Abstract
We are constantly exposed to the threat of fungal infection. The outcome—clearance, commensalism or infection—depends largely on the ability of our innate immune defences to clear infecting fungal cells versus the success of the fungus in mounting compensatory adaptive responses. As each seeks to gain advantage during these skirmishes, the interactions between host and fungal pathogen are complex and dynamic. Nevertheless, simply compromising the physiological robustness of fungal pathogens reduces their ability to evade antifungal immunity, their virulence, and their tolerance against antifungal therapy. In this article I argue that this physiological robustness is based on a ‘Resilience Network’ which mechanistically links and controls fungal growth, metabolism, stress resistance and drug tolerance. The elasticity of this network probably underlies the phenotypic variability of fungal isolates and the heterogeneity of individual cells within clonal populations. Consequently, I suggest that the definition of the fungal Resilience Network represents an important goal for the future which offers the clear potential to reveal drug targets that compromise drug tolerance and synergise with current antifungal therapies.
Keywords: antifungal drug resistance, antifungal immunity, fungal pathogenicity, fungal resilience, host–pathogen interactions, metabolic adaptation, stress resistance
1. INTRODUCTION
The interactions between fungal pathogens and their hosts are both complex and dynamic. The complexity arises largely from the evolutionary diversity of fungal pathogens, the numerous niches that these pathogens can occupy within us and from the multifarious types of host cell that contribute to antifungal immunity. The dynamism of host–fungus interactions is enhanced by host microenvironments that are in constant state of flux. These microenvironments change continuously as the fungus adapts and grows within them, as immune cells infiltrate to promote fungal killing and clearance and as the fungus then responds to these host defences. In this article, I contend that it is vital that we develop a deep understanding of host–fungus interactions, including their complexity and dynamism, because these interactions ultimately determine the outcome for the fungus and, consequently, the colonized host. Furthermore, a thorough understanding of these interactions is likely to reveal powerful therapeutic strategies that will favour positive outcomes for patients.
Bernhard Hube, a renowned expert in the field, has drawn an ineluctable conclusion about host–fungus interactions: he says ‘It's complicated!’. Paul Nurse also states that life is complex. He suggests that we first need to describe the complexity of living systems and then understand this complexity. 1 Nurse goes on to argue that we should seek simplicity from this complexity and that abstractions offered by systems‐based approaches may offer an approach towards achieving this. 1
Bearing this in mind, one major challenge seems clear: we need to understand ‘fungal resilience’. This view is based on the hypothesis that the expression of critical phenotypes that drive fungal pathogenicity and that compromise antifungal therapy—virulence factors, immune evasion strategies, the emergence of antifungal drug resistance and tolerance—are strongly influenced by the physiological status of the fungal cell. This ‘physiological status’ underlies the resilience of a fungal cell to environmental challenges such as changes in nutrient availability, the imposition of stresses and exposure to antifungal drugs.
By analogy with the metabolic network, the signalling pathways that regulate the growth, metabolism and drug and stress responses of fungal pathogens are all linked within a large ‘Resilience Network’ (Figure 1). Like the metabolic network, this Resilience Network contains hubs, such as the target of rapamycin (TOR), protein kinase A (PKA), protein kinase C (PKC), AMP kinase, cyclin‐dependent kinase, Hog1 and Hsp90, each of which exerts a strong influence over the growth status and physiological robustness of the fungal cell. Fungal pathogens differ with respect to their resistance to environmental challenges. 2 Nevertheless, there is strong evidence to indicate that compromising this Resilience Network attenuates fungal virulence, affects immune evasion strategies and diminishes antifungal drug resistance and tolerance. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 Therefore, in this perspective, I first set the scene by discussing the diversity of fungal pathogens and then describe the concept of the Resilience Network and the likely conservation of key components of this Network across fungal pathogens. The challenge to define the structure and dynamics of this network in fungal pathogens is then addressed, together with speculation about possible future advances that may empower this.
FIGURE 1.
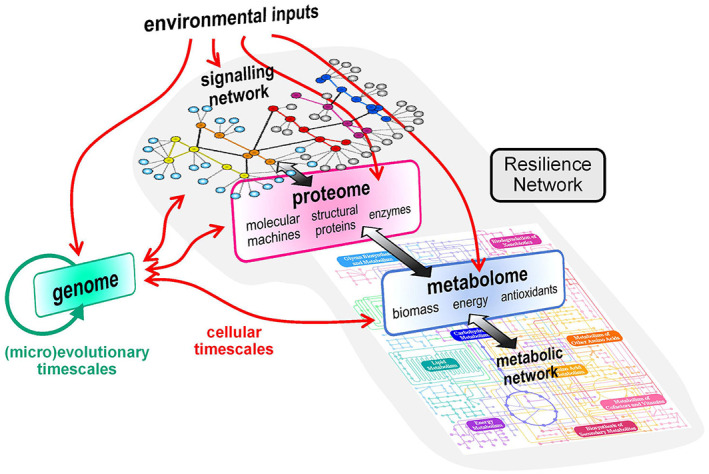
The concept of the ‘Resilience Network’. The fungal Resilience Network represents those features that promote the physiological robustness of the fungal cell as it responds to environmental inputs and genetic variation. In cellular timescales, environmental inputs such as stresses and changes in nutrient availability are perceived by the cell and these signals transduced via a signalling network. This signalling network ultimately links all signal transduction pathways (in the same way that all metabolic pathways are ultimately linked via the metabolic network) and this imparts elasticity and robustness through compensatory changes within the Resilience Network. The signalling network triggers changes in gene expression as well as modulating the activities of proteins within the proteome. This in turn mediates changes in the metabolic network and metabolome. Changes in the metabolome also feedback to the proteome, the signalling network and the genome. In (micro)evolutionary timescales, some environmental inputs cause mutations which, when combined with replication errors, lead to genetic variation. Some mutations will influence the functionality of the signalling network and proteome and thereby the functionality of the Resilience Network
2. UNDERLYING SIMILARITIES IN IMMUNE EVASION BETWEEN EVOLUTIONARILY DIVERSE FUNGAL PATHOGENS
Fungal pathogens display great evolutionary diversity and, thanks to their different lifestyles, are on different evolutionary trajectories. Pathogenicity traits that promote infections in animals and humans have emerged independently in different phylogenetic branches of the fungal tree of life. For example, Cryptococcus neoformans and Rhizopus delemar are members of the Basidiomycota and Mucoromycota, respectively and Pneumocystis jirovecii, Histoplasma capsulatum, Talaromyces marneffei, Sporothrix schenckii, Aspergillus fumigatus and Candida albicans belong to different families within the Ascomycota. Many of these species are saprotrophs, existing mainly in the environment and yet they have independently honed phenotypes that promote infection in humans (e.g. T. marneffei, S. schenckii, A. fumigatus). In contrast, other fungal pathogens appear to have co‐evolved with their host to become commensals (e.g. C. albicans) or even to the extent that they have become obligately dependent on their human host for their survival (e.g. P. jirovecii). Therefore, fungal pathogens are evolving in different niches, they display a high degree of genetic and phenotypic diversity and they differ significantly with respect to the degree to which they have co‐evolved with their human host. This complexity is further enhanced by the genetic and phenotypic variability displayed by individual isolates and even by the phenotypic heterogeneity of clonal populations of fungal pathogens. 13 , 14 , 15
This all suggests that fungal pathogens of humans have been subjected to quite different evolutionary pressures. Nevertheless, a core set of fundamental properties is required to colonize and infect humans and this includes the ability to avoid or evade antifungal immunity. How might environmental saprotrophs, which do not normally encounter humans, have evolved the ability to evade immunity? Some amoebae are able to feed on fungi, 16 , 17 which led to the attractive idea that the evolution of resistance to amoebic predation in the environment might have accidentally promoted phenotypes in C. neoformans that enhance its resistance to phagocytic killing in humans, 18 and this idea has been extended to other saprobic fungal pathogens. 19 , 20
Phagocytic killing can be avoided in a number of ways, for example, by activating stress responses that counter phagocytic killing mechanisms, by preventing phagolysosomal maturation and hence the activation of these killing mechanisms or by avoiding phagocytic uptake in the first place. These strategies resonate with those employed by many fungal pathogens in humans. Therefore, despite dramatic differences between niches in the environment and in humans, some common selective pressures are likely to have fortuitously promoted the pathogenicity of some saprobic fungi.
Predictably, given their evolutionary diversity, fungal pathogens have developed a diverse range of evasion strategies. For example, Blastomyces dermatitis generally evades amoebic uptake, whereas H. capsulatum is quickly engulfed by amoebae and then thrives intracellularly, while A. fumigatus is phagocytosed but subsequently kills the predatorial amoeba. 20 , 21 Casadevall argues that while some phenotypes that protect fungi against amoebic predation may promote virulence in humans, the reverse might not hold true. 22 For example, he suggests that fungal factors that modulate immune function in humans are unlikely to be relevant in amoebae given their lack of an immune system. 22 Interestingly, C. albicans is killed by amoebae, 23 indicating that the immune evasion strategies that this fungus has developed during its co‐evolution with the host are unlikely to protect it from amoebic predation in the environment.
2.1. Concealment
C. albicans has evolved an impressive armoury of immune evasion strategies some of which have parallels in other evolutionarily divergent fungal pathogens. 24 , 25 , 26 The first of these strategies involves concealment—mechanisms that limit fungal recognition by innate immune cells.
Much emphasis has been placed on the concealment of the proinflammatory pathogen‐associated molecular pattern (PAMP), β‐(1,3)‐glucan. The polymer, β‐(1,3)‐glucan, is an essential component of the cell walls of many fungi. 27 In C. albicans, most β‐(1,3)‐glucan is present in the inner cell wall, buried beneath the outer layer of mannan fibrils, 28 but some β‐(1,3)‐glucan becomes exposed at septal junctions between mother‐daughter cells, at bud scars and at punctate foci on the cell surface. 29 Also, β‐(1,3)‐glucan on the lateral cell wall can become exposed in vivo through stripping of the mannan outer layer by neutrophil attack. 30 To promote concealment, C. albicans secretes an exoglucanase (Xog1) and an endoglucanase (Eng1) that shave exposed β‐(1,3)‐glucan from the cell surface. 31 , 32 Interestingly, C. albicans induces β‐(1,3)‐glucan masking in response to host signals that potentially forewarn of impending attack by innate immune cells: lactate, hypoxia, iron limitation and ambient pH. 11 , 13 , 31 , 33 , 34 , 35 β‐(1,3)‐Glucan shaving from the cell surface correlates with reduced recognition by innate immune cells and attenuated cytokine responses. 11 , 13 , 34 This does, however, depend on the type of innate immune cell. While mannan acts to dampen β‐(1,3)‐glucan‐mediated antifungal responses in macrophages, mannan induces antifungal responses in monocytes. 36 Nevertheless, these observations have led to the idea that, over evolutionary time, C. albicans has developed a ‘memory’ of stresses that are likely to follow specific host signals and, consequently, has evolved the ability to anticipate immune attack and activate pre‐emptive protective responses. 35
PAMP concealment is also employed by other fungal pathogens. For example, H. capsulatum masks β‐(1,3)‐glucan beneath a layer of α‐(1,3)‐glucan and expresses an Eng1 endoglucanase that trims exposed β‐(1,3)‐glucan and helps this pathogen ‘fly under the radar’ of immune surveillance. 37 , 38 , 39 , 40 Meanwhile, C. neoformans synthesizes an extracellular capsule, which not only conceals cell wall PAMPs but also induces the anti‐inflammatory cytokine IL‐10, thereby dampening antifungal responses. 41 , 42 , 43 Likewise, A. fumigatus conceals the β‐(1,3)‐glucan in its spore cell wall underneath a layer of the RodA hydrophobin. 44 Clearly, PAMP concealment is a common strategy employed by evolutionary divergent fungal pathogens.
