Abstract
The severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) is the causative factor behind the 2019 global coronavirus pandemic (COVID-19). The main protease, known as Mpro, is encoded by the viral genome and is essential for viral replication. It has also been an effective target for drug development. In this review, we discuss the rationale for inhibitors that specifically target SARS-CoV-2 Mpro. Small molecules and peptidomimetic inhibitors are two types of inhibitor with various modes of action and we focus here on novel inhibitors that were only discovered during the COVID-19 pandemic highlighting their binding modes and structures.
Keywords: Antiviral, Coronavirus, COVID-19, Protease inhibitors, SARS-CoV-2
Introduction
Around 6.5 million people have died from, and over 600 million have been infected with, COVID-19 since SARS-CoV-2 was first discovered in Wuhan, China, in December 2019.1 The main clinical signs of COVID-19 are fever, coughing, and shortness of breath. These symptoms can quickly progress to respiratory and cardiac failure, which requires mechanical ventilation.1 Older, immunocompromised individuals, and those who have co-morbid metabolic, pulmonary, and cardiac diseases are more likely to die from COVID-19. It is suggested that the bat coronavirus RaTG13, which shares 96% of its sequence with SARS-CoV-2, is the closest strain.2 Over the years, numerous fatal virus epidemics have afflicted humanity, including those caused by the Ebola virus,3 Zika virus,4 Nipah virus,5 and, more recently, other coronaviruses (CoVs), such as SARS-CoV-16 and Middle East respiratory syndrome (MERS)-CoV.7 However, the most significant public health emergency since the Spanish influenza pandemic is the recent SARS-CoV-2 epidemic.8
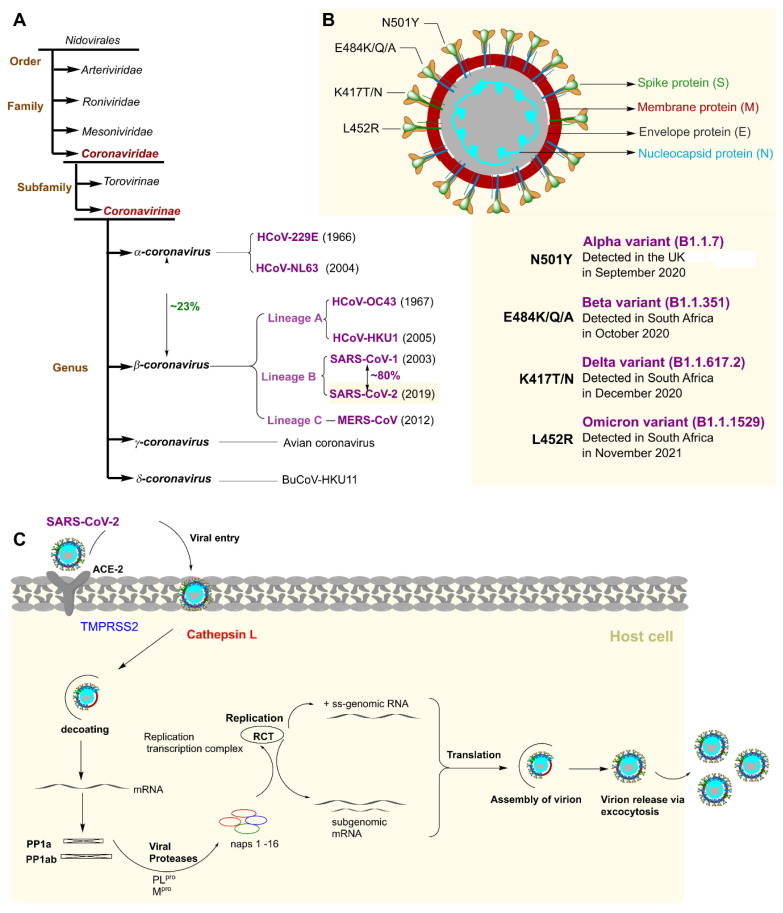
Even though successful vaccination programs against COVID-19 are available worldwide, little has so far been accomplished in the development of antivirals to treat COVID-19.9 The disparity in COVID-19 vaccination coverage, vaccine resistance, and the emergence of SARS-CoV-2 variants of concern (VOC) with increased transmission potential and the potential to evade both vaccination and acquired immunity emphasize the relevance of developing antiviral drugs to treat SARS-CoV-2 infections.10 Variant B.1.1.7, which was first identified in the UK, and B.1.617, which was identified in India, are SARS-CoV-2 variants that are more transmissible and can go beyond immune protection. B.1.617 has since been reported by 20 more countries.11 On November 26, 2021, the WHO received a report of a novel SARS-CoV-2 variant of concern: B.1.1.529 (Figure 1 a, b) with a significant number of mutations (https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern).
Figure 1.
Schematic classification of HCoV group and SARS-CoV-2 life cycle. (a) Schematic of human coronavirus (HCoV) taxonomy. The four genera, α-, β-, γ-, and δ, which contain the seven human coronaviruses, are highlighted in purple. The year of discovery of each HCoV is noted in brackets. (b) CoV structure that includes all of the functional proteins and various variations. The Alpha, Beta, Gamma, and Omicron variants all share the N501Y substitution. Whereas the L452R substitution is unique to the Delta variant, the E484K/Q/A and K417T/N substitutions are mostly found in the Beta, Gamma, and Omicron strains. (c) Life cycle of severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2). Abbreviations: ACE-2, angiotensin-converting enzyme 2; MERS, Middle East respiratory syndrome; TMPRSS2, transmembrane protease serine subtype 2.
SARS-CoV-2 contains a 3′ poly-A tail and a 5′ cap on its 29.9 kb single-stranded (ss), non-segmented RNA genome. The polyproteins pp1a and pp1ab are produced by two large open reading frames (ORF 1a/b) that are encoded by the 20 kb of the 5′ genome.9, 10, 12 The non-structural proteins (nsp) 1–16 are produced by the cleavage of polyproteins by the viral papain-like protease (PLpro) and the 3C-like cysteine protease (3CLpro), also known as Mpro. The LXGG recognition sites at nsp1, nsp2, and nsp3 are the cleavage site of PLpro, whereas Mpro cleaves the remaining downstream nsp4–16.13, 14
SARS-CoV-1 and SARS-CoV-2 share 100% of the same active site and Mpro sequences.15 To find promising antiviral leads against SARS-CoV-2, high-throughput tests have been successfully used for complete pharmacological screening of already-available drugs. Through a virtual structure-based, high-throughput screening of a library of over 10,000 chemicals, carbofur and ebselen were discovered to be inhibitors of SARS-CoV-2 infection of Vero cells.16, 17 A library of 12,000 small compounds in clinical development or with US Food and Drug Administration (FDA)-approved status led to the discovery of apilimod, MDL-28170, and ONO 5334, which inhibit SARS-CoV-2.16, 17, 18
The HIV drug combination of lopinavir–ritonavir inhibits protease activity19 and is used to both prevent and treat HIV infection. In nonhuman primates, lopinavir has been successful in enhancing the clinical outcome of MERS and has demonstrated in vitro action against SARS-CoV-1.20 A therapy regimen for COVID-19 could also involve the use of medications that directly target viral enzymes in conjunction with agents that target viral and host factors.21 It has been demonstrated that camostat mesylate inhibits TMPRSS2, a plasma membrane-associated host serine protease that is involved in SARS-CoV-1 cell entrance.22
Antivirals that target RNA-dependent and RNA polymerase (RdRp), such as remdesivir, favipiravir, and galidesivir, have demonstrated inhibitory effects against SARS-CoV-2.23, 24, 25, 26 The FDA awarded remdesivir emergency use permission for SARS-CoV-2 on May 1, 2020.27, 28 Remdesivir can assist patients with severe COVID-19 and minimize the duration of infections, but it does not significantly increase survival rates.29 Further, molnupiravir (MK-4482, EIDD-2801), an oral RdRp inhibitor, has been proven beneficial in treating individuals at the beginning of their illness. 30 (p2)
Nirmatrelvir, a peptidomimetic Mpro inhibitor, is now commercialized as a treatment for COVID-19 disease under the trade name Paxlovid. On December 22, 2021, the FDA approved the use of Paxlovid for high-risk patients with a confirmed SARS-CoV-2 infection. 31
Identification of potential drug targets
Designing drug compounds as small-molecule medications requires careful consideration of the target. SARS-CoV-2 is a positive-sense enveloped ssRNA virus. It is a member of the beta-genus coronavirus group, which also contains SARS-CoV-1, MERS-CoV, HCoV-OC43, and HCoV-HKU1 (Figure 1). Given tht they share ∼80% global sequence identity, most antiviral agents for SARS-CoV-2 were initially developed against SARS-CoV-1 or other related coronaviruses.32
Understanding the SARS-CoV-2 life cycle (Figure 1c) in detail is necessary to identify possible drug targets. SARS-CoV-2 exclusively infects cells that express angiotensin-converting enzyme 2 (ACE2) by merging with the cell surface or entering through the endosome. The host transmembrane protease serine subtype 2 (TMPRSS2) degrades the viral spike protein, which leads to the viral membrane immediately fusing with the host cell membrane.33 Endosomal entrance is made easier by cleavage of the viral spike protein by cathepsin L.34 The viral polyproteins pp1a and pp1b are created once the viral RNA is released into the cytoplasm. These viral polyproteins are then cleaved by PLpro and Mpro.
Once the viral proteins have been released, they join together to create a viral polymerase RdRp complex, which catalyzes the replication of viral RNA. Exocytosis allows the progeny virions to leave the infected cells and become ready for the subsequent infection. Interfering with viral entry by targeting ACE2, RNA-dependent RNA polymerase, and the two viral proteases PLpro and Mpro has been the focus of antiviral investigations using small-molecule inhibitors, even though all viral enzymes involved in coronavirus replication are potential candidates for therapeutic development, as reviewed for SARS-CoV-113, 14, 35 and SARS-CoV-2.36, 37, 38 Given its high conservation and specificity, Mpro has emerged as the most crucial target among these critical targets for the development of anti-COVID-19 drugs.
In this review, we discuss the rationale for small-molecule inhibitors that target the main protease of SARS-CoV-2. We highlight novel inhibitors that were only discovered during the COVID-19 pandemic, discussing their unique binding modes and structures. We provide a compilation of clinically tested Mpro inhibitors (Table 1 ) with up-to-date structural information for relevant inhibitors (Table S1 in the supplemental information online).
Table 1.
Potent Mpro inhibitors that have advanced to evaluation in in vivo or clinical studies.
| Drug | Mode of action | Delivery | Measure of efficacy |
Clinical trials | Refs | ||
|---|---|---|---|---|---|---|---|
| In vitro | In vivo | ||||||
| PF-07321332 (nirmatrelvir) | Covalent, reversible | Oral | Ki = 53.11 nM (FRET assay); EC50 = 77.9 nM (CPE assay in A549-ACE2 cells) | Prevention of weight loss in BALB/c mice and reduction of viral lung titer (by 1.4 and 1.9 CCID50 log10/ml for doses of 300 mg/kg and 1000 mg/kg, respectively) | 89% reduction of risk of hospitalization or death | Phase III/EUA by FDAb | 107, 110, 113 |
| PF-07304814 (lufotrelvir) | Covalent | Intravenous | IC50 = 0.27 nM (FRET assay); EC50 = 39.8 μM, (CPE assay in VeroE6-enACE2)c | Dose-dependent reduction in lung viral titers of 3 log10 in BALB/c infected mice | N.A. | Phase I | 105, 106 |
| PBI-0451 | Covalent, reversible | Oral | N.A. | N.A. | N.A. | Phase I | 114, 115 |
| EDP-235 | Not described | Oral | IC50 = 5.8 nM; EC90 = 33 nM in human airway epithelial cells | N.A. | N.A. | Phase I | 116, 117 |
| S-217622 | Noncovalent | Oral | IC50 = 0.013 mΜ; EC50 = 0.37 μΜ (CPE assay in Vero E6/ TMPRSS2 cells) | Dose-dependent inhibition of viral replication in lungs of infected mice | Antiviral effects | Phase IIb/III | 69, 118 |
| Atazanavir | Noncovalent | Oral | Ki = 703 nM (FRET-based assay); EC50 = 0.49 μM (antiviral assay in Calu-3 cells) | 30% increase of survival of K18-hACE2 transgenic mice | N.A. | Phase II | 119, 120 |
| Ebselen (SPI-1005) | Covalent | Oral | IC50 = 0.67 μM (FRET-based assay) | N.A. | N.A. | Phase II | 59, 121 |
| Lopinavir/ritonavir | Not described | Oral | IC50 = 10.9 μM (enzyme inhibition assay) | N.A. | No significant activity | Phase II | 122, 123, 124 |
| Danoprevir | Noncovalent | Oral | EC50 = 87 μM (antiviral assay in Vero E6 cells) | N.A. | Positive results | Phase IV | 125, 126, 127 |
| 13b | Covalent | Inhaled | IC50 = 0.67 μM (FRET-based assay) | N.A. | Preclinical | 15 | |
| GC-376 | Covalent | N.A. | IC50 = 0.19 μM (FRET-based assay) | 20% increase of survival, limitation of viral loads, inflammation and tissue lesions in K18-hACE2 transgenic mice | N.A. | Preclinical | 93 |
aAbbreviation: N.A.: not available.
Emergency use authorization from FDA.
Refer to PF-00835231, which is the active drug to which PF-07304814 is metabolized.
