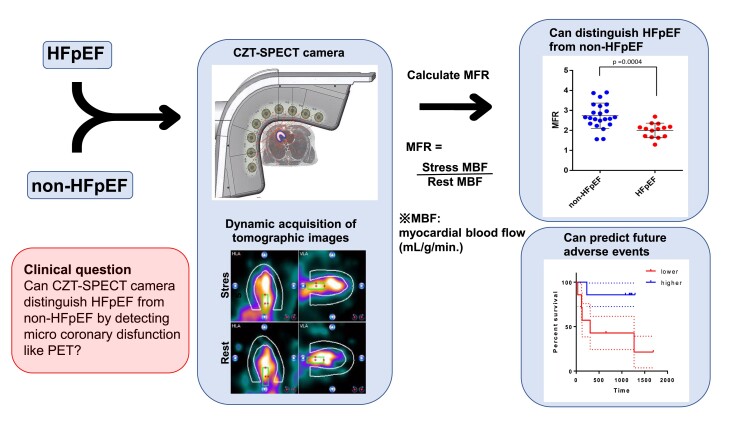
Abstract
Aims
Coronary microvascular dysfunction (CMD) is related to the pathophysiology, mortality, and morbidity of heart failure with preserved ejection fraction (HFpEF). A novel single-photon emission computed tomography (SPECT) camera with cadmium zinc telluride (CZT) detectors allows for the quantification of absolute myocardial blood flow and myocardial flow reserve (MFR) in patients with coronary artery disease. However, the potential of CZT-SPECT assessing for CMD has never been evaluated in patients with HFpEF.
Methods and results
The clinical records of 127 consecutive patients who underwent dynamic CZT-SPECT were retrospectively reviewed. Rest and stress scanning were started simultaneously with 3 and 9 MBq/kg of 99mTc-sestamibi administration, respectively. Dynamic CZT-SPECT imaging data were analysed using a net-retention model with commercially available software. Transthoracic echocardiography was performed in all patients. The MFR value was significantly lower in the HFpEF group (mean ± SEM = 2.00 ± 0.097) than that in the non-HFpEF group (mean ± SEM = 2.74 ± 0.14, P = 0.0004). A receiver operating characteristic analysis indicated that if a cut-off value of 2.525 was applied, MFR could efficiently distinguish HFpEF from non-HFpEF. Heart failure with preserved ejection fraction had a consistently low MFR, regardless of the diastolic dysfunction score. Heart failure with preserved ejection fraction patients with MFR values lower than 2.075 had a significantly higher incidence of heart failure exacerbation.
Conclusion
Myocardial flow reserve assessed by CZT-SPECT was significantly reduced in patients with HFpEF. A lower MFR was associated with a higher hospitalization rate in these patients. Myocardial flow reserve assessed by CZT-SPECT has the potential to predict future adverse events and stratify the severity of disease in patients with HFpEF.
Keywords: Myocardial blood flow, Myocardial flow reserve, Cadmium zinc telluride equipped single-photon emission computed tomography camera, Dynamic scanning, Heart failure with preserved ejection fraction, Coronary microvascular dysfunction
Graphical Abstract
Graphical abstract.
Introduction
Heart failure (HF) is a leading cause of mortality and morbidity worldwide.1 Approximately half the patients with HF symptoms do not have a marked reduction in left ventricular ejection fraction (LVEF).2 For a lengthy period, it remained uncovered; however, a new paradigm into the pathophysiology of HF with preserved ejection fraction (HFpEF) has been proposed over the last decade.3 The key component of this paradigm is that inflammation of the endothelium in the coronary micro-artery, which is compromised by comorbidities including obesity, hypertension, chronic obstructive lung disease, and diabetes, initiates a derangement of the NO-cGMP-PKG pathway, which induces cardiomyocyte hypertrophy or interstitial fibrosis, resulting in diastolic dysfunction.4–6 Increasing evidence also suggests that the presence of coronary microvascular dysfunction (CMD) is associated with the severity of symptoms7 or prognosis of patients with HFpEF.8,9 Therefore, alterations of MFR are associated with CMD in patients with HFpEF, which provides a potential physiologic marker of clinical risk and therapeutic efficacy.
Recently, several reports have shown that myocardial flow reserve (MFR) can be evaluated quantitatively using positron emission tomography (PET)10,11 and is impaired in patients with HFpEF.12,13 PET using oxygen-15-labelled water is widely accepted as the gold standard for evaluating myocardial perfusion. However, the use of this apparatus is largely limited to major academic centres or research laboratories.
Single-photon emission computed tomography (SPECT) cameras with cadmium zinc telluride (CZT) detectors (D-SPECT®), which are equipped with a high-speed and high-sensitivity CZT semiconductor camera, enable a dynamic acquisition of tomographic images suitable for the evaluation of absolute myocardial blood flow (MBF).14 It has also been reported that MFR assessed by a protocol using a 99mTc-labelled perfusion tracer and CZT-SPECT provided similar results compared with those with 15O-water PET and a high diagnostic value for detecting stable coronary artery disease (CAD).15,16
Here, we retrospectively evaluated the potential of CZT-SPECT with a 99mTc-labelled perfusion tracer to determine whether it can sensitively detect reduced MFR in patients with HFpEF, as previously reported with PET.
