Abstract
Spicy foods and chili peppers contain the primary ingredient capsaicin, which has potential health benefits. However, their efficacy in some health outcomes is also fiercely disputed, and some side effects have been confirmed. To assess the quality and strength of the associations between spicy food and chili pepper consumption and different health outcomes. An umbrella review is performed in humans. Eleven systematic reviews and meta‐analyses with a total of 27 findings are identified. The health effect of consuming spicy food and chili peppers is unclear. Furthermore, the characteristics and context of different world regions and populations should be carefully considered. Direct correlations exist in esophageal cancer, gastric cancer, and gallbladder cancer. However, negative connections are reported in metabolism, mortality, and cardiovascular disease. Dose–response analysis reveals a significant nonlinear relationship between gastric cancer risk and capsaicin intake. The consumption of spicy foods and chili peppers is typically safe. However, high‐quality proof is available to confirm this conclusion.
Keywords: capsicum consumption, health, meta‐analysis, spicy food consumption, uvmbrella review
To assess the quality and strength of the associations between spicy food and chili pepper consumption and different health outcomes. An umbrella review is performed in humans. Direct correlations exist in esophageal cancer, gastric cancer, and gallbladder cancer. However, negative connections are reported in metabolism, mortality, and cardiovascular disease.
1. Introduction
Chili pepper (Solanaceae) has been valued since ancient times as a food crop,[ 1 ] seasoning ingredient, natural dyestuff,[ 2 ] and traditional herbal medicine[ 3 ] and originated from approximately 9000 to 7000 B.P.[ 4 ] In Mexico. Spicy food, the flavor and aroma of food created by the use of chili peppers, not only offers a significant hedonic input in daily living but also has been linked to health benefits. Chili peppers contain a diverse mix of phytochemicals, such as capsaicin, dihydrocapsaicin, total phenolic compounds, and antioxidant activity,[ 5 ] which have broad therapeutic antimicrobial,[ 6 ] antiseptic,[ 3 ] antihypertensive,[ 7 ] antioxidant,[ 8 ] antiobesity,[ 9 ] antihyperglycemic,[ 10 ] and analgesic properties,[ 11 ] protect against cardiometabolic vascular diseases,[ 12 ] and have anticancer in vitro and animal studies.[ 13 ] Capsaicin, available in various topical applications, is FDA‐approved for the treatment of diabetic peripheral neuropathic pain (DPNP)[ 14 ] and neuropathic pain.[ 15 ] Remain aware that excessive dosages of capsaicin may have unanticipated physiological effects due to the widespread expression of transient receptor potential vanilloid 1 (TRPV1) receptors.[ 16 ]
To date, the benefits and risks of spicy food and chili pepper consumption and their effect on health have been investigated by the majority of observational and epidemiological studies.[ 17 ] However, there is no clear consensus in the literature about the effects of spicy foods and chili peppers on multiple health outcomes or no attempt to critically assess the quality and strength of the associations of spicy food and chili pepper consumption and different health outcomes. Chili pepper is one of the most commonly used condiments worldwide[ 5 ]; therefore, even potential minor effects could have profound implications for health at a population scale. Furthermore, substantial evidence exists to support the Mediterranean diet,[ 18 ] but the spicy dietary pattern has less well‐established associations with health outcomes. Moreover, dietary characteristics vary around the world, the Mediterranean diet preference is not suitable for other nations, and we should expand our research into regional localization diet strategies. Finally, we should pay attention to the deficiency of research into the multitudinous health outcomes of dietary flavor.
This study set out to provide a comprehensive perspective of the evidence landscape of spicy food and chili peppers and multiple health outcomes. The umbrella review, only considering incorporation of the highest level of evidence, allows the results of relevant reviews for a research topic to be compared and evaluated.[ 19 ] To the best of our knowledge, no umbrella review evaluates the validity and breadth of evidence available about spicy food and chili pepper consumption to all health outcomes in humans. Thus, we performed this study to address the problem and evolve our understanding of the effects that various dietary taste patterns and other food components play on health and disease.
2. Results
2.1. Characteristics of the Study
The flow diagram of the study selection process is shown in Figure 1 . A total of 275 articles existed in our initial search. Following the application of the predefined inclusion and exclusion criteria, 11 articles ultimately met our inclusion criteria. Figure 2 illustrates a map of health outcomes linked to spicy food and chili pepper intake, which includes 11 systematic reviews with 27 outcomes. Figure 3 shows the summary of AMSTAR and GRADE Classification of spicy food and chili pepper consumption on benefit and harm outcomes. Tables 1–3 show the associations between spicy food and chili pepper intake and health outcomes. The assessments of AMSTAR scores and GRADE classification are presented in Table 4 .
Figure 1.
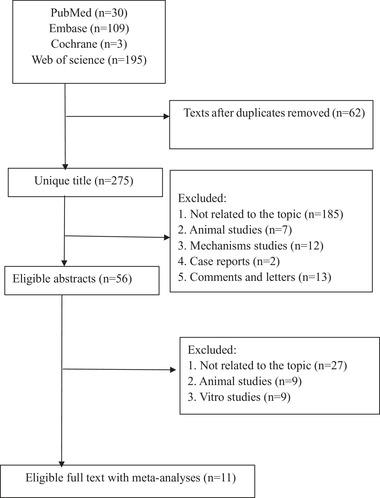
Flowchart of the selection process.
Figure 2.
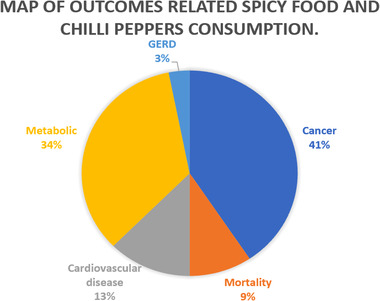
Map of outcomes related to spicy food and chili pepper consumption.
Figure 3.
