Highlights
-
•
A comprehensive, integrated clinical trial was beneficial for understanding the roles of the Fascin family across cancers.
-
•
Several noncanonical Fascin-1 functions have been associated with cancer progression.
-
•
A systematic molecular mechanism illustrates how tumor cells interact with their microenvironment through Fascin-1.
-
•
The potential treatment of Fascin-1 is beneficial for developing translational medicines to inhibit the progression of cancer.
Keywords: FSCN, Fascin, Cancer, Bioinformatics
Abstract
Filopodia are cellular protrusions that respond to a variety of stimuli. Filopodia are formed when actin is bound to the protein Fascin, which may play a crucial role in cellular interactions and motility during cancer metastasis. Significantly, the noncanonical features of Fascin-1 are gradually being clarified, including the related molecular network contributing to metabolic reprogramming, chemotherapy resistance, stemness ac-tivity, and tumor microenvironment events. However, the relationship between biological characteristics and pathological features to identify effective therapeutic strategies needs to be studied further. The pur-pose of this review article is to provide a broad overview of the latest molecular networks and multiomics research regarding fascins and cancer. It also highlights their direct and indirect effects on available cancer treatments. With this multidisciplinary approach, researchers and clinicians can gain the most relevant in-formation on the function of fascins in cancer progression, which may facilitate clinical applications in the future.
Graphical abstract
1. Introduction
Cellular plasticity makes cells respond to extracellular stimuli. This is accompanied by morphological changes to interactions or motility, which are involved in complex signal transduction to remodel the cytoskeleton. To attain such changes, cells rearrange actin filaments by actin-bundling proteins. For example, Fascin proteins, approximately 55–58 kDa, have been widely regarded as the molecules most involved in the regulation of the cytoskeleton. The first complete characterization was in 1979. It was discovered to be related to the morphology of filopodia formation in the sea urchin, opening up research into exploring the relationship between Fascin and the molecular mechanism of action of cells [1]. The protein is mainly composed of four β-trefoil structure loops connected, which exposes the F-actin-binding site. The crosslinking between Fascin and f-actin consequently changes cell morphology into filopodia, lamellipodia, stress fibers, and microspike structures, causing cells to undergo motility processes. Cellular migration is involved in multiple biological events, including embryogenesis, wound healing, and carcinogenesis. In cancer, migration and invasion have been defined as hallmarks that cause the patient to be diagnosed at an advanced stage with more rapid progression. The critical role of Fascin-1 has been recognized to contribute to cancer progression. However, most of the research is mainly based on Fascin-1, and other members, including Fascin-2 and Fascin-3, which are linked to pathological features, remain largely unknown. Therefore, this review summarizes the current research related to Fascin-1, enriched with omics-related data, to redefine their significance in cancer.
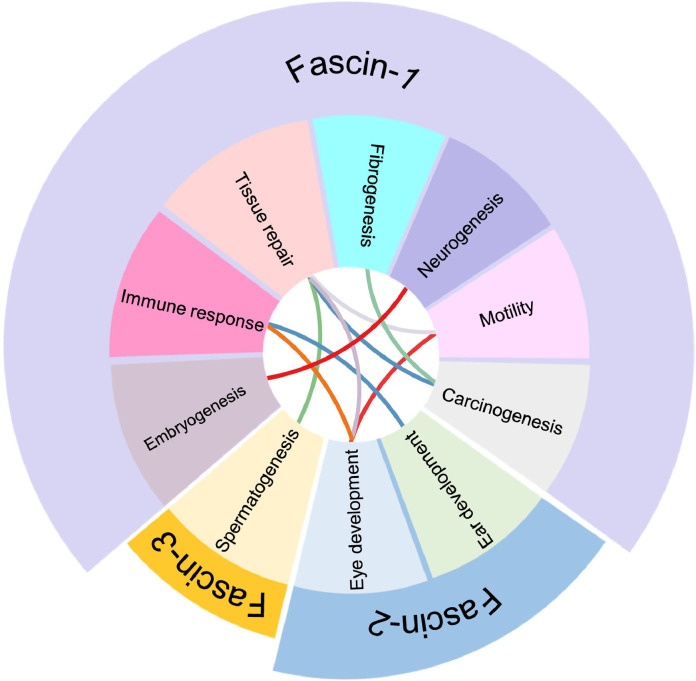
Currently, three members are classified by their relative sequence in the Fascin family. The related protein sequences between fascins revealed their possible evolutionary lineage relationship and diverse protein motif consensus sequence, including the beta-strand, helix, modified residue, turn, and similarity in humans (Fig. 1). They even share a similar protein sequence. However, the distribution and relative biology were distinct from each other. Based on the RNA and protein expression, Fascin-1 participates in more biological functions in the human body (Fig. 2). According to the distribution of Fascin-1 in organs, it is predictably involved in multiple biological functions.
Fig. 1.
Evolution lineage of the Fascin family.
Information on the Fascin family was generated from the UniProt website, and the consensus sequence or diverse protein motifs are highlighted with different colors.
Fig. 2.
The relative distribution of the Fascin family in human organs.
The relative Fascin 1-3 distributions are highlighted with different colors, including RNA and protein levels in human organs. Levels of RNA and protein were provided from the human protein atlas datasets and organized according to the related location. NX: Normalized expression, N/A: not applicable.
1.1. Fascin-1
1.1.1. Embryogenesis
During embryogenesis, oocyte meiotic maturation was associated with the interaction of FNNL3 and Fascin-1 [2,3]. A similar phenomenon was observed in endoderm formation, which can be regulated by the interaction of the TGF-β receptor and Fascin-1 to control receptor trafficking into early endosomes [4]. During neurogenesis, the mRNA expression of Fascin-1 can be observed to increase and is governed by CREB-binding protein and retinoic acid [5]. The related expression of Fascin-1 was associated with spermatid maturation, morphology, and motility [6,7]. A similar association was found in oral dysplasia and linked to carcinoma in situ [8]. In addition, the self-renewal ability of hematopoietic stem cells can be regulated by the TSC1/2-Fascin-1 axis [9]. Interestingly, the level of Fascin-1 is correlated with skeletogenesis and can serve as a meat production trait [10,11]. Most importantly, Fascin-1 contributes to hepatoblast and neuroblast migration into the proper location to form the liver and interneurons in the olfactory bulb [12,13].
1.1.2. Tissue repair
Fascin-1 is difficult to detect in normal colon tissues, and excessive Fascin-1 may be seen in inflammatory bowel disease tissues, which may be due to tissue injury or repair [14]. Wang et al. observed that the IL-6-mediated AKT/GSK 3β/β-catenin/Fascin-1 axis drove cell migration to remodel or repair the airway [15]. Therefore, targeting Fascin-1 may provide alternative strategies for asthma. A similar phenomenon was observed in spinal cord injury [16]. Miao et al. provided a possible mechanism in which SOX4 and SOX11 can reactivate Fascin-1 to undergo an epithelial and mesenchymal transition (EMT) to participate in the wound repair process [17].
1.1.3. Fibrogenesis
Such processes can be observed in tissue fibrosis. Zhang and Fu et al. observed that Fascin-1 participated in fibrogenesis processes and was regulated by miR-145-5p or miR-200b/c [18,19].
1.1.4. Motility
In addition, the phosphorylation of Fascin-1 at serine 39 can be induced by IL-17A, resulting in airway smooth muscle contraction [20].
1.1.5. Immune response
Moreover, Fascin-1 can serve as an immune response factor. The related expression of Fascin-1 was correlated with the level of autoimmune neuritis [21]. For example, the dendritic cell level is considered an inductor of allograft rejection when patients undergo transplantation. Yang et al. found that Fascin-1 presented in dendritic cells may contribute to related events [21] and may be used as a diagnostic indicator for patients treated with immunosuppression inhibitors [22]. A similar incidence of dendritic cells can be observed in allogeneic hematopoietic stem cell transplantation [23]. Targeting circFSCN1 can prevent alloimmune rejection in heart transplantations [24]. Conversely, Staphylococcus aureus-induced mastitis can be prevented by restoring Fascin-1 [25]. Moreover, the expression of Fascin-1 in dendritic cells responded to virus-infected events, such as human immunodeficiency virus-related lymphoid hyperplasias [26]. Interestingly, dendritic cell activity can be restricted by regulatory T cells through Fascin-1 [27].
1.1.6. Carcinogenesis
Most importantly, the expression of Fascin-1 in dendritic cells can serve as a distinguishing marker for Epstein–Barr virus-mediated Hodgkin's disease [28,29] and may contribute to its pathogenesis [30]. A similar phenomenon was observed in nasal inverted papilloma [31], which indicates that Fascin-1 links diverse diseases, including cancer progression.
1.2. Fascin-2
Fascin-2 has been found to be related to the functions of the ears and eyes.
1.2.1. Eye development
Fascin-2 has been found to be related to the functions of the ears and eyes. The genetic heterogeneity associated with retinitis pigmentosa, such as autosomal dominant retinitis pigmentosa and macular dystrophy, may be linked to mutations in Fascin-2 [32,33]. In a murine model, the 208delG/R109H mutation in Fascin-2 was positively linked to progressive photoreceptor degeneration [34]. Mechanistically, the phosphorylation of serine 39 can stabilize the actin cytoskeleton in the inner segment, consequently regulating photoreceptor disk morphogenesis [35].
1.2.2. Development
In addition, Lv et al. found that premRNA splicing factors may control the level of Fascin-2, causing retinitis pigmentosa [36]. In addition, mutation of Fascin-2 contributes to age-related hearing loss [37], [38], [39]. Such mutation can induce caspase-3-mediated apoptosis, resulting in progressive hair cell loss and spiral ganglion neuron degeneration [40].
1.3. Fascin-3
1.3.1. Spermatogenesis
Currently, testis-specific expressed Fascin-3 was found to be related to spermatogenesis and microfilament rearrangements [41]. It has also been demonstrated that Fascin-3-mediated spermatogenesis can be regulated by vitamin E [42], and treatment with methotrexate and platelet-rich plasma can upregulate the level of Fascin-3, which may be linked to the regeneration of testicular tissue [43].
As described above, the Fascin family plays an essential role from embryogenesis to disease development, in which Fascin-1 contributes to diverse biological processes after embryogenesis. However, Fascin-2 and Fascin-3 were assumed to only function during embryogenesis. Therefore, most studies have focused on Fascin-1. However, based on the Fascin family's biological distribution, they may partially share similar functions with each other (Fig. 3). Such hypotheses may be supported by Deng et al., who found that Fascin-1 and Fascin-2 were correlated with favorable overall survival in multiple myeloma, which indicated that the submembers might compensate for the functions [44]. Therefore, the unknown molecular interaction between the Fascin family may provide a possible niche and valuable biological processes that should be explored, especially in cancer biology.
Fig. 3.
Current understanding of the biological functions within the Fascin family.
The related biological functions of each Fascin submember were organized based on the works cited in the literature.
1.4. Fascin-1: Current understanding of the molecular network in cancers
The Cancer Genome Atlas Program datasets have been used to correlate cancer prognosis and provide a comparable analysis between family members. The correlation of Fascin and patient expression and overall survival rates across cancers are listed in Table 1, which provides insight into cancer development. Based on these correlations, it is suggested that Fascin-2 and Fascin-3 might be linked to cancer progression and worth exploring. However, no specific study has explored the relationship between them and cancer. Until now, Fascin-1 has been identified as being linked to cancer progression (Table 2). Therefore, the updated molecular networks of Fascin-1 that contribute to cancer are detailed in the following sections.
Table 1.
The relationship between the overall survival rate and FSCN family.
| FSCN1 |
FSCN2 |
FSCN3 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer type | Endpoint | Case | Cohort | p-value | Log-rank test statistics | Risk | p-value | Log-rank test statistics | Risk | p-value | Log-rank test statistics | Risk |
| Acute Myeloid Leukemia (LAML) | Overall survival | 161 | TCGA | 0.8556 | 0.03311 | High | 0.7654 | 0.08902 | High | 0.9216 | 0.009675 | Low |
| Adrenocortical Cancer (ACC) | Overall survival | 79 | TCGA | 0.000008006 | 19.94 | High | 0.01814 | 5.582 | High | 0.8998 | 0.01586 | High |
| Bile Duct Cancer (CHOL) | Overall survival | 45 | TCGA | 0.8954 | 0.01729 | High | 0.01858 | 5.541 | Low | 0.4946 | 0.4665 | High |
| Bladder Cancer (BLCA) | Overall survival | 425 | TCGA | 0.08484 | 2.97 | High | 0.7967 | 0.06637 | Low | 0.7141 | 0.1342 | Low |
| Breast Cancer (BRCA) | Overall survival | 1214 | TCGA | 0.7528 | 0.09919 | Low | 0.0003311 | 12.89 | Low | 0.6949 | 0.1538 | High |
| Cervical Cancer (CESC) | Overall survival | 307 | TCGA | 0.09286 | 2.824 | High | 0.7861 | 0.07362 | High | 0.234 | 1.416 | High |
| Colon and Rectal Cancer (COADREAD) | Overall survival | 250 | TCGA | 0.07314 | 3.211 | High | 0.0426 | 4.111 | High | 0.5161 | 0.4217 | High |
| Colon Cancer (COAD) | Overall survival | 326 | TCGA | 0.0301 | 4.704 | High | 0.03691 | 4.354 | High | 0.178 | 1.814 | High |
| Endometrioid Cancer (UCEC) | Overall survival | 189 | TCGA | 0.2443 | 1.356 | High | 0.1384 | 2.196 | High | 0.5209 | 0.4121 | High |
| Esophageal Cancer (ESCA) | Overall survival | 196 | TCGA | 0.8227 | 0.0502 | High | 0.5332 | 0.3883 | High | 0.8606 | 0.03085 | Low |
| Glioblastoma (GBM) | Overall survival | 166 | TCGA | 0.9675 | 0.001656 | Low | 0.2284 | 1.451 | High | 0.9988 | 0.000002288 | High |
| Head and Neck Cancer (HNSC) | Overall survival | 565 | TCGA | 0.0313 | 4.637 | High | 0.8648 | 0.02898 | High | 0.7678 | 0.08714 | High |
| Kidney Chromophobe (KICH) | Overall survival | 89 | TCGA | 0.3003 | 1.073 | Low | 0.5292 | 0.3959 | Low | 0.6487 | 0.2075 | Low |
| Kidney Clear Cell Carcinoma (KIRC) | Overall survival | 606 | TCGA | 0.03181 | 4.609 | High | 0.006612 | 7.375 | High | 0.0253 | 5.003 | High |
| Kidney Papillary Cell Carcinoma (KIRP) | Overall survival | 322 | TCGA | 0.3914 | 0.7346 | Low | 0.4047 | 0.6944 | Low | 0.7855 | 0.0741 | Low |
| Large B-cell Lymphoma (DLBC) | Overall survival | 48 | TCGA | 0.3779 | 0.7775 | Low | 0.3381 | 0.9175 | High | 0.6996 | 0.1489 | Low |
| Liver Cancer (LIHC) | Overall survival | 422 | TCGA | 0.1047 | 2.633 | High | 0.4772 | 0.5053 | High | 0.3422 | 0.902 | High |
| Lower Grade Glioma (LGG) | Overall survival | 528 | TCGA | 0.07948 | 3.075 | High | 0.001565 | 10 | High | 0.9924 | 0.00008962 | Low |
| lower grade glioma and glioblastoma (GBMLGG) | Overall survival | 694 | TCGA | 0.00E+00 | 42.8 | High | 0.00E+00 | 39.32 | High | 0.00E+00 | 35.64 | High |
| Lung Adenocarcinoma (LUAD) | Overall survival | 567 | TCGA | 0.004941 | 7.901 | High | 0.3183 | 0.9959 | Low | 0.003331 | 8.617 | Low |
| Lung Cancer (LUNG) | Overall survival | 1062 | TCGA | 0.005903 | 7.58 | High | 0.7667 | 0.08801 | High | 0.1034 | 2.653 | Low |
| Lung Squamous Cell Carcinoma (LUSC) | Overall survival | 495 | TCGA | 0.9843 | 0.0003853 | Low | 0.2124 | 1.555 | High | 0.9689 | 0.001519 | High |
| Melanoma (SKCM) | Overall survival | 457 | TCGA | 0.2627 | 1.255 | Low | 0.002391 | 9.222 | Low | 0.0715 | 3.248 | High |
| Mesothelioma (MESO) | Overall survival | 86 | TCGA | 0.00007813 | 15.6 | High | 0.4645 | 0.535 | High | 0.699 | 0.1496 | Low |
| Ocular melanomas (UVM) | Overall survival | 80 | TCGA | 0.9311 | 0.007473 | High | 0.3393 | 0.913 | High | 0.5495 | 0.3582 | Low |
| Ovarian Cancer (OV) | Overall survival | 307 | TCGA | 0.2355 | 1.408 | High | 0.1991 | 1.649 | High | 0.8905 | 0.01894 | Low |
| Pancreatic Cancer (PAAD) | Overall survival | 183 | TCGA | 0.06491 | 3.407 | High | 0.06578 | 3.385 | Low | 0.01428 | 6.004 | Low |
| Pheochromocytoma & Paraganglioma (PCPG) | Overall survival | 187 | TCGA | 0.07147 | 3.249 | Low | 0.01778 | 5.618 | Low | 0.5271 | 0.4 | Low |
| Prostate Cancer (PRAD) | Overall survival | 550 | TCGA | 0.9567 | 0.00295 | High | 0.3856 | 0.7528 | Low | 0.4765 | 0.5068 | high |
| Rectal Cancer (READ) | Overall survival | 58 | TCGA | 0.8169 | 0.05361 | Low | 0.2931 | 1.105 | High | 0.3266 | 0.9625 | Low |
| Sarcoma (SARC) | Overall survival | 265 | TCGA | 0.01546 | 5.864 | High | 0.08581 | 2.951 | Low | 0.01136 | 6.408 | Low |
| Stomach Cancer (STAD) | Overall survival | 443 | TCGA | 0.338 | 0.9179 | High | 0.7561 | 0.0965 | High | 0.8238 | 0.04955 | high |
| Testicular Cancer (TGCT) | Overall survival | 139 | TCGA | 0.5686 | 0.3249 | Low | 0.4313 | 0.6193 | High | 0.0644 | 3.42 | Low |
| Thymoma (THYM) | Overall survival | 121 | TCGA | 0.09627 | 2.766 | High | 0.4999 | 0.4551 | High | 0.3964 | 0.7191 | Low |
| Thyroid Cancer (THCA) | Overall survival | 572 | TCGA | 0.1477 | 2.096 | High | 0.2651 | 1.242 | High | 0.7129 | 0.1354 | high |
| Uterine Carcinosarcoma (UCS) | Overall survival | 57 | TCGA | 0.2991 | 1.078 | Low | 0.474 | 0.5126 | Low | 0.346 | 0.8879 | Low |
The correlation between the Fascin family and patients with cancer is listed based on “The Cancer Genome Atlas Program” generated from the XENA websites.
