on Thr156 is essential for the formation of coated vesicles during clathrin-mediated endocytosis. Earlier studies suggest that phosphorylation of µ2 subunit can increase the binding affinity between AP2 and membrane protein sorting signals [9]. AAK1 is a responsible kinase that phosphorylates the µ2 subunit of AP2 on Thr-156. The in vitro phosphorylation of µ2 subunit mediated by AAK1 is demonstrated to increase the binding affinity of AP2 to sorting signals [10]. Moreover, AAK1 localizes to the sites of endocytosis and copurifies with the AP-2 complex. All of this evidence strongly suggests that AAK1 is important in receptor-mediated endocytosis.
GAK, known as auxilin 2, is the ubiquitous homolog of the neuronal-specific protein auxilin 1, including an N-terminal kinase domain and a C terminal domain composed of the clathrin-binding domain, a J domain (DNA J domain), and a tensin domain [11]. GAK is reported to share some functions with AAK1. Similar to AAK1, GAK is also able to phosphorylate AP2 on Thr156 in vitro. But the kinase-dead mutant of GAK could rescue the phenotype caused by the knockdown of GAK in transferrin uptake assay, suggesting that the kinase activity of GAK is not essential to efficient endocytosis, or it is redundant by AAK1 [11]. Moreover, GAK is a cytosolic protein that is especially localized in the perinuclear area [12]. Its co-localization with AP1 and co-purification with the AP1 complex suggest that it may play an important role in the clathrin-dependent trafficking associated with the trans-Golgi network [11]. In addition, GAK binds to the heat shock cognate protein Hsc70 by its C terminus, playing an essential role in the uncoating of clathrin-coated vesicles as a cofactor to Hsc70 [12]. Both the clathrin-binding domain and the J domain in the C terminus of GAK are required for the clathrin uncoating activity of Hsc70. It is reported that the early stages of CME could be partially inhibited when GAK was silenced by specific siRNAs, and that GAK mutants without functional J domain cannot rescue this defect [11].
BMP2K is closely related to AAK1 in structure and also plays an important role in the regulation of clathrin-mediated endocytosis [13]. Shikha T. Ramesh et al. reported that BMP2K could regulate CME by phosphorylating AP2 on Thr-156 both in vivo and in vitro. They demonstrated that depletion of BMP2K obstructed AP2 phosphorylation resulting in defects in clathrin-coated pit morphology and internalization of cargo in Hela cells. BMP2K could bind with both α and β appendages of AP2 to be recruited to CCPs (clathrin-coated pits). However, it is the C terminus of BMP2K(561aa-1161aa) but not the kinase domain required for its interaction with AP2. BMP2K mutants without C terminus fail to interact with AP2 [14]. Moreover, BMP2K also interacts with the Numb and regulates the functions of the Numb in endocytosis, which is important for mammalian development [15].
Dynamic actin filaments are required for endocytosis. In Saccharomyces cerevisiae, endocytic vesicles have been demonstrated to move on the actin cable. Eps15-like protein Pan1 is essential both for actin cytoskeleton organization and endocytosis in yeast. On the one hand, Pan1 is involved in the activation of Arp2/3 which is the actin polymerization initiation complex. On the other hand, Pan1 could interact with multiple endocytic proteins and acts as a key scaffold protein in various events of receptor-mediated endocytosis. Studies show that Pan1 is a key regulator in the late stage of endocytosis in yeast [16]. Ark1/Prk1 kinases could regulate the interaction between actin cable and endocytic vesicles by phosphorylating coat proteins Pan1 and Ent1/2 [17, 18]. Phosphorylation of Pan1 by Ark1/Prk1 kinases leads to the disassembly of clathrin-coated vesicles. Sla1 is a key component of the endocytic machinery, which forms the Pan1-Sla1-End3 complex (yeast actin cytoskeleton regulatory complex) together with Pan1 and End3. Prk1 could regulate the dissociation of the Pan1-Sla1-End3 complex by phosphorylating Pan1 and Sla1. There is a functional overlap between Ark1 and Prk1. Depletion of ARK1 and PRK1 together results in severe abnormal actin cytoskeleton and endocytosis, whereas loss of neither Ark1 nor Prk1 causes significant defects [19, 20]. Although Ark1 and Prk1 share high identity in their kinase domain, there is almost no detectable similarity existing between the C terminal domains of Ark1 and Prk1. Studies show that the poly-P motif in the C terminus of Ark1 is responsible for its patch localization. A 21-aa motif in the C terminus of Prk1 is required for Prk1 to interact with Arp2 [19]. Similar to Prk1, Akl1 regulates endocytosis via phosphorylation of Pan1 in the Sla1-Pan1-End3 complex. Inhibition of the activity of Akl1 reduces the phosphorylation level of Pan1 and other endocytic coat proteins leading to slower endocytosis kinetics [21–23].
