Abstract
With a rich variety of chemical energy sources and steep physical and chemical gradients, hydrothermal vent systems offer a range of habitats to support microbial life. Cultivation-dependent and independent studies have led to an emerging view that diverse microorganisms in deep-sea hydrothermal vents live their chemolithoautotrophic, heterotrophic, or mixotrophic life with versatile metabolic strategies. Biogeochemical processes are mediated by microorganisms, and notably, processes involving or coupling the carbon, sulfur, hydrogen, nitrogen, and metal cycles in these unique ecosystems. Here, we review the taxonomic and physiological diversity of microbial prokaryotic life from cosmopolitan to endemic taxa and emphasize their significant roles in the biogeochemical processes in deep-sea hydrothermal vents. According to the physiology of the targeted taxa and their needs inferred from meta-omics data, the media for selective cultivation can be designed with a wide range of physicochemical conditions such as temperature, pH, hydrostatic pressure, electron donors and acceptors, carbon sources, nitrogen sources, and growth factors. The application of novel cultivation techniques with real-time monitoring of microbial diversity and metabolic substrates and products are also recommended.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42995-020-00086-4.
Keywords: Deep-sea hydrothermal vents, Cultivation, Diversity, Biogeochemical cycle
Introduction
The discovery of deep-sea hydrothermal vents in the late 1970s expanded our knowledge of the extent of life on Earth (Corliss et al. 1979). Hydrothermal-vent environments include positively buoyant and neutrally buoyant (lateral) hydrothermal plumes (∼2 °C), low-temperature hydrothermal fluids (∼5–100 °C), high-temperature hydrothermal fluids (∼150–400 °C), sulfide rock, basalt, and pelagic and metalliferous sediments. Active deep-sea hydrothermal vents, which are spread along mid-ocean ridges, back-arc basins, and volcanic arcs, occur in different geological contexts, characterized by the presence of different rock types, including basalts, peridotites, and felsic rocks. The associated hydrothermal fluids exhibit substantial chemical variability, depending largely on the compositional differences among the underlying rocks (Amend et al. 2011). Hydrothermal end-member fluids of basalt-hosted systems are usually characterized by greater sulfide than hydrogen concentrations, resulting from magma degassing and high-temperature-leaching of host rocks. In contrast, owing to serpentinization processes, the end-member fluids of ultramafic-hosted vent systems usually exhibit greater hydrogen (normal 10–20 mmol/L, some up to 154 mmol/L) and methane (at mmol/L levels) concentrations than sulfide concentrations (Adam and Perner 2018; Charlou et al. 2002; Perner et al. 2013). Because of the mixing processes with the oxygenated ambient seawater, deep-sea hydrothermal fluids of these ultramafic systems may contain numerous possible electron acceptors (primarily oxygen, nitrate, sulfate, elemental sulfur, and iron) (Adam and Perner 2018).
Deep-sea hydrothermal vents are representative areas of high biological productivity on the seafloor, fueled primarily by microbial chemoautotrophy, which is in contrast to most ecosystems fueled by photosynthesis (Reysenbach and Shock 2002). Hydrothermal vent microorganisms, including free-living cells, animal symbionts, and cells in microbial mats, use the energy produced by the oxidation of sulfur, hydrogen, methane, sulfide, and iron to fix carbon. In turn, this organic carbon supports dense animal communities, largely through symbiotic relationships with bacteria, but also via grazing or suspension-feeding and subsequent trophic transfer (Dick 2019).
This article reviews the current knowledge of microorganisms inhabiting deep-sea hydrothermal vents and, in particular, the isolates grown from this unique environment and their involvement in the biogeochemical cycles. In addition, it highlights the understanding of metabolically versatile microbial taxa and future challenges for microbiologists in the field of microbial culture.
Microbial diversity and biogeochemistry
Carbon cycle
The geological activity of hydrothermal vents produces a large number of carbon compounds (Jannasch and Mottl 1985). Hydrothermal vent plumes contain high concentrations of methane and carbon monoxide, with methane concentrations of up to 107 times that of the surrounding ocean water (Jannasch and Mottl 1985). Short-chain fatty acids, such as formate and acetate, have been repeatedly found in hydrothermal fluids. Autotrophic chemosynthetic microorganisms fix inorganic carbon and provide much of the organic carbon that supports heterotrophic life in the hydrothermal ecosystem (Dick 2019).
Carbon fixers
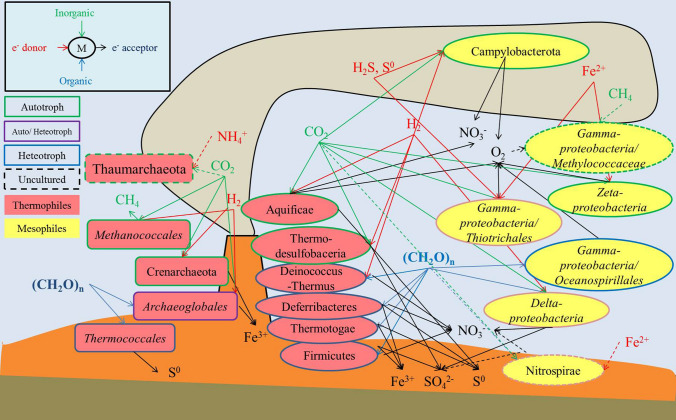
At hydrothermal sources, CO2 fixation can be carried out by members of the Alphaproteobacteria, Gammaproteobacteria, Deltaproteobacteria, Zetaproteobacteria, Campylobacterota (formerly Epsilonproteobacteria), Thermodesulfobacteria, Aquificae, Deferribacteres and by several members of the Archaea (Fig. 1, Table 1). Seven major metabolic pathways for carbon fixation are found in microbial vent communities including the Calvin–Benson–Bassham (CBB) cycle, reductive tricarboxylic acid (rTCA) cycle, 3-hydroxypropionate bicycle (3-HP), 3-hydroxypropionate/4-hydroxybutyrate cycle (3-HP/4-HB), reductive acetyl-coenzyme A (acetyl-CoA) pathway, dicarboxylate/4-hydroxybutyrate cycle, and the recently proposed reversible tricarboxylic acid cycle (“roTCA cycle”) (Minic and Thongbam 2011; Nakagawa and Takai 2008; Nunoura et al. 2018). The CBB cycle is the most common CO2 fixation pathway found among autotrophs. Ribulose bisphosphate carboxylase (RuBisCO), a key CO2 assimilation enzyme, has been identified in members of the microbial community, such as Zetaproteobacteria and Gammaproteobacteria, including the genera Thiomicrospira and Beggiatoa, and some endosymbionts of tubeworms, bivalves, and gastropods (Cerqueira et al. 2018). The rTCA cycle is preferred by anaerobes that inhabit anoxic zones of the hydrothermal vent system, for example, some members of Desulfobacterales, Aquificales, and Thermoproteales (Nakagawa and Takai 2008) and by many aerobic genera in the phylum Campylobacterota (Li et al. 2018). Most of the organisms using the 3-HP and 3-HP/4-HB cycle pathways are mixotrophs with the ability to utilize organic carbon in addition to carbon fixation, e.g., the deltaproteobacterial sulfate-reducer Desulfobacterium autotrophicum, acetogens, and methanogenic archaea (Nakagawa and Takai 2008). Deferribacter autotrophicus in the phylum Deferribacteres was found to use both the dicarboxylate/4-hydroxybutyrate cycle and the roTCA cycle (Slobodkin et al. 2019).
Fig. 1.
Schematic illustration of microbial metabolisms in deep-sea hydrothermal fields. Carbon
source in green or blue: CO2, CH4, (CH2O)n; Electron donors in red:·H2, H2S, S0, NH4+,Fe2+; Electron acceptors in black:·O2, S0, SO42−, NO3−, Fe3+
Table 1.
List of cultured genera in the domain of Bacteria and Archaea from the deep-sea hydrothermal vent environments and their metabolisms. Opt T, optimal growth temperature
| Categories | Genus (species number) | Opt T (°C) | Source | Main metabolism |
|---|---|---|---|---|
| Bacteria | ||||
| Aquificae | Balnearium (1) | 70–75 | Chimney | Anaerobe, autotroph, sulfur-oxidizer, denitrifier |
| Desulfurobacterium (5) | 65–75 | Chimney, sulfide, animal | Aerobe or anaerobe, autotroph, hydrogen-oxidizer, sulfur-reducer, denitrifier | |
| Hydrogenivirga (1) | 70–75 | Chimney | Aerobe or anaerobe, autotroph, sulfur-oxidizer, denitrifier | |
| Persephonella (1) | 73 | Chimney | Aerobe or anaerobe, autotroph, sulfur-oxidizer, denitrifier | |
| Thermovibrio (2) | 75–80 | Chimney | Anaerobe, autotroph, hydrogen-oxidizer, sulfur-reducer, denitrifier | |
| Thermosulfidibacter (1) | 70 | Sediment | Anaerobe, autotroph, hydrogen-oxidizer, sulfur-reducer | |
| Calditrichaeota | Caldithrix (1) | 60 | Chimney | Anaerobe, autotroph or heterotroph, hydrogen-oxidizer, denitrifier |
| Campylobacterota | Caminibacter (3) | 55–60 | Chimney | Aerobe or anaerobe, autotroph, hydrogen-oxidizer, sulfur-reducer, denitrifier |
| Cetia (1) | 55–60 | Chimney | Anaerobe, autotroph, hydrogen-oxidizer, sulfur-reducer, denitrifier | |
| Hydrogenimonas (1) | 55 | Fluid | Aerobe or anaerobe, autotroph, hydrogen-oxidizer, sulfur-reducer, denitrifier | |
| Lebetimonas (2) | 50–55 | Fluid, animal | Anaerobe, autotroph, hydrogen-oxidizer, sulfur-reducer, denitrifier, selenite-reducer | |
| Nautilia (4) | 40–60 | Chimney, animal | Anaerobe, autotroph or heterotroph, hydrogen-oxidizer, sulfur-reducer, denitrifier, selenite-reducer | |
| Nitratifractor (2) | 37 | Chimney | Aerobe or anaerobe, autotroph, hydrogen-oxidizer, sulfur-reducer, denitrifier | |
| Nitratiruptor (1) | 55 | Chimney | Aerobe or anaerobe, autotroph, hydrogen-oxidizer, sulfur-reducer, denitrifier | |
| Sulfurovum (4) | 28–35 | Chimney, sediment, rock, animal | Aerobe or anaerobe, autotroph, sulfur-oxidizer, hydrogen-oxidizer, denitrifier | |
| Sulfurimonas (3) | 25–33 | Chimney, sediment, animal | Aerobe or anaerobe, autotroph, sulfur-oxidizer, hydrogen-oxidizer, denitrifier | |
| Thiofractor (1) | 37 | Chimney | Anaerobe, autotroph, hydrogen-oxidizer, sulfur-reducer, denitrifier | |
| Deferribacteres | Deferribacter (3) | 60–65 | Chimney, fluid | Anaerobe, heterotroph, hydrogen-oxidizer, sulfur-reducer, denitrifier, iron-reducer, arsenate-reducer |
| Deinococcus-Thermus | Marinithermus (1) | 67.5 | Chimney | Aerobe, heterotroph, hydrogen-oxidizer |
| Oceanithermus (2) | 60 | Chimney | Aerobe, heterotroph, hydrogen-oxidizer | |
| Rhodothermus (2) | 70–75 | Chimney | Aerobe, heterotroph, sulfur-reducer, denitrifier | |
| Thermus (1) | 75 | Chimney | Aerobe, heterotroph, hydrogen-oxidizer | |
| Vulcanithermus (1) | 70 | Chimney | Aerobe, heterotroph, hydrogen-oxidizer | |
| Firmicutes | Anoxybacter (1) | 60 | Sulfide | Anaerobe, heterotroph, iron-reducer |
| Caloranaerobacter (2) | 55–60 | Chimney, sulfide | Anaerobe, heterotroph, iron-reducer | |
| Caminicella (1) | 55–60 | Animal | Anaerobe, heterotroph | |
| Desulfohalotomaculum (1) | 50 | Sediment | Anaerobe, heterotroph, sulfur-reducer | |
| Vulcanibacillus (1) | 55 | Sediment | Anaerobe, heterotroph, denitrifier | |
| Wukongibacter (1) | 30 | Sulfide | Anaerobe, heterotroph, iron-reducer | |
| Proteobacteria | ||||
| Alphaproteobacteria | Piezobacter (1) | 50 | Chimney | Aerobe or anaerobe, autotroph or heterotroph, hydrogen-oxidizer, sulfur-oxidizer, denitrifier |
| Gammaproteobacteria | Guyparkeria (1) | 35 | Chimney | Aerobe, autotroph or heterotroph, sulfur-oxidizer |
| Halomonas (4) | 20–35 | Fluid, plume, rock | Aerobe, heterotroph | |
| Hydrogenovibrio (2) | 25–40 | Fluid, animal | Aerobe, autotroph or heterotroph, sulfur-oxidizer, | |
| Salinisphaera (1) | 30–35 | Fluid | Aerobe, autotroph or heterotroph, sulfur-oxidizer | |
| Thiolapillus (1) | 40 | Chimney | Aerobe or anaerobe, autotroph, sulfur-oxidizer, denitrifier | |
| Thiomicrorhabdus (1) | 28 | Sea water | Aerobe or anaerobe, autotroph, sulfur-oxidizer | |
| Thioprofundum (2) | 39–40 | Chimney, rock | Aerobe or anaerobe, autotroph, sulfur-oxidizer, denitrifier | |
| Vibrio (1) | 40 | Animal | Aerobe, heterotroph | |
| Deltaproteobacteria | Deferrisoma (1) | 50 | Chimney | Anaerobe, heterotroph, sulfur-reducer, iron-reducers |
| Desulfonauticus (1) | 45 | Animal | Anaerobe, autotroph or heterotroph, hydrogen-oxidizer, sulfur-reducer | |
| Desulfothermus (1) | 50 | Chimney | Anaerobe, heterotroph, sulfur-reducer | |
| Desulfovibrio (1) | 35 | Chimney | Anaerobe, autotroph or heterotroph, hydrogen-oxidizer, sulfur-reducer | |
| Dissulfuribacter (1) | 61 | Chimney | Anaerobe, autotroph, sulfur-disproportionator | |
| Geothermobacter (1) | 55 | Fluid | Anaerobe, heterotroph, denitrifier, iron-reducers | |
| Pseudodesulfovibrio (1) | 30–35 | Rock | Anaerobe, heterotroph, hydrogen-oxidizer, sulfur-reducer | |
| Zetaproteobacteria | Ghiorsea (1) | 20 | Iron mat | Aerobe, autotroph, iron-oxidizers |
| Mariprofundus (2) | 25–30 | Iron mat, sediment | Aerobe, autotroph, iron-oxidizers | |
| Thermodesulfobacteria | Thermosulfurimonas (1) | 74 | Chimney | anaerobe, autotroph, sulfur-reducer, sulfur-disproportionator |
| Thermosulfuriphilus (1) | 65 | Chimney | Anaerobe, autotroph, sulfur-reducer, sulfur-disproportionator | |
| Thermodesulfatator (3) | 65–70 | Chimney | Aanaerobe, autotroph, hydrogen-oxidizer, sulfur-reducer | |
| Thermodesulfobacterium (1) | 75 | Sediment | Anaerobe, autotroph, hydrogen-oxidizer, sulfur-reducer | |
| Thermotogae | Marinitoga (4) | 55–65 | Chimney | Anaerobe, heterotroph, sulfur-reducer |
| Kosmotoga (1) | 70 | Sediment | Anaerobe, heterotroph, sulfur-reducer | |
| Thermosipho (4) | 65–72 | Chimney, fluid, animal | Anaerobe, heterotroph, sulfur-reducer | |
| Archaea | ||||
| Euryarchaeota | Aciduliprofundum (1) | 70 | Sulfide | Anaerobe, autotroph or heterotroph, sulfur-reducer, iron-reducer |
| Geoglobus (2) | 81–88 | Chimney | Anaerobe, autotroph or heterotroph, hydrogen-oxidizer, iron-reducer | |
| Methanocaldococcus (6) | 80–85 | Chimney, sediment, fluid | Anaerobe, autotroph, methanogen | |
| Methanofervidicoccus (1) | 70 | Chimney | Anaerobe, autotroph, methanogen | |
| Methanopyrus (1) | 98 | Chimney | Anaerobe, autotroph, methanogen | |
| Methanothermococcus (1) | 60–65 | Chimney | Anaerobe, autotroph, methanogen | |
| Methanotorris (1) | 75 | Chimney | Anaerobe, autotroph, methanogen | |
| Palaeococcus (2) | 80 | Chimney, sediment | Anaerobe, heterotroph, sulfur-reducer | |
| Pyrococcus (5) | 95–105 | Chimney, fluid | Anaerobe, heterotroph, sulfur-reducer | |
| Thermococcus (18) | 75–90 | Chimney, sediment, rock, fluid, animal | Anaerobe, heterotroph, sulfur-reducer, iron-reducer | |
| Crenarchaeota | Aeropyrum (1) | 85 | Chimney | Aerobe, heterotroph |
| Geogemma (1) | 100 | Chimney | Anaerobe, autotroph, hydrogen-oxidizer, iron-reducer | |
| Ignicoccus (1) | 90 | Chimney | Anaerobe, autotroph, hydrogen-oxidizer, sulfur -reducer | |
| Pyrodictium (1) | 90–92 | Chimney | Anaerobe, autotroph, hydrogen-oxidizer, denitrifier, iron-reducer | |
| Pyrolobus (1) | 106 | Chimney | Aerobe or anaerobe, autotroph, sulfur-reducer, denitrifier | |
In addition, at hydrothermal vents, geothermal light might drive photosynthetic reactions using the photosynthetic pigments found in a few microorganisms. An obligately photosynthetic green sulfur anaerobic bacterium is reported to grow anaerobically using light, H2S or elemental sulfur, and CO2, by capturing geothermal radiation with isorenieratene carotenoid pigments and chlorosome BChl e, a photosynthetic antennae complex (Beatty et al. 2005). Additionally, geo-chip surveys of the inside of an active chimney found abundant RubisCO genes of photosynthetic organisms closely related to cyanobacteria and green sulfur bacteria, further suggesting the presence of microorganisms that utilize photoradiation for energy (Wang et al. 2009). Perez et al. (2013) speculated that species that have very high efficiency in the use of infrared photons up to 1300 nm, can achieve good rates of photosynthesis in hydrothermal vents.
Methanogens
Thermophilic and hyperthermophilic methanogens that perform hydrogenotrophic methanogenesis are believed to be the numerically largest and most important group in higher temperature regimes. These methanogens require higher concentrations of hydrogen (e.g., ≥ 17 mmol/L according to the experiments carried out with hyperthermophilic Methanocaldococcus species) to support chemolithoautotrophic growth than organisms that use coupled hydrogen oxidation for respiration of alternative electron acceptors, for example, oxygen, nitrate, ferric iron, and sulfate (Ver Eecke et al. 2012). From mesophilic to hyperthermophilic methanogens, MGG (Huber et al. 1982), 141, and MMJ media (Takai et al. 2002) could be used for anaerobic cultivation techniques.
Archaea—Euryarchaeota
Among the archaea, the order Methanococcales is one of the most commonly found methanogen autotrophs in hydrothermal environments. They convert H2 and CO2 from degassing magma and water–rock reactions into CH4 and H2O. Many methanogens have been isolated from hydrothermal vents (Table 1, Supplementary Table S1), including Methanocaldococcus fervens (Jeanthon et al. 1999), M. indicus (L'Haridon et al. 2003a, b), M. infernus (Jeanthon et al. 1998), M. villosus (Bellack et al. 2011), M. vulcanius (Jeanthon et al. 1999), M. bathoardescens (Stewart et al. 2015), M. jannaschii (formerly Methanococcus jannaschii) (Jones et al. 1983), Methanopyrus kandleri (Kurr et al. 1991), Methanotorris formicicus (Takai et al. 2004a), Methanothermococcus okinawensis (Takai et al. 2002), and Methanofervidicoccus abyssi (Sakai et al. 2019). These marine organisms collectively have permissive growth temperatures ranging from 45–110 °C with respective optimums between 80 and 98 °C. They grow optimally at circumneutral pH (~ 6.5). Hydrogen and carbon dioxide can be used by all of these organisms to produce methane. Alternative substrates, such as formate, acetate, methanol, or methylamines, cannot be utilized. Tungsten, selenium, or yeast extract have always been found to significantly stimulate their growth. The methyl coenzyme-A reductase (McrA) gene is a key functional gene for methanogenesis present in all methanogenesis pathways (Luton et al. 2002).
Methane oxidizers
Hydrothermal ecosystems emit methane, which can originate from both geological and biological processes (Jannasch and Mottl 1985). Microbially mediated methane oxidation occurs with different electron acceptors, with sulfate being quantitatively the dominant electron acceptor for methane oxidation, because methane production occurs below the sulfate-rich zone in sediments. In hydrothermal vent communities, aerobic oxidation of methane (so-called methanotrophy) is also commonly found in microbial endosymbionts of vent animals (Nakagawa and Takai 2008) and the adjacent seawater (Valentine et al. 2001).
Gammaproteobacteria
The family Methylococcaceae in the class Gammaproteobacteria also uses methane as both an energy and carbon source and has been reported in deep-sea vents (Cerqueira et al. 2018).
Archaea—Euryarchaeota
Anaerobic oxidation of methane (AOM) is prevalent in methane-rich sediments found around some hydrothermal vents and may be responsible for consuming up to 75% of the methane produced by the vent (Wankel et al. 2012). Species that perform AOM include archaea of the phylum Euryarchaeota, referred to as anaerobic methane-oxidizing lineages (ANME). In the absence of pure cultures, high-throughput sequencing surveys, coupled with functional approaches and analytical monitoring of the metabolism based on δ 13C signatures of CH4 and sulfate reduction rates, have demonstrated the ability of different ANME lineages (ANME-1, 2a-c, and 3) to oxidize methane coupled to the reduction of sulfate, and the presence of these archaea has been reported in several hydrothermal vent fields (Biddle et al. 2012; Merkel et al. 2013; Wankel et al. 2012). Efforts to cultivate the hydrothermal microorganisms carrying out AOM deserve to be continued.
