Abstract
Excessive gestational weight gain contributes to adverse maternal and neonatal outcomes. Environmental exposures such as phthalates may lead to metabolic dysregulation, and studies suggest possible associations between maternal phthalate exposure and altered gestational weight gain. We assessed the association between nine maternal phthalate metabolites and measures of total gestational weight gain (pre-pregnancy to median 35.1 weeks of gestation) in a case-control study nested within LIFECODES (N = 379), a prospective birth cohort from Boston, Massachusetts (2006-2008). Our primary outcome was total gestational weight gain z score, a measure independent of gestational age that can provide a less biased estimate of this association. Our secondary outcomes were total gestational weight gain, rate of gestational weight gain, and adequacy ratio. The results were stratified by pre-pregnancy body mass index category. We found that concentrations of mono-(3-carboxypropyl) phthalate (MCPP) and mono-n-butyl phthalate (MBP) were positively associated with total gestational weight gain z scores among participants with obesity: adjusted mean difference (95% Confidence Interval [CI]) = 0.28 (0.03 – 0.46) and 0.11 (0.00 – 0.21) corresponding to an excess weight gain of 1.81 kilogram (kg) and 0.77 kg at 35 weeks of gestation per interquartile range-increase in MCPP and MBP, respectively. Also, among participants with obesity, MBP demonstrated a potential non-linear relationship with gestational weight gain in cubic spline models. These findings suggest that phthalates may be related to higher gestational weight gain, specifically, among individuals with pre-pregnancy obesity. Future research should investigate whether pregnant people with obesity represent a subpopulation with sensitivity to phthalate exposures.
Keywords: Pregnancy, Phthalates, Gestational weight gain, Maternal health
1. Introduction
Both inadequate and excessive gestational weight gain are associated with adverse maternal and neonatal health outcomes including gestational diabetes, preterm birth, and postpartum weight retention in the pregnant individual and large for gestational age and macrosomia in the offspring (Goldstein et al., 2017; Li et al., 2013; Pigatti Silva et al., 2019). Factors influencing gestational weight gain are multifactorial spanning sociodemographic, behavioral, and nutritional determinants (Bodnar et al., 2011; Campbell et al., 2016). Environmental chemical exposures are of emerging importance in our understanding of potentially modifiable factors influencing gestational weight gain.
Phthalates are a class of synthetic chemicals with potential obesogenic effects found in personal care products, medical supplies, building materials, and food packaging. Researchers observe ubiquitous exposure to phthalates within the general population (Zota et al., 2014). One way that phthalates are proposed to affect weight and metabolism is through the activation of peroxisome proliferator-activated receptors (PPARs) which have downstream effects on energy homeostasis through enhancing adipogenesis (Desvergne et al., 2009). Animal models also provide support for the obesogenic and metabolism-disrupting role of phthalates via activation of PPARs (Feige et al., 2010).
Outside of pregnancy, studies demonstrate an association between phthalate exposure and increased weight and weight gain (Buckley et al., 2019; Song et al., 2014; Trasande et al., 2013). Pregnancy represents a period of the life course with modified metabolic and physiologic adaptation; thus, pregnant individuals may be particularly sensitive to the impact of phthalates (Ferguson, McElrath, Ko, et al., 2014). Some prior studies support an association between maternal phthalate exposure and changes in weight gain during pregnancy. However, these studies have reported positive (Bellavia et al., 2017; Gao et al., 2021; James-Todd, Meeker, et al., 2016; Li et al., 2019; Tyagi, James-Todd, Minguez-Alarcon, et al., 2021; Zukin et al., 2021), negative (Deierlein et al., 2022; Pacyga et al., 2022) and null associations (Philips, Jaddoe, et al., 2020). Reports also differ with respect to the number and timing of phthalates measured and the phthalates implicated in the association with gestational weight gain. In addition, despite the fact that humans are co-exposed to numerous phthalates as a mixture, few studies have considered the cumulative effect of phthalate exposure on gestational weight gain (Deierlein et al., 2022; Gao et al., 2021; Pacyga et al., 2022).
Methods for quantifying gestational weight gain also vary between studies, complicating the comparability of results. Furthermore, preterm birth (i.e., delivery < 37 weeks of gestation) is often an exclusion criterion (Bellavia et al., 2017; James-Todd, Meeker, et al., 2016). Since prenatal phthalate exposure is associated with preterm birth (Ferguson, McElrath, & Meeker, 2014; Ferguson et al., 2019), this may exclude people with the greatest burden of exposure from assessment. Additionally, commonly used measures of gestational weight gain (e.g., total gestational weight gain, rate of gestational weight gain, gestational weight gain adequacy ratio) do not properly account for the natural correlation with gestational duration and may bias studies evaluating environmental exposures and total gestational weight gain when the exposure is also associated with the timing of delivery (Bodnar et al., 2015).
In this study, we sought to investigate the association between maternal phthalate exposure and gestational weight gain within a prospective birth cohort. Specifically, we measured the association between nine phthalate metabolites in maternal urine and their associations with gestational weight gain z scores, a measure of gestational weight gain that is uncorrelated with gestational age. In addition, we included analyses of other commonly used measures of total gestational weight gain (e.g., total gestational weight gain) to investigate whether results differed across these measures. Last, we used a mixtures-based approach, quantile g-computation, to examine the association between cumulative phthalate exposure and gestational weight gain.
2. Methods
2.1. Study sample
The study sample was drawn from the LIFECODES cohort, an on-going prospective birth cohort at Brigham and Women’s Hospital in Boston, Massachusetts (Ferguson, McElrath, & Meeker, 2014). Individuals are eligible to participate if they are 18 years or older, initiate prenatal care prior to 15 weeks of gestation, and plan to deliver at Brigham and Women’s Hospital. Participants attend four study visits, where questionnaires are administrated, which correspond to routinely scheduled prenatal care appointments (median: 9.7, 18.0, 26.1, and 35.1 weeks of gestation). Enrollment occurs during the first study visit. Gestational age is calculated using the last menstrual period and confirmed by ultrasound according to American College of Obstetrics and Gynecology guidelines (ACOG, 2014). Spot urine specimens are collected at each study visit. Following collection, urine specimens are stored at 4° C for a maximum of two hours prior to storage at −80° C until further chemical analysis.
We derived our study sample from participants with singleton pregnancies enrolled in the LIFECODES birth cohort between 2006-2008 (N = 1,181) that were included in a nested case-control study (N = 482). Details about the nested case-control have been described elsewhere (Ferguson, McElrath, & Meeker, 2014). Briefly, participants were selected for inclusion within the case-control study with an approximate ratio of 1:3 for preterm to term births. Preterm birth cases (N = 130) and term birth controls (N = 353) were randomly selected from cases and non-cases in the baseline LIFECODES study (N = 143 preterm and N = 1,039 term birth, respectively). We developed our study sample (Supplementary Figure 1) by further exclusion of individuals who developed preeclampsia or gestational diabetes from the analytical sample (N = 86) in accordance with recommendations for the best practices of studies evaluating maternal weight gain (Hutcheon & Bodnar, 2018), and because gestational weight gain trajectories differed among women with gestational diabetes and preeclampsia, compared to no events, in the current sample (Supplemental Figure 2). We excluded an additional 17 participants because they had one or more of the following: an intrauterine fetal demise (N = 1); missing gestational weight measures beyond the first trimester (N = 2); or missing covariates for insurance status or maternal education (N = 14). The final analytical sample comprised 379 participants.
2.2. Phthalate metabolite quantification
Nine phthalate metabolites were analyzed by NSF International (Ann Arbor, MI, USA) from the available urine samples collected at study visit 1 (N = 378), study visit 2 (N = 335), and study visit 3 (N = 322). Phthalate metabolites were also measured in samples from study visit 4, but due to the number of preterm births within the study sample, few were available, and we excluded these measurements from our analysis (Ferguson, McElrath, & Meeker, 2014). Phthalate metabolites were detected and quantified using enzymatic deconjugation of glucuronidated metabolites and solid-phase extraction coupled with high performance liquid chromatography-tandem mass spectrometry (Silva et al., 2007).Values below the limit of detection (LOD) were imputed as the LOD divided by the square root of 2 (Homung & Reed, 1990). The phthalate metabolites were specific gravity (SG)-corrected to adjust for urinary dilution with the formula: Pc = P[(1.015 – 1)/SG – 1], where Pc is the SG-corrected phthalate concentration, P is the observed phthalate concentration, SG is the observed specific gravity, and 1.015 is the median SG of the study population (Ferguson, McElrath, & Meeker, 2014). We calculated the geometric mean of each phthalate metabolite across the three study visits to estimate average phthalate exposure during pregnancy. A summary measure for the four metabolites of di(2-ethylhexyl) phthalate (DEHP) was created by summing their molar concentrations (ΣDEHP, micromoles/liter). Phthalate metabolite concentrations were natural log (ln)-transformed prior to analysis given their highly skewed nature.
