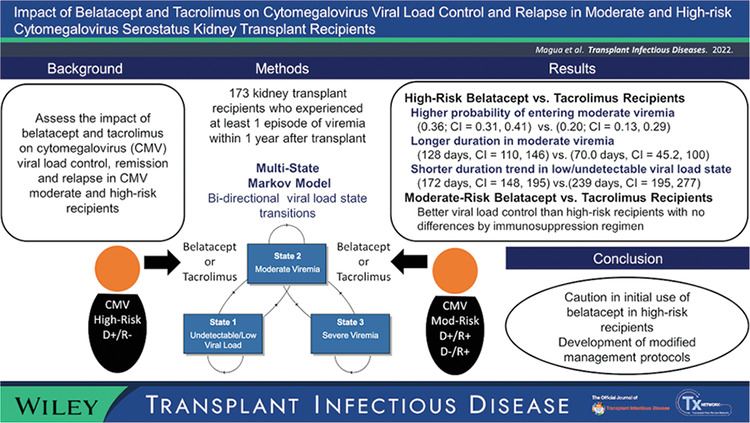
Abstract
Background
Belatacept improves long‐term graft survival, but control of some primary viral infections may be impaired. We evaluated the impact of belatacept and tacrolimus on cytomegalovirus (CMV) viral control, remission and relapse in CMV high‐risk and moderate‐risk recipients.
Methods
Using a multistate Markov model, we evaluated viral load state transitions of 173 kidney transplant recipients with at least one episode of viremia within 1 year after transplant: state 1, undetectable/low viral load; state 2, moderate viremia; and state 3, severe viremia.
Results
Among high‐risk recipients, belatacept‐treated recipients exhibited a significantly higher probability of entering moderate viremia (.36; 95% CI = .31, .41) than tacrolimus‐treated recipients (.20; 95% CI = .13, .29). The expected number of days in viremic states differed. High‐risk belatacept‐treated recipients persisted in moderate viremia for significantly longer (128 days, 95% CI = 110, 146) than did tacrolimus‐treated recipients (70.0 days, 95% CI = 45.2, 100) and showed a trend of shorter duration in low/undetectable viral load state (172 days, 95% CI = 148, 195) than did tacrolimus‐treated recipients (239 days, 95% CI = 195, 277). Moderate‐risk recipients showed better viral load control and with no differences by immunosuppression.
Conclusion
High‐risk belatacept‐treated recipients showed defects in sustaining viral control relative to tacrolimus‐treated recipients. Avoidance of initial use belatacept in high‐risk recipients or development of modified management protocols should be considered.
Keywords: belatacept, clinical research/practice, CMV bidirectional viral load state transitions, CMV control, CMV high‐risk, CMV moderate‐risk, CMV management strategies, CMV relapse, CMV serostatus risk, CMV viral infection and infectious agents, CMV viral load dynamics, CMV viral load volatility, cytomegalovirus, immunosuppression/immune modulation, immunosuppressive regimens—maintenance, infectious disease, kidney (allograft) function/disfunction, kidney transplant, length of stay in viral load state, Markov multistate, tacrolimus, viral load trajectory, viremia

Abbreviations
- AUC
area under the curve
- CMV
cytomegalovirus
- CNI
calcineurin inhibitor
- D/R
±donor/r±recipient serostatus
- EBV
Epstein–Barr virus
- MMF
mycophenolate mofetil
- MSMM
multistate Markov model
1. INTRODUCTION
Despite the development of advanced diagnostics, therapeutics and prophylaxis, 1 , 2 , 3 , 4 cytomegalovirus (CMV) persists as an important source of morbidity and mortality after transplantation. 5 , 6 Primary CMV exposure via transplantation of an organ from a seropositive donor to a seronegative recipient poses the highest risk of viremia, tissue‐invasive disease, and infection‐related mortality and leads to increased medical costs and inferior quality‐adjusted life years. 7 , 8 , 9 , 10 A systematic review of randomized trials evaluating the scope of infection outcomes in adult kidney transplant recipients reported CMV infection to be the most frequently reported organism‐specific outcome (∼62% of the trials). 11 When considering the site of infection, systemic infections were reported in ∼71% of the trials, over half of which were CMV related. CMV negative serostatus matched kidney transplant recipients have been shown to benefit in terms of increased long‐term survival even when their waitlist time is extended as a result of bypassing a mismatched organ. 10 In the absence of CMV serostatus matching in the allocation policy, increased CMV serostatus testing, viral load monitoring, drug resistance testing, and use of risk stratified antiviral prophylaxis and treatment remain essential for care and improved outcomes.
