Abstract
Intracerebral hemorrhage (ICH) is a non-traumatic hemorrhage caused by the rupture of blood vessels in the brain parenchyma, with an acute mortality rate of 30%‒40%. Currently, available treatment options that include surgery are not promising, and new approaches are urgently needed. Nanotechnology offers new prospects in ICH because of its unique benefits. In this review, we summarize the applications of various nanomaterials in ICH. Nanomaterials not only enhance the therapeutic effects of drugs as delivery carriers but also contribute to several facets after ICH such as repressing detrimental neuroinflammation, resisting oxidative stress, reducing cell death, and improving functional deficits.
Keywords: Intracerebral hemorrhage, nanomaterials, nanomicelle, hydrogel
Introduction
Intracerebral hemorrhage (ICH) is caused by the spontaneous rupture of blood vessels in the brain, thereby leading to primary and secondary brain injury. Primary brain injury is a mass effect induced by hematoma formation and increased pressure on adjacent brain tissues. A series of metabolic reactions generated in response to the primary brain injury, such as hemolysis and thrombin activation, then lead to secondary brain injury. 1 Neuroinflammation and oxidative stress are involved in secondary brain injury after ICH,2–4 and the extensive literature on these pathogenic mechanisms uncover new insights to counter the pathologic sequelae of ICH. At present, surgical or traditional treatments are still the main options,5,6 broad pre-clinical studies have been completed,7–9 and numerous researches are pouring in, 10 – 12 however, there is no widely recognized and effective method to ameliorate secondary brain injury 13 and improve the prognosis of ICH. 14 For example, applying hyperosmolar agents and external ventricular drain placement to cope with the decreased alertness caused by mass effect of ICH may be appropriate.15,16 Recent clinical trial which used tissue plasminogen activator to resolve hemorrhagic products turned out to be unsatisfactory in prognosis. 17 Numerous clinical trials targeting correcting elevated blood pressure and reversing coagulopathy are still ongoing.18–21
In the past few decades, nanotechnology has emerged as an important class in medical research, where it impacts molecular imaging, drug delivery, cancer therapy, and other aspects.22,23 Nanomedicine has attracted much attention as a safe and effective strategy because some materials possess special properties such as good biocompatibility, biodegradability, and low toxicity when they are modified into the nanoscale. Due to the multi-ligand valency, engineered nanomaterials offer other advantages such as long circulation time, crossing biological barriers, and enhanced targeting properties. 24 The different advantages of nanomaterials are highly dependent on their features which vary broadly from shape, size, and composition, to the surface charge, hydrophilicity, conductivity, and rigidity.25,26
Nanomaterials, mainly nanoparticles (NPs), have the potential to be applied in preclinical studies and clinical trials. They have exerted advantages in the studies of several neurological diseases such as Parkinson’s disease, Alzheimer’s disease, and ischemic stroke.27–29 Although access to the brain is often restricted for many drugs due to blood-brain barrier (BBB) and its selective transport of drugs, 30 nanomedicines improve the bioavailability, pharmacokinetics, blood circulation time and biological distribution of drugs into the central nervous system (CNS), and achieve the purpose of micro, high efficiency, stability and targeting compared to free drugs.31,32 The rapid development of nanotechnology has made it possible to consider its prospects to improve the therapeutic efficacy of drugs in ICH.
Roles of nanomaterials in ICH treatment
Neuroprotection still represents a major therapeutic goal in ICH in order to prevent the progression of ICH-triggered damage in the brain parenchyma. 33 Unfortunately, most protective therapeutic approaches in animal research have been disappointing when they were translated into clinical trials, mainly because of the side effects of the drugs, low BBB permeability, or narrow therapeutic window.34–36 The application of traditional medicines is often restricted by poor distribution and low selectivity. These problems may be solved by using nanotechnology, 37 which can conquer the main obstacles that hinder the implementation of neuroprotective approaches by transporting neurotherapeutic agents to the brain. 38 Multiple types of nanomaterials have been tested for delivering a variety of substances.38,39 Nanomaterials have been regarded as suitable carriers for overcoming pharmacokinetic limitations associated with conventional drug formulations. Nanotechnology has been mentioned as an emerging therapeutic strategy targeting ICH. 40 In this review, we summarize some nanomaterials which appear to exert influence on brain injury mechanisms of ICH including inhibiting brain cell death, resisting oxidative damage (Table 1), repressing neuroinflammation, and promoting brain tissue repair and functional recovery (Table 2), eventually improving neurological impairment and prognosis of ICH.
Table 1.
The strategies in targeted delivery of nanomedicines to inhibit cell death and resist oxidative stress after ICH.
| Action | Nanomedicine and intervention strategy | Traits | Achievements | References |
|---|---|---|---|---|
| Inhibit cell death | Deferoxamine-HCC-PEG | Prevents heme and iron-mediated toxicity; reduces neuronal aging and ferroptosis | Dharmalingam et al. 43 | |
| Minocycline-loaded keratose hydrogel | Good biocompatibility, porous property; promising for bone regeneration and nerve repair | Reduces ICH postoperative iron accumulation, edema; improves functional recovery and survival rate in rats | Luo et al. 14 | |
| Resveratrol-NPs | Have good biocompatibility; can cross physiological barriers; improve drug accumulation within the plasma and brain | Attenuate the progression of ICH-induced brain injury by inhibiting ferroptosis | Mo et al. 54 | |
| Polybutylcyanocr-ylate NPs | Protect neurons against apoptosis | Chung et al. 58 | ||
| Exosomes | Attenuate ferroptosis and neurologic injury; reduce neuronal apoptosis and inflammation | Yi and Tang, 62 Duan et al. 63 | ||
| Resist oxidative stress | Curcumin-nanoemulsions | Low toxicity; control the release of selenium; ensure favorable effects and reduce potential toxicity | Increase total antioxidant capacity; modulate antioxidant responses; reduce the size of the hematoma; recover locomotor activity | Marques et al. 75 |
| Quercetin-nanoemulsions | Galho et al. 76 | |||
| Selenium-SiO2 | Protects cells from ROS toxicity; alleviates brain edema; reduces BBB damage | Yang et al. 80 | ||
| PEG-CeNPs | Reduces ROS level; improves the anatomical integrity of myelinated fibers; ameliorates white matter injury | Zheng et al. 83 | ||
| RNPs | Extremely stable in vivo conditions; possess long blood circulation lifetimes | Minimize oxidative damage; decrease brain edema; decrease neurologic deficit | Krishna et al. 90 |
BBB: blood-brain barrier; HCC: hydrophilic carbon cluster; NPs: nanoparticles; PEG: poly (ethylene glycol); RNPs: Redox polymer self-assembled nanoparticles; ROS: reactive oxygen species;.
Table 2.
