Abstract
Outer membrane vesicles (OMVs) are spherical bilayered nanoparticles derived from the outer layer of Gram-negative bacteria. Bacteria communicate with nearby bacteria, their environment, and the cells of their host by secreting OMVs, which are essential for their survival. OMVs also play a critical role in bacterial pathogenesis since they are loaded with virulence factors, toxins, and enzymes. OMVs may modulate the immune response of the host by initiating inflammation through cytokine production and activating the innate immune response. OMVs also contribute to the resistance of bacteria to antibiotics by carrying antibiotic-degrading enzymes and acting as natural protection barriers. Concerns have also been raised regarding OMVs mediating the transfer of antibiotic resistance. Due to their advantageous properties, OMVs are attractive platforms for vaccine discovery and drug delivery research. In this review, we discuss the fundamental structure and biogenesis mechanisms of OMVs as well as their multifaceted roles in bacterial infection pathogenesis and host immune responses. We also discuss application examples of OMVs.
Keywords: Gram-negative bacteria, Outer membrane vesicles, Pathogenesis, Virulence
INTRODUCTION
Extracellular vesicles produced by both eukaryotes and prokaryotic cells continuously interact with their environment. Exosomes are microvesicles derived from eukaryotic cells. Their roles in intercellular communication and usage potential in clinical applications such as diagnostic methods and drug delivery vehicles have been widely investigated [1,2]. Extracellular vesicles of prokaryotic cells are called membrane vesicles. While membrane vesicles are secreted by both Gram-positive as well as Gram-negative bacterial cells, the term “outer-membrane vesicles” (OMVs) is specifically used to refer to vesicles from Gram-negative bacteria that are enclosed naturally by an outer membrane.
OMVs from Gram-negative bacteria were first described in Escherichia coli in 1965. Since then, OMVs of all Gram-negative bacterial species identified to date have been determined, and are now considered a ubiquitous secretion process [3,4]. Initially, OMVs were presumed to be merely lysed bacterial debris. However, their delicate production mechanism and function as a selective secretion process have since been proven [5]. OMVs of Neisseria meningitidis detected in the cerebrospinal fluid of an infant with meningococcus infection drew attention to the role of OMVs in the pathogenesis of Gram-negative organism infections [6,7]. However, general models of OMV biogenesis are still lacking and regulation and selective cargo mechanisms remain unknown despite decades of research in this area. A major challenge in research on OMVs is the difficulty of purifying true OMV from artifacts, which leads to low yields of OMVs from large bacterial culture volumes. However, bona fide OMVs are produced via an orchestrated process and play multifaceted roles in bacterial survival, and the clinical utility of OMVs as vaccines and drug delivery vehicles is actively investigated.
In this review, we discuss recent advances in our understanding of OMV biogenesis mechanisms as well as the roles of OMVs in bacterial infection pathogenesis and host immune responses. We also discuss application examples of OMVs.
STRUCTURE AND BIOGENESIS OF OMVs
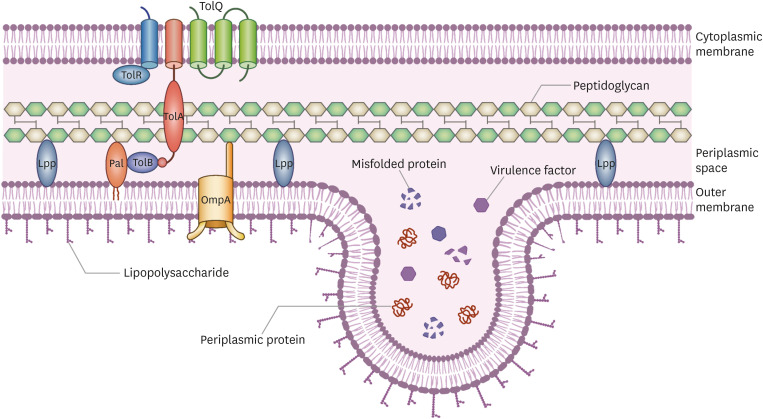
OMVs are single-membrane bilayered spherical particles ranging from 20 to 300 nm in size. OMVs are derived from the cell envelope of Gram-negative bacteria [8] (Fig. 1, 2). A good understanding of the cell wall structure of Gram-negative bacteria is therefore essential to understand the architecture of the OMVs. The cell envelope of Gram-negative bacteria consists of an outer membrane, a cytoplasmic membrane, and a periplasmic space. The outer membrane (OM) of Gram-negative bacteria is distinct from that of Gram-positive bacteria, in that it buds out and forms the OMV membrane. OM is asymmetrical, with the outer leaflet riched in lipopolysaccharide (LPS), and the inner leaflet primarily made up of phospholipids. The cytoplasmic membrane is composed of a phospholipid bilayer [9]. The transmembrane proteins of OM are referred to as outermembrane proteins (OMPs) and serve as porins or structural roles. Outer membrane protein A (OmpA) contains a periplasmic peptidoglycan (PG) binding site [10]. The space between the outer and cytoplasmic membranes is called the periplasmic space and contains a thin PG layer and periplasmic proteins. The PG layer serves as a skeleton for bacterial cells and protects against osmotic and shear stresses. Periplasmic proteins are densely packed in the periplasmic space, which is more viscous than the cytoplasm. Various crosslinks are present to maintain the stability of the cell envelope. Braun’s lipoprotein (Lpp) in the OM is covalently crosslinked with PG, and staples the PG layers together [11]. OmpA non-covalently binds with diaminopimelic acid (DAP) in the PG layer [12]. The transmembrane of the Tol-Pal complex enables OM to encompass the periplasmic space and cytoplasmic membrane [12,13,14].
