Abstract
Various therapeutic approaches, including supplemental nutritional support, have been tried for the treatment of atopic dermatitis (AD). Previous studies have reported the role of vitamin D in the treatment of AD with inconsistent results. The aim of this study was to evaluate the effectiveness of vitamin D in the treatment of AD, with considerations on the heterogeneities of AD. Randomized controlled trials (RCTs) on the efficacy of vitamin D supplementation for AD treatment, published before June 30, 2021 were identified in the PubMed, EMBASE, MEDLINE, and Cochrane Library databases. The quality of evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluation system. This meta-analysis included 5 RCTs with 304 cases of AD. We found that vitamin D supplementation did not decrease AD severity, even when AD was classified as severe vs non-severe. However, vitamin D supplementation was found to be effective in the treatment of AD in RCTs that included both children and adults, but not in those that included only children. Geographic location was associated with a significant difference in the therapeutic effect of vitamin D supplementation. Moreover, vitamin D supplementation of > 2,000 IU/day decreased AD severity, but supplementation ≤ 2,000 IU/day did not. Vitamin D supplementation, in general, was not effective for the treatment of AD. However, vitamin D supplementation might provide a therapeutic effect depending on the geographic location and dose of supplementation. The results of the present meta-analysis suggest that vitamin D supplementation might be targeted for patients with AD who may benefit from vitamin D supplementation.
Keywords: Atopic dermatitis, meta-analysis, vitamin D, treatment, randomized controlled trial, child, adult
INTRODUCTION
Atopic dermatitis (AD) is a common chronic inflammatory skin disease with a prevalence of approximately 20% in children and 10% in adults.1 The diverse AD phenotypes suggests different pathophysiologies underlying each AD phenotype and the need for targeted therapies and personalized medicine.2 Supplemental nutritional support has been considered as a potential adjuvant therapy for AD.3 Vitamin D might affect the clinical course of AD through the modulation of immune responses and skin barrier dysfunction.4,5,6 The previous studies on the effect of vitamin D supplementation on the treatment of AD have shown inconclusive results.7,8 Two previous meta-analyses showed the potential therapeutic effect of vitamin D on the treatment of AD without consideration of heterogeneities related to AD:3,9 one study included 4 randomized controlled trials (RCTs)9 and the other included 1 intervention study, 1 cross-sectional study, and 3 RCTs.3 In this systematic review and meta-analysis, we aimed to evaluate the efficacy of vitamin D supplementation in the treatment of AD. In addition, we compared the therapeutic effects of vitamin D supplementation for the treatment of AD according to age groups, disease severity, geographical regions, and duration and dose of vitamin D supplementation.
MATERIALS AND METHODS
Literature search strategy
A literature search of PubMed, EMBASE, MEDLINE, and Cochrane Library databases was conducted for articles published from database inception to June 30, 2021. The following keywords were used: ([Vitamin D OR calciferol* OR ergocalciferol*] or [treatment]) and (atopic dermatitis). The search was restricted to English language publications. The systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.10 No ethical approval was required for the analysis of publicly available anonymized data, and this systematic review was not registered.
Selection criteria and study selection
The inclusion criteria for study selection were as follows: 1) all study participants were patients with AD; 2) RCTs that reported data on the clinical efficacy of vitamin D supplementation in the treatment of AD; 3) oral administration of vitamin D; and 4) dose and duration of vitamin D supplementation clearly reported. The exclusion criteria were as follows: 1) case reports, reviews, letters, editorials, and publications that included overlapping study populations; 2) vitamin D prescribed as an ointment; 3) vitamin D supplementation combined with other vitamins; and (4) localized AD, such as hand eczema, and other types of eczema such as winter-related AD. Study selection was performed independently by 2 of the 4 reviewers (Sol IS, Park JS, Lee KS, or Lee E, and any disagreements were resolved by consensus.
