Abstract
Background
The KBindER (K+ Binders in Emergency Room and hospitalized patients) clinical trial is the first head-to-head evaluation of oral potassium binders (cation-exchange resins) for acute hyperkalemia therapy.
Methods
Emergency room and hospitalized patients with a blood potassium level ≥ 5.5 mEq/L are randomized to one of four study groups: potassium binder drug (sodium polystyrene sulfonate, patiromer, or sodium zirconium cyclosilicate) or nonspecific laxative (polyethylene glycol). Exclusion criteria include recent bowel surgery, ileus, diabetic ketoacidosis, or anticipated dialysis treatment within 4 h of treatment drug. Primary endpoints include change in potassium level at 2 and 4 h after treatment drug. Length of hospital stay, next-morning potassium level, gastrointestinal side effects and palatability will also be analyzed. We are aiming for a final cohort of 80 patients with complete data endpoints (20 per group) for comparative statistics including multivariate adjustment for kidney function, diabetes mellitus, congestive heart failure, metabolic acidosis, renin-angiotensin-aldosterone system inhibitor prescription, and treatment with other agents to lower potassium (insulin, albuterol, loop diuretics).
Discussion
The findings from our study will inform decision-making guidelines on the role of oral potassium binders in the treatment of acute hyperkalemia.
Trial registration
ClinicalTrials.gov Identifier: NCT04585542. Registered 14 October 2020.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-023-03145-x.
Keywords: Acute hyperkalemia, Cation-exchange resins, Sodium polystyrene sulfonate, Patiromer, Sodium zirconium cyclosilicate
Introduction
In the setting of normal kidney function, serum potassium (K+) is maintained within a tight range (3.5–5.3 mEq/L) via renal excretion and cellular redistribution [1]. Hyperkalemia is considered severe if K+ is ≥ 6.0 mEq/L, with or without symptomatic muscle weakness/paralysis or electrocardiogram changes. Severe hyperkalemia occurs in up to 10% of all hospitalized patients [2] and is an independent predictor of increased mortality [3] that is associated with prolonged hospital stays, higher healthcare costs, and a 30-day hospital readmission rate of 14.21% vs. 9.86% in non-hyperkalemic patients [4].
Risk factors for hyperkalemia include chronic kidney disease (CKD), diabetes mellitus [5–7], and use of certain medications such as renin-angiotensin-aldosterone system (RAAS) inhibitors and mineralocorticoid receptor antagonists [5, 8–10]. Historically, discontinuation of RAAS inhibitors occurred in ~75% of cases in response to drug-induced hyperkalemia [11], potentially leaving patients vulnerable to adverse cardiovascular events [12]. The emergence of the newer oral potassium binders (patiromer and sodium zirconium cyclosilicate [SZC]) has allowed maintenance of normokalemia in CKD patients receiving background therapy with RAAS inhibitors [13–15]. However, there is a lack of data on the potential utility of these binders for the treatment of acute hyperkalemia.
No universally accepted standard of care protocol has been established for the treatment of patients presenting with acute hyperkalemia, whether in the ambulatory or emergency room (ER) setting [2, 11, 16]. In a multicenter, prospective, observational analysis (n = 203), the REVEAL-ED study reported that 7 therapies (inhaled β2-agonists, intravenous [i.v.] bicarbonate, i.v. calcium, hemodialysis, i.v. diuretics, i.v. insulin/glucose, oral sodium polystyrene sulfonate) were utilized in 43 varying combinations in the 14 emergency departments surveyed [17].
Oral potassium binders hold particular appeal in patients with advanced CKD where diuretics may be less effective, and where dialysis has inherent risks and is costly. For over 50 years the only available oral potassium binder had been sodium polystyrene sulfonate (SPS, FDA approved in 1958). In recent years, two new agents have been approved by the FDA for chronic management of hyperkalemia (patiromer in 2015 and SZC in 2018) (Fig. 1). Clinical trials where oral potassium binders were studied against placebo are summarized in Table 1. To date, no studies have compared the efficacy of SPS, patiromer and SZC head-to-head in ER or hospitalized patients with acute hyperkalemia.
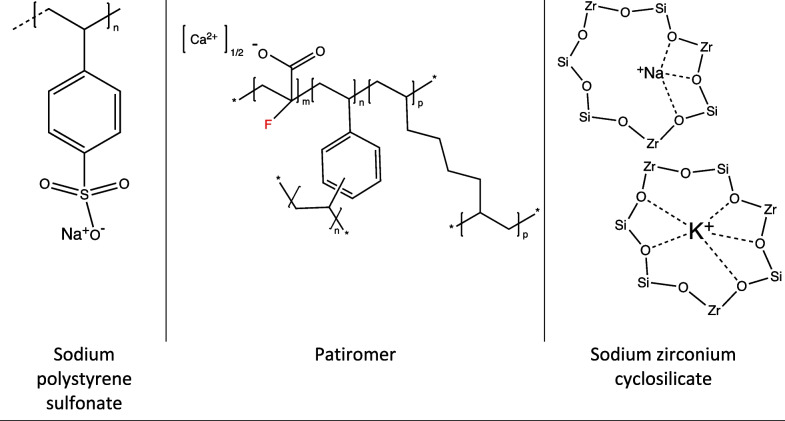
Fig. 1.