Table 2.
Related Fascin-1 studies in cancers.
| Cancer type | FSCNs expression | Biological relevance | Model | Year | Author | Reference |
|---|---|---|---|---|---|---|
| Breast cancer | FSCN1 | Diagnosis biomarker | Human Specimens | 2000 | A Grothey | [45] |
| Associated with tumors motility and malignancy | MDA-MB-435 | 2000 | A Grothey | [46] | ||
| Insulin-like growth factor-I receptor-induced Fascin projections | Cell lines (HMEC, BT474, MDA-MB-231, MCF-7) | 2000 | Marina A Guvakova | [47] | ||
| Diagnosis biomarker | Human Specimens | 2005 | Brian J Yoder | [48] | ||
| Diagnosis biomarker | Human Specimens | 2006 | Socorro María Rodríguez-Pinilla | [49] | ||
| Diagnosis biomarker | Human Specimens | 2010 | Y Zhang | [50] | ||
| Regulation of tumor cell proliferation and motility | Cell lines (MDA-MB-435, MDA-MB-231, T-47D) | 2011 | Peng Xing | [51] | ||
| Regulation of tumor cell motility | Human Specimens, Cell lines (MDA-MB-23, T-47D) | 2011 | Monther Al-Alwan | [52] | ||
| Diagnosis biomarker | Human Specimens | 2014 | Ashwini K Esnakula | [53] | ||
| Diagnosis biomarker | Human Specimens | 2014 | Nermeen Salah Youssef | [54] | ||
| Chemoresistant | Cell lines (MDA-MB-231, SK-BR-3, and T-47D) | 2014 | H Ghebeh | [55] | ||
| Regulation of tumor cell motility | Cell lines (MDA-MB-231) | 2014 | Marylynn Snyder | [56] | ||
| Diagnosis biomarker | Human Specimens | 2015 | Kyueng-Whan Min | [57] | ||
| Diagnosis biomarker | Human Specimens | 2015 | Ola M Omran | [58] | ||
| Diagnosis biomarker | Human Specimens | 2016 | Chao-Qun Wang | [59] | ||
| Stemness activity | Cell lines (MDA-MB-231, T-47D) | 2016 | Rayanah Barnawi | [60] | ||
| Regulation of tumor cell motility | Human Specimens, Cell lines (MDA-MB-231, MDA-MB-468, and MCF-7) | 2017 | Chao-Qun Wang | [61] | ||
| Diagnosis biomarker | Human Specimens | 2017 | Hye Jin Lee | [62] | ||
| Regulation of tumor cell metastasis | Cell lines (MDA-MB-231) | 2017 | Lisa S Heinz | [63] | ||
| Diagnosis biomarker | Human Specimens | 2019 | Ata Abbasi | [64] | ||
| Diagnosis biomarker | Human Specimens | 2019 | Ekaterini Christina Tampaki | [65] | ||
| Stemness activiry | Cell lines (MDA-MB-231, T-47D) | 2020 | Rayanah Barnawi | [66] | ||
| Ovarian cancer | FSCN1 | Diagnosis biomarker | Patient-derived cells, Human tissues | 2000 | W Hu | [67] |
| Diagnosis biomarker | Human Specimens | 2008 | Alexandros Daponte | [68] | ||
| Diagnosis biomarker | Human Specimens | 2008 | Chih-kung Lin | [69] | ||
| Diagnosis biomarker | Human Specimens | 2009 | Chih-Kung Lin | [69] | ||
| Diagnosis biomarker | Human Specimens | 2013 | Lars C Hanker | [69] | ||
| Regulation of tumor cell metastasis | Human Specimens, Cell lines (HeyA8, Ovcar5, Tyk-nu, Snu119, and Kuramochi) | 2019 | Sean McGuire | [70] | ||
| Regulation of tumor cell motility | Cell lines (ES-2, SK-OV-3 and NOE) | 2020 | Masato Yoshihara | [71] | ||
| Pancreatic cancer | FSCN1 | Diagnosis biomarker | Human Specimens | 2002 | Anirban Maitra | [72] |
| Diagnosis biomarker | Human Specimens | 2004 | Sharon L Swierczynski | [73] | ||
| Regulation of tumor cell motility | Cell lines (BxPC-3, MIAPaCa-2,AsPC-, PC-1, PC-4 and PC-7) | 2011 | Yan-Feng Xu | [74] | ||
| Diagnosis biomarker | Human Specimens | 2013 | Wen-Chiuan Tsai | [75] | ||
| Regulation of tumor cell metastasis | Human Specimens, PDAC mice model | 2014 | Ang Li | [76] | ||
| Regulation of tumor cell metastasis | Human Specimens, Cell lines (MiaPaCa-2, Aspc-1) | 2014 | Xiao Zhao | [77] | ||
| Diagnosis biomarker | Human Specimens | 2020 | Magdalena Misiura | [78] | ||
| Skin neoplasia | FSCN1 | Diagnosis biomarker | Human Specimens | 2002 | Viktor N Goncharuk | [79] |
| Lung cancer | FSCN1 | Diagnosis biomarker | Human Specimens | 2003 | G Pelosi | [80] |
| Diagnosis biomarker | Human Specimens | 2013 | Yu Teng | [81] | ||
| Diagnosis biomarker | Human Specimens | 2015 | Xiao-Ling Ling | [82] | ||
| Diagnosis biomarker | Human Specimens | 2015 | Aihua Luo | [83] | ||
| Diagnosis biomarker | Human Specimens | 2015 | Wei Zhao | [84] | ||
| Regulation of tumor cell growth and motility | Cell lines (A549, SPC-A-1) | 2016 | Zhigang Liang | [85] | ||
| Diagnosis biomarker | Human Specimens | 2016 | Juanjuan Zhang | [86] | ||
| Diagnosis biomarker | Human Specimens | 2017 | L Yang | [87] | ||
| Regulation of tumor cell motility | Cell lines (A549, H520) | 2018 | Da Zhao | [88] | ||
| Diagnosis biomarker | Human Specimens | 2018 | Yunxia Zhang | [89] | ||
| Regulation of tumor cell metastasis and recurrence | Human Specimens, Cell lines (H1650, H23, H292, LLC) mice animal model |
2019 | Shengchen Lin | [90] | ||
| Diagnosis biomarker | Human Specimens | 2020 | E S Kolegova | [91] | ||
| Regulation of tumor cell metastasis | Human Specimens, Cell lines (H1650, A549, H292, H23, LLC) | 2021 | Shengchen Lin | [92] | ||
| Cholangiocarcinoma | FSCN1 | Diagnosis biomarker | Human Specimens | 2004 | Sharon L Swierczynski | [73] |
| Diagnosis biomarker | Human Specimens | 2007 | Kohji Okada | [93] | ||
| Diagnosis biomarker | Human Specimens | 2009 | Young Hoon Roh | [94] | ||
| Diagnosis biomarker | Human Specimens | 2009 | Kyu Yeoun Won | [95] | ||
| Gastric cancer | FSCN1 | Diagnosis biomarker | Human Specimens | 2004 | Yosuke Hashimoto | [96] |
| Diagnosis biomarker | Human Specimens | 2007 | Wen-Chiuan Tsai | [97] | ||
| Regulation of tumor cell motility | Human Specimens, Cell lines (MKN-28) | 2010 | Seok-Jun Kim | [98] | ||
| Diagnosis biomarker | Human Specimens | 2013 | Hidetaka Yamamoto | [99] | ||
| Diagnosis biomarker | Human Specimens | 2012 | Su Jin Kim | [100] | ||
| Regulation of tumor cell motility | Cell lines (GES-1, MKN45, MKN28, BGC823, AGS and SGC7901) | 2014 | Jun Yao | [101] | ||
| Regulation of tumor cell growth and motility | (HGC-27, MGC-803, MKN-25 and SGC-7901 | 2014 | Lihua Guo | [102] | ||
| Regulation of tumor cell motility | AGS, MNK-45) | 2015 | Yunshan Yang | [103] | ||
| Diagnosis biomarker | In-silico analysis | 2017 | Hua-Chuan Zheng | [104] | ||
| Diagnosis biomarker | Human Specimens | 2018 | Byoung Kwan Son | [105] | ||
| Esophageal Cancer | FSCN1 | Diagnosis biomarker | Human Specimens, Cell lines (EC109, EC18, EC171, EC8712 and SHEEC) | 2006 | H Zhang | [106] |
| Diagnosis biomarker | Human Specimens | 2010 | Li-yan Xue | [107] | ||
| Diagnosis biomarker | Human Specimens | 2017 | Wen-Xia Chen | [108] | ||
| Regulation of tumor cell growth and motility | Cell lines (KYSE150, KYSE180) | 2017 | Fa-Min Zeng | [109] | ||
| Kidney cancer | FSCN1 | Diagnosis biomarker | Human Specimens | 2006 | Richard Zigeuner | [110] |
| Adrenocortical carcinoma | FSCN1 | Diagnosis biomarker | Human Specimens | 2015 | Giada Poli | [111] |
| Regulation of tumor cell growth and motility | Human Specimens, Cell lines (H295R) | 2019 | Giada Poli | [112] | ||
| Colon cancer | FSCN1 | Diagnosis biomarker | Human Specimens | 2006 | Yosuke Hashimoto | [113] |
| Regulation of tumor cell motility | Human Specimens, Cell lines (HT29, SW480, HCT116, CT26) | 2007 | Danijela Vignjevic | [114] | ||
| Regulation of tumor cell motility | Cell lines (SW480, SW1222, and DLD-1), subcutaneous xenograft model | 2007 | Yosuke Hashimoto | [115] | ||
| Diagnosis biomarker | Human Specimens | 2010 | Charles Chan | [116] | ||
| Diagnosis biomarker | Human Specimens | 2012 | Seung Yeop Oh | [117] | ||
| Diagnosis biomarker | Human Specimens | 2011 | Eun-Joo Jung | [118] | ||
| Diagnosis biomarker | Human Specimens | 2013 | Pablo Conesa-Zamora | [119] | ||
| Regulation of tumor growth and metastasis | Cell lines (SW480, HT29, LS174T, SW620, and LoVo) | 2014 | Y Feng | [120] | ||
| Regulation of tumor cell growth and motility | Transgenic mice | 2014 | Marie Schoumacher | [121] | ||
| Diagnosis biomarker | Human Specimens | 2015 | Josephine C Adams | [122] | ||
| Diagnosis biomarker | Human Specimens | 2018 | Barbara M Piskor | [123] | ||
| Diagnosis biomarker | Human Specimens | 2020 | Chao-Qun Wang | [124] | ||
| Diagnosis biomarker | In-silico analysis | 2020 | Shuai Shi | [125] | ||
| Diagnosis biomarker | Human Specimens | 2021 | Athanasios Tampakis | [126] | ||
| Prostate cancer | FSCN1 | Regulation of tumor cell motility | Human Specimens, Cell lines (DU145), Orthotopic prostate xenograft models |
2009 | Andrew D Darnel | [127] |
| Diagnosis biomarker | Human Specimens | 2014 | Daniel C Dim | [128] | ||
| Diagnosis biomarker | Human Specimens | 2017 | Matthew T Jefferies | [129] | ||
| Oral cancer | FSCN1 | Regulation of tumor cell motility | Cell lines (OECM-1, SCC-25) | 2009 | Su-Feng Chen | [130] |
| proteases activity and tumor cell motility | Cell lines (YD-10B, HSC-2, HSC-3, Ca9.22 OSCC) | 2018 | Min Kyeong Lee | [131] | ||
| Melanoma | FSCN1 | Regulation of tumor cell motility | Cell lines (A375MM, CHL-1, WM266.4, MV3) | 2010 | Ang Li | [132] |
| Regulation of tumor cell growth and motility | Fascin1 -/- mice | 2013 | Yafeng Ma | [133] | ||
| Regulation of tumor angiogenesis | Cell lines (B16F0) | 2013 | Yafeng Ma | [134] | ||
| Stemness activity | Cell lines (WM793, WM39) | 2018 | Jiaxin Kang | [135] | ||
| Regulation of tumor cell growth | Cell lines (WM793), subcutaneous xenograft model | 2021 | Byung-Soo Kang | [136] | ||
| FSCN family | Diagnosis biomarker | In-silico analysis | 2021 | Cong Deng | [44] | |
| Bladder cancer | FSCN1 | Regulation of tumor cell viability and motility | Human Specimens, Cell lines (BOY, T24m KK47) | 2010 | T Chiyomaru | [137] |
| Diagnosis biomarker | Human Specimens | 2013 | Mohamed Abd El-Rehim | [138] | ||
| Lymphoma | FSCN1 | Diagnosis biomarker | Human Peripheral blood mononuclear cells | 2011 | Andrea K Kress | [139] |
| Regulation of tumor cell motility | Cell lines (LCL-B, LCL-721, LCLs LCL-3 and LCL-4) | 2014 | Caroline F Mohr | [140] | ||
| Leukemia | FSCN1 | Diagnosis biomarker | Human Specimens | 2018 | Nabila El Kramani | [141] |
| Liver cancer | FSCN1 | Regulation of tumor cell viability and motility | Human Specimens, Cell lines (HLE, Hep3B, Huh7) | 2011 | Yoshihiro Hayashi | [142] |
| Diagnosis biomarker | Human Specimens | 2011 | Chih-Kung Lin | [143] | ||
| Diagnosis biomarker | Human Specimens | 2012 | Xiaodan Huang | [144] | ||
| Chemoresistant | Cell lines (SNU387, Huh7, Hep3B, and SNU449) | 2018 | Yuanbiao Zhang | [145] | ||
| Endometrioid carcinoma | FSCN1 | Diagnosis biomarker | Human Specimens | 2012 | Banu Dogan Gun | [146] |
| Head and neck cancers | FSCN1 | Diagnosis biomarker | Human Specimens | 2014 | Konstantinos Papaspyrou | [147] |
| Diagnosis biomarker | Human Specimens, Cell lines (OECM1, SAS, CGC8, and CGC9) | 2015 | Li-Yu Lee | [148] | ||
| Brain cancer | FSCN1 | Regulation of tumor cell motility | Cell lines (U251) | 2015 | Neil T Hoa | [149] |
| Cervical cancer | FSCN1 | Regulation of tumor growth | CaSki, subcutaneous xenograft model | 2018 | Xian Li | [150] |
| Diagnosis biomarker | Human Specimens | 2018 | Zohreh Yousefi Ghalejoogh | [151] | ||
| Cardia cancer | FSCN1 | Diagnosis biomarker | Human Specimens | 2019 | Li Wei | [152] |
As a well-known molecule, Fascin-1 can be used as a diagnostic marker and is essential for cell growth and motility in multiple cancer types. The table lists the Fascin-1-related studies from 2000 to 2021, including the biological relevance of cancer types, validated with human tissues or tumor cell lines, and established in which tumor cell model.