Definition of subgroups
The severity of AD was classified based on the SCORing atopic dermatitis (SCORAD) index (< 15, mild; 15 ≤ moderate < 40; ≥ 40, severe)11 or Eczema Area and Severity Index (EASI) score (0, almost clear; 0.1–1, clear; 1.1–7, mild; 7.1–21, moderate; 21.1–50, severe; 50–72 very severe).12 Subgroup analyses of the effects of vitamin D on the treatment of AD were performed according to age group (children vs. both children and adults), disease severity (severe, defined as any RCT that enrolled only severe AD patients; non-severe, defined as any RCT that enrolled mild to moderate AD patients; and total, defined as any RCT that enrolled mild to severe AD patients), World Health Organization (WHO) geographical regions (European Region [EUR], Eastern Mediterranean Region [EMR], Region of the Americas [AMR], and Western Pacific Region [WPR]) and duration (1–2 months vs. 3 months) and dose of vitamin D supplementation (≤ 2,000 IU/day vs. > 2,000 IU/day).
Assessment of risk of bias
Two authors independently assessed the included studies using the Cochrane risk of bias tool version 2 (Table).13
Table. Study and participant characteristics.
| Study | Countries | Study design | Vitamin D supplementation | Control | AD severity | Study population | Dosage of vitamin D, IU | Frequency of vitamin D supplementation, /day | Duration of vitamin D supplementation | Latitude | Overall bias | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age, yr, mean | No. | Age, yr, mean | ||||||||||
| Galli et al.14 2015 | Italy | RCT | 41 | 7.6 (range, 0.9–16.3) | 48 | 4.8 (range, 1.8–15) | SCORAD, mild–moderate | Children | 2,000 | 1 | 3 mon | 45° N | Some concerns |
| Javanbakht et al.15 2011 | Iran | RCT | 12 | 21.2 | 11 | 26.1 | SCORAD, mild–severe | Adults and children | 1,600 | 1 | 2 mon | 30° N | Low |
| Lara-Corrales et al.8 2019 | Canada | RCT | 21 | 8.1 | 24 | 8.5 | SCORAD, moderate | Children | 2,000 | 1 | 3 mon | 75° N | Low |
| Sanchez-Armendariz et al.16 2018 | Mexico | RCT | 29 | 12.9 | 29 | 12.2 | SCORAD, moderate–severe | Adults and children | 5,000 | 1 | 3 mon | 19° N | Low |
| Mansour et al.12 2020 | Egypt | RCT | 47 | 12 (median) | 42 | 11.0 (median) | EASI, severe | Adults and children | 1,600 | 1 | 3 mon | 30° N | Low |
AD, atopic dermatitis; RCT, randomized controlled trial; SCORAD, SCORing atopic dermatitis; EASI, Eczema Area and Severity Index.
Statistical analysis
Review Manager (Rev Man 5.3; Cochrane Collaboration, London, UK) was used to perform the meta-analyses. The I2 statistic was used to assess heterogeneity between the study outcomes. The meta-analyses were conducted using the random effects model. The effect size was calculated with the standardized mean difference (SMD). P values < 0.05 were considered as statistically significant. The statistical analyses were conducted using the R version 3.4.1.
RESULTS
Characteristics of the studies
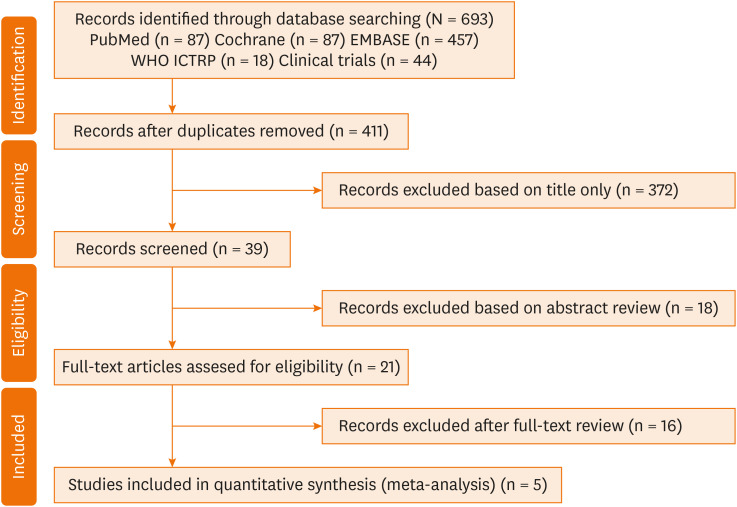
Five articles with 304 patients with AD were included in our systematic review and meta-analysis (Fig. 1).8,12,14,15,16 Two studies included only children,8,14 and the other 3 studies were performed in both children and adults (Table).12,15,16 One study15 included patients with mild to severe AD, and another12 included patients with severe AD. In 4 studies,8,12,14,15 ≤ 2,000 IU/day of vitamin D was administered, whereas in one study, 5,000 IU/day of vitamin D was administered.16 Vitamin D supplementation was administered for 3 months in 4 studies,8,12,14,15 whereas vitamin D was administered for 1-2 months in one study.15 Four RCTs8,14,15,16 reported the severity of AD using SCORAD index, and one RCT12 measured the severity of AD using EASI score.
