Abstract
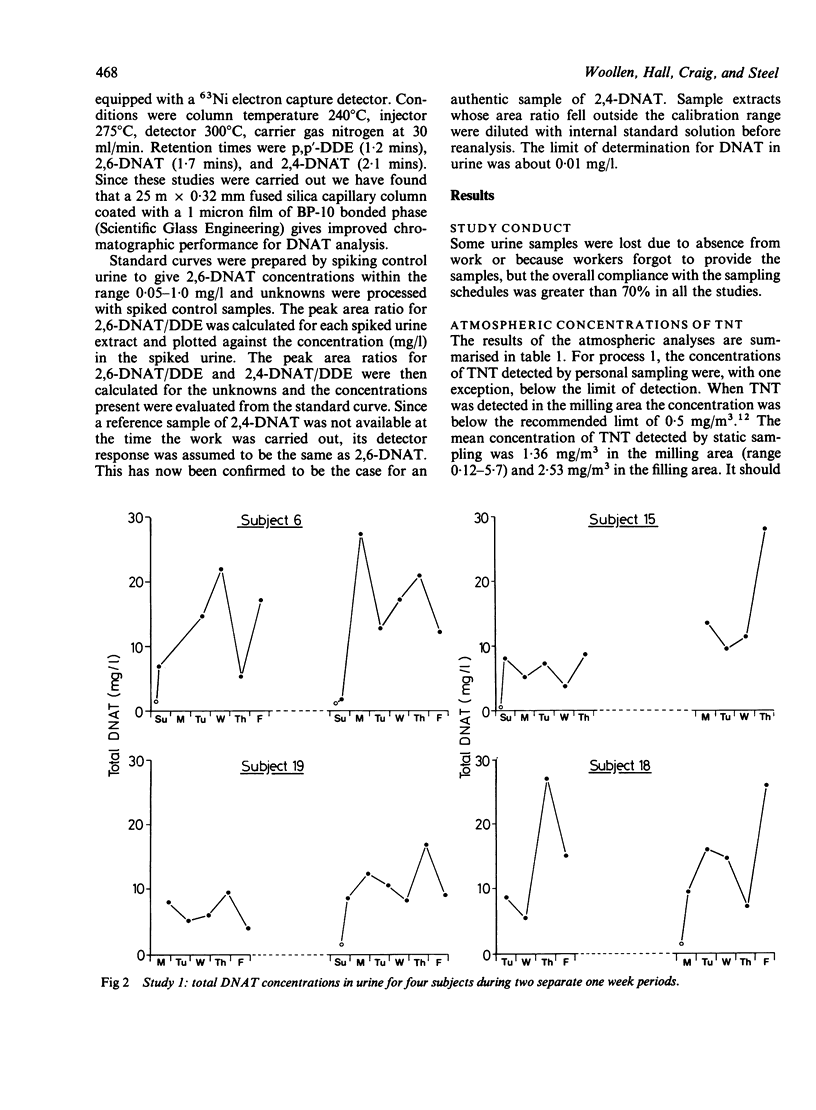
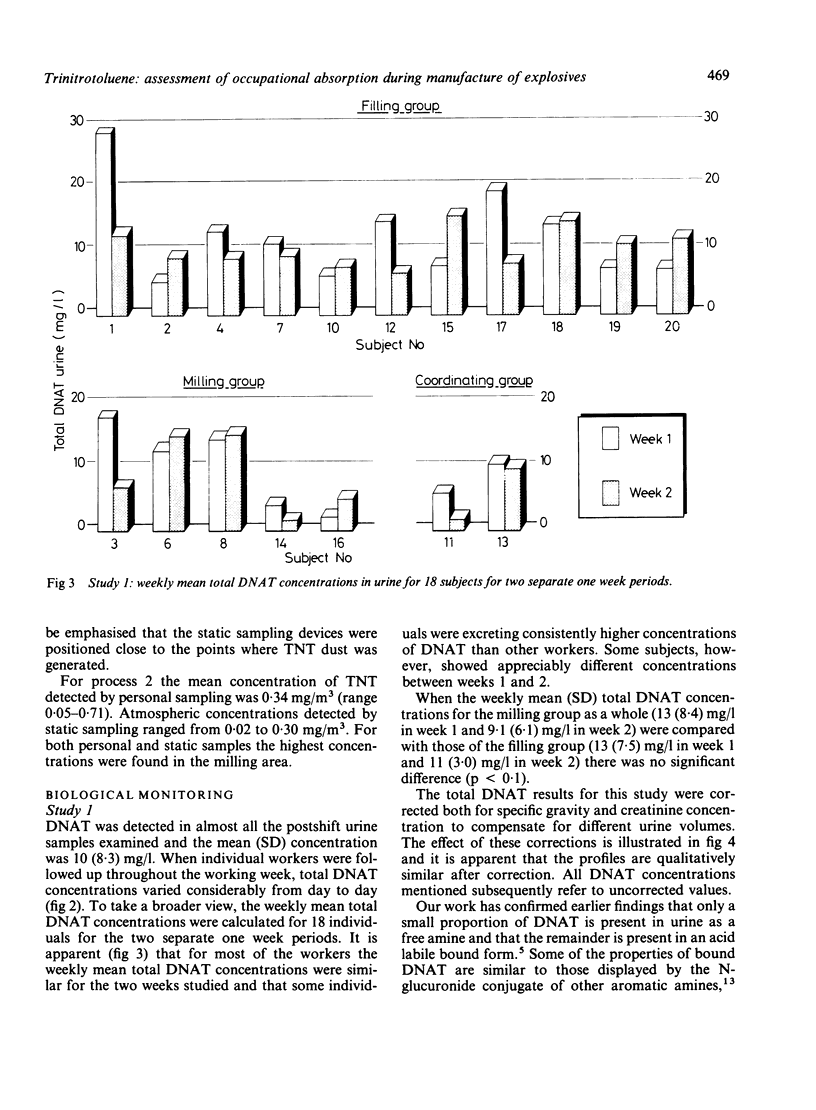
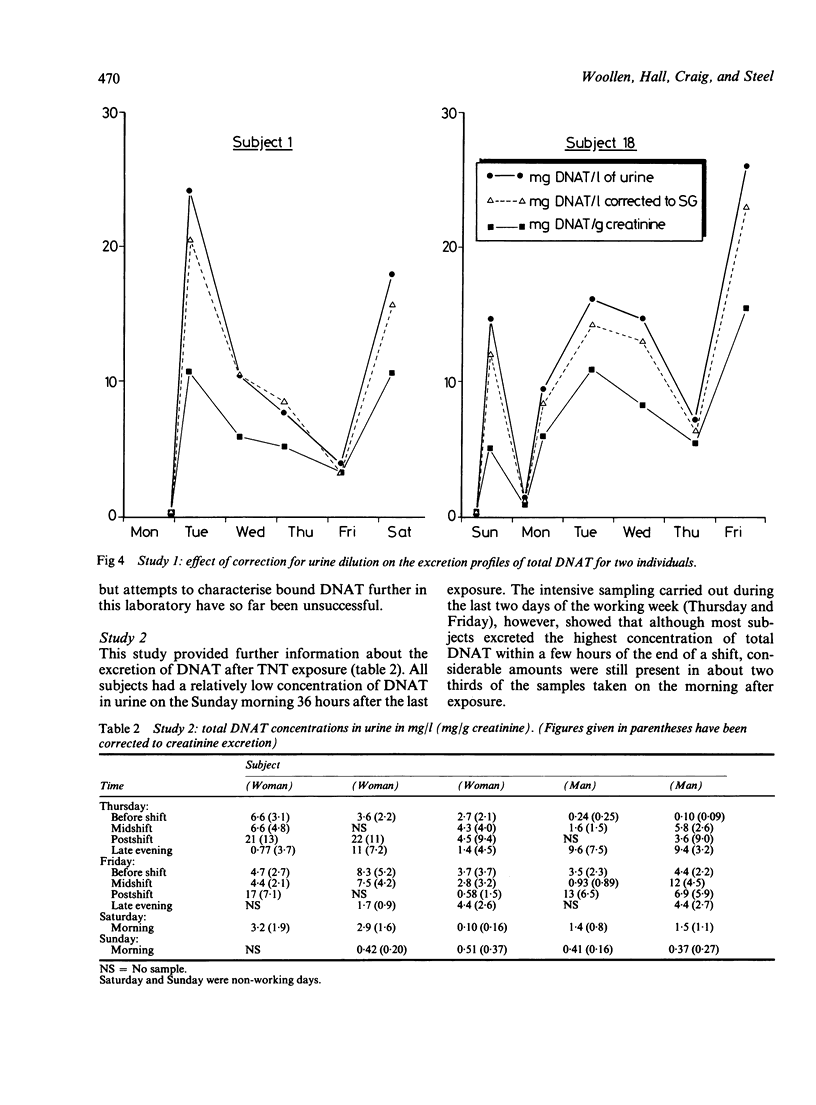
Trinitrotoluene (TNT) absorption was assessed in groups of workers at two explosives factories by measuring the urinary concentrations of dinitroaminotoluene (DNAT) metabolites. DNAT was detected in most of the urine samples analysed and for postshift samples the mean (SD) concentration was 9.7 (7.9) mg/l (range 0.1-44 mg/l (n = 219)). Individual workers showed substantial day to day variations in DNAT concentrations in postshift urine samples, but on a group basis the concentrations remained fairly constant throughout the working week. Preshift urine samples taken at the beginning of a working week showed low concentrations of DNAT which initially suggested that the elimination of TNT metabolites is fairly rapid. A survey carried out of preshift and postshift urine samples collected from a group of workers for a full working week showed wide variations in the rate of clearance of TNT metabolites from