Highlights
-
•
AFM measures and MD simulations show that quercetin reduces the elastic moduli of lipid bilayer membranes.
-
•
MD simulations show that membranes also get thinner as the acyl chains get more disordered, and lipid diffusion coefficients increase.
-
•
Changes in membrane properties can be interpreted in the light of quercetin localization and orientation in the membrane.
Keywords: Quercetin, Lipoid E80, Young modulus, Area compressibility modulus, Molecular dynamics, AFM
Abstract
Quercetin (3,3ʹ,4ʹ,5,7-pentahydroxyl-flavone) is a natural flavonoid with many valuable biological effects, but its solubility in water is low, posing major limitations in applications. Quercetin encapsulation in liposomes increases its bioavailability; the drug effect on liposome elastic properties is required for formulation development. Here, we quantify the effect of quercetin molecules on the rigidity of lipoid E80 liposomes using atomic force microscopy (AFM) and molecular dynamics (MD) simulations. AFM images show no effect of quercetin molecules on liposomes morphology and structure. However, AFM force curves suggest that quercetin softens lipid membranes; the Young modulus measured for liposomes encapsulating quercetin is smaller than that determined for blank liposomes. We then used MD simulations to interpret the effect of quercetin on membrane rigidity in terms of molecular interactions. The decrease in membrane rigidity was confirmed by the simulations, which also revealed that quercetin affects structural and dynamic properties: membrane thickness is decreased, acyl chains disorder is increased, and diffusion coefficients of lipid molecules are also increased. Such changes appear to be related to the preferential localization of quercetin within the membrane, near the interface between the hydrophobic core and polar head groups of the lipids.
Graphical abstract
Introduction
Quercetin (3,3ʹ,4ʹ,5,7-pentahydroxyl-flavone) is a common flavonoid occurring naturally in a variety of brightly colored plant-based foods (e.g., onion, apple, broccoli, tea, and red wine [1]). Quercetin has been the subject of many investigations because of its numerous beneficial properties, including antioxidant [1,2], antiviral [3,4], anticancer [5], antimicrobial [6,7], anti-inflammatory [8,9], and anti-obesity [10] properties. Currently there is growing interest for using quercetin as an ingredient in pharmaceutical preparations or food products. However, because of its low aqueous solubility (about 0.01 mg/mL at 25 C) [11], low bioavailability [12], and reduced chemical stability [11], the use of quercetin is still limited. The encapsulation of quercetin in liposomes could overcome the drawbacks related to the physico-chemical properties of this flavonoid [13], [14], [15]. Experimental works show that quercetin can be incorporated into liposomes [15,16]. When encapsulating drugs or other small molecules, it is important that liposome mechanical stability and elastic properties are preserved, since liposome elasticity impacts size, shape, membrane permeability, and drug loading efficiency [17], [18], [19], [20], and tissue targeting [17,21]. These aspects have been investigated by several research teams [4,18,[22], [23], [24]]. Liposome elasticity may be affected by the incorporation of quercetin, but the effect of quercetin on liposome's elasticity is still not known.
Here, we aim to characterize the elastic properties of liposomes encapsulating quercetin. To this end, we use both experimental and computational methods. We are particularly interested in characterizing the elasticity of nano-sized liposomes, with size below 100 nm, because they are the most useful for drug delivery purposes. Considering the small size, Atomic Force Microscopy (AFM) is a good choice for experimental measures of elasticity, as it can be used directly on nano-sized liposomes without additives. AFM can provide high-resolution images of a surface at nanometer scales, and it has been often used in the study of lipid vesicles [25], [26], [27]. Recently, we optimized the AFM experimental conditions for measuring the Young modulus of lipoid E80 vesicles [28]. The optimized conditions are applied in the present study. As for the choice of liposome composition, we demonstrated in a previous study that quercetin-loaded lipoid E80 liposomes exhibited better encapsulation efficiency compared to Phospholipon 90H and Lipoid S100 liposomes formulations [18]. Taking into consideration the yield of the encapsulation process, we use Lipoid E80 as the formulation of choice in the present study. We then interpret the AFM data using molecular dynamics (MD) simulations at the atomistic level, mimicking experimental conditions as closely as possible. Previous MD investigations probed the position and orientation of quercetin in lipid membranes, and showed that quercetin is found about half way between the hydrophobic core and the polar head groups [29], [30], [31]. Here, we use MD simulations to predict the effect of quercetin on membrane elastic properties, and particularly the area compressibility modulus. In addition, we use simulations to provide a detailed view of the structure and dynamics of bilayers incorporating quercetin, allowing for an interpretation of the experimental findings in terms of interactions at the atomic level.
Materials and methods
Experimental materials
Lipoid E80, purchased from Lipoid GmbH (Ludwigshafen, Germany) is constituted of 80-86% egg phosphatidylcholine (PC), 7-9.5% phosphatidylethanolamine (PE), 3% lyso-PC, 0.5% lyso-PE, 1-2% sphingomyelin, 2% water, 0.2% ethanol. Ethanol, quercetin (>95%), and cholesterol (95%) (Chol) were purchased from Sigma Aldrich (France).
Pyrex-nitride probes triangular cantilevers PNP-TR (NanoWord Innovative Technologies, Nano and More, Paris, France) integrating a sharpened pyramidal tip with a radius < 10 nm and a macroscopic half cone 35o angle, were used for imaging and indenting liposomes. Mica sheets (9 mm diameter and 0.1 mm thickness) were purchased from Nano and More (France).
Experimental setup: preparation of liposomes
The ethanol injection method was used to prepare liposomes according to the protocol described by Azzi et al. [18]. Two batches were prepared: 1, lipoid E80:Chol liposomes; 2, quercetin loaded lipoid E80:Chol liposomes. Briefly, the appropriate amounts of phospholipids, Chol, and quercetin, were dissolved in ethanol (10 ml) at concentrations of 40, 20, and 3.02 mg/ml, respectively. The lipoid E80:Chol molar ratio was of 100:98 and that of lipoid E80:Chol:quercetin was of 100:98:20. The organic solution was then injected, using a syringe pump into ultra-pure water (20 ml) under magnetic stirring (400 rpm). Ethanol was removed by rotary evaporation at 40°C under reduced pressure and the liposome suspensions were stored at 4°C. AFM measurements were realized during three months after the preparation of liposomes. The final composition was 100:74 (phospholipids:Chol) for blank liposomes and 100:55:15 (phospholids:Chol:quercetin) for quercetin-loaded liposomes.
AFM measurements
AFM imaging and force measurements were performed at room temperature using an Agilent 5420 microscope (Key sight, California, USA) equipped with the AFM probe described before. An aliquot of the liposomal suspension was diluted 10 times in ultrapure water. Then, 10 µl of the diluted formulation was deposited on freshly cleaved mica surface and left for 15 min in air at room temperature, for adsorption. The mica sheet was rinsed with ultrapure water to remove the non-adsorbed vesicles. The sample was then imaged in contact mode with a typical scan rate of 1 Hz, resolution of 512 × 512 pixels per image, 5 × 5, and 2 × 2 µm2 images sizes, scanning angle of 00, and a set point typically below 0.1 nN. For each sample, adsorbed vesicles were observed, and 200 force curves were recorded for 200 different liposomes (one curve per liposome), in force spectroscopy mode. Each curve was obtained by indenting centrally an adsorbed vesicle with a maximum force of 1.5 nN and a rate of 0.145 µm/s. Before the indentation measurement, the deflection sensitivity (deflection/voltage ratio) was calibrated on a clean mica surface. The force calibration plot was converted into force versus liposome deformation. This is possible by determining the zero force point, and the zero separation point between the tip and the sample. Zero force was determined where the separation tip-sample is large enough and the cantilever deflection attains its constant initial value was identified where the deflection of the cantilever was linear with the expansion of the piezo scanner.