Figure 1. This figure depicts cell wall envelope of the Gram-negative bacteria and biogenesis of Outer membrane vesicles (OMV). Gram-negative bacteria consists of two membrane. Outer leaflet of the outer membrane (OM) is composed of lipopolysaccharide (LPS). To stabilize membrane integrity, Braun's lipoprotein in the OM crosslinks OM layer and peptidoglycan (PG). Also Outer membrane protein A non-covalently binds with PG layer. The Tol-Pal complex which is consisted with cytoplasmic proteins, periplasmic protein, and outer membrane lipoprotein (peptidoglycan-associated lipoprotein) binds cell wall envelope together. OMV is generated from the OM by several mechanisms including reducing cell wall envelope crosslinks, LPS remodeling, and bilayer couple model by increasing OM fluidity. Periplasmic proteins including various virulence factors and cellular waste such as misfolded proteins are packaged into OMVs as cargos and secreted.
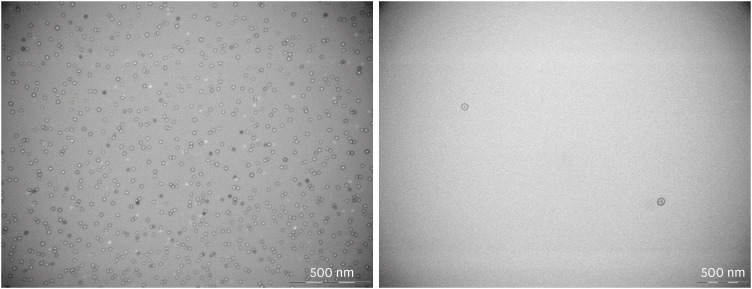
Figure 2. Transmission electron microscope-images of purified outer membrane vesicles derived from clinical isolates of Pseudomonas aeruginosa with magnification of × 80,000.
The OM should be free from crosslinks with PG, bulge outward to form budding vesicles, and finally detach to form OMVs. Several mechanisms have been proposed to explain OMV production and regulation; however, a definite mechanism still needs to be elucidated. Modulation of the envelope crosslink model is one of the earliest OMV biogenesis models proposed and has been widely investigated. Lpp and OmpA mutants in E. coli, Salmonella enterica, Vibrio cholera, and Pseudomonas aeruginosa were demonstrated to increase the fluidity of OM and lead to hypervesiculation [15,16]. In addition, the weakening of interactions between PG layers called DAP-DAP crosslinks leads to an increase in Lpp-PG crosslinks and thereby results in a decrease in OMV production [17,18]. Studies on the Lpp-independent OMVs production pathway have also been conducted. Porphyromonas gingivalis mutants lacking autolysin that cleaves PG amide bonds were found to lead to an increase in OMV production and thus motivated further studies on periplasmic accumulation [19]. Misfolded protein accumulation in the periplasmic space was also found to induce OMV production independent of Lpp crosslinks [18].
OMVs biogenesis based on alteration of the LPS content of the OM has also been reported in P. aeruginosa. This LPS remodeling process results in a selective type of anionic B-band LPS, which is detected in OMVs based on the mechanism of electronic charge repulsion [20,21]. Further studies on the modulation of OM lipid content have been carried out using the LPS-binding molecule Pseudomonas quinolone signal (PQS). PQS constitutes a quorum sensing system of P. aeruginosa and serves as a bacterial intercellular communication system. PQS is secreted to the outside of the cell and subsequently engages with the outer leaflet of OM to cause an expansion and changes in curvature thus resulting in increased production of OMVs [22,23,24].
The bilayer couple model is based on a mechanism of phospholipid accumulation in the outer leaflet of the outer membrane [25]. The VacJ/Yrb ATP-binding cassette (ABC) is a phospholipid transporter that prevents phospholipid accumulation in the outer leaflet of the OM [9]. Mutants that lack the VacJ/Yrb ABC transport system were found to show increased phospholipid accumulation in the cell and thus increased OMV production in Haemophilus influenzae and V. cholerae [26]. Furthermore, the activity of the VacJ/Yrb system was also found to be regulated by the presence of certain conditions, such as an iron-limited or bile-salt-enriched environment. This suggests that OMV production is regulated by environmental nutrient conditions [26,27].
FUNCTIONS OF OMVs in BACTERIAL INFECTION PATHOGENESIS
1. Delivery of virulence factors and toxins
Bacteria secrete OMVs that are loaded with various types of molecules, such as enzymes, proteins, and even genomic materials, to be delivered to distant sites. Investigation of OMV cargo revealed an abundance of OMPs (OmpA, OmpC, and OmpF), periplasmic proteins, virulence factors, and nucleic acids. OMVs also carry proteins from various subcellular locations as well [28]. Accordingly, periplasmic proteins are more likely to be included in OMVs compared to proteins bound to the inner membrane.
