Highlights
-
•
A systematic review for the characterization of lipidic cubic phases (LCPs) by solution NMR is presented.
-
•
Various aspects of LCPs readily accessible by solution NMR are covered in detail.
-
•
A brief discussion of future perspectives is also included.
Keywords: Diffusion, Dynamics, Hydration, Lipidic cubic phases, NMR, Structure
Abstract
Nuclear magnetic resonance (NMR) spectroscopy is well-established nowadays for the elucidation of the 3D structures of proteins and protein complexes, the evaluation of biomolecular dynamics with atomistic resolution across a range of time scales, the screening of drug candidates with site specificity, and for the quantitation of molecular translational diffusion. Lyotropic lipidic cubic phases (LCPs) are lipid bilayer-based materials with a complex geometry, formed via the spontaneous self-assembly of certain lipids in an aqueous environment at specific temperature ranges. LCPs have been successfully applied to the in meso crystallization of membrane proteins for structural studies by X-ray crystallography, and have also shown promising potential for serving as matrices for drug and nutrient delivery/release in vivo. The characterization of the structural and dynamics properties of LCPs is of significant interest for the application of these materials. Here we present a systematic review detailing the characterization of LCPs by solution NMR. Using LCPs formed by monoolein (MO) as an example, various aspects of LCPs readily accessible by solution NMR are covered, including spectral perturbation in the presence of additives, quantification of hydration levels, 13C relaxation-based measurements for studying atom-specific dynamics along the MO hydrocarbon chain, PGSE NMR measurement of translational diffusion and its correlation with release profiles, and the encapsulation of soluble proteins in LCPs. A brief discussion of future perspectives for the characterization of LCPs by solution NMR is also presented.
Abbreviations
- LCPs
lipidic cubic phases
- MO
monoolein
- NMR
nuclear magnetic resonance
- hetNOE
heteronuclear Overhauser effect
- PGSE
pulsed gradient spin echo
1. Introduction
Inverse bicontinuous lipidic cubic phases (LCPs), a subgroup of liquid-crystalline phases, are formed via the spontaneous self-assembly of certain lipids in an aqueous environment [1], [2], [3]. Their formation is temperature dependant, observed in membrane systems of wide-ranging composition, and is implicated in numerous biological processes, such as membrane fusion [4], fat digestion [5], and viral infection [6]. In the laboratory, LCPs can be readily produced from binary lipid-water mixtures, a typical example of which is the monoacylglycerol monoolein (MO, Mw = 356) in water, though much more complex formulations are possible. Accordingly, these materials have found a range of uses as biological membrane mimetics, matrices for drug encapsulation and release [7], [8], [9], [10], and as a means for restricting proton exchange for the study of hydration dynamics in soluble proteins. Perhaps their most impactful application has been in the crystallization of integral membrane proteins for structural determination by X-ray crystallography [11], so called in meso crystallization. LCPs provide both a stabilizing membrane mimetic environment in which the membrane protein can be reconstituted, as well as allowing self-diffusion of protein molecules throughout the sample matrix, enabling crystal nucleation and growth [12], [13], [14]. To date, over 200 membrane protein structures solved via X-ray crystallography have utilized this technique [14,15].
Structurally, LCPs are comprised of two interpenetrating yet unconnected networks of water channels with nanometre scale diameters, separated by a single lipid bilayer [16]. Three phase groups are commonly observed for inverse bicontinuous LCPs in binary lipid-water systems: the primitive (), the diamond (,) and the gyroid (), corresponding to space groups Im3m, Pn3m, and Ia3d, respectively [2]. Mathematically, these inverse bicontinuous LCPs have been treated successfully as infinite periodic minimal surfaces (IPMSs) [17]. Potential roles of membrane shape on protein properties have been reviewed recently [18]. These three commonly observed phase groups are presented as 3D illustrations in Fig. 1. This unique bilayer structure enables LCPs to encapsulate hydrophobic compounds within the bilayer environment, hydrophilic compounds within the water channels and amphiphilic compounds at the lipid-water interface. The utility of LCPs is further increased by their stability in excess water, resulting in the ability to form stable nanoparticles, termed cubosomes, with the addition of steric stabilizers such as Pluronic F127 [19].
Fig. 1.
Three-dimensional representations of three commonly observed LCP phase groups, (Im3m, left), (Pn3m; centre), and (la3d, right), in binary lipid-water systems.
Small angle X-ray scattering (SAXS), particularly with the assistance of synchrotron radiation, has proven to be a rapid and reliable technique to determine the space group and thus lipid phase, as well as the lattice parameter (a measure of unit cell size) of LCPs [20,21]. Other techniques, such as Small Angle Neutron Scattering (SANS) [22,23], Circular Dichroism (CD) [24,25], Dynamic Light Scattering (DLS) [26], and Fluorescence Recovery After Photobleaching (FRAP) [27,28], have also been used in the characterization of LCP-based materials. Recently, nuclear magnetic resonance (NMR) spectroscopy has been increasingly utilized to probe structural and dynamics properties of LCPs.
As a spectroscopic technique, NMR has seen applications across many disciplines, including 3D structure elucidation of proteins and protein complexes [29], [30], [31], and the compositional analysis of complex mixture in proteomics [32]. Furthermore, NMR has established itself as a principal experimental technology for investigating residue-specific dynamics of biomolecules [33,34], while pulsed gradient spin echo (PGSE) based NMR methods offer direct experimental quantitation of molecular translational diffusion [35], [36], [37], [38]. NMR is further utilized in the analysis of lipid and surfactant self-assembly as recently documented [39].
Attempts at exploring the microstructure of LCPs via NMR relaxation and translational diffusion measurements in combination with modelling have also been published [40], as have review articles focusing on the application of PGSE NMR to LCPs [41], [42], [43]. Herein we present a systematic review on the characterization of LCPs by solution NMR, primarily using LCPs formed by monoolein (MO) as an example. We cover various aspects of LCPs readily accessible by solution NMR, including the quantification of hydration levels, spectral perturbation induced by additives, 13C relaxation-based measurements for studying atom-specific dynamics along the MO hydrocarbon chain, PGSE NMR measurement of translational diffusion in LCPs and its correlation with release profiles, and the analysis of LCP encapsulated soluble proteins. A discussion on future perspectives for the characterization of LCPs using solution NMR is also presented.
2. Preparation of LCP samples for solution NMR measurements
LCP samples can be prepared using a variety of techniques and will form spontaneously with only minimal mixing of their constituent components given adequate time to equilibrate. For the purposes of NMR analysis, a high degree of compositional control can be achieved by using a coupled syringe mixing apparatus, as has been previously described [13,44]. Briefly, two gas-tight syringes of appropriate volume (typically 50-200 μL) are loaded with aqueous buffer and molten lipid, respectively, using a pipette and a laboratory balance to measure the appropriate mass of each (Fig. 2A). The syringes are then coupled together using a commercially available syringe fitting, designed specifically for this purpose. The two components are pushed back and forth, and forced through the narrow opening between the coupled syringes, resulting in rapid mixing of the components (Fig. 2 B&C). The subsequent formation of an LCP is often apparent by the visual change in the syringe contents, going from white/opaque to transparent. This approach also simplifies the handling process, as the sticky and viscous LCP can be dispensed directly from the syringe by removing the coupling and attaching a standard needle fitting.
Fig. 2.
Schematic illustrations of: (A) the initial stages of the syringe mixing process, with aqueous buffer and molten lipid loaded into each of the coupled syringes, (B) the cubic phase beginning to form after the components are combined, (C) the fully formed LCP after cycling the syringe plungers back and forth several times, and (D) the capillary-based sample setup typically employed for solution NMR analysis of LCPs. For the sake of illustration, the water/aqueous buffer, molten lipid, and LCP have been coloured blue, yellow and green, respectively, and the external deuterated solvent for the lock of the magnetic field has been coloured pink (D).
Depending on the sensitivity of the NMR spectrometers used for the study, one may encounter artefacts arising from radiation damping [45] in 1H based NMR of LCPs due to their high lipid content. This issue can be readily solved by using a micro-NMR tube instead, or simply using a glass capillary as described previously [46,47], to limit the active volume of the LCP for NMR measurements. To prepare such a sample, the LCP is injected via syringe and needle into a glass capillary (1-2 mm diameter), which is then flame sealed and placed inside a 5 mm NMR tube containing an external deuterated solvent needed for the lock of the magnetic field, as illustrated in Fig. 2D. Both internal and external chemical shift references can be used depending on the final sample configuration. If quantitation of hydration levels in LCP samples by NMR is of interest (see below), deuterated solvent (external) other than 2H2O should be considered for the lock of the magnetic field as residual H2O signal will complicate the quantitation of H2O in LCPs. All LCP samples should be allowed to equilibrate, typically for around 12 h, prior to NMR measurement. Standard pulse sequences, from the Bruker pulse sequence library used for the acquisition of spectra/data presented in the current review, are summarized in Table 1. It is worth mentioning that, where reduced sample volume leads to concerns around sensitivity such as for encapsulated proteins, advanced NMR methods that minimize the effects of radiation damping spectroscopically could be considered [45,48].
Table 1.