In our previous work, we demonstrated that Shell model provides the closest agreement between our AFM data and other experimental data for the membrane bending modulus comparing to Hertz model [28]. Hence, we fitted the force versus liposome deformation curves with Shell model to extract the Young modulus . The model was applied in the elastic region, when the indentation of the vesicle did not exceed 10% of its diameter. The Young modulus (E) was then determined using the following relationship, linking the measured force (F) with the measured deformation () [32]:
| (1) |
where is the radius of curvature of the indented liposomes, and is the membrane thickness. C is a correction factor introduced by Berry et al. for adsorbed vesicles on a rigid substrate [33]. The authors suggest that the measured indentation is the sum of indentions caused by both the indenter and the substrate [33]. The Poisson's ratio is assumed to be 0.5 and the membrane thickness 4 nm.
The bending modulus can, in principle, be derived from the following relation [34]:
| (2) |
Molecular simulations
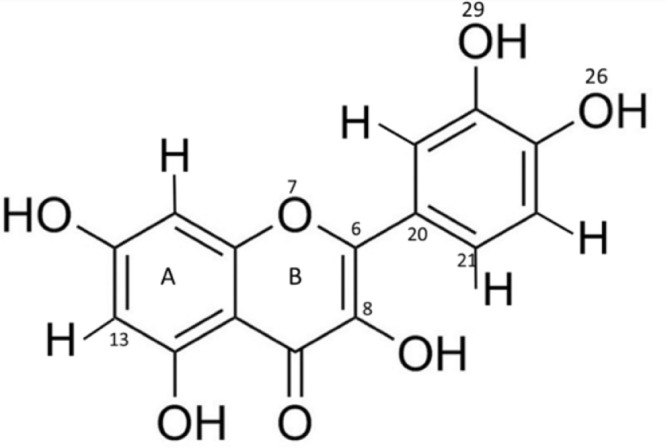
We built the topology for quercetin using an automatic server, the LigParGen web-based service [35], [36], [37], providing a complete topology for the OPLS-AA force field [38], compatible with the GROMACS software [39] that we used for all simulations. We used only the neutral form of the molecule, because the of quercetin is close to neutral pH [40]; also, we are mostly interested in studying the molecule in a membrane environment, where the will be higher, i.e., it will be more difficult to deprotonate the weak acid and the neutral form will be more likely [40]. The LigParGen server produces partial charges based on the precise geometry of the submitted molecule, so we used 2 conformations for quercetin, differing by the rotation of the main dihedral angle, corresponding to conformations (a) with the rings in the same plane and (b) rotated by 180 degrees. The partial charges produced by the server were similar but not identical, and we used the average value of the partial charge for each atom. The complete topology is provided in Supporting Information. Fig. 1, show the geometry of quercetin molecule.
Fig. 1.
Geometry and atom numbering of quercetin.
We then set up a simulation of one quercetin molecule in a box full of SPC (single point charge) water [41] of size 4 × 4 × 4 nm. We first performed energy minimization (steepest descent, 10000 steps), then equilibration for 20 ns in the NPT ensemble (298K and 1 bar), and finally a production run for 200 ns.
We used model membranes similar to the ones used in the AFM experiments, at the same temperature, to mimic AFM experimental conditions. We used the MemGen [42] software to generate starting structures for lipid bilayers, containing 138 lipid molecules (80 POPC, 10 POPE, and 48 Chol molecules) in a box full of water (4373 water molecules) with initial dimension of .
We used the force field by Berger for phospholipids [43], compatible with OPLS-AA [44,45] (used for quercetin molecules and cholesterol). The reliability of the Berger force field has been demonstrated in a number of previous works on the partitioning of small molecules in lipid membranes [44], [45], [46].
The Lennard-Jones and Coulomb interactions were cut off at 1.2 nm (, with the potentials shifted to zero at the cut off. The Lennard–Jones potential was shifted from to, while electrostatic interactions were shifted from to. A relative dielectric constant was used for explicit screening. Long-range electrostatic interactions were calculated with the Particle-Mesh Ewald algorithm [47,48], with Fourier spacing set to and the interpolation order (pme-order) set to 1.
Membrane systems were first energy minimized (steepest descent method, 10000 steps), then equilibrated for in the isothermic-isobaric ensemble , with the temperature set to and the pressure to . After equilibration, we generated five systems with different quercetin concentration, containing 0, 4, 8, 12, and 16 quercetin molecules. Quercetin molecules were inserted in the membrane in a symmetric fashion, with the same number of molecules in both leaflets. The initial orientation of quercetin was with the aromatic rings parallel to the membrane normal, and the phenyl group closer to the water region. Then, we minimized the energy and equilibrated each system in the isothermal-isobaric ensemble for , with the temperature and . Production runs were carried out for .
We performed all molecular dynamics simulations using the GROMACS software (v. 2016.4) [39]. In all simulations we used the leap-frog algorithm for the integration of the equation of motion, with an integration time step of in conjunction with the algorithm [49] to constrain the bonds involving hydrogen atoms. To maintain constant temperature, we used the Bussi-Donadio-Parrinello thermostat (named v-rescale in Gromacs [50]), with a time constant of and a reference temperature of . For pressure coupling, we used Berendsen [51] and Parinello-Rahman [52] method in equilibration and production run, respectively, with semi-isotropic coupling type, time constant of , and a compressibility of .
All analyses were performed over the last of simulation. We estimated the area per lipid () in the simulations from the total number of lipids per leaflet and the area of the membrane projected in the plane (obtained from the box dimensions), using the following equation:
| (3) |
, are respectively the and dimensions of the simulation box, and represents the number of lipids in the system. Statistical uncertainties were calculated using the GROMACS tool gmx analysis.
We calculated the membrane thickness as the difference between the average position (along the bilayer normal, i.e., the axis) of the phosphorous atoms in the lipids head groups. The latter was calculated using the GROMACS tool gmx density. The statistical uncertainty was estimated using block averaging, by dividing the simulation into 10 equal blocks and computing the difference between the highest and lowest values. This method is justified by the small size of the bilayers, leading to negligible undulations.
We calculated the angular distribution defined by the bilayer normal and the normal vector of the plane defined by the atoms of quercetin molecule using the GROMACS tool gmx gangle. The order parameter of C-H bonds in lipid bilayers was calculated (using the GROMACS tool gmx order) as:
| (4) |
where is the angle between the C−H bond vector and the bilayer normal, and angular brackets indicate ensemble and time averages.
The lateral diffusion coefficient, D, of POPC lipids was calculated using the Einstein relation, from the mean square displacement (MSD) versus time, using the GROMACS module gmx msd. The MSD was calculated for the phosphorus atoms, and least-squares fitted to a straight line (4), where is time and c is a fitting constant. Uncertainties were estimated using block averaging; we split the production run in two equal blocks, and the difference between average values was used as an estimate of the statistical uncertainty.
The isothermal compressibility modulus was calculated from area fluctuations:
| (5) |
where is Boltzmann constant, is the simulation temperature in Kelvin, is the average in-plane area of the membrane and is the instantaneous in-plane area. Statistical uncertainties were estimated from block averaging.
Results and discussion
AFM imaging and measurements
We prepared samples containing lipids and quercetin as detailed in Methods, then used AFM in contact mode to visualize the adsorbed vesicles on the mica substrate. Fig. 2 shows blank Lipoid E80 liposomes and Lipoid E80 liposomes loaded with quercetin, adsorbed on a clean mica sheets.
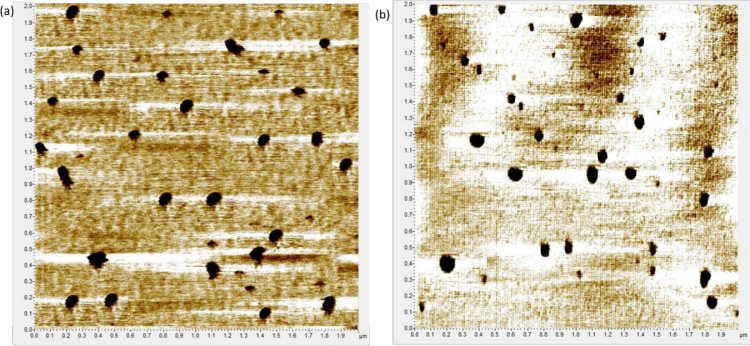
Fig. 2.