Fig. 1. PRISMA flow diagram.
Outcomes
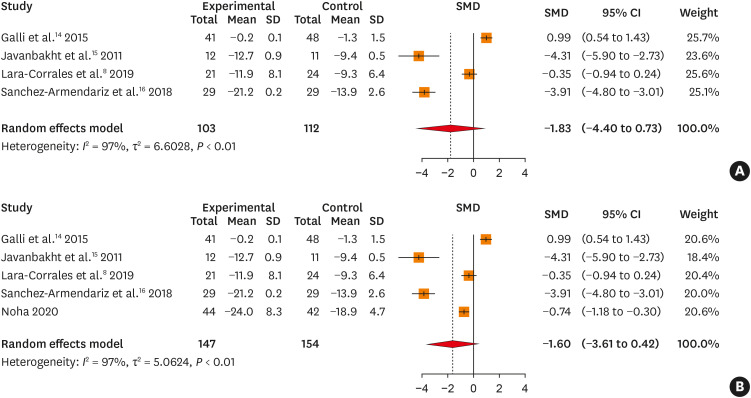
There was no significant reduction in the SCORAD index in the vitamin D group, compared to those in the placebo group (SMD, –1.835; 95% confidence interval [95% CI], –4.399 to 0.729, P = 0.161; Fig. 2A). Even when the severity of AD was assessed using a combination of the SCORAD index and EASI scores, there was no significant change in SMD in intervention with vitamin D for the treatment of AD, compared to that in the control group of AD patients (SMD, –1.595; 95% CI, –3.606 to 0.416, P = 0.120; Fig. 2B).
Fig. 2. (A) Forest plot for changes in SCORAD index in 4 RCTs. (B) Forest plot for changes in the SMD of the combination of SCORAD index and Eczema Area and Severity Index scores in 5 RCTs.
SD, standard deviation; SMD, standardized mean difference; CI, confidence interval; SCORAD, SCORing atopic dermatitis; RCT, randomized controlled trial.
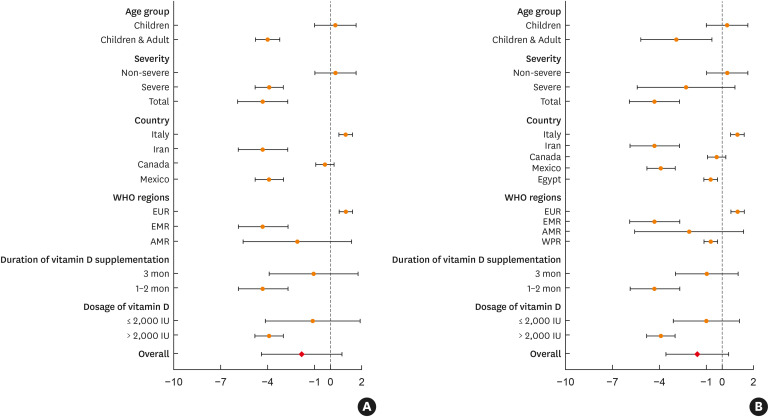
In studies that enrolled both children and adults, the SMD of the SCORAD index (SMD, –4.005; 95% CI, –4.786 to –3.223, P < 0.001), as well as the combination of the SCORAD index and EASI scores (SMD, –2.908; 95% CI, –5.176 to –0.639, P = 0.012) was significantly decreased in the intervention group with vitamin D supplementation compared to that in the placebo group (Fig. 3). However, there was no significant difference in SMD of the SCORAD index in RCTs that enrolled only children (SMD, 0.332; 95% CI, –0.981 to 1.645, P = 0.620).
