Abstract
Introduction:
Hearing, vision, and cognitive impairment commonly co-occur in older people. However, the rate of recognition and appropriate management of combined hearing and vision impairment in people with dementia impairment is low. The aim of this work was to codevelop internationally relevant, multidisciplinary practice recommendations for professionals involved in the diagnosis, care, and management of older people with these concurrent conditions.
Methods:
We applied consensus methods with professional and lay expert stakeholders, using an adapted version of the World Health Organization Handbook for Guideline Development. The development involved 4 phases and included: (1) collating existing evidence, (2) filling the gaps in evidence, (3) prioritising evidence, and (4) refining the final list of recommendations. Each phase encompassed various methodologies including a review of existing guidelines within the 3 clinical domains, systematic reviews, qualitative studies, a clinical professional consortium, surveys, and consensus meetings with interdisciplinary domain experts.
Results:
The task force evaluated an initial list of 26 recommendations, ranking them in the order of priority. A consensus was reached on 15 recommendations, which are classified into 6 domains of “awareness and knowledge,” “recognition and detection,” “evaluation,” “management,” “support,” and “services and policies.” Pragmatic options for implementation for each domain were then developed.
Conclusion:
This is the first set of international, interdisciplinary practice recommendations that will guide the development of multidisciplinary services and policy to improve the lives of people with dementia and hearing and vision impairment.
Keywords: Care, Dementia, Hearing impairment, Practice recommendations, Quality of life, Visual impairment
Introduction
Hearing and vision impairments increase in prevalence with increasing age and are among the most common comorbid conditions in people living with dementia (PwD) and other age-related cognitive impairment [1]. The added burden of poor sensory function in an individual with dementia exacerbates the impact of dementia on a range of outcomes, including cognitive and functional ability, behavioural disturbances, quality of life (QoL), overall level of independence, and mortality [2, 3]. In parallel, the presence of dementia has a deleterious effect on the ability to support people with sensory impairment as PwD may not be able to report their sensory impairment, or it may be overlooked by care providers [4, 5]. The impact on care partners (any person involved in the care of PwD) is also significant, and social isolation, depression, care burden, and relationship stress increase with concurrent sensory and cognitive problems in the care recipient [6, 7].
Hearing and vision interventions are relatively inexpensive, effective, and acceptable for PwD; thus, addressing sensory impairments in PwD represents a potentially cost-effective and acceptable opportunity to improve outcomes for PwD and their care partners [7] and may be relevant to people in all socio-economic spheres. Both dementia and sensory impairment (hearing, vision, or dual sensory loss) require timely and accurate detection and assessment to ensure the most appropriate care and management. Neuropsychological screening and assessment of cognition often relies on good hearing and/or vision functioning [8]. Similarly, cognitive impairment may hinder the accurate assessment of sensory impairment. Older people, and particularly PwD, have a low rate of access to vision and hearing services [8, 9]. For those who do access hearing and vision services, the uptake and adherence with corrective devices, such as hearing aids or spectacles, may be low [10] and have not been studied in individuals with both vision and hearing impairment [11]. Thus, a more holistic approach to adapting assessments and optimising sensory function in PwD is warranted [12].
The Need for Practice Recommendations
National or international care standards for the effective detection, assessment, treatment, and management of concurrent hearing (and/or), vision, and cognitive impairment are presently not widely available to our knowledge. Hearing, vision, and dementia care professionals have historically worked independently, and therefore, there is limited sharing of information of relevance to clinical care across these healthcare disciplines [12, 13]. Existing guidance for dementia care (i.e., the National Institute for Clinical Excellence, UK [14], and the Alzheimer’s Association, Chicago, IL, USA [15]) offers general statements regarding the evaluation of other morbidities including sensory impairments but contains no specific recommendations on how evaluations should be conducted nor how management of sensory impairments should be undertaken or supported. Recently, the World Health Organization (WHO) guideline for the integrated care of older people in the community [16] recommended the maintenance of sensory health as a means to preserve physical and mental capacity. This recommendation reinforces the need for more specific guidance on hearing and vision impairment in PwD.
Recently, studies exploring knowledge, attitudes, and practices regarding the detection and management of combined hearing and/or vision impairment in PwD (HVD) in different international settings have revealed that professionals are aware of the potential comorbidity of sensory and cognitive impairments but are less confident in identifying and managing concurrent sensory and cognitive impairments [12, 13, 17]. For example, in a 3-nation European study of the support care needs of 98 PwD and their care partners, evidence emerged that multidisciplinary, individualised care was lacking and was desired [7]. In-depth interviews revealed that critical areas of concern for PwD and their care partners were the impact of sensory impairment on dementia-related behavioural and psychological changes, as well as loneliness, social isolation, and care partner burden and strain. Thus, significant gaps remain in the following:
the knowledge of professionals working across the fields of hearing, vision, and dementia care with respect to the complementary areas [13];
the detection and diagnosis of concurrent hearing and vision impairment in PwD, and the detection of cognitive impairment in people with hearing and vision impairment [12, 18, 19];
timely and appropriate interventions to improve hearing and vision in PwD, which may impact other outcomes, such as QoL and care partner burden [19, 20].
Scope of the Practice Recommendations
Our objective was to provide the first set of practice recommendations for the detection, evaluation, management, and support of people living with HVD, as well as outlining steps for implementation. The practice recommendations are directed at clinicians and other professionals involved in the diagnosis, general care, management, and support of people living with HVD. The aim was to foster the highest quality care to enable people to live well with combined sensory and cognitive impairments. Addressing the issue of concurrent HVD offers an opportunity to improve outcomes for PwD, including QoL and, potentially, alter the trajectory of cognitive decline [21, 22]. The recommendations are not discipline-specific or exhaustive. Moreover, they do not provide advice or guidelines regarding the specific diagnostic procedures that should be undertaken by dementia, vision, or hearing professionals. The task force plans to revisit the evidence informing these recommendations in 2 years from the time of publication.
Materials and Methods
Approach
An initial rapid review of the literature undertaken by J.L. and I.L., revealed a scarcity of peer-reviewed published information, rendering a rigorous systematic review unfeasible. Thus, the task force adopted core elements of the WHO Handbook for Guideline Development and derived evidence to support the recommendations from recent studies pertinent to specific recommendations. Where published evidence was lacking, we relied on various methods for elicitation of expert knowledge, such as workshops and consultation [23, 24]. Since the topic area is novel and multidisciplinary, and evidence is still emerging, it was important to adopt this approach, which does not rely on a single class of evidence alone.
International Hearing, Vision, and Cognition Practice Recommendations Task Force
The task force was an international panel of 16 hearing, vision, and dementia professionals, outlined in Table 1. Members were from Australia, Canada, the USA, Cyprus, Ireland, and the UK. The initial discussions for this project arose from an invited keynote address on the topic of sensory-cognitive health (The Brucker Lecture, delivered by I.L.) at the American Congress of Rehabilitation Medicine’s 95th Annual Conference in Dallas, TX, USA, October 2018. Following this, task force members were self-appointed or nominated by colleagues in the field for their expertise and links to professional bodies in one or more of the 3 fields. Members were recruited between November 2018 and June 2019.
Table 1.
Professional roles of task force and consortium members
| Task force members (n = 16) | Professionals consortium members (n = 41) | ||
|---|---|---|---|
| Academic clinical audiologist | 2 | Care home manager | 1 |
| Academic clinical otologist | 1 | Dementia specialist | 10 |
| Academic optometrists/low-vision specialist | 3 | Hearing specialist | 13 |
| Academic speech and language pathologist, expertise in communication and dementia | 3 | Occupational or rehabilitation therapist | 7 |
| Geriatric psychiatrist, specialising in dementia | 2 | Social worker | 2 |
| Occupational therapist, specialising in sensory interventions for people with dementia | 1 | Unknown | 1 |
| Postdoctoral fellow in sensory cognitive health | 1 | Vision specialist | 7 |
| Rehabilitation gerontologist, expertise in neurodegeneration and sensory impairment | 2 | ||
| Rehabilitation neuropsychologist | 1 | ||
Principles Underlying the Recommendations
At the outset, the task force agreed on a set of guiding principles to underpin the recommendations. A key focus was the promotion of a person-centred approach that was pragmatic and internationally relevant. We considered various contextual levels (including professional care pathways), geographies, policies, and socio-economic factors. Accordingly, key principles underlying the recommendations were to: (1) be inclusive of people living with sensory impairment and dementia, their care partners, and professional stakeholders; (2) promote equity and mutual respect across the 3 fields; and (3) be pragmatic, implementable, and resource sparing.
