Abstract
Selenium is a trace mineral essential for life that acts physiologically through selenoproteins. Among other actions, the endogenous antioxidant selenoprotein glutathione peroxidase and the selenium transporter in blood, selenoprotein P, seem to play an important role in type 2 diabetes mellitus and insulin resistance by weakening the insulin signaling cascade through different mechanisms. Recent findings also suggest that selenoproteins also affect insulin biosynthesis and insulin secretion. This review discussed the role of selenium in type 2 diabetes and the complex interplay between selenoproteins and insulin pathways.
Keywords: Selenium, Diabetes, Insulin resistance, Metabolism, Antioxidants
Core Tip: In this review we explored the role of selenium in insulin resistance and β-cell secretory function. The response to selenium intake has a U-shaped dose-dependent effect so that when it is above the recommended dose, it causes hyperglycemia and hyperinsulinemia, which alters oxidative stress and the insulin signaling cascade and lipid and glucose metabolism. Recent findings also suggested that selenoproteins affect insulin biosynthesis and insulin secretion. Current evidence suggests that the ingestion of selenium supplements should be taken with caution considering the basal levels of selenium in daily food intake to avoid the development of type 2 diabetes mellitus.
INTRODUCTION
Type 2 diabetes mellitus incidence and global burden
A global increase in obesity, ageing population, sedentary lifestyles, physical inactivity, alcohol consumption, smoking and high fat and sugar intake has contributed to an unprecedented increase in the incidence of type 2 diabetes mellitus (T2DM), quadrupling between 1980 and 2004. In 2015, a total of 415 million people were estimated to have diabetes, more than 90% of whom were T2DM, making it the sixth leading cause of disability in the world[1].
T2DM is the most rapidly growing global health emergency. The incidence has already reached 9.3% among adults aged 20 years to 79 years in 2019[2]. Although advancing age is a risk factor, the increase in childhood obesity resulted in an alarming increase in T2DM in children and adolescents too[3].
T2DM is characterized by insulin action deficiency caused by pancreatic β-cell dysfunction and a consequent insulin resistance in target organs. The main organs involved in the development of T2DM are the pancreas, liver, muscle, brain, kidneys, adipose tissue and small intestine[1]. The state of chronic hyperglycemia and impaired carbohydrate, lipid and protein metabolism give rise to complications such as cardiovascular diseases, nephropathies or diabetic neuropathies[3]. Consequently, this situation leads to a 15% increased risk of mortality from any other disease, being twice as high in young patients[1].
Many studies have shown that it is not only lifestyle that causes T2DM, but it also results from an interaction between genetic factors, lifestyle, gut metagenome and different types of vitamins that have a potential role in controlling T2DM and insulin sensitivity[3].
Insulin resistance and β-cell dysfunction
Under normal conditions, insulin binds to the insulin receptor (IR) and promotes lipid and glucose uptake into adipose tissue. Any failure in this signaling cascade leads to an increase in circulating glucose (hyperglycemia) and lipids (hyperlipidemia), a phenomenon observed in T2DM[4,5].
Prospective studies in subjects with a high risk for T2DM or newly diagnosed have shown that in contrast to insulin resistance that remains relatively stable over time, β-cell functionality has a rapid and steady decline[6]. Indeed, another study performed in the Tanzanian adult population demonstrated that β-cell dysfunction is of greater contribution in T2DM compared to insulin resistance contribution[7].
Insulin resistance refers to a failure or impairment in the transduction of the insulin-mediated signaling cascade in certain tissues, especially in muscle, adipose tissue and liver. This leads to an elevated circulating glucose level that together with elevated hepatic glucose output results in very high plasma glucose levels. These glucose levels require a high demand for insulin production and secretion by the β-cells. When insulin resistance is prolonged over time, the β-cells are submitted to high glucose and lipid exposure that results in β-cell dysfunction and death[4,5].
On the other hand, β-cell organ dysfunction can develop before it can be clearly appreciated, and it is related to a reversible loss of β-cell functionality and β-cell content that only becomes irreversible when there is a large loss of cell mass due to β-cell apoptosis. If this loss of β-cell mass persists and the damage is significant enough, it results in β-cell organ failure. Thus, it highlights the need for an earlier preventive approach so that early pharmacological and dietary treatment can rescue the reduced and reversible β-cell organ dysfunction associated with T2DM[8].
Animal and human studies have demonstrated that the progressive failure of the β-cell insulin-secreting function is not only due to apoptosis and loss of cell mass but also to the phenomena of cellular dedifferentiation and conversion to other endocrine cells. Many studies have established that β-cells become dedifferentiated in response to hyperglycemia, reverting to a progenitor-like state, and then β-cell conversion to glucagon producing “α-like” cells takes places. This transdifferentiation process could explain the typical glucagon overproduction and hyperglucagonemia in T2DM[6,9,10].
Glucolipotoxicity is another added risk factor studied in rodent models because of excessive and chronic exposure to fatty acids, lipid storage and synthesis. This increase directly impairs glucose-stimulated insulin secretion resulting in β-cell stress and dysfunction[9,11].
Regarding other risk factors such as micronutrients, particularly selenium (Se) is a trace element that interferes with cellular antioxidant capacity through enzymes such as glutathione peroxidase (GPx), and it has been linked on several occasions to T2DM. However, there are many controversial studies on the beneficial or detrimental effects of Se on the risk of developing T2DM. This review discussed the role of Se in T2DM and the complex interplay between selenoproteins and insulin pathways reflecting the need for new knowledge and better mechanistic understanding.
THE CONTROVERSIAL ROLE OF SE IN T2DM
Se: An essential trace element
Se is a trace element that represents an essential micronutrient for humans, plants and microorganisms, and it is involved in a wide variety of physiological processes. Adequate levels of Se bioavailability in the organism are crucial for different aspects of human biology including the endocrine system, muscle function or the cardiovascular system[12].
In nature, inorganic Se is found in four different oxidation states: selenate; selenite; elemental Se; and selenide (in decreasing order of redox state). Biological systems are able to convert this inorganic Se into more bioavailable organic forms such as the two Se-containing amino acids, selenocysteine and selenomethionine, which will become part of proteins, the so-called selenoproteins[13].
Selenoproteins comprise a total of 25 proteins in the human proteome with different functions including, among others, protection against oxidative stress, Se storage and transport or redox signaling. Their crucial role in oxidative stress system is due to their ability to neutralize reactive oxygen and nitrogen species[14].
These selenoproteins can be differentiated by having enzymatic activity as the GPx family or by the absence of such activity as the selenoprotein K family[12].
