Abstract
High school students who participate in contact sports are vulnerable to sustaining multiple concussions and exhibit deficits in cognitive function in both the acute and chronic phases and in emotional behavior in the chronic phase. Further, boys are more likely to suffer cognitive problems whereas girls tend to report depression and anxiety. The effects of repetitive mild TBI in adolescent (35–40-day old) male and female Sprague-Dawley rats on object location and spatial working memory (hippocampal-dependent) and object recognition memory (hippocampal-independent) at 1-and-4-weeks post-injury along with trait-dependent anxiety- and depressive-like behaviors at 5 weeks were examined. Compared to sham-injured rats, male brain-injured rats demonstrated significant impairment in both hippocampal-dependent and - independent memory tasks at both time points, whereas female brain-injured rats only exhibited impairment in these tests at the 4-week time point. In contrast, depressive-like behaviors were present in the forced swim test in only the female brain-injured animals at 5 weeks post-injury; anxiety-like behaviors were not evident in either male or female brain-injured animals. Histological analysis at 6 weeks after injury revealed that repeated mild TBI in male and female adolescent rats resulted in increased reactivity of astrocytes and microglia within the corpus callosum below the impact site and in the stratum oriens and stratum pyramidale of the CA2 region of the dorsal hippocampus. Together, these data are indicative of the differences in the temporal pattern of post-traumatic behavioral deficits between male and female animals and that female animals may be more likely to develop deficits in the chronic post-traumatic period.
Keywords: Working memory, cognition, depression, reactive astrocytes, sports-related concussions
Introduction
Mild traumatic brain injury (TBI) comprises the greatest portion of all TBI cases and commonly manifests in adolescents through sports-related concussions (Gardner and Yaffe, 2016; Baldwin et al., 2018; Halstead et al., 2018). Over 7 million students participate in high school sports across the US annually (Pfister et al., 2015), and approximately 20% of these student-athletes experience concussions, many of which are repeated events (Gardner and Yaffe, 2016; Baldwin et al., 2018; Halstead et al., 2018). Sports-related concussions in boys and girls are associated with deficits in executive functioning and spatial memory in the days and weeks after injury, whereas depression and anxiety are more prevalent in the chronic period (Manley et al., 2017; Chong and Schwedt, 2018; Mustafi et al., 2018; Baldwin et al., 2018). Importantly, boys are thought to be more likely to exhibit cognitive deficits whereas girls typically present with affective disorders (Roberts et al., 2019; Bunt et al., 2020). These behavioral deficits are commonly associated with alterations in mean diffusivity of white matter tracts such as the corpus callosum and corona radiata (Mustafi et al., 2018). Resting-state functional magnetic resonance imaging studies have revealed reduced connectivity in regions necessary for cognitive function within the first few weeks after a concussion (Chong and Schwedt, 2018). An understanding of the cellular and network mechanisms underlying these acute and chronic behavioral deficits are necessary for the development of appropriate therapeutic strategies. Clinically relevant and age-appropriate preclinical models of repeated mild TBI are therefore critically important to elucidate these cellular mechanisms.
Existing preclinical models for repeated mild TBI in adolescent rats and mice vary with respect to injury type, frequency, and number of impacts (Weber, 2007; Bolton-Hall et al., 2019) with an impact either on the head (skin intact) or the exposed skull being the most common (Prins et al., 2013; Goddeyne et al., 2015; Semple et al., 2016; Mannix et al., 2017; Wright et al., 2017; Yamakawa et al., 2017; Wright et al., 2018; Meconi et al., 2018; McColl et al., 2018; Wu et al., 2018; Salberg et al., 2018; Wortman et al., 2018; Eyolfson et al., 2020). In adolescent rats, the frequency ranged from hours to 3–5 days apart and the number of impacts ranged from 2 to 5 (Prins et al., 2013; Goddeyne et al., 2015; Semple et al., 2016; Wright et al., 2017; Yamakawa et al., 2017; Wright et al., 2018; Meconi et al., 2018; Salberg et al., 2018; Wortman et al., 2018). Repeated mild TBI in adolescent rats resulted in deficits in locomotor activity at 24 hours (Wright et al., 2017; Yamakawa et al., 2017; Meconi et al., 2018; Salberg et al., 2018; Wortman et al., 2018). Deficits in executive functioning, by way of working memory and context mismatch memory, were observed in the first few hours to days after the last injury (Prins et al., 2013; Wright et al., 2017; Yamakawa et al., 2017; Meconi et al., 2018; Salberg et al., 2018), whereas anxiety and depressive-like behaviors were present around 1–2 weeks post-injury (Wright et al., 2017; Yamakawa et al., 2017; Salberg et al., 2018). Male brain-injured rats were more impaired in the working memory task compared to their female counterparts, (Meconi et al., 2018) although mild TBI in female rats led to a greater deficit in spatial learning (Yamakawa et al., 2017). Both male and female brain-injured rats exhibited anxiety-like behavior (Wright et al., 2017; Salberg et al., 2018), however, female rats were more likely to exhibit greater deficits in locomotor activity (Wright et al., 2017) and depressive-like behaviors (Wright et al., 2017; Yamakawa et al., 2017). Despite the sex differences in these behavioral outcomes, cellular pathologies such as increased reactivity of astrocytes and microglia in the prefrontal cortex and hippocampus occurred to similar extents in male and female rats whereas increased astrocytic reactivity was greater in the corpus callosum of female brain-injured rats (Goddeyne et al., 2015; Wright et al., 2017; Salberg et al., 2018; Wortman et al., 2018).
The present study sought to extend these observations of acute (hours to days) behavioral deficits following repeated mild TBI in adolescent rats into the chronic time-period (weeks). We utilized a well-established paradigm in the field which uses 3 impacts each spaced 3 days apart and has reported deficits in memory, anxiety, and depression over the first 2 weeks after injury (Wright et al., 2017; Yamakawa et al., 2017; Salberg et al., 2018). The hypothesis to be tested was that repeated mild TBI would result in greater deficits in cognition in male rats and deficits in affective behavior in female rats. We focused on hippocampal-dependent tests of cognition at 1- and 4-weeks post-injury as well as trait-dependent anxiety and tests of depressive-like behavior at 5–7 weeks. At the end of behavioral testing, brains were analyzed for degenerative cellular changes.
Materials and Methods
Study Design
Seventy female (30 sham-injured, 40 brain-injured) and 67 male (32 sham-injured, 35 brain-injured) adolescent Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were used in this study. Animals were randomly assigned to sham-injured and brain-injured groups and subsequently were randomly assigned to a battery of behaviors with no animal being used for more than 4 behaviors. Cognitive behaviors were tested at 1-and-4-weeks after the last impact, and affective behaviors tested beginning in the 5th week after the last impact. When necessary, the estrous phase of the female rat was determined using vaginal smears on the day(s) of the behavioral tests. At the end of behavioral testing (~6 weeks post-injury), a subset of animals, representing the various behavioral cohorts, was randomly selected, euthanized, and brains were harvested for histologic analysis. Animal numbers for each behavior test and histological analysis are reported in their perspective methods section below. Group sizes for each behavior were determined by a G*Power analysis.
Brain Injury
All surgical and behavioral procedures were done in accordance with the rules and regulations of the Institutional Animal Care and Use Committee of Drexel University College of Medicine and followed the Guide for the Care and Use of Animals. Brain injuries were induced in 35-day-old male and female Sprague-Dawley rats using the electronically driven controlled cortical impact device (Custom Design International, Richmond, VA), as previously described (Raghupathi and Huh, 2007; Giacometti et al., 2022). Animals were anesthetized with isoflurane delivered through a nose cone (5% induction, 3% maintenance). The head was supported by a soft foam pad to make it level with the body, the skull was exposed, and the animals were placed under the impact device. The convex metal indenter (5mm diameter) was aligned with bregma, zeroed on the skull over the midline suture and was then driven 2mm from the point of contact with the skull at a velocity of 5.5m/s and a dwell time of 100ms. Animals were injured ~45 seconds following the removal of anesthesia, and the total time from start of anesthesia to impact was 5–6 minutes; sham-injured animals were exposed to anesthesia for 4–5 minutes during surgical preparation and then the impactor tip was zeroed to the skull (but was not fired). Apnea times for brain-injured animals were determined from the time of impact to when the animal took its first breath. The loss of a righting reflex was measured as the time taken for the animal to return onto all four limbs after being placed on its side immediately after injury. Once the animal regained its righting reflex, it was anesthetized, the skull was evaluated for the presence of fracture and hematoma, and the scalp of the animal was then sutured shut. Brain-injured animals received 3 impacts each spaced 3 days apart whereas sham-injured animals were anesthetized 3 times.