2.2. Manipulation
The manipulation of antifungal immune defences provides a complementary strategy to concealment. 25 For example, C. albicans secretes aspartic proteases that inactivate complement proteins. 45 C. albicans, A. fumigatus and C. neoformans secrete proteins that interfere with the functionality of the complement system or complement receptors, thereby reducing phagocytic uptake. 46 , 47 , 48 , 49 , 50 , 51 Furthermore, C. albicans, Paracoccidioides brasiliensis, C. neoformans and A. fumigatus all express proteins that bind plasminogen to interfere with complement activation. 25 , 52 , 53 , 54
Fungal pathogens can manipulate host immunity even after phagocytic uptake, by inhibiting phagolysosomal maturation. For example, C. neoformans, Candida glabrata and H. capsulatum are able to persist within innate immune cells by blocking phagosomal maturation or acidification. 55 , 56 , 57 , 58 , 59 Although the outcome is similar, these pathogens exploit different strategies to achieve persistence inside macrophages. H. capsulatum and C. glabrata persist by blocking phagolysosomal acidification, whereas C. neoformans survives in acidic phagolysosomes. 55 , 58 , 60
C. albicans was thought to drive metabolic alkalinization to help maintain a neutral pH within the phagosome and escape phagocytic killing. 61 , 62 , 63 More recent data suggest that alternative mechanisms may drive alkalinization, 64 , 65 , 66 including yeast‐hypha morphogenesis which can lead to rupture of the phagosome and death of the macrophage. 67 , 68 , 69 Macrophages attempt to overcome hyphal development and fungal escape by physically folding hyphae. 70 However, C. albicans can trigger pyroptosis, inflammasome activation and death of the macrophage and can also promote immune cell death through competition for glucose. 71 , 72 , 73 , 74 , 75
2.3. Resistance
In addition to concealment and manipulation, fungal pathogens exploit resistance mechanisms to counteract antifungal killing mechanisms. 24 , 25 , 26 Innate immune cells exploit a battery of fungicidal mechanisms that include toxic chemicals, cationic fluxes, enzymes and antimicrobial peptides. 76 In particular, the respiratory burst generates high concentrations of reactive oxygen species (ROS) that would be lethal for many fungal species. 2 However, fungal pathogens such as C. albicans, C. glabrata, C. neoformans and H. capsulatum display robust responses to oxidative stress that provide some degree of protection against the respiratory burst. 2 , 7 , 77 , 78 , 79 Indeed, C. albicans and H. capsulatum even express superoxide dismutases on their cell surface to detoxify extracellular ROS. 79 , 80 , 81 In addition, fungal pathogens such as C. albicans have evolved mechanisms that protect against cationic fluxes, reactive nitrogen species and antimicrobial peptides. 7 , 82 , 83 , 84
To summarize, despite their evolutionary diversity and their different lifestyles, many fungal pathogens have developed similar approaches that help them to evade antifungal immunity. A second important point, which is pertinent to this review, is that many mechanisms that promote concealment, manipulation and resistance are environmentally, and in some cases, developmentally contingent. For example, the glucanases that conceal the proinflammatory PAMP β‐(1,3)‐glucan are regulated in response to specific environmental inputs and yeast‐hypha morphogenesis in C. albicans and, in H. capsulatum, are expressed by the pathogenic yeast form. 11 , 13 , 39 The robust stress responses that contribute to resistance against phagocytic killing are strongly influenced by carbon source in C. albicans. 85 , 86 , 87 These and many other observations indicate that the signalling mechanisms that regulate many immune evasion strategies are integral to the Resilience Network that governs responses to environmental change 11 , 13 , 86 , 87 , 88 , 89 (Figure 1).
3. RELATED FUNGAL VIRULENCE FACTORS ACTIVATED DURING ENVIRONMENTAL ADAPTATION
The same holds true for the ‘Virulence Factors’ that promote fungal infections in humans. The signalling pathways that control the expression of these factors are interconnected with those that drive the adaptive responses to environmental change. 90 This view is supported by numerous examples in many different fungal pathogens.
Cellular morphogenesis is a major virulence factor. The regulation of morphogenesis and virulence by environmental inputs is particularly obvious for dimorphic fungal pathogens such as H. capsulatum. 91 , 92 An increase in ambient temperature to 37°C triggers the morphological transition from the environmental filamentous form to the pathogenic yeast form and also the expression of the calcium binding protein, Cpb1 and the cell surface protein, Yps3, 93 both of which are required for virulence. The formation of large polyploid Titan cells by C. neoformans, which display altered PAMP exposure, physical evasion of phagocytosis and elevated drug resistance, is triggered by a variety of environmental stimuli, including nutrient starvation, hypoxia, bacterial peptidoglycan and quorum sensing molecules. 94 , 95 , 96 Significantly Titan formation is regulated by protein kinase A (PKA) signalling. 94 PKA signalling also drives yeast‐hypha morphogenesis in C. albicans in response to environmental signals that include temperature, serum, neutral pH, nutrient starvation, hypoxia, CO2, bacterial peptidoglycan and release from quorum sensing. 97 , 98 , 99 , 100 , 101 , 102 Significantly, key regulators of yeast‐hypha morphogenesis in C. albicans also control metabolism (e.g. Efg1, Nrg1, Tup1). 103 , 104 For example, Efg1 regulates genes involved in glycolysis and oxidative phosphorylation, 103 whereas Nrg1 and Tup1 repress genes required for the uptake of some carbon sources and the glyoxylate cycle. 104 Furthermore, hyphal development is controlled by intracellular signals such as the inhibition of cell cycle progression, as well as by extracellular stimuli, 105 and core stress and growth regulators modulate hyphal development (e.g. Tor1, Hog1). 3 , 106
The ability to switch between different phenotypic forms—white, opaque, grey—also promotes the fitness of C. albicans in different host niches. 102 , 107 , 108 , 109 , 110 Phenotypic switching is regulated by a complex transcriptional circuit in which Efg1 and Wor1 play central roles in controlling the different morphologies, mating capacities, cell walls and metabolism of white, grey and opaque cells. 111 , 112 Phenotypic switching is induced by environmental stimuli such as temperature, CO2, hypoxia and environmental stresses. 113 , 114 , 115
The control of morphogenesis and phenotypic switching highlights the interconnectedness of developmental, environmental and growth regulation in C. albicans and this concept extends to many other fungal virulence factors. The fungal cell wall is viewed as a virulence factor because it is the first point of physical contact between fungus and host and because it provides a robust shield against host‐imposed stresses. 27 The cell wall is a flexible and elastic structure that, in C. albicans, responds to genetic and environmental challenges via the cell wall remodelling, calcium and Hog1 signalling pathways. 86 , 116 It is also sensitive to nutrient and micronutrient availability. 11 , 86 , 117 , 118 In C. albicans, the secretion of aspartic proteases (SAPs) also depends upon nutrient availability. 119 Many adhesins (e.g. Als3, Hwp1), the Als3 invasin and the toxin candidalysin (encoded by ECE1) are expressed during hyphal development. 120 , 121 , 122 , 123 , 124 Therefore, the regulation of these virulence factors is intimately associated with the signals that trigger yeast‐hypha morphogenesis (above).
Analogous observations have been made about the regulation of virulence factors in other fungal pathogens. For example, capsule formation in C. neoformans is regulated by iron limitation, nutrient depletion and serum, in part by PKA and Rim101 signalling. 125 , 126 , 127 pH signalling via the transcription factor Rim101 in C. albicans and via its orthologue PacC in A. fumigatus, is critical for the virulence of these fungi. 128 , 129 , 130 , 131 Also, the expression of toxins in other fungal pathogens is linked to environmental and developmental regulation, A. fumigatus gliotoxin production being regulated by BrlA, AbaA and WetA and R. delemar mucoricin being synthesized under aerobic rather than anaerobic conditions. 132 , 133 , 134
4. COMMON FUNGAL FITNESS ATTRIBUTES THAT PROTECT AGAINST ENVIRONMENTAL CHALLENGES
Virulence factors are defined as fungal components that directly influence the host, 135 but fungal pathogenicity also depends upon the ‘Fitness Attributes’ that promote the physiological robustness of the fungal cell within host niches. 90 Fitness attributes include adaptive responses to fundamental environmental challenges that have been faced by microbes for billions of years. The challenges include, for example, changes in the availability of nutrients and essential micronutrients and fluctuations in ambient temperature, water balance, pH and oxygen levels. Consequently, evolutionarily divergent microbes share evolutionarily conserved responses to these ancient environmental challenges. They display the ability to switch between different carbon and nitrogen sources, to scavenge essential micronutrients such as iron and zinc from the surroundings, to assimilate osmolytes to restore turgor pressure, to synthesize antioxidants and detoxify reactive oxygen species and to repair damage to proteins and DNA. Furthermore, core regulators of these responses tend to be conserved across the fungal kingdom and some even in human cells. Examples include the Hog1 stress activated protein kinases (SAPKs) that activate osmotic and other stress responses, 2 , 7 , 136 the AP‐1‐like transcription factors and Skn7 response regulators that drive oxidative stress responses, 137 , 138 , 139 , 140 , 141 , 142 the Slt2/Mkc1 mitogen activated protein kinases (MAPKs) critical for cell wall remodelling, 143 , 144 , 145 the Zrt1/2 transporters involved in zinc scavenging, 146 the Snf1 AMP kinases and Mig1/CreA transcription factors that control carbon assimilation, 147 , 148 , 149 the Gcn4/CpcA transcription factors that regulate amino acid biosynthesis, 150 , 151 , 152 and the PKA orthologues that control growth, morphogenesis and stress adaptation. 5 , 153 , 154 , 155 , 156
Model fungi and fungal pathogens display differences in their adaptive responses. For example, they differ with respect to the sensitivities and amplitudes of their metabolic adaptation and stress responses. During their evolution there has also been regulatory rewiring which has led to the functional reassignment of certain regulators. Presumably these changes reflect the selective pressures that these fungal species have encountered in their respective niches. For example, the Hog1 SAPK, which activates the osmotic stress response in S. cerevisiae, has been co‐opted to regulate responses to oxidative stress, heavy metal stress, quorum sensing molecules and virulence in C. albicans. 3 , 7 , 157 The Gal4 transcription factor, which controls galactose assimilation in S. cerevisiae, regulates central carbon metabolism in C. albicans. 158 The Msn2/4 transcription factors activate the core transcriptional response to stress in S. cerevisiae but control the weak acid stress response in C. albicans. 159 , 160 , 161 , 162 , 163 Also, when S. cerevisiae cells are exposed to glucose, catabolite inactivation drives the ubiquitin‐mediated degradation of enzymes required for gluconeogenesis and the assimilation of alternative, less favourable carbon sources. 164 , 165 In contrast, catabolite inactivation appears to have been relaxed in C. albicans, allowing these enzymes to persist when glucose is available. 166 This means that while S. cerevisiae turns to alternative carbon sources only once available sugars have been depleted, C. albicans can simultaneously utilize sugars and alternative carbon sources such as fatty acids or lactate, 166 , 167 and this contributes to the virulence of this fungus. 167 Therefore, while evolutionarily conserved core signalling modules regulate stress and nutrient adaptation, these modules and their outputs have been tuned in response to niche‐specific evolutionary pressures.
It has been attractive to dissect specific signalling pathways in isolation with a view to defining their roles in controlling specific stress and metabolic responses. This can lead to the misleading impression that these signalling pathways act in isolation. However, there is abundant evidence to indicate that stress and metabolic signalling pathways are intimately linked. 168 For example, the core stress response in S. cerevisiae is downregulated in response to glucose via PKA signalling. 154 , 169 Key stress regulators in C. albicans also modulate metabolic genes (e.g. Hog1, Cap1, Hsf1) and core metabolic regulators also regulate stress genes (e.g. Gcn4, Mig1, Rbf1). 142 , 149 , 170 , 171 , 172 , 173 , 174 , 175 Furthermore, second messengers such as cyclic AMP and cyclic GMP are integral components of the metabolic network. Therefore, there is considerable crosstalk between the individual signalling pathways that control stress and metabolic adaptation. This means that, in reality, these pathways are intimately linked within a complex Resilience Network of signalling pathways (Figure 1). The core regulators described above and many others besides, represent hubs in this Resilience Network.