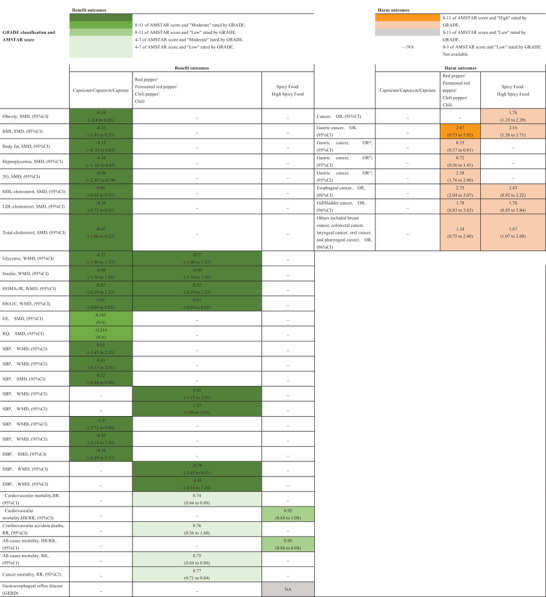
Summary of AMSTAR and GRADE classification of spicy food and chili pepper consumption on benefit and harm outcomes.
Table 1.
Associations between spicy food and chili pepper consumption and metabolic diseases
| Outcome | Category | Study | No. of cases/total | MA metric | Estimates | 95% CI | No. of studies in MA | Cohort | Case control | Cross‐sectional | Effects model | I 2 | Egger test p‐value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obesity | Capsicum | Jang 2020 | NA | SMD | −0.19 | −0.4–0.03 | 7 | NA | NA | NA | Random | 0% | NA |
| BMI | Capsicum | Jang 2020 | NA | SMD | −0.33 | −1.03–0.37 | 5 | NA | NA | NA | Random | 85% | NA |
| Body fat | Capsicum | Jang 2020 | NA | SMD | −0.15 | −0.35–0.05 | 9 | NA | NA | NA | Random | 18% | NA |
| Hyperglycemia | Capsicum | Jang 2020 | NA | SMD | −0.58 | −1.62–0.45 | 3 | NA | NA | NA | Random | 91% | NA |
| TG | Capsicum | Jang 2020 | NA | SMD | −0.96 | −2.67–0.74 | 3 | NA | NA | NA | Random | 95% | NA |
| HDL‐cholesterol | Capsicum | Jang 2020 | NA | SMD | 0.05 | −0.28–0.37 | 3 | NA | NA | NA | Random | 0% | NA |
| LDL‐cholesterol | Capsicum | Jang 2020 | NA | SMD | −0.39 | −0.72–0.07 | 3 | NA | NA | NA | Random | 13% | NA |
| Total‐cholesterol | Capsicum | Jang 2020 | NA | SMD | −0.47 | −1.06–0.12 | 3 | NA | NA | NA | Random | 71% | NA |
| Glycemic | Capsinoids and fermented red pepper | Reza Amini 2021 | NA/530 | WMD | −0.27 | −1.90–1.37 | 8 | NA | NA | NA | Random | 59.60% | 0.297 |
| Insulin | Capsinoids and fermented red pepper | Reza Amini 2021 | NA/530 | WMD | −0.09 | −1.76–1.57 | 4 | NA | NA | NA | Random | 77.20% | 0.076 |
| HOMA‐IR | Capsinoids and fermented red pepper | Reza Amini 2021 | NA/530 | WMD | 0.52 | −0.29–1.32 | 4 | NA | NA | NA | Random | 91.50% | 0.076 |
| HbA1C | Capsinoids and fermented red pepper | Reza Amini 2021 | NA/530 | WMD | 0.01 | −0.04–0.05 | 3 | NA | NA | NA | Random | NA | 0.955 |
| EE | Capsaicin and capsiate | Zsiborás 2018 | NA/255 | SMD | 0.245 | NA | 9 | NA | NA | NA | Fixed | NA | NA |
| RQ | Capsaicin and capsiate | Zsiborás 2018 | NA/256 | SMD | −0.216 | NA | 9 | NA | NA | NA | Fixed | NA | NA |
| EE | Capsaicin | Ludy 2012 | NA/255 | SMD | 0.11 | −0.06–0.29 | 7 | NA | NA | NA | Fixed | 16.60% | NA |
| RQ | Capsaicin | Ludy 2012 | NA/255 | SMD | −0.35 | −0.54–0.15 | 10 | NA | NA | NA | Fixed | 6% | NA |
| EE | Capsiate | Ludy 2012 | NA/255 | SMD | 0.4 | 0.22–0.59 | 13 | NA | NA | NA | Fixed | 0% | NA |
| RQ | Capsiate | Ludy 2012 | NA/255 | SMD | −0.31 | −0.54–0.07 | 9 | NA | NA | NA | Fixed | 26.80% | NA |
BMI, body mass index; EE, energy expenditure; HbA1C, hemoglobin A1C; HOMA‐IR, homeostasis model assessment of insulin resistance; NA, not available; RQ, respiratory quotient; SMD, standard mean difference; TG, triacylglycerol; WMD, weighted mean difference.
Table 4.
