Graphical abstract
Single-cell RNA-sequencing (scRNA-seq) was utilized to investigate the intratumoral heterogeneity of primary, recurrent, and metastatic osteosarcoma (OS) lesions. Briefly, following biopsy or surgical resection (1), tumor specimens are digested into a single-cell suspension (2) before encapsulation into emulsion droplets for scRNA-seq library construction using the 10X Genomics Chromium Controller. Libraries are evaluated for quality before sequencing (4). Standard pipelines and tools such as Cell Ranger are then used for pre-processing, integration, and cellular clustering before downstream expression analysis (5) [Figure designed at BioRender.com].
Keywords: Osteosarcoma, Single-cell, Sequencing, Recurrence, Metastasis, Microenvironment, Intratumoral heterogeneity
Highlights
-
•
scRNA-seq has provided invaluable insight into the heterogeneity of various tumors.
-
•
Review highlights scRNA-seq on OS primary, recurrent, or metastatic lesions.
-
•
Possible target identification and association with recurrent, metastatic potential.
-
•
TIGIT expression across immune cell populations suggests exhaustion in OS TME.
Abstract
While primary bone malignancies make up just 0.2% of all cancers, osteosarcoma (OS) is the third most common cancer in adolescents. Due to its highly complex and heterogeneous tumor microenvironment (TME), OS has proven difficult to treat. There has been little to no improvement in therapy for this disease over the last 40 years. Even the recent success of immunotherapies in other blood-borne and solid malignancies has not translated to OS. With frequent recurrence and lung metastases continuing to pose a challenge in the clinic, recent advancements in molecular profiling, such as single-cell RNA sequencing (scRNA-seq), have proven useful in identifying novel biomarkers of OS tumors while providing new insight into this TME that could potentially lead to new therapeutic options. This review combines the analyses of over 150,000 cells from 18 lesions ranging from primary, recurrent, and metastatic OS lesions, revealing distinct cellular populations and gene signatures that exist between them. Here, we detail these previous findings and ultimately convey the intratumoral heterogeneity that exists within OS tumor specimens.
1. Introduction
Osteosarcoma (OS) is a primary bone malignancy of mesenchymal origin. Although rare, this disease is the most common primary bone neoplasm in children/young adults. The incidence of OS presents bimodally, with the first peak in adolescents (4.4 cases per million individuals) and again amongst patients over 65 (4.2 cases per million individuals) [1]. Composed chiefly of malignant osteoblasts producing immature bone or osteoid, OS often develops in the metaphysis and diaphysis of long bones, resulting in the loss of balance between bone formation and reabsorption [2]. Both gross and histological classifications of OS can be divided into subtypes based on their location within the bone. These include surface (periosteal/cortical), extra-skeletal, and central (intramedullary). Both intramedullary and surface OS malignancies can be further classified by grade: intramedullary OS can be subdivided into low-grade and high-grade (conventional, telangiectatic, and small cell), while surface OS is further classified as low (parosteal), intermediate (periosteal), or high-grade [3], [4]. The most common presentation of OS (conventional) is high-grade, intramedullary, often located in the long bone metaphyseal region of the proximal humerus, tibia, or distal femur [4]. Histologically, conventional OS contains atypical osteoid-producing spindle-like cells. However, considering there are varying degrees in matrix composition (osteoid, cartilage, and fibrous tissue), lesions can be further classified by which cell type is proportionally greater than 50% of malignant cells. Based on this cellular composition, OS can then be divided into osteoblastic, chondroblastic, and fibroblastic designations [5].
Surgical resection in combination with chemotherapy has remained the standard of care for OS primary tumors since the 1980s. A three-drug regimen (Methotrexate, Adriamycin, and platin based therapies) is administered pre- and post-surgery. While five-year event-free survival (EFS) is over 78%, with 85% of OS presenting with only localized disease, metastasis remains the primary concern [6], [7]. In these metastatic patients, 74% present with metastasis to the lung, while another 9% present with metastatic spread to another site within the bone. In addition, OS recurrence occurs in 30–40% of cases. Most patients recur within two to three years of initial diagnosis and treatment [8]. In contrast to patients who only develop primary tumors, those with metastatic disease have a far less favorable outlook. Five-year overall survival decreases dramatically from 78% to 25% [9]. This difference in overall survival underscores the importance of developing new therapeutics to prevent OS disease progression and/or treat metastasis.
Although little has changed in OS treatment strategies, strides have been made regarding understanding the genomic and genetic factors underlying this disease. These efforts have mainly focused on the identification of “driver mutations” within tumor suppressor and/or oncogenes [10]. Two of these mutations that commonly exist in OS include the inactivation of the TP53 and RB genes. TP53 is the most frequently altered tumor suppressor gene in all cancers (20% in OS) [11]. This cell-cycle inhibitor is responsible for regulation of cell growth along with DNA replication and cell division [12]. Frequent mutations in the RB gene have also been implicated in pediatric OS cases, resulting in loss of heterozygosity and poorer prognosis [12], [13]. Additionally, mutation of the c-MYC gene, which plays a vital role in the MEK-ERK pathways, occurs in approximately 10% of OS cases. Elevated expression of MYC has been shown to promote cell proliferation and migration. Notably, increased MYC levels have been observed more commonly in metastatic than non-metastatic lesions. MYC alteration, therefore, likely indicates the presence of more aggressive cells and poorer prognosis [14], [15]. Advances in genomic sequencing have been critical for uncovering the mutations that exist in cancer cells. These genetic alterations ultimately influence the communication between cancerous cells and their surrounding environment. The resulting cellular and architectural changes that result from these interactions is referred to as the tumor microenvironment (TME).
The TME of OS is highly heterogeneous, forming a complex system composed of immune cells, fibroblasts, and tumor cells. The role of the TME in tumor progression and prognosis has been examined. The vast heterogeneity evident in OS malignancies has ultimately been associated with difficulties faced during treatment. For instance, cancer-associated fibroblasts (CAFs) have been shown to play an integral role in regulating metastasis through various signaling pathways that promote tumor progression, growth, invasion, as well as extracellular matrix construction. Activity of these CAFs and the resulting matrix produced thus creates a barrier for both therapies and immune cells from accessing the tumor [16]. Additionally, tumor-associated macrophages (TAMs), accounting for nearly 50% cellularity of solid neoplasms, are known to promote disease progression and therapy resistance through trophic and nutritional support to cancerous cells [17]. The specific role each cell plays within this microenvironment has been suggested through various analyses including bulk (bulkRNA-seq) and single-cell (scRNA-seq) RNA sequencing.
BulkRNA-seq of solid tumor specimens has been a standard method for gene expression analysis over the past decade. While this technique is relatively cost-effective, without intense computational deconvolution through bioinformatic methods, it only reflects averaged gene expression across all collected cells. Of these methods, CIBERSORT quantifies cellular populations from bulk tissue gene expression profiles (GEP). Using a machine-learning approach, this method estimates cell composition and associated immune pathways by comparing a signature matrix for the cell types of interest [18]. While innovative, this method still limits bulkRNA-seq to a cellular population analysis. By only viewing an averaged signal within each cell population, information regarding the heterogeneity of cells is, unfortunately, lost in translation [19], [20].
Advancements in sequencing technologies now allow for the identification of transcriptomic signatures at the single-cell resolution. Through scRNA-seq, the transcriptome of the complex OS TME can be analyzed at the level of individual cells, which is not possible with bulkRNA-seq. scRNA-seq allows investigators to identify distinct cellular mechanisms contributing to tumor progression and uncover potential crosstalk between tumor cells and their microenvironment [20], [21]. This comprehensive analysis allows investigation of cancer heterogeneity through molecular expression profiling at the cellular level, distinguishing cells with a highly specified phenotype to further infer cellular behavior [22]. Although scRNA-seq has proved advantageous in studying the TME, it is still a technique with inherent limitations. Due to technical (sample processing) and biological (patient-to-patient variability) factors, scRNA-seq data sets are often thwarted by high background noise, making their analysis far more complex than traditional bulkRNA-seq. Further, scRNA-seq is associated with low capture efficiency and high cellular dropouts due to specific transcripts measuring undetectable. With high dropout, weakly expressed genes could unfortunately be excluded from downstream analysis [19], [20].
In addition to these technical and biological factors, other confounding variables such as cellular apoptosis, batch effect, and the cell cycle can influence the analysis of scRNA-seq data. For example, gene expression heterogeneity amongst cells corresponds with the cell cycle stage at which the cell is captured. The cell cycle presents a confounding variable as the expression of a gene of interest, even within the same cellular cluster, can be influenced by each cell’s replicative state [23]. These effects become most apparent in the form of dropout events when discussing proliferative pathways, such as PI3K-AKT signaling, in the setting of proliferative cell clusters. Acknowledging these confounding factors is crucial for downstream analysis [24]. Alongside these technical hurdles, scRNA-seq comes at a high cost with added procedural complexity [25]. However, scRNA-seq is now widely recognized as a crucial method for in-depth analyses of TMEs in many forms of cancer.
scRNA-seq has provided invaluable insight into the heterogeneity of various tumors including lung cancer, breast cancer, pancreatic ductal adenocarcinoma (PDAC), and bladder cancer [26], [27], [28], [29], [30], [31]. In lung cancer, scRNA-seq was used to observe the cellular and molecular factors that increased metastatic potential in cancerous cells. Kim et al. identified a subtype of cancer cells more prevalent in metastatic lesions while uncovering the stromal and immune changes that created the pro-tumoral microenvironment [26]. Chung et al. utilized scRNA-seq to characterize the highly heterogenous TME of primary breast cancer, revealing the role that immune suppression plays in tumor progression while identifying potential targets for future immunotherapies [29]. Lastly, scRNA-seq was utilized to identify malignant cell types in primary PDAC tumors. Peng et al. identified numerous heterogenous malignant cell populations, characterizing their migratory and proliferative potential. This study also highlighted subsets of inactive tumor infiltrating lymphocytes (TILs), indicating the presence of immune cell exhaustion [30]. Ultimately, this form of analysis could lead to prediction of metastatic potential in future clinical practice.
While scRNA-seq has been deployed in several different cancers, this technique has been little utilized for characterization of OS. This review aims to provide a comprehensive atlas of the existing scRNA-seq data in OS. These scRNA-seq data lay the groundwork for future studies to investigate differences between the TMEs of primary, recurrent, and metastatic lesions. This comprehensive review particularly evaluates the works of five groups who have directly performed or re-analyzed scRNA-seq on OS primary, recurrent, or metastatic lesions. For clarity, the full names of the genes as well as their gene ontology (GO) classification highlighted throughout this review are summarized in Table 1.
Table 1.