Modulation of Notch pathway
Numb-associated kinases are also involved in the regulation of the Notch pathway, which is relevant to various cellular processes such as cell proliferation, differentiation, and cell fate assignation [6, 24]. The Notch signaling pathway is normally activated by the interaction between the Notch receptor and the Notch ligand. Following engaging with ligands, the extracellular domain of the Notch receptor is cleaved by a disintegrin and metalloprotease. Subsequently, the intracellular domain is cleaved from the transmembrane domain by multiprotein γ-secretase complex and then translocated to the nucleus to regulate the transcription of target genes [25, 26].
AAK1 is demonstrated to directly interact with the activated metalloprotease-cleaved Notch to positively modulate the Notch signaling pathway. It is reported that overexpression of AAK1 contributes to the stabilization of activated Notch, while depletion of AAK1 results in reduced Notch transcriptional activity [27]. Several proteins implicated in CME have been demonstrated to participate in Notch trafficking in mammals, such as α-adaptin, Numb, Eps15, and clathrin [27, 28]. Numb, a substrate of Numb-associated kinases, regulates the Notch pathway negatively via binding to AP2 [29–31]. Numb is shown to inhibit monoubiquitination of the Notch intermediate and promote polyubiquitination of the intracellular domain of the Notch receptor to regulate its degradation. Thus, AAK1 and Numb act antagonistically in the notch pathway [27, 32]. Thereby AAK1 plays dual roles in the Notch pathway: the activation and redistribution of Numb and activation of the Notch.
The Notch pathway is evolutionarily conserved in all metazoans. Elimination of Sel-5 activity in C.elegans leads to inhibition of the constitutive activity of lin-12(d) which is similar to the metalloprotease-cleaved form of Notch [33]. In Drosophila, auxilin is required for the internalization of the Notch ligand Delta, which is critical for the activation of the Notch receptor [34]. The auxilin mutations in Drosophila interact specifically with Notch and disrupt several processes mediated by Notch [35].
The role of Numb-associated kinases in autophagy
Autophagy is a eukaryotic highly conserved degradation and recycling process in which cytoplasmic components including protein aggregates and damaged organelles are engulfed by autophagosomes and delivered to lysosomes or vacuoles for degradation [36]. Dysregulation of autophagy results in various diseases such as neurodegenerative diseases, cancer, and infections. Recent studies indicate that Numb-associated kinases are also involved in the regulation of autophagy. Jaroslaw Cendrowski et al. have reported that the two splicing variants of BMP2K played opposite roles in autophagy to modulate erythroid maturation. They found that BMP2K-L and BMP2K-S regulate SEC16A-dependent COPII assembly differentially by binding to SEC16A to modulate autophagy. BMP2K-L promoted autophagic degradation, whereas BMP2K-S inhibited autophagic degradation [37]. In addition, Autophagy machinery has been demonstrated to be essential to the internalization of MHC class I molecules. AAK1 seems to contribute to the internalization of MHC class I molecules depending on its recruitment by LC3B [38]. Mitophagy is a kind of selective autophagy to clear abnormal mitochondria, which is essential to maintain cellular homeostasis. Dysfunction of GAK is discovered to positively regulate PRKN-independent mitophagy which is essential in physiology and stress conditions [39].