AFM 2 × 2 images of (a) blank Lipoid E80 liposomes and (b) quercetin-loaded Lipoid E80 liposomes, adsorbed on mica substrate
The presence of quercetin did not affect the morphology of the liposomes. For both blank and quercetin-loaded liposomes, we found an heterogeneous distribution of sizes, ranging from , and the same mean height of . Liposomes showed a mean size of for blank liposomes and for quercetin loaded liposomes. These distributions were confirmed by laser granulometry analysis (data not shown), and are in agreement with those obtained by Azzi et al. for Lipoid E80 liposomes containing cholesterol [18].
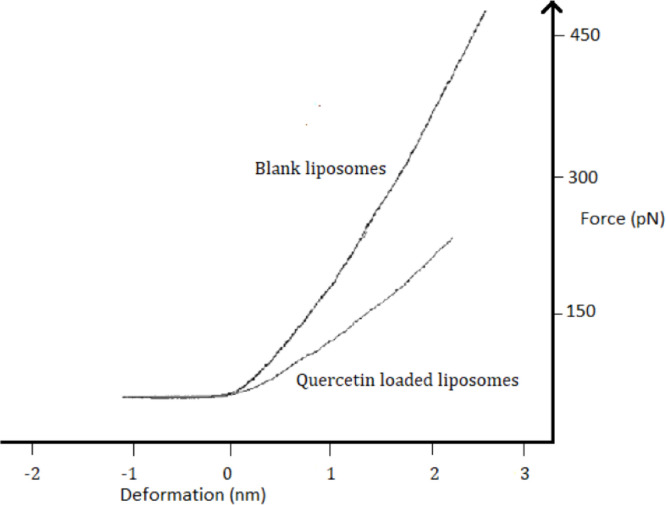
We used AFM to reveal the effect of quercetin on the liposome rigidity. Adsorbed vesicles from both samples were indented on a fix point on their upper surface, using a relatively small force, to avoid vesicle damage. We indented 200 liposomes from each sample, determined force vs deformation curves, and calculated the Young modulus from each curve (i.e., for each vesicle) as the slope of the force vs deformation curve in the elastic region (from see Fig. 3). We only collected the data on the elastic behavior when the degree of deformation of liposomes was small, i.e., the maximum indentation was about 10% of the measured liposome height. The curves were fitted using the modified Shell model (see Methods).
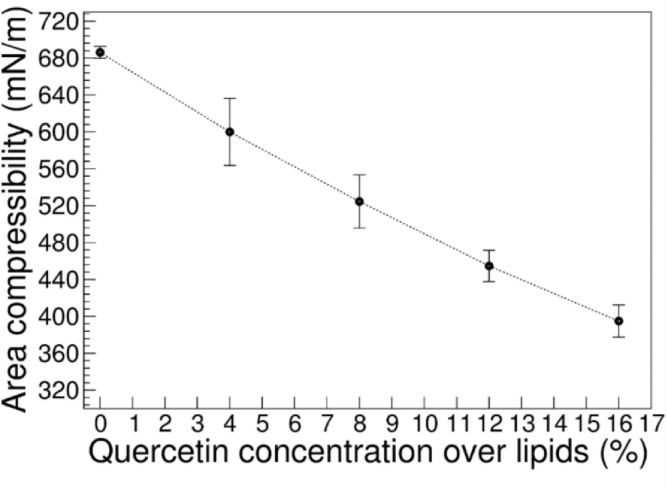
Fig. 4.
Area compressibility modulus of lipid membranes POPC+POPE:cholesterol:quercetin as a function of quercetin concentration (reported as molar fraction of quercetin over lipids).
Fig. 3.
Force-deformation curves, in the elastic region, obtained for blank and quercetin loaded lipoid E80 liposomes, during the approach of the tip to the sample.
As the example shows (Fig. 3), steeper force-deformation curves were generally obtained for blank liposomes, indicating that quercetin softens lipid membranes. Averaging over 200 vesicles for each composition, we obtained a value of for the Young modulus of blank liposomes, and for quercetin-loaded liposomes. These correspond to bending moduli of and for blank and quercetin-loaded liposomes, respectively (obtained from Eq. (2)). The bending modulus of blank liposomes (lipoid E80:Chol 100:74) is in reasonable agreement with previously reported values obtained via AFM by Takechi-Haraya et al. [53] on EggPC liposomes, containing cholesterol with molar ratio 50:50 (). The difference between bending moduli is around 16% and may be explained by the difference in cholesterol concentration (100:74 in our sample vs 50:50 in ref. 52), the AFM probe characteristics (cantilever force constant , and tip radius ), and the mathematical model (modified Shell vs Shell).
The reduction in elastic modulus in the system containing quercetin appears to be due to the presence of quercetin, and to the difference in cholesterol concentration (42 vs 32% in blank and quercetin-loaded liposomes, respectively). We remark here that the initial concentration of cholesterol in both systems (blank and quercetin loaded liposomes) during the preparation of liposomes was the same (20 mg/ml see paragraph 2.1), while the incorporation rate of cholesterol in liposomes was different in the two systems [18]. Due to differential incorporation of cholesterol, it is not clear to what extent the reduction in the elastic moduli should be ascribed to quercetin. Moreover, available experimental data does not allow to interpret the decrease in bending rigidity in terms of molecular structures and interactions, and how they change in the presence of quercetin. Such molecular interpretation can be afforded by MD simulations.
MD simulations
We used MD simulations at the atomistic level to understand the effect of quercetin on the properties of ternary lipid mixtures consisting of POPC:POPE:Chol. We simulated such ternary lipid mixtures in the presence of different concentrations of quercetin, from 4% to 16 % (molar fraction over phospholipids). We used the simulations to characterize membrane elastic properties by calculating the area compressibility modulus (). This is linked to the other elastic moduli of bilayer membranes [54], although not directly comparable to the Young modulus measured experimentally by AFM. First, we calculated the value of in the absence of quercetin, and compared it with values reported in the literature for similar membranes (see Table 1).
Table 1.
Values of area compressibility modulus determined in literature and in the present work for membranes containing POPC and Chol.
| Bilayer | Molar ratio (lipid:Chol) |
|
Temperature (K) |
Method | Reference |
|
POPC:ChoL |
70:30 |
562 87 | 288 | Box area fluctuation (CHARMM36) |
[55] |
| 757 87 | 288 | Local thickness fluctuations (LTF) (CHARMM36) | |||
|
POPC+POPE:ChoL |
70:30 |
401 ± 48 | 310 | Box area fluctuation (CHARMM36) | [56] |
| 457 ± 9 | 310 | Box area fluctuation (MARTINI v2.2) | |||
| 65:35 | 686 7 | 298 | Box area fluctuation (Berger lipids) |
Present work | |
| POPC:ChoL | 70:30 | 673 | 298 | Micropipette aspiration | [57] |
Our value of area compressibility modulus is in reasonable agreement with values previously obtained via simulations by Docktorova et al. for POPC:Chol (70:30) [55] using both box area fluctuation methods and local thickness fluctuations (see Table 1), and those obtained by Evans et al. for the same composition using micropipette aspiration [57]. Saeedimasine et al. reported lower values [56], easily explained by the difference in temperature (in our case vs by Saeedimasine et al.).
We then calculated the compressibility modulus in the presence of increasing amounts of quercetin. We found that KA decreases with increasing quercetin concentration, approximately in a linear fashion.
The trend is in qualitative agreement with the experimental results by AFM, indicating that quercetin softens lipid bilayers. A quantitative comparison is problematic, due to differences in cholesterol concentration in the samples used for AFM experiments.
