Abstract
Aim
Bioactive collagen peptides (CP) have been suggested to augment the functional, structural (size and architecture), and contractile adaptations of skeletal muscle to resistance training (RT), but with limited evidence. This study aimed to determine if CP vs. placebo (PLA) supplementation enhanced the functional and underpinning structural, and contractile adaptations after 15 weeks of lower body RT.
Methods
Young healthy males were randomized to consume either 15 g of CP (n = 19) or PLA (n = 20) once every day during a standardized program of progressive knee extensor, knee flexor, and hip extensor RT 3 times/wk. Measurements pre‐ and post‐RT included: knee extensor and flexor isometric strength; quadriceps, hamstrings, and gluteus maximus volume with MRI; evoked twitch contractions, 1RM lifting strength, and architecture (with ultrasound) of the quadriceps.
Results
Percentage changes in maximum strength (isometric or 1RM) did not differ between‐groups (0.684 ≤ p ≤ 0.929). Increases in muscle volume were greater (quadriceps 15.2% vs. 10.3%; vastus medialis (VM) 15.6% vs. 9.7%; total muscle volume 15.7% vs. 11.4%; [all] p ≤ 0.032) or tended to be greater (hamstring 16.5% vs. 12.8%; gluteus maximus 16.6% vs. 12.9%; 0.089 ≤ p ≤ 0.091) for CP vs. PLA. There were also greater increases in twitch peak torque (22.3% vs. 12.3%; p = 0.038) and angle of pennation of the VM (16.8% vs. 5.8%, p = 0.046), but not other muscles, for CP vs. PLA.
Conclusions
CP supplementation produced a cluster of consistent effects indicating greater skeletal muscle remodeling with RT compared to PLA. Notably, CP supplementation amplified the quadriceps and total muscle volume increases induced by RT.
Keywords: Magnetic resonance imaging, Muscle volume, Quadriceps femoris, Supplementation
1. INTRODUCTION
Human muscle, tendinous tissue, and their corresponding collagen‐rich extracellular matrix (ECM) produce and transmit muscular force to the skeleton and are sensitive to environmental factors such as mechanical 1 , 2 and nutritional 3 , 4 stimuli. Consequently, resistance training (RT), or a combination of RT and nutrition, facilitates remodeling these tissues to enhance physical function and resilience, 5 , 6 injury prevention/rehabilitation, 7 , 8 or healthy aging. 9 The musculotendinous remodeling that occurs after RT includes increases in muscle size (hypertrophy 10 ), force per unit muscle area (specific tension 11 ), muscle fascicle reorganization, 12 and enhanced tendon mechanics (stiffness 13 ). Collagen supplementation has been proposed as a valuable nutritional adjunct to RT, with the capacity to accentuate structural and functional adaptations, 14 , 15 but this possibility remains controversial. 16
Bioactive peptides are defined as peptide fragments with 2–45 amino acid residues that exert a beneficial effect on body functions and/or health. 17 , 18 While traditionally it was believed that all ingested peptides and proteins were digested down to their constituent amino acids in the human gut, it is now apparent that certain peptides can cross the intestinal epithelium under normal conditions, enter the circulation, and exert systemic effects including antihypertensive, antimicrobial, immunomodulatory, and antioxidant effects, among others. 19 , 20 , 21 Collagen is a well‐established human food source of peptides, some of which appear to enter the blood intact 22 , 23 and have a range of bioactive properties. 24
Collagen has been found to enhance synthesis or growth in a range of musculoskeletal tissues, such as cartilage, 25 ligament, 26 and bone. 27 , 28 Specific to musculotendinous tissues, collagen peptides have been shown to induce myoblast differentiation and myotube hypertrophy in mouse skeletal muscle cells in‐vitro by activating the mTOR signaling pathway, 29 upregulate human gene expression related to signal transduction pathways activated in muscular remodeling 30 and there is emerging evidence that collagen supplementation in humans can enhance the rehabilitation of injured tendons. 31 , 32 , 33 These benefits may be due to the ability of bioactive collagen peptides to upregulate the synthesis of ECM proteins in various tissues via a stimulatory cell effect, while also providing the specific amino acid building blocks for body collagens. 34 Furthermore, the skeletal muscle ECM is a dynamic environment that appears to have the capacity to regulate myogenesis and muscle regeneration (i.e., remodeling 35 ) and there is strong evidence for a role of ECM components (e.g., collagen VI 36 and fibronectin 37 ) in regulating the activity of muscle satellite stem cells, which are known to play a potent role in muscle remodeling and hypertrophy.
Collagen peptide (CP) supplementation has been found to augment RT‐induced strength gains in some 14 , 15 but not all studies. 38 , 39 Furthermore, these studies have consistently reported greater gains in fat‐free mass for CP vs. placebo, assessed with relatively crude indices (dual‐energy x‐ray absorptiometry or bioelectrical impedance analysis 14 , 15 , 38 ), with the magnitude of some of the reported gains causing some controversy among scientists. 16 The gold standard measures of muscle size are magnetic resonance imaging (MRI) or computed tomography, 40 but to date, no studies have assessed the efficacy of CP supplementation to augment RT‐induced increases in muscle size (volume or cross‐sectional area) using these techniques. The capacity of CPs to enhance the changes in muscle architecture (pennation angle and fascicle length) and maximum voluntary‐specific tension after RT also remains unknown but could be sensitive to changes in the ECM and/or lateral force transmission. 41
Therefore, the aim of this study was to determine if CP vs. placebo (PLA) supplementation enhanced primarily functional (strength), and secondarily muscle structural (size and architecture, assessed with MRI and ultrasound), mechanical (skeletal muscle‐specific tension), and contractile (evoked twitch) adaptations after 15 weeks of RT. We hypothesized that CP supplementation would accentuate the gains in muscle strength and size (volume) with RT, compared to placebo.
2. RESULTS
2.1. Group characteristics at baseline
There were no pre‐intervention differences (unpaired t‐test, 0.058 ≤ p ≤ 0.746) between‐groups for age (CP 27.0 ± 5.0; PLA 24.4 ± 3.2 y), height (CP 1.79 ± 0.09; PLA 1.80 ± 0.08 m), body mass (CP 73.3 ± 12.0; PLA 75.8 ± 11.5 kg), or habitual physical activity (IPAQ). Similarly, there were no pre‐intervention differences (unpaired t‐test, 0.332 ≤ p ≤ 0.661) between‐groups for knee extension maximum voluntary torque (MVT), knee extension one repetition maximum (1RM), or knee flexion MVT. Quadriceps volume (unpaired t‐test, p = 0.356), architecture (pennation angle (θ P) p = 0.053; fascicle length (L F ) p = 0.196), specific tension (p = 0.295), and evoked twitch responses (0.508 ≤ p ≤ 0.870) were similar for both groups pre‐intervention. Finally, volume of the hamstrings (unpaired t‐test, p = 0.593), gluteus maximus (p = 0.193), and total of the trained muscles (i.e., Σ of quadriceps, hamstrings, and gluteus maximus; p = 0.339) did not differ between‐groups at pre‐intervention.
2.2. Dietary assessment, physical activity, and body mass
There were no between‐group differences in absolute or relative (to body mass) energy or macronutrient intake (unpaired t‐test, 0.159 ≤ p ≤ 0.736; Table 1). After accounting for supplementation there were no differences between‐groups for energy, carbohydrate, or fat intake (absolute or relative; unpaired t‐test, 0.209 ≤ p ≤ 0.843), but protein intake was greater for CP than PLA (absolute and relative; 0.027 ≤ p ≤ 0.031; Table 1). Habitual physical activity (i.e., excluding supervised RT) of both groups was unchanged throughout the intervention period (paired t‐test, 0.239 ≤ p ≤ 0.592) and showed no between‐group interaction effect (ANOVA: group × time, p = 0.413; CP, Pre 2067 ± 1377, Week‐152 249 ± 1123; PLA, Pre 2220 ± 1542, Week‐153 030 ± 2781 MET‐min/wk). There was a within‐group increase in body mass in CP (paired t‐test, p = 0.001; Pre 73.3 ± 12.0; Post 75.4 ± 12.0 kg) but not PLA (p = 0.101; Pre 75.8 ± 11.5; Post 76.7 ± 10.0 kg), although no group × time (ANOVA, p = 0.138) or percentage change difference between‐groups (unpaired t‐test, p = 0.146) occurred.
TABLE 1.
Energy and macronutrient intake during the 15 weeks resistance training intervention period with supplementation of collagen peptides (CP, n = 18) or placebo (PLA, n = 20)
| Nutrition measure | CP | PLA | Unpaired t‐test |
|---|---|---|---|
| Energy intake | |||
| Absolute habitual diet (kcal.d−1) | 2471 ± 411 | 2250 ± 808 | p = 0.302 |
| Relative habitual diet (kcal.kg−1.d−1) | 34.10 ± 7.21 | 29.96 ± 11.94 | p = 0.210 |
| Absolute total diet (kcal.d−1) | 2533 ± 411 | 2311 ± 808 | p = 0.302 |
| Relative total diet (kcal.kg−1.d−1) | 34.95 ± 7.29 | 30. 77 ± 11.99 | p = 0.209 |
| Protein intake | |||
| Absolute habitual diet (g.d−1) | 119 ± 34 | 108 ± 36 | p = 0.352 |
| Relative habitual diet (g.kg−1.d−1) | 1.64 ± 0.53 | 1.44 ± 0.56 | p = 0.258 |
| Absolute total diet (g.d−1) | 134 ± 34 | 108 ± 36 | p = 0.031* |
| Relative total diet (g.kg−1.d−1) | 1.85 ± 0.54 | 1.44 ± 0.56 | p = 0.027* |
| Carbohydrate intake | |||
| Absolute habitual diet (g.d−1) | 275 ± 71 | 266 ± 97 | p = 0.736 |
| Relative habitual diet (g.kg−1.d−1) | 3.82 ± 1.15 | 3.52 ± 1.34 | p = 0.467 |
| Absolute total diet (g.d−1) | 275 ± 71 | 281 ± 97 | p = 0.843 |
| Relative total diet (g.kg−1.d−1) | 3.82 ± 1.15 | 3.72 ± 1.34 | p = 0.807 |
| Fat intake | |||
| Absolute habitual diet (g.d−1) | 97 ± 29 | 81 ± 42 | p = 0.178 |
| Relative habitual diet (g.kg−1.d−1) | 1.33 ± 0.43 | 1.08 ± 0.62 | p = 0.159 |
Note: Data are mean ± SD, and p values are displayed for unpaired t‐tests with statistical significance denoted by: *p < 0.05.
2.3. Strength
There were within‐group increases (paired t‐test, [all] p < 0.001) from pre to post in both groups for knee extension MVT (CP +21.4%; PLA +21.7%), knee extension 1RM (CP +29.7%; PLA +28.0%), and knee flexion MVT (CP +23.3%; PLA +21.3%; Table 2). However, there were no group × time (ANOVA, 0.596 ≤ p ≤ 0.743; Table 2; Figure 1A–C) or between‐group differences in percentage change for these maximum strength tests (unpaired t‐test, 0.703 ≤ p ≤ 0.929; 0.03 ≤ Effect size [ES] ≤ 0.13 [all] “Trivial”; Figure 1A–C).