The inclusion of toxins in OMVs plays a particularly significant role in bacterial infection pathogenesis. Bacteria can deliver virulence factors to distant sites via OMVs that protect them from degradation due to biochemical stress while avoiding cell-to-cell interactions [28,29]. Enterotoxigenic E. coli (ETEC) produces several toxins, including heat-labile enterotoxin (LT) and cytolysin A (ClyA) [28,30]. OMVs of ETEC deliver these toxins to mammalian cells and enhance their virulence by inducing oligomerization of toxins [31]. Leukotoxin and LPS that are tightly bound to the surface of OMVs also lead to more pronounced immunogenic effects on host cells than when presented alone [4,32]. Cytotoxin necrotizing factor type 1 (CNF1) and Shiga toxin are found in OMVs of uropathogenic E. coli and E. coli O157:H7, respectively [33,34,35]. V. cholerae is a well-known pathogen that releases cholera toxin (CT) and secretes virulence factors via OMVs [36]. P. aeruginosa OMVs carry multiple virulence factors that cause degradation and pore formation [20]. OMVs of P. aeruginosa were also shown to have bacteriolytic effects on both Gram-negative and Gram-positive bacteria [37]. Salmonella typhimurium translocates the virulence PhoP/PhoQ regulon into host cells via OMVs, and thereby attenuates virulence in mice [38].
OMVs also release adhesion molecules to increase the adherence of bacteria to host tissues [39]. For example, P. gingivalis OMVs include hemagglutinins and heat shock proteins that are deeply involved in host cell attachment and bacterial aggregation by causing dental plaque [40,41]. P. aeruginosa and Bacteroides fragilis OMVs contain aminopeptidase and hemagglutinin, respectively, and increase the adherence of bacteria to mammalian cells [42,43]. However, OMVs containing adhesins may also act to compete with bacterial cells as well, since bacteria and OMVs use identical host-bacteria interaction mechanisms. For example, Helicobacter pylori OMVs were found to include less abundant adhesion molecules than the outer membrane [44].
2. Immunomodulatory activities
OMVs are internalized by host epithelial cells via direct fusion or diverse endocytosis mechanisms. OMVs of P. aeruginosa and Legionella pneumophila are internalized by the host cell through the actin remodeling process, which causes direct fusion and delivery of OMV cargo directly into the cytoplasm of the host cell [45,46]. Clathrin- and caveola-mediated endocytosis pathways also participate in internalization, allowing large vesicles up to 80 - 120 nm to invade the host [47,48]. Lipid rafts, which are microdomains in cell membranes rich in sphingolipids, mediate endocytosis and are responsible for the uptake of H. inflenzae, P. aeruginosa, and P. gingivalis OMVs [29,42,48,49].
OMVs trigger an inflammatory response in epithelial cells following the invasion. Increased levels of pro-inflammatory cytokines after processing of OMVs into host cell cultures have been observed in various pathogens as well. H. pylori OMVs showed dose-dependent production of interleukin-8 (IL-8) in gastric epithelial cells [50]. Likewise, OMVs of P. aeruginosa and Klebsiella pneumoniae that induce IL-1β and IL-8 in human alveolar epithelial cells have been described [51,52]. OMVs that stimulate immune reactions have also been reported in mouse models. Acinetobacter baumannii OMVs were administered intratracheally to induce IL-1β and IL-6 cytokines in the lungs of mice [53].
The pro-inflammatory mechanism of OMV described above has been established based on the engagement of pathogen-associated molecular patterns (PAMPs) with host pattern recognition receptors (PRRs). PAMPs are highly conserved microbial determinants detected by host PRR, resulting in the induction of immune signaling. OMVs contain LPS, flagellin, peptidoglycan, lipoproteins, DNA, and RNA, which serve as PAMPs in the host, and activate PRR [54]. The signaling pathway of PRR differs between bacterial species depending on the components of the OMVs. E. coli OMVs induce Toll-like receptors 4 dependent IL-8 production [55]. Neisseria gonorrhoeae and H. pylori OMVs transduce signals in other PRRs such as nucleotide-binding oligomerization domain-containing protein 1 (NOD1) [56].
OMVs also exhibit immunosuppressive properties since inflammation induced by OMVs is not beneficial to bacteria. OMVs from P. gingivalis contain the cysteine proteinase gingipain, which degrades IL-8 [57]. In addition, OMVs of N. meningitidis induce the production of anti-inflammatory cytokines, such as IL-4, IL-10, and IL-13. However, N. meningitidis OMVs also induce the production of pro-inflammatory cytokines IL-8, IL-1β, IL-6, and TNF, indicating that OMVs play a multifaceted role in both inflammation and immunosuppression [58]. Furthermore, OMVs of commensal bacteria in the gut are considered to promote the maturation of the immune system. B. fragilis releases capsular polysaccharides via their OMVs and results in enhanced activation of regulatory T cells and anti-inflammatory cytokine production, and prevention of experimental colitis [59].