Steps in Developing the Recommendations
We have outlined our stepwise approach in Figure 1. In brief, this involved 4 phases: (I) collating existing evidence; (II) filling the gaps in evidence; (III) prioritising the evidence, and (IV) refining the final list of recommendations.
Fig. 1.
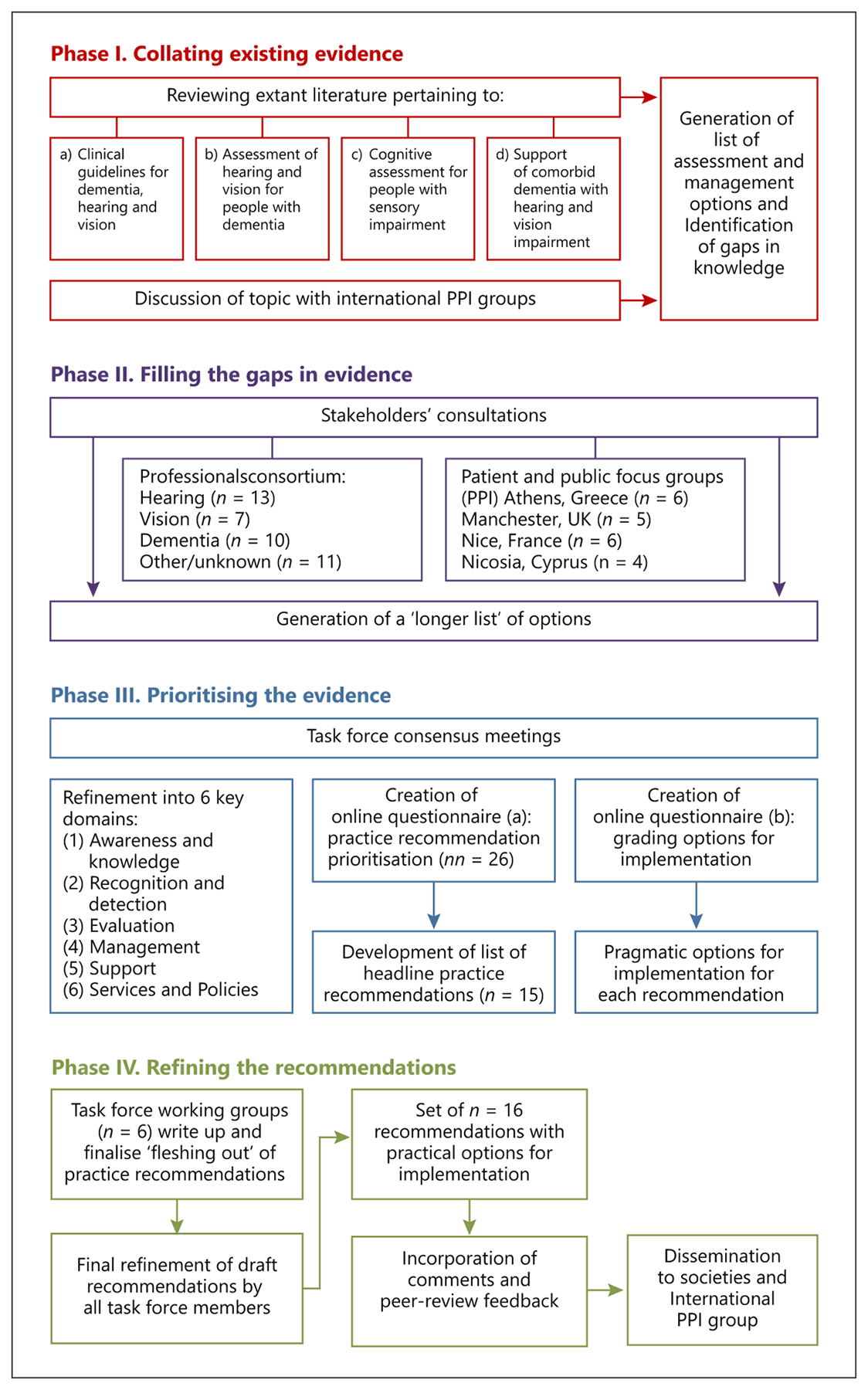
Steps to developing recommendations. PPI, patient and public involvement.
In phase I, we undertook scoping reviews of the extant literature and evidence regarding: (a) existing clinical guidelines in the 3 fields (hearing, vision, and dementia) to ascertain whether or not they refer to the dual or triple morbidity, (b) interventions to support hearing and vision impairment for PwD, (c) assessment of hearing and vision impairment in PwD, and (d) cognitive evaluation tools in PwD with hearing and/or vision impairment. The initial rapid review of this area highlighted a paucity of information, and thus, the search strategy was broadened to encompass all study designs and explore the breadth and depth of the evidence. We specified computer searches of electronic databases as well as hand-searching reference lists and identification of additional articles of interest by task force members. We did not include strict inclusion and exclusion criteria, except for practical reasons; only articles in English language were included. The existing clinical guidelines were identified through repositories such as the Guidelines International Network, CPG InfoBase: Clinical Practice Guidelines, and a search on PubMed. Search terms in PubMed for the literature (b–d) included dementia AND (sight OR vision OR hearing OR deaf) AND (intervention OR management OR rehabilitation OR assessment OR evaluation). For each article, 2 members of the task force graded the methodological quality of each study according to study design and risk of bias. Any differences in scores were resolved by discussion by the 2 graders and the lead researcher. Study design was reported descriptively according to the Oxford 2011 Levels of Evidence criteria [25]; and, if appropriate, the quality of intervention studies was assessed using Downs and Black’s checklist for randomised and non-randomised studies [26].
We then summarised and synthesised the findings from the literature to ascertain gaps in knowledge and generate a list of potential recommendations that broadly fell into one or more of the following 6 domains: awareness and knowledge, recognition and detection, evaluation, management, support, and services and policies. In phase II, we conducted a professional stakeholders’ consultation in the UK, held in Manchester on April 5, 2019, as a half-day event. We recruited 41 additional professionals, all of whom had experience in the diagnosis, management, and care of people living with impairment in one or more of the 3 fields (see Table 1). Following an initial didactic session on each of the 3 topics (dementia, hearing, and vision impairment), participants were divided into breakout groups to undertake facilitated discussions. The groups comprised 6–8 professionals as well as a member of the European SENSE-Cog research programme (www.sense-cog.eu; professionals, academics, and researchers), who facilitated each group by guiding the discussion and capturing feedback using field notes. We purposefully balanced the make-up of the groups a priori to include a mix of expertise, gender, and years of experience to ensure a range of views and perspectives. Within the groups, delegates were asked to express their views on a series of questions related to detection, evaluation, and management of HVC impairment in older people across different settings (home, clinic, and care home). Brief prototype case studies were used to prompt discussion and elicit feedback. The facilitator kept the participants on topic with guided questions and probed suggestions made by the group to achieve depth and clarity. Following the breakout sessions, the participants reconvened for a shared plenary discussion, led by an academic geriatric psychiatrist (I.L.), at which the facilitators fed back the discussion findings from each of the groups. This enabled group themes and differences to be highlighted and discussed and further summary points to be elicited and captured.