TABLE 2.
Maximum strength (knee extension and flexion), knee extension isometric explosive strength (absolute and relative to maximum voluntary torque [MVT]), and knee extension isometric evoked twitch torque properties pre and post 15 weeks of lower‐body resistance training, with collagen peptide (CP, n = 19) or placebo (PLA, n = 20) supplementation
| CP | PLA | ANOVA Interaction | Change (%) | Unpaired t‐test | Between‐group effect size | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | (g × t) | CP | PLA | |||
| Maximum strength | |||||||||
| Knee extension MVT (Nm) | 223 ± 47 | 269 ± 53*** | 234 ± 41 | 283 ± 49*** | p = 0.596 | 21.4 ± 7.8 | 21.7 ± 10.7 | p = 0.929 | 0.03 “Trivial” |
| Knee flexion MVT (Nm) | 105 ± 25 | 127 ± 24*** | 109 ± 30 | 129 ± 27*** | p = 0.671 | 23.3 ± 15.4 | 21.3 ± 17.6 | p = 0.703 | 0.12 “Trivial” |
| Knee extension 1RM (kg) | 50.2 ± 11.2 | 65.0 ± 14.8*** | 53.8 ± 11.7 | 68.0 ± 12.7*** | p = 0.743 | 29.7 ± 10.8 | 28.0 ± 15.3 | p = 0.684 | 0.13 “Trivial” |
| Absolute explosive strength (Nm) | |||||||||
| T50 | 49 ± 17 | 53 ± 17 | 59 ± 19 | 56 ± 17 | p = 0.054 | 11.2 ± 30.7 | −2.2 ± 20.8 | p = 0.117 | 0.51 “Moderate” |
| T100 | 134 ± 26 | 141 ± 29* | 137 ± 23 | 141 ± 19 | p = 0.423 | 5.3 ± 9.5 | 3.9 ± 9.1 | p = 0.636 | 0.15 “Trivial” |
| T150 | 175 ± 35 | 190 ± 38*** | 180 ± 29 | 193 ± 27*** | p = 0.474 | 9.3 ± 7.8 | 7.8 ± 7.2 | p = 0.550 | 0.19 “Trivial” |
| Relative explosive strength (%MVT) | |||||||||
| T50 | 22.9 ± 8.0 | 19.9 ± 5.4* | 25.0 ± 7.1 | 19.8 ± 5.6*** | p = 0.177 | −7.8 ± 27.4 | −19.5 ± 15.9 | p = 0.110 | 0.53 “Moderate” |
| T100 | 60.9 ± 8.6 | 52.7 ± 6.8*** | 59.0 ± 5.5 | 50.4 ± 5.8*** | p = 0.862 | −13.1 ± 8.7 | −14.4 ± 7.3 | p = 0.616 | 0.16 “Trivial” |
| T150 | 78.7 ± 5.4 | 70.8 ± 5.8*** | 77.4 ± 4.6 | 68.7 ± 4.9*** | p = 0.545 | −9.9 ± 5.9 | −11.2 ± 4.6 | p = 0.457 | 0.24 “Small” |
| Evoked twitch properties | |||||||||
| Twitch peak T (Nm) | 48 ± 12 | 58 ± 14*** | 51 ± 12 | 56 ± 14** | p = 0.076 | 22.3 ± 13.6 | 12.3 ± 15.4 | p = 0.038 | 0.69 “Moderate” |
| Twitch TPT (ms) | 80 ± 7 | 81 ± 5 | 79 ± 10 | 84 ± 10** | p = 0.077 | 1.5 ± 6.2 | 5.9 ± 8.7 | p = 0.080 | 0.58 “Moderate” |
Note: Data are mean ± SD. Within‐group pre to post effects were determined from paired t‐tests and are denoted by: *p < 0.05, **p < 0.01 or ***p < 0.001. p values are shown for ANOVA group × time (g × t) interactions and unpaired t‐tests on percentage change data. Effect size is calculated between‐groups using percentage change data.
Abbreviations: 1RM, one repetition maximum. Twitch Peak T, peak torque produced during twitch contractions. Twitch TPT, time from torque onset to peak twitch torque.
FIGURE 1.
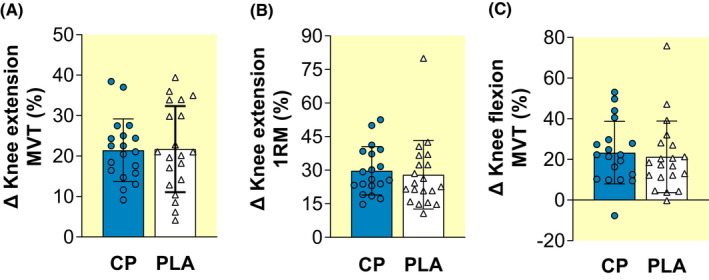
Pre to post percentage changes (Δ) in knee extension isometric maximum voluntary torque (MVT; A), knee extension one‐repetition maximum (1RM; B), and knee flexion MVT (C) after 15 weeks of lower‐body resistance training, with collagen peptide (CP, n = 19) or placebo (PLA, n = 20) supplementation. Data are mean ± SD with plots displaying individual participant data. No significant between‐group differences were detected from percentage change data (unpaired t‐test: 0.703 ≤ p ≤ 0.929) for MVT or 1RM.
Increases (pre to post) in absolute knee extension explosive torque at 100 ms (T100) and 150 ms (T150) after contraction onset occurred within the CP group (paired t‐test, 0.001 ≤ p ≤ 0.019) and at T150 within the PLA group (p < 0.001; Table 2). However, no within‐group increases were observed for absolute torque 50 ms after contraction onset (T50) in the CP group (paired t‐test, p = 0.200) or T50 and T100 in the PLA group (0.098 ≤ p ≤ 0.139). No group × time effects were observed for absolute T50, T100, or T150 (ANOVA, 0.054 ≤ p ≤ 0.474; Figure 2A). Pre to post decreases in knee extension relative explosive strength (i.e., %MVT) were observed across T50, T100, and T150 within both the CP (paired t‐test, 0.001 ≤ p ≤ 0.035) and PLA ([all] p < 0.001) groups (Table 2). No group × time effects were observed for relative T50, T100, or T150 (ANOVA, 0.177 ≤ p ≤ 0.862; Figure 2B).
FIGURE 2.
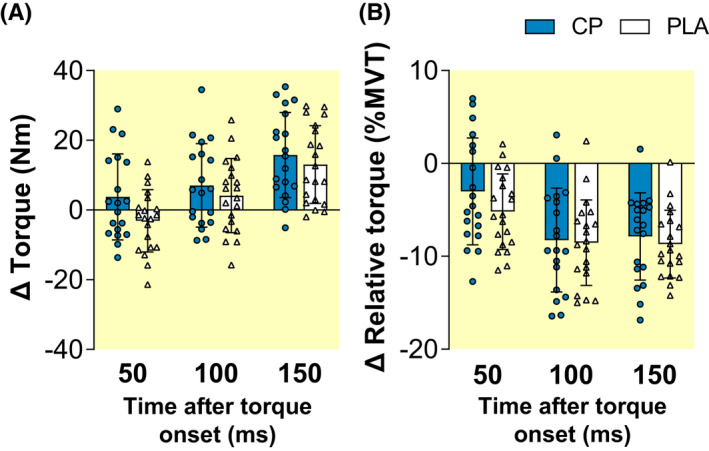
Pre to post absolute changes (Δ) in knee extension isometric explosive strength ([A] absolute torque and [B] torque expressed relative to maximum voluntary torque [MVT]) at 50 ms intervals after torque onset, after 15 weeks of lower‐body resistance training, with collagen peptide (CP, n = 19) or placebo (PLA, n = 20) Supplementation. Data are mean ± SD with plots displaying individual participant data. No significant between‐group differences occurred for absolute torque (ANOVA group × time: 0.054 ≤ p ≤ 0.474) or torque expressed relative to MVT (ANOVA group × time: 0.177 ≤ p ≤ 0.862).
2.4. Muscle volume
Pre to post volume of all three muscles (quadriceps, hamstrings and gluteus maximus) and their constituents, as well as total trained muscle volume, increased within both groups (paired t‐test: [all] p < 0.001; Table 3). There was a significantly greater increase in vastus medialis (VM) volume (determined by ANOVA group × time; p = 0.031; Table 3) as well as a tendency for greater increases in the volume of the whole quadriceps (p = 0.071; Table 3) for CP compared to PLA. Group × time interactions did not occur for vastus lateralis (VL), vastus intermedius (VI), and rectus femoris (RF) volume (0.102 ≤ p ≤ 0.446; Table 3). Greater percentage increases in the volume of the whole quadriceps (+15.2 vs. +10.3%; unpaired t‐test, p = 0.032; ES = 0.71 “Moderate” Figure 3A) and VM (+15.6 vs. +9.7%; p = 0.017; ES = 0.80 “Large”) were observed for CP compared to PLA and there were also tendencies for greater increases in VL (+17.6% vs. +12.8%; p = 0.069; ES = 0.60 “Moderate”; Table 3) and VI (+13.0% vs. +8.5%; p = 0.055; ES = 0.63 “Moderate”) volume for CP compared to PLA (Table 3). However, this was not the case for the RF volume (+14.1% vs. +9.9%; unpaired t‐test, p = 0.183; ES = 0.43 “Small”).
TABLE 3.
