Abstract
Measurement of the longitudinal relaxation time in the rotating frame of reference (T1ρ) is sensitive to the fidelity of the main imaging magnetic field (B0) and that of the RF pulse (B1). The purpose of this study was to introduce methods for producing continuous wave (CW) T1ρ contrast with improved robustness against field inhomogeneities and to compare the sensitivities of several existing and the novel T1ρ contrast generation methods with the B0 and B1 field inhomogeneities. Four hard‐pulse and four adiabatic CW‐T1ρ magnetization preparations were investigated. Bloch simulations and experimental measurements at different spin‐lock amplitudes under ideal and non‐ideal conditions, as well as theoretical analysis of the hard‐pulse preparations, were conducted to assess the sensitivity of the methods to field inhomogeneities, at low (ω1 << ΔB0) and high (ω1 >> ΔB0) spin‐locking field strengths. In simulations, previously reported single‐refocus and new triple‐refocus hard‐pulse and double‐refocus adiabatic preparation schemes were found to be the most robust. The mean normalized absolute deviation between the experimentally measured relaxation times under ideal and non‐ideal conditions was found to be smallest for the refocused preparation schemes and broadly in agreement with the sensitivities observed in simulations. Experimentally, all refocused preparations performed better than those that were non‐refocused. The findings promote the use of the previously reported hard‐pulse single‐refocus ΔB0 and B1 insensitive T1ρ as a robust method with minimal RF energy deposition. The double‐refocus adiabatic B1 insensitive rotation‐4 CW‐T1ρ preparation offers further improved insensitivity to field variations, but because of the extra RF deposition, may be preferred for ex vivo applications.
Keywords: Bloch simulation, contrast, field inhomogeneity, rotating frame of reference, T1ρ relaxation
New and existing state‐of‐the‐art CW‐T1ρ schemes were compared theoretically, numerically, and experimentally at 9.4 T. Of the CW‐T1ρ methods using hard pulses, the proposed triple‐focus and earlier single‐refocus methods were highly robust, while the scheme using adiabatic excitation and double refocusing with BIR‐4 was the most robust.
Abbreviations used
- AFP
adiabatic full passage
- AHP
adiabatic half passage
- BIR
B1 insensitive rotation
- B‐SL
balanced spin lock
- CW‐T1ρ
continuous wave T1ρ
- FSE
fast spin echo
- GRE
gradient echo
- HS
hyperbolic secant
- MNAD
mean normalized absolute deviation
- PSC‐SL
paired self‐compensated spin lock
- RF
radio frequency
- RMS
root mean square
- ROI
region of interest
- SAR
specific absorption rate
- SL
spin lock
- SNR
signal‐to‐noise ratio
- TE
echo time
- WASSR
water saturation shift referencing
- ω1
external field (B1)
- ωeff
effective field
1. INTRODUCTION
Relaxation in the rotating frame under the presence of an external spin‐locking radio frequency (RF) pulse, termed T1ρ relaxation, 1 has been under active research for the quantitative assessment of different tissue types, such as the central nervous system, 2 liver, 3 and articular cartilage. 4 , 5 For instance, in articular cartilage, T1ρ has been shown to be sensitive to the proteoglycan content, the collagen fiber network, and to degenerative changes in general. 5 , 6 , 7 , 8 T1ρ relaxation depends on the amplitude of the spin‐lock (SL) pulse, that is, the SL frequency, which in typical cases corresponds to the timescales of slow molecular motion. 9 In biological tissues, the processes affecting T1ρ relaxation include dipolar interaction, chemical exchange, and the motion of spins through field gradients; broadly, any local fluctuations in the magnetic field that are on the same or lower frequency scale as the SL frequency. 8 , 9 , 10 , 11 , 12 The relative importance of each mechanism varies with the SL frequency and the strength of the main magnetic field. 13 The standard T1ρ measurement uses on‐resonance continuous‐wave (CW) spin‐locking (CW‐T1ρ), and consists of tilting the magnetization 90 degrees and then locking the spins with a continuous RF pulse. 1 Several methods to produce T1ρ contrast at constant spin‐locking amplitude have been proposed, with variable sensitivity to the inhomogeneities of the main field (B0) and the RF field (B1).
Spin locking slows the relaxation process in the transverse plane by forcing the spins to rotate around the RF field. Because of the high sensitivity of the T1ρ measurement to field inhomogeneities, the design of the SL pulse is essential for high quality T1ρ‐weighted images and accurate quantification of the T1ρ relaxation time. 14 Typically, in the clinical setting, the amplitudes of the SL pulses (ω1 = γB1/2π, where γ is the gyromagnetic ratio) are between a few hundred and a thousand Hz, most often 400–500 Hz. To allow estimation of the T1ρ relaxation time, the same SL amplitude is maintained, while the SL durations are varied. The relaxation processes affecting T1ρ are modulated by the molecular makeup of the tissue, and thus T1ρ correlates with the properties of the tissues. 5
Various methods have been reported for compensating the inherent sensitivity of T1ρ measurement to field inhomogeneities. 14 , 15 , 16 Witschey et al. 14 introduced a T1ρ weighting method, which was demonstrated to be highly insensitive to variations in the B0 and B1 fields, in phantoms and in vivo human brains at 3 T. The sequence is a modification of the ΔB0 insensitive SL sequence proposed by Zeng et al., 17 with a change to the phase of the final 90° pulse, effectively inverting the magnetization at the end of the preparation. While the pulse sequence was proven to be highly robust against B0 and B1 field inhomogeneities, the authors noted that the downside of the sequence was that it would still require a perfect 180° refocusing pulse to fully compensate against field variations. Another attempt to alleviate the sensitivity of spin locking to field inhomogeneities with a single‐refocus pulse, termed paired self‐compensated SL (PSC‐SL), was proposed by Mitrea et al. 15 In their version, the spin‐locking periods were further split into pairs of opposite phases on either side of the refocusing pulse, making the SL pairs insensitive to B1 inhomogeneities; however, tiltin the magnetization back towards the positive z‐axis. The study demonstrated the sequence with phantom and small animal imaging at 7 T with gradient echo (GRE) and fast spin echo (FSE) readout sequences. A recent double‐refocusing pulse sequence, termed balanced SL (B‐SL), proposed by Gram et al., 18 applies an extra 180° refocusing pulse with opposite phase compensating for both inhomogeneities. The sequence was evaluated with simulations and demonstrated with an agarose phantom at 7 T. The authors concluded that B‐SL was superior in comparison with the existing single‐refocus sequence in which the magnetization is returned to the +Z axis, that is, the one presented by Zeng et al. 17 However, it remains unclear how the B‐SL sequence performs in comparison with the sequence presented by Witschey et al., 14 which inverts the magnetization at the end of the preparation, as this sequence was also shown to be superior in comparison with the noninverting T1ρ preparation.
Adiabatic pulses have also been used to improve the robustness of T1ρ imaging. Various studies used adiabatic half passage (AHP) pulses, coupled to CW spin locking to improve the B1 robustness of the measurements 16 , 19 , 20 , 21 , 22 , 23 The AHP pulses were utilized in these studies for tilting the magnetization to the transverse plane for the CW SL, followed by a reverse AHP to bring the magnetization back to the longitudinal axis. A dual acquisition method was proposed by Chen 16 to address the adverse effect from relaxation during the reverse AHP on T1ρ quantification. The method was demonstrated with phantom and human liver imaging at 3 T. Similar methods, using pulsed, fully adiabatic T1ρ preparation, have also been reported. 24 , 25 , 26
The purpose of this study was twofold; firstly, to perform a numerical, experimental, and partial theoretical comparison of the sensitivities of the different T1ρ contrast generation methods to the inhomogeneities in the B1 and B0 fields; and secondly, to introduce additional ways of producing T1ρ contrast with reduced sensitivity to the field inhomogeneities. We examined the different previously published and new T1ρ preparation methods via both Bloch simulations and experimentally. In the theoretical part, we focused on the different hard‐pulse implementations for T1ρ preparation.
2. MATERIALS AND METHODS
2.1. CW‐T1ρ preparation schemes
Here, we focus on the conventional non‐refocused hard‐pulse, single‐refocused ΔB0 and B1 insensitive preparation scheme presented by Witschey et al., 14 the double‐refocused B‐SL preparation scheme presented by Gram et al., 18 and on a novel triple‐refocused hard‐pulse CW‐T1ρ preparation scheme. Triple refocused hard‐pulse CW‐T1ρ attempts to account for the reported inability of the single‐refocus sequence presented by Witschey et al., 14 to fully compensate for the field variations if the single refocus is not a perfect 180° pulse (Figures 1, S1, and S2). Theoretical derivations on the sensitivities of the preparation are provided in the supporting information and in Witschey et al. 14 In addition, the ΔB0 and B1 insensitive T1ρ preparation presented by Mitrea et al.15 was considered in simulations.
FIGURE 1.