In this phase, we also consulted with lay stakeholders to ensure robust “patient and public involvement” (PPI) input. PPI is acknowledged as an important part of guideline development as it recognises that patients are experts by experience of their health conditions [27, 28]. We presented the 6 domains as areas of importance and asked for recommendations from 4 PPI focus groups in the UK (n = 5), Cyprus (n = 4), Greece (n = 6), and France (n = 6). All PPI members were experienced in providing feedback to clinical researchers regarding the assessment and management of PwD with hearing and/or vision problems. (A full description of the PPI methodology can be found in online suppl. material 1; for all online suppl. material, see www.karger.com/doi/10.1159/000515892.) Feedback was captured from all groups using field notes and added to the draft list of recommendations originating from phase I to create 26 recommendations across the 6 domains.
In phase III, we created 2 online surveys for a “prioritisation” exercise, which members of the task force (n = 16) completed. The methodology for both surveys was adapted from a robust decision-making method developed by the Child Health and Nutrition Research Initiative [29]. For the first survey (phase III a), we presented an initial draft list of 26 headline recommendations. These were generated by group-specific outputs from phases I and II according to the 6 domains. We asked respondents to rate each recommendation from the draft list as: (1) “yes,” (0) “no,” (0.5) “I do not know,” or “unsure,” for each of 4 scoring criteria. The criteria were: (a) potential for the recommendation to reduce the burden of dementia on those involved (i.e., people with dementia, care partners, and the society as a whole); (b) potential for the recommendation to be translated into practical impacts; (c) potential for the recommendation to achieve its stated outcome within a reasonable period of time; and (d) potential for the recommendation to be delivered an equitable way (considering socio-economic and demographic factors, particularly gender, education level, and geography). This led to the generation of intermediate scores calculated for each recommendation as the sum of scores divided by the number of scorers for each of the 4 scoring criteria. An overall score was then obtained for each recommendation by calculating the mean of the 4 intermediate scores, yielding a score with a possible range of 0–1. As 0.5 was the score given to responses “I don’t know or unsure” and 1 was the score given to “yes,” it was decided to be a priority recommendation, and thus, to be considered for further inclusion, it must have an overall score of ≥0.80.
For survey 2 (phase III b), each reference, recommendation, and idea generated in phases I and II were collated as “options,” duplications were removed and further explored against the 15 headline practice recommendations. This time, 3 criteria were chosen: (a) this option supports implementation of the recommendation; (b) this option has the opportunity to be delivered in an equitable way (considering socio-economic and demographic factors); and (c) this option can be delivered in a timely manner. Once more, the task force rated each option as: (1) “yes,” (0) “no,” (0.5) “I do not know,” or “unsure.” Again, for each option, there was an intermediate score for the 3 criteria, which was summed and averaged to yield overall scores.
For phase IV, the task force was then split into 6 working groups, each assigned a domain with the responsibility to discuss options for implementation of their respective domain from the prioritisation output, as well as further literature pertinent to that specific domain. Where gaps were identified and information was lacking, consensus meetings by all task force members enabled pragmatic solutions for implementation based on clinical and academic judgement. All members of the task force then critically appraised the final manuscript before submission.
Results
The output of phases I to III (a) in the form of draft recommendations, along with supporting evidence and the metrics, is collated in Tables 1–6 of online suppl. material 2. Overall scores from survey 1 ranged from 0.66 to 0.92. The task force critically evaluated the recommendations. Recommendations which yielded an overall score of <0.80 was interpreted as making the item a low priority and thus was discarded (n = 10). Wording of the recommendations was further refined for readability, clarity, and succinctness, leaving 15 headline practice recommendations.
Table 6.
Practice recommendations for domain 5 “support” with steps to implement the recommendation [38, 55, 69, 70]
| PR | Steps, options, or solutions to implement each recommendation |
|---|---|
| PR 5.1 Inform people living with HVC impairment and their care partners of community support services, resources, and opportunities for social inclusion | Commissioning and provision of opportunities that would be stimulating, interesting, and suitable for people who are living with HVC impairment to engage with (TF) |
| Self-referral/social prescribing to local resources and opportunities where training has been undertaken to understand the needs of people living with HVC impairment (PC/PPI) | |
| PR 5.2 Training in extra support (i.e., communication techniques) specific to the needs of people living with HVC impairment should be made available to stakeholders | Stakeholders (such as staff members in care settings) need to take responsibility to ensure that the person who is living with HVC impairment is encouraged to wear their corrective devices (e.g., hearing aids/spectacles) and that these are current, correct, and in good working order. They may need to support the person who is living with HVC impairment to find and use these devices (TF) |
| If the person living with HVC impairment is using assistive devices to aid communication (such as hearing aids or a “pocket talker”), stakeholders need to ensure that this is switched on and that the person living with HVC is supported to use it optimally [38, 55] | |
| Communication approaches need to be adjusted to optimise the person living with HVC impairment’s abilities. This includes ensuring that the person living with HVC impairment can see them well in order to elicit attention and support lip-reading and adjusting the content and delivery of their speech (TF) | |
| Identify suitable places and environments for communication, that is, with low noise and good lighting, soft-furnishings etc. (PC/PPI) (see PR 5.3) | |
| Distractions should be minimised (e.g., calm environment and turning the TV off during conversation) (PC/PPI) | |
| Stakeholders need to ensure that there is a balance of sensory demands across the day, including times of lower sensory stimulation (i.e., quieter times) [69] (TF) | |
| PR 5.3 The living environment of the person living with HVC impairment should be optimised to foster safety and independence | The acoustic environment should be checked and optimised. This includes both private and public/communal spaces if the person is living in a residential facility. The set-up of the furniture in rooms should facilitate face to face communication (TF) |
| The visual environment should be checked and optimised, with particular attention to lighting, signage, adjusting areas of glare or shadow, and removing obstacles [70] | |
| The eating environment should be adjusted to optimise the person living with HVC impairment’s function. This could include [70] Minimising noise of cutlery and crockery through using tablecloths Ensuring good quality lighting Introducing visual contrast such as using coloured, non-patterned crockery Verbal description of food being offered | |
| Environmental prompts (such as reminders to use hearing/vision aids) should be considered (PC/PPI) | |
| If the person living with HVC impairment is cohabiting with a family member (such as their spouse), the family member should be made aware of recommendations for environmental adjustments and supported to adapt to the person living with HVC impairment’s needs (PC/PPI) | |
| Environmental safety needs such as flashing lights and vibration for smoke or carbon monoxide detectors are important to identify for persons including care partners with sensory loss make sure emergency notifications flash in room and vibrate for hearing and visually impaired (TF) | |
| Ensure environmental modifications are extended to day service settings or anywhere person will be supported (PC/PPI) |
PR, practice recommendation; PC/PPI, professional consortia and/or patient and public involvement groups.
Overall scores from survey 2 (phase III b) ranged from 0.60 to 1.00. Again, options with overall scores under 0.80 were considered as low priority and thus were discarded (n = 58), leaving n = 83 “priorities” to be considered for implementation by the task force working groups in phase IV. The output from all phases is described below, according to domain and as steps to implement the headline recommendations (Tables 2–7). For transparency of idea/item generation, each is referenced with the published research or policy article. Where published evidence was lacking, “PC/PPI” describes where gaps were filled by professional consortia and/or PPI groups and “TF” if this came from the authors’ task force consensus meetings.
Table 2.
Practice recommendations for domain 1 “awareness and knowledge” with steps to implement the recommendation [53–55]
| PR | Steps, options, or solutions to implement each recommendation |
|---|---|
| PR 1.1 Awareness, knowledge, and skills to address under-detection of HVC impairment should be increased among care professionals | Inclusion of training modules in the alternate field (i.e., HVC) in all courses for health and social care professionals, as well as undergraduate and postgraduate training levels for clinicians (PC/PPI) |
| Professionals working in the primary care as well as HVC fields should ask questions on the patient’s medical history including HVC problems to understand how they impact on assessment (PC/PPI) | |
| Partnership working - HVC clinicians could train and transfer skills and knowledge through reciprocal team presentations and shadowing of practice [53] (PC/PPI) | |
| Conferences and congresses may be used for professionals’ awareness and training (PC/PPI) | |
| PR 1.2 Awareness of the nature and impact of HVC impairment in older people should be raised in the general public | Include accessible and easy to read/comprehend information on websites where people living with HVC impairments may visit (e.g., Alzheimer’s Society, UK), social media, and media campaigns (TF, PC/PPI) |
| Consider the role of technology and telehealth for accessing harder-to-reach communities which increases awareness [54] (PC/PPI) | |
| Community awareness campaigns, prevention workshops and education programmes delivered by third-sector or multidisciplinary clinicians [55] (PC/PPI) | |
| Inclusion of posters and leaflets at GP practices, and GP level screen for HVC health if patient concerned about one of the domains to help raise awareness HVC problems can coexist (PC/PPI) |
PR, practice recommendation; PC/PPI, professional consortia and/or patient and public involvement groups.