Heterotrophs
There is also a rich heterotrophic microbial life at hydrothermal vents. Versatile heterotrophic Alpha- and Gammaproteobacteria have been found, for example, in different venting areas of the Menez Gwen hydrothermal field; from the diffuse fluid discharge points through the mixing gradients to the plumes and the surrounding seawater (Meier et al. 2016). Generalist species belonging to the genera Marinobacter, Vibrio, Pseudoalteromonas, Halomonas, Pseudomonas, and Alcanivorax, among others, have been repeatedly isolated from hydrothermal vent samples (Kaye et al. 2004; Raguénès et al. 1997; Teske et al. 2000; Vetriani et al. 2005) and their abundance in vent fluids collected from the Pacific Ocean was estimated to be up to 28% of the total microorganisms (Kaye and Baross 2000). Strains of the phylum Deferribacteres are the heterotrophic taxa commonly found in deep-sea vents that can grow with organic acids and proteinaceous compounds as energy sources, for example, Caldithrix abyssi (Miroshnichenko et al. 2003b), Deferribacter abyssi (Miroshnichenko et al. 2003a), D. autotrophicus (Slobodkina et al. 2009a), D. desulfuricans (Takai et al. 2003a, b, c). A high fraction of heterotrophic microbial lineages related to cultivated members within the order Thermotogales and Bacteroidetes, and many novel lineages were also enriched using a novel in situ enrichment strategy (Stokke et al. 2020). Isolates in the phylum Deinococcus-Thermus have not only been isolated from hydrothermal vents but have often also been isolated from terrestrial hot springs. They include six genera, Thermus (Marteinsson 1999), Marinithermus (Sako et al. 2003), Oceanithermus (Miroshnichenko et al. 2003c; Mori et al. 2004), Vulcanithermus (Miroshnichenko et al. 2003d), Rhodothermus (Marteinsson et al. 2010) and Rhabdothermus (Steinsbu et al. 2011). Most of them are aerobic thermophilic heterotrophs capable of growing solely on complex organic substrates, while some can grow anaerobically with nitrate and elemental sulfur as electron acceptors.
At hydrothermal vent systems, alkanes are formed over relatively short geological time scales via thermogenic processes (including Fischer–Tropsch reactions and serpentinization) and often exist at high concentrations. Besides, the Guaymas Basin, is characterized by hydrothermal alterations that transform large amounts of organic carbon into methane, polycyclic aromatic hydrocarbons, low-molecular weight alkanes, and organic acids, allowing for diverse microbial communities to thrive (Dombrowski et al. 2017; He et al. 2013; Li et al. 2016a, b; Wang et al. 2019). Recently, the McrABG-like complex (Methyl coenzyme M reductase) involved in the oxidation of short-chain alkanes has been identified in various taxa, including Candidatus Syntrophoarchaeum spp., and closely related sequences have been detected in Guaymas Basin hydrothermal sediments (Wang et al. 2019). Gene coding for enzymes involved in the activation of hydrocarbons for anaerobic degradation was detected in Bacteroidetes, Chloroflexi, Latescibacteria and candidate bacteria KSB1 phyla (Dombrowski et al. 2017). The archaeal genome of GoM-Arc1 is also predicted to encode novel pathways for short-chain hydrocarbon oxidation by alkyl-coenzyme M formation (Dombrowski et al. 2017). Using discrete temperature (25, 55, and 75 °C) anaerobic batch reactor incubations of hydrothermal sediments supplemented with individual alkanes (C1–C4), a novel sulfate-reducing lineage of Deltaproteobacteria, was identified as the likely taxa mediating the oxidation of C2–C4 alkanes (Adams et al. 2013). The hyperthermophilic archaeon Geoglobus acetivorans can also degrade aromatic compounds (Mardanov et al. 2015). Recently, after enrichment at low temperatures under high hydrostatic pressure (HP), several chemoautotrophs previously recognized as sulfur oxidizers, such as the SAR324 group, clade SUP05, and representatives of the genus Sulfurimonas, were identified as bacteria capable of degrading hydrocarbons (Wang et al. 2020).
Fermentative microorganisms are often associated with methanogens, because methanogenic activity maintains a hydrogen concentration low enough to make the fermentation pathway energetically favorable. Many fermenters have been isolated under anaerobic culture conditions, in the domain Archaea, such as the Aeropyrum camini (Nakagawa et al. 2004), Thermococcales (Teske et al. 2009), Geoglobus species (Kashefi et al. 2002), Desulfurococcus species (Jannasch et al. 1988) and ‘Firmicutes’ such as Caminicella sporogenes (Alain et al. 2002b), Caloranaerobacter azorensis (Wery et al. 2001b), Caloranaerobacter ferrireducens (Zeng et al. 2015a), Anoxybacter fermentans (Zeng et al. 2015b), and Wukongibacter baidiensis (Li et al. 2016).
The distribution, abundance, and function of microbial eukaryotes in hydrothermal environments are still poorly understood but recent progress has revealed considerable diversity. Fungi can be associated with a wide variety of substrates in deep-sea hydrothermal vent systems, including seawater, sediments, minerals, and animals such as shrimps, mussels, tubeworms, corals, sponges, and gastropods (Luo and Pang 2014). At the Lucky Strike and Rainbow hydrothermal fields along the Mid-Atlantic Ridge, the presence of ciliates related to Stylonychia pustulata, fungi, and alvenellid-related metazoans were revealed through 18S rRNA gene surveys (Lopez-Garcia et al. 2003). Black yeast (order Chaetothyriales) was found in mussels (Bathymodiolus brevior) at the Mussel Hill hydrothermal vent in the Fiji Basin (Van Dover et al. 2007). Burgaud et al. (2009) focused on the occurrence and diversity of culturable filamentous fungi in deep-sea hydrothermal vents and isolated 62 filamentous Ascomycota fungi. Molecular methods revealed unsuspected species diversity in three of the five fungal phyla and discovered a new branch of Chytridiomycota, forming an ancient evolutionary lineage and many unknown species in Chytridiomycota, Ascomycota, and Basidiomycota (Calvez et al. 2009). Connell et al. (2009) investigated fungal diversity in actively growing Fe-oxide mats and basalt rock surfaces from deep-sea hydrothermal vents. A diverse fungal community, belonging to the genera Pichia, Cryptococcus, Rhodospordium, Rhodotorula, Dioszegia, Clavispora, Sporidiobolus, Aremonium, Geomyces, Aspergillus, and Fomitopsis, was recovered. All yeast species obtained can produce siderophores for Fe acquisition and utilization, and one isolate, Rhodotorula graminis, could oxidize Mn (II), suggesting that fungi in deep-sea hydrothermal vent ecosystems may play a role in biomineralization processes (Connell et al. 2009). Malassezia-like fungi and members of the order Malasseziales are exceedingly widespread and ecologically diverse, having even been found in hydrothermal vents (Amend et al. 2014). The study analyzed the species richness and abundance of fungi from a South Mid-Atlantic Ridge hydrothermal site, and found that the fungal community structures in the chimney samples were distinct from those in three sulfide samples, where the Ascomycota was significantly more abundant 2–3 orders) than the Basidiomycota (Xu et al. 2017). Xu et al. (2018) also found that there was a high fungal diversity, with 723 operational taxonomic units (OTUs) belonging to 79 taxa (Ascomycota and Basidiomycota contributed to 99% of all samples), in the deep-sea hydrothermal sediments of Southwest Indian ridge.
Sulfur cycle
Microbial communities at hydrothermal vents convert reduced sulfur, such as H2S, produced by geological activity into other more oxidized forms, such as sulfite, sulfate and elemental sulfur for energy production or assimilation into organic molecules (Sievert et al. 2008). Sulfide is abundant in hydrothermal vents, with concentrations ranging from one to several tens of mmol/L, whereas the surrounding ocean water generally has concentrations of only a few nanomolars (Radfordknoery et al. 2001).
Sulfur-oxidizers
Sulfide from hydrothermal vents is available to various sulfur-oxidizing microorganisms using oxygen (or nitrate) from the upper layers of the sea. Many reduced sulfur compounds, such as elemental sulfur (S0), hydrogen sulfide (H2S), methanethiol (CH3SH), dimethylsulfide [(CH3)2S], pyrrhotite (Fe1-xS), pyrite (FeS2), chalcopyrite (CuS), and sphalerite (ZnS) are used as electron donors by microorganisms (Orcutt et al. 2011). Sulfide and thiosulfate oxidation occur in sulfide-rich basalt-hosted hydrothermal fields, which provide the greatest energy yields of the hydrothermal vent biotopes (McCollom et al. 2007). Meier et al. (2017) found that representatives of the genus Sulfurovum in the phylum Campylobacterota thrive, mainly attached to surfaces exposed to diffuse venting, while the SUP05-clade dominated the bacterioplankton in highly diluted mixtures of vent fluids and seawater (Meier et al. 2017). Campylobacterota and Gammaproteobacteria also dominated the microbiota of chemosynthetic metazoans, such as polychaetes, mussels, gastropods, and shrimps (Nakagawa 2008). The sulfur-oxidizing Aquificae, however, generally occupy a narrow thermophilic niche (Reysenbach et al. 2001) of this singular ecosystem, and sulfur-oxidizing archaea (order Sulfolobales) are quite rare in the marine environment (Friedrich et al. 2005).
Bacterial sulfur-oxidation pathways include: sulfide oxidation to elemental sulfur by sulfide dehydrogenase (FccAB) or sulfide-quinone reductase (Sqr), which is further oxidized to sulfate by the reverse sulfate reduction pathway or by SoeABC in the cytoplasm. Thiosulfate can be oxidized to sulfate by the Sox system (SoxABCDXY) in the periplasm. These systems are classified into two different types: (1) the reverse sulfate reduction and (2) the Sox multienzyme system. Reverse sulfate reduction is a reversal of the sulfate reduction pathway in which sulfide is oxidized to sulfate via sulfite. This pathway uses the same families of enzymes as the sulfate reduction pathway, such as dissimilatory sulfite reductase (Dsr), adenylphosphosulfate reductase (Apr), and sulfate adenylyltransferase (Sat). Sox multienzyme complexes catalyze the oxidation of inorganic sulfur compounds. Four protein components, SoxYZ, SoxXA, SoxB, and SoxCD are required for the complete oxidation of thiosulfate to sulfate (Wasmund et al. 2017; Yamamoto and Takai 2011). However, in some strains, SoxCD was not necessary (Friedrich et al. 2005). The deep-sea Campylobacterota possesses versatile energy metabolisms: (i) they can couple the oxidation of hydrogen to the respiration of sulfur using hydrogenases and polysulfide reductase (Psr); (ii) they can couple the oxidation of sulfur compounds using the Sox multienzyme system, to the respiration of oxygen or nitrate; and (iii) they can also couple the oxidation of hydrogen to the reduction of oxygen or nitrate (Yamamoto and Takai 2011). Most of the chemotrophic sulfur-oxidizing Gammaproteobacteria harbor both incomplete Sox and complete Dsr systems, similar to the phototrophic gammaproteobacterial species of the family Chromatiaceae (Nakagawa and Takai 2008). Nevertheless, there are some exceptions, such as the species Thiomicrospira crunogena that possesses a complete Sox system but lacks the Dsr system (Scott et al. 2006).
Strains of the phyla Proteobacteria, Campylobacterota, Aquificae, and Thermodesulfobacteria that have so far been isolated from hydrothermal vents are shown in Fig. 1 and listed in Table 1 and Supplementary Table S1. They have been isolated under various conditions using different cultural methods, including thiosulfate-based solid media, oxygen-sulfide gradient tubes, or liquid media with controlled atmospheres, under lithoautotrophic conditions or heterotrophic conditions (Adair and Gundersen 1969; Ruby et al. 1981; Takai et al. 2003a, b, c; 2009).
Campylobacterota
Campylobacterota (formerly Epsilonproteobacteria), which are highly abundant in hydrothermal vents, take advantage of their versatile metabolisms to colonize different niches, where conditions are mesophilic to moderately thermophilic. Takai et al. (2003a, b, c) were the first to report that various kinds of sulfur-oxidizing Campylobacterota inhabit marine hydrothermal vents. Numerous novel mesophilic sulfur-oxidizers of this phylum have been isolated from these environments, including the most abundant genus Sulfurimonas (Inagaki et al. 2003), Sulfurovum species (Inagaki et al. 2004), and the genus Lebetimonas (Takai et al. 2005). Representatives of this genus have been isolated from hydrothermal vent lithological samples and vent animals. These include Sulfurovum lithotrophicum (Inagaki et al. 2004), Sulfurovum aggregans (Mino et al. 2014), Sulfurovum riftiae (Giovannelli et al. 2016), and Sulfurovum denitrificans (Mori et al. 2018). Most strains of the genus Sulfurovum grow chemolithoautotrophically using sulfur as an energy source, under mesophilic temperatures. The only exception is S. aggregans, which is a strictly hydrogen-oxidizing, sulfur-, nitrate-, and thiosulfate-reducing mesophilic bacteria (Mino et al. 2014). The genus Sulfurimonas has been reported to be one of the dominant groups in hydrothermal vent fields and may represent 70% and 20% of the bacterial abundance at sites in plume waters and diffuse fluids, respectively, e.g., in the Axial seamount in the Pacific Ocean (Akerman et al. 2013; Perner et al. 2013). The type species of this genus is Sulfurimonas autotrophica OK10, isolated from deep-sea hydrothermal vent sediments, can use molecular hydrogen, elemental sulfur, or thiosulfate as the energy source, carbon dioxide as the sole carbon source, ammonium or nitrate as the sole nitrogen source, and elemental sulfur, sulfide, or thiosulfate as the sulfur source (Inagaki et al. 2003). The species Sulfurimonas paralvinellae from polychaete nests and Sulfurimonas indica from sulfide chimney were also capable of growth using thiosulfate, sulfide or elemental sulphur as the sole energy source (Takai et al. 2006; Hu et al. 2020).
Proteobacteria
Several sulfur-oxidizing bacteria (SOB), belonging to the class Gammaproteobacteria, have also been isolated from hydrothermal habitats (Table 1, Supplementary Table S1). The main sulfur-oxidizing gammaproteobacterial taxa are in the order Thiotrichales, including Hydrogenovibrio crunogenus (formerly Thiomicrospira crunogena) (Jannasch et al. 1985), Hydrogenovibrio thermophile strain I78 (formerly T. thermophile) (Takai et al. 2004d), H. thermophilus strain S5 (Jiang et al. 2017), Thiomicrorhabdus indica (Liu et al. 2020); Thiomicrorhabdus sp. Kp2 is mentioned in the reclassification of these genera (Boden et al. 2017b). T. crunogena uses the energy of reduced sulfur compounds (sulfide, thiosulfate, and elemental sulfur) for carbon fixation and cellular maintenance (Jannasch et al. 1985). Its main carbon source is the CO2 released from hydrothermal vents. Given its changable environment, T. crunogena has a carbon concentrating mechanism that allows it to grow in the presence of low CO2 concentrations, by increasing cellular affinity for both HCO3− and CO2 to generate a high concentration of intracellular dissolved inorganic carbon under low CO2 conditions, such as seawater, near hydrothermal vents (Dobrinski et al. 2005).
Some thermophilic SOB of the order Chromatiales (Gammaproteobacteria) have also been isolated from pure cultures, such as Thioprofundum lithotrophica (Takai et al. 2009) and T. hispidum (Mori et al. 2011). Another example, Thiolapillus brandeum was isolated from a chimney fragment and grows on sulfur, thiosulfate, and tetrathionate, using oxygen and nitrate as terminal electron acceptors (Nunoura et al. 2014). Salinisphaera hydrothermalis strain EPR70 can grow under aerobic chemolithoautotrophic conditions in the presence of thiosulfate and CO2, or heterotrophically with acetate or n-alkanes as sole carbon and energy sources, and in complex artificial seawater medium (Crespomedina et al. 2009). Guyparkeria hydrothermalis (formerly Thiobacillus hydrothermalis) grows obligatorily under lithoautotrophic conditions using thiosulfate, tetrathionate, sulfide, and sulfur as electron donors (Boden 2017a; Durand et al. 1993). Using pressure cultivation approaches, Piezobacter thermophilus, affiliated with the family Rhodobacteraceae within the Alphaproteobacteria, was isolated. It is a facultative chemoautotroph, capable of both chemolithoautotrophic growth with H2 and S oxidations and organotrophic growth with complex organics or organic acids using nitrate and O2 as electron acceptors. Interestingly, chemolithoautotrophic growth is observed strictly under piezophilic conditions and under organotrophic growth conditions, this strain grows only at atmospheric pressure (0.1 MPa) (Takai et al. 2009).
In addition, giant microbial mats and SOB snowblowers have been identified as the filamentous bacteria Beggiatoa, such as Arcobacter sp. and as members of the SUP05-clade (Moussard et al. 2006; Nelson et al. 1989).
Aquificae
Species in the order Aquificales are thermophilic hydrogen- and/or sulfur-oxidizers. A variety of strains and environmental clones belong to the genera Aquifex (Reysenbach et al. 2002), Hydrogenivirga -including Hydrogenivirga okinawensis (Nunoura et al. 2008a) and Persephonella -including Persephonella marina, P. guaymasensis (Gotz et al. 2002) and P. hydrogeniphila (Nakagawa et al. 2003) have been isolated or detected in deep-sea hydrothermal environments. These can grow with hydrogen, elemental sulfur, or thiosulfate as electron donors and with nitrate or O2 as terminal electron acceptors.
Thermodesulfobacteria
An extremely thermophilic, anaerobic, chemolithoautotrophic bacterium Thermosulfuriphilus ammonigenes can grow anaerobically with inorganic carbon as a carbon source, with elemental sulfur as an electron donor and nitrate as an electron acceptor producing sulfate and ammonium. This strain is also able to grow by disproportionation of elemental sulfur, thiosulfate, and sulfite (Slobodkina et al. 2017).
Sulfur-reducers
Microorganisms that perform sulfur reduction coupled with the oxidation of H2, methane, small chain fatty acids, or other carbon compounds are one of the main drivers of the hydrothermal carbon cycle. Sulfate reduction is one of the most important processes from low temperature to high temperature (4–90 °C) in highly anoxic areas of the hydrothermal environment, for example, hydrothermal sediments (Frank et al. 2013), hydrothermal fluid, sulfide structures, and Alvinellid worm sheaths (Bonchosmolovskaya et al. 2011). Elemental sulfur can also be used to detoxify dihydrogen in fermentation processes carried out by many Thermococcales and some representatives of Desulfurococcales (Bonchosmolovskaya et al. 1994, 2011; Teske et al. 2009). Molecular inventories of Guaymas hydrothermal sediments, targeting the 16S rRNA and functional genes such as the dissimilatory sulfite reductase (dsrAB), revealed the existence of a major clade closely related to the acetate-oxidizing deltaproteobacterial genus Desulfobacter, and a few clones affiliated with Desulfobacterium niacini, Desulfobacula toluolica, and Desulfotignum balticum (Dhillon et al. 2003).
Numerous sulfate, thiosulfate, and sulfur reducers, isolated from hydrothermal vents, belong to different taxonomic groups: Archaea, Campylobacterota, Thermodesulfobacterium, Thermotogae, and Deltaproteobacteria (Table 1). For anaerobic, heterotrophic sulfur-reducing thermophiles, such as members of Thermococcales and Thermotogales, MJYPS medium, and Thermococcales Rich Medium (TRM) with sulfur as an electron acceptor are often used under an atmosphere of N2 (Takai et al. 2000; Zeng et al. 2009). For hydrogen-oxidizing, sulfur-reducing autotrophs and mixotrophs such as members of Desulfurococcales, Desulfurobacteriales, Nautiliales, and Deferribacteres, KA22 (Alain et al. 2002a), MJAIS-YTF (Nakagawa et al. 2005a, b), and MJAIS media (Takai et al. 2003a, b, c) are frequently used and incubated in an atmosphere of H2/CO2. For anaerobic dissimilatory Fe(III)- and/or sulfate-reducers, such as members of the Archaeoglobales, Deferribacteres, Thermodesulfobacteria, and Deltaproteobacteria, MJNS and MMJHFe media are often used (Takai et al. 2003a, b, c, 2008).
Campylobacterota
The genera Caminibacter, Nautilia, Lebetimonas, and Cetia of the order Nautiliales have cultured representatives that include autotrophs and mixotrophs, which couple the oxidation of hydrogen to the reduction of sulfur- or nitrogenous compounds to produce H2S and NH3. These moderately thermophilic bacteria have been found in association with invertebrates, chimney edifices, or in situ colonization devices. At present, the genus Caminibacter encompasses the species Caminibacter hydrogeniphilus (Alain et al. 2002a), C. mediatlanticus (Voordeckers et al. 2005), and C. profundus (Miroshnichenko et al. 2004). The genus Nautilia contains four species of anaerobic, thermophilic chemolithotrophs: Nautilia lithotrophica (Miroshnichenko et al. 2002), N. abyssi (Alain et al. 2009), N. profundicola (Smith et al. 2008), and N. nitratireducens (Perezrodriguez et al. 2010). The genus Lebetimonas encompasses two species: Lebetimonas acidiphila (Takai et al. 2005) and L. natsushimae (Nagata et al. 2017). The genus Cetia has been more recently cultivated and encompasses only the species Cetia pacifica, which branches separately from the three other genera that are close to each other, Caminibacter, Nautilia, and Lebetimonas, within the family Nautiliaceae (Grosche et al. 2015).
Hydrogenimonas thermophila, in the order Campylobacterales, can use molecular hydrogen as the sole energy source and carbon dioxide as the sole carbon source. Molecular oxygen, nitrate, or elemental sulfur (S0) can act as electron acceptors for its growth (Takai et al. 2004b). Thiofractor thiocaminus is a strict anaerobic, obligate chemolithoautotroph, which can grow using molecular hydrogen as its sole energy source, carbon dioxide as its sole carbon source, ammonium or nitrate as its sole nitrogen source, and elemental sulfur as a terminal electron acceptor. This isolate is distantly related to the taxa described above (Makita et al. 2012). Recently, Wang et al. (2020) reported that most species of Sulfurimonas have their potentials in sulfur reduction, with periplasmic and cytoplasmic polysulfide reductases (PsrA1B1CDE and PsrA2B2).