the body and in some cases higher concentrations of metabolites were seen in the samples taken the morning after exposure. When urine samples were collected from the same group of workers after 17 days away from the workplace DNAT was still detectable in samples from eight of the nine subjects, indicating that a proportion of TNT or its metabolites is slowly excreted. When five subjects were monitored more intensively during two workshifts TNT was shown to be absorbed rapidly during the exposure period. In most cases the highest concentrations were seen in the postshift urine samples but significant proportions were still present in samples taken the morning after exposure. Atmospheric levels of TNT were found to be too low to account for the observed excretion of DNAT and dermal uptake rather than inhalation appears to be the major route of absorption.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYLAND E., MANSON D., ORR S. F. The biochemistry of aromatic amines. II. The conversion of arylamines into arylsulphamic acids and arylamine-N-glucosiduronic acids. Biochem J. 1957 Mar;65(3):417–423. doi: 10.1042/bj0650417a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon H. J., Mills G. T., Williams R. T. The metabolism of 2:4:6-trinitrotoluene (alpha-T.N.T.). Biochem J. 1944;38(1):70–85. doi: 10.1042/bj0380070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway J. A. Trinitrotoluene: a review of reported dose-related effects providing documentation for a workplace standard. J Occup Med. 1977 May;19(5):341–345. [PubMed] [Google Scholar]
- Morton A. R., Ranadive M. V., Hathaway J. A. Biological effects of trinitrotoluene from exposure below the threshold limit value. Am Ind Hyg Assoc J. 1976 Jan;37(1):56–60. doi: 10.1080/0002889768507408. [DOI] [PubMed] [Google Scholar]
- Soter N. A., Wasserman S. I., Austen K. F. Cold urticaria: release into the circulation of histamine and eosinophil chemotactic factor of anaphylaxis during cold challenge. N Engl J Med. 1976 Mar 25;294(13):687–690. doi: 10.1056/NEJM197603252941302. [DOI] [PubMed] [Google Scholar]