Full names for gene IDs along with GO classification indicated throughout the text.
| Gene ID | Name | GO Classification | Reference |
|---|---|---|---|
| ACAN | aggrecan | extracellular matrix structural constituent, protein binding | PMID:1569188, PMID:17588949 |
| ACP5 | acid phosphatase 5 | ferrous iron binding, ferric iron binding | PMID:15993892 |
| ACTA2 | actin alpha 2 | ATP binding, hydrolase activity | GO_REF:0000043 |
| ALK2 | activin receptor-like kinase-2 | regulation of ossification | PMID:16642017 |
| APOC1 | apolipoprotein C1 | phospholipase inhibitor activity, fatty acid binding | PMID:2302419, PMID:17339654 |
| APOE | apolipoprotein E | amyloid-beta binding | PMID:11305869 |
| BMP9 | bone morphogenetic protein-9 | angiogenesis, osteoblast differentiation | GO_REF:0000043, GO_REF:0000107 |
| CADM1 | cell adhesion molecule 1 | signaling receptor binding, protein binding | PMID:12826663, PMID:12234973 |
| CCL2 | C-C motif chemokine ligand 2 | inflammatory response, cytoskeleton organization | PMID:21147091, PMID:10072545 |
| CCL3 | C-C motif chemokine ligand 3 | chemokine activity, protein binding | PMID:10072545, PMID:21784977 |
| CCL4 | C-C motif chemokine ligand 4 | protein binding, cytokine activity | PMID:2462251, PMID:25416956 |
| CCR7 | C-C motif chemokine receptor 7 | cell surface, C-C motif chemokine 19 and 21 receptor activity | PMID:18308860, PMID:9507024 |
| CDK1 | cyclin dependent kinase 1 | protein kinase activity, cyclin-dependent protein serine/threonine kinase activity | PMID:23574715, PMID:11069302 |
| CENPF | centromere protein F | chromatin binding, protein binding | PMID:9891037, PMID:17363900 |
| CLEC11A | C-Type lectin domain containing 11A | growth factor activity, extracellular region | PMID:11920266, PMID:9442024 |
| COL14A1 | collagen type XIV alpha 1 chain | extracellular matrix organization, extracellular matrix structural constituent | PMID:9427527, PMID:2187872 |
| COL18A1 | collagen type XVIII alpha 1 chain | cell adhesion, extracellular matrix | GO_REF:0000043, PMID:21873635 |
| COL1A1 | collagen type I alpha 1 chain | protein binding, skeletal system development | PMID:14749390, PMID:14976317 |
| COL2A1 | collagen type II alpha 1 chain | extracellular space, collagen-containing extracellular matrix | PMID:20603131, PMID:23658023 |
| COL6A1 | collagen type VI alpha 1 chain | osteoblast differentiation, collagen-containing extracellular matrix | PMID:16210410, PMID:23658023 |
| COL6A3 | collagen type VI alpha 3 chain | collagen-containing extracellular matrix, cell adhesion | PMID:23658023, GO_REF:0000043 |
| COL8A1 | collagen type VIII alpha 1 chain | cell adhesion, angiogenesis | GO_REF:0000043 |
| CTLA4 | cytotoxic T-lymphocyte associated protein 4 | adaptive immune response, cellular response to DNA damage stimulus | GO_REF:0000043, PMID:17875758 |
| CTSK | cathepsin K | protein binding, proteolysis involved in protein catabolic process | PMID:12504904, PMID:11082042 |
| CXCL10 | C-X-C motif chemokine ligand 10 | cytokine activity, chemotaxis | PMID:12782716, PMID:12782716 |
| CXCL12 | C-X-C motif chemokine ligand 12 | integrin binding, chemokine activity | PMID:29301984, PMID:12782716 |
| CXCL13 | C-X-C motif chemokine ligand 13 | chemokine activity, immune response | PMID:11554781 |
| CXCL14 | C-X-C motif chemokine ligand 14 | extracellular space, cell chemotaxis | GO_REF:0000043, GO_REF:0000002 |
| CXCL2 | C-X-C motif chemokine ligand 2 | inflammatory response, chemokine activity | PMID:21873635 |
| CXCL3 | C-X-C motif chemokine ligand 3 | chemokine activity, neutrophil chemotaxis | GO_REF:0000024 |
| CXCL9 | C-X-C motif chemokine ligand 9 | chemokine activity, regulation of cell population proliferation | PMID:12782716 |
| CYR61 | cysteine rich angiogenic inducer 61 | signal transduction, growth factor binding | GO_REF:0000004 |
| DES | desmin | protein binding | PMID:11353857 |
| ECM1P2 | Extracellular Matrix Protein 1 Pseudogene 2 | ossification, extracellular matrix | GO_REF:0000043, PMID:9367673 |
| FABP5 | fatty acid binding protein 5 | fatty acid binding, protein binding | PMID:8092987, PMID:12839573 |
| FOSB | FosB proto-oncogene, AP-1 transcription factor subunit | protein binding | PMID:28514442 |
| FOXP3 | forkhead box P3 | DNA binding, negative regulation of cytokine production | PMID:21458306, PMID:11483607 |
| GADD45B | growth arrest and DNA damage inducible beta | apoptotic process, cell differentiation | GO_REF:0000043 |
| G0S2 | G0/G1 Switch 2 | extrinsic apoptotic signaling pathway, protein binding | PMID:19706769 |
| GZMB | granzyme B | serine-type endopeptidase activity, cytolysis | PMID:11331782, GO_REF:0000043 |
| GZMH | granzyme H | serine-type endopeptidase activity, cyto-/proteolysis | PMID:2193684, GO_REF:0000043 |
| GZMK | granzyme K | serine-type endopeptidase activity, proteolysis | PMID:10480954, GO_REF:0000043 |
| HAPLN1 | hyaluronan and proteoglycan link protein 1 | hyaluronic acid binding, collagen-containing extracellular matrix | GO_REF:0000043, PMID:28327460 |
| HLA-DRA | major histocompatibility complex, class II, DR alpha | MHC class II protein complex binding, MHC class II receptor activity | PMID:20458337, PMID:6304715 |
| IBSP | integrin binding sialoprotein | osteoblast differentiation, extracellular matrix organization | PMID:16210410, GO_REF:0000024 |
| ICOS | Inducible T Cell Costimulator | immune response, extracellular region | PMID:9930702, GO_REF:0000044 |
| IFIT1 | interferon induced protein with tetratricopeptide repeats 1 | RNA binding, response to virus | PMID:21642987 |
| IFN-α | interferon alpha | pro-inflammatory | PMID:21873635 |
| IFN-γ | interferon gamma | pro-inflammatory | PMID:7605994 |
| IHH | Indian hedgehog signaling molecule | protein binding, skeletal system development | PMID:20519495, PMID:21537345 |
| IL17R | interleukin-17 receptor | inflammatory response, cell surface receptor signaling pathway | GO_REF:0000043, PMID:9367539 |
| IL2RA | interleukin 2 receptor subunit alpha | regulation of T cell tolerance induction, interleukin-2 receptor activity | PMID:23416241, PMID:2467293 |
| IL-4 | interleukin 4 | B cell differentiation, T cell activation | PMID:11418631, PMID:18337562 |
| ITGA1 | integrin subunit alpha 1 | collagen binding, collagen binding involved in cell-matrix adhesion | PMID:24823363, PMID:8428973 |
| ITGAE | integrin subunit alpha E | integrin binding, cell–cell adhesion | PMID:21873635 |
| JMJD3 | jumonji domain-containing protein-3 | cell cycle activity, inflammatory response | GO_REF:0000004 |
| JUND | JunD proto-oncogene, AP-1 transcription factor subunit | transcription cis-regulatory region binding, protein binding | PMID:1903194, PMID:17577209 |
| KRAS | KRAS proto-oncogene, GTPase | G protein activity, positive regulation of cell population proliferation | GO_REF:0000003, PMID:23698361 |
| LAG3 | lymphocyte activating protein 3 | negative regulation of regulatory T cell differentiation, adaptive immune response | GO_REF:0000043, GO_REF:0000024 |
| LGMN | legumain | cysteine-type endopeptidase activity, proteolysis | GO_REF:0000024, PMID:9065484 |
| LINC00662 | long intergenic non-protein coding RNA 662 | proliferation | PMID: 34354995 |
| LUM | lumican | extracellular matrix structural constituent, collagen binding | PMID:10892350, PMID:10892350 |
| MAF | MAF bZIP transcription factor | protein binding, regulation of chondrocyte differentiation | PMID:20102225, GO_REF:0000107 |
| MCAM | melanoma cell adhesion molecule | cell adhesion | GO_REF:0000043 |
| MCM5 | minichromosome maintenance complex component 5 | ATP binding, cell cycle | GO_REF:0000043 |
| MEF2C | myocyte enhancer factor 2C | osteoblast differentiation, DNA-binding transcription factor activity, RNA polymerase II-specific | GO_REF:0000024, PMID:8455629 |
| MERTK | MER proto-oncogene, tyrosine kinase | protein binding, positive regulation of phagocytosis, | PMID:20103767, PMID:18395422 |
| MIF | macrophage migration inhibitory factor | cytokine activity, innate immune response | PMID:12782713, GO_REF:0000043 |
| MKI67 | marker of proliferation Ki-67 | cell population proliferation, cell cycle | GO_REF:0000024, GO_REF:0000043 |
| MMP2 | matrix metallopeptidase 2 | collagen-containing extracellular matrix, metallopeptidase activity | PMID:28327460, PMID:24236012 |
| MMP9 | matrix metallopeptidase 9 | metalloendopeptidase activity, protein binding | PMID:16192646, PMID:16512877 |
| MRC1 | mannose receptor C-type 1 | virus receptor activity, cargo receptor activity | GO_REF:0000043, PMID:20035344 |
| MS4A1 | membrane spanning 4-domains A1 | immunoglobulin binding, B cell differentiation | PMID:18474602, PMID:3925015 |
| MT1X | metallothionein 1X | negative regulation of growth, protein binding | GO_REF:0000024, PMID:23251525 |
| MYC | MYC proto-oncogene, bHLH transcription factor | positive regulation of mesenchymal cell proliferation, transcription coregulator binding | GO_REF:0000024,PMID:15674325 |
| MYL9 | myosin light chain 9 | platelet aggregation | PMID:23382103 |
| MZB1 | marginal zone B and B1 cell specific protein | positive regulation of immunoglobulin production, regulation of B cell proliferation | GO_REF:0000024 |
| NR4A3 | nuclear receptor subfamily 4 group A member 3 | protein kinase binding, negative regulation of transcription by RNA polymerase II | PMID:25852083, GO_REF:0000024 |
| NT5E | 5′-nucleotidase ecto | nucleotide binding, 5′-deoxynucleotidase activity | GO_REF:0000043, PMID:22997138 |
| OGN | osteoglycin | growth factor activity | GO_REF:0000043 |
| OMD | osteomodulin | cell adhesion | GO_REF:0000043 |
| OPN | osteopontin | cytokine activity | GO_REF:0000043 |
| PCNA | proliferating cell nuclear antigen | chromatin binding | PMID:23277426 |
| PDCD1 | programmed cell death 1 | negative regulation of immune response, positive regulation of T cell apoptotic process | GO_REF:0000024, PMID:25281059 |
| PRDX2 | peroxiredoxin 2 | response to oxidative stress, regulation of apoptotic process | PMID:11904290, PMID:12943237 |
| PSMD4 | proteasome 26S subunit ubiquitin receptor, non-ATPase 4 | RNA binding, protein binding | PMID:22658674, PMID:15147878 |
| PTH1R | parathyroid hormone 1 receptor | parathyroid hormone receptor activity | PMID:8397094 |
| RB1 | RB transcriptional corepressor 1 | protein binding | PMID:15107404 |
| RUNX2 | RUNX family transcription factor 2 | ossification, osteoblast differentiation | PMID:12217689 |
| RUVBL1 | RuvB like AAA ATPase 1 | DNA helicase activity, DNA repair | GO_REF:0000043 |
| S100A10 | S100 calcium binding protein A10 | protein binding, positive regulation of GTPase activity | PMID:14699089, PMID:23129259 |
| S100A11 | S100 calcium binding protein A11 | signal transduction, | PMID:16130169 |
| S100A8 | S100 calcium binding protein A8 | leukocyte migration involved in inflammatory response, apoptotic process | PMID:12626582, GO_REF:0000043 |
| S100A9 | S100 calcium binding protein A9 | leukocyte migration involved in inflammatory response, neutrophil chemotaxis | PMID:12626582 |
| SDC1 | syndecan 1 | myoblastdevelopment | GO_REF:0000024 |
| SMPD3 | sphingomyelin phosphodiesterase 3 | sphingomyelin phosphodiesterase activity | GO_REF:0000024 |