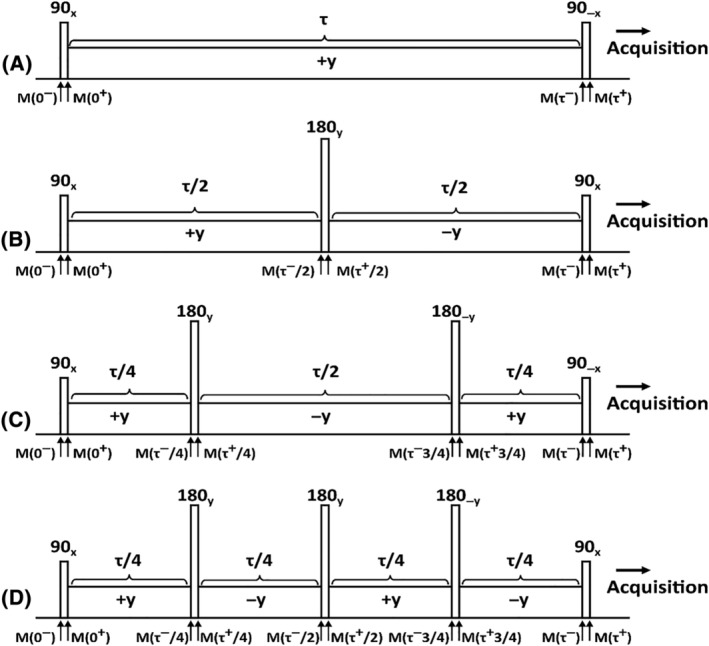
Hard‐pulse SL preparations. (A) Conventional SL, 14 (B) Single‐refocus ΔB0 and B1 insensitive SL, 14 (C) Double‐refocus “B‐SL”, 18 and (D) Triple‐refocus CW‐T1ρ schemes for T1ρ contrast preparation. M(0−) is the initial magnetization before the excitation pulse. The magnetization is flipped from the longitudinal plane (z‐axis) towards the transverse plane by the first pulse; M(0+) is the magnetization after the excitation. During the SL (of duration τ), the magnetization nutates about the SL field, along the z''‐axis; after n refocusing pulses and spin‐locking segments, the magnetization is M(τ−) before the final rewinder pulse. The final magnetization (M[τ+]) is returned back to the longitudinal axis by the rewinder pulse. Please note that (A) and (C) return the magnetization to the +z axis with a reverse 90° pulse, while (B) and (D) take the magnetization to the ‐z axis with another forward 90° pulse. B‐SL, balanced spin lock; CW‐T1ρ, continuous wave T1 ρ; SL, spin lock
Adiabatic pulses are amplitude‐ and frequency‐modulated RF pulses that are highly insensitive to B1 inhomogeneity and off‐resonance effects. 27 In adiabatic pulses, the amplitude of the effective field (ωeff [t]) of the pulse is the vectorial sum of the time‐dependent B1 and the off‐resonance component. The flip angle (φ) is largely independent of the applied B1 field, given that the adiabatic condition |ωeff (t)|>> |dφ / dt| is satisfied, that is, the sweep of the direction of the effective field (dφ/dt) is slow compared with its amplitude (ωeff). During an adiabatic sweep, spins at different resonances are primarily affected at different times of the pulse, in contrast to the CW‐pulse, which simultaneously affects the spins within its frequency bandwidth. Adiabatic pulses can be categorized as excitation, refocusing, and inversion pulses. 28 AHP pulses (Figure 2A) are employed to generate uniform excitation with a 90° flip on a defined frequency band, leaving the magnetization in the transverse plane, while reverse AHP pulses brings the magnetization back to the z axis from the transverse plane. 19 With the adiabatic excitation and CW‐SL, the SL continues from the same phase where the adiabatic excitation pulse ends, but the amplitude of the RF pulse is reduced to the desired spin‐lock amplitude (i.e., unlike in the adiabatic CW T1ρ reported by Chen, 16 where the amplitude of the SL equals the maximum amplitude of the AHP). Similarly, the reverse AHP starts from the phase where the SL ends, with amplitude ramped up to the maximum of the AHP. 16 , 19 , 22 , 24
FIGURE 2.
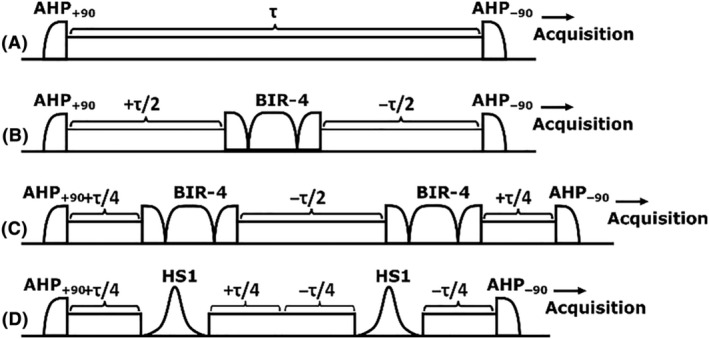
Adiabatic and CW SL preparations. (A) Conventional adiabatic CW‐T1ρ preparation, consisting of an AHP excitation, a SL of duration τ, and a reverse AHP. 19 Adiabatic CW‐T1ρ with (B) A single adiabatic BIR‐4 refocusing pulse, (C) With two BIR‐4 refocusing pulses, or (D) Using double refocusing with HS1 pulses. The negative sign in front of τ indicates a phase shift of 180°. AHP, adiabatic full passage; BIR, B1 insensitive rotation; CW, continuous wave; CW‐T1ρ, continuous wave T1ρ; HS, hyperbolic secant; SL, spin lock
Besides AHP excitation pulses, either B1 insensitive rotation (BIR)‐4 plane rotation pulses or adiabatic full passage (AFP) inversion pulses, such as hyperbolic secant (HS)n pulses, can be used for adiabatic refocusing/inversion during the spin‐locking train, both providing largely B1‐insensitive means for the refocusing/inversion. 28 , 29 As long as the adiabaticity can be sufficiently maintained during the pulses, inhomogeneities in the B1 field will not have an effect on the resulting flip angles using the adiabatic pulses. Here, we investigated four different CW‐T1ρ preparations utilizing AFP, AHP, BIR‐4, and HS1 adiabatic pulses, without refocusing 22 or using single or double BIR‐4 refocusing, or double AFP inversion, in between the SL (Figure 2).
2.2. Numerical simulations
Numerical Bloch simulations of the pulse trains were performed for ΔB0 and B1 field inhomogeneities of up to ±1 kHz and ±40%, respectively, to analyze the sensitivities of the sequences. The simulations for all the spin locking schemes were performed using SL durations of 8, 32, and 128 ms and SL amplitudes of 100 and 400 Hz. The duration of each of the hard 90° and 180° pulses was 200 μs. Maximum amplitudes of the adiabatic pulses were set to 2.5 kHz and the durations were 4, 3.03, and 5.17 ms for AHP, AFP, and BIR‐4, respectively. Additionally, conventional adiabatic CW T1ρ was simulated with a longer and lower maximum RF amplitude of 600‐Hz of the AHP pulses. 16 The following modulation functions were used for adiabatic pulses: the AHP and BIR‐4 pulses utilized tanh/tan modulations 30 and the AFP pulse was an HS1 pulse with a time‐bandwidth product value (R = 20). Relaxation effects were neglected in the simulations to focus on the effects of field inhomogeneity.
2.3. Sample preparation
Cylindrical osteochondral plugs (n = 4, diameter = 6 mm) were prepared from the patella of bovine knee joints obtained from a local grocery store. The samples were immersed in phosphate buffered saline containing enzyme inhibitors and frozen at −20°C. Prior to imaging, the samples were thawed and transferred into a custom‐built sample holder and test tube filled with perfluoropolyether (Galden HS‐240, Solvay Solexis, Italy). In addition to osteochondral plugs, cherry tomatoes (n = 2) and an agarose phantom (n = 1) were used as test samples. The cherry tomatoes were chosen such that they neatly fit within the RF coil. The cherry tomatoes were placed into the coil without immersion solution. The agarose phantom was prepared with 3% w/v agarose and water by heating the solution at 90°C. The agar solution was then transferred to a test tube and placed into a refrigerator (at ~ 5°C) for cooling and gel formation. The test tube was taken out of the refrigerator then allowed to settle to room temperature for 2 h prior to imaging.
2.4. MR imaging
MRI studies were performed using a 9.4‐T preclinical Varian/Agilent scanner (Vnmrj DirectDrive console v. 3.1) and a 19‐mm quadrature RF volume transceiver (Rapid Biomedical GmbH, Rimpar, Germany). A set of RF shapes for all the methods shown in Figures 1 and 2 for generating T1ρ contrast was created for the experiments. All the CW‐T1ρ measurements were conducted using a magnetization preparation block consisting of the RF train and a crusher gradient coupled to an FSE readout sequence. For each of the CW‐T1ρ methods, five SL amplitudes (γB1/2π = 0, 50, 100, 200, and 400 Hz) were used. Hard 90° and 180° pulses were both set to have a duration of 200 μs and the adiabatic refocusing/inversion pulses used were BIR‐4 and HS1, with durations of 5.17 and 3.03 ms, respectively. The AHP pulse duration was 4 ms. All the adiabatic pulses (Figure 2) were set to have a maximum B1 amplitude of 2.5 kHz. All the T1ρ measurements were performed using SL (CW) durations of 0, 4, 8, 16, 32, 64, 128, and 192 ms. In addition to T1ρ measurements, a B0 map was acquired using the same FSE readout sequence, coupled to a water saturation shift referencing (WASSR) 31 preparation module utilizing a saturation range of −300 to +300 Hz with a 50‐Hz step and saturation power of 30 Hz. Furthermore, the B1 field was estimated using a set of hard‐pulse saturation preparations around the expected 90° power (±40% from the expected power), coupled to a low‐resolution scan with the same FSE readout. The scan time for each of the aforementioned T1ρ setups was ~ 48 min, for WASSR ~ 8 min, and for the B1 scan ~ 13 min. The parameters of the readout FSE sequence varied slightly depending on the sample and its size (Table 1).
TABLE 1.