Methods
Study design and patients
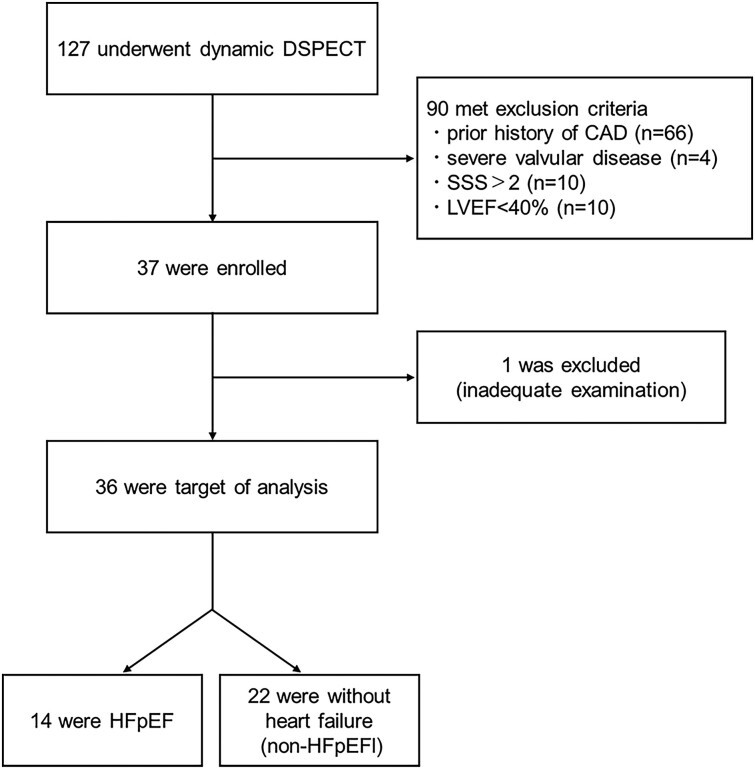
We retrospectively reviewed the clinical records of patients who underwent dynamic 99mTc-sestamibi perfusion SPECT with a D-SPECT camera for evaluation of CAD between October 2017 and October 2020 at the Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, Nagoya, Japan. Of the 127 cases reviewed, 90 patients were excluded from the current study due to prior CAD episodes (n = 66), severe valvular disease (n = 4), LVEF < 40% (n = 10), or a summed stress score (SSS) > 2 (n = 10) (Figure 1). One patient was excluded because of an inadequate examination. The remaining 36 cases were included in this study and divided into 2 groups. Fourteen patients assigned to the HFpEF group had a history of hospitalization due to HF (n = 11) or evidence of lung congestion diagnosed by chest radiography and CT in the outpatient clinic (n = 3). All 14 patients met the diagnostic criteria recently shown in the guidelines for treating HF patients.17 The remaining 22 patients, without signs or evidence of HF, were assigned to the non-HFpEF group. Nine patients among non-HFpEF group had chest pain but had no evidence of cardiac origin.
Figure 1.
Patient flow diagram. The medical records of 127 patients who underwent dynamic 99mTc-MIBI perfusion single-photon emission computed tomography using a D-single-photon emission computed tomography camera were retrospectively reviewed. Of these, 90 patients met the exclusion criteria and 1 was excluded because of inadequate examination. The resultant 36 patients were included in the current analysis.
Dynamic 99mTc-sestamibi cadmium zinc telluride-single-photon emission computed tomography acquisition
All patients were instructed to abstain from caffeine and methylxanthine-containing substrates and medications for at least 24 h before the scan. Rest and stress dynamic images were acquired in the list mode of D-SPECT® (Spectrum Dynamics Medical, Cesare, Israel). All imaging procedures were performed in the supine position. To identify the position of the heart in the scanner’s field of view, ∼37 MBq of 99mTc-sestamibi was administered pre-scanning. Rest scanning was started simultaneously with 3 MBq/kg 99mTc-sestamibi administration at a rate of 1 mL/s using an automatic injector (Nemoto, Tokyo, Japan) followed by a bolus injection of 30 mL saline and continued for 6 min. The gated perfusion images were acquired for 9 min. Maximum hyperaemia was induced by a continuous intravenous infusion of adenosine (PDRadiopharma Inc., Tokyo, Japan) at a rate of 120 µL/kg/min. Three minutes after the initiation of adenosine administration, a 9 MBq/kg dose of 99mTc-sestamibi was injected. The injection speed of the radioisotope and the scanning protocol were the same as those in the rest of the scan. Stress-gated perfusion images were also acquired following a stress dynamic scan (see Supplementary material online, Supplement S1). Data were parcellated into 32 frames (21 × 3, 1 × 9, 1 × 15, 1 × 21, 1 × 27, and 7 × 30 s frames). Images were reconstructed using an ordered subset expectation maximization algorithm with 4 iterations and 32 subsets.
Dynamic single-photon emission computed tomography image analysis
Details concerning the dynamic SPECT image analysis were previously described by Agostini et al.15 and are described only briefly here. The dynamic CZT-SPECT imaging data were analysed using a net-retention kinetic model18,19 using commercially available software (Corridor 4DM; Invia, Ann Arbor, MI, USA). A 2 pixel-wide × 6 pixel-long ROI was positioned at the basal valve plane (within the LV and LA) for blood pool sampling (see Supplementary material online, Supplement S2). The endocardial and epicardial borders of the LV wall were defined algorithmically using integrated images acquired during heart positioning. The mid-wall between the endocardial and epicardial borders was defined, and its surface was divided into 460 polar sectors. Time activity curves (TACs) were drawn according to the nearest point of each sector across all time frames. TACs globally and in each vessel region (left anterior descending artery, left circumflex artery and right coronary artery) were averaged from the polar map sectors.
Rest echocardiography
Transthoracic rest echo cardiography (EPIQ7; Philips, The Netherlands) was performed for all subjects within 1 month before or after SPECT scanning. The following standard parameters of cardiac function were recorded: septal wall thickness, LV posterior wall thickness, LV mass index, LV ejection fraction by the Simpson method, LA volume index, mitral inflow peak E- and A-wave velocities by pulse wave Doppler (E/A ratio), and tricuspid regurgitation (TR) systolic jet velocity. LV septal e′ velocity was measured using the tissue Doppler mode, and LV filling pressure (E/e′) was estimated. LV diastolic dysfunction was assessed using the following parameters recommended by the American Society of Echocardiography:20 average E/e′ > 14, septal e′ velocity <7 cm/s, TR systolic jet velocity >2.8 m/s, and left atrial volume index >34 mL/m2. Based on these parameters of LV diastolic dysfunction, each subject was classified into one of the following five grades: 0–4.
Statistical analysis
Continuous variables are expressed as mean ± SEM and were analysed by Student’s t-test or one-way analysis of variance (ANOVA) if they were compared between two groups or among three or more groups, respectively. When the P-value for ANOVA was statistically significant, Tukey’s test was conducted for post-hoc analysis. Fisher’s exact test was used to analyse categorical variables between the two groups. The accuracy of MFR in distinguishing HFpEF from non-HFpEF was evaluated using receiver operating characteristic (ROC) curve analysis. A single regression analysis was performed to evaluate the relationship between MFR and clinical variables. If the relationships were statistically significant, they were further evaluated by multiple regression analysis. The log-rank (Mantel–Cox) test was used to compare survival curves. All tests were two-tailed, and P-values ≤0.05 were considered statistically significant. Statistical analyses were performed using the Prism software (GraphPad Software, San Diego, CA, USA).
