Abstract
Affective experiences are commonly represented by either transient emotional reactions to discrete events or longer-term, sustained mood states that are characterized by a more diffuse and global nature. While both have considerable influence in shaping memory, their interaction can produce mood-congruent memory (MCM), a psychological phenomenon where emotional memory is biased towards content affectively congruent with a past or current mood. The study of MCM has direct implications for understanding how memory biases form in daily life, as well as debilitating negative memory schemas that contribute to mood disorders such as depression. To elucidate the factors that influence the presence and strength of MCM, here we systematically review the literature for studies that assessed MCM by inducing mood in healthy participants. We observe that MCM is often reported as enhanced accuracy for previously encoded mood-congruent content or preferential recall for mood-congruent autobiographical events, but may also manifest as false memory for mood-congruent lures. We discuss the relevant conditions that shape these effects, as well as instances of mood-incongruent recall that facilitate mood repair. Further, we provide guiding methodological and theoretical considerations, emphasizing the limited neuroimaging research in this area and the need for a renewed focus on memory consolidation. Accordingly, we propose a theoretical framework for studying the neural basis of MCM based on the neurobiological underpinnings of mood and emotion. In doing so, we review evidence for associative network models of spreading activation, while also considering alternative models informed by the cognitive neuroscience literature of emotional memory bias.
Keywords: mood-congruent memory, affective neuroscience, consolidation
Introduction
Life is not a neutral experience. We are constantly exposed to emotional content that can elicit a range of positive and negative reactions, which may shape our thoughts and behavior. There are also long-term consequences of these emotional experiences, as they tend to be the ones that we remember the best (LaBar & Cabeza, 2006). For several decades now, researchers have sought to explain why emotions hold such a special place in memory, and how this influence of emotion on memory relates to the debilitating memory biases that have been observed in mood disorders such as depression (Beck, 1967; Blaney, 1986). Modern neuroimaging techniques have also allowed for examination of the underlying neural mechanisms implicated in emotion, memory, and their interaction. Grounded in research by McGaugh et al. (1996), who demonstrated the importance of the amygdala in mediating the effects of heightened arousal on memory consolidation, studies on emotional memory often examine the relationship between temporarily-induced arousal and subsequent memory performance. Their findings have highlighted that arousal has a selective influence on what we remember (Mather & Sutherland, 2011), leading to both enhancement and impairment in memory via intricate neuromodulatory systems (Mather et al., 2016), and that the intrinsic value or valence of an emotional experience further moderates these effects (Bowen et al., 2018). These emotional influences can systematically shift the neurocognitive processes involved in long-term memory. For instance, recent work indicates that increased arousal shortly after encoding can even reverse systems consolidation by strengthening hippocampal dependency and reducing neocortical involvement in memory retrieval (Atucha et al., 2017; Krenz et al., 2021).
In real life, however, we are limited not only to these brief arousing experiences, but also sustained affective states that fluctuate at more protracted timescales and often have a less definable cause. These moods typically arise as a combination of experiences that together promote a feeling state lasting for minutes, hours or even days and are less specific to one particular stimulus or event (Beedie, 2007; Beedie et al., 2005; Frijda, 1994; Ketal, 1975; Ortony & Clore, 1989; Siemer, 2009). Moods have a profound influence on cognition by shaping the mental processes involved in attention (Sedikides, 1992; Tamir & Robinson, 2007; Wadlinger & Isaacowitz, 2006), cognitive control (Hsieh & Lin, 2019; E. A. Martin & Kerns, 2011), learning (Bower et al., 1978; Nadler et al., 2010), decision-making (Forgas, 1989; Hockey et al., 2000; Stanton et al., 2014), and memory (Blaney, 1986; Bower, 1981; Forgas & Eich, 2012). These effects can change significantly depending on the type of mood, such as positive moods facilitating an elaborative encoding style while negative moods promote more detail-oriented processing and careful monitoring of new information (Fiedler, 2001; Lench et al., 2011). Neuroimaging investigations have also begun to explore the neural underpinnings of induced and natural mood states in an effort to better understand why such multi-faceted influences are observed on cognition (Kirkby et al., 2018; Kohn et al., 2013). Clinically, the relationship between affective states and cognition is especially relevant for mood disorders, where biased focus towards negative material contributes to the onset and maintenance of depressive symptoms (Gotlib & Joormann, 2010).
Despite the relevance of both emotion and mood to memory biases in daily life and clinical disorders, we have a surprisingly limited understanding of how they combine to influence memory. Specifically, how does one’s mood state configure the way in which emotional content is remembered? Generally, we know that the congruence between moods and emotions modulates memory bias, such that we often remember positive or negative emotional information better when attending to or recalling that information in a congruent mood (Blaney, 1986). Beyond this general understanding, the specific neurocognitive mechanisms implicated in such an effect remain relatively underspecified and have received comparatively less attention than the more transient effects of emotion on memory. The lack of empirical research in this domain and integration with cognitive neuroscience perspectives is surprising, as MCM research can further progress our understanding of affective biases in memory that contribute to the development and maintenance of mood disorders. A particularly limited area of research, as we will further discuss in this review, is the influence of mood on the early consolidation of emotional content. Most evaluations of mood congruence focus on the online encoding or retrieval of information, but mood congruence may also influence the offline storage of recently encoded emotional experiences. This proposal integrates well with neurocognitive perspectives that have developed in the emotional memory literature, which consider post-encoding consolidation mechanisms as central to the long-term influence of emotion on memory.
Here we review evidence that mood selectively biases how emotional events are stored into memory. We examine a diverse set of behavioral investigations that have uncovered important insights into the psychological and methodological factors that determine the influence of induced mood on emotional memory, while also proposing opportunities for future research in this area. Further, we emphasize the need for examination of the neural mechanisms subserving mood experience, mood congruence, and subsequent memory effects to gain a more comprehensive understanding for how long-term emotional memory biases are formed and maintained.
Defining Affect: Emotions vs. Moods
To appreciate the independent and combined influences of emotion and mood on memory, we must first consider how these affective experiences are conceptualized. Here we follow a similar classification of affect, emotion, and mood as Bower & Forgas (2000), characterizing emotion as a reaction to an identifiable stimulus, event or thought; mood as a background mental state that casts a glow or shadow over our thoughts and behaviors; and affect as a broad term encompassing both emotions and moods. Moods are sustained affective experiences that last for an extended period of time, compared to the shorter-lived responses that constitute emotions (Ketal, 1975). Moods also tend to develop without awareness of a specific cause, while emotions typically result from more definable experiences (Ortony & Clore, 1989). These distinctions based on duration and remoteness to cause are common criteria used in the literature and also among laypersons (Beedie et al., 2005), preserving the consensus that mood states tend to be more diffuse and global than emotions (Frijda, 1994).
Moods can be further understood as temporary dispositions to have or generate particular emotional appraisals (Siemer, 2009), such as an angry mood facilitating feelings of anger to new experiences. That is, moods develop as internal mental states that have lasting effects beyond the immediate, emotional reaction to an event. In the subjective-contextual model, the distinction between an emotion or mood is defined by how an individual appraises their affect and if any specific actions are taken to resolve the cause or focus of one’s feelings (Beedie, 2007). Because emotions are more intentional than moods they signal the affective appraisal of a specific event or object, whereas moods either lack such a distinct appraisal or the appraised object has become diffuse and nonspecific (Clore et al., 2001). This non-intentional nature of moods allows them to endure over time despite changes to the surrounding environment. Note, however, that an emotional experience or collection of experiences may slowly evolve into a mood state. For example, when arguing with a close friend or partner, even if an individual can identify a cause for the initial emotional reaction (e.g., anger), the affect associated with the event can develop into a low-intensity negative mood that persists in the background if no action is taken to resolve or regulate that focus. Indeed, we note that the difference between emotions and moods can be less clear-cut in real-world scenarios that involve dynamically fluctuating social interactions and temporally extended events.
In sum, moods and emotions constitute related yet partially separable affective experiences that typically differ from one another with respect to duration, cause, and appraisal. As such, research on emotions and moods use different methodological approaches to induce the desired affect in study participants. An emotion-based study might present brief arousing images, whereas studies seeking to induce a particular mood might ask participants to watch a movie or read a series of self-referential statements that gradually induce an affective mental state over time. Consequently, researchers often measure emotional experiences by indexing immediate reactions to stimuli via subjective self-report of valence and arousal or measuring transient psychophysiological indices such as skin conductance response within seconds of the eliciting event (Christopoulos et al., 2019). In contrast, mood inductions are commonly measured by change in affective state from baseline to post-induction, whereby researchers administer more comprehensive questionnaires such as the profile of mood states (McNair et al., 1971) and measure slower-changing physiological signals continuously, such as skin conductance level or heart rate variability (Kop et al., 2011; Ribeiro et al., 2019).
Appreciating these differences between emotions and moods is fundamental to understanding their distinct, yet complementary, influence on memory. Transient emotional arousal has been shown to facilitate biased recollection of encoded material (LaBar & Cabeza, 2006; Mather & Sutherland, 2011) and recent work also suggests differential consolidation of positively- and negatively-valenced content (Bowen et al., 2018). However, moods may further bias, or filter, memory by specifically promoting one set of emotional stimuli (e.g., negative items) over others (e.g., positive items). Moods may also develop in a more discrete manner (e.g., a sad, angry, or fearful mood) and act in a category-specific fashion, targeting enhanced focus toward discrete emotions. Importantly, the sustained and diffuse nature of moods can allow for both online (encoding and retrieval) and offline (consolidation) effects that ultimately influence the long-term memory of emotional experiences. As the following section will review, this interaction is characterized by the congruency between internal mood state and external emotional information.
Mood and Memory
The influence of mood on cognition is extensive, with effects seen in multiple domains beyond just memory, such as attention (Sedikides, 1992; Tamir & Robinson, 2007; Wadlinger & Isaacowitz, 2006) and executive control (Dreisbach, 2006; Mitchell & Phillips, 2007). Moods have a profound influence in biasing the many cognitive operations that mediate long-term memory storage. While such multifaceted influences of mood on cognition are interesting in their own right, here we specifically focus on the interplay of mood with memory for emotional content. This interactive relationship among mood, memory, and emotions is commonly examined in the context of mood-congruent memory (MCM), which occurs when one’s mood selectively influences the storage or retrieval of affectively congruent material. MCM is closely associated with—yet distinct from—mood-dependent memory, a separate mood-related phenomenon that occurs when memory performance depends on the concordance between mood at encoding and retrieval. Together, MCM and mood-dependent memory constitute the foundation of mood and memory research, although only MCM explicitly involves an interaction between mood and emotion. As such, we will only briefly discuss mood-dependent memory before devoting the remainder of this review to MCM.
Mood-dependent memory implies that previously-experienced events are better remembered when re-experienced in the same mood state in which they were initially encoded, irrespective of the emotionality of the remembered information (Bower, 1981). Mood-dependent memory is an example of the encoding specificity principle (Tulving, 1983), whereby memory is enhanced when contextual factors at encoding (in this case mood) are reinstated at retrieval. Since mood-dependent memory is defined by an overlap in mood state and independent from the valence of the encoded material, researchers often test memory for neutral content in order to avoid the potential confound of any mood-congruent effects (Lewis & Critchley, 2003). Mood at encoding or retrieval could uniquely facilitate memory for affectively congruent content (as further discussed below), which may be difficult to dissociate from that of mood dependence. Although initial empirical investigations of mood dependence produced inconsistent findings (Bower & Mayer, 1985), ongoing work eventually helped establish the conditions that best facilitate mood-dependent memory. Specifically, mood dependence has been shown to be strengthened with free recall tasks that place greater reliance on participants’ internal contextual resources (i.e., mood) as opposed to external cues, allowing participants to generate their own target events during encoding (generative encoding), and providing longer delays between encoding and retrieval (Eich, 1995; Forgas & Eich, 2012). Recent empirical demonstrations of mood-dependent memory have further improved confidence in the reliability and validity of demonstrating this phenomenon in the lab (Thorley et al., 2016; Xie & Zhang, 2018).
In contrast to mood-dependent memory, MCM specifically reflects the interaction of internal mood state with the emotionality attributed to encoded or retrieved material (Blaney, 1986; Bower, 1981). For example, the presence of a sad mood at encoding can promote increased focus on sad material and subsequently enhance memory for that information, or a sad mood at retrieval can bias recall to previously encoded sad items in memory. Thus, mood dependence and mood congruence are not mutually exclusive, but rather represent two possible influences of mood on memory. Only MCM, however, reflects the influence of mood on memory at a single timepoint. In many ways, MCM is akin to schema-related memory processing whereby the encoding, consolidation, and retrieval of information is facilitated by a superordinate knowledge structure, or schema, that binds together closely-associated elements (Gilboa & Marlatte, 2017). Clinically depressed mood, for instance, is thought to activate negative schemas associated with the self, the world, and the future, which collectively facilitate biased attention and memory towards mood-congruent negative content (Beck, 2008; Bovy et al., 2020; Clark et al., 1999). Importantly, a related phenomenon known as mood-incongruent memory has also been observed, whereby memory recall is biased towards emotional content inconsistent with one’s mood state (Holland & Kensinger, 2010). Mood-incongruent memory may reflect mechanisms that suppress, impair, and/or override the generation of MCM. As we will further examine in the present review, a memory bias for mood-incongruent content seems to arise from automatic or instructed attempts at mood repair (for negative moods) or deeper processing of emotionally ambiguous stimuli.
In the lab, MCM is commonly studied by inducing a mood prior to encoding or retrieval and then examining the extent to which memory performance shifts in favor of mood-congruent external stimuli (Lewis & Critchley, 2003). MCM can also be studied by inducing mood prior to autobiographical recall and assessing the degree to which memory towards certain emotional material is favored (Holland & Kensinger, 2010). Our present review will focus on both approaches, which are assumed to derive from an associative network of connections among moods and emotional events. In what follows, we discuss this theoretical foundation.
A Network Theory of Memory and Emotion
Central to theoretical perspectives on mood and memory is the associative network theory proposed by Bower (1981), which suggests that affect exists as central units, or nodes, within a network of connections involving associated ideas and events. These network associations are initially formed during learning, whereby newly encoded material couples with nodes that are concurrently active. When a mood node is activated, the activation automatically spreads along these established links to neighboring nodes. For instance, mood-dependent memory is thought to result from mood that links with relevant information at encoding, subsequently lowering the activation threshold for retrieval of that information when the same mood is subsequently reactivated. Moods can also be present at either encoding or retrieval and still bias memory by spreading activation to mood-congruent emotional content, as is the case with MCM (Figure 1A). This proposal was built on the spreading activation theory of semantic processing (Collins & Loftus, 1975), which posits that semantic information is organized within a network of related concept nodes. In the case of mood and memory, however, an associative network specifically reflects connected links among affective and mnemonic information (Figure 1B).
Figure 1.
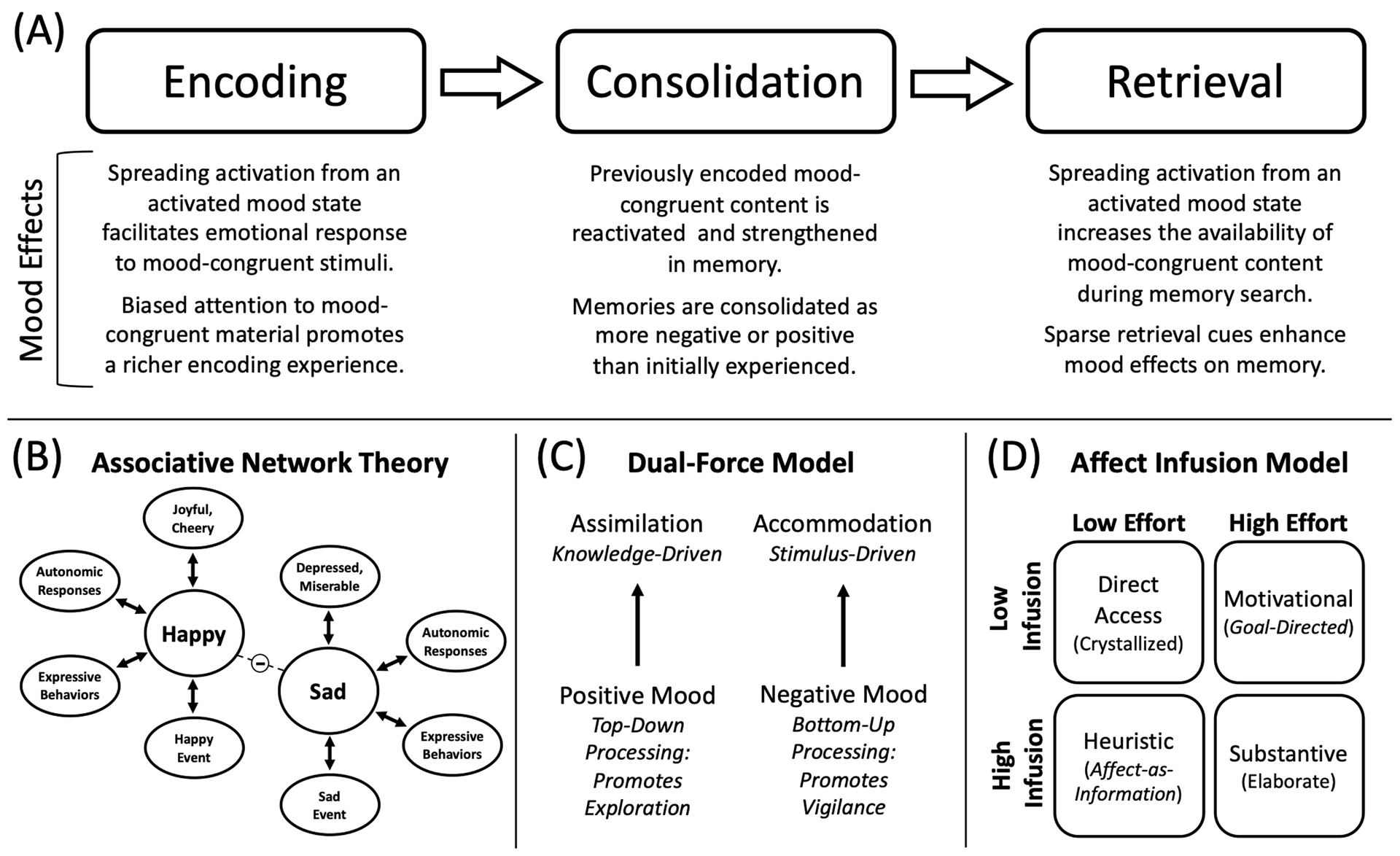
Models of Mood and Memory
Note. (A) Proposed influences of induced mood on each stage of the memory process. Mood selectively enhances attention, storage, and search processes to promote biased memory for mood-congruent material. Although most MCM research focuses on encoding or retrieval congruency, mood may also shift consolidation processes in a mood-congruent manner. (B) Illustration of the associative network theory of memory and emotion proposed by Bower (1981). Happy and sad affect are depicted alongside some of their associated nodes, including expressive behaviors, autonomic responses, verbal labels (e.g., joyful/cheery or depressed/miserable) and memories for mood-congruent events. When a mood node is activated, activation will spread along these established links to neighboring nodes. Emotions with opposing valence have inhibitory connections, such that a sad mood will inhibit happiness and its associated links. (C) The Dual-Force Model from Fiedler (1991, 2002) suggests that assimilative processes transform learned input into existing knowledge structures, whereas accommodative processes facilitate attentive and accurate encoding with relatively little transformation. Positive moods signal safety and activate assimilative processes that improve performance on generative tasks, whereas negative moods signal uncertainty and activate accommodative processes that facilitate item-specific processing. (D) The Affect Infusion Model from Forgas (1995) proposes four processing strategies that can be used when making judgements, which theoretically also influences memory. Affect infusion (mood-related effects) is most likely during heuristic processing (when mood is the direct source of judgement) and substantive processing (when elaborate, generative processes assimilate information into existing knowledge structures). By contrast, affect infusion is low during direct access processing (when evaluations are made from existing, crystallized judgements) or motivational processing (when specific goals guide task engagement, such as with mood repair).
Bower’s cognitive model was developed to aid in behavioral prediction, although the proposal that affective nodes can spread activation to linked information does make neural assumptions for how mood and memory are represented in the brain. Yet, as we will further discuss in this review, direct neuroscientific evidence of an associative network underlying MCM remains limited, mainly due to a lack of neuroimaging studies in this area. Nevertheless, research on the neural bases of mood, emotion, and memory have provided valuable insight to how the brain supports mood development and emotional responding more generally, as well as the complex neural interactions that produce long-term memory biases for emotional content. Furthermore, research on memory schemas have also helped to support the principal tenets of associative network theory with formal neural mechanisms. Recent work has shown, for instance, the consistent involvement of the ventromedial prefrontal cortex in identifying schema congruency (Ghosh et al., 2014; Spalding et al., 2015) and coordinating the activation of schematic representations in the posterior neocortex (Ghosh & Gilboa, 2014; Gilboa et al., 2009). Communication among these regions may also be responsible for recognizing mood-congruent content and then integrating that content with an existing representation of affect. We explore these points in more detail in The Cognitive Neuroscience of Mood-Congruent Memory.
Bower’s associative network theory helped to organize the field around a conceptual framework that would guide research on mood and memory. Specifically, the proposal that mood-related memory effects stem from spreading activation within a semantic network originally helped develop four primary hypotheses (Bower, 1981; Gilligan & Bower, 1985; Singer & Salovey, 1988). First, a matching of mood at encoding and retrieval will boost general memory performance (mood-dependent memory). This effect is theorized to reflect the formation of new associations among nodes during encoding, which subsequently lower the activation of content at retrieval when the appropriate mood node is reactivated. Mood-dependent memory is expected to be strengthened when external retrieval cues are sparse (e.g., during a free recall task), since having fewer external cues available places greater demand on the recapitulation of an internal contextual cue to facilitate retrieval. Given that we focus the remainder of this review on MCM, we refer readers to Forgas and Eich (2013) for further discussion of mood-dependent memory. Second, mood enhances emotionally congruent thoughts, judgments, and associations (referred to as retrieval congruency) since spreading activation increases the availability of emotionally congruent content during the memory search process. Third, mood improves the learning and retention of emotionally congruent material (referred to as encoding congruency) because a denser network representation is encoded for content closely affiliated with an activated mood node. Fourth, the influence of mood on learning scales with the intensity of affect experienced, given that associated network nodes are presumably more active when a mood is more intense.
Shortly after Bower proposed this network model, both Blaney (1986) and Singer and Salovey (1988) published comprehensive reviews to determine empirical support for the above hypotheses. When Bower initially proposed his network model, he was only able to reference a few instances of published, empirical evidence. But a steady influx of mood and memory experiments continued to be conducted in this area, and a review of the literature was needed to determine the reliability of the reported effects. Most notably, an increase in studies where the experimenter induced mood states in participants warranted further inspection to determine whether mood-related influences on memory could be properly manipulated within a controlled, laboratory setting. Such assessments provide a purer examination of the influence of mood on memory than individual difference analyses, since researchers can more precisely determine the unique influences of mood at specific stages of the memory process (Blaney, 1986). Yet, induction methods were highly variable, usually consisting of hypnosis or having participants read lists of elated or depressed self-referential statements, but also involving success/failure manipulations, music, autobiographical recall, or posturing. Could MCM be reliably demonstrated across such diverse manipulations?
Initial studies seemed to provide promising results, with MCM effects reliably demonstrated among both induced and naturally occurring mood states (Blaney, 1986; Singer & Salovey, 1988). Of the 29 mood induction articles reviewed by Blaney (1986), 25 reported evidence for MCM in measures of memory accuracy, recall latency, and/or phenomenological properties of memory recall. Many of these studies used autobiographical assessments, whereby participants were placed into elated or depressed mood and asked to recall past life experiences. Consequently, participants would recall more memories with emotional valence congruent with their mood state (e.g., Snyder & White, 1982; Teasdale & Taylor, 1981). Others assessed memory for content encoded in the lab setting (e.g., word lists, self-referent traits), finding that mood at both encoding (Brown & Taylor, 1986) and retrieval (Teasdale & Russell, 1983) could bias subsequent memory in a mood-congruent direction. Among the few studies with null findings, Blaney suggested methodological issues that obscured MCM, such as mood influences at both encoding and retrieval, small stimulus sets (e.g., a single narrative), or discouraged self-referential processing. This latter point was particularly emphasized, as Blaney observed a consistent pattern among assessments of natural mood (where participants were split by depression status, rather than induction) showing that MCM was more frequently obtained under conditions that encouraged participants to focus on stimulus applicability to their own lives (e.g., Bradley & Mathews, 1983). Compared to Blaney (1986), Singer and Salovey (1988) separately reviewed support for encoding congruency (mood induced prior to encoding) and retrieval congruency (mood induced prior to retrieval), but ultimately arrived at similar conclusions as those from Blaney (1986) noted above. In terms of differences between the two types of MCM, encoding congruency studies were far fewer in number, but all the induction paradigms in this set yielded significant MCM.
Regarding the intensity hypothesis, both reviews observed a lack of research on this proposal. Blaney (1986) highlighted a few studies that suggested mood intensity was unrelated to the extent of MCM, speculating that perhaps a certain intensity threshold is required, after which increased intensity has little or no influence. Interestingly, Singer and Salovey (1988) approached the mood intensity hypothesis differently from Blaney (1986), suggesting that intensely sad moods might actually impair memory for both congruent and incongruent items, provided that depressed mood diverts attention from external sources to internal ruminative thinking on failure and introspection. However, there was no direct empirical evidence to support this claim, other than general impairments in memory for neutral content observed with depressed mood (Ellis et al., 1985).
Despite both reviews concluding consistent empirical support for MCM, they also noted important caveats and suggestions for future work. Notably, mood induction procedures that instructed participants to self-generate the intended mood raised concerns over subject compliance and experimenter demand issues, whereby subjects might think they should respond on experimental tasks in a way that continues to perpetuate the intended mood, instead of a true influence of mood on memory. Relatedly, although hypnotic mood inductions were popular at the time, appropriately administering this technique required that the experimenter was properly trained in hypnosis, as well as willingness from the subject to comply with the induction procedures. Participants also needed to be carefully screened on hypnotic susceptibility, as individuals can vary considerably in their ability to enter the desired hypnotic state. Bower (1981) estimated that only 20–25% of people are highly susceptible to hypnosis, thus necessitating diligent screening for study inclusion. For these reasons, other induction techniques that do not require these same training and screening requirements began to gain in popularity shortly after the reviews of Blaney (1986) and Singer and Salovey (1988). Importantly, though, this is not to suggest that the use of hypnosis was problematic. When administered appropriately, hypnosis successfully induced strong and durable changes in mood state that allowed for testing both MCM and mood-dependent memory (Bower, 1981), and has remained a valuable technique to alter conscious experience (Kihlstrom, 2012). Other induction techniques, however, may be more accessible to a broader range of researchers.