2.3. Maternal weight measurements
Pre-pregnancy weight was self-reported at the first study visit, and participant height was measured by trained medical staff at the first study visit using instruments calibrated for clinical use. Maternal gestational weights were collected by trained medical staff at routine prenatal visits corresponding with study visit 1 (N = 376), study visit 2 (N = 353), study visit 3 (N = 348) and study visit 4 (N = 336). If maternal pre-pregnancy weight was unavailable, we substituted the weight measured at the first study visit (N = 4, median gestational age = 8.3 weeks). When available, we compared self-reported pre-pregnancy weight with first trimester weights to identify implausible values. The median difference in these weight measures was 1.8 kg (interquartile range [IQR]: 0.5 – 3.2 kg). Pre-pregnancy BMI was calculated from self-reported pre-pregnancy weight (kg) divided by the square of height (m). Maternal BMI (kg/m2) was categorized as underweight (< 18.5), normal weight (18.5 to < 25.0), overweight (25.0 to < 30.0), obese class I (30.0 to < 35.0), obese class II (35.0 to < 40.0), and obese class III (≥ 40.0) (CDC, 2022). In the stratified analysis, underweight and normal weight BMI categories were combined due to the small number of underweight women (N = 10). Additionally, participants in obesity classes I (N = 32), II (N = 17), and III (N = 6) were combined.
Our primary measure of gestational weight gain in this study was the total gestational weight gain z score, which was calculated using gestational age-specific weight gain reference charts constructed for people with normal pre-pregnancy BMI (Hutcheon et al., 2013) and those with underweight, overweight, and obese class I, II, and III pre-pregnancy BMI (Hutcheon et al., 2015). Total gestational weight gain in kg was calculated by subtracting pre-pregnancy weight from weight at the last available study visit. Gestational weight gain for gestational age z scores were calculated from the participants total gestational weight gain and gestational age at the time of the last weight measurement (median gestational age = 35.1 weeks, range: 15.7 weeks to 38.3 weeks).
As secondary measures of weight gain, we calculated: total gestational weight gain, rate of gestational weight gain, and the gestational weight gain adequacy ratio (Bodnar et al., 2011; IOM, 2009). Total gestational weight gain was calculated as previously described. The rate of gestational weight gain in kg per week was calculated from total gestational weight gain divided by the gestational age at the last available study visit. The adequacy ratio for gestational weight gain was calculated per the 2009 Institute of Medicine (IOM) guidelines (IOM, 2009). These guidelines provide a recommended amount of total weight gain across pregnancy based on pre-pregnancy BMI category with weekly rates of recommended weight gain in the second and third trimesters. The adequacy ratio was calculated using the observed total gestational weight gain divided by the IOM-recommended total gestational weight gain for each participant. This measure can be clinically applied to assess the adequacy of gestational weight gain in the second and third trimesters.
2.4. Covariates
Covariates were collected from information provided in the baseline questionnaire and from medical records. At study enrollment, participants filled out a demographic questionnaire that collected details about maternal race and ethnicity, factors related to socioeconomic status (e.g., education and health insurance provider), lifestyle factors (e.g., smoking and alcohol use during pregnancy), and medical history (e.g., number of previous pregnancies and existing chronic health conditions, such as thyroid disorders or diabetes mellitus). The questionnaire specifically asks about several racial identities (i.e., “Caucasian”, “Black”, “South Asian”, “East Asian”, “Native American/Pacific Islander”, “More than one race”, “Other”, “Unsure”, and “N/A”) and allowed participants to select multiple responses and provide free-form text response. Hispanic ethnicity was a separate question. Educational categories represented on the questionnaire included “Did not graduate high school”, “Graduated from high school”, “Attended technical school”, “Attended junior college or some college”, “Graduated from college”, “Attended or graduated from graduate school”. Additional information about their pregnancy (e.g., diagnosis with gestational diabetes, preeclampsia) and neonatal outcomes (e.g., timing of delivery and fetal sex) was abstracted from the medical record following delivery. Information abstracted from medical records was validated by medical record review conducted by two maternal fetal medicine specialists.
We used a directed acyclic graph (Supplementary Figure 3) to identify potential confounders. Within this directed acyclic graph, we interpret race and ethnicity to account for unmeasured cultural and social factors that may differ between groups leading to disparities in both exposures and reproductive health outcomes (James-Todd, Chiu, et al., 2016; Williams et al., 2016). The minimally sufficient adjustment set comprised maternal age, race and ethnicity, education, and insurance provider. Diet was also part of the minimally-sufficient adjustment set, but was unmeasured in this study. Pre-pregnancy BMI was included in our models for comparability purposes given that some measures of gestational weight gain include pre-pregnancy BMI in their calculation (e.g., gestational weight gain z-scores) and because it is a strong predictor of weight gain commonly accounted for in prior studies (Gao et al., 2021; Pacyga et al., 2023; Philips, Santos, et al., 2020; Tyagi, James-Todd, Mínguez-Alarcón, et al., 2021; Zukin et al., 2021). For other potential confounders not in the minimally-sufficient adjustment set (i.e., parity, maternal smoking during pregnancy, and alcohol consumption during pregnancy), we examined their influence on model estimates and retained them in final models if they changed the point estimate by more than 10% relative to the minimally sufficient model (Evans et al., 2012). The final covariates were expressed as: maternal age (years), race and ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and non-Hispanic other), insurance provider (private or public), maternal education (less than college or attended college), parity (nulliparous or multiparous), and pre-pregnancy BMI (kg/m2).
2.5. Statistical methods: single-pollutant
Analyses were performed using R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). Sample weights (1.1 and 2.95 for preterm cases and term controls, respectively) were incorporated into all analyses to account for sampling into the case-control study (Richardson et al., 2007), and data analysis was performed using the ‘survey’ package in R (Lumley, 2020).
Demographics and pregnancy characteristics of the study sample were tabulated as either N (%) or median (IQR). The 25th, 50th, 75th, and 95th percentiles of phthalate metabolites were also calculated and compared to levels reported among females in the 2007-2008 National Health and Nutrition Examination Survey (NHANES) cycle (CDC, 2009). Bivariate relationships between demographic variables and gestational weight gain z scores were explored and differences in gestational weight gain across these variables were tested using Wald tests. Correlations between the four gestational weight gain measures were assessed using Pearson correlation coefficients.
We used unadjusted and adjusted linear regression models to assess the relationship between an IQR-increase in urinary phthalate metabolites and gestational weight gain z scores. We stratified all models by pre-pregnancy BMI category since weight gain trajectories and total weight gain differ depending on pre-pregnancy BMI (Hutcheon & Bodnar, 2018; Riddell et al., 2017). Furthermore, the effect of weight gain on health outcomes during and after pregnancy can differ depending on pre-pregnancy BMI (Hutcheon & Bodnar, 2018), and others have found effect measure modification by pre-pregnancy BMI on the association between phthalates and gestational weight gain (Gao et al., 2021; Philips, Santos, et al., 2020).
