Abstract
Hot chilli (‘Bhut Jolokia’) (Capsicum chinense Jacq.) is the hottest chilli widely grown in the North-Eastern region of India for its high pungency. However, little information is available on its physiology, growth and developmental parameters including yield. Therefore, the present research was undertaken to study the physiological responses of Bhut Jolokia under elevated CO2 (eCO2) and temperature. Two germplasms from two different agro-climatic zones (Assam and Manipur) within the North-East region of India were collected based on the pungency. The present study explored the interactive effect of eCO2 [at 380, 550, 750 ppm (parts per million)] and temperature (at ambient, > 2 °C above ambient, and > 4 °C above ambient) on various physiological processes, and expression of some photosynthesis and capsaicin related genes in both the germplasms. Results revealed an increase (> 1–2 fold) in the net photosynthetic rate (Pn), carbohydrate content, and C: N ratio in ‘Bhut Jolokia’ under eCO2 and elevated temperature regimes compared to ambient conditions within the germplasms. Gene expression studies revealed an up-regulation of photosynthesis-related genes such as CsRuBPC2 (Ribulose biphosphate carboxylase 2) and CsSPS (Sucrose phosphate synthase) which, explained the higher Pn under eCO2 and temperature conditions. Both the germplasm showed better performance under CTGT-II (Carbon dioxide Temperature Gradient Tunnel having 550 ppm CO2 and temperature of 2 °C above ambient) in terms of various physiological parameters and up-regulation of key photosynthesis-related genes. An up-regulation of the Cs capsaicin synthase gene was also evident in the study, which could be due to the metabolite readjustment in ‘Bhut Jolokia’. In addition, the cultivar from Manipur (cv. 1) had less fruit drop compared to the cultivar from Assam (cv. 2) in CTGT II. The data indicated that 550 ppm of eCO2 and temperature elevation of > 2 °C above the ambient with CTGT-II favored the growth and development of ‘Bhut Jolokia’. Thus, results suggest that Bhut Jolokia grown under the elevation of CO2 up to 550 ppm and temperature above 2 °C than ambient may support the growth, development, and yield.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-023-01294-9.
Keywords: Bhut Jolokia, Capsicum chinense Jacq., Elevated CO2, Carbohydrate, Rubisco, Sugar metabolism
Introduction
Plant growth and development rely on carbon dioxide (CO2), the primary raw material for photosynthesis. The global atmospheric CO2 concentrations have risen from 280 ppm (μmol mol−1) to 418 ppm in 2021 (https://www.co2.earth/) due to the industrial revolution and are expected to rise by another 40% by 2050 (about 550 ppm) accompanied by a 2 °C rise in the global temperature (IPCC 2013). The increase in the global temperature is due to higher CO2 and increasing concentrations of other greenhouse gases (Hanse et al. 2010). It has been reported by some researchers that due to the emission of greenhouse gases, the ecosystems and agricultural activities have been adversely affecting biodiversity, the productivity of agriculture and food security have been adversely affected as a result of global climate change (Sloat et al. 2020) Due to the sessile lifestyle, plants must endure unfavorable weather events such as high temperature, cold, drought, flood, and salinity (Ohama et al. 2017). The key determinant of plant growth is CO2 (Craufurd et al. 2009), while the temperature is a key factor for the transition of various developmental phases, including flowering and yield (Zinta et al. 2018). Unfavorable weather events such as high temperature, cold, drought, flood, salinity, and an excess or deficit CO2 concentration in the atmosphere affect plant growth and productivity due to an array of adjustments that are needed in the physiological processes to adapt (Sarker and Oba, 2018a, b). Elevated CO2 (eCO2) influences photosynthesis, which is the entry point of carbon for plant metabolism. The expected rate of increase in photosynthesis is unclear because photosynthesis by CO2 is also dependent on leaf temperature and other plant growth conditions such as water and nutrients (Zhu et al. 2017). Also, high temperatures will have a profound impact on species distribution across the globe and may affect many biological and physiological processes of temperature-sensitive plants. Therefore, plant responses would likely be prominent under eCO2 and temperature due to their impacts on growth and flowering and it would be useful to study those responses in agriculturally important crops such as chilli. The impact of climate change and crop responses due to eCO2 have been documented in wheat (Dais de Oliveira et al. 2013), however, the mechanisms of plant responses to combined eCO2 and temperature are unclear (Zinta et al. 2018). Various workers have expressed their views regarding crop responses to climate change (Rojas-Downing et al. 2017).
Photosynthesis is a vital process necessary for crop growth, reproduction, productivity, and changes in CO2 concentration or eCO2 may have direct consequences which could be either positive or negative (Van der Kooi et al. 2016). Elevated CO2 may cause an increase in the rate of photosynthesis (Pn), which subsequently improves vegetative growth, crop biomass, and yield (Makino and Mae, 1999). This increased photosynthesis under eCO2 occurs mainly due to an increase in the activity of its key enzymes, ribulose-1,5-bisphosphate (RuBP) carboxylase/oxygenase (Rubisco). This bi-functional enzyme Rubisco catalyzes the carboxylation of RuBP and utilizes O2 as a substrate to oxygenate RuBP during photorespiration (Pan et al. 2018). Under eCO2 conditions, the rate of carboxylation will increase because, there will be a shift in the ratio of CO2: O2 around the enzyme, whereas the rate of oxygenation will decrease (Pan et al. 2018). In the growth and development of basil, a C3 plants, temperature and elevated CO2 are considered important factors (Yuan et al. 2017). The negative consequences of eCO2 would be related to an increase in temperature due to high levels of CO2. Photosynthesis is sensitive to heat stress and many crops adjust this process according to growth temperatures (Wen et al. 2005). The common stress-sensitive sites in the photosynthetic machinery are photosystem II (PSII) and its electron donor (OEC) and acceptor (QA, PQ) (Mittler et al. 2012). The plant can minimize the damage of heat stress by maintaining cellular homeostasis which enables them to function properly (Sakamoto and Murrata, 2002). The high temperature and eCO2 are known to have significant effects (both positive and negative) on the growth and development of peppers or chilli (Kumari et al. 2019). The bell peppers grown in the high altitudes of Northern India showed vigorous growth and yield under 550 ppm of CO2, however, a temperature of 1 °C above ambient negated the positive effects of eCO2. A few reports also suggested vegetative and reproductive organ abscission in chilli and other crops, which seems to be regulated by growth hormones such as ethylene and auxin (Huberman et al. 1997).
‘Bhut Jolokia’ (Capsicum chinense Jacq.) is an orphan Solanaceous crop of semi-perennial nature grown in the North-Eastern (NE) region of India. The crop is the hottest chilli in this region with a Scoville heat unit of 1,001,304 (Bosland and Baral, 2007). This is an important spice crop of NE India and fruits have been consumed by people for a long period of time and possibly originated through the natural hybridization of C. chinense and C. frutescens (Bosland and Baral, 2007). Generally, the crop is planted in the backyard for household consumption, The crop received global attention once it was listed in the Guinness Book of World Records in (2006) as the hottest chilli in the world and since then 'Bhut Jolokia' gained commercial importance as a high valued export crop in entire Northeast India. Moreover, this a triggered collection of various germplasms from NE India to study the variability and characterize the levels of capsaicin content within the available germplasm. Recently, two quantitative trait loci (QTLs) were identified in chromosomes 3 and 6 of ‘Bhut Jolokia’ (Lee et al. 2016). Heat stress-affected pepper transcripts, proteomes and metabolomes reported capsaicin biosynthesis genes which were positively correlated to capsaicin content (Liu et al. 2022). A study on the effects of different LED lightings on the post-harvest firmness and nutritional quality of chili peppers found that red and blue light increases the capsaicinoids content, while that of white and red light increases the amino acid content in pepper (Liu et al. 2022).
In the present study, the interactive effects of eCO2 and temperature on various plant physiological processes of ‘Bhut Jolokia’ were studied. The two germplasms with high pungency levels were collected from two different NE states of India, ie., Manipur and Assam, and were further grown under varying eCO2 (at 380, 550, 750 ppm) and temperature regimes (at ambient, > 2 °C from ambient, and > 4 °C from ambient) to understand the impacts of various treatments on photosynthesis, carbohydrate content, and other physiological parameters.
Material and methods
Plant material and growing conditions
This study was conducted in the Department of Crop Physiology, Assam Agricultural University, Jorhat, India. The experimental site was situated at 26°47′ N latitude, 94°12′ E longitude, and an elevation of 86.6 msl. The experimental site experiences a subtropical climate with hot humid summer and a relatively dry and cold winter.
Two germplasms of ‘Bhut Jolokia’ were collected from Manipur (cv Manipur/ cv.1) and Assam (cv. Assam / cv. 2). The plants were exposed to four different combinations of CO2 and temperature, including natural ambient conditions. The experiment included four treatments viz. T1 = Field (Ambient CO2 condition and temperature conditions), T2 = CTGT I (380 ppm CO2 + ambient temperature), T3 = CTGT II (550 ppm CO2 + 2 °C higher than ambient from flower bud initiation till maturity) and T4 = CTGT III (750 ppm CO2 + 4 °C higher than ambient from flower bud initiation till maturity). Plants were raised in various Carbon dioxide temperature Gradient Tunnels (CTGTs; marketed by Genesis Technologies, Maharashtra, India). In the field conditions, plants were grown in pots kept in the open field (ambient CO2 and temperature condition). In the CTGTs, the CO2 was maintained throughout the entire crop growth period from 9 A.M. to 2 P.M. regularly. The elevation of temperature was maintained through Infra Red Heater regulated by SCADA software.
