Abstract
For women with dense breast tissue, who are at much higher risk for developing breast cancer, the performance of mammography is at its worst. Consequently, many early cancers go undetected when they are the most treatable. Improved cancer detection for women with dense breasts would decrease the proportion of breast cancers diagnosed at later stages, which would significantly lower the mortality rate. The emergence of whole breast ultrasound provides good performance for women with dense breast tissue, and may eliminate the current trade-off between the cost effectiveness of mammography and the imaging performance of more expensive systems such as magnetic resonance imaging.
We report on the performance of SoftVue, a whole breast ultrasound imaging system, based on the principles of ultrasound tomography. SoftVue was developed by Delphinus Medical Technologies and builds on an early prototype developed at the Karmanos Cancer Institute. We present results from preliminary testing of the SoftVue system, performed both in the lab and in the clinic. These tests aimed to validate the expected improvements in image performance. Initial qualitative analyses showed major improvements in image quality, thereby validating the new imaging system design. Specifically, SoftVue’s imaging performance was consistent across all breast density categories and had much better resolution and contrast. The implications of these results for clinical breast imaging are discussed and future work is described.
Keywords: Breast imaging, tissue characterization, ultrasound tomography, ultrasound, SoftVue
1. INTRODUCTION
National cancer screening statistics indicate that only 51% of eligible women undergo annual mammograms1,2. Access, fear of radiation and discomfort are some of the factors that contribute to the low participation rate. Greater participation would lead to increased detection of breast cancer at an earlier stage, resulting in longer survival. Increased participation and improved breast cancer detection would have the greatest impact on the nearly 1 in 3 women who are diagnosed each year with later stage (regional or greater) breast cancer, totaling approximately 60,000 women per year in the United States. The net effect would be an increase in survival and a corresponding decrease in mortality rates.
For women with dense breast tissue, who are at much higher risk for developing breast cancer3-7, the performance of mammography is at its worst7. Consequently many early cancers go undetected when they are the most treatable. Improved cancer detection for women with dense breasts would decrease the proportion of breast cancers diagnosed at later stages, which would significantly lower the mortality rate.
Although emerging technologies such as tomosynthesis and Positron Emission Mammography (PEM) may improve upon some of the limitations of standard mammography, they are unlikely to create a paradigm shift in performance because of their generation of ionizing radiation. On the other hand, magnetic resonance imaging (MRI) can significantly improve on these limitations by virtue of its volumetric, radiation-free imaging capability. Studies have shown that MR can have a positive impact in the breast management continuum ranging from risk assessment to diagnosis and treatment monitoring.8-18 However, MR requires long exam times and intravenous contrast agents. Furthermore, MR has long been prohibitively expensive for routine use and there is a need for an equivalent low-cost alternative. Conventional sonography, which is inexpensive, comfortable and radiation-free, is not a practical alternative because of its operator dependence and the time needed to scan the whole breast. Therefore, it continues to play only an adjunctive role in breast imaging19. The emergence of whole breast ultrasound may eliminate the trade-off between the cost effectiveness of mammography and the imaging performance of MR.
We report on the performance of SoftVue, a whole breast ultrasound imaging system, based on the principles of ultrasound tomography (UST)20-27. SoftVue was developed by Delphinus Medical Technologies (DMT), and is based on an early prototype27-33 developed at the Karmanos Cancer Institute (KCI). The SoftVue imaging system was designed to provide enhanced performance relative to the prototype. The new design was predicated upon the early prototype’s clinical performance and pursued four aims for better image quality:
-
(i)
reducing the image slice thickness,
-
(ii)
improving the in-plane resolution,
-
(iii)
improving image contrast,
-
(iv)
artifact suppression.
The design goals were attained by increasing the transducer array density from 256 to 2048 and the central frequency from 2 to 3 MHz. Additional improvements focused on increasing patient throughput by integrating the reconstruction computer into the system, redesigning the water control system, increasing the number of transmit data channels from 11 to 512 and dramatically reducing the image reconstruction time from 9000 to 10 seconds per slice. The technical and clinical improvements are summarized in Table 1 below.
Table 1.