Small-molecule inhibitors
Covalent irreversible inhibitors
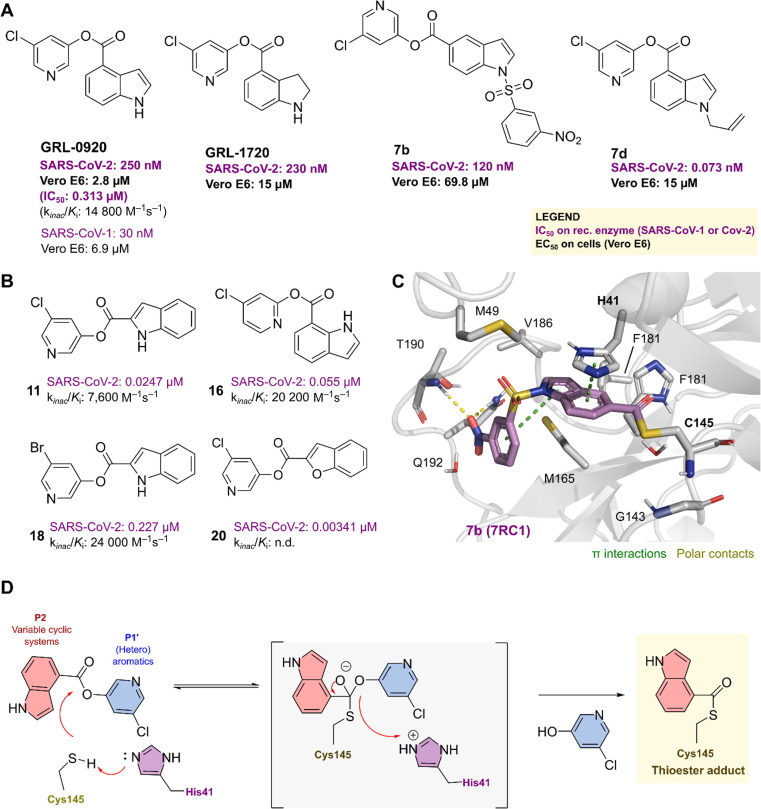
It was found that halopyridinyl esters are efficient SARS-CoV-1 Mpro inhibitors in the nanomolar range.39 For instance, GRL-0920 showed antiviral activity in cellular assays with an EC50 of 6.90 μM and enzymatic inhibitory activity with an IC50 of 30 nM (Figure 2 ). When SARS-CoV-2 was discovered in 2019, indane analogs GRL-1720 and GRL-0920 were repurposed for Mpro inhibition and subsequent antiviral activity. In Vero E6 cell-based assays, they exhibited potent antiviral activities against SARS-CoV-2 with EC50 values of 2.8 and 15 μM, respectively, and excellent SARS-CoV-2 Mpro inhibition with IC50 values of 250 and 320 nM, respectively (Figure 2a).40, 41
Figure 2.
Pyridinyl ester derivatives as inhibitors of the main protease (Mpro). Pyridinyl ester derivatives as inhibitors of the main protease (Mpro) inhibitors of (a) SARS-CoV-1 and (b) SARS-CoV-2. (c) The covalent binding mode of 7b with the main protease (Protein Data Bank ID: 7RC1). Polar interactions are depicted as dashed yellow lines, whereas pi-stacking is indicated by dashed green lines. (d) The inhibitory mechanism of Mpro by the inhibitor GRL-0920 or 13 highlights the final thioester adduct.
Two separate groups thoroughly examined the structure–activity relationships (SARs) of GRL-0920. First, a group led by Müller published several derivatives that were all identified through kinetic analysis as irreversible inhibitors of Mpro (Figure 2b). By first scanning the ester moiety position at the indole, they discovered that the strongest Mpro inactivation was observed with the ester moiety at position 7 (compound 16). Further improvement was then achieved when bromine was substituted for chlorine (13 versus 18). The Mpro inhibitory activity was significantly enhanced by switching from indole to benzofuran. Furthermore, at a 1 μM concentration, compounds 16, 17, and 20 completely inhibited Mpro activity in HEK293 and human lung epithelial A549 cell lysates.42 Second, a SAR study of GRL-0920 by Ghosh et al. led to compound 7d with improved Mpro inhibitory activity compared with its parent molecule, although it showed very weak antiviral activity in cellular assays.41
The SARS-CoV-2 Mpro -7b complex structure was determined by co-crystallization, with 7b covalently bound to the catalytic Cys145 in a thioester adduct (Figure 2c). The 5-chloropyridinyl ester is attacked by a nucleophile in the catalytic dyad (Cys145 and His41) to form a tetrahedral intermediate, which then releases the 5-chloropyridin-3-ol group and produces a covalent acylating product with Cys145 in the active site (e.g., GRL-0920 or 13). This positions the indole in the S2 pocket, where it performs a pi-stacking interaction with His41, validating the mechanism by which this class of compounds inhibits the virus (Figure 2d).
Expanding on the use of Mpro crystal structures, one can perform virtual screening to discover potential new scaffolds for later optimization. Pillaiyar et al. recently reported novel chemotypes as effective Mpro inhibitors.43 The Tübingen Kinase Inhibitor Collection (TüKIC), which at that time comprised more than 10,000 unique kinase inhibitors, was virtually screened by the researchers. This led to the development of several small molecules, including IV–VI (Figure 3 a), which had moderate potency against Mpro. Compounds IV–VI (Figure 3a, reactive groups highlighted in orange) are highly effective, selective Janus kinase (JAK) inhibitors, particularly the subtype JAK3. Given that COVID-19 involves the production of proinflammatory cytokines that result in a cytokine storm, one of the primary causes of COVID-19-related death, this is a promising strategy for the treatment of COVID-19. Therefore, dual inhibitors of Mpro and JAK would not only have direct antiviral effects, but also advantageously suppress the excessive cytokine production brought on by viral infection.
Figure 3.
Covalent inhibitors of Mpro identified from virtual screening.(a) Severe acute respiratory syndrome-coronavirus 2 main protease (SARS-CoV-2 Mpro) inhibitory activity (IC50) of hit compounds identified by high-throughput screening (A). (b) The proposed warhead or reactive group of the inhibitors highlighted in orange. Mpro active site [Protein Data Bank (PDB) ID: 7RC0] showing the catalytic Cys145 and the subsites; pi-stacking is depicted by dashed green lines. (c) Design of new thioesters by combining IPA-3 and LN5535. (d) The Mpro inhibitory activities and the antiviral activities of novel thioester molecules. (e) The crystal structure of 3w (PDB 7X6K) in complex with Mpro. pi-mediated interactions are depicted by dashed green lines.
Disulfide compounds with IC50 values of 2.35 and 4.03 μM, such as IPA-3 and JX-06, respectively, were recognized as hit molecules. IPA-3 is a dysregulated p21-activated kinase 1 (PAK1), which has been associated with oncogenesis, and a reversible covalent inhibitor, whereas JX-06 covalently inhibits 3-phosphoinositide-dependent protein kinase 1. A new molecule that inhibits Mpro with an IC50 of 2.1 μM has been discovered: a maleimide indole-2-carboxamide derivative (LN5535, Figure 3a).
A series of thioesters was designed by fusing the hit molecules IPA-3 and LN5535, guided by docking studies into the Mpro active site, and comparing them against known covalent inhibitors (Figure 3b, c). This resulted in a series of thioesters as novel, potent Mpro inhibitors with a potency range < 11 nM (Figure 3d; Figure S1). Excellent SARS-CoV-2 Mpro inhibition was demonstrated by compounds 3w and 3x, with kinac/K i values of 58 700 M−1s−1 (K i 0.0141 μM) and 27 200 M−1s−1 (K i 0.0332 μM), respectively. Mpro inhibitors were tested for their antiviral effectiveness in both Calu-3 and Vero76 cell lines, where numerous substances displayed antiviral activity in the nanomolar range without toxicity on the host cells. The fact that these potent SARS-CoV-2 Mpro inhibitors also inhibited the Mpro of SARS-CoV-1 and MERS-CoV suggests that they could be effective in treating a wider variety of coronaviral infections.
It was determined how the structure of Mpro interacted with 3w and 3af (Figure 3e; Figure S1 in the supplemental information online, respectively). The inhibitors were cleaved by proteases, and the crystal structures demonstrated that this resulted in a thioester-type enzyme–inhibitor complex. The pyrimidine thiolate served as a leaving group and left the active site, whereas the remaining fragment of the compound was covalently bound to the catalytic Cys145. The indole ring of 3w (Figure 3e) and the quinoline ring of 3af display contacts with nearby residues, such as His41, Met165, Asp187, Arg188, and Gln189. Specifically, the aromatic indole and quinoline rings of the inhibitors were clamped by the catalytic His41 and Met165.
Xu et al. identified the flavonoid myricetin as a nonpeptide and covalent inhibitor of SARS-CoV-2 Mpro.44 The structure-based optimization of myricetin led to the discovery of derivatives with potent antiviral properties (Figure 4 a). The study demonstrated the potential of pyrogallol as an alternative warhead in the design of targeted covalent ligands by showing that the covalent mode of action of pyrogallol-containing natural products can be a template for the design of nonpeptide covalent inhibitors against Mpro. The crystal structures of the protease bound to myricetin, and its derivatives, showed that the pyrogallol group functioned as an electrophile to covalently modify the catalytic cysteine following a well-determined mechanism (Figure 4b, c).
Figure 4.
Main protease (Mpro) inhibitory activity of natural polyphenolics.(a) Myricetin analogs and their potencies against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2). (b) The covalent binding mode of myricetin (Protein Data Bank ID: 7DPP). Polar interactions are depicted by dashed yellow lines, whereas pi-interactions are depicted by green dashed lines. (c) Covalent binding mechanism of myricetin to SARS-CoV-2.
The Shuanghuanglian preparation, which contains baicalin and baicalein as the two main ingredients, has been shown to have anti-SARS-CoV-2 activity.45 According to X-ray protein crystallography, the mode of binding of baicalein within SARS-CoV-2 Mpro (Figure 4b, c) differed significantly from that of other known Mpro inhibitors. The substance worked by serving as a ‘shield’ in front of the catalytic dyad in the active site to successfully block substrate access.
Among all small-molecule therapeutics, antibacterial medications with a β-lactam ring have historically been the most effective. The fact that human cathepsins can be inhibited by monocyclic β-lactams46, 47 indicates that viral cysteine proteases could be inhibited by spirocyclic β-lactams. In addition, that mycobacterial L,D-transpeptidases can be inhibited by bicyclic β-lactams demonstrates that β-lactams have the potential to be effective inhibitors of nucleophilic viral serine and cysteine proteases.48, 49, 50, 51
Schofield et al. recently published a solid-phase extraction coupled to MS (SPE-MS) Mpro inhibition assay.52 Using this assay, they identified a specific penicillin V derivative, 10, that inhibits Mpro by reacting with the active site cysteine residue, whereas, by contrast, the corresponding penicillin G derivative (11) was inactive (Figure 5 a). Despite the specific binding,52 penicillin V itself was not a potent Mpro inhibitor (IC50 > 50 μM), because of the lack of sulfone benzyl ester present in the active analogs. In the following study, a thorough SAR was carried out by synthesizing derivatives of penicillin, and their mode of inhibition was examined using mass spectrometrics, followed by structural crystallographic analyses.53
Figure 5.
The discovery of S-217622. Discovery process of S-217622 by (a) chemical change iterations, highlighting the initial identified hit binding modes (b) [Cpd 1, Protein Data Bank (PDB) ID: 7VTH] and S-217622 (c) (S217622 or Cpd 3, PDB ID: 7VU6).
The potency of 10 was increased by approximately a factor of ten, from an IC50 of 6.5 μM for 10 to an IC50 of 0.6 μM for 28, 31, and 32. In general, the penicillin sulfone oxidation state is preferred over the sulfoxide and sulfide oxidation states for effective Mpro inhibition. Additionally, the (6R)-configuration is preferred over the (6S)-configuration for penicillin containing a C6 amide side chain.
In contrast to penicillin G derivatives, which lack the ether oxygen, penicillin V sulfone derivatives are powerful Mpro inhibitors (Figure 5a, b). The structure suggests that the C6 phenoxy ether oxygen helps position the phenyl group of the C6 side chain to interact effectively with the His41 imidazole ring, although it may also be crucial in binding before the covalent reaction, resulting in the crystallographically observed complex, potentiating the reaction. Interestingly, the C6 dibromo-penicillin sulfones 28, 31, and 32 were among the most potent substances found in this research.
The binding mode of one of the penicillin derivatives (20e, IC50 3.6 μM) was investigated by analyzing its complex with Mpro (Figure S2a–c in the supplemental information online). It clearly demonstrated the active site Cys145 S-acylation in a manner similar to the covalent reactions of β-lactamases with penicillin and L,D-transpeptidases using a nucleophilic cysteine with carbapenems48, 50; it also provided evidence for active site Cys145 S-acylation. Additionally, the main chain amino groups of Cys145 and Cys143 interact with the thioester carbonyl moiety of 20e. Accordingly, the proposed inhibitory mechanism of inhibitor 20e is outlined in Figure S2d in the supplemental information online.