Numerous lines of evidence indicate that this concept extends to antifungal drug resistance and tolerance. For example, the genome sequencing of drug resistant clinical isolates that have evolved in patients undergoing protracted antifungal therapy has revealed that the evolved resistance is associated with mutations in nutrient assimilation and stress pathway genes as well as with mutations that affect drug targets and efflux pumps. 176 The imposition of stress has been shown to accelerate the rate at which C. albicans cells evolve resistance to antifungal drugs. 177 The stress induced molecular chaperone, Hsp90, promotes the emergence of drug resistance in evolutionarily diverse fungi, 8 and drug resistance is synergistically compromised when the cell wall integrity pathway, calcineurin signalling or Hsp90 functionality is perturbed. 9 , 12 Furthermore, cellular adaptation to changes in nutrients or stress imposition strongly influences the resistance of Candida to antifungal drugs. 8 , 118 Significantly, this is thought to be driven by the impact of stress pathways on drug tolerance. 178 Therefore, the fungal Resilience Network impacts drug tolerance and resistance as well as stress and nutrient adaptation (Figure 2).
FIGURE 2.

The Resilience Network influences the pathogenicity and therapeutic tractability of fungal pathogens. The Resilience Network responds to environmental inputs and its functionality is influenced by genetic variation (Figure 1). In addition to controlling stress resistance and metabolic flexibility, the Resilience Network regulates the expression of fungal virulence factors and the activation of fungal immune evasion strategies. These properties strongly influence fungal pathogenicity. In addition, the Resilience Network strongly influences the tolerance of fungal cells to antifungal drugs and drug tolerance permits the evolution of drug resistance. 8 , 14 Genetic variation between fungal species and between clinical isolates of these species influences the functionality of the Resilience Network and, consequently, their tolerance of and resistance to environmental stresses and antifungal drugs. Furthermore, the functionality of the Resilience Network is probably influenced by stochastic differences in the expression of key regulators and hence is likely to underlie the ability of subsets of fungal cells within clonal populations to evade antifungal immunity or tolerate antifungal drugs (see text)
To summarize, it is apparent that, despite the evolutionary diversity of fungal pathogens, they tend to display broadly similar fitness attributes that empower the physiological robustness of fungal pathogens during infection. Furthermore, the regulation of these virulence factors and fitness attributes and the therapeutic tractability of fungal strains, are intertwined within a Resilience Network that is based on the crosstalk between individual signalling modules. Because cell growth and division, metabolism and stress responses are fundamental processes that are evolutionarily conserved in most organisms, many features of the Resilience Network that links these processes are likely to be conserved across fungal pathogens of humans, despite their evolutionary diversity. In other words, although the relative strength of specific pathway connections will vary, the general architecture of Resilience Networks across divergent fungal pathogens is likely to display a reasonable level of conservation.
5. THE ARCHITECTURE OF THE RESILIENCE NETWORK
Based on the above arguments, a major challenge for the future is to define the architecture of the Resilience Network. But what is the Resilience Network? So far, I have largely inferred that this network represents the system of interconnected signalling pathways within a fungal pathogen. However, to underlie cellular fitness, this network must include structural proteins, repair and detoxification enzymes, cell wall enzymes and polymers, molecular machines such as the ribosome and respiratory chain, energy in the form of ATP, antioxidants such as glutathione and multifarious other molecules in addition to the signalling molecules that regulate their levels and activities. Therefore, the Resilience Network must ultimately incorporate information about the proteome and metabolome as well as the transcriptome (Figure 1). It must include data about the activities of key regulators and must include key molecular links between the individual metabolic, regulatory and structural modules that make up the network.
These individual modules, their interconnections and their relative contributions to the fitness of the fungal cell need to be defined. This type of information would help to elaborate the structure of the Resilience Network and its functionality under a given set of circumstances. Historically, the adaptive responses of fungal pathogens have generally been dissected under ‘standard’ laboratory conditions that bear little resemblance to microenvironments in host niches. 7 , 179 , 180 , 181 , 182 Therefore, as a starting point, the structure of the Resilience Network should be elaborated under defined in vitro conditions that relate to host niches, possibly in Roswell Park Memorial Institute (RPMI) medium, for example. The next step might be to compare this structure with those present in host niches. For C. albicans this might involve analysing the Resilience Network in cells colonizing the oral and vaginal epithelia, the colon and the bloodstream, whereas fungal cells from the lung and brain would be more appropriate for C. neoformans. These experiments would provide important insights into how the Resilience Network changes its architecture in fungal populations occupying these different host niches.
Great opportunities will arise from an understanding of the architecture of the fungal Resilience Network, how it adapts to different host niches and how this impacts fungal virulence and resilience towards innate immune defences and antifungal drugs. In particular, this will highlight nodes in the network that are critical for fungal resilience within host niches. These nodes, be they specific regulatory hubs, molecular machines or metabolic enzymes, may represent novel targets for therapeutic intervention (Figure 2). Some of these modules will interact with those that are targeted by antifungal drugs in clinical use, such as polyenes and azoles (ergosterol biosynthesis and membrane integrity) and echinocandins (β‐(1,3)‐glucan synthesis and cell wall functionality). 12 , 183 , 184 Inhibitors of these Resilience Network modules will have the potential to act synergistically with antifungal to inhibit the growth or kill fungal pathogens more effectively. There are clear precedents for this. For example, pharmacological inhibitors of Hsp90 or PKC signalling act synergistically with fluconazole to block the growth of C. albicans and, significantly, imparts this fungistatic drug with fungicidal activity. 9 Of course, many strict criteria must be fulfilled before a molecule can be used therapeutically. Nevertheless, it is work noting that some inhibitors of Hsp90 and PKC signalling are already in therapeutic use. 12
The holistic views of fungal adaptation mechanisms provided by the Resilience Network are likely to reveal additional examples of potentially useful approaches towards combination therapies. 12 This is important because combination therapies are proving effective in the treatment of complex conditions, can retard the emergence of drug resistance and may help to control drug resistant isolates. 185 , 186 , 187 , 188 In addition, the comparison of Resilience Networks between evolutionarily divergent fungi is likely to reveal common points of fragility that represent potential targets for broad spectrum antifungal drugs.
6. THE DYNAMISM OF THE RESILIENCE NETWORK
As discussed above, the microenvironments occupied by fungal pathogens are in a constant state of flux as niches are altered by the growth of fungal cells and the influx of immune infiltrates. Consequently, the Resilience Network itself must be in a constant state of flux as it detects and responds to these changes in the microenvironment. Dynamic changes in the Resilience Network are likely to provide mechanisms of fungal escape from pharmacological or genetic intervention, in the same way as elevated chitin synthesis provides a means of escaping the inhibitory effects of echinocandins. 189 , 190 , 191 These dynamics are also likely to provide escape from immune attack, passively through the modulation of cellular resistance to antifungal killing mechanisms and actively through pre‐emptive defensive responses against these mechanisms. 11 , 13 , 35 , 85 Therefore, having established the network architecture, the next challenge will be to define the network dynamics. Time‐series measurements of the activities of key regulators and the levels of critical metabolites would lie at the heart of this challenge.
Additional routes of escape from pharmacological insults to the Resilience Network exist for fungal pathogens. Clinical isolates of C. albicans display a high degree of genetic and phenotypic variability that includes differences in drug resistance and tolerance and the extent to which isolates are recognized by innate immune cells. 11 , 192 , 193 Also, when placed under selective pressure, fungal pathogens assimilate mutations that enhance their fitness under these conditions. 176 , 194 , 195 Defining mutations in clinical isolates or micro‐evolved strains that influence the functionality of the Resilience Network and that underlie their resistance, their tolerance phenotypes and their immune visibility, will enhance our understanding of these processes and, significantly the strengths and fragilities of the Resilience Network that underlies them.
In addition, individual cells within clonal populations of C. albicans display considerable heterogeneity with respect to their immune visibility and their tolerance to environmental stresses and antifungal drugs. 14 , 196 , 197 Stochastic differences between individual cells that affect the status of the Resilience Network, such as variations in the levels or activities of key regulators, are bound to contribute to this population heterogeneity. Therefore, determining which stochastic variations exert the greatest impact upon the Resilience Network will advance our awareness of the nodes that contribute most to its elasticity or that offer points of fragility.
7. TECHNOLOGICAL INNOVATIONS LIKELY TO EMPOWER A DEEP UNDERSTANDING OF RESILIENCE NETWORK ARCHITECTURE
Major advances in biology have often been dependent upon significant technological innovations. Paul Nurse describes how Robert Hooke's refinement of the compound microscope led to the discovery of the cell. 1 Fred Sanger's and Walter Gilbert's development of DNA sequencing technologies 198 , 199 opened the door the elaboration of gene structure and ultimately to the sequences of whole genomes. 200 , 201 , 202 The subsequent development of next generation sequencing 203 , 204 has led to dramatic advances in our understanding of fungal evolution, epidemiology and the emergence of drug resistance. 176 , 192 , 193 , 205 Clearly, future technical advances will accelerate the elaboration of Resilience Network architecture and enhance our understanding of how this network influences fungal resistance to antifungal immunity and therapeutic intervention.
Fungal pathogens are generally less experimentally tractable than model yeasts such as S. cerevisiae and S. pombe. Focusing on C. albicans, for many decades this major fungal pathogen was considered asexual, which severely limited the genetic dissection of its pathobiology. 206 , 207 This fungus was also found to display non‐standard codon usage, which constrained the exploitation of reporter‐based screens. 208 , 209 To a certain extent these challenges were circumvented by the development of codon‐optimized reporters and accurate tools for gene manipulation. 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 Significantly, the emergence of genome sequence data 219 empowered the development of transcript profiling and other genome‐wide tools for C. albicans. 170 , 171 , 220 , 221 , 222 , 223 , 224 , 225 Also, the discovery of efficient mating and concerted chromosome loss permitted the completion of a parasexual cycle for C. albicans. 207 , 226 , 227 , 228 , 229 The revelation of haploid forms of C. albicans and subsequent generation of transposon libraries also represented major steps forward. 230 , 231 , 232 Yet we still lack a complete collection of barcoded gene knockouts for C. albicans or for any fungal pathogen as far as I am aware. This kind of resource has proven invaluable for the S. cerevisiae research community permitting, for example, efficient comparisons of relative fitness for large pools of mutants in competition assays as well as global transcriptomic, proteomic and metabolomic analyses of these mutants. 233 , 234 Clearly, the availability of a complete collection of null mutants for C. albicans would dramatically accelerate the elaboration of the Resilience Network in this pathogen.