Chemical structure of the oral potassium binders (cation-exchange resins). Sodium zirconium cyclosilicate has a conformational change when sodium (Na+) is exchanged for potassium (K+)
Table 1.
Summary of clinical trials with oral potassium binders
| Clinical trial | Number of participants & dosage | Study population | Study type | Duration | Primary endpoints | Acute serum K+ findings | Limitations |
|---|---|---|---|---|---|---|---|
| Sodium polystyrene sulfonate | |||||||
| Randomized Clinical Trial of Sodium Polystyrene Sulfonate for the Treatment of Mild Hyperkalemia in CKD [18] Lepage et al. | 33 patients total; 16 assigned to SPS treatment group (30 g/day) and 17 assigned to placebo group | Adult patients in ambulatory setting with chronic kidney disease and baseline serum K+ of 5.0–5.9 mEq/L | Double-blind, randomized clinical controlled trial | 7 days | Comparison of serum K+ between groups from baseline to day 7 (after final treatment dose) | Decreased serum K+ levels by a mean of 1.25 mEq/L (SD: ± 0.56) in SPS group compared to 0.21 mEq/L (SD: ± 0.29) in placebo group over 7-day period; difference of -1.04 mEq/L (95% CI, -1.37 to -0.71); P < 0.001 | Small sample size; serum K+ of > 5.9 mEq/L was excluded; limited to 2 blood draws for serum K+ (day 0 and day 7) hence, unable to detect efficacy in acute setting |
| Patiromer | |||||||
| Patiromer for Treatment of Hyperkalemia in the Emergency Department: A Pilot Study [19] Rafique et al. | 30 patients total; 15 assigned to standard of care group, 15 assigned to patiromer group receiving single, 25.2 g dose | Adult emergency room patients with end-stage renal disease and baseline serum K+ ≥ 6.0 mEq/L | Single-center, randomized, open-label pilot study | 6 h | Difference in serum K+ between SOC group and treatment group at 6 h | Decreased serum K+ within 2 h of the patiromer-treated group (single-dose 25.2 g) compared to the SOC group (5.91 mEq/L vs. 6.51 mEq/L respectively; P < 0.009); no differences shown at 6 h | Small sample size |
| Patiromer Induces Rapid and Sustained Potassium Lowering in Patients with Chronic Kidney Disease and Hyperkalemia [13] Bushinsky et al. | 25 patients in single treatment group receiving 8.4 g/dose, 4 doses total | Adult patients hospitalized with serum K+ of 5.5–6.5 mEq/L; majority with chronic kidney disease and all taking at least one RAAS inhibitor | Multicenter, international, phase I, open-label, single-arm study conducted at 5 sites in Europe | 72-h K+/Na+ restricted diet phase followed by 48-h treatment phase and outpatient follow-up phase | Mean change in serum K+ from baseline until 48 h after initial dose | Decreased serum K+ of 0.21 mEq/L within 7 h of initial treatment compared to baseline (95% CI, -0.35 to -0.07) P = 0.004. Significant reductions occurred at all assessment points from 7–48 h P ≤ 0.004 at 10 h, P ≤ 0.001 at 12–48 h | Lack of comparative placebo control and small sample size. Those with abnormal electrocardiograph readings related to hyperkalemia were excluded |
| Sodium zirconium cyclosilicate | |||||||
| Emergency Potassium Normalization Treatment Including Sodium Zirconium Cyclosilicate: A Phase II, Randomized, Double-blind, Placebo-controlled Study (ENERGIZE) [16] Peacock et al. | 62 patients total; 29 assigned to SZC group (10 g, up to 3 doses); 33 assigned to placebo group | Adult patients admitted to the emergency room with baseline serum K+ of ≥ 5.8 mEq/L | Multicenter, international, randomized, double-blind, placebo-controlled, parallel-group, phase II study conducted at 33 sites in Denmark, Italy, Russia, and the United States | 10-h treatment period followed by a single follow-up visit 7 days later | Mean change in serum K+ from baseline until 4 h after initial dose | Greater reduction in baseline serum K+ within SZC treatment group compared to placebo group –0.72 (0.12) versus –0.36 (0.11) mEq/L (least squares mean difference –0.35 mmol/L; 95% CI = –0.68 to –0.02) | Missing lab values of serum K+ at 4 h for substantial amount of patients; small sample size of patients (N = 62); enrolled; high withdrawal rate (30.2% treatment group and 43.2% placebo group) |
| Sodium Zirconium Cyclosilicate in Hyperkalemia [20] Packham et al. | 754 patients assigned to 4 different SZC treatment groups (1.5 g, 2.5 g, 5 g, 10 g) or placebo group | Adult patients in the ambulatory setting with baseline serum K+ of 5.0 – 6.5 mEq/L | Multicenter, two-stage, randomized, double-blind, phase III study conducted at 65 sites in United States, Australia, and South Africa | Initial phase of 48 h, followed by a 12-day maintenance phase | Exponential rate of change in mean serum K+ at 48 h and between-group difference of serum K+ post 48 h during maintenance phase | Mean reduction of serum K+ with single dose of 10 g SZC within 1 h was 0.11 mEq/L compared to 0.01 mEq/L in placebo group (95% CI, -0.05 to 0.07) | Patients with serum K+ of > 6.5 mEq/L and those with abnormal electrocardiograph readings related to hyperkalemia were excluded; hospitalized patients were excluded |