Measurement parameters besides spin locking for the experimental samples
| Samples | Slice | TR | Resolution | FOV | B0 field inhomogeneity simulation | B1 field inhomogeneity simulation |
|---|---|---|---|---|---|---|
| Bone cartilage specimens | 1 mm | 5 s | 256 x 128 | 16 x 16 mm | a | b |
| Tomato 1 | 4 mm | 5 s | 256 x 128 | 25 x 25 mm | a | c |
| Tomato 2 | 1 mm | 5 s | 256 x 128 | 20 x 30 mm | a | d |
| Agarose phantom | 2 mm | 5 s | 256 x 128 | 40 x 15 mm | a | d |
a. As‐good‐as‐possible shimming and then setting the shim gradients deliberately to incorrect values along a specific axis (phase‐encoding direction).
b. B1 amplitude increased by 20% from the calibrated amplitude.
c. The sample was moved about 15 mm away from the RF center (uniform field about 25 mm) to introduce an inhomogeneous B1 field.
d. The sample was larger than the homogenous RF region.
The samples were scanned under two nominal conditions: (i) as homogenous B0 and B1 as possible; and (ii) altered B0 and B1 settings to introduce inhomogeneities. At the beginning of every session, manual shimming of B0 and a calibration of the B1 transmit power was performed.
The measurements were first conducted for case (i) with as good conditions and homogenous fields as possible, and subsequently for case (ii) with the shims deliberately set to an incorrect value along a specific axis to induce B0 variation of approximately ±250 Hz along the chosen direction (in‐plane, across the cartilage surface for osteochondral samples, and along the same axis for the other samples). Additionally, the B1 amplitude was either set to 20% lower or higher than the nominal calibrated value, or the specimen was pulled approximately 15 mm away from the RF center (approximately 50% of the RF visibility range) so that the B1 field along the sample became inhomogeneous. For those specimens that exceeded the homogenous region of the B1 field, no additional B1 inhomogeneities were introduced (Table 1).
2.5. Data analysis
The results of the simulations were evaluated visually and semiquantitatively. For ΔB0 response with a correct B1 value and for ΔB1 response with correct B0, a semiquantitative metric was estimated: the width of the flat region of the response, that is, the width of the relatively smooth and flat response around the on‐resonance condition after applying a moving average window of 50 Hz width and a threshold of 90% of the on‐resonance amplitude. The averaging window width was changed to 10 Hz for the nonrefocused schemes and simulations of ΔB0 response at 100‐Hz SL amplitude to obtain reliable estimates. The results were calculated and visualized using the absolute values of the simulated z magnetization to facilitate comparison between the preparation schemes, because some of them deliberately take the magnetization to the ‐z axis.
Relaxation time maps were fitted in a pixel‐wise manner using the three‐parameter monoexponential fit, using in‐house developed plugins for Aedes (http://aedes.uef.fi) in Matlab (Matlab R2019b; MathWorks, Natick, MA, USA). B0 maps were calculated using Lorenzian fits to the acquired WASSR saturation datasets 31 and the B1 maps were estimated via linear fitting to the acquired saturation datasets.
To compare the reliability and robustness of the different T1ρ preparation schemes, mean normalized absolute deviation (MNAD) values in large regions of interest (ROIs) were calculated for each of the preparation schemes between the relaxation times measured under ideal and non‐ideal conditions. The large ROIs for each specimen were defined on an average T1ρ map calculated over all the preparation schemes for the SL amplitude of 400 Hz. These ROIs, comprising areas with high SNR, were then used to extract the T1ρ values from all the measurements under both conditions for further computations. The MNADs of the relaxation times were calculated by
| (1) |
where i refers to an individual voxel within the ROIs under ideal and non‐ideal conditions. The MNAD value of 0.5 corresponds to a mean deviation of 50% of the T1ρ relaxation times under the nonideal conditions. For the comparison of the different T1ρ preparation schemes, MNAD values from all the samples available for a given preparation were averaged.
In addition to the primary spin‐locking pulse, each of the T1ρ preparation schemes requires other RF pulses to tilt and refocus the magnetization. Depending on the configuration, the RF power deposited by these additional pulses varies significantly. To assess the relative differences in RF energy deposition between the preparations, root mean square (RMS) integrals of the pulse trains with zero SL duration were calculated. To facilitate the comparison, the RMS values were normalized with that of the conventional CW‐T1ρ preparation.
3. RESULTS
Numerical simulations demonstrated variable sensitivity of the sequences to a range of offsets in the B0 and B1 fields (Figures 3, 4, and S3). 2D plots of the simulated responses on both ΔB0 and B1 offset axes demonstrate the differences in the sensitivities of the T1ρ preparations: adiabatic refocused schemes demonstrated the least B1‐dependent variation and especially the double‐refocused versions also minimal ΔB0‐dependent variation at all simulated SL amplitudes (100 and 400 Hz) and SL times (8, 32, and 128 ms) (Figures 3F‐H and 4F‐H).
FIGURE 3.
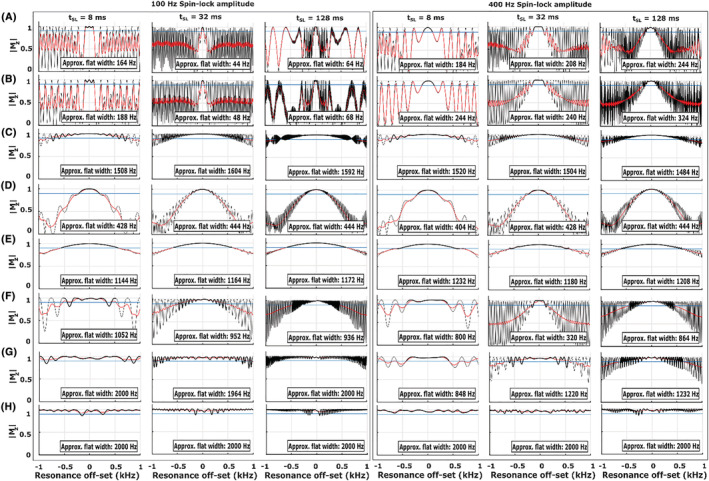
Simulations of the pulse sequences in Figures 1 and 2 with SL durations τ = 8, 32, and 128 ms and SL amplitudes of 100 and 400 Hz. The plots show Bloch simulations over ΔB0 (±1 kHz) at the correct B1 amplitude together with an estimated approximate flat response region (please see the Materials and Methods section) along the off‐resonance axis. (A) Conventional spin lock, (B) Conventional adiabatic CW‐T1ρ spin lock, (C) Single‐refocus ΔB0 and B1 insensitive spin lock, (D) Double‐refocus B‐SL, (E) Triple‐refocus CW‐T1ρ, (F) Adiabatic CW‐T1ρ with a single BIR‐4 refocusing, (G) Adiabatic CW‐T1ρ with double BIR‐4 refocusing, and (H) Adiabatic CW‐T1ρ with double HS1 refocusing. Absolute values of Mz are used to facilitate comparison between the sequences. B‐SL, balanced spin lock; BIR, B1 insensitive rotation; CW‐T1ρ, continuous wave T1ρ; HS, hyperbolic secant; SL, spin lock
FIGURE 4.

Simulations of the pulse sequences in Figures 1 and 2 with SL durations τ = 8, 32, and 128 ms and spin‐lock amplitudes of 100 and 400 Hz. The plots show Bloch simulations over ΔB1 (±40%) at the correct B0, together with an estimated approximate flat response region (please see the Materials and Methods section) along the relative B1 amplitude axis. (A) Conventional spin lock, (B) Conventional adiabatic CW‐T1ρ spin lock, (C) Single‐refocus ΔB0 and B1 insensitive spin lock, (D) Double‐refocus B‐SL, (E) Triple‐refocus CW‐T1ρ, (F) Adiabatic CW‐T1ρ with a single BIR‐4 refocusing, (G) Adiabatic CW‐T1ρ with double BIR‐4 refocusing, and (H) Adiabatic CW‐T1ρ with double HS1 refocusing. Absolute values of Mz are used to facilitate comparison between the sequences. B‐SL, balanced spin lock; BIR, B1 insensitive rotation; CW‐T1ρ, continuous wave T1ρ; HS, hyperbolic secant; SL, spin lock
Quantification of the flatness of the simulated ΔB0 and ΔB1 responses at the nominally correct B1 and B0 indicated that the non‐refocused schemes had a very poor B0 off‐resonance response with almost no flat region even at the correct B1, while the refocused versions showed significantly improved responses (Figures 3C‐H, 4C‐H, S7 and S8). However, the adiabatic CW pulse simulated at 600‐Hz maximum amplitude (Figure S3B) had a broader flat response for both B0 and B1 inhomogeneities at the higher SL amplitude (400 Hz) (Figures S3B and S9) when compared with the 2.5‐kHz maximum amplitude simulations of the pulse (Figures 3, 4, and S7‐S9).
The adiabatic double‐refocused schemes had the broadest ΔB0 robustness, with the flat range essentially covering the entire simulated range from −1 to +1 kHz (and beyond), while the single‐ and triple‐refocused preparations had the broadest flat responses among the hard‐pulse preparation schemes (Figures 3C,E and 4C,E), but with a slight drop at B1 amplitudes beyond ±31% of the nominally correct amplitude. The double‐refocused hard pulse was highly insensitive to a wide range of B1 offsets, but was more sensitive to B0 inhomogeneities, being the least robust among the refocused schemes (Figures 3D, 4D, S7 and S8).