Assessments of AMSTAR scores and GRADE classification
| Outcome | Category | Author | Year | AMSTAR | GRADE |
|---|---|---|---|---|---|
| Obesity | Capsicum | Jang | 2020 | 10 | Moderate |
| BMI | Capsicum | Jang | 2020 | 10 | Moderate |
| Hypertension: diastolic blood pressure (DBP) | Capsicum | Jang | 2020 | 10 | Moderate |
| Hypertension: systolic blood pressure (SBP) | Capsicum | Jang | 2020 | 10 | Moderate |
| Dyslipidemia: hyperglycemia | Capsicum | Jang | 2020 | 10 | Moderate |
| Dyslipidemia: triacylglycerol (TG) | Capsicum | Jang | 2020 | 10 | Moderate |
| Dyslipidemia: HDL‐cholesterol | Capsicum | Jang | 2020 | 10 | Moderate |
| Dyslipidemia: LDL‐cholesterol | Capsicum | Jang | 2020 | 10 | Moderate |
| Dyslipidemia: Total‐cholesterol | Capsicum | Jang | 2020 | 10 | Moderate |
| Insulin | Capsinoids and fermented red pepper | Reza Amini | 2021 | 11 | Moderate |
| HOMA‐IR, homeostasis model assessment of insulin resistance | Capsinoids and fermented red pepper | Reza Amini | 2021 | 11 | Moderate |
| Hemoglobin A1C (HbA1C) | Capsinoids and fermented red pepper | Reza Amini | 2021 | 11 | Moderate |
| Blood sugar | Capsinoids and fermented red pepper | Reza Amini | 2021 | 11 | Moderate |
| Hypertension: systolic blood pressure (SBP) | Red pepper/Capsaicin | Shirani | 2021 | 11 | Moderate |
| Hypertension: diastolic blood pressure (DBP) | Red pepper/Capsaicin | Shirani | 2021 | 11 | Moderate |
| Heart rate | Red pepper/Capsaicin | Shirani | 2021 | 11 | Moderate |
| Hypertension: systolic blood pressure (SBP) | Fermented red pepper | Reza Amini | 2021 | 11 | Moderate |
| Hypertension: diastolic blood pressure (DBP) | Fermented red pepper | Reza Amini | 2021 | 11 | Moderate |
| Energy expenditure, EE | Capsaicin and capsiate | Zsiborás | 2018 | 10 | Moderate |
| Respiratory quotient, RQ | Capsaicin and capsiate | Zsiborás | 2018 | 10 | Moderate |
| Energy expenditure, EE | Capsaicin | Ludy | 2012 | 5 | Moderate |
| Respiratory quotient, RQ | Capsaicin | Ludy | 2012 | 5 | Moderate |
| Gastric cancer | High Spicy Food | Chen | 2017 | 11 | Low |
| Esophageal cancer | High Spicy Food | Chen | 2017 | 11 | Low |
| Gallbladder cancer | High Spicy Food | Chen | 2017 | 11 | Low |
| Others included breast cancer, colorectal cancer, laryngeal cancer, oral cancer, and pharyngeal cancer. | High Spicy Food | Chen | 2017 | 11 | Low |
| Gastric cancer | Chili | Du | 2020 | 11 | High |
| All‐cause mortality | Chili‐pepper | Yamani | 2021 | 5 | Low |
| Cardiovascular mortality | Chili‐pepper | Yamani | 2021 | 5 | Low |
| Cancer mortality | Chili‐pepper | Yamani | 2021 | 5 | Low |
| Cerebrovascular accident deaths | Chili‐pepper | Yamani | 2021 | 5 | Low |
| All‐cause mortality | Spicy food | Ofori‐Asenso | 2021 | 10 | Low |
| Cardiovascular mortality | Spicy food | Ofori‐Asenso | 2021 | 10 | Low |
| Gastroesophageal reflux disease (GERD) | Spicy food | Castillo | 2015 | 3 | Very low |
2.2. Cancer Outcomes
Spicy food and pepper intake is positively related to various cancers: esophageal cancer,[ 20 ] gastric cancer,[ 21 ] gallbladder cancer,[ 20 ] and other cancers, including breast cancer, colorectal cancer, laryngeal cancer, oral cancer, and pharyngeal cancer.[ 20 ] Moderate to high pepper consumption in the Asian population was significantly linked to gastric cancer in Table 3 (OR = 2.24, 95% CI 1.88–2.67, p = 0.005); however, low pepper consumption did not appear to be related to an increased risk of gastric cancer in Table 3 (OR = 0.62, 95% CI 0.33–1.18, p = 0.144). Dose–response analysis found a significant nonlinear relationship between GC risk and capsaicin intake (p nonlinearity <0.05).[ 22 ]
Table 3.
Associations between spicy food and chili pepper consumption and cancer outcomes and GERD
| Outcome | Category | Study | No. of cases/total | MA metric | Estimates | 95% CI | No. of studies in MA | Cohort | Case control | Cross‐sectional | Effects model | I 2 | Egger test p‐value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer outcomes | |||||||||||||
| Cancer | High spicy food | Chen 2016 | 7884/18 026 | OR | 1.76 | 1.35–2.29 | 39 | 0 | 39 | 0 | Random | 88.30% | 0.714 |
| Gastric cancer | High spicy food | Chen 2017 | NA/18 026 | OR | 2.16 | 1.26–3.71 | 12 | 0 | 12 | 0 | Random | 91.30% | NA |
| Gastric cancer | Chili | Chen 2017 | NA/18 026 | OR | 2.07 | 0.73–5.91 | 6 | 0 | 6 | 0 | Random | 94.00% | NA |
| Esophageal cancer | High spicy food | Chen 2017 | NA/18 026 | OR | 1.43 | 0.92–2.22 | 9 | 0 | 9 | 0 | Random | 77.10% | NA |
| Esophageal cancer | Chili | Chen 2017 | NA/18 026 | OR | 2.75 | 2.04–3.70 | 4 | 0 | 4 | 0 | Random | 9.60% | NA |
| Gallbladder cancer | High spicy food | Chen 2017 | NA/18 026 | OR | 1.78 | 0.83–3.83 | 6 | 0 | 6 | 0 | Random | 75.00% | NA |
| Gallbladder cancer | Chili | Chen 2017 | NA/18 026 | OR | 1.78 | 0.83–3.83 | 6 | 0 | 6 | 0 | Random | 75.00% | NA |
| Others included breast cancer, colorectal cancer, laryngeal cancer, oral cancer, and pharyngeal cancer. | High spicy food | Chen 2017 | NA/18 026 | OR | 1.67 | 1.07–2.60 | 12 | 0 | 12 | 0 | Random | 90.00% | NA |
| Others included breast cancer, colorectal cancer, laryngeal cancer, oral cancer, and pharyngeal cancer. | Chili | Chen 2017 | NA/18 026 | OR | 1.34 | 0.75–2.40 | 7 | 0 | 7 | 0 | Random | 91.70% | NA |
| Gastric cancer | Chili | Du 2020 | 3095/7856 | OR | 1.96 | 1.59–2.42 | 13 | 0 | 13 | 0 | Random | 74.70% | 0.288 |
| Gastric cancer | Chili | Du 2020 | NA/7856 | ORa) | 0.55 | 0.37–0.81 | NA | NA | NA | NA | Random | NA | NA |
| Gastric cancer | Chili | Du 2020 | NA/7857 | ORb) | 0.72 | 0.36–1.41 | NA | NA | NA | NA | Random | NA | NA |
| Gastric cancer | Chili | Du 2020 | NA/7858 | ORc) | 2.28 | 1.76–2.96 | NA | NA | NA | NA | Random | NA | NA |
| GERD | Spicy food | Castillo 2015 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
GERD, gastroesophageal reflux disease; NA, not available; OR, odds ratio;
a)Low consumption (0–30 mg day‐1);
b)Moderate consumption (30–90 mg day‐1);
c)High consumption (90–250 mg day‐1).