Quadriceps, hamstrings, gluteus maximus, and total, muscle volume pre‐ and post‐15 weeks of lower‐body resistance training, with collagen peptide (CP, n = 19) or placebo (PLA, n = 20) supplementation
| CP | PLA | ANOVA interaction | Change (%) | Unpaired t‐test | Between‐group effect size | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | (g × t) | CP | PLA | |||
| Quadriceps (cm3) | |||||||||
| RF | 258 ± 70 | 292 ± 76*** | 275 ± 54 | 303 ± 69*** | p = 0.446 | 14.1 ± 11.0 | 9.9 ± 8.0 | p = 0.183 | 0.43 “Small” |
| VM | 473 ± 124 | 539 ± 113*** | 491 ± 77 | 538 ± 81*** | p = 0.031 | 15.6 ± 8.9 | 9.7 ± 5.4 | p = 0.017 | 0.80 “Large” |
| VI | 576 ± 126 | 645 ± 117*** | 619 ± 123 | 669 ± 119*** | p = 0.102 | 13.0 ± 8.5 | 8.5 ± 5.5 | p = 0.055 | 0.63 “Moderate” |
| VL | 617 ± 144 | 720 ± 155*** | 648 ± 114 | 729 ± 130*** | p = 0.154 | 17.6 ± 9.0 | 12.8 ± 7.0 | p = 0.069 | 0.60 “Moderate” |
| Q | 1924 ± 440 | 2196 ± 434*** | 2033 ± 318 | 2238 ± 339*** | p = 0.071 | 15.2 ± 8.2 | 10.3 ± 5.2 | p = 0.032 | 0.71 “Moderate” |
| Hamstrings (cm3) | |||||||||
| BFsh | 103 ± 32 | 121 ± 33*** | 108 ± 34 | 123 ± 33*** | p = 0.180 | 19.0 ± 11.2 | 15.4 ± 11.2 | p = 0.323 | 0.32 “Small” |
| BFlh | 187 ± 53 | 212 ± 57*** | 190 ± 41 | 210 ± 43*** | p = 0.220 | 14.4 ± 7.3 | 11.1 ± 8.0 | p = 0.186 | 0.43 “Small” |
| SM | 229 ± 51 | 249 ± 49*** | 228 ± 48 | 243 ± 48*** | p = 0.216 | 9.3 ± 6.0 | 6.8 ± 6.0 | p = 0.199 | 0.42 “Small” |
| ST | 187 ± 50 | 235 ± 60*** | 204 ± 48 | 245 ± 51*** | p = 0.190 | 26.7 ± 9.6 | 21.7 ± 9.5 | p = 0.111 | 0.52 “Moderate” |
| H | 706 ± 161 | 817 ± 174*** | 730 ± 137 | 821 ± 144*** | p = 0.122 | 16.5 ± 6.8 | 12.8 ± 6.1 | p = 0.089 | 0.56 “Moderate” |
| Gluteus maximus (cm3) | 894 ± 213 | 1035 ± 227*** | 973 ± 164 | 1097 ± 181*** | p = 0.346 | 16.6 ± 7.7 | 12.9 ± 5.4 | p = 0.091 | 0.55 “Moderate” |
| Total of the trained muscles (cm3) | 3523 ± 785 | 4048 ± 804*** | 3737 ± 582 | 4156 ± 618*** | p = 0.078 | 15.7 ± 7.2 | 11.4 ± 3.9 | p = 0.026 | 0.74 “Moderate” |
Note: Data are mean ± SD, Within‐group pre to post effects were determined from paired t‐tests and are denoted by: ***p < 0.001. p values are shown for ANOVA group × time (g × t) interactions and unpaired t‐tests on percentage change data. Effect size is calculated between‐groups using percentage change data. Total lower body muscle volume is the sum of quadriceps, hamstrings, and gluteus maximus muscle volumes.
Abbreviations: BFlh, biceps femoris long head; BFsh, biceps femoris short head; H, overall hamstrings; Q, overall quadriceps; RF, rectus femoris; SM, semimembranosus; ST, semitendinosus; VI, vastus intermedius; VL, vastus lateralis; VM, vastus medialis.
FIGURE 3.
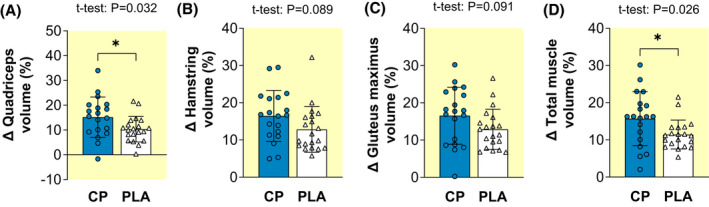
Pre to post percentage changes (Δ) in quadriceps (A), hamstrings (B), and gluteus maximus (C) and total muscle (Σ quadriceps, hamstrings, and gluteus maximus; (D) volume after 15 weeks of lower‐body resistance training, with collagen peptide (CP, n = 19) or placebo (PLA, n = 20) supplementation. Data are mean ± SD with plots displaying individual participant data. Unpaired t‐test p values are displayed when statistical tendencies (0.05 ≤ p ≤ 0.10) or significant (p < 0.05, denoted by *) differences between‐groups occurred.
There were no group × time interactions for whole hamstring volume (ANOVA, p = 0.122), any constituent hamstring muscle (0.180 ≤ p ≤ 0.220), or gluteus maximus volume (p = 0.346; Table 3). However, percentage increases in volume tended to be greater for CP than PLA for the gluteus maximus (+16.6% vs. +12.9%; unpaired t‐test, p = 0.091; ES = 0.55 “Moderate”; Figure 3C) and whole hamstrings (+16.5 vs. +12.8%; unpaired t‐test, p = 0.089; ES = 0.56 “Moderate”; Figure 3B) but this was not the case for any individual hamstring muscle (0.111 ≤ p ≤ 0.323; Table 3). Total volume of the trained muscles showed a tendency for a group × time interaction (ANOVA, p = 0.078; Table 3) and corresponding percentage change data showed greater increases for CP than PLA (+15.7% vs. +11.4%; unpaired t‐test, p = 0.026; ES = 0.74 “Moderate”; Figure 3D).
2.5. Muscle architecture and specific tension
θ P increased pre to post in both groups for the whole quadriceps and RF (paired‐test, 0.001 ≤ p ≤ 0.010), but in the VM (p = 0.001) only after CP supplementation (Table 4). In the VM there was a tendency for greater increases in θ P for CP vs. PLA (ANOVA: group × time, p = 0.060), but not any other constituent muscle (0.356 ≤ p ≤ 0.765) or the whole quadriceps (p = 0.112). Percentage increases in θ P were greater for CP than PLA within the VM (+16.8% vs. +5.8%; unpaired t‐test, p = 0.046; ES = 0.66 “Moderate”), but not the whole quadriceps (+10.5% vs. +5.7%; p = 0.111; ES = 0.52 “Moderate”) or any other individual quadriceps muscle (0.217 ≤ p ≤ 0.625; 0.16 ≤ ES≤0.40 “Trivial” to “Small”; Table 4). L F of the whole quadriceps and each constituent muscle was unchanged within both groups from pre to post (paired t‐test, 0.119 ≤ p ≤ 0.787), with no group × time interactions (ANOVA, 0.259 ≤ p ≤ 0.987; Table 4) and similar percentage changes of both groups (unpaired t‐test, 0.230 ≤ p ≤ 0.953; 0.02 ≤ ES≤0.39 “Trivial” to “Small”).
TABLE 4.
Muscle architecture (pennation angle [θP], fascicle length [L F ]) pre‐ and post‐15 weeks of lower‐body resistance training, with collagen peptide (CP, n = 19) or placebo (PLA, n = 20) supplementation
| CP | PLA | ANOVA Interaction | Change (%) | Unpaired | Between‐group effect size | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | (g × t) | CP | PLA | t‐test | ||
| Pennation angle (°) | |||||||||
| RF | 14.5 ± 1.7 | 16.2 ± 1.8** | 15.6 ± 2.2 | 16.8 ± 1.8** | p = 0.410 | 12.9 ± 15.0 | 9.1 ± 14.1 | p = 0.420 | 0.26 “Small” |
| VM | 22.7 ± 3.5 | 26.3 ± 4.7** | 25.0 ± 2.7 | 26.3 ± 3.6 | p = 0.060 | 16.8 ± 18.7 | 5.8 ± 14.3 | p = 0.046 | 0.66 “Moderate” |
| VI | 13.5 ± 2.3 | 14.5 ± 1.7 | 14.4 ± 2.6 | 14.7 ± 2.7 | p = 0.356 | 10.1 ± 20.8 | 3.3 ± 12.1 | p = 0.217 | 0.40 “Small” |
| VL | 17.7 ± 3.2 | 18.4 ± 3.9 | 17.9 ± 3.0 | 18.9 ± 3.0 | p = 0.765 | 4.0 ± 16.7 | 6.6 ± 16.3 | p = 0.625 | 0.16 “Trivial” |
| Q | 17.1 ± 1.4 | 18.8 ± 1.8*** | 18.2 ± 1.6 | 19.2 ± 1.4* | p = 0.112 | 10.5 ± 10.0 | 5.7 ± 8.4 | p = 0.111 | 0.52 “Moderate” |
| Fascicle length (mm) | |||||||||
| RF | 96.5 ± 5.8 | 93.3 ± 7.2 | 94.8 ± 12.2 | 92.9 ± 9.8 | p = 0.641 | −3.1 ± 8.8 | −1.4 ± 8.9 | p = 0.552 | 0.19 “Trivial” |
| VM | 109.1 ± 9.0 | 107.2 ± 9.2 | 103.8 ± 11.8 | 107.2 ± 13.6 | p = 0.298 | −1.0 ± 12.7 | 4.5 ± 17.7 | p = 0.269 | 0.36 “Small” |
| VI | 93.2 ± 8.5 | 92.4 ± 12.4 | 89.6 ± 11.4 | 90.6 ± 10.3 | p = 0.588 | −0.6 ± 12.1 | 1.8 ± 10.3 | p = 0.509 | 0.21 “Small” |
| VL | 105.7 ± 10.6 | 104.6 ± 11.0 | 105.4 ± 11.2 | 104.3 ± 10.6 | p = 0.987 | −0.8 ± 8.1 | −0.6 ± 9.1 | p = 0.953 | 0.02 “Trivial” |
| Q | 101.6 ± 4.6 | 99.4 ± 7.0 | 98.4 ± 8.1 | 98.8 ± 6.9 | p = 0.259 | −1.7 ± 5.5 | 0.6 ± 6.4 | p = 0.230 | 0.39 “Small” |
Note: Data are mean ± SD, Within‐group pre to post effects were determined from paired t‐tests and are denoted by: *p < 0.05, **p < 0.01 or ***p < 0.001. p values are shown for ANOVA group × time (g × t) interactions and unpaired t‐tests on percentage change data. Effect size is calculated between‐groups using percentage change data.
Abbreviations: Q, overall quadriceps; RF, rectus femoris; VI, vastus intermedius; VL, vastus lateralis; VM, vastus medialis.
Quadriceps maximum voluntary‐specific tension showed within‐group increases for PLA (+8.4 ± 13.0%; pre 29.8 ± 3.9, post 32.2 ± 4.7 N.cm2; paired t‐test, p = 0.008) but not CP (+3.9 ± 10.1%; pre 31.2 ± 4.5, post 32.3 ± 5.0 N.cm2; paired t‐test, p = 0.134), but there was no group × time interaction (ANOVA, p = 0.254) or a between‐group difference in percentage changes (unpaired t‐test, p = 0.239; ES = 0.38 “Small”).
2.6. Evoked twitch contractions
Twitch peak torque (PT) increased pre to post within both groups (paired t‐test, 0.001 ≤ p ≤ 0.005; Table 2), with a tendency for greater increases in Twitch PT following CP vs. PLA (ANOVA: group × time interaction, p = 0.076; Table 2) and greater Twitch PT percentage changes for CP vs. PLA (+22.3% vs. +12.3%; unpaired t‐test, p = 0.038; ES = 0.69 “Moderate”; Figure 4A). Twitch time to peak torque (TPT) increased (i.e., was longer/slower) after PLA (paired t‐test, p = 0.008) but not CP (p = 0.414; Table 2). Twitch TPT also showed tendencies toward both greater increases (i.e., a greater slowing) after PLA than CP (ANOVA group × time, p = 0.077; Table 2) and greater percentage changes after PLA than CP (unpaired t‐test, p = 0.080; ES = 0.58 “Moderate” Figure 4B).