Worldwide variation in Se intake
In contrast to other micronutrients, Se intake varies widely worldwide from deficiency to toxic concentrations leading to nail loss, hair loss, poor dental health or even nervous system or skin disorders. Recommended Se intake is around 55 μg/d, and it can be found in foods such as grains, meat, seafood, vegetables, nuts or dairy products[12].
Se intake from food depends not only on the Se content of the soil but also on factors that determine the availability of Se in food. In general, intake is higher in countries such as Venezuela, Canada, the United States and Japan. In Europe, on the other hand, intake is lower, and in countries such as New Zealand, Finland or Denmark it is especially low[15].
Dietary supplements containing Se are very common, especially in countries such as the United States where 50% of the population takes daily supplements. This extra intake of Se added to the daily food intake makes the average Se intake vary from 40 μg/d in Europe to 93 μg/d (in women) and 134 μg/d (in men) in the United States[15].
Se can have detrimental effects below and above the recommended intake range. A Se deficiency has been shown to be involved in the appearance of different pathologies such as Kaschin-Beck disease or Keshan disease. In the same way, a high and chronic exposure to Se can cause selenosis with severe manifestations in the organism[12,13].
Studies on Se supplementation effect
Many studies have demonstrated the beneficial effect of Se in different pathologies such as cancer, the immune system or hyperlipidemia.
Se supplementation in patients with Hashimoto’s thyroiditis has been associated with a decrease in thyroid autoantibodies and thyroid stimulating hormone levels due to its antioxidant capacity and overregulation of regulatory T cells[16]. Similarly, in patients with autoimmune thyroiditis, after 3 mo of Se supplementation, the levels of antithyroid peroxidase antibody decreased, revealing beneficial effects of the antioxidant capacity of Se[17].
In a study on leukocyte DNA integrity, they found that it was enhanced by Se supplementation depending on the interaction with dietary micronutrients. Particularly, Se supplementation was found to be beneficial when there were low folate and high methionine intake levels through increased homeostatic apoptosis[18].
On the other hand, in a study with septic patients it was seen that Se administration was beneficial in those patients with bronchopneumonia where there was greater oxidative stress in the lung pare-nchyma. The action of antioxidant molecules helped improvement. This was not the case in patients with persistent renal failure since continuous renal replacement therapy does not ensure GPx synthesis[19]. However, a separate study with septic patients showed that although it may seem logical to administer antioxidant elements because of the high oxidative stress and low Se levels in these patients, Se does not increase the release of cytokines or the activation of innate immune cells, suggesting a neutral effect of Se on the immune system[20].
The beneficial effect of Se on cancer is a controversial topic too. Many reviews have gathered a large amount of information and clinical trials to analyze and conclude that Se has no effect in preventing cancer overall, neither in prostate cancer (the cancer type with the most consistent association with antecedent Se exposure) nor in patients with low Se levels[21]. In addition, it has been shown that Se at the recommended daily intake concentrations is protective against cancer. Therefore, supplementing Se in people with Se deficiency improves prevention due to elimination of such deficiency, not as a result of elevated Se levels. In fact, increasing Se levels above the recommended dose does not improve cancer prevention and is not recommended since it favors the appearance of other diseases such as T2DM[22]. However, a recent study supports the use of different Se species as coadjuvant agents in cancer treatment due to their lower toxicity, higher selectivity and efficacy in inducing cell apoptosis[23].
In a cross-sectional study regarding the relationship between Se and hypertension in the Chinese population, a higher incidence of hypertension was found in the group with higher serum Se concentration, especially in women, as well as a higher amount of blood lipids[14]. On the other hand, in a 20-year cohort study also in a Chinese population, it was found that Se intake was inversely associated with the risk of hypertension in participants from the northern region and positively associated in participants from the southern region suggesting a protective factor for blood pressure in low-Se regions[24].
The relationship between plasma lipids and Se is also a controversial association. In a randomized trial with participants over 60 years, Se supplementation decreased total and non-high density lipoprotein cholesterol in their sample of relatively low Se status[25]. Similarly, a decrease in total cholesterol and an increase in high density lipoprotein were found in relation with increasing Se in an elderly Chinese population with low dietary Se intake[26]. However, in a cross-sectional study in a Spanish population, Se was positively associated with total and low-density lipoprotein cholesterol[27]. In addition, Se levels were higher in hyperlipidemic patients than in healthy volunteers[28]. Finally, in a cross-sectional study Se decreased lipid dysregulation caused by elevated toenail levels of mercury confirming the beneficial effects of Se against the harmful effects of mercury[29].
There are also studies on the effect of Se in the improvement of critically ill patients such as patients with cardiac surgery, major trauma or subarachnoid hemorrhage. It has been seen that the administration of Se-containing antioxidant supplements corrects the initial alterations and restores antioxidant defenses such as GPx activity but fails to achieve a significant improvement in organ dysfunction[30]. In a small observational study in patients who have undergone cardiopulmonary resuscitation, impr-ovement in neurological outcome and survival rate with early Se treatment was observed[31]. In a population-based study from four different geographic areas, high Se levels were associated with a greater probability of having depressive symptoms[32].
In a study with 7.5 years of follow-up, there was no benefit or adverse effect of multiple antioxidant supplementation on the incidence of metabolic syndrome[33]. Non-linear associations have been found between serum Se and the prevalence of nonalcoholic fatty liver disease. Only positive associations were found when serum Se level > 130 μg/L[34].
As explained above and shown in Table 1, Se supplementation is a controversial subject given the large number of factors (age, region, diet, genetic factors, diseases, etc) that influence the beneficial or adverse effect of Se.
Table 1.
Summary of a wide variety of studies suggesting a beneficial or detrimental role of selenium in different diseases and physiological processes
|
Ref.