Result
Baseline characteristics
The baseline characteristics of the 36 patients included in this study are shown in Table 1. The mean age of the patients was 71.2 ± 10.3 years; 65% were females, mean body mass index was 28.0 ± 32.5, which was similar between the groups. More cases of HFpEF were complicated by atrial fibrillation (AF) compared with non-HFpEF. No patients with diabetes were included in the HFpEF group. More patients with HFpEF were prescribed beta-blockers, mineral corticoid receptor antagonists, loop diuretics, and torvaptan compared with non-HFpEF. The mean blood pressure, heart rate, haemoglobin level, and renal function were not significantly different between the groups. The BNP levels in the HFpEF group were variably distributed, and the difference between the groups was not statistically significant.
Table 1.
Baseline patient characteristics
| Characteristics | Overall (n = 36) | Non-HF control (n = 22) | HFpEF (n = 14) | P-value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (years) | 71.2 ± 10.3 | 69.6 ± 9.6 | 73.7 ± 10.8 | 0.254 |
| Female, n (%) | 23 (64) | 14 (64) | 9 (64) | 0.626 |
| Body mass index (kg/m2) | 28.0 ± 32.5 | 24.1 ± 2.7 | 33.5 ± 51.3 | 0.378 |
| Comobidities, n (%) | ||||
| Hypertension | 29 (81) | 18 (82) | 11 (79) | 0.81 |
| Atrial fibrillation | 8 (22) | 2 (9) | 6 (43) | 0.018* |
| Diabetes | 9 (25) | 9 (41) | 0 (0) | 0.004* |
| Dyslipidaemia | 19 (53) | 13 (59) | 6 (43) | 0.342 |
| Chronic kidney disease | 23 (64) | 13 (59) | 10 (71) | 0.452 |
| Medications, n (%) | ||||
| Aspirin | 11 (31) | 10 (45) | 1 (7) | 0.015* |
| Statin | 16 (44) | 12 (55) | 4 (29) | 0.126 |
| Beta-blocker | 12 (33) | 3 (14) | 9 (64) | 0.002* |
| ACE inhibitor or ARB | 20 (56) | 12 (55) | 8 (57) | 0.878 |
| MRA | 7 (19) | 0 (0) | 7 (50) | <0.001* |
| Ca channel blocker | 13 (36) | 12 (55) | 1 (7) | 0.004* |
| Loop diuretic | 13 (36) | 2 (9) | 11 (79) | <0.001* |
| Torvaptan | 5 (14) | 1 (5) | 4 (29) | 0.042* |
| Vital signs and laboratory data | ||||
| Systolic blood pressure (mmHg) | 156.9 ± 22.9 | 160.5 ± 22.7 | 151.7 ± 22.2 | 0.277 |
| Diastolic blood pressure (mmHg) | 91.3 ± 15.5 | 94.1 ± 14.4 | 87.1 ± 16.2 | 0.198 |
| Heart rate (b.p.m.) | 72.1 ± 13.1 | 69.2 ± 13.3 | 76.7 ± 11.9 | 0.105 |
| Rest rate pressure product | 11 319.5 ± 2775.7 | 11 055.3 ± 2800.5 | 11 734.7 ± 2684.3 | 0.488 |
| Haemoglobin (g/dL) | 13.5 ± 3.9 | 13.3 ± 1.7 | 13.8 ± 5.7 | 0.687 |
| eGFR (mL/min/1.73 m2) | 52.4 ± 19.5 | 56.2 ± 21.2 | 46.7 ± 14.9 | 0.168 |
| Haemoglobin A1c (%) | 6.05 ± 0.82 | 6.35 ± 0.88 | 5.66 ± 0.53 | 0.022* |
| BNP (pg/mL) | 137.1 ± 204.9 | 76.9 ± 97.2 | 184.5 ± 249.9 | 0.208 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BNP, brain-type natriuretic peptide; eGFR, estimated glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; MRA, mineral corticoid receptor antagonist .
*P < 0.05.
Echocardiographic parameters
Mean LVEF was 63.7 ± 7.5% and 57.5 ± 11.6% for non-HFpEF and HFpEF groups, respectively (Table 2). LV mass index and LA volume index in HFpEF were significantly increased compared with the non-HFpEF groups; however, other parameters associated with diastolic dysfunction, such as TR jet velocity or E/A ratio, were not significantly different between the groups. In the same fashion, tissue Doppler indices were not significantly different between the groups. Among the parameters of diastolic dysfunction, only the LA volume index was significantly different between the groups. However, the HFpEF group had significantly higher diastolic dysfunction scores (DD scores) than the non-HFpEF group.
Table 2.
Echocardiographic parameters
| Echocardiographic parameters | Overall (n = 36) | Non-HF control (n = 22) | HFpEF (n = 14) | P-value |
|---|---|---|---|---|
| Left heart structure/function | ||||
| Septal wall thickness (cm) | 9.7 ± 1.9 | 9.8 ± 1.8 | 9.3 ± 1.8 | 0.44 |
| Posterior wall thickness (cm) | 9.7 ± 1.6 | 9.7 ± 1.4 | 9.8 ± 1.8 | 0.858 |
| LV mass index | 100.4 ± 26.5 | 92.7 ± 22.6 | 113.0 ± 27.5 | 0.031* |
| LV ejection fraction (%) | 61.2 ± 9.4 | 63.7 ± 7.5 | 57.5 ± 11.6 | 0.197 |
| LA volume index (mL/m2) | 40.4 ± 19.0 | 34.2 ± 12.4 | 49.8 ± 23.1 | 0.02* |
| E velocity (cm/s) | 77.8 ± 29.0 | 76.3 ± 22.9 | 80.2 ± 36.6 | 0.713 |
| E/A ratio | 0.95 ± 0.42 | 0.88 ± 0.32 | 1.10 ± 0.55 | 0.23 |
| TR systolic jet velocity | 2.29 ± 0.47 | 2.14 ± 0.44 | 2.51 ± 0.42 | 0.064 |
| Tissue Doppler indices | ||||
| LV septal e′ velocity (cm/s) | 6.3 ± 1.9 | 6.6 ± 1.8 | 5.6 ± 1.8 | 0.127 |
| LV E/e′ ratio | 12.6 ± 3.9 | 12.0 ± 3.9 | 13.7 ± 3.9 | 0.253 |
| Diastolic dysfunction score | 1.72 ± 1.28 | 1.18 ± 0.89 | 2.57 ± 1.29 | 0.00073* |
For the scoring of diastolic dysfunction, each case was classified into five grades: 0–4. The parameters used for scoring are average E/e′ > 14, septal e′ velocity <7 cm/s, tricuspid regurgitation systolic jet velocity >2.8 m/s and left atrial volume index >34 mL/m2.