List of pulse sequences used for the acquisition of spectra/data presented in the current review.
| Spectra/data names | Pulses sequences | Corresponding Fig.(s) |
|---|---|---|
| 1D 1H | zg30 | Fig. 3; Fig. 4 |
| 1D 13C | zgpg30 | Fig. 3; Fig. 5 |
| 2D multiplicity edited 1H-13C HSQC | hsqcedetgpsisp2.3 | Fig. 3 |
| 2D 1H-13C HSQC for 13C T1 | hsqct1etgpsi3d | Fig. 6; Fig. 7 |
| 2D 1H-13C HSQC for 13C-{1H} hetNOE | hsqcnoegpsi.2 | Fig. 6; Fig. 7 |
| Diffusion by PGSE | stegp1s | Fig. 8 |
| Diffusion by PGSE | stebpgp1s | Fig. 9 |
| 2D 1H-15N BEST-HMQC | sfhmqcf3gpph | Fig. 10 |
3. 1H and 13C spectral assignments of LCPs formed by monoolein
Due to the isotropic nature of LCPs, their solution NMR spectra often closely resemble those of their constituent lipids in organic solvent such as CDCl3 albeit with an increased linewidth of resonances, reflecting the formation of discrete domains. Both 1H and 13C spectra of LCPs formed by MO under various conditions have been reported previously [47,[49], [50], [51], [52]]. Nearly complete 1H and 13C spectral assignments of LCPs formed by MO are readily achievable via 1D and 2D homo- and heteronuclear NMR spectroscopy [47]. The spectral differences across different phase groups of LCPs formed by MO, i.e., , , are rather minor. Given the high content of lipids in LCPs, 3D 1H-13C based NOESY and TOCSY spectra [53] can also be recorded without the need for isotope enriched lipids. A 2D 1H-13C multiplicity edited HSQC spectrum of an LCP formed by MO at 40 wt% hydration is shown in Fig. 3, along with the chemical structure of MO.
Fig. 3.
(A) The chemical structure of MO, hydrogen carbons with degenerate chemical shifts due to their symmetry around the C9-C10 olefinic double bond are highlighted in colour (C9/C10 in red; C8/C11 in pink; and C4-C8/C11-C15 in blue), and (B) a 2D 1H-13C multiplicity edited HSQC spectrum (CH, CH3 in black and CH2 in red) of an LCP formed by MO at 40 wt% hydration. 1D 1H and 13C spectra are shown as 1D projections with all resonances labelled based on their atom numbers as indicated in (A). All spectra were acquired at 298 K on a Bruker 600 MHz Avance III spectrometer equipped with a TCI cryoprobe. Both 1H and 13C chemical shifts are referenced to CDCl3.
4. Assessment of hydration by quantitative 1D 1H NMR
One-dimensional 1H NMR spectroscopy has been widely applied for the quantitation of intra-molecular proton ratios in solution and has more recently seen substantial use in metabolomics for the quantitative analysis of multiple components in complex mixtures [54]. We have recently demonstrated that water content (wt%) in LCPs, , can be estimated reliably via their 1D 1H proton spectra by solving the following pair of equations [47]:
| (1a) |
| (1b) |
where MH2O and NH2O denote the mass and number of protons of solvent water, respectively. Similarly, Mlip and Nlip denote the mass and number of protons of the lipid molecules, respectively. For MO, Nlip = 40 and Mlip = 356. Iw and Ilip are integrals of water and lipid resonances, respectively, obtained directly from a 1D 1H spectrum, and M is the total weight of the LCP samples. As previously mentioned, deuterated solvent other than 2H2O (external) should be used for the lock of magnetic field in order to avoid the complication arising from signal overlap between H2O within the LCP (hydration water) and the residual H2O signal contained in 2H2O (lock solvent). A correlation plot of the water content () of MO based LCPs obtained using Eqs. 1a &1b versus their expected values (, those used for sample preparation) is shown in Fig. 4.
Fig. 4.
(A) 1D 1H spectrum of an LCP formed by MO at 35 wt% hydration recorded at 298 K with peak integrals indicated and (B) water content (hydration) in LCPs formed by MO derived from their 1D 1H spectra using Eqs. (1a) & (1b)versus expected values. Redrawn from Meikle et al. [47]. Lock of the magnetic field is achieved via CDCl3 surrounding the capillary in a standard 5 mm NMR tube (see Fig. 2D).
5. Spectral perturbation induced by additive molecules in LCPs
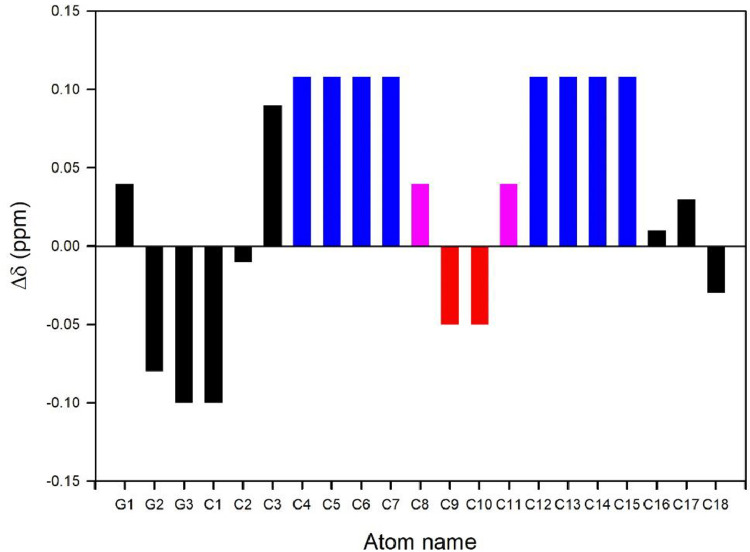
Chemical shift perturbation is a key indicator of structural changes upon self-association and/or ligand binding in structural studies of biomolecules by NMR spectroscopy [55]. Experimental evaluation of the structural impact of additive molecules in LCPs is of particular interest for the optimization of LCPs for specific applications. For example, the successful in meso crystallization of G-protein-coupled receptors (GPCRs) requires the presence of additives such as cholesterol [56,57]. A better understanding of the interactions which occur between additives and the LCP lipid bilayer thus enables more effective design and tailoring of crystallization trials. Consequently, the structural and functional impacts of cholesterol addition to lipid membranes, including LCPs, have been previously investigated [57,58]. The 13C chemical shifts of the hydrocarbon chain of cubic phase MO in the presence of cholesterol have also been reported [47]. The secondary 13C chemical shifts, along the hydrocarbon chain of MO in the presence of 20 wt% cholesterol, are shown in Fig. 5. While the structural symmetry related to the double bond of MO leads to the degeneracy of 13C chemical shifts for carbons around its double bond, well-resolved resonances from both the hydrophilic head group and hydrophobic tail provide a good probe for evaluating any potential structural perturbations in the presence of additives [59]. We expect that, with further experimentation in this area, stronger correlational data linking 13C chemical shift perturbation and structural/conformational impacts at the atomic level, upon the inclusion of additives in LCPs will be established.
Fig. 5.
13C chemical shift perturbation of the hydrocarbon chain of LCPs formed by MO at 40 wt% hydration, induced by the presence of 20 wt% cholesterol at 298 K. Note that the Δδ values for degenerate hydrocarbons, colour coded as in Fig. 3, are set equal and should be considered as an approximation only. Redrawn from Meikle et al. [47].
6. LCP hydrocarbon chain dynamics by 13C relaxation measurements
Relaxation measurements of quadrupolar spins at selective sites, such as 2H for isotope enriched lipids or 14N for nitrogen containing lipids, are a popular choice for exploring the structural, morphological and dynamic properties of lipids and biomembranes using solid-state NMR [40,60]. In addition, carbon relaxation parameters along the hydrocarbon chains, predominantly via 13C R1 ( 1/T1) and 13C-{1H} heteronuclear Overhauser effect (hetNOE), have also been widely used to explore the local dynamics of polymer chains [61,62], lipid bilayers [63], LCPs [64], [65], [66], and LCPs in the presence of additives such as hydrophilic and lipophilic compounds [67].
Unlike 13C relaxation-based studies of protein backbone/sidechain dynamics, measurements of 13C T2, particularly in a lipidic environment, are generally not attempted. This is due to the complicating dependence of T2 on complex motions at much slower time scales that contribute to the spectral density at zero frequency, J(0) [68,69] and to the very high power required for the CPMG (Carr-Purcell-Meiboom-Gill) pulse train [70,71] employed in T2 measurements that is exacerbated by the large bandwidth in 13C NMR.
-
(a)
Measuring 13C R1 and 13C-{1H} hetNOE of LCPs
In contrast to 2D 1H-13C HSQC based indirect detection schemes commonly used for measuring 13C relaxation parameters in studies of protein backbone/sidechain dynamics [72,73], 13C R1 and 13C-{1H} hetNOE of lipids (lipid aggregates) are generally obtained via 1D directly 13C detected methods. One-dimensional 13C R1 data, measured using the standard inversion recovery sequence with 1H decoupling during acquisition, are then analysed by fitting R1 relaxation weighted signals to the following three-parameter equation:
| (2a) |
Accordingly, the 13C-{1H} hetNOEs are calculated using the ratios of integrals of individual resonances, taken from 1D 13C spectra acquired with 1H decoupling, during acquisition, in the presence and absence of 1H saturation during the relaxation delay.
The advances in NMR instrumentation over the last couple of decades, particularly the introduction of the cryogenic probe-head, have made the acquisition of 2D spectra of LCPs at natural abundance practically feasible as far as sensitivity is concerned. Due to the structural symmetry of MO within its unsaturated hydrocarbon chain, 13C editing (as in 2D 1H-13C HSQC) here offers only limited gains in spectral resolution for the LCPs formed. However, the availability of 2D 1H-13C HSQC based indirect-detection schemes offer additional avenues for relaxation-based studies of lipid dynamics, where significant improvement of spectral resolution may be required for LCPs formed by lipids other than MO. In addition, 13C T1 is generally longer than the 1H T1, particularly for the hydrocarbon resonances towards the lipid tail, thus in order to allow full recovery of 13C magnetization for quantitative measurements, a sufficiently long relaxation delay would have to be used in 1D direct 13C relaxation measurements. When the overall acquisition time is of concern, one could consider using the non-uniform sampling scheme, which has gained popularity and become a standard practice in multidimensional NMR spectroscopy [74], [75], [76]. 13C R1 data measured using 1H-13C HSQC based indirect-detection schemes are analysed by fitting R1 relaxation weighted signals to a two-parameter exponential decay:
| (2b) |
Similar to the 1D version, the 13C-{1H} hetNOEs are then calculated from the ratios of 2D integrals of individual 13C resonances of spectra acquired in the presence and absence of 1H saturation during the relaxation delay. Representative 13C R1 relaxation data for the hydrocarbons in LCPs formed by MO at 35 wt% hydration, and a plot of resultant 13C relaxation rate R1 and 13C-{1H} hetNOE values along the hydrocarbon chain of MO, excluding the carboxylic carbon, are shown in Fig. 6. Both 13C relaxation rate R1 and 13C-{1H} hetNOE data were acquired using standard 2D 1H-13C HSQC based sequences (Table 1). As can be seen from Fig. 6, the overall profile of 13C R1 values along the hydrocarbon chain of MO agrees well with that reported previously using 1D direct 13C detection for LCPs formed by MO at a similar hydration value [65]. In contrast, somewhat uniform 13C-{1H} hetNOE values across the entire hydrocarbon chain of MO were observed with no clear evidence of increased flexibility (elevated 13C-{1H} hetNOE values) towards the hydrophobic tail.