Strategies in targeted delivery of nanomedicines to repress inflammation and promote tissue repair and functional recovery after ICH.
| Phase of cascade | Nanomedicine and intervention strategy | Traits | Achievements | References |
|---|---|---|---|---|
| Repress inflammation | Rosuvastatin nanomicelles | Good biocompatibility and nontoxicity | Inhibit the expression of downstream inflammatory factors; modulate the polarization of microglia/macrophages | Zi et al. 97 |
| Gelatin hydrogel | Excellent biodegradability, biocompatibility, and target activity | Attenuate postoperative neurological deficits; reduce neuron loss; repress inflammation | Xu et al. 104 | |
| Promote tissue repair and functional recovery | Gelatin-EGF-loaded hydrogel | Suitable for minimally invasive implantation | Fills irregularly shaped cavities; attracts cells for migration; improves recovery | Lim et al. 111 |
| RADA16mix | Reduces acute brain injury; decreases inflammatory response; promotes functional recovery and nerve fiber growth | Zhang et al. 118 |
Nanomaterials can improve the capacity of drugs to inhibit neuronal cell death
Drugs combined with nanomaterials can significantly reduce iron accumulation and ferroptosis
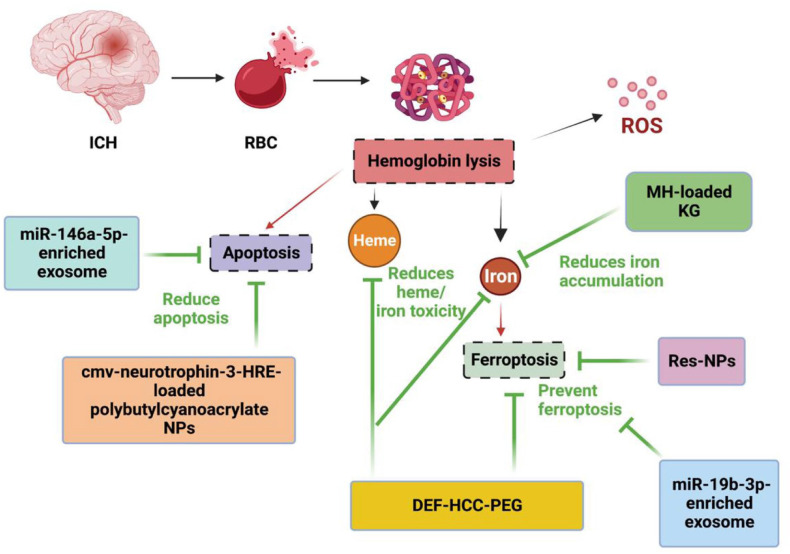
The blood components from hematoma after ICH including hemoglobin, heme, and free iron, have neurotoxic effects.41,42 However, the iron chelating agent deferoxamine (DEF) is dose-limited in human ICH trials due to its drawbacks of poor cellular absorption, toxicity, and short shelf life. A broad action antioxidant, poly (ethylene glycol)-conjugated hydrophilic carbon clusters (PEG-HCCs), was incapable of ameliorating the dual toxic effects of heme and iron and decreasing the susceptibility to ferroptosis. 43 The DEF-HCC-PEG, a synthesized, catalytic, multifunctional, and rapidly internalized carbon nanomaterial formed by PEG-HCC, covalently bonds to DEF. 43 The synthesized DEF-HCC-PEG was significantly more effective than PEG-HCCs, free DEF treatment alone, or combined but worked as a single drug, in reducing heme and iron-mediated toxicity, inhibiting neuronal aging and ferroptosis in culture. This combination strategy effectively protected neurons from the toxicity of ICH hemolysis (Figure 1). Using nanomaterials can greatly enhance the effect and decrease the dosage of drugs, and the optimal proportion of DEF and PEG-HCCs should be considered.
Figure 1.
Model illustrating the effects of nanomedicine on the ferroptosis and apoptosis after ICH. Deferoxamine-HCC-PEG is a complex of the iron chelator deferoxamine and antioxidant PEG-HCC, which can effectively reduce heme/iron toxicity, and prevent senescence and ferroptosis. Minocycline-loaded keratose hydrogel can reduce iron accumulation, resveratrol-NPs and miR-19b-3p-enriched exosome can inhibit ferroptosis. miR-146a-5p-enriched exosome and cmv-neurotrophin-3-HRE-loaded polybutylcyanoacrylate NPs can reduce apoptosis following ICH.
cmv: cytomegalovirus; DEF: deferoxamine; HCC: hydrophilic carbon cluster; HRE: hormone response element; KG: keratose hydrogel; MH: minocycline hydrochloride; NPs: nanoparticles; PEG: poly (ethylene glycol); RBC: red blood cell; ROS: reactive oxygen species.
Another iron chelator, minocycline, has been used to reduce ICH-induced brain edema, neuronal death, and neurological deficits.44,45 However, there were some adverse issues in clinical trials. 46 Keratose hydrogel, a kind of hydrogel formed from keratose, could delay hemoglobin-induced iron accumulation in rat primary neuronal culture owing to its adsorptive capacity, while minocycline hydrochloride-loaded keratose hydrogel displayed a stronger and more thorough cytoprotective effect than blank hydrogel (Figure 1). In vivo, the minocycline hydrochloride-loaded keratose hydrogel effectively reduced ICH postoperative iron accumulation and edema, and improved functional recovery and survival rate in rats compared to the systemic administration of minocycline. 14
Resveratrol, a widely used non-flavonoid polyphenol compound, 47 has neuroprotective roles in ICH.48–51 However, its poor oral bioavailability and difficulty in crossing physiological barriers52,53 limit its clinical application. NPs, as a commonly used drug delivery matrix material with good biocompatibility, can transport across physiological barriers and improve resveratrol accumulation in plasma and brain. Resveratrol-NPs safely and effectively attenuated the progression of ICH-induced brain injury by inhibiting ferroptosis 54 (Figure 1). In this condition, it can be considered to combine other agents which target necrosis or necroptosis, with nanomaterials, to investigate if they can effectively ameliorate the death of neurons post-ICH.
Using nanomaterials can remarkably reduce the neuronal cells apoptosis
Polybutylcyanoacrylate NPs have shown potential as an appropriate non-viral system for gene delivery,55,56 and they are also efficacious vectors for delivering large molecules to the injured brain. 57 A delivery system comprising polybutylcyanoacrylate NPs of plasmid neurotrophin-3 containing hormone response element (HRE) with a cytomegalovirus (CMV) promoter was used to treat ICH rats. 58 The results showed that polybutylcyanoacrylate NPs could raise the delivery of cmv-neurotrophin-3-HRE across the BBB in vivo, therefore increasing the expression of neurotrophin-3, and protecting neurons against apoptosis after ICH in vivo (Figure 1).
Exosomes are nanosized 30–100 nm diameter membranous vesicles released from diverse cell types, and they transfer biomolecules from one site to another. They are thought to play important functions in various biological pathways including cell-to-cell communication, tumor progression, and cellular waste disposal. 59 A large collection of recent research papers has revealed that exosomes released from various stem cells can improve multiple diseases,60,61 including ICH. For example, exosomes derived from miR-19b-3p-overexpressing adipose-derived stem cells attenuated ICH-induced ferroptosis and neuronal injury. 62 MiR-146a-5p-enriched exosomes released from bone marrow mesenchymal stem cells elicited neuroprotection and functional improvement after ICH by reducing neuronal apoptosis and neuroinflammation associated with the suppression of microglial pro-inflammatory polarization 63 (Figure 1).