| SOSTDC1 | sclerostin domain containing 1 | negative regulation of Wnt signaling pathway, negative regulation of myoblast differentiation | PMID:15020244 |
| SOX9 | SRY-box transcription factor 9 | skeletal system development, epithelial to mesenchymal transition | PMID:8640233, GO_REF:0000024 |
| SP7 | Sp7 transcription factor | osteoblast differentiation, DNA binding | PMID:11792318 |
| STAB1 | stabilin 1 | inflammatory response, negative regulation of angiogenesis | GO_REF:0000043, PMID:12077138 |
| STMN1 | stathmin 1 | negative regulation of microtubule polymerization | PMID:25198505 |
| TGFBI | transforming growth factor beta induced | response to stimulus, angiogenesis | GO_REF:0000043, PMID:11866539 |
| TGF-β | transforming growth factor beta | epithelial to mesenchymal transition, anti-inflammatory | PMID:25893292, PMID:21147091 |
| TIGIT | T cell immunoreceptor with Ig and ITIM domains | negative regulation of T cell activation | PMID:19011627 |
| TNF | tumor necrosis factor | positive regulation of cytokine production involved in inflammatory response | PMID:31824476 |
| TNFRSF14 | TNF receptor superfamily member 14 | adaptive immune response, T cell costimulation | GO_REF:0000043, PMID:10754304 |
| TNFRSF25 | TNF receptor superfamily member 25 | regulation of apoptotic process | PMID:9114039 |
| TNFSF11 | TNF superfamily member 11 | immune response, osteoclast differentiation | PMID:9312132, PMID:9568710 |
| TOP2A | DNA topoisomerase II alpha | DNA binding, positive regulation of apoptotic process | PMID:10788521, PMID:16611985 |
| TP53 | tumor protein p53 | regulation of cell cycle G2/M phase transition | PMID:10962037 |
| TXNIP | thioredoxin interacting protein | negative regulation of cell division, cellular response to tumor cell | PMID:18541147 |
| TYMS | thymidylate synthetase | mitochondrial matrix | PMID:21876188 |
| VEGFA | vascular endothelial growth factor A | angiogenesis | PMID:21771332 |
| VEGFR2 | vascular endothelial growth factor receptor 2 | positive regulation of cell population proliferation | PMID:15215251 |
| WISP2 | WNT1 inducible signaling pathway protein 2 | epithelial-mesenchymal transition, extracellular region | GO_REF:0000043 |
2. scRNA-seq of the osteosarcoma tumor microenvironment
ScRNA-seq analysis has been performed by two investigators to successfully analyze primary, metastatic, and recurrent OS lesions. Of these groups, Zhou et al. identified 100,987 cells in eleven patient samples (age range 11 – 38 years old), eight of which were from primary osteoblastic lesions and three from primary chondroblastic lesions. Of these lesions, seven were primary and accounted for most of the isolated cells (65,895 cells). The other four specimens were recurrent and metastatic lesions (two each). The seven patients with primary lesions received first-line adjuvant and neoadjuvant chemotherapy consisting of the four-drug regimen cisplatin, doxorubicin, ifosfamide, and methotrexate along with surgery. Both patients with lung metastasis received additional lung lobectomy. Both recurrent OS patients received gemcitabine and docetaxel in addition to the four-drug regimen [32] (Table 2). Guo et al., using the sequencing results from Zhou et al. (NCBI Gene Expression Omnibus database GS152048), identified 109,415 cells from these eleven patient samples. With the same dataset, Guo performed an in-depth analysis of a particular subcluster of OS cells and was thus included in this review [33]. Further, Liu et al. identified 29,278 cells from six primary lesions receiving no neoadjuvant chemotherapy (age range from 13 to 45 years old) [34]. Wang et al. focused on a single chemo-resistant lesion (Methotrexate, Adriamycin + Cisplatin), ultimately identifying nine clusters from 7,177 cells [35]. Finally, Huang analyzed scRNA-seq data sets from Zhou et al. and Liu et al., identifying differences in cancer-associated fibroblasts between recurrent and primary lesions [36] (Table 2). These data suggest that the OS TME is highly pleomorphic with thousands of distinct cell types. By analyzing lesions in different states (primary, recurrent metastatic), elucidating differences and similarities of these TMEs is possible, thus revealing potential areas of interest for targeted therapeutics. For clarity, the distinguishing genes, as well as those genes with increased or decreased expression within each cellular cluster, are provided in Fig. 1, Fig. 2.
Table 2.
Patient information including sex (male = M, female = F), age, treatment regimen, and lesion type for single-cell RNA-sequencing specimens highlighted in this review. Treatments indicate type of therapy received and type/order in which it was received (MTX- Methotrexate; AP- Doxorubicin + Cisplatin; IE- IFO + VP-16; IFO - Ifosulfamide; GT- Gemcitabine + Doxetaxel; VP- etoposide).
| Patient | Reference | Sex | Age | Tumor Type | Treatment Regimen | Histological Designation |
|---|---|---|---|---|---|---|
| 1 | Zhou et al. (2020), Guo et al. (2022), Huang et al. (2022) | M or F | 11–38 | Primary | 4 regimens (MTX, AP, IFO, MTX) | Conventional |
| 2 | Zhou et al. (2020), Guo et al. (2022), Huang et al. (2022) | M or F | 11–38 | Primary | 6 regimens (MTX, AP, MTX, AP, MTX, MTX) | Conventional |
| 3 | Zhou et al. (2020), Guo et al. (2022), Huang et al. (2022) | M or F | 11–38 | Primary | 3 regimens (MTX, IFO, AP) | Conventional |
| 4 | Zhou et al. (2020), Guo et al. (2022), Huang et al. (2022) | M or F | 11–38 | Primary | 3 regimens (MTX, IFO, AP) | Conventional |
| 5 | Zhou et al. (2020), Guo et al. (2022), Huang et al. (2022) | M or F | 11–38 | Metastasis (Lung) | 2 regimens (GT, GT) | Conventional |
| 6 | Zhou et al. (2020), Guo et al. (2022), Huang et al. (2022) | M or F | 11–38 | Recurrence | 3 regimens (GT, GT, GT) | Conventional |
| 7 | Zhou et al. (2020), Guo et al. (2022), Huang et al. (2022) | M or F | 11–38 | Primary | 4 regimens (IFO, AP, MTX, MTX) | Conventional |
| 8 | Zhou et al. (2020), Guo et al. (2022), Huang et al. (2022) | M or F | 11–38 | Metastasis (Lung) | 3 regimens (GT, GT, GT) | Chondroblastic |
| 9 | Zhou et al. (2020), Guo et al. (2022), Huang et al. (2022) | M or F | 11–38 | Recurrent | 4 regimens (AP, IE, AP, IE) | Chondroblastic |
| 10 | Zhou et al. (2020), Guo et al. (2022), Huang et al. (2022) | M or F | 11–38 | Primary | 4 regimens (MTX, AP, IFO, AP) | Intraosseous |
| 11 | Zhou et al. (2020), Guo et al. (2022), Huang et al. (2022) | M or F | 11–38 | Primary | 4 regimens (MTX, AP, IFO, MTX) | Chondroblastic |
| 1 | Liu et al. (2020) and Huang et al. (2022) | M | 16 | Primary | No Neoadjuvant Chemotherapy | Classical |
| 2 | Liu et al. (2020) and Huang et al. (2022) | F | 19 | Primary | No Neoadjuvant Chemotherapy | Classical |
| 3 | Liu et al. (2020) and Huang et al. (2022) | F | 45 | Primary | No Neoadjuvant Chemotherapy | Classical |
| 4 | Liu et al. (2020) and Huang et al. (2022) | M | 19 | Primary | No Neoadjuvant Chemotherapy | Classical |
| 5 | Liu et al. (2020) and Huang et al. (2022) | M | 14 | Primary | No Neoadjuvant Chemotherapy | Classical |
| 6 | Liu et al. (2020) and Huang et al. (2022) | M | 13 | Primary | No Neoadjuvant Chemotherapy | Classical |
| 1 | Wang et al. (2021) | M or F | N/A | Primary | 1 regimen (MTX, Adriamycin + Cisplatin, Adriamycin + IFO) | N/A |
Fig. 1.
Genes used to distinguish cellular clusters. (A) Genes used to differentiate and cluster by cell type from Zhou et al. (B) Genes used to differentiate and cluster by cell type from Liu et al.
Fig. 2.
Gene expression based on cell type and subcluster. (A) High and low expression of genes from scRNA-seq by cell type and subcluster from Liu et al. (B) High and low expression of genes from scRNA-seq by cell type and subcluster from Zhou et al.
3. Non-immune cells of OS lesions
3.1. Chondroblastic cells
Zhou et al. identified seven subclusters, six osteoblastic and one chondroblastic, from all three lesion types (primary, recurrent, and metastatic). First, chondroblastic cells were divided into four subclusters and were characterized by overall high expression of COL2A1, SOX9, and ACAN (Fig. 1). This differential state of chondrocytes is reflected by the structural genes (COL2A1 and ACAN) alongside the driving transcription factor SOX9. SOX9 is known to be key mediator of chondrogenesis, expressed in chondrosarcoma along with other forms of bone disease [37]. One cellular cluster was identified as proliferating malignant chondroblastic cells due to expression of TOP2A, PCNA, TYMS, and MKI67 (Fig. 2). These genes have been demonstrated to play major roles in the cell cycle. For example, TOP2A is essential for cell division and highly expressed in mitosis. Its dysregulation has been implicated in premature exit of the cell cycle [38]. TYMS and PCNA both contribute to DNA replication and repair, with TYMS displaying rate-limiting features essential for cellular proliferation [39], [40]. Further, MKI67 is expressed in interphase of mitotic cells and increases in expression during the G1 phase of the cell cycle [41]. Two other subclusters were described as hypertrophic, corresponding to elevated levels of MEF2C, PTH1R, and IHH (Fig. 2). Finally, the last subcluster, chondroblastic-trans cells, demonstrated upregulation of RUNX2, SPP1, and COL1A1, genes associated with trans-differentiation into osteoblastic cells. RUNx2 is crucial for the formation of osteoblasts as well as at later stages of chondrocyte differentiation and maturation. RUNx2 upregulates both COL1A1 and SPP1, major genes involved in the expression of bone matrix protein (BMP) during osteoblast differentiation [42], [43]. Structural genes, such as COL1A1, can often appear in more than one cellular cluster. Here, its gene expression is not only evident within chondroblastic cells, but also in cellular clusters identified as osteoblasts and fibroblasts. Since type I collagen supplies 90% of the organic component of bone matrix, and these three cellular clusters identify groups of matrix-supporting cells, overlap of structural gene expression is to be expected [44]. This overlap, however, further necessitates the identification of core or leading genes within each respective cluster/subcluster to infer differential cellular states and functions. Further, gene set enrichment analysis (GSEA) revealed higher expressions of the IL-2/STAT5, Hedgehog (Hh), and Notch pathways in the first hypertrophic subcluster. The second hypertrophic subcluster revealed elevated inflammatory signaling through expression of the IL-6/JAK/STAT3 pathway [32]. Increased levels of IL-6 are associated with the development of chronic inflammation in tissues. IL-6 also acts as a major mediator of inflammatory response by recruiting immune cells to the TME for further pro-inflammatory cytokine release [45]. The Hh pathway, with key regulatory function in tissue development and growth, has been implicated in the progression and metastasis of OS [46]. Additionally, the Notch pathway, which regulates cell-to-cell signal transduction, has been shown to promote differentiation and proliferation of mesenchymal stem cells (MSCs) by inducing BMP9/Smad signaling and upregulation of ALK2 [32], [47].