-
(a)
Interpretation of 13C relaxation data for LCPs – the two-step model
Fig. 6.
(A) Representative 13C relaxation data of hydrocarbons for LCPs formed by MO at 35 wt% hydration, and (B) plot of corresponding 13C R1 and 13C-{1H} hetNOE along the hydrocarbon chain, excluding the carboxylic carbon (C1, Fig. 3A), measured at 1H magnetic field strength of 600 MHz and 298 K. The pertaining minimum (1.15) and maximum (2.61) 13C-{1H} hetNOE values, at the field strength of the data acquired, expected for a completely rigid (S = 1) and flexible (S = 0) C-H bond respectively, are indicated by dotted lines. The same colour code as in Fig. 5 is adopted for hydrocarbons with degenerate 13C chemical shifts due to symmetry around the olefinic double bond.
Under the assumption that 13C spin relaxation of carbons along the hydrocarbon chain of lipids is dominated (solely) by dipole-dipole interactions with their directly bonded proton spins, i.e., that the contributions from chemical shift anisotropy, spin rotation, and cross-correlation effects are negligible, 13C R1 and 13C-{1H} hetNOE are given by:
| (3) |
| (4) |
where N is the number of protons bound to the 13C spin and with μ0 being the permeability of vacuum, γH and γC being the gyromagnetic ratios of proton and carbon, respectively, and h, rHC being Planck's constant and the C-H bond length, respectively.
Probing biomolecular dynamics via NMR relaxation measurements has become a routine practice nowadays [34,77]. In addition to the (reduced) spectral density approach [78], a so-called model-free formalism [79,80] is widely adopted for extracting residue-specific dynamics parameters. This model-free formalism actually follows the same theoretical approach as used in the dynamics study of lipids/lipid aggregates from 13C NMR relaxation data, known as the two-step model [81]. Both approaches were actually derived/established independently around the same time [39]. In the present review, the convention of symbols and terms commonly used in 13C relaxation studies of lipids/lipid aggregates, which differ slightly from those frequently seen in dynamics studies of protein/DNA via NMR relaxation measurements, are adopted. As its name suggests, two motions on different time scales are considered; the first, a slow motion, , accounts for the overall motion of the lipids/aggregates depending on the systems. The second, a fast motion, , accounts for the rapid motion of individual carbon atoms accompanied by an order parameter, S (ranging from 0 to 1), describing the amplitude of local spin-specific motion. In the two-step model [81], the reduced spectral density function, J(ω), takes the following form:
| (5a) |
where S is the order parameter and . If is sufficiently fast (typically in the order of tens of picoseconds) and typically in the order of a few tens of nanosecond, then and , Eq. (5a) reduces to
| (5b) |
It is worth noting that modified forms of the spectral density function (Eq. (5a) & (5b)) have been used for the analysis of 13C based relaxation parameters to accommodate the complexity of motions experienced by lipids/membranes [82]. For example, a restriction of freedom, Ss, for the lipids in vesicles might be incorporated into Eq. (5b) to give [83,84]:
| (5c) |
To a certain extent, Eq. (5c) could also be seen as an analogue to that adopted in the extended model-free formalism introduced by Clore et al. [85]. In the study of protein dynamics via NMR relaxation measurements, a global rotational correlation time, τm (similar to ,), or rotational diffusion tensor, Dr, for anisotropic overall motion is generally estimated initially from a subset of backbone 15N-1H pairs that exhibit significantly less localized motions, prior to the extraction of local residue-specific motional parameters following the model-free formalism [86]. In contrast, contributions to the slow correlation time depend strongly on the composition and properties of the lipid aggregates under investigation. In micelles and small vesicles, for instance, both rotational and translational diffusion of the aggregates, as well as the lateral diffusion of lipids, will contribute to the slow motions of the lipids. For lipids in LCPs, on the other hand, the dominant contributor is lateral (surface) diffusion as the translational and rotational diffusion of the aggregates (up to μm in size) is considered to be too large to make a significant contribution. A theoretical framework for spin relaxation in LCPs was presented in the 1990’s by Halle et al. [87].
For dynamic studies of lipids based on 13C R1 and 13C-{1H} hetNOE, an estimation of slow correlation time has traditionally been obtained from the relaxation parameters of quadrupolar spins, such as 14N or 2H, if available. In the absence of NMR active quadrupolar nuclei, a couple of alternative schemes have been employed in the literature. These include 13C relaxation measured at multiple fields to fit both slow and fast motion parameters simultaneously [67] and through computations, for example for micelles, accounting for both contributions from rotational tumbling and monomer translational diffusion in the micelle and over the micelle surface [69,88]. The resulting value of ca. 1.6 ns for a ternary system of MO/water/NaC10 (90.8/3.5/5.7; NaC10: Sodium decanoate) obtained by fitting and S simultaneously using relaxation data acquired at 13C field strength of 75 and 100 MHz, was rather low when compared with those reported in earlier studies [67]. The authors suggested including additional relaxation data sampled at lower field strength, as R1 rates at higher fields might not be sensitive enough to slow motions of lipids [67]. In fact, considering the linewidth in 1D 1H spectra of LCPs formed by MO (Fig. 2), one would expect an effective slow correlation time in the order of tens of ns to be more reasonable, in line with molecular rotation correlation times reported in NMR relaxation-based protein dynamics studies [89,90].
Recently, making use of the framework of relaxation theory in LCPs from Halle et al. [87], an exploration was reported relating slow correlation time with surface diffusion of lipids in LCPs (and subsequently, macroscopic diffusion coefficients as measured by PGSE NMR) [66]. This approach facilitates the evaluation of atom-specific LCP hydrocarbon chain dynamics without the need for isotope labelling of LCPs, such as those formed by MO, where NMR active quadrupolar spins are not present in its native form. This involves two steps, first converting the experimentally measured, macroscopic diffusion coefficient (Dmo) of the LCP lipids obtained by PGSE NMR (see below), to a (two-dimensional) surface diffusion coefficient of lipids, Ds, via the following [41,91]:
| (6) |
The slow correlation time of lipids, in LCPs can then be calculated from Ds using the relationship described by Halle et al. [87]:
| (7) |
where the constant ζ characterizes the reference IPMSs with exact values for three commonly observed phases of LCPs given in Halle et al. [87], a is the lattice parameter, and b is the displacement parameter or the thickness of the film [66].
MO forms a LCP with 35 wt% hydration at 298 K, with experimental SAXS data providing a lattice parameter and displacement parameter of 150 and 30 Å, respectively [47]. The calculated b values are close to the maximum b values (bmax/a of 0.1882) quoted for phase groups [87]. For certain LCPs, the contribution of b to the calculated was found to be minimal and could be ignored [66]. Using an experimentally measured macroscopic diffusion coefficient, Dmo, of 1.43 × 10−11 m2s−1 [92], a resultant value of 55 ns was obtained from Eqs. (6) and (7). This slow correlation time is more than an order of magnitude longer than that reported for the tertiary LCP system formed by MO/water/NaC10 using data recorded at two field strengths as mentioned earlier [67], but is comparable with a slow correlation time of 74 ns at 301 K for LCPs formed by dodecyl trimethylammonium chloride (DOTAC, Mw = 264) and water, resulting from 2H relaxation measurements [66]. To some extent, this variance reflects the complexity of motions that lipids might be experiencing in LCPs, similar to that seen in many forms of lipid nanostructures [82].
Extracting residue-specific dynamics parameters from experimentally measured relaxation parameters generally involves the minimization of the residual χ2 value measuring the difference between the observed and calculated values of 13C T1 and 13C-{1H} hetNOE, which is defined as:
| (8) |
With the use of the reduced spectral density given in Eq. (5a) and a slow correlation time of 55 ns, the resultant order parameters and fast correlation times of individual hydrocarbons of MO in LCPs (35 wt% hydration) are depicted in Fig. 7. From previous 13C relaxation-based studies, it was generally believed that carbons close to the polar head are more constrained (large S) than those near the hydrophobic tail because the surfactant molecules are anchored to the interface [39,64]. However, this trend is not evident in Fig. 7. Furthermore, in contrast to other hydrocarbons along the acyl chain, the residual error of fitting for the methyl carbon at the hydrophobic tail (C18) is significantly larger (Fig. 7C) suggesting that the chosen two-parameter model function here is less appropriate. Furthermore, the 13C R1 and 13C-{1H} hetNOE given in Eqs. (3) & (4) only consider the contribution from dipole-dipole interactions with their directly bonded protons. Overall contributions from chemical shift anisotropy, spin rotation and cross-correlation might be minor when compared with dipole-dipole interactions, but the degree of their contributions might vary along the hydrocarbon chain. This is particularly true for the methyl group at the end of the hydrophobic tail of MO, where fast rotation around the terminal C-C bond may also stimulate spin relaxation by the spin-rotation coupling mechanism [93,94], and thus could have a nonnegligible contribution to the 13C relaxation rates compared to the methylene groups along the hydrocarbon chain. In addition, a rotational decoupling between the hydrophilic and hydrophobic regions for lipid membranes formed by phospholipids has recently been reported [95], implying that a single slow correlation time, , might not correctly represent the slow motion experienced by both polar head and the hydrophobic tail.
Fig. 7.