Extensive literature, community guidelines, and clinical experience for calcineurin inhibitor (CNI)‐based regimens have led to a set of expectations of the likelihood of successful treatment and of the need for secondary treatment and/or alteration of immunosuppression in the management of CMV. In contrast, there is much less information about the behavior of CMV, patterns of viremia, and response to treatment for recipients treated with newer costimulation blockade‐based regimens such as belatacept. In phase III studies, belatacept demonstrated improved renal function and lower death and graft loss at 7 years among renal transplant recipients; however, there is limited information about CMV as an assessment of viremia was neither specified nor captured in these studies. 12 , 13 , 14 Recent analyses indicate that the clinical manifestations and consequences of CMV in belatacept‐based and standard CNI regiments may differ. 15 When considering CMV serostatus, we previously reported that high‐risk belatacept‐treated recipients (donor/recipient serostatus pair: D+/R−) showed a higher cumulative incidence of CMV viremia, higher area under the viral load curves (AUC), and a trend of high risk of graft loss than tacrolimus‐treated recipients. 7 Incidence of viremia, viral load peaks, and AUC have frequently been used in traditional statistical methods to characterize the burden of CMV viral load and predict CMV‐related outcomes. 5 , 7 , 16 Although these summary measures are relevant, they remain incomplete as they do not capture viral load dynamics, such as the frequency of change between viral load levels or states over time needed to capture viral load volatility. 17
A deeper understanding of viral control and relapse may guide the degree of clinical monitoring, choice of immunosuppression, and antiviral approaches needed to mitigate the risk of opportunistic infections and optimize long‐term graft survival. We sought to characterize CMV viral load control and relapse in belatacept‐treated and in traditional CNI‐treated adult kidney transplant recipients within CMV high‐risk (donor/recipient serostatus pair: D+/R−) and moderate‐risk (donor/recipient serostatus pair: D+/R+ or D−/R+) recipient groups. We use a continuous time‐homogeneous multistate Markov model (MSMM) to examine the impact of belatacept and tacrolimus‐based immunosuppression protocols on viral load control and recurrence within CMV serostatus groups in adult kidney transplant recipients who experienced viremia within 1 year after transplantation. MSMMs models handle interval‐censored data like longitudinal clinical viral load monitoring, similar to our study, and provide valid inference for such data. 18 , 19 MSM models have been widely applied in medical decision‐making to model the evolution of clinical states and is suitable for our study. 19 Our goal in this study is to gain a more detailed understanding of the impact of belatacept versus CNIs on viral load volatility in CMV moderate and high‐risk recipients who develop CMV viremia.
2. MATERIALS AND METHODS
2.1. Study population
The data were extracted from the Emory Transplant DataMart. The target population was a retrospective observational cohort of CMV high‐ and moderate‐risk renal transplant recipients over 18 years who underwent renal transplantation at Emory University Hospital between January 1, 2010 and December 31, 2017 and were treated with either a belatacept or tacrolimus‐based regimen. 7 To develop a study cohort of comparable belatacept and tacrolimus‐treated recipients, we excluded EBV (Epstein–Barr virus) seronegative recipients because belatacept is contraindicated in this subpopulation (Figure 1). Additionally, CMV low‐risk recipients, recipients enrolled in clinical trials, those with hematologic malignancies, or those receiving off‐protocol induction therapies were excluded. Among the remaining recipients, we selected those who had at least one episode of viremia (viral load ≥500 copies/ml) within 365 days after transplantation. A Qiagen Artus CMV assay limit of detection of 100 copies/ml with the limit of quantification of 300 copies/ml was used for CMV plasma viral load assessment from 2010 to 2018. The Roche Cobas assay lower limit of detection of 1 copy/ml with a limit of quantification of 35 copies/ml was used from 2018 onward. CMV risk status was defined by CMV immunoglobulin G combination of CMV donor (D)/recipient (R) serostatus pairs. The pair D+/R− identified high‐risk individuals and pairs D+/R+ or D−/R+ identified moderate‐risk individuals. This study was approved by Emory University Institutional Review Board (IRB00107934).
FIGURE 1.
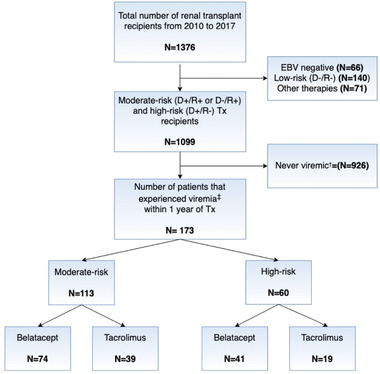
Flowchart illustrating the selection of the study cohort. †Recipients who remained negative for viremia consistently throughout the study period. ‡Recipients who experienced at least one episode of viremia within 1 year after transplant
2.2. Immunosuppression regimen
The composition of both belatacept‐ and tacrolimus‐based regimen included basiliximab induction, mycophenolate mofetil (MMF) 1 g twice a day, and intraoperative methylprednisolone 500 mg IV followed by 250 mg IV and 125 mg IV on day 1 and 2 respectively, and by prednisone 5 mg on day 3 onward. In addition, belatacept‐based regimen comprised (1) belatacept 10 mg/kg at day 0 and at weeks 4, 8, 12 followed by 5 mg/kg monthly and (2) a tapering dose of tacrolimus targeting trough level of 5–8 ng/ml in the first 6 months, 3–5 ng/ml on month 6–9, and a subsequent wean‐off phase to cessation by month 12. It is worth noting that the current tapering course of tacrolimus in the belatacept‐based regimen was developed systematically in response to the higher rates of acute T‐cell‐mediated rejection experienced by recipients treated with CNI‐free immunosuppression therapies. 12 On the other hand, tacrolimus‐based immunosuppression, in addition to basiliximab induction, MMF, and steroids comprised tacrolimus 8–12 ng/ml at 0–6 months with a subsequent maintenance dose of 5–8 ng/ml. There were two periods in which tacrolimus‐based regimen was standard of care at our center: (1) the early study period, January 2010 to July 2011 and (2) in 2017 when belatacept was unavailable.