Finally, while some form of MCM was reported among most studies, results were typically asymmetrical. Sometimes only the positive or negative mood induction would lead to MCM, or only the positive or negative emotional items were biased in memory. A pattern in this asymmetry seemed to emerge, whereby it was often the case that positive moods would promote MCM for positive material and inhibit negative material, whereas negative moods impaired memory for positive material but didn’t always boost memory for negative material (Singer & Salovey, 1988). Blaney suggested that this asymmetry could be due to a baseline positivity bias, whereby people tend to be in generally positive moods prior to any mood induction. Some induction techniques may then fail to overcome this initial offset, producing a weaker MCM effect for negative moods. Additionally, both reviews speculated that mood repair mechanisms might further prevent negative moods from facilitating a symmetrical bias in memory. Positive moods do not require regulation, and therefore MCM might be more automatic in positive states. In contrast, negative moods signal the need to deploy controlled processes that short circuit rumination on negative content, which may initiate positive, incongruent thoughts as a mechanism of mood repair. Indeed, depression can result from an impaired ability to implement these controlled processes (Joormann & Gotlib, 2010). Bower’s network model did not incorporate the possibility of regulatory mechanisms impeding the process of spreading activation, although this could help explain the observed asymmetrical MCM effects between positive and negative inductions (Blaney, 1986; Singer & Salovey, 1988).
As mentioned, induction studies attempted to further refine clinical perspectives of MCM by experimentally isolating the effect of mood on memory. The qualitative reviews by Blaney (1986) and Singer and Salovey (1988) suggested this was possible but did not quantitatively compare effect sizes between the two literatures. To address this gap, Matt et al. (1992) subsequently conducted a meta-analysis of studies on mood-congruent recall. Specifically, the authors assessed whether the difference in proportions of correctly recalled positive and negative stimuli (among studies that assessed and reported such effects) differed among naturally occurring mood states and induced ones. Their findings showed a shift in bias for recall of positively-valenced items in nondepressed individuals to symmetric recall of positive and negative items in subclinically depressed individuals, and to a bias for negatively-valenced items in those who were clinically depressed or induced into a depressed mood (Matt et al., 1992). While induced elated mood did produce a positivity bias, the confidence interval for the effect size estimate was not reliably different from zero. Note, however, that this initial analysis was only conducted in studies that measured the veridical recall of stimuli, which mostly consisted of memory for arbitrary word lists (i.e., not self-referential encoding). By comparison, a positivity bias was found to be reliable among studies that had used self-referential encoding, although these were few in number. Matt et al. (1992) also acknowledged that many studies were underpowered due to relatively small sample sizes and recommended that future researchers not only test with larger samples, but also define stimulus norming procedures and comprehensive reports of induction efficacy more clearly (for which the authors found a systematic lack of reporting in the reviewed studies). Thus, it remained unclear whether asymmetry between induction types truly reflected differences in MCM, or rather these methodological issues that introduced noise to the data. Nevertheless, this meta-analysis showed that memory biases observed in negative mood inductions were quantitatively similar to those observed in clinical depression.
In sum, the reviews by Blaney (1986) and Singer and Salovey (1988)—as well as a complementary quantitative assessment by Matt et al. (1992)—supported the feasibility of studying MCM effects among healthy participants exposed to experimentally manipulated mood. This finding was viewed as support for the basic tenets of network theory, although the reliability of certain mood inductions and the high prevalence of asymmetrical results suggested that (i) additional work was needed that utilized more experimentally sound induction techniques and (ii) a network model may be too simplistic to capture the many nuances of mood congruence, such as influences of mood repair and task engagement. Accordingly, several suggestions have been offered to help improve model predictions, which we will explore in the subsequent section.
Other Perspectives on Mood Congruence
An associative network theory is considered a memory-based theory of mood congruence since it assumes that mood experience can be traced to an underlying semantic architecture (Forgas & Eich, 2012). The implications of this theory, however, are not restricted to just the explicit memory domain. Mood congruence can also manifest in the judgements we make—when we are in a sad mood, we tend to judge the world around us as more negative. In these cases, a network theory proposes that mood increases the availability of affectively congruent concepts in memory, thereby promoting a biased interpretation or judgement of the object under consideration. This theoretical framework therefore suggests an indirect relationship between one’s mood and ultimate judgement, mediated by spreading activation. Alternatively, inferential theories such as the affect-as-information approach propose that moods can be a direct source of information when making evaluative judgements (Clore et al., 2001; Clore & Storbeck, 2012). That is, instead of cognitively appraising the emotional attributes of an object based on beliefs retrieved from memory, the affect-as-information approach suggests that we often base our judgements simply on how we feel about those objects, which may be directly informed from one’s mood state. This theory is based on the observation that people often rely on their current mood as a heuristic aid when making judgements, usually when they are unaware of the source of their mood state. Schwarz and Clore (1983), for instance, asked participants to judge their life satisfaction on either rainy or sunny days. Compared to rainy days, people experiencing sunny days tended to report greater life satisfaction and more positive moods. However, when the participants were first asked to elaborate on the weather, the difference in mood ratings remained but the difference in life satisfaction ratings disappeared (Schwarz & Clore, 1983). The authors interpreted these findings as suggesting that participants were more likely to misattribute the source of their mood to the object of judgement (life satisfaction)—and hence respond in a mood-congruent manner—when they were not made aware of the true source of their mood.
The affect-as-information hypothesis is primarily tailored towards explaining mood-congruent judgements in these instances of source misattribution, and as such it lacks specific memory predictions for MCM. The primary implication from this approach is that one’s awareness and appraisal of mood can influence the strength of mood congruence, in a similar vein as suggestions from Blaney (1986) and Singer and Salovey (1988) that network models need to account for both implicit and explicit regulatory mechanisms that can shape the course of mood development. Further, we also mention the affect-as-information approach here because this framework argues against the necessity of spreading activation from mood nodes in a semantic network to explain all mood congruent effects. In fact, according to this approach, affective experiences may not even exist as isolated nodes in declarative memory. Rather, people retain mood-relevant concepts, beliefs, and prepositions about affective experiences. In other words, people can produce affective reactions, but the reactions themselves are not distinctly represented (Wyer et al., 1999). This perspective aligns with constructionist views of affect, which suggest that the brain constructs a categorization for a sensory event based on past experiences, resulting in what individuals ultimately label as an emotion or mood (Feldman Barrett, 2017). In these proposals, emotions such as anger or sadness are not localized to dedicated neural systems, but rather emerge from an information flow process similar to visual or auditory perception. It is not yet clear whether this perspective is the correct depiction of the neuroscience of emotion, but if true, associative network theories of affect may inaccurately represent how the brain is perceiving and storing affective experiences since they assume the presence of dedicated nodes for discrete affective states, rather than conceptualizing affect as an emergent phenomenon.
While the above concerns relate to the appraisal of mood, the Dual-Force Model (Fiedler, 1991, 2001) further suggests that the type of task being performed may drive the presence or absence of any mood-related effects (Figure 1C). This model postulates that psychological functioning involves both the conservation of encoded stimulus representations (accommodation) and the active transformation of such input into existing knowledge structures (assimilation). Depending on the task at hand, accommodative and assimilative processes are differentially engaged. Tasks that require the active manipulation and generation of information are more sensitive to mood congruency effects because mood becomes an associative cue for affectively congruent content in memory. Accordingly, elaborative encoding tasks are more likely to produce MCM, as are free recall tasks because they place greater demand on reconstructing stored memory traces. Positive and negative moods may also differentially signal the need to implement these processing styles. Specifically, because positive moods tend to indicate that the present environment is familiar and safe, these moods may be more likely to facilitate top-down, assimilative processing that increases the scope of visuospatial attention to global features of a stimulus (Fredrickson & Branigan, 2005; Rowe et al., 2007). In contrast, negative moods often signify an uncertain situation, thereby promoting a bottom-up accommodative style that encourages careful, attentive, and item-specific stimulus processing (Fiedler, 2001; Storbeck & Clore, 2005). As a result, positive moods could be more susceptible to bias (i.e., mood congruence), specifically for tasks that require more assimilative processing, while negative moods might be less amenable to bias. As with the affect-as-information approach, though, evidence at the time for this theory was primarily based on the observed effects of mood on inferential tasks, while memory-related hypotheses were mostly speculative. For example, judging a person’s likelihood to engage in a particular type of behavior was shown to be primed by prior trait judgements only when participants were in a happy mood but not in a sad mood (Bless & Fiedler, 1995). Similarly, others have found that negative moods generally reduce judgmental biases by preventing stereotyping and gullibility (for further review, see Forgas, 2013). Whether these effects reliably translate into differential long-term memory biases is unclear.
The Affect Infusion Model (AIM; Forgas, 1995) also suggests that the type of information processing strategy someone uses will influence how mood impacts memory (Figure 1D). According to the AIM, four types of processing strategies determine the degree of affect infusion (i.e., mood bias) based on level of effort and constructive demand. These processing strategies include direct access (low-effort and non-constructive), motivated (effortful and non-constructive), heuristic (low-effort and constructive), and substantive processing (effortful and constructive). The AIM suggests that affect infusion is unlikely during the application of direct access or motivated processing strategies, as these strategies are either applied during highly familiar tasks in which elaborative thinking is not necessary (direct access) or tasks with a particular objective, such as mood control (motivational, goal-directed processing). Instead, affect infusion is likely to occur when tasks require constructive (i.e., elaborative) processing. Heuristic processing predominates when effort is low, as suggested by the affect-as-information approach. However, when constructive processing is effortful, we tend to rely more on the accessibility of thoughts and memories, by which affect infusion can then drive mood congruent biases. Again, evidence for this model was largely built on judgement-style tasks, whereby mood congruence was facilitated by having participants engage in more effortful and elaborative judgements, such as judgements of peripheral rather than central self-conceptions, atypical/unusual characters, and complex personal conflicts (for further review, see Forgas & Eich, 2012). Nonetheless, this proposal matches with observations in the mood-dependent memory and MCM literatures, where generative encoding and self-referential processing seem to boost mood-related effects (Blaney, 1986; Bower, 1981; Singer & Salovey, 1988).
In sum, the effects of mood on cognition—and memory in particular—are not universal. Rather, they are influenced by the way in which mood is appraised, the type of cognitive processing that is present, and potentially the type of mood that is felt (i.e., negative vs. positive). While an associative network perspective remains the foundational framework for research in this area, the perspectives outlined above have proved useful in defining contextual factors that seem to shape mood-related effects. Yet, despite continued growth of research on MCM within the past few decades, comparatively fewer theoretical adjustments have been proposed. We suspect that further refinement to models of MCM will ultimately require building a more comprehensive neurobiological understanding for how the brain is recruited during the MCM process and integrating these neuroscientific evaluations with existing behavioral evidence. To help achieve this goal, we believe an updated review on MCM is necessary, which we provide here.
The Clinical Relevance of MCM Research
Laboratory investigations of MCM have important implications for understanding processing biases in mood disorders such as depression. Before reviewing the current state of lab-based MCM research, we would be remiss to not properly acknowledge this clinical foundation, which further exemplifies the utility of studying MCM and its implications. Indeed, initial perspectives on MCM were largely derived from memory biases in depressed patients toward mood-congruent, negatively valenced material when compared to non-depressed controls (Blaney, 1986; Bower, 1981; Ingram, 1984; Singer & Salovey, 1988). Affective stimuli such as emotional words or stories were shown to be selectively remembered, such that depression facilitated an enhancement in memory for negative information and/or impairment for positive information (Breslow et al., 1981; Dunbar & Lishman, 1984; McDowall, 1984). Such biases were thought to contribute to the maintenance of depression by endorsing a triad of negative thoughts about the self, the world, and the future (Beck, 1967). Early clinical investigations also revealed that the self-relevance of encoded stimuli moderated the strength of MCM (Blaney, 1986), suggesting that mood congruence is strengthened by the overlap in personal significance between an internal mood and external source of information.
In more recent years, the presence of MCM in clinical depression has continued to receive empirical support, where depressed individuals demonstrate biased memory for negative past events and impaired recollection of positive experiences (Dalgleish & Werner-Seidler, 2014; Köhler et al., 2015). These negative memory biases associate with genetic susceptibilities to depression (Vrijsen et al., 2015; Woudstra et al., 2013) and prospectively predict increased depressive symptoms (Connolly & Alloy, 2018). MCM is also reflected in retrospective reports of daily affect, whereby depressed individuals overestimate the frequency of having experienced past negative emotions within the past day or the past week (Miron-Shatz et al., 2009; Urban et al., 2018). This memory-experience gap was recently shown to be disorder-specific. That is, when asked to recall affective experiences from the prior week, depressed individuals were most likely to overestimate having felt sad, and social phobics were most likely to overestimate having felt socially anxious; participants in a control group overestimated having felt happy (Rinner et al., 2019).
Observations of mood-congruent depressive biases are rather consistent for recalling autobiographical events (for further review, see Köhler et al., 2015), although the evaluation of MCM in depressed individuals for lab-learned stimuli has produced mixed findings. Yet, such investigations are surprisingly few in number, and evidence suggests that they are sensitive to the length of the encoding-recall delay (Bogie et al., 2019). That is, a more consistent MCM effect is observed in depressed versus control groups when memory is assessed at least a day later (Gotlib et al., 2004; Hamilton & Gotlib, 2008; Rinck & Becker, 2005), as opposed to shortly after encoding (e.g. Baños et al., 2001; Ellwart et al., 2003). This distinction between immediate and delayed effects underscores the notion that mood congruence is particularly influential in biasing the consolidation processes that store items into long-term memory. Similar effects are observed with emotional memory research, where biased memory for more arousing stimuli requires a time-dependent process involving the modulatory influence of amygdala activity on hippocampal storage (McGaugh, 1966, 2004). Indeed, the association between negative ruminative thinking and depressed mood likely facilitates MCM over time by consistently reactivating and strengthening mood-congruent thoughts (Spasojević & Alloy, 2001).
Although a majority of studies have focused on explicit, or conscious, recollection when testing MCM among depressed individuals, negative biases have also been demonstrated for implicit memories. Tests of implicit memory can be perceptually based, such as with word stem completion and lexical decision tasks, or conceptually based, such as with word generation or free association tasks. Meta-analytical evidence across such investigations indicates that clinical depression is reliably associated with a bias towards implicit recall of negative information, whereas nondepressed groups exhibit a bias towards implicit recall of positive information (Gaddy & Ingram, 2014). Importantly, this meta-analysis showed that negative implicit biases in depression are enhanced when the stimuli used are self-relevant, as well as when the nature of the task (perceptual vs. conceptual) is matched at encoding and retrieval, suggesting that similarity in processing demands at encoding and retrieval helps to facilitate MCM. Thus, in addition to explicit biases, depression is also associated with an automatic, implicit bias for negative content.
The clinical examination of MCM has shown that depressive mood contributes to long-term biases in memory for negative content, and hence the field of MCM research remains tightly bound to such clinical perspectives. But in line with the view of Blaney (1986), we acknowledge that studying MCM and detailing the underlying neurocognitive mechanisms within a clinical setting can be confounded by other factors influencing memory in clinically depressed patients, such as general cognitive impairments (McIntyre et al., 2013) or overgeneralized memories (Sumner, 2012). The diagnosis of major depressive disorder is also not solely dependent on the presence of depressed mood and may instead arise from a general loss of interest or pleasure (American Psychiatric Association, 2013). Further, affective memory biases are not unique to depression, but rather constitute a transdiagnostic cognitive marker across many mental disorders (Duyser et al., 2020). Collectively, these issues can interfere with experimentally isolating the influence of mood on memory, as opposed to a host of other cognitive factors that might have modulatory effects. Evaluating MCM in non-clinical samples with experimentally controlled affect can provide important insight to the factors that enhance or impair the strength of MCM, which may be able to advance treatment methods. By detailing the basic mechanisms of MCM, researchers will be able to more effectively pinpoint the neurocognitive processes that underlie how mood selectively targets emotional memories. Therefore, we have focused our subsequent systematic review on laboratory induction studies in healthy participants.
Revisiting Mood-Congruent Memory
We devote the remainder of this review to examining the present status of MCM research. To our knowledge, the qualitative assessments by Blaney (1986) and Singer and Salovey (1988), as well as the meta-analysis by Matt et al. (1992), remain the seminal peer-reviewed summaries on MCM via mood induction in healthy controls. Since these reviews were published, numerous studies have further examined MCM and provided a more nuanced perspective on the interaction of mood and emotional memory. Here we qualitatively review these investigations, focusing specifically on studies that used mood inductions to manipulate state-based affect during the encoding or retrieval of novel emotional stimuli or recall of autobiographical events.
To do so, we searched the PubMed, PsycINFO, and Web of Science databases for academic journal articles in English that were published since 1985 and not already referenced in Blaney (1986) or Singer and Salovey (1988) with the following search criteria: mood AND memory AND *congruen*. This search identified 333 articles from PubMed, 279 articles from PsycINFO, and 438 articles from Web of Science. After removing duplicate findings across the three databases, a total of 702 unique articles remained. The following eligibility criteria was applied to further screen for relevant MCM studies:
The study induced and measured a distinct psychological mood state in healthy, adult human subjects, excluding assessments focused on trait-based characteristics, natural mood reports, clinical disorders, or children. Further, we excluded studies with acute stress or sleep manipulations, as these produce specific physiological changes in cortisol levels compared to other types of mood inductions.
The study tested memory via autobiographical recall or designated encoding and retrieval tasks with valenced stimuli. We excluded studies that did not directly test emotional memory biases (e.g., mood-congruent attention, mood-congruent judgment, mood-congruent decision-making, mood regulation, or mood effects on memory for non-emotional stimuli).
The study administered a mood induction only once or, if multiple times, the mood induction consistently preceded either encoding or retrieval, thereby excluding studies better suited to evaluate mood-dependent memory. Note, however, that we did include one mood-dependent study that administered an autobiographical recall test after the first mood induction, thus allowing for examination of MCM (Eich et al., 1994).
In total, 65 articles were identified that matched the above criteria. Upon further review, we also found 13 additional articles referenced in these studies and other reviews that were not initially identified in our search. Together, a total of 48 articles evaluated MCM in the context of lab-based encoding and retrieval tasks, whereas 34 articles assessed MCM via autobiographical recall (note that 4 articles included experiments with both types of evaluations). A general overview of each study is presented in Supplementary Table 1 (encoding-retrieval tasks) and Supplementary Table 2 (autobiographical recall), detailing the induction method, stimulus materials, and MCM findings for all experiments.
Summary of Study Methods
Mood inductions varied considerably across both types of investigations obtained via our systematic search (Table 1). Studies used self-referential statements, memory recall, suggestive instruction, mental imagery, news articles/narratives, drug treatment, odors, feedback on a task, serially-presented images, music, film clips, natural/virtual environments, or combined techniques. The most common induction methods were film clips, music, or combined techniques (e.g., autobiographical recall and music). Mood inductions were, on average, biased towards inducing negative mood. That is, while 30 articles in the encoding-retrieval set and 19 studies in the autobiographical recall set reported inducing both positive and negative moods in their experiments, the other studies either only induced a negative mood, included a neutral induction in lieu of a positive one, or administered emotion regulation tasks after the negative induction. This is with the exception of one encoding-retrieval study that only administered a positive and neutral induction (Nielson & Lorber, 2009), and one autobiographical recall study that only administered a positive induction at retrieval (Piñeyro et al., 2018). If discrete mood inductions were mentioned, they were overwhelmingly labeled as happy/elated and sad/depressed, the exceptions being fear (Bland et al., 2016; Hansen & Shantz, 1995; Tesoriero & Rickard, 2012), anger (Bland et al., 2016; Hansen & Shantz, 1995), aggression (Gupta & Khosla, 2006), and calmness (Tesoriero & Rickard, 2012). Several studies induced mood with the use of comedic film clips, but changes in mood were still measured as generally happy/positive rather than amused (Kiefer et al., 2007; Liu et al., 2008; Nielson & Lorber, 2009; Wisco & Nolen-Hoeksema, 2009).
Table 1.
Mood induction methods used in mood-congruent memory studies
For encoding-retrieval assessments, we also observed some variability in the type of emotional stimuli that were used (Table 2). Most encoding tasks presented words or word lists, but others used sentences/narratives, images, facial expressions, memories, headlines, videos, social interactions, musical scales, and even foods.
Table 2.
Emotional stimuli used in mood-congruent memory studies (encoding-retrieval assessments)
Summary of Study Findings
Here we provide a brief summary of our general observations regarding memory effects. Subsequent sections will review more specific themes of research that we identified, such as the specificity of MCM to induced mood, the presence of valence asymmetry, mood-congruent false memory, mood-incongruent memory, and methodological considerations for future research. However, we initially aim to simply evaluate (i) the general consistency of MCM effects across studies and (ii) observed patterns in the literature that facilitate or hinder MCM.
Encoding-retrieval assessments.
Among the studies assessing memory for novel stimuli encoded in the lab (Table S1), we observed reports of MCM for at least one mood in 41 out of 66 total experiments (34 of the 48 articles), and also one instance of only mood-incongruent memory (Kaspar et al., 2015). When reported, recognition tests of memory accuracy showed that both positive and negative moods could enhance discriminability (d’) for congruent, compared to incongruent, information in the range of 0.85 – 1.17 (Hills et al., 2011; Houston & Haddock, 2007; Pliner & Steverango, 1994). Other experiments administered free recall tests, which suggested that mood can significantly boost percent accurate recall in the range of 5% – 30% for congruent, compared to incongruent or neutral, stimuli (e.g, Fitzgerald et al., 2011; Itoh, 2004; Knight et al., 2002; Nasby, 1996; Rinck et al., 1992; Rusting & DeHart, 2000). We note that in some instances, MCM effects were primarily driven by impairment for mood-incongruent content (e.g., Direnfeld & Roberts, 2006; Hills et al., 2011; Klaassen et al., 2002). For example, Hills et al. (2011) observed that happy mood induced before encoding reduced subsequent recognition accuracy for sad or neutral facial expressions compared to sad mood but did not enhance accuracy for happy expressions. Finally, we found a number of studies that reported enhanced false memories for mood-congruent material (Bland et al., 2016; Hansen & Shantz, 1995; Knott & Thorley, 2014; Ruci et al., 2009; Zhang et al., 2017, 2019), a relatively new development in the field that we will explore in more detail below (see Mood-Congruent False Memory).
Several experiments examined possible moderators of MCM. Supporting the conclusions from previous reviews (Blaney, 1986; Matt et al., 1992; Singer & Salovey, 1988), self-referent encoding was again found to enhance MCM. For example, Nasby (1996) administered elated, depressed, or neutral mood inductions prior to having participants read positive and negative adjectives. For each adjective, the participant was tasked with rating (yes/no) whether the adjective describes themselves, their best friend, or the experimenter. Memory for the adjectives was then tested with a free recall test after a short filler task. Compared to the neutral mood group, participants in the elated mood group better recalled positive adjectives they had rated as describing the self and their best friend, but not the experimenter. Alternatively, participants in the depressed mood group better recalled negative adjectives that they had rated as describing the self, but not their best friend or the experiment (Nasby, 1996). These findings replicated a previous assessment using identical procedures, except this study had compared self-referent with mother-referent judgements instead (Nasby, 1994). Note that in both studies, the effects were driven by differential judgements at encoding—elated subjects were more likely to endorse self-referent positive traits and depressed subjects were more likely to endorse self-referent negative traits, suggesting that selective elaboration on mood-congruent items fueled subsequent MCM. Notably, Itoh (2004) also produced this same pattern of results in a subsequent replication attempt, again supporting a moderating role for self-referential processing.
State and trait-level affective measures have also been found to moderate MCM. Rusting (1999) instructed participants to rate the valence of positive, negative, and neutral words after the induction of positive or negative mood with music. A free recall test was administered immediately after encoding. Higher ratings of positive/negative mood (post-induction) predicted improved recall for positive/negative words, and these effects were strengthened by average trait-level reports of positive and negative affectivity across multiple sessions. For example, individuals who reported higher levels of trait negative affect exhibited a stronger influence of state negative mood on subsequent recall performance. Additionally, another trait-level moderator found among the literature is self-esteem. Both Smith & Petty (1995) and Pereg & Mikulincer (2004) showed that only individuals measuring low in self-esteem exhibited a relationship between more negative mood (post-induction) and more negative recall. Thus, increased trait negative affect seems to boost the influence of mood on memory. We also observed a few instances where individual differences in the degree of mood change predicted stronger MCM effects (Pereg & Mikulincer, 2004; Rusting, 1999).
Surprisingly, 24 experiments (across 15 different articles) reported no interaction between mood and emotional memory. While still in the minority, repeated instances of null findings are nonetheless concerning. However, upon closer examination we did notice methodological patterns across several experiments that may have impeded the presence of MCM. Notably, most of these experiments involved either passive viewing or rating the valence of stimuli during encoding, while only two experiments specifically encouraged self-referential encoding (Hartig et al., 1999). Yet, these two experiments from Hartig et al. (1999) attempted to induce mood with environmental manipulations (i.e., placing participants in urban or natural settings) that produced only minor (Exp. 1) or no differences (Exp. 3) in reported affect. In a similar vein, Zhong et al. (2020) used virtual reality to construct pleasant or unpleasant (grisly) environments, which elicited slight shifts in valence ratings compared to neutral environments, but this induction method was also unable to produce subsequent MCM. The experiments from Liu (2008), Nielson and Lorber (2009), and Wang and Ren (2017) additionally failed to show MCM. These studies administered post-encoding inductions to evaluate the effects of mood during early memory consolidation, an exciting but relatively underdeveloped area in the field of MCM research. Unfortunately, although the inductions produced differences in mood ratings between groups, these experiments were designed to only display brief comedic or disturbing video clips intended as arousal manipulations. Further, Nielson and Lorber (2009) showed that even though affective ratings changed post-encoding/induction, they had returned to baseline by the end of the session. Hence, these manipulations were perhaps not strong enough to facilitate post-encoding biases, nor were they specifically matched to the encoded stimulus set (i.e., the stimuli were not normed to be amusing to match with the comedic clips). A similar concern is evident with the two experiments from Zhu et al. (2015), who attempted to induce mood by having participants read brief news articles that represented intergroup threats, which entailed having participants with low socioeconomic status reading condescending quotes from high socioeconomic individuals. It is not mentioned how long participants spent on these inductions, but both experiments in the study were reported as taking only ten minutes to fully complete. Accordingly, it is unclear whether affective ratings were indicative of sustained mood change, or rather just emotional responses to the news stories.
