Abstract
Endoscopic spine surgery (ESS) has evolved as a safe, effective, and efficient alternative for minimally invasive spine surgery (MISS). The innovation of full-endoscopic systems makes definitive decompression surgery through different approaches feasible. The approach can be determined according to the location of the target lesion or the surgeon's preference. During the past 2 decades, ESS has expanded its indications from lumbar to cervical spines. Except for decompression, endoscopy-assisted fusion surgery is also developing. However, ESS is still evolving and has a steep learning curve. The revolution of technologies and ESS techniques will enable surgeons to treat various spinal diseases more practically. In recent years, the application of the computer-assisted navigation system and augmented reality have reformed imaging quality and interpretation. The endoscopic rhizotomy techniques have opened a new way for MISS of chronic low back pain. This review introduces the current indications of ESS and its potential future expansion.
Keywords: Endoscopic, Spine, Indications, Minimally invasive, Rhizotomy
INTRODUCTION
Nowadays, minimally invasive spine surgeries (MISS) have been a trend in modern spine surgeries. The common goal of spinal surgeries is to improve the quality of life by relieving pain and restoring functional disability. Furthermore, MISS is devoted to the issue of enhanced recovery after surgery. As for MISS, endoscopic spine surgery (ESS) has developed as an emerging alternative in the recent 2 decades. The ESS is superior to conventional surgery in less soft tissue damage, reduced blood loss, lower complication rates [1], decreased damage to the epidural blood supply and consequent epidural fibrosis, shorter hospital stays, and shorter time to return to work [2-6]. In the 1990s, Kambin [7] reported a posterolateral approach for percutaneous lumbar discectomy with the assistance of the arthroscope. As technology has advanced, the endoscope with a working channel has evolved with different systems designed for various approaches [8]. The evolution of endoscopic equipment and techniques has expanded the indications of ESS [9,10]. Therefore, evidence of ESS has multiplied to catch more attention from spine surgeons worldwide.
The initial stage of ESS was to treat herniated intervertebral disc (HIVD) at the lumbar spine through the natural orifices, such as the intervertebral foramen or interlaminar window. Transforaminal and interlaminar approaches are the basis of the endoscopic techniques to remove HIVDs through the above 2 anatomical structures. However, at the initial stage, a full-endoscopic discectomy was mainly for nonmigrated or low-grade migrated disc herniation due to the limitation of the bony structures. Thus, the advent of the endoscopic burr and bone reamer was the game changer. Pioneers of ESS applied the endoscopic burr to conduct foraminoplasty or laminotomy to increase the space for entering the spinal canal [11]. The foraminoplasty can expand the working space of the transforaminal trajectory by widening the intervertebral foramen. Likewise, the interlaminar window can be enlarged by laminotomy to reach the target lesion. Following these modified full-endoscopic techniques, indications of ESS have expanded to all kinds of decompressive surgeries from lumbar to thoracic and cervical spines.
For the past 30 years, various endoscopic spinal procedures have been developed to solve the degenerative disease of the spine. Some case reports also demonstrated the effectiveness and safety of endoscopic procedures for infection or neoplasm of the spine. In 2020, the AOSpine MISS task force published a consensus regarding the nomenclature of endoscopic spinal procedures [12]. The consensus nomenclature summarized the current endoscopic procedures and classification based on the regions and techniques. In recent years, full-endoscopic techniques have been applied to treat various spinal diseases. The authors will give an overview of the current application and upcoming expansion of ESS in the article.
CURRENT INDICATIONS FOR ENDOSCOPIC SPINAL SURGERY
The location and level of the target lesion are essential factors in deciding the surgical approaches. The surgical anatomies at different spinal levels determine the ideal trajectory during the endoscopic approach. The cervical foramen is too narrow to allow the endoscope to pass through. Besides, critical arteries are located in the posterior aspect of the cervical intervertebral foramen and may be vulnerable to injury during the transforaminal approach. Therefore, the ESS is a mainly posterior or anterior approach at the cervical spine. At the thoracic spine level, the scapula may block the transforaminal route to the upper thoracic spine (T2 to T7 or T9) [13]. The thoracic cage and scapula will limit the posterolateral inclination of the endoscope during the transforaminal approach. Moreover, the imbricated thoracic lamina and a lack of a proper interlaminar window make the thoracic interlaminar endoscopic approach challenging. Therefore, modified techniques of ESS are necessary for the different target spinal levels.
The essential goal of the ESS is the decompression of neural structures that results from different pathologies. The evolution of ESS has expanded the indications to cervical and thoracic spine surgeries. Hence, the indication of ESS may be limited by the etiologies of spinal disease, and degenerative spinal diseases remain the most common indications for ESS. The decompression of neural structures by ESS can remove the pathologies, including HIVD, hypertrophic ligamentum flavum, facet joint cyst, overgrown facet joint, and osteophyte from subaxial cervical to the lumbar spine. The ESS can preserve collateral soft tissues and structures and avoid iatrogenic instability after an operation.
Another ideal indication for ESS is an infectious spinal disease, such as discitis with or without an epidural abscess. Patients who sustain infectious spondylodiscitis might have moderate to severe comorbidities or a high risk of surgeries, such as advanced age, immunocompromised, or unstable hemodynamics. Therefore, ESS has been applied to treat infectious spondylodiscitis with full-endoscopic discectomy, debridement, and drainage for infection control and restore neurological function. The endoscopic approach is beneficial to minimize surgical and anesthetic risks when it obtains causative organisms and directly decompresses the nerves by debridement and drainage of epidural abscess. The copious saline irrigation also decreases bacterial burden simultaneously.