Fig. 3. (A) Forest plot for changes in the SCORAD index in subgroup analyses in 4 RCTs. (B) Forest plot for changes in the SCORAD index and Eczema Area and Severity Index score in subgroup analyses in 5 RCTs.
EUR, European Region; EMR, Eastern Mediterranean Region; AMR, Region of the Americas; SCORAD, SCORing atopic dermatitis; RCT, randomized controlled trial.
The effect of vitamin D supplementation on the treatment of AD was found to differ according to the severity of AD. Vitamin D supplementation significantly decreased the SMD of the SCORAD index in patients with severe AD compared to that in the placebo group (SMD, –3.906; 95% CI, –4.803 to –3.008, P < 0.001). However, there was no significant decrease in SMD of the combination of SCORAD index and EASI scores (SMD, –2.296; 95% CI, –5.401 to 0.808, P = 0.147). One RCT,15 which enrolled patients with mild to severe AD, showed a significant decrease in SMD of the SCORAD index (SMD, –4.315; 95% CI, –5.904 to –2.725, P < 0.001) in the vitamin D supplementation group compared to that in the placebo group, whereas no significant difference was observed in SMD of the SCORAD index in another RCT,14 which enrolled patients with mild to moderate AD (SMD, 0.332; 95% CI, –0.981 to 1.645, P = 0.620).
The effects of vitamin D supplementation on the treatment of AD differed according to countries and geographic regions. The SMD of the SCORAD index significantly decreased in the vitamin D supplementation group compared to that in the control group in RCTs performed in Iran (SMD, –4.315; 95% CI, –5.904 to –2.725, P < 0.001) and Mexico (SMD, –3.906; 95% CI, –4.803 to –3.008, P < 0.001). The SMD of the SCORAD index significantly increased in the vitamin D supplement group compared to that in the placebo group in 1 RCT performed in Italy (SMD, 0.987; 95% CI, –0.545 to 1.430, P < 0.001). When the countries were classified according to the WHO geographic regions, there was no significant difference in SMD of the SCORAD index between the vitamin D supplementation and placebo groups in 2 RCTs performed in the AMR (SMD, –2.113; 95% CI, –5.594 to 1.369, P = 0.234).
The SMD of the SCORAD index was significantly decreased in the intervention group that had been taking vitamin D for 1–2 months compared to that in the placebo group (SMD, –4.315; 95% CI, –5.904 to –2.725, P < 0.001). When the RTCs were classified by dosage of vitamin D supplementation, the SMD of the SCORAD index was significantly decreased in the intervention group with >2000 IU/day of vitamin D compared to that in the placebo group (SMD, –3.906; 95% CI, –4.803 to –3.008, P < 0.001).
Publication bias
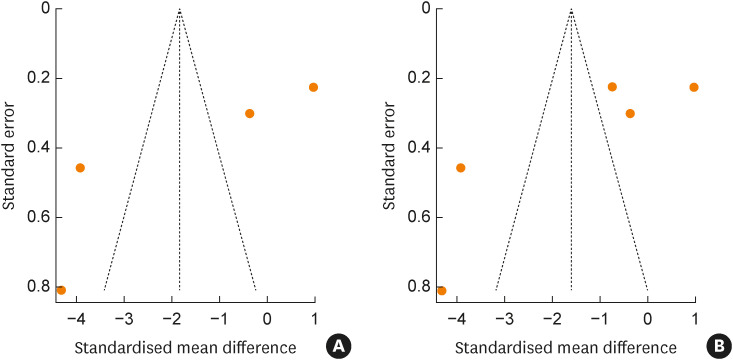
Both funnel plots were asymmetric, indicating the possibility of publication bias in the outcomes of the SCORAD index and combination of the SCORAD index and EASI scores (Fig. 4). However, Eggers test results on the SCORAD index and combination of SCORAD index and EASI scores were not significant (P = 0.105 and 0.120, respectively; data not shown), demonstrating the absence of significant publication bias.