In sum, we observed that most encoding-retrieval assessments reported MCM in the form of recall or recognition enhancement, although a few also indicated the presence of mood-congruent false memories. Several experiments assessed possible moderators of MCM and reported significant interactions with self-reference, trait affect, and self-esteem. We observed that most studies with null findings neglected to encourage self-referential processing and/or implemented relatively weak, non-specific affect inductions that may have impeded the presence of MCM. These concerns were similarly noted by previous reviews (Blaney, 1986; Matt et al., 1992; Singer & Salovey, 1988). A novel concern that we wish to particularly emphasize here is the lack of MCM studies with encoding-retrieval tasks separated by at least one day. We suspect that in many instances, MCM effects have not yet manifested within the brief (several minutes) delay between most encoding and retrieval tasks that were reviewed (Table S1). This concern is especially warranted, provided that emotional memory enhancements are known to be facilitated by consolidation mechanisms that require longer delays (Faul & LaBar, 2020; LaBar & Cabeza, 2006). We will further discuss this point in Methodological Considerations for MCM Research.
Autobiographical recall.
In autobiographical assessments, MCM was assessed via the free or cued recall of autobiographical events and commonly measured by the amount, recall latency, or emotional characteristics of valence-specific memories (Table S2). We found that most studies either reported mood-congruent recall bias (in 20 of 54 experiments; 17 of 34 articles) or both mood-congruent and mood-incongruent effects due to testing moderators (in 17 experiments; 10 articles). An additional 8 experiments (4 articles) only reported mood-incongruent memory.
Regarding specific examples of MCM effects, in one study sad mood participants were shown to be more than 3.5 times more likely than neutral mood participants to recall a negative autobiographical memory (Knight et al., 2002). Others observed increases in proportion rates for recalling positive/negative memories in a congruent versus incongruent mood in the range of .07 – 0.156 (e.g., Berntsen, 2002; Bullington, 1990; Ehrlichman & Halpern, 1988; Sakaki, 2007). Several studies have also indicated that mood shifts the valence of recalled memories in a mood-congruent direction (Drače, 2013; Drače et al., 2015; Drače & Desrichard, 2013), although such effects may not only influence memories for mood-congruent cues. Miranda and Kihlstrom (2005), for example, showed that memories recalled for positive, negative, or neutral cues were all rated as more or less pleasant depending on the induced mood state, indicating a generalized mood-congruent shift in phenomenological experience of memory recall. Further, mood-congruent effects on memory were shown to be strengthened by increasing both the intensity and self-relevance of the mood induction (Drače, 2013; Drače et al., 2015). However, most of the observed moderators of autobiographical MCM were individual differences in affect-related trait characteristics such as self-esteem or emotion regulatory abilities, with higher scores on these measures shifting recall bias from mood-congruent to mood-incongruent (e.g., Josephson et al., 1996; Rusting & DeHart, 2000; Smith & Petty, 1995). We further discuss this area of research below in Mood-Incongruent Memory and Mood Repair.
We found 9 experiments (across 6 articles) reported neither MCM nor mood-incongruent memory effects. Again, we observed several methodological factors that might help to explain the lack of effects, namely regarding induction techniques. For example, Eich et al. (1994; Exp. 1) observed no influence of mood on the number of autobiographical memories generated in response to cue words. In this experiment, however, the mood induction required the participant to self-generate and maintain a mood state while listening to pleasant or unpleasant music. The autobiographical assessment was completed at the end of the session after a separate word rating task and personality assessment, although participants were not allowed to proceed to the next task in the study unless they reached a specific rating threshold following additional rounds of self-generated mood change. Despite implementing these booster inductions, it is unclear whether participants were simply responding as the experimenter wished in order to proceed with the study. Since the autobiographical assessment occurred at the very end of the session, the effects of the initial induction were likely minimal by this time. Indeed, mood-congruent effects were observed when the task was changed so that event generation occurred first after the mood induction, with subjects in the pleasant mood condition recalling more positive and fewer negative events than those in the unpleasant mood condition (Eich et al., 1994; Exps. 2 & 3)
Hartig et al. (1999) also observed null effects, but the authors used the same natural or urban context manipulations as with their encoding-retrieval studies, where they observed that the manipulations produced negligible changes in mood. To evaluate the influence of rumination, Wisco and Nolen-Hoeksema (2009) added a rumination induction after a positive or negative mood induction, followed by an autobiographical recall task with positive, negative, or neutral cue words. Mood had no influence on the emotional ratings of memories, and regardless of cue, highly dysphoric (trait depressive) individuals recalled more negative memories. Importantly, though, while affect ratings changed in the expected direction immediately following the mood induction, they returned to baseline after the rumination induction and prior to the memory task. Thus, mood effects were no longer present during the recall task, which could explain the lack of MCM results. Greenberg & Meiran (2014a) assessed whether meditators and non-mediators differentially recalled mood-incongruent autobiographical memories but also did not observe a main effect of mood in their analyses. However, mood was manipulated within-subject during only a single session, and inductions were presented in a fixed order (sad mood first). Thus, there might have been order effects that obscured MCM. Moreover, the authors did not collect baseline measures of mood in all participants, and thus were unable to properly determine the extent of mood change following induction (Greenberg & Meiran, 2014a). Finally, Simpson & Sheldon (2020) were unable to reliably produce mood influences on recall, finding only in their first experiment that memories were generally rated as more positive in the happy, versus sad, mood condition. Instead, the authors observed that arousal and valence characteristics of the memory cues were more reliable predictors than induced mood for the reported tone and details of autobiographical memories recalled (Simpson & Sheldon, 2020). This is an important point, as researchers should be careful to norm cue words in recall tasks to the same extent as they norm stimulus materials in encoding-retrieval tasks. Specifically, high arousal cues increased the number of internal details for descriptions of past events, while the valence of cues often matched with the emotional tone of the recalled memories. But regarding the absence of mood-related influences, here we note that the authors also used a within-subjects design with mood inductions separated by only a brief, 10-minute break. Although order was counterbalanced, whichever induction came first could have impaired the efficacy of the subsequent induction. Moreover, in none of the three experiments was negative affect scores shown to significantly increase from pre to post induction for the sad mood group. Thus, the absence of mood-related memory effects may also reflect methodological factors.
While a general overview of autobiographical MCM again provides predominant support for MCM, the methodology and empirical focus across both the encoding-retrieval and autobiographical studies was diverse. To facilitate further review of the literature, we will now specifically elaborate on four themes of observations that group the implications of these studies together and expand an understanding for MCM: the validity and specificity of MCM, the impact of mood congruence on false memory, factors that shift mood-congruent to mood-incongruent recall, and methodological/theoretical considerations for future research based on our review of the literature.
On the Validity and Specificity of MCM
Subject compliance.
Early reviews of MCM noted a particular concern for demand characteristics and subject compliance when testing mood congruence (Blaney, 1986; Singer & Salovey, 1988). Likewise, we caution against suggestive induction techniques that instruct participants to self-generate and maintain the instructed mood state (Fiedler et al., 2003; Rinck et al., 1992), as participants may produce mood-related effects simply to accommodate experimenter demands. Most of the investigations that we reviewed, however, report clear statistical evaluations of mood change and we observed a general shift in the literature away from the use of suggestive techniques that may be particularly prone to demand characteristics. Most notably, the MCM literature now contains more studies using film clips, music, and/or autobiographical recall as induction methods, which require less instruction from the experimenter. Still, mood induced prior to encoding or recall may facilitate MCM on subsequent tasks simply because participants are motivated to try to maintain the mood, rather than a true memory effect. Only a few studies have directly attempted to address this concern. Parrott (1991) administered a musical mood induction to induce happy, sad, or neutral moods, followed by explicit instruction for participants to stop maintaining the mood. A subsequent autobiographical recall test still demonstrated MCM, suggesting that subject compliance is not a necessary condition for memory bias (Parrott, 1991). Other evidence also suggests that simulated moods (instructing participants to recall emotional memories as if they were in a particular mood) produce qualitatively distinct MCM compared to true inductions (Bullington, 1990; Eich & Macaulay, 2000). Although these studies provide some support that subject compliance may not fully account for past MCM findings, researchers should continue to utilize induction methods that best guard against such concerns by limiting experimenter demands.
Valence Asymmetry.
Another complicated feature of MCM is the presence of valence asymmetry. Both Blaney (1986) and Singer and Salovey (1988) observed that negative mood does not always promote enhancement for negative memories, whereas positive moods seemed to be more reliable in enhancing memory for positive content. However, Matt et al. (1992) showed that among experimental recall paradigms, negative shifts in mood-congruent recall were reliably demonstrated among induced negative moods in healthy participants (Matt et al., 1992). But mood-congruent effects were found to be stronger among naturally depressed groups, and Matt et al. (1992) noted a baseline positivity bias among non-induced, healthy controls that might influence the extent of negative MCM. For example, both trait dysphoria and experimentally induced negative mood have been shown to impair incidental memory for positive self-descriptive adjectives, but only trait dysphoria enhanced incidental memory for negative adjectives compared to a control group (Direnfeld & Roberts, 2006).
To investigate this matter further, we evaluated the extent to which reports of MCM have continued to demonstrate asymmetry. Among encoding-retrieval studies that utilized both positive and negative inductions and reported significant effects, we surprisingly observed a rather symmetrical pattern in MCM. Eleven of 19 papers reported mood-congruent enhancements in their dependent variable for both positive and negative moods (Fiedler et al., 2001; Houston & Haddock, 2007; Itoh, 2004; Nasby, 1994, 1996; Pliner & Steverango, 1994; Rinck et al., 1992; Ruci et al., 2009; Rusting, 1999; Tesoriero & Rickard, 2012; Zhang et al., 2019). Note, though, that in several instances mood-congruent effects were nevertheless shown to be stronger for positive moods (e.g., Fiedler, 2001; Tesoriero & Rickard, 2012). For those that only observed MCM for one mood and not the other, four reported only positive MCM (Fiedler et al., 2003; Fitzgerald et al., 2011; Hills et al., 2011; Kiefer et al., 2007), while four reported only negative MCM (Forgas, 1998; Lewis et al., 2005; Ridout et al., 2009; Zhang et al., 2017). Ridout et al. (2009), for instance, observed that negative mood enhanced the identification of sad faces and subsequent recognition memory of those faces, while no bias was observed for positive mood (Figure 1A). Hence, we did not observe systematic evidence for asymmetry in our qualitative review.
Moreover, in many instances it seems difficult to properly conclude whether reports of asymmetrical effects are representative of true asymmetry, or rather methodological factors. Differential MCM between positive and negative moods may result from the efficacy of the induced mood, which may not be symmetrical between positive and negative moods (e.g., Joseph et al., 2020; Kiefer et al., 2007; Lewis et al., 2005; Westermann et al., 1996). Asymmetrical effects may also reflect the structure of the task, rather than asymmetry in memory per se, given that positive and negative moods are suggested to facilitate unique assimilative or accommodative processing during initial encoding (Fiedler, 2001; Fiedler et al., 2003; Forgas & Eich, 2012). Incidental tasks where participants are unaware of a subsequent memory test have been shown to increase the presence of MCM (Direnfeld & Roberts, 2006; Hills et al., 2011), while intentional encoding paradigms where participants are required to actively study the material for an expected memory test may be more likely to produce just positive MCM.
Arousal, valence, and discrete moods.
A defining aspect of MCM involves the overlap in affective quality between a mood state and emotion, which is usually interpreted in terms of the valence of these elements – for instance, a match in positivity or negativity. Yet, mood inductions can also modulate arousal levels, which in turn may facilitate the encoding of emotionally arousing items. Improved memory for mood-congruent items could therefore involve a matching in arousal levels in addition to an overlap in valence (Gayle, 1997). The implications of this possibility warrant further discussion. First, even if the efficacy of induced mood is equated across negative and positive inductions, they may still differ in arousal levels, which could contribute to asymmetrical MCM, given the critical role of arousal in other emotional memory bias effects. Second, the extent to which arousal operates independently from valence is unclear. Can a sufficiently strong arousal induction facilitate improved memory for arousing emotional items independent of valence? Third, if only a combination of valence and arousal levels produce MCM, to what extent is MCM category specific when both valence and arousal are generally equated across moods (e.g., anger and fear)?
Among the first to address this issue, Varner & Ellis (1998) compared the effects of several different types of pre-encoding inductions on subsequent memory for words, including a negative mood induction via self-referential statements, a schema induction via statements about how to write and organize a paper, and an arousal induction via exercise. Their findings highlighted a specificity in memory bias for negative words that were encoded in a depressed mood, whereas the schema induction promoted better memory for organizational-type words. Importantly, the arousal induction did not promote an explicit bias for either sets of words. Thus, only congruency between internal mental state and external stimulus was predictive of subsequent memory performance. With a similar approach, Tesoriero & Rickard (2012) assessed subsequent recall of positive and negative narratives that were encoded in either a happy, fearful, calm, or sad mood induced with musical excerpts. An arousal-based account would suggest that more arousing affective states such as fear and happiness promote improved recall of the emotional narratives compared to less arousing states such as calmness and sadness, whereas a mood congruence account predicts enhanced memory when moods and emotions match based on valence. The results from this study only supported a mood congruence account whereby positive mood (happy and calm) improved recall of positively-valenced narratives and negative mood (fearful and sad) improved recall of negatively-valenced narratives (among individuals scoring high on trait mood characteristics), suggesting that arousal-based accounts were not sufficient in describing the observed effects.
There is an important caveat to consider, however, when evaluating the findings of Varner & Ellis (1998) and Tesoriero & Rickard (2012). In both of these studies, memory was tested immediately or soon after encoding and thus neglected to provide a sufficient delay for long-term emotional memory consolidation, which is considered the primary stage of memory influenced by arousal (McGaugh, 2004). By creating an internal affective context that facilitates encoding focus on congruent emotional stimuli, mood-congruence might be more sensitive to immediate effects on memory, whereas arousal operates in a more time-dependent manner on long-term memory storage. Whether the mood-congruent effects observed in these studies actually transferred to memory bias hours or even days later remains unclear since memory was only tested shortly after encoding. Nonetheless, these studies demonstrated that it is possible for mood congruence to act on emotional stimuli in a way that cannot be explained only by changes in arousal, at least in the short term.
In a more recent evaluation of MCM as an effect specific to the type of mood induced, Bland et al. (2016) examined two negative mood states similar on dimensional ratings of arousal and valence: fear and anger. In their study, Bland and colleagues studied the influence of a pre-encoding mood induction (film clips) on subsequent memory for word lists, with semantically related lures present during the memory test. Fearful and angry moods resulted in a greater likelihood of falsely recalling fearful and angry lures, respectively, demonstrating MCM even after experimentally controlling for valence and arousal among the induced moods (Figure 2B). Note that here MCM reflected increased false memory. A similar MCM specificity in false memory has also been observed for emotional video clips (Hansen & Shantz, 1995), where greater memory intrusions on a recognition test for anger clips occurred if an angry mood had been induced and for sad clips if a sad mood had been induced prior to encoding. This specificity in memory matched with similar emotion-specific mood-congruent judgements that were made during initial viewing of the stimuli (Hansen & Shantz, 1995). Collectively, these findings suggest the need to consider specific moods within an associative network, indicating that the strength of MCM might be influenced by the degree of congruence between moods and emotions. A significant number of MCM studies, however, only evaluate MCM in the context of generally positive or negative mood inductions and/or emotional stimuli (Table S1). Moreover, both Bland et al. (2016) and Hansen & Shantz (1995) only examined the occurrence of memory intrusions, which is rather atypical in the MCM literature (although still supported by network theory, as discussed in the next section). Future research should continue to evaluate whether the presence and strength of MCM is dependent upon a congruence in categorical affect.
Figure 2.
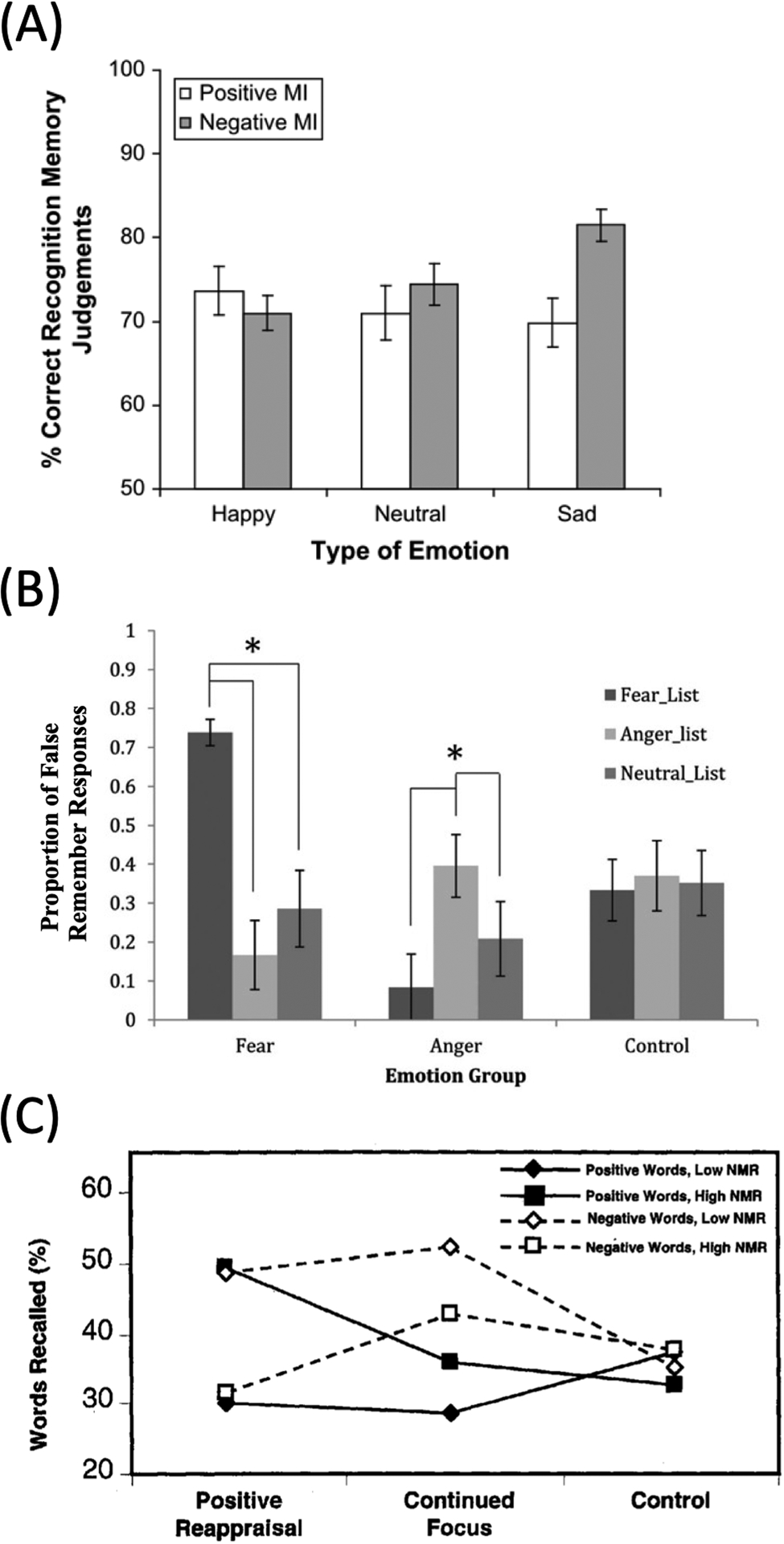
Mood Effects on Memory
Note. Three different ways in which mood congruence can impact memory. (A) Example of mood-congruent memory enhancement, reproduced from Ridout et al. (2009) with permission. Mood was induced before encoding. Participants in the negative mood group exhibited improved recognition of sad faces, while no bias was observed for the positive mood group. Negative mood also improved identification of sad faces at encoding (not shown). (B) Example of mood-congruent false memory, reproduced from Bland et al. (2016) with permission. Mood was induced before encoding. The fearful mood group exhibited a bias for falsely recalling critical lures from the fear word lists, while the anger mood group exhibited a bias for falsely recalling critical lures from the anger word lists, and the control group – who experienced no mood induction – showed no bias. (C) Example of both mood-congruent and mood-incongruent memory enhancement, reproduced from Rusting and DeHart (2000) with permission. Participants completed a sentence generation task, followed by a negative mood induction via autobiographical recall. After the induction, participants were instructed to either reappraise the content of the memories that produced their negative mood, continue focusing on the negative memories, or list whatever thoughts were going through their mind (control condition). Free recall performance showed that the positive reappraisal group exhibited mood-incongruent memory only for individuals high in negative mood regulation expectancies (NMR), such that these individuals recalled a greater percentage of positive than negative words from the sentence generation task. Individuals low in NMR, however, exhibited the opposite effect. In the continued focus group, both high and low NMR participants showed mood-congruent recall – as indicated by greater recall of negative words – while the control group showed no biases.
In sum, here we discussed the specificity and validity of MCM with regard to the induced mood state, including whether MCM reflects subject compliance, the symmetry among positive and negative moods, and whether discrete matches in mood and emotion can produce MCM. We found that several studies have attempted to address concerns over subject compliance, suggesting that compliance alone is not a sufficient driver of MCM, but also that the use of more rigorously controlled and validated induction techniques has placed greater confidence in reported MCM effects. Regarding asymmetry, we did not observe that positive moods were systematically more likely to produce MCM than negative moods, although evidence does suggest that assimilative (generative) tasks might be more amenable to positive MCM (Fiedler, 2001; Fiedler et al., 2001). Finally, several studies have shown that MCM is more specific to matches in valence than arousal, and MCM can arise from discretely matched moods and emotions, but research in this area is limited and requires further investigation.
Mood-Congruent False Memory
The findings of Bland et al. (2016) and Hansen & Shantz (1995) illustrate that MCM may sometimes manifest as false memory for mood-congruent content. This effect warrants close attention, given that researchers typically measure MCM in terms of improved memory accuracy (when tested as such in encoding-retrieval paradigms). Yet, a number of studies have consistently demonstrated the presence of mood-congruent false memory specifically in the Deese-Roediger-McDermott (DRM) paradigm (Deese, 1959; Roediger & McDermott, 1995), whereby participants falsely recognize semantically-related, mood-congruent lures when tested on a series of previously encoded word lists (Bland et al., 2016; Knott & Thorley, 2014; Ruci et al., 2009; Zhang et al., 2017, 2019). In these experiments, mood induction occurs prior to encoding, followed by listening to or reading several lists of closely related words (e.g., horror, scream, monster, panic, fright, etc.). At retrieval, participants complete old/new recognition tests containing some of the old words presented, new filler words unrelated to the word lists, and new critical lures that were never presented but semantically linked with the words lists from encoding (e.g., fear). Note that these experiments are explicitly designed to produce false memories, as high rates of false recognition are prevalent even without mood inductions (Roediger et al., 2001). But performance on the DRM task does associate with other types of memory intrusions (Qin et al., 2008; Unsworth & Brewer, 2010), suggesting that false MCM on the DRM task could indicate a general influence of moods on increasing the likelihood of misremembering affectively congruent, but not actually experienced, episodic events. Indeed, mood-congruent false memories are often reported by participants as remember judgements (mentally re-experiencing the original presentation of a word, as opposed to knowing the word was presented but not remembering its occurrence), demonstrating that mood has a powerful influence on promoting a confident, yet false, MCM (Knott & Thorley, 2014; Ruci et al., 2009).
Is an associative network account able to explain these instances of false memory? While not explicitly recognized by Bower (1981), upon closer examination his network perspective does support the presence of false memories. Because moods facilitate the activation of closely related concept nodes containing semantically and affectively linked information (Bower, 1981; Roediger et al., 2004), spreading activation from a mood node can increase the likelihood of falsely recalling mood-congruent items even if they were not explicitly encoded, an explanation commonly mentioned in these MCM-DRM studies. Moreover, it is well documented that emotion (without the presence of mood) can both enhance or impair memory based on what is selectively prioritized during consolidation (Faul & LaBar, 2020), that emotional memories can instill high confidence despite inaccuracy (Rimmele et al., 2011), and that increased false memory for emotional content might occur because of emotion-based grouping that confuses closely related emotional stimuli (Choi et al., 2013; Gallo et al., 2009). In the appropriate context, such as the DRM paradigm, mood might amplify these effects for congruent material.
Another competing perspective is also relevant to this discussion. The Fuzzy-Trace Theory (FTT) proposes that two types of stimulus representations are configured during encoding: a verbatim representation of item-specific features that encodes precise information of the stimulus, such as word length and list position, and a gist-based representation that encodes general semantic meaning (Brainerd & Reyna, 2002). While both representations can be present during retrieval, one might have more influence than the other. The DRM paradigm, for instance, generates particularly strong gist-based representations due to the grouping of words into semantically related word lists. So even if a word wasn’t seen during encoding, its general meaning might seem familiar at retrieval and produce a false memory. Extrapolating to MCM, mood further strengthens gist-based memories by providing an additional gist-level representation of affective meaning, thereby increasing false recognition of mood-congruent lures. Moreover, the competition between verbatim and gist-based representations may depend on the type of mood experienced, provided that positive moods tend to promote assimilative (relational/gist) processing and negative moods tend to promote accommodative (item-specific/verbatim) processing. Therefore, if false memories result from competing gist and verbatim representations, as suggested by FTT, then positive moods should produce greater instances of mood-congruent false memories than sad moods. Indeed, sad mood induced prior to encoding has been shown to subsequently decrease the occurrence of false memories compared to the induction of a happy mood or a control group with no induction (Storbeck & Clore, 2005, 2011). However, this effect was not specific to mood-congruent items. Moreover, in these studies happy mood was not shown to increase false memories compared to a control group, as would be expected. Further, false MCM on the DRM paradigm has been reliably demonstrated for both positive and negative moods, suggesting rather symmetrical effects (Bland et al., 2016; Ruci et al., 2009; Zhang et al., 2019). Thus, more research is needed in this area to test whether positive and negative mood states do indeed differentially enhance or impair false MCM.
While both theories remain plausible explanations for mood-congruent false memory, there is some evidence providing preferential support for the associative account. If a network of related affective elements is indeed activated during encoding, then a more “activated” list at retrieval (indicated by higher levels of true recognition for previously seen words) should associate with greater false recognition rates of semantically related lures. Accordingly, true recognition rates of positive, negative, and neutral word lists have been shown to correlate with corresponding false recognition rates across various mood conditions (Zhang et al., 2017). Moreover, mood-congruent false memory has been shown to consist of remember (highly confident) responses as opposed to the know responses that would be expected from FTT’s gist-based account (Ruci et al., 2009). This effect persists after a one-week delay (Knott & Thorley, 2014) which, speculatively, may result from consolidation processes that were selective for mood-associated concepts activated during encoding. The findings of discrete mood-emotion matches in MCM for fearful, angry, and sad moods further suggests an underlying associative network that is sensitive to the specificity of induced moods, rather than more generalized negative affect (Bland et al., 2016; Hansen & Shantz, 1995).