ROLES IN ANTIBIOTIC RESISTANCE
OMVs aid bacterial survival by hindering the activity of antibiotics. OMVs act as decoys that bind and absorb antimicrobial peptides or phages, and thereby function as physical barriers. Hence, antibiotic resistance is developed in bacteria. The addition of OMVs or hypervesiculating mutants of E. coli were found to result in the immediate development of resistance to the antimicrobial peptides polymyxin B, colistin, and phages [60]. In addition, the growth inhibitory effects of colistin and melittin on Pseudomoas syringae were found to be reversed by the addition of OMVs produced by the same organism. However, the protective effect of the vesicles on the organism was not observed against hydrophilic antibiotic streptomycin [61]. OMVs bind peptide antibiotics with high affinity, yet do not bind well to hydrophilic antibiotics [62,63]. β-lactamase producing organisms are considered serious threats especially in hematologic malignancy units and intensive care unit [64,65]. OMVs of these β-lactamase producing organisms carry hydrolases and protect bacteria from antibiotics [66]. Also β-lactamase associated with OMVs protects not only producer bacteria but also standing non-resistant pathogenic bacteria [67]. For example, OMVs from β-lactam-resistant E. coli degrade β-lactam antibiotics in a dose-dependent manner and rescue β-lactam-susceptible E. coli and other bacterial species from β-lactam antibiotic-induced growth inhibition [68,69]. Similar findings have been reported in amoxicillin-resistant Moraxella catarrhalis OMVs as well. Active β-lactamase from M. catarrhalis OMVs was found to promote the survival of H. influenzae and Streptococcus pneumoniae [70].
OMV-mediated genomic transfer between microbial communities is also a concern due to the high prevalence of antibiotic resistance even in healthy carriers [71]. OMVs of Acinetobacter spp. and E. coli O157:H7 that transfer double-stranded DNA to intra- and inter-species have been reported [72]. OMVs from A. baumannii are also capable of transferring the OXA-24 carbapenemase gene, leading to further dissemination of antibiotic resistance in bacteria [73]. Recent studies on K. pneumoniae OMVs in hypervirulent and multidrug-resistant strains have also demonstrated plasmid horizontal gene transfer [74]. Plasmid exchange via OMVs generates hybrid clones of hypervirulent strains showing a hypermucoid and multidrug-resistant phenotype [75].
Interestingly, antibiotic use increases the production of OMVs as well. Antibiotics, including ciprofloxacin, meropenem, fosfomycin, and polymyxin B, were found to induce the production of OMVs in E. coli [76]. In addition, a study on multidrug-resistant K. pneumoniae demonstrated that the use of carbapenems exacerbates the secretion of OMVs, which promotes the production of pro-inflammatory cytokines [77].
CURRENT APPLICATION OF OMVs
OMVs are not capable of self-replication; however, they mimic the immunogenic properties of the producing bacteria, making them an attractive vaccine platform. OMVs also have a size advantage, which enables their entry into lymph vessels and uptake by antigen-presenting cells (APCs), in addition to strong adjuvant properties that stimulate both innate and adaptive immune responses, and high stability against biochemical stress [56,78].
A successful OMV-based vaccine developed to date is the meningitis serogroup B vaccine BEXSERO® (GlaxoSmithKline, London, UK), which was approved by the Food and Drug Administration in 2016. Unlike other N. meningitidis serogroups that already have successful conjugate vaccines on the market, serogroup B capsules were not feasible since this polysaccharide was homologous to human brain tissue molecules [79]. To overcome this obstacle, researchers have focused on the outer membrane protein porin A (PorA), which is the main immunogenic molecule secreted by OMVs in N. meningitidis. Although PorA is highly variable between strains, OMVs derived from bioengineered strains expressing multiple PorA variants have been successfully developed [80]. OMV-based vaccines were also investigated for use against other pathogens, including V. cholerae, S. typhimurium, Burkholderia species, H. pylori, and Bordetella pertussis, yet none have progressed to clinical trials. B. pertussis acellular vaccine development was recently hampered by a reactogenic problem that prevents the effective reduction of pertussis infections. OMV-based vaccine from B. pertussis may provide a viable alternative to this end, due to the presence of many virulence factors in OMV of B. pertussis.
Besides bacterial vaccines, studies on the bioengineering of OMVs as cancer agents have also been carried out. OMVs can be engineered to express cancer-specific epitopes or to carry small non-coding RNAs [81]. The ability of OMVs to rapidly display antigens may lead to the development of personalized cancer vaccines [82]. Furthermore, OMVs induce a durable antitumor immune response and inhibit tumor growth in multiple tumor models by producing the antitumor cytokines and interferon-γ [83]. Their potential as drug delivery systems is also being investigated. Finally, doxorubicin-loaded K. pneumoniae OMVs showed tumor growth inhibition with favorable tolerability and pharmacokinetic profiles, thus showing great potential for tumor chemoimmunotherapy [84].
CONCLUSION
In summary, we discussed OMVs’ basic structure, suggested biogenesis mechanisms, and their functions and role in pathogenesis and antimicrobial resistance. To our most recent knowledge, the OMVs seem to serve a significant role in bacterial cells and bacterium-host interaction. However, current research on the therapeutic applications of OMV, such as vaccines or drug-delivery platforms, is still under progress. With deeper understanding of OMVs’ biogenesis and selective cargo mechanisms, the value of OMVs as a future therapeutic tool may become revealed.