Numb-associated kinases in dendrite morphogenesis, mitosis, cell differentiation
Other biological functions of Numb-associated kinases have also been reported in the relevant literature. AAK1 is shown to control dendrite development in hippocampal neurons as a substrate of NDR1 (Nuclear Dbf2-related kinase 1). Depletion of AAK1 with siRNA results in an increase of dendrite branching and length [40]. But how AAK1 contributes to limiting the growth of dendrites remains largely unknown. GAK can be implicated in mitotic progression by cooperating with clathrin. It is demonstrated that RNAi-mediated depletion of GAK causes activation of the spindle assembly checkpoint and multi-aster formation. The cell cycle will be arrested at metaphase in cells that lack GAK [41]. GAK is demonstrated to regulate microtubule outgrowth from kinetochores/chromatin via interaction with clathrin. Moreover, the absence of GAK results in a dramatical reduction of astral microtubules and defective spindle position during mitosis similar to the phenotype caused by the depletion of clathrin, indicating that GAK is essential for proper spindle positioning [42, 43]. The BMPs (bone morphogenic proteins) are reported to play a key role in skeletal development. Expression of BMP2K seems to be increased during osteoblast differentiation induced by BMP-2 in a mouse prechondroblastic cell line. When stably expressed, BMP2K negatively regulates osteoblast differentiation and mineral deposition. Thus, BMP2K, including a nuclear localization signal and a glutamine-rich region, is demonstrated to be implicated in the regulation of the cell differentiation process [44].
Numb-associated kinases as potential broad-spectrum antiviral targets
Numb-associated kinases are essential regulators of endocytosis which is a cellular process that could be hijacked by multiple viruses for their own benefit. Recent studies show that Numb-associated kinases participate in the life cycle of many viruses, thus they are proposed as potential host targets for the research and development of antiviral drugs. AAK1 is shown to be required for the infection of Rabies virus (RABV). AAK1 knockdown decreases, and AAK1 knockout inhibits, RABV infection in N2a cells [44]. Wang et al. reported that phosphorylation of AP2 by AAK1 was responsible for RABV entry. They also found that Sunitinib, an inhibitor of AAK1 and GAK, was able to increase the survival of mice challenged with RABV street virus [45]. Both AAK1 and GAK are reported to be implicated in the infection of HCV, DENV, and EBOV [46–48]. Gregory Neveu et al. have found that AAK1 and GAK could act on the assembly and entry of HCV in an AP2 phosphorylation-dependent manner [46, 47]. Moreover, Fei Xiao et al. demonstrated that AAK1 and GAK could regulate the cell-to-cell spread of HCV which is susceptibility to their inhibitors [49]. Furthermore, AAK1 and GAK are implicated in the entry and infectious virus production of multiple viruses such as DENV and EBOV depending on their binding partner APs. AAK1 or GAK inhibitors including sunitinib and erlotinib are demonstrated to have broad-spectrum antiviral potential to multiple RNA viruses such as HCV, DENV, and EBOV [48]. These data indicate that Numb-associated kinases are potential antiviral drug targets.