We performed several sensitivity analyses. First, we replicated our analysis using our secondary measures of gestational weight gain: total gestational weight gain (kg), rate of gestational weight gain (kg/wk), and the gestational weight gain adequacy ratio. When analyzing total gestational weight gain, we also adjusted our models for gestational age at the time of weight measurement (Hutcheon & Bodnar, 2018). In order to foster comparisons between these measures of gestational weight gain, we back transformed point estimates for the z scores, rate of gestational weight gain, and the gestational weight gain adequacy ratio onto the same scale (i.e., kg). To transform point estimates for gestational weight gain adequacy ratio, we calculated weight gain for a normal weight participant with a first and last documented gestational weight measurement recorded at the median timepoints for the study sample. Second, we recreated our analysis excluding weight measurements collected prior to the third trimester (n = 43). Third, in order to adequately capture temporality, we reconstructed our average phthalate exposure variables excluding phthalate measurements recorded at or after the final weight measurement (n = 68). Fourth, we included only the phthalate measurements taken at the first study visit (n = 377). Last, we explored the potential for non-linear relationships between phthalate exposure and gestational weight gain using natural cubic splines. The Rao-Scott likelihood ratio test was used to compare models containing splines and simple linear terms.
2.6. Statistical methods: multi-pollutant
We used quantile g-computation, a method for estimating the overall effect of chemical mixtures, to investigate the joint association between the mixture of phthalate metabolites and gestational weight gain z scores (Keil et al., 2020). Specifically, we estimated the mean difference (95% CI) in gestational weight gain z scores associated with a simultaneous one-quartile increase in each phthalate metabolite within the mixture. All models were adjusted using the same covariates as described in our single-pollutant approach in order to ensure comparability between approaches and stratified according to pre-pregnancy BMI. Although quantile g-computation allows for complex dose-response shapes and interactions, given the small sample sizes within strata of pre-pregnancy BMI, we assumed linearity and additivity of the phthalates within the mixture. Quantile g-computation was carried out using the ‘qgcomp’ package version 2.10.1 (Keil, 2022).
3. Results
3.1. Participant characteristics and phthalate measures
The sample characteristics are presented in Table 1. Participants in the study were predominantly non-Hispanic White (61.2%), attended or graduated from college (86.6%), and had private health insurance (80.6%). Few participants reported smoking during pregnancy (5.70%), had a thyroid disorder (8.10%), or had pre-gestational diabetes (1.30%). With the sample weights applied, 9.70% of the sample gave birth preterm.
Table 1.
Demographic characteristics of LIFECODES case-control study participants (N = 379).
| n | % or Median (IQR) |
|
|---|---|---|
| Maternal and Pregnancy Characteristics | ||
| Maternal age, years | 379 | 32.6 (28.7 - 35.9) |
| Race and Ethnicity | ||
| Non-Hispanic White | 233 | 61.7 |
| Non-Hispanic African American | 53 | 13.9 |
| Hispanic | 55 | 15.0 |
| Other1 | 38 | 9.35 |
| Education | ||
| Less than college | 50 | 13.4 |
| Attended or graduated from college | 329 | 86.6 |
| Health insurance | ||
| Private | 308 | 80.6 |
| Public | 71 | 19.4 |
| Smoking during pregnancy | ||
| No | 358 | 94.3 |
| Yes | 21 | 5.70 |
| Alcohol consumption during pregnancy | ||
| No | 368 | 94.5 |
| Yes | 19 | 5.50 |
| Parity | ||
| Nulliparous | 170 | 45.5 |
| Multiparous | 209 | 54.5 |
| History of Thyroid Disorder | ||
| No | 347 | 91.9 |
| Yes | 32 | 8.10 |
| Pre-gestational diabetes | ||
| No | 374 | 98.7 |
| Yes | 5 | 1.30 |
| Preterm birth | ||
| No | 294 | 90.3 |
| Yes | 85 | 9.70 |
| Neonatal Sex | ||
| Female | 162 | 42.4 |
| Male | 217 | 57.6 |
| Gestational Weight Gain Characteristics | ||
| Pre-pregnancy or first trimester BMI, kg/m2 | 379 | 23.8 (21.4 - 27.3) |
| Pre-pregnancy or first trimester BMI category | ||
| Underweight ( < 18.5 kg/m2) | 10 | 2.10 |
| Normal weight (18.5 kg/m2 - 24.9 kg/m2) | 219 | 58.0 |
| Overweight (25.0 kg/m2 - 29.9 kg/m2) | 95 | 25.5 |
| Obese (≥ 30.0 kg/m2) | 55 | 14.4 |
| Time of first measured weight during pregnancy, wk 2 | 4 | 8.30 (7.90 - 8.80) |
| Self-reported pre-pregnancy weight, kg 2 | 379 | 63.5 (57.6 - 74.4) |
| Last measured weight, kg 3 | 379 | 78.5 (70.3 - 88.0) |
| Gestational age at last recorded weight, wk | 379 | 35.1 (34.4 - 35.9) |
| Total gestational weight gain, kg | 379 | 12.7 (9.50 - 16.8) |
| Rate of gestational weight gain, kg/wk | 379 | 0.37 (0.28 - 0.47) |
| Gestational weight gain adequacy ratio | 379 | 1.28 (0.97 - 1.72) |
| Gestational weight gain z score, SD | 379 | −0.08 (ℒ0.68 - 0.501 |
Abbreviations: BMI body mass index, IQR interquartile range, SD standard deviation, kg kilogram, m meter, wk week
= Asian, Mixed, Other non-Hispanic
= first trimester weight used when no pre-pregnancy BMI available
Mean gestational age 35.1 weeks
A majority of the study sample was classified as having a normal pre-pregnancy BMI (60.1%), and the median pre-pregnancy BMI was 23.8 kg/m2. The median gestational age at the last recorded weight measurement was 35.1 weeks. The median total gestational weight gain, rate of gestational weight gain, and weight gain adequacy ratio was 12.7 kg, 0.37 kg/wk, and 1.28, respectively. The median gestational weight gain z score was −0.08, and the z score was highly correlated with the other measures of gestational weight gain (Supplementary Tables 1 & 2).
In our bivariate comparisons, we observed that participants with pre-gestational diabetes had a lower gestational weight gain z score (−1.09) compared to participants without (−0.15). We did not observe differences by maternal age, race and ethnicity, education, smoking status, preterm birth, and pre-pregnancy BMI category with respect to gestational weight gain z scores (Supplementary Table 1).
The SG-corrected geometric means of the urinary phthalate metabolites are presented in Table 2. Most metabolites were detected in greater than 95% of the samples. When compared to concentrations measured in women in NHANES (cycle year 2007 – 2008), LIFECODES study participants had higher levels of MEP and the individual metabolites of DEHP. Similar exposure levels were observed for the other phthalate metabolites.
Table 2.
Distribution of urinary phthalate metabolites in LIFECODES case-control study participants (N = 1035 samples) and in females from the National Health and Examination Nutrition Survey (NHANES) 2007 - 2008.
| Parent phthalate compound |
Phthalate metabolite |
LOD | N (%) < LOD |
LIFECODES percentiles a |
NHANES 2007 - 2008 percentiles b |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25 th |
50 th |
75 th |
95 th |
50th | 75th | 95th | ||||
| Diethyl phthalate (DEP) | Mono-ethyl phthalate (MEP), μg/L | 1.0 | 2 (0) | 58.0 | 12 9 | 29 7 | 11 73 | 82.4 | 233 | 1050 |
| Di-n-butyl phthalate (DnBP) | Mono-n-butyl phthalate (MBP), μg/L | 0.5 | 5 (0) | 10.9 | 16.0 | 23.0 | 52.0 | 20.8 | 42.5 | 132 |
| Benzyl butyl phthalate (BzBP) | Mono-n-benzyl phthalate (MBzP), μg/L | 0.2 | 12 (1) | 3.38 | 5.96 | 11.6 | 38.8 | 7.99 | 18.8 | 64.4 |
| Di-isobutyl phthalate (DiBP) | Mono-isobutyl phthalate (MiBP), μg/L | 0.1 | 0 (0) | 4.65 | 7.00 | 10.8 | 20.4 | 7.40 | 15.5 | 39.8 |
| Di-n-octyl phthalate (DnOP) | Mono-(3-carboxyprop yl)phthalate (MCPP), μg/L | 0.2 | 39 (4) | 1.16 | 1.82 | 3.06 | 10.2 | 2.60 | 5.90 | 15.4 |
| Di-2-ethylhexyl phthalate (DEHP) | Summed DEHP metabolites (ΣDEHP), μmol/L | — | — | 0.21 | 0.34 | 0.60 | 1.63 | — | — | — |
| Mono-(2-ethyl-5-hydroxyhex yl) phthalate (MEHHP), μg/L | 0.1 | 0 (0) | 18.6 | 32.8 | 56.8 | 16 3 | 19.9 | 51.0 | 223 | |
| Mono-(2-ethyl-5-ethyl-5-oxohexyl)phthalate (MEOHP), μg/L | 0.1 | 1 (0) | 10.0 | 16.4 | 30.1 | 77.0 | 12.5 | 25.9 | 114 | |
| Mono-(2-ethyl)-hexyl phthalate (MEHP), μg/L | 1.0 | 46 (4) | 5.70 | 10.9 | 17.8 | 58.1 | 2.00 | 5.10 | 26.4 | |
| Mono-(2-ethyl-5-carboxypent yl) phthalate (MECPP), μg/L | 0.2 | 0 (0) | 21.3 | 38.9 | 68.4 | 20 2 | 31.0 | 73.0 | 297 | |
Abbreviations: LOD; limit of detection; —, Not applicable.