Footnotes
Funding: This work was supported by the MSIT (Ministry of Science and ICT), Korea, under the ICT Creative Consilience program (IITP-2023-2020-0-01819) supervised by the IITP (Institute for Information & communications Technology Planning & Evaluation); and Government-wide R&D Fund for Infections Disease Research (GFID), funded by the Ministry of the Interior and Safety, Korea [Grant number: 20015024].
Conflict of Interest: No conflict of interest.
- Conceptualization: JYK.
- Data curation: JYK, JWS, JSK.
- Resources: JWS, JSK.
- Supervision: JWS, SBK, YKY.
- Writing - original draft: JYK.
- Writing - review & editing: JYK, JWS, JSK, SBK, YKY, JWS.
References
- 1.Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, Viganò L, Locatelli A, Sisto F, Doglia SM, Parati E, Bernardo ME, Muraca M, Alessandri G, Bondiolotti G, Pessina A. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262–270. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 2.Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32:2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop DG, Work E. An extracellular glycolipid produced by Escherichia coli grown under lysine-limiting conditions. Biochem J. 1965;96:567–576. doi: 10.1042/bj0960567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74:81–94. doi: 10.1128/MMBR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J Bacteriol. 2006;188:5385–5392. doi: 10.1128/JB.00498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephens DS, Edwards KM, Morris F, McGee ZA. Pili and outer membrane appendages on Neisseria meningitidis in the cerebrospinal fluid of an infant. J Infect Dis. 1982;146:568. doi: 10.1093/infdis/146.4.568. [DOI] [PubMed] [Google Scholar]
- 7.Brandtzaeg P, Bryn K, Kierulf P, Ovstebø R, Namork E, Aase B, Jantzen E. Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass-spectrometry, ultracentrifugation, and electron microscopy. J Clin Invest. 1992;89:816–823. doi: 10.1172/JCI115660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beveridge TJ. Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol. 1999;181:4725–4733. doi: 10.1128/jb.181.16.4725-4733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malinverni JC, Silhavy TJ. An ABC transport system that maintains lipid asymmetry in the gram-negative outer membrane. Proc Natl Acad Sci U S A. 2009;106:8009–8014. doi: 10.1073/pnas.0903229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeh YC, Comolli LR, Downing KH, Shapiro L, McAdams HH. The caulobacter Tol-Pal complex is essential for outer membrane integrity and the positioning of a polar localization factor. J Bacteriol. 2010;192:4847–4858. doi: 10.1128/JB.00607-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli . Biochim Biophys Acta. 1975;415:335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y. The function of OmpA in Escherichia coli . Biochem Biophys Res Commun. 2002;292:396–401. doi: 10.1006/bbrc.2002.6657. [DOI] [PubMed] [Google Scholar]
- 13.Gerding MA, Ogata Y, Pecora ND, Niki H, de Boer PA. The trans-envelope Tol-Pal complex is part of the cell division machinery and required for proper outer-membrane invagination during cell constriction in E. coli . Mol Microbiol. 2007;63:1008–1025. doi: 10.1111/j.1365-2958.2006.05571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cascales E, Bernadac A, Gavioli M, Lazzaroni JC, Lloubes R. Pal lipoprotein of Escherichia coli plays a major role in outer membrane integrity. J Bacteriol. 2002;184:754–759. doi: 10.1128/JB.184.3.754-759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wessel AK, Liew J, Kwon T, Marcotte EM, Whiteley M. Role of Pseudomonas aeruginosa peptidoglycan-associated outer membrane proteins in vesicle formation. J Bacteriol. 2013;195:213–219. doi: 10.1128/JB.01253-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernadac A, Gavioli M, Lazzaroni JC, Raina S, Lloubès R. Escherichia coli tol-pal mutants form outer membrane vesicles. J Bacteriol. 1998;180:4872–4878. doi: 10.1128/jb.180.18.4872-4878.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwechheimer C, Kuehn MJ. Synthetic effect between envelope stress and lack of outer membrane vesicle production in Escherichia coli . J Bacteriol. 2013;195:4161–4173. doi: 10.1128/JB.02192-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwechheimer C, Kulp A, Kuehn MJ. Modulation of bacterial outer membrane vesicle production by envelope structure and content. BMC Microbiol. 2014;14:324. doi: 10.1186/s12866-014-0324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi J, Hamada N, Kuramitsu HK. The autolysin of Porphyromonas gingivalis is involved in outer membrane vesicle release. FEMS Microbiol Lett. 2002;216:217–222. doi: 10.1111/j.1574-6968.2002.tb11438.x. [DOI] [PubMed] [Google Scholar]