LA, left atrium; LV, left ventricle; TR, tricuspid regurgitation.
*P < 0.05.
Dynamic 99mTc-MIBI perfusion single-photon emission computed tomography
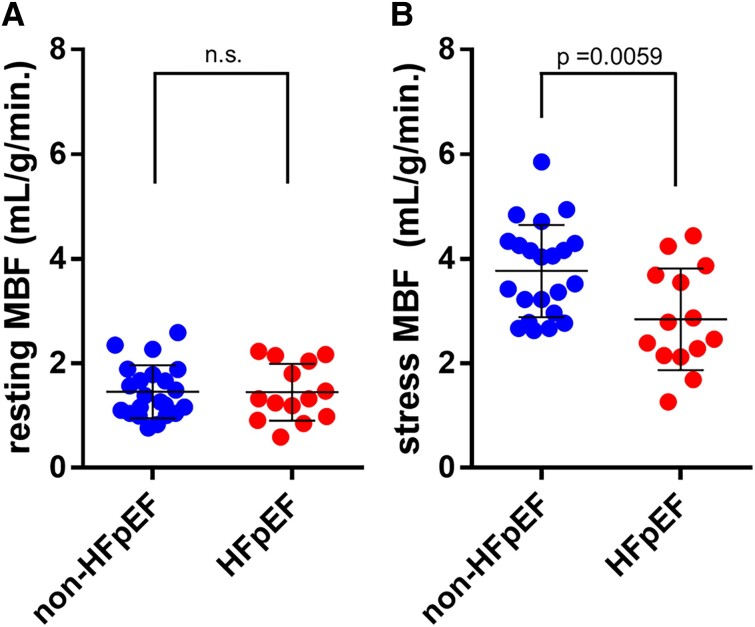
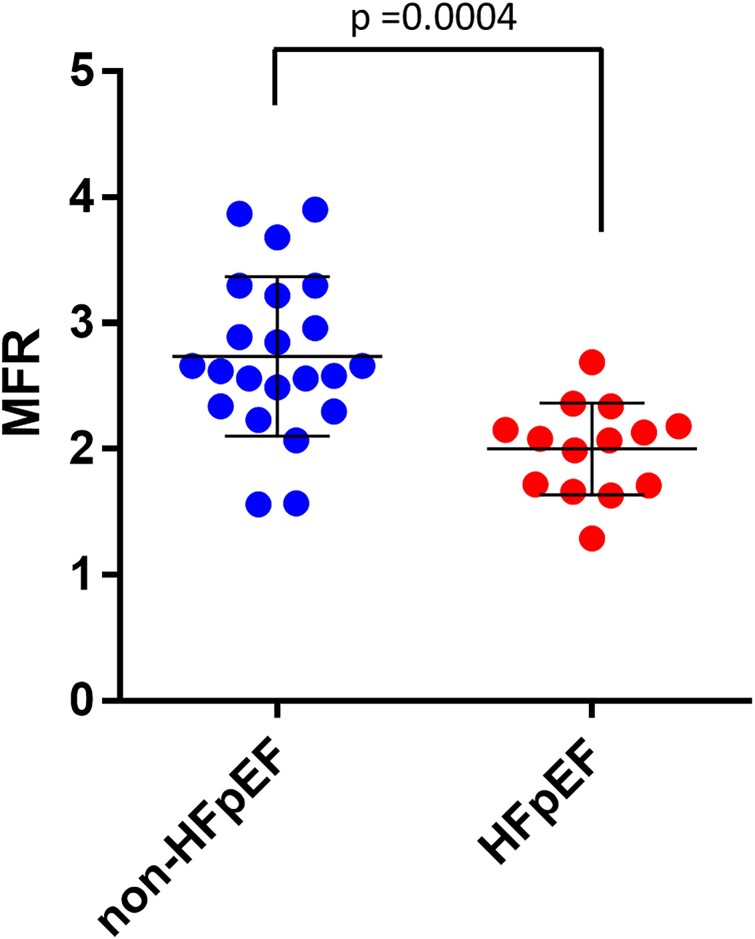
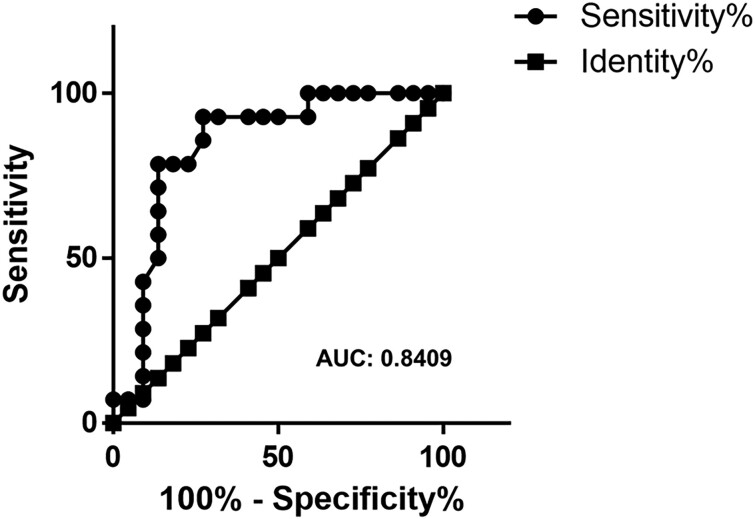
The resting MBF level was similar between the two groups (Figure 2A). In adenosine-induced hyperaemic conditions, the level of MBF in the HFpEF group (mean ± SEM = 2.84 ± 0.26) was significantly impaired compared with that in the non-HFpEF group (mean ± SEM = 3.77 ± 0.19; Figure 2B). Accordingly, the value of MFR, which was calculated as stress MBF/resting MBF in each case, was significantly lower in the HFpEF group (mean ± SEM = 2.00 ± 0.097) than in the non-HFpEF group (mean ± SEM = 2.74 ± 0.14, P = 0.0004; Figure 3). ROC analysis showed that the area under the curve was 0.8409, and the cut-off value was 2.525 (Figure 4), indicating that MFR can distinguish patients with HFpEF from the non-HFpEF group with high accuracy. Similar to the total MFR, the regional MFR in each coronary artery vessel was significantly reduced in patients with HFpEF compared with non-HFpEF (Table 3). It is reported that loss of atrial stroke performance in patients with AF can decrease cardiac output by up to 25%21 and could influence the MBF. Among the 36 subjects in the current study, the average of both rest and stress MBF was higher in patients without AF (rest: 1.51 ± 0.49, stress: 3.53 ± 0.88) compared with that with AF (rest: 1.26 ± 0.51, stress: 2.99 ± 1.25) but the difference was not statistically significant.