SoftVue characteristics relative to prototype
| Technical Feature | UST prototype | SoftVue | Improvement |
|---|---|---|---|
| Number of elements | 256 | 2048 | 8× |
| Number of receive channels | 256 | 512 | 2× |
| Number of transmit channels | 11 | 512 | 45× |
| Data resolution | 12 bits | 14 bits | 4× |
| Water usage | 48 gal. | 2.5-5 gal. | 10× |
| Reconstruction time per slice | 9,000 s | 10 s | 900× |
| Operating Frequency | 2 MHz | 3 MHz | 1.5× |
|
| |||
| Clinical Feature | UST prototype | SoftVue | Improvement |
|
| |||
| Image resolution (volume) | 5 × 0.5 × 0.5 mm | 2.5 × 0.3 × 0.3 mm | 5× |
| Max. breast diameter | 20 cm | 22 cm | 10% |
| Patient Throughput | 2/day | 4/hour | 16× |
Testing of the SoftVue system has been performed in the lab to determine and confirm parameters such as data acquisition time, breast scan time and image reconstruction time. Testing at the KCI breast center assessed clinical performance parameters such as patient throughput and image quality. The purpose of this paper is to describe the initial technical and clinical performance of SoftVue and to present a qualitative assessment of its performance.
2. METHODS
The SoftVue imaging system was first tested in DMT laboratories during August and September of 2012. The SoftVue imaging system and the earlier prototype imaging system collected data from an anthropomorphic phantom, built by Ernest Madsen from the University of Wisconsin. The phantom was seeded with a number of inclusions (i.e. “masses”) ranging in size from 4 to 12 mm, as well as a group of microcalcifications. The phantom was designed to mimic a “dense” breast and challenge the imaging system performance to its operating limits.
Following laboratory testing, SoftVue was installed at the KCI’s, Alexander J. Walt Comprehensive Breast Center. SoftVue was initially co-located with the UST prototype to allow comparative clinical imaging. Clinical images were then obtained in the last quarter of 2012 to test the performance of SoftVue with human subjects. Participants were consented and data acquired under Wayne State University’s IRB (approval number #040912M1F). Fourteen healthy patient volunteers were scanned with SoftVue and with the UST prototype in order to assess relative performance differences. SoftVue’s image reconstruction algorithm was used to generate cross-sectional reflection B-Mode images of the phantom from both SoftVue and prototype data. For both systems, reflection images were corrected for refraction and attenuation effects to maximize image quality. A set of 45 tomograms (image slices) were generated for each patient. Overall image contrast and the presence of artifacts in each image stack was assessed using the software package ImageJ.
3. RESULTS
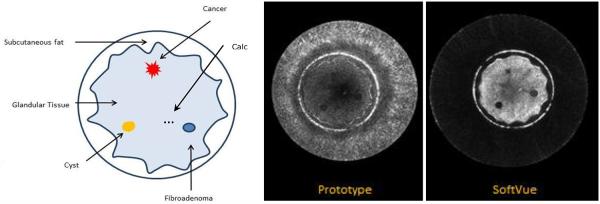
The SoftVue and prototype images produced from the phantom scans revealed the essential features of the phantom. Sample images are shown in Figure 1 with a schematic of the phantom components shown for comparison. The images shown reveal the skin, subcutaneous fat, glandular tissue, an 8mm cancer, a 12mm cyst, a 12mm fibroadenoma and a group of 500 micron sized microcalcifications. Both systems detected the relevant features but, as can be seen, in Figure 1, the SoftVue images have much greater contrast relative to background and greater signal to noise ratios for all 7 features. Furthermore, examination of the water background shows an almost complete elimination of artifacts relative to the prototype.
Figure 1.
Cross-sectional schematic of anthropomorphic breast phantom (left). The reconstruction from the early prototype is shown in the center. The SoftVue B-mode reconstruction is on the right.