COVID-19 caused by SARS-CoV-2 still threatens global health. Recent studies have shown the involvement of Numb-associated kinases in the infection of SARS-CoV-2. Multiple inhibitors of AAK1 among FDA-approved drugs, including baricitinib, sunitinib, and erlotinib, are shown to block the infection of SARS-CoV-2 in the BenevolentAI’s knowledge graph [50], suggesting that AAK1 is a potential target for the treatment of COVID-19. In the absence of AAK1, BIKE, GAK, and MPSK1, the infection of SARS-CoV-2 in human lung epithelial cells is proved to be suppressed. In addition to erlotinib and sunitinib, 7-oxozeaenol which inhibits the activity of Numb-associated kinases also suppresses SARS-CoV-2 infection. Furthermore, Combination treatment with sunitinib and erlotinib exhibits a synergistic effect against SARS-CoV-2 infection [7]. In addition, inhibition of AAK1 by Jaktinib hydrochloride which is also a broad-spectrum JAK (Janus kinase) inhibitor is shown to slow the proliferation of SARS-CoV-2 [51]. The expression level of AAK1 in COVID-19 patients is significantly decreased compared with normal subjects, suggesting that COVID-19 patients may consume a large amount of AAK1 in the late stage of the disease [52]. All of the evidence strongly suggests that Pharmacological suppression of Numb-associated kinases can be a potential strategy to treat COVID-19. Identifying new therapeutic drugs against COVID-19 targeting Numb-associated kinases is on-going. Recently, Qin Lin et al. design and synthesize an analogue of baricitinib which has strong activity against AAK1, JAK1, and JAK2 compared to baricitinib. Baricitinib, an inhibitor of JAK1/2 is an approved drug that can regulate cytokine levels. Besides, Baricitinib also shows anti-viral activity by inhibiting AAK1 and GAK [53]. Thus, baricitinib has been used in the clinical investigation against SARS-CoV-2 infection [54].
Discussion
The Numb-associated kinase family widely exists in mammals, yeast, Drosophila, and other model organisms. They have various functions including endocytosis, autophagy, regulation of the Notch pathway, osteoblast differentiation, and dendrite morphogenesis. Since family members are now largely determined by the homology within kinase domains, it is important to better understand how kinase domains cooperate with the divergent C terminal of these proteins. Numerous studies on Numb-associated kinases have been performed in the last several decades. Members of this kinase family are discovered to participate in a diversity of cellular processes. But there are still many problems that need to be solved. AAK1 is highly homology to Saccharomyces cerevisiae Prk1, so it is speculated that Prk1 may also be involved in autophagy in Saccharomyces cerevisiae. Further experiments are needed to confirm this. GAK has been reported to be involved in dendrite morphogenesis, mitosis, and cell differentiation, but the details of how GAK works need to be further studied. In addition, there are still novel functions of these proteins that remain undetermined. Though Numb-associated kinases have been proposed as promising host targets for the treatment of COVID-19, it is challenging to design and synthesize inhibitors with high specificity. Numb-associated kinases play essential roles in clathrin-mediated endocytosis. It is not known if the disruption of endocytosis will result in undesirable side effects. More work is needed to understand their cellular functions and related regulation mechanisms to have a more comprehensive understanding of those kinases.
Abbreviations
- AAK1
adaptor-associated kinase 1
- AP1
adaptor protein 1
- AP2
adaptor protein 2
- BMP2K
BMP-2-inducible kinase
- CCPs
Clathrin-coated pits
- CME
Clathrin-mediated endocytosis
- COVID-19
Coronavirus disease 2019
- DENV
dengue virus
- EBOV
Ebola virus
- GAK
cyclin G-associated kinase
- HCV
hepatitis C virus
- Hsc70
heat shock cognate protein
- JAK
Janus kinase
- MPSK1
myristoylated and palmitoylated serine/threonine kinase 1
- NAK
Numb-associated kinase
- NDR1
Nuclear Dbf2-related kinase 1
- RABV
Rabies virus
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- siRNA
Small interfering RNA
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing Interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cuicui Ji, Email: jicuicui2021@bjut.edu.cn.
Juan Wang, Email: juanwang16@163.com.