Topsoil and organic compost were collected, drenched with 0.1% Captan (WP), and covered with a plastic sheet. Later, Furadon 3G (3 g/m2) was mixed and covered for two days. Seeds of ‘Bhut Jolokia’ were also treated with Captan (@ 2.5 g/kg seed) fungicide agitating them for 5 min. in a container. Small earthen pots (28 cm in height and 30 cm in diameter) were filled with a mixture of soil and organic matter in an equal (50:50) ratio and the seeds were sown in those pots. Once the seedlings reached to 3–4 leaf stage, they were transplanted into big earthen pots (40 cm in height and diameter) filled with topsoil, sand, and cow dung in equal (1:1:1) parts. The pots were fertilized at the rate of N, P, and K in a ratio of 120: 60: 60 kg/ha. 60 kg N along with P and K as basal dose were applied and the remaining N fertilizer was applied as a top dressing in two split doses at 30 and 60 days after transplanting. A total of 5 pots per variety were kept in each treatment in a completely randomized manner. The data were collected from physiologically active leaves during the flowering stage and statistical analyses were performed from data obtained in the year I and II.
The structure of CTGTs were rectangular (10 m length, 2.5 m breadth, 2 m height) block, fabricated by metallic pipe and covered with polycarbonate sheet (100-micron gauge) for 85% transmission of light. Each tunnel was divided into four compartments of 2.5 m in length and within each chamber, 4 RTD temperature sensors were fitted. Humidity transmitters were placed in each chamber to get data in the control room through four core-shielded cables. Infrared heaters were mounted inside the CTGT. For measuring the ambient data, temperature sensors and humidity transmitters were placed outside the chamber. The humidity sensors were capable to measure humidity in the range of 0–100% relative humidity and CO2 gas cylinders were used for the supply of CO2 gas. An Air compressor was used to maintain the uniformity of CO2 gas.
CO2 and temperature control and monitoring
The system for monitoring and controlling the CO2 in CTGTs was fully automatic, and the desired level of CO2 was maintained in each tunnel. Data logger and the SCADA software were used to monitor and control appropriate CO2 levels in each chamber. The SCADA software facilitated the set of different concentrations of CO2 in the different CTGT chambers. Signals obtained from portable CO2 monitors were compared with an actual set value of CO2 level in ppm of each chamber. Most control actions were performed automatically by Remote Terminal Units or Data loggers. The CO2 in the chambers was controlled by a 12-channel measurement and control system using an ADC WA 526 IRGA (Infra-Red Gas Analyser). The system delivered (pure) CO2 to each chamber via 12 solenoid valves, according to a calculation based on the difference between the reading and the set point. The outlet of the CO2 regulator was connected to a manifold in which CO2 passes through distributing the system to respective CTGT. The manifold was used to distribute CO2 into hexagonal rings, and the solenoid valve is connected to each outlet of the manifold.
Measurement of various physiological and biochemical parameters
The rate of photosynthesis (Pn) of fully expanded ‘Bhut Jolokia’ leaves was measured using a portable Infrared Gas Analyzer [IRGA (Infrared Gas Analyzer, LI-6400. Lincoln, Nebraska, USA)] between 10.00 am to 12.00 noon. For leaf carbohydrate analyses, leaf samples were taken, and total soluble sugar (TSS) and total non-structural carbohydrate (TNSC) was measured based on the methods described in McCready et al. 1950.
Estimation of Carbon: Nitrogen ratio
The C: N ratio was calculated based on the per cent carbon (C) and nitrogen (N) content of the leaves. Carbon content was estimated following the Walkley–Black’s method (Walkley and Black, 1934). The dried plant samples were oxidized with a mixture of potassium dichromate and concentrated sulphuric acid using the heat of dilution of the acid. The unused potassium dichromate was estimated by back titration with ferrous ammonium sulphate.
Carbon content in plant parts was estimated using ground plant material. Fifty milligrams of plant sample was placed in a dry 500 mL conical flask. Ten milliliters of K2Cr2O7 solution were added to it. Flask was gently swirled, and 20 mL of concentrated sulphuric acid was rapidly added to it followed by immediate swirling so that the sample and the reagent were mixed. The flask was swirled several times and allowed to stand for 30 min. This was followed by the addition of 200 mL of distilled water and 10 mL of orthophosphoric acid. After adding 1 mL of diphenylamine indicator solution, the content was titrated with ferrous ammonium sulphate solution till the colour flashes from bluish-violet to green. A blank (without sample) titration was also carried out. The carbon content was calculated using the following formula and expressed in percentage.
where, A = Weight of the sample X = Volume (mL) of ferrous ammonium sulphate solution required for blank titration, Y = Volume (mL) of ferrous ammonium sulphate needed for the sample. The carbon percentage was obtained by multiplying this C per cent with a constant, 1.3, as only 77 per cent recovery was presumed from oxidation of the sample in this procedure.
Nitrogen content in plant parts was measured by Kjeltec Auto Analyzer. The dried plant material was digested with concentrated H2SO4 in the presence of a catalyst to convert the nitrogen to ammonium sulphate. Ammonia was liberated and collected in boric acid solution as ammonium borate by steam distillation of the salt in the presence of a strong alkali which is estimated against a standard acid titration.
Free proline content in the leaves was determined following the method of Bates et al. (1973).
Leaf sample (0.5 g) was homogenized in 5 mL of sulphosalicylic acid (3%) using a mortar and pestle. Two ml of extract was taken in a test tube, and 2 mL of glacial acetic acid and 2 ml of ninhydrin reagent were added. The reaction mixture was boiled in the water bath at 100° C for 30 min, and a brick-red colour was developed. After cooling the reaction mixtures, 6 mL of toluene was added and then transferred to a separating funnel. After thorough mixing, the chromophore containing toluene was separated and absorbance read at 520 nm in a spectrophotometer against a toluene blank. The concentration of the sample was estimated by referring to a standard curve and the result was expressed in mg g−1 FW.
Chlorophyll content
Leaf chlorophyll was estimated by a Dimethyl Sulphoxide (DMSO) based non-maceration method Hiscox and Israelstam, (1979). Fresh leaves (0.5 g) were placed in a test tube containing 5 mL of DMSO and kept in an oven at 65 °C for 4 h followed by the addition of 10 mL of DMSO. The optimal density (OD) of the extract was measured in a spectrophotometer at 663 nm and 645 nm. The chlorophyll content was determined by the formula suggested by Sarkar and Oba, 2018a, b). The amount of chlorophyll content was calculated using absorption coefficients and accordingly, the ratio of chlorophyll a/b was calculated.
Estimation of fruit drop
The fruit drop per cent was calculated by recording the number of fruit dropping at weekly intervals and the total was calculated cumulatively on per pot. The total number of fruit harvested was utilized for calculating the fruit drop per cent by the following formula.
The data obtained were transformed by the arcsine transformation, and using those values statistical analysis was performed. Meteorological data during the period of experimentation have been included (Supplementary Fig. 1 and Supplementary Fig. 2).
Statistical analysis
The experiment was laid out in a Completely Randomized Design (CRD) having 2 factors and was replicated thrice. Statistical analysis of all the parameters was done following the method of analysis of variance (ANOVA) given by Panse and Sukhatme, (1967). The critical difference (CD) values were calculated at 5 per cent probability level.
Semi qunatative reverse transcriptase-PCR for gene expression
RNA was isolated using the RNeasy Plus Minikit (Qiagen, USA) with a genomic DNA removal column. Gene-specific primers were designed using sequences from the NCBI databases. The primer design software used was Perfect Primer Design with Invitrogen’s Oligo Perfect TM Designer (www.invitrogen.org). Primers for the actin gene were used as an endogenous control. RNA extracted from plants grown in CTGT I, II, and III were subjected to semi-quantitative RT-PCR using gene-specific primers following the protocol outlined in the one-step RT-PCR kit (Qiagen, USA). The PCR plate was subjected to 30 cycles of the following conditions: Cycle 1: PCR activation at 50 °C for 30 min, Cycle 2: 94 °C for 90 s for denaturation Cycle 3: 94 °C for 30 s for denaturation Cycle 4: 55 °C for 30 s Cycle 5: 72 °C for 3 min for elongation Cycle 6: 72 °C for 5 min for final extension Cycle 7: 4 °C for storage. Cycle 3- Cycle 5 was repeated for 30 cycles. All the samples were run in triplicates on the RT-PCR system. PCR amplified product was mixed with gel loading buffer (1X 2 µl) and loaded into the wells of the submerged gel. The gel was run at 80 V for 1 ½ hours until the tracking dye reaches two-third of the bottom edge of the gel. The gel was visualized under UV and photographs were taken. The details of selected primers for the construction of cDNA is provided in Supplementary Table 1.
Results
Photosynthesis in ‘Bhut Jolokia’ plants under elevated CO2 and temperature
To understand the photosynthetic response in ‘Bhut Jolokia’ grown in eCO2 and temperature, the net photosynthetic rate (Pn) of leaves was measured using IRGA. The Pn for various treatments is summarized in Table 1. Photosynthesis was highest in both germplasms under CTGT II conditions. Pn was significantly higher in the cv. Manipur in CTGT II compared to the field conditions, CTGT I and CTGT III. The eCO2 of 550 ppm exhibited a stimulatory effect on the Pn in both the ‘Bhut Jolokia’ germplasms. On the contrary, 750 ppm of CO2 and 4 °C above ambient temperature (CTGT III) compromised Pn in plants of both germplasms when compared to those grown in the CTGT II. No significant differences were found in the Pn of the plants grown in the field and CTGT I.
Table 1.