Proteobacteria
Some Deltaproteobacteria, such as Pseudodesulfovibrio, Desulfovibrio, Desulfonauticus, and Desulfothermus species have been isolated from the hydrothermal ecosystem (Table 1, Supplementary Table S1). Pseudodesulfovibrio indicus was isolated from a serpentinized peridotite sample collected from a hydrothermal area of the Indian Ocean. This strain uses organic acid and hydrogen as energy sources and sulfate, thiosulfate, sulfite, fumarate, and nitrate as terminal electron acceptors (Cao et al. 2016). Desulfovibrio hydrothermalis, isolated from a chimney sample collected at 13° N on the East-Pacific Rise, can use H2/CO2, lactate, formate, ethanol, choline, and glycerol as electron donors. It can respire sulfate, sulfite, and thiosulfate (Alazard et al. 2003). Pseudodesulfovibrio indicus and Desulfovibrio hydrothermalis have both been reported as piezophilic taxa with an optimal growth pressure of 10 MPa. Desulfonauticus submarinus, isolated from a matrix of Alvinella and Riftia originating from the EPR 13° N, can grow on H2/CO2, oxidize formate, and use acetate as a carbon source. Sulfate, sulfite, thiosulfate, and elemental sulfur are used as terminal electron acceptors during hydrogen oxidation (Audiffrin et al. 2003). Desulfothermus okinawensis is a thermophilic and obligate heterotrophic sulfate-reducing bacterium that was isolated from a deep-sea hydrothermal field at the Yonaguni Knoll IV in the Southern Okinawa Trough (Nunoura et al. 2007a).
Nitrospirae
Frank et al. (2013) proposed that Thermodesulfovibro-like organisms may have a role in sulfate reduction in warmer parts of the hydrothermal ecosystem and that heterotrophic sulfate reduction may be quite common in deep-sea vents. Other studies based on metagenome and metaproteome analyses have shown that sulfate-reducing Nitrospirae dominate most inactive chimney communities (Meier et al. 2019).
Firmicutes
The gram-positive taxon Desulfohalotomaculum tongense (formerly Desulfotomaculum tongense) in the class Clostridia, isolated from hydrothermal sediment collected at the Tofua Arc in the Tonga Trench, was found to use pyruvate and H2 as electron donors and sulfate, sulfite, and thiosulfate as electron acceptors (Cha et al. 2013).
Aquificae
Aquificales represent a deeply rooted phylogenetic lineage within the bacterial domain and, with few exceptions, it includes thermophilic or hyperthermophilic lithoautotrophs utilizing H2, elemental sulfur, sulfide, or thiosulfate as energy sources and oxygen, nitrate, or elemental sulfur as electron acceptors. This metabolically versatile group plays a significant role in deep-sea hydrothermal ecosystems as primary producers of organic matter in anaerobic zones (Reysenbach et al. 2001). Members of the family Desulfurobacteriaceae, such as the genera Desulfurobacterium and Thermovibrio, are sulfur-reducing chemolithoautotrophs using hydrogen as the sole electron donor. This family includes the taxa Desulfurobacterium atlanticum (L’Haridon et al. 2006a), Desulfurobacterium pacificum (L’Haridon et al. 2006a), Desulfurobacterium thermolithotrophum (L’Haridon et al. 1998), ‘Desulfurobacterium crinifex’ (Alain et al. 2003), Desulfurobacterium indicum (Cao et al. 2017), and Therulfururobacterium guaymasensis (L’Haridon et al. 2006b), all of which have been isolated from deep-sea hydrothermal systems. For example, Desulfurobacterium indicum, which was isolated from a sulfide sample from an Indian Ocean hydrothermal vent, is an obligate chemolithoautotroph combining thiosulfate, sulfur, or nitrate respiration to H2 oxidation (Cao et al. 2017). Thermosulfidibacter takaii, which was isolated from a deep-sea hydrothermal field at the Southern Okinawa Trough, is an obligately anaerobic chemolithotroph coupling hydrogen oxidation coupled to sulfur reduction (Nunoura et al. 2008b).
Thermodesulfobacterium
In the Thermodesulfobacteria class, Thermodesulfobacterium hydrogeniphilum (Jeanthon et al. 2002), Thermodesulfatator indicus (Moussard et al. 2004), Thermodesulfatator atlanticus (Alain et al. 2010), and Thermodesulfatator autotrophicus (Lai et al. 2016) all are autotrophic sulfate-reducing bacteria; the growth of a few of these strains can be stimulated by organic compounds.
Thermotogae
In the Thermotogae phylum, the genera Thermosipho, Kosmotoga, and Marinitoga appear to be environmentally relevant in the deep-sea hydrothermal environment. These microorganisms can ferment a variety of carbohydrates as well as complex organic matter. The addition of yeast extract is often required for their growth. The products of glucose fermentation are acetate, H2, and CO2. H2 inhibits their growth, and these taxa often grow better in the presence of sulfur compounds, such as thiosulfate, elemental sulfur, cysteine, or thiosulfate, which is reduced to hydrogen sulfide, a less toxic compound than dihydrogen. The genus Marinitoga includes the species Marinitoga camini (Wery et al. 2001a), M. hydrogenitolerans (Postec et al. 2005), M. okinawensis (Nunoura et al. 2007b), and M. piezophila (Alain 2002c). Thermosipho spp. are also frequently isolated from deep-sea hydrothermal vents, for example, Thermosipho melanesiensis (Antoine et al. 1997), T. japonicus (Takai and Horikoshi 2000), T. atlanticus (Urios et al. 2004), T. affectus (Podosokorskaya et al. 2011), T. globiformans (Tomohiko et al. 2011), and T. activus (Podosokorskaya et al. 2014). Kosmotoga pacifica, in the genus Kosmotoga, was isolated from hydrothermal sediments mixed with fragments of inactive sulfide chimneys collected in the South Pacific Ocean (L'Haridon et al. 2013).
Deferribacteres
Some strains in the genus Deferribacter, such as Deferribacter desulfuricans, D. abyssi, and D. autotrophicus, can use elemental sulfur as a terminal electron acceptor (Miroshnichenko et al. 2003a; Takai et al. 2003a, b, c).
Archaea—Euryarchaeota
Deep-sea hydrothermal vent Euryarchaeota 2 (DHVE2) is extremely widespread in marine hydrothermal ecosystems and may account for up to 15% of the archaeal 16S rRNA gene sequences, suggesting that they are important players in deep-sea hydrothermal habitats (Flores et al. 2012). The only representative of this group, Aciduliprofundum boonei, is an obligate thermoacidophilic heterotrophic archaeon, which uses electron acceptors, such as elemental sulfur, sulfate, and ferric iron and is capable of growing from pH 3.3–5.8, and between 60 and 75 °C (Reysenbach et al. 2006).
Archaea of the order Thermococcales, distributed in three genera (i.e., Thermococcus, Pyrococcus, and Palaeococcus), have versatile physiologies to thrive in active hydrothermal vents, sediments, and even below the surface under hydrothermal vents (Teske et al. 2009; Zeng et al. 2013). Eighteen Thermococcus species, five Pyrococcus species, and two Palaeococcus species have been isolated from hydrothermal vents (Table 1, Supplementary Table S1), for example, Pyrococcus abyssi (Erauso et al. 1993), Pyrococcus horikoshii (González et al. 1998), Pyrococcus glycovorans (Barbier et al. 1999), Pyrococcus yayanosii (Birrien et al. 2011; Zeng et al. 2009), Pyrococcus kukulkanii (Callac et al. 2016), Palaeococcus ferrophilus (Takai et al. 2000), and Palaeococcus pacificus (Zeng et al. 2013). All Thermococcales species can ferment a variety of organic compounds, such as peptides, amino acids, organic acids, and diverse sugars, with S0 as the electron acceptor. The growth of many of them is better in the presence of sulfur compounds, which are reduced to hydrogen sulfide. Some strains, such as Pyrococcus kukulkanii or Palaeococcus ferrophilus, require sulfur or Fe (II) in the culture medium to grow, and some others can grow via carboxydotrophy. Thermococcales strains require high temperatures to grow; some of them also can survive for a long time in cold conditions, such as hydrothermal sediments and then quickly respond to the high temperature and energy supply by complex organic matter or elemental sulfur (Mora et al. 2014; Zeng et al. 2020). Piezophilic features are also widespread in this order (Dalmasso et al. 2016; Zeng et al. 2009) and some strains tolerate high doses of gamma radiation (Jolivet et al. 2004).
Archaea—Crenarchaeota
In the phylum Crenarchaeota, Pyrolobus fumarii is an archaeon that has long held records for high-temperature growth, with growth observed between 90 °C and 113 °C (optimum 106 °C) (Blochl et al. 1997). It is a facultatively aerobic obligate chemolithoautotroph, gaining energy by H2-oxidation. It can respire NO3–, S2O32–, and low concentrations of O2 (up to 0.3% v/v), which are, respectively, reduced to NH4+, H2S, and H2O (Blochl et al. 1997). In addition, another study on the colonization of hydrothermal chimneys of different ages showed that chemolithoautotrophic Crenarchaea of the genus Ignicoccus and their symbionts Nanoarchaeum dominated the initial colonization of high-temperature chimneys, while heterotrophs related to Thermococcales are more abundant in mature edifices (Mccliment et al. 2006). The taxa Ignicoccus islandicus and I. pacificus, isolated from the Kolbeinsey Ridge in northern Iceland and the Pacific Ocean, gain energy by the reduction of elemental sulfur to hydrogen sulfide using molecular hydrogen as an electron donor (Huber et al. 2000, 2002).
Sulfur-disproportionators
Disproportionation (dismutation) of inorganic sulfur compounds allows cells to gain energy for growth using a sulfur compound in an intermediate oxidation state as both an electron donor and acceptor, thus minimizing their needs relative to the chemical composition of the biotope (Slobodkin and Slobodkina 2019). It is a type of inorganic fermentation.
Sulfur disproportionators have recently been found in natural thermal ecosystems. Species known so far to carry out sulfur compound dismutation belong to the class Deltaproteobacteria within the phylum Proteobacteria, the class Clostridia within the phylum Firmicutes, and the phylum Thermodesulfobacteria (Slobodkin and Slobodkina 2019).
Thermodesulfobacteria
Thermosulfurimonas dismutans and Thermosulfuriphilus ammonigenes thrive in deep-sea hydrothermal vents of the Lau spreading center, in the Pacific (Slobodkin et al. 2012, 2017). Genome analyses revealed that Thermosulfurimonas dismutans and Thermosulfuriphilus ammonigenes contain a full set of genes for dissimilatory sulfate reduction, including ATP sulfurylase, the AprA and AprB subunits of adenosine-5′-phosphosulfate reductase, and dissimilatory sulfite reductase. While these species cannot grow via sulfate-reduction, these enzymes could be involved in the reductive path of dismutation. These genomes also contain several membrane-linked molybdopterin oxidoreductases that might also be involved in sulfur metabolism as well as subunits of thiosulfate, polysulfide, or tetrathionate reductases (Mardanov et al. 2016). The routes of dismutation of inorganic sulfur compounds are still unknown and there is likely to be more than one.
Deltaproteobacteria
Dissulfuribacter thermophilus can disproportionate all three types of sulpfur compounds (sulfide, thiosulfate, and elemental sulfur) (Slobodkin et al. 2013). Genomic and transcriptomic analyses of epibionts on the hydrothermal vent shrimp Rimicaris exoculata have revealed that “Candidatus Desulfobulbus rimicarensis” an uncultivated deltaproteobacterial epibiont, utilizes the Wood-Ljungdahl pathway for carbon assimilation and harvests energy via sulfur disproportionation (Jiang et al. 2020).
Hydrogen cycle
Hydrogen oxidation and methanotrophy in hydrogen-rich ultramafic-hosted systems are estimated to be the major sources of metabolic energy available in this type of hydrothermal setting (McCollom 2007). There is also evidence of hydrogen conversion capacity in sulfur-oxidizing metazoan symbionts belonging to Campylobacterota and Proteobacteria (Jiang et al. 2020; Miyazaki et al. 2020; Petersen et al. 2011). Terminal electron acceptors commonly used by hydrogen-oxidizing microorganisms are sulfate, iron (III), and nitrate (Vignais et al. 2007) but also elemental sulfur and different metals, such as Mn (III/IV), U (VI), Cr (VI), Co (III), and Tc (VII) (Liu et al. 2002; Nakagawa et al. 2008). Genes encoding hydrogenase have been selectively amplified (group 1 and F420-reducing [NiFe]-hydrogenases) in hydrothermal samples or mined from metagenomes and metatranscriptomes (consisting of the whole genetic information) of vent-derived samples (Adam and Perner 2018). According to the hydrogenase classification of Sondergaard et al. (2016), the [NiFe]-hydrogenases of Nautilia profundicola, Hydrogenovibrio crunogenus, and Desulfvibiro vulgaris belong to group 1b, that of Aquifex aeolicus belong to group 1d, and that of Geoglobus acetivorans belong to group 1k. The majority of the [NiFe]hydrogenase genes identified in these hydrothermal datasets could be phylogenetically affiliated with members of the Campylobacterota phylum, to other Proteobacteria (Gamma and Delta), and archaeal phyla (Adam and Perner 2018). Hydrogenase is also found in a variety of archaea and bacteria, such as Thermococcales and Firmicutes, for catalyzing hydrogen production.
Hydrogen-oxidizing microbes isolated from hydrothermal vents include Campylobacterota, Proteobacteria (Gamma- and Delta-), Aquificae, Thermodesulfobacteria, and Archaea (Fig. 1, Table 1). The enrichment and purification of hydrogen-oxidizing bacteria could be conducted, for example, in MMJHS medium with low or no oxygen content in the headspace (Takai et al. 2003a, b, c).
Campylobacterota
Campylobacterota plays a major role in hydrogen conversion and hydrogen-based primary production within hydrothermal vents (Adam and Perner 2018). Most isolates in the phylum Campylobacterota are characterized by versatile metabolisms and only a few isolates are strictly hydrogen oxidizers (i.e., they are not capable of using any other tested organic or inorganic electron donor), such as the mesophilic Sulfurovum aggregans (Mino et al. 2014).
Proteobacteria
Through metagenomic analysis of hydrogenases, it was found that H2-oxidizing Betaproteobacteria belonging to the order Burkholderiales thrive in shallow, aerobic to anoxic transition zones (Brazelton et al. 2012). Gammaproteobacteria has also been demonstrated to be relevant for microbially mediated hydrogen cycling (Adam and Perner 2018). Strains of the genera Hydrogenovibrio and Thiomicrospira have been shown to use hydrogen (Hansen et al. 2015,2016). Metagenomic and metatranscriptomic analyses revealed that the numerous deep-sea bacteria in clade SUP05 contain highly expressed genes coding for group 1 [NiFe]-hydrogenase for H2 oxidation (Anantharaman et al. 2013). Deltaproteobacteria may also contribute to hydrogen consumption in anoxic hydrothermal vent habitats (Adam and Perner 2018). The vent-originating autotrophic, hydrogen-oxidizing Deltaproteobacteria are nearly all characterized as thermophiles with optimal temperature between 50 and 61 °C, such as Desulfacinum hydrothermales or Dissulfuribacter thermophilus (Sievert and Kuever 2000; Slobodkin et al. 2013). One iron-oxiding Zetaproteobacteria, named Ghiorsea bivora, can also use molecular hydrogen (H2) as the sole electron donor and oxygen as a terminal electron acceptor for energy production (Mori et al. 2017).
Aquificae
Some members of the Aquificales, such as Thermovibrio ammonificans, T. guaymasensis, Balnearium lithotrophicum and members of the genus Desulfurobacterium, use hydrogen as the only energy source for autotrophic growth (L’Haridon et al. 2006a; b; Takai et al. 2003a, b, c; Vetriani et al. 2004).
Thermodesulfobacteria
In the phylum Thermodesulfobacteria, the thermophilic sulfate-reducing bacteria Thermodesulfobacterium hydrogeniphilum, Thermodesulfatator atlanticus, and Thermodesulfatator indicus are also known to use H2 as an electron donor and sulfate as an electron acceptor for autotrophic growth (Alain et al. 2010; Jeanthon et al. 2002; Moussard et al. 2004).
Archaea
Except for thermophilic methanogens of the order Methanococcales (mentioned in 2.1.2), other hydrogen-oxidizing hyperthermophilic archaea include Geoglobus ahangari (Kashefi et al. 2002), G. acetivorans (Slobodkina et al. 2009b), and Pyrodictium delaneyi (Lin et al. 2016), which thrive at lower hydrogen concentrations than methanogens.
Nitrogen cycle
Generally, high-temperature fluids (up to 350 °C) contain high levels of NH4+, while low-temperature diffuse fluids (< 50 °C) contain NO3− (Butterfield et al. 1997; Lam et al. 2004). These nitrogenous compounds are discharged from hydrothermal vents and serve as potentially important substrates and nitrogen sources for chemolithotrophic bacteria (Bourbonnais et al. 2012; Butterfield et al. 1997; Lam et al. 2004). The deep ocean also contains the largest reservoir of nitrogen available to hydrothermal vents with about 0.59 mmol/L of dissolved nitrogen gas (Bourbonnais et al. 2012).
Ammonium-oxidizers
Ammonium is the dominant species of dissolved inorganic nitrogen and can be produced by mixing water masses below hydrothermal vents and discharged in vent fluids. The quantities of available ammonium vary from one vent to another, from one end-member fluid to another, and with the geological settings/activity. NH4+ oxidation is prevalent in hydrothermal plumes, active smokers, animals, and microbial mats. A removal rate of 15 nmol/L NH4+ day−1 was observed for incubations carried out at 200 atm with plume samples from the Endeavor Segment of the Juan de Fuca Ridge (Cowen et al. 1998). Xu et al. (2014) found that ammonium oxidizing archaea (AOA) were much more diverse but less abundant than ammonium-oxidizing bacteria (AOB) in samples collected at the Mid-Atlantic Ridge and in the South Atlantic Ocean. By combining 16S rRNA gene sequence analyses, ladderane lipid analyses, and measurements of a 14N15N dinitrogen production in isotope-pairing experiments, it was found that active ammonium-oxidizers at deep-sea hydrothermal vents are related to known anammox bacteria (Byrne et al. 2009). No cultures of ammonium-oxidizing archaea (AOA), ammonium-oxidizing bacteria (AOB), or nitrite-oxidizing bacteria (NOB) have been reported from hydrothermal vents.
Archaea—Crenarchaeota
The proportion of the marine group I (MGI) Crenarchaeota, the group that includes the ammonium-oxidizing isolate “Candidatus Nitrosopumilus maritimus”, was found to vary from 2–28% of the total prokaryotic community within the first 10 m from the venting source, depending on the vent field and chimney (Takai et al. 2004c). Metagenomic and metatranscriptomic analyses of ammonium-rich hydrothermal plumes from the Guaymas Basin, showed that the most abundant and active lineages were also those of MGI archaea related to the cultured autotrophic ammonia-oxidizer, “Candidatus Nitrosopumilus maritimus”, but with key genomic and physiological differences (Baker et al. 2012).
Betaproteobacteria
AOB found in the plumes of the Endeavor Segment are predominantly Nitrosomonas spp., accounting for approximately 7% of the total microbial community (Lam et al. 2004).
Denitrifier
Nitrate and nitrite concentrations are relatively depleted at hydrothermal vents compared to the surrounding seawater. Few studies focusing on denitrifier communities have been conducted in deep-sea hydrothermal vents, but the limited data available suggest that chemolithotrophic denitrification would appear to be an important process given the high concentration of reduced sulfur species (notably H2S) in these environments (Bowles et al. 2012).
Campylobacterota
The organization of the Nap gene cluster, NapAGHBFLD, is conserved in numerous vent Campylobacterota, such as Sulfurimonas autotrophica, S. denitrificans, Sulfurovum sp. NBC37, Nitratiruptor sp. SB155, and Nitratiruptor salsuginis, whereas Campylobacter jejuni lacks the napF gene (Vetriani et al. 2014). NirS sequences have been retrieved from these systems, mainly from Campylobacterota (Bourbonnais et al. 2014). Most isolated strains have been characterized as denitrifiers (Table 1), such as Cetia pacifica (Grosche et al. 2015), Caminibacter spp. (Alain et al. 2002a; Miroshnichenko et al. 2004; Voordeckers et al. 2005), Sulfurovum spp. (Giovannelli et al. 2016; Inagaki et al. 2004; Mino et al. 2014; Mori et al. 2018) and Sulfurimonas spp. (Inagaki et al. 2003; Takai et al. 2006) and nitrous oxide (N2O) can be reduced at the final step of the microbial denitrification pathway. Enrichment of the N2O-reducing microorganisms in the HNN medium revealed that Hydrogenimonas, of the phylum Campylobacterota, is the most predominant bacterial group throughout the cultivation period and its relative abundance was greater than 98%, contributing to the consumption of N2O by the nitrous oxide reductase encoded by the NosZ gene (Mino et al. 2018).
Proteobacteria
Gammaproteobacteria also contain NirS (Bourbonnais et al. 2014). Some isolates in the phylum Proteobacteria, such as Pseudodesulfovibrio indicus, can also reduce nitrate (Cao et al. 2016). It has also been shown that sulfide-oxidizing bacteria can use nitrate as an electron acceptor for the oxidation of hydrogen sulfide in hydrothermal vent systems, for example, Beggiatoa spp. (McHatton 1996).
Aquificae
Most isolated strains from hydrothermal vents in the phylum Aquificae, have been characterized as denitrifiers (Table 1), e.g., Desulfurobacterium spp. (Alain et al. 2003; Cao et al. 2017; L’Haridon et al. 1998, 2006a, b), Thermovibrio spp. (L’Haridon et al. 2006a, b; Vetriani et al. 2004), H. okinawensis (Nunoura et al. 2008a) and Persephonella spp. (Gotz et al. 2002; Nakagawa 2003). In the Desulfurobacterium crinifex isolate, nitrate reduction supported higher growth rates than sulfur reduction.