Figure 2.
Resting and stress myocardial blood flow (mL/g/min). (A) Resting myocardial blood flow: no significant difference. (B) Stress myocardial blood flow: mean ± SEM of non-heart failure with preserved ejection fraction (n = 22) and heart failure with preserved ejection fraction (n = 14) are 3.77 ± 0.19 and 2.84 ± 0.26, respectively. 95% Confidence interval is −1.56 to −0.28. P = 0.0059 (two-tailed unpaired t-test).
Figure 3.
Myocardial flow reserve. Mean ± SEM of non-heart failure with preserved ejection fraction (n = 22) and heart failure with preserved ejection fraction (n = 14) are 2.74 ± 0.14 and 2.00 ± 0.097, respectively. 95% Confidence interval is −1.12 to −0.35. P = 0.0004 (two-tailed unpaired t-test).
Figure 4.
Receiver operating characteristic carve for myocardial flow reserve. AUC, area under the curve, the cut-off value for myocardial flow reserve is 2.525.
Table 3.
Regional myocardial flow reserve
| MFR | Overall (n = 36) | Non-HF control (n = 22) | HFpEF (n = 14) | P-value |
|---|---|---|---|---|
| LAD | 2.27 ± 0.62 | 2.63 ± 0.63 | 1.96 ± 0.30 | 0.001 |
| LCx | 2.38 ± 0.69 | 2.68 ± 0.68 | 1.92 ± 0.39 | 0.0008 |
| RCA | 2.44 ± 0.62 | 2.72 ± 0.57 | 2.01 ± 0.41 | 0.0004 |
LAD, left anterior descending artery; LCx, left circumflex artery; RCA, right coronary artery.
Relationship between myocardial flow reserve and clinical variables
To explore the relationship between MFR and clinical variables, a single regression analysis was performed. Clinical variables were selected from the patient’s background, laboratory data, gated SPECT, and cardiac echo parameters (Table 4). Among these variables, patient’s age, history of HF, tricuspid valve regurgitation (TVR) velocity, and DD score were significantly correlated with MFR. Variables that were significantly correlated with MFR in single regression analysis were selected for multiple regression analysis. TVR velocity and DD score were strongly correlated because TVR velocity is a component of DD score. Considering multicollinearity, TVR velocity was excluded from multiple regression analysis. The result is that history of HF (P = 0.0473) and DD score (P = 0.0150) were independently correlated with MFR and could pertly predict MFR (multiple R: 0.711, adjusted R2: 0.46, P* < 0.05). It is reported that peak filling rate (PFR) obtained by gated myocardial perfusion SPECT is correlated with echo LV diastolic parameters and could be an early indicator of LV dysfunction.22,23 In accordance with these reports, PFR showed a moderate correlation with E/e′ in our subjects (R = 0.519, P = 0.00196); however, it did not show a significant correlation with MFR (R = 0.228, P = 0.180).
Table 4.
Single regression analysis
| vs. MFR (n = 36) | R | P-value |
|---|---|---|
| Background | ||
| Age | 0.406 | 0.0138* |
| Gender | 0.282 | 0.0951 |
| BMI | 0.0432 | 0.802 |
| Smoke | 0.0773 | 0.653 |
| Hypertension | 0.0256 | 0.882 |
| DM | 0.227 | 0.181 |
| Heart failure | 0.558 | 0.000395* |
| AF | 0.295 | 0.0843 |
| Laboratory data | ||
| BNP | 0.238 | 0.161 |
| HbA1c | 0.165 | 0.335 |
| Gated SPECT parameter | ||
| PFR | 0.228 | 0.18 |
| 1/3MFR | 0.137 | 0.424 |
| TTPR/R-R | 0.144 | 0.403 |
| Echo parameter | ||
| EF | 0.121 | 0.478 |
| LVMI | 0.266 | 0.115 |
| LAD | 0.233 | 0.171 |
| E/e′ | 0.22 | 0.195 |
| e′ | 0.183 | 0.285 |
| TVR velocity | 0.544 | 0.000597* |
| DD score | 0.650523 | 0.0000174* |
AF, atrial fibrillation; BMI, body mass index; BNP, brain natriuretic peptide; DD, diastolic dysfunction; DM, diabetes mellitus; EF, ejection fraction; LAD, left atrial diameter; LVMI, LV mass index; PFR, peak filling rate; 1/3MFR, 1/3 mean filling rate; TTPF, time to peak filling; TVR, tricuspid valve regurgitation.
*P < 0.05.
Relationship between myocardial flow reserve and diastolic dysfunction score
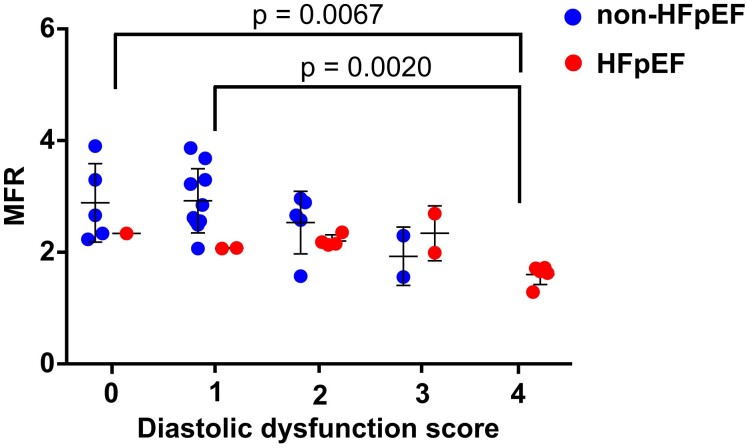
It has been reported that decreased MFR in patients with normal ejection fraction assessed by rubidium-82 PET imaging is associated with parameters of diastolic dysfunction on echocardiography.13 To further explore the relationship between MFR and LV diastolic dysfunction, MFR was compared with DD score (Figure 5), which was based on the following four parameters: average E/e′ > 14, septal e′ velocity <7 cm/s, TR systolic jet velocity >2.8 m/s and left atrial volume index >34 mL/m2. Patients with a higher DD score had a significantly lower MFR compared with those with a lower DD score (Figure 5, gathered HFpEF and non-HFpEF). Additionally, among patients with a lower DD score (0 to 2), MFR was significantly lower in patients with HFpEF than in non-HFpEF (P = 0.011), indicating that HF itself could be a factor deteriorating MFR.