Further, Guo et al. analyzed 53,441 OS cells and clustered them into nine subclusters. Two subclusters (OS_3 and OS_8) were deemed chondroblastic while the remaining seven were considered osteoblastic. Importantly, the OS_8 cluster (chondroblastic) revealed high expression of COL6A1, COL6A3, and MIF. All three genes have been previously associated with increased metastatic potential. Both COL6A1 and COL6A3 are involved in collagen degradation and synthesis. Increased expression of COL6A1 has been implicated in lung metastasis through STAT1 activation. Interestingly, COL6A1 demonstrated the ability to transform normal fibroblasts to cancer-associated fibroblasts using cytokines associated with inflammation [48]. Further, MIF has previously shown the ability to activate the RAS/MAPK pathway leading to both migratory and proliferative capacity of OS cells [49]. The GSEA analysis performed by Guo et al. revealed that the OS_8 cluster contained more aggressive cells. The aggressive nature of this cluster was associated with the upregulation of PI3K-AKT signaling [33]. MIF-induction in melanoma cells has been associated with increased proliferative capacity through direct activation of the PI3K-AKT pathway [50]. PI3K-AKT activation in OS_8 cells, possibly downstream of MIF, further indicates the metastatic nature of this cluster [51].
3.2. Osteoblastic cells
Further, Guo et al. identified seven osteoblastic subclusters. While Guo et al. associated the previous chondroblastic cluster OS_8 with metastasis, the highlighted osteoblastic clusters (OS_6 and OS_7) showed differing metastatic potential. OS_6 and OS_7 expressed the genes CADM1, LINC00662, and RUVBL1, previously associated with inhibited OS metastasis. Inhibition of the PI3K-AKT pathways, as seen in both OS_6 and OS_7, corresponded to enhanced anti-tumor activity, impaired tumor growth, and reduced migratory/metastatic potential. As a result, the OS_6 and OS_7 osteoblastic clusters were identified as less aggressive cells with limited metastatic potential [33].
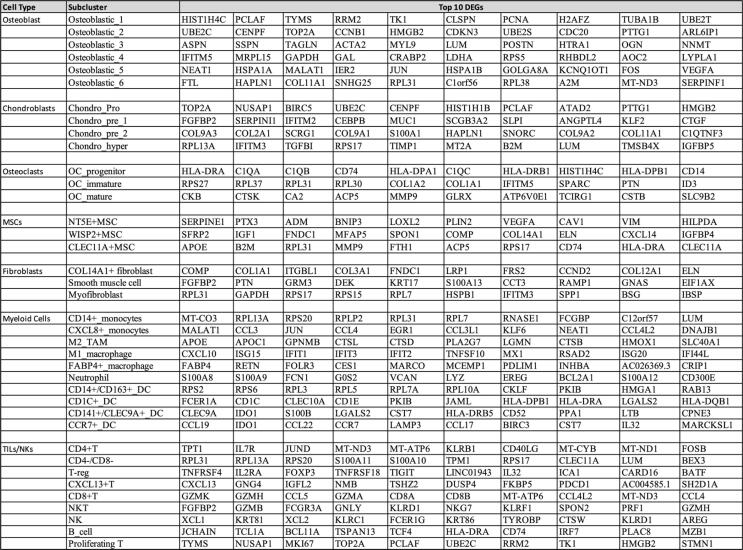
Zhou et al. subdivided the six osteoblastic subclusters by their proliferative nature and expression patterns. Two osteoblastic clusters (osteoblastic_1, osteoblastic_2) displayed higher levels of proliferation markers MKI67, PCNA, and TOP2, mirroring expression of proliferation in the chondroblastic populations (Fig. 2). Of these two proliferative clusters, there were measurable differences in their differentially expressed genes (DEGs). While osteoblastic_1 was found to express a higher level of mitotic S phase genes, osteoblastic_2 expressed increased levels in G2/M phase genes. The remaining osteoblastic clusters varied considerably in their signaling pathways. Interestingly, osteoblastic subcluster 3 revealed increased levels of interferon-gamma (IFN-γ), interferon alpha (IFN-α), and angiogenesis pathways. As for the remaining clusters, osteoblastic_4 revealed an increased level of oxidative phosphorylation and MYC signaling pathways; osteoblastic_5 showed elevated signaling pathways including P53, KRAS, transforming growth factor beta (TGF-β), and hypoxia; and osteblastic_6 exhibited pathways enriched in inflammation, immune rejection, and myogenesis [32]. These findings ultimately convey the highly heterogenous nature of osteoblastic cells within these lesions (Fig. 3).
Fig. 3.
Top 10 differentially expressed genes (DEGs) separated by cellular subclusters as identified by Zhou et al. DEG analysis identified the percent of cells positive for these indicated genes within each cluster. The percent positive cells within each cluster were then compared to cellular positivity in other clusters.
Importantly, Zhou et al. briefly indicated the top DEGs differing between metastatic and recurrent lesions. In recurrent OS lesions, genes consisting of MT1X, GADD45B, HAPLN1, and CYR61 were enriched alongside hypoxia, IL2-STAT5, and mTORC1 pathways. These pathways in recurrent OS specimens suggest a possible role in chemotherapeutic resistance and the recurrent nature of these lesions. However, in metastatic lung lesions, genes consisting of S100A11, S100A10, PRDX2, and PSMD4 were among the top expressed genes [32]. These genes are associated with metastasis and tumorigenesis in other cancers. In ovarian cancer cells, S100A10 has been implicated as an oncogene involved in proliferation and metastasis. Suppression of this gene indicates a more favorable outlook [52] Additionally, whereas PSMD4 overexpression in breast cancer is associated with poorer prognoses, cell growth is inhibited in PSMD4 knockdown models [53]. Contrary to the recurrent lesions, the pathways present in metastatic lesions included hypoxia, TGF-β, MYC, ROS, and oxidative phosphorylation. Tumor hypoxia leads to epithelial-mesenchymal transition (EMT), resulting in the ability for cancer cells to “turn-off” their epithelial features and activate mesenchymal ones. Undergoing EMT results in the ability of cellular migration from the primary site, increasing probability of metastasis [54], [55]. Although TGF-β is involved in many normal physiological roles, even tumor suppression, it has been demonstrated that overexpression of TGF-β in the TME is a hallmark of many cancers. Overexpression of TGF-β has been linked to metastasis. Its expression not only promotes immune suppression but drives both angiogenesis and tumor cell plasticity [56]. Verrecchia et al. discussed the failure of TGF-β to inhibit MSC proliferation in OS, further alluding to TGF-β’s pro-tumoral tendencies in OS [56]. As was indicated previously, MYC is implicated in OS progression by promoting cell division through activation of the MEK-ERK pathway. This activation is then associated with the upregulation of MMP-2/9 expression [15], [32]. MMP-2/9 have been associated with a poor prognosis in different cancer types, including OS. Its expression is associated with increased metastatic potential due to this enzyme’s ability to degrade both the basement membranes and extracellular matrices [57], [58], [59]. Liu et al. demonstrated upregulation of MMP-9 in bone metastases, where increased MMP-9 expression in the metaphysis was associated with increased cellular proliferation [60].
Further, Liu et al. identified five subclusters of osteoblastic cells (identified C1-C5) via uniform manifold approximation and projection (UMAP) analysis. This group further characterized these clusters by both marker genes and gene set variation analysis (GSVA) (Fig. 2). C1 cells expressed cell markers IL-17R, IL-4, and TNFSF11 and were associated with inflammation. Like Zhou et al., C2 was identified as a proliferative population containing an increased expression of TOP2A, CENPF, and CDK1, indicating cell-cycle proliferation. CDKs are involved in every part of the cell cycle from interphase to M−phase to cell cycle exit. CDK1 drives the cell cycle through G2-phase and mitosis and is crucial for cellular proliferation [61]. Further, C3 and C4 were associated with pathways of cellular metabolism (glucose catabolism and carbohydrate transport) and extracellular matrix (ECM) maintenance, with C3 expressing increased levels of both arginase-II and arginosuccinate synthetase-II (ASS) [34]. Both genes are implicated in metabolic pathways and immune responses, including negative regulation of CD4+ and CD8+ T cells, decreased tumor necrosis factor (TNF) signaling, as well as decreased interleukin (IL)-13,17 production [62]. The C4 cluster expressed elevated levels of ECM1P2 and COL18A1 alongside pathways involved in ECM regulation and collagen formation. In addition, the C5 cluster revealed increased expression of ossification markers SMPD3 and SOSTDC1, indicating trabecula morphogenesis and formation as well as replacement ossification. Upon analyzing the TARGET OS database, 518 genes were obtained and used to conduct a survival analysis. It was revealed that C1 and C5 subclusters displayed increased expression of 85 genes associated with poorer survival, with C5 containing the greatest expression. Survival analysis of the C5 cluster also revealed increased expression of SP7, previously implicated in the differentiation of mesenchymal stem cells (MSCs) into osteoblasts as well as the creation of treatment resistant tumors in breast cancer through lymphatic metastasis [34].
3.3. Osteoclasts (OC)
Osteoclasts (OCs), along with osteoblasts and osteocytes, are the three main cell types that make up bone tissue. In normal bone, the balance of osteoblasts and osteoclasts is crucial to bone homeostasis. While OCs play an essential role in osteolysis in normal bone, they are associated with tumor growth in OS tumors. In OS, genetic alterations occur causing incomplete differentiation of this cell population, leading to homeostatic imbalance and uncontrolled proliferation [63]. In general, unregulated osteoclast activity promotes cancer cell survival and proliferation. Together, Zhou et al. and Liu et al. identified a total of seven clusters of OCs. First, Zhou et al. identified three unique clusters: progenitor, immature, and mature OCs (Fig. 2). The progenitor cluster revealed expression of the myeloid markers CD74 and CD14, along with decreased levels of CTSK and ACP5. This phenotype appeared hyperproliferative due to its association with increased expression of TOP2A, a marker involved in cell cycle pathways and DNA binding. These data indicate that the progenitor OC cluster was likely influenced by osteogenic stimulation. Further, the immature cluster was positive for both OC and myeloid-lineage markers. Conversely, the mature cluster showed an inverse relationship, expressing high CTSK/ACP5 and low CD74/CD14 [32]. In mature osteoclasts, CTSK is secreted and involved in degradation of collagen during resorption of bone. ACP5 is needed for activation of osteopontin downstream of tartrate-resistant acid phosphatase type 5 (TRAP) enzyme formation. Osteopontin is required for osteoclast binding and later resorption of bone [64], [65].
Liu et al. identified four main clusters of OCs. Like Zhou et al., Liu et al. characterized cluster one as progenitor OCs by their elevated levels of CD74 and CD14 in addition to HLA-DRA and MKI67. Again, cluster two was characterized as mature OCs due to the expression of CTSK and ACP5 (Fig. 1). The two remaining clusters were termed hypo- and nonfunctional OCs. Interestingly, OCs were detected in both the metastatic lung and recurrent lesions, showing their importance in the TME of advanced OS [34]. Zhou et al. also revealed that compared to primary lesions, the proportion of mature OCs was decreased in both metastatic lung and recurrent OS lesions. Additionally, genes related to interferon response, antigen presentation, cell-cycle checkpoints, and even stem cell differentiation were decreased within this population. Conversely, genes associated with OC differentiation and bone remodeling/reabsorption were increased [32]. The significance of OC presence in recurrent and metastatic lesions likely requires further investigation.