|
Type of study
|
Number of participants
|
Effects on Se intake
|
| Hu et al[16], 2021 | Longitudinal study (6 mo) | 90 with HT; 36 healthy subjects | Reductions in thyroid autoantibodies and thyroid-stimulating hormone levels in HT patients |
| Karimi and Omrani[17], 2019 | Longitudinal study (3 mo) | 102 with AIT | Decreased antithyroid peroxidase antibody levels |
| Karunasinghe et al[18], 2016 | Longitudinal study (6 mo) | 572 males | Improved leukocyte DNA integrity through increased homeostatic apoptosis when folate intake levels were low and methionine intake levels were high |
| Kočan et al[19] 2014 | Longitudinal study (6 d) | 65 septic patients | Improvement of patients with acute lung injury and elevated oxidative stress |
| Guo et al[20], 2019 | Longitudinal study (21 d) | 76 severe septic patients | Neutral effect |
| Vinceti et al[21], 2018 | Review | - | Se had no effect in preventing cancer overall, including in patients with low Se levels |
| Rocourt and Cheng[22], 2013 | Review | - | Supplementing Se to people with Se deficiency improved cancer prevention due to elimination of such deficiency |
| Radomska et al[23], 2021 | Review | - | Se species as coadjuvant agents in cancer treatment due to their lower toxicity, higher selectivity and efficacy in inducing cell apoptosis |
| Wu et al[14], 2018 | Cross-sectional study | 8011 participants | Higher incidence of hypertension in the group with higher serum Se concentration |
| Xie et al[24], 2021 | Longitudinal study (20 yr) | 10025 participants | Protective factor for blood pressure in low-Se regions |
| Rayman et al[25], 2011 | Longitudinal study (6 mo) | 501 people aged 60 yr to 74 yr | Se supplementation decreased total and non-HDL cholesterol |
| Chen et al[26], 2015 | Longitudinal study (7 yr) | 2000 aged 65 and older | Decrease in total cholesterol and an increase in HDL in relation with increasing Se |
| González-Estecha et al[27], 2017 | Cross-sectional study | 372 participants | Positive association of Se with total and LDL cholesterol |
| Fülöp et al[28], 2013 | Study population | 81 hyperlipidemic patients; 43 healthy volunteers | Higher Se levels in hyperlipidemic patients |
| Park and Seo[29], 2017 | Cross-sectional study | 501 participants | Decreased lipid dysregulation caused by elevated toenail levels of mercury |
| Berger et al[30], 2008 | Longitudinal study (5 d) | 2000 cardiac surgery or major trauma or subarachnoid hemorrhage patients | Correction of initial alterations and restoration of antioxidant defenses |
| Fink and Busch[31], 2018 | Longitudinal study (24 h) | 28 resuscitated patients | Improvement in neurological outcome and survival rate with early Se treatment in patients after cardiopulmonary resuscitation |
| Colangelo et al[32], 2014 | Study population | 5115 participants | High Se levels were associated with a greater probability of having depressive symptoms |
| Czernichow et al[33], 2009 | Longitudinal study (7.5 yr) | 5220 participants | No benefit or adverse effect of multiple antioxidant supplementation on the incidence of metabolic syndrome |
| Wang et al[34], 2021 | Cross-sectional study | 3827 participants | Only positive associations when serum Se level > 130 μg/L in patients with NAFLD |
AIT: Autoimmune thyroiditis; HDL: High density lipoprotein; HT: Hashimoto’s thyroiditis; LDL: Low-density lipoprotein; NAFLD: Nonalcoholic fatty liver disease; Se: selenium.
Evidence showing higher levels of Se in T2DM
Among all the beneficial and harmful properties of Se, the association between high Se levels and the risk of developing T2DM is relatively recent. By analyzing non-experimental studies based on dietary and blood Se concentrations, a non-linear dose-response association with T2DM risk was determined, showing a dramatic increase from 80 μg of daily Se intake and above[35]. Another non-linear dose-response meta-analysis suggested that this positive association also occurred at low Se concentrations[36]. In fact, dose response to Se has been found to be U-shaped: Damage occurring both below and above the recommended concentration. In the case of increased risk of T2DM, an excess of Se promotes hyperinsulinemia, hyperglycemia and hyperlipidemia[22,37]. Increased selenoprotein levels have been found in T2DM patients, and its expression is reduced by the characteristic inflammatory response of T2DM[38]. However, there are some studies where negative associations have been found between Se dose and insulin resistance when Se intake is below 1.6 μg/kg/d[39].
It has also been suggested that the association between Se, obesity and T2DM may be due to abnormal metabolism in adipocytes by excessive release of fatty acids and/or hormones[40]. Moreover, a high Se intake (> 60 μg/d) in people without previous diabetes increases the risk of hospitalization for T2DM[41]. Similarly, the Hortega study found a positive association between plasma Se with prevalent and incident diabetes[42]. Nevertheless, in a cross-sectional study of the United States population, Se was found to be positively associated with diabetes but inversely associated with all-cause mortality[43].
Finally, in a randomized trial with T2DM patients, Se supplementation in those with deficient Se levels resulted in adverse effects on blood glucose homeostasis even when the Se concentration had reached the optimal level of antioxidant activity[44].
The vast majority of epidemiological studies (Table 2) support the evidence of an increased risk of T2DM when Se concentrations are elevated above the recommended levels, which emphasizes the need for special caution when supplementing with Se to obtain other beneficial effects given the possibility of developing T2DM.
Table 2.
Summary of evidence showing an association between elevated selenium levels and increased risk of type 2 diabetes mellitus
|
Ref.
|
Type of study
|
Number of participants
|
Evidence for T2DM risk
|
| Vinceti et al[35], 2021 | Dose-response meta-analysis | - | Non-linear dose-response association. Dramatically increase from 80 μg of daily Se intake and above |
| Wang et al[36], 2016 | Dose-response meta-analysis | - | Non-linear dose-response association with T2DM at low and high Se concentrations |
| Duntas and Benvenga[37], 2015; Rocourt and Cheng[22], 2013 | Reviews | - | U-shaped risk response. An excess of Se promotes hyperinsulinemia, hyperglycemia and hyperlipidemia |
| Rayman and Stranges[38], 2013 | Review | - | Increased selenoprotein levels in T2DM patients were reduced by the characteristic inflammatory response of T2DM |
| Wang et al[39], 2017 | Cross-sectional study | 2420 participants | Negative associations were found between Se dose and insulin resistance |
| Wongdokmai et al[40], 2021 | Cross-sectional study | 655 men | Abnormal metabolism in adipocytes by excessive release of fatty acids and/or hormones |
| Vinceti et al[41], 2021 | Prospective study | 24325 participants | High Se intake increased the risk of hospitalization for T2DM |
| Galan-Chilet et al[42], 2017 | Cross-sectional study | 1452 participants | Positive association between plasma Se with prevalent and incident diabetes |
| Hoque and Shi[43], 2022 | Cross-sectional study | 18932 participants | Positively associated with diabetes but inversely associated with all-cause mortality |
| Faghihi et al[44], 2014 | 3 mo | 60 T2DM patients | Se supplementation in T2DM patients with deficient Se levels resulted in adverse effects on blood glucose homeostasis |
Se: Selenium; T2DM: Type 2 diabetes mellitus.
Clinical research on the relationship between Se and treatment of diabetes
Despite the beneficial effect of many micronutrients on different diseases, including diabetes, there are many studies correlating the development of diabetes with Se[45].