Looking further ahead, the lack of a complete sexual cycle remains a major obstacle for the C. albicans research community. The ability to combine genetics with genomics in S. cerevisiae has proven extremely powerful. In particular, this facilitated the description of the genetic landscape of the yeast cell through the comprehensive dissection of synthetic genetic interactions. 235 , 236 Significantly, genetics has extended the reach of genomics beyond the analysis of gene deletions to include single nucleotide polymorphisms (SNPs) and small insertions and deletions (indels). This is important because while essential genes can be represented in mutant libraries by loss‐ and gain‐of‐function mutations caused by SNPs and indels, they are not included in the collections of gene deletion mutants that are generally exploited for genome‐wide loss‐of function screens. Yet essential genes tend to be more highly conserved than non‐essential genes, they tend to be more highly connected within networks and they represent attractive antifungal targets. 237 , 238 , 239 , 240 Therefore, approaches such as bulk segregant analysis (BSA), which screens for sequence polymorphisms that are associated with a specific phenotype, are likely to prove a rich source of information about the genes—essential or non‐essential—that contribute to fungal pathogenicity and drug resistance. Briefly, BSA involves sequential cycles of genetic crossing and the selection, at each stage of the process, for progeny with the phenotype of interest, whether it be drug or stress resistance, for example. After multiple rounds of crossing, the pooled genome sequence for the resistant segregants is compared with that for the pool of sensitive segregants with a view to identifying mutations that are significantly enriched in the resistant pool. 241 Importantly, this powerful approach has highlighted new genes that contribute to S. cerevisiae phenotypes that had already been studied in great detail. 242 , 243 , 244 , 245 Furthermore, BSA is capable of highlighting quantitative trait loci at single nucleotide resolution. 241
Clearly, the application of BSA to C. albicans pathobiology is hindered by the lack of a complete sexual cycle in this species. However, the ability to engineer a sexual cycle in C. glabrata 246 suggests that this might also be possible in C. albicans. An engineered sexual cycle, combined with approaches to enhance genetic recombination in C. albicans, 247 , 248 would open the door to BSA and other powerful genetics‐based approaches in this pathogen (Figure 3). These, together with new ultra‐fast proteomic 249 , 250 and, hopefully, metabolomic analyses of defined mutants would provide deep datasets that would inform the architecture of the Resilience Network. This would require, however, machine learning and advanced software capable of integrating genes, transcripts and proteins with the molecular machines they contribute to and/or the reactions they catalyse as well as the corresponding metabolites. 251 No doubt, in the future, the application of the accurate protein folding algorithms of DeepMind 252 will also be exploited to strengthen interactions within the Resilience Network and to provide evolutionary comparisons with analogous networks in other fungal pathogens. In this way, common points of fragility likely to affect fungal virulence, PAMP synthesis and exposure, stress resistance or drug tolerance may be identified.
FIGURE 3.
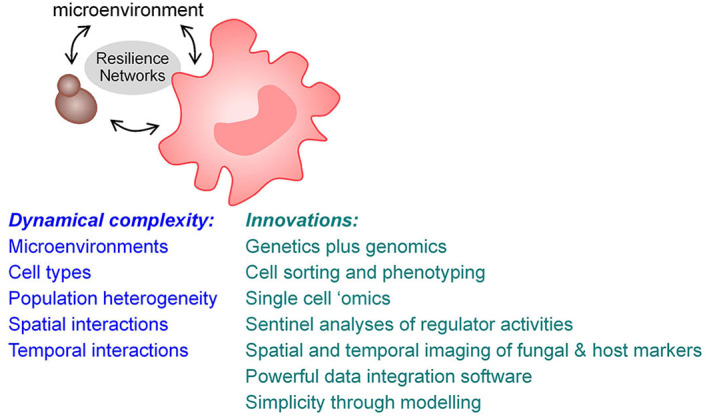
Challenges and potential ways forward. Host–fungus interactions are both complex and dynamic and, although the focus of this perspective is on the fungus, the Resilience Networks of both the fungus and the host lie at the heart of these interactions. The dynamic complexity of these interactions is influenced by the local microenvironment, the fungus, the host cell types, the degree of population heterogeneity of these cell types and the spatial organization and temporal dynamics of these interactions. Potential advances that will help to disentangle this enormous complexity are likely to include the engineering of sexual cycles in fungal pathogens to empower the application of fungal genetics alongside fungal genomics. In addition, cytometric cell sorting based on advanced markers of cell type and activity will permit accurate phenotyping of relevant cell types and populations. Single cell proteomics and possibly single cell metabolomics will complement single cell genomics and transcriptomics. Deep proteomic sentinel analyses will permit parallel quantification of the activities of Resilience Network modules within cells. Advanced immunohistochemical imaging will permit spatial and temporal relationships between specific host and fungal cell types within tissues to be elaborated. Powerful new software capable of integrating multidimensional ‘omic’ datasets will be required to achieve all of this and elegant modelling will be essential to reveal the simplicity that underlies this complexity (see text)
8. INNOVATIONS TO DEFINE THE DYNAMICS AND SPATIAL ACTIVATION OF THE NETWORK
The above innovations will permit the elaboration of Resilience Network architecture—the inputs, the regulators, the components and their interactions. The next challenges will be to define the dynamics of the Resilience Network, its spatial activation and its impact upon population heterogeneity in the context of host niches and the interactions between fungal cells and specific host cell types (Figure 3).
The definition of network dynamics will be dependent upon time‐series measurements of key regulators and enzymes, their activities and the levels of critical metabolites and for this, advanced, targeted proteomics and metabolomics will be required. The way forward may be offered by innovations that permit the multiplexing of high throughput sentinel assays that quantify the relative levels of post‐translationally modified, activated proteins. 253 , 254 , 255 , 256 Similarly, innovations that extend current metabolomic capabilities to follow the concentrations of multiple classes of metabolite in real time would be a major step forward. 257 , 258
Cytometry provides a classical approach to the analysis of population heterogeneity. Therefore, the development of a library of differentially labelled antibody fragments that are specific for the post‐translationally modified, active and native versions of key regulators would permit rapid analysis of the Resilience Network in individual cells within clonal fungal populations. By applying fluorescence activated cell sorting, the behaviours of individual cells could be examined in detail. The transcriptomes of individual cells are already accessible through single cell RNA sequencing, 259 , 260 and single cell proteomics is now feasible. 261 Therefore, the answers to questions such as these are within reach. How do different Network modules respond in individual cells to specific immunological, pharmacological or genetic insults? Are compensatory behaviours dependent on specific regulatory hubs? Can these hubs be targeted chemically to trigger synergistic killing of fungal cells?
The spatial definition of fungal Resilience Network behaviours in the context of host niches and local immune infiltrates is a major challenge for the future. Yet recent technological innovations appear to bring this exciting goal within reach (Figure 3). The MACSima imaging platform is reported to permit the analysis of hundreds of markers on single tissue sections. 262 Using this system, markers for specific immune cell types could be combined with markers for the activation of specific modules in the fungal Resilience Network. This approach would provide invaluable spatial information, within tissue samples, about the nature of immune responses to fungal colonization and how the fungal cells respond locally to immune attack. By including specific bacterial markers, this principle could be extended to examine the influence of local microbiotas upon host–fungus interactions. This information would move our understanding of host–fungus–microbiota interactions to a completely new dimension. 15
9. CONQUERING COMPLEXITY WITH SIMPLICITY
As Bernhard Hube has suggested, host–pathogen interactions are complicated! Indeed, in focussing on the fungal pathogen, this article has not addressed other important aspects of this complexity such as the diversity of immune cell types, their antifungal weaponry and their interactions. 15 , 76 , 263 , 264 Nevertheless, understanding the fungal Resilience Network will provide critical insights into how pathogenic fungi evade immunity, how they are adept at colonizing specific niches, why some fungal isolates are tolerant to antifungal drugs and why some fungal pathogens persist within specific niches. The fungal Resilience Network will also present valuable opportunities to develop new antifungal targets. Based on strong precedents, 9 , 12 , 184 inhibitors of some of these targets are likely to synergise with current antifungal drugs and hence empower the development of combination therapies strengthen our limited armoury of antifungal drugs 265 and possibly retard the emergence of antifungal drug resistance. 188
FUNDING INFORMATION
This work was funded by a programme grant from the UK Medical Research Council (www.mrc.ac.uk: MR/M026663/2) and by the Medical Research Council Centre for Medical Mycology at the University of Exeter (MR/N006364/2). The funder had no role in this study, the decision to publish or the preparation of the manuscript.
CONFLICT OF INTEREST
The author has declared no conflicts of interest for this article.
ACKNOWLEDGEMENTS
I am very grateful to many many friends, colleagues and collaborators who have helped to shape my outlandish ideas over the years. In particular, I thank Neil Gow, Gordon Brown, Frank Odds, Jan Quinn, Ken Haynes, Bernhard Hube, Christophe d'Enfert, Mihai Netea, Judy Berman, Jules Ene, Liz Ballou, Markus Ralser, Hiroji Chibana and this list goes on and on! The ideas expressed in this perspective have evolved from their insightful comments and suggestions, but the mistakes are all mine.
Brown AJP. Fungal resilience and host–pathogen interactions: Future perspectives and opportunities. Parasite Immunol. 2023;45(2):e12946. doi: 10.1111/pim.12946
Funding information Medical Research Council Centre for Medical Mycology at the University of Exeter, Grant/Award Number: MR/N006364/2; UK Medical Research Council, Grant/Award Number: MR/M026663/2
DATA AVAILABILITY STATEMENT
This review contains no new data.
REFERENCES
- 1. Nurse P. What Is Life?. David Fickling Books; 2020. ISBN: 9781788451406. [Google Scholar]
- 2. Nikolaou E, Agrafioti I, Stumpf M, Quinn J, Stansfield I, Brown AJP. Phylogenetic diversity of stress signalling pathways in fungi. BMC Evol Biol. 2009;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alonso‐Monge R, Navarro‐García F, Molero G, et al. Role of the mitogen‐activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans . J Bacteriol. 1999;181:3058‐3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cruz MC, Cavallo LM, Görlach JM, et al. Rapamycin antifungal action is mediated via conserved complexes with FKBP12 and TOR kinase homologs in Cryptococcus neoformans . Mol Cell Biol. 1999;19:4101‐4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bockmuhl DP, Krishnamurthy S, Gerads M, Sonneborn A, Ernst JF. Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans . Mol Microbiol. 2001;42:1243‐1257. [DOI] [PubMed] [Google Scholar]
- 6. Reinoso‐Martin C, Schueller C, Schuetzer‐Muehlbauer M, Kuchler K. The yeast protein kinase C cell integrity pathway mediates tolerance to the antifungal drug caspofungin through activation of Slt2p mitogen‐activated protein kinase signaling. Eukaryot Cell. 2003;2:1200‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith DA, Nicholls S, Morgan BA, Brown AJP, Quinn J. A conserved stress‐activated protein kinase regulates a core stress response in the human pathogen Candida albicans . Mol Biol Cell. 2004;15:4179‐4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cowen LE, Lindquist S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science. 2005;309:2185‐2189. [DOI] [PubMed] [Google Scholar]