Spatial working memory
Hippocampal-dependent spatial working memory was evaluated using the Morris water maze originally described by Hamm et al., (1996) and subsequently modified by Kline et al., (2002). For the 1-week time point, 14 male (7 sham-injured, 7 brain-injured) and 14 female (7 sham-injured, 7 brain-injured) animals were used, while 24 male (12 sham-injured, 12 brain-injured) and 15 female (8 sham-injured, 7 brain-injured) animals were used at 4 weeks. A platform was submerged 2cm below the surface of the water, made opaque using non-toxic Tempera white paint, and served as the hidden target. Animals underwent 8 pairs of trials per day and were given 120s to find the submerged platform with a 10s delay between the first (information) and the second (retention) trials. Rats were randomly assigned a starting location (North, South, East, and West) and a platform location (1, 2, 3, and 4), and each rat was tested twice from each start and for each goal location. A typical sequence would be N2, W1, E3, N4, S2, W4, S3 and E1; the order in which these trials were presented to the animal were randomly rearranged each day. Environmental cues were set up around maze to help animals orient themselves in the water. Once the animal found the platform in trial 1, it remained on it for 10s and if the animal did not find the platform, it was guided to it and allowed to stay on it for 10s. Spatial working memory was calculated as the difference in latency times between trials 1 and 2 for each pair of trials that an animal undergoes during the 5 days of testing and was averaged for each day (Kline et al., 2002). Trial 1 latency times were also independently analyzed as a measure of anterograde memory (Hamm et al., 1996).
Novel Object Recognition (NOR) Memory Test
The NOR test measures hippocampal-independent object recognition memory (Antunes and Biala, 2012, Oliveira et al., 2010) and was modified from that described by Prins and colleagues (2011). Separate groups of rats were tested at 1 (17 female- 8 sham-injured, 9 brain-injured; 12 male- 6 sham-injured, 6 brain-injured)- and 4 (17 female- 8 sham-injured, 9 brain-injured; 14 male-7 sham-injured, 7 brain-injured)- weeks following the third injury. The test apparatus consisted of a gray, open field arena that was large enough to maximize exploratory behavior while minimizing incidental contact with objects (Prins et al., 2011); testing was done under red-light conditions. Animals were habituated to the empty arena over 2 days (5 min/day). On the 3rd day, animals were allowed to explore two identical objects for 5 minutes (acclimation phase), and an hour later, animals were then put back into the arena and allowed to explore the two objects for 5 minutes (testing phase), one of which was the novel object. The time that each animal spent interacting with each object in the testing phase was determined, and a discrimination index (DI) was calculated for the testing phase (Antunes and Biala, 2012) to determine object recognition memory via this equation:
The DI ranges between +1 and −1, with positive scores indicating that the animal spent more time with the novel object.
Novel Object Location (NOL) Memory Test
The NOL test measures hippocampal-dependent memory via object location (spatial) memory and was conducted at 1-and-4-weeks following the third injury or sham surgery (Mclagan and Hales, 2019). For the 1-week time point, 17 (8 sham-injured, 9 brain-injured) males and 14 (7 sham-injured, 7 brain-injured) female rats were used. For the 4-week time point, 16 (8 sham-injured, 8 brain-injured) male and 19 (8 sham-injured, 11 brain-injured) female rats were used. The lighting conditions, testing arena, acclimation period, and testing times were as described above for the NOR test. Animals were given 5 minutes to explore the two identical objects during the acclimation phase and an hour later explored the same two objects except that one object was moved to a different location within the arena. The DI was calculated as follows:
The DI ranges between +1 and −1, with positive scores indicating that the animal spent more time with the object in the new location.
Forced Swim Test (FST)
The FST is a widely accepted measure of depressive-like behaviors (i.e., learned helplessness) in rats (Slattery and Cryan, 2012) and has been used in studies of single and repetitive mild TBI in adolescent rats (Wright et al., 2017; Yamakawa et al., 2017; Salberg et al., 2018; Giacometti et al., 2022). It was conducted at 5 weeks following the final injury or sham surgery. Twenty (10 sham-injured, 10 brain-injured) male rats and 39 (19 sham-injured, 20 brain-injured) female rats were used. Female rats were tested in estrus (11 sham-injured, 12 brain-injured,) and proestrus (8 sham-injured, 8 brain-injured) phases of the estrous cycle, as previous work in our lab demonstrates that depressive-like phenotypes may only be present during the estrus phase (Giacometti et al., 2022). The FST consisted of pretest (10min) and testing (5min) sessions which were 24 hours apart (Slattery and Cryan, 2012). The three main behaviors in the FST - swimming, climbing, and immobility – were quantified from the recorded videos by an experimenter blinded to the injury status and estrous phase of the animals. Climbing was defined as two paws out of the water and clearly moving up the side of the container, swimming as all limbs in water and visibly moving in the forward direction, and immobility as not moving, drifting, staying afloat (Giacometti et al., 2022).
Sucrose Preference Test (SPT)
The SPT is a measure for anhedonia, or lack of interest in pleasurable activities, which is a hallmark symptom of depression (Eagle et al., 2016; Liu et al., 2018). The test was conducted 7 weeks following the third injury using a two-bottle choice paradigm that takes place in the home cage of each rat (14 males (7 sham-injured, 7 brain-injured); 15 females (8 sham-injured, 7 brain-injured)). Starting at 5.5 weeks following the final injury, animals were singly housed for 1 week with regular food and water conditions followed by 3 days of acclimation to two bottles filled with water only. Starting on day 4, one of the bottles of water in each cage was replaced by a bottle with 0.25% sucrose in distilled water. The sipper tops of the bottles were equipped with ball stoppers to ensure that the liquid was not lost to leakage. Each bottle was weighed every 24 hours for a total of 5 days and its placement in the cage was switched to ensure that consumption was unbiased to cage side and lack of exploratory behavior, (Eagle et al., 2016; Liu et al., 2018). The preference for sucrose in each 24-hour period was calculated using the following formula:
The sucrose preference over the first two days was treated as the acclimation phase whereas the mean sucrose preference over the subsequent three days was treated as the test phase.
Light-Dark Box (LDB)
The LDB test is a measure of trait-dependent anxiety and capitalizes on rats’ innate conflict between the drive to explore novel spaces and the desire to avoid well-lit, open areas where they may be vulnerable to environmental threats (Calhoon and Tye, 2015). Twenty (10 sham-injured, 10 brain-injured) male rats and 39 (19 sham-injured, 20 brain-injured) female rats were used; female rats were tested in proestrus (11 sham-injured, 12 brain-injured) and estrus (8 sham-injured, 8 brain-injured) phases of the estrous cycle (Giacometti et al., 2022). The testing apparatus is divided into two parts: a dark, opaque covered chamber that is considered the “safe” compartment, and a well-lit, uncovered chamber that is aversive with an opening between the two chambers that allows for free movement (Malkesman et al., 2013). Animals were placed into the light side of the apparatus facing away from the opening to the dark chamber. Activity Monitor software was used to track the behavior of animals for 5 min and was automatically analyzed by the Activity Monitor which measures the time animals spend in the light and dark chambers and the times spent ambulating in each chamber.
Histology
Following behavioral testing, a subset of sham-injured and brain-injured animals were randomly selected to represent different behavioral cohorts, and their brains were processed for histologic analyses as previously described (Giacometti et al., 2022). One set of 40μm-thick coronal sections obtained between +3.20 mm and −7.64 mm relative to bregma (Paxinos and Watson, 1986) were mounted on gelatin-coated slides and stained with 0.016% Cresyl violet (Nissl) as previously described (Huh et al., 2008). Additional sets of sections were stained for neurodegeneration (FD NeuroSilver Kit 11, cat# PK301A), microglia (using anti-ionized calcium-binding molecule 1 (Iba-1), Wako, Richmond VA, 1:10,000), and astrocytes (using anti-glial fibrillary acidic protein (GFAP), Sigma-Aldrich, St. Louis, MO, 1:10,000). Primary antibody binding was detected using biotinylated donkey anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA, 1:500 for Iba-1) or biotinylated donkey anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA, 1:500 for GFAP). Antibody binding was visualized using the ABC Elite System with diaminobenzidine (Vector Laboratories, Burlingame CA).
Immunoreactivity for Iba-1 and GFAP in the corpus callosum below the impact site and in the stratum oriens and stratum pyramidale of the CA2 region within the dorsal hippocampus was quantified using densitometry with an automated thresholding approach (Donnelly et al., 2009; Giacometti et al., 2022). Images of the genu of the corpus callosum from 3 non-adjacent tissue sections taken from 0.7mm to −0.92mm from bregma (Paxinos and Watson, 1986) were digitized at 10X magnification and stitched together using Microsoft Image Composite Editor (GFAP: 5 sham-injured, 5 brain-injured males, 4 sham-injured, 4 brain-injured females; Iba-1: 5 sham-injured, 5 brain-injured males, 3 sham-injured, 4 brain-injured females). The percent area of immunoreactivity that exhibited staining intensity higher than the threshold for sham-injured animals was measured using ImageJ (Giacometti et al., 2022). For the stratum oriens of the CA2 region of the dorsal hippocampus, 3 non-adjacent images were taken from −2.80mm to −3.80mm from bregma (Paxinos and Watson, 1986), digitized at 10X magnification, stitched together, and immunoreactivity of GFAP and Iba-1 sections was quantified as the percent area of immunoreactivity using ImageJ (GFAP: 4 sham-injured, 5 brain-injured males, 4 sham-injured, 4 brain-injured females; Iba-1: 5 sham-injured, 5 brain-injured, 3 sham-injured, 4 brain-injured females; Giacometti et al., 2022).
