Abstract
One goal of origins of life research is to understand how primitive informational and catalytic biopolymers emerged and evolved. Recently, a number of sequencing techniques have been applied to analysis of replicating and evolving primitive biopolymer systems, providing a sequence-specific and high-resolution view of primitive chemical processes. Here, we review application of sequencing techniques to analysis of synthetic and primitive nucleic acids and polypeptides. This includes next-generation sequencing of primitive polymerization and evolution processes, followed by discussion of other novel biochemical techniques that could contribute to sequence analysis of primitive biopolymer driven chemical systems. Further application of sequencing to origins of life research, perhaps as a life detection technology, could provide insight into the origin and evolution of informational and catalytic biopolymers on early Earth or elsewhere.
Keywords: Origin of Life, Chemical Evolution, Astrobiology, Biopolymers, Next-Generation Sequencing, Display methods
Introduction
Understanding how life emerged on Earth requires a holistic understanding of the entire origin of life process, from the formation of Earth, to its early atmospheric composition and geology, to how this geology and atmospheric composition produced primitive chemicals, to how primitive chemicals reacted and self-assembled into biomolecules (or non-biomolecules [1]) and structures that evolved into membrane-enclosed cells [2], [3], [4], [5], [6], [7], [8]. Each step of this process is currently undergoing interdisciplinary investigation. Yet, there is still more to understand before a clear conclusion can be agreed upon by researchers in the field (and sometimes, researchers will not agree). Recent advances in understanding of the origins of life (OoL) have often been achieved through application of new technologies. For example, radio telescopes [9,10] have allowed observation of planetary formation [11] and detection of chemicals present in the interstellar medium [12]. Highly sensitive mass spectrometers used to analyze geochemical isotope distributions revealed the early Earth's atmospheric composition [13,14]. Atomic force microscopy-infrared spectroscopy revealed organic residues derived from billion-year-old organism remains within mineral inclusions [15], and advances in synthetic biology allow construction of artificial cells that mimic protocell structure and function [16,17]. As such, OoL researchers must continue to apply novel techniques to push the boundaries of understanding.
One major analytical technique that has gone through a technical advancement renaissance in the last decades is nucleic acid sequencing [18,19] (Table 1 contains a brief summary of the sequencing platforms mentioned in this article; we note that some platforms are omitted as summarizing all relevant sequencing platforms is beyond the scope of this review). Initially developed in the 1970s as a primer extension strategy, DNA Sanger Sequencing became one of the most used techniques in biology-related laboratories around the world [20], leading to a Nobel prize in 1980 and contributing to sequencing the human genome in 2001 [21]. However, the Human Genome Project took more than 10 years, partially limited by Sanger Sequencing's low throughput. In attempts to increase throughput, researchers began developing “next-generation” sequencing (NGS) methods in the 1990s and early 2000s, including nucleic acid sequencing by synthesis, ligation, and pyrosequencing [22,23]. These new NGS platforms, such as 454 (Roche), Genome Analyzer (Illumina), and SOLiD (Life Technologies), significantly increased DNA sequencing throughput (and allowed RNA sequencing), sometimes analyzing millions of sequence fragments per run, such that more whole-genome-based studies became possible [23].
Table 1.
Summary of sequencing platforms introduced in this mini-review, sorted by year of development.
| Technique | Initial Year Released | Run-time | Maximum Read-length (bp) | Maximum reads/run | Approx. Error Rate | Cost per bp (JPY) |
|---|---|---|---|---|---|---|
| Sanger Sequencing | 1977 | ∼5 h | 1000 | 1 | 0.0001 | 0.06 |
| Roche 454 | 2005 | 10–48 h | 700 | 1 million | 0.01 | 0.007 |
| Illumina Genome Analyzer | 2006 | 2–10 days | 75 (paired) | 168 million | 0.01 | 0.0002 |
| Life Technologies SOLiD | 2006 | 1–2 weeks | 85 | 1 billion | 0.0001 | 0.00001 |
| Illumina HiSeq | 2009 | 1–10 days | 150 (paired) | 5 billion | 0.008 | 0.00001 |
| Illumina MiSeq | 2011 | 27 h | 300 (paired) | 300 million | 0.008 | 0.00001 |
| Oxford Nanopore Technologies MinION | 2014 | 2–3 days | 5000 | 60,000 | 0.34 | 0.0001 |
While some of these platforms, which all require DNA/RNA amplification [22], underwent further development (for example, the Illumina HiSeq system can sequence hundreds of billions of bases per run, taking just a few days, while the Illumina MiSeq system provides faster readout for lower-capacity applications [24]), direct nucleic acid sequencing methods have also been developed. For example, Nanopore Sequencing (Oxford Nanopore Technologies) feeds nucleic acids through a nanopore protein embedded in an artificial membrane [25,26]. A sensor continuously measures the electrical current across this nanopore, and passage of single-stranded nucleic acids through the nanopore, which is modulated by a motor protein (the motor protein also unwinds duplexed nucleic acids into single-strands), results in changes in this current. Each base of the nucleic acid that passes through the nanopore will change the detected current in a specific way in real-time (unique to each base), resulting in the ability to detect which bases are passing through the nanopore in a specific order and, in turn, the identity and the order of the bases in the original nucleic acid. This system may provide the advantage of allowing sequencing of single DNA/RNA molecules while bypassing nucleic acid amplification, which may introduce sequencing error and bias [27,28]. To this day, sequencing technology is being optimized for application to a wide range of fields spanning from transcriptomics [29] to developmental biology [30] to OoL.
As the availability of sequencing techniques has increased over time (both in cost and accessibility), it behooves OoL researchers to utilize such a novel and accessible technology, and in particular to analyze biopolymer structure, function, and evolution. Modern life is composed of biopolymers which confer specific functions required for a living biological system (such as storing genetic information or catalyzing biochemical reactions). Similar polymers on early Earth may have been required for a primitive chemical system to evolve into primitive life [31]. For example, RNA can both store genetic information [32] and catalyze chemical reactions [33], and as such, RNA is believed to have been an essential biopolymer on primitive Earth (i.e., the RNA world theory) [34]. Of course, this theory neither guarantees that RNA was the first biopolymer on Earth (the existence of other nucleic acids such as proto-RNA [35] or XNA [36] or the co-existence of DNA, peptides, and/or RNA [37] on early Earth is possible) nor that biopolymers themselves were even necessarily required for life to have started [1,38,39]. Thus, at some point, functional nucleic acids likely existed on early Earth and sequencing techniques can be used to further increase understanding of primitive nucleic acid structure and function, in particular replication and evolution, which is important to understand the origin of life. For example, nanopore sequencing (one of the inventors of nanopore sequencing being Prof. David Deamer [40], [41], [42], a researcher in OoL) was applied to study geochemically driven nucleic acid polymerization [43], [44], [45]. In this mini-review, we highlight additional important recent studies on primitive nucleic acid replication (via template-directed nonenzymatic primer extension of RNA) and evolution that utilize sequencing techniques as a way to infuse a new viewpoint into OoL research, followed by a discussion on novel sequencing or sequencing-related technologies that could push our understanding even further.
Primitive replication via template-directed nonenzymatic primer extension of RNA
In order to evolve and produce more functional structures, a primitive biopolymer system would have been required to replicate its information with high efficiency and efficacy. In modern biology, DNA replication is facilitated by a suite of enzymes including a DNA polymerase [46]; RNA polymerase enzymes, which replicate RNA (i.e., an RNA-dependent RNA polymerase, or an RdRp), also exist in certain organisms, such as viruses [47]. While a primitive version of an RdRp may have existed at some point in life's history, enzymes likely did not exist early on at the origin of life and RdRp ribozymes (which also would have been quite complex in structure) were also fairly unlikely at the same time. Therefore, how did primitive nucleic acid-based biopolymer systems replicate before replicase or polymerase enzymes or ribozymes?
Recent research has focused on studying mechanisms by which primitive nucleic acids can polymerize via nonenzymatic, or chemical, methods. One plausible mechanism utilizes functionalization of nucleotide monophosphates with imidazole-groups on the phosphate group results in activated monomers, which can participate in template-directed polymerization of RNA strands through base addition to an elongating template-bound RNA primer [48]. However, these activated phosphorimidazolide were not very efficient at polymerization, and further optimization of this system led to activation with not only imidazole, but imidazole derivatives as well, including methylimidazoles [49,50] and 2-aminoimidazole [51], which both result in more efficient polymerization. While some have argued that such a replicating RNA system would not have been plausible on early Earth [52], [53], [54], research has shown the ability for such activated monomers to be synthesized under prebiotically plausible conditions [55,56], while the nonenzymatic polymerization reactions can also proceed (at variable yields) under a variety of potential early Earth environmental conditions [57,58]. Even though template-directed nonenzymatic RNA oligomerization via activated nucleotides is but a single possible mechanism of primitive oligomerization (other plausible mechanisms include, but are not limited to, dehydration synthesis of cyclical monomers [59,60], clay mineral catalysis [61], and ligation in thermophoretic rock pores [62]), such a nonenzymatically polymerizing RNA is one of the most well-studied model systems used in laboratory studies of primitive polymerization. However, there are still a number of remaining unanswered questions related to the efficiency of template-directed nonenzymatic RNA oligomerization which necessitate further exploration [63,64]; sequencing studies in particular have been applied to such detailed studies to increase understanding of the mechanism and plausibility of template-directed nonenzymatic RNA oligomerization at the origin of life.