Deferribacteres
Caldithrix abyssi, in the phylum Deferribacteres, grows chemoorganoheterotrophically or chemolithoheterotrophically utilizing molecular hydrogen as an electron donor and nitrate as an electron acceptor (Miroshnichenko et al. 2003b).
Nitrogen fixation
Mehta et al. (2003) provided the first evidence of the presence of NifH genes in deep-sea hydrothermal vents and found that methanogenic archaea are the dominant diazotrophs. Since then, proteobacterial diazotrophs have also been found to be abundant in deep-sea hydrothermal vents (Cao et al. 2015; Wu et al. 2014).
Iron cycle
In inactive chimney deposits, the dominant microbial processes involve metals (Orcutt et al. 2011). In iron- and manganese-oxide mats that form around diffuse flow venting, metal-cycling types of metabolism dominate, especially around high metal concentration seamounts (Kato et al. 2009; Emerson and Moyer 2002). Iron-oxidizing communities from deep-sea hydrothermal vents include chemolithotrophic Zetaproteobacteria and mixotrophic Gammaproteobacteria, which are generally isolated and characterized using the gradient tube method or in liquid media with low concentrations of Fe (II) and O2 (Emerson and Moyer 2002). At deep-sea hydrothermal vents, the iron-respiring species belong to the Proteobacteria, Deferribacteres, Archaea, and fermentative types of Firmicutes (Fig. 1, Table 1).
Iron-oxidizers
Chemosynthetic Fe-oxidizing communities are common in diffuse-flow hydrothermal vents throughout the world’s oceans (Makita et al. 2018).
Zetaproteobacteria
Zetaproteobacteria is primarily associated with ecosystems fed by ferrous iron, Fe (II) (Emerson et al. 2007). These strains produce iron oxide products with an aspect distinct from the usual stalks (xag operon), with filamentous and dread-like structures (Makita et al. 2018). Studies on ferro-oxidant taxa carried out on samples from the Southern Mariana Arc, the Hawaiian hot spot and the area of the Vai’lulu/Tonga Arc/East Lau Spreading Center/Kermadec Arc have shown that members of the Zetaproteobacteria fall into different 16S rRNA phylotypes of Zetaproteobacteria and include the microaerobic and chemolithotrophic iron-oxidizer Mariprofundus ferrooxydans (Singer et al. 2013). Mariprofundus ferrooxydans strains were isolated from the Loihi seamount vent field (strains PV-1 T and JV-1) in the first instance (Emerson and Moyer 2002; Emerson et al. 2007). M. micogutta ET2 was isolated from a deep-sea hydrothermal field at the Bayonnaise Knoll of the Izu-Ogasawara Arc (Makita et al. 2016). Ghiorsea bivora strains (TAG-1 and SV-108) were isolated from the Mid-Atlantic Ridge (TAG-1) and the Mariana back-arc (SV-108), which are unique in that they can utilize either Fe(II) or H2 as the sole electron donor and oxygen as the terminal electron acceptor for growth (Mori et al. 2017). Genome analysis of Mariprofundus ferrooxydans strain PV1 revealed a complete TCA cycle, the ability to fix CO2 with two sets of RuBisCo genes, and three carbonic anhydrase-encoding genes predicted to function in the rapid conversion of CO2 to bicarbonate, carbon-storage proteins, and a sugar phosphotransferase system (PTS). This genome is also found to contain a cytochrome cbb3 oxidase regulon (ccoNOP), two distinct cytochrome bd quinol oxidases and molybdopterin oxidoreductase for electron transport. Heme-containing cytochromes with peroxidase activity are likely to play a significant role in the oxidation of Fe(II) (Singer et al. 2011). Metagenomic analyses have also shown that nitrite reductases, sulfide: quinone oxidases, and RuBisCo are present in Zetaproteobacteria MAGs (Singer et al. 2013). In addition, genome and metatranscriptome analyses focusing on Zetaproteobacteria have shown that a homolog of the Fe oxidase Cyc2 of Acidithiobacillus ferrooxidans is highly expressed in incubation in vitro and in situ (McAllister et al. 2020).
Gammaproteobacteria
Some marine aerobic heterotrophic bacteria, such as members of the genus Marinobacter, have also been suggested to have physiological traits including nitrate-dependent Fe(II)-oxidation, Mn(II) oxidation, and arsenate and fumarate redox cycling properties (Handley and Lloyd 2013). A gammaproteobacterial strain (strain NP-6) of the genus Marinobacter was also isolated from aphyric, cryptocrystalline basalt over 300 m below the seafloor in the North Pond site of the Mid-Atlantic Ridge and found to have neutrophilic iron-oxidizing capabilities (Zhang et al. 2016). Sudek et al. (2009) reported that heterotrophs taxa (e.g., Pseudoalteromonas sp. and Pseudomonas sp.) isolated from a volcanic seamount, can also catalyze ferrous iron oxidation under micro-aerobic conditions. In addition, Alphaproteobacteria (e.g., Hyphomonas sp.) taxa from hydrothermal fields of the Juan de Fuca Ridge have been shown to oxidize Fe(II)(Edwards et al. 2003).
Finally, iron-oxidizing capacities have been reported for some sulfur-oxidizing Gammaproteobacteria, such as Thiomicrospira sp. (Barco et al. 2017) and Hydrogenovibrio sp. (Neely et al. 2018).
Iron-reducers
Dissimilatory iron-reducing microorganisms (DIRMs) can use extracellular Fe(III) as an electron acceptor and reduce Fe(III) to Fe(II) (Lovley et al. 1991). Deep-sea hydrothermal fields can be an ecological niche for DIRMs (Melton et al. 2014; Slobodkin et al. 2001), because they are rich in iron, and 60–70% of it is oxidized [Fe(III)] (Toner et al. 2012). So far, only a few DIRMs have been isolated from hydrothermal environments. Most of them belong to the phyla Proteobacteria and Deferribacteres, and are described as “respiratory-type.” Fermentative DIRMs have been reported in the Firmicutes and Euryarchaeota. In mesophilic bacteria, iron reduction by Geobacter and Shewanella species depends on polyheme c-type cytochrome proteins for electron transfer through the cell wall (Weber et al. 2006). Some hyperthermophilic archaea, such as Geoglobus ahangari and Pyrodictium delaneyi (strain Hulk), also require direct contact between the cell and the iron mineral to transfer respiratory electrons by c-type cytochromes to the outer surface of the cell (Demey et al. 2017; Manzella et al. 2013). Some DIRMs, such as Anoxybacter fermentans from hydrothermal sulfides, Desulfotomaculum reducens MI-1 from marine sediments, and Orenia metallireducens Z6 from deep underground, lack c-type cytochromes involved in extracellular electron transfer (EET) and these organisms could, therefore, have different iron reduction mechanisms (Dalla et al. 2013; Dong et al. 2016; Li et al. 2020).
To date, studies on the reduction of manganese, arsenic, and other heavy metals (copper, cadmium, and lead) and the resistance of microorganisms to these compounds in hydrothermal vents remain very limited, despite their important role in some areas (Hu et al. 2020; Mandernack and Tebo 1993).
Proteobacteria
Shewanella sp. PV-4, isolated from an iron-rich microbial mat in an active deep-sea hydrothermal vent, performs iron reduction using lactate, fumarate, and H2 as electron donors, leading to magnetite formation (Roh et al. 2006).
Deferribacteres
Other respiratory-type DIRMs isolated from hydrothermal vents include Deferribacter abyssi (Miroshnichenko et al. 2003a), D. autotrophicus (Slobodkina et al. 2009a), Geothermobacter ehrlichii (Kashefi et al. 2003), and Deferrisoma camini (Slobodkina et al. 2012).
Firmicutes
A few fermentative DIRMs have been reported in the Firmicutes phylum; they are Thermoanaerobacter siderophilus (Slobodkin et al. 1999), Thermovenabulum ferriorganovorum (Zavarzina et al. 2002), C. ferrireducens strain DY22619 (Zeng et al. 2015a), Anoxybacter fermentans strain DY22613 (Zeng et al. 2015b) and Wukongibacter baidiensis strain DY30321 (Li et al. 2016a, b). In Anoxybacter fermentans strain DY22613, growth is promoted by iron reduction. The mode of action of exogenous and endogenous redox molecules to co-mediate indirect EET was considered important in the process of iron reduction in this strain (Li et al. 2019, 2020). The dissimilatory iron reduction is also catalyzed by hyperthermophilic archaea in hydrothermal vents.
Archaea—Euryarchaeota
These hyperthermophilic iron-reducers belong mainly to the families Archaeoglobaceae and Pyrodictiaceae. Geoglobus ahangari, which was isolated from the Guaymas Basin (MOSC site), is capable of using H2 as the sole electron donor and reducing Fe(III) without the need for an organic carbon source and can also couple the oxidation of organic carbon, such as fatty acids and complex organic materials, to the reduction of Fe(III) at hyperthermophilic temperatures (Kashefi et al. 2002). Geoglobus acetivorans is also an obligate Fe(III) reducer of hydrothermal origin with an optimal growth temperature of 81 °C (Slobodkina et al. 2009b). Geogemma barossii strain 121, an obligate lithoautotroph archaeon isolated from an active ‘‘black smoker’’ from the Juan de Fuca Ridge, can utilize molecular hydrogen to reduce ferric iron and grow at 121 °C, the highest growth temperature known so far (Kashefi and Lovley 2003). The hyperthermophilic, autotrophic iron and nitrate reducer, Pyrodictium delaneyi strain Su06T isolated from an active deep-sea hydrothermal vent chimney on the Endeavor Segment in the northeastern Pacific Ocean, is an obligate anaerobic, hydrogenotroph reducing Fe(III) oxide to magnetite and NO3− to N2. The heterotroph Hyperthermus hephaesta Ro04 isolated from the same area is also able to reduce Fe(III) (Lin et al. 2014, 2016). Thermococcus indicus strain IOH1, T. celericrescens DSM 17994, and T. siculi DSM 12349 have also been reported to reduce soluble Fe(III)-citrate present in their growth medium, which improves their growth (Lim et al. 2020).
Virus
The limited abundance data available tend to indicate that deep-sea hydrothermal vents also host a large number of viruses, indicating high viral production (Ortmann and Curtis 2005). Samples from the Endeavor hydrothermal vents, located off the coast of southwest British Columbia, showed that active venting black smokers at these sites had viral abundances ranging from (1.45 × 105–9.90 × 107/ml) with a drop-off in abundance found in the hydrothermal-vent plume (3.5 × 106/ml) and outside the venting system (2.94 × 106/ml) (Ortmann et al. 2005). The metabolic compensation of hosts by viruses could be a very important aspect of virus-host interactions (He et al. 2017). The characterization of viruses from hydrothermal vents is limited so far with only eight viruses (two archaeal and eight bacterial viruses) described in detail, originating from archaeal and bacterial cultivated hosts (Lossouarn et al. 2015). Among them, one can cite, for example, the PAV1 from Pyrococcus abyssi (Geslin et al. 2003), the TPV1 from Thermococcus prieurii (Gorlas et al. 2012), and the viruses MPV1, MC1, and MC2 from Marinitoga piezophila and M. camini (Mercier et al. 2018). The high number of viruses and thus viral production (relative to the surrounding deep-sea waters) raises the question of whether viruses are an important source of microbial mortality at the vents and would have, in that case, a crucial ecological role in deep-sea vents, similar to other ecosystems. In our current state of knowledge (which is limited), the majority of viruses described from hydrothermal habitats are temperate and, therefore, do not lyse the host they infect. If they do not have a main role in population turnover, which remains to be clarified, these viruses probably have a very important role in the evolution of hydrothermal communities, as observed in other ecosystems, through gene transfers.
Perspectives
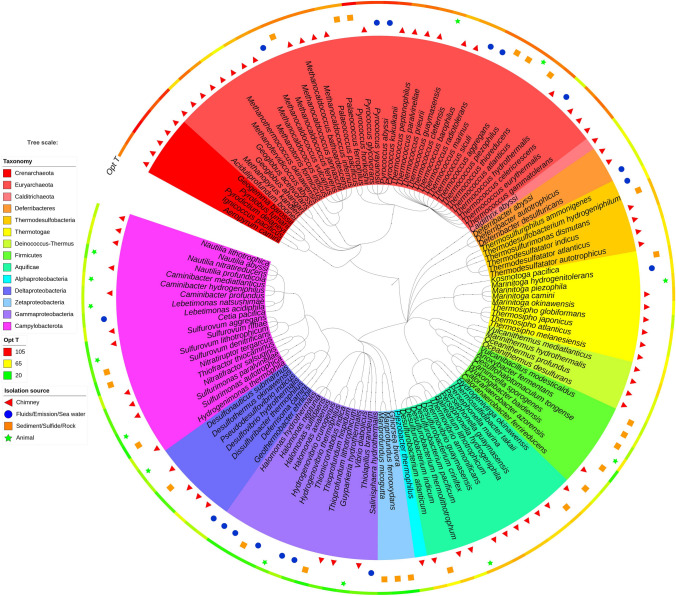
The potential impact of the environment on microbial communities within hydrothermal vent systems could be much more intense than in other habitats due to the dynamic and steep chemical and thermal gradients that prevail there. The characterization of isolates helps us to understand the microbial metabolism and their adaptation mechanisms. Many Campylobacterota genera grow chemolithoautotrophilically using a variety of electron donors and acceptors. Some sulfur-oxidizing lithoautotrophic Gammaproteobacteria, such as Thiomicrospira sp. and Hydrogenovibrio sp. are also iron-oxidizers. Gammaproteobacteria, such as Salinisphaera hydrothermalis, and the archaeon Pyrobaculum islandicum can live as autotrophs or heterotrophs. The chemolithoautotrophic growth of Piezobacter thermophiles is observed strictly under piezophilic conditions. This adaptation capability is important for effective competition within the hydrothermal ecosystem (Segerer et al. 1993). Furthermore, genomic comparisons of more isolates and metagenome-assembled genomes will also support the elucidation of microbial evolution strategies in hydrothermal vents. For example, Zhang et al. (2014) found that the pan-genome of mesophilic Epsilonproteobacteria includes niche-specific and lineage-specific genes and it is a complex process of gene acquisition, conservation, and removal, contributing to their metabolic diversity and versatility. The culturing studies to date have been biased towards hyperthermophiles, thermophiles, and mesophilic sulfur- and metal-metabolizing chemoautotrophs (Fig. 1, Fig. 2, Supplementary Table S1). They include: (1) thermophilic or hyperthermophilic archaeon with an optimal growth temperature upto 106 °C; (2) members of the phyla of Thermodesulfobacteria, Thermotogae, Deferribacteres, Calditrichaeota, Aquificae, Deinococcus-Thermus, which are thermophiles, mainly from chimney and sediments; (3) Campylobacterota and Proteobacteria are diverse with psychrophiles, mesophiles, and thermophiles, which are found in fluids, plumes, chimneys, sediment, rock, and animals.
Fig. 2.
Phylogenetic tree of 129 representative microbial isolates obtained from the deep-sea hydrothermal sources. The phylogenetic tree, based on 16S rRNA genes of isolates deposited in the NCBI taxonomy database, is constructed by phyloT and visualized by iTOL. The inner color-coded circle indicates the phylum to which the isolate belongs; symbols outside the inner ring indicate the matrix from which the isolate was obtained; the outer circle indicates the optimal growth temperature of the isolates. Opt T, optimal growth temperature
Recent discoveries in culture-independent metagenomic, metatranscriptomic, and metaproteomic approaches have led to a better understanding of microbial metabolic pathways and the identification of new microorganisms that have not been isolated (Baker et al. 2012; Cerqueira et al. 2018; Meier et al. 2019). For example, phylogenomic analysis of Juan de Fuca Ridge flank subsurface fluids revealed 53 bacterial and 45 archaeal genomes from metagenomes, of which nearly all are distantly related to known cultured isolates, including Chloroflexi, Nitrospirae, Acetothermia (OP1), EM3, Aminicenantes (OP8), Bathyarchaeota (formerly MCG), and Candidatus “Hydrothermarchaeota” (formerly Marine Benthic Group E, MBGE) (Jungbluth et al. 2017).
According to these results, new culture media and enrichment techniques could be developed to target detected uncultured taxa. We could design the medium, (i) mimicking, as closely as possible, the actual physical–chemical conditions in which these taxa are detected such as temperature, pH, and hydrostatic pressure (ii) multiplying the electron donor and acceptor pairs used, with a range of carbon sources, with different concentrations of oxygen, by high-throughput culturomic; or (iii) directing culturing based on available metagenomic data on target taxa (including metabolic pathways present and auxotrophy). Many studies have recently focused on cultivation. Using low-temperature and high-hydrostatic pressure incubations in the presence of hydrocarbons have been proven to be efficient for enrichment and isolation of certain groups of the yet-uncultivated SAR324 group and the SUP05 clade (Wang et al. 2020). The biopolymer lignin significantly stimulates the growth of the Bathyarchaeota subgroup Bathy-8 (Yu et al. 2018). By deploying in situ enrichment carriers in hot marine hydrothermal sediments, some thermophilic heterotrophic microorganisms that have not yet been cultured have been identified (Stokke et al. 2020). Enrichment cultures of electrotrophic communities are obtained on electrodes and show a specific enrichment of Archaea belonging to the Thermococcales and Archaeoglobales orders in microbial electrolysis cells (specially designed bio-electrochemical system) under hyperthermophilic conditions (80 °C) (Pillot et al. 2018). In an environmental context other than hydrothermal sources, high-potential cathodic experiments were also conducted to enrich microorganisms in North Pond crustal samples capable of extracellular electron transport (EET) (Jones et al. 2020). Subsequent analyses of these incubations, using meta-omic analysis, fluorescence in situ hybridization coupled to secondary ion mass spectrometry (FISH-SIMS), isotope labeling experiments, and chemical analysis were necessary to understand the microbial community and metabolic pathways. These innovative culture techniques and highly targeted enrichment culture methods or mimicking in situ conditions will make it possible to obtain new microbes from hydrothermal vents and to elucidate the keys to their physiological versatility and adaptations to the conditions of their habitat.
Extremophilic microorganisms produce novel organic compounds and stable biocatalysts that function under extreme conditions comparable to those prevailing in various industrial processes. Products from hydrothermal microorganisms that have undergone biotechnological development are quite limited considering the huge reservoir of diversity and molecules contained in hydrothermal sources. Among the best known are the Deep Vent® DNA polymerase (New England Biolabs) from Pyrococcus strain GB-D and the Vent® DNA polymerase (New England Biolabs) from Thermococcus litoralis used in molecular biology, the alpha-amylase Fuelzyme® (Verenium) from a deep hydrothermal sample used for starch liquefaction in the process of bioethanol production, or the Pyrolase® (Verenium) used for example in green chemistry to control viscosity in oil wells. In the future, more microbial products from hydrothermal microorganisms are expected to lead to other industrial applications. This potential for innovation deserves further exploration.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2018YFC0310701), the Chinese National Natural Science Foundation (No. 91951201), the Scientific Research Foundation of Third Institute of Oceanography, MNR (No. 2017003) and the Sino-French LIA/IRP 1211 MicrobSea.
Author contributions
XZ analyzed the data and wrote the paper. KA, ZS revised the paper. XZ, ZS offered the foundation. The final manuscript was approved by all the authors.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and human rights statement
This article does not contain any studies with human participants or animals performed by the authors.
Footnotes
SPECIAL TOPIC: Cultivation of uncultured microorganisms.
Edited by Chengchao Chen.