Statistical analysis
For statistical analysis, normal distributions and equal variances across groups justified the use of parametric tests using a random-effect statistical model. Body weights, latencies to regain righting reflex, and apnea times were compared using a two-way analysis of variance (2-way ANOVA) with injury status and sex as independent variables. The percent of animals exhibiting skull fractures were compared using a chi-square analysis. The difference between latencies in trials 1 and 2 in the spatial working memory test was compared using a 2-way repeated measures ANOVA with injury status, sex, and days as independent variables; data from the 1-week and 4-week time points were separately compared. A 2-way ANOVA (injury status and sex) was used to compare data from all other behavioral assays and histological analyses. Post-hoc analyses, when warranted, were performed using the Neuman-Keul’s test. A p value of 0.05 or less was used to determine significance. Data are presented in the graphs as box and whisker plots with the boxes representing the 25th and 75th quartiles and the whiskers denoting the 5th and 95th percentiles.
Results
Acute Responses to Injury
Impact to the intact skull of 5-week-old male and female rats resulted in an increased latency to recover righting reflexes compared to sham animals (F1,125=103.45, p=0.0000001, Table 1). Based on a significant interaction between injury status and day (F2,250=20.70, p=0.0000001), the loss of righting reflex was greater for brain-injured animals on the first day of injury compared to injury days 2 (p=0.000042) and 3 (p=0.000022) and between injury days 2 and 3 (p=0.023); no differences in the loss of righting reflex were observed in sham-injured across the 3 surgery sessions. Brain-injured animals also experienced a brief (1–4 s) period of apnea (F1,125=538.68, p=0.0000001, Table 1). There was a significant interaction effect between injury status and day (F 2,250=4.16, p=0.017) with apnea times on injury days 2 (p=0.012) and 3 (p=0.022) significantly lower compared to day 1. Impact to the intact skull was associated with a linear skull fracture and mild hematoma at the impact site typically after the first impact in 27% of male rats and 29% of female rats. Six female and two male brain-injured animals died immediately after the first or second impact (10.4%). None of these acute changes appeared to be dependent on sex suggesting that the injury severity was similar in male and female rats. Body weights of animals increased over the 3 surgery/injury days (F2,250=2002.68, p=0.0000001); the weights of female animals were significantly lower than that of their male counterparts (F1,125= 28.17, p=0.0000001).
Table 1. Acute variables for the animals used in the study.
Data are represented as mean values and standard errors of the mean. Male rats had a significantly greater body weight at injury compared to female rats (p=0.000009) and body weights increased on injury day 2 (p=0.000009) and injury day 3 (p=0.00002) compared to injury day 1 for both sexes. The apnea times (p=0.000009) and the times to recover righting reflex (p=0.000009) were greater in brain-injured animals and higher on injury day 1 (p=0.011 for apnea, p=0.00004 for righting reflex) compared to other injury days. NA, not applicable.
| Sex | Status | N | Body Weight at Injury (g) | Apnea (s) | Righting Reflex (s) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Injury 1 | Injury 2 | Injury 3 | Injury 1 | Injury 2 | Injury 3 | Injury 1 | Injury 2 | Injury 3 | |||
| Male | Sham-Injured | 32 | 176 ± 9 | 200 ± 9 | 225 ± 9 | NA | NA | NA | 267 ± 15 | 230 ± 13 | 236 ± 14 |
|
| |||||||||||
| Female | Sham-Injured | 30 | 148 ± 4 | 165 ± 3 | 179 ± 3 | NA | NA | NA | 265 ± 17 | 249 ± 16 | 207 ± 14 |
|
| |||||||||||
| Male | Brain-Injured | 33 | 177 ± 9 | 200 ± 9 | 225 ± 10 | 1.8 ± 0.2 | 1.4 ± 0.2 | 1.4 ± 0.1 | 438 ± 21 | 376 ± 19 | 335 ± 14 |
|
| |||||||||||
| Female | Brain-Injured | 34 | 151 ± 3 | 164 ± 3 | 178 ± 3 | 1.7 ± 0.2 | 1.5 ± 0.1 | 1.5 ± 0.1 | 422 ± 32 | 367 ± 15 | 347 ± 17 |
Cellular pathology following injury
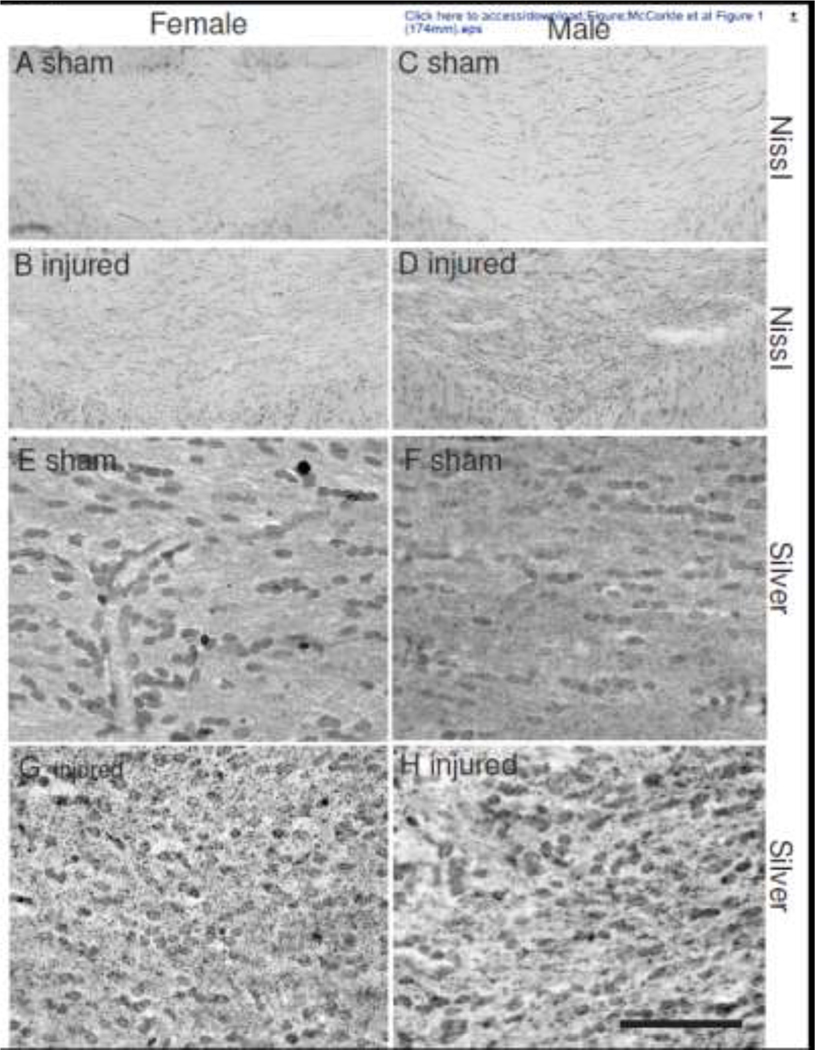
Repeated impacts to the intact skull of adolescent male and female rats did not result in overt lesions in the cortex below the impact site at 6 weeks post-injury (data not shown). This was also true in the subset of animals that exhibited hairline fractures of the skull. There was also no evidence of cellularity changes or neurodegeneration in any region of the cortex below the impact site (data not shown). However, brain-injured male and female animals exhibited structural alterations in the corpus callosum below the site of impact, characteristic of mild TBI pathology (Figure 1). Increased cellularity was observed in Nissl-stained sections within the genu of the corpus callosum (Figures 1B and 1D) compared to sections from sham-injured brains (Figures 1A and 1C). Silver staining within this region of the corpus callosum of brain-injured animals revealed minute puncta suggestive of axon degeneration (Figures 1F and 1H) which were not observed in corresponding sections from sham-injured brains (Figures 1E and 1G).
Figure 1. Cellular reactivity in the corpus callosum following repeated mild TBI in male and female adolescent rats.
Representative photomicrographs of the genu of the corpus callosum at 1mm posterior to bregma (center of the impact) illustrating cellular changes with Nissl (panels A-D) and silver stain (panels E-H). Sections were taken from female (panels A, B, E, F) and male (panels C, D, G, H) sham- (panels A, C, E, G) and brain-injured (panels B, D, F, H) animals. Note the increased cellularity (panels B, D) and the presence of punctate silver-impregnated profiles (panels F, H) in brain-injured animals. Scale bar represents 100μm for panels A-D and 25μm for panels E-H.