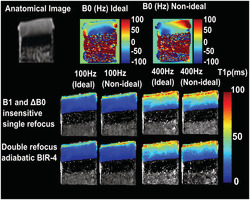
For the experimental measurements under as ideal as possible conditions, the T1ρ relaxation time maps of the cartilage bone samples, cherry tomatoes, and phantom were visually artifact‐free for all the preparation schemes for SL amplitudes above 100 Hz (Figures 5, 6, 7). Under the non‐ideal conditions, however, at SL amplitudes equal to and below ΔB0, the conventional and adiabatic non‐refocused T1ρ relaxation time maps showed banding artifacts (Figures 5, 6, 7). At higher SL amplitudes (ω1 > ΔB0), the artifacts were significantly suppressed for all methods. For the refocused preparation schemes that compensate for the B1 and B0 imperfections, the artifacts were significantly reduced even at the lower SL amplitudes. Suppression of artifacts was particularly effective by the preparation schemes utilizing either single or double refocusing with adiabatic BIR‐4 pulses (Figures 5, 6, 7). With the cherry tomato (as well as the phantom), marked banding artifacts are seen at the top and bottom of the samples, where both B1 and B0 fields deviated from the nominal values, even under the experimentally ideal conditions. At higher spin locking amplitudes, the artifacts due to the non‐homogenous fields were also suppressed in these samples, particularly with the adiabatic BIR‐4 refocusing.
FIGURE 5.
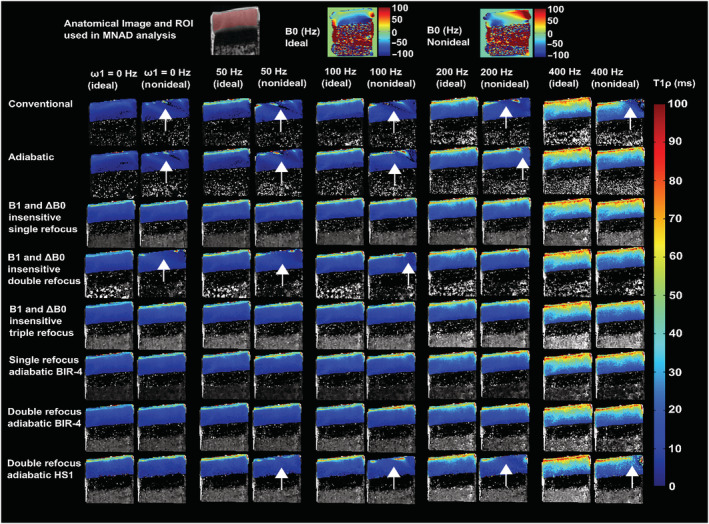
Representative examples of T1ρ relaxation time maps of cartilage bone specimens under ideal and non‐ideal conditions with inhomogeneous B0 and B1 fields, for SL amplitudes of 0–400 Hz acquired with the different methods. Anatomical reference (showing the MNAD analysis ROI with red shading) and the corresponding B0 maps are shown at the top. Under the ideal conditions, all the methods provided largely artifact‐free T1ρ relaxation time maps at all SL amplitudes. Under the non‐ideal conditions, the non‐refocused T1ρ methods in particular performed poorly at lower SL amplitudes, while the refocused methods provided mostly artifact‐free relaxation time maps at all SL amplitudes. In particular, the double‐refocused adiabatic BIR‐4 method was robust. The arrows indicate locations where differences (artifacts) can be noted between the conditions. BIR, B1 insensitive rotation; MNAD, mean normalized absolute deviation; ROI, region of interest; SL, spin lock
FIGURE 6.
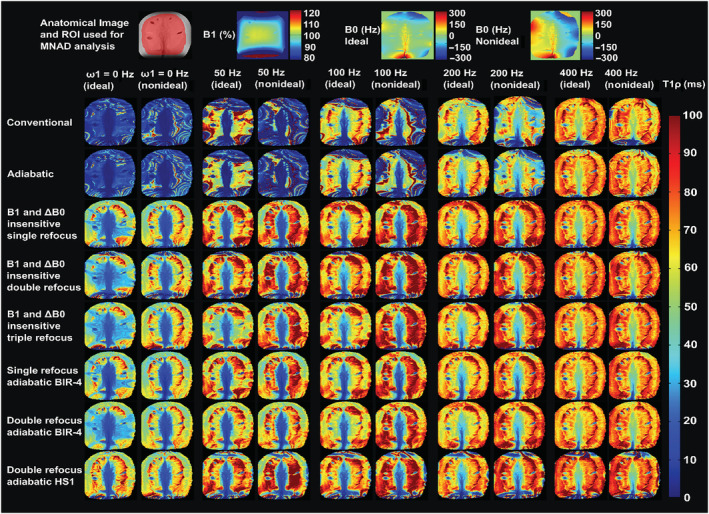
T1ρ relaxation time maps of a cherry tomato sample, under as ideal as possible conditions and under non‐ideal conditions, with inhomogeneous B0 field, for SL amplitudes of 0–400 Hz acquired with the different methods. Anatomical reference (showing the MNAD analysis ROI with red shading) and the corresponding B1 and B0 maps are shown at the top. Under the ideal conditions, all the refocused methods provided largely artifact‐free T1ρ relaxation time maps at all SL amplitudes, while the nonrefocused methods showed artifacts at the edges of the FOV at low SL amplitudes. Under the non‐ideal conditions, the nonrefocused T1ρ methods in particular performed poorly at lower SL amplitudes, while the refocused methods provided mostly artifact‐free relaxation time maps at all SL amplitudes. The differences between the ideal and nonideal conditions can particularly be seen at the top and bottom edges with more significant field inhomogeneities. FOV, field of view; MNAD, mean normalized absolute deviation; ROI, region of interest; SL, spin lock
FIGURE 7.
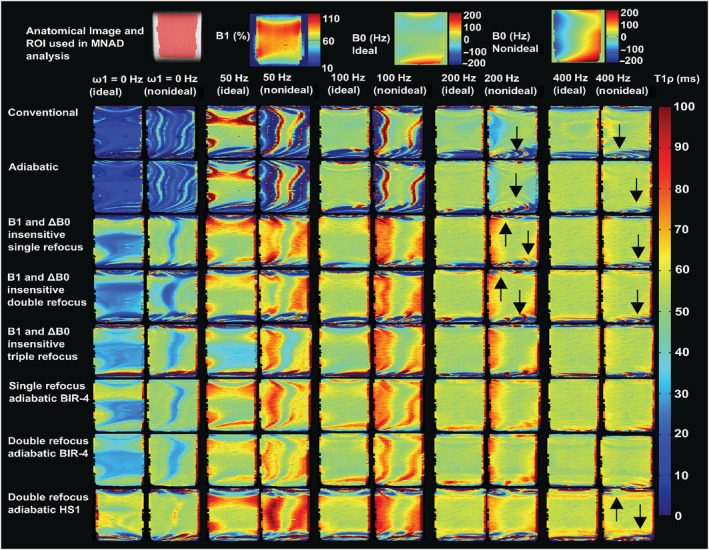
T1ρ relaxation time maps of agar phantom, under as ideal as possible conditions and under non‐ideal conditions, with the sample size covering the region away from the RF center and inhomogeneous B0 field, for SL amplitudes of 0–400 Hz acquired with the different methods. Anatomical reference (showing the MNAD analysis ROI with red shading) and the corresponding B1 and B0 maps are shown at the top. Under the ideal conditions, all the refocused methods provided largely artifact‐free T1ρ relaxation time maps at all SL amplitudes, while the non‐refocused methods showed artifacts at the edges of the FOV at low spin‐lock amplitudes. Under the non‐ideal conditions, the non‐refocused T1ρ methods performed poorly at lower SL amplitudes, while the refocused methods were able to mitigate the most severe artifacts, especially at the higher SL amplitudes. The arrows indicate locations where differences (artifacts) can be noted between the conditions. FOV, field of view; MNAD, mean normalized absolute deviation; RF, radio frequency; ROI, region of interest; SL, spin lock
The MNADs within the ROIs were generally the smallest for the refocused schemes (Figure 8), broadly in agreement with the sensitivities observed in the Bloch simulations. Among the refocused hard‐pulse and adiabatic preparation schemes, the double‐ and single‐refocus adiabatic BIR‐4 methods presented the smallest deviations in the experimental data (Figure 8). It should be noted that while all the scanned samples were used in the calculation of the average MNADs, the number of samples was not the same for all preparations because of the later inclusion of some of the preparation schemes in the study (Figure 8).
FIGURE 8.