Nanomaterials can improve the ability of drugs to resist oxidative stress
Curcumin has been described to prevent or delay the onset of ischemic stroke and multiple CNS diseases.64–70 Quercetin is known for its antioxidant activity and anti-inflammatory properties.71,72 However, they both have low bioavailability in their natural states.73,74 Nanoemulsions (NEs) are surfactant stabilized heterogeneous systems and their oil droplet sizes make them potential systems for improving drug delivery. 65 Studies have compared the possible therapeutic effect of these two free drugs versus their drug-loaded NEs in an ICH rat model.75,76 The results showed that the formulations of curcumin-NEs and quercetin-NEs increased total antioxidant capacity in ICH than them alone. Both drug-loaded NEs reduced the size of the hematoma, recovered locomotor activity, and attenuated weight loss caused by ICH, without obvious toxic effects. 71
Selenium is a cofactor of glutathione peroxidase and thioredoxin reductase, which are antioxidant enzymes that resist oxidative stress and maintain redox balance. 77 However, the use of selenium is restricted by the narrow gap between its beneficial and harmful effects. 78 The porous selenium-SiO2 nanocomposites with an average diameter of about 55 nm 79 could control the release of selenium, ensure favorable effects and reduce potential toxicity. 80 After intraperitoneal injection into ICH mice, the activity of glutathione peroxidase was increased, while the level of malonaldehyde was significantly decreased. By protecting cells from reactive oxygen species (ROS) toxicity, alleviating brain edema and reducing BBB damage, the porous selenium-SiO2 nanocomposites improved neurological functions of ICH mice.
Ceria NPs (CeNPs) are known to possess potent-free radical scavenging activity.81,82 One study found that PEG-CeNP, which was formed by CeNPs with a PEG coating, strongly reduced ROS levels in the brain tissue. It also modulated ROS-induced microglial polarization and astrocyte alteration improved the anatomical integrity of myelinated fibers and ameliorated white matter injury after ICH. 83
The nitroxide radical compound 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) is one of the strongest antioxidants that catalytically scavenge ROS.84,85 Under in vivo conditions, however, these low molecular weight nitroxide compounds have several problems, including nonspecific dispersion in normal tissues, preferential renal clearance and rapid reduction to the corresponding hydroxylamine form. Redox polymer self-assembled NPs (nitroxide radical-containing NPs [RNPs]) with diameters of approximately 40 nm were developed to solve these issues. 86 By contrast, RNPs are extremely stable in vivo and possess long blood circulation lifetimes.87,88 Nitroxide radicals in RNPs have catalytic ROS scavenging activity including for superoxide and hydroxyl radicals, 89 which is superior to that of superoxide dismutase (SOD). 90 Systemic RNP treatment at an early stage after ICH decreased levels of superoxide anion radicals and minimized ICH-induced oxidative damage to other molecules in addition to DNA. RNPs also decreased acute ICH-induced brain edema and neurologic deficit, probably by decreasing the hemorrhagic area.
Nanomaterials can improve the capacity of drugs to repress neuroinflammation
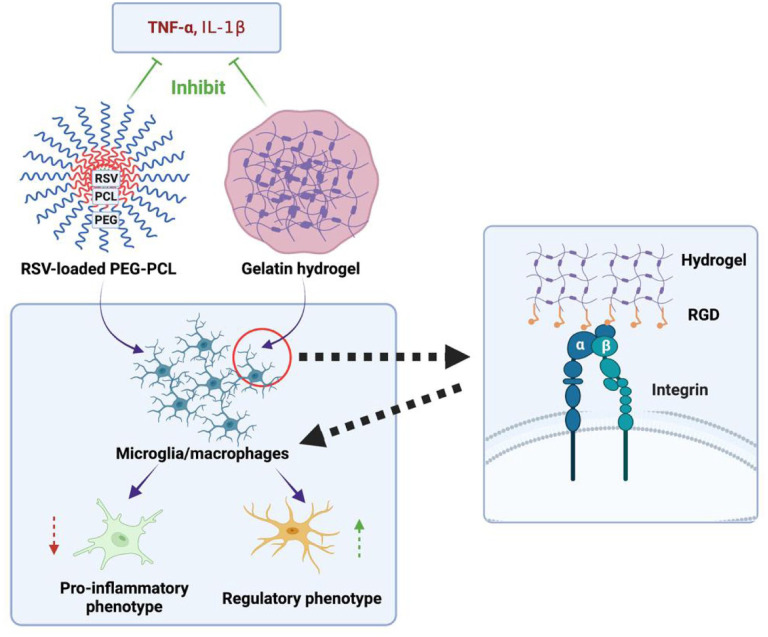
Statins are considered as neuroprotective agents that can reduce microglia activation and differentiation in many CNS diseases including ICH.91–93 Rosuvastatin is a synthetic, highly effective second-generation statin with poor water-solubility and low oral bioavailability. Polymeric nanomicelles as self-assembled copolymers are good candidates for poorly water-soluble or hydrophobic drugs.94–96 Rosuvastatin nanomicelles were prepared by cosolvent evaporation method using Poly (ethylene glycol)-poly (ε-caprolactone) (PEG-PCL) nanomicelles as nanocarrier 97 because of their good biocompatibility and nontoxicity.98–100 The rosuvastatin nanomicelles employed in the treatment after ICH inhibited the expression of downstream inflammatory factors such as tumor necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-1β), and promoted the polarization of microglia/macrophages toward a regulatory phenotype, effectively repressing neuroinflammation. 97
Injectable hydrogels are particularly suitable for ischemic and hemorrhagic stroke owing to their advantages of minimally invasive implantation.101,102 Hydrogels themselves can bind to specific cell-surface receptors of endogenous brain cells, inducing a variety of repair and anti-inflammatory cellular pathways. Gelatin is derived from denatured and partially degraded collagen. It has excellent biodegradability and biocompatibility, as well as adhesion to cells and lack of antigenicity, and can retain the cell adhesion motif of RGD. 103 There is an injectable hydrogel formed by Thiolated gelatin reacting with polyethylene glycol diacrylate. The in vivo experiment showed that the gelatin hydrogels interacted with different host cells, making it possible to attenuate postoperative neurological deficits, reduce neuronal loss, and repress the activation of astrocytes and microglia/macrophages. In addition, hydrogel injection reduced the release of inflammatory cytokines, IL-1β and TNF-α 104 (Figure 2).
Figure 2.
Schematic presentation of the effect of the injectable gelatin hydrogel and rosuvastatin nanomicelles on neuroinflammation after ICH. The implanted hydrogels bind to integrins on microglia/macrophages via cell adhesion RGD peptide, and then promote the regulatory phenotype polarization of the microglia/macrophages. Rosuvastatin-loaded PEG-PCL can also promote regulatory polarization.
PEG-PCL: Poly (ethylene glycol)-poly (ε-caprolactone); RGD: Arg-Gly-Asp.
Using nanomaterials can significantly promote brain tissue repair and functional recovery
Rebuilding the damaged CNS is a momentous goal for studies in neuroscience. Typical scaffolds are unsuitable in the damaged brain owing to massive manipulation of overlying tissues during implantation.105,106 Moreover, the advances of exogenous stem cells are still limited by some practical and ethical hurdles.107–110 In this light, injectable hydrogels are promising because they allow for a minimally invasive method that can eventually be compatible with current stereotactic procedures, as mentioned earlier.101,102 An injectable gelatin hydrogel containing epidermal growth factor (gelatin-EGF) as a therapy for ICH was evaluated. 111 This result indicated that growth factor-containing biomaterials could be safely introduced into an ICH cavity to rebuild tissue and potentially improve recovery in ICH rats. On this basis, the best time to inject the biomaterial and how long it will take to take effect after ICH still need exploration. Diverse recovery pathway and factors are involved in CNS recovery, 112 and given the good practices in brain ischemia, such as injectable hydrogels containing brain-derived neurotrophic factor was deposited into the cavity to promote repair and recovery,113–115 nanomaterials containing different growth factors or neuroprotective agents could be further studied.