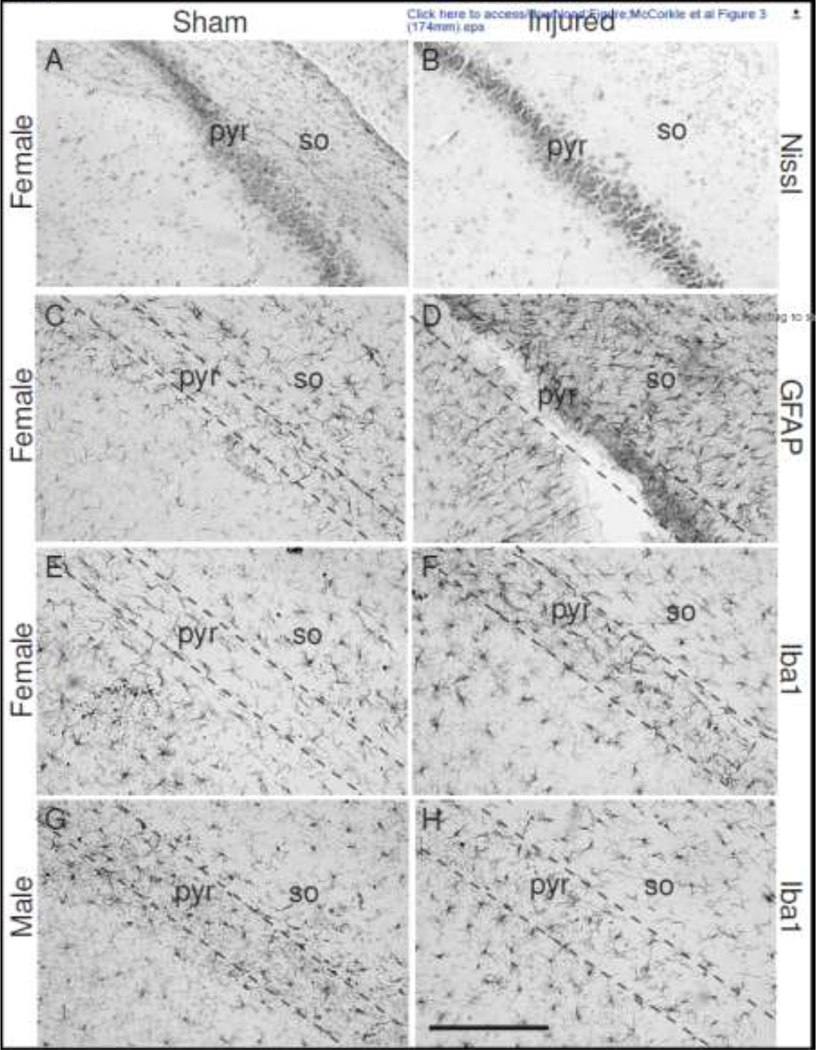
The cortex below the impact site did not contain reactive glia (data not shown). However, the increased cellularity observed in the Nissl-stained sections of the corpus callosum was accompanied by increased immunoreactivity for both GFAP (Figures 2B and 2D) and Iba-1 (Figures 2F and 2H). The cells exhibited the characteristic morphology of reactive glia with larger cell bodies and fewer processes (insets in Figures 2B, 2D, 2F and 2H) compared to the “resting” astrocytes and microglia present in sections from sham-injured animals (insets in Figures 2A, 2C, 2E and 2G). Little to no overt loss of pyramidal neurons was observed in CA2 region of the hippocampus from male (not shown) and female sham- and brain-injured animals (Figures 3A and 3B). However, an increased immunoreactivity for GFAP was observed in the stratum oriens and stratum pyramidale layers of the CA2 region in both female- (Figure 3D) and male (not shown) brain-injured animals. In contrast, compared to sham-injured female (Figure 3E) and male rats (Figure 3G), increased immunoreactivity for Iba-1 was only observed in CA2 stratum pyramidale of female brain-injured animals (Figure 3F) and not in their male counterparts (Figure 3H). None of the other areas of the dorsal hippocampus and the dentate gyrus exhibited any cellular alterations (data not shown).
Figure 2. Reactive glia in the corpus callosum following repeated mild TBI in male and female adolescent rats.
Representative photomicrographs of the genu of the corpus callosum at 1mm posterior to bregma (center of the impact) illustrating increased immunoreactivity for GFAP (panels A-D) and Iba1 (panels E-H). Sections were taken from female (panels A, B, E, F) and male (panels C, D, G, H) sham- (panels A, C, E, G) and brain-injured (panels B, D, F, H) animals. Note the changes in morphology of astrocytes (panels B, D) and microglia/macrophages (panels F, H) in brain-injured animals. Scale bar represents 100μm in all panels.
Figure 3. Cellular alterations in the dorsal hippocampus following repeated mild TBI in male and female adolescent rats.
Representative photomicrographs of the CA2 region of the dorsal hippocampus stained with Nissl (panels A and B), GFAP (panels C and D) and Iba1 (panels E-H). Note the absence of cell loss in the Nissl-stained sections and the increase in GFAP immunoreactivity in the stratum oriens in the brain-injured animals. Dotted lines represent the stratum pyramidale of the CA2 region. Scale bars represent 100μm in all panels. pyr, stratum pyramidale; so, stratum oriens.
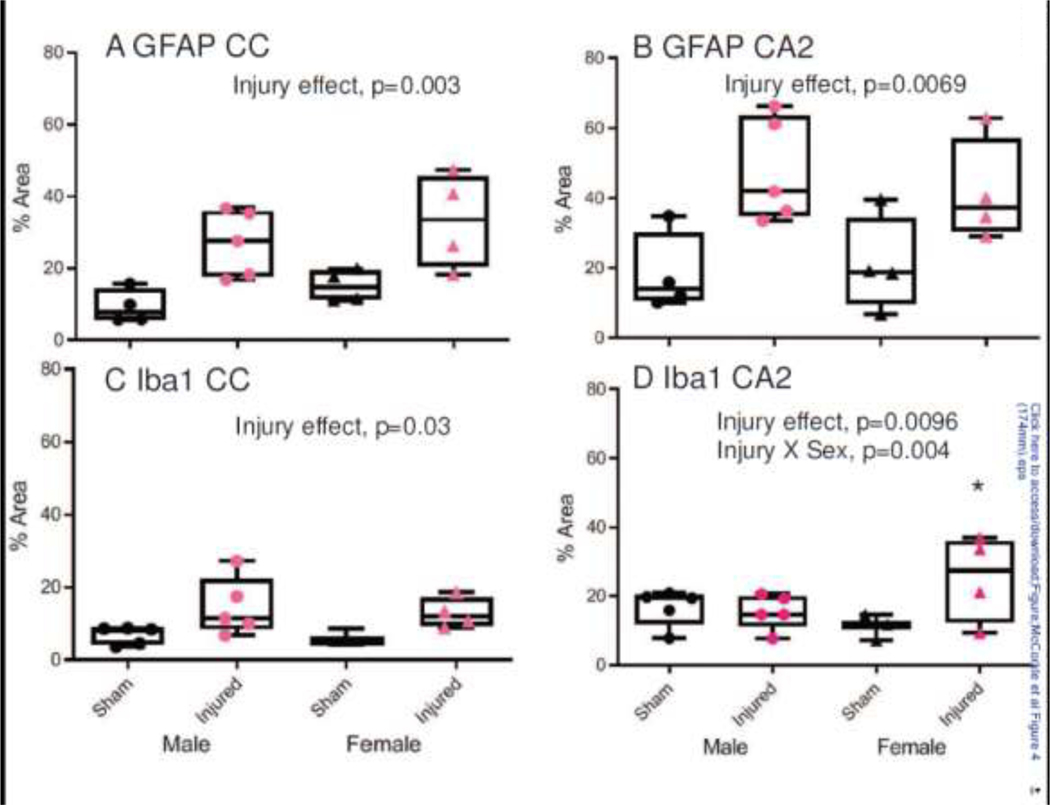
Densitometric analyses supported the qualitative assessments of increases in immunoreactivity of GFAP and Iba-1 (Figure 4). A 2-way ANOVA for the area of GFAP staining in the corpus callosum revealed a significant effect of injury (F1,13=17.53, p=0.001; Figure 4A) but not sex (F1,13=1.98, p=0.183). Similarly, there was a significant effect of injury for Iba-1 immunoreactivity (F1,13=8.16, p=0.01; Figure 4C), but not sex (F1,13=0.24, p=0.63). Immunoreactivity for GFAP in the CA2 region of the hippocampus also exhibited a significant effect of injury (F1,13 =13.88, p=0.0025; Figure 4B) but not sex (F1,13 =0.068, p=0.80). In contrast, a 2-way ANOVA of Iba-1 immunoreactivity within the CA2 revealed a significant effect of injury status (F1,12 =9.25, p=0.0096, Figure 4D) and an interaction between status and sex (F1,12 =12.57, p=0.004). A post-hoc comparison of the interaction effects demonstrated that female brain-injured animals exhibited increased immunoreactivity compared to their sham counterparts (p=0.0027), whereas male brain-injured animals did not differ from their sham-injured counterparts (p=0.745; Figure 4D).
Figure 4. Quantification of the immunoreactivity for GFAP and Iba1 following repeated mild TBI in male and female adolescent rats.
Box and whisker plots illustrating the densitometric analyses of the immunoreactivity for GFAP (panels A and B) and Iba1 (panels C and D) in the genu of the corpus callosum (panels A and C) and the stratum oriens of the CA2 region of the dorsal hippocampus (panels B and D). Area of increased intensity fo staining was measured using an automated thresholding approach in Image J. *, p<0.05 compared to sham-injured female animals.