Liposome softening is relevant in the context of drug delivery. The precise effect of liposome rigidity on the drug delivery process is still debated, but data is available on different drug delivery vectors. For instance, in vitro experiments on SUM159 cancer cells showed that softer (TA/PVPON) spherical particles are internalized more efficiently than their more rigid counterparts [58]. Hartmann et al. showed that softer (DextS/PLArg) and colloidal (PSS/PAH) particles were internalized and transported to lysosomes faster than more rigid particles in HeLa cells [59]. In the case of lipid vesicles, it is not clear whether their rigidity has an effect on internalization and intra-cellular transport. Sun et al. synthetized lipid‐covered polymeric nanoparticles (NPs) and varied water content and rigidity but with the same chemical composition, size, and surface properties [60]. They show that nanoparticles with a rigid lipid shell enter cells more easily than softer ones. Cellular uptake was tested by incubating the vesicles with HELA cells and human umbilical vein endothelial cells (HUVECs). Also, using MD simulations the authors showed that the soft NPs were deformed and thus energetically unfavorable for cellular uptake [60]. Yu et al. demonstrated, via both molecular-dynamics simulations and super-resolution microscopy, that liposomes with moderate rigidity displayed enhanced diffusivity through mucus and thus achieved an oral insulin delivery efficacy superior to that of both their soft and hard counterparts [61]. Also, they showed that liposomes with different transition temperatures (Tm) have different rigidities and further transform into various shapes, which results in different diffusion coefficients.
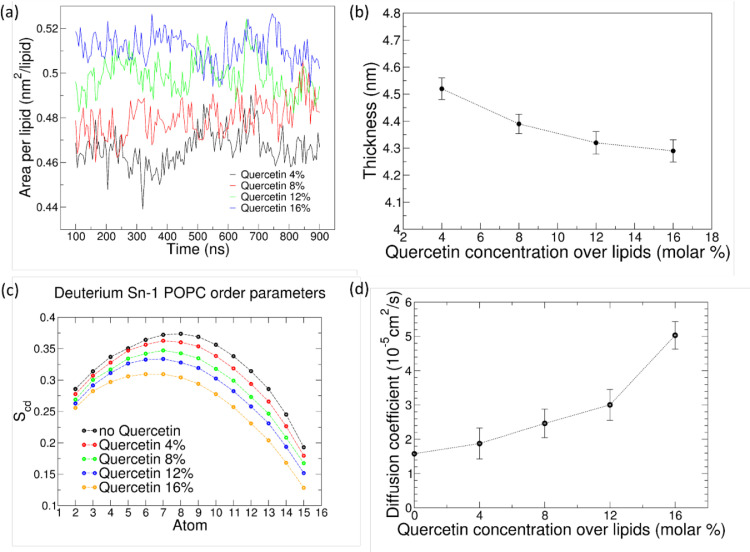
In order to interpret the membrane softening effect in terms of molecular interactions, we analyzed how quercetin affects membrane structural and dynamic properties, namely thickness, area per lipid, deuterium order parameter of sn-1 acyl chain of POPC lipids, and POPC diffusion coefficients (Fig. 5).
Fig. 5.
Variation of structural parameters of lipid membranes. (a). Area per lipid; (b) membrane thickness; (c) deuterium order parameter of sn-1 chain of POPC, and (d) diffusion coefficient of POPC lipids.
We found that the area per lipid increases with quercetin concentration. The estimate does not take into account the fraction of membrane area taken by quercetin. On the other hand, the average value of bilayer thickness decreases with increasing quercetin concentration. This is consistent with a decrease in the order parameter for POPC acyl chains (Fig. 5c). Also, the diffusion coefficient of POPC lipids increases in the presence of quercetin molecules, from 1.58 in the absence of quercetin to 5.03 in membranes containing 16% quercetin. The overall picture is very consistent: in the presence of quercetin, membranes get thinner and more disordered, hence softer; more space is available to each lipid, which increases their diffusion rates. Similar findings were obtained by Sanver et al. using X-ray scattering after encapsulating quercetin in DOPC membranes [62]: in their report it was shown that the presence of quercetin reduces bilayer thickness and increases bilayer undulations, leading to a membrane-fluidizing effect.
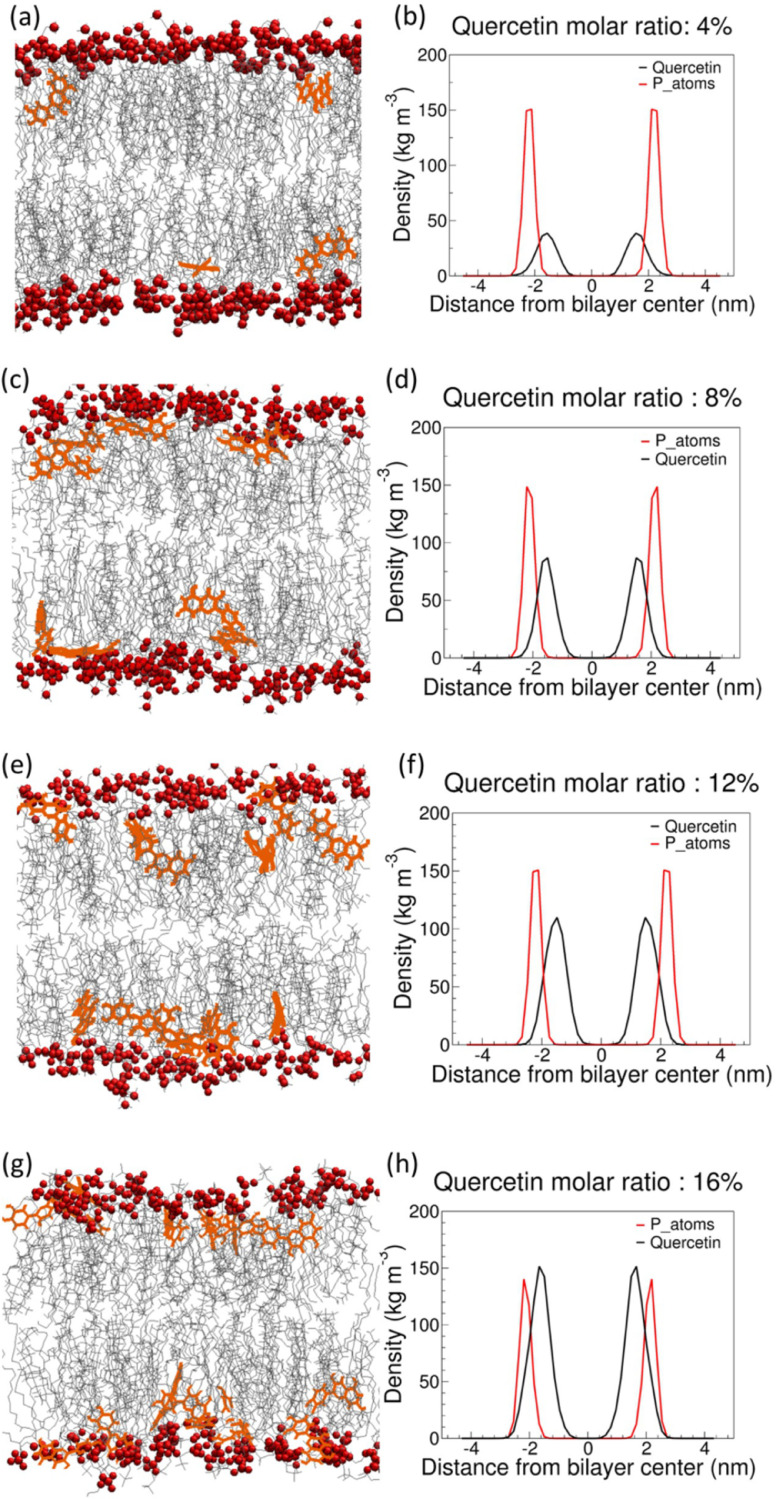
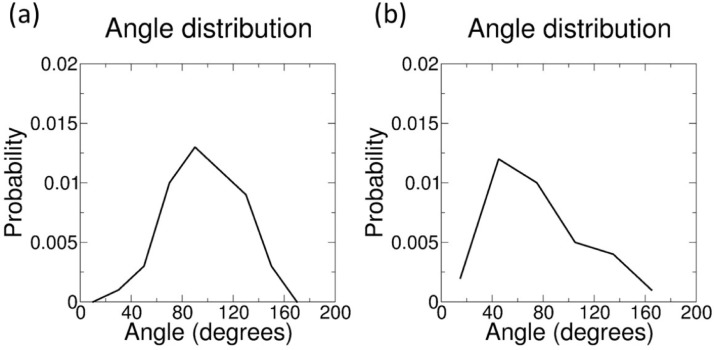
How can such softening effect be interpreted in terms of molecular interactions? We can use the same MD simulations to determine the interaction of quercetin with the lipids at the atomistic level. First, we verified by visual inspection that, even at the highest concentration, quercetin does not aggregate inside the bilayer (see Fig. 6). Then, we determined the position and orientation of quercetin in the membrane by calculating the symmetrized partial densities of quercetin molecules. We then calculated the distribution of the angle between the bilayer normal and the plane of the larger aromatic ring (Fig. 7), defined by atoms 6-8-13 (see Fig. 1).