The application of ESS in a spinal tumor is limited and challenging, especially in extensive, highly vascularized, or intradural tumors. Sharp and bimanual dissection of the tissue plane between the tumor and normal tissue is essential during microsurgery. The dissecting technique requires 2-hand cooperation. However, endoscopic surgery, either uniportal or biportal, is challenging in tumor dissection. Besides, hemostasis under endoscopic visualization can be difficult in highly vascularized tumors. Therefore, tumor biopsy for pathologic diagnosis or epidural tumor removal may be feasible by endoscopic approach in selected patients.
1. Indications of ESS for Lumbar Spine
Lumbar spinal diseases are usually suitable for ESS with different approaches. Endoscopic lumbar discectomy has been a standard MISS for all herniation types (Table 1). The transforaminal approach could be the first choice from L1 to L5, regardless of the disc location. At the L5-S1 level, the transforaminal approach can be restrictive by the high-iliac crest and narrowed foraminal area that results from a large L5 transverse process or hypertrophic facet joint [14,15]. Foraminoplasty might be necessary for the situation or a highly migrated disc at other levels [16]. Recently, Chen et al. [17] proposed a suprapedicular retrocorporeal technique to solve the highly downward migrated disc. The interlaminar window is wider at the caudal level of the lumbar spine. Therefore, the interlaminar endoscopic approach is also an alternative at the L5-S1 level or for the highly down-migrated or axillary-type lumbar disc at the rostral levels [18]. Endoscopic lumbar discectomy can be feasible in recurrent cases by experienced surgeons. The previous study demonstrated comparable clinical outcomes compared with microdiscectomy [19].
Table 1.
Indications for lumbar full-endoscopic spinal surgery
| Herniated intervertebral disc |
| Central |
| Paramedian |
| Foraminal |
| Extraforaminal |
| Migrated disc |
| Lumbar spinal stenosis |
| Lateral recess stenosis |
| Central canal stenosis |
| Ossification of ligamentum flavum |
| Foraminal stenosis |
| Infective spondylodiscitis |
| Pyogenic discitis |
| Epidural abscess |
| Revision surgery |
| Recurrent disc herniation |
| Cage displacement |
| Bone cement leakage into canal or foramen |
| Spondylolisthesis (≤grade 2) |
The endoscopic burr and bone reamer have brought ESS to a new era for treating lumbar spinal stenosis. For central canal or lateral recess stenosis, endoscopic surgeons can unilaterally decompress the thecal sac and traversing roots through an interlaminar approach. Complex pathologies, such as combined HIVD and spinal stenosis, can also be treated by full-endoscopic surgery [20]. The bilateral decompression of central and lateral recess stenosis is feasible by the “over-the-top” technique [21]. Recent studies showed comparable outcomes but fewer complications and shorter hospital stay after endoscopic surgeries [4,22,23]. One study reported that full-endoscopic decompression was effective for lumbar spinal stenosis with low-grade fixed spondylolisthesis (≥ 3 mm without motion translation on the dynamic radiography) or mild-to-moderate scoliosis(≥ 10° coronal Cobb angle) [24]. The functional outcome was better in the endoscopic group without a higher risk of revision for fusion in the early postoperative period. However, further studies for long-term outcomes are necessary.
Foraminal stenosis is a common pathology at the advanced stage of lumbar degeneration. Traditionally, decompression of intervertebral foramen has a risk of iatrogenic instability due to injury to the facet joint. Therefore, fusion surgery is usually indicated in the scenario. With the advent of ESS, transforaminal endoscopic lumbar foraminotomy has been studied. The preliminary studies showed favorable outcomes at 1-year follow-up [25,26]. For experienced surgeon, the technqiue can be useful for some iatrogenic problems. The previous studies have reported successful treatment of lumbar interbody cage migration after fusion surgery and intraspinal cement leakage after vertebroplasty by full-endoscopic decompression [27,28]. The minimally invasive revision can decompress the nerves without reopen in selected patients.
Full-endoscopic debridement and drainage are an alternative for infectious spondylitis, especially in pyogenic discitis or epidural abscess. Patients with pyogenic spondylitis usually have comorbidities causing poor constitutional factors or compromised immune, which preclude them from being candidates for surgical debridement [29]. Therefore, there are several benefits of full-endoscopic procedures for these patients. First, endoscopic debridement under local anesthesia can treat patients with high anesthetic risks. Second, the small incision and target-oriented approach avoid the physiological burden and iatrogenic injury to spinal structures during the operation. Third, continuous saline irrigation can significantly decrease the bacterial load of surgical sites. Effective spinal epidural abscess treatment is composed of identifying definite pathogens and adequate abscess evacuation. A previous study also reported that positive rates of bacterial culture were higher with percutaneous endoscopy than with computed tomography (CT)-guided biopsy (90% vs. 47%) [30]. Therefore, endoscopic debridement and drainage are beneficial for local control by drainage abscess and systemic control by identifying sensitive antibiotics for specific pathogens.
The endoscopy-assisted lumbar fusion surgery has been developing in recent years. Some pilot studies showed favorable outcomes in endoscopy-assisted transforaminal or posterior lumbar interbody fusion. For complex operations, prognostic factors are multiple, and there are diverse endoscopic and implant systems protocols. Many confounders, such as cage design (static or expandable), cage material, bone graft substitute, use of bone morphogenic protein, biomechanical profile, or endplate status, can affect the outcomes. The endoscopy-assisted lumbar interbody fusion techniques can minimize injuries to collateral soft tissue and endplate of vertebrae, which enhances postoperative recovery and shortens the hospital stay [31-33].