- 20.Kadurugamuwa JL, Beveridge TJ. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177:3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen TT, Saxena A, Beveridge TJ. Effect of surface lipopolysaccharide on the nature of membrane vesicles liberated from the Gram-negative bacterium Pseudomonas aeruginosa . J Electron Microsc (Tokyo) 2003;52:465–469. doi: 10.1093/jmicro/52.5.465. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Zhang L. The hierarchy quorum sensing network in Pseudomonas aeruginosa . Protein Cell. 2015;6:26–41. doi: 10.1007/s13238-014-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mashburn LM, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005;437:422–425. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- 24.Tashiro Y, Ichikawa S, Nakajima-Kambe T, Uchiyama H, Nomura N. Pseudomonas quinolone signal affects membrane vesicle production in not only gram-negative but also gram-positive bacteria. Microbes Environ. 2010;25:120–125. doi: 10.1264/jsme2.me09182. [DOI] [PubMed] [Google Scholar]
- 25.Schertzer JW, Whiteley M. A bilayer-couple model of bacterial outer membrane vesicle biogenesis. mBio. 2012;3:e00297–e00211. doi: 10.1128/mBio.00297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roier S, Zingl FG, Cakar F, Durakovic S, Kohl P, Eichmann TO, Klug L, Gadermaier B, Weinzerl K, Prassl R, Lass A, Daum G, Reidl J, Feldman MF, Schild S. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat Commun. 2016;7:10515. doi: 10.1038/ncomms10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lan Y, Zhou M, Li X, Liu X, Li J, Liu W. Preliminary investigation of iron acquisition in hypervirulent Klebsiella pneumoniae mediated by outer membrane vesicles. Infect Drug Resist. 2022;15:311–320. doi: 10.2147/IDR.S342368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 2004;23:4538–4549. doi: 10.1038/sj.emboj.7600471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharpe SW, Kuehn MJ, Mason KM. Elicitation of epithelial cell-derived immune effectors by outer membrane vesicles of nontypeable Haemophilus influenzae . Infect Immun. 2011;79:4361–4369. doi: 10.1128/IAI.05332-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horstman AL, Kuehn MJ. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J Biol Chem. 2000;275:12489–12496. doi: 10.1074/jbc.275.17.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wai SN, Takade A, Amako K. The release of outer membrane vesicles from the strains of enterotoxigenic Escherichia coli . Microbiol Immunol. 1995;39:451–456. doi: 10.1111/j.1348-0421.1995.tb02228.x. [DOI] [PubMed] [Google Scholar]
- 32.Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19:2645–2655. doi: 10.1101/gad.1299905. [DOI] [PubMed] [Google Scholar]
- 33.Kouokam JC, Wai SN, Fällman M, Dobrindt U, Hacker J, Uhlin BE. Active cytotoxic necrotizing factor 1 associated with outer membrane vesicles from uropathogenic Escherichia coli . Infect Immun. 2006;74:2022–2030. doi: 10.1128/IAI.74.4.2022-2030.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolling GL, Matthews KR. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Appl Environ Microbiol. 1999;65:1843–1848. doi: 10.1128/aem.65.5.1843-1848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia XX, Han MJ, Lee SY, Yoo JS. Comparison of the extracellular proteomes of Escherichia coli B and K-12 strains during high cell density cultivation. Proteomics. 2008;8:2089–2103. doi: 10.1002/pmic.200700826. [DOI] [PubMed] [Google Scholar]
- 36.Chatterjee D, Chaudhuri K. Association of cholera toxin with Vibrio cholerae outer membrane vesicles which are internalized by human intestinal epithelial cells. FEBS Lett. 2011;585:1357–1362. doi: 10.1016/j.febslet.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 37.Kadurugamuwa JL, Beveridge TJ. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J Bacteriol. 1996;178:2767–2774. doi: 10.1128/jb.178.10.2767-2774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon H, Ansong C, Adkins JN, Heffron F. Discovery of Salmonella virulence factors translocated via outer membrane vesicles to murine macrophages. Infect Immun. 2011;79:2182–2192. doi: 10.1128/IAI.01277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13:605–619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grenier D, Bélanger M. Protective effect of Porphyromonas gingivalis outer membrane vesicles against bactericidal activity of human serum. Infect Immun. 1991;59:3004–3008. doi: 10.1128/iai.59.9.3004-3008.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamaguchi A, Nakayama K, Ichiyama S, Nakamura R, Watanabe T, Ohta M, Baba H, Ohyama T. Effect of Porphyromonas gingivalis vesicles on coaggregation of Staphylococcus aureus to oral microorganisms. Curr Microbiol. 2003;47:485–491. doi: 10.1007/s00284-003-4069-6. [DOI] [PubMed] [Google Scholar]
- 42.Bauman SJ, Kuehn MJ. Pseudomonas aeruginosa vesicles associate with and are internalized by human lung epithelial cells. BMC Microbiol. 2009;9:26. doi: 10.1186/1471-2180-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patrick S, McKenna JP, O’Hagan S, Dermott E. A comparison of the haemagglutinating and enzymic activities of Bacteroides fragilis whole cells and outer membrane vesicles. Microb Pathog. 1996;20:191–202. doi: 10.1006/mpat.1996.0018. [DOI] [PubMed] [Google Scholar]