Fig. 6.
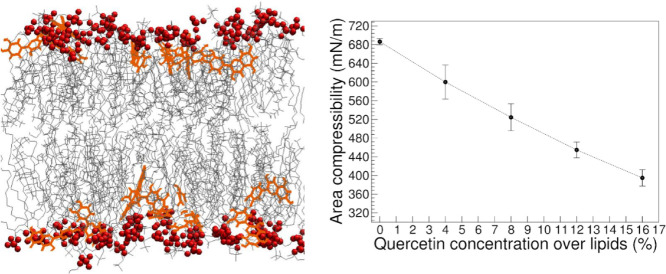
(a–h) Snapshots (left panels) and partial density profiles (right panels) of quercetin in membranes at different concentrations. Lipid head groups are in red, acyl chains in gray, and quercetin in orange. The partial density of phosphorus atoms in lipid head groups is reported for reference.
Fig. 7.
(a) Quercetin orientation in the membrane, quantified as angle distribution; the angle is defined by the bilayer normal (z-axis) and the normal to the larger aromatic ring (defined by atoms C6, C8 and C13 of quercetin, see Fig. 1). (b) Quercetin orientation in the membrane, quantified as the angle between the C13-C6 vector and the bilayer normal (z axis).
Partial density profiles indicate that quercetin is preferentially located below the lipid head group region, at the interface between the highly hydrated glycerol groups and the apolar acyl chains, at all concentrations. The preferred orientation of quercetin is with the aromatic rings in the plane containing the bilayer normal (Fig. 7a). The vector defined by atoms C13 and C16 (see Fig. 1), crossing the larger aromatic ring, is preferentially tilted with respect to the bilayer normal (Fig. 7b). Fluctuations in the molecular orientation are broad, but overall the orientation of aromatic rings allows atoms O29 and O26 (hydroxyl groups in the phenyl ring) to be in the proximity of the water interface, as confirmed by the average numbers of hydrogens bonds with water molecules ( hydrogen bonds per quercetin molecule on average, data not shown). The larger aromatic ring is embedded more deeply in the membrane compared to the smaller (phenyl) ring. Simulations also allow us to be more precise, and study the most favorable contacts within the membrane. For instance, atom C13 makes contact mostly with the first carbon atom of sn-1 chains (90 ± 4 % contact fraction, while the fraction of phospholipids in the system is only 65%), while contacts with the last carbon atom of acyl chains are infrequent (35± 20 %); this is consistent with partial density profiles (Fig. 6) and also suggests that quercetin is more often in contact with phospholipids than it is with cholesterol. Overall, the localization of quercetin agrees well with previous data obtained by electron paramagnetic resonance (EPR) measurements on DPPC (1,2-dipalmitoyl-snglycero- 3-phosphocholine) liposomes, and is consistent with fluorescence spectroscopy data on human skin fibroblast cells [16]. It is also compatible with MD simulations by Kosinova et al. [30], Fabre et al. [63], and by Sanver et al. [62], showing that quercetin localizes below the polar region of the membrane. We notice that the orientation of the aromatic rings reported by previous simulations is almost parallel to the membrane surface. This is different from our findings. However, the lipid composition used in previous studies is different (DOPC, while we used a POPC+POPE+CHOL mixture), and differences in the force fields can also contribute to differences in orientation; in particular, we notice that the partial charges for all oxygen atoms are higher in both Kosinova et al. [30] and Sanver et al. [62], which makes it more favorable for the molecule to be in contact with water – hence the preferential localization on the membrane surface.
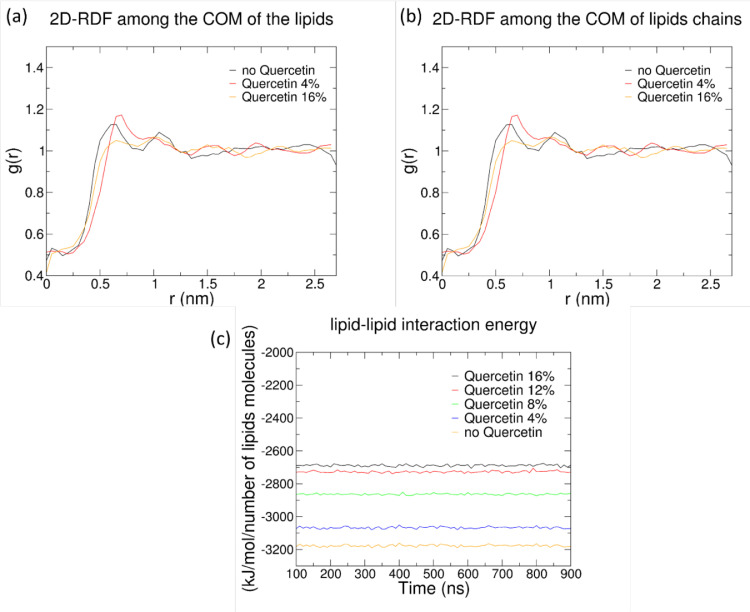
To better understand how quercetin localization affects lipid-lipid interactions, we calculated the 2D radial distribution function g(r) for the center of mass (COM) of lipid molecules, and for the COM of lipid acyl chains (Fig. 8). It is apparent that, with increasing quercetin concentrations, larger distances between the lipid molecules are more frequently sampled; in other words, lipid molecules are more frequently found farther apart from one another. This is due to the presence of the flavonoid, intercalating among lipid acyl chains. Looser packing of acyl chains is also demonstrated by lower order parameters, as reported above, and by lower lipid-lipid interaction energies (Fig. 8c), and appears to be correlated with membrane softening by quercetin.
Fig. 8.
Variation of 2D radial distribution functions as a function of quercetin concentration; (a) Center of mass (COM) of lipid molecules, and (b) COM of lipids tails. Only three compositions are reported, for the sake of clarity. (c) Lipid-lipid interaction energy in simulations with different concentration of quercetin.