(A) Order parameters, S, (B) fast correlation times, and, (C) residual χ2 value of individual hydrocarbons for LCPs formed by MO at 35 wt% hydration, from 13C NMR relaxation data depicted in Fig. 6, extracted by minimizing χ2 (Eq. (8)) using the Solver add-in in Microsoft Excel [88] with the reduced spectral density function given in Eq. (5a) and a slow correlation time, , of 55 ns. The same colour scheme used in Fig. 5 is adopted for hydrocarbons giving rise to degenerate 13C chemical shifts due to symmetry around the olefinic double bond.
Nevertheless, the two-step model (the model-free approach) remains the only formalism to extract dynamic parameters from 13C R1 and NOE data measured at a single field strength, even though caution is needed for the interpretation of dynamics of lipids in the absence of additional independent restraints. Alternatively, a so-called longitudinal study, such as in the absence and presence of additives or between bound and unbound (apo) states, might produce more reliable outcomes. A more diligent study with relaxation measurements as a function of frequency (field strength) could also be considered for detailed investigation of lipid dynamics [96]. Lastly, combining NMR relaxation measurements with MD simulation could be carried out for comprehensive investigation of hydrocarbons [97], [98], [99].
7. Translational diffusion of LCPs by PGSE NMR
Translational diffusion of both solvent water and lipids in LCPs is readily measurable using PGSE NMR [41]. Specifically, comprehensive studies of water and lipid diffusion in LCPs formed by MO using PGSE NMR have been reported [100,101]. The diffusion coefficients are generally determined by non-linear regression of diffusion weighted peak intensities (amplitudes) of non-exchangeable protons in LCPs to the following equation:
| (9) |
where γ is the gyromagnetic ratio of protons, and s, g, δ and Δ represent the shape factor, amplitude, duration and separation of the single pair of gradient pulses, respectively. As the diffusion of lipids in LCPs is approximately an order of magnitude slower than that of solvent water [92,100], the gradient duration, δ, and separation, Δ, must generally be adjusted to achieve optimal signal attenuation and yield reliable measurements for solvent water and lipid in LCPs, respectively. Fig. 8 depicts the translational diffusion induced signal attenuation in PGSE measurements of LCPs formed by MO at 40 wt% hydration in the absence and presence of cholesterol (20 wt%), for both water and lipid components. The measured translational diffusion coefficients of solvent water at 40 wt% hydration in the absence and presence of cholesterol are 6.37 and 6.55 × 10−10 m2s−1, respectively, indicating a small increase in apparent diffusion of water in the tertiary MO/cholesterol/water system. The corresponding diffusion coefficients of MO are 1.49 and 0.94 × 10−11 m2s−1, respectively, representing a significant slowing down of lipid translational motion, ca. 37%, in the presence of 20 wt% of cholesterol. For measurements of slow molecular diffusion, a dedicated probe-head capable of applying a strong field gradient is generally preferred. With lipid diffusion in LCPs at ambient temperature in the order of 10−11 m2s−1, a standard probe fitted with a gradient coil of strength ca. 50 Gcm−1 dedicated for coherence selection is possibly sufficient, provided a relatively long diffusion time, Δ, in the order of several hundred ms is used [92,102].
Fig. 8.
Translational diffusion induced signal attenuation in PGSE measurements of LCPs formed by MO at 40 wt% hydration in the absence and presence of cholesterol (20 wt%) for (A) solvent water, and (B) MO measured at 298 K.
In addition to the stimulated echo (STE, stegp1s, Bruker pulse sequence library) and bipolar-pulse pair STE (BPP-STE stegpbp1s, Bruker pulse sequence library) sequences (Table 1), an extensive library of PGSE sequences have been developed to meet with the broad scope of applications [103]. Compared with the standard STE sequence, the BPP-STE [104] is less susceptible to instrumental and spectral artefacts, such as eddy current [105,106], field lock signal perturbation and background field inhomogeneity [107,108], spin diffusion/NOE [109,110], chemical exchange [92,111], and gradient non-linearity [104]. In addition, a class of PGSE sequences constructed by replacing the single STE with a double STE format (dstebpgp3s, Bruker pulse sequence library) to minimise the influence of convection that might be present in the samples is also available [112,113].
8. Release profiles of LCPs by PGSE NMR
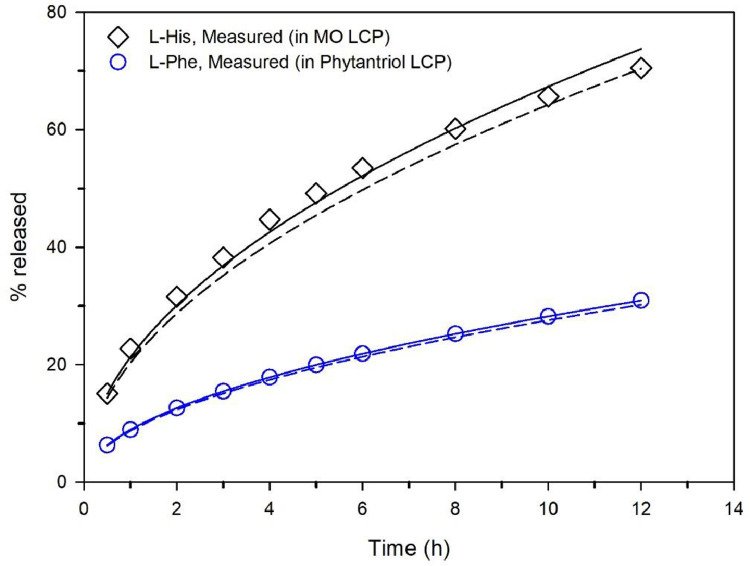
Controlling release from LCPs has been a major interest of research in the last two decades [114], [115], [116], [117]. As a result, various studies of the diffusion properties of drug-like molecules encapsulated in LCPs and their correlation with release profiles have been reported [44,[118], [119], [120]]. The release profile of small molecules from LCPs, a key measure for evaluating the efficiency and specificity of delivery, has been shown to correlate with restricted molecular diffusion behaviour [121]. Specifically, attempts have been made to evaluate correlation between translational diffusion coefficients of LCP encapsulated model drugs, measured by PGSE NMR, and their release profiles measured directly in vitro via the Higuchi equation [122]:
| (10) |
where Q (in mol) is the cumulative quantity of drug released per unit area (mol·cm−2) at time t, C0 is the initial molar concentration (mol·cm−3) of the drug molecule in the matrix, and D is the self-diffusion coefficient of the drug molecule. In our 2017 study, PGSE NMR was used to measure the diffusion coefficients of amino acids, serving as model drugs, in a number of different LCP compositions [44]. This data was then used in conjunction with Eq. (10) to generate predicted release profiles for various LCP formulations, and subsequently compared with the actual release profiles determined directly in vitro. The potential accuracy of predicted release rates is illustrated by Fig. 9, which shows both predicted and experimentally measured release profiles of L-histidine and L-phenylalanine encapsulated in MO and phytantriol based LCPs. This work also highlighted the correlation between the diameter of the LCP water channel and the diffusion coefficients of encapsulated molecules, and ultimately the release rates of model drugs. More broadly, the application of NMR PGSE based diffusion measurements for the characterization of nanomedicines has been reviewed recently with both in vitro and in vivo perspectives discussed [123].
Fig. 9.
Percentage of amino acids released from LCPs measured directly in vitro (symbols and solid lines) and predicted from NMR-derived translational diffusion coefficients using the Higuchi equation (dashed lines). Redrawn from Meikle et al. [44].
9. Encapsulation of soluble globular proteins
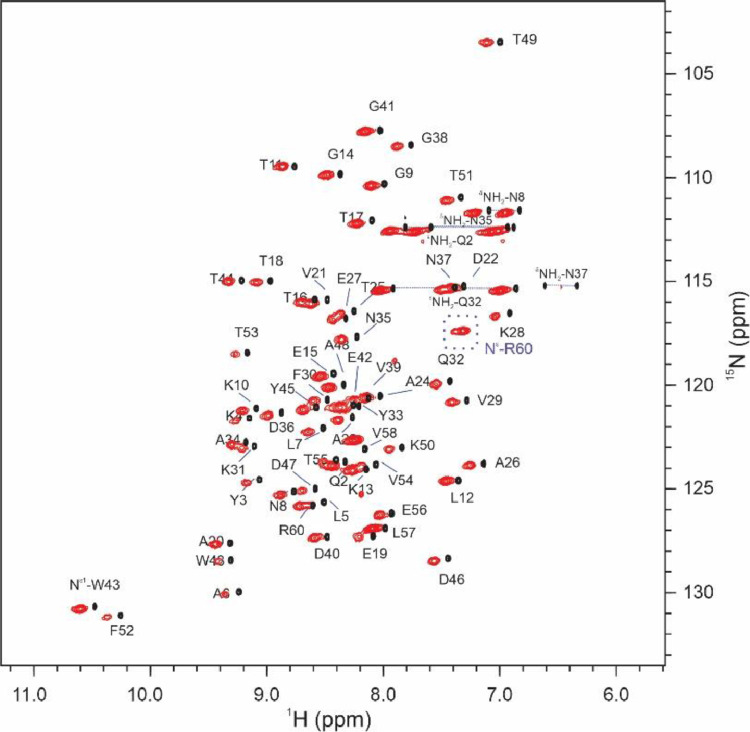
The membrane mimetic properties of LCPs have seen their use in a number of different applications, including in meso crystallization of membrane proteins and the encapsulation and delivery of therapeutic compounds [124,125]. We recently reported the successful encapsulation of a soluble protein, B1 immunoglobulin-binding domain of Streptococcal protein (GB1), and demonstrated that spectral features of a soluble protein are largely retained albeit with an increased linewidth and significantly reduced exchange between protein backbone amides and water [46]. This result highlights an alternative means for the study of protein hydration dynamics in a setting analogous to encapsulation in reverse micelles [126]. Importantly, LCPs have been demonstrated to be compatible with a broad range of temperatures and pH, as well as a variety of chemical reagents and detergents commonly utilized in structural/dynamics studies of proteins [127], [128], [129], [130]. Furthermore, through modifications to their lipid composition, the geometry of LCPs can be tuned to suit specific experimental requirements [131,132]. An overlay showing a comparison of 1H-15N HSQC of GB1 in aqueous solution and encapsulated in LCPs is depicted in Fig. 10.