One of the main issues in early template-directed nonenzymatic RNA oligomerization studies was the fact that certain activated monomers (i.e., C and G) were incorporated at a much faster rate than others (i.e., A, U) onto the elongating RNA strand [65,66]. If this process had occurred in nature at the OoL, then RNA on early Earth would have heavily favored compositions of high C and G rather than A and U, if polymerization rate was the only factor governing such a process (although we know that modern nucleic acids, if one believes that they originated from ancient RNA or RNA-like polymers, do in fact contain A and U). Thus, one of the earliest reports of applying NGS to template-directed nonenzymatic RNA oligomerization took place in 2015, where Heuberger and co-workers characterized an RNA primer-template system consisting of 2-thiouridine rather than uridine in order to observe whether a modified activated U monomer could be incorporated into nonenzymatically extending RNA primer strands at a faster rate than a modified canonical U monomer [67]. 2-thiouridine monomers confer greater stabilization to a base-pair with A than uridine, leading to greater thermodynamic stability between 2-thiouridine-containing RNA duplexes compared to uridine-containing RNA duplexes [68]. This increase in base-pairing stabilization and decrease in mismatch base-pairs was postulated to potentially increase the efficiency of the nonenzymatic RNA polymerization reaction, a process that is governed in part by the strength of the monomer-template base-pair binding strength [69] and inhibited by the presence of mismatched base-additions [70]. Indeed, activated 2-thiouridine monomers were incorporated into extending nonenzymatic RNA primer-template systems at a significantly enhanced rate than activated uridine monomers. NGS studies revealed that utilizing activated 2-thiouridine monomers in fact may have contributed to this increase in polymerization rate due to an increase in polymerization fidelity as compared to when using activated uridine monomers [67].
Since these studies, other pioneering studies have revealed even more aspects of template-directed nonenzymatic RNA oligomerization. These include discovery of a more efficient polymerizing activated monomer (2-aminoimidazole-activated monomers) [51] and the importance of using activated helper oligonucleotides to drive the polymerization reaction of all bases [71] due to the formation of a high-fidelity binding pocket for the adding activated monomer [72], modification of the RNA duplex into a structural form that is more amenable to polymerization [72], and promotion of the formation of an imidazolium-bridged dinucleotide intermediate which drives base addition [73] (among other studies). Most of these studies relied heavily on gel electrophoresis assays to analyze the polymerization process. While such assays are generally reliable, they suffer in throughput and the inability to readily detect mismatched bases. As a mechanism to increase the throughput of template-directed nonenzymatic RNA oligomerization analysis, a recent study incorporated information from all previous studies with NGS, and culminated in development of an NGS-based platform to evaluate template-directed nonenzymatic RNA oligomerization titled NERPE-Seq (Non-Enzymatic RNA Primer Extension-Seq) [74]. NERPE-Seq was designed to observe only dynamics resulting from the polymerization reaction, rather than various biases (such as enzymatic library preparation or variations in RNA secondary structure), and the library preparation strategy consists of adapter ligation of the RNA sample, followed by reverse transcription (RT) and barcoding polymerase chain reaction (PCR) (Fig. 1). NERPE-Seq was shown to be able to generate reliable and reproducible data across a variety of conditions on par with traditional gel electrophoresis strategies, with added mismatch detection ability and higher throughput, and was then applied to study why base mismatches occur in nonenzymatic RNA polymerization reactions [75]. Duzdevich and co-workers discovered that when a imidazolium-bridged dinucleotide intermediate was the predominant mechanism of polymerization [73], generally correctly-paired base additions resulted. However, in the absence of such an intermediate, direct reaction with an activated monomer nucleotide resulted in significantly more mismatched base additions. The presence of a mismatched terminal base also increased the probability that the next base addition was also a mismatch. These studies suggest that a different strategy for monomer activation which results in greater yields of the imidazolium-bridged dinucleotide intermediate, namely methyl isocyanide-mediated activation, could be incorporated into future template-directed nonenzymatic RNA oligomerization studies to potentially improve the fidelity of polymerization.
Fig. 1.
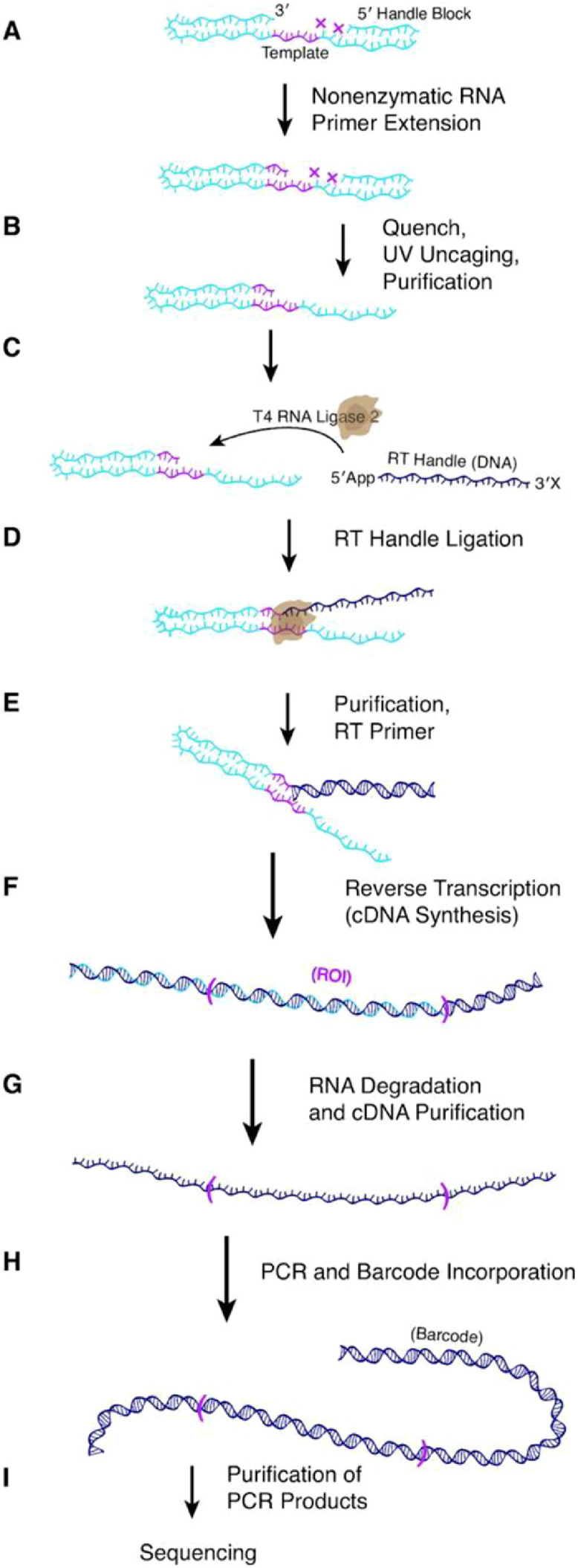
Library preparation strategy of NERPE-Seq. Figure reproduced with permission from D. Duzdevich, C.E. Carr, J.W. Szostak, Deep sequencing of non-enzymatic RNA primer extension, Nucleic Acids Res., 48 (2020) e70 [74] under a Creative Commons License.
While NERPE-Seq was designed specifically for analyzing activated nucleotide monomer-based template-directed nonenzymatic RNA oligomerization reactions, other nonenzymatic polymerization or ligation processes, such as those observed in nucleic acid systems assembled into supramolecular liquid crystal constructs [76,77] (liquid crystals themselves have been shown to co-assemble with potential primitive membraneless compartments [78], [79], [80]), could also benefit from NERPE-Seq analysis. Similarly, novel NGS-based sequencing techniques should be developed and applied to study other primitive nonenzymatic biopolymeric processes potentially relevant to OoL, such as ribosome-less translation processes [81].
In vitro selection and evolution
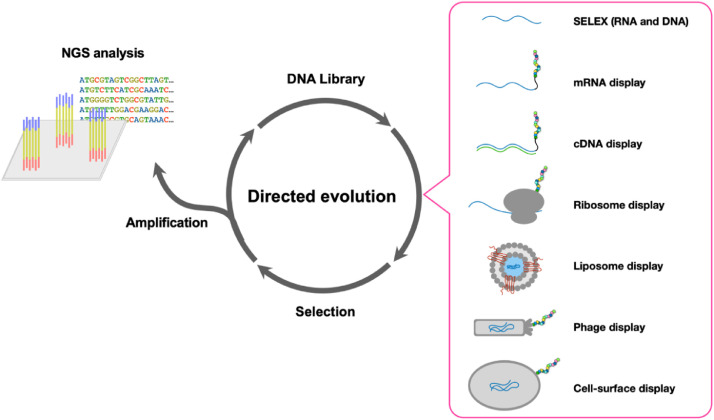
One of the key features of a primitive chemical system that allows it to eventually develop “life-like” properties is the ability to evolve. Assuming that such a primitive system consisted of genetic polymers, such as nucleic acids, studying how such polymers evolved into sequences with functional structures is necessary to understand the origins of life. One such method to observe direct sequence evolution is through in vivo and in vitro high-throughput laboratory simulations of biopolymer selection and evolution (Fig. 2). In vitro selection of nucleotide polymers was initially developed in 1990 by three different research groups, who each synthesized a large pool of RNA libraries to select for diverse classes of functional RNA, in this case aptamers and ribozymes [82], [83], [84]. Assuming that functional structures arose from a pool of random primitive biopolymers [85], this method, also known as SELEX (Systematic Evolution of Ligands by EXponential enrichment), enables multiple rounds of selection and amplification to obtain functional nucleic acid molecules. Since then, various high-affinity RNA ligands that bind to proteins and low molecular weight compounds, as well as ribozymes with specific activities (cleavage [86], ligation [87], addition and removal of phosphates (i.e., polynucleotide kinases) [88], etc.), have been evolved in vitro and subsequently identified through sequencing and experimental validation of selected/evolved sequences [89,90]. A similar approach has also been applied towards selecting double- and single-stranded DNA aptamers and DNAzymes [91,92].