Noncovalent, reversible inhibitors
Ebselen is a cytoprotective anti-inflammatory drug initially studied for its potential to cure hearing loss and bipolar disorder54, 55, 56, 57, 58 (Figure S3 in the supplemental information online). According to Renson et al., this substance has low cytotoxicity in rats, but it is still unknown whether it is safe for humans. Ebselen, which strongly binds to the SARS-CoV-2 Mpro active site (IC50 0.67 μM) and exhibits moderate antiviral activity (EC50 4.67 μM), is advised for the treatment of diseases brought on by coronaviruses.59 It was discussed as a potential drug for COVID-19 with promising inhibition of SARS-CoV-2 Mpro by Sies and Parnham, subject to the its in vivo antiviral activity being established.60 Yet with regard to the ebselen indication, caution is advised because this compound also inhibits other cysteine proteases that are unrelated to Mpro. When reducing reagents, such as dithiothreitol (DTT), are present, ebselen inhibition can be completely eliminated.61 Moreover, in a cell-based Mpro test, such as the FlipGFP-based,61 ebselen did not exhibit action. Nevertheless, it could be a useful chemical tool to understand the noncovalent bonding in Mpro. Qiao et al. further developed its SARs. As a result of the original publication, nine ebselen derivatives (EBs)62 with IC50 values between 0.07 and 0.38 μM were generated (Figure S3 in the supplemental information online). Two candidates, EB2-7 and EB2-19, had potent antiviral activity that was six and three times, respectively, more potent than that of ebselen, its equivalent. Further investigation of three derivatives showed that EB2-7, with an IC50 value of 4.1 μM in HPAepiC cells, provided the most powerful inhibition of SARS-CoV-2 viral replication compared with the prototype ebselen at 24.6 μM. The derivative EB2-7 functions in the LC-MS/MS test as a noncovalent Mpro inhibitor, similar to ebselen. However, Sun et al. found that ebsulfur and ebselen compounds were particularly potent covalent inhibitors of Mpro.63 The next-highest inhibitors of Mpro were ebselen 1i (IC50 0.074 μM) and ebsulfur 2k (IC50 0.11 μM), particularly the substances with furan substituents.
Cinanserin (Figure S3 in the supplemental information online), a well-known serotonin antagonist, showed a notable reduction in SARS-CoV-1 replication by blocking Mpro.64 According to Jin et al.,59 this drug showed a weak amount of inhibitory activity against SARS-CoV-2 Mpro (IC50 124 μM; EC50 20 μM), pointing to its potential use in preventing coronavirus diseases after specific modification. In addition, famotidine, which is a G-protein-coupled receptor antagonist, is undergoing clinical trials to treat COVID-19.65
Rossetti et al. investigated potential hits from previously performed in silico screens (that used REAL Space or ZINC chemicals) to identify Mpro inhibitors.66 Based on these approaches, 500 compounds from the top-ranking hits and subsequent synthesis and testing optimization identified two interesting chemotypes, which were further evaluated by the characterization of an additional 500 synthesis-on-demand analogs. The second chemotype bound to, and enhanced the melting temperature of, Mpro and the most active compound from this chemotype inhibited Mpro in vitro with an IC50 value of 1 μM and suppressed replication of SARS-CoV-2 in tissue culture cells. The structural determination of Mpro in complex with this inhibitor revealed its noncovalent binding mode. Additionally, the dihydroquinone moiety formed H-bond interactions with Glu166, His163, and His172 (Figure S4a–d in the supplemental information online), and carbonyl of the compound interacted with the thiol of Cys145 and the main chain of Glu166 through hydrogen bonds.67
Similarly, Santos et al. virtually screened 688 naphthoquinone compounds and derivatives against Mpro and PLpro.68 Among the finally selected candidates, four inhibited Mpro with IC50 values between 0.41 and 9.0 μM (Figure S4e in the supplemental information online), and three inhibited PLpro (IC50 1.9–3.3 μM). Given the quinone aggregation properties, experiments to exclude aggregation, high compound fluorescence, and inhibition by enzyme oxidation confirmed their specificity against the enzymes. Specifically, experiments developed in the presence or absence of reducing reagents showed no significant differences in the determined IC50's, which does not disregard the potential of these compounds as pan-cysteine protease inhibitors. Additional molecular dynamics simulations showed that suggested binding modes for Mpro inhibitors relied on interactions with the S1 and S2 pockets, whereas PLpro inhibitors displayed interactions in the S3 and S4 pockets.
Recently, Gao et al. reported the identification of strong noncovalent nonpeptide Mpro inhibitors with 1,2,4-trisubstituted piperazine scaffolds. To increase activity, the hit molecule MCULE-5948770040 underwent systematic modifications using structure-based rational design, multisite binding, and preferred structure assembly techniques. As a result, GC-14 was improved and showed good Mpro inhibition (IC50 0.40 μM), antiviral activity (EC50 1.1 μM), and minimal cytotoxicity (CC50. 100 μM). It also demonstrated exceptional selectivity for SARS-CoV-2 Mpro (IC50 > 50 μM for cathepsins B, F, K, L, and caspase 3). The X-ray co-crystal structures demonstrated that the inhibitors occupied several subpockets via essential noncovalent interactions.
Unoh et al. recently reported the discovery of S-217622, the first noncovalent, nonpeptidic SARS-CoV-2 Mpro inhibitor clinical candidate.69 This was achieved through the SAR studies and subsequent optimization of the hit compound 1 by means of virtual screening followed by biological screening. Briefly, the research team was focused on the virtual screening of the crystal structures of the Mpro and ML188-like noncovalent small molecules [Protein Data Bank (PDB) ID: 6W63]. The filter was developed based on known crystal structure complexes that included the H-bond acceptor site complementary to the side-chain NH of His163 in the S1 subsite, the lipophilic S2 subsite, and the H-bond acceptor site adjacent to the main-chain NH of Glu166. The resulting top 300 compounds were then assayed against SARS-CoV-2 Mpro using mass spectrometry.
The hit compound 1 (Figure 5a, b), with an IC50 of 8.6 μM, metabolic stability in human microsomes of 97%, CLrat of 7.3 ml/min/kg, t1/2(rat) of 2.1 h, and oral Frat of 111%, was selected as the initial lead. In the rat (71%) and human (97%) microsomes, this substance was found to be biologically stable. Additionally, a rat in vivo PK investigation showed that 1 had an oral bioavailability (F) of 111%, a low clearance of 7.3 ml/min/kg, and a favorable profile for the oral drug.
To improve the activity, compound 1 was co-crystallized with Mpro. The methylamide and 3,4,5-trifluorobenzene moieties were placed in the S1 and S2 pockets, respectively. The 4-difluoromethoxy-2-methylbenzene subunit was placed in the S1 pocket. Initially, the P1′ moiety was optimized for compound 2 (Figure 5a), which showed a 90-fold improvement in enzymatic inhibitory activity while maintaining a favorable drug metabolism and pharmacokinetics (DMPK) profile. Subsequently, the P1′ methyl-amide moiety was replaced with a range of heterocyclic compounds, thus yielding the clinical candidate S-217622 (Figure 5a, c). It exhibited a potent enzymatic IC50 of 0.013 μM, antiviral activity of EC50 of 0.37 μM, and favorable DMPK profiles for oral administration, such as high metabolic stability (96% and 88% in human and rat liver microsomes, respectively), high oral absorption (97%), and low clearance (1.70 ml/min/kg) in rats.
The 1-methyl-1H-1,2,4-triazole unit formed a hydrogen bond with the side-chain NH of His163 in the S1 pocket of the crystal structure of Mpro in association with S-217622. The 2,4,5-trifluorobenzylic moiety is located in the hydrophobic S2 pocket. The P1′ indazole moiety created a hydrophobic interaction with Met49 and a hydrogen bond with the main-chain NH of Thr26. S-217622 demonstrated significant in vitro antiviral activity against viruses, including VeroE6/TMPRSS2 cells infected with the SARS-CoV-2 Omicron strain, as well as several outbreaking variants. Additionally, S-217622 demonstrated broad-spectrum antiviral efficacy against various CoVs, including SARS-CoV-1 (EC50 0.21 μM), MERS-CoV (EC50 1.4 μM), HCoV-OC43 (EC90 0.074 μM), and HCoV-229E (EC50 5.5 μM), suggesting potential applications of S-217622 for upcoming emergent coronaviruses. S-217622 showed no inhibitory activity at concentrations up to 100 μM against host-cell proteases, such as caspase-2, chymotrypsin, cathepsin B/D/G/L, and thrombin, indicating a high selectivity for coronavirus proteases.
S-217622 significantly reduced SARS-CoV-2 intrapulmonary replication in mice infected with the SARS-CoV-2 Gamma strain, which is consistent with the favorable PK characteristics in vivo for a once-daily oral dosage. As a result, this brand-new noncovalent bond inhibitor could be used as a therapeutic oral drug to treat COVID-19.
The results of the analysis of a Phase IIa trial in Japan, which assessed the effectiveness and safety of a once-daily regimen of S-217622 over 5 days, were released by Shionogi on February 7, 2022.70 Compared with placebo, the positive virus titer fell by 60–80%, and there were no significant adverse effects. On February 25, 2022, approval for its production and sales in Japan was submitted after the primary endpoints of the Phase IIb clinical trial involving 428 infected individuals had been analyzed.71 A once-daily oral antiviral treatment was the subject of a global Phase III clinical investigation funded by the NIH.71 Although S-217622 belongs to the same pharmacological class as nirmatrelvir, it can be administered once daily and does not need to be used with ritonavir.
Han et al. reported noncovalent Mpro inhibitors of the SARS-CoV-2 derived from ML-300.72 Before the SARS-CoV-1 outbreak, the research team took part in efforts to develop inhibitors of the coronavirus Mpro, with an emphasis on the development of noncovalent inhibitors, which resulted in ML-300 and ML-188 (Figure 6 a–c). 73, 74 SARS-CoV-1 Mpro was significantly suppressed by ML-300, which had subpar in vitro absorption, distribution, metabolism, and excretion (ADME) characteristics and an IC50 of 4.45 μM. The team investigated the SARs of ML-300 to develop these benzotriazole class compounds against SARS-CoV-2. Initially, compounds 7 and 8, which were improved from ML-300 against SARS-CoV-1 Mpro, were tested against SARS-CoV-2 Mpro. The previously reported biphenyl derivative, 7,21 was significantly less effective and did not reach the reported value of 51 nM. Calculated SARS-CoV-1 IC50 values of 16.8 μM and SARS-CoV-2 IC50 values of 31.2 μM were based on the concentration–response curves of compound 7. The curves suggested that having a varied value for the solubility limit of 7 under various circumstances could be problematic. By contrast, 3-pyridyl congener 8 maintained an IC50 between 930 and 950 nM for both SARS-CoV-1 and SARS-CoV-2 enzymes, which reflected the initial report from 2013.
Figure 6.
Structure, activity, and binding mode for ML-188 and ML-300. Chemical structure of (a) ML-188 and (b, c) the binding modes of ML-188 and ML-300. (d, e) The structure–activity relationships of ML-300 derivatives and (f) the binding mode of 19.
Based on compound 8, the authors extensively investigated amide variants and heterocyclic modifications. As a result, a significant enhancement was found with the NH pyrazole and imidazole derivatives, 19 and 21, respectively, reaching an IC50 of 60 nM versus SARS-CoV-2 Mpro in the case of imidazole 21. Further optimization of the imidazole analog 21 led to the highly potent 41 (IC50 68 nM). The DMPK properties of these derivatives, including ML-300, showed an exceptionally high microsomal clearance, with hepatic clearance equivalent to hepatic blood flow in both humans and rats. The authors prophesied that the P1-benzotriazole group might be a source of significant metabolism. Similarly, although the unbound fraction and cellular permeability of these compounds are promising, a further hurdle these compounds must overcome is their strong inhibition of CYP enzymes at 10 μM (Figure 6d). Finally, the antiviral efficacy of compounds against SARS-CoV-2 in Vero E6 cells was evaluated by cytopathic effect (CPE) inhibition, which was evaluated by a plaque reduction assay. ML-300 displayed weak activity in the high micromolar range (CPE EC50 19.9 μM, plaque reduction EC50 28 μM, Figure 6e).
The pyrazole and imidazole P2c-containing derivatives, 19 and 21 (Figure 6d, f), respectively, which are among the more biochemically potent analogs of ML-300, showed improved EC50 values of between 1.7 and 8 μM in both assays. Interestingly, the potencies of the matched pair analogs 36 and 41 that substitute P2sp 3-thienyl for 3-chlorophenyl were greatly improved, with the EC50 of 41 achieving submicromolar activity in both assays. In addition, the 50% cytotoxic concentration of 41 (CC50) in the CPE antiviral assay was >50 μM, indicating an excellent selectivity index (Figure 6e). The efficacy of 41 was shown in these assays to be comparable to that of the clinically utilized polymerase inhibitor, remdesivir.
Later, Mpro- 23R complex crystal structure revealed a novel binding site in between the S2 and S4 pockets, occupied by a biphenyl moiety. Further SAR exploration of the S1′ pocket led to an extraordinary increase in potency for a noncovalent inhibitor (Figure S5 in the supplemental information online).75, 76, 77
Drug repurposing is a crucial choice for a speedy response to the COVID-19 epidemic. Jorgensen’s research group began using drug-repurposing techniques to find potent inhibitors against SARS-CoV-2 Mpro.78, 79 Their objectives were to find potential medications for drug repurposing and also to supply drug-like hits for lead optimization, which could generate highly effective antiviral therapeutics. Using a high-resolution (1.31 Å) structure of Mpro co-crystallized with a noncovalent small fragment hit (PDB ID: 5R82), they docked each drug after deleting the fragment. The virtual screening of ∼2,000 approved drugs followed by evaluation in a kinetic assay for Mpro inhibition produced 14 compounds (Figure S6 in the supplemental information online) that reduced the enzymatic activity at 100 μM concentration, and five provided IC50 values <40 μM: manidipine (IC50 4.8 μM), lercanidipine (IC50 16.2 μM), and efonidipine (IC50 38.5 μM), which are calcium channel blockers, as well as the anti-infectives bedaquiline (IC50 18.7 μM) and boceprevir (IC50 5.4 μM). Some of these inhibitors, most notably manidipine, were eventually declassified as Mpro inhibitors,61, 80 and colloidal aggregation was believed to be responsible for their activity.