Most importantly, awake surgery can be feasible with full-endoscopic lumbar interbody fusion technique [34]. However, endoscopic fusion surgeries are developing, and the ideal or newly designed instrument systems are on the way. The pilot studies mainly enrolled short segments of disease with low-grade spondylolisthesis. New technologies and further high-quality researches are necessary for the emerging application.
2. Indications of ESS for Thoracic Spine
Thoracic spine surgeries comprise less than 10% of spine surgeries [35]. Thoracic HIVD and spinal stenosis are possible etiologies for ESS. The epidemiologic study of thoracic spinal stenosis showed that ossification of ligamentum flavum (OLF) was the most common etiology and accounted for 41.5% of the cases, while 32.4% and 18.7% were diagnosed with thoracic HIVD and ossification of the posterior longitudinal ligament (OPLL), respectively [36]. Although the incidence of thoracic HIVD ranges from 7% to 37%, less than 1% of all TDH are symptomatic [37,38].
The indication of ESS for thoracic HIVD is the soft disc causing radiculopathy or myelopathy (Table 2). The paramedian or foraminal type of thoracic HIVD can be reached by interlaminar or translaminar approach. If the disc herniation is located at the central portion, a transforaminal approach with foraminoplasty or a transthoracic retropleural approach can be an alternative to remove the lesion [39,40]. However, the transthoracic retropleural approach is limited above the T5 level due to scapula or risks of injury to major vessels, such as the azygos vein or aorta, according to a cadaveric study [41]. Fortunately, thoracic HIVD is more common in the middle and lower levels of the thoracic spine. Besides, the transforaminal approach with foraminoplasty remains feasible at the upper thoracic HIVD in experienced hand [40]. The calcified or hard disc and OPLL are relative contraindications to the endoscopic approach, and the thoracoscopic approach may be an alternative. Thoracic spinal stenosis due to OLF can cause myelopathy and is indicated to be endoscopic decompression. The technique of unilateral laminotomy for bilateral decompression (ULBD) helps decompress the thoracic cord safely [42,43].
Table 2.
Common indications for thoracic full-endoscopic spinal surgery
| Herniated intervertebral disc: soft disc |
| Central |
| Paramedian |
| Migrated disc |
| Thoracic spinal stenosis |
| Central canal stenosis |
| Ossification of ligamentum flavum |
| Ossification of posterior longitudinal ligament |
| Infective spondylodiscitis |
| Pyogenic discitis |
| Epidural abscess |
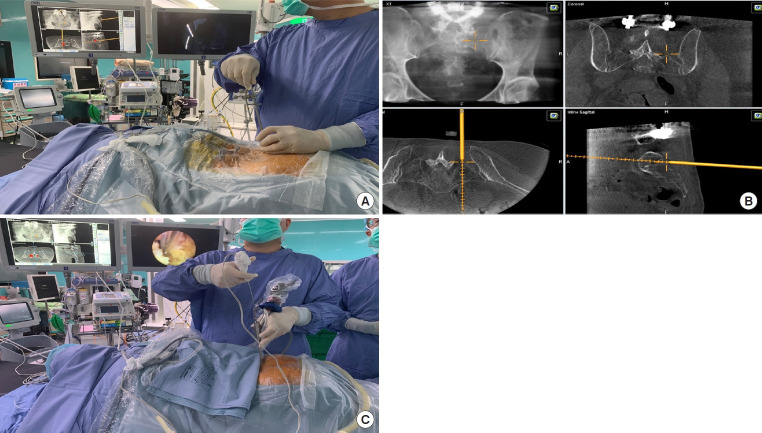
Intraoperative localization is a critical issue while conducting thoracic spine surgeries. C-arm fluoroscopy is the most common modality to localize the index level during operation. However, the thoracic cage and scapula might affect the visualization and confuse the interpretation of the intraoperative fluoroscopy. Recently, the integration of an intraoperative navigation system with endoscopic surgeries has been reported. The intraoperative scan of CT can quickly identify the index level (Fig. 1). Besides, computer-assisted navigation can guide the instruments in real-time during the operation without interruption for repeated scans. The evolution of imaging modality can help surgeons overcome the learning curve of thoracic ESS easily and safely.
Fig. 1.
The application of intraoperative navigation in full-endoscopic thoracic spine surgery. (A) Intraoperative computed tomography scan for localization and intraoperative neuronavigation. (B) The surgeon can localize the target and confirm real-time orientation during the operation.
3. Indications of ESS for Cervical Spine
Cervical HIVD, foraminal stenosis, and central canal stenosis are common indications for ESS (Table 3). The endoscopic approaches at the cervical spine are the anterior or posterior approach. As for cervical HIVD, conventional anterior cervical discectomy through the areolar plane between the esophagus and carotid artery results in minimal muscle trauma, and the risk of injuries to vessels and esophagus is low. Besides, there has been robust evidence of cervical arthroplasty showing favorable outcomes with artificial disc replacement [44]. Therefore, anterior endoscopic cervical discectomy (AECD) is usually considered when patients with a high risk of general anesthesia have cervical myelopathy or radiculopathy caused by soft disc herniation. The preliminary series showed comparable outcomes comparing the conventional anterior cervical discectomy with fusion [45]. The operative time, hospital stay, and time to return to work were shorter in the AECD group in a prospective cohort [46]. Patients having a calcified or hard disc, severe spondylosis with decreased intervertebral space (< 5 mm), OPLL, or spondylolisthesis with instability were not ideal candidates for AECD. Besides, When the disc herniation is in the paramedian or foraminal region, posterior endoscopic cervical discectomy or posterior endoscopic cervical foraminotomy (PECF) is a better solution to avoid fusion surgery and worsen disc degeneration. The endoscopic approach for the migrated disc in the cervical spine might be challenging because it is risky to retract the dural sac to reach the sequestrated fragment. Some recent reports proposed an anterior transcorporeal technique or posterior retrocorporeal technique to reach migrated fragments safely. However, the modified techniques are difficult for inexperienced surgeons. Further studies enrolling more cases with long-term results are necessary to evaluate the effectiveness and safety of these advanced techniques.