Figure 5.
Relationship between myocardial flow reserve and diastolic dysfunction score. Based on echocardiographic parameters, each case was classified into five grades: 0–4. The parameters used for scoring were average E/e′ > 14, septal e′ velocity <7 cm/s, TR systolic jet velocity >2.8 m/s and left atrial volume index >34 mL/m2. None of the patients in the non-heart failure with preserved ejection fraction group scored 4 points.
Myocardial flow reserve as a predictor of future events in heart failure with preserved ejection fraction
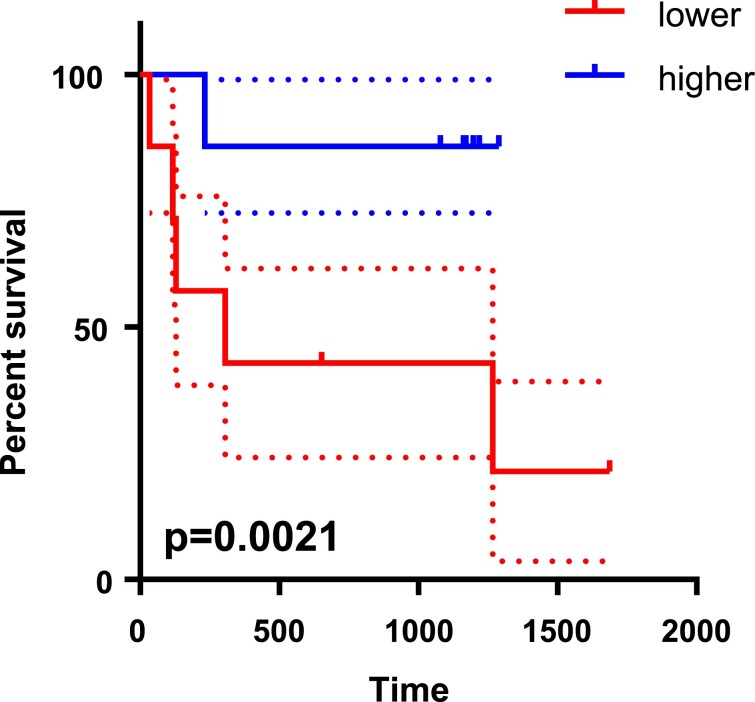
In the non-HFpEF group, no hospitalized cases or cardiovascular deaths were observed during the follow-up period. The average follow-up period was 3.5 years in HFpEF group (n = 14). During the follow-up period, six patients were hospitalized due to HF with lung congestion, and one patient died of lymphoma. The HFpEF group was divided into two groups according to the median value of MFR (2.075): higher (n = 7) and lower (n = 7) groups. Kaplan–Meier analysis showed that patients with HFpEF in the MFR-lower group were significantly exacerbated HF more frequently and were hospitalized more often than those in the MFR-higher group (Figure 6).
Figure 6.
Kaplan–Meier survival analysis for patients with heart failure with preserved ejection fraction. The heart failure with preserved ejection fraction group (n = 14) was divided into two groups according to the median value of myocardial flow reserve (2.075): higher (n = 7) and lower (n = 7) groups. An event was defined as hospitalization due to heart failure or cardiovascular death. The dotted lines show 95% confidence intervals for each group. The log-rank (Mantel–Cox) test was applied to compare survival curves.
Discussion
In the current study, we showed that MFR assessed by CZT-SPECT with a 99mTc-labelled perfusion tracer decreased in patients with HFpEF compared with non-HFpEF. Moreover, this method has high sensitivity and specificity, similar to PET imaging, and can distinguish patients with HFpEF from non-HFpEF with high accuracy. A significant incidence of adverse events was observed in the MFR-lower group compared with the MFR-higher group (Figure 6) and suggests the potential of CZT-SPECT MFR to stratify prognosis in these patients.
D-single-photon emission computed tomography is a novel SPECT system equipped with nine arrays of CZT detectors, which achieves better energy resolution, higher sensitivity, higher count rate, lower radiation exposure, and faster acquisition time than the conventional SPECT system.14 Several lines of evidence suggest that MBF assessed using CZT-SPECT provides comparable results with that assessed using PET and can be a surrogate modality for PET. Agostini et al. reported on over 30 patients with suspected CAD and found a significant correlation between CZT-SPECT and 15O-water PET for the assessment of global MFR.15,16 Other groups have also reported a significant correlation between dynamic 99mTc-sestamibi CZT-SPECT and 13N-ammonia PET.24–26 The correlation between MBF assessed by a dynamic CZT-SPECT scan and that assessed by an invasive coronary fractional flow reserve has also been reported. According to the report, a dynamic CZT-SPECT scan has sufficient ability to detect regional haemodynamic abnormalities.27 The evidence above indicates that MBF measurement with CZT-SPECT is reliable and can be a surrogate method for PET.
Increasing evidence suggests that a high prevalence of CMD is seen in HFpEF,8,28 up to 75% of patients with HFpEF also have impaired CFR without epicardial CAD.7 Furthermore, several reports have suggested that CMD might play a key role in the pathogenesis of HFpEF. Indeed, the systemic inflammatory state induced by risk factors of atherosclerosis such as hypertension, diabetes, or obesity causes endothelial dysfunction, resulting in impairment of NO production,3,29 which is followed by the derangement of the NO-cGMP-PKG pathway, inducing cardiomyocyte hypertrophy,30 interstitial fibrosis,30 or hypophosphorylation of titin,4 resulting in diastolic dysfunction. It has also been reported that coexisting CMD is associated with the prognosis of HFpEF. It is said that when CMD is defined as having a CFR of <2.5, it is independently associated with primally CV- and HF-specific events.9 For these reasons, it is important to evaluate CMD in patients with HFpEF.