- 44.Olofsson A, Vallström A, Petzold K, Tegtmeyer N, Schleucher J, Carlsson S, Haas R, Backert S, Wai SN, Gröbner G, Arnqvist A. Biochemical and functional characterization of Helicobacter pylori vesicles. Mol Microbiol. 2010;77:1539–1555. doi: 10.1111/j.1365-2958.2010.07307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bomberger JM, Maceachran DP, Coutermarsh BA, Ye S, O’Toole GA, Stanton BA. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 2009;5:e1000382. doi: 10.1371/journal.ppat.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jäger J, Keese S, Roessle M, Steinert M, Schromm AB. Fusion of Legionella pneumophila outer membrane vesicles with eukaryotic membrane systems is a mechanism to deliver pathogen factors to host cell membranes. Cell Microbiol. 2015;17:607–620. doi: 10.1111/cmi.12392. [DOI] [PubMed] [Google Scholar]
- 47.Rewatkar PV, Parton RG, Parekh HS, Parat MO. Are caveolae a cellular entry route for non-viral therapeutic delivery systems? Adv Drug Deliv Rev. 2015;91:92–108. doi: 10.1016/j.addr.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furuta N, Tsuda K, Omori H, Yoshimori T, Yoshimura F, Amano A. Porphyromonas gingivalis outer membrane vesicles enter human epithelial cells via an endocytic pathway and are sorted to lysosomal compartments. Infect Immun. 2009;77:4187–4196. doi: 10.1128/IAI.00009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ismail S, Hampton MB, Keenan JI. Helicobacter pylori outer membrane vesicles modulate proliferation and interleukin-8 production by gastric epithelial cells. Infect Immun. 2003;71:5670–5675. doi: 10.1128/IAI.71.10.5670-5675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bauman SJ, Kuehn MJ. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 2006;8:2400–2408. doi: 10.1016/j.micinf.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JC, Lee EJ, Lee JH, Jun SH, Choi CW, Kim SI, Kang SS, Hyun S. Klebsiella pneumoniae secretes outer membrane vesicles that induce the innate immune response. FEMS Microbiol Lett. 2012;331:17–24. doi: 10.1111/j.1574-6968.2012.02549.x. [DOI] [PubMed] [Google Scholar]
- 53.Jun SH, Lee JH, Kim BR, Kim SI, Park TI, Lee JC, Lee YC. Acinetobacter baumannii outer membrane vesicles elicit a potent innate immune response via membrane proteins. PLoS One. 2013;8:e71751. doi: 10.1371/journal.pone.0071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bielaszewska M, Marejková M, Bauwens A, Kunsmann-Prokscha L, Mellmann A, Karch H. Enterohemorrhagic Escherichia coli O157 outer membrane vesicles induce interleukin 8 production in human intestinal epithelial cells by signaling via Toll-like receptors TLR4 and TLR5 and activation of the nuclear factor NF-κB. Int J Med Microbiol. 2018;308:882–889. doi: 10.1016/j.ijmm.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Söderblom T, Oxhamre C, Wai SN, Uhlén P, Aperia A, Uhlin BE, Richter-Dahlfors A. Effects of the Escherichia coli toxin cytolysin A on mucosal immunostimulation via epithelial Ca2+ signalling and Toll-like receptor 4. Cell Microbiol. 2005;7:779–788. doi: 10.1111/j.1462-5822.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 56.Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15:375–387. doi: 10.1038/nri3837. [DOI] [PubMed] [Google Scholar]
- 57.Mikolajczyk-Pawlinska J, Travis J, Potempa J. Modulation of interleukin-8 activity by gingipains from Porphyromonas gingivalis: implications for pathogenicity of periodontal disease. FEBS Lett. 1998;440:282–286. doi: 10.1016/s0014-5793(98)01461-6. [DOI] [PubMed] [Google Scholar]
- 58.Mirlashari MR, Høiby EA, Holst J, Lyberg T. Outer membrane vesicles from Neisseria meningitidis: effects on cytokine production in human whole blood. Cytokine. 2001;13:91–97. doi: 10.1006/cyto.2000.0803. [DOI] [PubMed] [Google Scholar]
- 59.Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–520. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manning AJ, Kuehn MJ. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011;11:258. doi: 10.1186/1471-2180-11-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kulkarni HM, Swamy CV, Jagannadham MV. Molecular characterization and functional analysis of outer membrane vesicles from the antarctic bacterium Pseudomonas syringae suggest a possible response to environmental conditions. J Proteome Res. 2014;13:1345–1358. doi: 10.1021/pr4009223. [DOI] [PubMed] [Google Scholar]
- 62.Chattopadhyay MK, Jagannadham MV. Corrigendum: Vesicles-mediated resistance to antibiotics in bacteria. Front Microbiol. 2015;6:974. doi: 10.3389/fmicb.2015.00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uddin MJ, Dawan J, Jeon G, Yu T, He X, Ahn J. The role of bacterial membrane vesicles in the dissemination of antibiotic resistance and as promising carriers for therapeutic agent delivery. Microorganisms. 2020;8:670. doi: 10.3390/microorganisms8050670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi H, Ahn H, Lee R, Cho SY, Lee DG. Bloodstream infections in patients with hematologic diseases: Causative organisms and factors associated with resistance. Infect Chemother. 2022;54:340–352. doi: 10.3947/ic.2022.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flores-Paredes W, Luque N, Albornoz R, Rojas N, Espinoza M, Pons MJ, Ruiz J. Evolution of antimicrobial resistance levels of ESKAPE microorganisms in a peruvian IV-level hospital. Infect Chemother. 2021;53:449–462. doi: 10.3947/ic.2021.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ciofu O, Beveridge TJ, Kadurugamuwa J, Walther-Rasmussen J, Høiby N. Chromosomal beta-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa . J Antimicrob Chemother. 2000;45:9–13. doi: 10.1093/jac/45.1.9. [DOI] [PubMed] [Google Scholar]