3.4. Cancer-Associated Fibroblasts (CAFs)
In normal tissue and bone, fibroblasts are the most abundant cell type located in the stoma. These cells of mesenchymal origin, involved in a variety of functions pertaining to all stages of wound healing and inflammation, migrate to sites of injury through signaling by growth factors and cytokines [66]. Fibroblasts, while crucial in maintaining homeostasis of the extracellular matrix for tissues and organs, are influenced by cancerous cells within the TME. Cancer-associated fibroblasts (CAFs), a highly heterogeneous cell population in the TME of solid tumors, are associated with tumor progression, growth, and metastasis. In many cancers, increases in CAFs within the TME, alongside their ability to promote angiogenesis and immunosuppression, is correlated with worsening prognosis [67]. Zhou et al. identified three clusters of CAFs characterized by their DEGs. In general, all three clusters expressed elevated levels of LUM and COL1A1 (Fig. 1). Cluster one was characterized by the increased expression of COL14A1, demonstrating its association with COL14A1 + matrix fibroblasts. Cluster two expressed markers associated with smooth muscle activity, DES, along with decreased levels of ACTA2 and COL14A1. Finally, CAF cluster three displayed increased expression of MYL9 and LUM alongside ACTA2. It is important to note that this cluster showed no expression of DES and COL14A1, genes involved in collagen binding and ECM activity. These functions are inherently normal for fibroblasts [32], [62] and likely indicate the presence of a dysfunctional CAF population within these tumors. Notably, clusters one and three appeared to be the major CAFs in both primary and recurrent lesions. The seemingly dysfunctional CAFs in cluster two (lacking ACTA2 and COL14A1 expression) were predominately isolated from metastatic lung lesions [32]. Recently, ACTA2 expression in CAFs of gastric tumors was implicated as a positive indicator of immune checkpoint inhibitor (ICI) response [68]. Therefore, the increased presence of cluster two CAFs, with decreased expression of ACTA2, within metastatic lung lesions could indicate both ICI therapeutic resistance and poorer patient prognosis.
Similarly, Liu et al. identified three clusters of CAFs via UMAP analysis. Although both investigators identified common CAF markers, Liu et al. revealed that cluster one was also associated with MMP9 and MCAM expression, genes linked to tumor angiogenesis and invasion. Much like Zhou et al, Liu et al. also implicated CAF cluster two to be involved in osteoblast proliferation and ossification as evidenced by expression of OMD and OGN. Analysis of cluster three revealed expression of TOP2A and MKI67 (Fig. 1), genes associated with the cell cycle and proliferation [34]. Interestingly, Huang et al., using single-cell data sets from both Zhou et al. and Lui et al., identified a particular CAF cluster within recurrent OS lesions through GSEA. This CAF cluster exhibited increased EMT signaling and thus improved infiltration when compared to CAFs from primary lesions [36]. Ultimately, recurrent lesions are more likely to contain infiltrative CAFs with more aggressive tumor-promoting phenotypes. These CAFs could possibly be a target of next-generation therapeutics for treatment of locally recurrent OS disease.
3.5. Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells (MSCs) are non-hematopoietic precursor cells that, under normal conditions, are characterized by their multipotent nature and ability to differentiate into adipocytes, chondrocytes, and osteoblasts [69]. These cells play a crucial role in generating and repairing skeletal tissues. However, in the TME of OS, MSCs have long been implicated in drug resistance, metastatic potential, and tumor cell proliferation [70]. MSCs facilitate metastasis through the secretion of exosomes known to interact with cancer cells, influencing their ability to both proliferate and migrate. Zhou et al. identified MSCs based on the markers CD10, CD90, and CXCL12, with three MSC clusters then identified by differential expression of NT5E, WISP2, and CLEC11A (Fig. 2). The cluster characterized by NT5E expression was shown to be predominant in chondroblastic lesions and proposed to be associated with stimulation of angiogenesis and metastasis of tumor cells. This association was particularly due to their expression of VEGFA and TGFBI. OS patient tumors positive for VEGFA expression had poorer tumor-free survival as compared to patients with VEGFA negative tumors [71]. WISP2 + MSCs revealed high expressions of WISP2 as well as CXCL14 [32]. In previous studies, a secreted protein of WISP2 MSCs promoted further proliferation of MSCs, while the presence of CXCL14 was suggested to promote metastasis of OS cells [72]. Cluster three revealed increased expression of CLEC11A in conjunction with elevated levels of SSP1 and IBSP, differentiation markers of osteoblasts. Osteolectin, an associated protein with CLEC11A, particularly promotes differentiation into mature osteoblasts for bone maintenance [32]. Additionally, Wang et al. completed unsupervised trajectory analysis of chemo-resistant primary OS tumors. This analysis ultimately revealed that stem-like progenitor cells expressing the endothelial marker CD117 developed further into COL8A1 expressing chondrocyte-like cells enriched with both JMJD3 and MYC-associated VEGFR2. This MSC subset, characterized by JMJD3 and VEGFR2 positivity, was associated with chemo-resistant OS tumors [35]. In the future, identification of these MSCs within OS lesions could indicate chemotherapy resistance and necessity for differential therapy regimens in this patient subset.
4. Immune Cells of OS Lesions
4.1. Myeloid Cells: Tumor Associate Macrophages (TAMs), Monocytes, and Dendritic Cells (DCs)
Myeloid cells are yet another crucial immune component responsible for the vast heterogeneity of the OS TME. These granulocytic (neutrophils) and monocytic (monocytes) cells differentiate from hematopoietic stem cells within bone marrow. Upon their release into circulation, myeloid cells are recruited to tissues by the chemokine receptor axis where they play a major role in innate immune response through phagocytosis and release of inflammatory cytokines. Importantly, while some recruited monocytes transform into monocyte-derived macrophages, most tissue resident macrophages are fetal liver- or yolk sac-derived. Alongside monocytes, macrophages, and neutrophils, conventional dendritic cells, a myeloid lineage cell responsible for antigen presentation, play an integral role in myeloid-lymphoid interactions [73].
Zhou et al. identified ten unique clusters of myeloid cells represented as macrophages, monocytes, dendritic cells (DCs), and neutrophils (Fig. 2). Monocytes and macrophages accounted for 80% of the total myeloid cells that were identified. Firstly, tumor-associated macrophages (TAMs) were divided into three clusters, M1, M2, and M3 (Fig. 2). TAMs are known to play roles in both inflammatory response as well as tissue homeostasis. Infiltration of TAMs in solid tumors has correlated with poorer prognoses [17], [74]. The M1 cluster was characterized by the expression of inflammatory chemokines CCL2, CCL3, CCL4, CXCL2, and CXCL3. These inflammatory chemokines are known to attract natural killer (NK) cells, T cells, and DCs. Further, GSEA analysis revealed that M1-TAMs were likely under IFN-α and IFN-γ stimulation, suggesting that they were derived from a pro-inflammatory environment. Interestingly, M1-TAMs also demonstrated elevated expression of the TGF-β and Hh pathways, indicating a possible induction to an M2-like polarization. This induction of M1-like macrophages toward an M2-like phenotype was further supported by GSVA analysis which indicated that a portion of M2-TAMs also displayed high expression of M1 markers. Furthermore, the M2 cluster revealed increased expression of CD163, MRC1, MSA4, and MAF [32]. These macrophages were deemed the prominent anti-inflammatory TAMs and, unsurprisingly, were the dominant macrophage of metastatic lung lesions [75], [76]. Interestingly, the presence of CD163+ TAMs have been associated with increased T-cell suppression. This T-cell suppression is likely associated with the increased expression of PD-1 and TIM-3, markers of T-cell exhaustion. Decreases in CD163+ TAMs have also been associated with improved T-cell function [77]. M3-TAMs, identified by FABP4 positivity, were suggested to be alveolar macrophages isolated from lung specimens. These M3-TAMs displayed high levels of M1 markers, suggesting a possible role of these macrophages in the creation of the predominately pro-inflammatory environment of metastatic OS lesions. Lastly, isolated neutrophils were characterized by the expression of S100A8, S100A9, and GOS2. Primary OS lesions were associated with greater neutrophil infiltration than both recurrent and metastatic lesions. Zhou et al. also identified DCs, dividing them into four clusters characterized by CD14 + CD163+, cDC2, cDC1, and CCR7+ (Fig. 2). cDC1 DCs were found at a higher ratio in metastatic lung lesions than primary and recurrent lesions. This dendritic cell population has been identified as a possible therapeutic target due to its important role in antigen presentation to lymphoid cells [32]. CCR7 expression is associated with survival, chemotaxis, and endocytosis of DCs, proposing a role for this CCR7 + DC population in tumor metastatic potential [78], [79].
Through unsupervised clustering, Liu et al. divided 13,025 myeloid cells into twelve unique subclusters, including two monocytic, six macrophage, and two dendritic clusters. The six clusters of macrophages were separated based on positive expression of FABP5, NR4A3, TXNIP, IFIT1, MCM5, and MKI67 (Fig. 2). The FABP + cluster, characterized by expressions of APOC1, APOE, LGMN, and FABP5, demonstrated increased expression of genes associated with lipid metabolism. Interestingly, the NR4A3 + cluster expressed a hybrid phenotype with markers related to both M1 and M2 macrophages, including CCL3, CCL4, TNF, and CD163. This finding, again, indicates the highly plastic nature of the TAM population. A third cluster, identified by expression of TXNIP along with M2 markers MERTK, MRC1, STAB1, and CD163, were denoted to be the conventional anti-inflammatory M2-polarized macrophages within these lesions. The fourth cluster expressed elevated IFN signaling and pro-inflammatory genes CCL2/3/4, CXCL9, CXCL10, and TNF, likely the result of IFN stimulation within the lesions. An MKI67 + cluster, identified by the presence of integral cell-cycle genes, indicates a cluster of tissue resident macrophages with the ability to proliferate [34]. Whether inhibition or activation of this proliferative capacity could enhance or deter disease progression was not elucidated but deserves further investigation.
4.2. Tumor Infiltrating Lymphocytes (TILs)
Tumor-infiltrating lymphocytes (TILs) are vital components of the TME. TILs are proving to be critical targets of checkpoint blockade immunotherapies. TIL levels within the TME often predict both response and clinical outcome [80]. Zhou et al. identified 5,420 TILs placed into eight unique clusters characterized as CD4- CD8- T cells, CD8 + T cells, CD4 + T cells, T regulatory (Treg) cells, proliferating T cells, NK and NKT cells, as well as B cells (Fig. 2). Lower overall proportions of CD4 and CD8 cells were detected in recurrent and metastatic lesions, suggesting that T cell-targeted immunotherapies could be insufficient in this patient population. The CD8 + subcluster was characterized by the expression of markers of cytotoxicity, including GZMB, GZMK, and GZMH [32]. Interestingly, these cells also expressed exhaustion markers TIGIT and LAG3, with TIGIT being widely expressed across numerous cell types [81]. In addition, CD4 + T cells expressed costimulatory molecules TNFRSF14, TNFRSF25, and ICOS5, alongside the cytotoxicity gene GZMA. The expression of cytotoxic genes by CD4 + correlates with their ability to stimulate the cytotoxic activities of neighboring T cells. Like the CD8 + cluster, CD4 + T cells also widely expressed TIGIT [32]. Importantly, the TIGIT receptor plays a critical role in suppressing both adaptive and innate immune functions of T cells. Upregulation of this marker suggests an exhaustive immune phenotype, impeded T cell-tumor interaction, reduced T cell-dendritic cell interactions, and limited effector function [82]. Further, Tregs were characterized by the expression of FOXP3 and IL2RA. Enhanced expression of CTLA4 and TIGIT on these Tregs indicated suppression of anti-tumor immune responses in OS. Within the NKT cluster, analysis revealed cellular expression of GZMB, GZMA, and IFN-γ, indicating anti-tumor cytotoxic activities [32]. These tumor tissues were subjected to neoadjuvant cytotoxic chemotherapy before their analysis which likely indicates why TIL levels only constituted approximately 5% of all collected cells.