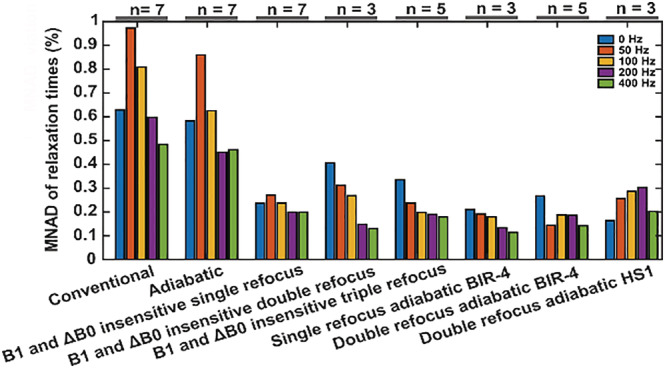
MNAD values of the relaxation times between the ideal and non‐ideal conditions in large ROIs (shown in Figures 5, 6, 7) over all the samples available for each measurement. The refocused preparation schemes stand out by showing the least variation between the ideal and nonideal cases, indicating their robustness against field inhomogeneities regardless of the SL amplitude. MNAD, mean normalized absolute deviation; ROI, region of interest; SL, spin lock
The conventional hard‐pulse CW‐T1ρ preparation with only two 90° pulses imposes the least additional RF energy deposition and thus produces the least specific absorption rate (SAR) (Figure 9). The preparations including adiabatic pulses add a constant adiabatic T1ρ weighting in addition to the T2 weighting from finite TE of the readout, and these pulses induce significantly higher RF energy deposition (the RMS integral of the 0‐ms SL pulse for the double‐refocus BIR‐4 is approximately 86 times that of the conventional T1ρ preparation) (Figure 9, Table S1). However, for a plain SL pulse (i.e., without the 90° or 180° pulses) of 50‐ms duration and 400‐Hz amplitude, the RMS integral is ~40 times that of the 0‐ms SL pulse of the conventional T1ρ preparation with the least extra RF. For increasing SL durations and amplitudes, relative differences in the energy deposition between the preparation schemes are reduced (an RMS integral ratio of a SL pulse of 64‐ms duration and 400‐Hz amplitude using double‐refocus BIR‐4 with respect to conventional is reduced from ~86 times to just under three times) (Figure 9, Table S1). The 0‐ms SL adiabatic CW T1ρ pulse, with a longer duration and a reduced maximum RF amplitude of 600 Hz of the AHP, was observed to have approximately one‐quarter of the RMS integral of the original pulse with a maximum amplitude of 2.5 kHz. With the same lower‐power AHP pulses, the RMS integral of a SL pulse of 64‐ms duration and 400‐Hz amplitude was reduced by a factor of approximately 1.5 compared with the original using 2.5‐kHz AHP pulses (Table S1, Figure S6).
FIGURE 9.
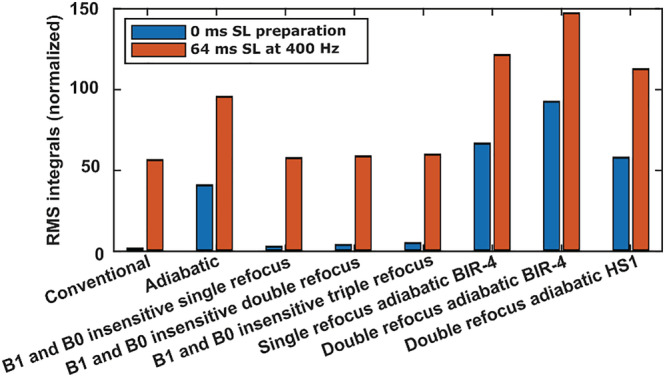
Comparison of the RMS integrals (RF power) of the zero SL duration RF pulse trains (blue bars) and 64‐ms SL duration RF pulse trains at 400‐Hz SL amplitude (orange bars) for the different T1ρ preparation methods normalized to the conventional SL method. All the hard‐pulse methods show negligible difference to the conventional SL method. On the other hand, the adiabatic pulses show significantly increased RF energy deposition. The RMS integrals of the zero SL duration adiabatic pulse schemes are approximately comparable with those of the conventional 64‐ms spin lock at 400 Hz. BIR, B1 insensitive rotation; HS, hyperbolic secant; RF, radio frequency; RMS, root mean square; SL, spin lock
4. DISCUSSION
T1ρ contrast remains interesting for various applications in the human body because of its sensitivity to low frequency molecular interactions that are often biologically important. 5 , 9 The different T1ρ contrast preparation methods, particularly at very low SL amplitude, are however sensitive to imperfections of the imaging field and the RF field. In this study, we proposed four new methods for generating T1ρ contrast and compared them experimentally and numerically with four existing methods for their sensitivity to the field inhomogeneities. The study builds on earlier reports introducing ΔB0 and B1 insensitive T1ρ preparation schemes, 15 , 18 , 19 , 22 particularly the one by Witschey et al., 14 and utilizes the same theoretical examination of the proposed hard‐pulse schemes (see the supporting information). The results of the study indicate that those methods employing a refocusing pulse are significantly more robust against field inhomogeneities than those methods which do not, and also that combining CW spin locking with fully adiabatic excitation and refocusing is the most robust method against field inhomogeneities. However, the fully adiabatic schemes have the additional cost of significantly increased RF energy deposition. Among the non‐adiabatic hard‐pulse refocusing sequences, the most robust ones were the single‐refocus hard‐pulse CW‐T1ρ method proposed by Witschey et al. 14 and the proposed triple‐refocus hard‐pulse CW‐T1ρ method. These two outperformed the double‐refocused B‐SL method proposed by Gram et al., 18 most likely because this method brings the magnetization back to the positive z axis, while the other two take the magnetization to the negative z axis and are thus more robust against variations in B0.
Recently, there has been an increase in interest towards T1ρ dispersion in cartilage, 13 , 32 , 33 , 34 , 35 , 36 because the measurement could provide information beyond a single amplitude T1ρ scan. However, especially lowering the SL amplitudes requires methods that are robust against field inhomogeneities. If the B0 variations exceed the spin‐locking amplitude, the locking becomes inefficient, resulting in spurious signal loss, which is further amplified with methods that do not compensate for field variations. 1 , 12
The theoretical considerations regarding the triple‐refocused hard‐pulse CW‐T1ρ preparation lead to the same conclusions that were found for the single‐refocused preparation scheme earlier by Witschey et al., 14 suggesting the methods should be approximately equal. The simulations showed a slightly broader flat response with respect to variations in B0 for the single‐refocus method, while the response of the triple‐refocused method was slightly smoother. The double‐refocused pulse scheme brings the magnetization back to the positive z axis; however, it appears to require nearly perfect 90° and 180° pulses, while the single‐ and triple‐refocused methods only require that the 180° pulses should be nearly perfect. Because of this difference, the single‐ or triple‐refocused schemes appeared more robust against field inhomogeneities, as confirmed by the simulations. In practice, however, all the refocused hard‐pulse options were observed to be very similar in soft tissues.
Adiabatic pulses are known for their excellent tolerance to RF inhomogeneity 28 and thus stand out as an interesting possibility to improve the robustness of CW T1ρ preparation. Furthermore, adiabatic T1ρ could be measured in fully adiabatic mode, using a train of AFP HS RF pulses, instead of a constant amplitude CW SL pulse in between AHP pulses. 22 , 24 , 29 , 37 , 38 In comparison with a CW SL with fixed B1 amplitude and orientation, the adiabatic T1ρ SL varies between off‐resonance and on‐resonance T1ρ during the adiabatic sweep, where the amplitude and frequency of the pulse are modulated during the time course of the pulse. 39 From the simulations, it was evident that the refocused adiabatic methods presented here are highly insensitive to ΔB0 and B1 field inhomogeneities. The robustness of the refocused adiabatic methods exceeded the simulated range of variation for the RF power, while the robustness against B0 variations depended on the specific scheme. The double‐refocused adiabatic BIR‐4 and HS1 versions were found to be the most robust in the simulations, while experimentally, the double‐refocused BIR‐4 scheme was found to be the most robust. The low‐powered (600‐Hz) adiabatic CW‐T1ρ, which had an AHP pulse approximately four times longer than the high‐powered (2.5‐kHz) AHP pulse, was highly insensitive to field inhomogeneities at the higher SL amplitude of 400 Hz in the simulations (Figure S3B). This simulation demonstrates that when the maximum B1 amplitude of the AHP pulses is brought closer to the spin‐locking amplitude, then adiabatic CW‐T1ρ becomes highly insensitive to B0 inhomogeneities that are of the order of or smaller than the spin‐locking amplitude.
The experimental findings under the ideal and non‐ideal conditions largely confirmed the observations of the simulations. The non‐refocused conventional and adiabatic schemes under the non‐ideal conditions fell behind all the other schemes that utilized refocusing, although under the ideal conditions, the relaxation time maps were mostly clean, particularly at higher SL amplitudes. The ΔB0 and B1 insensitive single‐refocus method 14 and the proposed triple‐refocus method were experimentally approximately equal, while the B‐SL method 18 exhibited poorer performance and more banding artifacts, especially at lower SL amplitudes. On the other hand, the adiabatic BIR‐4 refocused schemes produced very clean T1ρ maps compared with all other methods in all specimens, suppressing the most artifacts (Figure S4). The non‐refocused schemes showed severe banding artifacts in the T1ρ relaxation time maps under the non‐ideal conditions, at SL amplitudes equal to and below ΔB0. At higher SL amplitudes (ω1 > ΔB0, or ω1>> ΔB0), the banding artifacts were minimal for all the schemes, unless B1 variation was also present.
The differences in the sensitivities to field inhomogeneities between the preparation schemes were assessed by calculating the MNAD values between the measurements conducted at ideal versus non‐ideal conditions. This approach, while potentially dependent on the changes in the experimental conditions, provides a handle on the sensitivities of the methods, summarizing the results over all the measured samples. Among the hard‐pulse schemes, the non‐refocused preparations stood out with the largest deviations between the ideal and non‐ideal cases, while the refocused methods showed significantly smaller deviation between the cases at all SL amplitudes. The adiabatic refocused schemes were aligned with the hard‐pulse alternatives with similar small deviations. However, these analyses were conducted only in the tissues that had high SNR and were not clearly at off‐resonance (such as the fatty bone marrow tissue). Further experimental differences were seen at the extreme areas, such as the fat, or the edges of the coil‐visible region for the tomato specimen in Figures 5 and 6, and particularly in the phantom (Figure 7), where the non‐refocused methods, the B‐SL method, 18 and the double‐refocus adiabatic HS1 preparations showed signal loss and banding artifacts. The experimental performance of the adiabatic double‐refocus scheme incorporating HS1 inversion pulses was not as good as that of the BIR‐4 approach, despite providing the most promising simulation results. This could be because of the flip angle dispersion effects of the HS1‐AFP pulse on the magnetization components not being collinear with it, 28 as is the case here. Two HS1‐pulses were utilized to compensate for this effect, but the result remained inferior to that achieved by using an adiabatic plane rotation BIR‐4 pulse.