It has been observed that Se supplementation is protective against different pathologies such as cancer or autoimmune thyroid disorders when there is a deficiency of this trace element. In contrast, Se supplementation is not recommended when levels of this micronutrient are optimal because of its potential to promote the development of diabetes. Supplementation dose and duration should be carefully taken into account[22,46].
Human clinical trials have confirmed that Se supplementation does not help in the prevention of T2DM but that prolonged exposure to Se supplementation may increase the risk of this disease[47]. Indeed, Se supplementation to treat micro-albuminuria in diabetic patients has been found to be ineffective[48].
Oxidative stress as potential mediator of Se in T2DM
Elevated plasma Se concentrations are associated with biomarkers of diabetes as Se antagonizes the effects of insulin via GPx1 and selenoprotein P (SelP)[40].
Due to the low activity of enzymes such as catalase, superoxide dismutase and GPx, β-cells are protected from oxidative stress by peroxiredoxins, thioredoxins and thioredoxins reductases. As the activity of GPx and thioredoxin reductases depends on the bioavailability of Se, a deficiency of this element leads to oxidative damage of β-cells and a reduction of insulin secretion. However, an excessive amount of Se also leads to a dysregulation of insulin secretion resulting in hyperinsulinemia and a T2DM phenotype. In general, all these antioxidant enzymes, including selenoenzymes, play a role in cell differentiation and insulin secretion by interfering with critical redox signaling for these processes[49].
A supranutritional Se intake, leading to the aforementioned risk in T2DM, also results in endothelial dysfunction via apoptosis mechanisms activated by endoplasmic reticulum (ER) stress and excess reactive oxygen species (ROS) production[50].
It is known that an increase in oxidative stress caused by hyperglycemia leads to an increase in the production of inflammatory cytokines such as IL-6 and tumor necrosis factor alpha and in turn to an increase in the production of free radicals, an increase in insulin resistance in the adipose tissue, liver and muscle and β-cell failure in the pancreas. Therefore, excessive Se levels promote this chain of events that leads to cellular damage and a direct relationship with insulin resistance[51,52].
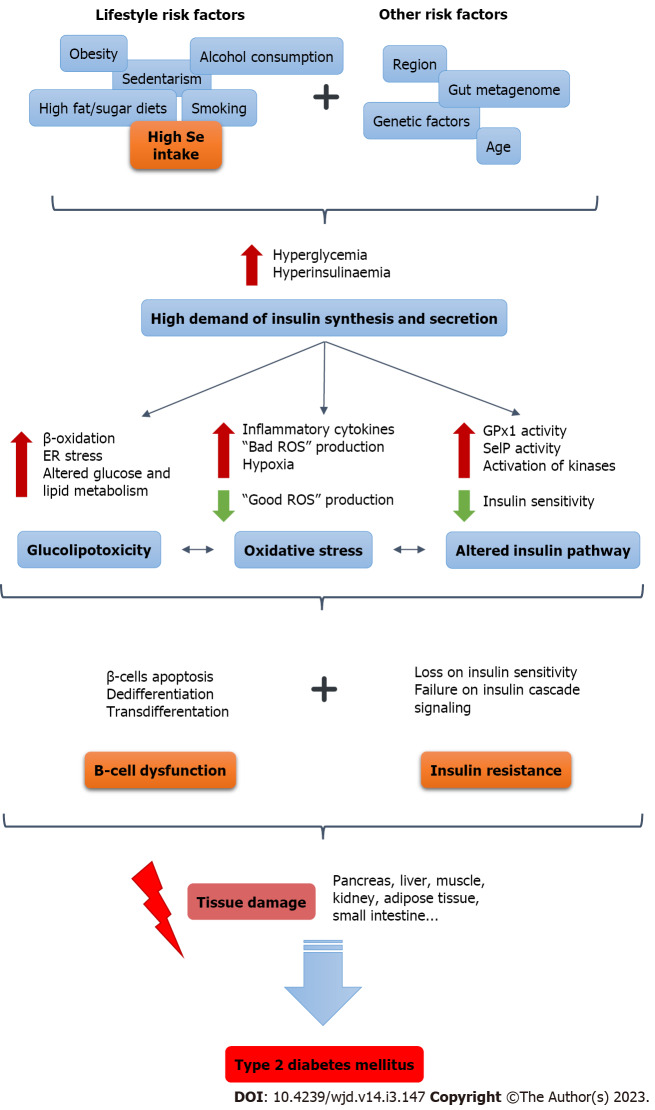
Excessive Se exposure results in hepatic insulin resistance through the opposite regulation of ROS. Certain levels of ROS (“good ROS”) generated at specific sites are essential for the signaling of different physiological processes such as the insulin signaling cascade. However, high levels of Se dysregulate this “good ROS” signaling in response to insulin through the overregulation of selenoproteins. Excess Se also increases the flux of fatty acids in the liver leading to increased production of “bad ROS” that impair insulin sensitivity[53] (Figure 1).
Figure 1.
Role of selenium as a risk factor in the chain of events that triggers the development of type 2 diabetes mellitus. ER: Endoplasmic reticulum; GPx: Glutathione peroxidase; ROS: Reactive oxygen species; Se: selenium; SelP: Selenoprotein P.
Relationship between Se and T2DM regarding the immune system and the inflammation process
Although there is much evidence from in vitro and animal studies on the role of Se in the immune system, there are few studies that support it in humans. Se supplementation appears to have immunostimulant effects by promoting the proliferation of activated T cells, increasing the activity of natural killer cells and increasing cytotoxic lymphocyte-mediated tumor cytotoxicity[15].
Selenoproteins are essential for the function of activated T cells since they are particularly sensitive to oxidative stress and selenoproteins aid promoting the suppression of ROS production. Moreover, human studies have correlated Se supplementation with lymphocyte proliferation preceded by increased expression of high-affinity IL-2 receptor[15]. Low-grade inflammation is involved in insulin resistance and increased SelP concentration has been positively correlated with high-sensitivity C-reactive protein, a biomarker of inflammation, in patients with prediabetes and diabetes[54].
In addition, the relationship between Se with T2DM via the inflammatory pathway has been described through SELENOS, a transmembrane protein located in the membrane of the ER and the plasma membrane. Its increase has been related, among other things, to a decrease in glucose uptake and glycogen biosynthesis as well as to an increase in circulating cytokines[55].