Finally, if gist-based representations, instead of an associative network, promote mood-congruent false memory and interact with the different processing styles of negative and positive moods, then the presentation of retrieval warnings that explicitly warn participants of the possibility for false memories on the DRM task should be successful in facilitating access to verbatim representations, thus reducing false memories especially for negative moods. However, this hypothesis was not supported in a recent study by Zhang et. al (2019), as both positive and negative moods showed higher levels of false memories compared to a neutral mood in the presence of such retrieval warnings. In other words, both positive and negative MCM were still observed even though the overall rate of false recognition was lowered. The authors interpreted these results as suggesting that mood primarily impedes source monitoring for mood-congruent lures via an activated associative network of closely affiliated content (Zhang et al., 2019).
When considering the influence of mood on false recognition rates it is important to re-emphasize that the DRM paradigm is specifically designed to facilitate false memories. In standard recognition tests without critical lures, MCM has been shown to increase general sensitivity to mood-congruent items while still maintaining a genuine memory advantage. That is, mood-congruent hits on memory recognition tests are not necessarily accompanied by an equal rate of mood-congruent false alarms, suggesting that moods do more than simply promote a heuristic response bias to both new and old mood-congruent items (Fiedler et al., 2001; but see Stea et al., 2013). Regardless, while moods may enhance memory for previously encoded, mood-congruent information, they can also in certain instances facilitate false recollection for affectively linked, but not actually experienced content.
Further examining the factors that drive this trade-off between memory enhancement and impairment will be important for future research to address, as it may have important clinical applications. For example, these instances of false memory are reminiscent of the tendency for depressed individuals to overestimate the number of past negative events experienced in their lives (Miron-Shatz et al., 2009; Rinner et al., 2019; Urban et al., 2018). Similar effects have also been observed in non-clinical samples. Sun et al. (2018), for instance, asked participants to rate how they remember feeling a month earlier during a job interview, finding that people who had a successful interview and were eventually offered the job remembered feeling more intense positive emotions and less intense negative emotions than what was actually reported immediately after the interview, whereas the opposite effect was found for people who had failed the interview. While the authors did not explicitly measure mood and thus can only infer MCM, their findings nonetheless help ground the study of MCM in real-life experiences. In fact, this result shows that experiences happening after-the-fact, such as eventually finding out about a job offer, can systematically change how past events are remembered. Further investigation into how the memory-experience gap is modulated by mood states remains an interesting and fruitful avenue for future research.
In sum, a growing body of research has provided evidence for increased false recognition of mood-congruent content, but these findings are mainly specific to performance on the DRM paradigm. Nonetheless, they suggest spreading activation can lead to biases that both enhance and impair subsequent memory, depending on the structure of the task and how emotional content is grouped together. While some have suggested that positive moods should lead to higher instances of false MCM than negative moods, research in this area has produced mixed findings. Positive moods do seem to facilitate greater instances of memory intrusions (Forgas et al., 2005; Storbeck & Clore, 2005, 2011), but this effect is independent of the valence of the stimuli (i.e., not MCM). Others have found that negative moods produce false MCM for discrete emotions (Bland et al., 2016) and increased false memory for negative lures compared to a neutral mood (Knott & Thorley, 2014), sometimes even at the same level as positive moods (Ruci et al., 2009). To provide further clarity to this issue, we encourage future researchers to explore other methods of testing false MCM, such as with misinformation paradigms (Forgas et al., 2005) or memories for false episodic events (Qin et al., 2008; Shaw & Porter, 2015). In particular, using adapted DRM paradigms where word lists are grouped orthographically (rather than semantically or conceptually), is a reasonable next step to test whether false MCM generalizes across different groupings of stimuli and lure distinctiveness (Choi et al., 2013).
Mood-Incongruent Memory and Mood Repair
An observation that obscures the consistency of MCM in autobiographical recall is the presence of mood-incongruent memory, particularly for negative moods. That is, when induced into a negative mood, people are sometimes more likely to recall positive memories of the past or think of positive memories faster than negative memories (e.g. Boden & Baumeister, 1997; Joormann & Siemer, 2004; Josephson et al., 1996; McFarland & Buehler, 1998; Öner & Gülgöz, 2018; Sakaki, 2007). These findings are often interpreted as an attempt by the participant to naturally repair or regulate negative mood. Accordingly, indices related to mood regulation ability have been noted to influence the likelihood of mood-incongruent recall. For example, recalling mood-incongruent events when in a negative mood is more likely for non-dysphoric individuals (Joormann & Siemer, 2004), those with higher self-esteem (Setliff & Marmurek, 2002; Smith & Petty, 1995), higher trait repressiveness (Boden & Baumeister, 1997), higher self-complexity (Sakaki, 2004), and higher mood-regulation expectancies (Rusting & DeHart, 2000). As noted, we did not observe mood-incongruent recall to be the norm in our literature search, but the prevalence of this effect requires further discussion. Mood-incongruent recall provides further evidence that the influence of mood on recall is particularly sensitive to individual differences in trait affect and mood regulation ability, which could have relevant clinical implications. Blaney (1986), for instance, suggested that depression may result when these controlled processes are unable to override the automaticity of mood-congruence, although at that point only one study was found to demonstrate mood-incongruent recall (D. M. Clark et al., 1983).
Though mood incongruent memory is more common in autobiographical recall, we note that these moderators of mood-incongruence have shown to also influence several non-autobiographical, explicit memory tasks. Smith & Petty (1995; Experiment 3), for example, observed that sad mood induced before the encoding of affectively toned news headlines led to better recall of negative headlines for individuals with lower self-esteem, while those with higher self-esteem shifted to more positive, mood-incongruent recall. Others have also observed moderating effects of mood regulation on subsequent memory performance, such that engaging in positive reappraisal of a recently experienced mood induction produced mood-incongruent memory, while continuing to focus on the negative mood produced MCM (Rusting & DeHart, 2000; Figure 2C). The emotional strength of encoded stimuli has also been suggested to drive mood-congruence vs. mood-incongruence. Following a positive or negative mood induction, Rinck et al. (1992) asked participants to rate the valence of pleasant and unpleasant words, followed by a surprise recall test of the words the following day (Experiment 1) or after a short distraction task (Experiment 2). In both experiments, words rated as strongly pleasant or strongly unpleasant produced mood-congruent recall, whereas words rated as only slightly pleasant or slightly unpleasant produced mood-incongruent recall. The authors reasoned that more emotionally intense words lead to MCM because their strong affective tone easily integrates with one’s mood. In contrast, less intense words are often emotionally ambiguous, especially if, say, the valence of a slightly negative word is rated in a strongly positive mood. The ambiguity of these slightly mood-incongruent words demands additional processing, which subsequently boosts memory for those items (Rinck et al., 1992). Note that this is a departure from the interpretation of mood incongruence as mood repair, rather suggesting that evaluative judgements made by participants could increase processing of items only slightly incongruent with a present mood state. Such accounts are not unique to mood-incongruent memory. A similar depth-of-processing explanation has been used, for instance, to account for improved recall of schema-incongruent information. In a study by Hastie & Kumar (1979) participants encoded sentences that described behaviors performed by fictional characters, with each set of behaviors preceded by a list of trait adjectives (e.g., intelligent, smart, knowledgeable, etc.). Behaviors incongruent with those trait adjectives were better recalled than congruent or neutral behaviors, which the authors reasoned was due to deeper processing of information that significantly adjusted the initial impressions of a character.
Several experiments have further demonstrated that the focus of the task configures whether mood-congruent or mood-incongruent memories are recalled. MCM is facilitated by the explicit instruction to ruminate on negative mood, whereas mood-incongruent memories are more likely when participants are instructed to reflect on and regulate their mood (Greenberg & Meiran, 2014b; McFarland & Buehler, 1998). Others have suggested that mood-incongruent recall is more likely when participants are less aware of how their mood states are relevant to the experiment, such as in studies that have measured natural mood resulting from exam performance or weather, although these results were specific to just the first memory recalled by participants (Parrott & Sabini, 1990). Moreover, when using cue words to help facilitate recall, words closely related to the negative mood induction (e.g. academic when the mood induction was based on failing a test) are more likely to produce MCM, whereas unrelated words (e.g. friends) are more likely to produce mood-incongruent memory (Sakaki, 2007). These findings suggest that the way in which someone is semantically primed might influence the likelihood of retrieving mood-regulating content. Finally, we note that mood-incongruent memory has also been observed in positive moods that enhanced the recall of negative headlines on websites (Kaspar et al., 2015), although this effect was interpreted as demonstrating that positive moods can sometimes increase attention and sensitivity to negative information when participants are allowed to conduct an unconstrained visual search of multiple emotional stimuli.
Understanding the factors that shift mood-congruence to mood-incongruence, especially in cases where mood repair overcomes negative MCM, can have useful clinical implications. For instance, the utility of mood-incongruent recall as a repair mechanism for negative moods resembles recent clinical examinations of memory bias training to promote rumination on positive, instead of negative, thoughts (Hertel et al., 2017; Vrijsen et al., 2016, 2019). Relatedly, the propensity for mood incongruence, or lack thereof, may be a reliable indicator of memory schemas that perpetuate depressed mood. Supporting this notion, clinically depressed patients who more frequently use positively-toned words when recalling a sad memory show less symptoms of depression at a 6-month follow-up (Brockmeyer et al., 2015). Future research should continue to explore individual difference factors that have a moderating role on this effect. It remains unclear whether the neuropsychological processes supporting mood-incongruent recall are similar or distinct to those supporting mood-congruent recognition, as mood-incongruent recall is generally considered a directed attempt at mood repair compared to the more automatic mechanisms that fuel MCM. Further, a multitude of emotional goals and cue characteristics can influence the type of emotional memories we recall (Holland & Kensinger, 2010; Simpson & Sheldon, 2020), all of which might uniquely interact with mood. More generally, studies of autobiographical MCM cannot rule out the possibility of mood-dependent memory effects, as the initial encoding of the emotional events is not experimentally manipulated or measured. Ongoing research in this area should therefore continue to examine how the controlled, mood-repairing process of mood-incongruent memory dissociates from the more automatic, mood-facilitating process of MCM.
Methodological Considerations for MCM Research
Examining MCM in the lab requires appropriate consideration of a multitude of experimental factors that can influence the presence or absence of memory bias. Here we propose relevant methodological considerations to help guide future MCM research based on observations we made upon reviewing the literature. In particular, we suggest that researchers carefully consider the nature of the tasks used in their experiments, such as how participants encode emotional stimuli, how/when moods are induced, and how/when memory performance is tested.
The encoding task.
Despite several studies corroborating evidence from clinical research that MCM is enhanced when participants engage with emotional stimuli in a self-referential manner (Hartig et al., 1999; Itoh, 2004; Nasby, 1994, 1996), we observed that many studies instruct participants to only read/listen to or memorize the emotional stimuli that were presented to them (see Table S1). If MCM emerges from an associated network of linked concepts, then careful attention should be devoted by the experimenter to an encoding task that properly engages the participant and encourages a self-referential mode of thinking. This engagement can also be achieved by presenting a more diverse stimulus set than commonly used emotional words or static pictures. The use of narratives, hypothetical vignettes, autobiographical memories, or dynamic video clips all provide opportunities to test MCM on stimuli that might better generalize to real-life, emotional episodic events. In fact, MCM has even been demonstrated for musical excerpts and food (Houston & Haddock, 2007; Pliner & Steverango, 1994). Empirical evidence for discrete moods acting upon discrete emotions further suggests that emotional stimuli should be carefully selected to foster specific emotional reactions, thereby providing a more congruent match between mood and emotion.
The mood induction method.
The mood inductions used in MCM studies may also benefit from incorporating more self-referential elements, although this view has received comparatively less attention in the literature. Recent studies have shifted to predominantly using movie clips that are known to effectively induce strong affect (Fernández-Aguilar et al., 2019; Joseph et al., 2020; Westermann et al., 1996), but the use of generic positive or negative clips may fail to induce specific congruence with emotional content. For example, a negative movie clip could include a combination of scenes evoking disgust, anger, and sadness, hindering the possibility of an induced mood state closely matching with a discrete-emotion stimulus set. Even if normed to represent specific affect, film clips depict emotional experiences that occur to other people. While such clips might stimulate the recollection of personal autobiographical events, they could also lead, for instance, to strong feelings of sympathy for others rather than a sad mood pertaining to the self (Fultz et al., 1988).
Researchers may need to consider combining mood induction techniques to achieve the appropriate balance of strength, relevance, and specificity for the induced mood. A meta-analysis of mood induction from Joseph et al. (2020) suggests that certain combinations (e.g., music and autobiographical recall) can outperform single inductions (e.g., music). Note, however, that combinations are not necessarily always better, depending on the technique used. In this same meta-analysis, the authors also quantitatively confirmed that the average participant already reports generally positive mood levels prior to induction, and that the effect size of negative inductions for inducing negative affect is nearly twice the size of positive inductions inducing positive affect. This matches with previous reports of researchers struggling to increase positive mood levels above an already positive baseline (Fitzgerald et al., 2011; Kiefer et al., 2007; Westermann et al., 1996). Yet despite having larger effect sizes, negative inductions are also not able to reliably overcome the initial positivity bias. For instance, experiments using the Positive and Negative Affect Schedule (Watson et al., 1988) show that average reports of negative affect still remain fairly low after a negative mood induction (1.91 on a 5-point scale), with average reports of positive mood dropping to comparable levels (2.37). Thus, it seems that negative inductions primarily produce equated levels of positive and negative mood in post-induction assessments because their effects are diminished by a predominantly positive mood pre-induction (Joseph et al., 2020). To boost effect sizes, Joseph et al. (2020) suggest carefully choosing the type of induction method based on the intended affect manipulation, as certain methods are stronger for some moods than others. Accordingly, a qualitative review provides specific recommendations on visual, musical, autobiographical recall, situational, and imagery induction techniques for discrete affect including anger, disgust, fear, sadness, surprise, and happiness (Siedlecka & Denson, 2019). To improve our understanding of mood sensitivity to certain inductions, researchers should make a point to assess the effectiveness of inductions with specific affect measures (e.g., happiness, pride, sadness, anger) as opposed to broad affect measures (e.g., general positive or negative affect) that are unable to provide the same level of specificity (Joseph et al., 2020).
When deciding on mood induction procedures, researchers should carefully consider whether the method properly induces a sustained affective state that can last for the intended duration, as opposed to a brief emotional reaction by the participant. We suggest researchers apply the distinctions between mood and emotion outlined at the beginning of this review regarding duration, remoteness to cause, and appraisal (Beedie, 2007; Beedie et al., 2005; Frijda, 1994; Ketal, 1975; Ortony & Clore, 1989; Siemer, 2009). In particular, these distinctions suggest that inductions should comprise a collection of experiences that together produce a mood state, slowly building over time. Surprisingly, though, a number of studies identified in our review used relatively short, approximately 5 minute-long induction procedures, often with only a specific stimulus such as an emotional video clip (e.g., Bland et al., 2016; Boden & Baumeister, 1997; Greenberg & Meiran, 2014b; Gupta & Khosla, 2006; Liu et al., 2008; Meeks et al., 2019; Nielson & Lorber, 2009; Tesoriero & Rickard, 2012; Wang & Ren, 2017; Zhang et al., 2018). The worry here is that these brief elicitations arising from just a single event may not fully produce the intended mood states. Affective measures obtained immediately after the induction may reflect an emotional reaction to the stimulus rather than the true presence of a sustained mood state. Researchers commonly assume that these brief inductions can still lead to lasting mood changes beyond the induction itself, but few studies have actually tested this proposal, and recent examinations suggest that mood effects actually diminish rather rapidly after only a few minutes post-induction when using film clips (Kuijsters et al., 2016), as well as autobiographical memories and music (Gillies & Dozois, 2021). This decay has significant consequences for affective states induced prior to encoding or retrieval, since the intended mood manipulation may not actually last throughout the entirety of the task if not properly administered.
Lastly, we observed that hypnotic mood inductions are absent from the recent literature on MCM, despite hypnosis having been used almost exclusively in the studies reviewed by Bower (1981) when developing an associative network theory of memory and emotion. This decline in use may, in part, have grown out of misconceptions surrounding hypnosis and myths that are perpetuated in popular media (Raz, 2011). Yet, empirical evidence shows that hypnosis is a powerful tool that can effectively alter conscious experience, especially in clinical applications such as pain management (Thompson et al., 2019). Ongoing work that details the underlying neural and physiological changes accompanying hypnosis have helped to further demonstrate the validity of this technique (Fernandez et al., 2022; Jiang et al., 2017; Terhune et al., 2017). Recent reviews comparing induction efficacies do not discuss the use of hypnosis (Joseph et al., 2020; Siedlecka & Denson, 2019), and therefore it remains unclear under which circumstances hypnosis might be preferred over other methods. Re-incorporating hypnosis into the study of MCM should be given consideration, given a renewed appreciation of its potential as a mood-induction technique (Kihlstrom, 2012).
The mood induction timing.
The timing of a mood induction in MCM studies—such as when the induction occurs in relation to the encoding and retrieval tasks—may shift the types of effects that are observed. Research on stress and emotional memory has in recent years addressed this topic by seeking to more precisely define how memory enhancement or impairment is configured by the proximity of a stress induction to encoding or retrieval, due to the time course of rising stress hormones (Schwabe et al., 2012). MCM research would benefit from a similar approach. For encoding-retrieval studies, we observed that moods were usually induced before encoding (see Table S1), but have also been induced prior to retrieval (e.g., Fiedler, 2001; Fiedler et al., 2003; Forgas et al., 2005; Gupta & Khosla, 2006; Lewis et al., 2005; Smith & Petty, 1995; Varner & Ellis, 1998), or maintained throughout experimental tasks with background music or repeated inductions (e.g., Fitzgerald et al., 2011; Itoh, 2004; Kiefer et al., 2007; Rafienia et al., 2008; Rusting, 1999). Since mood states differentially influence task engagement and processing strategies, the recency of an induced mood to encoding or retrieval likely influences if, and how, MCM will be observed. For instance, moods induced immediately prior to an encoding task are more likely to facilitate attentional bias to mood-congruent content and, consequently, might produce asymmetrical assimilative and accommodative processing of the stimuli (Fiedler, 2001). Moods induced prior to retrieval influence memory via a different mechanism, as here MCM results from biased access to mood-congruent content in memory rather than attentional focus at encoding. We note, however, that most investigations have used pre-encoding mood inductions and administer retrieval soon after encoding, often without measuring the extent to which induced mood lasted throughout the experiment. These limitations preclude a proper comparison of pre-encoding and pre-retrieval effects.
Alternatively, post-encoding mood inductions with retrieval occurring in a neutral mood might better isolate the effects of mood on emotional memory consolidation, although these types of investigations are few in number and have produced inconsistent findings. Liu et al. (2008) observed a memory enhancement effect of post-encoding arousal on emotional pictures compared to neutral pictures when memory was tested the following week, although Nielson & Lorber (2009) found that memory enhancement for emotional words was irrespective of the valence or arousal of the words. In contrast, others have shown that post-encoding negative mood impairs recollection memory for emotional stimuli when tested on the same day and also with a 24-hour delay, although even this finding was not consistently observed across experiments within the same study (Wang & Ren, 2017). Of note, none of these studies were specifically designed to test MCM, but rather the effect of general post-encoding arousal, hence explaining why they were not initially identified in our literature search. Heightened arousal was commonly induced with a 3-minute-long comedic video (Liu et al., 2008; Nielson & Lorber, 2009), or strongly aversive video depicting oral surgery (Liu et al., 2008) or violence against a pregnant woman (Wang & Ren, 2017, Experiments 1 & 5). This generalized state of heightened arousal will be less selective than a specific mood in enhancing memory for valence-specific content and may not be strong enough to sufficiently bias mood-congruent consolidation processes. While we did find another study that induced post-encoding happy and sad moods, other issues arose with the experimental design. Forgas et al. (2005) demonstrated across multiple experiments that happy moods enhanced and sad moods reduced the incorporation of misleading information into previously witnessed events, but this was irrespective of the valence of the original event (Forgas et al., 2005). However, the experienced events were few in number (usually just one positive and one negative event), the mood induction was prior to the presentation of the misleading information and far removed from the initial encoding period (at least 45 minutes and up to one week), and the affective tone of the misleading information was not manipulated. Thus, we note that this study also did not have an appropriate design to properly test for mood congruence resulting from a post-encoding induction.
The temporal proximity of a post-encoding mood induction is likely a critical factor contributing to any subsequent memory effects. Piñeyro et al. (2018) found that positive mood induced after recalling a negative autobiographical memory can modify subsequent recall a week and month later. Only an induction occurring 10 minutes after initially recalling the memory decreased the number of negative details subsequently recalled, compared to 6 hours after initial recall, recall with no mood induction, or no recall with a mood induction. This finding supports the presence of a time-dependent reconsolidation mechanism driving subsequent changes in memory recall (Piñeyro et al., 2018). Ultimately, though, our review of the literature has revealed a surprising neglect of mood effects during both the consolidation and reconsolidation stages of the memory process. Mood induction studies are often touted for providing a purer assessment of how mood influences memory compared to individual differences assessments or group comparisons between clinically depressed persons and healthy controls. Yet, induction studies can still confound shifts in mood with concomitant changes in attention, motivation, and subject compliance. Post-encoding inductions help to overcome these limitations by isolating the influence of mood on the consolidation stage and removing unintended confounds from encoding and retrieval. Theoretically, this approach also aligns well with existing models of emotional memory bias, which place the consolidation stage as central to long-term enhancements in memory for emotional content, as well as Bower’s network theory, which suggests that mood does not need to co-occur with encoding to activate and enhance the storage of affectively linked content. We thus propose that MCM research can greatly benefit from a renewed emphasis on consolidation and reconsolidation mechanisms.
The retrieval task.
How memory is queried is a crucial factor in MCM research. According to the Affect Infusion Model, moods are particularly sensitive to free recall tests given the ambiguous context that is created when asking participants to generate their own response, thus increasing reliance on accessible affective information in the search process (Bower & Forgas, 2000; Forgas, 1995). It seems that the literature has abided by this suggestion, with most studies administering free recall tests (Table S1), although we did not observe any systematic patterns that suggested old/new recognition paradigms were necessarily less sensitive to MCM. For example, Pliner and Steverango (1994), Fiedler et al. (2001), and Houston and Haddock (2007) all observed symmetrical MCM with old/new recognition assessments. The distinction between recall and recognition may be more apparent with greater delays between encoding and retrieval, although few MCM studies test memory at least a day after encoding.
MCM is clearly a more complex phenomenon than the number of emotional words one can remember in a designated timeframe, or the number of hits and misses on a recognition test. While such evaluations have simplified the study of MCM, future research should consider administering memory tests that more thoroughly evaluate how people remember the emotional past, instead of just the items they can remember. Does mood congruence induce a systematic shift in how individuals judge previous affective experiences? For instance, does depressed mood shift affective judgements of previously experienced events to be more negative than they actually were in the moment? Further probing this memory-experience gap might reveal important insight to how MCM perpetuates depressed mood by maintaining a skewed perspective of life events. Retrospective reports of emotional experiences are commonly evaluated with regard to dispositional affect, such as neuroticism, that shapes beliefs about emotions (Mill et al., 2016; Robinson & Clore, 2002), but state-based affective experiences might also contribute to a bias in self-report. For instance, higher peak ratings for a negative mood state over a two-week period is predictive of greater negativity bias in subsequent retrospective mood reports (Sato & Kawahara, 2011). This finding conceptually replicates the peak-end rule, whereby peak experienced state affect is predictive of subsequent global reports for emotional film clips, suggesting that retrospective reports consist of a weighted average of past emotional experiences (Fredrickson & Kahneman, 1993). Whether MCM for specific episodic events ultimately configures a sustained bias in subsequent memory remains an intriguing avenue for future research.
Relatedly, we observed a surprising lack of basic-science studies that have examined mood-congruent effects on implicit memory, despite reliable evidence across clinical investigations demonstrating a consistent negativity bias in implicit recall among depressed groups (Gaddy & Ingram, 2014). Theoretically, implicit memory may be more susceptible to mood-related effects than explicit memory, given that implicit memory tasks such as stem completion or free association involve minimal retrieval cues and thus place greater reliance on internal cues (i.e., mood) during retrieval. This same reasoning has been used, for instance, to suggest that recall tasks are more mood-sensitive than recognition tasks, given that strong retrieval cues may potentially overshadow the influence of mood on memory. When these retrieval cues are sparse or absent, mood becomes a stronger contextual cue to potentially bias recall (Forgas, 1995; Singer & Salovey, 1988). Although some early work in the field demonstrated mood-related effects on implicit memory, especially for mood-dependent memory (Tobias et al., 1992), more research is needed to confirm whether induced mood reliably biases implicit memory in healthy subjects, and how such effects compare to those on explicit memory.
As alluded to earlier, researchers will also need to incorporate longer delays between encoding and retrieval tasks to allow for a more thorough examination of the long-term biasing effect of mood on emotional memory. MCM studies primarily examine the effects of mood on attention and resource allocation when memory is tested immediately after encoding, which may not always extend to a maintained bias in memory. In fact, immediate and delayed emotional memory enhancements have a surprisingly low correlation, demonstrating a dissociation of emotional arousal effects depending on when memory is tested (Schümann et al., 2018). This dissociation may arise from unique cognitive resources deployed during encoding that contribute to an immediate, but not necessarily delayed, enhancement in emotional memory (Talmi, 2013). In a similar fashion, immediate and delayed MCM may reflect fundamentally different processes. According to the feeling-as-information theory and affect infusion model, people rely on their affective state as a source of information to make judgements in a task (Forgas, 1995; Schwarz, 2012). Memory tested in the same session as the mood induction and encoding phases may simply predispose a search for mood-congruent material during the memory test, which may not necessarily reflect the enhanced consolidation of that material into long-term memory.
Surprisingly, only a handful of MCM experiments have tested memory for previously encoded emotional stimuli with at least a 24 h delay (Bovy et al., 2020; Bullington, 1990; Klaassen et al., 2002; Knott & Thorley, 2014; Liu et al., 2008; Nielson & Lorber, 2009; Rinck et al., 1992; Wang & Ren, 2017). Bullington (1990) instructed participants to generate autobiographical events at an initial experimental session and then observed MCM in the percentage of the same emotional memories that were recalled after a mood induction the following day. Klaassen et al. (2002) evaluated how memory for learned words is influenced by the effects of tryptophan depletion, which lowers serotonin levels and, consequently, produces a more negative mood state. Compared to a placebo group, tryptophan depletion during encoding impaired the recall of positive words when tested the next day, even though tryptophan levels at that point had returned to baseline. This effect was only observed 24 hours, and not 6 hours, after encoding, suggesting that TRP depletion selectively impaired the consolidation of positive information. Note, though, that these effects were specific to positive words, while no enhancement effect was observed for mood-congruent negative words (Klaassen et al., 2002). As already mentioned, Knott & Thorley (2014) also found that mood-congruent false memory effects persist after a 1-week delay, whereas false memory for neutral lures was abolished by that time. Collectively, these studies suggest that MCM may be sensitive to the delay period between encoding and retrieval.