1.5. Transactivation factors of Fascin-1
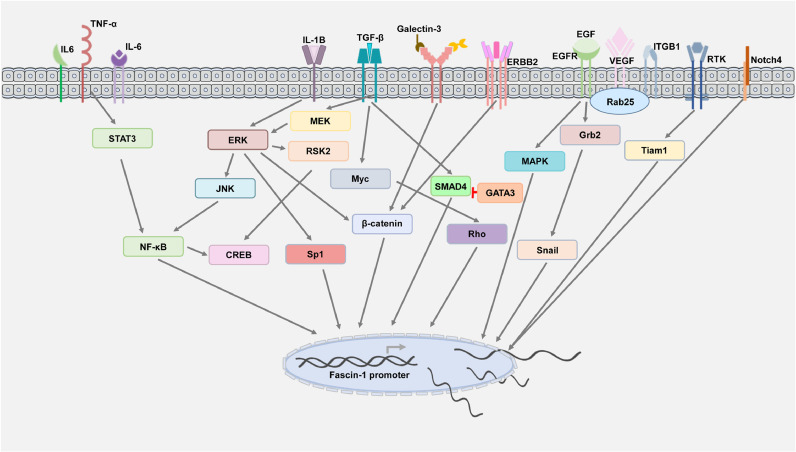
Stimulation by external factors, including physical or molecular factors, can trigger signal transduction to transactivate Fascin-1 expression. Currently, multiple upstream regulators have been identified to modulate the expression of Fascin-1 by interacting with the promoter region (Fig. 4).
Fig. 4.
The expression of Fascin-1 is regulated by diverse molecular pathways.
Inflammation-related factors, such as IL-1B, can induce Fascin-1 expression by the ERK/JNK/NF-κB/CREB cascade to promote oral squamous cell invasion, which can be reversed by treating cells with an antagonist of IL-1B [153]. Additionally, the evidence demonstrated that stimulation with IL-6 or TNF-α and Fas can induce STAT3-mediated Fascin-1 [56,101,103,154], in which the interaction of NF-κB and STAT3 may be required for binding to the Fascin-1 promoter. Similar to the response to viral infection and inflammatory situations, Fascin-1 can be found to be correlated with virus-related cancer progression.
Growth factors, such as EGF, have been found to induce the triple-negative breast cancer epithelial-to-mesenchymal (EMT) transition via MAPK/Fascin-1 [155]. As a downstream molecule of receptor tyrosine kinases, Tiam1 can regulate actin remodeling by targeting Fascin-1 [156]. Similar observations were described by Jeong et al., who found that Rab25 can activate EGFR/VEGF-A signaling by crosstalk; the integrin B1 receptor drives Snail to transactivate Fascin-1 expression, consequently promoting ovarian cancer malignancy [157]. In agreement with other reports, they demonstrated that ribosomal S6 kinase inhibitors can suppress Fascin-1 by blocking the RSK2-CREB axis [158]. Interestingly, the NF-κB consensus sequence shared a similar binding site for TCF/LEF. The report by Grothey et al. demonstrated that the ErbB2/HER2 tyrosine kinase receptor could stimulate the actin protrusion structure through the TCF/LEF motif to regulate breast cancer migration [46]. The TCF/LEF binding site is well known for the Wnt signaling cascade and can be activated with abnormal β-catenin.
For embryogenesis factors, related stimulation factors, such as TGF-β, were found to modulate the Fascin-1 level to control cell motility [159]. In addition, cell–cell interactions may trigger a similar phenomenon. According to a report by Qian, Notch4-mediated Fascin-1 contributes to cell motility in pancreatic cancer [160]. In agreement with other reports, extracellular factors, such as EGF and TGF-β, can transactivate Fascin-1 via the MER-REK-SP1 and Smad4 cascades [161]. Interestingly, Sun et al. found that TGF-β-induced Fascin-1 can be negatively regulated by the EMT-related factor GATA3 [162]. Similar observations were described for the Wnt/TGF-β downstream factor Myc. Myc can crosstalk with Rho signaling to serve as an essential transcription factor binding to the Fascin-1 promoter to promote filopodia formation [163].
The molecular network involved in Fascin-1 transactivation-related events, including extracellular stimulators, signaling pathways, and upstream regulators, was organized based on its current identification in cancer.
1.6. Downstream effectors of Fascin-1
1.6.1. Downstream effectors
1.6.1.1. MMP
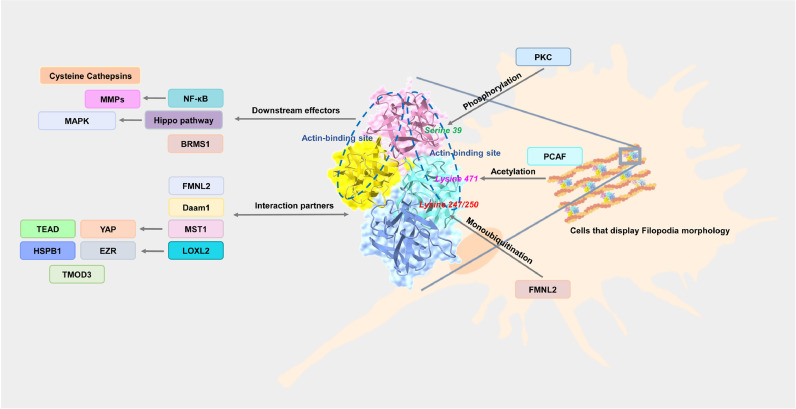
In addition to being transactivated by various stimulators, Fascin-1 is capable of regulating filopodia morphology by downstream effectors. A filopodia morphology can be easily observed when cells migrate, in which Fascin-1-mediated molecular regulation is needed. Currently, Fascin-1 has been identified as binding F-actin and diverse molecules to mediate filopodia formation. Additionally, molecular interactions can trigger signal transduction. Notably, Fascin-1-mediated cell motility can be regulated by posttranslational modifications (Fig. 5). It was found that in shRNA-based knockdown of Fascin-1 cells, multiple protease-related enzymes were decreased, including matrix metalloproteinase (MMP) and cysteine cathepsins [131]. Similar observations were described by Al-Alwan et al., who reported that Fascin-1 could regulate NF-κB and urokinase-type plasminogen activator as well as MMPs in breast cancer. In agreement with other reports demonstrating that invasiveness was correlated with Fascin-1 expression, one study showed that downregulation of Fascin-1 can decrease the EMT-related factors SNAI1/2 and MMPs and thus activate E-cadherin expression to suppress EMT [142].
Fig. 5.
Fascin-1-mediated molecular regulation contributed to filopodia morphology.
The related postmodification regulators of Fascin-1 and downstream effectors or interaction partners of Fascin-1 were organized. The simulated crystal structure (ID:1DFC) was generated from NCBI websites.
1.6.1.2. BRMS1
Notably, overexpression of Fascin-1 can abrogate the level of tumor suppressor breast cancer metastasis suppressor-1 (BRMS1) in the nucleus [164]. However, evidence suggests that BRMS1 can negatively regulate Fascin-1 as well, consequently suppressing metastasis ability [165].
1.6.1.3. Hippo-MAPK axis
Additionally, some studies have found that Fascin-1 regulates the Hippo and MAPK pathways to alter cell malignancy [88,166].
1.7. Protein–protein interaction
In general, Fascin-1 mediates filopodia by interacting with actin to contribute to cell motility. Therefore, the biological functions of Fascin-1 may depend on its protein–protein interaction partner. In cancer, the dynamic between Fascin-1 and F-actin interaction was observed, and Pfisterer et al. demonstrated that FMNL2 and Fascin-1 interaction could accelerate binding to F-actin and was essential to filopodia structure stabilization [167]. In addition, Fascin-1 interaction with the FH domain of Daam1 was required for filopodia formation in breast cancer [168]. In lung cancer, Fascin-1 has been shown to dominate the YAP/TEAD axis by interacting with MST1 to promote invasive cells [85]. Similar processes can be found in esophageal squamous cell carcinoma. The interaction complex between Fascin-1/LOXL2/EZR/HSPB1/TMOD3 contributes to cell cytoskeleton reconstruction during migration [169].
1.8. Posttranslational modification of Fascin-1
In addition to being transactivated by various stimulators, Fascin-1 is capable of regulating filopodia morphology by downstream effectors. A filopodia morphology can be easily observed when cells migrate, in which Fascin-1-mediated molecular regulation is needed. Currently, Fascin-1 has been identified as binding F-actin and diverse molecules to mediate filopodia formation. Additionally, molecular interactions can trigger signal transduction. Notably, Fascin-1-mediated cell motility can be regulated by posttranslational modifications (Fig. 5). Modifications of proteins can alter their structure to affect their stability and interaction ability. These modification processes can occur in Fascin-1, especially phosphorylation. Thus far, four phosphorylation sites have been identified in Fascin-1, including tyrosine 23, threonine 403 [170] and serine 38/39/274 [109]. A Fascin's ability to bundle actin is determined by its state of dephosphorylation [171]. For example, phosphorylation at serine 39 on Fascin-1 by protein kinase C (PKC) can deactivate its bundling activity to F-actin [172]. During the progression of esophageal cancer, Facin-mediated cell motility can be regulated by phosphorylation of threonine 403 by AKT2 [170]. On the other hand, studies demonstrated that serine to alanine substitution (S39A) of Fascin-1 could mimic dephosphorylation status [173]. Interestingly, such processes can be regulated by diverse molecules and contribute to cancer progression. Evidence demonstrated that nonphosphorylated Fascin-1 could bind to FMNL2, which accelerated the movement of Fascin-1 into filopodia from lamellipodia. In turn, FMNL2 can modulate the proportion of Fascin-1 between filopodia and lamellipodia [167]. Similar results can be observed in Zeng et al., who identified that the migration ability of esophageal squamous cancer cells could be regulated by the dephosphorylation of Fascin-1 in β-trefoil domain 1 (Tyrosine 23, Serine 38/39) and β-trefoil domain 3 (Serine 274) [109]. Studies have recently revealed that the acetylation of Fascin performs a similar function to phosphorylation. It has been reported that the acetyltransferase P300/CBP-associated factor (PCAF) can regulate esophageal tumor cell metastasis through acetylation of Fascin-1 at lysine 471 [174]. Additionally, Smurf1-mediated monoubiquitination of lysine 247/250 on Fascin-1 contributed to the interaction with F-actin but not protein stability; lysine to arginine substitution (K247R/K250R) of Fascin-1 can trigger the migration ability of colon cancer [175]. Therefore, various modification sites have been identified in Fascin-1, which may participate in protein stability and activity.
2. Noncanonical functions of Fascin-1 in cancer
It has been postulated that the molecular regulation of cell motility by Fascin may be divided into two types: those that are dependent on F-actin binding proteins and those that are independent. Many researchers have recently identified such independent actin-binding functions as noncanonical functions of Fascin [92,[176], [177], [178], [179], [180]]. Notably, Fascin-mediated EMT contributed to reprogramming events and was linked to clinical treatment issues. Fascin-1-independent F-actin binding biological functions have been identified to contribute to cancer progression, including metabolism reprogramming, chemotherapy resistance, and stemness activity. Significantly, such processes reprogram the tumor microenvironment and accelerate tumor aggressiveness (Fig. 6). The focus of this section was on the role of Fascin in promoting cancer progression through non-F-actin-binding events.
Fig. 6.
Noncanonical biological functions of Fascin-1 in cancer.
The F-actin-independent molecular regulation network of Fascin-1 was organized according to related events of metabolic reprogramming, chemoresistance, stemness activity, and the tumor microenvironment.