Previous studies have reported effects of flavonoids on membrane properties. For example, Ingólfsson et al. examined the membrane localization and bilayer-modifying effects of five phenolic phytochemicals—capsaicin, curcumin, epigallocatechin gallate, genistein, and resveratrol [64]. The authors used MD simulations using both Martini and all-atom force fields and found that phytochemicals localize into the bilayer/solution interface of POPC membrane (lipid headgroup and backbone region). The simulations showed that those phytochemicals have modest effects on the bilayer structural properties (increases in the average area per lipid or decreases in bilayer thickness) with little effect on average lipid order or bilayer compressibility, but they produced significant changes in the bilayer pressure profile – and hence on elastic properties [64]. Huh et al. used X-ray diffraction and NMR to show that tannic acids, naturally occurring polyphenolic compounds, also weaken bilayer integrity through an increase in membrane area [65]. Tamba et al. demonstrated (using both phase-contrast fluorescence microscopy and the single GUV method) that tea catechins, which are flavonoids, disrupt bilayers, rupture lipid vesicles, and induce cell lysis [66]. Sanver et al. studied the effect of quercetin and rutin on DOPC membrane structural parameters using both X-ray diffraction and MD simulations [62]. They showed that both molecules partitioned within lipid bilayers (at different locations), increased the lattice spacing, reduced bilayer thickness, and increased bilayer undulations. The influence of rutin was not as strong as for quercetin, due to the different location of both molecules in the membrane; quercetin is located more deeply in the membrane. Gharib et al. studied the interaction of DPPC bilayer membranes with a series of monoterpenes including eucalyptol, pulegone, α-terpineol, β-terpineol, γ-terpineol, δ-terpineol and thymol, using differential scanning calorimetry, Raman spectroscopy and fluorescence anisotropy [67]. They showed that monoterpens abolished the pre-transition of DPPC membrane and modified the intensity of the Raman peaks. The molecules also decreased the main transition temperature of DPPC bilayers, suggesting their interaction with the alkyl chains of DPPC membrane. Fluorescence anisotropy results suggested that monoterpenes fluidized the liposomal membrane at 25, 41, and 50°C. Kaddah et al., investigated the impact of a series of glucocorticoids molecules with different concentrations on the membrane fluidity of DPPC liposomes using electron paramagnetic resonance spectroscopy measurement [67]. They showed that the molecules concentration is a key parameter that affect the membrane fluidity of DPPC liposomes.
The relevance of our findings is threefold. First of all, softening of liposomes is relevant in the context of drug delivery, as shown in numerous previous studies. Indeed, membrane rigidity can dramatically alter cellular uptake efficiency for lipid-coated drug delivery vectors [17,60]. Second, softening of model membranes by quercetin indicates that probably a similar effect would be observed also in cell membranes, if sufficiently high concentrations of the flavonoid are (locally) reached. A recent study showed that membrane elasticity correlates with lipid ordering and viscosity in cells [68] – in agreement with our observations in model systems. Third, membrane softening and membrane thinning can have important effects on the activity of membrane proteins, as reported in a number of studies (for reviews, see [69], [70], [71]). For a protein in which the hydrophobic length of the active state is longer than that of the inactive state, the protein activity will increase when the average bilayer thickness increases (within a given range), and vice versa [69,70]. Also, softening the bilayer may shift the conformational equilibrium toward the state with higher hydrophobic mismatch [69,70]. Hence, bilayer softening could increase or decrease protein activity depending on the specific protein–bilayer structural and elastic properties. This is corroborated by the observation that lipid chain length is often found to modulate the function of integral membrane proteins; in numerous cases, the enzyme or transport activity reaches a maximum at a particular lipid chain length and is reduced in membranes with either shorter or longer lipid chains [70].
Conclusions
In this work, we investigated the effect of quercetin on the bending rigidity of lipid membranes using AFM measures and MD simulations. AFM data show that the average values of Young modulus decrease in the presence of quercetin, but the decrease is difficult to interpret due to the different cholesterol content of the quercetin-loaded liposomes, and the relatively large statistical uncertainty of the AFM measures. Atomistic MD simulations confirm that quercetin softens the bilayer membrane, and also indicate that it decreases membrane thickness and increases disorder of acyl chains. Consistent with such changes in structural properties, diffusion coefficients are also increased. Changes in structural and dynamic properties can be interpreted in the light of quercetin localization and orientation in the membrane: due to its amphipathic nature, quercetin is located preferentially at the interface between the hydrophobic core and polar head groups of the lipids, roughly parallel to the membrane normal; in such position and orientation, quercetin increases the average distance among phospholipids, reducing their mutual attraction and softening the membrane. Membrane softening and thinning have been found before to be relevant in the context of drug delivery vehicles, as well as for their potential effects on biological functions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
LM acknowledges funding from the Institut National de la Sante et de la Recherche Medical (INSERM). Calculations were performed in part at the French supercomputing center CINES, supported by Grand Equipement National de Calcul Intensif (GENCI, grant number A0080710138). JE acknowledges funding from the Association de coopération, développement et santé of Lebanon.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.bbadva.2021.100018.
Contributor Information
Alia Jraij, Email: ajraij@ul.edu.lb.
Luca Monticelli, Email: luca.monticelli@inserm.fr.
Appendix. Supplementary materials
References
- 1.Jan A.T., Kamli M.R., Murtaza I., Singh J.B., Ali A., Haq Q.M.R. Dietary flavonoid quercetin and associated health benefits—an overview. Food Rev. Int. 2010;26:302–317. [Google Scholar]
- 2.Boots A.W., Haenen G.R.M.M., Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Zandi K., Teoh B.-T., Sam S.-S., Wong P.-F., Mustafa M.R., AbuBakar S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011;8:560. doi: 10.1186/1743-422X-8-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coelho-dos-Reis J.G.A., Gomes O.A., Bortolini D.E., Martins M.L., Almeida M.R., Martins C.S., Carvalho L.D., Souza J.G., Vilela J.M.C., Andrade M.S., Barbosa-Stancioli E.F. Evaluation of the effects of quercetin and kaempherol on the surface of MT-2 cells visualized by atomic force microscopy. J. Virol. Methods. 2011;174:47–52. doi: 10.1016/j.jviromet.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Dajas F. Life or death: neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol. 2012;143:383–396. doi: 10.1016/j.jep.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Andrés S., Tejido M.L., Bodas R., Morán L., Prieto N., Blanco C., Giráldez F.J. Quercetin dietary supplementation of fattening lambs at 0.2% rate reduces discolouration and microbial growth in meat during refrigerated storage. Meat Sci. 2013;93:207–212. doi: 10.1016/j.meatsci.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Wu D., Kong Y., Han C., Chen J., Hu L., Jiang H., Shen X. d-Alanine:d-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents. 2008;32:421–426. doi: 10.1016/j.ijantimicag.