| Effect of Sodium Zirconium Cyclosilicate on Potassium Lowering for 28 Days Among Outpatients with Hyperkalemia – the HARMONIZE Randomized Clinical Trial [21] Kosiborod et al. | 258 patients assigned to 10 g SZC treatment group (open-label phase) followed by randomization to 3 different SZC dosage groups (5, 10, or 15 g) or placebo (28-day phase) | Adult patients in the ambulatory setting with baseline serum K+ of ≥ 5.1 mEq/L | Phase III, randomized, double-blind, placebo-controlled trial conducted at 44 sites in United States, Australia, and South Africa | Initial open-label phase of 48 h, followed by a randomized 28-day phase | Difference of mean serum K+ levels between 3 different SZC treatment groups and placebo group | Decreased serum K+ of 0.2 mEq/L within 1 h of single 10 g dose of SZC compared to baseline (95% CI, -0.3 to -0.2) P < 0.001. At 2 and 4 h after initial dose, mean change in serum K+ was -0.4 mEq/L (95% CI, -0.5 to -0.4 and -0.5 mEq/L (95% CI, -0.6 to -0.5); P < 0.001 for each time point | Hospitalized patients and those with severe arrythmias were excluded |
| A Phase 2 Study on the Treatment of Hyperkalemia in Patients with Chronic Kidney Disease Suggests that the Selective Potassium Trap, ZS-9, is Safe and Efficient [22] Ash et al. | 90 patients total; 60 assigned to 3 different SZC dosage groups (0.3, 3, or 10 g); 30 assigned to placebo group | Adult patients with stage 3 chronic kidney disease and baseline serum K+ of 5.0–6.0 mEq/L | Phase II, randomized, double-blind, placebo-controlled trial conducted at 9 sites in the United States | 48 h | Rate of serum K+ decline within the first 48 h | Decreased serum K+ of 0.11 mEq/L from baseline at 1 h following initial dose in 10 g SZC group compared to placebo (P = 0.02) | Small sample size of patients; serum K+ of > 6.0 mEq/L was excluded |
Sodium polystyrene sulfonate (SPS)
SPS was introduced to the market in 1958 prior to the Kefauver-Harris Drug Amendments, laws that established protocols for drug safety and efficacy [18]. SPS can be administered orally or rectally, and exchanges Na+ for K+ within the distal colon. The drug also has an affinity for divalent cations including calcium (Ca2+) and magnesium (Mg2+), thus there is a potential risk of hypocalcemia and hypomagnesemia. In rare cases, SPS has been associated with intestinal necrosis and increased mortality when combined with 70% sorbitol, resulting in a 2009 FDA black-box warning against long-term use for hyperkalemia management [23, 24]. Sorbitol was initially added for its laxative properties, to minimize bowel impaction with SPS. A review of case reports published between 1948–2011 identified 58 cases of SPS serious adverse events (41 preparations with sorbitol and 17 preparations without sorbitol); there was an associated 33% mortality rate [23]. This is a rare event; approximately 5 million doses of SPS are administered in the United States annually [25].
SPS was the only K+ lowering resin available for over 50 years, and remains widely used among clinicians today (without sorbitol or with much lower concentrations of the sugar-alcohol) [23]. There has been one randomized controlled trial which compared SPS vs. placebo in the outpatient setting, in 31 CKD patients with mild hyperkalemia (5.0–5.9 mEq/L). SPS without sorbitol dosed 30 g/day for 7 days was effective in decreasing serum K+ by an average of 1.04 mEq/L [18]. While SPS is widely used as an adjunct therapy for acute hyperkalemia, randomized controlled trials in the ER and hospital setting are lacking.
Patiromer
Patiromer was FDA-approved in 2015 and has been tested extensively in the management of chronic hyperkalemia [13, 14, 26, 27]. Patiromer is a non-absorbable spherical polymer that exchanges Ca2+ for K+ in the distal colon. In vitro animal studies conducted by Lingyun et al. demonstrated that patiromer had a 1.5–2.5-fold superior K+ binding capacity compared to SPS under colonic-like pH conditions [28]. Ca2+ loading in CKD patients may contribute to vascular calcification. However, in the 52-week study conducted by Bakris et al. (n = 304), there was no increase in serum Ca2+ amongst patients treated daily with patiromer [27]. Similar to SPS, binding of Mg2+ can occur and cases of patiromer-associated hypomagnesemia have been reported in CKD patients [29].
The OPAL-HK [14], PEARL-HF [30] and AMETHYST-DN [27] clinical trials demonstrated efficacy of patiromer for chronic management of hyperkalemia, in CKD patients with/without comorbidities such as heart failure, diabetes mellitus, and hypertension. Importantly, these studies showed that patiromer allowed safe continuation of RAAS inhibitor therapy with avoidance of drug-induced hyperkalemia.
To date, only one randomized controlled trial has investigated patiromer in the setting of acute hyperkalemia, in end-stage kidney disease patients. Rafique et al. reported significant decrease in serum K+ 2 h after patiromer therapy (n = 15 per group, baseline average serum K+ 6.32 mEq/L, follow up serum K+ of 5.91 mEq/L vs. 6.51 mEq/L in the standard of care group; P < 0.009) [19].