- 9. LaFayette SL, Collins C, Zaas AK, et al. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog. 2010;6:e1001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adler A, Park YD, Larsen P, et al. A novel specificity protein 1 (SP1)‐like gene regulating protein kinase C‐1 (Pkc1)‐dependent cell wall integrity and virulence factors in Cryptococcus neoformans . J Biol Chem. 2011;286:20977‐20990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pradhan A, Avelar GM, Bain JM, et al. Non‐canonical signalling mediates changes in fungal cell wall PAMPs that drive immune evasion. Nat Commun. 2019;10:5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berman J, Krysan DJ. Drug resistance and tolerance in fungi. Nat Rev Microbiol. 2020;18:319‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ballou ER, Avelar GM, Childers DS, et al. Lactate signalling regulates fungal β‐glucan masking and immune evasion. Nat Microbiol. 2016;2:16238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pradhan A, Herrero‐de‐Dios C, Belmonte RC, et al. Elevated catalase expression in a fungal pathogen is a double‐edged sword of iron. PLoS Pathog. 2017;13:e1006405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. d'Enfert C, Kaune AK, Alaban LR, et al. The impact of the fungus–host–microbiota interplay upon Candida albicans infections: current knowledge and new perspectives. FEMS Microbiol Rev. 2021;45:1‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nero LC, Tarver MG, Hedrick LR. Growth of Acanthamoeba castellani with the yeast Torulopsis famata . J Bacteriol. 1964;87:220‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seaton A, Robertson MD. Aspergillus, asthma, and amoebae. Lancet. 1989;1(8643):893‐894. [DOI] [PubMed] [Google Scholar]
- 18. Casadevall A, Steenbergen JN, Nosanchuk JD. ‘Ready made’ virulence and ‘dual use’ virulence factors in pathogenic environmental fungi—the Cryptococcus neoformans paradigm. Curr Opin Microbiol. 2003;6:332‐337. [DOI] [PubMed] [Google Scholar]
- 19. Casadevall A, Fu MS, Guimaraes AJ, Albuquerque P. The ‘amoeboid predator–fungal animal virulence’ hypothesis. J Fungi. 2019;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hillmann F, Novohradská S, Mattern DJ, et al. Virulence determinants of the human pathogenic fungus Aspergillus fumigatus protect against soil amoeba predation. Environ Microbiol. 2015;17:2858‐2869. [DOI] [PubMed] [Google Scholar]
- 21. Steenbergen JN, Nosanchuk JD, Malliaris SD, Casadevall A. Interaction of Blastomyces dermatitidis, Sporothrix schenckii, and Histoplasma capsulatum with Acanthamoeba castellanii . Infect Immun. 2004;72:3478‐3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Casadevall A. Amoeba provide insight into the origin of virulence in pathogenic fungi. Adv Exp Med Biol. 2012;710:1‐10. [DOI] [PubMed] [Google Scholar]
- 23. Steenbergen JN, Shuman HA, Casadevall A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc Natl Acad Sci U S A. 2001;98:15245‐15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Underhill DM. Escape mechanisms from the immune response. In: Brown GD, Netea MG, eds. Immunology of Fungal Infections. Springer; 2007:429‐442. [Google Scholar]
- 25. Marcos CM, de Oliveira HC, de Melo WC, et al. Anti‐immune strategies of pathogenic fungi. Front Cell Infect Microbiol. 2016;6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koenig A, Mueller R, Mogavero S, Hube B. Fungal factors involved in host immune evasion, modulation and exploitation during infection. Cell Microbiol. 2021;23:e13272. [DOI] [PubMed] [Google Scholar]
- 27. Erwig LP, Gow NAR. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol. 2016;14:163‐176. [DOI] [PubMed] [Google Scholar]
- 28. Lenardon MD, Sood P, Dorfmueller H, Brown AJP, Gow NAR. Scalar nanostructure of the Candida albicans cell wall; a molecular, cellular and ultrastructural analysis and interpretation. Cell Surf. 2020;6:100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Assis LJ, Bain JM, Liddle C et al. The nature of β‐1,3‐glucan exposing features on the Candida albicans cell wall and their modulation by protein kinase A, Sin3, Mig1 and Mig2, 2021. (submitted). [DOI] [PMC free article] [PubMed]
- 30. Wheeler RT, Kombe D, Agarwala SD, Fink GR. Dynamic, morphotype‐specific Candida albicans beta‐glucan exposure during infection and drug treatment. PLoS Pathog. 2008;4:e1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Childers DS, Avelar GM, Bain JM, et al. Epitope shaving promotes fungal immune evasion. mBio. 2020;11:e00984‐e00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang M, Solis NV, Marshall M, et al. Control of β‐glucan exposure by the endo‐1,3‐glucanase Eng1 in Candida albicans modulates virulence. PLoS Pathog. 2022;18(1):e1010192. doi: 10.1101/2020.09.07.285791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sherrington SL, Sorsby E, Mahtey N, et al. Adaptation of Candida albicans to environmental pH induces cell wall remodelling and enhances innate immune recognition. PLoS Pathog. 2017;13:e1006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pradhan A, Avelar G, Bain JM, et al. Hypoxia promotes immune evasion by triggering β‐glucan masking on the Candida albicans cell surface via mitochondrial and cAMP‐protein kinase A signalling. mBio. 2018;9:e01318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brown AJP, Gow NAR, Warris A, Brown GD. Memory in fungal pathogens promotes immune evasion, colonisation and infection. Trends Microbiol. 2019;27:219‐230. [DOI] [PubMed] [Google Scholar]
- 36. Yadav B, Mora‐Montes HM, Wagener J, et al. Differences in fungal immune recognition by monocytes and macrophages: N‐mannan can be a shield or activator of immune recognition. Cell Surf. 2020;6:100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edwards JA, Alore EA, Rappleye CA. The yeast‐phase virulence requirement for α‐glucan synthase differs among Histoplasma capsulatum chemotypes. Eukaryot Cell. 2011;10:87‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thind SK, Taborda CP, Nosanchuk JD. Dendritic cell interactions with Histoplasma and Paracoccidioides . Virulence. 2015;6:424‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garfoot AL, Shen Q, Wuthrich M, Klein BS, Rappleye CA. The Eng1 β‐glucanase enhances Histoplasma virulence by reducing β‐glucan exposure. mBio. 2016;7:e01388‐e01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ray SC, Rappleye CA. Flying under the radar: Histoplasma capsulatum avoidance of innate immune recognition. Semin Cell Dev Biol. 2019;89:91‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vecchiarelli A, Retini C, Pietrella D, et al. Downregulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin‐1 beta secretion from human monocytes. Infect Immun. 1995;63:2919‐2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vecchiarelli A, Retini C, Monari C, Tascini C, Bistoni F, Kozel TR. Purified capsular polysaccharide of Cryptococcus neoformans induces interleukin‐10 secretion by human monocytes. Infect Immun. 1996;64:2846‐2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chiapello LS, Baronetti JL, Aoki MP, Gea S, Rubinstein H, Masih DT. Immunosuppression, interleukin‐10 synthesis and apoptosis are induced in rats inoculated with Cryptococcus neoformans glucuronoxylomannan. Immunology. 2004;113:392‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aimanianda V, Bayry J, Bozza S, et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460:1117‐1121. [DOI] [PubMed] [Google Scholar]
- 45. Gropp K, Schild L, Schindler S, Hube B, Zipfel PF, Skerka C. The yeast Candida albicans evades human complement attack by secretion of aspartic proteases. Mol Immunol. 2009;47:465‐475. [DOI] [PubMed] [Google Scholar]
- 46. Luberto C, Martinez‐Marino B, Taraskiewicz D, et al. Identification of App1 as a regulator of phagocytosis and virulence of Cryptococcus neoformans . J Clin Invest. 2003;112:1080‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Behnsen J, Lessing F, Schindler S, et al. Secreted Aspergillus fumigatus protease Alp1 degrades human complement proteins C3, C4, and C5. Infect Immun. 2010;78:3585‐3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Luo S, Hartmann A, Dahse HM, Skerka C, Zipfel PF. Secreted pH‐regulated antigen 1 of Candida albicans blocks activation and conversion of complement C3. J Immunol. 2010;185:2164‐2173. [DOI] [PubMed] [Google Scholar]
- 49. Luo S, Skerka C, Kurzai O, Zipfel PF. Complement and innate immune evasion strategies of the human pathogenic fungus Candida albicans . Mol Immunol. 2013;56:161‐169. [DOI] [PubMed] [Google Scholar]
- 50. Luo S, Hipler UC, Munzberg C, Skerka C, Zipfel PF. Sequence variations and protein expression levels of the two immune evasion proteins Gpm1 and Pra1 influence virulence of clinical Candida albicans isolates. PLoS One. 2015;10:e0113192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dasari P, Shopova IA, Stroe M, et al. Aspf2 from Aspergillus fumigatus recruits human immune regulators for immune evasion and cell damage. Front Immunol. 2018;9:1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Crowe JD, Sievwright IK, Auld GC, Moore NR, Gow NAR, Booth NA. Candida albicans binds human plasminogen: identification of eight plasminogen‐binding proteins. Mol Microbiol. 2003;47:1637‐1651. [DOI] [PubMed] [Google Scholar]
- 53. Stie J, Bruni G, Fox D. Surface‐associated plasminogen binding of Cryptococcus neoformans promotes extracellular matrix invasion. PLoS One. 2009;4:e5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Behnsen J, Hartmann A, Schmaler J, Gehrke A, Brakhage AA, Zipfel PF. The opportunistic human pathogenic fungus Aspergillus fumigatus evades the host complement system. Infect Immun. 2008;76:820‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eissenberg LG, Goldman WE, Schlesinger PH. Histoplasma capsulatum modulates the acidification of phagolysosomes. J Exp Med. 1993;177:1605‐1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sebghati TS, Engle JT, Goldman WE. Intracellular parasitism by Histoplasma capsulatum: fungal virulence and calcium dependence. Science. 2000;290:1368‐1372. [DOI] [PubMed] [Google Scholar]
- 57. Johnston SA, May RC. The human fungal pathogen Cryptococcus neoformans escapes macrophages by a phagosome emptying mechanism that is inhibited by Arp2/3 complex‐mediated actin polymerisation. PLoS Pathog. 2010;6:e1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Seider K, Brunke S, Schild L, et al. The facultative intracellular pathogen Candida glabrata subverts macrophage cytokine production and phagolysosome maturation. J Immunol. 2011;187:3072‐3086. [DOI] [PubMed] [Google Scholar]
- 59. DeLeon‐Rodriguez CM, Casadevall A. Cryptococcus neoformans: tripping on acid in the phagolysosome. Front Microbiol. 2016;7:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Luberto C, Toffaletti DL, Wills EA, et al. Roles for inositol‐phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans . Genes Dev. 2001;15:201‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vylkova S, Lorenz MC. Modulation of phagosomal pH by Candida albicans promotes hyphal morphogenesis and requires Stp2p, a regulator of amino acid transport. PLoS Pathog. 2014;10:e1003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Danhof HA, Vylkova S, Vesely EM, Ford AE, Gonzalez‐Garay M, Lorenz MC. Robust extracellular pH modulation by Candida albicans during growth in carboxylic acids. mBio. 2016;7:e01646‐e01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Williams RB, Lorenz MC. Multiple alternative carbon pathways combine to promote Candida albicans stress resistance, immune interactions, and virulence. mBio. 2020;11:e03070‐e03019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Westman J, Moran G, Mogavero S, Hube B, Grinstein S. Candida albicans hyphal expansion causes phagosomal membrane damage and luminal alkalinization. mBio. 2018;9:e01226‐e01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Silao FGS, Ryman K, Jiang T, et al. Glutamate dehydrogenase (Gdh2)‐dependent alkalization is dispensable for escape from macrophages and virulence of Candida albicans . PLoS Pathog. 2020;16:e1008328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Austermeier S, Kasper L, Westman J, Gresnigt MS. I want to break free—macrophage strategies to recognize and kill Candida albicans, and fungal counter‐strategies to escape. Curr Opin Microbiol. 2020;58:15‐23. [DOI] [PubMed] [Google Scholar]
- 67. Lewis LE, Bain JM, Lowes C, et al. Stage specific assessment of Candida albicans phagocytosis by macrophages identifies cell wall composition and morphogenesis as key determinants. PLoS Pathog. 2012;8:e1002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ermert D, Niemiec MJ, Rohm M, Glenthoj A, Borregaard N, Urban CF. Candida albicans escapes from mouse neutrophils. J Leukoc Biol. 2013;94:223‐236. [DOI] [PubMed] [Google Scholar]