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Lin C.-F., Leu Y.-L., Al-Suwayeh S.A., Ku M.-C., Hwang T.-L., Fang J.-Y. Anti-inflammatory activity and percutaneous absorption of quercetin and its polymethoxylated compound and glycosides: the relationships to chemical structures. Eur. J. Pharm. Sci. 2012;47:857–864. doi: 10.1016/j.ejps.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 9.De Souza K.C.B., Bassani V.L., Schapoval E.E.S. Influence of excipients and technological process on anti-inflammatory activity of quercetin and Achyrocline satureioides (Lam.) D.C. extracts by oral route. Phytomedicine. 2007;14:102–108. doi: 10.1016/j.phymed.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Nabavi S.F., Russo G.L., Daglia M., Nabavi S.M. Role of quercetin as an alternative for obesity treatment: you are what you eat! Food Chem. 2015;179:305–310. doi: 10.1016/j.foodchem.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Aditya N.P., Macedo A.S., Doktorovova S., Souto E.B., Kim S., Chang P.-S., Ko S. Development and evaluation of lipid nanocarriers for quercetin delivery: a comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nanoemulsions (LNE) LWT - Food Sci. Technol. 2014;59:115–121. [Google Scholar]
- 12.Cai X., Fang Z., Dou J., Yu A., Zhai G. Bioavailability of quercetin: problems and promises. Curr. Med. Chem. 2013;20:2572–2582. doi: 10.2174/09298673113209990120. [DOI] [PubMed] [Google Scholar]
- 13.Hao J., Guo B., Yu S., Zhang W., Zhang D., Wang J., Wang Y. Encapsulation of the flavonoid quercetin with chitosan-coated nano-liposomes. LWT - Food Sci. Technol. 2017;85:37–44. [Google Scholar]
- 14.Tamjidi F., Shahedi M., Varshosaz J., Nasirpour A. Nanostructured lipid carriers (NLC): a potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg. Technol. 2013;19:29–43. [Google Scholar]
- 15.Toniazzo T., Peres M.S., Ramos A.P., Pinho S.C. Encapsulation of quercetin in liposomes by ethanol injection and physicochemical characterization of dispersions and lyophilized vesicles. Food Biosci. 2017;19:17–25. [Google Scholar]
- 16.Pawlikowska-Pawlęga B., Ignacy Gruszecki W., Misiak L., Paduch R., Piersiak T., Zarzyka B., Pawelec J., Gawron A. Modification of membranes by quercetin, a naturally occurring flavonoid, via its incorporation in the polar head group. Biochim. Biophys. Acta. 2007;1768:2195–2204. doi: 10.1016/j.bbamem.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Anselmo A.C., Mitragotri S. Impact of particle elasticity on particle-based drug delivery systems. Adv. Drug Del. Rev. 2017;108:51–67. doi: 10.1016/j.addr.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Azzi J., Jraij A., Auezova L., Fourmentin S., Greige-Gerges H. Novel findings for quercetin encapsulation and preservation with cyclodextrins, liposomes, and drug-in-cyclodextrin-in-liposomes. Food Hydrocolloids. 2018;81:328–340. [Google Scholar]
- 19.Briuglia M.-L., Rotella C., McFarlane A., Lamprou D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015;5:231–242. doi: 10.1007/s13346-015-0220-8. [DOI] [PubMed] [Google Scholar]
- 20.Takechi-Haraya Y., Sakai-Kato K., Goda Y. Membrane rigidity determined by atomic force microscopy is a parameter of the permeability of liposomal membranes to the hydrophilic compound calcein. AAPS PharmSciTech. 2017;18:1887–1893. doi: 10.1208/s12249-016-0624-x. [DOI] [PubMed] [Google Scholar]
- 21.Kloxin A.M., Benton J.A., Anseth K.S. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials. 2010;31:1–8. doi: 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frenzel M., Steffen-Heins A. Impact of quercetin and fish oil encapsulation on bilayer membrane and oxidation stability of liposomes. Food Chem. 2015;185:48–57. doi: 10.1016/j.foodchem.2015.03.121. [DOI] [PubMed] [Google Scholar]
- 23.Huang M., Su E., Zheng F., Tan C. Encapsulation of flavonoids in liposomal delivery systems: the case of quercetin, kaempferol and luteolin. Food Funct. 2017;8:3198–3208. doi: 10.1039/c7fo00508c. [DOI] [PubMed] [Google Scholar]
- 24.Phachonpai W., Wattanathorn J., Muchimapura S., Tong-Un T., Preechagoon D. Neuroprotective effect of quercetin encapsulated liposomes: a novel therapeutic strategy against Alzheimer's disease. Am. J. Appl. Sci. 2010;7:480–485. [Google Scholar]
- 25.Et-Thakafy O., Delorme N., Gaillard C., Mériadec C., Artzner F., Lopez C., Guyomarc'h F. Mechanical properties of membranes composed of gel-phase or fluid-phase phospholipids probed on liposomes by atomic force spectroscopy. Langmuir. 2017;33:5117–5126. doi: 10.1021/acs.langmuir.7b00363. [DOI] [PubMed] [Google Scholar]
- 26.Liang X., Mao G., Simon Ng K.Y. Probing small unilamellar EggPC vesicles on mica surface by atomic force microscopy. Colloids Surf. B. Biointerfaces. 2004;34:41–51. doi: 10.1016/j.colsurfb.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Storrs R.W., Tropper F.D., Li H.Y., Song C.K., Kuniyoshi J.K., Sipkins D.A., Li K.C.P., Bednarski M.D. Paramagnetic polymerized liposomes: synthesis, characterization, and applications for magnetic resonance imaging. J. Am. Chem. Soc. 1995;117:7301–7306. doi: 10.1002/jmri.1880050617. [DOI] [PubMed] [Google Scholar]
- 28.Eid J., Greige-Gerges H., Monticelli L., Jraij A. Elastic moduli of lipid membranes: reproducibility of AFM measures. Chem. Phys. Lipids. 2021;234 doi: 10.1016/j.chemphyslip.2020.105011. [DOI] [PubMed] [Google Scholar]
- 29.de Granada-Flor A., Sousa C., Filipe H.A.L., Santos M.S.C.S., de Almeida R.F.M. Quercetin dual interaction at the membrane level. Chem. Commun. 2019;55:1750–1753. doi: 10.1039/c8cc09656b. [DOI] [PubMed] [Google Scholar]
- 30.Košinová P., Berka K., Wykes M., Otyepka M., Trouillas P. Positioning of antioxidant quercetin and its metabolites in lipid bilayer membranes: implication for their lipid-peroxidation inhibition. J. Phys. Chem. B. 2012;116:1309–1318. doi: 10.1021/jp208731g. [DOI] [PubMed] [Google Scholar]
- 31.Sinha R., Gadhwal M., Joshi U., Srivastava S., Govil G., Nmr a., Research T., Mumbai India, Pharmacy b., Parade C. Interaction of quercetin with DPPC model membrane: molecular dynamic simulation, DSC and multinuclear NMR studies. J.-Indian Chem. Soc. 2011;88:1203–1210. [Google Scholar]
- 32.Reissner E. Stresses and small displacements of shallow spherical shells. II. J. Math. Phys. 1946;25:279–300. [Google Scholar]
- 33.Berry J.D., Mettu S., Dagastine R.R. Precise measurements of capsule mechanical properties using indentation. Soft Matter. 2017;13:1943–1947. doi: 10.1039/c6sm02841a. [DOI] [PubMed] [Google Scholar]
- 34.Evans E.A. Bending resistance and chemically induced moments in membrane bilayers. Biophys. J. 1974;14:923–931. doi: 10.1016/S0006-3495(74)85959-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dodda L.S., Cabeza de Vaca I., Tirado-Rives J., Jorgensen W.L. LigParGen web server: an automatic OPLS-AA parameter generator for organic ligands. Nucl. Acids Res. 2017;45:W331–W336. doi: 10.1093/nar/gkx312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dodda L.S., Vilseck J.Z., Tirado-Rives J., Jorgensen W.L. 1.14*CM1A-LBCC: localized bond-charge corrected CM1A charges for condensed-phase simulations. J. Phys. Chem. B. 2017;121:3864–3870. doi: 10.1021/acs.jpcb.7b00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jorgensen W.L., Tirado-Rives J. Potential energy functions for atomic-level simulations of water and organic and biomolecular systems. Proc. Natl. Acad. Sci. USA. 2005;102:6665. doi: 10.1073/pnas.0408037102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaminski G.A., Friesner R.A., Tirado-Rives J., Jorgensen W.L. Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J. Phys. Chem. B. 2001;105:6474–6487. [Google Scholar]
- 39.Abraham M.J., Murtola T., Schulz R., Páll S., Smith J.C., Hess B., Lindahl E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1-2:19–25. [Google Scholar]