Fig. 2.
Various directed evolution strategies for DNA, RNA, and peptides/proteins, each starting from a DNA library and involving selection, amplification, and eventually NGS analysis. The various strategies can be applied to DNA/RNA (SELEX, i.e., direct nucleic acid selection) or peptides/proteins (display coupling nucleotide information to peptide information) and encompass both in vitro techniques (mRNA, cDNA, and liposome display and SELEX) as well as in vivo techniques (phage and cell-surface display).
Together, the evolved functional nucleic acids derived from these studies (whether RNA or DNA) demonstrate the diverse function and the potential ancient role of nucleotide polymers during the origin and evolution of life. Previously, Sanger Sequencing was applied to identify the limited number of cloned isolates after selection; however, Sanger Sequencing contained a number of limitations in the context of understanding the distribution and overall population of functional polymers within the sequence space of the generated DNA and/or RNA library. Hence, the emergence of NGS provided a breakthrough in this field by clearly mapping the fitness landscape and small evolutionary network of in vitro-selected RNA sequences including GTP aptamers [93], by studying the effect of mineral surfaces on ribozyme evolution [94], and, more recently, by analyzing a complex reaction network catalyzed by a recombinase ribozyme [95]. In fact, further development of novel NGS techniques resulted in a method where catalytic activity could be coupled to sequence information (i.e., SCAPE: Sequencing to measure Catalytic Activity Paired with in vitro Evolution), which revealed the complex distribution of functional sequences of a 21-nucleotide region of self-aminoacylating RNA [96]. This study showed that evolution of the short self-aminoacylating RNA resulted in isolated fitness landscape peaks of high activity disconnected by vast valleys of low activity, suggesting that different “families” of evolved sequences may approach the same catalytic problem (in this case, self-aminoacylation) in different ways (i.e., mechanisms of action); however, none of the evolved sequences were the global optimum sequence for accomplishing the catalytic activity [96]. Further development of NGS-based methods of in vitro nucleic acid evolution analysis could lead to further understanding of evolution dynamics related to primitive genetic systems, such as understanding of host-parasite interactions and evolution gleaned by applying NGS analysis to compartmentalized RNA replicator pairs [97,98].
While primitive genetic systems likely relied on nucleic acid or nucleic acid-like polymers for information transfer, modern life primarily uses protein enzymes for catalytic activity, and as such, early life necessarily must have eventually evolved the ability to code for functional proteins; understanding the evolutionary dynamics of proteins would also reveal critical information about the development and evolution of early life. As proteins are subject to the central dogma, a protein's primary sequence of amino acids would be determined by the genetic (DNA) and transcriptomic (RNA) code that directed the organism to produce the protein, leading to traceability between the protein and the preceding nucleic acids. In this way, protein evolution must have necessarily been accompanied by the matching nucleic acid sequence changes, and both in vivo and in vitro laboratory techniques to select for functional proteins coupled with advances in NGS have resulted in various selection methods for functional protein sequences, known as display methods, being developed by directly coupling the genotype (nucleic acid) to protein phenotype [99,100]. Among these methods, mRNA (messenger RNA) and cDNA (DNA complementary to an RNA sequence) display are capable of screening the largest number of protein variants (∼ 1013) and have advantages towards molecular design and engineering given their in vitro natures [101]. mRNA and cDNA display have also been shown to have high compatibility with reconstituted cell-free protein synthesis and downstream NGS data analysis [102]. With regards to OoL and evolution of functional proteins, previously, random de novo protein sequence space was explored using mRNA display, which led to identification of four classes of novel ATP-binding proteins [103] and RNA ligase [103,104]. Furthermore, rationally designed protein libraries with a limited set of amino acids have been used to understand the function and foldability of primordial proteins [105], [106], [107], [108]. These early proteins may have relied on less (or different) amino acids compared to those used in modern biology [109,110]. Here, NGS has also been used to assure sequence quality of such constructed DNA libraries encoding proteins consisting of specific amino acid sets with precisely controlled composition [111,112]. Currently, several computational pipelines such as FASTAptamer [113] and EasyDIVER [114] exist to process NGS data for in vitro evolution studies of nucleotide polymers and peptides.
While the previous paragraph focused on the in vitro selection and evolution of a single polymer system (nucleic acid or polypeptide/protein), several research groups are experimentally testing the hypothesis that primitive polypeptides could have played a key role in the RNA world by studying combinations of such biopolymer systems. For example, in vitro selection of an RNA polymerase ribozyme (RPR) in the presence of basic peptides enabled the RPR to function at low magnesium ion concentration [115], which would have been relevant prebiotically as high magnesium concentrations may degrade certain primitive compartments such as fatty acid vesicles [116] as well as even RNA itself [117]. On the other hand, phage display was able to select hydrophobic-cationic peptide sequences that bind RPR and enhance its activity at high magnesium and ionic strength conditions [118]. Finally, with regards to the recent challenge to study and observe ribosome evolution in the laboratory, a library of 23S rRNA (ribosomal RNA) variants were screened by monitoring the translation activity of an in vitro reconstituted ribosome through ribosome display, resulting in finding multiple positive epistatic interactions on the ribosome [119]. Scanning of a functional L11 ribosomal protein variant library composed of just ten prebiotically plausible amino acids was then achieved using mRNA display, revealing a unique cation mediated RNA-protein interaction [120].
Although we have provided a few examples of recent experimental studies of co-existing and co-evolving biopolymer systems, such studies are in their infancy to support the molecular cooperation scenario for the OoL [121,122]. Further development of NGS tools, including the recent attempts on the direct protein readout and computational methods to allow more detailed and high-throughput analysis of polymer co-evolution, will lead to further understanding how primitive polymer systems eventually led to extant biology.
Sequencing or sequencing-related techniques relevant to OoL research
As presented here, novel sequencing technologies have contributed greatly to furthering our understanding of the origin and evolution of life, particularly related to primitive biopolymer structure and function. Nevertheless, sequencing and sequencing-related techniques are also “evolving” each day, and novel technologies/techniques in all facets of biosciences are being developed regularly; it behooves OoL researchers to attempt to incorporate such new techniques into further OoL studies. For example, one recently developed “up-and-coming” sequencing technology is the single-molecule protein sequencing system developed by Quantum-Si, Inc. (Connecticut, USA) [123], which utilizes a group of dye-labeled ClpS proteins (ATP-dependent Clp protease adaptor) to recognize different N-terminal amino acids of a single surface-immobilized peptide molecule. While not all 20 canonical amino acids are currently fully detectable, further development of this new technology could enable researchers to process and analyze the sequences of high complexity polypeptide samples that contain both canonical and non-canonical amino acids, including those derived from chemical evolution type experiments.
We have also briefly mentioned the potential importance of a modified nucleobase (2-thiouridine) to primitive replicating nucleic acids [67]. However, this is not the only non-canonical or modified nucleobase or nucleotide that has been investigated in OoL studies. Alternative nucleic acid backbone chemistries of xeno-nucleic acids (XNAs), such as replacing the ribose sugar with other sugars (like TNA [124]), have been investigated as potential proto-RNA nucleic acids, while researchers have also incorporated up to four additional non-canonical nucleobases into functional nucleic acids (such as AEGIS [125] or Hachimoji nucleic acids [126]) to show the potential for primitive nucleic acids to have contained more than four or five total potential nucleobases (i.e., A, G, C, T, and U); natural additional “Z” nucleotide (2-aminoadenine) has also been found in DNA in certain viruses [127], [128], [129]. Advances in sequencing techniques have thus allowed sequencing and analysis of nucleic acids which include such non-canonical bases [130,131]. Further advances in mass spectrometry-based “mass ladder” sequencing [132], [133], [134], [135] and direct single molecule nanopore sequencing [136], [137], [138] have the potential to be used for analysis of primitive nucleic acids containing non-canonical bases. Specifically, due the fact that nanopore sequencing uses changes in current as an indicator for molecular identity, it has not only been used to analyze proteins and peptides [139,140], but also has the potential for detailed of XNAs and other types of nucleic acids that may have been prebiotically relevant. In fact, nanopore-based sequencers have recently been investigated in the context of technologies to detect extraterrestrial life [141,142], which may not necessarily use the same genetic system as life on Earth. Such sequencers have even been included in the planned analytical suite for the Enceladus Orbilander mission concept [143] for in situ sequencing of potential life on Enceladus, a moon of Saturn and an important target for astrobiology programs in the search for evidence of extraterrestrial life [144,145].