2.1. Metabolism
Dysregulation of metabolism, including carbohydrates, lipids, and proteins, has contributed to cancer progression. Interestingly, cell motility can be affected by vitamin metabolism processes. For example, the All-trans-Retinoic acid metabolic-related enzyme CYP26A1 has been identified as being associated with cancer progression. An omics-based comparative analysis showed that Fascin-1 was upregulated in CYP26A1-overexpressing cells. Conversely, downregulation of Fascin-1 can abrogate cell growth and motility and subsequently induce apoptosis and senescence [181]. A similar approach can be observed in Lin et al.; metabolomics and proteomics-based analysis identified Fascin-1 links to the mitochondrial oxidative phosphorylation system by modulating mitochondrial Complex I and mitochondrial actin filaments [90], therefore demonstrating that Fascin-1 can regulate carbohydrate metabolism events to dominate the cell phenotype. However, as a mesenchymal-epithelial negative regulator, inositol has been reported to downregulate Fascin-1 expression via the PI3K/AKT axis, which suppresses breast cancer aggressiveness [182]. Furthermore, a similar proteomics-based approach identified that fatty acid synthase can bind to Fascin-1 directly to control the EMT phenotype of cells [183]. Such processes can be observed in breast cancer. Cells express more Fascin-1 via linoleic acid to promote cell migration and invasion [184]. This finding demonstrates that fatty acid metabolism-mediated Fascin-1 is a risk factor for cancer progression. In addition, dysregulation of amino acid metabolism has been found to be linked to cancer malignancy. Growing evidence shows that the expression of leucine aminopeptidase 3 is associated with cancer progression. The expression of leucine aminopeptidase 3 can regulate breast cancer cell motility via Fascin-1 [185]. Similar results can be observed in Fang et al., in which knockdown of leucine aminopeptidase 3 decreased Fascin-1 expression in ovarian cancer as well as cell migration ability [186].
2.2. Chemotherapy-resistance
Drug resistance enables cancer cells to survive treatment. Studies have shown that TS-1, as a standard treatment for oral and pancreatic cancer, induces resistance, and proteomics-based analysis revealed that Fascin-1 was upregulated in TS-1-resistant pancreatic cancer cells [187]. Treating cells with doxorubicin upregulated Fascin-1 expression in human KB carcinoma [188]. Mechanistically, the Fascin-derived PI3K/AKT axis can resist doxorubicin-induced apoptosis and is correlated with poor prognosis of breast cancer outcomes [189]. Growing evidence shows that various factors that can induce drug resistance require Fascin-1 expression. A roline to histidine substitution (P64H) of galectin-3 can promote nuclearization and accelerate the interaction with β-catenin to transactivate Fascin-1 [190]. Additional related stimulation factors can be observed in gastric and pancreatic cancer. Studies have found that knockdown of zinc finger protein 139 can downregulate Fascin-1 to induce drug sensitivity [191]. In pancreatic cancer, the expression of Notch4 was correlated with docetaxel resistance; knockdown of Notch4 can suppress Fascin-1 via the AKT/GSK3β axis to enhance cell sensitivity, consequently suppressing tumor cell drug resistance and motility [192].
2.3. Stemness activity
Self-renewal and embryonic features that characterize cell pluripotency are defined as “stemness” activity. In cancer, stemness may be contributed to by EMT processes, in which cells have more mesenchymal and stem-like marker expression as well as drug-resistant ability. According to a report by Barnawi et al., overexpression of Fascin-1 can induce stem-like markers and sphere formation via integrin-β1; coexpression of Fascin-1 and integrin-β1 provides a worse prognosis for breast cancer [193]. Under chronic inflammatory conditions, colonic polyp-derived differentiated adenomatous polyposis cells have been identified with an anchorage-independent growth phenotype and stemness-related marker expression, including CD44, CD166, and LGR5. Mechanistically, related chronic inflammatory factors, such as TNF-α- and IL-1β-mediated miR-146a expression, can increase Fascin-1 stability to promote stemness activity [194]. The same effect was observed by Barnawi and coworkers, who showed that the expression of Fascin-1 was correlated with the expression of EMT and stemness markers and that knockdown of Fascin-1 decreased tumorsphere formation and increased the sensitivity to doxorubicin [195]. In melanoma, knockout of Fascin-1 can reduce stemness characteristics. Interestingly, the Fascin-1 and MST2 interaction drives nonactin binding functions to increase Hippo pathway-related factor TAZ stability; consequently, there is dominant tumor cell sphere formation [196].
2.4. Immune evasion in the tumor microenvironment
Human T-cell leukemia virus type 1 was demonstrated to induce lymphoma by transfecting T cells, in which Fascin-1 serves as a downstream effector of the viral-related protein Tax and is transactivated by NF-кB [197]. In addition, staining Fascin-1 for circulating myeloid dendritic cells can be used as a specific indicator for diagnosis in pancreatic cancer [198]. In follicular lymphomas, the level of Fascin-1 in germinal center dendritic cells was decreased, which may explain why the tumor microenvironment can change the activity of germinal center dendritic cells to reduce antigen presentation into T cells to modulate the immune response [199]. Similar observations were described in Fascin-1/CXCL9-positive dendritic cells, where they are involved in stromal cells and can interact with T cells to modulate immune stimulation in gastric cancer [200]. A mouse model revealed that Fascin levels are related to conventional dendritic cell activity, and inhibition of Fascin can effectively expose tumor antigens to conventional dendritic cells and increase CD8+ T cells' antitumor immune responses [201].
This finding was in agreement with the fact that Fascin-1 can be linked to the tumor immune microenvironment. In addition, studies recently showed that cancer-associated fibroblasts could trigger cancer cells expressing Fascin-1 via TGF‑β1, EGF, or IL‑1β-mediated RhoA and NF-кB signaling [202] and may serve as a biomarker for cancer-associated fibroblasts [203]. Similarly, this effect can be observed in McGuire et al., who identified that Fascin-1 is expressed in both ovarian cancer and stromal cells, and downregulated Fascin-1 can abrogate tumor metastasis activity [70]. Additionally, Fascin-1 has been detected in serum, and the level of Fascin-1 can be used as a marker in lung cancer [204]. A similar approach was described in Chen et al., who revealed that the developed Fascin-1 autoantibodies could be used to distinguish the stage of esophageal squamous cell carcinoma [205]. Recently, extracellular vesicles have been considered critical mediators for tumor cells communicating with the microenvironment [206]. Thus, Fascin-1 can participate in the delivery of extracellular vesicles to promote cancer invasiveness [207]. In addition, the tumor microenvironment is considered to have more hypoxic conditions. Under this circumstance, HIF-1α was identified as a transcription factor and can bind to the Fascin-1 promoter to transactivate directly, consequently regulating cell motility [208]. Mechanistically, Fascin-1-mediated invasiveness with MMP [131] and filopodia morphology may contribute to angiogenesis [209]. In particular, Fascin-1 has been identified to participate in tumor self-seeding, which promotes tumor extravasation and related events [210].
3. Available inhibitors for Fascin-1
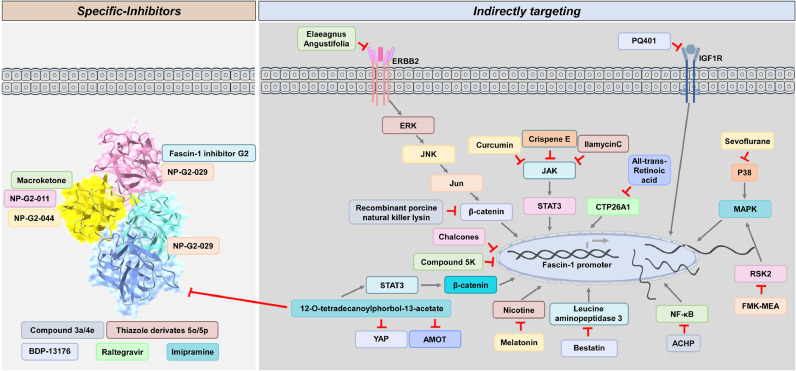
Currently, available inhibitors have been designed to target Fascin-1 and applied in cancer therapy. This section lists current strategies for targeting Fascin-1. Fascin-1 must be considered a critical target for cancer therapy. Therefore, multiple specific compounds have been designed to block Fascin-1 by directly interacting with its actin-binding sites. Interestingly, posttranslational modification can also change Fascin-1 activity. In addition, the evidence demonstrated that targeting upstream regulators of Fascin-1 may provide an alternative way to block Fascin-1-mediated cancer progression (Fig. 7).
Fig.7.
The available targeting strategies for Fascin-1.
Direct and indirect inhibitors of Fascin-1 are listed according to the current treatment in cancer.
3.1. Specific inhibitors
3.1.1. Macroketone
Fascin-1 is well known for its actin-binding ability. Therefore, targeting Fascin-1 by blocking its actin-binding sites would be efficient. Based on the crystal structure and molecular docking simulation analysis, macroketone can bind to Fascin-1 and shade actin-binding sites. Significantly, the glutamate to alanine substitution (E391A) or histidine to alanine substitution (H474A) of Fascin-1 can block macroketone binding. Interestingly, studies demonstrated that the H474A mutation could resist macroketone treatment and accelerate cell invasive activity [211].
3.1.2. N-(1-(4-(Trifluoromethyl)benzyl)-1H-indazol-3-yl)furan-2-carboxamide derivative compound
Huang and coworkers showed as an imaging-based assay that high throughput identification identified N-(1-(4-(trifluoromethyl)benzyl)-1H-indazol-3-yl)furan-2-carboxamide, also called Fascin-1 inhibitor G2, can bind to Serine 39 on Fascin-1 directly. The serine to aspartate substitution (S39D) of Fascin-1 can block Fascin-1 inhibitor G2 binding, in which the binding between Fascin-1 inhibitor G2 and Fascin-1 can modulate serum-stimulated filopodia morphology to block breast cancer metastasis [212]. This finding was in agreement with that previously reported by McGuire et al., who demonstrated that targeting Fascin-1 with the Fascin-1 inhibitor G2 in both ovarian cancer and cancer-associated fibroblasts can suppress metastasis [213]. A similar observation was described in colorectal cancer. Treating cells with the Fascin-1 inhibitor G2 can suppress cell migration and invasion activity [214]. Furthermore, to improve the efficiency of the Fascin-1 inhibitor G2, related derivatives, including NP-G2-011 and NP-G2-044, were developed to inhibit metastasis. Notably, in contrast to the G2 binding site of Fascin-1 inhibitors, NP-G2-044 was designed to target Fascin-1 by binding glutamate 391 to prevent actin bundling [215]. In particular, NP-G2-044 seems specific to activated B-cell subtype lymphoma. Mechanistically, targeting Fascin-1 with NP-G2-044 can modulate ERK/PLCγ1/PI3K/p65 to prevent metastasis [216]. Notably, recent studies have shown that NP-G2-044 can improve the survival rate and reactivate the immune response by modulating the Fascin-1 level in dendritic cells, and the antitumor ability has been evaluated by clinical trials (NCT05023486, NCT03199586) [201]. Similar to the Fascin-1 inhibitor G2-based derivatives, NP-G2-029 and its binding affinity can be changed by isoleucine to alanine substitution (I39A) and phenylalanine to alanine substitution (F216A). In contrast to NP-G2-044, NP-G2-029 had a low IC50 against diverse mutations of Fascin-1, including F14A, W101A, F14A/W101A, I93A, F216A, and I93A/F216A [217].
3.1.3. Thiazole derivative compound
A series of thiazole derivative compounds have been designed to target Fascin-1, in which the thiazole Compounds 5o and 5p showed powerful inhibition of cell motility and angiogenesis [218].
3.1.4. Others
Tetrahydropyrimidine derivatives have been designed to inhibit Fascin-1. Significantly, Compounds 3a and 4e can suppress Fascin-1-mediated cell motility [219]. Similar studies were reported by Francis et al., who designed a Fascin-1-specific inhibitor by modulating the structure of N-phenylacetamide with benzyl substituents and identified BDP-13176. Currently, BDP-13176 is used as an abatable commodity for targeting Fascin-1 [220]. Notably, the antiviral raltegravir [221] and the antidepressant imipramine [222], used for repurposing, effectively inhibited colorectal cancer metastasis in vitro and in vivo by binding to Fascin. In particular, Raltegravir has a similar binding site to NP-G2-029, but it may be more stable [221].
3.2. Indirect targeting
Currently, targeting upstream regulators of Fascin-1 can modulate the related expression and abrogate Fascin-1-mediated biological functions. Therefore, indirect targeting strategies were organized.
3.2.1. Natural products
Herbal medicines are considered to come from natural products and cause mild side effects in cells. Studies have demonstrated that the extract from Elaeagnus angustifolia has anticancer activity. Treating cells with Elaeagnus angustifolia extract can decrease Fascin-1 expression and downregulate the ErbB2/JNK/Jun/β-catenin axis as well as EMT-related markers to induce cell apoptosis, consequently abrogating Her2-positive breast cancer progression [223]. Another natural product, chalcones and their derivatives, has been identified to modulate the expression of Fascin-1 in KRAS-mutated colorectal cancers [214]. Similar effects can be observed with the herbal compound curcumin. STAT3 has been described as an upstream regulator of Fascin-1 [56,101,103,154], and curcumin can target the JAK/STAT3 axis to modulate Fascin-1 in ovarian cancer [224]. Extracts such as crispene E and ilamycin C were reported to downregulate Fascin-1 by abrogating STAT3 dimerization in breast cancer [225,226]. Therefore, targeting the upstream regulator of Fascin-1 may provide an alternative strategy.
3.2.2. Synthetic compound
The IKKβ inhibitor ACHP can suppress NF-κB-mediated Fascin-1 [227], sevoflurane can inhibit HIF-1α-mediated Fascin-1 [228], polyisoprenylated cysteinyl amide or salinomycin can block RhoA-mediated Fascin-1 [229,230], and FMK-MEA can abrogate RSK2/CREB-mediated Fascin-1 [231]. Meanwhile, the leucine aminopeptidase 3 inhibitor bestatin has been identified with identical effects [232]. In addition, the effects of thiazole derivatives have been described [218]. Zheng et al. identified that compound-5k could modulate Fascin-mediated filopodia morphology to control cell growth and motility [233]. The synthetic compound PQ401 was designed for IGF-1 receptor inhibition. Treating cells with PQ401 can suppress IGF-1-mediated Fascin-1 in skin cancer [234]. Notably, 12-O-tetradecanoylphorbol-13-acetate has been reported to phosphorylate Fascin-1 to inactivate its activity [235]. However, the controversial effects of 12-O-tetradecanoylphorbol-13-acetate have been described, which can induce the PKC/STAT3/β-catenin/Fascin-1 axis to promote breast cancer cell motility [236] and act as an anticancer reagent by suppressing YAP/AMOT in hepatocellular carcinoma [237].
3.2.3. Recombinant peptide
A similar process can be observed in recombinant porcine natural killer lysine, which was derived from porcine intestinal tissue. When receiving this peptide, Fascin-mediated β-catenin, as well as MMP2/9, were decreased, thus inhibiting hepatocellular carcinoma cell adhesion and migration ability [238]. Additionally, targeting metabolism-related events has been considered to efficiently block nutrient uptake to cancer. Treatment of cells with all-trans-retinoic acid can decrease CYP26A1-mediated Fascin expression, thus attenuating breast cancer aggressiveness [181].
3.2.4. Hormones
Melatonin is a hormone and can be generated from the human brain. Growing evidence has demonstrated that melatonin has anticancer activity. Studies have reported that supplements with melatonin suppress nicotine-mediated Fascin-1 in breast cancer [239].