Lastly, MCM in natural, daily life has also been assessed with the use of psychological and psychophysiological ambulatory monitoring of self-reported emotions and heart rate (Loeffler et al., 2013). Previous work by Mayer et al. (1995) demonstrated that natural mood states prior to completing cued category and association-retrieval tasks produced mood-congruent responses (e.g. thinking of a word for Attitude that begins with the letter p), although these generative response tasks were only completed at a single timepoint and subsequent memory was not tested. In the study conducted by Loeffler and colleagues, participants were remotely presented with lists of emotional words to memorize upon the first report of an emotional or neutral state, and recall was tested the following day and also a week later. The authors observed an interaction of MCM with arousal, such that recall of negative words encoded in a negative mood was enhanced with higher physiological arousal, whereas recall of positive words encoded in a positive mood was improved with lower arousal (Loeffler et al., 2013).While such experiments are unable to control the strength or duration of experienced mood, continued investigation of MCM in daily life may further elucidate the development of biased emotional memory over extended periods of time.
Summary of Behavioral MCM
Here we have reviewed more than three decades of empirical research on MCM to discern the current status of the field and provide guiding suggestions for future researchers. Despite observing varied methodology and a myriad of specific effects, the present review illustrates that MCM is a generally reliable and reproducible phenomenon among both sequential encoding-retrieval and autobiographical recall paradigms. However, evaluating MCM in lab settings is heavily influenced by methodological factors, most notably the efficacy of the mood induction technique which, when weak, may be insufficient to drive mood-related effects. Moreover, self-relevance, trait affect, and task paradigm (e.g., free recall) have also been suggested to moderate the presence and strength of mood-induced memory effects and thus should be carefully considered when designing an MCM study. A few investigations have suggested that MCM may be improved by facilitating discrete matches in mood and emotion, although such work is limited and requires further investigation.
We noticed a growing body of research examining MCM in the context of false memories. Evaluating the mechanisms by which mood can facilitate memory for events that were never actually experienced is a particularly novel development since previous reviews of MCM by Blaney (1986) and Singer and Salovey (1988). When assessed with the DRM task, induced mood seems to facilitate robust false memories for mood-congruent lures, which are often reported by participants as confidently remembered experiences. These findings provide novel support for associative network theories by demonstrating that semantically linked, mood-congruent content can be activated at encoding and better consolidated into memory, even if they were never actually experienced. The extent to which such memory intrusions generalize to false memories for real-word episodic events remains unclear but will be an interesting avenue for future research.
Regarding mood-incongruent memory, research indicates that individual differences in trait affect and emotion regulation abilities can shift recall from mood congruent to mood incongruent, seemingly as a mechanism of mood repair after a negative mood induction. These effects are mostly observed in studies that assess autobiographical recall after a mood induction, where shifting between mood-congruent and mood-incongruent memory reflects a change in the search process for affective content. For instance, positive memories may be more readily available if an emotion regulatory process is attempting to counteract a negative mood state. Importantly, this process may fundamentally differ from the more automatic mechanisms underlying the initial formation of MCMs in the context of encoding-retrieval tasks. Future research that further delineates the cognitive and neural factors underlying shifts between mood congruence and incongruence may be fundamental to improving therapeutic interventions that seek to curb ruminative thought patterns on mood-congruent content.
A few important adjustments in methodology have been made since previous reviews, namely in the choice of experimental materials. Mood induction techniques, for instance, have shifted away inductions that may be confounded by subject compliance and experimenter demands, with recent studies predominantly administering film, autobiographical, and/or music inductions. While encoding tasks still predominately employ emotional words, we did observe attempts to utilize more generalizable stimuli, such as images and short narratives. Moving forward, though, we suggest four key gaps in the literature for future researchers to consider when evaluating MCM. First, although few studies have examined discrete matches in mood and emotion (e.g., sadness) compared to more general congruence (e.g., negative), these investigations consistently indicate that MCM is sensitive to the specificity of the induced mood and the encoded stimuli. Thus, induction materials and stimulus sets should be carefully normed to ensure the proper mood and emotion is elicited for the purposes of the study. Second, little work has explored how the timing of a mood induction might influence MCM, whether it occurs before encoding, after encoding, or before retrieval. Administering post-encoding inductions that remove the influence of mood on motivation at encoding will be particularly useful in better pinpointing the specific influence of mood on memory consolidation. Third, MCM research is predominately limited to short-term memory effects, with surprisingly few studies evaluating persistent MCM effects that might last for at least one day. Future research in this area will greatly benefit by incorporating longer delays between encoding and retrieval. Doing so will more properly test the core tenets of network theory and integrate MCM findings with contemporary models of emotional memory, as the current literature impedes any formal conclusions on long-term memory bias. Fourth, few studies have attempted to integrate behavioral MCM findings with the brain, despite such an approach providing a means to test the neural implications of network theory and spreading activation among affect nodes. To discuss this point in more detail, the remainder of this review will specifically explore the cognitive neuroscience of mood, MCM, and avenues for future research in this area.
The Cognitive Neuroscience of Mood-Congruent Memory
We dedicate the final section of this review to examining the underlying neural processes that support MCM, a particularly underdeveloped area of research. Here we discuss neural investigations of mood and memory, emphasizing directions for future research and suggesting opportunities for the field to integrate with recent developments in the neurocognitive study of emotional memory consolidation.
Neural Investigations of Mood
Examining MCM in the brain firstly requires one to consider how mood states associate with activation and connectivity among neural regions. Emotional memory research, for instance, has reliably identified amygdala response to arousing stimuli, which modulates hippocampal-based storage (LaBar & Cabeza, 2006). However, measuring sustained affective states is challenging compared to the measurement of shorter-lived arousal responses due to the remoteness of moods to a definable cause and the dynamic nature by which moods develop over time.
Intuitively, enhanced or sustained activation in neural regions subserving transient emotional reactions may also underlie the experience of mood. Indeed, when viewing negative emotional stimuli, depressed people exhibit stronger activation in emotion processing regions such as the anterior cingulate cortex (ACC) and amygdala (Elliott et al., 2002; Stuhrmann et al., 2013). Likewise, in nondepressed participants, induced sad mood increases amygdala activation (Mitterschiffthaler et al., 2007), enhances amygdala response to mood-congruent sad stimuli (Wang et al., 2006), and even increases the unpleasantness of experienced pain by enhancing activity in the insula, hippocampus, and prefrontal cortex (Berna et al., 2010). Positive moods can have similar amplifying effects on neural activity in emotion processing regions, such as by increasing activity in corticostriatal regions while listening to happy music (Mitterschiffthaler et al., 2007) or anticipating reward (Young & Nusslock, 2016). Moreover, induced positive mood correlates with greater cerebellar, hippocampal, and amygdala activity (Kohn et al., 2013). Thus, the neural signature of a mood state may, in part, reflect enhanced or sustained activity in similar regions that support more transient emotional experiences.
The sustained affect that characterizes a mood may also arise from the coupling of emotionally reactive structures, such as the insula or amygdala, with higher-level cognitive regions that maintain a preparatory, affective mental state. For instance, sad mood increases resting-state functional connectivity within the paralimbic network, including the dorsal ACC and insula (Harrison et al., 2008). Similarly, sustained anxiety during unpredictable threat of shock enhances intrinsic functional coupling between the dorsomedial prefrontal cortex (dmPFC) and amygdala (Vytal et al. 2014). The amygdala typically activates in a transient fashion and habituates rather rapidly to threatening stimuli (Breiter et al., 1996), but synchronized activity with the PFC may function to maintain the amygdala in a primed state, ready for novel threat detection (Vytal et al., 2014). Indeed, enhanced connectivity between the dmPFC and amygdala during the viewing of emotional faces in an anxious state corresponds with faster reaction times to identify fearful faces, thus generating an adaptive threat bias (Robinson et al., 2012). Like anxiety, other moods may also manifest from the configuration of a preparatory, or primed, mental state that facilitates the processing of mood-congruent material and amplifies neural response in emotion processing centers. Consistent with this proposal, positive mood induced via humorous cartoons and positive performance feedback has been shown to increase activation in the ventral striatum while also shifting effective resting-state functional connectivity to a more reciprocal, or bidirectional, connection between the ventral striatum and anterior medial PFC (Admon & Pizzagalli, 2015). Thus, different moods may arise from PFC connections that modulate and sustain activity in dedicated neural systems for discrete emotions. We note, however, that such an interpretation remains controversial and ultimately depends on how affect is represented (e.g., categorically or dimensionally). Moreover, the neural representation of mood likely shifts depending on specificity. A general negative mood induction, such as via a stressor, may engage the brain differently compared to discrete moods such as anger or sadness.
Indeed, since moods can slowly develop over time from a collection of diverse emotional experiences, the PFC may instead integrate these multiple, contributing sources of information to construct an affective state. According to the appraisal-by-content model, regions within the PFC are specialized to appraise various affective inputs in parallel, such as exteroceptive sensations, episodic past or future events, viscero-motor and sensory signals, and self-related information (for a review, see Dixon et al., 2017). The subgenual ACC (sgACC), in particular, may be a core hub that monitors physiological arousal and regulates interactions among distributed neural systems subserving affective and cognitive processes that contribute to mood development. The sgACC exhibits differences in structural and functional properties in clinical depression (Drevets et al., 2008) and activation in this region associates with ruminating on self-referential content in both depressed (Cooney et al., 2010) and mood-induced, non-depressed individuals (Kohn et al., 2013). The sgACC also seems necessary for sustaining positive affect, as lesions in this area in macaque monkeys impairs elevated arousal during the anticipation of reward (Rudebeck et al., 2014), and the sgACC mediates dmPFC-amygdala connectivity during the upregulation of positive affect (Scharnowski et al., 2020). In fact, the sgACC is active in a diverse array of emotional paradigms eliciting fear, stress, and reward responses, suggesting a universal role in facilitating affect (Drevets et al., 2008). Regarding network connectivity, the sgACC is a core hub of the affective network with dense connections to limbic and deeper-brain structures such as the orbitofrontal cortex (OFC), amygdala, hypothalamus and periaqueductal gray (Bush et al., 2000; Dixon et al., 2017; Öngür et al., 2003). The sgACC is also closely situated to the medial prefrontal core of the default network (DN), and their connectivity is consistently implicated in maintaining ruminative thought and depression (Berman et al., 2011; Connolly et al., 2013; Zhou et al., 2020). In summary, given these findings, moods may arise from large-scale network interactions that are integrated in the PFC, rather than from amplified or modulated activity in discrete emotional networks.
Ultimately, an understanding for the brain basis of mood struggles with similar controversy surrounding broader emotion theory. Are feelings constructed from basic neuropsychological processes that combine to create the experience of emotion, or are they discretely represented in the brain by designated neural substrates (Lindquist, 2013)? Multivariate techniques provide promising means by which to evaluate the neural representation of discrete emotions (Kassam et al., 2013; Kragel & LaBar, 2016; Nummenmaa & Saarimäki, 2019), but this same approach has yet to be applied to mood states. Further uncovering the neural basis of mood may also benefit from studying how mood modulates emotional memory, as in the case of MCM, and whether designated neural systems facilitate memory for discretely matched, mood-congruent emotional stimuli. We thus continue our exploration of mood and the brain by specifically focusing on neural investigations of MCM.
Neural Investigations of MCM
Only a handful of neuroimaging studies have attempted to characterize the neural mechanisms underlying the influence of mood on emotional memory. Many of these investigations have sought to examine neural support for a network theory of MCM by evaluating whether mood promotes enhanced recruitment of the neural mechanisms that subserve semantic or schema-mediated memory. Here we provide an overview of the limited research in this area, while further illustrating the diversity of approaches that can be utilized to study MCM.
In an attempt to test the involvement of associative, semantic networks in mood-congruent encoding, Kiefer et al. (2007) recorded event-related potentials (ERPs) while participants encoded and immediately recalled positive and negative words in either positive or negative moods. The encoding task consisted of reading complete (read condition) or fragmented (generate condition) words in order to test whether the assimilative nature of positive moods facilitates performance on generative encoding and free recall, especially for mood-congruent positive content. Kiefer and colleagues assessed if mood-congruent stimuli are processed more efficiently by focusing on the N400, a centro-parietal, negative electrical potential sensitive to semantic processing and typically lower in amplitude when a congruent semantic context is already activated, thereby allowing newly-encoded stimuli to integrate with this existing semantic structure (Kiefer, 2002). Analyses indicated that positive moods, compared to negative moods, were more likely to produce mood-congruent recall and were most sensitive to valence-related modulations of the N400, which were subsequently source localized to inferior and ventral temporal cortices. These findings are consistent with the notion that the strength of MCM may be dependent upon the type of mood experienced. According to assimilative-accommodative processing theory, positive moods are more likely to activate associative semantic networks that strengthen mood-congruent generative encoding and, presumably, subsequent recall (Fiedler, 1991, 2001). Note, however, that in our behavioral review of MCM we did not observe consistent support for asymmetrical effects between positive and negative moods, so it remains unclear the extent to which this asymmetry exists outside of specific task contexts. Further, with a limited number of trials per condition and the recall task interspersed between multiple rounds of word encoding and mood induction, Kiefer et al. (2007) did not assess valence-related neural activity directly associated with subsequent memory performance. Thus, the longer-term influence of N400 modulation remains speculative. Arousal ratings for the words were also very low (less than 1 on a scale of 0–5) but slightly higher for positive words, an important caveat that may have contributed to the observed valence asymmetry (Kiefer et al., 2007).
A study by Egidi and Nusbaum (2012) also assessed how mood congruent processing modulates the N400. Instead of using words, these researchers used short narratives with positive or negative endings. This study also included a third, reference group with a neutral mood induction. Mood congruence was observed as the differential processing of stories with negative endings among the different mood conditions. Compared to the neutral condition, a happy mood enhanced N400 amplitudes for negative endings, suggesting more difficult processing, whereas a sad mood produced smaller amplitudes, suggesting more efficient processing (Egidi & Nusbaum, 2012). Interestingly, this pattern of mood-congruent and incongruent processing was mostly driven by the negative endings, whereas the processing of positive endings was not facilitated by positive mood or impaired by negative mood. Thus, in contrast to the findings from Kiefer et al. (2007), here mood-congruent processing was largely found in the negative mood condition. While the authors do not directly address this discrepancy, one potential source for the differential findings is the type of task that was used. Kiefer et al. (2007) asked participants to either read complete words or generate them from fragments during list learning and, in agreement with assimilative-accommodative accounts, observed memory and neural effects mostly in the generative condition. In contrast, Egidi & Nusbaum (2012) evaluated mood effects during discourse comprehension by asking participants to simply listen to emotional stories, which required less engagement from the participant. Moreover, Kiefer et al. (2007) administered recall tests after each block of mood induction and list learning (four in total) and thereby revealed to participants the nature of the study, while Egidi & Nusbaum (2012) administered a single recall test after the completion of both sets of encoding blocks. Unfortunately, Egidi & Nusbaum (2012) only assessed overall memory performance in order to exclude participants with poor performance; they did not report the effects of mood or valence on memory, or the relation of neural effects to subsequent recall performance. ERP investigations have thus provided limited support for associative network accounts of MCM by demonstrating that mood modulates the semantic processing signals of valence-related stimuli, but the predictive quality of this modulation on subsequent memory remains unclear and requires further investigation.
Several functional magnetic resonance imaging (fMRI) investigations have also assessed the neural mechanisms underlying MCM. In one of the first fMRI studies in this area, Lewis and colleagues (2005) instructed participants to read positive and negative words presented on a screen and indicate if the words are self-descriptive. Encoding was immediately followed by a series of memory tests where participants viewed mixed lists of old and new words and indicated whether they remembered the words (recollected), knew the words (familiar), or thought the words were new. Four separate lists were presented during this recognition test, with a brief happy or sad mood induction (in alternating order) before each list, consisting of happy or sad emotional faces with affectively congruent music playing in the background. Importantly, while subjective ratings indicated that these inductions were successful in promoting the desired mood before the memory test blocks, happy mood ratings remained positive throughout the block, whereas sad mood ratings returned to baseline, suggesting asymmetrical efficacy of the induction technique (Lewis et al., 2005).
With mood induced prior to retrieval, the goal of this study was to examine how mood states might activate associated links to previously encoded stimuli with similar valence. Behavioral findings were mixed, as only the negative mood condition showed a significant MCM effect in recollection responses for negative words, although overall memory for negative words was significantly worse than for positive words. Since mood was manipulated within-subject to allow each participant to complete happy and sad inductions throughout the retrieval test, this fluctuation in induced mood might have contributed to the mixed findings. For the neural data, the authors used a conjunction analysis to evaluate activation at encoding that matched with activation at retrieval for mood-congruent stimuli that were subsequently recollected. Recapitulated neural activity for mood-congruent stimuli in both inductions was found in regions commonly implicated in episodic memory retrieval, such as the superior parietal and dorsolateral prefrontal cortices. As well, specific reactivated regions were observed for happy and sad mood congruence. The sgACC was active during the encoding of subsequently recollected positive words and during the retrieval of those words when in a happy mood, while the right posterior lateral OFC was active for negative words during encoding and when recollected in a sad mood (Figure 3). The authors viewed these findings as support for the associative network theory, suggesting that the sgACC and OFC may represent emotion-specific nodes that facilitate MCM (Lewis et al., 2005). However, with moods induced immediately after encoding and prior to retrieval tests, this experiment was unable to fully assess the formation of longer-term, biased emotional memories. Moreover, a conjunction analysis only identifies direct correspondence in neural activity across conditions, thus limiting the detection of regions that might uniquely promote the encoding or retrieval of mood-congruent memories.
Figure 3.
Neural Regions Implicated in Mood-Congruent Memory in Healthy Subjects
Note. Neuroimaging investigations of MCM have consistently implicated the prefrontal cortex (PFC) in facilitating memory for mood-congruent content, including the subgenual anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC). Locations are marked based on the peak coordinate reported for the MCM effect (Fitzgerald et al., 2011; Lewis et al., 2005) or the site of stimulation (Bovy et al., 2020). Not shown here are the electroencephalography findings from Kiefer et al. (2007) and Egidi and Nusbaum (2012), which further demonstrate that the amplitude of the N400 event-related potential is lower for mood-congruent content due to more efficient processing.
To address some of these limitations, a different fMRI study assessed MCM resulting from moods induced before encoding (Fitzgerald et al., 2011). In this study, participants were instructed to read and memorize positive, negative, and neutral words, followed by free recall after a brief distraction task. This sequence of study, distraction, and test was repeated four times, with mood inductions (either sad or happy film clips) before each one. The same mood was repeatedly induced in a single session, although participants returned the following week to complete the task with the other type of mood induction. Positive words were recalled significantly better than negative words in a happy mood, while an MCM effect was not found for negative words, although results were still in the expected direction. Note that this finding is opposite to the asymmetrical effects from Lewis et al. (2005). As with the previous study, the different efficiencies of the mood inductions may have contributed to asymmetrical MCM. Participants in both groups started the session in generally positive moods, with the happy induction essentially maintaining this positive mood throughout the course of the study. In contrast, while the sad induction led to a more rapid decline in mood throughout the entirety of the experiment, average mood ratings were not significantly unpleasant (less than 0) until later task blocks.
To evaluate neural activation related to MCM, Fitzgerald et al. (2011) assessed the three-way interaction of mood, valence, and recall performance during the encoding of emotional words, which identified activation in the left OFC as representative of MCM. This effect was primarily driven by the sad mood condition, whereby OFC response increased for subsequently remembered negative words and decreased for positive words (Figure 3). The authors also identified the left inferior and middle frontal gyri as representative of successful mood-incongruent memory, such that these regions were generally more active during encoding for subsequently remembered words incongruent with the participant’s mood. These findings were suggested to indicate that mood-incongruent stimuli require greater conceptual processing since activitation in inferior and middle frontal gyri is often associated with semantic processing (Binder et al., 2009), similar to how semantic incongruity modulates the amplitude of the N400 (Kiefer, 2002; Kiefer et al., 2007). Fitzgerald and colleagues also observed a main effect of mood on amygdala activity, but no interaction of such activity with the emotionality of the stimuli or subsequent memory performance. Specifically, amygdala activity during encoding was generally greater for sad moods compared to happy moods. The authors suggested that this sustained activity may have obscured the detection of more transient responses to the emotional stimuli (Fitzgerald et al., 2011).
A third fMRI study investigated the effects of an antidepressant (duloxetine) in order to evaluate the mnemonic benefits of drug treatment on depressed mood (Tendolkar et al., 2011). Healthy participants were assigned to either a treatment or placebo control group, and during scanning were instructed to rate the valence and memorize a series of positive and negative pictures, which was immediately followed by a recognition memory test. A sad mood induction (film clip) was interspersed six times throughout the encoding and retrieval blocks in order to maintain a negative mood throughout the entire duration of the study. Note that the use of repeated mood inductions throughout the entire experiment prior to both encoding and retrieval tasks may have facilitated mood dependence and mood congruence, both of which could be impacted by duloxetine. This study was therefore excluded in our systematic review for this reason. The authors failed to identify MCM in the behavioral data, and no influence of duloxetine was observed on mood or memory performance. However, duloxetine administration did promote greater activation in the amygdala for happy pictures that were correctly recognized in a sad mood, suggesting duloxetine might facilitate mood-incongruent memory to help alleviate negative emotional memory bias (Tendolkar et al., 2011).
Finally, Ramel et al. (2007) evaluated MCM in mood-induced, never depressed participants compared to a group of remitted depressed individuals. Participants encoded self-referent adjectives before and after a sad mood induction via autobiographical recall and music, with each encoding period immediately followed by a free recall test. MCM was only observed for the remitted depressed group, such that the proportion of recalled positive self-referent words decreased after the mood induction compared to performance on the same task before the mood induction. Regarding neural effects, however, bilateral amygdala response during the encoding of negative words in a sad mood predicted a stronger subsequent recall bias for negative words, but this effect was primarily driven by a small subset of remitted depressed participants (n = 5). Thus, the behavioral and neural analyses produced a dissociated set of MCM findings for positive and negative words. The authors noted that further research is needed to confirm neural mechanisms modulating subsequent memory bias, provided that the neural analyses were primarily restricted to the amygdala, the analyses may have suffered from low statistical power, and words were only presented in valence-consistent blocks of encoding (Ramel et al., 2007).
In sum, surprisingly few neuroimaging studies have attempted to delineate the neural mechanisms subserving mood-induced MCM. Of the studies reviewed here, converging evidence implicates the N400 in mood congruent processing and MCM for linguistic stimuli (Egidi & Nusbaum, 2012; Kiefer et al., 2007) and activation in PFC regions that facilitate mood-congruent encoding and/or retrieval, in particular the OFC and sgACC (Fitzgerald et al., 2011; Lewis et al., 2005). In all these studies, memory was tested on the same day as encoding. A notable exception is a recent study that tested the influence of inhibitory transcranial magnetic stimulation (TMS) on the medial PFC after sad mood induction and prior to the encoding of emotional DRM word lists. On a memory test the next day, medial PFC inhibition decreased the false recognition of negative lures, suggesting that MCM was influenced by the TMS procedure (Bovy et al., 2020). This evidence lends support for a causal role of the medial PFC in promoting long-term negative memory schemas (Figure 3), although changes in neural activation or connectivity were not assessed.
While we focused this section primarily on MCM in nonclinical samples, the neural basis of MCM may also be informed by neuroimaging studies in clinical populations. Hamilton & Gotlib (2008), for instance, presented depressed and nondepressed participants with negative, neutral, and positive pictures. The depressed group remembered negative stimuli significantly better than the nondepressed group in an incidental memory recognition test a week later. Depression was also associated with hyperactive amygdala activation during the successful encoding of negative stimuli, as well as greater connectivity with the hippocampus and dorsal striatum, which may have enhanced negative memory consolidation (Hamilton & Gotlib, 2008). Similarly, compared to healthy controls, depressed people tend to exhibit greater activity in the left amygdala during the retrieval of negative autobiographical memories and less activity during the retrieval of positive autobiographical memories (Young et al., 2016). Accordingly, neurofeedback training that enhances amygdala responses during recall of positive memories has been shown to reduce depressive symptoms and increase subsequent recall of specific positive memories (Young et al., 2017). But identifying the neuropsychological foundation of MCM in clinical samples is challenging when multiple other factors can disrupt memory processing. For instance, chronic stress and anhedonia are linked with dopamine dysfunction, which impairs hippocampal consolidation for positive rewarding stimuli and promotes overgeneralized negative memories (Dillon & Pizzagalli, 2018). This trait-based neural mechanism may fundamentally differ from more state-based MCM effects studied via mood induction. Further research is therefore needed to better pinpoint how mood-related, long-term emotional memory biases are initially formed in the brain.
While an associative network model remains foundational to MCM, this broad theoretical account is unable to specify the precise neural mechanisms that underlie MCM. For instance, it remains unclear what a neural signature of ‘spreading activation’ is. As discussed earlier, some work has used event-related potentials that index semantic relatedness like the N400, but it is uncertain whether spreading activation in an affective sense would necessarily rely on the same mechanism. An understanding of how the brain supports MCM will need to better incorporate the complex interactions among hierarchical systems supporting affective experience and memory storage. As discussed, elucidating the categorical and/or dimensional representation of affect in the brain is a crucial step in this endeavor. Recent innovations in affective neurocomputation that apply machine-learning and graph-theoretic tools to identify emotion- and dimension-specific networks and hubs may provide novel insights in this regard (reviewed in Cowen & Keltner, 2021; Kragel & LaBar, 2016). In particular, these tools provide new ways to identify and track the neural correlates of background mood states over time that then can be related to memory network modulation and behavioral biases.
Likewise, further insight into the organization of memory in the brain may necessitate adjustments to associative network accounts of MCM. Neuroimaging research on memory schemas, for instance, suggests that schema instantiation involves communication between the ventromedial PFC (vmPFC) and the posterior neocortex (Hebscher & Gilboa, 2016). The involvement of the hippocampus in this process during memory formation remains unclear. Some models suggest that greater congruency between newly encoded content and existing schematic representations leads to a shift in memory processing from the hippocampus, which typically supports the binding of elements into a new episodic trace, to the vmPFC, which supports rapid learning of new connections within an existing framework of neocortical representations, (Van Kesteren et al., 2012). Alternatively, other models suggest that bidirectional communication between the hippocampus and PFC is required to facilitate memory integration (for further review, see Gilboa & Marlatte, 2017).