The hepatitis C virus protease inhibitor boceprevir had a previously reported IC50 of 4.13 μM (Figure S6 in the supplemental information online).81 With an IC50 of 18.7 μM, bedaquiline inhibits Mpro and is licensed for the treatment of multidrug-resistant TB. Interestingly, although its IC50 could not be estimated with accuracy because of its intrinsic fluorescence interfering with the fluorescence tests, perampanel appears to be the sixth most active molecule at 100 μM. These potencies are excellent for a virtual screening exercise, but they are probably insufficient for repurposing.
Subsequently, the research group redesigned the weak hit perampanel, an antiepileptic drug, to yield multiple noncovalent, nonpeptidic inhibitors with IC50 values of 20 nM in kinetic assays.82 Initially, the potential binding mode of perampanel, proposed by docking into the Mpro active site, suggested that the structure would resemble a cloverleaf motif with the three leaves occupying the binding pockets referred to as S1, S1′, and S2.83 Based on the docking pose, several modifications were proposed to the binding that appeared reasonable to pursue and, subsequently, FEP calculations were used to explore the possible benefits of such changes. These combined efforts led to the identification of compounds 2–4 with IC50 values of 10.0, 6.4, and 4.0 μM, respectively (Figure S6 in the supplemental information online).
Using compound 4 as a lead, compounds 18–24 were generated (IC50 0.018 – 0.037 μM), with the ortho-chloro analog 21 being the most potent (Figure S6 in the supplemental information online). SARS-CoV-2 replication in Vero E6 cells was investigated, in parallel with toxicity assessment in normal human bronchial epithelial cells. The most cytotoxic compounds were 21 and 23, 84 which also showed activity in the viral plaque assay but no antiviral activity in the MTT assay. Given that the disclosed compounds are structurally unique, nonpeptidic, noncovalent, and have low nanomolar activity, they stand out as relevant Mpro inhibitors.
By considering the positioning of substituted five-membered ring heterocycles in the S4 pocket of Mpro and the N-methylation of an uracil ring, the pyridine series was further developed.85 Selection of the heterocycles was guided by free energy perturbation simulations, and the intended S4 positioning was validated by protein crystallography. The compounds 19 and 21, which have IC50 values of 0.044–0.061 μM, EC50 values of ∼0.1 μM, strong aqueous solubility, and negligible cytotoxicity, are particularly intriguing as COVID-19 treatment options.
Recently, masitinib was shown to inhibit SARS-CoV-2 replication in a dose-dependent manner (IC50 3.2 μM) in human lung cells overexpressing ACE-2 and in mouse models. Masitinib inhibits Mpro in a similar activity range (IC50 2.5 μM) and has no effect on PLpro activity, which suggests this as its main mechanism of action.86 Masitinib is an orally bioavailable c-kit inhibitor87 that is already in use against mast cell tumours in dogs,88 and is being further evaluated in Phase II/III clinical trials in humans as an asthma treatment.89
The Carlsson group developed a further intriguing family of noncovalent Mpro inhibitors (Figure 7 )90 by applying two structure-based docking approaches to seek Mpro inhibitors in extremely vast chemical libraries. First, 235 million virtual molecules from a varied library were tested against the active site using structure-based docking. Second, 93 experimentally tested compounds and the docking of millions of developed molecules were used to guide and optimize a fragment that was found by crystallographic screening. Both sets of approaches generated initial hits, and structure-guided hit-to-lead optimization found effective inhibitors that had antiviral effects in cellular models.
Figure 7.
Noncovalent Mpro inhibitors identified from virtual screening. Main protease (Mpro) inhibitors with a noncovalent binding mode. (a) Schematic of the approaches used for screening and optimization from the Carlsson group, showing also the main developed compounds. Binding mode for (b) 18 [Protein Data Bank (PDB) ID: 7QBB] and (c) 21 (PDB ID: 7NBT).
To determine the ideal arrangement of substituents, variations in the S1 and S2 pockets were methodically investigated through several analog synthesis cycles. The highest increase in potency was observed in the S1 pocket for pyridine-based moieties based on compound 1. From a mild effect at 50 μM to IC50 values of 18, 7.5, and 1.3 μM for compounds 10, 11, and 12, respectively, pyridine elaborations of increasing size gradually increased the potency of derivatives of compound 1 (Figure 7). The highest efficacy in this series was achieved by the isoquinoline moiety, which the COVID Moonshot Consortium91 also recognized as a prospective extension of pyridine. Compounds 14 and 15, which are spirocyclic analogs of compound 3, were shown to be the best substituents in the S2 pocket (Figure 7).
By combining the best substitutes for the S1 and S2 pockets into a single chemical series by in-house synthesis, inhibitor efficacy and affinity both improved synergistically. In addition to having significant affinities for Mpro (KD values of 0.14 and 0.15 μM, respectively), compounds 16 and 17 had IC50 values of 0.46 and 0.33 μM, respectively (Figure 7). The authors added a phenyl ring at this location because it was difficult synthetically to further elaborate the aliphatic tails of compounds 16 and 17, and this inhibitor showed similar activity (IC50 0.39 μM and KD 0.17 μM). Further optimization was led by a crystal structure of Mpro in association with compound 18 (2.0 Å resolution, Figure 7b). Molecule 19 was produced by adding an o-chloro substituent to the phenyl. This compound had increased potency (IC50 0.077 μM, Figure 7) and affinity (KD 0.038 μM) over compounds 18 and 17. The potencies of GC-376 and PF-07321332 in the identical enzyme inhibition experiment were 0.073 and 0.033 μM, respectively.
Using the second approach, the docking model of compound 2 was used to optimize interactions in the S2 pocket for the phenylpiperazine scaffold. By fine-tuning interactions in the S2 pocket, the benzotriazole scaffold that was discovered by fragment elaboration was further improved. Consequently, compound 21 (IC50 2.1 μM) was discovered (Figure 7a, c).
The authors ran experiments in the presence of the reducing agent DTT for compounds 16 through 21 to determine whether inhibition involves covalent alteration of cysteine residues. The addition of DTT did not affect the IC50 values of compounds 16–21. By putting the inhibitors to the test against human cathepsin S, selectivity was determined. The peptidomimetics GC-376 and PF-07321332 showed cathepsin S inhibitory activity with IC50 values of 0.002 and 5.7 μM, respectively. GC-376 inhibited all tested Mpro and 3Cpro, including SARS-CoV-2 Mpro, SARS-CoV-1 Mpro, MERS-CoV Mpro, HCoV-OC43 Mpro, EV-A71 3Cpro, and EV-D68 3Cpro (IC50 ≤ 0.16 μM), but not the unrelated EV-A71 2Apro, EV-D68 2Apro, and SARS-CoV-2 PLpro (IC50 > 50 μM).75 In addition, GC-376 also inhibited the host cysteine proteases calpain 1, cathepsin K, and cathepsin L (IC50 ≤ 0.074 μM) but not the serine protease trypsin (IC50 > 50 μM) (Figure 7b; Table S1 in the supplemental information online). Compounds 16–21 also did not affect cathepsin S activity (IC50 > 50 μM). Nine additional human proteases were tested, and 19 showed selectivity against them; however, at a dose of 10 μM, 19 had no discernible inhibitory effect on these proteins.
Compounds 16, 17, and 19 were tested for their antiviral properties in SARS-CoV-2-infected cells. With EC50 values of 1.7, 1.6, and 0.077 μM, respectively and no detectable cytotoxicity at the highest tested concentration in Vero E6 cells (50% cytotoxicity concentration, CC50 > 20 μM), the three compounds demonstrated a dose-dependent reduction of the cytopathic effect (CPE). Their antiviral action was further supported by a yield reduction experiment, which measured their inhibition of viral replication using RT-qPCR. In these tests, compounds 16, 17, and 19 had EC50 values of 1.0, 0.9, and 0.044 μM, respectively, for inhibiting SARS-CoV-2 viral replication. In CPE-based experiments conducted in Huh7 cells, compound 19 and Pfizer’s clinical candidate (PF-07321332) were compared. Comparable potencies were discovered for both Mpro inhibitors, with EC50 values of 0.08 and 0.11 μM, respectively. SARS-CoV-1 (EC50 0.39 μM, Vero E6 cells) and MERS-CoV (EC50 0.20 μM, Huh7 cells) cytopathic effects were also suppressed by compound 19 (EC50 0.20 μM, Vero E6 cells).
The in vitro ADME characteristics of compound 19 were favorable, with good metabolic stability in the presence of human liver microsomes (intrinsic clearance CLint = 22 μL/min/mg) and plasma protein binding in human plasma (fraction unbound fu = 3.3%). The high permeability of 19 in a Caco-2 cell assay (Papp AB = 5.9 × 10–5 cm/s and Papp BA = 5.0 × 10–5 cm/s) and the presence of no efflux effect agreed with the strong antiviral action of 19 in cells (efflux ratio of 0.8).
Peptidomimetics as Mpro inhibitors
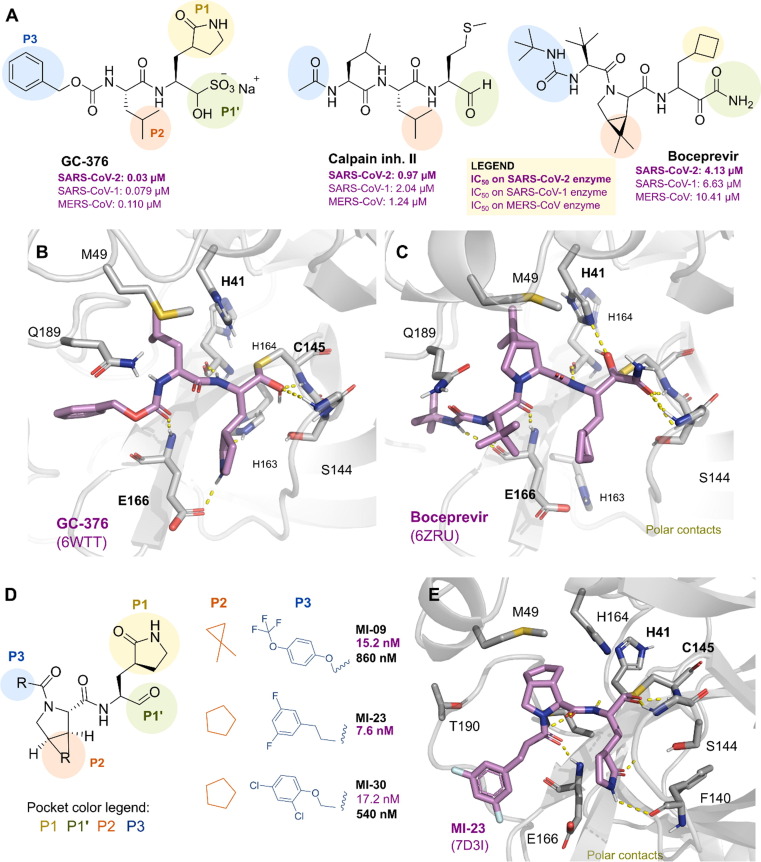
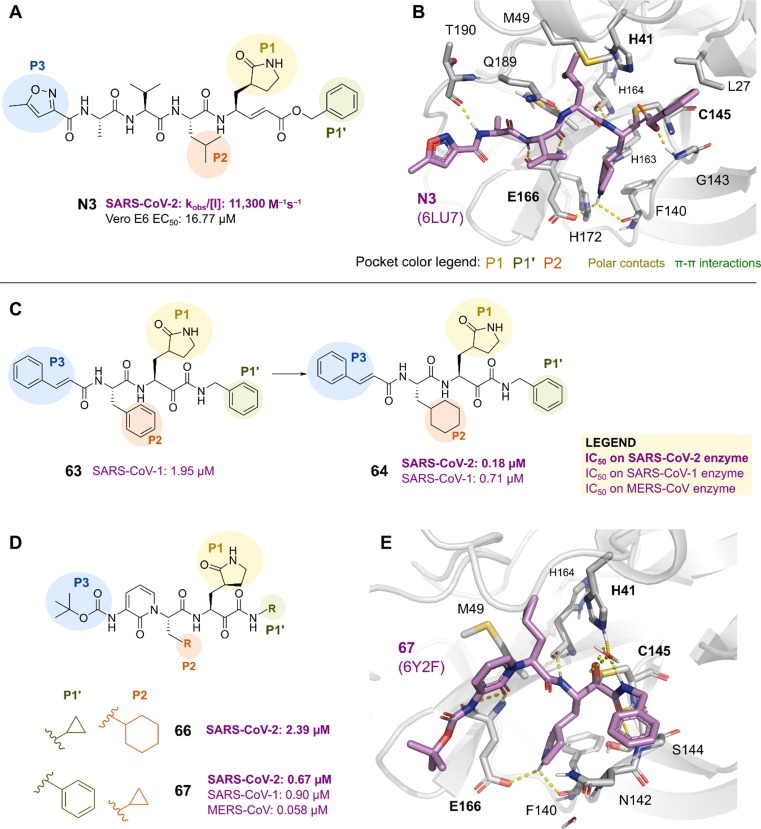
Repurposing approved inhibitors of related proteases was the first strategy used in the search for SARS-CoV-2 Mpro inhibitors. A study was conducted in April 2020 to find Mpro inhibitors using structure-based virtual and high-throughput screening. More than 10,000 compounds, including approved drugs, drug candidates in clinical trials, and other bioactive compounds, were analyzed.59 The Michael acceptor compound N3, which was previously reported to be an inhibitor of Mpro of SARS-CoV-1 and MERS-CoV, was discovered to be a time-dependent inhibitor of the SARS-CoV-2 Mpro with a kobs/[I] of 11 300 M−1s−1 (Figure 9a). N3 also demonstrated moderate antiviral activity in Vero E6 cells infected with SARS-CoV-2 (EC50 16.77 μM).