4. Conclusion
The biological functions of Fascin are linked to embryogenesis and carcinogenesis. It has been suggested that dysregulated cells might reactivate their expression to enhance cancer progression, especially for Fascin-1. This review systematically discusses the biological functions of Fascin-1 and its application value as a molecular target in tumor therapy. Notably, the noncanonical functions of Fascin may be distributed by specific conditions, subcellular locations, and interacting partners. With the leap forward to a single-cell level analysis, the roles of Fascin in the microenvironment will be unveiled. Most research focuses on fascin-1, and very little is known about fascin-2 and fascin-3, which can be explained by the distribution of expression in somatic cells. However, evidence indicates that they share similar functions and may compensate for each other. Recently, there is preliminary evidence highlighting the co-expression of Fascin-1 and Fascin-2 that has been found to be useful for diagnosing cancer patients [44] and may contribute to the regulation of novel non-coding RNAs [240]. Noteworthy, it has been found that metals–organic frameworks can affect mitotic processes by targeting F-actin-related molecules such as Fascin-2, thus causing cytotoxicity in tumor cells [241]. Evidence from these studies gradually supports the notion that Fascin-2 can be a potent target for improving the progression of cancer. Therefore, a comprehensive molecular characterization of Fascin-1, Fascin-2 and Fascin-3 might provide an alternative treatment strategy that targets the Fascin family more specifically.
Resource
Funding
None.
CRediT authorship contribution statement
Chien-Hsiu Li: Writing – original draft, Resources, Software, Writing – review & editing. Ming-Hsien Chan: Data curation, Writing – review & editing. Shu-Mei Liang: Data curation. Yu-Chan Chang: Writing – review & editing, Supervision. Michael Hsiao: Writing – review & editing, Supervision.
Declaration of Competing Interest
None.
Acknowledgments
This review study was supported by the Genomics Research Center of Academia Sinica, Taiwan to Michael Hsiao.
Contributor Information
Yu-Chan Chang, Email: jameskobe0@gmail.com.
Michael Hsiao, Email: mhsiao@gate.sinica.edu.tw.
References
- 1.Otto J.J., Kane R.E., Bryan J. Formation of filopodia in celomocytes - localization of Fascin, a 58,000 dalton actin cross-linking protein. Cell. 1979;17(2):285–293. doi: 10.1016/0092-8674(79)90154-5. [DOI] [PubMed] [Google Scholar]
- 2.Hu L.L., et al. FASCIN regulates actin assembly for spindle movement and polar body extrusion in mouse oocyte meiosis. J. Cell. Physiol. 2021 doi: 10.1002/jcp.30443. [DOI] [PubMed] [Google Scholar]
- 3.Pan M.H., et al. FMNL3 regulates FASCIN for actin-mediated spindle migration and cytokinesis in mouse oocytesdagger. Biol. Reprod. 2020;102(6):1203–1212. doi: 10.1093/biolre/ioaa033. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z.T., et al. Fscn1 is required for the trafficking of TGF-beta family type I receptors during endoderm formation. Nat. Commun. 2016:7. doi: 10.1038/ncomms12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Megiorni F., et al. Minor expression of Fascin-1 gene (FSCN1) in NTera2 cells depleted of CREB-binding protein. Neurosci. Lett. 2005;381(1–2):169–174. doi: 10.1016/j.neulet.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 6.Cheng Y., et al. Abnormal sperm in mice lacking the Taf7l gene. Mol. Cell. Biol. 2007;27(7):2582–2589. doi: 10.1128/MCB.01722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gungor-Ordueri N.E., Celik-Ozenci C., Cheng C.Y. Fascin 1 is an actin filament-bundling protein that regulates ectoplasmic specialization dynamics in the rat testis. Amer. J. Physiol. 2014;307(9):E738–E753. doi: 10.1152/ajpendo.00113.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimamura Y., et al. Immunohistochemical analysis of oral dysplasia: diagnostic assessment by Fascin and podoplanin expression. Acta Histochem. Cytochem. 2011;44(6):239–245. doi: 10.1267/ahc.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan B., et al. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc. Natl. Acad. Sci. U S A. 2008;105(49):19384–19389. doi: 10.1073/pnas.0810584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Zh L., et al. The chromosomal localization, expression pattern and polymorphism analysis of porcine FSCN1 gene differently expressed from LongSAGE library. Mol. Biol. Rep. 2010;37(5):2361–2367. doi: 10.1007/s11033-009-9742-9. [DOI] [PubMed] [Google Scholar]
- 11.Remsburg C., Testa M., Song J.L. Rab35 regulates skeletogenesis and gastrulation by facilitating actin remodeling and vesicular trafficking. Cells Dev. 2021;165 doi: 10.1016/j.cdev.2021.203660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi Y., et al. Expression of Fascin-1, an actin-bundling protein, in migrating hepatoblasts during rat liver development. Cell Tissue Res. 2008;334(2):219–226. doi: 10.1007/s00441-008-0683-8. [DOI] [PubMed] [Google Scholar]
- 13.Sonego M., et al. Fascin regulates the migration of subventricular zone-derived neuroblasts in the postnatal brain. J. Neurosci. 2013;33(30):12171–12185. doi: 10.1523/JNEUROSCI.0653-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qualtrough D., et al. The actin-bundling protein Fascin is overexpressed in inflammatory bowel disease and may be important in tissue repair. Bmc Gastroenterol. 2011;11 doi: 10.1186/1471-230X-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W.C., et al. IL-6 augmented motility of airway epithelial cell BEAS-2B via Akt/GSK-3beta signaling pathway. J. Cell. Biochem. 2012;113(11):3567–3575. doi: 10.1002/jcb.24235. [DOI] [PubMed] [Google Scholar]
- 16.Zhu B., et al. Dynamic proteome analysis of spinal cord injury after ischemia-reperfusion in rabbits by two-dimensional difference gel electrophoresis. Spinal Cord. 2013;51(8):610–615. doi: 10.1038/sc.2013.24. [DOI] [PubMed] [Google Scholar]
- 17.Miao Q., et al. SOX11 and SOX4 drive the reactivation of an embryonic gene program during murine wound repair. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-11880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang P., et al. Exosomes from microRNA-145-5p-modified HUCB-MSCs attenuate CCl4-induced hepatic fibrosis via down-regulating FSCN1 expression. Life Sci. 2021 doi: 10.1016/j.lfs.2021.119404. [DOI] [PubMed] [Google Scholar]
- 19.Fu H., et al. MiR-200b/c family inhibits renal fibrosis through modulating epithelial-to-mesenchymal transition via targeting Fascin-1/CD44 axis. Life Sci. 2020;252 doi: 10.1016/j.lfs.2020.117589. [DOI] [PubMed] [Google Scholar]
- 20.Bulek K., et al. IL-17A Recruits Rab35 to IL-17R to mediate PKCalpha-dependent stress fiber formation and airway smooth muscle contractility. J. Immunol. 2019;202(5):1540–1548. doi: 10.4049/jimmunol.1801025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Z., et al. Accumulation of Fascin+ cells during experimental autoimmune neuritis. Diagn. Pathol. 2013;8:213. doi: 10.1186/1746-1596-8-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs-Cacha C., et al. Fascin-1 is released from proximal tubular cells in response to calcineurin inhibitors (CNIs) and correlates with isometric vacuolization in kidney transplanted patients. Am. J. Transl. Res. 2017;9(9):4173. -+ [PMC free article] [PubMed] [Google Scholar]
- 23.Takebayashi M., et al. Blood dendritic cells are decreased in acute graft-versus-host disease. Bone Marrow Transplant. 2004;33(10):989–996. doi: 10.1038/sj.bmt.1704406. [DOI] [PubMed] [Google Scholar]
- 24.Wang B.W., et al. Preventing alloimmune rejection using circular RNA FSCN1-silenced dendritic cells in heart transplantation. J. Heart Lung Transplant. 2021;40(7):584–594. doi: 10.1016/j.healun.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z., et al. MicroRNA-145 regulates immune cytokines via targeting FSCN1 in Staphylococcus aureus-induced mastitis in dairy cows. Reproduct. Domest. Anim. 2019;54(6):882–891. doi: 10.1111/rda.13438. [DOI] [PubMed] [Google Scholar]
- 26.Said J.W., et al. The role of follicular and interdigitating dendritic cells in HIV-related lymphoid hyperplasia: localization of Fascin. Mod. Pathol. 1997;10(5):421–427. [PubMed] [Google Scholar]
- 27.Chen J., et al. Strong adhesion by regulatory T cells induces dendritic cell cytoskeletal polarization and contact-dependent lethargy. J. Exp. Med. 2017;214(2):327–338. doi: 10.1084/jem.20160620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinkus G.S., et al. Fascin, a sensitive new marker for Reed-Sternberg cells of hodgkin’s disease. Evidence for a dendritic or B cell derivation? Am. J. Pathol. 1997;150(2):543–562. [PMC free article] [PubMed] [Google Scholar]
- 29.Kempf W., et al. Fascin expression in CD30-positive cutaneous lymphoproliferative disorders. J. Cutan. Pathol. 2002;29(5):295–300. doi: 10.1034/j.1600-0560.2002.290507.x. [DOI] [PubMed] [Google Scholar]
- 30.Kluiver J., et al. Global correlation of genome and transcriptome changes in classical Hodgkin lymphoma. Hematol. Oncol. 2007;25(1):21–29. doi: 10.1002/hon.804. [DOI] [PubMed] [Google Scholar]
- 31.Yuan L., Lou W., Sang J. Expression and significances of FSCN1 and HGF in nasal inverted papilloma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;26(8):339–342. [PubMed] [Google Scholar]
- 32.Gamundi M.J., et al. Sequence variations in the retinal Fascin FSCN2 gene in a Spanish population with autosomal dominant retinitis pigmentosa or macular degeneration. Mol. Vis. 2005;11:922–928. [PubMed] [Google Scholar]
- 33.Ziviello C., et al. Molecular genetics of autosomal dominant retinitis pigmentosa (ADRP): a comprehensive study of 43 Italian families. J. Med. Genet. 2005;42(7):e47. doi: 10.1136/jmg.2005.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yokokura S., et al. Targeted disruption of FSCN2 gene induces retinopathy in mice. Invest. Ophthalmol. Vis. Sci. 2005;46(8):2905–2915. doi: 10.1167/iovs.04-0856. [DOI] [PubMed] [Google Scholar]
- 35.Lin-Jones J., Burnside B. Retina-specific protein Fascin 2 is an actin cross-linker associated with actin bundles in photoreceptor inner segments and calycal processes. Invest. Ophthalmol. Vis. Sci. 2007;48(3):1380–1388. doi: 10.1167/iovs.06-0763. [DOI] [PubMed] [Google Scholar]
- 36.Lv J.N., et al. Targeted RP9 ablation and mutagenesis in mouse photoreceptor cells by CRISPR-Cas9. Sci. Rep. 2017;7:43062. doi: 10.1038/srep43062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin J.B., et al. The R109H variant of Fascin-2, a developmentally regulated actin crosslinker in hair-cell stereocilia, underlies early-onset hearing loss of DBA/2J mice. J. Neurosci. 2010;30(29):9683–9694. doi: 10.1523/JNEUROSCI.1541-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson K.R., Longo-Guess C.M., Gagnon L.H. A QTL on Chr 5 modifies hearing loss associated with the Fascin-2 variant of DBA/2J mice. Mamm. Genome. 2015;26(7–8):338–347. doi: 10.1007/s00335-015-9574-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X., et al. Null Mutation of the Fascin2 Gene by TALEN Leading to Progressive Hearing Loss and Retinal Degeneration in C57BL/6J Mice. G3 (Bethesda) 2018;8(10):3221–3230. doi: 10.1534/g3.118.200405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L., et al. Attenuation of hearing loss in DBA/2J mice by anti-apoptotic treatment. Hear. Res. 2015;327:109–116. doi: 10.1016/j.heares.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Tubb B., et al. Testis Fascin (FSCN3): a novel paralog of the actin-bundling protein Fascin expressed specifically in the elongate spermatid head. Exp. Cell. Res. 2002;275(1):92–109. doi: 10.1006/excr.2002.5486. [DOI] [PubMed] [Google Scholar]
- 42.Qu Y.H., et al. Identification of candidate genes in regulation of spermatogenesis in sheep testis following dietary vitamin E supplementation. Anim. Reprod. Sci. 2019;205:52–61. doi: 10.1016/j.anireprosci.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Sayed W.M., Elzainy A. Impact of platelet-rich plasma versus selenium in ameliorating induced toxicity in rat testis: histological, immunohistochemical, and molecular study. Cell Tissue Res. 2021;385(1):223–238. doi: 10.1007/s00441-021-03439-2. [DOI] [PubMed] [Google Scholar]
- 44.Deng C., et al. Prognostic significance of FSCN family in multiple myeloma. J. Cancer. 2021;12(7):1936–1944. doi: 10.7150/jca.53675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grothey A., et al. Fascin, an actin-bundling protein associated with cell motility, is upregulated in hormone receptor negative breast cancer. Br. J. Cancer. 2000;83(7):870–873. doi: 10.1054/bjoc.2000.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grothey A., et al. C-erbB-2/HER-2 upregulates Fascin, an actin-bundling protein associated with cell motility, in human breast cancer cell lines. Oncogene. 2000;19(42):4864–4875. doi: 10.1038/sj.onc.1203838. [DOI] [PubMed] [Google Scholar]
- 47.Guvakova M.A., Boettiger D., Adams J.C. Induction of Fascin spikes in breast cancer cells by activation of the insulin-like growth factor-I receptor. Int. J. Biochem. Cell Biol. 2002;34(6):685–698. doi: 10.1016/s1357-2725(01)00160-1. [DOI] [PubMed] [Google Scholar]
- 48.Yoder B.J., et al. The expression of Fascin, an actin-bundling motility protein, correlates with hormone receptor-negative breast cancer and a more aggressive clinical course. Clin. Cancer Res. 2005;11(1):186–192. [PubMed] [Google Scholar]
- 49.Rodriguez-Pinilla S.M., et al. Prognostic significance of basal-like phenotype and Fascin expression in node-negative invasive breast carcinomas. Clin. Cancer Res. 2006;12(5):1533–1539. doi: 10.1158/1078-0432.CCR-05-2281. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J., et al. Rab35 controls actin bundling by recruiting Fascin as an effector protein. Science. 2009;325(5945):1250–1254. doi: 10.1126/science.1174921. [DOI] [PubMed] [Google Scholar]
- 51.Xing P., et al. Fascin, an actin-bundling protein, promotes breast cancer progression in vitro. Cell Biochem. Funct. 2011;29(4):303–310. doi: 10.1002/cbf.1750. [DOI] [PubMed] [Google Scholar]
- 52.Al-Alwan M., et al. Fascin is a key regulator of breast cancer invasion that acts via the modification of metastasis-associated molecules. PLoS One. 2011;6(11):e27339. doi: 10.1371/journal.pone.0027339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esnakula A.K., et al. Strong association of Fascin expression with triple negative breast cancer and basal-like phenotype in African-American women. J. Clin. Pathol. 2014;67(2):153–160. doi: 10.1136/jclinpath-2013-201698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Youssef N.S., Hakim S.A. Association of Fascin and matrix metalloproteinase-9 expression with poor prognostic parameters in breast carcinoma of Egyptian women. Diagn Pathol. 2014;9:136. doi: 10.1186/1746-1596-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghebeh H., et al. Fascin is involved in the chemotherapeutic resistance of breast cancer cells predominantly via the PI3K/Akt pathway. Br. J. Cancer. 2014;111(8):1552–1561. doi: 10.1038/bjc.2014.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snyder M., et al. A signal transducer and activator of transcription 3.Nuclear Factor kappaB (Stat3.NFkappaB) complex is necessary for the expression of Fascin in metastatic breast cancer cells in response to interleukin (IL)-6 and tumor necrosis factor (TNF)-alpha. J. Biol. Chem. 2014;289(43):30082–30089. doi: 10.1074/jbc.M114.591719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Min K.W., et al. Fascin expression predicts an aggressive clinical course in patients with advanced breast cancer. Oncol. Lett. 2015;10(1):121–130. doi: 10.3892/ol.2015.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Omran O.M., Al Sheeha M. Cytoskeletal Focal Adhesion Proteins Fascin-1 and paxillin are predictors of malignant progression and poor prognosis in human breast cancer. J. Environ. Pathol. Toxicol. Oncol. 2015;34(3):201–212. doi: 10.1615/jenvironpatholtoxicoloncol.2015013663. [DOI] [PubMed] [Google Scholar]