Fig. 10.
Overlay of 1H-15N HSQC spectra of GB1 in aqueous solution (black) and encapsulated in LCPs (red, 38 wt% hydration containing GB1 at 1.8 mM) at 298 K acquired using a Bruker 600 MHz Avance III spectrometer equipped with a TCI cryoprobe fitted with a single-axis gradient (Gz). Both spectra were acquired and processed with identical parameters except for the number of scans accumulated. The folded peak (inside dashed box) from the Nε- Hε group in the R60 sidechain only appears when encapsulated in LCPs, indicating a protection from fast exchange with water and/or sterically hindered rotation around the Nε-Cζ bond. Redrawn from Meikle et al. [46].
10. Outlook
LCPs serve as an attractive medium for the encapsulation of integral membrane proteins, facilitating structural and dynamics characterization [124]. A recent review describes both the fundamental advantages and limitations of these materials for the study of GPCRs [133]. The presence of additives, such as cholesterol, has been identified as playing a critical role in achieving the high-resolution crystal structures of GPCRs with NMR chemical shift theoretical calculations indicating that hydrogen bonding between MO and cholesterol produces stabilizing effects [57].
It was recently reported that exchange between the hydroxyl groups of MO and solvent water in LCPs could be readily quantified by 1D EXSY (exchange spectroscopy) experiments [92]. If MO and cholesterol are indeed involved in hydrogen bonding, then the exchange between hydroxyl groups of MO and solvent water would be expected to slow down. A study of the exchange properties of MO hydroxyl groups in LCPs using EXSY NMR, in the presence and absence of cholesterol, could potentially provide direct experimental evidence of the formation of hydrogen bonds involving the hydroxyl groups of MO. Such studies could also extend to membrane-active peptides encapsulated in LCPs where the formation of hydrogen bonds may stabilize their structures.
In summary, the structural, dynamic and translational diffusion properties of LCPs, which are readily accessible by solution NMR studies, enable greater characterization and optimization of these materials. The methods reviewed herein are expected to be equally applicable to more complicated LCPs, such as a quaternary lipid-water system [131], to cubosomes (a nanoparticle form of LCPs [19]), and to thermotropic cubic phases [134]. Lastly, the study of LCPs formed by lipids containing other NMR observable nuclei, such as 2H, 19F and 31P (as is frequently used for the study of lipids via solid-state NMR [135]) may provide additional insights. These experiments could be used to overcome limitations related to spectral dispersion and sensitivity, providing further complementary information, e.g. for relaxation-based dynamics analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge support from the Bio21 Institute NMR Facility, University of Melbourne. SY would like to thank Dr Marc-Antoine Sani (University of Melbourne) for helpful discussions. The authors would also like to thank two anonymous reviewers whose comments and suggestions helped improve and clarify the manuscript.
Data Availability
Data will be made available on request.
References
- 1.Mezzenga R., Seddon J.M., Drummond C.J., Boyd B.J., Schroder-Turk G.E., Sagalowicz L. Nature-Inspired Design and Application of Lipidic Lyotropic Liquid Crystals. Adv. Mater. 2019:31. doi: 10.1002/adma.201900818. [DOI] [PubMed] [Google Scholar]
- 2.Luzzati V., Tardieu A., Gulikkrzywicki T., Rivas E., Reisshus F. Structure of Cubic Phases of Lipid-Water Systems. Nature. 1968;220:485–488. doi: 10.1038/220485a0. [DOI] [PubMed] [Google Scholar]
- 3.Kaasgaard T., Drummond C.J. Ordered 2-D and 3-D nanostructured amphiphile self-assembly materials stable in excess solvent. Phys. Chem. Chem. Phys. 2006;8:4957–4975. doi: 10.1039/b609510k. [DOI] [PubMed] [Google Scholar]
- 4.Burger K.N.J. Greasing membrane fusion and fission machineries. Traffic. 2000;1:605–613. doi: 10.1034/j.1600-0854.2000.010804.x. [DOI] [PubMed] [Google Scholar]
- 5.Patton J.S., Carey M.C. Watching Fat Digestion. Science. 1979;204:145–148. doi: 10.1126/science.432636. [DOI] [PubMed] [Google Scholar]
- 6.Razinkov V.I., Melikyan G.B., Cohen F.S. Hemifusion between cells expressing hemagglutinin of influenza virus and planar membranes can precede the formation of fusion pores that subsequently fully enlarge. Biophys. J. 1999;77:3144–3151. doi: 10.1016/S0006-3495(99)77144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah J.C., Sadhale Y., Chilukuri D.M. Cubic phase gels as drug delivery systems. Adv. Drug. Deliv. Rev. 2001;47:229–250. doi: 10.1016/s0169-409x(01)00108-9. [DOI] [PubMed] [Google Scholar]
- 8.Zabara A., Mezzenga R. Controlling molecular transport and sustained drug release in lipid-based liquid crystalline mesophases. J Control Release. 2014;188:31–43. doi: 10.1016/j.jconrel.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 9.Milak S., Zimmer A. Glycerol monooleate liquid crystalline phases used in drug delivery systems. Int. J. Pharm. 2015;478:569–587. doi: 10.1016/j.ijpharm.2014.11.072. [DOI] [PubMed] [Google Scholar]
- 10.Drummond C.J., Fong C. Surfactant self-assembly objects as novel drug delivery vehicles. Curr. Opin. Colloid Interface Sci. 1999;4:449–456. [Google Scholar]
- 11.Zabara A., Meikle T.G., Trenker R., Yao S.G., Newman J., Peat T.S., Separovic F., Conn C.E., Call M.J., Call M.E., Landau E.M., Drummond C.J. Lipidic Cubic Phase-Induced Membrane Protein Crystallization: Interplay Between Lipid Molecular Structure, Mesophase Structure and Properties, and Crystallogenesis. Cryst. Growth Des. 2017;17:5667–5674. [Google Scholar]
- 12.Landau E.M., Rosenbusch J.P. Lipidic cubic phases: A novel concept for the crystallization of membrane proteins. P Natl Acad Sci USA, 1996;93:14532–14535. doi: 10.1073/pnas.93.25.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caffrey M., Cherezov V. Crystallizing membrane proteins using lipidic mesophases. Nat. Protoc. 2009;4:706–731. doi: 10.1038/nprot.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherezov V. Lipidic cubic phase technologies for membrane protein structural studies. Curr Opin Struc Biol. 2011;21:559–566. doi: 10.1016/j.sbi.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caffrey M. A comprehensive review of the lipid cubic phase or in meso method for crystallizing membrane and soluble proteins and complexes. Acta Crystallogr F. 2015;71:3–18. doi: 10.1107/S2053230X14026843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durr U.H.N., Gildenberg M., Ramamoorthy A. The Magic of Bicelles Lights Up Membrane Protein Structure. Chem. Rev. 2012;112:6054–6074. doi: 10.1021/cr300061w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyde S.T., Andersson S., Ericsson B., Larsson K. A cubic structure consisting of a lipid bilayer forming an infinite periodic minimum surface of the gyroid type in the glycerolmonooleate-water system. Zeitschrift Fur Kristallographie. 1984;168:213–219. [Google Scholar]
- 18.Bozelli J.C., Aulakh S.S., Epand R.M. Membrane shape as determinant of protein properties*. Biophys. Chem. 2021:273. doi: 10.1016/j.bpc.2021.106587. [DOI] [PubMed] [Google Scholar]
- 19.Barriga H.M.G., Holme M.N., Stevens M.M. Cubosomes: The next generation of smart lipid nanoparticles? Angewandte Chemie-International Edition. 2019;58:2958–2978. doi: 10.1002/anie.201804067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tenchov B., Koynova R. Cubic phases in membrane lipids. Eur Biophys J Biophy. 2012;41:841–850. doi: 10.1007/s00249-012-0819-3. [DOI] [PubMed] [Google Scholar]
- 21.Tenchov B., Koynova R. Cubic phases in phosphatidylethanolamine dispersions: Formation, stability and phase transitions. Chem. Phys. Lipids. 2017;208:65–74. doi: 10.1016/j.chemphyslip.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Angelov B., Angelova A., Garamus V.M., Lebas G., Lesieur S., Ollivon M., Funari S.S., Willumeit R., Couvreur P. Small-angle neutron and x-ray scattering from amphiphilic stimuli-responsive diamond-type bicontinuous cubic phase. J. Am. Chem. Soc. 2007;129:13474–13479. doi: 10.1021/ja072725+. [DOI] [PubMed] [Google Scholar]
- 23.van't Ha L., de Campo L., Tran N., Sokolova A., Trenker R., Call M.E., Call M.J., Garvey C.J., Leung A.E., Darwish T.A., Krause-Heuer A., Knott R., Meikle T.G., Drummond C.J., Mezzenga R., Conn C.E. Protein-Eye View of the in Meso Crystallization Mechanism. Langmuir. 2019;35:8344–8356. doi: 10.1021/acs.langmuir.9b00647. [DOI] [PubMed] [Google Scholar]