Liu et al., analyzing the same specimens, identified 5,239 TILs and divided them into five clusters by signature markers, dimensionality reduction, and clustering. The clusters were characterized similarly to the analysis of Zhou et al. and included CD8+, CD8-CD4-, NK, Treg, and mast cells. The CD8 + cluster, making up half of the TILs identified, was further subclustered into three additional groups (Fig. 2). CD8 + cluster one revealed an increased expression of JUND and FOSB with a lower expression of cytotoxicity genes. This expression profile was used to identify this subcluster as an early-stage CD8 + T cell. CD8 + cluster two was identified as cytotoxic T lymphocytes characterized by elevated expressions of genes involved in immune checkpoint pathways including PDCD1, CTLA4, LAG3, and TIGIT. This cluster also expressed CXCL13, ITGAE, and ITGA1, supporting likely T-cell exhaustion. The final CD8 + subcluster, characterized by expression of CD69, costimulatory genes, as well as genes associated with TNF secretion, was identified as an activated, cytotoxic CD8 + phenotype. This group also described a prominent B cell cluster. The 1,443 B cells were identified and placed into five subclusters. Clusters zero and four were identified as antibody secretory cells (expressing MZB1 and SDC1/CD138). B cell cluster two was identified as follicular B cells expressing MS4A1/CD20 and CD79A/B. Importantly, these follicular B cells are often found in the lymphoid follicles of tertiary lymphoid structures (TLSs) within OS lesions. Additionally, a naïve CD27- B cell population, exhibiting both IgD and IgM characteristics, was identified. This phenotype was associated with this population’s ability to migrate through germinal centers within these lesions [34]. TLSs have become an important marker of favorable outcomes, including immunotherapy response, in numerous cancers [83]. Further elucidation of TLS’s role in OS primary, recurrent, and metastatic lesions is warranted as presence of these structures could, in part, act as a clinical marker of immunotherapy candidacy [84].
5. Conclusion
This review details the efforts of five groups who characterized primary, recurrent, and metastatic OS lesions through scRNA-seq. We have summarized their findings into what we believe is the first comprehensive single-cell atlas in the field of OS, as determined by scRNA-seq. Analysis of these works revealed those gene expression patterns possibly relating to recurrent and metastatic potential. Identification of these patterns ultimately indicates possible targets for next-generation therapeutics. It appears that an initial pro-inflammatory response to tumor proliferation is present. Eventually, however, the surrounding microenvironment (including resident and infiltrating immune cells) becomes suppressed and exhausted. One postulation of why this occurs is that rapid growth of tumor cells, which then dominate cytokine and chemokine signaling within the TME, thwart potential immune responses by driving surrounding immune cells into an exhausted, nonfunctional state.
Guo et al. revealed the importance of the PI3K-AKT pathway in aggressive osteoblasts. Here, activation of this pathway produced a more aggressive osteoblast population with higher metastatic potential [33]. Zhou et al. identified the activation of both the Hh and Notch pathways within chondroblastic OS cells. Increased signaling of these pathways was previously implicated in metastasis and tumor progression. Identification of these populations in tumor specimens could help determine metastatic potential and ultimately guide therapeutic strategies within this patient population [32]. Further, Zhou et al. analyzed the top 20 DEGs by cellular subcluster, identifying the percent of cells positive for these indicated genes within each cluster (Fig. 3). The percent positive cells within each cluster were then compared to cellular positivity in other clusters. This subcluster analysis would have been even more powerful if each subcluster’s percent positivity were then associated with overall survival rates and clinical outcomes data [32]. Re-analysis of these DEGs, stratifying differences in DEG positivity amongst each subcluster to overall outcomes data, is warranted. Finally, both Liu et al. and Zhou et al. revealed the expression of TIGIT across numerous immune cell populations including CD4 + helper T cells, CD8 + cytotoxic T cells, as well as NK cells. Expression of TIGIT alongside LAG3 ultimately indicated the presence of T-cell exhaustion within the OS TME [32], [34]. Future studies inhibiting these surface receptors to enhance the cytotoxic activity of T cells, while preventing immunologic exhaustion, are warranted and are currently underway [85], [86], [87], [88].
Limitations ultimately exist in this reviewed literature. Differences in downstream data analysis led to discrepancies in the number of subclusters identified within each population, even with investigators analyzing the same data sets. Low agreement between clustering methods is a well-understood phenomena within the field and is associated with the high dimensionality of single-cell data [89]. For example, Liu et al. combined UMAP and GSVA for subcluster analysis [34]. Huang et al., analyzing the same data set, identified the top highly variable genes (HVGs) from the normalized expression matrix before performing a principal component analysis (PCA) [36]. A recent study investigated the use of several dimension reduction methods including PCA, UMAP, and t-distributed stochastic neighbor embedding (t-SNE) on single-cell RNAseq data sets. While PCA performed better than UMAP with regard to accuracy, UMAP exhibited superior stability over PCA in the setting of increased dropout rates [90]. While the use of different dimensionality reduction methods can ultimately confound cluster identification, comparisons between investigators is still warranted. Further, subjectivity in what is defined as a biologically relevant population can confound data analysis, particularly when identifying low abundance populations within larger cellular clusters [89]. It is also important to recognize the clinical differences between the specimens analyzed within this review. While Zhou et al. analyzed primary, recurrent, and metastatic lesions from patients receiving different treatments and with different histological designations [32], Liu et al. focused their analysis on primary lesions from patients with classical OS who had not received neoadjuvant therapy (Table 2) [34]. Numerous single-cell analyses have highlighted the effects of chemotherapy and immunotherapy, as well as the timing of sample collection during these therapeutic regimens, on both immune and non-immune cell populations [91], [92], [93], [94], [95], [96]. Therefore, identifying the clinical differences between those specimens analyzed within single-cell data sets is ultimately necessary for complete understanding of the prevalent cellular clusters examined.
These findings highlight the importance of single cell analysis in the field of cancer research. While the discussed studies successfully deployed scRNA-seq, processing and degradation of tissues into a single-cell suspension with this technique ultimately results in the loss of the tissue’s spatial architecture. Future investigations of the OS TME could also include techniques that combine proteomic and transcriptomic analysis while preserving tissue integrity. Spatial analysis would provide further insight into the cellular and stromal interactions within these tissues. For example, spatial RNA sequencing (spRNA-seq) through the 10X Genomics Visium system combines the transcriptomic abilities of RNA-seq with in situ hybridization to reveal the complex tissue organization within the TME. This tissue organization can then be analyzed for its potential influence on cellular communication, immune cell interactions, evasion, and metastasis [97], [98]. Through the identification of cellular neighborhoods, integral signaling networks between cell populations such as the proliferative osteoblastic cancer cells and surrounding macrophages and/or exhausted T cells can then be identified. Combining spRNA-seq with findings from these scRNA-seq studies could enhance our understanding of the OS TME while furthering the development of diagnostic tools to predict both metastatic potential and patient-specific therapeutic response.
Although scRNA-seq has been crucial for the discovery of complex gene expression patterns and cellular population changes within tumor microenvironments, its application in the clinical setting has not yet been fully determined. Advances in scRNA-seq, much like other sequencing technologies over the last two decades, will likely result in increased sample throughput alongside decreased cost and analysis complexity. While this form of analysis has yet to transition from bench-to-bedside, future translational capabilities are ultimately apparent and warrant continued investigation.
CRediT authorship contribution statement
Dylan D. Thomas: Formal analysis, Writing – original draft, Writing – review & editing. Ryan A. Lacinski: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing, Visualization. Brock A. Lindsey: Conceptualization, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
There are no acknowledgments for this review article.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data sets highlighted in this review are available on the Gene Expression Omnibus (GEO) at accession codes GSE152048 and GSE162454
References
- 1.Gill J., Gorlick R. Advancing therapy for osteosarcoma. Nat. Rev. Clin. Oncol. 2021;18(10):609–624. doi: 10.1038/s41571-021-00519-8. [DOI] [PubMed] [Google Scholar]
- 2.Yang C., Tian Y., Zhao F., Chen Z., Su P., Li Y., Qian A. Bone Microenvironment and Osteosarcoma Metastasis. Int. J. Mol. Sci. 2020;21(19):6985. doi: 10.3390/ijms21196985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.C.D.M. Fletcher, K.K. Unni, F. Mertens, Pathology and genetics of tumours of soft tissue and bone, 2002.
- 4.Messerschmitt P.J., Garcia R.M., Abdul-Karim F.W., Greenfield E.M., Getty P.J. Osteosarcoma. JAAOS – J. Am. Acad. Orthopaed. Surgeons. 2009;17(8):515–527. doi: 10.5435/00124635-200908000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Fox M.G., Trotta B.M. Osteosarcoma: review of the various types with emphasis on recent advancements in imaging. Semin. Musculoskelet. Radiol. 2013;17(2):123–136. doi: 10.1055/s-0033-1342969. [DOI] [PubMed] [Google Scholar]
- 6.Corre I., Verrecchia F., Crenn V., Redini F., Trichet V. The Osteosarcoma Microenvironment: A Complex But Targetable Ecosystem. Cells. 2020;9(4) doi: 10.3390/cells9040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaspar N., Marques Da Costa M.E., Fromigue O., Droit R., Berlanga P., Marchais A. Recent advances in understanding osteosarcoma and emerging therapies. Faculty Reviews. 2020;9 doi: 10.12703/r/9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luetke A., Meyers P.A., Lewis I., Juergens H. Osteosarcoma treatment – Where do we stand? A state of the art review. Cancer Treat. Rev. 2014;40(4):523–532. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Cascini C., Chiodoni C. The Immune Landscape of Osteosarcoma: Implications for Prognosis and Treatment Response. Cells. 2021;10(7) doi: 10.3390/cells10071668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 11.Hainaut P., Pfeifer G.P. Somatic TP53 Mutations in the Era of Genome Sequencing. Cold Spring Harb. Perspect. Med. 2016;6(11) doi: 10.1101/cshperspect.a026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czarnecka A.M., Synoradzki K., Firlej W., Bartnik E., Sobczuk P., Fiedorowicz M., Grieb P., Rutkowski P. Molecular Biology of Osteosarcoma. Cancers (Basel) 2020;12(8) doi: 10.3390/cancers12082130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rickel K., Fang F., Tao J. Molecular genetics of osteosarcoma. Bone. 2017;102:69–79. doi: 10.1016/j.bone.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.W. Feng, D.C. Dean, F.J. Hornicek, D. Spentzos, R.M. Hoffman, H. Shi, Z. Duan, Myc is a prognostic biomarker and potential therapeutic target in osteosarcoma, Therapeutic Advances in Medical Oncology 12 (2020) 1758835920922055. [DOI] [PMC free article] [PubMed] [Retracted]