2.3. Viral prophylaxis, monitoring, and treatment
Antiviral prophylaxis consisted of oral valganciclovir 450 mg daily for 3 months for moderate‐risk recipients and 450 mg daily for 6 months for high‐risk recipients with dosing adjustments based on renal function. 7 Viremia was treated with valganciclovir 900 mg twice daily, whereas foscarnet or cidofovir were administered for resistant or refractory CMV infection. 7 Recipient CMV viral load was systematically assessed monthly for the first year after transplantation as per our standard of care. 7 When viremia was detected, viral loads were monitored biweekly until clearance.
2.4. Analytic methods
We inspected individual viral load observations for emerging viral load trajectory patterns within each recipient immunosuppression and serostatus subgroup. We compared AUCs by immunosuppression within each serostatus stratum using nonparametric Mann–Whitney U test. We inspected viral load trajectories over time when recipient viral load AUCs were comparable. We used a continuous time MSM Markov model to examine CMV viral load dynamics. 17 , 19 In this framework, recipient viral loads are assumed to transition continuously through immediately adjacent states: State 1 indicates undetectable or low viral loads [0, 500) copies/ml; state 2 indicates moderate viremia with [500, 104) copies/ml; and state 3 indicates severe viremia (viral load >104). The MSM Markov model allows for irregularly spaced observation times, for interval‐censored data in which the exact transition among states is unobserved and is known to occur between two observations, and for the number and frequency of individual subject observations to vary. 20 , 21 , 22 MSM model analyses were conducted within each CMV serostatus stratum to evaluate whether serostatus modifies the effect of immunosuppression regimen on recipient viral load curve. 23 We estimated, using maximum likelihood estimators with 95% CI, the transition intensities as a function of immunosuppression protocol to examine the impact of belatacept‐ and tacrolimus‐based immunosuppression on viral load dynamics. Viral load state transition probabilities and state occupation probabilities were derived from transition intensities. The time occupied in viral load states was modeled using exponential distribution. We evaluated the model fit by comparing observed data with the model fitted expected values over time in all three states. Analyses were performed in R (version 3.6.2) and RStudio, 24 as well as R packages ggplot2, 25 ggstatsplot, 26 msm, 20 and diagram. 27
3. RESULTS
3.1. Study cohort
The study cohort was drawn from an initial population of 1376 individuals who underwent kidney transplantation between 2010 and 2017. Approximately 67.3% (n = 926) of the total number of recipients were identified as never viremic, 4.8% (n = 66) as EBV‐naïve, 10.2% (n = 140) as CMV low‐risk, and 5.2% (n = 71) as treated with nonstandard maintenance and hence did not meet the study inclusion criteria (Figure 1). As a result, the study cohort consisted of 173 CMV moderate‐risk (65.3%) and high‐risk (34.7%) recipients, who had at least one episode of viremia in any viremic states (viral load >500 copies/ml). Approximately two thirds ( of the recipients in each serostatus risk group were treated with belatacept‐based protocol. The proportion of male recipients, Black recipients, and recipients who received organs from deceased donors were similarly distributed by immunosuppression maintenance within each CMV serostatus group at statistical significance threshold .05 (Table 1). However, high‐risk belatacept‐treated recipients had a significantly higher mean age (M = 52.2, SD = 10.6) at transplant than did high‐risk tacrolimus‐treated recipients (M = 42.6, SD = 12.5).
TABLE 1.
Renal transplant–recipient characteristics stratified by cytomegalovirus (CMV) risk status and by immunosuppression protocols
| Moderate risk (n = 114, 65.1%) | High risk (n = 61, 34.9%) | Total (n = 173) | ||||
|---|---|---|---|---|---|---|
| Belatacept a (n = 74) | Tacrolimus (n = 39) | Belatacept (n = 41) | Tacrolimus (n = 19) | Belatacept (n = 115) | Tacrolimus (n = 58) | |
| Age at Tx | ||||||
| Mean (SD) | 52.1 (12.4) | 52.5 (12.5) | 52.2 (10.6) | 42.6 (12.5) | 52.1 (11.7) | 49.3 (13.2) |
| Median a [Min, Max] | 52.4 [23.8, 78.0] | 55.5 [29.4, 82.2] | 51.0 b [24.8, 70.2] | 43.5 b [25.4, 66.6] | 51.9 [23.8, 78.0] | 50.2 [25.4, 82.2] |
| Sex | ||||||
| Female | 32 (43.2%) | 22 (56.4%) | 10 (24.4%) | 7 (36.8%) | 42 (36.5%) | 29 (50.0%) |
| Male | 42 (56.8%) | 17 (43.6%) | 31 (75.6%) | 12 (63.2%) | 73 (63.5%) | 29 (50.0%) |
| Race | ||||||
| Black | 45 (60.8%) | 24 (61.5%) | 19 (46.3%) | 12 (63.2%) | 64 (55.7%) | 36 (62.1%) |
| Non‐Black | 29 (39.2%) | 15 (38.5%) | 22 (53.7%) | 7 (36.8%) | 51 (44.3%) | 22 (37.9%) |
| Donor type | ||||||
| Deceased | 53 (71.6%) | 25 (64.1%) | 29 (70.7%) | 12 (63.2%) | 82 (71.3%) | 37 (63.8%) |
| Living | 21 (28.4%) | 14 (35.9%) | 12 (29.3%) | 7 (36.8%) | 33 (28.7%) | 21 (36.2%) |
Note: All patients were transplanted at Emory Transplant Center between 2010 and 2017 and were considered to have entered a viremia states within 1 year after transplantation.