Table 3.
Indications for cervical full-endoscopic spinal surgery
| Herniated intervertebral disc: soft disc |
| Central |
| Paramedian |
| Migrated disc |
| Stand-alone cage fusion (single or 2 levels) |
| Cervical spinal stenosis |
| Foraminal stenosis with radiculopathy |
| Central canal stenosis with myelopathy |
| Ossification of ligamentum flavum |
| Ossification of posterior longitudinal ligament |
| Infective spondylodiscitis |
| Pyogenic discitis |
| Epidural abscess |
Foraminal stenosis with radiculopathy due to facet joint hypertrophy or osteophytes is an indication of PECF. PECF can decompress the existing root at the index level and preserve stability. Meanwhile, the herniated disc in the lateral canal or foraminal region can be removed. When patients present neck pain or myelopathy with OPLL or OLF causing central canal stenosis, PECF is not an ideal solution in such circumstances. Besides, patients with preoperative cervical kyphosis are not suitable for PECF.
Cervical spinal stenosis can result from hyperlordosis, shingling, and arthrosis with hypertrophic ligamentum flavum [47]. Myelopathy due to dural sac compression is usually the introductory presentation. The pathologies may include the combination of structures surrounding the spinal canal. The cervical spinal stenosis with myelopathy due to infolding of ligamentum flavum is an excellent indication for cervical endoscopic ULBD. For cervical OPLL with less than 50% canal occupancy and without significant kyphosis, posterior endoscopic decompression can be an alternative. A single incision can be used up to 3-level decompression. However, the cervical spinal cord is more vulnerable to water pressure and excessive manipulation. Besides, multilevel decompression for the endoscopic approach is timeconsuming and physically challenging for beginners. The evidence of cervical endoscopic ULBD for treating cervical spinal stenosis with myelopathy is insufficient. The decision-making depends on the patient’s factor and the surgeon’s experience. Further studies on the learning curve are necessary.
Full-endoscopic anterior cervical discectomy and fusion (ACDF) is an alternative for conventional ACDF. The outcomes are comparable according to small case series [48]. However, the full-endoscopic approach can only achieve stand-alone cage fusion, and there is a lack of a locking plate system for endoscopic surgery. Therefore, the full-endoscopic ACDF may be feasible in single- or 2-level disease without subluxation. Though ACDF has been the gold standard for cervical HIVD, arthroplasty with artificial disc replacement has rapidly risen in the recent decade [49]. The evidence supporting cervical arthroplasty has accumulated to change the trend of treatment for cervical HIVD. The fullendoscopic ACDF is still developing and lacks evidence. Newly designed implants suitable for endoscopic approaches are necessary to accentuate the advantages of full-endoscopic ACDF.
POTENTIAL FOR FUTURE EXPANSION
In recent years, endoscopic lumbar interbody fusion has been developed and studied. The conventional design of the static cage for lumbar interbody fusion is not suitable for passing through the endoscope’s working channel. Therefore, modification of the implant designs or endoscopic instruments is mandatory to overcome the limit. The customized expandable interbody device has been available for endoscopic fusion systems currently. Wang et al. [50] reported the technique of full-endoscopic transforaminal lumbar interbody fusion in awake patients. The preliminary outcomes of 100 patients with a minimum 1-year follow-up were favorable without nonunion. Endoscopic fusion enhances recovery in the ambulatory surgery setting, and this innovation enables lumbar fusion surgeries for those unable to undergo general anesthesia.
Endoscopic rhizotomy (ER) for different chronic low back pain (CLBP) has been an emerging alternative in recent years. The facet joint is innervated by the medial branch of the dorsal ramus, and facet arthropathy is a usual pain generator for CLBP. Percutaneous radiofrequency ablation is a routine intervention to manage the facet-oriented CLBP refractory to medical treatment [51]. The percutaneous lesioning intervention effectively reduces pain by more than 50% in most patients. However, the duration of pain relief is 7.3–9.0 months on average after a single intervention [52]. The intervention is based on fluoroscopic guidance and the patient’s report to localize the lesioning targets. Nerve regrowth can cause pain relapse, and repeated procedures may be necessary. On the contrary, ER of the nerve branches can ensure the rhizotomy under endoscopic visualization. The outcomes of the ER for facet joint syndrome were also superior to conventional radiofrequency lesioning regarding durability in previous studies [53,54]. For failed back surgery syndrome responding to the facet joint block treatment, ER can provide long-term relief of CLBP after previous spinal instrumentation [55].