Sodium zirconium cyclosilicate (SZC)
FDA-approved in 2018, SZC binds K+ in exchange for sodium and hydrogen ions within the small intestine. SZC mimics physiological K+ ion channels and has over 25-fold selectivity for K+ over other ions such as Ca2+ and Mg2+, in comparison to SPS which shows a 0.2–0.3 selectivity for K+ over these ions [31]. The safety and efficacy of SZC for the management of chronic hyperkalemia has been well documented in numerous randomized controlled trials in the ambulatory setting [20, 32, 33]. Its potential utility in acute hyperkalemia therapy was examined in a multicenter, phase three, double-blind study which randomized 753 patients to receive SZC (either 1.25 g, 2.5 g, 5 g, or 10 g) or placebo three times daily for 48 h [20]. SZC was demonstrated to lower serum K+ at 48 h from 5.3 mEq/L to 4.6 mEq/L (P < 0.001) while a clinically significant treatment effect was observed at 1 h utilizing the 10 g dose [20]. Kosiborod et al. demonstrated similar clinical significance at 1 h [21].
The ENERGIZE study is the only study to date analyzing SZC in the ER setting. In this randomized placebo-controlled trial (n = 70), SZC in addition to insulin and glucose incurred a greater reduction in serum K+ at 2 h compared to insulin and glucose alone (−0.72 ± 0.12 vs. -0.36 ± 0.11 mEq/L) [16].
Comparison of the mechanism of action, degree of K+ lowering, and potential side effects of the oral potassium binders is summarized in Table 2. As discussed above, data is limited regarding the potential role of the newer potassium binders (patiromer and SZC) for the therapy of acute hyperkalemia. We are addressing this knowledge gap with a head-to-head comparison of the oral potassium binders in the KBindER study.
Table 2.
Comparison of potassium exchange resins for the treatment of hyperkalemia
| Drug name | Sodium polystyrene sulfonate (SPS) | Patiromer | Sodium zirconium cyclosilicate (SZC) |
|---|---|---|---|
| Trade name | Kayexalate®, Kionex® | Veltessa® | Lokelma® |
| FDA approval | 1958 (prior to drug regulation laws) | 2015 | 2018 |
| Onset of action | 2–24 h | 2 h | 1 h |
| Mode of action | The sulfonate molecule exists as sulfonic acid in its ionic state and allows for interaction with a Na+ counterion. In this method, Na+ is exchanged for K+ within the gastrointestinal tract | Utilizes the high colonic K+ concentration and exchanges its Ca2+ counterion for it. The attached fluorine atom at the alpha-carbon position of patiromer causes electron-withdrawing effects and generates a lower pKa compared to colonic pH. This allows the resin to exist in an ionized state and bind K+ effectively | Selectively binds to K+ throughout the length of the gastrointestinal tract. SZC mimics physiological K+ ion channels by utilizing a thermodynamically favorable binding pocket unique to the size of K+ |
| Degree of K+ lowering | 1.04 mEq/La | 0.6 mEq/Lb | 0.4 mEq/Lc |
| Location of efficacy | Large intestine | Distal colon | Small intestine and large intestine |
| Toxicity | Na+ loading/fluid overload, hypomagnesemia, hypocalcemia, colonic necrosis | Hypomagnesemia | Edema |
| Side effects | Nausea, vomiting, diarrhea, constipation, abdominal pain | Mild-severe constipation, abdominal pain | Constipation, abdominal pain |
| Exchange ion | Na+ | Ca2+ | H+, Na+ |
| Selectivity | Non-specific, K+, Ca2+, Mg2+ | Specific, K+ (low specificity, Mg2+) | Highly specific, K+ and ammonium |
| Resin size | ~ 11–124 µm | ~ 100 µm | > 3 µm (non-absorbed) |
| Molecular composition | Polymer resin consisting of sulfonate and attached vinylbenzene (styrene) R group | Polymer resin with attached carboxylic acid and fluorine atom at alpha carbon position | Three-dimensional, seven-member ring lattice made up of alternating zirconium and silicate atoms with adjoining oxygen atoms |
a1.04 mEq/L mean decrease of serum K+ compared to placebo treatment; SPS was given at a dosage of 30 g/day for 7 days [18]
b0.6 mEq/L decrease of serum K+ 2 h post-treatment with a single dosage of 25.2 g [19]
c0.4 mEq/L decrease of serum K+ at 2 h post-treatment with dosage of 10 g 3 times daily; clinically significant decrease of K+ observed at 1 h [21]
Methods/design
The KBindER study (K+ Binders in Emergency Room and hospitalized patients) is the first trial to compare the efficacy of the 3 oral potassium binders (SPS, patiromer and SZC) in a head-to-head fashion, for the treatment of acute hyperkalemia. We propose a pragmatic study design whereby the cation-exchange resin is added on to standard hyperkalemia treatment per discretion of the treating physician. We have included a mock placebo group with a nonspecific laxative (polyethylene glycol 3350) to investigate the effects of increasing stool output without specific K+ exchange, since constipation has been associated with hyperkalemia risk [34].