- 40.Lemańska K., van der Woude H., Szymusiak H., Boersma M.G., Gliszczyńska-Świgło A., Rietjens I.M.C.M., Tyrakowska B. The effect of catechol o-methylation on radical scavenging characteristics of quercetin and luteolin—a mechanistic insight. Free Radical Res. 2004;38:639–647. doi: 10.1080/10715760410001694062. [DOI] [PubMed] [Google Scholar]
- 41.Berendsen H.J.C., Postma J.P.M., van Gunsteren W.F., Hermans J. In: Intermolecular Forces. Pullman B., editor. D. Reidel; Dordrecht, The Netherlands: 1981. Interaction models for water in relation to protein hydration; pp. 331–342. [Google Scholar]
- 42.Knight C.J., Hub J.S. MemGen: a general web server for the setup of lipid membrane simulation systems. Bioinformatics. 2015;31:2897–2899. doi: 10.1093/bioinformatics/btv292. [DOI] [PubMed] [Google Scholar]
- 43.Berger O., Edholm O., Jahnig F. Molecular dynamics simulations of a fluid bilayer of dipalmitoylphosphatidylcholine at full hydration, constant pressure, and constant temperature. Biophys. J. 1997;72:2002–2013. doi: 10.1016/S0006-3495(97)78845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tieleman D.P., MacCallum J.L., Ash W.L., Kandt C., Xu Z.T., Monticelli L. Membrane protein simulations with a united-atom lipid and all-atom protein model: lipid-protein interactions, side chain transfer free energies and model proteins. J. Phys.-Condens. Matter. 2006;18:S1221–S1234. doi: 10.1088/0953-8984/18/28/S07. [DOI] [PubMed] [Google Scholar]
- 45.MacCallum J.L., Bennett W.F.D., Tieleman D.P. Distribution of amino acids in a lipid bilayer from computer simulations. Biophys. J. 2008;94:3393–3404. doi: 10.1529/biophysj.107.112805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paloncýová M., DeVane R., Murch B., Berka K., Otyepka M. Amphiphilic drug-like molecules accumulate in a membrane below the head group region. J. Phys. Chem. B. 2014;118:1030–1039. doi: 10.1021/jp4112052. [DOI] [PubMed] [Google Scholar]
- 47.Darden T., York D., Pedersen L. Particle mesh Ewald: an N-log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993;98:10089–10092. [Google Scholar]
- 48.Essmann U., Perera L., Berkowitz M.L., Darden T., Lee H., Pedersen L.G. A smooth particle mesh Ewald potential. J. Chem. Phys. 1995;103:8577–8592. [Google Scholar]
- 49.Hess B. P-LINCS: a parallel linear constraint solver for molecular simulation. J. Chem. Theory Comput. 2008;4:116–122. doi: 10.1021/ct700200b. [DOI] [PubMed] [Google Scholar]
- 50.Bussi G., Donadio D., Parrinello M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007;126 doi: 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- 51.Berendsen H.J.C., Postma J.P.M., van Gunsteren W.F., di Nola A., Haak J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984;81:3684–3690. [Google Scholar]
- 52.Parrinello M., Rahman A. Polymorphic transitions in single crystals - a new molecular dynamics method. J. Appl. Phys. 1981;52:7182–7190. [Google Scholar]
- 53.Takechi-Haraya Y., Sakai-Kato K., Abe Y., Kawanishi T., Okuda H., Goda Y. Atomic force microscopic analysis of the effect of lipid composition on liposome membrane rigidity. Langmuir. 2016;32:6074–6082. doi: 10.1021/acs.langmuir.6b00741. [DOI] [PubMed] [Google Scholar]
- 54.Eid J., Razmazma H., Jraij A., Ebrahimi A., Monticelli L. On calculating the bending modulus of lipid bilayer membranes from buckling simulations. J. Phys. Chem. B. 2020;124:6299–6311. doi: 10.1021/acs.jpcb.0c04253. [DOI] [PubMed] [Google Scholar]
- 55.Doktorova M., LeVine M.V., Khelashvili G., Weinstein H. A new computational method for membrane compressibility: bilayer mechanical thickness revisited. Biophys. J. 2019;116:487–502. doi: 10.1016/j.bpj.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saeedimasine M., Montanino A., Kleiven S., Villa A. Role of lipid composition on the structural and mechanical features of axonal membranes: a molecular simulation study. Sci. Rep. 2019;9:8000. doi: 10.1038/s41598-019-44318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Evans E., Rawicz W., Smith B.A. Concluding remarks Back to the future: mechanics and thermodynamics of lipid biomembranes. Faraday Discuss. 2013;161:591–611. doi: 10.1039/c2fd20127e. [DOI] [PubMed] [Google Scholar]
- 58.Alexander J.F., Kozlovskaya V., Chen J., Kuncewicz T., Kharlampieva E., Godin B. Cubical shape enhances the interaction of layer-by-layer polymeric particles with breast cancer cells. Adv. Healthc. Mater. 2015;4:2657–2666. doi: 10.1002/adhm.201500537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hartmann R., Weidenbach M., Neubauer M., Fery A., Parak W.J. Stiffness-dependent in vitro uptake and lysosomal acidification of colloidal particles. Angew. Chem. Int. Ed. 2015;54:1365–1368. doi: 10.1002/anie.201409693. [DOI] [PubMed] [Google Scholar]
- 60.Sun J., Zhang L., Wang J., Feng Q., Liu D., Yin Q., Xu D., Wei Y., Ding B., Shi X., Jiang X. Tunable rigidity of (polymeric core)–(lipid shell) nanoparticles for regulated cellular uptake. Adv. Mater. 2015;27:1402–1407. doi: 10.1002/adma.201404788. [DOI] [PubMed] [Google Scholar]
- 61.Yu M., Song W., Tian F., Dai Z., Zhu Q., Ahmad E., Guo S., Zhu C., Zhong H., Yuan Y., Zhang T., Yi X., Shi X., Gan Y., Gao H. Temperature- and rigidity-mediated rapid transport of lipid nanovesicles in hydrogels. Proc. Natl. Acad. Sci. 2019;116:5362. doi: 10.1073/pnas.1818924116. USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanver D., Sadeghpour A., Rappolt M., Di Meo F., Trouillas P. Structure and dynamics of dioleoyl-phosphatidylcholine bilayers under the influence of quercetin and rutin. Langmuir. 2020;36:11776–11786. doi: 10.1021/acs.langmuir.0c01484. [DOI] [PubMed] [Google Scholar]
- 63.Fabre G., Bayach I., Berka K., Paloncýová M., Starok M., Rossi C., Duroux J.-L., Otyepka M., Trouillas P. Synergism of antioxidant action of vitamins E, C and quercetin is related to formation of molecular associations in biomembranes. Chem. Commun. 2015;51:7713–7716. doi: 10.1039/c5cc00636h. [DOI] [PubMed] [Google Scholar]
- 64.Ingólfsson H.I., Thakur P., Herold K.F., Hobart E.A., Ramsey N.B., Periole X., de Jong D.H., Zwama M., Yilmaz D., Hall K., Maretzky T., Hemmings H.C., Blobel C., Marrink S.J., Koçer A., Sack J.T., Andersen O.S. Phytochemicals perturb membranes and promiscuously alter protein function. ACS Chem. Biol. 2014;9:1788–1798. doi: 10.1021/cb500086e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huh N.W., Porter N.A., McIntosh T.J., Simon S.A. The interaction of polyphenols with bilayers: conditions for increasing bilayer adhesion. Biophys. J. 1996;71:3261–3277. doi: 10.1016/S0006-3495(96)79519-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tamba Y., Ohba S., Kubota M., Yoshioka H., Yoshioka H., Yamazaki M. Single GUV method reveals interaction of tea catechin (−)-epigallocatechin gallate with lipid membranes. Biophys. J. 2007;92:3178–3194. doi: 10.1529/biophysj.106.097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaddah S., Khreich N., Kaddah F., Khrouz L., Charcosset C., Greige-Gerges H. Corticoids modulate liposome membrane fluidity and permeability depending on membrane composition and experimental protocol design. Biochimie. 2018;153:33–45. doi: 10.1016/j.biochi.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 68.Steinkühler J., Sezgin E., Urbančič I., Eggeling C., Dimova R. Mechanical properties of plasma membrane vesicles correlate with lipid order, viscosity and cell density. Commun. Biol. 2019;2:337. doi: 10.1038/s42003-019-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andersen O.S., Koeppe R.E. Bilayer thickness and membrane protein function: an energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- 70.Marsh D. Protein modulation of lipids, and vice-versa, in membranes. Biochim. Biophys. Acta. 2008:1545–1575. doi: 10.1016/j.bbamem.2008.01.015. 1778. [DOI] [PubMed] [Google Scholar]
- 71.McIntosh T.J., Simon S.A. Roles of bilayer material properties in function and distribution of membrane proteins. Annu. Rev. Biophys. Biomol. Struct. 2006;35:177–198. doi: 10.1146/annurev.biophys.35.040405.102022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.