Interactive effect of elevated carbon dioxide (eCO2) and high temperature on the rate of photosynthesis and total nonstructural carbohydrate content in Bhut Jolokia’ germplasm
| Photosynthesis (µmol CO2 m−2 s−1) | Total Non structural Carbohydrate (mg g−1DW) | |||
|---|---|---|---|---|
| Treatments | ||||
| Field | 17.17 | 67.60 | ||
| CTGT-I (Amb) | 16.45 | 68.86 | ||
| CTGT-II | 20.87 | 91.61 | ||
| CTGT-III | 17.93 | 85.16 | ||
| SEd | 1.004 | 2.49 | ||
| CD (0.05%) | 2.128 | 5.28 | ||
| Cultivar | ||||
| cv. 1 (Manipur) | 18.88 | 81.28 | ||
| cv. 2 (Assam) | 17.33 | 75.33 | ||
| SEd | 0.71 | 1.76 | ||
| CD (0.05%) | 1.505 | NS | ||
| Treatment x Cultivar | ||||
| Field | cv. 1 | cv. 2 | cv. 1 | cv. 2 |
| 18.37 | 15.97 | 68.00 | 67.20 | |
| CTGT-I (Amb) | 17.80 | 15.10 | 68.57 | 69.16 |
| CTGT-II | 20.83 | 20.90 | 97.18 | 86.04 |
| CTGT-III | 18.50 | 17.37 | 91.37 | 78.94 |
| SEd | 1.42 | 0.557 | ||
| CD (0.05%) | NS | NS | ||
Accumulation of carbohydrate under elevated CO2 and temperature in ‘Bhut Jolokia’
The total soluble sugar (TSS) which indicates the active assimilation of photosynthates was measured using standard protocols. The TSS in the leaves of both the germplasms was significantly higher under eCO2 and the temperature of CTGT II. However, results also exhibited a significant decline in TSS with the increasing CO2 and temperature in the CTGT III compared to CTGT II. In comparison to cv. 2, the plants of cv. 1 grown under CTGT II accumulated more TSS (Fig. 1). In addition, the total non-structural carbohydrate content (TNSC) of plants grown in various eCO2 and temperature regimes was also measured. Results suggested that plants of both germplasms grown in CTGT II and III accumulated the highest TNSC compared to the plants grown in the field and ambient CO2 conditions (Table 1). However, cv. Manipur exhibited higher TNSC accumulation in the CTGT II and III compared to cv. 2 (Table 1).
Fig. 1.
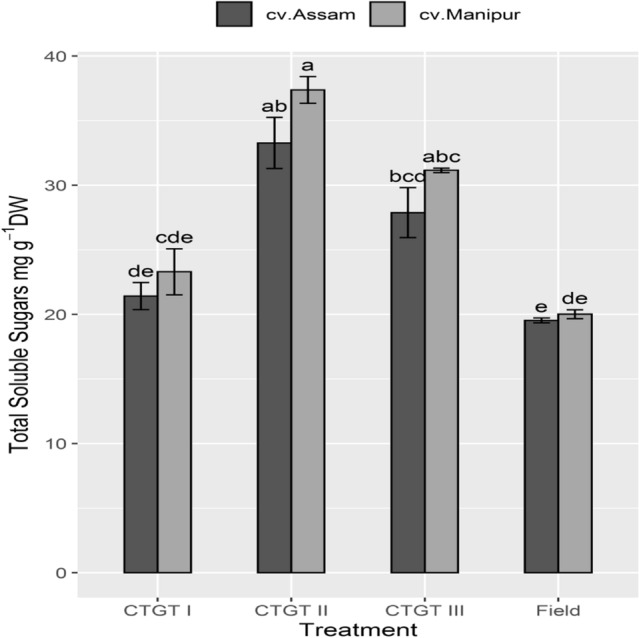
Interactive effect of elevated CO2 and temperature on total soluble sugar. Effects of different levels of carbon dioxide (CO2) and temperatures (oC) on the endogenous content of total soluble sugars (mg. g−1 dry weight) on two germplasm of Bhut Jolokia subjected to different treatments. Different treatments given were: CTGT I (380 ppm CO2 + ambient temperature), CTGT II (550 ppm CO2 + 2 °C higher than ambient from flower bud initiation till maturity) and CTGT III (750 ppm CO2 + 4 °C higher than ambient from flower bud initiation till maturity), normal field conditions. Bars with different letters are statistically different from each other (n = 6)
Carbon: nitrogen ratio in leaves of ‘Bhut Jolokia’
The C: N ratio in the leaves of two different germplasms of ‘Bhut Jolokia’ varied significantly under eCO2 and temperature. The leaf C: N ratio of both the germplasms was the highest in CTGT II and III compared to the plants grown in the field and ambient conditions. A significantly higher C: N ratio ranging from 6.92 to 5.91 was recorded in CTGT II and CTGT III, respectively (Fig. 2). A significant drop in the C: N values were recorded in Field and CTGT I compared to CTGT II and CTGT III. The eCO2 and temperature favored vigorous vegetative growth of ‘Bhut Jolokia’ that is reflected in the C: N values.
Fig. 2.
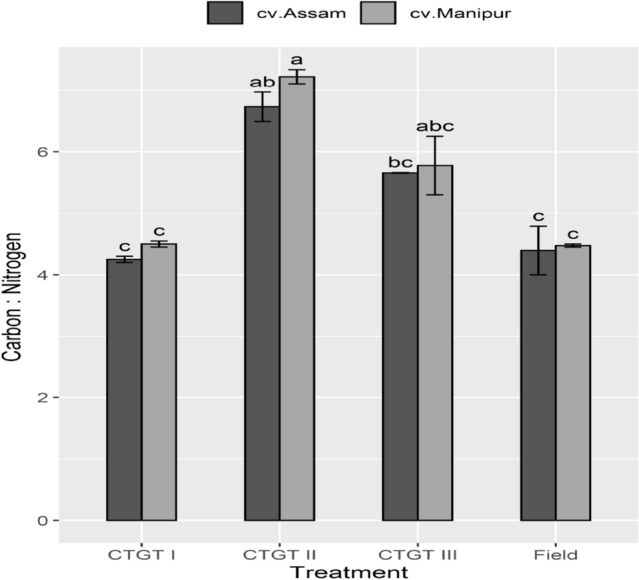
Interactive effect of elevated CO2 and temperature on C: N ratio. Effects of different levels of carbon dioxide (CO2) and temperatures (oC) on carbon, C: nitrogen, N (C:N) ratio of the two germplasm of Bhut Jolokia subjected to different treatments. Different treatments given were: CTGT I (380 ppm CO2 + ambient temperature), CTGT II (550 ppm CO2 + 2 °C higher than ambient from flower bud initiation till maturity) and CTGT III (750 ppm CO2 + 4 °C higher than ambient from flower bud initiation till maturity), normal field conditions. Bars with different letters are statistically different from each other (n = 6)
Leaf proline content in ‘Bhut Jolokia’
It is well known that the amino acid proline accumulates to protect plants from various stresses. Therefore, the proline levels in the plants grown in various CO2 and temperature regimes were measured. As expected, results showed higher levels of proline accumulation in the plants grown under eCO2 and temperature (CTGT II and III). The increment in the proline content of both the germplasms at CTGT II and CTGT III was significantly higher than that of CTGT I and Field. CTGT II and CTGT III recorded variations in proline accumulation within the germplasms. Interestingly, the proline content of leaves of ‘Bhut Jolokia’ cv. 1 was more compared to the cv. 2 under CTGT II and III (Fig. 3).
Fig. 3.
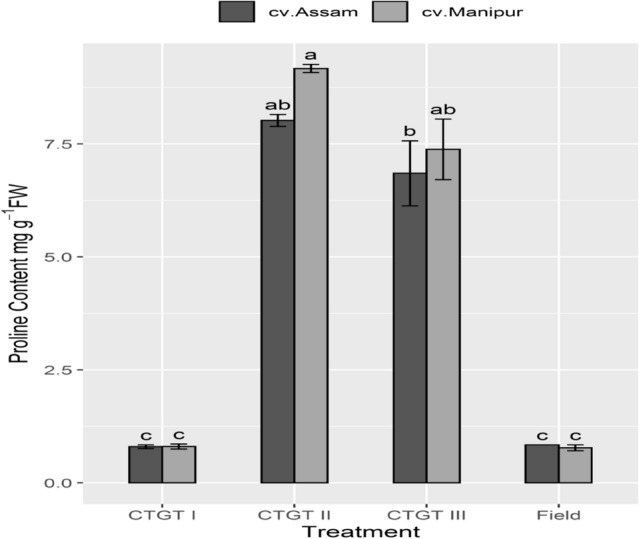
Elevated CO2 and temperature altered proline content (mg g−1fresh weight). Effects of different levels of carbon dioxide (CO2) and temperatures (oC) on proline content (mg. g−1fresh weight) of the two germplasm of Bhut Jolokia subjected to different treatments. Different treatments given were: CTGT I (380 ppm CO2 + ambient temperature), CTGT II (550 ppm CO2 + 2 °C higher than ambient from flower bud initiation till maturity) and CTGT III (750 ppm CO2 + 4 °C higher than ambient from flower bud initiation till maturity), normal field conditions. Bars with different letters are statistically different from each other (n = 6)
Chlorophyll content and chlorophyll a: b ratio of ‘Bhut Jolokia
The leaf chlorophyll content in the ‘Bhut Jolokia’ plant of both the germplasms under treatment was lower than those grown in the field and ambient conditions (Fig. 4). A significant decline in chlorophyll content under eCO2 and temperature was recorded. When compared with cv. 1, chlorophyll content in leaves of cv. 2 recorded a decrease of 8%. Stress-induced reduction in chlorophyll content ranged from 42% (CTGT II) to 47% (CTGT III) compared to ambient conditions. The interaction effect of treatments and germplasm also recorded a significant difference.