Geometric mean and specific gravity-corrected phthalate concentration over study visits one, two, three.
Percentiles from females in the NHANES 2007 - 2008 cycle. Not adjusted for specific gravity or creatinine.
3.2. Associations between phthalate metabolites and gestational weight gain
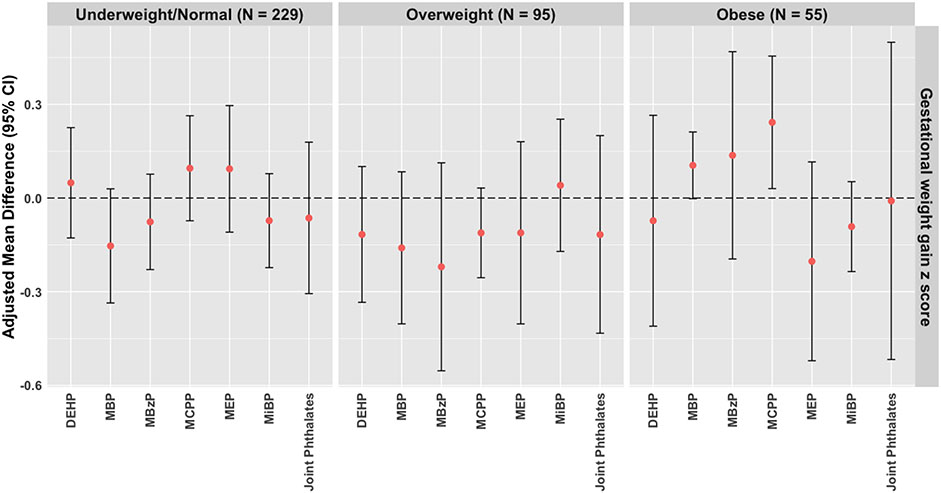
When stratified by pre-pregnancy BMI, we observed an association between mono-(3-carboxypropyl) phthalate (MCPP) and higher gestational weight gain z scores among participants with pre-pregnancy obesity in adjusted models (mean difference: 0.242, 95% CI: 0.030 – 0.455) (Figure 1; Supplementary Table 3). We also observed a positive association between mono-n-butyl phthalate (MBP) and higher gestational weight gain z scores among participants with pre-pregnancy obesity (mean difference: 0.105, 95% CI: 0.002 – 0.212) (Figure 1; Supplementary Table 3). These effect estimates correspond to an excess weight gain of 1.81 kg and 0.77 kg at 35 weeks of gestation per IQR-increase of MCPP and MBP, respectively. Associations for other phthalate metabolites and in other strata of pre-pregnancy BMI were null (Supplementary Table 3). In crude models, we did not observe associations between the phthalate metabolites and gestational weight gain z scores (Supplementary Table 4). After adjusting for potential confounders, our findings remained null for the association between phthalate metabolites and gestational weight gain z scores among the overall cohort.
Figure 1.
Adjusted mean difference (95% CI) of gestational weight gain z scores associated with an IQR-increase in urinary phthalate biomarkers or a 1-quartile increase in the phthalate mixture, stratified by pre-pregnancy BMI category in the LIFECODES cohort (n = 379).
Abbreviations: IQR, interquartile range; CI, Confidence Interval; BMI, Body Mass Index;DEHP, Summed Di-2-ethylhexyl phthalate metabolites; MBzP, Mono-benzyl phthalate; MBP, Mono-n-butyl phthalate; MiBP, Mono-isobutyl phthalate; MEP, Mono-ethyl phthalate; MCPP, Mono-(3-carboxypropyl) phthalate.
Models for rate of gestational weight gain, gestational weight gain adequacy ratio, and gestational weight gain z score adjusted for maternal age, insurance, race and ethnicity, education, parity, and pre-pregnancy BMI. Models for total gestation weight gain additionally adjusted for gestational age at last recorded weight. Phthalate metabolites were natural log transformed and IQR-standardized.
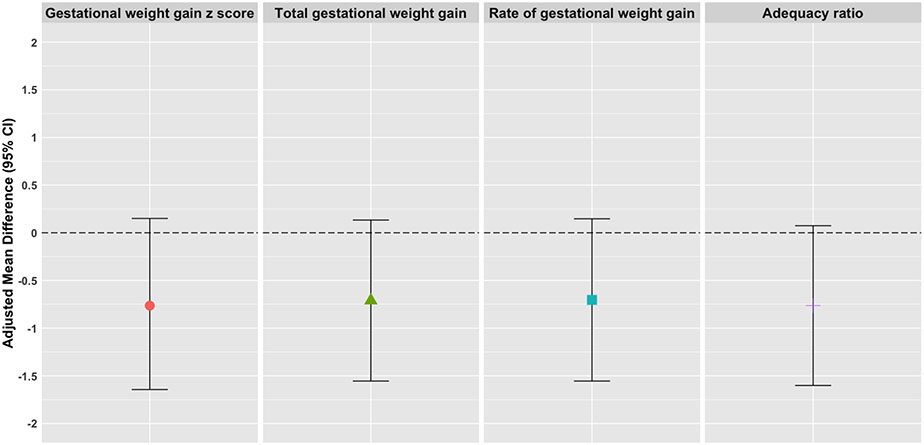
Results using our secondary measures of gestational weight gain (e.g., total gestational weight gain, rate of gestational weight gain, and the gestational weight gain adequacy ratio) were consistent with our primary analysis (Supplementary Table 3). For example, when point estimates for an association between MBP and gestational weight gain among normal pre-pregnancy BMI participants at 35 weeks of gestation were transformed onto the same scale (kg), the point estimates (95% CI) were extremely similar across all weight gain measures. Specifically, they were −0.76 (−1.64 – 0.15) for gestational weight gain z scores, −0.71 (−1.55 – 0.13) for total gestational weight gain, − 0.70 (−1.55 – 0.15) for the rate of gestational weight gain, and −0.76 (−1.60 – 0.07) for the gestational weight gain adequacy ratio (Figure 2).
Figure 2.
Adjusted mean difference (95% CI) per IQR unit increase of MBP for gestational weight gain z scores, total gestational weight gain, rate of gestational weight gain, and adequacy of gestational weight gain transformed to kilograms for a study participant with a normal pre-pregnancy BMI at 35 weeks of gestation.
Abbreviations: MBP, mono-n-butyl phthalate; BMI, Body Mass Index; CI, confidence interval; IQR, interquartile range.
Models for rate of gestational weight gain, gestational weight gain adequacy ratio, and gestational weight gain z score adjusted for maternal age, insurance, race and ethnicity, education, parity, and pre-pregnancy BMI. Models for total gestation weight gain additionally adjusted for gestational age at last recorded weight. Phthalate metabolites were natural log transformed and IQR-standardized.
In our sensitivity analyses, we restricted our models to participants with a final weight measurement taken during the third trimester (N = 336) (Supplementary Table 5). In addition, we reconstructed the pregnancy-averaged phthalate measurements to exclude measurements taken at or after the final weight measurement (Supplementary Table 6), and to only include phthalate measurements assessed at the first study visit (n = 377) (Supplementary Table 7). In these analyses, the point estimates remained consistent with our original models, although they were less precise.