SE AND INSULIN RESISTANCE
Se and its relationship with the insulin signaling pathway
The pancreas is essential for the control of metabolism and energy consumption. It is composed of the exocrine pancreas and the endocrine pancreas, which are morphologically and functionally different. The exocrine pancreas comprises ductal cells and acinar cells that are responsible for the production and release of digestive enzymes into the small intestine for the digestion of fats, carbohydrates and proteins for absorption. The endocrine pancreas is represented by the islets of Langerhans containing five different hormone-secreting cell types: Insulin (β-cells); glucagon (α-cells); somatostatin (δ-cells); pancreatic polypeptide (PP cells); and ghrelin (ε-cells). Among these, insulin and glucagon are secreted directly into the blood to control glucose levels. In contrast to diseases such as pancreatic cancer or pancreatitis, which are related to the exocrine pancreas, diabetes is related to the endocrine islets[56]. Existing data on β-cells show that not all behave in unison but rather exhibit considerable heterogeneity including differential vascular supply, local environment changes, neural innervation or pancreatic exocrine alterations[8].
Binding of insulin to its receptor initiates an intracellular insulin signaling cascade with a large number of molecules. Among them, insulin receptor substrate (IRS)-2, protein tyrosine phosphatase 1B, protein kinase B (serine/threonine kinase Akt), forkhead box class O1a transcription factor and its coactivator peroxisomal proliferator-activated receptor gamma coactivator 1α are of key importance. Dysregulation in the expression, localization or activity of any of these proteins results in insulin resistance. Indeed, elements such as Se can act as insulin-mimetic by activating Akt and other kinases of this signaling cascade[57].
It was also reported that Se acts as an insulin-mimic since at high concentrations it enhances glucose uptake in adipocytes by promoting the translocation of glucose transporters to the plasma membrane and activating serine/threonine kinases. Numerous animal studies have shown that high Se intake induces hyperinsulinemia, hyperglycemia, insulin resistance, glucose intolerance and altered lipid metabolism[58].
Se influence on glucose and lipid metabolism
Altered expression of key factors and enzymes of glycolysis, gluconeogenesis and lipogenesis is implicated in the prodiabetic effect of high Se intake. It has been shown that overintake of Se increases gene expression of forkhead box O1 and peroxisomal proliferator-activated receptor gamma coactivator 1α and reduces gene expression of glycolytic enzyme pyruvate kinase in muscle tissue essential in glucose metabolism. Likewise, adipose tissue is involved in lipid metabolism by increasing, among others, the expression of sterol regulatory element-binding transcription factor 1 and lipoprotein lipase[58] (Figure 1).
Moreover, in a study on the effect of Se supplementation on fatty acid metabolism in mice, an excess of Se increases the expression of genes of glucose transport and increases the β-oxidation of fatty acids associated to the accumulation of acylcarnitines and other lipid metabolites and in turn decreases bile acids metabolites. Thus, it suggests that an excess of Se alters the β-oxidation of fatty acids and creates an imbalance in acetyl-CoA-dependent metabolism[59].
Another study in pigs also confirmed this association of Se with lipid metabolism through the activation of AMPK and different selenoproteins depending on the tissue. In liver tissue, lipid accumulation caused by high Se intake has been associated with a stimulation of lipogenesis and gluconeogenesis as well as with the suppression of lipolysis with GPx3 playing an important role. In muscle tissue, it is SelP that contributes to the development of insulin resistance and hyperinsulinemia[60].
SE, β-CELLS AND INSULIN SECRETION
Effect of oxidative stress in β-cells
Compared to the liver, β-cells are particularly sensitive to oxidative stress given their high production of ROS and their low antioxidant capacity since they have 1% catalase, 2% GPx1 and 29% SOD1 activities[58]. A normal production of ROS derived from glucose metabolism, especially H2O2, are important for signaling. The binding of insulin to its receptor on the plasma membrane causes a transient release of ROS, which serves as secondary messengers promoting the phosphorylation of downstream molecules in the insulin signaling cascade[57].
However, just as elevated concentrations of glucose and cytokines mostly trigger β-cell apoptosis, overproduction of ROS leads to impaired insulin synthesis by affecting β-cell key regulators such as pancreatic duodenal homeobox transcription factor 1 (PDX1) or mitochondrial uncoupling protein 2. Moreover, an overproduction of ROS has a damaging effect by activating a variety of serine/threonine kinases that in turn phosphorylate a large number of targets such as IR and IRS proteins. Consequently, increased serine phosphorylation of IRS-1 decreases the insulin-stimulated threonine phosphorylation of IRS-1 leading to an insulin resistance response and the subsequent development of T2DM[58].
β-cell failure caused by inflammation, hyperglycemia or hyperlipidemia characteristic of diabetes is mainly explained by three linked phenomena: ER stress; mitochondrial dysfunction; and oxidative stress. The increased demand for insulin production and secretion under diabetic conditions saturates the ER folding capacity, and the amount of misfolded proinsulin increases. In response, β-cells activate two unfolded protein response (UPR) mechanisms: Adaptive UPR and apoptotic UPR. These response mechanisms fail when ER stress occurs due to a prolonged glucotoxicity and lipotoxicity. Concerning mitochondrial dysfunction, glucose stimulated insulin secretion is reduced in diabetic conditions, which results in the reduction of the ATP/ADP ratio and consequently decreasing the mitochondrial membrane potential and the expression of genes related to energy metabolism.
Finally, in β-cells, ROS and the antioxidant defense system (such as catalase or GPx) play a crucial role in insulin secretion. However, under chronic pathological conditions, the accumulation of ROS causes both oxidative stress and reduction in catalase and GPx1 expression leading to a high susceptibility of β-cells to ROS damage[4,11,61].
Moreover, under normal conditions, a high oxidative phosphorylation rate occurring in pancreatic islets relies on the availability of a constant oxygen supply. Nevertheless, high glucose exposure increases ROS and hypoxia, thereby activating mechanisms of apoptosis and necrosis[11].
Potential role of Se-dependent antioxidants in -cell function and insulin secretion
High Se intake increases the production of ROS involved in the molecular mechanisms for the insulin-like effects of Se consequently initiating different signaling cascades of programmed cell death, proinflammatory signaling and other adaptive system responses[62]. Particularly, elevated H2O2 attenuates oxidative inhibition of protein tyrosine phosphatases such as protein tyrosine phosphatase 1b or PTEN, suppressing insulin-stimulated IR/IRS/PI3-K/Akt signaling and stimulating the lipogenic pathway, aggravating insulin resistance[58] (Figure 1).