Fig. 4.
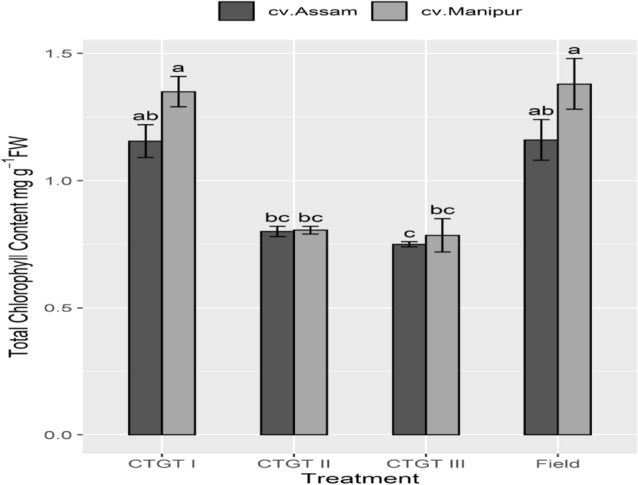
Total chlorophyll content changes under elevated CO2. Effects of different levels of carbon dioxide (CO2) and temperatures (oC) on total chlorophyll content (mg. g−1fresh weight) of the two germplasm of Bhut Jolokia subjected to different treatments. Different treatments given were: CTGT I (380 ppm CO2 + ambient temperature), CTGT II (550 ppm CO2 + 2 °C higher than ambient from flower bud initiation till maturity) and CTGT III (750 ppm CO2 + 4 °C higher than ambient from flower bud initiation till maturity), normal field conditions. Bars with different letters are statistically different from each other (n = 6)
In addition, eCO2 and temperature brought about a significant difference in chlorophyll a/b ratio in cv. 1 (Fig. 5). Cv. 1 recorded a significant variation in chlorophyll a/b ratio in CTGT III with that of CTGT I and the field. A non-significant variation within treatments was recorded in cv. 2.
Fig. 5.
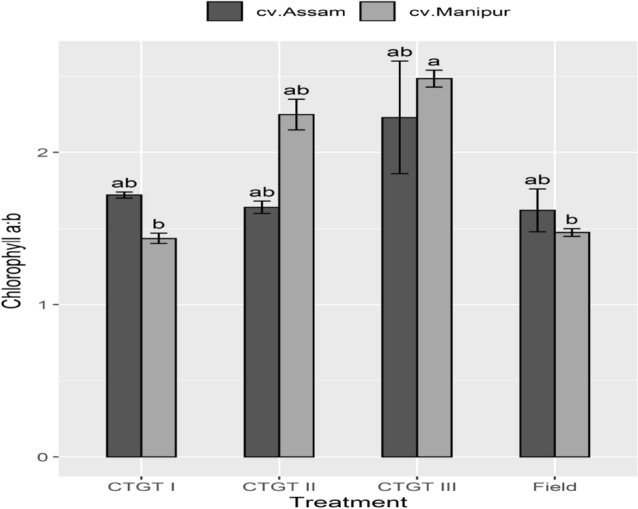
Interactive effect of elevated CO2 and temperature on chlorophyll a:b ratio. Effects of different levels of carbon dioxide (CO2) and temperatures (oC) on Chl a:b ratio of the two germplasm of Bhut Jolokia subjected to different treatments. Different treatments given were: CTGT I (380 ppm CO2 + ambient temperature), CTGT II (550 ppm CO2 + 2 °C higher than ambient from flower bud initiation till maturity) and CTGT III (750 ppm CO2 + 4 °C higher than ambient from flower bud initiation till maturity), normal field conditions. Bars with different letters are statistically different from each other (n = 6)
Fruit drop of ‘Bhut Jolokia’ under eCO2 and temperature
The elevated CO2 and temperature could also influence the yield. One of the major problems of the local ‘Bhut Jolokia’ germplasms is fruit drop; therefore, in the current study, the intensity of fruit drop was calculated due to various treatments. Results showed that the intensity of fruit drop was higher under elevated CO2 and temperature (CTGT III) compared to CTGT I and field. The percent fruit drop was 40% in cv. Assam and 35% in cv. Manipur grown under CTGT III (Fig. 6). Data revealed that 550 ppm of CO2 and 2ºC above ambient temperature had a marginal effect on per cent fruit drop of cv. Manipur.
Fig. 6.
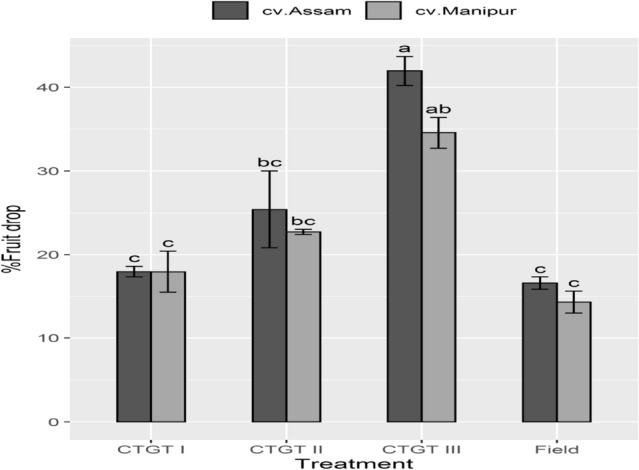
Interactive effect of elevated CO2 and temperature on percent fruit drop. Effects of different levels of carbon dioxide (CO2) and temperatures (oC) on %fruit drop of the two germplasm of Bhut Jolokia subjected to different treatments. Different treatments given were: CTGT I (380 ppm CO2 + ambient temperature), CTGT II (550 ppm CO2 + 2 °C higher than ambient from flower bud initiation till maturity) and CTGT III (750 ppm CO2 + 4 °C higher than ambient from flower bud initiation till maturity), normal field conditions. Bars with different letters are statistically different from each other (n = 6)
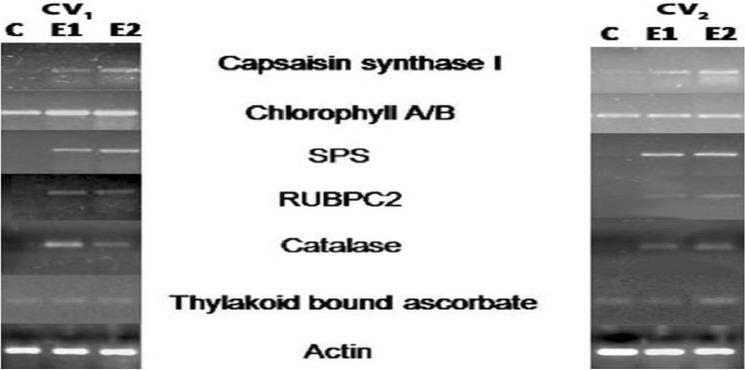
Expression of photosynthesis-related genes in ‘Bhut Jolokia’
The expression of four photosynthesis-related genes such as chlorophyll A/B, sucrose phosphate synthase (SPS), RuBPC2, thylakoid bound ascorbate peroxidase, and two other genes, catalase for defence-related and capsaicin synthase I for capsaicin were studied. Expression of the gene in plants grown CTGT II (E1) and CTGT III (E2) with CTGT I (control; C) was compared. Samples from plants grown in CTGT I was used as a control instead of plants grown in the field to avoid the additive effects of various environmental conditions, including soil on gene expression. The transcript abundance of the photosynthesis-related genes viz. chlorophyll A/B, SPS, RuBPC2, thylakoid bound ascorbate peroxidase revealed differences between the two germplasms. The expression of RuBPC2 was higher at eCO2 and temperatures in both germplasms. Under the elevated CO2, and temperature, RuBPC2 and SPS transcript was up regulated in comparison to the ambient. The gene expression of Chlorophyll A/B and thylakoid bound ascorbate peroxidase was not affected by the elevated climatic conditions in comparison to ambient. In both germplasms, the expression of SPS was highly up regulated under elevated conditions. The expression of catalase, an enzyme that expresses during stress was also studied. Up-regulation of catalase in both the germplasm was recorded (Fig. 7). The influence of eCO2 and temperature on the expression of capsaicin synthase I in 'Bhut Jolokia' showed up-regulation under CTGT II and III conditions compared to CTGT I.
Fig. 7.