3.3. Non-linear associations
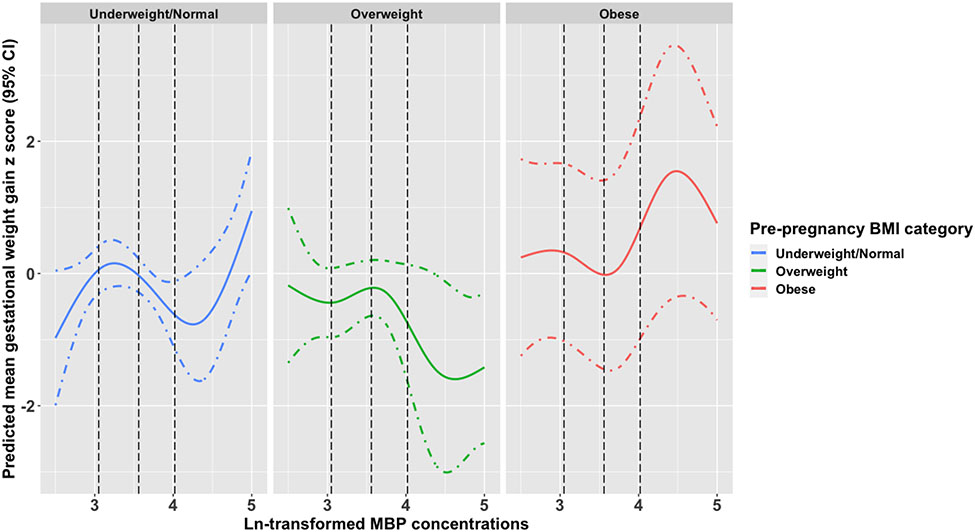
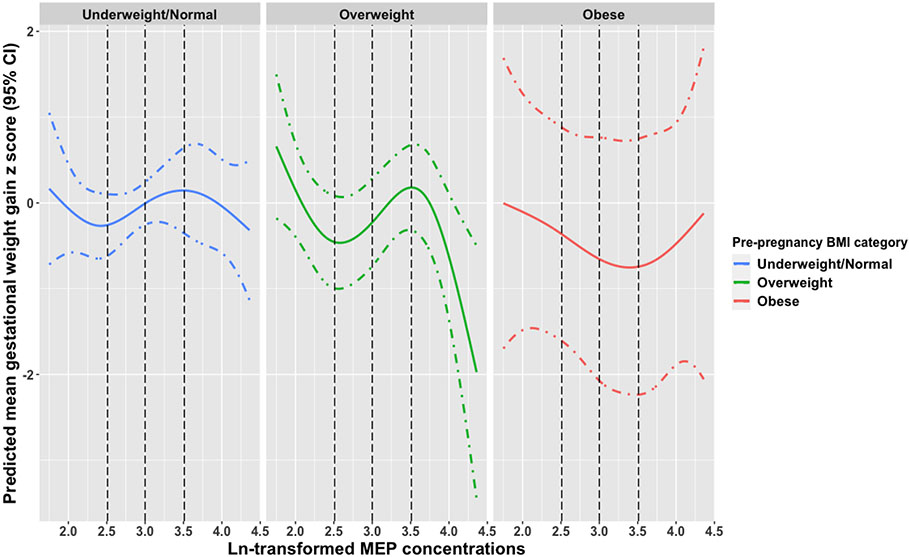
When potential non-linear associations were explored using natural cubic splines, both MBP and MEP demonstrated a non-linear association with gestational weight gain. Among participants with obesity, MBP concentrations above the median were associated with a higher gestational weight gain z score. Additionally, concentrations between the median and 75th percentile for participants with an underweight/normal BMI were associated with lower gestational weight gain z scores (Figure 3). MEP concentrations between the 25th and 75th percentiles showed an association with higher gestational weight gain z scores among overweight participants (Figure 4). We observed no evidence for strong non-linear associations between the other phthalate metabolites and gestational weight gain (data not shown).
Figure 3.
Predicted mean (95% CI) gestational weight gain z scores associated with ln-transformed MBP exposure using natural cubic splines stratified by pre-pregnancy BMI category.
Abbreviations: CI, Confidence Interval; ln, natural log; MBP, mono-n-butyl phthalate; BMI, Body Mass Index; LRT, likelihood ratio test; Black dashed lines (---) are the 25th, 50th, and 75th percentiles of overall exposure.
Models adjusted for maternal age, insurance, race and ethnicity, education, parity, and pre-pregnancy BMI. LRT p-values: underweight/normal (p = 0.01), overweight (p = 0.11), obese (p = 0.03).
Figure 4.
Predicted mean (95% CI) gestational weight gain z scores associated with ln-transformed MEP exposure using natural cubic splines stratified by pre-pregnancy BMI category.
Abbreviations: CI, Confidence Interval; ln, natural log; MEP, mono-ethyl phthalate; BMI, Body Mass Index; LRT, likelihood ratio test; Black dashed lines (---) are the 25th, 50th, and 75th percentiles of overall exposure.
Models adjusted for maternal age, insurance, race and ethnicity, education, parity, and pre-pregnancy BMI. LRT p-values: underweight/normal (p = 0.41), overweight (p = < 0.01), obese (p = 0.44).
3.4. Joint associations between phthalates and gestational weight gain
Using quantile g-computation, we observed a null association between a 1-quartile increase in all phthalate metabolites and gestational weight gain z scores (mean difference: −0.076, 95% CI: −0.251 – 0.098; Supplementary Table 3). Similarly, findings from models stratified by pre-pregnancy BMI were also null. Specifically, we observed that phthalates were jointly associated with a 0.064 (95% CI: −0.306 – 0.179), 0.117 (95% CI: −0.433 – 0.200), and 0.009 (95% CI: −0.517, 0.499) decrease in gestational weight gain z scores for participants with an underweight/normal, overweight, or obese pre-pregnancy BMI, respectively (Figure 1; Supplementary Table 3).
4. Discussion
In this study, we investigated associations between maternal phthalate exposure and weight gain during pregnancy in a subset of the LIFECODES cohort. In our primary analysis, we observed that urinary MCPP and MBP were associated with higher gestational weight gain z scores among participants with obesity. For example, we found that per IQR-increase of MCPP, this corresponded to an excess weight gain of 1.81 kg at 35 weeks of gestation for participants with pre-pregnancy obesity. Given that the recommended total weight gain for people with pre-pregnancy obesity is 5 to 9 kg (IOM, 2009), environmental factors, such as phthalate exposure, could contribute to a significant proportion of this weight gain. Moreover, our analyses suggested possible non-linear associations between some phthalate metabolites, including MBP, and gestational weight gain in overweight and obese participants. These findings remained robust in sensitivity analyses and were consistent across different measures of gestational weight gain. However, joint associations between phthalate metabolites and gestational weight gain z scores were null.
Previous studies on this topic have reported a variety of associations between phthalates and gestational weight gain. Consistent with the findings in our current study, the Environmental and Reproductive Health (EARTH) Study reported that MBP exposure was associated with increased second and third trimester weight gain (Tyagi, James-Todd, Minguez-Alarcon, et al., 2021). This analysis also examined exposure to a mixture of phthalates and phenols at multiple time points during pregnancy using Bayesian Kernel Machine Regression, although associations between phthalate metabolites and weight gain were null when accounting for the other compounds. Results from the Ma’anshan Birth Cohort also estimated that intake of the parent compounds di-n-butyl phthalate (DBP), diethyl phthalate (DEP), and several others, both individually and as a mixture, was associated with higher total gestational weight gain and weight gain during late pregnancy (Gao et al., 2021). Other studies have also reported associations between MEP and greater gestational weight gain. For example, in an analysis of total gestational weight gain among only term births in the LIFECODES study population, MEP was associated with higher odds of excessive gestational weight gain (James-Todd, Meeker, et al., 2016) and with higher weight gain during the first trimester for overweight and obese people (Bellavia et al., 2017). Similar observations for MEP exposure and excessive gestational weight gain were noted in a recent study of Latina individuals participating in the Center for Health Assessment of Mothers and Children of Salinas Valley (CHAMACOS) Study (Zukin et al., 2021). In contrast, a recent analysis from the I-KIDS cohort observed largely inverse associations between phthalates and gestational weight gain z scores, including for MCPP (Pacyga et al., 2022). Interestingly, Pacyga et al also found a joint inverse association between phthalate exposure and gestational weight gain z scores using both quantile g-computation and weighted quantile sums regression.