FIGURE 4.
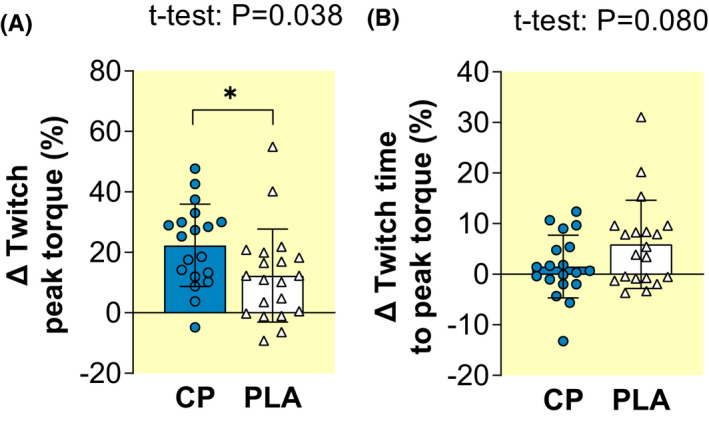
Pre to post percentage changes (Δ) in knee extension evoked twitch contraction peak torque (A) and time to peak torque (B) after 15 weeks of lower‐body resistance training, with collagen peptide (CP, n = 19) or placebo (PLA, n = 20) supplementation. Data are mean ± SD with plots displaying individual participant data. Unpaired t‐test p values are displayed when statistical tendencies (0.05 ≤ p ≤ 0.10) or significant (p < 0.05, denoted by *) differences between‐groups occurred.
3. DISCUSSION
The aim of this study was to determine if collagen peptide supplementation enhances functional, structural, mechanical, and contractile adaptations to resistance training in healthy young men. While CP supplementation did not enhance the strength gains of the knee extensors or flexors following 15 weeks of lower body RT, the primary outcomes of this study, a cluster of consistent effects were observed for greater adaptations in the underpinning skeletal muscle morphology and contractile properties compared to PLA. Specifically, with CP supplementation there were: greater percentage changes in total volume of the trained muscles, quadriceps volume, and evoked peak twitch torque; as well as a greater increase in VM muscle volume (group × time interaction), which was concomitant with a greater percentage change in θ P of this muscle that showed the greatest hypertrophic benefit from CP supplementation (61% greater muscle growth than PLA).
3.1. Maximum strength
Knee extension strength after 15 weeks of RT, improved by ~22% for MVT and ~29% for 1RM in both groups, which is consistent with previous knee extension‐focused RT studies (e.g., MVT +16% after 14 weeks 12 ; 1RM +26.9% after 12 weeks 42 ). The current study assessed a range of strength measures (isometric knee extension and flexion, and knee extension 1RM) in duplicate (i.e., twice before, and twice after the RT) to minimize measurement error and maximize the veracity of the measured changes. However, CP supplementation did not augment the strength gains in this study compared to PLA. Given the greater percentage gains in quadriceps muscle volume and evoked contractile torque after RT with CP versus PLA supplementation, it may be surprising that CP did not also augment strength changes. Strength gains after RT are known to be multifactorial and due to an extensive range of neural and morphological adaptations, including but not limited to changes in: agonist and antagonist neural activation, muscle fiber composition, muscle architecture, muscle size, as well as specific tension. 43 , 44 In this study, specific tension only increased pre–post intervention within the PLA group but without any between‐group differences. It is possible that the similarity of one or more of the previously mentioned factors obscured/diluted the expected influence of greater hypertrophy on strength gains. The strength gains after RT have been reported to be larger 14 for CP versus PLA supplementation, larger for some strength tasks but not others (grip strength, but not leg press 15 ) or similar. 38 , 39 Therefore, current evidence is ambiguous regarding the efficacy of CP supplementation to enhance strength gains following RT, although over time (i.e., a longer RT period) greater hypertrophy would be expected to benefit improvements in function.
3.2. Muscle growth
After RT, the CP group showed larger percentage increases in quadriceps volume (i.e., the primary muscle group targeted by the RT program employed in this study, +15.2% vs. +10.3%; p = 0.032) and the total volume of all the trained muscles (+15.7% vs. +11.4%; p = 0.026) with moderate effect sizes (0.71–0.74). There were also tendencies for greater growth of the muscles that were secondary targets of the training (i.e., hamstrings +16.5% vs. +12.8% [p = 0.089]; and gluteus maximus +16.6% vs. +12.9% [p = 0.091]). However, there were only tendencies (0.05 ≤ p ≤ 0.10) toward group × time interactions for whole quadriceps and total muscle volume. VM volume did show a significant group × time interaction, likely because this muscle showed a large effect size (0.80) and the greatest hypertrophic benefit of CP vs. PLA supplementation (+15.6% vs. +9.7%), which equates to 61% greater growth of this individual muscle with CP. The reason there were not larger between‐group statistical differences in muscle volume measures appears to be due to high within‐group variability (i.e., SDs equating to a between‐participant coefficient of variation of ~50%), which we attribute to extensive variability in individual hypertrophic adaptations, a well‐documented phenomenon. 45 Extensive individual variability appears a more plausible explanation than measurement error given the excellent between‐day reliability of our muscle volume measures (within‐participant coefficient of variation, CVW: 0.9% to 1.4%). Moreover, the rate of quadriceps hypertrophy (~1% increase in volume per training week) in the CP group was larger than any previous study we are aware of that has employed high‐resolution measures of muscle volume (e.g., MRI or computerized tomography: 0.62%–0.74% increase in volume per week 12 , 46 , 47 ; similar to our PLA group 0.66%/week) and resulted in superior muscle growth of +48% (quadriceps) and +38% (total trained muscles) for CP compared to placebo. Nonetheless, despite the greater percentage gains in muscle volume we found for the quadriceps and total of the trained muscles, the lack of consistent group × time interaction effects, places a statistical query over the efficacy of CP supplementation to augment muscle growth.
Our current muscle hypertrophy results are consistent with prior studies that reported greater gains in fat‐free mass (via dual‐energy x‐ray absorptiometry) for CP vs. PLA during RT. 14 , 15 , 38 However, to the best of our knowledge, the current study is the first to carefully assess muscle size across multiple muscles/muscle groups with high‐resolution MRI before and after RT with CP vs. PLA supplementation. It is possible that the effect of CP supplementation on the RT‐induced hypertrophy was due to the provision of additional amino acids, given that total dietary protein intake (i.e., including supplementation) was higher in CP than in PLA. However, the observed muscle growth effects of the current study appear out of proportion with the small amount of protein supplemented (15 g/day) in addition to the habitual protein intakes already greater than the recommended dietary allowance (>1.4 g/kg/day for both groups), and the relatively modest documented effect of supplementary protein on RT‐induced muscle growth. 48
Previous RT studies have suggested supplemental protein sources with sub‐optimal amino acid compositions (e.g., soy protein) are ineffective when consumed post‐exercise. 49 , 50 Thus, based on the small amount of protein and very low essential amino acid content of the CP supplementation within the current study, one might have expected CP supplementation to provide no increase in muscle growth compared to placebo. Therefore, it seems unlikely that a portion, if any of the muscle growth effects were due to amino acid provision per se. Rather the advantages we have observed may have been due to the bioactive properties of the collagen peptides. Collagen peptides have been shown to induce myoblast differentiation and myotube hypertrophy in mouse skeletal muscle cells in‐vitro, by activating the mTOR signaling pathway. 29 Bioactive collagen peptides may also upregulate the synthesis of ECM proteins 51 that in turn appear to have the capacity to regulate myogenesis and muscle regeneration (i.e., remodeling 35 ) via stimulation of muscle satellite stem cells 36 , 37 integral to significant hypertrophy. One conceivable explanation for the greater increase in muscle size, but not muscle strength, with CP supplementation is a disproportionate increase in muscle ECM and connective tissue content compared to PLA. 38 However, this may seem unlikely due to the consistent connective tissue fraction that usually exists within skeletal muscle for individuals with a wide range of muscle sizes and RT experience. 52 Nonetheless, the documented stimulatory effects of CP may explain the observed amplification of RT‐induced muscle growth with CP supplementation.
3.3. Muscle architecture and specific tension
The current study found a greater percentage change in θ P for one of the constituent quadriceps muscles (VM, +16.8 ± 18.7% vs. +5.8 ± 14.3%) after CP vs. PLA supplementation. This greater increase in θ P of the VM for CP vs. PLA is consistent with this muscle also showing the strongest hypertrophic effect (+61%) of CP vs. PLA, as increases in muscle size and θ P are widely regarded as simultaneous processes. These combined observations of augmented adaptations of separate, but related, phenomena (greater hypertrophy and θ P) for CP vs. PLA within the same muscle, indicate a systematic effect of CP supplementation on muscle remodeling after RT. θ P for the whole quadriceps increased in both groups after RT, but no group × time effects were observed. There were no within‐group changes or between‐group differences for L F , a finding that is in accordance with the majority of the short to moderate duration RT literature that has found no change in L F . 53 , 54
Quadriceps‐specific tension, a measure of force per unit area of muscle, showed a within‐group increase after RT only in the PLA group (+8.4%) and not CP (+3.9%), however, no between‐group differences were detected. There are very few reports of careful in vivo‐specific tension measurements with RT, although a substantial 17% increase after 9 weeks of RT has been reported, 46 and we recently found a much more modest 9% higher specific tension for long‐term RT vs. untrained individuals. 11
3.4. Evoked twitch contraction properties
The maximum evoked twitch response of the quadriceps, that reflects the intrinsic contractile properties (i.e., without voluntary nervous system input), showed increases in twitch peak torque for both groups. Indicating an improved contractile capacity for torque production after RT which is consistent with the increased contractile material (muscle volume) of both groups. Interestingly, however, CP supplementation produced a greater percentage increase in peak twitch torque than PLA (+22.3% vs. +12.3%). This between‐group difference is consistent with greater hypertrophy after CP supplementation and is a further independent measure supporting greater morphological adaptation for CP vs. PLA. Time to peak twitch torque, an index of the contractile speed of the muscle independent of torque amplitude, was longer (i.e., slower) after RT for the PLA group but was unchanged for the CP group. We have previously observed slowing of evoked contractile properties following longitudinal RT 10 and in long‐term resistance trained (≥3 years) compared to untrained individuals. 55 The slowing of evoked muscle contractile properties is likely due to decreased expression of myosin heavy‐chain type IIX fibers after RT. 56 , 57 Therefore, the tendency for less slowing of the muscle after CP supplementation could conceivably indicate type IIX fiber retention or greater type II muscle fiber hypertrophy in this group although greater type II fiber hypertrophy was not observed in a prior study that combined RT and CP supplementation. 38
3.5. Explosive strength
Using detailed analysis of explosive torque at multiple time points, we found 15 weeks of RT only enhanced the late phase of explosive contraction (150 ms) for PLA, and the middle and late phases (100 and 150 ms) for CP, with no changes in the early phase of contraction (50 ms) for either group. The finding that conventional heavy, slow RT (i.e., not specifically explosive) does not enhance early phase explosive strength/rate of force development is in accordance with previous isometric 10 , 57 and dynamic 63 , 64 RT studies. Relative explosive strength (i.e., relative to MVT) decreased in both CP and PLA groups with no between‐group differences detected. This reduction in relative explosive strength, while perhaps counter‐intuitive, is also consistent with many reports in the literature involving conventional, but not explosive, RT. 10 , 58 , 59 , 60 Specific to CP supplementation, no between‐group effects were observed for absolute or relative explosive strength after RT. In contrast, a recent report utilizing a less well‐controlled isometric squatting exercise reported combined hydrolyzed collagen and vitamin C supplementation to preserve explosive strength following a brief (3 weeks) power‐focused RT intervention 61 compared to PLA. Based on the findings of the current investigation it does not appear that CP supplementation enhances/maintains explosive strength following conventional RT beyond RT alone.