All study procedures were approved by the University of California, Irvine Institutional Review Board (protocol HS# 2020–5780, approval date August 6, 2020; latest version April 2, 2021). Inclusion and exclusion criteria are summarized in Table 3. Study participants are recruited from adult patients in the ER or main hospital (excluding the intensive care unit) at the University of California-Irvine Medical Center (Orange, CA). Inclusion criteria include plasma K+ ≥ 5.5 mEq/L, English or Spanish speaking (consent forms are available in these languages) and able to provide written informed consent. Exclusion criteria include impaired intestinal motility (recent bowel surgery, ileus or bowel obstruction), pregnancy, active psychiatric disorder impairing provision of informed consent, diabetic ketoacidosis, anticipated dialysis treatment within 4 h, or hypersensitivity to any of the study drugs. Patients with pseudohyperkalemia will be excluded: hemolyzed blood samples as reported by the clinical laboratory, severe leukocytosis (white cell count > 50 × 109/L) or severe thrombocytosis (platelet count > 500 × 109/L) [35]. Patients receiving sorbitol are also excluded, given the increased risk of intestinal necrosis if they were to be randomized to receive SPS [23].
Table 3.
Inclusion and exclusion criteria for the KBindER study
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
▪ Plasma K+ ≥ 5.5 mEq/L ▪ Age ≥ 18 years ▪ English or Spanish speaking ▪ Patient able to provide written informed consent |
▪ Recent Bowel Surgery ▪ Ileus or bowel obstruction ▪ Received a dose of an oral potassium binder in past 48 h ▪ Hemolyzed blood specimen as indicated by the laboratory ▪ Severe leukocytosis or thrombocytosis ▪ Pregnancy ▪ Active psychiatric disorder ▪ Diabetic ketoacidosis or hyperkalemia caused by any condition for which a therapy directed against the underlying cause is expected to correct the hyperkalemia ▪ Dialysis session expected within 4 h after randomization ▪ History or hypersensitivity to any of the study drugs ▪ Concurrent use of sorbitol (due to increased risk of intestinal necrosis when used with SPS) |
Study groups
Participants, physicians and house staff are blinded to the study group assignment. Participants undergo sequential randomization to one of 4 study groups:
SPS (Kayexalate, Kionex) one dose of 30 g
Patiromer (Veltassa) one dose of 25.2 g
Sodium zirconium cyclosilicate (Lokelma) one dose of 15 g
Polyethylene glycol 3350 (MiraLax) one dose of 17 g (nonspecific osmotic laxative)
Higher doses of the oral potassium binders were chosen to evaluate maximum impact on lowering blood K+ with one treatment dose. Given the focus on acute hyperkalemia therapy, it would not have been clinically meaningful to use the lowest starting dose.
Study procedures
Non-ICU and ER patients are screened remotely by research staff using the Epic Medical Records system to identify potential study candidates. Patients with a qualifying blood K+ level (≥ 5.5 mEq/L) are then screened for the inclusion and exclusion criteria (Table 3). Temporizing therapies for acute hyperkalemia (e.g., insulin/dextrose, IV fluids/loop diuretic or albuterol) will be administered per discretion of the attending physician regardless of enrollment status.
A physician listed on the consent form will perform a final criteria review and obtain signed informed consent. These study investigators include hospitalists, ER physicians, and nephrologists. To ensure that the patient and primary treating provider are blinded to the study drug, a nephrologist (WLL) will order the study drug that is dispensed by the Investigational Drug Services Pharmacy. The nurse dispending the study drug is advised not to disclose name of study drug to the participant.
Blood chemistries including basic metabolic panel, Mg2+ and phosphorus are drawn at 2 and 4 h following administration of the study drug. Participants complete a Symptom Assessment form and report a Palatability Score at 4 h following the medication dose (Supplementary Materials). If the 4-h blood K+ remains elevated ≥ 5.5 mEq/L then the primary hospital team will be notified for further hyperkalemia management per their discretion. Data on participant demographics, home and hospital-administered medications, comorbid conditions, lab parameters, and length of ER/hospital stay from chart review will be compiled in a secure REDCap database. Study procedures are summarized in Fig. 2.
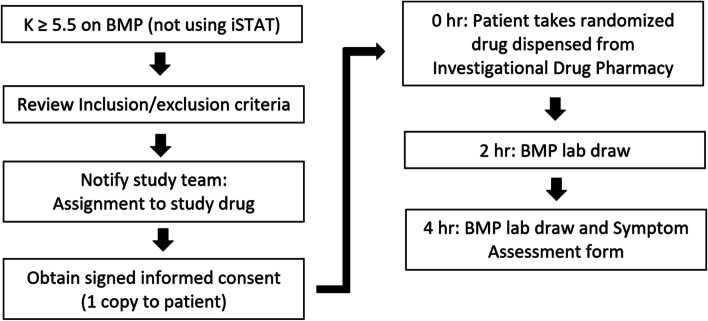
Fig. 2.
Flowchart of study procedures
An independent Data & Safety Monitoring Board will review potential adverse events every 3 months, or sooner if necessary. This data monitoring committee is comprised of a nephrologist, cardiologist and biostatistician who are not involved in the trial and have no conflict of interest pertaining to the study drugs.
Study endpoints
Primary endpoints include change in serum K+ at 2 and 4 h after the single dose of study medication, admission to hospital (yes/no) for the subgroup of participants enrolled from the ER, and length of ER or hospital stay. The timing of the follow up lab draws is directly communicated to the patient’s nurse. Secondary outcomes of interest include next morning serum K+ (for patients remaining in the hospital), need for dialysis within 8 h, change in blood Ca2+ and Mg2+, and side effects profile. Study endpoints are summarized in Table 4.