In the clinical setting, T1ρ relaxation measurements could provide important insights into disease diagnosis and progression. 33 , 40 , 41 , 42 However, small B0 and B1 variations are inevitable and interfere with the T1ρ quantification. This is particularly the case for low amplitude spin locking, because the threshold of B0 variation affecting measurements depends on the spin‐lockin amplitude. The double‐refocus adiabatic BIR‐4 scheme was found to be the most robust against field inhomogeneities for improving the T1ρ quantification. However, the most significant problem with this method is its significantly increased RF energy deposition: as realized here, the baseline zero SL pulse has a duration of approximately 18 ms at an RMS amplitude of 2.3 kHz, which is already well beyond what is typically even achievable on a clinical scanner (often the maximum transmit power is below 1 kHz, even for local transmit coils). 43 Besides the increased power requirements, such pulses are also likely to exceed SAR safety limits, 14 further limiting the use of such T1ρ preparations. Among the less RF‐intensive, yet ΔB0 and B1 insensitive T1ρ preparation schemes, the single‐refocus scheme 14 with minimal RF energy deposition appears to be the most feasible for in vivo imaging. However, because the magnetization after this preparation will be at the negative z axis, a spin‐echo type of readout sequence would be preferable over a gradient‐echo sequence with relatively small tip angles, which will drive the magnetization through zero if longer echo trains are collected. Alternatively, for a gradient‐echo readout sequence, an additional (adiabatic) inversion pulse could potentially be utilized at the end of the preparation to avoid this effect. Considering the overall scan duration, gradient‐echo sequences with short TR and RF cycling 44 or tailored flip angles 45 could be utilized to enable faster scans.
Other possibilities for improved T1ρ have been presented previously, such as the one by Mitrea et al. 15 Initial tests (Figure S5), however, suggested it to be more sensitive to field variations than the single‐refocus method reported by Witschey et al., 14 further supported by the simulations (see the supporting information). Another very promising approach utilizes adiabatic excitation and rewinder pulses at the same amplitude as the target SL amplitude. 16 , 46 , 47 Simulations with a nearly matched amplitude SL pulse 16 , 47 suggested that this non‐refocused adiabatic scheme performs very well against the field inhomogeneities (see the supporting information). However, this sequence is more akin to the adiabatic T1ρ method, 7 , 24 , 25 , 43 and is a combination of on‐resonance and off‐resonance T1ρ relaxation. Another potential challenge with this method is maintaining the adiabatic condition at very low SL amplitudes. Utilizing fully adiabatic spin locking 22 , 24 , 29 , 37 , 38 can further mitigate the effects of field inhomogeneities and even provide slice selectivity 37 as well as reduced orientation/magic angle dependence. 7 A variation of the double‐refocused hard‐pulse preparation scheme investigated here 18 was presented recently with promising results, but without direct comparison with other preparation methods. 48 Besides presenting a method for faster T1ρ acquisition by using tailored variable flip angle scheduling, Johnson et al. 45 also utilized a partially adiabatic variation of the single‐refocus method by Witschey et al., 14 replacing the hard 90° pulses with adiabatic pulses. This variation presents another interesting option for T1ρ preparation; however, no direct comparison with other T1ρ preparations with respect to sensitivity to inhomogeneities was provided.
The present study has certain limitations, including a limited selection of previously presented methods for the experimental generation of T1ρ contrast. The number of samples is limited, and all the experiments were carried out at 9.4 T and using a relatively high maximum B1 amplitude. However, the differences between the methods were generally confirmed with the simulations; similar practical differences may be expected with B0 and B1 variations regardless of the main field strength, although the practical in vivo importance is ultimately revealed with real measurements.
In conclusion, artifacts arising from the field inhomogeneities in CW‐T1ρ–weighted imaging can be efficiently suppressed by different refocused spin‐locking pulse schemes. In this numerical, experimental, and theoretical comparison of different T1ρ contrast preparation methods, the double‐refocus adiabatic BIR‐4 preparation was found to be the most robust. However, because of the excessive RF energy deposition of the adiabatic method, its use is likely restricted to the preclinical setting. Of the less RF‐intensive methods, the ΔB0 and B1 compensated single‐refocus hard‐pulse CW‐T1ρ method reported by Witschey et al. 14 and the proposed triple‐refocused method proved to be very robust against field inhomogeneities. The simulations confirm the increased robustness of the low‐power AHP CW spin locking, and both the experimental and the simulation findings promote the use of the previously reported hard‐pulse single‐refocus ΔB0 and B1 insensitive method for clinical use, while the adiabatic double‐refocused BIR‐4 method could be preferred for ex vivo experiments.
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.
Supporting information
Figure S1: Triple refocus magnetization path under on‐resonance conditions. The magnetization (M[0‐]) is flipped from the longitudinal axis (z‐axis) with an angle α, which does not need to be 90°, towards the transverse plane (y‐axis) (M[0+]) and nutates about the z1” axis (M[τ−/4]) and at time τ/4 is flipped by 2α about y‐axis (M[τ+/4]) and nutates (M[τ−/2]) around z2”, this trend of rotation and nutation is the same for the rest of the pulses in the train. The final magnetization (M[τ‐], after full spin‐locking) is brought to the negative longitudinal axis (M[τ+]) by another flip angle α. Artifacts due to imperfect 180° are compensated to an extent by the multiple refocuses.
Figure S2: Field insensitive spin lock with double refocusing for T1ρ relaxation measurements. Magnetization path under on‐resonance conditions. The magnetization (M[0‐]) is flipped from the longitudinal plane (z‐axis) with an angle α, which does not need to be 90° towards the transverse plane (y‐axis) (M[0+]) and nutates about the z1” axis (M[τ−/4]) and at time τ+/4 is flipped by 2α about y‐axis (M[τ+/4]) and nutates (M[τ‐3/4]) around z2”, this trend of rotation and nutation is same for the rest of the pulses in the train. The final magnetization (M [τ‐], after full spin‐locking) returns to the positive longitudinal axis (M [τ+]) with another flip angle ‐α. Artifacts due to imperfect 180° are compensated to an extent by the double refocusing.
Figure S3: Simulations for PSC‐SL (a) proposed by Mitrea et al1, and conventional adiabatic sequence with reduced maximum power of the AHP pulse to 600 Hz (b) with the same ΔB0 and B1 field inhomogeneity ranges of up to ±1 kHz and ±40% as presented in this manuscript, for spin‐lock durations of 32 ms and 128 ms, and for spin‐lock amplitudes of 100 Hz and 400 Hz.
Figure S4. Relative differences between the T1ρ maps acquired under the ideal and non‐ideal conditions for the worst performing conventional (Figure 1A), and for the two best performing, single‐refocus hard pulse (Figure 1B) and double‐refocus adiabatic BIR‐4 (Figure 2C) preparation schemes at spin‐lock amplitudes ranging from 0 to 400 Hz.
Figure S5. T1ρ relaxation time maps for paired self‐compensated single refocus PSC‐SL sequence proposed by Mitrea et al1, under the non‐ideal conditions for spin‐lock amplitudes of 0–400 Hz. Anatomical reference and the corresponding B0 map are shown at the top.
Figure S6 c. Comparison of the RMS integrals (RF power) of the zero spin‐lock duration RF pulse trains (blue bars) and 64 ms spin‐lock duration RF pulse trains at 400 Hz spin‐lock amplitude (orange bars) of the conventional pulse, paired self‐compensated PSC‐SL pulse sequence proposed by Mitrea et al1, and adiabatic CW pulse with 600 Hz maximum AHP power T1ρ preparation methods, normalized to the RMS integral of the zero spin‐lock duration conventional spin‐lock method.
Figure S7. Calculated approximate flat widths for the Bloch simulations of all the sequences in Figures 1 and 2 over ΔB0 (±1 kHz) at the correct B1 amplitude. Simulations were run with spin‐lock durations ranging from τ = 0 to 256 ms in 2 ms steps, and with spin‐lock amplitudes from 0 to 1,000 Hz in 10 Hz steps.
Figure S8. Calculated approximate flat widths for the Bloch simulations of all the sequences in Figures 1 and 2 over ΔB1 (±40%) at the correct B0 amplitude. Simulations were run with spin‐lock durations ranging from τ = 0 to 256 ms in 2 ms steps, and with spin‐lock amplitudes from 0 to 1,000 Hz in 10 Hz steps.
Figure S9: Calculated approximate flat widths for the Bloch simulations of the PSC‐SL proposed by Mitrea et al1, and for the adiabatic CW pulse with 600 Hz maximum AHP power T1ρ preparation methods with similar ΔB0 and B1 field inhomogeneities of up to ±1 kHz and ±40% as presented in this manuscript. Simulations were run with spin‐lock durations ranging from τ = 0 to 256 ms in 2 ms steps, and with spin‐lock amplitudes from 0 to 1,000 Hz in 10 Hz steps.