Whether and how such neural interactions might influence MCM via the detection of mood-congruent content remains to be explored. For instance, a PFC-MTL neural system may initially signal mood-emotion congruency, which subsequently assimilates congruent content into an existing network of affective information in the neocortex. The presence of such a domain-general congruence detector might also have important clinical implications. Depressed individuals often interpret ambiguous events with a negative meaning (Everaert et al., 2017) which is thought to have a neural basis in hyperactive responding within the medial PFC, amygdala, and hippocampus (Disner et al., 2011). Successful treatment methods may involve reducing the hypersensitive detection of mood-congruent material by targeting the neural system responsible for initially detecting congruence. Note that this proposal of a domain-general congruence detector differs from Bower’s network theory, which suggests that discrete affective nodes are first activated by one’s mood and then spread activation to closely linked event nodes, thereby lowering the threshold for activation, or detection, of mood-congruent content.
Finally, the cognitive neuroscience of emotional memory has greatly benefited from research assessing the influence of emotion at multiple timepoints in the memory formation process, but this same approach is surprisingly absent from the neuroimaging literature on MCM. Research on the cognitive neuroscience of MCM has yet to integrate with existing neural models of emotional memory bias that involve brainstem-amygdala-hippocampal interactions (LaBar & Cabeza, 2006; Mather et al., 2016; McGaugh, 2018), valence-specific memory storage (Bowen et al., 2018; Kark & Kensinger, 2019), and post-encoding tagging of relevant information (Dunsmoor et al., 2015; Patil et al., 2017; Ritchey et al., 2016). MCM effects are unlikely to be mediated solely by semantic networks in the brain, as these other neurobiological processes have repeatedly been shown to facilitate emotional memory bias. To further discuss these models and illustrate the feasibility of integrating them with our current understanding of MCM, we conclude this review with suggestions for neuroimaging researchers to directly target the influence of mood during emotional memory consolidation.
Mood as a Filter of the Emotional Past
The present review has consistently emphasized the importance of consolidation for MCM at both the behavioral and neural level, which is central to the neuroscience of emotional memory (Faul & LaBar, 2020; LaBar & Cabeza, 2006). However, none of the neuroimaging investigations reviewed here attempted to delineate the specific neural substrates subserving the consolidation of mood-induced, long-term emotional memory bias for encoded stimuli.
The influence of the amygdala on hippocampal storage is a well-documented neurobiological process (McGaugh, 2004) and we suggest that future MCM studies consider using longer delays between encoding and retrieval to accommodate this process. Doing so may be better able to evaluate how mood influences the time-dependent interactions among amygdala, hippocampal, and PFC activity to promote enhanced memory for specific emotional content. Importantly, the association between amygdala-hippocampal connectivity and subsequent emotional memory is strengthened with longer delays (Ritchey et al., 2008). Since mood can enhance memory for mood-congruent material while also impairing memory for mood-incongruent material, a longer delay may allow for these selective effects to emerge more clearly. The selective, competitive processing of information is supported by the release of norepinephrine (NE) from the locus coeruleus (LC) during increased arousal, which interacts with glutamate levels to enhance processing for high-priority stimuli and impair processing for low-priority stimuli (Mather et al., 2016). The PFC is an important regulator of the LC-NE system by evaluating the salience of incoming information, generating glutamate-NE feedback loops, or “hot spots”, that enhance the consolidation of highly-prioritized content (Mather et al., 2016). MCM may utilize a similar mechanism, whereby moods amplify the priority assigned to mood-congruent stimuli and lessen the priority of mood-incongruent stimuli, via this LC-NE system.
We also suggest researchers consider using post-encoding mood inductions to isolate the influence of mood on consolidation more efficiently. Heightened post-encoding arousal has been shown to significantly modulate consolidation processes by increasing hippocampal involvement in memory over time (Atucha et al., 2017; Krenz et al., 2021). More selective biases may also emerge after encoding via emotional experiences that retroactively tag-and-capture specific information to receive enhanced consolidation. A growing body of research on post-encoding conditioning supports this proposal. Dunsmoor et al. (2015) instructed participants to encode a series of neutral pictures in different categories (e.g. animals or tools), which was followed by a fear-conditioning task five minutes after encoding that reinforced one category as threatening and the other category as non-threatening for a completely new set of images. Their findings demonstrated memory enhancement for the neutral items at encoding that were conceptually congruent with whichever category was reinforced after encoding, but only if memory was tested at least six hours later or the next day. These effects were also observed in Hennings et al. (2021) and were recently shown to be mediated by post-encoding hippocampal-cortical functional connectivity (Clewett et al., 2022). Moreover, similar retroactive effects that selectively strengthen memory consolidation have been demonstrated with post-encoding reward manipulations (Braun et al., 2018; Patil et al., 2017).
However, recent attempts by Kalbe and Schwabe (2022) to replicate the retroactive memory effects resulting from aversive learning that were reported in Dunsmoor et al. (2015) and Hennings et al. (2021) have questioned the reliability of these effects. Across four experiments, Kalbe and Schwabe (2022) were consistently unable to find evidence for category-specific retroactive enhancements. Weak evidence was only found in one experiment and in a pooled analysis when focusing on high confidence hits and using corrected recognition scores, although the same analysis with memory sensitivity scores (d’) demonstrated no effect (Kalbe & Schwabe, 2022). Similarly, Oyarzún et al. (2016) were unable to demonstrate retroactive memory enhancement via post-encoding reward-based classical conditioning. Importantly, though, later findings of post-encoding reward effects from Patil et al. (2017) used an operant conditioning paradigm that was performance contingent, while Braun et al. (2018) used a maze exploration task that provided rewards at the end of each reinforced maze. Thus, reward may be more likely to selectively prioritize previously seen information if participants play a more active role in the study. Taken together, these studies therefore suggest that post-encoding fear or reward manipulations may selectively change consolidation processes, although more work is needed to clarify the boundary conditions that produce such retroactive enhancements in memory, which will ultimately help establish the reliability of these effects.
Individual differences in neural connectivity may further moderate how emotional information is selectively stored. Intrinsic post-encoding resting-state functional connectivity, for instance, has been shown to associate with subsequent emotional memory biases. Kark & Kensinger (2019) evaluated changes in resting-state functional connectivity after the encoding of negative, neutral, and positive scenes, finding that post-encoding amygdala-cortical connectivity predicted inter-individual differences in emotional memory bias the next day. Specifically, a negative memory bias associated with post-encoding amygdala-visuosensory connectivity, whereas a positive memory bias associated with amygdala-PFC connectivity. These associations could not be explained by differences in arousal ratings for the stimuli, nor individual differences in univariate activation and connectivity during encoding, emphasizing a valence-driven effect that only emerged in offline consolidation (Kark & Kensinger, 2019). The unique connectivity profile of positively- and negatively-valenced items is consistent with past research demonstrating enhanced consolidation of sensory details for negatively-valenced material which consequently contributes to greater sensory recapitulation at retrieval (Bowen et al., 2018).
That differential patterns of offline neural connectivity facilitate valence-specific biases in subsequent emotional memory suggests post-encoding mood may also moderate early consolidation mechanisms (Figure 1A). Mood may act as a filter on previously encoded emotional stimuli, enhancing the consolidation of mood-congruent items and impairing the consolidation of mood-incongruent items. In a similar vein, post-encoding stress has been shown to act as a mnemonic filter by shifting memory recollection towards greater dependence on amygdala and hippocampal response during encoding. Ritchey et al. (2017) induced stress via the cold-pressor test after the encoding of negative or neutral images. A surprise recognition test the following day revealed that participants who experienced larger increases in cortisol during the stress task were better at remembering items that elicited strong amygdala and hippocampal activity during encoding (Ritchey et al., 2017). Relatedly, when emotional content is tagged during encoding, post-encoding mood may specifically target previously encoded mood-congruent information to receive enhanced consolidation by reactivating the corresponding neural signature. As noted previously, several post-encoding manipulations were identified in our literature review, but most of these studies seemed ill-equipped to properly assess MCM either due to the induction of non-specific increases in arousal (Liu et al., 2008; Nielson & Lorber, 2009; Wang & Ren, 2017) or administering a mood induction far removed from the initial encoding period (Forgas et al., 2005). Post-encoding mood thus remains a particularly compelling area of research for neuroimaging investigators to further explore. Indeed, in light of the neural evidence reviewed here, the presence or absence of MCM resulting from post-encoding mood may provide strong corroborating or contradicting support, respectively, for the validity of associative network theories and tag-and-capture models, or for identifying necessary adjustments that must be made to these theoretical frameworks.
Studying the retroactive effect of mood on encoding may also better generalize to the experience of MCM in natural settings. Moods may not need to co-occur with encoding or retrieval to configure subsequently biased memory or retrospective reports of experienced affect. Natural fluctuations in mood throughout the day may have both retroactive and prospective effects on how emotional events are ultimately remembered. That moods facilitate recall of mood-congruent or mood-incongruent autobiographical events suggests that moods may reactivate memories in a labile state and modify the emotional appraisal of these memories to shift in emotional characteristics. Moods likely induce systematic memory biases by shaping both the consolidation and reconsolidation of emotional experiences (Nader et al., 2000; Sara, 2000). Consistent with this proposal, positive mood induced after negative autobiographical memory retrieval has been shown to decrease the number of negative details subsequently recalled (Piñeyro et al., 2018). Thus, moods may not only bias the initial encoding of an experienced event but may also repeatedly modify the affective qualities of a memory when reactivated in a particular mood.
Conclusions
Studying the interaction of mood and emotion on memory is undoubtedly a challenging endeavor, given the complexities that already arise when considering their independent effects. Despite this challenge, a wealth of research has demonstrated the significant impact of mood on human cognition. Memory is a particularly important construct to examine, as long-term emotional memory biases have clear relevance for psychopathology. Here we have reviewed MCM in mood-induced healthy adults, demonstrating a diverse array of methods that can be used to study its underlying neuropsychological profile. Compared to previous reviews, we found increased reports of MCM in false memory paradigms, suggesting that MCM may not necessarily confer a memory advantage but rather enhanced sensitivity to mood-congruent material. These memory intrusions are most likely to occur for content with a high degree of semantic and emotional overlap with previously encoded stimuli and may have important implications for real-life memory biases, such as overestimating the occurrence of past emotional events. An increased number of studies have also shown that MCM is sensitive to the structure of the encoding/retrieval task, as well as individual differences in trait affect and mood regulation ability. These individual differences may even contribute to shifts from mood-congruent to mood-incongruent memory, a regulatory mechanism that can help distract from negative ruminative thought patterns. Finally, while neural investigations remain limited, converging evidence has implicated the PFC, coupled with lower-level emotion processing circuitry and interactions with the DN, in subserving both mood development and MCM.
In reviewing this literature, we identified several crucial gaps for future research to address. In particular, MCM studies are heavily biased towards short-term effects of mood on memory, a surprising finding considering that enhanced memory for emotional content is known to be dependent upon consolidation mechanisms. As such, we note that many of the studies included in the present review may primarily reflect mood-congruent processing styles and not MCM per se. The reliability of long-term, mood-induced memory biases remains to be empirically confirmed. As such, ongoing work in this field must start incorporating longer delays between encoding and retrieval to allow for consolidation processes to properly unfold prior to memory testing.
Relatedly, to better capture the specific effects of mood on memory formation, more studies should consider administering inductions after encoding rather than immediately before encoding or before retrieval. Disentangling the influence of mood on attention and memory will be an important next step in MCM research but can only be achieved if mood is separated from initial exposure. This proposal builds on recent work that shows how emotional events can retroactively influence the way in which previously experienced content is remembered. Indeed, MCM is likely configured by a combination of both prospective and retrospective mood effects that collectively filter emotional information into memory. Addressing these gaps in the literature will better integrate our understanding of MCM with contemporary neuroscience models of emotional memory bias that are founded upon amygdala-hippocampal consolidation (McGaugh, 2004), glutamate-norepinephrine interactions (Mather et al., 2016), valence-specific neural recapitulation (Bowen et al., 2018), and post-encoding tag-and-capture mechanisms (Dunsmoor et al., 2015; Patil et al., 2017; Ritchey et al., 2017).
Careful consideration should be devoted towards choosing induction techniques that successfully elicit the intended mood, as recent qualitative and quantitative reviews on induction efficacy show that certain techniques work better for some moods than others. A pattern has emerged among induction studies whereby negative inductions are often unable to overcome a baseline positivity bias among healthy participants, while positive inductions may fail to reliably change mood at all. Using neutralizing mood inductions (e.g., watching a neutral movie) at the beginning of the task session may help to attenuate a baseline positivity bias. By targeting the components of mood that sets it apart from emotion—duration, cause, and appraisal—researchers will be better able to successfully manipulate and sustain negative or positive moods in the laboratory. Additionally, we suggest that increased attention should be devoted to using stimulus sets and mood induction materials that represent discrete emotional categories. Many of the studies we reviewed only assessed general positivity and negativity, which may obscure more nuanced effects of MCM for specific emotions. Instances of asymmetrical MCM findings may, for instance, be confounded by a lack of specificity in affect. Although research in this area is limited, exploring the specificity of MCM will help to clarify how the representation of affect contributes to mood congruence, and whether categorical and/or dimensional properties are shared across emotions and moods.
Finally, our review of the literature indicates a scarcity of neuroimaging studies on MCM, despite significant developments in the past two decades in our neurobiological understanding of emotional memory in humans. Implementing neuroimaging methods provides researchers with the opportunity to formally test whether mood indeed modulates the neural circuitry subserving processing and storage of emotional experiences, as well as pinpointing how the neural profile of a sustained mood state compares with that of an emotion. Improving our understanding of the neural substrates involved in MCM will provide evidence for or against associative network accounts, which remain foundational to theoretical perspectives on MCM.
As we have noted, it remains unclear whether affect is truly represented among discrete neural hubs, and whether activation spreads from these hubs to linked emotional content to produce MCM. Advancements in the multivariate decoding of emotional states provide a promising means with which to examine whether distinct categories of affective experience can be reliably separated from one another based on their activation patterns, and whether mood inductions specifically target these same patterns of activity. Graph theoretic approaches may also be able to isolate network hubs that coordinate the development and maintenance of mood states, which may ultimately moderate hippocampal-cortical interactions that selectively consolidate, retrieve, and/or reconsolidate memories for emotional events. Future studies that utilize post-encoding mood inductions will be better able to separate the neural mechanisms tracking mood development from those facilitating the initial encoding of emotional stimuli.
In conclusion, our review of more than three decades of MCM research provides an updated account for a field that is both highly diverse but also ripe for further discovery. Though a well-known phenomenon in the field of psychology, much remains to be explored regarding the fundamental mechanisms that subserve MCM. Continued investigation into the behavioral and neural underpinnings of MCM in the laboratory setting will also aid in developing more valid and generalizable models of mood-induced memory bias experienced in real life and in clinical disorders. Part of this discovery process will entail more properly detailing the separation of emotion and mood at both a behavioral and neural level. We also suggest that researchers build on foundational models of emotional memory formation to more directly target consolidation mechanisms involved in MCM by utilizing longer encoding-retrieval delays and/or incorporating post-encoding inductions to study designs. Doing so will bring a more nuanced cognitive neuroscience perspective to extant associative network models of MCM.
Supplementary Material
Acknowledgments
This work was supported by an NSF Graduate Fellowship award to L.F. and NIH grants R01 MH124112 and R01 MH113238 to K.S.L. This review was not preregistered.
References
- Admon R, & Pizzagalli DA (2015). Corticostriatal pathways contribute to the natural time course of positive mood. Nature Communications, 6. 10.1038/ncomms10065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). In Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. 10.1176/appi.books.9780890425596.893619 [DOI] [Google Scholar]
- Atucha E, Vukojevic V, Fornari RV, Ronzoni G, Demougin P, Peter F, Atsak P, Coolen MW, Papassotiropoulos A, McGaugh JL, De Quervain DJF, & Roozendaal B (2017). Noradrenergic activation of the basolateral amygdala maintains hippocampus-dependent accuracy of remote memory. Proceedings of the National Academy of Sciences of the United States of America, 114(34). 10.1073/pnas.1710819114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baños RM, Medina PM, & Pascual J (2001). Explicit and implicit memory biases in depression and panic disorder. Behaviour Research and Therapy, 39(1), 61–74. 10.1016/S0005-7967(99)00158-8 [DOI] [PubMed] [Google Scholar]
- Beck AT (1967). Depression: Clinical, experimental, and theoretical aspects. Harper & Row. [Google Scholar]
- Beck AT (2008). The evolution of the cognitive model of depression and its neurobiological correlates. In American Journal of Psychiatry (Vol. 165, Issue 8). 10.1176/appi.ajp.2008.08050721 [DOI] [PubMed] [Google Scholar]
- Beedie CJ (2007). In search of empirical distinctions between emotion and mood: A subjective contextual model. In Lane M (Ed.), Mood and human performance: Conceptual, measurement, and applied issues (pp. 63–88). Nova Science Publishers. [Google Scholar]
- Beedie Christopher J., Terry PC, & Lane AM (2005). Distinctions between emotion and mood. Cognition and Emotion, 19(6), 847–878. 10.1080/02699930541000057 [DOI] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, & Jonides J (2011). Depression, rumination and the default network. Social Cognitive and Affective Neuroscience, 6(5), 548–555. 10.1093/scan/nsq080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna C, Leknes S, Holmes EA, Edwards RR, Goodwin GM, & Tracey I (2010). Induction of Depressed Mood Disrupts Emotion Regulation Neurocircuitry and Enhances Pain Unpleasantness. Biological Psychiatry, 67(11), 1083–1090. 10.1016/j.biopsych.2010.01.014 [DOI] [PubMed] [Google Scholar]
- Berntsen D (2002). Tunnel memories for autobiographical events: Central details are remembered more frequently from shocking than from happy experiences. Memory and Cognition, 30(7), 1010–1020. 10.3758/BF03194319 [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, & Conant LL (2009). Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex, 19(12), 2767–2796. 10.1093/cercor/bhp055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland CE, Howe ML, & Knott L (2016). Discrete emotion-congruent false memories in the DRM paradigm. Emotion, 16(5), 611–619. 10.1037/emo0000153 [DOI] [PubMed] [Google Scholar]
- Blaney PH (1986). Affect and Memory. A Review. Psychological Bulletin, 99(2), 229–246. 10.1037/0033-2909.99.2.229 [DOI] [PubMed] [Google Scholar]
- Bless H, & Fiedler K (1995). Affective States and the Influence of Activated General Knowledge. Personality and Social Psychology Bulletin, 21(7). 10.1177/0146167295217010 [DOI] [Google Scholar]
- Boden JM, & Baumeister RF (1997). Repressive Coping: Distraction Using Pleasant Thoughts and Memories. Journal of Personality and Social Psychology, 73(1), 45–62. 10.1037/0022-3514.73.1.45 [DOI] [PubMed] [Google Scholar]
- Bogie BJM, Persaud MR, Smith D, Kapczinski FP, & Frey BN (2019). Explicit emotional memory biases in mood disorders: A systematic review. Psychiatry Research, 278, 162–172. 10.1016/j.psychres.2019.06.003 [DOI] [PubMed] [Google Scholar]
- Bovy L, Berkers RMWJ, Pottkämper JCM, Varatheeswaran R, Fernández G, Tendolkar I, & Dresler M (2020). Transcranial Magnetic Stimulation of the Medial Prefrontal Cortex Decreases Emotional Memory Schemas. Cerebral Cortex, 30(6), 3608–3616. 10.1093/cercor/bhz329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen HJ, Kark SM, & Kensinger EA (2018). NEVER forget: negative emotional valence enhances recapitulation. Psychonomic Bulletin and Review, 25(3), 870–891. 10.3758/s13423-017-1313-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower GH (1981). Mood and memory. American Psychologist, 36(2), 129–148. 10.1037/0003-066X.36.2.129 [DOI] [PubMed] [Google Scholar]
- Bower GH, & Forgas JP (2000). Affect, Memory, and Social Cognition. In Cognition and Emotion. [Google Scholar]
- Bower GH, & Mayer JD (1985). Failure to replicate mood-dependent retrieval. Bulletin of the Psychonomic Society, 23(1). 10.3758/BF03329773 [DOI] [Google Scholar]
- Bower GH, Monteiro KP, & Gilligan SG (1978). Emotional mood as a context for learning and recall. Journal of Verbal Learning and Verbal Behavior, 17(5), 573–585. 10.1016/S0022-5371(78)90348-1 [DOI] [Google Scholar]
- Bradley B, & Mathews A (1983). Negative self-schemata in clinical depression. British Journal of Clinical Psychology, 22(3), 173–181. 10.1111/j.2044-8260.1983.tb00598.x [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, & Reyna VF (2002). Fuzzy-trace theory and false memory. Current Directions in Psychological Science, 11(5), 164–169. 10.1111/1467-8721.00192 [DOI] [Google Scholar]
- Braun EK, Wimmer GE, & Shohamy D (2018). Retroactive and graded prioritization of memory by reward. Nature Communications, 9(1), 4886. 10.1038/s41467-018-07280-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, & Rosen BR (1996). Response and habituation of the human amygdala during visual processing of facial expression. Neuron, 17(5), 875–887. 10.1016/S0896-6273(00)80219-6 [DOI] [PubMed] [Google Scholar]
- Breslow R, Kocsis J, & Belkin B (1981). Contribution of the depressive perspective to memory function in depression. American Journal of Psychiatry, 138(2), 227–230. 10.1176/ajp.138.2.227 [DOI] [PubMed] [Google Scholar]
- Brockmeyer T, Kulessa D, Hautzinger M, Bents H, & Backenstrass M (2015). Mood-incongruent processing during the recall of a sad life event predicts the course and severity of depression. Journal of Affective Disorders, 187, 91–96. 10.1016/j.jad.2015.08.010 [DOI] [PubMed] [Google Scholar]
- Brown JD, & Taylor SE (1986). Affect and the processing of personal information: Evidence for mood-activated self-schemata. Journal of Experimental Social Psychology, 22(5). 10.1016/0022-1031(86)90044-2 [DOI] [Google Scholar]
- Bullington JC (1990). Mood congruent memory: A replication of symmetrical effects for both positive and negative moods. Journal of Social Behavior & Personality, 5(4), 123–134. http://psycnet.apa.org/psycinfo/1991-00341-001 [Google Scholar]
- Bush G, Luu P, & Posner MI (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222. 10.1016/S1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
- Choi HY, Kensinger EA, & Rajaram S (2013). Emotional content enhances true but not false memory for categorized stimuli. Memory and Cognition, 41(3). 10.3758/s13421-012-0269-2 [DOI] [PubMed] [Google Scholar]
- Christopoulos GI, Uy MA, & Yap WJ (2019). The Body and the Brain: Measuring Skin Conductance Responses to Understand the Emotional Experience. Organizational Research Methods, 22(1), 394–420. 10.1177/1094428116681073 [DOI] [Google Scholar]