Table 7.
Practice recommendations for domain 6 “services and policies” with steps to implement the recommendation
| PR 6.1 Services for the evaluation, management, and care of people living with HVC impairment should collaborate to establish communication and shared care pathways | All 3 disciplines should work together to develop assessment and interventions and build care pathways by means of cross-discipline training (TF) |
| All healthcare workers in the broader fields of older adult care need heightened awareness of added risks and burdens from overlapping cognitive and sensory impairments [13] | |
| Joint working is essential to maintaining individuals independence and QoL and ensuring the most appropriate forms/holistic rehabilitaion and support (PC/PPI). It can be undertaken through [53] (TF) Direct referrals for specialist assessments Joint assessments Community consultation service with a “named worker” specialising in the alternate field acting as liaison between HVC teams Knowledge transfer: shadowing of each other’s practice, team presentations, training, and skills | |
| PR 6.2 Health and care settings should have appropriate environments and adapted procedures to support people living with HVC impairment | Consider the patient and care partner journey/experience within your service setting or clinic for each type of appointment/engagement; taking into account how that journey/experience may be impacted by HVC impairments. Look for ways to improve or mitigate systems and processes so that people with HVC impairments can access your services and get the most from them (TF) |
| All 3 disciplines can adapt procedures for health appointments to support HVC (TF) (PC/PPI) Adapting appointment letters to have adequate font size and prompts to bring sensory-corrective devices if use them Availability of supportive equipment (i.e., low-cost amplification/acuity devices) in clinics to support the needs of people with HVC Consider whether additional time is needed for appointments, or whether more than 1, shorter appointments would be preferable - discuss options with PwD and their care partners If domiciliary visits are feasible within local funding models/service pathways, make sure people living with HVC are aware of this option Increase training of all staff patients will come into contact with regarding awareness of patients’ needs (see PR 1.1) | |
| Review environments and make changes to ensure fully accessible to people with HVC impairments, including both inside and outside of clinical environments (communal spaces, waiting areas etc) [70] (PC/PPI) Clean, pleasant, bright, comfortable, quiet, minimise hard surfaces, and disruptive noises Ensure adequate signage/directions to support people with HVC, that is, they should large, at appropriate height with good contrast | |
| Ensure all HVC health records are up to date and are available to clinicians and care providers working across the 3 fields as well as transfers to hospitals and care homes (PC/PPI) |
QoL, quality of life; PC/PPI, professional consortia and/or patient and public involvement groups; PwD, people living with dementia.
Domain 1: Awareness and Knowledge of the Links among HVD
To effectively recognise, manage, and support HVD, it is imperative that professionals and the public are aware of the implications of comorbid sensory and cognitive impairments in terms of prevalence, impact, identification, and treatment. Knowledge of the contribution of co-morbid sensory impairments is not consistent among dementia professionals, while knowledge of the functional aspects of dementia is low among hearing and vision professionals [13, 30]. Similarly, educating the public about dementia risk factors, such as sensory loss, is emerging as a good strategy to prevent or delay cases [31], and promoting good sensory health encourages active ageing in older adults with dementia [32]. PwD and their care partners may not realise communication or mobility limitations can be exacerbated by sensory impairments, and instead attribute such symptoms to dementia syndrome.
Two practice recommendations have been included for domain 1, aimed at increasing awareness of professionals, PwD, and caregivers to ensure individuals receive a timely diagnosis and treatment. These are outlined in Table 2, with suggestions to implement each recommendation.
Domain 2: Recognition and Detection of Hearing and Vision Impairment in People Living with Dementia
Hearing and vision impairments are common in PwD, with prevalence of hearing/vision impairment among those with cognitive impairment higher than that in the general population, whereas in the general population, approximately one-third of all adults older than 65 years have hearing loss [33] and 23% have vision loss [34]; one study reported 94% of attendees at a memory clinic had a hearing impairment, while 32.5% of people with dementia in a national sample had significant visual impairment [19, 20].
Hearing and vision impairments are under-recognised and under-treated in the general population and are particularly under-recognised among people with cognitive impairment [9, 35, 36]. Under-recognition of concurrent HVD is problematic for many reasons. Firstly, hearing/vision impairments may confound the results of cognitive assessments, which typically rely on good hearing/vision [37]. A person with unrecognised hearing/vision impairment may be incorrectly identified as having a cognitive impairment or may have the severity of cognitive impairment overestimated due to not being able to hear or see the test items.
Additionally, sensory impairments may reduce social engagement and QoL in PwD and increase dependency, behavioural and psychological symptoms, and rate of cognitive decline [38]. Sensory deprivation is associated with boredom and is classified as one of the most common unmet needs for PwD in care settings [39], where residents with sensory loss and dementia frequently have higher levels of inappropriate behaviours. Early sensory remediation may be preventative and has been shown to improve depression and neuropsychiatric symptoms in PwD [40, 41]. It is therefore imperative that hearing and vision impairments are recognised and detected among people with cognitive impairment and dementia [42].
Two practice recommendations have been included for domain 2, intended to identify hearing and vision loss in PwD with the aim to avoid the negative consequences associated with unrecognised HVD. These are outlined in Table 3, with suggestions to implement each recommendation.
Table 3.
Practice recommendations for domain 2 “recognition and detection” with steps to implement the recommendation [4, 40, 42, 56–59]
| PR | Steps, options, or solutions to implement each recommendation |
|---|---|
| PR 2.1 Accessible HVC screening tests should be widely available | Professionals should have access to validated and fast screening tools for the 3 domains, so that they can refer patients to other specialists if they identify impairments in another domains (PC/PPI) |
| Community clinics (in GP clinics or extra-care settings) should prioritise sensory screening, communicate the importance of undertaking screening and possible referral pathways for positive screens [4] (PC/PPI) | |
| Hearing and vision screening tests are widely available and can be undertaken through a range of self-administered questionnaires or mobile screening options. For examples see the NIH Toolbox* and Cochrane systematic review [56] | |
| Information on the validity and reliability of hearing and vision screening tests should be available for people with cognitive impairment and must consider the context of the screening. For example, tools, and strategies for adapting sensory screening for PwD have been discussed in detail [57] | |
| Cognitive screening tests are also widely available, but these may be impacted by hearing/vision impairment. There is a need for well-validated cognitive screening tests for people with hearing/vision impairment [58] | |
| PR 2.2 Specialist evaluations for cognitive decline should include hearing and vision screening initially and as part of regular clinical reviews, with referral to relevant specialist services if appropriate | The memory clinic referral system should request sight & hearing tests and embed in assessment paperwork for admissions [42, 59] (PC/PPI) |
| If sensory status is unknown, patients should be offered routine sensory screening as part of the memory clinic appointment before cognitive evaluation to ensure patient’s ability to properly perform during the evaluation [40, 42] | |
| For asymptomatic and nontreated patients, these could be basic vision (i.e., distance and near Snellen charts) and hearing (whispered voice, finger rub, and watch tick tests) screenings (TF) | |
| Professionals should recommend and encourage patients who fail screens to undertake full sensory assessments (PC/PPI) | |
| Direct referral pathways should be put into place (PC/PPI) |
PR, practice recommendation; PwD, people living with dementia; PC/PPI, professional consortia and/or patient and public involvement groups.