References
- 1.Sorrell FJ et al (2016) Family-wide structural analysis of Human Numb-Associated protein kinases. Structure 24(3) 401–11. 10.1016/j.str.2015.12.015 [DOI] [PMC free article] [PubMed]
- 2.Smythe E, Ayscough KR. The Ark1/Prk1 family of protein kinases. Regulators of endocytosis and the actin skeleton. EMBO Rep. 2003;4(3):246–251. doi: 10.1038/sj.embor.embor776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manandhar SP, et al. Saccharomyces cerevisiae Env7 is a novel serine/threonine kinase 16-related protein kinase and negatively regulates organelle fusion at the lysosomal vacuole. Mol Cell Biol. 2013;33(3):526–542. doi: 10.1128/mcb.01303-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kostich W, et al. Inhibition of AAK1 kinase as a Novel Therapeutic Approach to treat Neuropathic Pain. J Pharmacol Exp Ther. 2016;358(3):371–386. doi: 10.1124/jpet.116.235333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latourelle JC, et al. Genomewide association study for onset age in Parkinson disease. BMC Med Genet. 2009;10:98. doi: 10.1186/1471-2350-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Gualda B, Schols D, De Jonghe S (2013) A patent review of adaptor associated kinase 1 (AAK1) inhibitors ( Expert Opin Ther Pat, 2021. 31(10): p. 911–936. 10.1080/13543776.2021.1928637 [DOI] [PubMed]
- 7.Karim M, et al. Numb-associated kinases are required for SARS-CoV-2 infection and are cellular targets for antiviral strategies. Antiviral Res. 2022;204:105367. doi: 10.1016/j.antiviral.2022.105367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorensen EB, Conner SD. AAK1 regulates numb function at an early step in clathrin-mediated endocytosis. Traffic. 2008;9(10):1791–1800. doi: 10.1111/j.1600-0854.2008.00790.x. [DOI] [PubMed] [Google Scholar]
- 9.Fingerhut A, von Figura K, Honing S. Binding of AP2 to sorting signals is modulated by AP2 phosphorylation. J Biol Chem. 2001;276(8):5476–5482. doi: 10.1074/jbc.M009516200. [DOI] [PubMed] [Google Scholar]
- 10.Ricotta D, et al. Phosphorylation of the AP2 mu subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J Cell Biol. 2002;156(5):791–795. doi: 10.1083/jcb.200111068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang CX, et al. Multiple roles for cyclin G-associated kinase in clathrin-mediated sorting events. Traffic. 2005;6(12):1103–1113. doi: 10.1111/j.1600-0854.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- 12.Greener T, et al. Role of cyclin G-associated kinase in uncoating clathrin-coated vesicles from non-neuronal cells. J Biol Chem. 2000;275(2):1365–1370. doi: 10.1074/jbc.275.2.1365. [DOI] [PubMed] [Google Scholar]
- 13.Borner GH, et al. Multivariate proteomic profiling identifies novel accessory proteins of coated vesicles. J Cell Biol. 2012;197(1):141–160. doi: 10.1083/jcb.201111049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramesh ST, et al. BMP2K phosphorylates AP-2 and regulates clathrin-mediated endocytosis. Traffic. 2021;22(11):377–396. doi: 10.1111/tra.12814. [DOI] [PubMed] [Google Scholar]
- 15.Krieger JR, et al. Identification and selected reaction monitoring (SRM) quantification of endocytosis factors associated with Numb. Mol Cell Proteomics. 2013;12(2):499–514. doi: 10.1074/mcp.M112.020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enshoji M et al (2022) Eps15/Pan1p is a master regulator of the late stages of the endocytic pathway. J Cell Biol 221(10). 10.1083/jcb.202112138 [DOI] [PMC free article] [PubMed]