Figure 9.
Peptidomimetic inhibitors of Mpro. (a) Chemical structures of boceprevir, GC-376, and calpain inhibitors II and XII. Binding mode determined by crystal structures for (b) GC-376 [Protein Data Bank (PDB) ID: 6WTT] and (c) boceprevir (PDB ID: 6ZRU). (d) Main protease (Mpro) inhibitory and the antiviral activity of aldehyde inhibitors. (e) Co-crystal structure of Mpro in complex with MI-23.
The crystal structure of SARS-CoV-2 Mpro in complex with the N3 inhibitor was determined (2.1 Å resolution, PDB 6LU7, Figure 8 a, b). The electron density demonstrated the formation of a covalent bond between the Cys145 sulfur atom and the carbon atom of the vinyl group (1.8 Å C–S distance), supporting the function of N3 as a Michael-type inhibitor. The lactam rings of the inhibitor fit into the S1 site, which is also home to two ordered water molecules and is created by the side chains of the residues Phe140, Ser142, Glu166, His163, and His172 of protomer A (Figure 8b). The remaining residues, His41, Met49, Tyr54, Met165, and Asp187, were located around the isobutyl group of Leu at the S2 site. The isopropyl group of Val was solvent-exposed at the S3 site, and the residues Met165, Leu167, Phe185, Gln192, and Gln189 encircled alanine.59 Previous studies with long molecular dynamics simulations of Mpro bound to N1 (6Y2G) and N3 (6LU7),92 in both monomeric and dimeric states, corroborate observations that monomeric states would not be able to accommodate peptide-mimetic ligands because these simulations led to an unrealistically flexible active site. By contrast, the Mpro dimer displayed a stable oxyanion-loop conformation along the trajectory. In addition, ligand interactions with residues His41, Gly143, His163, Glu166, and Gln189 are postulated to significantly impact the inhibitory activity of the ligands.
Figure 8.
Discovery and binding mode of N3 and ketoamide derivatives. (a) Chemical structure of N3 and (b) its binding mode within the active site of severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) [Protein Data Bank (PDB) ID: 6LU7]. Chemical structures of further developed analogs (c) 63 and 64 and (d) 66 and 67. (e) Crystal structure of main protease (Mpro)-67 (PDB ID: 6Y2F).
The dipeptidic ketoamides 63 and 64 (Figure 9c) were described as powerful antiviral agents in April 2020 by Hilgenfeld et al. 15 They were effective against the 3C protease of enteroviruses as well as the primary proteases of humans and COVs. These substances were created using a previously identified compound that was effective against both MERS-CoV and SARS-CoV-1. For instance, a lipophilic cinnamoyl residue can be substituted for a less lipophilic tert-butyloxycarbonyl (Boc) group to increase plasma solubility and decrease plasma protein binding, or a pyridone ring can be used to mask the P2–P3 amide bond to increase plasma half-life. With an IC50 of 0.67 μM, compound 67 inhibited purified SARS-CoV-2 Mpro (Figure 9d). It was equally effective at inhibiting MERS-CoV Mpro and SARS-CoV-1 Mpro (IC50 of 0.90 μM and 0.58 μM, respectively). It successfully prevented SARS-CoV-1 replication with an EC50 value of 1.75 μM. In human Calu3 cells exposed to SARS-CoV-2, it prevented viral replication with an EC50 of 4–5 μM.
The crystal structures of the complex between compound 67 and SARS-CoV-2 Mpro show a thiohemiketal as a result of the thiol group on Cys145 attacking the keto carbonyl group of the ketoamide moiety of compound 67 through nucleophilic attack (Figure 8e). By creating a hydrogen bond between the amide oxygen and the amides in residues His41, Gly143, Cys145, and Ser144, thiohemiketal was stabilized in the oxyanion hole. When the carboxylate of Phe140 and carbonyl oxygen of Glu166 formed hydrogen bonds, the S1 site could accept the lactam ring, and when the imidazole and carbonyl oxygen of His163 did the same, the carbonyl oxygen could accept the lactam ring. Whereas the P2 cyclopropylmethyl moiety of 67 shrank to fit into the S2 subsite, the complex of 66 with SARS-CoV did not (Figure 8d, e). The carbonyl oxygen created a hydrogen bond with the amide of the main chain of Glu166, but the nitrogen of the pyridone ring did not. The Boc group was slightly shifted away from the S4 site and toward Pro168.15
Dai et al. designed and synthesized two novel peptidomimetic SARS-CoV-2 Mpro inhibitors, 11a and 11b (Figure S7a in the supplemental information online),83 which exhibited extremely high inhibitory activity on purified Mpro with IC50 values of 50 and 40 nM, respectively. Both substances contained an aldehyde group that served as a warhead to be attacked by the Cys145 of the active center and the glutamine substitute at the P1 site. To examine the impact of the ring, a cyclohexyl or 3-fluorophenyl ring was inserted into the P2 position. An indole group was added to the P3 site of both inhibitors to create new hydrogen bonds with S4 and enhance their drug-like properties. Furthermore, the group observed high antiviral activity of both compounds in cell-based assays (11a, EC50 0.42 μM; 11b, EC50 0.33 μM). Cytotoxicity assays revealed CC50 values of >100 μM. In terms of PK, both substances performed well. An in vivo toxicity study on rats and dogs found no obvious toxicity in either group, making 11a a strong candidate for additional clinical research.
X-ray structures were determined for both derivatives in complex with SARS-CoV-2 Mpro at 1.5 Å (11a and 11b, Figure S7b, c in the supplemental information online). The aldehyde carbon in 11a formed a covalent bond with the sulfur of Cys145, located in the active site of Mpro. The oxygen of the lactam ring interacted with the imidazole ring of His163, whereas the lactam NH group was hydrogen bonding with the main chain of Phe140. The P2-cychexyl or P2-m-fluorophenyl interacted with various hydrophobic residues at the S2 site (Figure S7b, c in the supplemental information online). Comparatively, the cyclohexyl moiety from 11a is more flexible and allowed the rearrangement of conserved water molecules around the site.
Another study used fluorescence resonance energy transfer (FRET)-based enzymatic assays to test 55 known peptidyl compounds against SARS-CoV-2 Mpro.81 These compounds are known inhibitors of the proteasome, aspartyl, serine, cysteine, and metalloproteases. The FDA-approved hepatitis C virus drug boceprevir, GC-376, and calpain inhibitors II and XII (in preclinical assays) (Figure 9a) had single-digit to submicromolar IC50 values against Mpro and also displayed low-micromolar EC50 values against SARS-CoV-2 viral replication in cells. Furthermore, they had low toxicity, with CC50 > 100 μM for boceprevir, GC-376, and calpain inhibitors II, and CC50 > 50 μM for calpain inhibitor XII. The ketoamide warhead group of calpain inhibitor XII serves as the calpain inhibitor XII and boceprevir warheads, respectively. A bisulfite adduct, known as inhibitor GC-376, functions as a prodrug for the active aldehyde form.
An aldehyde warhead and Cys145 combine to form a thiohemithioketal, as shown by the crystal structure of SARS-CoV-2 Mpro with inhibitor GC-376 (2.15 Å resolution, Figure 9b). The active site substrate bonding of Mpro was imitated by GC-376 (Figure 9b). The His163, Glu166, Phe140 main chain, and glutamine surrogate-lactam ring are all connected at the S1 site by hydrogen bonds. Position P2 of a leucine residue contained an isobutyl moiety that filled the hydrophobic space created by His41, Met49, and Met169. The carbamate group in GC-376 interacted with Glu166 via a hydrogen bond, and hydrophobic interactions complemented the benzyl group of the carbamate along the aliphatic S4 site. In a mouse model, GC-376 was recently shown to be effective for treating SARS-CoV-2.93
Qiao et al. described the design, synthesis, and characterization of 32 new SARS-CoV-2 Mpro inhibitors based on the crystal structures of Mpro, as well as co-crystal structures of Mpro in complex with two antihepatitis C virus drugs (boceprevir and telaprevir)94 (Figure 9c, depicts boceprevir binding mode within SARS-CoV-2 Mpro active site). For the first time, they showed that oral or intraperitoneal treatment with two compounds (MI-09 and MI-30) exhibited effective antiviral activity in a SARS-CoV-2 infection transgenic mouse model.
Novel boceprevir analogs were generated from its hybridization with other known inhibitors, such as GC-376 (UAWJ9d-36-3)95 and narlaprevir.96 UAWJ9d-36-3 (IC50: 0.054 μM on SARS-CoV-2 Mpro) was idealized based on the structural alignment of SARS-CoV-2 Mpro with GC-376 and boceprevir,95 which maintains the dimethylcyclopropylproline of boceprevir at P2, while including a pyrrolidone at P1 (Figure S8 in the supplemental information online). The extra pyrrolidone forms two or three hydrogen bonds with His163, Glu166, and Phe140, similarly to the MI-09 and MI-30 binding modes. UAWJ9d-36–3 is more selective against host cysteine proteases calpain I (IC50 > 20 μM) and cathepsin L (IC50 1.81 μM compared with 0.0044 μM from GC-376), but not cathepsin K, compared with the original GC-376. Boceprevir–narlaprevir hybrids96 were generated by shuffling moieties to the boceprevir scaffold, such as adding the pyrrolidone (P1) and changing its P1′ aldehyde to nitrile groups (generating the BBH-2 inhibitor) or to a benzothiazole (BBH-1). The Mpro-BBH-1 complex structure generated by neutron crystallography shows a specific adaptation of the ionization states and charge distribution within the pocket upon inhibitor binding. In addition, because of the bulkier nature of the benzothiazole of BBH-1, His41 is displaced from its normal position, the rotation of which leads to the displacement of the catalytic water, followed by the His164 to become deprotonated at the Nδ1 atom. However, both inhibitors displayed weak antiviral activity (BBH-1, EC50 16.1 μM and BBH-2 EC50 15.4 μM) in Vero E6 TMPRSS cells, when measured in absence of CP-100356. Their potency greatly increased with the addition of this P-gp inhibitor (BBH-1, EC50 > 1.54 μM and BBH-2, EC50 0.88 μM), suggesting that they are P-gp substrates.
To ensure the antiviral activity, P1 has an aldehyde as a warhead to form a covalent bond with the Mpro catalytic site Cys145 and a lactam derivative of Gln to occupy the S1 site. The S2 site is occupied in P2 by a bicycloproline moiety from either boceprevir or telaprevir, increasing in vivo exposure. P3 can occupy the S4 site with a variety of hydrophobic subgroups, which enhances its potency and PK characteristics. The inhibitory activities and Mpro-binding capacities of these compounds were then evaluated by Qiao et al. using differential scanning fluorimetry (DSF) and FRET. All 32 substances displayed strong Mpro binding and potent inhibition (IC50 5 7.67–48.5 nM). As anticipated, the P1, P2, and P3 fragments of MI-23, one of the most active compounds, occupied the S1, S2, and S4 sites of Mpro, respectively, and crystal structure analysis showed that the warhead of MI-23 forms a covalent bond with catalytic Cys145 (Figure 9d, e).
Qiao et al. further screened 20 compounds using the CCK8 assay, RT-qPCR, and cell protection assay in a variety of cell lines to determine the best candidate inhibitors for animal experiments (Vero E6, HPAEpiC, LO2, BEAS-2B, A549, and Huh7). Six substances demonstrated greater cell protection compared with the other substances, and two of them are known Mpro inhibitors (GC-376 and 11b). The authors then investigated the PK and toxicity of the two top candidates, MI-09 and MI-30 in rats (Figure 9d, e). Both substances demonstrated good PK characteristics and did not exhibit observable toxicity in animals at the tested doses. Although the oral bioavailability of MI-09 and MI-30 is >10%, their rat half-life (at a dose of 20 mg/kg) is <1 h.
Finally, using a hACE2 transgenic mouse model, Qiao et al. evaluated the in vivo antiviral activities of MI-09 and MI-30. Mice were administered a dose of SARS-CoV-2 [5 × 106 TCID50 virus/mouse, simulating a moderate infection (TCID, viral titration)] before receiving MI-09 (intraperitoneally or orally), MI-30 (intraperitoneally) or the vehicle (control) for 5 days. Compared with the vehicle treatment, all compound treatments reduced viral loads in lung tissues at 3 days post-infection (dpi). Histopathologically, compared with control mice, all compound-treated mice showed a slight increase in alveolar septal thickening and a decrease in inflammatory cell infiltration. In addition, compared with the vehicle treatment, all compound treatments decreased the expression of interferon (IFN)-beta, C-X-C motif chemokine ligand 10 (CXCL10), and the presence of neutrophils and macrophages in the lungs. The findings show that intraperitoneal or oral treatment with Mpro inhibitors can prevent SARS-CoV-2 replication and lessen the lung lesions that the virus causes in vivo.