- 24.Liu W., Caffrey M. Gramicidin structure and disposition in highly curved membranes. J. Struct. Biol. 2005;150:23–40. doi: 10.1016/j.jsb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Meikle T.G., Dharmadana D., Hoffmann S.V., Jones N.C., Drummond C.J., Conn C.E. Analysis of the structure, loading and activity of six antimicrobial peptides encapsulated in cubic phase lipid nanoparticles. J. Colloid. Interf. Sci. 2021;587:90–100. doi: 10.1016/j.jcis.2020.11.124. [DOI] [PubMed] [Google Scholar]
- 26.Nakano M., Teshigawara T., Sugita A., Leesajakul W., Taniguchi A., Kamo T., Matsuoka H., Handa T. Dispersions of liquid crystalline phases of the monoolein/oleic acid/Pluronic F127 system. Langmuir. 2002;18:9283–9288. [Google Scholar]
- 27.Cribier S., Gulik A., Fellmann P., Vargas R., Devaux P.F., Luzzati V. Cubic Phases of Lipid-Containing Systems - a Translational Diffusion Study by Fluorescence Recovery after Photobleaching. J. Mol. Biol. 1993;229:517–525. doi: 10.1006/jmbi.1993.1051. [DOI] [PubMed] [Google Scholar]
- 28.Cherezov V., Liu J., Griffith M., Hanson M.A., Stevens R.C. LCP-FRAP Assay for Pre-Screening Membrane Proteins for In Meso Crystallization. Cryst. Growth Des. 2008;8:4307–4315. doi: 10.1021/cg800778j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gronwald W., Kalbitzer H.R. Automated structure determination of proteins by NMR spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2004;44:33–96. [Google Scholar]
- 30.Wishart D. NMR spectroscopy and protein structure determination: Applications to drug discovery and development. Curr. Pharm. Biotechnol. 2005;6:105–120. doi: 10.2174/1389201053642367. [DOI] [PubMed] [Google Scholar]
- 31.Gobl C., Madl T., Simon B., Sattler M. NMR approaches for structural analysis of multidomain proteins and complexes in solution. Prog. Nucl. Magn. Reson. Spectrosc. 2014;80:26–63. doi: 10.1016/j.pnmrs.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Sugiki T., Kobayashi N., Fujiwara T. Modern Technologies of Solution Nuclear Magnetic Resonance Spectroscopy for Three-dimensional Structure Determination of Proteins Open Avenues for Life Scientists. Comput. Struct. Biotechnol. J. 2017;15:328–339. doi: 10.1016/j.csbj.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishima R., Torchia D.A. Protein dynamics from NMR. Nat. Struct. Biol. 2000;7:740–743. doi: 10.1038/78963. [DOI] [PubMed] [Google Scholar]
- 34.Palmer A.G. NMR characterization of the dynamics of biomacromolecules. Chem. Rev. 2004;104:3623–3640. doi: 10.1021/cr030413t. [DOI] [PubMed] [Google Scholar]
- 35.Stejskal E.O., Tanner J.E. Spin diffusion measurements: Spin echoes in the presence of a time-dependent field gradient. J. Chem. Phys. 1965;42:288–292. [Google Scholar]
- 36.Tanner J.E. Use of stimulated echo in nmr-diffusion studies. J. Chem. Phys. 1970;52:2523–2526. [Google Scholar]
- 37.Price W.S. Pulsed-field gradient nuclear magnetic resonance as a tool for studying translational diffusion .1. Basic theory. Concept. Magnetic Res. 1997;9:299–336. [Google Scholar]
- 38.Price W.S. Pulsed-field gradient nuclear magnetic resonance as a tool for studying translational diffusion: Part II. Experimental aspects. Concept. Magnetic Res. 1998;10:197–237. [Google Scholar]
- 39.Monduzzi M., Lindman B. Lipid and surfactant self-assembly: Significance of NMR in developing our understanding. Curr. Opin. Colloid Interface Sci. 2019;44:14–22. [Google Scholar]
- 40.Hakansson P., Westlund P.O. Nuclear magnetic relaxation study of the microstructure of a bicontinuous cubic phase. Phys. Chem. Chem. Phys. 2004;6:4321–4329. [Google Scholar]
- 41.Lindblom G., Oradd G. NMR studies of translational diffusion in lyotropic liquid-crystals and lipid-membranes. Prog. Nucl. Magn. Reson. Spectrosc. 1994;26:483–515. [Google Scholar]
- 42.Momot K.I., Kuchel P.W. Pulsed field gradient nuclear magnetic resonance as a tool for studying drug delivery systems. Concepts Magnetic Resonance Part A. 2003;19A:51–64. [Google Scholar]
- 43.Rajput S., Yao S., Keizer D., Sani M.-A., Separovic F. NMR spectroscopy of lipidic cubic phases. Biophys. Rev. 2022;14:67–74. doi: 10.1007/s12551-021-00900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meikle T.G., Yao S.G., Zabara A., Conn C.E., Drummond C.J., Separovic F. Predicting the release profile of small molecules from within the ordered nanostructured lipidic bicontinuous cubic phase using translational diffusion coefficients determined by PFG-NMR. Nanoscale. 2017;9:2471–2478. doi: 10.1039/c6nr07382d. [DOI] [PubMed] [Google Scholar]
- 45.Krishnan V.V., Murali N. Radiation damping in modern NMR experiments: Progress and challenges. Prog. Nucl. Magn. Reson. Spectrosc. 2013;68:41–57. doi: 10.1016/j.pnmrs.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meikle T.G., Sethi A., Keizer D.W., Babon J.J., Separovic F., Gooley P.R., Conn C.E., Yao S.G. Heteronuclear NMR spectroscopy of proteins encapsulated in cubic phase lipids. J. Magn. Reson. 2019;305:146–151. doi: 10.1016/j.jmr.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 47.Meikle T.G., Keizer D.W., Babon J.J., Drummond C.J., Separovic F., Conn C.E., Yao S. Physiochemical Characterization and Stability of Lipidic Cubic Phases by Solution NMR. Langmuir. 2020;36:6254–6260. doi: 10.1021/acs.langmuir.0c00949. [DOI] [PubMed] [Google Scholar]
- 48.Sklenar V. Suppression of radiation damping in multidimensional nmr experiments using magnetic-field gradients. J. Magn. Reson. Ser. A. 1995;114:132–135. [Google Scholar]
- 49.Pampel A., Strandberg E., Lindblom G., Volke F. High-resolution NMR on cubic lyotropic liquid crystalline phases. Chem. Phys. Lett. 1998;287:468–474. [Google Scholar]
- 50.Caboi F., Amico G.S., Pitzalis P., Monduzzi M., Nylander T., Larsson K. Addition of hydrophilic and lipophilic compounds of biological relevance to the monoolein/water system. I. Phase behavior. Chem. Phys. Lipids. 2001;109:47–62. doi: 10.1016/s0009-3084(00)00200-0. [DOI] [PubMed] [Google Scholar]
- 51.Murgia S., Caboi F., Monduzzi M., Ljusberg-Wahren H., Nylander T. Acyl migration and hydrolysis in monoolein-based systems. Prog. Coll. Pol. Sci. S. 2002;120:41–46. [Google Scholar]
- 52.Boyle-Roden E., Hoefer N., Dey K.K., Grandinetti P.J., Caffrey M. High resolution HNMR of a lipid cubic phase using a solution NMR probe. J. Magn. Reson. 2007;189:13–19. doi: 10.1016/j.jmr.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 53.Sattler M., Schleucher J., Griesinger C. Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog. Nucl. Magn. Reson. Spectrosc. 1999;34:93–158. [Google Scholar]
- 54.Bharti S.K., Roy R. Quantitative H-1 NMR spectroscopy. Trac-Trends in Analytical Chemistry. 2012;35:5–26. [Google Scholar]
- 55.Williamson M.P. Using chemical shift perturbation to characterise ligand binding. Prog. Nucl. Magn. Reson. Spectrosc. 2013;73:1–16. doi: 10.1016/j.pnmrs.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Stevens R.C., Cherezov V., Katritch V., Abagyan R., Kuhn P., Rosen H., Wuthrich K. The GPCR Network: a large-scale collaboration to determine human GPCR structure and function. Nat. Rev. Drug Discovery. 2013;12:25–34. doi: 10.1038/nrd3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gater D.L., Reat V., Czaplicki G., Saurel O., Milon A., Jolibois F., Cherezov V. Hydrogen Bonding of Cholesterol in the Lipidic Cubic Phase. Langmuir. 2013;29:8031–8038. doi: 10.1021/la401351w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gater D.L., Saurel O., Iordanov I., Liu W., Cherezov V., Milon A. Two Classes of Cholesterol Binding Sites for the beta(2)AR Revealed by Thermostability and NMR. Biophys. J. 2014;107:2305–2312. doi: 10.1016/j.bpj.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X.Q., Zhang Y., Gui S.Y., Huang J., Cao J.J., Li Z.G., Li Q., Chu X.Q. Characterization of Lipid-Based Lyotropic Liquid Crystal and Effects of Guest Molecules on Its Microstructure: a Systematic Review. AAPS PharmSciTech. 2018;19:2023–2040. doi: 10.1208/s12249-018-1069-1. [DOI] [PubMed] [Google Scholar]
- 60.Molugu T.R., Lee S., Brown M.F. Concepts and Methods of Solid-State NMR Spectroscopy Applied to Biomembranes. Chem. Rev. 2017;117:12087–12132. doi: 10.1021/acs.chemrev.6b00619. [DOI] [PubMed] [Google Scholar]
- 61.Karali A., Dais P., Heatley F. Carbon-13 nuclear magnetic relaxation study of solvent effects on chain local dynamics of poly(N-vinylcarbazole) in dilute solution. Macromolecules. 2000;33:5524–5531. [Google Scholar]
- 62.Kassaee M.Z., Heydari H., Hattami M., Nia A.F. 13C spin-lattice relaxation times and NOE related studies of hydroxyl-terminated polybutadiene (HTPB) Macromolecules. 2003;36:6773–6779. [Google Scholar]