Table S1: Pulse length, RMS integral and RMS amplitude values of preparation schemes with the zero spin‐lock (SL) pulses and with 64 ms SL at 400 Hz amplitude pulses.
ACKNOWLEDGMENTS
The authors acknowledge Assoc. Prof. Patrick Bolan, CMRR, University of Minnesota, for providing the pulse simulation tool. Financial support from the Academy of Finland (grant numbers: #285909, #319440, #325146, and #340761) and the Finnish Cultural Foundation (grant numbers: #00180787 and #65211960) is gratefully acknowledged.
Pala S, Hänninen NE, Nykänen O, Liimatainen T, Nissi MJ. New methods for robust continuous wave T1ρ relaxation preparation. NMR in Biomedicine. 2023;36(2):e4834. doi: 10.1002/nbm.4834
Funding information
Academy of Finland, Grant/Award Numbers: 285909, 319440, 325146, 340761; Finnish Cultural Foundation, Grant/Award Numbers: 00180787, 65211960
Funding information Academy of Finland, Grant/Award Numbers: #285909, #319440, #325146, #340761; Finnish Cultural Foundation, Grant/Award Numbers: #00180787, #65211960
1. DATA AVAILABILITY STATEMENT
All the data of the study are openly available at the Zenodo archives at http://doi.org/10.5281/zenodo.5547762.
REFERENCES
- 1. Sepponen RE, Pohjonen JA, Sipponen JT, Tanttu JI. A method for T1p imaging. J Comput Assist Tomogr. 1985;9(6):1007‐1011. doi: 10.1097/00004728-198511000-00002 [DOI] [PubMed] [Google Scholar]
- 2. Johnson CP, Follmer RL, Oguz I, et al. Brain abnormalities in bipolar disorder detected by quantitative T1ρ mapping. Mol Psychiatry. 2015;20(2):201‐206. doi: 10.1038/mp.2014.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang YXJ, Yuan J, Chu ESH, et al. T1ρ MR imaging is sensitive to evaluate liver fibrosis: An experimental study in a rat biliary duct ligation model. Radiology. 2011;259(3):712‐719. doi: 10.1148/radiol.11101638 [DOI] [PubMed] [Google Scholar]
- 4. Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T(1ρ)‐relaxation in articular cartilage: Effects of enzymatic degradation. Magn Reson Med. 1997;38(6):863‐867. doi: 10.1002/mrm.1910380602 [DOI] [PubMed] [Google Scholar]
- 5. Borthakur A, Mellon E, Niyogi S, Witschey W, Kneeland JB, Reddy R. Sodium and T1ρ MRI for molecular and diagnostic imaging of articular cartilage. NMR Biomed. 2006;19(7):781‐821. doi: 10.1002/nbm.1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Regatte RR, Akella SVS, Borthakur A, Reddy R. Proton spin‐lock ratio imaging for quantitation of glycosaminoglycans in articular cartilage. J Magn Reson Imaging. 2003;17(1):114‐121. doi: 10.1002/jmri.10228 [DOI] [PubMed] [Google Scholar]
- 7. Hänninen N, Rautiainen J, Rieppo L, Saarakkala S, Nissi MJ. Orientation anisotropy of quantitative MRI relaxation parameters in ordered tissue. Sci Rep. 2017;7(1):1‐11. doi: 10.1038/s41598-017-10053-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akella SVS, Regatte RR, Wheaton AJ, Borthakur A, Reddy R. Reduction of residual dipolar interaction in cartilage by spin‐lock technique. Magn Reson Med. 2004;52(5):1103‐1109. doi: 10.1002/mrm.20241 [DOI] [PubMed] [Google Scholar]
- 9. Gilani IA, Sepponen R. Quantitative rotating frame relaxometry methods in MRI. NMR Biomed. 2016;29(6):841‐861. doi: 10.1002/nbm.3518 [DOI] [PubMed] [Google Scholar]
- 10. Duvvuri U, Goldberg AD, Kranz JK, et al. Water magnetic relaxation dispersion in biological systems: The contribution of proton exchange and implications for the noninvasive detection of cartilage degradation. Proc Natl Acad Sci U S A. 2001;98(22):12479‐12484. doi: 10.1073/pnas.221471898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Knispel RR, Thompson RT, Pintar MM. Dispersion of proton spin‐lattice relaxation in tissues. J Magn Reson. 1974;14(1):44‐51. doi: 10.1016/0022-2364(74)90255-8 [DOI] [Google Scholar]
- 12. Mäkelä HI, Gröhn OHJ, Kettunen MI, Kauppinen RA. Proton exchange as a relaxation mechanism for T1 in the rotating frame in native and immobilized protein solutions. Biochem Biophys Res Commun. 2001;289(4):813‐818. doi: 10.1006/bbrc.2001.6058 [DOI] [PubMed] [Google Scholar]
- 13. Mlynárik V, Szomolányi P, Toffanin R, Vittur F, Trattnig S. Transverse relaxation mechanisms in articular cartilage. J Magn Reson. 2004;169(2):300‐307. doi: 10.1016/j.jmr.2004.05.003 [DOI] [PubMed] [Google Scholar]
- 14. Witschey WRT, Borthakur A, Elliott MA, et al. Artifacts in T1ρ‐weighted imaging: Compensation for B1 and B0 field imperfections. J Magn Reson. 2007;186(1):75‐85. doi: 10.1016/j.jmr.2007.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitrea BG, Krafft AJ, Song R, Loeffler RB, Hillenbrand CM. Paired self‐compensated spin‐lock preparation for improved T1ρ quantification. J Magn Reson. 2016;268:49‐57. doi: 10.1016/j.jmr.2016.04.017 [DOI] [PubMed] [Google Scholar]
- 16. Chen W. Artifacts correction for T1rho imaging with constant amplitude spin‐lock. J Magn Reson. 2017;274:13‐23. doi: 10.1016/j.jmr.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 17. Zeng H, Daniel G, Gatenby C, Zhao Y, Avison M, Gore J. A composite spin‐lock pulse for ∆ B 0 + B 1 insensitive T 1. Proc Int Soc Magn Reson Med. 2006;14:2356. [Google Scholar]
- 18. Gram M, Seethaler M, Gensler D, Oberberger J, Jakob PM, Nordbeck P. Balanced spin‐lock preparation for B1‐insensitive and B0‐insensitive quantification of the rotating frame relaxation time T1ρ. Magn Reson Med. 2021;85(5):2771‐2780. doi: 10.1002/mrm.28585 [DOI] [PubMed] [Google Scholar]
- 19. Gröhn HI, Michaeli S, Garwood M, Kauppinen RA, Gröhn OHJ. Quantitative T1ρ and adiabatic Carr‐Purcell T2 magnetic resonance imaging of human occipital lobe at 4 T. Magn Reson Med. 2005;54(1):14‐19. doi: 10.1002/mrm.20536 [DOI] [PubMed] [Google Scholar]
- 20. Gröhn OHJ, Mäkelä HI, Lukkarinen JA, et al. On‐ and off‐resonance T1ρ MRI in acute cerebral ischemia of the rat. Magn Reson Med. 2003;49(1):172‐176. doi: 10.1002/mrm.10356 [DOI] [PubMed] [Google Scholar]
- 21. Taheri S, Sood R. Spin‐lock MRI with amplitude‐ and phase‐modulated adiabatic waveforms: An MR simulation study. Magn Reson Imaging. 2006;24(1):51‐59. doi: 10.1016/j.mri.2005.10.020 [DOI] [PubMed] [Google Scholar]
- 22. Jokivarsi KT, Niskanen JP, Michaeli S, et al. Quantitative assessment of water pools by T1ρ and T 2ρ MRI in acute cerebral ischemia of the rat. J Cereb Blood Flow Metab. 2009;29(1):206‐216. doi: 10.1038/jcbfm.2008.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cobb JG, Xie J, Gore JC. Contributions of chemical exchange to T1ρ dispersion in a tissue model. Magn Reson Med. 2011;66(6):1563‐1571. doi: 10.1002/mrm.22947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mangia S, Liimatainen T, Garwood M, Michaeli S. Rotating frame relaxation during adiabatic pulses vs. conventional spin lock: simulations and experimental results at 4 T. Magn Reson Imaging. 2009;27(8):1074‐1087. doi: 10.1016/j.mri.2009.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ellermann J, Ling W, Nissi MJ, et al. MRI rotating frame relaxation measurements for articular cartilage assessment. Magn Reson Imaging. 2013;31(9):1537‐1543. doi: 10.1016/j.mri.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang J, Nissi MJ, Idiyatullin D, Michaeli S, Garwood M. Capturing fast relaxing spins with SWIFT adiabatic rotating frame spin – lattice relaxation (T 1 ρ) mapping. NMR Biomed. 2016;29:420‐430. doi: 10.1002/nbm.3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tannús A, Garwood M. Adiabatic pulses. NMR Biomed. 1997;10(8):423‐434. doi: [DOI] [PubMed] [Google Scholar]
- 28. Garwood M, DelaBarre L. The return of the frequency sweep: Designing adiabatic pulses for contemporary NMR. J Magn Reson. 2001;153(2):155‐177. doi: 10.1006/jmre.2001.2340 [DOI] [PubMed] [Google Scholar]
- 29. Michaeli S, Gröhn H, Gröhn O, et al. Exchange‐influenced T2ρ contrast in human brain images measured with adiabatic radio frequency pulses. Magn Reson Med. 2005;53(4):823‐829. doi: 10.1002/mrm.20428 [DOI] [PubMed] [Google Scholar]
- 30. Garwood M, Ke Y. Symmetric pulses to induce arbitrary flip angles with compensation for rf inhomogeneity and resonance offsets. J Magn Reson. 1991;94(3):511‐525. doi: 10.1016/0022-2364(91)90137-I [DOI] [Google Scholar]
- 31. Kim M, Gillen J, Landman BA, Zhou J, Van Zijl PCM. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med. 2009;61(6):1441‐1450. doi: 10.1002/mrm.21873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Keenan KE, Besier TF, Pauly JM, et al. T1ρ dispersion in articular cartilage: relationship to material properties and macromolecular content. Cartilage. 2015;6(2):113‐122. doi: 10.1177/1947603515569529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Borthakur A, Wheaton AJ, Gougoutas AJ, et al. In vivo measurement of T1ρ dispersion in the human brain at 1.5 Tesla. J Magn Reson Imaging. 2004;19(4):403‐409. doi: 10.1002/jmri.20016 [DOI] [PubMed] [Google Scholar]