- 59.Wang C.Q., et al. Fascin-1 as a novel diagnostic marker of triple-negative breast cancer. Cancer Med. 2016;5(8):1983–1988. doi: 10.1002/cam4.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnawi R., et al. Fascin is critical for the maintenance of breast cancer stem cell pool predominantly via the activation of the notch self-renewal pathway. Stem Cells. 2016;34(12):2799–2813. doi: 10.1002/stem.2473. [DOI] [PubMed] [Google Scholar]
- 61.Wang C.Q., et al. FSCN1 gene polymorphisms: biomarkers for the development and progression of breast cancer. Sci. Rep. 2017;7(1):15887. doi: 10.1038/s41598-017-16196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee H.J., et al. Fascin expression is inversely correlated with breast cancer metastasis suppressor 1 and predicts a worse survival outcome in node-negative breast cancer patients. J. Cancer. 2017;8(16):3122–3129. doi: 10.7150/jca.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heinz L.S., et al. Strong Fascin expression promotes metastasis independent of its F-actin bundling activity. Oncotarget. 2017;8(66):110077–110091. doi: 10.18632/oncotarget.22249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abbasi A., et al. Fascin overexpression is associated with higher grades of breast cancer. Pol. J. Pathol. 2019;70(4):264–268. doi: 10.5114/pjp.2019.93128. [DOI] [PubMed] [Google Scholar]
- 65.Tampaki E.C., et al. Combined Fascin-1 and MAP17 expression in breast cancer identifies patients with high risk for disease recurrence. Mol Diagn Ther. 2019;23(5):635–644. doi: 10.1007/s40291-019-00411-3. [DOI] [PubMed] [Google Scholar]
- 66.Barnawi R., et al. Fascin Activates beta-catenin signaling and promotes breast cancer stem cell function mainly through focal adhesion kinase (FAK): relation with disease progression. Front. Oncol. 2020;10:440. doi: 10.3389/fonc.2020.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu W., et al. Increased expression of Fascin, motility associated protein, in cell cultures derived from ovarian cancer and in borderline and carcinomatous ovarian tumors. Clin. Exp. Metastasis. 2000;18(1):83–88. doi: 10.1023/a:1026596609969. [DOI] [PubMed] [Google Scholar]
- 68.Daponte A., et al. Prognostic significance of Fascin expression in advanced poorly differentiated serous ovarian cancer. Anticancer Res. 2008;28(3B):1905–1910. [PubMed] [Google Scholar]
- 69.Lin C.K., et al. Association of cortactin, Fascin-1 and epidermal growth factor receptor (EGFR) expression in ovarian carcinomas: correlation with clinicopathological parameters. Dis. Markers. 2008;25(1):17–26. doi: 10.1155/2008/284382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McGuire S., et al. Inhibition of Fascin in cancer and stromal cells blocks ovarian cancer metastasis. Gynecol. Oncol. 2019;153(2):405–415. doi: 10.1016/j.ygyno.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshihara M., et al. Filopodia play an important role in the trans-mesothelial migration of ovarian cancer cells. Exp. Cell. Res. 2020;392(2) doi: 10.1016/j.yexcr.2020.112011. [DOI] [PubMed] [Google Scholar]
- 72.Maitra A., et al. Immunohistochemical validation of a novel epithelial and a novel stromal marker of pancreatic ductal adenocarcinoma identified by global expression microarrays: sea urchin Fascin homolog and heat shock protein 47. Am. J. Clin. Pathol. 2002;118(1):52–59. doi: 10.1309/3PAM-P5WL-2LV0-R4EG. [DOI] [PubMed] [Google Scholar]
- 73.Swierczynski S.L., et al. Analysis of novel tumor markers in pancreatic and biliary carcinomas using tissue microarrays. Hum. Pathol. 2004;35(3):357–366. doi: 10.1016/j.humpath.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 74.Xu Y.F., et al. Fascin promotes the motility and invasiveness of pancreatic cancer cells. World J. Gastroenterol. 2011;17(40):4470–4478. doi: 10.3748/wjg.v17.i40.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsai W.C., et al. The correlation of cortactin and Fascin-1 expression with clinicopathological parameters in pancreatic and ampulla of Vater adenocarcinoma. APMIS. 2013;121(3):171–181. doi: 10.1111/j.1600-0463.2012.02952.x. [DOI] [PubMed] [Google Scholar]
- 76.Li A., et al. Fascin is regulated by slug, promotes progression of pancreatic cancer in mice, and is associated with patient outcomes. Gastroenterology. 2014;146(5):1386–1396. doi: 10.1053/j.gastro.2014.01.046. e1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao X., et al. Hypoxia-inducible factor-1 promotes pancreatic ductal adenocarcinoma invasion and metastasis by activating transcription of the actin-bundling protein Fascin. Cancer Res. 2014;74(9):2455–2464. doi: 10.1158/0008-5472.CAN-13-3009. [DOI] [PubMed] [Google Scholar]
- 78.Misiura M., et al. Actin-bundling proteins (Actinin-4 and Fascin-1) are involved in the development of pancreatic intraepithelial neoplasia (PanIN) Am. J. Med. Sci. 2020;359(3):147–155. doi: 10.1016/j.amjms.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 79.Goncharuk V.N., Ross J.S., Carlson J.A. Actin-binding protein Fascin expression in skin neoplasia. J. Cutan. Pathol. 2002;29(7):430–438. doi: 10.1034/j.1600-0560.2002.290708.x. [DOI] [PubMed] [Google Scholar]
- 80.Pelosi G., et al. Independent value of Fascin immunoreactivity for predicting lymph node metastases in typical and atypical pulmonary carcinoids. Lung Cancer. 2003;42(2):203–213. doi: 10.1016/s0169-5002(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 81.Teng Y., et al. Serological investigation of the clinical significance of Fascin in non-small-cell lung cancer. Lung Cancer. 2013;82(2):346–352. doi: 10.1016/j.lungcan.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 82.Ling X.L., et al. Clinicopathological significance of Fascin-1 expression in patients with non-small cell lung cancer. Onco Targets Ther. 2015;8:1589–1595. doi: 10.2147/OTT.S84308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo A., et al. The clinical significance of FSCN1 in non-small cell lung cancer. Biomed. Pharmacother. 2015;73:75–79. doi: 10.1016/j.biopha.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 84.Zhao W., et al. Expression of Fascin-1 on human lung cancer and paracarcinoma tissue and its relation to clinicopathological characteristics in patients with lung cancer. Onco Targets Ther. 2015;8:2571–2576. doi: 10.2147/OTT.S81915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liang Z., et al. Fascin 1 promoted the growth and migration of non-small cell lung cancer cells by activating YAP/TEAD signaling. Tumour Biol. 2016;37(8):10909–10915. doi: 10.1007/s13277-016-4934-0. [DOI] [PubMed] [Google Scholar]
- 86.Zhang J., et al. Leucine-rich repeats and immunoglobulin-like domains protein 1 and Fascin actin-bundling protein 1 expression in nonsmall cell lung cancer. J. Cancer Res. Ther. 2016;12(Supplement):C248–C251. doi: 10.4103/0973-1482.200749. [DOI] [PubMed] [Google Scholar]
- 87.Yang L., et al. Clinical significance of Fascin-1 and laminin-5 in non-small cell lung cancer. Genet. Mol. Res. 2017;16(2) doi: 10.4238/gmr16029617. [DOI] [PubMed] [Google Scholar]
- 88.Zhao D., et al. Knockdown of Fascin-1 expression suppresses cell migration and invasion of non-small cell lung cancer by regulating the MAPK pathway. Biochem. Biophys. Res. Commun. 2018;497(2):694–699. doi: 10.1016/j.bbrc.2018.02.134. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Y., Liang B., Dong H. Expression of Fascin_1 protein in cancer tissues of patients with nonsmall cell lung cancer and its relevance to patients' clinicopathologic features and prognosis. J. Cancer Res. Ther. 2018;14(4):856–859. doi: 10.4103/jcrt.JCRT_732_17. [DOI] [PubMed] [Google Scholar]
- 90.Lin S., et al. Fascin controls metastatic colonization and mitochondrial oxidative phosphorylation by remodeling mitochondrial actin filaments. Cell Rep. 2019;28(11):2824–2836. doi: 10.1016/j.celrep.2019.08.011. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kolegova E.S., et al. Increases in mRNA and protein levels of the genes for the actin-binding proteins Profilin, Fascin, and Ezrin promote metastasis in non-small cell lung cancer. Mol. Biol. 2020;54(2):285–292. doi: 10.31857/S0026898420020068. [DOI] [PubMed] [Google Scholar]
- 92.Lin S., et al. Fascin promotes lung cancer growth and metastasis by enhancing glycolysis and PFKFB3 expression. Cancer Lett. 2021;518:230–242. doi: 10.1016/j.canlet.2021.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Okada K., et al. Fascin expression is correlated with tumor progression of extrahepatic bile duct cancer. Hepatogastroenterology. 2007;54(73):17–21. [PubMed] [Google Scholar]
- 94.Roh Y.H., et al. Fascin overexpression correlates with positive thrombospondin-1 and syndecan-1 expressions and a more aggressive clinical course in patients with gallbladder cancer. J. Hepatobiliary Pancreat. Surg. 2009;16(3):315–321. doi: 10.1007/s00534-009-0046-1. [DOI] [PubMed] [Google Scholar]
- 95.Won K.Y., et al. Prognostic significance of Fascin expression in extrahepatic bile duct carcinomas. Pathol. Res. Pract. 2009;205(11):742–748. doi: 10.1016/j.prp.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 96.Hashimoto Y., et al. The prognostic relevance of Fascin expression in human gastric carcinoma. Oncology. 2004;67(3–4):262–270. doi: 10.1159/000081327. [DOI] [PubMed] [Google Scholar]
- 97.Tsai W.C., et al. Association of cortactin and Fascin-1 expression in gastric adenocarcinoma: correlation with clinicopathological parameters. J. Histochem. Cytochem. 2007;55(9):955–962. doi: 10.1369/jhc.7A7235.2007. [DOI] [PubMed] [Google Scholar]
- 98.Kim S.J., et al. Galectin-3 increases gastric cancer cell motility by up-regulating Fascin-1 expression. Gastroenterology. 2010;138(3) doi: 10.1053/j.gastro.2009.09.061. 1035-45 e1-2. [DOI] [PubMed] [Google Scholar]
- 99.Yamamoto H., et al. Fascin-1 overexpression and miR-133b downregulation in the progression of gastrointestinal stromal tumor. Mod. Pathol. 2013;26(4):563–571. doi: 10.1038/modpathol.2012.198. [DOI] [PubMed] [Google Scholar]
- 100.Kim S.J., et al. Fascin expression is related to poor survival in gastric cancer. Pathol. Int. 2012;62(12):777–784. doi: 10.1111/pin.12012. [DOI] [PubMed] [Google Scholar]
- 101.Yao J., et al. Signal transducer and activator of transcription 3 signaling upregulates Fascin via nuclear factor-kappaB in gastric cancer: Implications in cell invasion and migration. Oncol. Lett. 2014;7(3):902–908. doi: 10.3892/ol.2014.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guo L., et al. The role of microRNA-133b and its target gene FSCN1 in gastric cancer. J. Exp. Clin. Cancer Res. 2014;33:99. doi: 10.1186/s13046-014-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang Y., et al. Fas signaling promotes gastric cancer metastasis through STAT3-dependent upregulation of Fascin. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0125132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng H.C., Zhao S. The meta and bioinformatics analysis of Fascin expression in gastric cancer: a potential marker for aggressiveness and worse prognosis. Oncotarget. 2017;8(62):105574–105583. doi: 10.18632/oncotarget.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Son B.K., et al. Smad4/Fascin index is highly prognostic in patients with diffuse type EBV-associated gastric cancer. Pathol. Res. Pract. 2018;214(4):475–481. doi: 10.1016/j.prp.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 106.Zhang H., et al. Fascin is a potential biomarker for early-stage oesophageal squamous cell carcinoma. J. Clin. Pathol. 2006;59(9):958–964. doi: 10.1136/jcp.2005.032730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xue L.Y., et al. Expression of Fascin and CK14 in different histological types of cancer and its differential diagnostic significance. Zhonghua Zhong Liu Za Zhi. 2010;32(11):838–844. [PubMed] [Google Scholar]
- 108.Chen W.X., et al. Tumor-associated autoantibodies against Fascin as a novel diagnostic biomarker for esophageal squamous cell carcinoma. Clin. Res. Hepatol. Gastroenterol. 2017;41(3):327–332. doi: 10.1016/j.clinre.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 109.Zeng F.M., et al. Fascin phosphorylation sites combine to regulate esophageal squamous cancer cell behavior. Amino Acids. 2017;49(5):943–955. doi: 10.1007/s00726-017-2398-1. [DOI] [PubMed] [Google Scholar]
- 110.Zigeuner R., et al. Biologic significance of Fascin expression in clear cell renal cell carcinoma: systematic analysis of primary and metastatic tumor tissues using a tissue microarray technique. Urology. 2006;68(3):518–522. doi: 10.1016/j.urology.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 111.Poli G., et al. 2D-DIGE proteomic analysis identifies new potential therapeutic targets for adrenocortical carcinoma. Oncotarget. 2015;6(8):5695–5706. doi: 10.18632/oncotarget.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Poli G., et al. Fascin-1 is a novel prognostic biomarker associated with tumor invasiveness in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2019;104(5):1712–1724. doi: 10.1210/jc.2018-01717. [DOI] [PubMed] [Google Scholar]
- 113.Hashimoto Y., et al. Prognostic significance of Fascin expression in advanced colorectal cancer: an immunohistochemical study of colorectal adenomas and adenocarcinomas. BMC Cancer. 2006;6:241. doi: 10.1186/1471-2407-6-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vignjevic D., et al. Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res. 2007;67(14):6844–6853. doi: 10.1158/0008-5472.CAN-07-0929. [DOI] [PubMed] [Google Scholar]
- 115.Hashimoto Y., Parsons M., Adams J.C. Dual actin-bundling and protein kinase C-binding activities of Fascin regulate carcinoma cell migration downstream of Rac and contribute to metastasis. Mol. Biol. Cell. 2007;18(11):4591–4602. doi: 10.1091/mbc.E07-02-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chan C., et al. Fascin expression predicts survival after potentially curative resection of node-positive colon cancer. Am. J. Surg. Pathol. 2010;34(5):656–666. doi: 10.1097/PAS.0b013e3181db36c0. [DOI] [PubMed] [Google Scholar]