- 69. Vylkova S, Lorenz MC. Phagosomal neutralization by the fungal pathogen Candida albicans induces macrophage pyroptosis. Infect Immun. 2017;85:e00832‐e00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bain JM, Alonso F, Childers DS, et al. Immune cells fold and damage fungal hyphae. Proc Natl Acad Sci U S A. 2021;118:e2020484118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wellington M, Koselny K, Sutterwala FS, Krysan DJ. Candida albicans triggers NLRP3‐mediated pyroptosis in macrophages. Eukaryot Cell. 2014;13:329‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. O'Meara TR, Veri AO, Ketela T, Jiang B, Roemer T, Cowen LE. Global analysis of fungal morphology exposes mechanisms of host cell escape. Nat Commun. 2015;6:6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kasper L, Konig A, Koenig PA, et al. The fungal peptide toxin candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat Commun. 2018;9:4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tucey TM, Verma J, Harrison PF, et al. Glucose homeostasis is important for immune cell viability during Candida challenge and host survival of systemic fungal infection. Cell Metab. 2018;27:988‐1006.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tucey TM, Verma J, Olivier FAB, et al. Metabolic competition between host and pathogen dictates inflammasome responses to fungal infection. PLoS Pathog. 2020;16:e1008695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol. 2011;29:1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cox GM, Harrison TS, McDade HC, et al. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect Immun. 2003;71:173‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Roetzer A, Klopf E, Gratz N, et al. Regulation of Candida glabrata oxidative stress resistance is adapted to host environment. FEBS Letts. 2011;585:319‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Youseff BH, Holbrook ED, Smolnycki KA, Rappleye CA. Extracellular superoxide dismutase protects Histoplasma yeast cells from host‐derived oxidative stress. PLoS Pathog. 2012;8:e1002713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fradin C, De Groot P, MacCallum D, et al. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol Microbiol. 2005;56:397‐415. [DOI] [PubMed] [Google Scholar]
- 81. Frohner IE, Bourgeois C, Yatsyk K, Majer O, Kuchler K. Candida albicans cell surface superoxide dismutases degrade host‐derived reactive oxygen species to escape innate immune surveillance. Mol Microbiol. 2009;71:240‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tillmann AT, Strijbis K, Cameron G, et al. Contribution of Fdh3 and Glr1 to glutathione redox state, stress adaptation and virulence in Candida albicans . PLoS ONE. 2015;10:e0126940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chiranand W, McLeod I, Zhou H, et al. CTA4 transcription factor mediates induction of nitrosative stress response in Candida albicans . Eukaryot Cell. 2008;7:268‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Szafranski‐Schneider E, Swidergall M, Cottier F, et al. Msb2 shedding protects Candida albicans against antimicrobial peptides. PLoS Pathog. 2012;8:e1002501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rodaki A, Bohovych IM, Enjalbert B, et al. Glucose promotes stress resistance in the fungal pathogen, Candida albicans . Mol Biol Cell. 2009;20:4845‐4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ene IV, Walker LA, Schiavone M, et al. Cell wall remodelling enzymes modulate fungal cell wall elasticity and osmotic stress resistance. mBio. 2015;6:e00986‐e00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kastora SL, Herrero‐de‐Dios C, Avelar GM, Munro CA, Brown AJP. Sfp1 and Rtg3 reciprocally modulate carbon source‐conditional stress adaptation in the pathogenic yeast Candida albicans . Mol Microbiol. 2017;105:620‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sentandreu M, Elorza MV, Sentandreu R, Fonzi WA. Cloning and characterization of PRA1, a gene encoding a novel pH‐regulated antigen of Candida albicans . J Bacteriol. 1998;180:282‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vylkova S, Carman AJ, Danhof HA, Collette JR, Zhou H, Lorenz MC. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. mBio. 2011;2:e00055‐e00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Brown AJP. Integration of metabolism with virulence in Candida albicans . In: Brown AJP, ed. Fungal Genomics. Mycota XIII. Springer‐Verlag; 2005:185‐203. [Google Scholar]
- 91. Beyhan S, Gutierrez M, Voorhies M, Sil A. A temperature‐responsive network links cell shape and virulence traits in a primary fungal pathogen. PLoS Biol. 2013;11:e1001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Beyhan S, Sil A. Sensing the heat and the host: virulence determinants of Histoplasma capsulatum . Virulence. 2019;10:793‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nguyen VQ, Sil A. Temperature‐induced switch to the pathogenic yeast form of Histoplasma capsulatum requires Ryp1, a conserved transcriptional regulator. Proc Natl Acad Sci U S A. 2008;105:4880‐4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dambuza IM, Drake T, Chapuis A, et al. The Cryptococcus neoformans Titan cell is an inducible and regulated morphotype underlying pathogenesis. PLoS Pathog. 2018;14:e1006978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hommel B, Mukaremera L, Cordero RJB, et al. Titan cells formation in Cryptococcus neoformans is finely tuned by environmental conditions and modulated by positive and negative genetic regulators. PLoS Pathog. 2018;14:e1006982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Trevijano‐Contador N, de Oliveira HC, Garcia‐Rodas R, et al. Cryptococcus neoformans can form titan‐like cells in vitro in response to multiple signals. PLoS Pathog. 2018;14:e1007007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Saporito‐Irwin SM, Birse CE, Sypherd PS, Fonzi WA. PHR1, a pH‐regulated gene of Candida albicans, is required for morphogenesis. Mol Cell Biol. 1995;15:601‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Enjalbert B, Whiteway M. Release from quorum‐sensing molecules triggers hyphal formation during Candida albicans resumption of growth. Eukaryot Cell. 2005;4:1203‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Klengel T, Liang WJ, Chaloupka J, et al. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol. 2005;15:2021‐2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Xu XL, Lee RTH, Fang HM, et al. Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe. 2008;4:28‐39. [DOI] [PubMed] [Google Scholar]
- 101. Lu Y, Su C, Unoje O, Liu H. Quorum sensing controls hyphal initiation in Candida albicans through Ubr1‐mediated protein degradation. Proc Natl Acad Sci U S A. 2014;111:1975‐1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Noble SM, Gianetti BA, Witchley JN. Candida albicans cell‐type switching and functional plasticity in the mammalian host. Nat Rev Microbiol. 2017;15:96‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Doedt T, Krishnamurthy S, Bockmuhl DP, et al. APSES proteins regulate morphogenesis and metabolism in Candida albicans . Mol Biol Cell. 2004;15:3167‐3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Murad AMA, Leng P, Straffon M, et al. NRG1 represses yeast‐hypha morphogenesis and hypha‐specific gene expression in Candida albicans . EMBO J. 2001;20:4742‐4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kornitzer D. Regulation of Candida albicans hyphal morphogenesis by endogenous signals. J Fungi. 2019;5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Su C, Lu Y, Liu H. Reduced TOR signaling sustains hyphal development in Candida albicans by lowering Hog1 basal activity. Mol Biol Cell. 2013;24:385‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Soll DR. High‐frequency switching in Candida albicans . Clin Microbiol Rev. 1992;5:183‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lohse MB, Johnson AD. Differential phagocytosis of white versus opaque Candida albicans by Drosophila and mouse phagocytes. PLoS One. 2008;3:e1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Soll DR. The role of phenotypic switching in the basic biology and pathogenesis of Candida albicans . J Oral Microbiol. 2014;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tao L, Du H, Guan G, et al. Discovery of a ‘white‐gray‐opaque’ tristable phenotypic switching system in Candida albicans: roles of non‐genetic diversity in host adaptation. PLoS Biol. 2014;12:e1001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zordan RE, Miller MG, Galgoczy DJ, Tuch BB, Johnson AD. Interlocking transcriptional feedback loops control white‐opaque switching in Candida albicans . PLoS Biol. 2007;5:e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hernday AD, Lohse MB, Fordyce PM, Nobile CJ, DeRisi JL, Johnson AD. Structure of the transcriptional network controlling white‐opaque switching in Candida albicans . Mol Microbiol. 2013;90:22‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ramırez‐Zavala B, Reuss O, Park YN, Ohlsen K, Morschhauser J. Environmental induction of white‐opaque switching in Candida albicans . PLoS Pathog. 2008;4:e1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Huang G, Srikantha T, Sahni N, Yi S, Soll DR. CO2 regulates white‐to‐opaque switching in Candida albicans . Curr Biol. 2009;19:330‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Alby K, Bennett RJ. Stress‐induced phenotypic switching in Candida albicans . Mol Biol Cell. 2009;20:3178‐3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Munro CA, Selvaggini S, de Bruijn I, et al. The PKC, HOG and Ca2+ signalling pathways coordinately regulate chitin synthesis in Candida albicans . Mol Microbiol. 2007;63:1399‐1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sosinska GJ, de Groot PW, Teixeira de Mattos MJ, et al. Hypoxic conditions and iron restriction affect the cell‐wall proteome of Candida albicans grown under vagina‐simulative conditions. Microbiology. 2008;154:510‐520. [DOI] [PubMed] [Google Scholar]
- 118. Ene IV, Adya AK, Wehmeier S, et al. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol. 2012;14:1319‐1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Naglik J, Challacombe S, Hube B. Candida albicans secreted aspartyl proteinases and their contribution to virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67:400‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Birse CE, Irwin MY, Fonzi WA, Sypherd PS. Cloning and characterization of ECE1, a gene expressed in association with cell elongation of the dimorphic pathogen Candida albicans . Infect Immun. 1993;61:3648‐3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hoyer LL, Payne TL, Bell M, Myers AM, Scherer S. Candida albicans ALS3 and insights into the nature of the ALS gene family. Curr Genet. 1998;33:451‐459. [DOI] [PubMed] [Google Scholar]
- 122. Phan QT, Myers CL, Fu Y, et al. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007;5:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Martin R, Albrecht‐Eckardt D, Brunke S, Hube B, Hünniger K, Kurzai O. A core filamentation response network in Candida albicans is restricted to eight genes. PLoS One. 2013;8:e58613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Moyes D, Wilson D, Richardson JP, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jung WH, Sham A, White R, Kronstad JW. Iron regulation of the major virulence factors in the AIDS‐associated pathogen Cryptococcus neoformans . PLoS Biol. 2006;4:e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. O'Meara TR, Norton D, Price MS, et al. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Pathog. 2010;6:e1000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Okagaki LH, Wang Y, Ballou ER, et al. Cryptococcal Titan cell formation is regulated by G‐protein signaling in response to multiple stimuli. Eukaryot Cell. 2011;10:1306‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Davis D, Edwards JE, Mitchell AP, Ibrahim AS. Candida albicans RIM101 pH response pathway is required for host–pathogen interactions. Infect Immun. 2000;68:5953‐5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bignell E, Negrete‐Urtasun S, Calcagno AM, Haynes K, Arst HN, Rogers T. The Aspergillus pH‐responsive transcription factor PacC regulates virulence. Mol Microbiol. 2005;55:1072‐1084. [DOI] [PubMed] [Google Scholar]
- 130. Nobile CJ, Solis N, Myers CL, et al. Candida albicans transcription factor Rim101 mediates pathogenic interactions through cell wall functions. Cell Microbiol. 2008;10:2180‐2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Bertuzzi M, Schrettl M, Alcazar‐Fuoli L, et al. The pH‐responsive PacC transcription factor of Aspergillus fumigatus governs epithelial entry and tissue invasion during pulmonary aspergillosis. PLoS Pathog. 2014;10:e1004413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gardiner DM, Howlett BJ. Bioinformatic and expression analysis of the putative gliotoxin biosynthetic gene cluster of Aspergillus fumigatus . FEMS Microbiol Lett. 2005;248:241‐248. [DOI] [PubMed] [Google Scholar]
- 133. Shin KS, Kim YH, Yu JH. Proteomic analyses reveal the key roles of BrlA and AbaA in biogenesis of gliotoxin in Aspergillus fumigatus . Biochem Biophys Res Commun. 2015;463:428‐433. [DOI] [PubMed] [Google Scholar]
- 134. Soliman SSM, Baldin C, Gu Y, et al. Mucoricin is a ricin‐like toxin that is critical for the pathogenesis of mucormycosis. Nat Microbiol. 2021;6:313‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Odds FC, Calderone RA, Hube B, Nombela C. Virulence in Candida species: views and suggestions from a peer‐group workshop. ASM News. 2003;69:54‐55. [Google Scholar]
- 136. Hohmann S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev. 2002;66:300‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Moye‐Rowley WS, Harshman KD, Parker CS. Yeast YAP1 encodes a novel form of the jun family of transcriptional activator proteins. Genes Dev. 1989;3:283‐292. [DOI] [PubMed] [Google Scholar]