A traditional self-assembly peptide nanofibrous (SAPNS) called RADA16-I, is frequently used in tissue engineering. 116 In an ICH model that incorporated stereotactic minimally invasive hematoma aspiration to provide space for local delivery of nanomaterials, 116 SAPNS served as a biocompatible material in the hemorrhagic brain cavity. It replaced the hematoma, reduced acute brain injury and brain cavity formation, and improved sensorimotor functional recovery. 117 With this ICH model, it has been found that RADA16-I could reduce acute brain injury and decrease the inflammatory response. In addition, a modified synthesized neutral SAPNS, which structure is RADA16-RGD (Arg-Gly-Asp), could overcome the disadvantages of RADA16-I, including poor biocompatibility caused by its acidity, very fine functional recovery and even no nerve fibers to ICH mice. 117 Another self-assembly peptide nanofibrous scaffold, RADA16mix, reduced acute brain injury, decreased inflammatory response, and promoted functional recovery and nerve fiber growth. 118
Potential applications of nanomaterials
The volume of hematoma is one of the most important predictors of functional outcome in ICH patients. 119 However, the clinical trials of hemostatic measures have turned out to be unsatisfactory. For example, intravenous administration of an activated recombinant factor VII did not improve functional outcomes after ICH, 120 and platelet transfusion was detrimental in patients taking antiplatelet therapy before ICH. 121 Compared to cell-derived microparticles, such as thrombosomes and synthocytes, which are ineffective at reducing bleeding and increase the risk of adverse immunoreaction, 122 nano-engineered agents have efficiently improved hemostasis with fewer side effects. 123 These are promising to improve the progress of ICH. Various topical NPs have been developed,124,125 but only a few have been tested in ICH. In this case, polymeric NPs are applied to stabilize the structure of some clot’s components such as platelets or fibrin, and reduce bleeding time. Synthetic platelets, made of a poly (lactic-coglycolic acid)-poly-L-lysine block copolymer core with PEG arms terminated with RGD functionalities, 126 bind to activated platelets and favor platelet aggregation, thus reducing bleeding time in arterial injury models. Further optimized NPs, which is closer to endogenous platelets owing to deformability characteristics, 127 could better accumulate at the wound site and improve the hemostatic functions of natural platelets. A similar approach, intravenous administration of a hemostatic polymer (PolySTAT), enhanced fibrin crosslinking and prevented clot degradation because of multiple binding sites for fibrin, and therefore improving survival by reducing blood loss in the trauma model. 128 The efficacy of these treatments for limiting bleeding in ICH remains to be confirmed. 129
In recent years, stem cell therapy has been a promising method for ICH treatment, aiming to replace the damaged neurons and therefore improve the functional recovery and prognosis post-ICH. 130 A magnetic resonance imaging (MRI)-visible PAsp (DMA)-Lys-(CA)2 polymer-based nanoscale polymeric micelle, has been used to deliver small interfering RNA/antisense oligonucleotides (siRNA/ASO) against Pnky long noncoding RNA (lncRNA) into neural stem cells. This multifunctional nanomedicine can direct stem cell differentiation in vivo and in vitro, as well as track the stem cells after transplantation in vivo in ischemic stroke. 131 If MRI-visible nanomaterials could be applied in ICH, it will be more targeted and more controllable for nanomaterials to play their roles and deliver therapeutic agents. Apart from delivering therapeutic genes into stem cells to regulate neuronal differentiation as vehicles, nanomaterials can exert their effects on stem cells in several ways as well. Nanomaterials can protect stem cells from a series of pathological processes such as inflammation, 132 and deliver growth-related factors to provide microenvironments for stem cells to survive and proliferate then form new tissue in the brain.89,133 Furthermore, nano-sized exosomes released by stem cells can reduce neuronal death and improve neurological function on their own.62,63 It is promising to develop nanomaterials-based stem cell therapies for ICH.
Based on the benefits of good biocompatibility, good targeting, and well-controlled drug release, 134 nanomaterials not only hold great prospects in treating primary and secondary injuries, but also in improving the neurological recovery after ICH. Meanwhile, many crossing strategies have combined nanotechnology with multiple substances such as cell-penetrating peptides, receptor, shuttle peptide, and cells to transport the therapeutic drugs through the BBB for improved precision of drug delivery, prolonged half-life, great stability, and increased drug loads. 135 In this condition, it is worthwhile to consider which nanomaterial to choose, whether nanomaterials need to be modified, and how to combine nanomaterials and agents when applied to ICH. More importantly, attention needs to be paid to whether the application of nanomaterials improves prognosis.
The concerns of nanomaterials
Toxicological analyses are essential before formulations or drugs are applying in clinical studies. The good news is that according to the pre-clinical experiments in ICH models, nanomaterials have not been found to cause renal or hepatic toxicity.54,75 However, despite the many advantages and advances of nanotechnology to improve neuroprotection, potential side effects and the complex metabolic processes of nanomaterials may limit their applications in clinical practice. For example, NPs based on pure copper, silver, or aluminum, can impair endothelial cell function and increase BBB permeability in rodents. 136 These processes are regarded as adverse in ICH, 137 although they might promote drugs into the brain. Furthermore, NPs are more easily identified as pathogens by the host than small molecules, resulting in rapid clearance and response of the complement system. 138 In addition to immune reactions that could be triggered by contact with blood components, nanomedicines may damage organelles such as mitochondria, endoplasmic reticulum or lysosome of macrophages, leading to the release of excessive ROS and pro-inflammatory mediators. 139 Since the nucleus is also exposed, DNA may be damaged, so extensive monitoring for genotoxicity is required. All these reasons should be noted in the application of ICH treatment, therefore, further in-depth assessment of the risks associated with the use of nanomaterials is necessary.
Summary
ICH is an intractable disorder. The emergence of nanotechnology provides a promising option for treating ICH. Compared to free drugs, nanomaterials improve the capacity of agents to cross the BBB, prolong the half-life of drugs, reduce adverse effects, and enhance drug stability. Citations within this review highlight that nanomaterials can exert protective role after ICH. However, current intervention approaches based on nanomedicine are far from being clinical applications. Moreover, we should consider their side effects. The toxicity of nanomaterials should be thoroughly investigated before they are applied to patient treatment. Fortunately, the toxicity of nanomaterials has received increasing attention, and more measures have been developed in the field of nanomedicine140–142 to support their eventual clinical translation. Furthermore, the application of nanomaterials should be based on clear drug targets and pathways.
Based on a series of conservative treatments, our attention has been turned to some innovative therapeutics targeting ICH. Among all, nanotechnology is undoubtedly a very efficient and effective method. More and more novel and useful nanomaterials are being developed to treat ICH and this has brought hope in improving the prognosis of this often-fatal form of stroke.