3.6. Limitations
This is the first careful assessment of the effect of CP supplementation on muscle growth after RT (i.e., with high‐resolution MRI) that has found a number of distinct significant effects, but also a number of statistical tendencies. Future studies would benefit from greater statistical power by determining sample sizes based on the muscle growth data in this report and/or by using more responsive interventions (e.g., upper body or longer duration RT). Furthermore, the current study examined tissue level adaptations and did not evaluate cellular or ECM adaptations to RT. Thus, the more detailed physiological mechanisms for the amplified hypertrophic response we observed remain elusive. It is also apparent that the optimal dose, frequency, and composition, in terms of amino acids and molecular weight, of CP supplementation has yet to be defined and hence the current findings may be specific to the exact supplement used in this study. Finally, the comparison of CP to other protein sources and controlling/matching total amino acid intake would be of value.
4. CONCLUSIONS
In conclusion, CP supplementation produced a cluster of consistent effects indicating greater skeletal muscle remodeling after RT compared to PLA. Notably, CP supplementation amplified: whole quadriceps and total muscle volume increases induced by RT; enhanced architectural remodeling (angle of fiber pennation) of the individual constituent quadriceps muscle (VM) that experienced the greatest hypertrophic benefit from CP supplementation; and augmented the intrinsic contractile capacity for evoked torque production (twitch peak torque). The lack of significant group × time interaction effects for many of these variables does place a statistical doubt over the efficacy of CP supplementation to amplify muscle morphological and contractile adaptations after RT. Furthermore, the beneficial effects of CP supplementation did not translate to greater strength gains. Overall, CP supplementation appears to augment the structural remodeling and contractile properties of skeletal muscle after RT and therefore may be a useful adjunct to RT for participants wishing to amplify the hypertrophic response. While there were no measurable effects of CP on the increases in voluntary function/strength in this study, over time greater hypertrophy would be expected to benefit improvements in function.
5. MATERIALS AND METHODS
5.1. Participants
Fifty‐two young, healthy men, aged 18–40 years, with no prior lower‐body injuries, no recent history (>18 months) of RT, a low‐moderate level of recreational physical activity, but not involved in regular systematic physical training, volunteered for this study and gave written informed consent. Ethical approval was granted by the Loughborough University Ethics Review Sub‐Committee (Reference Number: R19‐P030). Participants unwilling to consume an animal‐based protein supplement or with low habitual protein intake (i.e., <0.8 g/kg; based on 24 h food intake recall), were excluded from the study.
Thirteen participants did not complete the intervention period because of: changes in personal circumstance (×4); lack of enjoyment (×3); non‐attendance (×2); discomfort of one or both knees (×4). Consequently, thirty‐nine participants completed the study and no participants reported any side effects of either supplement. Familiarization session measurements were used to pair‐match participants for isometric knee extension maximum strength of their dominant leg and body mass, before being randomly assigned to either CP or PLA supplementation (CP group n = 19; PLA group: n = 20). An a priori statistical power calculation revealed a sample size of 18 per group based on β = 0.80 and α = 0.05 and estimated percentage changes (mean ± SD) after the training for isometric strength of 18.0 ± 8.5% (PLA) and 26.0 ± 8.5% (CP). These estimated changes for PLA were derived from several previous studies 10 , 62 , 63 and a strength gain that was more than twice as large after collagen supplementation than PLA in one previous study. 14
5.2. Overview
This study was a single‐center randomized double‐blind control trial with a per‐protocol analysis. Initially, participants completed a familiarization session involving all tasks to be performed in the main measurement sessions. Two duplicate main measurement sessions involving unilateral assessments of the dominant leg (determined from leg preference for kicking a ball) were completed both pre (sessions 3–5 days apart and prior to the first training session) and post (2–3 days after the last training session and 2–3 days later) 15 weeks of RT. Torque (all isometric contractions) or load were recorded during isometric knee extension maximum voluntary contractions (MVCs), explosive voluntary contractions, evoked twitch contractions; knee extension 1RM; and isometric knee flexion MVCs (completed in this order). Hamstring surface electromyography (EMG) was also recorded during isometric knee extension MVCs for the purposes of calculating quadriceps maximum voluntary‐specific tension. Prior to main measurement sessions participants were required to avoid caffeine consumption for 6 h. The time of main measurement sessions was standardized within participants pre‐ to post‐training, and all sessions for the whole cohort were completed between 12:00 and 20:00 h.
Musculoskeletal imaging of the dominant leg was also conducted pre‐ (5–7 days prior to the first training session) and post‐intervention (3–5 days after the final training session). T1 weighted MRI scans were used to assess volume of the quadriceps, hamstrings, and gluteus maximus muscles, as well as patellar tendon moment arm. Muscle architecture measurements (L F and θ P) were obtained with 2‐D ultrasonography scans of each constituent quadriceps muscle. Scan time was standardized within participants, who were instructed to eat and drink normally and avoid strenuous exercise and alcohol consumption for 36 h before all measurement sessions.
During the 15‐week intervention period participants consumed either CP or PLA supplements once per day (i.e., 105 daily doses) and completed a lower‐body RT program that primarily targeted the quadriceps, and secondarily the hamstrings and gluteus maximus three times per week (i.e., 45 sessions). Participants were instructed to maintain their habitual physical activity and usual diet throughout the study.
5.3. Habitual physical activity and dietary assessment
The International Physical Activity Questionnaire (IPAQ, short format) was administered at two time points: ~3 weeks prior to the intervention; and week 15 of the intervention. Participants also completed a 3‐day (2 weekdays, 1 weekend day) weighed food and fluid intake diary at two time points during the study (weeks 3 and 13 of the intervention) to estimate habitual energy and macronutrient intake. Food and fluid intake diaries were analyzed by one trained investigator with manual entry of any product not available in the software (Version 5.0, Nutritics). One participant in the CP group was excluded from the food and fluid intake diary analysis after failing to accurately complete their food diary, hence the CP group data for this measure has an n = 18.
5.4. Resistance training
During the 15‐week intervention period, all participants completed the same periodized and supervised lower‐body RT program three times per week (typically Monday, Wednesday, and Friday; Supplementary material 1). Each session involved completing 2–4 sets of each of three isoinertial (constant load) exercises, in the following order: unilateral knee extension (TechnoGym, Selection Leg Extension; Bracknell, UK) starting with the dominant leg and alternating sets between legs; bilateral knee flexion (Life Fitness, Seated Leg Curl SL40; Cambridgeshire, UK); bilateral leg press (Watson Gym Equipment, 45° Leg Press, Somerset, UK). A 2 min recovery period separated sets with the same leg/legs. The number of sets progressively increased during the first 6 weeks of the intervention period, with four sets being completed for all three exercises by week 7. The load and number of repetitions per set was varied according to an undulating periodized program between ~12 RM and ~6RM. Participants were required to perform each repetition through a specified range of motion (Supplementary material 2; approximate knee joint angles for each exercise, from the start to the end of the concentric phase, were: knee extension 85° to 140°, knee flexion 175° to 95°, leg press 85° to 150°; 180° = anatomical reference position) taking two seconds to lift and two seconds to lower the weight. The load for each exercise was increased if the participant could do all the specified repetitions on the penultimate set of an exercise, a common approach to achieve progressive overload. 57 , 63 , 64 , 65 Bilateral warm‐up sets were completed before the unilateral knee extension and bilateral knee flexion exercises with the same number of repetitions and with ~50% of the load to be lifted in the first main set.
5.5. Supplementation
Participants consumed 15 g of collagen peptides (CP; 10 g of ‘BodyBalance’ and 5 g of ‘Tendoforte’, Gelita AG) or placebo (PLA; 15 g of maltodextrin) dissolved in water once daily throughout the 15‐week intervention period. The CP supplement dose of 15 g of collagen peptides per day was the same as previous studies that reported a positive benefit of CP vs. PLA for functional and/or musculoskeletal outcomes after RT. 14 , 15 , 38 The CP supplement was the combination of two subtly different products with an identical amino acid composition (the amino acid composition of the overall CP supplement is shown in Supplementary Material 3) but different mean molecular weights (Bodybalance ~3.5 kD; Tendoforte ~2.0 kD) which may maximize absorption and bioactive properties. 66 CP and PLA supplements were provided pre‐packaged in identical sachets (except supplement code) to blind both participants and investigators. Prior to consumption, sachets were emptied into opaque shaker bottles, 350 ml of water added, and shaken vigorously (prepared by an investigator on training days and by the participant on non‐training days). On training days supplements were consumed immediately after RT sessions. On non‐RT days participants were instructed to consume their supplement mid‐afternoon (mid‐way between lunch and dinner). In all cases, participants were regularly instructed not to eat or drink anything, except water within 1 hour of supplement consumption. At the start of each training week, participants were provided with 4 supplement sachets for non‐RT days in the week ahead, returned empty sachets from the previous week, and signed that they had consumed each sachet on a non‐RT day.
5.6. Isometric dynamometer, torque, and EMG recordings
All isometric knee extension and flexion contractions were conducted while participants were seated on a rigid custom‐made isometric dynamometer (see figure 6B of 67 ) adjusted for consistent individualized positioning. Knee and hip joint angles during isometric knee extension and flexion contractions were 104° and 126° (180° = anatomical reference position). Tight restraints were fastened across the waist, shoulders, and lower thigh to prevent extraneous bodily movement during contraction. Knee extension and flexion force were measured with a low noise S‐beam strain gauge (Force Logic, Swallowfield, UK) mounted to the dynamometer perpendicular to, and either posterior (knee extension) or anterior (knee flexion) to the tibia. The strain gauge was positioned and secured at ~15% of tibial length above the medial malleolus with a reinforced canvas webbing ankle strap (35 mm width).