References
- Adair FW, Gundersen K. Chemoautotrophic sulfur bacteria in the marine environment. I. Isolation, cultivation, and distribution. Can J Microbiol. 1969;15:345–353. doi: 10.1139/m69-064. [DOI] [PubMed] [Google Scholar]
- Adams MM, Hoarfrost AL, Bose A, Joye SB, Girguis PR. Anaerobic oxidation of short-chain alkanes in hydrothermal sediments: potential influences on sulfur cycling and microbial diversity. Front Microbiol. 2013;14(4):110. doi: 10.3389/fmicb.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam N, Perner M. Microbially mediated hydrogen cycling in deep-sea hydrothermal vents. Front Microbiol. 2018;9:2873. doi: 10.3389/fmicb.2018.02873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerman NH, Butterfield DA, Huber JA. Phylogenetic diversity and functional gene patterns of sulfur-oxidizing subseafloor Epsilonproteobacteria in diffuse hydrothermal vent fluids. Front Microbiol. 2013;4:185–185. doi: 10.3389/fmicb.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain K, Marteinsson VT, Miroshnichenko ML, Bonchosmolovskaya EA, Prieur D, Birrien JL. Marinitoga piezophila sp. nov., a rod-shaped, thermo-piezophilic bacterium isolated under high hydrostatic pressure from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2002;52:1331–1339. doi: 10.1099/00207713-52-4-1331. [DOI] [PubMed] [Google Scholar]
- Alain K, Querellou J, Lesongeur F, Pignet P, Crassous P, Gerard R, Cueff V, Cambonbonavita M. Caminibacter hydrogeniphilus gen. nov., sp. nov., a novel thermophilic, hydrogen-oxidizing bacterium isolated from an East Pacific Rise hydrothermal vent. Int J Syst Evol Microbiol. 2002;52:1317–1323. doi: 10.1099/00207713-52-4-1317. [DOI] [PubMed] [Google Scholar]
- Alain K, Pignet P, Zbinden M, Quillevere M, Duchiron F, Donval J, Lesongeur F, Raguenes G, Crassous P, Querellou J, Cambonbonavita M. Caminicella sporogenes gen. nov., sp. nov., a novel thermophilic spore-forming bacterium isolated from an East-Pacific Rise hydrothermal vent. Int J Syst Evol Microbiol. 2002;52:1621–1628. doi: 10.1099/00207713-52-5-1621. [DOI] [PubMed] [Google Scholar]
- Alain K, Rolland S, Crassous P, Lesongeur F, Zbinden M, Le Gall C, Godfroy A, Page A, Juniper SK, Cambonbonavita M, Duchiron F, Querellou J. Desulfurobacterium crinifex sp.nov., a novel thermophilic, pinkish-streamer forming, chemolithoautotrophic bacterium isolated from a Juan de Fuca Ridge hydrothermal vent and amendment of the genus Desulfurobacterium. Extremophiles. 2003;7:361–370. doi: 10.1007/s00792-003-0329-4. [DOI] [PubMed] [Google Scholar]
- Alain K, Callac N, Guegan M, Lesongeur F, Crassous P, Cambonbonavita M, Querellou J, Prieur D. Nautilia abyssi sp. nov., a thermophilic, chemolithoautotrophic, sulfur-reducing bacterium isolated from an East Pacific Rise hydrothermal vent. Int J Syst Evol Microbiol. 2009;59:1310–1315. doi: 10.1099/ijs.0.005454-0. [DOI] [PubMed] [Google Scholar]
- Alain K, Postec A, Grinsard E, Lesongeur F, Prieur D, Godfroy A. Thermodesulfatator atlanticus sp. nov., a thermophilic, chemolithoautotrophic, sulfate-reducing bacterium isolated from a mid-atlantic ridge hydrothermal vent. Int J Syst Evol Microbiol. 2010;60:33–38. doi: 10.1099/ijs.0.009449-0. [DOI] [PubMed] [Google Scholar]
- Alazard D, Dukan S, Urios A, Frederic V, Bouabida N, Morel F, Thomas P, Garcia J, Ollivier B. Desulfovibrio hydrothermalis sp. nov., a novel sulfate-reducing bacterium isolated from hydrothermal vents. Int J Syst Evol Microbiol. 2003;53:173–178. doi: 10.1099/ijs.0.02323-0. [DOI] [PubMed] [Google Scholar]
- Amend AS. From dandruff to deep-sea vents: malassezia-like fungi are ecologically hyper-diverse. PLoS Pathog. 2014;10:e1004277. doi: 10.1371/journal.ppat.1004277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amend JP, Mccollom TM, Hentscher M, Bach W. Catabolic and anabolic energy for chemolithoautotrophs in deep-sea hydrothermal systems hosted in different rock types. Geochim Cosmochim Acta. 2011;75:5736–5748. [Google Scholar]
- Anantharaman K, Breier JA, Sheik CS, Dick G. Evidence for hydrogen oxidation and metabolic plasticity in widespread deep-sea sulfur-oxidizing bacteria. Proc Natl Acad Sci USA. 2013;110:330–335. doi: 10.1073/pnas.1215340110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine E, Cilia V, Meunier JR, Guezennec J, Lesongeur F, Barbier G. Thermosipho melanesiensis sp. nov., a new thermophilic anaerobic bacterium belonging to the order thermotogales, isolated from deep-sea hydrothermal vents in the southwestern Pacific Ocean. Int J Syst Bacteriol. 1997;47:1118–1123. doi: 10.1099/00207713-47-4-1118. [DOI] [PubMed] [Google Scholar]
- Audiffrin C, Cayol J, Joulian C, Casalot L, Thomas P, Garcia J. Desulfonauticus submarinus gen. nov., sp. nov., a novel sulfate-reducing bacterium isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2003;53:1585–1590. doi: 10.1099/ijs.0.02551-0. [DOI] [PubMed] [Google Scholar]
- Baker BJ, Lesniewski RA, Dick GJ. Genome-enabled transcriptomics reveals archaeal populations that drive nitrification in a deep-sea hydrothermal plume. ISME J. 2012;6:2269–2279. doi: 10.1038/ismej.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier G, Godfroy A, Meunier J, Querellou J, Cambon M, Lesongeur F, Grimont PAD, Raguenes G. Pyrococcus glycovorans sp. nov., a hyperthermophilic archaeon isolated from the East Pacific Rise. Int J Syst Evol Microbiol. 1999;49:1829–1837. doi: 10.1099/00207713-49-4-1829. [DOI] [PubMed] [Google Scholar]
- Barco RA, Hoffman CL, Ramírez GA, Toner BM, Edwards KJ, Sylvan JB. In-situ incubation of iron-sulfur mineral reveals a diverse chemolithoautotrophic community and a new biogeochemical role for Thiomicrospira. Environ Microbiol. 2017;19:1322–1337. doi: 10.1111/1462-2920.13666. [DOI] [PubMed] [Google Scholar]
- Beatty JT, Overmann J, Lince MT, Manske AK, Lang AS, Blankenship RE, Van Dover CL, Martinson TA, Plumley FG. An obligately photosynthetic bacterial anaerobe from a deep-sea hydrothermal vent. Proc Natl Acad Sci USA. 2005;102:9306–9310. doi: 10.1073/pnas.0503674102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellack A, Huber H, Rachel R, Wanner G, Wirth R. Methanocaldococcus villosus sp. nov., a heavily flagellated archaeon that adheres to surfaces and forms cell–cell contacts. Int J Syst Evol Microbiol. 2011;61:1239–1245. doi: 10.1099/ijs.0.023663-0. [DOI] [PubMed] [Google Scholar]
- Biddle JF, Cardman Z, Mendlovitz HP, Albert DB, Lloyd KG, Boetius A, Teske A. Anaerobic oxidation of methane at different temperature regimes in Guaymas Basin hydrothermal sediments. ISME J. 2012;6:1018–1031. doi: 10.1038/ismej.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrien J, Zeng X, Jebbar M, Cambonbonavita M, Querellou J, Oger P, Bienvenu N, Xiao X, Prieur D. Pyrococcus yayanosii sp. nov., an obligate piezophilic hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2011;61:2827–2881. doi: 10.1099/ijs.0.024653-0. [DOI] [PubMed] [Google Scholar]
- Blochl E, Rachel R, Burggraf S, Hafenbradl D, Jannasch HW, Stetter KO. Pyrolobus fumarii, gen. and sp. nov., represents a novel group of archaea, extending the upper temperature limit for life to 113°C. Extremophiles. 1997;1:14–21. doi: 10.1007/s007920050010. [DOI] [PubMed] [Google Scholar]
- Boden R. Reclassification of Halothiobacillus hydrothermalis and Halothiobacillus halophilus to Guyparkeria gen. nov. in the Thioalkalibacteraceae fam. nov., with emended descriptions of the genus Halothiobacillus and family Halothiobacillaceae. Int J Syst Evol Microbiol. 2017;67:3919–3928. doi: 10.1099/ijsem.0.002222. [DOI] [PubMed] [Google Scholar]
- Boden R, Scott KM, Williams J, Russel S, Antonen K, Rae AW, Hutt LP. An evaluation of Thiomicrospira, Hydrogenovibrio and Thioalkalimicrobium: reclassification of four species of Thiomicrospira to each Thiomicrorhabdus gen. nov. and Hydrogenovibrio, and reclassification of all four species of Thioalkalimicrobium to Thiomicrospria. Int J Syst Evol Microbiol. 2017;67:1140–1151. doi: 10.1099/ijsem.0.001855. [DOI] [PubMed] [Google Scholar]
- Bonchosmolovskaya EA. Bacterial sulfur reduction in hot vents. FEMS Microbiol Rev. 1994;15:65–77. [Google Scholar]
- Bonchosmolovskaya EA, Perevalova AA, Kolganova TV, Rusanov II, Jeanthon C, Pimenov N. Activity and distribution of thermophilic prokaryotes in hydrothermal fluid, sulfidic structures, and sheaths of Alvinellids (East Pacific Rise, 13°N) Appl Environ Microbiol. 2011;77:2803–2806. doi: 10.1128/AEM.02266-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbonnais A, Lehmann MF, Butterfield DA, Juniper SK. Subseafloor nitrogen transformations in diffuse hydrothermal vent fluids of the Juan de Fuca Ridge evidenced by the isotopic composition of nitrate and ammonium. Geochem Geophys Geosyst. 2012;13:Q02T01. [Google Scholar]
- Bourbonnais A, Juniper SK, Butterfield DA, Anderson RE, Lehmann MF. Diversity and abundance of bacteria and nirS-encoding denitrifiers associated with the Juan de fuca ridge hydrothermal system. Ann Microbiol. 2014;64:1691–1705. [Google Scholar]
- Bowles MW, Nigro LM, Teske AP, Joye SB. Denitrification and environmental factors influencing nitrate removal in Guaymas Basin hydrothermally altered sediments. Front Microbiol. 2012;3:377. doi: 10.3389/fmicb.2012.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelton W, Nelson B, Schrenk M. Metagenomic evidence for H2 oxidation and H2 production by serpentinite-hosted subsurface microbial communities. Front Microbiol. 2012;2:268. doi: 10.3389/fmicb.2011.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgaud G, Le Calvez T, Arzur D, Vandenkoornhuyse P, Barbier G. Diversity of culturable marine filamentous fungi from deep-sea hydrothermal vents. Environ Microbiol Rev. 2009;11:1588–1600. doi: 10.1111/j.1462-2920.2009.01886.x. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Jonasson IR, Massoth GJ, Feely RA, Roe KK, Embley RE, Holden JF, McDuff RE, Lilley JR. Seafloor eruptions and evolution of hydrothermal fluid chemistry. Philos Trans R Soc Lond A. 1997;355:369–386. [Google Scholar]
- Byrne N, Strous M, Crepeau V, Kartal B, Birrien J, Schmid M, Lesongeur F, Schouten S, Jaeschke A, Jetten MSM, Prieur D, Godfroy A. Presence and activity of anaerobic ammonium-oxidizin g bacteria at deep-sea hydrothermal vents. ISME J. 2009;3:117–123. doi: 10.1038/ismej.2008.72. [DOI] [PubMed] [Google Scholar]
- Callac N, Oger P, Lesongeur F, Rattray JE, Vannier P, Michoud G, Beauverger M, Gayet N, Rouxel O, Jebbar M, Godfroy A. Pyrococcus kukulkanii sp. nov., a hyperthermophilic, piezophilic archaeon isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2016;66:3142–3149. doi: 10.1099/ijsem.0.001160. [DOI] [PubMed] [Google Scholar]
- Calvez TL, Burgaud G, Mahe S, Barbier G, Vandenkoornhuyse P. Fungal diversity in deep-sea hydrothermal ecosystems. Appl Environ Microbiol. 2009;75:6415–6421. doi: 10.1128/AEM.00653-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Shao Z, Li J, Zhang W, Qian PY. Phylogenetic diversity of nitrogen-utilizing genes in hydrothermal chimneys from 3 middle ocean ridges. Extremophiles. 2015;19:1173–1182. doi: 10.1007/s00792-015-0788-4. [DOI] [PubMed] [Google Scholar]
- Cao J, Gayet N, Zeng X, Shao Z, Jebbar M, Alain K. Pseudodesulfovibrio indicus gen. nov., sp. nov., a piezophilic sulfate-reducing bacterium from the Indian Ocean and reclassification of four species of the genus Desulfovibrio. Int J Syst Evol Microbiol. 2016;66:3904–3911. doi: 10.1099/ijsem.0.001286. [DOI] [PubMed] [Google Scholar]
- Cao J, Birien T, Gayet N, Huang Z, Shao Z, Jebbar M, Alain K. Desulfurobacterium indicum sp. nov., a thermophilic sulfur-reducing bacterium from the Indian Ocean. Int J Syst Evol Microbiol. 2017;67:1665–1668. doi: 10.1099/ijsem.0.001837. [DOI] [PubMed] [Google Scholar]
- Cerqueira T, Barroso C, Froufe H, Conceição E, Raul B. Metagenomic signatures of microbial communities in deep-sea hydrothermal sediments of azores vent fields. Microb Ecol. 2018;76:387–403. doi: 10.1007/s00248-018-1144-x. [DOI] [PubMed] [Google Scholar]
- Cha I, Roh SW, Kim S, Hong H, Lee H, Lim W, Rhee S. Desulfotomaculum tongense sp. nov., a moderately thermophilic sulfate-reducing bacterium isolated from a hydrothermal vent sediment collected from the Tofua Arc in the Tonga Trench. Antonie Van Leeuwenhoek. 2013;104:1185–1192. doi: 10.1007/s10482-013-0040-0. [DOI] [PubMed] [Google Scholar]
- Charlou JL, Donval JP, Fouquet Y, Jean-Baptiste P, Holm N. Geochemistry of high H2 and CH4 vent fluids issuing from ultramafic rocks at the Rainbow hydrothermal field (36°14’, MAR) Chem Geol. 2002;191:345–359. [Google Scholar]
- Connell L, Barrett A, Templeton A, Staudigel H. Fungal diversity associated with an active deep sea volcano: Vailulu’u Seamount, Samoa. Geomicrobiol J. 2009;26:597–605. [Google Scholar]
- Corliss JB, Dymond J, Gordon LI, Edmond JM, Herzen RP, Ballard RD, Green K, Williams D, Bainbridge A, Crane K, Andel THV. Submarine thermal sprirngs on the galapagos rift. Science. 1979;203:1073–1083. doi: 10.1126/science.203.4385.1073. [DOI] [PubMed] [Google Scholar]
- Cowen JP, Wen XY, Jones RD, Thomson RE. Elevated NH4+ in a neutrally buoyant hydrothermal plume. Deep Sea Res Part A Oceanogr Res Pap. 1998;45:1891–1902. [Google Scholar]
- Crespomedina M, Chatziefthimiou AD, Cruzmatos R, Pérez-Rodríguez I, Barkay T, Lutz RA, Starovoytov V, Vetriani C. Salinisphaera hydrothermalis sp. nov., a mesophilic, halotolerant, facultatively autotrophic, thiosulfate-oxidizing gammaproteobacterium from deep-sea hydrothermal vents, and emended description of the genus Salinisphaera. Int J Syst Evol Microbiol. 2009;59:1497–1503. doi: 10.1099/ijs.0.005058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Vecchia E, Suvorova EI, Bernier-Latmani R. Fe(III) reduction during pyruvate fermentation by Desulfotomaculum reducens strain MI-1. Geobiology. 2013;12:48–61. doi: 10.1111/gbi.12067. [DOI] [PubMed] [Google Scholar]
- Dalmasso C, Oger P, Selva G, Courtine D, Lharidon S, Garlaschelli A, Roussel EG, Miyazaki J, Reveillaud J, Jebbar M, Takai K, Maignien L, Karine A. Thermococcus piezophilus sp. nov., a novel hyperthermophilic and piezophilic archaeon with a broad pressure range for growth, isolated from a deepest hydrothermal vent at the Mid-Cayman Rise. Syst Appl Microbiol. 2016;39:440–444. doi: 10.1016/j.syapm.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Demey LM, Miller CR, Manzella MP, Spurbeck RR, Sandhu SK, Reguera G, Kashefi K. The draft genome of the hyperthermophilic archaeon Pyrodictium delaneyi strain hulk, an iron and nitrate reducer, reveals the capacity for sulfate reduction. Stand Genomic Sci. 2017;12:47. doi: 10.1186/s40793-017-0260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon A, Teske A, Dillon JG, Stahl DA, Sogin ML. Molecular characterization of sulfate-reducing bacteria in the Guaymas Basin. Appl Environ Microbiol. 2003;69:2765–2772. doi: 10.1128/AEM.69.5.2765-2772.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick GJ. The microbiomes of deep-sea hydrothermal vents: distributed globally, shaped locally. Nat Rev Microbiol. 2019;17:271–283. doi: 10.1038/s41579-019-0160-2. [DOI] [PubMed] [Google Scholar]
- Dobrinski KP, Longo DL, Scott KM. The carbon-concentrating mechanism of the hydrothermal vent chemolithoautotroph Thiomicrospira crunogena. J Bacteriol. 2005;187:5761–5766. doi: 10.1128/JB.187.16.5761-5766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski N, Seitz KW, Teske A, Baker BJ. Genomic insights into potential interdependencies in microbial hydrocarbon and nutrient cycling in hydrothermal sediments. Microbiome. 2017;5:106–106. doi: 10.1186/s40168-017-0322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Sanford RA, Fouke BW. Orenia metallireducens sp. nov. strain Z6, a novel metal-reducing member of the phylum Firmicutes from the deep subsurface. Appl Environ Microbiol. 2016;82:6440–6453. doi: 10.1128/AEM.02382-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand P, Reysenbach A, Prieur D, Pace NR. Isolation and characterization of Thiobacillus hydrothermalis sp. nov., a mesophilic obligately chemolithotrophic bacterium isolated from a deep-sea hydrothermal vent in Fiji Basin. Arch Microbiol. 1993;159:39–44. [Google Scholar]
- Edwards KJ, Rogers DR, Wirsen CO, McCollom TM. Isolation and characterization of novel psychrophilic, neutrophilic, Feoxidizing, chemolithoautotrophic α- and γ-Proteobacteria from the deep sea. Appl Environ Microbiol. 2003;69:2906–2913. doi: 10.1128/AEM.69.5.2906-2913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson D, Moyer CL. Neutrophilic Fe-oxidizing bacteria are abundant at the loihi seamount hydrothermal vents and play a major role in Fe oxide deposition. Appl Environ Microbiol. 2002;68:3085–3093. doi: 10.1128/AEM.68.6.3085-3093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson D, Rentz JA, Lilburn T, Davis RE, Aldrich HC, Chan CS, Moyer CL. A novel lineage of proteobacteria involved in formation of marine fe-oxidizing microbial mat communities. PLoS ONE. 2007;2:e667. doi: 10.1371/journal.pone.0000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erauso G, Reysenbach AL, Godfroy A, Meunier JR, Crump B, Partensky F, Baross JA, Marteinsson VT, Barbier G. Pyrococcus abyssi sp. nov., a new hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Arch Microbiol. 1993;160:338–349. [Google Scholar]
- Flores GE, Wagner ID, Liu Y, Reysenbach AL. Distribution, abundance, and diversity patterns of the thermoacidophilc "deep-sea hydrothermal vent euryarchaeota 2". Front Microbiol. 2012;3:1–17. doi: 10.3389/fmicb.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank KL, Rogers DR, Olins HC, Vidoudez C, Girguis PR. Characterizing the distribution and rates of microbial sulfate reduction at Middle Valley hydrothermal vents. ISME J. 2013;7:1391–1401. doi: 10.1038/ismej.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich CG, Bardischewsky F, Rother D, Quentmeier A, Fischer J. Prokaryotic sulfur oxidation. Curr Opin Microbiol. 2005;8:253–259. doi: 10.1016/j.mib.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Geslin C, Le Romancer M, Erauso G, Gaillard M, Perrot G, Prieur D. PAV1, the first virus-like particle isolated from a hyperthermophilic euryarchaeote, Pyrococcus abyssi. J Bacteriol. 2003;185:3888–3894. doi: 10.1128/JB.185.13.3888-3894.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannelli D, Chung M, Staley J, Starovoytov V, Bris NL, Vetriani C. Sulfurovum riftiae sp. nov., a mesophilic, thiosulfate-oxidizing, nitrate-reducing chemolithoautotrophic epsilonproteobacterium isolated from the tube of the deep-sea hydrothermal vent polychaete Riftia pachyptila. Int J Syst Evol Microbiol. 2016;66:2697–2701. doi: 10.1099/ijsem.0.001106. [DOI] [PubMed] [Google Scholar]
- González JM, Masuchi Y, Robb FT, Ammerman JW, Maeder DL, Yanagibayashi M, Tamaoka J, Kato C. Pyrococcus horikoshii sp. nov., a hyperthermophilic archaeon isolated from a hydrothermal vent at the Okinawa Trough. Extremophiles. 1998;2:123–130. doi: 10.1007/s007920050051. [DOI] [PubMed] [Google Scholar]