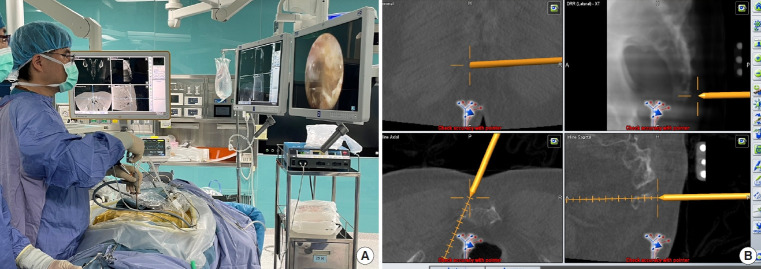
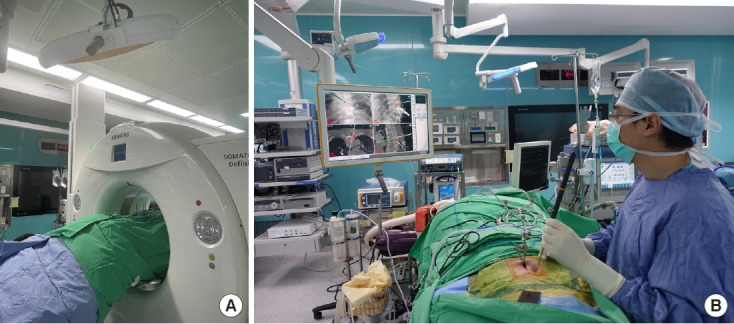
The ER is also effective in treating sacroiliac joint pain [56] or occipital neuralgia [57]. The preliminary study of endoscopic radiofrequency ablation of the sacroiliac joint complex revealed a favorable outcome with an 88.6% satisfaction rate in 17 patients during the 6-month follow-up [58]. Chen et al. [56] proposed a “cut-and-ablate” concept for the full-ER to ensure durable pain relief after the operation (Fig. 2). The preliminary study of the authors showed less relapsing pain during the 1-year follow-up in their technique compared to the cooled radiofrequency ablation treatment. However, the long-term outcomes remain further studies to prove which technique is better. Recently, the author also expands the indication of full-ER for coccydynia and the preliminary results were favorable (Fig. 3).
Fig. 2.
Navigation-guided full-endoscopic rhizotomy for sacroiliac joint pain treatment. (A) The endoscopic instrument with trackers guides the working sheath toward the target area. (B) The navigation screen shows the docking site of the working sheath. (C) Full-endoscopic rhizotomy of lateral branches of sacral dorsal ramus.
Fig. 3.
Navigation-guided full-endoscopic rhizotomy for coccydynia. (A) Endoscopic rhizotomy of the posterior ramus of the coccygeal nerve by radiofrequency ablation (Vantage Biotech Co., Ltd., Taoyuan, Taiwan). (B) Intraoperative navigation helps the localization of lesion sites.
Endoscopic surgery is seldom applied in treating neoplastic disease. The working space was narrowed, and it was challenging to manage brisk bleeding from the hypervascular tumor. Besides, bimanual dissection is not feasible with a full-endoscopic approach. Dural suture repair is technically demanding through the working channel of the uniportal endoscope [59]. Therefore, it may be feasible for the full-endoscopic approach to remove the extradural lesions, which are usually spinal metastasis with epidural invasion. Decompression of the neural structure by a full-endoscopic approach under local anesthesia has been reported in patients with radicular pain due to sacral metastasis [60]. For hypervascular tumors, transarterial embolization may help to control intraoperative bleeding [61]. The metastatic spinal tumors are usually extensive and unresectable. The goal of the surgery is to restore neurological function by separation of the tumor and dural sac for decompression.
As for intradural lesion, case report revealed that full-endoscopic approach may be a potential alternative for the resection of intradural extramedullary tumor [62] or the ligation of spinal dural arteriovenous fistula [63]. However, indications are limited to small size tumors without significant nerve roots or spinal cord compression. It is challenging to debulk and detatch the large-size tumor with full-endoscopic technique under limited visualization. Hybrid operation such as microscope-assisted endoscopic approach may be an alternative. Innovative tools or techniques are necessary to overcome the imperfection of full-endoscopic techniques in tumor dissection and dural repair.
CONCLUSION
Full-ESS is a diverse procedure with the evolution of instruments and the innovation of endoscopic techniques. Currently, ESS is suitable for the whole spine level. The indications of ESS have been expanded from discectomies to endoscopic fusion for lumbar degenerative disease. Full-ER for the denervation of branches of spinal nerves has been an emerging solution to treat CLBP or sacroiliac joint pain. The development of endoscopic tumor surgeries remains deficient due to its inherent limitation in instruments and dissection techniques. However, that does not influence the role of the ESS in the contemporary MISS. With innovative technologies and techniques, we look forward to breakthroughs in applying full-endoscopic spine systems for all kinds of spinal surgeries.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: JK, CC; Methodology: ML; Visualization: KC; Writing - original draft: KC, JK; Writing - review & editing: AH, ML, CC.