In relation to the influence of selenoproteins, an overexpression of GPx1 alters intracellular ROS, enhances β-cell mass and subsequent redox regulation of key events in insulin synthesis, secretion and function resulting in dysregulation of lipid and glucose metabolism mentioned above[58]. Overly diminishing intracellular ROS by overexpression of GPx1 desensitizes insulin signaling together with chronic hyperinsulinemia resulting from dysregulation of β-cell mass, insulin synthesis and secretion. This desensitization leads to insulin resistance[63] (Figure 1).
Specifically, overproduction of GPx1 overregulates PDX1 mRNA and protein levels and decreases its degradation. Elevated PDX1 functionality in islets results in hypertrophy of β-cells and increased pancreatic and plasma insulin concentrations. Overproduction of GPx1 downregulates uncoupling protein 2 and elevates mitochondrial membrane potential contributing to an accelerated increase in glucose stimulated insulin secretion and hyperinsulinemia[58]. While Se supplementation is not associated with a risk of T2DM in individuals with low concentrations or in individuals maintaining the recommended nutritional dose, excessive long-term Se exposure has been shown to increase the risk of T2DM due to excessive GPx1 activity[64] and a consequent impairment of insulin sensitivity[57] (Figure 1).
SelP1 specifically represents the most important selenoprotein for systemic Se homeostasis as it is involved in Se transport and supply[65]. Positive associations have been found between SelP levels and fasting plasma glucose, hemoglobin A1c and insulin resistance. The metabolic actions of SelP are due to the inactivation of AMPK severely affecting insulin sensitivity. The metabolic effect is similar to that produced by GPx overexpression without affecting insulin synthesis and secretion[58].
CONCLUSION
We highlighted the damaging role of Se in insulin resistance and β-cell secretory function when this element is taken in excess for a prolonged period of time. The response to Se intake has a U-shaped dose-dependent effect so that when it is above the recommended dose, it causes hyperglycemia and hyperinsulinemia, which alters oxidative stress and the insulin signaling cascade and lipid and glucose metabolism. This damage contributes to -cell dysfunction through apoptosis, dedifferentiation and transdifferentiation as well as insulin resistance in a large number of target organs such as the pancreas, liver, kidney and adipose tissue. Therefore, Se supplement ingestion should be taken with caution considering the basal levels of Se in daily food intake to avoid T2DM development. Finally, more studies are needed to better understand the mechanisms behind Se and insulin resistance and thus solve the controversy about the effects of this trace element.
Footnotes
Conflict-of-interest statement: There are no conflicts of interest to report.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 20, 2022
First decision: October 21, 2022
Article in press: February 9, 2023
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu QN, China; Zhu Y, China S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YL
Contributor Information
Pilar Casanova, Department of Pathology, University of Valencia, Valencia 46010, Spain.
Daniel Monleon, Department of Pathology, University of Valencia, Valencia 46010, Spain.
References
- 1.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 2.Yu M, Zhan X, Yang Z, Huang Y. Measuring the global, regional, and national burden of type 2 diabetes and the attributable risk factors in all 194 countries. J Diabetes. 2021;13:613–639. doi: 10.1111/1753-0407.13159. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci. 2014;11:1185–1200. doi: 10.7150/ijms.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keane KN, Cruzat VF, Carlessi R, de Bittencourt PI Jr, Newsholme P. Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and β-Cell Dysfunction. Oxid Med Cell Longev. 2015;2015:181643. doi: 10.1155/2015/181643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen MC, Shulman GI. Mechanisms of Insulin Action and Insulin Resistance. Physiol Rev. 2018;98:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cinti F, Bouchi R, Kim-Muller JY, Ohmura Y, Sandoval PR, Masini M, Marselli L, Suleiman M, Ratner LE, Marchetti P, Accili D. Evidence of β-Cell Dedifferentiation in Human Type 2 Diabetes. J Clin Endocrinol Metab. 2016;101:1044–1054. doi: 10.1210/jc.2015-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.PrayGod G, Filteau S, Range N, Kitilya B, Kavishe BB, Ramaiya K, Jeremiah K, Rehman AM, Changalucha J, Olsen MF, Andersen AB, Friis H, Krogh-Madsen R, Faurholt-Jepsen D. β-cell dysfunction and insulin resistance in relation to pre-diabetes and diabetes among adults in north-western Tanzania: a cross-sectional study. Trop Med Int Health. 2021;26:435–443. doi: 10.1111/tmi.13545. [DOI] [PubMed] [Google Scholar]
- 8.Charles MA, Leslie RD. Diabetes: Concepts of β-Cell Organ Dysfunction and Failure Would Lead to Earlier Diagnoses and Prevention. Diabetes. 2021;70:2444–2456. doi: 10.2337/dbi21-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White MG, Shaw JA, Taylor R. Type 2 Diabetes: The Pathologic Basis of Reversible β-Cell Dysfunction. Diabetes Care. 2016;39:2080–2088. doi: 10.2337/dc16-0619. [DOI] [PubMed] [Google Scholar]
- 10.Brereton MF, Rohm M, Ashcroft FM. β-Cell dysfunction in diabetes: a crisis of identity? Diabetes Obes Metab. 2016;18 Suppl 1:102–109. doi: 10.1111/dom.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber PA, Rutter GA. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid Redox Signal. 2017;26:501–518. doi: 10.1089/ars.2016.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avery JC, Hoffmann PR. Selenium, Selenoproteins, and Immunity. Nutrients. 2018;10 doi: 10.3390/nu10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangiapane E, Pessione A, Pessione E. Selenium and selenoproteins: an overview on different biological systems. Curr Protein Pept Sci. 2014;15:598–607. doi: 10.2174/1389203715666140608151134. [DOI] [PubMed] [Google Scholar]
- 14.Wu G, Li Z, Ju W, Yang X, Fu X, Gao X. Cross-sectional Study: Relationship Between Serum Selenium and Hypertension in the Shandong Province of China. Biol Trace Elem Res. 2018;185:295–301. doi: 10.1007/s12011-018-1272-7. [DOI] [PubMed] [Google Scholar]