- 34. Kajabi AW, Casula V, Sarin JK, et al. Evaluation of articular cartilage with quantitative MRI in an equine model of post‐traumatic osteoarthritis. J Orthop Res. 2021;39(1):63‐73. doi: 10.1002/jor.24780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kajabi AW, Casula V, Ojanen S, et al. Multiparametric MR imaging reveals early cartilage degeneration at 2 and 8 weeks after ACL transection in a rabbit model. J Orthop Res. 2020;38(9):1974‐1986. doi: 10.1002/jor.24644 [DOI] [PubMed] [Google Scholar]
- 36. Rautiainen J, Nissi MJ, Salo EN, et al. Multiparametric MRI assessment of human articular cartilage degeneration: Correlation with quantitative histology and mechanical properties. Magn Reson Med. 2015;74(1):249‐259. doi: 10.1002/mrm.25401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Andronesi OC, Bhat H, Reuter M, Mukherjee S, Caravan P, Rosen BR. Whole brain mapping of water pools and molecular dynamics with rotating frame MR relaxation using gradient modulated low‐power adiabatic pulses. Neuroimage. 2014;89:92‐109. doi: 10.1016/j.neuroimage.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sierra A, Michaeli S, Niskanen J, et al. Water spin dynamics during apoptotic cell death in glioma gene therapy probed by T1ρ and T2ρ. Magn Reson Med. 2008;1319:1311‐1319. doi: 10.1002/mrm.21600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen W. Errors in quantitative T1rho imaging and the correction methods. Quant Imaging Med Surg. 2015;5(4):583‐58391. doi: 10.3978/j.issn.2223-4292.2015.08.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao F, Zhou N, Wang JL, et al. Collagen deposition in the liver is strongly and positively associated with T1rho elongation while fat deposition is associated with T1rho shortening: An experimental study of methionine and choline‐deficient (MCD) diet rat model. Quant Imaging Med Surg. 2020;10(12):2307‐2321. doi: 10.21037/QIMS-20-651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kester BS, Carpenter PM, Yu HJ, et al. T1ρ/T2 mapping and histopathology of degenerative cartilage in advanced knee osteoarthritis. World J Orthop. 2017;8(4):350‐356. doi: 10.5312/wjo.v8.i4.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zarins ZA, Bolbos RI, Pialat JB, et al. Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthr Cartil. 2010;18(11):1408‐1416. doi: 10.1016/j.joca.2010.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Casula V, Autio J, Nissi MJ, et al. Validation and optimization of adiabatic T1ρ and T2ρ for quantitative imaging of articular cartilage at 3 T. Magn Reson Med. 2017;77(3):1265‐1275. doi: 10.1002/mrm.26183 [DOI] [PubMed] [Google Scholar]
- 44. Li X, Benjamin Ma C, Link TM, et al. In vivo T1ρ and T2 mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthr Cartil. 2007;15(7):789‐797. doi: 10.1016/j.joca.2007.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johnson CP, Thedens DR, Kruger SJ, Magnotta VA. Three‐dimensional GRE T1ρ mapping of the brain using tailored variable flip‐angle scheduling. Magn Reson Med. 2020;84(3):1235‐1249. doi: 10.1002/mrm.28198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jiang B, Chen W. On‐resonance and off‐resonance continuous wave constant amplitude spin‐lock and T1ρ quantification in the presence of B1 and B0 inhomogeneities. NMR Biomed. 2018;31(7):1‐17. doi: 10.1002/nbm.3928 [DOI] [PubMed] [Google Scholar]
- 47. Chen W, Karampinos DC. Chemical‐shift encoding–based water–fat separation with multifrequency fat spectrum modeling in spin‐lock MRI. Magn Reson Med. 2020;83(5):1608‐1624. doi: 10.1002/mrm.28026 [DOI] [PubMed] [Google Scholar]
- 48. Pang Y, Palmieri‐Smith RM, Maerz T. An efficient R1ρ dispersion imaging method for human knee cartilage using constant magnetization prepared turbo‐FLASH. NMR Biomed. 2021;34(6):1‐12. doi: 10.1002/nbm.4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Triple refocus magnetization path under on‐resonance conditions. The magnetization (M[0‐]) is flipped from the longitudinal axis (z‐axis) with an angle α, which does not need to be 90°, towards the transverse plane (y‐axis) (M[0+]) and nutates about the z1” axis (M[τ−/4]) and at time τ/4 is flipped by 2α about y‐axis (M[τ+/4]) and nutates (M[τ−/2]) around z2”, this trend of rotation and nutation is the same for the rest of the pulses in the train. The final magnetization (M[τ‐], after full spin‐locking) is brought to the negative longitudinal axis (M[τ+]) by another flip angle α. Artifacts due to imperfect 180° are compensated to an extent by the multiple refocuses.
Figure S2: Field insensitive spin lock with double refocusing for T1ρ relaxation measurements. Magnetization path under on‐resonance conditions. The magnetization (M[0‐]) is flipped from the longitudinal plane (z‐axis) with an angle α, which does not need to be 90° towards the transverse plane (y‐axis) (M[0+]) and nutates about the z1” axis (M[τ−/4]) and at time τ+/4 is flipped by 2α about y‐axis (M[τ+/4]) and nutates (M[τ‐3/4]) around z2”, this trend of rotation and nutation is same for the rest of the pulses in the train. The final magnetization (M [τ‐], after full spin‐locking) returns to the positive longitudinal axis (M [τ+]) with another flip angle ‐α. Artifacts due to imperfect 180° are compensated to an extent by the double refocusing.
Figure S3: Simulations for PSC‐SL (a) proposed by Mitrea et al1, and conventional adiabatic sequence with reduced maximum power of the AHP pulse to 600 Hz (b) with the same ΔB0 and B1 field inhomogeneity ranges of up to ±1 kHz and ±40% as presented in this manuscript, for spin‐lock durations of 32 ms and 128 ms, and for spin‐lock amplitudes of 100 Hz and 400 Hz.
Figure S4. Relative differences between the T1ρ maps acquired under the ideal and non‐ideal conditions for the worst performing conventional (Figure 1A), and for the two best performing, single‐refocus hard pulse (Figure 1B) and double‐refocus adiabatic BIR‐4 (Figure 2C) preparation schemes at spin‐lock amplitudes ranging from 0 to 400 Hz.
Figure S5. T1ρ relaxation time maps for paired self‐compensated single refocus PSC‐SL sequence proposed by Mitrea et al1, under the non‐ideal conditions for spin‐lock amplitudes of 0–400 Hz. Anatomical reference and the corresponding B0 map are shown at the top.
Figure S6 c. Comparison of the RMS integrals (RF power) of the zero spin‐lock duration RF pulse trains (blue bars) and 64 ms spin‐lock duration RF pulse trains at 400 Hz spin‐lock amplitude (orange bars) of the conventional pulse, paired self‐compensated PSC‐SL pulse sequence proposed by Mitrea et al1, and adiabatic CW pulse with 600 Hz maximum AHP power T1ρ preparation methods, normalized to the RMS integral of the zero spin‐lock duration conventional spin‐lock method.
Figure S7. Calculated approximate flat widths for the Bloch simulations of all the sequences in Figures 1 and 2 over ΔB0 (±1 kHz) at the correct B1 amplitude. Simulations were run with spin‐lock durations ranging from τ = 0 to 256 ms in 2 ms steps, and with spin‐lock amplitudes from 0 to 1,000 Hz in 10 Hz steps.
Figure S8. Calculated approximate flat widths for the Bloch simulations of all the sequences in Figures 1 and 2 over ΔB1 (±40%) at the correct B0 amplitude. Simulations were run with spin‐lock durations ranging from τ = 0 to 256 ms in 2 ms steps, and with spin‐lock amplitudes from 0 to 1,000 Hz in 10 Hz steps.
Figure S9: Calculated approximate flat widths for the Bloch simulations of the PSC‐SL proposed by Mitrea et al1, and for the adiabatic CW pulse with 600 Hz maximum AHP power T1ρ preparation methods with similar ΔB0 and B1 field inhomogeneities of up to ±1 kHz and ±40% as presented in this manuscript. Simulations were run with spin‐lock durations ranging from τ = 0 to 256 ms in 2 ms steps, and with spin‐lock amplitudes from 0 to 1,000 Hz in 10 Hz steps.
Table S1: Pulse length, RMS integral and RMS amplitude values of preparation schemes with the zero spin‐lock (SL) pulses and with 64 ms SL at 400 Hz amplitude pulses.
Data Availability Statement
All the data of the study are openly available at the Zenodo archives at http://doi.org/10.5281/zenodo.5547762.