The strain gauge signal was amplified (×370) and sampled at 2000 Hz with an external A/D converter (Micro 1401; CED Ltd., Cambridge, UK) and recorded with Spike 2 software (CED Ltd., Cambridge, UK). Prior to analysis, force data were low‐pass filtered at 500 Hz with a fourth‐order zero‐lag Butterworth filter, 67 gravity corrected via subtraction of baseline force, and then multiplied by lever length (the distance from the knee joint space to the center of the ankle strap) to calculate torque.
To facilitate calculation of quadriceps maximum voluntary‐specific tension, surface EMG was recorded from the lateral and medial hamstrings using a wireless EMG system (Trigno; Delsys Inc., Boston, MA). The skin beneath the sensors was prepared (shaved, abraded, and cleansed with 70% ethanol) before individual single differential (bipolar configuration) Trigno Standard EMG sensors were attached (using adhesive interfaces) over the lateral and medial hamstrings at 45% of thigh length above the popliteal fossa. Sensors were placed parallel to the presumed orientation of the underlying fibers. EMG signals were amplified at source (×300; 20‐ to 450‐Hz bandwidth) before further amplification (overall effective gain, ×909). EMG signals were sampled at 2000 Hz via the same A/D converter and computer software as the force signal, enabling data synchronization. Prior to analysis EMG signals were corrected for the 48‐ms delay inherent to the Trigno EMG system.
5.7. Isometric knee extension and flexion maximum voluntary contractions
Following incremental isometric knee extension or flexion warm‐up contractions of the dominant leg (~5 s contractions at 50% [×3], 75% [×3], and 90% [×1] of perceived maximum). Participants completed 3–4 MVCs. Prior to MVCs, participants received instruction to “push as hard as possible” (extension) or “pull as hard as possible” (flexion) for 3–5 s, with ≥30 s rest between each effort. Real‐time biofeedback was provided, via a computer monitor positioned in front of the participant displaying the torque‐time curve, with the greatest knee extension torque obtained within that session marked with a horizontal cursor. The greatest instantaneous torque achieved during any MVC within the measurement session was defined as knee extensor or flexor isometric MVT. Hamstrings root‐mean‐square (RMS) EMG amplitude of both sensors was measured during a 500‐ms epoch surrounding both knee extension and flexion MVT (250 ms on either side). RMS EMG amplitude of each sensor at knee extension MVT was normalized to RMS amplitude at knee flexion MVT before averaging the two sensors to produce an overall normalized antagonist EMG.
5.8. Isometric knee extension explosive voluntary contractions
Ten explosive voluntary contractions were completed by each participant. Instructions to perform each contraction “as fast and hard as possible” for ∼1 s, in order to exceed 80% MVT were provided. Between contractions participants had ≥15 s of rest. Peak rate of torque development (10 ms epoch) was displayed to provide participants with real‐time biofeedback. Changes in the baseline torque signal >0.34 N·m (i.e., in terms of pre‐tension or countermovement) in the 300 ms prior to contraction onset were discarded. The three explosive contractions with the highest torque at 100 ms were analyzed in detail with torque measured at 50, 100, and 150 ms from contraction onset (T50, T100, and T150), before taking a mean torque at each time point across the three contractions. Relative explosive torque (i.e., expressed as a proportion of MVT) was also calculated. Torque onset determination for explosive contractions was performed offline by one trained investigator using a systematic manual visual identification approach 68 , 69 considered to be more valid than automated methods. 69 Briefly, torque was initially viewed on an x‐axis scale of 300 ms prior to the contraction and y‐axis scales of 0.68 N·m before further magnification to determine the instant of the last peak or trough before the torque signal deflected away from the envelope of the baseline signal.
5.9. Isometric knee extension evoked twitch contractions
The dominant leg femoral nerve was electrically stimulated with a constant‐current variable‐voltage stimulator (DS7AH; Digitimer, Welwyn Garden City, UK) via a cathode (1‐cm diameter; Electro‐Medical Supplies, Wantage, UK) over the femoral nerve within the femoral triangle and an anode (7 × 10‐cm carbon rubber electrode; Electro‐Medical Supplies, Wantage, UK) over the greater trochanter. Cathode location within the femoral triangle was established by delivering single electrical impulses (square wave pulses of 0.2‐ms duration, 30–50 mA current intensity) to identify the position eliciting the greatest sub‐maximum twitch response. Thereafter, the cathode was taped in place and progressive 20 mA increases in current intensity were made until peak twitch torque plateaued (15 s apart). Finally, three further twitch contractions were evoked (15 s apart) at a supramaximal current (≥50% above the plateau level) to ensure maximal stimulation. During offline analysis, twitch torque onset during these maximal twitch contractions was identified using a systematic manual approach previously described. 10 Twitch Peak T and Twitch TPT were averaged across the three maximal twitch contractions.
5.10. One repetition maximum lifting strength
Unilateral knee extension 1RM was assessed using the same machine as during training, with participant's hips firmly strapped down and holding the handles either side of the seat. They completed incremental warm‐up sets (50% previous 1RM × 8 repetitions; 75% previous 1RM × 5 repetitions; 85% previous 1RM × 2 repetitions; with one min between sets) and then a series of single lifts separated by two min recovery. The first single lift was performed at the previous 1RM load, if successful the load was increased by 2.5 to 5.0 kg for the next lift. Typically, 3 to 4 attempts were required to establish 1RM to the nearest 2.5 kg, with additional attempts performed if necessary.
The knee extension machine was instrumented with a potentiometer (Rotary wirewound potentiometer, RS Components Ltd, Corby, UK), to standardize the range of movement required for a successful lift. The potentiometer signal was sampled at 2000 Hz using an external A/D converter (Micro 1401; CED Ltd., Cambridge, UK), displayed on a monitor in front of the participant (with the target extended position highlighted), and recorded using Spike 2 software. Each lift started from a standardized machine lever arm position (knee joint angle of 86 ± 3°; 180° = full extension). A successful lift was defined by moving the lever arm and attached load to an extended position (knee joint angle of 142 ± 4°).
5.11. Muscle architecture
L F and θ P of each constituent quadriceps muscle (VM, VL, VI, and RF) was measured using B‐mode ultrasonography (EUB‐8500, Hitachi Medical Systems UK Ltd, Northamptonshire, UK) using a 92 mm long, 10 MHz linear‐array transducer (EUP‐L53L) coated with water‐soluble transmission gel. Participants sat at rest in the isometric dynamometer, and imaging locations were marked. Images were recorded at two locations per muscle, at specific percentages of thigh length above the knee joint space: 30% and 50% (VI), 50% and 70% (VL); 20% and 40% (VM); 55% and 75% (RF) and in all cases at 50% of superficial medio‐lateral muscle width. Ultrasound recording locations were largely adopted from prior research incorporating ultrasound measurements at multiple sites along the length of each constituent quadriceps femoris muscle. 54 The transducer was positioned parallel to the thigh's long axis and perpendicular to the skin, so that the deep and superficial aponeuroses and the trajectory of several fascicles was clearly identifiable with minimal pressure applied to the dermal surface. Ultrasound video output was recorded by a PC (via an S‐video to USB video converter [VCAP302, ClimaxDigital, Spennymoor, UK] and Spike video recorder software [CED Ltd., Cambridge, UK]). Images were analyzed by one trained investigator using public domain software (Image J, v1.48, National Institutes of Health, Bethesda, USA).
θ P was measured as the angle of insertion of the muscle fascicles into the deep aponeurosis. L F was measured as the length of the fascicular path between the insertions into the superficial and deep aponeuroses (Supplementary material 4A). When the fascicular path extended beyond the field of view of the acquired image, the remaining portion of the fascicle was estimated by linear extrapolation, 70 and the visible proportion of L F was documented. When collapsed across quadricep measurement sites and then time points (pre and post) for each participant, a mean of 85.0 ± 3.2% of the measured L F was visible within ultrasound images across participants. Measurements (L F and θ P) of two individual fascicles at each measurement site were averaged, before also averaging across the two sites of each constituent muscle. Overall quadriceps L F and θ P were calculated as the mean of the constituent muscle values. Within‐participant between‐test session reliability (i.e., within‐participant coefficient of variation, CVW; [SD/mean] × 100) of L F and θ P assessed via ultrasound has been reported to range from 2.3% to 9.8% and 2.1% to 13.5%. 71 , 72
5.12. MRI scan and analysis
5.12.1. Muscle volume
Participants sat quietly for 15 min prior to MRI scans (3.0 T Discovery MR750w, GE Healthcare, Chicago, IL, USA). T1‐weighted axial and coronal plane MRI scans of the dominant thigh (between the anterior superior iliac spine to the lateral tibial condyle) were conducted with participants supine, arms folded across the chest, hip, and knee joints extended, and the ankle at ~90°. Using a receiver 8‐channel whole‐body coil, axial (time of repetition/time to echo 600/8.35 ms; image matrix 512 × 512; field of view 260 mm × 260 mm; pixel size 0.508 mm × 0.508 mm; slice thickness 5 mm; inter‐slice gap 0 mm) and coronal (time of repetition/time to echo 600/8.53 ms; image matrix 256 × 256, field of view 450 mm × 450 mm, pixel size 1.76 mm × 1.76 mm, slice thickness 5 mm, inter‐slice gap 0 mm) images were acquired in two overlapping blocks for both axial and coronal scans. Synchronization of coronal and axial plane scans permitted the objective alignment of blocks during analysis (Horos, version 3.3.6, www.thehorosproject.org).
Muscle volume of the quadriceps (RF, VL, VM, and VI), hamstrings (semitendinosus [ST], semimembranosus [SM], biceps femoris long head [BFlh] and short head [BFsh]) and gluteus maximus were analyzed (Supplementary material 4B,C). Specifically, one trained investigator segmented every third image (i.e., every 15 mm) along each muscle, from the first distal image in which that muscle appeared until the muscle was no longer visible. Additionally, based on visual inspection the seven contiguous slices around the suspected maximum anatomical cross‐sectional area (ACSAMAX; i.e., three either side of the suspected ACSAMAX) were assessed to ensure the single image with the highest ACSA (i.e., ACSAMAX) of that muscle was measured (and this was confirmed by inspection of the anatomical cross‐sectional area‐muscle length curve). The following number of images were segmented along the length of each muscle (mean of pre‐ and post‐image number before calculating a mean across all participants): RF, 27 (range 24–30); VM, 27 (range 24–30); VI, 29 (range 27–33); VL, 29 (range 25–31); BFsh, 23 (range 19–25); BFlh, 24 (range 20–29); SM, 23 (range 19–27); ST, 26 (range 23–32); gluteus maximus, 24 (range 20–27). The volume of each muscle was calculated as the area under the anatomical cross‐sectional area‐muscle length curve following cubic spline interpolation (1000 point; GraphPad Prism 8; GraphPad Software). Constituent muscle volumes were summed to determine whole quadriceps and hamstrings volumes, and total muscle volume was the sum of quadriceps, hamstrings, and gluteus maximus volumes. In a pilot trial of nine healthy young men with repeated MRI scans (5–14 days apart) using the above protocol and analysis we found within‐participant between‐day reliability (i.e., within‐participant coefficient of variation, CVW; [SD/mean] × 100) of quadriceps, hamstrings, gluteus maximus, and total muscle volume to be 0.9%, 1.0%, 1.4%, and 0.9%, respectively.