Notably, we observed associations between phthalates and gestational weight gain only among participants with obesity. These findings suggest that participants with obesity may represent a sensitive subpopulation to the effects of phthalates on gestational weight gain. One possible explanation for these observations could include that obesity is a condition characterized by higher inflammation, oxidative stress, and altered hormonal function (Catalano & Shankar, 2017; Fernandez-Sanchez et al., 2011), which could alter response to environmental chemicals such as phthalates. However, findings have been inconsistent across other studies that have examined pre-pregnancy BMI as an effect measure modifier of these associations. For example, while significant effect modification was observed based on pre-pregnancy BMI in the Generation R cohort, there was no association between early and mid-pregnancy phthalate exposure and total gestational weight gain (Philips, Jaddoe, et al., 2020). Yet, associations between phthalate exposure and gestational weight gain outcomes among participants with an obese pre-pregnancy BMI were not reported in the study, likely due to the small number in the study population (Philips, Jaddoe, et al., 2020). On the other hand, most phthalates were positively associated with gestational weight gain in the Ma’anshan Birth Cohort, though associations only reached statistical significance among participants with an underweight or normal pre-pregnancy BMI (Gao et al., 2021). Given that the distribution of pre-pregnancy BMI may vary widely across study populations and geographic locations, it may be important to stratify results based on this factor. Such differences may contribute to heterogeneity between studies and complicate the ability to make comparisons across populations.
There is toxicologic literature that supports a link between phthalate exposure and weight gain. For example, phthalates may selectively regulate the PPARγ isotype which can impact transcription of genes involved in lipid metabolism, energy homeostasis, and adipogenesis (Feige et al., 2007; Feige et al., 2010; Jia et al., 2016). In activation studies, higher molecular weight phthalates appear to potentiate the activation of PPARγ more strongly and efficiently with increasing side-chain length (Bility et al., 2004). As MCPP is a high molecular weight phthalate, this may explain why MCPP exposure was associated with higher gestational weight gain in our study. Phthalates may also impact weight gain during pregnancy through their endocrine disrupting effects. For example, in vitro studies have demonstrated that MBP may affect thyroid hormone signaling by acting as a triiodothyronine (T3) antagonist (Sugiyama et al., 2005). However, it should be noted that numerous phthalates interact with PPARγ and are known endocrine disrupting compounds. Therefore, further research is necessary to explain why some phthalates, and not others, may be related to weight gain during pregnancy. In addition, animal research remains conflicted around the role of phthalate exposure in weight gain during pregnancy, with prior studies reporting both null effects in mice (Hardin et al., 1987) and even inverse effects in rats (Furr et al., 2014; Gray et al., 2000; Howdeshell et al., 2008). However, there are challenges in making comparisons to animal models due to the relevance of exposure levels used and difficulty disentangling litter weight from other elements of weight gain during pregnancy (Weaver et al., 2020).
The lack of information about the participants’ delivery weights is a limitation of this study. In this sample, the median gestational age at the last study visit was 35 weeks of gestation, while the median gestational age at delivery was 39 weeks. However, given difficulties in appropriately accounting for the natural correlation between gestational weight gain and the timing of delivery, this approach may be less biased than those using delivery weights (Mitchell et al., 2016). In addition, the use of self-reported pre-pregnancy weights may also be subject to bias as underreporting is common and may vary according to important factors in this study, such as pre-pregnancy BMI (Headen et al., 2017; Sharma et al., 2021). The study sample was drawn from a high-risk population seeking maternal-fetal medicine services at a tertiary care center. This population is known to have a higher burden of maternal chronic diseases and adverse pregnancy outcomes, which may influence both phthalate exposure and gestational weight gain (Phthalates, 2008). Moreover, this population largely identified as non-Hispanic White and has a relatively high socioeconomic status. Given this, these results may not be fully generalizable to other low-risk obstetrics populations. However, it is worth noting that the observed exposure levels were consistent with levels reported in NHANES and our analysis excluded common pregnancy complications that affect gestational weight gain. We also lacked information about some potential sources of phthalates, such as diet. This may confound the relationship between phthalate exposure and gestational weight gain since phthalates, including those identified in this study, are commonly found in packaging materials as well as processed foods (Edwards et al., 2021; Schecter et al., 2013) and diet influences gestational weight gain (Donovan et al., 2020).
Our study does contain multiple strengths, including the use of gestational weight gain z scores as our primary measure of gestational weight gain. Z scores provide a method to account for the inherent correlation between gestational age and gestational weight gain. This is of additional importance in this study as phthalates have been shown to influence the timing of delivery (Ferguson, McElrath, & Meeker, 2014), which could result in bias when assessing total gestational weight gain (Hutcheon & Bodnar, 2018; Hutcheon et al., 2012). Nevertheless, we demonstrated that z scores produced similar results compared to our secondary measures of weight gain (e.g., total gestational weight gain, rate of gestational weight gain, and gestational weight gain adequacy ratio). While this result was unexpected, we believe this could be due to the fact that we lacked a measure of gestational weight at delivery and instead relied on a measure taken during late pregnancy (median 35.1 weeks gestation) to calculate our measures of gestational weight gain (Mitchell et al., 2016). We also incorporated a mixtures-based approach, using quantile-g computation to examine the joint association between phthalates and gestational weight gain z scores. Although our findings in this analysis were null, this study is among the first to examine the cumulative influence of phthalate exposure on gestational weight gain. Given the small sample size within strata of pre-pregnancy BMI, future studies may want to investigate additional questions about chemical mixtures in larger samples (e.g., chemical-chemical interactions or complex dose-response shapes). Last, phthalates are non-persistent and have short half-lives in the body. However, we derived our measure of phthalate exposure from the geometric mean of up to three exposure measurements taken during pregnancy, which represents a larger number of samples than several prior studies on this topic. Notably, averaging multiple measurements may reduce exposure misclassification (Braun et al., 2012; Yazdy et al., 2018).
5. Conclusions
Our results add to the existing body of research demonstrating an association between phthalate exposure during pregnancy and gestational weight gain. We observed potential associations between some phthalate metabolites and higher gestational weight gain among participants with obesity, suggesting possible sensitivity to phthalate exposure among these participants. Future studies should continue to investigate how the use of serial weight and exposure measurements impact this association. Furthermore, additional research is needed to determine if susceptibility may differ by pre-pregnancy BMI. Nevertheless, in light of evidence that phthalates contribute to a variety of adverse reproductive health outcomes, work to reduce exposure should continue.
Supplementary Material
Highlights.