Fig. 4. (A) Publication bias for RCTs using the SCORAD index. (B) RCTs using the combination of the SCORAD index and Eczema Area and Severity Index score.
RCT, randomized controlled trial; SCORAD, SCORing atopic dermatitis.
DISCUSSION
Our meta-analysis showed that vitamin D supplementation in patients with AD had no effect on the severity of AD. However, the subgroup analyses revealed that vitamin D supplementation can be effective in the treatment of AD depending on countries and WHO geographic regions, with the effective duration and dosage of vitamin D supplementation for the treatment of AD. The results of the present meta-analysis would be helpful in targeting patients in whom vitamin D supplementation is beneficial in treating AD, even without information on the levels of serum vitamin D.
Uncertain conclusions of the effect of vitamin D supplementation for the treatment of AD might be associated with heterogeneities between studies. Although the heterogeneities related to AD need to be considered when interpreting study results, most guidelines did not mention the effects of vitamin D supplementation for the treatment of AD based on the phenotypes of AD17,18,19; an exception is the severity of AD, which was mentioned in 1 consensus.19 Unlike other meta-analysis and review studies,3,9,20 our meta-analysis included only RCTs on the impact of vitamin D supplementation in the treatment of AD, regardless of the levels of serum vitamin D, with consideration on the heterogeneities of AD. All participants in our meta-analysis were patients with AD and specific types of AD, such as winter-related and localized AD, were excluded.9,20 The characteristics of AD patients, such as high-risk populations, and comparison groups, such as a healthy population, might affect the conclusions. These factors might partially explain the differences in the conclusions between the present and previous studies,3,9 which concluded that vitamin D supplementation might be beneficial in improving AD symptoms.
There have been no clear recommendations on the effective dose and duration of vitamin D supplementation for the treatment of AD. The present meta-analysis showed that supplementation of high-dose vitamin D (> 2,000 IU/day) can be effective for the treatment of AD, whereas a supplementation dosage of ≤ 2,000 IU/day of vitamin D has no effect on the treatment of AD. There may be concerns on the safety of high-dose vitamin D supplementation in children. In the present meta-analysis, 1 RCT, which administered 5,000 IU/day of vitamin D in children and adults for 12 weeks, reported no adverse reaction.16 In addition, vitamin D supplementation for 1–2 months was effective for the treatment of AD, whereas vitamin D supplementation for a longer period (e.g., 3 months) showed no significant therapeutic effects on the severity of AD. One RCT discussed short-term (e.g., 2 months) supplementation of vitamin D.15 Further studies on the proper duration of vitamin D supplementation for the treatment of AD based on age are required.
This study has several potential limitations. The RCTs that were included did not measure the serum vitamin D levels of the participants pre- and post-vitamin D supplementation. Therefore, the results of the present study were not associated with the serum vitamin D levels in patients with AD. Our meta-analysis did not consider diverse environmental factors, such as sun exposure, which could affect the levels of the active form of vitamin D. Lastly, the number of RCTs in our meta-analysis was relatively small, making it hard to reach a robust conclusion.