Semi-quantitative gene expression analysis of some photosynthesis and capsaicin-related genes under elevated CO2 and temperature. Effects of different levels of carbon dioxide (CO2) and temperatures (oC) on semiquantitative RT-PCR expression of key genes of photosynthesis Capsicum chinense chlorophyll A/B, Cccapsaisin synthase I, CcSPS, CcRUBP, Cccatalase of the two germplasm of Bhut Jolokia subjected to different treatments. Different treatments given were: CTGT I (380 ppm CO2 + ambient temperature), CTGT II (550 ppm CO2 + 2 °C higher than ambient from flower bud initiation till maturity) and CTGT III (750 ppm CO2 + 4 °C higher than ambient from flower bud initiation till maturity), normal field conditions. Bars with different letters are statistically different from each other (n = 6)
Discussion
Photosynthesis under elevated CO2 and temperature
An eCO2 can have both positive and negative effects on the productivity of major crops. One of the reasons for a positive impact is the fact that the carboxylation of RuBP is not saturated in the current CO2 levels in the atmosphere. The sensitivity of the rate of carboxylation of RUBP to temperatures > 35 °C has been reported (Perdomo et al. 2017). Therefore, an increase in the rate of photosynthesis can be expected with eCO2 combined with a moderately higher temperature regime. Data from our present study also indicate that eCO2 levels up to 550 ppm with a rise in temperature by 2 °C (CTGT-II) above ambient temperature favored photosynthetic rate (Pn) in hot chilli (‘Bhut Jolokia’). The Bhut jolokia, being a C3 plant species undergoes photorespiration; however, with eCO2 by way of suppression of photorespiration rate and Pn might have increased as noted in the current study. Elevated CO2 decreases the allocation of electron transport to photorespiration and increases the electron flow to RuBisCO carboxylation
(Pan et al. 2018). Such mechanisms potentially functioned in the current study, which may result in the promotion of photosynthesis. Ahammed et al. (2022), reported the effect of elevated atmospheric CO2 on physiological processes viz. photosynthesis, stomatal movement, and ethylene production as a result of response under abiotic stress. They have reported the role of elevated CO2 in delaying senescence. In current study, the CTGT III, the temperature-induced reduction in photosynthesis was observed with a regime of 750 ppm of CO2 and a temperature 4 °C above the ambient (CTGT-III). The reduced fixation rate of CO2 at high temperature could be due to a reduction in the stomatal aperture in ‘Bhut Jolokia’ as observed in the present investigation. Qaderi et al. (2006) opined that stomatal closure reduces CO2 diffusion to the leaf mesophyll which is the prime CO2 fixation site as observed in Brassica. Although a positive effect of eCO2 in ‘Bhut Jolokia’ was found in our study, adverse effects of eCO2 on Pn were reported when plants were exposed to eCO2 for a longer period in growth chambers (Warren et al. 2014). A study on clover plants exposed to 600 ppm of CO2 for 8 years showed a 37% increase in photosynthesis after acclimation (Ainsworth et al. 2003). This lower rate of Pn at CTGT III could also be due to the accompanying rise in temperature. Moore et al. (2021) reported that the inhibition of photosynthetic rate in plants grown under heat stress may be due to a reduction in RuBP regeneration rate and inactivation of oxygen-evolving enzymes. In both C3 and C4 plants, inhibition of net photosynthesis which is related to a decrease in active Rubisco accounts for the temperature response of net photosynthesis (Salvucci and Brandner, 2003). Increase in photosynthesis under eCO2 (500 ppm and 2 °C) elevation of temperature from ambient might also be linked to the proper maintenance of some physiological processes like stomatal conductance (Supplementary Table 2) with a reduced level of transpiration rate (Supplementary Table 3). Ahammed et al. (2022), reported the effect of elevated atmospheric CO2 on photosynthesis, stomatal movement, ethylene production. They have reported the role of elevated CO2 in delaying senescence. Habermann et al. (2019) also reported the association of stomatal properties with the positive combined effect of eCO2 (600 μmol mol−1) and elevated temperature (+ 2 °C) in a C3 plant Stylosanthes capitata. The eCO2 treatment up to 550 ppm could reduce the stomatal density, stomatal index, and stomatal conductance (gs), resulting in reduced transpiration rate, thus maintaining the favorable plant water status during the growing period. Elevated CO2 could stimulate photosynthesis, starch content, water use efficiency, and PSII photochemistry in the leaves (Habermann et al. 2019). According to them, both eCO2 and elevated temperature caused an increase in leaf temperature by negatively affecting transpiration, however, the stomatal functioning was independent of the rise in temperature. Das et al. (2016) reported higher carotenoid content in cv Manipur as compared to cv Assam under both the elevated CO2 and temperature regimes. Therefore cv Manipur showed better heat dissipation, thereby minimizing the impact of increased leaf temperature. Elevated CO2 and high temperature induce the expression of respiratory burst oxidase homologs (RBOHs) and reactive oxygen species (ROS) which plays a critical role as a signaling molecule in stomatal movement, for optimizing gas exchange and water use efficiency (Ahammed et al. 2021) The results of the present study are consistent with the findings of Habermann et al. (2019) in Tropical Forage Legume. Similarly, Zhang et al. (2019) in tomato and Chavan et al. 2019 in wheat, suggested that the combined been reported due to the influence effects of eCO2 and temperature not only control the stomatal opening but also improve functioning. Driesen et al. (2020) also reported that the varied stomatal response could be observed under changing environments, which could affect the stomatal conductance and photosynthetic rate. Stomatal development and its opening are significantly influenced by environmental factors both in the short and long term. A reduction in stomatal conductance might decrease the transpiration rate under eCO2, thereby conserving soil water (Purcell et al. 2018).
Carbohydrates accumulation under elevated CO2 and temperature
In the present study, an increase in total non-structural carbohydrate (TNSC) content has been reported due to the influence of eCO2 and temperature. Such an increase in TNSC might have increased the osmotic potential as a means to combat the high temperature stress by improving the osmoregulation of cells. This high sugar concentration could also change the temperature response of respiration by eliminating the increased respiration rate. Thus enhanced leaf sugar concentrations or enhanced osmotic potential may protect leaf cells from heat stress, i.e. higher sugar concentrations significantly modified the temperature-response curve of respiration by eliminating the increased respiration rate. Moreover, the increase in osmotic potential under the influence of sugars might protect the respiratory membranes and reduce starch degradation and consumption in respiratory processes (Huve et al. 2012). At CTGT III, the elevated temperature might have caused an impact on water stress due to higher temperature, which might be causally related to lower photosynthetic capacity in plants grown under CTGT III compared to that of CTGT II. Accordingly (Pan et al. 2018) high temperature might have a negative impact on, carboxylation rate, RuBP regeneration rate and maximal photochemical efficiency of PSII. Das et al. (2020b) recorded a significant increase in plant growth parameters viz. root: shoot ratio, leaf area index (LAI), leaf area duration (LAD), specific leaf weight (SLW) and a reduction in specific leaf area (SLA) under elevated CO2 and temperature in hot chilli due to elevated CO2. They explained that the negative effect of high temperature was counteracted by a higher level of CO2 when plants were grown under ambient conditions. Similar effect was also observed by Das (2020a) in Brassica species when grown under elevated CO2 under moisture stress conditions in free air CO2.
Soluble sugars act as signaling molecules and play an important role in the expression of various genes, hence controlling plant growth and metabolism (Li et al. 2018). Plants grown at elevated atmospheric CO2 require coordination of photosynthate production and sink demand. Elevated CO2 stimulates photosynthesis; this will lead to an increase in carbohydrate content and thereby respiratory metabolism. This leads to an increase in plant growth and biomass and economic yield (Leakey et al. 2009).
The higher accumulation of TSS and total non-structural carbohydrate in both the ‘Bhut Jolokia’ germplasm under CTGT II could have resulted from high Pn. Analyses showed a highly significant positive correlation between photosynthesis and TSS (r = 0.892) as shown in Table 2. The higher accumulation of sugar in ‘Bhut Jolokia’ in our study could be due to the stimulatory effect of elevated temperature as observed by Han et al. (2013) in lettuce. They reported that the accumulation of soluble sugar was higher in heat-resistant varieties than that in non-heat-resistant varieties.
Table 2.
Correlation study between Total non-structural carbohydrate (TNSC), total soluble sugar (TSS) and rate of photosynthesis
| Photosynthesis | TSS | |
|---|---|---|
| TNSC | 0.892* | 0.964* |
| TSS | 0.932 |
A large accumulation of non-structural carbohydrates (TNSC) in the leaf has been reported under elevated CO2 (Yoshinaga et al. 2020). During plant growth and metabolism, non-structural carbohydrate (NSC) has been reported to act as a substrate for respiration (Pan et al. 2002). NSC accumulation in the stem acts as a reserve and is directly related to sink strength and seed setting in rice (Fu et al. 2011). Due to the greater accumulation of soluble sugars, and non-structural carbohydrates in the cell sap, favourable water status could be maintained through osmoregulation. In the present study also, the higher accumulation of NSC in the CO2-enriched plant might have possibly helped in the maintenance of water status by osmoregulation. Long-term exposure of the plant to eCO2 could enhance growth and ameliorate the negative effect of stress (Wullschleger et al. 2002).
In the present study, higher NSC could have played a defensive role during the stress condition. Das (2020) reported in Brassica spp, that the increase in NSC might be related to the higher photosynthetic rate of the treated plants. Ulfat et al. (2021) reported a better assimilation rate of CO2 in plants grown under higher CO2 levels by enhancing antioxidant potential and due to a higher activities of carbohydrate metabolic enzymes. Under elevated CO2, high concentration of total NSC and N was reported in the stems when compared to ambient condition (Cao et al. 2020). From the present study, it was clear that the elevated CO2 could ameliorate the deleterious effects of high temperature on plants by augmented rates of net photosynthesis. This may lead to provide more substrate for osmotic adjustment during drought and high temperature. Zhuo et al. (2000) also reported that abundant soluble carbohydrates could enhance cell division and wall expansion under a higher level of CO2. There may be an alternation of C metabolism and growth due to interactive effects of elevated CO2 and high temperatures. Song et al. 2014, also reported that in C3 species for example, Kentucky bluegrass (Poa pratensis) under elevated CO2 could promote plant growth, whereas high temperature leads to an inhibitory effect when plants were exposed to a higher regime of temperatures (25 °C and above). Under such condition, a decrease in the rate of photosynthesis, shoot and root growth was evident along with an increase in leaf respiration rate leading to a negative C balance and a decline in soluble sugar content under ambient CO2. According to them, eCO2 could ameliorate the adverse effects of high temperatures (30 and 35 °C) which could be associated with the maintenance of a positive C balance and the accumulation of soluble sugars and TNSC by stimulating photosynthesis and suppressing respiration.