- 15.Rayman MP. Selenium and human health. Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Feng W, Chen H, Shi H, Jiang L, Zheng X, Liu X, Zhang W, Ge Y, Liu Y, Cui D. Effect of selenium on thyroid autoimmunity and regulatory T cells in patients with Hashimoto's thyroiditis: A prospective randomized-controlled trial. Clin Transl Sci. 2021;14:1390–1402. doi: 10.1111/cts.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karimi F, Omrani GR. Effects of selenium and vitamin C on the serum level of antithyroid peroxidase antibody in patients with autoimmune thyroiditis. J Endocrinol Invest. 2019;42:481–487. doi: 10.1007/s40618-018-0944-7. [DOI] [PubMed] [Google Scholar]
- 18.Karunasinghe N, Zhu S, Ferguson LR. Benefits of Selenium Supplementation on Leukocyte DNA Integrity Interact with Dietary Micronutrients: A Short Communication. Nutrients. 2016;8 doi: 10.3390/nu8050249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kočan L, Vašková J, Vaško L, Simonová J, Simon R, Firment J. Selenium adjuvant therapy in septic patients selected according to Carrico index. Clin Biochem. 2014;47:44–50. doi: 10.1016/j.clinbiochem.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Guo A, Srinath J, Feuerecker M, Crucian B, Briegel J, Boulesteix AL, Kaufmann I, Choukèr A. Immune function testing in sepsis patients receiving sodium selenite. J Crit Care. 2019;52:208–212. doi: 10.1016/j.jcrc.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Vinceti M, Filippini T, Del Giovane C, Dennert G, Zwahlen M, Brinkman M, Zeegers MP, Horneber M, D'Amico R, Crespi CM. Selenium for preventing cancer. Cochrane Database Syst Rev. 2018;1:CD005195. doi: 10.1002/14651858.CD005195.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rocourt CR, Cheng WH. Selenium supranutrition: are the potential benefits of chemoprevention outweighed by the promotion of diabetes and insulin resistance? Nutrients. 2013;5:1349–1365. doi: 10.3390/nu5041349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radomska D, Czarnomysy R, Radomski D, Bielawski K. Selenium Compounds as Novel Potential Anticancer Agents. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22031009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie C, Xian J, Zeng M, Cai Z, Li S, Zhao Y, Shi Z. Regional Difference in the Association between the Trajectory of Selenium Intake and Hypertension: A 20-Year Cohort Study. Nutrients. 2021;13 doi: 10.3390/nu13051501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rayman MP, Stranges S, Griffin BA, Pastor-Barriuso R, Guallar E. Effect of supplementation with high-selenium yeast on plasma lipids: a randomized trial. Ann Intern Med. 2011;154:656–665. doi: 10.7326/0003-4819-154-10-201105170-00005. [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Jin Y, Unverzagt FW, Cheng Y, Hake AM, Liang C, Ma F, Su L, Liu J, Bian J, Li P, Gao S. The association between selenium and lipid levels: a longitudinal study in rural elderly Chinese. Arch Gerontol Geriatr. 2015;60:147–152. doi: 10.1016/j.archger.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González-Estecha M, Palazón-Bru I, Bodas-Pinedo A, Trasobares E, Palazón-Bru A, Fuentes M, Cuadrado-Cenzual MÁ, Calvo-Manuel E. Relationship between serum selenium, sociodemographic variables, other trace elements and lipid profile in an adult Spanish population. J Trace Elem Med Biol. 2017;43:93–105. doi: 10.1016/j.jtemb.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Fülöp P, Seres I, Jenei Z, Juhász I, Paragh G. Increased hair selenium concentration in hyperlipidemic patients. J Cell Mol Med. 2013;17:350–355. doi: 10.1111/jcmm.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park K, Seo E. Toenail mercury and dyslipidemia: Interaction with selenium. J Trace Elem Med Biol. 2017;39:43–49. doi: 10.1016/j.jtemb.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Berger MM, Soguel L, Shenkin A, Revelly JP, Pinget C, Baines M, Chioléro RL. Influence of early antioxidant supplements on clinical evolution and organ function in critically ill cardiac surgery, major trauma, and subarachnoid hemorrhage patients. Crit Care. 2008;12:R101. doi: 10.1186/cc6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fink K, Busch HJ. Effect of selenium on early outcomes after cardiopulmonary resuscitation : A preliminary report from a retrospective data evaluation. Med Klin Intensivmed Notfmed. 2019;114:246–251. doi: 10.1007/s00063-018-0412-3. [DOI] [PubMed] [Google Scholar]
- 32.Colangelo LA, He K, Whooley MA, Daviglus ML, Morris S, Liu K. Selenium exposure and depressive symptoms: the Coronary Artery Risk Development in Young Adults Trace Element Study. Neurotoxicology. 2014;41:167–174. doi: 10.1016/j.neuro.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Czernichow S, Vergnaud AC, Galan P, Arnaud J, Favier A, Faure H, Huxley R, Hercberg S, Ahluwalia N. Effects of long-term antioxidant supplementation and association of serum antioxidant concentrations with risk of metabolic syndrome in adults. Am J Clin Nutr. 2009;90:329–335. doi: 10.3945/ajcn.2009.27635. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Seo YA, Park SK. Serum selenium and non-alcoholic fatty liver disease (NAFLD) in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2011-2016. Environ Res. 2021;197:111190. doi: 10.1016/j.envres.2021.111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinceti M, Filippini T, Wise LA, Rothman KJ. A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies. Environ Res. 2021;197:111210. doi: 10.1016/j.envres.2021.111210. [DOI] [PubMed] [Google Scholar]
- 36.Wang XL, Yang TB, Wei J, Lei GH, Zeng C. Association between serum selenium level and type 2 diabetes mellitus: a non-linear dose-response meta-analysis of observational studies. Nutr J. 2016;15:48. doi: 10.1186/s12937-016-0169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duntas LH, Benvenga S. Selenium: an element for life. Endocrine. 2015;48:756–775. doi: 10.1007/s12020-014-0477-6. [DOI] [PubMed] [Google Scholar]