- Gorlas A, Koonin EV, Bienvenu N, Prieur D, Geslin C. TPV1, the first virus isolated from the hyperthermophilic genus Thermococcus. Environ Microbiol. 2012;4:503–516. doi: 10.1111/j.1462-2920.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz D, Banta AB, Beveridge TJ, Rushdi AI, Simoneit BRT, Reysenbach A. Persephonella marina gen. nov., sp. nov. and Persephonella guaymasensis sp. nov., two novel, thermophilic, hydrogen-oxidizing microaerophiles from deep-sea hydrothermal vents. Int J Syst Evol Microbiol. 2002;52:1349–1359. doi: 10.1099/00207713-52-4-1349. [DOI] [PubMed] [Google Scholar]
- Grosche A, Sekaran H, Pérezrodríguez I, Starovoytov V, Vetriani C. Cetia pacifica gen. nov., sp. nov., a chemolithoautotrophic, thermophilic, nitrate-ammonifying bacterium from a deep-sea hydrothermal vent. Int J Syst Bacteriol. 2015;65:1144–1150. doi: 10.1099/ijs.0.000070. [DOI] [PubMed] [Google Scholar]
- Handley KM, Lloyd JR. Biogeochemical implications of the ubiquitous colonization of marine habitat and redox gradients by Marinobacter species. Front Microbiol. 2013;4:136. doi: 10.3389/fmicb.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Perner M. A novel hydrogen oxidizer amidst the sulfur oxidizing Thiomicrospira lineage. ISME J. 2015;9:696–707. doi: 10.1038/ismej.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Perner M. Hydrogenase gene distribution and H2 consumption ability within the Thiomicrospira lineage. Front Microbiol. 2016;7:99. doi: 10.3389/fmicb.2016.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Xiao X, Wang F. Metagenome reveals potential microbial degradation of hydrocarbon coupled with sulfate reduction in an oilimmersed chimney from Guaymas Basin. Front Microbiol. 2013;4:148. doi: 10.3389/fmicb.2013.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T, Li H, Zhang X. Deep-sea hydrothermal vent viruses compensate for microbial metabolism in virus-host interactions. mBio. 2017;8:E00893–E1817. doi: 10.1128/mBio.00893-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Wang S, Lai Q, Shao Z, Jiang L. Sulfurimonas indica sp. nov., a hydrogen- and sulfur- oxidizing chemolithoautotroph isolated from a hydrothermal sulfide chimney in the Northwest Indian Ocean. Int J Syst Evol Microbiol. 2020 doi: 10.1099/ijsem.0.004575. [DOI] [PubMed] [Google Scholar]
- Hu S, Barnes S, Pages A, Parr J, Binns RA, Verrall M, Quadir Z, Rickard WDA, Liu W, Fougerouse D, Grice K, Schoneveld L, Ryan C, Paterson DL. Life on the edge: microbial biomineralization in an arsenic-and lead-rich deep-sea hydrothermal vent. Chem Geol. 2020;533:119438. [Google Scholar]
- Huber H, Thomm M, Konig H, Thies G, Stetter KO. Methanococcus thermolithotrophicus, a novel thermophilic lithotrophic methanogen. Arch Microbiol. 1982;132:47–50. [Google Scholar]
- Huber H, Burggraf S, Mayer T, Wyschkony I, Rachel R, Stetter KO. Ignicoccus gen. nov., a novel genus of hyperthermophilic, chemolithoautotrophic Archaea, represented by two new species, Ignicoccus islandicus sp. nov. and Ignicoccus pacificus sp. nov. Int J Syst Evol Microbiol. 2000;50:2093–2100. doi: 10.1099/00207713-50-6-2093. [DOI] [PubMed] [Google Scholar]
- Huber HHM, Rachel R, Fuchs T, Wimmer VC, Stetter KO. A new phylum of Archaea represented by a nanosized hyperthermophilic symbiont. Nature. 2002;417:27–28. doi: 10.1038/417063a. [DOI] [PubMed] [Google Scholar]
- Inagaki F, Takai K, Kobayashi H, Nealson KH, Horikoshi K. Sulfurimonas autotrophica gen. nov., sp. nov., a novel sulfur-oxidizing ε -proteobacterium isolated from hydrothermal sediments in the Mid-Okinawa Trough. Int J Syst Evol Microbiol. 2003;53:1801–1805. doi: 10.1099/ijs.0.02682-0. [DOI] [PubMed] [Google Scholar]
- Inagaki F, Takai K, Nealson KH, Horikoshi K. Sulfurovum lithotrophicum gen. nov., sp. nov., a novel sulfur-oxidizing chemolithoautotroph within the ε-Proteobacteria isolated from Okinawa Trough hydrothermal sediments. Int J Syst Evol Microbiol. 2004;54:1477–1482. doi: 10.1099/ijs.0.03042-0. [DOI] [PubMed] [Google Scholar]
- Jannasch HW, Mottl MJ. Geomicrobiology of deep-sea hydrothermal vents. Science. 1985;229:717–725. doi: 10.1126/science.229.4715.717. [DOI] [PubMed] [Google Scholar]
- Jannasch HW, Wirsen CO, Nelson DC, Robertson LA. Thiomicrospira crunogena sp. nov., a colorless, sulfur-oxidizing bacterium from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 1985;35:422–424. [Google Scholar]
- Jannasch HW, Wirsen CO, Molyneaux SJ, Langworthy TA. Extremely thermophilic fermentative archaebacteria of the genus Desulfurococcus from deep-sea hydrothermal vents. Appl Environ Microbiol. 1988;54:1203–1209. doi: 10.1128/aem.54.5.1203-1209.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanthon C, L’Haridon S, Reysenbach AL, Vernet M, Messner P, Sleytr UB, Prieur D. Methanococcus infernus sp. nov., a novel hyperthermophilic lithotrophic methanogen isolated from a deep-sea hydrothermal vent. Int J Syst Bacteriol. 1998;48:913–919. doi: 10.1099/00207713-48-3-913. [DOI] [PubMed] [Google Scholar]
- Jeanthon C, Lharidon S, Reysenbach A, Corre E, Vernet M, Messner P, Sleytr UB, Prieur D. Methanococcus vulcanius sp. nov., a novel hyperthermophilic methanogen isolated from East Pacific Rise, and identification of Methanococcus sp. DSM 4213T as Methanococcus fervens sp. nov. Int J Syst Evol Microbiol. 1999;49:583–589. doi: 10.1099/00207713-49-2-583. [DOI] [PubMed] [Google Scholar]
- Jeanthon C, L'Haridon S, Cueff V, Banta A, Reysenbach AL, Prieur D. Thermodesulfobacterium hydrogeniphilum sp. nov., a thermophilic, chemolithoautotrophic, sulfate-reducing bacterium isolated from a deep-sea hydrothermal vent at Guaymas Basin, and emendation of the genus Thermodesulfobacterium. Int J Syst Bacteriol. 2002;52:765–772. doi: 10.1099/00207713-52-3-765. [DOI] [PubMed] [Google Scholar]
- Jolivet E, Corre E, Lharidon S, Forterre P, Prieur D. Thermococcus marinus sp. nov. and Thermococcus radiotolerans sp. nov., two hyperthermophilic archaea from deep-sea Hydrothermal vents that resist ionizing radiation. Extremophiles. 2004;8:219–219. doi: 10.1007/s00792-004-0380-9. [DOI] [PubMed] [Google Scholar]
- Jones WJ, Leigh JA, Mayer F, Woese CR, Wolfe RS. Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch Microbiol. 1983;136:254–261. [Google Scholar]
- Jiang L, Liu X, Dong C, Huang Z, Cambon-Bonavita MA, Alain K, Gu L, Wang S, Shao Z. Candidatus Desulfobulbus rimicarensis, an uncultivated deltaproteobacterial epibionts from the deep-sea hydrothermal vent shrimp Rimicaris exoculata. Appl Environ Microbiol. 2020;86:e02549-19. doi: 10.1128/AEM.02549-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Lyu J, Shao Z. Sulfur metabolism of Hydrogenovibrio thermophilus strain s5 and its adaptations to deep-sea hydrothermal vent environment. Front Microbiol. 2017;8:2513. doi: 10.3389/fmicb.2017.02513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Dangelo T, Orcutt BN. Using cathodic poised potential experiments to investigate extracellular electron transport in the crustal deep biosphere of North Pond. Mid-Atlantic Ridge Front Environ Sci. 2020;8:11. [Google Scholar]
- Jungbluth SP, Amend JP, Rappe MS. Metagenome sequencing and 98 microbial genomes from Juan de Fuca Ridge flank subsurface fluids. Sci Data. 2017;4:170037. doi: 10.1038/sdata.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashefi K, Lovley DR (2003) Extending the upper temperature limit for life science 301:934–934 [DOI] [PubMed]
- Kashefi K, Tor JM, Holmes DE, Gaw Van Praagh CV, Reysenbach AL, Lovley DR. Geoglobus ahangari gen. nov., sp. nov., a novel hyperthermophilic archaeon capable of oxidizing organic acids and growing autotrophically on hydrogen with Fe(III) serving as the sole electron acceptor. Int J Syst Evol Microbiol. 2002;52:719–728. doi: 10.1099/00207713-52-3-719. [DOI] [PubMed] [Google Scholar]
- Kashefi K, Holmes DE, Baross JA, Lovley DR. Thermophily in the Geobacteraceae: Geothermobacter ehrlichii gen. nov., sp. nov., a novel thermophilic member of the geobacteraceae from the" bag city" hydrothermal vent. Appl Environ Microbiol. 2003;69:2975–2984. doi: 10.1128/AEM.69.5.2985-2993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Hara K, Kasai H, Teramura T, Sunamura M, Ishibashi J, Kakegawa T, Yamanaka T, Kimura H, Marumo K, Urabe T, Yamagishi A. Spatial distribution, diversity and composition of bacterial communities in sub-seafloor fluids at a deep-sea hydrothermal field of the Suiyo Seamount. Deep Sea ResPart A Oceanogr Res Pap. 2009;56:1844–1855. [Google Scholar]
- Kaye JZ, Baross JA. High incidence of halotolerant bacteria in Pacific hydrothermal-vent and pelagic environments. FEMS Microbiol Ecol. 2000;32:249–260. doi: 10.1111/j.1574-6941.2000.tb00718.x. [DOI] [PubMed] [Google Scholar]
- Kaye JZ, Márquez MC, Ventosa A, Baross JA (2004) Halomonas neptunia sp. nov., Halomonas sulfidaeris sp. nov., Halomonas axialensis sp. nov. and Halomonas hydrothermalis sp. nov.: halophilic bacteria isolated from deep-sea hydrothermal-vent environments Int J Syst Evol Microbiol 54:499–511 [DOI] [PubMed]
- Kurr M, Huber R, Konig H , Jannasch H W,Fricke H,Trincone A, Kristjansson JK, Stetter KO (1991) Methanopyrus kandleri, gen. and sp. nov. represents a novel group of hyperthermophilic methanogens, growing at 110 C Arch Microbiol 156:239–247
- Lai Q, Cao J, Dupont S, Shao Z, Jebbar M, Alain K. Thermodesulfatator autotrophicus sp. nov., a thermophilic sulfate-reducing bacterium from the Indian Ocean. Int J Syst Evol Microbiol. 2016;66:3978–3982. doi: 10.1099/ijsem.0.001297. [DOI] [PubMed] [Google Scholar]
- Lam P, Cowen JP, Jone RD. Autotrophic ammonia oxidation in a deep-sea hydrothermal plume. FEMS Microbiol Ecol. 2004;47:191–206. doi: 10.1016/S0168-6496(03)00256-3. [DOI] [PubMed] [Google Scholar]
- L'Haridon S, Cilia V, Messner P, Raguenes G, Gambacorta A, Sleytr UB, Prieur D, Jeanthon C. Desulfurobacterium thermolithotrophum gen. nov., sp. nov., a novel autotrophic, sulphur-reducing bacterium isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 1998;48:701–711. doi: 10.1099/00207713-48-3-701. [DOI] [PubMed] [Google Scholar]
- L'Haridon S, Reysenbach AL, Banta A, Messner P, Schumann P, Stackebrandt E, Jeanthon C. Methanocaldococcus indicus sp. nov., a novel hyperthermophilic methanogen isolated from the Central Indian Ridge. Int J Syst Evol Microbiol. 2003;53:1931–1935. doi: 10.1099/ijs.0.02700-0. [DOI] [PubMed] [Google Scholar]
- L'Haridon S, Reysenbach A, Banta AB, Messner P, Schumann P, Stackebrandt E, Jeanthon C. Methanocaldococcus indicus sp. nov., a novel hyperthermophilic methanogen isolated from the Central Indian Ridge. Int J Syst Evol Microbiol. 2003;53:1931–1935. doi: 10.1099/ijs.0.02700-0. [DOI] [PubMed] [Google Scholar]
- L'Haridon S, Reysenbach A, Tindall BJ, Schonheit P, Banta AB, Johnsen U, Schumann P, Gambacorta A, Stackebrandt E, Jeanthon C. Desulfurobacterium atlanticum sp. nov., Desulfurobacterium pacificum sp. nov. and Thermovibrio guaymasensis sp. nov., three thermophilic members of the Desulfurobacteriaceae fam. nov., a deep branching lineage within the Bacteria. Int J Syst Evol Microbiol. 2006;56:2843–2852. doi: 10.1099/ijs.0.63994-0. [DOI] [PubMed] [Google Scholar]
- L'Haridon S, Miroshnichenko ML, Kostrikina NA, Tindall BJ, Spring S, Schumann P, Stackebrandt E, Bonchosmolovskaya EA, Jeanthon C. Vulcanibacillus modesticaldus gen. nov., sp. nov., a strictly anaerobic, nitrate-reducing bacterium from deep-sea hydrothermal vents. Int J Syst Evol Microbiol. 2006;56:1047–1053. doi: 10.1099/ijs.0.64012-0. [DOI] [PubMed] [Google Scholar]
- L'Haridon S, Jiang L, Alain K, Chalopin M, Jebbar M. Kosmotoga pacifica sp. nov., a thermophilic chemoorganoheterotrophic bacterium isolated from an East Pacific hydrothermal sediment. Extremophiles. 2013;18:81–88. doi: 10.1007/s00792-013-0596-7. [DOI] [PubMed] [Google Scholar]
- Li G, Zeng X, Liu X, Zhang X, Shao Z. Wukongibacter baidiensis gen. nov., sp. nov., an anaerobic bacterium isolated from hydrothermal sulfides, and proposal for the reclassification of the closely related Clostridium halophilum and Clostridium caminithermale within Maledivibacter gen. nov. and Paramaledivibacter gen. nov., respectively. Int J Syst Evol Microbiol. 2016;66:4355–4361. doi: 10.1099/ijsem.0.001355. [DOI] [PubMed] [Google Scholar]
- Li M, Jain S, Dick GJ. Genomic and transcriptomic resolution of organic matter utilization among deep-sea bacteria in guaymas basin hydrothermal plumes. Front Microbiol. 2016;7:1125. doi: 10.3389/fmicb.2016.01125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tang K, Zhang L, Zhao Z, Xie X, Chen C-TA, Wang D, Jiao N, Zhang Y. Coupled carbon, sulfur, and nitrogen cycles mediated by microorganisms in the water column of a shallow-water hydrothermal ecosystem. Front Microbiol. 2018;9:2718. doi: 10.3389/fmicb.2018.02718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zeng X, Qiu D, Zhang Z, Chen J, Shao Z. Dissimilatory iron [Fe (III)] reduction by a novel fermentative, piezophilic bacterium Anoxybacter fermentans DY22613T isolated from east pacific rise hydrothermal sulfides. Geomicrobiol J. 2019;36:291–302. [Google Scholar]
- Li X, Zeng X, Qiu D, Zhang Z, Zhang X, Shao Z. Extracellular electron transfer in fermentative bacterium Anoxybacter fermentans DY22613T isolated from deep-sea hydrothermal sulfides. Sci Total Environ. 2020;722:137723. doi: 10.1016/j.scitotenv.2020.137723. [DOI] [PubMed] [Google Scholar]
- Lim JK, Kim YJ, Yang JA, Namirimu T, Yang SH, Park MJ, Kwon YM, Lee HS, Kang SG, Lee JH, Kyoung KK. Thermococcus indicus sp. nov., a Fe(III)-reducing hyperthermophilic archaeon isolated from the onnuri vent field of the central indian ocean ridge. J Microbiol. 2020;58:260–267. doi: 10.1007/s12275-020-9424-9. [DOI] [PubMed] [Google Scholar]
- Lin TJ, Breves EA, Dyar MD, Ver Eecke H, Jamieson J, Holden J. Magnetite formation from ferrihydrite by hyperthermophilic archaea from E ndeavour Segment, Juan de Fuca Ridge hydrothermal vent chimneys. Geobiology. 2014;12:200–211. doi: 10.1111/gbi.12083. [DOI] [PubMed] [Google Scholar]
- Lin TJ, Sebae GK, Jung JH, Jung DH, Park CS, Holden JF (2016) Pyrodictium delaneyi sp. nov., a hyperthermophilic autotrophic archaeon that reduces Fe (III) oxide and nitrate. Int J Syst Evol Microbiol 66:3372 [DOI] [PMC free article] [PubMed]
- Liu C, Gorby YA, Zachara JM, Fredrickson JK, Brown CF. Reduction kinetics of Fe(III), Co(III), U(VI), Cr(VI), and Tc(VII) in cultures of dissimilatory metal-reducing bacteria. Biotechnol Bioeng. 2002;80:637–649. doi: 10.1002/bit.10430. [DOI] [PubMed] [Google Scholar]
- Liu X, Jiang L, Hu Q, Lyu J, Shao Z. Thiomicrorhabdus indica sp. nov., an obligately chemolithoautotrophic, sulfur-oxidizing bacterium isolated from a deep-sea hydrothermal vent environment. Int J Syst Evol Microbiol. 2020;70:234–239. doi: 10.1099/ijsem.0.003744. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia P, Philippe H, Gail F, Moreira D. (2003) Autochthonous eukaryotic diversity in hydrothermal sediment and experimental microcolonizers at the Mid-Atlantic Ridge. Proc Natl Acad Sci USA:697–702 [DOI] [PMC free article] [PubMed]
- Lossouarn J, Nesbø CL, Mercier C, Zhaxybayeva O, Johnson MS, Charchuck R, Farasin J, Bienvenu N, Baudoux A-C, Michoud G, Jebbar M, Geslin C. ‘Ménage à trois’: a selfish genetic element uses a virus to propagate within Thermotogales. Environ Microbiol. 2015;17:3278–3288. doi: 10.1111/1462-2920.12783. [DOI] [PubMed] [Google Scholar]
- Lovley DR. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Pang K. Fungi from substrates in marine environment. In: Misra JK, Tewari JP, Deshmukh SK, Vágvölgyi C, editors. Fungi from different substrates. Boca Raton: CRC press; 2014. pp. 97–114. [Google Scholar]
- Luton PE, Wayne JM, Sharp RJ, Riley PW. The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiology. 2002;148:3521–3530. doi: 10.1099/00221287-148-11-3521. [DOI] [PubMed] [Google Scholar]
- Makita H. Iron-oxidizing bacteria in marine environments: recent progresses and future directions. World J Microbiol Biotechnol. 2018;34:110–110. doi: 10.1007/s11274-018-2491-y. [DOI] [PubMed] [Google Scholar]
- Makita H, Nakagawa S, Miyazaki M, Nakamura K, Inagaki F, Takai K. Thiofractor thiocaminus gen. nov., sp. nov., a novel hydrogen-oxidizing, sulfur-reducing epsilonproteobacterium isolated from a deep-sea hydrothermal vent chimney in the Nikko Seamount field of the northern Mariana Arc. Arch Microbiol. 2012;194:785–794. doi: 10.1007/s00203-012-0814-1. [DOI] [PubMed] [Google Scholar]
- Makita H, Tanaka E, Mitsunobu S, Miyazaki M, Takai K. Mariprofundus micogutta sp. nov., a novel iron-oxidizing zetaproteobacterium isolated from a deep-sea hydrothermal field at the Bayonnaise knoll of the Izu-Ogasawara arc, and a description of Mariprofundales ord. nov. and Zetaproteobacteria classis nov. Arch Microbiol. 2016;199:335–346. doi: 10.1007/s00203-016-1307-4. [DOI] [PubMed] [Google Scholar]
- Mandernack KW, Tebo BM. Manganese scavenging and oxidation at hydrothermal vents and in vent plumes. Geochim Cosmochim Acta. 1993;57:3907–3923. [Google Scholar]
- Manzella MP, Reguera G, Kashefi K. Extracellular electron transfer to Fe(III) oxides by the hyperthermophilic archaeon Geoglobus ahangari via a direct contact mechanism. Appl Environ Microbiol. 2013;79:4694–4700. doi: 10.1128/AEM.01566-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardanov AV, Slododkina GB, Slobodkin AI, Beletsky AV, Gavrilov SN, Kublanov IV, Bonch-Osmolovskaya EA, Skryabin KG, Ravin NV. The geoglobus acetivorans genome: Fe(III) reduction, acetate utilization, autotrophic growth, and degradation of aromatic compounds in a hyperthermophilic archaeon. Appl Environ Microbiol. 2015;81:1003–1012. doi: 10.1128/AEM.02705-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardanov AV, Beletsky AV, Kadnikov VV, Slobodkin AI, Ravin NV. Genome Analysis of Thermosulfurimonas dismutans, the first thermophilic sulfur-disproportionating bacterium of the Phylum Thermodesulfobacteria. Front Microbiol. 2016;7:950–950. doi: 10.3389/fmicb.2016.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteinsson VT. Isolation and characterization of Thermus thermophilus Gy1211 from a deep-sea hydrothermal vent. Extremophiles. 1999;3:247–251. doi: 10.1007/s007920050123. [DOI] [PubMed] [Google Scholar]
- Marteinsson VT, Bjornsdottir SH, Bienvenu N, Kristjansson JK, Birrien J. Rhodothermus profundi sp. nov., a thermophilic bacterium isolated from a deep-sea hydrothermal vent in the Pacific Ocean. Int J Syst Evol Microbiol. 2010;60:2729–2734. doi: 10.1099/ijs.0.012724-0. [DOI] [PubMed] [Google Scholar]
- McAllister SM, Polson SW, Butterfield DA, Glazer BT, Sylvan JB, Chan CS. Validating the Cyc2 neutrophilic iron oxidation pathway using metaomics of Zetaproteobacteria iron mats at marine hydrothermal vents. Msystem. 2020;5:e00553–e1519. doi: 10.1128/mSystems.00553-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccliment EA, Voglesonger KM, Oday PA, Dunn EE, Holloway JR, Cary SC. Colonization of nascent, deep-sea hydrothermal vents by a novel Archaeal and Nanoarchaeal assemblage. Environ Microbiol. 2006;8:114–125. doi: 10.1111/j.1462-2920.2005.00874.x. [DOI] [PubMed] [Google Scholar]
- McCollom TM. Geochemical constraints on source of metabolic energy for chemolithoautotrophy in ultramafic-hosted deep-sea hydrothermal systems. Astrobiology. 2007;7:933–950. doi: 10.1089/ast.2006.0119. [DOI] [PubMed] [Google Scholar]