Domain 3: Evaluation (i.e., Specialist Assessment) of HVD
Most standard cognitive evaluations rely on items that require good hearing and vision, and inversely, evaluation of vision and hearing functioning depends on relatively intact cognitive functioning [43, 44]. Although highly prevalent, professionals and technicians administering cognitive assessments often do not identify or account for deficits in vision or hearing, and providers of vision and hearing services may not have adequate training or experience in evaluating individuals with cognitive impairments [13]. Therefore, to obtain valid assessment results, those carrying out the assessments need to take an individual’s sensory-cognitive health status into consideration.
Three practice recommendations, as outlined in Table 4, ensure other disciplines are considered when undertaking assessments. Some are general considerations for evaluating all hearing, vision, and cognitive impairments, with examples for how to adapt to the needs of specific services. More detailed advice regarding specific adaptations of assessments is beyond the scope of these recommendations.
Table 4.
Practice recommendations for domain 3 “evaluation” with steps to implement the recommendation [19, 47, 58, 60–68]
| PR | Steps, options, or solutions to implement each recommendation |
|---|---|
| PR 3.1 Professionals assessing cognitive function should take into account the impact of hearing and vision impairments on cognitive evaluations | Appointment letters should remind patients to bring their best-corrected hearing aids and glasses to the appointments (PPI/PC) |
| Increased awareness of environmental issues (see PR 6.2), that is, conduct testing in a quiet environment, one-on-one, and ensure the individual can see the test administrator’s face and gestures (TF) | |
| If not already undertaken, screening for hearing/vision should be undertaken (see PR 2.2) | |
| Consider which tests/assessment processes for evaluating PwD are suitable given HVC status - validated versions and grading systems of routine cognitive assessments that do not depend on vision and/or hearing are available and should be used if vision/hearing loss suspected [58, 60, 61] | |
| Routine provision of low-cost sensory-corrective devices (i.e., amplifiers and magnifiers) during cognitive testing may aid in obtaining accurate assessments [62, 63] | |
| Bring family members to help recall on the PwD’s history on vision and/or hearing problem (PC/PPI) | |
| Clinicians may need to raise awareness of other conditions and how they impact on assessment to the patient and care giver (PC/PPI) | |
| Give more time during the assessment (PC/PPI) | |
| PR 3.2 Professionals assessing hearing should take into account the impact of vision and cognitive impairments on hearing evaluations | Appointment letters should remind patients to bring their best-corrected glasses to appointments and provide information ahead of time about what to expect at the appointment for hearing assessment (PPI/PC) |
| Increased awareness of environmental issues (see PR 6.2) | |
| Allow adequate time for appointments with opportunities for breaks [19] (PC/PPI) | |
| Consider domiciliary evaluation if appropriate [64] | |
| Include caregiver/family members to help recall on the PwD’s history on hearing problem and allow to accompany during the whole appointment [64] (PC/PPI) | |
| Approach the assessment flexibly, it may be difficult for patients to follow instructions and do things which they once did [19] (PC/PPI) | |
| Consider alternative approaches to assess hearing abilities, such as presenting pulsed tones instead of continuous tones [64] | |
| If cognitive status is unknown and sufficient training has been undertaken, consider asking relevant questions to probe cognitive status or performing adapted or sensory-appropriate versions of cognitive screens before the hearing evaluation to find out the patient’s cognitive status ability to properly perform the evaluation (PC/PPI) | |
| PR 3.3 Professionals assessing vision should take into account the impact of hearing and cognitive impairments on evaluations of vision | Appointment letters should remind patients to bring their best-corrected hearing aids to appointments and provide information ahead of time about what to expect at the appointment for visual assessment (PPI/PC) |
| Increased awareness of environmental issues (see PR 6.2), that is, conduct testing in a quiet environment, one-on-one, and ensure the individual can see the test administrator’s face and gestures (TF) | |
| Allow adequate time for appointments with opportunities for breaks [19] (PC/PPI) | |
| Consider domiciliary evaluation visits if appropriate [19] | |
| Include caregiver/family members to help recall on the PwD’s history on hearing problem and allow to accompany during the whole appointment [64] (PC/PPI) | |
| Consider alternative approaches to assess visual acuity such as Teller Acuity Cards and ETDRS-letter chart that may work across a spectrum of cognitive impairment [65–67] | |
| Guidance for working with patients with visual loss and acquired cognitive impairment or dementia exists. For example, in the UK, the Royal College of Opthalmologists have produced quality statements [68] and the College of Optometrists have produced guidance for examining patients [47] | |
| Routine provision of low-cost amplification devices during testing may aid in obtaining accurate assessments, at minimum when providing instructions [62] | |
| Approach the assessment flexibly; it may be difficult for patients to follow instructions and do things which they once did; consider simple, shorter, objective tests, rather than subjective measures [19] (PC/PPI) | |
| If cognitive status is unknown, and sufficient training has been undertaken, consider asking relevant questions to probe cognitive status or performing adapted or sensory-appropriate versions of cognitive screens before the vision evaluation to find out the patient’s cognitive status ability to properly perform the evaluation (PC/PPI) |
PR, practice recommendation; PwD, people living with dementia; PC/PPI, professional consortia and/or patient and public involvement groups.
Domain 4: Management of Hearing, Vision, and Cognitive Impairment
A key theme that emerged from the evidence review and PPI groups is that for PwD, “diagnostic overshadowing” may occur, such that the focus for health and care management becomes the dementia, with other conditions or issues being missed, given lower priority, or ignored entirely. In addition, professionals providing care and health services to PwD may believe that due to dementia, there will be little that they can do to help an individual manage the hearing/vision condition. Care and health professionals involved in the overall care planning of PwD may lack specific knowledge about HVD and guidance on how to manage HVD impairments within the context of their specialism.
By providing recommendations for managing HVD, it is hoped that professionals and care partners will be enabled to better support PwD in managing their health and social care needs, maintaining their independence, and participating in activities of daily life. As outlined in Table 5, three practice recommendations have been included for domain 4 with suggestions to implement and promote person-centred and informed management approaches for HVD and their care partners.
Table 5.
Practice recommendations for domain 4 “management” with steps to implement the recommendation
| PR | Steps, options, or solutions to implement each recommendation |
|---|---|
| PR 4.1 Management approaches (strategies and devices) for people living with HVC impairment should be person-centred, interdisciplinary, and tailored to the needs of the person | When offering strategies and devices, consideration should be given to the persons communication needs in the context of their HVC impairments - alternate management approaches must be appraised, for example, for hearing loss, can the individual benefit from hearing aids or would other assistive listening devices be more appropriate in their specific circumstance [62] (PC/PPI) |
| Strategies to support adherence to devices need to be person-centred to help promote independence Two contrasting shades for spectacles that are for correction of different prescriptions (TF) Large/pictoral information may be beneficial, such as a picture of the individual wearing their glasses by the mirror or step-by-step instructions for routines (PC/PPI) Label devices with owner’s name or initials - ensure that PwD preferences are sought with regard to how items are labelled where possible (PC/PPI) | |
| Simulation of benefits for patient and care partners may help to facilitate cooperation, that is, asking patient to watch TV with and without the equipment so they can see the difference first hand (PC/PPI) | |
| PR 4.2 People living with HVC impairment and their care partners should have access to accessible information regarding the nature and effect of the overlapping conditions | Acknowledgement of overlapping conditions to people living with HVC and care partners (PC/PPI) (see also PR 1.2) |
| Highlight the benefits of undertaking sensory interventions, for example, help to reduce burden due to communication difficulties [42] (PC/PPI) | |
| Use of simple language with lived examples and clarifying questions to check understanding (PC/PPI) | |
| Health professionals should explore the scope for providing information in a range of formats (e.g., written hard copies, electronic files such as emails and audio files) (TF) | |
| Professionals should discuss format preferences with HVC and their care partners, and a note should be kept with their patient record. Where feasible and relevant, explore the scope for sending updates or reminders in the formats preferred (TF) | |
| PR 4.3 People living with HVC impairment and their care partners should have access to accessible information regarding the management approaches (strategies and devices) | Always check the PwD and care partner, understand the instructions, and ask them to repeat the instructions back, to ensure they fully understand how to use it. Provide device information cards with clear written instructions for PwD and care partners to take with them. Where possible these should be laminated matt, not glossy, to reduce glare (TF) |
| Clear information regarding maintenance should also be accessible, that is, importance of cleaning hearing aids, replacing batteries, and tubes etc. and steps for troubleshooting, for example, if PwD does not seem to be hearing with hearing aids check for wax in the ear or make sure the battery is live and hearing aid is on (TF) | |
| Provide checklists to aid all with utilising strategies and devices (PCI/PPI) |
PR, practice recommendation; PwD, people living with dementia; PC/PPI, professional consortia and/or patient and public involvement groups.