Median [minimum, maximum].
Belatacept‐treated recipients within the high‐risk group had a significantly higher medium age at transplant than did the tacrolimus‐treated recipients (p < .05).
Hence, our analytic data comprised 2386 viral load observations from 173 recipients in our study cohort with 58.5% (n = 1396) observations drawn from moderate‐risk recipients and 41.5% (n = 990) observations drawn from high‐risk recipients.
3.2. Viral load burden does not distinguish patterns of viremia
Viral load burden, quantified using AUC, was similarly distributed between the moderate‐risk cohort irrespective of the immunosuppression protocol the patients received (Figure 2). On the other hand, and consistent with our previous study, high‐risk belatacept‐treated recipients exhibited significantly higher AUCs than the tacrolimus‐treated recipients (Mann–Whitney U = 248, p = .03, n = 60). However, we found that among high‐risk recipients with comparable AUCs, different patterns of viral loads or volatility emerged with belatacept‐treated recipients exhibiting multi‐peak vial load curves compared to predominantly single‐peaked curves of tacrolimus‐treated recipients (Figure S1). These differences in viral transitions between states indicated divergent control, remission, and relapse pattern. These exploratory analyses suggested that a Markov multistate model would provide a fine‐grained insight into the patterns of viremia.
FIGURE 2.
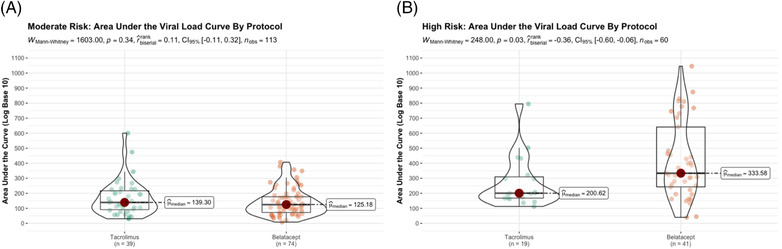
Area under the cytomegalovirus (CMV) viral curves (AUC) as a summary measure within CMV risk status (A moderate risk, B high risk) and by immunosuppression protocol using nonparametric Mann–Whitney U test
3.3. Comparable viral load state transitions in moderate‐risk recipients
Moderate‐risk recipients showed comparable viral load control, remission, and relapse pattern regardless of the immunosuppression regimen received (Figure 3). Moderate‐risk belatacept‐treated recipients emerged with a .19 (CI = .15, .22) probability of entering moderate viremic state and a .03 (CI = .02, .05) probability of entering severe viremic state. Similarly, moderate‐risk tacrolimus‐treated recipients had a .16 (CI = .12, .20) probability of entering moderate viremic state and a .04 (CI = .02, .07) probability of entering severe viremic state. Furthermore, moderate‐risk recipients exhibited a higher probability of entering into undetectable/low viral load state (belatacept‐treated: .78, CI = [.74, .82]; tacrolimus‐treated: .81, CI = [.75, .85]) than did high‐risk recipients (belatacept‐treated: .44, CI = [.38, .51]; tacrolimus‐treated; .63, CI = [.50, .75]).
FIGURE 3.
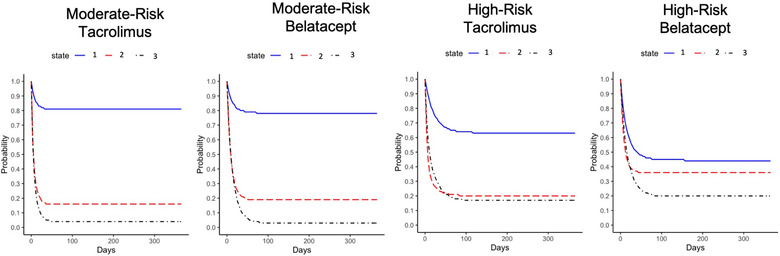
Estimated probability of remaining in a viral load state by immunosuppression protocol within cytomegalovirus (CMV) serostatus risk groups after the complete evolution of the Markov process
3.4. High‐risk belatacept‐treated recipients enter viremic states with high probability
High‐risk recipients showed divergent viral load control, remission, and relapse patterns by the immunosuppression regimen received (Figure 3). High‐risk belatacept‐treated recipients showed a significantly higher probability (.36; 95% CI = .31, .41) of entering the moderate viral load state than high‐risk tacrolimus‐treated recipients (.20; 95% CI = .13, .28). Furthermore, high‐risk belatacept‐treated recipients showed a trend of lower probability (.44; 95% CI = .38, .51) of remaining in undetectable/low viral load state than of tacrolimus‐treated recipients (.63; 95% CI = .50, .75). However, high‐risk recipients regardless of immunosuppression had comparable probabilities of entering the severe viral load state, with .20 (95% CI = .15, .25) and .17 (95% CI = .09, .27) probabilities in belatacept and tacrolimus‐treated recipients, respectively.