- 67.Lee J, Lee EY, Kim SH, Kim DK, Park KS, Kim KP, Kim YK, Roh TY, Gho YS. Staphylococcus aureus extracellular vesicles carry biologically active β-lactamase. Antimicrob Agents Chemother. 2013;57:2589–2595. doi: 10.1128/AAC.00522-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim SW, Park SB, Im SP, Lee JS, Jung JW, Gong TW, Lazarte JMS, Kim J, Seo JS, Kim JH, Song JW, Jung HS, Kim GJ, Lee YJ, Lim SK, Jung TS. Outer membrane vesicles from β-lactam-resistant Escherichia coli enable the survival of β-lactam-susceptible E. coli in the presence of β-lactam antibiotics. Sci Rep. 2018;8:5402. doi: 10.1038/s41598-018-23656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim SW, Lee JS, Park SB, Lee AR, Jung JW, Chun JH, Lazarte JMS, Kim J, Seo JS, Kim JH, Song JW, Ha MW, Thompson KD, Lee CR, Jung M, Jung TS. The importance of porins and β-lactamase in outer membrane vesicles on the hydrolysis of β-lactam antibiotics. Int J Mol Sci. 2020;21:2822. doi: 10.3390/ijms21082822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schaar V, Nordström T, Mörgelin M, Riesbeck K. Moraxella catarrhalis outer membrane vesicles carry β-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrob Agents Chemother. 2011;55:3845–3853. doi: 10.1128/AAC.01772-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alcedo K, Ruiz J, Ochoa TJ, Riveros M. High prevalence of bla CTX-M in fecal commensal Escherichia coli from healthy children. Infect Chemother. 2022;54:59–69. doi: 10.3947/ic.2021.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fulsundar S, Harms K, Flaten GE, Johnsen PJ, Chopade BA, Nielsen KM. Gene transfer potential of outer membrane vesicles of Acinetobacter baylyi and effects of stress on vesiculation. Appl Environ Microbiol. 2014;80:3469–3483. doi: 10.1128/AEM.04248-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rumbo C, Fernández-Moreira E, Merino M, Poza M, Mendez JA, Soares NC, Mosquera A, Chaves F, Bou G. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii . Antimicrob Agents Chemother. 2011;55:3084–3090. doi: 10.1128/AAC.00929-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hua Y, Wang J, Huang M, Huang Y, Zhang R, Bu F, Yang B, Chen J, Lin X, Hu X, Zheng L, Wang Q. Outer membrane vesicles-transmitted virulence genes mediate the emergence of new antimicrobial-resistant hypervirulent Klebsiella pneumoniae . Emerg Microbes Infect. 2022;11:1281–1292. doi: 10.1080/22221751.2022.2065935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jun JB. Klebsiella pneumoniae liver abscess. Infect Chemother. 2018;50:210–218. doi: 10.3947/ic.2018.50.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bauwens A, Kunsmann L, Karch H, Mellmann A, Bielaszewska M. Antibiotic-mediated modulations of outer membrane vesicles in enterohemorrhagic Escherichia coli O104:H4 and O157:H7. Antimicrob Agents Chemother. 2017;61:e00937–e00917. doi: 10.1128/AAC.00937-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ye C, Li W, Yang Y, Liu Q, Li S, Zheng P, Zheng X, Zhang Y, He J, Chen Y, Hua L, Yang Z, Li D, Ren Z, Yang Y, Qi J, Huang W, Ma Y. Inappropriate use of antibiotics exacerbates inflammation through OMV-induced pyroptosis in MDR Klebsiella pneumoniae infection. Cell Rep. 2021;36:109750. doi: 10.1016/j.celrep.2021.109750. [DOI] [PubMed] [Google Scholar]
- 78.Sartorio MG, Pardue EJ, Feldman MF, Haurat MF. Bacterial outer membrane vesicles: From discovery to applications. Annu Rev Microbiol. 2021;75:609–630. doi: 10.1146/annurev-micro-052821-031444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Finne J, Leinonen M, Mäkelä PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983;2:355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 80.van der Pol L, Stork M, van der Ley P. Outer membrane vesicles as platform vaccine technology. Biotechnol J. 2015;10:1689–1706. doi: 10.1002/biot.201400395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y, Fang Z, Li R, Huang X, Liu Q. Design of outer membrane vesicles as cancer vaccines: A new toolkit for cancer therapy. Cancers (Basel) 2019;11:1314. doi: 10.3390/cancers11091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cheng K, Zhao R, Li Y, Qi Y, Wang Y, Zhang Y, Qin H, Qin Y, Chen L, Li C, Liang J, Li Y, Xu J, Han X, Anderson GJ, Shi J, Ren L, Zhao X, Nie G. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology. Nat Commun. 2021;12:2041. doi: 10.1038/s41467-021-22308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH, Lee SW, Gho YS. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat Commun. 2017;8:626. doi: 10.1038/s41467-017-00729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuerban K, Gao X, Zhang H, Liu J, Dong M, Wu L, Ye R, Feng M, Ye L. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm Sin B. 2020;10:1534–1548. doi: 10.1016/j.apsb.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]