- 63.Brown M.F., Ribeiro A.A., Williams G.D. New view of lipid bilayer dynamics from 2H and 13C NMR relaxation time measurements. Proc. Natl. Acad. Sci. U S A, 1983;80:4325–4329. doi: 10.1073/pnas.80.14.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soderman O., Henriksson U. H-2 and C-13 nuclear magnetic-relaxation studies of the cubic liquid-crystalline phase-i1 in the sodium octanoate octane water-system. J. Chem. Soc. Faraday Trans. I. 1987;83:1515–1529. [Google Scholar]
- 65.Monduzzi M., Ljusberg-Wahren H., Larsson K.R. A C-13 NMR study of aqueous dispersions of reversed lipid phases. Langmuir. 2000;16:7355–7358. [Google Scholar]
- 66.Soderman O., Henriksson U. NMR studies of bicontinuous liquid crystalline phases of cubic symmetry: Interpretation of frequency-dependent relaxation rates. Langmuir. 2020;36:5927–5934. doi: 10.1021/acs.langmuir.0c00742. [DOI] [PubMed] [Google Scholar]
- 67.Murgia S., Caboi F., Monduzzi M. Addition of hydrophilic and lipophilic compounds of biological relevance to the mnonoolein/water system II - C-13 NMR relaxation study. Chem. Phys. Lipids. 2001;110:11–17. doi: 10.1016/s0009-3084(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 68.Ellena J.F., Lepore L.S., Cafiso D.S. Estimating lipid lateral diffusion in phospholipid-vesicles from c-13 spin spin relaxation. J. Phys. Chem. 1993;97:2952–2957. [Google Scholar]
- 69.Wong T.C., Ikeda K., Meguro K., Soderman O., Olsson U., Lindman B. Hydrocarbon chain conformation of bipolar surfactants in micelles - A magnetic-field dependent C-13 and N-14 nmr spin-lattice relaxation and nuclear overhauser effect study of N,N'-1,20-eicosanediylbis(triethylammonium bromide) J. Phys. Chem. 1989;93:4861–4867. [Google Scholar]
- 70.Carr H.Y., Purcell E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954;94:630–638. [Google Scholar]
- 71.Meiboom S., Gill D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958;29:688–691. [Google Scholar]
- 72.Nicholson L.K., Kay L.E., Baldisseri D.M., Arango J., Young P.E., Bax A., Torchia D.A. Dynamics of methyl-groups in proteins as studied by proton-detected C-13 NMR spectroscopy - Application to the leucine residues of staphylococcal nuclease. Biochemistry. 1992;31:5253–5263. doi: 10.1021/bi00138a003. [DOI] [PubMed] [Google Scholar]
- 73.Yang D. Probing protein side chain dynamics via 13C NMR relaxation. Protein Pept. Lett. 2011;18:380–395. doi: 10.2174/092986611794653932. [DOI] [PubMed] [Google Scholar]
- 74.Matsuki Y., Konuma T., Fujiwara T., Sugase K. Boosting Protein Dynamics Studies Using Quantitative Nonuniform Sampling NMR Spectroscopy. J. Phys. Chem. B. 2011;115:13740–13745. doi: 10.1021/jp2081116. [DOI] [PubMed] [Google Scholar]
- 75.Mobli M., Hoch J.C. Nonuniform sampling and non-Fourier signal processing methods in multidimensional NMR. Prog. Nucl. Magn. Reson. Spectrosc. 2014;83:21–41. doi: 10.1016/j.pnmrs.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jameson G., Hansen A.L., Li D.W., Bruschweiler-Li L., Bruschweiler R. Extreme Nonuniform Sampling for Protein NMR Dynamics Studies in Minimal Time. J. Am. Chem. Soc. 2019;141:16829–16838. doi: 10.1021/jacs.9b08032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palmer A.G. A dynamic look backward and forward. J. Magn. Reson. 2016;266:73–80. doi: 10.1016/j.jmr.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farrow N.A., Zhang O.W., Szabo A., Torchia D.A., Kay L.E. Spectral density-function mapping using N-15 relaxation data exclusively. J. Biomol. NMR. 1995;6:153–162. doi: 10.1007/BF00211779. [DOI] [PubMed] [Google Scholar]
- 79.Lipari G., Szabo A. Model-Free Approach to the Interpretation of Nuclear Magnetic-Resonance Relaxation in Macromolecules .1. Theory and Range of Validity. J. Am. Chem. Soc. 1982;104:4546–4559. [Google Scholar]
- 80.Lipari G., Szabo A. Model-Free Approach to the Interpretation of Nuclear Magnetic-Resonance Relaxation in Macromolecules .2. Analysis of Experimental Results. J. Am. Chem. Soc. 1982;104:4559–4570. [Google Scholar]
- 81.Wennerstrom H., Lindman B., Soderman O., Drakenberg T., Rosenholm J.B. C-13 magnetic-relaxation in micellar solutions - influence of aggregate motion on T1. J. Am. Chem. Soc. 1979;101:6860–6864. [Google Scholar]
- 82.Okamura E. Solution nmr to quantify mobility in membranes: Diffusion, protrusion, and drug transport processes. Chem. Pharm. Bull. (Tokyo) 2019;67:308–315. doi: 10.1248/cpb.c18-00946. [DOI] [PubMed] [Google Scholar]
- 83.Lepore L.S., Ellena J.F., Cafiso D.S. Comparison of the lipid acyl chain dynamics between small and large unilamellar vesicles. Biophys. J. 1992;61:767–775. doi: 10.1016/S0006-3495(92)81881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marbella L.E., Yin B.C., Spence M.M. Investigating the order parameters of saturated lipid molecules under various curvature conditions on spherical supported lipid bilayers. J. Phys. Chem. B. 2015;119:4194–4202. doi: 10.1021/jp510322t. [DOI] [PubMed] [Google Scholar]
- 85.Clore G.M., Szabo A., Bax A., Kay L.E., Driscoll P.C., Gronenborn A.M. Deviations from the Simple 2-Parameter Model-Free Approach to the Interpretation of N-15 Nuclear Magnetic-Relaxation of Proteins. J. Am. Chem. Soc. 1990;112:4989–4991. [Google Scholar]
- 86.Kay L.E., Torchia D.A., Bax A. Backbone Dynamics of Proteins as Studied by N-15 Inverse Detected Heteronuclear Nmr-Spectroscopy - Application to Staphylococcal Nuclease. Biochemistry. 1989;28:8972–8979. doi: 10.1021/bi00449a003. [DOI] [PubMed] [Google Scholar]
- 87.Halle B., Ljunggren S., Lidin S. Theory of spin relaxation in bicontinuous cubic liquid-crystals. J. Chem. Phys. 1992;97:1401–1415. [Google Scholar]
- 88.Doyle M.J., Marangoni D.G. Nuclear magnetic resonance investigation of the micellar properties of two-headed surfactant systems: The disodium 4-alkyl-3-sulfonatosuccinates. 2. The dynamics of the chains comprising the interior of two-headed surfactant micelles. Langmuir. 2004;20:2579–2583. doi: 10.1021/la034931a. [DOI] [PubMed] [Google Scholar]
- 89.Maciejewski M.W., Liu D.J., Prasad R., Wilson S.H., Mullen G.P. Backbone dynamics and refined solution structure of the N-terminal domain of DNA polymerase beta. Correlation with DNA binding and dRP lyase activity. J. Mol. Biol. 2000;296:229–253. doi: 10.1006/jmbi.1999.3455. [DOI] [PubMed] [Google Scholar]
- 90.Yao S., Keizer D.W., Babon J.J., Separovic F. NMR measurement of biomolecular translational and rotational motion for evaluating changes of protein oligomeric state in solution. Eur Biophys J Biophy. 2022;51:193–204. doi: 10.1007/s00249-022-01598-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anderson D.M., Wennerstrom H. Self-diffusion in bicontinuous cubic phases, l3 phases, and microemulsions. J. Phys. Chem. 1990;94:8683–8694. [Google Scholar]
- 92.Meikle T.G., Keizer D.W., Babon J.J., Drummond C.J., Separovic F., Conn C.E., Yao S.G. Chemical Exchange of Hydroxyl Groups in Lipidic Cubic Phases Characterized by NMR. J. Phys. Chem. B. 2021;125:571–580. doi: 10.1021/acs.jpcb.0c08699. [DOI] [PubMed] [Google Scholar]
- 93.Farrar T.C., Shoup R.R., Becker E.D., Druck S.J. Temperature-Dependent C-13 Relaxation Studies of Small Molecules. J. Am. Chem. Soc. 1972;94 699-&. [Google Scholar]
- 94.Shang X.Q., Fisher L.A., Rodriguez A.A. C-13 spin-lattice relaxation and molecular dynamics of C-60 in 1,2-dichlorobenzene-D-4. J. Phys. Chem. 1996;100:4361–4364. [Google Scholar]
- 95.Antila H.S., Wurl A., Ollila O.H.S., Miettinen M.S., Ferreira T.M. Rotational decoupling between the hydrophilic and hydrophobic regions in lipid membranes. Biophys. J. 2022;121:68–78. doi: 10.1016/j.bpj.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nevzorov A.A., Brown M.F. Dynamics of lipid bilayers from comparative analysis of H-2 and C-13 nuclear magnetic resonance relaxation data as a function of frequency and temperature. J. Chem. Phys. 1997;107:10288–10310. [Google Scholar]
- 97.Wohlert J., Edholm O. Dynamics in atomistic simulations of phospholipid membranes: Nuclear magnetic resonance relaxation rates and lateral diffusion. J. Chem. Phys. 2006:125. doi: 10.1063/1.2393240. [DOI] [PubMed] [Google Scholar]
- 98.Ferreira T.M., Ollila O.H.S., Pigliapochi R., Dabkowska A.P., Topgaard D. Model-free estimation of the effective correlation time for C-H bond reorientation in amphiphilic bilayers: H-1-C-13 solid-state NMR and MD simulations. J. Chem. Phys. 2015:142. doi: 10.1063/1.4906274. [DOI] [PubMed] [Google Scholar]
- 99.Shintani M., Yoshida K., Sakuraba S., Nakahara M., Matubayasi N. NMR-NOE and MD Simulation Study on Phospholipid Membranes: Dependence on Membrane Diameter and Multiple Time Scale Dynamics. J. Phys. Chem. B. 2011;115:9106–9115. doi: 10.1021/jp204051f. [DOI] [PubMed] [Google Scholar]