- 38.Rayman MP, Stranges S. Epidemiology of selenium and type 2 diabetes: can we make sense of it? Free Radic Biol Med. 2013;65:1557–1564. doi: 10.1016/j.freeradbiomed.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Lin M, Gao X, Pedram P, Du J, Vikram C, Gulliver W, Zhang H, Sun G. High dietary selenium intake is associated with less insulin resistance in the Newfoundland population. PLoS One. 2017;12:e0174149. doi: 10.1371/journal.pone.0174149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wongdokmai R, Shantavasinkul PC, Chanprasertyothin S, Panpunuan P, Matchariyakul D, Sritara P, Sirivarasai J. The Involvement of Selenium in Type 2 Diabetes Development Related to Obesity and Low Grade Inflammation. Diabetes Metab Syndr Obes. 2021;14:1669–1680. doi: 10.2147/DMSO.S303146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vinceti M, Bonaccio M, Filippini T, Costanzo S, Wise LA, Di Castelnuovo A, Ruggiero E, Persichillo M, Cerletti C, Donati MB, de Gaetano G, Iacoviello L Moli-sani Study Investigators. Dietary selenium intake and risk of hospitalization for type 2 diabetes in the Moli-sani study cohort. Nutr Metab Cardiovasc Dis. 2021;31:1738–1746. doi: 10.1016/j.numecd.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 42.Galan-Chilet I, Grau-Perez M, De Marco G, Guallar E, Martin-Escudero JC, Dominguez-Lucas A, Gonzalez-Manzano I, Lopez-Izquierdo R, Briongos-Figuero LS, Redon J, Chaves FJ, Tellez-Plaza M. A gene-environment interaction analysis of plasma selenium with prevalent and incident diabetes: The Hortega study. Redox Biol. 2017;12:798–805. doi: 10.1016/j.redox.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoque B, Shi Z. Association between selenium intake, diabetes and mortality in adults: findings from National Health and Nutrition Examination Survey (NHANES) 2003-2014. Br J Nutr. 2022;127:1098–1105. doi: 10.1017/S000711452100177X. [DOI] [PubMed] [Google Scholar]
- 44.Faghihi T, Radfar M, Barmal M, Amini P, Qorbani M, Abdollahi M, Larijani B. A randomized, placebo-controlled trial of selenium supplementation in patients with type 2 diabetes: effects on glucose homeostasis, oxidative stress, and lipid profile. Am J Ther. 2014;21:491–495. doi: 10.1097/MJT.0b013e318269175f. [DOI] [PubMed] [Google Scholar]
- 45.Sanmartin C, Plano D, Font M, Palop JA. Selenium and clinical trials: new therapeutic evidence for multiple diseases. Curr Med Chem. 2011;18:4635–4650. doi: 10.2174/092986711797379249. [DOI] [PubMed] [Google Scholar]
- 46.Drutel A, Archambeaud F, Caron P. Selenium and the thyroid gland: more good news for clinicians. Clin Endocrinol (Oxf) 2013;78:155–164. doi: 10.1111/cen.12066. [DOI] [PubMed] [Google Scholar]
- 47.Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, Cappuccio FP, Ceriello A, Reid ME. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 48.Ghadiri-Anari A, Jam-Ashkezari S, Fallah-Tafti B, Rahmanian M, Namiranian N. The effect of selenium on micro-albuminuria in diabetic patients: A randomized clinical trial. Iran J Diabetes and Obes. 2021;12 [Google Scholar]
- 49.Steinbrenner H, Duntas LH, Rayman MP. The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities. Redox Biol. 2022;50:102236. doi: 10.1016/j.redox.2022.102236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zachariah M, Maamoun H, Milano L, Rayman MP, Meira LB, Agouni A. Endoplasmic reticulum stress and oxidative stress drive endothelial dysfunction induced by high selenium. J Cell Physiol. 2021;236:4348–4359. doi: 10.1002/jcp.30175. [DOI] [PubMed] [Google Scholar]
- 51.Pouresmaeil V, Al Abudi AH, Mahimid AH, Sarafraz Yazdi M, Es-Haghi A. Evaluation of Serum Selenium and Copper Levels with Inflammatory Cytokines and Indices of Oxidative Stress in Type 2 Diabetes. Biol Trace Elem Res. 2023;201:617–626. doi: 10.1007/s12011-022-03191-w. [DOI] [PubMed] [Google Scholar]
- 52.Ogawa-Wong AN, Berry MJ, Seale LA. Selenium and Metabolic Disorders: An Emphasis on Type 2 Diabetes Risk. Nutrients. 2016;8:80. doi: 10.3390/nu8020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Zhang W, Chen H, Liao N, Wang Z, Zhang X, Hai C. High selenium impairs hepatic insulin sensitivity through opposite regulation of ROS. Toxicol Lett. 2014;224:16–23. doi: 10.1016/j.toxlet.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Yang SJ, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: implications for insulin resistance, inflammation, and atherosclerosis. J Clin Endocrinol Metab. 2011;96:E1325–E1329. doi: 10.1210/jc.2011-0620. [DOI] [PubMed] [Google Scholar]
- 55.Michalke B. Review about Powerful Combinations of Advanced and Hyphenated Sample Introduction Techniques with Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) for Elucidating Trace Element Species in Pathologic Conditions on a Molecular Level. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23116109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou Q, Melton DA. Pancreas regeneration. Nature. 2018;557:351–358. doi: 10.1038/s41586-018-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinbrenner H, Speckmann B, Pinto A, Sies H. High selenium intake and increased diabetes risk: experimental evidence for interplay between selenium and carbohydrate metabolism. J Clin Biochem Nutr. 2011;48:40–45. doi: 10.3164/jcbn.11-002FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou J, Huang K, Lei XG. Selenium and diabetes--evidence from animal studies. Free Radic Biol Med. 2013;65:1548–1556. doi: 10.1016/j.freeradbiomed.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu X, Chandler JD, Orr ML, Hao L, Liu K, Uppal K, Go YM, Jones DP. Selenium Supplementation Alters Hepatic Energy and Fatty Acid Metabolism in Mice. J Nutr. 2018;148:675–684. doi: 10.1093/jn/nxy036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao Z, Barcus M, Kim J, Lum KL, Mills C, Lei XG. High Dietary Selenium Intake Alters Lipid Metabolism and Protein Synthesis in Liver and Muscle of Pigs. J Nutr. 2016;146:1625–1633. doi: 10.3945/jn.116.229955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eguchi N, Vaziri ND, Dafoe DC, Ichii H. The Role of Oxidative Stress in Pancreatic β Cell Dysfunction in Diabetes. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brigelius-Flohé R, Flohé L. Selenium and redox signaling. Arch Biochem Biophys. 2017;617:48–59. doi: 10.1016/j.abb.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Huang JQ, Zhou JC, Wu YY, Ren FZ, Lei XG. Role of glutathione peroxidase 1 in glucose and lipid metabolism-related diseases. Free Radic Biol Med. 2018;127:108–115. doi: 10.1016/j.freeradbiomed.2018.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tabrizi R, Akbari M, Moosazadeh M, Lankarani KB, Heydari ST, Kolahdooz F, Mohammadi AA, Shabani A, Badehnoosh B, Jamilian M, Assarian A, Asemi Z. The Effects of Selenium Supplementation on Glucose Metabolism and Lipid Profiles Among Patients with Metabolic Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm Metab Res. 2017;49:826–830. doi: 10.1055/s-0043-119544. [DOI] [PubMed] [Google Scholar]
- 65.Steinbrenner H. Interference of selenium and selenoproteins with the insulin-regulated carbohydrate and lipid metabolism. Free Radic Biol Med. 2013;65:1538–1547. doi: 10.1016/j.freeradbiomed.2013.07.016. [DOI] [PubMed] [Google Scholar]