- 117.Oh S.Y., et al. Prognostic impact of Fascin-1 expression is more significant in advanced colorectal cancer. J. Surg. Res. 2012;172(1):102–108. doi: 10.1016/j.jss.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 118.Jung E.J., et al. Clinicopathologic significance of Fascin, extracellular matrix metalloproteinase inducer, and ezrin expressions in colorectal adenocarcinoma. Indian J. Pathol. Microbiol. 2011;54(1):32–36. doi: 10.4103/0377-4929.77320. [DOI] [PubMed] [Google Scholar]
- 119.Conesa-Zamora P., et al. Expression profiling shows differential molecular pathways and provides potential new diagnostic biomarkers for colorectal serrated adenocarcinoma. Int. J. Cancer. 2013;132(2):297–307. doi: 10.1002/ijc.27674. [DOI] [PubMed] [Google Scholar]
- 120.Feng Y., et al. MicroRNA-145 inhibits tumour growth and metastasis in colorectal cancer by targeting Fascin-1. Br. J. Cancer. 2014;110(9):2300–2309. doi: 10.1038/bjc.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 121.Schoumacher M., et al. Conditional expression of Fascin increases tumor progression in a mouse model of intestinal cancer. Eur. J. Cell Biol. 2014;93(10–12):388–395. doi: 10.1016/j.ejcb.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 122.Adams J.C. Fascin-1 as a biomarker and prospective therapeutic target in colorectal cancer. Expert Rev. Mol. Diagn. 2015;15(1):41–48. doi: 10.1586/14737159.2015.976557. [DOI] [PubMed] [Google Scholar]
- 123.Piskor B.M., et al. Immunohistochemical expression of Fascin-1 in colorectal cancer in relation to clinical and pathological parameters. Folia. Histochem. Cytobiol. 2018;1(2):106–112. doi: 10.5603/FHC.a2018.0011. [DOI] [PubMed] [Google Scholar]
- 124.Wang C.Q., et al. High expression of both resistin and Fascin-1 predicts a poor prognosis in patients with colorectal cancer. Biomed. Res. Int. 2020;2020 doi: 10.1155/2020/8753175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shi S., Zheng H.C., Zhang Z.G. Roles of Fascin mRNA expression in colorectal cancer: Meta-analysis and bioinformatics analysis. Mol. Clin. Oncol. 2020;13(2):119–128. doi: 10.3892/mco.2020.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tampakis A., et al. High Fascin-1 expression in colorectal cancer identifies patients at high risk for early disease recurrence and associated mortality. BMC Cancer. 2021;21(1):153. doi: 10.1186/s12885-021-07842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Darnel A.D., et al. Fascin regulates prostate cancer cell invasion and is associated with metastasis and biochemical failure in prostate cancer. Clin. Cancer Res. 2009;15(4):1376–1383. doi: 10.1158/1078-0432.CCR-08-1789. [DOI] [PubMed] [Google Scholar]
- 128.Dim D.C., et al. The usefulness of S100P, mesothelin, Fascin, prostate stem cell antigen, and 14-3-3 sigma in diagnosing pancreatic adenocarcinoma in cytological specimens obtained by endoscopic ultrasound guided fine-needle aspiration. Diagn. Cytopathol. 2014;42(3):193–199. doi: 10.1002/dc.21684. [DOI] [PubMed] [Google Scholar]
- 129.Jefferies M.T., et al. Analysis of Fascin-1 in relation to gleason risk classification and nuclear ets-related gene status of human prostate carcinomas: an immunohistochemical study of clinically annotated tumours from the wales cancer bank. Biomark Cancer. 2017;9 doi: 10.1177/1179299X17710944. 1179299X17710944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen S.F., et al. Effects of small interfering RNAs targeting Fascin on gene expression in oral cancer cells. J. Oral Pathol. Med. 2009;38(9):722–730. doi: 10.1111/j.1600-0714.2009.00769.x. [DOI] [PubMed] [Google Scholar]
- 131.Lee M.K., et al. Proteases are modulated by Fascin in oral cancer invasion. J Cancer Prev. 2018;23(3):141–146. doi: 10.15430/JCP.2018.23.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li A., et al. The actin-bundling protein Fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr. Biol. 2010;20(4):339–345. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ma Y., et al. Fascin 1 is transiently expressed in mouse melanoblasts during development and promotes migration and proliferation. Development. 2013;140(10):2203–2211. doi: 10.1242/dev.089789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ma Y., et al. Fascin 1 is dispensable for developmental and tumour angiogenesis. Biol. Open. 2013;2(11):1187–1191. doi: 10.1242/bio.20136031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kang J., et al. Fascin induces melanoma tumorigenesis and stemness through regulating the Hippo pathway. Cell Commun. Signal. 2018;16(1):37. doi: 10.1186/s12964-018-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kang B.S., Lim S.C. Fascin regulates the hippo pathway and is important for melanoma development. Anticancer Res. 2021;41(5):2403–2410. doi: 10.21873/anticanres.15015. [DOI] [PubMed] [Google Scholar]
- 137.Chiyomaru T., et al. miR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br. J. Cancer. 2010;102(5):883–891. doi: 10.1038/sj.bjc.6605570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.El-Rehim D.M., et al. Expression of extracellular matrix metalloproteinase inducer and Fascin in urinary bladder cancer: correlation with clinicopathological characteristics. Mol. Clin. Oncol. 2013;1(2):297–304. doi: 10.3892/mco.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kress A.K., et al. The tumor marker Fascin is strongly induced by the Tax oncoprotein of HTLV-1 through NF-kappaB signals. Blood. 2011;117(13):3609–3612. doi: 10.1182/blood-2010-09-305805. [DOI] [PubMed] [Google Scholar]
- 140.Mohr C.F., et al. The tumor marker Fascin is induced by the Epstein-Barr virus-encoded oncoprotein LMP1 via NF-kappaB in lymphocytes and contributes to their invasive migration. Cell Commun. Signal. 2014;12:46. doi: 10.1186/s12964-014-0046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.El Kramani N., et al. Clinical significance of the TNF-alpha receptors, TNFRSF2 and TNFRSF9, on cell migration molecules Fascin-1 and Versican in acute leukemia. Cytokine. 2018;111:523–529. doi: 10.1016/j.cyto.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 142.Hayashi Y., Osanai M., Lee G.H. Fascin-1 expression correlates with repression of E-cadherin expression in hepatocellular carcinoma cells and augments their invasiveness in combination with matrix metalloproteinases. Cancer Sci. 2011;102(6):1228–1235. doi: 10.1111/j.1349-7006.2011.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lin C.K., et al. Expression of LGR8 and related biomarkers in hepatocellular carcinoma: correlation with clinicopathological parameters. Chin. J. Physiol. 2011;54(3):161–168. doi: 10.4077/cjp.2011.amm021. [DOI] [PubMed] [Google Scholar]
- 144.Huang X., et al. Fascin and cortactin expression is correlated with a poor prognosis in hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2012;24(6):633–639. doi: 10.1097/MEG.0b013e3283515a18. [DOI] [PubMed] [Google Scholar]
- 145.Zhang Y., et al. FSCN1 increases doxorubicin resistance in hepatocellular carcinoma through promotion of epithelial-mesenchymal transition. Int. J. Oncol. 2018;52(5):1455–1464. doi: 10.3892/ijo.2018.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gun B.D., et al. Clinicopathological significance of Fascin and CD44v6 expression in endometrioid carcinoma. Diagn. Pathol. 2012;7:80. doi: 10.1186/1746-1596-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Papaspyrou K., et al. Fascin upregulation in primary head and neck squamous cell carcinoma is associated with lymphatic metastasis. Oncol. Lett. 2014;7(6):2041–2046. doi: 10.3892/ol.2014.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee L.Y., et al. Fascin is a circulating tumor marker for head and neck cancer as determined by a proteomic analysis of interstitial fluid from the tumor microenvironment. Clin. Chem. Lab. Med. 2015;53(10):1631–1641. doi: 10.1515/cclm-2014-1016. [DOI] [PubMed] [Google Scholar]
- 149.Hoa N.T., et al. Fascin-1 knock-down of human glioma cells reduces their microvilli/filopodia while improving their susceptibility to lymphocyte-mediated cytotoxicity. Am. J. Transl. Res. 2015;7(2):271–284. [PMC free article] [PubMed] [Google Scholar]
- 150.Li X., et al. Knocking down Fascin inhibits cervical cancer cell proliferation and tumorigenesis in nude mice. Nan Fang Yi Ke Da Xue Xue Bao. 2018;38(12):1409–1414. doi: 10.12122/j.issn.1673-4254.2018.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yousefi Ghalejoogh Z., et al. Human papilloma virus infection and Fascin over-expression in squamous cell carcinoma of the cervix. Med. J. Islam Repub. Iran. 2018;32:134. doi: 10.14196/mjiri.32.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wei L., Chang H., Huo S. Analyses on K-ras mutations and Fascin expression in patients with cardia cancer. Oncol. Lett. 2019;17(2):1807–1811. doi: 10.3892/ol.2018.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee M.K., et al. IL-1beta induces Fascin expression and increases cancer invasion. Anticancer Res. 2018;38(11):6127–6132. doi: 10.21873/anticanres.12964. [DOI] [PubMed] [Google Scholar]
- 154.Snyder M., Huang X.Y., Zhang J.J. Signal transducers and activators of transcription 3 (STAT3) directly regulates cytokine-induced Fascin expression and is required for breast cancer cell migration. J. Biol. Chem. 2011;286(45):38886–38893. doi: 10.1074/jbc.M111.286245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang C.Q., et al. EGFR conjunct FSCN1 as a novel therapeutic strategy in triple-negative breast cancer. Sci. Rep. 2017:7. doi: 10.1038/s41598-017-15939-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu L., et al. Proteomic analysis of Tiam1-mediated metastasis in colorectal cancer. Cell Biol. Int. 2007;31(8):805–814. doi: 10.1016/j.cellbi.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 157.Jeong B.Y., et al. Rab25 augments cancer cell invasiveness through a beta 1 integrin/EGFR/VEGF-A/Snail signaling axis and expression of Fascin (vol 50, e435, 2018) Exp. Mol. Med. 2018;50 doi: 10.1038/emm.2017.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li D., et al. The prometastatic ribosomal S6 kinase 2-cAMP response element-binding protein (RSK2-CREB) signaling pathway up-regulates the actin-binding protein Fascin-1 to promote tumor metastasis. J. Biol. Chem. 2013;288(45):32528–32538. doi: 10.1074/jbc.M113.500561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li L., et al. TGF-beta induces Fascin expression in gastric cancer via phosphorylation of smad3 linker area. Am. J. Cancer Res. 2015;5(6):1890–1896. [PMC free article] [PubMed] [Google Scholar]
- 160.Qian C.J., et al. Notch4 inhibition reduces migration and invasion and enhances sensitivity to docetaxel by inhibiting Akt/Fascin in pancreatic cancer cells. Oncol. Lett. 2016;12(5):3499–3505. doi: 10.3892/ol.2016.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lu X.F., et al. Specificity protein 1 regulates Fascin expression in esophageal squamous cell carcinoma as the result of the epidermal growth factor/extracellular signal-regulated kinase signaling pathway activation. Cell. Mol. Life Sci. 2010;67(19):3313–3329. doi: 10.1007/s00018-010-0382-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sun J., et al. GATA3 transcription factor abrogates Smad4 transcription factor-mediated Fascin overexpression, invadopodium formation, and breast cancer cell invasion. J. Biol. Chem. 2013;288(52):36971–36982. doi: 10.1074/jbc.M113.506535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Anderson S., et al. MYC-nick promotes cell migration by inducing Fascin expression and Cdc42 activation. Proc. Natl. Acad. Sci. U S A. 2016;113(37):E5481–E5490. doi: 10.1073/pnas.1610994113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Al-Alwan M., et al. Fascin is a key regulator of breast cancer invasion that acts via the modification of metastasis-associated molecules. PLoS One. 2011;6(11) doi: 10.1371/journal.pone.0027339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang S., Lin Q.D., Di W. Suppression of human ovarian carcinoma metastasis by the metastasis-suppressor gene, BRMS1. Int. J. Gynecol. Cancer. 2006;16(2):522–531. doi: 10.1111/j.1525-1438.2006.00547.x. [DOI] [PubMed] [Google Scholar]
- 166.Kang B.S., Lim S.C. Fascin regulates the hippo pathway and is important for melanoma development. Anticancer Res. 2021;41(5):2403–2410. doi: 10.21873/anticanres.15015. [DOI] [PubMed] [Google Scholar]
- 167.Pfisterer K., et al. FMNL2 regulates dynamics of Fascin in filopodia. J. Cell Biol. 2020;219(5) doi: 10.1083/jcb.201906111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hao L., et al. Formin homology domains of Daam1 bind to Fascin and collaboratively promote pseudopodia formation and cell migration in breast cancer. Cell Prolif. 2021;54(3):e12994. doi: 10.1111/cpr.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhan X.H., et al. LOXL2 upregulates phosphorylation of ezrin to promote cytoskeletal reorganization and tumor cell invasion. Cancer Res. 2019;79(19):4951–4964. doi: 10.1158/0008-5472.CAN-19-0860. [DOI] [PubMed] [Google Scholar]
- 170.Zhang Z.D., et al. AKT serine/threonine kinase 2-mediated phosphorylation of Fascin threonine 403 regulates esophageal cancer progression. Int. J. Biochem. Cell Biol. 2022;145 doi: 10.1016/j.biocel.2022.106188. [DOI] [PubMed] [Google Scholar]
- 171.Yamakita Y., et al. Phosphorylation of human Fascin inhibits its actin binding and bundling activities. J. Biol. Chem. 1996;271(21):12632–12638. doi: 10.1074/jbc.271.21.12632. [DOI] [PubMed] [Google Scholar]
- 172.Adams J.C., et al. Cell-matrix adhesions differentially regulate Fascin phosphorylation. Mol. Biol. Cell. 1999;10(12):4177–4190. doi: 10.1091/mbc.10.12.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Aratyn Y.S., et al. Intrinsic dynamic behavior of Fascin in filopodia. Mol. Biol. Cell. 2007;18(10):3928–3940. doi: 10.1091/mbc.E07-04-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cheng Y.W., et al. P300/CBP-associated factor (PCAF)-mediated acetylation of Fascin at lysine 471 inhibits its actin-bundling activity and tumor metastasis in esophageal cancer. Cancer Commun. 2021;41(12):1398–1416. doi: 10.1002/cac2.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lin S., et al. Monoubiquitination inhibits the actin bundling activity of Fascin. J. Biol. Chem. 2016;291(53):27323–27333. doi: 10.1074/jbc.M116.767640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lamb M.C., Tootle T.L. Fascin in cell migration: more than an actin bundling protein. Biology. 2020;9(11) doi: 10.3390/biology9110403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lamb M.C., et al. Fascin limits Myosin activity within Drosophila border cells to control substrate stiffness and promote migration. Elife. 2021;10 doi: 10.7554/eLife.69836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lin S.C., et al. Fascin controls metastatic colonization and mitochondrial oxidative phosphorylation by remodeling mitochondrial actin filaments. Cell Rep. 2019;28(11):2824. doi: 10.1016/j.celrep.2019.08.011. -+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen C., et al. Fascin enhances the vulnerability of breast cancer to erastin-induced ferroptosis. Cell Death. Dis. 2022;13(2) doi: 10.1038/s41419-022-04579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Saad A., et al. Insights into a novel nuclear function for Fascin in the regulation of the amino-acid transporter SLC3A2. Sci. Rep. 2016;6 doi: 10.1038/srep36699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Osanai M., Lee G.H. The retinoic acid-metabolizing enzyme CYP26A1 upregulates Fascin and promotes the malignant behavior of breast carcinoma cells. Oncol. Rep. 2015;34(2):850–858. doi: 10.3892/or.2015.4042. [DOI] [PubMed] [Google Scholar]