Spatial Working Memory
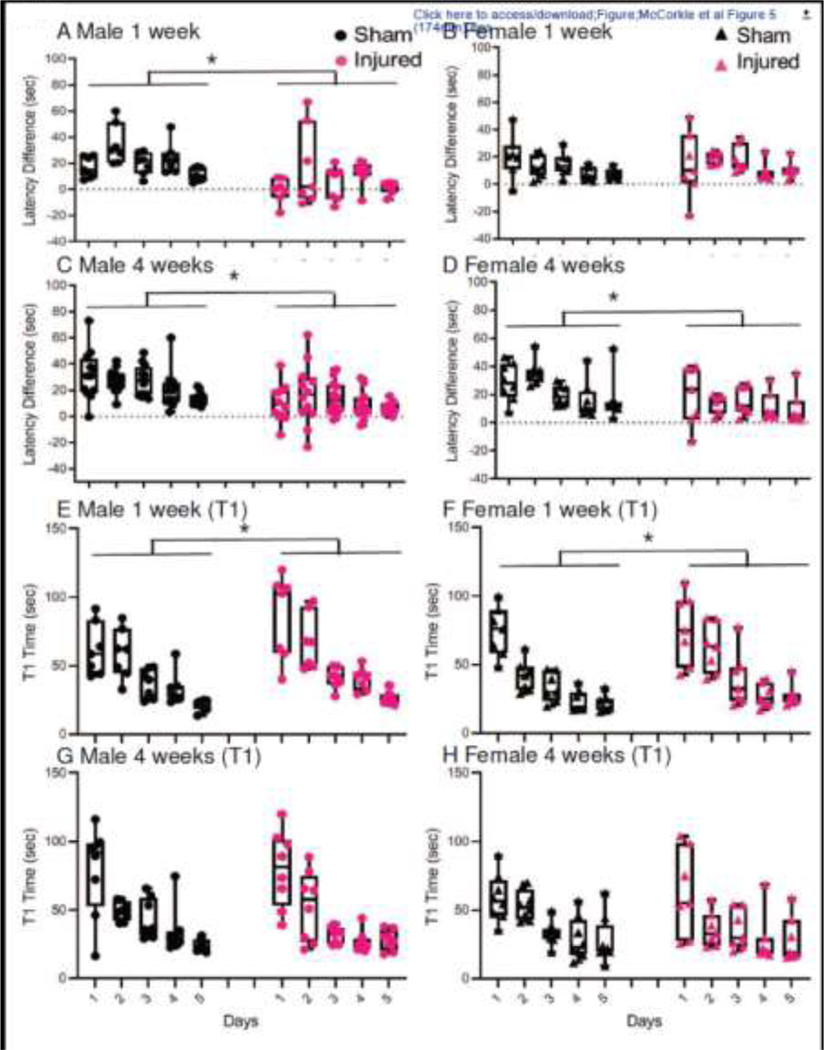
At 1-week post-injury, male (Figure 5A), but not female (Figure 5B), brain-injured rats exhibited impaired spatial working memory. In male rats, the difference in latencies between trials 1 and 2 was greater in sham-injured rats compared to brain-injured rats (Figure 5A), while the difference in latencies between the two trials was similar in sham- and brain-injured female rats (Figure 5B). A two-way repeated measures ANOVA on these latency differences between trial l and trial 2 over the five consecutive days revealed a significant injury effect (F1,24=8.91, p=0.0064) and an interaction between injury status and sex (F1,24=14.85, p=0.00076). The post-hoc analysis of the interaction effect revealed that male sham-injured animals, were different from their brain-injured counterparts (p=0.00049) whereas female sham-injured animals were not different from female brain-injured animals (p=0.54). In addition, female sham-injured animals were significantly worse at this task compared to their male counterparts (p=0.017). In contrast, at 4 weeks post-injury, both male and female brain-injured rats exhibited deficits in spatial working memory (Figures 5C and 5D, F1,35=24.95, p=0.00002) without a sex or an interaction effect.
Figure 5. Spatial working memory and anterograde memory deficits following repeated mild TBI in male and female adolescent rats.
Box and whisker plots represent the difference in latency times between Trials 1 and 2 in the spatial working memory test (panels A-D). Panels A and B represent male (A) and female (B) animals tested beginning at 1 week after the 3rd impact. Panels C and D represent male (C) and female (D) animals tested beginning at 4 weeks after the 3rd impact. Individual data points are superimposed over the box and whisker plots. Panels E-H denote box and whisker plots for anterograde memory as quantified by the average times to reach the submerged platform during the first trial (T1) of the spatial working memory test across the 5 testing days. Panels E and F represent male (E) and female (F) animals tested beginning at 1 week after the 3rd impact. Panels G and H represent male (G) and female (H) animals tested beginning at 4 weeks after the 3rd impact. Data from male and female animals are presented separately for ease of visualization although at each of the two time points in each of the two behavioral assays, a repeated measures (across days) 2-way ANOVA was carried out with sex and injury status as independent variables. *, p<0.05 compared to sham-injured animals.
Analysis of the latency times to find the submerged platform in Trial 1 of each pair of trials has been used to detect deficits in anterograde memory following TBI in adult animals (Hamm et al., 1996). At 1-week post-injury, this deficit was observed as brain-injured male and female rats took significantly longer to locate the hidden platform compared to the sham-injured rats (Figures 5E and 5F, 2-way repeated measures ANOVA, Injury effect, F1,24=5.51, p=0.028); all animals improved their latency times over the 5 training days (F4, 96=69.12, p=0.00001). At 4 weeks post-injury, there was no difference in the T1 latency times to locate the submerged platform between sham- and brain-injured male (Figure 5G) or female (Figure 5H) rats (F1,35=0.08, p=0.77).
Object Recognition Memory
Only male brain-injured rats exhibited impaired object recognition memory at 1-week post-injury (Figure 6A). The two-way ANOVA of the DI values revealed a significant effect of injury (F1,25 =29.74, p=0.000012) and sex (F1,25 =30.40, p=0.00001) and an interaction between injury and sex (F1,25 =28.09, p=0.000017). Post-hoc analysis of the interaction effect indicated that female sham-injured animals were not significantly different from female brain-injured animals (p=0.91, Figure 6A), whereas the DI values for male brain-injured animals were significantly lower compared to those in male sham-injured animals (p=0.0001, Figure 6A). At 4 weeks post-injury, both male and female brain-injured rats exhibited lower DI values compared to their sham-injured counterparts (Figure 6B, F1,27 =20.19, p=0.0001).
Figure 6. Novel object recognition and object location memories following repeated mild TBI in male and female adolescent rats.
Box and whisker plots represent discrimination index (DI) values for sham- and brain-injured male and female rats at 1 week for NOR (panel A), 4 weeks for NOR (panel B), 1 week for NOL (panel C) and 4 weeks for NOL (panel D). Individual data points are superimposed over the box and whisker plots. *, p<0.05 compared to sham-injured values; #, p<0.05 compared to female brain-injured animals.
At 1 week, brain-injured animals spent significantly less time interacting with the objects during the acclimation phase compared to sham-injured animals (2-way ANOVA, Injury effect, F1,25 =4.49, p=0.044) but this effect disappeared during the testing phase (2-way ANOVA Injury effect, F1,25=0.024, p=0.63; Table 2). Female animals spent more time interacting with the objects compared to their male counterparts during both acclimation (Sex effect, F 1,25=13.14, p=0.001) and testing (Sex effect, F1,25=12.80, p=0.001) phases (Table 2). At 4 weeks, there was no significant difference in the amount of time brain-injured and sham-injured animals spent with objects in the acclimation (2-way ANOVA Injury effect, F1,27=0.084, p=0.773) or test phases (2-way ANOVA Injury effect, F1,27=1.50, p=0.232) of the task. However, female animals, irrespective of injury status, interacted with the objects for longer times compared to their male counterparts during both acclimation (Sex effect, F1,27 =4.57, p=0.04) and testing phases (Sex effect, F1,27=6.91, p=0.01) (Table 2).
Table 2. Object interaction times in the NOR and NOL tests.