Footnotes
Author contributions: All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors acknowledge operating grant support from National Key Research and Development Program of China (grant no: 2018YFC1312200), the National Natural Science Foundation of China (grants no: 82071331, 81870942, and 81520108011), and from the Canadian Institutes of Health Sciences (VWY).
ORCID iD: Mengzhou Xue  https://orcid.org/0000-0002-4427-7285
https://orcid.org/0000-0002-4427-7285
References
- 1.Hua Y, Keep RF, Hoff JT, et al. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke 2007; 38: 759–762. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Khan S, Liu Y, et al. Modes of brain cell death following intracerebral hemorrhage. Front Cell Neurosci 2022; 16: 799753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai Q, Xue M, Yong VW.Microglia and macrophage phenotypes in intracerebral haemorrhage injury: therapeutic opportunities. Brain 2020; 143: 1297–1314. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Bai Q, Yong VW, et al. EMMPRIN promotes the expression of MMP-9 and exacerbates neurological dysfunction in a mouse model of intracerebral hemorrhage. Neurochem Res 2022; 47: 2383–2395. [DOI] [PubMed] [Google Scholar]
- 5.Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005; 365: 387–397. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Chen T, Mao G, et al. Clinical neurorestorative therapeutic guideline for brainstem hemorrhage (2020 China version). J Neurorestoratology 2020; 8: 232–240. [Google Scholar]
- 7.Zhang Y, Zhang X, Yong VW, et al. Vildagliptin improves neurological function by inhibiting apoptosis and ferroptosis following intracerebral hemorrhage in mice. Neurosci Lett 2022; 776: 136579. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Li Z, Khan S, et al. Neuroprotection of minocycline by inhibition of extracellular matrix metalloproteinase inducer expression following intracerebral hemorrhage in mice. Neurosci Lett 2021; 764: 136297. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Liu Y, Wei R, et al. The combination of deferoxamine and minocycline strengthens neuroprotective effect on acute intracerebral hemorrhage in rats. Neurol Res 2021; 43: 854–864. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Wang F, Li Z, et al. Neuroprotective effects of chlorogenic acid in a mouse model of intracerebral hemorrhage associated with reduced extracellular matrix metalloproteinase inducer. Biomolecules 2022; 12: 1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang F, Zhang X, Liu Y, et al. Neuroprotection by ozanimod following intracerebral hemorrhage in mice. Front Mol Neurosci 2022; 15: 927150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Zhang Y, Wang F, et al. Necrosulfonamide alleviates acute brain injury of intracerebral hemorrhage via inhibiting inflammation and necroptosis. Front Mol Neurosci 2022; 15: 916249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai Q, Sheng Z, Liu Y, et al. Intracerebral haemorrhage: from clinical settings to animal models. Stroke Vasc Neurol 2020; 5: 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo T, Guo T, Yang Q, et al. In situ hydrogels enhancing postoperative functional recovery by reducing iron overload after intracerebral haemorrhage. Int J Pharm 2017; 534:179–189. [DOI] [PubMed] [Google Scholar]
- 15.Hemphill Jc, 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46: 2032–2060. [DOI] [PubMed] [Google Scholar]
- 16.Wagner I, Hauer EM, Staykov D, et al. Effects of continuous hypertonic saline infusion on perihemorrhagic edema evolution. Stroke 2011; 42: 1540–1545. [DOI] [PubMed] [Google Scholar]
- 17.Hanley DF, Lane K, McBee N, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet 2017; 389: 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrag M, Kirshner H.Management of intracerebral hemorrhage: JACC focus seminar. J Am Coll Cardiol 2020; 75: 1819–1831. [DOI] [PubMed] [Google Scholar]
- 19.Anderson CS, Heeley E, Huang Y, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. New Engl J Med 2013; 368: 2355–2365. [DOI] [PubMed] [Google Scholar]
- 20.Qureshi AI, Palesch YY, Barsan WG, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. New Engl J Med 2016; 375: 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rehmani R, Han A, Hassan J, et al. Role of prothrombin complex concentrate (PCC) in acute intracerebral hemorrhage with positive CTA spot sign: an institutional experience at a regional and state designated stroke center. Emerg Radiol 2017; 24: 241–247. [DOI] [PubMed] [Google Scholar]
- 22.Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer 2017; 17: 20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lammers T, Aime S, Hennink WE, et al. Theranostic nanomedicine. Account Chem Res 2011; 44: 1029–1038. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Wang W, Yu DX, et al. Application of nanodiagnostics and nanotherapy to CNS diseases. Nanomed 2018; 13: 2341–2371. [DOI] [PubMed] [Google Scholar]
- 25.Farokhzad OC, Langer R.Impact of nanotechnology on drug delivery. ACS Nano 2009; 3: 16–20. [DOI] [PubMed] [Google Scholar]
- 26.Mishra D, Hubenak JR, Mathur AB.Nanoparticle systems as tools to improve drug delivery and therapeutic efficacy. J Biomed Mater Res A 2013; 101: 3646–3660. [DOI] [PubMed] [Google Scholar]
- 27.Lu X, Zhang Y, Wang L, et al. Development of L-carnosine functionalized iron oxide nanoparticles loaded with dexamethasone for simultaneous therapeutic potential of blood brain barrier crossing and ischemic stroke treatment. Drug Deliv 2021; 28: 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu R, Yang J, Liu L, et al. An “Amyloid-β Cleaner” for the treatment of Alzheimer’s disease by normalizing microglial dysfunction. Adv Sci 2020; 7: 1901555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim D, Yoo JM, Hwang H, et al. Graphene quantum dots prevent α-synucleinopathy in Parkinson’s disease. Nat Nanotechnol 2018; 13: 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaushik A, Jayant RD, Bhardwaj V, et al. Personalized nanomedicine for CNS diseases. Drug Discov Today 2018; 23: 1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanco E, Shen H, Ferrari M.Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 2015; 33: 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weissleder R, Kelly K, Sun EY, et al. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol 2005; 23: 1418–1423. [DOI] [PubMed] [Google Scholar]
- 33.Lo EH.A new penumbra: transitioning from injury into repair after stroke. Nat Med 2008; 14: 497–500. [DOI] [PubMed] [Google Scholar]
- 34.Braeuninger S, Kleinschnitz C.Rodent models of focal cerebral ischemia: procedural pitfalls and translational problems. Exp Transl Stroke Med 2009; 1: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul S, Candelario-Jalil E.Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies. Exp Neurol 2021; 335: 113518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacchetti ML.Is it time to definitely abandon neuroprotection in acute ischemic stroke? Stroke 2008; 39: 1659–1660. [DOI] [PubMed] [Google Scholar]