3.5. High‐risk belatacept‐treated recipients have longer duration in viremic states
The expected model‐estimated total lengths of stay in each state, when considering all viral load recovery and relapses, are presented in Figure 4. High‐risk belatacept‐treated recipients showed a trend of shorter total duration (172 days, 95% CI = 148, 195) in undetectable/low viral load state than did high‐risk tacrolimus‐treated recipients (239 days, 95% CI = 191, 276). Additionally, high‐risk belatacept‐treated recipients had a significantly longer total duration (128 days, 95% CI = 110, 145) in the moderate viremic state than did tacrolimus treated recipients (70.0 days, 95% CI = 46.3, 103). The total time spent in the severe viremic state remained comparable in the high‐risk recipient group irrespective of the immunosuppression treatment group (belatacept‐treated: 66.0 days, 95% CI = [48.5, 84.0]; tacrolimus‐treated: 56.2 days, 95% CI = [31.1, 89.4]), but these model‐estimated duration totals were approximately six and four times longer than those in the moderate‐risk belatacept‐treated and moderate‐risk tacrolimus‐treated recipients, respectively. Also, moderate‐risk recipients remained in the non‐viremic state for significantly longer than did than the high‐risk recipients, with the total duration remaining comparable across immunosuppression protocol groups (moderate‐risk belatacept‐treated: 289 days, 95% CI = [273, 301]; moderate‐risk tacrolimus‐treated: 296 days, 95% CI = [276, 312]).
FIGURE 4.
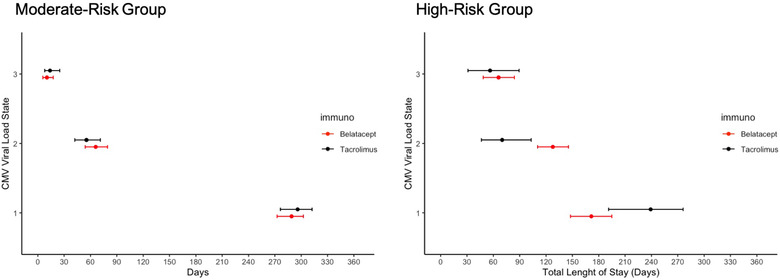
Estimated total length of stay (days) over the entire evolution of the Markov process in each state by immunosuppression protocol within recipient cytomegalovirus (CMV) risk status
3.6. Sensitivity analysis
To focus our model on the period of viremia, the viral load observation preceding the first measured instance of viremia was considered to be the first observation for each recipient. Approximately 5% of viremia high‐risk and moderate‐risk data were sparsely distributed during the first 4 months after transplantation. The model estimated observed percent prevalence and expected percent prevalence at all three viral load states were comparable, hence indicating a good model fit (Figure S2). We also compared unadjusted models with those adjusted for race, sex, and age of the recipient at transplant. We did not adjust for donor type because of the sample size. The direction and the conclusion of the study remained the same (Table S1).
4. DISCUSSION
We report the first detailed comparison of CMV viral load dynamics in CMV moderate‐ and high‐risk groups treated with belatacept‐ or tacrolimus‐based regimens using a study cohort of 173 adult recipients with at least one episode of viremia (>500 copies/ml) within the first year after kidney transplant. The analyses revealed divergent viral load patterns between moderate‐ and high‐risk recipients. Moderate‐risk recipients demonstrated significantly higher probabilities of remaining in the non‐viremic state and significantly shorter duration in viremic states in contrast to high‐risk recipients. Importantly, moderate‐risk recipients emerged with similar viral load control regardless of immunosuppression regimen. Conversely, despite use of the same antiviral prophylaxis and treatment strategy, high‐risk belatacept‐treated recipients exhibited volatile viral load trajectories, with a lower probability of remaining in low/undetectable viral load state and higher a probability of remaining in load states than high‐risk tacrolimus patients. The course of viremia is more protracted and control more difficult to achieve for belatacept recipients. A pattern of protracted and recurrent lower viral load has been associated with disease progression. 28 In the short run, difficulty controlling volatile and protracted viremia directly impacts patient experience, including increased duration of viral load monitoring, increased burden associated with appointments and treatments, longer exposure to toxicity of treatments, higher cost burden associated with increased care, and higher hospital utilzation. 10 , 29 , 30 In response to these data, our center now avoids belatacept in cmv high‐risk recipients and considers for‐cause conversion to belatacept only selectively after either sustained viral control has been achieved or if no viremia has been observed in the first‐year posttransplant.
The optimal methods for safe and effective use of belatacept have not yet been defined. Based on evidence from phase III clinical trials, belatacept is contraindicated for EBV‐naïve recipients. Considerable effort has been made to address the increased risk of early acute rejection associated with belatacept leading to modified belatacept regimens. 31 Yet, there is limited information to guide CMV management in belatacept‐treated recipients. The incidence of viremia above a specific threshold, peak viral load, and AUC among other metrics have been used to identify CMV risk factors to predict clinical outcomes and to inform treatment management in transplant recipients. 7 , 32 , 33 , 34 , 35 , 36 However, these traditional measures fail to capture viral load dynamics over time. 28 Access to granular clinical data creates the opportunity to leverage MSM models for additional insights on viral load dynamics such as probabilities of bidirectional viral load state transitions and duration in viral load states. Markov models have been used to examine the evolution of clinical states, including human immunodeficiency virus infection, cardiovascular disease, and aspects of CMV infection after hematopoietic stem‐cell transplant. 20 , 37 , 38 , 39
Given that increased morbidity and mortality are observed for CMV high‐risk recipients, 5 Lockridge et al. developed a pre‐transplant deceased donor kidney organ allocation protocol that incorporated CMV serostatus in organ matching. 40 Their preventative approach reduced the incidence of recipients falling into the CMV high‐risk category from 18.5% to 2.9% with minimal impact on transplant rates and waiting times. Similar approaches could be utilized for living donor exchange programs. Furthermore, increased frequency of CMV DNA monitoring and adoption of lower viral thresholds for initiating treatment can be considered. By minimizing the risks associated with CMV viremia, these approaches could maximize the reported benefits with respect to renal function, avoidance of sensitization, and survival benefits that have been associated with belatacept.