In the NOR test at 1 week, brain-injured animals spent significantly less time interacting with the objects during the acclimation phase (p=0.044) and female animals spent a greater time interacting with the objects compared to their male counterparts in both acclimation (p=0.001) and testing (p=0.001) phases. At 4 weeks, female animals spent more time interacting with the objects compared to the male animals in both acclimation (p=0.04) and testing (p=0.01) phases. In the NOL test, female animals spent more time with the objects in the acclimation phase compared to the male animals (p=0.001) at the 1-week time point. At 4 weeks, brain-injured animals spent less time than sham-injured animals with the objects in both phases (p=0.001 for acclimation and p=0.0003 for testing) and female animals spent more time than the male animals in both phases (p=0.0000001 for both phases). Female brain-injured rats spent less time interacting with the objects compared to their sham-injured counterparts in both acclimation (p=0.04) and testing phases (p=0.0019). Values presented are means ± standard errors of the mean.
| Sex | Status | NOR | NOL | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1 week | 4 weeks | 1 week | 4 weeks | ||||||
|
| |||||||||
| Acclimation | Testing | Acclimation | Testing | Acclimation | Testing | Acclimation | Testing | ||
|
| |||||||||
| Male | Sham | 68.0 ± 2.4 | 70.7 ± 3.6 | 66.4 ± 3.1 | 65.9 ± 3.3 | 79.8 ± 3.6 | 76.9 ± 6.0 | 55.8 ± 3.5 | 49.4 ± 2.8 |
|
| |||||||||
| Female | Sham | 85.6 ± 5.8 | 99.1 ± 5.7 | 72.4 ± 1.8 | 81.0 ± 3.7 | 97.6 ± 5.1 | 73.1 ± 7.4 | 85.6 ± 4.5 | 104.9 ± 5.6 |
|
| |||||||||
| Male | Injured | 57.7 ± 3.7 | 65.7 ± 8.5 | 63.6 ± 4.9 | 70.6 ± 7.3 | 69.7 ± 4.0 | 51.7 ± 4.7 | 50.5 ± 3.1 | 46.4 ± 1.6 |
|
| |||||||||
| Female | Injured | 75.3 ± 4.9 | 96.1 ± 10.2 | 78.0 ± 5.4 | 94.4 ± 10.5 | 90.9 ± 4.6 | 72.9 ± 7.7 | 65.5 ± 2.9 | 71.2 ± 5.3 |
Object Location Memory
As with object recognition memory, only male brain-injured rats showed a deficit in spatial memory through the NOL task 1-week post-injury (Figure 6C). A two-way ANOVA performed on the DIs calculated revealed significant main effects of injury status (F1,27 =5.54, p=0.026), sex (F1,27 =5.71, p=0.024), and an interaction between injury and sex (F1,27 =7.05, p=0.013). Post-hoc analysis of the interaction effect demonstrated that female sham-injured animals were not significantly different from female brain-injured animals (p=0.975); however, male sham-injured animals were significantly different than male brain-injured animals (p=0.004). Contrary to the 1-week time point, at 4-weeks post injury, both male and female brain-injured rats exhibited a deficit in spatial memory (Figure 6D). A two-way ANOVA revealed that the DI values for brain-injured animals was significantly lower compared to their sham counterparts (F1,31=32.58, p=0.000003). In addition, an injury status and sex interaction (F1,31=8.16, p=0.0076; Figure 6D) revealed that male brain-injured animals were significantly more impaired than female brain-injured animals (p=0.043).
At 1-week post-injury, a significant effect of sex was observed in the total time animals interacted with the object in the acclimation phase (Table 2, F1,27 =20.12, p=0.0001) with female animals interacting with the objects to a greater extent than their male counterparts; there was no significant difference between the sexes during the testing phase. A the 4-week time point, the total times animals interacted with the objects during the acclimation (Injury: F1,31=13.11, p=0.001; Sex: F1,31 =41.23, p=0.0000001) and testing (Injury: F1,31 =16.44 p=0.0003; Sex: F1,31 =78.76 p=0.0000001) phases were different based on injury status and sex (Table 2). Based on an interaction effect between injury and sex for both acclimation (F1,31 =4.49, p=0.04) and testing (F1,31 =11.51, p=0.0019) phases, female sham-injured animals spent more time with the objects than female brain-injured animals, but male sham-injured and brain-injured animals did not differ (Table 2).
Depression-like Behaviors
Only brain-injured female rats exhibited the learned helplessness phenotype of depression-like behavior in the FST (Figures 7A–7C). The time spent immobile (an indication of learned helplessness) was significantly higher in brain-injured rats (Figure 7A, F1,54=13.45, p=0.0005); an interaction effect between injury status and sex (F1,54=4.01, p=0.050) revealed that the injury effect was present in female rats (p=0.0006), but not in male rats (p=0.47). Brain-injured animals spent less time swimming (Figure 7B, F 1,54 =20.17, p=0.000038) and female animals swam for less time than male animals (F1,54 =7.49, p=0.0083) whereas climbing behavior was not different between sham- and brain-injured animals of both sexes (Figure 7C). Neither female nor male brain-injured animals exhibited a significantly lower preference for sucrose compared to their sham-injured counterparts in the SPT (Figure 7E, F1,25=2.60, p=0.120). There was also no effect seen during the acclimation phase (Figure 7D, F1,25=0.67, p=0.421). There was no significant difference between male and female animals in the acclimation (F1,25=0.688, p=0.415) or testing phases of the SPT (F1,25=0.052, p=0.821).
Figure 7. Depression-like and trait-dependent anxiety behaviors following repeated mild TBI in male and female adolescent rats.
Box and whisker plots represent immobility (panel A), swimming (panel B) and climbing (panel C) times in the Forced Swim Test. Panels D and E represent the preference for sucrose in the acclimation (panel D) and testing (panel E) phases. Panel F denotes the time spent in the light zone of the light dark box test. Individual data points are superimposed over the box and whisker plots. *, p<0.05 compared to sham-injured animals; #, compared to male animals
Anxiety-like behavior
Male and female sham- and brain-injured rats spent similar times in the light chamber in the LDB test (Figure 7F, F1,52=0.87, p=0.35) suggesting a lack of trait-dependent anxiety-like behaviors. However, female animals spent more time than male animals in the light chamber (Figure 7F, F1,52=62.76, p=0.0000001).
Discussion
Repeated mild TBI in adolescent rats resulted in cognitive impairment which manifested early (at 1 week) and was sustained (at 4 weeks) in male rats and was only evident at the chronic time point (4 weeks) in female rats. In addition, only female brain-injured rats exhibited learned helplessness behavior at 5 weeks post-injury. Neither trait-dependent anxiety nor anhedonia were observed in either male or female brain-injured rats. Cellular pathologic alterations, characterized by the presence of degenerating axons and reactive glia, were observed in the corpus callosum beneath the impact site and in the stratum oriens of the CA2 region within the dorsal hippocampus in both male and female brain-injured rats. Quantitative analyses of cellular pathology indicated increased area of glial reactivity in both sexes (with the exception of Iba-1 immunoreactivity in the stratum oriens of the CA2 region). Together, a lack of quantitative differences in the acute neurologic sequalae between brain-injured male and female rats suggests that both sexes experienced similar injury severities. However, the sex-dependent differences in behavioral consequences emphasize the importance of functional assessments in preclinical studies of repeated mild TBI.
The multifaceted nature of cognition necessitates the use of multiple assays for a comprehensive analysis of cognitive deficits (Shultz et al., 2020). In the present study, the Morris water maze was used for spatial working memory and the NOL task for spatial memory, both tasks that are dependent on hippocampal function, whereas the NOR task for object recognition memory is a hippocampal-independent test (Hamm et al., 1996, Antunes and Biala, 2012, Mclagan and Hales, 2019). Early and sustained cognitive deficits observed in male brain-injured animals are consistent with previous reports demonstrating persistent post-injury deficits in adolescent male mice (Mannix et al., 2017; Wu et al., 2018; Eyolfson et al., 2020) and impairments in the acute period in adolescent male rats (Prins et al., 2013; Wright et al., 2017; Yamakawa et al., 2017; Meconi et al., 2018). The latter studies also reported that cognitive deficits in female adolescent rats were observed within the first 3 days post-injury (Prins et al., 2013; Wright et al., 2017; Yamakawa et al., 2017; Meconi et al., 2018) suggesting that the current study may have missed the acute transient presence of memory deficits in brain-injured female rats by waiting until the end of the first week after injury to begin testing. It is also possible that because the female sham-injured animals performed poorly in this task at the 1-week time point compared to their male counterparts that we could not detect an injury-induced deficit; sex differences in water maze performance have been documented (reviewed by Jonasson, 2005). However, it is possible that the female animals improved with age such that we were able to detect deficits in spatial working memory in female brain-injured rats at the later time point which validates clinical studies that demonstrate that girls are at a higher risk of developing long-term impairment (Preiss-Farzanegan et al., 2009; Bazarian et al., 2010). It is possible that the hormonal state at the time of injury may contribute to the delayed development of cognitive deficits (Mihalik et al., 2009), although the estrous phase at the time of injury was not determined in the current study; most of the female rats achieved puberty (vaginal opening) at the time of injury, but they had not established the regular (5-day) cycle.
While NOR is a largely hippocampal-independent task, spatial working memory via the water maze involves the hippocampus, although the prefrontal cortex has also been implicated (Oliveira et al., 2010; Tamura et al., 2017). However, the dependence on environmental cues during this test highlight the need for hippocampal activation (Hamm et al., 1996; Tamura et al., 2017). The deficits observed in the NOL task, which is a purely hippocampal based behavior (Barker and Warburton, 2011), strongly supports damage to hippocampal circuitry. However, the CA1 region of the hippocampus, which is implicated in these behaviors, did not show any overt pathology following the repeated injuries. Although not evaluated in the present study (or in any other studies of repeated brain injuries in adolescent animals), our observations raise the possibility that hippocampal long-term potentiation may be impaired (Paterno et al., 2017; Wolf et al., 2017). The increased astrocytic and microglial reactivity in the CA2 region of the dorsal hippocampus does indicate cellular damage induced by the repeated impacts. The CA2 region of the hippocampus has been implicated in social recognition memory, although more recent findings suggest a role for regulating CA1-dependent working memory (Lehr et al 2021). Further, while not tested in the present study, deficits in prefrontal cortex-dependent executive functioning tests cannot be ruled out (Prins et al., 2013; Wright et al., 2017; Yamakawa et al., 2017; Meconi et al., 2018).