- 100.Eriksson P.O., Lindblom G. Lipid and Water Diffusion in Bicontinuous Cubic Phases Measured by Nmr. Biophys. J. 1993;64:129–136. doi: 10.1016/S0006-3495(93)81347-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Geil B., Feiweier T., Pospiech E.M., Eisenblatter J., Fujara F., Winter R. Relating structure and translational dynamics in aqueous dispersions of monoolein. Chem. Phys. Lipids. 2000;106:115–126. doi: 10.1016/s0009-3084(00)00136-5. [DOI] [PubMed] [Google Scholar]
- 102.Meikle T., Keizer D.W., Separovic F., Yao S.G. Water diffusion in complex systems measured by PGSE NMR using chemical shift selective stimulated echo: Elimination of magnetization exchange effects. J. Chem. Phys. 2021:155. doi: 10.1063/5.0073704. [DOI] [PubMed] [Google Scholar]
- 103.Yao S., Meikle T.G., Sethi A., Separovic F., Babon J.J., Keizer D.W. Measuring translational diffusion of (15)N-enriched biomolecules in complex solutions with a simplified (1)H-(15)N HMQC-filtered BEST sequence. Eur. Biophys. J. 2018;47:891–902. doi: 10.1007/s00249-018-1311-5. [DOI] [PubMed] [Google Scholar]
- 104.Wu D.H., Chen A.D., Johnson C.S. An improved diffusion-ordered spectroscopy experiment incorporating bipolar-gradient pulses. J. Magn. Reson. Ser. A. 1995;115:260–264. [Google Scholar]
- 105.Pelta M.D., Morris G.A., Stchedroff M.J., Hammond S.J. A one-shot sequence for high-resolution diffusion-ordered spectroscopy. Magn. Reson. Chem. 2002;40:S147–S152. [Google Scholar]
- 106.Wider G., Dotsch V., Wuthrich K. Self-compensating pulsed magnetic-field gradients for short recovery times. J. Magn. Reson. Ser. A. 1994;108:255–258. [Google Scholar]
- 107.Karlicek R.F., Lowe I.J. A modified pulsed gradient technique for measuring diffusion in the presence of large background gradients. J. Magn. Reson. 1980;37:75–91. [Google Scholar]
- 108.Latour L.L., Li L.M., Sotak C.H. Improved pfg stimulated-echo method for the measurement of diffusion in inhomogeneous fields. J. Magn. Reson. Ser. B. 1993;101:72–77. [Google Scholar]
- 109.Dvinskikh S.V., Furo I. Cross-relaxation effects in stimulated-echo-type PGSE NMR experiments by bipolar and monopolar gradient pulses. J. Magn. Reson. 2000;146:283–289. doi: 10.1006/jmre.2000.2158. [DOI] [PubMed] [Google Scholar]
- 110.Chou J.J., Baber J.L., Bax A. Characterization of phospholipid mixed micelles by translational diffusion. J. Biomol. NMR. 2004;29:299–308. doi: 10.1023/B:JNMR.0000032560.43738.6a. [DOI] [PubMed] [Google Scholar]
- 111.Chen A.D., Johnson C.S., Lin M., Shapiro M.J. Chemical exchange in diffusion NMR experiments. J. Am. Chem. Soc. 1998;120:9094–9095. [Google Scholar]
- 112.Jerschow A., Muller N. Suppression of convection artifacts in stimulated-echo diffusion experiments. Double-stimulated-echo experiments. J. Magn. Reson. 1997;125:372–375. [Google Scholar]
- 113.Jerschow A., Muller N. Convection compensation in gradient enhanced nuclear magnetic resonance spectroscopy. J. Magn. Reson. 1998;132:13–18. [Google Scholar]
- 114.Clogston J., Caffrey M. Controlling release from the lipidic cubic phase. Amino acids, peptides, proteins and nucleic acids. J Control Release. 2005;107:97–111. doi: 10.1016/j.jconrel.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 115.Negrini R., Sanchez-Ferrer A., Mezzenga R. Influence of Electrostatic Interactions on the Release, of Charged Molecules from Lipid Cubic Phases. Langmuir. 2014;30:4280–4288. doi: 10.1021/la5008439. [DOI] [PubMed] [Google Scholar]
- 116.Kulkarni C.V., Vishwapathi V.K., Quarshie A., Moinuddin Z., Page J., Kendrekar P., Mashele S.S. Self-Assembled Lipid Cubic Phase and Cubosomes for the Delivery of Aspirin as a Model Drug. Langmuir. 2017;33:9907–9915. doi: 10.1021/acs.langmuir.7b02486. [DOI] [PubMed] [Google Scholar]
- 117.Ghanbari R., Assenza S., Zueblin P., Mezzenga R. Impact of Molecular Partitioning and Partial Equilibration on the Estimation of Diffusion Coefficients from Release Experiments. Langmuir. 2019;35:5663–5671. doi: 10.1021/acs.langmuir.9b00510. [DOI] [PubMed] [Google Scholar]
- 118.Jeong S.W., O'Brien D.F., Oradd G., Lindblom G. Encapsulation and diffusion of water-soluble dendrimers in a bicontinuous cubic phase. Langmuir. 2002;18:1073–1076. [Google Scholar]
- 119.Larkin T.J., Garvey C.J., Shishmarev D., Kuchel P.W., Momot K.I. Na+ and solute diffusion in aqueous channels of Myverol bicontinuous cubic phase: PGSE NMR and computer modelling. Magn. Reson. Chem. 2017;55:464–471. doi: 10.1002/mrc.4432. [DOI] [PubMed] [Google Scholar]
- 120.Momot K.I., Kuchel P.W., Whittaker D. Enhancement of Na+ diffusion in a bicontinuous cubic phase by the ionophore monensin. Langmuir. 2004;20:2660–2666. doi: 10.1021/la0362371. [DOI] [PubMed] [Google Scholar]
- 121.Nazaruk E., Miszta P., Filipek S., Gorecka E., Landau E.M., Bilewicz R. Lyotropic Cubic Phases for Drug Delivery: Diffusion and Sustained Release from the Mesophase Evaluated by Electrochemical Methods. Langmuir. 2015;31:12753–12761. doi: 10.1021/acs.langmuir.5b03247. [DOI] [PubMed] [Google Scholar]
- 122.Higuchi T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961;50:874–875. doi: 10.1002/jps.2600501018. [DOI] [PubMed] [Google Scholar]
- 123.Franconi F., Lemaire L., Gimel J.C., Bonnet S., Saulnier P., diffusometry NMR. A new perspective for nanomedicine exploration. J Control Release. 2021;337:155–167. doi: 10.1016/j.jconrel.2021.07.025. [DOI] [PubMed] [Google Scholar]
- 124.Caffrey M. Crystallizing membrane proteins for structure determination: use of lipidic mesophases. Annu. Rev. Biophys. 2009;38:29–51. doi: 10.1146/annurev.biophys.050708.133655. [DOI] [PubMed] [Google Scholar]
- 125.Mulet X., Boyd B.J., Drummond C.J. Advances in drug delivery and medical imaging using colloidal lyotropic liquid crystalline dispersions. J Colloid Interf Sci. 2013;393:1–20. doi: 10.1016/j.jcis.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 126.Nucci N.V., Pometun M.S., Wand A.J. Site-resolved measurement of water-protein interactions by solution NMR. Nat. Struct. Mol. Biol. 2011;18:245–249. doi: 10.1038/nsmb.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van 't Hag L., Darmanin C., Le T.C., Mudie S., Conn C.E., Drummond C.J. In Meso Crystallization: Compatibility of Different Lipid Bicontinuous Cubic Mesophases with the Cubic Crystallization Screen in Aqueous Solution. Cryst. Growth Des. 2014;14:1771–1781. [Google Scholar]
- 128.Cherezov V., Fersi H., Caffrey M. Crystallization screens: Compatibility with the lipidic cubic phase for in meso crystallization of membrane proteins. Biophys. J. 2001;81:225–242. doi: 10.1016/S0006-3495(01)75694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Conn C.E., Darmanin C., Mulet X., Hawley A., Drummond C.J. Effect of lipid architecture on cubic phase susceptibility to crystallisation screens. Soft Matter. 2012;8:6884–6896. [Google Scholar]
- 130.Darmanin C., Conn C.E., Newman J., Mulet X., Seabrook S.A., Liang Y.L., Hawley A., Kirby N., Varghese J.N., Drummond C.J. High-Throughput Production and Structural Characterization of Libraries of Self-Assembly Lipidic Cubic Phase Materials. Acs. Comb. Sci. 2012;14:247–252. doi: 10.1021/co2001718. [DOI] [PubMed] [Google Scholar]
- 131.Sarkar S., Tran N., Rashid M.H., Le T.C., Yarovsky I., Conn C.E., Drummond C.J. Toward cell membrane biomimetic lipidic cubic phases: a high-throughput exploration of lipid compositional space. Acs Applied Bio Materials. 2019;2:182–195. doi: 10.1021/acsabm.8b00539. [DOI] [PubMed] [Google Scholar]
- 132.van t'Hag L., Gras S.L., Conn C.E., Drummond C.J. Lyotropic liquid crystal engineering moving beyond binary compositional space - ordered nanostructured amphiphile self-assembly materials by design. Chem. Soc. Rev. 2017;46:2705–2731. doi: 10.1039/c6cs00663a. [DOI] [PubMed] [Google Scholar]
- 133.Serebryany E., Zhu G.A., Yan E.C.Y. Artificial membrane-like environments for in vitro studies of purified G-protein coupled receptors. Biochimica Et Biophysica Acta-Biomembranes. 2012;1818:225–233. doi: 10.1016/j.bbamem.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 134.Imperor-Clerc M. Thermotropic cubic mesophases. Curr. Opin. Colloid Interface Sci. 2005;9:370–376. [Google Scholar]
- 135.Iskandar W., Salim M., Patrick M., Timimi B.A., Zahid N.I., Hashim R. Probing n-Octyl alpha-D-Glycosides Using Deuterated Water in the Lyotropic Phase by Deuterium NMR. J. Phys. Chem. B. 2021;125:4393–4408. doi: 10.1021/acs.jpcb.0c10629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.