The accumulation of soluble sugars, as well as NSC in the cell-sap, might have helped in the maintenance of osmoregulation in ‘Bhut Jolokia’ leaves and avoiding sink limitations by developing new sinks. This might have resulted in higher productivity under CTGT II conditions. Interestingly, the up-regulation of SPS transcript under eCO2 and temperature (CTGT-II) in ‘Bhut Jolokia’ suggests the enhanced activity of carbohydrate metabolizing enzymes such as sucrose phosphate synthase which might have resulted in greater accumulation and export of carbohydrates to the development of new sinks. This might be the reason behind high Pn in ‘Bhut Jolokia’ plants grown in CTGT II. As compared to CTGT II, plants grown at CTGT III experienced a decline in Pn and reduced accumulation of TSS and TNSC. The reason for the decline in Pn at 750 ppm could be related to the decreased mobilization of carbohydrates due to less number and size of the sink, which results in the inhibition of photosynthesis (feedback inhibition).
Enhanced biomass under elevated CO2 and temperature
The CO2 enrichment and elevated temperature brought about a significantly higher C: N ratio in ‘Bhut Jolokia’ grown in CTGT II. This higher C:N ratio indicates that plant tissue accumulated more carbohydrates compared to nitrogen, which may improve above-ground biomass, rate of decomposition and alter the balance of vegetative and reproductive growth (Fageria and Baligar, 2005). Marcos-Barbero et al. (2021) found that when eCO2 was combined with higher temperatures, it increased biomass and yield in wheat, counteracting the negative effects of temperature on grain yield. (Das 2021) reported the favourable effect of elevated CO2 on dry matter partitioning in the Brassica crops. The increment in C: N ratio in ‘Bhut Jolokia’ may be due to a higher accumulation of carbohydrates in the leaf tissues under eCO2 and temperature. Interestingly, the change in the C: N ratio in soybean (Glycine max) occurred due to increased structural and NSC and decreased protein content (Allen et al. 1988). The increase in C: N ratio could be due to a reduction in the relative concentration of N in plants compared to C when grown under eCO2. Under elevated CO2, inhibition in the assimilation of leaf nitrate has also been reported by Bloom et al. (2014). Enhanced CO2 raised the C:N ratio in rice and wheat crops, with the changes owing to an increase in C concentration and a decrease in N concentration, implying that the beneficial effect of elevated CO2 could offset the detrimental impact of warming on the C:N ratio Bloom et al. (2014). Due to eCO2, a depression in the glycolate pathway is apparent and because of that, there will be less production of the amino acids (glycine and serine) leading to alteration in C:N ratio. Moreover, a decline in the photo-respiratory pathway may lead to a reduced breakdown of stored carbon.
Effect of elevated CO2 and temperature on proline content
The amino acid proline accumulates in plants in almost all adverse growing conditions such as drought, high temperature, low temperature, nutrient deficiency, diseases, etc. (Sarker and Oba, 2018a, b). The accumulation of proline helps maintain the protoplasm and osmotic balance in the plant, which reduced water loss and provide an N reserve for the plant to recover from the stress (Sarker and Oba, 2018a, b). High-temperature stress also induces physiological and biochemical changes and accumulation of compatible osmolytes (Wang et al. 2008). A rapid increase in proline content with an increase in CO2 and temperature was reported in leaf lettuce (Lactuca sp) (Han et al. 2013). In the present study, an increase in proline and soluble sugar offered osmotic adjustment to the stressed plants. The increase in the concentration of compatible solutes facilitated the adaptability by maintaining membrane function at high temperatures. Results depicted that ‘Bhut Jolokia’ plants grown under eCO2 and temperature (CTGTII) can perform with better yield over CTGTIII. Das and Uprety (2006) reported that elevated CO2 reduced the accumulation of active oxy radicals and increased the activity of antioxidant enzymes ameliorating the drought-induced oxidative stress effects in Brassica species when grown under the FACE facility. Many scientists believe that the first reaction of most plants responds to drought stress by closing their stomata in a bid to conserve moisture. Stomatal closure can cause a progressive decline in PSII electron transport, triggering proline and malondialdehyde (MDA) accumulation in plants. Malondialdehyde is produced from membrane lipid oxidative damage, which causes an increase in the activity of antioxidant enzymes, namely POD and CAT Mardinata et al. (2021).
Effects of elevated CO2 and temperature on chlorophyll content and chlorophyll a:b ratio
The total amount of leaf chlorophyll content and the ratio of Chl a/b directly influence the photosynthetic capacity of plants (Li et al. 2018). In the present study, a decrease in chlorophyll content was recorded in CTGT III as compared to CTGT II. At the same time, the ambient condition showed the highest content of chlorophyll in both cultivars. There was an increase in chlorophyll a/b under eCO2 and high-temperature stress, which might be attributed to the faster degradation of chlorophyll b. A link between the decrease in chlorophyll content and the C: N ratio was reported by Lee et al. (2020).
In contrast, the concentration of chlorophyll declined in the later stages. Photosynthetic pigments also declined under unfavourable environments/environmental stress like drought and salinity, corroborating the present findings (Sarker and Oba, 2018a, b). An increase in the Pn indicates that NADP is reduced to NADPH, where the electron source is from an excited chlorophyll molecule. The higher chlorophyll content observed in the Bhut Jolokia in CTGT II appears to be at the cost of decreased synthesis of chlorophyll b. For accepting electrons, a more core complex of photo-system might have been produced in the lipid bilayer by converting chlorophyll b to chlorophyll a. Released chlorophyll b from LHC a/b-protein photo-system II can be converted to chlorophyll a for forming core complexes of photo-systems (Ohtsuka et al. 1997).
Expression of photosynthesis-related genes
One of the important observations is the expression of RuBPC2 under eCO2 and elevated temperature in both germplasms. A common response of C3 plants grown at elevated CO2 is photosynthetic acclimation, implemented by downregulation of the RbcS transcript abundance, compared with the RbcS transcript abundance of plants grown at ambient CO2. However, results indicated that both germplasms showed more RuBPC transcript under elevated climatic conditions. Ainsworth et al. (2003) reported that elevated temperature resulted in the transcription level of these genes as in soybean. Similar results were reported in Arabidopsis (Li et al. 2008). In the present study, the SPS transcript was up-regulated in CTGT II when compared to the ambient. A higher in vivo SPS activity and an increase in leaf blade elongation rate were observed under eCO2 where the growing elongated blades acted as strong carbohydrate sinks (Seneweera et al. 1995). The up-regulation of the catalase was observed, which could be due to prolonged exposure of plants to eCO2 concentrations. Some researchers conducted research on genome-wide gene expression analysis under heat stress in poplar, they observed a number of transcription factors and gene that were differentially expressed (Song et al. 2014 and Kim et al. 2021). In response to heat stress, these factors interact and regulate the expression of photosynthesis-related genes, affecting carbon fixation and stomatal conductance (Song et al. 2014). Capsaicin is the key metabolite in terms of its commercial importance of Bhut Jolokia, therefore the levels of the Capsaicin forming gene, capsaicin synthase I was studied and over-expression of capsaicin synthase I transcripts in plants grown under CTGT II and III was recorded. Elevated CO2 might have increased the capsaicin content (a secondary metabolite) thereby showing upregulation of the capsaicin synthase gene as a result of more incorporation of the CO2. A similar result of an increase in tea quality has been reported by Ahammed et al. (2021).
Fruit drop under elevated CO2 and temperature
The primary factor for the decrease in fruit production has been attributed to high temperatures. The decrease in fruit production is again attributed to flower and immature fruit abortion and not because of decreased flower initiation or plant growth. Amongst the studied germplasms, cultivar Assam showed higher fruit drop as compared to cultivar Manipur under CTGT-III. High-temperature stress is known to cause a profound impact on reproduction resulting in a reduced number of pollen grains, pollen infertility, and stigma deformation (Das et al. 2021) and lower fruit setting (Das et al. 2020b) and reducing yield in hot chilli (Das et al. 2020b) and reduced the fruit quality in hot chili (Das et al. 2016). Aloni et al. (1996) suggested that an accumulation of sugars and starch in flowers during daytime is an important factor in determining their retention and fruit set. Prasad et al. (2001) in their study on the effect of high temperature in peanut (Arachis hypogaea) has also reported that the plant was especially sensitive to elevated temperature from a few days before pollen maturation through fertilization of the ovule.
In the present investigation, an increase in premature fruit drop under CTGT III was observed as compared to CTGT II, which might be due to the formation of the lesser amounts of photosynthates in the form of TNSC leading to unbalanced nutrition and water transport under CTGTIII causing formation in abscission layer. The high temperature at CTGT III might have caused water stress, leading to abscisic acid (ABA) accumulation and subsequently causing senescence and abscission of immature fruits.
Conclusion
It is concluded that CO2 increment up to 550 ppm with a rise of 2 °C temperature has a positive effect on Capsicum chinense. A higher photosynthetic and carbohydrate status and lower fruit drop were observed in cv. Manipur at CTGT II. Both the germplasms showed up-regulation of transcripts related to photosynthesis, carbohydrate metabolism and capsaicin production, which correlates with biochemical responses in the present study. Germplasm Manipur (cv 1) was better adapted to the eCO2 and temperature compared to cv. Assam (cv.2) indicating its potentiality to adapt to the future climate change phenomenon. The present findings will help identify cultivars, develop the models, and in modifying cultivation and nutrient application technologies for future environments of higher CO2 and temperature stress.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Directorate of Post Graduate Studies (DPGS), Assam Agricultural University, Jorhat, Assam, India, and Technology Mission (MM-I) for providing financial support in conducting this Ph D. research work of SD. We are also grateful to the National Initiative on Climate Resilient Agriculture (NICRA) Project for providing the Carbon dioxide Temperature Gradient Tunnel facility which was required for experimentation.