- 17.Yoshida N, et al. Cooperative regulation of endocytic vesicle transport by yeast Eps15-like protein Pan1p and epsins. J Biol Chem. 2021;297(5):101254. doi: 10.1016/j.jbc.2021.101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson HA, et al. In vivo role for actin-regulating kinases in endocytosis and yeast epsin phosphorylation. Mol Biol Cell. 2001;12(11):3668–3679. doi: 10.1091/mbc.12.11.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin M, Cai M. A novel function of Arp2p in mediating Prk1p-specific regulation of actin and endocytosis in yeast. Mol Biol Cell. 2008;19(1):297–307. doi: 10.1091/mbc.e07-06-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cope MJ, et al. Novel protein kinases Ark1p and Prk1p associate with and regulate the cortical actin cytoskeleton in budding yeast. J Cell Biol. 1999;144(6):1203–1218. doi: 10.1083/jcb.144.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng G, Yu X, Cai M (2001) Regulation of yeast actin cytoskeleton-regulatory complex Pan1p/Sla1p/End3p by serine/threonine kinase Prk1p Mol Biol Cell, 12(12): p. 3759-72. 10.1091/mbc.12.12.3759 [DOI] [PMC free article] [PubMed]
- 22.Takahashi T, Furuchi T, Naganuma A. Endocytic Ark/Prk kinases play a critical role in adriamycin resistance in both yeast and mammalian cells. Cancer Res. 2006;66(24):11932–11937. doi: 10.1158/0008-5472.Can-06-3220. [DOI] [PubMed] [Google Scholar]
- 23.Bourgoint C, et al. Target of rapamycin complex 2-dependent phosphorylation of the coat protein Pan1 by Akl1 controls endocytosis dynamics in Saccharomyces cerevisiae. J Biol Chem. 2018;293(31):12043–12053. doi: 10.1074/jbc.RA117.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou B, et al. Notch signaling pathway: architecture, disease, and therapeutics. Signal Transduct Target Ther. 2022;7(1):95. doi: 10.1038/s41392-022-00934-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moldovan GE, Miele L, Fazleabas AT. Notch signaling in reproduction. Trends Endocrinol Metab. 2021;32(12):1044–1057. doi: 10.1016/j.tem.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, et al. The role of notch receptors in transcriptional regulation. J Cell Physiol. 2015;230(5):982–988. doi: 10.1002/jcp.24872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta-Rossi N, et al. The adaptor-associated kinase 1, AAK1, is a positive regulator of the notch pathway. J Biol Chem. 2011;286(21):18720–18730. doi: 10.1074/jbc.M110.190769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta-Rossi N, et al. Monoubiquitination and endocytosis direct gamma-secretase cleavage of activated notch receptor. J Cell Biol. 2004;166(1):73–83. doi: 10.1083/jcb.200310098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berdnik D, et al. The endocytic protein alpha-adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev Cell. 2002;3(2):221–231. doi: 10.1016/s1534-5807(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 30.Santolini E, et al. Numb is an endocytic protein. J Cell Biol. 2000;151(6):1345–1352. doi: 10.1083/jcb.151.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron. 1996;17(1):27–41. doi: 10.1016/s0896-6273(00)80278-0. [DOI] [PubMed] [Google Scholar]
- 32.McGill MA, et al. Numb regulates post-endocytic trafficking and degradation of Notch1. J Biol Chem. 2009;284(39):26427–26438. doi: 10.1074/jbc.M109.014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tax FE, et al. Identification and characterization of genes that interact with lin-12 in Caenorhabditis elegans. Genetics. 1997;147(4):1675–1695. doi: 10.1093/genetics/147.4.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eun SH, Banks SM, Fischer JA. Auxilin is essential for Delta signaling. Development. 2008;135(6):1089–1095. doi: 10.1242/dev.009530. [DOI] [PubMed] [Google Scholar]