The Hayashi research group reported a series of substrate-derived inhibitors of SARS-CoV-1 Mpro following the outbreak of SARS in 2003.97, 98, 99, 100, 101, 102 Inhibitors SH-5, YH-53, and YH-71 (previously designated 2i, 5 h, and 5n, respectively, Figure 10 a)101, 103 are of particular interest because they contain a unique benzothiazolyl ketone as the P1′-warhead moiety. They display potent SARS-CoV-1 Mpro inhibitory activities with K i values of 4.1 nM,103 6.3 nM,101 and 22 nM,101 respectively (Figure 10a). Cys145, the active site of SARS-CoV-1 Mpro, forms a reversible covalent bond with the electrophilic ketone warhead, forming a thiohemithioketal intermediate that momentarily inactivates the enzyme (Figure 10b). The nonspecific irreversible reaction of many mammalian thiols is less likely because of the reversibility of this inhibition, as is the likelihood of immunological or clinically unfavorable side effects. Given the highly structural identity of Mpro between SARS-CoV-1 and SARS-CoV-2, the potential of SARS-CoV-1 inhibitors as anti-SARS-CoV-2 drugs was investigated.100 Indeed, a compound with an indole moiety at the P3-position and the benzothiazolyl ketone as the reactive warhead as in YH-53 showed extremely potent inhibitory activity against the Mpro of SARS-CoV-2 and completely blocked viral replication in Vero E6 cells.
Figure 10.
Substrate-derived main protease (Mpro) inhibitors.(a) Development process starting from the natural peptide, optimizing its interactions into a dipeptide and ultimately leading to YH51/YH-53 compounds. (b) X-ray structure of YH-53 [Protein Data Bank (PDB) ID: 7E18] depicting its binding mode within the severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) binding site. (c) Chemical structure of hydroxymethyl ketone PF-00835231 and its prodrug PF-07304814. (d) crystal structure of Mpro with PF-00835231 (PDB ID: 6XHM).
The co-crystal structure of SARS-CoV-2 Mpro in complex with YH-53 was determined (Figure 10b), providing useful insights into the precise interaction between the structures of the inhibitors and the relevant substrate pockets of the viral protease. YH-53 forms covalent bonds with the catalytic cysteine and multiple hydrogen bonds with its backbone. The binding mode is also supported by the interaction of P1 with the Asn142 and Glu166 side-chains, as well as the Glu166 backbone interaction with the P3 residues. Additional information from computational and experimental ADME studies, as well as in vivo PK, and metabolic analysis of YH-53, indicates that it has a strong chance of succeeding as a leading contender treatment for COVID-19.
In September 2020, scientists from Pfizer reported compound PF-00835231 as a potent irreversible inhibitor of SARS-CoV-2 Mpro (Figure 10c, d). PF-00835231 is a hydroxymethyl ketone, which has already been identified for the treatment of SARS-CoV-1 Mpro. In addition, it has good drug-likeness, such as acceptable solubility, stability in blood plasma, and low in vitro and in vivo clearance, rendering it suitable for further development as an anti-coronavirus agent.104, 105 Importantly, the potent inhibition of SARS CoV-2 Mpro has prompted further preclinical investigations of PF-00835231 as a potential treatment for patients with COVID-19. The novel phosphate prodrug PF-07304814 was also described to increase the potential for the intravenous treatment of COVID-19.
The predicted human PK of PF-07304814 provides the ability to achieve systemic unbound concentrations of 0.5 μM (EC90) of PF-00835231 by delivering 500 mg as a continuous infusion over 24 h with infusion volumes < 0.25 L.105 Higher doses (up to and beyond 10 Ceff) also remain feasible if needed because of the high solubility of PF-07304814 (Figure 10c, d). Overall, PF-07304814 exhibits an encouraging preclinical profile that has SARS-CoV-2 antiviral activity, and an ADME profile and a safety profile that support progression to the clinic as a potential novel COVID-19 single-agent antiviral treatment or in combination with antivirals that target other possible critical stages of the coronavirus life cycle.106 The enhanced formulation and solubility profile of PF-07304814 provide clinical flexibility and a safety profile in the preclinical setting, which also support its progression to clinical evaluation.
Pfizer researchers investigated new Mpro inhibitors that had better oral absorption compared with compound PF-00835231 with the aim of developing an oral treatment for COVID-19.107 Given that the hydroxymethyl ketone moiety (P1′) is a hydrogen-bond donor and these kinds of group are known to be associated with low bioavailability, the warhead of the previous PF-00835231 could be altered. Benzothiazol-2-yl ketone103 and nitrile101 were two previously recognized cysteine protease warheads taken into consideration for the new Mpro inhibitors.107.
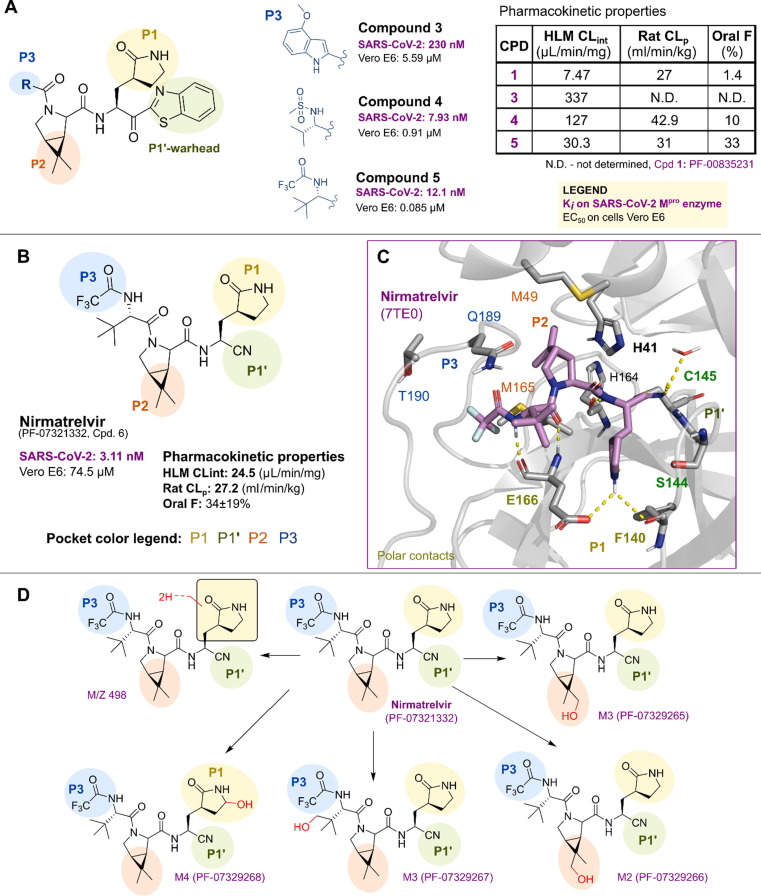
When a benzothiazolyl ketone served as the warhead at position P1′ and a cyclic leucine mimetic [6,6-dimethyl-3-azabicyclo(3.1.0)hexane] was introduced at position P2, the permeability of the resulting compounds was high (Figure 11 a), but the SARS-CoV-2 Mpro potency and metabolic stability were lower. Compound 4 (Figure 11a) improved potency and antiviral activity by substituting an indole ring (compound 3, Figure 11a) group at position P3 with a methanesulfonamide. Trifluoroacetamide was added at P3 to produce even greater antiviral activity (compound 5, Figure 11a). After adding nitrile compound 5 as a warhead over this scaffold, the best candidate (namely, compound 6 or PF-07321332, Figure 11b) was discovered. This substance is a powerful inhibitor of SARS-CoV-2 Mpro, and has strong antiviral activity and high oral bioavailability in rats (Figure 11b, c). Competitive assays with an irreversible inhibitor showed that it inhibits SARS-CoV-2 Mpro in a reversible manner. Given its improved solubility, simple synthetic scaling-up, and decreased tendency to epimerization at the P1 stereocenter, this substance was selected as a clinical candidate. As a biologically active component (‘warhead’), the structure includes a nitrile group that interacts with the cysteine residue of the catalytic dyad in the active site of Mpro. During the early stages of the disease, when the viral load is especially high but there are few symptoms and hospitalization is not yet required, oral administration as a capsule or tablet could enable the treatment of a SARS-CoV-2-infected individual.108.
Figure 11.
ADMET studies of PF-00835231 derivatives. (a) PF-00835231 derivatives (compounds 3–5) with different warheads and P3 moieties. Nirmatrevir (Compound 6) main protease (Mpro) inhibition; (b) pharmacokinetic properties and (c) binding mode from the crystal structure [Protein Data Bank (PDB) ID: 7TE0], with relevant interactions. (d) Metabolic studies of nirmatrelvir. The hydroxylation points are highlighted in red.
The metabolic stability of nirmatrelvir was studied in liver microsomes (fortified with NADPH) and cryopreserved hepatocytes from rats, monkeys, and humans using a substrate depletion assay format (Figure 11d).109 The metabolism of nirmatrelvir was qualitatively similar in liver microsomes and hepatocytes from rats, monkeys, and humans. In NADPH-supplemented HLM, PF-07321332 demonstrated moderate CLint (24.5 ml/min/mg). Reaction phenotyping studies in human liver microsomes revealed that CYP3A4 was primarily responsible for the oxidative metabolism of nirmatrelvir. The prominent metabolites arose via cytochrome P450 (CYP450)-mediated oxidation on the P1 pyrrolidinone ring (M4, PF-07329268), P2 6,6-dimethyl-3-azabicyclo(3.1.0)hexane, and the tertiary-butyl group at the P3 position. In clinical studies, nirmatrelvir was given along with a low dose of the HIV protease inhibitor ritonavir, which is already approved for antiretroviral therapy. The breakdown of nirmatrelvir is slowed by co-administration, allowing it to remain active at higher levels for longer and aid the body’s ability to fight off viruses.110 The cytochrome P450 system in the liver is inhibited by ritonavir.
The drug paxlovid, which combines nirmatrelvir with the protease inhibitor ritonavir, is now being commercialized as a treatment for COVID-19. On December 22, 2021, the FDA granted emergency use authorization for the administration of paxlovid to high-risk patients with confirmed SARS-CoV-2 infection.31
The validation of Mpro inhibitors
So far, numerous structurally diverse Mpro inhibitors have been reported from various sources, raising concerns about their target specificity. The target selectivity and cellular target engagement of purported Mpro inhibitors were addressed by several recent investigations using a variety of secondary assays.61, 111, 112 The compounds can be tested for their ability to inhibit unrelated cysteine proteases, such as the viral EV-A71 2Apro and EV-D68 2Apro, the host cathepsins B, L, and K, caspases, and calpains I, II, and III, among others, to determine their ability to specifically target Mpro. Most of the time, substances that block numerous cysteine proteases are promiscuous substances that might act by causing protein aggregation, redox cycling, or alkylating the catalytic cysteine residue Cys145. The Flip-GFP and protease-Glo luciferase assays for cellular target engagement can be suggested to forecast cellular antiviral activity. Recent reports revealed that previously known Mpro inhibitors, such as ebselen, disulfiram, tideglusib, carmofur, shikonin, and PX-12, are nonspecific inhibitors, and their inhibitory activity was abolished or considerably decreased in the presence of the reducing agent DTT. Several substances, including chloroquine, oxytetracycline, montelukast, candesartan, and dipyridamole, were excluded as Mpro inhibitors. Therefore, despite the urgent need for SARS-CoV-2 antivirals, the scientific community must be cautious about the impacts of promiscuous substances. Early secondary assays should also be carried out to sort hits with low translational potential.
Concluding remarks
Numerous molecules have been reported for viral and host targets since the COVID-19 outbreak caused by SARS-CoV-2. The main protease is one of the crucial viral targets for the researcher to combat the spread of the virus. Indeed, it has become a successful target. Targeting the Mpro of SARS-CoV-2 was greatly aided by understanding of, and structural insights into, the Mpro of previously emerging viruses, such as SARS-CoV-1 and MERS-CoV.
It is also important to confirm the target selectivity and cellular target engagement of Mpro inhibitors, the rapid identification of which has raised questions regarding their target specificity. It was discovered that several purported Mpro inhibitors were either nonspecific inhibitors or not indeed Mpro inhibitors. Therefore, despite the urgent need for SARS-CoV-2 antivirals, the scientific community must be cautious about the impacts of promiscuous substances. Early secondary assays should be performed to separate hits with low translational potential.
Many inhibitors have crystallized together with Mpro (Table S1 in the supplemental information online). Potent Mpro inhibitors were developed through virtual Mpro screening, ligand and structure-based drug design, lead generation, and subsequent optimizations; and some were tested in vivo or in clinical studies (Table 1). These inhibitors are typically divided into two groups: (i) small molecules derived from a variety of methods; and (ii) peptidomimetic derived from the natural peptide substrate. The latter typically have a glutamine surrogate at the P1 site, a leucine‐like residue at the P2, and an electrophilic group at the carboxyl end to react with Cys145.
Nirmatrelvir (PF-07321332) is Pfizer’s orally active drug targeting SARS-CoV-2 Mpro, which is co-administered with ritonavir to help prevent metabolic breakdown via CYP450. It is a covalent peptidomimetic inhibitor with a nitrile warhead that targets the active-site cysteine of Mpro. Conversely, S-217622 is a nonpeptidic, noncovalent inhibitor. The nonpeptidic character provides metabolic stability against proteolysis, and the lack of a covalent warhead reduces any potential off-target toxicity issues. Following the approach taken here, Unoh et al. 69 removed the bias for molecules containing an amide bond, thus avoiding potential metabolic degradation. Importantly, S-217622 shows efficacy similar to that of nirmatrelvir with only one daily dose and does not require the co-administration of ritonavir to block metabolic breakdown.
Authors’ contributions
T.K. and T.P. collected the data and wrote the manuscript, which was revised by T.K., T.P. and S.L.