REFERENCES
- 1.Yang CC, Chen CM, Lin MH, et al. Complications of fullendoscopic lumbar discectomy versus open lumbar microdiscectomy: a systematic review and meta-analysis. World Neurosurg. 2022;168:333–48. doi: 10.1016/j.wneu.2022.06.023. [DOI] [PubMed] [Google Scholar]
- 2.Ruetten S, Komp M, Merk H, et al. Surgical treatment for lumbar lateral recess stenosis with the full-endoscopic interlaminar approach versus conventional microsurgical technique: a prospective, randomized, controlled study. J Neurosurg Spine. 2009;10:476–85. doi: 10.3171/2008.7.17634. [DOI] [PubMed] [Google Scholar]
- 3.Ruetten S, Komp M, Merk H, et al. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008;33:931–9. doi: 10.1097/BRS.0b013e31816c8af7. [DOI] [PubMed] [Google Scholar]
- 4.Komp M, Hahn P, Oezdemir S, et al. Bilateral spinal decompression of lumbar central stenosis with the full-endoscopic interlaminar versus microsurgical laminotomy technique: a prospective, randomized, controlled study. Pain Physician. 2015;18:61–70. [PubMed] [Google Scholar]
- 5.Lee DY, Shim CS, Ahn Y, et al. Comparison of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for recurrent disc herniation. J Korean Neurosurg Soc. 2009;46:515–21. doi: 10.3340/jkns.2009.46.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim MJ, Lee SH, Jung ES, et al. Targeted percutaneous transforaminal endoscopic diskectomy in 295 patients: comparison with results of microscopic diskectomy. Surg Neurol. 2007;68:623–31. doi: 10.1016/j.surneu.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 7.Kambin P. Arthroscopic microdiskectomy. Mt Sinai J Med. 1991;58:159–64. [PubMed] [Google Scholar]
- 8.Yeung AT, Tsou PM. Posterolateral endoscopic excision for lumbar disc herniation: surgical technique, outcome, and complications in 307 consecutive cases. Spine (Phila Pa 1976) 2002;27:722–31. doi: 10.1097/00007632-200204010-00009. [DOI] [PubMed] [Google Scholar]
- 9.Chen KT, Jabri H, Lokanath YK, et al. The evolution of interlaminar endoscopic spine surgery. J Spine Surg. 2020;6:502–12. doi: 10.21037/jss.2019.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khandge AV, Sharma SB, Kim JS. The evolution of transforaminal endoscopic spine surgery. World Neurosurg. 2021;145:643–56. doi: 10.1016/j.wneu.2020.08.096. [DOI] [PubMed] [Google Scholar]
- 11.Choi G, Lee SH, Lokhande P, et al. Percutaneous endoscopic approach for highly migrated intracanal disc herniations by foraminoplastic technique using rigid working channel endoscope. Spine (Phila Pa 1976) 2008;33:E508–15. doi: 10.1097/BRS.0b013e31817bfa1a. [DOI] [PubMed] [Google Scholar]
- 12.Hofstetter CP, Ahn Y, Choi G, et al. AOSpine Consensus Paper on nomenclature for working-channel endoscopic spinal procedures. Global Spine J. 2020;10(2 Suppl):111S–121S. doi: 10.1177/2192568219887364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haneline MT, Cooperstein R, Young MD, et al. Determining spinal level using the inferior angle of the scapula as a reference landmark: a retrospective analysis of 50 radiographs. J Can Chiropr Assoc. 2008;52:24–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Mirkovic SR, Schwartz DG, Glazier KD. Anatomic considerations in lumbar posterolateral percutaneous procedures. Spine (Phila Pa 1976) 1995;20:1965–71. doi: 10.1097/00007632-199509150-00001. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Kang HS, Choi G, et al. Foraminoplastic ventral epidural approach for removal of extruded herniated fragment at the L5-S1 level. Neurol Med Chir (Tokyo) 2010;50:1074–8. doi: 10.2176/nmc.50.1074. [DOI] [PubMed] [Google Scholar]
- 16.Chen KT, Wei ST, Tseng C, et al. Transforaminal endoscopic lumbar discectomy for L5–S1 disc herniation with high iliac crest: technical note and preliminary series. Neurospine. 2020;17(Suppl 1):S81–7. doi: 10.14245/ns.2040166.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CM, Lin GX, Sharma S, et al. Suprapedicular retrocorporeal technique of transforaminal full-endoscopic lumbar discectomy for highly downward-migrated disc herniation. World Neurosurg. 2020;143:e631–9. doi: 10.1016/j.wneu.2020.08.038. [DOI] [PubMed] [Google Scholar]
- 18.Choi KC, Kim JS, Ryu KS, et al. Percutaneous endoscopic lumbar discectomy for L5-S1 disc herniation: transforaminal versus interlaminar approach. Pain Physician. 2013;16:547–56. [PubMed] [Google Scholar]
- 19.Ruetten S, Komp M, Merk H, et al. Recurrent lumbar disc herniation after conventional discectomy: a prospective, randomized study comparing full-endoscopic interlaminar and transforaminal versus microsurgical revision. J Spinal Disord Tech. 2009;22:122–9. doi: 10.1097/BSD.0b013e318175ddb4. [DOI] [PubMed] [Google Scholar]
- 20.Chen KT, Choi KC, Song MS, et al. Hybrid interlaminar endoscopic lumbar decompression in disc herniation combined with spinal stenosis. Oper Neurosurg (Hagerstown) 2021;20:E168–74. doi: 10.1093/ons/opaa360. [DOI] [PubMed] [Google Scholar]
- 21.Komp M, Hahn P, Merk H, et al. Bilateral operation of lumbar degenerative central spinal stenosis in full-endoscopic interlaminar technique with unilateral approach: prospective 2-year results of 74 patients. J Spinal Disord Tech. 2011;24:281–7. doi: 10.1097/BSD.0b013e3181f9f55e. [DOI] [PubMed] [Google Scholar]