- 15.Han G., Wang Y., Bi W. C-Myc overexpression promotes osteosarcoma cell invasion via activation of MEK-ERK pathway. Oncol. Res. 2012;20(4):149–156. doi: 10.3727/096504012x13522227232237. [DOI] [PubMed] [Google Scholar]
- 16.Vaziri N., Shariati L., Zarrabi A., Farazmand A., Haghjooy Javanmard S. Cancer-Associated Fibroblasts Regulate the Plasticity of Breast Cancer Stemness through the Production of Leukemia Inhibitory Factor. Life (Basel) 2021;11(12) doi: 10.3390/life11121298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cersosimo F., Lonardi S., Bernardini G., Telfer B., Mandelli G.E., Santucci A., Vermi W., Giurisato E. Tumor-Associated Macrophages in Osteosarcoma: From Mechanisms to Therapy. Int. J. Mol. Sci. 2020;21(15) doi: 10.3390/ijms21155207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen B., Khodadoust M.S., Liu C.L., Newman A.M., Alizadeh A.A. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol. Biol. 2018;1711:243–259. doi: 10.1007/978-1-4939-7493-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G., Ning B., Shi T. Single-Cell RNA-Seq Technologies and Related Computational Data Analysis. Front. Genet. 2019;10:317. doi: 10.3389/fgene.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y., Ma L., Wu D., Chen G. Advances in bulk and single-cell multi-omics approaches for systems biology and precision medicine. Brief. Bioinform. 2021;22(5):bbab024. doi: 10.1093/bib/bbab024. [DOI] [PubMed] [Google Scholar]
- 21.Huang R.H., Wang L.X., He J., Gao W. Application and prospects of single cell sequencing in tumors. Biomark Res. 2021;9(1):88. doi: 10.1186/s40364-021-00336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia Q., Chu H., Jin Z., Long H., Zhu B. High-throughput single-small es, Cyrillicell sequencing in cancer research. Signal Transduct. Target. Ther. 2022;7(1):145. doi: 10.1038/s41392-022-00990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stegle O., Teichmann S.A., Marioni J.C. Computational and analytical challenges in single-cell transcriptomics. Nat. Rev. Genet. 2015;16(3):133–145. doi: 10.1038/nrg3833. [DOI] [PubMed] [Google Scholar]
- 24.Buettner F., Natarajan K.N., Casale F.P., Proserpio V., Scialdone A., Theis F.J., Teichmann S.A., Marioni J.C., Stegle O. Computational analysis of cell-to-cell heterogeneity in single-cell RNA-sequencing data reveals hidden subpopulations of cells. Nat. Biotechnol. 2015;33(2):155–160. doi: 10.1038/nbt.3102. [DOI] [PubMed] [Google Scholar]
- 25.Whitley S.K., Horne W.T., Kolls J.K. Research Techniques Made Simple: Methodology and Clinical Applications of RNA Sequencing. J, Invest. Dermatol. 2016;136(8):e77–e82. doi: 10.1016/j.jid.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Kim N., Kim H.K., Lee K., Hong Y., Cho J.H., Choi J.W., Lee J.I., Suh Y.L., Ku B.M., Eum H.H., Choi S., Choi Y.L., Joung J.G., Park W.Y., Jung H.A., Sun J.M., Lee S.H., Ahn J.S., Park K., Ahn M.J., Lee H.O. Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nat. Commun. 2020;11(1):2285. doi: 10.1038/s41467-020-16164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng W., He M., Jiang X., Liu H., Xie T., Qin Z., Huang Q., Liao S., Lin C., He J., Xu J., Ma J., Liu Y., Wei Q. Single-Cell RNA Sequencing Reveals the Migration of Osteoclasts in Giant Cell Tumor of Bone. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.715552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee H.W., Chung W., Lee H.O., Jeong D.E., Jo A., Lim J.E., Hong J.H., Nam D.H., Jeong B.C., Park S.H., Joo K.M., Park W.Y. Single-cell RNA sequencing reveals the tumor microenvironment and facilitates strategic choices to circumvent treatment failure in a chemorefractory bladder cancer patient. Genome Med. 2020;12(1):47. doi: 10.1186/s13073-020-00741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung W., Eum H.H., Lee H.O., Lee K.M., Lee H.B., Kim K.T., Ryu H.S., Kim S., Lee J.E., Park Y.H., Kan Z., Han W., Park W.Y. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat. Commun. 2017;8:15081. doi: 10.1038/ncomms15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng J., Sun B.F., Chen C.Y., Zhou J.Y., Chen Y.S., Chen H., Liu L., Huang D., Jiang J., Cui G.S., Yang Y., Wang W., Guo D., Dai M., Guo J., Zhang T., Liao Q., Liu Y., Zhao Y.L., Han D.L., Zhao Y., Yang Y.G., Wu W. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 2019;29(9):725–738. doi: 10.1038/s41422-019-0195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen K., Wang Q., Li M., Guo H., Liu W., Wang F., Tian X., Yang Y. Single-cell RNA-seq reveals dynamic change in tumor microenvironment during pancreatic ductal adenocarcinoma malignant progression. EBioMedicine. 2021;66 doi: 10.1016/j.ebiom.2021.103315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y., Yang D., Yang Q., Lv X., Huang W., Zhou Z., Wang Y., Zhang Z., Yuan T., Ding X., Tang L., Zhang J., Yin J., Huang Y., Yu W., Wang Y., Zhou C., Su Y., He A., Sun Y., Shen Z., Qian B., Meng W., Fei J., Yao Y., Pan X., Chen P., Hu H. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-20059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo J., Tang H., Huang P., Guo J., Shi Y., Yuan C., Liang T., Tang K. Single-Cell Profiling of Tumor Microenvironment Heterogeneity in Osteosarcoma Identifies a Highly Invasive Subcluster for Predicting Prognosis. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.732862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Feng W., Dai Y., Bao M., Yuan Z., He M., Qin Z., Liao S., He J., Huang Q., Yu Z., Zeng Y., Guo B., Huang R., Yang R., Jiang Y., Liao J., Xiao Z., Zhan X., Lin C., Xu J., Ye Y., Ma J., Wei Q., Mo Z. Single-Cell Transcriptomics Reveals the Complexity of the Tumor Microenvironment of Treatment-Naive Osteosarcoma. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.709210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L., Huang X., You X., Yi T., Lu B., Liu J., Lu G., Ma M., Zou C., Wu J., Zhao W. Nanoparticle enhanced combination therapy for stem-like progenitors defined by single-cell transcriptomics in chemotherapy-resistant osteosarcoma. Signal Transduct. Target. Ther. 2020;5(1) doi: 10.1038/s41392-020-00248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang X., Wang L., Guo H., Zhang W., Shao Z. Single-cell transcriptomics reveals the regulative roles of cancer associated fibroblasts in tumor immune microenvironment of recurrent osteosarcoma. Theranostics. 2022;12(13):5877–5887. doi: 10.7150/thno.73714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zajac A.E., Kopec S., Szostakowski B., Spalek M.J., Fiedorowicz M., Bylina E., Filipowicz P., Szumera-Cieckiewicz A., Tysarowski A., Czarnecka A.M., Rutkowski P. Chondrosarcoma-from Molecular Pathology to Novel Therapies. Cancers (Basel) 2021;13(10) doi: 10.3390/cancers13102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uusküla-Reimand L., Wilson M.D. Untangling the roles of TOP2A and TOP2B in transcription and cancer. Sci. Adv. 2022;8(44):eadd4920. doi: 10.1126/sciadv.add4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strzalka W., Ziemienowicz A. Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Ann. Bot. 2011;107(7):1127–1140. doi: 10.1093/aob/mcq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burdelski C., Strauss C., Tsourlakis M.C., Kluth M., Hube-Magg C., Melling N., Lebok P., Minner S., Koop C., Graefen M., Heinzer H., Wittmer C., Krech T., Sauter G., Wilczak W., Simon R., Schlomm T., Steurer S. Overexpression of thymidylate synthase (TYMS) is associated with aggressive tumor features and early PSA recurrence in prostate cancer. Oncotarget. 2015;6(10):8377–8387. doi: 10.18632/oncotarget.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun X., Kaufman P.D. Ki-67: more than a proliferation marker. Chromosoma. 2018;127(2):175–186. doi: 10.1007/s00412-018-0659-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komori T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx 2. Int. J. Mol. Sci. 2019;20(7) doi: 10.3390/ijms20071694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010;339(1):189–195. doi: 10.1007/s00441-009-0832-8. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y., Yang S., Lovisa S., Ambrose C.G., McAndrews K.M., Sugimoto H., Kalluri R. Type-I collagen produced by distinct fibroblast lineages reveals specific function during embryogenesis and Osteogenesis Imperfecta. Nat. Commun. 2021;12(1):7199. doi: 10.1038/s41467-021-27563-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson D.E., O'Keefe R.A., Grandis J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018;15(4):234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao Z., Han L., Chen Y., He F., Sun B., Kamar S., Zhang Y., Yang Y., Wang C., Yang Z. Hedgehog signalling in the tumourigenesis and metastasis of osteosarcoma, and its potential value in the clinical therapy of osteosarcoma. Cell Death Dis. 2018;9(6):701. doi: 10.1038/s41419-018-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Celik B., Cicek K., Leal A.F., Tomatsu S. Regulation of Molecular Targets in Osteosarcoma Treatment. Int. J. Mol. Sci. 2022;23(20) doi: 10.3390/ijms232012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y., Liu Z., Yang X., Lu W., Chen Y., Lin Y., Wang J., Lin S., Yun J.P. H3K27 acetylation activated-COL6A1 promotes osteosarcoma lung metastasis by repressing STAT1 and activating pulmonary cancer-associated fibroblasts. Theranostics. 2021;11(3):1473–1492. doi: 10.7150/thno.51245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang C., Zhou X., Li W., Li M., Tu T., Ba X., Wu Y., Huang Z., Fan G., Zhou G., Wu S., Zhao J., Zhang J., Chen J. Macrophage migration inhibitory factor promotes osteosarcoma growth and lung metastasis through activating the RAS/MAPK pathway. Cancer Lett. 2017;403:271–279. doi: 10.1016/j.canlet.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira C.S., de Bock C.E., Molloy T.J., Sadeqzadeh E., Geng X.Y., Hersey P., Zhang X.D., Thorne R.F. Macrophage migration inhibitory factor engages PI3K/Akt signalling and is a prognostic factor in metastatic melanoma. BMC Cancer. 2014;14(1):630. doi: 10.1186/1471-2407-14-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J., Yu X.-H., Yan Y.-G., Wang C., Wang W.-J. PI3K/Akt signaling in osteosarcoma. ClinicaChimica Acta. 2015;444:182–192. doi: 10.1016/j.cca.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 52.Wang L., Yan W., Li X., Liu Z., Tian T., Chen T., Zou L., Cui Z. S100A10 silencing suppresses proliferation, migration and invasion of ovarian cancer cells and enhances sensitivity to carboplatin. J Ovarian Res. 2019;12(1):113. doi: 10.1186/s13048-019-0592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fejzo M.S., Anderson L., Chen H.W., Guandique E., Kalous O., Conklin D., Slamon D.J. Proteasome ubiquitin receptor PSMD4 is an amplification target in breast cancer and may predict sensitivity to PARPi. Genes Chromosom. Cancer. 2017;56(8):589–597. doi: 10.1002/gcc.22459. [DOI] [PubMed] [Google Scholar]
- 54.Santos Ramos F., Wons L., João Cavalli I., Ribeiro E.M.S.F. Epithelial-mesenchymal transition in cancer: An overview. Integr. Cancer Sci. Ther. 2017;4(3) [Google Scholar]