- 35.Kandachar V, Bai T, Chang HC. The clathrin-binding motif and the J-domain of Drosophila Auxilin are essential for facilitating notch ligand endocytosis. BMC Dev Biol. 2008;8:50. doi: 10.1186/1471-213X-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis S, Wang J, Ferro-Novick S. Crosstalk between the secretory and Autophagy Pathways regulates autophagosome formation. Dev Cell. 2017;41(1):23–32. doi: 10.1016/j.devcel.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cendrowski J, Miaczynska M. Splicing variants of an endocytic regulator, BMP2K, differentially control autophagic degradation in erythroid cells. Autophagy. 2020;16(12):2303–2304. doi: 10.1080/15548627.2020.1833501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loi M, et al. Macroautophagy proteins control MHC class I levels on dendritic cells and shape anti-viral CD8(+) T cell responses. Cell Rep. 2016;15(5):1076–1087. doi: 10.1016/j.celrep.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Munson MJ, et al. GAK and PRKCD are positive regulators of PRKN-independent mitophagy. Nat Commun. 2021;12(1):6101. doi: 10.1038/s41467-021-26331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ultanir SK, et al. Chemical genetic identification of NDR1/2 kinase substrates AAK1 and Rabin8 uncovers their roles in dendrite arborization and spine development. Neuron. 2012;73(6):1127–1142. doi: 10.1016/j.neuron.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimizu H et al (2009) GAK, a regulator of clathrin-mediated membrane traffic, also controls centrosome integrity and chromosome congression J Cell Sci, 122(Pt 17): p. 3145-52. 10.1242/jcs.052795 [DOI] [PubMed]
- 42.Tanenbaum ME et al (2010) Cyclin G-associated kinase promotes microtubule outgrowth from chromosomes during spindle assembly. Chromosoma 119(4) 415 – 24. 10.1007/s00412-010-0267-8 [DOI] [PMC free article] [PubMed]
- 43.Wolf B, Busso C, Gönczy P. Live imaging screen reveals that TYRO3 and GAK ensure accurate spindle positioning in human cells. Nat Commun. 2019;10(1):2859. doi: 10.1038/s41467-019-10446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo J et al (2020) Rhabdovirus infection is dependent on Serine/Threonine kinase AP2-Associated kinase 1. Life (Basel) 10(9). 10.3390/life10090170 [DOI] [PMC free article] [PubMed]
- 45.Wang C et al (2019) The Serine/Threonine kinase AP2-Associated kinase 1 plays an important role in rabies virus entry. Viruses 12(1). 10.3390/v12010045 [DOI] [PMC free article] [PubMed]
- 46.Neveu G, et al. Identification and targeting of an interaction between a tyrosine motif within hepatitis C virus core protein and AP2M1 essential for viral assembly. PLoS Pathog. 2012;8(8):e1002845. doi: 10.1371/journal.ppat.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neveu G, et al. AP-2-associated protein kinase 1 and cyclin G-associated kinase regulate hepatitis C virus entry and are potential drug targets. J Virol. 2015;89(8):4387–4404. doi: 10.1128/jvi.02705-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bekerman E, et al. Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. J Clin Invest. 2017;127(4):1338–1352. doi: 10.1172/jci89857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao F et al (2018) Interactions between the Hepatitis C Virus Nonstructural 2 protein and host adaptor proteins 1 and 4 Orchestrate Virus Release. mBio 9(2). 10.1128/mBio.02233-17 [DOI] [PMC free article] [PubMed]
- 50.Richardson P et al (2020) Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 395(10223). 10.1016/S0140-6736(20)30304-4. p. e30-e31 [DOI] [PMC free article] [PubMed]
- 51.Meng X, et al. Potential for jaktinib hydrochloride to treat cytokine storms in patients with COVID-19. Biosci Trends. 2020;14(3):161–167. doi: 10.5582/bst.2020.03106. [DOI] [PubMed] [Google Scholar]
- 52.Tang B, et al. The Landscape of Coronavirus Disease 2019 (COVID-19) and Integrated Analysis SARS-CoV-2 receptors and potential inhibitors in lung adenocarcinoma patients. Front Cell Dev Biol. 2020;8:577032. doi: 10.3389/fcell.2020.577032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin Q, et al. Design, synthesis, and biological evaluation of novel ruxolitinib and baricitinib analogues for potential use against COVID-19. Chem Biol Drug Des. 2023;101(3):760–771. doi: 10.1111/cbdd.14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalil AC, et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Umeda A, Meyerholz A, Ungewickell E. Identification of the universal cofactor (auxilin 2) in clathrin coat dissociation. Eur J Cell Biol. 2000;79(5):336–342. doi: 10.1078/s0171-9335(04)70037-0. [DOI] [PubMed] [Google Scholar]