In-Vivo Clinical Images
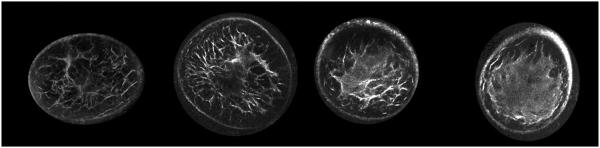
Figure 2 shows examples of reflection images obtained with the SoftVue imaging system. For increasing breast density on SoftVue reflection images, seen in Figure 2, underlying breast architecture of fibrous bands and/or Cooper’s ligaments become less and less visible as the enveloping parenchymal tissue contributes to the increasing radiographic density. In more fatty breasts, the specular reflectors of the fibrous bands are thus readily identified while the non-specular echo signals from the extended denser tissues dominate the images of the denser breasts. As shown below, masses are not obscured by the dense parenchyma, as in standard ultrasound, due to their differences in biomechanical, and therefore acoustic, properties.
Figure 2.
From left to right, cross-sectional coronal images of a fatty, scattered, heterogeneous and dense breast, as rendered by SoftVue.
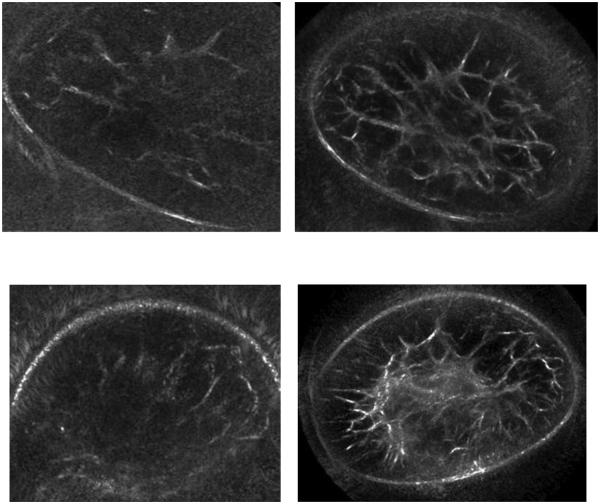
Figure 3 compares the reflection SoftVue images with those of the old prototype. The fibroglandular structures are revealed with greater sensitivity, contrast and resolution in the SoftVue images compared to the UST prototype. Despite the large contour of both breasts near the chest wall, the SoftVue images still visualize the entire breast with much better definition of the inner fibroglandular architecture. Even scanned at the same level per breast, the residual central parenchyma seen in the lower right SoftVue image is not even apparent on the degraded right prototype image. The 22cm diameter of the SoftVue ring array, compared with the 20cm prototype, allowed full visualization of breast skin boundary even near the chest wall. The much better noise reduction of the left SoftVue images also helps limit the near-field distortion that markedly degrades the prototype images.
Figure 3.
Coronal breast images show SoftVue images on the right and prototype images on the left. A more fatty breast (top row) displays much better architectural detail, while the breast with more scattered central density (bottom row) is now clearly defined. Despite both breasts being scanned near the chest wall, the larger diameter of the SoftVue ring array than the prototype allowed clear boundary definition and much less near-field distortion from the breasts contacting the ring array (obscured upper and lower aspects of the top and bottom left images, respectively).
4. DISCUSSION
The above phantom imaging results generated high resolution scans of a breast phantom that was designed to push both SoftVue and the UST prototype to their limits. In clinical terms, the phantom was designed to represent a very dense breast, corresponding to BIRADS category IV breast density, or over 75% dense parenchyma by mammography. In acoustic terms, the glandular tissue represented in the phantom has a sound speed of 1550 m/s and attenuation of ~1 dB/cm/MHz. At SoftVue’s operating frequency of 3MHz, the total attenuation along a straight-line path passing through the center of the phantom, at its widest point (13 cm) is ~ 40 dB. As indicated in Figure 1, this level of attenuation was a severe challenge for the UST prototype, despite operating at a lower frequency that should have allowed better penetration than SoftVue. Yet, the greater contrast resolution and noise reduction of SoftVue more than overcame any penetration deficit from the higher frequency. It is evident from SoftVue rendering of the glandular tissue, inclusions and the imbedded group of microcalcifications deep inside the phantom, that it performs well in this extreme case. The ability for whole breast ultrasound to perform well in dense breasts will be crucial for SoftVue to challenge mammography, which can miss up to 50% of cancers. Combined with the fact that women with dense breast tissue are much more likely to develop breast cancer, the clinical case for introducing whole breast ultrasound is compelling. If the SoftVue phantom results translate into the in-vivo domain for mass detection and discrimination above 5mm, the clinical case for SoftVue transitioning from diagnosis to screening may be made possible. We have begun scanning healthy volunteers and encouraging images were provided in the previous section.. These results demonstrated the ability to image breast architecture across the full range of breast density. Furthermore, it appears that the in-vivo images follow the phantom results, given the ability of SoftVue to penetrate even the densest breast tissues in a clinical setting.