Domain 5: Support for HVD (“Living Well with Dual or Triple Impairment”)
PwD who are living with hearing and vision impairment are at risk of reduced QoL and increased social isolation [38, 45]. They may find that their world “shrinks,” and they withdraw into themselves as they experience increased difficulty in communicating and engaging with the world around them. In order to support these individuals to live well, a multifaceted approach is warranted [7]. For example, many PwD and their care partners may not be aware of social inclusion opportunities that would meet their sensory needs. Equally, it is important that professionals and care partners offering treatment and care be trained to adapt their approaches and various environments to optimise the abilities of the individual with sensory and cognitive impairment. With the aim to promote safety, independence, and opportunities for social inclusion, 3 practice recommendations have been developed for domain 5 outlined in Table 6.
Domain 6: Services and Policies for People with HVC Impairment
Expansion of clinical competence in supporting HVD, as well as implementation and maintenance of change in health service provision over time, is only possible if developed in partnership with policy change. Traditionally, the fields of hearing, vision, and dementia care have been considered separately; however, recent health and social care trends for older adults indicate that sensory-cognitive ageing may transform from a multidisciplinary field (coming together of multiple separate health professions) to an interdisciplinary (several professions beginning to overlap), or even an emerging transdisciplinary field (several professions fusing to create an innovative and holistic care perspective). Only such a merged perspective can inform all care professionals involved and potentially open new pathways for research, treatment, and care provision. One example of this approach is the concept of frailty, where multiple changes in health status are considered in parallel, including changes in sensory and cognitive health [46].
At the policy level, few examples currently exist where inter- or transdisciplinary factors in HVD are acknowledged and/or incorporated. However, documents like the guidelines presented by the College of Optometrists in the UK on “Examining patients with dementia or other acquired cognitive impairment” [47] serve as guiding examples in bringing together professionals and their expertise in HVD. Ideally, these and other pioneering efforts to promote the importance of structuring and integrating HVD care (e.g., [48]) can then lead to the development and implementation of service delivery models that can appropriately accommodate the needs of persons with sensory-cognitive impairments.
Two practice recommendations have been included for the policy level. These are outlined in Table 7, with suggestions to implement each recommendation.
Discussion
Hearing and vision impairments commonly co-occur in PwD; however, their detection, diagnosis, and management represent an unmet need for older people with cognitive impairment or dementia. Based on the currently available health sciences literature, as well as the views of PwD and their care providers, this document presents the current consensus recommendations across 6 domains. Our approach, recommending a person-centred, multi-disciplinary path seeking to improve clinical and social care for HVD, is the first set of practice recommendations, which we hope will initiate an international exchange of ideas to move the development and refinement of care for this vulnerable population forward. We hope they will support all professionals working across health and social care to consider how they can adjust practice to better meet the needs of those living with combined sensory loss and dementia within the context in which they work.
The guidance presented here is only useful if it is accessible to the professionals and researchers for whom it has been designed. The task force, an interdisciplinary consortium of clinical and academic professionals, and the lay stakeholders with lived experience, who contributed to the development of the guidance, will support dissemination of the guidance to relevant groups, internationally. This will be supported by the national and international professional associations and institutions endorsing the work (outlined in acknowledgements) as well as snowballing by members of the wider sensory-cognitive community. This will include posting on partners’ Websites and linking with social media networks.
Although steps have been taken to ensure robust methodology and transparency regarding the development of these recommendations, there are some limitations. The main constraint is the lack of published literature across the 6 domains, rendering the level of evidence for the recommendations rather low. As this is the first set of recommendations for sensory-cognitive health, the practice recommendations rely heavily on expert professional opinion and the perspectives of those with lived experience, rather than substantive quantitative evidence. For example, the effects of hearing and vision impairment on QoL in PwD were excluded from a recent meta-analysis due to the lack of high-level evidence [49]. From our work with lay expert groups and professionals working in dementia care, we believe there is in fact a strong link between sensory loss and QoL in PwD and hope this gap in published evidence will be addressed by the findings from the ongoing large-scale randomised controlled trial in 5 countries, which is addressing the impact of hearing and vision rehabilitation on QoL in dementia [50]. As the field of sensory-health grows and the literature base emerges, future iterations of these recommendations will include higher level evidence to allow for systematically reviewing the literature and the production of more scientifically robust practice recommendations.
Further to this, methodological limitations from this study include the potential for selection bias by only including the literature published in English language and the threshold for which evidence was included during the prioritisation exercises (phase III). The task force chose an arbitrary, but pragmatic, cut-off point (of 0.80) for the generation of final practice recommendations which may have meant some examples and ideas were discarded.
In future iterations of this guidance, we may consider the topic of dementia prevention through sensory health, an area of emerging importance and growing evidence base [51]. In our preliminary guidance here, we purposefully omitted this area and focussed instead on the sensory health on individuals already living with dementia. However, as more evidence regarding the impact of hearing and/or vision remediation on prevention of dementia materialises, recommendations regarding prevention can be addressed.
The recommendations outlined here are intentionally broad, pragmatic and aimed at professionals working directly with individuals with sensory-cognitive challenges. As such, they will be relevant to the global health community. While we acknowledge that the task force, professional consortium, and lay stakeholder input have largely been generated from high-income countries, which may bias the recommendations towards better-resourced health and social care systems, evidence emerging from low- and middle-income countries, does appear to align with the recommendations we have made here [52]. Moreover, task force members span international boundaries and have the potential to activate global networks such as through the Global Brain Health Institute (GBHI; www.gbhi.org) whose aim is to work to decrease the burden of dementia globally. Further to this, the broad and pragmatic nature of the recommendations permits relevance across all socio-economic groups as they are low cost and relevant across different health systems and services. These recommendations are not intended to replace the many examples of specific dementia guidance that already exist. Instead, they are to be used in conjunction with the existing guidance to galvanise and empower professionals to focus their expertise on this vital area to fill the gap in practice. This guidance is preliminary and forms the foundation for future iterations as the transdisciplinary field of sensory-cognitive health in older adults develops and more robust evidence emerges. In the meantime, it can serve as a driver to improve services, rectify existing institutionalised dualism in which cognitive and sensory health are often disconnected and, ultimately, to better support PwD as well as sensory impairment.
Acknowledgement
The authors would like to thank people with lived, or caring, experience of dementia and sensory impairment for their invaluable input into this project, including the SENSE-Cog programme’s (www.sense-cog.eu) Research User Groups in Athens, Greece; Manchester, UK; Nice, France; Nicosia, Cyprus, and their PPI coordinators, Maria Alexaki and Maria Passa (Greece), Jahanara Miah (UK), Valeria Manera (France), and Anna Pavlina Charalambous (Cyprus). We also gratefully acknowledge support from the following international organisations: Deafblind International; International Society for Low Vision Research & Rehabilitation; and national organisations: Canadian Consortium on Neurode-generation in Aging (Canada); Deafness Support Network (UK); Ordre des Optometristes du Quebec (Canada); Royal College of Psychiatrists (UK); and the College of Optometrists (UK).