5.12.2. Moment arm
A lower extremity knee coil was used to acquire contiguous sagittal images of the knee joint between the medial and lateral tibial condyles (time of repetition/time to echo 600/9.83 ms; image matrix 512 × 512, field of view 160 mm × 160 mm, pixel size 0.313 mm × 0.313 mm, slice thickness 2 mm, inter‐slice gap 0 mm). A wedge‐shaped foam block was positioned under the knee to obtain the most flexed knee‐joint angle (143 ± 5°, 180° = full extension) possible within the constraints of the knee coil. Moment arm was measured at as flexed position as possible to minimize the correction required to derive an estimated moment arm at the knee joint angle of knee extension MVCs based on the measured moment arm. Patellar tendon moment arm length was measured from sagittal plane images as the perpendicular distance from the line of action of the patellar tendon to the tibio‐femoral contact point in the image closest to 40% of the distance between the most lateral (0%) and medial (100%) points of the femoral condyles as determined from synchronized axial images. 73 , 74
5.13. Calculation of specific tension
Specific tension was calculated as: maximum voluntary muscle force ([knee extensor MVT + estimated antagonist torque during knee extensor MVT]/estimated patellar tendon moment arm during knee extension MVC) divided by quadriceps effective physiological cross‐sectional‐area. 11 Antagonist torque during knee extensor MVT was estimated by multiplying overall normalized antagonist EMG amplitude by knee flexor MVT (assuming a linear relationship between EMG amplitude and knee flexion torque). Effective physiological cross‐sectional area (EFFPCSA) for each constituent muscle of the quadriceps was calculated as: muscle volume (from MRI scanning) divided by L F multiplied by the cosine of θ P (from ultrasonography). L F and θ P were mean values from the two ultrasound measurement sites on each individual constituent muscle of the quadriceps. Overall quadriceps EFFPCSA was the sum of the individual constituent muscle EFFPCSAs. Patellar tendon moment arm length measured from the MR image at a relatively extended knee‐joint angle (143 ± 5°), was corrected to the knee‐joint angle during knee extension MVC (104 ± 2°) using previously published data fitted with a quadratic function. 75
5.14. Statistical analysis
All data were anonymized prior to analysis to blind investigators to participant identity and supplement group allocation. Shapiro–Wilk tests were conducted to assess data normality at pre and post within each group. The majority of variables were normally distributed at pre‐test (86% or 30 out of 35 variables) when both groups were pooled. Therefore, parametric statistics were used to provide a consistent statistical approach across variables. Two‐way repeated measures ANOVA was used to assess group × time effects. Within‐group changes over time were also assessed with paired t tests. In addition to two‐way ANOVAs, unpaired t‐tests were also conducted on percentage change data to explore between‐group differences. 76 Percentage changes for each dependent variable were calculated as the change divided by the pre‐intervention value multiplied by 100. Between‐group effect size (ES) was calculated using percentage change data as previously described 77 and classified as: <0.20 = “trivial,” 0.20–0.49 = “small,” 0.50–0.80 = “moderate,” or >0.80 = “large”. Statistical significance was defined as p < 0.05 and statistical analysis was completed using SPSS version 27. All data are presented as mean ± standard deviation unless otherwise indicated.
FUNDING INFORMATION
This study was funded by the Collagen Research Institute (CRI); Kiel, Germany.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest and that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
PATIENT CONSENT STATEMENT
All participants in this study gave written informed consent prior to their participation.
Supporting information
Supplementary material 1
Supplementary material 2
Supplementary material 3
Supplementary material 4
ACKNOWLEDGMENTS
The authors would like to thank Jim Muddimer and Mike Myatt for their technical expertise, and radiographer Julie Thompson.
Balshaw TG, Funnell MP, McDermott E, et al. The effect of specific bioactive collagen peptides on function and muscle remodeling during human resistance training. Acta Physiol. 2023;237:e13903. doi: 10.1111/apha.13903
REFERENCES
- 1. Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84(2):649‐698. [DOI] [PubMed] [Google Scholar]
- 2. Narici MV, Maganaris CN. Plasticity of the muscle‐tendon complex with disuse and aging. Exerc Sport Sci Rev. 2007;35(3):126‐134. [DOI] [PubMed] [Google Scholar]
- 3. Tam CS, Chaudhuri R, Hutchison AT, Samocha‐Bonet D, Heilbronn LK. Skeletal muscle extracellular matrix remodeling after short‐term overfeeding in healthy humans. Metabolis. 2017;67:26‐30. [DOI] [PubMed] [Google Scholar]
- 4. Tack C, Shorthouse F, Kass L. The physiological mechanisms of effect of vitamins and amino acids on tendon and muscle healing: a systematic review. Int J Sport Nutr Exerc Metab. 2018;28(3):294‐311. [DOI] [PubMed] [Google Scholar]
- 5. McGuigan MR, Wright GA, Fleck SJ. Strength training for athletes: does it really help sports performance? Int J Sport Physiol. 2012;7(1):2‐5. [DOI] [PubMed] [Google Scholar]
- 6. Lum D, Barbosa TM. Effects of strength training on olympic time‐based sport performance: a systematic review and meta‐analysis of randomized controlled trials. Int J Sport Physiol. 2019;14(10):1‐13. [DOI] [PubMed] [Google Scholar]
- 7. Lauersen JB, Andersen TE, Andersen LB. Strength training as superior, dose‐dependent and safe prevention of acute and overuse sports injuries: a systematic review, qualitative analysis and meta‐analysis. Brit J Sport Med. 2018;52(24):1557‐1563. [DOI] [PubMed] [Google Scholar]
- 8. Kristensen J, Franklyn‐Miller A. Resistance training in musculoskeletal rehabilitation: a systematic review. Brit J Sport Med. 2011;46(10):719‐726. [DOI] [PubMed] [Google Scholar]
- 9. Chodzko‐Zajko WJ, Proctor DN, Singh MAF, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sport Exer. 2009;41(7):1510‐1530. [DOI] [PubMed] [Google Scholar]
- 10. Balshaw TG, Massey GJ, Maden‐Wilkinson TM, Tillin NA, Folland JP. Training specific functional, neural and hypertrophic adaptations to explosive‐ vs. sustained‐contraction strength training. J Appl Physiol. 2016;120(11):1364‐1373. [DOI] [PubMed] [Google Scholar]
- 11. Maden‐Wilkinson TM, Balshaw TG, Massey G, Folland JP. What makes long‐term resistance‐trained individuals so strong? A comparison of skeletal muscle morphology, architecture, and joint mechanics. J Appl Physiol. 2019;128(4):1000‐1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aagaard P, Andersen JL, Dyhre‐Poulsen P, et al. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol. 2001;534(2):613‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Massey GJ, Balshaw TG, Maden‐Wilkinson TM, Tillin NA, Folland JP. Tendinous tissue adaptation to explosive‐ vs. sustained‐contraction strength training. Front Physiol. 2018;9:1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zdzieblik D, Oesser S, Baumstark MW, Gollhofer A, König D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: a randomised controlled trial. Br J Nutrition. 2015;114(8):1237‐1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jendricke P, Centner C, Zdzieblik D, Gollhofer A, König D. Specific collagen peptides in combination with resistance training improve body composition and regional muscle strength in premenopausal women: a randomized controlled trial. Nutrients. 2019;11(4):892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Phillips SM, Tipton KD, van Loon LJC, Verdijk LB, Paddon‐Jones D, Close GL. Exceptional body composition changes attributed to collagen peptide supplementation and resistance training in older sarcopenic men. Br J Nutrition. 2016;116(3):569‐570. [DOI] [PubMed] [Google Scholar]
- 17. Kitts D, Weiler K. Bioactive proteins and peptides from food sources. applications of bioprocesses used in isolation and recovery. Curr Pharm Design. 2003;9(16):1309‐1323. [DOI] [PubMed] [Google Scholar]
- 18. Apostolopoulos V, Bojarska J, Chai T–T, et al. A global review on short peptides: frontiers and perspectives. Molecules. 2021;26(2):430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chakrabarti S, Guha S, Majumder K. Food‐derived bioactive peptides in human health: challenges and opportunities. Nutrients. 2018;10(11):1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rutherfurd‐Markwick KJ. Food proteins as a source of bioactive peptides with diverse functions. Brit J Nutr. 2012;108(S2):S149‐S157. [DOI] [PubMed] [Google Scholar]
- 21. Lundquist P, Artursson P. Oral absorption of peptides and nanoparticles across the human intestine: opportunities, limitations and studies in human tissues. Adv Drug Deliver Rev. 2016;106:256‐276. [DOI] [PubMed] [Google Scholar]
- 22. Ohara H, Matsumoto H, Ito K, Iwai K, Sato K. Comparison of quantity and structures of hydroxyproline‐containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J Agr Food Chem. 2007;55(4):1532‐1535. [DOI] [PubMed] [Google Scholar]
- 23. Shigemura Y, Suzuki A, Kurokawa M, Sato Y, Sato K. Changes in composition and content of food‐derived peptide in human blood after daily ingestion of collagen hydrolysate for 4 weeks: Hyp‐peptide in blood after daily ingestion of collagen hydrolysate. J Sci Food Agr. 2017;98(5):1944‐1950. [DOI] [PubMed] [Google Scholar]
- 24. Fu Y, Therkildsen M, Aluko RE, Lametsch R. Exploration of collagen recovered from animal by‐products as a precursor of bioactive peptides: successes and challenges. Crit Rev Food Sci. 2018;59(13):2011‐2027. [DOI] [PubMed] [Google Scholar]
- 25. McAlindon TE, Nuite M, Krishnan N, et al. Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: a pilot randomized controlled trial. Osteoarthr Cartilage. 2011;19(4):399‐405. [DOI] [PubMed] [Google Scholar]
- 26. Shaw G, Lee‐Barthel A, Ross ML, Wang B, Baar K. Vitamin C–enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am J Clin Nutrition. 2016;105(1):136‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Watanabe‐Kamiyama M, Shimizu M, Kamiyama S, et al. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J Agr Food Chem. 2010;58(2):835‐841. [DOI] [PubMed] [Google Scholar]
- 28. Guillerminet F, Fabien‐Soulé V, Even PC, et al. Hydrolyzed collagen improves bone status and prevents bone loss in ovariectomized C3H/HeN mice. Osteoporos Int. 2011;23(7):1909‐1919. [DOI] [PubMed] [Google Scholar]
- 29. Kitakaze T, Sakamoto T, Kitano T, et al. The collagen derived dipeptide hydroxyprolyl‐glycine promotes C2C12 myoblast differentiation and myotube hypertrophy. Biochem Bioph Res Commun. 2016;478(3):1292‐1297. [DOI] [PubMed] [Google Scholar]