Author contribution
SD performed experiments and analyzed data with the guidance from RD. SA helped to do semi quantative RT-PCR in her laboratory. SD and SA co-wrote the manuscript. RD obtained grants for this research work. SK, SP, PK and AJN wrote and reviewed the manuscript.
Funding
This work was supported by DPGS, AAU, JORHAT and National Initiative on Climate Resilient Agriculture (NICRA) Project provided the Carbon dioxide Temperature Gradient Tunnel facility which was required for experimentation.
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahammed GJ, Li X. Elevated carbon dioxide-induced regulation of ethylene in plants. Environ Exp Bot. 2022;2022:105025. doi: 10.1016/j.envexpbot.2022.105025. [DOI] [Google Scholar]
- Ahammed GJ, Guang Y, Yang Y, Chen J. Mechanisms of elevated CO2-induced thermotolerance in plants: the role of phytohormones. Plant Cell Rep. 2021;40(12):2273–2286. doi: 10.1007/s00299-021-02751-z. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A, Blum H, et al. Variation in acclimation of photosynthesis in Trifolium repens after eight years of exposure to Free Air CO2 Enrichment (FACE) J Experimental Botany. 2003;54(393):2769–2774. doi: 10.1093/jxb/erg309. [DOI] [PubMed] [Google Scholar]
- Allen JLH, Vu J, Valtte RR, Boote KJ, Jones PH. Non-structural carbohydrates and nitrogen of soybean grown under carbon dioxide enrichment. Crop Sci. 1988;28:84–94. doi: 10.2135/cropsci1988.0011183X002800010020x. [DOI] [Google Scholar]
- Aloni B, Karni L, Zaidman Z. Changes of carbohydrates in pepper (Capsicum annuum L.) flowers in relation to their abscission under different shading regimes. Ann Bot. 1996;78(2):163–168. doi: 10.1006/anbo.1996.0109. [DOI] [Google Scholar]
- Bates S, Waldren RP, Tear ID. Rapid determination of free proline in water stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bloom A, Burger M, Kimball BA, Pinter PJ., Jr Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat Clim Change. 2014;4:477–480. doi: 10.1038/nclimate2183. [DOI] [Google Scholar]
- Bosland PW, Baral J. Bhut Jolokia’—The World’s Hottest Known Chile) pepper is a putative naturally occurring interspecific hybrid. Hort Sci. 2007;42(2):222–224. [Google Scholar]
- Cao P, Sun W, Huang Y, et al. Effects of elevated CO2 concentration and nitrogen application levels on the accumulation and translocation of non-structural carbohydrates in Japonica rice. Sustainability. 2020;12:5386. doi: 10.3390/su12135386. [DOI] [Google Scholar]
- Chavan SG, Duursman RA, Tausz M. Ghannoum O (2019) Elevated CO2 alleviates the negative impact of heat stress on wheat physiology but not on grain yield. J Exp Bot. 2019;70(21):6447–6459. doi: 10.1093/jxb/erz386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craufurd PQ, Wheeler TR. Climate change and the flowering time of annual crops. J Exp Bot. 2009;60:2529–2539. doi: 10.1093/jxb/erp196. [DOI] [PubMed] [Google Scholar]
- Das R. Study on growth characteristics of some brassicaspecies under moisture stress and elevated Carbondioxide. Int J Environ Climate Change. 2020;10(12):373–389. doi: 10.9734/ijecc/2020/v10i1230313. [DOI] [Google Scholar]
- Das S, Das R. Genotypic variations in reproductive morphology and anatomy of hot chilli (Capsicum chinense Jacq.) Appl Biolo Res. 2021;23(1):60–69. doi: 10.5958/0974-4517.2021.00008.2. [DOI] [Google Scholar]
- Das R, Uprety DC. Interactive effect of moisture stress and elevated CO2 on the oxidative stress in Brassica species. J Food Agric Environ. 2006;4(2):298–305. [Google Scholar]
- Das S, Das R, Choudhury H, Saikia A. Interactive effect of elevated carbon dioxide and temperature on quality of hot chilli (Capsicum chinense Jacq) Int J Trop Agricul. 2016;34(7):1977–1981. [Google Scholar]
- Das S, Das R, Kalita P, Baruah U. Growth responses of hot chilli (Capsicum chinense Jacq.) to elevated carbondioxide and temperature. J Experiment Biol Agricult Sci. 2020;8(4):434–444. doi: 10.18006/2020.8(4).434.440. [DOI] [Google Scholar]
- Dias de Oliveira E, Bramley H, Siddique KHM, Henty S, Berger JD, Palta JA. Can elevated CO2 combined with high temperature ameliorate the effect of terminal drought in wheat? Funct Plant Biol. 2013;40:160–171. doi: 10.1071/FP12206. [DOI] [PubMed] [Google Scholar]
- Driesen E, Ende WV, Proft MD, Saeys W. Influence of environmental factors light, CO2, temperature, and relative humidity on stomatal opening and development. A Rev Agron. 2020;10:1975. doi: 10.3390/agronomy10121975. [DOI] [Google Scholar]
- Fageria NK, Baligar VC. Enhancing nitrogen use efficiency in crop plants. Adv Agron. 2005;88:97–185. doi: 10.1016/S0065-2113(05)88004-6. [DOI] [Google Scholar]
- Fu J, Huang Z, Wang Z, Yang J, Zhang J. Pre-anthesis non-structural carbohydrate reserve in the stem enhances the sink strength of inferior spikelets during grain filling of rice. Field Crops Res. 2011;123:170–182. doi: 10.1016/j.fcr.2011.05.015. [DOI] [Google Scholar]
- Guinness Book of World Records (2006) Hottest Spice. Available on www.guinnessworldrecords.com, accessed 13 Sept. 2006
- Habermann E, Dias de Oliveira EA, Contin DR, San Martin JAB, Curtarelli L, Gonzalez-Meler MA, Martinez CA. Stomatal development and conductance of a tropical forage legume are regulated by elevated CO2 under moderate warming. Front Plant Sci. 2019;10:609. doi: 10.3389/fpls.2019.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Fan S, Zhang Q. Effect of heat stress on the MDA, proline and soluble sugar content in leaf lettuce seedlings. Agril Sci. 2013;4:112–115. [Google Scholar]
- Hanse J, Ruedy R, Sto M, Lo K. Global surface temperature change. Rev Geophy. 2010;48:4004. [Google Scholar]
- Hiscox JD, Israelstam GF. A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot. 1979;57:1332–1334. doi: 10.1139/b79-163. [DOI] [Google Scholar]
- Huberman M, Riov J, Aloni B, Goren R. Role of ethylene biosynthesis and auxin content and transport in high temperature- induced abscission of pepper reproductive organs. J Plant Growth Reg. 1997;16:129–135. doi: 10.1007/PL00006986. [DOI] [Google Scholar]
- Huve K, Bichele I, Ivanova H, Keerberg O, Pärnik T, Rasulov B, Tobias M, Niinemets U. Temperature responses of dark respiration in relation to leaf sugar concentration. Physiol Plant. 2012;144(4):320–34. doi: 10.1111/j.1399-3054.2011.01562.x. [DOI] [PubMed] [Google Scholar]
- IPCC . Technical summary. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors. Climate change (2013) The physical science basis contribution of working group i to the fifth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- Kim TL, Chung H, Veerappan K, Lee WY, Park D, Lim H. Physiological and Transcriptome responses to elevated CO2 concentration in populus. Forests. 2021;12:980. doi: 10.3390/f12080980. [DOI] [Google Scholar]
- Kumari M, Verma SC, Bhardwaj SK. Effect of elevated CO2 and temperature on crop growth and yield attributes of bell pepper (Capsicum annuum L.) J. Agromet. 2019;21(1):1–6. doi: 10.54386/jam.v21i1.195. [DOI] [Google Scholar]
- Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot. 2009;60:2859–2876. doi: 10.1093/jxb/erp096. [DOI] [PubMed] [Google Scholar]
- Lee J, Park SJ, Hong SC, Han JH, Choi D, Yoon JB. QTL mapping for capsaicin and dihydrocapsaicin content in a population of Capsicum annuum ‘NB1’ × Capsicum chinense ‘Bhut Jolokia’. Plant Breed. 2016;135(3):376–383. doi: 10.1111/pbr.12355. [DOI] [Google Scholar]
- Lee YH, Sang WG, Baek JK, et al. The effect of concurrent elevation in CO2 and temperature on the growth, photosynthesis, and yield of potato crops. PLoS ONE. 2020;15(10):e0241081. doi: 10.1371/journal.pone.0241081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PH, Ainsworth EA, Leakey ADB. Arabidopsis transcript and metabolite profiles: ecotype-specific responses to open-air elevated CO2. Plant Cell Environ. 31: 1673–1687 Seneweera SP, Basra AS, Barlow EW, Conroy JP (1995) Diurna1 regulation of leaf blade elongation in Rice by CO2 1s it related to sucrose-phosphate synthase activity? Plant Physiol. 2008;108:1471–1477. [Google Scholar]
- Li Y, He N, Hou J, Xu L, Liu C, Zhang J, Wang Q, Zhang X, Wu X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front Ecol Evol. 2018;6:64. doi: 10.3389/fevo.2018.00064. [DOI] [Google Scholar]
- Liu C, Luo S, Zhao Y, et al. Multiomics analyses reveal high temperature-induced molecular regulation of ascorbic acid and capsaicin biosynthesis in pepper fruits. Environmen Experiment Botany. 2022;201:104941. doi: 10.1016/j.envexpbot.2022.104941. [DOI] [Google Scholar]