- 37.Wei R, Xu Y, Xue M.Hollow iron oxide nanomaterials: synthesis, functionalization, and biomedical applications. J Mater Chem B 2021; 9: 1965–1979. [DOI] [PubMed] [Google Scholar]
- 38.Ramos-Cabrer P, Campos F.Liposomes and nanotechnology in drug development: focus on neurological targets. Int J Nanomed 2013; 8: 951–960. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Da Silva-Candal A, Argibay B, Iglesias-Rey R, et al. Vectorized nanodelivery systems for ischemic stroke: a concept and a need. J Nanobiotechnol 2017; 15: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Z, Khan S, Liu Y, et al. Therapeutic strategies for intracerebral hemorrhage. Front Neurol 2022; 13: 1032343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xi G, Keep RF, Hoff JT.Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 2006; 5: 53–63. [DOI] [PubMed] [Google Scholar]
- 42.Cao JY, Dixon SJ.Mechanisms of ferroptosis. Cell Mol Life Sci 2016; 73: 2195–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dharmalingam P, Talakatta G, Mitra J, et al. Pervasive genomic damage in experimental intracerebral hemorrhage: Therapeutic Potential of a mechanistic-based carbon nanoparticle. ACS Nano 2020; 14: 2827–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu H, Wu T, Xu X, et al. Iron toxicity in mice with collagenase-induced intracerebral hemorrhage. J Cereb Blood Flow Metab 2011; 31: 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao F, Hua Y, He Y, et al. Minocycline-induced attenuation of iron overload and brain injury after experimental intracerebral hemorrhage. Stroke 2011; 42: 3587–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garton T, Keep RF, Hua Y, et al. Brain iron overload following intracranial haemorrhage. Stroke Vasc Neurol 2016; 1: 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou Y, Wang K, Wan W, et al. Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis 2018; 5: 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonsack F, Alleyne Ch, Jr, Sukumari-Ramesh S.Resveratrol attenuates neurodegeneration and improves neurological outcomes after intracerebral hemorrhage in mice. Front Cell Neurosci 2017; 11: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai JC, Liu W, Lu F, et al. Resveratrol attenuates neurological deficit and neuroinflammation following intracerebral hemorrhage. Exp Ther Med 2018; 15: 4131–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao R, Zhao K, Su H, et al. Resveratrol ameliorates brain injury via the TGF-β-mediated ERK signaling pathway in a rat model of cerebral hemorrhage. Exp Ther Med 2019; 18: 3397–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abd Aziz NAW, Iezhitsa I, Agarwal R, et al. Neuroprotection by trans-resveratrol against collagenase-induced neurological and neurobehavioural deficits in rats involves adenosine A1 receptors. Neurol Res 2020; 42: 189–208. [DOI] [PubMed] [Google Scholar]
- 52.Bano S, Ahmed F, Khan F, et al. Enhancement of the cancer inhibitory effect of the bioactive food component resveratrol by nanoparticle based delivery. Food Funct 2020; 11: 3213–3226. [DOI] [PubMed] [Google Scholar]
- 53.Duarte AC, Rosado T, Costa AR, et al. The bitter taste receptor TAS2R14 regulates resveratrol transport across the human blood-cerebrospinal fluid barrier. Biochem Pharmacol 2020; 177: 113953. [DOI] [PubMed] [Google Scholar]
- 54.Mo Y, Duan L, Yang Y, et al. Nanoparticles improved resveratrol brain delivery and its therapeutic efficacy against intracerebral hemorrhage. Nanoscale 2021; 13: 3827–3840. [DOI] [PubMed] [Google Scholar]
- 55.Duan J, Zhang Y, Chen W, et al. Cationic polybutyl cyanoacrylate nanoparticles for DNA delivery. J Biomed Biotechnol 2009; 2009: 149254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baier G, Musyanovych A, Landfester K, et al. DNA amplification via polymerase chain reaction inside miniemulsion droplets with subsequent poly(n-butylcyanoacrylate) shell formation and delivery of polymeric capsules into mammalian cells. Macromol Biosci 2011; 11: 1099–1109. [DOI] [PubMed] [Google Scholar]
- 57.Lin Y, Pan Y, Shi Y, et al. Delivery of large molecules via poly(butyl cyanoacrylate) nanoparticles into the injured rat brain. Nanotechnol 2012; 23: 165101. [DOI] [PubMed] [Google Scholar]
- 58.Chung CY, Yang JT, Kuo YC.Polybutylcyanoacrylate nanoparticles for delivering hormone response element-conjugated neurotrophin-3 to the brain of intracerebral hemorrhagic rats. Biomaterials 2013; 34: 9717–9727. [DOI] [PubMed] [Google Scholar]
- 59.Gurunathan S, Kang MH, Jeyaraj M, et al. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 2019; 8: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao Y, Geng F, Wang G, et al. Bone marrow-derived mesenchymal stem cells-derived exosomes prevent oligodendrocyte apoptosis through exosomal miR-134 by targeting caspase-8. J Cell Biochem 2019; 120: 2109–2118. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Zhao R, Liu D, et al. Exosomes derived from miR-214-Enriched bone marrow-derived mesenchymal stem cells regulate oxidative damage in cardiac stem cells by targeting CaMKII. Oxid Med Cell Longev 2018; 2018: 4971261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yi X, Tang X.Exosomes from miR-19b-3p-Modified ADSCs inhibit ferroptosis in intracerebral hemorrhage mice. Front Cell Dev Biol 2021; 9: 661317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duan S, Wang F, Cao J, et al. Exosomes derived from MicroRNA-146a-5p-Enriched bone marrow mesenchymal stem cells alleviate intracerebral hemorrhage by inhibiting neuronal apoptosis and microglial M1 polarization. Drug Des Devel Ther 2020; 14: 3143–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu A, Noble EE, Tyagi E, et al. Curcumin boosts DHA in the brain: Implications for the prevention of anxiety disorders. Biochim Biophys Acta 2015; 1852: 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang XS, Zhang ZR, Zhang MM, et al. Neuroprotective properties of curcumin in toxin-base animal models of Parkinson’s disease: a systematic experiment literatures review. BMC Complement Altern Med 2017; 17: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bielak-Zmijewska A, Grabowska W, Ciolko A, et al. The role of curcumin in the modulation of Ageing. Int J Mol Sci 2019; 20: eng1010927911422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lopresti AL, Drummond PD.Efficacy of curcumin, and a saffron/curcumin combination for the treatment of major depression: a randomised, double-blind, placebo-controlled study. J Affect Disord 2017; 207: 188–196. [DOI] [PubMed] [Google Scholar]
- 68.Tang M, Taghibiglou C.The mechanisms of action of curcumin in Alzheimer’s disease. J Alzheimers Dis 2017; 58: 1003–1016. [DOI] [PubMed] [Google Scholar]
- 69.Qureshi M, Al-Suhaimi EA, Wahid F, et al. Therapeutic potential of curcumin for multiple sclerosis. Neurol Sci 2018; 39: 207–214. [DOI] [PubMed] [Google Scholar]
- 70.Chongtham A, Agrawal N.Curcumin modulates cell death and is protective in Huntington’s disease model. Sci Rep 2016; 6: 18736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hädrich G, Vaz GR, Maidana M, et al. Anti-inflammatory effect and toxicology analysis of oral delivery quercetin nanosized emulsion in rats. Pharm Res 2016; 33: 983–993. [DOI] [PubMed] [Google Scholar]