- 22.Chen KT, Choi KC, Shim HK, et al. Full-endoscopic versus microscopic unilateral laminotomy for bilateral decompression of lumbar spinal stenosis at L4-L5: comparative study. Int Orthop. 2022;46:2887–95. doi: 10.1007/s00264-022-05549-0. [DOI] [PubMed] [Google Scholar]
- 23.McGrath LB, White-Dzuro GA, Hofstetter CP. Comparison of clinical outcomes following minimally invasive or lumbar endoscopic unilateral laminotomy for bilateral decompression. J Neurosurg Spine. 2019 Jan;11:1–9. doi: 10.3171/2018.9.SPINE18689. doi: . [Epub] [DOI] [PubMed] [Google Scholar]
- 24.Hasan S, McGrath LB, Sen RD, et al. Comparison of full-endoscopic and minimally invasive decompression for lumbar spinal stenosis in the setting of degenerative scoliosis and spondylolisthesis. Neurosurg Focus. 2019;46:E16. doi: 10.3171/2019.2.FOCUS195. [DOI] [PubMed] [Google Scholar]
- 25.Evins AI, Banu MA, Njoku I, Jr, et al. Endoscopic lumbar foraminotomy. J Clin Neurosci. 2015;22:730–4. doi: 10.1016/j.jocn.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Ahn Y, Park HB, Yoo BR, et al. Endoscopic lumbar foraminotomy for foraminal stenosis in stable spondylolisthesis. Front Surg. 2022;9:1042184. doi: 10.3389/fsurg.2022.1042184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Telfeian AE, Oyelese A, Fridley J, et al. Transforaminal endoscopic surgical treatment for posterior migration of polyetheretherketone transforaminal lumbar interbody fusion cage: case series. World Neurosurg. 2021;147:e437–43. doi: 10.1016/j.wneu.2020.12.080. [DOI] [PubMed] [Google Scholar]
- 28.Chu L, Yang JS, Yu KX, et al. Percutaneous endoscopic retrieval of intraspinal cement leakage: technical note. World Neurosurg. 2018;118:150–5. doi: 10.1016/j.wneu.2018.07.044. [DOI] [PubMed] [Google Scholar]
- 29.Lin GX, Kim JS, Sharma S, et al. Full endoscopic discectomy, debridement, and drainage for high-risk patients with spondylodiscitis. World Neurosurg. 2019;127:e202–11. doi: 10.1016/j.wneu.2019.02.206. [DOI] [PubMed] [Google Scholar]
- 30.Yang SC, Fu TS, Chen LH, et al. Identifying pathogens of spondylodiscitis: percutaneous endoscopy or CT-guided biopsy. Clin Orthop Relat Res. 2008;466:3086–92. doi: 10.1007/s11999-008-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He LM, Chen KT, Chen CM, et al. Comparison of percutaneous endoscopic and open posterior lumbar interbody fusion for the treatment of single-segmental lumbar degenerative diseases. BMC Musculoskelet Disord. 2022;23:329. doi: 10.1186/s12891-022-05287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HS, Wu PH, Sairyo K, et al. A narrative review of uniportal endoscopic lumbar interbody fusion: comparison of uniportal facet-preserving trans-kambin endoscopic fusion and uniportal facet-sacrificing posterolateral transforaminal lumbar interbody fusion. Int J Spine Surg. 2021;15(Suppl 3):S72–83. doi: 10.14444/8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgenstern C, Yue JJ, Morgenstern R. Full percutaneous transforaminal lumbar interbody fusion using the facet-sparing, Trans-Kambin approach. Clin Spine Surg. 2020;33:40–5. doi: 10.1097/BSD.0000000000000827. [DOI] [PubMed] [Google Scholar]
- 34.Butler AJ, Brusko GD, Wang MY. Awake endoscopic transforaminal lumbar interbody fusion: a technical note. HSS J. 2020;16:200–4. doi: 10.1007/s11420-020-09748-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi K, Ando K, Nishida Y, et al. Epidemiological trends in spine surgery over 10 years in a multicenter database. Eur Spine J. 2018;27:1698–703. doi: 10.1007/s00586-018-5513-4. [DOI] [PubMed] [Google Scholar]
- 36.Chen G, Fan T, Yang X, et al. The prevalence and clinical characteristics of thoracic spinal stenosis: a systematic review. Eur Spine J. 2020;29:2164–72. doi: 10.1007/s00586-020-06520-6. [DOI] [PubMed] [Google Scholar]
- 37.Carson J, Gumpert J, Jefferson A. Diagnosis and treatment of thoracic intervertebral disc protrusions. J Neurol Neurosurg Psychiatry. 1971;34:68–77. doi: 10.1136/jnnp.34.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stillerman CB, Chen TC, Couldwell WT, et al. Experience in the surgical management of 82 symptomatic herniated thoracic discs and review of the literature. J Neurosurg. 1998;88:623–33. doi: 10.3171/jns.1998.88.4.0623. [DOI] [PubMed] [Google Scholar]
- 39.Ruetten S, Hahn P, Oezdemir S, et al. Full-endoscopic uniportal decompression in disc herniations and stenosis of the thoracic spine using the interlaminar, extraforaminal, or transthoracic retropleural approach. J Neurosurg Spine. 2018;29:157–68. doi: 10.3171/2017.12.SPINE171096. [DOI] [PubMed] [Google Scholar]
- 40.Bae J, Chachan S, Shin SH, et al. Transforaminal endoscopic thoracic discectomy with foraminoplasty for the treatment of thoracic disc herniation. J Spine Surg. 2020;6:397–404. doi: 10.21037/jss.2019.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruetten S, Hahn P, Oezdemir S, et al. Decompression of the anterior thoracic spinal canal using a novel full-endoscopic uniportal transthoracic retropleural technique-an anatomical feasibility study in human cadavers. Clin Anat. 2018;31:716–23. doi: 10.1002/ca.23075. [DOI] [PubMed] [Google Scholar]
- 42.Kotheeranurak V, Pholprajug P, Lin GX, et al. Full-endoscopic decompression for thoracic myelopathy caused by ossification of the ligamentum flavum: patient series. J Neurosurg Case Lessons. 2021;1:CASE20138. doi: 10.3171/CASE20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miao X, He D, Wu T, et al. Percutaneous endoscopic spine minimally invasive technique for decompression therapy of thoracic myelopathy caused by ossification of the ligamentum flavum. World Neurosurg. 2018;114:8–12. doi: 10.1016/j.wneu.2018.02.152. [DOI] [PubMed] [Google Scholar]