- Clark DA, Beck AT, Alford BA, Bieling PJ, & Segal ZV (1999). Scientific Foundations of Cognitive Theory and Therapy of Depression. John Wiley & Sons. 10.1891/0889-8391.14.1.100 [DOI] [Google Scholar]
- Clark DM, Teasdale JD, Broadbent DE, & Martin M (1983). Effect of mood on lexical decisions. Bulletin of the Psychonomic Society, 21(3), 175–178. 10.3758/BF03334679 [DOI] [Google Scholar]
- Clewett D, Dunsmoor J, Bachman SL, Phelps EA, & Davachi L (2022). Survival of the salient: Aversive learning rescues otherwise forgettable memories via neural reactivation and post-encoding hippocampal connectivity. Neurobiology of Learning and Memory, 187. 10.1016/j.nlm.2021.107572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore GL, Gasper K, & Garvin E (2001). 4 - Affect as information. Handbook of Affect and Social Cognition. [Google Scholar]
- Clore GL, Wyer RS, Dienes B, Gasper K, Gohm C, & Isbell L (2001). Affective feelings as feedback: Some cognitive consequences. In Theories of mood and cognition: A user’s guidebook (pp. 27–62). [Google Scholar]
- Clore Gerald L., & Storbeck J (2012). Affect as information about liking, efficacy, and importance. In Affect in Social Thinking and Behavior. 10.4324/9780203720752 [DOI] [Google Scholar]
- Collins AM, & Loftus EF (1975). A spreading-activation theory of semantic processing. Psychological Review, 82(6), 407–428. 10.1037/0033-295X.82.6.407 [DOI] [Google Scholar]
- Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, Frank G, Hendren R, Max JE, Paulus MP, Tapert SF, Banerjee D, Simmons AN, & Yang TT (2013). Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biological Psychiatry, 74(12), 898–907. 10.1016/j.biopsych.2013.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SL, & Alloy LB (2018). Negative Event Recall as a Vulnerability for Depression: Relationship Between Momentary Stress-Reactive Rumination and Memory for Daily Life Stress. Clinical Psychological Science, 6(1), 32–47. 10.1177/2167702617729487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney RE, Joormann J, Eugène F, Dennis EL, & Gotlib IH (2010). Neural correlates of rumination in depression. Cognitive, Affective and Behavioral Neuroscience, 10(4), 470–478. 10.3758/CABN.10.4.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen AS, & Keltner D (2021). Semantic Space Theory: A Computational Approach to Emotion. In Trends in Cognitive Sciences (Vol. 25, Issue 2). 10.1016/j.tics.2020.11.004 [DOI] [PubMed] [Google Scholar]
- Dalgleish T, & Werner-Seidler A (2014). Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends in Cognitive Sciences, 18(11), 596–604. 10.1016/j.tics.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Deese J (1959). On the prediction of occurrence of particular verbal intrusions in immediate recall. Journal of Experimental Psychology, 58(1), 17–22. 10.1037/h0046671 [DOI] [PubMed] [Google Scholar]
- Demiray B, & Freund AM (2017). The psychological distance of memories: Examining causal relations with mood and self-esteem in young, middle-aged and older adults. Consciousness and Cognition, 49, 117–131. 10.1016/j.concog.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Dillon DG, & Pizzagalli DA (2018). Mechanisms of Memory Disruption in Depression. Trends in Neurosciences, 41(3), 137–149. 10.1016/j.tins.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Direnfeld DM, & Roberts JE (2006). Mood congruent memory in Dysphoria: The roles of state affect and cognitive style. Behaviour Research and Therapy, 44(9), 1275–1285. 10.1016/j.brat.2005.03.014 [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EAP, & Beck AT (2011). Neural mechanisms of the cognitive model of depression. In Nature Reviews Neuroscience (Vol. 12, Issue 8). 10.1038/nrn3027 [DOI] [PubMed] [Google Scholar]
- Dixon ML, Thiruchselvam R, Todd R, & Christoff K (2017). Emotion and the prefrontal cortex: An integrative review. Psychological Bulletin, 143(10), 1033–1081. 10.1037/bul0000096 [DOI] [PubMed] [Google Scholar]
- Drače Sasa. (2013). Evidence for the role of affect in mood congruent recall of autobiographic memories. Motivation and Emotion, 37(3), 623–628. 10.1007/s11031-012-9322-5 [DOI] [Google Scholar]
- Drače Saša, & Desrichard O (2013). Mood congruence effect in autobiographical recall: Is mood a mediator? Psihologija, 46(3), 217–228. 10.2298/PSI130525001D [DOI] [Google Scholar]
- Drače Saša, Efendić E, & Marić N (2015). The effect of negative mood intensity on autobiographical recall: Evidence for the underlying role of affect in mood congruence effect. Review of Psychology, 22(1–2), 3–9. 10.21465/rp0022.0001 [DOI] [Google Scholar]
- Dreisbach G (2006). How positive affect modulates cognitive control: The costs and benefits of reduced maintenance capability. Brain and Cognition, 60(1), 11–19. 10.1016/j.bandc.2005.08.003 [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, & Trimble M (2008). The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums, 13(8), 663–681. 10.1017/S1092852900013754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar GC, & Lishman WA (1984). Depression, recognition-memory and hedonic tone: a signal detection analysis. British Journal of Psychiatry, 144(4), 376–382. 10.1192/bjp.144.4.376 [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Murty VP, Davachi L, & Phelps EA (2015). Emotional learning selectively and retroactively strengthens memories for related events. Nature, 520(7547), 345–348. 10.1038/nature14106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyser FA, van Eijndhoven PFP, Bergman MA, Collard RM, Schene AH, Tendolkar I, & Vrijsen JN (2020). Negative memory bias as a transdiagnostic cognitive marker for depression symptom severity. Journal of Affective Disorders, 274, 1165–1172. 10.1016/j.jad.2020.05.156 [DOI] [PubMed] [Google Scholar]
- Egidi G, & Nusbaum HC (2012). Emotional language processing: How mood affects integration processes during discourse comprehension. Brain and Language, 122(3), 199–210. 10.1016/j.bandl.2011.12.008 [DOI] [PubMed] [Google Scholar]
- Ehrlichman H, & Halpern JN (1988). Affect and Memory: Effects of Pleasant and Unpleasant Odors on Retrieval of Happy and Unhappy Memories. Journal of Personality and Social Psychology, 55(5), 769–779. 10.1037/0022-3514.55.5.769 [DOI] [PubMed] [Google Scholar]
- Eich E (1995). Searching for Mood Dependent Memory. Psychological Science, 6(2), 67–75. 10.1111/j.1467-9280.1995.tb00309.x [DOI] [Google Scholar]
- Eich E, & Macaulay D (2000). Are real moods required to reveal mood-congruent and mood-dependent memory? Psychological Science, 11(3), 244–248. 10.1111/1467-9280.00249 [DOI] [PubMed] [Google Scholar]
- Eich E, Macaulay D, & Ryan L (1994). Mood Dependent Memory for Events of the Personal Past. Journal of Experimental Psychology: General, 123(2), 201–215. 10.1037/0096-3445.123.2.201 [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, & Dolan RJ (2002). The neural basis of mood-congruent processing biases in depression. Archives of General Psychiatry, 59(7), 597–604. 10.1001/archpsyc.59.7.597 [DOI] [PubMed] [Google Scholar]
- Ellis HC, Thomas RL, McFarland AD, & Lane JW (1985). Emotional Mood States and Retrieval in Episodic Memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 11(2). 10.1037/0278-7393.11.2.363 [DOI] [PubMed] [Google Scholar]
- Ellwart T, Rinck M, & Becker ES (2003). Selective memory and memory deficits in depressed inpatients. Depression and Anxiety, 17(4), 197–206. 10.1002/da.10102 [DOI] [PubMed] [Google Scholar]
- Erber R, & Erber MW (1994). Beyond mood and social judgment: Mood incongruent recall and mood regulation. European Journal of Social Psychology, 24(1), 79–88. 10.1002/ejsp.2420240106 [DOI] [Google Scholar]
- Everaert J, Podina IR, & Koster EHW (2017). A comprehensive meta-analysis of interpretation biases in depression. In Clinical Psychology Review (Vol. 58). 10.1016/j.cpr.2017.09.005 [DOI] [PubMed] [Google Scholar]
- Faul L, & LaBar KS (2020). Emotional Memory in the Human Brain. In Carew TJ (Ed.), The Oxford Handbook of the Neurobiology of Learning and Memory. Oxford University Press. 10.1093/oxfordhb/9780190069162.013.2 [DOI] [Google Scholar]
- Feldman Barrett L (2017). How emotions are made: The secret life of the brain. In How emotions are made: The secret life of the brain. [Google Scholar]
- Fernández-Aguilar L, Navarro-Bravo B, Ricarte J, Ros L, & Latorre JM (2019). How effective are films in inducing positive and negative emotional states? A meta-analysis. PLoS ONE, 14(11). 10.1371/journal.pone.0225040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A, Urwicz L, Vuilleumier P, & Berna C (2022). Impact of hypnosis on psychophysiological measures: A scoping literature review. In American Journal of Clinical Hypnosis (Vol. 64, Issue 1). 10.1080/00029157.2021.1873099 [DOI] [PubMed] [Google Scholar]
- Fiedler K (1991). On the Task, the Measures and the Mood in Research on Affect and Social Cognition. In Emotion and Social Judgments. 10.4324/9781003058731-6 [DOI] [Google Scholar]
- Fiedler K (2001). Affective states trigger processes of assimilation and accommodation. In Theories of mood and cognition: A user’s guidebook. [Google Scholar]
- Fiedler K, Nickel S, Asbeck J, & Pagel U (2003). Mood and the generation effect. Cognition and Emotion, 17(4), 585–608. 10.1080/02699930302301 [DOI] [PubMed] [Google Scholar]
- Fiedler K, Nickel S, Muehlfriedel T, & Unkelbach C (2001). Is Mood Congruency an Effect of Genuine Memory or Response Bias? Journal of Experimental Social Psychology, 37(3), 201–214. 10.1006/jesp.2000.1442 [DOI] [Google Scholar]
- Fitzgerald DA, Arnold JF, Becker ES, Speckens AEM, Rinck M, Rijpkema M, Fernández G, & Tendolkar I (2011). How mood challenges emotional memory formation: An fMRI investigation. NeuroImage, 56(3), 1783–1790. 10.1016/j.neuroimage.2011.02.061 [DOI] [PubMed] [Google Scholar]
- Forgas JP (1989). Mood effects on decision making strategies. Australian Journal of Psychology, 41(2), 197–214. 10.1080/00049538908260083 [DOI] [Google Scholar]
- Forgas JP (1995). Mood and judgment: The affect infusion model (AIM). Psychological Bulletin, 117(1), 39–66. 10.1037/0033-2909.117.1.39 [DOI] [PubMed] [Google Scholar]
- Forgas JP (1998). Asking nicely? The effects of mood on responding to more or less polite requests. Personality and Social Psychology Bulletin, 24(2), 173–185. 10.1177/0146167298242006 [DOI] [Google Scholar]
- Forgas JP, & Eich E (2012). Affective Influences on Cognition - Mood Congruence, Mood Dependence, and Mood Effects on Processing Strategies. In Handbook of psychology - Experimental psychology (pp. 61–82). [Google Scholar]
- Forgas JP, Laham SM, & Vargas PT (2005). Mood effects on eyewitness memory: Affective influences on susceptibility to misinformation. Journal of Experimental Social Psychology, 41(6), 574–588. 10.1016/j.jesp.2004.11.005 [DOI] [Google Scholar]
- Fredrickson BL, & Branigan C (2005). Positive emotions broaden the scope of attention and thought-action repertoires. Cognition and Emotion, 19(3), 313–332. 10.1080/02699930441000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, & Kahneman D (1993). Duration Neglect in Retrospective Evaluations of Affective Episodes. Journal of Personality and Social Psychology, 65(1), 45–55. 10.1037/0022-3514.65.1.45 [DOI] [PubMed] [Google Scholar]
- Frijda NH (1994). Varieties of affect: Emotions and episodes, moods, and sentiments. In The Nature of Emotion: Fundamental Questions (pp. 197–202). [Google Scholar]
- Fultz J, Schaller M, & Cialdini RB (1988). Empathy, Sadness, and Distress Three Related but Distinct Vicarious Affective Responses to Another’s Suffering. Personality and Social Psychology Bulletin, 14(2), 312–325. 10.1177/014616728801400201 [DOI] [PubMed] [Google Scholar]
- Gaddy MA, & Ingram RE (2014). A meta-analytic review of mood-congruent implicit memory in depressed mood. Clinical Psychology Review, 34(5), 402–416. 10.1016/j.cpr.2014.06.001 [DOI] [PubMed] [Google Scholar]
- Gallo DA, Foster KT, & Johnson EL (2009). Elevated False Recollection of Emotional Pictures in Young and Older Adults. Psychology and Aging, 24(4). 10.1037/a0017545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayle MC (1997). Mood-congruency in recall: The potential effect of arousal. Journal of Social Behavior and Personality, 12(2), 471–480. [Google Scholar]
- Ghosh VE, & Gilboa A (2014). What is a memory schema? A historical perspective on current neuroscience literature. In Neuropsychologia (Vol. 53, Issue 1). 10.1016/j.neuropsychologia.2013.11.010 [DOI] [PubMed] [Google Scholar]
- Ghosh VE, Moscovitch M, Colella BM, & Gilbo A (2014). Schema representation in patients with ventromedial PFC lesions. Journal of Neuroscience, 34(36). 10.1523/JNEUROSCI.0740-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A, Alain C, He Y, Stuss DT, & Moscovitch M (2009). Ventromedial prefrontal cortex lesions produce early functional alterations during remote memory retrieval. Journal of Neuroscience, 29(15). 10.1523/JNEUROSCI.5210-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A, & Marlatte H (2017). Neurobiology of Schemas and Schema-Mediated Memory. Trends in Cognitive Sciences, 21(8), 618–631. 10.1016/j.tics.2017.04.013 [DOI] [PubMed] [Google Scholar]
- Gillies JCP, & Dozois DJA (2021). How long do mood induction procedure (MIP) primes really last? Implications for cognitive vulnerability research. Journal of Affective Disorders, 292. 10.1016/j.jad.2021.05.047 [DOI] [PubMed] [Google Scholar]
- Gilligan S ., & Bower G . (1985). Cognitive consequences of emotional arousal. In Emotions, cognition, and behavior. (pp. 547–588). Cambridge University Press. [Google Scholar]
- Gotlib IH, & Joormann J (2010). Cognition and Depression: Current Status and Future Directions. Annual Review of Clinical Psychology, 6(1), 285–312. 10.1146/annurev.clinpsy.121208.131305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Kasch KL, Traill S, Arnow BA, Joormann J, & Johnson SL (2004). Coherence and specificity of information-processing biases in depression and social phobia. Journal of Abnormal Psychology, 113(3), 386–398. 10.1037/0021-843X.113.3.386 [DOI] [PubMed] [Google Scholar]
- Greenberg J, & Meiran N (2014a). Is mindfulness meditation associated with “Feeling Less?” Mindfulness, 5(5), 471–476. 10.1007/s12671-013-0201-2 [DOI] [Google Scholar]
- Greenberg J, & Meiran N (2014b). The role of emotional engagement and mood valence in retrieval fluency of mood incongruent autobiographical memory. Frontiers in Psychology, 5(FEB). 10.3389/fpsyg.2014.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, & Khosla M (2006). Is Mood Congruency an Effect of Affective State? National Academcy of Psychology, India, 51(4), 269–274. [Google Scholar]
- Hamilton JP, & Gotlib IH (2008). Neural Substrates of Increased Memory Sensitivity for Negative Stimuli in Major Depression. Biological Psychiatry, 63(12), 1155–1162. 10.1016/j.biopsych.2007.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CH, & Shantz CA (1995). Emotion-Specific Priming: Congruence Effects on Affect and Recognition Across Negative Emotions. Personality and Social Psychology Bulletin, 21(6), 548–557. 10.1177/0146167295216001 [DOI] [Google Scholar]
- Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, & Yücel M (2008). Modulation of brain resting-state networks by sad mood induction. PLoS ONE, 3(3). 10.1371/journal.pone.0001794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig T, Nyberg L, Nilsson LG, & Gärling T (1999). Testing for mood congruent recall with environmentally induced mood. Journal of Environmental Psychology, 19(4), 353–367. 10.1006/jevp.1999.0142 [DOI] [Google Scholar]
- Hastie R, & Kumar PA (1979). Person memory: Personality traits as organizing principles in memory for behaviors. Journal of Personality and Social Psychology, 37(1). 10.1037/0022-3514.37.1.25 [DOI] [Google Scholar]
- Hebscher M, & Gilboa A (2016). A boost of confidence: The role of the ventromedial prefrontal cortex in memory, decision-making, and schemas. Neuropsychologia, 90. 10.1016/j.neuropsychologia.2016.05.003 [DOI] [PubMed] [Google Scholar]
- Hennings AC, Lewis-Peacock JA, & Dunsmoor JE (2021). Emotional learning retroactively enhances item memory but distorts source attribution. Learning and Memory, 28(6). 10.1101/LM.053371.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel PT, Maydon A, Cottle J, & Vrijsen JN (2017). Cognitive Bias Modification: Retrieval Practice to Simulate and Oppose Ruminative Memory Biases. Clinical Psychological Science, 5(1), 122–130. 10.1177/2167702616649366 [DOI] [Google Scholar]
- Hills PJ, Werno MA, & Lewis MB (2011). Sad people are more accurate at face recognition than happy people. Consciousness and Cognition, 20(4), 1502–1517. 10.1016/j.concog.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Hockey GRJ, Maule AJ, Clough PJ, & Bdzola L (2000). Effects of negative mood states on risk in everyday decision making. Cognition and Emotion, 14(6), 823–856. 10.1080/02699930050156654 [DOI] [Google Scholar]
- Holland AC, & Kensinger EA (2010). Emotion and autobiographical memory. Physics of Life Reviews, 7(1), 88–131. 10.1016/j.plrev.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston D, & Haddock G (2007). On auditing auditory information: The influence of mood on memory for music. Psychology of Music, 35(2), 201–212. 10.1177/0305735607070303 [DOI] [Google Scholar]
- Hsieh S, & Lin SJ (2019). The Dissociable Effects of Induced Positive and Negative Moods on Cognitive Flexibility. Scientific Reports, 9(1). 10.1038/s41598-018-37683-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RE (1984). Toward an information-processing analysis of depression. Cognitive Therapy and Research, 8(5), 443–477. 10.1007/BF01173284 [DOI] [Google Scholar]
- Itoh M (2004). Mood-congruent encoding effect in self-and other-referent judgments. Psychologia, 47(3), 145–157. 10.2117/psysoc.2004.145 [DOI] [Google Scholar]
- Jiang H, White MP, Greicius MD, Waelde LC, & Spiegel D (2017). Brain activity and functional connectivity associated with hypnosis. Cerebral Cortex, 27(8). 10.1093/cercor/bhw220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, & Gotlib IH (2010). Emotion regulation in depression: Relation to cognitive inhibition. Cognition and Emotion, 24(2). 10.1080/02699930903407948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, & Siemer M (2004). Memory Accessibility, Mood Regulation, and Dysphoria: Difficulties in Repairing Sad Mood with Happy Memories? Journal of Abnormal Psychology, 113(2), 179–188. 10.1037/0021-843X.113.2.179 [DOI] [PubMed] [Google Scholar]
- Joseph DL, Chan MY, Heintzelman SJ, Tay L, Diener E, & Scotney VS (2020). The Manipulation of Affect: A Meta-Analysis of Affect Induction Procedures. Psychological Bulletin. 10.1037/bul0000224 [DOI] [PubMed] [Google Scholar]
- Josephson BR, Singer JA, & Salovey P (1996). Mood regulation and memory: Repairing sad moods with happy memories. Cognition and Emotion, 10(4), 437–444. 10.1080/026999396380222 [DOI] [Google Scholar]
- Kalbe F, & Schwabe L (2022). On the search for a selective and retroactive strengthening of memory: Is there evidence for category-specific behavioral tagging? Journal of Experimental Psychology. General, 151(1). 10.1037/xge0001075 [DOI] [PubMed] [Google Scholar]
- Kark SM, & Kensinger EA (2019). Post-Encoding Amygdala-Visuosensory Coupling Is Associated with Negative Memory Bias in Healthy Young Adults. The Journal of Neuroscience, 39(16), 3130–3143. 10.1523/jneurosci.2834-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar K, Gameiro RR, & König P (2015). Feeling good, searching the bad: Positive priming increases attention and memory for negative stimuli on webpages. Computers in Human Behavior, 53, 332–343. 10.1016/j.chb.2015.07.020 [DOI] [Google Scholar]
- Kassam KS, Markey AR, Cherkassky VL, Loewenstein G, & Just MA (2013). Identifying Emotions on the Basis of Neural Activation. PLoS ONE, 8(6). 10.1371/journal.pone.0066032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketal R (1975). Affect, mood, emotion, and feeling: semantic considerations. American Journal of Psychiatry, 132(11), 1215–1217. 10.1176/ajp.132.11.1215 [DOI] [PubMed] [Google Scholar]
- Kiefer M (2002). The N400 is modulated by unconsciously perceived masked words: Further evidence for an automatic spreading activation account of N400 priming effects. Cognitive Brain Research, 13(1), 27–39. 10.1016/S0926-6410(01)00085-4 [DOI] [PubMed] [Google Scholar]
- Kiefer M, Schuch S, Schenck W, & Fiedler K (2007). Mood states modulate activity in semantic brain areas during emotional word encoding. Cerebral Cortex, 17(7), 1516–1530. 10.1093/cercor/bhl062 [DOI] [PubMed] [Google Scholar]
- Kihlstrom JF (2012). The domain of hypnosis, revisited. In The Oxford Handbook of Hypnosis: Theory, Research, and Practice. 10.1093/oxfordhb/9780198570097.013.0002 [DOI] [Google Scholar]
- Kirkby LA, Luongo FJ, Lee MB, Nahum M, Van Vleet TM, Rao VR, Dawes HE, Chang EF, & Sohal VS (2018). An Amygdala-Hippocampus Subnetwork that Encodes Variation in Human Mood. Cell, 175(6), 1688–1700.e14. 10.1016/j.cell.2018.10.005 [DOI] [PubMed] [Google Scholar]
- Klaassen T, Riedel WJ, Deutz NEP, & Van Praag HM (2002). Mood congruent memory bias induced by tryptophan depletion. Psychological Medicine, 32(1), 167–172. 10.1017/S003329170100438X [DOI] [PubMed] [Google Scholar]
- Knight BG, Maines ML, & Robinson GS (2002). The effects of sad mood on memory in older adults: A test of the mood congruence effect. Psychology and Aging, 17(4), 653–661. 10.1037/0882-7974.17.4.653 [DOI] [PubMed] [Google Scholar]
- Knott LM, & Thorley C (2014). Mood-congruent false memories persist over time. Cognition and Emotion, 28(5), 903–912. 10.1080/02699931.2013.860016 [DOI] [PubMed] [Google Scholar]
- Köhler CA, Carvalho AF, Alves GS, McIntyre RS, Hyphantis TN, & Cammarota M (2015). Autobiographical Memory Disturbances in Depression: A Novel Therapeutic Target? Neural Plasticity, 2015. 10.1155/2015/759139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Falkenberg I, Kellermann T, Eickhoff SB, Gur RC, & Habel U (2013). Neural correlates of effective and ineffective mood induction. Social Cognitive and Affective Neuroscience, 9(6), 864–872. 10.1093/scan/nst055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kop WJ, Synowski SJ, Newell ME, Schmidt LA, Waldstein SR, & Fox NA (2011). Autonomic nervous system reactivity to positive and negative mood induction: The role of acute psychological responses and frontal electrocortical activity. Biological Psychology, 86(3), 230–238. 10.1016/j.biopsycho.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel PA, & LaBar KS (2016). Decoding the Nature of Emotion in the Brain. Trends in Cognitive Sciences, 20(6), 444–455. 10.1016/j.tics.2016.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenz V, Sommer T, Alink A, Roozendaal B, & Schwabe L (2021). Noradrenergic arousal after encoding reverses the course of systems consolidation in humans. Nature Communications, 12(1). 10.1038/s41467-021-26250-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijsters A, Redi J, de Ruyter B, & Heynderickx I (2016). Inducing sadness and anxiousness through visual media: Measurement techniques and persistence. Frontiers in Psychology, 7(AUG). 10.3389/fpsyg.2016.01141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Hemsley DR, Cotter PA, Checkley SA, & Gray JA (1998). Haloperidol-induced Mood and Retrieval of Happy and Unhappy Memories. Cognition and Emotion, 12(4), 497–508. 10.1080/026999398379538 [DOI] [Google Scholar]
- Kwiatkowski SJ, & Parkinson SR (1994). Depression, elaboration, and mood congruence: Differences between natural and induced mood. Memory & Cognition, 22(2), 225–233. 10.3758/BF03208893 [DOI] [PubMed] [Google Scholar]
- LaBar KS, & Cabeza R (2006). Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience, 7(1), 54–64. 10.1038/nrn1825 [DOI] [PubMed] [Google Scholar]
- Lench HC, Flores SA, & Bench SW (2011). Discrete emotions predict changes in cognition, judgment, experience, behavior, and physiology: A meta-analysis of experimental emotion elicitations. Psychological Bulletin, 137(5), 834–855. 10.1037/a0024244 [DOI] [PubMed] [Google Scholar]
- Lewis PA, & Critchley HD (2003). Mood-dependent memory. Trends in Cognitive Sciences, 7(10), 431–433. 10.1016/j.tics.2003.08.005 [DOI] [PubMed] [Google Scholar]
- Lewis PA, Critchley HD, Smith AP, & Dolan RJ (2005). Brain mechanisms for mood congruent memory facilitation. NeuroImage, 25(4), 1214–1223. 10.1016/j.neuroimage.2004.11.053 [DOI] [PubMed] [Google Scholar]
- Lindquist KA (2013). Emotions emerge from more basic psychological ingredients: A modern psychological constructionist model. Emotion Review, 5(4), 356–368. 10.1177/1754073913489750 [DOI] [Google Scholar]
- Liu DLJ, Graham S, & Zorawski M (2008). Enhanced selective memory consolidation following post-learning pleasant and aversive arousal. Neurobiology of Learning and Memory, 89(1), 36–46. 10.1016/j.nlm.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Loeffler SN, Myrtek M, & Peper M (2013). Mood-congruent memory in daily life: Evidence from interactive ambulatory monitoring. Biological Psychology, 93(2), 308–315. 10.1016/j.biopsycho.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Martin EA, & Kerns JG (2011). The influence of positive mood on different aspects of cognitive control. Cognition and Emotion, 25(2), 265–279. 10.1080/02699931.2010.491652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MA, & Metha A (1997). Recall of early childhood memories through musical mood induction. Arts in Psychotherapy. 10.1016/s0197-4556(97)00020-8 [DOI] [Google Scholar]
- Mather M, Clewett D, Sakaki M, & Harley CW (2016). Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behavioral and Brain Sciences, 39. 10.1017/S0140525X15000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, & Sutherland MR (2011). Arousal-biased competition in perception and memory. Perspectives on Psychological Science, 6(2), 114–133. 10.1177/1745691611400234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt GE, Vázquez C, & Campbell WK (1992). Mood-congruent recall of affectively toned stimuli: A meta-analytic review. Clinical Psychology Review, 12(2), 227–255. 10.1016/0272-7358(92)90116-P [DOI] [Google Scholar]
- Mayer JD, McCormick LJ, & Strong SE (1995). Mood-Congruent Memory and Natural Mood: New Evidence. Personality and Social Psychology Bulletin, 21(7), 736–746. 10.1177/0146167295217008 [DOI] [Google Scholar]
- McDowall J (1984). Recall of pleasant and unpleasant words in depressed subjects. Journal of Abnormal Psychology, 93(4), 401–407. 10.1037/0021-843X.93.4.401 [DOI] [PubMed] [Google Scholar]
- McFarland C, & Buehler R (1998). The impact of negative affect on autobiographical memory: the role of self-focused attention to moods. Journal of Personality and Social Psychology, 75(6), 1424–1440. 10.1037/0022-3514.75.6.1424 [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Cahill L, & Roozendaal B (1996). Involvement of the amygdala in memory storage: Interaction with other brain systems. Proceedings of the National Academy of Sciences, 93(24), 13508–13514. 10.1073/pnas.93.24.13508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh James L. (1966). Time-dependent processes in memory storage. Science, 153(3742), 1351–1358. 10.1126/science.153.3742.1351 [DOI] [PubMed] [Google Scholar]