2.3. Mortality
Chili‐pepper consumption could reduce the risk of all‐cause mortality with a risk ratio (RR) of 0.75 [95% CI: 0.64–0.88] compared to nonconsumers as well as a lower risk of cancer mortality moderate to high pepper consumption in the Asian population was significantly linked to gastric cancer in Table 2 (RR: 0.77; 95% CI: 0.71–0.84).[ 23 ] The consumption of spicy food is associated with a 12% lower risk of all‐cause mortality in Table 2 (HR/RR = 0.88, 95% CI, 0.86–0.90; I 2 = 0%).[ 24 ]
Table 2.
Associations between spicy food and chili pepper consumption and cardiovascular and mortality diseases
| Outcome | Category | Study | No. of cases/total | MA metric | Estimates | 95% CI | No. of studies in MA | Cohort | Case control | Cross‐sectional | Effects model | I 2 | Egger test p‐value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardiovascular outcomes | |||||||||||||
| DBP | Capsicum | Jang 2020 | NA | SMD | −0.16 | −0.49–0.17 | 3 | NA | NA | NA | Random | 0% | NA |
| SBP | Capsicum | Jang 2020 | NA | SMD | 0.22 | −0.44–0.88 | 4 | NA | NA | NA | Random | 81% | NA |
| SBP | Red pepper/Capsaicin | Shirani 2021 | NA | WMD | 0.43 | −1.15–2.01 | 8 | NA | NA | NA | Random | 72.50% | 0.267 |
| DBP | Red pepper/Capsaicin | Shirani 2021 | NA | WMD | −0.45 | −2.14–1.24 | 7 | NA | NA | NA | Random | 74.70% | 0.959 |
| Heart rate | Red pepper/Capsaicin | Shirani 2021 | NA | WMD | −0.6 | −1.97–0.78 | 5 | NA | NA | NA | Random | 46.80% | 0.001 |
| SBP | Fermented red pepper | Reza Amini 2020 | NA/363 | WMD | 1.83 | 1.03–2.63 | 8 | NA | NA | NA | Random | 54.30% | 0.228 |
| DBP | Fermented red pepper | Reza Amini 2020 | NA/363 | WMD | −0.78 | −1.45–0.01 | 7 | NA | NA | NA | Random | 66.50% | 0.409 |
| SBP | Capsinoids | Reza Amini 2020 | NA/363 | WMD | 0.55 | −1.45–2.55 | 8 | NA | NA | NA | Random | 54.30% | 0.228 |
| DBP | Capsinoids | Reza Amini 2020 | NA/363 | WMD | −1.9 | −3.72–0.09 | 7 | NA | NA | NA | Random | 66.50% | 0.409 |
| Cardiovascular mortality | Chili pepper | Yamani 2021 | NA/570 762 | RR | 0.74 | 0.64–0.88 | 4 | 4 | 0 | 0 | Random | 66% | NA |
| Cerebrovascular accident deaths | Chili pepper | Yamani 2022 | NA/570 763 | RR | 0.76 | 0.36–1.60 | 4 | 4 | 0 | 0 | Random | 93% | NA |
| Cardiovascular mortality | Spicy food | Ofori‐Asenso 2021 | NA/564 748 | HR/RR | 0.92 | 0.84–1.00 | 4 | 4 | 0 | 0 | Random | 0% | NA |
| Mortality | NA | ||||||||||||
| All‐cause mortality | Chili‐pepper | Yamani 2021 | NA/570 762 | RR | 0.75 | 0.64–0.88 | 4 | 4 | 0 | 0 | Random | 97% | NA |
| Cancer mortality | Chili‐pepper | Yamani 2021 | NA/570 762 | RR | 0.77 | 0.71; 0.84 | 4 | 4 | 0 | 0 | Random | 49% | NA |
| All‐cause mortality | Spicy food | Ofori‐Asenso 2021 | NA/564 748 | HR/RR | 0.88 | 0.86–0.90 | 4 | 4 | 0 | 0 | Random | 0% | NA |
DBP, diastolic blood pressure; HR, hazard ratio; NA, not available; RR, relative risk; SBP, systolic blood pressure; SMD, standard mean difference; WMD, weighted mean difference.
2.4. Cardiovascular Disease
Spicy food and pepper intake are associated with significant decreases in cardiovascular mortality[ 25 ] and cerebrovascular accident deaths in Table 2.[ 23 ] Furthermore, fermented red pepper supplementation may play a part in improving systolic blood pressure (SBP) and diastolic blood pressure (DBP).[ 26 ] However, there was no significant association between the intake of red pepper/capsaicin and SBP, DBP, and heart rate.[ 27 ]
2.5. Metabolic Outcome
Evidence has shown a beneficial effect of capsicum annuum supplementation on body weight [SMD = −0.19; 95% CI −0.40, 0.03], low density lipoprotein‑cholesterol in Table 1.[ 28 ] However, there was no significant association among triacylglycerol (TG), HDL‐cholesterol, total cholesterol,[ 28 ] blood glucose, insulin, homeostasis model assessment of insulin resistance (HOMA‐IR), and hemoglobin A1C (HbA1C) in Table 1.[ 29 ]
2.6. Other Outcomes
There is a lack of sufficient evidence to forbid eating spicy food for the prevention or treatment of gastroesophageal reflux disease (GERD) in Table 3.[ 26 ]
2.7. Side Effects
The spicy food and chili pepper consumption reported no serious adverse effects. However, some studies reported adverse events, including leg cramps,[ 30 ] bowel irregularities, skin rash,[ 31 ] heat sensation in the oral cavity1, and skin wheals.[ 32 ]
2.8. Heterogeneity
Six (12.5%) outcomes demonstrated low levels of heterogeneity (I 2 < 25%), 16 (33%) showed moderate‐to‐high levels of heterogeneity (I 2 = 25–75%), 13 (27%) indicated extremely high heterogeneity (I 2 > 75%), seven showed little heterogeneity (I 2 = 0%), and six (22.2%) did not reported heterogeneity.