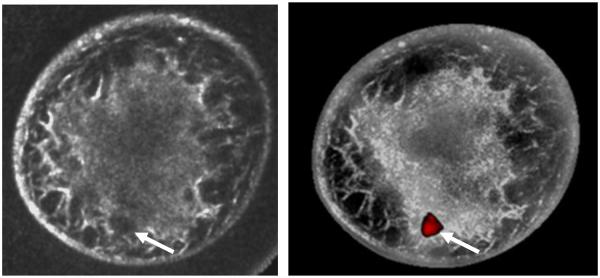
A major gap in these initial results arise from not addressing SoftVue’s clinical capability for mass detection and discrimination. To that end, we have begun a 300 patient study to quantify mass detectability and differentiation. The first cancer patient imaged thus far, presented with a 14 mm tumor (Figure 4). Figure 4 at least confirms that the cancer was detected within parenchymal tissue of a very dense breast. The reflection image was corrected using sound speed and attenuation data that was generated along with the reflection image. In the currently envisioned applications of SoftVue in the near term, reflection images will be used to provide diagnostic information using standard US-BIRADS criteria, pending FDA clearance to market. However, previous studies with the UST prototype suggest that sound speed and attenuation imaging can add diagnostic value that could augment the currently accepted US-BIRADS parameters. The ability of SoftVue to provide similar sound speed and attenuation information has not yet been tested but the 300 patient study will provide the needed clinical evaluation. A preliminary application of sound speed and attenuation data on the one cancer patient has successfully highlighted the mass and was based on a thresholding model that has been described fully in the literature32. The potential future of SoftVue imaging of breast cancer is shown in the right fusion image of Figure 4.
Figure 4.
SoftVue images show reflection-only reconstruction on left with an irregular but ill-defined 1.4cm invasive ductal carcinoma at the 6-7 o’clock position (arrows). The fusion image on the right used previously established thresholds to create a grayscale overlay which highlights the distribution of dense parenchyma. Moreover, the cancer is well seen when mass sound speed and attenuation thresholds were applied. Further work is needed to validate this highly encouraging initial fusion performance with other masses or suspicious foci.
5. CONCLUSIONS
A new UST imaging system has been designed with the goal of achieving clinical relevance in the area of breast cancer detection and tissue characterization. The main design driver was to bridge the gap between research and clinical practice by building and improving upon an earlier research prototype. The resulting imaging system, named SoftVue, has now been tested on a worst-case-scenario phantom and clinical evaluation is starting in a 300 patient study. The phantom results and the preliminary in-vivo data indicate a major improvement over the previous prototype, particularly in the area of dense breast imaging where mammography’s performance is at its weakest.
Successful implementation of this and other whole breast ultrasound technology will challenge existing paradigms. Current clinical practice is based on mammographic screening, with diagnostic follow-up and biopsy, frequently assisted by standard ultrasound and/or MR imaging. SoftVue has the potential to impact breast imaging and dramatically reduce the biopsy rate, which is the near term goal of SoftVue. In the long term, however, SoftVue may also challenge the screening paradigm and provide cost-efficacy by combining the screening and diagnostic steps. The current commercial version of SoftVue is the outcome of a platform UST technology whose performance could prove to be the equally effective, regardless of whether it is used for screening, diagnosis or future biopsy guidance. Furthermore, breast compression and use of ionizing radiation, or costly IV-enhanced MR would be averted.
Future near-term work is aimed at testing SoftVue’s diagnostic performance through a 300 patient study that began in January, 2013. In the longer term, multi-center trials are planned that will test the suitability of SoftVue for screening of both the high-risk and general population.
6. ACKNOWLEDGMENTS AND DISCLOSURES
The authors acknowledge the support of the National Cancer Institute through grant 1R44CA165320-01A1 and Susan G. Komen for the Cure through Grant KG100100. N. Duric and P. Littrup are co-founders of Delphinus Medical Technologies Inc. and are performing the clinical studies relating to SoftVue under a financial conflict of interest plan that has been approved by Wayne State University.