Table 4.
Primary and secondary endpoints
| Primary Endpoints | Secondary Endpoints |
|---|---|
|
▪ Change in serum potassium at 2 and 4 h ▪ Length of ER or hospital stay ▪ Admit to hospital (yes/no) for the subgroup of participants enrolled from the ER |
▪ Dialysis yes/no within 8 h ▪ Change in Ca2+ and Mg2+ ▪ Tolerability (gastrointestinal side effects) ▪ New lower extremity edema ▪ Palatability (patient subjective rating) ▪ Next morning serum potassium |
Sample size calculations
Power analysis was done using conservative estimates from patiromer data (Table 2) [19]. Aiming for 80% power to detect a difference in blood K+ lowering of 0.6 mEq/L between the nonspecific laxative and oral K+ binder, within-group standard deviation 0.5 mEq/L, with alpha error 0.01 (to adjust for multiple comparisons due to multiple randomization groups), at least 18 subjects per group are needed to detect a significant difference between the groups. We are aiming for 20 patients per group for final analysis to ensure adequate numbers for comparison and account for potential missing data (i.e., participants who do not complete the 2 timed lab draws). Our study is not intended to identify superiority of one oral K+ binder vs other binders.
Statistical analysis
Baseline characteristics between the four groups will be compared using Chi-square test for categorical variables or ANOVA with contrasts and Kruskal-Wallis test (non-parametric) for continuous variables. Variables of interest include acute kidney injury, CKD or chronic dialysis status; diabetes mellitus; congestive heart failure; RAAS inhibitor medication prescription; metabolic acidosis; and concurrent therapies for hyperkalemia (insulin/dextrose, diuretics, albuterol, bicarbonate).
Linear mixed effects models will be used to compare mean change in blood K+ from baseline between the four groups at 2 and 4 h, and next morning K+ (P set at 0.05). Multivariate adjustment will be done for kidney function, diabetes mellitus, congestive heart failure, metabolic acidosis, RAAS inhibitor prescription, and treatment with temporizing agents (insulin, albuterol, loop diuretics). For next morning K+ values, multivariate adjustment will include any hyperkalemia treatments that were done after the 4-h study protocol (including dialysis). A Cox proportional hazards model will be used to analyze the association between type of oral potassium binder and length of ER or hospital stay.
Safety and tolerability will be assessed using a Kruskal-Wallis test to globally test for a negative trend across all 4 study treatments (two-sided P < 0.05) and, if significant, further testing will be done using a two-sided Fisher Exact test compare each potassium binder vs. the nonspecific laxative control.
Discussion
To our knowledge, the KBindER trial is the first to evaluate the efficacy of SPS, patiromer and sodium zirconium cyclosilicate head-to-head in ER and hospitalized patients with acute hyperkalemia. The results will be highly relevant to patient outcomes and healthcare spending; patients who are admitted to the hospital with hyperkalemia spend on average 4 additional days in the hospital compared to matched, non-hyperkalemic inpatients and incur USD $15,606 higher in total health care costs over a one year period [4]. This study will be indirectly assessing health care costs by identifying if there is a difference in hospital length of stay amongst the K+ binders.
Based on available data (Table 1), we hypothesize that SZC will have the most rapid onset of action to decrease blood K+ via gastrointestinal excretion, if used as stand-alone therapy for acute hyperkalemia. However, it is important to test the oral potassium binders in the real-world setting where temporizing agents are commonly given to shift K+ intracellularly, to determine the degree of additional K+ lowering with the potassium binder. Further, we will evaluate whether patients treated with a potassium binder are more likely to maintain normokalemia on next-morning labs (for patients remaining in the hospital).
It is timely to re-evaluate the utility of SPS in the treatment of acute hyperkalemia. If SPS proves to have equivalent or lower efficacy in lowering K+ this may justify the higher cost of the newer oral binders (patiromer, SZC) to avoid the SPS-associated risk of colonic necrosis. Significant side effects such as hypercalcemia (patiromer) or edema (SZC) are not expected with a single dose of oral potassium binder. The oral K+ binders offer a favorable safety profile compared to some widely-used temporizing measures for hyperkalemia. For example, insulin can incur life-threatening hypoglycemia despite co-administration of glucose [11, 16], particularly in CKD patients who have reduced renal clearance of infused insulin and compromised gluconeogenic pathways [36]. In a retrospective analysis of late-stage CKD patients being treated with insulin (n = 221), 13% of patients developed hypoglycemia [36]. The use of i.v. bicarbonate in patients with metabolic acidosis is controversial due to the concern of displacing hydrogen ions from albumin, thus freeing up binding spots on albumin for Ca2+ and lowering ionized Ca2+. Further, i.v. bicarbonate can itself directly bind Ca2+ thus causing hypocalcemia [37] and incurring risk of myocardial destabilization.
Our study is not intended to identify superiority of one oral K+ binder vs other binders, and we acknowledge the limitation that differential efficacy of the oral K+ binders may be under-studied if the actual group difference is less than 0.6 mEq/L.