There are some limitations to our study. Despite our large experience with belatacept as a standard maintenance therapy, the number of recipients in our cohort remains limited. Our assumption of exponentially distributed state occupancy times is a simplifying assumption. Nonetheless, our models exhibited good fit to our data and hence offers valuable information on CMV viral load dynamics when considering immunosuppression regimen within CMV serostatus groups. Our study is neither randomized nor matched. Hence, despite adjusting our models for recipient characteristics, there remains a possibility of an imbalance in the distribution of clinical attributes that may impact viral load differences between treatment groups. Consideration should be given to incorporating MSM models into future immunosuppression clinical trials to examine viral load transitions as secondary or exploratory endpoints to provide more robust assessments of viremia than traditional adverse event or viremia incidence assessments. CMV DNA quantification was carried out in plasma and not whole blood samples and quantified in copies/ml. Hence, lower viral load detected by alternative assays and viral loads in IU/ml units may need to be validated. Additionally, future studies that address the impact of belatacept in other regimens such as belatacept with T‐cell depletion should be considered. It is possible that a valganciclovir prophylaxis dose 900 mg daily might help mitigate poor viral load control in belatacept‐treated recipients. However, we recommend caution and careful monitoring of belatacept‐treated CMV high‐risk recipients. In this study, we examined viral load dynamics observed only within the first year after transplantation and limited our study cohort to adult renal transplant recipients. Consequently, the results cannot be extrapolated to periods beyond a year or to pediatric populations. Future studies in pediatric cohorts should be undertaken.
Although this study is a retrospective analysis from a single center, the findings reported here provide nuanced data to inform the development of CMV management strategies in the setting of belatacept‐based immunosuppression regimen. Achieving optimal long‐term patient and transplant survival for individual transplant candidates involves balancing complex factors, including donor selection, donor–recipient matching (human leukocyte antigen, age, etc.), choice of initial and long‐term immunosuppression, prevention and treatment of infection and management of cardio‐metabolic risk reduction. Having shown an improved preservation of renal function, lower rates of de novo donor‐specific antibody formation and improved patient and graft survival in phase III trials, belatacept offers potential benefits toward the community's shared goal to provide personalized care protocols to deliver “One Transplant for Life.”
CONFLICT OF INTEREST
All authors in this manuscript declared no conflict of interest.
AUTHOR CONTRIBUTIONS
Wairimu Magua, Christian P. Larsen, Joseph B. Rickert, and Kirk A. Easley conceived the study plan and analytical framework. Geeta M. Karadkhele prepared and processed the data for the study. Wairimu Magua conducted the analysis. Wairimu Magua, Christian P. Larsen, and Kenneth A. Newell significantly contributed toward the interpretation of the results. All authors provided critical feedback needed to shape the research and development of the manuscript.
Supporting information
Figure S1 Comparison of the CMV viral load trajectories between high‐risk belatacept and tacrolimus‐treated recipients when the areas under the curves (AUC) are comparable. Belatacept‐treated recipients exhibited multipeak viral load dynamics even when AUCs were comparable.
Figure S2 Assessment of the extent to which the model can predict viral load observations by comparing expected with observed prevalence percent within CMV risk status.
Table S1 MSMM† hazard ratios associated with the effect of immunosuppression regimen on transition rates between adjacent state
Visual Abstract
ACKNOWLEDGMENTS
This research was supported by Carlos and Marguerite Mason Trust and James M. Cox Foundation.
Magua W, Johnson AC, Karadkhele GM, et al. Impact of belatacept and tacrolimus on cytomegalovirus viral load control and relapse in moderate and high‐risk cytomegalovirus serostatus kidney transplant recipients. Transpl Infect Dis. 2022;24:e13983. 10.1111/tid.13983
DATA AVAILABILITY STATEMENT
Deidentified data will be made available upon request to the corresponding author.