- 55.Muz B., de la Puente P., Azab F., Azab A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl) 2015;3:83–92. doi: 10.2147/HP.S93413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verrecchia F., Redini F. Transforming Growth Factor-beta Signaling Plays a Pivotal Role in the Interplay Between Osteosarcoma Cells and Their Microenvironment. Front. Oncol. 2018;8:133. doi: 10.3389/fonc.2018.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen W., Xi H., Wei B., Chen L. The prognostic role of matrix metalloproteinase 2 in gastric cancer: a systematic review with meta-analysis. J. Cancer Res. Clin. Oncol. 2014;140(6):1003–1009. doi: 10.1007/s00432-014-1630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H., Qiu Z., Li F., Wang C. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol. Lett. 2017;14(5):5865–5870. doi: 10.3892/ol.2017.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kunz P., Sahr H., Lehner B., Fischer C., Seebach E., Fellenberg J. Elevated ratio of MMP2/MMP9 activity is associated with poor response to chemotherapy in osteosarcoma. BMC Cancer. 2016;16:223. doi: 10.1186/s12885-016-2266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu B., Cui J., Sun J., Li J., Han X., Guo J., Yi M., Amizuka N., Xu X., Li M. Immunolocalization of MMP9 and MMP2 in osteolytic metastasis originating from MDA-MB-231 human breast cancer cells. Mol. Med. Rep. 2016;14(2):1099–1106. doi: 10.3892/mmr.2016.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown N.R., Korolchuk S., Martin M.P., Stanley W.A., Moukhametzianov R., Noble M.E.M., Endicott J.A. CDK1 structures reveal conserved and unique features of the essential cell cycle CDK. Nat. Commun. 2015;6(1):6769. doi: 10.1038/ncomms7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sayers E.W., Bolton E.E., Brister J.R., Canese K., Chan J., Comeau D.C., Connor R., Funk K., Kelly C., Kim S., Madej T., Marchler-Bauer A., Lanczycki C., Lathrop S., Lu Z., Thibaud-Nissen F., Murphy T., Phan L., Skripchenko Y., Tse T., Wang J., Williams R., Trawick B.W., Pruitt K.D., Sherry S.T. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022;50(D1):D20–D26. doi: 10.1093/nar/gkab1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodrigues J., Sarmento B., Pereira C.L. Osteosarcoma tumor microenvironment: the key for the successful development of biologically relevant 3D in vitro models. In vitro models. 2022;1(1):5–27. [Google Scholar]
- 64.Hayman A.R. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity. 2008;41(3):218–223. doi: 10.1080/08916930701694667. [DOI] [PubMed] [Google Scholar]
- 65.Lotinun S., Kiviranta R., Matsubara T., Alzate J.A., Neff L., Lüth A., Koskivirta I., Kleuser B., Vacher J., Vuorio E., Horne W.C., Baron R. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J. Clin. Invest. 2013;123(2):666–681. doi: 10.1172/JCI64840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Plikus M.V., Wang X., Sinha S., Forte E., Thompson S.M., Herzog E.L., Driskell R.R., Rosenthal N., Biernaskie J., Horsley V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell. 2021;184(15):3852–3872. doi: 10.1016/j.cell.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lavie D., Ben-Shmuel A., Erez N., Scherz-Shouval R. Cancer-associated fibroblasts in the single-cell era. Nature Cancer. 2022;3(7):793–807. doi: 10.1038/s43018-022-00411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.S. Park, J.D. Karalis, C. Hong, J.R. Clemenceau, M.R. Porembka, I.-H. Kim, S.H. Lee, S.C. Wang, J.-H. Cheong, T.H. Hwang, ACTA2 expression predicts survival and is associated with response to immune checkpoint inhibitors in gastric cancer, Clinical Cancer Research (2022) CCR-22-1897. [DOI] [PMC free article] [PubMed]
- 69.Hass R., Kasper C., Böhm S., Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011;9(1):12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng Y., Wang G., Chen R., Hua Y., Cai Z. Mesenchymal stem cells in the osteosarcoma microenvironment: their biological properties, influence on tumor growth, and therapeutic implications. Stem Cell Res. Ther. 2018;9(1) doi: 10.1186/s13287-018-0780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang J., Yang D., Sun Y., Sun B., Wang G., Trent J.C., Araujo D.M., Chen K., Zhang W. Genetic amplification of the vascular endothelial growth factor (VEGF) pathway genes, including VEGFA, in human osteosarcoma. Cancer. 2011;117(21):4925–4938. doi: 10.1002/cncr.26116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grünberg J.R., Hammarstedt A., Hedjazifar S., Smith U. The Novel Secreted Adipokine WNT1-inducible Signaling Pathway Protein 2 (WISP2) Is a Mesenchymal Cell Activator of Canonical WNT. J. Biol. Chem. 2014;289(10):6899–6907. doi: 10.1074/jbc.M113.511964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patel A.A., Ginhoux F., Yona S. Monocytes, macrophages, dendritic cells and neutrophils: an update on lifespan kinetics in health and disease. Immunology. 2021;163(3):250–261. doi: 10.1111/imm.13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han Y., Guo W., Ren T., Huang Y., Wang S., Liu K., Zheng B., Yang K., Zhang H., Liang X. Tumor-associated macrophages promote lung metastasis and induce epithelial-mesenchymal transition in osteosarcoma by activating the COX-2/STAT3 axis. Cancer Lett. 2019;440–441:116–125. doi: 10.1016/j.canlet.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 75.Luo Z.W., Liu P.P., Wang Z.X., Chen C.Y., Xie H. Macrophages in Osteosarcoma Immune Microenvironment: Implications for Immunotherapy. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.586580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vitale I., Manic G., Coussens L.M., Kroemer G., Galluzzi L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019;30(1):36–50. doi: 10.1016/j.cmet.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 77.Han Q., Shi H., Liu F. CD163+ M2-type tumor-associated macrophage support the suppression of tumor-infiltrating T cells in osteosarcoma. Int. Immunopharmacol. 2016;34:101–106. doi: 10.1016/j.intimp.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 78.Rodríguez-Fernández J.L., Criado-García O. The Chemokine Receptor CCR7 Uses Distinct Signaling Modules With Biased Functionality to Regulate Dendritic Cells. Front. Immunol. 2020;11:528. doi: 10.3389/fimmu.2020.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rizeq B., Malki M.I. The Role of CCL21/CCR7 Chemokine Axis in Breast Cancer Progression. Cancers (Basel) 2020;12(4) doi: 10.3390/cancers12041036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Casanova J.M., Almeida J.-S., Reith J.D., Sousa L.M., Fonseca R., Freitas-Tavares P., Santos-Rosa M., Rodrigues-Santos P. Tumor-Infiltrating Lymphocytes and Cancer Markers in Osteosarcoma: Influence on Patient Survival. Cancers. 2021;13(23):6075. doi: 10.3390/cancers13236075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ren X., Zhang L., Zhang Y., Li Z., Siemers N., Zhang Z. Insights Gained from Single-Cell Analysis of Immune Cells in the Tumor Microenvironment. Annu. Rev. Immunol. 2021;39:583–609. doi: 10.1146/annurev-immunol-110519-071134. [DOI] [PubMed] [Google Scholar]
- 82.Chauvin J.-M., Zarour H.M. TIGIT in cancer immunotherapy, Journal for ImmunoTherapy of. Cancer. 2020;8(2):e000957. doi: 10.1136/jitc-2020-000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kang W., Feng Z., Luo J., He Z., Liu J., Wu J., Rong P. Tertiary Lymphoid Structures in Cancer: The Double-Edged Sword Role in Antitumor Immunity and Potential Therapeutic Induction Strategies. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.689270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vanhersecke L., Brunet M., Guégan J.P., Rey C., Bougouin A., Cousin S., Moulec S.L., Besse B., Loriot Y., Larroquette M., Soubeyran I., Toulmonde M., Roubaud G., Pernot S., Cabart M., Chomy F., Lefevre C., Bourcier K., Kind M., Giglioli I., Sautès-Fridman C., Velasco V., Courgeon F., Oflazoglu E., Savina A., Marabelle A., Soria J.C., Bellera C., Sofeu C., Bessede A., Fridman W.H., Loarer F.L., Italiano A. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat Cancer. 2021;2(8):794–802. doi: 10.1038/s43018-021-00232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huo J.L., Wang Y.T., Fu W.J., Lu N., Liu Z.S. The promising immune checkpoint LAG-3 in cancer immunotherapy: from basic research to clinical application. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.956090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chocarro L., Blanco E., Arasanz H., Fernandez-Rubio L., Bocanegra A., Echaide M., Garnica M., Ramos P., Fernandez-Hinojal G., Vera R., Kochan G., Escors D. Clinical landscape of LAG-3-targeted therapy. Immunooncol Technol. 2022;14 doi: 10.1016/j.iotech.2022.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chiu D.K., Yuen V.W., Cheu J.W., Wei L.L., Ting V., Fehlings M., Sumatoh H., Nardin A., Newell E.W., Ng I.O., Yau T.C., Wong C.M., Wong C.C. Hepatocellular Carcinoma Cells Up-regulate PVRL1, Stabilizing PVR and Inhibiting the Cytotoxic T-Cell Response via TIGIT to Mediate Tumor Resistance to PD1 Inhibitors in Mice. Gastroenterology. 2020;159(2):609–623. doi: 10.1053/j.gastro.2020.03.074. [DOI] [PubMed] [Google Scholar]
- 88.Meftahpour V., Aghebati-Maleki A., Fotouhi A., Safarzadeh E., Aghebati-Maleki L. Prognostic significance and therapeutic potentials of immune checkpoints in osteosarcoma. EXCLI J. 2022;21:250–268. doi: 10.17179/excli2021-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vandenbon A., Diez D. A clustering-independent method for finding differentially expressed genes in single-cell transcriptome data. Nat. Commun. 2020;11(1):4318. doi: 10.1038/s41467-020-17900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xiang R., Wang W., Yang L., Wang S., Xu C., Chen X. A Comparison for Dimensionality Reduction Methods of Single-Cell RNA-seq Data. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.646936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ren Y., Li R., Feng H., Xie J., Gao L., Chu S., Li Y., Meng F., Ning Y. Single-cell sequencing reveals effects of chemotherapy on the immune landscape and TCR/BCR clonal expansion in a relapsed ovarian cancer patient. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.985187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carpen L., Falvo P., Orecchioni S., Mitola G., Hillje R., Mazzara S., Mancuso P., Pileri S., Raveane A., Bertolini F. A single-cell transcriptomic landscape of innate and adaptive intratumoral immunity in triple negative breast cancer during chemo- and immunotherapies. Cell Death Discovery. 2022;8(1):106. doi: 10.1038/s41420-022-00893-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qin T., Guo E., Lu F., Fu Y., Liu S., Xiao R., Wu X., Liu C., He C., Wang Z., Qin X., Hu D., You L., Li F., Li X., Huang X., Ma D., Xu X., Yang B., Fan J. Impact of chemotherapy and immunotherapy on the composition and function of immune cells in COVID-19 convalescent with gynecological tumors. Aging (Albany NY) 2021;13(23):24943–24962. doi: 10.18632/aging.203739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.K. Zhang, E.P. Erkan, S. Jamalzadeh, J. Dai, N. Andersson, K. Kaipio, T. Lamminen, N. Mansuri, K. Huhtinen, O. Carpén, S. Hietanen, J. Oikkonen, J. Hynninen, A. Virtanen, A. Häkkinen, S. Hautaniemi, A. Vähärautio, Longitudinal single-cell RNA-seq analysis reveals stress-promoted chemoresistance in metastatic ovarian cancer, Science Advances 8(8) eabm1831. [DOI] [PMC free article] [PubMed]
- 95.Chen Y., Yin J., Zhao L., Zhou G., Dong S., Zhang Y., Niu P., Ren H., Zheng T., Yan J., Li W., Ma P., Zhang C., Wei C., Church G., Li G., Zhao D. Reconstruction of the gastric cancer microenvironment after neoadjuvant chemotherapy by longitudinal single-cell sequencing. J. Transl. Med. 2022;20(1):563. doi: 10.1186/s12967-022-03792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Griffiths J.I., Wallet P., Pflieger L.T., Stenehjem D., Liu X., Cosgrove P.A., Leggett N.A., McQuerry J.A., Shrestha G., Rossetti M., Sunga G., Moos P.J., Adler F.R., Chang J.T., Sharma S., Bild A.H. Circulating immune cell phenotype dynamics reflect the strength of tumor-immune cell interactions in patients during immunotherapy. PNAS. 2020;117(27):16072–16082. doi: 10.1073/pnas.1918937117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mund A., Brunner A.D., Mann M. Unbiased spatial proteomics with single-cell resolution in tissues. Mol. Cell. 2022;82(12):2335–2349. doi: 10.1016/j.molcel.2022.05.022. [DOI] [PubMed] [Google Scholar]
- 98.Li J., Chen S., Pan X., Yuan Y., Shen H.-B. Cell clustering for spatial transcriptomics data with graph neural networks. Nat. Comput. Sci. 2022;2(6):399–408. doi: 10.1038/s43588-022-00266-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets highlighted in this review are available on the Gene Expression Omnibus (GEO) at accession codes GSE152048 and GSE162454