Acknowledgments
Acknowledgments
CMIF and TüCAD2 are funded by the Federal Ministry of Education and Research and the Baden-Württemberg Ministry of Science as part of the Excellence Strategy of the German Federal and State Governments. T.K. is funded by CMIF (Controlling Microbes to Fight Infections) and TüCAD2. The authors would like to thank CSC-Finland for generous computational resources provided in publications from our group cited within this work. The authors thank Kristine Schmidt for proof-reading (language) the manuscript.
Declaration of interest
The authors declare no competing financial interest.
Biographies

Thales Kronenberger received his PhD in biomedical sciences in 2017 under the supervision of Carsten Wrenger at the University of Sao Paulo (Brazil), after a secondment to the Fraunhofer – ScreeningPort (Germany). He then joined the University Hospital of Tübingen for a postdoctoral period working with Antti Poso and Lars Zender on identifying potential drug targets against cancer. He now works in the Department of Pharmaceutical/Medicinal Chemistry (University of Tübingen, Germany) developing modulators/inhibitors for a range of different drug targets and aiming to understand how protein dynamics influences their druggability.

Stefan Laufer studied pharmacy and completed his PhD at Regensburg University. After postdoctoral research in Frankfurt, he took a position in the pharmaceutical industry but maintained lectureships at Frankfurt and later at Mainz University, where he finished his habilitation in 1997. Since 1999, he has been a full professor (chair) of pharmaceutical and medicinal chemistry at Tübingen University. His research interests are protein kinase inhibitors and eicosanoid modulators.

Thanigaimalai Pillaiyar obtained his PhD under the supervision of Sang-Hun Jung at Pharmaceutical Institute, Chungnam National University, in 2011. As a Japanese Society for the Promotion of Science (JSPS) postdoctoral fellow (2011–2013) with Yoshio Hayashi at Tokyo University of Pharmacy and Life Science, he focused on the development of SARS-CoV-1 Mpro inhibitors. He was awarded an Alexander von Humboldt postdoctoral fellowship (AvH) (2013–2015) with Christa E. Müller and was a visiting scientist in Steven V. Ley’s laboratory, University of Cambridge. Currently, he is establishing a research group at the Pharmaceutical institute, University of Tübingen. His research interests include medicinal chemistry of GPCRs, and SARS-CoV-2 Mpro inhibitors.
Footnotes
Supplementary material to this article can be found online at https://doi.org/10.1016/j.drudis.2023.103579.
Appendix A. Supplementary material
The following are the Supplementary material to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Zhou P., et al. Addendum: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;588:E6. doi: 10.1038/s41586-020-2951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewnard J.A. Ebola virus disease: 11 323 deaths later, how far have we come? Lancet. 2018;392:189–190. doi: 10.1016/S0140-6736(18)31443-0. [DOI] [PubMed] [Google Scholar]
- 4.Weaver S.C., et al. Zika virus: history, emergence, biology, and prospects for control. Antiviral Res. 2016;130:69–80. doi: 10.1016/j.antiviral.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulkarni D.D., Tosh C., Venkatesh G., Senthil K.D. Nipah virus infection: current scenario. Indian J Virol. 2013;24:398–408. doi: 10.1007/s13337-013-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson R.M., et al. Epidemiology, transmission dynamics and control of SARS: the 2002–2003 epidemic. Philos Trans R Soc Lond B Biol Sci. 2004;359:1091–1105. doi: 10.1098/rstb.2004.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oboho I.K., et al. 2014 MERS-CoV outbreak in Jeddah–a link to health care facilities. N Engl J Med. 2015;372:846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taubenberger J.K., Morens D.M. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12:15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z., et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wibmer C.K., et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med. 2021;27:622–625. doi: 10.1038/s41591-021-01285-x. [DOI] [PubMed] [Google Scholar]
- 11.Mallapaty S. India’s massive COVID surge puzzles scientists. Nature. 2021;592:667–668. doi: 10.1038/d41586-021-01059-y. [DOI] [PubMed] [Google Scholar]
- 12.Hassan A.O., et al. A SARS-CoV-2 infection model in mice demonstrates protection by neutralizing antibodies. Cell. 2020;182:744–753. doi: 10.1016/j.cell.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Báez-Santos Y.M., St John S.E., Mesecar A.D. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grum-Tokars V., Ratia K., Begaye A., Baker S.C., Mesecar A.D. Evaluating the 3C-like protease activity of SARS-Coronavirus: recommendations for standardized assays for drug discovery. Virus Res. 2008;133:63–73. doi: 10.1016/j.virusres.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L., et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin Z., et al. Structural basis for the inhibition of SARS-CoV-2 main protease by antineoplastic drug carmofur. Nat Struct Mol Biol. 2020;27:529–532. doi: 10.1038/s41594-020-0440-6. [DOI] [PubMed] [Google Scholar]
- 17.Riva L., et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020;586:113–119. doi: 10.1038/s41586-020-2577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adedeji A.O., Sarafianos S.G. Antiviral drugs specific for coronaviruses in preclinical development. Curr Opin Virol. 2014;8:45–53. doi: 10.1016/j.coviro.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan J.F., et al. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon D.E., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y., et al. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res. 2015;116:76–84. doi: 10.1016/j.antiviral.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Wit E., et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2020;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 25.Pirzada R.H., Haseeb M., Batool M., Kim M., Choi S. Remdesivir and ledipasvir among the FDA-approved antiviral drugs have potential to inhibit SARS-CoV-2 replication. Cells. 2021;10:1052. doi: 10.3390/cells10051052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheahan T.P., et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eastman R.T., et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020;6:672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eastman R.T., et al. Correction to remdesivir: a review of its discovery and development leading to human clinical trials for treatment of COVID-19. ACS Cent Sci. 2020;6:1009. doi: 10.1021/acscentsci.0c00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grein J., et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer W.A., 2nd, et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med. 2022;14:eabl7430. doi: 10.1126/scitranslmed.abl7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.FDA. Frequently Asked Questions on the Emergency Use Authorization for Paxlovid for Treatment of COVID-19. www.fda.gov/media/155052. [Accessed March 28, 2023].
- 32.Liu C., et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann M., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu T., Luo S., Libby P., Shi G.P. Cathepsin L-selective inhibitors: a potentially promising treatment for COVID-19 patients. Pharmacol Ther. 2020;213 doi: 10.1016/j.pharmthera.2020.107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao S., et al. Medicinal chemistry strategies towards the development of effective SARS-CoV-2 inhibitors. Acta Pharmaceutica Sinica B. 2022;12:581–599. doi: 10.1016/j.apsb.2021.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan H., Hu Y., Jadhav P., Tan B., Wang J. Progress and challenges in targeting the SARS-CoV-2 papain-like protease. J Med Chem. 2022;65:7561–7580. doi: 10.1021/acs.jmedchem.2c00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghosh A.K., Mishevich J.L., Mesecar A., Mitsuya H. Recent drug development and medicinal chemistry approaches for the treatment of SARS-CoV-2 infection and COVID-19. ChemMedChem. 2022;17:e202200440. doi: 10.1002/cmdc.202200440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh A.K., et al. Structure-based design of highly potent HIV-1 protease inhibitors containing new tricyclic ring P2-ligands: design, synthesis, biological, and X-ray structural studies. J Med Chem. 2020;63:4867–4879. doi: 10.1021/acs.jmedchem.0c00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hattori S.I., et al. A small molecule compound with an indole moiety inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat Commun. 2021;12:668. doi: 10.1038/s41467-021-20900-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hattori S.I., et al. GRL-0920, an indole chloropyridinyl ester, completely blocks SARS-CoV-2 infection. mBio. 2020;11 doi: 10.1128/mBio.01833-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breidenbach J., et al. Targeting the main protease of SARS-CoV-2: from the establishment of high throughput screening to the design of tailored inhibitors. Angew Chem Int Ed Engl. 2021;60:10423–10429. doi: 10.1002/anie.202016961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pillaiyar T., et al. Small-molecule thioesters as SARS-CoV-2 main protease inhibitors: enzyme inhibition, structure-activity relationships, antiviral activity, and X-ray structure determination. J Med Chem. 2022;65:9376–9395. doi: 10.1021/acs.jmedchem.2c00636. [DOI] [PubMed] [Google Scholar]
- 44.Su H., et al. Identification of pyrogallol as a warhead in design of covalent inhibitors for the SARS-CoV-2 3CL protease. Nat Commun. 2021;12:3623. doi: 10.1038/s41467-021-23751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su H.X., et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol Sin. 2020;41:1167–1177. doi: 10.1038/s41401-020-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Setti E.L., Davis D., Chung T., McCarter J. 3,4-disubstituted azetidinones as selective inhibitors of the cysteine protease cathepsin K. Exploring P2 elements for selectivity. Bioorg Med Chem Lett. 2003;13:2051–2053. doi: 10.1016/s0960-894x(03)00304-4. [DOI] [PubMed] [Google Scholar]
- 47.Skiles J.W., McNeil D. Spiro indolinone beta-lactams, inhibitors of poliovirus and rhinovlrus 3C-proteinases. Tetrahedron Lett. 1990;31:7277–7280. [Google Scholar]
- 48.Cordillot M., et al. In vitro cross-linking of Mycobacterium tuberculosis peptidoglycan by L, D-transpeptidases and inactivation of these enzymes by carbapenems. Antimicrob Agents Chemother. 2013;57:5940–5945. doi: 10.1128/AAC.01663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Munnik M., Lohans C.T., Langley G.W., Bon C., Brem J., Schofield C.J. A fluorescence-based assay for screening β-lactams targeting the Mycobacterium tuberculosis transpeptidase Ldt(Mt2) Chembiochem. 2020;21:368–372. doi: 10.1002/cbic.201900379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar P., et al. Non-classical transpeptidases yield insight into new antibacterials. Nat Chem Biol. 2017;13:54–61. doi: 10.1038/nchembio.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steiner E.M., Schneider G., Schnell R. Binding and processing of β-lactam antibiotics by the transpeptidase LdtMt2 from Mycobacterium tuberculosis. FEBS J. 2017;284:725–741. doi: 10.1111/febs.14010. [DOI] [PubMed] [Google Scholar]
- 52.Malla T.R., et al. Mass spectrometry reveals potential of β-lactams as SARS-CoV-2 M(pro) inhibitors. Chem Commun (Camb) 2021;57:1430–1433. doi: 10.1039/d0cc06870e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malla T.R., et al. Penicillin derivatives inhibit the SARS-CoV-2 main protease by reaction with its nucleophilic cysteine. J Med Chem. 2022;65:7682–7696. doi: 10.1021/acs.jmedchem.1c02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kil J., et al. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2017;390:969–979. doi: 10.1016/S0140-6736(17)31791-9. [DOI] [PubMed] [Google Scholar]
- 55.Lynch E., Kil J. Development of ebselen, a glutathione peroxidase mimic, for the prevention and treatment of noise-induced hearing loss. Semin Hear. 2009;30:047–055. [Google Scholar]
- 56.Singh N., et al. A safe lithium mimetic for bipolar disorder. Nat Commun. 2013;4:1332. doi: 10.1038/ncomms2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Renson M, Etschenberg E, Winkelmann J. A Natterman und Cie GmbH. 2-Phenyl-1,2-benzisoselenazol-3(2H)-one containing pharmaceutical preparations and process for the treatment of rheumatic diseases. US4352799A.
- 58.Renson MP, Etschenberg ED, Winkelmann JD. A Natterman und Cie GmbH. 2-Phenyl-1.2-benzisoselenazol-3(2H)-one containing pharmaceutical preparations and their use. EP0044971A1.
- 59.Jin Z., et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 60.Sies H., Parnham M.J. Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Radic Biol Med. 2020;156:107–112. doi: 10.1016/j.freeradbiomed.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma C., Tan H., Choza J., Wang Y., Wang J. Validation and invalidation of SARS-CoV-2 main protease inhibitors using the Flip-GFP and Protease-Glo luciferase assays. Acta Pharm Sin B. 2022;12:1636–1651. doi: 10.1016/j.apsb.2021.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiao Z., et al. The Mpro structure-based modifications of ebselen derivatives for improved antiviral activity against SARS-CoV-2 virus. Bioorg Chem. 2021;117 doi: 10.1016/j.bioorg.2021.105455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun L.Y., et al. Ebsulfur and Ebselen as highly potent scaffolds for the development of potential SARS-CoV-2 antivirals. Bioorg Chem. 2021;112 doi: 10.1016/j.bioorg.2021.104889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen L., et al. Cinanserin is an inhibitor of the 3C-like proteinase of severe acute respiratory syndrome coronavirus and strongly reduces virus replication in vitro. J Virol. 2005;79:7095–7103. doi: 10.1128/JVI.79.11.7095-7103.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brennan C.M., et al. Oral famotidine versus placebo in non-hospitalised patients with COVID-19: a randomised, double-blind, data-intense, phase 2 clinical trial. Gut. 2022;71:879–888. doi: 10.1136/gutjnl-2022-326952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossetti G.G., et al. Non-covalent SARS-CoV-2 M(pro) inhibitors developed from in silico screen hits. Sci Rep. 2022;12:2505. doi: 10.1038/s41598-022-06306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao S., et al. Discovery and crystallographic studies of trisubstituted piperazine derivatives as non-covalent SARS-CoV-2 main protease inhibitors with high target specificity and low toxicity. J Med Chem. 2022;65:13343–13364. doi: 10.1021/acs.jmedchem.2c01146. [DOI] [PubMed] [Google Scholar]
- 68.Santos L.H., et al. Structure-based identification of naphthoquinones and derivatives as novel inhibitors of main protease M(pro) and papain-like protease PL(pro) of SARS-CoV-2. J Chem Inf Model. 2022;62:6553–6573. doi: 10.1021/acs.jcim.2c00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Unoh Y., et al. Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19. J Med Chem. 2022;65:6499–6512. doi: 10.1021/acs.jmedchem.2c00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shionogi. Shionogi Presents Phase 2/3 Clinical Trial Results (Phase 2a Part) for the COVID-19 Therapeutic Drug S-217622. www.shionogi.com/global/en/news/2022/2/e-20220207.html [Accessed March 28, 2023].