Currently, in patients without significant epicardial disease, MFR assessed using PET is considered a standard marker of microvascular function and a surrogate for coronary vascular health.31 Srivaratharajah et al.12 examined a total of 376 individuals, including 78 patients with HFpEF, and reported that MFR assessed by Rb-82 PET in patients with HFpEF was reduced (average = 2.16) compared with non-HF controls with hypertension (average = 2.54) or without hypertension (average = 2.89). Although our non-HFpEF subjects were not divided by blood pressure status, the MFR values of both HFpEF (average = 2.00) and non-HFpEF (average = 2.74) were comparable with the values assessed by Rb-82 PET. Another report evaluating the association between MFR and echocardiographic parameters of diastolic dysfunction in 73 subjects without a history of HF showed that reduced MFR was associated with higher E/e′ values, higher diastolic dysfunction scores, and decreased left atrial strain.13 As shown in Figure 6, MFR in non-HFpEF subjects appears to decrease as the diastolic dysfunction score increases; however, MFR in patients with HFpEF showed consistently low values regardless of the diastolic dysfunction score, indicating that the diastolic dysfunction score is less reliable than MFR for the evaluation of disease severity in patients with advanced HFpEF. Although the number of patients with HFpEF was only 14 in the current study, patients with lower MFR had a significantly higher incidence of hospitalization due to HF. A large-scale clinical trial is warranted to further clarify whether MFR assessed using D-SPECT can predict adverse cardiac events in patients with HFpEF.
Conclusions
Myocardial flow reserve assessed by CZT-SPECT is significantly reduced in patients with HFpEF compared with that in non-HFpEF subjects. The MFR value is similar to that previously reported for PET. A lower MFR may be associated with a higher hospitalization rate in patients with HFpEF. All data indicate that MFR assessed by CZT-SPECT has the potential to predict future adverse events and stratify the severity of disease in patients with HFpEF.
Supplementary Material
Acknowledgements
The authors appreciate the members of the Division of Nuclear Medicine and Clinical Examination, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital for cardiac SPECT and cardiac echo examination, respectively. The authors thank Honyaku Center Inc. for the English language editing.
Contributor Information
Satoya Yoshida, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan; Department Cardiology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, showa-ku, Nagoya, Aichi 466-8560, Japan.
Kazumasa Unno, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan; Department Cardiology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, showa-ku, Nagoya, Aichi 466-8560, Japan.
Mamoru Nanasato, Department of Cardiology, Sakakibara Heart Institute, 3-16-1 Asahi-cho, Fuchu, Tokyo 183-0003, Japan.
Takanaga Niimi, Division of Nuclear Medicine, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Sowa-ku, Nagoya, Aichi 466-8650, Japan.
Kohei Inukai, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan.
Hidenori Morisaki, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan.
Tomoki Hattori, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan.
Miku Hirose, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan; Department Cardiology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, showa-ku, Nagoya, Aichi 466-8560, Japan.
Takumi Hayashi, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan; Department Cardiology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, showa-ku, Nagoya, Aichi 466-8560, Japan.
Noriya Uchida, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan.
Masahiro Simoda, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan.
Hideo Oishi, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan; Department Cardiology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, showa-ku, Nagoya, Aichi 466-8560, Japan.
Monami Ando, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan.
Kenshi Hirayama, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan.
Masaki Takenaka, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan.
Mayuho Maeda, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan.
Ruka Yoshida, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan; Department Cardiology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, showa-ku, Nagoya, Aichi 466-8560, Japan.
Yasuhiro Ogura, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan.
Hirohiko Suzuki, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan.
Kenji Furusawa, Department Cardiology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, showa-ku, Nagoya, Aichi 466-8560, Japan.
Ryota Morimoto, Department Cardiology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, showa-ku, Nagoya, Aichi 466-8560, Japan.
Katsuhiko Kato, Department of Radiology, Nagoya University Hospital, 65 Tsurumai-cho, showa-ku, Nagoya, Aichi 466-8560, Japan.
Satoshi Isobe, Department Cardiology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, showa-ku, Nagoya, Aichi 466-8560, Japan.
Yukihiko Yoshida, Cardiovascular Center, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, 2-9 Myoken-cho, Showa-ku, Nagoya, Aichi 466-8650, Japan.
Toyoaki Murohara, Department Cardiology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, showa-ku, Nagoya, Aichi 466-8560, Japan.
Lead author biography
 Satoya Yoshida, MD, is a cardiologist and a candidate for PhD dedicating himself to basic cardiovascular research at Nagoya University Graduate School of Medicine, Japan. He graduated from the University of Tsukuba in 2014 and completed the training for a specialized doctor as a cardiologist after undergoing clinical resident training at the Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital. He is interested in a broad range of subjects and nurtures hopes that his research could contribute to the future growth of cardiology.
Satoya Yoshida, MD, is a cardiologist and a candidate for PhD dedicating himself to basic cardiovascular research at Nagoya University Graduate School of Medicine, Japan. He graduated from the University of Tsukuba in 2014 and completed the training for a specialized doctor as a cardiologist after undergoing clinical resident training at the Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital. He is interested in a broad range of subjects and nurtures hopes that his research could contribute to the future growth of cardiology.
Data availability
The data underlying this article will be shared on reasonable request with the corresponding author.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
None declared.
Conflict of interest: T.M. received lecture fees and unrestricted research grants from Bayer, Daiichi-Sankyo, Dainippon Sumitomo, Kowa, MSD, Mitsubishi Tanabe, Boehringer Ingelheim, Novartis, Pfizer, Sanofi-Aventis, Takeda, Astellas, Otsuka, and Teijin. All other authors declared no conflict of interest.