- 138. Morgan BA, Banks GR, Toone WM, Raitt D, Kuge S, Johnston LH. The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae . EMBO J. 1997;16:1035‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lee J, Godon C, Lagniel G, et al. Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J Biol Chem. 1999;274:16040‐16046. [DOI] [PubMed] [Google Scholar]
- 140. Alarco AM, Raymond M. The bZip transcription factor Cap1p is involved in multidrug resistance and oxidative stress response in Candida albicans . J Bacteriol. 1999;181:700‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Singh P, Chauhan N, Ghosh A, Dixon F, Calderone R. SKN7 of Candida albicans: mutant construction and phenotype analysis. Infect Immun. 2004;72:2390‐2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Znaidi S, Barker KS, Weber S, et al. Identification of the Candida albicans Cap1p regulon. Eukaryot Cell. 2009;8:806‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lee KS, Irie K, Gotoh Y, et al. A yeast mitogen‐activated protein kinase homolog (Mpk1) mediates signaling by protein kinase C. Mol Cell Biol. 1993;13:3067‐3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Martin H, Arroyo J, Sanchez M, Molina M, Nombela C. Activity of the yeast MAP kinase homolog Slt2 is critically required for cell integrity at 37°C. Mol Gen Genet. 1993;241:177‐184. [DOI] [PubMed] [Google Scholar]
- 145. Navarro‐Garcia F, Sanchez M, Pla J, Nombela C. Functional characterisation of the MKC1 gene of Candida albicans, which encodes a mitogen‐activated protein kinase homolog related to cell integrity. Mol Cell Biol. 1995;15:2197‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wilson D, Citiulo F, Hube B. Zinc exploitation by pathogenic fungi. PLoS Pathog. 2012;8:e1003034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Dowzer CE, Kelly JM. Analysis of the creA gene, a regulator of carbon catabolite repression in Aspergillus nidulans . Mol Cell Biol. 1991;11:5701‐5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hedbacker K, Carlson M. SNF1/AMPK pathways in yeast. Front Biosci. 2008;13:2408‐2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lagree K, Woolford CA, Huang MY, et al. Roles of Candida albicans Mig1 and Mig2 in glucose repression, pathogenicity traits, and SNF1 essentiality. PLoS Genet. 2020;16:e1008582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tripathi G, Wiltshire C, Macaskill S, Tournu H, Budge S, Brown AJP. CaGcn4 co‐ordinates morphogenetic and metabolic responses to amino acid starvation in Candida albicans . EMBO J. 2002;21:5448‐5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Krappmann S, Bignell EM, Reichard U, Rogers T, Haynes K, Braus GH. The Aspergillus fumigatus transcriptional activator CpcA contributes significantly to the virulence of this fungal pathogen. Mol Microbiol. 2004;52:785‐799. [DOI] [PubMed] [Google Scholar]
- 152. Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407‐450. [DOI] [PubMed] [Google Scholar]
- 153. Giacometti R, Kronberg F, Biondi RM, Passeron S. Catalytic isoforms Tpk1 and Tpk2 of Candida albicans PKA have non‐redundant roles in stress response and glycogen storage. Yeast. 2009;26:273‐285. [DOI] [PubMed] [Google Scholar]
- 154. Gorner W, Durchschlag E, Martinez‐Pastor MT, et al. Nuclear localisation of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 1998;12:586‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Thevelein JM, Cauwenberg L, Colombo S, et al. Nutrient‐induced signal transduction through the protein kinase A pathway and its role in the control of metabolism, stress resistance, and growth in yeast. Enzyme Microb Technol. 2000;26:819‐825. [DOI] [PubMed] [Google Scholar]
- 156. Cao C, Wu M, Bing J, et al. Global regulatory roles of the cAMP/PKA pathway revealed by phenotypic, transcriptomic and phosphoproteomic analyses in a null mutant of the PKA catalytic subunit in Candida albicans . Mol Microbiol. 2017;105:46‐64. [DOI] [PubMed] [Google Scholar]
- 157. Alonso‐Monge R, Navarro‐Garcia F, Roman E, et al. The Hog1 mitogen‐activated protein kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans . Eukaryot Cell. 2003;2:351‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Askew C, Sellam A, Epp E, et al. Transcriptional regulation of carbohydrate metabolism in the human pathogen Candida albicans . PLoS Pathog. 2009;5:e1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Gasch AP, Spellman PT, Kao CM, et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241‐4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Causton HC, Ren B, Koh SS, et al. Remodeling of yeast genome expression in response to environmental changes. Mol Biol Cell. 2001;12:323‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Enjalbert B, Nantel A, Whiteway M. Stress‐induced gene expression in Candida albicans: absence of a general stress response. Mol Biol Cell. 2003;14:1460‐1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Nicholls S, Straffon M, Enjalbert B, et al. Msn2/4‐like transcription factors play no obvious roles in the stress responses of the fungal pathogen, Candida albicans . Eukaryot Cell. 2004;3:1111‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ramsdale M, Selway L, Stead D, et al. The novel gene MNL1 regulates weak acid induced stress responses of the fungal pathogen Candida albicans . Mol Biol Cell. 2008;19:4393‐4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Gancedo JM, Gancedo C. Gluconeogenesis and catabolite inactivation. In: Zimmermann FK, Entian KD, eds. Yeast sugar metabolism. Technomic Publishing; 1997:359‐377. [Google Scholar]
- 165. Lopez‐Boado YS, Herrero P, Gascon S, Moreno F. Catabolite inactivation of isocitrate lyase from Saccharomyces cerevisiae . Arch Microbiol. 1987;147:231‐234. [DOI] [PubMed] [Google Scholar]
- 166. Sandai D, Yin Z, Selway L, et al. The evolutionary rewiring of ubiquitination targets has reprogrammed the regulation of carbon assimilation in the pathogenic yeast, Candida albicans . mBio. 2012;3:e00495‐e00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Childers DS, Raziunaite I, Mol Avelar G, et al. The rewiring of metabolic ubiquitination targets in a pathogenic yeast promotes metabolic flexibility, host colonization and virulence. PLoS Pathog. 2016;12:e1005566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Brown AJP, Brown GD, Netea MG, Gow NAR. Metabolism impacts Candida immunogenicity and pathogenicity at multiple levels. Trends Microbiol. 2014;22:614‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Garreau H, Hasa RN, Renault G, Estruch F, Boy‐Marcotte E, Jacquet M. Hyperphosphorylation of Msn2 and Msn4 in response to heat shock and the diauxic shift is inhibited by cAMP in Saccharomyces cerevisiae . Microbiology. 2000;146:2113‐2120. [DOI] [PubMed] [Google Scholar]
- 170. Murad AMA, d'Enfert C, Gaillardin C, et al. Transcript profiling in Candida albicans reveals new cellular functions for the transcriptional repressors, CaTup1, CaMig1 and CaNrg1. Mol Microbiol. 2001;42:981‐993. [DOI] [PubMed] [Google Scholar]
- 171. Yin Z, Stead D, Selway L, et al. Proteomic response to amino acid starvation in Candida albicans and Saccharomyces cerevisiae . Proteomics. 2004;4:2425‐2436. [DOI] [PubMed] [Google Scholar]
- 172. Enjalbert B, Smith DA, Cornell MJ, et al. Role of the Hog1 stress‐activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans . Mol Biol Cell. 2006;17:1018‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Nicholls S, Leach M, Priest C, Brown AJP. Role of the heat shock transcription factor, Hsf1, in a major fungal pathogen that is obligately associated with warm‐blooded animals. Mol Microbiol. 2009;74:844‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Leach MD, Farrer RA, Tan K, et al. Hsf1 and Hsp90 orchestrate temperature‐dependent global transcriptional remodelling and chromatin architecture in Candida albicans . Nat Commun. 2016;7:11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Khamooshi K, Sikorski P, Sun N, Calderone R, Li D. The Rbf1, Hfl1 and Dbp4 of Candida albicans regulate common as well as transcription factor‐specific mitochondrial and other cell activities. BMC Genomics. 2014;15:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Ford CB, Funt JM, Abbey D, et al. The evolution of drug resistance in clinical isolates of Candida albicans . Elife. 2015;4:e00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Chen G, Mulla WA, Kucharavy A, et al. Targeting the adaptability of heterogeneous aneuploids. Cell. 2015;160:771‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Rosenberg A, Ene IV, Bibi M, et al. Antifungal tolerance is a subpopulation effect distinct from resistance and is associated with persistent candidemia. Nat Commun. 2018;9:2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Navarro‐Garcia F, Sanchez M, Pla J, Nombela C. Functional characterization of the MKC1 gene of Candida albicans, which encodes a mitogen‐activated protein kinase homolog related to cell integrity. Mol Cell Biol. 1995;15:2197‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Stoldt VR, Sonneborn A, Leuker CE, Ernst J. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982‐1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Chauhan N, Inglis D, Roman E, et al. Candida albicans response regulator gene SSK1 regulates a subset of genes whose functions are associated with cell wall biosynthesis and adaptation to oxidative stress. Eukaryot Cell. 2003;2:1018‐1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Kaloriti D, Tillmann A, Cook E, et al. Combinatorial stresses kill pathogenic Candida species. Med Mycol. 2012;50:699‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Odds FC, Brown AJP, Gow NAR. Antifungal agents: mechanisms of action. Trends Microbiol. 2003;11:272‐279. [DOI] [PubMed] [Google Scholar]
- 184. Lee Y, Puumala E, Robbins N, Cowen LE. Antifungal drug resistance: molecular mechanisms in Candida albicans and beyond. Chem Rev. 2020;121:3390‐3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Pirrone V, Thakkar N, Jacobson JM, Wigdahl B, Krebs FC. Combinatorial approaches to the prevention and treatment of HIV‐1 infection. Antimicrob Agents Chemother. 2011;55:1831‐1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kerantzas CA, Jacobs WR. Origins of combination therapy for tuberculosis: lessons for future antimicrobial development and application. mBio. 2017;8:e01586‐e01516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Molloy SF, Kanyama C, Heyderman RS, et al. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med. 2018;378:1004‐1017. [DOI] [PubMed] [Google Scholar]
- 188. Zheng X, Berti AD, McCrone S, et al. Combination antibiotic exposure selectively alters the development of vancomycin intermediate resistance in Staphylococcus aureus . Antimicrob Agents Chemother. 2018;62:e02100‐e02117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Walker LA, Munro CA, de Bruijn I, Lenardon MD, McKinnon A, Gow NA. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 2008;4:e1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Walker LA, Gow NA, Munro CA. Elevated chitin content reduces the susceptibility of Candida species to caspofungin. Antimicrob Agents Chemother. 2013;57:146‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Walker LA, Lee KK, Munro CA, Gow NA. Caspofungin treatment of Aspergillus fumigatus results in ChsG‐dependent upregulation of chitin synthesis and the formation of chitin‐rich microcolonies. Antimicrob Agents Chemother. 2015;59:5932‐5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Ropars J, Maufrais C, Diogo D, et al. Gene flow contributes to diversification of the major fungal pathogen Candida albicans . Nat Commun. 2018;9:2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Hirakawa MP, Martinez DA, Sakthikumar S, et al. Genetic and phenotypic intra‐species variation in Candida albicans . Genome Res. 2015;25:413‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Cowen LE, Sanglard D, Calabrese D, Sirjusingh C, Anderson JB, Kohn LM. Evolution of drug resistance in experimental populations of Candida albicans . J Bacteriol. 2000;182:1515‐1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Ene IV, Farrer RA, Hirakawa MP, Agwamba K, Cuomo CA, Bennett RJ. Global analysis of mutations driving microevolution of a heterozygous diploid fungal pathogen. Proc Natl Acad Sci U S A. 2018;115:E8688‐E8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Gomes AC, Miranda I, Silva RM, et al. A genetic code alteration generates a proteome of high diversity in the human pathogen Candida albicans . Genome Biol. 2007;8:R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Hickman MA, Paulson C, Dudley A, Berman J. Parasexual ploidy reduction drives population heterogeneity through random and transient aneuploidy in Candida albicans . Genetics. 2015;200:781‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Maxam AM, Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977;74:560‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain‐terminating inhibitors. Proc Natl Acad Sci U S A. 1977;74:5463‐5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Goffeau A, Aert R, Agostini‐Carbone ML, et al. The yeast genome directory. Nature. 1997;387:5. [PubMed] [Google Scholar]