- McHatton SC, Barry JP, Jannasch HW, Nelson DC. High nitrate concentrations in vacuolate, autotrophic marine Beggiatoa spp. Appl Environ Microbiol. 1996;62:954–958. doi: 10.1128/aem.62.3.954-958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MPB, David A, Baross JA. Phylogenetic diversity of nitrogenase (nifH) genes in deep-sea and hydrothermal vent environments of the Juan de Fuca Ridge. Appl Environ Microbiol. 2003;69:960–970. doi: 10.1128/AEM.69.2.960-970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier D, Bach W, Girguis PR, Grubervodicka HR, Reeves EP, Richter M, Vidoudez C, Amann R, Meyerdierks A. Heterotrophic Proteobacteria in the vicinity of diffuse hydrothermal venting. Environ Microbiol. 2016;18:4348–4368. doi: 10.1111/1462-2920.13304. [DOI] [PubMed] [Google Scholar]
- Meier D, Pjevac P, Bach W, Hourdez S, Girguis PR, Vidoudez C, Amann R, Meyerdierks A. Niche partitioning of diverse sulfur-oxidizing bacteria at Hydrothermal vents. ISME J. 2017;11:1545–1558. doi: 10.1038/ismej.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier D, Pjevac P, Bach W, Markert S, Schweder T, Jamieson J, Petersen S, Amann R, Meyerdierks A. Microbial metal-sulfide oxidation in inactive hydrothermal vent chimneys suggested by metagenomic and metaproteomic analyses. Environ Microbiol. 2019;21:682–701. doi: 10.1111/1462-2920.14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton ED, Swanner ED, Behrens S, Schmidt C, Kappler A. The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle. Nature Rev Microbiol. 2014;12:797–808. doi: 10.1038/nrmicro3347. [DOI] [PubMed] [Google Scholar]
- Mercier C, Lossouarn J, Nesbo CL, Haverkamp TH, Baudoux A, Jebbar M, Bienvenu N, Thiroux S, Dupont S, Geslin C. Two viruses, MCV1 and MCV2, which infect Marinitoga bacteria isolated from deep-sea hydrothermal vents: functional and genomic analysis. Environ Microbiol. 2018;20:577–587. doi: 10.1111/1462-2920.13967. [DOI] [PubMed] [Google Scholar]
- Merkel AY, Huber JA, Chernyh NA, Bonchosmolovskaya EA, Lebedinsky AV. Detection of putatively thermophilic anaerobic methanotrophs in diffuse hydrothermal vent fluids. Appl Environ Microbiol. 2013;79:915–923. doi: 10.1128/AEM.03034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minic Z, Thongbam PD. The biological deep sea hydrothermal vent as a model to study carbon dioxide capturing enzymes. Mar Drugs. 2011;9:719–738. doi: 10.3390/md9050719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mino S, Kudo H, Arai T, Sawabe T, Takai K, Nakagawa S. Sulfurovum aggregans sp. nov., a hydrogen-oxidizing, thiosulfate-reducing chemolithoautotroph within the Epsilonproteobacteria isolated from a deep-sea hydrothermal vent chimney, and an emended description of the genus Sulfurovum. Int J Syst Evol Microbiol. 2014;64:3195–3201. doi: 10.1099/ijs.0.065094-0. [DOI] [PubMed] [Google Scholar]
- Mino S, Yoneyama N, Nakagawa S, Takai K, Sawabe T (2018) Enrichment and genomic characterization of a N2O-reducing chemolithoautotroph from a deep-sea hydrothermal vent. Front Bioeng Biotechnol 6:184 [DOI] [PMC free article] [PubMed]
- Miroshnichenko ML, Kostrikina N, Lharidon S, Jeanthon C, Hippe H, Stackebrandt E, Bonchosmolovskaya EA. Nautilia lithotrophica gen. nov., sp. nov., a thermophilic sulfur-reducing epsilon-proteobacterium isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2002;52:1299–1304. doi: 10.1099/00207713-52-4-1299. [DOI] [PubMed] [Google Scholar]
- Miroshnichenko ML, Slobodkin AI, Kostrikina NA, Lharidon S, Nercessian O, Spring S, Stackebrandt E, Bonchosmolovskaya EA, Jeanthon C. Deferribacter abyssi sp. nov., an anaerobic thermophile from deep-sea hydrothermal vents of the Mid-Atlantic Ridge. Int J Syst Evol Microbiol. 2003;53:1637–1641. doi: 10.1099/ijs.0.02673-0. [DOI] [PubMed] [Google Scholar]
- Miroshnichenko ML, Kostrikina NA, Chernyh NA, Pimenov NV, Tourova TP, Antipov AN, Spring S, Stackebrandt E, Bonchosmolovskaya EA. Caldithrix abyssi gen. nov., sp. nov., a nitrate-reducing, thermophilic, anaerobic bacterium isolated from a Mid-Atlantic Ridge hydrothermal vent, represents a novel bacterial lineage. Int J Syst Evol Microbiol. 2003;53:323–329. doi: 10.1099/ijs.0.02390-0. [DOI] [PubMed] [Google Scholar]
- Miroshnichenko ML, Lharidon S, Jeanthon C, Antipov A, Kostrikina NA, Tindall BJ, Schumann P, Spring S, Stackebrandt E, Bonchosmolovskaya EA. Oceanithermus profundus gen. nov., sp. nov., a thermophilic, microaerophilic, facultatively chemolithoheterotrophic bacterium from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2003;53:747–752. doi: 10.1099/ijs.0.02367-0. [DOI] [PubMed] [Google Scholar]
- Miroshnichenko ML, Lharidon S, Nercessian O, Antipov AN, Kostrikina NA, Tindall BJ, Schumann P, Spring S, Stackebrandt E, Jeanthon C. Vulcanithermus mediatlanticus gen. nov., sp nov., a novel member of the family Thermaceae from a deep-sea hot vent. Int J Syst Evol Microbiol. 2003;53:1143–1148. doi: 10.1099/ijs.0.02579-0. [DOI] [PubMed] [Google Scholar]
- Miroshnichenko ML, Lharidon S, Schumann P, Spring S, Bonch-Osmolovskaya EA, Jeanthon C, Stackebrandt E. Caminibacter profundus sp. nov., a novel thermophile of Nautiliales ord. nov. within the class 'Epsilonproteobacteria', isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2004;54:41–45. doi: 10.1099/ijs.0.02753-0. [DOI] [PubMed] [Google Scholar]
- Miyazaki J, Ikuta T, Watsuji T, Abe M, Yamamoto M, Nakagawa S, Takaki Y, Nakamura K, Takai K (2020) Dual energy metabolism of the Campylobacterota endosymbiont in the chemosynthetic snail Alviniconcha marisindica. ISME J 14:1–17 [DOI] [PMC free article] [PubMed]
- Mora M, Bellack A, Ugele M, Hopf J, Wirth R. The temperature gradient-forming device, an accessory unit for normal light microscopes to study the biology of hyperthermophilic microorganisms. Appl Environ Microbiol. 2014;80:4764–4770. doi: 10.1128/AEM.00984-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori JF, Scott JJ, Hager KW, Moyer CL, Küsel K, Emerson D. Physiological and ecological implications of an iron- or hydrogen-oxidizing member of the Zetaproteobacteria, Ghiorsea Bivora, gen. nov., sp. nov. ISME J. 2017;11:2624–2636. doi: 10.1038/ismej.2017.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Kakegawa T, Higashi Y, Nakamura K, Maruyama A, Hanada S. Oceanithermus desulfurans sp. nov., a novel thermophilic, sulfur-reducing bacterium isolated from a sulfide chimney in Suiyo Seamount. Int J Syst Evol Microbiol. 2004;54:1561–1566. doi: 10.1099/ijs.0.02962-0. [DOI] [PubMed] [Google Scholar]
- Mori K, Suzuki K, Urabe T, Sugihara M, Tanaka K, Hamada M, Hanada S (2011) Thioprofundum hispidum sp. nov., an obligately chemolithoautotrophic sulfur-oxidizing gammaproteobacterium isolated from the hydrothermal field on Suiyo Seamount, and proposal of Thioalkalispiraceae fam. nov. in the order Chromatiales. Int J Syst Bacteriol 61:2412–2418 [DOI] [PubMed]
- Mori K, Yamaguchi K, Hanada S. Sulfurovum denitrificans sp. nov., an obligately chemolithoautotrophic sulfur-oxidizing epsilonproteobacterium isolated from a hydrothermal field. Int J Syst Evol Microbiol. 2018;68:2183–2187. doi: 10.1099/ijsem.0.002803. [DOI] [PubMed] [Google Scholar]
- Moussard H, L’haridon S, Tindall BJ, Banta A, Schumann P, Stackebrandt E, Jeanthon C (2004) Thermodesulfatator indicus gen. nov., sp. nov., a novel thermophilic chemolithoautotrophic sulfate-reducing bacterium isolated from the Central Indian Ridge. Int J Syst Evol Microbiol 54:227-233 [DOI] [PubMed]
- Moussard H, Corre E, Cambon-Bonavita MA, Fouquet Y, Jeanthon C. Novel uncultured Epsilonproteobacteria dominate a filamentous sulphur mat from the 13°N hydrothermal vent field, East Pacific Rise. FEMS Microbiol Ecol. 2006;58:449–463. doi: 10.1111/j.1574-6941.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- Nagata R, Takaki Y, Tame A, Nunoura T, Muto H, Mino S, Sawayama S, Takai K, Nakagawa S. Lebetimonas natsushimae sp. nov., a novel strictly anaerobic, moderately thermophilic chemoautotroph isolated from a deep-sea hydrothermal vent polychaete nest in the Mid-Okinawa Trough. Syst Appl Microbiol. 2017;40:352–356. doi: 10.1016/j.syapm.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takai K. Deep-sea vent chemoautotrophs: diversity, biochemistry and ecological significance. FEMS Microbiol Ecol. 2008;65:1–14. doi: 10.1111/j.1574-6941.2008.00502.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takai K, Horikoshi K, Sako Y. Persephonella hydrogeniphila sp. nov., a novel thermophilic, hydrogen-oxidizing bacterium from a deep-sea hydrothermal vent chimney. Int J Syst Evol Microbiol. 2003;53:863–869. doi: 10.1099/ijs.0.02505-0. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takai K, Horikoshi K, Sako Y. Aeropyrum camini sp. nov., a strictly aerobic, hyperthermophilic archaeon from a deep-sea hydrothermal vent chimney. Int J Syst Evol Microbiol. 2004;54:329–335. doi: 10.1099/ijs.0.02826-0. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takai K, Inagaki F, Hirayama H, Nunoura T, Horikoshi K, Sako Y. Distribution, phylogenetic diversity and physiological characteristics of epsilon-Proteobacteria in a deep-sea hydrothermal field. Environ Microbiol. 2005;7:1619–1632. doi: 10.1111/j.1462-2920.2005.00856.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takai K, Inagaki F, Horikoshi K, Sako Y. Nitratiruptor tergarcus gen. nov., sp. nov. and Nitratifractor salsuginis gen. nov., sp. nov., nitrate-reducing chemolithoautotrophs of the e-Proteobacteria isolated from a deep-sea hydrothermal system in the Mid-Okinawa Trough. Int J Syst Evol Microbiol. 2005;55:925–933. doi: 10.1099/ijs.0.63480-0. [DOI] [PubMed] [Google Scholar]
- Neely C, Khalil CB, Cervantes A, Diaz R, Escobar A, Ho K, Hoefler S, Smith H, Abuyen K, Savalia P, Nealson KH, Emerson D, Tully BJ, Barco RA, Amend JP. Genome sequence of Hydrogenovibrio sp. strain SC-1, a chemolithoautotrophic sulfur and iron oxidizer. Genome Announc. 2018;6:e01581–e11517. doi: 10.1128/genomeA.01581-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DC, Wirsen CO, Jannasch HW. Characterization of large, autotrophic Beggiatoa spp. abundant at hydrothermal vents of the Guaymas Basin. Appl Environ Microbiol. 1989;55:2909–2917. doi: 10.1128/aem.55.11.2909-2917.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoura T, Oida H, Miyazaki M, Suzuki Y, Takai K, Horikoshi K. Desulfothermus okinawensis sp. nov., a thermophilic and heterotrophic sulfate-reducing bacterium isolated from a deep-sea hydrothermal field. Int J Syst Evol Microbiol. 2007;57:2360–2364. doi: 10.1099/ijs.0.64781-0. [DOI] [PubMed] [Google Scholar]
- Nunoura T, Oida H, Miyazaki M, Suzuki Y, Takai K, Horikoshi K. Marinitoga okinawensis sp. nov., a novel thermophilic and anaerobic heterotroph isolated from a deep-sea hydrothermal field, Southern Okinawa Trough. Int J Syst Evol Microbiol. 2007;57:467–471. doi: 10.1099/ijs.0.64640-0. [DOI] [PubMed] [Google Scholar]
- Nunoura T, Miyazaki M, Suzuki Y, Takai K, Horikoshi K. Hydrogenivirga okinawensis sp. nov., a thermophilic sulfur-oxidizing chemolithoautotroph isolated from a deep-sea hydrothermal field, Southern Okinawa Trough. Int J Syst Evol Microbiol. 2008;58:676–681. doi: 10.1099/ijs.0.64615-0. [DOI] [PubMed] [Google Scholar]
- Nunoura T, Oida H, Miyazaki M, Suzuki Y. Thermosulfidibacter takaii gen. nov., sp. nov., a thermophilic, hydrogen-oxidizing, sulfur-reducing chemolithoautotroph isolated from a deep-sea hydrothermal field in the Southern Okinawa Troug. Int J Syst Evol Microbiol. 2008;58:659–665. doi: 10.1099/ijs.0.65349-0. [DOI] [PubMed] [Google Scholar]
- Nunoura T, Takaki Y, Kazama H, Kakuta J, Shimamura S, Makita H, Hirai M, Miyazaki M, Takai K. Physiological and genomic features of a novel sulfur-oxidizing gammaproteobacterium belonging to a previously uncultivated symbiotic lineage isolated from a hydrothermal vent. PLoS ONE. 2014;19:e104959. doi: 10.1371/journal.pone.0104959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoura T, Chikaraishi Y, Izaki R, Suwa T, Sato T, Harada T, Mori K, Kato Y, Miyazaki M, Shimamura S, Yanagawa K, Shuto A, Ohkouchi N, Fujita N, Takaki Y, Atomi H, Takai K. A primordial and reversible TCA cycle in a facultatively chemolithoautotrophic thermophile. Science. 2018;359:559–563. doi: 10.1126/science.aao3407. [DOI] [PubMed] [Google Scholar]
- Orcutt BN, Sylvan JB, Knab NJ, Edwards K. Microbial ecology of the dark ocean above, at, and below the Seafloor. Microbiol Mol Biol Rev. 2011;75:361–422. doi: 10.1128/MMBR.00039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortmann ACS, Curtis A. High abundances of viruses in a deep-sea hydrothermal vent system indicates viral mediated microbial mortality. Deep Sea Res. Part I Oceanogr Res Pap. 2005;52:1515–1527. [Google Scholar]
- Perez N, Cardenas R, Martin O, Michel L. The potential for photosynthesis in Hydrothermal vents: a new avenue for life in the universe? J Astrophys Space Sci. 2013;346:327–331. [Google Scholar]
- Perezrodriguez I, Ricci J, Voordeckers JW, Starovoytov V, Vetriani C. Nautilia nitratireducens sp. nov., a thermophilic, anaerobic, chemosynthetic, nitrate-ammonifying bacterium isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2010;60:1182–1186. doi: 10.1099/ijs.0.013904-0. [DOI] [PubMed] [Google Scholar]
- Perner M, Hansen M, Seifert R, Strauss H, Koschinsky A, Petersen S. Linking geology, fluid chemistry, and microbial activity of basaltand ultramafic-hosted deep-sea hydrothermal vent environments. Geobiol. 2013;11:340–355. doi: 10.1111/gbi.12039. [DOI] [PubMed] [Google Scholar]
- Petersen JM, Zielinski FU, Pape T, Seifert R, Moraru C, Amann R, Hourdez S, Girguis PR, Wankel SD, Barbe V, Pelletier E, Fink D, Borowski C, Bach W, Dubilier N. Hydrogen is an energy source for hydrothermal vent symbioses. Nature. 2011;476(7359):176–180. doi: 10.1038/nature10325. [DOI] [PubMed] [Google Scholar]
- Pillot G, Frouin E, Pasero E, Godfroy A, Combetblanc Y, Davidson S, Liebgott P. Specific enrichment of hyperthermophilic electroactive Archaea from deep-sea hydrothermal vent on electrically conductive support. Bioresour Technol. 2018;259:304–311. doi: 10.1016/j.biortech.2018.03.053. [DOI] [PubMed] [Google Scholar]
- Podosokorskaya OA, Kublanov IV, Reysenbach A-L, Kolganova TV, Bonch-Osmolovskaya EA. Thermosipho affectus sp. nov., a thermophilic, anaerobic, cellulolytic bacterium isolated from a Mid-Atlantic Ridge hydrothermal vent. Int J Syst Evol Microbiol. 2011;61:1160–1164. doi: 10.1099/ijs.0.025197-0. [DOI] [PubMed] [Google Scholar]
- Podosokorskaya OA, Bonchosmolovskaya EA, Godfroy A, Gavrilov SN, Beskorovaynaya DA, Sokolova TG, Kolganova TV, Toshchakov SV, Kublanov IV. Thermosipho activus sp. nov., a novel thermophilic anaerobic hydrolytic bacterium isolated from a deep-sea sample Guaymas Basin, Gulf of California. Int J Syst Evol Microbiol. 2014;64:3307–3313. doi: 10.1099/ijs.0.063156-0. [DOI] [PubMed] [Google Scholar]
- Postec A, Breton CL, Fardeau M, Lesongeur F, Pignet P, Querellou J, Ollivier B, Godfroy A. Marinitoga hydrogenitolerans sp. nov., a novel member of the order Thermotogales isolated from a black smoker chimney on the Mid-Atlantic Ridge. Int J Syst Evol Microbiol. 2005;55:1217–1221. doi: 10.1099/ijs.0.63550-0. [DOI] [PubMed] [Google Scholar]
- Radfordknoery J, German CR, Charlou J, Donval JP, Fouquet Y. Distribution and behavior of dissolved hydrogen sulfide in hydrothermal plumes. Limnol Oceanogr. 2001;46:461–464. [Google Scholar]
- Raguénès G, Christen R, Guezennec J, Pignet P, Barbier G. Vibrio diabolicus sp. nov., a new polysaccharide-secreting organism isolated from a deep-sea hydrothermal vent polychaete annelid Alvinella pompejana. Int J Syst Bacteriol. 1997;47:989–995. doi: 10.1099/00207713-47-4-989. [DOI] [PubMed] [Google Scholar]
- Reysenbach AL. Aquificales. In: Boone DRGG, editor. Bergey’s manual of systematic bacteriology. Berlin Heidelberg New York: Springer; 2001. pp. 369–387. [Google Scholar]
- Reysenbach A, Shock EL. Merging genomes with geochemistry in hydrothermal ecosystems. Science. 2002;296:1077–1082. doi: 10.1126/science.1072483. [DOI] [PubMed] [Google Scholar]
- Reysenbach A, Gotz D, Banta AB, Jeanthon C, Fouquet Y. Expanding the distribution of the Aquificales to the deep-sea vents on Mid-Atlantic Ridge and Central Indian Ridge. Cah Biol Mar. 2002;43:425–428. [Google Scholar]
- Reysenbach A, Liu Y, Banta AB, Beveridge T, Kirshtein J, Schouten S, Tivey M, Von Damm K, Voytek M. A ubiquitous thermoacidophilic archaeon from deep-sea Hydrothermal vents. Nature. 2006;442:444–447. doi: 10.1038/nature04921. [DOI] [PubMed] [Google Scholar]
- Roh Y, Gao H, Vali H, Kennedy DW, Yang ZK, Gao W, Dohnalkova A, Stapleton RD, Moon J, Phelps TJ, Fredrickson JK, Zhou J. Metal reduction and iron biomineralization by a psychrotolerant Fe (III)-reducing bacterium, Shewanella sp. strain PV-4. Appl Environ Microbiol. 2006;72:3236–3244. doi: 10.1128/AEM.72.5.3236-3244.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby EG, Wirsen CO, Jannasch HW (1981) Chemolithotrophic sulfur-oxidizing bacteria from the galapagos rift hydrothermal vents.Appl Environ Microbiol 42:317–324 [DOI] [PMC free article] [PubMed]
- Sakai S, Takaki Y, Miyazaki M, Ogawara M, Yanagawa K, Miyazaki J, Takai K. Methanofervidicoccus abyssi gen. nov., sp. nov., a hydrogenotrophic methanogen, isolated from a hydrothermal vent chimney in the Mid-Cayman Spreading Center, the Caribbean Sea. Int J Syst Evol Microbiol. 2019;69:1225–1230. doi: 10.1099/ijsem.0.003297. [DOI] [PubMed] [Google Scholar]
- Sako Y, Nakagawa S, Takai K, Horikoshi K. Marinithermus hydrothermalis gen. nov., sp. nov., a strictly aerobic, thermophilic bacterium from a deep-sea hydrothermal vent chimney. Int J Syst Evol Microbiol. 2003;53:59–65. doi: 10.1099/ijs.0.02364-0. [DOI] [PubMed] [Google Scholar]
- Scott KM, Sievert SM, Abril FN, Ball LA, Barrett CJ, Blake RA, Boller AJ, Chain PS, Clark JA, Davis CR, Detter C, Do KF, Dobrinski KP, Faza BI, Fitzpatrick KA, Freyermuth SK, Harmer TL, Hauser LJ, Hügler M, Kerfeld CA, et al. The genome of deep-sea vent chemolithoautotroph Thiomicrospira crunogena XCL-2. PLoS Biol. 2006;4:e383. doi: 10.1371/journal.pbio.0040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerer AH, Burggraf S, Fiala G, Huber G, Huber R, Pley U, Stetter KO. Life in hot springs and hydrothermal vents. Origins Life Evol B. 1993;23:77–90. doi: 10.1007/BF01581992. [DOI] [PubMed] [Google Scholar]
- Sievert SM, Hugler M, Taylor CD, Wirsen CO (2008) Sulfur oxidation at deep-sea Hydrothermal vents. In: Dahl C FC (ed) Microbial sulfur metabolism. Springer, Berlin, Heidelberg, pp 238–258
- Singer E, Emerson D, Webb EA, Barco RA, Kuenen JG, Nelson WC, Chan CS, Comolli LR, Ferriera S, Johnson J, Heidelberg JF, Edwards KJ. Mariprofundus ferrooxydans PV-1 the first genome of a marine Fe(II) oxidizing Zetaproteobacterium. PLoS ONE. 2011;6:e25386. doi: 10.1371/journal.pone.0025386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer E, Heidelberg JF, Dhillon A, Edwards K. Metagenomic insights into the dominant Fe(II) oxidizing Zetaproteobacteria from an iron mat at Lō´ihi. Hawaii. Front Microbiol. 2013;4:52–52. doi: 10.3389/fmicb.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobodkin AI, Slobodkina GB. Diversity of sulfur-disproportionating microorganisms. J Microbiol. 2019;88:509–522. [Google Scholar]