- McGaugh James L. (2004). The Amygdala Modulates the Consolidation of Memories of Emotionally Arousing Experiences. Annual Review of Neuroscience, 27(1), 1–28. 10.1146/annurev.neuro.27.070203.144157 [DOI] [PubMed] [Google Scholar]
- McGaugh James L. (2018). Emotional arousal regulation of memory consolidation. Current Opinion in Behavioral Sciences, 19, 55–60. 10.1016/j.cobeha.2017.10.003 [DOI] [Google Scholar]
- McIntyre RS, Cha DS, Soczynska JK, Woldeyohannes HO, Gallaugher LA, Kudlow P, Alsuwaidan M, & Baskaran A (2013). Cognitive deficits and functional outcomes in major depressive disorder: Determinants, substrates, and treatment interventions. Depression and Anxiety, 30(6), 515–527. 10.1002/da.22063 [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, & Droppleman LF (1971). Manual for the POMS. Educational and Industrial Testing Service. [Google Scholar]
- Meeks JT, Taul ML, Rice RA, Posey ZW, & Harper NR (2019). Negative Mood Reduces Negative False Memories After a Brief Mindfulness Exercise. Mindfulness, 10(12), 2507–2521. 10.1007/s12671-019-01223-6 [DOI] [Google Scholar]
- Mill A, Realo A, & Allik J (2016). Retrospective ratings of emotions: The Effects of Age, Daily Tiredness, and Personality. Frontiers in Psychology, 6(JAN). 10.3389/fpsyg.2015.02020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, & Kihlstrom JF (2005). Mood congruence in childhood and recent autobiographical memory. Cognition and Emotion, 19(7), 981–998. 10.1080/02699930500202967 [DOI] [Google Scholar]
- Miron-Shatz T, Stone A, & Kahneman D (2009). Memories of Yesterday’s Emotions: Does the Valence of Experience Affect the Memory-Experience Gap? Emotion, 9(6), 885–891. 10.1037/a0017823 [DOI] [PubMed] [Google Scholar]
- Mitchell RLC, & Phillips LH (2007). The psychological, neurochemical and functional neuroanatomical mediators of the effects of positive and negative mood on executive functions. Neuropsychologia, 45(4), 617–629. 10.1016/j.neuropsychologia.2006.06.030 [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler MT, Fu CHY, Dalton JA, Andrew CM, & Williams SCR (2007). A functional MRI study of happy and sad affective states induced by classical music. Human Brain Mapping, 28(11), 1150–1162. 10.1002/hbm.20337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler RT, Rabi R, & Minda JP (2010). Better mood and better performance: Learning rule-described categories is enhanced by positive mood. Psychological Science, 21(12), 1770–1776. 10.1177/0956797610387441 [DOI] [PubMed] [Google Scholar]
- Nasby W (1994). Moderators of Mood-congruent Encoding: Self-/Other-reference and Affirmative/ Nonaffirmative Judgement. Cognition and Emotion, 8(3). 10.1080/02699939408408941 [DOI] [Google Scholar]
- Nasby W (1996). Moderators of mood-congruent encoding and judgement: Evidence that elated and depressed moods implicate distinct processes. Cognition and Emotion, 10(4), 361–377. 10.1080/026999396380187 [DOI] [Google Scholar]
- Nielson KA, & Lorber W (2009). Enhanced post-learning memory consolidation is influenced by arousal predisposition and emotion regulation but not by stimulus valence or arousal. Neurobiology of Learning and Memory, 92(1), 70–79. 10.1016/j.nlm.2009.03.002 [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, & Saarimäki H (2019). Emotions as discrete patterns of systemic activity. Neuroscience Letters, 693, 3–8. 10.1016/j.neulet.2017.07.012 [DOI] [PubMed] [Google Scholar]
- Öner S, & Gülgöz S (2018). Autobiographical remembering regulates emotions: a functional perspective. Memory, 26(1), 15–28. 10.1080/09658211.2017.1316510 [DOI] [PubMed] [Google Scholar]
- Öngür D, Ferry AT, & Price JL (2003). Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology, 460(3), 425–449. 10.1002/cne.10609 [DOI] [PubMed] [Google Scholar]
- Ortony A, & Clore GL (1989). Emotions, Moods, and Conscious Awareness Comment on Johnson-Laird and Oatley’s “The Language of Emotions: An Analysis of a Semantic Field.” Cognition and Emotion, 3(2), 125–137. 10.1080/02699938908408076 [DOI] [Google Scholar]
- Oyarzún JP, Packard PA, de Diego-Balaguer R, & Fuentemilla L (2016). Motivated encoding selectively promotes memory for future inconsequential semantically-related events. Neurobiology of Learning and Memory, 133. 10.1016/j.nlm.2016.05.005 [DOI] [PubMed] [Google Scholar]
- Parrott WG (1991). Mood Induction and Instructions to Sustain Moods: A Test of the Subject Compliance Hypothesis of Mood Congruent Memory. Cognition and Emotion, 5(1), 41–52. 10.1080/02699939108411022 [DOI] [Google Scholar]
- Parrott WG, & Sabini J (1990). Mood and Memory Under Natural Conditions: Evidence for Mood Incongruent Recall. Journal of Personality and Social Psychology, 59(2), 321–336. 10.1037/0022-3514.59.2.321 [DOI] [Google Scholar]
- Patil A, Murty VP, Dunsmoor JE, Phelps EA, & Davachi L (2017). Reward retroactively enhances memory consolidation for related items. Learning and Memory, 24(1), 65–69. 10.1101/lm.042978.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereg D, & Mikulincer M (2004). Attachment Style and the Regulation of Negative Affect: Exploring Individual Differences in Mood Congruency Effects on Memory and Judgment. Personality and Social Psychology Bulletin, 30(1), 67–80. 10.1177/0146167203258852 [DOI] [PubMed] [Google Scholar]
- Piñeyro M, Ferrer Monti RI, Díaz H, Bueno AM, Bustos SG, & Molina VA (2018). Positive emotional induction interferes with the reconsolidation of negative autobiographical memories, in women only. Neurobiology of Learning and Memory, 155, 508–518. 10.1016/j.nlm.2018.08.003 [DOI] [PubMed] [Google Scholar]
- Pliner P, & Steverango C (1994). Effect of induced mood on memory for flavors. Appetite, 22(2), 135–148. 10.1006/appe.1994.1013 [DOI] [PubMed] [Google Scholar]
- Qin J, Ogle CM, & Goodman GS (2008). Adults’ Memories of Childhood: True and False Reports. Journal of Experimental Psychology: Applied, 14(4), 373–391. 10.1037/a0014309 [DOI] [PubMed] [Google Scholar]
- Rafienia P, Azadfallah P, Fathi-Ashtiani A, & Rasoulzadeh-Tabatabaiei K (2008). The role of extraversion, neuroticism and positive and negative mood in emotional information processing. Personality and Individual Differences, 44(2), 392–402. 10.1016/j.paid.2007.08.018 [DOI] [Google Scholar]
- Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, & McQuaid JR (2007). Amygdala Reactivity and Mood-Congruent Memory in Individuals at Risk for Depressive Relapse. Biological Psychiatry, 61(2), 231–239. 10.1016/j.biopsych.2006.05.004 [DOI] [PubMed] [Google Scholar]
- Raz A (2011). Hypnosis: A twilight zone of the top-down variety. Few have never heard of hypnosis but most know little about the potential of this mind-body regulation technique for advancing science. In Trends in Cognitive Sciences (Vol. 15, Issue 12). 10.1016/j.tics.2011.10.002 [DOI] [PubMed] [Google Scholar]
- Rholes WS, Riskind JH, & Lane JW (1987). Emotional States and Memory Biases: Effects of Cognitive Priming and Mood. Journal of Personality and Social Psychology, 52(1), 91–99. 10.1037/0022-3514.52.1.91 [DOI] [PubMed] [Google Scholar]
- Ribeiro FS, Santos FH, Albuquerque PB, & Oliveira-Silva P (2019). Emotional induction through music: Measuring cardiac and electrodermal responses of emotional states and their persistence. Frontiers in Psychology, 10(MAR). 10.3389/fpsyg.2019.00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout N, Noreen A, & Johal J (2009). Memory for emotional faces in naturally occurring dysphoria and induced sadness. Behaviour Research and Therapy, 47(10), 851–860. 10.1016/j.brat.2009.06.013 [DOI] [PubMed] [Google Scholar]
- Rimmele U, Davachi L, Petrov R, Dougal S, & Phelps EA (2011). Emotion Enhances the Subjective Feeling of Remembering, Despite Lower Accuracy for Contextual Details. Emotion, 11(3). 10.1037/a0024246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinck M, & Becker ES (2005). A comparison of attentional biases and memory biases in women with social phobia and major depression. Journal of Abnormal Psychology, 114(1), 62–74. 10.1037/0021-843X.114.1.62 [DOI] [PubMed] [Google Scholar]
- Rinck M, Glowalla U, & Schneider K (1992). Mood-congruent and mood-incongruent learning. Memory & Cognition, 20(1), 29–39. 10.3758/BF03208251 [DOI] [PubMed] [Google Scholar]
- Rinner MTB, Meyer AH, Mikoteit T, Hoyer J, Imboden C, Hatzinger M, Bader K, Lieb R, Miché M, Wersebe H, & Gloster AT (2019). General or specific? The memory–experience gap for individuals diagnosed with a major depressive disorder or a social phobia diagnosis, and individuals without such diagnoses. Memory, 27(9), 1194–1203. 10.1080/09658211.2019.1640252 [DOI] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, & Cabeza R (2008). Role of amygdala connectivity in the persistence of emotional memories over time: An event-related fMRI investigation. Cerebral Cortex, 18(11), 2494–2504. 10.1093/cercor/bhm262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, McCullough AM, Ranganath C, & Yonelinas AP (2017). Stress as a mnemonic filter: Interactions between medial temporal lobe encoding processes and post-encoding stress. Hippocampus, 27(1), 77–88. 10.1002/hipo.22674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, Murty VP, & Dunsmoor JE (2016). Adaptive memory systems for remembering the salient and the seemingly mundane. The Behavioral and Brain Sciences, 39, e221. 10.1017/S0140525X15001922 [DOI] [PubMed] [Google Scholar]
- Robinson MD, & Clore GL (2002). Belief and feeling: Evidence for an accessibility model of emotional self-report. Psychological Bulletin, 128(6), 934–960. 10.1037/0033-2909.128.6.934 [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Charney DR, Overstreet C, Vytal K, & Grillon C (2012). The adaptive threat bias in anxiety: Amygdala-dorsomedial prefrontal cortex coupling and aversive amplification. NeuroImage, 60(1), 523–529. 10.1016/j.neuroimage.2011.11.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger HL, Balota DA, & Watson JM (2004). Spreading activation and arousal of false memories. The Nature of Remembering: Essays in Honor of Robert G. Crowder, 95–115. 10.1037/10394-006 [DOI] [Google Scholar]
- Roediger HL, & McDermott KB (1995). Creating False Memories: Remembering Words Not Presented in Lists. Journal of Experimental Psychology: Learning, Memory, and Cognition, 21(4), 803–814. 10.1037/0278-7393.21.4.803 [DOI] [Google Scholar]
- Roediger HL, Watson JM, Mcdermott KB, & Gallo DA (2001). Factors that determine false recall: A multiple regression analysis. Psychonomic Bulletin and Review, 8(3). 10.3758/BF03196177 [DOI] [PubMed] [Google Scholar]
- Rowe G, Hirsh JB, & Anderson AK (2007). Positive affect increases the breadth of attentional selection. Proceedings of the National Academy of Sciences of the United States of America, 104(1), 383–388. 10.1073/pnas.0605198104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruci L, Tomes J, & Zelenski J (2009). Mood-congruent false memories in the DRM paradigm. Cognition and Emotion, 23(6), 1153–1165. 10.1080/02699930802355420 [DOI] [Google Scholar]
- Rudebeck PH, Putnam PT, Daniels TE, Yang T, Mitz AR, Rhodes SEV, & Murray EA (2014). A role for primate subgenual cingulate cortex in sustaining autonomic arousal. Proceedings of the National Academy of Sciences of the United States of America, 111(14), 5391–5396. 10.1073/pnas.1317695111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusting CL (1999). Interactive effects of personality and mood on emotion-congruent memory and judgment. Journal of Personality and Social Psychology, 77(5), 1073–1086. 10.1037/0022-3514.77.5.1073 [DOI] [PubMed] [Google Scholar]
- Rusting CL, & DeHart T (2000). Retrieving positive memories to regulate negative mood: Consequences for mood-congruent memory. Journal of Personality and Social Psychology, 78(4), 737–752. 10.1037/0022-3514.78.4.737 [DOI] [PubMed] [Google Scholar]
- Sakaki M (2004). Effects of self-complexity on mood-incongruent recall. Japanese Psychological Research, 46(2), 127–134. 10.1111/j.0021-5368.2004.00244.x [DOI] [PubMed] [Google Scholar]
- Sakaki M (2007). Mood and recall of autobiographical memory: The effect of focus of self-knowledge. Journal of Personality, 75(3), 421–450. 10.1111/j.1467-6494.2007.00444.x [DOI] [PubMed] [Google Scholar]
- Salovey P, & Singer JA (1989). Mood congruency effects in recall of childhood versus recent memories. Journal of Social Behavior and Personality, 4, 99–120. [Google Scholar]
- Sato H, & Kawahara J ichiro. (2011). Selective bias in retrospective self-reports of negative mood states. Anxiety, Stress and Coping, 24(4), 359–367. 10.1080/10615806.2010.543132 [DOI] [PubMed] [Google Scholar]
- Scharnowski F, Nicholson AA, Pichon S, Rosa MJ, Rey G, Eickhoff SB, Van De Ville D, Vuilleumier P, & Koush Y (2020). The role of the subgenual anterior cingulate cortex in dorsomedial prefrontal–amygdala neural circuitry during positive-social emotion regulation. Human Brain Mapping, 41(11), 3100–3118. 10.1002/hbm.25001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schredl M, Hebel ME, Klütsch RC, & Liehe LJ (2009). The Role of Mood Congruency Memory Effects in Dream Recall: A Pilot Study. Dreaming, 19(2), 113–118. 10.1037/a0016295 [DOI] [Google Scholar]
- Schümann D, Bayer J, Talmi D, & Sommer T (2018). Dissociation of immediate and delayed effects of emotional arousal on episodic memory. Neurobiology of Learning and Memory, 148, 11–19. 10.1016/j.nlm.2017.12.007 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Joëls M, Roozendaal B, Wolf OT, & Oitzl MS (2012). Stress effects on memory: An update and integration. Neuroscience and Biobehavioral Reviews, 36(7), 1740–1749. 10.1016/j.neubiorev.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Schwarz N (2012). Feelings-as-information theory. In Handbook of Theories of Social Psychology : Volume 1 (pp. 289–308). 10.4135/9781446249215.n15 [DOI] [Google Scholar]
- Schwarz N, & Clore GL (1983). Mood, misattribution, and judgments of well-being: Informative and directive functions of affective states. Journal of Personality and Social Psychology, 45(3). 10.1037/0022-3514.45.3.513 [DOI] [Google Scholar]
- Sedikides C (1992). Mood as a Determinant of Attentional Focus. Cognition and Emotion, 6(2), 129–148. 10.1080/02699939208411063 [DOI] [Google Scholar]
- Setliff AE, & Marmurek HHC (2002). The mood regulatory function of autobiographical recall is moderated by self-esteem. Personality and Individual Differences, 32(4), 761–771. 10.1016/S0191-8869(01)00078-2 [DOI] [Google Scholar]
- Shaw J, & Porter S (2015). Constructing Rich False Memories of Committing Crime. Psychological Science, 26(3). 10.1177/0956797614562862 [DOI] [PubMed] [Google Scholar]
- Siedlecka E, & Denson TF (2019). Experimental Methods for Inducing Basic Emotions: A Qualitative Review. Emotion Review, 11(1), 87–97. 10.1177/1754073917749016 [DOI] [Google Scholar]
- Siemer M (2009). Mood experience: Implications of a dispositional theory of moods. Emotion Review, 1(3), 256–263. 10.1177/1754073909103594 [DOI] [Google Scholar]
- Simpson S, & Sheldon S (2020). Testing the impact of emotional mood and cue characteristics on detailed autobiographical memory retrieval. Emotion, 20(6), 965–979. 10.1037/emo0000603 [DOI] [PubMed] [Google Scholar]
- Singer JA, & Salovey P (1988). Mood and memory: Evaluating the network theory of affect. Clinical Psychology Review, 8(2), 211–251. 10.1016/0272-7358(88)90060-8 [DOI] [Google Scholar]
- Smith SM, & Petty RE (1995). Personality Moderators of Mood Congruency Effects on Cognition: The Role of Self-Esteem and Negative Mood Regulation. Journal of Personality and Social Psychology, 68(6), 1092–1107. 10.1037/0022-3514.68.6.1092 [DOI] [PubMed] [Google Scholar]
- Snyder M, & White P (1982). Moods and Memories: Elation, depression, and the remembering of the events of one’s life. Journal of Personality, 50(2). 10.1111/j.1467-6494.1982.tb01020.x [DOI] [PubMed] [Google Scholar]
- Spalding KN, Jones SH, Duff MC, Tranel D, & Warren DE (2015). Investigating the neural correlates of schemas: Ventromedial prefrontal cortex is necessary for normal schematic influence on memory. Journal of Neuroscience, 35(47). 10.1523/JNEUROSCI.2767-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasojević J, & Alloy LB (2001). Rumination as a Common Mechanism Relating Depressive Risk Factors to Depression. Emotion, 1(1), 25–37. 10.1037/1528-3542.1.1.25 [DOI] [PubMed] [Google Scholar]
- Stanton SJ, Reeck C, Huettel SA, & LaBar KS (2014). Effects of induced moods on economic choices. Judgment and Decision Making, 9(2), 167–175. [Google Scholar]
- Stea JN, Lee SM, & Sears CR (2013). Enhancement of false memory for negative material in dysphoria: Mood congruency or response bias? Cognitive Therapy and Research, 37(6), 1189–1200. 10.1007/s10608-013-9557-9 [DOI] [Google Scholar]
- Storbeck J, & Clore GL (2005). With sadness comes accuracy; with happiness, false memory: Mood and the false memory effect. Psychological Science, 16(10), 785–791. 10.1111/j.1467-9280.2005.01615.x [DOI] [PubMed] [Google Scholar]
- Storbeck J, & Clore GL (2011). Affect Influences False Memories at Encoding: Evidence from Recognition Data. Emotion, 11(4), 981–989. 10.1037/a0022754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhrmann A, Dohm K, Kugel H, Zwanzger P, Redlich R, Grotegerd D, Rauch AV, Arolt V, Heindel W, Suslow T, Zwitserlood P, & Dannlowski U (2013). Mood-congruent amygdala responses to subliminally presented facial expressions in major depression: Associations with anhedonia. Journal of Psychiatry and Neuroscience, 38(4), 249–258. 10.1503/jpn.120060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA (2012). The mechanisms underlying overgeneral autobiographical memory: An evaluative review of evidence for the CaR-FA-X model. Clinical Psychology Review, 32(1), 34–48. 10.1016/j.cpr.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Li A, Li B, & Wang H (2018). “Pain is forgotten where gain follows:” The masking effect of positive outcomes on emotional suffering. Asian Journal of Social Psychology, 21(3), 178–186. 10.1111/ajsp.12218 [DOI] [Google Scholar]
- Talmi D (2013). Enhanced Emotional Memory: Cognitive and Neural Mechanisms. Current Directions in Psychological Science, 22(6), 430–436. 10.1177/0963721413498893 [DOI] [Google Scholar]
- Tamir M, & Robinson MD (2007). The happy spotlight: Positive mood and selective attention to rewarding information. Personality and Social Psychology Bulletin, 33(8), 1124–1136. 10.1177/0146167207301030 [DOI] [PubMed] [Google Scholar]
- Teasdale JD, & Russell ML (1983). Differential effects of induced mood on the recall of positive, negative and neutral words. British Journal of Clinical Psychology, 22(3). 10.1111/j.2044-8260.1983.tb00597.x [DOI] [PubMed] [Google Scholar]
- Teasdale JD, & Taylor R (1981). Induced mood and accessibility of memories: An effect of mood state or of induction procedure? British Journal of Clinical Psychology, 20(1). 10.1111/j.2044-8260.1981.tb00494.x [DOI] [Google Scholar]
- Tendolkar I, Van Wingen G, Urner M, Jan Verkes R, & Fernández G (2011). Short-term duloxetine administration affects neural correlates of mood-congruent memory. Neuropsychopharmacology, 36(11), 2266–2275. 10.1038/npp.2011.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhune DB, Cleeremans A, Raz A, & Lynn SJ (2017). Hypnosis and top-down regulation of consciousness. In Neuroscience and Biobehavioral Reviews (Vol. 81). 10.1016/j.neubiorev.2017.02.002 [DOI] [PubMed] [Google Scholar]
- Tesoriero M, & Rickard NS (2012). Music-enhanced recall: An effect of mood congruence, emotion arousal or emotion function? Musicae Scientiae, 16(3), 340–356. 10.1177/1029864912459046 [DOI] [Google Scholar]
- Thompson T, Terhune DB, Oram C, Sharangparni J, Rouf R, Solmi M, Veronese N, & Stubbs B (2019). The effectiveness of hypnosis for pain relief: A systematic review and meta-analysis of 85 controlled experimental trials. In Neuroscience and Biobehavioral Reviews (Vol. 99). 10.1016/j.neubiorev.2019.02.013 [DOI] [PubMed] [Google Scholar]
- Thorley C, Dewhurst SA, Abel JW, & Knott LM (2016). Eyewitness memory: The impact of a negative mood during encoding and/or retrieval upon recall of a non-emotive event. Memory, 24(6), 838–852. 10.1080/09658211.2015.1058955 [DOI] [PubMed] [Google Scholar]
- Tobias BA, Kihlstrom JF, & Schacter DL (1992). Emotion and implicit memory. In The handbook of emotion and memory: Research and theory. (pp. 67–92). Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Tulving E (1983). Elements of Episodic Memory. Oxford University Press. [Google Scholar]
- Unsworth N, & Brewer GA (2010). Individual differences in false recall: A latent variable analysis. Journal of Memory and Language, 62(1), 19–34. 10.1016/j.jml.2009.08.002 [DOI] [Google Scholar]
- Urban EJ, Charles ST, Levine LJ, & Almeida DM (2018). Depression history and memory bias for specific daily emotions. PLoS ONE, 13(9). 10.1371/journal.pone.0203574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kesteren MTR, Ruiter DJ, Fernández G, & Henson RN (2012). How schema and novelty augment memory formation. In Trends in Neurosciences (Vol. 35, Issue 4). 10.1016/j.tins.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Varner LJ, & Ellis HC (1998). Cognitive activity and physiological arousal: Processes that mediate mood-congruent memory. Memory and Cognition, 26(5), 939–950. 10.3758/BF03201174 [DOI] [PubMed] [Google Scholar]
- Veenstra L, Schneider IK, & Koole SL (2017). Embodied mood regulation: the impact of body posture on mood recovery, negative thoughts, and mood-congruent recall. Cognition and Emotion, 31(7), 1361–1376. 10.1080/02699931.2016.1225003 [DOI] [PubMed] [Google Scholar]
- Vrijsen JN, Dainer-Best J, Witcraft SM, Papini S, Hertel P, Beevers CG, Becker ES, & Smits JAJ (2019). Effect of cognitive bias modification-memory on depressive symptoms and autobiographical memory bias: two independent studies in high-ruminating and dysphoric samples. Cognition and Emotion, 33(2), 288–304. 10.1080/02699931.2018.1450225 [DOI] [PubMed] [Google Scholar]
- Vrijsen JN, Hertel PT, & Becker ES (2016). Practicing Emotionally Biased Retrieval Affects Mood and Establishes Biased Recall a Week Later. Cognitive Therapy and Research, 40(6), 764–773. 10.1007/s10608-016-9789-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijsen JN, Vogel S, Arias-Vásquez A, Frankea B, Fernández G, Becker ES, Speckens A, & Oostrom I. van. (2015). Depressed patients in remission show an interaction between variance in the mineralocorticoid receptor NR3C2 gene and childhood trauma on negative memory bias. Psychiatric Genetics, 25(3), 99–105. 10.1097/YPG.0000000000000081 [DOI] [PubMed] [Google Scholar]
- Vytal KE, Overstreet C, Charney DR, Robinson OJ, & Grillon C (2014). Sustained anxiety increases amygdala-dorsomedial prefrontal coupling: A mechanism for maintaining an anxious state in healthy adults. Journal of Psychiatry and Neuroscience, 39(5), 321–329. 10.1503/jpn.130145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadlinger HA, & Isaacowitz DM (2006). Positive mood broadens visual attention to positive stimuli. Motivation and Emotion, 30(1), 87–99. 10.1007/s11031-006-9021-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, & Ren Y (2017). Effect of post-encoding emotion on recollection and familiarity for pictures. Quarterly Journal of Experimental Psychology, 70(7), 1236–1253. 10.1080/17470218.2016.1178311 [DOI] [PubMed] [Google Scholar]
- Wang L, LaBar KS, & McCarthy G (2006). Mood Alters Amygdala Activation to Sad Distractors During an Attentional Task. Biological Psychiatry, 60(10), 1139–1146. 10.1016/j.biopsych.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6). 10.1037//0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Westermann R, Spies K, Stahl G, & Hesse FW (1996). Relative effectiveness and validity of mood induction procedures: A meta-analysis. European Journal of Social Psychology, 26(4), 557–580. [DOI] [Google Scholar]
- Wisco BE, & Nolen-Hoeksema S (2009). The interaction of mood and rumination in depression: Effects on mood maintenance and mood-congruent autobiographical memory. Journal of Rational - Emotive and Cognitive - Behavior Therapy, 27(3), 144–159. 10.1007/s10942-009-0096-y [DOI] [Google Scholar]
- Woudstra S, van Tol MJ, Bochdanovits Z, van der Wee NJ, Zitman FG, van Buchem MA, Opmeer EM, Aleman A, Penninx BW, Veltman DJ, & Hoogendijk WJ (2013). Modulatory Effects of the Piccolo Genotype on Emotional Memory in Health and Depression. PLoS ONE, 8(4). 10.1371/journal.pone.0061494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyer RS, Clore GL, & Isbell LM (1999). Affect and Information Processing. Advances in Experimental Social Psychology, 31, 1–77. 10.1016/S0065-2601(08)60271-3 [DOI] [Google Scholar]
- Xie W, & Zhang W (2018). Mood-dependent retrieval in visual long-term memory: dissociable effects on retrieval probability and mnemonic precision. Cognition and Emotion, 32(4). 10.1080/02699931.2017.1340261 [DOI] [PubMed] [Google Scholar]
- Young CB, & Nusslock R (2016). Positive mood enhances reward-related neural activity. Social Cognitive and Affective Neuroscience, 11(6), 934–944. 10.1093/scan/nsw012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KD, Siegle GJ, Bodurka J, & Drevets WC (2016). Amygdala activity during autobiographical memory recall in depressed and vulnerable individuals: Association with symptom severity and autobiographical overgenerality. American Journal of Psychiatry, 173(1), 78–89. 10.1176/appi.ajp.2015.15010119 [DOI] [PubMed] [Google Scholar]
- Young KD, Siegle GJ, Zotev V, Phillips R, Misaki M, Yuan H, Drevets WC, & Bodurka J (2017). Randomized clinical trial of real-time fMRI amygdala neurofeedbackfor major depressive disorder: Effectson symptoms and autobiographical memory recall. American Journal of Psychiatry, 174(8), 748–755. 10.1176/appi.ajp.2017.16060637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Gross J, & Hayne H (2017). The effect of mood on false memory for emotional DRM word lists. Cognition and Emotion, 31(3), 526–537. 10.1080/02699931.2016.1138930 [DOI] [PubMed] [Google Scholar]
- Zhang W, Gross J, & Hayne H (2018). If You’re Happy and You Know It: Positive Moods Reduce Age-Related Differences in False Memory. Child Development, 89(4), e332–e341. 10.1111/cdev.12890 [DOI] [PubMed] [Google Scholar]
- Zhang W, Gross J, & Hayne H (2019). Mood impedes monitoring of emotional false memories: evidence for the associative theories. Memory, 27(2), 198–208. 10.1080/09658211.2018.1498107 [DOI] [PubMed] [Google Scholar]
- Zhong L, Wang Y, Kan H, & Ding J (2020). Virtual Reality Experiments on Emotional Face Recognition Find No Evidence of Mood-Congruent Effects. Frontiers in Psychology, 11. 10.3389/fpsyg.2020.00479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HX, Chen X, Shen YQ, Li L, Chen NX, Zhu ZC, Castellanos FX, & Yan CG (2020). Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. NeuroImage, 206. 10.1016/j.neuroimage.2019.116287 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhao Y, Ybarra O, Stephan WG, & Yang Q (2015). Enhanced memory for both threat and neutral information under conditions of intergroup threat. Frontiers in Psychology, 6(NOV). 10.3389/fpsyg.2015.01759 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