- 72.Lee J-C, Kim J, Park J-K, et al. The antioxidant, rather than prooxidant, activities of quercetin on normal cells: quercetin protects mouse thymocytes from glucose oxidase-mediated apoptosis. Exp Cell Res 2003; 291: 386–397. [DOI] [PubMed] [Google Scholar]
- 73.Ma Z, Wang N, He H, et al. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J Control Release 2019; 316: 359–380. [DOI] [PubMed] [Google Scholar]
- 74.Yousef MI, Omar SA, El-Guendi MI, et al. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem Toxicol 2010; 48: 3246–3261. [DOI] [PubMed] [Google Scholar]
- 75.Marques MS, Cordeiro MF, Marinho MAG, et al. Curcumin-loaded nanoemulsion improves haemorrhagic stroke recovery in wistar rats. Brain Res 2020; 1746: 147007. [DOI] [PubMed] [Google Scholar]
- 76.Galho AR, Cordeiro MF, Ribeiro SA, et al. Protective role of free and quercetin-loaded nanoemulsion against damage induced by intracerebral haemorrhage in rats. Nanotechnol 2016; 27: 175101. [DOI] [PubMed] [Google Scholar]
- 77.Zachara BA.Selenium and selenium-dependent antioxidants in chronic kidney disease. Adv Clin Chem 2015; 68: 131–151. [DOI] [PubMed] [Google Scholar]
- 78.Zhai X, Zhang C, Zhao G, et al. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J Nanobiotechnol 2017; 15: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X, Deng G, Wang Y, et al. A novel and facile synthesis of porous SiO2-coated ultrasmall Se particles as a drug delivery nanoplatform for efficient synergistic treatment of cancer cells. Nanoscale 2016; 8: 8536–8541. [DOI] [PubMed] [Google Scholar]
- 80.Yang Y, Deng G, Wang P, et al. A selenium nanocomposite protects the mouse brain from oxidative injury following intracerebral hemorrhage. Int J Nanomedicine 2021; 16: 775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bao Q, Hu P, Xu Y, et al. Simultaneous Blood-brain barrier crossing and protection for stroke treatment based on edaravone-loaded ceria nanoparticles. ACS Nano 2018; 12: 6794–6805. [DOI] [PubMed] [Google Scholar]
- 82.Jeong HG, Cha BG, Kang DW, et al. Ceria nanoparticles synthesized with aminocaproic acid for the treatment of subarachnoid hemorrhage. Stroke 2018; 49: 3030–3038. [DOI] [PubMed] [Google Scholar]
- 83.Zheng J, Lu J, Mei S, et al. Ceria nanoparticles ameliorate white matter injury after intracerebral hemorrhage: microglia-astrocyte involvement in remyelination. J Neuroinflammation 2021; 18: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hahn SM, Lepinski DL, DeLuca AM, et al. Neurophysiological consequences of nitroxide antioxidants. Can J Physiol Pharmacol 1995; 73: 399–403. [DOI] [PubMed] [Google Scholar]
- 85.Samuni A, Krishna CM, Riesz P, et al. A novel metal-free low molecular weight superoxide dismutase mimic. J Biol Chem 1988; 263: 17921–17924. [PubMed] [Google Scholar]
- 86.Chonpathompikunlert P, Fan C-H, Ozaki Y, et al. Redox nanoparticle treatment protects against neurological deficit in focused ultrasoundinduced intracerebral hemorrhage. Nanomed 2012; 7: 1029–1043. [DOI] [PubMed] [Google Scholar]
- 87.Maeda H, Wu J, Sawa T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 2000; 65: 271–284. [DOI] [PubMed] [Google Scholar]
- 88.Maeda H, Fang J, Inutsuka T, et al. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int Immunopharmacol 2003; 3: 319–328. [DOI] [PubMed] [Google Scholar]
- 89.Chonpathompikunlert P, Yoshitomi T, Han J, et al. Chemical nanotherapy: nitroxyl radical-containing nanoparticle protects neuroblastoma SH-SY5Y cells from aβ-induced oxidative stress. Ther Deliv 2011; 2: 585–597. [DOI] [PubMed] [Google Scholar]
- 90.Krishna MC, Russo A, Mitchell JB, et al. Do nitroxide antioxidants act as scavengers of O2-. Or as SOD mimics? J Biol Chem 1996; 271: 26026–26031. [DOI] [PubMed] [Google Scholar]
- 91.Youssef S, Stüve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 2002; 420: 78–84. [DOI] [PubMed] [Google Scholar]
- 92.Cheng SM, Lai JH, Yang SP, et al. Modulation of human T cells signaling transduction by lovastatin. Int J Cardiol 2010; 140: 24–33. [DOI] [PubMed] [Google Scholar]
- 93.Bagheri H, Ghasemi F, Barreto GE, et al. The effects of statins on microglial cells to protect against neurodegenerative disorders: a mechanistic review. Biofactors 2020; 46: 309–325. [DOI] [PubMed] [Google Scholar]
- 94.Ding J, Chen J, Gao L, et al. Engineered nanomedicines with enhanced tumor penetration. Nano Today 2019; 29: 19. [Google Scholar]
- 95.Feng X, Xu W, Li Z, et al. Immunomodulatory nanosystems. Adv Sci 2019; 6: 1900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Han S, Huang K, Gu Z, et al. Tumor immune microenvironment modulation-based drug delivery strategies for cancer immunotherapy. Nanoscale 2020; 12: 413–436. [DOI] [PubMed] [Google Scholar]
- 97.Zi L, Zhou W, Xu J, et al. Rosuvastatin nanomicelles target neuroinflammation and improve neurological deficit in a mouse model of intracerebral hemorrhage. Int J Nanomed 2021; 16: 2933–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu C, Xu Q, Chen X, et al. Delivery luteolin with folacin-modified nanoparticle for glioma therapy. Int J Nanomed 2019; 14: 7515–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y, Xie J, Ai Z, et al. Nobiletin-loaded micelles reduce ovariectomy-induced bone loss by suppressing osteoclastogenesis. Int J Nanomed 2019; 14: 7839–7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang L, Zhang Z, Hou J, et al. Targeted delivery of ginsenoside compound K using TPGS/PEG-PCL mixed micelles for effective treatment of lung cancer. Int J Nanomed 2017; 12: 7653–7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dimatteo R, Darling NJ, Segura T.In situ forming injectable hydrogels for drug delivery and wound repair. Adv Drug Deliv Rev 2018; 127: 167–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang C, Feng N, Chang F, et al. Injectable cholesterol-enhanced stereocomplex polylactide thermogel loading chondrocytes for optimized cartilage regeneration. Adv Healthc Mater 2019; 8: e1900312. [DOI] [PubMed] [Google Scholar]
- 103.Echave MC, Saenz del Burgo L, Pedraz JL, et al. Gelatin as biomaterial for tissue engineering. Curr Pharm Des 2017; 23: 3567–3584. [DOI] [PubMed] [Google Scholar]
- 104.Xu J, Duan Z, Qi X, et al. Injectable gelatin hydrogel suppresses inflammation and enhances functional recovery in a mouse model of intracerebral hemorrhage. Front Bioeng Biotechnol 2020; 8: 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deguchi K, Tsuru K, Hayashi T, et al. Implantation of a new porous gelatin-siloxane hybrid into a brain lesion as a potential scaffold for tissue regeneration. J Cereb Blood Flow Metab 2006; 26: 1263–1273. [DOI] [PubMed] [Google Scholar]
- 106.Elias PZ, Spector M.Treatment of penetrating brain injury in a rat model using collagen scaffolds incorporating soluble Nogo receptor. J Tissue Eng Regen Med 2015; 9: 137–150. [DOI] [PubMed] [Google Scholar]