- 56.Lin AW, et al. Chemical genetic identification of GAK substrates reveals its role in regulating na(+)/K(+)-ATPase. Life Sci Alliance. 2018;1(6):e201800118. doi: 10.26508/lsa.201800118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yabuno Y, et al. Clathrin heavy chain phosphorylated at T606 plays a role in proper cell division. Cell Cycle. 2019;18(16):1976–1994. doi: 10.1080/15384101.2019.1637201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naito Y, et al. Cyclin G-associated kinase regulates protein phosphatase 2A by phosphorylation of its B’γ subunit. Cell Cycle. 2012;11(3):604–616. doi: 10.4161/cc.11.3.19114. [DOI] [PubMed] [Google Scholar]
- 59.Schor S, et al. The cargo adapter protein CLINT1 is phosphorylated by the Numb-associated kinase BIKE and mediates dengue virus infection. J Biol Chem. 2022;298(6):101956. doi: 10.1016/j.jbc.2022.101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ligos JM, et al. Cloning, expression analysis, and functional characterization of PKL12, a member of a new subfamily of ser/thr kinases. Biochem Biophys Res Commun. 1998;249(2):380–384. doi: 10.1006/bbrc.1998.9163. [DOI] [PubMed] [Google Scholar]
- 61.Stairs DB, et al. Cloning and characterization of Krct, a member of a novel subfamily of serine/threonine kinases. Hum Mol Genet. 1998;7(13):2157–2166. doi: 10.1093/hmg/7.13.2157. [DOI] [PubMed] [Google Scholar]
- 62.Berson AE, et al. Identification and characterization of a myristylated and palmitylated serine/threonine protein kinase. Biochem Biophys Res Commun. 1999;259(3):533–538. doi: 10.1006/bbrc.1999.0811. [DOI] [PubMed] [Google Scholar]
- 63.Eswaran J, et al. Structure of the human protein kinase MPSK1 reveals an atypical activation loop architecture. Structure. 2008;16(1):115–124. doi: 10.1016/j.str.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeng G, Cai M. Regulation of the actin cytoskeleton organization in yeast by a novel serine/threonine kinase Prk1p. J Cell Biol. 1999;144(1):71–82. doi: 10.1083/jcb.144.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang B, et al. Negative regulation of the actin-regulating kinase Prk1p by patch localization-induced autophosphorylation. Traffic. 2009;10(1):35–41. doi: 10.1111/j.1600-0854.2008.00842.x. [DOI] [PubMed] [Google Scholar]
- 66.Henry KR, et al. The actin-regulating kinase Prk1p negatively regulates Scd5p, a suppressor of clathrin deficiency, in actin organization and endocytosis. Curr Biol. 2003;13(17):1564–1569. doi: 10.1016/s0960-9822(03)00579-7. [DOI] [PubMed] [Google Scholar]
- 67.Wang J, Neo SP, Cai M (2009) Regulation of the yeast formin Bni1p by the actin-regulating kinase Prk1p Traffic, 10(5): p. 528–35. 10.1111/j.1600-0854.2009.00893.x [DOI] [PubMed]
- 68.Roelants FM et al (2017) Complex 2-Regulated protein kinase Fpk1 stimulates endocytosis via inhibition of Ark1/Prk1-Related protein kinase Akl1 in Saccharomyces cerevisiae. Mol Cell Biol 37(7). 10.1128/mcb.00627-16 [DOI] [PMC free article] [PubMed]
- 69.Shan Z, et al. Basal condensation of Numb and Pon complex via phase transition during Drosophila neuroblast asymmetric division. Nat Commun. 2018;9(1):737. doi: 10.1038/s41467-018-03077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wrobel AG, et al. Temporal ordering in endocytic clathrin-coated vesicle formation via AP2 phosphorylation. Dev Cell. 2019;50(4):494–508. doi: 10.1016/j.devcel.2019.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.