While new sequencing technologies have the ability to contribute to future OoL research, sequencing platforms are not the only techniques required for sequencing analyses, which may also require sample extraction, library preparation, and data analysis steps, to name a few. Recent developments in single-cell sequencing [146,147], sample barcoding techniques [148,149] (such as digital nucleic acid sequencing [27]), or a combination of both [150] have offered significant advances in sample extraction, library preparation, and data analysis. In particular, novel single-cell sequencing methods are of interest for analysis of functional and evolving protocells, which are primitive cells that can potentially encapsulate or transfer primitive genetic materials (like nucleic acids) [151], [152], [153], due to the similarity in structure between modern cells and protocells. These protocells (or other primitive compartments) were essential for primitive evolution and speciation [152,154], and could have been assembled from a number of different prebiotic chemical architectures, such from fatty acid/lipid bilayers [155], [156], [157] or from phase-separated droplets [158] (such as coacervates [159], [160], [161], aqueous two-phase systems [162], [163], [164], or polyester microdroplets [165,166]), both of which are present in modern cells (the latter in the form of membraneless organelles). In fact, single-exosome (which are simple extracellular vesicles) sequencing techniques have been proposed [167], while single-coacervate sequencing methods are being developed [168], suggesting their potential future application to single protocell sequencing.
It is still unclear whether nucleic acid-based or peptide-based life (or a combination of both) was what the first life on Earth was based upon, as a number of other OoL theories (such as composome-based [38,169], metabolism-based [170,171], or “non-biomolecule”-based [1] theories, among others) have been presented; nevertheless, primitive biopolymers necessarily became interwoven with life at some point in its evolution. Here, we have presented a number of demonstrations of how current sequencing methods have been applied to or could potentially be applied to analysis of such primitive nucleic acid and polypeptide biopolymers. We hope that these demonstrations show the enormous potential of sequencing in various aspects of OoL research, and encourage OoL researchers to utilize these (and other yet-to-be-developed) sequencing techniques in the future.
CRediT authorship contribution statement
Tony Z. Jia: Writing – original draft, Writing – review & editing. Shota Nishikawa: Writing – original draft, Writing – review & editing. Kosuke Fujishima: Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
T.Z.J. is a co-inventor of a provisional patent filing related to MS-based direct RNA modification sequencing and is also a co-inventor of patents and patent applications related to single cell nucleic acid sequencing and detection (but T.Z.J. has not actively worked in the field of single cell sequencing since 2012).
Acknowledgements
S.N. is a student and K.F. and T.Z.J. are researchers at the Earth-Life Science Institute (ELSI) of Tokyo Institute of Technology, which is sponsored by a grant from the Japan Ministry of Education, Culture, Sports, Science and Technology (MEXT) as part of the World Premier International Research Center Initiative (WPI). K.F. is supported by the ELSI‐First Logic Astrobiology Donation Program. T.Z.J. is further supported by Japan Society for the Promotion of Science (JSPS) Grants-in-aid 18K14354 and 21K14746, the Tokyo Institute of Technology Yoshinori Ohsumi Fund for Fundamental Research, and by the Assistant Staffing Program by the Gender Equality Section, Diversity Promotion Office, Tokyo Institute of Technology.
References
- 1.Chandru K., Mamajanov I., Cleaves H.J., II, Jia T.Z. Polyesters as a model system for building primitive biologies from non-biological prebiotic chemistry. Life. 2020;10:6. doi: 10.3390/life10010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazcano A., Miller S.L. The origin and early evolution of life: prebiotic chemistry, the pre-RNA world, and time. Cell. 1996;85:793–798. doi: 10.1016/s0092-8674(00)81263-5. [DOI] [PubMed] [Google Scholar]
- 3.Sasselov D.D., Grotzinger J.P., Sutherland J.D. The origin of life as a planetary phenomenon. Sci. Adv. 2020;6:eaax3419. doi: 10.1126/sciadv.aax3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakashima S., Kebukawa Y., Kitadai N., Igisu M., Matsuoka N. Geochemistry and the origin of life: from extraterrestrial processes, chemical evolution on earth, fossilized life's records, to natures of the extant life. Life. 2018;8:39. doi: 10.3390/life8040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scharf C., Virgo N., Cleaves H.J., II, Aono M., Aubert-Kato N., Aydinoglu A., Barahona A., Barge L.M., Benner S.A., Biehl M., Brasser R., Butch C.J., Chandru K., Cronin L., Danielache S., Fischer J., Hernlund J., Hut P., Ikegami T., Kimura J., et al. A strategy for origins of life research. Astrobiology. 2015;15:1031–1042. doi: 10.1089/ast.2015.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleaves H.J., II Prebiotic chemistry: what we know, what we don't. Evol. Educ. Outreach. 2012;5:342–360. [Google Scholar]
- 7.Kitadai N., Maruyama S. Origins of building blocks of life: A review. Geosci. Front. 2018;9:1117–1153. [Google Scholar]
- 8.Sarkar S., Das S., Dagar S., Joshi M.P., Mungi C.V., Sawant A.A., Patki G.M., Rajamani S. Prebiological membranes and their role in the emergence of early cellular life. J. Membr. Biol. 2020;253:589–608. doi: 10.1007/s00232-020-00155-w. [DOI] [PubMed] [Google Scholar]
- 9.García Yus J.F., Dent B., Brisbin D., Chang C.-S., Gómez L., Nakos T. Observatory Operations: Strategies, Processes, and Systems VIII. International Society for Optics and Photonics; 2020. Towards the processing, review, and delivery of 80% of the ALMA data by the Joint ALMA Observatory (JAO) [Google Scholar]
- 10.Prestage R.M. In: Ground-Based and Airborne Telescopes. Stepp L.M., editor. SPIE; 2006. The Green Bank telescope. [Google Scholar]
- 11.Teague R., Bae J., Bergin E.A. Meridional flows in the disk around a young star. Nature. 2019;574:378–381. doi: 10.1038/s41586-019-1642-0. [DOI] [PubMed] [Google Scholar]
- 12.McGuire B.A., Loomis R.A., Burkhardt A.M., Lee K.L.K., Shingledecker C.N., Charnley S.B., Cooke I.R., Cordiner M.A., Herbst E., Kalenskii S., Siebert M.A., Willis E.R., Xue C., Remijan A.J., McCarthy M.C. Detection of two interstellar polycyclic aromatic hydrocarbons via spectral matched filtering. Science. 2021;371:1265–1269. doi: 10.1126/science.abb7535. [DOI] [PubMed] [Google Scholar]
- 13.Hattori S., Kamezaki K., Yoshida N. Constraining the atmospheric OCS budget from sulfur isotopes. Proc. Natl. Acad. Sci. USA. 2020;117:20447–20452. doi: 10.1073/pnas.2007260117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasting J.F., Howard M.T. Atmospheric composition and climate on the early Earth. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361:1733–1741. doi: 10.1098/rstb.2006.1902. discussion 1741–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassenkam T., Andersson M.P., Dalby K.N., Mackenzie D.M.A., Rosing M.T. Elements of Eoarchean life trapped in mineral inclusions. Nature. 2017;548:78–81. doi: 10.1038/nature23261. [DOI] [PubMed] [Google Scholar]
- 16.Buddingh’ B.C., van Hest J.C.M. Artificial cells: synthetic compartments with life-like functionality and adaptivity. Acc. Chem. Res. 2017;50:769–777. doi: 10.1021/acs.accounts.6b00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martín N.Y., Valer L., Mansy S.S. Toward long-lasting artificial cells that better mimic natural living cells. Emerg. Top. Life Sci. 2019;3:597–607. doi: 10.1042/ETLS20190026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shendure J., Balasubramanian S., Church G.M., Gilbert W., Rogers J., Schloss J.A., Waterston R.H. DNA sequencing at 40: past, present and future. Nature. 2017;550:345–353. doi: 10.1038/nature24286. [DOI] [PubMed] [Google Scholar]
- 19.Shendure J., Ji H. Next-generation DNA sequencing. Nat. Biotechnol. 2008;26:1135–1145. doi: 10.1038/nbt1486. [DOI] [PubMed] [Google Scholar]
- 20.Sanger F., Nicklen S., Coulson A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venter J.C., Adams M.D., Myers E.W., Li P.W., Mural R.J., Sutton G.G., Smith H.O., Yandell M., Evans C.A., Holt R.A., Gocayne J.D., Amanatides P., Ballew R.M., Huson D.H., Wortman J.R., Zhang Q., Kodira C.D., Zheng X.H., Chen L., Skupski M., et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 22.Heather J.M., Chain B. The sequence of sequencers: The history of sequencing DNA. Genomics. 2016;107:1–8. doi: 10.1016/j.ygeno.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L., Li Y., Li S., Hu N., He Y., Pong R., Lin D., Lu L., Law M. Comparison of next-generation sequencing systems. J. Biomed. Biotechnol. 2012;2012 doi: 10.1155/2012/251364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulski J.K. Next Generation Sequencing–Advances, Applications and Challenges. InTech; 2016. Next-generation sequencing — an overview of the history, tools, and “omic” applications. [Google Scholar]