- 107.Lam J, Lowry WE, Carmichael ST, et al. Delivery of iPS-NPCs to the stroke cavity within a hyaluronic acid matrix promotes the differentiation of transplanted cells. Adv Funct Mater 2014; 24: 7053–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Borlongan CV.Preliminary reports of stereotaxic stem cell transplants in chronic stroke patients. Mol Ther 2016; 24: 1710–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhong J, Chan A, Morad L, et al. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil Neural Repair 2010; 24: 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Steinberg GK, Kondziolka D, Wechsler LR, et al. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a Phase 1/2a study. Stroke 2016; 47: 1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lim TC, Mandeville E, Weng D, et al. Hydrogel-based therapy for Brain Repair after intracerebral hemorrhage. Transl Stroke Res 2020; 11: 412–417. [DOI] [PubMed] [Google Scholar]
- 112.Kokaia Z, Martino G, Schwartz M, et al. Cross-talk between neural stem cells and immune cells: the key to better brain repair? Nat Neurosci 2012; 15: 1078–1087. [DOI] [PubMed] [Google Scholar]
- 113.Nih LR, Carmichael ST, Segura T.Hydrogels for brain repair after stroke: an emerging treatment option. Curr Opin Biotechnol 2016; 40: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lim TC, Rokkappanavar S, Toh WS, et al. Chemotactic recruitment of adult neural progenitor cells into multifunctional hydrogels providing sustained SDF-1α release and compatible structural support. FASEB J 2013; 27: 1023–1033. [DOI] [PubMed] [Google Scholar]
- 115.Cook DJ, Nguyen C, Chun HN, et al. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J Cereb Blood Flow Metab 2017; 37: 1030–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sang Y-H, Liang Y-X, Liu LG, et al. Rat model of intracerebral hemorrhage permitting hematoma aspiration plus intralesional injection. Exp Anim 2013; 62: 63–69. [DOI] [PubMed] [Google Scholar]
- 117.Hua Sang LY. A self-assembling nanomaterial reduces acute brain injury and enhances functional recovery in a rat model of hypertensive intracerebral hemorrhage. J Nanomed Nanotech 2015; 05: 611–620. [DOI] [PubMed] [Google Scholar]
- 118.Zhang N, Luo Y, He L, et al. A self-assembly peptide nanofibrous scaffold reduces inflammatory response and promotes functional recovery in a mouse model of intracerebral hemorrhage. Nanomed 2016; 12: 1205–1217. [DOI] [PubMed] [Google Scholar]
- 119.Broderick JP, Brott TG, Duldner JE, et al. Volume of intracerebral hemorrhage A powerful and Easy-to-Use predictor of 30-Day mortality. Stroke 1993; 24: 987–993. [DOI] [PubMed] [Google Scholar]
- 120.Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. New Engl J Med 2008; 358: 2127–2137. [DOI] [PubMed] [Google Scholar]
- 121.Baharoglu MI, Cordonnier C, Salman RA-S, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet 2016; 387: 2605–2613. [DOI] [PubMed] [Google Scholar]
- 122.Shoffstall AJ, Atkins KT, Groynom RE, et al. Intravenous hemostatic nanoparticles increase survival following blunt trauma injury. Biomacromolecules 2012; 13: 3850–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hangge P, Stone J, Albadawi H, et al. Hemostasis and nanotechnology. Cardiovasc Diagn Ther 2017; 7: S267–S275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luo Z, Wang S, Zhang S.Fabrication of self-assembling D-form peptide nanofiber scaffold d-EAK16 for rapid hemostasis. Biomaterials 2011; 32: 2013–2020. [DOI] [PubMed] [Google Scholar]
- 125.Gu BK, Park SJ, Kim MS, et al. Fabrication of sonicated chitosan nanofiber mat with enlarged porosity for use as hemostatic materials. Carbohydr Polym 2013; 97: 65–73. [DOI] [PubMed] [Google Scholar]
- 126.Bertram JP, Williams CA, Robinson R, et al. Intravenous hemostat: nanotechnology to halt bleeding. Sci Transl Med 2009; 1: 11ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Anselmo AC, Modery-Pawlowski CL, Menegatti S, et al. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano 2014; 8: 11243–11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chan LW, Wang X, Wei H, et al. A synthetic fibrin cross-linking polymer for modulating clot properties and inducing hemostasis. Sci Transl Med 2015; 7: 277ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bonnard T, Gauberti M, Martinez de Lizarrondo S, et al. Recent advances in nanomedicine for ischemic and hemorrhagic stroke. Stroke 2019; 50: 1318–1324. [DOI] [PubMed] [Google Scholar]
- 130.Wei L, Wei ZZ, Jiang MQ, et al. Stem cell transplantation therapy for multifaceted therapeutic benefits after stroke. Prog Neurobiol 2017; 157: 49–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lin B, Lu L, Wang Y, et al. Nanomedicine directs neuronal differentiation of neural stem cells via silencing long noncoding RNA for stroke therapy. Nano Lett 2021; 21: 806–815. [DOI] [PubMed] [Google Scholar]
- 132.Ahmad A, Fauzia E, Kumar M, et al. Gelatin-coated polycaprolactone nanoparticle-mediated naringenin delivery rescue human mesenchymal stem cells from oxygen glucose deprivation-induced inflammatory stress. ACS Biomater Sci Eng 2019; 5: 683–695. [DOI] [PubMed] [Google Scholar]
- 133.Liu Z, Yuan X, Fernandes G, et al. The combination of nano-calcium sulfate/platelet rich plasma gel scaffold with BMP2 gene-modified mesenchymal stem cells promotes bone regeneration in rat critical-sized calvarial defects. Stem Cell Res Ther 2017; 8: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Torres FG, Troncoso OP, Pisani A, et al. Natural polysaccharide nanomaterials: an overview of their immunological properties. Int J Mol Sci 2019; 20: 5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang Y, Guo P, Ma Z, et al. Combination of cell-penetrating peptides with nanomaterials for the potential therapeutics of central nervous system disorders: a review. J Nanobiotechnol 2021; 19: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sharma HS, Hussain S, Schlager J, et al. Influence of nanoparticles on blood-brain barrier permeability and brain edema formation in rats. Acta Neurochir Suppl 2010; 106: 359–364. [DOI] [PubMed] [Google Scholar]
- 137.Tschoe C, Bushnell CD, Duncan PW, et al. Neuroinflammation after intracerebral hemorrhage and potential therapeutic targets. J Stroke 2020; 22: 29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pallardy MJ, Turbica I, Biola-Vidamment A.Why the immune system should Be concerned by nanomaterials? Front Immunol 2017; 8: 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alexander A, Agrawal M, Uddin A, et al. Recent expansions of novel strategies towards the drug targeting into the brain. Int J Nanomed 2019; 14: 5895–5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Croissant JG, Fatieiev Y, Khashab NM.Degradability and clearance of silicon, organosilica, silsesquioxane, silica mixed oxide, and mesoporous silica nanoparticles. Adv Mater (Deerfield Beach, Fla.) 2017; 29: 1604634. [DOI] [PubMed] [Google Scholar]
- 141.Bonnard T, Jayapadman A, Putri JA, et al. Low-Fouling and biodegradable protein-based particles for thrombus imaging. ACS Nano 2018; 12: 6988–6996. [DOI] [PubMed] [Google Scholar]
- 142.Luk BT, Zhang L.Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release 2015; 220: 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]