Our results suggest vitamin D supplementation in general was not effective for the treatment of AD. However, vitamin D supplementation might provide a therapeutic effect depending on the geographic location and dose of supplementation. The results of the present meta-analysis suggest that vitamin D supplementation might be targeted for patients with AD who may benefit from vitamin D supplementation.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2022R1A2C2011078).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345–360. doi: 10.1016/S0140-6736(20)31286-1. [DOI] [PubMed] [Google Scholar]
- 2.Nomura T, Kabashima K. Advances in atopic dermatitis in 2019–2020: endotypes from skin barrier, ethnicity, properties of antigen, cytokine profiles, microbiome, and engagement of immune cells. J Allergy Clin Immunol. 2021;148:1451–1462. doi: 10.1016/j.jaci.2021.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Hattangdi-Haridas SR, Lanham-New SA, Wong WH, Ho MH, Darling AL. Vitamin D deficiency and effects of vitamin D supplementation on disease severity in patients with atopic dermatitis: a systematic review and meta-analysis in adults and children. Nutrients. 2019;11:1158. doi: 10.3390/nu11081854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4:1151–1165. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roider E, Ruzicka T, Schauber J. Vitamin d, the cutaneous barrier, antimicrobial peptides and allergies: is there a link? Allergy Asthma Immunol Res. 2013;5:119–128. doi: 10.4168/aair.2013.5.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dombrowski Y, Peric M, Koglin S, Ruzicka T, Schauber J. Control of cutaneous antimicrobial peptides by vitamin D3. Arch Dermatol Res. 2010;302:401–408. doi: 10.1007/s00403-010-1045-4. [DOI] [PubMed] [Google Scholar]
- 7.Samochocki Z, Bogaczewicz J, Jeziorkowska R, Sysa-Jędrzejowska A, Glińska O, Karczmarewicz E, et al. Vitamin D effects in atopic dermatitis. J Am Acad Dermatol. 2013;69:238–244. doi: 10.1016/j.jaad.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Lara-Corrales I, Huang CM, Parkin PC, Rubio-Gomez GA, Posso-De Los Rios CJ, Maguire J, et al. Vitamin D level and supplementation in pediatric atopic dermatitis: a randomized controlled trial. J Cutan Med Surg. 2019;23:44–49. doi: 10.1177/1203475418805744. [DOI] [PubMed] [Google Scholar]
- 9.Kim G, Bae JH. Vitamin D and atopic dermatitis: a systematic review and meta-analysis. Nutrition. 2016;32:913–920. doi: 10.1016/j.nut.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willemsen MG, van Valburg RW, Dirven-Meijer PC, Oranje AP, van der Wouden JC, Moed H. Determining the severity of atopic dermatitis in children presenting in general practice: an easy and fast method. Dermatol Res Pract. 2009;2009:357046. doi: 10.1155/2009/357046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansour NO, Mohamed AA, Hussein M, Eldemiry E, Daifalla A, Hassanin S, et al. The impact of vitamin D supplementation as an adjuvant therapy on clinical outcomes in patients with severe atopic dermatitis: a randomized controlled trial. Pharmacol Res Perspect. 2020;8:e00679. doi: 10.1002/prp2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minozzi S, Cinquini M, Gianola S, Gonzalez-Lorenzo M, Banzi R. The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J Clin Epidemiol. 2020;126:37–44. doi: 10.1016/j.jclinepi.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Galli E, Rocchi L, Carello R, Giampietro PG, Panei P, Meglio P. Serum Vitamin D levels and Vitamin D supplementation do not correlate with the severity of chronic eczema in children. Eur Ann Allergy Clin Immunol. 2015;47:41–47. [PubMed] [Google Scholar]
- 15.Javanbakht MH, Keshavarz SA, Djalali M, Siassi F, Eshraghian MR, Firooz A, et al. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatolog Treat. 2011;22:144–150. doi: 10.3109/09546630903578566. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez-Armendáriz K, García-Gil A, Romero CA, Contreras-Ruiz J, Karam-Orante M, Balcazar-Antonio D, et al. Oral vitamin D3 5000 IU/day as an adjuvant in the treatment of atopic dermatitis: a randomized control trial. Int J Dermatol. 2018;57:1516–1520. doi: 10.1111/ijd.14220. [DOI] [PubMed] [Google Scholar]
- 17.Wollenberg A, Christen-Zäch S, Taieb A, Paul C, Thyssen JP, de Bruin-Weller M, et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34:2717–2744. doi: 10.1111/jdv.16892. [DOI] [PubMed] [Google Scholar]
- 18.Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850–878. doi: 10.1111/jdv.14888. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Kim JE, Park GH, Bae JM, Byun JY, Shin MK, et al. Consensus update for systemic treatment of atopic dermatitis. Ann Dermatol. 2021;33:497–514. doi: 10.5021/ad.2021.33.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng JC, Yew YW. Effect of vitamin D serum levels and supplementation on atopic dermatitis: a systematic review and meta-analysis. Am J Clin Dermatol. 2022;23:267–275. doi: 10.1007/s40257-022-00677-0. [DOI] [PubMed] [Google Scholar]