References
- 1. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e895–e1032. [DOI] [PubMed] [Google Scholar]
- 2. Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res 2019;124:1598–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 4. van Heerebeek L, Hamdani N, Falcao-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012;126:830–839. [DOI] [PubMed] [Google Scholar]
- 5. Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschope C, Leite-Moreira AF, Musters R, Niessen HW, Linke WA, Paulus WJ, Hamdani N. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail 2016;4:312–324. [DOI] [PubMed] [Google Scholar]
- 6. Hamdani N, Bishu KG, von Frieling-Salewsky M, Redfield MM, Linke WA. Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc Res 2013;97:464–471. [DOI] [PubMed] [Google Scholar]
- 7. Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink-Nelson L, Ljung Faxen U, Fermer ML, Broberg MA, Gan LM, Lund LH. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 2018;39:3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, Di Carli MF. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hage C, Svedlund S, Saraste A, Faxen UL, Benson L, Fermer ML, Gan LM, Shah SJ, Lam CSP, Lund LH. Association of coronary microvascular dysfunction with heart failure hospitalizations and mortality in heart failure with preserved ejection fraction: a follow-up in the PROMIS-HFpEF study. J Card Fail 2020;26:1016–1021. [DOI] [PubMed] [Google Scholar]
- 10. Camici PG, Rimoldi OE. The clinical value of myocardial blood flow measurement. J Nucl Med 2009;50:1076–1087. [DOI] [PubMed] [Google Scholar]
- 11. Gewirtz H, Dilsizian V. Integration of quantitative positron emission tomography absolute myocardial blood flow measurements in the clinical management of coronary artery disease. Circulation 2016;133:2180–2196. [DOI] [PubMed] [Google Scholar]
- 12. Srivaratharajah K, Coutinho T, deKemp R, Liu P, Haddad H, Stadnick E, Davies RA, Chih S, Dwivedi G, Guo A, Wells GA, Bernick J, Beanlands R, Mielniczuk LM. Reduced myocardial flow in heart failure patients with preserved ejection fraction. Circ Heart Fail 2016;9:e002562. [DOI] [PubMed] [Google Scholar]
- 13. Konerman MC, Greenberg JC, Kolias TJ, Corbett JR, Shah RV, Murthy VL, Hummel SL. Reduced myocardial flow reserve is associated with diastolic dysfunction and decreased left atrial strain in patients with normal ejection fraction and epicardial perfusion. J Card Fail 2018;24:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erlandsson K, Kacperski K, van Gramberg D, Hutton BF. Performance evaluation of D-SPECT: a novel SPECT system for nuclear cardiology. Phys Med Biol 2009;54:2635–2649. [DOI] [PubMed] [Google Scholar]
- 15. Agostini D, Roule V, Nganoa C, Roth N, Baavour R, Parienti JJ, Beygui F, Manrique A. First validation of myocardial flow reserve assessed by dynamic (99 m)Tc-sestamibi CZT-SPECT camera: head to head comparison with (15)O-water PET and fractional flow reserve in patients with suspected coronary artery disease. The WATERDAY study. Eur J Nucl Med Mol Imaging 2018;45:1079–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Otaki Y, Manabe O, Miller RJH, Manrique A, Nganoa C, Roth N, Berman DS, Germano G, Slomka PJ, Agostini D. Quantification of myocardial blood flow by CZT-SPECT with motion correction and comparison with (15)O-water PET. J Nucl Cardiol 2021;28:1477–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 18. Leppo JA, Meerdink DJ. Comparison of the myocardial uptake of a technetium-labeled isonitrile analogue and thallium. Circ Res 1989;65:632–639. [DOI] [PubMed] [Google Scholar]
- 19. Yoshida K, Mullani N, Gould KL. Coronary flow and flow reserve by PET simplified for clinical applications using rubidium-82 or nitrogen-13-ammonia. J Nucl Med 1996;37:1701–1712. [PubMed] [Google Scholar]
- 20. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston T, Oslo N, Phoenix A, Nashville T, Hamilton OC, Uppsala S, Ghent LB, Cleveland O, Novara I, Rochester M, Bucharest R, St. Louis M. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–1360. [DOI] [PubMed] [Google Scholar]
- 21. Kotecha D, Piccini JP. Atrial fibrillation in heart failure: what should we do? Eur Heart J 2015;36:3250–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akincioglu C, Berman DS, Nishina H, Kavanagh PB, Slomka PJ, Abidov A, Hayes S, Friedman JD, Germano G. Assessment of diastolic function using 16-frame 99mTc-sestamibi gated myocardial perfusion SPECT: normal values. J Nucl Med 2005;46:1102–1108. [PubMed] [Google Scholar]
- 23. Nakajima K, Taki J, Kawano M, Higuchi T, Sato S, Nishijima C, Takehara K, Tonami N. Diastolic dysfunction in patients with systemic sclerosis detected by gated myocardial perfusion SPECT: an early sign of cardiac involvement. J Nucl Med 2001;42:183–188. [PubMed] [Google Scholar]
- 24. Yamamoto A, Nagao M, Ando K, Nakao R, Matsuo Y, Sakai A, Momose M, Kaneko K, Hagiwara N, Sakai S. First validation of myocardial flow reserve derived from dynamic (99 m)Tc-sestamibi CZT-SPECT camera compared with (13)N-ammonia PET. Int Heart J 2022;63:202–209. [DOI] [PubMed] [Google Scholar]
- 25. Giubbini R, Bertoli M, Durmo R, Bonacina M, Peli A, Faggiano I, Albano D, Milan E, Stern E, Paghera B, Rodella C, Cerudelli E, Gazzilli M, Dondi F, Bertagna F, Camoni L. Comparison between N(13)NH3-PET and (99 m)Tc-tetrofosmin-CZT SPECT in the evaluation of absolute myocardial blood flow and flow reserve. J Nucl Cardiol 2021;28:1906–1918. [DOI] [PubMed] [Google Scholar]
- 26. de Souza A, Harms HJ, Martell L, Bibbo C, Harrington M, Sullivan K, Hainer J, Dorbala S, Blankstein R, Taqueti VR, Foley Kijewski M, Park MA, Meretta A, Breault C, Roth N, Poitrasson-Riviere A, Soman P, Gullberg GT, Di Carli MF. Accuracy and reproducibility of myocardial blood flow quantification by single photon emission computed tomography imaging in patients with known or suspected coronary artery disease. Circ Cardiovasc Imaging 2022;15:e013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zavadovsky KV, Mochula AV, Boshchenko AA, Vrublevsky AV, Baev AE, Krylov AL, Gulya MO, Nesterov EA, Liga R, Gimelli A. Absolute myocardial blood flows derived by dynamic CZT scan vs invasive fractional flow reserve: correlation and accuracy. J Nucl Cardiol 2021;28:249–259. [DOI] [PubMed] [Google Scholar]
- 28. Rush CJ, Berry C, Oldroyd KG, Rocchiccioli JP, Lindsay MM, Touyz RM, Murphy CL, Ford TJ, Sidik N, McEntegart MB, Lang NN, Jhund PS, Campbell RT, McMurray JJV, Petrie MC. Prevalence of coronary artery disease and coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. JAMA Cardiol 2021;6:1130–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal 2008;10:1115–1126. [DOI] [PubMed] [Google Scholar]
- 30. Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med 2005;11:214–222. [DOI] [PubMed] [Google Scholar]
- 31. Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging 2010;3:623–640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request with the corresponding author.