2.9. GRADE Classification and AMSTAR Score
Overall, three (27.2%) of evidence were classified as “very low” or “low” level of qualities by GRADE, seven (64%) as “moderate” and one (9.0%) as “high” level of the quality. The mean AMSTAR score was 9.32 (range 3.0–11.0). The detailed AMSTAR scores and GRADE classifications for each study are presented in Table 4.
3. Discussion
3.1. Main Findings and Interpretation
We examined 11 systematic reviews in the umbrella review to evaluate the relationship between spicy food and chili pepper consumption and health outcomes. Overall, the health effects of consuming spicy foods and chili peppers are uncertain. The following outcomes had a direct correlation with spicy food and capsicum intake: esophageal cancer, gastric cancer, or gallbladder cancer. However, there was a negative correlation between the intake of spicy food and capsicum and blood pressure, energy expenditure, respiratory quotient, obesity, all‐cause mortality, cardiovascular mortality, or cerebrovascular accident deaths. No significant association exists in blood glucose, insulin, homeostasis model assessment of insulin resistance (HOMA‐IR), hemoglobin A1C (HbA1C), or gastroesophageal reflux disease (GERD). A significant nonlinear relationship between GC risk and capsaicin intake revealed by dose–response analysis. GRADE classified over half (64%) of the evidence quality as “moderate.”
Capsaicin and spicy food consumption and metabolism were some of the most apparent links found in this study. Growing evidence indicates that capsaicin and spicy food consumption favor metabolism. Further studies on metabolic syndrome indicated that capsaicin is a potent dietary supplement in the treatment of obesity and insulin resistance.[ 33 ] On the other hand, capsaicin has also been shown to manifest antihyperglycemic and antiobesity effects by modulating the gut‐brain axis and inhibiting the enterohepatic FXR‐FGF15 axis.[ 34 ] Recent findings about the association between capsaicin and gut microbiota composition, abundance, and function have reported the possible mechanisms by which capsaicin exerts its influence.[ 34 ] An epidemiological study announced that the consumption of capsaicin‐containing foods is connected with a lower prevalence of obesity.[ 35 ] Rural Thais consume food containing 0.014% capsaicin. Rodents administered a diet containing 0.014% capsaicin showed no difference in calorie consumption, although there was a significant decline in visceral (perirenal) fat weight of 24%[ 36 ] and 29%.[ 37 ] This evidence suggests that in general, capsaicin and spicy foods may provide fresh insight into the prevention and management of metabolic syndrome. However, when confronted with different world regions and populations, this conclusion requires more caution in its application. Because of the factors contributing to MetS, such as genetic, environmental, and lifestyle factors, the risk of metabolic syndrome (MetS) varies significantly between populations and world regions.[ 38 ] According to some research, the gut microbiome may be the genesis of the pathways leading to MetS risk factors.[ 39 , 40 ] The constitution and behavior of gut microbiome from different populations could exert an effect on the emergence of low‐grade inflammation,[ 41 ] insulin resistance, obesity, and dyslipidemia.[ 42 ] This was clearly evidenced in Martha's study.[ 39 ] Furthermore, additional discussion should be made of different world regions and populations the unique metabolic digestion of nutrients, genetics, lifestyle habits.
Research applying the national data of the China Health and Nutrition Survey (CHNS) demonstrated that even though individuals have the same preference for spicy foods, different food consumption will have different metabolic impacts.[ 43 ] The spicy food preference is more of a psychological attitude‐correlated choice than a behavior, so it is not clear what effect it has on the metabolism. For example, intakes of dietary sugars make several contributions to Mets, Chinese children consumed 26 g d−1 of total and added sugars, Mexican children 92 g d−1, and US children 124 g d−1.[ 44 ] These data indicated that we were able to evaluate the food categories of daily intake across nations, thereby establishing the interaction between dietary preferences and health. Hypertension and Mets are interrelated and co‐occurrence,[ 45 ] the incidence changes in each country that was due to changes in population size, age composition, and age‐specific prevalence under various world regions and populations. For instance, changing demographics alone will increase the number of individuals in need of care for hypertension by 319.7 million, varying from a relative increase of 55% in China to a relative increase of 151% in Mexico.[ 46 ]
The results in this review showed no significant association among triacylglycerol (TG), HDL‐cholesterol, total cholesterol, blood glucose, and insulin.[ 28 , 30 ] Moreover, when used in the medical field, it necessitates strict adherence to a certain dosage, which has not been proven to be practical.[ 47 ] It is possible, therefore, that the results are affected by some restrictions (DNA, habitat, climate, lifestyle, and energy expenditure).[ 48 ] A range of Asian‐based diets contain spicy food compared with Western countries; similarly, the prevalence of obesity is as low as 3%, but it is well over 10% in most Western countries.[ 49 ] A large‐scale nationwide Internet data‐based study in China including 212 314 708 individuals reported that dietary preferences ranged by geographical distribution, with higher altitude regions covering large proportions of spicy food; likewise, spicy food appetite was inversely connected to diabetes risk.[ 50 ] Further studies are needed to clarify the interaction between dietary preferences and environmental health. Some people prefer spicy flavors based on a demand to release pressure and stimulate appetite, which is accompanied by an increase in energy intake. Therefore, in the case of consistent energy intake and food intake, adopting a spicy diet will bring certain metabolic benefits.