- Liu C, Wan H, Yang Y, Ye Q, Zhou G, Wang X, Ahammed GJ, Cheng Y. Post-Harvest LED Light Irradiation Affects Firmness, Bioactive Substances, and Amino Acid Compositions in Chili Pepper (Capsicum annum L.) Foods. 2022;11:2712. doi: 10.3390/foods11172712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Mae T. Photosynthesis and plant growth at elevated levels of CO2. Plant Cell Physiol. 1999;40(10):999–1006. doi: 10.1093/oxfordjournals.pcp.a029493. [DOI] [Google Scholar]
- Marcos-Barbero EL, Pérez P, Martínez-Carrasco R, Arellano JB, Morcuende R. Screening for higher grain yield and biomass among sixty bread wheat genotypes grown under elevated CO2 and high-temperature conditions. Plants. 2021;10:1596. doi: 10.3390/plants10081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardinata Z, Edy ST, Ulpah S. Biochemical responses and leaf gas exchange of fig (Ficus carica L.) to water stress, short-term elevated CO2 levels and brassinolide application. Horticulturae. 2021;7:73. doi: 10.3390/horticulturae7040073. [DOI] [Google Scholar]
- McCready RM, Gugglog J, Silviera V. Determination of starch and amylase in vegetables. Anal Chem. 1950;22:156–158. doi: 10.1021/ac60045a016. [DOI] [Google Scholar]
- Mittler R, Finka A, Goloubinoff P. How do plants feel the heat? Trends in Biochem Sci. 2012;37:118–125. doi: 10.1016/j.tibs.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Moore CE, Katherine M, Pauline L, et al. The effect of increasing temperature on crop photosynthesis: from enzymes to ecosystems. J Experiment Botany. 2021;72(8):2822–2844. doi: 10.1093/jxb/erab090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017;22:53–65. doi: 10.1016/j.tplants.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Ito H, Tanaka A. Conversion of Chlorophyll b to Chlorophyll a and the Assembly of Chlorophyll with Apoproteins by isolated chloroplasts. Plant Physiol. 1997;113:137–147. doi: 10.1104/pp.113.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Han X, Bai Y, Yang J. Advances in physiology and ecology studies on stored non-structure carbohydrates in plants. Chin Bull Bot. 2002;19:30–38. [Google Scholar]
- Pan J, Huang D, Guo Z, Kuang Z, Zhang H, Xie X, Ma Z, Gao S, Lerdau M, Chu C, Li L. Overexpression of microRNA408 enhances photosynthesis, growth, and seed yield in diverse plants. J Integ Plant Biol. 2018;60(4):323–340. doi: 10.1111/jipb.12634. [DOI] [PubMed] [Google Scholar]
- Panse VG, Sukhatme PT. Statistical methods for agricultural workers. 2. New Delhi: Indian Council of Agricultural Research; 1967. [Google Scholar]
- Perdomo JA, Capó-Bauçà S, Carmo-Silva E, Galmés J. Rubisco and Rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water Deficit. Front Plant Sci. 2017;8:490. doi: 10.3389/fpls.2017.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad PVV, Craufurd PQ, Kakani VG. Influence of high temperature during pre and post-anthesis stages of floral development on fruit-set and pollen germination in peanut. Funct Plant Biol. 2001;28(3):233–240. doi: 10.1071/PP00127. [DOI] [Google Scholar]
- Purcell C, Batke SP, Yiotis C, Caballero SWR, Murray M, McElwain JC. Increasing stomatal conductance in response to rising atmospheric CO2. Ann Bot. 2018;121(7):1427. doi: 10.1093/aob/mcy023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaderi MM, Kurepin LV, Reid DM. Growth and physiological responses of canola (Brassica napus) to three components of global climate change: temperature, carbon dioxide and drought. Plant Physiol. 2006;128:710–721. doi: 10.1111/j.1399-3054.2006.00804.x. [DOI] [Google Scholar]
- Das R (2021) Effect of Elevated CO2 on Dry Mater Partitioning in Brassica Species Under Moisture Stress Condition Agriways 9(1): 26–32
- Rojas-Downing MM, Nejadhashemi AP, Harrigan T, Woznicki SA. Climate change and livestock: impacts, adaptation, and mitigation. Clim Risk Manag. 2017;16:145–163. doi: 10.1016/j.crm.2017.02.001. [DOI] [Google Scholar]
- Sakamoto A, Murata N. The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ. 2002;25:163–171. doi: 10.1046/j.0016-8025.2001.00790.x. [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Brandner SJC. Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plantarum. 2003;120:179–186. doi: 10.1111/j.0031-9317.2004.0173.x. [DOI] [PubMed] [Google Scholar]
- Sarker U, Oba S. Augmentation of leaf color parameters, pigments, vitamins, phenolic acids, flavonoids and antioxidant activity in selected Amaranthus tricolor under s alinity stress. Sci Rep. 2018;8:12349. doi: 10.1038/s41598-018-30897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker U, Oba S. Response of nutrients, minerals, antioxidant leaf pigments, vitamins, polyphenol, flavonoid and antioxidant activity in selected vegetable amaranth under four soil water content. Food Chem. 2018;252:72–83. doi: 10.1016/j.foodchem.2018.01.097. [DOI] [PubMed] [Google Scholar]
- Seneweera SP, Basra AS, Barlow EW, Conroy JP. Diurna1 regulation of leaf blade elongation in rice by CO2 1s it related to sucrose-phosphate synthase activity? Plant Physiol. 1995;108:1471–1477. doi: 10.1104/pp.108.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloat LL, Davis SJ, Gerber JS, et al. (2020) Climate adaptation by crop migration. Nat Commun. 2020;11:1243. doi: 10.1038/s41467-020-15076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Yu J, Huang B. Elevated CO2-mitigation of high temperature stress associated with maintenance of positive carbon balance and carbohydrate accumulation in Kentucky bluegrass. PLoS ONE. 2014;9(3):e89725. doi: 10.1371/journal.pone.0089725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfat A, Shokat S, Li X, et al. Elevated carbon dioxide alleviates the negative impact of drought on wheat by modulating plant metabolism and physiology. Agric Water Manag. 2021 doi: 10.1016/j.agwat.2021.106804. [DOI] [Google Scholar]
- Van der Kooi CJ, Reichb M, Low M, De Kok LJ, Tausz M. Growth and yield stimulation under elevated CO2 and drought: A meta-analysis on crops Environ. Exp Bot Environ Exp Bot Environ Exp Bot Environ Exp Bot Environ Exp Bot. 2016;122:150–157. [Google Scholar]
- Walkley A, Black CA. An exogenous degtjareff method for determining soil organic matter and a proposed modification of the chromic acid and titration method. Soil Sci. 1934;37:29–38. doi: 10.1097/00010694-193401000-00003. [DOI] [Google Scholar]
- Wang HM, Bao WK, Li FL. Physiological and biochemical responses of two-years-old Sophora davidii seedling leaves to different water stresses. Chin J Appl Environ Biol. 2008;14(6):757–762. [Google Scholar]
- Warren JM, Jensen AM, Medlyn BE, Norby RJ, Tissue DT. Carbon dioxide stimulation of photosynthesis in Liquidambar styraciflua is not sustained during a 12-year field experiment. AoB Plants. 2014;5:1–13. doi: 10.1093/aobpla/plu074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Gong H, Lu C. Heat stress induces a reversible inhibition of electron transport at the acceptor side of photosystem II in a cyanobacterium Spirulina platensis. Plant Sci. 2005;168:1471–1476. doi: 10.1016/j.plantsci.2005.01.015. [DOI] [Google Scholar]
- Wullschleger SD, Tschaplinski TJ, Norby RJ. Plant water relations at elevated CO2 – implications for water-limited environments. Plant, Cell Environ. 2002;25:319–331. doi: 10.1046/j.1365-3040.2002.00796.x. [DOI] [PubMed] [Google Scholar]
- Yoshinaga S, Tokida T, Usui Y, et al. Analysis of factors related to varietal differences in the yield of rice (Oryza sativa L.) under Free-Air CO2 Enrichment (FACE) conditions. Plant Prod Sci. 2020;23:19–27. doi: 10.1080/1343943X.2019.1683455. [DOI] [Google Scholar]
- Yuan L, Yuan Y, Liu S, et al. (2017) Influence of high temperature on photosynthesis, antioxidative capacity of chloroplast, and carbon assimilation among heat-tolerant and heat-susceptible genotypes of nonheading chinese cabbage. HortScience. 2017;52:1464–1470. doi: 10.21273/HORTSCI12259-17. [DOI] [Google Scholar]
- Zhang H, Pan C, Gu S, Ma Q, Zhang Y, Li X. Shi K (2019) Stomatal movements are involved in elevated CO2 -mitigated high temperature stress in tomato. Physiol Plant. 2019;165(3):569–583. doi: 10.1111/ppl.12752. [DOI] [PubMed] [Google Scholar]
- Zhu P, Zhuang Q, Ciais P, Welp L, Li W, Xin Q. Elevated atmospheric CO2 negatively impacts photosynthesis through radiative forcing and physiology mediated climate feedback. Geophy Res Lett. 2017;44:1956–1963. [Google Scholar]
- Zhuo X, Misaghi IJ, Hawes MC. Stimulation of border cell production in response to increased carbon dioxide level. Plant Physiol. 2000;122:181–188. doi: 10.1104/pp.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinta G, Abdelgawad H, Peshev D, Weedon JT, Van Den Ende W, Nijs I. Dynamics of metabolic responses to periods of combined heat and drought in Arabidopsis thaliana under ambient and elevated atmospheric CO2. J Exp Bot. 2018;69:2159–2170. doi: 10.1093/jxb/ery055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.