In summary, the KBindER trial is the first head-to-head evaluation of oral potassium binders in a real-world setting where temporizing therapies will be given per discretion of the medical team. The findings from our study will inform decision-making guidelines for the treatment of acute hyperkalemia.
Supplementary Information
Additional file 1. 4-hour symptom survey form.
Acknowledgements
Not applicable.
Disclosures
OD has received speaker fees for Bristol Myer Squibb and Pfizer. KKZ has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, American Society of Nephrology, Astra-Zeneca, AVEO Oncology, Chugai, DaVita, Fresenius, Genentech, Haymarket Media, Hofstra Medical School, International Federation of Kidney Foundations, International Society of Hemodialysis, International Society of Renal Nutrition & Metabolism, Japanese Society of Dialysis Therapy, Hospira, Kabi, Keryx, Novartis, National Institutes of Health, National Kidney Foundation, OPKO, Pfizer, Relypsa, Resverlogix, Sandoz, Sanofi, Shire, Vifor, UpToDate, and ZSPharma. WLL has received honoraria and/or support from Fresenius, Hub Therapeutics, Roche, Sanofi, and ZS Pharma.
The other authors declare that they have no conflict of interests.
Abbreviations
- CKD
Chronic kidney disease
- ER
Emergency Room
- ICU
Intensive care unit
- K+
Potassium
- Na+
Sodium
- RAAS
Renin-angiotensin-aldosterone system
- SPS
Sodium polystyrene sulfonate
- SZC
Sodium zirconium cyclosilicate
Authors’ contributions
AEC, HRT and WLL drafted the original manuscript. WLL is the lead investigator who designed the trial, and is responsible for sequential randomization of participants and oversight of data collection and analysis. WLL is responsible for reporting significant protocol modifications to the IRB and the ClinicalTrials.gov trial registry. HRT, ST and WLL drafted IRB documents. LJ is the lead biostatistician. AEC, HRT, LJ and WLL will have access to the final trial dataset. WLL will be responsible for dissemination of de-identified study results at professional meetings and through research publications. Eligible co-authors are individuals who participated in study design and/or participant enrollment. All authors contributed edits, reviewed and approved the final manuscript.
Authors’ information
Not applicable.
Funding
The trial is supported by University of California-Irvine Division of Nephrology faculty research funds. Funds are used to purchase study drugs, and cover Investigational Drugs Pharmacy personnel effort and stationery costs. The funding body has no role in the design of the study and collection, analysis, and interpretation of data nor in writing the manuscript.
Availability of data and materials
The de-identified dataset from the current study will be available as a supplementary file with the manuscript, and will be available immediately following publication. De-identified study findings will be reported on ClinicalTrials.gov.
Declarations
Ethics approval and consent to participate
All research procedures were approved by the University of California, Irvine Institutional Review Board (HS# 2020-5780). Informed written consent is required for participation in the clinical trial.
Consent for publication
Not applicable.
Competing interests
KKZ has received honoraria and/or support from Astra-Zeneca, Relypsa, and ZSPharma. WLL has received honoraria and/or support from ZS Pharma. The remaining authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004;43:155–161. doi: 10.1016/j.jacc.2003.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Mahoney BA, Smith WA, Lo DS, Tsoi K, Tonelli M, Clase CM. Emergency interventions for hyperkalaemia. Cochrane Database Syst Rev. 2005;2005(2):CD003235. [DOI] [PMC free article] [PubMed]
- 3.Luo J, Brunelli SM, Jensen DE, Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. CJASN. 2016;11:90–100. doi: 10.2215/CJN.01730215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betts KA, Woolley JM, Mu F, Xiang C, Tang W, Wu EQ. The cost of hyperkalemia in the United States. Kidney Int Rep. 2018;3:385–393. doi: 10.1016/j.ekir.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer BF. Managing hyperkalemia caused by inhibitors of the renin–angiotensin–aldosterone system. N Engl J Med. 2004;351:585–592. doi: 10.1056/NEJMra035279. [DOI] [PubMed] [Google Scholar]
- 6.Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovesdy CP, Matsushita K, Sang Y, Brunskill NJ, Carrero JJ, Chodick G, et al. Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta-analysis. Eur Heart J. 2018;39:1535–1542. doi: 10.1093/eurheartj/ehy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. CJASN. 2006;1:940–951. doi: 10.2215/CJN.00240106. [DOI] [PubMed] [Google Scholar]
- 9.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 10.Reardon LC, Macpherson DS. Hyperkalemia in outpatients using angiotensin-converting enzyme inhibitors: how much should we worry? Arch Intern Med. 1998;158:26–32. doi: 10.1001/archinte.158.1.26. [DOI] [PubMed] [Google Scholar]
- 11.Hundemer GL, Talarico R, Tangri N, Leon SJ, Bota SE, Rhodes E, et al. Ambulatory treatments for RAAS inhibitor-related hyperkalemia and the 1-year risk of recurrence. CJASN. 2021;16:365–373. doi: 10.2215/CJN.12990820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sica DA. Hyperkalemia risk in chronic kidney disease. Hypertension. 2009;53:749–750. doi: 10.1161/HYPERTENSIONAHA.108.128017. [DOI] [PubMed] [Google Scholar]