Our umbrella review found sufficient evidence that capsaicin and spicy food consumption were also positively correlated with cancer outcomes. Some evidence presented thus far supports the idea that a study reported that topical capsaicin treatment caused more skin papillomas in TRPV1 knockout mice than in TRPV1 wild‐type animals.[ 51 ] Then, capsaicin induced EGFR‐tyrosine phosphorylation. These findings suggest that capsaicin might act as a cocarcinogen in a TRPV1‐independent but EGFR‐dependent manner.[ 52 ] Furthermore, capsaicin has also been reported to boost the proliferation and survival of androgen‐responsive prostate cancer LNCaP cells, which corresponds to enhanced androgen receptor expression.[ 53 ]
However, research data contradictory to the results in this review showed that capsaicin can serve as a cancer resistance agent. First, TRPV1 is a possible treatment for a wide range of illnesses, such as inflammation, cancer, and autoimmune diseases. Some studies are increasingly turning attention to the association between TRPV1 activation by capsaicin and anticancer effects.[ 54 ] Second, in many studies, capsaicin's anticancer activity against various cancers has been established to delay tumor progression, limit metastasis, and improve survival rates.[ 55 ] Finally, according to mouse models of HCC, CCA, pancreatic cancer, and colorectal cancer, capsaicin inhibits the development of cancer cells in vivo by acting on cancer‐related genes and signaling pathways.[ 56 ] Conflicting evidence offers further support for the hypothesis that the ultimate effects of capsaicin may be based on the dosage. Furthermore, capsaicin seems to have a biphasic function, supporting growth at low dosages and causing apoptosis at concentrations greater than 200 µM.[ 57 ] In addition, the contamination of spicy foods with carcinogenic chemicals such as aflatoxin complicates their interpretation.
Another association identified in our umbrella review was about cardiovascular outcomes. TRPV1 possesses multiple mechanisms for cardiovascular protection. Capsaicin induces subsequent physiological processes by activating TRPV1. Moreover, TRPV1 can play a potential role in protecting cardiac metabolism, implying that TRPV1 may be a promising target for the treatment of cardiac metabolic diseases.[ 58 , 59 ]
3.2. Safety
People who suffer from gastrointestinal discomfort should limit the intake of spicy food. Other physiological effects, such as neurotoxicity, redness, and allergies, result in high doses of capsaicin consumption.[ 60 ]
3.3. Strengths and Limitations
Umbrella reviews, only considering incorporation of the highest level of evidence, are studies that identify, assess, and synthesize the outcomes of diverse research on a certain issue, resulting in credible data in a useable format. The associations between capsaicin and spicy food consumption and various health outcomes evaluated in this review comprehensively and systematically. However, there are some potential limitations that should be emphasized in this review. First, the evidence has a low level of strength, and almost 43.7% of GRADE's evidence had a “very low” or “low” level of quality. Second, we cannot address this relationship between chili peppers and spicy food intake and health outcomes going further because of the lack of dosage response analysis. Finally, some important but not in accordance with the inclusion criteria studies might be missed, such as valuable animal experimental studies.
4. Conclusions
In conclusion, the effect of capsaicin and spicy food consumption to a range of health outcomes in humans is less clear. The evidence, moreover, was not of extremely high quality. In addition, high‐quality researches are further required to appraise the validity of evidence. Notwithstanding these limitations, this study offers comprehensive insights into spicy food and chili peppers and multiple health outcomes.
5. Experimental Section
Umbrella Review Methods and Literature Search
This umbrella review thoroughly searched and analyzed available evidence from systematic reviews and meta‐analyses of multiple healthy outcomes associated with spicy food and capsicum consumption. Four databases, including PubMed, Web of Science, the Cochrane Database, and Embase, were searched from inception until November 9, 2021. The following search strategy was used: (capsicum) AND (systematic review* or meta‐analys*). The study also searched references from all eligible articles. Any disagreement was resolved by discussion with the authors.
Eligibility Criteria
Systematic reviews assessing the relevance of capsicum and spicy food and multiple health outcomes were included. Chinese and English were not limited. If there was more than one similar article, only the latest could be included. The study excluded articles such as animal, in vitro, research mechanism studies, comments, and letters.
Data Extraction
The following data were extracted by two authors: 1) outcomes involved any types of health in humans, 2) type of spicy food and chili peppers, 3) dosage and frequency of spicy food and chili peppers, 4) the first author, journal, 5) publication year, 6) number of total studies, 7) number of total participants, 8) the kind of study design, 9) the type of effect model, 10) relative estimated effect (OR, RR, SMD, WMD, HR, HR/RR), 11) the 95% confidence intervals (CIs), 12) heterogeneity, 13) publication bias, and 14) dose–response analyses. Any disagreement was resolved by discussion with the authors.
Evidence Evaluation and Grading
The methodological quality was assessed by two review authors independently using the AMSTAR checklist with 11 elements.[ 61 ] Authors performed GRADE to assess the strength of the evidence.[ 62 ]
Data Analysis
The study extracted the estimated summary effect and the 95% CI from every published systematic review. The heterogeneity among studies was evaluated by Cochran's Q test and the I 2 metric. The random effects methods were taken into account if the I 2 statistic was high over 50%. Egger's method was conducted to calculate publication bias, with a p value compared with fewer than 0.1 judged significant for small‐study effects and heterogeneity. Otherwise, the significance criterion was set to p < 0.05. Dose–response analyses were abstracted when data were available.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
Z.A. and H.L. designed the study. Z.A., H.L, and Z.H. conducted data extraction and analysis. Z.A. wrote the manuscript. Any disagreement was resolved by discussion with the authors (Z.A., Z.H., and H.L.). The manuscript was reviewed and approved by all authors.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No. 82172909).
Biographies
Zhimin Ao, a graduate student in the Surgery Department of Integrated Traditional Chinese and Western Medicine, West China Hospital, Sichuan University. She focuses on improving and treating metabolic diseases by using alternative therapies to regulate intestinal flora and digestive function.

Zongyue Huang, a graduate student at the Sixth Medical Center of PLA General Hospital, PLA Medical College. He focuses on the neuroelectrophysiological effects of acupuncture.

Hong Liu, Doctor of Medicine, Vice Chief physician, Vice Director of Surgery Department of Integrated Traditional Chinese and Western Medicine, West China Hospital, Sichuan University. He focuses on the alleviative effect of nutritional intervention on metabolic diseases via improving the structure of the gut microbiota.