- 201. Wood V, Gwilliam R, Rajandream MA, et al. The genome sequence of Schizosaccharomyces pombe . Nature. 2002;415:871‐880. [DOI] [PubMed] [Google Scholar]
- 202. International Human Genome Sequencing Consortium . Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931‐945. [DOI] [PubMed] [Google Scholar]
- 203. Hall N. Advanced sequencing technologies and their wider impact in microbiology. J Exp Biol. 2007;209:1518‐1525. [DOI] [PubMed] [Google Scholar]
- 204. Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next‐generation sequencing technologies. Nat Rev Genet. 2016;17:333‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Stajich JE. Fungal genomes and insights into the evolution of the kingdom. Microbiol Spectr. 2017;5:1‐15. doi: 10.1128/microbiolspec.FUNK-0055-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Hull CM, Johnson AD. Identification of a mating type‐like locus in the asexual pathogenic yeast Candida albicans . Science. 1999;285:1271‐1275. [DOI] [PubMed] [Google Scholar]
- 207. Bennett RJ, Johnson AD. Mating in Candida albicans and the search for a sexual cycle. Annu Rev Microbiol. 2005;59:233‐255. [DOI] [PubMed] [Google Scholar]
- 208. Santos MAS, Keith G, Tuite MF. Non‐standard translational events in Candida albicans mediated by an unusual seryl‐tRNA with a 5′‐CAG‐3′ (leucine) anticodon. EMBO J. 1993;12:607‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Santos MAS, Tuite MF. The CUG codon is decoded in vivo as serine and not leucine in Candida albicans . Nucleic Acids Res. 1995;23:1481‐1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans . Genetics. 1993;134:717‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Cormack B, Bertram G, Egerton M, Gow NAR, Falkow S, Brown AJP. Yeast enhanced green fluorescent protein (yEGFP): a reporter of gene expression in Candida albicans . Microbiology. 1997;143:303‐311. [DOI] [PubMed] [Google Scholar]
- 212. Wilson RB, Davis D, Enloe BM, Mitchell AP. A recyclable Candida albicans URA3 cassette for PCR product‐directed gene disruptions. Yeast. 2000;16:65‐70. [DOI] [PubMed] [Google Scholar]
- 213. Uhl MA, Johnson AD. Development of Streptococcus thermophilus lacZ as a reporter gene for Candida albicans . Microbiology. 2001;147:1189‐1195. [DOI] [PubMed] [Google Scholar]
- 214. Enjalbert B, Rachini A, Vediyappan G, et al. A multifunctional, synthetic Gaussia princeps luciferase reporter for live imaging of Candida albicans infections. Infect Immun. 2009;77:4847‐4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Zeng G, Wang YM, Chan FY, Wang Y. One‐step targeted gene deletion in Candida albicans haploids. Nat Protoc. 2014;9:464‐473. [DOI] [PubMed] [Google Scholar]
- 216. Shahana S, Childers DS, Ballou ER, et al. New Clox systems for rapid and efficient gene disruption in Candida albicans . PLoS One. 2014;9:e100390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Vande Velde G, Kucharíkova S, Schrevens S, Himmelreich U, Van Dijck P. Towards non‐invasive monitoring of pathogen–host interactions during Candida albicans biofilm formation using in vivo bioluminescence. Cell Microbiol. 2014;16:115‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Vyas VK, Barrasa MI, Fink GR. A Candida albicans CRISPR system permits genetic engineering of essential genes and gene families. Sci Adv. 2015;1:e1500248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Jones T, Federspiel NA, Chibana H, et al. The diploid genome sequence of Candida albicans . Proc Natl Acad Sci U S A. 2004;101:7329‐7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Nantel A, Dignard D, Bachewich C, et al. Transcription profiling of Candida albicans cells undergoing the yeast‐to‐hyphal transition. Mol Biol Cell. 2002;13:3452‐3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Lorenz MC, Bender JA, Fink GR. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell. 2004;3:1076‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Garcia‐Sanchez S, Aubert S, Iraqui I, Janbon G, Ghigo JM, d'Enfert C. Candida albicans biofilms: a developmental state associated with specific and stable gene expression patterns. Eukaryot Cell. 2004;3:536‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Bruno VM, Wang Z, Marjani SL, et al. Comprehensive annotation of the transcriptome of the human fungal pathogen Candida albicans using RNA‐seq. Genome Res. 2010;20:1451‐1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Willger SD, Liu Z, Olarte RA, et al. Analysis of the Candida albicans phosphoproteome. Eukaryot Cell. 2015;14:474‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Gil‐Bona A, Parra‐Giraldo CM, Hernaez ML, et al. Candida albicans cell shaving uncovers new proteins involved in cell wall integrity, yeast to hypha transition, stress response and host–pathogen interaction. J Proteomics. 2015;127:340‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Hull CM, Raisner RM, Johnson AD. Evidence for mating of the ‘asexual’ yeast Candida albicans in a mammalian host. Science. 2000;289:307‐310. [DOI] [PubMed] [Google Scholar]
- 227. Magee BB, Magee PT. Induction of mating in Candida albicans by construction of MTLa and MTLα strains. Science. 2000;289:310‐313. [DOI] [PubMed] [Google Scholar]
- 228. Miller MG, Johnson AD. White‐opaque switching in Candida albicans is controlled by mating‐type locus homeodomain proteins and allows efficient mating. Cell. 2002;110:293‐302. [DOI] [PubMed] [Google Scholar]
- 229. Bennett RJ, Johnson AD. Completion of a parasexual cycle in Candida albicans by induced chromosome loss in tetraploid strains. EMBO J. 2003;22:2505‐2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Hickman MA, Zeng G, Forche A, et al. The ‘obligate diploid’ Candida albicans forms mating‐competent haploids. Nature. 2013;494:55‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Shtifman Segal E, Gritsenko V, Levitan A, et al. Gene essentiality analyzed by in vivo transposon mutagenesis and machine learning in a stable haploid isolate of Candida albicans . mBio. 2018;9:e02048‐e02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Li Z, Wang H, Cai C, et al. Genome‐wide piggyBac transposon‐based mutagenesis and quantitative insertion‐site analysis in haploid Candida species. Nat Protoc. 2020;15:2705‐2727. [DOI] [PubMed] [Google Scholar]
- 233. Gavin AC, Bosche M, Krause R, et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature. 2002;415:141‐147. [DOI] [PubMed] [Google Scholar]
- 234. Giaever G, Chu AM, Ni L, et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387‐391. [DOI] [PubMed] [Google Scholar]
- 235. Tong AH, Lesage G, Bader GD, et al. Global mapping of the yeast genetic interaction network. Science. 2004;303:808‐813. [DOI] [PubMed] [Google Scholar]
- 236. Costanzo M, Baryshnikova A, Bellay J, et al. The genetic landscape of a cell. Science. 2010;327:425‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Roemer T, Jiang B, Davison J, et al. Large‐scale essential gene identification in Candida albicans and applications to antifungal drug discovery. Mol Microbiol. 2003;50:167‐181. [DOI] [PubMed] [Google Scholar]
- 238. Mnaimneh S, Davierwala AP, Haynes J, et al. Exploration of essential gene functions via titratable promoter alleles. Cell. 2004;118:31‐44. [DOI] [PubMed] [Google Scholar]
- 239. Vavierwala AP, Haynes J, Li Z, et al. The synthetic genetic interaction spectrum of essential genes. Nat Genet. 2005;37:1147‐1152. [DOI] [PubMed] [Google Scholar]
- 240. Carlson MRJ, Zhang B, Fang Z, Mischel PS, Horvath S, Nelson SF. Gene connectivity, function, and sequence conservation: predictions from modular yeast co‐expression networks. BMC Genomics. 2006;7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Liti G, Louis EJ. Advances in quantitative trait analysis in yeast. PLoS Genet. 2012;8:e1002912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Wenger JW, Schwartz K, Sherlock G. Bulk segregant analysis by high‐throughput sequencing reveals a novel xylose utilization gene from Saccharomyces cerevisiae . PLoS Genet. 2010;6:e1000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Song Q, Johnson C, Wilson TE, Kumar A. Pooled segregant sequencing reveals genetic determinants of yeast pseudohyphal growth. PLoS Genet. 2014;10:e1004570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Hou J, Sigwalt A, Fournier T, et al. The hidden complexity of Mendelian traits across natural yeast populations. Cell Rep. 2016;16:1106‐1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Salinas F, Cubillos FA, Soto D, et al. The genetic basis of natural variation in oenological traits in Saccharomyces cerevisiae . PLoS One. 2012;7:e49640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Usher J, Day A, Berman J, Quinn J, Haynes K. Resurrecting a sexual cycle in the human fungal pathogen Candida glabrata . Nature Commun. 2022; (in press). [Google Scholar]
- 247. Murakami H, Nicolas A. Locally, meiotic double‐strand breaks targeted by Gal4BD‐Spo11 occur at discrete sites with a sequence preference. Mol Cell Biol. 2009;29:3500‐3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Anderson MZ, Thomson GJ, Hirakawa MP, Bennett RJ. A ‘parameiosis’ drives depolyploidization and homologous recombination in Candida albicans . Nat Commun. 2019;10:4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Vowinckel J, Zelezniak A, Bruderer R, Mulleder M, Reiter L, Ralser M. Cost‐effective generation of precise label‐free quantitative proteomes in high‐throughput by microLC and data‐independent acquisition. Sci Rep. 2018;8:4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Messner CB, Demichev V, Bloomfield N, et al. Ultra‐fast proteomics with scanning SWATH. Nat Biotech. 2021;39:846‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Zelezniak A, Vowinckel J, Capuano F, et al. Machine learning predicts the yeast metabolome from the quantitative proteome of kinase knockouts. Cell Syst. 2018;7:269‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Tunyasuvunakool K, Adler J, Wu Z, et al. Highly accurate protein structure prediction for the human proteome. Nature. 2021;596:590‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Soste M, Hrabakova R, Wanka S, et al. A sentinel protein assay for simultaneously quantifying cellular processes. Nat Methods. 2014;11:1045‐1048. [DOI] [PubMed] [Google Scholar]
- 254. Kusebauch U, Campbell DS, Deutsch EW, et al. Human SRMAtlas: a resource of targeted assays to quantify the complete human proteome. Cell. 2016;166:766‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Ball B, Bermas A, Carruthers‐Lay D, Geddes‐McAlister J. Mass spectrometry‐based proteomics of fungal pathogenesis, host–fungal interactions, and antifungal development. J Fungi. 2019;5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Wang Y, Wirekoh Arthur E, Liu N, et al. iTRAQ‐based quantitative proteomics analysis of HeLa cells infected with Chlamydia muridarum TC0668 mutant and wild‐type strains. Front Microbiol. 2019;10:2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Zheng X, Yu J, Cairns TC, et al. Comprehensive improvement of sample preparation methodologies facilitates dynamic metabolomics of Aspergillus niger . Biotechnol J. 2019;14:e1800315. [DOI] [PubMed] [Google Scholar]
- 258. Burgain A, Tebbji F, Khemiri I, Sellam A. Metabolic reprogramming in the opportunistic yeast Candida albicans in response to hypoxia. mSphere. 2020;5:e00913‐e00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Papalexi E, Satija R. Single‐cell RNA sequencing to explore immune cell heterogeneity. Nat Rev Immunol. 2018;18:35‐45. [DOI] [PubMed] [Google Scholar]
- 260. Deng W, Su Z, Liang P, et al. Single‐cell immune checkpoint landscape of PBMCs stimulated with Candida albicans . Emerg Microbes Infect. 2021;10:1272‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Kelly RT. Single‐cell proteomics: progress and prospects. Mol Cell Proteomics. 2020;19:1739‐1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Schaefer D, Tomiuk S, Kuster LN, et al. Identification of CD318, TSPAN8 and CD66c as target candidates for CAR T cell based immunotherapy of pancreatic adenocarcinoma. Nat Commun. 2021;12:1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Netea MG, Balkwill F, Chonchol M, et al. A guiding map for inflammation. Nat Immunol. 2017;18:826‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Lionakis MS, Levitz SM. Host control of fungal infections: lessons from basic studies and human cohorts. Annu Rev Immunol. 2018;36:139‐173. [DOI] [PubMed] [Google Scholar]
- 265. Krysan DJ. The unmet clinical need of novel antifungal drugs. Virulence. 2017;8:135‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This review contains no new data.