- 25.Kono N., Arakawa K. Nanopore sequencing: review of potential applications in functional genomics. Dev. Growth Differ. 2019;61:316–326. doi: 10.1111/dgd.12608. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y., Zhao Y., Bollas A., Wang Y., Au K.F. Nanopore sequencing technology, bioinformatics and applications. Nat. Biotechnol. 2021;39:1348–1365. doi: 10.1038/s41587-021-01108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiroguchi K., Jia T.Z., Sims P.A., Xie X.S. Digital RNA sequencing minimizes sequence-dependent bias and amplification noise with optimized single-molecule barcodes. Proc. Natl. Acad. Sci. USA. 2012;109:1347–1352. doi: 10.1073/pnas.1118018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aird D., Ross M.G., Chen W.-S., Danielsson M., Fennell T., Russ C., Jaffe D.B., Nusbaum C., Gnirke A. Analyzing and minimizing PCR amplification bias in Illumina sequencing libraries. Genome Biol. 2011;12:R18. doi: 10.1186/gb-2011-12-2-r18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang B., Kumar V., Olson A., Ware D. Reviving the Transcriptome studies: an insight into the emergence of single-molecule transcriptome sequencing. Front. Genet. 2019;10:384. doi: 10.3389/fgene.2019.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffiths J.A., Scialdone A., Marioni J.C. Using single-cell genomics to understand developmental processes and cell fate decisions. Mol. Syst. Biol. 2018;14:e8046. doi: 10.15252/msb.20178046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koonin E.V. Why the central dogma: on the nature of the great biological exclusion principle. Biol. Direct. 2015;10:52. doi: 10.1186/s13062-015-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poltronieri P., Sun B., Mallardo M., Viruses RNA. RNA roles in pathogenesis, coreplication and viral load. Curr. Genomics. 2015;16:327–335. doi: 10.2174/1389202916666150707160613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanner N.K. Ribozymes: the characteristics and properties of catalytic RNAs. FEMS Microbiol. Rev. 1999;23:257–275. doi: 10.1111/j.1574-6976.1999.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert W. Origin of life: the RNA world. Nature. 1986;319:618. –618. [Google Scholar]
- 35.Hud N.V. Searching for lost nucleotides of the pre-RNA World with a self-refining model of early Earth. Nat. Commun. 2018;9:5171. doi: 10.1038/s41467-018-07389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murayama K., Okita H., Kuriki T., Asanuma H. Nonenzymatic polymerase-like template-directed synthesis of acyclic L-threoninol nucleic acid. Nat. Commun. 2021;12:804. doi: 10.1038/s41467-021-21128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saad N.Y. A ribonucleopeptide world at the origin of life. J. Syst. Evol. 2018;56:1–13. [Google Scholar]
- 38.Lancet D., Zidovetzki R., Markovitch O. Systems protobiology: origin of life in lipid catalytic networks. J. R. Soc. Interface. 2018;15 doi: 10.1098/rsif.2018.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandru K., Jia T.Z., Mamajanov I., Bapat N., Cleaves H.J., II Prebiotic oligomerization and self-assembly of structurally diverse xenobiological monomers. Sci. Rep. 2020;10:17560. doi: 10.1038/s41598-020-74223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasianowicz J.J., Brandin E., Branton D., Deamer D.W. Characterization of individual polynucleotide molecules using a membrane channel. Proc. Natl. Acad. Sci. USA. 1996;93:13770–13773. doi: 10.1073/pnas.93.24.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deamer D., Akeson M., Branton D. Three decades of nanopore sequencing. Nat. Biotechnol. 2016;34:518–524. doi: 10.1038/nbt.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akeson M., Branton D., Kasianowicz J.J., Brandin E., Deamer D.W. Microsecond time-scale discrimination among polycytidylic acid, polyadenylic acid, and polyuridylic acid as homopolymers or as segments within single RNA molecules. Biophys. J. 1999;77:3227–3233. doi: 10.1016/S0006-3495(99)77153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva L.Da, Maurel M.-C., Deamer D. Salt-promoted synthesis of RNA-like molecules in simulated hydrothermal conditions. J. Mol. Evol. 2015;80:86–97. doi: 10.1007/s00239-014-9661-9. [DOI] [PubMed] [Google Scholar]
- 44.DeGuzman V., Vercoutere W., Shenasa H., Deamer D. Generation of oligonucleotides under hydrothermal conditions by non-enzymatic polymerization. J. Mol. Evol. 2014;78:251–262. doi: 10.1007/s00239-014-9623-2. [DOI] [PubMed] [Google Scholar]
- 45.Rajamani S., Vlassov A., Benner S., Coombs A., Olasagasti F., Deamer D. Lipid-assisted synthesis of RNA-like polymers from mononucleotides. Orig. Life Evol. Biosph. 2008;38:57–74. doi: 10.1007/s11084-007-9113-2. [DOI] [PubMed] [Google Scholar]
- 46.Langston L.D., O'Donnell M. DNA replication: keep moving and don't mind the gap. Mol. Cell. 2006;23:155–160. doi: 10.1016/j.molcel.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 47.Machitani M., Yasukawa M., Nakashima J., Furuichi Y., Masutomi K. RNA-dependent RNA polymerase, RdRP, a promising therapeutic target for cancer and potentially COVID-19. Cancer Sci. 2020;111:3976–3984. doi: 10.1111/cas.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weimann B.J., Lohrmann R., Orgel L.E., Schneider-Bernloehr H., Sulston J.E. Template-directed synthesis with adenosine-5’-phosphorimidazolide. Science. 1968;161:387. doi: 10.1126/science.161.3839.387. [DOI] [PubMed] [Google Scholar]
- 49.Inoue T., Orgel L.E. Oligomerization of (guanosine 5′-phosphor)-2-methylimidazolide on poly(C) J. Mol. Biol. 1982;162:201–217. doi: 10.1016/0022-2836(82)90169-3. [DOI] [PubMed] [Google Scholar]
- 50.Sosson M., Pfeffer D., Richert C. Enzyme-free ligation of dimers and trimers to RNA primers. Nucleic Acids Res. 2019;47:3836–3845. doi: 10.1093/nar/gkz160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L., Prywes N., Tam C.P., O'Flaherty D.K., Lelyveld V.S., Izgu E.C., Pal A., Szostak J.W. Enhanced nonenzymatic RNA copying with 2-Aminoimidazole activated nucleotides. J. Am. Chem. Soc. 2017;139:1810–1813. doi: 10.1021/jacs.6b13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernhardt H.S. The RNA world hypothesis: the worst theory of the early evolution of life (except for all the others) Biol. Direct. 2012;7:23. doi: 10.1186/1745-6150-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wills P.R., Carter Jr C.W. Insuperable problems of the genetic code initially emerging in an RNA world. Biosystems. 2018;164:155–166. doi: 10.1016/j.biosystems.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Vay K., Mutschler H. The difficult case of an RNA-only origin of life. Emerg. Top Life Sci. 2019;3:469–475. doi: 10.1042/ETLS20190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oró J., Basile B., Cortes S., Shen C., Yamrom T. The prebiotic synthesis and catalytic role of imidazoles and other condensing agents. Orig. Life. 1984;14:237–242. doi: 10.1007/BF00933663. [DOI] [PubMed] [Google Scholar]
- 56.Fahrenbach A.C., Giurgiu C., Tam C.P., Li L., Hongo Y., Aono M., Szostak J.W. Common and potentially prebiotic origin for precursors of nucleotide synthesis and activation. J. Am. Chem. Soc. 2017;139:8780–8783. doi: 10.1021/jacs.7b01562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin L., Engelhart A.E., Zhang W., Adamala K., Szostak J.W. Catalysis of template-directed nonenzymatic RNA copying by Iron(II) J. Am. Chem. Soc. 2018;140:15016–15021. doi: 10.1021/jacs.8b09617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanavarioti A., Monnard P.A., Deamer D.W. Eutectic phases in ice facilitate nonenzymatic nucleic acid synthesis. Astrobiology. 2001;1:271–281. doi: 10.1089/15311070152757465. [DOI] [PubMed] [Google Scholar]
- 59.Dagar S., Sarkar S., Rajamani S. Geochemical influences on nonenzymatic oligomerization of prebiotically relevant cyclic nucleotides. RNA. 2020;26:756–769. doi: 10.1261/rna.074302.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wunnava S., Dirscherl C.F., Výravský J., Kovařík A., Matyášek R., Šponer J., Braun D., Šponer J.E. Acid-catalyzed RNA-oligomerization from 3’,5′-cGMP. Chemistry. 2021;27:17581–17585. doi: 10.1002/chem.202103672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferris J.P. Montmorillonite-catalysed formation of RNA oligomers: the possible role of catalysis in the origins of life. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361:1777–1786. doi: 10.1098/rstb.2006.1903. discussion 1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mast C.B., Schink S., Gerland U., Braun D. Escalation of polymerization in a thermal gradient. Proc. Natl. Acad. Sci. USA. 2013;110:8030–8035. doi: 10.1073/pnas.1303222110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szostak J.W. The eightfold path to non-enzymatic RNA replication. J. Syst. Chem. 2012;3:2. [Google Scholar]
- 64.Szostak J.W. The narrow road to the deep past: in search of the chemistry of the origin of life. Angew. Chem. Int. Ed Engl. 2017;56:11037–11043. doi: 10.1002/anie.201704048. [DOI] [PubMed] [Google Scholar]
- 65.Hill Jr A.R., Orgel L.E., Wu T. The limits of template-directed synthesis with nucleoside-5’-phosphoro(2-methyl)imidazolides. Orig. Life Evol. Biosph. 1993;23:285–290. doi: 10.1007/BF01582078. [DOI] [PubMed] [Google Scholar]
- 66.Wu T., Orgel L.E. Nonenzymatic template-directed synthesis on hairpin oligonucleotides 3 Incorporation of adenosine and uridine residues. J. Am. Chem. Soc. 1992;114:7963–7969. doi: 10.1021/ja00047a001. [DOI] [PubMed] [Google Scholar]