Associations between phthalates and gestational weight gain were modified by pre-pregnancy BMI
MCPP and MBP are associated with higher gestational weight gain among women with obesity
Some phthalates, including MBP, had non-linear associations with gestational weight gain
Acknowledgements
This research was funded, in part, by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health (ZIAE103321). Additional funding was provided by the National Institute of Environmental Health Sciences, National Institutes of Health (R01ES018872, R01ES031591, P30ES000002) and the National Heart, Blood, and Lung Institute, National Institutes of Health (T32HL007024).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- ACOG. (2014, Oct). Committee opinion no 611: method for estimating due date. Obstet Gynecol, 124(4), 863–866. 10.1097/01.AOG.0000454932.15177.be [DOI] [PubMed] [Google Scholar]
- Bellavia A, Hauser R, Seely EW, Meeker JD, Ferguson KK, McElrath TF, & James-Todd T (2017, Nov). Urinary phthalate metabolite concentrations and maternal weight during early pregnancy. Int J Hyg Environ Health, 220(8), 1347–1355. 10.1016/j.ijheh.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bility MT, Thompson JT, McKee RH, David RM, Butala JH, Vanden Heuvel JP, & Peters JM (2004, Nov). Activation of mouse and human peroxisome proliferator-activated receptors (PPARs) by phthalate monoesters. Toxicol Sci, 82(1), 170–182. 10.1093/toxsci/kfh253 [DOI] [PubMed] [Google Scholar]
- Bodnar LM, Hutcheon JA, Parisi SM, Pugh SJ, & Abrams B (2015, Jan). Comparison of gestational weight gain z-scores and traditional weight gain measures in relation to perinatal outcomes. Paediatr Perinat Epidemiol, 29(1), 11–21. 10.1111/ppe.12168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar LM, Hutcheon JA, Platt RW, Himes KP, Simhan HN, & Abrams B (2011, Jul 15). Should gestational weight gain recommendations be tailored by maternal characteristics? Am J Epidemiol, 174(2), 136–146. 10.1093/aje/kwr064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, & Hauser R (2012, May). Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ Health Perspect, 120(5), 739–745. 10.1289/ehp.1104139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Kim H, Wong E, & Rebholz CM (2019, Oct). Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013-2014. Environ Int, 131, 105057. 10.1016/j.envint.2019.105057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EE, Dworatzek PD, Penava D, de Vrijer B, Gilliland J, Matthews JI, & Seabrook JA (2016, Nov). Factors that influence excessive gestational weight gain: moving beyond assessment and counselling. J Matern Fetal Neonatal Med, 29(21), 3527–3531. 10.3109/14767058.2015.1137894 [DOI] [PubMed] [Google Scholar]
- Catalano PM, & Shankar K (2017, Feb 8). Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ, 356, j1. 10.1136/bmj.j1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2009). Fourth Report on Human Exposure to Environmental Chemicals. https://www.cdc.gov/exposurereport/
- CDC. (2022). Defining Adult Overweight & Obesity. https://www.cdc.gov/obesity/basics/adult-defining.html
- Deierlein AL, Wu H, Just AC, Kupsco AJ, Braun JM, Oken E, Soria-Contreras DC, Cantoral A, Pizano ML, McRae N, Tellez-Rojo MM, Wright RO, & Baccarelli AA (2022, Jun). Prenatal phthalates, gestational weight gain, and long-term weight changes among Mexican women. Environ Res, 209, 112835. 10.1016/j.envres.2022.112835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvergne B, Feige JN, & Casals-Casas C (2009, May 25). PPAR-mediated activity of phthalates: A link to the obesity epidemic? Mol Cell Endocrinol, 304(1-2), 43–48. 10.1016/j.mce.2009.02.017 [DOI] [PubMed] [Google Scholar]
- Donovan S, Dewey K, Novotny R, Stang J, Taveras E, Kleinman R, Raghavan R, Nevins J, Scinto-Madonich S, Kim JH, Terry N, Butera G, & Obbagy J (2020). In Dietary Patterns during Pregnancy and Gestational Weight Gain: A Systematic Review. 10.52570/NESR.DGAC2020.SR0201 [DOI] [PubMed] [Google Scholar]
- Edwards L, McCray NL, VanNoy BN, Yau A, Geller RJ, Adamkiewicz G, & Zota AR (2021, Oct 27). Phthalate and novel plasticizer concentrations in food items from U.S. fast food chains: a preliminary analysis. J Expo Sci Environ Epidemiol. 10.1038/s41370-021-00392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D, Chaix B, Lobbedez T, Verger C, & Flahault A (2012, Oct 11). Combining directed acyclic graphs and the change-in-estimate procedure as a novel approach to adjustment-variable selection in epidemiology. BMC Med Res Methodol, 12, 156. 10.1186/1471-2288-12-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige JN, Gelman L, Rossi D, Zoete V, Metivier R, Tudor C, Anghel SI, Grosdidier A, Lathion C, Engelborghs Y, Michielin O, Wahli W, & Desvergne B (2007, Jun 29). The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J Biol Chem, 282(26), 19152–19166. 10.1074/jbc.M702724200 [DOI] [PubMed] [Google Scholar]
- Feige JN, Gerber A, Casals-Casas C, Yang Q, Winkler C, Bedu E, Bueno M, Gelman L, Auwerx J, Gonzalez FJ, & Desvergne B (2010, Feb). The pollutant diethylhexyl phthalate regulates hepatic energy metabolism via species-specific PPARalpha-dependent mechanisms. Environ Health Perspect, 118(2), 234–241. 10.1289/ehp.0901217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Ko YA, Mukherjee B, & Meeker JD (2014, Sep). Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int, 70, 118–124. 10.1016/j.envint.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, & Meeker JD (2014, Jan). Environmental phthalate exposure and preterm birth. JAMA Pediatr, 168(1), 61–67. 10.1001/jamapediatrics.2013.3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Rosen EM, Rosario Z, Feric Z, Calafat AM, McElrath TF, Velez Vega C, Cordero JF, Alshawabkeh A, & Meeker JD (2019, Nov). Environmental phthalate exposure and preterm birth in the PROTECT birth cohort. Environ Int, 132, 105099. 10.1016/j.envint.2019.105099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, Esquivel-Soto J, Morales-Gonzalez A, Esquivel-Chirino C, Durante-Montiel I, Sanchez-Rivera G, Valadez-Vega C, & Morales-Gonzalez JA (2011). Inflammation, oxidative stress, and obesity. Int J Mol Sci, 12(5), 3117–3132. 10.3390/ijms12053117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr JR, Lambright CS, Wilson VS, Foster PM, & Gray LE Jr. (2014, Aug 1). A short-term in vivo screen using fetal testosterone production, a key event in the phthalate adverse outcome pathway, to predict disruption of sexual differentiation. Toxicol Sci, 140(2), 403–424. 10.1093/toxsci/kfu081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Zhu BB, Huang K, Zhu YD, Yan SQ, Wu XY, Han Y, Sheng J, Cao H, Zhu P, & Tao FB (2021, Oct). Effects of single and combined gestational phthalate exposure on blood pressure, blood glucose and gestational weight gain: A longitudinal analysis. Environ Int, 155, 106677. 10.1016/j.envint.2021.106677 [DOI] [PubMed] [Google Scholar]
- Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, Li N, Hu G, Corrado F, Rode L, Kim YJ, Haugen M, Song WO, Kim MH, Bogaerts A, Devlieger R, Chung JH, & Teede HJ (2017, Jun 6). Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA, 317(21), 2207–2225. 10.1001/jama.2017.3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE Jr., Ostby J, Furr J, Price M, Veeramachaneni DN, & Parks L (2000, Dec).Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci, 58(2), 350–365. 10.1093/toxsci/58.2.350 [DOI] [PubMed] [Google Scholar]
- Hardin BD, Schuler RL, Burg JR, Booth GM, Hazelden KP, MacKenzie KM, Piccirillo VJ, & Smith KN (1987). Evaluation of 60 chemicals in a preliminary developmental toxicity test. Teratog Carcinog Mutagen, 7(1), 29–48. 10.1002/tcm.1770070106 [DOI] [PubMed] [Google Scholar]
- Headen I, Cohen AK, Mujahid M, & Abrams B (2017, Mar). The accuracy of self-reported pregnancy-related weight: a systematic review. Obes Rev, 18(3), 350–369. 10.1111/obr.12486 [DOI] [PubMed] [Google Scholar]
- Hornung RW, & Reed LD (1990). Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg, 5, 46–51. [Google Scholar]
- Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, Hotchkiss AK, & Gray LE Jr. (2008, Sep). A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicol Sci, 105(1), 153–165. 10.1093/toxsci/kfn077 [DOI] [PubMed] [Google Scholar]