REFERENCES
- 1. López‐Oliva MO, Flores J, Madero R, et al. Cytomegalovirus infection after kidney transplantation and long‐term graft loss. Nefrologia. 2017;37(5):515‐525. 10.1016/j.nefro.2016.11.018 [DOI] [PubMed] [Google Scholar]
- 2. Kotton CN, Kumar D, Caliendo AM, et al. The third international consensus guidelines on the management of cytomegalovirus in solid‐organ transplantation. Transplantation. 2018;102(6):900‐931. 10.1097/TP.0000000000002191 [DOI] [PubMed] [Google Scholar]
- 3. Xin W, Hui Y, Xiaodong Z, Xiangli C, Shihui W, Lihong L. Effectiveness of valganciclovir 900 mg versus 450 mg for cytomegalovirus prophylaxis in renal transplantation: a systematic review and meta‐analysis. J Pharm Pharm Sci. 2017;20(0):168‐183. doi: 10.18433/J3805B [DOI] [PubMed] [Google Scholar]
- 4. Heldenbrand S, Li C, Cross RP, et al. Multicenter evaluation of efficacy and safety of low‐dose versus high‐dose valganciclovir for prevention of cytomegalovirus disease in donor and recipient positive (D+/R+) renal transplant recipients. Transpl Infect Dis. 2016;18(6):904‐912. 10.1111/tid.12609 [DOI] [PubMed] [Google Scholar]
- 5. McBride JM, Sheinson D, Jiang J, et al. Correlation of cytomegalovirus (CMV) disease severity and mortality with CMV viral burden in CMV‐seropositive donor and CMV‐seronegative solid organ transplant recipients. Open Forum Infect Dis. 2019;6(2):ofz003. 10.1093/ofid/ofz003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Selvey LA, Lim WH, Boan P, et al. Cytomegalovirus viraemia and mortality in renal transplant recipients in the era of antiviral prophylaxis. Lessons from the western Australian experience. BMC Infect Dis. 2017;17(1):501. 10.1186/s12879-017-2599-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karadkhele G, Hogan J, Magua W, et al. CMV high‐risk status and posttransplant outcomes in kidney transplant recipients treated with belatacept. Am J Transplant. 2021;21:208‐221. Published online 2020. [DOI] [PubMed] [Google Scholar]
- 8. Leeaphorn N, Garg N, Thamcharoen N, Khankin EV, Cardarelli F, Pavlakis M. Cytomegalovirus mismatch still negatively affects patient and graft survival in the era of routine prophylactic and preemptive therapy: a paired kidney analysis. Am J Transplant. 2019;19(2):573‐584. 10.1111/ajt.15183 [DOI] [PubMed] [Google Scholar]
- 9. Desai R, Collett D, Watson CJE, Johnson PJ, Moss P, Neuberger J. Impact of cytomegalovirus on long‐term mortality and cancer risk after organ transplantation. Transplantation. 2015;99(9):1989‐1994. 10.1097/TP.0000000000000641 [DOI] [PubMed] [Google Scholar]
- 10. Axelrod DA, Chang SH, Lentine KL, et al. The clinical and economic benefit of CMV matching in kidney transplant: a decision analysis. Transplantation. 2022;106(6):1227‐1232. 10.1097/TP.0000000000003887 [DOI] [PubMed] [Google Scholar]
- 11. Chan S, Au E, Johnson DW, et al. Range and consistency of infection outcomes reported in trials conducted in kidney transplant recipients: a systematic review. Transplantation. 2021;105(12):2632‐2638. 10.1097/TP.0000000000003723 [DOI] [PubMed] [Google Scholar]
- 12. Adams AB, Goldstein J, Garrett C, et al. Belatacept combined with transient calcineurin inhibitor therapy prevents rejection and promotes improved long‐term renal allograft function. Am J Transplant. 2017;17(11):2922‐2936. 10.1111/ajt.14353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long‐term outcomes in kidney transplantation. N Engl J Med. 2016;374(4):333‐343. 10.1056/NEJMoa1506027 [DOI] [PubMed] [Google Scholar]
- 14. Garcia VD, Meinerz G, Keitel E. A safety evaluation of belatacept for the treatment of kidney transplant. Expert Opin Drug Saf. 2016;15(8):1125‐1132. 10.1080/14740338.2016.1202236 [DOI] [PubMed] [Google Scholar]
- 15. Bertrand D, Chavarot N, Gatault P, et al. Opportunistic infections after conversion to belatacept in kidney transplantation. Nephrol Dial Transplant. 2020;35(2):336‐345. 10.1093/ndt/gfz255 [DOI] [PubMed] [Google Scholar]
- 16. Basso G, Felipe CR, Cristelli MP, et al. The effect of anti‐thymocyte globulin and everolimus on the kinetics of cytomegalovirus viral load in seropositive kidney transplant recipients without prophylaxis. Transpl Infect Dis. 2018;20(4):e12919. 10.1111/tid.12919 [DOI] [PubMed] [Google Scholar]
- 17. Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making Int J Soc Med Decis Making. 1993;13(4):322‐338. 10.1177/0272989;9301300409 [DOI] [PubMed] [Google Scholar]
- 18. Commenges D. Inference for multi‐state models from interval‐censored data. Stat Methods Med Res. 2002;11(2):167‐182. 10.1191/0962280202sm279ra [DOI] [PubMed] [Google Scholar]
- 19. Wan L, Lou W, Abner E, Kryscio RJ. A comparison of time‐homogeneous Markov chain and Markov process multi‐state models. Commun Stat: Case Stud Data Anal Appl. 2016;2(3‐4):92‐100. 10.1080/23737484.2017.1361366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson C. Multi‐state models for panel data: the MSM package for R. J Stat Softw. 2011;38(1):1‐28. doi: 10.18637/jss.v038.i08 [DOI] [Google Scholar]
- 21. Andersen PK, Keiding N. Multi‐state models for event history analysis. Stat Methods Med Res. 2002;11(2):91‐115. 10.1191/0962280202SM276ra [DOI] [PubMed] [Google Scholar]
- 22. Sutradhar R, Barbera L, Seow H, Howell D, Husain A, Dudgeon D. Multistate analysis of interval‐censored longitudinal data: application to a cohort study on performance status among patients diagnosed with cancer. Am J Epidemiol. 2011;173(4):468‐475. 10.1093/aje/kwq384 [DOI] [PubMed] [Google Scholar]
- 23. Corraini P, Olsen M, Pedersen L, Dekkers OM, Vandenbroucke JP. Effect modification, interaction and mediation: an overview of theoretical insights for clinical investigators. Clin Epidemiol. 2017;9:331‐338. 10.2147/CLEP.S129728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5(3):299‐314. [Google Scholar]
- 25. Wickham H. ggplot: an implementation of the grammar of graphics in R, 2006. R package version 04 0. Published online 2006.