7. REFERENCES
- 1. SEER website. http://seer.cancer.gov/
- 2.American Cancer Society . Cancer Prevention & Early Detection Facts & Figures 2009. American Cancer Society; Atlanta, GA: 2009. pp. 34–37. Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli. [Google Scholar]
- 3.Chen J, Pee D, Ayyagari R, Graubard B, Schairer C, Byrne C, Benichou J, Gail MH. Projecting absolute invasive breast cancer risk in white women with a model that includes mammographic density. J Natl Cancer Inst. 2006;98:1215–1226. doi: 10.1093/jnci/djj332. [DOI] [PubMed] [Google Scholar]
- 4.Ursin G, Hovanessian-Larsen L, Parisky YR, Pike MC, Wu AH. Greatly increased occurrence of breast cancers in areas of mammographically dense tissue. Breast Cancer Res. 2005;7:R605–R608. doi: 10.1186/bcr1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin LJ, Boyd N. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10:1–14. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong K, Moye E, Williams S, Berlin JA, Reynolds EE. Screening mammography in women 40 to 49 years of age: a systematic review for the American College of Physicians. Ann Intern Med. 2007;146:516–26. doi: 10.7326/0003-4819-146-7-200704030-00008. [DOI] [PubMed] [Google Scholar]
- 7.A, Minkin S, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 8.Turnbull LW. Dynamic contrast-enhanced MRI in the diagnosis and management of breast cancer. J NMR Biomed. 2008 doi: 10.1002/nbm.1273. [DOI] [PubMed] [Google Scholar]
- 9.Jansen SA, Fan X, Karczmar GS, Abe H, Schmidt RA, Newstead GM. MAGNETIC RESONANCE IN MEDICINE. 4. Vol. 59. John Wiley & Sons, Ltd; 2008. Differentiation between benign and malignant breast lesions detected by bilateral dynamic contrast-enhanced MRI: A sensitivity and specificity study; p. 747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhl CK, Schrading S, Bieling HB, Wardelmann E, Leutner CC, Koenig R, Kuhn W, Schild HH. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370:485–92. doi: 10.1016/S0140-6736(07)61232-X. [DOI] [PubMed] [Google Scholar]
- 11.Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, Morris E, Pisano E, Schnall M, Sener S, Smith RA, Warner E, Yaffe M, Andrews KS, Russell CA, American Cancer Society Breast Cancer Advisory Group American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 12.Chen JH, et al. MRI evaluation of pathologically complete response and residual tumors in breast cancer after neoadjuvant chemotherapy. Cancer. 2008;112(1):17–26. doi: 10.1002/cncr.23130. [DOI] [PubMed] [Google Scholar]
- 13.Sharma U, et al. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed. 2009;22(1):104–13. doi: 10.1002/nbm.1245. [DOI] [PubMed] [Google Scholar]
- 14.Bando H, et al. Imaging evaluation of pathological response in breast cancer after neoadjuvant chemotherapy by real-time sonoelastography and MRI. European Journal of Cancer-Supplement. 2008;6(7):66–66. [Google Scholar]
- 15.Bhattacharyya M, et al. Using MRI to plan breast-conserving surgery following neoadjuvant chemotherapy for early breast cancer. Br J Cancer. 2008;98(2):289–93. doi: 10.1038/sj.bjc.6604171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Partridge S. Recurrence Rates After DCE-MRI Image Guided Planning for Breast-conserving Surgery Following Neoadjuvant Chemotherapy for Locally Advanced Breast Cancer Patients. Breast Diseases: A Year Book Quarterly. 2008;19(1):91–91. doi: 10.1016/j.ejso.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Tozaki M. Diagnosis of breast cancer: MDCT versus MRI. Breast Cancer. 2008;15(3):205–211. doi: 10.1007/s12282-008-0049-9. [DOI] [PubMed] [Google Scholar]
- 18.Partridge S, et al. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. Am Roentgen Ray Soc. 2002:1193–1199. doi: 10.2214/ajr.179.5.1791193. [DOI] [PubMed] [Google Scholar]
- 19.Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, Pisano ED, Jong RA, Evans WP, Morton MJ, Mahoney MC, Hovanessian Larsen L, Barr RG, Farria DM, Marques HS, Boparai K, the ACRIN 6666 Investigators Combined Screening With Ultrasound and Mammography vs Mammography Alone in Women at Elevated Risk of Breast Cancer. JAMA. 2008;299(18):2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carson PL, Meyer CR, Scherzinger AL, Oughton TV. Breast imaging in coronal planes with simultaneous pulse echo and transmission ultrasound. Science. 1981 Dec 4;214(4525):1141–3. doi: 10.1126/science.7302585. [DOI] [PubMed] [Google Scholar]
- 21.Andre MP, Janee HS, Martin PJ, Otto GP, Spivey BA, Palmer DA. High-speed data acquisition in a diffraction tomography system employing large-scale toroidal arrays. International Journal of Imaging Systems and Technology. 1997;8(1):137–147. [Google Scholar]
- 22.Johnson SA, Borup DT, Wiskin JW, Natterer F, Wuebbling F, Zhang Y, Olsen C. Apparatus and Method for Imaging with Wavefields using Inverse Scattering Techniques. 1999 United States Patent 6,005,916.