- 30. Centner C, Jerger S, Mallard A, et al. Supplementation of specific collagen peptides following high‐load resistance exercise upregulates gene expression in pathways involved in skeletal muscle signal transduction. Front Physiol. 2022;13:838004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Balius R, Álvarez G, Baró F, et al. A 3‐arm randomized trial for achilles tendinopathy: eccentric training, eccentric training plus a dietary supplement containing mucopolysaccharides, or passive stretching plus a dietary supplement containing mucopolysaccharides. Curr Ther Res Clin Exp. 2016;78:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baar K. Stress relaxation and targeted nutrition to treat patellar tendinopathy. Int J Sport Nutr Exerc Metab. 2019;29:453‐457. [DOI] [PubMed] [Google Scholar]
- 33. Praet SFE, Purdam CR, Welvaert M, et al. Oral supplementation of specific collagen peptides combined with calf‐strengthening exercises enhances function and reduces pain in achilles tendinopathy patients. Nutrients. 2019;11(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Siebert H‐C, Burg‐Roderfeld M, Eckert T, et al. Interaction of the α2A domain of integrin with small collagen fragments. Protein Cell. 2010;1(4):393‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840(8):2506‐2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Urciuolo A, Quarta M, Morbidoni V, et al. Collagen VI regulates satellite cell self‐renewal and muscle regeneration. Nat Commun. 2013;4(1):1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. 2013;12(1):75‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kirmse M, Oertzen‐Hagemann V, de Marées M, Bloch W, Platen P. Prolonged collagen peptide supplementation and resistance exercise training affects body composition in recreationally active men. Nutrients. 2019;11(5):1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zdzieblik D, Jendricke P, Oesser S, Gollhofer A, König D. The influence of specific bioactive collagen peptides on body composition and muscle strength in middle‐aged, untrained men: a randomized controlled trial. Int J Environ Res Public Health. 2021;18(9):4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cruz‐Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bloch RJ, Gonzalez‐Serratos H. Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev. 2003;31(2):73‐78. [DOI] [PubMed] [Google Scholar]
- 42. Wakahara T, Ema R, Miyamoto N, Kawakami Y. Inter‐ and intramuscular differences in training‐induced hypertrophy of the quadriceps femoris: association with muscle activation during the first training session. Clin Physiol Funct Imaging. 2015;37(4):405‐412. [DOI] [PubMed] [Google Scholar]
- 43. Folland JP, Williams AG. The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med. 2007;37(2):145‐168. [DOI] [PubMed] [Google Scholar]
- 44. Balshaw TG, Massey GJ, Maden‐Wilkinson TM, et al. Changes in agonist neural drive, hypertrophy and pre‐training strength all contribute to the individual strength gains after resistance training. Eur J Appl Physiol. 2017;117(4):631‐640. [DOI] [PubMed] [Google Scholar]
- 45. Petrella JK, Kim J‐S, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell‐mediated myonuclear addition: a cluster analysis. J Appl Physiol. 2008;104(6):1736‐1742. [DOI] [PubMed] [Google Scholar]
- 46. Erskine RM, Jones DA, Williams AG, Stewart CE, Degens H. Inter‐individual variability in the adaptation of human muscle specific tension to progressive resistance training. Eur J Appl Physiol. 2010;110(6):1117‐1125. [DOI] [PubMed] [Google Scholar]
- 47. Mitchell CJ, Churchward‐Venne TA, West DWD, et al. Resistance exercise load does not determine training‐mediated hypertrophic gains in young men. J Appl Physiol. 2012;113(1):71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morton RW, Murphy KT, McKellar SR, et al. A systematic review, meta‐analysis and meta‐regression of the effect of protein supplementation on resistance training‐induced gains in muscle mass and strength in healthy adults. Brit J Sport Med. 2018;52(6):376‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hartman JW, Tang JE, Wilkinson SB, et al. Consumption of fat‐free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutrition. 2007;86(2):373‐381. [DOI] [PubMed] [Google Scholar]
- 50. Volek JS, Volk BM, Gómez AL, et al. Whey protein supplementation during resistance training augments lean body mass. J Am Coll Nutr. 2013;32(2):122‐135. [DOI] [PubMed] [Google Scholar]
- 51. Sivaraman K, Muthukumar K, Shanthi C. A potential bioactive peptide candidate for biomaterial and tissue engineering applications. Life Sci. 2019;226:140‐148. [DOI] [PubMed] [Google Scholar]
- 52. MacDougall JD, Sale DG, Alway SE, Sutton JR. Muscle fiber number in biceps brachii in bodybuilders and control subjects. J Appl Physiol. 1984;57(5):1399‐1403. [DOI] [PubMed] [Google Scholar]
- 53. Blazevich AJ, Cannavan D, Coleman DR, Horne S. Influence of concentric and eccentric resistance training on architectural adaptation in human quadriceps muscles. J Appl Physiol. 2007;103(5):1565‐1575. [DOI] [PubMed] [Google Scholar]
- 54. Ema R, Wakahara T, Miyamoto N, Kanehisa H, Kawakami Y. Inhomogeneous architectural changes of the quadriceps femoris induced by resistance training. Eur J Appl Physiol. 2013;113(11):2691‐2703. [DOI] [PubMed] [Google Scholar]
- 55. Balshaw TG, Massey GJ, Maden‐Wilkinson TM, Lanza MB, Folland JP. Effect of long‐term maximum strength training on explosive strength, neural, and contractile properties. Scand J Med Sci Spor. 2022;32(4):685‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Adams GR, Hather BM, Baldwin KM, Dudley GA. Skeletal muscle myosin heavy chain composition and resistance training. J Appl Physiol. 1993;74(2):911‐915. [DOI] [PubMed] [Google Scholar]
- 57. Andersen JL, Aagaard P. Myosin heavy chain IIX overshoot in human skeletal muscle. Muscle Nerve. 2000;23(7):1095‐1104. [DOI] [PubMed] [Google Scholar]
- 58. Buckthorpe M, Erskine RM, Fletcher G, Folland JP. Task‐specific neural adaptations to isoinertial resistance training. Scand J Med Sci Spor. 2015;25(5):640‐649. [DOI] [PubMed] [Google Scholar]
- 59. Erskine RM, Fletcher G, Folland JP. The contribution of muscle hypertrophy to strength changes following resistance training. Eur J Appl Physiol. 2014;114(6):1239‐1249. [DOI] [PubMed] [Google Scholar]
- 60. Tillin NA, Folland JP. Maximal and explosive strength training elicit distinct neuromuscular adaptations, specific to the training stimulus. Eur J Appl Physiol. 2014;114(2):365‐374. [DOI] [PubMed] [Google Scholar]
- 61. Lis DM, Jordan M, Lipuma T, Smith T, Schaal K, Baar K. Collagen and vitamin C supplementation increases lower limb rate of force development. Int J Sport Nutr Exerc Metab. 2021;32:65‐73. [DOI] [PubMed] [Google Scholar]
- 62. Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre‐Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol. 2002;93(4):1318‐1326. [DOI] [PubMed] [Google Scholar]
- 63. Erskine RM, Fletcher G, Hanson B, Folland JP. Whey protein does not enhance the adaptations to Elbow Flexor Resistance Training. Med Sci Sports Exerc. 2012;44(9):1791‐1800. [DOI] [PubMed] [Google Scholar]
- 64. Häkkinen K, Pakarinen A, Kraemer WJ, Newton RU, Alen M. Basal concentrations and acute responses of serum hormones andstrength development during heavy resistance training in middle‐aged andelderly men and women. J Gerontol A Biol Sci Med Sci. 2000;55(2):B95‐B105. [DOI] [PubMed] [Google Scholar]
- 65. Trezise J, Blazevich AJ. Anatomical and neuromuscular determinants of strength change in previously untrained men following heavy strength training. Front Physiol. 2019;10:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hong H, Fan H, Chalamaiah M, Wu J. Preparation of low‐molecular‐weight, collagen hydrolysates (peptides): current progress, challenges, and future perspectives. Food Chem. 2019;301:125222. [DOI] [PubMed] [Google Scholar]
- 67. Maffiuletti NA, Aagaard P, Blazevich AJ, Folland J, Tillin N, Duchateau J. Rate of force development: physiological and methodological considerations. Eur J Appl Physiol. 2016;116(6):1091‐1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tillin NA, Jimenez‐Reyes P, Pain MTG, Folland JP. Neuromuscular performance of explosive power athletes versus untrained individuals. Med Sci Sports Exerc. 2010;42(4):781‐790. [DOI] [PubMed] [Google Scholar]
- 69. Tillin NA, Pain MTG, Folland JP. Identification of contraction onset during explosive contractions. Response to Thompson et al. “Consistency of rapid muscle force characteristics: influence of muscle contraction onset detection methodology” [J Electromyogr Kinesiol 2012;22(6):893–900]. J Electromyogr Kinesiol. 2013;23(4):991‐994. [DOI] [PubMed] [Google Scholar]
- 70. Massey G, Evangelidis P, Folland J. Influence of contractile force on the architecture and morphology of the quadriceps femoris. Exp Physiol. 2015;100(11):1342‐1351. [DOI] [PubMed] [Google Scholar]
- 71. Kwah LK, Pinto RZ, Diong J, Herbert RD. Reliability and validity of ultrasound measurements of muscle fascicle length and pennation in humans: a systematic review. J Appl Physiol. 2013;114(6):761‐769. [DOI] [PubMed] [Google Scholar]
- 72. Hooren BV, Teratsias P, Hodson‐Tole EF. Ultrasound imaging to assess skeletal muscle architecture during movements: a systematic review of methods, reliability, and challenges. J Appl Physiol. 2020;128(4):978‐999. [DOI] [PubMed] [Google Scholar]
- 73. Blazevich AJ, Coleman DR, Horne S, Cannavan D. Anatomical predictors of maximum isometric and concentric knee extensor moment. Eur J Appl Physiol. 2009;105(6):869‐878. [DOI] [PubMed] [Google Scholar]
- 74. Seynnes OR, Erskine RM, Maganaris CN, et al. Training‐induced changes in structural and mechanical properties of the patellar tendon are related to muscle hypertrophy but not to strength gains. J Appl Physiol. 2009;107(2):523‐530. [DOI] [PubMed] [Google Scholar]
- 75. Kellis E, Baltzopoulos V. In vivo determination of the patella tendon and hamstrings moment arms in adult males using videofluoroscopy during submaximal knee extension and flexion. Clin Biomech. 1999;14(2):118‐124. [DOI] [PubMed] [Google Scholar]
- 76. Andrade ITD, Gualano B, Hevia‐Larrain V, et al. Leucine supplementation has no further effect on training‐induced muscle adaptations. Med Sci Sports Exerc. 2020;52(8):1809‐1814. [DOI] [PubMed] [Google Scholar]
- 77. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t‐tests and ANOVAs. Front Psychol. 2013;4:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Supplementary material 3
Supplementary material 4