- 13.Bushinsky DA, Williams GH, Pitt B, Weir MR, Freeman MW, Garza D, et al. Patiromer induces rapid and sustained potassium lowering in patients with chronic kidney disease and hyperkalemia. Kidney Int. 2015;88:1427–1433. doi: 10.1038/ki.2015.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 15.Georgianos PI, Agarwal R. Revisiting RAAS blockade in CKD with newer potassium-binding drugs. Kidney Int. 2018;93:325–334. doi: 10.1016/j.kint.2017.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peacock WF, Rafique Z, Vishnevskiy K, Michelson E, Vishneva E, Zvereva T, et al. Emergency potassium normalization treatment including sodium zirconium cyclosilicate: a phase ii, randomized, double-blind, placebo-controlled study (ENERGIZE) Acad Emerg Med. 2020;27:475–486. doi: 10.1111/acem.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peacock WF, Rafique Z, Clark CL, Singer AJ, Turner S, Miller J, et al. Real world evidence for treatment of hyperkalemia in the emergency department (REVEAL–ED): a multicenter, prospective, observational study. J Emerg Med. 2018;55:741–750. doi: 10.1016/j.jemermed.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Lepage L, Dufour A-C, Doiron J, Handfield K, Desforges K, Bell R, et al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015;12:2136–2142. doi: 10.2215/CJN.03640415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rafique Z, Liu M, Staggers KA, Minard CG, Peacock WF. Patiromer for treatment of hyperkalemia in the emergency department: a pilot study. Acad Emerg Med. 2020;27:54–60. doi: 10.1111/acem.13868. [DOI] [PubMed] [Google Scholar]
- 20.Packham DK, Rasmussen HS, Lavin PT, El-Shahawy MA, Roger SD, Block G, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372:222–231. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- 21.Kosiborod M, Rasmussen HS, Lavin P, Qunibi WY, Spinowitz B, Packham D, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312:2223–2233. doi: 10.1001/jama.2014.15688. [DOI] [PubMed] [Google Scholar]
- 22.Ash SR, Singh B, Lavin PT, Stavros F, Rasmussen HS. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int. 2015;88:404–411. doi: 10.1038/ki.2014.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126:264.e9–264.e24. doi: 10.1016/j.amjmed.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Kayexalate (sodium polystyrene sulfonate) powder. 2009. http://web.archive.org/web/20091104011812/http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm186845.htm. Accessed 18 Aug 2021.
- 25.Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21:733. doi: 10.1681/ASN.2010010079. [DOI] [PubMed] [Google Scholar]
- 26.Ali W, Bakris G. Evolution of patiromer use: a review. Curr Cardiol Rep. 2020;22:94. doi: 10.1007/s11886-020-01342-w. [DOI] [PubMed] [Google Scholar]
- 27.Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, et al. Effect of Patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314:151–161. doi: 10.1001/jama.2015.7446. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Harrison SD, Cope MJ, Park C, Lee L, Salaymeh F, et al. Mechanism of action and pharmacology of patiromer, a nonabsorbed cross-linked polymer that lowers serum potassium concentration in patients with hyperkalemia. J Cardiovasc Pharmacol Ther. 2016;21:456–465. doi: 10.1177/1074248416629549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haller H, Bianchi S, McCafferty K, Arthur S, Quinn CM, Budden J, et al. Safety and efficacy of patiromer in hyperkalemic patients with CKD: a pooled analysis of three randomized trials. Kidney360. 2022;3:2019–26. doi: 10.34067/KID.0001562022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang I-Z, et al. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32:820–828. doi: 10.1093/eurheartj/ehq502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stavros F, Yang A, Leon A, Nuttall M, Rasmussen HS. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS One. 2014;9:e114686. doi: 10.1371/journal.pone.0114686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amin AN, Menoyo J, Singh B, Kim CS. Efficacy and safety of sodium zirconium cyclosilicate in patients with baseline serum potassium level ≥ 5.5 mmol/L: pooled analysis from two phase 3 trials. BMC Nephrol. 2019;20:440. doi: 10.1186/s12882-019-1611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anker SD, Kosiborod M, Zannad F, Piña IL, McCullough PA, Filippatos G, et al. Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur J Heart Fail. 2015;17:1050–1056. doi: 10.1002/ejhf.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sumida K, Yamagata K, Kovesdy CP. Constipation in CKD. Kidney Int Rep. 2020;5:121–134. doi: 10.1016/j.ekir.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranjitkar P, Greene DN, Baird GS, Hoofnagle AN, Mathias PC. Establishing evidence-based thresholds and laboratory practices to reduce inappropriate treatment of pseudohyperkalemia. Clin Biochem. 2017;50:663–669. doi: 10.1016/j.clinbiochem.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Apel J, Reutrakul S, Baldwin D. Hypoglycemia in the treatment of hyperkalemia with insulin in patients with end-stage renal disease. Clin Kidney J. 2014;7:248–250. doi: 10.1093/ckj/sfu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dépret F, Peacock WF, Liu KD, Rafique Z, Rossignol P, Legrand M. Management of hyperkalemia in the acutely ill patient. Ann Intensive Care. 2019;9:32. doi: 10.1186/s13613-019-0509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. 4-hour symptom survey form.
Data Availability Statement
The de-identified dataset from the current study will be available as a supplementary file with the manuscript, and will be available immediately following publication. De-identified study findings will be reported on ClinicalTrials.gov.