- 182.Dinicola S., et al. Inositol induces mesenchymal-epithelial reversion in breast cancer cells through cytoskeleton rearrangement. Exp. Cell. Res. 2016;345(1):37–50. doi: 10.1016/j.yexcr.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 183.Huang J., et al. Identification of the fatty acid synthase interaction network via iTRAQ-based proteomics indicates the potential molecular mechanisms of liver cancer metastasis. Cancer Cell Int. 2020;20:332. doi: 10.1186/s12935-020-01409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gonzalez-Reyes C., et al. Migration and invasion induced by linoleic acid are mediated through Fascin in MDA-MB-231 breast cancer cells. Mol. Cell. Biochem. 2018;443(1–2):Cp6–C10. doi: 10.1007/s11010-017-3205-8. [DOI] [PubMed] [Google Scholar]
- 185.Wang X., et al. Vimentin plays an important role in the promotion of breast cancer cell migration and invasion by leucine aminopeptidase 3. Cytotechnology. 2020;72(5):639–647. doi: 10.1007/s10616-020-00402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang X., et al. Inhibition of leucine aminopeptidase 3 suppresses invasion of ovarian cancer cells through down-regulation of Fascin and MMP-2/9. Eur. J. Pharmacol. 2015;768:116–122. doi: 10.1016/j.ejphar.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 187.Yoshida K., et al. Proteomic differential display analysis for TS-1-resistant and -sensitive pancreatic cancer cells using two-dimensional gel electrophoresis and mass spectrometry. Anticancer Res. 2011;31(6):2103–2108. [PubMed] [Google Scholar]
- 188.Alaeddini M., et al. Expression of Fascin protein and mRNA in the KB carcinoma cell line following treatment with doxorubicin. J. Cancer Res. Ther. 2011;7(4):427–432. doi: 10.4103/0973-1482.92009. [DOI] [PubMed] [Google Scholar]
- 189.Ghebeh H., et al. Fascin is involved in the chemotherapeutic resistance of breast cancer cells predominantly via the PI3K/Akt pathway. Br. J. Cancer. 2014;111(8):1552–1561. doi: 10.1038/bjc.2014.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kim S.J., et al. Galectin-3 germline variant at position 191 enhances nuclear accumulation and activation of beta-catenin in gastric cancer. Clin. Exp. Metastasis. 2011;28(8):743–750. doi: 10.1007/s10585-011-9406-8. [DOI] [PubMed] [Google Scholar]
- 191.Hao Y.J., et al. Role of RNA-interference-induced zinc finger protein 139 suppression in gastric cancer cell sensitivity to chemotherapeutic agents. Oncol. Lett. 2015;10(3):1333–1338. doi: 10.3892/ol.2015.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Qian C.J., et al. Notch4 inhibition reduces migration and invasion and enhances sensitivity to docetaxel by inhibiting Akt/Fascin in pancreatic cancer cells. Oncol. Lett. 2016;12(5):3499–3505. doi: 10.3892/ol.2016.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Barnawi R., et al. beta 1 Integrin is essential for Fascin-mediated breast cancer stem cell function and disease progression. Int. J. Cancer. 2019;145(3):830–841. doi: 10.1002/ijc.32183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kanda Y., et al. Fascin protein stabilization by miR-146a implicated in the process of a chronic inflammation-related colon carcinogenesis model. Inflamm. Res. 2018;67(10):839–846. doi: 10.1007/s00011-018-1175-2. [DOI] [PubMed] [Google Scholar]
- 195.Barnawi R., et al. Fascin is critical for the maintenance of breast cancer stem cell pool predominantly via the activation of the notch self-renewal pathway. Stem Cells. 2016;34(12):2799–2813. doi: 10.1002/stem.2473. [DOI] [PubMed] [Google Scholar]
- 196.Kang J.X., et al. Fascin induces melanoma tumorigenesis and stemness through regulating the Hippo pathway. Cell Commun. Signal. 2018;16 doi: 10.1186/s12964-018-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mohr C.F., et al. Regulation of the tumor marker Fascin by the viral oncoprotein Tax of human T-cell leukemia virus type 1 (HTLV-1) depends on promoter activation and on a promoter-independent mechanism. Virology. 2015;485:481–491. doi: 10.1016/j.virol.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 198.Yamamoto T., et al. Circulating myeloid dendritic cells as prognostic factors in patients with pancreatic cancer who have undergone surgical resection. J. Surg. Res. 2012;173(2):299–308. doi: 10.1016/j.jss.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 199.Said J.W., et al. Alterations in Fascin-expressing germinal center dendritic cells in neoplastic follicles of B-cell lymphomas. Mod. Pathol. 1998;11(1):1–5. [PubMed] [Google Scholar]
- 200.Ohtani H., et al. Abundant expression of CXCL9 (MIG) by stromal cells that include dendritic cells and accumulation of CXCR3(+) T cells in lymphocyte-rich gastric carcinoma. J. Pathol. 2009;217(1):21–31. doi: 10.1002/path.2448. [DOI] [PubMed] [Google Scholar]
- 201.Wang Y.F., et al. Fascin inhibitor increases intratumoral dendritic cell activation and anti-cancer immunity. Cell Rep. 2021;35(1) doi: 10.1016/j.celrep.2021.108948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang X.L., et al. Fascin is involved in cancer cell invasion and is regulated by stromal factors. Oncol. Rep. 2019;41(1):465–474. doi: 10.3892/or.2018.6847. [DOI] [PubMed] [Google Scholar]
- 203.Yamada Y., et al. Fascin as a useful marker for cancer-associated fibroblasts in invasive lung adenocarcinoma. Medicine. 2021;100(35) doi: 10.1097/MD.0000000000027162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Teng Y., et al. Serological investigation of the clinical significance of Fascin in non-small-cell lung cancer. Lung Cancer. 2013;82(2):346–352. doi: 10.1016/j.lungcan.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 205.Chen W.X., et al. Tumor-associated autoantibodies against Fascin as a novel diagnostic biomarker for esophageal squamous cell carcinoma. Clin. Res. Hepatol. Gastroenterol. 2017;41(3):327–332. doi: 10.1016/j.clinre.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 206.Rubenich D.S., et al. Small extracellular vesicle-mediated bidirectional crosstalk between neutrophils and tumor cells. Cytokine Growth Factor Rev. 2021 doi: 10.1016/j.cytogfr.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Beghein E., et al. Cortactin and Fascin-1 regulate extracellular vesicle release by controlling endosomal trafficking or invadopodia formation and function. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-33868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhao X., et al. Hypoxia-inducible factor-1 promotes pancreatic ductal adenocarcinoma invasion and metastasis by activating transcription of the actin-bundling protein Fascin. Cancer Res. 2014;74(9):2455–2464. doi: 10.1158/0008-5472.CAN-13-3009. [DOI] [PubMed] [Google Scholar]
- 209.Ma Y.F., et al. Fascin 1 is dispensable for developmental and tumour angiogenesis. Biol. Open. 2013;2(11):1187–1191. doi: 10.1242/bio.20136031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kim M.Y., et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen L., et al. Migrastatin analogues target Fascin to block tumour metastasis (vol 464, pg 1062, 2010) Nature. 2011;476(7359) doi: 10.1038/nature08978. 240-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Huang F.K., et al. Targeted inhibition of Fascin function blocks tumour invasion and metastatic colonization. Nat. Commun. 2015;6 doi: 10.1038/ncomms8465. [DOI] [PubMed] [Google Scholar]
- 213.McGuire S., et al. Inhibition of Fascin in cancer and stromal cells blocks ovarian cancer metastasis. Gynecol. Oncol. 2019;153(2):405–415. doi: 10.1016/j.ygyno.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Montoro-Garcia S., et al. Novel anti-invasive properties of a Fascin1 inhibitor on colorectal cancer cells. J. Mol. Med. 2020;98(3):383–394. doi: 10.1007/s00109-020-01877-z. [DOI] [PubMed] [Google Scholar]
- 215.Han S.Q., et al. Improving Fascin inhibitors to block tumor cell migration and metastasis. Mol. Oncol. 2016;10(7):966–980. doi: 10.1016/j.molonc.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang Y.F., Zhang J.J., Huang X.Y. Anti-metastasis Fascin inhibitors decrease the growth of specific subtypes of cancers. Cancers. 2020;12(8) doi: 10.3390/cancers12082287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Huang J.Y., et al. Structural insights into the induced-fit inhibition of Fascin by a small-molecule inhibitor. J. Mol. Biol. 2018;430(9):1324–1335. doi: 10.1016/j.jmb.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zheng S.L., et al. Modification and biological evaluation of thiazole derivatives as novel inhibitors of metastatic cancer cell migration and invasion. J. Med. Chem. 2014;57(15):6653–6667. doi: 10.1021/jm500724x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Riahi N., et al. Design, synthesis and molecular docking studies of some tetrahydropyrimidine derivatives as possible Fascin inhibitors. Chem. Biodivers. 2019;16(2) doi: 10.1002/cbdv.201800339. [DOI] [PubMed] [Google Scholar]
- 220.Francis S., et al. Structure-based design, synthesis and biological evaluation of a novel series of isoquinolone and pyrazolo[4,3-c]pyridine inhibitors of Fascin 1 as potential anti-metastatic agents. Bioorg. Med. Chem. Lett. 2019;29(8):1023–1029. doi: 10.1016/j.bmcl.2019.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Alburquerque-Gonzalez B., et al. The FDA-approved antiviral raltegravir inhibits Fascin1-dependent invasion of colorectal tumor cells in vitro and in vivo. Cancers. 2021;13(4) doi: 10.3390/cancers13040861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Alburquerque-Gonzalez B., et al. New role of the antidepressant imipramine as a Fascin1 inhibitor in colorectal cancer cells. Exp. Mol. Med. 2020;52(2):281–292. doi: 10.1038/s12276-020-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jabeen A., et al. Elaeagnus angustifolia plant extract inhibits epithelial-mesenchymal transition and induces apoptosis via HER2 inactivation and JNK pathway in HER2-positive breast cancer cells. Molecules. 2020;25(18) doi: 10.3390/molecules25184240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kim M.J., et al. The inhibitory effect of curcumin via Fascin suppression through JAK/STAT3 pathway on metastasis and recurrence of ovary cancer cells. Bmc Womens Health. 2020;20(1) doi: 10.1186/s12905-020-01122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mantaj J., et al. Crispene E, a cis-clerodane diterpene inhibits STAT3 dimerization in breast cancer cells. Org. Biomol. Chem. 2015;13(13):3882–3886. doi: 10.1039/c5ob00052a. [DOI] [PubMed] [Google Scholar]
- 226.Xie Q., et al. Ilamycin C induces apoptosis and inhibits migration and invasion in triple-negative breast cancer by suppressing IL-6/STAT3 pathway. J. Hematol. Oncol. 2019;12 doi: 10.1186/s13045-019-0744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mohr C.F., et al. The tumor marker Fascin is induced by the Epstein-Barr virus-encoded oncoprotein LMP1 via NF-kappa B in lymphocytes and contributes to their invasive migration. Cell Commun. Signal. 2014;12 doi: 10.1186/s12964-014-0046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Liang H., et al. Sevoflurane suppresses hypoxia-induced growth and metastasis of lung cancer cells via inhibiting hypoxia-inducible factor-1alpha. J. Anesth. 2015;29(6):821–830. doi: 10.1007/s00540-015-2035-7. [DOI] [PubMed] [Google Scholar]
- 229.Lamango N.S., et al. Polyisoprenylated cysteinyl amide inhibitors: a novel approach to controlling cancers with hyperactive growth signaling. Curr. Med. Chem. 2021;28(18):3476–3489. doi: 10.2174/0929867327666201111140825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Schenk M., et al. Salinomycin inhibits growth of pancreatic cancer and cancer cell migration by disruption of actin stress fiber integrity. Cancer Lett. 2015;358(2):161–169. doi: 10.1016/j.canlet.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 231.Li D., et al. The prometastatic ribosomal S6 Kinase 2-cAMP response element-binding protein (RSK2-CREB) signaling pathway up-regulates the actin-binding protein Fascin-1 to promote tumor metastasis. J. Biol. Chem. 2013;288(45):32528–32538. doi: 10.1074/jbc.M113.500561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yang H.L., et al. Inhibition of the proliferation, migration, and invasion of human breast cancer cells by leucine aminopeptidase 3 inhibitors derived from natural marine products. Anticancer Drugs. 2020;31(1):60–66. doi: 10.1097/CAD.0000000000000842. [DOI] [PubMed] [Google Scholar]
- 233.Mahmoud A., et al. Novel polymethoxylated chalcones as potential compounds against KRAS-mutant colorectal cancers. Curr. Pharm. Des. 2020;26(14):1622–1633. doi: 10.2174/1381612826666200206095400. [DOI] [PubMed] [Google Scholar]
- 234.Alyoussef A. The therapeutic effects of blocking IGF-R1 on mice model of skin cancer. J. Dermatol. Treatm. 2020 doi: 10.1080/09546634.2019.1708243. [DOI] [PubMed] [Google Scholar]
- 235.Cohan C.S., et al. Role of the actin bundling protein Fascin in growth cone morphogenesis: localization in filopodia and lamellipodia. Cell Motil. Cytoskeleton. 2001;48(2):109–120. doi: 10.1002/1097-0169(200102)48:2<109::AID-CM1002>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 236.Lii C.K., et al. Docosahexaenoic acid inhibits 12-O-tetradecanoylphorbol-13-acetate-induced Fascin-1-dependent breast cancer cell migration by suppressing the PKC delta- and Wnt-1/beta-catenin-mediated pathways. Oncotarget. 2016;7(18):25162–25179. doi: 10.18632/oncotarget.7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhu G.Q., et al. 12-O-Tetradecanoylphorbol-13-acetate (TPA) is anti-tumorigenic in liver cancer cells via inhibiting YAP through AMOT. Sci. Rep. 2017;7 doi: 10.1038/srep44940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Khan A., et al. Recombinant porcine NK-lysin inhibits the invasion of hepatocellular carcinoma cells in vitro. Int. J. Biol. Macromol. 2019;140:1249–1259. doi: 10.1016/j.ijbiomac.2019.08.212. [DOI] [PubMed] [Google Scholar]
- 239.Proietti S., et al. Increase in motility and invasiveness of MCF7 cancer cells induced by nicotine is abolished by melatonin through inhibition of ERK phosphorylation. J. Pineal Res. 2018;64(4) doi: 10.1111/jpi.12467. [DOI] [PubMed] [Google Scholar]
- 240.Zhang C., Ge C. A simple competing endogenous rna network identifies novel mRNA, miRNA, and lncRNA markers in human cholangiocarcinoma. Biomed. Res. Int. 2019;2019 doi: 10.1155/2019/3526407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chen D., et al. Pristine Cu-MOF induces mitotic catastrophe and alterations of gene expression and cytoskeleton in ovarian cancer cells. ACS Appl. Bio Mater. 2020;3(7):4081–4094. doi: 10.1021/acsabm.0c00175. [DOI] [PubMed] [Google Scholar]