- 23.Marmarelis VZ, Kim T, Shehada RE. Proceedings of the SPIE: Medical Imaging; Ultrasonic Imaging and Signal Processing; 2003. Paper 5035-6. [Google Scholar]
- 24.Liu D-L, Waag RC. Propagation and backpropagation for ultrasonic wavefront design. IEEE Trans. on Ultras. Ferro. and Freq. Contr. 1997;44(1):1–13. doi: 10.1109/58.585184. [DOI] [PubMed] [Google Scholar]
- 25.Gemmeke H, Ruiter N. 3D ultrasound computer tomography for medical imaging. Nuclear instruments and methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2007;580(2):1057–1065. [Google Scholar]
- 26.Ruiter Nicole V., Göbel Georg, Berger Lutz, Zapf Michael, Gemmeke Hartmut. Realization of an optimized 3D USCT. Proc. SPIE. 2011;7968:796805. [Google Scholar]
- 27.Duric N, Littrup P, Poulo L, Babkin A, Pevzner R, Holsapple E, Rama O, Glide C. Detection of Breast Cancer With Ultrasound Tomography: First Results with the Computerized Ultrasound Risk Evaluation (C.U.R.E) Prototype. Medical Physics. 2007 Feb;34(2):773–785. doi: 10.1118/1.2432161. [DOI] [PubMed] [Google Scholar]
- 28.Glide CK, Duric N, Littrup P. A new method for quantitative analysis of mammographic density. Med Phys. 2007 Nov;3411:4491–4498. doi: 10.1118/1.2789407. [DOI] [PubMed] [Google Scholar]
- 29.Glide-Hurst C, Duric N, Littrup P. Volumetric breast density evaluation from ultrasound tomography images. Med Phys. 2008;35(9):3988–3997. doi: 10.1118/1.2964092. [DOI] [PubMed] [Google Scholar]
- 30.Duric N, Li C, Littrup PJ, Huang L, Glide-Hurst C, Rama O, Bey-Knight L, Schmidt S, Xu Y, Lupinacci J. Detection and characterization of breast masses with ultrasound tomography: clinical results. Proc. SPIE. 2008;6920:6920–28. [Google Scholar]
- 31.Li C, Duric N, Huang LJ. Clinical breast imaging using sound-speed reconstructions of ultrasound tomography data. Proc. SPIE. 2008;6920:6920–09. [Google Scholar]
- 32.Ranger B, Littrup P, Duric N, Chandiwala-Mody P, Li C, Schmidt S, Lupinacci J. Breast ultrasound tomography versus magnetic resonance imaging for clinical display of anatomy and tumor rendering: Preliminary results. AJR Am J Roentgenol. 2012 Jan;198(1):233–9. doi: 10.2214/AJR.11.6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lupinacci J, Duric N, Li C, Littrup P, Wang D, Rama O, Schmidt S. Monitoring of breast masses with ultrasound tomography for patients undergoing neoadjuvant chemotherapy. Proc. SPIE. 2009;7265:7265–43. [Google Scholar]