Ao Z., Huang Z., Liu H., Spicy Food and Chili Peppers and Multiple Health Outcomes: Umbrella Review. Mol. Nutr. Food Res. 2022, 66, 2200167. 10.1002/mnfr.202200167
References
- 1. Barboza G. E., de Bem Bianchetti L., Stehmann J. R., PhytoKeys 2020, 140, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arimboor R., Natarajan R. B., Menon K. R., Chandrasekhar L. P., Moorkoth V., J. Food Sci. Technol. 2015, 52, 1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Batiha G. E., Alqahtani A., Ojo O. A., Shaheen H. M., Wasef L., Elzeiny M., Ismail M., Shalaby M., Murata T., Zaragoza‐Bastida A., Rivero‐Perez N., Magdy Beshbishy A., Kasozi K. I., Jeandet P., Hetta H. F., Int. J. Mol. Sci. 2020, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kraft K. H., Brown C. H., Nabhan G. P., Luedeling E., Luna Ruiz Jde J., Coppens d'Eeckenbrugge G., Hijmans R. J., Gepts P., Proc. Natl. Acad. Sci. USA 2014, 111, 6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bogusz S. Jr., Libardi S. H., Dias F. F., Coutinho J. P., Bochi V. C., Rodrigues D., Melo A. M., Godoy H. T., J. Sci. Food Agric. 2018, 98, 217. [DOI] [PubMed] [Google Scholar]
- 6. Afroz M., Akter S., Ahmed A., Rouf R., Shilpi J. A., Tiralongo E., Sarker S. D., Göransson U., Uddin S. J., Front Pharmacol. 2020, 11, 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alam F., Shafique Z., Amjad S. T., Bin Asad M. H. H., Phytother. Res. 2019, 33, 41. [DOI] [PubMed] [Google Scholar]
- 8. Deng S., Rong H., Tu H., Zheng B., Mu X., Zhu L., Zhou X., Peng W., Wu M., Zhang E., Li X., Shen H., Biomed. Pharmacother. 2019, 112, 108696. [DOI] [PubMed] [Google Scholar]
- 9. Meghvansi M. K., Siddiqui S., Khan M. H., Gupta V. K., Vairale M. G., Gogoi H. K., Singh L., J. Ethnopharmacol. 2010, 132, 1. [DOI] [PubMed] [Google Scholar]
- 10. Munjuluri S., Wilkerson D. A., Sooch G., Chen X., White F. A., Obukhov A. G., Cells 2021, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nava‐Ochoa A. E., Antunes‐Ricardo M., Guajardo‐Flores D., J. Biotechnol. 2021, 333, 77. [DOI] [PubMed] [Google Scholar]
- 12. Sun F., Xiong S., Zhu Z., Nutrients 2016, 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oyagbemi A. A., Saba A. B., Azeez O. I., Indian J. Cancer 2010, 47, 53. [DOI] [PubMed] [Google Scholar]
- 14. Selvarajah D., Kar D., Khunti K., Davies M. J., Scott A. R., Walker J., Tesfaye S., Lancet Diabetes Endocrinol. 2019, 7, 938. [DOI] [PubMed] [Google Scholar]
- 15. Finnerup N. B., Attal N., Haroutounian S., McNicol E., Baron R., Dworkin R. H., Gilron I., Haanpää M., Hansson P., Jensen T. S., Kamerman P. R., Lund K., Moore A., Raja S. N., Rice A. S., Rowbotham M., Sena E., Siddall P., Smith B. H., Wallace M., Lancet Neurol. 2015, 14, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCarty M. F., DiNicolantonio J. J., O'Keefe J. H., Open Heart 2015, 2, e000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lv J., Qi L., Yu C., Yang L., Guo Y., Chen Y., Bian Z., Sun D., Du J., Ge P., Tang Z., Hou W., Li Y., Chen J., Chen Z., Li L., BMJ 2015, 351, h3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ofori‐Asenso R., Mohsenpour M. A., Nouri M., Faghih S., Liew D., Mazidi M., Angiology 2021, 72, 625. [DOI] [PubMed] [Google Scholar]
- 19. Aromataris E., Fernandez R., Godfrey C. M., Holly C., Khalil H., Tungpunkom P., Int. J. Evid. Based Healthc. 2015, 13, 132. [DOI] [PubMed] [Google Scholar]
- 20. Chen Y. H., Zou X. N., Zheng T. Z., Zhou Q., Qiu H., Chen Y. L., He M., Du J., Lei H. K., Zhao P., Chin. Med. J. (Engl) 2017, 130, 2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Du Y., Lv Y., Zha W., Hong X., Luo Q., Nutr. Cancer 2021, 73, 45. [DOI] [PubMed] [Google Scholar]
- 22. Yamani N., Musheer A., Gosain P., Sarfraz S., Qamar H., Waseem M. M., Arshad M. S., Almas T., Figueredo V., Ann. Med. Surg. (Lond.) 2021, 70, 102774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amini M. R., Sheikhhossein F., Bazshahi E., Hajiaqaei M., Shafie A., Shahinfar H., Azizi N., Eghbaljoo Gharehgheshlaghi H., Naghshi S., Fathipour R. B., Shab‐Bidar S., Clin. Nutr. 2021, 40, 1767. [DOI] [PubMed] [Google Scholar]
- 24. Shirani F., Foshati S., Tavassoly M., Clark C. C. T., Rouhani M. H., Phytother. Res. 2021, 35, 6080. [DOI] [PubMed] [Google Scholar]
- 25. Jang H. H., Lee J., Lee S. H., Lee Y. M., Sci. Rep. 2020, 10, 20912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hosseinkhani A., Lankarani K. B., Mohagheghzadeh A., Long C., Pasalar M., Curr. Drug. Discov. Technol. 2018, 15, 305. [DOI] [PubMed] [Google Scholar]
- 27. Galgani J. E., Ravussin E., Am. J. Clin. Nutr. 2010, 92, 1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Snitker S., Fujishima Y., Shen H., Ott S., Pi‐Sunyer X., Furuhata Y., Sato H., Takahashi M., Am. J. Clin. Nutr. 2009, 89, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim Y., Park Y. J., Yang S. O., Kim S. H., Hyun S. H., Cho S., Kim Y. S., Kwon D. Y., Cha Y. S., Chae S., Choi H. K., Nutr. Res. 2010, 30, 455. [DOI] [PubMed] [Google Scholar]