Funding Sources
J. Littlejohn is funded by Deafness Support Network (www.dsnonline.co.uk), DSN fellowship 2018-2022. I. Leroi’s contribution to this work was supported in part by the European Union’s Horizon 2020 research and innovation program (grant agreement No. 668648) for the SENSE-Cog Project (www.sense-cog.eu). C. Nieman was supported in part by the National Institute on Aging (K23AG059900).
Footnotes
Statement of Ethics
Ethical approval was not required for this study as it involved groups of professionals and members of the public acting as specialist advisors providing knowledge and expertise based on their professional or lived experience of the health conditions and as such are exempt according to the UK Policy Framework for Health and Social Care Research.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- 1.Guthrie DM, Davidson JGS, Williams N, Campos J, Hunter K, Mick P, et al. Combined impairments in vision, hearing and cognition are associated with greater levels of functional and communication difficulties than cognitive impairment alone: analysis of interRAI data for home care and long-term care recipients in Ontario. PLoS One. 2018;13(2):e0192971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nyman SR, Innes A, Heward M. Social care and support needs of community-dwelling people with dementia and concurrent visual impairment. Aging Ment Health. 2017;21(9): 961–7. [DOI] [PubMed] [Google Scholar]
- 3.Mitoku K, Masaki N, Ogata Y, Okamoto K. Vision and hearing impairments, cognitive impairment and mortality among long-term care recipients: a population-based cohort study. BMC Geriatr. 2016;16(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campos JL, Höbler F, Bitton E, Labreche T, McGilton KS, Wittich W. Screening for vision impairments in individuals with dementia living in long-term care: a scoping review. J Alzheimers Dis. 2019;68(3):1039–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGilton KS, Höbler F, Campos J, Dupuis K, Labreche T, Guthrie DM, et al. Hearing and vision screening tools for long-term care residents with dementia: protocol for a scoping review. BMJ Open. 2016;6(7): e011945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leroi I, Simkin Z, Hooper E, Wolski L, Abrams H, Armitage CJ, et al. Impact of an intervention to support hearing and vision in dementia: the SENSE-Cog field trial. Int J Geriatr Psychiatry. 2020;35(4):348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leroi I, Wolski L, Hann M. Support care needs of people with hearing and vision impairment in dementia: a European cross-national perspective. In: American society of rehabilitation medicine. Chicago: American Society of Rehabilitation Medicine; 2019. [DOI] [PubMed] [Google Scholar]
- 8.Smeeth L, Fletcher AE, Ng ES, Stirling S, Nunes M, Breeze E, et al. Reduced hearing, ownership, and use of hearing aids in elderly people in the UK: the MRC trial of the assessment and management of older people in the community: a cross-sectional survey. Lancet. 2002;359(9316):1466–70. [DOI] [PubMed] [Google Scholar]
- 9.Evans BJ, Rowlands G. Correctable visual impairment in older people: a major unmet need. Ophthalmic Physiol Opt. 2004;24(3): 161–80. [DOI] [PubMed] [Google Scholar]
- 10.Lorenzini MC, Wittich W. Factors related to the use of magnifying low vision aids: a scoping review. Disabil Rehabil. 2019;1–13. [DOI] [PubMed] [Google Scholar]
- 11.Wittich W, Jarry J, Groulx G, Southall K, Gagné J-P. Rehabilitation and research priorities in deafblindness for the next decade. J Visual Impairment Blindness. 2016;110(4):219–31. [Google Scholar]
- 12.Wolski L, Leroi I, Regan J, Dawes P, Charalambous AP, Thodi C, et al. The need for improved cognitive, hearing and vision assessments for older people with cognitive impairment: a qualitative study. BMC Geriatr. 2019; 19(1):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leroi I, Himmelsbach I, Wolski L, Littlejohn J, Jury F, Parker A, et al. Assessing and managing concurrent hearing, vision and cognitive impairments in older people: an international perspective from healthcare professionals. Age Ageing. 2019;48(4):580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence, (NICE). Dementia: supporting people with dementia and their carers in health and social care. NICE Guideline CG42; 2006. (Updated 2016). [Google Scholar]
- 15.Jack CR Jr, Albert MS, Knopman DS, McK-hann GM, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thiyagarajan JA, Araujo de Carvalho I, Peña-Rosas JP, Chadha S, Mariotti SP, Dua T, et al. Redesigning care for older people to preserve physical and mental capacity: WHO guidelines on community-level interventions in integrated care. PLoS Med. 2019;16(10):e1002948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowen M, Zutshi H, Cordiner M, Crutch S, Shakespeare T. Qualitative, exploratory pilot study to investigate how people living with posterior cortical atrophy, their carers and clinicians experience tests used to assess vision. BMJ Open. 2019;9(3):e020905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gold M, Lightfoot LA, Hnath-Chisolm T. Hearing loss in a memory disorders clinic. A specially vulnerable population. Arch Neurol. 1996;53(9):922–8. [DOI] [PubMed] [Google Scholar]
- 19.Bowen M, Edgar DF, Hancock B, Haque S, Shah R, Buchanan S, et al. The prevalence of visual impairment in people with dementia (the PrOVIDe study): a cross-sectional study of people aged 60–89 years with dementia and qualitative exploration of individual, carer and professional perspectives. In: Heath services and delivery research. Southampton, UK: NIHR Journals Library; 2016. [PubMed] [Google Scholar]
- 20.Allen NH, Burns A, Newton V, Hickson F, Ramsden R, Rogers J, et al. The effects of improving hearing in dementia. Age Ageing. 2003;32(2):189–93. [DOI] [PubMed] [Google Scholar]
- 21.Maharani A, Dawes P, Nazroo J, Tampubolon G, Pendleton N; Longitudinal relationship between hearing aid use and cognitive function in older Americans. J Am Geriatr Soc. 2018;66(6):1130–6. [DOI] [PubMed] [Google Scholar]
- 22.Maharani A, Dawes P, Nazroo J, Tampubolon G, Pendleton N. Cataract surgery and age-related cognitive decline: a 13-year follow-up of the English Longitudinal Study of Ageing. PLoS One. 2018;13(10):e0204833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coughlan J, Macredie RD. Effective communication in requirements elicitation: a comparison of methodologies. Requir Eng. 2002; 7(2):47–60. [Google Scholar]
- 24.Hadorn D, Kvizhinadze G, Collinson L, Blakely T. Use of expert knowledge elicitation to estimate parameters in health economic decision models. Int J Technol Assess Health Care. 2014;30(4):461–8. [DOI] [PubMed] [Google Scholar]
- 25.OCEBM Levels of Evidence Working Group. The Oxford 2011 levels of evidence. Oxford Centre for Evidence-Based Medicine; 2011. [Google Scholar]
- 26.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and nonrandomised studies of health care interventions. J Epidemiol Community Health. 1998; 52(6):377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boote J, Telford R, Cooper C. Consumer involvement in health research: a review and research agenda. Health Policy. 2002;61(2): 213–36. [DOI] [PubMed] [Google Scholar]
- 28.Krahn M, Naglie G. The next step in guideline development: incorporating patient preferences. JAMA. 2008;300(4):436–8. [DOI] [PubMed] [Google Scholar]
- 29.Rudan I, Yoshida S, Chan KY, Cousens S, Sridhar D, Bahl R, et al. Setting health research priorities using the CHNRI method: I. Involving funders. J Glob Health. 2016;6(1): 010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrence V, Murray J, Banerjee S, Ffytche D. The experiences and needs of people with dementia and serious visual impairment: a qualitative study. London: Thomas Pocklington Trust; 2008. [Google Scholar]
- 31.Grand JH, Caspar S, Macdonald SW. Clinical features and multidisciplinary approaches to dementia care. J Multidiscip Healthc. 2011;4: 125–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hopper T, Hinton P. Hearing loss among individuals with dementia: barriers and facilitators to care. Can J Speech Lang Pathol Audiol. 2012;36(4):302–13. [Google Scholar]
- 33.World Health Organization, W.H. Deafness and hearing loss. 2020. [cited 2020 Aug 25]. Available from: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss.