Depression is a frequent long-term deficit (Ellis et al., 2015; Bunt et al., 2020) that has a higher post-injury prevalence in girls compared to boys (Bunt et al., 2020). The observation of increased immobility in brain-injured female rats in the present study support the observation of learned helplessness in singly injured female adolescent rats (Giacometti et al., 2022) and in the acute post-traumatic period (Yamakawa et al., 2017). The depressive phenotype observed in the female animals in the present study was independent of the phase of the estrous cycle in contrast to a recent observation of increased learned helplessness in the estrus phase following a single mild TBI (Giacometti et al., 2022). The contributions of menstrual cycle on symptom severity and outcome in high school and college athletes have not been clearly defined and underscore the need for detailed preclinical studies (Mihalik et al., 2009; Wunderle et al., 2014). Although exhibiting learned helplessness, female brain-injured animals did not show anhedonia. This measure of depressive-like behaviors has not been investigated in previous literature on repeated mild TBI in adolescent animals. Therefore, it is possible that females injured at this age and frequency, do not show impairments in motivated behaviors. Further, failing to see a depressive phenotype in our male brain-injured animals is consistent with data from adolescent male mice following repeated mild TBI (Wu et al., 2018; Eyolfson et al., 2020) and adolescent male rats following a single mild TBI (Giacometti et al., 2022) but contrasts data from adult rats subjected to repeated mild TBI, suggesting that age might be a contributing factor to the apparent resilience to depression-like behavior (Tan et al., 2016). No overt pathology was observed in the nucleus accumbens (data not shown), a region that has been implicated in depression and motivation behaviors (Gebara et al., 2021), suggesting that repeated mild injury may induce abnormal neuronal activity in this circuit in female brain-injured animals, thereby contributing to the depressive-like behaviors seen through learned helplessness.
Anxiety is a common long-term symptom and has a higher prevalence in girls compared to boys (Bunt et al., 2020). Anxiety-like behaviors in rats and mice have typically used the elevated plus maze which measures state-dependent anxiety (Walf and Frye, 2007; Semple et al., 2016; Mannix et al., 2017; Wright et al., 2017; Yamakawa et al., 2017; Meconi et al., 2018; McColl et al., 2018; Wu et al., 2018; Salberg et al., 2018). Both male and female rats exhibited anxiety-like behaviors in the acute post-injury period (Wright et al., 2017; Salberg et al., 2018) whereas male mice did not demonstrate signs of long-term anxiety-like behaviors (Mannix et al., 2017; McColl et al., 2018; Wu et al., 2018). Brain-injured animals in the present study did not exhibit trait-dependent anxiety-like behaviors at 5-weeks following injury which validates a previous report in adolescent male and female mice at 10 days post-injury (Eyolfson et al., 2020). Investigating impairments in state-dependent anxiety at both early and late time points post-injury can help determine if the anxiety-like behaviors exhibited in the acute period in previous literature persist.
White matter injury is a defining feature of mild TBI, and alterations in white matter tracts can last for years post-injury in adolescent male and female athletes (Chong and Schwedt, 2018; Terry et al., 2019). The increased astrocytosis and microgliosis observed in the corpus callosum replicate the neuropathology observed following mild TBI in adult and adolescent animals (Dixon et al., 1997; Mannix et al., 2017; Ferguson et al., 2017; Wright et al., 2017; McColl et al., 2018; Salberg et al., 2018). Abnormalities in white matter tracts post-injury have been observed at both acute and chronic time points (Goddeyne et al., 2015; Mannix et al., 2017; Wright et al., 2018; Wortman et al., 2018). The lack of overt neurodegeneration in the present study validates the typical pathology of clinical mild TBI whereas the white matter damage suggests that the changes in neurocircuitry may be the functional basis for the observed behavioral deficits.
The utilization of a battery of tests for cognitive, affective, and structural impairments following repeated mild TBI strengthens confidence in the observed outcomes. They also reflect numerous symptoms seen in adolescent athletes suffering from sports-related concussions. Ongoing studies are focused on investigating the underlying mechanisms and sex differences involved in impairments in hippocampal-based cognition and depressive-like behaviors seen following repeated mild TBI with a view to advancing translational drug research (Dixon et al., 1997; Pike and Hamm, 1997; McCorkle et al., 2021; Giacometti et al., 2022).
Male brain-injured adolescent rats exhibited spatial working memory deficits at 1 and 4 weeks
Female brain-injured rats developed these deficits only at the 4 week time point
Female, but not male, brain-injured rats exhibited depressive-like behaviors at 5 weeks
Reactive astrocytes and microglia were present in the corpus callosum and dorsal hippocampus in both sexes.
Acknowledgements.
These studies were supported in part by a grant from the NIH (R01 NS1108098) to RR which includes a Research Supplement to Promote Diversity in Health Related Research to TAM, and grants from the PA Department of Health (SAP 410–007-9710 and SAP 410–007-7079) to RR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antunes M, and Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13:93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin GT, Breiding MJ, and Dawn Comstock R (2018) Epidemiology of sports concussion in the United States. Handb Clin Neurol 158:63–74. [DOI] [PubMed] [Google Scholar]
- Barker GRI, and Warburton EC (2011) When is the hippocampus involved in recognition memory? J Neurosci 31:10721–10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian JJ, Blyth B, Mookerjee S, He H, and McDermott MP (2010) Sex differences in outcome after mild traumatic brain injury. J Neurotrauma 27:527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton-Hall AN, Hubbard WB, and Saatman KE (2019) Experimental designs for repeated mild traumatic brain injury: Challenges and considerations. J Neurotrauma 36:1203–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt SC, Didehbani N, Tarkenton T, Rossetti H, Hicks C, Vargas B, Silver C, Nakonezny P et al. (2020) Sex differences and reporting of scat-5 concussion symptoms in adolescent athletes. Clin J Sport Med 31:229–234. [DOI] [PubMed] [Google Scholar]
- Calhoon GG, and Tye KM (2015) Resolving the neural circuits of anxiety. Nat Neurosci 18:1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong CD, and Schwedt TJ (2018) Research imaging of brain structure and function after concussion. Headache 58:827–835. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Flinn P, Bao J, Venya R, and Hayes RL (1997) Nerve growth factor attenuates cholinergic deficits following traumatic brain injury in rats. Exp Neurol 146:479–490. [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Gensel JC, Ankeny DP, van Rooijen N, and Popovich PG. (2009). An efficient and reproducible method for quantifying macrophages in different experimental models of central nervous system pathology. J Neurosci Methods 181:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle AL, Mazei-Robison M, and Robison AJ (2016) Sucrose preference test to measure stress-induced anhedonia. Bio Protocol 6:1686–1698. [Google Scholar]
- Ellis MJ, Ritchie LJ, Koltek M, Hosain S, Cordingley D, Chu S, Selci E, Leiter J et al. (2015) Psychiatric outcomes after pediatric sports-related concussion. J Neurosurg Pediatr 16:709–718. [DOI] [PubMed] [Google Scholar]
- Eyolfson E, Carr T, Khan A, Wright DK, Mychasiuk R, and Lohman AW (2020) Repetitive mild traumatic brain injuries in mice during adolescence cause sexually dimorphic behavioral deficits and neuroinflammatory dynamics. J Neurotrauma 37:2718–2732. [DOI] [PubMed] [Google Scholar]
- Ferguson S, Mouzon B, Paris D, Aponte D, Abdullah L, Stewart W, Mullan M, and Crawford F (2017) Acute or delayed treatment with anatabine improves spatial memory and reduces pathological sequelae at late time-points after repetitive mild traumatic brain injury. J Neurotrauma 34:1676–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, and Yaffe K (2016) Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci 66:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebara E, Zanoletti O, Ghosal S, Grosse J, Schneider BL, Knott G, Astori S, and Sandi C (2021) Mitofusin-2 in the nucleus accumbens regulates anxiety and depression-like behaviors through mitochondrial and neuronal actions. Biol Psychiatry 89:1033–1044. [DOI] [PubMed] [Google Scholar]
- Giacometti LL, Huh JW, and Raghupathi R (2022) Sex and estrous-phase dependent alterations in depressive-like behavior following mild traumatic brain injury in adolescent rats. J Neurosci Res 2:490–505. [DOI] [PubMed] [Google Scholar]