- 26. Patil I. Visualizations with statistical details: the “ggstatsplot” approach. PsyArxiv. Published online 2021. https://psyarxiv.com/p7mku/
- 27. Soetaert K. Diagram: functions for visualising simple graphs (networks), plotting flow diagrams. Published online September 30, 2020. https://CRAN.R‐project.org/package=diagram
- 28. Regoes RR, Frances Bowen E, Cope AV, et al. Modelling cytomegalovirus replication patterns in the human host: factors important for pathogenesis. Proc R Soc B: Biol Sci. 2006;273(1596):1961‐1967. 10.1098/rspb.2006.3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lorenz EC, Egginton JS, Stegall MD, et al. Patient experience after kidney transplant: a conceptual framework of treatment burden. J Patient‐Rep Outcomes. 2019;3(1):8. 10.1186/s41687-019-0095-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khan S, Tighiouart H, Kalra A, Raman G, Rohrer RJ, Pereira BJG. Resource utilization among kidney transplant recipients. Kidney Int. 2003;64(2):657‐664. 10.1046/j.1523-1755.2003.00102.x [DOI] [PubMed] [Google Scholar]
- 31. Kirk AD, Adams AB, Durrbach A, et al. Optimization of de novo belatacept‐based immunosuppression administered to renal transplant recipients. Am J Transplant. 2021;21(5):1691‐1698. 10.1111/ajt.16386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schäfer P, Tenschert W, Cremaschi L, Schröter M, Zöllner B, Laufs R. Area under the viraemia curve versus absolute viral load: utility for predicting symptomatic cytomegalovirus infections in kidney transplant patients. J Med Virol. 2001;65(1):85‐89. [PubMed] [Google Scholar]
- 33. McCrea JB, Macha S, Adedoyin A, et al. Pharmacokinetic drug‐drug interactions between letermovir and the immunosuppressants cyclosporine, tacrolimus, sirolimus, and mycophenolate mofetil. J Clin Pharmacol. 2019;59(10):1331‐1339. 10.1002/jcph.1423 [DOI] [PubMed] [Google Scholar]
- 34. Shendi AM, Hung RKY, Caplin B, Griffiths P, Harber M. The use of sirolimus in patients with recurrent cytomegalovirus infection after kidney transplantation: a retrospective case series analysis. Saudi J Kidney Dis Transplant. 2019;30(3):606‐614. 10.4103/1319-2442.261333 [DOI] [PubMed] [Google Scholar]
- 35. Marrero WJ, Naik AS, Friedewald JJ, et al. Predictors of deceased donor kidney discard in the United States. Transplantation. 2017;101(7):1690‐1697. 10.1097/TP.0000000000001238 [DOI] [PubMed] [Google Scholar]
- 36. Eshraghi H, Hekmat R. Which CMV viral load threshold should be defined as CMV infection in kidney transplant patients? Transplant Proc. 2015;47(4):1136‐1139. 10.1016/j.transproceed.2014.11.066 [DOI] [PubMed] [Google Scholar]
- 37. Webb BJ, Harrington R, Schwartz J, et al. The clinical and economic impact of cytomegalovirus infection in recipients of hematopoietic stem cell transplantation. Transpl Infect Dis. 2018;20(5):e12961. 10.1111/tid.12961 [DOI] [PubMed] [Google Scholar]
- 38. Gentleman RC, Lawless JF, Lindsey JC, Yan P. Multi‐state Markov models for analysing incomplete disease history data with illustrations for HIV disease. Stat Med. 1994;13(8):805‐821. 10.1002/sim.4780130803 [DOI] [PubMed] [Google Scholar]
- 39. Piñana JL, Perez‐Pitarch A, Guglieri‐Lopez B, et al. Sirolimus exposure and the occurrence of cytomegalovirus DNAemia after allogeneic hematopoietic stem cell transplantation. Am J Transplant. 2018;18(12):2885‐2894. 10.1111/ajt.14754 [DOI] [PubMed] [Google Scholar]
- 40. Lockridge J, Roberts D, Olyaei A, et al. Cytomegalovirus serologic matching in deceased donor kidney allocation optimizes high‐ and low‐risk (D+R‐ and D‐R‐) profiles and does not adversely affect transplant rates. Am J Transplant. 2020;20:3502‐3508. Published online May 6, 2020. 10.1111/ajt.15976 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Comparison of the CMV viral load trajectories between high‐risk belatacept and tacrolimus‐treated recipients when the areas under the curves (AUC) are comparable. Belatacept‐treated recipients exhibited multipeak viral load dynamics even when AUCs were comparable.
Figure S2 Assessment of the extent to which the model can predict viral load observations by comparing expected with observed prevalence percent within CMV risk status.
Table S1 MSMM† hazard ratios associated with the effect of immunosuppression regimen on transition rates between adjacent state
Visual Abstract
Data Availability Statement
Deidentified data will be made available upon request to the corresponding author.