- Hutcheon JA, & Bodnar LM (2018, Mar). Good Practices for Observational Studies of Maternal Weight and Weight Gain in Pregnancy. Paediatr Perinat Epidemiol, 32(2), 152–160. 10.1111/ppe.12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon JA, Bodnar LM, Joseph KS, Abrams B, Simhan HN, & Platt RW (2012, Mar). The bias in current measures of gestational weight gain. Paediatr Perinat Epidemiol, 26(2), 109–116. 10.1111/j.1365-3016.2011.01254.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, & Bodnar LM (2013, May). A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. Am J Clin Nutr, 97(5), 1062–1067. 10.3945/ajcn.112.051706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, & Bodnar LM (2015, Mar). Pregnancy weight gain charts for obese and overweight women. Obesity (Silver Spring), 23(3), 532–535. 10.1002/oby.21011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM. (2009). In Rasmussen KM & Yaktine AL (Eds.), Weight Gain During Pregnancy: Reexamining the Guidelines. 10.17226/12584 [DOI] [PubMed] [Google Scholar]
- James-Todd TM, Chiu YH, & Zota AR (2016, Jun). Racial/ethnic disparities in environmental endocrine disrupting chemicals and women's reproductive health outcomes: epidemiological examples across the life course. Curr Epidemiol Rep, 3(2), 161–180. 10.1007/s40471-016-0073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd TM, Meeker JD, Huang T, Hauser R, Ferguson KK, Rich-Edwards JW, McElrath TF, & Seely EW (2016, Nov). Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ Int, 96, 118–126. 10.1016/j.envint.2016.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Liu T, Zhou L, Zhu J, Wu J, Sun D, Xu J, Wang Q, Chen H, Xu F, Zhang Y, Zhang T, Liu H, & Ye L (2016, Nov 4). Effects of Di-(2-ethylhexyl) Phthalate on Lipid Metabolism by the JAK/STAT Pathway in Rats. Int J Environ Res Public Health, 13(11). 10.3390/ijerph13111085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A (2022). qgcomp: Quantile G-Computation. In https://CRAN.R-project.org/package=qgcomp
- Keil AP, Buckley JP, O'Brien KM, Ferguson KK, Zhao S, & White AJ (2020, Apr). A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ Health Perspect, 128(4), 47004. 10.1289/EHP5838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Qian X, Zhao H, Zhou Y, Xu S, Li Y, Xiang L, Shi J, Xia W, & Cai Z (2019, Nov 30). Determinants of exposure levels, metabolism, and health risks of phthalates among pregnant women in Wuhan, China. Ecotoxicol Environ Saf, 184, 109657. 10.1016/j.ecoenv.2019.109657 [DOI] [PubMed] [Google Scholar]
- Li N, Liu E, Guo J, Pan L, Li B, Wang P, Liu J, Wang Y, Liu G, Baccarelli AA, Hou L, & Hu G (2013). Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS One, 8(12), e82310. 10.1371/journal.pone.0082310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley T (2020). survey: Analysis of Complex Survey Samples. In (Version R package 4.0) http://r-survey.r-forge.r-project.org/survey/ [Google Scholar]
- Mitchell EM, Hinkle SN, & Schisterman EF (2016, Mar). It's About Time: A Survival Approach to Gestational Weight Gain and Preterm Delivery. Epidemiology, 27(2), 182–187. 10.1097/EDE.0000000000000413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacyga DC, Patti MA, Papandonatos GD, Haggerty DK, Calafat AM, Gardiner JC, Braun JM, Schantz SL, & Strakovsky RS (2022, Sep 16). Associations of individual and cumulative urinary phthalate and replacement biomarkers with gestational weight gain through late pregnancy. Sci Total Environ, 855, 158788. 10.1016/j.scitotenv.2022.158788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacyga DC, Patti MA, Papandonatos GD, Haggerty DK, Calafat AM, Gardiner JC, Braun JM, Schantz SL, & Strakovsky RS (2023, 2023/January/10/). Associations of individual and cumulative urinary phthalate and replacement biomarkers with gestational weight gain through late pregnancy. Science of The Total Environment, 855, 158788. https://doi.org/ 10.1016/j.scitotenv.2022.158788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, Jaddoe VWV, Deierlein A, Asimakopoulos AG, Kannan K, Steegers EAP, & Trasande L (2020, Nov). Exposures to phthalates and bisphenols in pregnancy and postpartum weight gain in a population-based longitudinal birth cohort. Environ Int, 144, 106002. 10.1016/j.envint.2020.106002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, Santos S, Steegers EAP, Asimakopoulos AG, Kannan K, Trasande L, & Jaddoe VWV (2020, Feb). Maternal bisphenol and phthalate urine concentrations and weight gain during pregnancy. Environ Int, 135, 105342. 10.1016/j.envint.2019.105342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Record #73 is using a reference type undefined in this output style.]
- Pigatti Silva F, Souza RT, Cecatti JG, Passini R Jr., Tedesco RP, Lajos GJ, Nomura ML, Rehder PM, Dias TZ, Oliveira PF, Silva CM, & Brazilian Multicenter Study on Preterm Birth study, g. (2019, Sep 11). Role of Body Mass Index and gestational weight gain on preterm birth and adverse perinatal outcomes. Sci Rep, 9(1), 13093. 10.1038/s41598-019-49704-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DB, Rzehak P, Klenk J, & Weiland SK (2007, Jul). Analyses of case-control data for additional outcomes. Epidemiology, 18(4), 441–445. 10.1097/EDE.0b013e318060d25c [DOI] [PubMed] [Google Scholar]
- Riddell CA, Platt RW, Bodnar LM, & Hutcheon JA (2017, Mar). Classifying Gestational Weight Gain Trajectories Using the SITAR Growth Model. Paediatr Perinat Epidemiol, 31(2), 116–125. 10.1111/ppe.12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Lorber M, Guo Y, Wu Q, Yun SH, Kannan K, Hommel M, Imran N, Hynan LS, Cheng D, Colacino JA, & Birnbaum LS (2013, Apr). Phthalate concentrations and dietary exposure from food purchased in New York State. Environ Health Perspect, 121(4), 473–494. 10.1289/ehp.1206367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AJ, Bulkley JE, Stoneburner AB, Dandamudi P, Leo M, Callaghan WM, & Vesco KK (2021, Aug). Bias in Self-reported Prepregnancy Weight Across Maternal and Clinical Characteristics. Matern Child Health J, 25(8), 1242–1253. 10.1007/s10995-021-03149-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL Jr., Reidy JA, Needham LL, & Calafat AM (2007, Dec 1). Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci, 860(1), 106–112. 10.1016/j.jchromb.2007.10.023 [DOI] [PubMed] [Google Scholar]
- Song Y, Hauser R, Hu FB, Franke AA, Liu S, & Sun Q (2014, Dec). Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. Int J Obes (Lond), 38(12), 1532–1537. 10.1038/ijo.2014.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S, Shimada N, Miyoshi H, & Yamauchi K (2005, Dec). Detection of thyroid system-disrupting chemicals using in vitro and in vivo screening assays in Xenopus laevis. Toxicol Sci, 88(2), 367–374. 10.1093/toxsci/kfi330 [DOI] [PubMed] [Google Scholar]
- Trasande L, Attina TM, Sathyanarayana S, Spanier AJ, & Blustein J (2013, Apr). Race/ethnicity-specific associations of urinary phthalates with childhood body mass in a nationally representative sample. Environ Health Perspect, 121(4), 501–506. 10.1289/ehp.1205526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi P, James-Todd T, Mínguez-Alarcón L, Ford JB, Keller M, Petrozza J, Calafat AM, Hauser R, Williams PL, & Bellavia A (2021, Mar). Identifying windows of susceptibility to endocrine disrupting chemicals in relation to gestational weight gain among pregnant women attending a fertility clinic. Environ Res, 194, 110638. 10.1016/j.envres.2020.110638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi P, James-Todd T, Minguez-Alarcon L, Ford JB, Keller M, Petrozza J, Calafat AM, Hauser R, Williams PL, Bellavia A, & team E. s. (2021, Mar). Identifying windows of susceptibility to endocrine disrupting chemicals in relation to gestational weight gain among pregnant women attending a fertility clinic. Environ Res, 194, 110638. 10.1016/j.envres.2020.110638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JA, Beverly BEJ, Keshava N, Mudipalli A, Arzuaga X, Cai C, Hotchkiss AK, Makris SL, & Yost EE (2020, Dec). Hazards of diethyl phthalate (DEP) exposure: A systematic review of animal toxicology studies. Environ Int, 145, 105848. 10.1016/j.envint.2020.105848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Priest N, & Anderson NB (2016, Apr). Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychol, 35(4), 407–411. 10.1037/hea0000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdy MM, Coull BA, Gardiner JC, Aguiar A, Calafat AM, Xiaoyun Y, Schantz SL, & Korrick SA (2018, Sep). A possible approach to improving the reproducibility of urinary concentrations of phthalate metabolites and phenols during pregnancy. J Expo Sci Environ Epidemiol, 28(5), 448–460. 10.1038/s41370-018-0050-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, & Woodruff TJ (2014, Mar). Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001-2010. Environ Health Perspect, 122(3), 235–241. 10.1289/ehp.1306681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukin H, Eskenazi B, Holland N, & Harley KG (2021, Mar). Prenatal exposure to phthalates and maternal metabolic outcomes in a high-risk pregnant Latina population. Environ Res, 194, 110712. 10.1016/j.envres.2021.110712 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.