- 67.Heuberger B.D., Pal A., Del Frate F., Topkar V.V., Szostak J.W. Replacing uridine with 2-thiouridine enhances the rate and fidelity of nonenzymatic RNA primer extension. J. Am. Chem. Soc. 2015;137:2769–2775. doi: 10.1021/jacs.5b00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larsen A.T., Fahrenbach A.C., Sheng J., Pian J., Szostak J.W. Thermodynamic insights into 2-thiouridine-enhanced RNA hybridization. Nucleic Acids Res. 2015;43:7675–7687. doi: 10.1093/nar/gkv761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Izgu E.C., Fahrenbach A.C., Zhang N., Li L., Zhang W., Larsen A.T., Blain J.C., Szostak J.W. Uncovering the thermodynamics of monomer binding for RNA replication. J. Am. Chem. Soc. 2015;137:6373–6382. doi: 10.1021/jacs.5b02707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rajamani S., Ichida J.K., Antal T., Treco D.A., Leu K., Nowak M.A., Szostak J.W., Chen I.A. Effect of stalling after mismatches on the error catastrophe in nonenzymatic nucleic acid replication. J. Am. Chem. Soc. 2010;132:5880–5885. doi: 10.1021/ja100780p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prywes N., Blain J.C., Del Frate F., Szostak J.W. Nonenzymatic copying of RNA templates containing all four letters is catalyzed by activated oligonucleotides. Elife. 2016;5:e17756. doi: 10.7554/eLife.17756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang W., Tam C.P., Zhou L., Oh S.S., Wang J., Szostak J.W. Structural rationale for the enhanced catalysis of nonenzymatic RNA Primer extension by a downstream oligonucleotide. J. Am. Chem. Soc. 2018;140:2829–2840. doi: 10.1021/jacs.7b11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walton T., Pazienza L., Szostak J.W. Template-Directed catalysis of a multistep reaction pathway for nonenzymatic RNA primer extension. Biochemistry. 2019;58:755–762. doi: 10.1021/acs.biochem.8b01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duzdevich D., Carr C.E., Szostak J.W. Deep sequencing of non-enzymatic RNA primer extension. Nucleic Acids Res. 2020;48:e70. doi: 10.1093/nar/gkaa400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duzdevich D., Carr C.E., Ding D., Zhang S.J., Walton T.S., Szostak J.W. Competition between bridged dinucleotides and activated mononucleotides determines the error frequency of nonenzymatic RNA primer extension. Nucleic Acids Res. 2021;49:3681–3691. doi: 10.1093/nar/gkab173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Todisco M., Fraccia T.P., Smith G.P., Corno A., Bethge L., Klussmann S., Paraboschi E.M., Asselta R., Colombo D., Zanchetta G., Clark N.A., Bellini T. Nonenzymatic polymerization into long linear RNA templated by liquid crystal self-assembly. ACS Nano. 2018;12:9750–9762. doi: 10.1021/acsnano.8b05821. [DOI] [PubMed] [Google Scholar]
- 77.Fraccia T.P., Smith G.P., Zanchetta G., Paraboschi E., Yi Y., Walba D.M., Dieci G., Clark N.A., Bellini T. Abiotic ligation of DNA oligomers templated by their liquid crystal ordering. Nat. Commun. 2015;6:6424. doi: 10.1038/ncomms7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fraccia T.P., Jia T.Z. Liquid crystal coacervates composed of short double-stranded DNA and cationic peptides. ACS Nano. 2020;14:15071–15082. doi: 10.1021/acsnano.0c05083. [DOI] [PubMed] [Google Scholar]
- 79.Jia T.Z., Fraccia T.P. Liquid crystal peptide/DNA coacervates in the context of prebiotic molecular evolution. Crystals. 2020;10:964. [Google Scholar]
- 80.Jia T.Z., Fraccia T.P. Proceedings of the ALIFE 2021: The 2021 Conference on Artificial Life. 2021. Liquid crystal phase assembly in peptide-DNA coacervates as a mechanism for primitive emergence of structural complexity; p. 56. [Google Scholar]
- 81.Jash B., Tremmel P., Jovanovic D., Richert C. Single nucleotide translation without ribosomes. Nat. Chem. 2021;13:751–757. doi: 10.1038/s41557-021-00749-4. [DOI] [PubMed] [Google Scholar]
- 82.Ellington A.D., Szostak J.W. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 83.Robertson D.L., Joyce G.F. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature. 1990;344:467–468. doi: 10.1038/344467a0. [DOI] [PubMed] [Google Scholar]
- 84.Tuerk C., Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 85.Kudella P.W., Tkachenko A.V., Salditt A., Maslov S., Braun D. Structured sequences emerge from random pool when replicated by templated ligation. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2018830118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thomson J.B., Sigurdsson S.T., Zeuch A., Eckstein F. In vitro selection of hammerhead ribozymes containing a bulged nucleotide in stem II. Nucleic Acids Res. 1996;24:4401–4406. doi: 10.1093/nar/24.22.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ekland E.H., Szostak J.W., Bartel D.P. Structurally complex and highly active RNA ligases derived from random RNA sequences. Science. 1995;269:364–370. doi: 10.1126/science.7618102. [DOI] [PubMed] [Google Scholar]
- 88.Lorsch J.R., Szostak J.W. In vitro evolution of new ribozymes with polynucleotide kinase activity. Nature. 1994;371:31–36. doi: 10.1038/371031a0. [DOI] [PubMed] [Google Scholar]
- 89.Gold L., Brown D., He Y.-Y., Shtatland T., Singer B.S., Wu Y. From oligonucleotide shapes to genomic SELEX: Novel biological regulatory loops. Proc. Natl. Acad. Sci. USA. 1997;94:59–64. doi: 10.1073/pnas.94.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lorsch J.R., Szostak J.W. Chance and necessity in the selection of nucleic acid catalysts. Acc. Chem. Res. 1996;29:103–110. doi: 10.1021/ar9501378. [DOI] [PubMed] [Google Scholar]
- 91.Breaker R.R., Joyce G.F. A DNA enzyme that cleaves RNA. Chem. Biol. 1994;1:223–229. doi: 10.1016/1074-5521(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 92.Hollenstein M., Catalysis DNA. The chemical repertoire of DNAzymes. Molecules. 2015;20:20777–20804. doi: 10.3390/molecules201119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiménez J.I., Xulvi-Brunet R., Campbell G.W., Turk-MacLeod R., Chen I.A. Comprehensive experimental fitness landscape and evolutionary network for small RNA. Proc. Natl. Acad. Sci. USA. 2013;110:14984–14989. doi: 10.1073/pnas.1307604110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stephenson J.D., Popović M., Bristow T.F., Ditzler M.A. Evolution of ribozymes in the presence of a mineral surface. RNA. 2016;22:1893–1901. doi: 10.1261/rna.057703.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jeancolas C., Matsubara Y.J., Vybornyi M., Lambert C.N., Blokhuis A., Alline T., Griffiths A.D., Ameta S., Krishna S., Nghe P. RNA diversification by a self-reproducing ribozyme revealed by deep sequencing and kinetic modelling. Chem. Commun. 2021;57:7517–7520. doi: 10.1039/d1cc02290c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pressman A.D., Liu Z., Janzen E., Blanco C., Müller U.F., Joyce G.F., Pascal R., Chen I.A. Mapping a systematic ribozyme fitness landscape reveals a frustrated evolutionary network for self-aminoacylating RNA. J. Am. Chem. Soc. 2019;141:6213–6223. doi: 10.1021/jacs.8b13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Furubayashi T., Ueda K., Bansho Y., Motooka D., Nakamura S., Mizuuchi R., Ichihashi N. Emergence and diversification of a host-parasite RNA ecosystem through Darwinian evolution. eLife. 2020;9:e56038. doi: 10.7554/eLife.56038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bansho Y., Furubayashi T., Ichihashi N., Yomo T. Host–parasite oscillation dynamics and evolution in a compartmentalized RNA replication system. Proc. Natl. Acad. Sci. USA. 2016;113:4045–4050. doi: 10.1073/pnas.1524404113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bozovičar K., Bratkovič T. Evolving a peptide: library platforms and diversification strategies. Int. J. Mol. Sci. 2019;21:215. doi: 10.3390/ijms21010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Obexer R., Walport L.J., Suga H. Exploring sequence space: harnessing chemical and biological diversity towards new peptide leads. Curr. Opin. Chem. Biol. 2017;38:52–61. doi: 10.1016/j.cbpa.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 101.Newton M.S., Cabezas-Perusse Y., Tong C.L., Seelig B. In vitro selection of peptides and proteins-advantages of mRNA display. ACS Synth. Biol. 2020;9:181–190. doi: 10.1021/acssynbio.9b00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reyes S.G., Kuruma Y., Fujimi M., Yamazaki M., Eto S., Nishikawa S., Tamaki S., Kobayashi A., Mizuuchi R., Rothschild L., Ditzler M., Fujishima K. PURE mRNA display and cDNA display provide rapid detection of core epitope motif via high-throughput sequencing. Biotechnol. Bioeng. 2021;118:1736–1749. doi: 10.1002/bit.27696. [DOI] [PubMed] [Google Scholar]
- 103.Keefe A.D., Szostak J.W. Functional proteins from a random-sequence library. Nature. 2001;410:715–718. doi: 10.1038/35070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seelig B., Szostak J.W. Selection and evolution of enzymes from a partially randomized non-catalytic scaffold. Nature. 2007;448:828–831. doi: 10.1038/nature06032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Riddle D.S., Santiago J.V., Bray-Hall S.T., Doshi N., Grantcharova V.P., Yi Q., Baker D. Functional rapidly folding proteins from simplified amino acid sequences. Nat. Struct. Biol. 1997;4:805–809. doi: 10.1038/nsb1097-805. [DOI] [PubMed] [Google Scholar]
- 106.Kamtekar S., Schiffer J.M., Xiong H., Babik J.M., Hecht M.H. Protein design by binary patterning of polar and nonpolar amino acids. Science. 1993;262:1680–1685. doi: 10.1126/science.8259512. [DOI] [PubMed] [Google Scholar]
- 107.Akanuma S., Kigawa T., Yokoyama S. Combinatorial mutagenesis to restrict amino acid usage in an enzyme to a reduced set. Proc. Natl. Acad. Sci. USA. 2002;99:13549–13553. doi: 10.1073/pnas.222243999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.V. Tretyachenko, J. Vymětal, T. Neuwirthová, J. Vondrášek, K. Fujishima, K. Hlouchová, Structured proteins are abundant in unevolved sequence space, bioRxiv, (2021) 2021.08.29. 458031.