- Goddeyne C, Nichols J, Wu C, and Anderson T (2015) Repetitive mild traumatic brain injury induces ventriculomegaly and cortical thinning in juvenile rats. J Neurophysiol 113:3268–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead ME, Walter KD, and Moffatt K (2018) Sport-related concussion in children and adolescents. Pediatrics 142 doi: 10.1542/peds.2018-3074. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Temple MD, Pike BR, O’Dell DM, Buck DL, and Lyeth BG (1996) Working Memory Deficits following Traumatic Brain Injury in the Rat. J Neurotrauma 13:317–323. [DOI] [PubMed] [Google Scholar]
- Huh JW, Widing AG, and Raghupathi R (2008) Midline brain injury in the immature rat induces sustained cognitive deficits, bihemispheric axonal injury and neurodegeneration. Exp Neurol 213:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson Z (2005) Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci Biobehav Rev 28:811–825. [DOI] [PubMed] [Google Scholar]
- Kline AE, Massucci JL, Marion DW, and Dixon CE (2002) Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J Neurotrauma 19:415–425. [DOI] [PubMed] [Google Scholar]
- Lehr AB, Kumar A, Tetzlaff C, Hafting T, Fyhn M, Stӧber TM (2021) CA2 beyond social memory: Evidence for a fundamental role in hippocampal information processing. Neurosci Biobehav Rev 126:398–412. [DOI] [PubMed] [Google Scholar]
- Liu M-Y, Yin C-Y, Zhu L-J, Zhu X-H, Xu C, Luo C-X, Chen H, Zhu D-Y et al. (2018) Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc 13:1686–1698. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Tucker LB, Ozl J, and McCabe JT (2013) Traumatic brain injury-modeling neuropsychiatric symptoms in rodents. Front Neurol 4 doi: 10.3389/fneur.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley G, Gardner AJ, Schneider KJ, Guskiewicz KM, Bailes J, Cantu RC, Castellani RJ, Turner M et al. (2017) A systematic review of potential long-term effects of sport-related concussion. Br J Sports Med 51:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannix R, Berkner J, Mei Z, Alcon S, Hashim J, Robinson S, Jantzie L, Meehan WP et al. (2017) Adolescent mice demonstrate a distinct pattern of injury after repetitive mild traumatic brain injury. J Neurotrauma 34:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl TJ, Brady RD, Shultz SR, Lovick L, Webster KM, Sun M, McDonald SJ, O’Brien TJ et al. (2018) Mild traumatic brain injury in adolescent mice alters skull bone properties to influence a subsequent brain impact at adulthood: A pilot study. Front Neurol 9 doi: 10.3389/fneur.2018.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorkle TA, Barson JR, Raghupathi R (2021) A Role for the Amygdala in Impairments of Affective Behaviors Following Mild Traumatic Brain Injury. Front Behav Neurosci 15 doi: 10.3389/fnbeh.2021.601275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclagan AN, and Hales JB (2019) Displaced Object Recognition Memory in Rats. Bio Protoc 9 doi: 10.21769/BioProtoc.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meconi A, Wortman RC, Wright DK, Neale KJ, Clarkson M, Shultz SR, and Christie BR (2018) Repeated mild traumatic brain injury can cause acute neurologic impairment without overt structural damage in juvenile rats. PLoS One 13 doi: 10.1371/journal.pone.0197187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalik JP, Ondrak KS, Guskiewicz KM, and McMurray RG (2009) The effects of menstrual cycle phase on clinical measures of concussion in healthy college-aged females. J Sci Med Sport 12:383–387. [DOI] [PubMed] [Google Scholar]
- Mustafi SM, Harezlak J, Koch KM, Nencka AS, Meier TB, West JD, Giza CC, DiFiori, JP et al. (2018) Acute white-matter abnormalities in sports-related concussion: a diffusion tensor imaging study from the NCAA-DoD CARE consortium. J Neurotrauma 22:2653–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AMM, Hawk JD, Abel T, and Havekes R (2010) Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem 3:155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterno R, Folweiler KA, and Cohen AS (2017) Pathophysiology and treatment of memory dysfunction after traumatic brain injury. Curr Neurol Neurosci Rep 17:52. doi: 10.1007/211910-017-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G and Watson C (1986) The Rat Brain in Stereotaxic Coordinates: Second Edition. Academic Press. [Google Scholar]
- Pfister T, Pfister K, Hagel B, Ghali WA, and Ronksley PE (2015) The incidence of concussion in youth sports: A systematic review and meta-analysis. Br J Sports Med 50:292–297. [DOI] [PubMed] [Google Scholar]
- Pike BR, and Hamm RJ (1997) Activating the posttraumatic holinergic system for the treatment of cognitive impairment following traumatic brain injury. Pharmacol Biochem Behav 57:785–791. [DOI] [PubMed] [Google Scholar]
- Preiss-Farzanegan SJ, Chapman B, Wong TM, Wu J, and Bazarian JJ (2009) The relationship between gender and postconcussion symptoms after sport-related mild traumatic brain injury. PM R 1:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML, Alexander D, Giza CC, and Hovda DA (2013) Repeated mild traumatic brain injury: Mechanisms of cerebral vulnerability. J Neurotrauma 30:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML, Hales A, Reger M, Giza CC, and Hovda DA (2011). Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev Neurosci 32:510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupathi R, and Huh JW (2007) Diffuse brain injury in the immature rat: evidence for an age- at-injury effect on cognitive function and histopathologic damage. J Neurotrauma 24:1596–1608. [DOI] [PubMed] [Google Scholar]
- Roberts AL, Pascual-Leone A, Speizer FE, Zafonte RD, Baggish AL, Taylor H, Nadler LM, Courtney TK et al. (2019) Exposure to American football and Neuropsychiatric health in former National Football League PLAYERS: Findings from the football PLAYERS Health Study. Am J Sports Med 47:2871–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salberg S, Christensen J, Yamakawa GR, Lengkeek C, Malik H, Tabor J, Hazari A, and Mychasiuk R (2018) A bump on the head or late to Bed: Behavioral AND Pathophysiological effects of sleep deprivation AFTER REPETITIVE mild traumatic brain injury in Adolescent Rats. J Neurotrauma 35:1895–1905. [DOI] [PubMed] [Google Scholar]
- Semple BD, Sadjadi R, Carlson J, Chen Y, Xu D, Ferriero DM, and Noble-Haeusslein LJ (2016) Long-term anesthetic-dependent hypoactivity after repetitive mild traumatic brain injuries in adolescent mice. Dev Neurosci 38:220–238. [DOI] [PubMed] [Google Scholar]
- Shultz SR, McDonald SJ, Corrigan F, Semple BD, Salberg S, Zamani A, Jones NC, and Mychasiuk R (2020) Clinical relevance of behavior testing in animal models of traumatic brain injury. J Neurotrauma 37:2381–2400. [DOI] [PubMed] [Google Scholar]
- Slattery DA, and Cryan JF (2012) Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc 7:1009–1014. [DOI] [PubMed] [Google Scholar]
- Tamura M, Spellman TJ, Rosen AM, Gogos JA, and Gordon JA (2017) Hippocampal-prefrontal theta-gamma coupling during performance of a spatial working memory task. Nat Commun 8 doi: 10.1038/s41467-017-02108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan XL, Wright DK, Liu S, Hovens C, O’Brien TJ, Shultz SR (2016) Sodium selenate, a protein phosphatase 2A activator, mitigates hyperphosphorylated tau and improves repeated mild traumatic brain injury outcomes. Neuropharmacology 108:382–93. [DOI] [PubMed] [Google Scholar]
- Terry DP, Mewborn CM, and Miller LS (2019) Repeated sport-related concussion shows only minimal white matter differences many years after playing high school football. J Int Neuropsychol Soc 25:950–960. [DOI] [PubMed] [Google Scholar]
- Walf AA, and Frye CA (2007) The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2:322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JT (2007) Experimental models of repetitive brain injuries. Prog Brain Res 161:253–261. [DOI] [PubMed] [Google Scholar]
- Wolf JA, Johnson BN, Johnson VE, Putt ME, Browne KD, Mietus CJ, Brown DP, Wofford KL et al. (2017) Concussion Induces Hippocampal Circuitry Disruption in Swine. J Neurotrauma 14:23032314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortman RC, Meconi A, Neale KJ, Brady RD, McDonald SJ, Christie BR, Wright DK, and Shultz SR (2018). Diffusion MRI abnormalities in adolescent rats given repeated mild traumatic brain injury. Ann Clin Transl Neurol 5:1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DK, O’Brien TJ, Mychasiuk R, and Shultz SR (2018) Telomere length and advanced diffusion mri as biomarkers for repetitive mild traumatic brain injury in adolescent rats. NeuroImage Clin 18:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DK, O’Brien TJ, Shultz SR, and Mychasiuk R (2017) Sex matters: Repetitive mild traumatic brain injury in adolescent rats. Ann Clin Transl Neurol 4:640–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Chung JY, Saith S, Tozzi L, Buckley EM, Sanders B, Franceschini MA, Lule S et al. (2018) Repetitive head injury in adolescent mice: A role for vascular inflammation. J Cereb Blood Flow Metab 39:2196–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderle K, Hoeger KM, Wasserman E, and Bazarian JJ (2014) Menstrual phase as predictor of outcome after mild traumatic brain injury in women. J Head Trauma Rehabil 29:E1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa GR, Lengkeek C, Salberg S, Spanswick SC, and Mychasiuk R (2017) Behavioral and Pathophysiological outcomes associated with caffeine consumption and repetitive mild traumatic brain Injury (rmtbi) in Adolescent rats. PLoS One 12 doi: 10.1371/journal.pone.0187218. [DOI] [PMC free article] [PubMed] [Google Scholar]