- 71.Shionogi. Shionogi Files for Approval of S-217622, a Therapeutic Drug for COVID-19, in Japan. www.shionogi.com/global/en/news/2022/2/220225.html. [Accessed March 28, 2023].
- 72.Han S.H., et al. Structure-based optimization of ML300-derived, noncovalent inhibitors targeting the severe acute respiratory syndrome coronavirus 3CL protease (SARS-CoV-2 3CL(pro)) J Med Chem. 2022;65:2880–2904. doi: 10.1021/acs.jmedchem.1c00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacobs J., et al. Discovery, synthesis, and structure-based optimization of a series of N-(tert-butyl)-2-(N-arylamido)-2-(pyridin-3-yl) acetamides (ML188) as potent noncovalent small molecule inhibitors of the severe acute respiratory syndrome coronavirus (SARS-CoV) 3CL protease. J Med Chem. 2013;56:534–546. doi: 10.1021/jm301580n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turlington M., et al. Discovery of N-(benzotriazol-1-yl)-N-(benzyl)acetamido)phenyl) carboxamides as severe acute respiratory syndrome coronavirus (SARS-CoV) 3CLpro inhibitors: identification of ML300 and noncovalent nanomolar inhibitors with an induced-fit binding. Bioorg Med Chem Lett. 2013;23:6172–6177. doi: 10.1016/j.bmcl.2013.08.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kitamura N., et al. Expedited approach toward the rational design of noncovalent SARS-CoV-2 main protease inhibitors. J Med Chem. 2022;65:2848–2865. doi: 10.1021/acs.jmedchem.1c00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stille J.K., et al. Design, synthesis and in vitro evaluation of novel SARS-CoV-2 3CLpro covalent inhibitors. Eur J Med Chem. 2022;229 doi: 10.1016/j.ejmech.2021.114046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma C., et al. Discovery of di- and trihaloacetamides as covalent SARS-CoV-2 main protease inhibitors with high target specificity. J Am Chem Soc. 2021;143:20697–20709. doi: 10.1021/jacs.1c08060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pushpakom S., et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 79.Ghahremanpour M.M., et al. Identification of 14 known drugs as inhibitors of the main protease of SARS-CoV-2. ACS Med Chem Lett. 2020;11:2526–2533. doi: 10.1021/acsmedchemlett.0c00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Donnell HR, Tummino TA, Bardine C, Craik CS, Shoichet BK. Colloidal aggregators in biochemical SARS-CoV-2 repurposing screens. J Med Chem. Published online November 23, 2021: 10.1021/acs.jmedchem.1c01547. [DOI] [PMC free article] [PubMed]
- 81.Ma C., et al. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020;30:678–692. doi: 10.1038/s41422-020-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang C.H., et al. Potent noncovalent inhibitors of the main protease of SARS-CoV-2 from molecular sculpting of the drug perampanel guided by free energy perturbation calculations. ACS Cent Sci. 2021;7:467–475. doi: 10.1021/acscentsci.1c00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dai W., et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Meyer S., et al. Lack of antiviral activity of darunavir against SARS-CoV-2. Int J Infect Dis. 2020;97:7–10. doi: 10.1016/j.ijid.2020.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang C.H., et al. Optimization of triarylpyridinone inhibitors of the main protease of SARS-CoV-2 to low-nanomolar antiviral potency. ACS Med Chem Lett. 2021;12:1325–1332. doi: 10.1021/acsmedchemlett.1c00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Drayman N., et al. Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2. Science. 2021;373:931–936. doi: 10.1126/science.abg5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dubreuil P., et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS ONE. 2009;4:e7258. doi: 10.1371/journal.pone.0007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hahn K.A., et al. Masitinib is safe and effective for the treatment of canine mast cell tumors. J Vet Intern Med. 2008;22:1301–1309. doi: 10.1111/j.1939-1676.2008.0190.x. [DOI] [PubMed] [Google Scholar]
- 89.Humbert M., et al. Masitinib, a c-kit/PDGF receptor tyrosine kinase inhibitor, improves disease control in severe corticosteroid-dependent asthmatics. Allergy. 2009;64:1194–1201. doi: 10.1111/j.1398-9995.2009.02122.x. [DOI] [PubMed] [Google Scholar]
- 90.Luttens A., et al. Ultralarge virtual screening identifies SARS-CoV-2 main protease inhibitors with broad-spectrum activity against coronaviruses. J Am Chem Soc. 2022;144:2905–2920. doi: 10.1021/jacs.1c08402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Consortium TCM, Chodera J, Lee A, London N, Delft F von. Open science discovery of oral non-covalent SARS-CoV-2 main protease inhibitors. bioRxiv. Published online March 2, 2023. 10.1101/2020.10.29.339317. [DOI]
- 92.Ferreira G.M., Kronenberger T., Tonduru A.K., Hirata R.D.C., Hirata M.H., Poso A. SARS-COV-2 M(pro) conformational changes induced by covalently bound ligands. J Biomol Struct Dyn. 2022;40:12347–12357. doi: 10.1080/07391102.2021.1970626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cáceres C.J., et al. Efficacy of GC-376 against SARS-CoV-2 virus infection in the K18 hACE2 transgenic mouse model. Sci Rep. 2021;11:9609. doi: 10.1038/s41598-021-89013-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qiao J., et al. SARS-CoV-2 M(pro) inhibitors with antiviral activity in a transgenic mouse model. Science. 2021;371:1374–1378. doi: 10.1126/science.abf1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xia Z., et al. Rational design of hybrid SARS-CoV-2 main protease inhibitors guided by the superimposed cocrystal structures with the peptidomimetic inhibitors GC-376, telaprevir, and boceprevir. ACS Pharmacol Transl Sci. 2021;4:1408–1421. doi: 10.1021/acsptsci.1c00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kneller D.W., et al. Covalent narlaprevir- and boceprevir-derived hybrid inhibitors of SARS-CoV-2 main protease. Nat Commun. 2022;13:2268. doi: 10.1038/s41467-022-29915-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sydnes M.O., et al. Synthesis of glutamic acid and glutamine peptides possessing a trifluoromethyl ketone group as SARS-CoV 3CL protease inhibitors. Tetrahedron. 2006;62:8601–8609. doi: 10.1016/j.tet.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bacha U., Barrila J., Gabelli S.B., Kiso Y., Mario Amzel L., Freire E. Development of broad-spectrum halomethyl ketone inhibitors against coronavirus main protease 3CL(pro) Chem Biol Drug Des. 2008;72:34–49. doi: 10.1111/j.1747-0285.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dai W., et al. New developments for the design, synthesis and biological evaluation of potent SARS-CoV 3CL(pro) inhibitors. Bioorg Med Chem Lett. 2009;19:2722–2727. doi: 10.1016/j.bmcl.2009.03.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Konno S., et al. 3CL Protease inhibitors with an electrophilic arylketone moiety as anti-SARS-CoV-2 agents. J Med Chem. 2022;65:2926–2939. doi: 10.1021/acs.jmedchem.1c00665. [DOI] [PubMed] [Google Scholar]
- 101.Thanigaimalai P., et al. Development of potent dipeptide-type SARS-CoV 3CL protease inhibitors with novel P3 scaffolds: design, synthesis, biological evaluation, and docking studies. Eur J Med Chem. 2013;68:372–384. doi: 10.1016/j.ejmech.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thanigaimalai P., et al. Design, synthesis, and biological evaluation of novel dipeptide-type SARS-CoV 3CL protease inhibitors: structure-activity relationship study. Eur J Med Chem. 2013;65:436–447. doi: 10.1016/j.ejmech.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Konno S., et al. Design and synthesis of new tripeptide-type SARS-CoV 3CL protease inhibitors containing an electrophilic arylketone moiety. Bioorg Med Chem. 2013;21:412–424. doi: 10.1016/j.bmc.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mahase E. Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375 doi: 10.1136/bmj.n2713. [DOI] [PubMed] [Google Scholar]
- 105.Boras B., et al. Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID19. Nat Commun. 2021;12:6055. doi: 10.1038/s41467-021-26239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pfizer. A Phase 1b, 2-part, double-blind, placebo-controlled, sponsor-open study, to evaluate the safety, tolerability and pharmacokinetics of single ascending (24-hour, part 1) and multiple ascending (120-hour, part 2) intravenous infusions of pf-07304814 in hospitalized participants with COVID-19. https://clinicaltrials.gov/ct2/show/NCT04535167. [Accessed March 28, 2023].
- 107.Owen D.R., et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- 108.Avoxa-Mediengruppe Deutscher Apotheker. Covid-19-Medikamente: Oraler Proteasehemmer gegen Corona geht in klinische Prüfung. www.pharmazeutische-zeitung.de/oraler-proteasehemmer-gegen-corona-geht-in-klinische-pruefung-124794/. [Accessed March 28, 2023].
- 109.Eng H., et al. Disposition of nirmatrelvir, an orally bioavailable inhibitor of SARS-CoV-2 3C-like protease, across animals and humans. Drug Metab Dispos. 2022;50:576–590. doi: 10.1124/dmd.121.000801. [DOI] [PubMed] [Google Scholar]
- 110.Pfizer. Pfizer’s novel COVID-19 oral antiviral treatment candidate reduced risk of hospitalization or death by 89% in interim analysis of Phase 2/3 EPIC-HR study. www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate. [Accessed March 28, 2023].
- 111.Fischer C., Feys J.R. SARS-CoV-2 Mpro inhibitors: achieved diversity, developing resistance and future strategies. Future Pharmacology. 2023;3:80–107. [Google Scholar]
- 112.Tan H., Ma C., Wang J. Invalidation of dieckol and 1,2,3,4,6-pentagalloylglucose (PGG) as SARS-CoV-2 main protease inhibitors and the discovery of PGG as a papain-like protease inhibitor. Med Chem Res. 2022;31:1147–1153. doi: 10.1007/s00044-022-02903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.FDA. Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19. [Accessed March 28, 2023].
- 114.Pardes BioSciences. Pipeline. www.pardesbio.com/pipeline/. [Accessed March 28, 2023].
- 115.Pardes BioSciences. Pardes Biosciences announces FDA clearance of IND application for PBI-0451, an oral antiviral drug candidate for the treatment and prevention of SARS-CoV-2 infections. https://ir.pardesbio.com/news-releases/news-release-details/pardes-biosciences-announces-fda-clearance-ind-application-pbi/. [Accessed March 28, 2023].
- 116.Enanta Pharmaceuticals. Pipeline overview. www.enanta.com/pipeline/pipeline-overview/. [Accessed March 28, 2023].
- 117.Enanta. New preclinical data for EDP-235, Enanta’s oral coronavirus protease inhibitor designed for the treatment of COVID-19, to be presented at IDWeek™ 2022. https://ir.enanta.com/news-releases/news-release-details/new-preclinical-data-edp-235-enantas-oral-coronavirus-protease/. [Accessed March 28, 2023].
- 118.Shionogi. Notice regarding the progress of S-217622 to fight COVID-19. www.shionogi.com/global/en/news/2022/01/e-220120.html. [Accessed March 28, 2023].
- 119.Chaves O.A., Sacramento C.Q., Ferreira A.C., Mattos M., Fintelman-Rodrigues N., Temerozo J.R., et al. Atazanavir is a competitive inhibitor of SARS-CoV-2 Mpro, impairing variants replication in vitro and in vivo. Pharmaceuticals (Basel) 2021;15:21. doi: 10.3390/ph15010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hospital do Coracao. Antiviral for adult patients hospitalized for SARS-CoV-2 infection: a Randomized, Phase 2/3, multicenter, placebo controlled, adaptive, multi-arm, multi-stage clinical trial. https://clinicaltrials.gov/ct2/show/NCT04468087. [Accessed March 28, 2023].
- 121.Sound Pharmaceuticals. A phase 2, randomized, double-blind, placebo-controlled, dose escalation study to evaluate the safety and efficacy of SPI-1005 in moderate COVID-19 patients. https://clinicaltrials.gov/ct2/show/NCT04484025. [Accessed March 28, 2023].
- 122.Rice T. Trial of early therapies during non-hospitalized outpatient window (TREAT NOW) for COVID-19. https://clinicaltrials.gov/ct2/show/NCT04372628. [Accessed March 28, 2023]. [DOI] [PMC free article] [PubMed]
- 123.Cao B., et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jang M, et al. Lopinavir-ritonavir is not an effective inhibitor of the main protease activity of SARS-CoV-2 in vitro. bioRxiv. Published online September 16, 2020. 10.1101/2020.09.16.299800. [DOI]
- 125.Chen H., et al. First clinical study using HCV protease inhibitor danoprevir to treat COVID-19 patients. Medicine (Baltimore) 2020;99:e23357. doi: 10.1097/MD.0000000000023357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gammeltoft K.A., et al. Hepatitis C virus protease inhibitors show differential efficacy and interactions with remdesivir for treatment of SARS-CoV-2 in vitro. Antimicrobial Agents Chemotherapy. 2021;65 doi: 10.1128/AAC.02680-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.The Ninth Hospital of Nanchang. An open clinical trial to evaluate ganovo (danoprevir) combined with ritonavir in the treatment of SARS-CoV-2 infection. https://clinicaltrials.gov/ct2/show/NCT04291729. [Accessed March 28, 2023].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.