- 109.Higgs P.G., Pudritz R.E. A thermodynamic basis for prebiotic amino acid synthesis and the nature of the first genetic code. Astrobiology. 2009;9:483–490. doi: 10.1089/ast.2008.0280. [DOI] [PubMed] [Google Scholar]
- 110.Trifonov E.N. Consensus temporal order of amino acids and evolution of the triplet code. Gene. 2000;261:139–151. doi: 10.1016/s0378-1119(00)00476-5. [DOI] [PubMed] [Google Scholar]
- 111.Tretyachenko V., Voráček V., Souček R., Fujishima K., Hlouchová K. CoLiDe: combinatorial library design tool for probing protein sequence space. Bioinformatics. 2021;37:482–489. doi: 10.1093/bioinformatics/btaa804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fujishima K., Venter C., Wang K., Ferreira R., Rothschild L.J. An overhang-based DNA block shuffling method for creating a customized random library. Sci. Rep. 2015;5:1–5. doi: 10.1038/srep09740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alam K.K., Chang J.L., Burke D.H. FASTAptamer: A Bioinformatic toolkit for high-throughput sequence analysis of combinatorial selections. Mol. Ther. Nucleic Acids. 2015;4:e230. doi: 10.1038/mtna.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blanco C., Verbanic S., Seelig B., Chen I.A. EasyDIVER: a pipeline for assembling and counting high-throughput sequencing data from in vitro evolution of nucleic acids or peptides. J. Mol. Evol. 2020;88:477–481. doi: 10.1007/s00239-020-09954-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tagami S., Attwater J., Holliger P. Simple peptides derived from the ribosomal core potentiate RNA polymerase ribozyme function. Nat. Chem. 2017;9:325–332. doi: 10.1038/nchem.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Adamala K., Szostak J.W. Nonenzymatic template-directed RNA synthesis inside model protocells. Science. 2013;342:1098–1100. doi: 10.1126/science.1241888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.AbouHaidar M.G., Ivanov I.G. Non-enzymatic RNA hydrolysis promoted by the combined catalytic activity of buffers and magnesium ions. Z. Naturforsch. C. 1999;54:542–548. doi: 10.1515/znc-1999-7-813. [DOI] [PubMed] [Google Scholar]
- 118.P. Li, P. Holliger, S. Tagami, Hydrophobic-cationic peptides enhance RNA polymerase ribozyme activity by accretion, bioRxiv, (2021) 2021.02.22.432394. [DOI] [PMC free article] [PubMed]
- 119.Hammerling M.J., Fritz B.R., Yoesep D.J., Kim D.S., Carlson E.D., Jewett M.C. In vitro ribosome synthesis and evolution through ribosome display. Nat. Commun. 2020;11:1108. doi: 10.1038/s41467-020-14705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Giacobelli V.G., Fujishima K., Lepšík M., Tretyachenko V., Kadavá T., Makarov M., Bednárová L., Novák P., Hlouchová K. In vitro evolution reveals non-cationic protein-RNA interaction mediated by metal ions. Mol. Biol. Evol. 2022:msac032. doi: 10.1093/molbev/msac032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lahav N. The RNA-world and co-evolution hypotheses and the origin of life: implications, research strategies and perspectives. Orig. Life Evol. Biosph. 1993;23:329–344. doi: 10.1007/BF01582084. [DOI] [PubMed] [Google Scholar]
- 122.Carter C. What RNA world? Why a peptide/RNA partnership merits renewed experimental attention. Life. 2015;5:294–320. doi: 10.3390/life5010294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.B.D. Reed, M.J. Meyer, V. Abramzon, O. Ad, P. Adcock, F.R. Ahmad, G. Alppay, J.A. Ball, J. Beach, D. Belhachemi, A. Bellofiore, M. Bellos, J.F. Beltrán, A. Betts, M.W. Bhuiya, K. Blacklock, R. Boer, D. Boisvert, N.D. Brault, A. Buxbaum, et al., Real-time dynamic single-molecule protein sequencing on an integrated semiconductor device, bioRxiv, (2022) 2022.01.04.475002. [DOI] [PubMed]
- 124.Yu H., Zhang S., Chaput J.C. Darwinian evolution of an alternative genetic system provides support for TNA as an RNA progenitor. Nat. Chem. 2012;4:183–187. doi: 10.1038/nchem.1241. [DOI] [PubMed] [Google Scholar]
- 125.Sefah K., Yang Z., Bradley K.M., Hoshika S., Jiménez E., Zhang L., Zhu G., Shanker S., Yu F., Turek D., Tan W., Benner S.A. In vitro selection with artificial expanded genetic information systems. Proc. Natl. Acad. Sci. USA. 2014;111:1449–1454. doi: 10.1073/pnas.1311778111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hoshika S., Leal N.A., Kim M.-J., Kim M.-S., Karalkar N.B., Kim H.-J., Bates A.M., Watkins N.E., Jr, SantaLucia H.A., Meyer A.J., DasGupta S., Piccirilli J.A., Ellington A.D., SantaLucia J., Jr, Georgiadis M.M., Benner S.A. Hachimoji DNA and RNA: a genetic system with eight building blocks. Science. 2019;363:884–887. doi: 10.1126/science.aat0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sleiman D., Garcia P.S., Lagune M., Loc'h J., Haouz A., Taib N., Röthlisberger P., Gribaldo S., Marlière P., Kaminski P.A. A third purine biosynthetic pathway encoded by aminoadenine-based viral DNA genomes. Science. 2021;372:516–520. doi: 10.1126/science.abe6494. [DOI] [PubMed] [Google Scholar]
- 128.Zhou Y., Xu X., Wei Y., Cheng Y., Guo Y., Khudyakov I., Liu F., He P., Song Z., Li Z., Gao Y., Ang E.L., Zhao H., Zhang Y., Zhao S. A widespread pathway for substitution of adenine by diaminopurine in phage genomes. Science. 2021;372:512–516. doi: 10.1126/science.abe4882. [DOI] [PubMed] [Google Scholar]
- 129.Pezo V., Jaziri F., Bourguignon P.-Y., Louis D., Jacobs-Sera D., Rozenski J., Pochet S., Herdewijn P., Hatfull G.F., Kaminski P.-A., Marliere P. Noncanonical DNA polymerization by aminoadenine-based siphoviruses. Science. 2021;372:520–524. doi: 10.1126/science.abe6542. [DOI] [PubMed] [Google Scholar]
- 130.Yang Z., Chen F., Alvarado J.B., Benner S.A. Amplification, mutation, and sequencing of a six-letter synthetic genetic system. J. Am. Chem. Soc. 2011;133:15105–15112. doi: 10.1021/ja204910n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Biondi E., Lane J.D., Das D., Dasgupta S., Piccirilli J.A., Hoshika S., Bradley K.M., Krantz B.A., Benner S.A. Laboratory evolution of artificially expanded DNA gives redesignable aptamers that target the toxic form of anthrax protective antigen. Nucleic Acids Res. 2016;44:9565–9577. doi: 10.1093/nar/gkw890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang N., Shi S., Jia T.Z., Ziegler A., Yoo B., Yuan X., Li W., Zhang S. A general LC-MS-based RNA sequencing method for direct analysis of multiple-base modifications in RNA mixtures. Nucleic Acids Res. 2019;47:e125. doi: 10.1093/nar/gkz731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang N., Shi S., Yuan X., Ni W., Wang X., Yoo B., Jia T.Z., Li W., Zhang S. A general LC-MS-based method for direct and de novo sequencing of rna mixtures containing both canonical and modified nucleotides. Methods Mol. Biol. 2021;2298:261–277. doi: 10.1007/978-1-0716-1374-0_17. [DOI] [PubMed] [Google Scholar]
- 134.Zhang N., Shi S., Yoo B., Yuan X., Li W., Zhang S. 2D-HELS MS Seq: a general LC-MS-based method for direct and de novo sequencing of RNA mixtures with different nucleotide modifications. J. Vis. Exp. 2020;161:e61281. doi: 10.3791/61281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang N., Shi S., Wang X., Ni W., Yuan X., Duan J., Jia T.Z., Yoo B., Ziegler A., Russo J.J., Li W., Zhang S. Direct sequencing of tRNA by 2D-HELS-AA MS Seq reveals its different isoforms and dynamic base modifications. ACS Chem. Biol. 2020;15:1464–1472. doi: 10.1021/acschembio.0c00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu L., Seki M. Recent advances in the detection of base modifications using the Nanopore sequencer. J. Hum. Genet. 2020;65:25–33. doi: 10.1038/s10038-019-0679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Girgis H.S., DuPai C.D., Lund J., Reeder J., Guillory J., Durinck S., Liang Y., Kaminker J., Smith P.A., Skippington E. Single-molecule nanopore sequencing reveals extreme target copy number heterogeneity in arylomycin-resistant mutants. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2021958118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goto Y., Akahori R., Yanagi I., Takeda K.-I. Solid-state nanopores towards single-molecule DNA sequencing. J. Hum. Genet. 2020;65:69–77. doi: 10.1038/s10038-019-0655-8. [DOI] [PubMed] [Google Scholar]