- 30. Okla M., Kim J., Koehler K., Chung S., Adv. Nutr. 2017, 8, 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Varghese S., Kubatka P., Rodrigo L., Gazdikova K., Caprnda M., Fedotova J., Zulli A., Kruzliak P., Büsselberg D., Int. J. Food Sci. Nutr. 2017, 68, 392. [DOI] [PubMed] [Google Scholar]
- 32. Panchal S. K., Bliss E., Brown L., Nutrients 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanati S., Razavi B. M., Hosseinzadeh H., Iran J. Basic Med. Sci. 2018, 21, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosca A. E., Iesanu M. I., Zahiu C. D. M., Voiculescu S. E., Paslaru A. C., Zagrean A. M., Molecules 2020, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wahlqvist M. L., Wattanapenpaiboon N., Lancet 2001, 358, 348. [DOI] [PubMed] [Google Scholar]
- 36. Kawada T., Hagihara K., Iwai K., J. Nutr. 1986, 116, 1272. [DOI] [PubMed] [Google Scholar]
- 37. Ohnuki K., Haramizu S., Oki K., Watanabe T., Yazawa S., Fushiki T., Biosci. Biotechnol. Biochem. 2001, 65, 2735. [DOI] [PubMed] [Google Scholar]
- 38. Lemieux I., Després J. P., Nutrients 2020, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guevara‐Cruz M., Flores‐López A. G., Aguilar‐López M., Sánchez‐Tapia M., Medina‐Vera I., Díaz D., Tovar A. R., Torres N., J. Am. Heart Assoc. 2019, 8, e012401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dabke K., Hendrick G., Devkota S., J. Clin. Invest. 2019, 129, 4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thaiss C. A., Levy M., Grosheva I., Zheng D., Soffer E., Blacher E., Braverman S., Tengeler A. C., Barak O., Elazar M., Ben Zeev R., Lehavi Regev D., Katz M. N., Pevsner Fischer M., Gertler A., Halpern Z., Harmelin A., Aamar S., Serradas P., Science 2018, 359, 1376. [DOI] [PubMed] [Google Scholar]
- 42. Bishehsari F., Voigt R. M., Keshavarzian A., Nat. Rev. Endocrinol. 2020, 16, 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Canfora E. E., Jocken J. W., Blaak E. E., Nat. Rev. Endocrinol. 2015, 11, 577. [DOI] [PubMed] [Google Scholar]
- 44. Sun S., He J., Fan X., Nutrients 2019, 11. [Google Scholar]
- 45. Afeiche M. C., Koyratty B. N. S., Wang D., Jacquier E. F., Lê K. A., Pediatr. Obes. 2018, 13, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fahed G., Aoun L., Bou Zerdan M., Allam S., Bou Zerdan M., Bouferraa Y., Assi H. I., Int. J. Mol. Sci. 2022, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sudharsanan N., Geldsetzer P., Hypertension 2019, 73, 770. [DOI] [PubMed] [Google Scholar]
- 48. Diepvens K., Westerterp K. R., Westerterp‐Plantenga M. S., Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R77. [DOI] [PubMed] [Google Scholar]
- 49. Spence C., Curr. Res. Food Sci. 2022, 5, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vesnina A., Prosekov A., Kozlova O., Atuchin V., Genes (Basel) 2020, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhao Z., Li M., Li C., Wang T., Xu Y., Zhan Z., Dong W., Shen Z., Xu M., Lu J., Chen Y., Lai S., Fan W., Bi Y., Wang W., Ning G., J. Diabetes 2020, 12, 270. [DOI] [PubMed] [Google Scholar]
- 52. Cho S. C., Lee H., Choi B. Y., Food Sci. Biotechnol. 2017, 26, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ziglioli F., Frattini A., Maestroni U., Dinale F., Ciufifeda M., Cortellini P., Acta Biomed. 2009, 80, 13. [PubMed] [Google Scholar]
- 54. Malagarie‐Cazenave S., Olea‐Herrero N., Vara D., Díaz‐Laviada I., FEBS Lett. 2009, 583, 141. [DOI] [PubMed] [Google Scholar]
- 55. Bujak J. K., Kosmala D., Szopa I. M., Majchrzak K., Bednarczyk P., Front. Oncol. 2019, 9, 1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Popescu G. D. A., Scheau C., Badarau I. A., Dumitrache M. D., Caruntu A., Scheau A. E., Costache D. O., Costache R. S., Constantin C., Neagu M., Caruntu C., Molecules 2020, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Díaz‐Laviada I., Rodríguez‐Henche N., Prog. Drug Res. 2014, 68, 181. [DOI] [PubMed] [Google Scholar]
- 58. Díaz‐Laviada I., Future Oncol. 2010, 6, 1545. [DOI] [PubMed] [Google Scholar]
- 59. Rubino A., Burnstock G., Cardiovasc. Res. 1996, 31, 467. [PubMed] [Google Scholar]
- 60. Vaishnava P., Wang D. H., Curr. Med. Chem. Cardiovasc. Hematol. Agents 2003, 1, 177. [DOI] [PubMed] [Google Scholar]
- 61. Mózsik G., Vincze A., Szolcsányi J., J. Gastroenterol. Hepatol. 2001, 16, 1093. [DOI] [PubMed] [Google Scholar]
- 62. Shea B. J., Grimshaw J. M., Wells G. A., Boers M., Andersson N., Hamel C., Porter A. C., Tugwell P., Moher D., Bouter L. M., BMC Med. Res. Methodol. 2007, 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Guyatt G., Oxman A. D., Akl E. A., Kunz R., Vist G., Brozek J., Norris S., Falck‐Ytter Y., Glasziou P., DeBeer H., Jaeschke R., Rind D., Meerpohl J., Dahm P., Schünemann H. J., J. Clin. Epidemiol. 2011, 64, 383. [DOI] [PubMed] [Google Scholar]