- Slobodkin AI, Tourova T, Kuznetsov B, Kostrikina N, Chernyh N, Bonch-Osmolovskaya E. Thermoanaerobacter siderophilus sp. nov., a novel dissimilatory Fe (III)-reducing, anaerobic, thermophilic bacterium. Int J Syst Evol Microbiol. 1999;49:1471–1478. doi: 10.1099/00207713-49-4-1471. [DOI] [PubMed] [Google Scholar]
- Slobodkin AI, Campbell BJ, Cary SC, Bonch-Osmolovskaya EA, Jeanthon C. Evidence for the presence of thermophilic Fe(III)-reducing microorganisms in deep-sea Hydrothermal vents at 13。N(East Pacific Rise) FEMS Microbiol Ecol. 2001;36:235–243. doi: 10.1111/j.1574-6941.2001.tb00844.x. [DOI] [PubMed] [Google Scholar]
- Slobodkin AI, Reysenbach A, Slobodkina GB, Baslerov RV, Kostrikina NA, Wagner ID, Bonchosmolovskaya EA. Thermosulfurimonas dismutans gen. nov., sp. nov., an extremely thermophilic sulfur-disproportionating bacterium from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2012;62:2565–2571. doi: 10.1099/ijs.0.034397-0. [DOI] [PubMed] [Google Scholar]
- Slobodkin AI, Reysenbach A, Slobodkina GB, Kolganova TV, Kostrikina NA, Bonchosmolovskaya EA. Dissulfuribacter thermophilus gen. nov., sp. nov., a thermophilic, autotrophic, sulfur-disproportionating, deeply branching deltaproteobacterium from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2013;63:1967–1971. doi: 10.1099/ijs.0.046938-0. [DOI] [PubMed] [Google Scholar]
- Slobodkin AI, Slobodkina GB, Allioux M, Alain K, Jebbar M, Shadrin V, Kublanov IV, Toshchakov SV, Bonchosmolovskaya EA. Genomic insights into the carbon and energy metabolism of a thermophilic deep-sea bacterium Deferribacter autotrophicus revealed new metabolic traits in the Phylum Deferribacteres. Genes. 2019;10:849. doi: 10.3390/genes10110849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobodkina G, Kolganova T, Chernyh N, Querellou J, Bonch-Osmolovskaya E, Slobodkin AI. Deferribacter autotrophicus sp. nov., an iron (III)-reducing bacterium from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2009;59:1508–1512. doi: 10.1099/ijs.0.006767-0. [DOI] [PubMed] [Google Scholar]
- Slobodkina G, Kolganova T, Querellou J, Bonch-Osmolovskaya E, Slobodkin AI. Geoglobus acetivorans sp. nov., an iron (III)-reducing archaeon from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2009;59:2880–2883. doi: 10.1099/ijs.0.011080-0. [DOI] [PubMed] [Google Scholar]
- Slobodkina G, Reysenbach A-L, Panteleeva A, Kostrikina N, Wagner I, Bonch-Osmolovskaya E, Slobodkin AI. Deferrisoma camini gen. nov., sp. nov., a moderately thermophilic, dissimilatory iron (III)-reducing bacterium from a deep-sea hydrothermal vent that forms a distinct phylogenetic branch in the Deltaproteobacteria. Int J Syst Evol Microbiol. 2012;62:2463–2468. doi: 10.1099/ijs.0.038372-0. [DOI] [PubMed] [Google Scholar]
- Slobodkina G, Reysenbach A, Kolganova TV, Novikov AA, Bonchosmolovskaya EA, Slobodkin AI. Thermosulfuriphilus ammonigenes gen. nov., sp. nov., a thermophilic, chemolithoautotrophic bacterium capable of respiratory ammonification of nitrate with elemental sulfur. Int J Syst Evol Microbiol. 2017;67:3474–3479. doi: 10.1099/ijsem.0.002142. [DOI] [PubMed] [Google Scholar]
- Smith JL, Campbell BJ, Hanson TE, Zhang CL, Cary SC. Nautilia profundicola sp. nov., a thermophilic, sulfur-reducing epsilonproteobacterium from deep-sea hydrothermal vents. Int J Syst Evol Microbiol. 2008;58:1598–1602. doi: 10.1099/ijs.0.65435-0. [DOI] [PubMed] [Google Scholar]
- Sondergaard D, Pedersen CN, Greening C. HydDB: a web tool for hydrogenase classification and analysis. Sci Rep. 2016;6:34212. doi: 10.1038/srep34212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinsbu BO, Tindall BJ, Torsvik V, Thorseth IH, Daae FL, Pedersen RB. Rhabdothermus arcticus gen. nov., sp. nov., a member of the family Thermaceae isolated from a hydrothermal vent chimney in the Soria Moria vent field on the Arctic Mid-Ocean Ridge. Int J Syst Evol Microbiol. 2011;61:2197–2204. doi: 10.1099/ijs.0.027839-0. [DOI] [PubMed] [Google Scholar]
- Stewart LC, Jung J, Kim Y, Kwon S, Park C, Holden JF. Methanocaldococcus bathoardescens sp. nov., a hyperthermophilic methanogen isolated from a volcanically active deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2015;65:1280–1283. doi: 10.1099/ijs.0.000097. [DOI] [PubMed] [Google Scholar]
- Stokke R, Reeves EP, Dahle H, Fedøy A-E, Viflot T, Lie Onstad S, Vulcano F, Pedersen RB, Eijsink VGH, Steen IH. Tailoring hydrothermal vent biodiversity toward improved biodiscovery using a novel in situ enrichment strategy. Front Microbiol. 2020;11:249. doi: 10.3389/fmicb.2020.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudek LA, Templeton AS, Tebo BM, Staudigel H. Microbial ecology of Fe (hydr)oxide mats and basaltic rock from Vailulu’u Seamount, American Samoa. Geomicrobiol J. 2009;26:581–596. [Google Scholar]
- Takai K, Horikoshi K. Thermosipho japonicas sp. nov., an extremely thermophilic bacterium isolated from a deep-sea hydrothermal vent in Japan. Extremophiles. 2000;4:9–17. doi: 10.1007/s007920050002. [DOI] [PubMed] [Google Scholar]
- Takai K, Sugai A, Itoh Y, Horikoshi K. Palaeococcus ferrophilus gen. nov., sp. nov., a barophilic, hyperthermophilic archaeon from a deep-sea hydrothermal vent chimney. Int J Syst Evol Microbiol. 2000;50:489–500. doi: 10.1099/00207713-50-2-489. [DOI] [PubMed] [Google Scholar]
- Takai K, Inoue A, Horikoshi K. Methanothermococcus okinawensis sp. nov., a thermophilic, methane-producing archaeon isolated from a Western Pacific deep-sea hydrothermal vent system. Int J Syst Evol Microbiol. 2002;52:1089–1095. doi: 10.1099/00207713-52-4-1089. [DOI] [PubMed] [Google Scholar]
- Takai K, Inagaki F, Nakagawa S, Hirayama H, Nunoura T, Sako Y. Isolation and phylogenetic diversity of members of previously uncultivated ε-Proteobacteria in deep-sea hydrothermal fields. FEMS Microbiol Lett. 2003;218:167–174. doi: 10.1111/j.1574-6968.2003.tb11514.x. [DOI] [PubMed] [Google Scholar]
- Takai K, Kobayashi H, Nealson KH, Horikoshi K. Deferribacter desulfuricans sp. nov., a novel sulfur-, nitrate- and arsenate-reducing thermophile isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2003;53:839–846. doi: 10.1099/ijs.0.02479-0. [DOI] [PubMed] [Google Scholar]
- Takai K, Nakagawa S, Sako Y, Horikoshi K. Balnearium lithotrophicum gen. nov., sp. nov., a novel thermophilic, strictly anaerobic, hydrogen-oxidizing chemolithoautotroph isolated from a black smoker chimney in the Suiyo Seamount hydrothermal system. Int J Syst Evol Microbiol. 2003;53:1947–1954. doi: 10.1099/ijs.0.02773-0. [DOI] [PubMed] [Google Scholar]
- Takai K, Nealson KH, Horikoshi K. Methanotorris formicicus sp. nov., a novel extremely thermophilic, methane-producing archaeon isolated from a black smoker chimney in the Central Indian Ridge. Int J Syst Evol Microbiol. 2004;54:1095–1100. doi: 10.1099/ijs.0.02887-0. [DOI] [PubMed] [Google Scholar]
- Takai K, Nealson KH, Horikoshi K. Hydrogenimonas thermophila gen. nov., sp. nov., a novel thermophilic, hydrogenoxidizing chemolithoautotroph within the ε-Proteobacteria, isolated from a black smoker in a Central Indian Ridge hydrothermal field. Int J Syst Evol Microbiol. 2004;54:25–32. doi: 10.1099/ijs.0.02787-0. [DOI] [PubMed] [Google Scholar]
- Takai K, Oida H, Suzuki Y, Hirayama H, Nakagawa S, Nunoura T, Inagaki F, Nealson KH, Horikoshi K. Spatial distribution of marine Crenarchaeota group I in the vicinity of deep-sea hydrothermal systems. Appl Environ Microbiol. 2004;70:2404–2413. doi: 10.1128/AEM.70.4.2404-2413.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai K, Hirayama H, Nakagawa T, Suzuki Y, Nealson KH, Horikoshi K. Thiomicrospira thermophila sp. nov., a novel microaerobic, thermotolerant, sulfur-oxidizing chemolithomixotroph isolated from a deep-sea hydrothermal fumarole in the TOTO caldera, Mariana Arc, Western Pacific. Int J Syst Evol Microbiol. 2004;54:2325–2333. doi: 10.1099/ijs.0.63284-0. [DOI] [PubMed] [Google Scholar]
- Takai K, Hirayama H, Nakagawa T, Suzuki Y, Nealson KH, Horikoshi K. Lebetimonas acidiphila gen. nov., sp. nov., a novel thermophilic, acidophilic, hydrogen-oxidizing chemolithoautotroph within the 'Epsilonproteobacteria', isolated from a deep-sea hydrothermal fumarole in the Mariana Arc. Int J Syst Evol Microbiol. 2005;55:183–189. doi: 10.1099/ijs.0.63330-0. [DOI] [PubMed] [Google Scholar]
- Takai K, Suzuki M, Nakagawa S, Miyazaki M, Suzuki Y, Inagaki F, Horikoshi K. Sulfurimonas paralvinellae sp. nov., a novel mesophilic, hydrogen- and sulfur-oxidizing chemolithoautotroph within the Epsilonproteobacteria isolated from a deep-sea hydrothermal vent polychaete nest, reclassification of Thiomicrospira denitrificans as Sulfurimonas denitrificans comb. nov. and emended description of the genus Sulfurimonas. Int J Syst Evol Microbiol. 2006;56:1725–1733. doi: 10.1099/ijs.0.64255-0. [DOI] [PubMed] [Google Scholar]
- Takai K, Nunoura T, Ishibashi J, Lupton J, Suzuki R, Hamasaki H. Variability in the microbial communities and hydrothermal fluid chemistry at the newly-discoverred Mariner hydrothermal field, southern Lau Basin. J Geophys Res. 2008;113:G02031. [Google Scholar]
- Takai K, Miyazaki M, Hirayama H, Nakagawa S, Querellou J, Godfroy A. Isolation and physiological characterization of two novel, piezophilic, thermophilic chemolithoautotrophs from a deep-sea hydrothermal vent chimney. Environ Microbiol. 2009;11:1983–1997. doi: 10.1111/j.1462-2920.2009.01921.x. [DOI] [PubMed] [Google Scholar]
- Teske A, Brinkhoff T, Muyzer G, Moser DP, Rethmeier J, Jannasch HW. Diversity of thiosulfate-oxidizing bacteria from marine sediments and hydrothermal vents. Appl Environ Microbiol. 2000;66:3125–3133. doi: 10.1128/aem.66.8.3125-3133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teske AE, Rivers P, Thompson AR, Gomez JR, Molyneaux SJ, Wirsen CO. A molecular and physiological survey of a diverse collection of hydrothermal vent Thermococcus and Pyrococcus isolates. Extremophiles. 2009;13:905–915. doi: 10.1007/s00792-009-0278-7. [DOI] [PubMed] [Google Scholar]
- Tomohiko K, Akitomo K, Ikuko U, Akihiko S. Thermosipho globiformans sp. nov., an anaerobic thermophilic bacterium that transforms into multicellular spheroids with a defect in peptidoglycan formation. Int J Syst Evol Microbiol. 2011;61:1622–1627. doi: 10.1099/ijs.0.025106-0. [DOI] [PubMed] [Google Scholar]
- Toner BM, Marcus MA, Edwards KJ, Rouxel O, German CR. Measuring the form of iron in hydrothermal plume particles. Oceanography. 2012;25:209–212. [Google Scholar]
- Urios L, Cueff-Gauchard V, Pignet P, Postec A, Barbier G. Thermosipho atlanticus sp. nov., a novel member of the Thermotogales isolated from a Mid-Atlantic Ridge hydrothermal vent. Int J Syst Evol Microbiol. 2004;54:1953–1957. doi: 10.1099/ijs.0.63069-0. [DOI] [PubMed] [Google Scholar]
- Valentine DL, Blanton DC, Reeburgh WS, Kastner M. Water column methane oxidation adjacent to an area of active hydrate dissociation, Eel River Basin. Geochim Cosmochim Acta. 2001;65:2633–2640. [Google Scholar]
- Van Dover CL, Ward ME, Scott JL, Underdown J, Anderson B, Gustafson C, Whalen MA, Carnegie RB. A fungal epizootic in mussels at a deep-sea hydrothermal vent. Marine Ecol. 2007;28:54–62. [Google Scholar]
- Ver Eecke HC, Butterfield DA, Huber JA, Lilley MD, Olson EJ, Roe KK. Hydrogen-limited growth of hyperthermophilic methanogens at deep-sea hydrothermal vents. Proc Natl Acad Sci USA. 2012;109:13674–13679. doi: 10.1073/pnas.1206632109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetriani C, Speck M, Ellor SV, Lutz RA, Starovoytov V. Thermovibrio ammonificans sp. nov., a thermophilic, chemolithotrophic, nitrate-ammonifying bacterium from deep-sea hydrothermal vents. Int J Syst Evol Microbiol. 2004;54:175–181. doi: 10.1099/ijs.0.02781-0. [DOI] [PubMed] [Google Scholar]
- Vetriani C, Chew YS, Miller SM, Yagi J, Coombs J, Lutz RA, Barkay T. Mercury adaptation among bacteria from a deep-sea hydrothermal vent. Appl Environ Microbiol. 2005;71:220–226. doi: 10.1128/AEM.71.1.220-226.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetriani C, Voordeckers JW, Crespomedina M, Obrien CE, Giovannelli D, Lutz RA. Deep-sea hydrothermal vent Epsilonproteobacteria encode a conserved and widespread nitrate reduction pathway (Nap) ISME J. 2014;8:1510–1521. doi: 10.1038/ismej.2013.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais PM, Billoud B. Occurrence, classification, and biological function of hydrogenases: an overview. Chem Rev. 2007;107:4206–4272. doi: 10.1021/cr050196r. [DOI] [PubMed] [Google Scholar]
- Voordeckers JW, Starovoytov V, Vetriani C. Caminibacter mediatlanticus sp. nov., a thermophilic, chemolithoautotrophic, nitrate-ammonifying bacterium isolated from a deep-sea hydrothermal vent on the Mid-Atlantic Ridge. Int J Syst Evol Microbiol. 2005;55:773–779. doi: 10.1099/ijs.0.63430-0. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhou H, Meng J, Peng X, Jiang L, Sun P, Zhang C, Nostrand JDV, Deng Y, He Z, Wu L, Zhou J, Xiao X. GeoChip-based analysis of metabolic diversity of microbial communities at the Juan de Fuca Ridge hydrothermal vent. Proc Natl Acad Sci USA. 2009;106:4840–4845. doi: 10.1073/pnas.0810418106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Feng X, Natarajan VP, Xiao X, Wang F. Diverse anaerobic methane- and multi- carbon alkane- metabolizing archaea coexist and show activity in Guaymas Basin hydrothermal sediment. Environ Microbiol. 2019;21:1344–1355. doi: 10.1111/1462-2920.14568. [DOI] [PubMed] [Google Scholar]
- Wang S, Jiang L, Hu Q, Liu X, Yang S, Shao Z. Elemental sulfur reduction by a deep-sea hydrothermal vent Campylobacterium Sulfurimonas sp. NW10. Environ microbial. 2020 doi: 10.1111/1462-2920.15247. [DOI] [PubMed] [Google Scholar]
- Wang W, Li Z, Zeng L, Dong C, Shao Z. The oxidation of hydrocarbons by diverse heterotrophic and mixotrophic bacteria that inhabit deep-sea hydrothermal ecosystems. ISME J. 2020;14:1994–2006. doi: 10.1038/s41396-020-0662-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wankel SD, Adams MM, Johnston DT, Hansel CM, Joye SB, Girguis PR. Anaerobic methane oxidation in metalliferous hydrothermal sediments: influence on carbon flux and decoupling from sulfate reduction. Environ Microbiol. 2012;14:2726–2740. doi: 10.1111/j.1462-2920.2012.02825.x. [DOI] [PubMed] [Google Scholar]
- Wasmund K, Musmann M, Loy A. The life sulfuric: microbial ecology of sulfur cycling in marine sediments. Environ Microbiol Rep. 2017;9:323–344. doi: 10.1111/1758-2229.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KA, Achenbach LA, Coates JD. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol. 2006;4:752–764. doi: 10.1038/nrmicro1490. [DOI] [PubMed] [Google Scholar]
- Wery N, Lesongeur F, Pignet P, Derennes V, Barbier G. Marinitoga camini gen. nov., sp. nov., a rod-shaped bacterium belonging to the order Thermotogales, isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2001;51:495–504. doi: 10.1099/00207713-51-2-495. [DOI] [PubMed] [Google Scholar]
- Wery N, Moricet J, Cueff V, Jean J, Pignet P, Lesongeur F, Cambonbonavita M, Barbier G. Caloranaerobacter azorensis gen. nov., sp. nov., an anaerobic thermophilic bacterium isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2001;51:1789–1796. doi: 10.1099/00207713-51-5-1789. [DOI] [PubMed] [Google Scholar]
- Wu Y, Cao Y, Wang C, Wu M, Aharon O, Xu X. Microbial community structure and nitrogenase gene diversity of sediment from a deep-sea hydrothermal vent field on the Southwest Indian Ridge. Acta Oceanol Sinica. 2014;33:94–104. [Google Scholar]
- Xu W, Li M, Ding J, Gu J, Luo Z. Bacteria dominate the ammonia-oxidizing community in a hydrothermal vent site at the mid-atlantic ridge of the South Atlantic Ocean. Appl Microbiol Biotechnol. 2014;98:7993–8004. doi: 10.1007/s00253-014-5833-1. [DOI] [PubMed] [Google Scholar]
- Xu W, Guo S, Pang KL, Luo Z. Fungi associated with chimney and sulfide samples from a South Mid-Atlantic Ridge hydrothermal site: distribution, diversity and abundance. Deep-Sea Res Part I. 2017;123:48–55. [Google Scholar]
- Xu W, Gong LF, Pang KL, Luo Z. Fungal diversity in deep-sea sediments of a hydrothermal vent system in the Southwest Indian Ridge. Deep-Sea Res Part I. 2018;131:16–26. [Google Scholar]
- Yamamoto M, Takai K. Sulfur metabolisms in epsilon- and gamma-proteobacteria in deep-sea hydrothermal fields. Front Microbiol. 2011;2:192. doi: 10.3389/fmicb.2011.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Wu W, Liang W, Lever MA, Hinrichs K-U, Wang F. Growth of sedimentary bathyarchaeota on lignin as an energy source. Proc Natl Acad Sci USA. 2018;115:6022–6027. doi: 10.1073/pnas.1718854115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavarzina D, Tourova T, Kuznetsov B, Bonch-Osmolovskaya E, Slobodkin A. Thermovenabulum ferriorganovorum gen. nov., sp. nov., a novel thermophilic, anaerobic, endospore-forming bacterium. Int J Syst Evol Microbiol. 2002;52:1737–1743. doi: 10.1099/00207713-52-5-1737. [DOI] [PubMed] [Google Scholar]
- Zeng X, Birrien J, Fouquet Y, Cherkashov G, Jebbar M, Quérellou J, Oger P, Cambon-Bonavita MA, Xiao X, Prieur D. Pyrococcus CH1, an obligate piezophilic hyperthermophile: extending the upper pressure–temperature limits for life. ISME J. 2009;3:873–876. doi: 10.1038/ismej.2009.21. [DOI] [PubMed] [Google Scholar]
- Zeng X, Zhang X, Jiang L, Alain K, Jebbar M, Shao Z. Palaeococcus pacificus sp. nov., an archaeon from deep-sea hydrothermal sediment. Int J Syst Evol Microbiol. 2013;63:2155–2159. doi: 10.1099/ijs.0.044487-0. [DOI] [PubMed] [Google Scholar]
- Zeng X, Zhang Z, Li X, Jebbar M, Alain K, Shao Z. Caloranaerobacter ferrireducens sp. nov., an anaerobic, thermophilic, iron (III)-reducing bacterium isolated from deep-sea hydrothermal sulfide deposits. Int J Syst Evol Microbiol. 2015;65:1714–1718. doi: 10.1099/ijs.0.000165. [DOI] [PubMed] [Google Scholar]
- Zeng X, Zhang Z, Li X, Cao J, Zhang X, Jebbar M, Alain K, Shao Z. Anoxybacter fermentans gen. nov., sp. nov., a piezophilic, thermophilic, anaerobic, fermentative bacterium isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2015;65:710–715. doi: 10.1099/ijs.0.068221-0. [DOI] [PubMed] [Google Scholar]
- Zeng X, Zhang X, Shao Z. Metabolic adaptation to sulfur of hyperthermophilic Palaeococcus pacificus DY20341T from deep-sea hydrothermal sediments. Int J Mole Sci. 2020;21:368. doi: 10.3390/ijms21010368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sievert SM. Pan-genome analyses identify lineage- and niche-specific markers of evolution and adaptation in Epsilonproteobacteria. Front Microbiol. 2014;5:110. doi: 10.3389/fmicb.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Fang J, Bach W, Edwards KJ, Orcutt BN, Wang F. Nitrogen stimulates the growth of subsurface basalt-associated microorganisms at the Western flank of the mid-Atlantic Ridge. Front Microbiol. 2016;3:633. doi: 10.3389/fmicb.2016.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.