- 34.van der Pols JC, Bates CJ, McGraw PV, Thompson JR, Reacher M, Prentice A, et al. Visual acuity measurements in a national sample of British elderly people. Br J Ophthalmol. 2000;84(2):165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen-Mansfield J, Infeld DL. Hearing aids for nursing home residents: current policy and future needs. Health Policy. 2006;79(1): 49–56. [DOI] [PubMed] [Google Scholar]
- 36.McCreedy EM, Weinstein BE, Chodosh J, Blustein J. Hearing loss: why does it matter for nursing homes? J Am Med Dir Assoc. 2018; 19(4):323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pye A, Charalambous AP, Leroi I, Thodi C, Dawes P. Screening tools for the identification of dementia for adults with age-related acquired hearing or vision impairment: a scoping review. Int Psychogeriatr. 2017; 29(11):1771–84. [DOI] [PubMed] [Google Scholar]
- 38.Mamo SK, Oh E, Lin FR. Enhancing communication in adults with dementia and age-related hearing loss. Semin Hear. 2017;38(2): 177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen-Mansfield J, Dakheel-Ali M, Marx MS, Thein K, Regier NG. Which unmet needs contribute to behavior problems in persons with advanced dementia? Psychiatry Res. 2015;228(1):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mamo SK, Nirmalasari O, Nieman CL, McNabney MK, Simpson A, Oh ES, et al. Hearing care intervention for persons with dementia: a Pilot Study. Am J Geriatr Psychiatry. 2017;25(1):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmer C, Adams SW, Bourgeois M, Durrant J, Rossi M. Reduction in caregiver-identified problem behaviors in patients with Alzheimer disease post-hearing-aid fitting. J Speech Lang Hear Res. 1999;42(2):312–28. [DOI] [PubMed] [Google Scholar]
- 42.Ismail Z, Black SE, Camicioli R, Chertkow H, Herrmann N, Laforce R, et al. Recommendations of the 5th Canadian Consensus Conference on the diagnosis and treatment of dementia. Alzheimers Dement. 2020;16(8): 1182–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dupuis K, Pichora-Fuller MK, Chasteen AL, Marchuk V, Singh G, Smith SL. Effects of hearing and vision impairments on the montreal cognitive assessment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2015; 22(4):413–37. [DOI] [PubMed] [Google Scholar]
- 44.Behrman S, Chouliaras L, Ebmeier KP. Considering the senses in the diagnosis and management of dementia. Maturitas. 2014;77(4): 305–10. [DOI] [PubMed] [Google Scholar]
- 45.Maharani A, Pendleton N, Leroi I. Hearing impairment, loneliness, social isolation, and cognitive function: longitudinal analysis using English Longitudinal Study on Ageing. Am J Geriatr Psychiatry. 2019;27(12):1348–56. [DOI] [PubMed] [Google Scholar]
- 46.Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology. 2009;55(5):539–49. [DOI] [PubMed] [Google Scholar]
- 47.The College of Optometrists. Examining patients with dementia or other acquired cognitive impairment. 2016. [cited 2020 Jul 2]. Available from: https://guidance.college-optometrists.org/guidance-contents/knowledge-skills-and-performance-domain/examining-patients-with-dementia.
- 48.Whitson HE, Cronin-Golomb A, Cruickshanks KJ, Gilmore GC, Owsley C, Peelle JE, et al. American geriatrics society and national institute on aging bench-to-bedside conference: sensory impairment and cognitive decline in older adults. J Am Geriatr Soc. 2018; 66(11):2052–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martyr A, Nelis SM, Quinn C, Wu YT, Lamont RA, Henderson C, et al. Living well with dementia: a systematic review and correlational meta-analysis of factors associated with quality of life, well-being and life satisfaction in people with dementia. Psychol Med. 2018;48(13):2130–9. [DOI] [PubMed] [Google Scholar]
- 50.Regan J, Frison E, Collin F, Dawes P, Hann M, Himmelsbach I, et al. Individualised sensory intervention to improve quality of life in people with dementia and their companions (SENSE-Cog trial): study protocol for a randomised controlled trial. Trials. 2019;20(1): 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet commission. Lancet. 2020; 396(10248):413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prince MJ, Acosta D, Castro-Costa E, Jackson J, Shaji KS. Packages of care for dementia in low- and middle-income countries. PLoS Med. 2009;6(11):e1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawrence V, Murray J. Balancing independence and safety: the challenge of supporting older people with dementia and sight loss. Age Ageing. 2010;39(4):476–80. [DOI] [PubMed] [Google Scholar]
- 54.Swanepoel DW, Clark JL, Koekemoer D, Hall JW 3rd, Krumm M, Ferrari DV, et al. Tele-health in audiology: the need and potential to reach underserved communities. Int J Audiol. 2010;49(3):195–202. [DOI] [PubMed] [Google Scholar]
- 55.Hubbard HI, Mamo SK, Hopper T. Dementia and hearing loss: interrelationships and treatment considerations. Semin Speech Lang. 2018;39(3):197–210. [DOI] [PubMed] [Google Scholar]
- 56.Clarke EL, Evans JR, Smeeth L. Community screening for visual impairment in older people. Cochrane Database Syst Rev. 2018;2(2): CD001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wittich W, Höbler F, Jarry J, McGilton KS. Recommendations for successful sensory screening in older adults with dementia in long-term care: a qualitative environmental scan of Canadian specialists. BMJ Open. 2018; 8(1):e019451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dawes P, Pye A, Reeves D, Yeung WK, Sheikh S, Thodi C, et al. Protocol for the development of versions of the montreal cognitive assessment (MoCA) for people with hearing or vision impairment. BMJ Open. 2019;9(3): e026246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.National Institute for Health and Care Excellence (NICE). Assessment and diagnosis [NG97] Dementia: assessment, management and support for people living with dementia and their carers. 2018. [PubMed]
- 60.Wittich W, Phillips N, Nasreddine ZS, Chertkow H. Sensitivity and specificity of the montreal cognitive assessment modified for individuals who are visually impaired. J Visual Impairment Blindness. 2010;104(6):360–8. [Google Scholar]
- 61.Busse A, Sonntag A, Bischkopf J, Matschinger H, Angermeyer MC. Adaptation of dementia screening for vision-impaired older persons: administration of the mini-mental state examination (MMSE). J Clin Epidemiol. 2002; 55(9):909–15. [DOI] [PubMed] [Google Scholar]
- 62.Kricos PB. Audiologic management of older adults with hearing loss and compromised cognitive/psychoacoustic auditory processing capabilities. Trends Amplif. 2006;10(1): 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen J, Sherman M, Souza PE. Test administration methods and cognitive test scores in older adults with hearing loss. Gerontology. 2019;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lemke U Hearing impairment in dementia: how to reconcile two intertwined challenges in diagnostic screening. Audiol Res. 2011; 1(1):e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kergoat H, Law C, Chriqui E, Leclerc BS, Ker-goat MJ. Tool for screening visual acuity in older individuals with dementia. Am J Alzheimers Dis Other Demen. 2017;32(2):96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ciocler Froiman P, Dantas PE. Assessment of visual acuity in patients with dementia using teller acuity cards. Strabismus. 2013;21(2): 93–7. [DOI] [PubMed] [Google Scholar]
- 67.Chriqui E, Kergoat M-J, Champoux N, Leclerc B-S, Kergoat H. Assessment of visual acuity of older individuals with dementia. Invest Ophthalmol Visual Sci. 2012;53(14):4790. [Google Scholar]
- 68.The Royal College of Opthalmologists. Quality standard for people with sight loss and dementia in an ophthalmology department. London, England: The Royal College of Opthalmologists; 2015. [Google Scholar]
- 69.Canevelli M, Valletta M, Trebbastoni A, Sarli G, D’Antonio F, Tariciotti L, et al. Sundowning in dementia: clinical relevance, patho-physiological determinants, and therapeutic approaches. Front Med. 2016;3:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Social Care Institiute for Excellence (SCIE). Dementia. London, UK: 2015. Available from: https://www.scie.org.uk/dementia/. [Google Scholar]