- 44.Peng Z, Hong Y, Meng Y, et al. A meta-analysis comparing the short- and mid- to long-term outcomes of artificial cervical disc replacement (ACDR) with anterior cervical discectomy and fusion (ACDF) for the treatment of cervical degenerative disc disease. Int Orthop. 2022;46:1609–25. doi: 10.1007/s00264-022-05318-z. [DOI] [PubMed] [Google Scholar]
- 45.Ruetten S, Komp M, Merk H, et al. Full-endoscopic anterior decompression versus conventional anterior decompression and fusion in cervical disc herniations. Int Orthop. 2009;33:1677–82. doi: 10.1007/s00264-008-0684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahn Y, Keum HJ, Shin SH. Percutaneous endoscopic cervical discectomy versus anterior cervical discectomy and fusion: a comparative cohort study with a five-year follow-up. J Clin Med. 2020;9:371. doi: 10.3390/jcm9020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Epstein JA. The surgical management of cervical spinal stenosis, spondylosis, and myeloradiculopathy by means of the posterior approach. Spine (Phila Pa 1976) 1988;13:864–9. doi: 10.1097/00007632-198807000-00031. [DOI] [PubMed] [Google Scholar]
- 48.Yao N, Wang C, Wang W, et al. Full-endoscopic technique for anterior cervical discectomy and interbody fusion: 5-year follow-up results of 67 cases. Eur Spine J. 2011;20:899–904. doi: 10.1007/s00586-010-1642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang CJ, Liu YF, Hsiao YM, et al. Comparison of anterior cervical discectomy and fusion versus artificial disc replacement for cervical spondylotic myelopathy: a meta-analysis. J Neurosurg Spine . 2012 Apr;22:1–10. doi: 10.3171/2022.2.SPINE211500. doi: 10.3171/2022.2.SPI NE211500. [Epub] [DOI] [PubMed] [Google Scholar]
- 50.Kolcun JPG, Brusko GD, Basil GW, et al. Endoscopic transforaminal lumbar interbody fusion without general anesthesia: operative and clinical outcomes in 100 consecutive patients with a minimum 1-year follow-up. Neurosurg Focus. 2019;46:E14. doi: 10.3171/2018.12.FOCUS18701. [DOI] [PubMed] [Google Scholar]
- 51.Du R, Xu G, Bai X, et al. Facet joint syndrome: pathophysiology, diagnosis, and treatment. J Pain Res. 2022;15:3689–710. doi: 10.2147/JPR.S389602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smuck M, Crisostomo RA, Trivedi K, et al. Success of initial and repeated medial branch neurotomy for zygapophysial joint pain: a systematic review. PM R. 2012;4:686–92. doi: 10.1016/j.pmrj.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Du T, Lu G, Li J, et al. Pain-free survival after endoscopic rhizotomy versus radiofrequency for lumbar facet joint pain: a real-world comparison study. Pain Physician. 2022;25:E87–94. [PubMed] [Google Scholar]
- 54.Xue Y, Ding T, Wang D, et al. Endoscopic rhizotomy for chronic lumbar zygapophysial joint pain. J Orthop Surg Res. 2020;15:4. doi: 10.1186/s13018-019-1533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang SJ, Hsiao MC, Lee JH, et al. How I do it? Full endoscopic lumbar rhizotomy for chronic facet joint pain due to failed back surgery syndrome. Acta Neurochir (Wien) 2022;164:1233–7. doi: 10.1007/s00701-021-05042-4. [DOI] [PubMed] [Google Scholar]
- 56.Lee JH, Chen KT, Chang KS, et al. How I do it? Fully endoscopic rhizotomy assisted with three-dimensional robotic C-arm navigation for sacroiliac joint pain. Acta Neurochir (Wien) 2021;163:3297–301. doi: 10.1007/s00701-020-04682-2. [DOI] [PubMed] [Google Scholar]
- 57.Yu Y, Jiang Y, Xu F, et al. Percutaneous full-endoscopic C2 ganglionectomy for the treatment of intractable occipital neuralgia: technical note. Oper Neurosurg (Hagerstown) 2021;21:E472–8. doi: 10.1093/ons/opab228. [DOI] [PubMed] [Google Scholar]
- 58.Choi WS, Kim JS, Ryu KS, et al. Endoscopic radiofrequency ablation of the sacroiliac joint complex in the treatment of chronic low back pain: a preliminary study of feasibility and efficacy of a novel technique. Biomed Res Int. 2016;2016:2834259. doi: 10.1155/2016/2834259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin JK, Youn MS, Seong YJ, et al. Iatrogenic dural tear in endoscopic lumbar spinal surgery: full endoscopic dural suture repair (Youn's technique) Eur Spine J. 2018;27(Suppl 3):544–8. doi: 10.1007/s00586-018-5637-6. [DOI] [PubMed] [Google Scholar]
- 60.Tsai SH, Wu HH, Cheng CY, et al. Full endoscopic interlaminar approach for nerve root decompression of sacral metastatic tumor. World Neurosurg. 2018;112:57–63. doi: 10.1016/j.wneu.2018.01.075. [DOI] [PubMed] [Google Scholar]
- 61.Şentürk S, Ünsal ÜÜ. Percutaneous endoscopic interlaminar decompression of hypervascular spinal metastases. World Neurosurg. 2020;134:182–6. doi: 10.1016/j.wneu.2019.10.175. [DOI] [PubMed] [Google Scholar]
- 62.Şentürk S, Ünsal ÜÜ. Percutaneous full-endoscopic removal of lumbar intradural extramedullary tumor via translaminar approach. World Neurosurg. 2019;125:146–9. doi: 10.1016/j.wneu.2019.01.206. [DOI] [PubMed] [Google Scholar]
- 63.Wang C, Chen CM, Shen F, et al. Microscope-assisted endoscopic interlaminar ligation of spinal arteriovenous fistulas: technical note. J Neurosurg Spine. 2016;25:394–7. doi: 10.3171/2015.12.SPINE15366. [DOI] [PubMed] [Google Scholar]