- 139.Alfaro J.A., Bohländer P., Dai M., Filius M., Howard C.J., van Kooten X.F., Ohayon S., Pomorski A., Schmid S., Aksimentiev A., Anslyn E.V., Bedran G., Cao C., Chinappi M., Coyaud E., Dekker C., Dittmar G., Drachman N., Eelkema R., Goodlett D., et al. The emerging landscape of single-molecule protein sequencing technologies. Nat. Methods. 2021;18:604–617. doi: 10.1038/s41592-021-01143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brinkerhoff H., Kang A.S.W., Liu J., Aksimentiev A., Dekker C. Multiple rereads of single proteins at single-amino acid resolution using nanopores. Science. 2021;374:1509–1513. doi: 10.1126/science.abl4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sutton M.A., Burton A.S., Zaikova E., Sutton R.E., Brinckerhoff W.B., Bevilacqua J.G., Weng M.M., Mumma M.J., Johnson S.S. Radiation tolerance of nanopore sequencing technology for life detection on Mars and Europa. Sci. Rep. 2019;9:5370. doi: 10.1038/s41598-019-41488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carr C.E., Bryan N.C., Saboda K.N., Bhattaru S.A., Ruvkun G., Zuber M.T. Nanopore sequencing at Mars, Europa, and microgravity conditions. NPJ Microgravity. 2020;6:24. doi: 10.1038/s41526-020-00113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.MacKenzie S.M., Neveu M., Davila A.F., Lunine J.I., Craft K.L., Cable M.L., Phillips-Lander C.M., Hofgartner J.D., Eigenbrode J.L., Hunter Waite J., Glein C.R., Gold R., Greenauer P.J., Kirby K., Bradburne C., Kounaves S.P., Malaska M.J., Postberg F., Patterson G.Wesley, Porco C., et al. The enceladus orbilander mission concept: balancing return and resources in the search for life. Planet. Sci. J. 2021;2:77. [Google Scholar]
- 144.Neveu M., Anbar A.D., Davila A.F., Glavin D.P., MacKenzie S.M., Phillips-Lander C.M., Sherwood B., Takano Y., Williams P., Yano H. Returning samples from Enceladus for life detection. Front. Astron. Space Sci. 2020;7:26. [Google Scholar]
- 145.Kahana A., Schmitt-Kopplin P., Lancet D. Enceladus: first observed primordial soup could arbitrate origin-of-life debate. Astrobiology. 2019;19:1263–1278. doi: 10.1089/ast.2019.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kashima Y., Sakamoto Y., Kaneko K., Seki M., Suzuki Y., Suzuki A. Single-cell sequencing techniques from individual to multiomics analyses. Exp. Mol. Med. 2020;52:1419–1427. doi: 10.1038/s12276-020-00499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stuart T., Satija R. Integrative single-cell analysis. Nat. Rev. Genet. 2019;20:257–272. doi: 10.1038/s41576-019-0093-7. [DOI] [PubMed] [Google Scholar]
- 148.Shokralla S., Gibson J.F., Nikbakht H., Janzen D.H., Hallwachs W., Hajibabaei M. Next-generation DNA barcoding: using next-generation sequencing to enhance and accelerate DNA barcode capture from single specimens. Mol. Ecol. Resour. 2014;14:892–901. doi: 10.1111/1755-0998.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ogawa T., Kryukov K., Imanishi T., Shiroguchi K. The efficacy and further functional advantages of random-base molecular barcodes for absolute and digital quantification of nucleic acid molecules. Sci. Rep. 2017;7:13576. doi: 10.1038/s41598-017-13529-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tambe A., Pachter L. Barcode identification for single cell genomics. BMC Bioinformatics. 2019;20:32. doi: 10.1186/s12859-019-2612-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Joyce G.F., Szostak J.W. Protocells and RNA Self-Replication. Cold Spring Harb. Perspect. Biol. 2018;10 doi: 10.1101/cshperspect.a034801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schrum J.P., Zhu T.F., Szostak J.W. The origins of cellular life. Cold Spring Harb. Perspect. Biol. 2010;2 doi: 10.1101/cshperspect.a002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Froese T., Campos J.I., Fujishima K., Kiga D., Virgo N. Horizontal transfer of code fragments between protocells can explain the origins of the genetic code without vertical descent. Sci. Rep. 2018;8:3532. doi: 10.1038/s41598-018-21973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jia T.Z., Caudan M., Mamajanov I. Origin of Species before origin of life: the role of speciation in chemical evolution. Life. 2021;11:154. doi: 10.3390/life11020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sakuma Y., Imai M. From vesicles to protocells: the roles of amphiphilic molecules. Life. 2015;5:651–675. doi: 10.3390/life5010651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen I.A., Walde P. From self-assembled vesicles to protocells. Cold Spring Harb. Perspect. Biol. 2010;2 doi: 10.1101/cshperspect.a002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang A., Szostak J.W. Lipid constituents of model protocell membranes. Emerg Top Life Sci. 2019;3:537–542. doi: 10.1042/ETLS20190021. [DOI] [PubMed] [Google Scholar]
- 158.Yoshizawa T., Nozawa R.-S., Jia T.Z., Saio T., Mori E. Biological phase separation: cell biology meets biophysics. Biophys. Rev. 2020;12:519–539. doi: 10.1007/s12551-020-00680-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mann S. Systems of creation: the emergence of life from nonliving matter. Acc. Chem. Res. 2012;45:2131–2141. doi: 10.1021/ar200281t. [DOI] [PubMed] [Google Scholar]
- 160.Ghosh B., Bose R., Tang T.-Y.D. Can coacervation unify disparate hypotheses in the origin of cellular life? Curr. Opin. Colloid Interface Sci. 2021;52 [Google Scholar]
- 161.Poudyal R.R., Pir Cakmak F., Keating C.D., Bevilacqua P.C. Physical principles and extant biology reveal roles for RNA-containing membraneless compartments in origins of life chemistry. Biochemistry. 2018;57:2509–2519. doi: 10.1021/acs.biochem.8b00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Keating C.D. Aqueous phase separation as a possible route to compartmentalization of biological molecules. Acc. Chem. Res. 2012;45:2114–2124. doi: 10.1021/ar200294y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pir Cakmak F., Keating C.D. Combining catalytic microparticles with droplets formed by phase coexistence: adsorption and activity of natural clays at the aqueous/aqueous interface. Sci. Rep. 2017;7:3215. doi: 10.1038/s41598-017-03033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jia T.Z., Hentrich C., Szostak J.W. Rapid RNA exchange in aqueous two-phase system and coacervate droplets. Orig. Life Evol. Biosph. 2014;44:1–12. doi: 10.1007/s11084-014-9355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jia T.Z., Chandru K., Hongo Y., Afrin R., Usui T., Myojo K., Cleaves H.J., II Membraneless polyester microdroplets as primordial compartments at the origins of life. Proc. Natl. Acad. Sci. USA. 2019;116:15830–15835. doi: 10.1073/pnas.1902336116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jia T.Z., Bapat N.V., Verma A., Mamajanov I., Cleaves H.J., II, Chandru K. Incorporation of basic α-Hydroxy acid residues into primitive polyester microdroplets for RNA segregation. Biomacromolecules. 2021;22:1484–1493. doi: 10.1021/acs.biomac.0c01697. [DOI] [PubMed] [Google Scholar]
- 167.Veziroglu E.M., Mias G.I. Characterizing extracellular vesicles and their diverse RNA contents. Front. Genet. 2020;11:700. doi: 10.3389/fgene.2020.00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.D. Wollny, B. Vernot, J. Wang, M. Hondele, A. Hyman, K. Weis, J.G. Camp, T.-Y. Dora Tang, B. Treutlein, Characterization of RNA content in individual phase-separated coacervate microdroplets, bioRxiv, (2021) 2021.03.08.434405. [DOI] [PMC free article] [PubMed]
- 169.Segré D., Ben-Eli D., Lancet D. Compositional genomes: prebiotic information transfer in mutually catalytic noncovalent assemblies. Proc. Natl. Acad. Sci. USA. 2000;97:4112–4117. doi: 10.1073/pnas.97.8.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Muchowska K.B., Varma S.J., Moran J. Nonenzymatic metabolic reactions and life's origins. Chem. Rev. 2020;120:7708–7744. doi: 10.1021/acs.chemrev.0c00191. [DOI] [PubMed] [Google Scholar]
- 171.Kitadai N., Kameya M., Fujishima K. Origin of the reductive tricarboxylic acid (rTCA) cycle-type CO2 fixation: A perspective. Life. 2017;7:39. [Google Scholar]